Open Access Article
Open Access ArticleSolid acid catalyzed esterification of dicyclopentadiene with organic acids to bio-based functional monomers†
Sang-Ho
Chung‡
 ,
Marilena
Demetriou
,
Marilena
Demetriou
 ,
Hongqi
Wang
,
Hongqi
Wang
 and
N. Raveendran
Shiju
and
N. Raveendran
Shiju
 *
*
Van ‘t Hoff Institute for Molecular Sciences, University of Amsterdam, P.O. Box 94157, 1090 GD Amsterdam, The Netherlands. E-mail: n.r.shiju@uva.nl
First published on 29th February 2024
Abstract
Here, we report the synthesis of new functional monomers of dicyclopentadiene (DCPD) by esterification with carboxylic acids that can be derived from biomass. We demonstrate that the esterification of DCPD proceeds via a reaction intermediate, that is, cydecanol (DCPD-OH), and propose an esterification mechanism over solid acid catalysts.
Dicyclopentadiene (DCPD) is one of the interesting cyclic olefins for polymer chemistry.1 DCPD is mostly used in the form of cross-linked DCPD (poly-DCPD), exhibiting excellent impact strength and chemical resistance.2 Yet the lack of chemical functionality of poly-DCPD has restricted its broader application. For example, poly-DCPD cannot be applied as a paint source without oxidizing the surface,3 due to its hydrophobicity and disagreeable odor.4
A functionalized DCPD polymer can be obtained by sequential reactions of (i) functionalization of the DCPD monomer and then (ii) polymerization.4–10 For example, Wang et al. reported the direct addition of aliphatic acid with DCPD using sulfamic acid as a catalyst.10 Saha et al. reported a method for the derivatization of DCPD to obtain alcohol, esters, and ethers.4 For example, treatment of DCPD with SeO2 in a 1,4-dioxane/water medium gave endo-α-1-hydroxydicyclopentadiene in the presence of triethylamine, while ether derivatives were obtained by nucleophilic substitution of alkyl halides in the presence of sodium hydride in dry dimethylfuran.4
Considering the limited amounts of crude oil and the environmental impact of petrochemical processing, a shift in the chemical industry to a sustainable and environmentally friendly solution is necessary.11–15 Thus, reacting petroleum derived olefins with biomass derived acids is an interesting option,16,17 which can help achieve sustainability thresholds.18
Here, we report the functionalization of DCPD with carboxylic acids, which can be derived from renewable feedstocks such as biomass.19 It is known that homogeneous catalysts such as sulfuric acid and hydrochloric acid can catalyze esterification of carboxylic acids with olefins.20,21 However, there are drawbacks to homogeneous acids such as corrosion of equipment and difficult separation of target products from the reaction mixture.22,23 These limitations can be resolved by the employment of heterogeneous catalysts,24,25 providing a corrosion-free atmosphere, easy separation and effective recovery of the catalysts.26 Cationic ion-exchange resins, one of the industrial heterogeneous acid catalysts, are versatile catalysts, particularly with their macroreticular pore structures. Amongst them, Amberlyst resins are strongly acidic catalysts with surface sulfonic groups that can be utilized for esterifications.27,28 In terms of scale up, Amberlyst has some advantages as it is commercially available in large quantities and is relatively cheap. Its properties are well understood and it is used in commercial processes already.
We used heterogeneous Amberlyst resin catalysts for liquid phase esterification of DCPD with levulinic acid (LA), which is obtained from biomass29–32 (Fig. 1a). After 4 h of reaction, the DCPD-LA ester was obtained and its structure was analyzed by 1H nuclear magnetic resonance spectroscopy (NMR) and gas chromatography coupled with mass spectrometry (GC-MS) (Fig. 1b, S1 and S2, ESI†). After esterification, the disappearance of the proton of the carboxylic acid group in levulinic acid (δ1H 11.1 ppm) and the double bond in the norbornene ring of DCPD (5.3–6.0 ppm) are observed (Fig. 1b). Among the possible isomers of DCPD-LA esters, the simulated 1H NMR spectra elucidated that the double bond in the norbornene side reacted to form DCPD-LA ester during the esterification between DCPD and LA, in line with the experimental results (Fig. S2, ESI†). It has been reported that steric repulsion of substituted bicyclic structures in DCPD is the main reason for the difference in reactivity between the two double bonds of DCPD.9,33,34 Because of its more strained structure, the double bond in the norbornene ring in DCPD is more reactive than the bond in the cyclopentene ring.33,34 The GC-MS analysis also indicated the formation of DCPD-LA ester, showing a molecular mass of 248 g mol−1 with the corresponding fragmentation MS patterns (Fig. S1, ESI†).
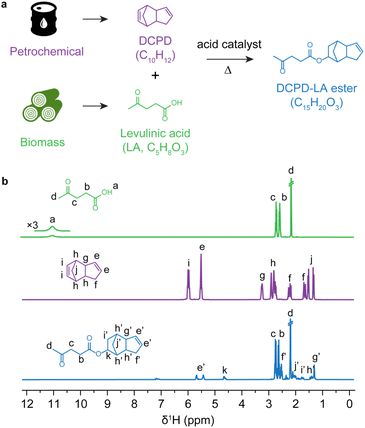 | ||
| Fig. 1 (a) Scheme of the esterification between DCPD and LA. (b) 1H NMR spectrum of DCPD, LA, and the DCPD-LA ester after purification using column chromatography. | ||
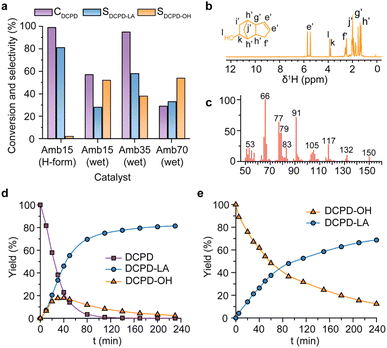
As the physicochemical properties of Amberlyst resin catalysts could play crucial roles in acid catalyzed esterification,35 four different types of Amberlyst resin catalysts were tested for the DCPD esterification with LA. The properties of the Amberlyst resins such as the acidity, the surface area, and the moisture content are summarized in Table S1, ESI.†Fig. 2a shows the results of the DCPD esterification with LA over the Amberlyst resin catalysts. Amberlyst-15 (hydrogen form) showed the highest yield of DCPD-LA ester (80%). However, the wet resin catalysts showed lower DCPD conversion (Fig. 2a). For example, in the case of Amberlyst-15 (wet form), which only differs in the water content compared to Amberlyst-15 (hydrogen form) (Table S1, ESI†), much lower DCPD conversion and DCPD-LA ester selectivity were observed (57% and 28% for CDCPD and SDCPD-LA, respectively). Due to the presence of water in their macroreticular pore system, the rate of esterification can be slowed down, and even the reverse reaction of the esterification, that is, the hydrolysis of the ester to the alcohol, can be promoted.36 Meanwhile, among the wet resin catalysts, the higher acid site concentration leads to higher DCPD conversion and DCPD-LA selectivity, suggesting the importance of the acid amount for the esterification (Fig. 2a, Table S1, ESI††).
Interestingly, the formation of cydecanol (DCPD-OH) was observed during the esterification of DCPD with LA (Fig. 2b and c). During the esterification, DCPD-OH formation was mostly observed for the wet resin catalysts, but Amberlyst-15 (hydrogen form) also produced DCPD-OH. For example, Fig. 2d illustrates the time course of the esterification between DCPD and LA with Amberlyst-15 (hydrogen form). DCPD was consumed completely after 2 h, while the DCPD-LA ester was formed gradually to the maximum yield throughout 4 h of reaction. The formation of DCPD-OH reached maximum at 40 min (18% yield) and then gradually decreased, suggesting that the esterification between DCPD and LA proceeds via a reaction intermediate, that is, DCPD-OH. The kinetic analysis also suggested that the esterification between DCPD and LA is a consecutive reaction of two elementary steps, that is, the hydration of DCPD to form DCPD-OH, followed by the esterification of DCPD-OH and LA (Fig. S3 and S4, ESI†). We deliberately synthesized DCPD-OH (∼95% purity after column chromatography) and performed the esterification of DCPD-OH with LA under identical reaction conditions to DCPD (Fig. 2e). The consumption of DCPD-OH and the simultaneous formation of DCPD-LA ester were observed, which clearly suggests that DCPD-OH acts as an intermediate during the esterification between DCPD and LA.
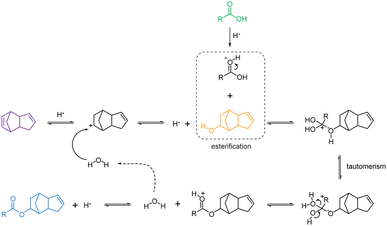
Based on the results, we propose the reaction mechanism for the esterification of DCPD and carboxylic acids (Fig. 3). In the presence of cation-exchange resin, which acts as a proton donor, the addition of a proton to the DCPD molecule can form a dicyclopentadienyl carbonium ion as a transition state, especially in the double bond in the norbornene ring of DCPD.37 The carbonium ion simultaneously reacts with the lone pair of the oxygen atom of the H2O to form DCPD-OH. Then, the esterification between DCPD-OH and the protonated carboxylic acid takes place according to the Fischer esterification reaction.38–40 Under acidic conditions, the addition of a proton to the carbonyl oxygen can lead to a more reactive electrophile carbon. Subsequently, the nucleophilic substitution of the alcohol (1,2-addition by the alcohol) on the electrophile carbon gives a tetrahedral intermediate and one of these hydroxyl groups is eliminated as a water molecule after a proton shift (tautomerism). The protonated ester would be subsequently deprotonated, yielding the ester of DCPD and acids.
It is interesting to note how water is supplied for the hydration step during the esterification. For example, Amberlyst-15 (hydrogen form) only contains less than 1.6% of moisture, which can only give 12% yield of DCPD-OH theoretically, and the amount of water is not sufficient for the observed 80% yield of the DCPD-LA ester (Fig. 2a). We thus believe that the water formed during the esterification is again utilized for the hydration of DCPD. Moreover, we expect the effective removal of water to form DCPD-OH in the system drives the chemical equilibrium towards the completion of the esterification reaction.41
To compare the reactivity of other acids which can be potentially derived from biomass, we further carried out the esterification of DCPD with different carboxylic acids, such as formic acid,42 acetic acid,43 acrylic acid,44 butyric acid45 and hexanoic acid46 (Table 1). The formation of each ester was verified by GC-MS (Fig. S5–S9†) and 1H NMR (Fig. S10–S14†). DCPD was fully consumed with shorter chain carboxylic acids (formic, acetic and acrylic acids), while the DCPD conversion was lower for butyric and hexanoic acids. We expect two contributions to the observed lower product yield for longer chain carboxylic acids. The inductive effect could result in an increase in the electron-releasing ability of the acid with a longer alkyl chain.47 Although the inductive effect facilitates the protonation of the carbonyl oxygen, it also lowers the electrophilicity of the carbonyl carbon, resulting in a more energy-hindered nucleophilic attack by the alcohol36 (DCPD-OH in our case). Furthermore, the steric hindrance can also increase with the molecular size, inducing electronic repulsion between nonbonded atoms of reacting molecules. This repulsive hindrance lowers electron density in the intermolecular region and disturbs bonding interactions. Therefore, as the alkyl chain in the carboxylic acid increases, its steric effect can increase as well.36
| Acid | Chemical structure of acid | Product structure | DCPD conversion (%) | Product yield (%) |
|---|---|---|---|---|
a Reaction conditions: 80 °C, mole ratio (DCPD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carboxylic acid) = 1 carboxylic acid) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, catalyst loading = 20 g-dry resin L−1. 1, catalyst loading = 20 g-dry resin L−1.
|
||||
| Formic acid |
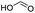
|
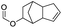
|
>99 | 86 |
| Acetic acid |
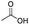
|
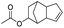
|
>99 | 78 |
| Acrylic acid |
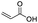
|
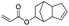
|
>99 | 62 |
| Butyric acid |
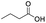
|
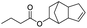
|
97 | 35 |
| Hexanoic acid |
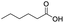
|
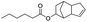
|
72 | 25 |
To compare with other resins, we tested Dowex 50WX8 for the reaction under identical conditions. However, the conversion was low. We think that the different structures (gel-type Dowex and macroreticular-type Amberlyst) and the surface textures after coming into contact with water (or other solvents) give different reactivities.48 Kuzminska et al. suggested using get-type resins in a polar medium in order to induce their swelling and to provide good accessibility to active sites.48 For our system, we used a non-polar solvent (toluene). The test indicates that not all resins will give the same reactivity for this reaction.
To test the possible leaching of sulfonic acid groups, we carried out a leaching test using Amberlyst-15. After 20 min of reaction, the catalyst was removed quickly by filtration, and the reaction was continued in the absence of the catalyst. No further conversion of DCPD was observed after removal of the catalyst from the reaction mixture (at 20 min), indicating that the active species of the resin catalyst were not leached out (Fig. S15†). We believe that leaching is avoided because the reaction temperature (80 °C) is much lower than the maximum operating temperature of Amberlyst-15 (120 °C) and the reaction is performed in the non-polar solvent, toluene.
We noted a small increase in the mass of the catalyst after the reaction, suggesting that the pores may contain the reaction solvent, reactants or products. Therefore, the reactivation and regeneration of the catalyst are necessary before the recycling test. For this, we carefully washed the resin catalyst with minimum amounts of water, ethanol and acetone and dried at 112 °C. A couple of recycling tests were then carried out using this procedure which gave similar results, indicating the restoration of the activity.
In summary, we report the direct esterification of DCPD with acids that can be derived from biomass, using Amberlyst resin catalysts. During the esterification, we found that DCPD-OH was formed as a key reaction intermediate and the proposed reaction mechanism includes the acid catalyzed formation of a dicyclopentadienyl carbonium ion to form DCPD-OH by the lone pair of the oxygen atom of H2O. Finally, conventional Fischer esterification between DCPD-OH and the protonated carboxylic acids can yield DCPD-esters.
Author contributions
N. R. S. conceptualized the work, acquired the funding and administered the project. S. C. performed preliminary experiments to validate the concept. M. D. conducted catalytic tests and formal analysis. H. W. conducted additional experiments for the revision. M. D. wrote the original draft, and S. C. and N. R. S. reviewed and edited the draft. All the authors discussed the experiments, analysed the data and contributed to the writing of the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support of the EFRO-Project Green Innovation Cluster and GF Biochemicals. We thank N. J. Geels, P. F. Collignon, E. Zuidinga and M. C. Mittelmeijer–Hazeleger (University of Amsterdam) for the technical support.Notes and references
-
T. T. P. Cheung, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2001, vol. 2 Search PubMed
.
- T. A. Davidson, K. B. Wagener and D. B. Priddy, Macromolecules, 1996, 29, 786–788 CrossRef CAS
.
- J. Chen, F. P. Burns, M. G. Moffitt and J. E. Wulff, ACS Omega, 2016, 1, 532–540 CrossRef CAS PubMed
.
- S. Saha, Y. Ginzburg, I. Rozenberg, O. Iliashevsky, A. Ben-Asuly and N. G. Lemcoff, Polym. Chem., 2016, 7, 3071–3075 RSC
.
- Q. Deng, X. Zhang, L. Wang and J.-J. Zou, Chem. Eng. Sci., 2015, 135, 540–546 CrossRef CAS
.
- H. Ren, J. Z. Sun, B. J. Wu and Q. Y. Zhou, Polymer, 2006, 47, 8309–8316 CrossRef CAS
.
- M. Worzakowska, Polym. Bull., 2012, 68, 775–787 CrossRef CAS
.
- J. V. Crivello and S. Y. Song, Chem. Mater., 2000, 12, 3674–3680 CrossRef CAS
.
- S. H. Chung, G. H. Park, N. Schukkink, H. Lee and N. R. Shiju, Chem. Commun., 2023, 59, 756–759 RSC
.
- B. Wang, Y. L. Gu, L. M. Yang, J. S. Suo and O. Kenichi, Catal. Lett., 2004, 96, 70–74 Search PubMed
.
- J. Mors and N. Raveendran Shiju, Sustainable Chemistry for Climate Action, 2023, 2, 100013 CrossRef
.
- S. Shirazimoghaddam, I. Amin, J. A. Faria Albanese and N. R. Shiju, ACS Eng. Au, 2023, 3, 37–44 CrossRef CAS PubMed
.
- S. H. Chung, S. de Haart, R. Parton and N. Raveendran Shiju, Sustainable Chemistry for Climate Action, 2022, 1, 100004 CrossRef
.
- W. Zhang, G. Innocenti, P. Oulego, V. Gitis, H. Wu, B. Ensing, F. Cavani, G. Rothenberg and N. R. Shiju, ACS Catal., 2018, 8, 2365–2374 CrossRef CAS PubMed
.
- E. Schuler, M. Morana, N. R. Shiju and G.-J. M. Gruter, Sustainable Chemistry for Climate Action, 2022, 1, 100001 CrossRef
.
- T. Willke and K. D. Vorlop, Appl. Microbiol. Biotechnol., 2004, 66, 131–142 CrossRef CAS PubMed
.
- R. Beerthuis, G. Rothenberg and N. R. Shiju, Green Chem., 2015, 17, 1341–1361 RSC
.
- M. Bachmann, C. Zibunas, J. Hartmann, V. Tulus, S. Suh, G. Guillen-Gosalbez and A. Bardow, Nat Sustainability, 2023, 6, 599–610 CrossRef
.
- E. M. Karp, R. M. Cywar, L. P. Manker, P. O. Saboe, C. T. Nimlos, D. Salvachua, X. Q. Wang, B. A. Black, M. L. Reed, W. E. Michener, N. A. Rorrer and G. T. Beckham, ACS Sustainable Chem. Eng., 2018, 6, 15273–15283 CrossRef CAS
.
- R. Altschul, J. Am. Chem. Soc., 1946, 68, 2605–2609 CrossRef CAS
.
-
G. I. Badea, G. L. Radu, Carboxylic acid – Key role in life sciences, InTechOpen, 2018, ch. 2 Search PubMed
.
- N. S. Babu, R. Sree, P. S. S. Prasad and N. Lingaiah, Energy Fuels, 2008, 22, 1965–1971 CrossRef CAS
.
-
G. Kalavathy, A. Pandey, E. Gnansounou and B. Gurunathan, in Biofuels and Bioenergy, ed. B. Gurunathan and R. Sahadevan, Elsevier, 2022, pp. 627–652, DOI: DOI:10.1016/B978-0-323-90040-9.00012-6
.
- G. Jyoti, A. Keshav, J. Anandkumar and S. Bhoi, Int. J. Chem. Kinet., 2018, 50, 370–380 CrossRef CAS
.
- Y. Li, S. Zhang, Z. Li, H. Zhang, H. Li and S. Yang, Fuel, 2022, 329, 125467 CrossRef CAS
.
- M. M. Sharma, React. Funct. Polym., 1995, 26, 3–23 CrossRef CAS
.
- J. Gangadwala, S. Mankar, S. Mahajani, A. Kienle and E. Stein, Ind. Eng. Chem. Res., 2003, 42, 2146–2155 CrossRef CAS
.
- M. Rat, M. H. Zahedi-Niaki, S. Kaliaguine and T. O. Do, Microporous Mesoporous Mater., 2008, 112, 26–31 CrossRef CAS
.
- H. Li, Z. Fang, R. L. Smith and S. Yang, Prog. Energy Combust. Sci., 2016, 55, 98–194 CrossRef
.
- W. Luo, U. Deka, A. M. Beale, E. R. H. van Eck, P. C. A. Bruijnincx and B. M. Weckhuysen, J. Catal., 2013, 301, 175–186 CrossRef CAS
.
- D. M. Alonso, S. G. Wettstein, M. A. Mellmer, E. I. Gurbuz and J. A. Dumesic, Energy Environ. Sci., 2013, 6, 76–80 RSC
.
- W. Luo, M. Sankar, A. M. Beale, Q. He, C. J. Kiely, P. C. A. Bruijnincx and B. M. Weckhuysen, Nat. Commun., 2015, 6, 6540 CrossRef CAS PubMed
.
- S. Tsuchiya, I. Imai, A. Ohshima and H. Hayashi, J. Jpn. Pet. Inst., 1984, 27, 26–30 CrossRef CAS
.
- G. Zanchin, G. Leone, I. Pierro, A. Rapallo, W. Porzio, F. Bertini and G. Ricci, Macromol. Chem. Phys., 2017, 218, 1600602 CrossRef
.
- R. Tesser, M. Di Serio, L. Casale, G. Carotenuto and E. Santacesaria, Can. J. Chem. Eng., 2010, 88, 1044–1053 CrossRef CAS
.
- Y. J. Liu, E. Lotero and J. G. Goodwin, J. Catal., 2006, 243, 221–228 CrossRef CAS
.
- K. Roth, Z. fur Naturforsch. - B, 1977, 32, 76–81 CrossRef
.
- E. Fischer and A. Speier, Ber. Dtsch. Chem. Ges., 1895, 28, 3252–3258 CrossRef CAS
.
- S. Samanta and R. Srivastava, Sustainable Energy Fuels, 2017, 1, 1390–1404 RSC
.
- V. Russo, C. Rossano, E. Salucci, R. Tesser, T. Salmi and M. Di Serio, Chem. Eng. Sci., 2020, 228, 115974 CrossRef CAS
.
- C. H. Tsai, H. T. Chen, S. M. Althaus, K. M. Mao, T. Kobayashi, M. Pruski and V. S. Y. Lin, ACS Catal., 2011, 1, 729–732 CrossRef CAS
.
- F. Valentini, V. Kozell, C. Petrucci, A. Marrocchi, Y. Gu, D. Gelman and L. Vaccaro, Energy Environ. Sci., 2019, 12, 2646–2664 RSC
.
- T. Sarchami, N. Batta and F. Berruti, Biofuels, Bioprod. Biorefin., 2021, 15, 1912–1937 CrossRef CAS
.
- S. Yang, M. Kim, S. Yang, D. S. Kim, W. J. Lee and H. Lee, Catal. Sci. Technol., 2016, 6, 3616–3622 RSC
.
- D. Salvachúa, P. O. Saboe, R. S. Nelson, C. Singer, I. McNamara, C. del Cerro, Y.-C. Chou, A. Mohagheghi, D. J. Peterson, S. Haugen, N. S. Cleveland, H. R. Monroe, M. T. Guarnieri, E. C. D. Tan, G. T. Beckham, E. M. Karp and J. G. Linger, Cell Rep. Phys. Sci., 2021, 2, 100587 CrossRef
.
- Q. Wu, X. Bao, W. Guo, B. Wang, Y. Li, H. Luo, H. Wang and N. Ren, Biotechnol. Adv., 2019, 37, 599–615 CrossRef CAS PubMed
.
- J. H. Clark, R. Luque and A. S. Matharu, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 183–207 CrossRef CAS PubMed
.
- M. Kuzminska, R. Backov and E. M. Gaigneaux, Appl. Catal., A, 2015, 504, 11–16 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01019h |
| ‡ Current address: KAUST Catalysis Center (KCC), King Abdullah University of Science and Technology, Thuwal 23955, Saudi Arabia. |
| This journal is © The Royal Society of Chemistry 2024 |