Thermally stable NIR broad emission of Cr3+ doping phosphor with a high output power†
Zhishan
Chen
d,
Shaoan
Zhang
 *bc,
Zhenzhang
Li
b,
Huacong
Ye
d,
Haoran
Yan
b,
Jialong
Xu
d,
Ling
Gao
a,
Yang
Li
*bc,
Zhenzhang
Li
b,
Huacong
Ye
d,
Haoran
Yan
b,
Jialong
Xu
d,
Ling
Gao
a,
Yang
Li
 *acd and
Shizhen
Zhang
*a
*acd and
Shizhen
Zhang
*a
aDepartment of Neurosurgery and Neurosurgical Disease Research Centre, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, 510006, China. E-mail: zhangshizhen2024@yeah.net
bInstitute of Light+X Science and Technology, Faculty of Electrical Engineering and Computer Science, Ningbo University, Ningbo, 315211, China. E-mail: zsagdut2016@yeah.net; lychris@sina.com
cSchool of Mathematics and Systems Science, Guangdong Polytechnic Normal University, Guangzhou, 510665, China
dSchool of Biomedical Engineering, Guangzhou Medical University, Guangzhou, 510006, China
First published on 22nd August 2024
Abstract
The development of high-performance near-infrared (NIR) luminescent materials remains a significant challenge, particularly in enhancing thermal stability. Herein, we observed an anti-thermal quenching effect in the YGa1.5Al1.5(BO3)4:Cr3+ phosphor, with its emission intensity reaching 104% at 423 K and 101% at 483 K compared to room temperature. This anti-thermal quenching is mainly attributed to thermal-induced emission compensation resulting from excited electrons trapped at crystal defects, as confirmed by density functional theory computation. Additionally, YGa1.5Al1.5(BO3)4:Cr3+ exhibits a broad NIR emission peaking at 760 nm with a full width at half maximum of 135 nm and a high photoluminescence quantum yield (86%). As a proof-of-concept, we fabricated an NIR phosphor-converted light-emitting diode device that achieves an NIR output power of 59.67 mW with an input working current of 150 mA, demonstrating a photoelectric conversion efficiency of 13.6%. The utilization of this high-power NIR light-emitting diode device as a lighting source enables a penetration depth of up to 15 mm, demonstrating the potential applications of Cr3+-doped YGa1.5Al1.5(BO3)4:Cr3+ phosphors for non-invasive detection of biological tissue.
1. Introduction
Integrating near-infrared (NIR) optical imaging technology into compact, portable smart devices and wearable optical gadgets has been an ongoing pursuit in recent years, aiming to achieve real-time monitoring of human health.1–6 In the foreseeable future, silicon-based detectors integrated into smartphones will be extensively utilized for measuring oxygen content in blood, monitoring blood pressure, and detecting heart rate by leveraging the accuracy and user-friendliness of NIR technology.2–4 While traditional NIR halogen lamps and semiconductor light-emitting diode (LED) arrays with their wide spectral range covering nearly the entire near-ultraviolet to NIR region face limitations such as high-power consumption, bulky size, and high operating temperature that restrict their application in miniaturized and portable devices.5,6 In contrast to these traditional NIR sources, NIR phosphor-converted light-emitting diodes (pc-LEDs) exhibit more promising prospects due to their compact structure, long lifespan, low energy consumption, convenient lightweight design, especially with adjustable NIR spectrum distribution.4–7 The rapid advancement in the research of NIR phosphors, activated by Cr3+ ions and regarded as ideal candidates, along with their efficient absorption of blue light, make them highly promising luminescent materials for applications in pc-LED devices.8,9 However, the significant difference in energy between the excitation and emission spectra leads to a strong decrease in brightness for Cr3+-doped NIR phosphors when exposed to high temperatures from blue LED chips.10,11 Therefore, it is imperative to ensure optimal thermal stability while simultaneously ensuring high photoluminescence quantum efficiency.As we know, the design of wideband NIR Cr3+-activated phosphors poses a high-dimensional, multi-scale, and intricate scientific conundrum, wherein the performance of NIR Cr3+-activated phosphors is not solely contingent upon the intrinsic properties of Cr3+ luminescent center but also intricately linked to the composition of elements, atomic arrangement, crystal structure, and particularly intertwined with the local structure of the luminescent center.12–15 Henceforth, the selection of Cr3+ doped potential candidates assumes paramount significance. The double borates RX3(BO3)4, where R represents a rare earth ion and X denotes Al or Ga, exhibit heterogeneous isomorphism with the mineral huntite CaMg3(CO3)4 and possess a layered structure comprising diverse sequences of Y- and Ga/Al-rich layers.16,17 Adjacent [Ga/AlO6] octahedra are interconnected through edges to form stable helical chains along the c-axis direction. These helical chains are linked with [YO6] trigonal prisms and [BO3] triangular planes by sharing bridging oxygen atoms, resulting in an exceptionally rigid and steadfast framework.16,17 In addition to their remarkable structural rigidity, the unique structure of YGa1.5Al1.5(BO3)4, belonging to the RX3(BO3)4 group, suggests its exceptional suitability for incorporating Cr3+ doping sites, positioning it as an outstanding candidate with great potential.
In our study, we conducted both experimental and theoretical calculations to investigate YGa1.5Al1.5(BO3)4:Cr3+ phosphors. Moving on to experimental results, YGa1.5Al1.5(BO3)4:Cr3+ exhibits a broad NIR emission with a peak at 760 nm and a full width at half maximum (FWHM) of 135 nm. Additionally, YGa1.5Al1.5(BO3)4:Cr3+ demonstrated an impressive photoluminescence quantum yield (PLQY) of 86% and excellent thermal stability even at elevated temperatures (104%@423 K). The anti-thermal quenching observed in this study can be attributed to the defect level, which is caused by a coupling between the doped ion Cr3+ and the oxygen vacancy (VO). To showcase its potential applications, we fabricated an NIR phosphor-converted LED device by coating our prepared YGa1.5Al1.5(BO3)4:Cr3+ phosphors onto commercially available blue LED chips as a proof-of-concept demonstration. Remarkably, this LED device achieved significant NIR output power of up to 59.67 mW with an input working current of 150 mA while maintaining a remarkable photoelectric conversion efficiency of 13.6%. The utilization of this high-power NIR LED device as a lighting source enables a penetration depth of up to 15 mm, showcasing the potential applications of Cr3+-doped YGa1.5Al1.5(BO3)4:Cr3+ phosphors for non-invasive detection of biological tissue.
2. Experiments section
2.1. Synthesis
The YGa1.5Al1.5(BO3)4:Cr3+ phosphor was synthesized using high-purity raw materials, including yttrium oxide (Y2O3, 99.99%), aluminum oxide (Al2O3, 99.99%), boron trioxide (B2O3, 99.95%), chromium(III) oxide (Cr2O3, 99.95%), and gallium oxide (Ga2O3, 99.99%) via the conventional solid-state reaction technique. Stoichiometric proportions of the precursors were measured based on the YGa1.5Al1.5(BO3)4:Cr3+ composition with an additional 10 wt% boron trioxide to compensate for evaporation during the calcination process. The materials were thoroughly blended by milling in an agate mortar before transferring to a corundum crucible for further processing at a preheating stage of 500 °C for 2 hours in a muffle furnace followed by calcination at 1100 °C for 6 hours. After completion of the sintering process, the products were allowed to cool naturally to room temperature.2.2. Measurements and characterizations
In this paper, the YGa1.5Al1.5(BO3)4:0.06Cr3+ sample exhibited the highest PL intensity. Therefore, a doping concentration of 0.06 for Cr3+ was selected for the following experiment and characterization. The X-ray diffraction (XRD) patterns of the prepared Cr3+ doping phosphor were obtained using a Bruker D8 Advance X-ray diffractometer with Cu Kα1 radiation, operating at 40 kV and 20 mA. The photoluminescence (PL) and photoluminescence excitation (PLE) experiments were conducted using an FLS1000 fluorescence spectrophotometer (Edinburgh Instruments Ltd, UK). A 450 W ozone-free xenon was used as an excitation source for the steady-state measurements. Photoluminescence quantum efficiency (PLQY) of our prepared phosphors was measured on a quantum yields measurement system (C9920-02, Hamamatsu Photonics K.K., Japan). The high-temperature PL spectra of 300 K–600 K were measured by the Jobin Yvon Triax 320 fluorescence spectrometer equipped with a double excitation monochromator and a homemade high-temperature sample heater. The morphology and size of the particles were characterized by transmission electron microscope (TEM) with a FEI Talos F200S.2.3. NIR LED fabrication
The NIR LEDs were created by utilizing the synthesized YGa1.5Al1.5(BO3)4:Cr3+ NIR-emitting phosphor in combination with a blue LED from Shanghai Blu-ray Technology Co., Ltd, China. The epoxy resin to NIR phosphor mass ratio remained constant at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The electroluminescence spectrum (EL) of NIR LEDs was measured using a fiber spectrophotometer (Nova high-sensitivity spectrometer, Idea Optics, China). Output power and conversion efficiency were determined using an ATA 100 photoelectric measuring system (EVERFINE, China). Images were captured using an industrial night-vision camera (MVCA050-20GN, Hikvision, China). Meanwhile, the temperatures of the operating NIR LEDs were recorded with a thermal infrared camera (FOTRIC 280).
1. The electroluminescence spectrum (EL) of NIR LEDs was measured using a fiber spectrophotometer (Nova high-sensitivity spectrometer, Idea Optics, China). Output power and conversion efficiency were determined using an ATA 100 photoelectric measuring system (EVERFINE, China). Images were captured using an industrial night-vision camera (MVCA050-20GN, Hikvision, China). Meanwhile, the temperatures of the operating NIR LEDs were recorded with a thermal infrared camera (FOTRIC 280).
2.4. DFT calculations
In this investigation, we employed density functional theory (DFT) in conjunction with the projector-augmented wave (PAW) method as implemented in the Vienna Ab initio Simulation Package (VASP).18 The Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) framework was utilized for exchange–correlation energy calculations.19 The choice of the PBE functional is well-established for providing a good balance between computational efficiency and accuracy for a wide range of materials, including the types studied in this work.To ensure computational accuracy, we initially validated the authenticity of the pseudopotentials used in our calculations. This involved comparing the calculated properties of well-known reference materials to experimental data and high-accuracy theoretical results. The basis functions of the PAW method were defined with an energy cutoff of 550 eV, ensuring sufficient plane-wave basis set completeness for reliable results.18,19
For Brillouin zone sampling, we employed the Monkhorst–Pack method with a 5 × 5 × 5 K-point grid, providing a fine sampling mesh that ensures accurate integration over the reciprocal space. To achieve convergence of defect formation energies within a margin of ±0.2 eV, we carefully adjusted the supercell dimensions, K-point grids, and cutoff energies. This process included systematic testing and convergence studies to confirm that the chosen parameters yield reliable and accurate defect formation energies.
Additionally, the internal atomic positions within each supercell were allowed to relax until the forces exerted on the atoms were reduced below 0.01 eV Å−1. This relaxation ensures that the structures are at their lowest energy configurations, which is critical for accurate defect energy calculations.
DFT calculations were performed to investigate the lattice occupancy of Cr3+ doping in YGa1.5Al1.5(BO3)4. The crystal structure data was obtained from the materials project database (no. mp-1216259, 40 atoms), where the lattice constants were determined as a = 5.9841 Å, b = 6.00002 Å, c = 11.3040 Å, α = 98.2333°, β = 98.0532°, and γ = 104.1277°, respectively. Initially, we optimized the structure of the supercell and obtained optimized lattice constants of a = 5.9825 Å, b = 6.0064 Å, c = 11.3200 Å, α = 98.2818°, β = 98.1212°, and γ = 104.0709°, which showed excellent agreement with results from the crystal library analysis. After performing optimization, the monomer cell was expanded to create a supercell with dimensions of 2 × 2 × 1, containing a total of 160 atoms. The coordination and atomic radii (RCr = 1.18 Å, RAl = 1.18 Å, RGa = 1.26 Å, RY = 1.62 Å) imply the potential for substitution of Al and Ga sites with Cr3+ ions in the YGa1.5Al1.5(BO3)4 crystal lattice. Thus, an analysis was conducted on the distribution of Cr3+ on various sites within the supercell: specifically, the Al site (Cr–Al), Ga site (Cr–Ga), and Y site (Cr–Y). Considering that Al and Ga have similar local structures within the crystal lattice, simultaneous occupation of Cr on both Al and Ga systems (Cr–Al/Ga) was analyzed. Initially, we optimized the structure of the doped system within the supercell followed by computation of its corresponding formation energy:20,21
The chemical potentials for B (uB), Y (uY), Al (uAl), Ga (uGa), and Cr (uCr) are obtained from their respective bulk metallic phases. The baseline energy for an oxygen molecule is expressed as uO, which is defined as half of the total energy for a single O2 molecule (uO = 1/2uO2). When undergoing processes deviating from equilibrium, it is crucial to ensure that the chemical potential for any given atom does not exceed its elemental ground state chemical potential. Additionally, the formation energy (Δ) of the stable phase YGa1.5Al1.5(BO3)4 is characterized by a negative value, indicating that Δ = EH (YGa1.5Al1.5(BO3)4) − uY (bulk) − 1.5uGa (bulk) − 1.5uAl (bulk) − 4uB (bulk) − 12uO < 0. In this framework, we equate uO in its gaseous state to uO in the solid state. Hence, the determined values for uY, uAl, uGa, uB, and uO must comply with the following conditions:20,21
| Δ + uY (bulk) ≤ uY ≤ uY (bulk), |
| Δ + 1.5uGa (bulk) ≤ 1.5uGa ≤ 1.5uGa (bulk), |
| Δ + 1.5uAl (bulk) ≤ 1.5uAl ≤ 1.5uAl (bulk), |
| Δ + 4uB (bulk) ≤ 4uB ≤ 4uB (bulk), |
| Δ + 12uO (gas) ≤ 12uO ≤ 12uO (gas). |
3. Results and discussion
3.1. XRD refinements and crystal structure
To investigate the impact of Cr3+ doping concentration on the crystal structure, Fig. 1a presents the XRD patterns of as-prepared YGa1.5Al1.5(BO3)4:xCr3+ (x = 0.02, 0.04, 0.06, and 0.08) samples. The diffraction peaks in Fig. 1a exhibit excellent agreement with the standard card ISCD no. 91963, indicating the incorporation of Cr3+ into the YGa1.5Al1.5(BO3)4 host due to their similar ionic radii with Ga3+/Al3+.11,17 The main diffraction peak at 33° in Fig. 1a exhibits a blue shift, indicating the influence of Cr3+ on the crystal structure of the YGa1.5Al1.5(BO3)4 host. To gain insights into the effect of Cr3+ substitution on the crystal structure of YGa1.5Al1.5(BO3)4, the Rietveld refinement results were performed on both YGa1.5Al1.5(BO3)4 host and YGa1.5Al1.5(BO3)4:0.06Cr3+ sample. The crystal structure of YGa1.5Al1.5(BO3)4 was initially chosen as a model for structural refinements. Fig. 1b and c display the experimental, calculated, and difference patterns of YGa1.5Al1.5(BO3)4 host and YGa1.5Al1.5(BO3)4:0.06Cr3+ sample, respectively. The structural refinements, including space group, unit cell parameters and R factors, for both YGa1.5Al1.5(BO3)4 host and YGa1.5Al1.5(BO3)4:0.06Cr3+ sample are presented (Table S1†). Low R factors indicate an excellent fit between experimental and calculated XRD patterns. Based on crystallographic parameters of YGa1.5Al1.5(BO3)4 host, the corresponding crystal structure was constructed. Furthermore, the atomic coordinate parameters of YGa1.5Al1.5(BO3)4 host and YGa1.5Al1.5(BO3)4:0.06Cr3+ sample are presented in Table S2.†As shown in Fig. 1d, the crystal structure of YGa1.5Al1.5(BO3)4 host consists of three polyhedral YO6, Ga/AlO6 and BO3. The unique feature of YGa1.5Al1.5(BO3)4 host can be characterized as a framework layer, which is formed by two Ga/AlO6 octahedra sharing corners (Fig. 1e). Notably, Ga/Al ions are situated in a distorted octahedral coordination environment, potentially enhancing the optical absorption intensity of Cr3+ ions when Cr3+ substitutes for the Ga/Al site. In Fig. 1f, TEM analysis reveals that the particle size measures approximately 200 nm, exhibiting excellent dispersion characteristics.
3.2. Photoluminescence performance
As illustrated in Fig. 2a, YGa1.5Al1.5(BO3)4 host exhibits a high diffuse reflectance within the visible region, spanning 350 to 800 nm; however, there is a significant reduction within the ultraviolet (UV) region (200–300 nm), which is ascribed to the bandgap absorption of YGa1.5Al1.5(BO3)4 host. YGa1.5Al1.5(BO3)4:0.06Cr3+ sample exhibits two broad absorption bands at 430 and 600 nm, arising from the ground state 4A2g to 4T1g and 4T2g of Cr3+ ions, respectively. The bandgap of the YGa1.5Al1.5(BO3)4 host can be determined by analyzing the UV absorption edge. Assuming the YGa1.5Al1.5(BO3)4 host to be a direct bandgap material, the corresponding optical bandgap (Eg) was estimated using the following equation:22,23| (αhν)2 = A(hν − Eg) | (1) |
 | (2) |
The reflectance of YGa1.5Al1.5(BO3)4 host is denoted as R∞. The Tauc plot in Fig. 2b illustrates the optical bandgap of approximately 4.6 eV for the YGa1.5Al1.5(BO3)4 host.
In Fig. 2c, a broad band between 350–500 nm results from the transition of Cr3+ from the ground state 4A2g to the first excited state 4T2g, while another broad band centered at 611 nm is ascribed to the 4A2g → 4T1g transition of Cr3+ ions.24,25 It is worth noting that the FWHM of the PLE broad bands at 430 nm is calculated to be 81 nm, with a wide FWHM being suitable for the stimulation of commercial blue LED chips. Under 430 nm blue light stimulation, Fig. 2c shows a broad NIR emission extending from the red to the NIR region (625–850 nm), which originates from the 4T2g (4F) → 4A2g (4F) transition of Cr3+ with a FWHM as wide as 135 nm. A line emission at 683 nm is attributed to the spin-forbidden 2Eg → 4A2g transitions (R-lines) of Cr3+.24,25 PL intensity of YGa1.5Al1.5(BO3)4:Cr3+ in Fig. 2d exhibits a dependence on the doping concentration of Cr3+, with the optimal value observed at a doping concentration of 0.06 for Cr3+. The presence of both 2E → 4A2 transitions and 4T2g (4F) → 4A2g (4F) of Cr3+ indicates the existence of an intermediate crystal field.1,26,27 Whether the emission from Cr3+ is broadband or a sharp line depends on the interaction between Cr3+ and its surrounding ligands, which can be evaluated by crystal field strength Dq as well as two Racah parameters B and C. The values of Dq, B, and C parameters were calculated by the following equations:28,29
| 10Dq = E(4A2g − 4T2g)−ΔS/2 | (3) |
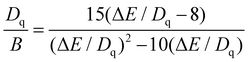 | (4) |
| ΔE = E(4A2g → 4T1g) − E(4A2g → 4T2g) | (5) |
Fig. 2f and g show the temperature-dependent PL spectrum and integrated intensity of YGa1.5Al1.5(BO3)4:0.06Cr3+ sample ranging from room temperature to 573 K. The PL intensity of YGa1.5Al1.5(BO3)4:0.06Cr3+ sample in Fig. 2g reached its maximum value (104%) at 423 K, and 101% at 483 K compared to that at room temperature, and subsequently declined to 74.4% of room temperature as the temperature rose to 573 K. The anti-thermal quenching of Cr3+ in YGa1.5Al1.5(BO3)4 can be attributed to the rigid structure of the host material, YGa1.5Al1.5(BO3)4, on one hand, and to the trapping of excited electrons at trap defects on the other (Fig. 2h). In Fig. 2h, the thermoluminescence (TL) spectrum confirmed the existence of trap defects in the YGa1.5Al1.5(BO3)4:0.06Cr3+ sample. Based on the empirical equation Etrap = T/500 eV, the trap depth (Etrap) was estimated at 0.78 and 1.16 eV, which might be attributed to trap levels.31–33 The anti-thermal quenching behavior of YGa1.5Al1.5(BO3)4:Cr3+ sample can be explained as follows: when the electrons on the 4T2g ground state of Cr3+ ions are excited to the 4A2g excited state, they undergo two competitive processes: radiative emission and non-radiative cross-relaxation, thermal ionization or energy transfer to quenching centers. At elevated temperatures, the non-radiative probability increases leading to the thermal quenching in PL intensity.34–36 However, electrons released thermally from trap levels and recombined by the Cr3+–4A2g state compensate for this loss at high temperatures, resulting in dynamic equilibrium between non-radiative and recombination processes that achieve anti-thermal quenching behavior.37,38 In the following, we will discuss in detail the attribution of defect levels through DFT.
3.3. DFT calculations of YGa1.5Al1.5(BO3)4:Cr3+
Based on DFT calculations, the formation energies of Cr–Al, Cr–Ga, Cr–Al/Ga, and Cr–Y are determined to be −2.39 eV, −3.46 eV, −3.06 eV, and 5.99 eV, respectively. It is evident that the formation energy of Cr–Al is comparatively low, indicating a strong preference for Cr3+ to occupy the Al3+ site within the crystal lattice. Furthermore, these findings suggest a pronounced affinity between Cr3+ and Al3+ atoms in this specific crystal structure due to their similar atomic radii and coordination environments which facilitate their mutual incorporation into the lattice. However, upon considering both the Cr–Ga and Cr–Al/Ga systems, it becomes evident that their formation energies closely resemble that of Cr–Al, implying a potential coexistence within the crystal structure.39–41The impact of Cr3+ on the bandgap and density-of-states (DOS) of the YGa1.5Al1.5(BO3)4 host was investigated through DFT calculations. As shown in Fig. 3a, the YGa1.5Al1.5(BO3)4 host exhibits a direct band gap of 4.93 eV. It is noteworthy that the conduct band (CB) primarily consists of DOS contributed by Y atoms, and the valence band (VB) mainly comprises DOS from O atoms. In Fig. 3b–d, there is a significant influence on the band gap, with calculated values of 4.97 eV, 5.00 eV, and 5.01 eV for Cr3+ ions occupying Al, Ga or Al/Ga sites, respectively. Furthermore, the introduction of Cr3+ ions in Fig. 3b–d results in one defect level of 2.64 eV, 2.63 eV and 2.66 eV above VB maximum (VBM + 1), while the other defect level of 0.40 eV, 0.46 eV and 0.46 eV below CB minimum (CBM − 1), respectively; this phenomenon can be attributed to explain the energy level transition of Cr3+ in YGa1.5Al1.5(BO3)4.42–45
The doped lattice structure of Cr3+ generally exhibits a propensity for oxygen vacancy defects, which in turn play a crucial role in the formation of trap levels. Therefore, to delve deeper into the specific mechanism underlying trap level formation, we will simulate the scenario where Cr3+ occupies the Al3+ lattice and experiences localized structural oxygen loss, as this closely resembles real-world conditions (Fig. 3e). In Fig. 3f, when oxygen defects (VO) form at the doping position, there is an increase in the band gap (5.08 eV), resulting in two new energy levels located at 1.20 eV above VBM (VBM + 1) and at 2.05 eV below CBM (CBM + 2). VBM + 1 primarily arises from the coupling between Cr3+ and VO, while CBM + 2 mainly originates from Cr atoms due to electron or hole release when Cr–O bonds break. The other two energy levels (CBM − 1 and VBM + 2) exhibit similarities with those of the Cr–Al system (CBM − 1 and VBM + 1). Therefore, we can conclude that the attribution of the defect level that determines the thermal stability of Cr3+ is not only caused by the oxygen vacancy but also by the coupling of the doped ion Cr3+ and the VO.
Compared with Fig. 4a, introducing Cr3+ into the Al3+ sites in Fig. 4b significantly disturbs the band structure and electronic structures, leading to alteration in the depth of defect levels at the VBM due to variations in its DOS curve shape while still maintaining dominance in composition within VBM's oxygen orbital component. This can be attributed to substituting Cr3+ ions in octahedral Al3+ sites, which impacts the electronic orbital distribution of O atoms near the original Al lattice site. The CBM is minimally affected and remains composed of Y, Ga, B, and O. The defect energy levels of VBM + 1 and VBM + 2 primarily result from Cr doping, with electron orbitals mainly consisting of Cr along with minor amounts of O, B, and Y components. However, the DOS of CBM − 1 is significantly higher than that of VBM + 1.
When both Al and Ga are occupied by Cr3+ (Fig. 4d), the energy levels of the VBM and CBM exhibit a remarkable similarity to those of Al. In the defect level within the band gap, VBM + 1 exhibits a morphology highly resembling that of the Cr–Al system (Fig. 4b), while CBM − 1 displays a morphology similar to that of the Cr–Ga system. Notably, CBM − 1 possesses a higher charge density compared to VBM + 1. The dominant similarity lies in the components related to Cr3+, whereas the O, B, and Y components demonstrate relatively minor similarities. When Cr3+ simultaneously occupies the Al and Ga sites, the charge density of the resulting energy level is comparable to that of Cr–Ga or Cr–AI, with the exception that the charge density of Cr3+ increases. This can be interpreted as a superposition of the charge coupling between Cr–Ga and Cr–AI. In the presence of an oxygen vacancy near the Cr–AI system, significant disturbances occur in both O and Ga atoms due to the breakage of Cr–O bonds.45,46 Consequently, a new energy level VBM + 1 is predominantly formed by disturbances from Cr, Ga, and O; while CBM + 2 is primarily influenced by disturbances from Cr and O. The electron cloud states for VBM + 2 and CBM + 1 resemble those observed in Cr–Ga or Cr–AI systems (Fig. 4e).
3.4. NIR performance of LED chips
The electroluminescence (EL) spectra of the NIR pc-LED device, fabricated by coating YGa1.5Al1.5(BO3)4:Cr3+ NIR phosphors onto a commercial blue LED chip, are presented in Fig. 5a. As depicted in Fig. 5a, the electroluminescence intensity of our NIR pc-LEDs exhibits an upward trend with increasing working current. Fig. 5b and c show the output optical power and conversion photoelectric efficiency of the NIR pc-LEDs, respectively. Notably, the output power surged from 40.8 mW at 100 mA to an impressive 59.67 mW at 150 mA (Fig. 5b). Furthermore, when operated at high currents up to 100 mA and 150 mA in Fig. 5c, the exceptional NIR pc-LED chip demonstrates a commendable conversion photoelectric efficiency of 10.9% and 13.6%, respectively as depicted in Table S3.†The real-time temperature rise of LED devices under maximum operating current was continuously monitored to ensure their long-term stability, as shown in Fig. 5d. The dependence of EL intensity on working temperature for LED devices driven at a current of 300 mA. After three hours of uninterrupted operation under this maximum current (300 mA), the working temperature reached its peak value at approximately 355.7 K. However, due to the outstanding thermal stability exhibited by YGa1.5Al1.5(BO3)4:Cr3+, only a marginal five percent decrease in EL intensity was observed for the LED device (Fig. 5e). The utilization of NIR pc-LED offers significant advantages due to its exceptional tissue penetration capability without causing any harm or discomfort to the individual. As depicted in Fig. 5f, by employing this high-power NIR LED device, it becomes feasible to achieve an impressive depth of pork tissue penetration up to 15 mm, thereby emphasizing the vast potential applications of Cr3+-doped YGa1.5Al1.5(BO3)4:Cr3+ phosphors for non-invasive detection of biological tissue.43,46
4. Conclusions
In this paper, the YGa1.5Al1.5(BO3)4 host is a direct band gap material with a band gap of 4.93 eV. In comparison to other occupying sites, Cr3+ ions exhibit a preference for occupying Al sites due to their lower formation energy. Notably, the introduction of Cr3+ doping in Al sites results in the emergence of two defect levels near 2.64 eV (VBM + 1) and 0.40 eV (CBM − 1) within the band gap, which are closely associated with the luminescent properties exhibited by Cr3+. Experimental results show that the YGa1.5Al1.5(BO3)4 host has an optical bandgap of approximately 4.6 eV, which is in agreement with the DFT calculation result. YGa1.5Al1.5(BO3)4:Cr3+ exhibits a broad NIR emission peaking at 760 nm with a FWHM of 135 nm. Furthermore, YGa1.5Al1.5(BO3)4:Cr3+ phosphors show a high PLQY (86%), and excellent thermal stability (104%@423 K and 101%@483 K). As a proof-concept demonstration, we fabricated an NIR phosphor-converted LED device by coating our prepared YGa1.5Al1.5(BO3)4:Cr3+ phosphors onto commercial blue LED chips. This device achieves significant NIR output power up to 59.67 mW when the input working current reaches 150 mA, along with a remarkable photoelectric conversion efficiency of 13.6%. By utilizing this high-power NIR LED device, it becomes possible to achieve a remarkable penetration depth of up to 15 mm in biological tissue, thereby highlighting the immense potential applications of Cr3+-doped YGa1.5Al1.5(BO3)4:Cr3+ phosphors for non-invasive detection of biological tissue.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant No. 52172083, 82203887), International Science & Technology Cooperation Program of Guangdong (Grant No. 2021A0505030078), and Guangzhou Key Research and Development Program (Grant No. 2023B03J1239), China Postdoctoral Science Foundation (No. 2022M720919) and Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110393, 2022A1515110463).References
- M. Zhang, P. Dang, Y. Wan, Y. Wang, Z. Zeng, D. Liu, Q. Zhang, G. Li and J. Lin, Tailoring Ultra-Wide Visible-NIR Luminescence by Ce3+/Cr3+/Yb3+-alloying Sc-Based Oxides for Multifunctional Optical Applications, Adv. Opt. Mater., 2024, 12, 2302941 CrossRef CAS.
- Y. Wei, P. Dang, Z. Dai, G. Li and J. Lin, Advances in Near-Infrared Luminescent Materials without Cr3+: Crystal Structure Design, Luminescence Properties, and Applications, Chem. Mater., 2021, 33, 5496–5526 CrossRef CAS.
- F. Y. Zhao, Z. Song and Q. L. Liu, Advances in Chromium-Activated Phosphors for Near-Infrared Light Sources, Laser Photonics Rev., 2022, 16, 2200380 CrossRef CAS.
- P. Dang, Y. Wei, D. Liu, G. Li and J. Lin, Recent Advances in Chromium-Doped Near-Infrared Luminescent Materials: Fundamentals, Optimization Strategies, and Applications, Adv. Opt. Mater., 2022, 11, 2201739 CrossRef.
- G. N. A. De Guzman, M.-H. Fang, C.-H. Liang, Z. Bao, S.-F. Hu and R.-S. Liu, Near-infrared phosphors and their full potential: A review on practical applications and future perspectives, J. Lumin., 2020, 219, 116944 CrossRef CAS.
- M. D. Mehare, C. M. Mehare, H. C. Swart and S. J. Dhoble, Recent development in color tunable phosphors: A review, Prog. Mater. Sci., 2023, 133, 101067 CrossRef CAS.
- W. Yan, S. T. Chen, Y. X. Liu, Z. Y. Gao, Y. Wei and G. G. Li, Giant Photoluminescence Improvement and Controllable Emission Adjustment in Bi3+-Activated Ca4Zr3GeO12 Phosphors for High-Quality White Light-Emitting Diodes, ACS Appl. Electron. Mater., 2019, 1, 1970–1980 CrossRef CAS.
- Q. Zhang, X. Wei, J. Zhou, B. Milićević, L. Lin, J. Huo, J. Li, H. Ni and Z. Xia, Thermal Stability Improvement of Cr3+-Activated Broadband Near-Infrared Phosphors via State Population Optimization, Adv. Opt. Mater., 2023, 11, 2300310 CrossRef CAS.
- A. Meijerink, Ultrabright near-infrared light, Light: Sci. Appl., 2024, 13, 73 CrossRef CAS PubMed.
- G. C. Liu, W. B. Chen, Z. Xiong, Y. Z. Wang, S. Zhang and Z. G. Xia, Laser-driven broadband near-infrared light source with watt-level output, Nat. Photonics, 2024, 18, 562–568 CrossRef CAS.
- T. Wang, Y. Wang, W. Chen and Z. Xia, Crystallization of Na2SrGe6O14:Cr3+,Yb3+ Glass Ceramics Enabling a Watt-Level Output Power NIR-I/NIR-II Lighting Source, Laser Photonics Rev., 2023, 18, 2300784 CrossRef.
- M. Zhao, S. Liu, H. Cai, F. Zhao, Z. Song and Q. Liu, Efficient broadband near-infrared phosphor Sr2ScSbO6:Cr3+ for solar-like lighting, Sci. China Mater., 2021, 65, 748–756 CrossRef.
- T. Liu, H. Cai, N. Mao, Z. Song and Q. Liu, Efficient near-infrared pyroxene phosphor LiInGe2O6:Cr3+ for NIR spectroscopy application, J. Am. Ceram. Soc., 2021, 104, 4577–4584 CrossRef CAS.
- G. G. Li, C. C. Lin, W. T. Chen, M. S. Molokeev, V. V. Atuchin, C. Y. Chiang, W. Z. Zhou, C. W. Wang, W. H. Li, H. S. Sheu, T. S. Chan, C. G. Ma and R. S. Liu, Photoluminescence Tuning via Cation Substitution in Oxonitridosilicate Phosphors: DFT Calculations, Different Site Occupations, and Luminescence Mechanisms, Chem. Mater., 2014, 26, 2991–3001 CrossRef CAS.
- C. Dou, C. X. Cai, Z. Song and Q. L. Liu, Highly Quantum Efficient and Thermally Stable Near-Infrared-Emitting K-β-Al2O3:Cr3+ Phosphor, Adv. Opt. Mater., 2023, 12, 2301579 CrossRef.
- D. Santamaria-Perez, A. Otero-de-la-Roza, J. Ruiz-Fuertes, R. Chulia-Jordan, T. Marqueño, S. MacLeod and C. Popescu, Pressure and Temperature Effects on Low-Density Mg3Ca(CO3)4 Huntite Carbonate, J. Phys. Chem. C, 2019, 124, 1077–1087 CrossRef.
- B. Malysa, A. Meijerink and T. Jüstel, Temperature dependent luminescence Cr3+-doped GdAl3(BO3)4 and YAl3(BO3)4, J. Lumin., 2016, 171, 246–253 CrossRef CAS.
- G. Kresse and J. Furthmuller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter, 1996, 54, 11169–11186 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- Z. Li, B. Zhong, Y. Cao, S. Zhang, Y. Lv, Z. Mu, Z. Hu and Y. Hu, Energy transfer and tunable luminescence properties in Y3Al2Ga3O12: Tb3+, Eu3+ phosphors, J. Alloys Compd., 2019, 787, 672–682 CrossRef CAS.
- J. P. Perdew, A. Ruzsinszky, G. I. Csonka, O. A. Vydrov, G. E. Scuseria, L. A. Constantin, X. L. Zhou and K. Burke, Restoring the density-gradient expansion for exchange in solids and surfaces, Phys. Rev. Lett., 2008, 100, 136406 CrossRef PubMed.
- W. Xiao, E. T. Basore, G. Zheng, X. Liu, B. Xu and J. Qiu, Suppressed Concentration Quenching Brightens Short-Wave Infrared Emitters, Adv. Mater., 2023, 35, e2306517 CrossRef PubMed.
- E. T. Basore, W. G. Xiao, X. F. Liu, J. H. Wu and J. R. Qiu, Broadband Near-Infrared Garnet Phosphors with Near-Unity Internal Quantum Efficiency, Adv. Opt. Mater., 2020, 8, 2000296 CrossRef CAS.
- V. Rajendran, W. T. Huang, K. C. Chen, H. Chang and R. S. Liu, Energy-saving chromium-activated garnet-structured phosphor-converted near-infrared light-emitting diodes, J. Mater. Chem. C, 2022, 10, 14367–14378 RSC.
- G. N. A. De Guzman, S. F. Hu and R. S. Liu, Enticing applications of near-infrared phosphors: Review and future perspectives, J. Chin. Chem. Soc., 2020, 68, 206–215 CrossRef.
- W. T. Huang, K. C. Chen, M. H. Huang and R. S. Liu, Tunable Spinel Structure Phosphors: Dynamic Change in Near-Infrared Windows and Their Applications, Adv. Opt. Mater., 2023, 11, 2301166 CrossRef CAS.
- M. H. Chan, W. T. Huang, K. C. Chen, T. Y. Su, Y. C. Chan, M. Hsiao and R. S. Liu, The optical research progress of nanophosphors composed of transition elements in the fourth period of near-infrared windows I and II for deep-tissue theranostics, Nanoscale, 2022, 14, 7123–7136 RSC.
- Y. Tanabe and S. Sugano, On the absorption spectra of complex ions II, J. Phys. Soc. Jpn., 1954, 9, 766–779 CrossRef CAS.
- A. Trueba, P. Garcia-Fernandez, J. M. García-Lastra, J. A. Aramburu, M. T. Barriuso and M. Moreno, A Cr3+-Doped Fluorides and Oxides: Role of Internal Fields and Limitations of the Tanabe-Sugano Approach, J. Phys. Chem. A, 2011, 115, 1423 CrossRef CAS PubMed.
- S. Xu, J. Feng, D. Zhang, B. Zhang, D. Wen, M. Wu and J. Li, Quantifying rigidity for thermally stable Cr3+ phosphors, Phys. Chem. Chem. Phys., 2023, 25, 29303–29309 RSC.
- F. Zhu, Y. Gao, C. Zhao, J. Pi and J. Qiu, Achieving Broadband NIR-I to NIR-II Emission in an All-Inorganic Halide Double-Perovskite Cs2NaYCl6:Cr3+ Phosphor for Night Vision Imaging, ACS Appl. Mater. Interfaces, 2023, 15, 39550–39558 CrossRef CAS PubMed.
- C. Zhang, L. Huang, J. Wang, T. Zhou and R.-J. Xie, Screening of Broadband Near-Infrared Cr3+-Activated Phosphors Using Ce3+ as a Probe, Chem. Mater., 2023, 35, 2038–2046 CrossRef CAS.
- P. Luo, D. Sun, Z. Lyu, S. Wei, Z. Lu, L. Zhou, X. Zhang, S. Shen and H. You, Remote Control and Noninvasive Detection Enabled by a High-performance NIR pc-LED, Inorg. Chem., 2024, 63, 2655–2662 CrossRef CAS PubMed.
- S. Saikia, A. Ghosh and A. Nag, Broad Dual Emission by Codoping Cr3+ (d→d) and Bi3+ (s→p) in Cs2Ag0.6Na0.4 InCl6 Double Perovskite, Angew. Chem., Int. Ed., 2023, 62, e202307689 CrossRef CAS PubMed.
- Y. B. Ma, W. F. Liao, B. B. Quan, Z. H. Kong, M. S. Molokeev, A. Zolotov, M. Cheng, X. Y. Chen, Z. Zhou and M. Xia, Solid solution structural engineering enhances the luminescence of SrMgAl10O17:Cr3+for agricultural lighting, J. Lumin., 2024, 270, 120553 CrossRef CAS.
- C. Wang, X. Zhang, C. Zhong, X. Wu, Y. Xu, S. Yin, Q. Yang, L. Zhou and H. You, Enhanced quantum efficiency and thermal stability by crystal-field engineering in a Y(Ga,Al)3(BO3)4:Cr3+,Yb3+ phosphor for diverse short-wave infrared applications, J. Mater. Chem. C, 2024, 12, 3515–3525 RSC.
- Y. Wang, Y. Sun, Z. Xu, X. Xing and M. Shang, Two-Site Occupation for Constructing Double Perovskite BaLaMgNbO6:Cr3+ Ultrabroadband NIR Phosphors, Inorg. Chem., 2024, 63, 8899–8907 CrossRef CAS PubMed.
- G. Chen, Y. Jin, L. Yuan, B. Wang, J. Huo, H. Suo, H. Wu, Y. Hu and F. Wang, Unlocking Cr3+-Cr3+ Coupling in Spinel: Ultrabroadband Near-Infrared Emission beyond 900 nm with High Efficiency and Thermal Stability, ACS Appl. Mater. Interfaces, 2024, 16, 30185–30195 CrossRef CAS PubMed.
- R. Li, Y. Liu, C. Jin, L. Zhang, J. Zhang, X. J. Wang, G. Chen and J. Jiang, Boosting Applications with High-Performance Near-Infrared Phosphor-Converted Light-Emitting Diodes, Laser Photonics Rev., 2023, 18, 2300608 CrossRef.
- Z. Song, P. A. Tanner and Q. Liu, Host Dependency of Boundary between Strong and Weak Crystal Field Strength of Cr3+ Luminescence, J. Phys. Chem. Lett., 2024, 15, 2319–2324 CrossRef CAS PubMed.
- A. Balhara, S. K. Gupta, B. Modak, M. Abraham, A. K. Yadav, H. V. Annadata, S. Das, N. S. Rawat and K. Sudarshan, Synergy between structural rigidity and cluster defects in a bright near-infrared Cr3+-based phosphor for excellent thermal stability and long afterglow, J. Mater. Chem. C, 2024, 12, 9716–9732 RSC.
- N. Majewska, Y. T. Tsai, X. Y. Zeng, M. H. Fang and S. Mahlik, Advancing Near-Infrared Light Sources: Enhancing Chromium Emission through Cation Substitution in Ultra-Broadband Near-Infrared Phosphors, Chem. Mater., 2023, 35, 10228–10237 CrossRef CAS PubMed.
- V. Rajendran, K. C. Chen, W. T. Huang, M. Kaminski, M. Grzegorczyk, S. Mahlik, G. Leniec, K. M. Lu, D. H. Wei, H. Chang and R. S. Liu, Unraveling Luminescent Energy Transfer Pathways: Futuristic Approach of Miniature Shortwave Infrared Light-Emitting Diode Design, ACS Energy Lett., 2023, 8, 2395–2400 CrossRef CAS.
- D. Liu, G. Li, P. Dang, Q. Zhang, Y. Wei, L. Qiu, H. Lian, M. Shang and J. Lin, Valence conversion and site reconstruction in near-infrared-emitting chromium-activated garnet for simultaneous enhancement of quantum efficiency and thermal stability, Light: Sci. Appl., 2023, 12, 248 CrossRef CAS PubMed.
- S. W. Yuan, F. G. Wu, J. Wang, L. L. Lou, S. Zhao, D. Y. Zhu and Z. F. Mu, Ultra-broadband near infrared phosphor with wide spectral range and long peak wavelength achieved by double-site occupation, J. Rare Earths, 2023, 41, 1670–1677 CrossRef CAS.
- S. Guo, L. Ma, M. Abudureyimu, R. Wei, F. Lu, F. Hu and H. Guo, Improving and broadening luminescence in Gd2−xAlxGaSbO7:Cr3+ phosphors for NIR LED applications, Inorg. Chem. Front., 2023, 10, 2197–2205 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi01728e |
| This journal is © the Partner Organisations 2024 |






