Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Flexible electrochemical energy storage devices and related applications: recent progress and challenges
Bo-Hao
Xiao
ab,
Kang
Xiao
 *a,
Jian-Xi
Li
a,
Can-Fei
Xiao
a,
Shunsheng
Cao
*a,
Jian-Xi
Li
a,
Can-Fei
Xiao
a,
Shunsheng
Cao
 *b and
Zhao-Qing
Liu
*b and
Zhao-Qing
Liu
 *a
*a
aSchool of Chemistry and Chemical Engineering/Institute of Clean Energy and Materials/Key Laboratory for Clean Energy and Materials, Guangzhou University, Guangzhou 510006, China. E-mail: kxiao@gzhu.edu.cn; lzqgzu@gzhu.edu.cn
bSchool of Materials Science & Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: sscao@ujs.edu.cn
First published on 28th June 2024
Abstract
Given the escalating demand for wearable electronics, there is an urgent need to explore cost-effective and environmentally friendly flexible energy storage devices with exceptional electrochemical properties. However, the existing types of flexible energy storage devices encounter challenges in effectively integrating mechanical and electrochemical performances. This review is intended to provide strategies for the design of components in flexible energy storage devices (electrode materials, gel electrolytes, and separators) with the aim of developing energy storage systems with excellent performance and deformability. Firstly, a concise overview is provided on the structural characteristics and properties of carbon-based materials and conductive polymer materials utilized in flexible energy storage devices. Secondly, the fabrication process and strategies for optimizing their structures are summarized. Subsequently, a comprehensive review is presented regarding the applications of carbon-based materials and conductive polymer materials in various fields of flexible energy storage, such as supercapacitors, lithium-ion batteries, and zinc-ion batteries. Finally, the challenges and future directions for next-generation flexible energy storage systems are proposed.
1. Introduction
The rapid consumption of fossil fuels in the world has led to the emission of greenhouse gases, environmental pollution, and energy shortage.1,2 It is widely acknowledged that sustainable clean energy is an effective way to solve these problems, and the use of clean energy is also extremely important to ensure sustainable development on a global scale.3–5 Over the past 30 years, great progress has been made in the development of electrochemical energy storage (EES) devices, such as rechargeable lithium-ion batteries (LIBs) and supercapacitors (SCs), which have been used in portable devices, electric vehicles, and stationary energy storage systems.6–8 With the increasing demand for energy storage devices, research on flexible electronic devices with flexible, folding and stretching functions is imminent, such as wearable electronic devices, electronic paper, smart clothing, electronic skins, displays, flexible smartphones, implantable medical devices, etc.9–11 To power these devices, it is necessary to develop flexible, scalable energy storage systems that can withstand mechanical deformation while maintaining their electrochemical properties.12,13 In general, LIBs and SCs are composed of several main components such as a positive electrode, negative electrode, isolation layer, electrolyte, fluid collector and packaging material.14 Due to the limitations of molding technology and traditional structure, the commercial LIBs and SCs currently used have high hardness and weight.15–18 Conventional electrodes are prepared by coating an electrode slurry (including active materials, conductive materials, and polymer adhesives) on a collector, then drying and pressing tightly.19,20 As a result, electrode materials easily fall off from the collector. As for the metal collectors, it is difficult for them to return to the initial state after repeated deformation, which leads to the deterioration of energy storage performance.21 In addition, the delaminated electrode material may penetrate the isolation layer, leading to short circuits and thermal runaway.22 Additionally, conventional liquid electrolytes inevitably exhibit leakage under deformation, thus necessitating the utilization of solid/quasi-solid gel electrolytes possessing superior mechanical properties and adhesion as an optimal solution to this issue.23For flexible and scalable LIBs and SCs, all components are inevitably subjected to repeated elastic deformation, but traditional components do not have this advantage, so all components in LIBs and SCs should be selected to withstand continuous deformation.24,25 Among these components, the electrodes and electrolytes play a pivotal role in determining the mechanical and electrochemical properties of the system. Previous research has predominantly focused on investigating these two crucial elements.26–29Fig. 1a presents a comprehensive timeline illustrating the evolution and development of deformable electrodes and electrolytes for energy storage devices, as well as their applications in wearable electronics.30–48 The timeline categorizes these advancements based on material composition and functionality, showcasing the diverse progress achieved using carbon-based materials and organo-gels for developing wearable energy storage devices. The increasing number of published papers and patent applications (Fig. 1b) reflects that flexible energy storage has gradually attracted extensive attention from academic and industrial circles in recent years. These technological advancements are poised to revolutionize wearable electronics by enhancing their comfort, durability, and multifunctionality, ultimately paving the way for their commercialization. However, it is still in the early stages of research. Therefore, the exploration of novel material and structural design solutions for flexible and scalable EES remains an urgent and thorny challenge.
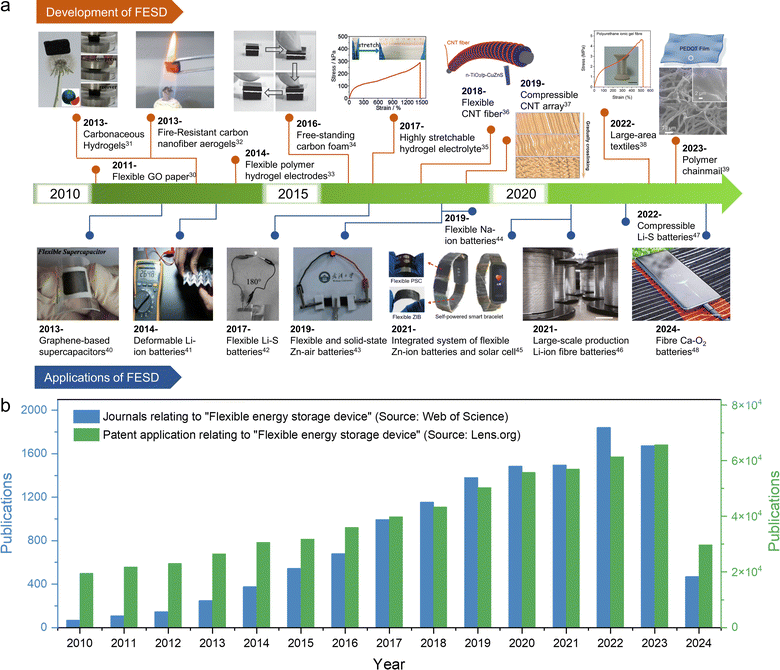 | ||
| Fig. 1 (a) Timeline showing the key development of flexible energy storage devices and their applications in wearable electronics.30–48 Reproduced with permission. (b) Summary of the publication records pertaining to “flexible energy storage device” in the Web of Science and Lens.org databases, with a search date of June 2024. | ||
In this review, we review the design, synthesis strategies, and recent advances of electrode and electrolyte materials for various flexible energy storage devices (Fig. 2). The review begins with a detailed discussion of synthetic strategies for flexible electrode materials and gel electrolytes in Section 2. Subsequent sections provide a comprehensive discourse on electrochemical energy storage systems currently employed in wearable electronics: SCs in Section 3, zinc-ion batteries (ZIBs) in Section 4, metal–air batteries in Section 5 within an aqueous system, lithium-ion batteries in Section 6, lithium–sulfur batteries (LSBs) in Section 7, and sodium ion batteries (SIBs) as well as potassium ion batteries (PIBs) in Section 8 within the organic system. Finally, we provide a comprehensive overview of strategies aimed at optimizing flexible electrode and electrolyte materials, as well as integrating flexible energy storage devices, to expedite the realization of next-generation flexible electronic devices.
2. Material design for flexible electrochemical energy storage devices
In general, the electrodes and electrolytes of an energy storage device determine its overall performance, including mechanical properties (such as maximum tensile/compressive strain, bending angle, recovery ability, and fatigue resistance) and electrochemical properties (including capacity, rate performance, and long-term cycling stability). To cater to the requirements of flexible electronic devices, it is imperative for electrodes and electrolytes to retain their original mechanical and electrochemical properties across diverse deformation states.2.1 Flexible electrodes
There are two primary approaches to the design of flexible electrode materials: one involves transforming non-flexible materials into flexible ones through structural engineering, while the other entails combining active electrode materials with flexible substrates. Both strategies require further development to effectively address the increasing demand for wearable electronics.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 20% strain), and remarkable electrochemical stability across varying strains (Fig. 3a). The recombination strategy proposed by Tao et al.49 enables the construction of independent and flexible MXene thin film electrodes. The Ti3C2Tx microgels, isolated from 3D structured hydrogels, are reassembled with individual Ti3C2Tx nanosheets in varying mass ratios to form a densely packed 3D network in microscale, and a film morphology in macroscale. The resulting material demonstrates excellent bending properties (180°) and exhibits a high capacity of 736 F cm−3 at an ultrahigh scan rate of 2.0 V s−1 (Fig. 3b). Yin et al.50 developed a flexible LIB that draws inspiration from the helical structure of DNA. The battery architecture primarily consists of multiple rigid, thick energy stacks and some flexible, thin connectors. The thick energy stacks serve as the main storage units for energy, while the thin connectors are responsible for accommodating deformation. This design allows for stress buffering in the spiral strain region within the cell slot. As a result, this battery exhibits satisfactory capacity retention and stable energy output even under static, dynamic, and harsh in situ stress loads (Fig. 3c).
000 cycles at 20% strain), and remarkable electrochemical stability across varying strains (Fig. 3a). The recombination strategy proposed by Tao et al.49 enables the construction of independent and flexible MXene thin film electrodes. The Ti3C2Tx microgels, isolated from 3D structured hydrogels, are reassembled with individual Ti3C2Tx nanosheets in varying mass ratios to form a densely packed 3D network in microscale, and a film morphology in macroscale. The resulting material demonstrates excellent bending properties (180°) and exhibits a high capacity of 736 F cm−3 at an ultrahigh scan rate of 2.0 V s−1 (Fig. 3b). Yin et al.50 developed a flexible LIB that draws inspiration from the helical structure of DNA. The battery architecture primarily consists of multiple rigid, thick energy stacks and some flexible, thin connectors. The thick energy stacks serve as the main storage units for energy, while the thin connectors are responsible for accommodating deformation. This design allows for stress buffering in the spiral strain region within the cell slot. As a result, this battery exhibits satisfactory capacity retention and stable energy output even under static, dynamic, and harsh in situ stress loads (Fig. 3c).
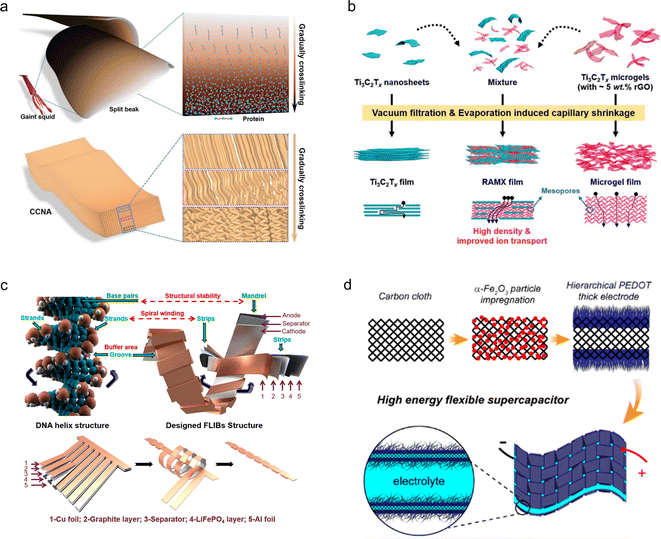 | ||
| Fig. 3 (a) Schematic illustration of a biomimetic freestanding carbon nanotube array designed to replicate the gradually crosslinked structure found in the beak of a giant squid.37 Reproduced with permission from ref. 37. Copyright 2019, Wiley-VCH GmbH. (b) Schematic representation of the preparation of Ti3C2Tx films.49 Reproduced with permission from ref. 49. Copyright 2021, Wiley-VCH GmbH. (c) Schematic representation of a helix-inspired battery designed with reference to the DNA structure.50 Reproduced with permission from ref. 50. Copyright 2022, American Chemical Society. (d) Schematic representation of in situ synthesis of polymer PEDOT on carbon cloth.51 Reproduced with permission from ref. 51. Copyright 2021, American Chemical Society. | ||
2.2 Flexible hydrogel electrolytes
Another crucial element of energy storage devices is the electrolyte, comprising inorganic salts and solvents with high conductivity. Within an electrolyte, the conductive salt undergoes dissociation into charge-carrying ions and shuttles between the positive and negative electrodes to facilitate charge transport. The electrochemical properties, such as conductivity, mobility rate, and diffusion coefficient, are determined by factors including ion concentration within the electrolyte, solvent type (inorganic/organic), and electrode interactions.Gel electrolytes have been extensively utilized in the field of flexible energy storage due to their excellent mechanical stability and conductivity. To develop electrolytes suitable for flexible energy storage devices, it is imperative to modify the physical state of the electrolyte to a solid or quasi-solid form, thereby preventing any leakage during mechanical deformation. The commonly employed raw materials for gel preparation mainly include polyvinyl alcohol, polyacrylamide, polyethylene glycol, and polyacrylic acid. Gel electrolytes can be categorized into ionic gel electrolytes, hydrogel electrolytes, and organic gel electrolytes. For instance, C. Hersam et al.60 have reported on the development of an ion-gel electrolyte based on layered heterostructures utilizing two imidazole ionic liquids. This unique layered architecture exhibits a significantly expanded electrochemical window while maintaining exceptional ionic conductivity. In comparison to conventional ion-gel electrolytes, the heterogeneous nature of this layered ion-gel electrolyte enhances both the cycling stability and mechanical properties of LIBs (Fig. 4a). To solve the problems of low conductivity of solid gel electrolyte and poor interfacial stability of the Zn anode, Zhi et al.61 developed a water-deficient hydrogel electrolyte as an intermediate layer. The polymer backbone of the hydrogel features a polymeric zwitterion, with sulfonate end groups that exhibit both hydrophilic and zincophilic properties, while the coordination unit is provided by the zinc salt. This unique structure enables impressive ion conductivity (2.6 × 103 S cm−1) even under water-poor conditions, demonstrating excellent long-term cycling stability (91% at 5C for 4000 cycles), as well as strong adhesion and strain tolerance (Fig. 4b).
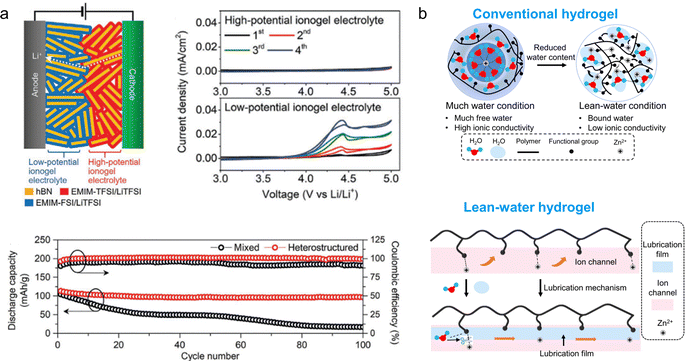 | ||
| Fig. 4 (a) Schematic of a solid-state lithium-ion battery using the layered heterostructure ionic gel electrolyte with two different ionic liquids and hexagonal boron nitride nanoplatelets and the electrochemical properties.60 Reproduced with permission from ref. 60. Copyright 2021, Wiley-VCH GmbH. (b) Schematic illustration of the ionic transportation mechanism of hydrogels.61 | ||
3. Flexible supercapacitors
SCs represent a highly promising candidate for flexible/wearable energy storage devices owing to their high power density, long cycle life and fast charge/discharge rates.62 Categorized based on the energy storage mechanism, they can be classified into electrical double layer capacitors and pseudo-capacitors.63 Electrical double layer capacitors store charge through the electrostatic adsorption process that occurs at the interface between the electrode and electrolyte, while pseudo-capacitors store charge through electron gain/loss via a rapid and reversible redox reaction on the surface of the electrode. Although the flexible electrode materials and hydrogel electrolytes investigated thus far exhibit commendable mechanical properties, there remains space for improvement in the electrochemical performance of these devices. The electrode materials for flexible SCs are classified and discussed in this section, encompassing three categories: compressible electrodes, foldable electrodes, and stretchable electrodes. Furthermore, a concise overview is provided on the electrolytes and current collectors employed in flexible SCs.3.1 Flexible electrodes
Zhang and his colleagues66 prepared cellulose nanofibrils/carbon nanotubes/reduced graphene oxide (CNF/CNTs/rGO) composite carbon aerogels through bi-directional freezing and annealing treatments. By introducing cellulose nanofibers and carbon nanotubes into the reduced graphene oxide, a regular layered porous structure was formed, effectively inhibiting the stacking of graphene sheets. Therefore, the CNF/CNTs/rGO exhibited excellent performance with a capability to undergo 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 50% compressive strain. Furthermore, it demonstrated outstanding area capacitance (109.4 mF cm−2 at 0.4 mA cm−2) as well as long-term cycling stability (88% retention after 5000 cycles at 50% compression strain). The CNF/CNTs/rGO can be used as a strain sensor to capture biological signals from the human body (Fig. 5a). Ye et al.57 synthesized nitrogen-doped carbon aerogels (C-NGDs) through a biomass-mediated approach, utilizing cost-effective glucose and dicyandiamide as raw materials instead of the expensive graphene oxide and carbon nanotubes. Owing to the highly stable wave-layered structure of the aerogels, it can withstand up to 95% compressive strain. Moreover, when assembled into a symmetric device, it exhibits an impressive specific capacity of 220.2 F g−1 at a current density of 0.5 A g−1, thus establishing itself as a versatile material for applications in biosensing, flexible electronics, and energy storage and conversion devices (Fig. 5b). However, despite the 3D carbon aerogel's potential as a SC electrode material, it cannot meet the required capacity demands, hence the need for introducing pseudocapacitive materials to enhance their electrochemical performance. M. Razal et al.67 developed a super-elastic and highly loaded MXene/rGO composite aerogel through reduction, freeze-drying and annealing processes. The highly loaded MXene was attached to the graphene wall to form a robust cellular scaffold (Fig. 5c). Due to the synergistic effect of the pseudocapacitive material MXene and graphene, the composite aerogel exhibits not only excellent mechanical stability (60% strain for 1000 cycles) but also high specific capacity (397 F g−1 at 0.5 A g−1). Similarly, Liu and his colleagues39 proposed a “polymer chainmail” strategy, wherein electrochemically stabilized anion-doped PEDOT is synthesized on a 3D nitrogen-doped carbon foam skeleton through chemical in situ polymerization (Fig. 5d). Due to the film-forming characteristics during the PEDOT polymerization process and excellent electrochemical performances, the resulting composites exhibit a synergistic enhancement of mechanical properties (1000 cycles of compression at 80% strain) and electrochemical properties (604.6 F g−1 at 3 mA cm−3).
000 cycles at 50% compressive strain. Furthermore, it demonstrated outstanding area capacitance (109.4 mF cm−2 at 0.4 mA cm−2) as well as long-term cycling stability (88% retention after 5000 cycles at 50% compression strain). The CNF/CNTs/rGO can be used as a strain sensor to capture biological signals from the human body (Fig. 5a). Ye et al.57 synthesized nitrogen-doped carbon aerogels (C-NGDs) through a biomass-mediated approach, utilizing cost-effective glucose and dicyandiamide as raw materials instead of the expensive graphene oxide and carbon nanotubes. Owing to the highly stable wave-layered structure of the aerogels, it can withstand up to 95% compressive strain. Moreover, when assembled into a symmetric device, it exhibits an impressive specific capacity of 220.2 F g−1 at a current density of 0.5 A g−1, thus establishing itself as a versatile material for applications in biosensing, flexible electronics, and energy storage and conversion devices (Fig. 5b). However, despite the 3D carbon aerogel's potential as a SC electrode material, it cannot meet the required capacity demands, hence the need for introducing pseudocapacitive materials to enhance their electrochemical performance. M. Razal et al.67 developed a super-elastic and highly loaded MXene/rGO composite aerogel through reduction, freeze-drying and annealing processes. The highly loaded MXene was attached to the graphene wall to form a robust cellular scaffold (Fig. 5c). Due to the synergistic effect of the pseudocapacitive material MXene and graphene, the composite aerogel exhibits not only excellent mechanical stability (60% strain for 1000 cycles) but also high specific capacity (397 F g−1 at 0.5 A g−1). Similarly, Liu and his colleagues39 proposed a “polymer chainmail” strategy, wherein electrochemically stabilized anion-doped PEDOT is synthesized on a 3D nitrogen-doped carbon foam skeleton through chemical in situ polymerization (Fig. 5d). Due to the film-forming characteristics during the PEDOT polymerization process and excellent electrochemical performances, the resulting composites exhibit a synergistic enhancement of mechanical properties (1000 cycles of compression at 80% strain) and electrochemical properties (604.6 F g−1 at 3 mA cm−3).
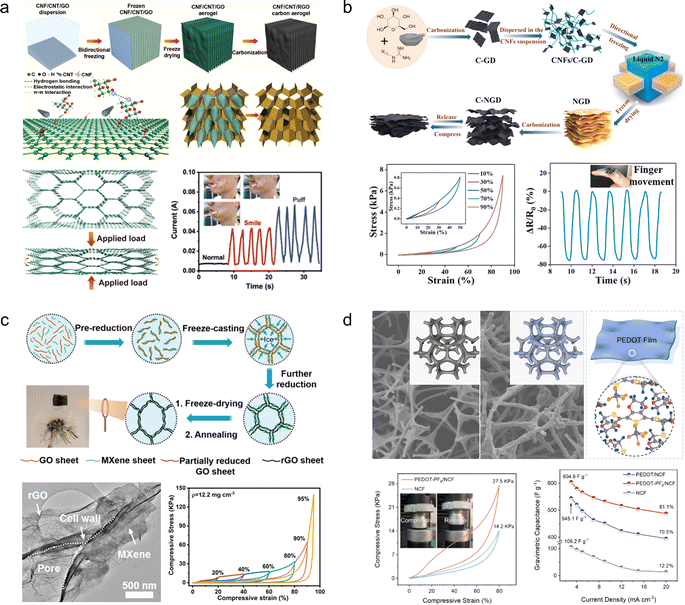 | ||
| Fig. 5 (a) Schematic diagram for the preparation of CNF/CNT/rGO carbon aerogels and their practical applications.66 Reproduced with permission from ref. 66. Copyright 2022, Wiley-VCH GmbH. (b) Preparation process of C-NGD carbon aerogels and their mechanical and electrochemical properties.57 Reproduced with permission from ref. 57. Copyright 2021, Elsevier. (c) Schematic diagram and digital photographs of the fabrication process for the MXene/rGO; SEM image and galvanostatic charge–discharge curves of MXene/rGO.67 Reproduced with permission from ref. 67. Copyright 2021, American Chemical Society. (d) Schematic illustration of the synthesis process of PEDOT-PF-6, along with the mechanical and electrochemical properties of PEDOT-PF-6.39 Reproduced with permission from ref. 39. Copyright 2023, Wiley-VCH GmbH. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), while maintaining proper functionality across diverse bending angles.
000 cycles), while maintaining proper functionality across diverse bending angles.
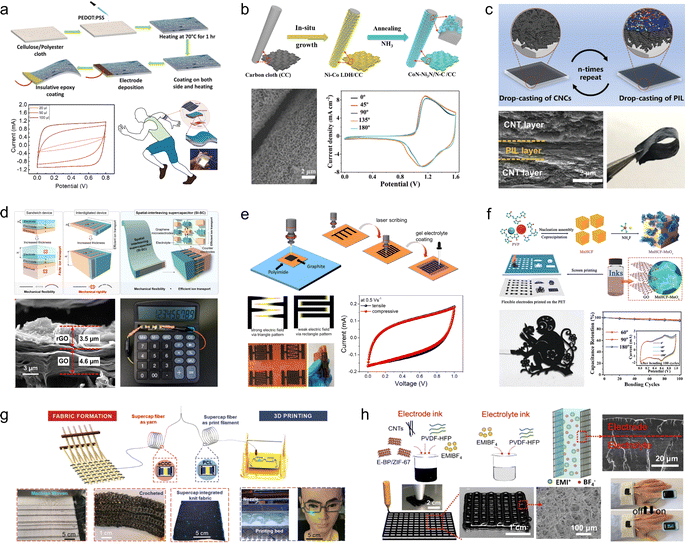 | ||
| Fig. 6 (a) Scheme for the fabrication of PEDOT:PSS-based electrodes on cloth.68 (b) Schematic diagram of the synthesis process for CoN–Ni3N/N–C/CC.69 Reproduced with permission from ref. 69. Copyright 2021, Wiley-VCH GmbH. (c) Schematic illustration of the preparation process and digital photographs of (CNC/PIL)-nL.70 Reproduced with permission from ref. 70. Copyright 2022, Wiley-VCH GmbH. (d) Spatial-interleaving SCs based on graphene microelectrodes.71 Reproduced with permission from ref. 71. Copyright 2022, American Chemical Society. (e) Fabrication of interdigitated carbon-based electrodes.72 (f) Schematic representation of the synthesis steps for MnHCF–MnOx, the photograph of large-scale printed patterned electrodes, and characterization of their electrochemical properties.73 Reproduced with permission from ref. 73. Copyright 2020, Wiley-VCH GmbH. (g) Schematic diagram of machine-weaving and 3D printing from thermally drawn supercapacitor fibers.74 Reproduced with permission from ref. 74. Copyright 2020, Wiley-VCH GmbH. (h) Illustration of the 3D printing of all-integrated solid-state flexible supercapacitors.75 Reproduced with permission from ref. 75. Copyright 2021, Wiley-VCH GmbH. | ||
In addition to commercially available fabrics, a wide range of homemade flexible 2D thin-film carbon-based electrode materials have been extensively developed for application in foldable SCs. These materials can be tailored with diverse internal structures based on specific requirements using techniques such as laser cutting, repeated drop-casting, and 3D printing, thereby overcoming the limitations imposed by the original structure of commercial flexible substrates. Pan et al.70 prepared CNC/PEDOT:PSS flexible films with hierarchical structure by alternating deposition of carbon nanocoils and PEDOT:PSS (Fig. 6c). The interconnected CNCs within the porous structure serve as high-speed channels for ion transport, while the presence of the PEDOT:PSS conductive film ensures structural stability and enhances interlayer electron transfer kinetics. Consequently, these thin-film electrodes exhibit excellent bendability and electrochemical properties, achieving an area capacitance of 1402.5 mF cm−2 at 0.25 mA cm−2. Moreover, when assembled into solid-state SCs based on CNC/PEDOT:PSS, they demonstrate a wide potential window of 2.0 V and maintain initial performances even under various bending angles. Li and his coworkers71 developed a spatially interleaved SC using the laser cutting method (Fig. 6d). In this approach, graphene microelectrodes are reversely stacked layer by layer in 3D space, with narrow gaps strategically incorporated to enhance ion transport within the electrolyte. The unique interlaced structure of graphene microelectrodes imparts excellent mechanical flexibility to the spatially interleaved SCs, which exhibits negligible capacity decay even after undergoing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending tests. Moreover, it achieves an impressive area-ratio capacitance of 36.46 mF cm−2 within a compact thickness of only 100 μm, thereby showcasing its potential for seamless integration into wearable electronic devices. Lee et al.72 improved the process of laser engraving to design flexible SCs, wherein the electrode layer was designed with intersecting sharp edges to induce a pronounced electric field at the corners of the electrode fingers, thereby augmenting charge accumulation near the electrode surface (Fig. 6e). Wu et al.73 employed screen-printing technology to fabricate electrode materials composed of manganese hexacyanoferrate–manganese oxide and electrochemically reduced graphene oxide composites (MnHCF–MnOx/ErGO), which can be tailored into diverse patterns as required (Fig. 6f). At a current density of 1.0 A g−1, a high specific capacity of 467 F g−1 was achieved, and the capacitance remained stable for 100 cycles under different bending angles (60°, 90°, and 180°).
000 bending tests. Moreover, it achieves an impressive area-ratio capacitance of 36.46 mF cm−2 within a compact thickness of only 100 μm, thereby showcasing its potential for seamless integration into wearable electronic devices. Lee et al.72 improved the process of laser engraving to design flexible SCs, wherein the electrode layer was designed with intersecting sharp edges to induce a pronounced electric field at the corners of the electrode fingers, thereby augmenting charge accumulation near the electrode surface (Fig. 6e). Wu et al.73 employed screen-printing technology to fabricate electrode materials composed of manganese hexacyanoferrate–manganese oxide and electrochemically reduced graphene oxide composites (MnHCF–MnOx/ErGO), which can be tailored into diverse patterns as required (Fig. 6f). At a current density of 1.0 A g−1, a high specific capacity of 467 F g−1 was achieved, and the capacitance remained stable for 100 cycles under different bending angles (60°, 90°, and 180°).
In recent years, the widespread utilization of 3D printing technology in the domain of flexible energy storage devices has been attributed to its capability to design electrode materials or energy storage devices with diverse geometries based on specific requirements. This addresses the issues related to limited scalability, flexibility, and adaptability encountered by flexible energy storage devices. Fink et al.74 pioneered the fabrication of fiber supercapacitors through a top-down approach, wherein macroscopic prefabricated components were thermally stretched into 100 meter-long energy storage fibers possessing excellent mechanical strength and moisture resistance (Fig. 6g). Wu et al.75 developed a heterogeneous nanostructured black phosphorus/metal–organic framework hybrid (E-BP/ZIF-67), which exhibited high specific surface area and remarkable electrochemical activity (Fig. 6h). The highly active nanohybrid along with the poly(vinylidene difluoride–hexafluoropropylene) binder functioned as stabilizing ink constituents, enabling successful construction of this electrode material using 3D printing technology in a fully integrated manner. The resulting solid-state flexible supercapacitor demonstrated an ultra-high volumetric energy density of 109.8 mW h cm−3, long-term stability over 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and exceptional deformable performance.
000 cycles, and exceptional deformable performance.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at −35 °C).
000 cycles at −35 °C).
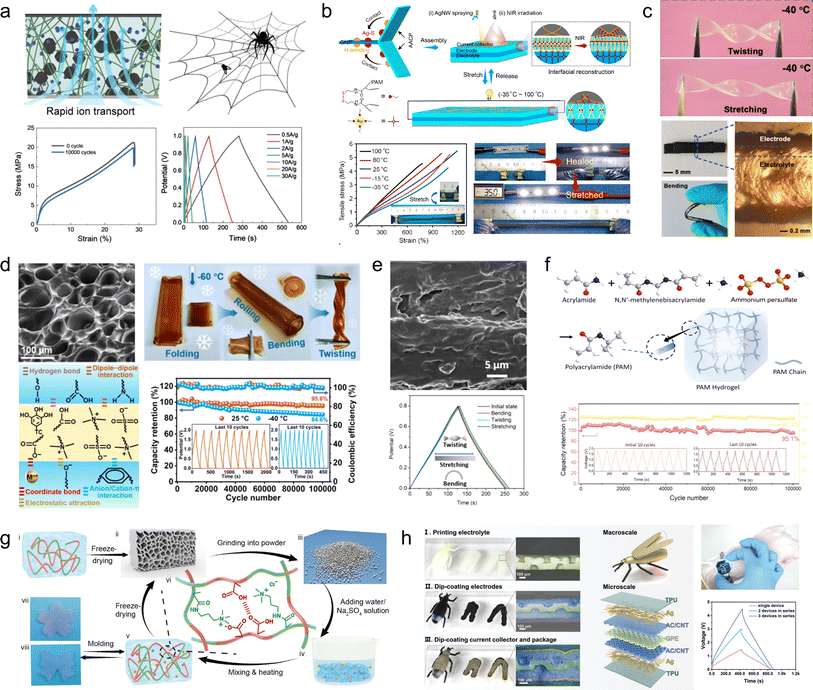 | ||
| Fig. 7 (a) Electrodes based on the preparation of 3D structural adhesives inspired by spider webs.78 Reproduced with permission from ref. 78. Copyright 2021, Wiley-VCH GmbH. (b) Schematic illustrations of the preparation of the AACP hydrogel electrode.79 Reproduced with permission from ref. 79. Copyright 2022, American Chemical Society. (c) Exhibition of twisting and stretching for hydrogel webs.80 Reproduced with permission from ref. 80. Copyright 2021, Wiley-VCH GmbH. (d) Anti-freezing and self-adhesive polyzwitterionic hydrogel electrolyte.81 Reproduced with permission from ref. 81. Copyright 2021, American Chemical Society. (e) Morphology and electrochemical performance of the MMT/PVA membrane.82 Reproduced with permission from ref. 82. Copyright 2020, American Chemical Society. (f) Illustration of the synthesis of the polyacrylamide (PAM) hydrogel and its cycle stability.83 Reproduced with permission from ref. 83. Copyright 2020, Wiley-VCH GmbH. (g) Fabrication scheme for the regenerated hydrogel electrolyte.84 Reproduced with permission from ref. 84. Copyright 2021, Springer Nature. (h) Optical photographs, structural schematics, and electrochemical properties of double-layer supercapacitors assembled based on gel-like polymer electrolytes.85 Reproduced with permission from ref. 85. Copyright 2023, Wiley-VCH GmbH. | ||
3.2 Flexible electrolytes
Hydrogel electrolytes have attracted a great deal of attention in flexible and safe SCs due to the risk of electrolyte leakage from SC devices under deformation or bending. However, the interfacial contact problem between the hydrogel electrolyte and electrodes as well as the environmental instability are the key factors restricting the development of hydrogel supercapacitors. Therefore, in recent years, people have been committed to developing new high-performance hydrogel electrolytes. Gao et al.80 developed a nucleotide-tackified adhesive organo-hydrogel electrolyte (P(AM-co-DMAEMA)/gelatine) that facilitates intimate electrode-hydrogel electrolyte contact while reducing the interfacial contact resistance (Fig. 7c). Moreover, the hydrogel exhibited excellent mechanical properties and freezing resistance, ensuring the absence of delamination and displacement between the electrolyte and electrodes even under severe deformation conditions. Additionally, these SCs operated reliably for 5000 cycles at temperatures as low as −20 °C. However, further reduction in temperature leads to a significant decline in both the conductivity and mechanical properties of these SCs, thereby severely compromising interfacial adhesion. To address this issue, Yang and his colleagues81 developed an anti-freezing and self-adhesive polyzwitterionic hydrogel electrolyte (PZHE) using an autocatalytic nano-enhancement strategy (Fig. 7d). The PZHE maintained good interfacial adhesion even at a low temperature of −60 °C. Additionally, the ZnCl2-filled salt-in-water type PZHE facilitated ion migration channels and enhanced the reversibility of the Zn metal electrodes, effectively reducing side reactions and prolonging cycle life. As a result, the Zn2+ ion hybrid capacitor achieved a high energy density of 80.5 W h kg−1 with excellent cycle stability of up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. On the other hand, to address the issue of instability exhibited by conventional hydrogel electrolytes under high temperature, Chen et al.82 developed a montmorillonite/polyvinyl alcohol (MMT/PVA) hydrogel electrolyte, which exhibited enhanced thermal stability due to the presence of montmorillonite and a reduced freezing point by incorporating dimethyl sulfoxide into the solution (Fig. 7e). As a result, the electrolyte demonstrated high conductivity ranging from 0.17 × 10−4 to 0.76 × 10−4 S cm−1 over a wide temperature range (−50 °C to 90 °C). Moreover, the assembled devices exhibited excellent capacity performance and mechanical flexibility. In addition to its exceptional temperature adaptability, it is crucial not to overlook the influence of flexible gel electrolyte modification on the cycling stability of devices. Chen et al.83 have successfully developed a mechanically flexible water-in-salt hydrogel electrolyte based on a chloride ion-facilitated de-solvation mechanism within hydrated ZnCl+–(H2O)n−1 (with n = 1–6) clusters (Fig. 7f). This innovative approach not only enhances the energy storage capacity of porous carbon materials but also improves the reversibility of Zn metal electrodes. The resulting zinc-ion hybrid capacitor exhibits an impressive energy density of 217 W h kg−1 and demonstrates outstanding cycle life with up to 100
000 cycles. On the other hand, to address the issue of instability exhibited by conventional hydrogel electrolytes under high temperature, Chen et al.82 developed a montmorillonite/polyvinyl alcohol (MMT/PVA) hydrogel electrolyte, which exhibited enhanced thermal stability due to the presence of montmorillonite and a reduced freezing point by incorporating dimethyl sulfoxide into the solution (Fig. 7e). As a result, the electrolyte demonstrated high conductivity ranging from 0.17 × 10−4 to 0.76 × 10−4 S cm−1 over a wide temperature range (−50 °C to 90 °C). Moreover, the assembled devices exhibited excellent capacity performance and mechanical flexibility. In addition to its exceptional temperature adaptability, it is crucial not to overlook the influence of flexible gel electrolyte modification on the cycling stability of devices. Chen et al.83 have successfully developed a mechanically flexible water-in-salt hydrogel electrolyte based on a chloride ion-facilitated de-solvation mechanism within hydrated ZnCl+–(H2O)n−1 (with n = 1–6) clusters (Fig. 7f). This innovative approach not only enhances the energy storage capacity of porous carbon materials but also improves the reversibility of Zn metal electrodes. The resulting zinc-ion hybrid capacitor exhibits an impressive energy density of 217 W h kg−1 and demonstrates outstanding cycle life with up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
To accommodate supercapacitor electrode materials with customizable external geometries obtained through 3D printing preparation, studies on gel electrolytes prepared using 3D printing technology have also been reported. Hydrogel electrolytes, due to their excellent mechanical strength, reproducibility, and plasticity, can serve as a suitable substrate for 3D printing. For instance, Wang et al.84 developed a robust hydrogel by random copolymerization of 3-(methacryloylamino)-propyl trimethylammonium chloride and methacrylic acid, utilizing various interactions such as hydrophilic hydrogen bonding and polyanionic interactions along with entanglement of hydrophobic polymer chains (Fig. 7g). The resulting hydrogel exhibits mechanical stability and self-repairing properties owing to the interplay between the hydrogen bonding network and polyanions/cations. When employed in flexible supercapacitors, this electrolyte can also function as a binder that integrates active materials into the polymer network while significantly reducing interfacial resistance between the electrolyte and electrodes. Consequently, the assembled device maintains stable mechanical and electrochemical properties even after undergoing over 100 consecutive bending cycles. Zhou et al.85 proposed an innovative fabrication method for flexible supercapacitors wherein a gel electrolyte template was initially prepared via 3D printing followed by assembly of electrodes, collectors, and encapsulation materials through the dip-coating technique, thus enabling realization of flexible supercapacitors possessing both an internal three-dimensional structure and overall 3D geometry that can be easily integrated into diverse electronic products (Fig. 7h).
4. Flexible zinc-ion batteries
Compared to LIBs employing organic electrolytes, aqueous ZIBs have garnered attention due to their superior safety profile, cost-effectiveness, and simplified assembly process (eliminating the need for glovebox operation).86 Since the Zn anode enables two-electron participation in the electrochemical reaction, it exhibits a remarkable specific capacity of 820 mA h g−1 alongside a low redox potential of −0.76 V (vs. the standard hydrogen electrode).87 Therefore, ZIBs hold great promise as highly efficient energy storage devices for next-generation wearable electronics.884.1 Foldable electrodes
Manganese-based or vanadium-based oxides, such as V2O5, VO2, MnO2, and Mn3O4, are commonly employed as cathode materials for aqueous ZIBs.89,90 However, the limited electrical conductivity inherent in these metal oxides poses challenges to their rate performance and cycling stability. To address this issue, these active materials are usually compounded with various carbon-based materials, such as carbon nanotubes, reduced graphene oxide and carbon cloth.91,921D carbon nanotubes can serve as linking materials for transition metal oxides, thereby addressing the issue of mechanical instability in the direction perpendicular to 2D nanosheets. For instance, Wang et al.93 developed a strategy involving the use of 1D carbon nanotubes stitched with Zn3(OH)2V2O7·2H2O (CNT-stitched ZVO) 2D nanosheets directly grown on oxidized carbon nanotube fibers (Fig. 8a). The incorporation of stitched carbon nanotubes onto the ZVO nanosheets not only imparted electrical conductivity but also enhanced mechanical properties. Consequently, even when subjected to bending at 180°, the composites exhibited no active material detachment and retained their original properties. Moreover, assembling a full cell with Zn NSs@CNT fibers (carbon nanotube fibers electrodeposited with zinc nanosheets) as anodes demonstrated exceptional performance stability over extended cycling periods (69.7% capacity retention after a 100-fold increase in current density and 88.6% capacity retention after 2000 cycles).
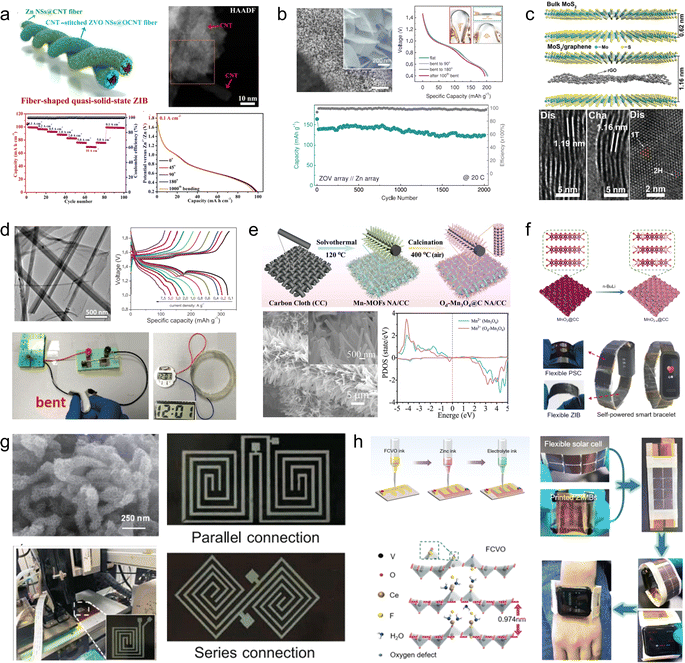 | ||
| Fig. 8 (a) Schematic illustration of the fiber-shaped quasi-solid-state Zn//ZVO ZIB and its electrochemical properties.93 Reproduced with permission from ref. 93. Copyright 2020, American Chemical Society. (b) Morphology and electrochemical properties of a ZVO array cathode.94 Reproduced with permission from ref. 94. Copyright 2018, Wiley-VCH GmbH. (c) Crystal structures of MoS2/graphene and its phase transition.95 Reproduced with permission from ref. 95. Copyright 2021, Wiley-VCH GmbH. (d) Morphology and electrochemical properties of the MnO2/rGO nanocomposite.59 Reproduced with permission from ref. 59. Copyright 2020, Wiley-VCH GmbH. (e) Illustration of the synthesis procedures of the Od-Mn3O4@C NA/CC nanostructure.96 Reproduced with permission from ref. 96. Copyright 2020, Wiley-VCH GmbH. (f) Schematic fabrication process of the MnO2−x@CC and its practical applications.45 Reproduced with permission from ref. 45. Copyright 2021, American Chemical Society. (g) Photograph of the multinozzle printing system and a heating plate of the 3D printing equipment printer.97 (h) Schematic of the fabrication process and optical photographs of the flexible zinc ion micro-batteries through 3D printing.98 | ||
2D graphene nanosheets are commonly used as flexible and conductive scaffolds for layered transition metal oxides. For instance, Fan et al.94 fabricated layered Zn orthovanadate and Zn nanosheets on a 3D porous graphene substrate to serve as the cathode and anode, respectively, in flexible ZIBs (Fig. 8b). Due to the ordered structure of the active material, the full cell assembled with quasi-solid hydrogel electrolyte exhibited exceptional rate capability at 50C and demonstrated excellent cycling stability (2000 cycles at a rate of 20C), effectively suppressing dendrite growth. Furthermore, the fully functional cell displayed remarkable mechanical flexibility within a bending angle range of 0 to 180°. Li et al.95 innovatively embedded graphene nanosheets into MoS2 channels (Fig. 8c). The sandwich structured MoS2/graphene nanosheets promoted the diffusion of Zn2+ as well as the penetration of the electrolyte solution, along with strong structural stability. The composite thus demonstrated high-rate performance (285.4 mA h g−1 at 0.05 A g−1 with 141.6 mA h g−1 at 5 A g−1) and long-term cycling stability (88.2% capacity retention after 1800 cycles). Through a series of ex situ characterizations, the authors validated that the introduction of graphene nanosheets enabled highly reversible phase transition between 2H-MoS2 and 1T-MoS2, thus elucidating the energy storage mechanism. Similarly, Wei et al.59 fabricated a composite material comprising of ultra-long manganese dioxide nanowires and graphene nanosheets as a flexible cathode for ZIBs (Fig. 8d). Benefiting from the strong electrochemical synergy provided by the unique 1D/2D nanostructures, the MnO2/rGO composite demonstrates an enhanced specific capacity (317 mA h g−1) in its conventional state and maintains normal operation even when folded at 180°.
Commercial carbon cloths are also commonly used to prepare cathode materials for flexible ZIBs. Wang et al.96 synthesized oxygen-deficient Mn3O4@C nanorod arrays (Od-Mn3O4@C NA/CC) on carbon cloth by carbonizing the manganese metal–organic framework (Fig. 8e). In addition to enhancing the conductivity of Mn3O4, they successfully improved the stability of the MnO6 octahedral structure, effectively inhibiting Mn2+ dissolution. As a result, the assembled full cell achieved an impressive capacity retention of 84.1 mA h g−1 (up to 95.7% of the initial capacity) after 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 5 A g−1. Similarly, Tan et al.45 fabricated a cathode material for ZIBs by growing defective MnO2−x nanosheets on carbon cloth (Fig. 8f). A Li treatment during the preparation process was employed to expand the layer spacing of MnO2 and induce oxygen vacancies. Compared to pristine MnO2/CC, the utilization capacity, rate capability, and cycling stability of MnO2−x/CC as a cathode were significantly enhanced. Even at an ultra-high mass loading of 25.5 mg cm−2, the specific capacity reached 3.63 mA h cm−2. Self-powered wristbands based on flexible ZIBs assembled with MnO2−x/CC and integrated with solar cells demonstrated their potential in powering commercial wearable electronic devices.
000 cycles at 5 A g−1. Similarly, Tan et al.45 fabricated a cathode material for ZIBs by growing defective MnO2−x nanosheets on carbon cloth (Fig. 8f). A Li treatment during the preparation process was employed to expand the layer spacing of MnO2 and induce oxygen vacancies. Compared to pristine MnO2/CC, the utilization capacity, rate capability, and cycling stability of MnO2−x/CC as a cathode were significantly enhanced. Even at an ultra-high mass loading of 25.5 mg cm−2, the specific capacity reached 3.63 mA h cm−2. Self-powered wristbands based on flexible ZIBs assembled with MnO2−x/CC and integrated with solar cells demonstrated their potential in powering commercial wearable electronic devices.
The 3D printing technology has been utilized for the fabrication of carbon-based cathode materials in flexible ZIBs. For instance, Ma et al.97 developed a bendable electrode material by employing the 3D printing technique to prepare manganese dioxide-coated carbon nanotube (CNT@MnO2) ink. The resulting Zn//CNT@MnO2 flexible ZIBs exhibited a stable capacity of 63 μA h cm−2 at 0.4 mA cm−2, with only a maximum capacity loss of 2.72% when subjected to bending in different states (Fig. 8g). Tan et al.98 reported an F/Ce co-doped V2O5 printing ink that demonstrated improved specific capacity, rate performance, and long-term cycling stability due to increased layer spacing and introduction of oxygen defects in V2O5 after co-doping (Fig. 8h). The electrodes fabricated using the 3D printing technology displayed high-quality loading and excellent mechanical properties, leading to ultra-high capacity (10.1 mA h cm−2) and energy density (8.1 mW h cm−2) in assembled flexible ZIBs.
The primary obstacle currently impeding the advancement of ZIBs lies in the uncontrolled growth of dendrites on the anode Zn electrode, which is primarily attributed to excessive local current density and electric field imbalance during Zn deposition. This flaw is inevitably exacerbated under conditions of device bending and deformation. Consequently, it is imperative to consider the issue of dendrite growth when designing flexible anode materials for ZIBs.
Generally, there are two design concepts for flexible ZIB anodes. The first involves employing structural engineering techniques to make the Zn anode flexible. For example, Duan et al.99 have developed a topology-optimized bionic honeycomb Zn anode that effectively enhances the distribution of current, stress, and thermal fields within the anode (Fig. 9a). This innovative structure achieves a multi-field modulation effect, leading to improved electrochemical and mechanical stability. Notably, the electrode exhibits an impressive cycle life of 2000 h (at a current density of 30 mA cm−2) and demonstrates exceptional rate performance at 20C. Furthermore, this anode stands out for its remarkable performance while maintaining cost-effectiveness.
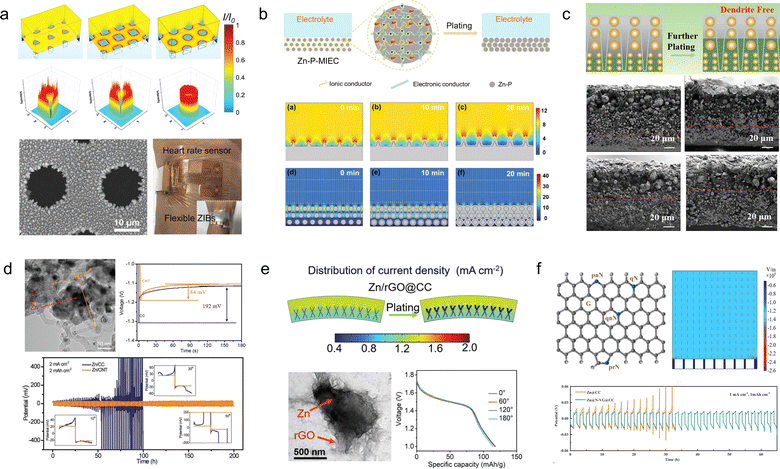 | ||
| Fig. 9 (a) Theoretical simulations of current density redistributions and morphology of the biomimetic honeycomb anode.99 Reproduced with permission from ref. 99. Copyright 2023, Wiley-VCH GmbH. (b) Schematic illustrations and electric field simulation on the mixed ionic–electronic conductor scaffold for Zn-powder.100 Reproduced with permission from ref. 100. Copyright 2022, Wiley-VCH GmbH. (c) Schematic diagram of desirable bottom-up and dendrite-free Zn deposition behavior in the Zn powder-based anode with gradient particle size.101 Reproduced with permission from ref. 101. Copyright 2023, Wiley-VCH GmbH. (d) TEM images and electrochemical performances of the Zn/CNT sample.102 Reproduced with permission from ref. 102. Copyright 2019, Wiley-VCH GmbH. (e) Electric field simulation, TEM image, and electrochemical performances of Zn/rGO@CC electrodes.103 Reproduced with permission from ref. 103. Copyright 2023, Wiley-VCH GmbH. (f) Schematic diagram and models of the electric field distributions for the Zn@N-VG@CC electrode.104 Reproduced with permission from ref. 104. Copyright 2021, Wiley-VCH GmbH. | ||
Flexible Zn anode materials can also be prepared using Zn powder as a raw material by the tape-casting technology. Liang et al.100 mixed Zn powder, ethylene vinyl acetate copolymer and carbon nanotubes sufficiently and then cast them onto a film, which was peeled off from the mold after drying to obtain a Zn-P-MIEC film (Fig. 9b). This freestanding flexible electrode material consists of a hybrid ionic–electronic conductor scaffold formed by carbon nanotubes and ethylene vinyl acetate copolymer, providing flexibility to the material while protecting the Zn powder from corrosion in aqueous solutions. The resulting zinc anode enables homogenization of the Zn2+ flux during repetitive galvanizing/stripping processes for stable and reversible cycling of Zn2+. Remarkably, even after 1270 h of cycling, only a minimal increase in voltage hysteresis was observed in batteries assembled with Zn-P-MIEC. Guan et al.101 employed a similar method to fabricate thin-film anode materials based on Zn powder, but they introduced a novel concept of gradient particle size (Fig. 9c). By reducing the particle size of the Zn powder and electrode porosity with increasing depth, a vertical gradient particle flux is generated, facilitating bottom-up Zn deposition and exfoliation while impeding dendrite growth. Consequently, these prepared electrodes exhibit exceptional cycling stability even under high current density and capacity conditions (5 mA cm−2, 5 mA h cm−2), achieving a cycle life of up to 130 h.
A more streamlined and efficient approach to mitigating dendrite growth in ZIBs involves the construction of a flexible 3D conductive framework, which serves as a galvanized/stripped support. Lu et al.102 utilized 3D carbon nanotubes as a flexible substrate and a galvanized support, resulting in a notable enhancement in the coulombic efficiency of the Zn/CNTs compared to the pristine deposited Zn electrode (Fig. 9d). This improvement can be attributed to the preparation of Zn/CNTs with reduced Zn nucleation overpotential and a more uniform distribution of electric field. Moreover, it exhibited excellent stability, operating at 2 mA cm−2 for 200 h without any observed dendrite growth. Lu et al.103 fabricated a Zn/3D reduced graphene oxide composite on carbon cloth substrates via one-step co-electrodeposition (Fig. 9e). The incorporation of a 3D reduced graphene oxide network not only enhanced the electrical conductivity and wettability of the electrode materials but also resulted in a more uniform electric field distribution and lower local current density, effectively suppressing the growth of zinc dendrites. The electrode material exhibited excellent long-term cycling stability for 1000 h at 1 mA cm−2, while the assembled full battery with MnO2 demonstrated remarkable rate performance and maintained its original properties even under bending conditions. Further optimization was conducted by Guan and his colleagues,104 resulting in the proposal of an in situ growth method for 3D nitrogen-doped vertical graphene nanosheets on carbon cloth (Fig. 9f). This approach also enables the realization of flexible Zn anodes without dendrite growth. The introduction of nitrogen-containing groups into the 3D graphene nanosheets significantly enhances the interaction between Zn2+ and the carbon substrate, thereby reducing the overpotential for Zn nucleation and facilitating uniform distribution of Zn nuclei on the surface of the anode.
4.2 Stretchable electrodes
Compared to foldable batteries, stretchable batteries play an equally crucial role in wearable electronic devices. Chen et al.105 designed a stretchable ZIB which is based on a V2CTx cathode and a Zn-modified Ti3C2Tx film composition (Fig. 10a). During electrode fabrication, both MXene-based electrodes were intentionally designed with a wrinkle-like micro-texture, enabling reversible folding/unfolding behaviour upon stretching to effectively mitigate in-plane stress. Consequently, the assembled device exhibits remarkable tensile strain resistance up to 50% while experiencing only minimal capacity attenuation (from 118.5 to 103.6 mA h g−1) under tensile conditions. However, the tensile properties of MXene films are not inherent, thereby limiting the maximum tensile strain of this electrode material. Guan and his colleagues106 have reported an intrinsically stretchable liquid metal fiber anode (LM@Zn) (Fig. 10b). The inherent stretchability and liquid properties of LM@Zn ensure that the fiber ZIBs possess excellent tensile properties. When subjected to a tensile strain of 50%, the fiber ZIBs based on the LM@Zn anode retain 83% of their initial capacity. Furthermore, owing to its remarkable zincophilic and alloying effect, the LM@Zn anode exhibits a more uniform Zn deposition process along with enhanced Zn (002) crystal orientation. Consequently, the assembled ZIB demonstrates exceptional cycling stability, enabling continuous operation for over 800 h. Wei et al.107 prepared NiCo2S4−x arrays with tunable sulfur vacancies grown on carbon yarn (NiCo2S4−x@CY) through a simple hydrothermal method, utilizing carbon yarns as substrate materials that possess intrinsic stretchable properties (Fig. 10c). The introduction of sulfur vacancies was found to promote the surface remodelling and phase transition of NiCo2S4−x, leading to enhanced conductivity and charge storage kinetics. As a cathode material for ZIBs, the composite exhibited favourable discharge capacity (271.7 mA h g−1) and excellent cycling performance (70.9% capacity retention at 5 A g−1). Moreover, when integrated into stretchable ZIBs as the base material, NiCo2S4−x@CY demonstrated exceptional tensile stability and durability under various mechanical deformations. However, during the process of the device being stretched, the individual components within it may separate from each other, thus affecting the overall performance of the cell. In order to solve this problem, Niu et al.108 successfully developed a stretchable ultrathin all-in-one ZIB through a blade coating and rolling assembly process, wherein the PANI/SWCNTs cathode, Zn/SWCNTs anode, and PVA/Zn(CF3SO3)2 gel electrolyte were seamlessly integrated into a single unit (Fig. 10d). This innovative integrated structure effectively prevents any relative displacement or detachment between adjacent components, ensuring continuous and efficient ion and/or load transfer capability even under external deformation while maintaining exceptional structural and electrochemical stability. Moreover, this battery can be customized into various shapes and structures to meet specific requirements.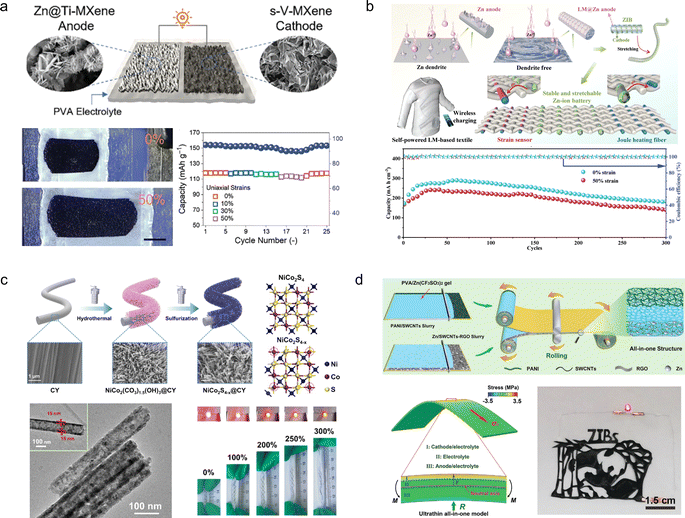 | ||
| Fig. 10 (a) Schematic illustration (left) and digital photographs at different deformation stages (right) of a stretchable Zn2+ ion hybrid battery.105 Reproduced with permission from ref. 105. Copyright 2021, Wiley-VCH GmbH. (b) Scheme of a self-powered LM-based electronic textile integrating a ZIB with a strain sensor, a Joule heating fiber, and a wireless charging part.106 Reproduced with permission from ref. 106. Copyright 2023, Wiley-VCH GmbH. (c) The fabrication schematic diagram of NiCo2S4−x@CY.107 Reproduced with permission from ref. 107. Copyright 2023, Springer Nature. (d) The design of ultrathin all-in-one ZIBs.108 Reproduced with permission from ref. 108. Copyright 2021, Wiley-VCH GmbH. | ||
4.3 Flexible electrolytes
A solid gel electrolyte is required to replace the aqueous zinc salt solution in flexible ZIBs. The high-performance gel electrolyte not only enhances device deformability but also improves anti-freezing properties, inhibits anode Zn dendrite formation and side reactions to a certain extent, thereby enhancing cycling stability. Therefore, the development of flexible hydrogel electrolytes holds significant importance in assembling flexible ZIBs.Zhi et al.109 developed a polyacrylamide-based hierarchical polymer electrolyte (HPE) by grafting polyacrylamide (PAM) onto gelatine chains embedded in a network of electrostatic spinning fiber membranes made from polyacrylonitrile (PAN), which enhanced the mechanical strength and conductivity of the polymer electrolyte (Fig. 11a). The assembled full cell with an MnO2/CNTs cathode demonstrated remarkable specific capacity (306 mA h g−1) and excellent cycling stability (97% capacity retention after 1000 cycles at 2772 mA g−1). Due to the exceptional mechanical stability of the layered hydrogel, the battery exhibits outstanding wear resistance and safety, enabling normal operation under extreme conditions such as cutting, bending, beating, etc. Subsequently, Zhi et al.110 have developed a high-performance, stretchable zinc-ion battery with a double-helical structure utilizing an HPE as a gel electrolyte and MnO2/CNTs as electrodes. The cell exhibits exceptional programmability and customizability while being able to withstand 300% tensile strain (Fig. 11b).
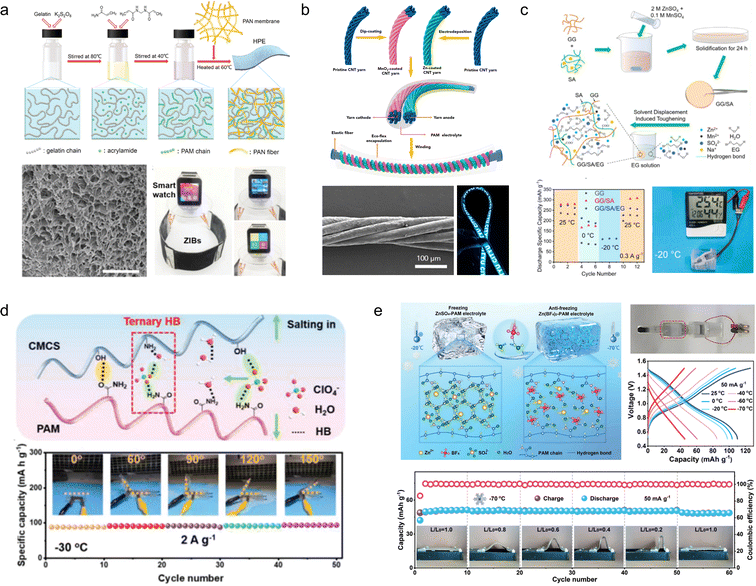 | ||
| Fig. 11 (a) Synthesis diagram, morphology and application of an HPE.109 (b) Schematic diagram of the fabrication and encapsulation of the yarn ZIBs.110 Reproduced with permission from ref. 110. Copyright 2018, American Chemical Society. (c) Schematic illustration of the fabrication of GG/SA/EG hydrogel electrolytes.111 Reproduced with permission from ref. 111. Copyright 2021, Elsevier. (d) Schematic diagram and performance of the CSAM-C hydrogel.112 Reproduced with permission from ref. 112. Copyright 2022, Wiley-VCH GmbH. (e) Schematic diagram and mechanism of the anti-freezing hydrogel electrolytes.113 Reproduced with permission from ref. 113. Copyright 2023, Wiley-VCH GmbH. | ||
However, conventional hydrogel electrolytes exhibit a significant loss of flexibility at low temperatures and a notable decrease in conductivity, resulting in the attenuation of electrochemical properties. To address this issue, Zhou et al.111 developed GG/SA/EG hydrogels by combining two natural polymers, guar gum (GG) and sodium alginate (SA), which possess excellent antifreeze properties. The inclusion of ethylene glycol (EG) forms stable molecular clusters with H2O molecules and competes with hydrogen bonds in water, leading to a reduction in the saturated vapor pressure of water and consequently lowering the freezing point. Moreover, the GG/SA/EG hydrogels demonstrate remarkable mechanical properties with capacity retention rates of 87.2% and 78.3%, respectively, after undergoing 1000 bending cycles (Fig. 11c). Zhang et al.114 fabricated a highly flexible polysaccharide hydrogel incorporating a low concentration of Zn(ClO4)2 salt, wherein the ClO4− anion, water, and polymer chains establish ternary and weak hydrogen bonds. This interaction enhances the mechanical properties of the polymer chains while disrupting the hydrogen bonding network among water molecules, leading to a significant reduction in electrolyte solidification point. Simultaneously, it diminishes free water content and effectively suppresses hydrogen precipitation side reactions as well as dendrite growth at the Zn anode (Fig. 11d). In order to achieve enhanced freezing resistance and mechanical properties, Niu et al.113 developed a hydrogel electrolyte (Zn(BF4)2–PAM) based on Zn(BF4)2 and PAM. The BF4− anions interact with water molecules through strong O–H⋯F hydrogen bonds due to the highly electronegative nature of fluorine atoms, replacing the weak O–H⋯O hydrogen bonds between water molecules and effectively inhibiting ice crystal formation at low temperatures. As a result, the Zn(BF4)2–PAM hydrogel remains unfrozen even at −70 °C, enabling the construction of freeze-resistant flexible ZIBs that exhibit excellent cycling stability and consistent electrochemical performance under low temperature conditions as well as during bending (Fig. 11f).
5. Flexible metal–air batteries
The rapid advancement of portable and wearable electronic devices has led to widespread interest in flexible metal–air batteries due to their safe operation, low cost, high energy density, and eco-friendliness.55 However, the ORR and OER of the air electrode often experience sluggish kinetics under significant polarization conditions that rely heavily on efficient and stable catalysts.115 Additionally, conventional electrolytes face significant challenges in semi-open systems such as water evaporation at high temperatures or gel freezing at low temperatures leading to battery failure in harsh environments.116 To promote the development of flexible metal–air batteries, researchers have recently developed various new flexible air cathode catalysts and gel electrolytes, which are mainly used in Li–air batteries (LABs), Zn–air batteries (ZABs), and Al–air batteries (AABs).5.1 Li–air batteries
Due to their reasonable theoretical energy density (∼400 W h kg−1) and energy efficiency (>90%), LABs have garnered significant attention in the realm of metal–air batteries.117 Despite the synthesis of air electrodes with porous structures and organic/aqueous electrolytes, conventional lithium–air batteries are manufactured with inflexible integral architectures that fail to meet the demands of wearable electronic devices. Furthermore, LABs still encounter substantial challenges such as side reactions, limited recyclability, and electrolyte leakage.To develop an electrochemical energy storage system compatible with wearable electronics, Peng et al.118 fabricated a novel fibrous LAB featuring a coaxial structure that integrates an external carbon nanotube cathode and an internal Li wire anode within a polymer gel electrolyte, rendering it fibrous in nature. The incorporation of the gel electrolyte effectively hinders air diffusion onto the Li anode, thereby lowering its corrosion rate. Consequently, the resulting LAB exhibits excellent flexibility, braiding capability, and cycling stability (Fig. 12a).
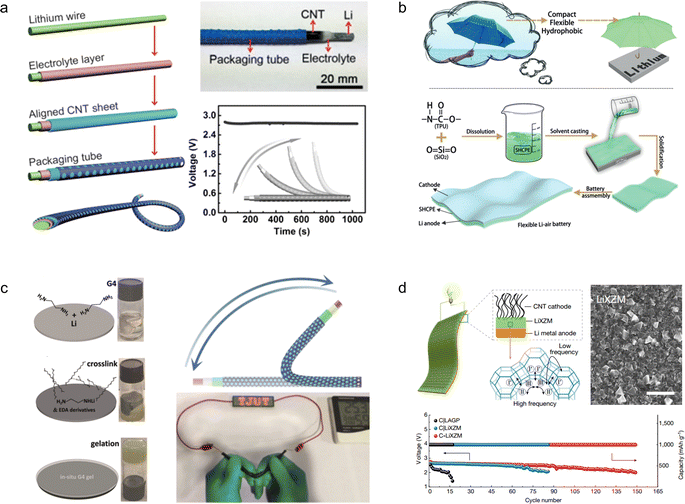 | ||
| Fig. 12 (a) Schematic fabrication of a fibrous lithium–air battery and its strain stability.118 Reproduced with permission from ref. 118. Copyright 2016, Wiley-VCH GmbH. (b) Schematic diagram illustrating the protection of the lithium anode and the fabrication process of a flexible LAB.119 Reproduced with permission from ref. 119. Copyright 2019, Wiley-VCH GmbH. (c) Schematic diagram illustrating the in situ preparation process of gel electrolyte and physical demonstration showcasing the normal operation of the flexible lithium–air battery assembled using this procedure in a bent state.120 Reproduced with permission from ref. 120. Copyright 2019, Wiley-VCH GmbH. (d) Schematic diagram of the Li+ ion conduction mechanism, morphology, and long-term cycling stability of Li+ ion exchange zeolites.121 Reproduced with permission from ref. 121. Copyright 2021, Springer Nature. | ||
To enhance the protection of the anode in lithium–air batteries, novel gel electrolytes with superior mechanical properties have been developed. For instance, Zhang et al.119 drew inspiration from the umbrella structure to develop a robust hydrophobic composite polymer electrolyte film that exhibits exceptional flexibility, hydrophobicity, and stability for safeguarding the lithium anode. The electrolyte is composed of thermoplastic polyurethane and hydrophobic silica nanoparticles, effectively preventing cell deformation and flooding. By mitigating the risks of puncture and heat-induced short-circuit fires, this innovation significantly enhances the safety profile of wearable LABs. Furthermore, the in situ preparation of the electrolyte film ensures its uniform distribution on the surface of the Li anode, thereby substantially retarding corrosion and dendrite growth while leveraging its inherent hydrophobic properties to prevent undesired side reactions (Fig. 12b). The tetraethylene glycol dimethyl ether gel electrolyte was prepared in situ on the lithium anode surface by Ding et al. through a crosslinking reaction.120 Notably, the inclusion of a polymer-free additive allows for the maintenance of high ionic conductivity within the gel, while the in situ preparation method ensures excellent interfacial contact between the electrodes. Thanks to this enhanced conductivity, the LABs assembled with this gel electrolyte exhibit stable operation for 1175 h in air (Fig. 12c).
The inherent mechanical stability of the organic gel electrolyte effectively safeguards the Li anode, but its low conductivity remains a limiting factor in its development. Despite efforts to enhance conductivity through the incorporation of inorganic electrolyte additives, they have not yet resolved the issue of unstable performance in air. To solve this problem, Yu et al.121 introduced a flexible solid zeolite electrolyte—a thin Li+ ion exchange zeolite membrane—through an innovative in situ assembly strategy that integrated it with both the Li anode and carbon nanotubes at the cathode (Fig. 12d). Due to the inherent chemical stability in air as well as high conductivity of zeolites, this all-solid-state LAB exhibited remarkable specific capacity (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 mA h g−1 for carbon nanotubes) and cycle stability (149 cycles at 500 mA g−1 and 1 mA h g−1).
020 mA h g−1 for carbon nanotubes) and cycle stability (149 cycles at 500 mA g−1 and 1 mA h g−1).
5.2 Zinc–air batteries
Since Zn participates in a redox reaction involving the transfer of two electrons, the theoretical specific energy density of Zn–air batteries reaches 1086 W h kg−1, which surpasses that of LABs.122 Moreover, ZABs exhibit cost-effectiveness, safety, and environmental friendliness, positioning them as potential candidates for next-generation electrochemical energy storage and conversion systems.123,124 However, the sluggishness of the oxygen reduction reaction at the air cathode and the vulnerability of water electrolytes and Zn anodes to external conditions impede their further advancement.125,126 Therefore, developing affordable, high-performance, and stable cathode catalysts along with temperature-responsive gel electrolytes is essential for practical implementation of flexible ZABs in wearable electronic devices.127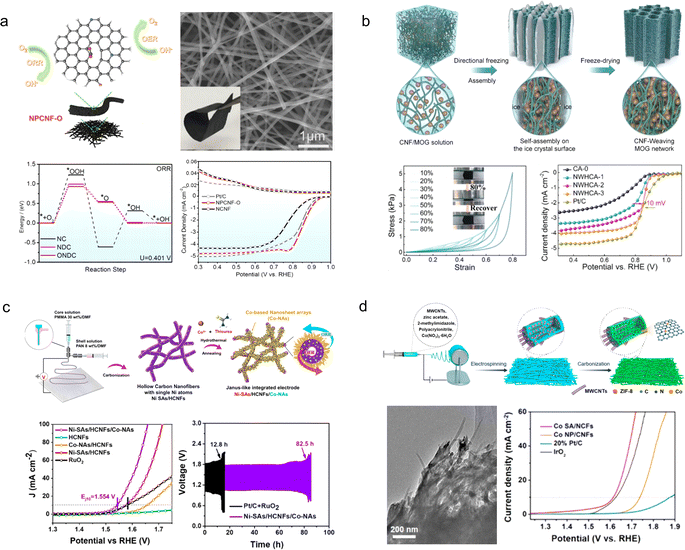 | ||
| Fig. 13 (a) Schematic illustration and electrochemical properties of the NPCNF–O catalyst.129 Reproduced with permission from ref. 129. Copyright 2022, American Chemical Society. (b) Schematic of the preparation procedure of the CNF-MOG by the nanofiber weaving strategy.56 Reproduced with permission from ref. 56. Copyright 2023, Wiley-VCH GmbH. (c) Illustration for the preparation of Ni-SAs/HCNFs/Co-NAs.130 Reproduced with permission from ref. 130. Copyright 2022, American Chemical Society. (d) Schematic illustration of the electrospinning process for the fabrication of Co SA/NCFs.131 Reproduced with permission from ref. 131. Copyright 2022, American Chemical Society. | ||
Moreover, the compressive stress retained 94% of the initial stress even after undergoing 5000 cycles of compression and release. When used as an ORR electrocatalyst, NWHCA displayed exceptional catalytic activity with an onset potential of 0.93 V vs. RHE, a half-wave potential of 0.84 V vs. RHE, and outstanding electrochemical stability.
To further enhance the catalytic activity of the air cathode in flexible ZABs, cost-effective and high-performance ORR/OER catalysts can be integrated onto flexible porous carbon nanofibers. For instance, Liu et al.130 developed Ni single atoms coordinated with O and N for OER activity, as well as a Co3O4@Co1−xS nanosheet array for ORR activity, which were integrated on the inner and outer walls of flexible hollow carbon nanofibers (Ni-SAs/HCNFs/Co-NAs), respectively (Fig. 13c). As stand-alone bifunctional oxygen catalysts with flexibility, Ni-SAs/HCNFs/Co-NAs demonstrate remarkable ORR (E1/2 = 0.89 V vs. RHE) and OER (EJ=10 = 1.554 V vs. RHE) activities, rapid electron/ion transfer kinetics, large electrochemically active specific surface area, and excellent mechanical flexibility. The assembled flexible all-solid-state ZABs exhibit outstanding cycling stability exceeding 80 h. Zhang et al.131 successfully fabricated N-doped porous carbon nanofibers interconnected by nanotubes, incorporating Co single atoms through electrospinning (Fig. 13d). The atomically dispersed Co species provide a plethora of catalytic sites for ORR/OER reactions with exceptional reactivity. The abundant pyridine N moieties not only serve as active sites but also effectively anchor the Co atoms, forming a stable Co–N4 configuration that enhances the activity and stability of the catalyst. Moreover, the internal CNTs enhance the flexibility and mechanical strength of the porous fibers. As an air cathode without binders, the prepared catalyst exhibits remarkable durability over 600 hours at a current density of 10 mA cm−2.
Carbon cloth, composed of a cluster of carbon filaments, exhibits remarkable electrical conductivity, mechanical strength, and flexibility, causing it to be extensively employed in flexible ZABs. Nevertheless, commercially available pristine carbon cloths typically exhibit limited specific surface areas and lack electrochemically active sites on their surfaces.132 In order to enhance the electrochemical properties of carbon cloth for direct utilization as a catalyst-free air cathode material in flexible ZABs, Zhang et al.133 developed a highly electrochemically active carbon cloth (Fig. 14a). The activated carbon cloth was prepared through simple electrochemical oxidation/reduction of commercial carbon cloth in an NH4Cl solution. Following the electrochemical treatment, a graphene-like exfoliated carbon layer formed on the surface of the carbon cloth, introducing oxygen-containing groups and doping N and Cl atoms to significantly improve its electrochemical performance. The flexible ZABs assembled on the treated carbon cloth exhibited an energy density of up to 690 W h kg−1 with no degradation under the bending state.
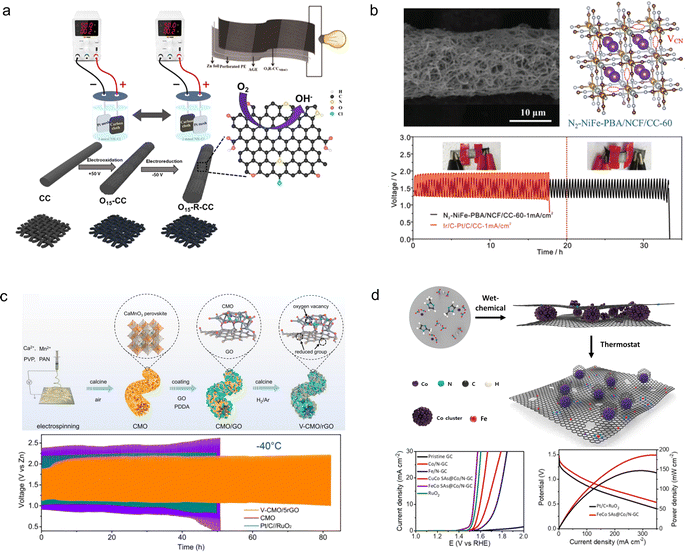 | ||
| Fig. 14 (a) Schematic diagram illustration of the electro-activation of carbon cloth.133 (b) Schematic diagram of the synthesis of N2–NiFe-PBA/NCF/CC-60 and cycle stability.134 (c) Schematic diagram of the synthesis process of the V-CMO/rGO.135 Reproduced with permission from ref. 135. Copyright 2023, Wiley-VCH GmbH. (d) Fabrication scheme of FeCo SAs@Co/N-GC catalysts.136 Reproduced with permission from ref. 136. Copyright 2021, American Chemical Society. | ||
To further enhance the electrocatalytic performance of carbon cloths, it is imperative to introduce catalysts with exceptional electroactivity on their surface; however, this inevitably results in the incorporation of adhesives. The presence of a poorly conductive binder would cover the catalyst's surface, diminishing its reactive sites and increasing electrode impedance.137 Moreover, a conventional binder may degrade when exposed to air for extended periods, significantly impacting the cycle life of the air electrode. Therefore, there is an urgent need to design novel binder-free and efficient OER/ORR catalysts.138 Jiang et al.134 synthesized a flexible and self-supporting bifunctional cathode material by combining a Ni–Fe Prussian blue analogue with N-doped carbon fibers grown on carbon cloth (N2–NiFe-PBA/NCF/CC-60) (Fig. 14b). The in situ formation of CN vacancies in NiFe-PBA enhanced the catalytic activity of the oxygen evolution reaction (OER) while suppressing the loss of elemental Fe during the OER process. The 3D interconnecting network structure formed by NiFe-PBA and carbon fibers significantly increased specific surface area and electrical conductivity, ensuring sufficient and stable active sites for ORR/OER reactions. As a result, N2–NiFe-PBA/NCF/CC-60 exhibited excellent OER performance (overpotential of 270 mV at 50 mA cm−2) and ORR performance (0.89 V at 5 mA cm−2), enabling stable operation of ZABs assembled based on this electrode for up to 2000 hours. Peng et al.135 fabricated reduced graphene oxide-coated oxygen-rich void porous perovskite (CaMnO3) nanofibers (V-CMO/rGO) as an air electrode catalyst on a carbon cloth support (Fig. 14c). The incorporation of the graphene oxide coating enhanced the conductivity of the perovskite, thereby promoting reaction kinetics within the 3D network structure. Consequently, this catalyst achieved a half-wave potential of 0.86 V vs. RHE, enabling a high peak power density of 56 mW cm−2 and long cycling stability exceeding 80 h. Lee et al.136 reported a graphitic carbon nitride framework catalyst (FeCo SAs@Co/N-GC) for growing Fe, Co single atoms (Fig. 14d). The Co active sites were integrated by an electronic structure modulation method to enhance the OER and ORR performance of the catalyst. Compared with commercial Pt/C, FeCo SAs@Co/N-GC demonstrated a lower overpotential (0.29 V at EJ=10) and Tafel slope (56.6 mV dec−1). The catalyst coated on a carbon cloth carrier can be directly used as an air cathode for flexible ZABs. The assembled solid-state flexible ZABs demonstrated an energy density of 1017 W h kgZn−1 and an endurance of 680 cycles at 50 mA cm−2.
Taking inspiration from the intricate structure of animal dermis and the high water retention capacity of plant cells, Chen et al.144 developed a gel electrolyte with a dynamic double-permeable network structure by creating an OH− conductive ionomer network in situ within a hollow polymer microcapsule-modified hydrogel polymer network (Fig. 15a). The resulting electrolyte exhibited excellent water retention (107 g g−1), ionic conductivity (215 mS cm−1), and ultra-high tensile strength (1800%). When used to assemble a flexible ZAB, this electrolyte demonstrated long-term cycling stability for up to 320 h without significant performance degradation even when bent at 180°.
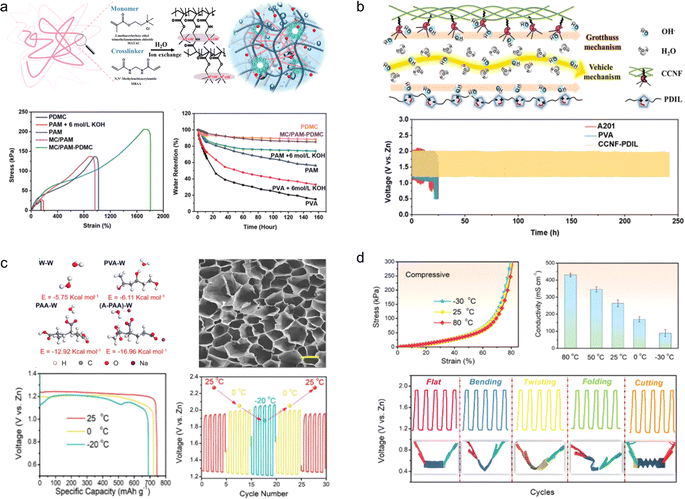 | ||
| Fig. 15 (a) PDMC ionomer network, mechanical properties, and water retention properties.144 Reproduced with permission from ref. 144. Copyright 2022, Wiley-VCH GmbH. (b) Schematic illustration of the ion transport mechanism via combination of the Grotthuss mechanism and the vehicle mechanism by synergy of channels along the CCNF surface and within PDIL.145 Reproduced with permission from ref. 145. Copyright 2022, Wiley-VCH GmbH. (c) Morphology and performances at low temperatures of A-PAA hydrogel.146 Reproduced with permission from ref. 146. Copyright 2020, Wiley-VCH GmbH. (d) Mechanical and electrochemical properties of the A-PAA hydrogel at high temperature.147 | ||
An ionic liquid is a kind of non-volatile, high conductivity and heat stable electrolyte. It is promising to use it to design new gel electrolytes for flexible ZABs. Inspired by the reinforced concrete structure, Chen et al.145 constructed a CCNF-PDIL gel electrolyte with hierarchical nanostructures by confining the polymerization (PDIL) of a double cationic ionic liquid to a robust 3D porous cationic cellulose nanofiber (CCNF) matrix (Fig. 15b). Owing to the synergistic effect of the robust CCNF skeleton and strong noncovalent interactions, as well as the high hydrophilicity of PDIL, the CCNF-PDIL gel electrolyte exhibits high OH− conductivity (286.5 mS cm−1), good mechanical properties (withstanding 105 MPa tensile stress), and appropriate water retention (27 g g−1). Assembled from CCNF-PDIL gel electrolyte ZABs have realized the cycle life of 240 h.
Although the newly designed and prepared hydrogel electrolyte exhibits excellent mechanical properties and electrical conductivity, it is susceptible to deactivation under extreme temperature conditions.148 Chen et al.146 developed a cold-resistant hydrogel electrolyte, demonstrating that the polarity of the end group plays a crucial role in enhancing its antifreeze performance (Fig. 15c). Motivated by this finding, they significantly increased the interaction energy between alkalized polyacrylic acid (A-PAA) and water molecules by neutralizing the carboxyl group into salt using an alkali. Consequently, the assembled flexible air cell maintained 92.7% of its initial capacity at −20 °C while retaining satisfactory flexibility. In contrast, Chen et al.147 discovered that the incorporation of a conventional low-vapor-pressure glycerol substance could ameliorate the desiccation of the water-saturated A-PAA hydrogel, thereby augmenting the stability of the hydrogel under elevated temperatures (Fig. 15d). Consequently, flexible zinc–air batteries fabricated with the modified A-PAA hydrogel can function seamlessly at temperatures as high as 80 °C.
5.3 Al–air batteries
Aluminium is the most abundant metal on earth, with a low redox potential (−1.67 V vs. SHE), high volumetric capacity (8056 mA h cm−3), low cost, and safety in aqueous solutions.149 Therefore, AABs have received increasing attention. However, the low activity of the air electrode and the formation of by-products, Al oxide/hydroxide or hydrogen, lead to a low practical specific energy of ∼1000 W h kg−1.150 Therefore, the development of efficient air cathode catalysts is of great importance.Peng et al.151 fabricated a fibrous AAB by sequentially coating a gel electrolyte and wrapping cross-stacked carbon nanotube/silver-nanoparticle hybrid sheets as the air cathode onto a spring-like Al substrate, achieving a specific capacity of 935 mA h g−1 and an energy density of 1168 W h kg−1. The fiber shaped AAB exhibits flexibility and stretchability, rendering it suitable for integration into wearable textiles and large-scale applications (Fig. 16a). To enhance the deformation performance of AABs, Shim et al.152 proposed a shape-reconfigurable cell for assembling flexible AABs with deformable components made of micro-meter scale or nanoscale composite materials. They fabricated an Al foil anode and a carbon composite cathode using cellulose as a flexible current collector, enabling the assembled device to withstand folding, wrinkling, and 3D expansion. The AAB demonstrates a specific capacity of 496 mA h g−1 and can deliver a voltage of 10.3 V when connected in series with 16 cells (Fig. 16b).
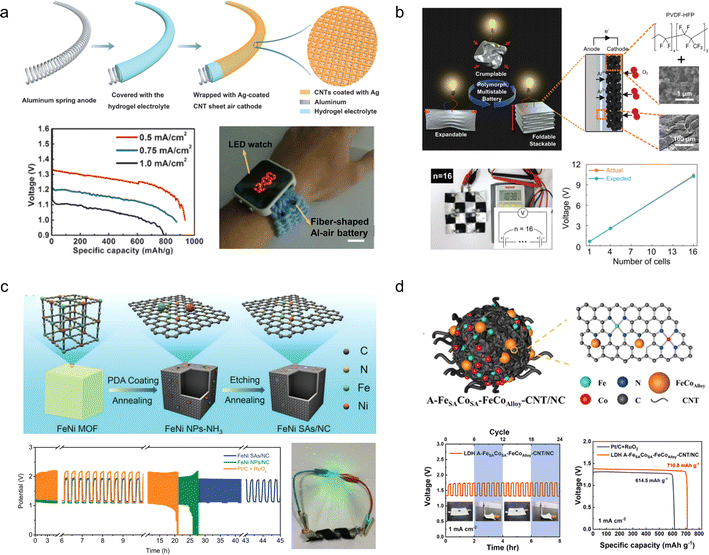 | ||
| Fig. 16 (a) Schematic diagram of the preparation and performance of the fibrous Al–air batteries.151 Reproduced with permission from ref. 151. Copyright 2016, Wiley-VCH GmbH. (b) Concept drawing of a shape-reconfigurable Al–air battery and the output voltage of 16 cell units in series.152 Reproduced with permission from ref. 152. Copyright 2017, Wiley-VCH GmbH. (c) Schematic of FeNi SAs/NC catalyst preparation, cycle stability and electronic photographs of the flexible device.153 Reproduced with permission from ref. 153. Copyright 2021, Wiley-VCH GmbH. (d) Schematic diagram of the NiFe LDH A–FeSACoSA–FeCoalloy–CNT/NC cathode catalyst structure and electrochemical properties.154 Reproduced with permission from ref. 154. Copyright 2023, Elsevier. | ||
The improvement of the flexible AAB cathode catalyst performance has also been documented. For instance, Peng et al.153 synthesized a N-doped carbon matrix embedded with bimetallic MOF-derived Fe–Ni nanoparticles (FeNi SAs/NC). Density functional theory calculations revealed the presence of a stable Fe–Ni–N6 structure in this system, which exhibited enhanced stability and high intrinsic electrocatalytic activity. Furthermore, the hollow porous carbon matrix facilitated improved contact between the catalyst and electrolyte, leading to enhanced atomic utilization. As a result, FeNi SAs/NC demonstrated remarkable ORR activity (with an onset potential of 0.98 V and half-slope potential of 0.84 V). When incorporated into an AAB configuration, it displayed a specific capacity of 1576.4 mA h g−1 and maintained normal operation even under bending conditions (Fig. 16c). Li et al.154 synthesized the air cathode catalyst material (NiFe LDH A–FeSACoSA–FeCoalloy–CNT/NC) by immobilizing an iron–cobalt alloy and iron–cobalt double single-atoms in a N-doped carbon framework that contains carbon nanotubes, which exhibited a half-wave potential of 0.84 V and an OER potential of 1.56 V (EJ = 10 mA cm−2). The excellent electrocatalytic performance of this catalyst can be attributed to the augmentation of charge density on the material surface by metal/alloy nanoparticles, as well as the synergistic effect between Fe–Co dual single atoms and Ni–Fe LDH. An AAB assembled using this catalyst achieved a power density of 145 mW cm−2 at a current density of 157 mA cm−2 (Fig. 16d).
In summary, the primary challenges faced by flexible metal–air batteries are how to mitigate metal anode fracture during device deformation and maintain electrolyte stability in air. Utilizing gel electrolytes with excellent mechanical stability is a favourable option for safeguarding the metal anode against mechanical fractures and reducing its corrosion rate. Additionally, further advancements are required in developing all-solid-state metal–air batteries to prevent degradation of electrochemical properties caused by exposure to air.
The electrolytes utilized in the flexible aqueous energy storage devices (SCs, ZIBs, and metal–air batteries) are hydrogel electrolytes that possess non-volatile and non-flammable properties. Consequently, there is no risk of fire or explosion resulting from electrolyte leakage or device short-circuiting. Moreover, even if these energy storage devices endure mechanical damage caused by external forces, the internal materials can be easily collected and recycled without any significant impact on human health or the environment. Additionally, it is crucial to consider the biocompatibility of flexible electronic devices intended for prolonged interaction with human skin as this greatly influences user experience. Therefore, it is imperative to fabricate an encapsulation shell for such devices that exhibits skin-friendly characteristics. However, there remains a scarcity of relevant research in this field at present.
6. Flexible lithium-ion batteries
Since its introduction in 1991, LIBs with high energy density and stable output voltage have become the primary power supply system for everyday electronic products.155 The energy storage mechanism of LIBs is similar to that of ZIBs. By facilitating the transmission of Li+ ions between electrolytes and enabling the intercalation/deintercalation process of the cathode and anode, the charging and discharging process of LIBs can be achieved.156 When used in wearable electronic devices, LIBs ensure prolonged durability.157,158 However, due to the utilization of organic electrolytes in these batteries, it is essential to consider safety aspects during the development of flexible electrodes and electrolytes while ensuring stability amidst device deformation.159,1606.1 Flexible electrodes
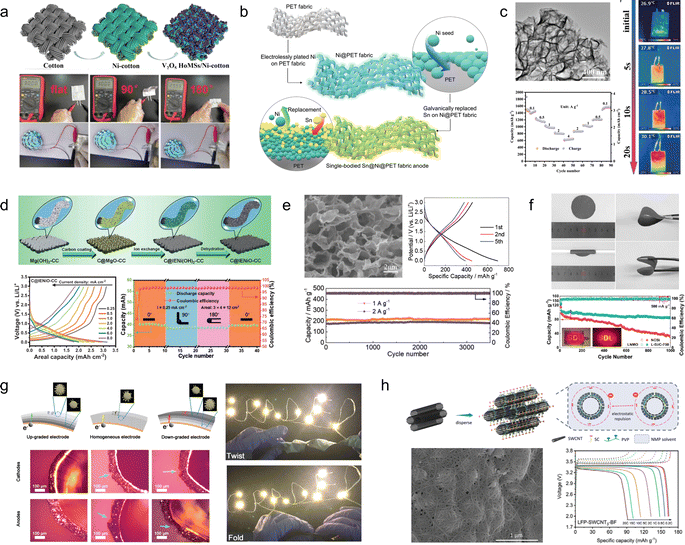 | ||
| Fig. 17 (a) Schematic of the fabrication process of the V2O5 hollow multishelled structures/Ni-cotton fabric electrode and the electrode performances. Reproduced with permission.161 Reproduced with permission from ref. 161. Copyright 2020, Wiley-VCH GmbH. (b) A schematic of the stepwise fabrication of the single-bodied Sn@Ni fabric electrode. Reproduced with permission.162 Reproduced with permission from ref. 162. Copyright 2020, Wiley-VCH GmbH. (c) CoP@CC anode.163 Reproduced with permission from ref. 163. Copyright 2022, Wiley-VCH GmbH. (d) Schematic illustration of fabrication of the C@IENiO-CC freestanding electrode.164 Reproduced with permission from ref. 164. Copyright 2021, Wiley-VCH GmbH. (e) TEM and performances of 3D porous MXene foams.165 Reproduced with permission from ref. 165. Copyright 2021, Wiley-VCH GmbH. (f) Digital photographs and cycle stability of MXene/L-Si/C.166 Reproduced with permission from ref. 166. Copyright 2020, American Chemical Society. (g) The gradient distribution of binders enables simultaneous optimization of bendability and electronic conductivity in the upgraded electrode.167 Reproduced with permission from ref. 167. Copyright 2022, Wiley-VCH GmbH. (h) Schematic diagram, SEM and rate performance of LFP/SWCNTs.168 | ||
In addition to metallic fabrics, low-cost and easy-to-handle 2D carbon materials can also serve as suitable flexible substrates. When used as a substrate for transition metal compound anode materials with high theoretical specific capacity, it can significantly expedite lithium-ion diffusion and enhance electron transport kinetics, thereby optimizing the multiplicity performance and cycling stability of the active materials. Yang et al.163 successfully synthesized arrays of CoP nanosheets on carbon cloth through a facile one-step electrodeposition and in situ phosphorylation strategy, which served as a binder-free anode material for LIBs (Fig. 17c). The CoP nanosheets exhibited excellent interfacial bonding properties with the carbon cloth substrate, effectively preventing agglomeration and exposing more reaction sites. Moreover, the 3D lattice morphology of the electrodeposited CoP nanosheets provided sufficient space to accommodate volume expansion during charging. The CoP@CC demonstrated a high discharge capacity of 1866.9 mA h g−1 (3.8 mA h cm−2). Furthermore, the assembled full-battery device exhibited slow warming rates during external short circuits, ensuring enhanced safety. In comparison to the conventional direct carbon coating method, Lu et al.164 employed an indirect carbon cladding approach to synthesize a carbon-covered NiO composite material on a carbon cloth (Fig. 17d). This novel method ensured the formation of a well-graphitized carbon cladding layer while preventing the reduction of transition metal oxides into metal monomers at elevated temperatures. The resulting anode composites exhibited a high areal capacity of 3.08 mA h cm−2 at 0.25 mA cm−2 and demonstrated excellent flexibility.
The 2D transition metal carbides and nitrides, called MXene, are currently under development as anode materials for flexible LIBs. Xu et al.165 successfully fabricated 3D MXene foams with a well-defined porous structure using a simple sulfur templating method, which effectively prevents the aggregation and re-stacking of MXene nanosheets while providing abundant active sites for Li+ storage (Fig. 17e). The resulting self-supporting foam exhibits excellent bendability and high electrical conductivity, delivering a capacity of 455.5 mA h g−1 at 50 mA g−1 when directly employed as a LIB anode. Moreover, it demonstrates outstanding rate performance and long-term cycling stability. Feng et al.166 synthesized the layered Si/C materials using MXene films as flexible substrates (Fig. 17f). Incorporating carbon with a reasonable degree of graphitization can effectively mitigate the volume expansion of Si and enhance its conductivity. Moreover, the integration of monolayer Si and C facilitates the transport of Li+ within the active material. Consequently, this composite material exhibits remarkable mechanical robustness while maintaining a capacity retention of 82.85% over 3200 cycles at 5 A g−1.
Layered oxides (LiNi1−xMxO2, M = Co, Mn, and Al), spinel oxides (LiNi0.5Mn1.5O4), and polyanionic compounds (phosphates, sulfates, silicates) play a crucial role as cathode materials in LIBs.169 In the process of assembling flexible LIBs, they are generally coated on flexible conductive substrates as active materials. In general, the easiest way to increase the energy density of a battery is to increase the load of the active material. Nevertheless, such electrodes typically become more susceptible to mechanical fracture under deformation conditions like bending and folding compared to thin and lightweight electrodes with lower loading.170 Consequently, designing flexible LIBs that strike a balance between mechanical properties and energy density becomes challenging. To tackle this issue, Chen and his colleagues167 proposed a mechanically graded electrode featuring a gradient distribution of maximum allowable strain to mitigate the mismatch between the mechanical properties of the electrode material and energy density (Fig. 17g). The LiNi1/3Mn1/3Co1/3O2 cathode fabricated based on this concept demonstrated exceptional flexibility withstanding a bending radius of 400 μm, while maintaining 92.3% of its initial performance after undergoing 550 consecutive bending tests. Wang et al.168 developed a method for the massive production of ultra-long single-walled carbon nanotubes in N-methyl-2-pyrrolidone solution by harnessing electrostatic dipole interactions and spatial site resistance of dispersant molecules, while incorporating a low concentration of LiFePO4 as a conductive additive (Fig. 17h). The composite electrode material exhibits a stress tolerance of 7.2 MPa and can withstand various degrees of bending and folding. A high-quality loaded electrode with a mass loading of 39.1 mg cm−2 was successfully fabricated, demonstrating rate capacities of 161.5 mA h g−1 at 0.5C and 130.2 mA h g−1 at 5C, respectively, while maintaining a capacity retention rate of 87.4% after undergoing 200 cycles at 2C.
 | ||
| Fig. 18 (a) Schematic illustration of the fabrication of the stretchable lithium-ion battery based on micro-honeycomb structures of graphene–carbon nanotube (CNT)/active materials.171 Reproduced with permission from ref. 171. Copyright 2020, American Chemical Society. (b) Scanning electron microscope images and mechanical properties of pristine PVDF/LFP.172 Reproduced with permission from ref. 172. Copyright 2022, American Chemical Society. (c) Schematic illustration of the fabrication process of the 3D-printed TPU-based electrodes.173 Reproduced with permission from ref. 173. Copyright 2023, Wiley-VCH GmbH. (d) Schematic illustration of different types of 3D compressible electrodes.174 Reproduced with permission from ref. 174. Copyright 2021, Wiley-VCH GmbH. (e) Preparation and characterization of GPE.175 Reproduced with permission from ref. 175. Copyright 2021, Wiley-VCH GmbH. (f) Schematic diagram of the preparation process of functionalized BN nanosheet added ion gel.176 Reproduced with permission from ref. 176. Copyright 2020, Wiley-VCH GmbH. (g) Optical photograph and characterization of a c-PEGR gel.177 (h) High flexibility and fire resistance of the PIA separator.178 Reproduced with permission from ref. 178. Copyright 2021, Wiley-VCH GmbH. (i) The characterization of MGFs.179 Reproduced with permission from ref. 179. Copyright 2023, Wiley-VCH GmbH. (j) Schematics and morphology characterization of the SBCIF.180 Reproduced with permission from ref. 180. Copyright 2022, American Chemical Society. | ||
The 3D printing technology demonstrates significant potential in the production of flexible and customizable high-performance LIBs, which are essential for the upcoming era of smart and ubiquitous energy. However, there still exists a notable performance disparity between 3D printed electrodes and conventional electrodes, particularly regarding cycling stability. This limitation severely hinders the practical implementation of 3D printed batteries. To address this issue, Chen et al.173 have developed a series of intricate 3D printed electrodes based on thermoplastic polyurethane (TPU) (Fig. 18c). By leveraging TPU's exceptional stress-buffering properties, these electrodes not only exhibit adaptability to volume changes during charge–discharge cycling and prevent microstructure collapse in 3D printed electrodes but also demonstrate remarkable resistance to significant bending angles. Moreover, they showcase an impressive capacity retention rate close to 100% after undergoing 300 cycles at a rate of 1C, thereby providing novel insights for bridging the performance gap between 3D printed batteries and conventional LIBs.
6.2 Flexible electrolytes, separators, and current collectors
The utilization of flexible LIBs in wearable electronic devices inevitably entails the risk of electrolyte leakage, which is further compounded by the flammable and explosive properties of commonly employed organic electrolytes. To enhance the safety of flexible LIB electrolytes, an innovative design approach becomes necessary. The primary research concept revolves around designing polymer gel electrolytes as alternatives to conventional liquid counterparts; however, this often leads to a compromise in the electrochemical performance of LIBs, thereby presenting ongoing challenges in the design and preparation of electrolytes for flexible applications.Hu et al.175 developed a non-flammable gel polymer electrolyte that effectively addresses the fundamental issue of battery safety (Fig. 18e). The gel electrolyte (GPE-3) was synthesized through in situ polymerization of tri(acryloyloxyethyl) phosphate and triethylene glycol dimethacrylate within the electrolyte solution, with only 7.5 wt% flame retardant added to ensure minimal impact on electrochemical performance. Even when exposed to fire for over 10 s, GPE-3 remained non-combustible. Furthermore, graphite//GPE-3//LiFePO4 cells exhibited normal operation under various deformation conditions and demonstrated exceptional long-term cycling stability (average capacity degradation rate: 0.057%).
In addition to the necessity of ensuring electrolyte safety, the electrolyte also plays a pivotal role in ion diffusion rate within batteries. Despite their exceptional mechanical properties, polymer gels often suffer from sluggish diffusion kinetics, which consequently impacts overall device capacity. To address this concern, Chen and colleagues176 developed ionic gel electrolytes incorporating amine-functionalized boron nitride nanosheets (AFBNNSs) through thermal polymerization processes, achieving an impressive maximum tensile strain of 72% with a tensile strength of 2.22 MPa (Fig. 18f). Importantly, the strong interaction between amine groups on boron nitride nanosheets and bis(trifluoromethanesulfonyl)imide anions significantly reduces anion mobility while enhancing Li+ mobility. LIBs assembled using this electrolyte demonstrated stable lithium deposition for over 600 hours. Wang et al.177 have synthesized cross-linked poly(ethylene glycol)-based resin (c-PEGR) gel electrolytes prepared by the ring-opening reactions of poly(ethylene glycol)diglycidyl ether (PEGDE) with epoxy groups and poly(ether amine) (PEA) with amines (Fig. 18g). These gel electrolytes exhibited exceptional Li+ transport and compatibility, along with enhanced flexibility and improved oxidative stability. Due to the confinement of hydroxyl groups within the crosslinked structure, their movement is restricted, resulting in an oxidation potential of 4.36 V. Consequently, this c-PEGR gel electrolyte holds great promise for utilization in high-voltage flexible LIB assemblies.
In addition to the electrolyte, the separator and current collector are pivotal components of LIBs, exerting a critical influence on both thermal safety and electrochemical stability; however, there is a paucity of research in this domain. For example, Cheng et al.178 fabricated a novel polyimide aerogel (PIA) separator with high-temperature resistance through spin-coating, which exhibits excellent foldability and bendability without any damage (Fig. 18h). The PIA diaphragm possesses the remarkable characteristics of an aerogel, including a high porosity of up to 78.35%, thereby enhancing its wettability. Moreover, its unique 3D mesh structure can significantly improve the rate capability and cycling stability of LIBs. Additionally, the chemical cross-linking method employed in the preparation process has successfully enhanced its thermal stability (>500 °C). In summary, this gel-based diaphragm holds great potential for flexible LIB applications.
Current collectors are crucial components that facilitate electron transport and provide mechanical support for the electrodes. However, since they do not contribute to the capacity, minimizing the mass of the current collector enhances the mass energy density of the cell. In line with this concept, Zheng et al.179 introduced a novel type of low-density (2.9 mg cm−2) current collector composed of 3D metal glass-fiber fabrics (MGFs) (Fig. 18i). These MGFs were fabricated using various metal foils (Cu, Ni, Ag), exhibiting exceptional tensile strength (>150 MPa) and negligible impedance alteration even after enduring 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles. Moreover, their flexibility was significantly improved compared to conventional metal foils due to their pliable nature. Additionally, owing to the fire-resistant properties conferred by glass fibers, MGFs exhibit non-combustibility even at surface temperatures as high as 500 °C. Furthermore, these collectors demonstrate electrochemical compatibility with a wide range of mainstream LIB cathodes and anodes. Deng et al.180 proposed a simple and versatile bio-self-growth strategy for the preparation of flexible LIBs (Fig. 18j), in which the electrodes and the diaphragm (SBCIF) are essentially integrated into a single whole, capable of adapting to various active materials. Meanwhile, owing to the good self-generated corrugated structure that helps to disperse the stress, no rupture or electrode/diaphragm separation was observed after 20
000 bending cycles. Moreover, their flexibility was significantly improved compared to conventional metal foils due to their pliable nature. Additionally, owing to the fire-resistant properties conferred by glass fibers, MGFs exhibit non-combustibility even at surface temperatures as high as 500 °C. Furthermore, these collectors demonstrate electrochemical compatibility with a wide range of mainstream LIB cathodes and anodes. Deng et al.180 proposed a simple and versatile bio-self-growth strategy for the preparation of flexible LIBs (Fig. 18j), in which the electrodes and the diaphragm (SBCIF) are essentially integrated into a single whole, capable of adapting to various active materials. Meanwhile, owing to the good self-generated corrugated structure that helps to disperse the stress, no rupture or electrode/diaphragm separation was observed after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending cycles at a bending angle of 165° and a bending radius of 3 mm. The flexible LIBs assembled based on SBCIF maintained 95% of their initial capacity after 10
000 bending cycles at a bending angle of 165° and a bending radius of 3 mm. The flexible LIBs assembled based on SBCIF maintained 95% of their initial capacity after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bending/compression cycles.
000 bending/compression cycles.
In summary, to design deformable LIBs with both mechanical and electrochemical properties, it is essential to employ electrically active flexible substrates for electrode material preparation or directly design the active materials to be deformable. Furthermore, enhancing the synergistic effect of the composite is crucial. Additionally, while ensuring the device's electrochemical performance remains uncompromised, efforts should be made to maximize fire resistance in electrolyte design and eliminate safety hazards.
7. Flexible lithium–sulfur batteries
The unstable electrochemical properties and high flammability of LIBs under mechanical deformation conditions have hindered the widespread utilization of LIBs in flexible electronics.181 LSBs are anticipated to replace LIBs due to their high economic viability, non-toxic nature, and abundant availability of active materials for cathodes.47,182 Considering the two-electron system during the complete electrochemical reaction, the sulfur cathode exhibits a theoretical capacity of up to 1672 mA h g−1, which is approximately 2 times higher than that of conventional LIB active materials.183However, challenges persist in the application of LSBs for flexible energy storage. Firstly, the volumetric changes experienced by the sulfur cathode during charge and discharge processes result in continuous capacity degradation.184 Secondly, the low conductivity exhibited by the sulfur cathode leads to sluggish electrochemical kinetics.185 Lastly, the dissolution of polysulfides in electrolytes gives rise to the phenomenon known as the “shuttle effect”.186 In order to tackle these issues, researchers have made efforts towards designing and synthesizing various novel sulfur cathodes, separators, and electrolytes.
7.1 Flexible electrodes
Despite the high theoretical specific capacity and energy density of LSBs, sulfur cathodes still encounter several challenges, including low conductivity, instability of conventional polymer binders, and the shuttling phenomenon induced by Li2Sx in the electrolyte.187 Therefore, when designing cathode materials for flexible LSBs, it remains imperative to address these aforementioned issues in order to optimize their electrochemical performances. The preparation of sulfur cathodes has been attempted using carbon-based and conductive polymer-based materials. | ||
| Fig. 19 (a) Designed functions of the electrode skeleton for addressing the critical issues of volume change and LiPS shuttle effects in composite sulfur electrodes.190 (b) Synthesis process and high temperature resistance test of S@ACNTs materials.191 Reproduced with permission from ref. 191. Copyright 2022, Wiley-VCH GmbH. (c) Structures of the flexible LSBs and infiltration behaviour of the liquid electrolyte on a PE membrane.192 Reproduced with permission from ref. 192. Copyright 2022, Wiley-VCH GmbH. (d) Schematic diagram of MoSe2in situ phase transformation processes, and its morphology and properties.193 | ||
 | ||
| Fig. 20 (a) Schematic illustration of polysulfides anchored by PANI-MnO2 to form a thiosulfate (polythiosulfate) complex and the electrochemical properties.195 (b) Digital images and rate performance of the Li2S6-rDIB.196 Reproduced with permission from ref. 196. Copyright 2021, American Chemical Society. (c) Morphology and properties of the SVPA cathode.197 Reproduced with permission from ref. 197. Copyright 2021, Elsevier. (d) The microfluidic assembly process and cycle stability of PPy@S/rGOFs.198 Reproduced with permission from ref. 198. Copyright 2022, Wiley-VCH GmbH. | ||
In the pursuit of higher specific capacity and charge/discharge rates, Park et al.197 successfully synthesized SVPA microparticles containing various sulfur isomers via a rapid (30 min) one-pot reaction involving elemental sulfur and vinyl phosphonic acid (Fig. 20c). The well-ordered sulfur domains within these microparticles contribute to the formation of a large surface area, thereby enhancing the lithium diffusion coefficient and facilitating polysulfide conversion kinetics in Li–S batteries. Moreover, the abundant presence of phosphonate molecules on both the surface and interface of these microparticles serves as effective chemical anchors for lithium polysulfides, effectively mitigating the shuttle effect and extending cycle life at different rates. Consequently, when assembled into a full cell configuration, this system exhibits an impressive discharge capacity of 1529 mA h g−1 at 0.05C along with excellent rate performance (721 mA h g−1 at 7C). To further enhance the mechanical properties of the electrode materials, Bao et al.198 proposed a synergistic interfacial bonding enhancement strategy by uniformly implanting polypyrrole@sulfur (PPy@S) nanospheres into the built-in cavities of self-assembled reduced graphene oxide fibers (rGOFs) using a simple microfluidic assembly method (Fig. 20d). The PPy@S/rGOFs composites exhibited excellent adsorption capacity, rapid reaction kinetics, and remarkable mechanical flexibility due to the synergistically enhanced interfacial chemical bonding between the carbon interface and polymer interface. Moreover, these composites demonstrated outstanding cycling stability with 81.9% capacity retention over 200 cycles at 0.1 A g−1 and exceptional rate performance with a specific capacity of 523 mA h g−1 at 5 A g−1.
7.2 Flexible electrolytes, separators, and current collectors
Polysulfide shuttling in liquid electrolytes, lithium dendrite growth, and contact issues during bending are the primary factors contributing to capacity degradation, potential safety concerns, and limited flexibility. To address these challenges, gel polymer electrolytes are considered a superior choice for flexible LSBs due to their significantly reduced polysulfide solubility compared to liquid electrolytes, enhanced inhibition of lithium dendrite formation, and improved ability to maintain component connectivity under bending conditions.199 Furthermore, their lower interfacial resistance relative to ceramic solid-state electrolytes is better suited for mitigating the sluggish kinetics associated with the sulfur reaction.200 For example, Zhou et al.201 developed a (polyethylene oxide–polyacrylonitrile) (PEO–PAN) copolymer membrane electrolyte, where PAN fibers served as both fillers and cross-linkers (Fig. 21a). This unique structure not only enhanced the ionic conductivity, mechanical strength, and lithium dendrite suppression ability but also effectively hindered polysulfide shuttling due to the strong adsorption of polysulfides by the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O functional groups formed during PEO and PAN crosslinking. Consequently, this electrolyte exhibited improved safety, cycling stability, and rate performance when employed in LSBs. The exceptional mechanical properties and bonding capability of the electrolyte enabled the flexible battery to retain over 96% of its capacity after undergoing 1000 bending cycles.
N–O functional groups formed during PEO and PAN crosslinking. Consequently, this electrolyte exhibited improved safety, cycling stability, and rate performance when employed in LSBs. The exceptional mechanical properties and bonding capability of the electrolyte enabled the flexible battery to retain over 96% of its capacity after undergoing 1000 bending cycles.
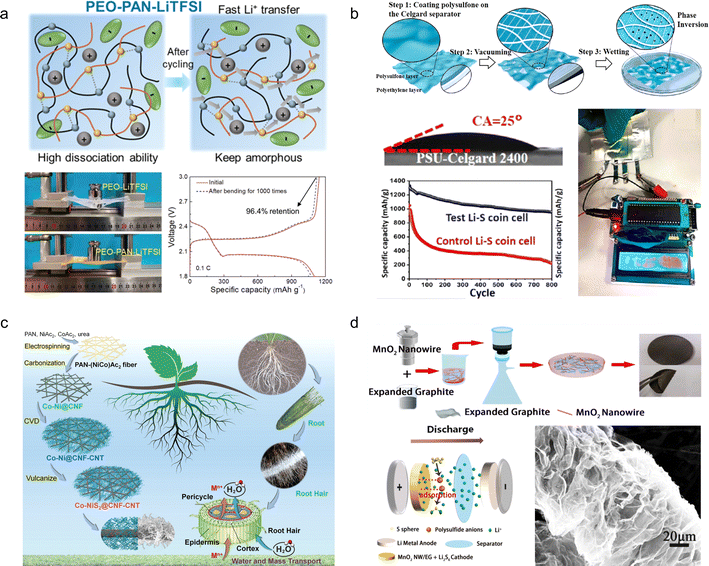 | ||
| Fig. 21 (a) Mechanical and electrochemical performance of PEO–PAN–LiTFSI.201 Reproduced with permission from ref. 201. Copyright 2022, Wiley-VCH GmbH. (b) Schematic of the synthetic procedures of PSU-Celgard separators.202 Reproduced with permission from ref. 202. Copyright 2021, American Chemical Society. (c) Schematic illustrations of the fabrication of hierarchical fibrous Co–NiS2@CNF–CNT with grass root hair structure as an interlayer for LSBs.203 Reproduced with permission from ref. 203. Copyright 2023, Wiley-VCH GmbH. (d) Schematic illustration for the synthesis of the MnO2 NW/EG current collector.204 Reproduced with permission from ref. 204. Copyright 2021, Elsevier. | ||
Modification of commercial diaphragms is also a viable strategy for suppressing the polysulfide shuttle effect. For instance, Chen et al.202 employed a phase transition approach to functionalize commercial diaphragms with polysulfone (Fig. 21b). The incorporation of PSU not only enhances the mechanical properties and thermal stability of the diaphragm but also improves the overall safety of the battery system. Moreover, reducing the pore size of the diaphragm effectively restricts the occurrence of the shuttle effect, thereby enabling excellent cycling stability in flexible LSBs. In fact, even in its folded state, this flexible full cell can achieve an impressive lifetime of up to 800 cycles. Inspired by the grassroots system, Chen and his colleagues203 designed and fabricated a flexible hierarchical carbon nanofiber–carbon nanotube membrane modified with doped NiS2 nanoparticles for LSB separators (Fig. 21c). DFT calculations demonstrated that Co doping induced an electron-deficient region at the doping site of NiS2, thereby enhancing chemisorption and catalytic activity towards lithium polysulfides. The intercalation of Co–NiS2@CNF–CNT in the battery exhibited exceptional performance with a high specific capacity of 951.4 mA h g−1 at 3C, a reversible capacity of 944.1 mA h g−1 after 500 cycles at 0.2C, and an impressive cycle life of up to 3000 cycles at 5C.
It is crucial to design a flexible collector that is compatible with the sulfur cathode. Shao et al.204 have developed a stable and flexible collector composed of ultra-long MnO2 nanowires and expanded graphite nanosheets (MnO2 NW/EG) with excellent electronic conductivity (Fig. 21d). This self-supporting cathode, in combination with Li2S6, demonstrates high performance for flexible LSBs. The ultra-long MnO2 nanowires enhance the adsorption and catalytic conversion ability of lithium polysulfides through their polar active surfaces. Moreover, the incorporation of MnO2 NW/EG, which exhibits remarkable mechanical flexibility, effectively mitigates the volume expansion of sulfur during charge–discharge cycles. Notably, even at a sulfur loading as high as 7 mg cm−2, an impressive capacity of up to 2.5 mA h cm−2 can be achieved.
8. Other flexible batteries
LIBs and LSBs are currently one of the most widely used types of batteries, but due to the shortage of Li resources and increasing costs, alternative energy storage systems such as Na+ ion and K+ ion batteries have been researched.205 SIBs and PIBs have shown great potential due to their relatively low cost, abundant reserves, and comparable performance to that of LIBs.206 However, due to the larger ionic radii of Na+ and K+ ions compared to Li ions, their diffusion in the electrolyte is impeded, resulting in diminished rate performance and cycling stability. Additionally, repeated insertion/extraction of large radius examples can cause severe volume changes in the electrode material that accelerate battery capacity decay. To solve these problems, various attempts have been made to design and prepare new flexible electrodes and electrolytes.8.1 Sodium ion batteries
 | ||
| Fig. 22 (a) Schematic illustration of the preparation of H–TiO2@CFC, in situ XRD patterns, and photograph of the battery.207 Reproduced with permission from ref. 207. Copyright 2020, Wiley-VCH GmbH. (b) SEM image and electrochemical properties of CCFSF.208 Reproduced with permission from ref. 208. Copyright 2020, Wiley-VCH GmbH. (c) Schematic diagram of the free-standing NVO–rGO heterointerface with the V–O–C bond and its anomalous Na+ storage principle and the cycle stability.209 Reproduced with permission from ref. 209. Copyright 2023, Wiley-VCH GmbH. (d) Schematic representation of the nanocellulose gel electrolyte fabrication.210 Reproduced with permission from ref. 210. Copyright 2022, Wiley-VCH GmbH. | ||
In addition, reduced graphene oxide can also serve as a flexible electrode material substrate. To enhance the diffusion kinetics of Na+ ions, Sun et al.209 prepared a heterojunction composite of Na5V12O32/rGO (Fig. 22c). Within this heterostructure, rGO not only acts as a flexible substrate but also undergoes chemical transformation through interfacial electronic interactions with V–O bonds of NVO, resulting in controllable V–O–C bonds at the interface. The interfacial synergy between excellent bonding properties and inherent stress fields at the heterogeneous interface simultaneously reduces Na+ diffusion resistance and promotes charge transfer. As a result, the composite exhibits excellent rate performance (100 mA h g−1 at 10C) and cycling stability (95.4% retention after 3000 cycles).
To construct high-performance flexible SIBs, it remains a challenge to simultaneously achieve high electrical conductivity and good cycling stability while maintaining the high mechanical strength of carbon matrix composites as well as gel electrolytes. The rational design and optimization of the layered structure and interfacial properties of carbon-based electrode materials play a crucial role in this endeavour.
8.2 Potassium ion batteries
Electrode materials for flexible KIBs can be prepared by combining various carbon materials with electroactive substances. For instance, Lu et al.211 synthesized free-standing and flexible anode materials (CDs@rGO) for PIBs by incorporating carbon dots onto the surface of reduced graphene oxide (Fig. 23a). Due to the abundant defects and oxygen-containing functional groups provided by the carbon dots, CDs@rGO exhibited exceptional multiplicity performance and prolonged cycle life. However, as CDs@rGO is an unmodified pure carbon matrix composite, its capacity at 0.1 A g−1 is only 310 mA h g−1, which is unsatisfactory. | ||
| Fig. 23 (a) Schematic diagram and long-term cycling performance at 200 mA h g−1 of CDs@rGO.211 (b) Schematic illustration for the synthesis, TEM, and long-term cycling performance of the CNT/SNCF composite.212 Reproduced with permission from ref. 212. Copyright 2021, American Chemical Society. (c) Physical demonstration, rate performance and in situ XRD patterns of SnS2@C.213 (d) Schematic illustration of the preparation process and practical application of the V2C–VO2 multi-heterostructure.214 Reproduced with permission from ref. 214. Copyright 2022, Wiley-VCH GmbH. | ||
In order to achieve a higher specific capacity, Chen et al.212 proposed a unique multi-channeling carbon-based electrode material composed of N,S co-doped carbon nanotubes and carbon nanofibers (Fig. 23b). The co-doping of S,N co-doping not only significantly enhances the electrical conductivity of the composite material but also increases the interlayer spacing in the carbon material, effectively mitigating volume changes during cycling caused by K+ ion intercalation/deintercalation processes and thereby enhancing cycling stability. Based on this, Ci et al.213 further confined SnS2 within N,S co-doped carbon nanofibers as a flexible anode material for PIBs, achieving a remarkable reversible capacity of 457.4 mA h g−1 at 0.05 A g−1 (Fig. 23c). Through in situ XRD tests, the authors confirmed that the ultra-high reversible capacity can be attributed to the successive transformation alloying reactions and the enhanced capacitive behaviour exhibited by the SnS2 active material.
To achieve synergistic enhancement of capacity and mechanical properties, Xiao et al.214 fabricated a 3D multi-heterostructure composed of V2C–VO2 nanoribbons wrapped around nanosheets with abundant heterointerfaces and heterojunctions, which not only facilitates ion diffusion but also enhances the mechanical stability of the material (Fig. 23d). The authors further demonstrate that the V2C–VO2 multi-heterojunction can self-regulate the spin polarization density of its constituent monomers (V2C and VO2), thereby promoting K+ ion dynamics during charge/discharge cycling. As a result, V2C–VO2 exhibits satisfactory specific capacity (372 mA h g−1 at 0.2 A g−1) and excellent flexibility.
The LIBs, LSBs, SIBs and PIBs belong to the organic system of secondary batteries, which possess a wider voltage range and larger specific capacity than those employing hydrogel electrolytes; however, they also entail the risk of spontaneous combustion. To enhance the safety of flexible devices, numerous related strategies have been reported such as preparing non-flammable organic gel electrolytes and modifying fire-resistant coatings on electrode surfaces; nevertheless, these approaches inevitably result in certain electrochemical performance sacrifices for the devices. Furthermore, components utilized in organic system secondary batteries are poorly biocompatible and harmful to the human body; therefore, it is necessary to develop suitable encapsulation fabrics with skin-friendly properties to prevent direct contact between internal materials and human skin.
9. Conclusions and outlook
The advent of the smart electronics era necessitates the development of environmentally friendly, electrochemically superior, and lightweight flexible energy storage devices. However, the current performance of the developed flexible energy storage devices still falls short in meeting practical application demands. To advance wearable electronic device development, this review provides a comprehensive review on the research progress in various flexible energy storage systems. This includes novel design and preparation of flexible electrode materials, gel electrolytes, and diaphragms as well as interfacial engineering between different components.The energy storage systems applied to wearable electronic devices in this review are categorized into two groups: water-based systems and organic-based systems. Water-based systems include SCs, ZIBs, and metal–air batteries, while organic-based systems consist of LIBs, LSBs, SIBs, and PIBs. Considering the direct contact between wearable electronic devices and human skin, safety is the primary concern. From a safety perspective, energy storage systems utilizing water gel electrolytes are safer than those using organic gel electrolytes. Moreover, hydrogels are more environmentally friendly and cost-effective compared to organic gels. However, these aqueous electrochemical energy storage devices have their own advantages and disadvantages in terms of performance: SCs offer fast charging and discharging but lack sufficient endurance; ZIBs exhibit higher energy storage efficiency but suffer from poor cycle life due to zinc dendrite growth, which is a defect that becomes amplified in flexible ZIBs; metal–air batteries exhibit satisfactory energy density and power density, but because the system is in direct contact with air, there are effects on the long-term stability of the hydrogel electrolyte, such as extreme temperatures leading to evaporation/freezing of water and side reactions between the electrolyte and oxygen in the air. On the other hand, despite the inadequate safety of organic energy storage devices, numerous strategies have been proposed to address this issue, including incorporating flame retardant additives into the system and utilizing non-combustible battery housings. Due to their utilization of organic gel electrolytes, these secondary batteries can circumvent hydrogen evolution and oxygen evolution reactions, thereby exhibiting a wide active voltage range and high energy density. While LIBs and LSBs encounter escalating costs, efforts are currently being made to substitute them with SIBs/PIBs; however, this comes at the expense of battery capacity.
Extensive research has been dedicated to employing diverse advanced material synthesis strategies for developing carbon-based/polymer-based composites with both mechanical and electrochemical properties. Nonetheless, there exist several significant bottlenecks impeding the commercialization of flexible energy storage devices:
(1) The energy storage performance of most flexible energy storage devices developed so far falls significantly short compared to that of non-flexible energy storage devices in similar systems, including cycle life, energy density, and power density. The primary reasons for this disparity are as follows: firstly, the preparation strategy for flexible electrode materials typically involves composite flexible substrates with active materials. However, most of these substrates exhibit low specific capacity and mass loading of active materials. Therefore, it is critical to develop flexible substrates with high specific surface area and exceptional electrochemical properties or design substrate-free flexible electrode materials. Secondly, the active material and the flexible substrate are connected by an unstable binder, serving as a mass transfer bridge, which results in a reduced power density. Additionally, there exists a potential risk of active material shedding caused by volumetric variations during the charge and discharge cycles. Furthermore, material deformation exacerbates the existing challenges faced by various energy storage systems such as Zn dendrite growth in ZIBs, the polysulfide-induced “shuttle effect” in LSBs, and electrolyte volatilization in ZABs, leading to degradation in cycle life.
(2) Currently, the research focus in the field of flexible energy storage devices primarily lies in the development of novel electrode materials, often overlooking other crucial components such as electrolytes, separators, and current collectors. However, it is important to recognize that these components do not operate independently; any performance mismatch among them may pose potential safety hazards during practical device utilization. Therefore, further advancements are required for non-electrode components of energy storage devices. Moreover, the concept of “all-in-one” energy storage devices holds great promise as it enables seamless integration between individual components.
(3) The majority of flexible energy storage devices are currently limited to laboratory production and have not yet been commercialized. To achieve cost-effective and large-scale fabrication of these devices, it is imperative to investigate simplified and controllable device integration technologies. Furthermore, to meet the practical requirements of wearable electronic devices, energy storage devices must be seamlessly integrated into flexible, breathable textiles. Therefore, efficient weaving techniques need to be developed as a replacement for manual laboratory weaving. Furthermore, the utilization of 3D printing technology enables the fabrication of flexible electrode materials and their integration into electronic device systems with intricate geometries tailored to specific requirements, thereby minimizing raw material wastage. In recent years, numerous studies have reported the application of 3D printing for electrochemical energy storage and conversion electrodes/devices due to its rapid prototyping capabilities and cost-effectiveness. However, ensuring both mechanical stability and device capacity remains a pressing challenge as the feedstock used in 3D printing often incorporates non-conductive substrates (e.g., acrylonitrile butadiene styrene and polylactic acid), which can adversely impact device performance.
(4) With the increasing demand for wearable/portable electronics, the development of flexible energy storage devices has been progressively advancing towards versatility, intelligence, and scalability. However, most of the current flexible carbon-based composites possess a singular functionality and are limited to specific application scenarios (compression, tensile strain, and bending). Consequently, integrating multiple functionalities into a unified carbon matrix composite remains a significant challenge. Furthermore, exploring novel conductive polymer gel electrode materials could offer potential solutions. Recent efforts have focused on developing self-repairing, extreme temperature-resistant, and fatigue-resistant gel electrodes that hold promise for future wearable electronic devices.
(5) Regarding the safety of wearable electronics, apart from addressing issues such as electrolyte leakage and mechanical damage-induced leakage, there is also a growing focus on the comfort and biocompatibility aspects. Skin-friendly touch panels are essential for electronic devices and wearable electronics to prevent skin rashes or itching caused by prolonged usage. However, achieving touch interaction panels that are truly compatible with human skin remains a challenge in current designs of wearable electronics.
In the past decade, the interdisciplinary field of flexible electronics has experienced significant growth by surmounting the limitations associated with rigidity. It is anticipated that this domain will find extensive applications across various facets of human life, encompassing wearable sensors for monitoring human behavioral patterns, miniature mobile power sources with flexibility for portable exoskeletons, and implantable electronic devices for minimally invasive surgeries or diagnostic medical imaging in pathology. The convergence of flexible sensors and display technologies holds immense potential to facilitate the advancement of smart home and smart city planning. The capabilities offered by flexible sensors and Internet of Things devices could empower robots to play a pivotal role in disaster response and agricultural monitoring, as well as enabling real-time road traffic condition surveillance. Moreover, the development of flexible energy storage devices not only drives technological progress on Earth but also supports space exploration within the aerospace industry, ultimately enabling humanity's venture into cosmic realms.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.Author contributions
All authors contributed to the writing and editing of this review article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22278094), Guangdong Graduate Education Innovation Program (No. 2023JGXM_102), Jiangxi Province “Double Thousand Plan” (No. jxsq2023102142), Basic and Applied Basic Research Program of Guangzhou (No. SL2024A03J00499), University Innovation Team Scientific Research Project of Guangzhou (No. 202235246), Science and Technology Research Project of Guangzhou (No. 202201020214), Guangdong University Student Science and Technology Innovation Climbing Program (No. pdjh2022b0415), and 2022 Innovation Training Program for College Students (No. s202211078133).Notes and references
- X. Hu, D. Zuo, S. Cheng, S. Chen, Y. Liu, W. Bao, S. Deng, S. J. Harris and J. Wan, Chem. Soc. Rev., 2023, 52, 1103–1128 RSC.
- H. Xiang, Z. Li, H. Liu, T. Chen, H. Zhou and W. Huang, npj Flexible Electron., 2022, 6, 15 CrossRef.
- T. Xu, X. Ding, H. Cheng, G. Han and L. Qu, Adv. Mater., 2024, 36, e2209661 CrossRef PubMed.
- H. Chen, Y. Zheng, J. Li, L. Li and X. Wang, ACS Nano, 2023, 17, 9763–9792 CrossRef CAS PubMed.
- H. Li, X. Zhou, W. Zhai, S. Lu, J. Liang, Z. He, H. Long, T. Xiong, H. Sun, Q. He, Z. Fan and H. Zhang, Adv. Energy Mater., 2020, 10, 2002019 CrossRef CAS.
- Z. Hui, L. Zhang, G. Ren, G. Sun, H. D. Yu and W. Huang, Adv. Mater., 2023, 35, e2211202 CrossRef PubMed.
- V. Vijayakumar, B. Anothumakkool, S. Kurungot, M. Winter and J. R. Nair, Energy Environ. Sci., 2021, 14, 2708–2788 RSC.
- R. Xu, M. She, J. Liu, S. Zhao, J. Zhao, X. Zhang, L. Qu and M. Tian, ACS Nano, 2023, 17, 8293–8302 CrossRef CAS PubMed.
- J. Min, J. Tu, C. Xu, H. Lukas, S. Shin, Y. Yang, S. A. Solomon, D. Mukasa and W. Gao, Chem. Rev., 2023, 123, 5049–5138 CrossRef CAS PubMed.
- Y. Ding, J. Jiang, Y. Wu, Y. Zhang, J. Zhou, Y. Zhang, Q. Huang and Z. Zheng, Chem. Rev., 2024, 124, 1535–1648 CrossRef CAS PubMed.
- L. Li, Y. Wang, X. Wang, R. Lin, X. Luo, Z. Liu, K. Zhou, S. Xiong, Q. Bao, G. Chen, Y. Tian, Y. Deng, K. Xiao, J. Wu, M. I. Saidaminov, H. Lin, C.-Q. Ma, Z. Zhao, Y. Wu, L. Zhang and H. Tan, Nat. Energy, 2022, 7, 708–717 CrossRef CAS.
- A. Nawaz, L. Merces, L. M. M. Ferro, P. Sonar and C. C. B. Bufon, Adv. Mater., 2023, 35, e2204804 CrossRef PubMed.
- G. Li, M. Zhang, S. Liu, M. Yuan, J. Wu, M. Yu, L. Teng, Z. Xu, J. Guo, G. Li, Z. Liu and X. Ma, Nat. Electron., 2023, 6, 154–163 CrossRef.
- T. Xu, H. Du, H. Liu, W. Liu, X. Zhang, C. Si, P. Liu and K. Zhang, Adv. Mater., 2021, 33, e2101368 CrossRef PubMed.
- R. Zhu, Z. Wang, X. Hu, X. Liu and H. Wang, Adv. Funct. Mater., 2021, 31, 2101487 CrossRef CAS.
- H. Huo, J. Gao, N. Zhao, D. Zhang, N. G. Holmes, X. Li, Y. Sun, J. Fu, R. Li, X. Guo and X. Sun, Nat. Commun., 2021, 12, 176 CrossRef CAS PubMed.
- Z. Zhou, P. Li, Z. Man, X. Zhu, S. Ye, W. Lu, G. Wu and W. Chen, Angew. Chem., Int. Ed., 2023, 62, e202301618 CrossRef CAS PubMed.
- J. Ma, S. Zheng, L. Chi, Y. Liu, Y. Zhang, K. Wang and Z. S. Wu, Adv. Mater., 2022, 34, 2205569 CrossRef CAS PubMed.
- H. Dong, J. Li, J. Guo, F. Lai, F. Zhao, Y. Jiao, D. J. L. Brett, T. Liu, G. He and I. P. Parkin, Adv. Mater., 2021, 33, e2007548 CrossRef PubMed.
- X. Zhu, C. Hu, R. Amal, L. Dai and X. Lu, Energy Environ. Sci., 2020, 13, 4536–4563 RSC.
- A. Suresh, S. J. Rowan and C. Liu, Adv. Mater., 2023, 35, 2206416 CrossRef CAS PubMed.
- W. Zhao, M. Jiang, W. Wang, S. Liu, W. Huang and Q. Zhao, Adv. Funct. Mater., 2020, 31, 2009136 CrossRef.
- J. He, L. Cao, J. Cui, G. Fu, R. Jiang, X. Xu and C. Guan, Adv. Mater., 2024, 36, e2306090 CrossRef PubMed.
- S. Jiang, X. Liu, J. Liu, D. Ye, Y. Duan, K. Li, Z. Yin and Y. Huang, Adv. Mater., 2022, 34, e2200070 CrossRef PubMed.
- X. Wan, T. Mu and G. Yin, Nano–Micro Lett., 2023, 15, 99 CrossRef CAS PubMed.
- X. Xiao, Z. Zheng, X. Zhong, R. Gao, Z. Piao, M. Jiao and G. Zhou, ACS Nano, 2023, 17, 1764–1802 CrossRef CAS PubMed.
- L. Hu, P. L. Chee, S. Sugiarto, Y. Yu, C. Shi, R. Yan, Z. Yao, X. Shi, J. Zhi, D. Kai, H. D. Yu and W. Huang, Adv. Mater., 2023, 35, e2205326 CrossRef PubMed.
- Z. Zhao, K. Xia, Y. Hou, Q. Zhang, Z. Ye and J. Lu, Chem. Soc. Rev., 2021, 50, 12702–12743 RSC.
- D. Xu, M. Liang, S. Qi, W. Sun, L. P. Lv, F. H. Du, B. Wang, S. Chen, Y. Wang and Y. Yu, ACS Nano, 2021, 15, 47–80 CrossRef CAS PubMed.
- H. Gwon, H.-S. Kim, K. U. Lee, D.-H. Seo, Y. C. Park, Y.-S. Lee, B. T. Ahn and K. Kang, Energy Environ. Sci., 2011, 4, 1277–1283 RSC.
- X. Wu, T. Wen, H. Guo, S. Yang, X. Wang and A. Xu, ACS Nano, 2013, 7, 3589–3597 CrossRef CAS PubMed.
- Y. Xu, Z. Lin, X. Huang, Y. Liu, Y. Huang and X. Duan, ACS Nano, 2013, 7, 4042–4049 CrossRef CAS PubMed.
- Z. Chen, J. W. F. To, C. Wang, Z. Lu, N. Liu, A. Chortos, L. Pan, F. Wei, Y. Cui and Z. Bao, Adv. Energy Mater., 2014, 4, 1400207 CrossRef.
- K. Xiao, L. X. Ding, G. Liu, H. Chen, S. Wang and H. Wang, Adv. Mater., 2016, 28, 5997–6002 CrossRef CAS PubMed.
- Y. Huang, M. Zhong, F. Shi, X. Liu, Z. Tang, Y. Wang, Y. Huang, H. Hou, X. Xie and C. Zhi, Angew. Chem., Int. Ed., 2017, 56, 9141–9145 CrossRef CAS PubMed.
- X. Xu, J. Chen, S. Cai, Z. Long, Y. Zhang, L. Su, S. He, C. Tang, P. Liu, H. Peng and X. Fang, Adv. Mater., 2018, 30, e1803165 CrossRef PubMed.
- Y. Zhao, J. Cao, Y. Zhang and H. Peng, Adv. Funct. Mater., 2019, 30, 1902971 CrossRef.
- X. Shi, Y. Zuo, P. Zhai, J. Shen, Y. Yang, Z. Gao, M. Liao, J. Wu, J. Wang, X. Xu, Q. Tong, B. Zhang, B. Wang, X. Sun, L. Zhang, Q. Pei, D. Jin, P. Chen and H. Peng, Nature, 2021, 591, 240–245 CrossRef CAS PubMed.
- B. Xiao, J. Li, H. Xu, J. Huang, Y. Luo, K. Xiao and Z. Liu, Angew. Chem., Int. Ed., 2023, 62, e202309614 CrossRef CAS PubMed.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2013, 7, 174–182 CrossRef CAS PubMed.
- Z. Song, T. Ma, R. Tang, Q. Cheng, X. Wang, D. Krishnaraju, R. Panat, C. K. Chan, H. Yu and H. Jiang, Nat. Commun., 2014, 5, 3140 CrossRef PubMed.
- P. Xiao, F. Bu, G. Yang, Y. Zhang and Y. Xu, Adv. Mater., 2017, 29, 1703324 CrossRef PubMed.
- W. Wang, M. Tang, Z. Zheng and S. Chen, Adv. Energy Mater., 2019, 9, 1803628 CrossRef.
- Y. Liu, Y. Fang, Z. Zhao, C. Yuan and X. W. Lou, Adv. Energy Mater., 2019, 9, 1803052 CrossRef.
- J. Zhao, Z. Xu, Z. Zhou, S. Xi, Y. Xia, Q. Zhang, L. Huang, L. Mei, Y. Jiang, J. Gao, Z. Zeng and C. Tan, ACS Nano, 2021, 15, 10597–10608 CrossRef CAS PubMed.
- J. He, C. Lu, H. Jiang, F. Han, X. Shi, J. Wu, L. Wang, T. Chen, J. Wang, Y. Zhang, H. Yang, G. Zhang, X. Sun, B. Wang, P. Chen, Y. Wang, Y. Xia and H. Peng, Nature, 2021, 597, 57–63 CrossRef CAS PubMed.
- J. Xia, R. Gao, Y. Yang, Z. Tao, Z. Han, S. Zhang, Y. Xing, P. Yang, X. Lu and G. Zhou, ACS Nano, 2022, 16, 19133–19144 CrossRef CAS PubMed.
- L. Ye, M. Liao, K. Zhang, M. Zheng, Y. Jiang, X. Cheng, C. Wang, Q. Xu, C. Tang, P. Li, Y. Wen, Y. Xu, X. Sun, P. Chen, H. Sun, Y. Gao, Y. Zhang, B. Wang, J. Lu, H. Zhou, Y. Wang, Y. Xia, X. Xu and H. Peng, Nature, 2024, 626, 313–318 CrossRef CAS PubMed.
- Z. Wu, X. Liu, T. Shang, Y. Deng, N. Wang, X. Dong, J. Zhao, D. Chen, Y. Tao and Q. H. Yang, Adv. Funct. Mater., 2021, 31, 2102874 CrossRef CAS.
- Q. Meng, C. Kang, J. Zhu, X. Xiao, Y. Ma, H. Huo, P. Zuo, C. Du, S. Lou and G. Yin, Nano Lett., 2022, 22, 5553–5560 CrossRef CAS PubMed.
- H. Wang, H. Yang, Y. Diao, Y. Lu, K. Chrulski and J. M. D'Arcy, ACS Nano, 2021, 15, 7799–7810 CrossRef CAS PubMed.
- H. Yao, H. Yu, Y. Zheng, N. W. Li, S. Li, D. Luan, X. W. Lou and L. Yu, Angew. Chem., Int. Ed., 2023, 62, e202315257 CrossRef CAS PubMed.
- S. Dong, W. Shin, H. Jiang, X. Wu, Z. Li, J. Holoubek, W. F. Stickle, B. Key, C. Liu, J. Lu, P. A. Greaney, X. Zhang and X. Ji, Chem, 2019, 5, 1537–1551 CAS.
- F. Liu, L. Xie, L. Wang, W. Chen, W. Wei, X. Chen, S. Luo, L. Dong, Q. Dai, Y. Huang and L. Wang, Nano–Micro Lett., 2020, 12, 17 CrossRef CAS PubMed.
- C. Lai, M. Li, Y. Shen, M. Zhou, W. Wang, K. Jiang, H. Li and K. Wang, Energy Environ. Mater., 2023, 7, e12541 CrossRef.
- H. Guo, Q. Fei, M. Lian, T. Zhu, W. Fan, Y. Li, L. Sun, F. Jong, K. Chu, W. Zong, C. Zhang and T. Liu, Adv. Mater., 2023, 35, e2301418 CrossRef PubMed.
- S. Long, Y. Feng, F. He, J. Zhao, T. Bai, H. Lin, W. Cai, C. Mao, Y. Chen, L. Gan, J. Liu, M. Ye, X. Zeng and M. Long, Nano Energy, 2021, 85, 105973 CrossRef CAS.
- F. Qu, D. Jiang, Z. Liu, A. Zhang, Y. Zheng, Q. Jia, C. Li and J. Liu, Electrochim. Acta, 2020, 353, 136600 CrossRef CAS.
- J. Wang, J. G. Wang, H. Liu, Z. You, Z. Li, F. Kang and B. Wei, Adv. Funct. Mater., 2020, 31, 2007397 CrossRef.
- W. J. Hyun, C. M. Thomas, N. S. Luu and M. C. Hersam, Adv. Mater., 2021, 33, e2007864 CrossRef PubMed.
- Y. Wang, Q. Li, H. Hong, S. Yang, R. Zhang, X. Wang, X. Jin, B. Xiong, S. Bai and C. Zhi, Nat. Commun., 2023, 14, 3890 CrossRef CAS PubMed.
- K. Wang, L. Huang, N. Eedugurala, S. Zhang, M. A. Sabuj, N. Rai, X. Gu, J. D. Azoulay and T. N. Ng, Adv. Energy Mater., 2019, 9, 1902806 CrossRef CAS.
- S. Xu, J. Wu, X. Wang and Q. Zhang, Chem. Sci., 2023, 14, 13601–13628 RSC.
- M. Zhao, Y. Li, F. Lin, Y. Xu, L. Chen, W. Jiang, T. Jiang, S. Yang and Y. Wang, J. Mater. Chem. A, 2020, 8, 1829 RSC.
- Y. Yang, Y. Liu, Y. Li, B. Deng, B. Yin and M. Yang, J. Mater. Chem. A, 2020, 8, 17257–17265 RSC.
- H. Liu, T. Xu, C. Cai, K. Liu, W. Liu, M. Zhang, H. Du, C. Si and K. Zhang, Adv. Funct. Mater., 2022, 32, 2113082 CrossRef CAS.
- D. Jiang, J. Zhang, S. Qin, Z. Wang, K. A. S. Usman, D. Hegh, J. Liu, W. Lei and J. M. Razal, ACS Nano, 2021, 15, 5000–5010 CrossRef CAS PubMed.
- L. Manjakkal, A. Pullanchiyodan, N. Yogeswaran, E. S. Hosseini and R. Dahiya, Adv. Mater., 2020, 32, e1907254 CrossRef PubMed.
- K. Li, B. Zhao, H. Zhang, H. Lv, J. Bai, H. Ma, P. Wang, W. Li, J. Si, X. Zhu and Y. Sun, Adv. Funct. Mater., 2021, 31, 2103073 CrossRef CAS.
- H. Huang, Y. Zhao, T. Cong, C. Li, N. Wen, X. Zuo, Y. Guo, H. Zhang, Z. Fan and L. Pan, Adv. Funct. Mater., 2022, 32, 2110777 CrossRef CAS.
- L. Wang, H. Yao, F. Chi, J. Yan, H. Cheng, Y. Li, L. Jiang and L. Qu, ACS Nano, 2022, 16, 12813 CrossRef CAS PubMed.
- J. Kim, S. M. Wi, J. G. Ahn, S. Son, H. Lim, Y. Park, H. J. Eun, J. B. Park, H. Lim, S. Pak, A. R. Jang and Y. W. Lee, Energy Environ. Mater., 2023, 6, e12581 CrossRef CAS.
- J. Liang, B. Tian, S. Li, C. Jiang and W. Wu, Adv. Energy Mater., 2020, 10, 2000022 CrossRef CAS.
- T. Khudiyev, J. T. Lee, J. R. Cox, E. Argentieri, G. Loke, R. Yuan, G. H. Noel, R. Tatara, Y. Yu, F. Logan, J. Joannopoulos, Y. Shao-Horn and Y. Fink, Adv. Mater., 2020, 32, e2004971 CrossRef PubMed.
- T. Wu, Z. Ma, Y. He, X. Wu, B. Tang, Z. Yu, G. Wu, S. Chen and N. Bao, Angew. Chem., Int. Ed., 2021, 60, 10366–10374 CrossRef CAS PubMed.
- J. Nan, G. Zhang, T. Zhu, Z. Wang, L. Wang, H. Wang, F. Chu, C. Wang and C. Tang, Adv. Sci., 2020, 7, 2000587 CrossRef CAS PubMed.
- L. Li, Y. Zhang, H. Lu, Y. Wang, J. Xu, J. Zhu, C. Zhang and T. Liu, Nat. Commun., 2020, 11, 62 CrossRef CAS PubMed.
- Q. F. Guan, Z. C. Ling, Z. M. Han, T. T. Luo, H. B. Yang, K. P. Yang, C. H. Yin and S. H. Yu, Adv. Funct. Mater., 2021, 31, 2105070 CrossRef CAS.
- J. Dai, H. Qin, W. Dong, H. Cong and S. Yu, Nano Lett., 2022, 22, 6444–6453 CrossRef CAS PubMed.
- Q. Zhang, X. Hou, X. Liu, X. Xie, L. Duan, W. Lu and G. Gao, Small, 2021, 17, e2103091 CrossRef PubMed.
- Q. Fu, S. Hao, L. Meng, F. Xu and J. Yang, ACS Nano, 2021, 15, 18469–18482 CrossRef CAS PubMed.
- C. Lu and X. Chen, Nano Lett., 2020, 20, 1907–1914 CrossRef CAS PubMed.
- C. Wang, Z. Pei, Q. Meng, C. Zhang, X. Sui, Z. Yuan, S. Wang and Y. Chen, Angew. Chem., Int. Ed., 2021, 60, 990–997 CrossRef CAS PubMed.
- X. He, D. Wu, Y. Shang, H. Shen, S. Xi, X. Wang, W. Li and Q. Wang, Sci. China Mater., 2021, 65, 115–123 CrossRef.
- D. Liu, Z. Wang, Q. Qian, J. Wang, J. Ren, H. Chen, W. Xing and N. Zhou, Adv. Funct. Mater., 2023, 33, 202214301 Search PubMed.
- R. Ma, Z. Xu and X. Wang, Energy Environ. Mater., 2023, 6, e12464 CrossRef CAS.
- H. Liu, J. Li, X. Zhang, X. Liu, Y. Yan, F. Chen, G. Zhang and H. Duan, Adv. Funct. Mater., 2021, 31, 2106550 CrossRef CAS.
- Y. Zhang, X. Zheng, N. Wang, W. H. Lai, Y. Liu, S. L. Chou, H. K. Liu, S. X. Dou and Y. X. Wang, Chem. Sci., 2022, 13, 14246–14263 RSC.
- M. S. Javed, H. Lei, Z. Wang, B.-t. Liu, X. Cai and W. Mai, Nano Energy, 2020, 70, 104573 CrossRef CAS.
- N. Xu, C. Yan, W. He, L. Xu, Z. Jiang, A. Zheng, H. Wu, M. Chen and G. Diao, J. Power Sources, 2022, 533, 231358 CrossRef CAS.
- L. Chen, H. Yue, Z. Zhang, Y. Ma, Y. Wang, M. Xu, Y. Huang and G. Yuan, Chem. Eng. J., 2023, 455, 140679 CrossRef CAS.
- W. Yang, W. Yang, Y. Huang, C. Xu, L. Dong and X. Peng, Chin. Chem. Lett., 2022, 33, 4628–4634 CrossRef CAS.
- Z. Pan, J. Yang, J. Yang, Q. Zhang, H. Zhang, X. Li, Z. Kou, Y. Zhang, H. Chen, C. Yan and J. Wang, ACS Nano, 2020, 14, 842–853 CrossRef CAS PubMed.
- D. Chao, C. R. Zhu, M. Song, P. Liang, X. Zhang, N. H. Tiep, H. Zhao, J. Wang, R. Wang, H. Zhang and H. J. Fan, Adv. Mater., 2018, 30, e1803181 CrossRef PubMed.
- S. Li, Y. Liu, X. Zhao, Q. Shen, W. Zhao, Q. Tan, N. Zhang, P. Li, L. Jiao and X. Qu, Adv. Mater., 2021, 33, e2007480 CrossRef PubMed.
- Q. Tan, X. Li, B. Zhang, X. Chen, Y. Tian, H. Wan, L. Zhang, L. Miao, C. Wang, Y. Gan, J. Jiang, Y. Wang and H. Wang, Adv. Energy Mater., 2020, 10, 2001050 CrossRef CAS.
- Y. Ren, F. Meng, S. Zhang, B. Ping, H. Li, B. Yin and T. Ma, Carbon Energy, 2022, 4, 446–457 CrossRef CAS.
- W. Yan, X. Cai, F. Tan, J. Liang, J. Zhao and C. Tan, Chem. Commun., 2023, 59, 1661–1664 RSC.
- H. Liu, J. Li, D. Wei, X. Liu, Z. Cai, H. Zhang, Z. Lv, L. Chen, H. Li, H. Luo, Y. Zhao, H. Yu, X. Wang, F. Chen, G. Zhang and H. Duan, Adv. Funct. Mater., 2023, 33, 2300419 CrossRef CAS.
- M. Zhang, P. Yu, K. Xiong, Y. Wang, Y. Liu and Y. Liang, Adv. Mater., 2022, 34, 2200860 CrossRef CAS PubMed.
- X. Zhao, Y. Gao, Q. Cao, F. Bu, J. Pu, Y. Wang and C. Guan, Adv. Energy Mater., 2023, 13, 2301741 CrossRef CAS.
- Y. Zeng, X. Zhang, R. Qin, X. Liu, P. Fang, D. Zheng, Y. Tong and X. Lu, Adv. Mater., 2019, 31, e1903675 CrossRef PubMed.
- Q. Wang, J. Zhao, J. Zhang, X. Xue, M. Li, Z. Sui, X. Zhang, W. Zhang and C. Lu, Adv. Funct. Mater., 2023, 33, 2306346 CrossRef CAS.
- Q. Cao, H. Gao, Y. Gao, J. Yang, C. Li, J. Pu, J. Du, J. Yang, D. Cai, Z. Pan, C. Guan and W. Huang, Adv. Funct. Mater., 2021, 31, 2103922 CrossRef CAS.
- Y. Li, H. Yang, T. Zhang, S. Li, S. Li, S. He, T. Chen, J. Y. Lee, Y. Zhao and P. Y. Chen, Adv. Energy Mater., 2021, 11, 202101862 Search PubMed.
- J. Pu, Q. Cao, Y. Gao, Q. Wang, Z. Geng, L. Cao, F. Bu, N. Yang and C. Guan, Adv. Mater., 2023, 36, e2305812 CrossRef PubMed.
- X. Cheng, X. Yang, Y. Zhang, P. Lv, J. Yang, F. Huang and Q. Wei, Adv. Fiber Mater., 2023, 5, 650–661 CrossRef CAS.
- M. Yao, Z. Yuan, S. Li, T. He, R. Wang, M. Yuan and Z. Niu, Adv. Mater., 2021, 33, e2008140 CrossRef PubMed.
- H. Li, C. Han, Y. Huang, Y. Huang, M. Zhu, Z. Pei, Q. Xue, Z. Wang, Z. Liu, Z. Tang, Y. Wang, F. Kang, B. Li and C. Zhi, Energy Environ. Sci., 2018, 11, 941–951 RSC.
- H. Li, Z. Liu, G. Liang, Y. Huang, Y. Huang, M. Zhu, Z. Pei, Q. Xue, Z. Tang, Y. Wang, B. Li and C. Zhi, ACS Nano, 2018, 12, 3140–3148 CrossRef CAS PubMed.
- J. Wang, Y. Huang, B. Liu, Z. Li, J. Zhang, G. Yang, P. Hiralal, S. Jin and H. Zhou, Energy Storage Mater., 2021, 41, 599–605 CrossRef.
- S. Huang, L. Hou, T. Li, Y. Jiao and P. Wu, Adv. Mater., 2022, 34, e2110140 CrossRef PubMed.
- Y. Shi, R. Wang, S. Bi, M. Yang, L. Liu and Z. Niu, Adv. Funct. Mater., 2023, 33, 2214546 CrossRef CAS.
- Q. Liu, Z. Yu, Q. Zhuang, J. K. Kim, F. Kang and B. Zhang, Adv. Mater., 2023, 35, e2300498 CrossRef PubMed.
- J. Kuang, Y. Shen, Y. Zhang, J. Yao, J. Du, S. Yang, S. Zhang, Y. Fang and X. Cai, Small, 2023, 19, e2207413 CrossRef PubMed.
- X. Zhong, Z. Zheng, J. Xu, X. Xiao, C. Sun, M. Zhang, J. Ma, B. Xu, K. Yu, X. Zhang, H. M. Cheng and G. Zhou, Adv. Mater., 2023, 35, e2209980 CrossRef PubMed.
- P. Tan, B. Chen, H. Xu, H. Zhang, W. Cai, M. Ni, M. Liu and Z. Shao, Energy Environ. Sci., 2017, 10, 2056–2080 RSC.
- Y. Zhang, L. Wang, Z. Guo, Y. Xu, Y. Wang and H. Peng, Angew. Chem., Int. Ed., 2016, 55, 4487–4491 CrossRef CAS PubMed.
- T. Liu, X. L. Feng, X. Jin, M. Z. Shao, Y. T. Su, Y. Zhang and X. B. Zhang, Angew. Chem., Int. Ed., 2019, 58, 18240–18245 CrossRef CAS PubMed.
- X. Lei, X. Liu, W. Ma, Z. Cao, Y. Wang and Y. Ding, Angew. Chem., Int. Ed., 2018, 57, 16131–16135 CrossRef CAS PubMed.
- X. Chi, M. Li, J. Di, P. Bai, L. Song, X. Wang, F. Li, S. Liang, J. Xu and J. Yu, Nature, 2021, 592, 551–557 CrossRef CAS PubMed.
- Y. Li, M. Cui, Z. Yin, S. Chen and T. Ma, Chem. Sci., 2020, 11, 11646–11671 RSC.
- Y. H. Zhu, X. Y. Yang, T. Liu and X. B. Zhang, Adv. Mater., 2020, 32, e1901961 CrossRef PubMed.
- S. Lee, J. Choi, M. Kim, J. Park, M. Park and J. Cho, Chem. Sci., 2022, 13, 6159–6180 RSC.
- X. Yang, F. Wang, Z. Jing, M. Chen, B. Wang, L. Wang, G. Qu, Y. Kong and L. Xu, Small, 2023, 19, e2301985 CrossRef PubMed.
- Z. Wu, H. Wang, P. Xiong, G. Li, T. Qiu, W. B. Gong, F. Zhao, C. Li, Q. Li, G. Wang and F. Geng, Nano Lett., 2020, 20, 2892–2898 CrossRef CAS PubMed.
- H. W. Go, T. T. Nguyen, Q. P. Ngo, R. Chu, N. H. Kim and J. H. Lee, Small, 2023, 19, e2206341 CrossRef PubMed.
- Y. Ma, D. Chen, W. Li, Y. Zheng, L. Wang, G. Shao, Q. Liu and W. Yang, Energy Environ. Mater., 2022, 5, 543–554 CrossRef CAS.
- F. Qiang, J. Feng, H. Wang, J. Yu, J. Shi, M. Huang, Z. Shi, S. Liu, P. Li and L. Dong, ACS Catal., 2022, 12, 4002–4015 CrossRef CAS.
- Y. Chen, S. Qiao, Y. Tang, Y. Du, D. Zhang, W. Wang, H. Zhang, X. Sun and C. Liu, ACS Nano, 2022, 16, 15273–15285 CrossRef CAS PubMed.
- Y. Han, H. Duan, C. Zhou, H. Meng, Q. Jiang, B. Wang, W. Yan and R. Zhang, Nano Lett., 2022, 22, 2497–2505 CrossRef CAS PubMed.
- Y. Zhang, Y.-P. Deng, J. Wang, Y. Jiang, G. Cui, L. Shui, A. Yu, X. Wang and Z. Chen, Energy Storage Mater., 2021, 35, 538–549 CrossRef.
- R. Manjunatha, J. Yuan, L. Hongwei, S. Q. Deng, E. R. Ezeigwe, Y. Zuo, L. Dong, A. Li, W. Yan, F. Zhang and J. Zhang, Carbon Energy, 2022, 4, 762–775 CrossRef CAS.
- C. Lai, H. Li, Y. Sheng, M. Zhou, W. Wang, M. Gong, K. Wang and K. Jiang, Adv. Sci., 2022, 9, e2105925 CrossRef PubMed.
- H. Huang, A. Huang, D. Liu, W. Han, C. H. Kuo, H. Y. Chen, L. Li, H. Pan and S. Peng, Adv. Mater., 2023, 35, e2303109 CrossRef PubMed.
- N. K. Wagh, D. H. Kim, S. H. Kim, S. S. Shinde and J. H. Lee, ACS Nano, 2021, 15, 14683–14696 CrossRef CAS PubMed.
- M. Yang, X. Shu, W. Pan and J. Zhang, Small, 2021, 17, e2006773 CrossRef PubMed.
- Y. Rao, S. Chen, Q. Yue and Y. Kang, ACS Catal., 2021, 11, 8097–8103 CrossRef CAS.
- Z. Song, J. Ding, B. Liu, X. Liu, X. Han, Y. Deng, W. Hu and C. Zhong, Adv. Mater., 2020, 32, e1908127 CrossRef PubMed.
- X. Hui, P. Zhang, J. Li, D. Zhao, Z. Li, Z. Zhang, C. Wang, R. Wang and L. Yin, Adv. Energy Mater., 2022, 12, 2201393 CrossRef CAS.
- Y. Zuo, W. Zhang, M. Wei, P. Zhang, S. Zhao, P. Pei, L. Qiu, H. Wang, Z. Meng and K. Wang, Energy Storage Mater., 2022, 53, 136–147 CrossRef.
- X. Fan, R. Zhang, S. Sui, X. Liu, J. Liu, C. Shi, N. Zhao, C. Zhong and W. Hu, Angew. Chem., Int. Ed., 2023, 62, e202302640 CrossRef CAS PubMed.
- M. Jiao, L. Dai, H. R. Ren, M. Zhang, X. Xiao, B. Wang, J. Yang, B. Liu, G. Zhou and H. M. Cheng, Angew. Chem., Int. Ed., 2023, 62, e202301114 CrossRef CAS PubMed.
- H. Dou, M. Xu, Y. Zheng, Z. Li, G. Wen, Z. Zhang, L. Yang, Q. Ma, A. Yu, D. Luo, X. Wang and Z. Chen, Adv. Mater., 2022, 34, e2110585 CrossRef PubMed.
- M. Xu, H. Dou, Z. Zhang, Y. Zheng, B. Ren, Q. Ma, G. Wen, D. Luo, A. Yu, L. Zhang, X. Wang and Z. Chen, Angew. Chem., Int. Ed., 2022, 61, e202117703 CrossRef CAS PubMed.
- Z. Pei, Z. Yuan, C. Wang, S. Zhao, J. Fei, L. Wei, J. Chen, C. Wang, R. Qi, Z. Liu and Y. Chen, Angew. Chem., Int. Ed., 2020, 59, 4793–4799 CrossRef CAS PubMed.
- Z. Pei, L. Ding, C. Wang, Q. Meng, Z. Yuan, Z. Zhou, S. Zhao and Y. Chen, Energy Environ. Sci., 2021, 14, 4926–4935 RSC.
- Y. Zhang, D. Wu, F. Huang, Y. Cai, Y. Li, H. Ke, P. Lv and Q. Wei, Adv. Funct. Mater., 2022, 32, 2203204 CrossRef CAS.
- Z. Zhao, Z. Zhang, T. Xu, W. Wang, B. Wang and X. Yu, J. Am. Chem. Soc., 2024, 146, 2257–2266 CrossRef CAS PubMed.
- J. Ryu, M. Park and J. Cho, Adv. Mater., 2019, 31, e1804784 CrossRef PubMed.
- Y. Xu, Y. Zhao, J. Ren, Y. Zhang and H. Peng, Angew. Chem., Int. Ed., 2016, 55, 7979–7982 CrossRef CAS PubMed.
- S. Choi, D. Lee, G. Kim, Y. Y. Lee, B. Kim, J. Moon and W. Shim, Adv. Funct. Mater., 2017, 27, 1702244 CrossRef.
- D. Yu, Y. Ma, F. Hu, C. C. Lin, L. Li, H. Y. Chen, X. Han and S. Peng, Adv. Energy Mater., 2021, 11, 2101242 CrossRef CAS.
- W.-X. Hong, W.-H. Wang, Y.-H. Chang, H. Pourzolfaghar, I. H. Tseng and Y.-Y. Li, Nano Energy, 2024, 121, 109236 CrossRef CAS.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 CrossRef PubMed.
- Z. Fang, S. Duan, H. Liu, Z. Hong, H. Wu, F. Zhao, Q. Li, S. Fan, W. Duan and J. Wang, Small, 2022, 18, e2105172 CrossRef PubMed.
- A. Chen, X. Guo, S. Yang, G. Liang, Q. Li, Z. Chen, Z. Huang, Q. Yang, C. Han and C. Zhi, Energy Environ. Sci., 2021, 14, 3599–3608 RSC.
- A. Kothuru, A. Cohen, G. Daffan, Y. Juhl and F. Patolsky, Carbon Energy, 2024, e507 CrossRef.
- W. Shen, K. Li, Y. Lv, T. Xu, D. Wei and Z. Liu, Adv. Energy Mater., 2020, 10, 1904281 CrossRef CAS.
- A. Kuwabara, M. Enomoto, E. Hosono, K. Hamaguchi, T. Onuma, S. Kajiyama and T. Kato, Chem. Sci., 2020, 11, 10631–10637 RSC.
- Y. Zhu, M. Yang, Q. Huang, D. Wang, R. Yu, J. Wang, Z. Zheng and D. Wang, Adv. Mater., 2020, 32, e1906205 CrossRef PubMed.
- S. G. Woo, S. Yoo, S. H. Lim, J. S. Yu, K. Kim, J. Lee, D. Lee, J. H. Kim and S. Y. Lee, Adv. Funct. Mater., 2020, 30, 1908633 CrossRef CAS.
- Y. Yang, J. Xia, X. Guan, Z. Wei, J. Yu, S. Zhang, Y. Xing and P. Yang, Small, 2022, 18, e2204970 CrossRef PubMed.
- S. Chen, R. Tao, J. Tu, P. Guo, G. Yang, W. Wang, J. Liang and S. Y. Lu, Adv. Funct. Mater., 2021, 31, 2101199 CrossRef CAS.
- Q. Zhao, Q. Zhu, J. Miao, P. Zhang, P. Wan, L. He and B. Xu, Small, 2019, 15, e1904293 CrossRef PubMed.
- Y. An, Y. Tian, Y. Zhang, C. Wei, L. Tan, C. Zhang, N. Cui, S. Xiong, J. Feng and Y. Qian, ACS Nano, 2020, 14, 17574–17588 CrossRef CAS PubMed.
- X. Ge, S. Cao, Z. Lv, Z. Zhu, Y. Tang, H. Xia, H. Zhang, J. Wei, W. Zhang, Y. Zhang, Y. Zeng and X. Chen, Adv. Mater., 2022, 34, e2206797 CrossRef PubMed.
- M. Guo, Z. Cao, Y. Liu, Y. Ni, X. Chen, M. Terrones and Y. Wang, Adv. Sci., 2023, 10, e2207355 CrossRef PubMed.
- W. Li, B. Song and A. Manthiram, Chem. Soc. Rev., 2017, 46, 3006–3059 RSC.
- X. Yu, J. Deng, X. Yang, J. Li, Z.-H. Huang, B. Li and F. Kang, Nano Energy, 2020, 67, 104256 CrossRef CAS.
- S. Kang, S. Y. Hong, N. Kim, J. Oh, M. Park, K. Y. Chung, S. S. Lee, J. Lee and J. G. Son, ACS Nano, 2020, 14, 3660–3668 CrossRef CAS PubMed.
- S. Y. Hong, S. M. Jee, Y. Ko, J. Cho, K. H. Lee, B. Yeom, H. Kim and J. G. Son, ACS Nano, 2022, 16, 2271–2281 CrossRef CAS PubMed.
- X. Hu, Y. Chen, W. Xu, Y. Zhu, D. Kim, Y. Fan, B. Yu and Y. Chen, Small, 2023, 19, e2301604 CrossRef PubMed.
- Z. Wang, Y. Wang, Y. Chen, H. Wu, Y. Wu, X. Zhao, R. P. S. Han and A. Cao, Small, 2021, 17, e2100911 CrossRef PubMed.
- X. Mu, X. Li, C. Liao, H. Yu, Y. Jin, B. Yu, L. Han, L. Chen, Y. Kan, L. Song and Y. Hu, Adv. Funct. Mater., 2022, 32, 2203006 CrossRef CAS.
- D. Kim, X. Liu, B. Yu, S. Mateti, L. A. O'Dell, Q. Rong and Y. Chen, Adv. Funct. Mater., 2020, 30, 1910813 CrossRef CAS.
- Z. Fang, Y. Luo, H. Liu, Z. Hong, H. Wu, F. Zhao, P. Liu, Q. Li, S. Fan, W. Duan and J. Wang, Adv. Sci., 2021, 8, e2100736 CrossRef PubMed.
- Y. Deng, Y. Pan, Z. Zhang, Y. Fu, L. Gong, C. Liu, J. Yang, H. Zhang and X. Cheng, Adv. Funct. Mater., 2021, 32, 2106176 CrossRef.
- J. Shang, W. Yu, L. Wang, C. Xie, H. Xu, W. Wang, Q. Huang and Z. Zheng, Adv. Mater., 2023, 35, e2211748 CrossRef PubMed.
- R. Ke, L. Du, B. Han, H. Xu, H. Meng, H. Zeng, Z. Zheng and Y. Deng, Nano Lett., 2022, 22, 9327–9334 CrossRef CAS PubMed.
- T. Yang, J. Xia, Z. Piao, L. Yang, S. Zhang, Y. Xing and G. Zhou, ACS Nano, 2021, 15, 13901–13923 CrossRef CAS PubMed.
- H. Yuan, T. Liu, Y. Liu, J. Nai, Y. Wang, W. Zhang and X. Tao, Chem. Sci., 2019, 10, 7484–7495 RSC.
- H. Dong, S. Qi, L. Wang, X. Chen, Y. Xiao, Y. Wang, B. Sun, G. Wang and S. Chen, Small, 2023, e2300843 CrossRef PubMed.
- X. Zhong, D. Wang, J. Sheng, Z. Han, C. Sun, J. Tan, R. Gao, W. Lv, X. Xu, G. Wei, X. Zou and G. Zhou, Nano Lett., 2022, 22, 1207–1216 CrossRef CAS PubMed.
- L. Niu, T. Wu, D. Zhou, J. Qi and Z. Xiao, Energy Storage Mater., 2022, 45, 840–850 CrossRef.
- L. Chen, Y. Yuan, R. Orenstein, M. Yanilmaz, J. He, J. Liu, Y. Liu and X. Zhang, Energy Storage Mater., 2023, 60, 102817 CrossRef.
- R. Gao, Q. Zhang, Y. Zhao, Z. Han, C. Sun, J. Sheng, X. Zhong, B. Chen, C. Li, S. Ni, Z. Piao, B. Li and G. Zhou, Adv. Funct. Mater., 2021, 32, 2110313 CrossRef.
- Y. Luo, L. Wang, Z. Wei, Q. Huang, Y. Deng and Z. Zheng, Adv. Energy Mater., 2022, 13, 2203621 CrossRef.
- Y. An, C. Luo, D. Yao, S. Wen, P. Zheng, S. Chi, Y. Yang, J. Chang, Y. Deng and C. Wang, Nano–Micro Lett., 2021, 13, 84 CrossRef CAS PubMed.
- Z. Zhang, J. Mo, P. Yu, L. Feng, Y. Wang, Y. Lu and W. Yang, Adv. Sci., 2022, 9, e2201881 CrossRef PubMed.
- H. A. Hoang and D. Kim, Small, 2022, 18, e2202963 CrossRef PubMed.
- S. C. Jo, J. W. Hong, I. H. Choi, M. J. Kim, B. G. Kim, Y. J. Lee, H. Y. Choi, D. Kim, T. Kim, K. J. Baeg and J. W. Park, Small, 2022, 18, e2200326 CrossRef PubMed.
- Y. Wang, Y. Zhao, K. Liu, S. Wang, N. Li, G. Shao, F. Wang and P. Zhang, Carbon Energy, 2022, 5, e255 CrossRef.
- H.-J. Peng, J.-Q. Huang and Q. Zhang, Chem. Soc. Rev., 2017, 46, 5237–5288 RSC.
- Y. Zhang, X. Liu, L. Wu, W. Dong, F. Xia, L. Chen, N. Zhou, L. Xia, Z.-Y. Hu, J. Liu, H. S. H. Mohamed, Y. Li, Y. Zhao, L. Chen and B.-L. Su, J. Mater. Chem. A, 2020, 8, 2741–2751 RSC.
- F. Dong, C. X. Peng, H. Y. Xu, Y. X. Zheng, H. F. Yao, J. H. Yang and S. Y. Zheng, ACS Nano, 2021, 15, 20287–20299 CrossRef CAS PubMed.
- H. Kang and M. J. Park, Nano Energy, 2021, 89, 106459 CrossRef CAS.
- L. Huang, T. Guan, H. Su, Y. Zhong, F. Cao, Y. Zhang, X. Xia, X. Wang, N. Bao and J. Tu, Angew. Chem., Int. Ed., 2022, 61, e202212151 CrossRef CAS PubMed.
- J. Zhou, T. Wu, Y. Pan, J. Zhu, X. Chen, C. Peng, C. Shu, L. Kong, W. Tang and S. L. Chou, Adv. Funct. Mater., 2021, 32, 2106966 CrossRef.
- P. Zhu, C. Yan, J. Zhu, J. Zang, Y. Li, H. Jia, X. Dong, Z. Du, C. Zhang, N. Wu, M. Dirican and X. Zhang, Energy Storage Mater., 2019, 17, 220–225 CrossRef.
- J. Sheng, Q. Zhang, C. Sun, J. Wang, X. Zhong, B. Chen, C. Li, R. Gao, Z. Han and G. Zhou, Adv. Funct. Mater., 2022, 32, 2203272 CrossRef CAS.
- B. Yu, Y. Fan, S. Mateti, D. Kim, C. Zhao, S. Lu, X. Liu, Q. Rong, T. Tao, K. K. Tanwar, X. Tan, S. C. Smith and Y. I. Chen, ACS Nano, 2021, 15, 1358–1369 CrossRef CAS PubMed.
- X. Dai, G. Lv, Z. Wu, X. Wang, Y. Liu, J. Sun, Q. Wang, X. Xiong, Y. Liu, C. Zhang, S. Xin, Y. Chen and T. Zhou, Adv. Energy Mater., 2023, 13, 2300452 CrossRef CAS.
- Q. Li, Z. Ma, J. Zhao, K. Shen, T. Shi, Y. Xie, Y. Fan, X. Qin and G. Shao, J. Power Sources, 2022, 521, 230929 CrossRef CAS.
- L. Ren and B. W. Zhang, Exploration, 2022, 2, 20210182 CrossRef CAS PubMed.
- E. J. Kim, P. R. Kumar, Z. T. Gossage, K. Kubota, T. Hosaka, R. Tatara and S. Komaba, Chem. Sci., 2022, 13, 6121–6158 RSC.
- C. Wang, J. Zhang, X. Wang, C. Lin and X. S. Zhao, Adv. Funct. Mater., 2020, 30, 2002629 CrossRef CAS.
- Z. H. Sun, D. Y. Qu, D. X. Han, Z. Y. Gu, J. Z. Guo, X. X. Zhao, Y. M. Ma, B. L. Zhao, Z. Q. Song, X. L. Wu and L. Niu, Adv. Mater., 2024, 36, 2308987 CrossRef CAS PubMed.
- X. Song, X. Li, H. Shan, J. Wang, W. Li, K. Xu, K. Zhang, H. M. K. Sari, L. Lei, W. Xiao, J. Qin, C. Xie and X. Sun, Adv. Funct. Mater., 2024, 34, 2303211 CrossRef CAS.
- N. Mittal, S. Tien, E. Lizundia and M. Niederberger, Small, 2022, 18, 2107183 CrossRef CAS PubMed.
- E. Zhang, X. Jia, B. Wang, J. Wang, X. Yu and B. Lu, Adv. Sci., 2020, 7, 2000470 CrossRef CAS PubMed.
- D. Chen, Z. Huang, S. Sun, H. Zhang, W. Wang, G. Yu and J. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 44369–44378 CrossRef CAS PubMed.
- D. Li, L. Dai, X. Ren, F. Ji, Q. Sun, Y. Zhang and L. Ci, Energy Environ. Sci., 2021, 14, 424–436 RSC.
- M. Xu, D. Zhou, T. Wu, J. Qi, Q. Du and Z. Xiao, Adv. Funct. Mater., 2022, 32, 2203263 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |