Open Access Article
Open Access ArticlePolymeric biomaterials for periodontal tissue engineering and periodontitis
Gizem
Yürük†
 ,
Yağmur Damla
Demir†
,
Yağmur Damla
Demir†
 ,
Şevra
Vural
,
Şevra
Vural
 and
Nermin Seda
Kehr
and
Nermin Seda
Kehr
 *
*
Department of Chemistry, Izmir Institute of Technology, 35430, Urla/Izmir, Turkey. E-mail: sedakehr@iyte.edu.tr
First published on 17th April 2024
Abstract
The periodontium is one of the most complex tissues in the body because its structure is formed by a hierarchical combination of soft and hard tissues. Due to its complex architecture, the treatment and regeneration of damaged periodontal tissue caused by diseases is still a challenge in biomedicine. The most common disease of the periodontium is periodontitis, which occurs when the periodontium becomes infected and inflamed as a bacterial biofilm forms in the mouth. Recently, various biocompatible biomaterials made of natural and synthetic polymers have been developed for periodontal tissue regeneration or treatment due to their superior properties such as controlled drug and bioactive molecule delivery, mimicking the 3D network of tissue, biocompatibility, antibacterial and mechanical properties. In particular, biomaterials designed for drug delivery, such as hydrogels, scaffolds, films, membranes, micro/nanoparticles and fibers, and additively manufactured biomaterials have undergone in vitro and in vivo testing to confirm their potential clinical utility in periodontal regeneration and periodontitis treatment. This review explores recent advances in the use of biomaterials for the prevention and/or treatment of periodontal regeneration and periodontitis. Specifically, it emphasizes advancements in drug/biomolecule delivery and the use of additively manufactured biomaterials for addressing periodontal issues.
1. Introduction
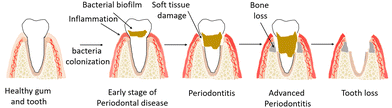
The periodontium includes hard and soft tissues as it consists of gingiva, periodontal ligament (PDL), cementum and alveolar bone.1,2 The most common disease of the periodontium is periodontitis, an infection-related inflammatory disease that affects about 20–50% of the world's population and is common in both developed and developing countries. Periodontitis results from the formation of a biofilm or, in other words, the accumulation of plaque at the gum line, which triggers systemic inflammation and damages the periodontium,3 including PDL and alveolar bone. In the late stages, the affected tooth may fall out of the jaw and the damage in periodontium can be irreversible (Fig. 1).Current treatments rely on daily oral hygiene and professional temporary removal of the microbial biofilm, but these treatments cannot prevent the biofilm from returning. Lost teeth can be replaced with implants, and jawbone can be replaced with surgical bone grafts or artificial tissue constructs. However, regeneration of the entire periodontium remains a challenge because the complex and hierarchical architecture of the periodontium requires highly synchronous spatiotemporal regeneration.
In this context, recently, the engineering of biomaterials for periodontal regeneration has attracted considerable interest and has the potential to be utilized clinically in a wide range of applications. Therefore, this review discusses the latest developments regarding the use of biomaterials in periodontal regeneration and in the prevention and/or treatment of periodontitis. This review by no means covers all biomaterial systems currently used for dental applications; the focus is on the drug delivery and/or additively manufactured biomaterials used for periodontal regeneration and periodontitis.
2. The periodontium and the periodontal diseases
The periodontium, a complex but hierarchical connective tissue, is composed of soft and hard tissues including the gingiva, alveolar bone, cementum, and PDL. These four different tissue types have different functions in the periodontium and differ in their cellular composition, extracellular components, and degree of metabolic activity, mineralization, and vascularization.Cementum is an avascular, calcified tissue covering the root of a tooth and allows the attachment of the tooth to the alveolar bone by supporting insertion of PDL fibers that hold the tooth to the alveolar bone within the socket. In addition, cementum inhibits root resorption during remodeling of the periodontium.
The alveolar bone, a highly mineralized tissue, is the part of the jawbone that surrounds the teeth and forms the tooth sockets. It also provides the attachment of the fibers of the PDL to hold the teeth tightly in the tooth socket. Moreover, blood vessels and nerves run through the alveolar bone supply the PDL. Alveolar bone is excreted by osteoblasts, osteocytes, and osteoclasts, while cementoblasts are responsible for the homeostasis and functions of the cementum.
The PDL is a unique vascular and connective tissue consisting of a series of aligned fibers. The PDL lies between the cementum and the alveolar bone and connects the tooth to the surrounding alveolar bone. The most abundant cells in the PDL are fibroblasts, which are responsible for the development, function and regeneration of the system that connects the tooth to the alveolar bone.
The gingiva, a part of the oral mucosa, is the covering tissue of the periodontium and surrounds the neck of the tooth. The gingiva protects the teeth and the underlying bone and provides additional attachment of the tooth. The fibroblasts of the gingiva are the most abundant cells in the gingiva, regulating tissue repair and the inflammatory response. The main functions of the periodontium are therefore to support the tooth and its attachment to the bone, to protect the tooth from injury by mechanical forces and microbes, and to enable sensation to environmental factors such as touch, temperature, and pressure.
The bacteria in the mouth form a biofilm around the tooth, or, in other words, plaque is accumulated at the supragingival line (above the gum line). If the bacteria remain on the teeth long enough, the biofilm becomes harder (tartar) and spreads to the subgingival (below the gum line) areas. Subgingival biofilms trigger the host's immune defenses and lead to an inflammatory response in the gingiva that initiates periodontal destruction. The damaging effects of biofilms spread from the gums to the tooth. Inflammations first affect the gums, which swell and bleed. This stage of periodontal disease is called gingivitis. If inflammation and infection are not prevented, periodontitis develops, and the gums may retract from the tooth. Since periodontitis does not cause pain, it goes unnoticed for a long time. Therefore, often, periodontitis is not discovered until the tooth is already damaged. Chronic periodontitis can lead to bone loss, which is irreversible and leads to loss of the periodontium and in the final stage teeth may fall out. Different stages of periodontal disease can occur in both adults and children, but chronic periodontitis mostly affects adults. This pathophysiological situation continues until the affected tooth is extracted or the bacterial biofilm is therapeutically removed by a dentist and the periodontal disease process is stopped. Periodontitis thus affects both the hard and soft tissues of the periodontium, threatens the integrity of these tissues, and induces irreversible damage.
2.1. Current approaches for periodontal therapy
The most common treatment for periodontal disease4 is regular professional cleaning of biofilms and tartar or in order words, scaling, and root planning. Thus, gingivitis can be kept biofilm-free for a while by good oral hygiene on a daily basis and removal of biofilms by professionals, but these treatments cannot prevent the biofilm from returning. Severe periodontitis, on the other hand, requires surgical treatment procedures such as deep cleaning of the tooth root surfaces and reshaping or replacement of the damaged tissues. On the other hand, lost teeth can be replaced with implants, and jawbone can be replaced with surgical bone grafts or artificial tissue constructs.Dental implants currently on the market are primarily made of titanium, gold, zirconia, and ceramics; the main disadvantage of these materials is their low elasticity. Therefore, polymeric materials such as polymethyl methacrylate and polytetrafluoroethylene are increasingly used in dentistry. Polymeric materials are also attractive due to their biological inertness, high mechanical strength, elasticity, and good fatigue resistance, and they can be used as drug delivery systems. Despite their advantages, it is difficult to find an ideal biomaterial that has excellent mechanical stability, can mimic the three-dimensional (3D) complexity and functionality of tooth, jawbone or PDL, and has surface properties that inhibit biofilm formation and immunological reactions and that support adhesion to living tissue.
As an alternative to implants, autografts, allografts, or xenografts are used as jawbone grafts. They have disadvantages related to donor site morbidity, a second surgical procedure at the donor site, difficulty in matching the graft to the defect site for optimal function, and a limited amount of graft tissue. In addition, current grafts are not combined with other regenerative approaches. They are limited to the regeneration of the alveolar bone and do not support the regeneration of the entire periodontium.
Moreover, in the presence of autografts or implants in the body can adversely affect the immune system environment and lead to chronic inflammation, causing cell death and tissue damage. After implantation, the surface of an implant is coated with serum and tissue proteins, which promote bacterial adhesion. After their initial colonization, bacteria undergo a transition from the planktonic to the biofilm stage. Bacteria in biofilm formation differentiate into a non-growing phenotype that is highly tolerant to antibiotics and is associated with chronic infections and can cause infection of the tissue around implants. All this leads to further damage and loss of periodontal tissues and inhibits the regeneration process. While surgical debridement of the infected tissue and implant can be performed to stop the chronic inflammation, this has serious consequences for affected patients. In addition, surgical debridement significantly limits the regenerative capacity of the affected tissue. Under these conditions, lost or damaged periodontal tissue cannot easily repair itself even though it has self-healing potential.
Traditional regeneration methods for the regeneration of the periodontium5 such as guided tissue regeneration (GTR)6–9 are efficient in repairing periodontal defects when the goal is to provide support and stability to the teeth. This technique uses artificial membranes to prevent the migration of soft tissue into the defect site and instead promotes the slower-moving bone-producing cells to settle and grow there. However, this technique is not able to achieve functional regeneration.10 Recent regeneration-supporting methods11 are based on transplanting engineered stem cells to the area of damage but that presents several difficulties, including an inadequate stem cell source, poor survival of stem cells upon transplantation, safety issues, and time and financial costs for cell culture and storage.11 Various biomolecules, such as growth factors (GFs), antibodies, chemokines, cytokines and cell-adhesive molecules, have been used to promote the migration of autologous stem cells to the damaged tissue and, thus, encourage tissue regeneration. However, these studies are not suitable for translation to the clinic, and complete periodontal regeneration is still not achievable. Furthermore, they do not address periodontal tissue vascularization during regeneration and healing. The formation of new blood vessels from the existing vasculature (angiogenesis) is an essential step for achieving successful tissue regeneration and healing.
Even though, the current methods show significant progress in the regeneration of alveolar bone and gingival soft tissue, the repair and restoration of the physiological function of the PDL has limited success due to the complex and hierarchical architecture of the periodontium that requires highly synchronized spatiotemporal regeneration.
In this context, the engineering of biomaterials and/or manipulating endogenous stem cells in vivo via biomaterials and/or biomolecules for periodontal regeneration has attracted considerable interest and has the potential to be utilized clinically in a wide range of applications. The in vivo manipulation of stem cells via 3D biomaterials is a more promising approach for stimulating and accelerating healing and regeneration of periodontal tissues. Moreover, biomaterials exhibit tunable physicochemical properties, able to provide 3D (multiphasic) networks, and allow controlled delivery of bioactive molecules to the defect site in a time- and site-dependent manner,12 enabling control of the complex spatiotemporal regeneration of the periodontium. This capability greatly enhances the regenerative effect of biomaterials on the complex and hierarchical architecture of the periodontium.
3. Biomolecule delivery and additive manufacturing for periodontal regeneration and periodontitis
Periodontal treatment has two main objectives: the inhibition and elimination of biofilm formation and biofilm-induced inflammation, and the 3D repair and replacement of damaged periodontal tissue, including the formation of new bone and cementum and the restoration of the integration of the PDL with the bone and cementum.13,14 Therefore, to improve treatment outcomes, local biomolecule delivery methods and additively manufactured cell incorporated 3D network structures have been used to reduce and eliminate periodontal inflammation, mimicking the 3D complexity of the periodontium, and 3D repair and replacement of damaged periodontal tissue by supporting the functional integration of various tissue components and their synchronized spatiotemporal regeneration in a 3D matrix.3.1. Biomolecule delivery biomaterials for periodontal regeneration and periodontitis
In addition to the clinical approaches that have been used for many years in the treatment of periodontitis and the elimination of inflammation with the help of various drugs, regenerative methods have started to be used. Regenerative approaches, which include engineered stem cells or gene and growth factor therapies applied to support wound healing and tissue regeneration in the area where biofilm is formed and tissue loss occurs, also include the use of oxygen transport systems, various antimicrobial materials integrated with drug transport systems, and aim to eliminate the oxygen deficiency caused by the inflammatory condition in the area. In recent years, studies in this field have increased, especially with the integration of various biomaterials15–19 that will be discussed in section 4.Antibiotics are the primary treatment to prevent bacterial infection due to biofilm formation in the periodontium. Despite its long-term clinical importance is still controversial, most periodontal therapies demonstrate a substantial microbiological advantage due to the application of additional agents’ delivery of systemic antibiotics in conjunction with short-term nonsurgical mechanical treatments.20
In the past 20 years, studies have shown that antibiotic treatment also carries its own risk. In certain cases, patients may develop resistance to these drugs. Rams et al. studied chronic periodontitis patients for the effect of antibiotic treatment. In the United States, 400 people with chronic periodontitis had their inflammatory deep periodontal cavities’ subgingival biofilm samples removed before receiving therapy. Results showed that subgingival periodontal infections resistant to a minimum of one of the examined antibiotics were found in 74.2% of the chronic periodontitis patients overall and it was common for patients with chronic periodontitis to develop resistance to the moderate amount of antibiotics in the therapy.21 That is why dental specialists must be educated on antibiotic treatments and only administer antibiotics in situations when all possible criteria have been properly established. Therefore, alternative treatments to the antibiotics are needed.
Anti-virulence factors are one of the alternative treatments for periodontal diseases used by periodontal pathogens to start and advance periodontal disease. Anti-virulence substances may be used as new techniques as able to prevent the periodontal bacteria's harmful effects.22,23 Targeting the virulence factors and regulatory systems that regulate pathogenicity in infections offers an appealing replacement for antibiotic therapy. A strategy like this is aimed at limiting bacterial virulence factors to prevent risky bacterial infections from causing harm to the host. The pathogenic activities of periodontal bacteria may be stopped by substances with anti-virulence capabilities. Anti-virulence medications have the potential to maintain healthy, neutral flora without compromising bacterial viability. They can also lower the need for antibiotics as well as the development of antibiotic resistance.24 Kumbar et al. demonstrated the virulence of P. gingivalis was inhibited by curcumin through the downregulation of genes that code for important virulence factors, such as adhesions and proteinases. In both minimum inhibitory and minimum bactericidal concentrations, curcumin could decrease the amount of living bacteria, indicating that it has strong anti-biofilm and bactericidal qualities. Consequently, curcumin is a useful substance for reducing periodontal gingivalis biofilm and it may be a straightforward and affordable therapeutic option for the treatment of periodontal disease since curcumin is nature-based and abundant.25
Another alternative is the quorum sensing method that bacteria use to communicate with one another which has been demonstrated through decades of research, and the list of compounds that bacteria use to do this is continuously expanding. Many bacterial pathogens in people, plants, and animals are controlled by quorum sensing, and quorum-sensing-interference is one of the most well-researched ways of preventing sickness brought on by antibiotic-resistant bacteria. By preventing bacterial communication, a crucial component of biofilm development, and the expression of virulence factors, quorum quenching presents a promising method of treating periodontal bacteria.26,27 The quorum-sensing gene regulation systems of many Gram-negative bacteria species that interact with eukaryotic organisms depend on Acyl-homoserine lactones (AHLs), which are significant signaling molecules. Chai et al. tested AHL analogs against tongue cancer and oral squamous cell carcinoma. Through its stimulation of apoptosis, the active analogs of AHL demonstrated clearly both radiation-sensitizing and antiproliferative properties.28
Periodontal diseases can also be effectively treated with immune-modulating therapy. Through regulating the osteolytic and inflammatory, it can prevent periodontal disease and may be beneficial to minimizing bone loss. Periodontal disease is highly dependent on the immunological surroundings of the affected tissue. Infection can be effectively treated by improved leukocyte infiltration and a discharge of inflammatory compounds. The alveolar bone, and the periodontal tissues, can suffer severe damage if this inflammatory response occurs in excess. In addition to complementing existing periodontal disease treatments, altering this microenvironment may also encourage periodontal regeneration.29 Numerous scientific studies propose that cytokines are essential for preserving tissue homeostasis.30,31 A study indicated that the cytokine network may have a role in the development of human periodontitis by demonstrating the expression of inflammatory cytokine mRNA in inflamed gingiva.32 Besides immune-modulating medication therapy other methods, such as, gene therapy, and stem cell therapy, are currently used to control leukocytes as well as inflammatory cytokines.33
Reactive oxygen species (ROS) is a molecule that is part of normal metabolism, excessive production of which causes various diseases and provides defense against bacteria.34 The antibacterial property of ROS is based on its ability to break double stranded deoxyribonucleic acid (DNA), oxidation of lipids and proteins.
Probiotic therapy has been recommended recently in studies to treat periodontal disorders. By immunity control and pathogen-colonization resistance, probiotics are thought to contribute to regulating diseases. The colonization of cariogenic bacteria may be changed by probiotics, hence preventing dental caries.35,36 Tekce et al. suggested that tablets containing L. reuteri might be beneficial when used to treat chronic periodontitis by enhancing patient outcomes and preventing recolonization.37
Another method involves transplanting healthy donors’ microbiota into patients with disease. This method is known as microbiota replacement therapy. According to the study, BCS3-L1 is a good candidate to act as an activator strain in oral cavity replacement treatment. Its genetic stability, selection advantage in colonizing disease-prone tissues, and greatly decreased pathogenic potential all contribute to its unique qualities.38 It is anticipated that more useful and effective microbiota replacement therapy techniques for periodontal diseases will be accessible in the future as the understanding of the human microbiome and the connection between oral microbiota and oral diseases improves.39
Plant-based therapies are also very common applications. There have been numerous studies on the inhibitory activity of lavandula, manuka, rosemary, eucalyptus, and tea tree oils against periodontal bacteria.40–46 Studies showed that chewing sticks may be used as toothbrushes when it is correctly applied because of their mechanical brushing and improved salivation effects.47 According to Huh et al. safflower seed extract stimulates the mineralization pathway by increasing the alkaline phosphatase (ALP) production and proliferation of human PDL fibroblasts (hPDLF) and osteoblasts.48 Green tea is suggested as well for the inhibition of dental plaque and its anti-cariogenic properties.49,50 In addition, to showing promise against oral infections, the essential oil of L. sidoides and its main constituents also points to the plant's potential utility in preventing oral microbial development.51 Turkish Gall, a self-regenerating phenol used in the treatment of various diseases due to its antibacterial, anti-inflammatory, and antioxidant properties.
Platelet-rich plasma (PRP) is a readily available source of GFs to support bone and soft tissue healing, obtained using methods that concentrate autologous platelets. Platelet-rich fibrin (PRF) is a platelet and immune concentrate containing all the necessary elements in the blood for healing and immunity, which can be obtained by simply centrifuging the blood without any addition.52 PRP is known as the first-generation autologous platelet concentrate, while PRF is known as the second-generation. These concentrates activate platelets in the wound-healing process and contribute positively to the secretion of growth and differentiation factors. It has been observed that PRP delivers GFs to the target area more quickly.53 Furthermore, it has been shown that in centrifugation of PRF, naturally occurring, gradual polymerization has a critical point in the 3D organization of fibrin structure. Cell migration and the production of cytokines are impacted by these flexible and fine fibrin structures.54
Concentrated growth factor (CGF) is a relatively new platelet concentration that is generally used in periodontal tissues and oral operations. Unlike PRF and PRP, it has higher viscosity, tensile, and adhesion strength.55 The tight fibrin structures of CGF allow the support of scaffolding structures used in the migration, differentiation, and proliferation of cells in the maintenance or regeneration of the initial bone volume.56 Li et al. design a study to understand the role of CGF in the proliferation of human PDL cells (hPLDC) in an inflammatory environment showed a significant increase in hPLDC after 72 hours. In this study, with tumour necrosis factor-alpha (TNF-α), which is generally responsible for inflammation in periodontal tissues, CGF was found to promote osteogenic differentiation, increase ALP levels, and increase Runt-related homeobox2 (RUNX2) and Osterix (OSX) gene expressions responsible for tissue regeneration and proliferation.57
On the other hand, platelet-derived growth factor (PDGF) is one of the GFs that has been extensively studied until today. Since its discovery in the late 1980s, nearly 100 studies have been published on PDL, alveolar bone cells, and periodontium regeneration.58 In addition to the safety and efficacy of using PDGF with B-tricalcium phosphate, the use of the recombinant form promotes the regeneration of bone, ligament, and cementum.59,60
Like other GFs involved in the regeneration of periodontal tissues, the transforming growth factor (TGF) is produced by many cells and has divergent functions such as proliferation and differentiation of cells and regulation and control of immune system responses. TGF-β1 also acts in key roles such as regulating chemotactic and mitogenic activities of PDL fibroblasts and regulation of extracellular matrix (ECM) materials such as collagen, fibronectin, and proteoglycans.61 Matarese et al. study with PDL and gingival tissues with chronic periodontitis showed that TGF-β1 production is a factor that protects against periodontitis and accelerates remodelling and angiogenesis of connective tissues.62
Bone morphogenic proteins (BMPs) is an important family of proteins involved in the formation and regeneration of bone and cartilage tissue in the body, including the regeneration, repair, and rebuilding of periodontal tissues. Although it is a crowded protein family, the most researched members in periodontal tissues today are bone morphogenic protein-2 (BMP-2) and bone morphogenic protein-7 (BMP-7). Wei et al. study with dogs with chronic periodontitis, it was observed that the defective area treated with BMP-2 incorporated biomimetic calcium phosphate barrier membrane treatment had more regenerated periodontal tissues compared to other experimental groups.63 The dose, flap management and carrier system of the members of the BMP group, which supports the formation of new bone and cementum in the treatment of periodontal defects, have an important role in the regeneration of periodontal tissues. Lee et al. was done successful 1.5 mg mL−1 dose of rhBMP-2 carried by the collagen matrix was found to be successful for new bone formation. In the same study, fluorescence labelled cell analyses showed that mineralized bone tissue was more in low dose rhBMP-2 specimens than in the control group and there was no significant difference between high dose rhBMP-2.64 Ebe et al. found that studies conducted with IL-1β, which is found in high amounts in areas with inflammation, and BMP-9, it was observed that BMP-9 increased ALP level by 46% and mineralization by 85% on the 12th day compared to the control group.65 Badr et al. study, as a result of histological analyses, the optimal dose for the use of BMP-7 in periodontal regeneration was found to be 100 ng mL−1.66
Enamel matrix derivative (EMD) is a heat-treated heterogeneous mixture consisting of 90% amelogenins and its derivatives, which contain various proteins and bind to the periodontium during tooth formation. It is an important substance involved in Runx2 and OSX transcription.67,68 Takeda et al. study conducted with diabetic and non-diabetic rats, the antioxidant properties of EMD were investigated. As a result of this study, it was observed that oxidative stress and inflammation were significantly reduced in vivo and in vitro studies in diabetic and non-diabetic rats treated with EMD and it was concluded that EMD supports early periodontal tissue regeneration.69 Mizutani et al. designed a 3-year study of type 2 diabetic and non-diabetic patients treated with minimally invasive surgical technique and EMD, significant clinical attachment level (CAL) gain and probing depth reduction were observed independent of diabetes.70 Between 1999 and 2012, a study of patients with periodontal intrabony defects treated with EDM and regenerative therapy, which lasted approximately 10 years, showed a significant reduction in gingival recession and a significant increase in CAL gain between 6 months and 8 years. This study has shown that EDM treatment with a regenerative approach alone provides a successful treatment for about 10 years.71 Kobayashi et al. showed that a study of relatively newly developed liquid delivery systems for EMD with better physicochemical properties and absorption capabilities, EMD-liquid was studied in vivo with natural bone mineral (NBM). A statistically significant increase in mineralized tissue was observed in white rabbits treated with NBM + EMD-liquid.72 According to the results of a study published in 2019 by Lee et al. it was also observed that the use of EMD in patients undergoing periodontal procedures greatly reduces the amount of pain and swelling felt and patients have a more comfortable post-operation period.73
In addition to GFs that have been researched, there are GFs such as epidermal growth factor (EGF), insulin-like growth factor (IGF), stromal cell derived factor-1 (SDF-1), which have been less researched but have a positive effect on periodontal tissue regeneration. Although the EGF has been investigated in the healing of dermal wounds from past to present, it has also given positive results in the healing and regeneration of oral wounds. Ben Amara et al. observed that rhEGf applied topically at 1 and 10 μg g−1 accelerated wound healing by increasing keratinocyte activity in the epithelial layer and reducing inflammation in the subepithelial region.74 Studies have also shown that EGF is an effective stem cell-based therapy for ECM mineralization, which is important for periodontal tissue regeneration. Del Angel-Mosqueda et al. studies with dental pump stem cells (DPSCs), it was proved that cells treated with 10 ng mL−1 EGF increased ALP messenger ribonucleic acid (mRNA)expression, could form calcium deposits and could be osteogenic.75 IGF and its binding proteins (IGFBP1-6), like other GFs, have a significant role in osteoblast function and bone formation. One of these binding proteins, IGFBP-5, has proven effects on cell growth, remodelling, and bone repair.76 Han et al. study using PDLSCs, IGFBP-5 was found to promote osteogenic differentiation in mesenchymal stem cells viac-Jun N-terminal kinase (JNK) and MEK/Erk signalling. It was also concluded that 0.5 ng mL−1 IGFBP-5 applied to PDLSCs in TNF-α-induced inflammatory environment increased cell migration, chemotaxis and cell proliferation and restored their impaired functions.77
Especially in recent years, research on the use of different carrier systems in periodontal tissue regeneration has increased. GF release is affected by the kind of delivery device used, the rate at which it deteriorates, and the degree to which quickly GFs diffuse through scaffold pores.78 Therefore, various GFs incorporated injected platelet-rich fibrin and the double network hydrogel prepared with i-PRF, and gelatine nanoparticles was used to be mechanically stronger and bioactive system that can provide long term release of various GFs.
GFs and signalling molecules have a pivotal role in periodontal regeneration and treatment of periodontitis, cell differentiation, proliferation, and regeneration of complex periodontal tissues. However, application and transport methods, the dose to be applied and the preparation and application of appropriate combinations are important clinical points to be considered.
Gene delivery strategies can be categorized as in vivo and ex vivo gene delivery.98 Both of the strategies are important and useful in periodontal regeneration. In vivo strategy is a short process including the implantation of scaffolds into the tissue or direct injection into the targeted sites. Although it is a simple and preferred procedure in practice, there are drawbacks such as difficulties in targeting cells, standardizing, and maintaining high efficiency.99 On the other side, the ex vivo strategy has more steps including cell harvesting from the host. Harvested cells are being expanded, manipulated, and targeted by specific cells to achieve gene delivery. Ex vivo strategy might be a safer option but compared to in vivo, it is a time-consuming process, not cost-effective, and contaminations may occur. Take into consideration, that ex vivo also has the advantage as it is possible to target specific cells for the tissue.100
GFs are important reagents in periodontal regeneration. Gene delivery can be used to support GFs to improve regeneration.101 GFs have short half-lives and they spread into the environment resulting in fast degradation and lack of precise delivery.102 Gene delivery may achieve the difficulties that GFs have.
The process is possibly practicable to introduce genetic material using vectors into specific PDL cell types, causing these genes to be transcribed and cementoblasts to develop and differentiate, forming new attachments.103 Kawai et al. studied rat maxillae by electroporation of BMP-2/7 gene expression into the target sites. Results show that it is a viable non-surgical method for alveolar bone regeneration therapy that combines the BMP-2/7 non-viral vector with in vivo electroporation.104 Lai et al. found BMP-4 adenoviral gene transfer via the bone marrow promotes prior implant stability and peri-implant bone repair in osteoporotic rabbit femurs.105 Kanzaki et al. study of periodontal disease is effectively halted from continuing with the delivery of AAV2/1-TNFR:Fc, showing an additional method of inhibiting the generation of cytokines that are caused by pathogenic lipopolysaccharide.106 The transfer of PDGF genes into animal cells and the subsequent increase in cementoblastic activity is an illustration of gene delivery.107 In an animal model, this approach has been researched, and it was found that gene delivery of PDGF generated greater cementoblasts production and enhanced regeneration relative to a simple delivery of recombinant PDGF.108 Nevertheless, gene delivery is an encouraging system for periodontal diseases and is yet to be studied in future studies.109
Materials that support oxygen release, such as calcium peroxide (CaO2), produce hydrogen peroxide (H2O2) when exposed to water and then decompose into the end product oxygen. Encapsulation is used to reduce the toxic effect of by-products that may occur.
Hyperbaric oxygen therapy (HBO) is another therapy used in periodontal regeneration and to provide oxygen to the periodontitis area. HBO therapy is based on the principle of inhalation of highly concentrated oxygen gas with a pressure higher than 1 atm. HBO therapy is known to improve CAL gain, probe depth reduction and leading parameters in probing. In a published case report, it was found to be effective in preventing inflammation caused by anaerobic bacteria.113 Giacon et al. study with anaerobic Bacteroides Meaninogenicus, in which the effect of HBO therapy on aggressive periodontitis was investigated, it was observed that the number of bacteria decreased significantly in those treated with HBO therapy compared to the control group (increased from 96.5% to 34.5%).114 Gajendrareddy et al. observed that type 1 collagen expression measured at 15 and 28 days was higher and collagen accumulation, maturation and thickness were better in rats treated with 2 hours of HBO treatment per day.115 HBO treatment is known to have a favourable effect on wound healing because it breaks the hypoxia cycle at every stage. It is a crucial factor of angiogenesis because it promotes Vascular endothelial growth factors (VEGF) expression and oxygen is at the key point for vascularization.116
Another system used to carry oxygen to periodontal tissues is ozone application. There are basically 3 methods of application of ozone. These are ozonized water, ozone gas and ozonized oil. Ozonized water is used as an ultrasonic water reservoir rinse before scaling, root planning and sulcus treatment, as well as for non-surgical pocket cleaning with syringe and cannula. This procedure reduces the initial pathogenic load on the patient locally and systemically.117,118 In subgingival application of ozonized water, Issac et al. observed that the number of anaerobic bacteria in periodontal pockets decreased, and recolonization was prevented.119 The effect of ozone therapy used in gas form on periodontal pathogens was investigated with biofilm cultures (P. gingivalis, T. forsythia and P. micra) and ozone in gas form was found effective at a 54 g m−3 density.120 Pires et al. observed that wound closure was completely realized on the 15th day and the inflammation process was not observed, muscle regeneration was completed, and the density of connective tissues increased in rats treated with ozone gas compared to the control group.121 Ozonated olive oil is a product obtained by exposing extra virgin olive oil to high concentrations of unstable ozone gas obtained by energizing oxygen. Yousef et al. study with rabbits with periodontal defects, it was observed that new bone formation in those treated with ozone oil was significantly higher than those who were not. In the same study, it was observed that the application of ozone therapy increased osteoblastic and fibroblastic activities and the density of collagen fibers.122 In one study with human gingival fibroblasts, it was observed that 0.5 ppm ozone ointment did not have toxic effects and positively affected the production of type 1 collagen involved in cell proliferation.123 In case studies done by Anand et al., it was found that ozone therapy affects periodontal abscesses, pockets and bone deterioration in regenerative and antibacterial terms.124
Alsherif et al. study with rats on intrabony three-wall periodontal disorders, the effects of both ozone and PRF were histologically examined. As a result of the research, it was observed that periodontium regeneration was almost complete and observed at the end of 4 weeks with rats treated using PRF or ozone.125
Arenicola marina hemoglobin is an extracellular oxygen carrier with high molecular weight and oxygen binding capacity, derived from the blood of the marine sandworm. M101, an extracellular soluble hemoglobin, has antioxidative properties due to its high oxygen binding and prevents ROS-induced oxygen deficiency with degradation of hemoglobin, which is associated with potentially harmful free radicals.126,127P. gingivalis is the major anaerobic pathogen responsible for periodontitis. In a proof-of-concept study examining the healing effects of M101 on P. gingivalis, it was found to increase some anti-inflammatory markers and some interleukin synthesis (PDGF-BB, TGF-B1, IL-10). It was also proven to significantly kill the living P. gingivalis pathogen.126 Batool et al. study on P. gingivalis-induced hypoxia, it was reported that epithelial cells treated with 1 g L−1 M101 showed complete recovery after 24 hours and regression was observed in hypoxic conditions created with P. gingivalis + CoCl2, CoCl2 and P. gingivalis alone in the experiment. In this study, it was also observed that M101 increased antioxidant ability in these hypoxic conditions.128
In addition to this, ozonated oil is also used to support periodontal regeneration by bringing it into gel form using different chemical compounds. In a study conducted with commercially available ozonated gels, it was observed that the percentage of bone regeneration was 62% at 6 months and 77. 7% at 9 months after the surgical procedure.129
The continuous secretion of oxygen to tissues with hypoxia in specific doses is an area that is still being studied and its limitations are still being overcome. Apart from the mentioned oxygen transport system and methods, new approaches such as oxygen high level laser therapy, which have been less studied, are available in the literature. In an article published by Caccianiga et al., it was observed that periodontal pockets were treated with oxygen high level laser therapy without any complications and that the plaque index decreased by an average of 75%, bleeding on probing by an average of 62% and probing depth by an average of 1.8 mm 6 months after treatment.130 Based on all these studies and approaches, oxygen deficiency caused by periodontal defects and periodontitis is reduced by means of oxygen transport and release systems and has an important place in the regeneration of periodontal tissues. Studies in this field continue with developing technology and new approaches.
3.2. Additive manufacturing for periodontal regeneration and periodontitis
Periodontal tissue, consisting of hard and soft tissue, therefore, presents a 3D complex biological and hierarchical structure that demonstrates the functional integration of different tissue components. Therefore, it is difficult to regenerate damaged periodontal tissue via mimicking its functional and 3D structure with traditional approaches including using grafts, root surface conditioning, membranes, cell sheets, biomolecule induced stem cell differentiation, and gene therapy.In early attempts of periodontal tissue engineering includes cell sheet engineering. This method includes non-enzymatic harvesting of cells which are deposited in ECM to form cell sheet that is transplanted into the side of the defect to promote periodontal regeneration. However, the formed cell sheet shows lack of biomechanical stability and ability to regenerate periodontal defects which have complex 3D structure and mechanical and biological composition. Therefore, in the later approaches, cell-incorporated biomaterials were layer-by-layer printed into 3D structures or several cell sheets were layer-by-layer combined or supported by hydrogel network or cells were embedded into 3D layered (composite) scaffolds to maintain ECM integrity and subsequent periodontal tissue regeneration.
Achieving compatibility between the produced object and natural tissue is relatively easier with 3D bioprinting, affording greater control over the material during production. Additionally, 3D bioprinting can rapidly adapt to specific requirements, resulting in time and cost savings without the need for intricate modeling procedures.
Furthermore, temporary crowns produced using 3D printing demonstrated an enhanced fit compared to other methods such as milling and compression molding. When the longevity and success of the implants were tracked, a success rate of 94.3% was observed. It is understood that implants produced through 3D bioprinting hold potential in the long term within this context.131,132
Although implants and membranes are frequently used, scaffold structures have recently been extensively studied to achieve periodontal regeneration. The porous structure of scaffolds supports regeneration in terms of combining with materials with various properties, delivering various agents such as drugs or growth factors to the area to be regenerated and creating composite structures.
3D printing is an important method for gingival soft tissue regeneration as well, as it can mimic the natural structure of gingival tissue and optimize its mechanical functions with proper alignment. It can be used to create tissues which are like their natural states133 Obtaining structures like the original cells through 3D bioprinting is important because the similarity of these structures to the originals implies a potentially better compatibility within the body. Their resemblance can lead to greater acceptance by the body, enabling them to mimic biological processes more effectively. It may contribute to faster tissue healing and support regeneration.
3D bioprinting can also be used in combination with different printing techniques such as melt electro writing, electrospinning, inkjet printing to obtain the properties required by the application. Melt electro writing is one of the techniques that can be used in scaffold production. Primarily using thermoplastic materials, this method can work with biocompatible polymers.
Another advantageous ease of use of 3D bio-printers is that the variety of bio-ink used can be selected to have the required properties in the area to be applied. A bioink must degrade gradually so that it supports the newly formed tissue's integration with its environment and the tissue regeneration. It also must be biocompatible, ensuring it is safe and suitable for use in living cells. In relatively new and still under development, natural or polymer-based hydrogels are defined as polymer materials that can absorb water. In addition to the properties that bioinks should have, they should also have a water retention capacity that will provide the moist environment necessary for the growth of cells.
To sum up, 3D bioprinting holds significant potential in dental regeneration despite being a relatively new technology. In the reconstruction and treatment of dental tissues, it can offer new treatment options as an alternative to traditional methods for individuals, so 3D bioprinting can be considered a promising research field in the topic of dental regeneration.
In tissue engineering studies targeting periodontal tissue healing, composite scaffolds are created to provide parameters such as flexibility, biodegradability, biocompatibility, swelling, biomolecule loading, and controlled release capabilities of the material used and to modify and control structural properties such as porosity.
Scaffolds are an important component in the repair and regeneration of damaged tissue. These temporary structures provide mechanical support to the affected area while the body heals. Scaffolds are highly preferred structures because they facilitate the creation of an environment with the necessary characteristics for complete periodontal regeneration. While scaffolds are used to regenerate periodontal tissues and deliver materials to damaged tissues with their carrying capacity, they must also meet some specific criteria. They must have a porous structure depending on the area where they are applied and the content they carry. Their porous structure must be of a size that will allow the passage of substances, support the migration, proliferation and growth of cells that can provide a suitable environment for cell cultivation. The volume of the holes should be between 50–90% to encourage cell adhesion, and the porosity should be high enough to allow the pores to connect and the surface. However, balancing high porosity and pore size with scaffold strength is essential. The pore size and porosity of the scaffolds is a critical feature in initiating tissue repair and stabilizing blood clot formation by allowing blood to leak into the implanted area. Ashworth et al. examined pore size, pore wall alignment and pore transport pathways (percolation diameter) in a study on collagen scaffold structures. As a result of this study, it was observed that reducing the pore size from 100 to 52 μm did not make a difference due to the optimum pore size of 100 μm and that pore walls and pore size provided the most uniform distribution at 100 μm.134 Micropores smaller than 100 μm may inhibit cell growth due to inadequate blood supply, but macropores between 100 and 700 μm specifically promote vascularization at the implanted sites.
A scaffold must also be biodegradable, biocompatible, and made of an appropriate biomaterial that can be absorbed by surrounding tissues with minimal immunological and inflammatory response. Additionally, the scaffold should have a high bioactivity level to promote close integration with the surrounding tissue. Integrating bioactive components like hydroxyapatite (HA) into polymer matrices presents bioactive hybrid composites that have the potential to significantly improve biocompatibility, mechanical robustness, and hydrophilicity. HA nanoparticles enhance bone cells differentiation and growth rate, accumulating excessive calcium minerals in the scaffold. This leads to the accelerated development of new bone tissues within a brief timeframe. Scaffolds should be sturdy enough for surgical handling and have mechanical properties consistent with their anatomical placement.135 Scaffolds provide structural support and spatial guidance for cells, forming the foundation for tissue-engineered structures.
ECM, also known as intercellular matrix in biology, is a network of extracellular macromolecules and minerals, including collagen, enzymes, glycoproteins, and HA, which biochemically and structurally support neighbouring cells, functioning as a scaffold in nature and having an amorphous porous structure. This matrix acts as a scaffold that allows fibroblasts, blood vessels and epithelium to develop in various tissues. Therefore, the scaffolds developed should mimic the ECM structure.136 Decellularized ECM is often used as an exogenous complete form of ECM to replicate a 3D microenvironment at implanted sites for tissue repair and regeneration.
Various traditional and innovative scaffold fabrication techniques have been explored, including melt electro-writing,137 salt leaching and freeze drying,138 decellularization.139 These methods aim to produce biomimetic scaffolds that replicate the hierarchical organization of native periodontal tissues in an ex vivo environment. Rapid prototyping (RP) or 3D printing is also commonly used in the fabrication of scaffolds for tissue engineering. Due to the high flexibility and low costs, 3D printing is becoming increasingly important in the manufacture of highly individual scaffolds.140
Polymeric biomaterials are commonly used in scaffold design and fabrication for periodontal regeneration. Some of the polymers used in scaffold design for periodontal regeneration include chitosan, alginate, gelatin, or collagen as natural polymers and poly(lactic acid) (PLA), polycaprolactone (PCL), and their copolymers as synthetic polymers. These polymers have been shown to promote cell proliferation and differentiation and have good biocompatibility and biodegradability. Natural biomaterials derived from plants and animals that are mostly of polysaccharide origin (such as chitin and alginate) or protein-derived (such as collagen or gelatin, a degraded form of collagen) have distinct advantages over the conventional synthetic polymers, including biocompatibility, processability, comprehensive availability, and exceptional biological activities.141
Scaffolds can be produced as monolithic or multilayer scaffolds that can have variable physical architecture with mechanical and/or biochemical properties in single or multiple compartments of the scaffold produced to mimic natural periodontal tissue.
The monolithic scaffolds contain just one compartment, which fulfils the prerequisites for periodontal regeneration: stability of bone defects, selective cell proliferation, and spatiotemporal control of periodontal repair. Single monophasic scaffolds can straight induce periodontal healing. Additionally, monophasic scaffolds can operate as vectors for the release of bioactive stimuli and for the transport of cells.
To achieve periodontal regeneration completely, multiple tissues need to be hierarchically oriented. To do this more effectively, a biphasic or triphasic design is used to direct progenitors to specific cell types. This is achieved by layering components with distinct characteristics, such as material composition, architecture, and functionalization. Many combinations are possible, and while there is no perfect combination, several proof-of-concept studies have developed prototype designs that favour the regeneration of hierarchical structures. A highly porous and topographically nano fibered chitosan/polyvinyl alcohol scaffold impregnated with plant proteolytic enzyme using electrospinning method with conjugated magnesium-added HA nano particles as an example of bilayer scaffolds was produced.142
3D printing technology has recently emerged as a promising strategy to produce multilayered constructs for tissue engineering. With the help of 3D computer-aided design modelling software, structures can be created layer by layer in a way tailored to patients and treatments. A significant benefit of using 3D printing to create scaffolds is its design flexibility. By controlling factors such as porosity, pore size, interconnectivity, and strand alignment pattern, it is possible to create a structural gradient within the construct to guide tissue regeneration.143 This technique has immense potential in orthopaedic applications, particularly in developing biomimetic structures for bone and soft tissue grafts. However, periodontal regeneration presents a unique challenge, requiring integrating soft and hard tissue elements within their environment. To overcome this challenge, experts recommend using a multilayered scaffold architecture as it can improve the connection between soft and hard tissue and facilitate more effective regeneration.144
4. Biomaterials used for biomolecule delivery and additive manufacturing for periodontal regeneration and periodontitis
Various biocompatible biomaterials and their additively manufactured 3D scaffolds made of natural and synthetic polymers such as hydrogels, scaffolds, films, membranes, micro/nanoparticles and fibers have been developed and tested in vitro and in vivo for their effect in periodontal tissue regeneration and periodontitis due to their superior properties.145–149 The general requirements for biomaterials are mainly based on biocompatibility, biodegradability, coupled with suitable biomechanical properties, antibacterial properties, and porosity. In addition, 3D network structure and the ability to deliver drugs that respond to stimuli are the most advanced properties for improved performance of biomaterials in periodontal tissue regeneration and inhibiting and/or treating periodontitis. These properties can be achieved by regulating the chemical composition and microarchitecture of materials. Several examples of natural and synthetic polymers and their 3D scaffolds loaded with drug or bioactive molecules (e.g., antibiotics, antimicrobials, anti-inflammatory agents, GFs) have been used for periodontal tissue regeneration and drug delivery application including as PLA, polyethylene glycol diacrylate (PEGDA), poly(lactic-co-glycolic acid) (PLGA), alginate, collagen, chitosan, hyaluronic acid (HA), PCL, fibrin, gelatine-based hydrogels, scaffolds, films, nano- and micro-particles.4.1. Nano- and micro-particles
Nano- and micro-particles display unique advantages in the treatment of periodontal diseases and damage due to their ability to provide controlled biomolecule delivery via external stimuli, encapsulate both hydrophobic and hydrophilic biomolecules with their tuneable chemical structures, possess high biomolecule loading efficiency thanks to their large surface area, and their easy surface functionalization that allow targeted biomolecule and oxygen delivery and antibacterial properties.Therefore, various nano- and micro-particles have been utilized for inhibiting bacterial biofilm formation, to reduce inflammation, to improve cell proliferation and differentiation and so accelerate wound healing and new tissue formation at the damaged periodontal tissues. However, the low loading efficiency and limited ability of controlled drug delivery hinder their clinical application. In this respect, Song et al. used abalone-inspired microparticles (Fig. 2) as an adhesive and photosensitive microparticle (MP) delivery system to achieve controlled and effective drug release for the treatment of periodontitis. The structure of the MP was created by an electrostatic spray microfluidic strategy and inspired by abalones, which have enhanced adhesive properties due to their suction cup-like prolegs. The MP was composed of alginate/PEGDA microparticles loaded with minocycline hydrochloride as antibiotic and near infrared (NIR)-sensitive black phosphorus. That was prepared as discs in calcium chloride solution through a combination of microfluidic and ultraviolet (UV) irradiation strategy. The results showed that the photosensitized abalone-inspired MP significantly reduced bacterial growth and caused minimal colony formation after 10 days on the tooth due to effective NIR-induced drug release.150
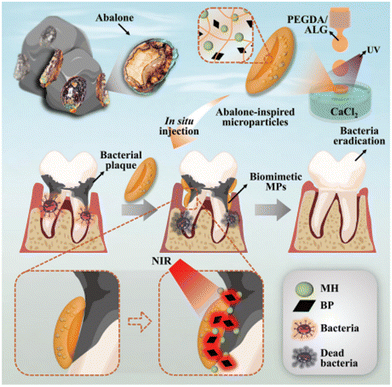 | ||
| Fig. 2 The graphical illustration of the design and preparation of abalone-inspired microparticles and encapsulation of BPs and MH to realize antibacterial photothermal treatment of teeth with controlled drug release. Reproduced from ref. 150 with permission from Wiley-VCH GmbH, copyright 2022. | ||
Although growth factors such as PDGF could enhance periodontal therapy, their effect is limited due to a lack of continuous supply over a required period. To overcome this obstacle, nano-sized calcium phosphate particles (NCaPP) were used as non-viral vector by Elangovan et al. for sustained PDGF-B gene delivery in fibroblasts.151 NCaPP has attracted great interest in dentistry as a targeted and efficient gene delivery to cells due to its small size, large surface area and easy surface modification via adhesive polymers, enabling high biomolecule encapsulation and continuous delivery. NCaPP prepared in combination with PDGF plasmids has been successfully transferred into fibroblasts for up to 96 hours. This demonstrated a higher level of biocompatibility and usability in non-viral gene therapy for periodontal applications by enhancing fibroblast proliferation. Similarly, sustained, and targeted administration of antibacterial and anti-inflammatory agents is crucial for the treatment of periodontitis. Therefore, Qi et al. investigated the conversion of the active ingredient of Turkish Gall into nanoparticles (T-NPs) via oxidative self-polymerization and encapsulation of the formed T-NPs in a thermosensitive hydrogel. The advantages of the T-NPs used are their ability to reach and adhere to the periodontal pocket, demonstrating sustained release of antioxidant and antibacterial polyphenol extracted from Turkish Gall in the alkaline environment of the tooth. The study revealed that under the presence of periodontitis, T-NPs was continuously released over 96 hours, with the alkaline oral environment and exhibited a 50% and 25% reduction in the minimum inhibitory concentration against P. gingivalis and A. viscous, respectively.152
In another study for enhanced controlled drug delivery, Wang et al. designed a dual-sensitive nanocarrier system. This system was composed of a hydrophobic core of alpha-lipoic acid (ALA) loaded with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-poly (ethylene glycol) (DSPE-PEG) and the hydrophilic outer wall synthesized with poly (amidoamine) dendrimer (PAMAM) which electrostatically adsorbs minocycline. The unique structure was prepared to target the release of the antimicrobial minocycline in a controlled, low pH inflammation microenvironment. The results showed that in the presence of lipase and under acidic conditions, lipase induced ALA release and minocycline was released faster at pH 5.5 than at pH 7.4, thus reducing the inflammation process by decreasing ROS production. Furthermore, the minimal inhibitory concentration value was reduced to 100 μg mL−1 for S. aureus and 300 μg mL−1 for E. coli, while osteogenic differentiation of cells was enhanced, and periodontal bone resorption was improved.153
Another dual-responsive nanomaterial was described by Zhang et al. to address the effective antimicrobial release via photothermal exposure and antimicrobial drugs in the treatment of periodontitis. A nano-antibiotic platform (TC-PCM@GNC-PND) containing the antibiotic tetracycline (TC) was developed by combining NIR photosensitive gold nanocages (GNC) with phase change materials (PCM) and the thermosensitive polymer poly (N-isopropylacrylamide-codiethylaminoethyl methacrylate) (PND), which can have a coil-granule transition with temperature change. In the in vitro antibacterial activity evaluation, it was observed that the GNC-PND group exhibited sustained release of TC when irradiated with NIR and at elevated temperature, thus effectively destroying bacteria with a MIC value of 10 ppm. Furthermore, examination of bacterial growth curves revealed that bacterial growth was significantly slower in the GNC-PND and NIR-assisted treatment compared to the other groups.154
Topically applied agents’ challenge in avoiding immediate clearance from interfaces and the association of exopolysaccharide matrix and biofilm microenvironments’ acidification with cariogenic biofilm virulence led Horev et al. to develop a nanoparticle drug release system sensitive to acidic pH, capable of binding to exopolysaccharides. High-capacity nanoparticle-mediated delivery of a hydrophobic antibacterial agent, farnesol, was shown to boost therapeutic efficacy against planktonic S. mutans cells, but to have minimal action against cariogenic biofilms after topical applications. These nanoparticles, composed of diblock copolymers of 2-(dimethyl amino) ethyl methacrylate (DMAEMA), butyl methacrylate (BMA), and 2-propylacrylic acid (PAA) (p(DMAEMA)-b-p(DMAEMA-co-BMA-co-PAA)), load the hydrophobic antibacterial drug farnesol at 22%, providing release durations of t1/2 = 7 and 15 hours at pH 4.5 and 7.2, respectively. In the same study, farnesol-loaded nanoparticles reduced biofilm virulence when applied twice daily in a rodent dental caries model.155
Oxygen is an important biomolecule, like antibacterial agents and growth factors, which is effective in wound healing and tissue regeneration. However, long-term oxygen utilization with materials that produce, or release oxygen remains as a challenge. In this context, Sun et al. synthesized oxygen-generating nanocomposite materials that can reduce the deficient oxygen concentration in the periodontal pocket and increase ROS production to evaluate antibacterial properties and inhibition of bacteria-induced periodontal disease. Antimicrobial photodynamic therapy (aPDT), which is used as an antibacterial treatment method especially in deep periodontal pockets where the oxygen rate is relatively lower and difficult to access, was combined with encapsulated Fe3O4 nanoparticles to catalyse the conversion of H2O2 to O2. The nanoplatform created for this purpose offered oxygen generation by MnO2 catalysis, magnetic target capability with Fe3O4 nanoparticles, fulfilling its function for aPDT. As a result of the research, the amount of P. gingivalis and F. nucleatum pathogens, which have a prominent place in inflammatory periodontal diseases, decreased from 66.21% to 51.61% in the group treated with irradiated control.156
In addition, Han et al. developed a printable biomaterial with specially selected demineralized dentin matrix particles (DDMp) after extracting minerals from human dentins and created a personalized 3D tooth-like cellular structure with computer-aided design. It was proved that 95% of DPSCs were viable and odontogenic differentiation improved as the amount of DDMp increased.157
4.2. Polymer-based hydrogels and 3D scaffolds
Hydrogels and their 3D scaffolds consisting of natural polymers such as chitosan, HA, collagen, alginate and synthetic polymers such as GelMA, PEG, PCL and PLGA provides advanced solutions for periodontitis treatment in terms of inhibiting the growth of periodontal pathogens, reducing the inflammation at the periodontal damaged tissue and accelerating wound healing and periodontal tissue regeneration (Table 1). Hydrogels provide biocompatibility, biodegradability, injectability, as well as controlled drug delivery via external stimuli, and can mimic soft tissue microenvironment. While 3D composite scaffolds can mimic both soft and hard tissue microenvironment of periodontal tissue and act as an 3D network to support adhesion, proliferation, and differentiation of cells to generate periodontal ligament and alveolar bone-specific compartments.| Polymer | Applications & outcomes | Studied cells, bacteria, and biomolecules | Ref. |
|---|---|---|---|
| Collagen | Collagen significantly promoted bone formation within one month when used to deliver growth factors. It has been observed to contribute to the release of other growth factors for up to two weeks, with benefits for longer periods. When supplemented with bioprinting, it has also been shown to contribute to PDL regeneration. | PDL cells, FGF-2, TGF-B1, and PDGF-BB | Ogawa et al.,159 Nica et al.160 and Lin et al.161 |
| Gelatin | Gelatin enhanced cell growth and adhesion in the form of GelMA/nanohydrocyapatite (nHA) microgel. As a bioink, it also facilitated even distribution of cells and provided a high cell motility, maintaining their viability for more than a week. | hPDLFs, PDL fibroblasts, osteoblasts, dental follicle cells, PDLSCs, bioactive glass | Chen et al.,162 Vurat et al.,163 Mei et al.165 and Ma et al.166 |
| Chitosan | Chitosan showed an antimicrobial effect on P. Gingivalis as a component of injectable hydrogels. It also enhanced the mechanical and physical properties of the scaffolds as a part of the composite scaffold. | hPDLCs, PDLSCs and P. gingivalis, non-steroidal anti-inflammatory drugs such as aspirin, ibuprofen, and antimicrobial drugs, GFs, EPO, BMP-7 and ORN, rhAm, bFGF | Zang et al.,168 Suo et al.,169 Liao et al.170 and Akman et al.171 |
| HA | Upon encapsulation of CaO2 in HA, this system was observed to release sufficient oxygen at a to reduce the growth of P. gingivalis. Furthermore, when combined with M101 in a hydrogel, HA exhibited an antibacterial effect against this pathogen. | Primary human fibroblasts, osteoblasts, HUVECs and P. gingivalis, M101, bFGF, rhAm, O2 | Özçelik et al.,110 Liao et al.,170 Akman et al.171 and Müller-Heupt172 |
| PCL | Combining PCL with various bioactive molecules promoted bone regeneration, while coating it with materials such as bioceramics improved its surface properties. | hPDLSC PDL cells, F/CaP, heparin, β-tricalcium phosphate | Xu et al.,175 Liu et al.176 and Daghrery et al.137 |
| Alginate | Due to its high viscosity and biocompatibility, alginate is widely used as a hydrogel and scaffold in drug delivery systems and for the transport of bioactive molecules. Combined with different materials such as gelatin and dentin matrix, alginate has shown success in the delivery of anti-inflammatory drugs and in increasing the strength of hydrogels under stress. | Metronidazole, spermidine | Athirasala et al.,178 Zusmann et al.179 and Zhang et al.180 |
| PLGA | PLGA, an FDA-approved polymer used in tissue engineering and drug delivery systems, has been shown in studies to reduce biofilm and inflammation when combined with bioactive molecules (e.g. quercetin). It has also been shown to support osteoblast cell growth with controlled drug release such as minocycline. | Osteoblast cells, S. mutants/qercetin, minocycline, pFGF-2, nBGC and cementum protein 1, platelet rich plasma | Jiang et al.,183 Sowmya et al.184 and Lian et al.185 |
| PEGDA | When PEDGA is used in a hydrogel structure, the migration capacity of cells is increased, the growth of P. gingivalis is reduced and inflammation is decreased. It is also used in a membrane structure to achieve higher durability and better mechanical properties. | Stromal cells and P. gingivalis/antimicrobial peptide, SDF-1 | Liu et al.97 and Wang et al.186 |
Since the retention and continuous delivery of signalling molecules in the 3D matrix is crucial for periodontal tissue engineering, Ogawa et al. used a collagen scaffold as a growth factor carrier system in one study. In this study, collagen scaffold incorporated with beta-tricalcium phosphate and fibroblast growth factor-2 (FGF-2) bioactive molecule was applied to a dog with periodontitis with infra-bone defects. Four weeks after implantation, the GF-loaded scaffold was observed to support a significant amount of new bone formation. In FGF-2-treated tissues, the increase in cementum and PDL-like tissues was 5.1-fold and 5.5-fold higher than that treated with collagen scaffold alone.159 For a similar purpose, Nica et al. prepared different porcine-derived collagen matrices loaded with various GFs. It was found that the release of TGF-B1, FGF-2 and PDGF-BB continued until day 13, indicating the beneficial use of such scaffolds for long-term tissue regeneration.160
In addition to their ability to protect and deliver signalling molecules, scaffolds should be able to provide fiber-like structure to mimic the natural microarchitecture of PDL and withstand functional load to aid PDL regeneration. In this context, Lin et al. investigated a collagen-based wave-shaped microfibrous scaffold using an extrusion-based bioprinter. The loaded PDL cells were found to maintain their viability under shear stress of 6 dynes per cm2 with large cytoskeleton expansion and adhesion. The PDL-loaded wave-shaped scaffold was also found to up-regulate periostin, a mechanosensitive molecule required for ECM and tissue morphogenesis.161
3D bioprinting-based structures are widely used, especially in therapies applied for the regeneration of periodontal tissues. For example, in the study conducted by Vurat et al. used GelMA to bioprint the hPDLFs and thus to mimic the periodontal ligament layer, while GelMa and HAp-magnetic iron oxide composite bioink was used to bioprint the human osteoblasts to mimic the alveolar bone. The results showed that the prepared 3D matric supported the homogeneous distribution of cells and their viability for more than a week. As well as the immunofluorescence analysis proved the interaction of human PDL fibroblasts containing a PDL layer and osteocalcin human osteoblasts containing a mineralized layer over time.163
GelMA bioink is also used in the regeneration of various periodontal tissues by supporting the encapsulation of dental cells. In the study by Yang et al., a bioink composed of GelMA combined with dental cells and decellularized extracellular matrix (dECM) was designed for PDL and alveolar bone regeneration. It was observed that the mobility (migration rate) of cells and the highest number of viable cells were observed in the GelMA/dECM matrix within 7 days. The viability and fibrogenesis and osteogenic differentiation of dental follicle cells was improved resulted in the restoration of bone-ligament interfaces and orientation of PDL fibers.164
Mei et al. investigated the effects on osteogenic and cementogenic differentiation in PDLSCs by adding bioactive glass (BG) NPs to the macroporous scaffold, which is difficult to form with GelMA alone, and printing it with extrusion-based 3D printer. It was observed that the addition of BG NPs stabilized the shape of the scaffold structure and increased its stiffness and bioactivity. At the same time, the release of inorganic bioactive ions during the degradation of the scaffold structure had positive effects on hPDLSCs and the macroporous scaffold structure was successfully printed.165
In a 3D printed microarray study with GelMA/PEG hydrogel developed by Ma et al., the viability of PDLSCs encapsulated in the hydrogel under different ratios of GelMA and PEG was investigated. Cell viability 3 days after printing was found to be 82.5% ± 4.1% in GelMA/PEG hydrogel synthesized at 5/0 ratio and 30% in GelMA/PEG hydrogel synthesized at 0/5 ratio. In the same study, as a result of 3-day culture, it was observed that cells proliferated and elongated by establishing connections with neighboring cells at higher concentrations of GelMA and PDLSCs proliferation decreased with increasing PEG concentration.166
For example, in one study, injectable thermosensitive hydrogel containing chitosan, b-sodium glycerophosphate (b-GP) and gelatin prepared by Xu et al. was loaded with aspirin and growth factor erythropoietin (EPO) and its release was investigated for effective inhibition of inflammation. It was observed that aspirin and EPO were released within the first 8 days. EPO release continued until day 21, at the same time aspirin was released faster and increased the effect of EPO.47 Zang et al. added BMP-7 and ornidazole (ORN) to the thermosensitive chitosan/β-glycerophosphate (CS/β-GP) hydrogel and investigated its release profile and periodontal regeneration in small dogs with class 3 furcation injury. It was observed that ORN-loaded hydrogels had antimicrobial properties on P. gingivalis, 67% ORN release occurred in the first 80 minutes and at the end of 8 weeks, the damaged area was largely filled with regenerated tissue and both groups supported regeneration.168 Suo et al. also investigated the antibacterial effect of 3D printed carbon nanotube/chitosan/sodium alginate (CNT/CS/AL) composite scaffold structure on the proliferation of hPDLCs and P. gingivalis. As a result of the study, it was observed that the CNT/CS/AL composite scaffold increased the number of hPDLCs and decreased the number of viable bacteria as the CNT amount increased, indicating CNT amount-dependent bacteriostatic properties.169
In various studies, a composite structure was formed by producing a mesoporous HA/chitosan (mHA/CS) composite scaffold. The mechanical limitations of mHA material alone have been overcome by combining it with materials with high elasticity and porous structures such as chitosan. For example, Liao et al. evaluated the effect of mesoporous hydroxyapatite/chitosan (mHA/CS) scaffold on periodontal regeneration. The scaffold was loaded with recombinant human amelogenin (rhAm), which improves osteogenic differentiation of human PDL stem cells (PDLSCs) and reduces inflammation. This study aimed to enhance the effect of rhAm with the mHA/CS scaffold, which can increase the amount of rhAm loaded on the large surface area of HA coated with chitosan and thus sustained rhAm release. mHA/CS scaffold was found to be able to reduce bacterial growth and exhibit sustained release of rhAm. This in turn increased ALP activity and enhanced bone and cementum-like tissue formation in vivo.170 HA-particle chitosan scaffolds were also prepared by Akman et al. These scaffolds were prepared by freeze-drying method and loading the scaffold with bFGF. It was observed that the bFGF, was continued to be released up to 168 hours and the analysis showed that the HA-chitosan scaffold provided the 3D environment necessary for mineralization, proliferation and support of the cellular structure.171
PCL is commonly utilized to produce composite structures, as it can be combined with different natural or synthetic polymers to address its low bioactivity and hydrophilicity limitations to enhance its mechanical properties, or PCL can be coated to improve it surface quality based on the specific requirements of its usage area.
In a recent study involving PCL, Batool et al. discovered that by incorporating bioactive molecules and drugs, such as hydroxyapatite and antibiotics, into composite scaffolds through electrospinning, they were able to stimulate new bone formation and improve the mechanical properties of mesenchymal stem cells.173 As the predominant inorganic component of the bone matrix, nHA possesses a distinctive attraction towards diverse adhesion proteins and plays a critical role in the differentiation and mineralization of bone cells. This makes it a highly suitable option for integration into PCL for the purpose of bone regeneration. In the study by Daghrery et al., which aimed to increase osteogenic capacity and tissue-based regeneration by coating PCL, with bioceramics such as fluorinated calcium phosphate (F/CaP), F/CaP coated scaffolds were found to have better Young's modulus and tensile strength. It was also observed that coating PCL scaffolds with F/CaP had a positive effect on the formation of new bone tissue and promoted the formation of more mineralized tissue.137
This polymer is also well suited for creating 3D bioprinted scaffolds and implants due to its user-friendly nature, low cost, especially its application to obtain bone and hard tissue. It is generally not used on its own but rather mixed with other substances for better results. For example, the response of hPDLSC cells to scaffolds prepared with different concentrations of PCL/PLGA bioink was investigated by Peng et al. It was found that PLGA is a fast degrading and brittle material, but PCL is a slow degrading and flexible material and the composite bioink obtained had an optimal degradation of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio and the expression of ALP and RunX at this concentration was higher at 7 and 14 days compared to other groups. In the same study, hPDLSCs were found to be denser in layers with 0.5 PCL/0.5 PLGA scaffold structure.174
50 ratio and the expression of ALP and RunX at this concentration was higher at 7 and 14 days compared to other groups. In the same study, hPDLSCs were found to be denser in layers with 0.5 PCL/0.5 PLGA scaffold structure.174
In the study conducted by Xu et al. involved the creation of a scaffold structure through the combination of electrospun gelatin and PCL, selected for their high hydrophilicity and mechanical strength, respectively. Through both in vivo and in vitro studies, it was determined that this combination had superior cytocompatibility and degradation properties. The results indicated that the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 PCL/gel structure exhibited superior surface hydrophilicity compared to the pure form of PCL. In the same study, it was discovered that the membrane with dispersed fibers had the highest ALP activity, with the membrane containing aligned fibers having the highest Periostin gene ratio.175 Another study conducted by Liu et al. focused on developing a multifunctional bilayer scaffold structure through electrospinning. The resulting membrane was composed of heparin-conjugated PCL/Gel nanofibers, with the upper layer serving as a barrier and the lower layer being printed with poly (glycolic acid) (PGH) using 3D bioprinting. The aim was to facilitate bone tissue regeneration by dispersing the upper layer. The double-layer scaffold design displayed superior outcomes in comparison to the PGH scaffold alone, exhibiting enhanced integration with neighboring tissues and the most robust new bone growth after 5 and 20 weeks of implantation, as indicated by in vivo experiments.176 Vaquette et al. used fused deposition and electrospun techniques to regenerate the alveolar bone and PDL complex. In their study, they produced the two-layer scaffold structure composed of PCL containing β-tricalcium Phosphate. The prepared scaffold displayed a layer that mimic bone fabricated by fused deposition, while the second layer generated by electrospun technique represents periodontal layer due to its flexibility. The results showed that the scaffold structure implanted in the subcutaneous and dentin block regions was more stable for the cell sheet and cementum deposition on the dentin slice surface. Furthermore, two layered scaffolds were able to combine PDL cell sheets and deliver cells for bone, PDL and cementum regeneration.177
50 PCL/gel structure exhibited superior surface hydrophilicity compared to the pure form of PCL. In the same study, it was discovered that the membrane with dispersed fibers had the highest ALP activity, with the membrane containing aligned fibers having the highest Periostin gene ratio.175 Another study conducted by Liu et al. focused on developing a multifunctional bilayer scaffold structure through electrospinning. The resulting membrane was composed of heparin-conjugated PCL/Gel nanofibers, with the upper layer serving as a barrier and the lower layer being printed with poly (glycolic acid) (PGH) using 3D bioprinting. The aim was to facilitate bone tissue regeneration by dispersing the upper layer. The double-layer scaffold design displayed superior outcomes in comparison to the PGH scaffold alone, exhibiting enhanced integration with neighboring tissues and the most robust new bone growth after 5 and 20 weeks of implantation, as indicated by in vivo experiments.176 Vaquette et al. used fused deposition and electrospun techniques to regenerate the alveolar bone and PDL complex. In their study, they produced the two-layer scaffold structure composed of PCL containing β-tricalcium Phosphate. The prepared scaffold displayed a layer that mimic bone fabricated by fused deposition, while the second layer generated by electrospun technique represents periodontal layer due to its flexibility. The results showed that the scaffold structure implanted in the subcutaneous and dentin block regions was more stable for the cell sheet and cementum deposition on the dentin slice surface. Furthermore, two layered scaffolds were able to combine PDL cell sheets and deliver cells for bone, PDL and cementum regeneration.177
Athirasala et al. isolated dentin matrix obtained from human molars and combined it with alginate, a natural polymer, at different concentrations to form a gel and cross-linked with calcium chloride. During the process of fabricating scaffolds using a 3D bio-printer, it was noted that scaffolds containing a higher proportion of alginate exhibited more defined edges and well-ordered lines. Alg-dentin scaffold produced in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was found to be optimal in terms of structural properties and had the highest cell viability after 5 days.178 In another study, Zussman et al. loaded metronidazole into the hydrogel formed with gelatin, which has natural adhesive properties, cross-linked with N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride, which has a less toxic effect, and alginate to take advantage of its viscosity property, and found that alginate used even in small amounts can have a large effect on the hydrogel structure. They observed that 83–92% of the metronidazole was released within the first 6 hours and spread at a constant rate for the remaining 24 hours, and the burst effect decreased, although the high crosslinking level and gelation time were negatively affected by increasing the concentration of alginate in the hydrogel.179 Zhang et al. added spermidine (Spd) with anti-inflammatory properties and other benefits such as immunomodulation to alginate gel modified with phenylboronic acid (PBA) and tested it in various mechanical and in vivo experiments for periodontal ligament regeneration. PBA interacts with cis-diols present in the alginate structure, resulting in self-gelation and the development of viscoelastic and injectable properties, ultimately enhancing alginate's adaptability. The PBA-Alg/Spd hydrogel displayed a more rapid stress relaxation rate under 10% stress compared to the typical Alg/Ca2+ hydrogel. Furthermore, it enhanced the deposition of periodontal collagen and thus promoted the repair of periodontal damage.180
1 ratio was found to be optimal in terms of structural properties and had the highest cell viability after 5 days.178 In another study, Zussman et al. loaded metronidazole into the hydrogel formed with gelatin, which has natural adhesive properties, cross-linked with N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride, which has a less toxic effect, and alginate to take advantage of its viscosity property, and found that alginate used even in small amounts can have a large effect on the hydrogel structure. They observed that 83–92% of the metronidazole was released within the first 6 hours and spread at a constant rate for the remaining 24 hours, and the burst effect decreased, although the high crosslinking level and gelation time were negatively affected by increasing the concentration of alginate in the hydrogel.179 Zhang et al. added spermidine (Spd) with anti-inflammatory properties and other benefits such as immunomodulation to alginate gel modified with phenylboronic acid (PBA) and tested it in various mechanical and in vivo experiments for periodontal ligament regeneration. PBA interacts with cis-diols present in the alginate structure, resulting in self-gelation and the development of viscoelastic and injectable properties, ultimately enhancing alginate's adaptability. The PBA-Alg/Spd hydrogel displayed a more rapid stress relaxation rate under 10% stress compared to the typical Alg/Ca2+ hydrogel. Furthermore, it enhanced the deposition of periodontal collagen and thus promoted the repair of periodontal damage.180
Qercetin (QUE) is a natural flavonoid useful for human health and used in the treatment of various periodontal and systematic diseases. In the study conducted by Cristo et al., different concentrations of QUE were added to PLA nanofibers developed using the electrospun technique to release them in acidic microenvironment containing oral bacteria. As a result of the examination of antibiofilm activity by crystal violet staining, it was observed that it strongly reduced biofilm formation on PAO1 and S. mutants at the end of 9 hours. In the same study, when the cytokine regulation before inflammation was examined, it was observed that PLA/QUE with 10% w/w ratio slowed down the inflammation process more than PLA/QUE with 5% w/w.43
Minocycline, an antibiotic belonging to the tetracycline group and known to support bone formation, was loaded at different concentrations onto electrospun PLGA membranes and its effect on periodontitis was investigated. As a result of the study, Ma et al. concluded that the 2% minocycline/PLGA membrane had the highest cell connectivity and was suitable for osteoblast cell growth and adhesion. Although an initial burst effect was observed at the beginning of the experiment due to the amount of drug that may have settled on the fiber surfaces, it exhibited a controlled regular release profile with diffusion and matrix degradation.182 Jiang et al. used PLGA to carry the growth factor DNA encoding fibroblast growth factor-2 (pFGF-2) for periodontal ligament regeneration. While there was no difference in human periodontal ligament cell viability between the scaffold made with PLGA alone and the scaffold made with PLGA/pFGF-2 on days 1 and 3, it was observed that the PLGA/pFGF-2 scaffold showed better cell viability at the end of day 7 and positively influenced the periodontal ligament regeneration in vivo model by continuously secreting the growth factor.183
Sowmya et al. developed a three-layer scaffold structure that targets different parts of periodontal tissue. Specific tissue regeneration was targeted by using a different polymer and growth factor in each layer. The Fig. 3 displays the preparation of three layers. The first layer used chitin-PLGA/nanobioactive glass ceramic (nBGC) and cementum protein 1, the middle layer used chitin-PLGA/fibroblast growth factor 2, and the last layer used chitin-PLGA/(nBGC)/platelet rich plasma. These layers were obtained in scaffold structures through lyophilization, aiming at regenerating cementum, PDL, and alveolar bone. Complete healing and wound closure were observed in the areas where scaffolds with a pore size of 100–350 μm were applied, and spongy bone formation was well realized in the scaffolds to which GFs were added.184
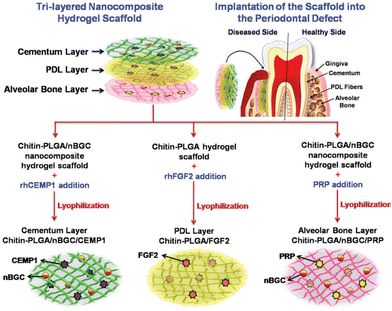 | ||
| Fig. 3 Schematic description of the development of a three-layer nanocomposite hydrogel scaffold and its implantation into a periodontal defect for concurrent and complete periodontal regeneration. Reproduced from ref. 184 with permission from Wiley-VCH GmbH, copyright 2017. | ||
PLGA hydrogel, which has different preparation techniques, is usually obtained by physical mixing with different materials, covalent cross-linking, or entrapment in hydrogel junctions. PLGA, which is used for targeted use by combining with different biomaterials due to limitations such as the low mechanical strength of its pure form and the need to prepare PLA and PGA ratios in accordance with the area of use and target, was used to form scaffolds by combining gelatine with copper-loaded mesoporous silica metal nanoparticles in a study by Lian et al. The solution electro-writing technique was used to create a porous and loose structure, while the solution electro-spinning technique was used to create a denser and more compact layer. Thus, the dense layer inhibited bacterial growth and the loose layer prepared the environment for the subsequent osteogenesis of local cells. Concurrently, the double-layer scaffolds that were prepared demonstrated enhanced mechanical properties, while also exhibiting a prolonged and consistent release of Cu ions from the PLGA/gelatin composite scaffolds of varying densities.185
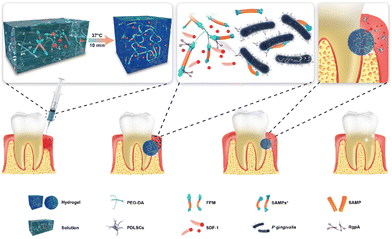 | ||
| Fig. 4 Schematic illustration of the preparation and application of the multifunctional hydrogel. Gingipain-sensitized thermosensitive hydrogel was cross-linked with PEG-DA and FPM and loaded with SDF-1. The prepared hydrogel inhibits the growth of periodontal pathogens, while the loaded SDF-1 is released to recruit host stem cells and promote osteogenesis. Reproduced from ref. 97 with permission from American Chemical Society, copyright 2021. | ||
5. Conclusion and future perspectives
Periodontal disease and associated damage to periodontal tissue is a major public health problem due to the limitations of current therapies, which rely on daily oral hygiene practices and periodic professional removal of microbial biofilm but are insufficient to prevent recurrence and associated damage. Moreover, the complex and hierarchical structure of the periodontium poses a contemporary medical challenge requiring synchronized and spatiotemporal regeneration. Therefore, in the field of biomedicine, discovering effective therapies to treat the disease and facilitate the regeneration of periodontal tissue is a very important goal. Hence, there is a great interest in engineering various biomaterials that involve the customization of their chemical composition and microarchitecture and show promise for a wide range of clinical applications.Recent approaches focus on drug and/or biomolecule delivery, including antibacterial biomolecules, oxygen, gene and growth factor therapies applied to inhibit biofilm formation or to promote wound healing at the site of biofilm formation and tissue loss, and to improve regeneration of damaged or lost tissues. Furthermore, additively manufactured biomaterials have been used as advanced systems due to their ability to mimic the 3D complexity of the periodontium, supporting functional integration of various tissue components and synchronized spatiotemporal regeneration within the 3D matrix. Thus, this ability greatly promotes 3D repair and replacement of damaged periodontal tissue.
Notwithstanding these significant advances, there are still numerous biological, technical, and clinical hurdles to be overcome: despite these significant advances, complete regeneration of the periodontium still remains a considerable challenge today due to its complex structure and characteristics. There are many biological, technical, and clinical barriers to overcome in this area. For example, lack of understanding of the molecular and signalling pathways that control cell differentiation and subsequent wound healing and tissue engineering, inability to adequately mimic the cell microenvironment in vivo, failure to restore fine fiber structures, horizontal alveolar bone loss and long-term stability of regenerated periodontal tissues, inability to control the release of bioactive molecules such as bioactive factors to promote tissue regeneration, the inability to produce the natural 3D extracellular matrix scaffold of periodontal tissue and the clinical challenges in cell-based and/or cell-associated biomaterial-based periodontal therapy, such as immune rejection of the administration of stem cells and biomaterials to the host after treatment.
Therefore, the design of future biomaterials should consider the production of 3D multiphase biomaterials that provide different but complementary properties to control the complex spatiotemporal regeneration of the periodontium. In addition, spatiotemporal and continuous drug and/or bioactive molecule delivery to the site of action in a time- and site-dependent manner should be incorporated into biomaterials as tunable physicochemical properties to promote controlled stem cell differentiation, reduce side effects of drugs and biomolecules on healthy tissues, and provide long-term therapy.
Author contributions
All authors contributed to the writing of the article. N. S. K. determined the content and directed the writing process. All authors have read and accepted the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.References
- D. F. Kinane, P. G. Stathopoulou and P. N. Papapanou, Nat. Rev. Dis. Primers, 2017, 22, 17038 CrossRef PubMed.
- E. Könönen, M. Gursoy and U. K. Gursoy, J. Clin. Med., 2019, 8, 1135 CrossRef PubMed.
- M. Martínez-García and E. Hernández-Lemus, Front. Physiol., 2021, 12, 709438 CrossRef PubMed.
- M. M. Haque, K. Yerex, A. Kelekis-Cholakis and K. Duan, BMC Oral Health, 2022, 22, 492 CrossRef PubMed.
- A. Upadhyay, S. Pillai, P. Khayambashi, H. Sabri, K. T. Lee, M. Tarar, S. Zhou, I. Harb and S. D. Tran, Biomimetics, 2020, 5, 51 CrossRef CAS PubMed.
- A. H. Melcher, J. Periodontol., 1976, 47, 256–260 CrossRef CAS PubMed.
- W. V. Giannobile, J. Periodontol., 2014, 85, 1151–1154 CrossRef PubMed.
- L. Larsson, A. M. Decker, L. Nibali, S. P. Pilipchuk, T. Berglundh and W. V. Giannobile, J. Dent. Res., 2015, 95, 255–266 CrossRef PubMed.
- S. Dabra, K. Chhina, N. Soni and R. Bhatnagar, J. Dent. Res., 2012, 9, 671–680 CAS.
- T. Kwon, I. B. Lamster and L. Levin, Int. Dent. J., 2021, 71, 462–476 CrossRef PubMed.
- X.-Y. Xu, X. Li, J. Wang, X.-T. He, H.-H. Sun and F.-M. Chen, Stem Cells Transl. Med., 2018, 8, 392–403 CrossRef PubMed.
- F. Rizzo and N. S. Kehr, Adv. Healthcare Mater., 2020, 10, 2001341 CrossRef PubMed.
- W. B. Swanson, Y. Yao and Y. Mishina, Genesis, 2022, 60, e23499 CrossRef CAS PubMed.
- I. Roato, B. Masante, G. Putame, D. Nada and F. Mussano, Nanomaterials, 2022, 12, 3878 CrossRef CAS PubMed.
- R. J. Genco, J. Periodontol., 1996, 67, 1041–1049 CAS.
- M. J. McDevitt, H.-Y. Wang, C. Knobelman, M. G. Newman, F. S. di Giovine, J. Timms, G. W. Duff and K. S. Kornman, J. Periodontol., 2000, 71, 156–163 CrossRef CAS PubMed.
- P. P. Katz, M. R. Wirthlin Jr., S. M. Szpunar, J. V. Selby, S. J. Sepe and J. A. Showstack, Diabetes Care, 1991, 14, 375–385 CrossRef CAS PubMed.
- J. M. Albandar, Dent. Clin. North Am., 2005, 49, 517–532 CrossRef PubMed.
- H. Nilsson, J. S. Berglund and S. Renvert, Clin. Oral Investig., 2017, 22, 2103–2109 CrossRef PubMed.
- T. Ramich, A. Asendorf, K. Nickles, G. M. Oremek, R. Schubert, L. Nibali, M. Wohlfeil and P. Eickholz, Clin. Oral Investig., 2018, 22, 3079–3089 CrossRef PubMed.
- T. E. Rams, J. E. Degener and A. J. van Winkelhoff, J. Periodontol., 2014, 85, 160–169 CrossRef PubMed.
- B. Heras, M. J. Scanlon and J. L. Martin, Br. J. Clin. Pharmacol., 2015, 79, 208–215 CrossRef CAS PubMed.
- X. Tong, X. Qi, R. Mao, W. Pan, M. Zhang, X. Wu, G. Chen, J. Shen, H. Deng and R. Hu, Carbohydr. Polym., 2020, 245, 116585 CrossRef CAS PubMed.
- H. Ogawara, J. Antibiot., 2021, 74, 24–21 CrossRef PubMed.
- V. Kumbar, M. R. Peram, M. Kugaji, T. Shah, S. P. Patil, U. M. Muddapur and K. Bhat, Odontology, 2020, 109, 18–28 CrossRef PubMed.
- T. Defoirdt, Trends Microbiol., 2018, 26, 313–328 CrossRef CAS PubMed.
- A. K. Bhardwaj, K. Vinothkumar and N. Rajpara, Recent Pat. Anti-Infect. Drug Discovery, 2013, 8, 68–83 CrossRef CAS PubMed.
- H. Chai, M. Hazawa, N. Shirai, J. Igarashi, K. Takahashi, Y. Hosokawa, H. Suga and I. Kashiwakura, Invest. New Drugs, 2010, 30, 157–163 CrossRef PubMed.
- C. Sima, A. Viniegra and M. Glogauer, J. Leukocyte Biol., 2018, 105, 473–487 CrossRef PubMed.
- A. B. Molofsky, A. K. Savage and R. M. Locksley, Immunity, 2015, 42, 1005–1019 CrossRef CAS PubMed.
- S. Rutz, X. Wang and W. Ouyang, Nat. Rev. Immunol., 2014, 14, 783–795 CrossRef CAS PubMed.
- Y. Matsuki, T. Yamamoto and K. Hara, PubMed, 1992, 76, 42–47 CAS.
- A. Chaves, K. Ishikawa, M. R. Messora and M. Pinto, Adv. Exp. Med. Biol., 2022, 353–375 Search PubMed.
- M. Saita, J. Kaneko, T. Sato, S. S. Takahashi, S. Wada-Takahashi, R. Kawamata, T. Sakurai, M. C. Il Lee, N. Hamada, K. Kimoto and Y. Nagasaki, Biomaterials, 2016, 76, 292–301 CrossRef CAS PubMed.
- D. R. Silva, J. C. O. Sardi, N. de S. Pitangui, S. M. Roque, A. C. Barbosa da Silva and P. L. Rosalen, J. Funct. Foods, 2020, 73, 104080 CrossRef CAS.
- J. H. Meurman, Eur. J. Oral Sci., 2005, 113, 188–196 CrossRef PubMed.
- M. Tekce, G. Ince, H. Gursoy, S. Dirikan-Ipci, G. Cakar, T. Kadir and S. Yılmaz, J. Clin. Periodontol., 2015, 42, 363–372 CrossRef PubMed.
- J. D. Hillman, T. A. Brooks, S. M. Michalek, C. C. Harmon, J. L. Snoep and C. C. van der Weijden, Infect. Immun., 2000, 68, 543–549 CrossRef CAS PubMed.
- R. P. Allaker and C. W. I. Douglas, Int. J. Antimicrob. Agents, 2009, 33, 8–13 CrossRef CAS PubMed.
- U. K. Gursoy, M. Gursoy, O. V. Gursoy, L. Cakmakci, E. Könönen and V.-J. Uitto, Anaerobe, 2009, 15, 164–167 CrossRef CAS PubMed.
- H. J. English and P. C. Molan, J. Int. Acad. Periodontol., 2004, 6, 63–67 Search PubMed.
- G. Castellino, F. Mesa, F. Cappello, C. Benavides-Reyes, G. A. Malfa, I. Cabello and A. Magan-Fernandez, Appl. Sci., 2021, 11, 9563 CrossRef.
- F. Di Cristo, A. Valentino, I. De Luca, G. Peluso, I. Bonadies, A. Calarco and A. Di Salle, Molecules, 2022, 27, 2205 CrossRef CAS PubMed.
- S. Soukoulis and R. Hirsch, Aust. Dent. J., 2004, 49, 78–83 CrossRef CAS PubMed.
- C. Salvatori, Oral Implantol., 2017, 10, 59 CrossRef CAS PubMed.
- C. D. Wu, I. A. Darout and N. Skaug, J. Periodontal Res., 2001, 36, 275–284 CrossRef CAS PubMed.
- X. Xu, Z. Gu, X. Chen, C. Shi, C. Liu, M. Liu, L. Wang, M. Sun, K. Zhang, Q. Liu, Y. Shen, C. Lin, B. Yang and H. Sun, Acta Biomater., 2019, 86, 235–246 CrossRef CAS PubMed.
- J. Huh, J.-H. Kang, Y. Yoo, C. S. Kim, K.-S. Cho and S.-H. Choi, J. Korean Acad. Periodontol., 2001, 31, 833 CrossRef.
- M. Hirasawa, K. Takada, M. Makimura and S. Otake, J. Periodontal Res., 2002, 37, 433–438 CrossRef CAS PubMed.
- J. M. T. Hamilton-Miller, J. Med. Microbiol., 2001, 50, 299–302 CrossRef CAS PubMed.
- M. A. Botelho, N. A. P. Nogueira, G. M. Bastos, S. G. C. Fonseca, T. L. G. Lemos, F. J. A. Matos, D. Montenegro, J. Heukelbach, V. S. Rao and G. A. C. Brito, Braz. J. Med. Biol. Res., 2007, 40, 349–356 CrossRef CAS PubMed.
- H. Saluja, V. Dehane and U. Mahindra, Ann. Maxillofac. Surg., 2011, 1, 53 CrossRef PubMed.
- E. Mijiritsky, H. D. Assaf, O. Peleg, M. Shacham, L. Cerroni and L. Mangani, Biology, 2021, 10, 317 CrossRef CAS PubMed.
- A. Suchetha, P. Lakshmi, D. Bhat, D. B. Mundinamane, K. V. Soorya and G. A. Bharwani, Contemp. Clin. Dent., 2015, 6, 510–516 CrossRef CAS PubMed.
- P. Kumar Y, J. Dent. Health Oral Disord. Ther., 2018, 9, 350–352 Search PubMed.
- Y. Xu, J. Qiu, Q. Sun, S. Yan, W. Wang, P. Yang and A. Song, Med. Sci. Monit., 2019, 25, 4384–4389 CrossRef PubMed.
- X. Li, H. Yang, Z. Zhang, Z. Yan, H. Lv, Y. Zhang and B. Wu, Mol. Med. Rep., 2019, 19, 943–950 CAS.
- K. Thakare, M. L. Bhongade, P. Charde, P. Jaiswal, N. Shah and A. Deshpande, Case Rep. Dent., 2013, 2013, 1–4 Search PubMed.
- R. T. Kao and S. E. Lynch, Clin. Adv. Periodontics, 2011, 1, 132–141 CrossRef PubMed.
- M. Nevins, R. T. Kao, M. K. McGuire, P. K. McClain, J. E. Hinrichs, B. S. McAllister, M. S. Reddy, M. L. Nevins, R. J. Genco, S. E. Lynch and W. V. Giannobile, J. Periodontol., 2013, 84, 456–464 CrossRef CAS PubMed.
- V. Vikram, T. Ramakrishnan, K. Anilkumar and N. Ambalavanan, J. Clin. Diagn. Res., 2015, 9, ZC13–ZC16 CAS.
- G. Matarese, G. Isola, G. P. Anastasi, G. Cutroneo, G. Cordasco, A. Favaloro, G. Vita, G. Vermiglio, D. Milardi, V. L. Zizzari, S. Tetè and L. Perillo, Eur. J. Inflammation, 2013, 11, 479–488 CrossRef CAS.
- L. Wei, F. Teng, L. Deng, G. Liu, M. Luan, J. Jiang, Z. Liu and Y. Liu, J. Clin. Periodontol., 2019, 46, 1254–1263 CrossRef CAS PubMed.
- J. Lee, J. Yun, K. H. Kim, K. T. Koo, Y. J. Seol and Y. M. Lee, J. Craniofac. Surg., 2020, 31, 1602–1607 CrossRef PubMed.
- Y. Ebe, T. Nakamura, K. Hasegawa-Nakamura and K. Noguchi, Eur. J. Oral Sci., 2021, 129, e12792 CrossRef CAS PubMed.
- A. M. Badr, H. K. Shalaby, M. A. Awad and M. A. Hashem, Saudi Dent. J., 2023, 35, 760–767 CrossRef PubMed.
- H. D. Amin, I. Olsen, J. C. Knowles, M. Dard and N. Donos, Acta Biomater., 2013, 9, 4796–4805 CrossRef CAS PubMed.
- L. Fan and D. Wu, J. Healthcare Eng., 2022, 2022, 8661690 Search PubMed.
- K. Takeda, K. Mizutani, T. Matsuura, D. Kido, R. Mikami, P. Buranasin, N. Saito, H. Kominato, S. Takemura, K. Nakagawa and T. Iwata, J. Periodontol., 2022, 93, 1206–1217 CrossRef CAS PubMed.
- K. Mizutani, H. Shioyama, T. Matsuura, R. Mikami, K. Takeda, Y. Izumi, A. Aoki and T. Iwata, J. Periodontol., 2021, 92, 1262–1273 CrossRef PubMed.
- S. P. De Ry, A. Roccuzzo, N. P. Lang, A. Sculean and G. E. Salvi, J. Periodontol., 2022, 93, 548–559 CrossRef PubMed.
- E. Kobayashi, M. Fujioka-Kobayashi, N. Saulacic, B. Schaller, A. Sculean and R. J. Miron, Clin. Oral Implants Res., 2019, 30, 542–549 CrossRef PubMed.
- J. H. Lee, Y. S. Park, Y. T. Kim, D. H. Kim and S. N. Jeong, Clin. Oral Investig., 2020, 24, 229–237 CrossRef PubMed.
- H. B. Amara, D. S. Thoma, F. Schwarz, H. Y. Song, J. Capetillo and K. T. Koo, J. Clin. Periodontol., 2019, 46, 105–117 CrossRef PubMed.
- C. Del Angel-Mosqueda, Y. Gutiérrez-Puente, A. P. López-Lozano, R. E. Romero-Zavaleta, A. Mendiola-Jiménez, C. E. Medina-De la Garza, M. Márquez-M and M. A. De la Garza-Ramos, Head Face Med., 2019, 11, 29 CrossRef PubMed.
- Y. Wang, Z. Jia, S. Diao, X. Lin, X. Lian, L. Wang, R. Dong, D. Liu and Z. Fan, Cell Proliferation, 2016, 49, 618–627 CrossRef CAS PubMed.
- N. Han, F. Zhang, G. Li, X. Zhang, X. Lin, H. Yang, L. Wang, Y. Cao, J. Du and Z. Fan, Stem Cell Res. Ther., 2017, 8, 210 CrossRef PubMed.
- J. Taba, Q. Jin, J. V. Sugai and W. V. Giannobile, Orthod. Craniofacial Res., 2005, 8, 292–302 CrossRef PubMed.
- R. Mulligan, Science, 1993, 260, 926–932 CrossRef CAS PubMed.
- T. Wirth, N. Parker and S. Ylä-Herttuala, Gene, 2013, 525, 162–169 CrossRef CAS PubMed.
- G. A. R. Gonçalves and R. de M. A. Paiva, Einstein, 2017, 15, 369–375 CrossRef PubMed.
- J. A. Roth and R. J. Cristiano, JNCI, J. Natl. Cancer Inst., 1997, 89, 21–39 CrossRef CAS PubMed.
- T. Friedmann and R. Roblin, Science, 1972, 175, 949–955 CrossRef CAS PubMed.
- B. A. Bunnell and R. A. Morgan, Clin. Microbiol. Rev., 1998, 11, 42–56 CrossRef CAS PubMed.
- A. M. Badr, H. K. Shalaby, M. A. Awad and M. A. Hashem, Saudi Dent. J., 2023, 35, 760–767 CrossRef PubMed.
- H. D. Amin, I. Olsen, J. C. Knowles, M. Dard and N. Donos, Acta Biomater., 2013, 9, 4796–4805 CrossRef CAS PubMed.
- L. Fan and D. Wu, J. Healthcare Eng., 2022, 2022, 8661690 Search PubMed.
- K. Takeda, K. Mizutani, T. Matsuura, D. Kido, R. Mikami, P. Buranasin, N. Saito, H. Kominato, S. Takemura, K. Nakagawa and T. Iwata, J. Periodontol., 2022, 93, 1206–1217 CrossRef CAS PubMed.
- K. Mizutani, H. Shioyama, T. Matsuura, R. Mikami, K. Takeda, Y. Izumi, A. Aoki and T. Iwata, J. Periodontol., 2021, 92, 1262–1273 CrossRef PubMed.
- S. P. De Ry, A. Roccuzzo, N. P. Lang, A. Sculean and G. E. Salvi, J. Periodontol., 2022, 93, 548–559 CrossRef PubMed.
- E. Kobayashi, M. Fujioka-Kobayashi, N. Saulacic, B. Schaller, A. Sculean and R. J. Miron, Clin. Oral Implants Res., 2019, 30, 542–549 CrossRef PubMed.
- J. H. Lee, Y. S. Park, Y. T. Kim, D. H. Kim and S. N. Jeong, Clin. Oral Investig., 2020, 24, 229–237 CrossRef PubMed.
- M. Ramamoorth, J. Clin. Diagn. Res., 2015, 9, GE01–GE06 Search PubMed.
- P. L. Felgner, Y. J. Tsaı, L. Sukhu, C. J. Wheeler, M. Manthorpe, J. Marshall and S. H. Cheng, Ann. N. Y. Acad. Sci., 1995, 772, 126–139 CrossRef CAS PubMed.
- D. Schaffert and E. Wagner, Gene Ther., 2008, 15, 1131–1138 CrossRef CAS PubMed.
- M. A. Barry, W. J. Dower and S. A. Johnston, Nat. Med., 1996, 2, 299–305 CrossRef CAS PubMed.
- S. Liu, Y. N. Wang, B. Ma, J. Shao, H. Liu and S. Ge, ACS Appl. Mater. Interfaces, 2021, 13, 36880–36893 CrossRef CAS PubMed.
- C. Evans, Int. Orthop., 2014, 38, 1761–1769 CrossRef PubMed.
- F. Goker, L. Larsson, M. Del Fabbro and F. Asa'ad, Int. J. Mol. Sci., 2019, 20, 3551 CrossRef CAS PubMed.
- F.-M. Chen, Z.-W. Ma, Q.-T. Wang and Z.-F. Wu, Curr. Gene Ther., 2009, 9, 248–266 CrossRef CAS PubMed.
- S. Sood, S. Gupta and A. Mahendra, Med. Oral Patol. Oral Cir. Bucal, 2012, 17, e301–e310 CrossRef CAS PubMed.
- P. M. Bartold, S. Gronthos, S. Ivanovski, A. Fisher and D. W. Hutmacher, J. Periodontal Res., 2015, 51, 1–15 CrossRef PubMed.
- L. L. Southwood, D. D. Frisbie, C. E. Kawcak and C. W. Mcilwraith, Vet. Surgery, 2004, 33, 565–578 CrossRef PubMed.
- M. Kawai, Y.-H. Kataoka, J. Sonobe, H. Yamamoto, M. Inubushi, T. Ishimoto, T. Nakano, H. Maruyama, J.-I. Miyazaki, T. Yamamoto, K. Bessho and K. Ohura, J. Periodontol., 2017, 89, 1–18 Search PubMed.
- Y. Lai, N.-C. Kuo, W.-K. Hsiao, T.-L. Yew, S. Y. Lee and H.-L. Chen, J. Periodontol., 2011, 82, 1043–1050 CrossRef PubMed.
- H. Kanzaki, M. Chiba, I. Takahashi, N. Haruyama, M. Nishimura and H. Mitani, J. Dent. Res., 2004, 83, 920–925 CrossRef CAS PubMed.
- O. Anusaksathien, S. A. Webb, Q.-M. Jin and W. V. Giannobile, Tissue Eng., 2003, 9, 745–756 CrossRef CAS PubMed.
- P.-C. Chang, J. A. Cirelli, Q. Jin, Y. Seol, J. V. Sugai, N. J. D'Silva, T. E. Danciu, L. A. Chandler, B. A. Sosnowski and W. V. Giannobile, Hum. Gene Ther., 2009, 20, 486–496 CrossRef CAS PubMed.
- C. Vaquette, S. P. Pilipchuk, P. M. Bartold, D. W. Hutmacher, W. V. Giannobile and S. Ivanovski, Adv. Healthcare Mater., 2018, 7, 1800457 CrossRef PubMed.
- H. Özçelik, F. Batool, M. Corre, A. Garlaschelli, G. Conzatti, C. Stutz, C. Petit, E. Delpy, F. Zal, E. Leize-Zal and O. Huck, Int. J. Pharm., 2021, 615, 120810 CrossRef PubMed.
- R. Niveda and G. Kaarthikeyan, J. Pharm. Res. Int., 2020, 32, 75–82 CrossRef.
- D. M. Dos Santos, L. M. Dias, A. K. Surur, D. A. De Moraes, A. C. Pavarina, C. R. Fontana and D. S. Correa, ACS Appl. Nano Mater., 2022, 5, 14425–14436 CrossRef CAS.
- T. L. Chen, B. Xu, J. C. Liu, S. G. Li, D. Y. Li, G. C. Gong, Z. F. Wu, S. L. Lin and Y. J. Zhou, J. Indian Soc. Periodontol., 2012, 16, 492–497 CrossRef PubMed.
- T. A. Giacon, F. Giancola, M. Paganini, C. Tiengo, E. M. Camporesi and G. Bosco, Int. J. Environ. Res. Public Health, 2021, 18, 413 CrossRef PubMed.
- P. K. Gajendrareddy, R. Junges, G. Cygan, Y. Zhao, P. T. Marucha and C. G. Engeland, J. Periodontal Res., 2017, 52, 644–649 CrossRef CAS PubMed.
- K. Uslu, H. D. Tansuker, A. Tabaru, S. E. Egeren, K. K. Kulahci, P. Bulut, F. Emre and M. F. Oktay, Eur. Arch. Oto-Rhino-Laryngol., 2020, 277, 1771–1777 CrossRef PubMed.
- K. Srinivasan and S. Chitra, Scholars J. Dent. Sci., 2015, 2, 373–377 Search PubMed.
- M. D’Amario, M. Di Cario, S. M. Natale, L. Memè, G. Marzo, G. Matarazzo and M. Capogreco, Appl. Sci., 2022, 12, 11100 CrossRef.
- A. V. Issac, J. J. Mathew, M. Ambooken, A. J. Kachappilly, P. K. Ajithkumar, T. Johny, V. K. Linith and A. Samuel, J. Clin. Diagn. Res., 2015, 9, ZC29–ZC33 CAS.
- K. C. Huth, M. Quirling, S. Lenzke, E. Paschos, K. Kamereck, K. Brand, R. Hickel and N. Ilie, Eur. J. Oral Sci., 2011, 119, 204–210 CrossRef CAS PubMed.
- J. R. Pires, A. M. Karam, V. G. Garcia, F. S. Riberio, A. E. F. Pontes, C. R. de Andrade and E. C. Zuza, Rev. Odontol. UNESP, 2021, 50, e20210046 CrossRef.
- M. Yousef, M. Shoukheba, D. Amel, M. Ezzat, M. M. Shoukheba, A. M. Ezzatabdel-Hamid and E. Abo-Shady, Egypt. Dent. J., 2014, 60, 144–149 Search PubMed.
- P.-L. Wang, Y. Tachi, K. Masuno, N. Okusa and Y. Imamura, J. Hard Tissue Biol., 2018, 27, 209–212 CrossRef CAS.
- N. Anand, S. C. Chandrasekaran, N. Alam, G. Shankar and A. Nithya, Biomedicine, 2014, 34, 426–429 Search PubMed.
- A. A. Alsherif, H. M. Eltokhey and D. A. Taiema, J. Oral Biol. Craniofacial Res., 2020, 10, 639–649 CrossRef PubMed.
- F. Batool, C. Stutz, C. Petit, N. Benkirane-Jessel, E. Delpy, F. Zal, E. Leize-Zal and O. Huck, Sci. Rep., 2020, 10, 14725 CrossRef PubMed.
- F. Lemaire, S. Sigrist, E. Delpy, J. Cherfan, C. Peronet, F. Zal, K. Bouzakri, M. Pinget and E. Maillard, J. Cell. Mol. Med., 2019, 23, 8025–8034 CrossRef CAS PubMed.
- F. Batool, C. Petit, C. Stutz, H. Özçelik, P. Y. Gegout, N. Benkirane-Jessel, E. Delpy, F. Zal, E. Leize-Zal and O. Huck, J. Periodontol., 2022, 93, 1712–1724 CrossRef CAS PubMed.
- N. Mnasour, Egypt. Dent. J., 2022, 68, 213–224 CrossRef.
- G. Caccianiga, G. Rey, A. Paiusco, D. Lauritano, F. M. Curá, Z. Ormianer and F. Carinci, J. Biol. Regul. Homeostatic Agents, 2016, 30, 87–97 CAS.
- S. Tunchel, A. Blay, R. Kolerman, E. Mijiritsky and J. A. Shibli, Int. J. Dent., 2016, 2016, 8590971 Search PubMed.
- U. B. Sheela, P. G. Usha, M. M. Joseph, J. S. Melo, S. T. Thankappan Nair and A. Tripathi, in 3D Printing in Medicine and Surgery, Elsevier, Amsterdam, The Netherlands, 2021, pp. 83–104 Search PubMed.
- D. Nesic, S. Durual, L. Marger, M. Mekki, I. Sailer and S. S. Scherrer, Bioprinting, 2020, 20, e00100 CrossRef.
- J. C. Ashworth, M. Mehr, P. G. Buxton, S. M. Best and R. E. Cameron, J. Mater. Sci. Mater. Med., 2018, 29, 166 CrossRef PubMed.
- K. Pluta, D. Malina and A. Sobczak-Kupiec, Tech. Trans., 2015, 1, 89–97 Search PubMed.
- L. Tavelli, M. K. McGuire, G. Zucchelli, G. Rasperini, S. E. Feinberg, H. L. Wang and W. V. Giannobile, J. Periodontol., 2020, 91, 17–25 CrossRef PubMed.
- A. Daghrery, J. A. Ferreira, J. Xu, N. Golafshan, D. Kaigler, S. B. Bhaduri, J. Malda, M. Castilho and M. C. Bottino, Bioact. Mater., 2023, 19, 268–281 CAS.
- S. Yamada, S. Shanbhag and K. Mustafa, Dent. Clin. North Am., 2022, 66, 111–130 CrossRef PubMed.
- H. Son, M. Jeon, H. J. Choi, H. S. Lee, I. H. Kim, C. M. Kang and J. S. Song, PLoS One, 2019, 14, e0221236 CrossRef CAS PubMed.
- W. Zhang, I. Ullah, L. Shi, Y. Zhang, H. Ou, J. Zhou, M. W. Ullah, X. Zhang and W. Li, Mater. Des., 2019, 180, 107946 CrossRef CAS.
- G. A. N. Atia, H. K. Shalaby, M. Zehravi, M. M. Ghobashy, H. A. N. Attia, Z. Ahmad, F. S. Khan, A. Dey, N. Mukerjee, A. Alexiou, M. H. Rahman, J. Klepacka and A. Najda, Polymers, 2022, 14, 3192 CrossRef CAS PubMed.
- E. Shoba, R. Lakra, M. S. Kiran and P. S. Korrapati, J. Mech. Behav. Biomed. Mater., 2020, 109, 103822 CrossRef CAS PubMed.
- F. Batool, D. N. Morand, L. Thomas, I. M. Bugueno, J. Aragon, S. Irusta, L. Keller, N. Benkirane-Jessel, H. Tenenbaum and O. Huck, Materials, 2018, 11, 580 CrossRef PubMed.
- N. Abedi, N. Rajabi, M. Kharaziha, F. Nejatidanesh and L. Tayebi, J. Oral Biol. Craniofacial Res., 2022, 12, 782–797 CrossRef PubMed.
- R. Deng, Y. Xie, U. Chan, T. Xu and Y. Huang, J. Dent. Res. Dent. Clin. Dent. Prospects, 2022, 16, 1–10 CrossRef PubMed.
- G. Iviglia, S. Kargozar and F. Baino, J. Funct. Biomater., 2019, 10, 3 CrossRef CAS PubMed.
- J. Luan, R. Li, W. Xu, H. Sun, Q. Li, D. Wang, S. Dong and J. Ding, Acta Pharm. Sin. B, 2023, 13, 2310–2333 CrossRef CAS PubMed.
- M. Huang, Y.-S. Huang, H. Liu, Z. Tang, Y. Chen, Z. Huang, S. Xu, J. Du and B. Jia, Biomater. Sci., 2022, 10, 6413–6446 RSC.
- S. Ye, B. Wei and L. Zeng, Gels, 2022, 8, 302 CrossRef CAS PubMed.
- C. Song, D. Huang, C. Zhao and Y. Zhao, Adv. Sci., 2022, 25, 2202829 CrossRef PubMed.
- S. Elangovan, S. Jain, P.-C. Tsai, H. C. Margolis and M. M. Amiji, J. Periodontol., 2013, 84, 117–125 CrossRef CAS PubMed.
- Y. Qi, J. Yang, Y. Chi, P. Wen, Z. Wang, S. Yu, R. Xue, J. Fan, H. Li, W. Chen, X. Wang, Y. Zhang, G. Guo and B. Han, Mol. Biomed., 2022, 3, 28 CrossRef CAS PubMed.
- Y. Wang, Z. Jia, S. Diao, X. Lin, X. Lian, L. Wang, R. Dong, D. Liu and Z. Fan, Cell Proliferation, 2016, 49, 618–627 CrossRef CAS PubMed.
- L. Zhang, Y. Wang, C. Wang, M. He, J. Wan, Y. Wei, J. Zhang, X. Yang, Y. Zhao and Y. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 3354–3362 CrossRef CAS PubMed.
- B. Horev, M. I. Klein, G. Hwang, Y. Li, D. Kim, H. Koo and D. S. W. Benoit, ACS Nano, 2015, 9, 2390–2404 CrossRef CAS PubMed.
- X. Sun, J. Sun, Y. Sun, C. Li, J. Fang, T. Zhang, Y. Wan, L. Xu, Y. Zhou, L. Wang and B. Dong, Adv. Funct. Mater., 2021, 31, 2101040 CrossRef CAS.
- J. Han, W. Jeong, M. K. Kim, S. H. Nam, E. K. Park and H. W. Kang, Polymers, 2021, 13, 1294 CrossRef CAS PubMed.
- T. Binlateh, P. Thammanichanon, P. Rittipakorn, N. Thinsathid and P. Jitprasertwong, Biomimetics, 2022, 7, 34 CrossRef CAS PubMed.
- K. Ogawa, H. Miyaji, A. Kato, Y. Kosen, T. Momose, T. Yoshida, E. Nishida, S. Miyata, S. Murakami, H. Takita, B. Fugetsu, T. Sugaya and M. Kawanami, J. Periodontal Res., 2016, 51, 758–767 CrossRef CAS PubMed.
- C. Nica, Z. Lin, A. Sculean and M. B. Asparuhova, Materials, 2020, 13, 2635 CrossRef CAS PubMed.
- H. H. Lin, P. H. G. Chao, W. C. Tai and P. C. Chang, Int. J. Mol. Sci., 2022, 22, 7725 CrossRef PubMed.
- X. Chen, S. Bai, B. Li, H. Liu, G. Wu, S. Liu and Y. Zhao, Int. J. Nanomed., 2016, 11, 4707–4718 CrossRef CAS PubMed.
- M. T. Vurat, Ş. Şeker, Ö. Lalegül-Ülker, M. Parmaksiz, A. E. Elçin and Y. M. Elçin, Genes Dis., 2022, 9, 1008–1023 CrossRef CAS PubMed.
- X. Yang, Y. Ma, X. Wang, S. Yuan, F. Huo, G. Yi, J. Zhang, B. Yang and W. Tian, Adv. Sci., 2023, 10, 2205041 CrossRef CAS PubMed.
- N. Mei, Y. Wu, B. Chen, T. Zhuang, X. Yu, B. Sui, T. Ding and X. Liu, Front. Bioeng. Biotechnol., 2022, 10, 950970 CrossRef PubMed.
- Y. Ma, Y. Ji, G. Huang, K. Ling, X. Zhang and F. Xu, Biofabrication, 2015, 7, 044105 CrossRef PubMed.
- H. N. Woo, Y. J. Cho, S. Tarafder and C. H. Lee, Bioact. Mater., 2021, 6, 3328–3342 CAS.
- S. Zang, R. Mu, F. Chen, X. Wei, L. Zhu, B. Han, H. Yu, B. Bi, B. Chen, Q. Wang and L. Jin, Mater. Sci. Eng., C, 2019, 99, 919–928 CrossRef CAS PubMed.
- L. Suo, H. Wu, P. Wang, Z. Xue, J. Gao and J. Shen, J. Biomed. Mater. Res., Part B, 2023, 111, 73–84 CrossRef CAS PubMed.
- Y. Liao, H. Li, R. Shu, H. Chen, L. Zhao, Z. Song and W. Zhou, Front. Cell. Infect. Microbiol., 2020, 10, 180 CrossRef CAS PubMed.
- A. C. Akman, R. S. Tiǧli, M. Gumusderelioglu and R. M. Nohutcu, J. Biomed. Mater. Res., Part A, 2010, 92, 953–962 CrossRef PubMed.
- L. K. Müller-Heupt, N. Wiesmann-Imilowski, S. Schröder, J. Groß, P. C. Ziskoven, P. Bani, P. W. Kämmerer, E. Schiegnitz, A. Eckelt, J. Eckelt, U. Ritz, T. Opatz, B. Al-Nawas, C. V. Synatschke and J. Deschner, Int. J. Mol. Sci., 2023, 24, 5936 CrossRef PubMed.
- F. Batool, D.-N. Morand, L. Thomas, I. Bugueno, J. Aragon, S. Irusta, L. Keller, N. Benkirane-Jessel, H. Tenenbaum and O. Huck, Materials, 2018, 11, 580 CrossRef PubMed.
- C. Peng, J. Zheng, D. Chen, X. Zhang, L. Deng, Z. Chen and L. Wu, Mol. Med. Rep., 2018, 18, 1335–1344 CAS.
- X. Xu, Y. Zhou, K. Zheng, X. Li, L. Li and Y. Xu, ACS Appl. Mater. Interfaces, 2022, 14, 46145–46160 CrossRef CAS PubMed.
- J. Liu, Q. Zou, C. Wang, M. Lin, Y. Li, R. Zhang and Y. Li, Mater. Des., 2021, 210, 110047 CrossRef CAS.
- C. Vaquette, S. P. Pilipchuk, P. M. Bartold, D. W. Hutmacher, W. V. Giannobile and S. Ivanovski, Adv. Healthcare Mater., 2018, 7, 1800457 CrossRef PubMed.
- A. Athirasala, A. Tahayeri, G. Thrivikraman, C. M. Franca, N. Monteiro, V. Tran, J. Ferracane and L. E. Bertassoni, Biofabrication, 2018, 10, 024101 CrossRef PubMed.
- M. Zussman and M. Zilberman, J. Biomater. Appl., 2022, 37, 166–179 CrossRef CAS PubMed.
- S. Zhang, Y. Jia, J. Liu, F. Feng, Z. Wei, M. Zhang and F. Xu, Regener. Biomater., 2023, 10, rbad009 CrossRef CAS PubMed.
- Y. Lu, D. Cheng, B. Niu, X. Wang, X. Wu and A. Wang, Pharmaceuticals, 2023, 16, 454 CrossRef CAS PubMed.
- Y. Ma, J. Song, H. N. S. Almassri, D. Zhang, T. Zhang, Y. Cheng and X. Wu, Drug Delivery, 2020, 27, 151–160 CrossRef CAS PubMed.
- L. Jiang, Z. Ding, S. Xia, Y. Liu, S. Lei, M. Zhong and X. Chen, J. Periodontal Res., 2020, 55, 488–495 CrossRef CAS PubMed.
- S. Sowmya, U. Mony, P. Jayachandran, S. Reshma, R. A. Kumar, H. Arzate, S. V. Nair and R. Jayakumar, Adv. Healthcare Mater., 2017, 6, 1601251 CrossRef PubMed.
- M. Lian, Y. Han, B. Sun, L. Xu, X. Wang, B. Ni, W. Jiang, Z. Qiao, K. Dai and X. Zhang, Acta Biomater., 2020, 118, 83–99 CrossRef CAS PubMed.
- Y. Wang, M. Ma, J. Wang, W. Zhang, W. Lu, Y. Gao, B. Zhang and Y. Guo, Materials, 2018, 11, 1345 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |