Trinitromethyl- and nitramino-substituted triazolo-pyridazines: synthesis and energetic performance†
Sagar
Nehe‡
 a,
Abhishek Kumar
Yadav‡
a,
Abhishek Kumar
Yadav‡
 a,
Vikas D.
Ghule
a,
Vikas D.
Ghule
 b and
Srinivas
Dharavath
b and
Srinivas
Dharavath
 *a
*a
aEnergetic Materials Laboratory, Department of Chemistry, Indian Institute of Technology Kanpur, Kanpur-208016, Uttar Pradesh, India. E-mail: srinivasd@iitk.ac.in
bDepartment of Chemistry, National Institute of Technology Kurukshetra, Kurukshetra-136119, Haryana, India
First published on 21st May 2025
Abstract
We synthesized a series of ring-fused triazolo-pyridazine energetic compounds with trinitromethyl, nitramine, and hydrazine groups and thoroughly characterised them using NMR, IR, mass spectrometry, and TGA–DSC analysis. Furthermore, single-crystal X-ray analysis supports the structure of compounds 3 and 7. Among all, compound 6 shows exceptional thermal stability (Td = 353 °C), good density (1.77 g cm−3), good VOD (6710 m s−1), and high insensitivity towards impact (40 J) and friction (360 N), which makes it a super heat-resistant explosive.
High energy density materials (HEDMs) are essential to modern technology and industry, offering high performance with quick energy release, efficiency, and precision.1,2 Their role spans critical sectors such as defense, space exploration, construction, mining, and safety, contributing to advancements in science, technology, and engineering while maintaining safety and operational control.3–5 Balancing the trade-off between high energy and low sensitivity in the design of new energetic molecules is a critical challenge in the field of energetic materials.5–7 Over the past few decades, various strategies have been devised to develop advanced HEDMs including using energetic salts, energetic zwitterionic salts, cocrystals, energetic coordination compounds and energetic metal–organic frameworks (EMOFs).8–12 In recent years, bicyclic or polycyclic fused heterocyclic compounds have been studied as building blocks to construct new HEDMs.13,14 Nitrogen-rich energetic materials with conjugated fused rings containing favourable aromaticity have garnered attention due to their promising balance between energy output, sensitivity, and thermal stability.15–17 Compared to traditional high explosives like cyclo-1,3,5-trimethylene-2,4,6-trinitamine (RDX) and 1,3,5,7-tetranitrotetraazacyclooctane (HMX), fused-ring energetic compounds are typically planar, conjugated molecules.18 These compounds often exhibit high density and superior thermal stability, owing to strong hydrogen bonding and π–π interactions.13 Additionally, the high nitrogen content of the N-heterocyclic fused ring skeleton ensures a significant presence of C–N, and N–N bonds within the molecular structure, which contributes to a high positive heat of formation in the target energetic molecules.19,20 Therefore, the development of fused-ring energetic materials is considered a promising approach to address the energy-safety trade-off, offering a balanced combination of thermostability, and insensitive next-generation HEDMs. Over the past few years, numerous fused nitrogen-rich heterocycles, such as imidazolo-pyridazine,21 tetrazolo-pyridazine,22 triazolo-pyridazine,23 and tetrazino-tetrazine24 have been reported. Energy-rich functional groups, such as nitro (–NO2), nitrato (–ONO2), nitramino (–NHNO2), geminal dinitro (–CH(NO2)2), and trinitromethane (–C(NO2)3) are commonly introduced in HEDMs as they enhance key properties like density, nitrogen content, and oxygen balance, thereby boosting the overall energetic performance.25–30 The trinitromethane group (–C(NO2)3) is highly valued in the field of energetic materials due to its ability to significantly enhance the energy density and oxidizing potential.28 One of the key advantages is its high oxygen content, as it contains three nitro groups that contribute a substantial amount of oxygen, thereby improving the oxygen balance of compounds. This leads to more efficient combustion and detonation, making it ideal for high-performance explosives and propellants.
Keeping this in mind, we propose the synthesis of a series of triazolo-pyridazine fused energetic compounds functionalized with –NH2, –NHNH2, –NHNO2, and –C(NO2)3 groups derived from commercially available 3-chloro-6-hydrazineylpyridazine. Among the synthesized compounds, compound 3 with –C(NO2)3 functionality reveals good energetic performance with an impact sensitivity (IS) of 2.5 J, suggesting its potential as a metal-free primary explosive. Furthermore, compound 6 with –NHNO2 functionality displays exceptional thermal stability up to 353 °C, respectively, highlighting its potential as a super heat-resistant explosive.
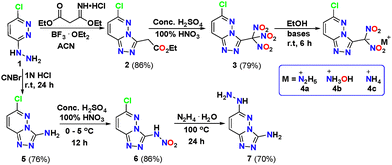
Ethyl 2-(6-chloro-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)acetate (2) was synthesized via the cyclization of 3-chloro-6-hydrazineylpyridazine (1) with 3-ethoxy-3-iminopropionic acid ethyl ester hydrochloride in acetonitrile, using BF3·OEt2 as a catalyst. The nitration of compound 2 was performed using conc. sulfuric acid and 100% nitric acid, resulting in 6-chloro-3-(trinitromethyl)-[1,2,4]triazolo[4,3-b]pyridazine (3) in a 79% yield. Finally, compound 3 was treated with nitrogen-rich bases such as hydrazine hydrate, hydroxylamine hydrate, and aqueous ammonia in ethanol at room temperature for 6 hours to obtain hydrazine (4a), hydroxylamine (4b), and ammonia (4c) salts in quantitative yields as shown in Scheme 1.
Furthermore, compound 1 reacted with cyanogen bromide (CNBr) in 1N HCl at room temperature for 24 hours, affording 6-chloro-[1,2,4]triazolo[4,3-b]pyridazin-3-amine (5) with a 76% yield. The nitration of compound 5 was subsequently performed using concentrated H2SO4 and 100% HNO3, producing N-(6-chloro-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)nitramide (6) with an 86% yield. When compound 6 was treated with 80% hydrazine monohydrate at 100 °C overnight, the reaction unexpectedly yielded 6-hydrazineyl-[1,2,4]triazolo[4,3-b]pyridazin-3-amine (7) in a 70% yield, instead of the anticipated N-(6-hydrazineyl-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)nitramide. This result highlights a preferential reaction pathway involving the removal of the nitro group, likely driven by the reducing environment provided by hydrazine under these conditions.
Crystals suitable for X-ray single crystal diffraction analysis of compounds 3 and 7 were obtained through slow evaporation at room temperature. For compound 3, a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol-to-dimethyl sulfoxide solution was used, while for compound 7, dimethyl sulfoxide alone served as the solvent. Compound 3 crystallizes in the triclinic crystal system and belongs to the P
1 methanol-to-dimethyl sulfoxide solution was used, while for compound 7, dimethyl sulfoxide alone served as the solvent. Compound 3 crystallizes in the triclinic crystal system and belongs to the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group having a crystal density of 1.89 g cm−3 at 100 K. In the asymmetric unit, four molecules of compound 3 are present, as shown in Table S1 (ESI†). All the ring atoms and C6 are coplanar to each other. The trinitromethyl groups linked to C5 are arranged in a tetrahedral geometry. The interlayer distance between the two planes is 3.557 Å, as depicted in Fig. 1b.
space group having a crystal density of 1.89 g cm−3 at 100 K. In the asymmetric unit, four molecules of compound 3 are present, as shown in Table S1 (ESI†). All the ring atoms and C6 are coplanar to each other. The trinitromethyl groups linked to C5 are arranged in a tetrahedral geometry. The interlayer distance between the two planes is 3.557 Å, as depicted in Fig. 1b.
 | ||
| Fig. 1 (a) Crystal structure of 3. (b) Interlayer distance between two layers of compound 3. (c) Crystal packing along the a-axis for compound 3. | ||
Compound 7 crystallizes in the orthorhombic space group Pbca with a crystal density of 1.63 g cm−3 at 100 K. It has eight molecules per unit cell, as shown in Table S2 (ESI†). The molecular structure of compound 7 is planar, as depicted in Fig. 2c. A strong hydrogen bonding interaction between the hydrogen atom of the hydrazine (–NHNH2) group and the nitrogen atom of the triazole ring leads to the formation of a helical-like packing diagram extended via a hydrogen bonding network along the b-axis (Fig. 2b).
Additionally, along the a-axis, the molecules exhibit well-ordered wave-like layer-by-layer stacking with an interlayer distance of approximately 3.540 Å (Fig. 2d). These strong hydrogen bonds and π–π stacking interactions contribute to the enhanced molecular stability and insensitivity of compound 7.
The physicochemical and energetic properties of all the synthesized compounds, such as density, thermal stability, detonation performance, and mechanical sensitivity, were comprehensively evaluated and are presented in Table 1. Density is one of the most important factors in determining the performance of energetic compounds. Experimental densities were determined using a gas pycnometer (Ultrapyc 5000 with meso cells) in a helium atmosphere at 25 °C. Each compound was measured three times to ensure accuracy, and the average values are reported in Table 1. The densities of the synthesized compounds range from 1.60 to 1.84 g cm−3. All geometry optimizations were performed using the Gaussian 09 suite of programs31 and heats of formation (HOFs) were computed based on the isodesmic reaction approach, revealing values between −156.31 and 565.5 kJ mol−1. Detonation velocity (VOD) and detonation pressure (DP) were calculated using EXPLO5 v7.01.01 software, based on the experimental density and computed HOF data. The synthesized compounds exhibited moderate performance, with VODs ranging from 6624 to 7554 m s−1 and DP from 16.35 GPa to 20.43 GPa. Thermal stability, crucial for the safe handling of energetic compounds during synthesis, storage, and transport was assessed using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) at a heating rate of 5 °C min−1 in a nitrogen atmosphere. The onset decomposition temperatures of the compounds 3, 4a–c, 6, and 7 ranged from 126 to 353 °C. Among the synthesized compounds, compound 6 (Td = 353 °C) demonstrated notable thermal stability, surpassing the benchmark heat-resistant explosive HNS (Td = 318 °C) and closely rivalling PYX (Td = 357 °C).
| Compound | T d [°C] | D [g cm−3] | HOFc [kJ mol−1] | VODd [ms−1] | DPe [GPa] | ISf [J] | FSg [N] |
|---|---|---|---|---|---|---|---|
| a Onset decomposition temperature (DSC, 5 °C min−1). b Density measured by a gas pycnometer (25 °C). c Computed HOF. d Detonation velocity. e Detonation pressure. f Impact sensitivity. g Friction sensitivity. h Ref. 15. i Ref. 32. j Ref. 33. | |||||||
| 3 | 126 | 1.84 | −156.31 | 7188 | 20.43 | 2.5 | 254 |
| 4a | 194 | 1.80 | −4.14 | 7025 | 18.48 | 25 | 360 |
| 4b | 156 | 1.73 | −92.8 | 6798 | 17.60 | 10 | 360 |
| 4c | 197 | 1.79 | −142.7 | 6624 | 16.35 | 20 | 360 |
| 6 | 353 | 1.77 | 377.67 | 6710 | 17.68 | 40 | 360 |
| 7 | 183 | 1.60 | 565.5 | 7554 | 20.34 | 40 | 360 |
| LAh | 315 | 4.80 | 450.1 | 5920 | 33.8 | 2.5–4 | 0.1–1 |
| DDNPh | 157 | 1.72 | 321 | 6900 | 24.2 | 2.2j | 24.7 |
| PYXi | 357 | 1.76 | 43.7 | 7448 | 24.2 | 15.4j | 360 |
| HNSi | 318 | 1.75 | 78.2 | 7164 | 21.6 | 10.7j | 240 |
Impact and friction sensitivities were evaluated using BAM Fall Hammer and Friction Tester instruments. These sensitivities are crucial safety parameters, as they indicate how easily an energetic material can be accidentally initiated during impact and friction. The measured impact sensitivities of the compounds 3, 4a–c, 6 and 7 range from 2.5 to 40 J, while their friction sensitivities fall between 254 and 360 N. Among these compounds, the neutral compound 3 exhibits relatively higher susceptibility to impact (IS = 2.5 J) owing to the presence of a –C(NO2)3 functionality; however, it still demonstrates better impact resistance than DDNP (IS = 2.2 J). Importantly, all compounds display insensitivity to friction, with FS values consistently in the range of 254 to 360 N.
To gain deeper insight into how non-bonded interactions affect the physical properties of crystalline materials, we conducted a detailed analysis using Hirshfeld surfaces, 2D fingerprints, and the percentage contribution of individual close contacts of compounds 3 and 7via Crystal Explorer Software (version 17).34 The results from the Hirshfeld surface analysis were visually represented with colour codes: blue signified distances longer than the van der Waals radii, red indicated shorter distances, and white denoted distances equal to the sum of the van der Waals radii. The red and blue regions correspond to high and low populations of close contacts, respectively. Specifically, red spots highlighted intermolecular O⋯H/H⋯O and N⋯H/H⋯N interactions. In compound 3, O⋯H interactions contributed 19%, while N⋯H interactions accounted for 9%. In contrast, compound 7 exhibited a significantly higher contribution of N⋯H interactions at 45%, highlighting the strength of intermolecular hydrogen bonding, beneficial to lowering the impact sensitivity. Additionally, compound 3 displayed various other interactions, including N⋯O (16%), Cl⋯N (4%), C⋯O (8%), O⋯O (16%), and Cl⋯O (15%). For compound 7, notable interactions included N⋯C (7%), N⋯N (5%), and H⋯H (36%), as illustrated in Fig. 3.
In summary, we successfully synthesized compounds 3, 6, and 7, along with the energetic salts 4a–4c derived from compound 3, using commercially available and cost-effective starting materials with good yields. All the newly synthesized compounds were fully characterized with IR, multinuclear NMR, and TGA–DSC measurements. Additionally, the structure of compounds 3 and 7 is further supported by X-ray diffraction analysis. All the synthesized compounds showed good density (1.60–1.84 g cm−3), moderate VOD (6624–7554 m s−1) and DP (16.35–20.43 GPa), good thermal decomposition (126–353 °C) and acceptable sensitivity toward impact (2.5–40 J) and friction (FS > 254 N). Compound 3 has good density (1.84 g cm−3) and detonation velocity (7188 m s−1) with high impact sensitivity (2.5 J), and can be utilised as a metal-free primary explosive. Additionally, compound 6 exhibits remarkable thermal stability (Td = 353 °C), underscoring its suitability as a super heat-resistant explosive. We have demonstrated the potential of a fused triazolo-pyridazine backbone substituted with trinitromethyl (–C(NO2)3), nitramine (–NHNO2), and hydrazine (–NHNH2) groups and hope that our contribution will further encourage researchers to extend their studies in the energetic materials field with nitrogen-rich fused molecular frameworks.
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.
S. N. thanks IIT Kanpur for funding and infrastructure. A. K. Y. thanks IIT Kanpur for the FARE Fellowship and infrastructure. S. D. is grateful for the financial support from the core research grant (ANRF-CRG/2023/000007), the Anusandhan National Research Foundation-Science and Engineering Research Board, Department of Science and Technology, Government of India.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Notes and references
-
T. M. Klapötke, Chemistry of High-Energy Materials, Walter de Gruyter GmbH, Berlin/Boston, 2nd edn, 2022 Search PubMed
.
-
J. P. Agrawal and R. D. Hodgson, Organic Chemistry of Explosives, Wiley, 2006 Search PubMed
.
-
J. C. Oxley, Explosive Effects and Applications, 1998, 137–172 Search PubMed
.
- A. K. Yadav, V. D. Ghule and S. Dharavath, ACS Appl. Mater. Interfaces, 2022, 14, 49898–49908 Search PubMed
.
- A. K. Sikder and N. Sikder, J. Hazard. Mater., 2004, 112, 1–15 Search PubMed
.
- S. Thangadurai, K. P. S. Kartha, D. R. Sharma and S. K. Shukla, Sci. Technol. Energy Mater., 2004, 65, 215–226 CAS
.
- M. Anniyappan, M. B. Talawar, R. K. Sinha and K. P. S. Murthy, Combust., Explos. Shock Waves, 2020, 56, 495–519 Search PubMed
.
- S. Banik, V. D. Ghule and S. Dharavath, J. Org. Chem., 2024, 89, 14038–14049 Search PubMed
.
- X. Miao, J. Yu, Y. Li and S. Pang, Org. Lett., 2024, 26(47), 10085–10089 Search PubMed
.
- M. Jujam, R. Rajak and S. Dharavath, Adv. Funct. Mater., 2025, 35, 2412638 Search PubMed
.
- J. Zhang, J. Zhang, G. H. Imler, D. A. Parrish and J. M. Shreeve, ACS Appl. Energy Mater., 2019, 2, 7628–7634 Search PubMed
.
- M. Xu, T. Wang, C. Zhang, B. L. Kuang, Z. M. Xie, Z. X. Yi, Z. J. Lu, Y. Li, S. Zhu and J. G. Zhang, Inorg. Chem., 2023, 62, 21371–21378 CrossRef CAS PubMed
.
- H. Gao, Q. Zhang and J. M. Shreeve, J. Mater. Chem. A, 2020, 8, 4193–4216 RSC
.
- J. Li, Y. Liu, W. Ma, T. Fei, C. He and S. Pang, Nat. Commun., 2022, 13, 5697 CrossRef CAS PubMed
.
- M. Deng, Y. Feng, W. Zhang, X. Qi and Q. Zhang, Nat. Commun., 2019, 10, 1339 CrossRef PubMed
.
- Y. Tang, C. He, P. Yin, G. H. Imler, D. A. Parrish and J. M. Shreeve, Eur. J. Org. Chem., 2018, 2273–2276 CrossRef CAS
.
- Y. Hu, W. S. Dong, Z. J. Lu, H. Zhang and J. G. Zhang, Chem. Commun., 2023, 59, 9864 Search PubMed
.
- Y. Liu, M. Lv, G. Zhang, Z. Dong and Z. Ye, Molecules, 2024, 29, 353 Search PubMed
.
- Y. Liu, X. Zhang, J. Li, A. Kou, H. Ding, S. Pang and C. He, Org. Lett., 2024, 26, 5488–5492 CrossRef CAS PubMed
.
- S. Kukreja, A. K. Yadav, S. Nehe and S. Dharavath, Org. Lett., 2024, 26, 10611–10615 CrossRef CAS PubMed
.
- L. Hu, C. He, S. Pang and J. M. Shreeve, Org. Lett., 2021, 23, 7860–7864 Search PubMed
.
- S. Chen, Y. Liu, Y. Feng, X. Yang and Q. Zhang, Chem. Commun., 2020, 56, 1493–1496 RSC
.
-
(a) L. Hu, R. J. Staples and J. M. Shreeve, Chem. Eng. J., 2021, 420, 129839 CrossRef CAS
; (b) S. Nehe, A. K. Yadav, V. D. Ghule and S. Dharavath, Org. Lett., 2025, 27, 5165–5169 CrossRef PubMed
.
- N. V. Muravyev, Chem. Eng. J., 2023, 472, 145032 Search PubMed
.
- A. K. Yadav, M. Jujam, V. D. Ghule and S. Dharavath, J. Heterocycl. Chem., 2024, 61, 401–406 CrossRef CAS
.
- J. Tang, H. Wei Yang and G. Bin Cheng, Energy Mater. Front., 2023, 4, 110–122 CrossRef CAS
.
- X. Yu, J. Tang, C. Lei, C. Xue, H. Yang, C. Xiao and G. Cheng, J. Mater. Chem. A, 2024, 12, 29638–29644 RSC
.
- C. Li, T. Zhu, J. Tang, G. Yu, Y. Yang, H. Yang, C. Xiao and G. Cheng, Chem. Eng. J., 2024, 479, 147355 CrossRef CAS
.
- W. Zhang, Y. Yang, Y. Wang, T. Fei, Y. Wang, C. Sun and S. Pang, Chem. Eng. J., 2023, 452, 138609 CrossRef
.
- P. Chen, H. Dou, J. Zhang, C. He and S. Pang, ACS Appl. Mater. Interfaces, 2023, 15, 4144–4151 CrossRef CAS PubMed
.
-
M. J. Frisch, et al., Gaussian 09, Revision B. 01, Gaussian, Inc., Wallingford, 2009 Search PubMed
.
- S. Banik, V. D. Ghule and S. Dharavath, Mater. Chem. Phys., 2023, 301, 12767 CrossRef
.
-
(a) S. V. Bondarchuk, J. Phys. Chem. A, 2018, 122, 5455–5463 CrossRef CAS PubMed
; (b) W. S. Wilson, D. E. Bliss, S. L. Christian and D. J. Knight, Explosive Properties of Polynitroaromatics, NWC/TP-7073, NavalWeapons Center, China Lake, CA, 1990
; (c) C. B. Storm, J. R. Stine and J. F. Kramer, Chem. Phys, Energy Mater., 1989, 309, 605–639 Search PubMed
.
- P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, J. Appl. Crystallogr., 2021, 54, 1006–1011 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2441109 and 2441110. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc02368h |
| ‡ These authors (SN and AKY) contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |