Open Access Article
Open Access ArticleGallium incorporation in blue-emitting In1−xGaxP alloy quantum dots facilitated by monomeric gallium precursors†
Taewan
Kim
a,
Se-Yup
Kim
a,
Sooho
Lee
b,
Ji-Sang
Park
c,
Hyeonjun
Lee
*d and
Doh C.
Lee
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, KAIST Institute for the Nanocentury, Energy and Environmental Research Center (EERC), KAIST Graduate School of Semiconductor Technology, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: dclee@kaist.edu
bSamsung Display Research Center, Samsung Display, Yongin, Republic of Korea
cSKKU Advanced Institute of Nanotechnology (SAINT), Sungkyunkwan University (SKKU), Suwon 16419, Republic of Korea
dDepartment of Energy Science and Center for Artificial Atoms, Sungkyunkwan University, Suwon 16419, Republic of Korea. E-mail: lhj9269@skku.edu
First published on 18th March 2025
Abstract
Demand for environmentally friendly quantum dots (QDs) in wide color-gamut displays has led to successful development of red- and green-emitting InP QDs with outstanding optical properties. While progress in developing blue-emitting variants remains challenging, In1−xGaxP alloy QDs have recently garnered attention as blue emitters. However, Ga incorporation in these In1−xGaxP QDs is hindered by the limited reactivity of conventional gallium halide-derived precursors having a dimeric molecular structure. Here, we adopt trimethylgallium which yields monomeric gallium carboxylates as a Ga precursor in the colloidal synthesis of In1−xGaxP QDs. This approach promotes efficient Ga incorporation into In1−xGaxP QDs with narrow size distributions. The use of zinc chloride and oleylamine for ZnS shell growth on the In1−xGaxP cores further adjusts the photoluminescence (PL) wavelength to the blue range and enhances PL quantum yield. The resulting In1−xGaxP/ZnS core/shell QDs exhibit a peak emission at 470 nm, 67% of photoluminescence quantum yield, and 40 nm of emission linewidth. Successful employment of these QDs into light-emitting diodes demonstrates their potential as a blue electroluminescent emitter for future QD displays.
1 Introduction
As colloidal quantum dot (QD) emitters gain prominence in wide color-gamut display technology, efforts to develop environmentally friendly QDs have rapidly intensified.1,2 Consequently, red- and green-emitting Cd-free QDs have achieved remarkable progress,3–5 driving the successful commercialization of photoluminescence (PL)-based QD display technology.6,7 QD display technology is set to advance beyond its current photoluminescence-based applications toward electroluminescent devices, underscoring the need for environmentally friendly blue-emitting QDs.8,9One prominent outcome of the efforts for developing blue-emitting QDs is the emergence of Zn chalcogenide-based QDs. ZnSe QDs, with a bulk band gap (Eg) of 2.7 eV, typically emit in the near-ultraviolet region, and various strategies have been employed to shift this emission into the blue range. These include controlling nanocrystal growth chemistry to synthesize ZnSe QDs with near-bulk dimensions (∼10 nm),10 and alloying ZnSe with ZnTe (with a bulk Eg of 2.26 eV) to synthesize ZnSe1−xTex alloy QDs.11,12 Despite these advances, Zn chalcogenide QDs continue to face critical challenges; ZnSe1−xTex QDs exhibit emissions from localized states caused by inhomogeneous Te doping, and Zn chalcogenide QDs demonstrate relatively low photo-13 or electro-chemical stability.14,15
III–V group QDs with a covalent nature, such as In1−xGaxP alloy QDs, are emerging as an alternative composition for blue QD emitters. Single-composition InP (with a bulk Eg of 1.35 eV and an exciton Bohr radius of ∼10 nm) is theoretically available for blue emission with a sub-2 nm diameter16 at which, the nanocrystals are not only thermodynamically unstable due to a very high surface-to-volume ratio but also energetically competitive with metastable magic size clusters.17 Uniform alloying of GaP into the InP lattice can address these thermodynamic limitations. The incorporation of GaP with a wider Eg (2.24 eV) successfully shifts the Eg of InP QDs into the blue emission region.18
However, a key challenge remains in achieving the incorporation of Ga ions within In1−xGaxP QDs. Conventional gallium halide-mediated precursors, widely used in bottom-up colloidal synthesis,19 generally exhibit lower chemical reactivity compared to their In counterparts, leading to very low Ga incorporation20,21 or nonuniform GaP alloying that results in a core/shell-like structure.22 Wegner et al. pointed out the low reactivity of the gallium halide-mediated precursors due to their dimeric structure, which features bridging halides and requires high activation energy for decomposition.20 However, it remains unclear how the structural characteristics of gallium precursors affect the synthesis of In1−xGaxP QDs.
A top-down synthesis of In1−xGaxP QDs via cation exchange reaction is considered a potential strategy to address the reactivity differences between In and Ga precursors.18,23–25 In the cation exchange process, Ga3+ ions substitute In3+ ions in the pre-synthesized host lattice of InP QDs, which is driven by Pearson's hard–soft acid–base theory.26–28 However, this method poses challenges in achieving spatial control of guest cations within the host lattice, as the incorporation of the guest cation has to be initiated from the surface of QDs.29,30 In addition, it is hard to directly integrate this post-synthetic process into the current mass production systems for QDs, based on the bottom-up colloidal synthesis. Such limitations speak volumes about the necessity for appropriate Ga precursors, enabling efficient Ga incorporation in the colloidal synthesis of In1−xGaxP QDs.
Herein, we addressed the challenge of the low reactivity of gallium halide-based precursors during the bottom-up colloidal synthesis of blue-emitting In1−xGaxP QDs by employing monomeric Ga precursors derived from organometallic trimethylgallium (TMGa). Unlike gallium carboxylates produced from gallium iodide, which maintain a dimeric structure with bridging iodides and di-substituted carboxylate groups, gallium carboxylates derived from TMGa adopt a monomeric structure with fully substituted carboxylate groups. These monomeric precursors react with indium carboxylates and phosphorus precursors at 300 °C to produce In1−xGaxP alloy QDs with a narrow size distribution and uniform composition. The photoluminescence properties of the In1−xGaxP QDs were further enhanced through ZnS shell growth utilizing zinc chloride and oleylamine. The resulting In1−xGaxP/ZnS core/shell QDs were successfully integrated into electroluminescent devices, demonstrating their potential to be widely applied in electroluminescent QD displays.
2 Experimental methods
2.1 Materials
Zinc stearate (Zn(St)2, 99%), lauric acid (LA, 98%), oleylamine (OAm, 70%), 1-dodecanethiol (DDT, ≥98%), 2-ethylhexanoic acid (2-EHA, 99%), sodium hydroxide (NaOH, ≥98%), gallium(III) iodide (GaI3, 99.99%), zinc acetate dihydrate (Zn(Ac)2·2H2O, ≥99%), magnesium acetate tetrahydrate (Mg(Ac)2·4H2O, ≥99%), tetramethylammonium hydroxide (TMAH, ≥99%), and molybdenum(VI) oxide (MoO3, ≥99.9%) were purchased from Sigma-Aldrich. Anhydrous zinc chloride (ZnCl2, 98%) and oleic acid (OA, 90%) were purchased from Alfa Aesar. Tris(trimethylsilyl)phosphine (TMS3P, 97%) was purchased from LABCARE. Trimethylgallium (TMGa, 99%) was obtained from ICHEMS. Indium acetate (In(Ac)3, 99%), zinc acetate (Zn(Ac)2, ≥99%), 1-octadecene (ODE, 98%), and trioctylphosphine (TOP, ≥97.5%) were supplied by UNIAM. Aluminum (Al, ≥99.9%) and 4,4′-bis(N-carbazolyl)-1,1′-biphenyl (CBP, ≥99.5%) were purchased from Lumtec. All other organic solvents were obtained from Samchun Chemicals. All chemicals were used as received without further purification.2.2 Ex situ synthesis of gallium carboxylates using GaI3 and TMGa
To synthesize gallium carboxylates, 2 mmol of GaI3 were dissolved in 20 mL of anhydrous methanol under stirring at room temperature. Subsequently, desired equivalents of 2-EHA and NaOH relative to GaI3 were sequentially added into the solutions. The mixture was allowed to react at room temperature for 1 hour, during which the formation of a white precipitate of gallium carboxylates was observed. The precipitate was collected, washed five times with anhydrous methanol, and dried under vacuum for subsequent analysis.To synthesize gallium carboxylates using TMGa, TMGa dissolved in TOP was reacted with a desired equivalent of 2-EHA, and the reaction was performed at 200 °C for 1 hour. The obtained gallium carboxylates were filtered out using methanol and further washed with methanol ten times. The precipitate was dried under vacuum for subsequent analysis.
2.3 Synthesis of In1−xGaxP alloy QDs
All syntheses were conducted using standard Schlenk line equipment. Indium laurate (In(LA)3) was prepared by reacting 2 mmol of In(Ac)3 with 6 mmol of LA in 6 mL of ODE under vacuum (≤200 mTorr) for 2 hours. Gallium laurate (Ga(LA)3) was prepared by reacting 5 mmol of 1.0 M TMGa dissolved in TOP with 15 mmol of LA in 10 mL of ODE at 200 °C for 30 minutes under a N2 atmosphere.To synthesize In1−xGaxP cores for blue emission, 2 mmol of Zn(St)2 was dissolved in 16 mL of ODE and degassed at 110 °C for 30 minutes.31 Subsequently, 2 mmol each of In(LA)3 and Ga(LA)3 were added to the reaction flask and further degassed at 110 °C for 30 minutes. The reaction flask was then heated to 300 °C under a N2 atmosphere and held for 1 hour to complete the formation of metal carboxylates. After the reaction temperature was lowered to 110 °C, 3 mL of 0.33 M TMS3P dissolved in TOP was injected and held for 20 minutes. The reaction flask was then reheated to 300 °C, yielding In1−xGaxP cores. After the reaction, the flask was rapidly cooled down to room temperature. The solution was purified twice using a precipitation/redispersion method with anhydrous EtOH/toluene. The purified cores were redispersed in toluene and stored in a glove box for shell growth.
2.4 ZnS shell growth on In1−xGaxP cores
0.5 M ZnCl2 solution was prepared by dissolving 5 mmol of ZnCl2 in 10 mL of TOP or OAm under stirring at 100 °C in a nitrogen-filled glove box. 0.5 M Zn(OA)2 was prepared by degassing 5 mmol of Zn(Ac)2 and 10 mmol of OA in 6.8 mL of TOA at 160 °C for 2 hours.To grow ZnS shells on In1−xGaxP QD cores, 0.37 mL of In1−xGaxP cores (optical density of 1.55 at 380 nm) were dissolved in 10 mL of ODE in a three-necked round-bottom flask and degassed at 110 °C for 30 minutes. For surface-treatment of the cores, 1 mL of 0.5 M ZnCl2 dissolved in TOP was injected into the flask and further degassed for 30 minutes. Backfilling N2, 1.5 mL of ZnCl2 dissolved in OAm and 0.19 mL of DDT were added. The reaction flask was heated up to 300 °C and held for 2 hours, which yields the ZnS inner shell. Then, for the growth of the ZnS thick shell, 2.4 mL of 0.5 M Zn(OA)2 and 0.29 mL of DDT were dropwise injected into the flask at 300 °C and the reaction was continued for another 20 minutes. After the shell growth was finished, the solution was rapidly cooled down to room temperature and purified twice using a precipitation/redispersion method with acetone/toluene. The purified In1−xGaxP/ZnS QDs were dispersed in n-octane and stored in a glove box for further use.
2.5 Fabrication of QLEDs
Zinc magnesium oxide (ZnMgO) nanoparticles, used as an electron transport layer (ETL), were synthesized with minor modifications based on a literature method.32 Specifically, 5.1 mmol of Zn(Ac)2·2H2O and 0.9 mmol of Mg(Ac)2·4H2O were dissolved in 60 mL of DMSO at 60 °C until completely dissolved. Then, 10 mmol of 0.5 M TMAH dissolved in EtOH was rapidly injected into the DMSO solution and reacted for 1 hour, yielding ZnMgO nanoparticles. The nanoparticles were collected by washing twice with acetone and redispersed in EtOH.To fabricate QLEDs, 33 nm-thick ZnMgO nanoparticle ETLs were spin-coated onto indium-tin-oxide (ITO) substrates at 4000 rpm for 30 seconds, followed by annealing at 150 °C for 25 minutes. QD emissive layers of 1.5 monolayers were deposited by spin-coating an In1−xGaxP/ZnS QD solution at 4000 rpm for 30 seconds, followed by annealing at 90 °C for 25 minutes. The spin-coated substrates were then transferred to a thermal evaporator, where 60 nm of CBP, 10 nm of MoO3, and 100 nm of an Al electrode were deposited under a vacuum of 1 × 10−6 Torr at evaporation rates of 1.5, 0.2, and 2.0 Å s−1, respectively. The fabricated devices were encapsulated with UV-curable resin and glass lids, and then stored in a glove box for further characterization.
2.6 Characterization
Elemental analysis of gallium carboxylates was performed using a Thermo Fisher Scientific FlashSmart elemental analyzer. X-ray photoelectron spectroscopy (XPS) was conducted with Thermo VG Scientific K-alpha. Fourier transform infrared (FTIR) spectroscopy was performed with Thermo Fisher Nicolet iS50. Laser desorption ionization time of flight (LDI-TOF) mass spectrometry was conducted with Bruker autoflex maX. UV-vis absorption spectroscopy was conducted with a Shimadzu UV-2600i spectrophotometer. PL spectroscopy and PL quantum yield (QY) measurements were carried out using a Hamamatsu absolute PL quantum yield spectrometer C11347 at an excitation wavelength of 365 nm. Time-resolved PL measurement was performed using a light conversion femtosecond laser (PHAROS) coupled with a PicoQuant time-correlated single-photon counting system. The laser output was converted to the excitation wavelength of 365 nm using a light conversion collinear optical parametric amplifier (ORPHEUS). Transmission electron microscopy (TEM) was conducted using an FEI Tecnai F20 microscope operating at 200 kV. Inductively coupled plasma optical emission spectrometry (ICP-OES) was performed with an Agilent ICP-OES 5110. For ICP-OES analysis, samples were digested in aqua regia (a mixture of hydrochloric acid and nitric acid in a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and diluted with deionized water. X-ray diffraction (XRD) spectroscopy was conducted with a RIGAKU Ultima IV system under Cu Kα irradiation. Ultraviolet photoelectron spectroscopy (UPS) measurements were performed with a Thermo Scientific Nexsa G2 using a He I photon source (21.2 eV). The electroluminescence characteristics of blue-emitting quantum dot light-emitting diodes (QLEDs) were measured using a Konica Minolta CS-2000 spectroradiometer coupled with a Keithley 2450 SourceMeter.
1) and diluted with deionized water. X-ray diffraction (XRD) spectroscopy was conducted with a RIGAKU Ultima IV system under Cu Kα irradiation. Ultraviolet photoelectron spectroscopy (UPS) measurements were performed with a Thermo Scientific Nexsa G2 using a He I photon source (21.2 eV). The electroluminescence characteristics of blue-emitting quantum dot light-emitting diodes (QLEDs) were measured using a Konica Minolta CS-2000 spectroradiometer coupled with a Keithley 2450 SourceMeter.
2.7 Energy change calculation in the reaction of gallium precursors using density functional theory (DFT)
We performed first-principles density functional theory calculations by using the projector augmented wave method (PAW)33 as implemented in the Vienna ab initio simulation package (VASP).34 We used an exchange–correlation functional parametrized by Perdew, Burke, and Ernzerhof35 and the DFT-D3 method with Becke–Johnson damping function for a better description of dispersion interaction.36 The plane waves were expanded up to 400 eV. The atomic structures were optimized until the residual forces became less than 0.01 eV Å−1.3 Results and discussion
3.1 Structures of gallium carboxylates derived from gallium iodide and TMGa
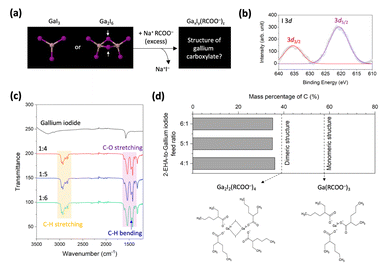
Solid-state gallium halides are predominantly stable as a structure of Ga2X6 dimers (X = Cl, Br, or I) rather than GaX3 monomers in equilibrium (Fig. 1a).37,38 Our first-principles DFT calculations support that Ga2I6 dimers are more preferred than monomeric GaI3. The energy change (ΔE) in the dimerization of GaI3 is calculated to be −0.839 eV (Fig. S1a†). Previous studies have suggested that the reaction of these Ga2X6 dimers with carboxylic acids results in the partial substitution of only two halogen atoms with carboxylate groups.20 To quantitatively examine the carboxylate substitution, we ex situ synthesized gallium carboxylates derived from gallium iodide by reacting them with 2-EHA in a methanol medium (Fig. 1a, see Experimental methods).39 To ensure complete carboxylate substitution, an excess amount of sodium 2-ethylhexanoate, exceeding three equivalents, was reacted with gallium iodide.We first conducted XPS measurements for the I 3d core-level to assess the substitution of iodide to the carboxylate group. The XPS spectra demonstrate the I 3d orbitals, revealing that the iodine atoms still remain in gallium carboxylates (Fig. 1b).40 The FTIR spectra for gallium carboxylates exhibit distinctive vibrational peaks corresponding to C–H stretching (2990–2850 cm−1), C–O stretching (1600–1500 cm−1 and 1430–1400 cm−1), and C–H bending (1462 cm−1), indicative of carboxylate groups (Fig. 1c).41 These results suggest that not all the iodide groups in gallium iodide were fully replaced by carboxylate groups, but were only partially substituted.
The elemental analysis result demonstrates that the weight percent of carbon in gallium carboxylates derived from gallium iodide was approximately 36%, regardless of the excess amount of reacting 2-EHA (Fig. 1d and Table S1†). This value is significantly lower than the theoretical carbon content of monomeric gallium tri-2-ethylhexanoate (57.7%, right lower panel in Fig. 1d). In the dimeric structure of gallium halides, the halogen atoms bridging the two gallium centers, known as bridging halides, are strongly bound to the Ga atoms.37,38 The large activation energy required for the dissociation of bridging halides contributes to the formation of dimeric and partially substituted precursors with a structure of Ga2I2(RCOO−)4 (left lower panel in Fig. 1d). This structure theoretically has a carbon mass percentage of 39%, which is close to the 36% carbon content in the analyzed gallium 2-ethylhexanoates derived from gallium iodide. We attribute the discrepancy in the carbon mass percentage to the contribution from a minor portion of mono-substituted gallium carboxylates.
To investigate the carboxylate substitution of a monomeric Ga compound and thereby to circumvent the challenges of bridging halide dissociation in gallium halides, we selected TMGa as a suitable candidate. While various organometallic compounds (e.g., diethylzinc or dimethylcadmium) have been employed in the colloidal synthesis of QDs,42–44 the application of TMGa in this field remains largely unexplored.
We also performed first-principles DFT calculations to evaluate the energy change in dimerization (Fig. S1b†) and gallium tri-2-ethylhexanoate formation reaction (Fig. S1c†) from monomeric TMGa. The energy change in the dimerization of TMGa is calculated to be 0.934 eV, which is thermodynamically unfavorable (Fig. S1b†). In contrast, the formation of gallium tri-2-ethylhexanonoates is highly favorable, with an energy change of −3.769 eV. These results suggest that monomeric TMGa would enable efficient carboxylate substitution, leading to the formation of gallium tri-carboxylates.
The FTIR spectra of TMGa and its derivative, gallium 2-ethylhexanoate, both exhibit identical vibrational peaks corresponding to C–H stretching and C–H bending of the methyl and 2-EHA groups (Fig. S2†). However, following the carboxylate substitution reaction, gallium 2-ethylhexanoate exhibits a distinctive C–O stretching peak, indicating that the carboxylate groups have replaced the methyl groups in TMGa. The LDI-TOF analysis of gallium 2-ethylhexanoates derived from TMGa reveals a sharp peak at 497.52 m/z (Fig. S3†) which greatly approximates the molar mass of ∼500 Da of gallium tri-2-ehtylhexanoate. These results suggest that gallium 2-ethylhexanoate derived from TMGa has a monomeric structure, with the methyl groups fully substituted by carboxylates (Fig. 1d, lower right).
3.2 Synthesis of In1−xGaxP alloy QDs using monomeric and dimeric gallium carboxylates
We synthesized In1−xGaxP QDs using monomeric and dimeric gallium carboxylates derived from TMGa and gallium iodides, respectively (Fig. 2a). The QD synthetic scheme was modified from the well-established bottom-up colloidal synthesis of InP QDs, which utilizes metal carboxylates and TMS3P as precursors (Fig. 2a, see the Experimental methods).45 To investigate the optical properties of the QDs as a function of Ga content, we varied the Ga-to-In feed ratios to 1, 2, and 3 while maintaining the total amount of Ga and In precursors constant.The absorption spectra demonstrate that the 1S transition peak wavelength of the QDs blue-shifted with increasing Ga-to-In feed ratios, regardless of the type of gallium carboxylate used (Fig. 2b). However, the 1S transition peak wavelength of QDs using monomeric Ga(LA)3 was 30–40 nm shorter than that of QDs using dimeric Ga2I2LA4. This suggests that for the same Ga-to-In feed ratio, Ga incorporation into QDs was more efficient when using monomeric Ga(LA)3 compared to that with dimeric Ga2I2LA4. The half-width at half-maximum (HWHM) of the absorption peaks of monomeric precursor-based QDs was significantly narrower (with a smaller valley-to-peak ratio in the absorption spectra) compared to that of dimeric precursor-based QDs with similar 1S transition energies (Fig. S4†). This result indicates that the monomeric gallium precursor produces QDs with a narrow size distribution (inset image in Fig. 2b).
We attribute the superior optical properties of TMGa-mediated In1−xGaxP QDs to the monomeric structure of gallium carboxylates. Monomeric Ga(LA)3 could effectively decompose during the early stages of the nucleation and subsequent growth of QDs, enabling uniform incorporation of Ga. In contrast, the decomposition of bridging halides in the dimeric gallium carboxylate requires high temperatures close to 300 °C,46 at which point a vast majority of indium and phosphorus precursors are already consumed by QD formation. The robust bonding configurations of bridging halides exacerbate the reactivity difference between indium and gallium precursors, leading to nonuniform Ga incorporation. To additionally testify whether the monomeric structure in gallium precursors plays a decisive role in efficient Ga incorporation into QDs, we also synthesized In1−xGaxP QDs using monomeric gallium acetylacetonate (Ga(acac)3) as a replacement of TMGa. The resulting optical properties were similar to those of QDs synthesized with TMGa (Fig. S5†).
In order to investigate how the gallium precursors affect the crystal properties of In1−xGaxP QDs, we performed XRD analysis on the samples (Fig. 2c). Both samples exhibited distinctive diffraction peaks corresponding to the (111), (220), and (311) planes (Fig. 2c, dashed lines), which were positioned between the standard peak positions of zinc blende InP (black vertical lines in Fig. 2c) and GaP (red vertical lines in Fig. 2c). While the diffraction peaks of gallium iodide-mediated QDs were closer to the standard peak positions of InP, the peaks of TMGa-derived QDs shifted toward higher 2θ, closer to the GaP standard peaks. This shift suggests higher Ga content incorporated into In1−xGaxP QDs derived from TMGa, resulting in a reduction in d-spacing.
3.3 Composition analysis of In1−xGaxP QDs according to the extent of carboxylate substitution from TMGa
Previous analyses demonstrate that the monomeric structure of gallium carboxylates facilitates Ga incorporation in In1−xGaxP QDs. However, a question remains as to how the under-substitution of carboxylate (e.g., di-substitution) from TMGa affects the Ga incorporation into In1−xGaxP QDs. To address this question, we synthesized In1−xGaxP QDs using di-substituted gallium laurate [i.e., intentionally reacting 2 equivalents of LA with TMGa to produce Ga(CH3)(LA)2]. Interestingly, the absorption spectra reveal that the wavelength at the 1S transition peaks was seldom changed regardless of the Ga-to-In feed ratio, with only the HWHM of the absorption peaks varying (Fig. S6a†). This result implies that the amount of di-substituted gallium laurate has little influence on the extent of the Ga incorporation into In1−xGaxP QDs.To quantitatively characterize the composition in In1−xGaxP QDs, we performed ICP-OES analysis of the QD samples synthesized using Ga(LA)3 and Ga(CH3)(LA)2 (Table S2† and Fig. 3). When Ga(LA)3 was used, the incorporated Ga-to-In ratio in In1−xGaxP linearly increased to In0.6Ga0.4P, In0.4Ga0.6P, and In0.3Ga0.7P as the feed Ga-to-In ratio increased by 1, 2, and 3, respectively (red squares in Fig. 3). This tendency corresponds to the widening of the optical Eg observed as the Ga-to-In feed ratio increased (Fig. 2b). The reaction yield of Ga (i.e., the incorporated Ga-to-In ratio divided by the fed Ga-to-In ratio) for Ga(LA)3 reached ∼71%. This value is significantly higher compared to the ∼40% reaction yield typically achieved with gallium halide-based precursors.47
When Ga(CH3)(LA)2 was used with a feed Ga-to-In ratio of 1, In0.5Ga0.5P QDs were produced with a reaction yield of Ga approaching 100% (leftmost triangle in Fig. 3). However, as the Ga-to-In feed ratio increased to 2 and 3, the detected Ga-to-In ratios exceeded the fed Ga-to-In ratio. Considering that TMS3P is the limiting reagent, this result indicates that TMS3P selectively reacted with Ga(CH3)(LA)2 rather than In(LA)3, producing GaP QDs apart from In0.5Ga0.5P QDs. This result is in line with the consistent 1S transition peaks regardless of the amount of Ga(CH3)(LA)2 (Fig. S6a†). Notably, we could synthesize GaP QDs using only Ga(CH3)(LA)2 and TMS3P (Fig. S6a†), even though GaP QDs were typically known to require high formation energies.48
To sum up, the under-substitution of carboxylates in monomeric gallium precursors exhibits higher reactivity compared to their fully substituted counterparts. While Ga incorporation into In1−xGaxP QDs was more efficient at a low Ga-to-In feed ratio (Ga-to-In ratio of 1) using under-substituted precursors, a separate formation of GaP QDs simultaneously occurred at higher Ga-to-In feed ratios (Ga-to-In ratios of 2 and 3).
3.4 Optical enhancement of In1−xGaxP/ZnS core/shell QDs using ZnCl2 and their electroluminescence applications
The as-synthesized In1−xGaxP QDs exhibit negligible photoluminescence (PL QY of ∼1%) due to the nonradiative surface defects.49,50 Undercoordinated and oxidized surface metal and phosphorus atoms have been generally identified as the primary culprits for nonradiative recombination centers in metal phosphide QDs.51,52 To address the surface issue of In1−xGaxP core QDs, we employed a surface treatment method using zinc halides. The zinc halides act as Z-type ligands, effectively passivating the undercoordinated surface atoms prior to the shell growth (Fig. 4a).53,54Interestingly, we found that the use of ZnCl2 not only passivates the surface but also helps adjust the PL peak wavelength of the In1−xGaxP/ZnS QDs. Achieving a PL peak within the range of 460 to 475 nm for blue emitters is critical to meet the spectral requirements of the National Television System Committee (NTSC) while avoiding severe damage to the human eye from short-wavelength emissions.55–57 The use of Zn(OA)2, as a conventional Zn precursor, during ZnS shell growth resulted in QDs with an emission peak exceeding 480 nm (Fig. S7a†), requiring a blue shift of PL by at least 5 nm. This longer PL peak wavelength could be attributed to the binding behavior of the oleate ligand in Zn(OA)2, which acts as a quasi-bidentate ligand due to its chelating geometry.58 The chelating nature promotes the formation of strong hybrid molecular orbitals with the shell material, leading to enhanced exciton delocalization into the shell.59,60
To address the chelation issue, we utilized a ZnCl2–OAm precursor (ZnCl2 dissolved in OAm) as a Zn precursor for shell growth, replacing Zn(OA)2 with a monodentate ligand.61,62 The monodentate OAm ligand minimized the exciton delocalization by chelation, resulting in a hypsochromic shift of approximately 10 nm, which could then align with the NTSC requirements (solid line in Fig. S7b†). However, after the purification process for the integration into QLEDs, the PL QY of the In1−xGaxP/ZnS QDs was reduced to nearly half of its initial value (dashed line in Fig. S7b†) due to the detachment of weakly bound OAm ligands on the ZnS shell during the purification process.63 To prevent ligand detachment after purification, an additional ZnS shell was grown using Zn(OA)2 (Fig. 4b) to ensure effective surface passivation. This modification produced In1−xGaxP/ZnS core/shell QDs with an average diameter of 6.34 nm, consisting of 2.3 nm In1−xGaxP cores and a 2.0 nm ZnS shell (Fig. 5a). The resulting QDs exhibited remarkable optical properties, with an emission peak at 470 nm, a narrow linewidth of 40 nm, and a PL QY of 67% after purification (Fig. 5b). The XRD spectra of the In1−xGaxP/ZnS QDs were compared with those of the In1−xGaxP cores (Fig. 5c). The (111), (220), and (311) diffraction peaks of the zinc blende In1−xGaxP cores shifted to higher 2θ values after the growth of ZnS shells. This shift indicates the uniform growth of ZnS thick shell on the In1−xGaxP cores, which is responsible for the robust PL properties and narrow linewidths of the In1−xGaxP/ZnS QDs.
 | ||
| Fig. 5 Characterization of the In1−xGaxP/ZnS core/shell QDs and their electroluminescence properties. (a) TEM image of In1−xGaxP/ZnS QDs. (b) UV-vis absorption and PL emission spectra of In1−xGaxP/ZnS QDs. The PL peak is located at 470 nm, with a FWHM of 40 nm and a PL QY of 67%. The inset photograph demonstrates the In1−xGaxP/ZnS QDs irradiated with a UV light of 380 nm. (c) XRD spectra of In1−xGaxP cores and In1−xGaxP/ZnS core/shell QDs (lower panel). Standard diffraction peaks for InP (JCPDS #70-2902), GaP (JCPDS #72-4949), and ZnS (JCPDS #86-8464) are provided in the upper panels. Dashed lines indicate the peaks corresponding to the (111), (220), and (311) planes of In1−xGaxP cores and In1−xGaxP/ZnS QDs, respectively. (d) Energy level diagram with respect to a vacuum level for the In1−xGaxP/ZnS QD-employed QLEDs. The energy levels of In1−xGaxP/ZnS QDs were characterized using UPS analysis (Fig. S8†). (e) Current (J)–voltage (V)–luminance (L) characteristics of In1−xGaxP/ZnS QD-employed QLEDs. The inset shows the PL and EL spectra of the QLEDs where the EL spectrum is measured at 6 V. | ||
The resulting QDs exhibited one of the narrowest linewidths reported to date among blue-emitting In1−xGaxP QDs (Table S4†), highlighting their potential for blue-color reproduction in display technologies. However, the underlying reason for the limited PL QY (67%) of In1−xGaxP/ZnS QDs, even after uniform ZnS shell growth, remains unclear. We attribute the limited PL QY primarily to the increased contribution of indirect Eg transition arising from high Ga content in In1−xGaxP/ZnS QDs.25 We synthesized In1−xGaxP/ZnS QDs with varying Ga-to-In feed ratios of 1, 2, and 3 during core synthesis and analyzed their PL characteristics (Fig. S8†). As the Ga-to-In feed ratio increases, the PL spectra blue-shifts (Fig. S8a†), indicating a widened Eg due to a higher amount of Ga incorporated. Concurrently, the PL QY becomes lower (Fig. S8b†). To further elucidate the relationship between Ga content and PL characteristics, we measured time-resolved PL of the samples (Fig. S8c–S8e†). Biexponential fitting of the PL decay curves revealed two distinct decay components (Table S3†): a trap-associated recombination component (1.5–1.6 ns) and a radiative recombination component (45.9–58.7 ns). While the former was relatively consistent regardless of the Ga-to-In ratio, the latter became longer as the ratio increased. The prolonged radiative recombination lifetime, accompanied by a decrease of PL QY, is a distinctive feature of enhanced indirect band gap transition in In1−xGaxP/ZnS QDs.25 These results emphasize that precise control of Ga incorporation into In1−xGaxP QDs is essential for optimizing both PL QY and emission wavelength to attain efficient blue emission.
Finally, we incorporated the In1−xGaxP/ZnS QDs into QLEDs with an inverted structure. The device architecture consisted of an ITO cathode, a ZnMgO electron transport layer (ETL), an In1−xGaxP/ZnS QD emissive layer, a CBP hole transport layer (HTL), and a MoO3/Al anode (Fig. 5d and Fig. S9†).18,64,65 Current (J)–voltage (V)–luminance (L) characteristics show that the QLEDs exhibit a low turn-on voltage of 2.6 V which is close to the optical Eg of the QDs (Fig. 5e). The EL peak of the QLEDs exhibits a moderate extent of red shift from 470 nm to 477 nm due to the quantum-confined Stark effect at a high operation voltage of 6 V (Fig. 5c).66 These electrical advantages are attributed to the well-matched energy levels of In1−xGaxP/ZnS QDs to those of charge transport layers, leading to the minimal accumulation of drift charges with low carrier injection barriers.67 Based on the EL properties of our devices (Fig. S10 and Table S5†), we could highlight the potential of In1−xGaxP/ZnS QDs as promising electroluminescent emitters for future blue QLEDs.
4 Conclusions
In summary, we revealed the structural effect of Ga precursors on the Ga incorporation into the In1−xGaxP QDs. Due to the strong bonding of bridge halides in Ga2I6, Ga2I2(RCOO−)4 with a dimeric structure is produced after the carboxylate substitution of Ga2I6. These dimeric Ga precursors exhibit low reactivity during the synthesis of In1−xGaxP QDs, limiting the Ga incorporation. In contrast, monomeric TMGa produced monomeric gallium carboxylates with tri-substituted carboxylates of Ga(RCOO−)3, facilitating Ga incorporation during the synthesis of In1−xGaxP QDs. Meanwhile, partially substituted gallium carboxylates from TMGa (i.e., Ga(CH3)(RCOO−)2) led to the formation of separate GaP QDs due to their higher reactivity compared to that of their indium counterparts.The use of ZnCl2 and OAm in the early stage of ZnS shell growth improved the optical properties of In1−xGaxP/ZnS core/shell QDs. The ZnCl2–OAm precursors adjusted the emission wavelength to the blue range due to their monodentate coordination characteristics. Additional ZnS outer shell growth further passivated the In1−xGaxP/ZnS QDs, resulting in efficient and robust PL characteristics, including an emission peak at 470 nm, a linewidth of 40 nm, and a PLQY of 67%. Consequently, the resulting In1−xGaxP/ZnS QDs were successfully incorporated into the QLEDs. The In1−xGaxP/ZnS QD-based QLEDs show efficient charge injection characteristics due to the well-balanced energy levels of the QDs with the charge transport layers.
These results highlight the promising operational performance of these devices and reinforce the potential of In1−xGaxP QDs as environmentally friendly materials for optoelectronic applications. While further advancements in QD synthesis and device engineering are necessary for commercialization, we believe that this work establishes a solid foundation for the deployment of In1−xGaxP-based QDs in next-generation display technologies.
Author contributions
T. Kim: investigation, conceptualization, methodology, data curation, writing – original draft, and writing – review and editing. S.-Y. Kim: methodology, data curation, and writing – review and editing. S. Lee: conceptualization, funding acquisition, and writing – review and editing. J.-S. Park: methodology, software, and writing – review and editing. H. Lee: supervision, investigation, conceptualization, methodology, data curation, writing – original draft, and writing – review and editing. D. C. Lee: supervision, conceptualization, project administration, funding acquisition, writing – original draft, and writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (RS-2021-NR057412 and RS-2022-NR070840). Additional support was provided by the Korea Planning & Evaluation Institute of Industrial Technology (KEIT) funded by the Korean government (MOTIE) (20019417 and RS-2024-00440884) and the Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (RS-2023-KI002692, HRD Program for Industrial Innovation). We also acknowledge support from Samsung Display Co., Ltd.References
- Y. U. Kim, D. Y. Kim, J. W. Park and B. G. Jeong, Heavy-Metal-Free Heterostructured Nanocrystals for Light-Emitting Applications, Korean J. Chem. Eng., 2024, 41, 3303–3315 CrossRef CAS.
- Y. Sung, J. Chang, S. Choi and S. Jeong, Synthesis Strategies and Applications of Non-toxic Quantum Dots, Korean J. Chem. Eng., 2024, 41, 3317–3343 CrossRef CAS.
- Y.-H. Won, O. Cho, T. Kim, D.-Y. Chung, T. Kim, H. Chung, H. Jang, J. Lee, D. Kim and E. Jang, Highly efficient and stable InP/ZnSe/ZnS quantum dot light-emitting diodes, Nature, 2019, 575, 634–638 CrossRef CAS PubMed.
- J. H. Jo, D. Y. Jo, S. W. Choi, S. H. Lee, H. M. Kim, S. Y. Yoon, Y. Kim, J. N. Han and H. Yang, Highly bright, narrow emissivity of InP quantum dots synthesized by aminophosphine: effects of double shelling scheme and Ga treatment, Adv. Opt. Mater., 2021, 9, 2100427 CrossRef CAS.
- H. J. Lee, S. Im, D. Jung, K. Kim, J. A. Chae, J. Lim, J. W. Park, D. Shin, K. Char, B. G. Jeong, J.-S. Park, E. Hwang, D. C. Lee, Y.-S. Park, H.-J. Song, J. H. Chang and W. K. Bae, Coherent heteroepitaxial growth of I-III-VI2 Ag(In,Ga)S2 colloidal nanocrystals with near-unity quantum yield for use in luminescent solar concentrators, Nat. Commun., 2023, 14, 3779 CrossRef PubMed.
- J. Yang, M. Lee, S. Y. Park, M. Park, J. Kim, N. Sitapure, D. Hahm, S. Rhee, D. Lee, H. Jo, Y. H. Jo, J. Lim, J. Kim, T. J. Shin, D. C. Lee, K. Kwak, J. S. Kwon, B. Kim, W. K. Bae and M. S. Kang, Nondestructive Photopatterning of Heavy-Metal-Free Quantum Dots, Adv. Mater., 2022, 34, 2205504 CrossRef CAS PubMed.
- B. G. Jeong, J. H. Chang, D. Hahm, S. Rhee, M. Park, S. Lee, Y. Kim, D. Shin, J. W. Park, C. Lee, D. C. Lee, K. Park, E. Hwang and W. K. Bae, Interface polarization in heterovalent core–shell nanocrystals, Nat. Mater., 2022, 21, 246–252 CrossRef CAS PubMed.
- S. Cho, Y. Kim, S. Lee and J. Y. Woo, Recent Progress in Blue-Emitting Semiconductor Nanocrystal Quantum Dots for Display Applications, Korean J. Chem. Eng., 2024, 41, 3359–3370 CrossRef CAS.
- Y. Jeon, H. Ryu and H. Lee, Recent Progress on Blue Quantum Dot Light-Emitting Diodes from Materials to Device Engineering, Korean J. Chem. Eng., 2024, 41, 3483–3500 CrossRef CAS.
- M. Gao, H. Yang, H. Shen, Z. Zeng, F. Fan, B. Tang, J. Min, Y. Zhang, Q. Hua, L. S. Li, B. Ji and Z. Du, Bulk-like ZnSe Quantum Dots Enabling Efficient Ultranarrow Blue Light-Emitting Diodes, Nano Lett., 2021, 21, 7252–7260 CrossRef CAS PubMed.
- B. J. Lee, T. Y. Kim, I. Kim, J. Y. Ryu, S. Jung, J.-U. Park, D. H. Yoon, Y. Choi, S. Y. Lee and T. Kim, Bright and Stable ZnSeTe Core/Shell Quantum Dots Enabled by Surface Passivation with Organozinc Halide Ligands, Chem. Mater., 2023, 36, 471–481 CrossRef.
- C. Cheng, B. Yu, F. Huang, L. Gao, K. Cao, P. Zang, K. Zheng and J. Tian, Near–Unity Quantum Yield ZnSeTe Quantum Dots Enabled by Controlling Shell Growth for Efficient Deep–Blue Light–Emitting Diodes, Adv. Funct. Mater., 2024, 2313811 CrossRef CAS.
- S. Kim, J.-A. Kim, T. Kim, H. Chung, S. Park, S.-M. Choi, H.-M. Kim, D.-Y. Chung and E. Jang, Efficient blue-light-emitting Cd-free colloidal quantum well and its application in electroluminescent devices, Chem. Mater., 2020, 32, 5200–5207 CrossRef CAS.
- Y. Bi, S. Cao, P. Yu, Z. Du, Y. Wang, J. Zheng, B. Zou and J. Zhao, Reducing Emission Linewidth of Pure–Blue ZnSeTe Quantum Dots through Shell Engineering toward High Color Purity Light–Emitting Diodes, Small, 2023, 19, 2303247 CAS.
- E.-P. Jang, C.-Y. Han, S.-W. Lim, J.-H. Jo, D.-Y. Jo, S.-H. Lee, S.-Y. Yoon and H. Yang, Synthesis of alloyed ZnSeTe quantum dots as bright, color-pure blue emitters, ACS Appl. Mater. Interfaces, 2019, 11, 46062–46069 CAS.
- E. Cho, H. Jang, J. Lee and E. Jang, Modeling on the size dependent properties of InP quantum dots: a hybrid functional study, Nanotechnology, 2013, 24, 215201 Search PubMed.
- H. H. Ripberger, S. F. Sandeno, F. W. Eagle, H. A. Nguyen and B. M. Cossairt, Structure and Reactivity of II–VI and III–V Magic-Sized Clusters: Understanding and Expanding the Scope of Accessible Form and Function, Acc. Mater. Res., 2024, 5, 726–738 Search PubMed.
- K.-H. Kim, J.-H. Jo, D.-Y. Jo, C.-Y. Han, S.-Y. Yoon, Y. Kim, Y.-H. Kim, Y. H. Ko, S. W. Kim, C. Lee and H. Yang, Cation-Exchange-Derived InGaP Alloy Quantum Dots toward Blue Emissivity, Chem. Mater., 2020, 32, 3537–3544 CAS.
- H. Shin, D. Hong, H. Cho, H. Jang, G. Y. Kim, K. M. Song, M.-J. Choi, D. Kim and Y. S. Jung, Indirect-to-direct bandgap transition in GaP semiconductors through quantum shell formation on ZnS nanocrystals, Nat. Commun., 2024, 15, 8125 CAS.
- K. D. Wegner, S. Pouget, W. L. Ling, M. Carrière and P. Reiss, Gallium–a versatile element for tuning the photoluminescence properties of InP quantum dots, Chem. Commun., 2019, 55, 1663–1666 CAS.
- Y. Kim, K. Yang and S. Lee, Highly luminescent blue-emitting In 1− x Ga x P@ ZnS quantum dots and their applications in QLEDs with inverted structure, J. Mater. Chem. C, 2020, 8, 7679–7687 Search PubMed.
- J. P. Park, J.-J. Lee and S.-W. Kim, Highly luminescent InP/GaP/ZnS QDs emitting in the entire color range via a heating up process, Sci. Rep., 2016, 6, 30094 CAS.
- V. Srivastava, V. Kamysbayev, L. Hong, E. Dunietz, R. F. Klie and D. V. Talapin, Colloidal chemistry in molten salts: Synthesis of luminescent In1−x Ga x P and In1−x Ga x As quantum dots, J. Am. Chem. Soc., 2018, 140, 12144–12151 CAS.
- M. H. Hudson, A. Gupta, V. Srivastava, E. M. Janke and D. V. Talapin, Synthesis of In1−x Ga x P Quantum Dots in Lewis Basic Molten Salts: The Effects of Surface Chemistry, Reaction Conditions, and Molten Salt Composition, J. Phys. Chem. C, 2022, 126, 1564–1580 Search PubMed.
- A. Gupta, J. C. Ondry, K. Lin, Y. Chen, M. H. Hudson, M. Chen, R. D. Schaller, A. J. Rossini, E. Rabani and D. V. Talapin, Composition-Defined Optical Properties and the Direct-to-Indirect Transition in Core–Shell In1−x Ga x P/ZnS Colloidal Quantum Dots, J. Am. Chem. Soc., 2023, 145, 16429–16448 CAS.
- B. J. Beberwyck, Y. Surendranath and A. P. Alivisatos, Cation exchange: a versatile tool for nanomaterials synthesis, J. Phys. Chem. C, 2013, 117, 19759–19770 CAS.
- R. G. Pearson, Absolute electronegativity and hardness: application to inorganic chemistry, Inorg. Chem., 1988, 27, 734–740 CAS.
- D. Lee, W. D. Kim, S. Lee, W. K. Bae, S. Lee and D. C. Lee, Direct Cd-to-Pb exchange of CdSe nanorods into PbSe/CdSe axial heterojunction nanorods, Chem. Mater., 2015, 27, 5295–5304 Search PubMed.
- L. De Trizio and L. Manna, Forging colloidal nanostructures via cation exchange reactions, Chem. Rev., 2016, 116, 10852–10887 CrossRef CAS PubMed.
- S. Koh, W. D. Kim, W. K. Bae, Y. K. Lee and D. C. Lee, Controlling ion-exchange balance and morphology in cation exchange from Cu3−x P nanoplatelets into InP crystals, Chem. Mater., 2019, 31, 1990–2001 CrossRef CAS.
- S. Koh, T. Eom, W. D. Kim, K. Lee, D. Lee, Y. K. Lee, H. Kim, W. K. Bae and D. C. Lee, Zinc–phosphorus complex working as an atomic valve for colloidal growth of monodisperse indium phosphide quantum dots, Chem. Mater., 2017, 29, 6346–6355 Search PubMed.
- Y.-B. Eun, G.-P. Jang, J.-H. Yang, S.-Y. Kim, Y.-B. Chae, M.-Y. Ha, D.-G. Moon and C.-K. Kim, Performance improvement of quantum dot light-emitting diodes using a ZnMgO electron transport layer with a core/shell structure, Materials, 2023, 16, 600 CrossRef CAS PubMed.
- P. E. Blöchl, Projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 Search PubMed.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- A. F. Wells, Structural inorganic chemistry, Oxford University Press, USA, 2012 Search PubMed.
- C. Brünig, S. Locmelis, E. Milke and M. Binnewies, Chemischer Transport fester Lösungen. 27. Mischphasenbildung und chemischer Transport im System ZnSe/GaAs, Z. Anorg. Allg. Chem., 2006, 632, 1067–1072 CrossRef.
- S. Mishra, S. Daniele and L. G. Hubert-Pfalzgraf, Metal 2-ethylhexanoates and related compounds as useful precursors in materials science, Chem. Soc. Rev., 2007, 36, 1770–1787 RSC.
- J. L. Bourque, M. C. Biesinger and K. M. Baines, Chemical state determination of molecular gallium compounds using XPS, Dalton Trans., 2016, 45, 7678–7696 RSC.
- P. J. Larkin and A. Jackson, Interpretation of the Infrared Spectra of Metal-Stearate Salts, Appl. Spectrosc. Pract., 2024, 2(2) DOI:10.1177/27551857241253834.
- C. Murray, D. J. Norris and M. G. Bawendi, Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- J. H. Yu, J. Joo, H. M. Park, S.-I. Baik, Y. W. Kim, S. C. Kim and T. Hyeon, Synthesis of quantum-sized cubic ZnS nanorods by the oriented attachment mechanism, J. Am. Chem. Soc., 2005, 127, 5662–5670 CrossRef CAS PubMed.
- J. Bang, J. Park, J. H. Lee, N. Won, J. Nam, J. Lim, B. Y. Chang, H. J. Lee, B. Chon, J. Shin, J. B. Park, J. H. Choi, K. Cho, S. M. Park, T. Joo and S. Kim, ZnTe/ZnSe (Core/Shell) Type-II Quantum Dots: Their Optical and Photovoltaic Properties, Chem. Mater., 2010, 22, 233–240 CrossRef CAS.
- Y. Kim, S. Ham, H. Jang, J. H. Min, H. Chung, J. Lee, D. Kim and E. Jang, Bright and uniform green light emitting InP/ZnSe/ZnS quantum dots for wide color gamut displays, ACS Appl. Nano Mater., 2019, 2, 1496–1504 CrossRef CAS.
- N. J. DeYonker and S. A. Shah, The role of core–valence electron correlation in gallium halides: a comparison of composite methods, Theor. Chem. Acc., 2014, 133, 1–11 Search PubMed.
- Y. Kim and S. Lee, Investigating the role of zinc precursor during the synthesis of the core of III–V QDs, Chem. Commun., 2022, 58, 875–878 CAS.
- Y. Choi, C. Choi, J. Bae, J. Park and K. Shin, Synthesis of gallium phosphide quantum dots with high photoluminescence quantum yield and their application as color converters for LEDs, J. Ind. Eng. Chem., 2023, 123, 509–516 CAS.
- P. Liu, Y. Lou, S. Ding, W. Zhang, Z. Wu, H. Yang, B. Xu, K. Wang and X. W. Sun, Green InP/ZnSeS/ZnS core multi–shelled quantum dots synthesized with aminophosphine for effective display applications, Adv. Funct. Mater., 2021, 31, 2008453 CrossRef CAS.
- D. Hahm, J. H. Chang, B. G. Jeong, P. Park, J. Kim, S. Lee, J. Choi, W. D. Kim, S. Rhee, J. Lim, D. C. Lee, C. Lee, K. Char and W. K. Bae, Design Principle for Bright, Robust, and Color-Pure InP/ZnSexS1−x/ZnS Heterostructures, Chem. Mater., 2019, 31, 3476–3484 CAS.
- K. C. Dumbgen, J. Leemans, V. De Roo, M. Minjauw, C. Detavernier and Z. Hens, Surface Chemistry of InP Quantum Dots, Amine–Halide Co-Passivation, and Binding of Z-Type Ligands, Chem. Mater., 2023, 35, 1037–1046 CAS.
- G. Almeida, R. F. Ubbink, M. Stam, I. du Fossé and A. J. Houtepen, InP colloidal quantum dots for visible and near-infrared photonics, Nat. Rev. Mater., 2023, 8, 742–758 CrossRef CAS.
- H.-L. Hu, H. Hao, X. Ren, Z.-Y. Chen, M. Liu, Y. Liu and F.-L. Jiang, Bright InP quantum dots by mid-synthetic modification with zinc halides, Inorg. Chem., 2023, 62, 2877–2886 CAS.
- J. J. Calvin, J. K. Swabeck, A. B. Sedlak, Y. Kim, E. Jang and A. P. Alivisatos, Thermodynamic investigation of increased luminescence in indium phosphide quantum dots by treatment with metal halide salts, J. Am. Chem. Soc., 2020, 142, 18897–18906 CAS.
- M. F. Prodanov, V. V. Vashchenko and A. K. Srivastava, Progress toward blue-emitting (460–475 nm) nanomaterials in display applications, Nanophotonics, 2021, 10, 1801–1836 CAS.
- J. Zhang, L. Wang, X. Zhang, G. Xie, G. Jia, J. Zhang and X. Yang, Blue light-emitting diodes based on halide perovskites: Recent advances and strategies, Mater. Today, 2021, 51, 222–246 CrossRef CAS.
- C. Luo, C. Yan, W. Li, F. Chun, M. Xie, Z. Zhu, Y. Gao, B. Guo and W. Yang, Ultrafast thermodynamic control for stable and efficient mixed halide perovskite nanocrystals, Adv. Funct. Mater., 2020, 30, 2000026 CrossRef CAS.
- S. Ghosh, K. Das, K. Chakrabarti and S. De, Effect of oleic acid ligand on photophysical, photoconductive and magnetic properties of monodisperse SnO 2 quantum dots, Dalton Trans., 2013, 42, 3434–3446 RSC.
- J.-Y. Yoo, S. A. Park, W. H. Jung, C. W. Lee, J. S. Kim, J.-G. Kim and B. D. Chin, Effect of dithiocarbamate chelate ligands on the optical properties of InP/ZnS quantum dots and their display devices, Mater. Chem. Phys., 2020, 253, 123415 CrossRef CAS.
- M. T. Frederick and E. A. Weiss, Relaxation of exciton confinement in CdSe quantum dots by modification with a conjugated dithiocarbamate ligand, ACS Nano, 2010, 4, 3195–3200 CrossRef CAS PubMed.
- X. Yin, M. Shi, J. Wu, Y.-T. Pan, D. L. Gray, J. A. Bertke and H. Yang, Quantitative analysis of different formation modes of platinum nanocrystals controlled by ligand chemistry, Nano Lett., 2017, 17, 6146–6150 CrossRef CAS PubMed.
- Y.-J. Choi, C. W. Lee and J. S. Kim, Improvement of quantum dot light-emitting device efficiency by using multi-functional bipyridine ligands, J. Korean Phys. Soc., 2020, 76, 1121–1126 Search PubMed.
- M. Liu, Y.-Y. Wang, Y. Liu and F.-L. Jiang, Thermodynamic implications of the ligand exchange with alkylamines on the surface of CdSe quantum dots: The importance of ligand–ligand interactions, J. Phys. Chem. C, 2020, 124, 4613–4625 CrossRef CAS.
- H. Yu, Color Tuning for Perovskite Light-Emitting Diodes, Linköping University Electronic Press, 2020 Search PubMed.
- S. Wang, Y. Guo, D. Feng, L. Chen, Y. Fang, H. Shen and Z. Du, Bandgap tunable Zn 1− x Mg x O thin films as electron transport layers for high performance quantum dot light-emitting diodes, J. Mater. Chem. C, 2017, 5, 4724–4730 Search PubMed.
- S. A. Empedocles and M. G. Bawendi, Quantum-confined stark effect in single CdSe nanocrystallite quantum dots, Science, 1997, 278, 2114–2117 CrossRef CAS PubMed.
- H. Lee, B. G. Jeong, W. K. Bae, D. C. Lee and J. Lim, Surface state-induced barrierless carrier injection in quantum dot electroluminescent devices, Nat. Commun., 2021, 12, 5669 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00302d |
| This journal is © the Partner Organisations 2025 |