Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Integrated microfluidic platforms for heavy metal sensing: a comprehensive review
Sharmila Sajankila
Nadumane
a,
Rajib
Biswas
b and
Nirmal
Mazumder
 *a
*a
aDepartment of Biophysics, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka, India-576104
bApplied Optics and Photonics Laboratory, Department of Physics, Tezpur University, Tezpur, Assam, India -784028
First published on 1st April 2024
Abstract
Heavy metals are found naturally; however, anthropogenic activities such as mining, inappropriate disposal of industrial waste, and the use of pesticides and fertilizers containing heavy metals can cause their unwanted release into the environment. Conventionally, detection of heavy metals is performed using atomic absorption spectrometry, electrochemical methods and inductively coupled plasma-mass spectrometry; however, they involve expensive and sophisticated instruments and multistep sample preparation that require expertise for accurate results. In contrast, microfluidic devices involve rapid, cost-efficient, simple, and reliable approaches for in-laboratory and real-time monitoring of heavy metals. The use of inexpensive and environment friendly materials for fabrication of microfluidic devices has increased the manufacturing efficiency of the devices. Different types of techniques used in heavy metal detection include colorimetry, absorbance-based, and electrochemical detection. This review provides insight into the detection of toxic heavy metals such as mercury (Hg), cadmium (Cd), lead (Pb), and arsenic (As). Importance is given to colorimetry, optical, and electrochemical techniques applied for the detection of heavy metals using microfluidics and their modifications to improve the limit of detection (LOD).
Introduction
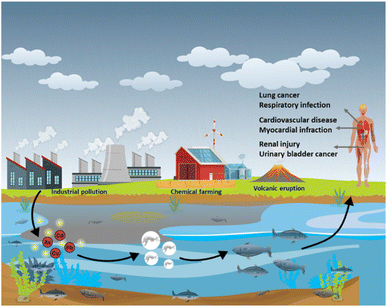
Toxicity of heavy metals such as mercury (Hg), manganese (Mn), cadmium (Cd), lead (Pb), arsenic (As) and nickel (Ni), is caused due to their accumulation inside the organs of the human body. Although heavy metals naturally occur, they are also introduced into the environment through various human activities that include disposal of unprocessed industrial waste into water sources, mining, and the use of chemical-based fertilizers in agriculture (Fig. 1).1–4 Of these heavy metals, As, Cd, Pb and Hg are recognized as the most toxic. Cd is a silvery white metal with a bluish tinge of atomic number 48 which exists as compounds with organic amines, sulphur groups, and chlorine groups. The stable isotopes of Cd are 106Cd, 108Cd, 110Cd, 111Cd, 112Cd, 113Cd, 114Cd, and 116Cd.5 Cd enters the human body through food, water, and dust or through dermal absorption and causes cancers of the lung, breast, prostate, pancreas, urinary bladder, and nasopharynx.5Pb is a bluish-grey metal that is found in the range between 10 and 30 mg kg−1 in the Earth's crust. Naturally Pb occurs as compounds such as PbS, PbSO4, PbCO3 and they exist in two ionic forms: +2 and +4. Pb gains access to the human body through the inhalation of polluted dust or through contaminated food or water.6 Even at extremely low levels, Pb can show adverse effects in the human body, such as saturnism or plumbism that mainly affect the gastrointestinal system and nervous system.7 With an atomic number of 33, it is the twentieth most abundant heavy metalloid found in the Earth's crust.8 Pb is commonly found as sulfide-bearing ores.9,10 In nature, weathering causes arsenic sulphides to convert to arsenic trioxide, which enters the arsenic cycle as dust or through dissolution in water. Excessive exposure to As affects the kidney, lungs, cardiovascular system, and respiratory system.11 Hg is a heavy metal of the d-block of the periodic table. In nature, Hg exists in an elemental, inorganic format and possesses diverse toxicity and bioavailability.12 Hg usually exists in Hg2+, Hg2++, Hg, or in the alkylated form. The intake of these mercuric forms causes Minamata disease that affects the nervous system, mainly cerebellar cortices and peripheral sensory nerves.13Table 1 lists the sources, effects, and permissible concentrations of various heavy metals. The amount of heavy metals on the surface and in groundwater has increased during the last few years; consequently, there is a need for regular water quality assessments.14 Frequently used techniques for heavy metal analysis include atomic absorption spectroscopy (AAS),15 atomic fluorescence spectroscopy,16 and inductively coupled plasma-mass spectrometry (ICP-MS).17
| Heavy metals | Sources | Permissible amount (ppb) | Effect | Reference |
|---|---|---|---|---|
| Arsenic (As) | Mining, smelting, arsenic-containing pesticides, timber preservatives, and electronics production | 10 | Keratosis, skin cancer, and internal organ cancer | 88 and 121–125 |
| Cadmium (Cd) | Electroplating, battery, petroleum products, and synthetic chemicals | 3 | Lung, breast, prostate, pancreas, urinary bladder, and nasopharynx cancers | 126 and 127 |
| Mercury (Hg) | Iron and steel industry and chloro-alkali industry | 6 | Itai-itai disease, renal injury, cardiovascular disease, and myocardial infarction | 86 and 128–132 |
| Lead (Pb) | Mining, smelting, waste incineration, coal burning, leaded gasoline, dust, batteries vent, and lead paint | 10 | Saturnism or plumbism, mainly affecting the gastrointestinal and nervous systems and severe damage to the brain and kidneys | 133–136 |
| Chromium (Cr) | Metallurgy, electroplating, production of paints and pigments, tanning, and wood preservation | 50 | Lower IQ, hearing loss, anemia, kidney failure, blindness, hallucination, cardiovascular disorder, impair development, allergic contact dermatitis, cardiovascular disorder, hepatotoxicity, and respiratory infections | 137 and 138 |
| Nickel (Ni) | Ni alloy industry, pigment manufacturing industries, and tannery industry | 70 | Allergic contact dermatitis, cardiovascular disorder, hepatotoxicity, and respiratory infection | 139–143 |
| Cobalt (Co) | Coloring agent for glass, pottery, and jewelry | 50 | Cardiomyopathy, occupational asthma, allergic alveolitis, and occupational contact dermatitis | 144–146 |
High versatility toward simultaneous metal detection, sensitivity, specificity, accurate detection, and detection limits in the femtomolar range are a few advantages of these methods18 (Table 2). However, they show certain drawbacks, such as requiring expensive and sophisticated instruments with the need for multistep sample preparation that requires expertise for accurate results.19,20 As a result, the need for quick, economically beneficial, simple, and reliable approaches for in-laboratory and real-time monitoring of heavy metals has increased, prompting the advancement of sensors.6,10,21 Lately, sensing of heavy metal ions using microfluidics has grabbed the attention of the global community. Researchers are now resorting to this fast-sensing scheme where minimal logistics can be deployed with enhanced accuracy. Accordingly, several articles on heavy metal detection using microfluidic-based devices have been published.22–24 However, available literature caters to different adaptations of microfluidics to achieve sensing. There is a need for a comprehensive review of all these adaptations where readers can grasp the basics as well as an understanding of the potential of this growing branch of sensing. With this aim, the current review is an attempt to provide an overview of microfluidics in heavy metal detection. It will highlight the integration of techniques such as colorimetry, absorbance, fluorescence and electrochemical detection with microfluidics for detecting heavy metals. Accordingly, the following sections summarize the different routes adopted for microfluidic-based heavy metal sensing along with potentialities.
| Heavy metals | Technique | Limit of detection | Reference |
|---|---|---|---|
| Mercury (Hg) | Atomic absorption spectroscopy (AAS) | 0.0155 μg L−1 | 15 |
| Atomic fluorescence spectrometry (AFS) | Hg2+ – 0.007 μg L−1 | 16 | |
| CH3Hg+ – 0.018 μg L−1 | |||
| Graphite Furnace Atomic Absorption Spectrometry (GF-AAS) | Hg – 0.017 μg L−1 | 147 | |
| SWASV | 0.1 μg L−1 | 148 | |
| ICP-MS | 0.09 ng g−1 | 17 | |
| Lead (Pb) | GF-AAS | Pb – 0.009 μg L−1 | 147 |
| ICP-MS | 0.031 ng L−1 | 149 | |
| Potentiometry | 31 μg L−1 | 150 | |
| Amperometry | 2 ppb | 151 | |
| High resolution continuum source graphite furnace atomic absorption spectrometry (HR-CS-GFF-AAS) | 200 ng L−1 | 152 | |
| Arsenic (As) | ICP-MS | As(III) – 0.008 μg kg−1 | 28 and 153 |
| As(V) – 0.013 μg kg−1 | |||
| Cyclic voltammetry | 4.64 μM | 154 | |
| Differential pulse anodic stripping voltammetry (DPASV) | 11.39 pM | 155 | |
| Cadmium (Cd) | HR-CS-GFF-AAS | 100 ng L−1 | 152 |
| SWASV | 0.062 ppb | 156 | |
| AFS | 0.05 μg L−1 | 157 | |
| ICP-MS | 0.008 μg L−1 | 158 | |
| Nickel (Ni) | ICP-MS | 1.2 pg mL−1 | 159 |
| AAS | 0.305 μg L−1 | 160 | |
| GF-AAS | |||
| Chromium (Cr) | Flame atomic absorption spectrometry (FAAS) | 0.21 μg L−1 | 161 |
| Ion chromatography-inductively coupled plasma-mass spectrometry (IC-ICP-MS) | Cr(III) – 0.09 μg L−1 | 162 | |
| Cr(VI) – 0.03 μg L−1 | |||
| Amperometry | 0.0016 μM | 163 |
Microfluidic devices for heavy metal detection
Microfluidic devices involve the precise handling of samples in micro- or nano liters.25 Microfluidic devices are integrated with microchannels, micropumps, and microchips that manipulate the properties of the liquid. Microfluidic systems exhibit important fluid properties, such as laminar airflow, which introduces micromachining and microoperation that cannot be incorporated into conventional techniques.26 The experimental procedures used in the research laboratory, such as preparation of the sample, chemical reaction, separation, and detection, can be replicated in the microscale devices using a microfluidic chip, therefore referred to as a lab on a chip.10 Compared to conventional techniques, microfluidics provides faster reaction time, minimum waste generation, and reduced sample and reagent consumption. Microfluidic devices have found applications in various research fields, including chemistry,27 microelectronics,10 material biology,28 biomedical engineering,29 and fluid dynamics.30Microfluidic devices can be fabricated using different types of materials such as glass,31 silicon,32 polydimethylsiloxane (PDMS), thermoplastics,33 paper,34etc. Silicon was the first material used in the fabrication of microfluidic channels.35 The surface of silicon is made up of silanol groups (–Si–OH–) which can be easily modified. Its semiconducting properties, chemical resistance and flexibility made silicon one of the most widely used materials in fabrication.36 Silicon glass was the most widely used material in microfluidic devices due to its properties such as high transparency, low fluorescence background,37 and high resistance to temperature (>500–1500 °C).38 However, fabrication of glass and silicon based microfluidic devices requires a cleanroom facility which makes fabrication expensive. It also requires the use of hazardous chemicals such as HF. Consequently, this has limited the use of glass and silicon.39,40 PDMS is the most common type of elastomer used in the fabrication of microfluidics because of its high elasticity, cost-effectiveness and simple fabrication steps.41 PDMS enables fabrication of multilayered microchannels by stacking multiple layers.42 However, PDMS shows certain drawbacks such as incompatibility towards certain organic solvents and adsorption of biomolecules due to its hydrophobic properties.43,44 Thermoplastics show better solvent compatibility compared to PDMS. They include polystyrene (PS), polyethylene terephthalate (PET), polyvinyl chloride (PVC) and polymethyl methacrylate (PMMA).45 However, the major drawback of thermoplastics is their inability to adhere to other surfaces.46 Paper is one of the cheapest, portable, nature friendly, highly porous cellulose based materials, and is widely used as a microfluidic material.47 The capillary movement of a liquid along a paper-based microfluidic device liquid simplifies the fabrication of microchannels.48 The major limitation of paper is its reusability, as it can be used only once. The selection of material for fabrication depends upon the type of samples used, nature of chemical reagents and application. Microfluidic devices exhibit some unique features compared to macroscale devices, such as a high surface-to-volume ratio and laminar flow; hence, the selection of suitable materials for the fabrication of microfluidic devices is crucial. Various types of sensors, such as electrochemical, optical, hybrid, and biosensors, are integrated into microfluidic devices to detect heavy metals.
Optical detection
The detecting components react with the analyte resulting in optical variations, which is identified by optical detection. This method is a basic, cost-effective technique that uses electromagnetic radiation with wavelengths ranging from 200 nm to 1 mm. The electromagnetic domain is further divided into the UV, visible, and near-infrared regions.49 The widely used optical detection methods include colorimetry, absorbance-based detection and fluorescence detection. Heavy metals will interact with chemical reagents that exhibit optical properties, including nanoparticles, fluorescent proteins, synthetic dyes, and quantum dots. The optical signal will undergo changes due to the interaction between these chemical reagents and varying concentrations of heavy metals, enabling the quantification of each heavy metal through recorded measurements.50 Metallic nanoparticles such as AuNPs and AgNPs exhibit a phenomenon known as SPR, which is used for heavy metal detection. SPR is widely used due to its specific features, such as sensitivity toward the detection of analytes under very dilute conditions, high selectivity, and label-free sensing.51,52 SPR is a quantum optical-electrical occurrence that occurs during the interaction of light and metal surfaces. This technique involves generation of plasmonic waves between the metal layer and the dielectric medium.22 It employs singular and p-polarized light to generate surface plasmons. As the momentum of the SPR wave matches that of the incoming light, the intensity of the reflected light starts to diminish because of the resonance. The angle at which the intensity diminishes is called the resonance angle. The resonance angle is determined by the refractive index of the metal surface.52 The intensity of the reflected light starts to diminish due to resonance photons from incident light at a specific angle of incidence called the resonance angle, exciting the electrons on the metal surface layer, which upon excitation propagate in a direction parallel to the metal surface.23Colorimetry detection
Heavy metal detection utilizing microfluidics and colorimetry is a semiquantitative process. In colorimetric detection, the chemical reaction between the solution or the substrate containing the heavy metal and the chemical reagent employed for its detection results in a colour change which can be observed for confirming the presence of the heavy metal, and the concentration can be determined using an optical system. Specific dyes or chemical reagents for the metal are employed when detecting various heavy metals in water.Development of a paper-based microfluidic device for detecting Pb2+ using the colorimetric method was reported by Wisang et al. The device consisted of two zones: a sample zone and a detection zone. To indicate the presence of Pb2+, sodium rhodizonate was employed as the indicator. When Pb2+ was introduced to sodium rhodizonate, the colour changed from yellow to pink because of the formation of a Pb–rhodizonate complex. The limit of detection (LOD) was found to be 0.756 mg L−1 (756 ppb) (Fig. 2).22
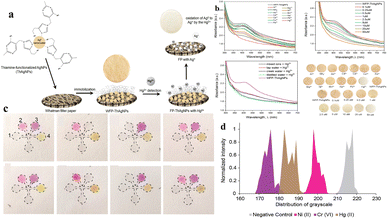 | ||
| Fig. 2 (a) A schematic representation of the possible mechanism involved in the detection of Hg2+ through a color change using ThAgNPs anchored on filter paper. (b) UV-vis spectra of WFP-ThAgNPs exposed to different ions and various concentrations of Hg2+ (reproduced from Budlyan et al., 2022, Environmental Nanotechnology, Monitoring and Management).57 (c) μPAD showing the color development after the application of DI water, 5 ppm of Ni2+, Cr6+, or Hg2+, and heavy metal ion spiked lake-water samples. (d) Graphical representation of the distribution of grayscale vs. normalized intensity (reproduced from Devadhasan and Kim, 2018, with permission from Elsevier).54 | ||
Substituting the need for individual devices that are specific to different heavy metals with a single device capable of simultaneously detecting multiple heavy metals is both cost-effective and time saving. A paper-based microfluidic device for simultaneous detection of Ni2+, Cu2+ and Fe2+ was developed by Aryal et al. for the substrate Whatman grade 1 was used with five folds. The top three layers of the fold were used as detection pads, and the bottom two layers were used as waste pads. The detection of Ni2+ was achieved using Chugaev's method; briefly, it involved the reaction of Ni2+ ions with dimethylglyoxime (DMG), a bidentate organic ligand, in the presence of alkaline ammonium solution. A pink-colored complex known as nickel dimethylglyoxime (NiDMG2) was formed as a result of the reaction. Cu2+ was identified using a precise and highly responsive Cu–bathocuproine test. An orange-colored compound was formed when Cu2+ interacted with bathocuproine in an acidic environment (pH 4.5). Fe2+ was identified utilizing bathophenanthroline. Under acidic conditions (pH 4.5), bathophenanthroline reacts with Fe2+ to produce a complex with a red color. The LODs of Ni2+, Cu2+ and Fe2+ were found to be 2 ppm (2000 ppb), 6.67 ppm (6670 ppb), and 1 ppm (1000 ppb), respectively.53 Multiple heavy metals, such as Ni2+, Cr3+, and Hg2+ were detected by the paper-based microfluidic device. The substrate was a chromatographic paper with detection zones for three different heavy metals. The surface of each detection zone was functionalized with three different functional groups: amine (–NH2), carboxyl (–COOH), and thiol (–SH). The substrate was subjected to chemical reactions for the immobilization of these functional groups on its surface. The immobilization of the functionalized groups was followed by the addition of heavy-metal-specific colorimetric reagents such as dimethylglyoxime, 1,5-diphenylcarbazide, and Michler's thioketone to this detection zone for the detection of Ni2+, Cr3+ and Hg2+ respectively. The interaction of the chemical reagent with the heavy metals resulted in the development of bluish-purple, orange, and yellow colours, which were analyzed using the colours developed by standard solutions; analysis of the images was performed to obtain the grayscale for quantification. The LODs of Ni2+, Cr3+, and Hg2+ were found to be 0.24 ppm (240 ppb), 0.18 ppm (180 ppb), and 0.19 ppm (190 ppb), respectively.54 Even though the use of colorimetric assays or dyes in optical detection of heavy metals is easy, economically effective, and rapid, these assays tend to lack sensitivity due to inherent limitations in the low extinction coefficients of the dyes.55,56 The sensitivity of detection can be improved by using nanosized particles of gold (AuNPs) and silver (AgNPs). The aggregation of the nanoparticles by heavy metals can be associated with their optical and surface plasmon resonance (SPR) characteristics. The change of colour in the solution due to the aggregation of nanoparticles enhances the sensitivity of this method of detection. Budlayan et al. used thiamine functionalized AgNPs in the detection of Hg2+. Thiamine-functionalized AuNPs were immobilized on filter paper. Thiamine shows selective coordination with Hg2+, and the AgNPs demonstrate highly adjustable optical absorption and reactivity. When the Hg containing water sample is poured on the Whatman filter paper with thiamine functionalized AuNPs, a colour change from yellow to faint yellow to white is observed, and the changed intensity corresponds to the quantity of tested Hg2+. The change in the colour of the filter paper was a result of a reduction in the absorbance peak at approximately 425 nm in the UV-vis spectrum. ImageJ software was utilized to assess the red, green, and blue (RGB) colour intensity profiles of the obtained digital images. This characterization method offers a semiquantitative assessment of the colorimetric response. High intensities were recorded from red and green colors, resulting in the yellow color of the filter paper. The LOD of this sensor was found to be 0.5 μM (0.5 ppb).57 In their study, Sahu et al. employed glucose-functionalized AuNPs to detect As3+ and Pb2+. The presence of As3+ and Pb2+ was detected by glucose-functionalized AuNPs with a change in color from pink to purple and bluish gray, respectively. The change in colour due to the interaction of glucose-functionalized AuNPs with the heavy metals can be visualized through the naked eye. To measure the intensity of this change in colour, a UV-vis spectrometer was used. The limit of detection of As3+ and Pb2+ was found to be 5.6 μg L−1 and 7.7 μg L−1, respectively.58 Despite its simplicity, rapidity, and portability as a method for detecting heavy metals, colorimetry is considered a basic detection approach due to its semiquantitative nature and limitations in terms of the LOD. Table 3 provides a summary of the various microfluidic-based devices used for heavy metal detection using colorimetry.
| Heavy metals | Microfluidic device | Indicator | LOD | Reference |
|---|---|---|---|---|
| Pb2+ | Paper-based | Sodium rhodizonate | 756 ppb | 22 |
| Ni2+, Cu2+ and Fe2+ | Paper-based | Dimethylglyoxime (DMG) | Ni2+ – 2000 ppb | 53 |
| Bathocuproine | Cu2+ – 6670 ppb | |||
| Bathophenanthroline | Fe2+ – 1000 ppb | |||
| Ni2+, Cr3+, and Hg2+ | Paper-based | Dimethylglyoxime, 1,5-diphenylcarbazide, and Michler's thioketone | Ni2+ – 240 ppb | 54 |
| Cr3+ – 180 ppb | ||||
| Hg2+ – 190 ppb | ||||
| Hg2+ | Paper-based | Thiamine functionalized AgNPs | 0.5 ppb | 57 |
| As3+ and Pb2+ | Paper-based | Glucose-functionalized AuNPs | As3+ – 5.6 μg L−1 | 58 |
| Pb2+ – 7.7 μg L−1 | ||||
| Cu2+ | Paper-based | Chrome azurol S and pyrocatechol violet | Chrome azurol S – 1700 ppb | 164 |
| Pyrocatechol violet – 1900 ppb | ||||
| Cu2+, Co2+, Ni2+, Hg2+, Mn2+ | Paper-based silver nanoparticles were modified with pyrrolidine-1-dithiocarboxylic acid ammonium salt | Bathocuproine (BC) | Cu2+ – 320 ppb | 165 |
| Dimethylglyoxime (DMG) | Co2+ – 590 ppb | |||
| Dithizone (DTZ) | Ni2+ – 5870 ppb | |||
| 4-(2-Pyridylazo)resorcinol (PAR) | Hg2+ – 200 ppb | |||
| Mn2+ – 110 ppb | ||||
| Cr3+ and Al3+ | PMMA | AgNPs modified with pyrrolidine-1-dithiocarboxylic acid ammonium salt | Cr3+ – 0.010 ppb | 166 |
| Al3+ – 0.003 ppb | ||||
| Ni2+, Cu2+, Cr6+ | Paper-based | Ni2+ – dimethylglyoxime | Ni2+ – 4.8 mg L−1 | 167 |
| Cu2+ – bathocuproine | Cu2+ – 1.6 mg L−1 | |||
| Cr6+ – 1,5-diphenylcarbazide | Cr6+ – 0.18 mg L−1 |
Absorbance-based detection
In absorbance-based detection, light-emitting diodes (LEDs) and LASER (Light Amplification by Stimulated Emission of Radiation) are used as a light source. LEDs have high durability, low cost, low power consumption, high energy conversion efficiency, small size, and broad spectral band from UV to NIR, which makes them some of the most frequently used light sources in optofluidic detection of heavy metals.59 LED-based sensors eliminate the need for optical couplers or monochromators because LEDs emit a relatively narrow range of wavelengths. Additionally, LEDs can be easily electronically modulated for intensity, eliminating the necessity for a separate mechanical chopper. Light from the light source enters the device's microchannel, where the light and the heavy metals in water interact. The light will be absorbed by the metal ions, which then emit at different intensities recorded by the detectors.60Lace et al. used a green dye, leucomalachite, for the detection of As3+ using optical detection where the LED was used as a light source with a photodiode detector (Fig. 3). The microfluidic device was made up of poly(methyl methacrylate) (PMMA). The chip was made up of serpentine channels used for the mixing of the reagents. The reaction of dye with As3+ produced a green-colored complex, giving an absorption peak at 617 nm. The LOD was found to be 0.19 mg L−1. A method was developed to detect As3+ using iron oxide nanoparticles by Chauhan et al., where the iron oxide surface was modified with cysteine. A filter paper with a hydrophobic zone was used as the substrate where the cysteine-modified iron oxide nanoparticles reacted with As3+. The LOD was found to be 10 ppb.61 A polydimethylsiloxane (PDMS)-based microfluidic device for the detection of Hg2+ was developed by Li and Lin. In this device, an LED served as the light source with a wavelength of 525 nm, while a photomultiplier functioned as the detector. For precise detection of Hg2+, the AuNPs were modified with 3-mercaptopropionic acid (3-MPA), leading to aggregation of AuNPs. A UV-vis spectrophotometer was used to quantify Hg2+, revealing an LOD of 200 ppb.27 To identify a specific heavy metal from a mixture of heavy metals in water, metal-specific compounds or reagents can be used.27,61 A microfluidic device where the microchannels were modified with –SH groups was developed by Karakuzu et al. The As from the water source adhered to these –SH groups, with unaltered AuNPs serving as markers that bind to free –SH groups. The intensity of absorbance recorded at 530 nm, for the AuNPs was inversely proportional to the As concentration. The LOD was determined to be 2.2 μg L−1 (2.2 ppb).62Table 4 provides an overview of optical-based microfluidic devices for detecting heavy metals.
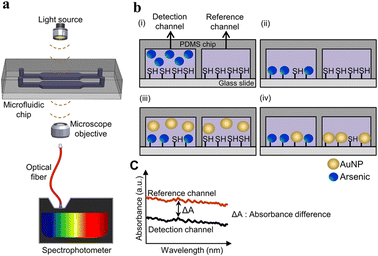 | ||
| Fig. 3 Detection of As(III) in the microfluidic chip. (a) Representation of a microfluidic device in As(III) detection; (b) illustration of the As(III) detection procedure: (i) injection of As into the detection channel and injection of reference solutions into reference channels, (ii) washing of the channel with distilled water, (iii) introduction of AuNPs in the microchannels and (iv) washing of the microchannels; (c) measurement of absorbance from the reference channel and detection channel containing As and reference solutions, respectively. The difference in absorbance in the channel was used to calculate the concentration of As (reproduced from Karakuzu et al., 2021, with permission from Elsevier).62 | ||
| Heavy metal | Type of detection | Device | LOD | Reference |
|---|---|---|---|---|
| As3+ | Absorbance based detection | PMMA | 190 ppb | 61 |
| As3+ | Absorbance based detection | PDMS | 10 ppb | 99 |
| Hg2+ | Absorbance based detection | PDMS | 200 ppb | 27 |
| As3+ | Absorbance-based | PDMS | 2.2 ppb | 62 |
| Hg2+ | Fluorescence | PDMS | 2 nM | 66 |
| Cr 3+ | Fluorescence | PDMS | 0.094 nM | 67 |
| Hg2+ and Pb2+ | Fluorescence | PDMS | Hg2+ – 0.70 ppb | 69 |
| Pb2+ – 0.53 ppb | ||||
| Cu2+, Mn2+, and Hg2+ | Fluorescence | Paper-based | Hg2+ – 5.4 nM | 72 |
| Mn2+ – 8.1 nM | ||||
| Cu2+ – 7.3 nM | ||||
| Cd2+ | Fluorescence | PDMS | 0.26 μg L−1 | 77 |
| Hg2+ and Pb2+ | Fluorescence | Cloth-based | Hg2+ – 0.18 ppb | 73 |
| Pb2+ – 0.07 ppb | ||||
| Cd2+ and Pb2+ | Fluorescence | Paper-based | Cd2+ – 0.245 ppb | 76 |
| Pb2+ – 0.335 ppb | ||||
| Hg2+ | LSPR | PDMS | 2.7 pM | 87 |
Fluorescence-based detection
Fluorescence-based detection of heavy metals is one of the simplest methods with high sensitivity and fast response time. Fluorescence detection involves the emission of higher wavelength light from the sample surface after it has been exposed to low wavelength light. As the concentration of the analyte increases, the intensity of light decreases. Typically, in this approach, fluorescent probes are combined with the analyte of interest. Various probes are employed for fluorescence detection. The analyte can be detected as the fluorescence signal alters upon the attachment of the probe to the specific analyte. The fluorescent probes can be further classified based on their optical performance into “off–on” probes and “on–off” probes. The binding of fluorescent probes to analytes may enhance the fluorescence (off–on) or quench the fluorescence (on–off). Rhodamine dye and its derivatives exhibit longer excitation wavelengths, high fluorescence quantum yield, and high photostability, rendering them the prevailing choice for fluorescent probes. Rhodamine and its derivatives are being used in detecting heavy metals like Cd2+ and Pb2+, Cd2+ and Hg2+.63–65A PDMS-based microfluidic device was fabricated by Karthikeyan and Sujatha for the detection of Hg2+ where the fluorescent sensing probe was a gold nanofluid surface functionalized with rhodamine 6G and L-arginine amino acid. The device was incorporated with two inlets and an outlet for fluid entry and exit, respectively. The device was also incorporated with a herringbone type of micromixer that enabled the mixing of the fluids. The analysis of Hg2+ with a concentration of 0–16 nM showed an increase in fluorescence intensity as the concentration of Hg2+ increased. The maximum detectable concentration of Hg2+ was found to be 16 nM, beyond which the intensity decreased. The LOD was determined to be 2 nM.66 Peng et al. used rhodamine B derivatives for the detection of Hg2+. For simultaneous analysis of the fluorescence intensity, a portable fibre-optic spectrophotometer was coupled with a fabricated microchip. 0.094 nM was the LOD found.67 The accuracy and sensitivity of heavy metal detection can be improved using fluorescent aptamers. Aptamers are target-specific DNAs or RNAs that show excellent stability compared to antibodies; therefore, they can be utilized in heavy metal detection. For example, thymine (T) nucleotides show greater specificity toward Hg2+ than other heavy metals.68 Similarly, Pb can be detected using quadruplexes. Fluorescent sensors coupled with aptamers were used for sensitive detection of Hg2+ and Pb2+. Fluorescent dyes such as FAM and HEX were used in the labelling of the aptamer sequences and mixed with GO solution and 500 ppm of heavy metals. The fluorescence produced by FAM and HEX was quenched by GO. The interaction of Hg2+ and Pb2+ with the aptamer led to the restoration of fluorescence. An increase in fluorescence was observed with an increase in heavy metal concentration with a LOD of 0.70 ppb and 0.53 ppb for Hg2+ and Pb2+, respectively.69
Zero-dimensional particles with dimensions of 2–100 nm, called quantum dots (QDs) can also be used as nanosensors. QDs show unique properties, such as wide absorption ranges, precise and adjustable emission ranges, extended fluorescence duration, exceptional resilience to light-induced decay, and resistance to photodegradation, making them an alternative for organic and protein fluorescent dyes.70 A paper-based device (PAD) by Yue et al. uses QDs for detection of heavy metals such as Cu2+, Mn2+, and Hg2+ by the colorimetric technique of detection. The PAD consists of three different layers, a sample area, three channels, and a testing area. The different testing areas were surface-modified with O-phenylenediamine (OPD), sodium 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonate (TOOS), 4-aminoantipyrine (4-AAP) mixture, and 3,3′,5,5′-tetramethylbenzidine (TMB) followed by the addition of C-NH2QDs, C-COOH QDs and CdSe QDs. The concentrations of Hg2+, Mn2+, and Cu2+ were determined by photocatalytic oxidation of TMB, the TOOS-4-AAP mixture, and OPD using C-COOH QDs, CdSe QDs, and NH2QDs, respectively. After the reaction, using the photo of the PAD, the testing areas were analysed. The LODs of Hg2+, Mn2+, and Cu2+ were found to be 5.4 nM, 8.1 nM, and 7.3 nM, respectively. CdTe/CdS QDs have applicability in the detection of Cd2+.71 A three-dimensional origami ion imprinted polymer paper-based microfluidic device was developed for the detection of Cu2+ and Hg2+. The fluorescence quenching mechanism was employed through formation of Cu2+ or the Hg2+ IIP and CdTe QD complex, where there was a transfer of photo luminescence energy of QDs to the complex. The platform enabled simultaneous detection of Cu2+ and Hg2+ with a LOD of 0.11 μg L−1 to 58.0 μg L−1.72 Similarly, a cloth/paper hybrid was used as a substrate for detection of Hg2+, Pb2+ and Cr6+ using QDs. The device was integrated with a fluorescent sensing cloth-based component and paper-based μPAD. The sensing component of the device was prepared by grafting the QDs on the surface of the cloth followed by the modification with ion imprinted polymers (IIPs). The detection of Hg2+ and Pb2+ was carried out using fluorescence quenching. The LOD achieved was found to be 0.18 μg L−1 for Pb2+ and 0.07 μg L−1 for Hg2.73 Wang et al. used tetrasodium iminodisuccinate (IDS) in the etching of CdTe/CdS QDs. Chemical etching caused fluorescence quenching of the CdTe/CdS QDs, enabling sensing of Cd2+ ions and consequent alterations in fluorescence emission. These changes were captured by a fluorescent E-eye comprising an excitation source, an optical lens, and a smartphone. The LOD achieved in this study was determined to be 0.26 μg L−1.74 The uniform distribution of QDs on the substrate can be achieved by grafting onto the surface of the nanoparticles. In the study by Han et al. the QDs were grafted onto the surface of the silica nanoparticles and the uniform distribution was achieved. The grafted QDs were used in the detection of Hg2+ in water. The Hg2+ was detected using fluorescence quenching of QDs. The fluorescence signals were captured using a smartphone and grayscale data were obtained. The LOD was found to be 2.83 μg L−1.75 Although CdTE/CdS QDs are sensitive in fluorescence detection, they are highly toxic to the environment. ZeSe QDs show less toxicity than CdTe QDs. Hence, Zhou et al. used ZnSe QDs for the detection of Cd2+ and Pb2+ ions. The microfluidic device used was known as 3D rotary μPADs modified with ZnSe QD-wrapped ion-imprinted polymers. The LODs of Cd2+ and Pb2+ were found to be 0.245 μg L−1 and 0.335 μg L−1, respectively.76 A rhodamine B-graphed paper-based microfluidic device was fabricated by Liu et al., 2022 to detect Fe3+. The addition of Fe3+ to the device changed the colour from colourless to pink, whereas the addition of other heavy metals, such as Pb2+, Cu2+, Ni2+, and Hg2+, did not result in any significant colour change (Fig. 4).83
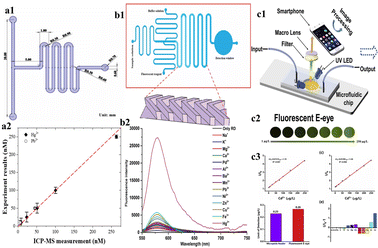 | ||
| Fig. 4 (a1) Schematic representation of the microchannel of the microfluidic device; (a2) Graphical representation of a comparison of results obtained by ICP-MS and the microfluidic device (reproduced with permission from Huang et al., 2021);69 (b1) schematic representation of a microchannel; (b2) graphical representation of fluorescence intensity of various heavy metals (reproduced with permission from Peng et al., 2018);67 (c1) schematic representation of a procedure for Cd2+ detection using a fluorescent E-eye with QDs; (c2) FL images of different concentrations of Cd2+ by using a fluorescent E-eye; (c3) the calibration curve for different concentrations of Cd2+ using a microplate reader and fluorescent E-eye (reproduced from Wang et al., 2020, with permission from Elsevier).77 | ||
Electrochemical detection
The electrochemical method of heavy metal detection is a strong alternative due to its various advantages, such as selectivity toward metals, broader linear dynamic range, high sensitivity78 portability, and easy sample preparation steps.79 In electrochemical detection, the analytes interact with the electrode or probe, producing electrical signals.80 There are three kinds of electrodes in the electrochemical technique, the working electrode (WE), counter electrode (CE), and reference electrode (RE). The WE generate measurable current, potential, charge, or frequency differences as a result of the electrochemical reaction.10 The fabrication of the electrochemical electrode is important. Frequently, electrode fabrication involves the hybridization of two or more materials. These include materials with improved conductivity to facilitate electron transmission, materials with high porosity that provide a larger surface area containing a large number of active sites for the binding of active components or heavy metals and materials containing the functional group on the surface area that enhance metal attachment and selectivity for a particular target metal ion. The most common materials used include carbon-based materials, bismuth-based materials, and polymer-based materials.81,82 Carbon nanotubes (CNTs), graphene, and fullerenes are some of the carbon-based materials.25 Carbon nanomaterials are considered the most versatile materials that are environment friendly. They possess specific properties like high electrical conductivity and high stability, and the surface of carbon can be easily modified.82 A low-cost disposable graphene-based sensor for the detection of heavy metals such as Cd2+, Cu2+ and Pb2+ was developed by Yue et al. The WE, RE, and CE were graphene-ferrocene-doped graphene and Chit-Fc, respectively.71Bismuth shows minimal toxicity and is hence regarded as one of the best choices for a heavy metal sensor. It is partially insensitive to dissolved oxygen (DO). Hwang et al. developed a modified nanoporous bismuth electrode (modified-NPBiE) for the detection of heavy metals such as Cd2+ and Pb2+.84 Printed electrochemical sensors are considered economical analytical detection methods for single-use and disposable sensors.85 The advancement of microelectronics resulted in the easy accession of electrochemical sensors. Inkjet printing, 3D printing, and screen printing are the most common methods for printing electrodes and are widely employed to create planar electrodes for electrochemical sensing.86,87 Screen-printed electrodes (SPEs) are single-use electrodes that are cost-effective and are fabricated in large quantities. These electrodes are user-friendly and do not require any preprocessing or specialized personnel.88 The steps involved in the fabrication are as follows: transfer of electrode design onto the substrate, mask creation by eliminating the undesired sections of the mask, application of the electrode ink onto the mask followed by drying, and finally, removal of the mask to acquire electrodes of its shape.89 The fundamental constituents of conductive screen-printing ink include solvents, conductive nanoparticles, organic binders, and conductive agents.90 Electrode sensitivity can be improved by integrating modified substances into the printing ink. These include inorganic materials such as gold, silver organic materials such as chitosan, carbon-based materials such as graphene, and carbon nanotubes.91 Inkjet printing is one of the most common techniques in microstructure fabrication and involves a dispensing unit for the deposition of liquid material on the surface of the substrate. The major advantages of inkjet printing include uniform deposition of the material on the substrate, maskless fabrication of electrodes, minimum sample consumption, and cost-effectiveness.92,93 Ink-jet printing is an automated process that digitally manages the deposition of ink on predetermined spots on a substrate. This process enables exceptional accuracy, ensuring consistent reproducibility of the printed electrodes.94 The distinct designs of the electrodes were created using graphic design software and are printed. The sensitivity of detection can be improved by mixing the biomolecules or nanomaterials with the printing ink.85 The limitation of the fabrication of a single layer of the electrode by inkjet printing was resolved by adopting 3D inkjet printing, enabling the production of multiple layers of electrodes. The advantages of 3D printing include large-scale manufacturing capabilities, personalized electrode design, and streaming of the fabrication process into a single step.95,96 The classification of different electrochemical techniques involved in heavy metal detection is shown in Fig. 5.
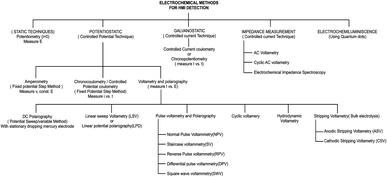 | ||
| Fig. 5 List of different electrochemical-based techniques (reproduced from Bansod et al., 2017, with permission from Elsevier).97 | ||
Among these techniques, the most widely used electrochemical techniques for heavy metal analysis are potentiometry, amperometry, and voltammetry techniques. The potentiometric method is based on the continuous measurement of the potential difference between the electrodes, which, in turn, facilitates the quantification of analyte concentrations.98 Potentiometry-based detection is a simple technique that is widely used because of its cost-effectiveness and high selectivity. Voltammetry techniques are widely adopted in microfluidic-based heavy metal detection due to their sensitivity, simplicity, and ability to provide real-time electrochemical information about analytes.99 These techniques involve applying a potential across the electrodes and measuring the resulting current, allowing for the characterization and quantification of various electroactive species, including heavy metal ions.97 In microfluidics, these techniques are particularly advantageous as they can be integrated with miniaturized devices, enhancing sensitivity and reducing sample and reagent consumption. Here is a brief overview of some common techniques used in microfluidic-based heavy metal detection:
Microfluidics allows for precise control of fluid flow, which is crucial for electrochemical measurements. Microfluidic channels can be designed for enhancing the mass transport to the electrode surface, improving the sensitivity and response time of voltammetry techniques.110 Additionally, microfabrication techniques can be employed to create miniaturized electrodes and electrode arrays, enhancing the analytical capabilities of these techniques.111 Microfluidic-based voltammetry offers several advantages that include minimum usage of the sample and reagent, rapid analysis, and the potential for automation and integration with other analytical techniques.112 It has been successfully applied to heavy metal detection in various environmental samples, including water, soil, and biological fluids. However, challenges such as electrode fouling and interference from complex matrices still need to be addressed for accurate and reliable heavy metal quantification in real-world samples. Certainly, there are a few scientific studies that demonstrate how voltammetry techniques are employed in microfluidic-based heavy metal detection. Kokkinos et al. developed a paper-based electrochemical device with tin (Sn) as the WE, platinum (Pt) as the CE, and silver (Ag) as the RE. The device was integrated with a microfluidic channel, and the three electrodes were deposited onto the paper substrate using a sputtering process. The developed device was used in the voltammetric detection of Cd2+ and Zn2+ using ASV. The LOD was found to be 0.9 ppb and 1.1 ppb for Cd2+ and Zn2+ respectively (Fig. 6).113
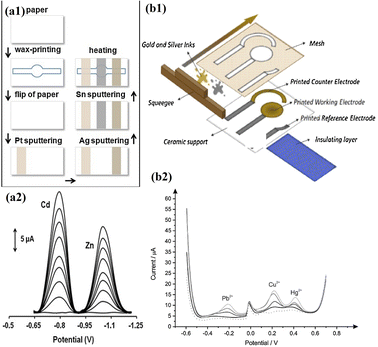 | ||
| Fig. 6 (a1) Schematic representation of steps involved in fabrication of an ePAD; (a2) SWASV voltammogram of Zn2+ and Cd2+ (0–40 μg L−1) (reproduced with permission from Kokkinos et al., 2018);113 (b1) schematic representation of the fabrication of a screen-printed electrode. Schematic representation of steps involved in the fabrication of ePADs; (b2) SWASV voltammograms and calibration plots of 0–40 μg L−1 Zn2+ and Cd2+ (reproduced from Bernalte et al., 2020, with permission from Elsevier).114 | ||
Mohan et al. fabricated a microfluidic electroanalytical device for simultaneous detection of Cu and Hg. The device was fabricated using maskless lithography where PDMS was used as the substrate. ITO was used as the WE and CE, and Ag/AgCl deposited on the tip of the third ITO electrode was used as the RE. The device was connected to a portable potentiostat and smartphone. Hg and Cu underwent oxidation at −0.4 V and 0.1 V, respectively. The device was tested with samples of tap water, lake water, and blood. The LODs of Cu and Hg were 0.4 μM and 3.19 μM, respectively.115 Santangelo et al. introduced an innovative technique for the electrochemical analysis of heavy metals, which involved a graphene-based sensor integrated with a 3D-printed microfluidic chip. EG/SiC, Ag/AgCl, and Pt were used as the WE, CE, and RE, respectively. A portable potentiostat was used for the electrochemical analysis. The developed device was used for the detection of Cd2+ and Pb2+.116 Subramanian et al. developed a microfluidic device integrated with a radial microchannel to electrodes. Linear sweep voltammetry was conducted for the detection of various heavy metal ions. The LOD of As3+ was found to be 1 ppb. Specific peaks for Cu2+, Fe2+, Mn2+, and Pb2+ were also observed in the voltammogram.117 A point-of-care testing (POCT) electrochemical-based device was developed by Xu et al. for the simultaneous detection of multiple heavy metals, such as Cd2+, Cu2+, Hg2+ and Pb2+, using DPV. The LODs for Cd2+, Cu2+, Hg2+ and Pb2+ were found to be 0.29 μM, 0.055 μM, 0.351 μM and 0.025 μM, respectively.108 An integrated microfluidic-based electrochemical sensor was fabricated by Dai et al. for Pb2+ detection. The sensor was made up of three layers, and the RE was fabricated on the first layer. The third layer was integrated with a Au micropillar array (3D) WE and a planar Au CE on a glass substrate. This three-layered sensor, composed of glass–silicon–glass, was formed through the bonding of two electrode layers and a silicon layer. The quantification of Pb2+ was achieved by SWASV. The Cd2+, Cu2+, and Hg2+ heavy metals were found to cause interference in the detection of Pb2+, which can be minimized in the presence of the masking agents. The LOD was found to be 0.13 ppb.118 A screen-printed electrode was fabricated by Bernalte et al. for the simultaneous detection of heavy metals such as Cu, Pb2+, and Hg2+. The WE, RE, and CE were fabricated using gold ink and silver ink, respectively. Using SWASV, the heavy metals were quantified. The LODs of Cu2+, Pb2+ and Hg were found to be 1.3 mg L−1, 0.015 mg L−1 and 0.002 mg L−1, respectively.114 The modification of the WE with various materials will increase the sensitivity of the electrochemical sensor toward detecting specific heavy metals.97 For example, multiwalled carbon nanotube–PANI nanocomposites were used in the surface modification of glassy carbon electrodes for Pb2+ detection.119 The electrodes were modified with AuNPs and AgNPs to improve the LOD. A paper-based electrochemical device was developed by Pungjunun et al., where a boron-doped diamond electrode modified with AuNPs (AuNP/BDD) was used in As3+ detection. Screen-printed carbon and Ag/AgCl were used as the CE and RE, respectively. Interference from Cu2+ was detected in the procedure but was successfully eliminated using ferricyanide. The LOD was determined to be 20 ng mL−1.120
Conclusion
This review has highlighted the role of devices using microfluidics to detect heavy metals. The field of microfluidic-based heavy metal detection is rapidly advancing and finding applications not only in environmental monitoring but also in other fields of research, such as food technology and health care. These devices are being developed to compensate for existing complex and expensive procedures. The development of real-time devices, that can provide quick results and can be operated without experienced people, has become a new trend in the field of microfluidics. Most of the developed devices are believed to have the same sensitivity and accuracy as the current detection methods. In this review, we have discussed various techniques used in detecting different heavy metals, such as colorimetry, absorbance-based techniques, and electrochemical detection. The in situ and real-time monitoring of these techniques is crucial for the identification and mitigation of heavy metal contamination. Microfluidic technologies will continue to advance, offering great sensitivity, selectivity, and portability. However, there are few shortcomings which need to be addressed. Most of the existing microfluidics devices focus on the detection of single heavy metals; simultaneous detection remains a challenge. This can be improved by integrating multiple detection techniques such as colorimetry, absorbance-based detection and electrochemical detection in a single device that in turn improves the sensitivity. A prominent challenge in analysis is that the LOD can differ across different heavy metal samples and can be improved by optimization of the device and improving the surface chemistry of the sensors. Even though microfluidic-based devices are portable and used in real time detection, data interpretation and analysis are time consuming. The integration of artificial intelligence (AI) and machine learning (ML) in detection not only improves the data analysis, but it can also reduce the interference from other elements present in the real samples.Author contributions
N. M. created the outline of this review. S. S. N. wrote the manuscript and designed the figures. N. M. and R. B. reviewed and edited the manuscript.Conflicts of interest
The authors do not have any conflict of interest.Acknowledgements
NM thanks the Department of Science and Technology (DST) (Project Number – SERB/MTR/2020/000058) and the Indian Council of Medical Research (ICMR) (Project Number – ITR/Ad hoc/43/2020-21, ID No. 2020-3286) Government of India, India, for financial support. RB thanks the Biotechnology Industry Research Assistance Council (BIRAC), Department of Biotechnology, Government of India, India (Proposal number: BIRAC/KIIT01165/BIG-17/20), for financial support. NM and SSN thank the Manipal School of Life Sciences, Manipal Academy of Higher Education (MAHE), Manipal, Karnataka, India, for providing the infrastructure. SSN thanks MAHE, Manipal, Karnataka, India for the Dr T. M. A. Pai PhD fellowship.References
- S. H. A. Koop and C. J. van Leeuwen, Environ. Dev. Sustain., 2017, 19, 385–418 CrossRef.
- P. Lentini, L. Zanoli, A. Granata, S. S. Signorelli, P. Castellino and R. Dellaquila, Mol. Med. Rep., 2017, 15, 3413–3419 CrossRef CAS PubMed.
- M. Balali-Mood, K. Naseri, Z. Tahergorabi, M. R. Khazdair and M. Sadeghi, Front. Pharmacol, 2021, 12, 643972 CrossRef CAS PubMed.
- A. Bahrami, M. R. Arabestani, M. Taheri, A. Farmany, F. Norozzadeh, S. M. Hosseini, H. Nozari and F. Nouri, Biol. Trace Elem. Res., 2022, 200, 2639–2650 CrossRef CAS PubMed.
- J. Hogervorst, M. Plusquin, J. Vangronsveld, T. Nawrot, A. Cuypers, E. Van Hecke, H. A. Roels, R. Carleer and J. A. Staessen, Environ. Res., 2007, 103, 30–37 CrossRef CAS PubMed.
- S. Mishra, R. N. Bharagava, N. More, A. Yadav, S. Zainith, S. Mani and P. Chowdhary, in Springer eBooks, 2018, pp. 103–125 Search PubMed.
- E. G. C. Clarke, J. Small Anim. Pract., 1973, 14, 183–194 CrossRef CAS PubMed.
- W. R. Cullen and K. J. Reimer, Chem. Rev., 1989, 89, 713–764 CrossRef CAS.
- E. Diesel, M. Schreiber and J. R. van der Meer, Anal. Bioanal. Chem., 2009, 394, 687–693 CrossRef CAS PubMed.
- A. G.-M. Ferrari, P. Carrington, S. J. Rowley-Neale and C. E. Banks, Environ. Sci., 2020, 6, 2676–2690 Search PubMed.
- G. Matta and L. Gjyli, J. Chem. Pharm. Sci., 2016, 9, 718–725 CAS.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- K. Eto, Neuropathology, 2000, 20, 14–19 CrossRef PubMed.
- G. S. Dheri, M. S. Brar and S. S. Malhi, Commun. Soil Sci. Plant Anal., 2007, 38, 1353–1370 CrossRef CAS.
- A. Thongsaw, R. Sananmuang, Y. Udnan, R. J. Ampiah-Bonney and W. C. Chaiyasith, Anal. Sci., 2019, 35, 1195–1202 CrossRef CAS PubMed.
- H. Zheng, J. Hong, X. Luo, S. Li, M. Wang, B. Yang and M. Wang, Microchem. J., 2019, 145, 806–812 CrossRef CAS.
- T. Narukawa, T. Iwai and K. Chiba, Talanta, 2020, 210, 120646 CrossRef CAS PubMed.
- H. K. Sunaina, N. Kumari, A. Sharma, M. Sachdeva and V. Mutreja, Mater. Today: Proc., 2022, 48, 1673–1679 CAS.
- L.-L. Shen, G.-R. Zhang, W. Li, M. Biesalski and B. J. M. Etzold, ACS Omega, 2017, 2, 4593–4603 CrossRef CAS PubMed.
- J. Hwang, Y. H. Cho, M. S. Park and B. H. Kim, Int. J. Precis. Eng. Manuf., 2019, 20, 479–495 CrossRef.
- G. Aragay and A. Merkoçi, Electrochim. Acta, 2012, 84, 49–61 CrossRef CAS.
- Y. F. Wisang, H. Sulistyarti, U. Andayani and A. Sabarudin, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 546, 032033 CAS.
- A. Lace and J. Cleary, Chemosensors, 2021, 9, 60 CrossRef CAS.
- M. K. Filippidou, A. I. Kanaris, E. Aslanidis, A. Rapesi, D. Tsounidi, S. Ntouskas, E. Skotadis, G. Tsekenis, D. Tsoukalas, A. Tserepi and S. Chatzandroulis, Micromachines, 2023, 14, 1595 CrossRef PubMed.
- G. Chen, X. Wang and L. Wang, Int. J. Electrochem. Sci., 2020, 15, 4252–4263 CrossRef CAS.
- D. Janasek, J. Franzke and A. Manz, Nature, 2006, 442, 374–380 CrossRef CAS PubMed.
- D.-E. Li and C.-H. Lin, RSC Adv., 2018, 8, 16139–16145 RSC.
- D. Wu, S. Yang, F. Li, T. Zhu and H. Chen, Anal. Chem., 2020, 92, 14309–14313 CrossRef CAS PubMed.
- B.-H. Chen, S.-J. Jiang and A. C. Sahayam, Food Chem., 2020, 324, 126698 CrossRef CAS PubMed.
- N. Burshtein, S. T. Chan, K. Toda-Peters, A. Q. Shen and S. J. Haward, Curr. Opin. Colloid Interface Sci., 2019, 43, 1–14 CrossRef CAS.
- H. Wang, H. Rao, M. Luo, X. Xue, X. Zhang and X. Lu, Coord. Chem. Rev., 2019, 398, 113003 CrossRef CAS.
- F. Zhu, Y. He, Z. Lu, H. Fan and T. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 37589–37597 CrossRef CAS PubMed.
- A. H. McMillan, M. Roeffaers and S. C. Lesher-Pérez, Thermoplastic elastomer microfluidic devices forbiology and chemistry, University of Vienna, 2021 Search PubMed.
- E. L. Fava, T. A. Silva, T. M. do Prado, F. C. de Moraes, R. C. Faria and O. Fatibello-Filho, Talanta, 2019, 203, 280–286 CrossRef CAS PubMed.
- S. C. Terry, J. H. Jerman and J. B. Angell, IEEE Trans. Electron Devices, 1979, 26, 1880–1886 Search PubMed.
- W. H. Grover, R. H. C. Ivester, E. C. Jensen and R. A. Mathies, Lab Chip, 2006, 6, 623 RSC.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550–2583 CrossRef CAS PubMed.
- S. Aralekallu, R. Boddula and V. Singh, Mater. Des., 2023, 225, 111517 CrossRef CAS.
- S. Funano, N. Ota and Y. Tanaka, Lab Chip, 2021, 21, 2244–2254 RSC.
- K. Ren, J. Zhou and H. Wu, Acc. Chem. Res., 2013, 46, 2396–2406 CrossRef CAS PubMed.
- J. C. McDonald, D. C. Duffy, J. R. Anderson, D. T. Chiu, H. Wu, O. J. A. Schueller and G. M. Whitesides, Electrophoresis, 2000, 21, 27–40 CrossRef CAS PubMed.
- H. Wu, T. W. Odom, D. T. Chiu and G. M. Whitesides, J. Am. Chem. Soc., 2003, 125, 554–559 CrossRef CAS PubMed.
- J. Shim, G. Cristobal, D. R. Link, T. Thorsen, Y. Jia, K. Piattelli and S. Fraden, J. Am. Chem. Soc., 2007, 129, 8825–8835 CrossRef CAS PubMed.
- G. T. Roman, T. Hlaus, K. J. Bass, T. G. Seelhammer and C. T. Culbertson, Anal. Chem., 2005, 77, 1414–1422 CrossRef CAS PubMed.
- H. Becker and C. Gärtner, Anal. Bioanal. Chem., 2008, 390, 89–111 CrossRef CAS PubMed.
- C.-W. Tsao and D. L. DeVoe, Microfluid. Nanofluid., 2009, 6, 1–16 CrossRef CAS.
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., 2007, 119, 1340–1342 CrossRef.
- S. A. Klasner, A. K. Price, K. W. Hoeman, R. S. Wilson, K. J. Bell and C. T. Culbertson, Anal. Bioanal. Chem., 2010, 397, 1821–1829 CrossRef CAS PubMed.
- D. Wu, S. Yang, F. Li, T. Zhu and H. Chen, Anal. Chem., 2020, 92, 14309–14313 CrossRef CAS PubMed.
- W. Cui, Z. Ren, Y. Song and C. L. Ren, Sens. Actuators, A, 2022, 344, 113733 CrossRef CAS.
- S. B. D. Borah, T. Bora, S. Baruah and J. Dutta, Groundw. Sustain. Dev., 2015, 1, 1–11 CrossRef.
- W. M. E. M. M. Daniyal, Y. W. Fen, N. I. M. Fauzi, H. S. Hashim, N. S. M. Ramdzan and N. A. S. Omar, Sens. Mater., 2020, 32, 4191 CAS.
- P. Aryal, E. Brack, T. Alexander and C. S. Henry, Anal. Chem., 2023, 95, 5820–5827 CrossRef CAS PubMed.
- J. P. Devadhasan and J. Kim, Sens. Actuators, B, 2018, 273, 18–24 CrossRef CAS.
- H. Wang, H. Rao, M. Luo, X. Xue, Z. Xue and X. Lu, Coord. Chem. Rev., 2019, 398, 113003 CrossRef CAS.
- M. J. Kangas, R. M. Burks, J. Atwater, R. M. Lukowicz, P. Williams and A. E. Holmes, Crit. Rev. Anal. Chem., 2017, 47, 138–153 CrossRef CAS PubMed.
- M. L. Budlayan, J. Dalagan, J. P. Lagare-Oracion, J. Patricio, S. Arco, F. Latayada, T. Vales, B. Baje, A. Alguno and R. Capangpangan, Environ. Nanotechnol., Monit. Manage., 2022, 18, 100736 CAS.
- B. Sahu, R. Kurrey, M. K. Deb, K. Shrivas, I. Karbhal and B. R. Khalkho, RSC Adv., 2021, 11, 20769–20780 RSC.
- P. Yeh, N. Yeh, C.-H. Lee and T.-J. Ding, Renewable Sustainable Energy Rev., 2017, 75, 461–468 CrossRef CAS.
- S. A. A. Razavi and A. Morsali, Coord. Chem. Rev., 2020, 415, 213299 CrossRef CAS.
- A. Lace, D. Ryan, M. Bowkett and J. Cleary, Anal. Methods, 2019, 11, 5431–5438 RSC.
- B. Karakuzu, Y. Gulmez and H. C. Tekin, Microelectron. Eng., 2021, 247, 111583 CrossRef CAS.
- X. Xie, M. Pan, L. Hong, K. Liu, J. Yang, S. Wang and S. Wang, J. Agric. Food Chem., 2021, 69, 7209–7217 CrossRef CAS PubMed.
- Y. C. Reyes R, T. B. Rouf, O. E. Torres and E. E. González, ACS Agric. Sci. Technol., 2022, 2, 144–152 CrossRef CAS.
- S. Singh, B. Coulomb, J.-L. Boudenne, D. Bonne, F. Dumur, B. Simon and F. Robert-Peillard, Talanta, 2021, 224, 121909 CrossRef CAS PubMed.
- K. Karthikeyan and L. Sujatha, IEEE Sens. J., 2018, 18, 5225–5231 CAS.
- G. Peng, Y. Chen, R. Deng, Q. He, D. Liu, Y. Lu and J.-M. Lin, Spectrochim. Acta, Part A, 2018, 204, 1–6 CrossRef CAS PubMed.
- H. Yuan, G. Sun, W. Peng, W. Ji, S. Chu, Q. Liu and Y. Liang, Nanomaterials, 2021, 11, 397 CrossRef CAS PubMed.
- W. Huang, V. Phung, R.-Y. Wu, K.-L. Yeh and R.-J. Yang, Micromachines, 2021, 12, 1283 CrossRef PubMed.
- A. Biranje, N. Azmi, A. Tiwari and A. Chaskar, J. Fluoresc., 2021, 31, 1241–1250 CrossRef CAS PubMed.
- J. Yue, Q. Lv, W. Wang and Q. Zhang, Talanta Open, 2022, 5, 100099 CrossRef.
- J. Qi, B. Li, X. Wang, Z. Zhang, Z. Wang, J. Han and L. Chen, Sens. Actuators, B, 2017, 251, 224–233 CrossRef CAS.
- L. Wang, B. Li, J. Wang, J. Qi, J. Li, J. Ma and L. Chen, J. Hazard. Mater., 2022, 428, 128165 CrossRef CAS PubMed.
- L. Wang, B. Li, J. Li, J. Qi, Z. Zhang and L. Chen, Analyst, 2022, 147, 3756–3763 RSC.
- J. Han, H. Liu, J. Qi, J. Xiang, L. Fu, X. Sun, L. Wang, X. Wang, B. Li and L. Chen, Sensors, 2023, 23, 3094 CrossRef CAS PubMed.
- J. Zhou, B. Li, A. Qi, Y. Shi, J. Qi, H. Xu and L. Chen, Sens. Actuators, B, 2020, 305, 127462 CrossRef CAS.
- X. Wang, L. Kong, Y. Gan, T. Liang, S. Zhou, J. Sun, H. Wan and P. Wang, Anal. Chim. Acta, 2020, 1131, 126–135 CrossRef CAS PubMed.
- M. Lu, Y. Deng, Y. Luo, J. Lv, T. Li, J. Xu, S.-W. Chen and J. Wang, Anal. Chem., 2019, 91, 888–895 CrossRef CAS PubMed.
- R. M. El-Shishtawy, H. A. Al-Ghamdi, M. M. Alam, Z. M. Al-amshany, A. M. Asiri and M. M. Rahman, Chem. Eng. J., 2018, 352, 225–231 CrossRef CAS.
- N. Wongkaew, P. He, V. Kurth, W. Surareungchai and A. J. Baeumner, Anal. Bioanal. Chem., 2013, 405, 5965–5974 CrossRef CAS PubMed.
- H. Hou, K. M. Zeinu, S. Gao, B. Liu, J. Yang and J. Hu, Energy Environ. Mater., 2018, 1, 113–131 CrossRef CAS.
- T. D. Nguyen, M. T. N. Nguyen and J. S. Lee, Inorganics, 2023, 11, 81 CrossRef CAS.
- S. Liu, T. Wu, F. Li, Q. Zhang, X. Dong and L. Niu, Anal. Methods, 2018, 10, 1986–1992 RSC.
- J.-H. Hwang, X. Wang, D. Zhao, M. M. Rex, H. J. Cho and W. H. Lee, Electrochim. Acta, 2019, 298, 440–448 CrossRef CAS.
- P. B. Deroco, D. Wachholz Junior and L. T. Kubota, Chemosensors, 2021, 9, 61 CrossRef CAS.
- F. Wang, S. Wang, L. Zhang, H. Yang, W. Gao, Q. Wu and J. Hao, J. Environ. Sci., 2016, 43, 293–301 CrossRef CAS PubMed.
- W. Zhang, G. Liu, J. Bi, K. Bao and P. Wang, Sens. Actuators, A, 2023, 349, 114074 CrossRef CAS.
- Y. Li, F. Ye, A. Wang, D. Wang, B. Yang, Q. Zheng, G. Sun and X. Gao, Int. J. Environ. Res. Public Health, 2016, 13, 133 CrossRef PubMed.
- R. A. G. de Oliveira, E. M. Materon, M. E. Melendez, A. L. Carvalho and R. C. Faria, ACS Appl. Mater. Interfaces, 2017, 9, 27433–27440 CrossRef CAS PubMed.
- S. Singh, J. Wang and S. Cinti, ECS Sens. Plus, 2022, 1, 023401 CrossRef.
- N. Zavanelli and W.-H. Yeo, ACS Omega, 2021, 6, 9344–9351 CrossRef CAS PubMed.
- H. Shamkhalichenar and J.-W. Choi, ECS Meeting Abstracts, 2017, MA2017-01, p. 1879 Search PubMed.
- K. Zub, S. Hoeppener and U. S. Schubert, Adv. Mater., 2022, 34, 2105015 CrossRef CAS PubMed.
- S. Diaz-Amaya, L.-K. Lin, R. E. DiNino, C. Ostos and L. A. Stanciu, Electrochim. Acta, 2019, 316, 33–42 CrossRef CAS.
- A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC.
- R. M. Cardoso, C. Kalinke, R. G. Rocha, P. L. dos Santos, D. P. Rocha, P. R. Oliveira, B. C. Janegitz, J. A. Bonacin, E. M. Richter and R. A. A. Munoz, Anal. Chim. Acta, 2020, 1118, 73–91 CrossRef CAS PubMed.
- B. Bansod, T. Kumar, R. Thakur, S. Rana and I. Singh, Biosens. Bioelectron., 2017, 94, 443–455 CrossRef CAS PubMed.
- G. Lisak, Environ. Pollut., 2021, 289, 117882 CrossRef CAS PubMed.
- S. Chauhan and L. S. B. Upadhyay, J. Ravishankar Univ., Part B, 2019, 32, 23–26 CrossRef.
- G. Le Guillanton, Q. T. Do and D. Elothmani, J. Electrochem. Soc., 1996, 143, L223–L225 CrossRef CAS.
- J. Zhou, K. Ren, Y. Zheng, J. Su, Y. Zhao, D. Ryan and H. Wu, Electrophoresis, 2010, 31, 3083–3089 CrossRef CAS PubMed.
- G.-L. Wen, W. Zhao, X. Chen, J.-Q. Liu, Y. Wang, Y. Zhang, Z.-J. Huang and Y.-C. Wu, Electrochim. Acta, 2018, 291, 95–102 CrossRef CAS.
- H. A. Hamid, Z. Lockman, N. M. Nor, N. D. Zakaria and K. A. Razak, Mater. Chem. Phys., 2021, 273, 125148 CrossRef CAS.
- H. Men, S. Zou, Y. Li, Y. Wang, X. Ye and P. Wang, Sens. Actuators, B, 2005, 110, 350–357 CrossRef CAS.
- B. S. Sherigara, Y. Shivaraj, R. J. Mascarenhas and A. K. Satpati, Electrochim. Acta, 2007, 52, 3137–3142 CrossRef CAS.
- W. Jung, A. Jang, P. L. Bishop and C. H. Ahn, Sens. Actuators, B, 2011, 155, 145–153 CrossRef CAS.
- D. B. Sheth and M. Gratzl, Anal. Bioanal. Chem., 2013, 405, 5539–5547 CrossRef CAS PubMed.
- Z. Xu, Z. Liu, M. Xiao, L. Jiang and C. Yi, Chem. Eng. J., 2020, 394, 124966 CrossRef CAS.
- G. Luka, A. Ahmadi, H. Najjaran, E. Alocilja, M. DeRosa, K. Wolthers, A. Malki, H. Aziz, A. Althani and M. Hoorfar, Sensors, 2015, 15, 30011–30031 CrossRef CAS PubMed.
- Y. Hong, M. Wu, G. Chen, Z. Dai, Y. Zhang, G. Chen and X. Dong, ACS Appl. Mater. Interfaces, 2016, 8, 32940–32947 CrossRef CAS PubMed.
- A. M. Baracu and L. A. Dinu Gugoasa, J. Electrochem. Soc., 2021, 168, 037503 CrossRef CAS.
- H.-F. Li and J.-M. Lin, Anal. Bioanal. Chem., 2009, 393, 555–567 CrossRef CAS PubMed.
- C. Kokkinos, A. Economou and D. Giokas, Sens. Actuators, B, 2018, 260, 223–226 CrossRef CAS.
- E. Bernalte, S. Arévalo, J. Pérez-Taborda, J. Wenk, P. Estrela, A. Avila and M. Di Lorenzo, Sens. Actuators, B, 2020, 307, 127620 CrossRef.
- J. M. Mohan, S. Dudala, K. Amreen, A. Javed, S. K. Dubey and S. Goel, IEEE Trans. NanoBiosci., 2023, 22, 881–888 CAS.
- M. F. Santangelo, I. Shtepliuk, D. Filippini, D. Puglisi, M. Vagin, R. Yakimova and J. Eriksson, Sensors, 2019, 19, 2393 CrossRef CAS PubMed.
- V. Subramanian, S. Lee, S. Jena, S. K. Jana, D. Ray, S. J. Kim and P. Thalappil, Sens. Actuators, B, 2020, 304, 127340 CrossRef CAS.
- J. Dai, W. Gao, J. Yin, L. Liang, J. Zou and Q. Jin, Anal. Chim. Acta, 2021, 1164, 338511 CrossRef CAS PubMed.
- Z. Wang, E. Liu, D. Gu and Y. Wang, Thin Solid Films, 2011, 519, 5280–5284 CrossRef CAS.
- K. Pungjunun, S. Chaiyo, I. Jantrahong, S. Nantaphol, W. Siangproh and O. Chailapakul, Microchim. Acta, 2018, 185, 324 CrossRef PubMed.
- R. A. Schwartz, Int. J. Dermatol., 1997, 36, 241–250 CrossRef CAS PubMed.
- H. Garelick, H. Jones, A. Dybowska and E. Valsami-Jones, Rev. Environ. Contam. Toxicol., 2009, 17–60 Search PubMed.
- L. Joseph, B.-M. Jun, J. R. V. Flora, C. M. Park and Y. Yoon, Chemosphere, 2019, 229, 142–159 CrossRef CAS PubMed.
- D. L. Alonso, R. Pérez, C. K. Y. A. Okio and E. Castillo, J. Environ. Manage., 2020, 264, 110478 CrossRef CAS PubMed.
- W. Ramos, A. G. Ortega-Loayza, J. Díaz, J. A. De La Cruz-Vargas, M. Tello, G. Ronceros, M. Loayza and E. L. Gutierrez, Clin., Cosmet. Invest. Dermatol., 2022, 15, 2407–2414 CrossRef CAS PubMed.
- T. C. Nguyen, P. Loganathan, T. V. Nguyen, S. Vigneswaran, J. Kandasamy and R. Naidu, Chem. Eng. J., 2015, 270, 393–404 CrossRef CAS.
- M. Mezynska and M. M. Brzóska, Environ. Sci. Pollut. Res., 2018, 25, 3211–3232 CrossRef CAS PubMed.
- T. Umemura and Y. Wako, J. Toxicol. Pathol., 2006, 19, 69–74 CrossRef.
- L. Järup, Nephrol., Dial., Transplant., 2002, 17, 35–39 CrossRef PubMed.
- L. Patrick, Alternative Med. Rev., 2003, 8, 106–128 Search PubMed.
- C. J. Everett and I. L. Frithsen, Environ. Res., 2008, 106, 284–286 CrossRef CAS PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- B. Tripathy, A. Dash and A. P. Das, Crit. Rev. Anal. Chem., 2022, 1–11 CrossRef PubMed.
- I. Manisalidis, E. Stavropoulou, A. Stavropoulos and E. Bezirtzoglou, Front. Public Health, 2020, 8, 505570 Search PubMed.
- E. G. C. Clarke, J. Small Anim. Pract., 1973, 14, 183–194 CrossRef CAS PubMed.
- A. L. Wani, A. Ara and J. A. Usmani, Interdiscip. Toxicol., 2015, 8, 55–64 CrossRef CAS PubMed.
- K. Raj and A. P. Das, Environ. Chem. Ecotoxicol., 2023, 5, 79–85 CrossRef CAS.
- Z. Rahman and V. P. Singh, Environ. Monit. Assess., 2019, 191, 419 CrossRef PubMed.
- C. Lidén, Br. J. Dermatol., 2013, 169, 733 CrossRef PubMed.
- Q. Y. Chen, J. Brocato, F. Laulicht and M. Costa, in Molecular and Integrative Toxicology, 2017, vol. 8, pp. 181–197 Search PubMed.
- W. Dong, Y. Zhang and X. Quan, Chemosphere, 2020, 242, 125113 CrossRef CAS PubMed.
- S. Albanese, M. Sadeghi, A. Lima, D. Cicchella, E. Dinelli, P. Valera, M. Falconi, A. Demetriades and B. De Vivo, J. Geochem. Explor., 2015, 154, 81–93 CrossRef CAS.
- M. Babaahmadifooladi, L. Jacxsens, B. De Meulenaer and G. Du Laing, Food Addit. Contam.: Part A, 2020, 37, 607–621 CrossRef CAS PubMed.
- L. Leyssens, B. Vinck, C. Van Der Straeten, F. Wuyts and L. Maes, Toxicology, 2017, 387, 43–56 CrossRef CAS PubMed.
- W. Uter, R. Rühl, A. Pfahlberg, J. Geier, A. Schnuch and O. Gefeller, Ann. Occup. Hyg., 2004, 48, 21–27 CAS.
- P. N. Bezerra, A. G. A. Vasconcelos, L. L. A. Cavalcante, V. B. d. V. Marques, T. N. A. G. Nogueira and M. A. Holanda, J. Bras. Pneumol., 2009, 35, 1254–1258 CrossRef PubMed.
- B. Zhao, M. He, B. Chen and B. Hu, Spectrochim. Acta, Part B, 2022, 196, 106524 CrossRef CAS.
- M. Ghanei-Motlagh and M. Baghayeri, Mater. Chem. Phys., 2022, 285, 126127 CrossRef CAS.
- Y. Xing, J. Han, X. Wu, D. T. Pierce and J. X. Zhao, Microchim. Acta, 2020, 187, 56 CrossRef CAS PubMed.
- D. Mishra, A. Krause, H. S. Sahni and S. Chatterjee, Diamond Relat. Mater., 2023, 137, 110156 CrossRef CAS.
- G. Vyas, S. Bhatt and P. Paul, ACS Omega, 2019, 4, 3860–3870 CrossRef CAS PubMed.
- H. R. Cadorim, M. Schneider, J. Hinz, F. Luvizon, A. N. Dias, E. Carasek and B. Welz, Anal. Lett., 2019, 52, 2133–2149 CrossRef CAS.
- A. Bhat, T. O. Hara, F. Tian and B. Singh, Environ. Sci.: Adv., 2023, 2, 171–195 CAS.
- T. Agustiany, M. Khalil, Y. Einaga, P. K. Jiwanti and T. A. Ivandini, Mater. Chem. Phys., 2020, 244, 122723 CrossRef CAS.
- C. G. A. Maria, A. S. Agnihotri, A. Varghese, T. Fatima and S. Hameed, New J. Chem., 2023, 47, 5179–5192 RSC.
- J. You, J. Li, Z. Wang, M. Baghayeri and H. Zhang, Chemosphere, 2023, 335, 139133 CrossRef CAS PubMed.
- J. Zhou, D. Deng, Y. Su and Y. Lv, Microchem. J., 2019, 146, 359–365 CrossRef CAS.
- P. Montoro-Leal, J. C. García-Mesa, M. T. Siles Cordero, M. M. López Guerrero and E. Vereda Alonso, Microchem. J., 2020, 155, 104796 CrossRef CAS.
- S. Chen, J. Yan, J. Li and D. Lu, Microchem. J., 2019, 147, 232–238 CrossRef CAS.
- L. A. Malik, A. Bashir, A. Qureashi and A. H. Pandith, Environ. Chem. Lett., 2019, 17, 1495–1521 CrossRef CAS.
- Y. A. Ghorbani, S. M. Ghoreishi and M. Ghani, Microchem. J., 2020, 155, 104786 CrossRef CAS.
- S. Sel, F. A. Erulaş, F. Turak and S. Bakırdere, Anal. Lett., 2019, 52, 761–771 CrossRef CAS.
- A. Karthika, S. Nikhil, A. Suganthi and M. Rajarajan, Adv. Powder Technol., 2020, 31, 1879–1890 CrossRef CAS.
- H. Sharifi, J. Tashkhourian and B. Hemmateenejad, Anal. Chim. Acta, 2020, 1126, 114–123 CrossRef CAS PubMed.
- P. Kamnoet, W. Aeungmaitrepirom, R. F. Menger and C. S. Henry, Analyst, 2021, 146, 2229–2239 RSC.
- H. Taheri and G. Khayatian, Spectrochim. Acta, Part A, 2022, 272, 121000 CrossRef CAS PubMed.
- X. Sun, B. Li, A. Qi, C. Tian, J. Han, Y. Shi, B. Lin and L. Chen, Talanta, 2018, 178, 426–431 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |