 Open Access Article
Open Access ArticleNanozymes with biomimetically designed properties for cancer treatment
Ke
Xu†
ab,
Yujie
Cui†
c,
Bin
Guan†
de,
Linlin
Qin
bf,
Dihao
Feng
g,
Abudumijiti
Abuduwayiti
ab,
Yimu
Wu
ab,
Hao
Li
h,
Hongfei
Cheng
 *c and
Zhao
Li
*b
*c and
Zhao
Li
*b
aSchool of Medicine, Tongji University, Shanghai 200092, China
bDepartment of Thoracic Surgery, Shanghai Pulmonary Hospital, School of Medicine, Tongji University, Shanghai 200433, China. E-mail: lizhao@tongji.edu.cn
cShanghai Key Laboratory for R&D and Application of Metallic Functional Materials, Institute of New Energy for Vehicles, School of Materials Science and Engineering, Tongji University, Shanghai 201804, China. E-mail: cheng_hongfei@tongji.edu.cn
dCenter Laboratory, Jinshan Hospital, Fudan University, Shanghai 201508, China
eDepartment of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China
fDepartment of Thoracic Surgery, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, Shanghai 200081, China
gSchool of Art, Shaoxing University, Shaoxing 312000, Zhejiang, China
hDepartment of Organ Transplantation, Xiang'an Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen 361005, Fujian, China
First published on 12th March 2024
Abstract
Nanozymes, as a type of nanomaterials with enzymatic catalytic activity, have demonstrated tremendous potential in cancer treatment owing to their unique biomedical properties. However, the heterogeneity of tumors and the complex tumor microenvironment pose significant challenges to the in vivo catalytic efficacy of traditional nanozymes. Drawing inspiration from natural enzymes, scientists are now using biomimetic design to build nanozymes from the ground up. This approach aims to replicate the key characteristics of natural enzymes, including active structures, catalytic processes, and the ability to adapt to the tumor environment. This achieves selective optimization of nanozyme catalytic performance and therapeutic effects. This review takes a deep dive into the use of these biomimetically designed nanozymes in cancer treatment. It explores a range of biomimetic design strategies, from structural and process mimicry to advanced functional biomimicry. A significant focus is on tweaking the nanozyme structures to boost their catalytic performance, integrating them into complex enzyme networks similar to those in biological systems, and adjusting functions like altering tumor metabolism, reshaping the tumor environment, and enhancing drug delivery. The review also covers the applications of specially designed nanozymes in pan-cancer treatment, from catalytic therapy to improved traditional methods like chemotherapy, radiotherapy, and sonodynamic therapy, specifically analyzing the anti-tumor mechanisms of different therapeutic combination systems. Through rational design, these biomimetically designed nanozymes not only deepen the understanding of the regulatory mechanisms of nanozyme structure and performance but also adapt profoundly to tumor physiology, optimizing therapeutic effects and paving new pathways for innovative cancer treatment.
1. Introduction
Nanozymes represent a groundbreaking and distinctive class of nanomaterials that have come to the forefront with the evolution of nanoscience. These materials showcase the ability to catalyze reactions, much like enzymes, challenging the old view of nanomaterials as merely inert in biological systems.1,2 Now, they stand on par with proteins and nucleic acids as key players in biological catalysis. What sets nanozymes apart from traditional synthetic enzyme mimics is their unique nanostructure, which is the key to their high and adjustable catalytic efficiency. Research has consistently shown that tweaking these nanostructures, such as size and shape, can dramatically boost their catalytic performance.3 The versatility of these structures also allows some nanozymes to possess multiple enzymatic activities. The hallmark of nanozymes lies in their multifunctionality, blending their catalytic prowess with the distinct physical and chemical properties of nanomaterials, like magnetic and photothermal features. This fusion makes nanozymes a versatile tool with promising applications in the realm of biomedical science.Nanocatalysis in medicine is an exciting field that links nanomaterials, chemistry, enzymology, and medicine,4 and is progressively being applied in research areas ranging from in vitro testing to the diagnosis and treatment of cancer,5 inflammation,6 infectious diseases,7 and degenerative diseases.8 These interdisciplinary studies between medicine and engineering have validated the feasibility of utilizing nanocatalysis to intervene in the pathophysiology of diseases. Thanks to a deep understanding of tumor oxidative stress regulation mechanisms and the characteristics of the tumor microenvironment (TME), nanocatalytic therapy, by modulating intracellular and extracellular redox levels, induces reactive oxygen species (ROS) including hydroxyl radicals (˙OH), singlet oxygen (1O2), and superoxide radicals (O2˙−), thereby directly killing tumor cells.9 This opens a new paradigm for cancer treatment. However, the effectiveness of solo nanocatalytic therapy is limited, with weak anti-tumor responses, and it cannot fundamentally suppress the development, and recurrence of tumors.10 Recent research indicates that there are complex biological interactions between cancer cells and the TME. Tumors can alter the TME, and the TME can also affect the growth and invasion of tumors.11 Tumor cells have an antioxidant system that can resist and adapt to oxidative stress damage through pentose phosphate pathway, either by producing more nicotinamide adenine dinucleotide phosphate (NADPH) or upregulating antioxidant transcription factors.12,13 The unique biochemical and metabolic characteristics of the TME,14 such as hypoxia, lower pH, high concentrations of glutathione (GSH), as well as immune tolerance and immunosuppressive mechanisms in the immune cells of the TME.15 These conditions significantly reduce the stability, sustainability, and effectiveness of nanocatalytic therapy. Therefore, the ideal nanocatalysts for future anti-tumor therapy should improve catalytic activity, control reaction stability and sustainability, and possess multifunctionality to enhance tumor specificity, alleviating tumor hypoxia, blocking immune checkpoints, and re-regulating tumor metabolism to enhance the therapeutic effect of nanocatalytic therapy.
Developing effective strategies for designing high-performance nanozymes has always been a central issue in nanozyme research. Unlike natural enzymes, nanozymes can be synthesized from scratch, offering the advantage of controlled design and synthesis.16 In recent years, driven by practical applications, the design and synthesis of nanozymes have gradually evolved bionic design strategies by deeply analyzing and understanding the key structural sites, spatial conformations, mechanisms of reaction processes, and the catalytic activity and specificity involved in natural enzymes.17,18 Drawing inspiration from the structure of natural enzymes, biomimetic single-atom nanozymes have been engineered to mimic their metal–Nx active sites.19 These nanozymes boast customizable geometrical structures and electronic coordinations, distinctive quantum size effects, and optimal atomic utilization.20 Additionally, biological enzyme cascade reactions are known for their high substrate concentration, efficient mass transfer, and minimized intermediate breakdown.21 This has guided the development of biomimetic cascade reaction systems using nanozymes, focusing on their catalytic processes.22,23 However, understanding nanozyme catalytic activity and physical–chemical properties indicates that merely enhancing their enzyme-like functions is not enough to tackle the complexities of the TME effectively.24,25 It is also essential to combine the inherent characteristics of nanomaterials to endow nanozymes with new biomimetic functional requirements, such as combining with other treatment methods26 or reshaping the TME27 through catalytic reactions to build a cyclic reaction system.28 Ultimately, this improves the specificity and effectiveness of cancer treatment. To our knowledge, a theoretical summary of the design and application of nanozymes based on biomimetic design strategies is still lacking.
Therefore, this review focuses on nanozymes capable of biomimetic design from de novo, systematically summarizing the design strategies of structural biomimetics, process biomimetics, and functional biomimetics of nanozymes, as well as their application progress in anti-tumor diagnosis and treatment. We believe that a deep analysis of the relationship between structural components, reaction processes, multifunctionality, and catalytic therapy performance in the biomimetic design philosophy of nanozymes will lay a theoretical foundation for the evidence-based biomimetic design of nanozymes for cancer diagnosis and treatment. This is of great significance in breaking through the bottleneck in the biomedical transformation research of nanozymes.
2. Biomimetic design of nanozymes
The strategy of biomimetic design has proven to be both viable and efficient in enhancing the enzyme-like catalytic activity and therapeutic efficacy of nanozymes.29,30 Initially, nanozyme research primarily depended on arbitrary synthesis and sequential activity testing to discover highly active nanozymes.31 However, due to lacking solid theoretical guidance and high-throughput screening techniques, turned out to be cumbersome and inefficient, and it did not significantly advance the catalytic efficiency of nanozymes.32 Moreover, the diverse composition, complex structure, and unclear catalytic sites of nanomaterials have left the mechanism of nanozymes in catalytic reactions still unclear. Past studies suggested that higher catalytic activity in nanozymes often requires a smaller size, larger surface area, higher specific surface area, more open and exposed crystal faces and dangling bonds, higher nanozyme density, unsaturated surface coordination, high-energy surfaces, low surface energy, and specific surface modifications.33,34 In recent years, backed by research into the catalytic mechanisms of nanozymes, researchers have started designing and optimizing nanozymes for specific applications from scratch by mimicking the structure, process, and function of natural enzymes.35,36 This approach in structural biomimetics, process biomimetics, and functional biomimetics has paved new ways for designing and synthesizing nanozymes with enhanced catalytic activity, specificity, and selectivity.17 The concept of biomimetic design is expected to actively expand the application of nanozymes in the biomedical field, especially in integrated platforms for anti-tumor diagnosis and therapy.2.1. Structural biomimicry of nanozymes
Reactive enzymes in living organisms have gradually evolved into the most compatible substrate-active center-specific catalytic pairs over a long period. The intrinsic catalytic activities of natural enzymes are highly dependent on their structures. For example, N-acyl homoserine lactones amide hydrolase has a hydrophobic substrate-binding pocket that confers C12 fatty acid-like chain recognition.37 Lignin peroxidase can catalyze substrate oxidation with a high redox potential, as in the case of the low electron density heme active site.38 This characteristic of natural enzymes has inspired the structural biomimetic design of artificial nanozymes. We believe that biomimetic-based enzyme structure design is function-guided rather than the trial-and-error approach of random synthesis and batch screening of nano-enzymes. Currently, the widely reported methods for structural bionics can be classified into three categories: active center engineering, surface modifications, as well as geometric and chiral structure modulation (Fig. 1). Meanwhile, multiple biomimetic approaches are often combined in practical synthesis in pursuit of better results.39–42 This chapter focuses on biomimetic designs inspired by the structures of natural enzymes, with emphasis on the aforementioned three kinds of strategies.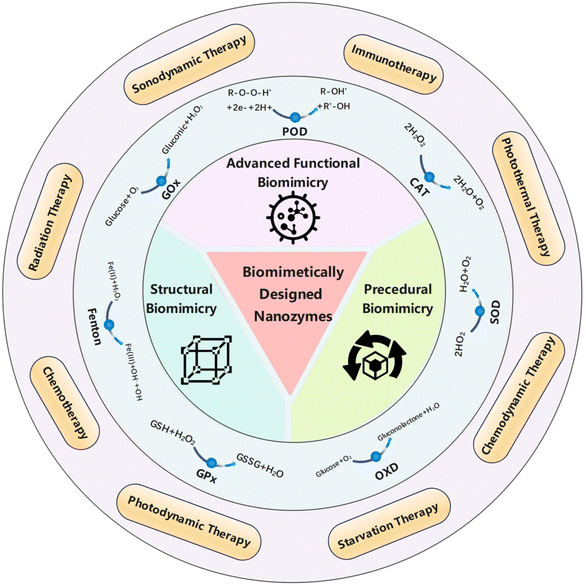 | ||
| Fig. 1 Basic aspects of the structure, procedure and advanced functions of biomimetically designed nanozymes and their biomedical applications in anti-tumor treatments. | ||
2.1.1.1 Classical metallic and non-metallic site structures. Metal sites in enzymes play important roles in catalysis, such as stabilizing charge, stabilizing free radicals, increasing system acidity, and so on.43 A class of porous nanomaterials possessing a structure similar to that of metalloproteinases in biomass are metal–organic frameworks (MOFs), which consist of metal ions or metal clusters connected by organic linkers. In addition to the biomimetic structures, MOFs also have some inherent characteristics that can aid the improvement of the catalytic performance, such as high specific surface area, tunable pore size, abundant and uniformly dispersed active sites, and multiple interconnected channels.44
MOFs such as those based on iron,45 copper,46 and zirconium47 have been developed as biomimetic nano-enzymes and the catalytic ability can be enhanced by doping various metal elements or incorporating multi-element alloy nanoparticles as active sites.48 We can either mimic the MOF system for better catalytic performance or take full advantage of its robust restriction framework as a carrier for biomimetic enzymes. MOFs with copper centers are often developed as biomimetic nano-enzymes. By mimicking the trinuclear isocenter structure of natural catechol oxidase, Li et al.49 prepared MOF-818, which selectively and efficiently catalyzes the oxidation of catechol to the corresponding o-quinone structure, illustrating its superiority to conventional oxidases (OXDs) (Fig. 2A). In addition to traditional crystalline MOFs, Liang et al.46 synthesized an amorphous multicopper-centered MOF nano-enzyme using nucleotides as ligands, which structurally and functionally mimics the natural multi-copper metalloenzymes, namely laccases. The nucleotide itself as a ligand brought about asymmetry and the high flexibility of copper ions, resulting in a partially amorphous MOF structure. Compared to natural laccases, this MOF-based laccase-mimic exhibited a higher catalytic rate, better stability over long-term storage, and stronger robustness in harsh conditions, such as extreme pH, temperature, and salt. MOFs also play an important role as a carrier, where enzymes are dispersed within the frameworks to achieve high reaction activity and stability. Scaffolds for natural enzymes are usually composed of biomolecular polymers such as peptides. They provide a relatively confined environment, support the active sites, and provide specific spatial organization. The solid support of MOF allows the enzyme to exert better activity. For example, Wang group anchored Pt single atoms as active catalytic sites in MOFs and encapsulated them to bring about high catalase (CAT) mimetic activity.50
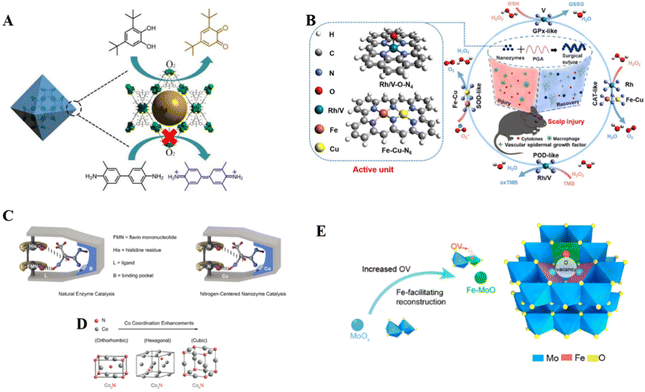 | ||
| Fig. 2 Active site engineering. (A) Schematic illustration of selective catalysis by biomimetic MOF-818 nanozymes. Reproduced from ref. 49 with permission from American Chemical Society, copyright 2022. (B) Rh/V–O–N4 and Fe–Cu–N6 active unit. Reproduced from ref. 55 with permission from Springer Nature, copyright 2022. (C) Comparison of natural enzyme catalytic models and N-center nano-enzymatic catalytic models; (D) crystallographic transition due to change in Co coordination number. Reproduced from ref. 61 with permission from the American Chemical Society, copyright 2023. (E) Fe doping causes substitution defects and oxygen vacancies (OVs) in Fe–MoOv nanozymes. Reproduced from ref. 62 with permission from the American Chemical Society, copyright 2021. | ||
We believe that constructing MOF structures will continue to be an important approach for future nanozyme mimicry and there is huge room awaiting to be explored. For example, the pore structure of MOFs can be further modified to improve mass transfer capacity, and multi-catalytic centers can be constructed to achieve synergistic effects, or amorphous and crystalline MOF heterostructure can also be attempted to synthesize.
Unlike traditional complex enzyme systems, single-atom nanomaterials with well-defined geometries and electronic structures can mimic the highly evolved catalytic centers of natural enzymes to achieve the highest atom utilization efficiencies, such as iron-nitrogen core single-atom nanozymes that mimic the local coordination environment of heme.51 They usually exist in the form of isolated active metal centers anchored to suitable carriers, which allows the catalytic potential of the mimetic enzyme to be fully realized. It is important to note that the active centers of single-atom nanozymes are not simply zero-valent metal atoms, but rather the metal centers are coordinated with other atoms (such as nitrogen and phosphorus) of the carrier. The final microstructure formation of single-atom nanozymes is inextricably linked to the synthesis process, and the carriers should ensure close integration with the enzyme body and have a certain degree of stability. For example, Liu et al.40 prepared Fe single-atom nanozymes by using Fe(NO3)3@ZIF-8 as the precursor and subsequent carrier for better iron source adsorption and site distribution. Huang et al.51 developed an oxidoreductase-like single-atom nanozymes with a FeN5 active center (FeN5 SA), which resembles the structure of a cellular P450 active center. Specifically, a carbon nano-framework was used to restrict the formation of Fe sites, which were further reconstructed into FeN4 at high temperatures, and ligated to form thermodynamically stable FeN5/C sites. Similarly, Xu et al.52 just replaced the nitrogen source to obtain the FeN5 structure directly. In addition to single N coordination, other non-metallic elements such as B53 and P54 can be introduced to bring more variable microstructures.
Various metals have been explored as the active centers of single-atom nano-enzymes in addition to Fe and Cu that are common in biological enzymes, such as Ir,55 Zn,53 and Mn.56 As shown in Fig. 2B, Zhang group55 prepared RhN4 and VN4 single-atom nano-enzymes, which displayed much higher CAT-like and glutathione peroxidase (GPx)-like activities compared with the corresponding natural enzymes, respectively.
Carbon materials are excellent candidates for single-atom nanozyme carriers. For example, environmentally friendly and biocompatible carbon dots have abundant dangling bonds, such as –NH2, –OH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, thus can easily anchor metal ions and prevent the agglomeration of metal sites. Zhang et al.57 used carbon dots as carriers and developed Pt single-atom nano-enzymes that can be easily assembled and disassembled. N-doped hollow carbon spheres can be used as carriers for Cu single-atom nanozymes, in which the active center is CuN4, as demonstrated in the work of Lu et al.58
O, thus can easily anchor metal ions and prevent the agglomeration of metal sites. Zhang et al.57 used carbon dots as carriers and developed Pt single-atom nano-enzymes that can be easily assembled and disassembled. N-doped hollow carbon spheres can be used as carriers for Cu single-atom nanozymes, in which the active center is CuN4, as demonstrated in the work of Lu et al.58
There are also some interesting structures derived from traditional metal-site structured enzymes or single-atom nanozymes. For example, inspired by the natural heme copper oxidase with a dinuclear active site, Jiao et al.59 made the first attempt to construct the Fe2NC diatomic nanozymes, which have an Fe2N6 active site that accelerates the O–O cleavage. The Fe–Cu–N6 active unit synthesized by Jana et al.60 is a large expansion of traditional single-atom nanozymes, because the lattice strain as well as the electron coordination effect brought about by such alloying contributes to the enhancement of the enzyme-like activity and the increase in the number of reaction sites.
Although most enzymes use metallic elements as active centers, some enzymes use non-metallic elements as catalytic centers, such as lactate oxidase (LOX). Given that the nitrogen atom plays an important role in natural enzymes as a catalytic center with electron-rich properties, Zhao et al.61 were inspired to optimize the electronic configuration around the N center (Fig. 2C), and the performance of LOX was sufficiently approached by adjusting the number of Co atoms coordinated to the N center. As shown in the simulated model in Fig. 2D, N-centered components borrowed from the structure of natural enzymes, and underwent different lattice transitions, causing changes in electronic structure. This non-metallic element center mimicry enriches the family of mimetic nanozymes.31
2.1.1.2 Defect-derived multiple active sites. Defects usually have higher energy than conventional ordered structures, and the artificial introduction of vacancies, substitutions, or dopants in nanomaterials can effectively mimic the distribution of active sites in natural enzymes and their spatial co-operation.31 Unlike the well-defined structural metallic or non-metallic sites discussed in the previous section, defect engineering brings about highly dispersed active sites, which act synergistically with each other to trigger multiple enzyme activities. Wu et al.63 discussed in detail and summarized the implementation strategies of nano-enzyme defect engineering, such as the formation of OVs, metal element doping, and amorphization of the enzyme to make the man-made enzyme mimic the binding and catalytic properties of the natural enzyme. Among them, the construction of OVs can effectively regulate various free radicals, so that these ROS can efficiently kill cancer cells.64 Specifically, oxygen vacancy defects bring about an optimized energy band structure that can interact with oxygen-containing substances, assisting the production of ROS and greatly enhancing the catalytic effect.
Yu et al.62 pioneered in utilizing defect engineering to develop structural biomimetic nano-enzymes, which provided the cognitive basis for subsequent defect-based studies. They used MoOx as a starting material to prepare Fe-MoOv nanozymes with substitution defects and OVs by Fe doping (Fig. 2E). Similarly, Cu doping has also been reported.39 The OVs acted as substrate pockets, and the Fe-substituted metal sites promoted the optimization of the adsorption energy of the ROS intermediates, which ultimately resulted in enhanced redox catalysis. Dong's team41 also used defect engineering to integrate active sites. They doped CeO2 with Fe to obtain a Fe–CeOv defective structure, in which Fe and O act as key synergistic active centers. Unlike the previous work, they used a hollow mesoporous structural template, and subsequently additional modifications were made. At the same time, such as doping and vacancy-induced defects, the mechanistic explanation of their promotion of catalytic reactions often requires the aid of theoretical DFT. In future work, multiple defect construction methods could be used jointly to obtain higher defect density and catalytic efficacy.
2.1.2.1 Loading of functional groups. The surface of nanozymes is the main site where catalytic reactions occur. Surface functional groups determine the affinity for the substrate substance and the nature of the enzyme surface charge. Loading functional groups on the enzyme surface by physical adsorption or chemical bonding is an intuitive and easy way to mimic the natural enzymes.
Modification groups can considerably increase the reaction rate by enhancing the interaction between substrates and reaction sites. For instance, Fan et al.35 carried out pioneering work on surface modification mimicry. By mimicking the catalytic mechanism of horseradish peroxidase (HRP), in which imidazole His42 assists the substrate to reach the active site cavity for subsequent reaction through hydrogen bonding, they introduced histidine residues as modifying groups on the surface of Fe3O4 nanozymes, which brought about hydrogen bonding to help the adsorption of hydrogen peroxide (H2O2) on the enzyme and the cleavage of the O–O bond of H2O2, thus significantly enhancing the hydroperoxide affinity on the enzyme. Consequently, the H2O2 affinity as well as the catalytic efficiency were significantly improved (Fig. 3A and B). The flexible state of surface modifiers affects the effectiveness of the modification, and past studies have shown that ligands with an upright conformation on the nanomaterial surface achieve the maximum distribution density and improve the bioactivity.65 Zhou group66 made the first attempt to use deoxyribonucleic acid (DNA) modification to functionalize CuO nanozymes and achieved controlled polar adsorption of DNA (Fig. 3C). The fluorescence quenching ability of CuO in this study promoted DNA loading, and DNA fragments not only possess the flexibility to curl up to achieve high distribution density, but also stabilize CuO colloids, resulting in better enzyme stability.
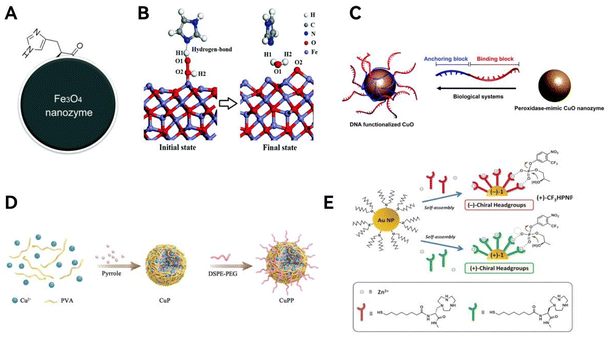 | ||
| Fig. 3 Loading of functional groups. (A) Macroscopic schematic of histidine-modified nanozymes; (B) mechanistic simulation diagram of the catalytic effect of histidine. Reproduced from ref. 35 with permission from The Royal Society of Chemistry, copyright 2017. (C) Schematic diagram of dual DNA-functionalized CuO nanozymes. Reproduced from ref. 66 with permission from The Royal Society of Chemistry, copyright 2021. (D) Schematic introduction of surface modifying groups by polyethylene glycolization. Reproduced from ref. 69 with permission from Wiley-VCH, copyright 2022. (E) Modification of surface with chiral zinc ion complexes to form chiral selective nanozymes. Reproduced from ref. 74 with permission from Wiley-VCH, copyright 2016. | ||
Enzymes for oncology therapy need to be highly biocompatible, a property that can be conferred by the modifying groups on the surface. Most of the works use amino acid-based modifying groups for better biocompatibility.67,68 Other modification groups are also available. Zeng et al.69 used polypyrrole doped with copper and then polyethylene glycolised to produce nano-enzymes that fully bind to immune checkpoint blockers and exhibit strong immune efficacy (Fig. 3D).
Folic acid, as an appealing tumor-targeting molecule, was used by Nwahara et al.70 for modification of phthalocyanine nanozymes, where the enzyme outer liposomes could be selectively conjugated with folic acid to improve in vitro photodynamic therapy (PDT) activity under hypoxic conditions. This is an example of a surface modification that improves the selectivity of the reaction. To take it a step further, the enantioselectivity of the enzyme can likewise be complemented by the introduction of appropriate surface modifiers.71–74 It is worth noting that this kind of selectivity usually originates from a chiral structure, some chiral amino acids or metal ionic organics are commonly used to introduce chirality. Chen et al.74 self-assembled thiols containing chiral zinc ion-binding headgroups on Au nanoparticles to obtain nanozymes possessing a chiral Au–Zn bimetallic catalytic site, which can subsequently enantioselectively discriminate and catalyze substrates, producing opposed activities for different chiral substrates (Fig. 3E). In another research, Chen et al.73 used arginine as a capping agent to obtain chiral ruthenium nanozymes (denoted as D/L-arginine@Ru), which mimic both OXD and nitric oxide (NO) synthase activities and thus can simultaneously produce sufficient ROS and NO to induce tumor cell apoptosis and ferroptosis. It was found that L-arginine@Ru was more effective in this study because of the chirality-induced higher natural homology, catalytic activity, and cellular uptake.
In conclusion, surface modifications aim to improve enzyme catalytic reaction kinetics, stability, biocompatibility, and enantioselectivity. The selection of the modifying moiety can draw on the optimal interaction between the enzyme and substrate to achieve a biomimetic effect. We believe that surface modification will become an important part of biomimetic design by the simple post-treatment process.
2.1.2.2 Encapsulation in membranes. Apart from modifying the nano-enzyme surface with individual molecules, surface modification can also be achieved by wrapping nano-enzymes in integrated membranes. This biomimetic structural design is mainly inspired by the structure of biological cell membranes, which can bring about good biocompatibility and aid anti-tumor drug delivery. Meanwhile, this coated membrane can enhance the adhesion of the enzyme on tumor tissues, thus improving therapeutic stability.
The surface cladding can firstly enhance the target recognition ability and bring precise treatment. Such modifications can enable nanozymes that do not have targeting capabilities but have other advantages to show unexpected therapeutic effects. A representative work is that Duo et al.75 wrapped the hollow MnO2 enzyme in a circulating platelet (PLT) membrane to form a complete enzyme–membrane system. This PLT-wrapped MnO2 enzyme fully combines the high drug-loading characteristics of the hollow material with the in vivo tumor-targeting ability of the membrane to enable the MnO2 enzyme to play a greater role, and this modification work is easy to operate (Fig. 4A).
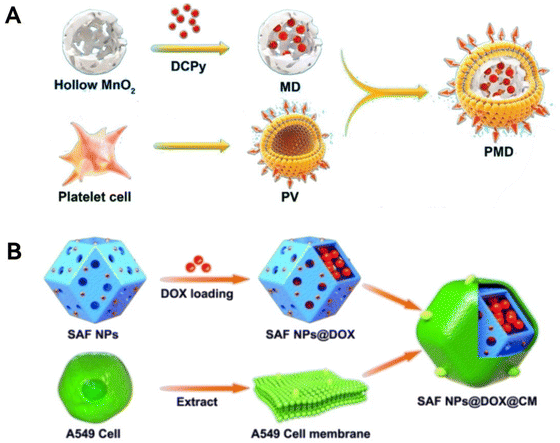 | ||
| Fig. 4 Encapsulation in membranes. (A) A Scheme showing platelet cell membrane coated hollow MnO2 that combines long-lasting therapeutic effect and high drug loading characteristics. Reproduced from ref. 75 with permission from American Chemical Society, copyright 2022. (B) Schematic representation of the single-atom nanozymes encapsulated in cell membranes. Reproduced from ref. 40 with permission from European Chemical Societies Publishing, copyright 2021. | ||
Besides, the cell membrane encapsulation structure can protect the encapsulated nano-enzymes from being cleared by the immune system, thus achieving long-lasting tumor therapy to a certain extent, as cell membranes possess certain biorecognition information.40,76,77 The mechanism of this protection is the communication of endogenous information through cell membranes, which has a camouflaging effect and thus slows down metabolism and clearance in the body. Specifically speaking, Liu et al.40 synthesized an iron-monoatomic type nano-enzyme encapsulated by A549 cell membranes, and this was used for the treatment of tumors in a long-lasting manner. The enhanced biocompatibility of the enzyme encapsulated by A549 cell membranes is reflected in the fact that it can achieve homologous binding to tumor tissues, preventing premature clearance by the body (Fig. 4B). In another case, the membrane spontaneously bends to fit the shape of the enzyme during synthesis. The structural design of cell membrane-encapsulated nanozyme is undoubtedly an excellent example of bionics, and this encapsulated structure brings enhanced targeting ability, therapeutic stability, and long-lasting efficacy, which is worth further development.
As shown in the diagram of Fig. 4B, the extraction of the cell membrane from the cell and the coating of the cell membrane onto the body of the nano-enzymes are two key steps in the realization of membrane coating, and it is particularly important to confirm the adaptability of different cell membranes for the extraction and the coating effect. It is also important to note that when surface modification is performed, the catalytically active sites should not be overly covered and obstructed, which can lead to a decrease in enzyme activity.78 How to make a balance between wrapping and ensuring the number of active sites is a challenge for future work. Besides, the modifying agents need to be firmly anchored onto nanoparticle surface, otherwise they may gradually fall off as nanozymes circulate in the human body. Finally, the modifying agents need to be rationally selected in order to circumvent the involvement of harmful elements and substances to the human body.
The geometry of the mimetic enzyme should be designed in such a way that the active site is in a pocket of the substrate channel so that the center of redox can be spatially separated from the host solution.80 Qileng et al.81 attempted to construct a Pt nano-enzyme in which the active site is distributed in an internally segregated substrate channel, which exhibits a concave half-moon shape, and the reaction channel is artificially constructed by chemical etching (Fig. 5A and B). In addition to enzyme-specific geometries, the geometric features of microscopic organisms can also be mimicked. Inspired by the process of viral invasion into cells, Zhao et al.42 modified silica matrix nano-enzymes into the shape of viruses to enable rapid response and meanwhile utilize the dense tentacles to achieve large specific surface area for high drug loading and drug delivery efficiency (Fig. 5C). The shape of the virus is suitable for cell adhesion and will aid cancer therapy. To summarize, specific geometries bring about specific confinement effects or specific biological interactions.
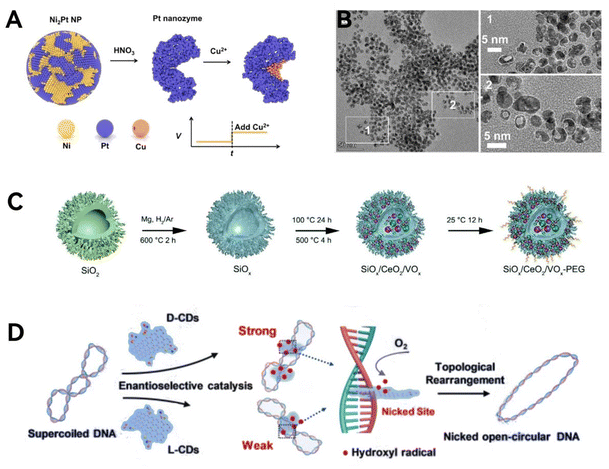 | ||
| Fig. 5 Geometric structure mimicry. (A) Mimicking the concave side of the meniscus in natural enzymes. (B) Scanning electron microscope (SEM) images of the half-moon geometry structure. Reproduced from ref. 81 with permission from Elsevier, copyright 2022. (C) Schematic diagram of nano-enzymes modified into the shape of a virus. Reproduced from ref. 42 with permission from The Royal Society of Chemistry, copyright 2022. (D) Schematic illustration of the enantioselective chiral carbon dot mediated topological rearrangement of supercoiled DNA. Reproduced from ref. 83 with permission from Wiley-VCH, copyright 2020. | ||
Chirality, as an element of geometric symmetry and a significant bio-structural analytical feature, is also included in the biomimetic design of nanozymes. Chirality is prevalent in nature, and the chirality of enzymes endows them with stereospecificity, which allows the enzyme to selectively act on only one specific stereoisomer when faced with different stereoisomers.82 This property can be utilized to selectively kill tumor cells without attacking normal cells, so the study of chirality and geometrical mimicry has practical medical applications. Most of the chiral introduction methods of nanozymes are based on surface modification of chiral ligands and achieve good enantioselective catalytic effects, which have been discussed above.71–73 In addition, Li et al.83 demonstrated that chiral carbon dots, synthesized from cysteine, exhibit topoisomerase I mimetic activity. These carbon dots can mediate the topological rearrangement of supercoiled DNA in an enantioselective manner (Fig. 5D). More specifically, the intercalative binding of D-CDs with DNA double helix is stronger than that of L-CDs, thus D-CDs perform more efficiently in catalyzing the rearrangement of DNA. In summary, structural chirality has been introduced into nano-enzymes to improve the low enantioselectivity of the original artificial enzyme, thus achieving artificially controllable selective catalysis.
Unfortunately, geometrical mimicry of enzymes is relatively less studied at present compared with other mimicry strategies, partly because of the difficulty in investigating the mechanism of enhanced catalytic activity, and partly due to the complexity of synthesizing sophisticated structures. However, it is still valuable to biomimetic design some natural enzymes with well-defined geometrical conformational relationships and pay attention to their morphological changes during catalysis.
2.2. Procedural biomimicry of nanozymes
In biological systems, an enzyme often participates in multiple catalytic reactions, or various enzymes form complexes to create complex catalytic networks,84 which carry out a series of crucial biochemical reactions, ensuring the normal physiological functions of the organism. For example, human cells contain various antioxidant enzymes like superoxide dismutase (SOD), CAT, and GPx, which together establish a comprehensive defense system based on antioxidant enzymes.85 This system is essential for maintaining the redox balance within cells and protecting the body from oxidative damage.Inspired by enzyme cascade reactions, nanozymes are designed to replicate the complex enzyme systems found in organisms, generating a series of synergistic and cascading enzymatic reactions.86 This approach, known as process biomimicry, involves participating in and simulating specific life processes. Nanozymes with process biomimetic characteristics are based on multi-enzyme systems and often possess the synergistic catalytic activities of multiple enzymes,87 mediating cascading catalytic reactions88 or responding to specific environmental factors to regulate the catalytic process.89 Compared to nanozymes with single enzyme activity, the concept of process biomimetic design offers tighter spatial structures and shorter diffusion paths for multi-enzyme catalytic reactions.22 This broadens the types of catalytic reactions, facilitates smooth transitions between reaction steps, improves catalytic efficiency, and enhances therapeutic effects.
Multi-enzyme synergistic activity and multi-enzyme cascade reactions are classified based on the number of enzymes involved in the reaction. Multi-enzyme synergistic activity depends on one enzyme that exhibits two types of activities to convert substrates into products. Multi-enzyme cascade reactions involve two or more enzymes that catalyze different types of reactions, which can be categorized into five main types: linear, parallel, orthogonal, cyclic, and triangular. While current understanding of how nanozymes exhibit multi-enzyme catalytic activities is still evolving. The catalytic capabilities of nanozymes are influenced by various intrinsic characteristics, such as size, shape, surface modifications, and composition. Nanomaterials with multiple catalytic abilities can be customized for specific purposes through from-scratch design. An effective method for developing multi-enzyme mimetic nanocatalysts with varied activities is to hybridize two or more different materials. For example, graphene quantum dots/silver nanoparticles hybrids have shown high peroxidase (POD)-like activity and OXD properties. A hollow mesoporous Mn/Zr co-doped CeO2 tandem nanocatalyst (PHMZCO-AT) exhibits tunable multi-enzymatic activities, specifically enhanced SOD- and POD-like activities while inhibiting CAT-like activity.90 By encapsulating bimetallic nanocatalysts (Fe2NC) within a selenium MOF (Se-MOF) shell, a bimetallic nanocatalyst with multi-enzymatic cascade capabilities has been constructed.91
Biomimetic design principles offer a clue: by studying the active centers of natural enzymes and applying these insights to the rational design of nanozymes, it is feasible to combine the active centers of various nanozymes into one. This innovative approach holds promise for creating nanozymes that demonstrate targeted synergistic activities, sequential cascade catalysis, and adaptability to environmental changes. Such advancement in nanozyme technology could significantly enhance their functionality and application potential in anti-tumor treatment fields.
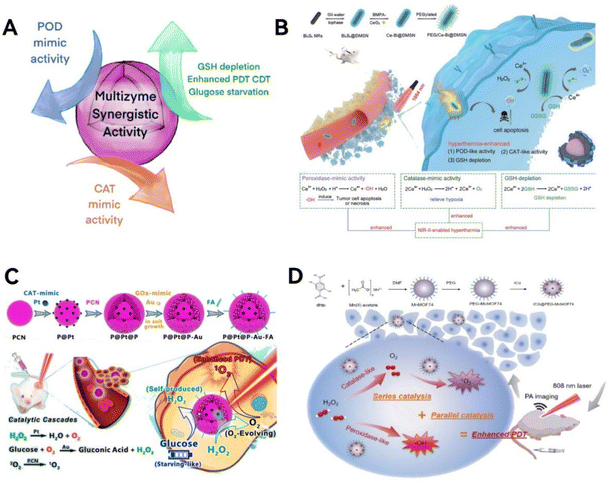 | ||
| Fig. 6 Multi-enzyme synergistic activity. (A) Scheme illustration of the mechanism of multi-enzyme synergistic activity. (B) The PEG/Ce-Bi@DMSN nanozymes, featuring synergistic dual enzyme-mimic catalytic activities and GSH depletion, are designed for in vivo synergistic photothermal-enhanced nanocatalytic cancer therapy. Reproduced from ref. 92 with permission from WILEY, copyright 2020. (C) Pt NPs effectively reduce tumor hypoxia by catalyzing H2O2 into O2, enhancing oxygen-dependent PDT, and are complemented by Au NPs that accelerate β-D-glucose consumption, synergizing with H2O2 for improved starvation therapy. Reproduced from ref. 94 with permission from the American Chemical Society, Copyright 2019. (D) The synthesis process of ICG@PEG-MnMOF74 nanoparticles with improved PDT capabilities that are realized through a series–parallel catalysis process. Reproduced from ref. 95 with permission from WILEY, copyright 2021. | ||
Lin group92 has developed a novel approach by first coating Bi2S3 nanorods with dendritic mesoporous silica (Bi2S3@DMSN) and then infusing these structures with ultra-tiny cerium dioxide nanozymes (Fig. 6B). This innovative design endows the nanozymes with the ability to mimic two key enzymes: POD and CAT. These nanozymes are particularly effective in adjusting the TME in acidic conditions. They have the dual capability of increasing oxidative stress while simultaneously reducing hypoxia, offering a promising strategy for targeted cancer therapy. Chen group93 has created a LaFeO3 perovskite nanozyme with a unique set of capabilities, mimicking four different enzymes: OXD, POD, GPx, and CAT. This nanozyme triggers a series of reactions that not only reverse the low-oxygen conditions often found in tumors but also deplete the natural antioxidant, GSH in cancer cells. This process leads to a continuous production of harmful ROS, effectively inducing cell death in densely packed breast cancer cells through pyroptosis.
To targeting overexpressed H2O2 and glucose within tumors. Liu et al.94 investigated the potential advantages of platinum nanoparticles (Pt NPs) mimicking CAT and ultra-small “exposed” gold nanoparticles (Au NPs) mimicking GOx in tumor synergistic catalytic therapy (Fig. 6C). The porous porphyrin metal organic framework (PCN) with PDT and fluorescence imaging capabilities is the cornerstone supporting synergistic catalytic activity of non-toxic nanozymes. The production of ample oxygen by Pt NPs, catalyzed by endogenous H2O2, enhances the generation of cytotoxic 1O2 during PDT induced by PCN under light exposure. This process also hastens the oxygen-dependent decomposition of glucose in tumors by Au NPs. Furthermore, catalytic therapy synergizes with starvation therapy by using the self-generated H2O2 as a substrate for the Pt NPs. The deployment of PCN-supported dual nanozyme systems to simulate catalytic reactions is poised to provide enhanced, TME-specific, and synergistic therapeutic outcomes.
To provide sufficient oxygen in the relatively hypoxic TME, Roy and colleagues95 developed a Mn-doped MOF-based nanozyme, indocyanine green (ICG)@PEG-MnMOF74, composed of three key components: Mn-doped MOF74, the photosensitizer ICG, and the stabilizer polyethylene glycol (PEG) (Fig. 6D). It exhibits catalytic activities similar to POD and CAT, not only supplying oxygen for the production of 1O2 in PDT but also generating ˙OH, thereby inducing Fenton reactions to enhance anticancer effects. This synergistic ‘series–parallel catalytic pathway’ significantly improves the therapeutic efficiency of PDT.
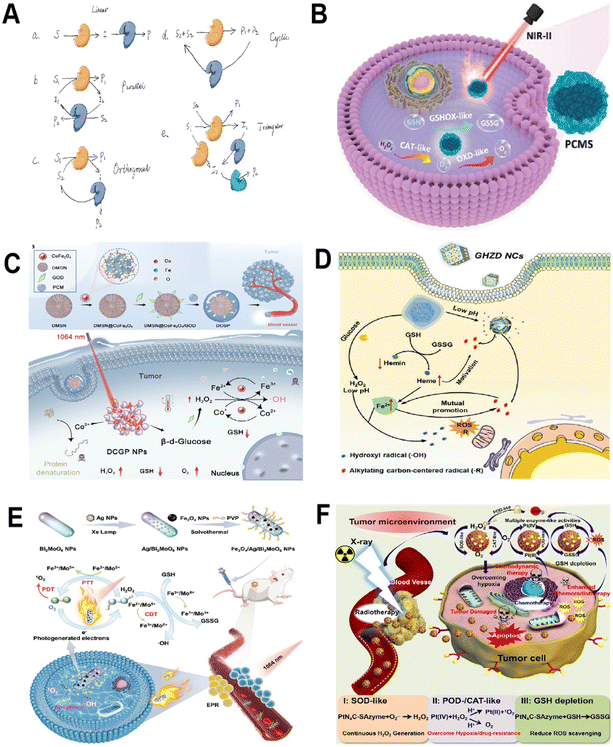 | ||
| Fig. 7 Multi-enzyme cascade catalysis. (A) Scheme illustration of the reaction types of cascade catalysis system. (B) The mechanism of PCMS for photothermal-enhanced cascade catalytic therapy. Reproduced from ref. 96 with permission from WILEY, copyright 2023. (C) Under NIR-II illumination, CoFe2O4 nanozymes initiate a cascade catalytic reaction. Within this Co/Fe dual-cycle system, highly toxic ˙OH are produced. This reaction sequence is further intensified by the in situ consumption of GSH and the alleviation of hypoxia within the tumor environment, leading to a significant amplification of oxidative stress. Reproduced from ref. 97 with permission from American Chemical Society, Copyright 2022. (D) GHZD NC nanozymes significantly amplify endogenous oxidative stress within the TME by facilitating cascade reactions. This strategic augmentation of biocatalytic therapy, when used in conjunction with checkpoint blockade therapy, effectively orchestrates an anti-tumor immune response. Reproduced from ref. 98 with permission from WILEY, copyright 2021. (E) Fe3O4/Ag/Bi2MoO6 NPs utilize their multiple nanozyme activities and photodynamic properties to initiate and sustain cascade nano-catalytic reactions in the TME. Reproduced from ref. 99 with permission from WILEY, copyright 2021. (F) PtN4C-SAzyme and its cascaded catalytic reaction in TME for synergistic enhanced CDT and chemoradiotherapy of tumor. Reproduced from ref. 100 with permission from Ivyspring International Publisher, copyright 2022. | ||
Inspired by natural enzyme cascades, researchers are trying to design nanozymes with biomimetic cascade reaction characteristics. Nanozymes with multi-enzymatic activities are the material basis for simulating multi-enzyme cascade catalysis. Since different reactions in these cascades naturally lack affinity under identical conditions, immobilizing enzymes become a vital step in constructing these systems. Numerous effective enzyme immobilization techniques have been developed, including physical adsorption, covalent grafting, and in situ encapsulation.
Liu group96 developed a polyethylene glycolated CuxMnySz (PCMS) featuring multiple oxidation states of manganese (Mn2+/3+/4+) and copper (Cu1+/2+) (Fig. 7B). PCMS showcases remarkable CAT-like and OXD-like cascade catalytic activities, efficiently converting endogenous H2O2 into O2 and subsequently catalyzing O2 in the TME to generate toxic O2˙−. Furthermore, PCMS possesses GOx-like activity, capable of depleting GSH, which amplifies its chemodynamic therapy (CDT) effectiveness. Remarkably, under near-infrared II (NIR-II) 1064 nm laser exposure, PCMS achieves an impressive photothermal conversion efficiency of 56.7%, enhancing its catalytic performance. In vivo studies have demonstrated PCMS's ability to effectively suppress tumor growth, highlighting its potential as a multifaceted agent for cancer therapy. Additionally, Yang and his team97 developed an innovative nano-platform (DMSN@CoFe2O4/GOD-PCM) that integrates nanozymes and natural enzymes (Fig. 7C). This platform is crafted by embedding cobalt ferrite (CoFe2O4) bimetallic oxide nanozymes and GOx, a natural enzyme, into the pores of dendritic mesoporous silica (DMSN). When subjected to NIR-II illumination, the CoFe2O4 nanozymes produce heat. This heat, in turn, triggers the phase change material to release GOD. Upon its release, GOD initiates changes in the TME through the modulation of glucose metabolism pathways. This alteration leads to highly acidic conditions and the production of a significant amount of H2O2, setting off a series of catalytic reactions that can target tumor cells. Furthermore, Lin group98 introduced an innovative approach by developing a novel nanozyme–drug conjugate known as GHZD NCs. They ingeniously combined GOx, trivalent iron protoporphyrin (hemin, Fe3+), and dihydroartemisinin (DHA) within a zeolitic imidazolate framework 8 (ZIF-8) using a one-pot reaction method (Fig. 7D). This unique combination endows GHZD NCs with sustained enzymatic activities, including POD-, GOx-, and GPx-like functions. These activities are particularly effective in cancer cells that rely on glucose as their energy source. The GOx-driven glycolysis generates adequate H2O2 to sustain the creation of toxic ˙OH. Fe3+ serves a dual role, both as a POD generating ˙OH and as a consumer of the GSH, crucial for GPx. This process transforms Fe3+ into ferrous (Fe2+), disrupting iron balance and triggering DHA to produce carbon-centered radicals. This cascade, fueled by tumor-activated Fe2+, perpetuates a lethal cycle of accumulating carbon-centered radicals. The resulting depletion of GSH and the formation of radicals amplify oxidative stress, intensifying the immunogenic cell death (ICD) effect and combating immunosuppressive tumors. This customized strategy improves the targeted action and efficacy in treating particular cancer cells. It refines the accuracy of ROS-mediated treatments while simultaneously reducing the inadvertent oxidative damage to healthy cells and tissues.
The effectiveness of catalytic therapy largely stems from the synergistic interplay between cascade nano-catalytic reactions and multi-enzymatic activities. This coupling ensures that the cascade reactions are both sustainable and capable of self-replenishment, crucial for maintaining their therapeutic efficiency. To address the challenge of sustaining cascade reactions within the TME, in an innovative approach, Dong and colleagues99 developed a multifunctional nanoparticle, denoted as Fe3O4/Ag/Bi2MoO6 NPs. This particle showcases a range of nanozyme actions, emulating the functions of enzymes like POD, CAT, SOD, and GOx (Fig. 7E). Triggered by the TME, a series of nanocatalytic reactions continuously produce cytotoxic ˙OH and 1O2. The sustainability and self-replenishing nature of these reactions are ensured through a synergy of light activation, catalytic reactions, nanozyme activities, and the dynamic interchange between Fe2+/Mo5+ and Fe3+/Mo6+ ions. Moreover, Yong and colleagues100 proposed a novel TME-responsive PtN4C single atom nanozyme (SAzyme), capable of continuously self-replenishing H2O2, promoting the release of O2 and Pt2+, and depleting GSH, for tumor-specific cascade catalytic therapy (Fig. 7F). This system has the following advantages: (a) SAzyme can significantly enhance the production of OH and O2 through POD- and SOD-like activities, as well as X-ray deposition capability, which helps oxygenate the TME for intensified CDT and O2-dependent radiochemotherapy; (b) simultaneously, the self-recycling valence state change of Pt(IV) and Pt(II) leads to the continuous depletion of overexpressed GSH in cells and the release of a large amount of Pt2+, ultimately overwhelming the antioxidant defense and enhancing tumor-specific therapy; (c) more importantly, PtN4C SAzyme can also convert O2˙− into H2O2, achieving sustainable replenishment of H2O2 at the tumor site, which can further react with PtN4C SAzyme to realize the cyclic accumulation of ˙OH and O2.
To sum up, it is recognized that nanozymes, crafted from metal elements or amino acids that facilitate electron transfer, can replicate the catalytic reactions facilitated by various oxidoreductase enzymes.101 The oxidation states of these metal elements play a crucial role in determining the multi-enzymatic activities of nanozymes.102 A deeper understanding of these multi-enzyme mechanisms will be instrumental in designing nanozymes with enhanced activity and catalytic efficiency. Such advancements will not only boost the efficiency of nanozyme catalysis but also bolster their use in biomimetic processes, providing a strong theoretical foundation for future applications.
Nanozymes with multi-enzymatic activities are more likely to exhibit environmentally responsive catalytic activities. Combining the characteristics of nanomaterials, they can mediate changes in enzyme-like catalytic reactions based on physicochemical factors such as pH, temperature, light, or magnetism.108–111 Moreover, ideally, these nanozymes are designed to be highly selective, and activated by specific triggers related to the TME or external stimuli. This targeted approach enables them to precisely attack tumors or specific cancer cells, thus reducing the possibility of damaging healthy cells or tissues. Deng group112 conducted pioneering work in introducing nitrogen-doped graphene materials (N-GNM) as catalysts to biomedical fields. The N-GNM they developed are characterized by strong biocatalytic properties and specifically tailored for targeted catalytic therapy of tumors (Fig. 8A). N-GNM functions as a nanozyme that emulates POD, boasting a unique structure and robust catalytic activity. It is designed to be activated in response to the mildly acidic TME and react specifically to endogenous H2O2 concentrations of 100 μM to 1 mM. This specificity allows N-GNM to utilize endogenous H2O2 to generate ˙OH, selectively inducing tumor cell death at minimal lethal doses. In acidic conditions, N-GNM demonstrates the catalytic behavior of POD mimics and actively engages in modifying the TME, while it remains inert in normal tissues with neutral pH.
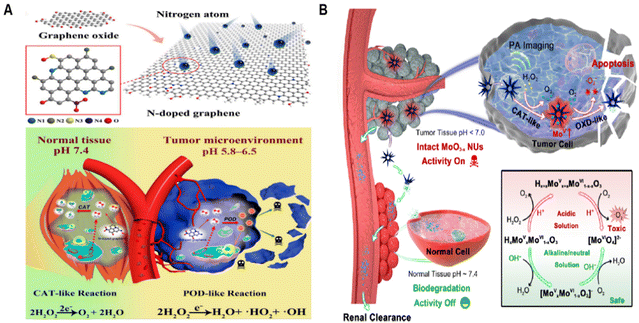 | ||
| Fig. 8 Microenvironmental response initiation. (A) The synthesis process of N-GNMS and the application for pH-triggered tumor-specific catalytic treatment. Reproduced from ref. 112 with permission from MDPI, copyright 2021. (B) Biodegradable and enzyme-activity-tunable MoO3−x NUs exhibit high specificity for tumor tissues due to their multi-enzymatic stepwise cascade catalysis in the acidic TME. Reproduced from ref. 114 with permission from American Chemical Society, copyright 2020. | ||
Qian group113 has innovated a straightforward polyol method to create AgBiS2 nanozymes with a unique hollow structure. These nanozymes, made up of small, poorly crystalline nanoparticles, become highly active under near-infrared light as well as without it. This is achieved through a rapid formation and ion exchange process in a mildly polar solvent, leading to a targeted toxic effect on cancer cells. The approach effectively lowers the energy barrier for producing highly reactive ˙OH in the TME. Thanks to the surface-catalyzed conversion of abundant H2O2 in the TME into reactive ˙OH, the AgBiS2 hollow-structured nanozymes demonstrate a marked increase in their ability to specifically target and kill cancer cells, a result that has been proven both in lab settings and within living organisms, particularly when subjected to NIR laser illumination at 808 nm.
Developing smart nanozymes that accurately perform in the TME without harming nearby normal tissues is a formidable challenge. Drawing inspiration from the fact that the activity of biological enzymes is tied to their specific structures, researchers have developed a method to control the active/inactive states of nanozymes by altering their nanostructures through biodegradation. This technique paves the way for catalytic therapies that are responsive to tumors, capable of differentiating between diseased and healthy states, thereby ensuring compatibility with normal tissues. Ling group114 innovated by creating MoO3−x nanourchins (NUs), whose enzymatic activities are dependent on their structure (Fig. 8B). Characterized by their expansive active surface and the presence of numerous Mo5+ species, these MoO3−x NUs exhibit remarkable CAT-like behavior in the acidic milieu of tumors. They efficiently convert H2O2 into oxygen, followed by activation of OXD-like properties that facilitate electron transfer to oxygen, resulting in the formation of harmful O2˙−. Crucially, these NUs rapidly lose their enzymatic properties in the physiological pH of 7.4, undergoing pH-responsive biodegradation. This process leads to the formation of kidney-clearable and biocompatible molybdate ions. This rapid biodegradation in normal physiological conditions allows MoO3−x NUs to target tumors with highly specific cascading catalytic activity while preserving the integrity of normal tissues.
2.3. Advanced functional biomimicry of nanozymes
Due to various adverse factors in the dynamically changing TME, there are limitations to the expected catalytic therapeutic effects of nanozymes. For instance, high levels of intracellular GSH (about 10 × 10−3 M) can neutralize the produced ROS, and the typically low levels of H2O2 are insufficient to sustain enzyme reactions.14,115 To overcome these obstacles, the biomimetic design of nanozymes, in addition to achieving basic functional biomimicry, i.e., mimicking the catalytic activity of natural enzymes and enhancing catalytic performance through the aforementioned structural and process biomimicry, still needs to develop advanced nanozymes with more profound and comprehensive biomimetic functions.Based on the understanding of tumor biology, biomimetic nanozymes applied for anti-tumor therapy need to meet the following new advanced functional requirements: (a) reshape the microenvironment through biocatalysis to obtain better anti-tumor therapeutic effects; (b) disrupt the balance of the oxidative stress regulation network in the TME to achieve cyclical catalytic reactions; (c) construct biologically orthogonal catalytic systems for in situ synthesis of biological prodrugs. All these advanced functional biomimetics highlight key regulatory mechanisms in the realm of nanozyme-mediated anti-tumor therapy. They form the cornerstone for enhancing the efficacy of nanozyme-catalyzed therapies, combined with drug delivery, conventional or novel anti-tumor treatments, or regulating the TME. Such comprehensive methods are essential in profoundly expanding our understanding of the synergistic roles and underlying mechanisms of nanozymes in combating cancer.
Yang group,119 inspired by the enhanced activity achieved by adding axial nitrogen coordination in single-atom catalysts, created an innovative Ir–N5 single-atom nanozyme (Ir–N5 SA) (Fig. 9A). This nanozyme not only emulates the activities of natural OXDs, POD-like, and CAT-like enzymes but also possesses the ability to convert NADH into H2O2, similar to nitric oxide synthase (NOS). Its effective CAT-like traits enable the conversion of H2O2 into oxygen at tumor sites, relieving tumor hypoxia. At the same time, Ir–N5 SA, which functions similarly to NOS, catalyzes the conversion of NADH into H2O2. It also blocks the mitochondrial electron transport chain and hinders aerobic respiration, thereby reducing the consumption of oxygen within cells. This combined effect notably increases both oxygen and H2O2 concentrations at tumor locations, boosting the nanozyme's capacity to produce ROS, leading to irreversible oxidative harm to tumor cells. Importantly, NADH and NAD+ are essential redox coenzymes in fundamental metabolic processes. The consumption of NADH disrupts their equilibrium, hindering adenosine triphosphate (ATP) synthesis through both glycolysis and oxidative phosphorylation. While Ir–N5 SA interferes with the energy metabolism of tumor cells, these cells can adapt through metabolic reprogramming, relying on fatty acid oxidation (FAO) for survival in the nutrient-depleted TME. To address this adaptation, cerulenin (Cer), an inhibitor of fatty acid synthase, was integrated into Ir–N5 SA to further weaken the FAO metabolism in tumor cells. In summary, the combined Ir–N5 SA/Cer formulation substantially enhances tumor treatment by significantly upsetting the tumor's redox balance and metabolic homeostasis.
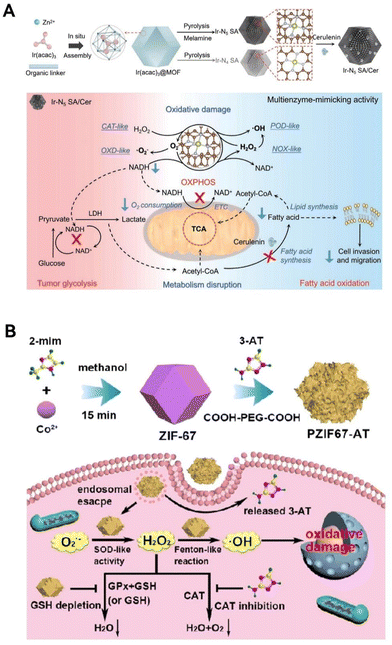 | ||
| Fig. 9 Metabolism imbalanced function. (A) The Ir–N5 SA/Cer nanozyme has the capability to disturb both the redox balance and the metabolic equilibrium within tumor regions through emulating a series of enzymatic cascade reactions. Reproduced from ref. 119 with permission from WILEY, copyright 2022. (B) The PZIF67-AT nanozyme enhances intracellular H2O2 levels by promoting H2O2 production and inhibiting its elimination, thereby intensifying CDT. Reproduced from ref. 121 with permission from American Chemical Society, Copyright 2020. | ||
In cancer cells, H2O2 levels are delicately balanced between production and elimination, regulated by the antioxidant system that includes enzymes like SOD, CAT, GPx, and molecules such as GSH.120 Capitalizing on these findings, the research team led by Qu121 has engineered a novel H2O2 disruptor, termed PZIF67-AT. This disruptor is an innovative modification of zeolitic imidazolate framework 67 (ZIF-67) nanoparticles, achieved by incorporating 3-amino-1,2,4-triazole (3-AT) and PEG (Fig. 9B). These nanoparticles are structured by the interlinking of 2-methylimidazole (2-mim) with cobalt ions, resulting in a sodalite zeolitic formation. This newly created nanozyme demonstrates SOD-like behavior, effectively converting O2˙− into H2O2 and thereby promoting its accumulation. Moreover, under slightly acidic conditions, it releases molecules that inhibit CAT, reducing H2O2 decomposition. Significantly, the disruptor exhausts GSH, hindering GSH-mediated H2O2 clearance irrespective of GPx presence. By targeting the H2O2 homeostasis in tumor cells and disturbing this balance, this study achieves H2O2 accumulation in cancer cells, thereby enhancing CDT effectiveness through the Fenton reaction.
Biorthogonal reactions could produce therapeutic drugs in situ, greatly minimizing off-target effects. Despite the challenges posed by the lysosomal membrane, which serves as a cellular barrier impeding drug delivery to targeted sites.124 Sun group125 has made a groundbreaking advancement (Fig. 10A). They developed a protein-based nanozyme platform incorporating transition metals, alongside a novel cage-like composite fluorophore group system. This innovative system facilitated the screening of compatible nanozyme/protective group pairs. By integrating various transition metal nanoparticles directly into protein frameworks, they were able to create a comprehensive library of nanozymes. This library proved to be an excellent resource for selecting the ideal pairs for cleavable bonds in nanozyme-driven processes. Among their discoveries, a Pd-based nanozyme exhibits activities akin to mutant P450(BM3), which specifically targets propargyl ether groups. Leveraging a multi-enzyme synergistic strategy, this Pd nanozyme achieves in situ biorthogonal catalysis and can permeate the lysosomal membrane due to its POD-like properties. This innovation has been effectively utilized in the development of prodrugs for cancer treatment, laying a foundational framework for the creation of lysosome-targeted prodrugs in oncological therapies.
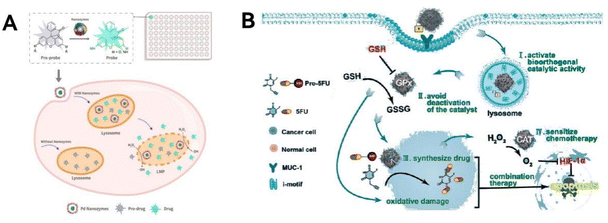 | ||
| Fig. 10 Biorthogonal catalytic function. (A) The prodrugs are activated by the biorthogonal catalysis of the Pd nanozymes, thereby significantly boosting effective drug delivery to target cancer cells. Reproduced from ref. 125 with permission from WILEY, copyright 2022. (B) A DNA-gated and self-protected biorthogonal catalyst is crafted by utilizing highly programmable DNA molecules to modify the nanozyme Pd0. Reproduced from ref. 127 with permission from WILEY, copyright 2023. | ||
Transition metal catalysts show promise in activating prodrugs, but their constant ‘always-on’ catalytic state and struggle to operate in complex cellular environments have raised concerns about their safety and effectiveness in therapy.126 To address these issues, Qu and team127 ingeniously used programmable DNA molecules to modify nanozyme-Pd0, creating a DNA-gated, self-protective biorthogonal catalyst (Fig. 10B). This innovation has been effectively applied in synthesizing drugs within cells and treating cancer. The catalyst single DNA layer serves a dual purpose: it targets cancer cells specifically and also acts as a ‘gatekeeper’, selectively triggering prodrugs in these cells. Additionally, the graphite nitrogen-doped carbon nanozymes, which mimic GPx and CAT enzymes, help create a more favorable environment for the catalyst inside cells. This prevents the catalyst from becoming inactive and increases the effectiveness of subsequent chemotherapy treatments. This research represents a significant step forward in developing safe and efficient nanozyme-based biorthogonal catalysis systems for medical applications.
Overcoming the immunosuppressive environment within tumors ranks among the toughest challenges in cancer treatment.130 Tumor-associated macrophages have been recognized for their role in advancing malignant tumor growth, drug resistance, and unfavorable outcomes.131 Drawing inspiration from natural bodily responses, Liu's team73 has innovatively used arginine, a precursor to NO, as a capping agent to create two types of chiral ruthenium nanozymes (D/L-arginine@Ru) (Fig. 11A). These nanozymes mimic both OXD and NOS activities, rapidly producing 1O2 and O2. They then catalyze arginine to generate enough NO, which plays a crucial role in boosting macrophage M1 polarization, a key factor in reversing the immune suppression in tumors. Moreover, these chiral Ru nanozymes harness the combined anti-tumor powers of 1O2 and NO, leading to a ‘cocktail therapy’ effect that triggers tumor cell death through apoptosis and ferroptosis. This research paves the way for new approaches in tumor catalytic therapy, offering a viable strategy to modulate the immune microenvironment by leveraging self-catalytic cascade reactions that activate macrophage M1 polarization via ROS and NO. Zeng group69 has created an innovative copper-doped polypyrrole nanozyme (CuP) possessing three enzyme-like activities: CAT, GPx, and POD (Fig. 11B). This nanozyme distinctively increases O2 and ˙OH levels via a straightforward process, simultaneously decreasing GSH within the TME. This mechanism results in irreversible oxidative damage to tumor cells, disrupting their redox balance. The CuP nanozyme enhanced with polyethylene glycol, denoted as CuPP, is particularly effective in reversing the immunosuppressive nature of the TME. It alleviate tumor hypoxia and converts M2 macrophages, which promote tumor growth, back into the M1 phenotype that combats tumors. Crucially, CuPP also demonstrates enhanced catalytic and immunomodulatory activities when combined with thermotherapy. When used alongside the immune checkpoint inhibitor PD-L1, CuPP facilitates potent immune responses and delivers outstanding anti-tumor results.
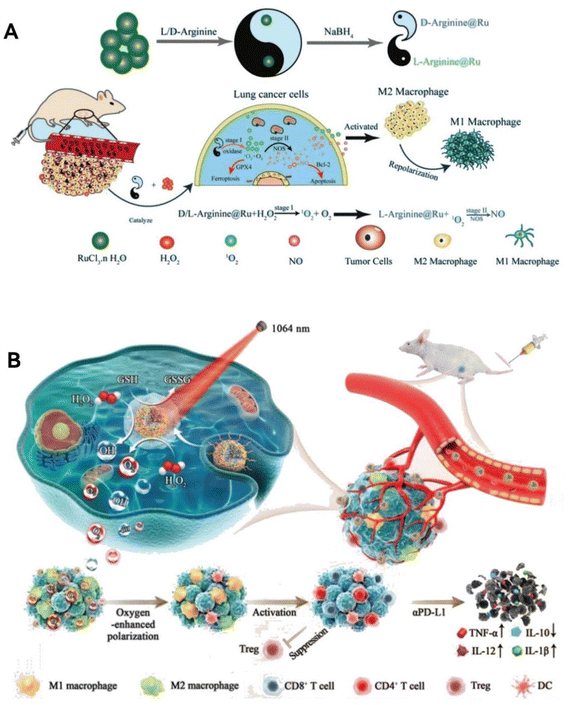 | ||
| Fig. 11 Environment Reprogramming Function. (A) Chiral ruthenium nanozymes (D/L-arginine@Ru) with OXD- and NOS-mimic activities play a crucial role in enhancing the M1 polarization of macrophages, thereby reversing the immunosuppressive environment in tumors. By leveraging the combined anti-tumor effects of 1O2 and NO, these chiral Ru nanozymes induce apoptosis and ferroptosis in tumor cells. Reproduced from ref. 73 with permission from WILEY, copyright 2023. (B) CuP nanozymes specifically promote an increase in O2 and ˙OH, and a decrease in GSH, causing oxidative stress damage to tumor cells and reversing the redox balance. CuPP overcomes tumor hypoxia and re-educates macrophages from M2 to M1 phenotype, reversing the immunosuppressive TME. Reproduced from ref. 69 with permission from WILEY, copyright 2022. | ||
Reversing the immunosuppressive nature of the TME and reactivating the capability of immune system to fight tumors is a key therapeutic strategy.132 Certain immune cells in the TME, like T cells, dendritic cells, and macrophages, become dysfunctional upon sensing high extracellular lactate levels through MCT1 and MCT2.133 LDH, crucial in the lactate metabolism pathway, catalyzes the conversion of lactate to pyruvate.134 Wang and colleagues135 harnessed this by synthesizing SnSe nanosheets (SnSe NSs) with lactate adsorbed on their surface, creating an effective adsorption structure. SnSe NSs were found to mimic LDH, efficiently consuming lactate both in lab settings and in living organisms. Importantly, the lactate degradation facilitated by SnSe NSs effectively counteracts the immunosuppression induced by elevated lactate levels in the TME, leading to the inhibition of tumor growth in different mouse models. This suggests that SnSe NSs play a significant role in enhancing the acidic and immunosuppressive TME. They achieve this by promoting the consumption of lactate within tumors, which in turn revitalizes the immune system's ability to combat cancer.
3. Applications in cancer treatment
The TME is a sophisticated network comprising tumor cells, stromal cells, and the extracellular matrix, which profoundly influences tumor development, growth, and spread. This influence is exerted through metabolic, secretory, immune, structural, and functional changes, making the TME a pivotal factor in the distribution and biological effects of nanoparticles. In the TME, hypoxia arises not only from the rapid proliferation of tumor cells, which drastically increases their oxygen demand but also from the inadequate oxygen supply due to the abnormal vasculature of tumor.115 This lack of oxygen is further compounded by an imbalance in pH levels, a direct consequence of altered glucose metabolism.136 In such hypoxic conditions, tumor cells shift towards glycolysis, resulting in distinct pH values both inside and outside the cells. Moreover, the TME is characterized by elevated levels of ROS, H2O2, and GSH.137 Tumor cells adapt by enhancing their antioxidative systems to effectively manage these increased internal ROS levels. This unique feature of the TME, with its higher redox potential compared to normal tissues, presents an opportunity for nanozyme-catalyzed therapeutic strategies, offering targeted and efficient options for tumor therapy.Reflecting these mechanisms, extensive research has been conducted on the use of nanozymes with biomimetic designs in anti-tumor treatments, encompassing both single-type and multi-enzymatic nanozymes. This section will delve into the advancements in research on various cancers, as well as the integration of catalytic therapy with other complementary therapies like radiotherapy, PDT, and sonodynamic therapy (SDT), in the realm of tumor catalytic therapy.
3.1. Catalytic therapy
The therapeutic action of nanozymes in cancer treatment is largely driven by their ability to induce the production of ROS, which are key in killing tumor cells. The main members of the ROS family are O2˙−, 1O2, and ˙OH. Based on the number of enzyme-like catalytic activities mediated, nanozyme-based catalytic therapy can be categorized into single-enzyme activity catalysis and multi-enzyme activity catalysis, with the latter further classified into bi-enzymic, tri-enzymic, and quarter-enzymic nanozymes. Most single-enzyme activity nanozymes generate or scavenge reactive ROS based on their inherent POD, SOD, CAT, OXD, GOx, and GPx mimetic activities. For multi-enzyme activity nanozymes, the nanomaterials may exhibit stronger ROS-producing or clearing effects through synergistic or cascading catalytic reactions of multi-enzyme-like activities, displaying more complex functionalities. This could lead to more effective outcomes in the application of anti-tumor diagnosis and therapy.Zhang et al.140 synthesized ultrasmall Pt (nPt) nanozymes within the restricted domains of the worm-like pore channels of gold–nanobipyramide–mesoporous silica nanocomposites to prepare AP-mSi nanozyme carriers with photo-enhanced POD ability (Fig. 12A). Next, based on the prepared AP-mSi, a nanozyme probe (AP-HAI) for lung cancer therapy was prepared by removing SiO2, modifying human serum albumin, and loading atovaquone molecules and IR780. Upon irradiation with near-infrared light, the internal Au particles and IR780 interact in a photothermal process, thereby promoting a POD-like catalytic process of H2O2. The photo-enhancing ability of IR780 nanozyme for PDT and nPt PODs gives the probe high ROS performance, which can be used to induce anti-tumor immune responses that destroy tumor tissue.
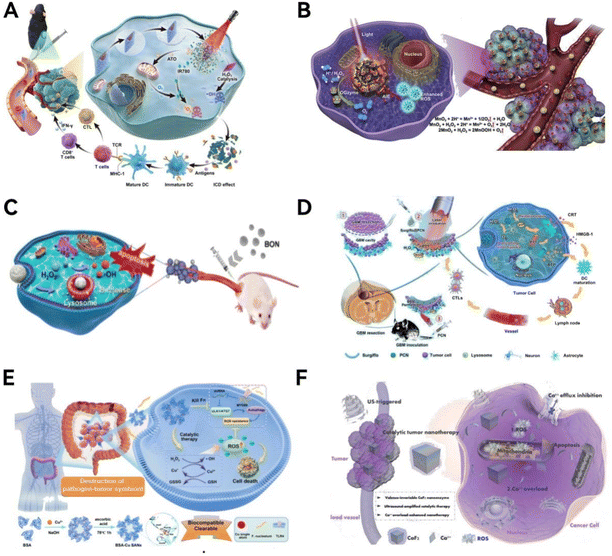 | ||
| Fig. 12 Biomimetic-designed nanozymes for caner catalytic therapy. (A) Under near-infrared light irradiation, the internal Au particles and IR780 with photo-enhancing ability work synergistically to promote the POD-like catalytic process of H2O2. Reproduced from ref. 140 with permission from WILEY, copyright 2023. (B) Nanozymes possess POD-like activity and can efficiently penetrate directly into the hypoxic areas of tumor tissues to provide oxygen based on catalytic reactions within the TME. Reproduced from ref. 143 with permission from Elsevier Ltd, copyright © 2020. (C) BON nanozymes catalyze the production of cytotoxic ˙OH, inducing apoptosis in 4T1 cancer cells. Reproduced from ref. 144 with permission from WILEY, copyright 2021. (D) The catalytic and photothermal therapeutic effect of Surgiflo@PCN in GBM treatment. Reproduced from ref. 146 with permission from American Chemical Society, copyright 2023. (E) The catalytic therapeutic effect of BSA-Cu SAN in killing colorectal cancer cells. Reproduced from ref. 150 with permission from Springer Nature, copyright 2023. (F) The (CaF2) nanozyme with ultrasound-enhanced POD-mimicking activity are used for hepatoma therapy. Reproduced from ref. 153 with permission from WILEY, copyright 2022. | ||
Addressing the pivotal challenge of effectively promoting M1 macrophage polarization in lung cancer treatment, due to the critical role of macrophages in tumor-mediated immunosuppression, has led to significant advancements. Drawing inspiration from natural biochemical processes, Chen et al.73 innovated a dual-activation approach for M1 macrophage polarization, harnessing the synergistic effects of ROS and NO through a self-propagating cascade reaction. This method employs NO precursor-arginine as a capping element, facilitating the creation of two distinct enantiomeric forms of ruthenium-based nanozymes (D/L-arginine@Ru). These chiral Ru nanozymes are adept at rapidly generating 1O2 and O2, simultaneously mimicking OXD and NOS activities. Subsequently, they catalyze arginine, leading to the production of ample NO, thereby boosting M1 macrophage polarization and counteracting tumor immunosuppression.
As oxygen deprivation is a major obstacle to hypoxia-related theranostics, much research has been done to develop methods to utilize the hypoxic response properties of nanoparticles to deliver oxygen.142 Nonetheless, effectively achieving nanoparticle penetration into the hypoxic zones of tumors remains an unresolved issue. In a recent study, Gao et al.143 developed a biomimetic ferritin nanocages with CAT-like activity and can penetrate into a hypoxic region in tumor tissue to deliver oxygen based on a catalytic response in the TME (Fig. 12B), which significantly alleviated tumor hypoxia by almost three times compared to untreated tumor tissue. Moreover, this nanosystem facilitates multimodal tumor imaging and alters the TME, enhancing PDT through a series of therapeutic agents. The result is a marked improvement in inhibiting tumor growth and preventing lung metastasis.
Nanomaterials with enzyme-like activity have been identified as potentially important self-therapeutic nanomedicines. Zeng et al.144 have pioneered a novel biodegradable boroxynitride (BON) based POD nanozyme for an advanced, multimodal approach to breast cancer treatment (Fig. 12C). This BON nanozyme efficiently produces cytotoxic ˙OH, leading to a significant reduction in 4T1 cancer cell viability—up to 82% within 48 hours. In vivo studies revealed that this nanozyme curbs breast tumor growth by 97% over a 14-day treatment period, outperforming inert and B-releasing boron nitride nanospheres and by 10 times and 1.3 times, respectively. This study underscores the potential of integrating BON nanoclay within the boron nitride nanomedicine framework for breast cancer treatment.
Wang et al.150 developed an innovative approach to target and eradicate the intratumoral pathogen F. nucleatum, thereby disrupting its symbiotic relationship with colon cancer cells (Fig. 12E). This method employs a novel monoatomic protein-assisted copper nanozyme (BSA-Cu SAN), designed based on natural enzyme structures with metal elements as active sites. The BSA-Cu SAN demonstrates catalytic efficiency by producing ROS and degrading GSH. This action leads to the elimination of the pathogenic F. nucleatum within the tumor, disrupting its symbiotic interactions and effectively contributing to the destruction of colorectal cancer cells.
Zhu et al.151 successfully synthesized hollow Ru@CeO2 nanozymes (Ru@CeO2YSNs) utilizing a one-pot synthesis method. They innovatively loaded both the anticancer drugs ruthenium complex (RBT) and resveratrol (Res) into these Ru@CeO2YSNs. A dual-layered structure was then created by incorporating polyethylene glycol, forming a sophisticated dual drug delivery system (Ru@CeO2-RBT/Res-DPEG) designed for controlled drug release. This double outer layer structure not only enhanced the biocompatibility of Ru@CeO2YSNs but also significantly extended their circulation time in the bloodstream. The efficacy of Ru@CeO2-RBT/Res-DPEG was demonstrated in reducing tumor hypoxia and in the inhibition of metastasis and recurrence in both orthotopic and subcutaneous models of colorectal cancer.
Dong et al.153 ingeniously engineered a calcium fluoride (CaF2) nanozyme with varying valences, featuring ultrasound-enhanced POD-like activity (Fig. 12F). This innovation marks the first instance of calcium-based nanozymes being used in catalytic cancer therapy. The design leverages the release of exogenous Ca2+ ions from CaF2 nanocrystals, coupled with the generation of harmful ROS amplified by the POD-mimicking effects of ultrasound. This mechanism promotes intracellular calcium accumulation, leading to Ca2+ overload and subsequent mitochondrial dysfunction, which is instrumental in the therapeutic effectiveness.
The Hepatitis C virus (HCV) stands as a primary contributor to various liver-related conditions, including chronic hepatitis, cirrhosis, and hepatocellular carcinoma.154 Presently, the prevalent interferon-based treatments only successfully eradicate the virus in roughly half of the patients and are not specifically tailored to HCV, leading to considerable side effects. Given the lack of an effective vaccine, there is an urgent need for more targeted antiviral treatments. RNA interference plays a crucial role in gene regulation through the RNA-induced silencing complex. Wang et al.155 highlighted that a specially engineered nanoparticle complex can adeptly imitate the cellular RISC, facilitating targeted RNA cleavage. Their findings reveal that this nanozyme, custom-designed for combating HCV, can precisely and effectively slice HCV RNA in a sequence-specific way.
The use of exosome-expressed proteins as liquid biopsy biomarkers for cancer diagnosis has shown considerable promise.156 Nevertheless, profiling these exosomal proteins accurately is still technically challenging. Di et al.157 introduced a novel approach called the nanozyme-assisted immunoassay (NAISA) for the sensitive, rapid, and multiplexed profiling of exosomal proteins. This innovative NAISA system employs POD-like nanozymes attached to the phospholipid membranes of exosomes, eliminating the requirement for post-labeling with detection antibodies. Thanks to the NAISA nanozyme, the efficient profiling of a wide range of exosomal proteins has been made possible, potentially revolutionizing early detection of hepatocellular carcinoma and other cancers.
Cutaneous squamous cell carcinoma (cSCC) ranks as the second most prevalent form of non-melanoma skin cancer, contributing to 20% of all skin cancer deaths. Lately, local PDT has gained favor for its safety and effectiveness against non-melanoma skin cancers.160 Yet, the therapy's impact is often curtailed by the poor skin penetration and bioavailability of photosensitizers. Addressing this, Tao and colleagues161 have introduced a microneedle patch containing MnO2/Cu2O nanosheets and Cabotegravir A4, designed for precise drug delivery to tumors. The innovative use of MnO2/Cu2O enables the catalysis of glucose into H2O2, which, in combination with released copper, triggers a Fenton-like reaction to produce ˙OH, offering an effective CDT approach. Additionally, the ability of MnO2/Cu2O to convert light into heat not only directly attacks cancer cells but also boosts the Fenton-like reaction's efficiency, presenting a promising multi-modal treatment strategy for enhancing cSCC therapy.
3.2. Combination therapy
Anti-tumor treatments encompass conventional methods like surgery, chemotherapy, and radiotherapy, alongside novel approaches such as immunotherapies, targeted treatments, PDT, and PTT. However, they usually lack targeted and sustained drug release, and the accompanying drug resistance and enrichment with systemic toxicity limit their application.138,162 Owing to the constraints inherent in single-modality therapies, current research is increasingly directed towards multi-modal approaches for enhanced, synergistic tumor treatment.163 In this context, nanozymes are gaining prominence for their augmented therapeutic impact across various cancer treatments. With superior catalytic activity, enhanced stability, and greater ease of modification compared to natural enzymes, nanozymes have made significant strides in diverse treatment modalities including chemotherapy, radiotherapy, phototherapy, immunotherapy, CDT, PDT, sonodynamic therapy, and starvation therapy.The TME is characterized by a mildly acidic nature, an excess of H2O2, hypoxic conditions, and reduced activity of specific enzymes like CAT.169,170 These factors are instrumental in fostering the nourishment, proliferation, and metastatic progression of cancer cells, as well as aiding their escape from immune-mediated destruction.171,172 Consequently, leveraging nanozymes to modulate the TME presents a promising strategy for cancer therapy. Nanozymes can induce notable alterations in the TME via various established or potential mechanisms. These include catalyzing Fenton-like reactions, converting prodrugs into active chemotherapeutics locally, breaking down tumor-promoting oncogenes, and boosting the function of enzymes such as CAT.103,173,174 Such nanozyme-mediated adjustments to the TME can either directly eradicate cancer cells or augment the efficacy of other treatments like chemotherapy, PTT, and PDT. Cai et al.103 have identified that combining TME cascade catalytic therapy with chemotherapy offers a promising avenue for effective tumor treatment (Fig. 13A). They engineered a unique single-atom nanozyme specifically designed to initiate a series of enzymatic reactions within the TME, targeting tumor cells precisely. This nanozyme, a single-atom cobalt structure supported on N-doped porous carbon (Co-SAs@NC), exhibits CAT-like properties, breaking down endogenous H2O2 in cells to produce O2. It then demonstrates OXD-like activity by transforming O2 into toxic O2˙−, which are highly effective in eradicating tumor cells. Additionally, incorporating doxorubicin (DOX) into this treatment protocol markedly enhances its antitumor efficacy in vivo. Furthermore, Li et al.175 engineered an innovative nanosystem, H-MnO2-DOX-Col NPs, using mesoporous manganese dioxide (H-MnO2) as a base, encapsulating DOX within its core, and coating the surface with collagenase (Col) (Fig. 13B). Additionally, these NPs were enveloped in a fusion membrane (FM) composed of RAW264.7, an inflammation-specific cell membrane, and pH-sensitive liposomes, creating a biomimetic structure termed MP@H-MnO2-DOX-Col. The study findings suggest that MP@H-MnO2-DOX-Col enhances the effectiveness of DOX while significantly reducing its cardiotoxicity. This is achieved through its multifunctional capabilities, including efficient tumor tissue penetration, TME hypoxia reduction, pH-responsive drug release, and targeted DOX delivery. This comprehensive strategy not only amplifies DOX therapeutic impact but also markedly diminishes the risk of cardiac damage often associated with the drug. Song et al.47 constructed Zr/Ce-MOFs/DOX/MnO2, which serves as a new type of nanosome for combined chemotherapy and catalytic treatment (Fig. 13C). Zr/Ce-MOFs can produce ˙OH to mimic TME, and MnO2 on the surface can deplete GSH, further stimulating ˙OH production. The dual stimulation of pH/GSH accelerates the release of the anticancer drug DOX into tumor tissue to enhance cancer chemotherapy.
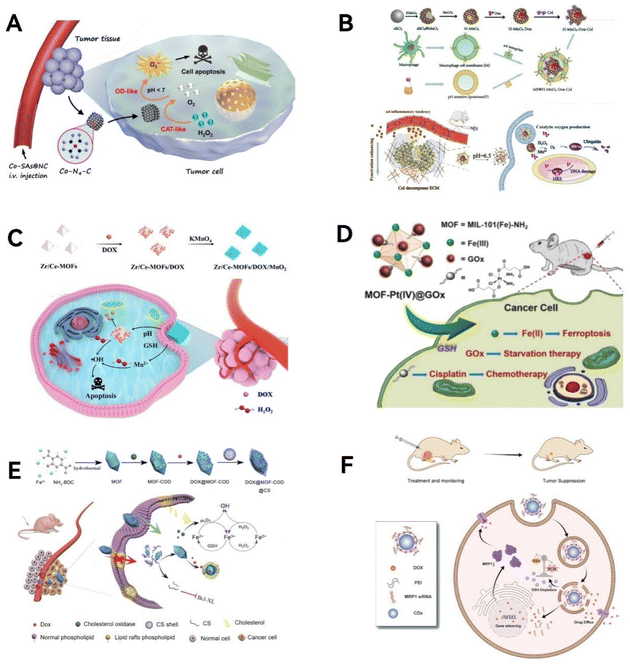 | ||
| Fig. 13 Biomimetic-designed nanozymes for cancer chemotherapy. (A) The Co-SAs@NC nanozyme with dual enzymatic activities are paired with the chemotherapy drug DOX for effective cancer therapy. Reproduced from ref. 103 with permission from WILEY, copyright 2022. (B) The H-MnO2-DOX-Col nano-system possesses multifunctional capabilities that maximize the therapeutic efficacy of DOX. Reproduced from ref. 175 with permission from Springer Nature, copyright 2023. (C) The Zr/Ce-MOFs/DOX/MnO2 nanosystem serves as a new type of nanosome for combined chemotherapy and catalytic treatment. Reproduced from ref. 47 with permission from Royal Society of Chemistry, copyright 2023. (D) The MOF-Pt(IV)@GOx nanozyme provides remarkable anti-tumor efficacy through synergistic trimodal therapy with ferroptosis, starvation therapy, and chemotherapy. Reproduced from ref. 177 with permission from Elsevier, copyright 2023. (E) By immobilizing cholesterol oxidase (COD) onto an NH2-MIL-88B MOF through an amide reaction, this system can catalyze the conversion of cholesterol into H2O2, which then leverages the POD-like activity of the MOF to generate toxic ˙OH. Reproduced from ref. 178 with permission from Springer Nature, copyright 2022. (F) CD-PEI is capable of loading and delivering siMRP1 and DOX into tumors, increasing DOX accumulation and significantly enhancing the cell vitality inhibition induced by CD-PEI-DOX to counteract chemotherapy resistance. Reproduced from ref. 179 with permission from Dove Press, copyright 2023. | ||
Fan et al.176 addressed the challenge posed by low H2O2 levels in the TME, which often limits the effectiveness of POD nanozyme therapy. They developed a mesoporous nanozyme with dual functionality: it not only demonstrates POD-like activity but also carries an anti-tumor drug, thereby facilitating synergistic cancer treatment. This nanozyme consists of iron-doped mesoporous silica nanoparticles (FeMSN). Iron inclusion imbues FeMSN with POD-like properties, allowing it to break down H2O2 into OH− in acidic environments. Additionally, the mesoporous structure of FeMSN serves as a carrier for the chemotherapeutic agent DOX, enhancing the chemotherapy by generating H2O2 and creating a synergistic effect that improves overall cancer treatment efficacy. Wu et al.177 tackled the challenge in chemotherapy of utilizing Fenton chemistry in the TME for ROS generation, which is often hindered by low H2O2 levels and insufficient acidity in tumors. They engineered a MOF containing iron, integrating a cisplatin prodrug (Pt(IV) prodrug) and incorporating a GOx biocatalyst, resulting in the MOF-Pt(IV)@GOx nanozyme designed for cascade reactions (Fig. 13D). In this arrangement, the Pt(IV) prodrug attached to the MOF significantly enhances GOx loading and chemotherapy. The abundant GSH in the TME reduces Fe(III) to Fe(II) for the Fenton reaction and transforms the Pt(IV) prodrug into active cisplatin, which targets DNA and generates H2O2. Additionally, the GOx-catalyzed glucose oxidation not only depletes glucose for starvation therapy but also amplifies intracellular acidity and H2O2 availability, thus boosting the Fenton reaction. Both in vitro and in vivo experiments demonstrate that MOF-Pt(IV)@GOx achieves remarkable tumor inhibition through a synergistic tri-modal approach combining ferroptosis, starvation therapy, and chemotherapy.
Multiple resistance remains a barrier to cancer treatment. Most research focuses on the inhibition of P-glycerin activity during drug transport, but its effect is also very unsatisfactory. Du et al.178 developed an enzyme-driven DOX@MOF-COD@CS nanosystem (Fig. 13E). This system carries the drug DOX on MOF-COD nanoparticles, where a chondroitin sulfate (CS) shell with disulfide bonds reacts with GSH, triggering drug release, catalyzing a series of reactions, and causing cell death. The focus of the study is on overcoming drug resistance by using cholesterol oxidase to reduce cholesterol in resistant cell membranes, enhancing drug absorption. Furthermore, cholesterol oxidase converts cholesterol into H2O2, which is then transformed into ˙OH by MOF nanozymes, aiding in combating resistance and effectively destroying cancer cells. Finally, the CS shell suppresses the production of COX enzymes, leading to a reduction in the levels of the anti-apoptotic protein Bcl-XL, making tumor cells more susceptible to chemotherapy. Yu and team179 employed a CD-PEI system for loading siRNA aimed at MRP1, addressing the resistance of lung cancer to DOX treatment (Fig. 13F). They utilized siRNA to lower MRP1 levels, curtailing drug expulsion and boosting the drug concentration within lung cancer cells. The CD-PEI simultaneously transports siRNA and chemotherapeutic drugs to tumors, enhancing drug absorption in cells by blocking the drug outflow channels and managing oxidative stress, thus concentrating more drugs near the nucleus. Crucially, the co-delivery system combats MRP1 activity by promoting GSH suppression through increased oxidation and ROS. Their approach of delivering DOX via CD-PEI, alongside a dual strategy of MRP1 inhibition and GSH depletion, underscores the significant potential of this combined system in improving the efficacy of chemotherapy and siRNA treatments for challenging lung cancer.
To overcome these radiotherapy resistance that may lead to local recurrence and treatment failure,183 Zhou et al.184 reported the synthesis of a covalent organic framework (COF) saturated with iodine and ferrocene (TADI-COF-Fc) at room temperature to improve the efficacy of radiotherapy in the treatment of radioresistant oesophageal cancer (Fig. 14A). Iodine atoms in the COF scaffold not only directly affected radiotherapy and increased X-ray absorption to enhance the therapeutic effect, but also promoted aqueous radiolysis, which increased ROS generation. In addition, ferrocene surface finishing increased the levels of hydroxyl and lipid peroxyl radicals and decreased intracellular antioxidants, which disrupted redox homeostasis.
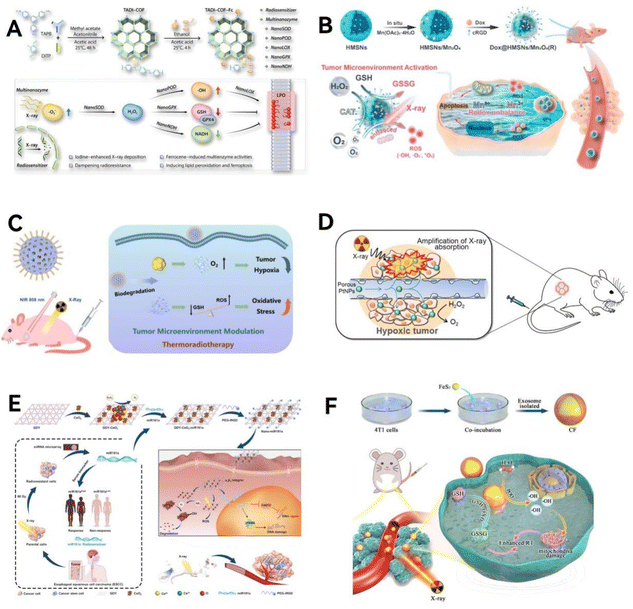 | ||
| Fig. 14 Biomimetic designed nanozymes for cancer radiotherapy. (A) The TADI-COF-Fc is used to enhance the radiotherapy efficacy for esophageal cancer therapy. Reproduced from ref. 184 with permission from American Chemical Society, copyright 2023. (B) A TME-sensitive DOX@HMSN/Mn3O4(R) nanosome for effective radio-chemotherapy. Reproduced from ref. 187 with permission from Elsevier Ltd, copyright 2022. (C) BiPt-PFA acts as a radiosensitizer to enhance the absorption of X-rays at tumor sites, also triggering reactions related to the TME. Reproduced from ref. 188 with permission from American Chemical Society, copyright 2020. (D) Porous platinum nanoparticles exhibit the ability to deposit X-ray radiation energy effectively within cancer cells and enhance tumor oxygenation. Reproduced from ref. 189 with permission from Elsevier Ltd, copyright 2019. (E) The POD-mimicking GDY-CeO2 nanocomposites, integrated with miR181a, are conjugated to iRGD-grafted polyoxyethylene glycol, augmenting their radiotherapeutic efficacy. Reproduced from ref. 194 with permission from WILEY, copyright 2021. (F) A biomimetic nanozyme system (CF) was developed by encapsulating FeS2 into exosomes derived from tumor cells, designed to enhance low-dose radiotherapy. Reproduced from ref. 193 with permission from Springer Nature, copyright 2021. | ||
Ionizing radiation therapy often leads to H2O2 radiolysis, generating substantial ROS that can alter DNA.185 However, the hypoxic conditions in solid tumors can diminish the effectiveness of radiotherapy due to the reduced capacity of oxygen to facilitate DNA damage and ROS generation.186 Addressing these challenges, new strategies have been developed. For instance, Yuan et al.187 devised a TME-sensitive DOX@HMSN/Mn3O4(R) nanosome. This nanosome, responding to the TME, undergoes an in situ Mn3+/Mn2+ transition, disrupting redox balance and catalyzing excessive ROS production (Fig. 14B). It also interacts with excess GSH in the TME to produce oxidized glutathione (GSSG), neutralizing ROS. The OXD-like activity of nanosome fosters ˙OH from O2 and enhances the TME oxidative state.188 When combined with radiotherapy, the outer layer electrons of the nanosome are excited by high-energy X-rays, contributing to the OXD-like reaction and further boosting ROS levels, thereby amplifying the efficacy of radiochemotherapy. Besides, an innovative BiPt-PFA nanocomposite was created by embedding platinum nanoparticles into a mesoporous bismuth (Bi)-based nanomaterial, followed by acid modification with amphiphilic polyethylene glycol (PFA) (Fig. 14C). BiPt-PFA functions as an effective radiosensitizer, enhancing X-ray absorption specifically in tumor areas and responding to the TME due to the material accumulation in these regions. During its action, the Bi component of the nanocomposite interacts with GSH, disrupting the oxidative stress equilibrium through resonance. Simultaneously, the platinum nanoparticles facilitate the breakdown of H2O2 into O2, thereby mitigating the hypoxic state of the tumor. Li et al.189 introduced porous platinum nanoparticles as an innovative single-agent nanomedicine platform, addressing dual challenges in cancer treatment (Fig. 14D). Leveraging the high atomic number and oxygen generation capabilities of these nanoparticles, they significantly enhance radiation-induced DNA damage, ROS stress, and cell cycle arrest in cancer cells by effectively concentrating X-ray energy. Moreover, these porous platinum nanoparticles boost tumor oxidation by transforming endogenous H2O2 into O2, thus markedly enhancing the efficacy of radiotherapy while demonstrating no apparent in vivo toxicity in animal models.
Nanocatalysts can be engineered to “reprogram” the TME by changing how certain biomolecules are expressed, leading to significantly improved radiotherapy outcomes. The designed multifunctional nanocatalyst system, CuPy-Au@EM, which mimics tumor cell exosomes, acts as a radiosensitizer.190 Its exosome membrane proteins on the surface ensure targeted delivery to tumor sites, with a core made of CuPy nanocatalyst embedded with gold nanoparticles (AuNPs). These AuNPs boost H2O2 levels in a manner similar to GOx, while CuPy-Au@EM further lowers cellular GSH and through its GPx and POD activities, generates a vast number of ˙OH, expanding the effectiveness of radiotherapy. Additionally, an innovative method uses AuNPs-modified iron SOD-mimic (FeSAE@Au) for a self-propelling catalytic effect in radiotherapy.191 In this dual-nanocatalyst strategy, AuNPs serve as a GOx mimic, enabling FeSAE@Au to produce H2O2, which boosts its POD-like activity. This leads to a marked increase in ˙OH within cells, thereby enhancing radiotherapy's effectiveness.
The effectiveness of conventional radiotherapy is often limited by inadequate radiation energy deposition and collateral damage to healthy tissues.192 To address these issues, recent advancements have focused on multifunctional nanoformulations and synergistic therapies, aiming to boost both the efficacy and safety of radiotherapy. Hu et al.192 introduced a bimetallic nanozyme, RuCu NPs (copper-modified ruthenium nanoparticles), incorporating the high atomic number element ruthenium as a novel radiosensitizer. This nanozyme demonstrates ultrasensitive POD-like and CAT-like activities, offering an ideal approach for radiotherapy sensitization. DFT calculations further elucidated the optimal POD-like catalytic ratio of RuCu NPs and shed light on its superior catalytic activity. Under X-ray irradiation, RuCu NPs coated with PEG effectively enhanced ROS production and alleviated tumor hypoxia in the acidic TME, showing significant therapeutic efficacy in the MDA-MB-231 breast cancer model. Besides, Huang et al.193 developed a biomimetic nanozyme system (CF) by encapsulating pyrite (FeS2) into tumor-derived exosomes (Fig. 14F). This CF system endows FeS2 with immune evasion and homologous targeting capabilities. Post-administration, CF, exhibiting both GSH-OXD and POD activities, notably reduces GSH content in tumors and catalyzes intracellular H2O2 to generate abundant ˙OH, disrupting intracellular redox balance and destroying mitochondria, thus mitigating radiotherapy resistance. Both in vivo and in vitro studies have demonstrated that CF, combined with radiotherapy (2Gy), significantly curbs tumor proliferation.
Building on the complex yet effective link between immunotherapy and tumor catalytic therapy, Yang group introduced a groundbreaking tumor treatment method,77 this approach combines MnOx nanocatalysts that mimic multiple enzymes, coated with tumor cell membranes (CM@Mn), and PD-1 monoclonal antibodies (αPD-1) (Fig. 15A). The aim is to kickstart the TME for a manganese-enhanced catalytic immunotherapy that works in tandem with PD-1 checkpoint inhibition. These CM@Mn nanocatalysts are capable of generating a significant amount of ROS to eliminate tumor cells and counteract tumor hypoxia via CAT activity, thereby transforming the tumor immune microenvironment. Additionally, the release of DAMPs by tumor cells boosts the tumor's immunogenicity. The TME facilitates the generation of Mn2+, further boosting immune activation within the tumor. Ultimately, with the support of PD-1 checkpoint inhibition, this strategy can unleash a robust anti-tumor response, converting an immunosuppressive TME into an immune-activated environment.
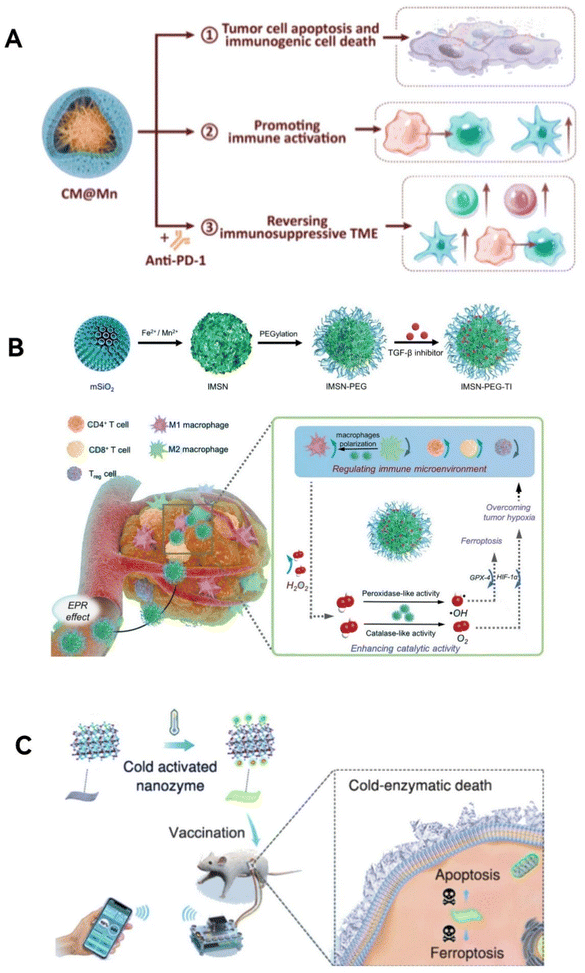 | ||
| Fig. 15 Biomimetic-designed nanozymes for cancer immunotherapy. (A) In the acidic TME, the CM@Mn nanozyme, exhibits POD and OXD-like activities, designed for killing tumor cells killing and evoking immunogenic cell death. Reproduced from ref. 77 with permission from American Chemical Society, copyright 2020. (B) The IMSN-PEG-TI nanozyme, exhibiting both POD-like and CAT-like activities in the acidic TME, facilitates a synergistic interaction between nanozyme efficiency and TME modulation, significantly enhancing immunotherapy outcomes. Reproduced from ref. 196 with permission from WILEY, copyright 2020. (C) Bi2Fe4O9 nanosheets, serving as in situ vaccines, successfully activate systemic anti-tumor immunity, effectively inhibiting tumor metastasis and recurrence. Reproduced from ref. 198 with permission from American Chemical Society, copyright 2022. | ||
Current trends in immunotherapy, particularly its combination with chemotherapy and radiotherapy, aim to enhance treatment outcomes. However, the adverse effects of chemotherapy and radiotherapy necessitate the discovery of safer, more effective complementary methods. In this context, Xu et al.196 devised a technique to trigger tumor cell pyroptosis and modulate the expression levels of PD-L1 by controlling tumor glycometabolism (Fig. 15B). They synthesized nanoparticles with dual enzymatic activities using a biomineralization-like approach. These nanoparticles are capable of self-amplifying the regulation of tumor cell glycometabolism, inducing pyroptosis, and elevating PD-L1 expression in tumor cells. When these nanoparticles are used in conjunction with anti-PD-L1 therapy, there is a significant suppression in tumor growth and a notable increase in the survival rates of mice. This combination therapy not only demonstrates a pronounced immunological memory effect but also effectively prevents tumor recurrence and metastasis.
Accurately triggering antitumor immune responses is a complex challenge. Nguyen et al.197 developed a novel nanocomposite polymer immunomodulator, the drug-free polypyrrole–polyethyleneimine nanozyme (PPY-PEI NZ). This nanozyme specifically responds to the B-cell lymphoma TME, aiming for precise cancer immunotherapy. It rapidly binds to various B-cell lymphoma cells due to early endocytosis, effectively inhibiting B-cell colony growth in vitro and inducing cytotoxic apoptosis. PPY-PEI NZ-triggered cell death involves mitochondrial dysfunction, downregulation of antiapoptotic proteins, and caspase-dependent apoptosis, with altered AKT and ERK signaling pathways contributing to apoptosis. Additionally, these nanozymes can disrupt lysosomal membranes while preventing endosomal acidification, offering protection against lysosomal apoptosis. Notably, PPY-PEI NZs can selectively target and eliminate malignant B cells in mixed cultures with healthy leukocytes, showing effectiveness ex vivo without harming wild-type mice. In vivo, they significantly inhibit the growth of B-cell lymphoma-driven nodules in a xenograft model.
Moreover, a cold-activated artificial enzyme based on Bi2Fe4O9 nanosheets (NSs) is introduced (Fig. 15C).198 These NSs exhibit glutathione oxidase-like activity at low temperatures due to their pyroelectric properties. They induce tumor cell death via apoptosis and ferroptosis while minimizing toxicity to normal tissues. An innovative device also allows for the intelligent, remote control of the enzymatic activity of Bi2Fe4O9 NSvia a smartphone. Serving as an in situ vaccine, these NSs activate systemic antitumor immunity, effectively suppressing metastasis and relapse. Blood biochemistry and histological analyses confirm the high biosafety of Bi2Fe4O9 NSs for in vivo use.
However, CDT has been constrained by the demanding conditions of Fenton reaction (pH = 3–4) and slow kinetics. To address this, Jana's team60 introduced a minuscule trimetallic alloy nanozyme (PCF-a NE), comprising Pd, Cu, and Fe (Fig. 16A). This nanozyme leverages dynamic active site synergy, enabling cascading GPx and POD-like activities at a neutral pH. Notably, PCF-a NE exhibits photothermally enhanced POD characteristics and an impressive photothermal conversion efficiency of 62%, aiding in the apoptosis of tumor cells. Furthermore, ultrasound application can boost the mass transfer at the nanozyme active sites, speeding up the tumor-specific CDT through a Fenton-like reaction. This innovation outlines a biomimetic approach to designing alloy nanozymes that amplify ROS within tumors in response to external stimuli, showing marked efficiency in suppressing tumor growth both in lab settings and live models.
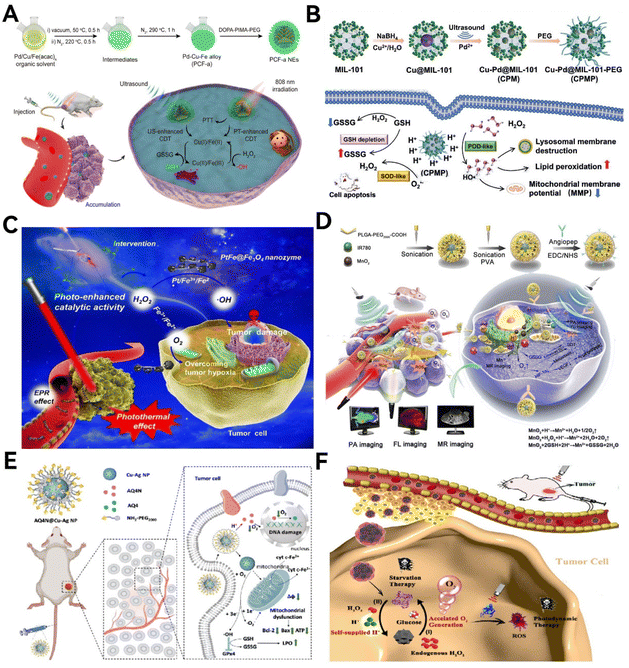 | ||
| Fig. 16 (A) PCF-a NE targets cancer with a two-pronged approach: one method boosts CDT using ultrasound and depletes GSH, and the other enhances CDT through heat generated by PTT while also reducing GSH. Reproduced from ref. 60 with permission from American Chemical Society, copyright 2021. (B) PEG-modified Cu–Pd@MIL-101 (Cu–Pd@MIL-101-PEG, CPMP) exhibits the highest POD-like and SOD-like activities, as well as GSH depletion characteristics, demonstrating strong tumor CDT effects. Reproduced from ref. 48 with permission from WILEY, copyright 2021. (C) PtFe@Fe3O4 significantly enhances catalytic activity under near-infrared laser irradiation, and by utilizing a photointervention device, implements an in situ photo-enhanced catalytic therapy combined with a photothermal effect strategy. Reproduced from ref. 200 with permission from American Chemical Society, copyright 2022. (D) AIMP nanoparticles are designed for crossing the blood–brain barrier (BBB) and specifically targeting tumors and mitochondria, offering deep tissue penetration and improved SDT outcomes. Reproduced from ref. 201 with permission from Royal Society of Chemistry, copyright 2021. (E) Nanozymes made of a copper–silver alloy, carrying AQ4N, have the unique capability to trigger cell starvation, induce ferroptosis, and deliver targeted prodrug treatments all at once. Reproduced from ref. 202 with permission from WILEY, copyright 2022. (F) rMGB achieves self-supply of H+ and accelerates the generation of O2, alleviating tumor hypoxia, enhancing PDT, and starvation therapy for hypoxic tumors. Reproduced from ref. 203 with permission from American Chemical Society, copyright 2022. | ||
Yang's team48 advanced this field by creating ultrafine, evenly distributed nanozymes within MOF structures for CDT in cancer. Using high-intensity ultrasound, they produced various ultrafine alloy nanoparticles like Ni–Ru@MIL-101 and Cu–Pd@MIL-101 (Fig. 16B). Among these, the Cu–Pd@MIL-101 modified with a 9.5% alloy (Cu–Pd@MIL-101-PEG, or CPMP) showed exceptional ability in generating toxic ˙OH and depleting GSH, making it highly effective in CDT. Both lab and animal studies confirmed that Cu–Pd@MIL-101-PEG was highly effective in producing toxic radicals within the TME, leading to tumor cell death, and hindering tumor growth. Additionally, these ultrafine nanoparticles were found to be biocompatible and safe for biological use.
Li's team200 introduced an innovative nanozyme (PtFe@ Fe3O4) that showcases dual enzymatic-like activities, offering a promising approach for effective tumor catalytic therapy (Fig. 16C). In the acidic milieu of the TME, PtFe@Fe3O4 demonstrates intrinsic photothermal effects alongside light-amplified POD and CAT activities. This dual functionality enables it to efficiently eliminate tumor cells and address the issue of tumor hypoxia. Furthermore, their research delved into the electron transfer dynamics between PtFe nanorods, Fe3O4 nanoparticles, and H2O2 molecules to explore the potential light-enhanced synergistic catalytic mechanism.
Sun's team203 developed an innovative biomimetic hybrid nanozyme, rMGB, by harnessing GOx and BSA-Ce6 on MnO2 nanoparticles, forming the cornerstone of this hybrid nanozyme system (Fig. 16F). They cleverly utilized a red blood cell membrane coating on the MGB NPs to minimize the systemic toxicity associated with GOx and MnO2. The rMGB is particularly effective against hypoxic tumors, catalyzing the conversion of endogenous H2O2 and H+ into O2 right at the tumor site. This process supplies the necessary oxygen for GOx, thereby facilitating cancer starvation therapy. Additionally, H+ production by GOx aids MnO2 in further boosting oxygen generation, mitigating tumor hypoxia, and consequently amplifying the effectiveness of PDT. Therefore, rMGB, through the synergistic action of GOx and MnO2, significantly improves the efficacy of PDT and starvation therapy for hypoxic tumors by enhancing oxygen production in the tumor environment.
4. Perspectives
4.1. Catalytic specificity
Nanozymes, while offering synergistic and multifunctional enzyme-like activities, lack the precise catalytic specificity and pathway selectivity of natural enzymes.204 This restricts their use in highly selective applications, especially in critical biological processes. The variety and number of nanozymes known for catalytic activity are significantly fewer than natural enzymes. Progress in enhancing nanozyme selectivity is notable, yet it falls short of the stringent specificity found in natural enzymes. Enhancing the catalytic selectivity of nanozymes is crucial, especially for targeted and personalized approaches in cancer therapy.Improving nanozyme specificity hinges on boosting the ability of their structural domains to specifically recognize substrates and finely control catalytic sites. Unlike natural enzymes, many nanozymes have exposed catalytic sites, lacking the specific binding sites and precise environments needed for effective substrate interaction and catalysis. Selecting appropriate ligands as binding domains can increase substrate recognition and binding, thereby enhancing activity for particular substrates. For example, lactate, a key signaling molecule, is crucial in the progression of tumor malignancy, blood vessel formation, immune suppression, and resistance to treatment. Targeting lactate has emerged as a promising approach to improve cancer therapies. Developing nanozymes with effective LOX mimicry could advance lactate-responsive cancer treatments, yet oxidizing the α-C–sp3–H bond of lactate to generate pyruvate under gentle conditions remains a complex challenge. Drawing inspiration from nature, advancements in nanozymes mimicking LOX should focus on enhancing the electronic configuration near electron-rich nitrogen (N) sites. Zhao group61 introduced a strategy that mimics LOX by adjusting the electronic characteristics of the N center in Co(x)–N nanocomposites, by controlling the number of Co atoms near the N. This results in Co4N/C having specific sites for accurately recognizing lactate and its intermediates, fine-tuning the absorption energy of intermediates, thus facilitating the conversion of lactate's α-C–sp3–H bond into a ketone. This optimized nanozyme markedly improves its anti-cancer properties by altering the TME high lactate and immunosuppressive conditions, leading to significant inhibition of tumor growth and metastasis.
Future research should aim at designing nanozymes that closely mimic enzyme active sites for selective substrate interaction and high catalytic efficiency. Understanding the structure–activity relationships of nanozymes that dictate substrate transformation pathways is also vital. These research directions are key to enhancing substrate selectivity and determining the specificity of nanozyme-catalyzed reactions.
4.2. Functional versatility
Nanozymes offer distinct advantages over natural enzymes: they are cost-effective, stable, easily modifiable, and can self-assemble. Their activity can be tuned through size and structural factors. Crucially, nanozymes blend the unique physical and chemical properties of nanomaterials into their functionality.205 For example, Fe3O4 nanozymes not only act as PODs but also have superparamagnetic features.206 Similarly, molybdenum disulfide (MoS2) nanoparticles combine catalytic activity with near-infrared photothermal properties, making nanozymes versatile in function.207 In cancer therapy, this allows for an innovative approach: beyond just combining catalytic treatments with other cancer therapies, the catalytic properties of nanozymes can be integrated with their physical and chemical attributes. This fusion opens up new, groundbreaking possibilities for unified cancer diagnostics and treatment strategies, leveraging the multifaceted nature of nanozymes. For instance, Song group208 have developed an innovative manganese semiconductor polymer-based nanozyme (MSPN), designed for specific cancer treatment in acidic tumor environments. What sets MSPN apart is its ability to self-report its activity, providing real-time near-infrared fluorescence-photoacoustic (NIRF-PA) feedback. The core of MSPN is a mix of manganese oxide (MnOx) and semiconductor polymer (PFODBT), linked to oxidase-responsive molecules (ORM) through amide bonds. In acidic conditions, MnOx triggers the breakdown of ORM, which changes the emission signals at 695 nm and 825 nm due to effective fluorescence resonance energy transfer between PFODBT and ORM. Importantly, MSPN maintains a consistent internal PA signal at 680 nm while the responsive PA signal at 780 nm drops, creating a distinct PA signal ratio (PA680/PA780). This functionality allows MSPN to not only actively treat cancer in the body but also to monitor the efficiency of its OXD-like catalysis in real-time through dual-mode NIRF-PA imaging. This cutting-edge method merges nanozyme catalysis with tumor imaging, providing a novel strategy for evaluating and tracking the success of cancer treatments.Multifunctional nanozymes are adept at combining diagnostic and therapeutic functions. Chen group209 developed a versatile Ti3C2–MXene–Au nano-platform. This platform integrates photothermal, enzymatic, and anti-tumor immune therapies for treating triple-negative breast cancer (TNBC) by coupling Ti3C2–MXene–Au nanodrugs with OX40 mAb. Thanks to the nanostructure's enhanced permeability and retention effect, the Ti3C2–MXene–Au nanocomposite specifically accumulates at tumor sites post-intravenous injection. It also facilitates thermal/PA dual-mode imaging within the body. Upon laser exposure, the localized heating at the tumor site not only ablates the tumor but also boosts catalytic reactions to produce ˙OH, leading to tumor cell death. Furthermore, introducing OX40 mAb strengthens T-cell-mediated anti-tumor immunity and mitigates immune suppression caused by tumor cells.
Multifunctional nanozymes, serving as versatile molecules, hold potential in areas like medical diagnosis, therapy, and monitoring. Given the broad and yet-to-be-fulfilled clinical needs for diagnosing and treating diverse diseases, exploring and applying the multifaceted capabilities of nanozymes is a key focus for future research. Moreover, before these nanozymes can be clinically applied, it's crucial to rigorously investigate and test them, particularly concerning issues like their clinical safety, stability, and how their catalytic processes work. This thorough examination is vital to ensure their efficacy and safety in medical applications.
4.3. Artificial intelligence-aided design
AlphaFold, a ground-breaking artificial intelligence (AI) program by DeepMind, has revolutionized the field with its ability to predict human protein structures, a long-standing challenge in biology and medicine.210 This achievement underscores the immense potential of AI in advancing medical research. More than just handling vast datasets, this cutting-edge technology can unravel complex biological patterns that elude direct human observation. Inspired by this, AI-driven data analysis and performance prediction have become essential tools for assessing material properties and spearheading new material developments.211,212 In contrast to the traditional, resource-intensive methods of developing nanozymes—relying on trial and error or intuition and experience, which are often inefficient and costly. Using AI algorithms, including machine learning (ML) and deep learning (DL), presents a more effective alternative.212 leveraging existing data through artificial intelligence algorithms such as ML and DL as a reliable tool can uncover the hidden relationships between the physicochemical characteristics of nanozymes and their enzyme-like functions. This approach holds tremendous research and practical value, opening new frontiers in material science.213Researchers are finding innovative uses of ML in pinpointing the best activities for nanozymes. At the outset, ML helps sift through nanozymes by examining their kinetic features—like KM, Vmax, kcat, and kcat/KM ratios—offering a clear picture of their performance and specificity.213 ML also steps in to forecast the energy barriers and thermodynamic aspects of nanozyme-catalyzed reactions, evaluating how challenging it is to these reactions.214 Such thermodynamic insights, typically gleaned from DFT computations that demand high-performance computing and significant time,215 can now be more efficiently predicted by ML. This leap is possible because ML models, trained on precise quantum or experimental data, might even outperform hybrid DFT analyses in accuracy.216 Moreover, the scope of ML extends to classifying nanozymes based on their enzymatic actions.213 It does so by digitizing their catalytic activities and leveraging intrinsic and extrinsic nanozyme markers for direct predictions, or by using physical descriptors to foretell the enzyme type and specificity.217 ML is also viable for analyzing the biomimetic structures of nanozymes. For instance, ML can predicate the spatial arrangement and the type and distribution of metal atoms at SAN active sites, which are key to their catalytic process, showcasing the broad applicability of ML in advancing nanozyme research.
Li group218 applied an innovative approach, combining stochastic surface walking technology based on global neural network potentials and DFT calculations, to fast-track exploring the global potential energy surface of catalysts. Through this method, they successfully mapped out the phase diagrams for PdAg catalysts, both in bulk and on surfaces, under actual reaction conditions. This exploration shed light on how variations in phase composition and surface structures influence the catalyst effectiveness. From their findings on how in situ surface structures affect PdAg catalysts, they developed a new catalyst, Pd1Ag3, supported by rutile. This catalyst exhibited exceptional performance, achieving a remarkable 496% conversion rate and 485% selectivity, setting a new standard in catalytic efficiency. Moreover, Huang's group213 conducted a detailed analysis on a diverse dataset of nanozymes, extracted from over 300 research papers. They focused on datasets that included at least one variable from the Michaelis–Menten equation, aiming at further refinement for data mining purposes. The analysis highlighted eight key internal factors, namely, the type and valency of metals, the quantity of metals, size, shape, non-metal elements, surface modifications, and types of enzyme mimicry. Additionally, external conditions such as buffer pH, temperature, and substrate type were identified as critical variables influencing nanozyme characteristics. The researchers then developed 14 sophisticated deep neural network (DNN)-based models, both qualitative and quantitative, designed to accurately predict the enzymatic activities of nanozymes. The classification models were tasked with identifying the types of enzyme mimetics, whereas the quantitative models focused on measuring the levels of enzymatic activity. Remarkably, the predictions made by these models were found to align closely with actual observed data, showcasing the potential of DNN in advancing nanozyme research. In summary, these studies reveal the strength of ML in the development of nanozymes.
5. Conclusion
In summary, this review provides a comprehensive overview of the pioneering research in the field of nanozymes, specifically those that are biomimetically engineered from the ground up for anti-tumor treatment. It emphasizes the intricate processes involved in their creation, including structural biomimicry, process biomimicry, and the nuances of advanced functional biomimicry. This exploration sheds light on the fundamental mechanisms that amplify their catalytic properties and examines their extensive biomedical applications in the fight against tumors, thereby underscoring the remarkable progress achieved in this cutting-edge area of study.Abbreviations
| 2-mim | 2-Methylimidazole |
| 3-AT | 3-Amino-1,2,4-triazole |
| ATP | Adenosine triphosphate |
| Ang | Angiopep-2 |
| AI | Artificial intelligence |
| BON | Boroxynitride |
| CAT | Catalase |
| Cer | Cerulenin |
| CDT | Chemodynamic therapy |
| CS | Chondroitin sulfate |
| CuP | Copper-doped polypyrrole nanozyme |
| COF | Covalent organic framework |
| DL | Deep learning |
| DNN | Deep neural network |
| DMSN | Dendritic mesoporous silica |
| DFT | Density functional theory |
| DNA | Deoxyribonucleic acid |
| DHA | Dihydroartemisinin |
| DOX | Doxorubicin |
| FAO | Fatty acid oxidation |
| Fe2+ | Ferrous |
| FM | Fusion membrane |
| GBM | Glioblastoma |
| GOx | Glucose oxidase |
| GSH | Glutathione |
| GPx | Glutathione peroxidase |
| Fe3+ | Hemin |
| HCV | Hepatitis C virus |
| HRP | Horseradish peroxidase |
| H2O2 | Hydrogen peroxide |
| ˙OH | Hydroxyl radicals |
| ICB | Immune checkpoint blockade |
| ICD | Immunogenic cell death |
| ICG | Indocyanine green |
| FeMSN | Iron-doped mesoporous silica nanoparticles |
| LDH | Lactate dehydrogenase |
| LOX | Lactate oxidase |
| ML | Machine learning |
| MOFs | Metal–organic frameworks |
| NPs | Nanoparticles |
| NUs | Nanourchins |
| NAISA | Nanozyme-assisted immunoassay |
| NIR-II | Near-infrared II |
| NADH/NAD+ | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| N-GNM | Nitrogen-doped graphene materials |
| OXD | Oxidase |
| GSSG | Oxidized glutathione |
| O2 | Oxygen |
| OVs | Oxygen vacancies |
| PCNs | Palladium–copper nanoclusters |
| POD | Peroxidase |
| PDT | Photodynamic therapy |
| PTT | Photothermal therapy |
| PLT | Platelet |
| PEG | Polyethylene glycol |
| PD-1/PD-L1 | Programmed cell death protein 1/programmed cell death 1 ligand 1 |
| ROS | Reactive oxygen species |
| Rh | Rhodium |
| Ru | Ruthenium |
| SAzyme | Single atom nanozyme |
| 1O2 | Singlet oxygen |
| SOD | Superoxide dismutase |
| O2˙− | Superoxide radicals |
| TNBC | Triple-negative breast cancer |
| TIME | Tumor immune microenvironment |
| TME | Tumor microenvironment |
| ZIF | Zeolitic imidazolate framework |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support from the Fundamental Research Funds for the Central Universities, conducted at Tongji University.References
- H. Wang, K. Wan and X. Shi, Recent Advances in Nanozyme Research, Adv. Mater., 2019, 31(45), e1805368 CrossRef PubMed.
- J. Golchin, K. Golchin, N. Alidadian, S. Ghaderi, S. Eslamkhah and M. Eslamkhah, et al., Nanozyme applications in biology and medicine: an overview, Artif. Cells, Nanomed., Biotechnol., 2017, 45(6), 1–8 Search PubMed.
- S. Fu, S. Wang, X. Zhang, A. Qi, Z. Liu and X. Yu, et al., Structural effect of Fe(3)O(4) nanoparticles on peroxidase-like activity for cancer therapy, Colloids Surf., B, 2017, 154, 239–245 CrossRef CAS PubMed.
- Q. Wang, J. Jiang and L. Gao, Nanozyme-based medicine for enzymatic therapy: progress and challenges, Biomed. Mater., 2021, 16(4), 042002 CrossRef CAS PubMed.
- M. Huo, L. Wang, Y. Chen and J. Shi, Tumor-selective catalytic nanomedicine by nanocatalyst delivery, Nat. Commun., 2017, 8(1), 357 CrossRef PubMed.
- Q. Li, Y. Liu, X. Dai, W. Jiang and H. Zhao, Nanozymes Regulate Redox Homeostasis in ROS-Related Inflammation, Front. Chem., 2021, 9, 740607 CrossRef PubMed.
- T. Qin, R. Ma, Y. Yin, X. Miao, S. Chen and K. Fan, et al., Catalytic inactivation of influenza virus by iron oxide nanozyme, Theranostics, 2019, 9(23), 6920–6935 CrossRef CAS PubMed.
- Y. Zhao, M. J. Haney, V. Mahajan, B. C. Reiner, A. Dunaevsky and R. L. Mosley, et al., Active Targeted Macrophage-mediated Delivery of Catalase to Affected Brain Regions in Models of Parkinson’s Disease, J. Nanomed. Nanotechnol., 2011, S4, 003 Search PubMed.
- Y. Ai, Z.-N. Hu, X. Liang, H.-b. Sun, H. Xin and Q. Liang, Recent Advances in Nanozymes: From Matters to Bioapplications, Adv. Funct. Mater., 2022, 32(14), 2110432 CrossRef CAS.
- T. Chen, Q. Chu, M. Li, G. Han and X. Li, Fe(3)O(4)@Pt nanoparticles to enable combinational electrodynamic/chemodynamic therapy, J. Nanobiotechnol., 2021, 19(1), 206 CrossRef CAS PubMed.
- K. Maffuid and Y. Cao, Decoding the Complexity of Immune-Cancer Cell Interactions: Empowering the Future of Cancer Immunotherapy, Cancers, 2023, 15(16), 4188 CrossRef CAS PubMed.
- J. D. Hayes, A. T. Dinkova-Kostova and K. D. Tew, Oxidative Stress in Cancer, Cancer Cell, 2020, 38(2), 167–197 CrossRef CAS PubMed.
- S. Arfin, N. K. Jha, S. K. Jha, K. K. Kesari, J. Ruokolainen and S. Roychoudhury, et al., Oxidative Stress in Cancer Cell Metabolism, Antioxidants, 2021, 10(5), 642 CrossRef CAS PubMed.
- I. Elia and M. C. Haigis, Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism, Nat. Metab., 2021, 3(1), 21–32 CrossRef PubMed.
- D. H. Munn and V. Bronte, Immune suppressive mechanisms in the tumor microenvironment, Curr. Opin. Immunol., 2016, 39, 1–6 CrossRef CAS PubMed.
- S. Singh, Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives, Front. Chem., 2019, 7, 46 CrossRef CAS PubMed.
- R. Zhang, K. Fan and X. Yan, Nanozymes: created by learning from nature, Sci. China: Life Sci., 2020, 63(8), 1183–1200 CrossRef PubMed.
- R. Zhang, X. Yan and K. Fan, Nanozymes Inspired by Natural Enzymes, Acc. Mater. Res., 2021, 2(7), 534–547 CrossRef CAS.
- B. Jiang, Z. Guo and M. Liang, Recent progress in single-atom nanozymes research, Nano Res., 2023, 16(2), 1878–1889 CrossRef CAS PubMed.
- H. Zhang, X. F. Lu, Z.-P. Wu and X. W. D. Lou, Emerging Multifunctional Single-Atom Catalysts/Nanozymes, ACS Cent. Sci., 2020, 6(8), 1288–1301 CrossRef CAS PubMed.
- Y. Zhang and H. Hess, Toward Rational Design of High-efficiency Enzyme Cascades, ACS Catal., 2017, 7(9), 6018–6027 CrossRef CAS.
- P. B. O'Mara, P. Wilde, T. M. Benedetti, C. Andronescu, S. Cheong and J. J. Gooding, et al., Cascade Reactions in Nanozymes: Spatially Separated Active Sites inside Ag-Core–Porous-Cu-Shell Nanoparticles for Multistep Carbon Dioxide Reduction to Higher Organic Molecules, J. Am. Chem. Soc., 2019, 141(36), 14093–14097 CrossRef PubMed.
- C. Zhu, Z. Zhou, X. J. Gao, Y. Tao, X. Cao and Y. Xu, et al., Cascade nanozymatic network mimicking cells with selective and linear perception of H2O2, Chem. Sci., 2023, 14(24), 6780–6791 RSC.
- L. Yang, S. Dong, S. Gai, D. Yang, H. Ding and L. Feng, et al., Deep Insight of Design, Mechanism, and Cancer Theranostic Strategy of Nanozymes, Nano-Micro Lett., 2023, 16(1), 28 CrossRef PubMed.
- J. Ma, J. Qiu and S. Wang, Nanozymes for Catalytic Cancer Immunotherapy, ACS Appl. Nano Mater., 2020, 3(6), 4925–4943 CrossRef CAS.
- W. Du, W. Chen, J. Wang, H. Zhang, L. Song and Y. Hu, et al., A dual-nanozyme-loaded black phosphorus multifunctional therapeutic platform for combined photothermal/photodynamic/starvation cancer therapy, J. Mater. Chem. B, 2023, 11(23), 5185–5194 RSC.
- W. Dong, M. Chen, C. Chang, T. Jiang, L. Su and C. Chen, et al., Remodeling of Tumor Microenvironment by Nanozyme Combined cGAS-STING Signaling Pathway Agonist for Enhancing Cancer Immunotherapy, Int. J. Mol. Sci., 2023, 24(18), 13935 CrossRef CAS PubMed.
- S. Lei, J. Zhang, N. T. Blum, M. Li, D. Y. Zhang and W. Yin, et al., In vivo three-dimensional multispectral photoacoustic imaging of dual enzyme-driven cyclic cascade reaction for tumor catalytic therapy, Nat. Commun., 2022, 13(1), 1298 CrossRef CAS PubMed.
- T. Wang, X. Bi, L. Wang, M. Liu, W. W. Yu and Z. Zhu, et al., Biomimetic design of graphdiyne supported hemin for enhanced peroxidase-like activity, J. Colloid Interface Sci., 2022, 607(Part 1), 470–478 CrossRef CAS PubMed.
- W. Wu, Q. Wang, J. Chen, L. Huang, H. Zhang and K. Rong, et al., Biomimetic design for enhancing the peroxidase mimicking activity of hemin, Nanoscale, 2019, 11(26), 12603–12609 RSC.
- Y. Xu, Z. Zhou, N. Deng, K. Fu, C. Zhu and Q. Hong, et al., Molecular insights of nanozymes from design to catalytic mechanism, Sci. China: Chem., 2023, 66(5), 1318–1335 CrossRef CAS PubMed.
- M. Wen, J. Li, W. Zhong, J. Xu, S. Qu and H. Wei, et al., High-Throughput Colorimetric Analysis of Nanoparticle-Protein Interactions Based on the Enzyme-Mimic Properties of Nanoparticles, Anal. Chem., 2022, 94(24), 8783–8791 CrossRef CAS PubMed.
- L. Hou, G. Jiang, Y. Sun, X. Zhang, J. Huang and S. Liu, et al., Progress and Trend on the Regulation Methods for Nanozyme Activity and Its Application, Catalysts, 2019, 9(12), 1057 CrossRef CAS.
- R. Zhang, H. Zhao and K. Fan, Structure-Activity Mechanism of Iron Oxide Nanozymes. Nanozymes: Design, Synthesis, and Applications. ACS Symposium Series. 1422: American Chemical Society, 2022. p. 1–35.
- K. Fan, H. Wang, J. Xi, Q. Liu, X. Meng and D. Duan, et al., Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site, Chem. Commun., 2017, 53(2), 424 RSC.
- T. Tian, L. Ai, X. Liu, L. Li, J. Li and J. Jiang, Synthesis of hierarchical FeWO4 architectures with {100}-faceted nanosheet assemblies as a robust biomimetic catalyst, Ind. Eng. Chem. Res., 2015, 54(4), 1171 CrossRef CAS.
- M. Bokhove, P. Nadal Jimenez, W. J. Quax and B. W. Dijkstra, The quorum-quenching N-acyl homoserine lactone acylase PvdQ is an Ntn-hydrolase with an unusual substrate-binding pocket, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(2), 686–691 CrossRef CAS PubMed.
- D. W. Wong, Structure and action mechanism of ligninolytic enzymes, Appl. Biochem. Biotechnol., 2009, 157(2), 174–209 CrossRef CAS PubMed.
- D. Jana, B. He, Y. Chen, J. Liu and Y. Zhao, A Defect-Engineered Nanozyme for Targeted NIR-II Photothermal Immunotherapy of Cancer, Adv. Mater., 2024, 36(10), 2206401 CrossRef CAS PubMed.
- Y. Liu, M. Yao, W. Han, H. Zhang and S. Zhang, Construction of a Single-Atom Nanozyme for Enhanced Chemodynamic Therapy and Chemotherapy, Chemistry, 2021, 27(53), 13418–13425 CrossRef CAS PubMed.
- S. Dong, Y. Dong, Z. Zhao, J. Liu, S. Liu and L. Feng, et al., “Electron Transport Chain Interference” Strategy of Amplified Mild-Photothermal Therapy and Defect-Engineered Multi-Enzymatic Activities for Synergistic Tumor-Personalized Suppression, J. Am. Chem. Soc., 2023, 145(17), 9488–9507 CrossRef CAS PubMed.
- R. Zhao, R. Zhang, L. Feng, Y. Dong, J. Zhou and S. Qu, et al., Constructing virus-like SiOx/CeO2/VOx nanozymes for 1064 nm light-triggered mild-temperature photothermal therapy and nanozyme catalytic therapy, Nanoscale, 2022, 14(2), 361–372 RSC.
- Y. Valasatava, A. Rosato, N. Furnham, J. M. Thornton and C. Andreini, To what extent do structural changes in catalytic metal sites affect enzyme function?, J. Inorg. Biochem., 2018, 179, 40–53 CrossRef CAS PubMed.
- X. Zhang, G. Li, D. Wu, X. Li, N. Hu and J. Chen, et al., Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy, Biosens. Bioelectron., 2019, 137, 178–198 CrossRef CAS PubMed.
- X. Ma, X. Ren, X. Guo, C. Fu, Q. Wu and L. Tan, et al., Multifunctional iron-based Metal-Organic framework as biodegradable nanozyme for microwave enhancing dynamic therapy, Biomaterials, 2019, 214, 119223 CrossRef CAS PubMed.
- H. Liang, F. Lin, Z. Zhang, B. Liu, S. Jiang and Q. Yuan, et al., Multicopper Laccase Mimicking Nanozymes with Nucleotides as Ligands, ACS Appl. Mater. Interfaces, 2017, 9(2), 1352–1360 CrossRef CAS PubMed.
- Q. Song, B. Chi, H. Gao, J. Wang, M. Wu and Y. Xu, et al., Functionalized nanozyme with drug loading for enhanced tumour combination treatment of catalytic therapy and chemotherapy, J. Mater. Chem. B, 2023, 11(29), 6889–6895 RSC.
- P. Yang, J. Tao, F. Chen, Y. Chen, J. He and K. Shen, et al., Multienzyme-Mimic Ultrafine Alloyed Nanoparticles in Metal Organic Frameworks for Enhanced Chemodynamic Therapy, Small, 2021, 17(7), 2005865 CrossRef CAS PubMed.
- M. Li, J. Chen, W. Wu, Y. Fang and S. Dong, Oxidase-like MOF-818 Nanozyme with High Specificity for Catalysis of Catechol Oxidation, J. Am. Chem. Soc., 2020, 142(36), 15569–15574 CrossRef CAS PubMed.
- D. Wang, L. Zhang, C. Wang, Z. Cheng, W. Zheng and P. Xu, et al., Missing-Linker-Confined Single-Atomic Pt Nanozymes for Enzymatic Theranostics of Tumor, Angew. Chem., Int. Ed., 2023, 62(19), 202217995 CrossRef PubMed.
- L. Huang, J. Chen, L. Gan, J. Wang and S. Dong, Single-atom nanozymes, Sci. Adv., 2019, 5(5), eaav5490 CrossRef CAS PubMed.
- B. Xu, S. Li, L. Zheng, Y. Liu, A. Han and J. Zhang, et al., A Bioinspired Five-Coordinated Single-Atom Iron Nanozyme for Tumor Catalytic Therapy, Adv. Mater., 2022, 34(15), 2107088 CrossRef CAS PubMed.
- M. Feng, Q. Zhang, X. Chen, D. Deng, X. Xie and X. Yang, Controllable synthesis of boron-doped Zn-N-C single-atom nanozymes for the ultrasensitive colorimetric detection of p-phenylenediamine, Biosens. Bioelectron., 2022, 210, 114294 CrossRef CAS PubMed.
- S. Ji, B. Jiang, H. Hao, Y. Chen, J. Dong and Y. Mao, et al., Matching the kinetics of natural enzymes with a single-atom iron nanozyme, Nat. Catal., 2021, 4, 407–411 CrossRef CAS.
- S. Zhang, Y. Li, S. Sun, L. Liu, X. Mu and S. Liu, et al., Single-atom nanozymes catalytically surpassing naturally occurring enzymes as sustained stitching for brain trauma, Nat. Commun., 2022, 13(1), 4744 CrossRef CAS PubMed.
- Q. Feng, G. Wang, L. Xue, Y. Wang, M. Liu and J. Liu, et al., Single-Atom Nanozyme Based on Mn-Center with Enhanced Peroxidase-like Activity for Organic Dye Degradation, ACS Appl. Nano Mater., 2023, 6(6), 4844–4853 CrossRef CAS.
- L. Zhang, Q. Dong, Y. Hao, Z. Wang, W. Dong and Y. Liu, et al., Drug-Primed Self-Assembly of Platinum-Single-Atom Nanozyme to Regulate Cellular Redox Homeostasis Against Cancer, Adv. Sci., 2023, 10(30), 2302703 CrossRef CAS PubMed.
- X. Lu, S. Gao, H. Lin, L. Yu, Y. Han and P. Zhu, et al., Bioinspired Copper Single-Atom Catalysts for Tumor Parallel Catalytic Therapy, Adv. Mater., 2020, 32(36), 2002246 CrossRef CAS PubMed.
- L. Jiao, W. Ye, Y. Kang, Y. Zhang, W. Xu and Y. Wu, et al., Atomically dispersed N-coordinated Fe-Fe dual-sites with enhanced enzyme-like activities, Nano Res., 2022, 15(2), 959–964 CrossRef CAS.
- D. Jana, D. Wang, A. K. Bindra, Y. Guo, J. Liu and Y. Zhao, Ultrasmall Alloy Nanozyme for Ultrasound- and Near-Infrared Light-Promoted Tumor Ablation, ACS Nano, 2021, 15(4), 7774–7782 CrossRef CAS PubMed.
- S. Zhao, H. Li, R. Liu, N. Tao, L. Deng and Q. Xu, et al., Nitrogen-Centered Lactate Oxidase Nanozyme for Tumor Lactate Modulation and Microenvironment Remodeling, J. Am. Chem. Soc., 2023, 145(18), 10322–10332 CrossRef CAS PubMed.
- B. Yu, W. Wang, W. Sun, C. Jiang and L. Lu, Defect Engineering Enables Synergistic Action of Enzyme-Mimicking Active Centers for High-Efficiency Tumor Therapy, J. Am. Chem. Soc., 2021, 143(23), 8855–8865 CrossRef CAS PubMed.
- Y. Wu, W. Xu, L. Jiao, Y. Tang, Y. Chen, W. Gu and C. Zhu, Defect engineering in nanozymes, Mater. Today, 2022, 52, 327–347 CrossRef CAS.
- G. Li, G. R. Blake and T. T. M. Palstra, Vacancies in functional materials for clean energy storage and harvesting: the perfect imperfection, Chem. Soc. Rev., 2017, 46, 1693–1706 RSC.
- B. Liu, P. Wu, Z. Huang, L. Ma and J. Liu, Bromide as a Robust Backfiller on Gold for Precise Control of DNA Conformation and High Stability of Spherical Nucleic Acids, J. Am. Chem. Soc., 2018, 140(13), 4499–4502 CrossRef CAS PubMed.
- Y. Meng, Y. Chen, J. Zhu, Y. Qi, J. Ding and W. Zhou, Polarity control of DNA adsorption enabling the surface functionalization of CuO nanozymes for targeted tumor therapy, Mater. Horiz., 2021, 8, 972–986 RSC.
- X. Zhang, D. Wu, Y. Wu and G. Li, Bioinspired nanozyme for portable immunoassay of allergenic proteins based on A smartphone, Biosens. Bioelectron., 2021, 172, 112776 CrossRef CAS PubMed.
- L. Yang, X. Du, Y. Qin, X. Wang, L. Zhang, Z. Chen, Z. Wang, X. Yang, M. Lei and Y. Zhu, Biomimetic multifunctional nanozymes enhanced radiosensitization for breast cancer via an X-ray triggered cascade reaction, J. Mater. Chem. B, 2022, 10, 3667–3680 RSC.
- W. Zeng, M. Yu, T. Chen, Y. Liu, Y. Yi, C. Huang, Jia Tang, H. Li, M. Ou, T. Wang, M. Wu and L. Mei, Polypyrrole Nanoenzymes as Tumor Microenvironment Modulators to Reprogram Macrophage and Potentiate Immunotherapy, Adv. Sci., 2022, 9(23), 2201703 CrossRef CAS PubMed.
- N. Nwahara, G. Abrahams, E. Prinsloo and T. Nyokong, Folic acid-modified phthalocyanine-nanozyme loaded liposomes for targeted photodynamic therapy, Photodiagnosis Photodyn Ther., 2021, 36, 102527 CrossRef CAS PubMed.
- P. Zhan, Z.-G. Wang, N. Li and B. Ding, Engineering Gold Nanoparticles with DNA Ligands for Selective Catalytic Oxidation of Chiral Substrates, ACS Catal., 2015, 5(3), 1489–1498 CrossRef CAS.
- S. Hu, Q. Shuai, Y. Lin, Y. Fu and M. Li, Chiral Fe(x)Cu(y)Se nanoparticles as peroxidase mimics for colorimetric detection of 3, 4-dihydroxy-phenylalanine enantiomers, Nanotechnology, 2022, 33(13), 135503 CrossRef PubMed.
- X. Chen, Y. Yang, G. Ye, S. Liu and J. Liu, Chiral Ruthenium Nanozymes with Self-Cascade Reaction Driven the NO Generation Induced Macrophage M1 Polarization Realizing the Lung Cancer “Cocktail Therapy”, Small, 2023, 19(28), 2207823 CrossRef CAS PubMed.
- J. L. Chen, C. Pezzato, P. Scrimin and L. J. Prins, Chiral Nanozymes-Gold Nanoparticle-Based Transphosphorylation Catalysts Capable of Enantiomeric Discrimination, Chemistry, 2016, 22(21), 7028–7032 CrossRef CAS PubMed.
- Y. Duo, M. Suo, D. Zhu, Z. Li, Z. Zheng and B. Z. Tang, AIEgen-Based Bionic Nanozymes for the Interventional Photodynamic Therapy-Based Treatment of Orthotopic Colon Cancer, ACS Appl. Mater. Interfaces, 2022, 14(23), 26394–26403 CrossRef CAS PubMed.
- M. Jia, W. Ren, Y. Liu, C. Wang, X. Zheng and D. Zhang, et al., Messenger Nanozyme for Reprogramming the Microenvironment of Rheumatoid Arthritis, ACS Appl. Mater. Interfaces, 2023, 15(1), 338–353 CrossRef CAS PubMed.
- Z. Zhao, S. Dong, Y. Liu, J. Wang, L. Ba and C. Zhang, et al., Tumor Microenvironment-Activable Manganese-Boosted Catalytic Immunotherapy Combined with PD-1 Checkpoint Blockade, ACS Nano, 2022, 16(12), 20400–20418 CrossRef CAS PubMed.
- J. Mou, X. Xu, F. Zhang, J. Xia and Z. Wang, Promoting Nanozyme Cascade Bioplatform by ZIF-Derived N-Doped Porous Carbon Nanosheet-based Protein/Bimetallic Nanoparticles for Tandem Catalysis, ACS Appl. Bio Mater., 2021, 3(1), 664–672 CrossRef PubMed.
- Y.-X. Han, J.-H. Cheng and D.-W. Sun, Changes in activity, structure and morphology of horseradish peroxidase induced by cold plasma, Food Chem., 2019, 301, 125240 CrossRef CAS PubMed.
- I. Wheeldon, S. D. Minteer, S. Banta, S. C. Barton, P. Atanassov and M. Sigman, Substrate channelling as an approach to cascade reactions, Nat. Chem., 2016, 8(4), 299–309 CrossRef CAS PubMed.
- A. Qileng, S. Chen, H. Liang, H. Shen, M. Chen and W. Liu, et al., Bionic structural design of Pt nanozymes with the nano-confined effect for the precise recognition of copper ion, Chem. Eng. J., 2023, 455, 140769 CrossRef CAS.
- R. Zhang, Y. Zhou, X. Yan and K. Fan, Advances in chiral nanozymes: a review, Mikrochim. Acta, 2019, 186(12), 782 CrossRef CAS PubMed.
- F. Li, S. Li, X. Guo, Y. Dong, C. Yao and Y. Liu,
et al., Chiral Carbon Dots Mimicking Topoisomerase
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I To Mediate the Topological Rearrangement of Supercoiled DNA Enantioselectively, Angew. Chem., Int. Ed., 2020, 59(27), 11087–11092 CrossRef CAS PubMed.
I To Mediate the Topological Rearrangement of Supercoiled DNA Enantioselectively, Angew. Chem., Int. Ed., 2020, 59(27), 11087–11092 CrossRef CAS PubMed. - J. M. Sperl and V. Sieber, Multienzyme Cascade Reactions—Status and Recent Advances, ACS Catal., 2018, 8(3), 2385–2396 CrossRef CAS.
- R. Tian, J. Xu, Q. Luo, C. Hou and J. Liu, Rational Design and Biological Application of Antioxidant Nanozymes, Front. Chem., 2020, 8, 831 CrossRef CAS PubMed.
- J. Sheng, Y. Wu, H. Ding, K. Feng, Y. Shen and Y. Zhang, et al., Multienzyme-Like Nanozymes: Regulation, Rational Design, and Application, Adv. Mater., 2024, 36(10), 2211210 CrossRef CAS PubMed.
- H. Wang, L. Cheng, S. Ma, L. Ding, W. Zhang and Z. Xu, et al., Self-Assembled Multiple-Enzyme Composites for Enhanced Synergistic Cancer Starving-Catalytic Therapy, ACS Appl. Mater. Interfaces, 2020, 12(18), 20191–20201 CrossRef CAS PubMed.
- R. Xu, D. Zhang, J. Tan, N. Ge, D. Liu and J. Liu, et al., A multifunctional cascade bioreactor based on a layered double oxides composite hydrogel for synergetic tumor chemodynamic/starvation/photothermal therapy, Acta Biomater., 2022, 153, 494–504 CrossRef CAS PubMed.
- N. Alizadeh and A. Salimi, Multienzymes activity of metals and metal oxide nanomaterials: applications from biotechnology to medicine and environmental engineering, J. Nanobiotechnol., 2021, 19(1), 26 CrossRef CAS PubMed.
- S. Dong, Y. Dong, B. Liu, J. Liu, S. Liu and Z. Zhao, et al., Guiding Transition Metal-Doped Hollow Cerium Tandem Nanozymes with Elaborately Regulated Multi-Enzymatic Activities for Intensive Chemodynamic Therapy, Adv. Mater., 2022, 34(7), 2107054 CrossRef CAS PubMed.
- D. Zheng, S. Sato, H. Arima, E. Heeley, C. Delcourt and Y. Cao, et al., Estimated GFR and the Effect of Intensive Blood Pressure Lowering After Acute Intracerebral Hemorrhage, Am. J. Kidney Dis., 2016, 68(1), 94–102 CrossRef PubMed.
- S. Dong, Y. Dong, T. Jia, S. Liu, J. Liu and D. Yang, et al., GSH-Depleted Nanozymes with Hyperthermia-Enhanced Dual Enzyme-Mimic Activities for Tumor Nanocatalytic Therapy, Adv. Mater., 2020, 32(42), 2002439 CrossRef CAS PubMed.
- M. Chang, Z. Wang, C. Dong, R. Zhou, L. Chen and H. Huang, et al., Ultrasound-Amplified Enzyodynamic Tumor Therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis, Adv. Mater., 2023, 35(7), 2208817 CrossRef CAS PubMed.
- C. Liu, J. Xing, O. U. Akakuru, L. Luo, S. Sun and R. Zou, et al., Nanozymes-Engineered Metal-Organic Frameworks for Catalytic Cascades-Enhanced Synergistic Cancer Therapy, Nano Lett., 2019, 19(8), 5674–5682 CrossRef CAS PubMed.
- Z. Cao, L. Zhang, J. Liu, D. Wang, K. Liang and Y. Chen, et al., A dual enzyme-mimicking radical generator for enhanced photodynamic therapy via series-parallel catalysis, Nanoscale, 2021, 13(41), 17386–17395 RSC.
- Y. Zhu, Y. Pan, Z. Guo, D. Jin, W. Wang and M. Liu, et al., Photothermal Enhanced and Tumor Microenvironment Responsive Nanozyme for Amplified Cascade Enzyme Catalytic Therapy, Adv. Healthcare Mater., 2023, 12(7), 2202198 CrossRef CAS PubMed.
- J. Chang, X. Qin, S. Li, F. He, S. Gai and H. Ding, et al., Combining Cobalt Ferrite Nanozymes with a Natural Enzyme to Reshape the Tumor Microenvironment for Boosted Cascade Enzyme-Like Activities, ACS Appl. Mater. Interfaces, 2022, 14(40), 45217–45228 CrossRef CAS PubMed.
- Y. Zhao, X. Xiao, M. Zou, B. Ding, H. Xiao and M. Wang, et al., Nanozyme-Initiated In Situ Cascade Reactions for Self-Amplified Biocatalytic Immunotherapy, Adv. Mater., 2021, 33(3), 2006363 CrossRef CAS PubMed.
- C. Cao, H. Zou, N. Yang, H. Li, Y. Cai and X. Song, et al., Fe3O4/Ag/Bi2 MoO6 Photoactivatable Nanozyme for Self-Replenishing and Sustainable Cascaded Nanocatalytic Cancer Therapy, Adv. Mater., 2021, 33(52), 2106996 CrossRef CAS PubMed.
- Q. Xu, Y. Zhang, Z. Yang, G. Jiang, M. Lv and H. Wang, et al., Tumor microenvironment-activated single-atom platinum nanozyme with H2O2 self-supplement and O2-evolving for tumor-specific cascade catalysis chemodynamic and chemoradiotherapy, Theranostics, 2022, 12(11), 5155–5171 CrossRef CAS PubMed.
- J. Xi, R. Zhang, L. Wang, W. Xu, Q. Liang and J. Li, et al., A Nanozyme-Based Artificial Peroxisome Ameliorates Hyperuricemia and Ischemic Stroke, Adv. Funct. Mater., 2021, 31(9), 2007130 CrossRef CAS.
- Q. Wang, C. Cheng, S. Zhao, Q. Liu, Y. Zhang and W. Liu, et al., A Valence-Engineered Self-Cascading Antioxidant Nanozyme for the Therapy of Inflammatory Bowel Disease, Angew. Chem., Int. Ed., 2022, 61(27), 202201101 CrossRef PubMed.
- S. Cai, J. Liu, J. Ding, Z. Fu, H. Li and Y. Xiong, et al., Tumor-Microenvironment-Responsive Cascade Reactions by a Cobalt-Single-Atom Nanozyme for Synergistic Nanocatalytic Chemotherapy, Angew. Chem., Int. Ed., 2022, 61(48), 202204502 CrossRef PubMed.
- H. Meng, W. Leong, K. W. Leong, C. Chen and Y. Zhao, Walking the line: The fate of nanomaterials at biological barriers, Biomaterials, 2018, 174, 41–53 CrossRef CAS PubMed.
- R. Yang, S. Fu, R. Li, L. Zhang, Z. Xu and Y. Cao, et al., Facile engineering of silk fibroin capped AuPt bimetallic nanozyme responsive to tumor microenvironmental factors for enhanced nanocatalytic therapy, Theranostics, 2021, 11(1), 107–116 CrossRef CAS PubMed.
- S. Fu, R. Yang, L. Zhang, W. Liu, G. Du and Y. Cao, et al., Biomimetic CoO@AuPt nanozyme responsive to multiple tumor microenvironmental clues for augmenting chemodynamic therapy, Biomaterials, 2020, 257, 120279 CrossRef CAS PubMed.
- Y. Su, X. Zhang, L. Lei, B. Liu, S. Wu and J. Shen, Tumor Microenvironment-Activatable Cyclic Cascade Reaction to Reinforce Multimodal Combination Therapy by Destroying the Extracellular Matrix, ACS Appl. Mater. Interfaces, 2021, 13(11), 12960–12971 CrossRef CAS PubMed.
- L. Li, Z. Lin, X. Xu, W. Wang, H. Chen and Z. Feng, et al., A pH/GSH/Glucose Responsive Nanozyme for Tumor Cascade Amplified Starvation and Chemodynamic Theranostics, ACS Appl. Mater. Interfaces, 2023, 15(35), 41224–41236 CrossRef CAS PubMed.
- A. A. P. Mansur, H. S. Mansur, A. G. Leonel, I. C. Carvalho, M. C. G. Lage and S. M. Carvalho, et al., Supramolecular magnetonanohybrids for multimodal targeted therapy of triple-negative breast cancer cells, J. Mater. Chem. B, 2020, 8(32), 7166–7188 RSC.
- S. Ali, S. Sikdar, S. Basak, D. Roy, D. Das and M. S. Haydar, et al., Intrinsic Light-Activated Oxidase Mimicking Activity of Conductive Polyaniline Nanofibers: A Class of Metal-Free Nanozyme, ACS Appl. Bio Mater., 2022, 5(12), 5518–5531 CrossRef CAS PubMed.
- M. Chang, Z. Hou, M. Wang, C. Yang, R. Wang and F. Li, et al., Single-Atom Pd Nanozyme for Ferroptosis-Boosted Mild-Temperature Photothermal Therapy, Angew. Chem., Int. Ed., 2021, 60(23), 12971–12979 CrossRef CAS PubMed.
- D. Liang, Y. Yang, G. Li, Q. Wang, H. Chen and X. Deng, Endogenous H(2)O(2)-Sensitive and Weak Acidic pH-Triggered Nitrogen-Doped Graphene Nanoparticles (N-GNMs) in the Tumor Microenvironment Serve as Peroxidase-Mimicking Nanozymes for Tumor-Specific Treatment, Materials, 2021, 14(8), 1933 CrossRef CAS PubMed.
- B. Chen, C. Zhang, W. Wang, Z. Chu, Z. Zha and X. He, et al., Ultrastable AgBiS(2) Hollow Nanospheres with Cancer Cell-Specific Cytotoxicity for Multimodal Tumor Therapy, ACS Nano, 2020, 14(11), 14919–14928 CrossRef CAS PubMed.
- X. Hu, F. Li, F. Xia, X. Guo, N. Wang and L. Liang, et al., Biodegradation-Mediated Enzymatic Activity-Tunable Molybdenum Oxide Nanourchins for Tumor-Specific Cascade Catalytic Therapy, J. Am. Chem. Soc., 2020, 142(3), 1636–1644 CrossRef CAS PubMed.
- H. T. Nia, L. L. Munn and R. K. Jain, Physical traits of cancer, Science, 2020, 370(6516), eaaz0868 CrossRef CAS PubMed.
- N. N. Pavlova, J. Zhu and C. B. Thompson, The hallmarks of cancer metabolism: Still emerging, Cell Metab., 2022, 34(3), 355–377 CrossRef CAS PubMed.
- L. E. Navas and A. Carnero, NAD+ metabolism, stemness, the immune response, and cancer, Signal Transduction Targeted Ther., 2021, 6(1), 2 CrossRef CAS PubMed.
- M. Jaganjac, L. Milkovic, S. B. Sunjic and N. Zarkovic, The NRF2, Thioredoxin, and Glutathione System in Tumorigenesis and Anticancer Therapies, Antioxidants, 2020, 9(11), 1151 CrossRef CAS PubMed.
- Y. Liu, B. Wang, J. Zhu, X. Xu, B. Zhou and Y. Yang, Single-Atom Nanozyme with Asymmetric Electron Distribution for Tumor Catalytic Therapy by Disrupting Tumor Redox and Energy Metabolism Homeostasis, Adv. Mater., 2023, 35(9), 2208512 CrossRef CAS PubMed.
- E. Panieri and M. M. Santoro, ROS homeostasis and metabolism: a dangerous liason in cancer cells, Cell Death Dis., 2016, 7(6), e2253 CrossRef CAS PubMed.
- Y. Sang, F. Cao, W. Li, L. Zhang, Y. You and Q. Deng, et al., Bioinspired Construction of a Nanozyme-Based H(2)O(2) Homeostasis Disruptor for Intensive Chemodynamic Therapy, J. Am. Chem. Soc., 2020, 142(11), 5177–5183 CrossRef CAS PubMed.
- S. He, Y. Feng, Q. Sun, Z. Xu and W. Zhang, Charge-Switchable CuxO Nanozyme with Peroxidase and Near-Infrared Light Enhanced Photothermal Activity for Wound Antibacterial Application, ACS Appl. Mater. Interfaces, 2022, 14(22), 25042–25049 CrossRef CAS PubMed.
- C. Keum, C. M. Hirschbiegel, S. Chakraborty, S. Jin, Y. Jeong and V. M. Rotello, Biomimetic and bioorthogonal nanozymes for biomedical applications, Nano Convergence, 2023, 10(1), 42 CrossRef CAS PubMed.
- X. Zhang, Y. Liu, S. Gopalakrishnan, L. Castellanos-Garcia, G. Li and M. Malassiné, et al., Intracellular Activation of Bioorthogonal Nanozymes through Endosomal Proteolysis of the Protein Corona, ACS Nano, 2020, 14(4), 4767–4773 CrossRef CAS PubMed.
- Z. Sun, Q. Liu, X. Wang, J. Wu, X. Hu and M. Liu, et al., Bioorthogonal catalytic nanozyme-mediated lysosomal membrane leakage for targeted drug delivery, Theranostics, 2022, 12(3), 1132–1147 CrossRef CAS PubMed.
- W. Wang, X. Zhang, R. Huang, C.-M. Hirschbiegel, H. Wang and Y. Ding, et al., In situ activation of therapeutics through bioorthogonal catalysis, Adv. Drug Delivery Rev., 2021, 176, 113893 CrossRef CAS PubMed.
- Y. Zhang, L. Zhang, W. Wang, Q. Deng, M. Liu and Z. Zhu, et al., A DNA-Gated and Self-Protected Bioorthogonal Catalyst for Nanozyme-Assisted Safe Cancer Therapy, Angew. Chem., Int. Ed., 2023, 62(32), 202306395 CrossRef PubMed.
- Y. Chang, X. Wu, S. Lu, J. Du, Y. Long and Y. Zhu, et al., Engineered procyanidin-Fe nanoparticle alleviates intestinal inflammation through scavenging ROS and altering gut microbiome in colitis mice, Front. Chem., 2023, 11, 1089775 CrossRef CAS PubMed.
- X. Qian, R. Shi, J. Chen, Y. Wang, X. Han and Y. Sun, et al., The single-atom iron nanozyme mimicking peroxidase remodels energy metabolism and tumor immune landscape for synergistic chemodynamic therapy and photothermal therapy of triple-negative breast cancer, Front. Bioeng. Biotechnol., 2022, 10, 1026761 CrossRef PubMed.
- G. W. Tormoen, M. R. Crittenden and M. J. Gough, Role of the immunosuppressive microenvironment in immunotherapy, Adv. Radiat. Oncol., 2018, 3(4), 520–526 CrossRef PubMed.
- E. Cendrowicz, Z. Sas, E. Bremer and T. P. Rygiel, The Role of Macrophages in Cancer Development and Therapy, Cancers, 2021, 13(8), 1946 CrossRef CAS PubMed.
- M. A. M. Ayala, Z. Li and M. DuPage, Treg programming and therapeutic reprogramming in cancer, Immunology, 2019, 157(3), 198–209 CrossRef PubMed.
- I. Manoharan, P. D. Prasad, M. Thangaraju and S. Manicassamy, Lactate-Dependent Regulation of Immune Responses by Dendritic Cells and Macrophages, Front. Immunol., 2021, 12, 691134 CrossRef CAS PubMed.
- X. Li, Y. Yang, B. Zhang, X. Lin, X. Fu and Y. An, et al., Lactate metabolism in human health and disease, Signal Transduction Targeted Ther., 2022, 7(1), 305 CrossRef CAS PubMed.
- H. Wang, B. Wang, J. Jiang, Y. Wu, A. Song and X. Wang, et al., SnSe Nanosheets Mimic Lactate Dehydrogenase to Reverse Tumor Acid Microenvironment Metabolism for Enhancement of Tumor Therapy, Molecules, 2022, 27(23), 8552 CrossRef CAS PubMed.
- J. Barar and Y. Omidi, Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy, BioImpacts, 2013, 3(4), 149–162 Search PubMed.
- D. Yang, M. Ding, Y. Song, Y. Hu, W. Xiu and L. Yuwen, et al., Nanotherapeutics with immunoregulatory functions for the treatment of bacterial infection, Biomater. Res., 2023, 27(1), 73 CrossRef PubMed.
- R. L. Siegel, K. D. Miller, H. E. Fuchs and A. Jemal, Cancer statistics, 2022, CA-Cancer J. Clin., 2022, 72(1), 7–33 CrossRef PubMed.
- A. Ashrafi, Z. Akter, P. Modareszadeh, P. Modareszadeh, E. Berisha and P. S. Alemi, et al., Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance, Cancers, 2022, 14(19), 4562 CrossRef CAS PubMed.
- F. Duan, Q. Jia, G. Liang, M. Wang, L. Zhu, K. J. McHugh, L. Jing, M. Du and Z. Zhang, Schottky Junction Nanozyme Based on Mn-Bridged Co-Phthalocyanines and Ti3C2Tx Nanosheets Boosts Integrative Type I and II Photosensitization for Multimodal Cancer Therapy, ACS Nano, 2023, 17(12), 11290–11308 CrossRef CAS PubMed.
- O. A. Martin, R. L. Anderson, K. Narayan and M. P. MacManus, Does the mobilization of circulating tumour cells during cancer therapy cause metastasis?, Nat. Rev. Clin. Oncol., 2017, 14(1), 32–44 CrossRef CAS PubMed.
- M. Z. Zou, W. L. Liu, H. S. Chen, X. F. Bai, F. Gao and J. J. Ye, et al., Advances in nanomaterials for treatment of hypoxic tumor, Natl. Sci. Rev., 2021, 8(2), nwaa160 CrossRef CAS PubMed.
- F. Gao, J. Wu, H. Gao, X. Hu, L. Liu and A. C. Midgley, et al., Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics, Biomaterials, 2020, 230, 119635 CrossRef CAS PubMed.
- L. Zeng, Y. Han, Z. Chen, K. Jiang, D. Golberg and Q. Weng, Biodegradable and Peroxidase-Mimetic Boron Oxynitride Nanozyme for Breast Cancer Therapy, Adv. Sci., 2021, 8(16), 2101184 CrossRef CAS PubMed.
- T. F. Cloughesy, A. Y. Mochizuki, J. R. Orpilla, W. Hugo, A. H. Lee and T. B. Davidson, et al., Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma, Nat. Med., 2019, 25(3), 477–486 CrossRef CAS PubMed.
- D. Nie, Y. Ling, W. Lv, Q. Liu, S. Deng and J. Shi, et al., In Situ Attached Photothermal Immunomodulation-Enhanced Nanozyme for the Inhibition of Postoperative Malignant Glioma Recurrence, ACS Nano, 2023, 17(14), 13885–13902 CrossRef CAS PubMed.
- S. M. Carvalho, A. A. P. Mansur, I. B. da Silveira, T. F. S. Pires, H. F. V. Victória and K. Krambrock, et al., Nanozymes with Peroxidase-like Activity for Ferroptosis-Driven Biocatalytic Nanotherapeutics of Glioblastoma Cancer: 2D and 3D Spheroids Models, Pharmaceutics, 2023, 15(6), 1702 CrossRef CAS PubMed.
- J. Wu, Q. Li and X. Fu, Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity, Transl. Oncol., 2019, 12(6), 846–851 CrossRef PubMed.
- S. Bullman, C. S. Pedamallu, E. Sicinska, T. E. Clancy, X. Zhang and D. Cai, et al., Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer, Science, 2017, 358(6369), 1443–1448 CrossRef CAS PubMed.
- X. Wang, Q. Chen, Y. Zhu, K. Wang, Y. Chang and X. Wu, et al., Destroying pathogen-tumor symbionts synergizing with catalytic therapy of colorectal cancer by biomimetic protein-supported single-atom nanozyme, Signal Transduction Targeted Ther., 2023, 8(1), 277 CrossRef CAS PubMed.
- X. Zhu, Y. Gong, Y. Liu, C. Yang, S. Wu and G. Yuan, et al., Ru@CeO(2) yolk shell nanozymes: Oxygen supply in situ enhanced dual chemotherapy combined with photothermal therapy for orthotopic/subcutaneous colorectal cancer, Biomaterials, 2020, 242, 119923 CrossRef CAS PubMed.
- V. Bhandari, C. Hoey, L. Y. Liu, E. Lalonde, J. Ray and J. Livingstone, et al., Molecular landmarks of tumor hypoxia across cancer types, Nat. Genet., 2019, 51(2), 308–318 CrossRef CAS PubMed.
- C. Dong, X. Dai, X. Wang, Q. Lu, L. Chen and X. Song, et al., A Calcium Fluoride Nanozyme for Ultrasound-Amplified and Ca2+ -Overload-Enhanced Catalytic Tumor Nanotherapy, Adv. Mater., 2022, 34(43), 2205680 CrossRef CAS PubMed.
- W. L. Tsai and R. T. Chung, Viral hepatocarcinogenesis, Oncogene, 2010, 29(16), 2309–2324 CrossRef CAS PubMed.
- Z. Wang, H. Liu, S. H. Yang, T. Wang, C. Liu and Y. C. Cao, Nanoparticle-based artificial RNA silencing machinery for antiviral therapy, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(31), 12387–12392 CrossRef CAS PubMed.
- K. Boriachek, M. N. Islam, A. Möller, C. Salomon, N. T. Nguyen and M. S. A. Hossain, et al., Biological Functions and Current Advances in Isolation and Detection Strategies for Exosome Nanovesicles, Small, 2018, 14(6), 1702153 CrossRef PubMed.
- H. Di, Z. Mi, Y. Sun, X. Liu, X. Liu and A. Li, et al., Nanozyme-assisted sensitive profiling of exosomal proteins for rapid cancer diagnosis, Theranostics, 2020, 10(20), 9303–9314 CrossRef CAS PubMed.
- J. Ritter and S. S. Bielack, Osteosarcoma, Ann. Oncol., 2010, 21(Suppl 7), vii320–vii325 CrossRef PubMed.
- Y. Liang, C. Liao, X. Guo, G. Li, X. Yang and J. Yu, et al., RhRu Alloy-Anchored MXene Nanozyme for Synergistic Osteosarcoma Therapy, Small, 2023, 19(22), 2205511 CrossRef CAS PubMed.
- L. Fania, D. Didona, F. R. Di Pietro, S. Verkhovskaia, R. Morese and G. Paolino, et al., Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches, Biomedicines, 2021, 9(2), 171 CrossRef CAS PubMed.
- E. Ju, M. Peng, Y. Xu, Y. Wang, F. Zhou and H. Wang, et al., Nanozyme-integrated microneedle patch for enhanced therapy of cutaneous squamous cell carcinoma by breaking the gap between H2O2 self-supplying chemodynamic therapy and photothermal therapy, J. Mater. Chem. B, 2023, 11(28), 6595–6602 RSC.
- C. Holohan, S. Van Schaeybroeck, D. B. Longley and P. G. Johnston, Cancer drug resistance: an evolving paradigm, Nat. Rev. Cancer, 2013, 13(10), 714–726 CrossRef CAS PubMed.
- T. D. Eubank, R. D. Roberts, M. Khan, J. M. Curry, G. J. Nuovo and P. Kuppusamy, et al., Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages, Cancer Res., 2009, 69(5), 2133–2140 CrossRef CAS PubMed.
- K. Bukowski, M. Kciuk and R. Kontek, Mechanisms of Multidrug Resistance in Cancer Chemotherapy, Int. J. Mol. Sci., 2020, 21(9), 3233 CrossRef CAS PubMed.
- M. Kartal-Yandim, A. Adan-Gokbulut and Y. Baran, Molecular mechanisms of drug resistance and its reversal in cancer, Crit. Rev. Biotechnol., 2016, 36(4), 716–726 CrossRef CAS PubMed.
- M. Tang, Z. Zhang, C. Ding, J. Li, Y. Shi and T. Sun, et al., Two birds with one stone: innovative ceria-loaded gold@platinum nanospheres for photothermal-catalytic therapy of tumors, J. Colloid Interface Sci., 2022, 627, 299–307 CrossRef CAS PubMed.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nanocarriers as an emerging platform for cancer therapy, Nat. Nanotechnol., 2007, 2(12), 751–760 CrossRef CAS PubMed.
- G. Chen, I. Roy, C. Yang and P. N. Prasad, Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy, Chem. Rev., 2016, 116(5), 2826–2885 CrossRef CAS PubMed.
- Q. Chen, C. Liang, X. Sun, J. Chen, Z. Yang and H. Zhao, et al., H2O2-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay, Proc. Natl. Acad. Sci. U. S. A., 2017, 114(21), 5343–5348 CrossRef CAS PubMed.
- M. Nishikawa, A. Tamada, H. Kumai, F. Yamashita and M. Hashida, Inhibition of experimental pulmonary metastasis by controlling biodistribution of catalase in mice, Int. J. Cancer, 2002, 99(3), 474–479 CrossRef CAS PubMed.
- T. Y. Reynolds, S. Rockwell and P. M. Glazer, Genetic instability induced by the tumor microenvironment, Cancer Res., 1996, 56(24), 5754–5757 CAS.
- T. L. Whiteside, The tumor microenvironment and its role in promoting tumor growth, Oncogene, 2008, 27(45), 5904–5912 CrossRef CAS PubMed.
- S. Thangudu and C.-H. Su, Peroxidase Mimetic Nanozymes in Cancer Phototherapy: Progress and Perspectives, Biomolecules, 2021, 11(7), 1015 CrossRef CAS PubMed.
- A. Sahu, I. Kwon and G. Tae, Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia, Biomaterials, 2020, 228, 119578 CrossRef CAS PubMed.
- J. Li, C. Gong, X. Chen, H. Guo, Z. Tai and N. Ding, et al., Biomimetic liposomal nanozymes improve breast cancer chemotherapy with enhanced penetration and alleviated hypoxia, J. Nanobiotechnol., 2023, 21(1), 123 CrossRef CAS PubMed.
- X. Fan, X. Gong, F. Zhou, B. Chen, S. Tan and H. Xu, et al., Mesoporous peroxidase nanozyme for synergistic chemodynamic therapy and chemotherapy, Colloids Surf., B, 2022, 216, 112603 CrossRef CAS PubMed.
- P. H. Wu, P. F. Cheng, W. Kaveevivitchai and T. H. Chen, MOF-based nanozyme grafted with cooperative Pt(IV) prodrug for synergistic anticancer therapy, Colloids Surf., B, 2023, 225, 113264 CrossRef CAS PubMed.
- B. Du, M. Zheng, H. Ma, J. Huang, Q. Jiao and Y. Bai, et al., Nanozyme-natural enzymes cascade catalyze cholesterol consumption and reverse cancer multidrug resistance, J. Nanobiotechnol., 2022, 20(1), 209 CrossRef CAS PubMed.
- H. Yu, K. Tang, Z. Cai, X. Lin, Y. Huang and T. Yu, et al., Carbon Dots-Based Nanozyme for Drug-Resistant Lung Cancer Therapy by Encapsulated Doxorubicin/siRNA Cocktail, Int. J. Nanomed., 2023, 18, 933–948 CrossRef CAS PubMed.
- C. Zhang, L. Yan, Z. Gu and Y. Zhao, Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy, Chem. Sci., 2019, 10(29), 6932–6943 RSC.
- R. Serra-Maia, M. Bellier, S. Chastka, K. Tranhuu, A. Subowo and J. D. Rimstidt, et al., Mechanism and Kinetics of Hydrogen Peroxide Decomposition on Platinum Nanocatalysts, ACS Appl. Mater. Interfaces, 2018, 10(25), 21224–21234 CrossRef CAS PubMed.
- Y. Dou, Y. Liu, F. Zhao, Y. Guo, X. Li and M. Wu, et al., Radiation-responsive scintillating nanotheranostics for reduced hypoxic radioresistance under ROS/NO-mediated tumor microenvironment regulation, Theranostics, 2018, 8(21), 5870–5889 CrossRef CAS PubMed.
- L. Tang, F. Wei, Y. Wu, Y. He, L. Shi and F. Xiong, et al., Role of metabolism in cancer cell radioresistance and radiosensitization methods, J. Exp. Clin. Cancer Res., 2018, 37(1), 87 CrossRef PubMed.
- L.-L. Zhou, Q. Guan, W. Zhou, J.-L. Kan, K. Teng and M. Hu, et al., A Multifunctional Covalent Organic Framework Nanozyme for Promoting Ferroptotic Radiotherapy against Esophageal Cancer, ACS Nano, 2023, 17(20), 20445–20461 CrossRef CAS PubMed.
- A. K. Holley, L. Miao, D. K. St Clair and W. H. St Clair, Redox-modulated phenomena and radiation therapy: the central role of superoxide dismutases, Antioxid. Redox Signaling, 2014, 20(10), 1567–1589 CrossRef CAS PubMed.
- A. Menegakis, R. Klompmaker, C. Vennin, A. Arbusà, M. Damen and B. van den Broek, et al., Resistance of Hypoxic Cells to Ionizing Radiation Is Mediated in Part via Hypoxia-Induced Quiescence, Cells, 2021, 10(3), 610 CrossRef CAS PubMed.
- Z. Yuan, X. Liu, J. Ling, G. Huang, J. Huang and X. Zhu, et al., In situ-transition nanozyme triggered by tumor microenvironment boosts synergistic cancer radio-/chemotherapy through disrupting redox homeostasis, Biomaterials, 2022, 287, 121620 CrossRef CAS PubMed.
- J. Zhang, Y. Liu, X. Wang, J. Du, K. Song and B. Li, et al., Nanozyme-Incorporated Biodegradable Bismuth Mesoporous Radiosensitizer for Tumor Microenvironment-Modulated Hypoxic Tumor Thermoradiotherapy, ACS Appl. Mater. Interfaces, 2020, 12(52), 57768–57781 CrossRef CAS PubMed.
- Y. Li, K. H. Yun, H. Lee, S. H. Goh, Y. G. Suh and Y. Choi, Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo, Biomaterials, 2019, 197, 12–19 CrossRef CAS PubMed.
- F. Rodríguez, P. Caruana, N. Fuente, P. Español, M. Gámez and J. Balart, et al., Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges, Biomolecules, 2022, 12, 784 CrossRef PubMed.
- P. Qi, C. Luo, Y. Pan, S. Ding, X. Li and K. Qiao, et al., Self-cascade catalytic single-atom nanozyme for enhanced breast cancer low-dose radiotherapy, Colloids Surf., B, 2023, 227, 113347 CrossRef CAS PubMed.
- B. Hu, X. Xiao, P. Chen, J. Qian, G. Yuan and Y. Ye, et al., Enhancing anti-tumor effect of ultrasensitive bimetallic RuCu nanoparticles as radiosensitizers with dual enzyme-like activities, Biomaterials, 2022, 290, 121811 CrossRef CAS PubMed.
- C. Huang, Z. Liu, M. Chen, L. Du, C. Liu and S. Wang, et al., Tumor-derived biomimetic nanozyme with immune evasion ability for synergistically enhanced low dose radiotherapy, J. Nanobiotechnol., 2021, 19(1), 457 CrossRef CAS PubMed.
- X. Zhou, M. You, F. Wang, Z. Wang, X. Gao and C. Jing, et al., Multifunctional Graphdiyne-Cerium Oxide Nanozymes Facilitate MicroRNA Delivery and Attenuate Tumor Hypoxia for Highly Efficient Radiotherapy of Esophageal Cancer, Adv. Mater., 2021, 33(24), 2100556 CrossRef CAS PubMed.
- D. Lindau, P. Gielen, M. Kroesen, P. Wesseling and G. J. Adema, The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells, Immunology, 2013, 138(2), 105–115 CrossRef CAS PubMed.
- B. Xu, Y. Cui, W. Wang, S. Li, C. Lyu and S. Wang, et al., Immunomodulation-Enhanced Nanozyme-Based Tumor Catalytic Therapy, Adv. Mater., 2020, 32(33), 2003563 CrossRef CAS PubMed.
- T. T. Nguyen, E. Y. Chuang, Y. P. Chen, P. C. Tseng, M. K. Jhan and C. Y. Lai, et al., Anticancer polypyrrole-polyethylenimine drug-free nanozyme for precise B-cell lymphoma therapy, Biomed. Pharmacother., 2023, 160, 114397 CrossRef CAS PubMed.
- Y. Zou, B. Jin, H. Li, X. Wu, Y. Liu and H. Zhao, et al., Cold Nanozyme for Precise Enzymatic Antitumor Immunity, ACS Nano, 2022, 16(12), 21491–21504 CrossRef CAS PubMed.
- C. Zhang, W. Bu, D. Ni, S. Zhang, Q. Li and Z. Yao, et al., Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction, Angew. Chem., Int. Ed., 2016, 55(6), 2101–2106 CrossRef CAS PubMed.
- S. Li, L. Shang, B. Xu, S. Wang, K. Gu and Q. Wu, et al., A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy, Angew. Chem., Int. Ed., 2019, 58(36), 12624–12631 CrossRef CAS PubMed.
- L. Zhu, J. Liu., G. Zhou, T.-M. Liu, Y. Dai, G. Nie and Q. Zhao, Remodeling of Tumor Microenvironment by Tumor-Targeting Nanozymes Enhances Immune Activation of CAR T Cells for Combination Therapy, Small, 2021, 17(43), 2102624 CrossRef CAS PubMed.
- C. Cao, N. Yang, Y. Su, Z. Zhang, C. Wang and X. Song, et al., Starvation, Ferroptosis, and Prodrug Therapy Synergistically Enabled by a Cytochrome c Oxidase like Nanozyme, Adv. Mater., 2022, 34(29), 2203236 CrossRef CAS PubMed.
- X. Yang, Y. Yang, F. Gao, J. J. Wei, C. G. Qian and M. J. Sun, Biomimetic Hybrid Nanozymes with Self-Supplied H+ and Accelerated O2 Generation for Enhanced Starvation and Photodynamic Therapy against Hypoxic Tumors, Nano Lett., 2019, 19(7), 4334–4342 CrossRef CAS PubMed.
- X. Li, H. Zhu, P. Liu, M. Wang, J. Pan and F. Qiu, et al., Realizing selective detection with nanozymes: Strategies and trends, TrAC, Trends Anal. Chem., 2021, 143, 116379 CrossRef CAS.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li and Y. Zhu, et al., Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II), Chem. Soc. Rev., 2019, 48(4), 1004–1076 RSC.
- Y. Huang, J. C. Hsu, H. Koo and D. P. Cormode, Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle, Theranostics, 2022, 12(2), 796–816 CrossRef CAS PubMed.
- W. Feng, L. Chen, M. Qin, X. Zhou, Q. Zhang and Y. Miao, et al., Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy, Sci. Rep., 2015, 5(1), 17422 CrossRef CAS PubMed.
- L. Teng, X. Han, Y. Liu, C. Lu, B. Yin and S. Huan, et al., Smart Nanozyme Platform with Activity-Correlated Ratiometric Molecular Imaging for Predicting Therapeutic Effects, Angew. Chem., Int. Ed., 2021, 60(50), 26142–26150 CrossRef CAS PubMed.
- X. Chang, Q. Wu, Y. Wu, X. Xi, J. Cao and H. Chu, et al., Multifunctional Au Modified Ti(3)C(2)-MXene for Photothermal/Enzyme Dynamic/Immune Synergistic Therapy, Nano Lett., 2022, 22(20), 8321–8330 CrossRef CAS PubMed.
- J. Jumper, R. Evans, A. Pritzel, T. Green, M. Figurnov and O. Ronneberger, et al., Highly accurate protein structure prediction with AlphaFold, Nature, 2021, 596(7873), 583–589 CrossRef CAS PubMed.
- J. Zhuang, A. C. Midgley, Y. Wei, Q. Liu, D. Kong and X. Huang, Machine-Learning-Assisted Nanozyme Design: Lessons from Materials and Engineered Enzymes, Adv. Mater., 2024, 36(10), 2210848 CrossRef CAS PubMed.
- Y. Li, R. Zhang, X. Yan and K. Fan, Machine learning facilitating the rational design of nanozymes, J. Mater. Chem. B, 2023, 11(28), 6466–6477 RSC.
- Y. Wei, J. Wu, Y. Wu, H. Liu, F. Meng and Q. Liu, et al., Prediction and Design of Nanozymes using Explainable Machine Learning, Adv. Mater., 2022, 34(27), 2201736 CrossRef CAS PubMed.
- Y. Yu, Y. Jiang, C. Zhang, Q. Bai, F. Fu and S. Li, et al., Machine Learning Assisted Graphdiyne-Based Nanozyme Discovery, ACS Mater. Lett., 2022, 4, 2134–2142 CrossRef CAS.
- Z. W. Ulissi, A. J. Medford, T. Bligaard and J. K. Nørskov, To address surface reaction network complexity using scaling relations machine learning and DFT calculations, Nat. Commun., 2017, 8, 14621 CrossRef PubMed.
- F. A. Faber, L. Hutchison, B. Huang, J. Gilmer, S. S. Schoenholz and G. E. Dahl, et al., Prediction Errors of Molecular Machine Learning Models Lower than Hybrid DFT Error, J. Chem. Theory Comput., 2017, 13(11), 5255–5264 CrossRef CAS PubMed.
- X. J. Gao, J. Yan, J.-J. Zheng, S. Zhong and X. Gao, Clear-Box Machine Learning for Virtual Screening of 2D Nanozymes to Target Tumor Hydrogen Peroxide, Adv. Healthc. Mater., 2023, 12(10), 2202925 CrossRef CAS PubMed.
- X.-T. Li, L. Chen, C. Shang and Z.-P. Liu, In Situ Surface Structures of PdAg Catalyst and Their Influence on Acetylene Semihydrogenation Revealed by Machine Learning and Experiment, J. Am. Chem. Soc., 2021, 143(16), 6281–6292 CrossRef CAS PubMed.
Footnote |
| † Ke Xu, Yujie Cui and Bin Guan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |

