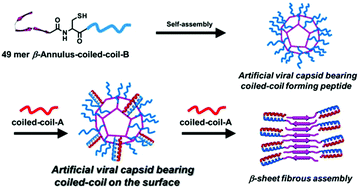
In order to construct artificial viral capsids bearing complementary dimeric coiled-coils on the surface, a β-annulus peptide bearing a coiled-coil forming sequence at the C-terminus (β-annulus-coiled-coil-B) was synthesized by a native chemical ligation of a β-annulus-SBn peptide with a Cys-containing coiled-coil-B peptide. Dynamic light scattering (DLS) measurements and transmission electron microscopy (TEM) images revealed that the β-annulus-coiled-coil-B peptide self-assembled into spherical structures of about 50 nm in 10 mM Tris-HCl buffer. Circular dichroism (CD) spectra indicated the formation of the complementary coiled-coil structure on the spherical assemblies. Addition of 0.25 equivalent of the complementary coiled-coil-A peptide to the β-annulus-coiled-coil-B peptide showed the formation of spherical assemblies of 46 ± 14 nm with grains of 5 nm at the surface, whereas addition of 1 equivalent of the complementary coiled-coil-A peptide generated fibrous assemblies.