DOI:
10.1039/C5RA09812B
(Paper)
RSC Adv., 2015,
5, 72132-72141
Bile salt-surface active ionic liquid mixtures: mixed micellization and solubilization of phenothiazine†
Received
25th May 2015
, Accepted 17th August 2015
First published on 18th August 2015
Abstract
The present work deals with the mixed micellization behavior of bile salts (sodium cholate, NaC and sodium deoxycholate, NaDC) with surface active imidazolium ionic liquid, (SAIL) 1-dodecyl-3-methylimidazolium bromide (C12mimBr). Surface tension and steady state fluorescence measurements were employed to gain a detailed picture of the interactions between NaC/NaDC and C12mimBr molecules in the mixed monolayer and in the mixed micelles, where the interactions have been found to be highly synergistic. The bile salt–SAIL mixtures have been thoroughly characterized through the evaluation of various micellar and interfacial parameters like critical micellar concentration (cmc), micellar interaction parameter (βm), surface excess concentration (Гmax), minimum area per molecule (Amin), surface pressure at cmc (πcmc) and hydrodynamic radius (Rh). Dynamic light scattering (DLS) measurements revealed the formation of the biggest mixed micelles at a mole fraction of 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 of NaC/NaDC
0.6 of NaC/NaDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C12mimBr. Furthermore the solubilization of phenothiazine (Pz) has been studied in pure bile salts as well as in mixed micelles of bile salts + C12mimBr employing UV-visible measurements. From the evaluation of various solubilization parameters viz. molar solubilization ratio (MSR, χ), micelle-water partition coefficient (Km) and standard free energy of solubilization (ΔG°s), it was established that the solubilization capacity of bile salts for Pz were enhanced when mixed with C12mimBr, the effect being more pronounced for NaDC than NaC.
C12mimBr. Furthermore the solubilization of phenothiazine (Pz) has been studied in pure bile salts as well as in mixed micelles of bile salts + C12mimBr employing UV-visible measurements. From the evaluation of various solubilization parameters viz. molar solubilization ratio (MSR, χ), micelle-water partition coefficient (Km) and standard free energy of solubilization (ΔG°s), it was established that the solubilization capacity of bile salts for Pz were enhanced when mixed with C12mimBr, the effect being more pronounced for NaDC than NaC.
1. Introduction
Phenothiazine, a tricyclic aromatic compound, consists of a central six membered ring bearing sulfur and nitrogen atoms and is flanked by two benzene rings on either side. Its derivatives commonly substituted in the 2 or 10 position are largely employed as tranquilizers and neuroleptics in psychiatry.1 But a major limitation of its poor solubility in water largely restricts its use in various applications involving aqueous media. Thus enhancing the solubility of a basic phenothiazine moiety will not only increase the oral absorption and bioavailability of many phenothiazine based drugs but also will be beneficial in several other research areas e.g. as reagents for chemical analysis, in the formation of ion-association complexes etc.
Solubilization of poorly soluble drugs using surface active agents has been more popular among other methods viz. formation of water soluble salts, micronization of drug particles, addition of solubilizing agents, enhancing the wettability of drug powder etc.2 Surfactants generally form micelles above their critical micellar concentrations (cmc), which provides a hydrophobic environment for the solubilization of these poorly water soluble solutes.3–6 Along with hydrophobic environment, factors such as temperature, structure and size of solubilizer (surfactant) as well as solubilizate, aggregation number and geometry of the micelles play an important role in the solubilization process.7 Various reports regarding the solubilization of poorly water soluble compounds such as aromatic hydrocarbons and drugs in various cationic, anionic and nonionic surfactants have been earlier published.8–13 However synergism in mixed surfactant systems generally improves the micellar properties of their pure counterparts and thus are expected to play a better role in the solubilization process too.14–16
With this aim, we chose to investigate the solubilization of phenothiazine in mixed micellar media comprising of bile salts viz. sodium cholate (NaC) and sodium deoxycholate (NaDC) and surface active ionic liquid (SAIL), 1-dodecyl-3-methylimidazolium bromide (C12mimBr). SAILs possess inherent amphiphilicity, are greener have antimicrobial activity, low toxicity, enhanced solubility in various media and better surface behavior than conventional surfactants and thus recently replacing them in many research areas.17–24 Bile salts are well known, important biological surfactants, produced in the liver from cholesterol and play an important role in emulsification of lipids, fats, fats droplets or fat soluble vitamins in the body for they help in the absorption of lipids and cholesterol molecules in the body.25,26 These are also amphiphilic having a hydrophobic steroid nucleus and hydrophilic hydroxyl groups, thus structurally differing from commonly known conventional surfactants.27 Bile salts have been found to improve the solubility and hence bio-availability of poorly water soluble drugs as reported in literature.28 Very few studies focussing the solubilization of drugs in mixed micellar media have been reported, but no such studies concerning the solubilization of drugs in mixed micelles of bile salts with SAILs appear in literature.
The work presented in this paper has been carried in two parts. Firstly, the mixed micellization of bile salts (NaC and NaDC) with C12mimBr has been investigated for the various micellar, interfacial and thermodynamic parameters. Secondly, we studied the solubilization of phenothiazine in pure bile salts and their mixed micelles with C12mimBr. Various techniques such as surface tension, fluorescence and dynamic light scattering (DLS) have been used to study the physiochemical properties of mixed micellar media comprising of C12mimBr and bile salts. The solubility of phenothiazine in pure bile salts and their mixed micellar system with C12mimBr has been determined using UV-visible spectroscopic measurements.
2. Experimental
2.1 Materials
The surface active ionic liquid, 1-dodecyl-3-methylimidazolium bromide (C12mimBr) was synthesized by procedure reported elsewhere29 and the procedure involved the alkylation of 1-methyl imidazole with 1-bromododecane and the mixture was stirred at 75–80 °C for 48 h. The solidified product was purified by recrystallization from ethyl acetate at least four to five times and then dried under vacuum for 1 day. The purity of the synthesized IL was ascertained by 1H NMR in CDCl3 and spectrum is given in ESI (Fig. S1†). Bile salts viz. sodium cholate (NaC, purity ≥ 99%) and sodium deoxycholate (NaDC, purity ≥ 97%), 1-methyl imidazole (purity ≥ 98%), 1-bromododecane (purity ≥ 97%), ethanol (purity ≥ 99.8%) and pyrene (purity ≥ 98%) were purchased from Sigma Aldrich. Phenothiazine (Pz, purity ≥ 98%) was purchased from Alfa Aesar. All the chemicals were of analytical grade and used without further purification. Double distilled water having a specific conductivity in the range of 1–2 μS was used in all the measurements. A Sartorius analytical balance with a precision of ±0.0001 g was utilized for weighing different substances. The chemical structures of the bile salts (NaC and NaDC), C12mimBr and Pz are given in Fig. S2.†
2.2 Methods
2.2.1 Surface tension measurements. The surface tension values (γ) were measured using Kruss (Hamburg, Germany) Easy dyne tensiometer employing ring detachment method at 298.15 K. The platinum ring was washed and cleaned thoroughly by washing with double distilled water followed by methanol and heating through flame till red hot. The surface tension of doubly distilled water (72 mN m−1) was used to calibrate the tensiometer. The γ values of pure NaC, NaDC, C12mimBr and their binary mixtures at various mole fractions were measured by adding their concentrated stock solutions in double distilled water. The measured γ values were corrected according to the procedure of Harkins and Jordan inbuilt in the instrument software. The reproducibility of γ values is estimated to be within ±0.15 mN m−1.
2.2.2 Steady state fluorescence measurements. The fluorescence measurements were performed using Hitachi F-4600 fluorescence spectrophotometer using a 10 mm path length quartz cuvette at 298.15 K. Pyrene was used as fluorescent probe and the concentration of pyrene used in all measurements is equal to 1 × 10−6 M, to avoid its interference in micelle formation process. At concentration (1 × 10−6 M) below its solubility limit (3 × 10−6 M) pyrene probe fails to dimerize.30 For the cmc determination of pure NaC, NaDC, C12mimBr and their binary mixtures at various mole fractions, pyrene was excited at 335 nm and emission spectra were recorded between 350 nm to 550 nm. The plot of ratio of first vibronic peak and third vibronic peak of pyrene (i.e. I1/I3) vs. concentration of the respective amphiphiles was used to obtain critical micellar concentrations.
2.2.3 Dynamic light scattering (DLS) measurements. Information regarding the micellar size in terms of hydrodynamic radius (Rh) of NaC, NaDC, C12mimBr and their mixtures at various mole fractions was sought from DLS measurements. All measurements were taken at 173° angle in a Malvern Zetasizer Nanoseries NanoZS instrument with a He–Ne laser (λ = 632.8 nm) at 298.15 K. The temperature was maintained by in-built temperature controller having an accuracy of ±0.1 K. The samples were properly filtered through 0.2 μm filters (Acrodisc) to avoid interference from dust particles.
2.2.4 UV-visible measurements. A Shimadzu (model UV-1800) UV-visible double beam spectrophotometer with a quartz cuvette having a path length of 1 cm was used to study solubility of phenothiazine (Pz) in bile salts (NaC and NaDC) and their mixed micelles with C12mimBr. The extinction coefficient (ε) was determined from the calibration curve of concentration vs. absorbance of ethanolic solution of Pz. Aqueous solutions of amphiphiles covering a range of concentrations below and above cmc were prepared using doubly distilled water and added to the 15 mL glass vials containing excess amount of Pz to make saturated Pz loaded solutions. The samples were stirred overnight, centrifuged and filtered using millipore (0.45 μm) to remove excess of unsolubilized drug. The concentration of Pz solubilized in the micelles was then determined from the absorbance of solution at 252 nm (which is wavelength of maximum absorption, λmax).
3. Results and discussion
3.1 Analysis of NaC/NaDC and C12mimBr interactions in the mixed micelles
To characterize the aqueous bile salts–SAIL mixed micellar systems, the cmc values of pure NaC, NaDC, C12mimBr and their binary aqueous mixtures at various mole fractions of bile salts have been determined from both surface tension and fluorescence measurements and listed in Table 1. As deduced from the plots of γ vs. log of amphiphilic concentration [Fig. S3 in ESI†] and I1/I3 vs. amphiphilic concentration [Fig. S4 in ESI†], the cmc values of pure NaC, NaDC and C12mimBr have been found to be in good conformity with the literature values.29,31,32 Similar plots for the aqueous bile salt–SAIL mixtures at various mole fractions of the respective bile salts have been given in Fig. 1(a) and (b) for NaC + C12mimBr mixtures and Fig. 2(a) and (b) for NaDC + C12mimBr mixtures. As per Table 1, the cmc values from surface tension measurements have been found to be somewhat lower than that from fluorescence measurements because of method dependent variations.33 Among bile salts, NaC has higher cmc than NaDC because of the former having three hydroxyl groups being more hydrophilic than the latter with two hydroxyl groups, although the hydrophobic steroid nucleus is the same in both the cases. The cmc values of the various NaC/NaDC + C12mimBr aqueous mixtures are found to be quite lower than their pure counterparts. This is highly expected as the mixtures involve oppositely charged amphiphiles (bile salts being negatively and SAIL being positively charged) and hence the interactions in the mixed micelles would be attractive because of the enhanced newer electrostatic and hydrophobic forces. Among mixtures, it can be observed that the cmc values first decreases (from αBileSalts = 0.2 to αBileSalts = 0.4) and then increases (from αBileSalts = 0.4 to αBileSalts = 0.6) with increase in the mole fraction of the bile salt. This change in behavior of oppositely charged surfactant mixtures at equimolarity has been exemplified earlier.34–38
Table 1 Micellar parameters: experimental cmc determined from fluorescence (Flu) and surface tension (S.T), ideal cmc (cmc*), micellar mole fraction (Xm1), ideal mole fraction (Xideal), micellar interaction parameter (βm), activity coefficients (fm1 and fm2) and hydrodynamic radius (Rh) of binary mixtures of bile salts (NaC and NaDC) with SAIL (C12mimBr)
| αBileSalts |
cmca (mM) (Flu) |
cmcb (mM) (S.T) |
cmc* (mM) |
Xm1 |
Xideal |
βm |
fm1 |
fm2 |
Rh (nm) |
| Maximum uncertainty limit ±0.02 mM. Maximum uncertainty limit ±0.01 mM. Size of the larger aggregates formed by the bile salts. |
| NaC + C12mimBr |
| 0.0 |
10.00 |
10.50 |
— |
— |
— |
— |
— |
— |
1.4 ± 0.2 |
| 0.2 |
1.48 |
0.88 |
10.76 |
0.42 |
0.14 |
−9.18 |
0.045 |
0.198 |
2.6 ± 0.4 |
| 0.4 |
1.28 |
0.72 |
11.65 |
0.47 |
0.30 |
−9.45 |
0.070 |
0.123 |
4.1 ± 0.6 |
| 0.6 |
1.40 |
0.80 |
12.70 |
0.50 |
0.49 |
−8.88 |
0.108 |
0.108 |
3.2 ± 0.5 |
| 0.8 |
1.86 |
0.95 |
13.96 |
0.55 |
0.72 |
−8.62 |
0.174 |
0.073 |
1.8 ± 0.3 |
| 1.0 |
15.50 |
10.00 |
— |
— |
— |
— |
— |
— |
1.1 ± 0.2 (209.0 ± 10.4)c |
| NaDC + C12mimBr |
| 0.0 |
10.00 |
10.50 |
— |
— |
— |
— |
— |
— |
1.4 ± 0.2 |
| 0.2 |
0.92 |
0.52 |
9.37 |
0.46 |
0.25 |
−10.05 |
0.053 |
0.119 |
3.0 ± 0.6 |
| 0.4 |
0.73 |
0.40 |
8.82 |
0.50 |
0.47 |
−10.21 |
0.077 |
0.077 |
39.4 ± 3.8 |
| 0.6 |
0.83 |
0.42 |
8.33 |
0.54 |
0.66 |
−9.90 |
0.123 |
0.055 |
8.1 ± 2.1 |
| 0.8 |
0.99 |
0.44 |
7.89 |
0.58 |
0.84 |
−9.65 |
0.182 |
0.038 |
1.5 ± 0.2 |
| 1.0 |
7.50 |
3.07 |
— |
— |
— |
— |
— |
— |
1.2 ± 0.1 (244.0 ± 12.2)c |
 |
| | Fig. 1 Plots of (a) surface tension (γ) vs. the logarithm of total amphiphilic concentration (Ct) and (b) pyrene intensity (I1/I3) ratio vs. total amphiphilic concentration (Ct) of binary mixture of NaC + C12mimBr at various mole fractions of NaC (αNaC). | |
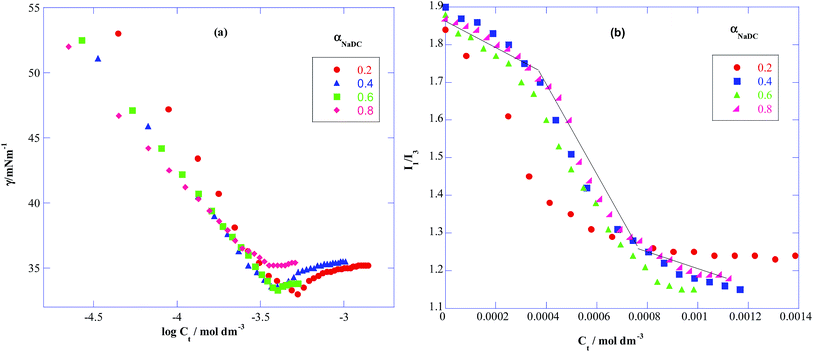 |
| | Fig. 2 Plots of (a) surface tension (γ) vs. the logarithm of total amphiphilic concentration (Ct) and (b) pyrene intensity (I1/I3) ratio vs. total amphiphilic concentration (Ct) of binary mixture of NaDC + C12mimBr at various mole fractions of NaDC (αNaDC). | |
Further insights in the aqueous bile salt–SAIL mixtures have been made to determine the ideality of mixed micelles as per Clint's eqn (1).39
| |
 | (1) |
where cmc
1 and cmc
2 are the cmc values of NaC/NaDC and C
12mimBr respectively,
α1 is the mole fraction of NaC/NaDC. It is to be mentioned here that in evaluating the various micellar parameters, we used the cmc values determined from fluorescence measurements. As per
Table 1, much lower values of cmc (experimental) than cmc* (ideal) illustrates the non-ideal behavior of NaC/NaDC + C
12mimBr aqueous mixtures and also hints at the presence of synergistic interactions between the oppositely charged head groups of mixtures.
40
Various other micellar parameters such as micellar mole fraction (Xm1) and micellar interaction parameter (βm) have also been calculated using Rubingh's regular solution theory41 as per eqn (2) and (3) respectively. This Xm1 can be further compared with the micellar mole fraction of mixed systems in ideal state (Xideal) employing Motomura's approximation42 from eqn (4)
| |
 | (2) |
| |
 | (3) |
| |
 | (4) |
The βm values are a measure of the favorable or unfavorable interactions between the mixed micelles formed. According to Rubingh's, a negative value of βm indicates the attractive interactions in binary mixtures of mixed micelles and hence micellization process is synergistic, whereas positive value of βm indicates the presence of repulsive interactions and micellization process is antagonistic. From the data given in the Table 1, it is clear that the mixtures behave synergistically at all mole fractions of bile salts, however the values show a transition from decrease to increase with increase in the mole fraction of the bile salts in mixtures with C12mimBr. In anionic rich region, increase in cmc values of mixtures can be due to the bulky structure of bile salt molecules and the role of different interactions playing there such as ion-dipole attractive interactions, van der Waals attractive interactions and repulsive interactions among their similarly charged head groups and their tails.43–46 It has been reported earlier that bile salts, being facial amphiphiles possess large, bulky stiffed steroid nucleus, so when the concentration of bile salts in mixtures increases, they tend to lie in a flat fashion between the head groups of the aggregates formed and causes steric hindrance there,47–49 which could be responsible for the lessening of attractive interactions in mixtures with SAIL. It can be further noted that the highest synergism in mixtures is obtained when the mole fraction of the bile salt (NaC/NaDC) in mixtures is 0.4. Analysis of other parameters suggest that with increase in mole fraction of NaC and NaDC, Xm1 also increases and hence, favouring the mixed micelles formation. In case of binary mixtures of NaC and NaDC with C12mimBr, Xm1 values are higher than Xideal at lower mole fraction of bile salts indicating that mixed micelles are enriched with bile salts, but with further increase in mole fractions of bile salts, Xideal value becomes higher than Xm1, expressing the more transfer of C12mimBr from solution to mixed micellar phase which might be due to the fact that in anionic rich region more concentration of NaC and NaDC molecules causes steric hindrance among the bile salts molecules thus their transfer to mixed micelles lessens. Similarly Barry and Gray50 have earlier reported the lowering in cmc in mixed micellization of tetradecyl-trimethylammonium bromide (TTAB) with NaC and NaDC due to reduction in unfavorable electrostatic interactions. The decrease in cmc values in NaC/NaDC + TTAB binary mixtures continues as the mole percent of TTAB increases but in more cationic rich region cmc values again starts to increases. They also observed the formation of smaller mixed micelles in NaC + TTAB whereas larger mixed micelles in TTAB + NaDC mixtures. Another group Panda et al.40 have reported the mixed micellization of NaC/NaDC with alkyltrimethylammonium bromides (CnTABr, n = 12, 14 and 16) and also observed the formation of larger aggregates in NaDC + CnTABr and small spherical micelles in NaC + CnTABr mixtures. In their work they confirmed that the increase of mole fraction of NaC/NaDC leads to the formation of ion-pair amphiphiles which supports the present work.
Further, the activity coefficients for both the components in the mixed micelles have also been calculated as per eqn (5) and (6)
| | |
fm1 = exp[βm(1 − Xm1)2]
| (5) |
fm1 and
fm2 are the activity coefficients of NaC/NaDC and C
12mimBr respectively. From
Table 1 it can be observed that
fm1 and
fm2 values are less than unity, illustrating the non-ideal behavior of mixed micelles formed. Comparisons of all these parameters for the two bile salts (NaC and NaDC) clearly signify that the mixed micelles with greater synergistic activity are obtained for NaDC + C
12mimBr mixtures.
3.2 DLS measurements
Further information regarding the size of the micelles was sought from DLS measurements providing us the hydrodynamic radius (Rh) of both pure NaC, NaDC, C12mimBr micelles and their mixed micelles as listed in Table 1. Both the bile salts (NaC/NaDC) and C12mimBr can be observed to form spherical micelles with the size range 1–2 nm. However bile salts form larger aggregates as well in the range of 150–270 nm and C12mimBr also seen to form larger aggregates as well as unilamellar vesicles at very high concentration.51,52 These smaller and larger aggregates formed can be ascribed to primary and secondary micelles respectively.53–55 Regarding mixtures, the mixed micellar hydrodynamic radius has been found to be larger than the pure micelles for both NaC + C12mimBr and NaDC + C12mimBr systems at all mole fractions, the values however show a change from increase to decrease with increase in the mole fraction of the bile salt. This is because initially at lower mole fractions bile salts get intercalated in the micelles enhancing their size, but as the concentration of the bulky NaC/NaDC molecules increases within the micelles, the repulsive interactions start dominating leading to their breakdown to smaller micelles. It is clear from the Fig. 3(a) and (b) that the highest Rh value has been found for the bile salts–SAIL mixtures at a mole fraction of 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, this Rh value being much larger for NaDC + C12mimBr system in comparison to NaDC + C12mimBr mixture. This might be due to the difference in the nature of the two bile salts used, with NaC being more hydrophilic preferring to remain flat on the surface of the micelles leading to smaller micelles, and NaDC being more hydrophobic getting incorporated into the interior of the micelles, thus forming larger micelles.
0.6, this Rh value being much larger for NaDC + C12mimBr system in comparison to NaDC + C12mimBr mixture. This might be due to the difference in the nature of the two bile salts used, with NaC being more hydrophilic preferring to remain flat on the surface of the micelles leading to smaller micelles, and NaDC being more hydrophobic getting incorporated into the interior of the micelles, thus forming larger micelles.
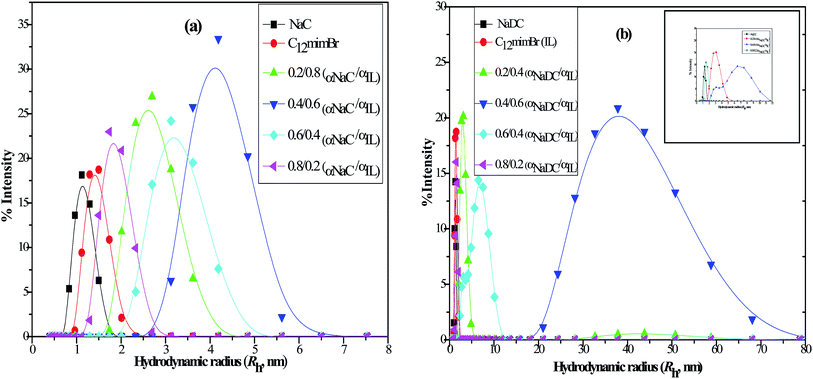 |
| | Fig. 3 Plots of % intensity vs. hydrodynamic radius (Rh, nm) of (a) pure NaC, C12mimBr and their mixtures at various mole fraction of NaC (b) pure NaDC, C12mimBr and their mixtures at various mole fraction of NaDC (inset shows Rh values in the range of 1–12 nm). | |
3.3 Analysis of NaC/NaDC and C12mimBr interactions at air–water interface
The adsorption behavior and surface properties of the aqueous mixtures of NaC/NaDC with C12mimBr have been studied using surface tension technique. When the mixtures of NaC/NaDC with C12mimBr are added into water, the amphiphilic molecules of both type get adsorbed at the air–water interface, form mixed monolayer and leads to a decrease in surface tension (γ) of water by lowering cohesive interactions among the water molecules. The decrease in γ value continues with increase in concentration of mixed solution and then reaches a constant value giving the cmc of the mixture as explained in the previous section. The adsorption efficiency of the amphiphilic molecules at the air/water interface is usually described in terms of their bulk concentration which generates a surface tension reduction of 20 m Nm−1 (C20) from pure solvent i.e. water. The higher values of their negative logarithm (pC20) denote the higher adsorption efficiency. As per Table 2, the pC20 value of NaDC is more than NaC because of its greater hydrophobicity. The mixtures of NaC/NaDC with C12mimBr have been found to be more surface active than their individual components as indicated by higher pC20 values. Various other interfacial parameters such as surface excess concentration (Гmax), minimum area per molecule (Amin) and surface pressure (πcmc) have been evaluated for the various bile salt–SAIL mixtures by use of eqn (7)–(9) respectively and listed in Table 2.| |
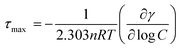 | (7) |
| |
 | (8) |
where (∂γ/∂![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) is taken as maximum slope from γ vs. log of surfactant concentration plot, R is universal gas constant (R = 8.314 J K−1 mol−1), NA is the Avogadro's number, T is the temperature, γ0 and γcmc are surface tension values of pure solvent and mixtures at cmc respectively and n is the number of chemical species whose concentration at the interface changes with bulk phase concentration. The value of n has been taken as 2 because of the both components being ionic.56 The Гmax and Amin values for pure C12mimBr and bile salts (NaC and NaDC) have been found to be in conformity with literature values.32,57,58 In accordance with the bulky head groups of the bile salts in comparison to C12mimBr, the respective Amin values have been found to be larger in case of former but with the presence of a larger alkyl chain in C12mimBr, the Гmax values are greater for C12mimBr. Among bile salts, however Amin values are almost the same although NaC is more hydrophilic than NaDC. In bile salts–SAIL mixtures, the Гmax values and Amin values are found to lie in between those of the values for pure components reflecting the formation of mixed monolayer at the air/water interface. Further, both these parameters are observed to undergo a very slight change with the mixture composition. This might be due to possibility of formation of C12mim+cholate−/C12mim+deoxycholate− ion pairs in solution which are held together by strong electrostatic as well as hydrophobic interactions. As expected, the aqueous NaC/NaDC + C12mimBr mixtures possess higher πcmc values as compared to their pure counterparts, although their efficiency to bring about surface tension reduction of water varies only slightly with the mixture composition. However, an appraisal of the various interfacial parameters for both bile salts–SAIL mixtures at various mole fractions reveals that NaDC + C12mimBr mixtures are more effectively adsorbed than NaC + C12mimBr.
C) is taken as maximum slope from γ vs. log of surfactant concentration plot, R is universal gas constant (R = 8.314 J K−1 mol−1), NA is the Avogadro's number, T is the temperature, γ0 and γcmc are surface tension values of pure solvent and mixtures at cmc respectively and n is the number of chemical species whose concentration at the interface changes with bulk phase concentration. The value of n has been taken as 2 because of the both components being ionic.56 The Гmax and Amin values for pure C12mimBr and bile salts (NaC and NaDC) have been found to be in conformity with literature values.32,57,58 In accordance with the bulky head groups of the bile salts in comparison to C12mimBr, the respective Amin values have been found to be larger in case of former but with the presence of a larger alkyl chain in C12mimBr, the Гmax values are greater for C12mimBr. Among bile salts, however Amin values are almost the same although NaC is more hydrophilic than NaDC. In bile salts–SAIL mixtures, the Гmax values and Amin values are found to lie in between those of the values for pure components reflecting the formation of mixed monolayer at the air/water interface. Further, both these parameters are observed to undergo a very slight change with the mixture composition. This might be due to possibility of formation of C12mim+cholate−/C12mim+deoxycholate− ion pairs in solution which are held together by strong electrostatic as well as hydrophobic interactions. As expected, the aqueous NaC/NaDC + C12mimBr mixtures possess higher πcmc values as compared to their pure counterparts, although their efficiency to bring about surface tension reduction of water varies only slightly with the mixture composition. However, an appraisal of the various interfacial parameters for both bile salts–SAIL mixtures at various mole fractions reveals that NaDC + C12mimBr mixtures are more effectively adsorbed than NaC + C12mimBr.
Table 2 Interfacial parameters: interaction parameter at interface (βσ), activity coefficients (fσ1 and fσ2), interfacial mole fraction at interface (Xσ), surface tension at cmc (γcmc), surface excess (Гmax), minimum area per molecule (Amin) and pC20a of binary mixtures of bile salts (NaC and NaDC) with SAIL (C12mimBr)
| αBileSalts |
βσ |
Xσ |
fσ1 |
fσ2 |
γcmc (mN m−1) |
Πcmc (mN m−1) |
106 Гmax (mol m−2) |
Amin (Å2) |
pC20 |
| Maximum uncertainty limits in Гmax, Amin and πcmc are ±0.02, ±0.06 and ±0.07 respectively. |
| NaC + C12mimBr |
| 0.0 |
— |
— |
— |
— |
33.4 |
38.6 |
2.61 |
63.61 |
2.59 |
| 0.2 |
−16.90 |
0.46 |
0.007 |
0.027 |
35.9 |
36.1 |
1.31 |
126.70 |
4.14 |
| 0.4 |
−15.71 |
0.49 |
0.016 |
0.023 |
35.8 |
36.1 |
1.38 |
120.30 |
4.25 |
| 0.6 |
−15.39 |
0.51 |
0.024 |
0.018 |
36.2 |
35.7 |
1.19 |
139.50 |
4.32 |
| 0.8 |
−13.93 |
0.54 |
0.052 |
0.017 |
36.0 |
35.9 |
1.47 |
112.90 |
3.99 |
| 1 |
— |
— |
— |
— |
44.6 |
27.3 |
1.05 |
157.30 |
2.57 |
| |
| NaDC + C12mimBr |
| 0.0 |
— |
— |
— |
— |
33.4 |
38.6 |
2.61 |
63.61 |
2.59 |
| 0.2 |
−13.40 |
0.52 |
0.045 |
0.026 |
32.8 |
39.1 |
1.64 |
101.2 |
4.29 |
| 0.4 |
−14.55 |
0.55 |
0.052 |
0.012 |
32.9 |
39.0 |
1.49 |
111.4 |
4.47 |
| 0.6 |
−14.22 |
0.57 |
0.072 |
0.009 |
32.5 |
39.4 |
1.43 |
116.1 |
4.56 |
| 0.8 |
−13.73 |
0.60 |
0.111 |
0.007 |
33.7 |
38.3 |
1.47 |
112.9 |
4.46 |
| 1.0 |
— |
— |
— |
— |
41.8 |
30.1 |
0.95 |
174.7 |
3.45 |
From the evaluation of various surface parameters, it was established that the formation of mixed monolayer occurs at the interface in aqueous bile salts–SAIL mixtures. Hence, the nature of interactions between the components of the mixed monolayer was derived using Rosen's equation replacing micellar parameters (Xm1, cmc1, cmc2, cmc12, βm) in eqn (2) and (3) by interfacial parameters (Xσ1, Cσ1, Cσ2, Cσ12, βσ). The values of interfacial parameter (βσ), interfacial mole fraction (Xσ) and activity coefficients at interface (fσ1 and fσ2) for the aqueous NaC/NaDC + C12mimBr mixtures at various mole fraction ratios have been given in Table 2. The interactions between NaC/NaDC and C12mimBr monomers at the interface have also been found to be synergistic, even greater than in the mixed micelles. This has been earlier ascribed to the lessening of the electrostatic repulsions between similarly charged head groups and easier accommodation of the two hydrophobic groups of the mixtures at the planar interface.59 Because of this strong synergism, the mixtures turn out to behave non ideally as indicated by the values of interfacial activity coefficients for both NaC/NaDC (fσ1) and C12mimBr (fσ2) being lesser than unity.
The phenomena of adsorption and mixed micellization of NaC/NaDC + C12mimBr can be quantified with the help of various thermodynamic parameters which are detailed in ESI.†
3.4 Solubilization of phenothiazine in NaC/NaDC + C12mimBr mixed micelles
To determine the practical applicability of our bile salts–SAIL mixtures, we aimed to explore the solubilization of a poorly water soluble drug, phenothiazine (Pz) in pure bile salts as well as in mixed micellar media comprising NaC/NaDC + C12mimBr mixtures (at a mole fraction ratio of 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6). This was inspired by the fact that bile salts are widely used as solubilizers and Pz derivatives finds numerous applicability in various drug formulations. The solubilization of Pz in micellar media was thus carried out with UV-visible measurements.
0.6). This was inspired by the fact that bile salts are widely used as solubilizers and Pz derivatives finds numerous applicability in various drug formulations. The solubilization of Pz in micellar media was thus carried out with UV-visible measurements.
The UV-visible spectrae of Pz at various concentrations were first recorded in ethanolic solution as shown in Fig. 4(a). Using absorbance (λmax) at 252 nm, the plot of absorbance (A252) vs. Pz concentration [Fig. 4(b)] were linear, from which the molar extinction coefficient (ε) of Pz was determined to be 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 mol−1 dm3 cm−1 in conformity with the literature value.1 The solubilisation of Pz was studied in following four micellar medias: (a) pure NaC (b) pure NaDC (c) binary mixture of NaC + C12mimBr at 0.4 mole fraction of NaC (d) binary mixture of NaDC + C12mimBr at 0.4 mole fraction of NaDC. So, to determine the solubility of Pz, first saturated solution of Pz were prepared in above micellar medias covering a range of concentrations below and above cmc (1–50 mM for pure bile salts and 0.1–5 mM for NaC/NaDC + C12mimBr mixtures). The samples were stirred overnight followed by centrifugation. After the centrifugation samples were filtered using millipore filters (0.45 μm) to remove unsolubilized drug. The absorption spectra of Pz loaded C12mimBr solutions at concentrations (above cmc) gives broad bands instead of sharp peaks, so the solubilization of Pz was not determined in pure C12mimBr whereas, the mixed micelles of C12mimBr with NaC/NaDC acted as good solubilizing media. The absorption spectra of Pz in the above mentioned micellar media at different concentrations (higher than the cmc) were thus recorded and have been given in Fig. S5–S8 (ESI†) respectively. From these spectra, thus the amount of Pz solubilized in the micelles was determined by the use of molar extinction coefficient (ε) of Pz (calculated earlier) and the respective plots of amount of Pz solubilized vs. the total surfactant concentration for pure NaC/NaDC micelles and for NaC/NaDC + C12mimBr mixed micelles have been illustrated in Fig. 5(a) and (b) respectively. A linear enhancement in the solubilization of Pz with increase in concentration of the respective surfactant was observed in all the cases. The slope of these curves was further utilized to determine the molar solubilization capacity (χ),12,60,61 as per eqn (10).
353 mol−1 dm3 cm−1 in conformity with the literature value.1 The solubilisation of Pz was studied in following four micellar medias: (a) pure NaC (b) pure NaDC (c) binary mixture of NaC + C12mimBr at 0.4 mole fraction of NaC (d) binary mixture of NaDC + C12mimBr at 0.4 mole fraction of NaDC. So, to determine the solubility of Pz, first saturated solution of Pz were prepared in above micellar medias covering a range of concentrations below and above cmc (1–50 mM for pure bile salts and 0.1–5 mM for NaC/NaDC + C12mimBr mixtures). The samples were stirred overnight followed by centrifugation. After the centrifugation samples were filtered using millipore filters (0.45 μm) to remove unsolubilized drug. The absorption spectra of Pz loaded C12mimBr solutions at concentrations (above cmc) gives broad bands instead of sharp peaks, so the solubilization of Pz was not determined in pure C12mimBr whereas, the mixed micelles of C12mimBr with NaC/NaDC acted as good solubilizing media. The absorption spectra of Pz in the above mentioned micellar media at different concentrations (higher than the cmc) were thus recorded and have been given in Fig. S5–S8 (ESI†) respectively. From these spectra, thus the amount of Pz solubilized in the micelles was determined by the use of molar extinction coefficient (ε) of Pz (calculated earlier) and the respective plots of amount of Pz solubilized vs. the total surfactant concentration for pure NaC/NaDC micelles and for NaC/NaDC + C12mimBr mixed micelles have been illustrated in Fig. 5(a) and (b) respectively. A linear enhancement in the solubilization of Pz with increase in concentration of the respective surfactant was observed in all the cases. The slope of these curves was further utilized to determine the molar solubilization capacity (χ),12,60,61 as per eqn (10).
| |
 | (10) |
where
St is the total apparent solubility of Pz,
Scmc is the apparent solubility of Pz at cmc,
Ct is the total surfactant concentration taken. The
χ value, which is defined as the number of moles of the solute (Pz) that can be solubilized by one mole of micellar media have been given in
Table 3.
 |
| | Fig. 4 Plots of (a) UV-visible spectra of phenothiazine in ethanol at various concentrations (b) absorbance (at λmax 252 nm, A252) vs. phenothiazine concentration (Cpz). | |
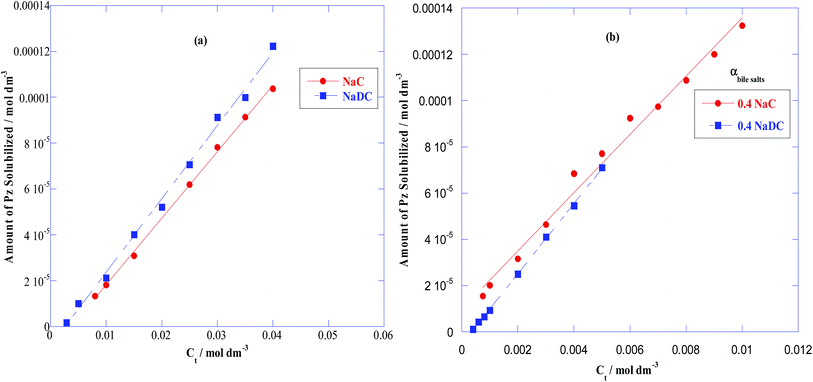 |
| | Fig. 5 Plots of amount of phenothiazine solubilized in (a) pure NaC and NaDC (b) binary mixture of NaC/NaDC + C12mimBr at 0.4 mole fraction of NaC/NaDC. | |
Table 3 Solubilization parameters: molar solubilization capacity (χ), partition coefficient (Km) and standard free energy of solubilization (ΔG°s) for Pz in NaC, NaDC and mixed micelles of NaC and NaDC with C12mimBr
| Surfactant |
103χ |
10−3Km |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km Km |
ΔG°s (kJ mol−1) |
| NaC |
2.89 |
6.31 |
3.80 |
−11.65 |
| NaDC |
3.19 |
17.61 |
4.24 |
−14.20 |
| 0.4αNaC/0.6αSAIL |
12.60 |
31.87 |
4.50 |
−15.75 |
| 0.4αNaDC/0.6αSAIL |
15.17 |
215.74 |
5.33 |
−20.45 |
There are number of factors on which solubilization depends such as structure and nature of surfactants as well as solubilizates, presence of additives, temperature etc.62 The locus of the solubilizates has also been reported to be influenced by the shapes, structure and nature of interactions among surfactants and solubilizates. It can be observed that the binary mixtures of NaC/NaDC with C12mimBr possess higher χ values than pure bile salts, due to the presence of more stable and larger micelles in mixtures. Among NaC + C12mimBr and NaDC + C12mimBr mixtures, the χ values are higher for the latter in conjunction with our previous discussion. As Pz is basically hydrophobic, it will prefer to get solubilized in the hydrophobic core of micelles, thus its solubilization in micellar media is expected to be guided largely by the hydrophobic interactions of Pz with the micellar components and the size of the micelles determining the incorporation of Pz in the micelles. Both these factors would thus favor the solubilization of Pz in mixed micellar media of NaDC + C12mimBr.
The results are also supported from the evaluation of certain other parameters like partition coefficient (Km) and standard free energy of solubilization (ΔG°s), computed by the use of eqn (11) and (12) respectively, where Vm represents the molar volume of water (0.01805 dm3 mol−1).
| |
 | (11) |
| |
 | (12) |
The values of Km and ΔG°s for the various systems understudy have been listed in Table 3. Negative values of ΔG°s indicate that solubilization of Pz in pure bile salts micelles and mixed micelles of bile salts with C12mimBr is spontaneous. Further, the values of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km and ΔG°s have been found to follow the same trend as χ values, revealing that the partition coefficient depends upon the hydrophobicity and as the hydrophobicity increases, the spontaneity of the solubilization process also increases. Thus from the χ values and log
Km and ΔG°s have been found to follow the same trend as χ values, revealing that the partition coefficient depends upon the hydrophobicity and as the hydrophobicity increases, the spontaneity of the solubilization process also increases. Thus from the χ values and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km values it becomes clear that pure NaDC and its binary mixture with C12mimBr act as better solubilizer media than pure NaC and its binary mixture due to its comparatively greater hydrophobicity.
Km values it becomes clear that pure NaDC and its binary mixture with C12mimBr act as better solubilizer media than pure NaC and its binary mixture due to its comparatively greater hydrophobicity.
4. Conclusions
The present study aimed at determining the solubilization of poorly soluble drug, phenothiazine in the mixed micellar media comprising of bile salts (NaC/NaDC) and SAIL (C12mimBr). For this the interactions between NaC/NaDC and C12mimBr molecules were explored through steady state fluorescence, surface tension and dynamic light scattering measurements. The binary aqueous mixtures of NaC/NaDC and C12mimBr exhibited strongly synergistic activity both in the mixed micelles and at the air/water interface in lieu of the generation of newer stronger hydrophobic and electrostatic forces between the components. The feasibility and the stability of the mixed micelles and mixed monolayer was also affirmed through the negative values of free energies of micellization and adsorption. Although the mixtures revealed almost similar adsorption tendencies at various mole fractions of the bile salts, yet a notable difference in their hydrodynamic radius was observed through DLS measurements, with highest Rh for the mixtures containing 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 mole fraction of NaC/NaDC and C12mimBr respectively. Among the two bile salts used, NaDC exhibited better synergism, enhanced adsorption and larger hydrodynamic radius in mixtures with C12mimBr. This has been ascribed to the more hydrophobic nature of NaDC which allows its molecules to get deeply intercalated in the mixed micelles in comparison to NaC, whose molecules prefer to lay flat on the micellar surface because of three hydroxyl groups preferring aqueous environment. Further regarding solubilization of phenothiazine, the solubility of Pz in micellar media has been found to be dependent on the hydrophobicity and the size of the micelles, with solubility increasing in the order: NaC < NaDC < NaC + C12mimBr < NaDC + C12mimBr. To sum up, bile salt–SAIL mixtures can be advantageously employed for the solubilization of phenothiazine and its derivatives. Since the bile salts and C12mimBr have already been proved to be important biologically, the results of this study are expected to be quite useful in various research areas especially drug delivery.
0.6 mole fraction of NaC/NaDC and C12mimBr respectively. Among the two bile salts used, NaDC exhibited better synergism, enhanced adsorption and larger hydrodynamic radius in mixtures with C12mimBr. This has been ascribed to the more hydrophobic nature of NaDC which allows its molecules to get deeply intercalated in the mixed micelles in comparison to NaC, whose molecules prefer to lay flat on the micellar surface because of three hydroxyl groups preferring aqueous environment. Further regarding solubilization of phenothiazine, the solubility of Pz in micellar media has been found to be dependent on the hydrophobicity and the size of the micelles, with solubility increasing in the order: NaC < NaDC < NaC + C12mimBr < NaDC + C12mimBr. To sum up, bile salt–SAIL mixtures can be advantageously employed for the solubilization of phenothiazine and its derivatives. Since the bile salts and C12mimBr have already been proved to be important biologically, the results of this study are expected to be quite useful in various research areas especially drug delivery.
Acknowledgements
This work was financially supported by the Department of Science and Technology (DST) New Delhi, India as a part of Project No. SR/S1/PC-02/2011. Rajni Vashishat is thankful to UGC-BSR for the award of Research Fellow.
References
- J. Karpinska, B. Starczewska and H. P. Tarasiewicz, Anal. Sci., 1996, 12, 161–170 CrossRef CAS.
- P. A. Bhat, A. A. Dar and G. M. Rather, J. Chem. Eng. Data, 2008, 53, 1271–1277 CrossRef CAS.
- S. Parla and P. K. Yuet, Ind. Eng. Chem. Res., 2006, 45, 3552–3558 CrossRef.
- D. A. Edwards, R. G. Luthy and Z. Llu, Environ. Sci. Technol., 1991, 25, 127 CrossRef CAS.
- P. Mukerjee, in Solution chemistry of surfactants, ed. K. L. Mittal, Plenum Press, New York, 1979, pp. 153–174 Search PubMed.
- D. E. Kile and C. T. Chiou, Environ. Sci. Technol., 1989, 23, 832 CrossRef CAS.
- D. Attwood and A. T. Florence, Surfactants systems: their chemistry, pharmacy and biology, Chapmann and Hall, New York, 1983 Search PubMed.
- A. R. T. Bagha, R. G. Singh and K. Holmberg, J. Colloid Interface Sci., 2012, 376, 112–118 CrossRef PubMed.
- Y. Kadam, U. Yerramilli and A. Bahadur, Colloids Surf., B, 2009, 72, 141–147 CrossRef CAS PubMed.
- T. Farias, L. C. de Menorval, J. Zajac and A. Rivera, Colloids Surf., A, 2009, 345, 51–57 CrossRef CAS PubMed.
- I. Xiarchos and D. Doulia, J. Hazard. Mater., 2006, 136, 882–888 CrossRef CAS PubMed.
- A. Raval, A. Parmar, A. Raval and P. Bahadur, Colloids Surf., B, 2012, 93, 180–187 CrossRef CAS PubMed.
- M. M. Swe, L. E. Yu, K. Hung and B. Hung, J. Surfactants Deterg., 2006, 9, 237–244 CrossRef CAS.
- J. Lakra, D. Tikariha, T. Yadav, S. Das, S. Ghosh, M. L. Satnami and K. K. Ghosh, Colloids Surf., A, 2014, 451, 56–65 CrossRef CAS PubMed.
- A. A. Dar, G. M. Rather and A. R. Das, J. Phys. Chem. B, 2007, 111, 3122–3132 CrossRef CAS PubMed.
- M. Maswal and A. A. Dar, Colloids Surf., A, 2013, 436, 704–713 CrossRef CAS PubMed.
- S. Pandey, Anal. Chim. Acta, 2006, 556, 38–45 CrossRef CAS PubMed.
- G. A. Baker, S. N. Baker, S. Pandey and F. V. Bright, Analyst, 2005, 130, 800–808 RSC.
- K. A. Fletcher and S. Pandey, Langmuir, 2004, 20, 33–36 CrossRef CAS.
- K. Behera, P. Dahiya and S. Pandey, J. Colloid Interface Sci., 2007, 307, 235–245 CrossRef CAS PubMed.
- P. D. Galgano and O. A. El Seoud, J. Colloid Interface Sci., 2011, 361, 186–194 CrossRef CAS PubMed.
- D. Zhao, M. Wu, Y. Kou and E. Min, Catal. Today, 2002, 74, 157–189 CrossRef CAS.
- H. D. Williams, Y. Sahbaz, L. Ford, T. H. Nguyen, P. J. Scammells and C. J. H. Porter, Chem. Commun., 2014, 50, 1688–1690 RSC.
- A. Cornellas, L. Perez, F. Comelles, I. Ribosa, A. Manresa and M. T. Garcia, J. Colloid Interface Sci., 2011, 355, 164–171 CrossRef CAS PubMed.
- R. Holm, A. Mullertz and H. Mu, Int. J. Pharm., 2013, 453, 44–55 CrossRef CAS PubMed.
- J. Maldonado-Valderrama, P. Wilde, A. Macierzanka and A. Mackie, Adv. Colloid Interface Sci., 2011, 165, 36–46 CrossRef CAS PubMed.
- S. Gouin and X. X. Zhu, Langmuir, 1998, 14, 4025–4029 CrossRef CAS.
- S. Selvam, J. Photochem. Photobiol., B, 2012, 116, 105–113 CrossRef CAS PubMed.
- R. Sanan and R. K. Mahajan, J. Colloid Interface Sci., 2013, 394, 346–352 CrossRef CAS PubMed.
- G. B. Ray, I. Chakraborty, S. Ghosh and S. P. Moulik, J. Colloid Interface Sci., 2007, 307, 543–553 CrossRef CAS PubMed.
- S. Reis, C. G. Moutinho, C. Matos, B. de Castro, P. Gameiro and J. L. F. C. Lima, Anal. Biochem., 2004, 334, 117–126 CrossRef CAS PubMed.
- P. K. Jana and S. P. Moulik, J. Phys. Chem., 1991, 95, 9525–9532 CrossRef CAS.
- S. Mahajan and R. K. Mahajan, J. Colloid Interface Sci., 2012, 387, 194–204 CrossRef CAS PubMed.
- A. Yousefi, S. Javadian, H. Gharibi, J. Kakemam and M. R. Alavijeh, J. Phys. Chem. B, 2011, 115, 8112–8121 CrossRef CAS PubMed.
- S. Prevost and M. Gradzielski, J. Colloid Interface Sci., 2009, 337, 472–484 CrossRef CAS PubMed.
- E. F. Marques, O. Regev, A. Khan, M. da, G. Miguel and B. Lindman, J. Phys. Chem. B, 1998, 102, 6746–6758 CrossRef CAS.
- K. Tsuchiya, J. Ishikake, T. S. Kim, T. Ohkubo, H. Sakai and M. Abe, J. Colloid Interface Sci., 2007, 312, 139–145 CrossRef CAS PubMed.
- B. F. B. Silva, E. F. Marques and U. Olsson, J. Phys. Chem. B, 2007, 111, 13520–13526 CrossRef CAS PubMed.
- J. H. Clint, J. Chem. Soc., Faraday Trans., 1975, 171, 1327 RSC.
- K. Manna, C. H. Chang and A. K. Panda, Colloids Surf., A, 2012, 415, 10–21 CrossRef CAS PubMed.
- D. N. Rubingh and K. L. Mittal, Solution Chemistry of Surfactants, Plenum Press, New York, 1979, vol. 1 Search PubMed.
- K. Motumura and M. Aratono, Mixed Surfactant Systems, Marcel Decker, New York, 1993 Search PubMed.
- R. Sanan and R. K. Mahajan, Ind. Eng. Chem. Res., 2011, 50, 7319–7325 CrossRef CAS.
- T. Yoshimura, A. Ohno and K. Esumi, J. Colloid Interface Sci., 2004, 272, 191–196 CrossRef CAS PubMed.
- L. S. Hao, Y. T. Deng, L. S. Zhou, H. Ye, Y. Q. Nan and P. Hu, J. Phys. Chem. B, 2012, 116, 5213–5225 CrossRef CAS PubMed.
- G. Kume, M. Gallotti and G. Nunes, J. Surfactants Deterg., 2008, 11, 1–11 CrossRef CAS.
- L. Jiang, K. Wang, M. Deng, Y. Wang and J. Huang, Langmuir, 2008, 24, 4600–4606 CrossRef CAS PubMed.
- J. Ulmius, G. Lindblom, H. Wennerstrom, L. B. A. Johansson, K. Fontell, O. Soderman and G. Arvidson, Biochemistry, 1982, 21, 1553–1560 CrossRef CAS.
- S. H. Tung, Y. E. Huang and S. R. Raghavan, J. Am. Chem. Soc., 2006, 128, 5751–5756 CrossRef CAS PubMed.
- B. W. Barry and G. M. T. Gray, J. Colloid Interface Sci., 1975, 55(2), 327–339 CrossRef.
- N. A. Mazer, M. C. Carey, R. F. Kwasnick and G. B. Benedek, Biochemistry, 1979, 18(14), 3064–3075 CrossRef CAS.
- H. Wang, L. Zhang, J. Wang, Z. Li and S. Zhang, Chem. Commun., 2013, 49, 5222–5224 RSC.
- M. Posa and A. Sebenji, Biochim. Biophys. Acta, 2014, 1840, 1072–1082 CrossRef CAS PubMed.
- A. Jover, F. Meijide, E. R. Nunez and J. V. Tato, Langmuir, 1997, 13, 161–164 CrossRef CAS.
- D. B. Warren, D. K. Chalmers, K. Hutchison, W. Dang and C. W. Pouton, Colloids Surf., A, 2006, 280, 182–193 CrossRef CAS PubMed.
- M. S. Vethamuthu, M. Almgren, P. Hansson and J. Zhao, Langmuir, 1996, 12, 2186–2189 CrossRef.
- B. Dong, N. Li, L. Zheng, L. Yu and T. Inoue, Langmuir, 2007, 23, 4178–4182 CrossRef CAS PubMed.
- M. E. Haque, J. A. R. Das and S. P. Moulik, J. Phys. Chem., 1995, 99, 14032–14038 CrossRef CAS.
- Q. Zhou and M. J. Rosen, Langmuir, 2003, 19, 4555–4562 CrossRef CAS.
- Y. Morol, K. Sato and R. Matuura, J. Phys. Chem., 1982, 86, 2463–2468 CrossRef.
- Y. Kadam, U. Yerramilli, A. Bahadur and P. Bahadur, Colloids Surf., B, 2011, 83, 49–57 CrossRef CAS PubMed.
- M. J. Rosen, Surfactant and interfacial phenomena, Wiley, New York, 2nd edn, 1984 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra09812b |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 of NaC/NaDC
0.6 of NaC/NaDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C12mimBr. Furthermore the solubilization of phenothiazine (Pz) has been studied in pure bile salts as well as in mixed micelles of bile salts + C12mimBr employing UV-visible measurements. From the evaluation of various solubilization parameters viz. molar solubilization ratio (MSR, χ), micelle-water partition coefficient (Km) and standard free energy of solubilization (ΔG°s), it was established that the solubilization capacity of bile salts for Pz were enhanced when mixed with C12mimBr, the effect being more pronounced for NaDC than NaC.
C12mimBr. Furthermore the solubilization of phenothiazine (Pz) has been studied in pure bile salts as well as in mixed micelles of bile salts + C12mimBr employing UV-visible measurements. From the evaluation of various solubilization parameters viz. molar solubilization ratio (MSR, χ), micelle-water partition coefficient (Km) and standard free energy of solubilization (ΔG°s), it was established that the solubilization capacity of bile salts for Pz were enhanced when mixed with C12mimBr, the effect being more pronounced for NaDC than NaC.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6, this Rh value being much larger for NaDC + C12mimBr system in comparison to NaDC + C12mimBr mixture. This might be due to the difference in the nature of the two bile salts used, with NaC being more hydrophilic preferring to remain flat on the surface of the micelles leading to smaller micelles, and NaDC being more hydrophobic getting incorporated into the interior of the micelles, thus forming larger micelles.
0.6, this Rh value being much larger for NaDC + C12mimBr system in comparison to NaDC + C12mimBr mixture. This might be due to the difference in the nature of the two bile salts used, with NaC being more hydrophilic preferring to remain flat on the surface of the micelles leading to smaller micelles, and NaDC being more hydrophobic getting incorporated into the interior of the micelles, thus forming larger micelles.
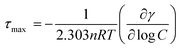

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C) is taken as maximum slope from γ vs. log of surfactant concentration plot, R is universal gas constant (R = 8.314 J K−1 mol−1), NA is the Avogadro's number, T is the temperature, γ0 and γcmc are surface tension values of pure solvent and mixtures at cmc respectively and n is the number of chemical species whose concentration at the interface changes with bulk phase concentration. The value of n has been taken as 2 because of the both components being ionic.56 The Гmax and Amin values for pure C12mimBr and bile salts (NaC and NaDC) have been found to be in conformity with literature values.32,57,58 In accordance with the bulky head groups of the bile salts in comparison to C12mimBr, the respective Amin values have been found to be larger in case of former but with the presence of a larger alkyl chain in C12mimBr, the Гmax values are greater for C12mimBr. Among bile salts, however Amin values are almost the same although NaC is more hydrophilic than NaDC. In bile salts–SAIL mixtures, the Гmax values and Amin values are found to lie in between those of the values for pure components reflecting the formation of mixed monolayer at the air/water interface. Further, both these parameters are observed to undergo a very slight change with the mixture composition. This might be due to possibility of formation of C12mim+cholate−/C12mim+deoxycholate− ion pairs in solution which are held together by strong electrostatic as well as hydrophobic interactions. As expected, the aqueous NaC/NaDC + C12mimBr mixtures possess higher πcmc values as compared to their pure counterparts, although their efficiency to bring about surface tension reduction of water varies only slightly with the mixture composition. However, an appraisal of the various interfacial parameters for both bile salts–SAIL mixtures at various mole fractions reveals that NaDC + C12mimBr mixtures are more effectively adsorbed than NaC + C12mimBr.
C) is taken as maximum slope from γ vs. log of surfactant concentration plot, R is universal gas constant (R = 8.314 J K−1 mol−1), NA is the Avogadro's number, T is the temperature, γ0 and γcmc are surface tension values of pure solvent and mixtures at cmc respectively and n is the number of chemical species whose concentration at the interface changes with bulk phase concentration. The value of n has been taken as 2 because of the both components being ionic.56 The Гmax and Amin values for pure C12mimBr and bile salts (NaC and NaDC) have been found to be in conformity with literature values.32,57,58 In accordance with the bulky head groups of the bile salts in comparison to C12mimBr, the respective Amin values have been found to be larger in case of former but with the presence of a larger alkyl chain in C12mimBr, the Гmax values are greater for C12mimBr. Among bile salts, however Amin values are almost the same although NaC is more hydrophilic than NaDC. In bile salts–SAIL mixtures, the Гmax values and Amin values are found to lie in between those of the values for pure components reflecting the formation of mixed monolayer at the air/water interface. Further, both these parameters are observed to undergo a very slight change with the mixture composition. This might be due to possibility of formation of C12mim+cholate−/C12mim+deoxycholate− ion pairs in solution which are held together by strong electrostatic as well as hydrophobic interactions. As expected, the aqueous NaC/NaDC + C12mimBr mixtures possess higher πcmc values as compared to their pure counterparts, although their efficiency to bring about surface tension reduction of water varies only slightly with the mixture composition. However, an appraisal of the various interfacial parameters for both bile salts–SAIL mixtures at various mole fractions reveals that NaDC + C12mimBr mixtures are more effectively adsorbed than NaC + C12mimBr.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6). This was inspired by the fact that bile salts are widely used as solubilizers and Pz derivatives finds numerous applicability in various drug formulations. The solubilization of Pz in micellar media was thus carried out with UV-visible measurements.
0.6). This was inspired by the fact that bile salts are widely used as solubilizers and Pz derivatives finds numerous applicability in various drug formulations. The solubilization of Pz in micellar media was thus carried out with UV-visible measurements.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 mol−1 dm3 cm−1 in conformity with the literature value.1 The solubilisation of Pz was studied in following four micellar medias: (a) pure NaC (b) pure NaDC (c) binary mixture of NaC + C12mimBr at 0.4 mole fraction of NaC (d) binary mixture of NaDC + C12mimBr at 0.4 mole fraction of NaDC. So, to determine the solubility of Pz, first saturated solution of Pz were prepared in above micellar medias covering a range of concentrations below and above cmc (1–50 mM for pure bile salts and 0.1–5 mM for NaC/NaDC + C12mimBr mixtures). The samples were stirred overnight followed by centrifugation. After the centrifugation samples were filtered using millipore filters (0.45 μm) to remove unsolubilized drug. The absorption spectra of Pz loaded C12mimBr solutions at concentrations (above cmc) gives broad bands instead of sharp peaks, so the solubilization of Pz was not determined in pure C12mimBr whereas, the mixed micelles of C12mimBr with NaC/NaDC acted as good solubilizing media. The absorption spectra of Pz in the above mentioned micellar media at different concentrations (higher than the cmc) were thus recorded and have been given in Fig. S5–S8 (ESI†) respectively. From these spectra, thus the amount of Pz solubilized in the micelles was determined by the use of molar extinction coefficient (ε) of Pz (calculated earlier) and the respective plots of amount of Pz solubilized vs. the total surfactant concentration for pure NaC/NaDC micelles and for NaC/NaDC + C12mimBr mixed micelles have been illustrated in Fig. 5(a) and (b) respectively. A linear enhancement in the solubilization of Pz with increase in concentration of the respective surfactant was observed in all the cases. The slope of these curves was further utilized to determine the molar solubilization capacity (χ),12,60,61 as per eqn (10).
353 mol−1 dm3 cm−1 in conformity with the literature value.1 The solubilisation of Pz was studied in following four micellar medias: (a) pure NaC (b) pure NaDC (c) binary mixture of NaC + C12mimBr at 0.4 mole fraction of NaC (d) binary mixture of NaDC + C12mimBr at 0.4 mole fraction of NaDC. So, to determine the solubility of Pz, first saturated solution of Pz were prepared in above micellar medias covering a range of concentrations below and above cmc (1–50 mM for pure bile salts and 0.1–5 mM for NaC/NaDC + C12mimBr mixtures). The samples were stirred overnight followed by centrifugation. After the centrifugation samples were filtered using millipore filters (0.45 μm) to remove unsolubilized drug. The absorption spectra of Pz loaded C12mimBr solutions at concentrations (above cmc) gives broad bands instead of sharp peaks, so the solubilization of Pz was not determined in pure C12mimBr whereas, the mixed micelles of C12mimBr with NaC/NaDC acted as good solubilizing media. The absorption spectra of Pz in the above mentioned micellar media at different concentrations (higher than the cmc) were thus recorded and have been given in Fig. S5–S8 (ESI†) respectively. From these spectra, thus the amount of Pz solubilized in the micelles was determined by the use of molar extinction coefficient (ε) of Pz (calculated earlier) and the respective plots of amount of Pz solubilized vs. the total surfactant concentration for pure NaC/NaDC micelles and for NaC/NaDC + C12mimBr mixed micelles have been illustrated in Fig. 5(a) and (b) respectively. A linear enhancement in the solubilization of Pz with increase in concentration of the respective surfactant was observed in all the cases. The slope of these curves was further utilized to determine the molar solubilization capacity (χ),12,60,61 as per eqn (10).


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km
Km

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km and ΔG°s have been found to follow the same trend as χ values, revealing that the partition coefficient depends upon the hydrophobicity and as the hydrophobicity increases, the spontaneity of the solubilization process also increases. Thus from the χ values and log
Km and ΔG°s have been found to follow the same trend as χ values, revealing that the partition coefficient depends upon the hydrophobicity and as the hydrophobicity increases, the spontaneity of the solubilization process also increases. Thus from the χ values and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Km values it becomes clear that pure NaDC and its binary mixture with C12mimBr act as better solubilizer media than pure NaC and its binary mixture due to its comparatively greater hydrophobicity.
Km values it becomes clear that pure NaDC and its binary mixture with C12mimBr act as better solubilizer media than pure NaC and its binary mixture due to its comparatively greater hydrophobicity.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 mole fraction of NaC/NaDC and C12mimBr respectively. Among the two bile salts used, NaDC exhibited better synergism, enhanced adsorption and larger hydrodynamic radius in mixtures with C12mimBr. This has been ascribed to the more hydrophobic nature of NaDC which allows its molecules to get deeply intercalated in the mixed micelles in comparison to NaC, whose molecules prefer to lay flat on the micellar surface because of three hydroxyl groups preferring aqueous environment. Further regarding solubilization of phenothiazine, the solubility of Pz in micellar media has been found to be dependent on the hydrophobicity and the size of the micelles, with solubility increasing in the order: NaC < NaDC < NaC + C12mimBr < NaDC + C12mimBr. To sum up, bile salt–SAIL mixtures can be advantageously employed for the solubilization of phenothiazine and its derivatives. Since the bile salts and C12mimBr have already been proved to be important biologically, the results of this study are expected to be quite useful in various research areas especially drug delivery.
0.6 mole fraction of NaC/NaDC and C12mimBr respectively. Among the two bile salts used, NaDC exhibited better synergism, enhanced adsorption and larger hydrodynamic radius in mixtures with C12mimBr. This has been ascribed to the more hydrophobic nature of NaDC which allows its molecules to get deeply intercalated in the mixed micelles in comparison to NaC, whose molecules prefer to lay flat on the micellar surface because of three hydroxyl groups preferring aqueous environment. Further regarding solubilization of phenothiazine, the solubility of Pz in micellar media has been found to be dependent on the hydrophobicity and the size of the micelles, with solubility increasing in the order: NaC < NaDC < NaC + C12mimBr < NaDC + C12mimBr. To sum up, bile salt–SAIL mixtures can be advantageously employed for the solubilization of phenothiazine and its derivatives. Since the bile salts and C12mimBr have already been proved to be important biologically, the results of this study are expected to be quite useful in various research areas especially drug delivery.



