NHC catalysed trimethylsilylation of terminal alkynes and indoles with Ruppert's reagent under solvent free conditions†
Panjab Arde,
Virsinha Reddy and
Ramasamy Vijaya Anand*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Mohali, Sector 81, Knowledge City, S.A.S. Nagar Manauli (PO), Mohali, Punjab – 140306, India. E-mail: rvijayan@iisermohali.ac.in; Tel: +91 172 2293190
First published on 29th September 2014
Abstract
An organo-catalytic protocol for the trimethylsilylation of terminal alkynes employing Ruppert's reagent (CF3SiMe3) as a trimethylsilyl source has been developed under solvent and fluoride free conditions. This method was found to be very effective as a variety of terminal alkynes bearing aliphatic or aromatic substituents underwent smooth transformation to their corresponding silylated products in excellent yields within a few minutes using N-heterocyclic carbene as an organo-catalyst. This methodology was also applied to the chemospecific N-silylation of indoles.
Alkynylsilicon reagents are considered to be valuable synthons due to their widespread applications in carbon–carbon bond forming reactions such as metal catalysed cross-coupling reactions,1 alkynylation reactions2 and metathesis reactions.3 Alkynylsilicon compounds also serve as versatile intermediates in the synthesis of some biologically important natural products.4 Apart from the traditional method for the preparation of alkynylsilicon compounds,5 which involves deprotonation of terminal acetylene with organolithium or Grignard reagent followed by quenching with a silyl electrophile, some other methods have also been developed which include metallic Zn6 or Zinc salts mediated7 and Zn(OTf)2 catalysed8 trimethylsilylation of terminal alkynes with suitable silyl electrophiles. Few iridium complexes are also known to catalyse silylation of terminal acetylenes.9 Another fascinating approach to alkynyl silyl compounds encompasses metal catalyzed dehydrogenative coupling of terminal alkynes with silyl hydrides.10 Apart from the use of conventional silylating agents, Ishizaki's group has demonstrated fluoride catalysed trimethylsilylation of terminal alkynes using an unconventional reagent, CF3SiMe3 (Ruppert's reagent), as a silyl source.11
Although most of the above mentioned methods show good functional group compatibility, limitations such as use of metal or a fluoride source and longer reaction times render these methods from practicality. Hence, development of an efficient, metal and fluoride free method for the preparation of alkynylsilyl compounds is highly desired.
Being an integral part of organocatalysis, N-heterocyclic carbene (NHC) catalysis is emerging as a powerful tool for carbon–carbon and carbon–heteroatom bond forming reactions.12,13 It is well documented in the literature that NHC forms a hypervalent complex with silicon compounds.14 This distinctive reactivity of NHC towards silicon has been explored in fundamental transformations such as cyanosilylation of carbonyl compounds and imines,15 aziridine ring opening16 and Mukaiyama aldol reactions.17 Recently, Song's group reported NHC catalysed trifluoromethylation of aldehydes and ketones,18 in which the CF3 anion was utilized as a nucleophile (eqn (1), Scheme 1). While developing NHC catalysed trifluoromethylation of various functional groups with CF3SiMe3, we envisioned that CF3 anion, generated in situ during the reaction of NHC with CF3SiMe3, could be used as a traceless base to deprotonate acetylenic proton and the resulting acetylide anion could be trapped with electrophilic silicon. Based on this idea, we have developed an efficient and alternative protocol for the preparation of alkynyl trimethylsilanes from terminal acetylenes and CF3SiMe3 (Ruppert's reagent)19 using NHC as a catalyst (eqn (2), Scheme 1).
The optimisation studies were performed using phenyl acetylene (1) and CF3SiMe3 (2). A wide range of NHCs derived from NHC precursors 4–9 were utilised for this purpose (Table 1). When the experiment was conducted using 2 mol% of NHC, derived from 5 and NaH in THF, and 2 as a silyl source the expected product 3 was obtained in 50% yield. Encouraged by this observation, further optimisation experiments were carried out under different conditions with or without solvent (Table 1).
| S. no | Catalyst | Base | Solvent | Time [min] | Yielda [%] |
|---|---|---|---|---|---|
| a Isolated yield; RT = 31–33 °C. | |||||
| 1 | 5 | NaH | THF | 20 | 50 |
| 2 | 5 | NaH | DCM | 20 | 76 |
| 3 | 5 | NaH | Et2O | 20 | 30 |
| 4 | 5 | NaH | — | 7 | 98 |
| 5 | 5 | DBU | — | 360 | 72 |
| 6 | 5 | — | — | 360 | — |
| 7 | 4 | NaH | — | 60 | 50 |
| 8 | 6 | NaH | — | 20 | 81 |
| 9 | 7 | NaH | — | 30 | 50 |
| 10 | 8 | NaH | — | 120 | 23 |
| 11 | 9 | NaH | — | 120 | 7 |
| 12 | — | NaH | — | 360 | Trace |
| 13 | 5 | NaNH2 | — | 20 | 85 |
| 14 | 5 | KOtBu | — | 15 | 92 |
Surprisingly, the reaction rate and the yield of the product 3 were found to be higher when the reaction was performed under solvent free conditions. Out of several conditions screened, the best result was obtained (98% yield in 7 min) using NHC derived from 5 under neat conditions, thus chosen as standard condition (Entry 4). No product or only traces of product was observed if the reaction was conducted without base (Entry 6) or without NHC (Entry 12, Table 1). We also tried the silylation of phenyl acetylene (1) with other silyl pro-nucleophiles (see ESI† for more information) including Et3SiH20 using 5 mol% of NHC derived from 5 as a catalyst under various conditions. Unfortunately, the desired silylated product was not observed in any of the cases.
To generalise this protocol, a variety of terminal alkynes bearing aromatic and aliphatic substituents were subjected to silylation reaction under optimised reaction conditions and the results are summarised in Table 2. It is apparent from Table 2 that, irrespective of the nature of the substituents, most alkynes gave the corresponding trimethylsilylated products in quantitative yields. Almost in all the cases, the reaction was completed within a few minutes. Phenyl acetylenes with electron-withdrawing substituents on the aromatic ring (10j–10m) reacted at relatively faster rates than the alkynes attached with electron rich aromatic groups (10a–10i). Alkynes substituted with aliphatic groups also underwent smooth conversion to the corresponding silylated alkynes (10n–10u) under the standard conditions. This method was found to be efficient for the trimethysilylation of alkynes bearing hetero-aromatic substituents as well (10v and 10w).
At this stage, our attention was shifted towards elucidating a suitable mechanism for this transformation. Careful monitoring of the reaction revealed that the reaction was exothermic and some gas evolution was taking place in the reaction vessel when CF3SiMe3 was added to NHC and phenyl acetylene. Since fluoroform is the only by-product possible in this methodology and also it is a gas, we presumed that the evolved gas was CHF3. To confirm this, the reaction mixture was analyzed by 19F NMR spectroscopy. The spectrum showed a peak at −78.6 ppm (please see ESI† for 19F spectrum), which clearly confirms the presence of CHF3 in the reaction mixture.21
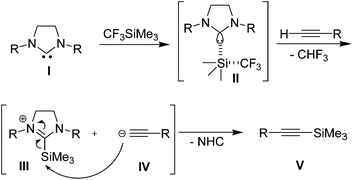
Based on the above experimental investigations, a plausible mechanism has been proposed (Scheme 2). We presume that NHC(I) reacts with CF3SiMe3 and generates a penta co-ordinated silicon complex II, which immediately deprotonates the terminal alkyne to give the intermediate III along with acetylide anion IV and CHF3. Nucleophillic attack of IV on the electrophillic silicon center of III gives the silylated product V with the expulsion of NHC (Scheme 2).
Although fluoroform is considered to be a potential green house gas,22 it has been widely employed as a trifluoromethyl source to introduce CF3 group in organic molecules.23,24 Since CHF3 is the by-product in our methodology, in order to make our process sustainable, we thought of utilising it for the regeneration of CF3SiMe3 using a protocol reported by Surya Prakash and co-workers.24 Scheme 3 portrays the experimental set up adapted for the synthesis of CF3SiMe3. The fluoroform (2.1 equiv.) generated during the reaction was purged in to another reaction flask containing KHMDS (1 equiv.) and Me3SiCl (1 equiv.) in toluene at −85 °C. The reaction proceeded smoothly and the product CF3SiMe3 was obtained in 58% yield. It was confirmed by 19F and 29Si NMR spectroscopy. The yield of the product was assigned based on 19F NMR using an internal standard (please refer ESI† for more details).
While developing the silylation of terminal acetylenes, we were also interested to utilise this methodology for N-silylation of indoles, which is considered to be an important transformation, because N-silylated indoles serve as prevalent intermediates for the synthesis of indole based natural products.25 Besides, chemoselective N-silylation of indoles still remains as a challenge. The conventional method for the preparation of N-silyl indoles involves deprotonation of indole using a bit excess of a strong base followed by treatment with Me3SiCl.26 The drawback associated with this method was the formation of equimolar quantities of salt and the requirement of strong base such as nBuLi or NaH. In order to over come these issues, few metal catalyzed dehydrogenative Si–N coupling methods have been recently developed.27 Hartwig's group reported silyl directed site selective borylation of N-silyl indoles, where N-silyl indoles were prepared in situ through Ruthenium catalyzed dehydrogenative silylation.28 Very recently, Oestreich group has also developed ruthenium catalysed dehydrogenative Si–N coupling of amines including indoles using hydrosilanes under base free conditions.29 But, so far, organo-catalytic version for the synthesis of N-silylated indoles is not reported in the literature. It is highly preferred to develop an organo-catalytic N-silylation of indoles to avoid the use of expensive or toxic metal catalysts.
Since we have demonstrated that the NHC–CF3SiMe3 combination was very effective for the silylation of terminal alkynes, we thought of utilising the same recipe for the N-silylation of indoles under solvent free conditions. To our surprise, when indole was treated with 2 equiv. of CF3SiMe3 and a NHC (2 mol%) derived from 5, the silylated product 12a was obtained in 93% isolated yield after 3 h under solvent free condition at room temperature (Entry 12a, Table 3). A couple of optimisation experiments were carried out using other NHCs, but the yield of the N-silylated indole was inferior when compared to the above mentioned condition (please refer ESI† for more details). To demostrate the scope of this methodology, a wide range of substituted indoles were subjected to N-silylation reaction under the above mentioned reaction conditions and the results are presented in Table 3.
| a CF3SiMe3 was added at 0 °C. |
|---|
 |
It is clear from Table 3 that, most the indoles tried underwent smooth conversion to their corresponding N-silylated products in moderate to good yields at room temperature. Surprisingly, 2- and 3-methyl substituted indoles (12b and 12c) reacted at faster rate when compared to indole. In the case of 5-bromo indole (12d), the product was obtained in moderate yield. The reaction also worked well in the case of pyrrole and the silylated product 12i was obtained in 76% yield. Unfortunately, 5-nitroindole failed to give the corresponding silylated product 12h even after 24 h. The silylation reaction of 5-nitroindole was even tried in the presence of solvents such as THF, DMF and 1,4-dioxane, but in all those cases the product 12h was not observed. We believe that the mechanism of N-silylation reaction is similar to silylation of acetylenes. A general observation in N-silylation reactions was that the reaction time was longer when compared to silylation of acetylenes. This could be due to the less nucleophilicity of indole anion (when compared with acetylide anion) towards reaction with NHC–silicon complex III (Scheme 2). This reaction was found to be chemo-specific as no C-3 or C-2 silylated products were observed in any of the cases.
In conclusion, an efficient and metal free process for the synthesis of trimethylsilyl acetylenes has been developed using NHC as a catalyst under solvent free conditions. We have shown that the by-product, fluoroform, can be effectively utilised for the regeneration of CF3SiMe3. We have also demonstrated the first organocatalytic N-silylation of indoles using NHC as a catalyst. High yield of the products, low catalyst loading (2 mol%), less reaction time and simple work-up procedure are the prominent features of this methodology.
Acknowledgements
The authors sincerely acknowledge the Department of Science and Technology (DST), New Delhi for financial support and IISER Mohali for providing infrastructure. PA and VR thank CSIR for a research fellowship. The NMR and HRMS facilities of IISER Mohali are gratefully acknowledged.References
- (a) Y. Hatanaka and T. Hiyama, J. Org. Chem., 1988, 53, 918–920 CrossRef CAS; (b) Y. Hatanaka and T. Hiyama, Tetrahedron Lett., 1990, 31, 2719–2722 CrossRef CAS; (c) Y. Hatanaka and T. Hiyama, Synlett, 1991, 845–853 CrossRef CAS PubMed; (d) Y. Nishihara, K. Ikegashira, A. Mori and T. Hiyama, Tetrahedron Lett., 1998, 39, 4075–4078 CrossRef CAS; (e) S. Chang, S. Yang and P. Lee, Tetrahedron Lett., 2001, 42, 4833–4835 CrossRef CAS; (f) Y. Nishihara, K. Ikegashira, K. Hirabayashi, J. Ando, A. Mori and T. Hiyama, J. Org. Chem., 2000, 65, 1780–1787 CrossRef CAS PubMed; (g) S. E. Denmark and S. A. Tymonko, J. Org. Chem., 2003, 68, 9151–9154 CrossRef CAS PubMed; (h) R. Severin, J. Reimer and S. Doye, J. Org. Chem., 2010, 75, 3518–3521 CrossRef CAS PubMed.
- (a) K. Utimoto, M. Tanaka, M. Kitai and H. Nozaki, Tetrahedron Lett., 1978, 19, 2301–2304 CrossRef; (b) T. H. Chan and I. Fleming, Synthesis, 1979, 761–786 CrossRef CAS; (c) M. Hayashi, A. Inubushi and T. Mukaiyama, Bull. Chem. Soc. Jpn., 1988, 61, 4037–4042 CrossRef CAS; (d) V. R. Chintareddy, H. Wadhwa and J. G. Verkade, J. Org. Chem., 2011, 76, 4482–4488 CrossRef CAS PubMed.
- (a) M. Kim, S. Park, S. V. Maifeld and D. Lee, J. Am. Chem. Soc., 2004, 126, 10242–10243 CrossRef CAS PubMed; (b) J. Wang, Y. Gurevich, M. Botoshansky and M. S. Eisen, J. Am. Chem. Soc., 2006, 128, 9350–9351 CrossRef CAS PubMed.
- (a) S. E. Denmark and S.-M. Yang, J. Am. Chem. Soc., 2004, 126, 12432–12440 CrossRef CAS PubMed; (b) B. M. Trost, M. U. Frederiksen, J. P. N. Pappilon, P. E. Harrington, S. Shin and B. T. Shireman, J. Am. Chem. Soc., 2005, 127, 3666–3667 CrossRef CAS PubMed; (c) D. Mujahidin and S. Doye, Eur. J. Org. Chem., 2005, 2689–2693 CrossRef CAS; (d) G. W. O'Neil and A. G. Phillips, J. Am. Chem. Soc., 2006, 128, 5340–5341 CrossRef PubMed.
- (a) T. E. Kedar, M. W. Miller and L. S. Hegedus, J. Org. Chem., 1996, 61, 6121–6126 CrossRef CAS PubMed; (b) Y. Tobe, N. Nakagawa, K. Naemura, T. Wakabayashi, T. Shida and Y. Achiba, J. Am. Chem. Soc., 1998, 120, 4544–4545 CrossRef CAS; (c) T. Shibata, K. Yamashita, T. Ohta and K. Soai, Tetrahedron, 2000, 56, 9259–9267 CrossRef CAS.
- (a) H. Sugita, Y. Hatanaka and T. Hiyama, Tetrahedron Lett., 1995, 36, 2769–2772 CrossRef CAS; (b) H. Sugita, Y. Hatanaka and T. Hiyama, Synlett, 1996, 637–639 CrossRef PubMed.
- (a) A. A. Andreev, V. V. Konshin, N. V. Komarov, M. Rubin, C. Brouwer and V. Gevorgyan, Org. Lett., 2004, 6, 421–424 CrossRef CAS PubMed; (b) H. Jiang and S. Zhu, Tetrahedron Lett., 2005, 46, 517–519 CrossRef CAS PubMed.
- R. J. Rahaim Jr and J. T. Shaw, J. Org. Chem., 2008, 73, 2912–2915 CrossRef PubMed.
- I. Kownacki, B. Marciniec, B. Dudziec and M. Kubicki, Organometallics, 2011, 30, 2539–2545 CrossRef CAS.
- (a) K. Takaki, M. Kurioka, T. Kamata, K. Takehira, Y. Makioka and Y. Fujiwara, J. Org. Chem., 1998, 63, 9265–9269 CrossRef CAS; (b) R. Shimuzu and T. Fuchikami, Tetrahedron Lett., 2000, 41, 907–910 CrossRef; (c) T. Tsuchimoto, M. Fujii, Y. Iketani and M. Sekine, Adv. Synth. Catal., 2012, 354, 2959–2964 CrossRef CAS; (d) K. Yamaguchi, Y. Wang, T. Oishi, Y. Kuroda and N. Mizuno, Angew. Chem., Int. Ed., 2013, 52, 5627–5630 CrossRef CAS PubMed.
- M. Ishizaki and O. Hoshino, Tetrahedron, 2000, 56, 8813–8819 CrossRef CAS.
- (a) For reviews, please see: D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed; (b) E. M. Phillips, A. Chan and K. A. Scheidt, Aldrichimica Acta, 2009, 42, 55–66 CAS; (c) J. L. Moore and T. Rovis, Top. Curr. Chem., 2009, 291, 77–144 CrossRef; (d) P. C. Chiang and J. W. Bode, TCIMAIL, 2011, 149, 2–17 Search PubMed; (e) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336–5346 RSC; (f) A. T. Biju, N. Kuhl and F. Glorius, Acc. Chem. Res., 2011, 44, 1182–1195 CrossRef CAS PubMed; (g) A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314–325 CrossRef CAS PubMed; (h) H. U. Vora, P. Wheeler and T. Rovis, Adv. Synth. Catal., 2012, 354, 1617–1639 CrossRef CAS PubMed; (i) X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC; (j) J. Izquierdo, G. E. Hutson, D. T. Cohen and K. A. Scheidt, Angew. Chem., Int. Ed., 2012, 51, 11686–11698 CrossRef CAS PubMed; (k) S. J. Ryan, L. Candish and D. W. Lupton, Chem. Soc. Rev., 2013, 42, 4906–4917 RSC; (l) D. J. Nelson and S. P. Nolan, Chem. Soc. Rev., 2013, 42, 6723–6753 RSC.
- (a) P. Arde, B. T. Ramanjaneyulu, V. Reddy, A. Saxena and R. V. Anand, Org. Biomol. Chem., 2012, 10, 848–851 RSC; (b) B. T. Ramanjaneyulu, V. Reddy, P. Arde, S. Mahesh and R. V. Anand, Chem.–Asian J, 2013, 8, 1489–1496 CrossRef CAS PubMed.
- (a) For X-ray structure of NHC–Si pentaco-ordinate complexes, please see: (i) T. Böttcher, B. S. Bassil, L. Zhechkov, T. Heine and G.-V. Röschenthaler, Chem. Sci., 2013, 4, 77–83 RSC (ii) T. Böttcher, S. Steinhauer, B. Neumann, H.-G. Stammler, G.-V. Röschenthaler and B. Hoge, Chem. Commun., 2014, 50, 6204–6206 RSC; (b) For reviews on NHC–Silicon complexes: (i) W. A. Herrmann and C. Köcher, Angew. Chem., Int. Ed. Engl., 1997, 36, 2162–2187 CrossRef PubMed (ii) M. J. Fuchter, Chem.–Eur. J., 2010, 16, 12286–12294 Search PubMed (iii) C. E. Willians, Organomet. Chem., 2010, 36, 1–28 Search PubMed; (c) For a review of hypervalent silicon complexes: R. R. Holmes, Chem. Rev., 1996, 96, 927–950 Search PubMed; (d) N. Khun, T. Kratz, D. Blaser and R. Boese, Chem. Ber., 1995, 128, 245–250 Search PubMed; (e) T. E. Reynolds, C. A. Stern and K. A. Scheidt, Org. Lett., 2007, 9, 2581–2584 Search PubMed; (f) J. Raynaud, A. Ciolino, A. Caceiredo, M. Destarac, F. Bonnette, T. Kato, Y. Gnanou and D. Taton, Angew. Chem., Int. Ed., 2008, 47, 5390–5393 Search PubMed; (g) Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. von R. Schleyer and G. H. Robinson, Science, 2008, 321, 1069–1071 Search PubMed; (h) J. Raynaud, Y. Gnanou and D. Taton, Macromolecules, 2009, 42, 5996–6005 Search PubMed; (i) J. Raynaud, N. Liu, Y. Gnanou and D. Taton, Macromolecules, 2010, 43, 8853–8861 Search PubMed.
- (a) J. J. Song, F. Gallou, J. T. Reeves, Z. Tan, N. K. Yee and C. H. Senanayake, J. Org. Chem., 2006, 71, 1273–1276 CrossRef CAS PubMed; (b) Y. Fukuda, Y. Maeda, S. Ishii, K. Kondo and T. Aoyama, Synthesis, 2006, 589–590 CAS; (c) Y. Suzuki, A. Bakar, K. Muramatsu and M. Sato, Tetrahedron, 2006, 62, 4227–4231 CrossRef CAS PubMed; (d) T. Kano, K. Sasaki, T. Konishi, H. Mii and K. Maruoka, Tetrahedron Lett., 2006, 47, 4615–4618 CrossRef CAS PubMed.
- J. Wu, X. Sun, S. Ye and W. Sun, Tetrahedron Lett., 2006, 47, 4813–4816 CrossRef CAS PubMed.
- J. J. Song, Z. Tan, J. T. Reeves, N. K. Yee and C. H. Senanayake, Org. Lett., 2007, 9, 1013–1016 CrossRef CAS PubMed.
- J. J. Song, Z. Tan, J. T. Reeves, F. Gallou, N. K. Yee and C. H. Senanayake, Org. Lett., 2005, 7, 2193–2196 CrossRef CAS PubMed.
- (a) I. Ruppert, K. Schlich and W. Volbach, Tetrahedron Lett., 1984, 25, 2195–2198 CrossRef CAS; (b) G. K. S. Prakash, R. Krishnamurti and G. A. Olah, J. Am. Chem. Soc., 1989, 111, 393–395 CrossRef CAS; (c) For a review, see: G. K. S. Prakash and A. K. Yudin, Chem. Rev., 1997, 97, 757–786 CrossRef CAS PubMed.
- For NHC catalysed dehydrogenative O-silylation of alcohols with Et3SiH, see: D. Gao and C. Cui, Chem.–Eur. J., 2013, 19, 11143–11147 CrossRef CAS PubMed.
- G. K. S. Prakash, P. V. Jog, P. T. D. Batamack, G. A. Olah, PCT Patent Appln. WO 2012/148772 A1, 2012.
- For a recent article: W. Han, Y. Chen, B. Jin and H. Liu, Greenhouse Gases: Sci. Technol., 2014, 4, 121–130 CrossRef CAS.
- (a) T. Shono, M. Ishifune, T. Okada and S. Kashimura, J. Org. Chem., 1991, 56, 2–4 CrossRef CAS; (b) J. Russell and N. Roques, Tetrahedron, 1998, 54, 13771–13782 CrossRef CAS; (c) B. R. Langlois and T. Billard, Synthesis, 2003, 2, 185–194 CrossRef PubMed; (d) A. Zanardi, M. A. Novikov, E. Martin, J. Benet-Buchholz and V. V. Grushin, J. Am. Chem. Soc., 2011, 133, 20901–20913 CrossRef CAS PubMed; (e) I. Popov, S. Lindeman and O. Daugulis, J. Am. Chem. Soc., 2011, 133, 9286–9289 CrossRef CAS PubMed; (f) P. Novák, A. Lishchynskyi and V. V. Grushin, Angew. Chem., Int. Ed., 2012, 51, 7767–7770 CrossRef PubMed; (g) P. Novák, A. Lishchynskyi and V. V. Grushin, J. Am. Chem. Soc., 2012, 134, 16167–16170 CrossRef PubMed.
- G. K. S. Prakash, P. V. Jog, P. T. D. Batamack and G. A. Olah, Science, 2012, 338, 1324–1327 CrossRef CAS PubMed.
- (a) A. B. Smith III and H. Cui, Org. Lett., 2003, 5, 587–590 CrossRef PubMed; (b) C.-G. Yang, G. Liu and B. Jiang, J. Org. Chem., 2002, 67, 9392–9396 CrossRef CAS PubMed; (c) M. Kim and E. Vedejs, J. Org. Chem., 2004, 69, 7262–7265 CrossRef CAS PubMed; (d) H.-J. Borschberg, Curr. Org. Chem., 2005, 9, 1465–1491 CrossRef CAS; (e) K. R. Buszek, N. Brown and D. Luo, Org. Lett., 2009, 11, 201–204 CrossRef CAS PubMed; (f) G.-Y. J. Im, S. M. Bronner, A. E. Goetz, R. S. Paton, P. H.-Y. Cheong, K. N. Houk and N. K. Garg, J. Am. Chem. Soc., 2010, 132, 17933–17944 CrossRef CAS PubMed.
- (a) R. J. Sundberg and H. F. Russell, J. Org. Chem., 1973, 38, 3324–3330 CrossRef CAS; (b) D. Dhanak and C. B. Reese, J. Chem. Soc., Perkin Trans. 1, 1986, 1, 2181–2186 RSC; (c) P. G. M. Wuts and T. W. Greene, Greene's Protective Groups in Organic Synthesis, Wiley, 4th edn, New York, 2007 Search PubMed.
- (a) T. Tsuchimoto, Y. Iketani and M. Sekine, Chem.–Eur. J., 2012, 18, 9500–9504 CrossRef CAS PubMed; (b) S. Itagaki, K. Kamata, K. Yamaguchi and N. Mizuno, Chem. Commun., 2012, 48, 9269–9271 RSC.
- D. W. Robbins, T. A. Boebel and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 4068–4069 CrossRef CAS PubMed.
- C. D. F. Königs, M. F. Müller, N. Aiguabella, F. T. Hendrick, H. F. T. Klare and M. Oestreich, Chem. Commun., 2013, 49, 1506–1508 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and copies of NMR spectra. See DOI: 10.1039/c4ra08727e |
| This journal is © The Royal Society of Chemistry 2014 |