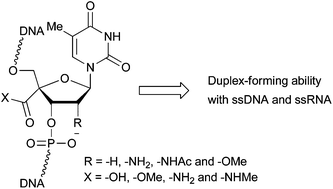
Oligonucleotides containing 4′-carboxy-, 4′-methoxycarbonyl-, 4′-carbamoyl-, and 4′-methylcarbamoyl-thymidines, and their 2′-methoxy, 2′-amino or 2′-acetamido analogs were prepared. Their duplex-forming ability with DNA and RNA complements was evaluated by UV melting experiments. Interestingly, 4′-carboxythymidine existing in the S-type sugar conformation was found to lead to an increase in the stability of the duplex formed with RNA complements compared to natural thymidine.