Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in aza Friedel–Crafts reaction: strategies for achiral and stereoselective synthesis
Ameni
Hadj Mohamed
 and
Nicolas
Masurier
and
Nicolas
Masurier

Institut des Biomolécules Max Mousseron, UMR 5247, CNRS, Université de Montpellier, ENSCM, UFR des Sciences Pharmaceutiques et Biologiques, 34293 Montpellier, France. E-mail: nicolas.masurier@umontpellier.fr
First published on 28th February 2023
Abstract
The aza-Friedel–Crafts (aza-FC) reaction is a very powerful tool for forming C–C and C–N bonds, based on an acid-catalyzed addition of electron-rich aromatic compounds to imines. This reaction has been particularly useful for synthesizing polysubstituted amines bearing various aromatic groups, as this structural motif is present in many biologically active and pharmacologically relevant compounds. Through representative examples, recent improvements achieved on the aza-FC reaction are presented, classified according to the type of catalyst used. Particular emphasis has been put on the different catalysts used to obtain optically active compounds, as the stereoselective addition of a C(sp2)–H bond to an imine allows access to chiral amines in a completely atom-economical way.
1. Introduction
The aza Friedel–Crafts (aza-FC) reaction is a very powerful tool to form C–C and C–N bonds based on acid-catalysed addition of electron-rich aromatic compounds to imines.1–3 Therefore, this reaction involves the use of an amino compound (primary or secondary amine, sulfonamide, primary amide, (thio)urea, etc.), an aldehyde or a ketone and an electron-rich aromatic compound.4 This reaction allows access to polysubstituted amines bearing various aromatic groups, such structural motifs being found in many biologically active and pharmacologically relevant compounds.3,5 The importance and efficiency of aza-FC reactions in organic and medicinal chemistry has pushed researchers to perform a tremendous amount of work, seeking to improve their operating conditions in order to decrease toxic waste and to generate the desired product in the highest yields.Moreover, the stereoselective addition of an sp2 C–H bond to an imine allows access to optically active amines in a completely atom-economical way, making this reaction an efficient way to access complex chiral molecules. Therefore, controlling and increasing the stereoselectivity of the reaction has become a major concern of many research groups. Thus, a wide variety of catalytic systems have been studied, such as Lewis and Brønsted acids, and chiral organic and organometallic catalysts, with the main objective of accessing polysubstituted amines with excellent enantioselectivity and regioselectivity.6–8
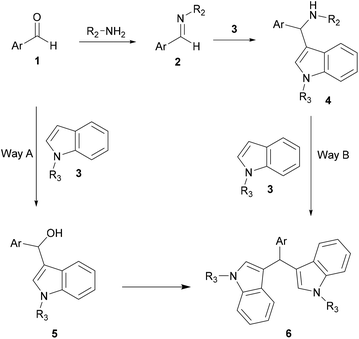
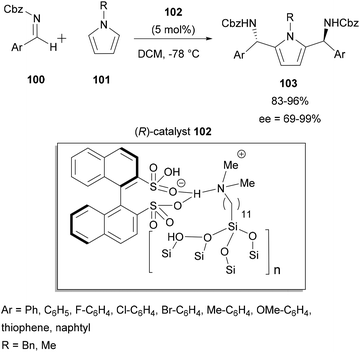
Two types of aza-FC reaction could be considered: intermolecular and intramolecular reactions. In the intermolecular type, electron-rich compounds such as naphthol,9–12 indole4 and pyrrole13,14 are commonly used as aza-FC donors and could react with a variety of imines. Regarding the intramolecular aza-FC reaction, they have been extensively investigated for years.15–19 Seminal work describes several types of intramolecular synthesis of tetrahydro-β-carbolines, from tryptamines and various aldehydes or ketones (known as the Pictet Spengler reaction), and this reaction will not be discussed here.20–27
In this current review, we present an overview of the most relevant works and the improvement achieved in the aza-FC reaction, classified in the following sections according to the type of catalyst used. As this reaction led to the formation of a new chiral center, a particular focus will be on the different catalysts used to obtain optically active compounds.
2. Achiral aza Friedel–Crafts reactions
2.1. Brønsted acid catalyzed aza Friedel–Crafts reactions
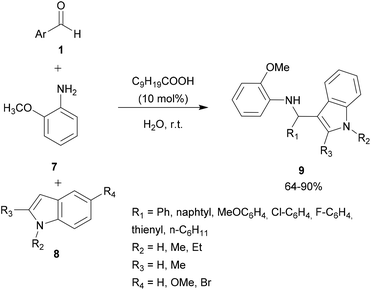
Acid assistance protons are quite versatile catalysts of organic reactions and several Brønsted acids have been reported to efficiently catalyze aza FC reactions, involving a large variety of electron-rich compounds and imines.In an attempt to solve these shortcomings, Shirakawa and Kobayashi undertook the search for a suitable carboxylic acid as a catalyst that avoids the formation of the unwanted bis-indolyl product 6.28 Thus, several carboxylic acids were tested at 5 mol%. The results showed that in the presence of TFA and AcOH, no reaction took place. However, using scandium tris(dodecyl sulfate) and dodecylbenzenesulfonic acid, both the desired product 4 and the undesired product 6 were obtained. Other carboxylic acids containing different lengths of alkyl chains were also studied, showing that the length of the alkyl chains of the carboxylic acids was crucial for this reaction. Interestingly, decanoic acid (C9H19COOH) was found to be the most efficient catalyst, giving a good yield of the expected product without any formation of the unwanted product 6. Optimization of the catalytic amount of the catalyst concluded that 10 mol% afforded the best yield of the expected product, which was recovered by recrystallization. Indeed, it is noteworthy that this strategy constitutes an easy and simple approach for the development of 3-substituted indoles compounds 9 without any need for chromatographic purification (Scheme 2).
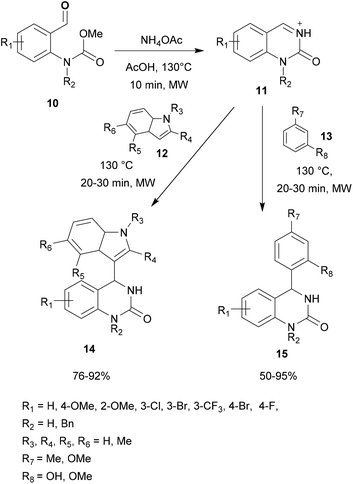
The applicability of the microwave-assisted aza-FC arylation of N-acyliminium ions was also investigated using substituted electron-rich phenyl derivatives 13. This study demonstrates that this protocol tolerated a wide range of arenes affording the corresponding desired products 15 with yields up to 95% (Scheme 3).
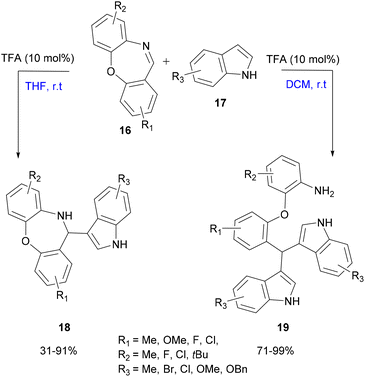 | ||
| Scheme 4 Selectivity difference depending on the solvent in the TFA-catalyzed aza-FC reaction of cyclic imine and indole. | ||
Structurally diverse indole derivatives and cyclic imines as the substrates provided the targeted compounds in high yields, using mild conditions, solvent-controlled efficient selectivity and easily accessible TFA (10 mol%) as a catalyst.35
2.2. Lewis acid catalyzed aza Friedel–Crafts reactions
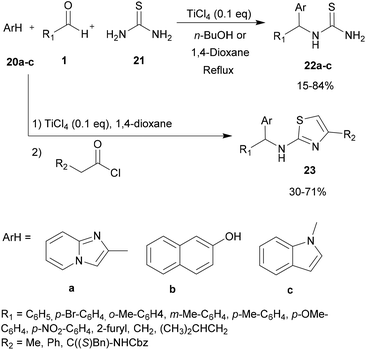
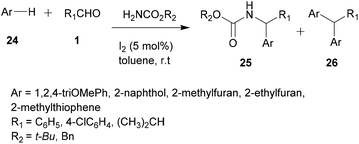
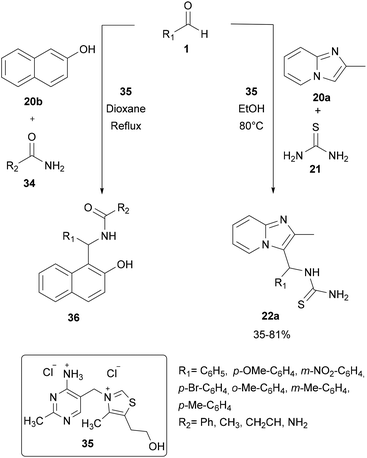
For a long time, transition metal-catalyzed multi-component reactions (MCRs) have attracted great interest because of their ability to achieve many difficult transformations using a one-pot method. In particular, metal catalysts which are derived from the VIII–X group show remarkable efficiency for the formation of carbon–carbon and carbon–heteroatom bonds. Specifically, transition metals have been shown to be effective and widely used, especially in aza-FC coupling.16The development of the experimental protocol involved several optimizations of the catalyst, the solvent, the reaction concentration and the temperature used. The aza-FC reaction was first optimized with 20a as the electron-rich aromatic ring. Several Brønsted and Lewis acids were tested and TiCl4 (0.1 eq.) in 1,4-dioxane or in n-butanol was shown to be the most efficient condition to access mono-substituted thiourea derivatives 22, in 15 to 84% yields, depending on the aldehyde used. These conditions proved to be compatible to further involve the monosubstituted thiourea in a one-pot tandem Hantzsch cyclization with an α-chloromethylketone, leading to the formation of the polysubstituted aminothiazole derivatives 23 in 30 to 71% isolated yields.
In order to study the scope of this reaction, a variety of substituted aliphatic and aromatic aldehydes, as well as several arenes and heteroarenes, were tested. Results showed that, using the conditions described above, the corresponding expected products 25 were recovered in good yields, up to 90% (Scheme 6). However, the reaction leads to a second final product 26 resulting from a double addition of the arene/heteroarene, which is still recovered in a minor quantity.
In 2006, Carretero and co-workers performed the development of symmetric and asymmetric diarylmethanes and triarylmethanes using the aza-FC reaction. In their works, the authors highlighted the use of copper-based catalysts and stated the influence of the source of the copper catalyst as well as the nature of the sulfonyl imines used on the proceeding of the reaction.43 Using phenyl-derived, thienyl or dimethylamine sulfonyl imines 27a–d, N-methylindole and Cu(OTf) as the catalyst, the aza-FC reaction proceeded smoothly and led selectively to the monosubstituted product 28, whereas the use of the more reactive Cu(OTf)2 led to bis-indole 6 (Scheme 7). Interestingly, when 2-pyridylsulfonyl imines 27e were used with indoles 29, both catalysts led only to the mono-Friedel–Crafts products 30 and no bis-indoles 6 were formed. The most electrophilic CuII system provided 30 in good yields (70–90%), with very short reaction times (5 to 25 min). Finally, the further addition of another electron-rich arene allowed the preparation of various triarylmethanes 31 in good yields (72–85%). However, it was observed that the one-pot reaction occurred only when Ar2 was a highly reactive indolyl derivative. To access more diversity, the second aromatic electrophilic substitution could be performed on isolated sulfonamides in the presence of Sc(OTf)3 (10 mol%) in ACN at 60 °C.
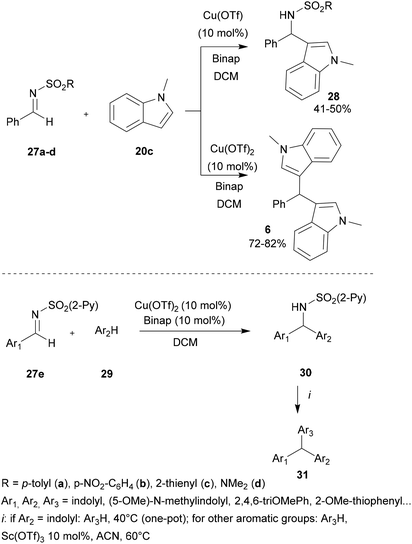 | ||
| Scheme 7 One-pot synthesis of unsymmetrical triarylmethanes through a copper-catalyzed aza-FC reaction. | ||
This study showed that the copper catalyst system Cu(OTf)2 applied in a sequential one-pot synthesis promotes a controlled double electrophilic aromatic substitution which provides access to unsymmetrical triarylmethanes with wide structural diversity and good yields (Scheme 7).
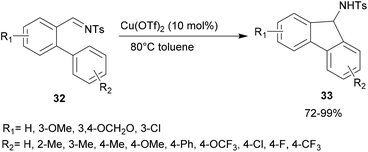
In 2011, Lu and co-workers studied a variety of Lewis acids to perform an intramolecular aza-FC reaction on O-arylated N-tosylbenzaldimines 32 (Scheme 8).44 Whereas palladium or iron complexes did not show any catalytic activity, the use of 10 mol% of Cu(OTf)2 as the catalyst, in toluene at 80 °C, was shown to be the most efficient condition for the intramolecular aza-FC reaction. The expected product 33 was obtained in high yield (Scheme 8). Interestingly, this reaction occurred smoothly with electron-donating or withdrawing groups at R2.
2.3. Organocatalysts for achiral aza Friedel–Crafts reactions
The use of thiamine·HCl 35 as a catalyst in 1,4-dioxane was also explored by Chaubet and co-workers in 2011.12 This catalyst proved to be efficient as an alternative of titanium tetrachloride (see part 2.2.1) to promote the aza-FC reaction between thiourea, an aromatic aldehyde and imidazo[1,2-a]pyridine 20a. This process allows the generation of the expected mono-substituted thiourea 22a with moderate to good yields (Scheme 9). However, some aldehydes such as 4-nitrobenzaldehyde or isovaleraldehyde were unreactive under these conditions, while titanium tetrachloride led to the desired product, suggesting that this catalyst is less effective than Lewis acids to catalyse aza-FC reactions.
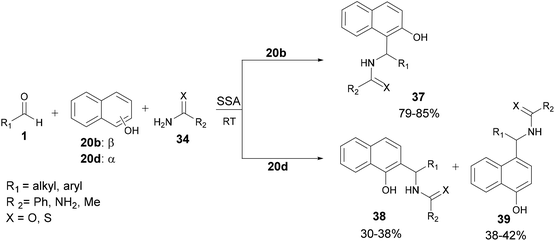 | ||
| Scheme 10 Aza-FC reaction between aldehydes, α-or β-naphthols, and (thio)amides (thio)urea under silica supported sulfuric acid (SSA) catalysis. | ||
Unlike some other catalytic systems, this strategy proves effective even with aliphatic aldehydes and appears to be suitable for large-scale operations as well.
3. Stereoselective aza Friedel–Crafts reactions
3.1. Brønsted acid-catalyzed aza-FC reactions
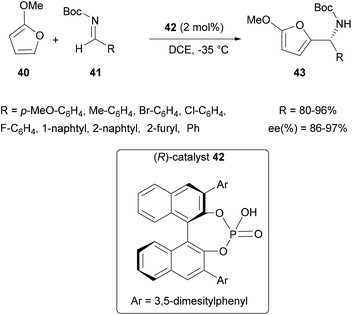
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 using 2-methoxyfuran 40 with N-Boc aldimines 41 and 2 mol% of the chiral phosphoric acid 42 (Scheme 11). This synthesis has been shown to be effective, even on substituted aldimines containing various alkylated and halogenated aromatic groups, with good yields and high ee (up to 97%). Since this first report, many teams have developed such catalysts to access chiral amines, using the aza-FC reaction. These works have been the subject of excellent reviews,3,58 and we only report the most recent developments in this field.
2 using 2-methoxyfuran 40 with N-Boc aldimines 41 and 2 mol% of the chiral phosphoric acid 42 (Scheme 11). This synthesis has been shown to be effective, even on substituted aldimines containing various alkylated and halogenated aromatic groups, with good yields and high ee (up to 97%). Since this first report, many teams have developed such catalysts to access chiral amines, using the aza-FC reaction. These works have been the subject of excellent reviews,3,58 and we only report the most recent developments in this field.
In 2009, Nakamura's team reported the enantioselective organocatalytic aza-FC reaction of unprotected pyrrole 45 with imines containing an heteroaryl-sulfonyl group 44 (Scheme 12A).59 Interestingly, the imine bearing a 2-pyridinylsulfonyl group has two points of coordination with the catalyst, which can effectively enhance the reactivity and enantioselectivity of the reaction by controlling the transition states or intermediates by chelation (Scheme 12B). The catalyst 46 employed was a monophosphoric binaphthol which led to high enantioselectivity up to 95%.
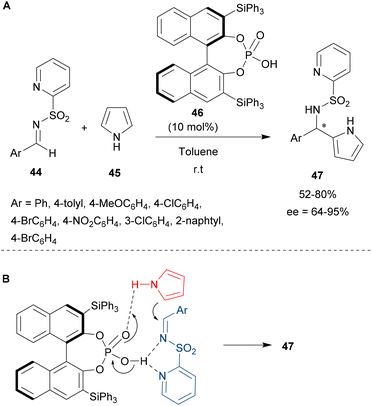 | ||
| Scheme 12 Enantioselective aza-FC reaction of N-(2-pyridinylsulfonyl) imines with unprotected pyrrole. Scope of the reaction (A) and assumed transition state of the reaction (B). | ||
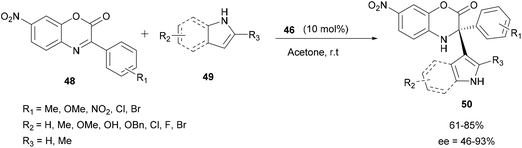
In 2017, Zhao and co-workers described the enantioselective reaction of aza-FC of indoles with hindered cyclic aryl α-ketimino esters 48 catalyzed by the same chiral silylated phosphoric acid 46, to access chiral α,α-disubstituted derivatives of unnatural α-amino esters (Scheme 13).60 Despite the steric hindrance of the imine used, this reaction carried out in acetone at room temperature efficiently gave access to the corresponding products 50 with a tetrasubstituted α-stereogenic center in moderate to high yields (up to 85%) and with high enantioselectivities (up to 93% ee). This methodology was also applied to unprotected pyrroles, and it provided the expected products in good yields, but this time with moderate enantioselectivities. Using N-methylindole or N-substituted pyrroles, no reaction was achieved under the same conditions used. This result demonstrated that in this reaction, the presence of a N–H bond was crucial for the success of the process.
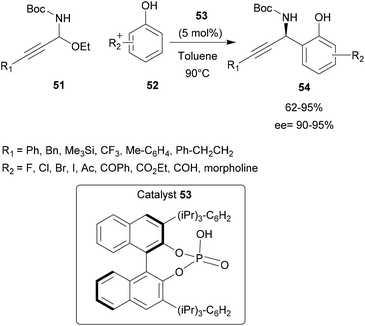
Chiral phosphoric acids have also proved to be efficient for the synthesis of non-poly-aromatic compounds. Shao and co-workers described an unusual synthesis of chiral propargylimine compounds 51 using low electron starting compounds such as phenol derivatives 52.61 However, the use of electron-neutral phenols in the asymmetric aza-FC reaction remained elusive due to its lower nucleophilicity compared with naphthols and indoles. Herein the reaction could occur at two different reaction sites (ortho and para positions).
In the presence of the chiral phosphoric acid catalyst 53 ((R)-TRIP), the reaction between propargyl derivative 51 and substituted phenol 52 proceeded successfully, providing the desired 1,2-addition aza-FC product 54 with good yields (up to 95%). This study has shown that the aza-FC reaction can be applied to non-electron-rich aromatic reagents, thus opening up perspectives for this reaction (Scheme 14).
Some authors have further explored and developed the range of applications of phosphoric acid catalysts on the aza-FC reaction, using chiral imidazoline-phosphoric acid derivatives. These catalysts were developed to act as dual activating organocatalysts, by enhancing the electrophilicity of the imine and the nucleophilicity of the unprotected indole or pyrrole by hydrogen bonding (Scheme 15).62,63
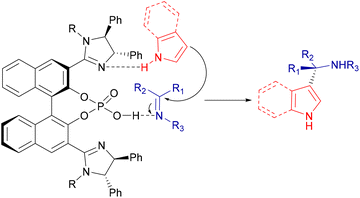 | ||
| Scheme 15 Possible interactions between imidazoline-phosphoric acid derivatives and substrates of the aza-FC reaction. | ||
Thus in 2016, Nakamura's group studied such catalysts in the enantioselective aza-FC reaction of 2-substituted 3H-indol-3-ones 55 with unprotected pyrrole 45 (Scheme 16A). Several organocatalytic catalysts have been screened to address the difficulty of this reaction and the bis(imidazoline)phosphoric acid catalyst 56 offered the highest yield and enantioselectivity, even when only 2 mol% of the catalyst were used.63
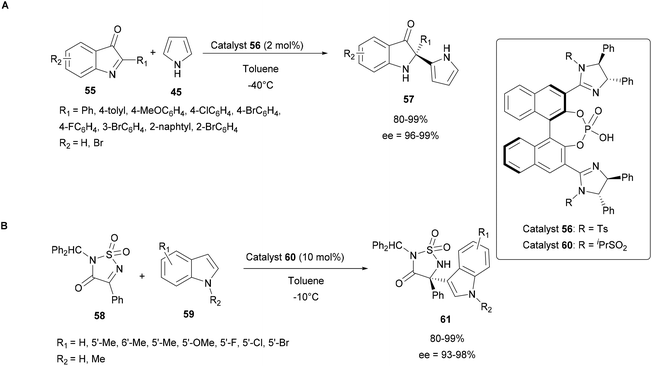 | ||
| Scheme 16 Organocatalytic enantioselective aza-FC reactions using imidazoline/phosphoric acids and 3H-indol-3-ones (A) or cyclic ketimines (B). | ||
In 2018, they also demonstrated that the imidazoline-phosphoric acid derivative 60 can efficiently catalyze the aza-FC reaction between indoles 59 and 4-aryl-3-oxo-1,2,5-thiadiazol-1,1-oxides 58 as cyclic ketimines (Scheme 16B). Here, it was found that the reaction proved to be efficient, which opens up prospects for the use of this catalyst with more complex substrates.62
Since their development in 2012 by Čorić and List,64 imidodiphosphoric acids have been extensively used by Zhang's group to catalyse a range of reactions including aza-FC reactions.65–67
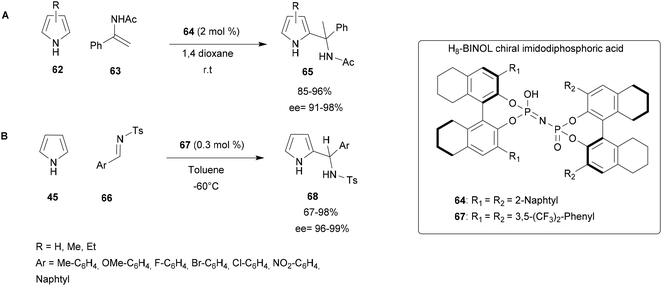
Since 2014, this group has been working on chemo-, regio- and enantioselective aza-FC reactions catalysed by H8-BINOL-type chiral imidodiphosphoric acid.65,67 In 2015, this catalyst was investigated in an aza-FC reaction between pyrroles and imines/enamides. A multiparametric optimization of this reaction (solvent, amount of catalyst, temperature, …) concluded that the reaction proceeds successfully in the presence of 2 mol% of the catalyst in dioxane at room temperature. These conditions were applied to a range of substrates. First α-phenyl substituted enamides 63 with electron-withdrawing or electron-donating groups at the ortho, meta or para position of the phenyl ring (Scheme 17A) led to compounds 65 with high yields and excellent enantioselectivity (85–96% yield, 91–98% ee).66
Encouraged by these successful results, the procedure was then applied to the reaction between pyrroles 45 and imines 66. During this process, a range of enantio-enriched substituted aryl-(2-pyrrolyl)methanamine scaffolds 68 were obtained in high yields (up to 98%) with excellent enantioselectivities (up to 99% ee) using lower catalyst loadings of 0.3 mol% (Scheme 17B).67
Bis(phosphoric acid) derivatives have recently been designed and reported in the literature as multiprotic acids. These catalysts containing a hydrogen bonding network are found to be important not only for the enhancement of catalytic activity, but also for the fine control of the conformation of substrates, reactants and catalysts due to acid–base cooperative interactions.
In 2018, Ishihara and co-workers developed the chiral C1-symmetric BINOL-derived bis(phosphoric acid) catalyst (R)-70, which has OP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(OH)2 and OP(
O)(OH)2 and OP(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(OH)(Oi-Pr) moieties at the 2,2′-positions. This catalyst was investigated in an aza-FC reaction between 2-methoxyfuran and various substituted aryl α-ketimino esters using 5 mol% of catalyst 70, in dichloromethane at −78 °C. The scope of this reaction has been studied and these conditions revealed to be suitable for electron-withdrawing and electron-donating substituted aryl α-ketimino esters. Targeted compounds 71 were obtained with excellent yields and enantioselectivity up to 99% (Scheme 18A).68
O)(OH)(Oi-Pr) moieties at the 2,2′-positions. This catalyst was investigated in an aza-FC reaction between 2-methoxyfuran and various substituted aryl α-ketimino esters using 5 mol% of catalyst 70, in dichloromethane at −78 °C. The scope of this reaction has been studied and these conditions revealed to be suitable for electron-withdrawing and electron-donating substituted aryl α-ketimino esters. Targeted compounds 71 were obtained with excellent yields and enantioselectivity up to 99% (Scheme 18A).68
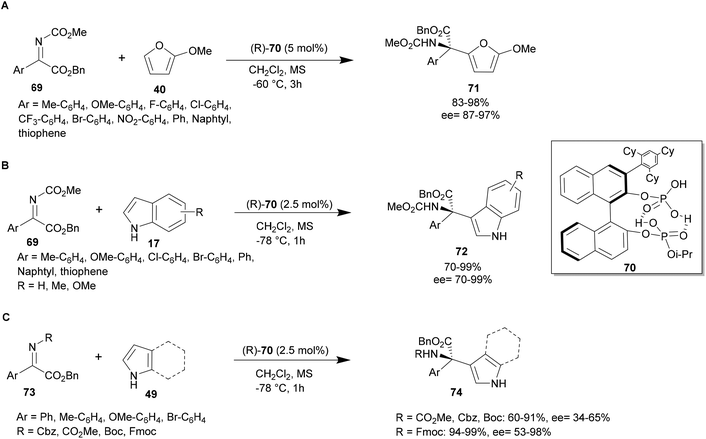 | ||
| Scheme 18 Bis(phosphoric acid)-catalyzed aza-FC type reaction of α-ketimino esters with furan, indole and pyrrole. | ||
In 2020, the team of Ishihara and co-workers studied the bis-(phosphoric) catalyst 70 with pyrroles and indoles as substrates. An aza-FC reaction of indole 17 with aryl-α-ketimino ester 69 was performed in dichloromethane using only 2.5 mol% of catalyst 70 at −78 °C. Various substituted aryl or heteroaryl-α-ketimino esters 69, bearing electron-donating or electro-withdrawing groups, are tolerated and give access to the desired compound in high yields and ee (up to 99%) (Scheme 18B). Aza-FC on pyrroles was also achieved using α-ketimino esters protected by a methylester, a benzylcarbonyl or a Boc group in the same conditions. With these substrates, the reaction led to moderate yields and enantioselectivity, which were improved when an Fmoc-group was used as a protective group (yields up to 99% and ee up to 98%, Scheme 18C).48 Interestingly, using either indole or pyrrole, the catalyst is effectively recovered after column chromatography in 99% yield. However, this reaction is limited to aromatic α-ketimino esters and is not applicable to non-aromatic imines, probably due to a lack of attractive hydrogen bonding and/or π–π stacking interactions among the substrates and catalyst 70.
3.2. Organocatalytic catalysis of aza Friedel–Crafts reactions
Asymmetric organocatalysis has emerged as an effective and safe alternative to synthesize optically pure compounds without using any precious or toxic metals.69 Organocatalysis offers several notable advantages over their metal homologue processes. For example, catalysis can be environmentally harmless, reactions can be operated under aerobic conditions and the catalysts are generally inexpensive. Organocatalytic and metal-free catalysis proved to be able to catalyze several types of C–C and C–X bond-forming reactions with high chemical and stereochemical efficiency, thereby complementing other major organocatalytic asymmetric approaches. Due to their unique molecular recognition ability, several organocatalysts attracted much attention in asymmetric aza-FC reactions.10,70–74 In this section, we report the most outstanding examples of organocatalyzed aza-FC reactions.In 2011, Chimni and co-workers have explored the catalytic potential of 6′-OH Cinchona alkaloids for an aza-FC reaction of naphthol with N-sulfonyl aldimines.10 Substituted 2-naphthols 75 with electron-donating or electron-withdrawing groups at C6 were successfully reacted with aromatic N-tosylimine 66 derivatives to give aza-FC adducts 77 with good to excellent ee, using only 5 mol% of the catalyst 76 (Scheme 19). This reaction is also suitable for scale-up. Thus, the gram scale carried out under the same conditions on unsubstituted β-naphthol proved to be efficient and led to the expected product in good yield (86%) and good enantiomeric excess (91%).
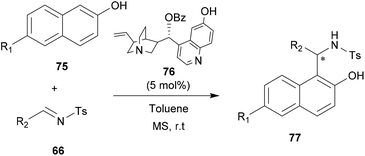 | ||
| Scheme 19 Alkaloid-modified derivatives-catalyzed aza-FC type reaction of naphthol derivatives with N-tosylimines. | ||
In 2019, Zhou and co-workers employed Cinchona alkaloid-based organocatalysts for asymmetric aza-FC reactions between seven-membered cyclic N-sulfonylimines 78 and α-naphthols 79 in order to access chiral seven-membered cyclic sulfonamides (ε-sultams) 81,80 compounds known for their attractive biological activities.83 In this work, several conditions were evaluated, such as the solvent, the alkaloid derivative catalyst used and the temperature. Among several alkaloid derivatives tested, it was found that the catalyst 80 in chloroform allowed the efficient synthesis of cyclic sulfonamide derivatives 81 in 90–98% yields with ee up to 92% (Scheme 20).80
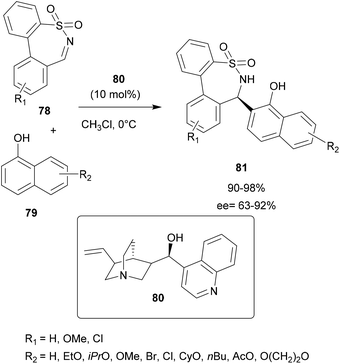 | ||
| Scheme 20 The alkaloid derivative-catalyzed asymmetric aza-FC reaction of N-sulfonylimines with naphthols. | ||
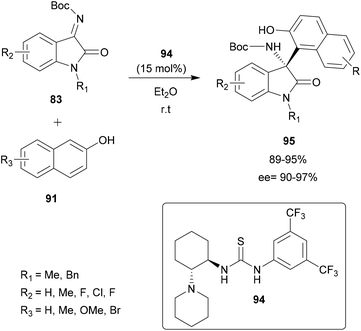
Pedro and co-workers presented in 2016 a regio- and enantioselective aza-FC reaction to functionalize hydroxy-substituted indoles (Scheme 21).82 Interestingly, this method allowed the functionalization of the carbocyclic ring instead of the “classical” C-3 position of the indole. The reaction was performed on hydroxyindoles 82 with isatin-derived ketimines 83, using a squaramide-based quinine derivative 84 as a bifunctional organocatalyst (catalyst amount 0.1–5 mol%). In toluene at room temperature, the reaction led to highly enantioenriched tetrasubstituted 3-aminooxindoles 85 in high yields and ee. Interestingly, this strategy allows the introduction of substituents in all positions of the indole carbocyclic ring in a regioselective manner, by simply switching the position of the hydroxy group activating/directing the regioselectivity. Moreover, the authors showed that, after functionalization, the activating/directing hydroxy group could be removed or used for further transformations. Thus, this approach enables structural diversity to be achieved according to research needs.
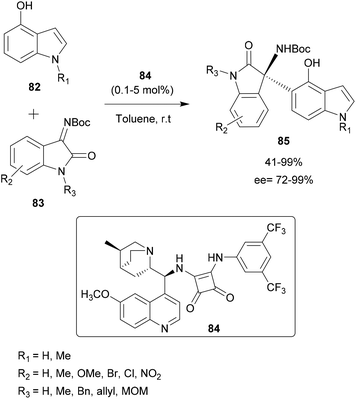 | ||
| Scheme 21 Regio and enantioselective aza-FC catalyzed by a quinine skeleton as a bifunctional organocatalyst. | ||
In 2019, the same team applied a similar strategy with cyclic electrophilic imines, benzoxathiazine 2,2-dioxide derivatives 87 and 4- to 6-hydroxyindoles 86 to prepare chiral indolyl sulfamidates 89a–c. The aza-FC reaction was realized in DCM in the presence of a small amount of quinine-derived bifunctional organocatalyst 88 (2 mol%) (Scheme 22).84 The substitution occurred in the α-position of the hydroxyl group and compounds 89 were obtained in moderate to good yields and enantioselectivities.
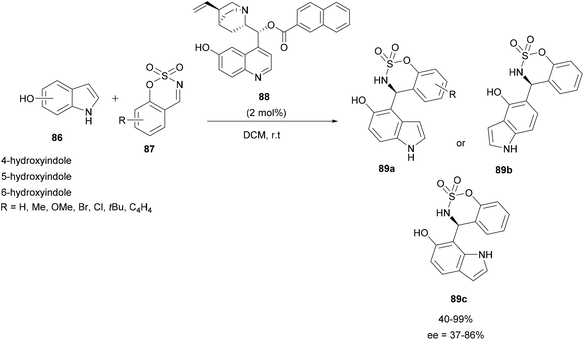 | ||
| Scheme 22 Aza-FC catalyzed by bifunctional organocatalyst for the development chiral indolyl sulfamidates. | ||
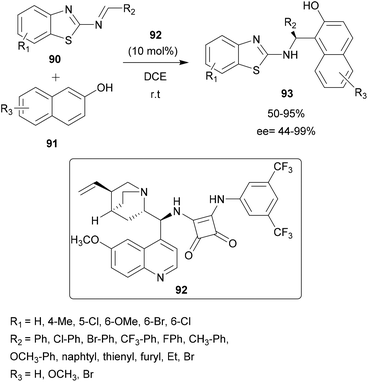
Using the Cinchona-derived squaramide organocatalyst 92, Wang and co-workers described the enantioselective aza-FC reaction between benzothiazolimines 90 and 2-naphthols 91.85 25 examples of 2′-aminobenzothiazolomethyl naphthols 93 with potential antiproliferative and anthelmintic activities were obtained with moderate to high yields and enantioselectivity (Scheme 23). The induction is explained by the spatial conformation of the catalyst which allows the formation of hydrogen bonds between the two NH groups of the squaramide catalyst and the two nitrogen atoms of the benzothiazolimine derivative, allowing the stereo-controlled nucleophilic attack of the β-naphthol.85
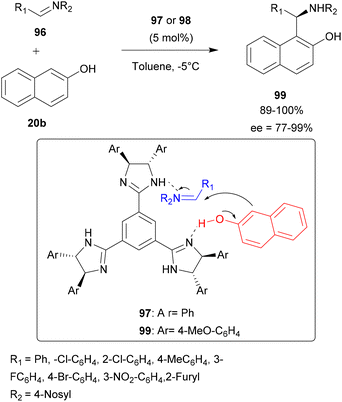
Sasai and co-workers have reported the first imidazoline-mediated aza-FC type reaction between nosyl-aldimines 96 and β-naphthol 20b. The choice of chiral trisimidazoline was related to the assumption that one imidazoline group could function as a Brønsted base interacting with the hydroxyl group of 20b, while the other imidazoline could give its proton to the imine derivative 96 (Scheme 25). The reaction was carried out in the presence of 5 mol% of chiral trisimidazoline 97 or 98 in toluene at −5 °C to give sulfonamides 99 with good yields and moderate to high enantioselectivities (72 to 99%), depending on the aldimine used.87
3.3. Solid-supported acid for aza-FC catalysis
The immobilization of both of chiral and achiral catalysts on a solid support has made significant progress in recent years and it represents an attractive approach to increase the durability of organic synthesis. This immobilization approach of chiral catalysts is increasingly important in the catalysis of asymmetric reactions.88–90In order to reduce the conformational flexibility of immobilized chiral or achiral Brønsted acid catalysts, some researchers adopted a simple adsorption of an acid molecule by an immobilized base through acid–base interactions. In most of the cases, the immobilized support adopted is silica because of its structural characteristics such as a large surface area and abundant silanol groups that allows chemical interactions and the easy adsorption of various compounds at its surface. Moreover, this type of catalyst immobilized on a solid support often has the advantage of easy removal and reuse.91
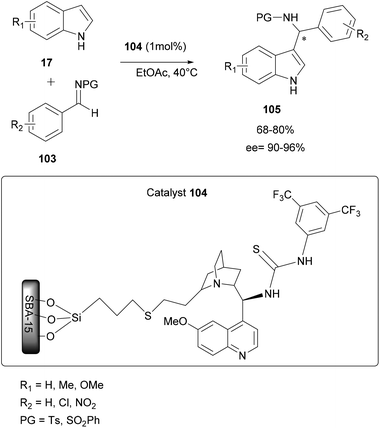 | ||
| Scheme 27 Asymmetric aza-FC reaction of indoles with imines using 9-thiourea epi-quinine immobilized on mesoporous silica (SBA-15). | ||
In addition, this heterogeneous catalyst 104 has the advantage of being easily removable from the reaction medium by simple filtration and it can be regenerated just by washing with an organic solvent and reused up to 4 times without any loss of enantioselectivity (ee up to 96%).
3.4. Organometallic complex catalysts for the aza Friedel–Crafts reaction
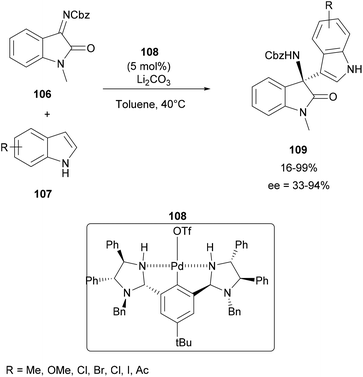
In 2019, Kakino and co-workers described the aza-FC reaction of indoles with isatin-derived N-Cbz-ketimines 106 using a chiral bis(imidazolidine)-containing NCN-pincer palladium catalyst 108. In the presence of lithium carbonate in toluene at 40 °C, this asymmetric optimized synthesis pathway leads to several indolyl-amino-2-oxindoles 109, substituted by electron-withdrawing or donating groups, with yields in the range of 16–99% and a moderate to good enantioselectivity between 33–94% (Scheme 28).94,95
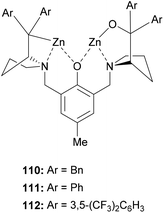
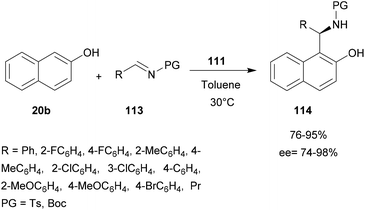
A dinuclear zinc complex-catalyzed aza-FC (Fig. 1) was investigated by Hui and co-workers in 2010 (Fig. 1).9 In this study, three chiral zinc-based complexes 110, 111 and 112 were tested with 2-naphthol and tosylimines (Scheme 29).
The reaction between N-tosyl-phenyl-1-imine and β-naphthol in the presence of dinuclear zinc complexes 110 or 112 led to 114 with low enantioselectivities (85% and 54% respectively). Using the complex 111, the desired product was obtained in a good 90% yield and with high enantioselectivity (ee = 96%) in toluene at 30 °C, but only when using 1 equivalent of catalyst. This reaction is also efficient with N-Boc phenyl-1-imine and the corresponding aza-FC product 114 was obtained in 88% yields and 91% ee.
Then, the efficacy of catalyst 111 was evaluated using a wide variety of aliphatic or aryl-aldimine substrates, possessing either electron-withdrawing or electron-donating groups. With all compounds 113, the reaction was successful and the highest enantioselectivities (90 to 98%) were obtained with sterically hindered 4-substituted arylimines.
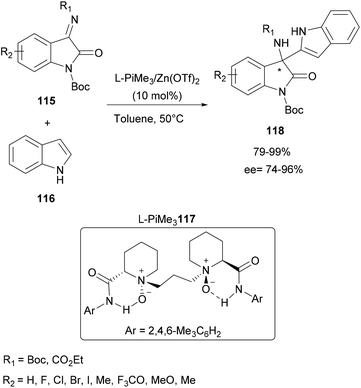
Another study using an organozinc complex has been reported by Feng and co-workers in 2016. This time, the aza-FC reaction between isatin-derived ketimines and free protected indoles was investigated in the presence of a N,N′-dioxides-zinc(II) complex catalytic system (Scheme 30).97 Initially, several metals were screened to catalyze this aza-FC coupling reaction in the presence of various chiral N,N′-dioxide ligands. Complexes containing Sc(OTf)3, Cu(OTf)2 and Ni(OTf)2 allowed obtaining of the expected products with average yields but with low ee. Fortunately, the use of Zn(OTf)2 allowed good results. This study is followed by the optimization of the chiral ligand in order to improve the ee. It was found that L-PiMe3117 improved considerably the enantioselectivity. These conditions are then retained to prepare a series of final enantiomerically enriched 3-indolyl-3-amino-oxindoles 118 containing different electron-withdrawing and electron-donating groups in up to 99% yield and 96% ee.
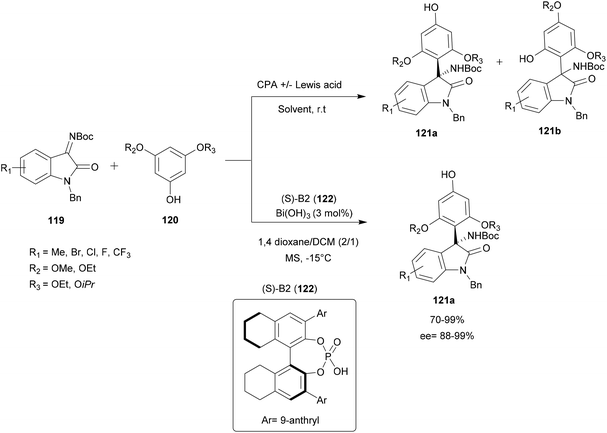 | ||
| Scheme 31 Bismuth-catalyzed enantio and regioselective aza-FC of phenol and isatin-derived ketimines. | ||
A screening of several Lewis acids in the presence of CPA with different solvents was performed. Under most conditions tested, the improvement in regioselectivity of this reaction is not significant. However, using Bi(OH)3/(S)-B2 122 catalyst, in dioxane/DCM (2/1) with 5 Å molecular sieves at −15 °C, provides a series of 3-substituted functional 3-amino-2-oxindoles 121a with good yield (up to 99%), excellent enantioselectivity (ee up to 99%) and moderate to very good regioselectivity (p/o up to 25/1) (Scheme 31).
After its synthesis, this catalyst was able to catalyze a succession of one-pot reactions: aerobic oxidation reactions of benzyl alcohols 123 to the corresponding aldehyde 125, followed by an intramolecular aza-FC reaction with N-aminoethylpyrroles 126. The reaction was carried out with 4-substituted benzyl alcohols with a wide range of functional groups, such as F, Cl, CF3, CO2Me and CN, to provide the corresponding chiral pyrrolopiperazines 127 in good yields and enantioselectivities (Scheme 32).
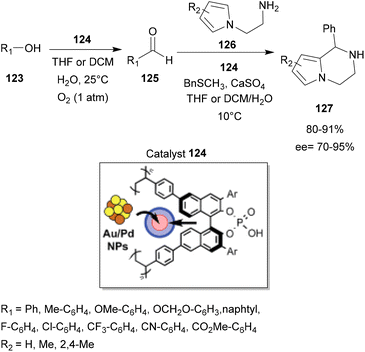 | ||
| Scheme 32 Sequential aerobic oxidation of benzyl alcohol and asymmetric aza-FC reaction catalyzed by chiral bifunctional heterogeneous catalysts Au/Pd. | ||
Moreover, the designed chiral heterogeneous catalyst could be recovered and reused several times without significant loss of activity or enantioselectivity.
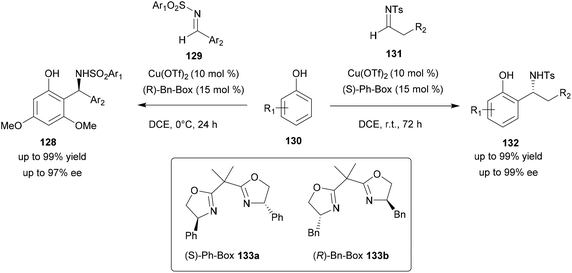
Using Cu(OTf)2, several conditions were evaluated such as the chiral ligands as well as the solvent used. Thus, Cu(OTf)2 associated with various chiral bis(oxazoline) ligands was screened for the reactivity and enantioselectivity. Among all tested ligands, the (S)-Ph-Box ligand 133a afforded the best reactivity and enantiomeric excess. Several solvents such as DCM, dichloroethane (DCE), THF, ethyl acetate, xylene and toluene were also screened and DCE led to the best yield and enantiomeric excess. These conditions were then tested with N-tosyl arylimines 129 but led to poor regioselectivity and enantioselectivity. With these substrates, (R)-Bn-Box 133b with Cu(OTf)2 in DCE was found to be effective and led to compounds 128 with significant yields and ee (up to 99%) (Scheme 33). The scope of these two reactions was studied with compounds containing electron-withdrawing or electron-donating groups. In all cases, the aza-FC product was obtained in high yield and ee (>99%).
In order to generate a catalyst with eco-compatible, easily recoverable and recyclable properties while retaining its role in enantioselective catalysis, Bajaj and co-workers designed and synthesized recyclable chiral tridentate Schiff base ligands incorporating donor atoms and different (achiral and chiral) linkers (trigol, piperazine and diethyl D-(−)-tartrate).101 Several sources of copper were also tested and Cu(OTf)2 was found to be the most reliable. Among all the tested copper catalysts, the dinuclear copper catalyst derived from ligand 136, containing a chiral diphenyl amino-alcohol moiety and diethyl D-tartrate as a chiral linker was found to be the most effective catalyst for the asymmetric aza-FC reaction of tosyl aldimine 135 with 1-naphthol.
The reaction was carried out in DCM using only 2.5 mol% Cu(OTf)2 catalyst coupled to the ligand 136 under anhydrous conditions. The conditions proved to be efficient and applicable using various substituted tosyl imines 135 and several substituted α- or β-naphthols 134. The expected products 137 or 138 were obtained in high yields (up to 98%) and high enantioselectivity up to 99% (Scheme 34).
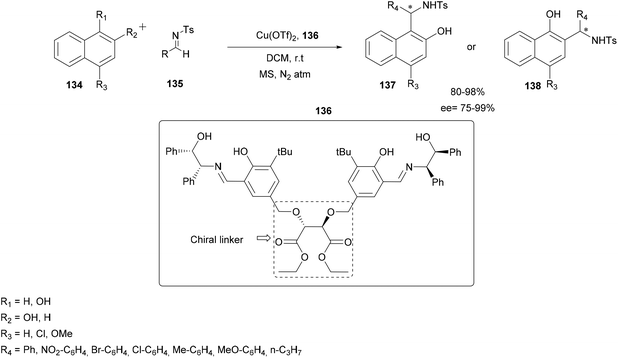 | ||
| Scheme 34 Enantioselective addition of 1- or 2-naphthol with tosyl aldimine with a dinuclear Cu(II) complex catalyst. | ||
Usually, under homogeneous conditions, catalysts are not recyclable due to their inherent problem of solubility and thus their separation during post-catalytic processing. However, in this work, the authors recovered the catalyst according to the following process. After the first catalytic run, the amount of solvent was reduced and an excessive amount of n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (9
ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the reaction mixture. The catalyst precipitated out of the reaction mixture, and the unreacted substrate and product remained in the solvent supernatant, which was then removed by centrifugation. At each catalyst recycling post-treatment step, 98–99% of the initial amount of catalyst is recovered. The recovered catalyst retains its catalytic efficiency for the asymmetric aza-FC reaction of naphthols 134 with tosyl aldimines 135 without any noticeable decrease of its reactivity and enantioselectivity up to six cycles.
1) was added to the reaction mixture. The catalyst precipitated out of the reaction mixture, and the unreacted substrate and product remained in the solvent supernatant, which was then removed by centrifugation. At each catalyst recycling post-treatment step, 98–99% of the initial amount of catalyst is recovered. The recovered catalyst retains its catalytic efficiency for the asymmetric aza-FC reaction of naphthols 134 with tosyl aldimines 135 without any noticeable decrease of its reactivity and enantioselectivity up to six cycles.
4. Conclusion
The rich variety of aromatic organic compounds being compatible with the aza-FC reaction has made it one of the most important C–N–C bond-forming reactions in synthetic organic chemistry, allowing the synthesis of polyfunctionalized amines.Several Lewis acids, Brønsted acids and organic catalysts have been employed for the synthesis of achiral compounds via relatively affordable synthesis routes that we have reported in the first part of this review.
Despite the success of this reaction, some limitations such as the selectivity of the aza-FC-derived products could be a constraining feature. For this reason, great progress has been made in the design and the applications of new catalysts and ligands to overcome the regio- and stereoselectivity of the aza-FC reaction. A large variety of chiral catalysts have been then developed, including binaphthyl phosphoric acids, Cinchona-derived catalysts, and thiourea or imidazoline compounds. The novelty of the catalyst, its suitability for applications and its potential to generate excellent selectivity are among the most attractive requirements. The influence of the architecture and the nature of the ligand on the outcome of the aza-FC was the major discussion point in this review.
Whether achiral or chiral, the aza-FC reaction is compatible with a wide variety of electron-rich aromatic groups and has a high tolerance for different substituents, such as halogens, and electron-withdrawing and electron-donating groups. This makes this reaction crucial in the synthesis of complex compounds or in the preparation of biologically relevant molecules.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by a grant from the Agence Nationale de la Recherche (ANR-16-IDEX-0006).References
- M. Terada and K. Sorimachi, Enantioselective Friedel-Crafts Reaction of Electron-Rich Alkenes Catalyzed by Chiral Brønsted Acid, J. Am. Chem. Soc., 2007, 129, 292–293, DOI:10.1021/ja0678166.
- D. Uraguchi, K. Sorimachi and M. Terada, Organocatalytic Asymmetric Aza-Friedel-Crafts Alkylation of Furan, J. Am. Chem. Soc., 2004, 126, 11804–11805, DOI:10.1021/ja046185h.
- S. L. You, Q. Cai and M. Zeng, Chiral Brønsted Acid Catalyzed Friedel–Crafts Alkylation Reactions, Chem. Soc. Rev., 2009, 38, 2190–2201, 10.1039/b817310a.
- M. Terada, S. Yokoyama, K. Sorimachi and D. Uraguchi, Chiral Phosphoric Acid-Catalyzed Enantioselective Aza-Friedel-Crafts Reaction of Indoles, Adv. Synth. Catal., 2007, 349, 1863–1867, DOI:10.1002/adsc.200700151.
- Y. Rokade and N. Dongare, Synthesis and Antimicrobial Activity of Some Azetidinone Derivatives with the β-Naphthol, Rasayan J. Chem., 2010, 3, 641–645 CAS.
- S. Saaby, P. Bayón, P. S. Aburel and K. A. Jørgensen, Optically Active Aromatic and Heteroaromatic α-Amino Acids by a One-Pot Catalytic Enantioselective Addition of Aromatic and Heteroaromatic C-H Bonds to α-Imino Esters, J. Org. Chem., 2002, 67, 4352–4361, DOI:10.1021/jo0256787.
- M. Johannsen, An Enantioselective Synthesis of Heteroaromatic N -Tosyl a -Amino Acids Tosyl a -Amino Acids Has Been Developed Which Uses Readily of Enantioselection, Giving the a -Amino Acids with up to 96%, Chem. Commun., 1999, 88, 2233–2234 RSC.
- G. Zhao, S. S. Samanta, J. Michieletto and S. P. Roche, A Broad Substrate Scope of Aza-Friedel-Crafts Alkylation for the Synthesis of Quaternary α-Amino Esters, Org. Lett., 2020, 22, 5822–5827, DOI:10.1021/acs.orglett.0c01895.
- L. F. Niu, Y. C. Xin, R. L. Wang, F. Jiang, P. F. Xu and X. P. Hui, Asymmetric Aza-Friedel-Crafts Reaction of 2-Naphthol with Tosylimines Catalyzed by a Dinuclear Zinc Complex, Synlett, 2010, 765–768, DOI:10.1055/s-0029-1219390.
- P. Chauhan and S. S. Chimni, Asymmetric Organocatalytic Aza-Friedel-Crafts Reaction of Naphthols with N-Sulfonyl Imines, Eur. J. Org. Chem., 2011, 1636–1640, DOI:10.1002/ejoc.201001653.
- D. Wu, X. Zhang, Y. Xu, Y. Xue, J. Li, W. Wang and J. Zhu, Organocatalytic Enantioselective Friedel-Crafts Reaction of 1-Naphthols with Isatins and an Unexpected Spontaneous Dehydration Process, Asian J. Org. Chem., 2014, 3, 480–486, DOI:10.1002/ajoc.201300170.
- G. Chaubet, L. T. Maillard, J. Martinez and N. Masurier, A Tandem Aza-Friedel-Crafts Reaction/Hantzsch Cyclization: A Simple Procedure to Access Polysubstituted 2-Amino-1,3-Thiazoles, Tetrahedron, 2011, 67, 4897–4904, DOI:10.1016/j.tet.2011.04.090.
- S.-G. Wang and S.-L. You, Hydrogenative Dearomatization of Pyridine and an Asymmetric Aza-Friedel-Crafts Alkylation Sequence, Angew. Chem., 2014, 126, 2226–2229, DOI:10.1002/ange.201309876.
- D. Gaviña, M. Escolano, J. Torres, G. Alzuet-Piña, M. Sánchez-Roselló and C. del Pozo, Organocatalytic Enantioselective Friedel-Crafts Alkylation Reactions of Pyrroles, Adv. Synth. Catal., 2021, 363, 3439–3470, DOI:10.1002/adsc.202100313.
- M. Zaghouani, L. A. K. Bögeholz, E. Mercier, W. Wintermeyer and S. P. Roche, Total Synthesis of (±)-Fumimycin and Analogues for Biological Evaluation as Peptide Deformylase Inhibitors, Tetrahedron, 2019, 75, 3216–3230, DOI:10.1016/j.tet.2019.03.037.
- Y. Li and M. H. Xu, Lewis-Acid-Catalyzed Intramolecular Aza-Friedel-Crafts Reaction of N-Tert-Butanesulfinyl Imines: Efficient Synthesis of Optically Active 9-Aminofluorene Derivatives, Asian J. Org. Chem., 2013, 2, 50–53, DOI:10.1002/ajoc.201200161.
- H. G. Cheng, J. Miguélez, H. Miyamura, W. J. Yoo and S. Kobayashi, Integration of Aerobic Oxidation and Intramolecular Asymmetric Aza-Friedel-Crafts Reactions with a Chiral Bifunctional Heterogeneous Catalyst, Chem. Sci., 2017, 8, 1356–1359, 10.1039/C6SC03849B.
- Y. He, M. Lin, Z. Li, X. Liang, G. Li and J. C. Antilla, Direct Synthesis of Chiral 1,2,3,4-Tetrahydropyrrolo[1,2-a]Pyrazines via a Catalytic Asymmetric Intramolecular Aza-Friedel-Crafts Reaction, Org. Lett., 2011, 13, 4490–4493, DOI:10.1021/ol2018328.
- S. G. Wang, W. Zhang and S. L. You, Construction of Spiro -Tetrahydroquinolines via Intramolecular Dearomatization of Quinolines: Free of a Preinstalled Activation Group, Org. Lett., 2013, 15, 1488–1491, DOI:10.1021/ol4002416.
- M. J. Wanner, R. N. S. Van Der Haas, K. R. De Cuba, J. H. Van Maarseveen and H. Hiemstra, Catalytic Asymmetric Pictet-Spengler Reactions via Sulfenyliminium Ions, Angew. Chem., Int. Ed., 2007, 46, 7485–7487, DOI:10.1002/anie.200701808.
- M. S. Taylor and E. N. Jacobsen, Highly Enantioselective Catalytic Acyl-Pictet-Spengler Reactions, J. Am. Chem. Soc., 2004, 126, 10558–10559, DOI:10.1021/ja046259p.
- I. T. Raheem, P. S. Thiara, E. A. Peterson and E. N. Jacobsen, Enantioselective Pictet-Spengler-Type Cyclizations of Hydroxylactams: H-Bond Donor Catalysis by Anion Binding, J. Am. Chem. Soc., 2007, 129, 13404–13405, DOI:10.1021/ja076179w.
- M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, Enantioselective Brønsted Acid-Catalyzed N-Acyliminium Cyclization Cascades, J. Am. Chem. Soc., 2009, 131, 10796–10797, DOI:10.1021/ja9024885.
- C. Zhao, S. B. Chen and D. Seidel, Direct Formation of Oxocarbenium Ions under Weakly Acidic Conditions: Catalytic Enantioselective Oxa-Pictet-Spengler Reactions, J. Am. Chem. Soc., 2016, 138, 9053–9056, DOI:10.1021/jacs.6b05225.
- C. A. Holloway, M. E. Muratore, R. L. Storer and D. J. Dixon, Direct Enantioselective Brønsted Acid Catalyzed N-Acyliminium Cyclization Cascades of Tryptamines and Ketoacids, Org. Lett., 2010, 12, 4720–4723, DOI:10.1021/ol101651t.
- I. T. Raheem, P. S. Thiara and E. N. Jacobsen, Regio-and Enantioselective Catalytic Cyclization of Pyrroles onto N-Acyliminium Lons, Org. Lett., 2008, 10, 1577–1580, DOI:10.1021/ol800256j.
- R. S. Klausen and E. N. Jacobsen, Weak Brønsted Acid - Thiourea Co-Catalysis: Enantioselective, Catalytic Protio-Pictet - Spengler Reactions, Org. Lett., 2009, 11(4), 887–890, DOI:10.1021/ol802887h.
- S. Shirakawa and S. Kobayashi, Carboxylic Acid Catalyzed Three-Component Aza-Friedel-Crafts Reactions in Water for the Synthesis of 3-Substituted Indoles, Org. Lett., 2006, 8, 4939–4942, DOI:10.1021/ol062031q.
- Y. X. Jia, J. H. Xie, H. F. Duan, L. X. Wang and Q. L. Zhou, Asymmetric Friedel-Crafts Addition of Indoles to N-Sulfonyl Aldimines: A Simple Approach to Optically Active 3-Indolyl-Methanamine Derivatives, Org. Lett., 2006, 8, 1621–1624, DOI:10.1021/ol0602001.
- X. Mi, S. Luo, J. He and J. P. Cheng, Dy(OTf)3 in Ionic Liquid: An Efficient Catalytic System for Reactions of Indole with Aldehydes/Ketones or Imines, Tetrahedron Lett., 2004, 45, 4567–4570, DOI:10.1016/j.tetlet.2004.04.039.
- B. Ke, Y. Qin, Q. He, Z. Huang and F. Wang, Preparation of Bisindolylalkanes from N-Tert-Butanesulfinyl Aldimines, Tetrahedron Lett., 2005, 46, 1751–1753, DOI:10.1016/j.tetlet.2004.12.140.
- M. L. Deb and P. J. Bhuyan, An Efficient and Clean Synthesis of Bis(Indolyl)Methanes in a Protic Solvent at Room Temperature, Tetrahedron Lett., 2006, 47, 1441–1443, DOI:10.1016/j.tetlet.2005.12.093.
- R. T. Sawant, M. Y. Stevens and L. R. Odell, Microwave-Assisted Aza-Friedel-Crafts Arylation of N-Acyliminium Ions: Expedient Access to 4-Aryl 3,4-Dihydroquinazolinones, ACS Omega, 2018, 3, 14258–14265, DOI:10.1021/acsomega.8b02298.
- Y. Q. Wang, Z. S. Wei, C. Q. Zhu, Y. Y. Ren and C. Wu, Solvent-Controlled and Selective Synthesis of Mono- and Bis-Indolyl Products in Brønsted Acid Catalyzed Aza-Friedel–Crafts Reactions of Indoles with Cyclic Imines, Tetrahedron, 2016, 72, 4643–4654, DOI:10.1016/j.tet.2016.06.037.
- C. A. Holloway, M. E. Muratore, R. L. Storer and D. J. Dixon, Direct Enantioselective Brønsted Acid Catalyzed N-Acyliminium Cyclization Cascades of Tryptamines and Ketoacids, Org. Lett., 2010, 12, 4720–4723, DOI:10.1021/ol101651t.
- J. Jaratjaroonphong, S. Krajangsri and V. Reutrakul, Iodine-Catalyzed, One-Pot, Three-Component Aza-Friedel-Crafts Reaction of Electron-Rich Arenes with Aldehyde/Carbamate Combinations, Tetrahedron Lett., 2012, 53, 2476–2479, DOI:10.1016/j.tetlet.2012.03.024.
- J. Jaratjaroonphong, S. Krajangsri and V. Reutrakul, Iodine-Catalyzed, One-Pot, Three-Component Aza-Friedel-Crafts Reaction of Electron-Rich Arenes with Aldehyde/Carbamate Combinations, Tetrahedron Lett., 2012, 53, 2476–2479, DOI:10.1016/j.tetlet.2012.03.024.
- T. R. Li, M. M. Zhang, B. C. Wang, L. Q. Lu and W. J. Xiao, Synthesis of 3,3′-Biindoles through a Copper-Catalyzed Friedel-Crafts Propargylation/Hydroamination/Aromatization Sequence, Org. Lett., 2018, 20, 3237–3240, DOI:10.1021/acs.orglett.8b01100.
- Y. Sun, Y. Qiao, H. Zhao, B. Li and S. Chen, Construction of 9H-Pyrrolo[1,2-a]Indoles by a Copper-Catalyzed Friedel–Crafts Alkylation/Annulation Cascade Reaction, J. Org. Chem., 2016, 81, 11987–11993, DOI:10.1021/acs.joc.6b02143.
- Y.-S. Zhu, B.-B. Yuan, J.-M. Guo, S.-J. Jin, H.-H. Dong, Q.-L. Wang and Z.-W. Bu, A Copper-Catalyzed Friedel–Crafts Alkylation/Cyclization Sequence: An Approach to Functionalized Pyrrolo[1,2-a]Indole Spirooxindoles and 9H-Pyrrolo[1,2-a]Indoles, J. Org. Chem., 2017, 82, 5669–5677, DOI:10.1021/acs.joc.7b00488.
- C. Li, F. Guo, K. Xu, S. Zhang, Y. Hu, Z. Zha and Z. Wang, Copper-Catalyzed Enantioselective Friedel–Crafts Alkylation of Pyrrole with Isatins, Org. Lett., 2014, 16, 3192–3195, DOI:10.1021/ol501086q.
- P. Kumari, P. K. Bera, N. U. H. Khan, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, Asymmetric Friedel-Crafts Addition of Indoles to N-Sulfonyl Aldimines Catalyzed by Cu(Ii) Chiral Amino Alcohol Based Schiff Base Complexes, Catal. Sci. Technol., 2014, 4, 563–568, 10.1039/c3cy00629h.
- J. Esquivias, R. G. Arrayás and J. C. Carretero, A Copper(II)-Catalyzed Aza-Friedel-Crafts Reaction of N-(2-Pyridyl)Sulfonyl Aldimines: Synthesis of Unsymmetrical Diaryl Amines and Triaryl Methanes, Angew. Chem., Int. Ed., 2006, 45, 629–633, DOI:10.1002/anie.200503305.
- M. Lei, L. Ma and L. Hu, Thiamine hydrochloride as a efficient catalyst for the synthesis of amidoalkyl naphthols, Tetrahedron Lett., 2009, 50, 6393–6397, DOI:10.1016/j.tetlet.2009.08.081.
- G. Srihari, M. Nagaraju and M. M. Murthy, Solvent-Free One-Pot Synthesis of Amidoalkyl Naphthols Catalyzed by Silica Sulfuric Acid, Helv. Chim. Acta, 2007, 90, 1497–1504, DOI:10.1002/hlca.200790156.
- G. Srihari, M. Nagaraju and M. M. Murthy, Solvent-Free One-Pot Synthesis of Amidoalkyl Naphthols Catalyzed by Silica Sulfuric Acid, Helv. Chim. Acta, 2007, 90, 1497–1504, DOI:10.1002/hlca.200790156.
- Y. Zhao, L. Wang and J. Zhao, Chiral Phosphoric Acid Catalyzed Aza-Friedel-Crafts Alkylation of Indoles with Cyclic Aryl α-Ketimino Esters, Tetrahedron Lett., 2017, 58, 213–217, DOI:10.1016/j.tetlet.2016.12.012.
- M. Hatano, K. Toh and K. Ishihara, Enantioselective Aza-Friedel-Crafts Reaction of Indoles and Pyrroles Catalyzed by Chiral C1-Symmetric Bis(Phosphoric Acid), Org. Lett., 2020, 22, 9614–9620, DOI:10.1021/acs.orglett.0c03662.
- Y. S. Cheng, S. H. Chan, G. A. Rao, R. Gurubrahamam and K. Chen, Enantioselective Aza-Friedel-Crafts Reaction of Heteroarenes with in Situ Generated Isoxazolium Ions via Chiral Phosphoric Acid Catalysis, Adv. Synth. Catal., 2021, 363, 3502–3506, DOI:10.1002/adsc.202100408.
- Q. Kang, Z. A. Zhao and S. L. You, Highly Enantioselective Friedel-Crafts Reaction of Indoles with Imines by a Chiral Phosphoric Acid, J. Am. Chem. Soc., 2007, 129, 1484–1485, DOI:10.1021/ja067417a.
- J. Feng, W. Yan, D. Wang, P. Li, Q. Sun and R. Wang, The Highly Enantioselective Addition of Indoles and Pyrroles to Isatins-Derived N -Boc Ketimines Catalyzed by Chiral Phosphoric Acids, ChemComm, 2012, 8003–8005, 10.1039/c2cc33200k.
- K. F. Zhang, J. Nie, R. Guo, Y. Zheng and J. A. Ma, Chiral Phosphoric Acid-Catalyzed Asymmetric Aza-Friedel-Crafts Reaction of Indoles with Cyclic N-Acylketimines: Enantioselective Synthesis of Trifluoromethyldihydroquinazolines, Adv. Synth. Catal., 2013, 355, 3497–3502, DOI:10.1002/adsc.201300534.
- G. Li, G. B. Rowland, E. B. Rowland and J. C. Antilla, Organocatalytic Enantioselective Friedel - Crafts Reaction of Pyrrole Derivatives with Imines, Org. Lett., 2007, 9, 4065–4068, DOI:10.1021/ol701881j.
- M. Miyagawa, M. Yoshida, Y. Kiyota and T. Akiyama, Enantioselective Friedel–Crafts Alkylation Reaction of Heteroarenes with N-Unprotected Trifluoromethyl Ketimines by Means of Chiral Phosphoric Acid, Chem. – Eur. J., 2019, 25, 5677–5681, DOI:10.1002/chem.201901020.
- H. Liu, Y. Yan, M. Li and X. Zhang, An Enantioselective Aza-Friedel-Crafts Reaction of 5-Aminoisoxazoles with Isatin-Derived: N -Boc Ketimines, Org. Biomol. Chem., 2021, 19, 3820–3824, 10.1039/d1ob00374g.
- E. Xie, A. Rahman and X. Lin, Asymmetric Synthesis of CF3- and Indole-Containing Tetrahydro-β-Carbolines: Via Chiral Spirocyclic Phosphoric Acid-Catalyzed Aza-Friedel-Crafts Reaction, Org. Chem. Front., 2017, 4, 1407–1410, 10.1039/c7qo00229g.
- M. Hatano, T. Mochizuki, K. Nishikawa and K. Ishihara, Enantioselective Aza-Friedel-Crafts Reaction of Indoles with Ketimines Catalyzed by Chiral Potassium Binaphthyldisulfonates, ACS Catal., 2018, 8, 349–353, DOI:10.1021/acscatal.7b03708.
- D. Gaviña, M. Escolano, J. Torres, G. Alzuet-Piña, M. Sánchez-Roselló and C. del Pozo, Organocatalytic Enantioselective Friedel-Crafts Alkylation Reactions of Pyrroles, Adv. Synth. Catal., 2021, 363, 3439–3470, DOI:10.1002/adsc.202100313.
- S. Nakamura, Y. Sakurai, H. Nakashima, N. Shibata and T. Toru, Organocatalytic Enantioselective Aza-Friedel-Crafts Alkylation of Pyrroles with N-(Heteroarenesulfonyl)Imines, Synlett, 2009, 1639–1642, DOI:10.1055/s-0029-1217324.
- Y. Zhao, L. Wang and J. Zhao, Chiral Phosphoric Acid Catalyzed Aza-Friedel-Crafts Alkylation of Indoles with Cyclic Aryl α-Ketimino Esters, Tetrahedron Lett., 2017, 58, 213–217, DOI:10.1016/j.tetlet.2016.12.012.
- Y. Wang, L. Jiang, L. Li, J. Dai, D. Xiong and Z. Shao, An Arylation Strategy to Propargylamines: Catalytic Asymmetric Friedel-Crafts-Type Arylation Reactions of C-Alkynyl Imines, Angew. Chem., 2016, 128, 15366–15370, DOI:10.1002/ange.201608918.
- S. Nakamura, T. Furukawa, T. Hatanaka and Y. Funahashi, Enantioselective Aza-Friedel-Crafts Reaction of Cyclic Ketimines with Indoles Using Chiral Imidazoline-Phosphoric Acid Catalysts, Chem. Commun., 2018, 54, 3811–3814, 10.1039/c8cc00594j.
- S. Nakamura, N. Matsuda and M. Ohara, Organocatalytic Enantioselective Aza-Friedel–Crafts Reaction of Cyclic Ketimines with Pyrroles Using Imidazolinephosphoric Acid Catalysts, Chem. – Eur. J., 2016, 22, 9478–9482, DOI:10.1002/chem.201601573.
- I. Čorić and B. List, Asymmetric Spiroacetalization Catalysed by Confined Brønsted Acids, Nature, 2012, 483, 315–319, DOI:10.1038/nature10932.
- Y. S. Fan, Y. J. Jiang, D. An, D. Sha, J. C. Antilla and S. Zhang, H8-BINOL Chiral Imidodiphosphoric Acids Catalyzed Enantioselective Synthesis of Dihydroindolo-/-Pyrrolo[1,2- a ]Quinoxalines, Org. Lett., 2014, 16, 6112–6115, DOI:10.1021/ol502965p.
- K. Wu, Y. J. Jiang, Y. S. Fan, D. Sha and S. Zhang, Double Axially Chiral Bisphosphorylimides Catalyzed Highly Enantioselective and Efficient Friedel-Crafts Reaction of Indoles with Imines, Chem. – Eur. J., 2013, 19, 474–478, DOI:10.1002/chem.201202900.
- K. Wu, M. H. Zhuo, D. Sha, Y. S. Fan, D. An, Y. J. Jiang and S. Zhang, H8-BINOL Chiral Imidodiphosphoric Acid Catalyzed Highly Enantioselective Aza-Friedel-Crafts Reactions of Pyrroles and Enamides/Imines, Chem. Commun., 2015, 51, 8054–8057, 10.1039/c5cc00685f.
- M. Hatano, H. Okamoto, T. Kawakami, K. Toh, H. Nakatsuji, A. Sakakura and K. Ishihara, Enantioselective Aza-Friedel–Crafts Reaction of Furan with α-Ketimino Esters Induced by a Conjugated Double Hydrogen Bond Network of Chiral Bis(Phosphoric Acid) Catalysts, Chem. Sci., 2018, 9, 6361–6367, 10.1039/C8SC02290A.
- V. Balzani and S. Campagna, Topics in Current Chemistry: Preface, 2007, vol. 280. ISBN: 3540733469 Search PubMed.
- B. R. Buckley, Organocatalysis, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2007, 103, 90–106, 10.1039/b614410c.
- C. Palomo, M. Oiarbide and R. López, Asymmetric Organocatalysis by Chiral Brønsted Bases: Implications and Applications, Chem. Soc. Rev., 2009, 38, 632–653, 10.1039/b708453f.
- U. Kaya, P. Chauhan, S. Mahajan, K. Deckers, A. Valkonen, K. Rissanen and D. Enders, Squaramide-Catalyzed Asymmetric Aza-Friedel–Crafts/N,O-Acetalization Domino Reactions Between 2-Naphthols and Pyrazolinone Ketimines, Angew. Chem., Int. Ed., 2017, 56, 15358–15362, DOI:10.1002/anie.201709224.
- Z. T. Yang, W. L. Yang, L. Chen, H. Sun and W. P. Deng, Organocatalytic Enantioselective Aza-Friedel-Crafts Reactions of Pyrazolinone Ketimines with Hydroxyindoles and Electron-Rich Phenols, Adv. Synth. Catal., 2018, 360, 2049–2054, DOI:10.1002/adsc.201800181.
- E. Dündar and C. Tanyeli, Enantioselective Friedel-Crafts Alkylation of Indole with Nitroalkenes in the Presence of Bifunctional Squaramide Organocatalysts, Tetrahedron Lett., 2021, 73, 16–19, DOI:10.1016/j.tetlet.2021.153153.
- H. M. R. Hoffmann and J. Frackenpohl, Recent Advances in Cinchona Alkaloid Chemistry, Eur. J. Org. Chem., 2004, 4293–4312, DOI:10.1002/ejoc.200400294.
- M. Shi, Z.-Y. Lei, M.-X. Zhao and J.-W. Shi, A Highly Efficient Asymmetric Michael Addition of Anthrone to Nitroalkenes with Cinchona Organocatalysts, Tetrahedron Lett., 2007, 48, 5743–5746, DOI:10.1016/j.tetlet.2007.06.107.
- L. Cheng, L. Liu, H. Jia, D. Wang and Y.-J. Chen, Enantioselective Organocatalytic Anti-Mannich-Type Reaction of N-Unprotected 3-Substituted 2-Oxindoles with Aromatic N-Ts-Aldimines, J. Org. Chem., 2009, 74, 4650–4653, DOI:10.1021/jo9006688.
- X. Liu, H. Li and L. Deng, Highly Enantioselective Amination of α-Substituted α-Cyanoacetates with Chiral Catalysts Accessible from Both Quinine and Quinidine, Org. Lett., 2005, 7, 167–169, DOI:10.1021/ol048190w.
- X. Liu, B. Sun and L. Deng, Catalytic Enantioselective Electrophilic Aminations of Acyclic α-Alkyl β-Carbonyl Nucleophiles, Synlett, 2009, 1685–1689 CAS.
- Z. B. Zhao, L. Shi, Y. Li, F. J. Meng and Y. G. Zhou, Facile Synthesis of Chiral ε-Sultams: Via an Organocatalytic Aza-Friedel-Crafts Reaction, Org. Biomol. Chem., 2019, 17, 6364–6368, 10.1039/c9ob01158g.
- P. Chauhan and S. S. Chimni, Asymmetric Addition of Indoles to Isatins Catalysed by Bifunctional Modified Cinchona Alkaloid Catalysts, Chem. – Eur. J., 2010, 16, 7709–7713, DOI:10.1002/chem.201000846.
- M. Montesinos-Magraner, C. Vila, A. Rendón-Patiño, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, Organocatalytic Enantioselective Friedel-Crafts Aminoalkylation of Indoles in the Carbocyclic Ring, ACS Catal., 2016, 6, 2689–2693, DOI:10.1021/acscatal.6b00260.
- L. Kiefer, T. Gorojankina, P. Dauban, H. Faure, M. Ruat and R. H. Dodd, Design and Synthesis of Cyclic Sulfonamides and Sulfamates as New Calcium Sensing Receptor Agonists, Bioorg. Med. Chem. Lett., 2010, 20, 7483–7487, DOI:10.1016/j.bmcl.2010.10.006.
- C. Vila, A. Tortosa, G. Blay, M. C. Muñoz and J. R. Pedro, Organocatalytic Enantioselective Functionalization of Indoles in the Carbocyclic Ring with Cyclic Imines, New J. Chem., 2019, 43, 130–134, 10.1039/c8nj05577g.
- C.-Y. Li, M. Xiang, J. Zhang, W. Li, Y. Zou, F. Tian and L.-X. Wang, Organocatalytic Enantioselective Aza-Friedel-Crafts Reaction between Benzothiazolimines and 2-Naphthols for Preparation of Chiral 2′-Aminobenzothiazolomethyl Naphthols, Org. Biomol. Chem., 2021, 19, 7690–7694, 10.1039/d1ob01443a.
- Z. Chen, T. Zhang, Y. Sun, L. Wang and Y. Jin, Organocatalytic Enantioselective Aza-Friedel–Crafts Alkylation of β-Naphthols and Isatin-Derived Ketimines via a Takemoto-Type Catalyst, New J. Chem., 2021, 45, 10481–10487, 10.1039/D1NJ01421H.
- S. Takizawa, S. Hirata, K. Murai, H. Fujioka and H. Sasai, C3-Symmetric Chiral Trisimidazoline-Catalyzed Friedel-Crafts (FC)-Type Reaction, Org. Biomol. Chem., 2014, 12, 5827–5830, 10.1039/c4ob00925h.
- M. Bartók, Advances in Immobilized Organocatalysts for the Heterogeneous Asymmetric Direct Aldol Reactions, Catal. Rev., 2015, 57, 192–255, DOI:10.1080/01614940.2015.1039432.
- S. Itsuno and M. M. Hassan, Polymer-Immobilized Chiral Catalysts, RSC Adv., 2014, 4, 52023–52043, 10.1039/c4ra09561h.
- S. Itsuno, M. M. Parvez and N. Haraguchi, Polymeric Chiral Organocatalysts, Polym. Chem., 2011, 2, 1942–1949, 10.1039/c1py00083g.
- A. Corma and H. Garcia, Silica-Bound Homogenous Catalysts as Recoverable and Reusable Catalysts in Organic Synthesis, Adv. Synth. Catal., 2006, 348, 1391–1412, DOI:10.1002/adsc.200606192.
- M. Hatano, X. Zhao, T. Mochizuki, K. Maeda, K. Motokura and K. Ishihara, Reusable Silica-Supported Ammonium BINSate Catalysts for Enantio- and Diastereoselective Friedel–Crafts-Type Double Aminoalkylation of N-Alkylpyrroles with Aldimines, Asian J. Org. Chem., 2021, 10, 360–365, DOI:10.1002/ajoc.202000603.
- P. Yu, J. He and C. Guo, 9-Thiourea Cinchona Alkaloid Supported on Mesoporous Silica as a Highly Enantioselective, Recyclable Heterogeneous Asymmetric Catalyst, Chem. Commun., 2008, 2355–2357, 10.1039/b800640g.
- T. Arai, K. Araseki and J. Kakino, Catalytic Asymmetric Aza-Friedel-Crafts-Type Reaction of Indoles with Isatin-Derived N-Cbz-Ketimines Using a Chiral Bis(Imidazolidine)-Containing NCN-Pincer Palladium Catalyst, Org. Lett., 2019, 21, 8572–8576, DOI:10.1021/acs.orglett.9b03148.
- T. Arai and J. Kakino, Catalytic Asymmetric Synthesis of 3-Indolyl Methanamines Using Unprotected Indoles and N-Boc Imines under Basic Conditions, Angew. Chem., Int. Ed., 2016, 55, 15263–15267, DOI:10.1002/anie.201607679.
- D. Ferraris, B. Young, T. Dudding and T. Lectka, Catalytic, Enantioselective Alkylation of α-Imino Esters Using Late Transition Metal Phosphine Complexes as Catalysts, J. Am. Chem. Soc., 1998, 120, 4548–4549, DOI:10.1021/ja9802450.
- X. Zhang, J. Zhang, L. Lin, H. Zheng, W. Wu, X. Liu and X. Feng, Chiral N,N′-Dioxide-Zinc(II) Complex-Catalyzed Asymmetric Aza-Friedel–Crafts Reaction of Isatin-Derived Ketimines with Indoles, Adv. Synth. Catal., 2016, 358, 3021–3026, DOI:10.1002/adsc.201600630.
- L. Cai, X. Liu, J. Wang, L. Chen, X. Li and J. P. Cheng, Enantioselective and Regioselective Aza-Friedel-Crafts Reaction of Electron-Rich Phenols with Isatin-Derived Ketimines, Chem. Commun., 2020, 56, 10361–10364, 10.1039/d0cc04966b.
- H. G. Cheng, J. Miguélez, H. Miyamura, W. J. Yoo and S. Kobayashi, Integration of Aerobic Oxidation and Intramolecular Asymmetric Aza-Friedel-Crafts Reactions with a Chiral Bifunctional Heterogeneous Catalyst, Chem. Sci., 2017, 8, 1356–1359, 10.1039/C6SC03849B.
- J. M. Shikora and S. R. Chemler, Synthesis of Benzyl Amines via Copper-Catalyzed Enantioselective Aza-Friedel-Crafts Addition of Phenols to N -Sulfonyl Aldimines, Org. Lett., 2018, 20, 2133–2137, DOI:10.1021/acs.orglett.8b00282.
- P. Kumari, A. Jakhar, N. H. Khan, R. Tak, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, Recyclable Chiral Dinuclear Copper(II) Complexes Catalyzed Asymmetric Friedel–Crafts Alkylation of 1-Naphthol Using N-Tosyl Aldimine, Catal. Commun., 2015, 69, 138–142, DOI:10.1016/j.catcom.2015.06.002.
| This journal is © the Partner Organisations 2023 |