Open Access Article
Open Access ArticleEnhancing the mechanical strength and toughness of epoxy resins with linear POSS nano-modifiers†
Hong
Chi
 a,
Guocheng
Zhang
a,
Ning
Wang
a,
Yaoguang
Wang
*a,
Tianduo
Li
a,
FuKe
Wang
a,
Guocheng
Zhang
a,
Ning
Wang
a,
Yaoguang
Wang
*a,
Tianduo
Li
a,
FuKe
Wang
 *b and
Chen
Ye
c
*b and
Chen
Ye
c
aShandong Provincial Key Laboratory of Molecular Engineering, School of Chemistry and Chemical Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China 250353. E-mail: wangyaoguang@qlu.edu.cn
bPolymeric Materials Department, Institute of Materials Research and Engineering, Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, #08-03 Innovis, Singapore 138634. E-mail: wangf@imre.a-star.edu.sg
cSchool of Chemistry and Chemical Engineering, University of Jinan, No. 336, West Road of Nan Xinzhuang, China 250022
First published on 7th January 2022
Abstract
Glass transition temperature (Tg) always deteriorates while improving the strength of epoxy resins which inherently suffer from brittleness. Herein, novel linear polyhedral oligomeric silsesquioxane (POSS)-epoxy nano-modifiers are synthesized with variable contents of POSS. The thermomechanical properties and chemical structure study of the POSS-epoxy indicates significant differences of the rigid POSS content in the linear nano-modifiers. By taking advantage of the synergistic effect of nanofillers and linear polymers, the modifiers disperse at the molecular level when POSS-epoxy is utilized as a co-curing agent for epoxy resins, allowing the applied force to be transferred into the polymer matrix. A good balance of Tg, stiffness, and fracture toughness can be obtained. At 5 wt% of the nano-modifier, the resultant epoxy resins showed 27% enhancement in the Young's modulus relative to the neat epoxy. In addition, the Tg and strength of epoxy thermosets are improved due to the increased cross-linking density, rough surface and tortuous path that resulted in good dispersion of energy during crack propagation.
1. Introduction
Epoxy resin is one of the most important thermosetting polymers. Because of its good mechanical and thermal properties and high chemical resistance, it is widely used as a composite matrix for adhesives and structural materials in the aerospace and electronics industries.1 However, epoxy resins are inherently brittle and notch sensitive due to their highly cross-linked structure. Various materials have been utilized to improve the toughness of epoxy resins, including inorganic nanoparticles,2 carbon based nanomaterials,3,4 synthetic rubber,5 dendrimers,6 block copolymers7 and natural macromolecules.8 At present, the influence of inorganic nanoparticles and heterogeneous polymers on the modification of epoxy resins is being extensively studied. However, it is difficult for inorganic nanoparticles to be uniformly dispersed in the polymer matrix. Therefore, in addition to the optimization ratio and facile preparation method, the more crucial thing is to consider whether the compatibility between materials and glass transition temperature is reduced at a cost.In order to improve the compatibility of materials without sacrificing thermodynamic and mechanical properties, the structure of nanofillers must be rationally designed. In our previous study, azobenzene modified graphene oxide and rigid star-shaped cyclophosphazene have been reported to show good compatibility and reinforcement effect toward polyurethane.9,10 Due to their unique nanoscale structure and excellent mechanical and thermal properties, polyhedral oligomeric silsesquioxanes (POSS) have been suggested to be an ideal reinforcing reagent. If we combine rigid nanoscale POSS and flexible polymer chains, it would be able to toughen epoxy resin without deteriorating the physical properties. POSS is a cage-like structure composed of inorganic SiO1.5 units and organic functional groups.11 The terminal functional groups of POSS can be modulated by various strategies, thus, the interaction and compatibility between POSS and epoxy resins can be improved through physical or chemical mixing.12–14
Herein, we report the synthesis of a new series of linear nano-modifiers that can act effectively as molecular modifiers and co-curing agents with a large aspect ratio for high performance epoxy resins. As shown in Fig. 1, the POSS-epoxy modifiers were designed with the purpose of increasing the miscibility with the epoxy resins. The highly aromatic backbone containing phenylfluorene units was chosen to offer good thermal stability, mechanical properties and chemical resistance. Most importantly, dual-substituted POSS was linearly incorporated into the polymer backbones at higher ratios to achieve the purpose of combining nanoparticle reinforcement and linear polymer toughening together. We found that the curing performance of POSS-epoxy conforms to the autocatalytic Sestak–Berggren (SB) model,15 which enhances the interfacial interaction between the POSS-epoxy filler and epoxy resin matrix, resulting in high thermal stability and improved mechanical properties compared with neat epoxy resins.
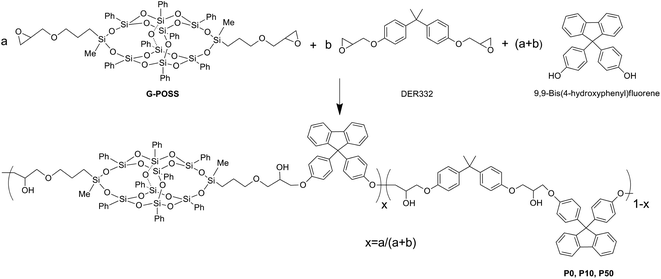 | ||
| Fig. 1 The synthesis of thermoplastic modifiers G-POSS, P0, P10, and P50 (n in Pn denotes the weight percent of G-POSS used in the feed). | ||
2. Experiments and methods
2.1 Synthesis of thermoplastic modifiers
All thermoplastic modifiers were synthesized via step polymerization between diglycidyl ether resin (DER332) and G-POSS (ESI†) with 9,9-bis(4-hydroxyphenyl)fluorine by varing the ratio of G-POSS. Different nano-modifiers Pn (n denotes the weight percent of G-POSS) were obtained by adjusting the feeding ratio of DER332 and G-POSS. In a typical synthesis of P10, G-POSS (0.11 g, 0.08 mmol), 9,9-bis(4-hydroxyphenyl)fluorene (1.0 g, 2.86 mmol), DER332 (0.95 g, 2.78 mmol), 0.3 wt% tetrabutylammoniumbromide (TBAB) and 1,4-dioxane (6.0 mL) were added into a flask. The mixture was allowed to react at 120 °C for 5 h. After that, 1,4-dioxane was removed by distillation. The reaction was further continued for an additional 16 h at 150 °C. A pale solid was obtained after washing 3 times with methanol. After drying at 60 °C in a vacuum oven for 24 h, 1.65 g product was obtained with a yield of ∼80%.2.2 Preparation of epoxy resins with the modifiers
A series of epoxy resins labeled ER-X (where X = P0, P10, P50 or POSS) were prepared by adding 5 wt% of various modifiers X. Thus, 0.75 g of the modifier was mixed with 2.96 g of LC100, 11.29 g of DER332, and 10 mL of THF. The mixture was stirred at room temperature for 2 hours. After removal of the solvent via evaporation, the mixture was poured carefully into a glass mold and cured using the temperature program: 130 °C for 1 hour, 160 °C for 2 hours and 230 °C for 4 hours.3. Results and discussion
3.1 Synthesis and properties of the nano-modifiers
The bifunctional G-POSS was synthesized via a hydrosilylation reaction between ally glycidyl ether and 3,13-dihydrooctaphenyl POSS following a previously reported method with a minor modification.16 Fig. S1† shows the 1H-NMR spectrum of G-POSS and the other modifiers prepared. The resonance peak of Si–H at 4.98 ppm disappeared, suggesting the complete hydrosilylation reaction. New peaks that appeared at 0.75, 1.68, 2.43, 2.65, 2.97, 3.17, 3.47 and 3.34 ppm were assigned to the glycidyloxypropyl groups of G-POSS. 13C-NMR was used to further confirm the chemical structure of G-POSS (see the ESI, Fig. S2†). The MALDI-TOF MS spectrum of the sample showed peaks at m/z = 1381.4 (1404.45 − 22.99), which is in agreement with the calculated value at 1380.2 (see the ESI, Fig. S3†).Linear thermoplastic epoxies can be obtained via an epoxy–phenol reaction, catalyzed by quaternary ammonium salts as the Lewis base.17 In this work, polymerization between DER332, G-POSS epoxy groups and 9,9-bis(4-hydroxyphenyl)fluorene was used to produce linear thermoplastic modifiers, labelled P0, P10 and P50. The viscosity of the reaction system increased during the reaction, and the resulting thermoplastics were transparent and homogeneous. All these thermoplastics could be readily dissolved in chloroform, THF and dioxane. Typical 1H-NMR spectra of P0 and P50 are displayed in Fig. S1.† Resonance peaks of benzene rings in G-POSS, DER332 and 9,9-bis(4-hydroxyphenyl)fluorene appeared at 6.75–7.75. New peaks of methylene groups appeared at 4.1 and 4.3 ppm. Ethyloxy groups were found to shift from 3.35 ppm to 3.75 ppm and methyl silicate groups shifted from 0.36 ppm to 0.30 ppm after polymerization. 1H-NMR spectra confirmed the formation of G-POSS and the linear thermoplastic polymers. The molecular weight and polydispersity of the resultant polymers as presented in Table S1† indicated that higher ratios of DER332 lead to higher molecular weights. This observation can be attributed to the large steric hindrance of POSS, which greatly decreases the coupling possibility and reaction kinetics in P10 and P50 samples.
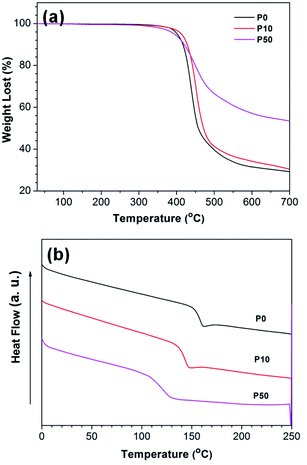
The thermal stability of the nano-modifiers was evaluated by TGA (Fig. 2a). All samples exhibited similar decomposition temperature (Td) at ∼400 °C, indicating that the thermal stability is mainly determined by the chemical structure of the polymer backbones rather than the POSS content. It has been proposed that polymer chain scission is sensitive to the molecular weight and its distribution during the mass loss in TGA.18 Comparing the trend in the TGA curves, P50 degraded much slower than P0, suggesting that the G-POSS moiety has prohibited the random-chain scissions. A higher percentage of residue was detected in the P50 sample as expected from the higher inorganic content present. The percentage of residue from P10 was rather similar to that of P0 due to the low feed amount of G-POSS incorporated.
DSC analysis (Fig. 2b) confirmed that all the modifiers displayed composition-dependent glass transition temperature. The Tg of P0 was found to be ∼159 °C. It decreased to ∼130 °C in P10 and further decreased to ∼121 °C in P50. It has been suggested that the introduction of nano-POSS into the main chain will increase the free volume between polymer chains, thereby reducing the bulk density of glassy thermoplastics. As a result, the polymer chain rotates more freely, resulting in a lower Tg value.
3.2 Properties of the thermosetting epoxy resins
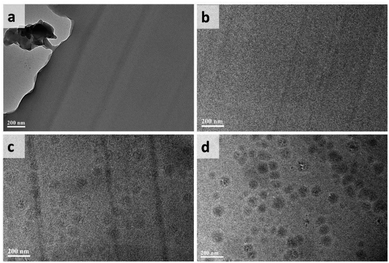
Fig. 3 presents the TEM images of the epoxy resins added without (Fig. 3a) or with 5 wt% of the modifiers (Fig. 3b–d). The dark lines in each image are cutting marks made by the ultramicrotome. TEM observation indicated a smooth surface and a homogeneous phase throughout the ER, ER-P0 and ER-P10 samples. Aggregation was noticed to a small extent, however, when the POSS content was increased further in ER-P50 as shown in Fig. 3c. The aggregation or phase separation became even more obvious when 100% POSS was used in the sample ER-POSS (Fig. 3d). This phase separation induced by the high POSS content may be attributed to the hydrophobic nature of POSS, which may lead to aggregation of the thermoplastics during curing.
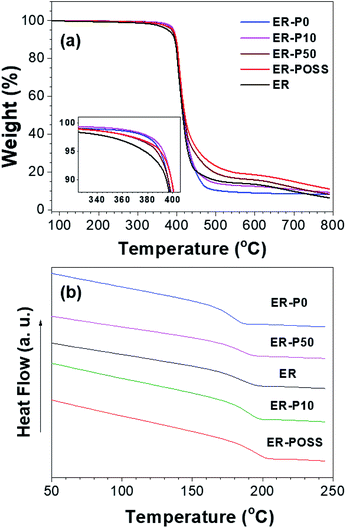
From Fig. 4a, all the samples showed closer decomposition rates which is in agreement with the small amount of modifiers added (5 wt%), the intrinsic properties of the epoxy resin are not altered. The DSC thermographs of all the samples are presented in Fig. 4b. A single glass transition was detected, suggesting completely cured structures and uniform dispersion of the modifiers in the epoxy resins. An increment in Tg may be attributed to the reduction of matrix chain mobility in the presence of the modifiers.20 During the fabrication of the epoxy resins, we believe that the thermoplastics bridged into the matrix with their dangling hydroxyl groups and thus a strong interfacial interaction was produced. This strong interaction confined the mobility of the chains and contributed to the increased Tg.
It is known, however, that aggregation or phase separation of the thermoplastics in the system would lead to weakening of interactions at the interfaces and thus a decrease in Tg. This was indeed observed for the ER-P50 sample. It is worthwhile to note here that G-POSS is a macromolecule containing two end functional groups. Thus, comparing with P50, the same amount (weight) of G-POSS can provide more than tenfold functional groups than that in P50. Hence, we propose that the higher Tg in ER-POSS may be attributed to the strong interfacial interaction between G-POSS and the epoxy matrix. On the other hand, the lower Tg in ER-P0 was probably due to the plasticizer effect,21 which has been reported in similar P0 doped polymers.
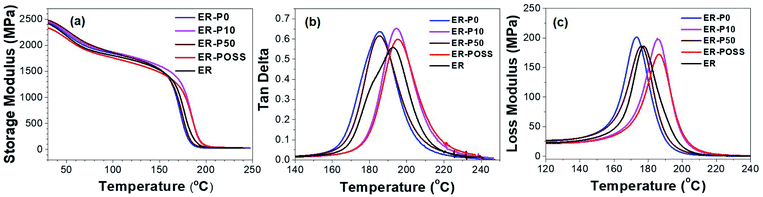 | ||
Fig. 5 Temperature plots of (a) storage modulus, (b) loss factor tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, and (c) loss modulus of the neat and modified epoxy resins. δ, and (c) loss modulus of the neat and modified epoxy resins. | ||
DMA also characterizes Tg at the point when there is sufficient vibrational energy for the rearrangement of cross-linked chains. As shown in Fig. 5b, the Tg value obtained from DMA is consistent with the Tg value obtained from DSC. This can be attributed to the nano-sized POSS and the active hydroxyl groups on the modifiers. The multifunctional groups on POSS cages could limit the mobility of polymer chains. Active hydroxyl side groups on the modifier can participate in the curing reaction, leading to the formation of covalent bonds between the POSS-epoxy and the polymer matrix. It was found that the Tg in ER-P10 and ER-POSS samples was 194.5 °C and 195.1 °C, respectively. On the other hand, the bulky POSS could also increase the free volume of the system, resulting in a depression of Tg. Thus, the higher POSS content in ER-P50 provided higher free volume between the chains, leading to a decrease of Tg to 185.4 °C. The maximum loss moduli of the modified epoxy resins are all higher than that of the control. It increased in the sequence from ER to ER-P50, ER-P10, ER-P0 (Fig. 5c), indicating that the mobility of the polymer chains was enhanced during the glass transition process.
The mechanical properties of thermoset blends depend on the morphological type of nanofillers. The toughening effect of rigid particles depends on the functional groups, dispersibility, amount of loading, particle size and shape.22,23 By direct mixing, POSS molecules with different substituents (methacryloyl, glycidyl, and trisilanol phenyl) were added to the epoxy resin at a loading amount of 0.5 to 8% by weight. The results showed that the addition of POSS increased the fracture toughness of the epoxy resin by 130%, while the elastic modulus and strength did not change significantly. Furthermore, the glass transition temperature decreases with the increase of POSS content due to aggregation.24 In order to improve the dispersibility of nanofillers, a hyperbranched POSS-NH2 grafted onto the surface of carbon fiber was fabricated for the toughening epoxy resin. The results showed that the number of amino groups, the hydrophilicity of the interface and the number of covalent bonds formed are all related to the toughening effect. The glass transition temperature of the composite material increased to about 145 degrees.25 At the same time, the crosslink density also has a great influence on the material properties. Within a certain content range, the lower the crosslink density, the stronger the toughening effect. It is reported that the nanoparticles cavitation could induce shear banding of the epoxy resin matrix.26 The results of fracture toughness and tensile tests showed that all modified epoxy resins exhibit higher elongation at breaks except ER-POSS (Fig. 6a), indicating that the modified resin has higher stress absorption capacity. Under the synergistic effect of the rigid nanocore and rubbery linear chain of the modifiers, the modified resin exhibited high strength and toughness, and Young's modulus (Fig. 6b). The G-POSS modified sample showed a maximum average modulus of 3716 MPa, which was an increase of about 26.9% compared with neat epoxy resin. The Young's modulus and strength of the linear polymer modified resin were both increased. This is because the thermoplastic modifier acts as a stress release center, allowing energy to be dispersed throughout the resin, resulting in higher elongation. Therefore, unlike other nanoparticle toughened epoxy resins,27 POSS-based linear modifiers can toughen epoxy resins without sacrificing the stiffness and glass transition temperature of the resulting material. The stress–strain curve is integrated to obtain the toughness value as shown in Fig. 6c. All modifiers showed toughening effects on epoxy resins, conforming the synergistic effect of nano-POSS and rubbery linear chains. Among them, samples with a higher proportion of conjugated segments have better toughening effects, which are related to the reactive hydroxyl groups.
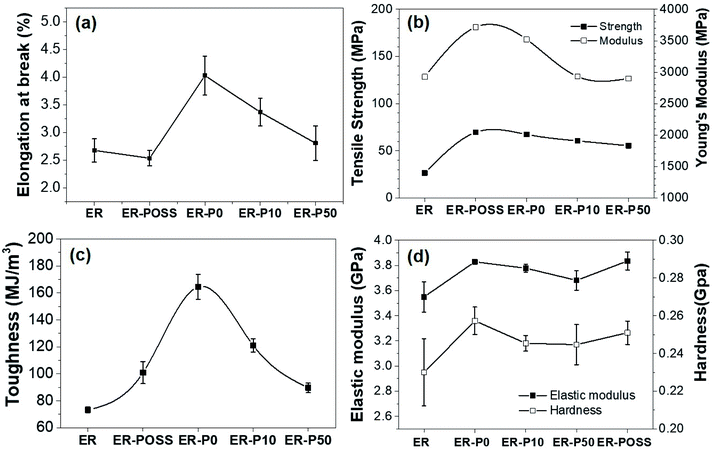 | ||
| Fig. 6 Plots of (a) elongation at breaks, (b) tensile strength, (c) toughness and (d) hardness for the neat and modified epoxy resins. | ||
Hardness is a measure of how resistant a solid material is when a force is applied. In nanoindentation hardness measurement, the resistance and deformation of the sample towards constant compression from a sharp object were monitored. As an important engineering material, the modified epoxy resins also showed higher hardness and elastic modulus. As shown in Fig. 7d, the addition of G-POSS resulted in high hardness due to increased crosslinking density. Surprisingly, in the modifier, the resin hardness decreases from ER-P0 to ER-P50. This indicates that the hardness is more sensitive to the soft interface surrounding the core of the POSS. Elastic modulus is used to describe the elastic deformation of a substance when a force is applied. It is defined as the slope of the stress versus strain curve in the elastic deformation region. So, a stiffer material will have higher elastic modulus. Therefore, harder materials will have a higher modulus of elasticity. This is consistent with our indentation results.
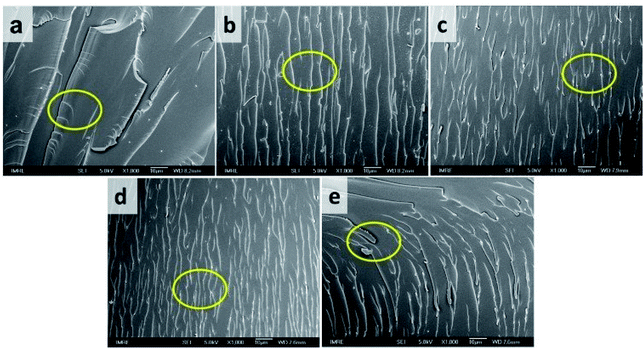 | ||
| Fig. 7 Fracture surfaces of the tensile test samples: (a) ER, (b) ER-POSS, (c) ER-P50, (d) ER-P10, and (e) ER-P0. | ||
Our indentation results were in agreement with the observation that all the prepared thermoplastics showed the toughening effect compared with the neat ER.
4. Conclusions
A new series of linear POSS-containing epoxy thermoplastics were designed and used as reactive modifiers to enhance the toughness and hardness of epoxy resins. With only 5 wt% addition of our thermoplastics, a 27% increment of Young's modulus could be achieved. It was possible to enhance both the toughness and thermal stability at the same time. The results revealed that the toughening effect was owing to the effective increase of crack propagation energy due to the synergistic effect of the rigid POSS core and the flexible polymer chain.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51702178); the Jinan Science and Technology Bureau (Grant No. 2019GXRC021); the Program for Scientific Research Innovation Team in Colleges and universities of Shandong Province; and a project of Shandong Province Higher Educational Science and Technology Program (No. J18KA073).Notes and references
- F.-L. Jin, X. Li and S.-J. Park, Synthesis and application of epoxy resins: A review, J. Ind. Eng. Chem., 2015, 29, 1–11 CrossRef CAS.
- J. Ai, W. Cheng, P. Wang, W. Qian and Q. Chen, Silica solid particles toughening, strengthening and anti-aging on epoxy resin, J. Appl. Polym. Sci., 2021, 138, 50331 CrossRef CAS.
- J. Munoz-Guijosa, G. F. Zapico, O. Peña-Rodríguez and H. Akasaka, Toughening of graphene oxide-epoxy nanocomposites by means of very high pressures and shear rates, Compos. Sci. Technol., 2020, 199, 108354 CrossRef CAS.
- Z. Li, Y. Wang, J. Cao, X. Meng, R. M. Aamir and W. Lu, et al., Effects of loading rates on mode I interlaminar fracture toughness of carbon/epoxy composite toughened by carbon nanotube films, Composites, Part B, 2020, 200, 108270 CrossRef CAS.
- X. Ren, Z. Tu, J. Wang, T. Jiang, Y. Yang and D. Shi, et al., Critical rubber layer thickness of core–shell particles with a rigid core and a soft shell for toughening of epoxy resins without loss of elastic modulus and strength, Compos. Sci. Technol., 2017, 153, 253–260 CrossRef CAS.
- C. Ma, S. Qiu, B. Yu, J. Wang, C. Wang and W. Zeng, et al., Economical and environment-friendly synthesis of a novel hyperbranched poly (aminomethylphosphine oxide-amine) as co-curing agent for simultaneous improvement of fire safety, glass transition temperature and toughness of epoxy resins, Chem. Eng. J., 2017, 322, 618–631 CrossRef CAS.
- W. Wang, G. Zhou, B. Yu and M. Peng, New reactive rigid-rod aminated aromatic polyamide for the simultaneous strengthening and toughening of epoxy resin and carbon fiber/epoxy composites, Composites, Part B, 2020, 197, 108044 CrossRef CAS.
- S. Pruksawan, S. Samitsu, Y. Fujii, N. Torikai and M. Naito, Toughening Effect of Rodlike Cellulose Nanocrystals in Epoxy Adhesive, ACS Appl. Polym. Mater., 2020, 2, 1234–1243 CrossRef CAS.
- M. Karthika, H. Chi, T. Li, H. Wang and S. Thomas, Super-hydrophobic graphene oxide-azobenzene hybrids for improved hydrophobicity of polyurethane, Composites, Part B, 2019, 173, 106978 CrossRef.
- X. Song, H. Chi, Z. Li, T. Li and F. Wang, Star-Shaped Crosslinker for Multifunctional Shape Memory Polyurethane, Polymers, 2020, 12, 740 CrossRef CAS PubMed.
- R. M. Laine and M. F. Roll, Polyhedral Phenylsilsesquioxanes, Macromolecules, 2011, 44, 1073–1109 CrossRef CAS.
- Y. Han, F. Wang, C. Y. Lim, H. Chi, D. Chen and F. Wang, et al., High-Performance Nano-Photoinitiators with Improved Safety for 3D Printing, ACS Appl. Mater. Interfaces, 2017, 9, 32418–32423 CrossRef CAS PubMed.
- H. Shi, J. Yang, M. You, Z. Li and C. He, Polyhedral oligomeric silsesquioxanes (POSS)-based hybrid soft gels: molecular design, material advantages, and emerging applications, ACS Mater. Lett., 2020, 2, 296–316 CrossRef CAS.
- M. Wang, H. Chi, K. S. Joshy and F. Wang, Progress in the synthesis of bifunctionalized polyhedral oligomeric silsesquioxane, Polymers, 2019, 11, 2098 CrossRef CAS PubMed.
- J. Šesták and G. Berggren, Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures, Thermochim. Acta, 1971, 3, 1–12 CrossRef.
- H. Chi, S. L. Lim, F. Wang, X. Wang, C. He and W. S. Chin, Pure Blue-Light Emissive Poly(oligofluorenes) with Bifunctional POSS in the Main Chain, Macromol. Rapid Commun., 2014, 35, 801–806 CrossRef CAS PubMed.
- S. Zheng, J. Huang, Z. Zhong, G. He and Q. Guo, A polymer of bisphenol A and bisphenol A diglycidyl ether and its blends with poly(styrene-co-acrylonitrile): In situ polymerization preparation, morphology, and mechanical properties, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 525–532 CrossRef CAS.
- L. Wang, C. Zhang and S. Zheng, Organic–inorganic poly(hydroxyether of bisphenol A) copolymers with double-decker silsesquioxane in the main chains, J. Mater. Chem., 2011, 21, 19344 RSC.
- L. Matějka, A. Strachota, J. Pleštil, P. Whelan, M. Steinhart and M. Šlouf, Epoxy Networks Reinforced with Polyhedral Oligomeric Silsesquioxanes (POSS). Structure and Morphology, Macromolecules, 2004, 37, 9449–9456 CrossRef.
- H. Xu, S.-W. Kuo, J.-S. Lee and F.-C. Chang, Thermal Properties, and Tg Increase Mechanism of Inorganic/Organic Hybrid Polymers Based on Polyhedral Oligomeric Silsesquioxanes, Preparations, Macromolecules, 2002, 35, 8788–8793 CrossRef CAS.
- D. J. Hourston and J. M. Lane, The toughening of epoxy resins with thermoplastics: 1. Trifunctional epoxy resin-polyetherimide blends, Polymer, 1992, 33, 1379–1383 CrossRef CAS.
- Y. Zhang, L. Li, K. Nie and S. Zheng, Thermomechanical, surface and shape memory properties of thermosetting blends of epoxy with Poly(ethylene oxide): An impact of POSS microdomain formation, Mater. Chem. Phys., 2020, 240, 122183 CrossRef CAS.
- J. Wang, X. Zhang, L. Jiang and J. Qiao, Advances in toughened polymer materials by structured rubber particles, Prog. Polym. Sci., 2019, 98, 101160 CrossRef CAS.
- K. Mishra, G. Pandey and R. P. Singh, Enhancing the mechanical properties of an epoxy resin using polyhedral oligomeric silsesquioxane (POSS) as nano-reinforcement, Polym. Test., 2017, 62, 210–218 CrossRef CAS.
- L. Ma, Y. Zhu, M. Wang, X. Yang, G. Song and Y. Huang, Enhancing interfacial strength of epoxy resin composites via evolving hyperbranched amino-terminated POSS on carbon fiber surface, Compos. Sci. Technol., 2019, 170, 148–156 CrossRef CAS.
- J. D. Liu, H. J. Sue, Z. J. Thompson, F. S. Bates, M. Dettloff, G. Jacob, N. Verghese and H. Pham, Effect of crosslink density on fracture behavior of model epoxies containing block copolymer nanoparticles, Polymer, 2009, 50, 4683–4689 CrossRef CAS.
- R. Bagheri, B. T. Marouf and R. A. Pearson, Rubber-Toughened Epoxies: A Critical Review, Polym. Rev., 2009, 49, 201–225 CrossRef CAS.
- W. Liu, R. Zhou, H. L. S. Goh, S. Huang and X. Lu, From Waste to Functional Additive: Toughening Epoxy Resin with Lignin, ACS Appl. Mater. Interfaces, 2014, 6, 5810–5817 CrossRef CAS PubMed.
- R. A. Pearson and A. F. Yee, Toughening mechanisms in thermoplastic-modified epoxies: 1. Modification using poly(phenylene oxide), Polymer, 1993, 34, 3658–3670 CrossRef CAS.
- R. Daniel, M. Meindlhumer, W. Baumegger, J. Zalesak, B. Sartory and M. Burghammer, et al., Grain boundary design of thin films: Using tilted brittle interfaces for multiple crack deflection toughening, Acta Mater., 2017, 122, 130–137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00757b |
| This journal is © The Royal Society of Chemistry 2022 |