Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorization of plastic waste via chemical activation and carbonization into activated carbon for functional material applications
Rachel
Blanchard
and
Tizazu H.
Mekonnen
 *
*
Department of Chemical Engineering, Institute of Polymer Research, Waterloo Institute of Nanotechnology, University of Waterloo, N2V 0E6, Waterloo, ON, Canada. E-mail: tmekonnen@uwaterloo.ca
First published on 19th March 2024
Abstract
Addressing the complex issue of plastic waste disposal requires a nuanced approach, as no single solution proves universally effective. This review advocates for a comprehensive strategy, combining mechanical recycling and chemical methods to manage plastic waste while emphasizing the transformative potential of carbonization and activation processes specifically. With a focus on chemical activation, this review explores the synthesis of high surface area activated carbon (AC) from diverse plastic sources including polyesters (e.g., polyethylene terephthalate), polyolefins (e.g., polyethylene, polypropylene), and non-recyclable thermoset resins (e.g., epoxy, phenolics). The resulting AC products exhibit notable potential, with high surface areas exceeding 2000 m2 g−1 in some cases. Furthermore, the adsorptive behavior of the plastic derived ACs are discussed with respect to common pollutants such as dyes and CO2 in addition to emerging pollutants, such as micro/nano-plastics. Overall, this work highlights carbonization and chemical activation as important upcycling methods for plastic wastes that may otherwise end up in landfills or spills into the environment. Given the urgency of plastic waste disposal, it is recommended that the feasibility and scalability of plastic-derived AC production is explored in future work for the potential replacement of conventional AC feedstocks derived from coal or biomass.
1. Introduction
The use of plastic products has been continuously increasing due to their lightweight, appealing cost structure, ease of processing, durability, and flexibility for various applications.1 They are mass produced from hydrocarbons refined from petroleum, using coal powered plants resulting in large carbon footprints. Additionally, they do not biodegrade in the natural environment in a reasonable time frame. As a result, greenhouse gas (GHG) emissions from plastic production are rising in conjunction with plastic waste accumulation in landfills and spill to the environment accelerated by the quick disposal of most products after a single use consuming the global carbon budget.2 Though plastics do not totally degrade in a short time, they can undergo fragmentation due to environmental factors causing the formation of microplastics3 and even nano-plastics.4 These highly mobile plastic fragments pollute marine environments, agricultural ecosystems, and other terrestrial and freshwater systems4,5 which can affect drinking water sources. Therefore, the effective recycling of all plastics and alleviation of this pollution is a major topic of discussion.Currently, common methods used for plastic waste management include landfilling, incineration, mechanical recycling, chemical recycling, and thermal cracking.2 Recycling mainly refers to thermo-mechanical recycling, in which plastics are collected, sorted, cleaned, grinded, extruded and pelletized to form new products. This method helps extend the lifetime of plastics, but the resulting decline of product properties limits its continued product value. Both landfilling and incineration are also widely used disposal options but cause burdens on the environment due to the negative effects on soil environments and air pollution respectively. Thermal cracking to form fuels and valuable chemicals is a type of chemical recycling, which has received substantial interest, helps to reutilize plastics while favoring reduced emissions.2 This review highlights an alternate method of reutilizing plastic waste by controlled carbonization to produce high value carbonaceous products. Carbonization differs from thermal cracking through pyrolysis as it focuses on the production of solid residue with high carbon content1 as opposed to liquid or volatile fractions which are major pyrolysis products (oil and gas).6 It can generate valuable products such as carbon nanomaterials, carbon fibers, adsorbents and energy storage devices.1,2 A particular product of interest in this work is activated carbon (AC), which is differentiated through additional activation processes to develop a high surface area.
Because the conversion of polymers to carbonaceous products has drawn attention in recent years, a variety of reviews have covered this topic. Chen et al. (2020) provided an overview of the types of carbonizations and the methods used for various plastics in addition to analyzing its feasibility as a plastic waste disposal process.7 Another review by Gong et al. (2019) discussed the conversion of plastic waste to carbon but focused more on the production of carbon nanomaterials,8 as was also the focus of a separate review by Zhuo et al. (2014).9 Choi et al. (2022) covered the upcycling of plastic waste more broadly by addressing processes other than carbonization in addition to the applications of these products.1 The content of this review is centered around the conversion of plastic into carbon-based products but with a focus specifically on the production of high surface area AC ideal for application as an adsorbent. Unlike the review by Choi et al. (2022), this work does not solely speak on the upcycling of plastic to carbon products but also touches on the existing recycling methods and where carbonization lies within this framework.
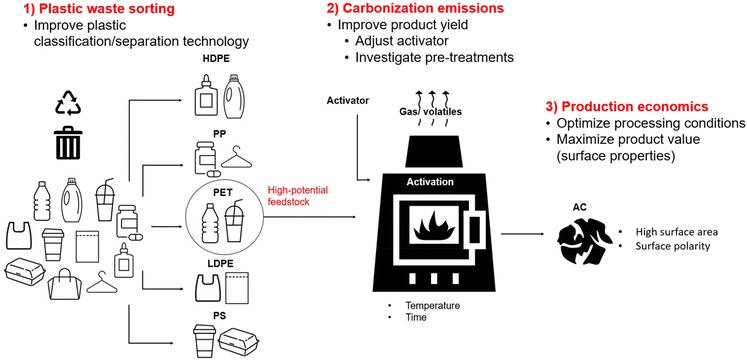
To highlight the need for alternative treatment options like carbonization, this work begins with a discussion of plastic recycling and upcycling methods in relation to key recycling challenges (section 2). Section 3 focuses on the carbonization of plastics, including the various carbon products obtained from plastic precursors and the pre-treatments necessary for the conversion. This is followed by an overview of the activation of plastics to produce high surface area AC (section 4) and an in-depth review of AC production from common plastics (section 5), with a focus on chemical activation processes. Some key applications of these plastic derived AC adsorbents are also reviewed in section 6 to emphasize the potential impact of these high-capacity products, followed by key future prospectives in section 7.
2. Recycling and upcycling of plastic waste
There are a variety of methods for reutilization of plastic waste, which generally can be categorized into recycling by mechanical methods, chemical methods and incineration for energy recovery. As shown in Fig. 1b, these can also be categorized according to ASTM D5033 definitions of primary, secondary tertiary and quaternary recycling. Primary recycling consists of mechanical recycling of products in a closed loop system, while secondary recycling is mechanical recycling into products with different purposes, often downgraded polymeric materials. Tertiary recycling refers to the use of waste polymers for generation of lower molecular weight materials such as monomers and valuable chemicals.10 This depolymerization can be carried out by numerous chemical methods such as hydrolysis, ammonolysis, pyrolysis etc. For the purpose of this study, chemical recycling was divided into solvolysis methods used for monomer regeneration, and pyrolysis to produce oil and gas. Furthermore, carbonization was identified separately from pyrolysis because the production of carbon materials (mainly activated carbon) will be the focus of this review. Lastly, a final resort is the quaternary recycling of plastics by combustion with recovery of energy.10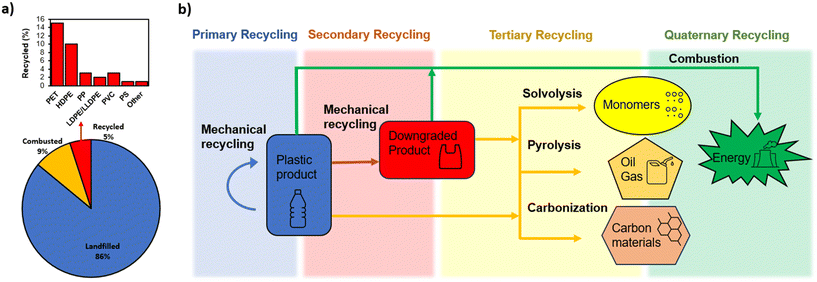 | ||
| Fig. 1 (a) Breakdown of plastic waste management pathways in the US in 2019. Data obtained from ref. 11; (b) schematic of various plastic recycling methods which are discussed and compared. | ||
Based on the breakdown of plastic waste disposal methods in the US (Fig. 1a), only a small proportion (5%) of plastic is recycled, although certain plastics such as PET are recycled at rates as high as 15%. This is due to the challenges associated with recycling, which will be discussed in this section and assessed with respect to the recycling methods outlined in Fig. 1b. Jung et al. (2023) outlined factors hindering recycling and upcycling of plastic waste. The major challenges include difficulty in the separation and classification of plastic wastes, variability of additives and coatings in various plastic streams, contaminations with food and other products, and the presence of thermoset polymers, which are incapable to be melted or dissolved.12 Therefore, the various recycling methods outlined in this section (Mechanical, solvolysis, pyrolysis, carbonization, and incineration) will be discussed in general and with respect to sorting issues, additives and contamination, and the processing of thermoset materials.
2.1. Mechanical recycling
The most widely employed recycling technique is the mechanical recycling by melt processing of used plastic waste to form new products. This is conducted through sorting, washing and drying, crushing and compounding. It is a relatively simple and economical recycling technique but is limited by various shortcomings. Mainly, the effects of heat, light, oxidation, and mechanical shear lead to degradation of plastic products during their lifetime and during the mechanical reprocessing.13 Additionally, post consumer plastics are usually laden with contaminants, co-blend partners, additives, and mixed with other plastics or non-plastics (e.g., paper), resulting in the need for additional costly washing, sorting, and separation processes. As a result, mechanical recycling can only be carried out for a few cycles. A very common example is poly(ethylene terephthalate) (PET) bottles, which are usually only recycled once into textiles. A small portion of mechanical recycling consists of primary recycling using the purest and cleanest streams while most mechanical recycling is downcycling.14 Therefore, the other recycling techniques illustrated in Fig. 1b (tertiary and quaternary) are required as complementary recycling options.One of the main factors contributing to the difficulty in achieving pure polymer products from mechanical recycling is obstacles associated with plastic waste sorting. Currently, there are methods for sorting different types of plastic such as Near infrared (NIR) and X-ray fluorescence based identification and sorting technology, which can identify polymers based on their unique spectrum leading to the subsequent separation process. There are also techniques to sort plastic granules based on their specific density in air (air sorting) and in fluid (sink float sorting). Electrostatic methods are employed to separate plastics according to their triboelectric charge in addition to melting of polymers with varying melting temperatures.15 Despite these techniques, they all have their limitations, such as NIR being ineffective for dark plastics,16 X-ray fluorescence being constrained to polymers that fluoresce and applied for the identification of limited polymers, such as PVC.17 Other challenges include the difficulty in controlling and maintaining the purity of density sorting techniques due to overlapping density ranges,18 the inability to sort coarse granules by triboelectrostatic methods,19 and the applicability of melt separation to two plastics of varying melt temperatures such as mixtures of polypropylene (Tm = 160 °C) and poly(ethylene terephthalate) (Tm = 260 °C).20 Therefore, sorting remains a challenging and time-consuming aspect of recycling.
For mechanical recycling, there is potential to produce blend polymers in systems where complete sorting is not possible.10 However, many polymers are immiscible and incompatible, such as polyolefins (PO) with polystyrene (PS) and must be compatibilized to produce stable blends. Compatibilization is commonly achieved using hydrogenated styrene butadiene rubber copolymers (e.g. SEBS), and other more cost-effective alternatives, such as styrene butadiene block copolymers, isotactic polybutene among others. For example, SEBS and PP grafted with styrene,21 styrene butadiene styrene (SBS),21 and ethylene-propylene-diene terpolymer (EPDM)-g-maleic anhydride and SEBS-g-MAH22 were used to stabilize mixed waste blends of plastics including PS, PP, PE, and PVC. Additionally, fillers are often required to maintain the physical properties including elongation, young's modulus and impact strength of recycled plastics and blends. For example, organic fillers such as starch, cellulose, lignin, chitin etc. are used as compatibilizers in blends.23 Other common fillers are cheap materials like glass fibre, CaCO3, and talc,24 in addition to nanoparticles, most notably modified montmorillonite clay.25–27
An important consideration when employing polymer blends to retain value from incomplete plastic sorting is that reprocessing is often not addressed. For this reason, the complete investigation of polymer blend degradation mechanisms and the influence of polymer blends on waste management systems is necessary.28 Secondly, not all plastics can be blended due to extreme incompatibility or variation in processing parameters. For example, PET and PVC can not be processed together because the high temperatures required for PET processing accelerates the dehydrochlorination degradation of PVC.29 This also means that mechanical recycling cannot be used for treatment of multilayer packaging materials due to the chemically incompatible layers.30
Next, additives are a big issue in mechanical recycling since most plastic products contain additives (e.g., impact modifiers, plasticizers, compatibilizers, pigments) to enhance material properties, such as stiffness, flexibility, thermal stability, and barrier properties.31,32 Additionally, fillers and other performance or processing enhancing additives are incorporated during the recycling process to overcome the reduced physical properties of recycled materials.13,33 However, the use of filler and additives must be planned cautiously because their presence can increase the processing viscosity, causing increased risk to equipment and greater energy requirements. In some cases, the filler may even need to be removed before recycling due to differing recyclability of filler compared to the polymer.28
Contamination also complicates the recycling process and decrease the final product quality. For example, pigments can accelerate plastic degradation during extrusion, ink components from labels introduce volatile components, and lubricants used on plastic bags can produce unwanted odors.34–36 The effects of these contaminants are often combatted by introducing virgin polymer into the recycling stream, which is employed for PET bottles with a virgin to recyclate ratio of 70/30.37 Other methods to improve the quality of recycled PET is through molecular weight improvement using solid state post condensation38 and chain extenders.39
Additives and contaminants may also be dealt with extraction or dissolution and precipitation methods. During extraction, the waste plastic is washed by an appropriate solvent or supercritical fluid, while the dissolution/precipitation method consists of dissolving the polymer to separate it from insoluble impurities followed by precipitation in antisolvents.14 Despite these existing techniques, the reduction in quality due to additives, inks and remaining traces of incompatible polymer is an inevitable occurrence. This contributes to the stream of plastic waste that must be downgraded to products of less demanding quality (plastic containers, wood plastic composites for fences, agricultural applications, such as silage wraps and mulch films),34 as shown by the secondary recycling in Fig. 1b.
Lastly, the processing of thermoset plastic wastes must be discussed. Thermoset plastics such as epoxies, polyurethanes, silicones, and polyesters account for around 12% of the global plastic production and is projected to grow over time.40 However, they cannot undergo melt processing due to the covalent bond between the thermoset chains leading to most thermoset wastes being incinerated, sent to landfills or grinded for use as filler.41,42 With respect to mechanical recycling, the only method that can be employed for thermoset plastics is pulverization into powder to be used as fillers in thermoplastic and thermoset polymers.43 Although the thermoset waste must be cleaned and sorted before processing, this method is economical and therefore being used commercially albeit as a small scale.44 Overall, recycling of thermoset plastic waste by mechanical recycling is extremely limited and therefore requires chemical methods for full reutilization. An example of a successful commercial recycling technology for thermoset wastes is the devulcanization of waste tires by critical CO2, introduced by Tyromer in Canada.45
2.2. Chemical recycling (solvolysis)
Because mechanical recycling can only be used for a fraction of plastic markets, chemical recycling is necessary in cases where mechanical recycling can not deliver the required mechanical performance or purity. As shown in Fig. 2a, some plastics are more easily depolymerized into monomers while others can only be cracked through the more intense pyrolysis process to form hydrocarbon materials. In this mapping adapted from Lange (2021), the horizontal axis identifies the plastics which are easy to depolymerize based on heat of polymerization while the vertical axis indicates the incentive to recover the monomer based on the mass of resources consumed for its production.14 Resultingly, the condensation polymers, such as PET and polyamides (PA) in the upper left quadrant are recommended to be depolymerized into monomers through various solvent methods (solvolysis).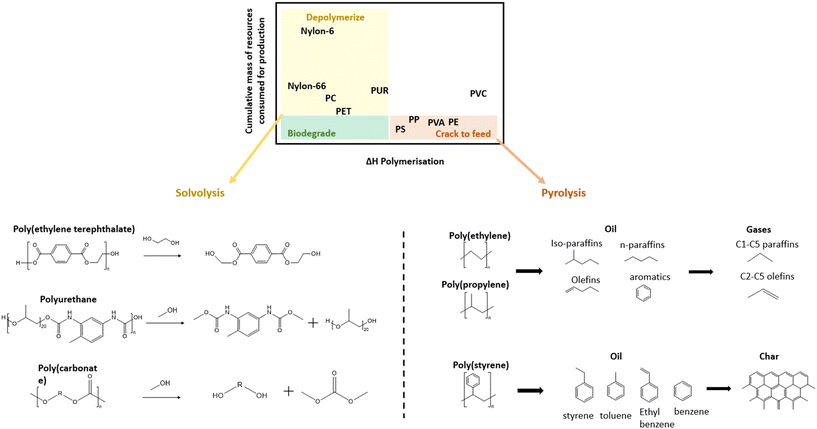 | ||
| Fig. 2 (a) Mapping of the recommended chemical recycling technique for different plastics;14 (b) schematic of the glycolysis of PET and methanolysis of PUR and PC; (c) general pyrolysis products of PE, PP and PS. | ||
These condensation polymers in the upper left quadrant of Fig. 2a consist of monomers connected through bonds, such as ester, amide, urethane linkages etc., which are susceptible to chain-scission through various reactions. In Fig. 2b select chemical approaches are illustrated for the depolymerization of PET, polyurethane (PUR), and PC. PET, which is often chemically recycled, can be depolymerized using various methods including methanolysis, glycolysis, hydrolysis, aminolysis, etc. depending on the chemical used.13 However, glycolysis is the simplest method and is practiced by many large companies, such as DuPont, DOW Chemical Company, and Goodyear.46 PURs can also be depolymerized through similar methods (alcoholysis, glycolysis, and hydrolysis) but rather than yielding its original monomers it results in high molecular weight polyols and aromatic oligomers.14 For PC, these methods are used to break the carbonate bonds and regenerate BPA monomers in addition to organic carbonates, urea (ammonolysis) and methanol (hydrogenolysis).47
For chemical recycling, waste sorting is an important operation, since high purity monomers are desired. For the chemical recycling of PET, it is generally kept as a pure mono stream with only around 16 ppm PVC and 29 ppm of other contaminants.48 In other cases, solvolysis can be used to separate different plastic constituents. For example, PLA can be separated from PET through hydrolysis which can selectively depolymerize the PLA component followed by glycolysis to depolymerize the PET.14 Polymers can also be selectively dissolved from a mixture such as polyolefin dissolution in hydrocarbons, which has been commercialized in multiple processes (e.g., Newcycling process, CreaSolv process) in Europe and Asia.48,49 For the separation of components from multilayer films, Walker et al. (2020) reported a method for separation of PET, PVOH and PE from real films using solvent targeted recovery.50 The authors reported that targeted selective dissolution and precipitation process was able to separate polymers from a commercial multilayer film with a reasonable cost, and close to 100% material efficiency, and high material quality.
Next, the effects of additives and contamination must be considered as well. Compared to mechanical recycling, the cleaning process may not be as consequential, as solvolysis can remove additives and foreign polymers. However, it may add significantly to the cost and complexity of the process if the purification stages must remove high proportions of additives and comonomers.14 With respect to the components added during the recycling process, only catalysts may be required. Resultingly, difficulties in catalyst separation can limit the monomer purity and direct usability, as has been an issue for BHET obtained from PET glycolysis.51,52
Lastly, chemical recycling can provide disposal options for thermoset plastics. Due to the inability to mechanically recycle thermoset plastics, a common approach is to apply solvolysis methods to break the crosslinked bonds. Although solvent processes in general are difficult to scale, this method can recover unaltered fillers from thermoset composites. This is important because some reinforcing agents and fillers used in thermosets, such as carbon fiber reinforced resin materials are often used as substitutes for metals in a variety of industries (construction, energy, transportation),44,53 and it is of interest to recover these filler materials without degrading their physical properties. Solvolysis methods can be performed on thermoset plastics due to the presence of degradable functional groups such as esters,53–55 carbonates,56 acetal,57–59 sulfur groups,60–62 and furans and maleimides63–65 which have been degraded under generally mild conditions.66 Therefore, solvolysis is used as a commercially feasible method for recycling carbon fiber reinforced plastic composites to recover filler materials.67 For these reinforced materials, various chemicals, such as ethanol,68 supercritical methanol69 and even water70,71 have been used for degradation. Although the resin portion of the composites undergo degradation, this method generally focuses on the recovery of reinforcing fiber rather than the recycling of the thermoset matrix.
2.3. Pyrolysis based chemical recycling
As illustrated in Fig. 2a, the chemical recycling of most polyolefins must also consider pyrolysis as a suitable alternative due to the strength of the constituent hydrocarbon bonds. Pyrolysis is a tertiary recycling method which converts high molecular weight polymers into oil, gases and char by high temperature decomposition under an inert atmosphere.72 The oil and gas products are desirable since they are used as precursors to valuable fuels and chemicals. A recent study by Wang et al. (2023) also showed that the oil and gas products of plastic waste pyrolysis can be converted to valuable hydrogen and solid carbon products through subsequent thermolysis.73 The produced hydrogen fuel can generate clean electricity and the solid carbon has many applications including the investigated use as a reinforcing agent.In the pyrolysis of polyethylene, it is understood that degradation occurs by free radical initiation, random scission, followed by recombination of various chains through termination.74,75 The pyrolysis results in gas products consisting of C1–C4 olefins and oil products consisting of C5–C20 olefins and aromatics.76 Das and Tiwari (2018) reported similar pyrolysis products after slow pyrolysis of PE and PP plastics, which consist of paraffins, olefins and some aromatics. However, the proportion of branched paraffins (iso-paraffin) was higher in PP compared to PE. The gaseous products consisted of light paraffins and olefins, mainly propylene, ethane, methane etc.77
For PS, the degradation is also known to occur through free radical reactions.78,79 The pyrolysis results in complete conversion to oil products at 350 °C, but as temperature is increased char production is promoted with very small proportions of gas (max 2.5 wt%). Therefore, the products are mainly oil (toluene, ethylbenzene, benzene, and styrene) and char due to the predominant presence of aromatic degradation products leading to char formation by condensation of aromatic rings. In comparison, LDPE began degradation at 450 °C, but increasing pyrolysis temperature promoted conversion to gases rather than char.80 This phenomenon is shown in Fig. 2c, in which the pyrolysis oils and following gaseous products are illustrated for the pyrolysis of PE and PP, while the aromatic oil products and following char at increased temperature are illustrated for the pyrolysis of PS.
One of the key obstacles is that mixed plastics complicate the pyrolysis process compared to individual plastics due to the unique product compositions for each type of plastic. As mentioned previously, the pyrolysis of PE and PP produces oil/wax, light hydrocarbon gas, and negligible char, while polystyrene produces an aromatic oil product81,82 and char at high temperature.80 On the contrary, PVC pyrolysis produces hydrogen chloride, along with aromatic oil and char,81,82 and PET pyrolysis yields CO2, CO and char.81,83 As a result, the pyrolysis products obtained from mixed plastic wastes are expected to vary greatly depending on composition. Furthermore, the compositions of pyrolysis products from mixed plastics have been reported to deviate from what is expected based on individual plastic pyrolysis data likely due to complex reactions during the process. For example, Williams and Williams (1998) reported that introducing PS to other common plastics resulted in a significantly greater gas yield than would be expected.84 It has also been reported that PS reduces the time required to produce maximum oil yield from PE.85 Generally, Wenning (1993) has reported variations in product compositions of 40–55 wt% oil/wax, 30–50 wt% gas, and 5–15 wt% char from pyrolysis of different plastic mixtures of PE, PP, PVC, PET and PA.86 Therefore, if plastic wastes cannot be sorted properly, it is close to impossible to understand the expected product yields from mixed pyrolysis processes.
Next, additives are somewhat of a concern because pigments and by-products in plastic waste can lead to issues in pyrolysis oil.87 Therefore, there can be a need for removal of contaminants by pre-treatments and washing before pyrolysis similar to mechanical recycling. Additionally, the presence of chlorine from PVC and other halogens that can be introduced from pigments and coatings88,89 can result in many harmful products (dioxins, HCl).87 This issue necessitates that the chlorine content in the oil product must be reduced below 10 ppm through post-treatment before use as a feedstock.87
With respect to the cleaning process, Genuino et al. (2023) has investigated the effects of washing on the pyrolysis of a mixed plastic waste stream containing PE, PP, PET, PS, acrylonitrile butadiene styrene (ABS), paper and aluminum laminates. Washing using a combination of cold and hot water resulted in significant cleaning (11.7 wt% reduction) which mainly affected the ash content in the pyrolysis product. The washed and unwashed batches produced similar wax and oil yields (66–69%), with the difference in the solid product aligning exactly with the ash removal by cleaning.90 Therefore, washing of plastic waste before pyrolysis is very helpful for reducing the ash content caused by inorganic contaminants.
Lastly, one of the major advantages of pyrolysis is that it can be easily employed for thermoset plastics. For the treatment of filler reinforced thermosets, the polymer portion is decomposed to form liquids and gases while the filler portion can be separated and reused.91 However, to ensure that the filler is not significantly damaged the processing conditions must be considered. At very low temperatures (<300 °C) the resin component does not degrade properly, while at high temperatures (>600 °C) the reinforcing fibres are degraded.92 To overcome this issue, a two-stage process has been reported for glass fiber recovery from thermoset plastic which led to improved tensile strength of glass fibers.93 In terms of carbon reinforced thermosets, pyrolysis is more suitable. At the lowest temperature of 400 °C, the tensile strength reduction of recycled carbon fibres is much less (5–20%) compared to that of glass fibers (>50%).94
2.4. Carbonization chemical recycling
The carbonization of plastic waste is considered a distinct treatment compared with pyrolysis, because it is specific to the production of value-added carbon materials rather than fuels and chemicals. For carbonization processes, a slow heating rate is employed to promote the production of solid products containing maximum carbon content from the precursor. This slow heating rate leads to a more sequential conversion of the feedstock into a carbonized material through many reactions. Additionally, higher temperatures of 600–1200 °C are used in carbonization compared to pyrolysis for oil products generation, which occurs at temperatures around 500 °C.1 During carbonization, the plastic is heat treated at high temperature under an inert atmosphere to produce carbon material through aromatization, while some gases (H2O, CO2, CH4, NH3etc.) are released through decomposition of the plastic constituents.2Carbonization at different conditions (catalysts, templates, and pressures) can result in varying carbon products. As a result, different structures are obtained, including activated carbon, carbon fibres, carbon nanotubes, carbon spheres, and graphene.95 In terms of the plastic precursors, polyolefins such as PP and PE are ideal for producing carbon nanotubes, carbon spheres, and graphene because they form light hydrocarbons, which are catalyzed to form these structures during the carbonization process.95,96 This can be achieved using combined catalysts, which act as both degradation and carbonization catalysts. The degradation catalyst helps to promote the formation of the required low molecular weight compounds, while the carbonization catalyst facilitates the degradation process such that carbon materials can be formed.97 Templates may also be employed, in which a removable mold is used to create controlled voids in the material. Some materials used as rigid templates include silica, clays, MgO and CaCO3.98
During the carbonization of PET and PS containing benzene rings in their structure, aromatics and oil products are formed which then lead to the formation of amorphous carbon.95 This is due the occurrence of cyclization, aromatization and crosslinking rather than degradation into small molecules.96 As a result, polyolefins, such as PP and PE are considered non-charring while aromatic plastics such as PET and PC are considered charring plastics, as shown in Fig. 3. Non-charring plastics are beneficial for producing ordered carbon materials (graphene, carbon nanotubes etc.) through catalysis while charring plastics can produce amorphous carbon material. Activation by chemical or physical methods can then be used to enhance the surface area and porosity of the products.
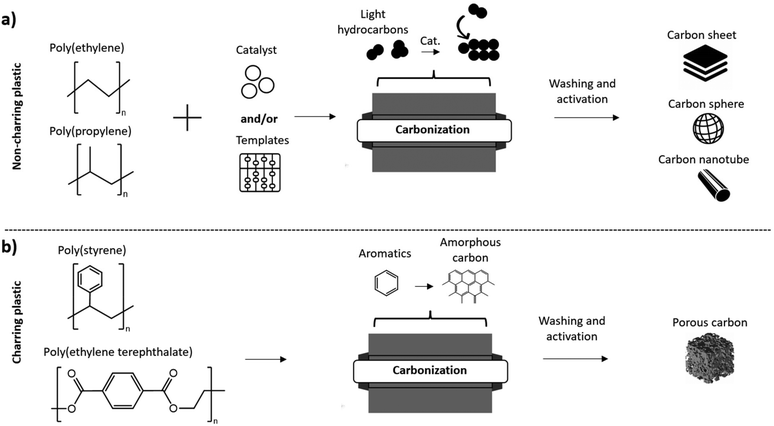 | ||
| Fig. 3 (a) Carbonization of non-charring plastics to produce carbon sheets/carbon spheres/carbon nanotubes; (b) carbonization of charring plastics to produce porous carbon. | ||
Sorting of plastic waste prior to carbonization is an important consideration because of the variation in products that can be obtained based on the type of plastic. For example, PET plastic is the most frequently used feedstock to produce porous carbon, but this is aided by the already established systems in place for collection and recycling of PET bottles and other products.99 On the other hand, the low fixed carbon content of polyolefins doesn't allow for porous carbon to be produced from these common waste plastics.100 Pretreatments of polyolefins enabling the production of porous carbon will be discussed in the following section, but still require different procedures compared to charring plastics. Therefore, sorting must be performed before carbonization processes to ensure the feed consists of the appropriate type of plastic for the intended product such as porous carbon.
It has been reported that mixed waste plastics containing polyolefins can be used to produce carbon sheets and spheres through template methods employing organically modified montmorillonite (OMMT). The acidic sites on OMMT promote dehydrogenation and aromatization of plastics and catalyzes the carbonization such that graphene or carbon spheres can be grown on its surface.8 Using this template, mixed plastics have been converted into hollow carbon spheres101 and porous carbon nanosheets102,103 by incorporating a final activation step. Plastic mixtures have also been used to produce carbon nanosheets on magnesium oxide104 and carbon nanotubes on silicone, glass, and carbon paper substrates105 and over a Co–Mo–MgO catalyst.106 However, it remains a challenge to directly produce porous carbon products from polyolefins without the use of template or catalyst methods, limiting the production of porous carbon from mixed wastes.
Next, the consideration of additives and contamination is relevant to the production of carbon nanomaterials such as nanosheets and nanotubes, as these impurities can interfere with the catalytic process of carbon growth.8 Therefore, cleaning pretreatments may produce higher quality products. With respect to general carbonization, most research has neglected the impact of impurities like plasticizers, metals, antioxidants etc. This area should be investigated further since additives may affect the carbon conversion process.96 However, it is expected that the effects of washing on carbonization would be similar to that of pyrolysis, in which the ash content is reduced greatly.
Lastly, carbonization technology has the advantage of being able to process thermoset plastics by conversion into carbon material. Specifically, phenolic formaldehyde resin (PFR) is the most used resin for producing amorphous carbon and exhibits a high carbon yield (61.8%).107 Another area of research is in the conversion of epoxy resin to carbon materials, as it is used in many products including electronics (circuit boards) and composite materials (windmills, aircrafts). The production of high surface area activated carbon has been reported using physical108 and chemical activation109 of the epoxy resin component in waste circuit boards. Additionally, the conversion of cured epoxy to activated carbon has been investigated and applied as an adsorbent material110 and for supercapacitors.111 The upcycling of thermoset waste to high value carbon materials is a promising area, but more work is required to investigate more precursors and conversion methods. One prominent concern with respect to epoxy waste, is the separation of the metal component in waste circuit boards before treatment.
2.5. Energy recovery
Lastly, energy recovery through combustion is a common method used to deal with large quantities of plastic waste. As illustrated in Fig. 1b, combustion for energy recovery is a quaternary recycling method that used as a last resort for producing value from unutilized waste streams. It takes advantage of the high energy density of plastic waste, which can supply large amounts of thermal and electric power (e.g. PE: 43 MJ kg−1).10 It is a simple and inexpensive method of disposing plastic waste; however, it results in large emissions of harmful chemicals (dioxins, furans, greenhouse gases etc.).2 Still, combustion is a very useful alternative to landfilling plastics which are difficult to recycle.It is known that various plastics have different calorific values. For example, PVC, PET and PA have much lower carlorific energy than PP, PE and PS, which are similar to conventional fuels. Therefore, some plastics are not as suitable for an incineration process due to their efficiency.112 As a result, variations in compositions can cause significant fluctuations in energy output, making it ideal for sorting operations to be applied before treatment.10 However, combustion is still used for disposing mixed wastes which would otherwise be landfilled.
Next, the incineration of materials containing additives may contribute to the ash content in the product. This may pose concerns if the waste contains heavy metals, because these can be released into the environment upon incineration.2 Regardless, incineration is known to release toxic emissions, which is why it is used as a last resort and the impact of additives and extraneous agents need to be considered.
Lastly, combustion can be a last resort option for disposal of hard to recycle thermosets. It is specifically helpful in the recovery of precious metals from electronic wastes, because of the difficulty in removing the plastic resin portions. Therefore, the metals are extracted by incineration of the resin, or alternatively by using solvent methods,12 as discussed previously for the removal of reinforcing agents in epoxy.
3. Anoxic pyrolysis carbonization of plastics
With respect to the various disposal methods, the carbonization route is a very promising method for producing both amorphous and graphitic carbon. To better understand the application of this technology, the effect of plastic composition should be further investigated. In section 2.4., it was outlined that non-charring polyolefin plastics required catalytic conversion to produce structured carbon materials while aromatic plastics can be directly converted to amorphous carbon (Fig. 3). This section focuses on anoxic pyrolysis carbonization, which refers to the direct heat treatment of plastics under an inert atmosphere rather than the catalytic carbonization of polyolefins for producing nanomaterials.2 Although polyolefin plastics (PP, PE) produce light hydrocarbons upon heat treatment, there are stabilization treatments that allow these non-charring plastics to undergo anoxic pyrolysis carbonization. Additionally, the subsequent activation of carbon materials to produce high surface area activated carbon from plastic precursors will be discussed in the next section (section 4).3.1. Stabilization pre-treatments
It is generally known that carbonization of aromatic plastics leads to the formation of oils and aromatics which enhance char formation.95 However, the oxygen content in the polymer also plays an important role in its conversion to carbon material. Plastic which contains oxygen, such as PET and epoxy resins, are more easily carbonized through heat treatment, while non-oxygen containing plastics may require a stabilization pre-treatment. The preliminary treatment allows for an increased yield of carbon residues rather than gaseous organic molecules.2 Therefore, polyolefins can be converted into amorphous carbons (e.g. activated carbon, carbon fibres) if a stabilization through oxidation or other chemical treatment is preformed before carbonization.In terms of stabilization treatments, sulfonation and oxidation treatments are commonly used. For LLDPE, Choi et al. (2017) has shown that oxidation in air introduces C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and C–O bonds, which occur in the main chain and as ether bonds bridging chains. As temperature increases, the linear chains are converted to a crosslinked structure with an increasing composition of oxygen. The cyclized structure can then be carbonized at higher temperature under inert atmosphere to yield a carbonaceous product.113 Alternatively, PE can be sulfonated using sulfuric acid to result in sulfonic acid groups among other sulfur containing groups (sultones, sulfates). Subsequently during carbonization, unsaturated polyolefin is obtained through release of sulfur and oxygen, and carbonized material can then be achieved. The sulfonation mechanism is investigated in detail by Younker et al. (2013).114 The general mechanism of the oxidation and sulfonation pre-treatments are illustrated in Fig. 4a.
O bonds and C–O bonds, which occur in the main chain and as ether bonds bridging chains. As temperature increases, the linear chains are converted to a crosslinked structure with an increasing composition of oxygen. The cyclized structure can then be carbonized at higher temperature under inert atmosphere to yield a carbonaceous product.113 Alternatively, PE can be sulfonated using sulfuric acid to result in sulfonic acid groups among other sulfur containing groups (sultones, sulfates). Subsequently during carbonization, unsaturated polyolefin is obtained through release of sulfur and oxygen, and carbonized material can then be achieved. The sulfonation mechanism is investigated in detail by Younker et al. (2013).114 The general mechanism of the oxidation and sulfonation pre-treatments are illustrated in Fig. 4a.
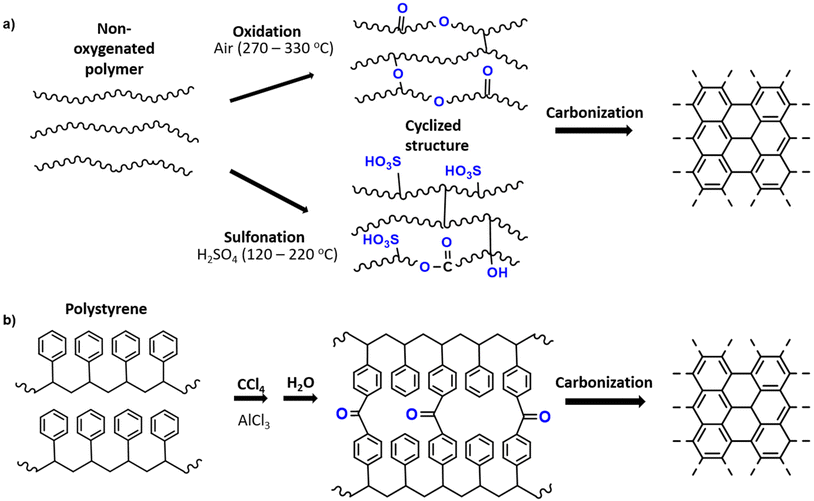 | ||
| Fig. 4 (a) General schematic of oxidation and sulfonation pretreatments for carbonization of plastics based on the mechanisms of PE stabilizations;2 (b) Friedel–Crafts reaction as a pretreatment for polystyrene carbonization.116 | ||
Sulfonation can also be used for PS, as was reported by Hines et al. (2004) to produce porous carbon.115 Additionally, PS can be stabilized by the Friedel–Crafts reaction in which crosslinking occurs through carbonyl bridging using a carbon tetrachloride reagent and Lewis acid catalyst (Fig. 4b). This leads to enhanced carbonization due to the crosslinked structure and increased oxygen content.116 In the case of PVC, stabilization usually occurs through heat treatment in air, during which oxygen functional groups are introduced during this treatment, leading to crosslinking and aromatization.117 A list of various carbon materials obtained from plastic precursors with or without pre-treatments is presented in Table 1. Evidently, the sulfonation of PE is a very common method, especially to produce carbon fibers.114,118,119 Carbon fibres are often used in polymer composites reinforcement as alternatives to heavier construction materials like steel and are mainly produced from poly(acrylonitrile) (PAN) by melt spinning and oxidation pre-treatment.118 Alternatives like PE are of interest due to the costs of PAN precursor and conversion yield, which limit the applications in industries requiring lower cost products.118
| Precursor | Stabilization treatment | Carbon product | Ref. |
|---|---|---|---|
| LLDPE | Oxidation | Graphitic carbon | 113 |
| LLDPE | Chlorosulfonation | Carbon fiber | 119 |
| LDPE | Sulfonation | Carbon scaffold | 120 |
| PS | Sulfonation | Porous carbon | 115 |
| PS | Crosslinking (Friedel–Crafts) | Porous carbon | 116–121 |
| PE | Sulfonation | Carbon fiber | 114–118 |
| PE | Sulfonation | Porous carbon | 122 |
| PE | Sulfonation | Amorphous carbon chips | 123 |
| PVC | Oxidation | Porous carbon | 124 |
| PVC | — | Char | 125 and 126 |
4. Activation of plastics
4.1. Activation methods
As seen in the previous section, porous carbons are often produced from plastic precursors (Table 1). These products are very valuable due to their high surface area (SA) and pore volume, which allow them to be used as high-capacity adsorbents. However, activation processes are often used to further improve the SA of the carbonized materials to produce activated carbons (ACs), which are defined by large SA, porosity, and adsorption capacity.127 The activation process involves reactions between carbon and an activating agent to produce new pores and open existing pores in the carbon structure through physical or chemical methods. The specific SAs of commercial ACs are in the range of 500–1500 m2 g−1,128 which is determined by the Brunauer–Emmett–Teller (BET) method of SA analysis through N2 adsorption.Physical activation involves heat treatment with an oxidizing gas such as O2, CO2 or steam at high temperature (800–1200 °C). This takes place after carbonization of the material under an inert atmosphere, such that a two-stage process is required.129 It can be considered a more environmentally friendly approach due to the lack of chemicals, but it has the downsides of long activation times and high energy consumption.130 Chemical activation on the other hand involves impregnation of the precursor with an oxidizing and dehydrating chemical, heat treatment at temperatures between 400 to 900 °C, and subsequent washing (e.g., HCl) to remove the chemical. In this case, the carbonization and activation can occur simultaneously such that a single stage process can be employed.130 However, many studies also employ carbonization prior to activation, using a lower temperature of around 600 °C for carbonization where most mass loss occurs, followed by chemical activation at temperatures from 400 to 1000 °C.127 This two-stage chemical activation is illustrated in comparison to physical activation in Fig. 5a.
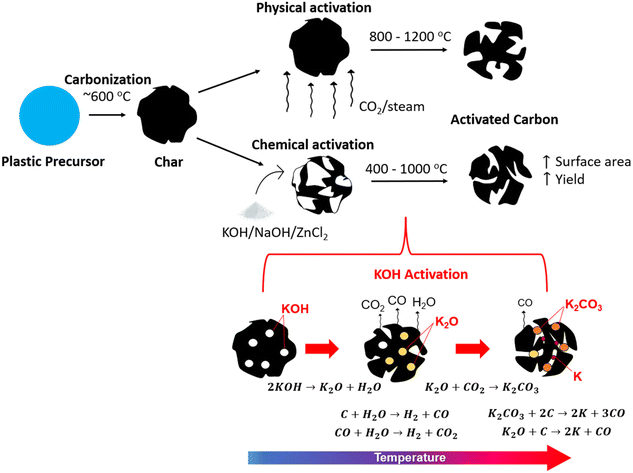 | ||
| Fig. 5 (a) Illustration of activated carbon production through physical versus chemical activation; (b) KOH activation mechanism adapted from ref. 110. | ||
The main parameters affecting activation include the activating agent, treatment temperature, time, and the impregnation ratio (IR), which is the mass ratio of chemical activating agent to precursor in the case of chemical activation. In general, chemical activation is preferred compared to physical activation due to the advantages of lower activation times and temperatures, generation of high specific SAs, and high carbon yield.131 The most used chemical activating agents include alkaline chemicals, such as KOH, NaOH and K2CO3, acidic chemicals such as H3PO4 and H2SO4, and metal salts such as ZnCl2.130 Of all activating agents, KOH is known to be the most effective due to its capacity to produce high SAs in AC.129 As a result, much of the work that will be explored has focused on activation using KOH.
With respect to the feedstock, commercial AC is mainly produced from charcoal, lignite, wood, peat shells and coconut, but any carbonaceous organic material are viable precursors.130 Therefore, plastics are an enticing option for AC feedstocks since they possess high carbon content, and the utilization of plastic waste is of high concern. Currently, the production of AC from plastic waste has not been commercialized, although it is under ongoing investigation. For example, an Australian company called ByGen has reported the success in converting plastics including PET into AC,132 although they do not yet produce any AC products at full scale. Therefore, this section will reflect on the current understanding of AC production from plastics based on the relevant research studies.
4.2. Chemical activation mechanisms
First, the activation mechanism of KOH will be outlined in detail, as it is the most well studied chemical activating agent. The mechanism of pore formation using KOH activation occurs through physical activation by the evolved CO2 and H2O, redox reactions between potassium compounds and carbon, and through the formation of potassium metal at high temperatures,133 as illustrated in Fig. 6b. Gases such as CO and CO2 are formed through the reaction of carbon with surface and internally bound water (eqn (1) and (2)), and water is released through the dehydration of KOH (eqn (3)). As the decomposition continues, K2CO3 is produced due to the transformation of K2O (eqn (4)). At high temperatures exceeding 700 °C, metallic potassium is formed through the reduction of K2O and K2CO3 (eqn (5) and (6)).129,133,134 The potassium metal is important for the continued pore formation due to its ability to penetrate and expand the carbon structure. It also helps to form more active sites for reaction with carbon and improves the wettability of the surface. These effects are unique and can not be achieved through activation by acidic or neutral activating agents.129 This is why strong alkali activating agents are very effective, especially KOH.| C + H2O → H2 + CO | (1) |
| CO + H2O → H2 + CO2 | (2) |
| 2KOH → K2O + H2O | (3) |
| K2O + CO2 → K2CO3 | (4) |
| K2O + C → 2K + CO | (5) |
| K2CO3 + 2C → 2K + 3CO | (6) |
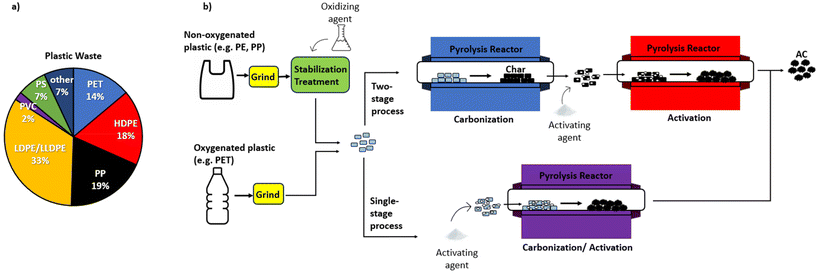 | ||
| Fig. 6 (a) Composition of plastic waste managed in the US in 2019. Data obtained from ref. 11; (b) illustration of various pathways for the chemical activation of oxygenated and non-oxygenated plastics. | ||
Although KOH is most well-known, there are other activating agents which also are effective in producing high SA products. These chemicals include NaOH, K2CO3, ZnCl2 and acids such as H3PO4, which are all employed in some of the studies referenced in section 5. Firstly, NaOH is a common alternative to KOH, as it is another alkaline hydroxide that can melt without decomposition at high temperature, allowing reaction with carbon at high temperature.135 The NaOH activation mechanism follows the same global activation mechanism (eqn (7)) at high temperature, in which M refers to either K or Na.135,136 Although the activation mechanisms are very similar, KOH is seen to have a greater activation effect due to the lower temperatures required for reactions to occur in the case of KOH.136
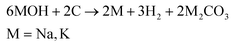 | (7) |
Another well-known activating agent is K2CO3, as it has a high activating effect and is a non-harmful alternative to the corrosive metal hydroxides. It has a powerful activating agent because it is the effective activating agent in the KOH activation mechanism at high temperatures,137 based on eqn (6). At lower temperatures the K2CO3 activator does not participate in any reactions as it is stable below 700 °C during activation.137 This makes K2CO3 very effective in SA development because it only participates in high temperature activation reactions; however, the lack interaction with the feedstock at low temperature can lead to low carbon yield, as was reported in the case of Epoxy activation.110
Acidic activating agents such as H3PO4 are also employed due to its multiple activation effects outlined by Gao et al. (2020).129 Firstly, H3PO4 acts as a dehydrating agent to draw out hydrogen and oxygen in the form of water rather than carbon volatiles. Secondly, it diffuses into the starting material to produce a homogenous incorporation that enables uniform heating during activation, acts as a framework for the carbon network, and lowers the carbonization temperature due to the higher thermal conductivity of H3PO4 compared to the alternative heating media (air, water or CO2). Above 200 °C polyphosphoric acid is produced, which facilitates oxidation and carbonization of volatile components. Additionally, polycondensation, cross-linking and cyclization reactions lead to the formation of a polycondensation structure with the organic material. With increasing temperature, these polyphosphate esters and polyphosphoric acids are also converted into P2O5, which contributes to the pore development through its reaction with carbon to form C–O–P structures.
Lastly, ZnCl2 is often employed as an activating agent, especially for cellulosic feedstocks.130 It is considered as a neutral activating agent, which generally performs through the reduction reactions between the positive ion (e.g. Zn2+) and carbon.129 This consumes the carbon to leave behind pores containing carbon bonded components to be removed during the washing process. The metal species may also play a role in the catalysis of CO2 and CO release, as were observed by neutral activators FeCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138 and KMnO4.139 This process contributes to surface area development by the additional physical activation.
138 and KMnO4.139 This process contributes to surface area development by the additional physical activation.
5. Progress in plastic conversion to AC
The conversion of plastics to AC can occur through various methods. As discussed previously, the composition of the plastic can necessitate preliminary stabilization treatments (section 3.1) and the activation process itself can follow various paths. In this section, the conversion process for common plastics will be reviewed, with a focus on chemical activation methods. The BET SAs are investigated as the main indication of AC quality due to the wide range of applications in the investigated works. The SAs achieved in ACs through activation of various thermoplastics (Table 2), chemical activation of PET (Table 3), and chemical activation of thermosets (Table 4) have been tabulated in the following sections. Based on these tables, it is evident that most research focuses on KOH activation of plastics in both single stage and two stage carbonization/activation processes. The various process pathways utilized in these works have been illustrated in Fig. 6b to showcase the combination of steps required to produce chemically activated AC from plastics.| Precursor | Stabilization treatment | Activation type | Activating agent | AC surface area (m2 g−1) | Ref. |
|---|---|---|---|---|---|
| PE | Sulfonation | Chemical | KOH | 156–1803 | 148 |
| PVC | Oxidation | Physical | Steam | 1096–2096 | 153 |
| PVC | Oxidation | Physical | CO2 | 528–1211 | 117 |
| PVC | Oxidation | Chemical | KOH | 4–2507 | 158 |
| PVC | — | Chemical | KOH | 2666 | 155 |
| PS | Sulfonation | Physical | Air, steam | 567, 842 | 149 |
| PS | — | Chemical | KOH | 2109–2712 | 152 |
| PS | — | Chemical | KOH | 393–1250 | 151 |
| PS | Crosslinking (Friedel–Crafts) | Chemical | KOH | 2637 | 150 |
| PC | — | Chemical | KOH | Max. 2098.7 | 156 |
| PC | — | Chemical | NaOH | 348–815 | 157 |
| Carbonization conditions | Activating agent | IR | Activation conditions | AC surface area (m2 g−1) | Ref. |
|---|---|---|---|---|---|
| — | ZnCl2 | 1 | 500 °C 2 h | 700 | 169 |
| — | K2CO3 | 0.25–1 | 800 °C 2 h | 680–1390 | |
| — | K2CO3 | 2 | 800 °C | 1439 | 170 |
| KOH | 1206 | ||||
| — | ZnCl2 | 1 | 400 °C 1 h then 800 °C 1 h | 682 | 171 |
| H3PO4 | 1223 | ||||
| H2SO4 | 583 | ||||
| KOH | 1338 | ||||
| — | KOH | 1 | 700–800 °C 1–2 h | 625–1214 | 159 |
| — | KOH | 2 | 700 °C 30 min | 1418 | 176 |
| — | KOH | 2 | 700 °C | 1334 | 177 |
| — | KOH | 2 | 700–850 °C 1 h | 566–1002 | 165 |
| 800 °C 0.25–2 h | 666–844 | ||||
| — | KOH | 1–5 | 800 °C 1 h | 817–1889 | 172 |
| — | KOH | 5 | 900–1100 °C 1 h | 1092–1808 | 173 |
| 600 °C 1 h | KOH | 2 | 850 °C 1.5 h | 2831 | 155 |
| 600 °C 2 h | KOH | 2 | 700–1000 °C 1 h | 1689–2006 | 168 |
| NaOH | 1926–2060 | ||||
| 600 °C 1 h | KOH | 2 | 600–1000 °C 1 h | 1636–1937 | 174 |
| 1–3 | 700 °C 1 h | 736–2650 | |||
| 700 °C 2 h | KOH | 1–4 | 700 °C 2 h | 591–1690 | 178 |
The plastic precursors investigated in this study were chosen based on the abundance in waste streams as represented in Fig. 6a in addition to thermoset plastics, which are non-recyclable. With respect to the relevant thermoplastics, each possess differing structures and properties, prompting their high demand for specific industrial applications. To provide some background of the importance of these plastics leading to their usage and disposal, the structures and applications of the commodity thermoplastics outlined in Fig. 6a are presented in Table 2.
Polyethylene (PE), which is available in both high density (HDPE) and low density (LDPE) grades, has a very linear structure with a low degree of branching. It can be easily processed into a variety of forms including films, and blow-moulded containers.141 Polypropylene (PP) also has very good processability but exhibits increased hardness due to the methyl group in its repeating structure. It also has improved temperature resistance, leading to its usage in containers and bottles.144 In comparison, polystyrene exhibits a phenyl group in place of the methyl in polypropylene, leading it to an amorphous and clear plastic when extruded.145 However, most PS products are expanded PS, a lightweight material ideal for insulation and foam products such as cups/trays.141,146 Polyvinyl chloride (PVC) is different from other thermoplastics due to its chlorine content. This makes PVC non combustible and suitable for use in buildings and furniture.141 Lastly, poly(ethylene terephthalate) (PET) is a semi-crystalline polyester leading to a balance of properties including strength and stiffness in addition to resistance to gas and water permeation.141,147 These properties combined with its transparency has led it to become the main containment material for beverages including water and carbonated drinks. These plastics are reviewed with respect to their conversion into activated carbon due to their high consumption. Additionally, polycarbonate (PC), a strong thermoplastic containing carbonate groups was also investigated, as it is a good precursor for carbonization due to its oxygen content.
5.1. Polypropylene (PP) and polyethylene (PE)
In terms of carbon materials, PP waste is mainly transformed into carbon nanotubes.1 As discussed in section 2.4, polypropylene decomposes into light hydrocarbon gases which can be used as building blocks for carbon nanomaterials through a catalyzed process. Although PE also produces similar pyrolysis products, stabilization pre-treatments have been heavily investigated for the successful anoxic pyrolysis carbonization of PE. As shown in Table 1, sulfonation of PE is often employed to produce a variety of carbon materials such as carbon fibres and porous carbon. PP and PE are not often used to produce activated carbon; however, Yang et al. (2022) reported the subsequent activation of sulfonated PE using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 KOH IR and an activation temperature of 900 °C.148 The ACs exhibited a maximum SA of 1803 m2 g−1 using a carbonization temperature of 900 °C, which decreased upon further increases in carbonization temperature up to 2400 °C.
4 KOH IR and an activation temperature of 900 °C.148 The ACs exhibited a maximum SA of 1803 m2 g−1 using a carbonization temperature of 900 °C, which decreased upon further increases in carbonization temperature up to 2400 °C.
5.2. Polystyrene
To produce carbon materials from polystyrene (PS) chemical stabilizations can be performed prior to carbonization, but direct carbonization/activation has also been reported in the production of AC (Table 3). Gonsalvesh et al. (2016) employed sulfonation of PS using sulfuric acid prior to carbonization (at 600 °C) and activation using air or steam.149 The capacity of the steam activated AC based on iodine number increased with temperature. However, 850 °C was determined as optimal due to the decreasing carbon yield with respect to temperature. For activation in air, a temperature of 350 °C was used due to the significant decrease in iodine number beyond this temperature. The steam activation was more effective, as it produced a greater SA (842 m2 g−1) compared to the air activated AC (567 m2 g−1).Stabilization of PS by Friedel–Crafts reaction was also employed by Gatti et al. (2019) prior to carbonization at 600 °C and activation by KOH using an IR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.150 Activation at 800 °C for 1 h resulted in a very large increase in SA from 739 m2 g−1 to 2637 m2 g−1. Based on these studies, the Friedel–Crafts reaction combined with KOH activation was more effective for producing AC, as the carbonized PS had a much greater SA (739 m2 g−1) compared to the sulfonated PS (267 m2 g−1) under the same carbonization temperature. Additionally, a much greater increase in SA was achieved through KOH activation compared to the physical activation at the same temperature (800 °C).
3.150 Activation at 800 °C for 1 h resulted in a very large increase in SA from 739 m2 g−1 to 2637 m2 g−1. Based on these studies, the Friedel–Crafts reaction combined with KOH activation was more effective for producing AC, as the carbonized PS had a much greater SA (739 m2 g−1) compared to the sulfonated PS (267 m2 g−1) under the same carbonization temperature. Additionally, a much greater increase in SA was achieved through KOH activation compared to the physical activation at the same temperature (800 °C).
Although stabilization treatments of PS improve the carbonization, it is not completely necessary due to its aromatic structure which can aid char formation. For example, Deka et al. (2020) produced AC from PS through direct chemical activation using KOH.151 Using an IR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, it was observed that the SA increased from 393 m2 g−1 to 1250 m2 g−1 alongside increases in activation temperature from 600 °C–800 °C. Additionally, de Paula et al. (2018) investigated KOH activation of PS after carbonization at 10 bar and 530 °C for 5 h.152 Under the same activation temperatures as the previous study and an IR of 1
3, it was observed that the SA increased from 393 m2 g−1 to 1250 m2 g−1 alongside increases in activation temperature from 600 °C–800 °C. Additionally, de Paula et al. (2018) investigated KOH activation of PS after carbonization at 10 bar and 530 °C for 5 h.152 Under the same activation temperatures as the previous study and an IR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the ACs exhibited higher SAs in the range of 2109–2712 m2 g−1. This large difference is likely attributed to the separation of carbonization and activation stages in the study by Paula et al. (2018). However, the carbon yield and cost of such a process must be considered as well.
4, the ACs exhibited higher SAs in the range of 2109–2712 m2 g−1. This large difference is likely attributed to the separation of carbonization and activation stages in the study by Paula et al. (2018). However, the carbon yield and cost of such a process must be considered as well.
5.3. Poly(vinyl chloride)
Poly(vinyl chloride) (PVC) is not often upcycled to carbonaceous products,1 but it has been reported to produce porous carbons using a stabilization pre-treatment124 and char in the absence of pretreatment.125,126 Activation of carbonized PVC has been investigated to produce AC fibres through carbonization and activation of melt spun PVC.117,153 Both studies utilized a two-stage heat treatment to produce spinnable PVC pitch, which was then stabilized in air up to 320 °C prior to carbonization and activation. Qiao et al. (2004) utilized steam activation at 900 °C and found that the resulting AC SA increased from 1096 m2 g−1 to 2096 m2 g−1 as activation time increased from 30 to 90 min.153 Their later study using CO2 as an activating agent reported lower AC SAs of 528 m2 g−1 to 1211 m2 g−1 using greater temperatures of 900 °C to 1000 °C and an activation time of 1 h.117 PVC has also been chemically activated by KOH after stabilization in air. Liu et al. (2022) reported a maximum SA of 2507 m2 g−1 under activation conditions of 800 °C for 1 h and a KOH IR of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.154 A similarly high SA of 2666 m2 g−1 was achieved by Lian et al. (2012) using a lower KOH IR of 2
1.154 A similarly high SA of 2666 m2 g−1 was achieved by Lian et al. (2012) using a lower KOH IR of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and elevated activation conditions of 850 °C for 1.5 h.155 At these conditions, it was also shown that a higher SA AC (2831 m2 g−1) was achieved using PET as a precursor while the PVC AC displayed a narrower pore distribution. Based on these studies, it seems that the chemical activation of PVC by KOH can produce higher SAs while using lower activation temperatures compared to the physical activation processes.
1 and elevated activation conditions of 850 °C for 1.5 h.155 At these conditions, it was also shown that a higher SA AC (2831 m2 g−1) was achieved using PET as a precursor while the PVC AC displayed a narrower pore distribution. Based on these studies, it seems that the chemical activation of PVC by KOH can produce higher SAs while using lower activation temperatures compared to the physical activation processes.
5.4. Polycarbonate
Polycarbonate (PC) has also been reported as a feedstock to produce AC without the need for stabilization pre-treatment. The KOH activation of PC has been used to achieve AC with SAs as high as 2098 m2 g−1 and with yields of greater than 40%.156 As an alternative to KOH, NaOH was also investigated due to its lower cost and corrosivity. Li et al. (2014) investigated NaOH activation of PC at 500 °C using a central composite design and produced ACs with a maximum SA of 815 m2 g−1 using an IR of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 150 min activation treatment.157 Evidently, the NaOH activator was not as effective in developing SA as KOH but may be preferred in some applications as a less corrosive/hazardous option.
4 and 150 min activation treatment.157 Evidently, the NaOH activator was not as effective in developing SA as KOH but may be preferred in some applications as a less corrosive/hazardous option.
5.5. Poly(ethylene terephthalate)
Poly(ethylene terephthalate) (PET) plastic is a special example because it is the most researched plastic feedstock for AC production. PET is an ideal precursor to AC due to its aromatic and oxygenated structure, high carbon content (> 60 wt%) and high char yield compared to other waste plastics.159 Most studies have employed chemical activation for the synthesis of PET AC, which is the focus of this section. The various carbonization conditions, chemical activation conditions, and resulting AC SAs have been summarized in Table 4, and the trends with respect to operational conditions will be discussed.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160–164 and steam165–167 as activating agents. For CO2 activation, Esfandiari et al. (2012) found optimal conditions of 975 °C for 240 min to produce ACs with a SA of 790 m2 g−1.163 Compared to much of the chemical activation results presented in Table 4, the CO2 activation requires a higher temperature and longer treatment time to produce a product of lesser SA. Mandoza-Carrasco et al. (2016) compared steam activation (8.33 mL min−1) of PET to KOH activation at an IR of 2.165 It was found that KOH activation caused an optimal SA (1002 m2 g−1) at 850 °C, while steam activation resulted in a higher SA (1235 m2 g−1) at 800 °C under the same treatment time of 1 h. Although the results are influenced by the levels of activating agent used, this showcases that steam activation can produce similar SAs as KOH at a reasonable IR of 2.
160–164 and steam165–167 as activating agents. For CO2 activation, Esfandiari et al. (2012) found optimal conditions of 975 °C for 240 min to produce ACs with a SA of 790 m2 g−1.163 Compared to much of the chemical activation results presented in Table 4, the CO2 activation requires a higher temperature and longer treatment time to produce a product of lesser SA. Mandoza-Carrasco et al. (2016) compared steam activation (8.33 mL min−1) of PET to KOH activation at an IR of 2.165 It was found that KOH activation caused an optimal SA (1002 m2 g−1) at 850 °C, while steam activation resulted in a higher SA (1235 m2 g−1) at 800 °C under the same treatment time of 1 h. Although the results are influenced by the levels of activating agent used, this showcases that steam activation can produce similar SAs as KOH at a reasonable IR of 2.
5.6. Thermoset resins
Another important category of plastic precursors is thermoset plastics, which are often found in composite materials. Thermosetting resins are used as a matrix to hold a structural filler in place and are chemically cross-linked such that they cannot be reprocessed or reshaped after curing. Therefore, conversion of thermoset resins to carbon is a promising treatment as they cannot be recycled by the conventional thermo-mechanical process. Some examples of AC production from commonly used phenolic and epoxy resins are summarized in Table 5.| Precursor | Stabilization treatment | Carbonization conditions | Activating agent | IR | Activation conditions | AC surface area (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Epoxy | — | 500 °C 1 h | KOH | 3 | 600 °C 3 h | 1353.78 | 183 |
| Epoxy (PCB) | — | 650 °C 2.5 h | KOH | 3 | 800 °C 1 h | 2573 | 109 |
| Epoxy | — | — | KOH | 1–2 | 600–800 °C 2 h | 788.1–1728.5 | 110 |
| Phenolic resin | — | — | K2FeO4 | 19.8 | 750–950 °C 1 h | 416–1086 | 179 |
| Phenol formaldehyde resin | — | — | KOH | 5 | 750 °C 1 h | 2653 | 181 |
| Phenol–melamine–formaldehyde resin | — | — | KOH | 2 | 800 °C 1 h | 2376 | 180 |
| K2CO3 | 800 °C 1 h | 1610 | |||||
| ZnCl2 | 700 °C 1 h | 1296 | |||||
| Phenol formaldehyde resin | Oxidation (pre-impregnation) | — | KOH | 1–4 | 700 °C 2 h | 960–2800 | 182 |
| — | 1200–2200 |
Phenolic resin (PR) is an example of thermosetting resin material which is commonly used to make fiber reinforced composites.2 It is a good candidate for carbonization due to its complex structure of phenol and aldehydes that facilitate high carbon yield.179 Dong et al. investigated the production of PR derived AC for application in supercapacitors using K2FeO4 as both an activating and graphitization agent.179 An activation temperature of 950 °C was required to produce an electrode material with a SA above 1000 m2 g−1; however, this was not the only parameter of importance given its application. With respect to SA development alone, KOH has been shown to be most effective compared to K2CO3 and ZnCl2 in the activation of phenol–melamine–formaldehyde resin.180 Within this study, the benefit of PR was also apparent due to the excellent SA of 2376 m2 g−1 achieved, which is superior to that of PET ACs synthesized using similar single-stage activation conditions (Table 4). Additionally, Zheng & Gao (2011) produced a PR derived AC of even higher SA (2653 m2 g−1) using an increased IR of 5.181 Similar to PET, PRs do not require a stabilization step; however, the impact of an oxidative pretreatment was investigated by Teng & Wang (2000). Oxidation after impregnation was found to increase the carbon yield and enhance SA but only at high KOH levels (IR = 4).182
Epoxy resin is another very commonly used thermosetting plastic. In addition to its application in composite materials it is also a large component of electronics, specifically printed circuit boards (PCBs). The non-metallic portions of PCBs, which comprise 70% of the material, are mainly composed of epoxy resin (∼60%).108 Therefore, it has been of interest to convert the non-metallic portions of waste PCBs to high SA AC using KOH activation109 and steam activation.108 Both studies employed carbonization prior to activation at 800 °C. However, the KOH activation (IR = 3) produced a much higher SA (2573 m2 g−1) using a shorter treatment time of 1 h compared to steam activation, which required 1.5 h to produce ACs of SA = 803 m2 g−1. Epoxy ACs were also produced for application in supercapacitors using a similar two-stage process with the same proportion of KOH.183 However, a lower SA of 1353 m2 g−1 was reported, likely due to the lower activation temperature of 600 °C. A single-stage KOH activation process was also investigated by Blanchard & Mekonnen (2023) with increases in IR from 1 to 2 and activation temperature from 600 to 800 °C.110 This resulted in a maximum SA of 1728 m2 g−1, which is lower compared to the two-stage KOH activation processes but still comparable and takes advantage of a much simpler process design.
6. Applications of plastic derived AC
Activated carbon (AC) is mainly used as an adsorbent for a variety of pollutant molecules. At its origins in ancient Egypt (1500 BC) it was used for water purifications, and during the first world war it was implemented in gas masks to remove toxic gases.184 In recent times, AC is often applied for purification of air and color removal from industrial wastes. For many applications, the most important property of the AC adsorbent is the specific surface area, which refers to the total internal and external surface area (SA) of the material per unit mass. Other parameters affecting adsorption include the pore structure, surface functional groups and elemental composition.185 However, it is also dependent on the treatment conditions and the specific mechanism of adsorption.Various adsorption isotherm models are used to describe the adsorption process, specifically the relationship between adsorbate concentration (liquid phase) or pressure (gas phase) and the resulting equilibrium adsorption (mg adsorption/g adsorbent). For example, it is generally known that the equilibrium adsorption of dyes increases with dye concentration until the binding sites are filled and the adsorbent is saturated with dye.186 This adsorption point is called the monolayer capacity (qm) and is a relevant parameter in many adsorption models, such as the Langmuir model, which is a very popular isotherm describing the adsorption of a single layer of solute on an adsorbent surface (Fig. 7). It is a very simple model assuming adsorption onto homogenous surfaces but can accurately predict the adsorption behavior of a wide variety of molecules. As shown in Tables 6 and 7 outlining various plastic derived AC adsorption capacities, most plastic derived ACs exhibit adsorption behaviors which have been described by the Langmuir model. Additionally, the adsorption of key solutes methylene blue and CO2 surpass that of commercial ACs in all relevant studies.
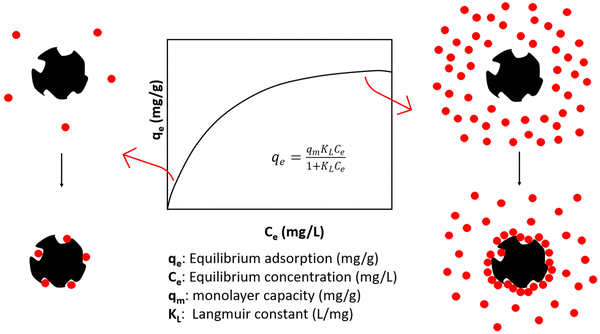 | ||
| Fig. 7 Langmuir adsorption isotherm and corresponding illustrations of adsorption onto AC at low and high solute concentrations.187 | ||
| Precursor | Activating agent | Adsorbate | AC surface area (m2 g−1) | q m (mg g−1) | Isotherm model | Ref. |
|---|---|---|---|---|---|---|
| PVC | KOH | Trichloroethylene | 2666 | 1418.9 | Polanyi-Dubinin-Manes | 155 |
| Dichlorobenzene | 1308.3 | |||||
| Dinitrobenzene | 1193.6 | |||||
| Hexachlorocyclohexane | 2326.5 | |||||
| PET | Trichloroethylene | 2831 | 1510.9 | |||
| Dichlorobenzene | 1381.8 | |||||
| Dinitrobenzene | 1277.6 | |||||
| Hexachlorocyclohexane | 2471.9 | |||||
| PS | Steam | Ni(II) | 842 | 40.82 | Langmuir | 149 |
| PET | KOH | Methylene blue | 1334 | 326.2 | Langmuir | 177 |
| Chloromethylphenoxyacetic acid | 298.9 | |||||
| PET | K2CO3 | Methylene blue | 1390 | 625 | Langmuir | 169 |
| Victoria blue | 137 | |||||
| ZnCl2 | Methylene Blue | 700 | 333 | |||
| Victoria blue | 196 | |||||
| PET | KOH | Methylene blue | 1124 | 335 | Langmuir | 159 |
| PET | KOH | Phenol | 1418 | 207 | Langmuir | 176 |
| Nitrophenol | 278 | |||||
| Epoxy | KOH | Methylene blue | 2572 | 737.19 | Langmuir | 109 |
| Epoxy | KOH | PET nano-plastic | 1705 | 325 | AD-Langmuir | 110 |
| Commercial AC | Methylene Blue | 900 | 303 | Langmuir | 169 | |
| Precursor | Activating agent | Adsorbate | Surface area (m2 g−1) | Monolayer capacity (mmol g−1) | Isotherm model | Ref. | |
|---|---|---|---|---|---|---|---|
| PET | KOH | CO2 | 1338 | 8.65 | Langmuir | 171 | |
| CH4 | 5.60 | ||||||
| H3PO4 | CO2 | 1223 | 8.50 | ||||
| CH4 | 5.30 | ||||||
| ZnCl2 | CO2 | 682 | 5.73 | ||||
| CH4 | 3.38 | ||||||
| H2SO4 | CO2 | 583 | 4.80 | ||||
| CH4 | 2.94 | ||||||
| PET | KOH | CO2 | 1812 | 10.32 | Langmuir | 168 | |
| NaOH | 1707 | 8.18 | |||||
| PET | KOH | CF4 | 1771 | 6.76 | Langmuir | 174 | |
| PET | KOH | CO2 | 1690 | 3.81 | Langmuir | 178 | |
| PVC | KOH | CO2 | 2507 | Site A | 21.36 | Dual-site Langmuir | 158 |
| Site B | 0.78 | ||||||
| Commercial AC | CO 2 | 856 | 4.50 | Langmuir | 194 | ||
6.1. Dye adsorption
One very large application of AC is in the treatment of dye contaminated wastewater produced from textile industries. For this application an additional process consideration is the pH due to its effect on the ionization degree of the adsorbate dye in addition to the chemical state of the AC.186 This is important because textile dyes are often charged molecules, so electrostatic interaction with the AC plays a large role in the adsorption process. For example, the adsorption of a cationic dye such as methylene blue (MB) is enhanced by a negatively charged AC surface.159 As shown in Fig. 8b, a basic pH improves MB adsorption as explained by the development of negative charge on AC through deprotonation of acidic functional groups such as hydroxyls (Fig. 8a). Therefore, not only does the pH influence the electrostatic interaction with charged dyes but also the amount and types of AC surface functional groups. Kuang et al. (2020) reported increased MB adsorption onto AC modified by anionic surfactants, while AC modified by a cationic surfactant reduced MB adsorption (Fig. 8d).188 The effect of dye type as it relates to charge is exemplified in Fig. 8c in which PET ACs selectively adsorb cationic MB dye over anionic methyl orange (MO) dye (Fig. 8c). The monolayer capacity (qm) of MB dye and other aqueous pollutants by plastic derived ACs can be seen in Table 5 alongside their corresponding AC surface areas.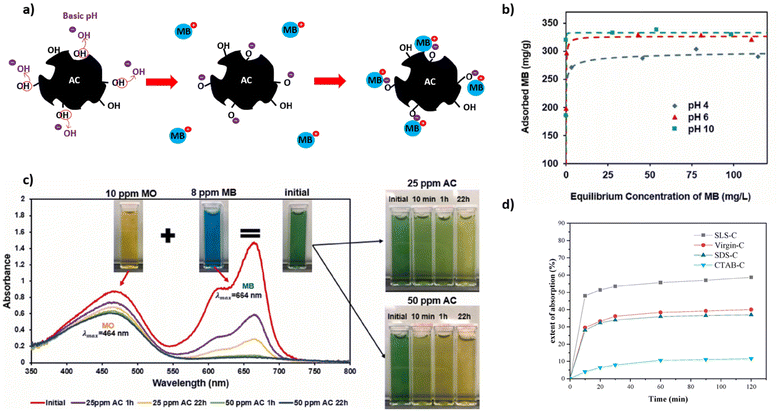 | ||
Fig. 8 (a) Adsorption of methylene blue (MB) by AC in basic solution with hydroxyls as representative surface functional groups facilitating electrostatic interactions; (b) adsorption isotherms of MB onto PET AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 KOH IR, 800 °C) under various solution pHs; (c) UV-vis spectra of a mixed dye solution containing methyl orange (MO) and MB over time after treatment with PET AC (1 1 KOH IR, 800 °C) under various solution pHs; (c) UV-vis spectra of a mixed dye solution containing methyl orange (MO) and MB over time after treatment with PET AC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 KOH IR, 800 °C) and corresponding solution images. Adapted from ref. 159 with permission from Elsevier. Copyright ©2022; (d) adsorption isotherm of MB onto unmodified AC (Virgin-C), and AC modified with anionic sodium lauryl sulfate (SLS-C), anionic sodium dodecyl sulfonate (SDS-C), and cationic hexadecyl trimethyl ammonium bromide (CTAB-C). Adapted from ref. 188. 1 KOH IR, 800 °C) and corresponding solution images. Adapted from ref. 159 with permission from Elsevier. Copyright ©2022; (d) adsorption isotherm of MB onto unmodified AC (Virgin-C), and AC modified with anionic sodium lauryl sulfate (SLS-C), anionic sodium dodecyl sulfonate (SDS-C), and cationic hexadecyl trimethyl ammonium bromide (CTAB-C). Adapted from ref. 188. | ||
6.2. Carbon dioxide adsorption
AC is also commonly used for the adsorption of gas molecules such as volatile organic compounds (VOCs)189 and CO2,190 especially considering the increasing concerns related to climate change. The adsorption process primarily occurs through physical adsorption, which is influenced heavily by the AC surface functional groups. AC can be comprised of various functional groups such as carboxylic acid, phenolic and lactone groups,190 but they also may be imparted through treatments with ammonia, nitric acid, metal hydroxides etc.189 For CO2 adsorption specifically, the introduction of nitrogen191 and oxygen192 functional groups are known to enhance the adsorption. Both elements are more electronegative than the surrounding carbon and can pull electrons to increase the polarity and attract CO2 molecules192 through dipole–dipole forces, as shown by the red hashed bonds in Fig. 9a. Additionally, Yuan et al. (2020) found that the pore size plays a large role in CO2 adsorption by PET ACs.168 As displayed in Fig. 9c, the pore volume of narrow pores (<0.8 nm) was correlated closely with CO2 uptake rather than total pore volume. Therefore, the trend in CO2 adsorption with increasing activation temperature (Fig. 9d) did not align with the BET surface area which was optimal at 800 °C. On the other hand, PET ACs produced at 700 °C followed CO2 adsorption trends aligning with the overall textural properties (BET SA, pore volume) which were maximized at an IR of 3 (Fig. 9b).178 The qm of CO2 and other gaseous pollutants by plastic derived ACs can be seen in Table 6 alongside their corresponding AC surface areas.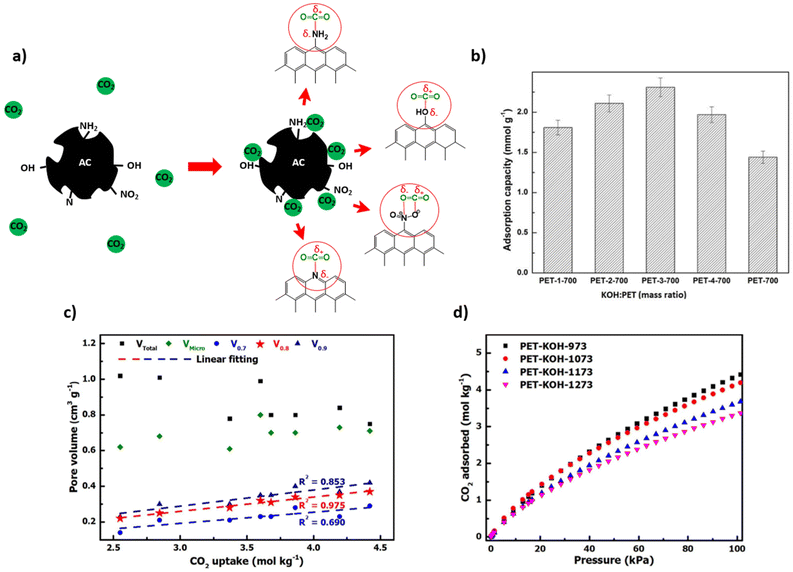 | ||
| Fig. 9 (a) Adsorption of CO2 on AC with oxygen and nitrogen surface functional groups facilitating polar interactions (red hashed bonds);193 (b) adsorption capacities of PET ACs prepared at 700 °C using varying KOH IRs. Adapted from ref. 175 with permission from Elsevier. Copyright ©2019; (c) CO2 uptake in relation to pore volumes of PET AC prepared using a KOH IR of 2 and (d) the CO2 adsorption isotherms using AC of various activation temperatures. Adapted from ref. 168 with permission from Elsevier. Copyright ©2020. | ||
6.3. Micro- and nano-plastic adsorption
Another noteworthy application of AC is in the adsorption of micro- and nano-plastic (NP) pollution from wastewater. This waste is caused by human activities such as laundering and exfoliant products which introduce small plastic particles into wastewater effluents.4 These particles breakdown into microplastics (MPs) and further into nano-plastics (NPs), which can escape wastewater treatment operations much more easily.195 With respect to MPs, various studies have investigated biochar within columns196–198 and AC filters.199 Similarly, the adsorption of the more difficult to treat NPs using biochar200,201 and commercial AC202 have been studied using batch mixing experiments. Recently, a plastic epoxy precursor has been activated and investigated for NP adsorption.110 Unlike the other studies which employed charged PS NPs as the adsorbate, this work used neutrally charged PET NPs. Due to the surface charges, the PS NPs followed monolayer adsorption which was described well by the Langmuir model (Fig. 10a and b), while the more neutral PET NPs could form multiple layers on the AC which was described best by the multilayer AD-Langmuir adsorption isotherm model (Fig. 10c). | ||
| Fig. 10 Adsorption isotherm curves of (a) PS NPs onto sugarcane Bagasse biochar at varying adsorption temperatures. Adapted from ref. 200 with permission from Elsevier. Copyright ©2021; (b) PS NPs onto oxidized and non-oxidized corncob biochar. Adapted from ref. 201 with permission from Elsevier. Copyright ©2021; and (c) PET NPs onto epoxy AC. Adapted from ref. 110 with permission from Elsevier. Copyright ©2023. | ||
The NP adsorption capacity of the various carbonaceous adsorbents and the maximum percentage recovery of NPs are reported in Table 8. Based on the monolayer capacities, the Epoxy AC clearly has a much higher capacity for adsorption of NPs, which may be attributed to its high surface area of 1705 m2 g−1. However, the NP recoveries (%) at low NP concentration are important to discuss as it is relevant to its application in wastewater treatment. While the epoxy AC showed superior adsorption under relatively high NP concentrations (100–350 mg L−1), it struggled to achieve percentage recoveries above 95% as was observed for biochar200 and commercial AC202 at lower NP concentrations. This may be attributed to the oppositely charged NP and adsorbent combinations in these studies (Table 8), which enhance the adsorption even at low solute concentrations. Therefore, adsorbents derived from plastics like epoxy have good potential for applications in NP recovery but may require surface treatments and further process optimization to improve their interaction with neutral plastic particles.
| NP type | Adsorbent type | Adsorbent surface area (m2 g−1) | [NP] (mg L−1) | pH | NP zeta potential (mV) | AC zeta potential (mV) | Monolayer capacity (mg g−1) | Isotherm model | Maximum NP recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PS | Bagasse biochar | 540.36 | 10–50 | 5.5 | −39.8 | +2.85 | 44.9 | Langmuir | >99 | 200 |
| PS | Corncob biochar | 36.3 | 50–1000 | 7 | −48 | −45.1 | 20.89 | Langmuir | — | 201 |
| PS | Commercial AC | 1150 | 5–40 | 7.4 | ∼(+40) | ∼(−28) | 2.15 | Langmuir | 98 | 202 |
| PET | Epoxy AC | 1705 | 100–350 | 7 | −0.074 | −33.8 | 325 | AD-Langmuir | 94 | 110 |
6.4. Comparison to existing products
The capacities of other activated products were also compiled to contextualize the properties of the plastic derived ACs. Activated products of various feedstocks are compared in Table 9 with respect to their surface areas and methylene blue adsorption. Methylene blue (MB) was chosen as a reference adsorbate because it is a common parameter for measuring adsorptive capacity. The most common methods include the iodine adsorption for indication of small pore (<2 nm) adsorption and molasses adsorption for indication of large pore (>50 nm), while MB adsorption is related to both mesopore and macropore adsorption.203 Therefore, MB adsorption data is available for many commercial and synthesized AC products, which can be compared to the aqueous adsorption data obtained for plastic derived ACs (Table 6) containing mainly MB studies.| AC source | AC surface area (m2 g−1) | Langmuir monolayer capacity (mg g−1) | Ref. |
|---|---|---|---|
| Commercial AC (Filtrasorb) | 1050 | 299 | 203 |
| Commercial AC | 950–1050 | 355 | 204 |
| Commercial AC (DARCO) | 900 | 303 | 169 |
| Commercial AC (Merck) | 950 | 200 | 205 |
| Peach stone | 1298 | 412 | 206 |
| Bamboo dust | — | 143 | 207 |
| Coconut shell | — | 278 | |
| Groundnut shell | — | 165 | |
| Rice husk | — | 344 | |
| Straw | — | 472 | |
| Oil palm shell | 596 | 244 | 208 |
| Tire char | 602 | 227 | 209 |
| Rubber seed coat | 1225 | 227 | 210 |
| Desert plant | 1178 | 130 | 205 |
| Cola nut shell | 648 | 87 | 211 |
| Pea shell | — | 270 | 212 |
| Tea seed shell | 1530 | 325 | 213 |
| Chitosan flakes | 318 | 144 | 214 |
When comparing Tables 9–6 it can be stated that plastic derived ACs have very similar SA and MB capacities to the commercial AC products. In terms of the alternative products, there is a range of adsorption capacities generally between 100–400 mg g−1. However, most of these values are also in line with commercial ACs which are observed to be ≥200 mg g−1. As observed in Table 9, the products of lower adsorption capacities are not necessarily explained by the trends in SA due to the effect of the initial feedstock on the final AC surface chemistry. In comparison, the consistently high MB adsorption capacities of the plastic derived products in Table 6 (∼300–700 mg g−1) indicate that these products may exhibit a more acidic surface property, as was reported for PET activated by solid KOH.159,215 This means that the AC surface may contain oxygenated groups with acidic characteristics (e.g. Hydroxyl) which can become deprotonated to produce negative surface charge for better interaction with cationic MB dye.159 Therefore, not only do the plastic derived ACs exhibit competitive SAs with commercial products, but there is also indication of significant oxygen functionalities which can generally improve interaction with polar molecules.
7. Future prospectives
Although research has shown significant potential for the conversion of both thermoplastic and thermoset plastic to activated carbon with substantial adsorption capabilities, there are challenges which have inhibited the implementation of full-scale production. These issues are outlined in Fig. 11. To begin, the first barrier to production of AC from plastics involves the already existing challenges for plastics recycling including sorting, cleaning, and additives. Waste segregation is specifically important due to the concerns surrounding carbonization of mixed plastic streams as mentioned in section 2.4. Therefore, the upcycling of plastic waste will likely begin with plastic that are more easily separated, such as PET bottles which are one of the first plastic wastes to be recycled at high levels.216 PET is also one of the best plastic feedstocks for a carbonization process, with the most amount of research available on its conversion to activated carbon, as compiled in section 5.5.With respect to the conversion process itself, the main factor limiting the large-scale production of AC is the cost.217 Therefore, proper optimization of activation temperatures and times are important to reduce the amount of energy required while producing a product of highest quality and value.184 Additionally, it is vital to address the typically low production yield for the conversion of plastic to AC. For example, the mass yield of carbon material from PET is only around 17%.159,171 This can be improved by doping the plastic precursor with chemicals such as increased levels of chemical activating agents, which has shown to inhibit volatilization during heat treatment.110,159 Lastly, there are environmental concerns surrounding the potential release of hazardous volatile compounds during the carbonization process, such as benzoic and terephthalic acids in the case of PET.218 This issue reinforces the need to improve AC yield such that gaseous emissions are minimized. Overall, upcycling plastic waste into AC would be a very helpful waste diversion method; however, the feasibility must be fully investigated based on the supply of segregated waste streams, energy requirements, and the resulting product value and yield.
8. Conclusions
There is no single solution to the plastic waste issue, as this waste may vary in its composition and each disposal option has its advantages and disadvantages. Relying solely on mechanical recycling proves insufficient, yielding recycled products of limited quality and offering no remedy for thermoset plastics. To address this challenge, a combination of chemical recycling and mechanical recycling is imperative. In addition to the depolymerization of plastics into monomers, it is essential to integrate upcycling methods such as pyrolysis processes to extract value from underutilized waste streams. Notably, this study underscores the significance of carbonization, akin to pyrolysis but tailored to optimize carbon material synthesis. Both pyrolysis and carbonization processes must overcome key recycling issues, such as accommodating mixed waste streams and contamination. However, they offer viable solutions for recycling thermoset waste, which are notoriously challenging to recycle. Furthermore, carbonization processes exhibit the potential to yield high-value products like nanomaterials, carbon fibers, and specifically activated carbon (AC), achievable by introducing an activating agent during carbonization.This review discussed the current progress in the conversion of plastics to AC, with a specific focus on chemical activation. It was evident that KOH is by far the most common activating agent and produced very high surface area ACs. Although many studies successfully produce high surface area ACs through simultaneous carbonization and activation processes, the use of two separate process stages resulted in extremely high surface areas surpassing 2000 m2 g−1. It was also seen that there is much more research available on the production of AC from PET plastic among all other commercial plastics. PET is a good AC precursor as it is a charring plastic due to its aromatic structure and does not require stabilization pre-treatments due to the presence of oxygen. Other plastics like polyolefins do require stabilization treatments but produce similarly high surface area ACs through activation. Thermoset resins are also very good precursors due to their aromatic and oxygenated structure, in addition to the greater need for disposal of these non-recyclable plastics. Both epoxy and phenolic resin have shown good potential for producing ACs with application in supercapacitors, CO2 adsorption and even nano-plastic adsorption.
A very important aspect of the carbonization and activation conversion processes is the value of the resulting AC product. It was shown that plastic derived ACs exhibit very high surface areas, which translates into substantial capacities for the adsorption of pollutants, such as dyes and CO2. There is also potential for these ACs to treat a wider range of pollutants including micro and nano-plastics, which are an increasing pollution concern. However, more research investigating this area is necessary, especially with respect to plastic derived ACs. Overall, this work communicates that alternative plastic waste disposal options such as carbonization can potentially divert a portion of plastic waste from landfilling or incineration. It displayed the success in converting many types of plastics to high surface AC and their application in various adsorption processes. The feasibility of employing these carbonization and activation processes should be considered in detail in future analyses. However, due to the dire need to dispose of plastic wastes, they should not be overlooked as precursors to AC products that are conventionally produced from coal or biomass sources.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The financial support of the University of Waterloo's Engineering Excellence Fellowship Scholarship and NSERC is greatly appreciated.References
- J. Choi, I. Yang, S.-S. Kim, S. Y. Cho and S. Lee, Macromol. Rapid Commun., 2022, 43, 2100467 CrossRef CAS PubMed.
- S. Chen, Z. Liu, S. Jiang and H. Hou, Sci. Total Environ., 2020, 710, 136250 CrossRef CAS PubMed.
- D. K. A. Barnes, F. Galgani, R. C. Thompson and M. Barlaz, Philos. Trans. R. Soc., B, 2009, 364, 1985–1998 CrossRef CAS PubMed.
- K. Boyle and B. Örmeci, Water, 2020, 12, 2633 CrossRef.
- M. Cole, P. Lindeque, C. Halsband and T. S. Galloway, Mar. Pollut. Bull., 2011, 62, 2588–2597 CrossRef CAS PubMed.
- S. D. Anuar Sharuddin, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, Energy Convers. Manage., 2016, 115, 308–326 CrossRef CAS.
- S. Chen, Z. Liu, S. Jiang and H. Hou, Sci. Total Environ., 2020, 710, 136250 CrossRef CAS PubMed.
- J. Gong, X. Chen and T. Tang, Prog. Polym. Sci., 2019, 94, 1–32 CrossRef CAS.
- C. Zhuo and Y. A. Levendis, J. Appl. Polym. Sci., 2014, 131, DOI:10.1002/APP.39931.
- A. Dorigato, Adv. Ind. Eng. Polym. Res., 2021, 4, 53–69 CAS.
- A. Milbrandt, K. Coney, A. Badgett and G. T. Beckham, Resour., Conserv. Recycl., 2022, 183, 106363 CrossRef.
- H. Jung, G. Shin, H. Kwak, L. T. Hao, J. Jegal, H. J. Kim, H. Jeon, J. Park and D. X. Oh, Chemosphere, 2023, 320, 138089 CrossRef CAS PubMed.
- K. Ragaert, L. Delva and K. Van Geem, Waste Manage., 2017, 69, 24–58 CrossRef CAS PubMed.
- J.-P. Lange, ACS Sustainable Chem. Eng., 2021, 9, 15722–15738 CrossRef CAS.
- B. Ruj, V. Pandey, P. Jash and V. Srivastava, Int. J. Appl. Sci. Eng. Res., 2022, 180, 106217, DOI:10.6088/ijaser.04058.
- H. Masoumi, S. M. Safavi and Z. Khani, Int. J. Mech. Ind. Eng., 2012, 6, 213–220 Search PubMed.
- M. Sadat-Shojai and G.-R. Bakhshandeh, Polym. Degrad. Stab., 2011, 96, 404–415 CrossRef CAS.
- G. M. Richard, M. Mario, T. Javier and T. Susana, Resour., Conserv. Recycl., 2011, 55, 472–482 CrossRef.
- G. Wu, J. Li and Z. Xu, Waste Manage., 2013, 33, 585–597 CrossRef PubMed.
- B. Chappell, A. Pramanik, A. K. Basak, P. K. Sarker, C. Prakash, S. Debnath and S. Shankar, Cleaner Mater., 2022, 6, 100158 CrossRef CAS.
- L. A. Utracki and C. A. Wilkie, Polymer blends handbook, Kluwer academic publishers Dordrecht, 2002, vol. 1 Search PubMed.
- C. Ha, H. Park, Y. Kim, S. Kwon and W. Cho, Polym. Adv. Technol., 1996, 7, 483–492 CrossRef CAS.
- P. J. Herrera-Franco and A. Valadez-González, Composites, Part A, 2004, 35, 339–345 CrossRef.
- C. Fonseca-Valero, A. Ochoa-Mendoza, J. Arranz-Andrés and C. González-Sánchez, Composites, Part A, 2015, 69, 94–104 CrossRef CAS.
- R. M. Rosnan and A. Arsad, J. Polym. Eng., 2013, 33, 615–623 CAS.
- A. Causa, M. C. Mistretta, D. Acierno and G. Filippone, AIP Conf. Proc., 2014, 1599, 414–417 CrossRef CAS.
- C. Fang, L. Nie, S. Liu, R. Yu, N. An and S. Li, Composites, Part B, 2013, 55, 498–505 CrossRef CAS.
- Z. O. G. Schyns and M. P. Shaver, Macromol. Rapid Commun., 2021, 42, 2000415 CrossRef CAS PubMed.
- R. Moller and U. Jeske, Recycling PVC. Basics, state of the art, options for action, Research report/preprint Karlsruhe Institute of Technology (KIT), 1995. DOI:10.5445/IR/270037438.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2020, 6, eaba7599 CrossRef PubMed.
- S. Wagner and M. Schlummer, Resour., Conserv. Recycl., 2020, 158, 104800 CrossRef.
- B. D. Vogt, K. K. Stokes and S. K. Kumar, ACS Appl. Polym. Mater., 2021, 3, 4325–4346 CrossRef CAS.
- I. A. Ignatyev, W. Thielemans and B. Vander Beke, ChemSusChem, 2014, 7, 1579–1593 CrossRef CAS PubMed.
- O. Horodytska, F. J. Valdés and A. Fullana, Waste Manage., 2018, 77, 413–425 CrossRef CAS PubMed.
- J. N. Hahladakis and E. Iacovidou, J. Hazard. Mater., 2019, 380, 120887 CrossRef CAS PubMed.
- G. Faraca and T. Astrup, Waste Manage., 2019, 95, 388–398 CrossRef CAS PubMed.
- M. K. Eriksen, J. D. Christiansen, A. E. Daugaard and T. F. Astrup, Waste Manage., 2019, 96, 75–85 CrossRef CAS PubMed.
- F. Welle, Resour., Conserv. Recycl., 2011, 55, 865–875 CrossRef.
- F. Awaja and D. Pavel, Eur. Polym. J., 2005, 41, 1453–1477 CrossRef CAS.
- E. Morici and N. Tz. Dintcheva, Polymer, 2022, 14, 4153 CAS.
- S. Leszczyński and B. Brzychczyk, Pol. J. Chem. Technol., 2007, 9, 122–126 CrossRef.
- X. Xue, S.-Y. Liu, Z.-Y. Zhang, Q.-Z. Wang and C.-Z. Xiao, J. Reinf. Plast. Compos., 2021, 41, 459–480 CrossRef.
- J. Guo, J. Guo and Z. Xu, J. Hazard. Mater., 2009, 168, 567–590 CrossRef CAS PubMed.
- X. Xue, S.-Y. Liu, Z.-Y. Zhang, Q.-Z. Wang and C.-Z. Xiao, J. Reinf. Plast. Compos., 2021, 41, 459–480 CrossRef.
- M. Meysami, C. Tzoganakis, P. Mutyala, S. H. Zhu and M. Bulsari, Int. Polym. Process., 2017, 32, 183–193 CrossRef CAS.
- J. Scheirs, Polymer Recycling: Science, Technology and Applications, J. Wiley & Sons, Chichester, UK, 1998 Search PubMed.
- Y. Liu and X.-B. Lu, J. Polym. Sci., 2022, 60, 3256–3268 CrossRef CAS.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423 CrossRef CAS PubMed.
- A. Maeurer, M. Schlummer and O. Beck, Method for recycling plastic materials and use thereof, Publication numberUS20080281002A1, 2012. Fraunhofer-Gesellschaft Zur Forderung Der Angewandten Forschung E.V Search PubMed.
- T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn and G. W. Huber, Sci. Adv., 2023, 6, eaba7599 CrossRef PubMed.
- J. Xin, Q. Zhang, J. Huang, R. Huang, Q. Z. Jaffery, D. Yan, Q. Zhou, J. Xu and X. Lu, J. Environ. Manage., 2021, 296, 113267 CrossRef CAS PubMed.
- E. Sert, E. Yılmaz and F. S. Atalay, J. Polym. Environ., 2019, 27, 2956–2962 CrossRef CAS.
- S. Ma, D. C. Webster and F. Jabeen, Macromolecules, 2016, 49, 3780–3788 CrossRef CAS.
- T. Liu, C. Hao, L. Wang, Y. Li, W. Liu, J. Xin and J. Zhang, Macromolecules, 2017, 50, 8588–8597 CrossRef CAS.
- C. Yu, Z. Xu, Y. Wang, S. Chen, M. Miao and D. Zhang, ACS Omega, 2018, 3, 8141–8148 CrossRef CAS PubMed.
- G. Huh, K. Kwon, S. Cha, S. Yoon, M. Y. Lee and J. Lee, J. Appl. Polym. Sci., 2009, 114, 2093–2100 CrossRef CAS.
- T. Hashimoto, H. Meiji, M. Urushisaki, T. Sakaguchi, K. Kawabe, C. Tsuchida and K. Kondo, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3674–3681 CrossRef CAS.
- S. Ma, J. Wei, Z. Jia, T. Yu, W. Yuan, Q. Li, S. Wang, S. You, R. Liu and J. Zhu, J. Mater. Chem. A, 2019, 7, 1233–1243 RSC.
- A. Yamaguchi, T. Hashimoto, Y. Kakichi, M. Urushisaki, T. Sakaguchi, K. Kawabe, K. Kondo and H. Iyo, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 1052–1059 CrossRef CAS.
- L. Zhao, Y. Liu, Z. Wang, J. Li, W. Liu and Z. Chen, Polym. Degrad. Stab., 2013, 98, 2125–2130 CrossRef CAS.
- J. Canadell, H. Goossens and B. Klumperman, Macromolecules, 2011, 44, 2536–2541 CrossRef CAS.
- A. Rekondo, R. Martin, A. R. de Luzuriaga, G. Cabañero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC.
- N. Bai, K. Saito and G. P. Simon, Polym. Chem., 2013, 4, 724–730 RSC.
- J. Bai, H. Li, Z. Shi and J. Yin, Macromolecules, 2015, 48, 3539–3546 CrossRef CAS.
- A. Gandini, Prog. Polym. Sci., 2013, 38, 1–29 CrossRef CAS.
- M. Shen, H. Cao and M. L. Robertson, Annu. Rev. Chem. Biomol. Eng., 2020, 11, 183–201 CrossRef CAS PubMed.
- S. Utekar, V. K. Suriya, N. More and A. Rao, Composites, Part B, 2021, 207, 108596 CrossRef CAS.
- T. Liu, X. Guo, W. Liu, C. Hao, L. Wang, W. C. Hiscox, C. Liu, C. Jin, J. Xin and J. Zhang, Green Chem., 2017, 19, 4364–4372 RSC.
- I. Okajima, M. Hiramatsu, Y. Shimamura, T. Awaya and T. Sako, J. Supercrit. Fluids, 2014, 91, 68–76 CrossRef CAS.
- R. Piñero-Hernanz, C. Dodds, J. Hyde, J. García-Serna, M. Poliakoff, E. Lester, M. J. Cocero, S. Kingman, S. Pickering and K. H. Wong, Composites, Part A, 2008, 39, 454–461 CrossRef.
- G. Oliveux, J.-L. Bailleul and E. L. G. La Salle, Composites, Part A, 2012, 43, 1809–1818 CrossRef CAS.
- S. H. Gebre, M. G. Sendeku and M. Bahri, ChemistryOpen, 2021, 10, 1202 CrossRef CAS PubMed.
- Y. Wang, B. P. Chang, A. Veksha, A. Kashcheev, A. ling Y. Tok, V. Lipik, R. Yoshiie, Y. Ueki, I. Naruse and G. Lisak, J. Hazard. Mater., 2024, 464, 132996 CrossRef CAS PubMed.
- S. Kumar, A. K. Panda and R. K. Singh, Resour., Conserv. Recycl., 2011, 55, 893–910 CrossRef.
- T. Ueno, E. Nakashima and K. Takeda, Polym. Degrad. Stab., 2010, 95, 1862–1869 CrossRef CAS.
- G. Elordi, M. Olazar, G. Lopez, M. Artetxe and J. Bilbao, Ind. Eng. Chem. Res., 2011, 50, 6650–6659 CrossRef CAS.
- P. Das and P. Tiwari, Waste Manage., 2018, 79, 615–624 CrossRef CAS PubMed.
- T. M. Kruse, O. S. Woo, H.-W. Wong, S. S. Khan and L. J. Broadbelt, Macromolecules, 2002, 35, 7830–7844 CrossRef CAS.
- J. Zhou, Y. Qiao, W. Wang, E. Leng, J. Huang, Y. Yu and M. Xu, Fuel, 2016, 182, 333–339 CrossRef CAS.
- J. A. Onwudili, N. Insura and P. T. Williams, J. Anal. Appl. Pyrolysis, 2009, 86, 293–303 CrossRef CAS.
- I. Aminu, M. A. Nahil and P. T. Williams, Energy Fuels, 2022, 36, 3788–3801 CrossRef CAS.
- U. R. Gracida-Alvarez, M. K. Mitchell, J. C. Sacramento-Rivero and D. R. Shonnard, Ind. Eng. Chem. Res., 2018, 57, 1912–1923 CrossRef CAS.
- Y. Zhang, J. Huang and P. T. Williams, Energy Fuels, 2017, 31, 8497–8504 CrossRef CAS.
- P. T. Williams and E. A. Williams, Energy Fuels, 1999, 13, 188–196 CrossRef CAS.
- J.-K. Koo and S.-W. Kim, Waste Manag. Res., 1993, 11, 515–529 CrossRef CAS.
- H. P. Wenning, J. Anal. Appl. Pyrolysis, 1993, 25, 301–310 CrossRef CAS.
- M. Roosen, N. Mys, M. Kusenberg, P. Billen, A. Dumoulin, J. Dewulf, K. M. Van Geem, K. Ragaert and S. De Meester, Environ. Sci. Technol., 2020, 54, 13282–13293 CrossRef CAS PubMed.
- Z. Yuan, J. Zhang, P. Zhao, Z. Wang, X. Cui, L. Gao, Q. Guo and H. Tian, ACS Omega, 2020, 5, 11291–11298 CrossRef CAS PubMed.
- J. Yu, L. Sun, C. Ma, Y. Qiao and H. Yao, Waste Manage., 2016, 48, 300–314 CrossRef CAS PubMed.
- H. C. Genuino, M. P. Ruiz, H. J. Heeres and S. R. A. Kersten, Fuel Process. Technol., 2022, 233, 107304 CrossRef CAS.
- E. Morici and N. Dintcheva, Polymer, 2022, 14, 4153 CAS.
- X. Xue, S.-Y. Liu, Z.-Y. Zhang, Q.-Z. Wang and C.-Z. Xiao, J. Reinf. Plast. Compos., 2021, 41, 459–480 CrossRef.
- R. Ginder and S. Ozcan, Recycling, 2019, 4, 24 CrossRef.
- S. J. Pickering, Composites, Part A, 2006, 37, 1206–1215 CrossRef.
- Y. Luo, X. Lin, E. Lichtfouse, H. Jiang and C. Wang, Environ. Chem. Lett., 2023, 21, 3127–3158 CrossRef CAS.
- S. Ren, X. Xu, K. Hu, W. Tian, X. Duan, J. Yi and S. Wang, Carbon Res., 2022, 1, 15 CrossRef.
- J. Gong, X. Chen and T. Tang, Prog. Polym. Sci., 2019, 94, 1–32 CrossRef CAS.
- L. Dai, O. Karakas, Y. Cheng, K. Cobb, P. Chen and R. Ruan, Chem. Eng. J., 2023, 453, 139725 CrossRef CAS.
- J. Y. Q. Teo, A. Ong, T. T. Y. Tan, X. Li, X. J. Loh and J. Y. C. Lim, Green Chem., 2022, 24, 6086–6099 RSC.
- W. A. Algozeeb, P. E. Savas, Z. Yuan, Z. Wang, C. Kittrell, J. N. Hall, W. Chen, P. Bollini and J. M. Tour, ACS Nano, 2022, 16, 7284–7290 CrossRef CAS PubMed.
- J. Gong, J. Liu, Z. Jiang, X. Chen, X. Wen, E. Mijowska and T. Tang, Appl. Catal., B, 2014, 152–153, 289–299 CrossRef CAS.
- J. Gong, B. Michalkiewicz, X. Chen, E. Mijowska, J. Liu, Z. Jiang, X. Wen and T. Tang, ACS Sustainable Chem. Eng., 2014, 2, 2837–2844 CrossRef CAS.
- J. Gong, J. Liu, X. Chen, Z. Jiang, X. Wen, E. Mijowska and T. Tang, J. Mater. Chem. A, 2015, 3, 341–351 RSC.
- J. Ma, J. Liu, J. Song and T. Tang, RSC Adv., 2018, 8, 2469–2476 RSC.
- X. Wu, W. H. Tu, A. Veksha, W. Chen and G. Lisak, Chemosphere, 2024, 349, 140769 CrossRef CAS PubMed.
- A. Veksha, W. Chen, L. Liang and G. Lisak, J. Hazard. Mater., 2022, 435, 128949 CrossRef CAS PubMed.
- H.-W. Wong, J. Peck, R. E. Bonomi, J. Assif, F. Panerai, G. Reinisch, J. Lachaud and N. N. Mansour, Polym. Degrad. Stab., 2015, 112, 122–131 CrossRef CAS.
- Y. Kan, Q. Yue, B. Gao and Q. Li, Mater. Lett., 2015, 159, 443–446 CrossRef CAS.
- Y. Kan, Q. Yue, B. Gao and Q. Li, J. Taiwan Inst. Chem. Eng., 2016, 68, 440–445 CrossRef CAS.
- R. Blanchard and T. H. Mekonnen, Sep. Purif. Technol., 2023, 326, 124755 CrossRef CAS.
- C. Zhao, S. Chen, N. Sun, W. Jiang, W. Cai and C. Zhao, J. Phys. Chem. C, 2023, 127, 18821–18831 CrossRef CAS.
- F. Zhang, Y. Zhao, D. Wang, M. Yan, J. Zhang, P. Zhang, T. Ding, L. Chen and C. Chen, J. Cleaner Prod., 2021, 282, 124523 CrossRef CAS.
- D. Choi, D. Jang, H.-I. Joh, E. Reichmanis and S. Lee, Chem. Mater., 2017, 29, 9518–9527 CrossRef CAS.
- J. M. Younker, T. Saito, M. A. Hunt, A. K. Naskar and A. Beste, J. Am. Chem. Soc., 2013, 135, 6130–6141 CrossRef CAS PubMed.
- D. Hines, A. Bagreev and T. J. Bandosz, Langmuir, 2004, 20, 3388–3397 CrossRef CAS PubMed.
- C. Zou, D. Wu, M. Li, Q. Zeng, F. Xu, Z. Huang and R. Fu, J. Mater. Chem., 2010, 20, 731–735 RSC.
- W. M. Qiao, S. H. Yoon, I. Mochida and J. H. Yang, Waste Manage., 2007, 27, 1884–1890 CrossRef CAS PubMed.
- B. E. Barton, J. Patton, E. Hukkanen, M. Behr, J.-C. Lin, S. Beyer, Y. Zhang, L. Brehm, B. Haskins, B. Bell, B. Gerhart, A. Leugers and M. Bernius, Carbon, 2015, 94, 465–471 CrossRef CAS.
- A. R. Postema, H. De Groot and A. J. Pennings, J. Mater. Sci., 1990, 25, 4216–4222 CrossRef CAS.
- P. J. Kim, H. D. Fontecha, K. Kim and V. G. Pol, ACS Appl. Mater. Interfaces, 2018, 10, 14827–14834 CrossRef CAS PubMed.
- X. Yang, C. Li, G. Zhang and C. Yang, J. Mater. Sci., 2015, 50, 6649–6655 CrossRef CAS.
- C. Li, H. Zhu, N. V. Salim, B. L. Fox and N. Hameed, Polym. Degrad. Stab., 2016, 134, 272–283 CrossRef CAS.
- S. Villagómez-Salas, P. Manikandan, S. F. Acuña Guzmán and V. G. Pol, ACS Omega, 2018, 3, 17520–17527 CrossRef PubMed.
- W. M. Qiao, Y. Song, S.-H. Yoon, Y. Korai, I. Mochida, S. Yoshiga, H. Fukuda and A. Yamazaki, Waste Manage., 2006, 26, 592–598 CrossRef CAS PubMed.
- T. Saito, J. Appl. Phys., 2009, 105, 013902 CrossRef.
- Y. Bai, Z. Wang, C. Wu, R. Xu, F. Wu, Y. Liu, H. Li, Y. Li, J. Lu and K. Amine, ACS Appl. Mater. Interfaces, 2015, 7, 5598–5604 CrossRef CAS PubMed.
- R. Bhattacharya, J. Environ. Manage., 2023, 325, 116613 CrossRef CAS PubMed.
- A. Sharma, J. Jindal, A. Mittal, K. Kumari, S. Maken and N. Kumar, Environ. Chem. Lett., 2021, 19, 875–910 CrossRef CAS.
- Y. Gao, Q. Yue, B. Gao and A. Li, Sci. Total Environ., 2020, 746, 141094 CrossRef CAS PubMed.
- Z. Heidarinejad, M. H. Dehghani, M. Heidari, G. Javedan, I. Ali and M. Sillanpää, Environ. Chem. Lett., 2020, 18, 393–415 CrossRef CAS.
- T. S. Hui and M. A. A. Zaini, Carbon Lett., 2015, 16, 275–280 CrossRef.
- A. Spence, Activated carbon breakthrough offers solution to global plastic crisis, The Lead News. https://theleadsouthaustralia.com.au/industries/technology/activated-carbon-breakthrough-offers-solution-to-global-plastic-crisis/, (accessed 12 December 2023).
- F. Hussin, M. K. Aroua, M. A. Kassim and U. F. Md. Ali, Energies, 2021, 14, 8421 CrossRef CAS.
- T. Otowa, R. Tanibata and M. Itoh, Gas Sep. Purif., 1993, 7, 241–245 CrossRef CAS.
- A. Linares-Solano, M. A. Lillo-Ródenas, J. P. Marco-Lozar, M. Kunowsky and A. J. Romero-Anaya, Int. J. Energy Environ. Econ., 2012, 20, 59–91 Search PubMed.
- M. A. Lillo-Ródenas, D. Cazorla-Amorós and A. Linares-Solano, Carbon, 2003, 41, 267–275 CrossRef.
- Y. Xi, D. Yang, X. Qiu, H. Wang, J. Huang and Q. Li, Ind. Crops Prod., 2018, 124, 747–754 CrossRef CAS.
- Z. Xu, Y. Zhou, Z. Sun, D. Zhang, Y. Huang, S. Gu and W. Chen, Chemosphere, 2020, 241, 125120 CrossRef CAS PubMed.
- D. Qiu, N. Guo, A. Gao, L. Zheng, W. Xu, M. Li, F. Wang and R. Yang, Electrochim. Acta, 2019, 294, 398–405 CrossRef CAS.
- L. Desidery and M. Lanotte, in Plastic Waste for Sustainable Asphalt Roads, ed. F. Giustozzi and S. Nizamuddin, Woodhead Publishing, Cambridge, UK, 2022, pp. 3–28 Search PubMed.
- A. L. Andrady and M. A. Neal, Philos. Trans. R. Soc., B, 2009, 364, 1977–1984 CrossRef CAS PubMed.
- D. Jubinville, G. Chen and T. H. Mekonnen, Polym. Degrad. Stab., 2023, 211, 110342 CrossRef CAS.
- S. Saikrishnan, D. Jubinville, C. Tzoganakis and T. H. Mekonnen, Polym. Degrad. Stab., 2020, 182, 109390 CrossRef CAS.
- H. Maddah, Am. J. Polym. Sci., 2016, 6, 1–11 CAS.
- T. Terashima, in Encyclopedia of Polymeric Nanomaterials, ed. S. Kobayashi and K. Müllen, Springer Berlin Heidelberg, Berlin, Germany, 2015, pp. 2077–2091 Search PubMed.
- G. R. Koerner, Y. G. Hsuan and R. M. Koerner, in Geosynthetics in Civil Engineering, ed. R. W. Sarsby, Woodhead Publishing, Cambridge, UK, 2007, pp. 36–65 Search PubMed.
- E. A. Campo, in Selection of Polymeric Materials, ed. E. A. Campo, William Andrew Publishing, Norwich, NY, USA, 2008, pp. 1–39 Search PubMed.
- I. Yang, J. H. Mok, M. Jung, J. Yoo, M.-S. Kim, D. Choi and J. C. Jung, Macromol. Rapid Commun., 2022, 43, 2200006 CrossRef CAS PubMed.
- L. Gonsalvesh, S. P. Marinov, G. Gryglewicz, R. Carleer and J. Yperman, Fuel Process. Technol., 2016, 149, 75–85 CrossRef CAS.
- G. Gatti, M. Errahali, L. Tei, E. Mangano, S. Brandani, M. Cossi and L. Marchese, Nanomaterials, 2019, 9, 726 CrossRef CAS PubMed.
- N. Deka, J. Barman, S. Kasthuri, V. Nutalapati and G. K. Dutta, Appl. Surf. Sci., 2020, 511, 145576 CrossRef CAS.
- F. G. F. de Paula, M. C. M. de Castro, P. F. R. Ortega, C. Blanco, R. L. Lavall and R. Santamaría, Microporous Mesoporous Mater., 2018, 267, 181–184 CrossRef CAS.
- W. M. Qiao, S. H. Yoon, Y. Korai, I. Mochida, S. Inoue, T. Sakurai and T. Shimohara, Carbon, 2004, 42, 1327–1331 CrossRef CAS.
- X. Liu, F. Yang, M. Li, S. Wang and C. Sun, Sci. Total Environ., 2022, 833, 154894 CrossRef CAS PubMed.
- F. Lian, C. Chang, Y. Du, L. Zhu, B. Xing and C. Liu, J. Environ. Sci., 2012, 24, 1549–1558 CrossRef CAS PubMed.
- Z. Li, R. Wang, J. Ye, F. Liu and Z. Sha, CHISA, 17th International Congress of Chemical and Process Engineering, 2006.
- Z. Li, K. Wang, J. Song, Q. Xu and N. Kobayashi, J. Mater. Cycles Waste Manag., 2014, 16, 359–366 CrossRef CAS.
- X. Liu, F. Yang, M. Li, S. Wang and C. Sun, Sci. Total Environ., 2022, 833, 154894 CrossRef CAS PubMed.
- R. Blanchard and T. H. Mekonnen, J. Environ. Chem. Eng., 2022, 10, 108810 CrossRef CAS.
- L. Wei, N. Yan and Q. Chen, Environ. Sci. Technol., 2011, 45, 534–539 CrossRef CAS PubMed.
- J. B. Parra, C. O. Ania, A. Arenillas, F. Rubiera and J. J. Pis, Appl. Surf. Sci., 2004, 238, 304–308 CrossRef CAS.
- J. B. Parra, C. O. Ania, A. Arenillas, F. Rubiera, J. M. Palacios and J. J. Pis, J. Alloys Compd., 2004, 379, 280–289 CrossRef CAS.
- A. Esfandiari, T. Kaghazchi and M. Soleimani, J. Taiwan Inst. Chem. Eng., 2012, 43, 631–637 CrossRef CAS.
- W. Bratek, A. Świątkowski, M. Pakuła, S. Biniak, M. Bystrzejewski and R. Szmigielski, J. Anal. Appl. Pyrolysis, 2013, 100, 192–198 CrossRef CAS.
- R. Mendoza-Carrasco, E. M. Cuerda-Correa, M. F. Alexandre-Franco, C. Fernández-González and V. Gómez-Serrano, J. Environ. Manage., 2016, 181, 522–535 CrossRef CAS PubMed.
- K. László, A. Bóta and L. G. Nagy, Carbon, 2000, 38, 1965–1976 CrossRef.
- E. Lorenc-Grabowska, M. A. Diez and G. Gryglewicz, J. Colloid Interface Sci., 2016, 469, 205–212 CrossRef CAS PubMed.
- X. Yuan, J. G. Lee, H. Yun, S. Deng, Y. J. Kim, J. E. Lee, S. K. Kwak and K. B. Lee, Chem. Eng. J., 2020, 397, 125350 CrossRef CAS.
- C. S. de Castro, L. N. Viau, J. T. Andrade, T. A. P. Mendonça and M. Gonçalves, New J. Chem., 2018, 42, 14612–14619 RSC.
- I. P. da Paixão Cansado, C. R. Belo and P. A. Mira Mourão, Environ. Nanotechnol. Monit. Manag., 2019, 12, 100261 Search PubMed.
- M. Adibfar, T. Kaghazchi, N. Asasian and M. Soleimani, Chem. Eng. Technol., 2014, 37, 899–1079, DOI:10.1002/ceat.201200719.
- Ç. Sarıcı-Özdemir and Y. Önal, Fullerenes, Nanotubes Carbon Nanostruct., 2018, 26, 451–457 CrossRef.
- S. Ayyalusamy, S. Mishra and V. Suryanarayanan, Sci. Rep., 2018, 8, 13151 CrossRef PubMed.
- X. Yuan, M.-K. Cho, J. G. Lee, S. W. Choi and K. B. Lee, Environ. Pollut., 2020, 265, 114868 CrossRef CAS PubMed.
- B. Kaur, J. Singh, R. K. Gupta and H. Bhunia, J. Environ. Manage., 2019, 242, 68–80 CrossRef CAS PubMed.
- I. P. P. Cansado, P. A. M. Mourão, A. I. Falcão, M. M. L. R. Carrott and P. J. M. Carrott, Fuel Process. Technol., 2012, 103, 64–70 CrossRef CAS.
- I. P. P. Cansado, C. Galacho, Á. S. Nunes, M. L. R. Carrott and P. J. M. Carrott, Adsorpt. Sci. Technol., 2010, 28, 807–821 CrossRef CAS.
- B. Kaur, J. Singh, R. K. Gupta and H. Bhunia, J. Environ. Manage., 2019, 242, 68–80 CrossRef CAS PubMed.
- X. Dong, J. Wang, M. Yan, B. Ren, J. Miao, L. Zhang, Z. Liu and Y. Xu, Ceram. Int., 2021, 47, 5998–6009 CrossRef CAS.
- X. Xiang, E. Liu, H. Xie, Y. Tian, Y. Wu, Z. Wu and Y. Zhu, J. Solid State Electrochem., 2012, 16, 2661–2666 CrossRef CAS.
- Z. Zheng and Q. Gao, J. Power Sources, 2011, 196, 1615–1619 CrossRef CAS.
- H. Teng and S.-C. Wang, Ind. Eng. Chem. Res., 2000, 39, 673–678 CrossRef CAS.
- C. Zhao, S. Chen, N. Sun, W. Jiang, W. Cai and C. Zhao, J. Phys. Chem. C, 2023, 127, 18821–18831 CrossRef CAS.
- M. A. Tadda, A. Ahsan, A. Shitu, M. Elsergany, A. Thirugnanasambantham, B. Jose, M. Razzaque and N. Norsyahariati, J. Adv. Civ. Eng. Pract. Res., 2016, 2, 7–13 Search PubMed.
- X. Wang, H. Cheng, G. Ye, J. Fan, F. Yao, Y. Wang, Y. Jiao, W. Zhu, H. Huang and D. Ye, Chemosphere, 2022, 287, 131995 CrossRef CAS PubMed.
- S. Husien, R. M. El-taweel, A. I. Salim, I. S. Fahim, L. A. Said and A. G. Radwan, Curr. Res. Green Sustainable Chem., 2022, 5, 100325 CrossRef CAS.
- J. Wang and X. Guo, Chemosphere, 2020, 258, 127279 CrossRef CAS PubMed.
- Y. Kuang, X. Zhang and S. Zhou, Water, 2020, 12, 1–19 CrossRef.
- S. Zhang, Q. Chen, M. Hao, Y. Zhang, X. Ren, F. Cao, L. Zhang, Q. Sun and R. Wennersten, Surf. Sci., 2023, 736, 122352 CrossRef CAS.
- A. Saadallah, M. Alsultan, A. Sabah and G. (Gerry) Swiegers, J. Compos. Sci., 2023, 7, 179 CrossRef.
- D. Liu, J. Li, J. Dong, S. Li, W. Feng and B. Jia, Processes, 2019, 7, 801 CrossRef CAS.
- B. Liu, H. Li, X. Ma, R. Chen, S. Wang and L. Li, RSC Adv., 2018, 8, 38965–38973 RSC.
- B. Petrovic, M. Gorbounov and S. Masoudi Soltani, Carbon Capture Sci. Technol., 2022, 3, 100045 CrossRef CAS.
- J. Sreńscek- Nazzal, U. Narkiewicz, A. Morawski, R. Wróbel and B. Michalkiewicz, Acta Phys. Pol., A, 2016, 129, 394–401 CrossRef.
- A. Murray and B. Örmeci, Water, 2020, 12, 635 CrossRef CAS.
- V. Siipola, S. Pflugmacher, H. Romar, L. Wendling and P. Koukkari, Appl. Sci., 2020, 10, 788 CrossRef CAS.
- M. Tong, L. He, H. Rong, M. Li and H. Kim, Water Res., 2020, 169, 115284 CrossRef CAS PubMed.
- Z. Wang, M. Sedighi and A. Lea-Langton, Water Res., 2020, 184, 116165 CrossRef CAS PubMed.
- Z. Wang, T. Lin and W. Chen, Sci. Total Environ., 2020, 700, 134520 CrossRef CAS PubMed.
- Z. A. Ganie, N. Khandelwal, E. Tiwari, N. Singh and G. K. Darbha, J. Hazard. Mater., 2021, 417, 126096 CrossRef CAS PubMed.
- A. S. I. Abdoul Magid, Md. S. Islam, Y. Chen, L. Weng, J. Li, J. Ma and Y. Li, Sci. Total Environ., 2021, 784, 147115 CrossRef CAS PubMed.
- L. Ramirez Arenas, S. Ramseier Gentile, S. Zimmermann and S. Stoll, Sci. Total Environ., 2021, 791, 148175 CrossRef CAS PubMed.
- F. Raposo, M. A. De La Rubia and R. Borja, J. Hazard. Mater., 2009, 165, 291–299 CrossRef CAS PubMed.
- J. H. Potgieter, J. Chem. Educ., 1991, 68, 349 CrossRef CAS.
- B. Bestani, N. Benderdouche, B. Benstaali, M. Belhakem and A. Addou, Bioresour. Technol., 2008, 99, 8441–8444 CrossRef CAS PubMed.
- A. A. Attia, B. S. Girgis and N. A. Fathy, Dyes Pigm., 2008, 76, 282–289 CrossRef.
- N. Kannan and M. M. Sundaram, Dyes Pigm., 2001, 51, 25–40 CrossRef CAS.
- I. A. W. Tan, A. L. Ahmad and B. H. Hameed, Desalination, 2008, 225, 13–28 CrossRef CAS.
- Y. R. Lin and H. Teng, Microporous Mesoporous Mater., 2002, 54, 167–174 CrossRef CAS.
- B. H. Hameed and F. B. M. Daud, Chem. Eng. J., 2008, 139, 48–55 CrossRef CAS.
- J. Ndi Nsami and J. Ketcha Mbadcam, J. Chem., 2013, 2013, 1–7 CrossRef.
- Ü. Geçgel, G. Özcan and G. Ç. Gürpnar, J. Chem., 2013, 1, 1–9 Search PubMed.
- J. Gao, Y. Qin, T. Zhou, D. Cao, P. Xu, D. Hochstetter and Y. Wang, J. Zhejiang Univ., Sci., B, 2013, 14, 650 CrossRef CAS PubMed.
- F. Marrakchi, M. J. Ahmed, W. A. Khanday, M. Asif and B. H. Hameed, Int. J. Biol. Macromol., 2017, 98, 233–239 CrossRef CAS PubMed.
- V. Gómez-Serrano, M. Adame-Pereira, M. Alexandre-Franco and C. Fernández-González, Environ. Sci. Pollut. Res., 2020, 28, 24342–24354 CrossRef PubMed.
- S. E. Selke, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan and P. Veyssière, Elsevier, Oxford, 2001, pp. 8075–8079 Search PubMed.
- J. Hopewell, R. Dvorak and E. Kosior, Philos. Trans. R. Soc., B, 2009, 364, 2115–2126 CrossRef CAS PubMed.
- Y. Sakata, M. A. Uddin, K. Koizumi and K. Murata, Polym. Degrad. Stab., 1996, 53, 111–117 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |