DOI:
10.1039/C5RA24774H
(Paper)
RSC Adv., 2016,
6, 3446-3457
Designed preparation of 3D hierarchically porous carbon material via solvothermal route and in situ activation for ultrahigh-efficiency dye removal: adsorption isotherm, kinetics and thermodynamics characteristics
Received
23rd November 2015
, Accepted 15th December 2015
First published on 18th December 2015
Abstract
Herein, the first preparation of a novel 3D hierarchically porous carbon (3D HPC) via precarbonization and in situ alkali activation, wherein mesoporous polydivinylbenzene (meso-PDVB) synthesized by a facile and general solvothermal route was used as the carbon precursor, is reported. Its physico-chemical properties were characterized by SEM, TEM, XRD, FT-IR, elemental analysis, Raman spectroscopy and N2 adsorption–desorption isotherm. 3D HPC exhibited a very large SSA of 3131.32 m2 g−1 and a pore volume of 1.462 cm3 g−1. Moreover, 3D HPC was used to eliminate a dye from wastewater for the first time. The effects of the contact time, initial dye concentration, temperature and solution pH on the adsorption of methylene blue (MB) onto 3D HPC were investigated by batch techniques. The isotherm and kinetics data were well described by a Langmuir model and a pseudo-second-order kinetic model, respectively. 3D HPC had a high adsorption affinity for MB over a broad pH range. 3D HPC displayed remarkably strong adsorption of MB, with a maximum adsorption capacity of 717.77 mg g−1 at 308 K. The adsorption process was endothermic and spontaneous, and the kinetics was controlled by film and intra-particle diffusion. The mechanism of the adsorption of MB was mainly attributed to van der Waals forces, π–π stacking and electrostatic interactions. In addition, 3D HPC displayed excellent reusability and exhibited promising potential for the treatment of dyes in wastewater.
1. Introduction
Dyes have so far been extensively used as colorants in large numbers of industries such as leather, textiles, pulp and paper, and pharmaceuticals.1 During the process of industrial production, numerous dye-containing colored wastewaters are commonly directly discharged into natural water environments without any extra treatment.2 Unfortunately, textile industry wastewater, which includes various dyes, organic compounds and toxicants, can result in huge damage to the natural environment and human health, due to their teratogenic, carcinogenic, and toxic effects.3,4 Moreover, it is obvious that residual dyes frequently appear in water resources.5 Among these, methylene blue (MB), which is a typical thiazine dye, is most usually used for coloring paper and dyeing cottons, hair (as a temporary colorant), and wools6,7 and is therefore found easily in industrial wastewater. In spite of the fact that MB is not treated as an acutely toxic substance, it still has a variety of harmful effects. Once inhaled, MB could cause temporary difficulty in breathing, a burning sensation, nausea, vomiting, and even gastritis problems.8 Other than these adverse effects, almost all people cannot aesthetically accept colored wastewater.9 Therefore, it is urgent to develop low-cost and highly effective methods for the removal of MB from wastewater.
Although microbial degradation is one of the best choices for the elimination of organic dyes in a natural ecological system, their degradation products may be more carcinogenic and toxic, due to their residual complex aromatic structures, and environmental self-purification processes are very slow. Also, owing to their high chemical and photolytic stability, it is consequently difficult, time-consuming and ineffective to achieve the complete removal of dyes via biodegradation or biological treatment.10,11 The reduction of dye concentrations to meet emissions standards before discharge is of great necessity.1,5 In recent years, a variety of conventional artificial treatment techniques have been developed to remove dyes, such as chemical coagulation, electrochemical techniques, activated sludge, oxidation, photochemical degradation, trickling filters and adsorption.12–17 Among these techniques, adsorption has been proved to be a general, facile treatment method to remove organic chemical pollutants in practice, because of its ease of use, high efficiency, low cost and safety when performed.18,19 Based on researchers' efforts, various porous adsorbents, e.g., silicas, resins, metal oxides/hydroxides, natural clays and carbons, have been widely used in attempts to remove organic dyes from wastewaters.20,21
Porous carbons (PCs) exhibit great potential as superior adsorbents for applications in environmental remediation, due to their excellent thermal/chemical stability, large specific surface area, abundant porosity and tunable microstructure.11,22–24 For the adsorptive removal of MB, it has been reported that several kinds of PCs have been prepared and used, which were derived from rice husk,11 rambutan peel,22 polypropylene23 and waste plastic.24 In order to promote the diffusion of target molecules into internal surfaces, especially for adsorption, it is desirable that PCs possess a mesoporous structure. Conventionally, mesoporous carbons are synthesized via a nanocasting approach (hard-templating) or organic–organic self-assembly (soft-templating). The former is time-consuming, high-cost, and limited for scaled-up production. However, the latter suffers from the utilization of expensive amphiphilic block copolymers, e.g., P123 and F127, and hazardous reactants such as formaldehyde and phenol during the preparation of mesoporous polymers.25 Therefore, facile, cost-effective approaches for the mass production of mesoporous polymers employed as carbon precursors are strongly desirable.
To the best of our knowledge, the preparation of 3D hierarchically porous carbon (3D HPC) derived from mesoporous polydivinylbenzene (meso-PDVB) synthesized by a solvothermal reaction via precarbonization and in situ KOH activation is first reported. This novel 3D HPC was systematically characterized by elemental analysis, scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy, Raman spectroscopy and nitrogen adsorption/desorption. A study of the adsorption capacity of 3D HPC for MB from an aqueous medium was carried out by a batch technique. The effects of the initial concentration, contact time, pH and reaction temperature were also investigated. The adsorption isotherms, kinetics, thermodynamics and regeneration properties of 3D HPC were also analyzed in detail.
2. Experimental
2.1 Materials
MB (C16H18ClN3S·3H2O, ≥95%), hydrochloric acid (HCl, 36%), potassium hydroxide (KOH, ≥98%), absolute ethanol, acetone and ethyl acetate were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Divinylbenzene (DVB, ≥98%) and azobisisobutyronitrile (AIBN, ≥98%) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Deionized ultrapure water was purified by a Purelab Ultra (Organo, Japan). All other reagents were used as obtained without any purification.
2.2 Instruments
FT-IR spectroscopy was performed on a Nexus-470 spectrophotometer (Thermo Nicolet, USA) with KBr pellets. Morphology was observed using SEM (S-4800, Hitachi, Japan) and TEM (IEM-200CX, JEOL, Japan). N2 adsorption–desorption measurements were made by a Quantachrome Autosorb-iQC volumetric instrument at 77 K. XRD analysis was carried out on a powder X-ray diffractometer (SmartLab, Rigaku, Japan). The elemental composition was determined by an elemental analyzer (FLASH1112A, CE, Italy) and Raman spectra were recorded with a laser Raman spectrometer (DXR, Thermo Fisher, USA).
2.3 Preparation of mesoporous polydivinylbenzene (meso-PDVB)
Meso-PDVB was prepared via a facile solvothermal route according to the previous literature.26 Typically, 2.5 mL DVB and 50 mg AIBN were dissolved in 15 mL ethyl acetate and vigorously stirred at room temperature for 0.5 h. The above solution was sealed in a Teflon-lined stainless-steel autoclave (25 mL capacity) and maintained at 100 °C for 24 h. After cooling to room temperature, the black products were washed with ethanol several times and dried at 60 °C for 12 h.
2.4 Preparation of 3D hierarchically porous carbon (3D HPC)
A 5.0 g sample of meso-PDVB was put into a porcelain boat and calcined in a tube furnace under a N2 atmosphere at 500 °C for 2.0 h at a heating ramp rate of 5.0 °C min−1 from room temperature. After cooling to room temperature, black meso-PDVB-derived char (meso-PDVBC) was obtained and the yield was about 49 wt%. Subsequently, 2.0 g meso-PDVBC was mixed with KOH in a weight ratio of 1/4 and activated at 850 °C for 1.0 h at a heating rate of 5.0 °C min−1 under a N2 atmosphere. The complex mixture was reacted with 1.0 M HCl solution in order to remove generated potassium compounds, rinsed with hot deionized water, and finally dried at 60 °C overnight to obtain the novel 3D HPC. The yield was about 24 wt%.
2.5 Batch adsorption experiments
In order to investigate the effects of the temperature and initial concentration on the adsorption kinetics, 3.0 mg 3D HPC was added to 10 mL MB solutions with initial concentrations of 50, 100 and 150 mg L−1, respectively. After the reaction was carried out for different times at three temperatures, 298, 308 and 318 K, the supernatant was obtained by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3.0 min. Meanwhile, the concentration of free MB was determined by a UV-vis spectrophotometer (UV 2450, Shimadzu, Japan) at a maximum absorbance wavelength of 664 nm, while the pH of these solutions was not adjusted. To study adsorption isotherms, 3.0 mg 3D HPC was added to 10 mL MB solutions with initial concentrations ranging from 50 to 300 mg L−1 at temperatures of 298, 308 and 318 K, respectively. In addition, the effect of the solution pH on the adsorption of MB was also tested by the addition of 3D HPC (3.0 mg) to 10 mL solutions of 200 mg L−1 MB with various initial pH values ranging from 2.0 to 12 at 298 K. To test its reusability, 3.0 mg 3D HPC was added to a 10 mL solution of 50 mg L−1 MB to reach saturated adsorption at 298 K and then MB adsorbed onto 3D HPC was desorbed by acetone at room temperature. The washed 3D HPC was used to adsorb free MB solution (50 mg L−1) to study its capacity at 298 K again. The adsorption–desorption cycle was performed under the same conditions eight times. Besides, the amount of MB adsorbed at equilibrium (Qe, mg g−1) and at time t (Qt, mg g−1) was calculated according to the difference between the initial and residual amounts of MB in the supernatant by the following equations:
000 rpm for 3.0 min. Meanwhile, the concentration of free MB was determined by a UV-vis spectrophotometer (UV 2450, Shimadzu, Japan) at a maximum absorbance wavelength of 664 nm, while the pH of these solutions was not adjusted. To study adsorption isotherms, 3.0 mg 3D HPC was added to 10 mL MB solutions with initial concentrations ranging from 50 to 300 mg L−1 at temperatures of 298, 308 and 318 K, respectively. In addition, the effect of the solution pH on the adsorption of MB was also tested by the addition of 3D HPC (3.0 mg) to 10 mL solutions of 200 mg L−1 MB with various initial pH values ranging from 2.0 to 12 at 298 K. To test its reusability, 3.0 mg 3D HPC was added to a 10 mL solution of 50 mg L−1 MB to reach saturated adsorption at 298 K and then MB adsorbed onto 3D HPC was desorbed by acetone at room temperature. The washed 3D HPC was used to adsorb free MB solution (50 mg L−1) to study its capacity at 298 K again. The adsorption–desorption cycle was performed under the same conditions eight times. Besides, the amount of MB adsorbed at equilibrium (Qe, mg g−1) and at time t (Qt, mg g−1) was calculated according to the difference between the initial and residual amounts of MB in the supernatant by the following equations:| |
 | (1) |
| |
 | (2) |
where Ce and Ct (mg L−1) are the dye concentrations at equilibrium and at time t (min), respectively, V (L) is the volume of the solution and m (mg) is the mass of the adsorbent.
3. Results and discussion
3.1 Structural characterization
SEM and TEM images of meso-PDVBC and 3D HPC are shown in Fig. 1 to give clear morphological and structural information. Meso-PDVBC possessed a three-dimensional foam-like structure with holes with a size of several microns (see Fig. 1a) and there were not too many obvious nanopores in the TEM image in Fig. 1b. During the conversion process from the mesoporous organic polymer to carbon, the framework shrank and was stacked closely and meanwhile gas bubbles were formed due to the release of a considerable amount of gases. For 3D HPC, as shown in Fig. 1c, the 3D foam-like structure still remained after KOH activation and the carbon skeleton clearly became thinner. It is obvious that numerous nanopores can be observed in the TEM image in Fig. 1d.
 |
| | Fig. 1 SEM and TEM images of meso-PDVBC (a and b) and 3D HPC (c and d). | |
Fig. 2 displays FT-IR spectra of meso-PDVB, meso-PDVBC and 3D HPC. As can be seen in the spectrum of meso-PDVB, the peaks at 1509, 1487 and 1448 cm−1 were ascribed to the stretching vibrations of benzene.27 The stretching vibration of vinyl groups occurred at 1603 cm−1. The peaks at 2924 and 2869 cm−1 originated from C–H symmetric and asymmetric stretching vibrations of methylene groups. The characteristic stretching vibrations of the C–H bonds of vinyl groups and benzene appeared at 3018 and 3054 cm−1, respectively.28,29 After carbonization at 500 °C, the intensity of the peaks for C–H bonds dramatically decreased, which indicated the removal of hydrogen element. In the spectrum of 3D HPC, there were some broad peaks mainly assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C bonds. The results from FT-IR were matched with those from elemental analysis, as listed in Table 1. The contents of both hydrogen and oxygen obviously decreased during the activation process.
C and C–C bonds. The results from FT-IR were matched with those from elemental analysis, as listed in Table 1. The contents of both hydrogen and oxygen obviously decreased during the activation process.
 |
| | Fig. 2 FT-IR spectra of meso-PDVB, meso-PDVBC and 3D HPC. | |
Table 1 Elemental analysis of meso-PDVB and 3D HPC
| Sample |
Elemental composition (wt%) |
| H |
C |
Oa |
| Oxygen was assessed by the difference. |
| meso-PDVB |
4.6915 |
80.0292 |
15.2793 |
| 3D HPC |
0.5781 |
96.9693 |
2.4526 |
Fig. 3a presents an XRD pattern of 3D HPC with two peaks at around 24.3° (002) and 43.2° (101), which correspond to hexagonal graphite and the honeycomb lattice in single-layer graphene, respectively. Moreover, Raman spectroscopy is a powerful technique that is widely used to test the degree of crystallization of carbons.30 For meso-PDVBC and 3D HPC, as shown in Fig. 3b, there were two characteristic peaks at 1348 cm−1 (D band) and 1590 cm−1 (G band), which were attributed to lattice defects of carbon and the E2g stretching vibration in sp2 carbon domains, respectively.31 The ratio of the intensity of the D band to that of the G band (ID/IG) can indicate the degree of graphitization of carbons.30 The values of ID/IG were 0.746 for meso-PDVBC and 0.953 for 3D HPC, respectively, which indicated a decrease in the degree of graphitization after the activation reaction and an increase in defects, which provided more active sites to enhance adsorption.
 |
| | Fig. 3 XRD pattern of 3D HPC (a) and Raman spectra of meso-PDVBC and 3D HPC (b). | |
The nitrogen adsorption–desorption isotherms and pore size distribution of 3D HPC are illustrated in Fig. 4. As can be observed in Fig. 4a, the adsorption isotherm of 3D HPC exhibited a combination of types I and IV, according to the IUPAC classification,32 which indicated a large amount of micropores and some small mesopores. The hysteresis loops, which belonged to type H4, were connected with slit pores and a wide range of micropores. The pore size distribution of 3D HPC (Fig. 4b) determined by a DFT method showed that the size of pores was mainly less than 2.0 nm and there were also some pores in the size range of 2.0–4.0 nm. The textural properties of 3D HPC are listed in Table 2. The very large BET surface area and total pore volume of 3D HPC were 3131.32 m2 g−1 and 1.462 cm3 g−1, respectively. According to a t-plot method, the surface area and pore volume of micropores in 3D HPC were 2923.14 m2 g−1 and 1.280 cm3 g−1, respectively. From the results of the analysis of the nitrogen adsorption–desorption isotherms, it was proved that this novel carbon architecture was a hierarchically porous structure with an average pore diameter of 1.868 nm and exhibited great potential for excellent performance in the adsorption of MB from the water phase.
 |
| | Fig. 4 Nitrogen adsorption–desorption isotherms (a) and pore size distribution (b) of 3D HPC. | |
Table 2 Textural properties of 3D HPCa
| Sample |
SBET (m2 g−1) |
Smicro (m2 g−1) |
Vtotal (cm3 g−1) |
Vmicro (cm3 g−1) |
Vmicro/Vtotal (%) |
daverage (nm) |
| SBET: Brunauer–Emmett–Teller surface area. Smicro: surface area of micropores. Vtotal: total pore volume. Vmicro: pore volume of micropores. daverage: BET average pore diameter. |
| 3D HPC |
3131.32 |
2923.14 |
1.462 |
1.280 |
87.55 |
1.868 |
3.2 Effect of solution pH on MB adsorption
The solution pH can affect the surface charge of 3D HPC and degree of ionization of MB molecules, which play an important role in the adsorption of dyes. Fig. 5 shows the effect of the solution pH on the adsorption of MB onto 3D HPC at 298 K with a dye concentration of 200 mg L−1. MB is a typical cationic dye in its molecular structure, as observed in the inset illustration. The uptake amounts of MB dye changed significantly over the pH range of 2.0–12. With an increase in the solution pH, the equilibrium adsorbed amount of MB gradually increased. At a lower pH, abundant protons in solution could competitively occupy the active sites of 3D HPC, which resulted in a decrease in the adsorption of MB. As the solution pH increased, 3D HPC with more negative charge could adsorb more MB molecules by electrostatic attraction. Similar adsorption behavior has also been observed in the previous literature.33–35 However, the quantities of MB that were adsorbed at pH values of 2 and 12 were 471.21 and 487.42 mg g−1, respectively, with a small difference in value, which indicated that electrostatic attraction was not the dominant adsorption mechanism.
 |
| | Fig. 5 Effect of solution pH on the adsorption of MB onto 3D HPC and chemical structure of MB (inset). | |
3.3 Adsorption kinetics
The effects of the contact time, initial concentration and reaction temperature on the adsorption of MB onto 3D PHC from aqueous solutions were investigated and are shown in Fig. 6. The adsorption of MB occurred quickly at the beginning due to the rich empty pore structure, and the filled amount of MB increased obviously with an increase in the contact time. However, the speed of adsorption was reduced when the reaction approached equilibrium, because there were few empty sites waiting for free MB and MB adsorbed onto 3D PHC could repel free dye molecules via charge repulsion. It is also observed that an increase in the initial concentration obviously enhanced adsorption. For example, at 298 K the adsorption increased from 165.78 mg g−1 for 50 mg L−1 and 329.63 mg g−1 for 100 mg L−1 to 477.71 mg g−1 for 150 mg L−1. A higher initial concentration provides a larger driving force to overcome resistance to the mass transfer of dye molecules from the aqueous phase to the solid phase. In addition, an increase in the contact temperature favored adsorption, which suggested that the adsorption of the dye by 3D HPC was an endothermic reaction.
 |
| | Fig. 6 Effects of contact time and initial dye concentration on the adsorption of MB onto 3D HPC at 298 (a), 308 (b) and 318 K (c). | |
In order to understand the character and mechanism of the adsorption process between MB dye and 3D HPC in the aqueous phase, pseudo-first-order and pseudo-second-order kinetic models were used to simulate the adsorption kinetics in this research. The linear equations of the pseudo-first-order and pseudo-second-order models were as follows, respectively:36
| |
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (3) |
| |
 | (4) |
where
k1 (min
−1) and
k2 (g mg
−1 min
−1) are the adsorption rate constants of the pseudo-first-order and pseudo-second-order models, respectively.
The linear plots of two kinetics models for the adsorption of MB are presented in Fig. 7 and the fitting parameters are listed in Table 3. All the values of R2 (>0.999) for the pseudo-second-order model were higher than those for the pseudo-first-order model under the same initial conditions and the values of Qe,cal from the pseudo-second-order model were near to the values of Qe,exp, which indicated that the pseudo-second-order model was more suitable for describing the real adsorption process. From the values of k2 in Table 3, at the same initial dye concentration an increase in temperature could accelerate the molecular dynamics and increase the opportunity for collisions between dye molecules and the novel 3D HPC adsorbent, resulting in an increase in the adsorption rate. Also, at the same temperature, a higher dye concentration required a longer time to achieve adsorption equilibrium, thus decreasing the kinetic constant k2, as clearly observed in Table 3.
 |
| | Fig. 7 Linear fitting kinetics curves for the adsorption of MB onto 3D HPC by pseudo-first-order (a, c, and e) and pseudo-second-order (b, d, and f) rate models with various initial dye concentrations at 298 (a and b), 308 (c and d) and 318 K (e and f). | |
Table 3 Kinetic constants for the adsorption of MB onto 3D HPC
| Model |
Pseudo-first-order |
Pseudo-second-order |
| T (K) |
C0 (mg L−1) |
Qe,exp (mg g−1) |
Qe,cal (mg g−1) |
k1 (min−1) |
R2 |
Qe,cal (mg g−1) |
k2 × 1000 (g mg−1 min−1) |
R2 |
| 298 |
50 |
166.14 |
80.68 |
0.0073 |
0.9560 |
170.09 |
0.3457 |
0.9998 |
| 100 |
329.64 |
346.69 |
0.0127 |
0.9013 |
340.90 |
0.1068 |
0.9995 |
| 150 |
477.72 |
556.40 |
0.0141 |
0.8897 |
493.61 |
0.0771 |
0.9998 |
| 308 |
50 |
166.25 |
93.42 |
0.0114 |
0.9572 |
169.90 |
0.3464 |
0.9999 |
| 100 |
332.74 |
269.14 |
0.0121 |
0.9231 |
341.44 |
0.1414 |
0.9997 |
| 150 |
492.25 |
289.15 |
0.0087 |
0.9542 |
505.33 |
0.0916 |
0.9998 |
| 318 |
50 |
166.61 |
85.55 |
0.0119 |
0.8994 |
170.09 |
0.3485 |
0.9999 |
| 100 |
333.27 |
222.37 |
0.0126 |
0.9581 |
341.94 |
0.1616 |
0.9999 |
| 150 |
499.07 |
378.25 |
0.0114 |
0.9202 |
511.51 |
0.0969 |
0.9998 |
In addition, the intra-particle diffusion model that was proposed was used to identify the diffusion mechanism of the adsorption process and its linear equation is given as follows:37
where
kp (mg g
−1 min
−1/2) and
Ci (mg g
−1) are the diffusion rate constants.
Fitting curves of the intra-particle diffusion model for the adsorption of MB onto 3D HPC at different initial dye concentrations and reaction temperatures are shown in Fig. 8. If the value of Ci from the intra-particle diffusion model is zero, the adsorption rate is controlled by intra-particle diffusion during the whole adsorption process. It can be seen that all the curves display three linear stages, which indicates a multi-stage adsorption process.38 The first stage was governed by surface diffusion with the fastest diffusion rate, whereas the second stage was controlled by intra-particle diffusion and displayed a slower diffusion, and the diffusion rate was reduced gradually to zero at the third stage until equilibrium was reached. The diffusion constants of each portion for the adsorption of MB onto 3D HPC are listed in Table 4. The results show that the values of both kp1 and kp2 increased with an increase in the initial dye concentration, which was possibly due to the higher concentration gradient produced by the higher initial concentration. Therefore, both intra-particle and film diffusion mainly governed the adsorption process of MB.
 |
| | Fig. 8 Intra-particle diffusion plots of the adsorption of MB onto 3D HPC at 298 (a), 308 (b) and 318 K (c) at various initial dye concentrations. | |
Table 4 Intra-particle diffusion constants for the adsorption of MB onto 3D HPC
| T (K) |
C0 (mg L−1) |
k1 (mg g−1 min−1/2) |
C1 (mg g−1) |
R2 |
k2 (mg g−1 min−1/2) |
C2 (mg g−1) |
R2 |
C3 (mg g−1) |
R2 |
| 298 |
50 |
10.54 |
34.15 |
0.9446 |
1.641 |
126.9 |
0.9443 |
165.8 |
1.000 |
| 100 |
20.41 |
50.85 |
0.9612 |
4.708 |
218.9 |
0.9604 |
329.6 |
1.000 |
| 150 |
41.73 |
18.30 |
0.9788 |
8.251 |
289.7 |
0.9074 |
477.7 |
1.000 |
| 308 |
50 |
14.17 |
24.37 |
0.9656 |
2.432 |
115.8 |
0.9295 |
166.2 |
1.000 |
| 100 |
28.24 |
36.36 |
0.9572 |
4.656 |
226.2 |
0.9367 |
332.7 |
1.000 |
| 150 |
42.44 |
44.77 |
0.9794 |
7.336 |
324.8 |
0.9324 |
491.9 |
1.000 |
| 318 |
50 |
14.66 |
23.39 |
0.9522 |
2.219 |
119.8 |
0.9057 |
166.6 |
1.000 |
| 100 |
29.29 |
30.11 |
0.9728 |
5.556 |
220.5 |
0.8733 |
333.3 |
1.000 |
| 150 |
41.97 |
59.41 |
0.9684 |
7.151 |
335.8 |
0.9243 |
499.0 |
1.000 |
3.4 Adsorption isotherms
An adsorption isotherm is commonly used to describe experimental data to understand the essence of the interaction between the adsorbate and adsorbent comprehensively. As shown in Fig. 9, the adsorption capacity of MB onto 3D HPC increased with an increase in the initial concentration and gradually reached a maximum value. Also, an increase in temperature enhanced the adsorption ability, which matched the results from adsorption kinetics in Section 3.3. The maximum equilibrium adsorbed quantities of MB were 569.91, 657.25 and 717.91 mg g−1 at 298, 308 and 318 K, respectively, which indicated excellent adsorption affinity. Furthermore, to optimize the adsorption characteristics, Langmuir and Freundlich isotherm models in nonlinear forms were used to simulate the adsorption of MB onto 3D HPC from an aqueous solution.
 |
| | Fig. 9 Isotherm fitting curves of the adsorption of MB onto 3D HPC by the Langmuir and Freundlich models at three different temperatures. | |
The Langmuir isotherm is a classical model where the experimental process is regarded as an idealized monolayer adsorption with no other intermolecular interaction, the nonlinear form of which is defined as follows:39
| |
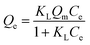 | (6) |
where
Qm (mg g
−1) is the maximum monolayer adsorption capacity and
KL (L mg
−1) is the Langmuir constant.
Moreover, the essential characteristic of the Langmuir isotherm, the separation factor (RL), can be expressed by a dimensionless constant, which is usually written as follows:40
| |
 | (7) |
where
C0 (mg L
−1) is the initial dye concentration in solution. The value of
RL shows the shape of the isotherm as follows: when
RL > 1, unfavorable; when
RL = 1, linear; when 0 <
RL < 1, favorable; when
RL = 0, irreversible.
The Freundlich isotherm is used as an empirical equation to describe the adsorption process when it is heterogeneous and its isotherm equation is written as follows:41
where
n and
KF ((mg g
−1) (L mg
−1)
1/n) are the Freundlich isotherm constants.
Langmuir and Freundlich isotherm fitting curves for the adsorption of MB onto 3D HPC at different temperatures are shown in Fig. 9 and the detailed isotherm parameters are presented in Table 5. As can be seen, higher correlation coefficients (≥0.9997) were obtained by fitting the experimental data with the Langmuir model as compared with the Freundlich isotherm model (≥0.7812). Moreover, the fitting curves for Qe calculated according to the Langmuir model agreed well with the experimental values of Qe,exp, which suggested that the Langmuir model could describe the adsorption data more suitably. Therefore, 3D HPC possessed a homogeneous surface and monolayer adsorption played a dominant role in adsorbing MB. Also, the values of RL that were calculated were in the range between 0 and 1, which indicated that the designed experimental conditions were favorable for the adsorption of MB by 3D HPC at the three temperatures. Furthermore, with an increase in the initial concentration of MB from 50 to 300 mg L−1, the values of RL for MB decreased from 0.0161 to 0.0027 at 298 K, from 0.0104 to 0.0018 at 308 K, and from 0.0061 to 0.0010 at 318 K, respectively: see Fig. 10. This phenomenon suggested that the adsorption process could be more favorable at a high initial concentration and contact temperature. From Table 6, we could also find that the maximum monolayer adsorption capacities (Qm) of MB from aqueous solutions were 574.42, 662.42 and 717.77 mg g−1 at 298, 308 and 318 K, respectively. As compared with other adsorbents listed in Table 6,7,11,42–48 our synthesized 3D HPC exhibited a very high adsorption capacity for MB from aqueous solutions, due to its large specific surface area and pore volume, which suggested that 3D HPC has remarkable potential for applications in the treatment of dyes in wastewater.
Table 5 Isotherm model parameters for the adsorption of MB onto 3D HPC
| Model |
Langmuir |
Freundlich |
| T (K) |
Qm (mg g−1) |
KL (L mg−1) |
R2 |
KF ((mg g−1) (L mg−1)1/n) |
1/n |
R2 |
| 298 |
574.42 |
1.222 |
0.9999 |
293.32 |
0.1783 |
0.8173 |
| 308 |
662.42 |
1.899 |
0.9999 |
365.79 |
0.1706 |
0.7812 |
| 318 |
717.77 |
2.797 |
0.9997 |
483.30 |
0.1201 |
0.9057 |
 |
| | Fig. 10 Effect of the initial concentration of MB on the value of RL of the Langmuir isotherm. | |
Table 6 Comparison of adsorption capacities of MB onto various adsorbents
| Adsorbent |
Qm (mg g−1) |
SBET (m2 g−1) |
T (K) |
Reference |
| Bone charcoal |
5.0 |
100 |
298 |
7 |
| Rice husk activated carbon |
9.83 |
180.50 |
303 |
11 |
| GNS/Fe3O4 |
43.82 |
— |
298 |
42 |
| CoFe2O4/AC |
100 |
1096.85 |
293 |
43 |
| Graphene nanosheet |
111.6 |
— |
293 |
44 |
| Mn/MCM-41 |
131.6 |
921 |
298 |
45 |
| Fe/ordered mesoporous carbon |
316 |
731 |
298 |
46 |
| P-CSCNT-4 |
319.1 |
558.7 |
293 |
47 |
| h-XG/SiO2 |
497.5 |
398.0 |
323 |
48 |
| 3D HPC |
574.42 |
3131.32 |
298 |
This work |
| 662.42 |
308 |
| 717.77 |
318 |
3.5 Thermodynamics analysis
To study the behavior and mechanism of adsorption, it is important to analyze its thermodynamic properties. Here, the effect of the reaction temperature on the adsorption of MB onto 3D HPC was tested and a series of related thermodynamic parameters including the enthalpy (ΔHθ), changes in entropy (ΔSθ) and Gibbs free energy (ΔGθ) were calculated. The value of ΔGθ (kJ mol−1) can be calculated as follows:49| |
ΔGθ = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd Kd
| (9) |
where R (8.3145 J mol−1 K−1) is the gas constant, T (K) is the temperature, Kd is the distribution coefficient (Kd = Qs/Cs), Qs (mg g−1) is the amount of MB adsorbed per gram of 3D HPC and Cs (mg L−1) is the equilibrium dye concentration. Moreover, the values of ΔHθ (kJ mol−1) and ΔSθ (J mol−1 K−1) can be calculated as follows:| |
 | (10) |
Fig. 11 presents a van't Hoff plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T and the thermodynamic parameters are listed in Table 7. As can be seen, the values of ΔHθ and ΔSθ for the adsorption of MB were 7.299 kJ mol−1 and 42.93 J mol−1 K−1, respectively, and the values of ΔGθ at the three different temperatures were all negative, which proves the endothermic and spontaneous nature of the adsorption process.
Kd vs. 1/T and the thermodynamic parameters are listed in Table 7. As can be seen, the values of ΔHθ and ΔSθ for the adsorption of MB were 7.299 kJ mol−1 and 42.93 J mol−1 K−1, respectively, and the values of ΔGθ at the three different temperatures were all negative, which proves the endothermic and spontaneous nature of the adsorption process.
 |
| | Fig. 11 van't Hoff plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T (initial MB concentrations: 50, 100 and 150 mg L−1). Kd vs. 1/T (initial MB concentrations: 50, 100 and 150 mg L−1). | |
Table 7 Thermodynamic parameters for the adsorption of MB onto 3D HPC
| T (K) |
Kd |
ΔGθ (kJ mol−1) |
ΔHθ (kJ mol−1) |
ΔSθ (J mol−1 K−1) |
| 298 |
9.219 |
−5.493 |
7.299 |
42.93 |
| 308 |
10.01 |
−5.922 |
| 318 |
11.10 |
−6.351 |
3.6 Adsorption mechanism
Generally, various factors, such as the physicochemical properties of carbon (e.g., surface properties, structure and pore size) and the mass transfer process, can greatly affect the adsorption mechanism of dyes. The graphene surface of 3D HPC possessed a high van der Waals index,50 which was proved by the result of XRD and Raman spectroscopy, and MB is a planar cyclic molecule. Therefore, strong van der Waals forces were likely to occur between MB and the graphene surface of 3D HPC. Moreover, MB could thus be easily adsorbed onto 3D HPC via π–π stacking interactions between the aromatic structure of the MB dye and the hexagonal skeleton of 3D HPC. However, interactions other than van der Waals forces and π–π stacking interactions could also contribute to adsorption. It has been demonstrated that MB, which is positively charged, could be adsorbed onto the negatively charged surface of 3D HPC, which suggests that electrostatic attraction was partially responsible for the adsorption of MB onto this novel carbon adsorbent.
3.7 Reusability study
The reusability of adsorbents is very important for the practical treatment of wastewater. To test its reusability, an identical batch of 3D HPC was used in adsorption–desorption for eight cycles, as described in Section 2.5 in detail, and the results are presented in Fig. 12. As can be observed obviously, the adsorption of MB dye decreased a little after eight cycles from an initial value of 166.14 mg g−1 to 156.31 mg g−1, which suggested that 3D HPC possessed excellent regeneration capacity and high chemical stability, with great potential for applications in water treatment.
 |
| | Fig. 12 Reusability of 3D HPC toward MB. | |
4. Conclusions
In summary, a novel 3D HPC adsorbent material, with a large specific surface area of 3131.32 m2 g−1 and a total pore volume of 1.462 cm3 g−1, was fabricated successfully via a two-step precarbonization and in situ alkali activation method, using solvothermally synthesized meso-PDVB as a carbon source. Batch experiments on the adsorption of MB onto 3D HPC from aqueous solutions suggested that the adsorption capacity for MB increased with increasing initial concentration, contact time, temperature and solution pH. The adsorption process was endothermic and spontaneous and the isotherm and kinetics data were well described by a Langmuir model and a pseudo-second-order kinetic model, respectively. The maximum adsorption capacity of 3D HPC reached 717.77 mg g−1 at 308 K. The adsorption rate was mainly controlled by film and intra-particle diffusion. The remarkably strong adsorption of MB onto 3D HPC could be attributed to strong adsorptive interactions, including van der Waals forces, π–π stacking interactions and electrostatic attraction. In addition, 3D HPC exhibited excellent regeneration performance, with promising potential for the treatment of dyes in wastewater and other organic contaminants.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 21176107, 21174057, 21277063, 21446015 and U1407123), the National Basic Research Program of China (973 Program, 2012CB821500), Natural Science Foundation of Jiangsu Province (BK20140534), PhD. Innovation Programs Foundation of Jiangsu Province (No. CXZZ13_0668), Research Fund for the Doctoral Program of Higher Education of China (20133227110022 and 20133227110010) and Jiangsu Planned Projects for Postdoctoral Research Funds (1102119C).
References
- B. Kayranli, Adsorption of textile dyes onto iron based waterworks sludge from aqueous solution; isotherm, kinetic and thermodynamic study, Chem. Eng. J., 2011, 173, 782–791 CrossRef CAS.
- B. K. Nandi, A. Goswami and M. K. Purkait, Removal of cationic dyes from aqueous solutions by kaolin: kinetic and equilibrium studies, Appl. Clay Sci., 2009, 42, 583–590 CrossRef CAS.
- T. Akar and M. Divriklioglu, Biosorption application of modified fungal biomass for decolourization of reactive red 2 contaminated solutions: batch and dynamic flow model studies, Bioresour. Technol., 2010, 101, 7271–7277 CrossRef CAS PubMed.
- W. Somasiri, X. Li, W. Ruan and C. Jian, Evolution of efficacy of upflow anaerobic sludge blanket reactor in removal of colour and reduction of COD in real textile wastewater, Bioresour. Technol., 2008, 99, 3692–3699 CrossRef CAS PubMed.
- P. Nigam, G. Armour, I. Banat, D. Singh and R. Marchant, Physical removal of tex-tile dyes and solid state fermentation of dye adsorbed agricultural residues, Bioresour. Technol., 2000, 272, 219–226 CrossRef.
- L. Liu, Z. Y. Gao, X. P. Su, X. Chen, L. Jiang and J. M. Yao, Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent, ACS Sustainable Chem. Eng., 2015, 3, 432–442 CrossRef CAS.
- G. Ghanizadeh and G. Asgari, Adsorption kinetics and isotherm of methylene blue and its removal from aqueous solution using bone charcoal, React. Kinet., Mech. Catal., 2011, 102, 127–142 CrossRef CAS.
- B. H. Hameed, A. T. M. Din and A. L. Ahmad, Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies, J. Hazard. Mater., 2007, 141, 819–825 CrossRef CAS PubMed.
- J. Y. Song, W. H. Zou, Y. Y. B., F. Y. Su and R. P. Han, Adsorption characteristics of methylene blue by peanut husk in batch and column modes, Desalination, 2011, 265, 119–125 CrossRef CAS.
- M. Feng, W. You, Q. Chen and H. Zhan, Mildly Alkaline Preparation and Methylene Blue Adsorption Capacity of Hierarchical Flower-like Sodium Titanate, ACS Appl. Mater. Interfaces, 2013, 5, 12654–12662 CAS.
- C. Yogesh Sharma and Uma, Optimization of parameters for adsorption of methylene blue on a low-cost activated carbon, J. Chem. Eng. Data, 2010, 55, 435–439 CrossRef.
- Y. Xu, R. Lebrun, P. J. Gallo and P. Blond, Treatment of textile dye plant effluent by nano-filtration membrane, Sep. Sci. Technol., 1999, 34, 2501–2519 CrossRef CAS.
- T. Bechtold, E. Burtscher and A. Turcanu, Cathodic decolorisation of textile wastewater containing reactive dyes using multicathode electrolyser, J. Chem. Technol. Biotechnol., 2001, 76, 303–311 CrossRef CAS.
- M. Basibuyuk, T. Yilmaz, B. Kayranli, A. Yuceer and C. F. Forster, The use of water-works sludge for the treatment of dye wastes, Environ. Technol., 2002, 23, 345–351 CrossRef CAS PubMed.
- F. Xia, E. Ou, L. Wang and J. Wang, Photocatalytic degradation of dyes over cobalt doped mesoporous SBA-15 under sunlight, Dyes Pigm., 2008, 76, 76–81 CrossRef.
- N. K. Amin, Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: adsorption equilibrium and kinetics, J. Hazard. Mater., 2009, 165, 52–62 CrossRef CAS PubMed.
- Z. Belala, M. Jeguirim, M. Belhachemi, F. Addoun and G. Trouvé, Biosorption of basic dye from aqueous solutions by Date Stones and Palm-Trees Waste: kinetic, equilibrium and thermodynamic studies, Desalination, 2011, 271, 80–87 CrossRef CAS.
- J. D. Dai, J. S. He, A. T. Xie, L. Gao, J. M. Pan, X. Chen, Z. P. Zhou, X. Wei and Y. S. Yan, Novel Pitaya-Inspired Well-Defined Core-Shell Nanospheres with Ultrathin Surface Imprinted Nanofilm from Magnetic Mesoporous Nanosilica for Highly Efficient Chloramphenicol Removal, Chem. Eng. J., 2016, 284, 812–822 CrossRef CAS.
- J. D. Dai, J. M. Pan, L. C. Xu, X. X. Li, Z. P. Zhou, R. X. Zhang and Y. S. Yan, Preparation of molecularly imprinted nanoparticles with superparamagnetic susceptibility through atom transfer radical emulsion polymerization for the selective recognition of tetracycline from aqueous medium, J. Hazard. Mater., 2012, 205–206, 179–188 CrossRef CAS PubMed.
- M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Dye and its removal from aqueous solution by adsorption: A review, Adv. Colloid Interface Sci., 2014, 209, 172–184 CrossRef CAS PubMed.
- C. A. P. Almeida, N. A. Debacher, A. J. Downs, L. Cottet and C. A. D. Mello, Removal of methylene blue from colored effluents by adsorption on montmorillonite clay, J. Colloid Interface Sci., 2009, 332, 46–53 CrossRef CAS PubMed.
- V. O. Njoku, K. Y. Foo, M. Asif and B. H. Hameed, Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption, Chem. Eng. J., 2014, 250, 198–204 CrossRef CAS.
- G. McKay, A. Mesdaghinia, S. Nasseri, M. Hadi and M. S. Aminabad, Optimum isotherms of dyes sorption by activated carbon: fractional theoretical capacity & error analysis, Chem. Eng. J., 2014, 251, 236–247 CrossRef CAS.
- J. Gong, J. Liu, X. C. Chen, Z. W. Jiang, X. Wen, E. Mijowskac and T. Tang, Converting real-world mixed waste plastics into porous carbon nanosheets with excellent performance in the adsorption of an organic dye from wastewater, J. Mater. Chem. A, 2015, 3, 341–351 CAS.
- J. Wei, D. D. Zhou, Z. K. Sun, Y. H. Deng, Y. Y. Xia and D. Y. Zhao, A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 capture and supercapacitors, Adv. Funct. Mater., 2013, 23, 2322–2328 CrossRef CAS.
- F. J. Liu, W. P. Kong, C. Z. Qi, L. F. Zhu and F. S. Xiao, Design and synthesis of mesoporous polymer-based solid acid catalysts with excellent hydrophobicity and extraordinary catalytic activity, ACS Catal., 2012, 2, 565–572 CrossRef CAS.
- L. R. Wen, M. Li and G. L. Zhao, Application of Low-Temperature Decomposition in Identification of Plastic Products by FTIR Spectra, Chin. J. Spectrosc. Lab., 2004, 2, 244–247 Search PubMed.
- H. Y. Shen, Y. Zhu, X. E. Wen and Y. M. Zhuang, Preparation of Fe3O4-C18 nano-magnetic composite materials and their cleanup properties for organophosphorous pesticides, Anal. Bioanal. Chem., 2007, 387, 2227–2237 CrossRef CAS PubMed.
- S. F. Lim, Y. M. Zheng, S. W. Zou and J. P. Chen, Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study, Environ. Sci. Technol., 2008, 42, 2551–2556 CrossRef CAS PubMed.
- C. X. Gui, Q. Q. Wang, S. M. Hao, J. Qu, P. P. Huang, C. Y. Cao, W. G. Song and Z. Z. Yu, Sandwichlike magnesium silicate/reduced graphene oxide nanocomposite for enhanced Pb2+ and methylene blue adsorption, ACS Appl. Mater. Interfaces, 2014, 6, 14653–14659 CAS.
- D. Geng, Y. Chen, Y. Chen, Y. Li, R. Li, X. Sun, S. Ye and S. Knights, High oxygen-reduction activity and durability of nitrogen-doped graphene, Energy Environ. Sci., 2011, 4, 760–764 CAS.
- S. Brunauer, L. S. Deming, W. E. Deming and E. Teller, On a theory of the van der Waals adsorption of gases, J. Am. Chem. Soc., 1940, 62, 1723–1732 CrossRef CAS.
- B. Royer, N. F. Cardoso, E. C. Lima, J. C. P. Vaghetti, N. M. Simon, T. Calvete and R. C. Veses, Applications of Brazilian pine-fruit shell in natural and carbonized forms as adsorbents to removal of methylene blue from aqueous solutions: kinetics and equilibrium study, J. Hazard. Mater., 2009, 164, 1213–1222 CrossRef PubMed.
- K. Y. Foo and B. H. Hameed, Preparation of oil palm (Elaeis) empty fruit bunch activated carbon by microwave-assisted KOH activation for the adsorption of methylene blue, Desalination, 2011, 275, 302–305 CrossRef CAS.
- J. Ma, F. Yu, L. Zhou, L. Jin, M. X. Yuan, J. S. Luan, H. B. Fan, Z. W. Yuan and J. H. Chen, Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes, ACS Appl. Mater. Interfaces, 2012, 4, 5749–5760 CAS.
- S. H. Chen, Q. Y. Yue, B. Y. Gao and X. Yu, Equilibrium and kinetic adsorption study of the adsorptive removal of Cr(VI) using modified wheat residue, J. Colloid Interface Sci., 2010, 349, 256–264 CrossRef CAS PubMed.
- P. Luo, Y. F. Zhao, B. Zhang, J. D. Liu, Y. Yang and J. F. Liu, Study on the adsorption of neutral red from aqueous solution onto halloysite nanotubes, Water Res., 2010, 44, 1489–1497 CrossRef CAS PubMed.
- W. Y. Huang, D. Li, Z. Q. Liu, Q. Tao, Y. Zhu, J. Yang and Y. M. Zhang, Kinetics, isotherm, thermodynamic, and adsorption mechanism studies of La(OH)3-modified exfoliated vermiculites as highly efficient phosphate adsorbents, Chem. Eng. J., 2014, 236, 191–201 CrossRef CAS.
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- T. W. Weber and R. K. Chakkravorti, Pore and solid diffusionmodels for fixed-bed adsorbers, AIChE J., 1974, 20, 228–238 CrossRef CAS.
- H. M. F. Freundlich, Over the adsorption in solution, J. Phys. Chem., 1906, 57, 385–470 CAS.
- L. H. Ai, C. Y. Zhang and Z. L. Chen, Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite, J. Hazard. Mater., 2011, 192, 1515–1524 CrossRef CAS PubMed.
- J. C. Xu, P. H. Xin, Y. Q. Gao, B. Hong, H. X. Jin, D. F. Jin, X. L. Peng, J. Li, J. Gong, H. L. Ge and X. Q. Wang, Magnetic properties and methylene blue adsorptive performance of CoFe2O4/activated carbon nanocomposites, Mater. Chem. Phys., 2014, 147, 915–919 CrossRef CAS.
- W. Fan, W. Gao, C. Zhang, W. T. Wen, J. S. Pan and T. X. Liu, Hybridization of graphene sheets and carbon-coated Fe3O4 nanoparticles as a synergistic adsorbent of organic dyes, J. Mater. Chem., 2012, 22, 25108–25115 RSC.
- Y. M. Shao, X. Wang, Y. Kang, Y. H. Shu, Q. Q. Sun and L. S. Li, Application of Mn/MCM-41 as an adsorbent to remove methyl blue from aqueous solution, J. Colloid Interface Sci., 2014, 429, 25–33 CrossRef CAS PubMed.
- X. M. Peng, D. P. Huang, T. Odoom-Wubah, D. F. Fu, J. L. Huang and Q. D. Qin, Adsorption of anionic and cationic dyes on ferromagnetic ordered mesoporous carbon from aqueous solution: Equilibrium, thermodynamic and kinetics, J. Colloid Interface Sci., 2014, 430, 272–282 CrossRef CAS PubMed.
- J. Gong, J. D. Feng, J. Liu, Z. W. Jiang, X. C. Chen, E. Mijowska, X. Wen and T. Tang, Catalytic carbonization of polypropylene into cup-stacked carbon nanotubes with high performances in adsorption of heavy metallic ions and organic dyes, Chem. Eng. J., 2014, 248, 27–40 CrossRef CAS.
- S. Ghorai, A. Sarkar, M. Raoufi, A. B. Panda, H. Schönherr and S. Pal, Enhanced removal of methylene blue and methyl violet dyes from aqueous solution using a nanocomposite of hydrolyzed polyacrylamide grafted xanthan gum and incorporated nanosilica, ACS Appl. Mater. Interfaces, 2014, 6, 4766–4777 CAS.
- A. Sari and M. Tuzen, Biosorption of Pb(II) and Cd(II) from aqueous solution using green alga (Ulva lactuca) biomass, J. Hazard. Mater., 2008, 152, 302–308 CrossRef CAS PubMed.
- L. L. Ji, W. Chen, L. Duan and D. Q. Zhu, Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite
as adsorbents, Environ. Sci. Technol., 2009, 43, 2322–2327 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3.0 min. Meanwhile, the concentration of free MB was determined by a UV-vis spectrophotometer (UV 2450, Shimadzu, Japan) at a maximum absorbance wavelength of 664 nm, while the pH of these solutions was not adjusted. To study adsorption isotherms, 3.0 mg 3D HPC was added to 10 mL MB solutions with initial concentrations ranging from 50 to 300 mg L−1 at temperatures of 298, 308 and 318 K, respectively. In addition, the effect of the solution pH on the adsorption of MB was also tested by the addition of 3D HPC (3.0 mg) to 10 mL solutions of 200 mg L−1 MB with various initial pH values ranging from 2.0 to 12 at 298 K. To test its reusability, 3.0 mg 3D HPC was added to a 10 mL solution of 50 mg L−1 MB to reach saturated adsorption at 298 K and then MB adsorbed onto 3D HPC was desorbed by acetone at room temperature. The washed 3D HPC was used to adsorb free MB solution (50 mg L−1) to study its capacity at 298 K again. The adsorption–desorption cycle was performed under the same conditions eight times. Besides, the amount of MB adsorbed at equilibrium (Qe, mg g−1) and at time t (Qt, mg g−1) was calculated according to the difference between the initial and residual amounts of MB in the supernatant by the following equations:
000 rpm for 3.0 min. Meanwhile, the concentration of free MB was determined by a UV-vis spectrophotometer (UV 2450, Shimadzu, Japan) at a maximum absorbance wavelength of 664 nm, while the pH of these solutions was not adjusted. To study adsorption isotherms, 3.0 mg 3D HPC was added to 10 mL MB solutions with initial concentrations ranging from 50 to 300 mg L−1 at temperatures of 298, 308 and 318 K, respectively. In addition, the effect of the solution pH on the adsorption of MB was also tested by the addition of 3D HPC (3.0 mg) to 10 mL solutions of 200 mg L−1 MB with various initial pH values ranging from 2.0 to 12 at 298 K. To test its reusability, 3.0 mg 3D HPC was added to a 10 mL solution of 50 mg L−1 MB to reach saturated adsorption at 298 K and then MB adsorbed onto 3D HPC was desorbed by acetone at room temperature. The washed 3D HPC was used to adsorb free MB solution (50 mg L−1) to study its capacity at 298 K again. The adsorption–desorption cycle was performed under the same conditions eight times. Besides, the amount of MB adsorbed at equilibrium (Qe, mg g−1) and at time t (Qt, mg g−1) was calculated according to the difference between the initial and residual amounts of MB in the supernatant by the following equations:

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C bonds. The results from FT-IR were matched with those from elemental analysis, as listed in Table 1. The contents of both hydrogen and oxygen obviously decreased during the activation process.
C and C–C bonds. The results from FT-IR were matched with those from elemental analysis, as listed in Table 1. The contents of both hydrogen and oxygen obviously decreased during the activation process.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t
Qe − k1t



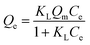

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd
Kd

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd vs. 1/T and the thermodynamic parameters are listed in Table 7. As can be seen, the values of ΔHθ and ΔSθ for the adsorption of MB were 7.299 kJ mol−1 and 42.93 J mol−1 K−1, respectively, and the values of ΔGθ at the three different temperatures were all negative, which proves the endothermic and spontaneous nature of the adsorption process.
Kd vs. 1/T and the thermodynamic parameters are listed in Table 7. As can be seen, the values of ΔHθ and ΔSθ for the adsorption of MB were 7.299 kJ mol−1 and 42.93 J mol−1 K−1, respectively, and the values of ΔGθ at the three different temperatures were all negative, which proves the endothermic and spontaneous nature of the adsorption process.







