Reaction mechanism and kinetics of oxygen reduction reaction on the iron–nickel dual atom catalyst†
Abstract
Dual-atom catalysts (DACs) have recently emerged as promising and high-activity catalysts for the oxygen reduction reaction (ORR), a key process in many electrochemical energy conversion devices. However, the ORR mechanism and kinetics on DACs has not yet been established. To address this problem, we employed grand canonical potential kinetics (GCP-K) with CANDLE solvation. The behavior of the free energy and grand canonical potential for ORR and hydrogen evolution reaction (HER) intermediates (OO*, OOH*, O*, OH*, and H*) and their corresponding transition states at constant charges were calculated and converted to free energy as a function of applied potential to predict current density as a function of applied potential for ORR (through the associative pathway) and the competitive HER on iron–nickel DAC (FeNiN6-DAC). We find a Tafel slope of 281 mV dec−1 for ORR, comparable with the experimental Tafel slope of 169 mV dec−1 at the current density of −1.7 mA cm−2. The change in concentration of ORR reaction intermediates on FeNiN6-DAC as a function of applied potentials indicates that the dominant intermediate is OH* at potentials >0.25 V vs. RHE. The charge transfer and spin density of Fe active sites reaches a maximum during each proton transfer step. The partial density of states of 3d orbitals on the Fe site indicates that the 3dx2−y2 orbital is near the Fermi level, while the position of the 3dz2 orbital and Fermi level reaches a minimum along the first reaction step of 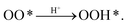 These insights into the fundamental aspects of ORR on DACs provide guidance for the design of efficient catalysts.
These insights into the fundamental aspects of ORR on DACs provide guidance for the design of efficient catalysts.

- This article is part of the themed collection: 2023 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...
