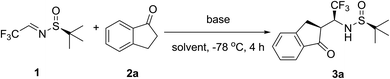
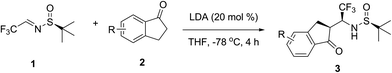
LDA-promoted asymmetric synthesis of β-trifluoromethyl-β-amino indanone derivatives with virtually complete stereochemical outcome†
Chen Xiea,
Haibo Meiab,
Lingmin Wua,
Vadim A. Soloshonokcd,
Jianlin Han*ab and
Yi Pana
aSchool of Chemistry and Chemical Engineering, State of Key Laboratory of Coordination, Nanjing University, Nanjing, 210093, China. E-mail: hanjl@nju.edu.cn; yipan@nju.edu.cn; Fax: +86-25-83593153; Tel: +86-25-83593153
bInstitute for Chemistry & BioMedical Sciences, Nanjing University, Nanjing, 210093, China
cDepartment of Organic Chemistry I, Faculty of Chemistry, University of the Basque Country UPV/EHU, 20018 San Sebastian, Spain
dIKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain
First published on 25th November 2013
Abstract
We demonstrate that reactions between various 1-indanones and (SS)-N-tert-butanesulfinyl-(3,3,3)-trifluoroacetaldimine, conducted in the presence of catalytic amounts of LDA, occur with virtually complete stereochemical outcome, offering reliable and generalized access to biologically relevant β-trifluoromethyl-β-amino indanone derivatives. The products can be isolated in diastereomerically pure form simply by washing the crude reaction mixture with hexanes, underscoring practicality of the present method.
Introduction
β-Amino ketones represent one of the most extraordinary important classes of nitrogen-containing biologically relevant compounds.1 Their chemistry has been studied by many generations of organic chemists leading to discovery and development of numerous commercial pharmaceuticals.2 β-Amino ketones are also of great importance as synthetic intermediates as they can be easily converted into the corresponding γ-amino alcohols, β-amino esters, 1,3-diamines and many other multifunctional compounds.3 In particular, Mannich-type addition reactions of ketones with imino compounds, or their precursors, is one of the most straightforward synthetic approaches to β-amino carbonyl compounds.4 While this reaction is well-studied, its application for preparation of fluorinated β-amino carbonyl compounds has been quite limited, in particular in asymmetric mode, due to restricted availability of proper fluorine containing imines.5Fluorine substitution for hydrogen is currently an established strategy in the development of new drugs with improved metabolic stability, bioavailability and protein–ligand interactions.6,7 In particular, CF3–CH(NH2)-structural feature8 is generally used as a pharmacophore in the design of bioactive compounds. In this regard, fluorinated β-amino carbonyl compounds, especially the β-trifluoromethylated β-amino ketones were shown to play a critical role in the field of biochemistry and pharmacology.7 (SS)-N-tert-Butanesulfinyl (3,3,3)-trifluoroacetaldimine 1 (Table 1), an analogue of Ellman-type imine reagents,9 displays high reactivity, asymmetric induction and is commercially available in both enantiomeric forms.10 In the recent years, application of reagent 1 for introduction of CF3–CH(NH2)-pharmacophore unit in numerous biologically relevant molecules has been quite actively pursued, as evidenced by the dramatic increase in research disclosures in the literature.11 However, to the best of our knowledge, there were no reports on the reactions of imine 1 with 1-indanone derivatives. One may agree that asymmetric modification of indanone skeleton with CF3–CH(NH2)-group would be of great interest offering a new class of biologically relevant fluorinated β-amino ketones. In this Communication, we report a preliminary study on the addition reactions between indanone derived enolates and imine reagent 1. We demonstrate that the reactions under study proceed with virtually complete stereochemical outcome proving a generalized and practical access to the target β-trifluoromethyl β-amino ketones.
| Entry | Base (mol%) | Solvent | Yield (%)b | %de of 3ac |
|---|---|---|---|---|
| a Reaction condition: 1-indanone 2a (1.1 mmol), sulfinylimine 1 (1.0 mmol), base, solvent (5 mL).b Isolated yields of diastereomerically pure 3a.c Isomer ratio was determined by 19F NMR on crude reaction mixtures.d Sulfinylimine 1 was pre-cooled to −78 °C, and then transferred via cannula to the reaction mixture. | ||||
| 1 | LDA (100) | THF | 95 | 88 |
| 2 | n-BuLi (100) | THF | 42 | 88 |
| 3 | LiHMDS (100) | THF | 83 | 86 |
| 4 | LDA (100) | Et2O | 81 | 30 |
| 5 | LDA (100) | Toluene | 89 | 65 |
| 6d | LDA (100) | THF | 96 | >98 |
| 7d | LDA (50) | THF | 96 | >98 |
| 8d | LDA (20) | THF | 94 | >98 |
Results and discussions
At the outset of this study, we selected unsubstituted 1-indanone 2a as a model substrate to optimize the reaction conditions (Table 1). Preliminary experiments have shown that complete conversion of imine 1 can be achieved with as little excess as 1.1 equivalents of ketone 2a. Using THF as solvent at −78 °C (entries 1–3) we screened the bases such as LDA, n-BuLi and LiHMDS. While the diastereoselectivity was quite encouraging in all cases, LDA was clearly superior in terms of chemical yield, giving rise to the desired product 3a in 95% yield (entry 1). With a goal to improve the stereochemical outcome, we studied the reactions in different solvents. Unfortunately, these attempts gave negative results, as application of any other solvent than THF led to noticeable decrease in the stereoselectivity. Two representative examples are given in entries 4 and 5. Detailed analysis of numerous peculiarities of the experimental procedures allowed us to determine that mode of the addition of imine 1 to a solution of preformed enolate of 2a has a profound effect on the stereochemical outcome. Thus, we found that sulfinylimine 1 has to be pre-cooled to −78 °C, and then transferred via cannula to the reaction mixture (entry 6). This modification of the addition procedure allowed preparation of the desired product 3a in 96% yield and with virtually complete diastereoselectivity (de > 98%). While studying various aspects of the enolate formation, we made another surprising observation. In particular, we found that LDA can be used in substoichiometric amounts. As shown in entry 7, application of 0.5 equivalents of LDA gave the same (entry 7 vs. 6) stereochemical outcome of the addition reaction under study. Furthermore, we determined that LDA can be used in as low as 0.2 equivalents without any noticeable effect on the reaction outcome (entry 8 vs. 7 and 6). Of particular importance is that the product 3a can be isolated in diastereomerically pure form without column chromatography and recrystallization. Drawing from the GAP chemistry (group-assisted purification) reported by Li group,12 we used the recommended procedure by washing the crude mixture with hexanes.Having optimized the reaction conditions, we studied next the generality of this method using series of 1-indanones bearing various substituents on the aromatic ring (Table 2). As shown in Table 2, electron-donating, -withdrawing and sterically bulky substituents in position 6 (entries 2–7) as well as in position 5 (entries 8–11) had very little effect on the stereochemical outcome of the addition reactions. Similar results were also obtained with substrates 2l, m bearing two substituents in the positions 5,7 or 5,6. These results clearly suggest that these reactions have a good range of generality as various substituents can be introduced on the aromatic ring for systematic biological studies. It should be emphasised that the yields reported in Tables 1 and 2 are those obtained for diastereomerically pure compounds 3a–m prepared by washing the crude products with hexanes, without application of column chromatography.
| Entry | R | Product | Yield (%)b | de (%)c |
|---|---|---|---|---|
| a Reaction condition: 1-indanone 2 (1.1 mmol), sulfinylimine 1 (1.0 mmol), LDA (0.2 mmol), THF (5 mL).b Isolated yields.c Isomer ratio was determined by 19F NMR. | ||||
| 1 | H | 3a | 94 | 98 |
| 2 | 6-Me | 3b | 91 | 96 |
| 3 | 6-tBu | 3c | 92 | 96 |
| 4 | 6-OMe | 3d | 92 | 93 |
| 5 | 6-F | 3e | 93 | 98 |
| 6 | 6-Cl | 3f | 92 | 98 |
| 7 | 6-Br | 3g | 93 | 98 |
| 8 | 5-OMe | 3h | 89 | 98 |
| 9 | 5 F | 3i | 95 | 98 |
| 10 | 5-Cl | 3j | 91 | 96 |
| 11 | 5-Br | 3k | 90 | 96 |
| 12 | 5,7-di-Cl | 3l | 85 | 98 |
| 13 | 5,6-di-OMe | 3m | 93 | 98 |
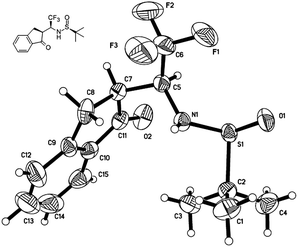
To assign the absolute configuration of the two newly created stereogenic centres we performed crystallographic analysis of 3a (Fig. 1). As shown in Fig. 1, both of the stereogenic centers are of (S) absolute configuration. The absolute configuration of the rest of products 3b–m in this study were assigned based on the similarity of their spectroscopic and chiroptical properties.
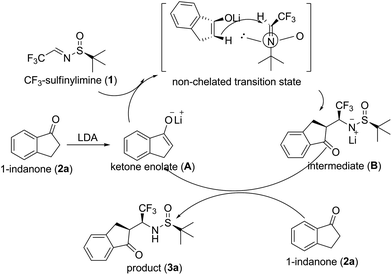
To account for the observed preference for the absolute configuration of the products 3, we propose a plausible mechanism shown in Scheme 1. Initially, ketone enolate (A) is generated by the reaction of 1-indanone 2a with LDA. Then Mannich addition between ketone derived enolate (A) and CF3-sulfinylimine 1 occurred via an open non-chelated transition state model to afford the intermediate (B). In this model, enolate (A) preferably added to the imine from the less hindered face to afford (SS,S,S)-3a as major diastereomer. One may agree that, the intermediate (B) can react with another molecule of 1-indanone 2a to generate the final product 3a as well as ketone enolate (A) to carry over the catalytic cycle.
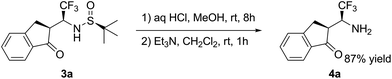
As the final goal of this study, we demonstrated deprotection of the addition products 3a. The N-tert-butylsulfinyl group can be conveniently cleaved by treatment with aqueous HCl in methanol under mild condition (Scheme 2).13 Using this standard procedure, free β-trifluoromethylated β-amino ketone 4a was obtained in high isolated yield of 87%.
Conclusion
In summary, this work reveals that various β-trifluoromethylated β-amino indanone derivatives can be prepared in enantiomerically pure form via asymmetric addition of 1-indanone to CF3-sulfinylimine. These addition reactions occur with virtually complete stereochemical outcome and can tolerate electron-withdrawing, -donating or sterically bulky substituents. Unusual feature of these reactions is that they can be conducted in the presence of catalytic (0.2 equiv.) amounts of LDA. Importantly, the purifications of the resulting β-trifluoromethylated β-amino indanone products 3a–m are performed via the economical and simple operation which involved merely washing the crude mixtures with hexanes, thus emphasising practicality and scalability of this method.Experimental section
General information
All reagents were obtained from commercial suppliers and used without further purification. The reactions were conducted in a closed system with an atmosphere of N2 and were monitored by TLC. Solvents were dried and distilled prior to use. Flash chromatography was performed using silica gel 60 (200–300 mesh). Thin layer chromatography was carried out on silica gel 60 F-254 TLC plates of 20 cm × 20 cm.Proton NMR spectra were recorded on a 400 spectrometer. Proton chemical shifts are reported in ppm (δ) relative to internal tetramethylsilane (TMS, δ 0.0 ppm) or with the solvent reference relative to TMS employed as the internal standard (CDCl3, δ 7.26 ppm). Data are reported as follows: chemical shift (multiplicity [singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m)], coupling constants [Hz], integration). Carbon NMR spectra were recorded at 101 MHz with complete proton decoupling. Carbon chemical shifts are reported in ppm (δ) relative to TMS with the respective solvent resonance as the internal standard (CDCl3, δ 77.0 ppm). 19F NMR spectra were recorded with a Bruker ARX 400 spectrometer. Melting points are uncorrected. Values of optical rotation were measured on Rudolph Automatic Polarimeter A21101. Infrared spectra were obtained on Bruker Vector 22 in KBr pellets. HRMS were recorded on an Agilent 6540Q-TOF LC/MS equipped with electrospray ionization (ESI) probe operating in positive or negative ion mode.
Typical procedure for asymmetric addition of sulfinylimine
Into an oven-dried reaction vial flushed with N2 were taken ketone 2 (1.1 mmol) and anhydrous THF (5.0 mL). The reaction vial was cooled to −78 °C and LDA (2 M in THF, 0.1 mL) was added dropwise with stirring. After 40 min at −78 °C, sulfinylimine 1 (1.0 mmol) dissolved in anhydrous THF (2 mL) was pre-cooled to −78 °C, then added dropwise to the reaction mixture. Stirring was continued at −78 °C for 4 h, then the reaction was quenched with saturated NH4Cl (3.0 mL), followed by H2O (5.0 mL) and the mixture was brought to room temperature. The organic layer was taken and the aqueous layer was extracted with EtOAc (2 × 20 mL). The combined organic layers were washed with water (1 × 30 mL) and brine solution (1 × 30 mL) and dried over anhydrous Na2SO4. The solvent was evaporated, and the crude mixture was co-concentrated with hexane. The solid products were washed with a minimum amount of hexanes to afford pure product without column chromatography.Conversion of 3a affording free β-amino ketone 4a
3a (0.5 mmol) and MeOH (5 mL) were placed in a 25 mL round-bottom flask and aq HCl (36%, 1 mL) was added. The reaction was stirred at rt for 8 h, during which the cleavage was monitored by TLC. Volatiles were removed under reduced pressure. The residue was dissolved in CH2Cl2 (10 mL) and Et3N (15 mmol) was added. The mixture was stirred at rt for 1 h, then H2O (10 mL) was added. The organic layer was taken, washed with H2O (2 × 10 mL), dried with anhydrous Na2SO4, filtered and the solvent was removed to give the crude product, which was purified by TLC plate.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (no. 21102071) and the Fundamental Research Funds for the Central Universities (no. 1107020522 and no. 1082020502). The Jiangsu 333 program (for Pan) and Changzhou Jin-Feng-Huang program (for Han) are also acknowledged.Notes and references
- (a) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS PubMed; (b) R. P. Cheng, S. H. Gellman and W. F. Degrado, Chem. Rev., 2001, 101, 3219 CrossRef CAS PubMed.
- (a) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef; (b) S. F. Martin, Acc. Chem. Res., 2002, 35, 895 CrossRef CAS PubMed; (c) B. Das, P. Balasubramanyam, B. Veeranjaneyulu and G. C. Reddy, J. Org. Chem., 2009, 74, 9505 CrossRef CAS PubMed; (d) F. A. David, M. Song, H. Qiu and J. Chai, Org. Biomol. Chem., 2009, 7, 5067 RSC.
- (a) M. Liu and M. P. Sibi, Tetrahedron, 2002, 58, 7991 CrossRef CAS; (b) M. J. Gaunt and J. B. Spencer, Org. Lett., 2001, 3, 25 CrossRef CAS; (c) N. Srivastava and B. K. Banik, J. Org. Chem., 2003, 68, 2109 CrossRef CAS PubMed; (d) N. B. Ambhaikar, J. P. Snyder and D. C. Liotta, J. Am. Chem. Soc., 2003, 125, 3690 CrossRef CAS PubMed.
- (a) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS PubMed; (b) S. Číhalová, M. Remeš, I. Císařová and J. Veselý, Eur. J. Org. Chem., 2009, 6277 CrossRef; (c) M. Hatano, T. Horibe and K. Ishihara, Org. Lett., 2010, 12, 3502 CrossRef CAS PubMed.
- (a) A. E. Sorochinsky and V. A. Soloshonok, J. Fluorine Chem., 2010, 131, 127 CrossRef CAS PubMed; (b) V. P. Kukhar, A. E. Sorochinsky and V. A. Soloshonok, Future Med. Chem., 2009, 1, 793 CrossRef CAS PubMed.
- (a) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed; (b) D. O'Hagan, Chem. Soc. Rev., 2008, 37, 308 RSC; (c) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (d) K. Muller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (e) Y. H. Pan, Y. J. Zhao, T. Ma, Y. Y. Yang, H. J. Liu, Z. Y. Jiang and C. H. Tan, Chem.–Eur. J., 2010, 16, 779 CrossRef CAS PubMed; (f) X. L. Qiu, W. D. Meng and F. L. Qing, Tetrahedron, 2004, 60, 6711 CrossRef CAS PubMed; (g) N. N. Sergeeva, A. S. Golubev, L. Hennig, M. Findeisen, E. Paetzold, G. Oehme and K. Burger, J. Fluorine Chem., 2000, 111, 41 CrossRef; (h) H. Wang, X. M. Zhao, Y. H. Li and L. Lu, Org. Lett., 2006, 8, 1379 CrossRef CAS PubMed; (i) H. Mimura, K. Kawada, T. Yamashita, T. Sakamoto and Y. Kikugawa, J. Fluorine Chem., 2010, 131, 477 CrossRef CAS PubMed; (j) S. Fustero, C. D. Pozo, S. Catalàn, J. Alemán, A. Parra, V. Marcos and J. L. G. Ruano, Org. Lett., 2009, 11, 641 CrossRef CAS PubMed.
- (a) I. Ojima, J. R. McCarthy and J. T. Welch, Biomedical Frontiers of Fluorine Chemistry, American Chemical Society, Washington, DC, 1996, ACS Symposium Series, p. 639 Search PubMed; (b) R. Filler, Y. Kobayashi and Y. L. Yagulpolskii, Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications, Elsevier, Amsterdam, 1993 Search PubMed; (c) J.-P. Bégué and D. Bonnet-Delpon, Bioorganic and medicinal chemistry of fluorine, John Wiley & Sons, Inc., Hoboken, USA, 2008 Search PubMed.
- (a) V. A. Soloshonok, A. G. Kirilenko, V. P. Kukhar and G. Resnati, Tetrahedron Lett., 1994, 35, 3119 CrossRef CAS; (b) H. Ohkura, D. O. Berbasov and V. A. Soloshonok, Tetrahedron, 2003, 59, 1647 CrossRef CAS; (c) V. A. Soloshonok, H. Ohkura and M. Yasumoto, J. Fluorine Chem., 2006, 127, 930 CrossRef CAS PubMed.
- M. T. Robak, M. A. Herbage and J. A. Ellman, Chem. Rev., 2010, 110, 3600 CrossRef CAS PubMed.
- H. Mimura, K. Kawada, T. Yamashita, T. Sakamoto and Y. Kikugawa, J. Fluorine Chem., 2010, 131, 477 CrossRef CAS PubMed.
- (a) V. L. Truong, M. S. Ménard and I. Dion, Org. Lett., 2007, 9, 683 CrossRef CAS PubMed; (b) V. L. Truong and J. Y. Pfeiffer, Tetrahedron Lett., 2009, 50, 1633 CrossRef CAS PubMed; (c) H. Mei, Y. Xiong, J. Han and Y. Pan, Org. Biomol. Chem., 2011, 9, 1402 RSC; (d) H. Zhang, Y. Li, W. Xu, W. Zheng, P. Zhou and Z. Sun, Org. Biomol. Chem., 2011, 9, 6502 RSC; (e) G.-V. Röschenthaler, V. P. Kukhar, I. B. Kulik, M. Y. Belik, A. E. Sorochinsky, E. B. Rusanov and V. A. Soloshonok, Tetrahedron Lett., 2012, 53, 539 CrossRef PubMed; (f) N. Shibata, T. Nishimine, N. Shibata, E. Tokunaga, K. Kawada, T. Kagawa, A. E. Sorochinsky and V. A. Soloshonok, Chem. Commun., 2012, 48, 4124 RSC; (g) K. V. Turcheniuk, K. O. Poliashko, V. P. Kukhar, A. B. Rozhenko, V. A. Soloshonok and A. E. Sorochinsky, Chem. Commun., 2012, 48, 11519 RSC; (h) M. V. Shevchuk, V. P. Kukhar, G.-V. Roeschenthaler, B. S. Bassil, K. Kawada, V. A. Soloshonok and A. E. Sorochinsky, RSC Adv., 2013, 3, 6479 RSC.
- (a) P. Kaur, S. Pindi, W. Wever, T. Rajale and G. Li, J. Org. Chem., 2010, 75, 5144 CrossRef CAS PubMed; (b) H. Sun, H. Zhang, J. Han, Y. Pan and G. Li, Eur. J. Org. Chem., 2013, 4744 CrossRef CAS; (c) S. Pindi, J. Wu and G. Li, J. Org. Chem., 2013, 78, 4006 CrossRef CAS PubMed.
- (a) Z. J. Liu and J. T. Liu, Chem. Commun., 2008, 5233 RSC; (b) V. L. Truong and J. Y. Pfeiffer, Tetrahedron Lett., 2009, 50, 1633 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, full spectroscopic data for new compounds 3, 4 and copies of 1H NMR and 13C NMR spectra. CCDC 951194. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra45773g |
| This journal is © The Royal Society of Chemistry 2014 |