Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An integrated green process for the extraction of triterpenic acids from Eucalyptus globulus leaves after hydrodistillation
Cátia S. D.
Oliveira
 a,
Patrícia
Moreira
a,
Patrícia
Moreira
 bc,
Maria T.
Cruz
bc,
Maria T.
Cruz
 bc,
Cláudia M. F.
Pereira
bc,
Cláudia M. F.
Pereira
 bd,
Alexandre
Gaspar
e,
Carlos Pascoal
Neto
bd,
Alexandre
Gaspar
e,
Carlos Pascoal
Neto
 e,
Paula C. R. O.
Pinto
e,
Paula C. R. O.
Pinto
 e,
Pedro Costa
Branco
e,
Pedro Costa
Branco
 e,
Artur M. S.
Silva
e,
Artur M. S.
Silva
 f,
Sónia A. O.
Santos
f,
Sónia A. O.
Santos
 *a and
Armando J. D.
Silvestre
*a and
Armando J. D.
Silvestre
 a
a
aCICECO – Aveiro Institute of Materials, University of Aveiro, Aveiro 3810-193, Portugal. E-mail: santos.sonia@ua.pt
bCNC − Center for Neuroscience and Cellular Biology, University of Coimbra, Coimbra 3004-504, Portugal
cFaculty of Pharmacy, University of Coimbra, Coimbra 3000-548, Portugal
dFaculty of Medicine, University of Coimbra, Coimbra 3000-548, Portugal
eRAIZ – Forest and Paper Research Institute, Quinta de S. Francisco, Rua José Estevão, Eixo 3800-783, Portugal
fLAQV-REQUIMTE, Department of Chemistry, University of Aveiro, Aveiro 3810-193, Portugal
First published on 6th June 2023
Abstract
In this study the viability of the integrated exploitation of E. globulus leaf essential oil and triterpenic acids was evaluated through the development of a novel extraction process that can be implemented sequentially in a biorefinery context. Thus, essential oil (EO) collected by hydrodistillation was used for the first time as a bio-based solvent to recover triterpenic acids (TTAs) from the residue resulting from the EO recovery (from the hydrodistilled leaves). Ursolic, oleanolic, betulonic and betulinic acids were successfully extracted with EO with, for comparison purposes, its major component, 1,8-cineole (CO), showing TTAs extraction yields of 2.8 and 2.7% dw, respectively. Both EO and CO were particularly efficient in extracting ursolic (18.3 and 17.9 g kg−1 dw) and oleanolic (6.0 and 5.7 g kg−1 dw, respectively) acids, the major components of crude extracts. In addition, cytotoxicity evaluation showed that EO and CO crude extracts are non-toxic to macrophage cell lines at concentrations less than or equal to 0.04 and 0.08 mg mL−1, respectively. Crude extracts dissolved in the EO and CO also showed higher anti-inflammatory activity than a synthetic mixture representative of the TTAs detected, demonstrating the synergistic effect between EO or CO and the extracted components. In summary, the EO is a potential bio-based solvent, which could be applied in biorefinery processes, replacing organic solvents such as n-hexane in the recovery of TTAs, without environmental side effects, and even with potential applications of the crude extracts themselves in the nutraceutical and pharmaceutical fields.
Sustainability spotlightLarge amounts of by-products are generated from Eucalyptus globulus exploitation, which are considered as valuable sources of high-value compounds. However, only essential oils from leaves have been intensively exploited, with other promising components such as triterpenic acids being neglected. In addition, the current extraction methodologies to recover these components are challenged by low selectivity, use of organic solvents and high extraction time. In this vein, the viability of the integrated exploitation of E. globulus leaf essential oil and triterpenic acids was studied, using the essential oil as a bio-based solvent to extract triterpenic acids from the hydrodistilled leaves. Our work emphasizes the importance of the UN's industry, innovation and infrastructure sustainable development goal. |
Introduction
Eucalyptus is a genus of the myrtaceae family, native to Australia and Tasmania.1,2 This genus has been successfully introduced in many countries, in the tropics, subtropics and Mediterranean regions, including Asia, America, Africa and Europe.1,3 It is estimated that a total area of about 16–19 million ha is occupied by Eucalyptus ssp. plantations, mainly in Brazil, India, China, South Africa, Spain and Portugal.2,4E. globulus in particular has been extensively cultivated in Portugal and Spain due to its rapid growth and excellent properties for the production of high-quality pulp and paper. E. globulus forests are usually managed in short rotation periods (10–12 years) and the biomass collected after thinning is considered an unseasonal renewable raw material, since it is produced throughout the year.5–9 The by-products of E. globulus forest management and tree harvesting, such as bark and leaves, are widely described as rich sources of value-added compounds, which could add substantial value to this chain if adequately explored.
E. globulus leaves have thus been intensively exploited as a source of essential oil (EO), normally obtained by hydrodistillation, which is mainly composed of monoterpenic and sesquiterpenic compounds.10 The major components present in E. globulus leaf EO are the monoterpenic compounds 1,8-cineole (CO), also known as eucalyptol (17.2–86.5%) (Fig. 1), α-pinene (2.8–52.7%), and limonene (6.6–28.0%), as well as the sesquiterpenic compounds aromadendrene (3.7–7.1%) and globulol (2.4–9.8%).10–12 In addition, E. globulus leaves, as well as its bark, are also rich in pentacyclic triterpenic compounds (Fig. 1). Betulonic, betulinic, oleanolic, ursolic, 3-acetyloleanolic and 3-acetylursolic acids and β-amyrin have been identified in the bark and leaves,7,9,13,14 while 3-acetylbetulinic acid, α-amyrin and lupeol have only been reported in the bark.7,14
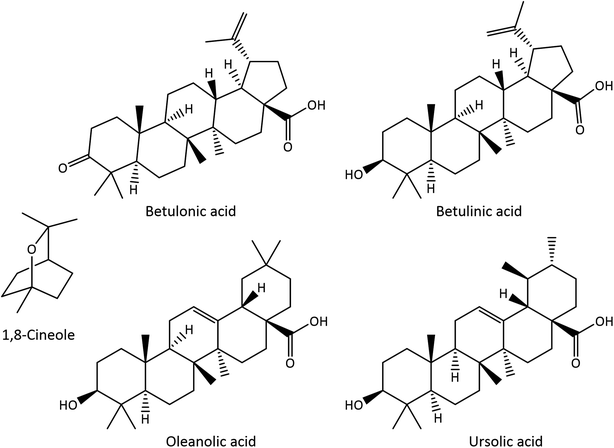 | ||
| Fig. 1 Structures of 1,8-cineole (CO) and triterpenic acids (TTAs) identified in E. globulus leaves. | ||
Apart from the production of EO, from a minor fraction of the available raw material, the sustainable (and integrated) exploitation of eucalyptus biomass as a source of other value-added compounds, such as triterpenic acids (TTAs), is still unexplored.11,15
Due to its medicinal properties, E. globulus EO and leaf extracts are used in pharmaceutical, sanitary, agricultural, cosmetic, and food applications.5,16 Additionally, several biological activities have also been reported for the EO obtained from E. globulus, such as anti-inflammatory, antitumor, antibacterial, antifungal, antioxidant, acaricidal and insecticide activities.10,12,15,17–21 Moreover, eucalyptus leaf lipophilic extracts are also known for their antibacterial activity,19 and their main components, TTAs, are known to exhibit a wide range of biological properties, such as antimicrobial, antitumor, anti-inflammatory, hepatoprotective, anti-allergic, anti-HIV and antimalarial properties, among many others,3,13,22 with potential applications in the pharmaceutical, cosmetic and food industries.
In recent years, diverse extraction methodologies have been explored to isolate these compounds from plant biomass, including eucalyptus bark,13,22–25 while only a few studies have focused on eucalyptus leaves (with the exception of EO).4,13
As mentioned above, EO is most commonly isolated by an environmentally safe process, hydrodistillation, that uses only steam, although other alternative methodologies have been suggested, such as ultrasound- or microwave-assisted extractions,26,27 whereas TTAs are usually extracted through solid–liquid extractions using polar or non-polar organic solvents or different mixtures of (predominantly volatile, flammable and often toxic) organic solvents such as n-hexane,28,29 dichloromethane,7,9 ethanol30,31 and chloroform.32 However, currently the challenge is to replace these solvents with more sustainable alternatives.33,34 The most studied alternatives include water, supercritical and subcritical fluids, ionic liquids, deep eutectic solvents, and bio-based solvents.35,36 Supercritical CO2 extraction is an attractive alternative for the extraction of triterpenic compounds, because it is a clean and green extraction procedure; however, it is associated with high initial costs (associated with high pressure equipment/operation costs), and also has high energy consumption.36,37 Ionic liquids have also been considered, and despite their successful use in the extraction of triterpenic acids from other biomass sources,22 their use is still limited given the complexity and high cost of their production, and often the associated recycling problems.35,36,38
Bio-based solvents might play an important role in the replacement of petrochemical solvents. These solvents are produced from biomass feedstocks by extraction, or chemical or biochemical transformations and have high solvent power, and are most often non-toxic and biodegradable.33,34 EO and the compounds obtained thereof have already been used as solvents.33,34 For example, α-pinene and/or limonene, obtained from pine and citrus oils, respectively, due to their low polarity and high solvent power have been used for the extraction of fats and oils.34,39–41 Moreover, CO has already been used in the extraction of phenolic compounds.39 Notwithstanding, no studies were found in the literature reporting the use of these components, and ultimately the eucalyptus EO itself as a bio-based solvent for the extraction of TTAs from biomass.
Considering the high relevance of developing low cost and sustainable integrated extraction processes that would allow a sequential exploitation of different fractions of the same biomass, i.e., in the present case, for sequentially obtaining EO and triterpenic compound rich fractions, maximizing the generation of added value, and allowing a decrease in the use of organic solvents, and a decrease in the costs involved in the process, with respect to the recovery of solvents, and reducing environmental impacts, this work aimed to develop an extraction process for the recovery of TTAs from E. globulus leaves, which could be integrated with the EO hydrodistillation step. For the reasons mentioned before, isolated EO was selected as a bio-based solvent to recover an extract rich in TTAs from the remaining biomass E. globulus hydrodistilled leaves (EgHDL). For comparison purposes, the results obtained in the extraction with the EO were also compared with the extraction with CO. The extracts obtained from the EgHDL were analyzed and quantified by gas chromatography-mass spectroscopy (GC-MS). The cytotoxicity and anti-inflammatory activity of crude extracts dissolved in the EO and CO were also evaluated and compared with a synthetic mixture representative of the TTAs present in the crude extracts.
Materials and methods
Reagents
Dichloromethane (DCM) (p.a., ≥99% purity) was supplied by Fisher Scientific (Thermo Fisher Scientific, Waltham, Massachusetts, USA), and 1,8-cineole (99% purity) was purchased from Acros Organics. Essential oil (80–85% of cineole) was provided by Socidestilda (Socidestilda, Aldeia de Paio Pires, Portugal). Pyridine (p.a., ≥99.5% purity), N,O-bis(trimethylsilyl)trifluoroacetamide (99% purity), trimethylchlorosilane (99% purity), tetracosane (99% purity) and ursolic acid (≥98% purity) were supplied by Sigma Chemical Co. (Madrid, Spain). Betulonic (BoA), oleanolic (OA), betulinic (BA) and ursolic (UA) acid standards (≥98% purity) were acquired from Chemos GmbH (Germany). Dulbecco's Modified Eagle's Medium (DMEM), sodium bicarbonate, sodium pyruvate and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) and penicillin-streptomycin were obtained from Gibco (Carlsbad, CA, USA).Materials
E. globulus leaves, representative of harvesting biomass residues, were sampled from 6-year-old E. globulus trees, in October 2018, randomly selected from a property of “The Navigator Company”, Braçal (GPS coordinates 40°44′5.388N, 8°23′53.97W), region of Sever do Vouga, Portugal.Hydrodistillation
Fresh E. globulus leaves, with a moisture content of ∼30% weight (evaluated by drying at 105 °C for 72 h), were subjected to hydrodistillation, as described by Moreira et al.42 to collect the EO. The yield, as obtained in a previous study,42 was 1.7% (v/w, based on the fresh leaf weight) and the main components of EO were CO (72.3%), α-pinene (9.4%), E-pinocarveol (3.6%), limonene (2.3%), globulol (1.6), pinocarvone (1.4%) and α-terpinyl acetate (1.2%).42 Hydrodistilled leaves, resulting from the hydrodistillation process performed by Moreira et al.,42 were air dried and lyophilized. After lyophilization, the leaves were ground using a Kenwood ch580 450W grinder in order to reach biomass with a particle size between 2 and 3 mm.Soxhlet extraction
Dried samples of E. globulus hydrodistilled leaves (EgHDL) (∼10 g) were subjected, in triplicate, to Soxhlet extraction with DCM for a period of 8 h. The solvent was evaporated to dryness, at low pressure and 35 °C and the results were expressed as a percentage of dry weight (dw) biomass. Soxhlet extraction with DCM, which is a very selective solvent for extracting lipophilic compounds from biomass,9 was performed for comparison purposes.Solid–liquid extraction
Dried samples of EgHDL (∼7 g) were extracted with E. globulus EO or with CO (solid–liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/v, dw EgHDL) at room temperature, protected from light and with stirring for up to 24 h. The biomass and the liquid fraction (TTAs enriched extracts dissolved in EO or CO, from now on denoted as EO or CO crude extracts, respectively) were separated by pressing. The extracts were prepared in triplicate.
4 w/v, dw EgHDL) at room temperature, protected from light and with stirring for up to 24 h. The biomass and the liquid fraction (TTAs enriched extracts dissolved in EO or CO, from now on denoted as EO or CO crude extracts, respectively) were separated by pressing. The extracts were prepared in triplicate.
GC-MS analysis
For GC-MS analysis, aliquots of EO or CO crude extracts were derivatized. Aliquots of 200 μL of crude extracts were dissolved in 250 μL of pyridine containing 0.6 mg of tetracosane (internal standard) and then 250 μL of N,O-bis(trimethylsilyl)trifluoroacetamide and 50 μL of trimethylchlorosilane were added. The mixture was kept at 70 °C for 30 min. By adding these last two reagents, the hydroxyl and carboxyl groups of the extracts' components were converted into trimethylsilyl (TMS) ethers and esters, respectively.GC-MS analysis was carried out in a GC–MS-QP2010 Ultra (Shimadzu, Japan), and compounds were separated in a DB-1 J&W capillary column (30 m × 0.32 mm inner diameter, 0.25 μm film thickness), using helium as the carrier gas (40 cm s−1). The chromatographic conditions were as follows: initial temperature, 80 °C for 5 min; temperature rate, 4 °C min−1 up to 260 °C, 2 °C min−1 up to 285 °C, which was maintained for 15 min; injector temperature, 250 °C; transfer-line temperature, 290 °C; split ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The mass spectrometer was operated in the electron impact mode with an energy of 70 eV, and data were collected at a rate of 1 scan per s over a range of m/z 35–900. The ion source was kept at 250 °C.43 A solvent delay of 30 min was used to avoid the solvent's (eucalyptus EO) intense chromatographic peaks.
50. The mass spectrometer was operated in the electron impact mode with an energy of 70 eV, and data were collected at a rate of 1 scan per s over a range of m/z 35–900. The ion source was kept at 250 °C.43 A solvent delay of 30 min was used to avoid the solvent's (eucalyptus EO) intense chromatographic peaks.
TTAs were identified by comparing their mass spectra (MS) with a mass spectral library (Wiley-NIST Mass Spectral Library, 2014) and with literature data, and also based on their characteristic retention times under the same experimental conditions.13,44,45
TTAs were quantified by their peak areas in comparison with the internal standard (tetracosane). The response factor (average of six runs) in relation to tetracosane was determined using UA. TTAs abundance was expressed as g kg−1 dw. One aliquot of each extract (triplicates) was injected in duplicate and the results presented the average of the concordant values obtained with a variation of less than 5% (both between injections of the same aliquot and between triplicate extracts of the same sample).
Cell culture
The mouse leukaemic macrophage cell line (RAW 264.7, ATCC TIB-71, Manassas, VA, USA) was cultured in DMEM supplemented with 10% (v/v) non-inactivated FBS, 1% (v/v) antibiotic solution (from a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U per mL penicillin and 10
000 U per mL penicillin and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg per mL streptomycin stock), 1.5 g per L sodium bicarbonate, and 1 mM sodium pyruvate. The cells were cultured in 75 cm2 flasks and maintained in a humidified 5% CO2–95% air atmosphere at 37 °C, and the medium was changed every 2–3 days. The macrophages were mechanically detached with a cell scraper for passage and sub-culturing when the cells reached 70–80% confluence.
000 μg per mL streptomycin stock), 1.5 g per L sodium bicarbonate, and 1 mM sodium pyruvate. The cells were cultured in 75 cm2 flasks and maintained in a humidified 5% CO2–95% air atmosphere at 37 °C, and the medium was changed every 2–3 days. The macrophages were mechanically detached with a cell scraper for passage and sub-culturing when the cells reached 70–80% confluence.
Cell viability evaluation
To evaluate the cell viability, the MTT reduction assay was performed. RAW 264.7 cells were seeded in 96-well plates at a density of 9.6 × 104 and allowed to stabilize for 24 h. On the day of the experiment, the culture medium was replaced by exposure medium [DMEM supplemented with 1% (v/v) non-inactivated FBS]. The cells were incubated with 0–2.5 mg mL−1 EO, CO, EO crude extract or CO crude extract, for 24 h at 37 °C. For comparison purposes and to evaluate the interference of EO and CO (benefits or harms), two synthetic mixtures of pure TTAs, consisting of BoA, OA, BA and UA, were also tested, where the same proportions of TTAs determined in the EO or CO crude extracts by GC-MS were used. All samples and stock solutions were prepared in dimethyl sulphoxide (DMSO), and were stored at −20 °C, and consequently each experiment included a solvent control [0.2% (v/v) DMSO prepared in exposure medium]. After the incubation period, the medium was removed and a solution of 0.5 mg per mL MTT prepared in Krebs medium (140 mM NaCl, 5 mM KCl, 1 mM NaH2PO4, 1 mM MgCl2, 9.6 mM glucose, 20 mM HEPES, 1.5 mM CaCl2, pH 7.4) was added. The cells were incubated with this solution, protected from the light, for 30 min at 37 °C, the MTT solution was aspirated, and DMSO was added to dissolve the formazan crystals. The absorbance was measured, after 10 min of shaking, at 570 nm, using a SpectraMax Plus 384 spectrophotometer (Molecular Devices, San Jose, CA, USA). The results of at least three independent experiments carried out in triplicate were expressed as a percentage (%) of the absorbance value obtained in the control, which was considered 100%, and were graphically represented as the percentage of cell viability versus the concentration of samples.Measurement of nitric oxide production
RAW 264.7 cells were seeded in 96-well plates at the same density of cell viability experiments, and were allowed to stabilize for 24 h. Then, the cells were incubated in exposure medium (control) or exposed to 1 μg mL−1 lipopolysaccharide (LPS), in the absence or in the presence of 0.04 mg per mL EO or EO crude extract, 0.08 mg per mL CO or CO crude extract or the corresponding mixture of TTAs, for 24 h. The nitric oxide (NO) production was evaluated by measuring its stable secreted metabolite nitrite. For that, equal volumes of the Griess reagent [0.1% (w/v) N-(1-naphthyl)-ethylenediamine dihydrochloride and 1% (w/v) sulfanilamide containing 5% (w/v) H3PO4] were added to the cells' supernatants, and were incubated protected from light at room temperature for 30 min. The absorbance was measured at 550 nm with a SpectraMax Plus 384 spectrophotometer, using the culture medium as a blank. The results of at least three independent experiments made in triplicate were expressed as the nitrite production (μM) by the cells.Statistical analysis
The results are represented as the mean ± standard error of the mean of the given number of experiments. The D'Agostino and Pearson and Shapiro–Wilk normality tests were used to evaluate the normality of the data distribution. Statistical comparisons between groups were performed by one-way analysis of variance (ANOVA) followed by Dunnett's and Sidak's multiple comparison tests. Significance was accepted at p values < 0.05. The statistical analysis was made using GraphPad Prism software (8.0.2, GraphPad Software Inc., San Diego, CA, USA).Results and discussion
Triterpenic acids content of E. globulus hydrodistilled leaves
EgHDL were first submitted to a Soxhlet extraction using DCM in order to evaluate their TTAs composition for comparison purposes, since DCM is widely used in the characterization of lipophilic fraction, including triterpenic compounds, of forest biomass.13,44,45 The identification and quantification of the main TTAs, namely BoA, OA, BA and UA, were performed by GC-MS analysis using pure UA as a reference for retention time and quantification (Table 1). As previously reported,13,14 lipophilic extracts from E. globulus leaves are mainly composed of TTAs, followed by smaller amounts of mono- and sesquiterpenic compounds, fatty acids, long-chain aliphatic alcohols, and sterols (namely β-sitosterol), not reported here as they are not the main focus of the study. In addition, it was already verified that hydrodistillation does not affect the TTAs content of E. globulus leaves.46| Soxhlet DCM extract | Essential oil crude extract | 1,8-Cineole crude extract | |
|---|---|---|---|
| Betulonic acid (BoA) | 8.41 | 2.65 | 2.34 |
| Oleanolic acid (OA) | 7.57 | 6.04 | 5.71 |
| Betulinic acid (BA) | 1.70 | 1.39 | 1.42 |
| Ursolic acid (UA) | 27.01 | 18.29 | 17.93 |
| Total | 44.69 | 28.36 | 27.41 |
The EgHDL extract resulting from the Soxhlet DCM extraction showed a total TTAs content of 44.7 g kg−1 dw (Table 1). These values are higher than those reported by Rodrigues et al.13 for the extraction of eucalyptus leaves with DCM (values ranging from 7.5 to 12.4 g kg−1 dw of E. globulus leaves), but lower than the values presented in the same study for the Soxhlet extraction of eucalyptus leaves with ethanol and methanol (54.2 and 75.5 g kg−1 dw of E. globulus leaves, respectively).13 Although the differences between DCM extracts might partially result from the different extraction and edaphoclimatic conditions of the samples used,13 the most probable cause for the higher extraction yield in the present work is the impact of the hydrodistillation process, through the disruption of the leaves' cellular structures, that might facilitate the extractability of TTAs.
In the present study, UA was the major TTA in the DCM extract, with a content of 27.0 g kg−1 dw of EgHDL, followed by BoA with a content of 8.4 g kg−1 dw, OA with 7.6 g kg−1 dw and lastly BA which accounted for 1.7 g kg−1 dw.
The order of abundance of each TTA in the DCM extract (UA > BoA > OA > BA) is not fully in agreement with the amounts of each TTA obtained by the DCM Soxhlet extraction by Rodrigues et al.13 where the OA content was higher than the BoA content (UA > OA > BoA > BA). In the same extract,13 the UA content ranged from 4.8 to 7.8 g kg−1 dw of E. globulus leaves for Soxhlet extractions with DCM and 39.6 and 53.7 g kg−1 dw for Soxhlet extractions with ethanol and methanol, respectively; meanwhile, the OA content ranged from 1.5 to 3.8 g kg−1 dw for Soxhlet extractions with DCM and to 11.1 and 15.8 g kg−1 dw for ethanol and methanol Soxhlet extractions, respectively, followed by BoA with content ranging from 0.6 to 1.0, 2.6 and 3.9 g kg−1 dw for DCM, ethanol and methanol Soxhlet extractions, respectively, and lastly BA with content ranging from 0.2 to 0.5, 0.8 and 2.1 g kg−1 dw for DCM, ethanol and methanol Soxhlet extractions, respectively.
Extraction of TTAs with essential oil (EO) and 1,8-cineole (CO)
The extraction of TTAs from EgHDL using EO and CO resulted in crude EO and CO extracts. In terms of chemical composition, these extracts are comparable to the DCM extract. The EO and CO crude extracts show similar profiles in the amount of TTAs extracted with a total TTAs content of 28.4 and 27.4 g kg−1 dw respectively, yet lower than the yield obtained with DCM (Table 1). Since no significant differences were observed in the extraction of TTAs between EO and CO crude extracts, the extraction efficiency is expected to be mainly governed by CO, the major component of E. globulus EO. In this situation and considering only the extraction yield aspect the best choice would be to use the EO as extraction media, provided that the cost of purifying CO would be eliminated.With the EO and CO as bio-based solvents, the extracts presented relatively lower TTAs contents compared to the DCM extract, however UA remained the most abundant TTA and BA the least (UA > OA > BoA > BA). The CO crude extract showed a UA content of 17.9 g kg−1 dw while the EO crude extract presented 18.3 g kg−1 dw of EgHDL. In this case, both CO and EO solvents were more efficient in extracting OA, with a content of 5.7 and 6.0 g kg−1 dw of EgHDL, respectively, than in extracting BoA which presented a lower content (2.3 and 2.6 g kg−1 dw of EgHDL, respectively). BA was the least abundant TTA in all extracts, with a content of 1.4 g kg−1 dw of EgHDL for both the EO and CO crude extracts.
In the literature only a single alternative methodology has been reported to extract TTAs from E. globulus leaves, namely supercritical CO2 extraction, which has a cost disadvantage. However considerably lower contents of these compounds were observed (1.1–4.3 g kg−1 dw of E. globulus leaves) with these technique13 than those obtained with EO or CO, which clearly emphasizes the advantage of the current approach. In addition, the relative contents of each TTA in EO and CO crude extracts are considerably different from those obtained with supercritical CO2 extraction. Extraction with EO and CO not only led to vastly higher contents of TTAs to be extracted but also allowed obtainment of extracts enriched in UA, followed by OA, in contrast to supercritical CO2 extracts, which presented quite similar contents between the different TTAs, namely BoA (ranging from 0.63 to 1.89 g kg−1 dw of E. globulus leaves), OA (0.18–1.51 g kg−1 dw of E. globulus leaves), UA (0.20–0.41 g kg−1 dw of E. globulus leaves) and BA (0.06–0.47 g kg−1 dw of E. globulus leaves).13 Considering the higher number of biological activities and health benefits reported for UA and OA,3 compared to BoA and BA, it is clear that CO and in particular EO have the potential to be used as bio-based solvents of extraction to valorize E. globulus leaves in pharmaceutical, cosmetic and food applications.
The use of alternative solvents in the extraction of TTAs from E. globulus has only been investigated for the bark. Silva et al.22 firstly investigated the solubility of UA in different bio-based solvents (e.g., limonene, menthol, thymol, γ-valerolactone and α-pinene), with the eutectic solvent thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (1
menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) being selected to proceed with the extraction of TTAs from the E. globulus bark. Despite the promising extraction yields for thymol
2) being selected to proceed with the extraction of TTAs from the E. globulus bark. Despite the promising extraction yields for thymol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) menthol (1
menthol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the approach proposed in the present study is more effective and attractive as it allows researchers to rely, not on pure compounds, but on an EO which additionally can be obtained in an integrated way from the same plant source and easily recovered at the end. Furthermore, the intense odor of both thymol and menthol would represent an additional limitation for the use of the latter.
2), the approach proposed in the present study is more effective and attractive as it allows researchers to rely, not on pure compounds, but on an EO which additionally can be obtained in an integrated way from the same plant source and easily recovered at the end. Furthermore, the intense odor of both thymol and menthol would represent an additional limitation for the use of the latter.
Regarding the biological activities described both for the EO, where CO is one of the major components, and for the TTAs, it is pertinent to evaluate the potential synergistic effect between EO or CO and TTAs and to understand whether it might be feasible to use the crude extracts instead of purified ones, thus avoiding a separation process of EO/CO from TTAs.
Macrophages' viability in the presence of crude extracts and mixture of TTAs
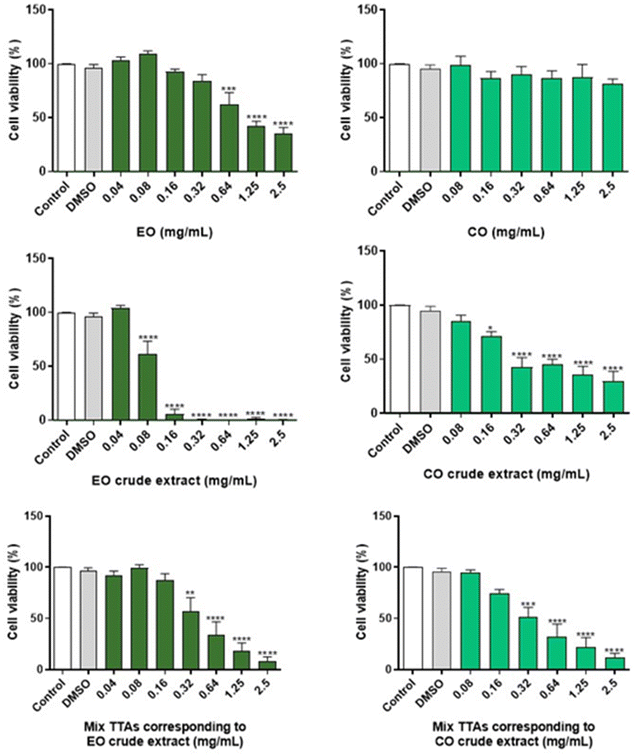
In order to assess the safety of EO and CO crude extracts, the cell viability of cultured macrophages was evaluated by the MTT assay (Fig. 2). For comparison purposes, the cell viability of a mixture of TTAs prepared in the same proportions as present in the crude extracts was evaluated. The cell viability in the presence of EO and pure CO was also studied to assess the interference of the extraction solvent in the toxicity of the extract. Regarding the EO crude extract, the absence of cytotoxicity was observed after a 24 h treatment at concentrations below 0.04 mg mL−1, while for the corresponding mixture of TTAs and EO (extraction solvent) a similar result was detected below 0.16 mg mL−1 and 0.32 g mL−1, respectively, as cell viability was preserved. In the case of the CO crude extract, non-toxic effects were observed at 24 h for concentrations below 0.08 mg mL−1, and similar results were obtained for concentrations of the corresponding mixture of TTAs below 0.16 mg mL−1, and all of the tested concentrations of CO were free of toxicity.The safe concentrations of EO and CO crude extracts were revealed in innate immune cells (macrophages). In this study we tested the cytotoxicity of the solvents used to perform the extraction of the crude extracts, and effectively low toxicity of EO (below 0.32 mg mL−1) and CO (below 2.5 mg mL−1) was found, with EO exhibiting higher toxicity than CO. These results are in accordance with previous results showing that 0.3–0.5 mg per mL EO did not significantly reduce the viability of macrophages.42,47 CO did not adversely affect the viability of macrophages below 2.5 mg mL−1 concentration, although previous results demonstrated its safety in macrophages between 0.2 and 0.5 mg mL−1.48,49 Moreover, the EO crude extract, non-toxic at concentrations less than 0.04 mg mL−1, demonstrated higher toxicity than the CO crude extract, non-toxic at concentrations below 0.08 mg mL−1. In addition, the corresponding mixture of TTAs of the EO crude extract revealed lower toxicity than the crude extract, namely safe concentrations below 0.16 mg mL−1 were identified for the EO crude extract, which can be a result of EO toxicity that was used as extraction solvent. In the case of the mixture of TTAs corresponding to the CO crude extract, no differences in terms of toxicity were observed when compared with the CO crude extract. The mixture of TTAs is composed of BoA, OA, BA and UA, which are devoid of toxicity in macrophages taking into consideration the literature reports.50–52 In conclusion, this study revealed concentrations of EO and CO crude extracts that are not deleterious to macrophages, providing support to evaluate their biological activities.
Anti-inflammatory activity
The anti-inflammatory effect of the EO and CO crude extracts was evaluated by determining their ability to inhibit the production of the pro-inflammatory mediator NO in LPS-stimulated macrophages. The results obtained demonstrated that 0.04 mg per mL EO crude extract and 0.08 mg per mL CO crude extract decrease NO levels by 25.95% and 35.07% in LPS-treated cells, respectively, in comparison with cells exposed to LPS alone (Fig. 3). The anti-inflammatory effect was higher than that of the respective mixture of TTAs, which reduced the NO production by 18.31% and 24.59% (Mix EO + LPS and Mix CO + LPS, respectively). On the other hand, the same concentration of the corresponding extraction solvents did not demonstrate any effect.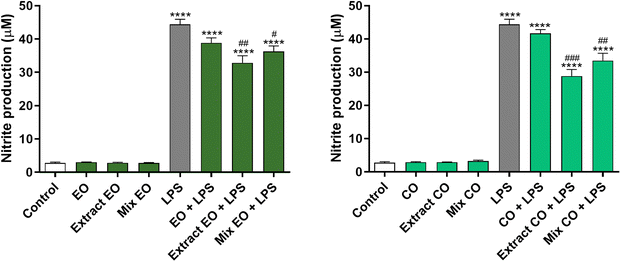 | ||
| Fig. 3 Effect of EO and CO crude extracts on nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. | ||
Several in vitro and in vivo studies support the view that EO possesses potent anti-inflammatory activity.42,47,53 These studies attributed the EO's anti-inflammatory activity to the presence of CO, which is a strong suppressor of cytokine release.54 In accordance, there is also information in the literature reporting BoA, OA, BA and UA as anti-inflammatory agents.55,56
Taken together these results show the strong anti-inflammatory effect of the EO and CO crude extracts, which is higher than that of the pure compounds, suggesting their potential beneficial effect for several conditions associated with deregulated responses to inflammation.
Conclusions
The need to extract value-added compounds points to the importance of a continuous search for economically and ecologically viable extraction technologies. This work demonstrated the potential of an integrated extraction process that maximizes the use of a renewable raw material, E. globulus leaves. In this vein, EO obtained from E. globulus leaves by hydrodistillation was for the first time used as a bio-based solvent in the subsequent extraction of TTAs from hydrodistilled leaves. UA, OA, BoA and BA were successfully extracted with EO and, for comparison purposes, with its major component, CO. No significant differences in the yields obtained with EO and CO were observed, and so EO can be used advantageously (no energy consumption in the purification steps to obtain pure CO).The cytotoxicity assays on cells of the innate immune system (macrophages) showed that the non-toxic concentrations of the EO and CO crude extracts are less than or equal to 0.04 and 0.08 mg mL−1, respectively. Additionally, the CO crude extract showed higher anti-inflammatory activity than the EO crude extract and lastly, these extracts exhibited higher activity than their respective mixture of TTAs, which is indicative of the potential beneficial synergistic effects of EO and CO with TTAs.
Still, if required EO or CO could be removed from the crude extracts, by hydrodistillation, allowing the recycling of the bio-based solvents, or their routing into other applications, preserving the potential of the TTAs extracts. Finally, from an integrated process perspective, it is important to mention that after extraction of TTAs with EO or CO, the remaining biomass can then be routed to a subsequent extraction step with more polar solvent to recover other value-added components, such as phenolic compounds.
With this study, we expect to contribute to the modernization of current biorefineries, leading to a fully integrated biorefinery model. However, it is important to note that for the implementation of this process it will still be necessary to optimize the process, preferably at a pilot scale, and perform an evaluation of the technical and economic aspects, and not least, of the environmental impact of this process.
Author contributions
Cátia S. D. Oliveira and Patrícia Moreira were responsible for the methodology, investigation, formal analysis and writing of the original draft. Cláudia M. F. Pereira, Sónia A. O. Santos and Armando J. D. Silvestre were responsible for the conceptualization. Cláudia M. F. Pereira, Maria T. Cruz, Alexandre Gaspar, Carlos P. Neto, Paula C. R. O. Pinto, Pedro C. Branco, Sónia A. O. Santos and Armando J. D. Silvestre were responsible for the design of the work. Cláudia M. F. Pereira, Maria T. Cruz, Artur M. S. Silva, Sónia A. O. Santos and Armando J. D. Silvestre supervised the work. The manuscript was written through contributions of all authors. All authors have read, reviewed and given approval to the final published version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work has one Portuguese (20222004117200) and one European (EP22020513.2) patent pending. This work was carried out under the Project InPacTus – Innovative products and technologies from eucalyptus, Project No. 21874 (POCI-01-0247-FEDER-021874), funded by Portugal 2020 through the European Regional Development Fund (ERDF) in the frame of COMPETE 2020 no. 246/AXIS II/2017, and projects CICECO–Aveiro Institute of Materials (UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020), LAQV-REQUIMTE (UIDB/50006/2020 & UIDP/50006/2020) and CIBB (UIDB/04539/2020 & UIDP/04539/2020), financed by national funds through the FCT/MEC (PIDDAC). FCT is also acknowledged for the research contract under Scientific Employment Stimulus to S. Santos (2021.03348.CEECIND).References
- J. J. W. Coppen, Eucalyptus: The genus Eucalyptus, Taylor & Francis, London, 2002 Search PubMed.
- M. I. H. Brooker and D. Kleinig, Field Guide to Eucalypts, Bloomings Books Pty Ltd, Melbourne, South Eastern Australia, Australia, 3rd edn, 2006, vol. 1 Search PubMed.
- R. Domingues, A. Guerra, M. Duarte, C. Freire, C. Neto, C. Silva and A. Silvestre, Mini-Rev. Org. Chem., 2014, 11, 382–399 CrossRef CAS.
- V. H. Rodrigues, M. M. R. de Melo, I. Portugal and C. M. Silva, Sep. Purif. Technol., 2018, 191, 207–213 CrossRef.
- B. Gullón, A. Muñiz-Mouro, T. A. Lú-Chau, M. T. Moreira, J. M. Lema and G. Eibes, Ind. Crops Prod., 2019, 138, 111473 CrossRef.
- T. Silva-Fernandes, L. C. Duarte, F. Carvalheiro, S. Marques, M. C. Loureiro-Dias, C. Fonseca and F. Gírio, Bioresour. Technol., 2015, 183, 203–212 CrossRef CAS PubMed.
- C. S. R. Freire, A. J. D. Silvestre, C. P. Neto and J. A. S. Cavaleiro, Holzforschung, 2002, 56, 372–379 CAS.
- D. M. Neiva, S. Araújo, J. Gominho, A. C. de Carneiro and H. Pereira, Ind. Crops Prod., 2018, 123, 262–270 CrossRef CAS.
- R. M. A. Domingues, D. S. J. Patinha, G. D. A. Sousa, J. Villaverde, C. M. Silva, C. S. R. Freire, A. J. D. Silvestre and C. P. Neto, Cellul. Chem. Technol., 2011, 45, 475–481 CAS.
- L. C. A. Barbosa, C. A. Filomeno and R. R. Teixeira, Molecules, 2016, 21, 1671 CrossRef PubMed.
- A. J. D. Silvestre, J. S. Cavaleiro, B. Delmond, C. Filliatre and G. Bourgeois, Ind. Crops Prod., 1997, 6, 27–33 CrossRef CAS.
- Â. Luís, A. Duarte, J. Gominho, F. Domingues and A. P. Duarte, Ind. Crops Prod., 2016, 79, 274–282 CrossRef.
- V. H. Rodrigues, M. M. R. de Melo, I. Portugal and C. M. Silva, J. Supercrit. Fluids, 2018, 135, 263–274 CrossRef CAS.
- R. M. A. Domingues, G. D. A. Sousa, C. S. R. Freire, A. J. D. Silvestre and C. P. Neto, Ind. Crops Prod., 2010, 31, 65–70 CrossRef CAS.
- C. Chinnarasu, A. Montes, M. T. Fernandez-Ponce, L. Casas, C. Mantell, C. Pereyra, E. J. M. de la Ossa and S. Pattabhi, J. Supercrit. Fluids, 2015, 101, 161–169 CrossRef CAS.
- S. H. Nile and Y. S. Keum, Indian J. Exp. Biol., 2018, 56, 734–742 CAS.
- L. Harkat-Madouri, B. Asma, K. Madani, Z. Bey-Ould Si Said, P. Rigou, D. Grenier, H. Allalou, H. Remini, A. Adjaoud and L. Boulekbache-Makhlouf, Ind. Crops Prod., 2015, 78, 148–153 CrossRef CAS.
- R. G. Bachir and M. Benali, Asian Pac. J. Trop. Biomed., 2012, 2, 739–742 CrossRef PubMed.
- V. Pereira, C. Dias, M. C. Vasconcelos, E. Rosa and M. J. Saavedra, Ind. Crops Prod., 2014, 52, 1–7 CrossRef CAS.
- G. R. Vilela, G. S. de Almeida, M. A. B. R. D'Arce, M. H. D. Moraes, J. O. Brito, M. F. das G. F. da Silva, S. C. Silva, S. M. de Stefano Piedade, M. A. Calori-Domingues and E. M. da Gloria, J. Stored Prod. Res., 2009, 45, 108–111 CrossRef CAS.
- N. Salem, S. Kefi, O. Tabben, A. Ayed, S. Jallouli, N. Feres, M. Hammami, S. Khammassi, I. Hrigua, S. Nefisi, A. Sghaier, F. Limam and S. Elkahoui, Ind. Crops Prod., 2018, 124, 115–125 CrossRef CAS.
- N. H. C. S. Silva, E. S. Morais, C. S. R. Freire, M. G. Freire and A. J. D. Silvestre, Molecules, 2020, 25, 210 CrossRef CAS PubMed.
- R. M. A. Domingues, G. D. A. Sousa, C. M. Silva, C. S. R. Freire, A. J. D. Silvestre and C. P. Neto, Ind. Crops Prod., 2011, 33, 158–164 CrossRef CAS.
- L. Bennie, J. Coetzee, E. Malan, D. Slade, J. P. J. Marais and D. Ferreira, Phytochemistry, 2004, 65, 215–220 CrossRef CAS PubMed.
- R. M. A. Domingues, M. M. R. De Melo, E. L. G. Oliveira, C. P. Neto, A. J. D. Silvestre and C. M. Silva, J. Supercrit. Fluids, 2013, 74, 105–114 CAS.
- I. D. Febriana, H. S. Kusuma, S. Galan and M. Mahfud, ASEAN J. Chem. Eng., 2016, 16, 45 CrossRef.
- H. S. Kusuma and M. Mahfud, in AIP Conference Proceedings, American Institute of Physics Inc., 2016, vol. 1755, p. 050001 Search PubMed.
- D. M. Neiva, Â. Luís, J. Gominho, F. Domingues, A. P. Duarte and H. Pereira, Wood Sci. Technol., 2020, 1–27 Search PubMed.
- V. Brezáni, V. Leláková, S. Hassan, K. Berchová-Bímová, P. Nový, P. Klouček, P. Maršík, S. Dall'Acqua, J. Hošek and K. Šmejkal, Viruses, 2018, 10, 360 CrossRef PubMed.
- P. Kaur, R. C. Gupta, A. Dey and D. Kumar Pandey, Ind. Crops Prod., 2019, 130, 537–546 CrossRef CAS.
- Â. Luís, D. Neiva, H. Pereira, J. Gominho, F. Domingues, A. Duarte, Â. Luís, D. Neiva, H. Pereira, J. Gominho, F. Domingues and A. P. Duarte, Molecules, 2014, 19, 16428–16446 CrossRef PubMed.
- L. Novotny, M. E. Abdel-Hamid, H. Hamza, I. Masterova and D. Grancai, J. Pharm. Biomed. Anal., 2003, 31, 961–968 CrossRef CAS PubMed.
- M. Vian, C. Breil, L. Vernes, E. Chaabani and F. Chemat, Curr. Opin. Green Sustain. Chem., 2017, 5, 44–48 CrossRef.
- F. Chemat, M. A. Vian and G. Cravotto, Int. J. Mol. Sci., 2012, 13, 8615–8627 CrossRef CAS PubMed.
- M. Cvjetko Bubalo, S. Vidović, I. Radojčić Redovniković and S. Jokić, J. Chem. Technol. Biotechnol., 2015, 90, 1631–1639 CrossRef CAS.
- S. Armenta, F. A. Esteve-Turrillas, S. Garrigues and M. de la Guardia, Green Anal. Chem., 2022, 1, 100007 CrossRef.
- A. Khoddami, M. Wilkes, T. Roberts, A. Khoddami, M. A. Wilkes and T. H. Roberts, Molecules, 2013, 18, 2328–2375 CrossRef CAS PubMed.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS.
- S. Hamiche, N. Bouzidi, Y. Daghbouche, A. Badis, S. Garrigues, M. de la Guardia and M. El Hattab, Sustain. Chem. Pharm., 2018, 10, 97–102 CrossRef.
- C. Dejoye Tanzi, M. Abert Vian, C. Ginies, M. Elmaataoui and F. Chemat, Molecules, 2012, 17, 8196–8205 CrossRef PubMed.
- M. Aissou, Z. Chemat-Djenni, E. Yara-Varón, A. S. Fabiano-Tixier and F. Chemat, Comptes Rendus Chim., 2017, 20, 346–358 CrossRef CAS.
- P. Moreira, F. J. Sousa, P. Matos, G. S. Brites, M. J. Gonçalves, C. Cavaleiro, A. Figueirinha, L. Salgueiro, M. T. Batista, P. C. Branco, M. T. Cruz and C. F. Pereira, Pharm, 2022, 14, 561 CAS.
- S. A. O. Santos, C. S. D. Oliveira, S. S. Trindade, M. H. Abreu, S. S. M. Rocha and A. J. D. Silvestre, J. Appl. Phycol., 2016, 28, 3151–3158 CrossRef CAS.
- D. J. S. Patinha, R. M. A. Domingues, J. J. Villaverde, A. M. S. Silva, C. M. Silva, C. S. R. Freire, C. P. Neto and A. J. D. Silvestre, Ind. Crops Prod., 2013, 43, 340–348 CrossRef CAS.
- S. A. O. Santos, C. Vilela, R. M. A. Domingues, C. S. D. Oliveira, J. J. Villaverde, C. S. R. Freire, C. P. Neto and A. J. D. Silvestre, Ind. Crops Prod., 2017, 95, 357–364 CrossRef CAS.
- C. S. D. Oliveira, P. Moreira, M. T. Cruz, C. M. F. Pereira, A. M. S. Silva, S. A. O. Santos and A. J. D. Silvestre, Int. J. Mol. Sci., 2023, 24, 6226 CrossRef CAS PubMed.
- S. Aazza, B. Lyoussi, C. Megías, I. Cortés-Giraldo, J. Vioque, A. C. Figueiredo and M. G. Miguel, Nat. Prod. Commun., 2014, 9, 587–594 CrossRef CAS PubMed.
- R. C. Santana, A. D. S. Rosa, M. H. S. da Mateus, D. C. Soares, G. Atella, A. C. Guimarães, A. C. Siani, M. F. S. Ramos, E. M. Saraiva and L. H. Pinto-da-Silva, J. Ethnopharmacol., 2020, 259, 112981 CrossRef CAS PubMed.
- M. F. Zaccaro Scelza, L. R. Lima Oliveira, F. B. Carvalho and S. Côrte-Real Faria, Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol., 2006, 102, e24–e27 CrossRef PubMed.
- K.-S. Kim, D.-S. Lee, D.-C. Kim, C.-S. Yoon, W. Ko, H. Oh and Y.-C. Kim, Molecules, 2016, 21, 1206 CrossRef PubMed.
- Y. Han, C. Yuan, X. Zhou, Y. Han, Y. He, J. Ouyang, W. Zhou, Z. Wang, H. Wang and G. Li, Int. J. Mol. Sci., 2021, 22, 12009 CrossRef CAS PubMed.
- M.-H. Kim, J. N. Kim, S. N. Han and H.-K. Kim, Immunopharmacol. Immunotoxicol., 2015, 37, 228–235 CrossRef CAS PubMed.
- T.-C. Lin, Chin. J. Physiol., 2018, 61, 257–265 CrossRef CAS PubMed.
- U. R. Juergens, M. Stöber and H. Vetter, Eur. J. Med. Res., 1998, 3, 508–510 CAS.
- D. Kashyap, A. Sharma, H. S. Tuli, S. Punia and A. K. Sharma, Recent Pat. Inflammation Allergy Drug Discovery, 2016, 10, 21–33 CrossRef CAS.
- H. Lou, H. Li, S. Zhang, H. Lu and Q. Chen, Molecules, 2021, 26, 5583 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |