Extending the structural landscape of Mo(vi) hydrazonato inorganic–organic POM-hybrids: an experimental and computational study†
Abstract
Five hexamolybdate and five octamolybdate-based organic–inorganic hybrid assemblies, with dioxomolybdenum(VI) hydrazonato complex cations, were synthesized from the [MoO2(acac)2] and isonicotinoyl hydrazone ligands bearing OH (H2L1,2) and OMe (H2L3,4) electron-donating groups. They can be classified into four categories including one known (i) [MoO2(HL1–4)(D)]2[Mo6O19]·xMeCN·yH2O, where D = H2O or MeCN, and three novel (ii) [Mo2O2(μ-O)2(HL3)2][Mo6O19], (iii) [MoO2(HL1,2)(H2O)]4[Mo8O26]·xMeCN·yH2O, and (iv) [MoO2(H2L2,4)(H2O)]2[Mo8O26]·xMeCN·yH2O, thus extending the structural landscape of Mo-complex–POM hybrids. Characterization of the hybrids, employing X-ray crystallography, IR-ATR, and TG analysis, was carried out. The effects of the reaction conditions and coordination nature of the Mo(VI) complex cations on the structure of the hybrid compounds were investigated. Relative differences in the standard Gibbs energies of formation (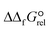 ) of the structures were calculated to determine the possibility of developing the covalently anchored POM–Mo-complex hybrids. The impact of the metal–organic components and various solvents on the particular type of hybrid formation was evaluated.
) of the structures were calculated to determine the possibility of developing the covalently anchored POM–Mo-complex hybrids. The impact of the metal–organic components and various solvents on the particular type of hybrid formation was evaluated.

- This article is part of the themed collection: Coordination Networks


 Please wait while we load your content...
Please wait while we load your content...