Metal-free synthesis of N-fused quinazolino-quinazoline-diones as a MALAT1 RNA triple helix intercalator†
Vijay Babu
Pathi
ab,
Pranotosh
Das
ab,
Abhyuday
Guin
a,
Manish
Debnath
 ab and
Biswadip
Banerji
ab and
Biswadip
Banerji
 *ab
*ab
aOrganic and Medicinal Chemistry Division, CSIR-Indian Institute of Chemical Biology, Kolkata-700032, India. E-mail: biswadip.banerji@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
First published on 22nd October 2024
Abstract
The development of chemical scaffolds that target highly conserved MALAT1 RNA received attention due to its significance in splicing, nuclear organization, and gene expression in disease progression pathways. Here, we synthesized a series of N-fused quinazolino-quinazoline-diones via a PIDA-induced C–N coupling methodology to target MALAT1. Interestingly, compound 2z binds to the UUG pocket of a MALAT1 RNA triple-helix through intercalation, evidenced from molecular docking studies, fluorescence-based assay and CD experiments. 2z exhibited cytotoxicity towards MALAT1 overexpressing cancer cells (SKOV-3, IC50 of 8.0 ± 0.4 μM). These findings demonstrated 2z as a MALAT1 RNA triple-helix intercalator with therapeutic potential, offering an important chemical scaffold to understand MALAT1 activity in disease development pathways.
Ribonucleic acids (RNAs) are vital for many biological functions, either encoding proteins (messenger RNAs) or performing tasks directly (non-coding RNAs). Non-coding RNAs are increasingly seen as drug targets for metastatic and drug-resistant cancers.1 The metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a highly conserved long non-coding RNA involved in nuclear organization, alternative splicing, and gene regulation linked to cancer progression.2MALAT1 is overexpressed in many cancers, including colon, ovarian, breast, prostate, and lung cancers, and is essential for the proliferation, migration, and invasion of cells. It is reported to induce chemotherapy resistance in a variety of malignancies.3MALAT1 down-regulation effectively inhibited the growth of non-small cell lung cancer and ovarian cancer.4 Moreover, it has been observed that reducing MALAT1 expression is not fatal to normal cells in culture, and its knockout in mice does not impair typical growth and development.5 The 3′-end of MALAT1 RNA exists in an equilibrium of a duplex and a unique triple helix structure, with U–A·U and U–U·A base triplets. This region stabilizes MALAT1 RNA and regulates its 3′ end processing, affecting nuclear localization and function. Due to its critical role in metastasis and drug resistance, MALAT1 has huge potential as a therapeutic target for cancer.6 Despite this, only a few molecules have been reported to interact with structures like T–A–T and U–A–U, which includes diphenyl furamidine (DPF),7 quercetin,8 and N-(3-methoxybenzyl)-5-(4-methoxyphenyl)-1-methyl-1H-imidazol-2-amine.9 Thus, the development of small molecules that can interact with the triple helix of MALAT1 represents a significant advancement in the recognition of RNA by chemical scaffolds and provides new opportunities for investigating the therapeutic implications of MALAT1 (Fig. 1).
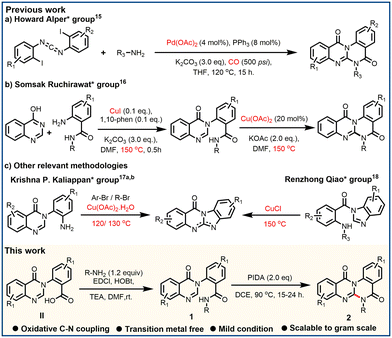
In recent times, N-heterocycles have gained importance in the field of drug discovery because of their frequent presence in several drugs and natural products.10 In particular, quinazolinone belongs to a privileged class of N-heterocycles due to its vast abundance in various natural products and pharmaceuticals, which exhibit a wide range of biological activities, including anticancer.11 In particular, halofugione12 (tRNA inhibitor) and 2-((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[3,4-d]pyrimidin-4(3H)-one13 (microRNA-21 inhibitor) possess quinazolinone as a key motif in their structures (Fig. 1). Both tRNA and microRNA are non-coding RNAs like MALAT1,1 and are directly involved in cellular functions. So, based on this literature, we were interested in making quinazolinone fused motifs such as N-fused quinazolino-quinazoline-diones to target MALAT1. N-fused planar aromatic compounds can be inserted between base pairs of nucleic acids. After insertion, these structures form hydrogen-bonding and van der Waals interactions with the base pairs of nucleic acids thereby hindering their functions.14 Quinazolino-quinazoline-dione molecules were first synthesized by the Howard Alper group in 2010 by a difficult procedure,15 using Pd-catalyst and poisonous CO gas, at a high temperature (Scheme 1a). Subsequently, in 2023, the Somsak Ruchirawat group described a Cu-catalyzed methodology,16 conducted at high temperature (Scheme 1b). Furthermore, structurally relevant quinazolinone fused polyheterocycles, benzimidazo-quinazolinones, were synthesized by the Krishna P. Kaliappan group17 and Renzhong Qiao group18 from 3-aryl quinazolinones and 1-benzoyl benzimidazoles, respectively, but these methodologies also require the use of a metal catalyst and operated at high temperatures. As a consequence, development of a mild and less tedious methodology to access quinazolino-quinazoline-diones is highly desirable. Herein, we report a PIDA-induced intramolecular oxidative C–N coupling process to obtain quinazolino-quinazoline-diones in moderate to good yields with broad functional group tolerance. Unlike the previous literature, our method avoids the use of expensive metal catalysts and poisonous CO gas and can be operated at comparatively lower temperatures.
The optimized reaction condition was performed using methyl (2-(4-oxoquinazolin-3(4H)-yl)benzoyl)glycinate (1a) as the model substrate (Table 1). The reaction of 1a with 1.5 eq. of PIDA (phenyl iodine diacetate) in HFIP (hexafluoroisopropanol) produced our desired compound (2a) in 28% yield (entry 1). However, the use of TFE (trifluoroethanol) and DCE (dichloroethane) as solvents produced 2a in 17% and 67%, respectively (entries 2 and 3). Delightfully, 2a was formed with a maximum yield of 84% with 2.0 eq. of PIDA in DCE at 90 °C (entry 5). Further increase of PIDA quantity to 2.5 equivalents didn't improve the yield of 2a (entry 6). Screening of different solvents like acetic acid (AcOH), trifluoracetic acid (TFA), acetonitrile (ACN), chloroform (CHCl3), and carbon tetrachloride (CCl4) didn't give improved results. Reagents like PIFA gave 2a in 41%, and further use of K2S2O8, m-CPBA, and TBHP didn't produce 2a (entries 12–15).
| Entry | Reagent (eq.) | Solvent | T (°C) | Yieldb (%) 2a |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.15 mmol), PIDA (2.0 eq.), DCE, 0 °C, 15–24 h. b Yields of isolated products, T = temperature. | ||||
| 1 | PIDA (1.5) | HFIP | 70 | 28 |
| 2 | PIDA (1.5) | TFE | 80 | 17 |
| 3 | PIDA (1.5) | DCE | 80 | 67 |
| 4 | PIDA (1.5) | DCE | 90 | 71 |
| 5 | PIDA (2.0) | DCE | 90 | 84 |
| 6 | PIDA (2.5) | DCE | 90 | 85 |
| 7 | PIDA (2.0) | AcOH | 90 | 15 |
| 8 | PIDA (2.0) | TFA | 90 | 10 |
| 9 | PIDA (2.0) | ACN | 90 | Trace |
| 10 | PIDA (2.0) | CHCl3 | 80 | 49 |
| 11 | PIDA (2.0) | CCl4 | 80 | 42 |
| 12 | PIFA (2.0) | DCE | 90 | 41 |
| 13 | K2S2O8 (2.0) | DCE | 90 | Trace |
| 14 | m-CPBA (2.0) | DCE | 90 | Trace |
| 15 | TBHP (2.0) | DCE | 90 | 0 |
With the optimized conditions, we next studied the substrate scope of this C–N coupling methodology (Scheme 2). Initially, we studied the substrate scope of R substitutions. N-substituted alkyl substrates with electron-withdrawing group substitution reacted smoothly and produced desired products in good yields (2a and 2x). N-substituted alkyl chains gave the desired products in moderate yields (2b, 2c and 2y). N-substituted benzyl and phenethyl substrates with electron-withdrawing group substitutions (–F, –CO2Me, –CN, –NO2, –CF3 and –OCF3) on the phenyl ring produced desired products in good to excellent yields (62–87%) and substitution of electron donating groups (−Me, –OMe) on the phenyl ring produced compounds in low yields (30–59%). Picolylamine substrates furnished desired products in good yields (2m, 2w and 2aa). Naphthylamine derivative produced compound 2n in 52% yield. Aniline and aniline with methyl substitution on the ortho position gave desired products in low yields compared with substitutions like –Cl and –CF3. Gratifyingly, the dimer molecule 2z was formed in 38% yield. Next, we studied the scope of R1 substitutions on the aryl ring. Substitutions like –Me, –OMe, and –Br groups on aryl ring gave cyclized products in low yields (51–78%, 2ab and 2ad–2af) compared with –Cl substitution (80–82%, 2ac and 2ag). Fortunately, structures of compounds 2h, 2m, 2aa, and 2ab were also established by using single crystal X-ray diffraction studies (ESI,† S36–S40). To study the practical applicability of this methodology, we performed a gram scale reaction with 1a, and this method delivered 2a in 81% yield (ESI,† S9).
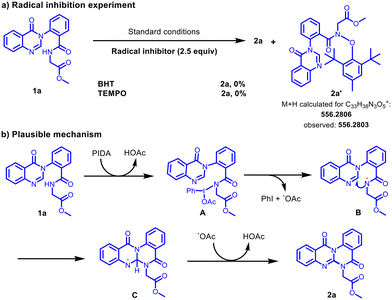
We performed some control experiments to understand the plausible mechanism of this C–N coupling. We observed that the reaction was inhibited upon the addition of radical scavengers like BHT (2,6-di-tert-butyl-4-methylphenol) and TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) to the standard reaction conditions (Scheme 3a). BHT adduct 2a′ was confirmed using the ESI mass spectrum. These results suggested that this coupling process proceeds via a radical mechanism. Based on the reported literature19 and the above control experiments, a plausible mechanism is depicted in Scheme 3b. Initially, PIDA reacts with 1a to generate the N-iodoamido species (A), which consequently undergoes thermal homolytic cleavage to provide the amidyl radical (B). Then, the intramolecular reaction of the resulting amidyl radical (B) at the C-3 position of quinazolinone provides the radical intermediate (C), which, upon a radical abstraction/oxidation, could deliver 2a.
To investigate the binding of thirty-three (33) quinazolino-quinazoline-diones to the MALAT1 triple helix (PDB ID: 4PLX)6 and their three-dimensional shape recognition, molecular modeling was employed (Table S1, ESI,† S5). The in silico binding analysis revealed that the molecules have overall nucleic acid binding capabilities, and 8 molecules, namely 2z, 2n, 2h, 2g, 2l, 2s, 2d, and 2j, showed affinity towards MALAT1 with binding affinity scores less than −10.0 kcal mol−1. Interestingly, 2z demonstrated the most favourable binding affinity score (−12.5 kcal mol−1). The MALAT1 RNA triple helix structure consists of a duplex complementary base pairing region near the 3′-end, following which there is a poly-A tail that wraps around and inserts into the duplex structure, forming the triple helix segment. Docking results indicate that 2z (Fig. 2) localizes to the UUG pocket near the triple helical domain through hydrogen bonding with uracil and guanine molecules. Notably, the size of 2z may be a perfect fit for the UUG pocket, which is larger than the duplex segment and hence explains the favourable intercalation of the molecule with MALAT1 RNA.
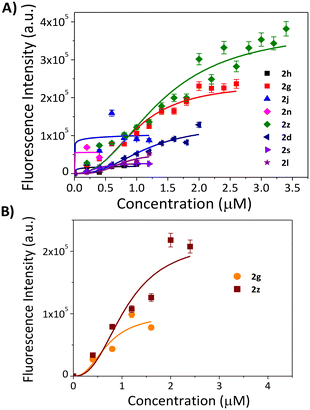
The ability of these eight (8) molecules to interact with nucleic acids was studied using a fluorescence-based screening assay with two nucleic acid structures: A) duplex and B) triple helix (Fig. 3). Binding of the molecules with the fluorescent nucleic acid molecules resulted in alterations in the fluorescence spectra, providing a tangible depiction of the hit molecules forging interactions. As seen from Fig. 3A, 2z exhibited the highest spectral gap (ΔFI) with EC50 values of 1.4 μM, for the duplex, which indicates 1.31-fold higher affinity over 2g (ΔFI = 0.76) and above 10-fold affinity over other tested molecules (Table 2). The remaining molecules exhibited negligible binding at lower concentrations as no 50% alteration in fluorescence intensity of the duplex was observed before the precipitation limit of the molecules, consequently, the EC50 values were not found (see ESI,† S5 and S6). The screening analysis with the MALAT1 triple helix showed no appreciable ΔFI values for molecules other than 2z and 2g (see ESI,† S7). 2z exhibited a significant 2-fold higher affinity for the MALAT1 triple helix over 2g (ΔFI = 0.504) with the EC50 value of 1.1 μM (Table 2). The EC50 values obtained for the 2z and 2g with MALAT1 triple helix were comparable to that of the standard MALAT1 RNA binder diphenyl furamidine (DPF)7 (EC50 = 0.6 μM) in a similar fluorescence-based screening assay. To further validate the binding of 2z with the MALAT1 triple helix, temperature-dependent CD experiments were performed (see the ESI,† S7 and S8). Adding 1 and 2 equivalents of 2z to the annealed RNA exhibited change in ellipticity of the folded RNA, but does not significantly alter the conformation of the triple helical structure, indicating that 2z binds to the MALAT1 triple helix without altering its conformation significantly (Fig. 4).
| Ligand | ΔFI (dsDNA) | EC50 (μM) | ΔFI (MALAT1) | EC50 (μM) |
|---|---|---|---|---|
| 2h | 0.079 | — | — | — |
| 2g | 0.76 | 1 | 0.504 | 0.8 |
| 2j | 0.29 | — | — | — |
| 2n | 0.014 | — | — | — |
| 2z | 1 | 1.4 | 1 | 1.1 |
| 2d | 0.426 | — | — | — |
| 2s | 0.084 | — | — | — |
| 2l | 0.172 | — | — | — |
To investigate the biological consequence of MALAT1 intercalator 2z, a cytotoxic assessment of 2z in MALAT1 overexpressing human cancer cell lines such as SKOV-3 (ovarian cancer cell line, obtained from ATCC, USA) and a standard cancer cell line (cervical cancer, HeLa, obtained from NCCS, Pune, India) was performed. 2z showed a lower IC50 value of 8.0 ± 0.4 μM in the SKOV-3 cell line compared to the HeLa cell line (IC50 = 63.21 ± 3.16 μM). 2z displayed negligible toxicity against normal fibroblast cells L929 (obtained from NCCS, Pune, India), signifying that it has higher cytotoxicity in SKOV-3 cell lines over other tested cells. The IC50 values obtained for 2z were higher than that of the standard nucleic acid binder doxorubicin against the HeLa and SKOV-3 cell line (IC50 = 1.39 μM and 0.534 μM, respectively)20 but lower than the standard MALAT1 binder DPF (IC50 = 90.0 μM)21 in cell cytotoxicity analysis.
In summary, PIDA-induced intramolecular C–N coupling methodology was developed to synthesize quinazolino-quinazoline-diones in moderate to good yields. Gratifyingly, 2z demonstrated good binding affinity towards triple-helical MALAT1 RNA evidenced by molecular docking studies, fluorescence-based assay and CD experiments. 2z exhibited good cytotoxicity against MALAT1 overexpressing ovarian cancer cells (SKOV-3) over normal fibroblast cells. One of the possible mechanisms by which 2z induces cancer cell death may involve its interaction with specific nucleic acid structures, warranting further detailed investigation through structure–activity relationship studies. We believe that quinazolino-quinazoline-dione motif lays the foundation for developing more effective RNA triple helix recognition ligands for their therapeutic intervention.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
BB and MD thank CSIR, New Delhi for financial support. VB, PD and AG thank CSIR for fellowship. The authors also thank Dr. E. Padmanaban, Sr. Tech. Officer, and Mr. Gautam karmakar for recording the NMR; Mr. Sandip Chowdhury, Sr. Tech. Officer, for recording the EI-HRMS spectra, Mr. Soumik Laha, for recording the ESI-HRMS spectra; Mr. Sandip Kundu, Technical officer, for recording X-ray crystal data (CIF, CSIR-IICB).Notes and references
- (a) K. V. Morris and J. S. Mattick, Nat. Rev. Genet., 2014, 15, 423–437 CAS; (b) G. Arun, S. D. Diermeier and D. L. Spector, Trends Mol. Med., 2018, 24, 257–277 CAS; (c) T. R. Cech and J. A. Steitz, Cell, 2014, 157, 77–94 CAS.
- V. Tripathi, Z. Shen, A. Chakraborty, S. Giri, S. M. Freier, X. Wu, Y. Zhang, M. Gorospe, S. G. Prasanth, A. Lal and K. V. Prasanth, PLoS Genet., 2013, 9, e1003368 CrossRef CAS PubMed.
- (a) P. Ji, S. Diederichs, W. Wang, S. Böing, R. Metzger, P. M. Schneider, N. Tidow, B. Brandt, H. Buerger, E. Bulk, M. Thomas, W. E. Berdel, H. Serve and C. Müller-Tidow, Oncogene, 2003, 22, 8031–8041 CrossRef; (b) M. Majidinia and B. Yousefi, DNA Repair, 2016, 45, 25–33 CrossRef CAS; (c) T. Gutschner, M. Hämmerle, M. Eißmann, J. Hsu, Y. Kim, G. Hung, A. Revenko, G. Arun, M. Stentrup, M. Groß, M. Zörnig, A. R. MacLeod, D. L. Spector and S. Diederichs, Cancer Res., 2013, 73, 1180–1189 CrossRef CAS PubMed; (d) J. Hou, G. Zhang, X. Wang, Y. Wang and K. Wang, Biomark. Res., 2023, 11, 1–11 CrossRef.
- (a) L. Bai, A. Wang, Y. Zhang, X. Xu and X. Zhang, Exp. Cell Res., 2018, 366, 161–171 CAS; (b) J. Huang, C. Lin, H. Dong, Z. Piao, C. Jin, H. Han and D. Jin, Cancer Chemother. Pharmacol., 2020, 86, 663–672 CAS; (c) S. Liu, X. Jiang, W. Li, D. Cao, K. Shen and J. Yang, Oncol. Lett., 2016, 11, 3686–3692 CAS.
- (a) S. Nakagawa, J. Y. Ip, G. Shioi, V. Tripathi, X. Zong, T. Hirose and K. V. Prasanth, RNA, 2012, 18, 1487–1499 CAS; (b) B. Zhang, G. Arun, Y. S. Mao, Z. Lazar, G. Hung, G. Bhattacharjee, X. Xiao, C. J. Booth, J. Wu, C. Zhang and D. L. Spector, Cell Rep., 2012, 2, 111–123 CrossRef CAS.
- J. A. Brown, D. Bulkley, J. Wang, M. L. Valenstein, T. A. Yario, T. A. Steitz and J. A. Steitz, Nat. Struct. Mol. Biol., 2014, 21, 633–640 CrossRef CAS PubMed.
- A. Donlic, B. S. Morgan, J. L. Xu, A. Liu, C. Roble and A. E. Hargrove, Angew. Chem., Int. Ed., 2018, 57, 13242–13247 CrossRef CAS.
- I. Rakheja, A. H. Ansari, A. Ray, D. Chandra Joshi and S. Maiti, Mol. Ther.--Nucleic Acids, 2022, 30, 241–256 CrossRef CAS.
- F. A. Abulwerdi, W. Xu, A. A. Ageeli, M. J. Yonkunas, G. Arun, H. Nam, J. S. Schneekloth, T. K. Dayie, D. Spector, N. Baird and S. F. J. Le Grice, ACS Chem. Biol., 2019, 14, 223–235 CrossRef CAS PubMed.
- (a) M. M. Heravi and V. Zadsirjan, RSC Adv., 2020, 10, 44247–44311 Search PubMed; (b) A. Amin, T. Qadir, P. K. Sharma, I. Jeelani and H. Abe, Open Med. Chem. J., 2022, 16, 1–27 Search PubMed.
- (a) A. M. Alsibaee, H. M. Al-Yousef and H. S. Al-Salem, Molecules, 2023, 28, 978 CAS; (b) R. Karan, P. Agarwal, M. Sinha and N. Mahato, ChemEngineering, 2021, 5, 73 CrossRef CAS; (c) S. N. Murthy Boddapati, H. Babu Bollikolla, K. Geetha Bhavani, H. Singh Saini, N. Ramesh and S. Babu Jonnalagadda, Arabian J. Chem., 2023, 16, 105190 CrossRef CAS.
- Z. Cai, B. Chen, Y. Yu, J. Guo, Z. Luo, B. Cheng, J. Xu, Q. Gu and H. Zhou, J. Med. Chem., 2022, 65, 5800–5820 CAS.
- M. D. Shortridge, B. Chaubey, H. J. Zhang, T. Pavelitz, V. Vidadala, C. Tang, G. L. Olsen, G. A. Calin and G. Varani, ACS Chem. Biol., 2023, 18, 237–250 CAS.
- V. Sharma, M. Gupta, P. Kumar and A. Sharma, Curr. Pharm. Des., 2020, 27, 15–42 Search PubMed.
- F. Zeng and H. Alper, Org. Lett., 2010, 12, 3642–3644 CrossRef CAS PubMed.
- N. Palavong, P. Arunkirirote, J. Tummatorn, C. Thongsornkleeb and S. Ruchirawat, Asian J. Org. Chem., 2023, 12, e202300188 CrossRef CAS.
- (a) A. Banerjee, P. Subramanian and K. P. Kaliappan, J. Org. Chem., 2016, 81, 10424–10432 CrossRef CAS; (b) G. G. Dake, N. Blanchard and K. P. Kaliappan, ACS Omega, 2024, 9, 33805–33814 CrossRef CAS PubMed.
- L. Chen, C. Li, X. Bi, H. Liu and R. Qiao, Adv. Synth. Catal., 2012, 354, 1773–1779 CAS.
- (a) S. Mondal, S. Samanta, S. Jana and A. Hajra, J. Org. Chem., 2017, 82, 4504–4510 CrossRef CAS PubMed; (b) W. Wimonsong and S. Yotphan, Tetrahedron, 2021, 81, 131919 CAS.
- (a) N. Bellance, F. Furt, S. Melser, C. Lalou, D. Thoraval, L. Maneta-Peyret, D. Lacombe, P. Moreau and R. Rossignol, Int. J. Mol. Sci., 2020, 21, 1317 CAS; (b) T. Ittiudomrak, S. Puthong, S. Roytrakul and C. Chanchao, Toxicol. Res., 2019, 35, 167–179 CAS.
- K. Bielawski, S. Wolczynski and A. Bielawska, Biol. Pharm. Bull., 2001, 24, 704–706 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2356874, 2356882, 2356885 and 2356891. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4md00614c |
| This journal is © The Royal Society of Chemistry 2025 |