Open Access Article
Open Access ArticleCarbon-based nanocomposites for biomedical applications
Minkyu Shin†
a,
Joungpyo Lim†
a,
Yongseon Park
a,
Ji-Young Leea,
Jinho Yoon
*b and
Jeong-Woo Choi
 *a
*a
aDepartment of Chemical & Biomolecular Engineering, Sogang University, 35 Baekbeom-ro, Mapo-gu, Seoul 04107, Republic of Korea. E-mail: jwchoi@sogang.ac.kr
bDepartment of Biomedical-Chemical Engineering, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu, Bucheon-si, Gyeonggi-do 14662, Republic of Korea. E-mail: jyoon@catholic.ac.kr
First published on 28th February 2024
Abstract
Carbon nanomaterials have attracted significant attention in the biomedical field, including for biosensing, drug delivery, and tissue engineering applications. Based on their inherent properties such as their unique structure and high conductivity, carbon nanomaterials can overcome the current limitations in biomedical research such as poor stability of biomolecules, low sensitivity and selectivity of biosensors, and difficulty in precise drug delivery. In addition, recently, several novel nanomaterials have been integrated with carbon nanomaterials to develop carbon-based nanocomposites for application in biomedical research. In this review, we discuss recent studies on carbon-based nanocomposites and their biomedical applications. First, we discuss the representative carbon nanomaterials and nanocomposites composed of carbon and other novel nanomaterials. Next, applications of carbon nanomaterials and nanocomposites in the biomedical field are discussed according to topics in the biomedical field. We have discussed the recent studies on biosensors, drug delivery, and tissue engineering. In conclusion, we believe that this review provides the potential and applicability of carbon nanomaterials and their nanocomposites and suggests future directions of the application of carbon-based nanocomposites in biomedical applications.
1. Introduction
In the field of biology, the research topics receiving the most attention recently are biomedical applications including accurate biosensing, drug delivery, and tissue engineering or regeneration.1,2 Significant research is being conducted to achieve various goals, such as higher sensitivity and selectivity of biosensors, precise delivery of drugs, and rapid and effective recovery of damaged tissue from the perspective of tissue engineering.3–5 However, several obstacles exist in effectively achieving each research goal. For instance, achieving a high accuracy and selectivity using biomolecule-based biosensors is challenging because of the intrinsic limitations of biomolecules including nucleic acids and enzymes. Furthermore, the precise control of drug release rate and its localization is difficult to achieve well. Accordingly, to address these obstacles, several strategies and materials have been introduced into the biomedical field.6–8Recently, several nanomaterials and nanotechnologies have been introduced to overcome the limitations and disadvantages of conventional techniques and conduct efficient biomedical research.9,10 In particular, nanomaterials have several advantages in terms of biomedical applications, including a unique nanoscale structure such as nanoporosity, a large surface area, superconductivity, and unique plasmonic properties.11,12 Among the various nanomaterials, carbon has attracted considerable attention because of its exorbitant and broad applicability; therefore, it has been utilized in various fields from the development of battery electrodes to the piezoelectric field.13–15 Particularly, it has huge potential in biomedical field. Therefore, carbon is considered a promising material for the development of sensors with high sensitivity/selectivity, effective drug delivery and release control, and excellent tissue regeneration. For example, research is being conducted on the development of highly sensitive biosensors using excellent the conductivity or quenching properties of carbon, the production of carbon-based drug delivery platform, and the development of implementable nanomaterials for tissue regeneration based on carbon supports which exhibit excellent biocompatibility.16–18 Moreover, in recent years, through the development of carbon-based nanocomposites that combine carbon and other functional nanomaterials, such as transition metal dichalcogenide (TMD) and MXene, these nanocomposites, which have superior properties compared to carbon alone and can be used in the biomedical field, are being studied.19,20 It is expected that these efforts will contribute to the effective utilization of carbon nanomaterials into biomedical applications.
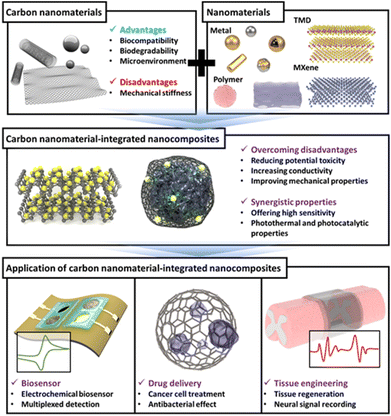
In this review, we discuss carbon nanomaterials, their integration with other nanomaterials, and their applications in biomedical fields, with a selective overview of recent studies. First, several carbon nanomaterials, such as carbon nanotubes (CNTs), graphene, carbon dots (CDs), and carbon-based nanocomposites composed of other newly reported nanomaterials are discussed. Next, based on specific topics in the biomedical field that are currently being researched the most, recent literature on applications of carbon-based nanocomposites is discussed in the order of biosensors, drug delivery, and tissue engineering (Fig. 1). We believe that this review will highlight the superiority and potential of carbon nanomaterials and suggest the future direction of carbon-based nanocomposites and their application in new biomedical fields.
2. Carbon-based nanocomposites
Carbon nanomaterials are one of the most attractive nanomaterials that are composed of carbon atoms and have a unique nanoscale structure. Carbon nanomaterials exhibit superior electrical, mechanical, and optical properties because of their unique geometry and arrangement.21 Specifically, carbon nanomaterials including CNTs, graphene, graphene oxide (GO), and CDs have been widely researched for biological applications because of the electrical conductivity, biocompatibility, and optical properties.22 For example, CDs are carbon nanoparticles less than 10 nm in size that have excellent photoluminescence properties and biocompatibility, low toxicity, and, most notably, exhibit a quenching effect, which allows them to emit their own fluorescent signal, making them an attractive candidate for bioimaging and drug delivery applications.23 The two main approaches to CD synthesis are top-down and bottom-up.24 Top-down methods involve the cutting and exfoliation of carbon sources such as graphite. Various methods are used in top-down approaches such as oxidative cleavage, hydrothermal/melting heat synthesis, electrochemical synthesis, and microwave and ultrasonic-assisted methods. Bottom-up methods include pyrolyzing small organic molecules, such as glucose, and can be mixed with other molecules that provide heteroatomic doping atoms.One of the most commonly used carbon nanomaterials in the biomedical field is CNTs. Methods for synthesizing CNTs include chemical vapor deposition (CVD), arc discharge, and laser ablation.25 Of these, CVD, which involves the decomposition of hydrocarbon gases such as methane or ethylene at high temperatures in the presence of a metal catalyst (e.g., iron, nickel), is the most commonly used. CNTs are categorized into single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) based on their structure. SWCNTs are composed of a single layer of graphene rolled into a cylindrical tube (Fig. 2a).26 The diameter typically ranges from 0.4 to 3 nm, and their length can be several micrometers. They exhibit remarkable electrical conductivity and can be either metallic or semiconducting depending on the rolling angle (chirality) of the graphene sheet. They also have extraordinary tensile strength and thermal conductivity. Because of these properties, SWCNTs have been explored for application in nanoelectronics and field-effect transistors (FETs) and as fillers in composite materials. In contrast, MWCNTs consist of multiple layers of graphene sheets rolled into concentric cylinders and have diameters ranging from 2 to 100 nm.27 Their properties are similar to those of SWCNTs, such as high mechanical strength and electrical conductivity, but they typically have lower electrical and thermal conductivities compared to SWCNTs due to interlayer electron scattering. MWCNTs are used in various applications, including energy storage, biosensors, and reinforced nanocomposite materials. Graphene is also widely used in biomedical applications. Graphene is a single layer of carbon atoms arranged in a two-dimensional honeycomb lattice.
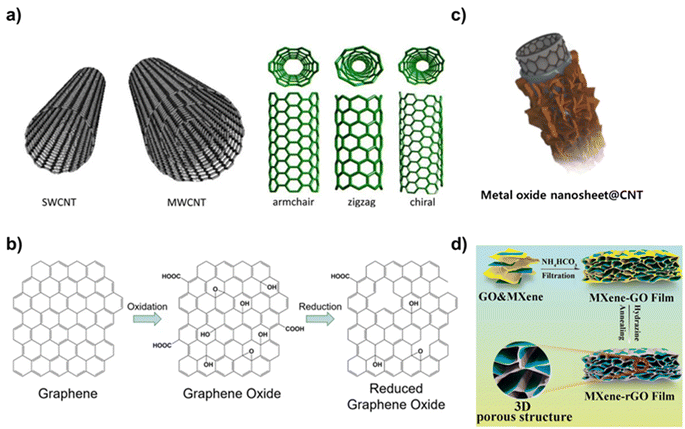 | ||
| Fig. 2 Representative carbon nanomaterials and carbon-based nanocomposites. (a) Schematic diagram of SWCNT and MWCNT and the various forms of SWCNTs. Reproduced from ref. 26 with permission under the terms of the CC-BY Creative Commons attribution 4.0. (b) Scheme of chemical structures of graphene, graphene oxide, and reduced graphene oxide and their synthetic processes. Reproduced from ref. 28 with permission under the terms of the CC-BY Creative Commons attribution 4.0. (c) Schematic illustration of metal oxide nanosheet@CNT nanocomposites. Reproduced from ref. 30 with permission under the terms of the CC-BY Creative Commons attribution 4.0, and (d) schematic diagram of a structure of MXene-rGO film. Reproduced from ref. 32 with permission under the terms of the CC-BY Creative Commons attribution 4.0. | ||
Graphene is known for its exceptional strength, flexibility, electrical and thermal conductivity, and large surface area.28 Graphene is also transparent and impermeable to most gases and liquids. These properties make graphene suitable for use in bioelectronic devices, supercapacitors, and as a reinforcing agent in nanocomposite materials (Fig. 2b). A variety of synthesis techniques are used to synthesize graphene, including epitaxial growth, liquid phase exfoliation, electrochemical exfoliation, mechanical exfoliation, and CVD.29 In CVD, the hot iridium surface is exposed to ethylene. The ethylene thermally decomposes on the surface, and the carbon forms a graphene monolayer by adsorbing on the surface. In graphene oxide (GO), graphene is chemically modified with oxygen-containing groups such as hydroxyl, epoxide, and carboxyl groups.30 These functional groups make GO hydrophilic and dispersible in aqueous solutions, although they disrupt the sp2 bonding network, diminishing its electrical conductivity compared to pristine graphene. GO is used for water purification, in drug delivery, and as a precursor for preparing other graphene-based materials. The Hummers' method is the most common method to synthesize GO.31 It involves treating graphite powder with a strong oxidizing agent, such as potassium permanganate or sulfuric acid. This process introduces oxygen-containing groups into the graphite structure, making it hydrophilic and capable of exfoliating. Similarly, reduced graphene oxide (rGO) is derived from GO, in which oxygen-containing functional groups are partially removed through chemical, thermal, or other reduction methods.32 For example, chemical reduction method involves treating GO with reducing agents like hydrazine, sodium borohydride, or ascorbic acid to remove oxygen-containing groups and restore the graphene structure.33 The reduction process restores the sp2 carbon network, improving its electrical conductivity compared to the GO while maintaining some functional groups that aid in solubility and processability. rGO is used in applications in which a conductive material is required, such as in energy storage, biosensors, and nanocomposites. Each of these carbon nanomaterials possesses a unique set of properties that make them suitable for specific applications, from bioelectronics and energy storage to biomedical applications and biosensors. The material choice often depends on the required balance between conductivity, mechanical strength, surface chemistry, and biocompatibility for the intended application.
As shown, carbon nanomaterials are available in various structures and have related properties, making them easy to apply in biomedical applications. In addition, with the recent significant advances in the field of materials, carbon-based nanocomposites, which introduce other kinds of nanomaterials, have attracted considerable attention because of their wide range of application potential.34 In particular, carbon nanomaterials can be easily integrated with various nanomaterials due to the versatility of modifying their structure and adding surface functional groups.35 Integration of these additional nanomaterials to carbon nanomaterials can not only compensate for the weaknesses of carbon nanomaterials but also maximize their intrinsic strengths and impart new properties. Studies have been conducted on the introduction of various types of nanomaterials, including metals. Several metal nanomaterials such as gold, silver, and iron oxide nanoparticles can be combined with carbon nanomaterials to enhance imaging and targeting capabilities.36 For example, gold nanoparticles can support imaging and photothermal therapy, and iron oxide nanoparticles can add magnetic properties useful for magnetic resonance imaging (MRI) and drug targeting (Fig. 2c).30 These modifications improve imaging capabilities and exhibit additional therapeutic functions.37 They also improve the targeting ability of nanocomposites. Polymers such as polyacrylonitrile and polyvinyl alcohol (PVA) are often used to coat carbon nanomaterials.38
These coatings provide a biocompatible barrier around carbon nanomaterials, reducing their potential toxicity and improving biocompatibility. The rate of drug release from nanocomposites can also be precisely controlled by adjusting the mechanical properties of the polymer.39 Among polymers, hydrogels are the most commonly used.40 Hydrogels are hydrophilic polymer networks that can hold large amounts of water and can mimic the properties of the extracellular matrix. When a nanocomposite is introduced into a cell or tissue, it can be coated with a hydrogel to induce the desired effect while maintaining the function of the tissue.41 In addition, silica dioxide (SiO2) can be used to coat carbon nanomaterials.42 Silica coating can improve the structural integrity of carbon nanomaterials, reduce toxicity, and provide a platform for further functionalization with therapeutic agents. By incoporating transition metal dichalcogenides (TMDs) or MXenes into carbon nanomaterials, hybrid nanocomposites with enhanced properties and functionalities can be developed.43,44 TMDs such as MoS2, WS2, and MXenes (a family of 2D inorganic compounds, composed of atomically thin layers of transition metal carbides, nitrides, or carbonitrides) are known for their unique electronic, optical, and catalytic properties.45 When combined with carbon nanomaterials such as graphene or carbon nanotubes, the resulting composites exhibit synergistic properties that are beneficial for various biomedical applications (Fig. 2d).32 Specifically, because TMDs and MXene have excellent electrical conductivity, hybrid nanocomposites can be excellent candidates for biosensors, offering high sensitivity and selectivity for detecting biomolecules.46 In addition, photothermal and photocatalytic properties of TMDs and MXene enable various applications.47 Because of these properties, the nanomaterials can be applied to drug delivery, photothermal therapy, and medical imaging. In the following chapter, various types of carbon-based nanocomposites with their applications in the biomedical field including biosensors, drug delivery, and tissue engineering will be discussed (Table 1).
| Nanomaterials | Properties | Ref. | |
|---|---|---|---|
| Carbon nanomaterials | Carbon dot | - Excellent photoluminescence properties, biocompatibility, low toxicity, and, quenching effect | 23 |
| SWCNT | - Remarkable electrical conductivity | 26 | |
| MWCNT | - High mechanical strength and electrical conductivity | 27 | |
| Graphene | - Exceptional strength, flexibility, electrical and thermal conductivity, and large surface area | 28 | |
| Graphene oxide | - Hydrophilic and dispersible in aqueous solutions | 30 | |
| Reduced graphene oxide | - Improved electrical conductivity compared to GO while maintaining some functional groups | 32 | |
| Carbon-based nanocomposites | Metal/carbon nanomaterials | - Supporting imaging and photothermal properties, and magnetic properties | 36 |
| Polymer/carbon nanomaterials | - Providing a biocompatible barrier, reducing potential toxicity, and improving biocompatibility | 39 | |
| Silica/carbon nanomaterials | - Improving the structural integrity of carbon nanomaterials, reducing toxicity, and providing a platform for further functionalization | 42 | |
| TMD-MXene/carbon nanomaterials | - Unique electronic, optical, and catalytic properties, with photothermal and photocatalytic properties | 46 |
3. Development of biosensors by carbon-based nanocomposites
Among the various biomedical fields in which carbon nanomaterials can be used, carbon nanomaterials are attracting attention as a core component for biosensor development. Based on their unique properties, carbon nanomaterials can improve the sensing ability of biosensors and are being utilized to develop highly sensitive and selective biosensors capable of detecting important or harmful target molecules precisely. Particularly, because of their unique properties, they can be directly applied to develop electrochemical and fluorescent biosensors, which are the most actively researched among numerous types of biosensors including lateral flow assay (LFA), colorimetric, and surface-enhanced Raman spectroscopic (SERS) biosensors.48,49 Carbon nanomaterials can amplify the electrochemical or fluorescent signals generated from target detection by biosensors or it can block the fluorescent signals because of their quenching properties. In addition, some carbon nanomaterials can emit fluorescent signals themselves. In this section, recent studies focussing on carbon-based nanocomposite-based electrochemical and fluorescent biosensors are discussed.Among the various techniques used for the development of biosensors such as SERS, LFA, optical, and piezoelectric techniques, an electrochemical technique has considerable potential to be used in point-of-care testing (POCT) applications because of its advantages such as fast response and sophisticated and easy diagnosis. Therefore, it is widely used in biosensor development.
Furthermore, the COVID-19 pandemic has led to considerable research being conducted on the development of POCT biosensors, with electrochemical biosensors attracting significant attention.50 During the operation of electrochemical biosensors, changes in electrochemical signals caused by the interaction between targets and probes of biosensors enable the quantitative and qualitative detection of targets. Here, the excellent conductive property of carbon nanomaterials can contribute to the development of highly conductive electrodes for biosensors, thereby allowing the biosensor to detect even the smallest changes in electrochemical signals. Therefore, carbon nanomaterials have garnered considerable attention in the development of electrodes for electrochemical biosensors.51 In one study, carboxylated SWCNTs were introduced on carbon-based screen-printed electrodes (SPEs) to develop an electrochemical biosensor for the detection of point-of-care antibodies (Anti-S protein) against SARS-CoV-2 (Fig. 3a).52 Here, after the introduction of SWCNT on the SPEs, p-phenylenediamine was modified to make the amine groups for SARS-CoV-2 Spike protein immobilization capable of capturing SARS-CoV-2 antibodies. Because of the highly conductive electrode composed of SWCNTs, the developed biosensor based on electrochemical impedance spectroscopy (EIS) exhibited a sensitive limit of detection of 0.7 pg mL−1 with a wide linear response ranging from 1.0 pg mL−1 to 10 ng mL−1. It successfully detected SARS-CoV-2 antibodies in human positive sera at a detection level of 2.3 μg mL−1. In another study, conductive hydrogel-based electrochemical biosensors composed of polypyrrole and sulfonated multi-walled CNT (MWCNT) were developed to detect dopamine released from PC12 cells.53 By introducing conductive CNT to hydrogel, the developed conductive hydrogel exhibited a high conductivity and biocompatibility. In addition to CNT, graphene is also used to develop electrochemical biosensors. Moreover, size control of graphene is relatively easy; therefore, it can be integrated with other functional nanomaterials. For instance, the graphene sponge (GS) was combined with glucose oxidase (GOx) and chitosan (CTS) to develop a flexible enzymatic electrochemical glucose biosensor (Fig. 3b).54 The sponge structure of graphene has attracted attention in the biomedical field due to its properties including large surface area and loading capacity, high conductivity, biocompatibility, and mechanical strength. After producing GS from the pure graphene using a hydrothermal method, GOx and CTS were loaded onto the porous structure of the GS. The GS structure could absorb, disperse, and protect the loaded GOx well. The prepared GS-based complex was then prepared on the flexible polyimide electrode and used for the detection of glucose. The developed flexible biosensor exhibited excellent bending properties and detected glucose in human sweat with a low detection sensitivity of 2.45 μM through amperometric sensing; this result was more sensitive than that of biosensors prepared using CNT. In the other studies, to address the conductivity issue of GO, conductive metal nanoparticles were introduced to reduced GO (rGO) or the nanoribbon structure of the GO and used for the detection of miRNA-21 and Human papillomavirus-16 (HPV-16) in an electrochemical manner.55,56 In addition, to maximize the conductivity of the rGO, a nanocomposite was developed by combining rGO and molybdenum disulfide (MoS2) nanoparticles, a TMD nanomaterial and was used to develop an electrochemical biosensor.57 In this study, a carbon-based nanocomposite composed of rGO and MoS2 nanoparticles was further combined with a conductive polymer [poly(3,4-ethoxylenedioxythiophene), PEDOT] to develop an electrochemical parathyroid hormone biosensor. In addition to several viruses and glucose, important nucleic acids including miRNAs which are required to be monitored have been detected using biosensors for broadening the application areas of carbon-based nanocomposites.58,59 For example, the carbon-based nanocomposite composed of gold and rGO was employed as an electrode for detection of miRNA-122 in an electrochemical manner.60 In the other study, a probe DNA was modified on the gold-decorated laser-induced graphene electrode for detection of miRNA-141 using differential pulse voltammetry (DPV) technique.61 Besides, these recent studies for development of electrochemical biosensors based on carbon-based nanocomposites have been applied for patent registration such as SWCNT-based microscale biosensor (European patent registration number: 03030891) and hormone monitoring biosensor composed of carbon-based SPEs (US patent registration number: 11801000). In commercial market field, still, most products and companies including Abbott and SD BIOSENSOR hugely focus on the monitoring of glucose level which is important to maintain the human health. Although most of commercialized electrochemical biosensors focus on detection of glucose level based on non-carbon materials (Gold), several efforts are being conducted to achieve the commercialization of carbon nanomaterials or carbon-based nanocomposite assisted electrochemical biosensors such as laser-induced graphene based wearable electrochemical glucose biosensor.62 As seen in this study, recently, several newly reported nanomaterials are being combined with carbon nanomaterials to improve the conductivity and facilitate electron transfer reactions. For example, MXene and nickel oxide were incorporated with rGO and used as an electrode of an electrochemical biosensor for the detection of influenza viruses (H1N1 and H5N2) and viral surface protein hemagglutinin.63
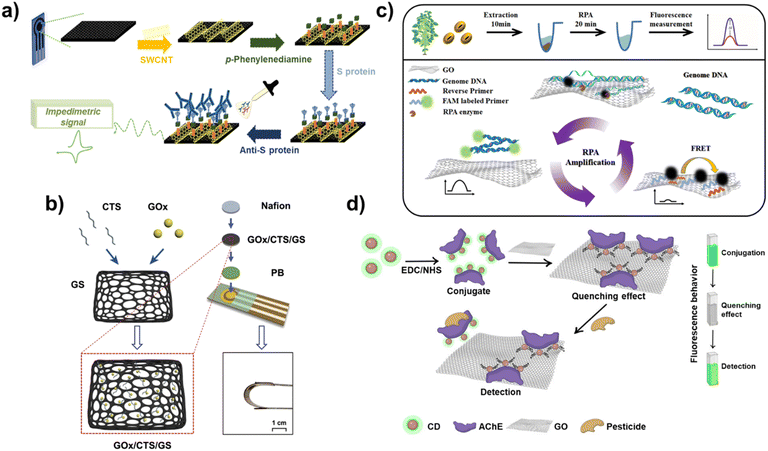 | ||
| Fig. 3 Electrochemical and fluorescent biosensors based on carbon-based nanocomposites. (a) Schematic diagram of an electrochemical SARS-CoV-2 antibody biosensor by using SPE with SWCNT. Reproduced from ref. 52 with permission from Elsevier, copyright 2022, (b) scheme of flexible electrochemical glucose biosensor composed of GOx, CTS, and GS. Reproduced from ref. 54 with permission from Elsevier, copyright 2022, (c) schematic illustration of RPA-mediated fluorescent biosensor composed of the GO and FAM-labeled primers for detection of RNAi transgenic plants. Reproduced from ref. 64 with permission from Elsevier, copyright 2021, and (d) schematic diagram of a fluorescent biosensor composed of AChE, CD and GO for detection of organophosphate pesticide. Reproduced from ref. 68 with permission under the terms of the CC-BY Creative Commons attribution 4.0. | ||
In addition to electrochemical biosensors, the fluorescent technique has also attracted attention to develop biosensors using the unique fluorescence properties of carbon nanomaterials. Because of the ease of verification of biosensing and its capability for multiplexed detection, fluorescent biosensors have been studied in detail. In the operation of fluorescent biosensors, carbon nanomaterials are used to realize the appearance and disappearance of fluorescence signals of the fluorescent probe of biosensor through diagnosis of target molecules. The first characteristic of carbon nanomaterials that can be used in the development of fluorescent biosensors is their quenching property. When fluorescent molecules are located on the surface of carbon nanomaterials, the emission of fluorescence signals is blocked by the quenching property of carbon nanomaterials. However, through the detection of target molecules, if the fluorescent molecules are released from the carbon nanomaterials, their fluorescence signals are recovered. Based on this biosensing mechanism, several fluorescent biosensors have been reported. For instance, recombinase polymerase amplification (RPA)-mediated fluorescent biosensors have been developed using the quenching property of GO and FAM-labelled primers capable of detecting RNA interference (RNAi) transgenic plants.64 In this fluorescent biosensor, GO was introduced to achieve two goals. The first goal was to improve the RPA efficiency by absorption of molecules on the GO to induce local aggregation because of its unique blanket-like structure. The second goal was to block fluorescent signals from FAM-labelled primers in the absence of target sequences. As shown in Fig. 3c, in the absence of target DNA sequences, this biosensor did not emit fluorescent signals; however, in the presence of targets, fluorescent signals from FAM were recovered due to binding with the targets and release from GO. Furthermore, due to the enhanced RPA efficiency by the GO, highly sensitive detection could be achieved. These results indicate that this biosensor could perform on-site detection of RNAi soybeans with high sensitivity and a detection limit of 1.5 ng genomic DNA in a rapid manner (within 20 min). In another study, a GO-assisted fluorescent biosensor was developed with the sulf-1 aptamer to detect sulfameter, the reason for drug resistance, allergic, or toxic reactions, in milk using the quenching property of GO.65
In addition to the quenching property of carbon nanomaterials, some carbon nanomaterials have their own fluorescent signal emission properties that can be used for developing fluorescent biosensors. For example, CDs, also known as carbon quantum dots, are carbon nanoparticles with a size of 10 nm or less that can emit their own fluorescent signals. Furthermore, fluorescence emission wavelengths of the CD can be varied depending on its size.66 Using this unique fluorescence property of CDs, various fluorescent biosensors have been developed. In one study, CD was used to evaluate UV-induced DNA damage through changes in the fluorescent signals of the CD due to quenching by the formation of a CD- and UV-damaged DNA complex.67 In another study, by combining two fluorescence properties of carbon nanomaterials mentioned here, a fluorescent biosensor composed of acetylcholinesterase (AChE), fluorescent signal emitting CD, and GO capable of quenching was developed to detect organophosphate pesticides (Fig. 3d).68 To develop this biosensor, first, AChE and CD were conjugated using EDC-NHS binding between amine groups of AChE and carboxyl groups of the CD, and then the prepared conjugates were attached on the GO. In the absence of pesticide, the fluorescent signals of CD are quenched by the GO, but they can be recovered in the presence of pesticide due to the release of the CD from GO. Therefore, by measuring the fluorescence recovery of the CD, quantitative analysis of pesticides can be successfully performed with a detection limit of 0.14 ppb for the detection of chlorpyrifos, which is an organophosphate pesticide. Besides, several studies have been applied for patent registration to preoccupy the techniques recently such as SWCNT-based fluorescent steroid hormone biosensor (US patent registration number: 11698372) and degradable CNT-based biosensor for clinical markers detection related to bone or tissue (US patent registration number: 10881332). In addition, recently, research on the conjugation of carbon nanomaterials and the other newly developed functional nanomaterials is being conducted which will contribute to the development of ultrasensitive biosensors for POCT applications. As discussed in this section, carbon nanomaterials are being used to develop biosensors both on their own and in combination by fusion with other nanomaterials. Such theranostic research is expected to achieve both diagnosis and treatment in the future.
4. Development of drug delivery system by carbon-based nanocomposites
Nanomaterial-based drug delivery is a cutting-edge approach in the field of medicine that utilizes nanoscale materials to transport therapeutic agents directly to the targeted cells or tissues.69 This research field aims to improve the efficacy and safety of treatments, particularly for diseases such as cancer in which traditional methods can have significant side effects.70 Nanocarriers for drug delivery are designed to have specific properties such as size, shape, surface charge, and hydrophobicity/hydrophilicity, which are critical for their function.71 Drugs can be loaded on the nanocarriers by physical encapsulation, adsorption on the surface, or chemical conjugation.72 Furthermore, the surface of nanocarriers is often modified with targeting ligands, such as antibodies, peptides, or small molecules, that can specifically bind to receptors or antigens expressed on the target cells.73 This modification enhances the ability of the nanocarriers to selectively accumulate in the target tissue. When they reach the target site, such as a tumor, nanocarriers can release their drugs. The release mechanisms can be passive or active (triggered by pH, temperature, enzymes, or other stimuli present in the target environment).74 In particular, carbon-based nanocomposites offer numerous advantages for maximizing the effectiveness of drug delivery.75 For example, targeted delivery by the carbon-based nanocomposites leads to higher concentrations of the drug at the disease site, potentially increasing the therapeutic efficacy.76 In addition, carbon-based nanocomposites can be designed to release their drugs over a sustained period, offering prolonged therapeutic effects and reducing the need for frequent dosing.77 Some carbon-based nanocomposites have been designed for diagnostic purposes in addition to drug delivery, enabling simultaneous treatment and disease monitoring.78Graphitic carbon nitride with a 2D nanosheet structure has significant potential to be used as a functional component of nanocarriers because of its excellent properties including high thermal and chemical stability, good biocompatibility, and high body tissue permeability. In particular, the large surface area of the graphitic carbon nitride allows it to be used for efficient drug encapsulation. As an example of taking advantage of these beneficial properties of graphitic carbon nitride, in one study, a pH-sensitive nanocarrier composed of chitosan, agarose, graphitic carbon nitride, and curcumin was developed to deliver curcumin to breast cancer cells (Fig. 4a).79 The nanocarrier was fabricated by the water-in-oil-in-water emulsification method using curcumin entrapped in a chitosan/agarose/graphitic carbon nitride hydrogel. Nanocarriers only comprising chitosan and agarose, without graphitic carbon nitride as a control, were prepared and analysed for drug delivery capability. The introduction of graphitic carbon nitride increased the entrapment efficiency from 83% to 94% and the loading efficiency from 42% to 54%, overcoming the low solubility and bioavailability issues that have been a challenge in utilizing curcumin for drug delivery. Furthermore, the developed nanocarriers released more curcumin than the control in an acidic medium (Tumor cell environment) because of the pH-sensitive nature of graphitic carbon nitride, resulting in a 77.8% cell death rate of breast cancer cells (MCF-7).
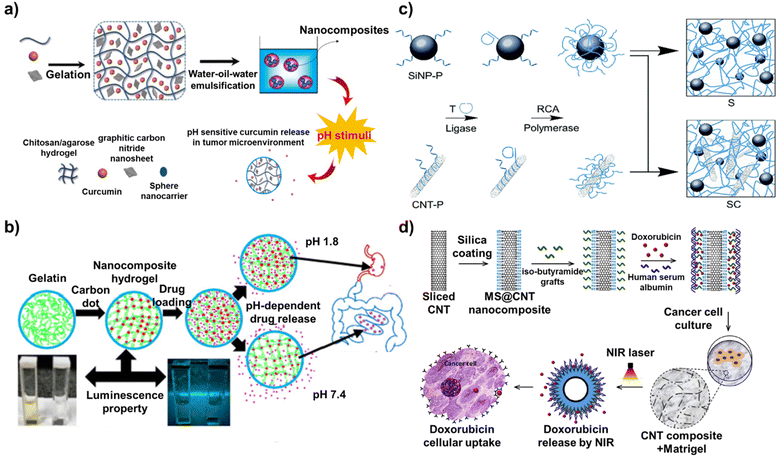 | ||
| Fig. 4 Mechanism of drug delivery based on carbon-based nanocomposites. (a) Schematic diagram of a pH-sensitive nanocarrier composed of chitosan, agarose, graphitic carbon nitride, and curcumin. Reproduced from ref. 79 with permission from Elsevier, copyright 2022, (b) scheme of carbon dot-gelatin nanocomposite hydrogel for delivery of cefadroxil. Reproduced from ref. 80 with permission from American Chemical Society, copyright 2020, (c) schematic illustration of programmable drug delivery system based on silica nanoparticle/CNT-DNA nanocomposites. Reproduced from ref. 81 with permission under the terms of the CC-BY Creative Commons attribution 4.0, and (d) schematic diagram of a mesoporous silica (MS)-modified CNT nano-platform. Reproduced from ref. 82 with permission from Elsevier, copyright 2020. | ||
One of the most commonly used factors for delivering drugs to these target cells or tissues is the pH. This is an important property for maintaining the physiological function of cells and tissues. It is also easy to utilize for drug delivery because different organs in the body have different pH values. The pH is also altered by diseases such as cancer. A similar study exploited this characteristic. A carbon dot-gelatin nanocomposite hydrogel was developed through simple solvent-casting methods for simultaneous intestinal drug delivery and near-neutral pH sensing (Fig. 4b).80 Here, the carbon dot played a dual role as both a crosslinker and a chromophore, avoiding the use of toxic crosslinkers. With the introduction of carbon dots into gelatin, the disadvantages of using gelatin as a carrier such as fast dissolution in the water medium, weak mechanical strength, and dimensional stability of neat gelatin could be overcome. In addition, carbon dots have size-dependent photoluminescence properties, offering a unique excitation-dependent emission. The developed nanocomposite hydrogel showed excellent pH-dependent behavior in terms of the photoluminescence and drug delivery. The nanocomposite hydrogel swelled less at low pH and swelled more at high pH, releasing the entrapping drugs. Furthermore, its ability to receive light at 350 nm and emit light at 431 nm allowed near-neutral pH sensing. Cefadroxil, an antimicrobial agent, was loaded onto the developed nanocomposite hydrogel and the in vitro drug release was analyzed under gastric pH (1.2) and intestinal pH (7.4). Over 24 hours of analysis, modest and continuous release of cefadroxil was observed at pH 7.4 compared to that under an acidic medium (pH 1.2), resulting in up to 77% inhibition of bacterial growth. Furthermore, the cefadroxil release profiles also showed that the rate of drug release can be controlled as desired by adjusting the pH of the release medium and the CD cross-linking ratio. Furthermore, cell viability assays revealed that all nanocomposite hydrogel samples, including the cefadroxil-loaded nanocomposite hydrogel, exhibited acceptable cytocompatibility and non-toxicity due to the excellent biocompatibility of CDs. In addition, several studies have been patented including a carbon dots for diagnostic analysis and drug delivery (WIPO (PCT) application number: PCT/US2017/016743) and preparation method of carbon dot drug-loading system (CN application number: CN201810640613.XA).
In another study, a programmable drug delivery system based on silica nanoparticle/CNT-DNA nanocomposites was developed (Fig. 4c).81 These nanocomposites were synthesized in a highly modular manner from DNA-functionalized CNT and silica nanoparticles via enzymatic rolling circle amplification. The ability of the developed silica nanoparticle/CNT-DNA nanocomposites to recognize target biomolecules was achieved through DNA sequence design. Specifically, this was implemented by incorporating GC/CG-rich stem loops for efficient loading of intercalating drugs and aptamer motifs for selective binding of cell surface receptors. This was possible because CNTs exhibit characteristics such as a large surface area, physical and chemical stability, and ease of surface modification. Furthermore, the utilization of GC/CG-rich stem loops with CNT increased the stability of the drug-loading capacity. In aqueous solutions with various conditions (PBS, acetate buffer, and cell culture medium), the silica nanoparticle/CNT-DNA nanocomposites still contained more than 80% of the drug even after 24 hours. Furthermore, doxorubicin, an anthracycline drug, was conjugated with a fluorescent tag, Cy5, to trace drug delivery to the cells. The silica nanoparticle/CNT-DNA nanocomposites loaded with doxorubicin were used to target HeLa cancer cells to demonstrate the utility of this approach. By simultaneously analyzing the fluorescence signal from doxorubicin itself and the signal from Cy5, the authors confirmed that doxorubicin penetrated the nucleus of HeLa cells and effectively induced their death.
Similar to this study, other studies have reported CNT-based nanocomposites for drug delivery as well as phototherapy. CNTs exhibit a strong absorbance in the near infrared (NIR) region (in the range of 750 nm to 1400 nm) and can convert NIR light to localized heat. Taking advantage of these properties, a mesoporous silica (MS)-modified CNT nano-platform was designed in one study (Fig. 4d).82 MS-modified CNTs were then coated with iso-butyramide grafts as non-covalent linkers, which enabled a very high drug-loading capacity (more than 80 wt%) of the anti-cancer drug doxorubicin. Finally, the MS@CNT nanocomposite was fabricated by adsorption of human serum albumin for the formation of a biocompatible interface and drug gate-keeping function. Here, the iso-butyramide grafts played an important role as a thermo-responsive interface that released the loaded drugs from the surface upon photo-induced local heat generated on the surface. Moreover, because of the large surface area of CNT, the drug loading capacity reached 68% with a drug loading efficacy of 23%. Regarding the antitumor activity, because of the near-infrared photothermal effect induced by the developed MS@CNT nanocomposites, D2A1 cancer cells could be effectively killed. Furthermore, drug delivery mediated by near-infrared light could efficiently kill tumor cells through synergistic effects with additional anticancer effects. Especially, even when the MS@CNT nanocomposites were embedded within a hydrogel that mimicked the extracellular matrix, they released the drug to D2A1 cancer cells cultured on the hydrogel in response to near-infrared light. Thus, CNTs, in particular, have been actively used in various forms to fabricate drug delivery platforms because of their numerous advantages over other carbon-based nanomaterials. Recent studies have reported that by maximizing the properties of CNTs, not only drug delivery but also gene delivery is possible. For example, a MWCNT/Fe3O4-based nanocarrier was developed for the delivery of drugs and genes simultaneously.83 Doxorubicin and pCRISPR were loaded on the developed MWCNT/Fe3O4-based nanocarrier, and then, various cell lines (MCF-7, HEK-293, and PC-12) and mice were treated with the nanocarriers. The MWCNT/Fe3O4-based nanocarrier showed antimicrobial activity, high delivery efficiency, and non-toxicity both in vitro and in vivo. Moreover, there are also patents pending on CNTs, including drug delivery by carbon nanotube arrays (US application number: US13/224287) and method of drug delivery by carbon nanotube-chitosan nanocomplexes (US application number: US12/105884).
As such, the structural specificity and ease of modifying the surface functional groups of the carbon-based nanocomposites have led to active research on the delivery of not only drugs but also various substances to the desired cells and tissues. However, there are still several challenges that need to be addressed to perform effective drug delivery. For example, carbon-based nanocomposites can overcome the stability issues that can lead to aggregation in biological fluids. This can be addressed by inducing dispersive properties in aqueous solutions through appropriate surface modifications.84 In addition, immune responses should also be considered if carbon-based nanocomposite-based systems are applied directly to the human body in the future. The body's immune system may recognize carbon-based nanocomposites as foreign objects, triggering an immune response. This can lead to the rapid clearance of these particles from the body, reducing their effectiveness in drug delivery. In addition, long-term effects of nanocomposites injected into the human body must also be studied. For these reasons, especially since the carbon-based nanocomposites need to be present inside the human body for drug delivery, it is still very difficult to implement. Moreover, it is also not feasible to analyze the changes in physiological signals in the human body, so there are no clinically approved carbon-based nanocomposites-based drug delivery platform. Nevertheless, carbon-based nanocomposites are expected to make a significant contribution to the development of relevant drug delivery systems in the future because of their excellent properties related to the field of drug delivery.
5. Tissue engineering based on carbon-based nanocomposites
In recent years, the unique physicochemical properties of carbon nanomaterials make them promising candidates to enhance various aspects of tissue engineering, from scaffold design and mechanical support to cell interaction and regeneration. Specifically, the biocompatibility and bioactivity of carbon nanomaterials promote cell adhesion, proliferation, and differentiation for application in tissue interaction and regeneration. Furthermore, carbon nanomaterials can be applied to fabricate scaffolds because of their excellent electrical conductivity for electrophysiological signal transmission of muscle and neural tissue as well as the control of cell fate. Using carbon nanomaterial-based scaffolds, research has been actively conducted to develop structures that can be adapted to the characteristics of each tissue and apply them for transplantation. In this section, the recent studies on carbon-based nanocomposites for tissue engineering are discussed.The carbon nanomaterial-based nanocomposites have been studied for the differentiation of cells due to their unique structural and physiochemical properties. The functional groups of carbon nanomaterial-based nanocomposites can easily be combined with other biomaterials that can promote cell differentiation, and this advantage was actively used to control stem-cell differentiation. For example, polycaprolactone (PCL) nanofibers with CNT were developed for modulation of multiple cell interactions.85 The surfaces of the CNTs were functionalized by acid treatment to combine CNTs on the PCL nanofiber, and the negative charge of the functionalized CNT reacted with the alkaline-modified PCL nanofiber. In addition, PCL nanofibers with CNT induced the human mesenchymal stem cells (hMSCs) differentiation to an osteogenic lineage because the accelerated adhesion by the CNT mediated signalling processes to determine the subsequent osteogenic differentiation potential of hMSCs. In another study, a 3D printed porous scaffold composed of aligned MWCNT and nanohydroxyapatite (nHA) was developed to mimic the natural bone tissue.86 The aligned MWCNT with nHA provided abundant nucleation sites for cell proliferation and differentiation, and the aligned MWCNT mimicked the highly aligned fibril collagen.
These effects of MWCNT promoted osteogenic differentiation because of the stiffer surface for modulating the mechano-transduction pathway. In addition, 3D structures of vertically aligned CNT (VA-CNT) have been developed to modulate neural stem cell (NSCs) differentiation.87 For this, a VA-CNT 3D structure was prepared by gentle pilling-off of a Fe/Al2O3/SiO2/Si substrate (Fig. 5a). The structural stability and adaptability of the VA-CNT induced direct differentiation of human neural precursor cells. In addition, to optimize the impact of the 3D structure of VA-CNT on neural differentiation, free-standing VA-CNT forests and capillary-driven VA-CNT forests were developed. NSC differentiation was confirmed by the protein levels of the neuritogenic markers βIII-tubulin, MAP2, and Gαo. The protein levels of βIII-tubulin, MAP2, and Gαo were 1.8, 1.9, and 1.5 times higher in NSCs grown on capillary-driven VA-CNT forests compared to those on free-standing VA-CNT forests, respectively.
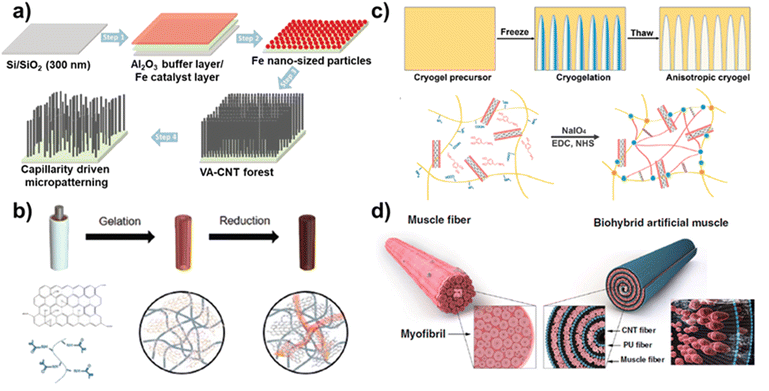 | ||
| Fig. 5 Tissue engineering based on carbon-based nanocomposites. (a) Schematic diagram of a 3D structures of vertically aligned carbon nanotube for neural stem cell differentiation. Reproduced from ref. 87 with permission from John Wiley and Sons, copyright 2023, (b) schematic diagram of conductive hydrogel composed of rGO and GelMA for peripheral nerve regeneration. Reproduced from ref. 90 with permission from John Wiley and Sons, copyright 2020, (c) schematic illustration of 3D aligned conductive tubular cryogel scaffold composed of polydopamine coated CNTs and gelatin for skeletal muscle tissue regeneration. Reproduced from ref. 92 with permission from Elsevier, copyright 2022, and (d) schematic diagram of hydrophilic polyurethane/CNT nanofibers based biohybrid artificial muscle. Reproduced from ref. 93 with permission from Springer Nature, copyright 2021. | ||
In addition to cell differentiation, carbon nanomaterial-based nanocomposites have recently been applied to the field of tissue regeneration. Carbon nanomaterials have excellent mechanical strength and stiffness and can strengthen tissue scaffolds and provide structural support, especially in load-bearing tissues such as the bone, tendon, and cartilage. For example, a 3D high-porosity chitosan/honeycomb porous carbon (HPC)/nHA scaffold was developed for enhanced bone regeneration.88 The porous chitosan/HPC/nHA scaffold was prepared by a vacuum freeze-drying method and exhibited excellent porosity of 91.87 ± 2.516%. The porous structure of HPC provided a suitable site for bone-cell regeneration with enhanced nutrient transport and angiogenesis, and the nHA was uniformly dispersed on the surface of HPC. In addition, mRNA expressions of the bone-related marker alkaline phosphatase (ALP), OPN, and VEGF were 4.1, 3.5, and 4.2 times higher in chitosan/HPC/nHA scaffold compared to those on the chitosan scaffold, respectively, after 2 weeks. By culturing mouse bone marrow mesenchymal stem cells on the scaffold, we verified that the scaffold promoted its growth and differentiation through osteogenesis. In another study, iron-doped carbon dots (Fe-CDs) were developed for efficient wound healing.89 Because of the excellent optical and catalytic properties of CDs, they could be applied for antibacterial therapy. Antibacterial experiments with Fe-CDs indicated that the combination of peroxidase (POD)-like activity and local heating of Fe-CDs resulted in antibacterial rates of 99.85% and 99.68% against E. coli and S. aureus, respectively. Furthermore, Fe-CDs exhibited photothermal effects upon NIR laser irradiation, indicating bacterial death. Furthermore, the excellent electrical conductivity of carbon nanomaterial-based nanocomposites plays a significant role in neural growth and differentiation. For example, multifunctional nerve guidance conduits (NGCs) were developed using rGO/gelatin-methacrylate (GelMA) for the regeneration of injured peripheral nerves.90 The developed rGO/GelMA was suitable for use as NGCs because it exhibits excellent flexibility, electrical conductivity, and permeability. Hydrogels synthesized using GelMA have been extensively utilized on tissue engineering applications, with a particularly positive impact on nerve regeneration. GO has been used in combination with GelMA to provide electrical conductivity to the hydrogel and was further chemically reduced to rGO to increase the electrical properties of the devleoped hydrogel (Fig. 5b). The impedance values of rGO/GelMA indicated with 10 ± 1 kΩ, and compared with other hydrogels, rGO/GelMA exhibited a significantly lower resistivity value due to rGO. Furthermore, PC12 neuronal cells were cultured on the prepared hydrogel to confirm neural growth and neurite outgrowth. The cell number and mean neurite length of PC12 neuronal cells grown on rGO/GelMA were 3.2 ± 0.6 × 104 cm−2 and 52 μm, respectively, which were higher than those of GelMA and GO/GelMA, and these results indicated that the rGO/GelMA supported neurite growth. To evaluate the functional recovery of sciatic nerve, electromyography (EMG) was performed on an animal model after rGO/GelMA implantation. The rGO/GelMA group had a significantly enhanced sciatic function index (−72.7 ± 0.8) compared to the other groups (GelMA and GO/GelMA), which had a similar sciatic function index to the autograft (−67.7 ± 0.9) group. These results indicated that the developed rGO/GelMA has the potential to be applied to the regeneration of electroactive tissue.
In addition to neural cell regeneration, artificial muscle fibers developed using carbon nanomaterials have attracted significant attention because of the excellent properties such as good electrical conductivity and similar structure. The integration of muscle cells into carbon nanomaterial-based scaffolds is an outstanding candidate to mimic muscle function. For example, a carbon-based hierarchical scaffold was developed for myoblast differentiation.91 To develop the hierarchical scaffold using CNT, tailored carpet-like arrays of CNT were grown on the micro-pore walls of foam. Because of the microscale features and surface nanofunctionalization of the developed scaffold, the differentiation of mouse myoblasts was promoted to multinucleated myotubes. In another study, a 3D aligned conductive tubular cryogel scaffold was developed for muscle-cell alignment.92 To prepare the 3D aligned conductive tubular cryogel, polydopamine (PDA)-coated CNTs (PCNTs) were mixed with gelatin (GT)-PDA-PCNTs, and scaffolds were fabricated using unidirectional freeze casting technology. The GT-PDA-PCNT cryogel scaffold mimicked the microenvironment of native skeletal muscle cells, providing a suitable platform for skeletal muscle tissue regeneration due to its aligned microstructure and excellent conductivity (Fig. 5c). However, most research on artificial muscle fibers has only used 2D scaffolds to induce muscle cell differentiation, or they have been applied for muscle tissue regeneration. In other studies, to overcome the challenges of the artificial muscle structure, a carbon nanomaterial-based 3D structure was introduced to muscle cells to induce contractive behaviors in response to stimulation. For example, biohybrid artificial muscle was developed using hydrophilic polyurethane (HPU)/CNT nanofibers.93 To induce alignment of the C2C12 myoblast cells on the developed nanofibers, HPU was fabricated using electrospinning methods and coated with CNTs to develop a strong and flexible scaffold. In addition, the CNTs provided enhanced electrical properties and mechanical structure stability to the artificial muscle. The C2C12 myoblast cells seeded on the planner shaped HPU/CNT nanofiber were rolled into a natural fiber shape, and the C2C12 myoblast cells were induced to undergo further proliferation and differentiation (Fig. 5d). The biohybrid artificial muscle exhibited the maximum contractile activity with ∼2.73 ± 0.27 μm by the developed HPU/CNT nanofiber scaffolds. Similar to CNTs, GO and rGO have recently been used to develop artificial muscles. Three types of hybrid hydrogels were prepared by mixing CNTs, GO, and rGO in GelMA were developed, and the differentiation degree and action potential of muscle cells according to the mixed carbon nanomaterials were confirmed.94 Because of the properties of carbon nanomaterial such as superior mechanical properties, pore size, and roughness, the muscle cell seeded onto the different hybrid hydrogel exhibited enhanced cell morphology, maturation, and functionality. The electrically conductive hybrid hydrogel acted as an electrical nanobridge between cardiomyocytes, increasing the expression levels of cardiac markers. Furthermore, as confirmed by the duration and action potential morphology, cardiomyocytes seeded on CNT-GelMA and GO-GelMA were more similar to ventricular and atrial cardiomyocytes, respectively, while those seeded on rGO-GelMA showed intermediate characteristics between ventricular and atrial phenotypes. Besides, several studies have been applied to patent for the techniques of tissue engineering recently such as carbon nanotube and graphene patches and implants for biological tissue (WIPO (PCT) application number: WO0214143925A1) and preparation method of biological conductive nanofiber tissue engineering scaffold (CN application number: CN111686307A). As discussed in this section, carbon nanomaterials are being used to apply tissue engineering for cell differentiation, regeneration, and implantation.95,96 In particular, the structural stability and easy functionalization of carbon nanomaterials enable their use in the regeneration of hard tissues such as bone and tendon, and their excellent electrical conductivity could be actively employed in the future for electrophysiological signaling of nerve and muscle cells and tissue regeneration.
6. Conclusions and future perspective
Carbon nanomaterial-based nanocomposites are receiving considerable attention because of their excellent electrical properties, structural stability, and biocompatibility.97 While various types of carbon nanomaterials are being researched for biomedical applications such as biosensors, drug delivery, and tissue engineering, there still remain several challenges that need to be considered.98 For example, pristine carbon nanomaterials such as MWCNTs have low biocompatibility, which can induce cytotoxic effects on cells, and the difficulty of uniform synthesis of nanomaterial results in low binding affinity, which limits their effectiveness in targeted applications. Furthermore, carbon-based nanocomposites are difficult to develop the marketed products for clinical trials due to the non-uniform structure and functions. Therefore, recent patents and products using carbon nanomaterials are mostly focused on biosensors. To address these disadvantages, various types of nanomaterials and biomolecules are being combined with carbon nanomaterials for development of nanobiohybrid nanomaterials. In addition, the introduction of other materials such as polymers, metal nanomaterials, and TMD can enhance the properties of carbon nanomaterials to realize biological applications with improved structures and excellent function.99–101This review highlights the recent research on carbon-based nanocomposites for biomedical application. First, the structures and characteristics of carbon nanomaterials and carbon-based nanocomposites combined with metal nanomaterials, polymers, and TMDs were discussed. Subsequently, the biological applications of carbon-based nanocomposites were discussed based on biosensors, drug delivery, and tissue engineering. The biological application based on carbon-based nanocomposites discussed in this review are summarized in Table 2. In addition to the subjects discussed here, carbon-based nanocomposites are being researched for other biological applications. For example, a 3D nanobiohybrid hydrogel composed of motor neuron spheroid and carboxylated CNT (CNT-COOH) was developed for a biosensing system for drug evaluation of amyotrophic lateral sclerosis (ALS).102 The CNT induced the neural differentiation, and enhanced neurite length in hydrogel. The developed 3D nanobiohybrid was connected with muscle cells for neuromuscular junction (NMJ), and muscle bundle contraction was enhanced by neurotransmitter treatment. Another study reported CNT-GelMA hydrogel for the development of a biohybrid robot.103,104 As the muscle cells on the surface of the CNT-GelMA hydrogel differentiate, contractile motion is generated, and the developed biohybrid robot indicated forward motion. In addition, it is expected that in the future, carbon nanomaterials will be applied to various scientific fields beyond biological applications. For example, carbon nanomaterial can be used to develop the soft robotics and actuator.105–107 Graphene is a single layer of carbon atoms arranged in a hexagonal lattice, giving it exceptional strength, electrical conductivity, and flexibility. Graphene-based actuators often utilize graphene's excellent electrical conductivity and piezoelectric effect. These applications using the carbon nanomaterials can be used for analysis of the external stimulation (light, electric, and humidity), detection of chemical such as NO or H+, and soft robotics for human like motion. In conclusion, this review discussed the recent advances in carbon-based nanocomposites and their applications in biosensor, drug delivery, and tissue engineering. This achievement is expected to be a breakthrough in providing an innovative and perceptive approach to the development of next-generation biological application and biohybrid robotic systems.
| Nanomaterials | Applications | Ref. | |
|---|---|---|---|
| Biosensor | The carboxylated SWCNTs | Electrochemical SARS-CoV-2 biosensor | 52 |
| The graphene sponge (GS) | Electrochemical glucose biosensor | 54 | |
| The graphene oxide (GO) | Fluorescent RNAi biosensor | 64 | |
| The carbon dot (CD) and GO | Fluorescent organophosphate pesticide biosensor | 68 | |
| Drug delivery | Chitosan/agarose/graphitic carbon nitride | MCF7 cancer cell treatment | 79 |
| Gelatin/carbon dot | Antibacterial drug release | 80 | |
| Silica nanoparticle/CNT-DNA | HeLa cancer cell treatment | 81 | |
| Mesoporous silica-modified CNT | D2A1 cancer cell treatment | 82 | |
| Tissue engineering | VA-CNT | Neural stem cell differentiation | 87 |
| rGO/GelMA | Peripheral nerve regeneration | 90 | |
| GT-PDA-PCNTs | Skeletal muscle regeneration | 92 | |
| HPU/CNT fiber | Artificial muscle actuation | 93 |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by National R&D Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (NRF-2022M3H4A1A01005271), by GRDC Cooperative Hub through the National Research Foundation of Korea funded by the Ministry of Science and ICT (Grant number RS-2023-00259341), and by the Basic Science Research Program through the NRF funded by the Ministry of Education (No. 2016R1A6A1A03012845).Notes and references
- P. Mohankumar, J. Ajayan, T. Mohanraj and R. Yasodharan, Measurement, 2021, 167, 108293 CrossRef.
- A. M. Vargason, A. C. Anselmo and S. Mitragotri, Nat. Biomed. Eng., 2021, 5, 951–967 CrossRef PubMed.
- M. Ouyang, D. Tu, L. Tong, M. Sarwar, A. Bhimaraj, C. Li, G. L. Cote and D. Di Carlo, Biosens. Bioelectron., 2021, 171, 112621 CrossRef CAS.
- C. Li, J. Wang, Y. Wang, H. Gao, G. Wei, Y. Huang, H. Yu, Y. Gan, Y. Wang, L. Mei, H. Chen, H. Hu, Z. Zhang and Y. Jin, Acta Pharm. Sin. B, 2019, 9, 1145–1162 CrossRef.
- S. J. Forbes and N. Rosenthal, Nat. Med., 2014, 20, 857–869 CrossRef CAS PubMed.
- I. S. Kucherenko, O. O. Soldatkin, D. Y. Kucherenko, O. V. Soldatkina and S. V. Dzyadevych, Nanoscale Adv., 2019, 1, 4560–4577 RSC.
- S. Song, H. Shen, Y. Wang, X. Chu, J. Xie, N. Zhou and J. Shen, Colloids Surf., B, 2020, 185, 110596 CrossRef CAS PubMed.
- Y. Hui, Z. Yan, H. Yang, X. Xu, W. E. Yuan and Y. Qian, ACS Appl. Bio Mater., 2022, 5, 4741–4759 CrossRef CAS PubMed.
- A. Murali, G. Lokhande, K. A. Deo, A. Brokesh and A. K. Gaharwar, Mater. Today, 2021, 50, 276–302 CrossRef CAS PubMed.
- S. S. Ray and J. Bandyopadhyay, Nanotechnol. Rev., 2021, 10, 728–743 CrossRef CAS.
- A. Thakur, R. Bharti and R. Sharma, Mater. Today: Proc., 2022, 50, 2256–2268 CAS.
- N. Bellier, P. Baipaywad, N. Ryu, J. Y. Lee and H. Park, Biomater. Res., 2022, 26, 65 CrossRef CAS PubMed.
- M. Tebyetekerwa, T. T. Duignan, Z. Xu and X. S. Zhao, Adv. Energy Mater., 2022, 12, 2202450 CrossRef CAS.
- S. X. Yu, B. B. Guo, T. B. Zeng, H. Q. Qu, J. L. Yang and J. M. Bai, Composites, Part B, 2022, 246, 110232 CrossRef CAS.
- N. Afsarimanesh, A. Nag, M. E. E. Alahi, S. Sarkar, S. Mukhopadhyay, G. S. Sabet and M. E. Altinsoy, Sens. Actuators, A, 2022, 344, 113743 CrossRef CAS.
- H. M. Fahmy, E. S. Abu Serea, R. E. Salah-Eldin, S. A. Al-Hafiry, M. K. Ali, A. E. Shalan and S. Lanceros-Mendez, ACS Biomater. Sci. Eng., 2022, 8, 964–1000 CrossRef CAS.
- J. Ackermann, J. T. Metternich, S. Herbertz and S. Kruss, Angew Chem. Int. Ed. Engl., 2022, 61, e202112372 CrossRef CAS.
- S. Zheng, Y. Tian, J. Ouyang, Y. Shen, X. Wang and J. Luan, Front. Chem., 2022, 10, 990362 CrossRef CAS PubMed.
- J. Yoon, T. Lee, G. B. Bapurao, J. Jo, B. K. Oh and J. W. Choi, Biosens. Bioelectron., 2017, 93, 14–20 CrossRef CAS PubMed.
- M. Qin, C. Merzougui, Y. M. Su, Y. F. Li, W. Y. Chen and D. Huang, New Res. Carbon Mater., 2023, 38, 496–506 CrossRef CAS.
- C. Yang, M. E. Denno, P. Pyakurel and B. J. Venton, Anal. Chim. Acta, 2015, 887, 17–37 CrossRef CAS.
- F. R. Baptista, S. A. Belhout, S. Giordani and S. J. Quinn, Chem. Soc. Rev., 2015, 44, 4433–4453 RSC.
- M. Kurian and A. Paul, Carbon Trends, 2021, 3, 100032 CrossRef CAS.
- D. Ozyurt, M. Al Kobaisi, R. K. Hocking and B. Fox, Carbon Trends, 2023, 100276 CrossRef CAS.
- J. Prasek, J. Drbohlavova, J. Chomoucka, J. Hubalek, O. Jasek, V. Adam and R. Kizek, J. Mater. Chem., 2011, 21, 15872–15884 RSC.
- Z. A. ALOthman and S. M. Wabaidur, Arabian J. Chem., 2019, 12, 633–651 CrossRef CAS.
- K. K. Gangu, S. Maddila, S. B. Mukkamala and S. B. Jonnalagadda, J. Energy Chem., 2019, 30, 132–144 CrossRef.
- N. Bellier, P. Baipaywad, N. Ryu, J. Y. Lee and H. Park, Biomater. Res., 2022, 26, 65 CrossRef CAS.
- Q. Abbas, P. A. Shinde, M. A. Abdelkareem, A. H. Alami, M. Mirzaeian, A. Yadav and A. G. Olabi, Mater., 2022, 15, 7804 CrossRef CAS PubMed.
- H. Zhou, L. Zhang, D. Zhang, S. Chen, P. R. Coxon, X. He, M. Coto, H. K. Kim, K. Xi and S. Ding, Sci. Rep., 2016, 6, 37752 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS.
- Z. Y. Ma, X. F. Zhou, W. Deng, D. Lei and Z. P. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 3634–3643 CrossRef CAS.
- S. Abdolhosseinzadeh, H. Asgharzadeh and H. Seop Kim, Sci. Rep., 2015, 5, 10160 CrossRef CAS PubMed.
- D. Veeman, M. V. Shree, P. Sureshkumar, T. Jagadeesha, L. Natrayan, M. Ravichandran and P. Paramasivam, J. Nanomater., 2021, 2021, 1–21 CrossRef.
- R. Paul, F. Du, L. Dai, Y. Ding, Z. L. Wang, F. Wei and A. Roy, Adv. Mater., 2019, 31, 1805598 CrossRef.
- B. Bayatsarmadi, Y. Zheng, A. Vasileff and S. Z. Qiao, Small, 2017, 13, 1700191 CrossRef PubMed.
- L. Fritea, F. Banica, T. O. Costea, L. Moldovan, L. Dobjanschi, M. Muresan and S. Cavalu, Mater., 2021, 14, 6319 CrossRef CAS PubMed.
- Y. Liu and S. Kumar, ACS Appl. Mater. Interfaces, 2014, 6, 6069–6087 CrossRef CAS.
- G. Cirillo, S. Hampel, U. G. Spizzirri, O. I. Parisi, N. Picci and F. Iemma, BioMed Res. Int., 2014, 2014, 825017 Search PubMed.
- A. A. Adewunmi, S. Ismail and A. S. Sultan, J. Inorg. Organomet. Polym. Mater., 2016, 26, 717–737 CrossRef CAS.
- M. Shin, J. Lim, J. An, J. Yoon and J. W. Choi, Nano Convergence, 2023, 10, 8 CrossRef CAS PubMed.
- R. R. Castillo and M. Vallet-Regi, Int. J. Mol. Sci., 2019, 20, 929 CrossRef CAS PubMed.
- D. Tsikritzis, N. Tsud, T. Skála and L. Sygellou, Appl. Surf. Sci., 2022, 599, 153896 CrossRef CAS.
- L. P. Yu, X. H. Zhou, L. Lu, L. Xu and F. J. Wang, ChemSusChem, 2021, 14, 5079–5111 CrossRef CAS.
- J. Yoon, J. Lim, M. Shin, J. Y. Lee and J. W. Choi, Biosens. Bioelectron., 2022, 212, 114427 CrossRef CAS.
- Y. Xu, Y. S. Ang, L. Wu and L. K. Ang, Nanomaterials, 2019, 9, 165 CrossRef CAS.
- Z. Xie, Y. Duo, Z. Lin, T. Fan, C. Xing, L. Yu, R. Wang, M. Qiu, Y. Zhang, Y. Zhao, X. Yan and H. Zhang, Adv. Sci., 2020, 7, 1902236 CrossRef CAS PubMed.
- N. Zare-Shehneh, F. Mollarasouli and M. Ghaedi, Crit. Rev. Anal. Chem., 2023, 53, 520–536 CrossRef CAS PubMed.
- X. Xiao, Y. Zhang, L. Zhou, B. Li and L. Gu, Nanomaterials, 2022, 12, 2444 CrossRef CAS PubMed.
- S. Madhurantakam, S. Muthukumar and S. Prasad, ACS Omega, 2022, 7, 12467–12473 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, E. B. Noruzi, E. Chidar, M. Jafari, F. Davoodi, A. Kashtiaray, M. G. Gorab, S. M. Hashemi, S. Javanshir, R. A. Cohan, A. Maleki and M. Mahdavi, Chem. Eng. J., 2022, 442, 136183 CrossRef CAS.
- A. R. Cardoso, J. F. Alves, M. F. Frasco, A. M. Piloto, V. Serrano, D. Mateus, A. I. Sebastiao, A. M. Matos, A. Carmo, T. Cruz, E. Fortunato and M. G. F. Sales, Mater. Today Bio, 2022, 16, 100354 CrossRef CAS PubMed.
- M. Yang, X. N. Ren, T. T. Yang, C. Xu, Y. Q. Ye, Z. W. Sun, L. H. Kong, B. Wang and Z. Q. Luo, Chem. Eng. J., 2021, 418, 129483 CrossRef CAS.
- B. Li, X. Wu, C. Shi, Y. Dai, J. Zhang, W. Liu, C. Wu, Y. Zhang, X. Huang and W. Zeng, Surf. Interfaces, 2023, 36, 102525 CrossRef CAS.
- M. T. Deng, J. L. Feng, D. Tao, H. H. Yan, J. J. Ding, N. Jaffrezic-Renault and Z. Z. Guo, Bioelectrochemistry, 2022, 148, 108256 CrossRef CAS PubMed.
- S. Pareek, U. Jain, M. Bharadwaj, K. Saxena, S. Roy and N. Chauhan, Anal. Biochem., 2023, 663, 115015 CrossRef CAS PubMed.
- R. S. Juang, C. T. Hsieh, C. M. Liu, T. A. Lin and Y. A. Gandomi, Colloids Surf., A, 2023, 666, 131336 CrossRef CAS.
- H. S. Dinani, M. Pourmadadi, F. Yazdian, H. Rashedi, S. A. S. Ebrahimi, J. S. Shayeh and M. Ghorbani, Eng. Life Sci., 2022, 22, 519–534 CrossRef CAS.
- C. Pothipor, J. Jakmunee, S. Bamrungsap and K. Ounnunkad, Analyst, 2021, 146, 4000–4009 RSC.
- S. Kasturi, Y. Eom, R. Torati and C. Kim, J. Ind. Eng. Chem., 2021, 93, 186–195 CrossRef CAS.
- S. R. Torati, B. Hanson, M. Shinde and G. Slaughter, IEEE Sens. J., 2024, 24, 2154–2161 Search PubMed.
- J. Zhu, S. Liu, Z. Hu, X. Zhang, N. Yi, K. Tang, M. G. Dexheimer, X. Lian, Q. Wang, J. Yang, J. Gray and H. Cheng, Biosens. Bioelectron., 2021, 193, 113606 CrossRef CAS PubMed.
- Y. V. M. Reddy, J. H. Shin, J. Hwang, D. H. Kweon, C. H. Choi, K. Park, S. K. Kim, G. Madhavi, H. Y. M. Yi and J. P. Park, Biosens. Bioelectron., 2022, 214, 114511 CrossRef.
- K. Li, Z. Lei, C. Zhang, L. Zhu, K. Huang, Y. Shang and W. Xu, Anal. Chim. Acta, 2022, 1189, 339222 CrossRef CAS PubMed.
- Y. He, J. Zhang, R. Tian, Y. Lu, L. Pan and Y. Zhang, Luminescence, 2023, 38, 518–526 CrossRef CAS PubMed.
- M. Pourmadadi, E. Rahmani, M. Rajabzadeh-Khosroshahi, A. Samadi, R. Behzadmehr, A. Rahdar and L. F. R. Ferreira, J. Drug Delivery Sci. Technol., 2023, 80, 104156 CrossRef CAS.
- G. Jabeen, M. H. Ahmad, M. Aslam, S. Riaz, A. Hayat and M. H. Nawaz, RSC Adv., 2022, 12, 22458–22464 RSC.
- M. I. Gaviria, K. Barrientos, J. P. Arango, J. B. Cano and G. A. Peñuela, Front. Environ. Sci., 2022, 10, 825112 CrossRef.
- M. Abd Elkodous, S. Olojede, M. Morsi and G. S. El-Sayyad, RSC Adv., 2021, 11, 26463–26480 RSC.
- A. P. Singh, A. Biswas, A. Shukla and P. Maiti, Signal Transduction Targeted Ther., 2019, 4, 33 CrossRef.
- S. Hossen, M. K. Hossain, M. K. Basher, M. N. H. Mia, M. T. Rahman and M. J. Uddin, J. Adv. Res., 2019, 15, 1–18 CrossRef CAS PubMed.
- N. Wang, X. Cheng, N. Li, H. Wang and H. Chen, Adv. Healthcare Mater., 2019, 8, e1801002 CrossRef PubMed.
- N. Osman, N. Devnarain, C. A. Omolo, V. Fasiku, Y. Jaglal and T. Govender, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1758 CAS.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- M. Mohajeri, B. Behnam and A. Sahebkar, J. Cell. Physiol., 2018, 234, 298–319 CrossRef PubMed.
- R. Y. Zhang and H. Olin, Mater. Sci. Eng. C., 2012, 32, 1247–1252 CrossRef CAS.
- K. Kokubun, S. Matsumura, M. Yudasaka, S. Iijima and K. Shiba, Int. J. Nanomed., 2018, 13, 1643–1652 CrossRef CAS PubMed.
- N. Panwar, A. M. Soehartono, K. K. Chan, S. Zeng, G. Xu, J. Qu, P. Coquet, K. T. Yong and X. Chen, Chem. Rev., 2019, 119, 9559–9656 CrossRef CAS PubMed.
- M. Rajabzadeh-Khosroshahi, M. Pourmadadi, F. Yazdian, H. Rashedi, M. Navaei-Nigjeh and B. Rasekh, J. Drug Delivery Sci. Technol., 2022, 74, 103443 CrossRef CAS.
- S. K. Bhattacharyya, M. Dule, R. Paul, J. Dash, M. Anas, T. K. Mandal, P. Das, N. C. Das and S. Banerjee, ACS Biomater. Sci. Eng., 2020, 6, 5662–5674 CrossRef CAS PubMed.
- Y. Hu and C. M. Niemeyer, J. Mater. Chem. B, 2020, 8, 2250–2255 RSC.
- B. Li, S. Harlepp, V. Gensbittel, C. J. Wells, O. Bringel, J. G. Goetz, S. Begin-Colin, M. Tasso, D. Begin and D. Mertz, Mater. Today Chem., 2020, 17, 100308 CrossRef CAS.
- M. Bagherzadeh, M. Safarkhani, H. Daneshgar, F. Radmanesh, F. Taghavimandi, A. M. Ghadiri, M. Kiani, Y. Fatahi, N. Safari-Alighiarloo, S. Ahmadi and N. Rabiee, J. Drug Delivery Sci. Technol., 2022, 78, 103917 CrossRef CAS.
- V. Biju, Chem. Soc. Rev., 2014, 43, 744–764 RSC.
- K. D. Patel, T.-H. Kim, N. Mandakhbayar, R. K. Singh, J.-H. Jang, J.-H. Lee and H.-W. Kim, Acta Biomater., 2020, 108, 97–110 CrossRef CAS PubMed.
- B. Huang, C. Vyas, J. J. Byun, M. El-Newehy, Z. Huang and P. Bártolo, Mater. Sci. Eng. C., 2020, 108, 110374 CrossRef CAS PubMed.
- L. Nascimento, C. Fernandes, R. M. Silva, Â. Semitela, B. M. de Sousa, P. A. Marques, S. I. Vieira, R. F. Silva, N. Barroca and G. Gonçalves, Adv. Healthcare Mater., 2023, 12, 2300828 CrossRef CAS PubMed.
- C. Dai, Y. Li, W. Pan, G. Wang, R. Huang, Y. Bu, X. Liao, K. Guo and F. Gao, ACS Biomater. Sci. Eng., 2019, 6, 575–586 CrossRef PubMed.
- Y. Liu, B. Xu, M. Lu, S. Li, J. Guo, F. Chen, X. Xiong, Z. Yin, H. Liu and D. Zhou, Bioact. Mater., 2022, 12, 246–256 CAS.
- J. Park, J. Jeon, B. Kim, M. S. Lee, S. Park, J. Lim, J. Yi, H. Lee, H. S. Yang and J. Y. Lee, Adv. Funct. Mater., 2020, 30, 2003759 CrossRef CAS.
- A. Patel, S. Mukundan, W. Wang, A. Karumuri, V. Sant, S. M. Mukhopadhyay and S. Sant, Acta Biomater., 2016, 32, 77–88 CrossRef CAS PubMed.
- T. Hu, M. Shi, X. Zhao, Y. Liang, L. Bi, Z. Zhang, S. Liu, B. Chen, X. Duan and B. Guo, Chem. Eng. J., 2022, 428, 131017 CrossRef CAS.
- Y. Jang, S. M. Kim, E. Kim, D. Y. Lee, T. M. Kang and S. J. Kim, Microsyst. Nanoeng., 2021, 7, 70 CrossRef CAS PubMed.
- J. Lee, V. Manoharan, L. Cheung, S. Lee, B.-H. Cha, P. Newman, R. Farzad, S. Mehrotra, K. Zhang and F. Khan, ACS Nano, 2019, 13, 12525–12539 CrossRef CAS PubMed.
- Q. Huang, Y. Cai, X. Zhang, J. Liu, Z. Liu, B. Li, H. Wong, F. Xu, L. Sheng and D. Sun, ACS Appl. Mater. Interfaces, 2021, 13, 112–122 CrossRef CAS PubMed.
- J. Zhang, X. Zhang, C. Wang, F. Li, Z. Qiao, L. Zeng, Z. Wang, H. Liu, J. Ding and H. Yang, Adv. Healthcare Mater., 2021, 10, 2000604 CrossRef CAS PubMed.
- Z. Peng, X. Liu, W. Zhang, Z. Zeng, Z. Liu, C. Zhang, Y. Liu, B. Shao, Q. Liang, W. Tang and X. Yuan, Environ. Int., 2020, 134, 105298 CrossRef CAS PubMed.
- R. Kour, S. Arya, S.-J. Young, V. Gupta, P. Bandhoria and A. Khosla, J. Electrochem. Soc., 2020, 167, 037555 CrossRef CAS.
- W. Li, C. Wang and X. Lu, J. Mater. Chem. A, 2021, 9, 3786–3827 RSC.
- Z. Li, L. Wang, Y. Li, Y. Feng and W. Feng, Compos. Sci. Technol., 2019, 179, 10–40 CrossRef CAS.
- L. H. Madkour and L. H. Madkour, Nanoelectronic Materials: Fundamentals and Applications, 2019, pp. 165–245 Search PubMed.
- T. Ha, S. Park, M. Shin, J.-Y. Lee, J.-H. Choi and J.-W. Choi, Chem. Eng. J., 2023, 463, 142284 CrossRef CAS.
- H. Tetsuka, L. Pirrami, T. Wang, D. Demarchi and S. R. Shin, Adv. Funct. Mater., 2022, 32, 2202674 CrossRef CAS PubMed.
- S. R. Shin, B. Migliori, B. Miccoli, Y. C. Li, P. Mostafalu, J. Seo, S. Mandla, A. Enrico, S. Antona, R. Sabarish, T. Zheng, L. Pirrami, K. Zhang, Y. S. Zhang, K. T. Wan, D. Demarchi, M. R. Dokmeci and A. Khademhosseini, Adv. Mater., 2018, 30, 1704189 CrossRef PubMed.
- L. Chang, D. Wang, A. Jiang and Y. Hu, Chempluschem, 2022, 87, e202100437 CrossRef CAS PubMed.
- L. Kong and W. Chen, Adv. Mater., 2014, 26, 1025–1043 CrossRef CAS PubMed.
- Y. L. Li, W. W. Liu, X. L. Gao, T. Zou, P. Y. Deng, J. Zhao, T. Zhang, Y. D. Chen, L. Y. He, L. H. Shao, Z. Y. Yan and X. G. Zhang, Sens. Actuators, A, 2023, 354, 114277 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work as the first authors. |
| This journal is © The Royal Society of Chemistry 2024 |