Open Access Article
Open Access ArticleA comparative techno-economic assessment of blue, green, and hybrid ammonia production in the United States
Matthias
Mersch
 abc,
Nixon
Sunny
ac,
Roghayeh
Dejan
ac,
Anthony Y.
Ku
d,
Gregory
Wilson
e,
Sean
O'Reilly
e,
Grigorii
Soloveichik
f,
John
Wyatt
g and
Niall
Mac Dowell
*acd
abc,
Nixon
Sunny
ac,
Roghayeh
Dejan
ac,
Anthony Y.
Ku
d,
Gregory
Wilson
e,
Sean
O'Reilly
e,
Grigorii
Soloveichik
f,
John
Wyatt
g and
Niall
Mac Dowell
*acd
aCentre for Environmental Policy, Imperial College London, London SW7 2AZ, UK. E-mail: niall@imperial.ac.uk
bClean Energy Processes (CEP) Laboratory, Department of Chemical Engineering, Imperial College London, London SW7 2AZ, UK
cThe Sargent Centre for Process Systems Engineering, Imperial College London, London SW7 2AZ, UK
dXiron Global Ltd, London W1W 5PF, UK
eJERA Americas Inc., Houston, TX, USA
fSolEXS Consulting, Houston, TX, USA
gCET Consulting, NJ, USA
First published on 15th February 2024
Abstract
Alternatives to fossil fuels as energy carriers are required to reach global climate targets. Hydrogen and ammonia are promising candidates that are carbon emission-free at point of combustion. Ammonia is critically important for fertiliser production and thus global food production. Additionally, low-carbon ammonia is a potentially valuable fuel for shipping, power generation, and industry. However, ammonia production today accounts for about 2% of total global carbon dioxide emissions. Conventional ammonia production based on methane reforming can be decarbonised by using carbon capture and storage, creating so-called blue ammonia. Alternatively, low-carbon electricity from additional clean energy sources can be used for electrolytic (green) hydrogen and ammonia production. Production tax credits (PTC) for clean hydrogen production via the 45V and carbon sequestration via the 45Q under the Inflation Reduction Act (IRA) in the United States have sparked interest in large-scale commercial low-carbon ammonia projects. In this work, we analyse different blue and electrolytic low-carbon ammonia production processes under economic and practical considerations. We propose and evaluate two novel designs: integrating a biomethane supply into the reformer; and combining blue and green ammonia production processes. Results show that all low-carbon ammonia plants can significantly reduce emissions compared to the conventional process. With the production tax credits, blue ammonia is likely to be the most economical production route in the near-term, being cheaper than conventional ammonia which is not eligible for any credits. The economics of electrolytic ammonia depend heavily on the price of reliable low-carbon electricity. A levelised cost of electricity of about 35 $/MWh and lower is required for electrolytic ammonia to be competitive with blue ammonia at average gas prices and upstream emissions. Of the two novel process designs, blending in biomethane shows promise as it can lead to carbon-neutral or even carbon-negative ammonia with a near-zero cost of production when supported by 45V. Blue–green ammonia on the other hand can improve economics if upstream emissions are small and low-carbon electricity is cheap. Overall, the IRA tax credits improve the economics of low-carbon ammonia production significantly and result in it being competitive with conventional ammonia production, enabling significant carbon emission reduction.
Introduction
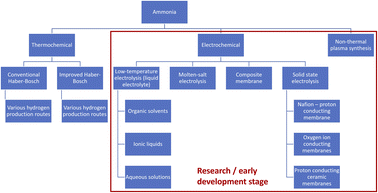
Ammonia (NH3) is an essential feedstock for the fertilizer industry and a promising low-carbon fuel to replace e.g., diesel for heavy goods vehicles or shipping, or natural gas for power generation, in the future. As with hydrogen, no carbon emissions are generated when burning ammonia. However, ammonia is easier to transport and store than hydrogen, and significant infrastructure for ammonia shipping and bunkering already exists worldwide, thus reducing barriers to scale-up of this technology.1 Both hydrogen and ammonia are expected to have a useful function as chemical energy vectors in reaching net zero.2 When comparing hydrogen and ammonia as low-carbon fuels, attention needs to be paid to practical considerations such as the availability of equipment, roundtrip efficiencies, and other emissions such as NOx, which are potentially higher when burning ammonia.3,4 In industrial combustion processes, NOx emissions will have to be maintained at existing permit levels, which can be achieved through a combination of burner design and upgraded selective catalytic reaction (SCR) systems to clean the exhaust gas.Most of the nearly 180 Mt of ammonia produced globally every year are generated via reforming of natural gas (72%) or coal (26%), resulting in approximately 500 Mt of carbon dioxide (CO2) emissions.5 The ammonia industry alone accounts for almost 2% of global CO2 emissions, 90% of which are associated with the production of the hydrogen (H2) required for the ammonia synthesis.5 Proposed low-carbon ammonia production routes involve either capturing and storing CO2 emissions from natural gas reforming (blue ammonia), electrolytic hydrogen production using renewable energy sources (green ammonia), or novel electrochemical ammonia synthesis routes.6–8 An overview of different ammonia synthesis routes compiled based on academic literature9–11 is provided in Fig. 1.
Traditionally, ammonia is synthesised from nitrogen and hydrogen via the thermochemical route using the Haber–Bosch process. The Haber–Bosch technology is mature. It has been continuously optimised over the last century and is commercially used at the megatonne scale.7 Conventional Haber–Bosch reactors use an iron-based catalyst and require pressures of 15 to 25 MPa and temperature of 400 to 450 °C. Typical single-pass conversion efficiencies are only about 15%, thus a large recycling loop is required.7 Improved Haber–Bosch reactors mainly use improved catalysts to achieve higher conversion efficiencies at lower pressures and temperatures. Ruthenium-based catalysts, especially in combination with different transition metals and metal hydrides show high catalytic activity at pressures of 1 to 10 bar and temperatures of 200 to 350 °C.11 However, such advanced designs account for less than 5% of global ammonia production, as they either yield ammonia at very low partial pressures, making the separation challenging, or are more energy- and cost-intensive than the conventional process.7
Various electrochemical ammonia production routes are being pursued as potential low-carbon alternatives to the traditional thermochemical synthesis. The ambition is to utilise clean energy directly for ammonia synthesis rather than using green hydrogen as an intermediate product. Key technologies under development include low-temperature, molten-salt or solid-state electrolysis as well as non-thermal plasma synthesis. However, these technologies are still in research or early development stage and not yet commercially mature. For detailed reviews the reader is referred to Ghavam et al.9 and Dincer et al.10 The focus of this work lies on low-carbon ammonia production projects for the near future; hence we focus on commercially available technologies.
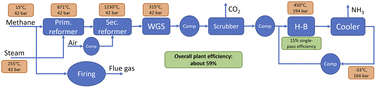
Key to decarbonising Haber–Bosch ammonia is the hydrogen supply, which is normally sourced from a steam-methane reformer (SMR) (see Fig. 2 for a simplified process flow diagram). Natural gas and steam are reformed at high temperatures in an SMR to syngas containing hydrogen (H2), carbon monoxide (CO), and carbon dioxide (CO2). In a secondary reformer, air is introduced to the system for further reforming and to provide the nitrogen required for the ammonia synthesis. A water gas shift (WGS) reactor further converts CO and steam to more H2 and CO2. The CO2 is then separated from the product gas stream and vented. It accounts for about two-thirds of process emissions.5 The SMR reactor requires heat input to attain the high temperatures required for the process, which is typically provided by natural gas furnaces. The flue gas stream from these furnaces constitutes the largest share of the remaining process emissions.5
Since the CO2 from the process stream is already being separated during normal operation of the hydrogen production plant (but typically simply vented), storing it in secure underground storage is a relatively low-cost solution to reduce emissions associated with ammonia production. This is a low-hanging fruit to reduce emissions by about two thirds. However, for deep decarbonisation it is necessary to also capture and store CO2 emissions from the flue gas stream.12 Large-scale systems can use chemical absorption of CO2 by solvents with high CO2 capture rates to reduce the concentration of CO2 in the flue gas. However, the costs of the required carbon capture facility are likely to be significant.
Alternatives to conventional SMRs are autothermal reformers (ATRs), which introduce pure oxygen in the reformer to partially oxidise the feed and provide heat, thereby eliminating the need for external firing.13 One key benefit of ATRs is that the CO2 is contained in the process gas stream at higher partial pressures and concentrations and the nitrogen is only introduced at a later stage, thereby reducing the size of the carbon capture unit. On the other hand, ATRs require an air separation unit (ASU) to provide the oxygen. However, the availability of nitrogen from the ASU, which is required for the ammonia synthesis, can be an advantage. Large-scale ATR-based ammonia production facilities are already commercially available, which can reduce emissions by up to 99%.14,15
While process emissions from natural gas-based ammonia production can be significantly reduced using carbon capture and storage (CCS), upstream methane emissions from natural gas production and transportation can still be a concern.5 In contrast, water electrolysis using e.g., alkaline electrolysers, solid oxide electrolyser cells (SOECs) or polymer electrolyte membrane (PEM) electrolysers, can produce emission-free hydrogen, if power from renewables or nuclear is used.
Nosherwani and Costa Neto16 performed a techno-economic assessment of commercial ammonia production in Germany, considering conventional SMR-based ammonia production without CCS as well as green ammonia production using an onshore wind farm and PEM or alkaline electrolysers. It was found that either capital cost reductions or an increase in carbon tax is required for the low carbon processes to be cost-competitive. A variety of techno-economic assessments of non-fossil-based ammonia production have been published, focussing for example on green ammonia production from unspecified renewable energy in Chile,17 offshore wind in the US,18 wind and solar in Chile, Denmark and Australia19 and Iran,20 and integrated biomass gasification.21 Tunå et al.22 compared ammonia production from water electrolysis using renewable energy, steam reforming of biogas from anaerobic digesters, and biomass gasification, finding the latter to perform best economically, but none of the processes to be competitive with conventional ammonia production. Bose et al.23 investigate the spatial variation of green ammonia production in the US, while Salmon and Banares-Alcantara24 map offshore green ammonia production costs globally.
Arnaiz del Pozo and Cloete8 performed a techno-economic assessment of blue and green ammonia production, comparing blue ammonia production using KBR, Linde and gas switching reforming processes with green ammonia production from wind and solar with battery and hydrogen storage in Germany, Spain and Saudi Arabia. They found green ammonia to be 70 to 150% more expensive than the best blue ammonia process. Mayer et al.25 conducted a techno-economic analysis and life-cycle assessment of an SMR-based blue ammonia plant and a PV-based green ammonia plant in Saudi Arabia, though the green hydrogen production is not explicitly modelled for the technoeconomic assessment. The results show favourable economics for the blue ammonia process, but also highlight that the climate change impact of the blue ammonia process is very sensitive to methane leakage rates. At low methane leakage rates, blue ammonia was found to be an attractive and commercially available solution to mitigate environmental impacts of commercial ammonia production.
Studies in literature highlight that low-carbon ammonia production processes struggle to compete with the conventional process economically. However, new investment incentives in the form of tax credits for carbon sequestration under section 45Q of the US tax code and hydrogen production under section 45V of the US tax code have sparked significant interest in low-carbon ammonia production in the United States (US). These incentives, further explained below, are meant to improve the economic competitiveness of low-carbon technologies compared to the incumbent. However, the extent to which different incentives can make low-carbon ammonia processes commercially attractive compared to the conventional grey ammonia production needs to be evaluated.
In this study, we present a comparative analysis of various methane reforming-based (blue) and water electrolysis-based (green) low-carbon ammonia production pathways under economic and practical considerations, considering performance metrics such as capital and operational costs, effective ammonia production costs and carbon intensity of the processes. We also discuss the practicality of implementing such low-carbon ammonia processes and discuss how to best utilise available subsidies. Finally, we propose and investigate two novel processes: (i) integrating biomethane into blue ammonia production processes to further reduce emissions and to potentially create carbon-negative ammonia; and (ii) using synergies between blue and green ammonia processes in a combined blue–green ammonia production process.
Key contributions of this work are a comprehensive techno-economic assessment and comparison of conventional, blue and electrolytic ammonia production processes, explicitly considering the newly available tax credits. We study the economic and emissions characteristics of the different ammonia production pathways with a focus on practical applications in the next few years and explore how the available tax credits can be best utilised. Additionally, we propose two novel processes that may improve the economics and environmental impact of commercial low-carbon ammonia production.
The investigation is carried out for a representative 3300 tons per day ammonia production facility located in Texas, United States. Texas is a promising location for ammonia production and export, as it offers low natural gas prices,26 significant existing oil and gas infrastructure and expertise,27 good potential for CCS,28 and a high availability of renewable energy sources.29 Four different systems are considered: conventional SMR-based ammonia production, SMR-based ammonia production with CCS, ATR-based ammonia production with CCS, and electrolytic ammonia production. Additionally, the two novel processes introduced above are investigated. Details on the methodology are provided in the following section.
Methodology
Ammonia production process models
Techno-economic models of five ammonia production processes were developed:(1) Conventional SMR-based ammonia production without CCS.
(2) SMR-based blue ammonia production with process and flue gas CCS.
(3) ATR-based blue ammonia production with CCS.
(4) PEM electrolyser based (green) ammonia production.
(5) ATR & PEM electrolyser based blue–green ammonia production.
The techno-economic models were developed in Microsoft Excel, using the CoolProp package30 to calculate all thermo-physical properties. Underlying are rate-based models for the SMR and ATR reactions,31,32 and an assumed single-pass efficiency for the Haber–Bosch reactor as outlined in the following subsection.
Primary and secondary reformer are designed to yield a stochiometric H2–N2 ratio in the product suitable for ammonia production. This corresponds to a methane conversion rate of about 82% in the primary reformer. The steam-to-methane ratio is assumed to be 3.1 mole H2O per mole CH4. The steam is available as saturated steam at 42.4 bar, which corresponds to a temperature of 255 °C. The air for the secondary reformer is compressed from ambient conditions to 42.4 bar and then pre heated using heat from the primary reformer to attain the high temperature in the secondary reformer.
Auxiliary systems such as the natural gas desulphurisation, the pre-reformer, and feedwater treatment systems are not explicitly modelled or shown in the simplified process drawings, but considered in the costing of the SMR system.
Following the reformers, the syngas stream containing H2, N2, CO, CO2, and steam is cooled down in syngas coolers before entering the water gas shift (WGS) reactors at 315 °C. The heat from the syngas coolers is integrated into the steam generation system described below.
In the WGS reactors, any remaining CO is reacted with steam to H2 and CO2 to increase hydrogen yield. At the outlet, the stream is further cooled down to condense out the water, which is recycled to the steam generation system. The process stream then enters the CO2 separation unit where all CO2 is removed. It remains a stochiometric stream of H2 and N2 which is fed into the ammonia synthesis loop.
The Haber–Bosch reactor is assumed to operate at 450 °C and 194 bar. The single pass conversion efficiency is assumed to be 15%,5,7 while the pressure loss over the reactor is assumed to be 30 bar. The feed stream is compressed to reactor pressure using two compressors with intercooling and then mixed with the recycling stream. After the Haber–Bosch reactor, the product stream is cooled down in a heat exchanger, heating up the feed. It is then refrigerated to −33 °C to condense and separate the ammonia. A recycling compressor makes up for the pressure drop over reactor and coolers.
The flue gas capture system is modelled as an amine scrubber. In an absorber column, the flue gas is contacted with a lean amine solution which absorbs the CO2. The rich solution then passes through a stripper column, where steam is used to regenerate the solvent. A stream of concentrated CO2 is available from the stripper, which can then be compressed and pumped to storage sited. A water lean solvent with a reboiler duty of 2.35 GJ per tCO2 captured34 is used, representing best-available technology. In line with industrial best practice, the default flue gas CO2 capture rate is assumed to be 95%, though this is varied throughout the analysis. The steam for the solvent regeneration is provided by the steam system and additional firing, if necessary.
Not all methane is reformed in GHR and ATR, it is assumed that about 3.5% unreacted methane remains in the off gas.35 This unreacted methane in the off gas is burned in auxiliary burners, resulting in residual emissions. However, 96.5% of carbon is contained in the process gas stream and then separated and stored, as in the SMR-based blue ammonia process.
The O2 required in the ATR and well as the N2 required for the ammonia synthesis are provided by an air separation unit (ASU). It is sized according to the O2 demand and produces excess N2. The specific power demand of the ASU, which is assumed to be a cryogenic unit, is 0.37 kWh/kgN2.36
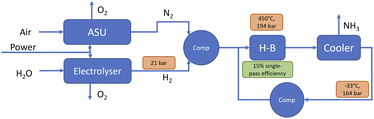 | ||
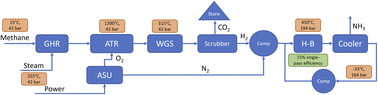
| Fig. 4 Simplified process drawing of the PEM-based electrolytic ammonia synthesis process. “H–B” is the Haber–Bosch reactor, “ASU” the air separation unit, and “Comp” are compressors. | ||
We distinguish between “electrolytic” and “green” hydrogen and ammonia in this paper. “Electrolytic” refers to any hydrogen and ammonia produced via electrolysis, using any electricity input. “Green” hydrogen and ammonia on the other hand is explicitly produced using renewable energy.
To be independent of electricity price assumptions in the blue ammonia processes, it is assumed that all power for compressors and the ASU, if present, is generated from steam onsite. For that purpose, the saturated steam at 42.4 bar and 255 °C is superheated and then expanded.
In the SMR-based processes, all required additional energy is provided from the natural gas firing of the SMR. In the ATR case however, additional natural gas firing is to be avoided to reduce emissions. For that reason, a share of about 19% of the produced hydrogen is used for additional firing with the off gas.
All compressors are modelled as turbomachines with a fixed isentropic efficiency of 90%. A variety of additional components are not explicitly modelled but accounted for in the costing as much as possible.
| CAPEX | Fixed OPEX | Variable OPEX | |
|---|---|---|---|
| SMR reactor without CCS, including auxiliary systems | 453 $/(kgH2/day) | 22.0 $/(kgH2/day)/year | 0.087 $/kgH2 |
| SMR reactor with CCS, including auxiliary systems | 1233 $/(kgH2/day) | 48.3 $/(kgH2/day)/year | 0.242 $/kgH2 |
| ATR with CCS, including auxiliary systems | 929 $/(kgH2/day) | 37.5 $/(kgH2/day)/year | 0.36 $/kgH2 |
| Water–gas shift | 26 $/(kgH2/day) | 4% of CAPEX | — |
| Process CO2 removal | 60 $/(kgH2/day) | 4% of CAPEX | — |
The study by Ganzer and Mac Dowell38 is used for the costing of Haber–Bosch process and ASU. The capital costs of the Haber–Bosch system are:
CHaber–Bosch = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ($) + 4220 ($/(kgNH3/hour)) × capacity 000 ($) + 4220 ($/(kgNH3/hour)) × capacity |
While those of the ASU are:
CASU = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 777 777![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ($) + 789 ($/(kgN2/hour)) × capacity 000 ($) + 789 ($/(kgN2/hour)) × capacity |
For both, annual fixed operation and maintenance costs are assumed to be 4% of capital costs.
Estimates of electrolyser costs vary widely in literature, and costs are expected to fall significantly in future. The International Energy Agency (IEA) reports current PEM electrolyser costs of 1100 to 1800 $/kW,39 while the US Department of Energy (DOE) estimates current uninstalled costs of 975 to 1200 $/kW, but expects these costs to fall to 380 to 450 $/kW by 2030.40 Another DOE report puts “current central” electrolyser capital costs at 515.2 $/kW with fixed operational costs of 31.65 $/kW, which includes a stack replacement every 7 years.37 The latter values are used in our analysis, though it should be noted that they are likely closer to nths of a kind (NOAK) costs than first of a kind (FOAK) costs.
General assumptions, feedstocks, and project finance
The analysis is based on 3300 t NH3 per day production trains situated in Texas, US. The process is assumed to operate constantly throughout the year, yielding an annual ammonia production of 1.2 Mt NH3. The project is assumed to be 30% equity financed, costed at 12%, and 70% debt financed, costed at 3%, yielding a weighted-average cost of capital of 5.7%. The construction time is assumed to be 4 years, and capital recovery is required within 12 years of project start. The effective ammonia production costs calculated in this work are the revenues required to reach a net present value (NPV) of 0. These figures will vary with variations in the project lifetime, and the financing conditions (debt-equity splits, interest rates, etc.). In line with the NETL report,33 a December 2018 cost basis is used.Natural gas is a key feedstock to the blue ammonia processes. The average Henry Hub natural gas price was about 3.5 $/MMBTU,41 which is used as a baseline for the analysis. However, recognising that fuel prices are very volatile, the effect of gas prices varying from 0 to 8 $/MMBTU on effective ammonia production costs is investigated. A gas price of 0 $/MMBTU is not practical, the lowest historical monthly average Henry Hub prices were around 1.6 $/MMBTU. However, it establishes a theoretical lower bound and shows how much capital and maintenance costs contribute to overall production costs.
Another important parameter are upstream emissions from natural gas production, which are emissions associated with blue ammonia processes that cannot be reduced by any carbon capture mechanism at the plant. As baseline value of 0.28 kgCO2 eq. per kgCH4, the average value for US natural gas,42 is assumed in the analysis. However, the actual value of fugitive methane emissions will depend highly on the location, the equipment used and the maintenance. By locating the ammonia plant close to natural gas production sites and applying measures to reduce methane leakage, upstream methane emissions can be reduced significantly. On the other hand, long transport distances and leaky equipment can result in higher emissions. Recognising this, values from 0 to 0.5 kgCO2 eq. per kgCH4 are considered in the analysis.
Electricity for the electrolytic ammonia process should ideally be supplied from renewables or low-carbon electricity sources. However, as reference, average values from the ERCOT grid in Texas are considered as well. The average industrial electricity price from January 2018 to January 2021 was just over 50 $/MWh.43 The monthly average industrial electricity prices were stable for most of the time period, with a distinct spike to about 70 $/MWh during the heat wave in August 2019, and another spike to 120 $/MWh during the winter crisis in February 2021. At the same time, the emissions intensity of the Texas power grid in 2021 was 427 kgCO2 eq. per MWh.44 With (mostly) renewable electricity supply to the electrolytic ammonia process, the emission intensity would be significantly lower, but, depending on capacity factors, cost may be significantly higher.
The carbon dioxide separated in the blue ammonia processes needs to be compressed for transport, piped to a suitable location, and injected into secure geological storage. The energy demand of the CO2 compressors is accounted for in the process simulation. It is assumed that the CO2 must be compressed to a pressure of 150 bar. To account for transport and storage costs, effective costs of 20 $/tCO2 are assumed in the model. This value may be on the conservative side for CCS projects in the US and specifically Texas, which are often quoted with transport and storage costs of around 10 $/tCO2.45,46 On the other hand, it may be optimistic for new, first-of-a-kind projects.47 A true carbon transport and storage costs will again strongly depend on the siting, transport distances and local geography, as well as permitting.
For a high-level validation, energy intensity and emission factors calculated from the models used in this analysis are compared to average values estimated in the low-carbon ammonia roadmap report,5 as shown in Table 2. The process models show good agreement with the values reported in literature. As the reference report does not include any technical detail, it is not possible to evaluate the reasons for the small discrepancies, but potential reasons may be different plant scales or process assumptions.
| This analysis | Low-carbon ammonia report5 | |||
|---|---|---|---|---|
| Energy intensity [GJ/tNH3] | Emission intensity [t CO2/tNH3] | Energy intensity [GJ/tNH3] | Emission intensity [tCO2/tNH3] | |
| Conventional | 31.9 | 1.9 | 32 | 1.8 |
| SMR + CCS | 31.9 | 0.2 | 32 | 0.1 |
| ATR + CCS | 28.7 | 0.2 | 27.9 | 0.1 |
Results and discussion
Blue and green ammonia have significantly lower carbon intensities compared to conventional ammonia
Both blue and electrolytic ammonia have the potential to significantly reduce emissions associated with the ammonia production. However, the carbon intensity depends heavily on upstream emissions due to methane leakage (blue ammonia) or emissions associated with the power production (electrolytic ammonia).Table 3 shows the estimated carbon intensity of the two blue ammonia production processes compared to the conventional process for different values of upstream emissions. For the conventional process, these upstream emissions are relatively small, as the majority of emissions stem from the process itself. The blue ammonia processes, on the other hand, avoid process emissions by 97% and more, such that residual emissions are dominated by upstream emissions. Overall, the blue ammonia processes achieve a carbon avoidance of 85% (high upstream emissions) to 93% (low upstream emissions).
| −50% upstream emissions | US average upstream emissions | +50% upstream emissions | ||||
|---|---|---|---|---|---|---|
| kgCO2 eq. per kgNH3 | kgCO2 eq. per kgH2 | kgCO2 eq. per kgNH3 | kgCO2 eq. per kgH2 | kgCO2 eq. per kgNH3 | kgCO2 eq. per kgH2 | |
| Conventional ammonia | 1.80 | 10.11 | 1.89 | 10.62 | 1.98 | 11.14 |
| SMR-based blue ammonia | 0.12 | 0.67 | 0.21 | 1.18 | 0.30 | 1.70 |
| ATR-based blue ammonia | 0.14 | 0.78 | 0.22 | 1.23 | 0.30 | 1.68 |
| Grid-based electrolytic ammonia | 4.51 (kg CO2 eq. per kg NH3)/25.4 (kgCO2 eq. per kgH2) (assuming grid emissions of 427 kgCO2 eq. per MWh (ref. 44)) | |||||
Table 3 also shows specific emissions on a hydrogen basis, which are relevant for 45V production tax credits (PTCs) under the Inflation Reduction Act (IRA) in the US. These credits are tiered depending on the carbon intensity of the produced hydrogen, as shown in Table 4. Additionally, labour standards such as paying workers the prevailing wage depending on region and occupation for the first 10 years of the project and hiring a certain number of apprentices must be met to qualify for the full PTCs.48 It is apparent that blue ammonia projects are unlikely to reach the highest subsidy tier due to upstream emissions, giving more motivation to explore alternatives such as incorporating biomethane or green hydrogen.
| Hydrogen emission intensity | Production tax credit (assuming labour requirements are met) |
|---|---|
| <0.45 kgCO2 eq. per kgH2 | 3 $/kgH2 |
| 0.45–1.5 kgCO2 eq. per kgH2 | 1 $/kgH2 |
| 1.5–2.5 kgCO2 eq. per kgH2 | 0.75 $/kgH2 |
| 2.5–4 kgCO2 eq. per kgH2 | 0.6 $/kgH2 |
| >4 kgCO2 eq. per kgH2 | No credit available |
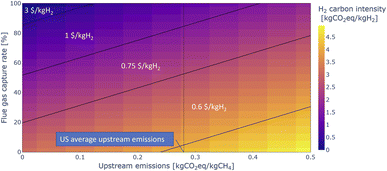
The solution space between upstream emissions, carbon capture rate, and available 45V PTCs is further explored for the SMR-based blue ammonia process in Fig. 6. It is assumed that all the process emissions are captured, while flue gas carbon capture rate and upstream emissions are varied. The figure shows that reaching the highest PTC tier requires upstream emissions less than 0.1 kgCO2- eq. per kgCH4 and flue gas carbon capture rates higher than 90%. At US average upstream emissions, blue ammonia projects can comfortably reach the 1 $/kgH2 range with flue gas carbon capture rates of 85% and higher. The oil and gas climate initiative (OGCI) aims for zero methane emissions from their operations by 2030,49 and the PTC tiers provide an additional financial incentive to do so, as lower upstream emissions can lead to significantly higher PTCs being available to projects.
Note that Fig. 6 also shows that without flue gas capture, at US-average upstream emissions blue hydrogen projects are not eligible for any support under 45V, while still 45Q tax credits for carbon sequestration can be claimed. The trade-off between saving the capital costs of the post combustion capture units vs. only being able to claim 45Q tax credits is not further explored here, as the focus is on very low carbon ammonia.
The carbon intensity of electrolytic ammonia depends on the carbon intensity of the electricity used for the H2 and N2 production. If carbon-free electricity is used, the electrolytic ammonia is also carbon-free (neglecting emissions related to construction, etc.). At grid-average carbon intensity, on the other hand, which is around 430 kgCO2-eq. per MWh in Texas in 2021,44 emissions associated with electrolytic ammonia production are 4.5 kgCO2-eq. per kgNH3 (25.4 kgCO2-eq. per kgH2), much worse than the conventional SMR-based ammonia production process without CCS. At an electricity carbon intensity of 20 kgCO2-eq. per MWh, electrolytic ammonia emissions are on par with blue ammonia with average upstream emissions, and a maximum electricity carbon intensity of 7 kgCO2-eq. per MWh is required to reach the highest 45V subsidy tier.
Significant discussion50–52 is currently ongoing regarding the definition of additionality of renewable energy production for powering electrolysers. Hourly,53 weekly,53 monthly54 or annual53 matching requirements of renewable energy production and electrolyser power consumption are being deliberated. Hourly matching is asserted to assure the lowest emissions and seems to be gaining traction but is likely more expensive and may be challenging to implement initially.53–57 Such requirements will likely impact the economics of any green hydrogen or ammonia project, though it has been argued that available 45V credits are sufficient to make up for the added costs under an hourly-matching framework.53
Production tax credits under the inflation reduction act make low carbon ammonia cost competitive with the conventional production process
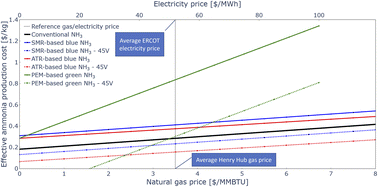
Available PTCs under the IRA shift the economics of ammonia production significantly, as shown in Fig. 7. Without tax credits (solid lines), the conventional ammonia production process is approximately 30% cheaper than blue ammonia processes regardless of the natural gas price. However, assuming that a 45V credit of 1 $/kgH2 is available for blue ammonia projects, both the SMR-based and the ATR-based blue ammonia processes become more cost-effective than the counterfactual. With the PTCs, blue ammonia is now cheaper than the carbon-intensive conventional process regardless of natural gas price.The production costs of electrolytic ammonia are a first order function of the electricity price. However, even with free electricity, without subsidies it is always more expensive than the conventional ammonia production process. Assuming electrolytic ammonia can benefit from the highest 45V PTC tier of 3 $/kgH2, which is the case if the emission intensity of the power is below 7 kgCO2-eq per MWh, the economics of electrolytic ammonia improve drastically. At baseline electrolyser costs, electrolytic ammonia with 45V tax credits is cheaper than conventional ammonia for electricity prices below about 50 $/MWh, the current average electricity price in Texas. Note however that this electricity is required to be virtually emission-free to access to highest PTC tier.
Blue ammonia with 45V subsidies appears to be more economical than electrolytic ammonia for wide ranges of natural gas and electricity prices. Only if electrolytic ammonia can indeed access the highest 45V PTC ties and blue ammonia is only eligible for the second-highest tier, carbon-free electricity prices below about 35 $/MWh are required for electrolytic ammonia to be more economical than blue ammonia at average natural gas prices.
Electrolyser capital costs are highly uncertain. According to the International Energy Agency (IEA), today's cost of PEM electrolysers can be as high as 1800 $/kWe, while future cost may be as low as 200 $/kWe.58 However, such cost reductions require a scale-up of the technology, and near-term inflationary realities suggest that declines in electrolyser cost may not be straight-line. The impact of different electrolyser costs in addition to different electricity prices is shown in Fig. 8. Note that the estimated costs here include 45V PTCs of 3 $/kgH2, which as mentioned above requires very low-carbon electricity supply. The electricity price remains the dominant driver for electrolytic ammonia production costs, but if electrolyser capital cost reductions can be achieved by large scale deployment of the technology, the economics of electrolytic ammonia production can be significantly improved.
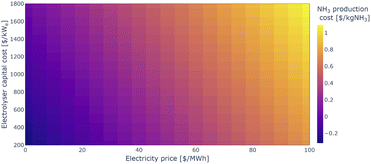 | ||
| Fig. 8 Estimated electrolytic ammonia production costs depending on electricity price and electrolyser capital cost. | ||
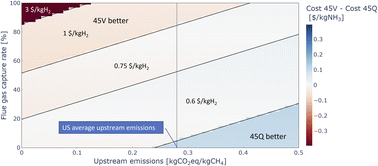
The IRA not only offers PTCs for clean hydrogen production under tax code 45V, but also tax credits of up to 85 $/tCO2 for permanent carbon sequestration under tax code 45Q, which may be attractive for blue ammonia projects. As they are not “stackable”,59 this leads to the question of finding the most suitable tax code for low-carbon ammonia producers. To explore this question, a comparison of estimated effective ammonia production costs with 45V tax credits and 45Q tax credits is shown in Fig. 9. Note that the 45V eligibility period is currently 10 years, while 45Q is available for 12 years, which may have an impact on the longer-term financials of a project.
The data shows that for a wide range of upstream emissions and flue gas carbon capture rates, and especially in the most desirable region of low upstream emissions and high carbon capture rates, claiming 45V credits is more economical than claiming 45Q credits. Only when upstream emissions of the natural gas are so high and flue gas carbon capture rates are so low that no 45V credits are available, 45Q offers benefits to low-carbon ammonia projects. Note that regardless of the flue gas carbon capture rate, it is assumed that in any case all the process emissions are captured and stored.
Incorporating a share of biomethane can lead to better economics and carbon neutral or carbon negative ammonia
Fig. 6 shows how challenging it is to reach the highest 45V PTC tier with blue ammonia production processes. One potential way to reach this subsidy tier and to improve the carbon intensity of the ammonia as well as the economics of blue ammonia projects is to incorporate a share of biomethane into the natural gas supply.Biomethane is typically produced from anaerobic digestion (AD). AD takes agricultural residues, animal waste, food waste, wastewater sludge or other organic matter as feedstock and converts it into biogas, which is then upgraded to biomethane. This involves a desulphurisation step as well as the removal of water and CO2 among other elements.60 Biomethane from different sources is already readily available in the US, though the market is still growing. According to the US Department of Energy, as of mid-2022, 538 biogas from landfill projects were operational in the US, as well as 330 anaerobic digesters producing biogas from livestock farms and 1200 anaerobic digesters installed at wastewater treatment facilities.61
The costs of biomethane vary depending on feedstock and location. Based on the global biomethane supply curve developed by the International Energy Agency (IEA),62 a biomethane price of 15 $/MMBTU is assumed for the initial analysis presented here, which is about 4 to 5 times higher than the baseline natural gas price.41 Regarding emissions, based on the median whole-site measurements presented by Bakkaloglu et al.63 upstream biomethane emissions are assumed to be 1.37 kgCO2-eq. per kgCH4. However, the CO2 arising from the combustion of biomethane is carbon neutral, as the biomass has absorbed carbon over its lifetime which would otherwise also be released during the decomposition. Any carbon captured from biomethane and stored in geological reservoirs is therefore counted as negative emission.64
Fig. 10 shows how increasing the share of biomethane in the feedstock to blue ammonia plants can drastically reduce the carbon intensity of the hydrogen and ammonia. A share of 10 to 20% biomethane, depending on upstream emissions, results in carbon-neutral ammonia. Beyond that, carbon-negative ammonia is being generated with considerably greater costs. These negative emissions can potentially be monetised by selling carbon removal credits on voluntary carbon markets. However, this option is not evaluated in the analysis presented here.
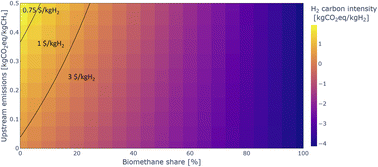 | ||
| Fig. 10 Carbon intensity of blue hydrogen used for ammonia production depending on upstream emissions and biomethane share. The IRA 45V subsidy tiers are also shown in the figure. | ||
The figure also shows that blending in a share of biomethane can allow blue ammonia projects to comfortably reach the highest 45V subsidy tier. At US default upstream emissions, a share of about 15% biomethane can reduce emissions sufficiently. For the reference 3300 tNH3 per day ammonia plant, this corresponds to about 270 t of biomethane per day, which likely requires access to multiple AD facilities.
Despite the significantly higher price and upstream emissions of biomethane, economics of blue ammonia projects can be improved if the higher-tier 45V PTCs are reached. An analysis of effective ammonia production costs depending on the share of biomethane and for US average upstream natural gas emissions is shown in Fig. 11. The costs drop suddenly at 15% biomethane blend as the highest 45V PTC tier is reached. Increasing the biomethane share beyond that point offers no obvious monetary benefits, effective ammonia production costs increase as more and more expensive biomethane is used. However, if indeed carbon-negative ammonia is being produced, carbon dioxide removal (CDR) credits sold on voluntary carbon markets may become available as an additional revenue stream, which has not been considered in the analysis presented here.
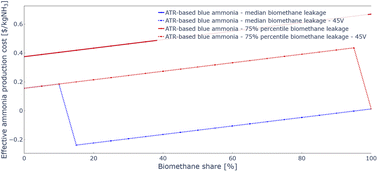 | ||
| Fig. 11 Estimated effective blue ammonia production costs with 45V production tax credit depending on biomethane share. As biomethane leakage values vary significantly between facilities, results from 25% and 75% biomethane leakage percentiles were also analysed in addition to the median biomethane leakage values. The 25% case yields the same cost curve as the median, but the 75% case is also plotted. All leakage values are based on Bakkaloglu et al.63 The resolution of the x axis is 5 percentage points of biomethane share. | ||
Biomethane leakage values vary widely between sites.63 In addition to median leakage values, 25% and 75% percentiles of leakage were also investigated. The biomethane blending cost curve of the former coincides with the one of the median leakage case, while the latter is also plotted in Fig. 11. In that case, due to the high biomethane leakage the highest 45V PTC tier is only reached at 100% biomethane. While the 100% biomethane blend still achieves lower effective production costs than the baseline natural gas-only case, for any other biomethane blend percentage production costs are significantly higher. Additionally, sourcing sufficient biomethane to run the entire ammonia production may prove challenging. Fig. 11 shows that reducing biomethane leakage is crucial not just to reduce emissions, but also to improve the economics of the ammonia production.
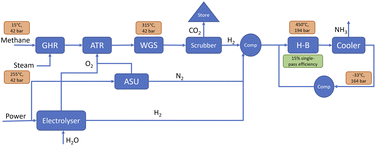
Blue–green ammonia can use synergies to improve economics under specific conditions
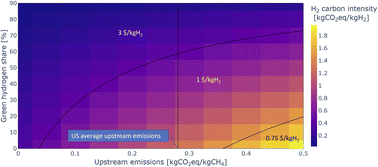
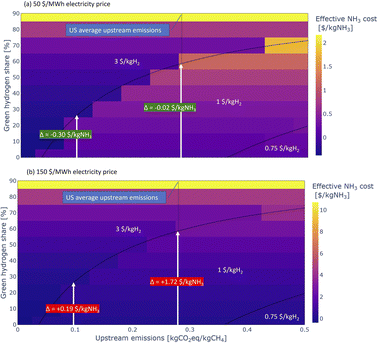
Another way to reduce the carbon intensity of blue ammonia production is to use a share of electrolytic hydrogen production. Additional potential synergies are using oxygen from the electrolyser in the ATR and thus reducing the size of the ASU required by the ATR process. For the analysis presented here, it is assumed that the electrolyser only runs on carbon free electricity, hence no emissions are associated with the green hydrogen. Further, it is assumed that the blue–green ammonia plants run baseload with a fixed share of green hydrogen production. Allowing for the share of green hydrogen to vary with e.g., the availability of renewable energy sources could unlock significant added benefits which are not evaluated here but are an interest topic for future work.Fig. 12 shows the overall carbon intensity of the hydrogen used for blue–green ammonia production depending on upstream emissions and share of green hydrogen, as well as the 45V PTC tiers. At US average upstream emissions, a blend of about 60% green hydrogen and 40% blue hydrogen is required to reach overall emissions low enough to claim 3 $/kgH2. The required share of green hydrogen reduces with reducing the emissions from the gas supply chain, but nonlinearly, such that upstream emissions of half the US average still require about 40% green hydrogen.
Depending on electricity price and upstream emissions, blue–green ammonia can be more cost-effective than blue ammonia in specific cases. The added costs from the green hydrogen production compete with the increase in available subsidies. Fig. 13 shows estimated effective ammonia production costs for electricity prices of 50 $/MWh and 150 $/MWh depending on upstream emissions and share of green hydrogen. The former value is indicative of the levelised-cost-of-energy of onshore wind and utility-scale PV projects without storage,65 while the latter represents the cost of reliable renewable energy production with storage at 90% capacity factor, determined using the γ-AWE tool developed by Ganzer and Mac Dowell.38
In the case of low electricity price (approximately on par with current wholesale prices in Texas), the blue–green ammonia process offers marginal cost savings over the ATR-based blue ammonia process at US average upstream emissions, but shows significantly lower costs with decreasing upstream emissions, as less and less expensive green hydrogen is required. At the higher electricity price however, which is more representative of reliable renewable energy production with storage, the blue–green ammonia process offers no cost savings over the ATR-based blue ammonia process, as the electrolytic hydrogen production is too expensive.
The real value of the proposed blue–green ammonia process likely lies in a more flexible operation, using cheap renewable electricity for green hydrogen production when available and higher shares of blue hydrogen at times of low wind and solar availability. A more flexible operation of the ammonia synthesis reactor might add additional benefits. The drawback of more flexible operation is that the benefits from using oxygen from the electrolyser for the ATR are reduced, as a backup system is needed for times when the electrolyser is running at lower load or shut off. Additional analysis is required to explore this trade off further.
Conclusions
A variety of mature blue and electrolytic low carbon ammonia production processes has been compared and evaluated under economical and practical considerations, considering large scale production facilities in Texas, US. Additionally, two novel processes, one integrating biomethane into blue ammonia production and one combining blue and green hydrogen for blue–green ammonia production, have been proposed and evaluated.Results show that both blue and electrolytic ammonia production processes can significantly reduce carbon emissions compared to the conventional process, which can be a major contribution to global decarbonisation. As process emissions from blue ammonia production are reduced, natural gas upstream emissions start to become dominant in the emission profile and should be reduced as much as possible. However, even with US average upstream emissions, blue ammonia plants are about 90% less carbon intensive than conventional ammonia plants. For electrolytic ammonia production, the emissions associated with the electricity production are key. With the current grid mix in Texas, electrolytic ammonia production is significantly more carbon intensive than the conventional process. With purely renewable electricity however, green ammonia has the potential to be carbon neutral (neglecting construction emissions).
Production tax credits for low carbon hydrogen under the inflation reduction act change the economics of low carbon ammonia production significantly. While blue ammonia is estimated to be about 50% more expensive than conventional ammonia without the subsidies, it becomes the cheapest production pathway even if only the second highest credit tier of 1 $/kgH2 is reached. Electrolytic ammonia production costs are very sensitive to the cost of low carbon electricity, but higher than blue ammonia production costs for many combinations of gas and electricity prices.
Blending a share of biomethane into the feedstock of blue ammonia production plants can lead to carbon neutral or even carbon negative ammonia and improve economics further. A share of 10 to 20% biomethane is likely sufficient to reach the highest production tax credit tier and lead to a significant reduction in effective ammonia production costs despite biomethane being much more expensive than natural gas. Higher shares of biomethane can have additional climate benefits of carbon dioxide removal, which may also enable additional revenue streams from selling carbon dioxide removal credits.
Blue–green ammonia uses synergies between green hydrogen production and ATR-based blue ammonia production. This can lead to lower emissions compared to blue ammonia plants when renewable electricity is used, and lower costs than pure green ammonia plants. Compared to pure blue ammonia plants, economic benefits may be achieved by reaching the highest production tax credit tier. These benefits depend on the natural gas upstream emissions, which determine the share of green hydrogen production required, as well as the electricity price. Additional benefits may be enabled by operating the plant more flexibly, however further analysis is required to quantify this.
The analysis shows that low carbon ammonia production in Texas is possible and, thanks to the production tax credit provided by the IRA, also economical. The emission reduction potential is significant, though it comes at a cost to US taxpayers. On the other hand, it appears that IRA will enable significant investments in low carbon industry in the US.
Limitations of the study
In this conceptual analysis, process flow diagrams have been simplified and many auxiliary systems are not explicitly modelled, though their costs were included in the analysis. Chemical reactions are not modelled in detail, but typical product compositions have been assumed. The plants are assumed to operate constantly at nominal capacity and under steady state conditions.Estimating costs of chemical plants is difficult, and costs will naturally vary depending on the project. It was attempted to use representative data from literature, and as natural gas and electricity prices are volatile, a range of values was explored. Still, the estimated ammonia production costs are indicative only. Front end engineering design (FEED) studies, which are much more detailed than the work presented here, are required to evaluate the economics of specific projects.
Emissions from biomethane supply chains vary widely between facilities and were shown to have a strong impact on the overall emissions associated with ammonia production incorporating a share of biomethane. For any application this needs to be considered carefully on a case-by-case basis.
Author contributions
Conceptualization, G. W., S. O. R., and N. M. D.; methodology, M. M., N. S., A. Y. K., and N. M. D.; software, M. M., and N. S.; validation, M. M., N. S., A. Y. K., G. W., S. O. R., G. S., J. W., and N. M. D.; investigation, M. M., N. S., and R. D.; resources, G. W., S. O. R., and N. M. D.; writing – original draft, M. M.; writing – review & editing, M. M., N. S., R. D., A. Y. K., G. W., S. O. R., G. S., J. W., and N. M. D; visualization, M. M., and N. S.Conflicts of interest
JERA Americas have announced a collaboration to supply clean ammonia from the US Gulf Coast to Germany, as well as MOUs and feasibility studies on clean ammonia supply with a number of other companies. JERA Americas sponsored this work. The authors all consult widely for a range of public and private organisations focused on the energy sector and net zero transitions.Acknowledgements
M. M., N. S., R. D., A. Y. K., and N. M. D. would like to thank JERA Americas for funding this work.Notes and references
- The Royal Society, Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store, 2020, https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf Search PubMed.
- The Royal Society, The Role of Hydrogen and Ammonia in Meeting The Net Zero Challenge, 2021, https://royalsociety.org/-/media/policy/projects/climate-change-science-solutions/climate-science-solutions-hydrogen-ammonia.pdf Search PubMed.
- C. Walter, L. William Huellmantel and H. R. Mitchell, Ammonia as an Engine Fuel, SAE Trans., 1966, 74, 300–326 Search PubMed . https://www.jstor.org/stable/44460524.
- H. Kobayashi, A. Hayakawa, K. D. K. A. Somarathne and E. C. Okafor, Science and technology of ammonia combustion, Proc. Combust. Inst., 2019, 37(1), 109–133, DOI:10.1016/j.proci.2018.09.029.
- D. Sandalow, R. Aines, Z. Fan, J. Friedmann and C. McCormick, Ann-Kathrin Merz and Corinne Scown, Low-Carbon Ammonia Roadmap, ICEF Innovation Roadmap Project, 2022 Search PubMed.
- N. Morlanes, S. P. Katikaneni, S. N. Paglieri, A. Harale, B. Solami, S. M. Sarathy and J. Gascon, A technological roadmap to the ammonia energy economy: Current state and missing technologies, Chem. Eng. J., 2021, 408, 127310, DOI:10.1016/j.cej.2020.127310.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape, Energy Environ. Sci., 2021, 13, 331–344, 10.1039/C9EE02873K.
- C. Arnaiz del Pozo and S. Cloete, Techno-economic assessment of blue and green ammonia as energy carriers in a low-carbon future, Energy Convers. Manage., 2022, 255, 115312, DOI:10.1016/j.enconman.2022.115312.
- S. Ghavam, M. Vahdati, I. A. G. Wilson and P. Styring, Sustainable Ammonia Production Processes, Front. Energy Res., 2021, 9, 580808, DOI:10.3389/fenrg.2021.580808.
- I. Dincer, D. Erdemir, M. I. Aydin, H. Karasu and G. Vezina, Ammonia Production, in Ammonia Energy Technologies, Lecture Notes in Energy, Springer, Cham, 2023, vol. 91, DOI:10.1007/978-3-031-13532-3_2.
- G. Soloveichik, Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process, Nat. Catal., 2019, 2, 377–380, DOI:10.1038/s41929-019-0280-0.
- G. Collodi, G. Azzaro, N. Ferrari and S. Santos, Techno-economic Evaluation of Deploying CCS in SMR Based Merchant H2 Production with NG as Feedstock and Fuel, Energy Procedia, 2017, 114, 2690–2712, DOI:10.1016/j.egypro.2017.03.1533.
- A. O. Oni, K. Anaya, G. Di Lullo and A. Kumar, Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions, Energy Convers. Manage., 2022, 254, 115245, DOI:10.1016/j.enconman.2022.115245.
- TOPSOE, Blue Ammonia. Tomorrow's Fuel. Ready Today, https://www.topsoe.com/blueammonia Search PubMed.
- KBR, Ammonia & Fertilizer Technologies, https://www.kbr.com/en-gb/what-we-do/technologies/clean-process-technologies/ammonia-fertilizers-technologies Search PubMed.
- S. A. Nosherwani and R. Costa Neto, Techno-economic assessment of commercial ammonia synthesis methods in coastal areas of Germany, J. Hydrog. Storage, 2021, 34, 102201, DOI:10.1016/j.est.2020.102201.
- C. Funez Guerra, L. Reyes-Bozo, E. Vyhmeister, M. Jaen Caparros, J. L. Salazar and C. Clemente-Jul, Technical-economic analysis for a green ammonia production plant in Chile and its subsequent transport to Japan, Renewable Energy, 2020, 157, 404–414, DOI:10.1016/j.renene.2020.05.041.
- E. R. Morgan, J. F. Manwell and J. G. McGowan, Sustainable Ammonia Production from U.S. Offshore Wind Farms: A Techno-Economic Review, ACS Sustain. Chem. Eng., 2017, 5(11), 9554–9567, DOI:10.1021/acssuschemeng.7b02070.
- N. Campion, H. Nami, P. R. Swisher, P. V. Hendriksen and M. Münster, Techno-economic assessment of green ammonia production with different wind and solar potentials, Renewable Sustainable Energy Rev., 2023, 173, 113057, DOI:10.1016/j.rser.2022.113057.
- A. Kakavand, S. Sayadi, G. Tsatsaronis and A. Behbahaninia, Techno-economic assessment of green hydrogen and ammonia production from wind and solar energy in Iran, Int. J. Hydrogen Energy, 2023, 48(38), 14170–14191, DOI:10.1016/j.ijhydene.2022.12.285.
- J. Anderson and J. Lundgren, Techno-economic analysis of ammonia production via integrated biomass gasification, Appl. Energy, 2014, 130, 484–490, DOI:10.1016/j.apenergy.2014.02.029.
- P. Tunå, C. Hulteberg and S. Ahlgren, Techno-economic assessment of nonfossil ammonia production, Environ. Prog. Sustainable Energy, 2013, 33(4), 1290–1297, DOI:10.1002/ep.11886.
- A. Bose, N. Lazouski, M. L. Gala, K. Manthiram and D. S. Mallapragada, Spatial Variation in Cost of Electricity-Driven Continuous Ammonia Production in the United States, ACS Sustain. Chem. Eng., 2022, 10(24), 7862–7872, DOI:10.1021/acssuschemeng.1c08032.
- N. Salmon and R. Banares-Alcantara, A global, spatially granular techno-economic analysis of offshore green ammonia production, J. Cleaner Prod., 2022, 367, 133045, DOI:10.1016/j.jclepro.2022.133045.
- P. Mayer, A. Ramirez, G. Pezzella, B. Winter, S. M. Sarathy, J. Gascon and A. Bardow, Blue and green ammonia production: A techno-economic and life cycle assessment perspective, iScience, 2023, 26(8), 107389, DOI:10.1016/j.isci.2023.107389.
- International Energy Agency (IEA), Natural Gas Prices in Europe, Asia and the United States, Jan 2020-February 2022, 2022, https://www.iea.org/data-and-statistics/charts/natural-gas-prices-in-europe-asia-and-the-united-states-jan-2020-february-2022 Search PubMed.
- Railroad Commission of Texas (RRC), Public GIS Viewer (Map), https://www.rrc.texas.gov/resource-center/research/gis-viewer/ Search PubMed.
- D. Blackmon, Why Texas, Louisiana Are Poised To Win The Carbon Capture Sweepstakes, Forbes, 2023. https://www.forbes.com/sites/davidblackmon/2023/04/24/why-texas-louisiana-are-poised-to-win-the-carbon-capture-sweepstakes/?sh=789d3f042532 Search PubMed.
- B. Chang and K. Starcher, Evaluation of wind and solar energy investments in Texas, Renewable Energy, 2019, 132, 1348–1359, DOI:10.1016/j.renene.2018.09.037.
- I. H. Bell, J. Wronski, S. Quoilin and B. Lemort, Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp, Ind. Eng. Chem. Res., 2014, 53(6), 2498–2508, DOI:10.1021/ie4033999 , http://www.coolprop.org/.
- S. Z. Abbas, V. Dupont and T. Mahmud, Kinetics study and modelling of steam methane reforming process over a NiO/Al2O3 catalyst in an adiabatic packed bed reactor, Int. J. Hydrogen Energy, 2017, 42(5), 2889–2903, DOI:10.1016/j.ijhydene.2016.11.093.
- G. Collodi, G. Azzaro, N. Ferrari and S. Santos, Techno-economic Evaluation of Deploying CCS in SMR Based Merchant H2 Production with NG as Feedstock and Fuel, Energy Procedia, 2017, 114, 2690–2712, DOI:10.1016/j.egypro.2017.03.1533.
- National Energy Technology Laboratory (NETL), Comparison of Commercial, State of the Art, Fossil Based Hydrogen Production Technologies, 2022, DOE/NETL-2022/3241 Search PubMed.
- Y. Jiang, P. M. Mathias, C. J. Freeman, J. A. Swisher, R. F. Zheng, G. A. Whyatt and D. J. Heldebrant, Techno-economic comparison of various process configurations for post-combustion carbon capture using a single-component water-lean solvent, Int. J. Greenhouse Gas Control, 2021, 106, 103279, DOI:10.1016/j.ijggc.2021.103279.
- M. Johnson, LCH™ Process for the Production of Blue Hydrogen, 2022 Search PubMed.
- M. Aneke and M. Wang, Potential for improving the energy efficiency of cryogenic air separation unit (ASU) using binary heat recovery cycles, Appl. Therm. Eng., 2015, 81, 223–231, DOI:10.1016/j.applthermaleng.2015.02.034.
- D. Peterson, J. Vickers and D. DeSantis, Hydrogen Production Cost From PEM Electrolysis – 2019, DOE Hydrogen and Fuel Cells Program Record, 2020 Search PubMed.
- C. Ganzer and N. Mac Dowell, A comparative assessment framework for sustainable production of fuels and chemicals explicitly accounting for intermittency, Sustainable Energy Fuels, 2020, 4, 3888–3903, 10.1039/C9SE01239G.
- International Energy Agency (IEA), Electolysers, 2022, https://www.iea.org/reports/electrolysers Search PubMed.
- US Department of Energy (DOE), Pathways to Commercial Liftoff: Clean Hydrogen, 2023, https://liftoff.energy.gov/wp-content/uploads/2023/03/20230320-Liftoff-Clean-H2-vPUB.pdf Search PubMed.
- CME Group, Henry Hub Natural Gas – Futures and Options, https://www.cmegroup.com/markets/energy/natural-gas/natural-gas.quotes.html Search PubMed.
- International Energy Agency (IEA), Methane Emissions from Oil and Gas Operations, 2022, https://www.iea.org/reports/methane-emissions-from-oil-and-gas-operations Search PubMed.
- U.S. Energy Information Administration (EIA), Average Texas Electricity Prices Were Higher in February 2021 Due to a Severe Winter Storm, 2021, https://www.eia.gov/todayinenergy/detail.php?id=47876 Search PubMed.
- U.S. Energy Information Administration (EIA), Texas Electricity Profile 2021, 2022, https://www.eia.gov/electricity/state/texas/ Search PubMed.
- National Energy Technology Laboratory (NETL), Carbon Dioxide Transport and Storage Costs in NETL Studies, 2019, DOE/NETL-2019/2044 Search PubMed.
- International Energy Agency (IEA), Is carbon capture too expensive?, 2021, https://www.iea.org/commentaries/is-carbon-capture-too-expensive Search PubMed.
- E. Smith, J. Morris, H. Kheshgi, G. Teletzke, H. Herzog and S. Paltsev, The cost of CO2 transport and storage in global integrated assessment modeling, Int. J. Greenhouse Gas Control, 2021, 109, 103367, DOI:10.1016/j.ijggc.2021.103367.
- Evergreen Action, What Are Clean Energy Tax Credits and How Do They Work?, 2023, https://www.evergreenaction.com/blog/what-are-clean-energy-tax-credits Search PubMed.
- Oil and Gas Climate Initiative, OGCI Members Aim for Zero Methane Emissions from Oil and Gas Operations by 2030, 2022, https://www.ogci.com/news/ogci-members-aim-for-zero-methane-emissions-from-oil-and-gas-operations-by-2030 Search PubMed.
- N. Kaufman and A.-S. Corbeau, The Battle for the US Hydrogen Production Tax Credits, Center on Global Energy Policy at Columbia, 2023, https://www.energypolicy.columbia.edu/the-battle-for-the-us-hydrogen-production-tax-credits/ Search PubMed.
- B. King, G. Hiltbrand, M. Tamba, W. Jones and J. Larsen, Scaling Green Hydrogen in a Post-IRA World, Rhodium Group, 2023, https://rhg.com/research/scaling-clean-hydrogen-ira/ Search PubMed.
- M. Vargas, K. McNutt and C. Seiple, Annual Matching Requirements For New IRA Tax Credits Could Kick-Start Economically Competitive Green Hydrogen Production, Forbes, 2023, https://www.forbes.com/sites/woodmackenzie/2023/03/09/annual-matching-requirements-for-new-ira-tax-credits-could-kick-start-economically-competitive-green-hydrogen-production/?sh=6e71a5014702 Search PubMed.
- W. Ricks, Q. Xu and J. D. Jenkins, Minimizing emissions from grid-based hydrogen production in the United States, Environ. Res. Lett., 2023, 18, 014025, DOI:10.1088/1748-9326/acacb5.
- Montel Group, EC Proposes Monthly Matching in Hydrogen Additionality Rules, 2023, https://www.montelnews.com/news/1442222/ec-proposes-monthly-matching-in-hydrogen-additionality-rules Search PubMed.
- Clean Air Task Force, Re: Implementation of the IRA 45V Clean Hydrogen Tax Credits as it Relates to Guidelines for Emissions Accounting of Grid-Connected Electrolyzers, 2023, https://www.nrdc.org/sites/default/files/2023-03/joint-letter-45v-implementation-20230223.pdf Search PubMed.
- D. Esposito, E. Gimon and M. O'Boyle, Smart Design of 45V Hydrogen Production Tax Credit Will Reduce Emissions and Grow the Industry, Energy Innovation Policy & Technology LLC, 2023, https://energyinnovation.org/wp-content/uploads/2023/04/Smart-Design-Of-45V-Hydrogen-Production-Tax-Credit-Will-Reduce-Emissions-And-Grow-The-Industry.pdf Search PubMed.
- A. Bergman and K. Rennert, Emissions Effects of Differing 45V Crediting Approaches, Resources for the Future, 2023, https://www.rff.org/documents/4057/Report_23-07_noEM5zX.pdf Search PubMed.
- International Energy Agency (IEA), The Future of Hydrogen, 2019, https://www.iea.org/reports/the-future-of-hydrogen Search PubMed.
- S. Kamyans, Decoding the Tax and Climate Law's ‘Green’ Credit Complexities, Bloomberg Tax, 2023, https://news.bloombergtax.com/tax-insights-and-commentary/decoding-the-tax-and-climate-laws-green-credit-complexities Search PubMed.
- E. Ryckebosch, M. Drouillon and H. Vervaeren, Techniques for transformation of biogas to biomethane, Biomass Bioenergy, 2011, 35(5), 1633–1645, DOI:10.1016/j.biombioe.2011.02.033.
- US Department of Energy (DOE), Alternative Fuels Data Center, https://afdc.energy.gov/fuels/natural_gas_renewable.html Search PubMed.
- International Energy Agency (IEA), Cost Curve of Potential Global Biomethane Supply by Region, 2018, https://www.iea.org/data-and-statistics/charts/cost-curve-of-potential-global-biomethane-supply-by-region-2018 Search PubMed.
- S. Bakkaloglu, J. Cooper and A. Hawkes, Methane emissions along biomethane and biogas supply chains are underestimated, One Earth, 2022, 5(6), 724–736, DOI:10.1016/j.oneear.2022.05.012.
- S. E. Tanzer and A. Ramirez, When are negative emissions negative emissions?, Energy Environ. Sci., 2019, 12, 1210–1218, 10.1039/C8EE03338B.
- Lazard, 2023 Levelized Cost of Energy+, 2023, https://www.lazard.com/research-insights/2023-levelized-cost-of-energyplus/ Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |