Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of fused-ring carbazole derivatives as emitter and/or host materials in organic light emitting diode (OLED) applications
Saliha
Oner
 and
Martin R.
Bryce
and
Martin R.
Bryce
 *
*
Department of Chemistry, Durham University, Stockton Road, Durham DH1 3LE, UK. E-mail: m.r.bryce@durham.ac.uk
First published on 28th June 2023
Abstract
This review focuses on fused-ring carbazole derivatives, their molecular design, electronic and photophysical properties, and in particular their applications as the emitter and/or the host material in the emitting layer of organic light emitting diodes (OLEDs), with emphasis on recent developments. This review is timely because of the rapidly expanding research into fused-ring carbazoles, predominantly indolocarbazole, indenocarbazole, benzofurocarbazole, benzothienocarbazole and diindolocarbazole derivatives. To our knowledge this class of materials has not been reviewed previously. The appeal of fused-ring carbazoles is their extended π-electron systems with good thermal stability, tunable frontier orbital energies that enable a wide gamut (red, green, blue and white) emission colour, high photoluminescence quantum yields, and versatility for chemical functionalisation at different sites, leading to outstanding OLED efficiencies. This review is divided into sections according to the molecules’ role in OLEDs: namely, as conventional luminescent emitters – especially in the deep-blue region; as state-of-the-art hosts for phosphorescent iridium-based emitters; as thermally activated delayed fluorescence (TADF) emitters with high external quantum efficiency; and as multiresonance (MR) emitters with unprecedented high colour purity. We conclude by highlighting the challenges and the great opportunities for fused-ring carbazole derivatives in OLEDs and other optoelectronic applications.
1. Introduction
Relevant general background information will be summarised before focusing on fused-ring carbazoles. Carbazole is a versatile building block for compounds that have been a wide variety of electronic applications such as organic light-emitting diodes (OLEDs),1,2 solar cells,3,4 sensors,5,6 organic field effect transistors (OFETs),7,8 molecular wires,9 and electrochromic devices.10,11 Carbazole is an electron-rich unit, providing good hole transporting properties,12 high thermal and electrochemical stability,13,14 high photoluminescence quantum yield (PLQY),15 ease of functionalisation through different sites and tunable optical and electronic properties.16 (Co)polymerisation of substituted carbazoles is also readily achieved yielding carbazole as a main-chain unit in luminescent and electroactive polymers.17 However, it is important to note that commercial carbazole can contain trace amounts of isomeric impurities, identified as 1H-benzo[f]indole18 and 5H-benzo[b]carbazole,19 which have profound effects on the luminescent properties of the sample and derived products, notably giving enhanced PLQY and ultralong phosphorescence.Through rational molecular design many carbazole derivatives have been developed as emitter and/or host materials for organic light emitting diode (OLED) applications.1,2,20,21 OLED technology is growing rapidly in areas such as consumer electronics, flat panel displays, flexible screens, and solid-state lighting.22 Further development of efficient and cheap OLED materials is still necessary to fully exploit the technology. As a prime example, there is a continued need for stable blue emitters and hosts.23,24 First generation OLEDs were based on fluorescence which has a theoretical maximum of only 25% internal quantum efficiency (IQE) because spin statistics dictate that emissive singlets and non-emissive triplets are formed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, thereby severely limiting the external quantum efficiency (EQE) of the OLEDs.25 Second generation OLEDs utilised phosphorescent emitters which can theoretically achieve 100% IQE by harnessing both singlet and triplet excitons.26 In phosphorescent OLEDs (PhOLEDs) organometallic complexes (predominantly Ir and Pt) can increase the spin–orbit coupling (SOC) due to the heavy atom effect and have favourable intersystem crossing (ISC) rates. By ISC, singlet excitons are transformed into the triplet state and are deactivated radiatively via phosphorescence. In PhOLEDs, the emitter (guest) is doped in a host material to prevent concentration quenching and triplet–triplet annihilation (TTA) processes that decrease the quantum efficiency.27,28 Small-molecule and polymeric carbazole derivatives are ubiquitous host materials for PhOLEDs.29–32 Regarding PhOLEDs, the low natural abundance of the heavy metals makes these complexes more expensive and less attractive for commercialisation compared to pure organic materials. Also, the complexes or their precursors may be toxic because of the presence of heavy metals, and their chemical modification is more limited, with some complexes being unstable especially for blue emission. Because of the above reasons, there is a great demand for organic compounds which are cheaper, more abundant, easy to modify and have high light emission efficiency. Emission mechanisms that are utilised to harvest both singlet and triplet excitons radiatively in pure organic materials include: triplet–triplet annihilation (TTA),33,34 hybridised local and charge transfer (HLCT) excitation35 and triplet–polaron annihilation (TPA).36
3 ratio, thereby severely limiting the external quantum efficiency (EQE) of the OLEDs.25 Second generation OLEDs utilised phosphorescent emitters which can theoretically achieve 100% IQE by harnessing both singlet and triplet excitons.26 In phosphorescent OLEDs (PhOLEDs) organometallic complexes (predominantly Ir and Pt) can increase the spin–orbit coupling (SOC) due to the heavy atom effect and have favourable intersystem crossing (ISC) rates. By ISC, singlet excitons are transformed into the triplet state and are deactivated radiatively via phosphorescence. In PhOLEDs, the emitter (guest) is doped in a host material to prevent concentration quenching and triplet–triplet annihilation (TTA) processes that decrease the quantum efficiency.27,28 Small-molecule and polymeric carbazole derivatives are ubiquitous host materials for PhOLEDs.29–32 Regarding PhOLEDs, the low natural abundance of the heavy metals makes these complexes more expensive and less attractive for commercialisation compared to pure organic materials. Also, the complexes or their precursors may be toxic because of the presence of heavy metals, and their chemical modification is more limited, with some complexes being unstable especially for blue emission. Because of the above reasons, there is a great demand for organic compounds which are cheaper, more abundant, easy to modify and have high light emission efficiency. Emission mechanisms that are utilised to harvest both singlet and triplet excitons radiatively in pure organic materials include: triplet–triplet annihilation (TTA),33,34 hybridised local and charge transfer (HLCT) excitation35 and triplet–polaron annihilation (TPA).36
However, the mechanism that has had the most dramatic impact on the OLED field in the last decade is thermally activated delayed fluorescence (TADF) leading to third-generation organic electroluminescent materials. In this mechanism, T1 excitons are converted into S1 excitons (the subscript 1 denotes the lowest energy T and S states) by a reverse intersystem crossing (RISC) process, causing a delayed fluorescence, which allows theoretical 100% IQE. The basic TADF mechanism in organic materials was known since the 1960s (named “E-type delayed fluorescence”).37 However, its potential had lain dormant until the first report in 2009 from Adachi's group of a TADF organometallic complex in electroluminescence.38 A pure organic compound (indolocarbazole based) was subsequently demonstrated as a TADF emitter in OLEDs, again by Adachi's group.39 To obtain an efficient TADF emitter, a second-order vibronically coupled RISC mechanism is achieved by a molecular design which requires a small S1–T1 energy gap (ΔEST < ≈200 meV). TADF emitters generally have a highly twisted D–A structure (D = electron donor; A = electron acceptor), with optimised dihedral angle and distance between the D and A units, induced by steric hindrance and/or a molecular bridge. Thereby the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO, respectively) are predominantly localised on the D and A units respectively, but at the expense of low values of oscillator strength.
For efficient TADF the emitter should have a small ΔEST, a large SOC, a fast kRISC ≈ 106 s−1 and a high photoluminescence quantum yield (PLQY). Additionally, for good colour purity the emission peak should have narrow full-width at half-maximum (FWHM). The latter is very challenging to achieve as the delayed fluorescence comes from a charge transfer (CT) excited state, which typically features significant structural relaxations leading to broadened emission (FWHM ca. 70–100 nm). According to El-Sayed's rules the direct conversion from T1 to S1 is not allowed because the large CT character of both excited states leads to vanishing SOC. However, an energetically close state (usually a locally excited D or A triplet state, 3LE) comes to the rescue and an efficient spin–flip process can occur within the manifold of 1CT, 3CT and 3LE states. This LE state is tuned by intramolecular vibrations which enhance the SOC, thereby enabling fast ISC and RISC to occur.40
An alternative to the standard D–A design of TADF molecules was first reported in 2016 by Hatakeyama et al.41 The molecules are polycyclic aromatic compounds with electron-deficient boron and electron-rich nitrogen atoms embedded within the rigid carbon framework. Short-range charge transfer occurs through atomically separated frontier molecular orbitals (FMOs) leading to multi-resonance TADF (MR-TADF). Benefitting from the MR effect, the radiative decay constant of fluorescence (kF) of some MR-TADF emitters is ≈107 s−1 which is much higher than most TADF emitters. Narrow FWHM of the emission is achieved due to the presence of FMOs that suppress the vibrational relaxations that occur upon excitation. However, OLEDs based on such BN-based MR emitters tend to show serious efficiency roll-off at higher brightness, and poor device operation stability: a main reason is slow kRISC rates which are generally within the range ≈103–105 s−1.
For thin film photophysical measurements and for OLED fabrication, the emitter molecules are typically dispersed in a host material to limit quenching mechanisms such as triplet–triplet annihilation (TTA) and aggregation caused quenching (ACQ).42,43 A suitable host should possess: (i) higher triplet energies than those of the guest molecules to confine triplet excitons within the emitting layer (EML) by preventing exothermic reverse energy transfer; (ii) energy levels appropriately aligned with those of the neighbouring active layers for efficient charge carrier injection into the EML to achieve a low operating voltage; (iii) balanced charge carrier properties for holes and electrons; (iv) high glass transition temperature (Tg) to favour uniform and stable amorphous films and to minimise the possibility of phase separation upon heating, thus prolonging the operational lifetime of the device.
Four classes of hosts have been reported: (i) hole transporting, based on electron-donating units such as carbazole, arylamine and phenothiazine; (ii) electron transporting, based on electron-withdrawing units, such as triazine, oxadiazole, cyanoarene and phosphine oxide; (iii) bipolar, with both hole transporting and electron transporting units in the same molecule; (iv) mixed hosts that form intermolecular exciplexes. The latter two classes achieve a better balance of holes and electrons in the EML. A drawback of bipolar hosts can be a reduction of the HOMO–LUMO gap due to intramolecular charge transfer, which will lower the S and T energies, and consequently lead to unwanted back-transfer of energy from the guest to the host. Exciplex forming co-hosts have been used especially for PhOLEDs, but also for TADF-OLEDs and hyperfluorescent (TADF-sensitised fluorescence) OLEDs.44,45 Compared to a single bipolar host molecule, an exciplex co-host has more spacially separated frontier molecular orbital distribution which results in reduced intramolecular charge transfer and a smaller ΔEST.46 It is a challenge to fine tune the substituents and steric effects to design molecules with a high T energy which also possess a HOMO–LUMO gap appropriate for injection of both holes and electrons into the doped emitter layer from adjacent layers. It should be noted that ET values can vary significantly between doped films and neat films (by as much as 0.24 eV higher in dilute doped film) ascribed to conformational demands of the molecule in the solid state. Therefore, caution should be used in comparing literature data obtained in different media.47
Classical hole-transporting host materials that are frequently used to benchmark the performance of new emitters or new hosts are 4,4′-bis(9-carbazolyl)biphenyl (CBP) and 1,3-bis(N-carbazolyl)benzene (mCP). CBP has ET 2.56 eV48 and its Tg is 103 °C49 determined by a melt-blending method, correcting earlier reported values of 62 and 112 °C from differential scanning calorimetry (DSC). mCP has significantly higher ET 2.90 eV50 but lower Tg 62 °C (with previous reports quoting 55, 60, 65 and 66 °C).49 However, these hosts are prone to crystallisation, especially when the dopant concentration is low. The ET and Tg values have been raised in conformationally restricted bi(carbazole) derivatives.51,52 Established carbazole-based ambipolar hosts with sufficiently high triplet energies (>2.7 eV) for sky-blue and blue emitters include derivatives with electron accepting pyridine29,53 or phosphine oxide54 substituents. The large S1–T1 gap in conventional host materials causes a high triplet exciton density, which facilitates TTA, triplet–polaron annihilation (TPA) and electric field induced quenching processes, which contribute to detrimental efficiency roll-off in PhOLEDs.55 Therefore, TADF host materials with small ΔEST and efficient RISC rate can overcome these drawbacks, by converting triplet excitons to the singlet state.
Fused-ring carbazole derivatives are having a major impact in the OLED field. Their structures lead to increased rigidity which has the benefit of restricting molecular deformations in the excited state and suppressing nonradiative energy dissipation. Section 2 considers their applications as conventional fluorescent emitters. Section 3 gives examples where fused carbazoles are hosts for phosphorescent iridium complexes. Section 4 is a major part of this review, showcasing how fused carbazoles are key components of some of the most efficient TADF emitters, imparting rigidity,56 narrow band emission,57 small ΔEST, high PLQY,58,59 good thermal stability, and structural versatility. Most notably, indolocarbazole, indenocarbazole, benzofurocarbazole, benzothienocarbazole and diindolocarbazole derivatives are highlighted. Section 5 concerns the current hot topic of multi-resonance (MR) fused carbazoles. In Section 6 we conclude with perspectives on the future of this very active and expanding field. Throughout this article we have retained the nomenclature and abbreviations used for the compounds in the cited publications to facilitate cross-checking with the original data. Selected figures are included to illustrate key optoelectronic properties of the molecules. Synthetic routes will not be considered. In the chemical structures the carbazole frameworks in the fused rings are shown in blue.
2. Fused-ring carbazole derivatives as conventional fluorescent emitters
The emphasis of this section is on stable blue/deep-blue electroluminescence. An initial report that aryl substituted indolo[2,3-a]carbazole derivatives give blue/deep blue fluorescence in solution and in neat thin film set the stage. However, OLEDs were not reported in this work.60 Subsequently, indeno[2,1-b]carbazole derivatives IDC-PA and IDC-Py were studied (Fig. 1a).61λPLmax was 450/439 nm in DCM solution, and 453/443 nm, respectively, in films. The negligible red-shifts indicated that there were almost no intermolecular interactions in the films and aggregation was suppressed by the twisted structures induced by the bulky anthracene and pyrene groups. The PLQYs were 0.84 and 0.78 (DCM solution). The Tgs were 186 and 157 °C for IDC-PA and IDC-Py, respectively. Non-doped OLEDs with a neat EML exhibited efficiencies of up to 4.80 and 4.13 cd A−1, and EQEmax values of 4.41% and 6.08% with deep-blue Commission Internationale de l'Éclairage (CIEx,y) coordinates (0.15, 0.10) and (0.15, 0.08) for IDC-PA and IDC-Py devices, with FWHM of 58 and 56 nm, respectively. There was negligible efficiency roll-off, even at increased voltage (Fig. 1b) and at a brightness of 5000 cd m−2 (EQEs 4.35% and 5.35%).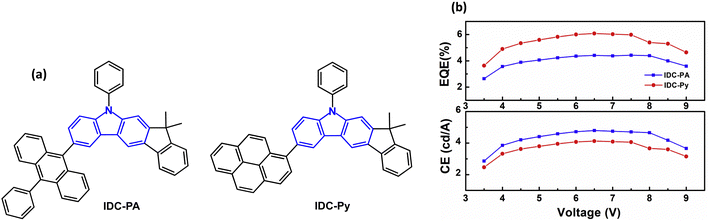 | ||
| Fig. 1 (a) Molecular structures of IDC-PA and IDC-Py. (b) Current efficiency and EQE versus voltage.61 Copyright 2018 Elsevier. | ||
The bis(indolo[3,2-b]carbazole) derivative BEDCE (Fig. 2a) was studied.62 Differential scanning calorimetry (DSC) exhibited a weak exothermic peak at 20 °C, corresponding to a glass transition temperature (Tg), which resulted in an elastomeric material at room temperature. The oligo-oxyethylene chains introduced large steric hindrance, modulating the strong π–π stacking tendency of the fused carbazole skeletons, and provided an amorphous structure. BEDCE showed λPLmax at 426 and 531 nm, with a PLQY of 88% (THF solution). Devices with a solution-processed, non-doped emitter layer had EQEmax 1.34% with CEmax of 4.56 cd A−1 and Lmax of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 cd m−2 with greenish-yellow EL, CIE(0.36, 0.59).
320 cd m−2 with greenish-yellow EL, CIE(0.36, 0.59).
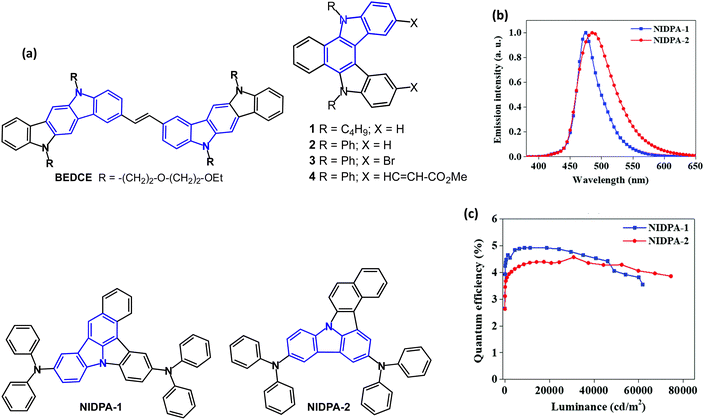 | ||
| Fig. 2 (a) Molecular structures of BEDCE, 1–4, NIDPA-1 and NIDPA-2. (b) EL spectra. (c) EQE–luminance curves of doped OLEDs.64 Copyright 2020 The Royal Society of Chemistry. | ||
The indolo[2,3-c]carbazoles 1–4 (Fig. 2) have λPLmax (undoped films) of 437, 447, 451 and 539 nm, respectively.63 The PL measurements at 77 K (in MeTHF) indicated a red-shifted and structured emission for 1–3 which was attributed to phosphorescence. This behaviour was not observed at room temperature. Monoexponential decays were observed for 1 and 2, indicating that only the S1 state was emitting. Two components were obtained for compound 3, due to fluorescence from the S1 state and a limited degree of dimer formation. For compound 4, all the decays were multiexponential and solvent-dependent. Two components were attributed to different torsional conformations of the ester group, and the third component with a longer lifetime was attributed to the small contribution of a CT state. EL spectra of single- and double-layer devices mostly resembled the PL spectra, but with additional contributions from electromers or electroplexes.
Isomeric benzo[c]carbazole chromophores NIDPA-1 and NIDPA-2 (Fig. 2) showed λPLmax at 473/484 nm with PLQYs of 51/54% (under N2) and FWHMs of 46/71 nm (2 wt% doped in PANB host film) respectively.64 The emission of both compounds in the neat film (489/502 nm) was red-shifted compared to the doped film due to strong intermolecular interactions. The excited-state lifetime of the NIDPA-2 was longer than for NIDPA-1 (26.6 vs. 12.6 ns). The compounds are conventional fluorescent emitters with ΔEST 0.67 and 0.89 eV (PL measurements in PANB host). OLEDs had a PANB:emitter (2 wt%) EML. The NIDPA-1 device exhibited blue EL [λELmax 475 nm, CIE(0.13, 0.22)], EQEmax 4.9% and narrow FWHM of 41 nm. The NIDPA-2 device had broader EL [λELmax 487 nm, CIE(0.16, 0.34)] and EQEmax 4.6%. High values of Lmax 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/74
000/74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cd m−2 were obtained for NIDPA-1 and NIDPA-2 devices, respectively. Both devices showed excellent stability with a very low drop of EQE at high brightness (Fig. 2b and c).
500 cd m−2 were obtained for NIDPA-1 and NIDPA-2 devices, respectively. Both devices showed excellent stability with a very low drop of EQE at high brightness (Fig. 2b and c).
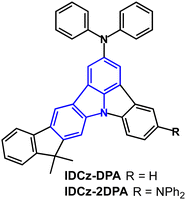
The related indolocarbazole derivatives IDCz-DPA and IDCz-2DPA (Fig. 3) have λPLmax 451 and 457 nm, respectively, in toluene solution.65 PLQYs were 48% and 56% with narrow FWHM of 41 and 37 nm doped in α-ADN films (under N2). Using the same device architecture as for NIDPA-1 and NIDPA-2 (above) IDCz-2DPA showed higher OLED performance than IDCz-DPA, with EQEmax of 5.6% (vs. 5.3%), Lmax 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cd m−2 (vs. 23
600 cd m−2 (vs. 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 cd m−2), FWHM 35 nm (vs. 41 nm), CIE(0.13, 0.15) [vs. (0.14, 0.09)] with little EQE roll-off at high luminance. Lifetimes at 80% (LT80) of the initial luminance (L0) were 142 h and 50 h for IDCz-2DPA and IDCz-DPA devices, respectively. The longer lifetime for IDCz-2DPA was explained by the higher current efficiency and red-shifted colour coordinate (i.e., lower emission energy) reducing stress on the device.
700 cd m−2), FWHM 35 nm (vs. 41 nm), CIE(0.13, 0.15) [vs. (0.14, 0.09)] with little EQE roll-off at high luminance. Lifetimes at 80% (LT80) of the initial luminance (L0) were 142 h and 50 h for IDCz-2DPA and IDCz-DPA devices, respectively. The longer lifetime for IDCz-2DPA was explained by the higher current efficiency and red-shifted colour coordinate (i.e., lower emission energy) reducing stress on the device.
In 2021 indeno[2,1-b]carbazole derivative m-FLDID (Fig. 4a) was reported as a violet emitter.66 The HOMO/LUMO energies were calculated by density functional theory (DFT) as 5.20/1.38 eV, resulting in a wide energy gap (Eg) of 3.82 eV. Electrochemical measurements gave Eg 3.32 eV. m-FLDID showed λPLmax 400 nm in THF solution and 404 nm as 1 wt% doped in mixed mCP:TSPO1 host, with FWHM values of 23 and 22 nm, respectively. These values are comparable to the best multi-resonance (MR) type emitters (see Section 5).67,68 The remarkably small FWHM, small Stokes shift (6 nm), and sharp emission of m-FLDID were attributed to an MR structure with alternating HOMO and LUMO distribution. S1/T1/ΔEST energies are 3.08/2.72/0.36 eV. m-FLDID had PLQY 71% in mixed mCP:TSPO1 host, which remained unchanged in air and nitrogen atmosphere, confirming that there was no contribution from triplet excitons (i.e. no TADF); τPF was 15.5 ns without a delayed component. OLEDs with mCP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TSPO1 host
TSPO1 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m-FLDID (25
m-FLDID (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X wt%) EML had EQEmax values of 4.4–5.2% with a narrow FWHM of 17–27 nm, depending on the doping concentration, and CIEy < 0.03 (Fig. 4b). However, there was extensive efficiency roll-off to 2.7–3.2% at 100 cd m−2.
X wt%) EML had EQEmax values of 4.4–5.2% with a narrow FWHM of 17–27 nm, depending on the doping concentration, and CIEy < 0.03 (Fig. 4b). However, there was extensive efficiency roll-off to 2.7–3.2% at 100 cd m−2.
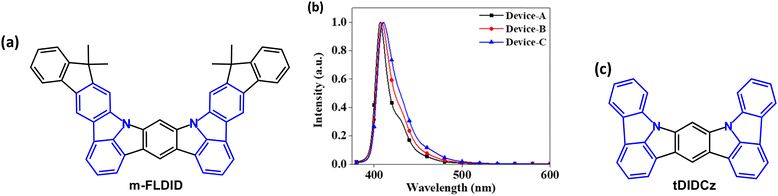 | ||
| Fig. 4 (a) Molecular structure of m-FLDID. (b) EL spectra of m-FLDID Devices A (1 wt%) B (3 wt%) and C (5 wt%).66 Copyright 2021 The American Chemical Society. (c) Molecular structure of tDIDCz. | ||
A similar core structure tDIDCz (Fig. 4c) gave violet-emitting OLEDs with CIE(0.164, 0.018) and remarkably narrow FWHM of only 14 nm in mCBP:TSPO1 host. The EQEmax was 3.3% with extensive efficiency roll off to ≈1% at 100 cd m−2.69
3 Fused-ring carbazole derivatives as host materials
Molecules that are both conventional fluorescent emitters and hosts are covered in this section. In many cases 1,3,5-triazine derivatives are prominent acceptors in these types of bipolar molecules. Two indolocarbazole donors were combined with a triazine acceptor to obtain BICT, PBICT, POBICT and BBICT (Fig. 5).70 An additional electron-withdrawing or -donating group (substituent R) on the triazine tuned the energy levels, the transporting ability and the ΔEST values. Intramolecular π–π stacking was observed between the two indolocarbazole units (in single-crystal X-ray structures). DFT calculations indicated the dihedral angles between the D and A groups were in the range 41–44° for the four compounds. Tg values were in the range 164–202 °C. λPLmax red shifted sequentially from deep-blue to green for POBICT, BICT, PBICT and BBICT (412, 425, 469 and 516 nm in DCM solution). The T1 energy decreased in the order of BICT ≈ POBICT (2.70 eV) > PBICT (2.66 eV) > BBICT (2.47 eV) (PL measurements). The ΔEST values varied between 0.34 eV for POBICT and 0.06 eV for BBICT. The compounds were host materials for PhOLEDs with the orange emitter iridium(III)bis(4-phenylthieno[3,2-c]pyridinato-N,C2′) acetylacetonate (PO-01) as the guest with 10 wt% doping in the EML. The highest EQEmax of 24.5% was obtained with PBICT host. All the devices showed small efficiency roll-off with 90% of the initial luminance at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 which was attributed to the wide charge recombination zone in the EML due to the bipolar hosts.
000 cd m−2 which was attributed to the wide charge recombination zone in the EML due to the bipolar hosts.
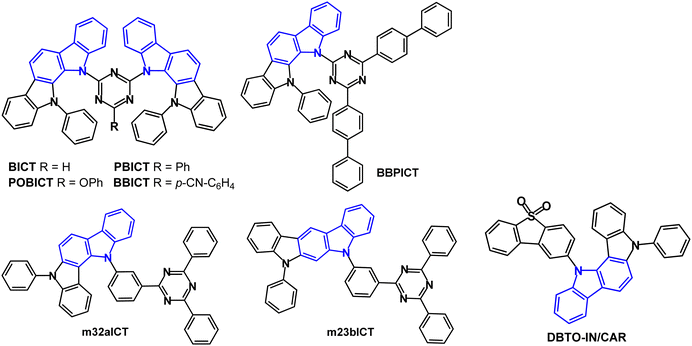 | ||
| Fig. 5 Molecular structures of indolocarbazole derivatives BICT, PBICT, POBICT, BBICT, BBPICT, m32aICT, m23bICT and DBTO-IN/CAR. | ||
BBPICT (Fig. 5) is a host for red PhOLEDs using the emitter Ir(mphmq)2(tmd).71BBPICT exhibited a broad peak at λPLmax 510 nm (toluene solution); S1/T1/ΔEST levels were 2.74/2.58/0.16 eV (PL measurements). The device structure used BBPICT:Ir(mphmq)2(tmd) (2 wt%) dopant as the EML. EQEmax 20.9% was obtained. The devices exhibited small efficiency roll-off of 1.7% at 1000 cd m−2, and a long operational half-life LT50 of 1390 h at a brightness of 1000 cd m−2. Comparable devices with CBP host had much higher efficiency roll-off of 17.3% at 1000 cd m−2 and shorter LT50 of 57 h, attributed to CBP's unbalanced carrier mobility and larger ΔEST, compared to BBPICT.
The isomeric indolo[3,2-a] and indolo[2,3-b]carbazole derivatives m32aICT and m23bICT (Fig. 5) are also efficient host materials.72 They exhibit broad, featureless emission at λPLmax 493/482 nm (toluene solution), respectively. S1/T1/ΔEST levels were 2.90/2.89/0.01 eV and 2.92/2.72/0.20 eV, respectively (toluene solution). PL transient decay curves (pristine films) indicated the presence of delayed components for m32aICT related to its small ΔEST, whereas for m23bICT only a negligible delayed component was observed. The OLED structure used DACT-II (a carbazole–phenylene–triazine derivative) as a TADF sensitiser and PhtBuPAD as a conventional anthracene-based fluorescent emitter, together with the new host in the EML. The m32aICT host device had green emission EQEmax/PEmax values of 23.2%/76.9 lm W−1, remaining at 20.0% and 46.4 lm W−1 at 5000 cd m−2, which was more efficient than the m23bICT device.
Dibenzothiophene-S,S-dioxide served as the acceptor in the bipolar indolocarbazole host DBTO-IN/CAR (Fig. 5) in PhOLEDs with the archetypal green emitter Ir(ppy)3.73DBTO-IN/CAR has Tg 156 °C and ET 2.71 eV (in toluene). PhOLEDs with a DBTO-IN/CAR:Ir(ppy)3 (5 wt%) EML exhibited maximum PEmax/CEmax/EQEmax of 45.36 lm W−1, 51.98 cd A−1 and 19.03%, which were only slightly higher than the reference device with CBP host (42.01 lm W−1, 50.81 cd A−1, 17.72%). DBTO-IN/CAR was also used as a non-doped cyan fluorescence emitter in devices which exhibited 3.0 lm W−1, 4.30 cd A−1 and EQEmax 2.47%, CIE(0.23, 0.41).
Bipolar indenocarbazoles have also been studied. DPDDC (Fig. 6a) is a host for green PhOLEDs.74DPDDC has high thermal stability with a decomposition temperature (Td) of 441 °C (corresponding to 5% weight loss), and Tg of 140 °C. The λPLmax was 475 nm (thin solid film) and ET/ΔEST values were 2.79/0.18 eV, respectively (in 2Me-THF solution). Transient decay times of 10 wt% doped films of DPDDC in mCP were τPF 46.4 ns and τDF 12.3 μs. Green PhOLEDs with an EML comprising DPDDC:(mdppy)2Ir(acac) (5 wt%) exhibited EQEmax 23.6% with low efficiency roll-off of 5.5% at 5000 cd m−2. The devices operated at room temperature and at a constant current density for an initial luminance of 5000 cd m−2 showed an improved lifetime LT90 of ca. 665 min compared to a device with CBP host (LT90 = 31 min). The long operating lifetime was attributed to the minimised interactions between the triplets on the Ir dopant and the polarons on the DPDDC host molecules.
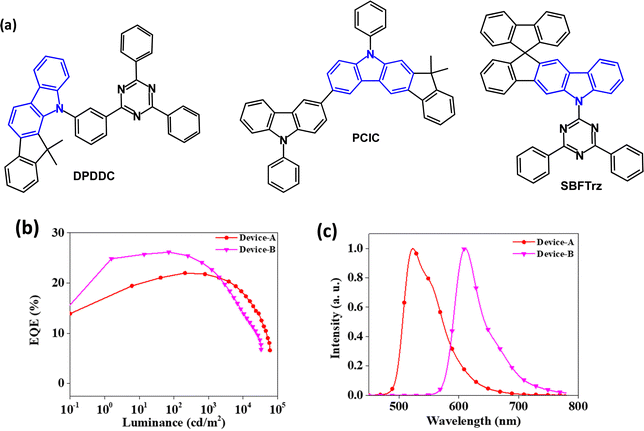 | ||
| Fig. 6 (a) Molecular structures of indenocarbazoles DPDDC, PCIC and SBFTrz. (b) EQE–L data for SBFTrz-Ir(ppy)2acac (Device-A) and SBFTrz-Ir(mphmq)2tmd (Device-B).76 (c) EL spectra of Device-A and Device-B.76 Copyright 2021 Elsevier. | ||
A hole transporting indenocarbazole derivative PCIC (Fig. 6a) was developed.75 The T1 level is 2.75 eV (PL data in toluene) Td 482 °C (TGA, 5% weight loss) and Tg (DSC) 98 °C. PCIC served as a host for the emitter Ir(mppy)3 in green PhOLEDs with a solution processed EML. A lifetime of 1300 hours to LT50, and CE 66.2 cd A−1 (at 1000 cd m−2) was obtained for a device structure that included a bipolar exciton blocking layer with a high triplet level to suppress exciton migration from the green emissive layer. EQE data were not reported.
Theoretical studies of SBFTrz (Fig. 6a) indicated bipolar CT character with the spiro unit enforcing a highly twisted geometry.76 The Tg of SBFTrz is 134 °C and S1/T1/ΔEST energies are 3.63/2.74/0.89 eV. Based on data from hole-only and electron-only devices, SBFTrz was treated as a predominantly electron transporting host and was combined with p-type BPBPCz (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) to develop an exciplex co-host system with improved charge balance in the EML. Green and red PhOLEDs had Ir(ppy)2acac and Ir(mphmq)2tmd emitters, respectively, at 5 wt% doping, with SBFTrz:BPBPCz:dopant as the EML. EQEmax values were 22.0 and 26.2%, λELmax 523 nm, CIE(0.33, 0.63) and 610 nm, CIE(0.65, 0.35), and low efficiency roll-off up to 1000 cd m−2 (Fig. 6b and c). There was three- and five-fold enhancement in the operational lifetime of the exciplex-host green and red PhOLEDs, compared to the corresponding SBFTrz single-host devices.
1 ratio) to develop an exciplex co-host system with improved charge balance in the EML. Green and red PhOLEDs had Ir(ppy)2acac and Ir(mphmq)2tmd emitters, respectively, at 5 wt% doping, with SBFTrz:BPBPCz:dopant as the EML. EQEmax values were 22.0 and 26.2%, λELmax 523 nm, CIE(0.33, 0.63) and 610 nm, CIE(0.65, 0.35), and low efficiency roll-off up to 1000 cd m−2 (Fig. 6b and c). There was three- and five-fold enhancement in the operational lifetime of the exciplex-host green and red PhOLEDs, compared to the corresponding SBFTrz single-host devices.
Indolo[3,2,1-jk]carbazole derivatives ICz-PPI and 2ICz-PPI (Fig. 7a) were studied as conventional fluorescent emitters and as hosts for PhOLEDs.77 DFT studies indicated a small overlap between HOMO and LUMO with the ICz unit acting as the acceptor (Fig. 7b). ICz-PPI and 2ICz-PPI are violet-blue emitters with λPLmax 398/414 nm in DCM and PLQYs of 72.5/63.9%, respectively. PL measurements in different polarity solvents suggested a weak ICT interaction. The emissions showed small red shifts (24 and 28 nm, respectively) in solid thin films, indicating that the π–π interactions in the solid state are suppressed by the twisted molecular structures. The T1 energies were 2.48/2.80 eV (PL measurements). Introducing the additional ICz group (2ICz-PPI) significantly increased the T1 energy and slightly decreased the PLQY. Non-doped blue-emitting OLEDs used ICz-PPI and 2ICz-PPI as the EML. The ICz-PPI device had EQEmax 2.47%, CIE(0.153, 0.121), while the 2ICz-PPI device had EQEmax 1.94%, CIE(0.161, 0.102). Based on data for hole-only and electron-only devices, ICz-PPI could be a better bipolar host material compared with 2ICz-PPI.
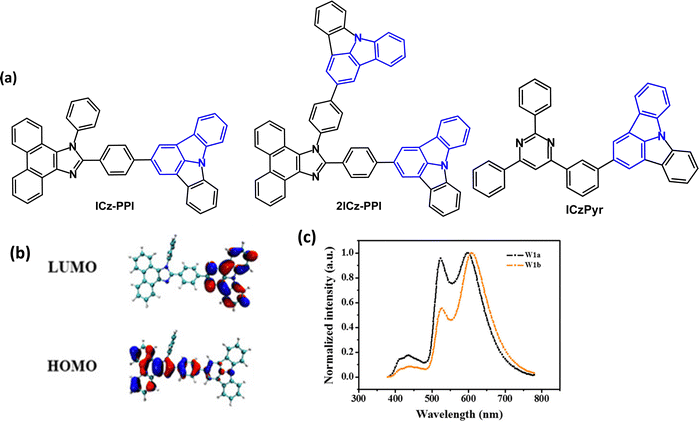 | ||
| Fig. 7 (a) Molecular structures of ICz-PPI, 2ICz-PPI and ICzPyr. (b) HOMO and LUMO plots for ICz-PPI.77 (c) EL spectra of warm-white devices using Ir(MDQ)2(acac), Ir(ppy)3 and ICz-PPI in two different device configurations (W1a and W1b).77 Copyright 2019 Wiley-VCH. | ||
ICz-PPI and 2ICz-PPI were tested as hosts in green and red PhOLEDs, with host:Ir(ppy)3 (8 wt%) or Ir(MDQ)2acac (10 wt%) as the EMLs. The ICz-PPI devices were more efficient with CEmax/EQEmax 62.8 cd A−1 and 17.8% for Ir(ppy)3, and 25.7 cd A−1 and 19.4% for Ir(MDQ)2acac. The ICz-PPI:Ir(MDQ)2acac device retained EQE of 14.9% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. Furthermore, ICz-PPI served as both host and emitter in a warm-white OLED, CIE(0.427, 0.468) with EQEmax 14.4%, Lmax 42
000 cd m−2. Furthermore, ICz-PPI served as both host and emitter in a warm-white OLED, CIE(0.427, 0.468) with EQEmax 14.4%, Lmax 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cd m−2, through simultaneous emission from three layers in which Ir(MDQ)2(acac), Ir(ppy)3 and ICz-PPI were the red, green and blue components, respectively (Fig. 7c).
500 cd m−2, through simultaneous emission from three layers in which Ir(MDQ)2(acac), Ir(ppy)3 and ICz-PPI were the red, green and blue components, respectively (Fig. 7c).
ICzPyr (Fig. 7a) is a host for the green TADF emitter 4CzIPN.78 Theoretical calculations indicated that the HOMO was localised on the indolo[3,2,1-jk]carbazole unit while the LUMO was localised on the pyrimidine and peripheral phenyl units. The Tg of ICzPyr is 110 °C and S1/T1/ΔEST levels are 3.17/2.83/0.34 eV (PL data in solution). The PL spectrum of ICzPyr showed a very narrow FWHM of 22 nm. Green TADF-OLEDs with ICzPyr:4CzIPN (10 wt%) as the EML had EQE 10.0% at 100 cd m−2 and 13.9% at 1000 cd m−2. The device using ICzPyr showed a lower driving voltage, higher luminous efficiency, and a significantly longer lifetime of LT50 5.5 h than using a conventional mCBP/4CzIPN emitter layer (LT50 1.0 h) which was ascribed to the bipolar characteristics of ICzPyr.
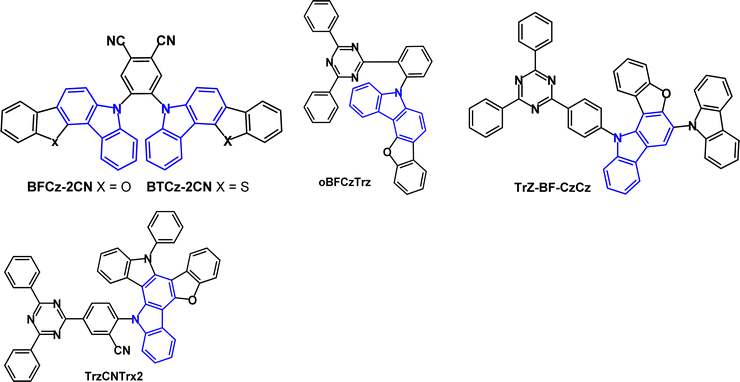
Benzofuro- and benzothieno-carbazole derivatives BFCz and BTCz (Fig. 8) were designed as analogues of mCP.50 The Tg of both compounds was significantly increased compared to mCP, to 147 °C (BFCz) and 157 °C (BTCz) due to the higher rigidity of the fused rings which had a positive effect on the film forming properties. The photophysical and device properties of BFCz and BTCz were very similar. The λPLmax was 365 and 362 nm, respectively (in solid polystyrene). The HOMO was distributed over the heteroaromatic core with the LUMO predominantly on the central phenylene ring, giving some bipolar character. The high T1 energies of 2.94 and 2.93 eV enabled their use as hosts for sky-blue PhOLEDs with FIrpic guest emitter in the EML. The EQEmax values were 15.0 and 16.1%; the power efficiencies were 14.9 and 14.8 lm W−1, respectively, with CIE(0.16, 0.37).
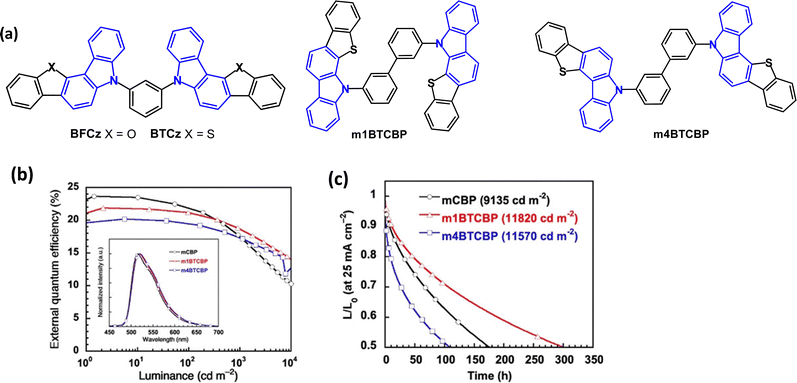 | ||
| Fig. 8 (a) Molecular structures of BFCz, BTCz, m1BTCBP and m4BTCBP. (b) EQE–L characteristics.79 (c) Operational lifetime at LT50 with Ir(ppy)3 emitter.79 Copyright 2021 Wiley-VCH. | ||
The isomeric benzothieno[3,2-c]carbazole (BTCz) derivatives m1BTCBP and m4BTCBP (Fig. 8a) were studied as host materials.79 DFT calculations showed that the HOMO and LUMO were distributed on the phenylcarbazole units and biphenyl linkers, respectively. The π-expanded carbazole units provided deeper LUMO levels and shallower HOMO levels, compared to mCBP. Also, compared to mCBP, the BTCz group increased the bond dissociation energy (BDE) of the Cphenylene–N bond by 10% in the anionic state. The T1 energies were calculated as 2.87 and 2.94 eV for m1BTCBP, and m4BTCBP, respectively. Thermal studies showed considerably higher Tg values (166–167 °C) compared to mCP. PhOLEDs using host:Ir(ppy)3 emitter (12 wt%) as the EML, gave EQEmax > 20% and low turn-on voltages around 3.0 V at 1 cd m−2 and 4.0 V at 1000 cd m−2. A comparable mCBP-based device had higher EQEmax (24%), but also higher efficiency roll-off (Fig. 8b). m1BTCBP:[Ir(ppy)3] OLEDs exhibited LT50 300 h at an initial luminance of ca. 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 (Fig. 8c). The LT50 at 1000 cd m−2 was estimated to be ≈ 23
000 cd m−2 (Fig. 8c). The LT50 at 1000 cd m−2 was estimated to be ≈ 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h which was higher than that of mCBP-based OLEDs (LT50 ≈ 8500 h).
000 h which was higher than that of mCBP-based OLEDs (LT50 ≈ 8500 h).
The lifetime improvement of green PhOLEDs with Ir(ppy)3 emitter was demonstrated by using an exciplex host based on a mixture of a fused-ring pyrrolocarbazole hole transport component with a triazine type electron transport component.80 The lifetime of the exciplex-host device to LT80 was ≈30 h which was >15 times longer than that of single-host devices.
4 Fused carbazole derivatives as TADF emitters
4.1 Indolocarbazole, indenocarbazole, benzofurocarbazole, benzothienocarbazole and pyrrolocarbazole derivatives
The rather weak electron donating ability of carbazole limits it use in TADF emitters. Alternative six-membered nitrogen heterocyclic donors such as phenothiazine, phenoxazine and 9,9-dimethyl-9,10-dihydroacridine have been successful, but their TADF efficiency can be complicated by different conformers in which the nitrogen lone pairs are oriented differently with respect to the acceptor.81–83 Processes that decrease the efficiency of TADF emitters are concentration quenching and exciton annihilations, such as triplet–triplet annihilation (TTA), singlet–triplet annihilation (STA), and triplet–polaron annihilation (TPA).84,85 Designs that combine two or more (non-fused) carbazole units,86 or fused-ring carbazole units, have been widely exploited to impart increased donor strength and molecular rigidity, especially in very efficient green and blue TADF emitters. Fused-ring systems will now be considered.Indolocarbazole derivatives are popular because of their ease of synthesis and systematic structural variation. Indeed, indolocarbazole has a special distinction as a component of the first all-organic TADF emitter PICTRZ used for OLEDs by Adachi's group in 2011 (Fig. 9a).39 The two indolocarbazole units increase the steric hindrance between the D and A units of PICTRZ (compared to carbazole) leading to a well-separated HOMO and LUMO. The PL spectrum showed green emission with a large Stokes shift, indicating an efficient intramolecular charge transfer. S1/T1/ΔEST values were 2.66/2.55/0.11 eV for mCP:PICTRZ (6 wt%) film. The small ΔEST together with a high fluorescence radiative decay rate (kr ≈ 107) allowed the up-conversion from T1 → S1 and efficient TADF (Fig. 9b). Transient decay times were τPF ≈ 10 ns and τDF ≈ 230 μs. The PLQYs for PICTRZ in non-degassed/degassed toluene solution were 10 ± 3%/35 ± 3%. The OLED with mCP host:PICTRZ (6 wt%) as the EML had EQEmax 5.3%. This landmark paper initiated a surge of interest in all-organic TADF-OLEDs.
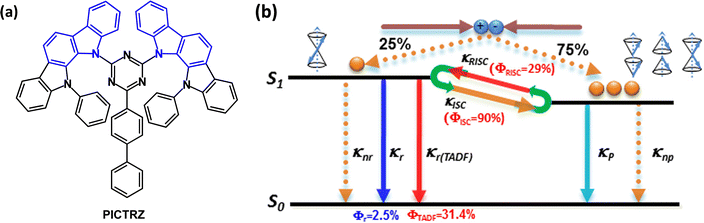 | ||
| Fig. 9 (a) Molecular structure of PICTRZ. (b) A first approximation of the electroluminescence process involving TADF with efficient intersystem crossing from T1 → S1.39 Copyright 2011 The American Institute of Physics. | ||
The isomeric indolocarbazole–triazine D–A molecules IndCzpTr-1 and IndCzpTr-2 (Fig. 10) were reported in 2018.58 The calculated HOMO/LUMO values were −5.16/−1.91 and −4.92/−1.90 eV for IndCzpTr-1 and IndCzpTr-2. The shallower HOMO of IndCzpTr-2 indicates the stronger electron donating ability of indolo[3,2-b]carbazole compared to the [2,3-a] unit in IndCzpTr-1. λPLmax values were 480 and 490 nm (toluene solution); S1/T1/ΔEST values were 2.94/2.81/0.13 eV for IndCzpTr-1 and 2.77/2.66/0.11 eV for IndCzpTr-2 (PL measurements). PLQYs were >70% for IndCzpTr-1 and IndCzpTr-2 (mCBP doped films in air). OLEDs had an mCBP:emitter (10–20 wt%) EML. The IndCzpTr-1 OLEDs exhibited EQEmax 14.5% with CEmax 28.1 cd A−1 (sky-blue emission) whereas IndCzpTr-2 OLEDs had EQEmax 30.0% with CEmax of 82.6 cd A−1 (green emission) without any optical outcoupling technology. The high efficiency of the latter was explained by the more linear structure of IndCzpTr-2 imparting a preferential horizontal dipole orientation which could enhance carrier mobility.
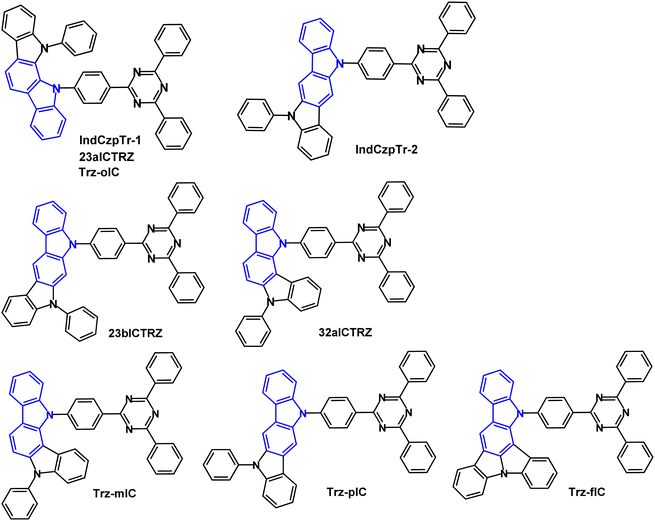 | ||
| Fig. 10 Molecular structures of IndCzpTr-1/23aICTRZ/Trz-oIC, IndCzpTr-2, 23bICTRZ, 32aICTRZ, Trz-mIC, Trz-pIC and Trz-fIC. | ||
Another 2018 report on IndCzpTr-1 (re-named as 23aICTRZ), 23bICTRZ and 32aICRTZ (Fig. 10) focused on the importance of the phenylene group between the D and A units.87 This work illustrates that well-separated HOMO/LUMO distributions are required to obtain a small ΔEST, although completely separated HOMO and LUMO leads to a relatively low radiative decay rate (kr, usually 106 s−1) according to Fermi's golden rule.87,88 The enlarged separation between D and A increases the dipole moment change in the charge-transfer (CT) transition89 and also controls the dihedral angle between D and A to modulate the FMO distributions. As a result, the compounds with a phenylene bridge have the potential to achieve high kr and small ΔEST simultaneously. The analogue PIC-TRZ2, without a phenylene spacer, showed completely separated HOMO/LUMO distributions88 whereas the FMOs of 23aICTRZ, 23bICTRZ and 32aICTRZ showed some overlap resulting in higher oscillator strengths (f) of 0.123, 0.161, and 0.025, respectively, compared to PIC-TRZ2 (f 0.0012). Tg values were in the range 155–159 °C; T1 energies were 2.69, 2.70 and 2.80 eV (PL in toluene) for 23aICTRZ, 23bICTRZ, and 32aICTRZ, respectively, giving ΔEST values of 0.25, 0.27, and 0.15 eV. The lower T1 value of the first two compounds indicates a stronger interaction between D and A, compared to 32aICTRZ. Significant DF emission was observed for 32aICTRZ (consistent with the smaller ΔEST) while DF was negligible for the other derivatives. The kr values were very high, around 108 s−1, compared to ca. 105 s−1 for PIC-TRZ288 which was attributed to the favoured FMO distributions of 23aICTRZ, 23bICTRZ and 32aICTRZ. TADF-OLEDs with DPEPO host:32aICTRZ (10 wt%) EML exhibited EQEmax 25.1% and 21.5% at 5000 cd m−2 with λELmax 512 nm. The devices based on 23aICTRZ and 23bICTRZ had much lower EQEmax 13.6% and 9.8%, respectively, with significant efficiency roll-off. Additionally, by utilising 32aICTRZ as the host for green TADF-OLEDs with DACT-II as the emitter (15 wt%) impressively high EQE/PE of 26.2%/69.7 lm W−1 at a brightness of 5000 cd m−2 was obtained.
The TADF properties of the isomeric indolocarbazole derivatives IndCzpTr-1 (re-named as Trz-oIC), Trz-mIC and Trz-pIC, and the fused ring analogue Trz-fIC, (Fig. 10) were subsequently reported.90 The absorption spectrum of Trz-pIC was red-shifted compared to other isomers, because of a stronger ICT interaction. The non-fused carbazole analogue (PhCzTrz) had decreased donor strength and reduced ICT.91 ΔEST increased in the sequence Trz-mIC (0.13 eV) < Trz-oIC (0.16 eV) < Trz-pIC (0.29 eV) < Trz-fIC (0.45 eV). The PLQY (DBFPO doped films) was highest for Trz-mIC (90%), which also showed the highest DF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF component ratio (62
PF component ratio (62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28) in the series. KRISC rates in the series were in the range 4.03–11.90 × 10−5 s−1. OLEDs with a DBFPO host:emitter (20 wt%) EML had EQEmax that increased in the order Trz-fIC (10.6%) < Trz-oIC (23.2%) < Trz-pIC (26.8%) < Trz-mIC (28.0%) with maximum current efficiencies of 18.4, 46.1, 66.8 and 56.5 cd A−1, respectively. The devices showed sky-blue to green emission with λELmax 470–507 nm and FWHM 75–85 nm (Fig. 11).
28) in the series. KRISC rates in the series were in the range 4.03–11.90 × 10−5 s−1. OLEDs with a DBFPO host:emitter (20 wt%) EML had EQEmax that increased in the order Trz-fIC (10.6%) < Trz-oIC (23.2%) < Trz-pIC (26.8%) < Trz-mIC (28.0%) with maximum current efficiencies of 18.4, 46.1, 66.8 and 56.5 cd A−1, respectively. The devices showed sky-blue to green emission with λELmax 470–507 nm and FWHM 75–85 nm (Fig. 11).
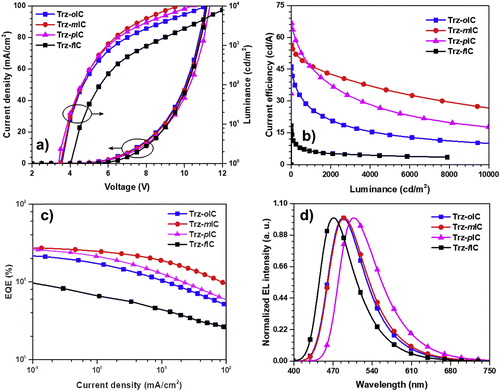 | ||
| Fig. 11 Electroluminescence properties: (a) J–V–L plot; (b) current efficiency; (c) EQE and (d) EL spectra of Trz-oIC, Trz-mIC, Trz-pIC and Trz-fIC.90 Copyright 2019 Elsevier. | ||
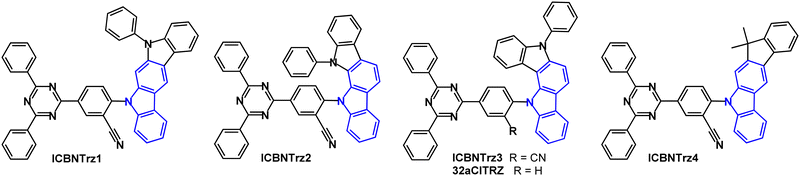
A series of green indolo- and indeno-carbazole TADF emitters reported in 2022 have diphenyltriazine as the main acceptor group and benzonitrile as a secondary acceptor which served to increase the bond dissociation energy (BDE) and the kRISC for TADF emission (Fig. 12).92 Compared to the non-CN analogue (32aCITRZ), ICBNTrz3 has a much smaller ΔEST because the CN group stabilises the CT singlet state. ICBNTrz3 was the most successful emitter of the series with PLQY 85% (5 wt% doped film in CzTrz), kRISC 3.15 × 105 s−1, ΔEST 0.04 eV (1 wt% doped film in polystyrene). Devices had CzTrz host:emitter (20 wt%) as the EML. The EQEmax of ICBNTrz3 devices was 18.5%. The device lifetime was extended by more than 20 times compared to the 32aCITRZ devices. The ICBNTrz3 device had LT90 = 532 h at 1000 cd m−2 which is among the longest lifetimes achieved to date in green TADF OLEDs. The long lifetime correlates with the high kRISC which reduces triplet exciton density.
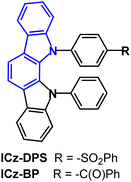
The indolo[2,3-a]carbazole D–A emitters ICz-DPS and ICz-BP with sulfonyl and carbonyl units in the acceptor unit (Fig. 13) show both TADF and aggregation induced emission (AIE) properties.93 AIE can be beneficial in OLEDs as some non-doped devices can show high efficiencies.94–96 Large dihedral angles (>70°) between the D–A groups prevent intermolecular π–π stacking and the phenylene spacer between the D–A units interrupts the HOMO–LUMO spatial overlap. ΔEST was reduced from 0.21 eV (ICz-DPS) to 0.10 eV (ICz-BP): the deeper LUMO level of ICz-BP reflects the stronger electron-accepting ability of BP. ICz-DPS and ICz-BP have λPLmax 438 nm and 481 nm; PLQY 53% and 62%; kRISC 30.5 and 39.7 × 105 s−1 (neat film). The excited state lifetimes were: ICz-DPSτPF = 22.7 ns, τDF = 616 ns; ICz-BPτPF = 21.1 ns, τDF = 448 ns (10 wt% films in DPEPO). OLEDs with a DPEPO host:emitter (10 w%) EML had λELmax 435 nm (0.15, 0.08) and 475 nm (0.17, 0.28) with EQEmax/CEmax of 11.6%/30.1 cd A−1 and 17.7%/44.8 cd A−1 for the ICz-DPS and ICz-BP devices, respectively. Non-doped OLEDs based on ICz-DPS had efficiencies of 11.7% and 30.1 cd A−1. The deep blue emission of ICz-DPS is notable.
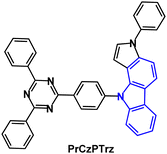
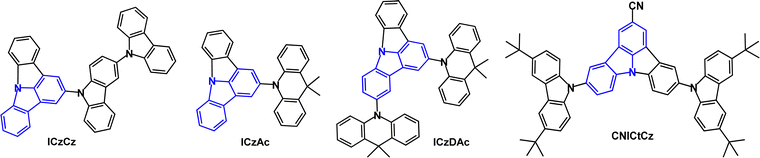
The TADF emitter PrCzPTrz (Fig. 14) contains an unusual pyrrolocarbazole donor.97 Fusion of the pyrrole unit increased the donor character compared to the parent carbazole, as indicated by the shallower calculated HOMO energy of PrCz. The PLQYs for PrCzPTrz were 0.13/0.80 under oxygen/nitrogen with ΔEST 0.03 eV (toluene solution) and τDF 15.8 μs. OLEDs with mCP host:PrCzPTrz (10–50 wt%) EML had EQEmax 17.7% with green emission, CIE(0.26, 0.47). The EQE was much higher than for a comparable device with a non-fused carbazole donor (EQEmax 4%) although lower than the dimethylacridine analogue DMAC-TRZ using an alternative device structure (EQEmax 26.5%).98 The LT50 of the PrCzPTrz device (≈0.7 h) was improved compared to the DMAC analogue (<0.1 h).
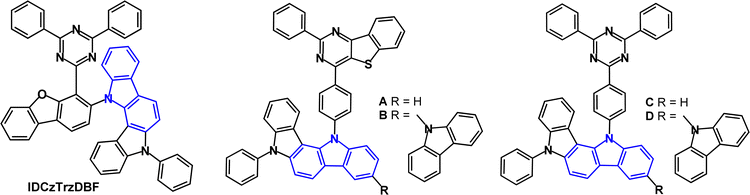
A series of indolocarbazole derivatives with additional heterocyclic functionality in the bridge or in the acceptor has been reported. IDCzTrzDBF (Fig. 15) has dibenzofuran as a linker chosen for its rigidity, steric effect and high stability.99 The HOMO and LUMO were largely separated with a small overlap on dibenzofuran. Theoretical calculations revealed a dihedral angle of 77° between the D and A units. S1/T1/ΔEST levels were 2.92/2.87/0.05 eV (PL, toluene solution). The PLQY under air/nitrogen was 77.3/85.4% (5 wt% doped in mCBPTrz). The transient PL decay showed τDF 2.8 μs. Green TADF-OLEDs with mCBPTrz host:IDCzTrzDBF (5 wt%) EML had EQEmax 12.2%, which was lower than analogues with di or tri(carbazole) instead of IDCz (EQE > 20%). The relatively low EQEmax of IDCzTrzDBF, despite its high PLQY, was attributed to the poor carrier balance in the EML as shown in the hole- and electron-only device measurements. The LT85 of the IDCzTrzDBF devices at an initial luminance (L0) of 3000 cd m−2 was 56.9 h; the distorted structure of the molecule reduces the device lifetime compared to carbazole analogues.
Blue/sky-blue emitters A–D (Fig. 15) include two with the unusual thienopyrimidine acceptor (A, B) instead of triazine (C, D).100 The calculated triplet energies were between 2.55 and 2.67 eV. PLQY measurements (toluene solution) indicated that C and D had slightly higher values (≈0.8) than A and B (≈0.7). The τDF values were 71.9, 108.6, 6.2 and 6.1 μs for A to D, respectively. Predictably, hole-only devices of B and D exhibited higher current densities than those of A and C due to the improved hole-transporting ability from the extra carbazole unit of B and D. Electron-only devices indicated that TRZ (in C and D) is a more efficient electron transport unit than thienopyrimidine (in A and B). OLEDs had a DPEPO host:emitter (15 wt%) EML. The EQEmax values for the C and D devices were slightly higher (21.4% and 22.3%) than the A and B devices (EQEmax 17.8% and 19.0%). Devices A and B showed high efficiency roll-off at 500 cd m−2. λELmax values were 492, 475, 484 and 475 nm for A–D, respectively. The higher EQEmax and lower efficiency roll-off of the C and D devices were attributed to the higher PLQY, smaller ΔEST and shorter exciton lifetimes of C and D compared with A and B. In summary, thienopyrimidine was less efficient than triazine in this series.
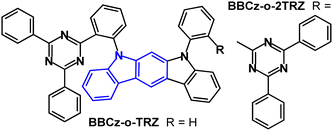
Indolo[2,3-b]carbazole TADF emitters BBCz-o-TRZ and BBCz-o-2TRZ feature an o-phenylene bridge between D and A units (Fig. 16).101 PLQY values were 0.46/0.41 in toluene and 0.83/0.86 for 10 wt%-doped PYD2 films. S1/T1/ΔEST energies were 2.89/2.7/0.17 eV and 2.85/2.73/0.12 eV, respectively (PL in toluene). TADF components were observed with τDF 7.2/4.8 μs in the doped films. OLEDs with PYD2 host:emitter (30 wt%) as the EML gave bluish-green EL with EQEmax 15.3% and 16.6% for BBCz-o-TRZ and BBCz-o-2TRZ devices, which was comparable to analogous carbazole derivatives. The EQE roll-off was higher for the indolocarbazole devices than for the carbazole analogues. The half-life (LT50) for BBCz-o-TRZ and BBCz-o-2TRZ devices was 345 h and 262 h, respectively, at an initial luminance (L0) of 1000 cd m−2. These data show that in this case increasing the number of acceptors in the emitter reduces the stability of the device, although it improves the balance between hole and electron transfer in the EML.
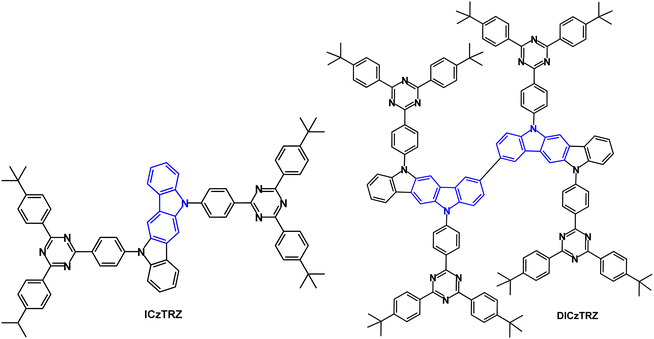
The A–D–A TADF emitter ICzTRZ (Fig. 17) had λPLmax 462 nm (toluene solution), 479 nm (5 wt% doped in mCBP) and 470 nm (10 wt% doped in PMMA).102 S1/T1/ΔEST levels were 2.85/2.62/0.23 eV (5 wt% in mCBP); PLQY under air/N2 was 59/70%. Devices with mCBP host:ICzTRZ (5 wt%) EML gave sky-blue emission with λELmax 483 nm, CIE(0.17, 0.32), EQEmax 22.1% at 16 cd m−2, EQE100 17.3% and Lmax 7805 cd m−2. Simulations demonstrated that for a PLQY of 70% if the molecules were randomly orientated in the film the device EQE would be ≈15%. Therefore, it was inferred that there is strong horizontal orientation of the emitter molecules in the device, in line with polarization- and angle-dependent PL thin film experiments in DPEPO, mCP and mCBP hosts, leading to enhanced light outcoupling and higher EQE in the OLEDs.
The bis(indolocarbazole) twin (A–D–A)2 TADF emitter DICzTRZ (Fig. 17) was subsequently reported.103 The PLQY of DICzTRZ was 60% in degassed toluene and 44% after exposure to oxygen. S1/T1/ΔEST values were 2.80/2.59/0.21 eV (PL data). λPLmax in (9-(4-t-butylphenyl)-3,6-bis(triphenylsilyl)-9H-carbazole) (CzSi) host (30 wt% doping) was 488 nm, and only slightly red-shifted compared to that in toluene, and PLQY was 57%. The τDF values were 156.1 and 69.49 μs with ΔEST 0.03 and 0.19 eV for the doped films in PMMA and CzSi, respectively. OLEDs with a solution-deposited EML of CzSi host:DICzTRZ (20 wt%) had EQEmax 8.4%, λELmax 494 nm; CIE(0.22, 0.47). The device efficiency of DICzTRZ was lower compared to the non-twin derivative ICzTRZ (EQEmax = 11.6%; λELmax 485 nm; CIE(0.19, 0.37), solution deposited EML)103 which was attributed to the lower PLQY and unfavourable vertical orientation of the transition dipole moment of DICzTRZ in the EML.
Triarylboron derivatives have been widely used as acceptor groups in TADF emitters.104–112 The vacant p orbital on boron imparts the electron accepting properties. For example, TDBA-TPDICz (Fig. 18a) has λPLmax 447 nm and ΔEST 0.41 eV (in toluene solution); ΔEST 0.29 eV in DBFPO host.56 OLEDs with DBFPO:TDBA-TPDICz (20 wt%) as the EML gave EQEmax 16.9%, λELmax 462 nm, CIE(0.14, 0.14). The results showed that the weaker cyclic boron acceptor significantly blue shifted the emission compared to a triazine analogue [λELmax 509 nm, CIE(0.25, 0.53)].
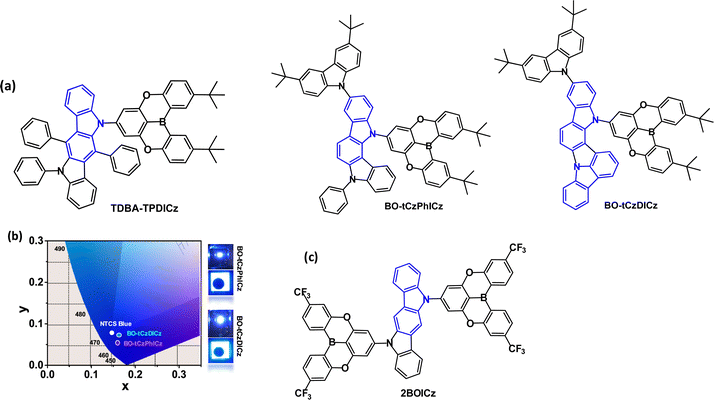 | ||
| Fig. 18 (a) Molecular structures of TPDA-TPDICz, BO-tCzPhICz and BOtCzDICz. (b) CIE diagram and images of EL of devices of BO-tCzPhICz and BOtCzDICz.111 Copyright 2021 The American Chemical Society. (c) Molecular structure of 2BOICz. | ||
Indolo[3,2-a]carbazole derivatives BO-tCzPhICz and BOtCzDICz (Fig. 18a) with the same multi-resonance boron-based acceptor are deep-blue TADF emitters.111BO-tCzPhICz has a larger dihedral angle (68.3 vs. 59.8°) and smaller ΔEST (76.6 vs. 153 meV, film state) compared to BO-tCzDICz. Both compounds showed TADF with λPLmax ≈ 450 nm (thin film), FWHM ≈ 50 nm and τDF 0.22–0.23 μs. OLEDs had a solution processed mCP host:emitter EML. The smaller ΔEST, faster RISC rate (34.4 × 105vs. 20.9 × 105 s−1) and higher PLQY (92.7 vs. 77.3%, film) of BO-tCzPhICz resulted in a higher EQEmax of 17.8% vs. 14.8% for BOtCzDICz. The devices have among the best EL performance for solution-processed deep-blue TADF-OLEDs with CIEy < 0.1 and close to the National Television System Committee (NTSC) standard blue (0.14, 0.08) (Fig. 18b). A narrow FWHM of 56 nm was retained in the BO-tCzPhICz device.
Two equivalent acceptors functionalised with CF3 substituents have been attached to a sterically-uncrowded indolo[3,2-b]carbazole core to afford the A–D–A molecule 2BOICz (Fig. 18c) as a fluorescent emitter with hybrid long- and short-range CT excitations.113 The well-balanced rate constants of kr ≫ kISC ≈ kRISC > 106 s−1 lead to a short τDF of ≈ 0.88 μs. The quasi-planar structure of 2BOICz gives a favoured dipole orientation for a large outcoupling efficiency. The OLEDs with mBPBC host:2BOICz (20 wt%) had green emission with EQEmax 40.4%. The LT50 of the device was ≈ 300 h at L0 5000 cd m−2.
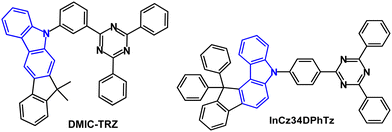
Studies have addressed indenocarbazole donors as components of TADF emitters. For example, in 2017 it was reported that DMIC-TRZ (Fig. 19) had λPLmax was 466 nm (toluene solution) with ΔEST 0.14 eV (PL measurements).114 The PL decay curve showed prompt and delayed components, and kRISC was calculated to be 1.8 × 105 s−1. An OLED based on pure DMIC-TRZ film as the EML showed a low EQEmax of 1.93%. DMIC-TRZ was more successful as a host with sky-blue (5TCzBN), green (DMAC-BP) and orange (4TCzTPN) guest TADF emitters. The PLQYs of the doped films (6 wt% emitter) were ≈70% with τDF of 1.84, 2.67, and 4.07 μs for 5TCzBN, DMAC-BP, and 4TCzTPN, respectively. The sky-blue and green TADF devices had EQEmax values of 19.2% and 21.0%, respectively. Both devices showed small efficiency roll-offs: EQEs were 18.0% at 2000 cd m−2 and 14.2% at 5000 cd m−2 for 5TCzBN, and comparably 19.5% and 14.2% for DMAC-BP. Furthermore, the orange 4TCzTPN host:DMIC-TRZ OLEDs achieved EQEmax of 23.2%, (18.9% at 5000 cd m−2) which was higher that a reference device with CBP host (EQEmax 15.4%). These data demonstrated the benefits of more balanced charge injection into DMIC-TRZ with similar hole and electron carrier mobilities within the EML, leading to a wide recombination zone and suppressed exciton annihilation rates in the DMIC-TRZ-guest EMLs.
A series of indenocarbazole-triazine blue TADF emitters were reported in 2019 of which InCz34DPhTz (Fig. 19) is the most noteworthy.115 S1/T1/ΔEST levels were 2.90/2.79/0.11 eV. PL data indicated only a small TADF contribution due to a slow kRISC (1.6 × 104 s−1). TADF was confirmed by a linear dependence of DF with excitation power and slope ≈1, signifying a unimolecular process. The PLQY was 97.9% (doped in DPEPO film). TADF OLEDs with a DPEPO host:InCz34DPhTz (10 wt%) EML had EQEmax 25.9% although with extensive roll-off to 14.2% at 100 cd m−2; λELmax 472 nm; CIE(0.15, 0.24).
Benzofurocarbazole and benzothienocarbazole donors have been incorporated into TADF emitters, notably by J.-Y. Lee's group. Two established ways to increase kRISC are: (i) to reduce ΔEST116,117 and (ii) to enhance the SOC between S and T states of a TADF molecule. Introducing heteroatoms such as S, N or O into a TADF molecule can benefit the SOC and kRISC.118–123 For example, the blue TADF emitters BFCz-2CN and BTCz-2CN (Fig. 20)124 both have ET 2.46 eV (frozen THF), leading to ΔEST values 0.17 and 0.13 eV, respectively. PLQY values were ≈94% (mCP doped films), which is higher than the non-fused carbazole analogue 2CzPN.125 Transient PL decay curves for BFCz-2CN and BTCz-2CN indicated prompt and delayed emissions with τDF 2.60 and τDF 1.98 μs, which are shorter than 2CzPN (τDF 166 μs) because of the restricted molecular motion of the fused carbazole derivatives. OLEDs with an EML of mCP host:BFCz-2CN or BTCz-2CN (1 wt%) had λELmax 486, FWHM 73 nm, EQEmax ≈ 12% for both emitters, compared with 5% for 2CzPN.
Benzofuro[3,2-c]carbazole donor and triazine acceptor have been linked via an o-phenylene bridge in the emitter oBFCzTrz (Fig. 20).126oBFCzTrz has a very small experimental ΔEST value of 0.002 eV (compared to 0.191 and 0.302 eV for the meta- and para-phenylene isomers), τDF 5.4 μs and PLQY of 97.9% (under nitrogen). OLEDs with a DPEPO:oBFCzTrz (20 wt%) EML had EQE of 20.0% and 17.7% at 100 and 1000 cd m−2, respectively with sky-blue emission and CIE(0.18, 0.31). Device lifetime studies gave LT95 of ≈3 h at a fixed current density of 5 mA cm−2. Subsequently, different workers reported that OLEDs with a DPEPO host:oBFCzTrz (50 wt%) EML had EQEmax 25.5%, and a neat oBFCzTrz EML had EQEmax 14.0%.127 Inspired by these results, a theoretical study suggested that o-linked D–A molecules may be an efficient design strategy for TADF emitters free of concentration quenching effects.128
The related benzofuro[3,2-a]carbazole derivative Trz-BF-CzCz (Fig. 20) had kRISC 6.37 × 104 s−1 (doped film in DPEPO host) and gave OLEDs with EQEmax 23.3% and CIE(0.18, 0.32) using 20 wt% doping in DPEPO as the EML.129
TrzCNTrx2 (Fig. 20) was designed with enhanced donor rigidity to favour a faster RISC process.130 The HOMO was mainly located on the Trx donor unit whereas the LUMO was on the diphenyltriazine acceptor and benzonitrile linker, with slight HOMO–LUMO overlap inside the donor unit. S1/T1/ΔEST levels were 2.83/2.75/0.08 eV (PL spectra in THF); PLQY 91% (doped film in CzTrz host) and kRISC was raised to 5.13 × 105 s−1. Green emitting OLEDs with an EML of CzTrz:TrzCNTrx2 (5 wt%) had EQEmax 20.6% and CIE(0.30, 0.55) with very low roll-off to EQE 20.2% at 3000 cd m−2. The devices had a lifetime up to 90% of initial luminance (LT90) at 3000 cd m−2 of 141 h.
pBFCz-26DPPM and pBTCz-26DPPM (Fig. 21) are deep-blue TADF emitters based on benzofuro- and benzothieno-carbazole.131 The HOMOs are localised on the BFCz and BTCz donors, while the LUMOs are predominantly on the 4,6-diphenylpyrimidine acceptor with some overlap on the phenylene bridge. The S1/T1/ΔEST values were 2.97/2.70/0.27 eV for pBFCz-26DPPM and 3.06/2.72/0.34 eV for pBTCz-26DPPM. The PLQYs were 71/75% for 10% doped films in DPEPO and 38/42% for the neat films for pBFCz-26DPPM and pBTCz-26DPPM, respectively. Transient PL data including temperature dependence indicated TADF emission for both compounds (10 wt% doped in DPEPO). OLEDs with DPEPO host:emitter (10 wt%) EML had low EQEmax 6.20, CIE(0.154, 0.054) and 5.36%, CIE(0.154, 0.052) for pBFCz-26DPPM and pBTCz-26DPPM, respectively.
 | ||
| Fig. 21 Molecular structures of pBFCz-26DPPM, pBTCz-26DPPM, BFCZPZ1, BTCZPZ1, BFCZPZ2, BTCZPZ2, BFCZPZ3, 12BTCzTPN and 2BT12CzINN. | ||
The D–A–D bis(benzofurocarbazole) and bis(benzothienocarbazole) derivatives with a 2,5-pyrazine linkage were subsequently studied as blue TADF emitters (Fig. 21).132λPLmax values were 415 (BFCZPZ1), 444 (BFCZPZ2, BFCZPZ3 and BTCZPZ2) and 456 nm (BTCZPZ1) (toluene solution) demonstrating a 41 nm red shift upon changing benzofuran to benzothiophene (BFCZPZ1 to BTCZPZ1). The FWHMs were in the range 51–73 nm, which is narrower than a non-fused carbazole analogue (76 nm). BTCZPZ1 had the highest SOCME 0.980 cm−1, lowest ΔEST (0.24 eV, doped film in PPT host) and highest kRISC (8.5 × 104 s−1) of the five compounds. Devices with a PPT host:emitter (7 wt%) EML had EQEmax 21.1%, CIE(0.16, 0.17) for BTCZPZ1 and EQEmax 21.3%, CIE(0.15, 0.10) for BFCZPZ2 as the highest efficiencies in the series. The LT50 of the BTCZPZ1 device (5.0 h) was the highest in the series.
Efficient hyperfluorescent OLEDs constitute a high ET host co-doped with a TADF material and the final emitter. A fast Förster resonance energy transfer (FRET) from the S1 excited state of the TADF material to S1 of the fluorescent emitter is needed to activate the hyperfluorescence. 12BTCzTPN (Fig. 21)133 has increased kRISC of 3.67 × 106 s−1 due to the heavy atom effect of the two sulfur atoms compared to 4CzTPN (kRISC 0.80 × 106 s−1). 12BTCzTPN served as the assistant dopant in a red hyperfluorescent device with 4tBuMB as the fluorescent emitter giving EQEmax 19.9% and CIE(0.64, 0.36). The pyridyl analogue 2BT12CzINN (Fig. 21) has kRISC of 10.9 × 105 s−1. With the polycyclic aromatic hydrocarbon DBP as the fluorescent emitter hyperfluorescent 2BT12CzINN:DBP devices in a PBICT:DBTTP1 host had EQEmax 14.7% and CIE(0.60, 0.39).134 The EQE and the lifetime LT90 of 41.8 h at L0 3000 cd m−2 were improved compared to a reference 4CzTPN:DBP device (EQEmax 11.3%; LT90 27.8 h).
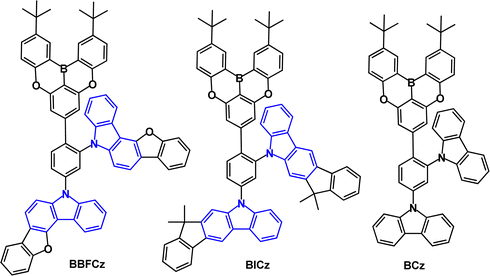
Benzofuro[3,2-c]carbazole and indeno[2,1-b]carbazole have been combined with an oxygen-bridged triarylboron acceptor to give D2–π–A emitters BBFCz and BICz (Fig. 22; compare with Fig. 18a).112 The structures are highly twisted with the HOMO and LUMO mainly distributed on the ortho-, para-substituted donors and the boron acceptor, respectively, and slightly extended to the adjacent phenylene core. PL data showed that BBFCz and BICz have very high T1 values of 2.97 and 3.07 eV (toluene solution), ΔEST values of 0.26 and 0.15 eV leading to TADF, confirmed by temperature dependent studies. In contrast, the non-fused carbazole derivative (BCz) had ΔEST 0.40 eV and showed only conventional fluorescence. The electron donating ability increased in the order of BCz < BBFCz < BICz. The PLQYs were much higher in films than in solution, which was ascribed to AIE effects. Devices with a solution-processed neat EML gave deep-blue emission with λELmax 412, 416 and 424 nm and CIE(0.18, 0.10), (0.17, 0.07) and (0.16, 0.08) and FWHM of 33, 42 and 61 nm, for BCz, BBFCz and BICz respectively. BICz, having the stronger donor and smaller ΔEST, gave the highest EQEmax of 10.1% in the series.
Blue/deep-blue emitters using indolo[3,2,1-jk]carbazole as the acceptor unit with carbazole and acridine donors have been reported (Fig. 23).135 The λPLmax values for ICzCz, ICzAc and ICzDAc were 400, 439 and 449 nm, respectively (in toluene solution) and ΔEST values were 0.38, 0.20 and 0.17 eV (in polystyrene film). Transient PL experiments showed only prompt fluorescence for ICzCz, whereas ICzAc and ICzDAc had both prompt and delayed components, with τDF 9.86 μs and τDF 8.46 μs, respectively. The PLQYs of ICzCz, ICzAc and ICzDAc, as doped films in DPEPO under oxygen/nitrogen, were 0.40/0.53, 0.32/0.95 and 0.49/0.96. OLEDs with a DPEPO host:emitter (10 wt%) EML gave EQEmax values for ICzCz, ICzAc, and ICzDAc of 2.3%, 13.7% and 19.5%, with FWHM 49, 56, and 58 nm, and CIE(0.17, 0.04), (0.15, 0.09) and (0.15, 0.16) respectively.
The TADF emitter CNICtCz has enhanced acceptor character due to the cyano-functionalised indolocarbazole unit (Fig. 22).136 DF was observed with τDF 6.25 μs (10 wt% in DPEPO film) and PLQYs were 0.33/0.50 under oxygen/nitrogen. The ΔEST of CNICtCz was 0.19 eV. Blue-emitting OLEDs with a DPEPO host:CNICtCz (10 wt%) EML had EQEmax 16.0% (EQE 10.7% at 100 cd m−2), λELmax 456 nm, CIE(0.14, 0.13), FWHM 60 nm. The t-Bu substituents on CNICtCz significantly improved the device efficiency and slightly red-shifted the emission.
4.2 Diindolocarbazole (triazatruxene) derivatives
In the last few years, the C3 symmetric, diindolocarbazole framework, also named triazatruxene, has become a popular near-planar, π-extended unit for TADF derivatives with a spacially large HOMO. Different D–A ratios can be obtained by attachment of an acceptor unit at one, two or three of the nitrogen atoms. Prominent examples will now be considered.The D–A3 structure TAT-3DBTO2 (Fig. 24a) was reported in 2018.137 In the ground state geometry all the A units are tilted by 57° with respect to the TAT core.138 After excitation the A units can rotate around the C-N bonds to minimise the orbital overlap. Calculations showed ten possible conformers of TAT-3DBTO2 all within 0.03 eV of each other, and ΔEST 0.01 eV was obtained from PL studies, with kRISC as fast as 1 × 107 s−1 and PLQY ≈ 1, doped in BCPO film. Devices with a BCPO host:TAT-3DBTO2 (17 wt%) EML gave green emission, CIE(0.26, 0.46), EQEmax 30.9% and roll-off to 16.5% at 1000 cd m−2; CEmax 50.8 cd A−1.
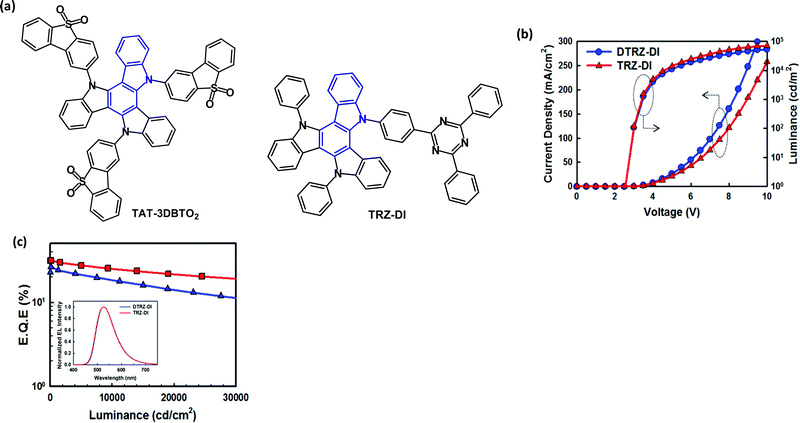 | ||
| Fig. 24 (a) Molecular structures of TAT-3DBTO2 and TRZ-DI. (b) Current density–voltage–luminance (J–V–L) data. (c) EQE–L data; inset EL spectra for TRZ-DI and DTRZ-DI devices.140 Copyright 2018 The Royal Society of Chemistry. | ||
The isomeric D–A3 structure with the sulfone groups meta to the nitrogen atoms has a much larger ΔEST 0.28 eV compared to TAT-3DBTO2.139 As expected, this larger ΔEST strongly reduced the RISC rates. Calculations showed that the meta isomer is less conformationally restricted and rotation of its acceptor units lead to a lower T1 energy, further away from the region of high density of states where larger vibronic coupling is found, favouring RISC.
The D–A molecule TRZ-DI (Fig. 24a) with a triazine acceptor has ΔEST 0.023 eV from PL studies, with kRISC 1.63 × 106 s−1 and PLQY 0.87, doped in mixed TCTA:Bepp2 film.140 Devices with TCTA:Bepp2 host:TRZ-DI (25 wt%) as the EML gave green emission, CIE(0.32, 0.58), EQEmax 31.4%. The analogous D–A2 structure (DTRZ-DI) gave less efficient devices EQEmax 26.2% (Fig. 24b). It was noted that the high Tg values of TRZ-DI (203 °C) and DTRZ-DI (239 °C) should favour good morphological stability and electroluminescent lifetime.
An intricate triazatruxene-triazine cage molecule (Cage DA-1) is a TADF macrocyclic exciplex emitter. The cofacial D–A architecture induces efficient intramolecular through-space charge transfer with PLQY 35% in degassed toluene and a ΔEST of 0.049 eV. OLEDs were not reported.141
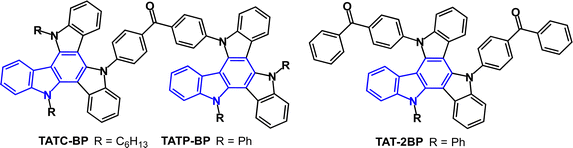
D–A–D molecules TATC-BP and TATP-BP (Fig. 25) exhibit multifunctional luminescence properties, namely, TADF, AIE and mechanoluminescence in the crystalline and powdered states.142 PLQYs were very low in solution (0.8–1.9%) and were enhanced to 22–24% in neat films. From PL data ΔEST values were 0.125 and 0.129 eV, with τDF values 0.94 and 0.91 μs for TATC-BP and TATP-BP, respectively. Non-doped OLEDs with a solution-processed EML gave EQEmax ≈ 6%. Doped devices in H2 host (a dendritic polycarbazole derivative) with H2:TATC-BP (30 wt%) gave EQEmax 15.9% with low roll-off to 15.3% at 1000 cd m−2 and green emission, CIE(0.37, 0.53). For comparison the D–A2 analogue TAT-2BP (Fig. 25) gave non-doped OLEDs with EQEmax 9.8% and very low roll-off to EQE 9.6% at 2000 cd m−2.143
The D–A–D molecule TAT-DBPZ and the analogue TAT-FDBPZ with the more electron accepting fluorine-substituted dibenzophenazine unit have been reported (Fig. 26a).144 Unusual features of this work are that both compounds show AIE behaviour in neat films and red emission, with PLQYs 76% and 62% doped in CBP films. The large steric hindrance between the TAT and DBPZ moieties gave small ΔEST 0.16 and 0.10 eV for TAT-DBPZ and TAT-FDBPZ, respectively, leading to τDF values 2.30 and 1.51 μs and kRISC 1.00 and 1.71 × 106 s−1. The fluorine substituents in the FDBPZ unit imparted red-shifted emission and shorter DF lifetime and slightly decreased PLQY compared to TAT-DBPZ. The TAT-DBPZ device (20 wt% in CBP host as a solution processed EML) exhibited higher efficiency because of its higher PLQY and faster kr giving EQEmax 15.4%; λELmax 626 nm; CIE(0.61, 0.38) (Fig. 26b–d).
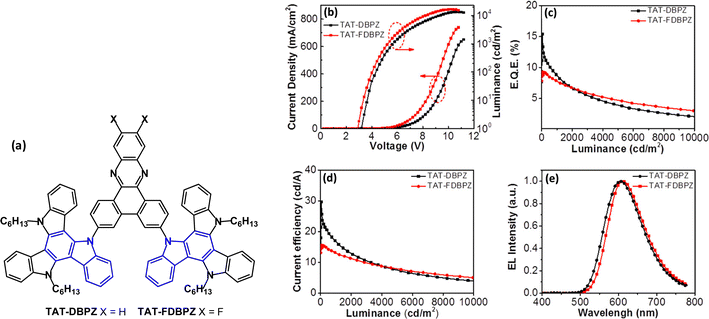 | ||
| Fig. 26 (a) Molecular structures of TAT-DBPZ and TAT-FDBPZ. (b) Current density–voltage–luminance characteristics. (c) EQE–L curves. (d) CE–L curves. (e) EL spectra at 1000 cd m−2.144 Copyright 2020 The American Chemical Society. | ||
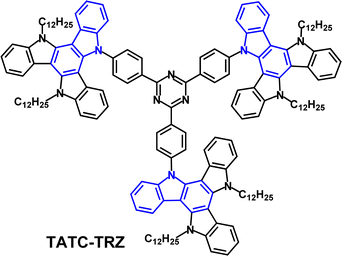
The D3–A structure TATC-TRZ (Fig. 27) has flexible alkyl chains to enhance solubility, film forming ability and morphological stability that are beneficial for solution processed non-doped EMLs.145 S1/T1/ΔEST values were 2.53/2.57/−0.04 eV (PL in neat films) and ΔEST 0.1 eV in doped films. The authors suggested that the very unusual negative ΔEST value in neat films might be due to a different geometrical arrangement in the fully relaxed S and T states. (Negative ΔEST values are a topic of current experimental and theoretical debate).146 The PLQY of TATC-TRZ was only 2% in dilute toluene solution; this was raised to 22% in neat films, consistent with AIE. TADF characteristics were observed with τPF 17.5 ns, τDF 0.6 μs and kRISC 2.9 × 106 s−1 in neat films. Green OLEDs fabricated with a neat TATC-TRZ EML had EQEmax 7.5%; λELmax 560 nm. An EML of mCP host:TATC-TRZ (5 wt%) gave EQEmax 8.6%, lower roll-off (EQE 6.7% at 1000 cd m−2) and λELmax 530 nm.
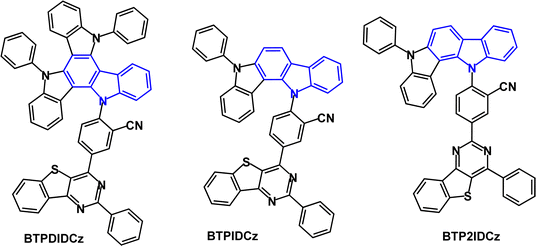
Superior TADF behaviour has been demonstrated for the diindolocarbazole BTPDIDCz compared with the indolocarbazole analogues BTPIDCz and BTP2IDCz, all incorporating a thienopyrimidine acceptor (Fig. 28).147 Cyclic voltammetric data gave a shallower HOMO level for BTPDIDCz indicating the stronger electron donating ability of the diindolocarbazole group, compared to indolocarbazole. The S1/T1/ΔEST levels were 2.76/2.75/0/01 eV, 2.91/2.82/0.09 eV and 3.16/2.78/0.38 eV (PL measurements, THF solution) for BTPDIDCz, BTPIDCz and BTP2IDCz, respectively. Diindolocarbazole BTPDIDCz showed the smallest ΔEST and shortest τDF (4.1 μs) indicating its superior TADF behaviour. Conversely, the data for BTP2IDCz indicated only weak DF. PLQY values for the series were between 0.83–0.86. TADF-OLEDs had CzTrz host:emitter (5 or 20 wt%) EML. The best performance was achieved for BTPDIDCz with EQEmax 24.5%, λELmax 530 nm; CIE(0.34, 0.57) and low efficiency roll-off to EQE 20.3% at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. The lifetimes of the devices were assessed by operating them at 5 mA cm−2. In the series the BTPDIDCz device had the longest LT85 value of 93.4 h. This long lifetime can be correlated with the emitter's fast kRISC of ≈1.0 × 106 s−1 which limits triplet exciton-triggered degradation mechanisms such as TTA and TPA which induce device failure.
000 cd m−2. The lifetimes of the devices were assessed by operating them at 5 mA cm−2. In the series the BTPDIDCz device had the longest LT85 value of 93.4 h. This long lifetime can be correlated with the emitter's fast kRISC of ≈1.0 × 106 s−1 which limits triplet exciton-triggered degradation mechanisms such as TTA and TPA which induce device failure.
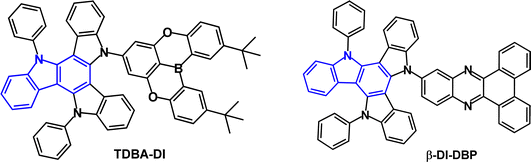
To achieve colour tuning, alternative acceptors have been attached to diindolocarbazole. For examples, the D–A structures TDBA-DI and β-DI-DBP (Fig. 29) have sky-blue and orange/red emission, respectively. TDBA-DI has λPLmax 456 nm with FWHM of 55 nm (toluene solution).104 The large dihedral angle between the D and A groups enables a small overlap between the HOMO and LUMO; S1/T1/ΔEST values are 3.06/2.95/0.11 eV (PL measurements). The PLQY of TDBA-DI as 20 wt% doped film in DBFPO host was 0.99. OLEDs with DBFPO host:TDBA-DI (20 wt%) as the EML had remarkably high EQEmax 38% (FWHM 65 nm; CIE 0.15, 0.28) while the analogous emitter with non-fused dimethylacridine as the D unit had EQEmax 26%, CIE(0.14, 0.15). The outstanding efficiency of TDBA-DI was attributed to its highly conjugated and rigid structure, which provided a high PLQY. Evidence was obtained that the horizontal molecular dipole orientation of TDBA-DI also contributed to the increased light out-coupling efficiency and high EQE. The operating lifetime of the DBFPO:TDBA-DI device was <2 h at 500 cd m−2.
Theoretical studies of β-DI-DBP indicated a dihedral angle between D and A of 64.3°.148β-DI-DBP showed a broad emission with λPLmax 578 nm (toluene solution). The PLQY was 96.1% for a 5 wt% doped DIC-TRZ film with τDF 14.0 μs; S1/T1/ΔEST levels were 2.48/2.36/0.12 eV. β-DI-DBP showed a slightly faster kRISC compared to the 9,9-dimethylacridine analogue β-DMAC-DBP, (1.79 × 105 s−1, versus 1.53 × 105 s−1) which may be due to the higher rigidity of the diindolocarbazole donor. OLEDs with DIC-TRZ host:β-DI-DBP (5 wt%) as the EML had EQEmax 23.8% with orange/red emission λELmax 587 nm, CIE(0.53, 0.47) and broad FWHM of 102 nm. EQEmax was slightly higher and the efficiency roll-off at 1000 cd m−2 was considerably improved compared to β-DMAC-DBP devices (EQEmax 21.8%).
4.3 Carbazole fused with 6-membered rings
An interesting structural variant is the fused carbazole–acridine unit in the isomeric D–A emitters 12AcCz-PM and 23AcCz-PM (Fig. 30a) which have remarkably different photophysical behaviour.14912AcCz-PM has large steric hindrance between the D and A units leading to a quasi-axial acridine conformation (Fig. 30b) with no TADF character. In contrast, the reduced steric hindrance in 23AcCz-PM allows a more favourable orthogonal D–A structure with a quasi-equatorial acridine conformation (Fig. 30b) and efficient TADF. DFT indicated a large HOMO–LUMO overlap in 12AcCz-PM, whereas the HOMO and LUMO were well separated in 23AcCz-PM. S1/T1/ΔEST values were 3.06/2.67/0.39 eV and 2.73/2.67/0.06 eV for 12AcCz-PM and 23AcCz-PM, respectively (doped films in DPEPO). λPLmax values were 440 and 516 nm, with PLQYs of 78 and 95% for 12AcCz-PM and 23AcCz-PM. OLEDs had a DPEPO host:emitter (X wt%) EML. The 12AcCz-PM doped device (20 wt%) had deep blue emission with CIE(0.15, 0.05) and EQEmax 6.4%. The 30 wt% 23AcCz-PM device had blue-green emission with CIE(0.26, 0.55), EQEELmax 26.1%, 24.8% at 1000 cd m−2 and 20.7% at 5000 cd m−2, indicating low efficiency roll-off and high operational stability.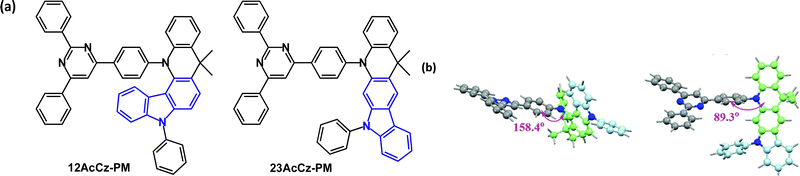 | ||
| Fig. 30 (a) Molecular structures of 12AcCz-PM, 23AcCz-PM and (b) DFT computed optimal structures of 12AcCz-PM, 23AcCz-PM.149 Copyright 2019 The Royal Society of Chemistry. | ||
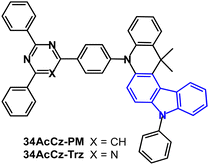
A comparison of pyrimidine and triazine acceptors in 34AcCz-PM and 34AcCz-Trz (Fig. 31) showed superior devices for the pyrimidine analogue.150 S1/T1/ΔEST levels were 2.72/2.57/0.15 eV and 2.62/2.50/0.12 eV (PL, doped films in CBP) for 34AcCz-PM and 34AcCz-Trz, respectively. λPLmax was 520/538 nm and PLQYs were 88/69% (doped films in CBP) and 67/42% (neat films) for 34AcCz-PM and 34AcCz-Trz, respectively. Both compounds showed DF with τDF of 0.73 and 0.88 μs (doped films in CBP). PL spectra in THF/water mixtures indicated that both compounds are AIE active. Doped and non-doped devices were reported. The EML of the doped devices for 34AcCz-PM was 10 wt% in CBP; for 34AcCz-Trz 5 wt% in CBP. The device with 34AcCz-PM exhibited EQEmax 22.6% and CEmax 73.3 cd A−1 with 20.1% and 65.5 cd A−1 at 5000 cd m−2, CIE(0.33, 0.58). The higher efficiency of the 34AcCz-PM devices was attributed to its higher PLQY and higher kRISC resulting from more prominent AIE compared to 34AcCz-Trz. A non-doped OLED of 34AcCz-PM exhibited EQEmax 14.1% and CEmax 45.2 cd A−1.
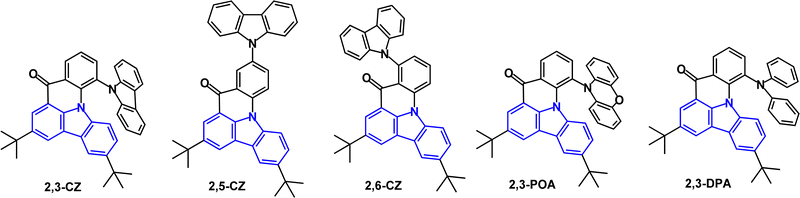
A series of unusual fused di-t-Bu-carbazole derivatives with peripheral carbazole (2,3-, 2,5- and 2,6-CZ), phenoxazine (2,3-POA) and diphenylamine (2,3-DPA) (Fig. 32) has been studied.21 DFT calculations indicated that the HOMOs were mainly located on the peripheral donor units, whereas the LUMOs resided mainly on the central carbazole/carbonyl skeleton. 2,3-POA had the shallowest HOMO, indicating its strong donor character. λPLmax ranged from blue (449 nm; 2,3-CZ) to yellow-green (547 nm; 2,3-POA) (toluene solution). All the compounds showed TADF with FWHM values increasing in the order 2,3-CZ 36 nm < 2,5-CZ 41 nm < 2,3-DPA 57 nm < 2,6-CZ 80 nm < 2,3-POA 92 nm, with the narrow values ascribed to a potential multiresonance (MR) effect. ΔEST values were 0.26, 0.29, 0, 0.19 and 0.01 eV for 2,3-CZ, 2,5-CZ, 2,6-CZ, 2,3-DPA and 2,3-POA, respectively. PLQYs ranged from 39.5% (2,3-CZ) to 82.5% (2,3-POA) (3.5 wt% doped in mCBP film). OLEDs used mCBP or 26DCzPPy as hosts in the EML. Notably, the pure-blue OLED with 2,5-CZ had EQEmax 22.3% with CIE(0.13, 0.13) and FWHM 48 nm with significant efficiency roll-off. The yellow-green 2,3-POA device had EQEmax 21.7%, CIE(0.30, 0.62) and FWHM 68 nm with low efficiency roll-off.
CBZANQ has a D–σ–A structure and orthogonal D and A units enforced by the spiro linkage (Fig. 33).151 The molecule exhibited weak CT character and low PLQY of 18% (30 wt% doped in PMMA host) and ΔEST of 0.05 eV (in DPEPO host). OLEDs with DPEPO host:CBZANQ as the EML had low EQEmax 6.7% with λELmax 492 nm. For comparison, devices with DPEPO:PXZANQ (Fig. 33) as the EML had EQEmax 22.1% and λELmax 528 nm. The improved performance of this device was attributed to the increased donor strength of phenoxazine compared to carbazole.
Very recently TADF emitter CCO-2 (Fig. 33) and close analogues152 with different aryl substituents on the carbazole nitrogen atom have been reported. The molecular design combines a weak and rigid electron donor spiro[acridine-9,9′-xanthene] and a weak electron acceptor chromeno[3,2-c]carbazole that is also rigid. The molecules have high PLQY, high transition dipole ratios, fast kr and fast kRISC values. OLEDs fabricated with DPEPO host:CCO-2 (15 wt%) gave blue emission with λELmax 462 nm, CIE(0.14, 0.15) and EQEmax > 40% and roll-off to EQE 22.4% at 1000 cd m−2. For 30–50 wt% devices there was a slight red shift to λELmax 470 nm and reduced roll-off to EQE 31.3–33.7% at 1000 cd m−2. Deep-blue and green hyperfluorescent OLEDs with CCO-2 as sensitiser for multi-resonance delated fluorescent emitters had EQEmax 32.5%, CIE(0.14, 0.10) and EQEmax 37.6%, CIE(0.32, 0.64), respectively. The LT50 of the blue and green devices at L0 100 cd m−2 was 1099 h and 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 669 h, respectively.
669 h, respectively.
5. Multiresonance (MR) fused carbazole derivatives
5.1 Boron-containing molecules
As noted in the introduction section, multiresonance (MR) emitters are currently attracting great attention. Nitrogen and oxygen bridged triarylboron systems in D–A molecules show efficient TADF with narrow FWHM and small Stokes shifts because of their rigid structure and MR effects.41,153 The MR-TADF mechanism is complex and is currently believed to involve crossing between T1 and an upper T2 (or higher) state via reverse internal conversion, followed by RISC from T2 to S1.154 In 2018 it was reported that a carbazole-containing MR emitter with enhanced oscillator strength and faster kRISC leads to pure-blue OLEDs with EQEmax of 32.1% and FWHM of only 27 nm.67 The initially assigned molecular structure of this emitter has subsequently been corrected.155 It was, therefore, logical to explore analogues with carbazole units embedded within a polycyclic B–C–N framework and this is now a fast-moving topic.In 2019 3F-BN (Fig. 34) was reported to exhibit narrowband green emission (λPLmax 499 nm) and a very small Stokes shift (27 nm) suggesting a small change in the electronic configuration between ground and excited states, typical of the MR effect.156 The PLQY was 83.4% (6 wt% doped in mCPBC films); S1/T1/ΔEST levels were 2.48/2.40/0.08 eV; kF 4.66 × 107 s−1; kRISC 0.39 × 105 s−1. PL decay curves of the films showed both PF and DF with τDF 16.7 μs. OLEDs with an EML of 5TCzBN host:3F-BN (6 wt%) gave EQEmax 22.7% with small efficiency roll-off (21.1% at 1000 cd m−2), PEmax 72.2 lm W−1, λELmax 499 nm, FWHM 38.5 nm and CIE(0.20, 0.58).
The related tri-carbazole MR emitter NBNP (Fig. 34) was reported in 2022.157 S1/T1/ΔEST levels for NBNP are 2.48/2.39/0.09 eV; kRISC 3.0 × 105 s−1. Large SOC matrix elements were also beneficial for activating TADF in NBNP. The PLQY was 93% with τPF 7.6 ns and τDF 3.8 μs (4 wt% doped in mCBP film). Green OLEDs used mCBP host:NBNP (4 wt%) as the EML giving EQEmax 28.0% with λELmax 502 nm, FWHM 33 nm, CIE(0.12, 0.62), and maintaining EQE 18.8% at 5000 cd m−2.
Tetra-carbazole derivative R-TBN (Fig. 34) is a deep-red emitter with λPLmax 692 nm (toluene solution) with ΔEST 0.16 eV; τDF 46.4 μs.158 The PLQY is 100% both in solution and as 3 wt% doped in CBP film. OLEDs with CBP host:R-TBN EML gave EQEmax 24.7%. Because of the relatively long DF lifetime of R-TBN in doped film (0.71 ms) the EML also adopted a ternary system of CBP:Ir(mphmq)2tmd (30 wt%):R-TBN (3 wt%) where Ir(mphmq)2tmd is a phosphorescent sensitiser to assist exciton recycling. These devices gave EQEmax 27.6%, λELmax 686 nm, FWHM 49 nm and CIE(0.721, 0.278). The devices with and without sensitiser gave identical EL spectra, confirming that EL is solely from R-TBN. The significant efficiency roll-off was partly attributed to the slow kRISC 2.5 × 104 s−1 of R-TBN.
The new multi-carbazole MR green emitters VTCzBN and VCz-VTCzBN (Fig. 35) were reported in 2022.159 In particular, the pendant carbazole groups of VCz-VTCzBN extend the CT delocalisation leading to λPLmax 521 nm with FWHM of 29 nm in toluene solution and ΔEST < 0.01 eV. The PLQY of both VTCzBN and VCz-VTCzBN in doped films was 98%, with kRISC 1.0 × 106 s−1 which is relatively high for MR B–N systems. Both compounds show prompt and delayed emissions with τPF 3.3 ns and τDF 9.9 μs for VTCzBN, and τPF 13.2 ns and τDF 8.7 μs for VCz-VTCzBN. OLEDs with an EML comprising 2,6-DCzppy host:emitter (4 wt%) gave high efficiencies of 91.0 cd A−1, EQEmax 31.7% for VTCzBN, and 129.3 cd A−1, EQEmax 32.2% for VCz-VTCzBN with FWHM 37–38 nm. There was considerable roll-off to EQE 24.8% and 18.0% at 100 cd m−2. The peripheral carbazole units of VCz-VTCzBN red shifted the emission to λELmax 524 nm, CIE(0.22, 0.71), compared to 499 nm, CIE(0.14, 0.56) for VTCzBN.
Molecules for full-colour narrow-band and high efficiency EL by modular design combining carbazole and tricoordinate boron fragments, were reported (Fig. 36).160 PLQY values were in the range 85–98%; kRISC values were in the range 1.2–18 × 104 s−1; OLEDs with mCP as host achieved EQEmax values of 29.3%, 31.8% and 22.0% for blue, green and red, respectively.
 | ||
| Fig. 36 Modular design for full-colour emitters 1–5.160 Copyright 2020 American Chemical Society. | ||
Devices comprising Cz2DABNA-NP-M/TB and CzB2-M/P (Fig. 37) as emitters (1 wt% in DOBNA-Tol host) exhibited sky-blue and green emission at λELmax 477 and 497 nm with FWHM 27 and 29 nm, respectively, and EQEmax of 21.8 and 26.7%. The LT80 value of the Cz2DABNA-NP-M/TB device was 91 h at L0 100 cd m−2, whereas the LT80 value of the CzB2-M/P device was 74 h at L0 500 cd m−2.155
The emitters BN-ICz-1 and BN-ICz-2 (Fig. 37) fuse an MR fragment onto an indolo[3,2-b]carbazole skeleton to achieve extended π-conjugation, increased molecular rigidity and reduced vibrational frequency.161 The devices in mCBP host showed ultra-pure green emission with λmax 523 nm and FWHM 23 nm; CIE(0.22, 0.74) and (0.21, 0.73) for BN-ICz-1 and BN-ICZ-2, with EQEmax values ≈30%.
TCZ-F-DABNA (Fig. 38a) has a linearly extended π-system and emission shifted to the orange-red region.162 The DABNA core of TCZ-F-DABNA imparts the MR properties and a small ΔEST of 0.12 eV (S1/T1 2.21/2.09 eV) for efficient TADF. The HOMO and LUMO distributions are similar to other DABNA derivatives and are less distributed on the fused carbazoles (Fig. 38b), although some extension of the HOMO introduces weak CT interaction with the DABNA core leading to a narrower band gap. TCZ-F-DABNA has a significantly twisted conformation (X-ray crystal structure and theoretical calculations) which can prevent molecular close packing and relieve concentration quenching of the emission. The PLQY was 99% with τPF 7.9 ns and τDF 20.2 μs (8 wt% doped in PhCzBCz film). This host was selected because of its high triplet level (2.96 eV) and balanced bipolar characteristics. OLEDs had PhCzBCz host:TCZ-F-DABNA (X wt%) as the EML. In the doping range 1–30%, EQEmax values were >30%, peaking at 39.2% for 8 wt%, with λELmax 588 nm, CIE(0.54, 0.44) and FWHM 61 nm (Fig. 38c). A high horizontal molecular orientation ratio of 96% helped to boost the outcoupling efficiency and hence raise the EQEmax. Nonetheless, all the devices exhibited serious efficiency roll-off ascribed to the rather slow kRISC of 7.80 × 104 s−1.
 | ||
| Fig. 38 (a) Molecular structure of TCZ-F-DABNA; (b) optimised molecular geometry and HOMO and LUMO distributions from DFT calculations; (c) EQE–L characteristics; inset shows the EL spectrum.162 Copyright 2022 Wiley-VCH. (d) Molecular structure of CzB4-oPh. | ||
Very recently multi-carbazole-fused BN-embedded nonacene, tridecacene and heptadecacene with more extended ladder frameworks were constructed.163 The molecules exhibited ultra-narrowband green emission with FWHM 12–16 nm and kRISC of ≈105 s−1 doped in polystyrene films. The nonacene derivative CzB4-oPh (Fig. 38d) was used as an emitter in OLEDs with EQEmax 28.7% and EQE1000 25.8%; λmax 490 nm; FWHM 21 nm. The operational lifetime LT80 was 43 h at L0 1000 cd m−2.
In the search for new blue MR-TADF emitters, mICz-DABNA and BFCz-DABNA have been studied (Fig. 39).164 PLQY values were ≈93%, ΔEST 0.20 eV and τDF ≈ 18 μs for 3 wt% emitters doped in mCBP-CN film. OLEDs had EQEmax 26.4 and 28.0% for mICz-DABNA and BFCz-DABNA, respectively, with λELmax 466 and 463 nm; CIE(0.13, 0.11) and (0.13, 0.09) and very narrow FWHM of 26 nm. There was, however, severe efficiency roll-off to ≤8% at 400 cd m−2 due to the slow kRISC ≈ 104 s−1. Very recently it has been reported that integrating meta-xylene rotors in tri-mx-CzDABNA (Fig. 39) leads to an enhanced kRISC of 2.85 × 105 s−1 (in mCBP host). Devices gave pure blue emission with λELmax 472 nm; CIE(0.13, 0.19), FWHM 34 nm and reduced efficiency roll-off with EQEmax 26.9%; EQE 24.8% at 100 cd m−2.165
It is well established that MR emitters as TADF molecules can achieve high efficiency OLEDs with narrow-band emission. However, device durability can be limited because of the instability of triplet states due to the rather slow kRISC and long triplet lifetimes. Instead of striving to shorten the DF lifetime by molecular design, the quick scavenging of triplets has been achieved by a new strategy of attaching a pyrene unit with a low-lying T1 level onto the MR emitter.166CzBNPyr (Fig. 40) is TADF-inactive with experimental ΔEST 0.62 eV (compared to unsubstituted and naphthalene-substituted CzBN analogues which are TADF-active with ΔEST 0.15 eV). Blue hyperfluorescent-OLEDs based on CzBNPyr gave EQEmax 19.4% and a substantial improvement in device stability to LT95 29.1 h at L0 1000 cd m−2, compared to analogous devices with standard MR emitters. The authors note that this strategy should be applicable to enhancing device lifetimes of other HF-OLEDs with blue, green and red MR emitters without sacrificing efficiency and colour purity.
Chalcogen atoms have been incorporated into fused carbazole MR emitters in the series CzBO, CzBS and CzBSe (Fig. 41a).167 The motivation for this work was to utilise a heavy atom effect in CzBS and CzBSe to enhance the SOC between the T and S states and thereby boost kRISC. Indeed, kRISC is claimed to be as high a 1.8 × 108 s−1 for CzBSe, accelerated by ≈800 and ≈20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times compared to CzBS and CzBO, respectively. In contrast to CzBO and CzBS, the PF component was negligible in CzBSe demonstrating that the typical kRISC ≪ kF situation that gives rise to both PF and DF components is not applicable for CzBSe. The ΔEST values for CzBO, CzBS and CzBSe were all in the range 0.14–0.16 eV (PL in 1 wt% doped in mCBP film) with PLQY values of 98–99%. Narrowband emission was observed in the doped films with λPLmax 448, 472 and 479 nm for CzBO, CzBS and CzBSe, respectively, with FWHM ≈ 30 nm (Fig. 41b). OLEDs with an EML of mCBP host:emitter (1 wt%) exhibited deep-blue to sky-blue emission with λELmax 448, 473 and 481, CIE(0.15, 0.05), (0.11, 0.16), and (0.10, 0.24), FWHM 30–33 nm for CzBO, CzBS and CzBSe, respectively. These devices gave EQE100 8.4, 21.3 and 23.4%, which was increased with the use of a microlens array to enhance light outcoupling (Fig. 41c). The CzBSe-based device exhibited a longer operational lifetime of LT50 7.48 h than the CzBS- and CzBO-devices (4.23 h and 0.16 h, respectively) at L0 100 cd m−2. The data clearly demonstrate interesting trends on implanting a chalcogen atom into MR-TADF emitters.
000 times compared to CzBS and CzBO, respectively. In contrast to CzBO and CzBS, the PF component was negligible in CzBSe demonstrating that the typical kRISC ≪ kF situation that gives rise to both PF and DF components is not applicable for CzBSe. The ΔEST values for CzBO, CzBS and CzBSe were all in the range 0.14–0.16 eV (PL in 1 wt% doped in mCBP film) with PLQY values of 98–99%. Narrowband emission was observed in the doped films with λPLmax 448, 472 and 479 nm for CzBO, CzBS and CzBSe, respectively, with FWHM ≈ 30 nm (Fig. 41b). OLEDs with an EML of mCBP host:emitter (1 wt%) exhibited deep-blue to sky-blue emission with λELmax 448, 473 and 481, CIE(0.15, 0.05), (0.11, 0.16), and (0.10, 0.24), FWHM 30–33 nm for CzBO, CzBS and CzBSe, respectively. These devices gave EQE100 8.4, 21.3 and 23.4%, which was increased with the use of a microlens array to enhance light outcoupling (Fig. 41c). The CzBSe-based device exhibited a longer operational lifetime of LT50 7.48 h than the CzBS- and CzBO-devices (4.23 h and 0.16 h, respectively) at L0 100 cd m−2. The data clearly demonstrate interesting trends on implanting a chalcogen atom into MR-TADF emitters.
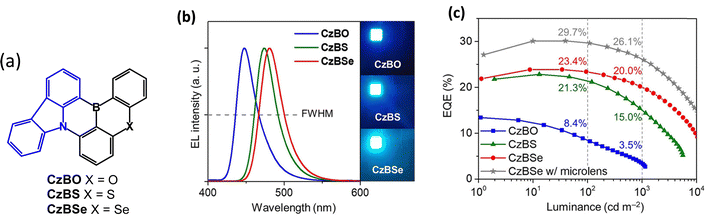 | ||
| Fig. 41 (a) Molecular structures of CzBO, CzBS and CzBSe. (b) EL spectra and device images. (c) EQE–L data for the OLEDs.167 Copyright 2022 Wiley-VCH. | ||
A more extended MR skeleton incorporating a selenium atom was designed (Fig. 42a).168BN-Se exhibited higher PLQY (99%), and faster kRISC (1.6 × 106 s−1) compared to the previously reported sulfur analogue Cz-PTN-BN (91%; 0.81 × 105 s−1).169 The ΔEST value for BN-Se was 0.08 eV. OLEDs with an EML of DMICTRZ host:BN-Se (1 wt%) exhibited λELmax 506 nm, CIE(0.15, 0.62) with FWHM 45 nm. Without any outcoupling optimisation, EQEmax 32.6%, CEmax 95.9 cd A−1 and PEmax 103.9 lm W−1 were obtained with ultralow efficiency roll-off to EQE 32.2% at 1000 cd m−2 (Fig. 42b). For comparison OLEDs with emitter Cz-PTN-BN had enhanced roll-off. The operational lifetime of the Cz-PTN-BN device was LT50 10.3 h at L0 500 cd m−2; the data were not reported for BN-Se. Furthermore, BN-Se was proposed as a photosensitiser for solar-powered applications such as photocatalysis.
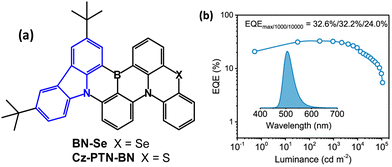 | ||
| Fig. 42 (a) Molecular structures of BN-Se and Cz-PTN-BN. (b) EQE–L data for the BN-Se OLEDs.168 Copyright 2022 The American Chemical Society. | ||
In the context of OLEDs, the benefits of sulfur and selenium may be compound-specific and not universal. There is a very recent suggestion that the similar size of oxygen and carbon atoms may optimise narrowband MR emission compared to a sulfur analogue, as the larger sulfur atom can reduce both the π-conjugation and the molecular rigidity, thereby increasing geometrical relaxation energies which broaden the FWHM.170 Also, there is evidence that device operational lifetimes can be adversely affected by the heavier chalcogen atoms,171 perhaps due to the higher chemical reactivity of C-X bonds (X = S, Se), although this is not the case with the CzBO, CzBS and CzBSe series (Fig. 41a).
5.2 Boron-free MR fused-ring carbazole derivatives
This section will highlight recent reports of boron-free MR-TADF emitters with no standard acceptor groups present in the molecules. Indolocarbazole systems embedded within the π-framework have been prominent in this regard.A rational molecular design strategy focused on the synergistic effect of para-positioned nitrogen atoms to enhance electronic coupling and to decrease the emitting energy gap.57 Accordingly, pICZ and pICZ-TPA (Fig. 43a) are deep-blue emitters with λPLmax 441 and 447 nm, FWHM of 18 and 21 nm and PLQY ≈ 90% in toluene solution. HOMO–LUMO distributions throughout the core confirm the MR character of these molecules (Fig. 43b). PL decay curves showed a single exponential feature with τPF 8.37 ns and 6.70 ns for pICZ and pICZ-TPA, respectively, suggesting their non-TADF character, which is consistent with their ΔEST values of 0.29 and 0.32 eV. Nonetheless, impressive EL performance was obtained using an EML with a ternary composition, namely pICZ or pICZ-TPA (fluorophor):PPF (a wide energy gap host):DPAc-DtCzBN (a TADF sensitiser to assist exciton recycling). Recombination of charges on the TADF sensitizer, will be followed by T1 → S1 upconversion, then energy transfer to the fluorophore for emission. EQEmax values as high as 32% and 34.7% were obtained with pICZ or pICZ-TPA, respectively, with CIEy ≤ 0.10 and FWHM 41 and 30 nm (Fig. 43b and c). Efficiency was enhanced by the high horizontal dipole ratio (0.84–0.85) of the emitters in the doped films. The large efficiency roll-off was ascribed mainly to the insufficient RISC rate (kRISC ≈ 105 s−1) of the sensitiser.
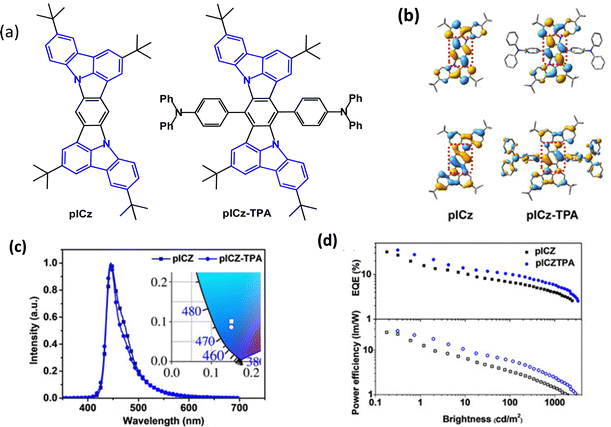 | ||
| Fig. 43 (a) Molecular structures of pICZ and pICZ-TPA. (b) Calculated HOMO and LUMO distributions of pICZ and pICZ-TPA. (c) EL spectra of the OLEDs recorded at 1000 cd m−2. (d) EQE–brightness and power efficiency–brightness curves.57 Copyright 2021 Wiley-VCH. | ||
ICz-Mes3 and DiICz-Mes4 are based on indolocarbazole units (Fig. 44a).172λPLmax was 387 and 441 nm, with very small FWHM values of 21 and 17 nm (toluene solution) for ICz-Mes3 and DICz-Mes4, respectively. The PLQY values were 66/70% under N2 and 43/47% in air saturated solutions, and the corresponding ΔEST values were 0.39/0.26 eV. ICzMes3 did not show TADF due to its large ΔEST, whereas DiICzMes4 showed weak, but unambiguous TADF. OLEDs had an EML of DPEPO host:DiICzMes4 (10 wt%). The very slow RISC rate of DiICzMes4 (kRISC 1.8 × 102 s−1) was insufficient to enable triplet harvesting even at low current densities, and DiICzMes4 acted as a fluorescent dopant by harvesting only the singlet excitons, giving EQEmax < 5%. Therefore, DiICzMes4 was utilised as a terminal emitter in a hyperfluorescent OLED with a D–A–D TADF co-host which was co-evaporated at 35 wt% in the EML, alongside 1% DiICzMes4 and bulk host DPEPO. The resulting OLEDs showed deep-blue emission from DiICzMes4 [CIE of (0.15, 0.11)] with an EQEmax 16.5% enabled by triplet harvesting of the D–A–D co-host.
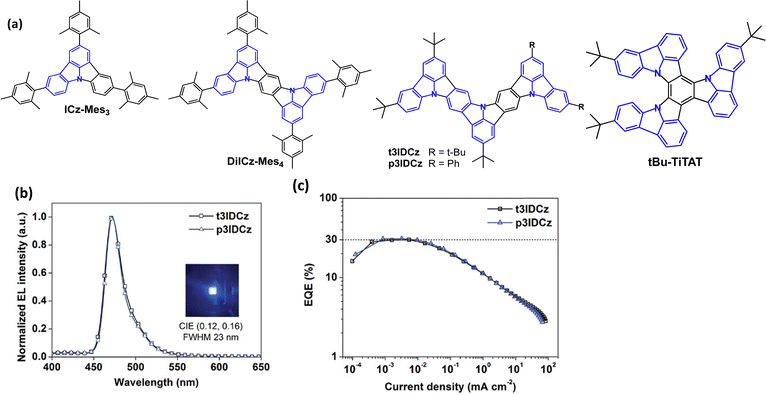 | ||
| Fig. 44 (a) Molecular structures of ICz-Mes3 and DiICz-Mes4, t3IDCz, p3IDCz and tBu-TiTAT. (b) EL spectra of t3IDCz and p3IDCz. (c) EQE–current density curves.173 Copyright 2022 Wiley-VCH. | ||
The related tricarbazole molecules t3IDCz and p3IDCz (Fig. 44a) were reported very recently.173 Blue photoluminescence was obtained with PLQYs 92–100% and λPLmax 470 nm (doped film). The calculated kRISC values were in the range 0.68–1.00 × 104 s−1, which are consistent with the experimental thin film data. ΔEST values were ≈0.20 eV (in frozen THF). Using an EML of mixed mCP:mCBP-1CN host:emitter (1 wt%) both t3IDCz and p3IDCz gave blue OLEDs (λELmax 472 nm, FWHM 23–28 nm) with EQEmax > 30%, but with extensive roll-off at 100 cd m−2 (Fig. 44b and c). The tricarbazole derivative tBu-TiTAT (Fig. 44a) has ΔEST 0.36 eV in toluene, PLQY 76% (1% doped in mCP film) and gave blue OLEDs (λELmax 454 nm, FWHM 55 nm) with EQEmax 12.6% and EQE 7.1% at 100 cd m−2.174
The MR effect has been exploited to induce narrow emission in the indoloacridine derivatives pSFlAc1 and pSFlAc2 (Fig. 45a).175 Both the emitters show deep-blue emission with λPLmax 443 and 450 nm and FWHM 18 nm in toluene solution, and ΔEST 0.29 and 0.31 eV. The PLQY values were >80% in DPEPO host films. OLEDs with an EML of α,β-ADH host:emitter (X wt%) gave EQEmax ≈ 9% and only limited roll-off to 7.3% and 8.2% at 5000 cd m−2 (Fig. 45c). The emission was deep-blue with CIE(0.147, 0.054) and (0.142, 0.066) for pSFlAc1 and pSFlAc2 devices, respectively, with FWHM 19 nm for both devices (Fig. 45b). All devices showed the same EL spectra within the dopant range 1–15% which was assigned to alleviated intermolecular interactions due to the orthogonal spirofluorene units. Furthermore, TADF-sensitised devices with an EML of mCBP:m4TCzPhBN (30 wt%):pSFlAc1 or pSFlAc2 (1–3 wt%) gave EQEmax 24.9% and 31.4%, respectively, retaining deep-blue emission, but with extensive efficiency roll-off at high brightness.
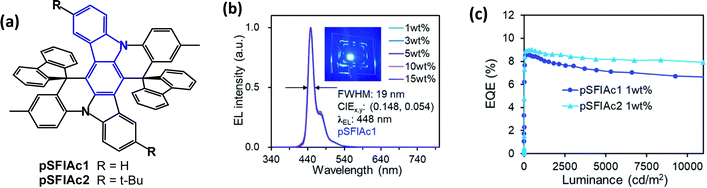 | ||
| Fig. 45 (a) Molecular structures of pSFlAc1 and pSFlAc2. (b) EL spectra and (c) EQE–luminance curves.175 Copyright 2022 The Royal Society of Chemistry. | ||
5 Conclusions and outlook
This review has demonstrated how in the last decade fused-ring carbazole derivatives have had a major impact in all the key aspects of OLED materials development – namely, traditional fluorophores, stable bipolar hosts for PhOLEDs, TADF emitters and MR emitters. These advances are set to continue at a rapid pace with a combination of precise molecular engineering, detailed photophysical analysis, high-level theory and device fabrication strategies. Fused carbazoles offer the benefits of inherent molecular rigidity, narrow band emission, small ΔEST, high PLQY, good thermal stability and structural versatility. MR-TADF emitters are currently stealing the show and further striking advances are no doubt already in the pipeline: improving kRISC is a key issue for limiting the OLED efficiency roll-off. As noted recently,172 a direct comparison between reported RISC rates from different research teams is challenging because of the different methods that can be used to obtain the data. Moreover, TD-DFT calculations which are routinely used to probe the S and T states of D–A TADF emitters, may not be reliable for MR-TADF emitters, thus hindering computationally guided molecular design.176 The race to achieve ever higher EQE values is only part of the story. As highlighted in recent reviews, a critical goal is to achieve long-lifetime OLEDs at higher brightness and lower operating voltage for global commercialisation in phones, laptops, smart consumer devices and lighting, especially in the blue region where there are critical issues with short operational lifetimes.177,178 To this end, further work is needed in understanding material degradation phenomena and device failure mechanisms under electrical stress. The continued development of narrowband pure-red emitters to meet the BT.2020 colour standard for ultra-high-definition displays is also imperative, with high colour purity especially important to offset the reduced sensitivity of humans’ eyes in the red region.We are not aware of any reports of fused carbazoles as units in emissive polymers, although TADF polymers, some of which incorporate (non-fused) carbazole, are prominent materials in OLEDs. Doped or non-doped polymer EMLs have been tailored for orange-red, green or blue emission.179–182 Solution-processing and ink-jet printing of polymers has a high material utilisation rate and does not require harsh fabrication conditions for the EML. The technology, therefore, offers advantages over vacuum evaporation techniques, especially for large-area production on flexible substrates, as reviewed recently.183 TADF-active polymer nanoparticles and films also have great potential in sensing and imaging applications.184 Indeed, recent conferences have featured exciting new directions that are at the frontiers of electroluminescent materials including wearable and implantable light emitters for healthcare monitoring, biophotonics, and information and quantum optics.185–187 TADF molecules have also been applied in X-ray scintillation and radiation detection.188 Fused-ring carbazoles should be readily adaptable by smart synthetic chemistry to play enhanced roles in all these cutting-edge topics. Indolocarbazoles have very recently been demonstrated as efficient afterglow materials for proof-of-concept data protection, further realising their promise as versatile building blocks.189
In conclusion, we hope that readers are inspired by the menu of fused-ring carbazole derivatives that is now on the banquet table of optoelectronic materials, and that many colleagues will join the party and contribute to the exciting new interdisciplinary science that is on offer!
Author contributions
S. O. and M. R. B. contributed equally to writing this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. O. thanks the Scientific and Technical Research Council of Turkey (TUBITAK) for the award of an International Postdoctoral Research Fellowship to visit Durham University.References
- B. Wex and B. R. Kaafarani, Perspective on carbazole-based organic compounds as emitters and hosts in TADF applications, J. Mater. Chem. C, 2017, 5, 8622–8653 RSC.
- P. Ledwon, Recent advances of donor-acceptor type carbazole-based molecules for light emitting applications, Org. Electron., 2019, 75, 105422 CrossRef CAS.
- G. Sathiyan, E. K. T. Sivakumar, R. Ganesamoorthy, R. Thangamuthu and P. Sakthivel, Review of carbazole based conjugated molecules for highly efficient organic solar cell application, Tetrahedron Lett., 2016, 57, 243–252 CrossRef CAS.
- L. Gao, T. H. Schloemer, F. Zhang, X. Chen, C. Xiao, K. Zhu and A. Sellinger, Carbazole-Based Hole-Transport Materials for High-Efficiency and Stable Perovskite Solar Cells, ACS Appl. Energy Mater., 2020, 3, 4492–4498 CrossRef CAS.
- L. Yang, J. Wang, L. Yang, C. Zhang, R. Zhang, Z. Zhang, B. Liu and C. Jiang, Fluorescent paper sensor fabricated by carbazole-based probes for dual visual detection of Cu2+ and gaseous H2S, RSC Adv., 2016, 6, 56384–56391 RSC.
- A. Pundi, C.-J. Chang, J. Chen, S.-R. Hsieh and M.-C. Lee, A chiral carbazole based sensor for sequential “on-off-on” fluorescence detection of Fe3+ and tryptophan/histidine, Sens. Actuators, B, 2021, 328, 129084 CrossRef CAS.
- M. Más-Montoya, J. P. Cerón-Carrasco, S. Hamao, R. Eguchi, Y. Kubozono, A. Tárraga and D. Curiel, Synthesis and characterization of carbazolo[2,1-a]carbazole in thin film and single crystal field-effect transistors, J. Mater. Chem. C, 2017, 5, 7020–7027 RSC.
- C.-H. Chen, Y. Wang, T. Michinobu, S.-W. Chang, Y.-C. Chiu, C.-Y. Ke and G.-S. Liou, Donor–Acceptor Effect of Carbazole-Based Conjugated Polymer Electrets on Photoresponsive Flash Organic Field-Effect Transistor Memories, ACS Appl. Mater. Interfaces, 2020, 12, 6144–6150 CrossRef CAS PubMed.
- L. J. O’Driscoll, X. Wang, M. Jay, A. S. Batsanov, H. Sadeghi, C. J. Lambert, B. J. Robinson and M. R. Bryce, Carbazole-Based Tetrapodal Anchor Groups for Gold Surfaces: Synthesis and Conductance Properties, Angew. Chem., Int. Ed., 2020, 59, 882–889 CrossRef PubMed.
- E. G. Cansu-Ergun and A. M. Önal, Carbazole based electrochromic polymers bearing ethylenedioxy and propylenedioxy scaffolds, J. Electroanal. Chem., 2018, 815, 158–165 CrossRef CAS.
- Z. Xu, Y. Zhang, B. Wang, Z. Liu, J. Zhao and Y. Xie, Yellow-to-blue switching of indole[3,2-b]carbazole-based electrochromic polymers and the corresponding electrochromic devices with outstanding photopic contrast, fast switching speed, and satisfactory cycling stability, Electrochim. Acta, 2019, 302, 373–384 CrossRef CAS.
- P. Gratia, A. Magomedov, T. Malinauskas, M. Daskeviciene, A. Abate, S. Ahmad, M. Grätzel, V. Getautis and M. K. Nazeeruddin, A Methoxydiphenylamine-Substituted Carbazole Twin Derivative: An Efficient Hole-Transporting Material for Perovskite Solar Cells, Angew. Chem., Int. Ed., 2015, 54, 11409–11413 CrossRef CAS PubMed.
- J. Ji, P. Li, Q. Tian, W. Feng and C. Wu, Three new carbazole derivatives with high thermal stability as host for efficient green phosphorescent organic-light emitting diodes, Dyes Pigm., 2019, 171, 107670 CrossRef CAS.
- F. Liu, J. Zou, Q. He, C. Tang, L. Xie, B. Peng, W. Wei, Y. Cao and W. Huang, Carbazole end-capped pyrene starburst with enhanced electrochemical stability and device performance, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4943–4949 CrossRef CAS.
- X.-D. Zhu, Q.-S. Tian, Q. Zheng, X.-C. Tao, Y. Yuan, Y.-J. Yu, Y. Li, Z.-Q. Jiang and L.-S. Liao, A sky-blue thermally activated delayed fluorescence emitter based on multimodified carbazole donor for efficient organic light-emitting diodes, Org. Electron., 2019, 68, 113–120 CrossRef CAS.
- I. A. Pocock, A. M. Alotaibi, K. Jagdev, C. Prior, G. R. Burgess, L. Male and R. S. Grainger, Direct formation of 4,5-disubstituted carbazoles via regioselective dilithiation, Chem. Commun., 2021, 57, 7252–7255 RSC.
- J.-F. Morin, M. Leclerc, D. Adès and A. Siove, Polycarbazoles: 25 Years of Progress, Macromol. Rapid Commun., 2005, 26, 761–778 CrossRef.
- C. Chen, K. C. Chong, Y. Pan, G. Qi, S. Xu and B. Liu, Revisiting Carbazole: Origin, Impurity, and Properties, ACS Mater. Lett., 2021, 3, 1081–1087 CrossRef CAS.
- H. Xiao, D.-S. Zheng, L.-Y. Zhang, L.-J. Xu and Z.-N. Chen, Ultra-Long Room Temperature Phosphorescence with the Efficiency Over 64% Induced by 1‰ Impurity Doping, Adv. Funct. Mater., 2023, 2214241 CrossRef CAS.
- N. A. Kukhta, T. Matulaitis, D. Volyniuk, K. Ivaniuk, P. Turyk, P. Stakhira, J. V. Grazulevicius and A. P. Monkman, Deep-Blue High-Efficiency TTA OLED Using Para- and Meta-Conjugated Cyanotriphenylbenzene and Carbazole Derivatives as Emitter and Host, J. Phys. Chem. Lett., 2017, 8, 6199–6205 CrossRef CAS PubMed.
- X.-F. Luo, F.-L. Li, J.-W. Zou, Q. Zou, J. Su, M.-X. Mao and Y.-X. Zheng, A Series of Fused Carbazole/Carbonyl Based Blue to Yellow-Green Thermally Activated Delayed Fluorescence Materials for Efficient Organic Light-Emitting Diodes, Adv. Opt. Mater., 2021, 9, 2100784 CrossRef CAS.
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Bräse, A Brief History of OLEDs—Emitter Development and Industry Milestones, Adv. Mater., 2021, 33, 2005630 CrossRef CAS PubMed.
- M. Godumala, S. Choi, M. J. Cho and D. H. Choi, Thermally activated delayed fluorescence blue dopants and hosts: from the design strategy to organic light-emitting diode applications, J. Mater. Chem. C, 2016, 4, 11355–11381 RSC.
- A. Monkman, Why Do We Still Need a Stable Long Lifetime Deep Blue OLED Emitter?, ACS Appl. Mater. Interfaces, 2022, 14, 20463–20467 CrossRef CAS.
- M. A. Baldo, D. F. O’Brien, M. E. Thompson and S. R. Forrest, Excitonic singlet-triplet ratio in a semiconducting organic thin film, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 14422–14428 CrossRef CAS.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, Nearly 100% internal phosphorescence efficiency in an organic light-emitting device, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- Y. Kawamura, J. Brooks, J. J. Brown, H. Sasabe and C. Adachi, Intermolecular Interaction and a Concentration-Quenching Mechanism of Phosphorescent Ir(III) Complexes in a Solid Film, Phys. Rev. Lett., 2006, 96, 17404 CrossRef PubMed.
- J. H. Yun, J. Lim, W. J. Chung and J. Y. Lee, Exciton stabilizing high triplet energy n-type hosts for blue phosphorescent organic light-emitting diodes, Dyes Pigm., 2021, 190, 109297 CrossRef CAS.
- C.-J. Zheng, J. Ye, M.-F. Lo, M.-K. Fung, X.-M. Ou, X.-H. Zhang and C.-S. Lee, New Ambipolar Hosts Based on Carbazole and 4,5-Diazafluorene Units for Highly Efficient Blue Phosphorescent OLEDs with Low Efficiency Roll-Off, Chem. Mater., 2012, 24, 643–650 CrossRef CAS.
- M. Hong, M. K. Ravva, P. Winget and J.-L. Brédas, Effect of Substituents on the Electronic Structure and Degradation Process in Carbazole Derivatives for Blue OLED Host Materials, Chem. Mater., 2016, 28, 5791–5798 CrossRef CAS.
- W. Qu, Z. Gao, W. Li, X. Fan, Y. Shi, Y. Miao, Z. Wu, J. Huang, H. Wang and B. Wei, Carbazole/triazine based host materials for high-performance green PhOLEDs, Dyes Pigm., 2022, 199, 110086 CrossRef CAS.
- M.-J. Yang and T. Tsutsui, Use of Poly(9-vinylcarbazole) as Host Material for Iridium Complexes in High-Efficiency Organic Light-Emitting Devices, Jpn. J. Appl. Phys., 2000, 39, 828 CrossRef.
- C.-J. Chiang, A. Kimyonok, M. K. Etherington, G. C. Griffiths, V. Jankus, F. Turksoy and A. P. Monkman, Ultrahigh Efficiency Fluorescent Single and Bi-Layer Organic Light Emitting Diodes: The Key Role of Triplet Fusion, Adv. Funct. Mater., 2013, 23, 739–746 CrossRef CAS.
- V. Jankus, C.-J. Chiang, F. Dias and A. P. Monkman, Deep Blue Exciplex Organic Light-Emitting Diodes with Enhanced Efficiency; P-type or E-type Triplet Conversion to Singlet Excitons?, Adv. Mater., 2013, 25, 1455–1459 CrossRef CAS PubMed.
- Y. Liu, H. Liu, Q. Bai, C. Du, A. Shang, D. Jiang, X. Tang and P. Lu, Pyrene[4,5-d]imidazole-Based Derivatives with Hybridized Local and Charge-Transfer State for Highly Efficient Blue and White Organic Light-Emitting Diodes with Low Efficiency Roll-Off, ACS Appl. Mater. Interfaces, 2020, 12, 16715–16725 CrossRef CAS PubMed.
- A. Obolda, Q. Peng, C. He, T. Zhang, J. Ren, H. Ma, Z. Shuai and F. Li, Triplet–Polaron-Interaction-Induced Upconversion from Triplet to Singlet: a Possible Way to Obtain Highly Efficient OLEDs, Adv. Mater., 2016, 28, 4740–4746 CrossRef CAS PubMed.
- C. A. Parker and C. G. Hatchard, Triplet–singlet emission in fluid solutions. Phosphorescence of eosin, Trans. Faraday Soc., 1961, 57, 1894–1904 RSC.
- A. Endo, M. Ogasawara, A. Takahashi, D. Yokoyama, Y. Kato and C. Adachi, Thermally Activated Delayed Fluorescence from Sn4+–Porphyrin Complexes and Their Application to Organic Light Emitting Diodes—A Novel Mechanism for Electroluminescence, Adv. Mater., 2009, 21, 4802–4806 CrossRef CAS.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes, Appl. Phys. Lett., 2011, 98, 083302 CrossRef.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Revealing the spin–vibronic coupling mechanism of thermally activated delayed fluorescence, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- A. Chaskar, H.-F. Chen and K.-T. Wong, Bipolar Host Materials: A Chemical Approach for Highly Efficient Electrophosphorescent Devices, Adv. Mater., 2011, 23, 3876–3895 CrossRef CAS PubMed.
- Y. Tao, C. Yang and J. Qin, Organic host materials for phosphorescent organic light-emitting diodes, Chem. Soc. Rev., 2011, 40, 2943–2970 RSC.
- Q. Wang, Q.-S. Tian, Y.-L. Zhang, X. Tang and L.-S. Liao, High-efficiency organic light-emitting diodes with exciplex hosts, J. Mater. Chem. C, 2019, 7, 11329–11360 RSC.
- T. B. Nguyen, H. Nakanotani, T. Hatakeyama and C. Adachi, The Role of Reverse Intersystem Crossing Using a TADF-Type Acceptor Molecule on the Device Stability of Exciplex-Based Organic Light-Emitting Diodes, Adv. Mater., 2020, 32, 1906614 CrossRef CAS PubMed.
- C.-C. Lee, N. R. Al Amin, J.-J. Xu, B.-C. Wang, D. Luo, K. Sutanto, S. Biring, S.-W. Liu and C.-H. Chen, Structural effect of phenylcarbazole-based molecules on the exciplex-forming co-host system to achieve highly efficient phosphorescent OLEDs with low efficiency roll-off, J. Mater. Chem. C, 2021, 9, 9453–9464 RSC.
- I. A. Wright, A. Danos, S. Montanaro, A. S. Batsanov, A. P. Monkman and M. R. Bryce, Conformational Dependence of Triplet Energies in Rotationally Hindered N- and S-Heterocyclic Dimers: New Design and Measurement Rules for High Triplet Energy OLED Host Materials, Chem. – Eur. J., 2021, 27, 6545–6556 CrossRef CAS.
- M.-H. Tsai, Y.-H. Hong, C.-H. Chang, H.-C. Su, C.-C. Wu, A. Matoliukstyte, J. Simokaitiene, S. Grigalevicius, J. V. Grazulevicius and C.-P. Hsu, 3-(9-Carbazolyl)carbazoles and 3,6-Di(9-carbazolyl)carbazoles as Effective Host Materials for Efficient Blue Organic Electrophosphorescence, Adv. Mater., 2007, 19, 862–866 CrossRef CAS.
- Y. Tsuchiya, N. Nakamura, S. Kakumachi, K. Kusuhara, C.-Y. Chan and C. Adachi, A convenient method to estimate the glass transition temperature of small organic semiconductor materials, Chem. Commun., 2022, 58, 11292–11295 RSC.
- M.-S. Gong, J.-R. Cha and C. W. Lee, Synthesis and device properties of mCP analogues based on fused-ring carbazole moiety, Org. Electron., 2017, 42, 66–74 CrossRef CAS.
- M. Gantenbein, M. Hellstern, L. Le Pleux, M. Neuburger and M. Mayor, New 4,4′-Bis(9-carbazolyl)–Biphenyl Derivatives with Locked Carbazole–Biphenyl Junctions: High-Triplet State Energy Materials, Chem. Mater., 2015, 27, 1772–1779 CrossRef CAS.
- I. A. Wright, H. A. Al-Attar, A. S. Batsanov, A. P. Monkman and M. R. Bryce, Conformationally-restricted bicarbazoles with phenylene bridges displaying deep-blue emission and high triplet energies: systematic structure–property relationships, Phys. Chem. Chem. Phys., 2018, 20, 11867–11875 RSC.
- S.-J. Su, H. Sasabe, T. Takeda and J. Kido, Pyridine-Containing Bipolar Host Materials for Highly Efficient Blue Phosphorescent OLEDs, Chem. Mater., 2008, 20, 1691–1693 CrossRef CAS.
- D. Kim, L. Zhu and J.-L. Brédas, Electronic Structure of Carbazole-Based Phosphine Oxides as Ambipolar Host Materials for Deep Blue Electrophosphorescence: A Density Functional Theory Study, Chem. Mater., 2012, 24, 2604–2610 CrossRef CAS.
- S. Lee, K.-H. Kim, D. Limbach, Y.-S. Park and J.-J. Kim, Low Roll-Off and High Efficiency Orange Organic Light Emitting Diodes with Controlled Co-Doping of Green and Red Phosphorescent Dopants in an Exciplex Forming Co-Host, Adv. Funct. Mater., 2013, 23, 4105–4110 CrossRef CAS.
- J. H. Maeng, D. H. Ahn, H. Lee, Y. H. Jung, D. Karthik, J. Y. Lee and J. H. Kwon, Rigid indolocarbazole donor moiety for highly efficient thermally activated delayed fluorescent device, Dyes Pigm., 2020, 180, 108485 CrossRef CAS.
- J. Wei, C. Zhang, D. Zhang, Y. Zhang, Z. Liu, Z. Li, G. Yu and L. Duan, Indolo[3,2,1-jk]carbazole Embedded Multiple-Resonance Fluorophors for Narrowband Deep-blue Electroluminescence with EQE ≈ 34.7% and CIEy ≈ 0.085, Angew. Chem., Int. Ed., 2021, 60, 12269–12273 CrossRef CAS PubMed.
- S. Xiang, X. Lv, S. Sun, Q. Zhang, Z. Huang, R. Guo, H. Gu, S. Liu and L. Wang, To improve the efficiency of thermally activated delayed fluorescence OLEDs by controlling the horizontal orientation through optimizing stereoscopic and linear structures of indolocarbazole isomers, J. Mater. Chem. C, 2018, 6, 5812–5820 RSC.
- R. Braveenth, H. Lee, J. D. Park, K. J. Yang, S. J. Hwang, K. R. Naveen, R. Lampande and J. H. Kwon, Achieving Narrow FWHM and High EQE Over 38% in Blue OLEDs Using Rigid Heteroatom-Based Deep Blue TADF Sensitized Host, Adv. Funct. Mater., 2021, 31, 2105805 CrossRef CAS.
- S. Yang, D. Guan, M. Yang, J. Tian, W. Chu and Z. Sun, Synthesis and characterization of novel butterfly-shaped aryl-substituted indolo[2,3-a]carbazole derivatives, Tetrahedron Lett., 2015, 56, 2223–2227 CrossRef CAS.
- Y.-X. Zhang, Y. Yuan, Q. Wang, Y. Hu, A. Khan, Z.-Q. Jiang and L.-S. Liao, Highly efficient non-doped deep-blue organic light-emitting diodes by employing a highly rigid skeleton, Dyes Pigm., 2018, 158, 396–401 CrossRef CAS.
- R. Mai, X. Wu, Y. Jiang, Y. Meng, B. Liu, X. Hu, J. Roncali, G. Zhou, J.-M. Liu, K. Kempa and J. Gao, An efficient multi-functional material based on polyether-substituted indolocarbazole for perovskite solar cells and solution-processed non-doped OLEDs, J. Mater. Chem. A, 2019, 7, 1539–1547 RSC.
- F. de Jong, M. Daniels, L. Vega-Castillo, K. Kennes, C. Martín, G. de Miguel, M. Cano, M. Pérez-Morales, J. Hofkens, W. Dehaen and M. Van der Auweraer, 5,10-Dihydrobenzo[a]indolo[2,3-c]carbazoles as Novel OLED Emitters, J. Phys. Chem. B, 2019, 123, 1400–1411 CrossRef CAS PubMed.
- V. V. Patil, K. H. Lee and J. Y. Lee, Isomeric fused benzocarbazole as a chromophore for blue fluorescent organic light-emitting diodes, J. Mater. Chem. C, 2020, 8, 8320–8327 RSC.
- V. V. Patil, K. H. Lee and J. Y. Lee, A novel fluorene–indolocarbazole hybrid chromophore to assemble high efficiency deep-blue fluorescent emitters with extended device lifetime, J. Mater. Chem. C, 2020, 8, 3051–3057 RSC.
- V. V. Patil, J. Lim and J. Y. Lee, Strategic Synchronization of 7,7-Dimethyl-5,7-dihydroindeno[2,1-b]carbazole for Narrow-Band, Pure Violet Organic Light-Emitting Diodes with an Efficiency of >5% and a CIEy Coordinate of <0.03, ACS Appl. Mater. Interfaces, 2021, 13, 14440–14446 CrossRef CAS.
- X. Liang, Z.-P. Yan, H.-B. Han, Z.-G. Wu, Y.-X. Zheng, H. Meng, J.-L. Zuo and W. Huang, Peripheral Amplification of Multi-Resonance Induced Thermally Activated Delayed Fluorescence for Highly Efficient OLEDs, Angew. Chem., Int. Ed., 2018, 57, 11316–11320 CrossRef CAS PubMed.
- D. Sun, S. M. Suresh, D. Hall, M. Zhang, C. Si, D. B. Cordes, A. M. Z. Slawin, Y. Olivier, X. Zhang and E. Zysman-Colman, The design of an extended multiple resonance TADF emitter based on a polycyclic amine/carbonyl system, Mater. Chem. Front., 2020, 4, 2018–2022 RSC.
- H. L. Lee, W. J. Chung and J. Y. Lee, Narrowband and Pure Violet Organic Emitter with a Full Width at Half Maximum of 14 nm and y Color Coordinate of Below 0.02, Small, 2020, 16, 1907569 CrossRef CAS PubMed.
- D. Zhang, L. Duan, Y. Li, H. Li, Z. Bin, D. Zhang, J. Qiao, G. Dong, L. Wang and Y. Qiu, Towards High Efficiency and Low Roll-Off Orange Electrophosphorescent Devices by Fine Tuning Singlet and Triplet Energies of Bipolar Hosts Based on Indolocarbazole/1,3,5-Triazine Hybrids, Adv. Funct. Mater., 2014, 24, 3551–3561 CrossRef CAS.
- Y. Li, D. Zhang and L. Duan, High efficiency red phosphorescent organic light-emitting diodes with low dopant concentration, low roll-off and long lifetime based on a novel host material with thermally activated delayed fluorescent properties, Org. Electron., 2018, 57, 53–59 CrossRef CAS.
- X. Song, D. Zhang, Y. Lu, C. Yin and L. Duan, Understanding and Manipulating the Interplay of Wide-Energy-Gap Host and TADF Sensitizer in High-Performance Fluorescence OLEDs, Adv. Mater., 2019, 31, 1901923 CrossRef PubMed.
- R. Braveenth, I.-J. Bae, K. Raagulan, S. Kim, S. Kim, M. Kim and K. Y. Chai, Dibenzothiophene-indolocarbazole-based bipolar material: Host for green phosphorescent OLEDs and non-doped fluorescent emitter, Dyes Pigm., 2019, 162, 466–474 CrossRef CAS.
- K. Guo, H. Wang, Z. Wang, C. Si, C. Peng, G. Chen, J. Zhang, G. Wang and B. Wei, Stable green phosphorescence organic light-emitting diodes with low efficiency roll-off using a novel bipolar thermally activated delayed fluorescence material as host, Chem. Sci., 2017, 8, 1259–1268 RSC.
- E. Y. Park, D. H. Lee, T. N. Le, C.-M. Shin, J. Lee and M. C. Suh, An indenocarbazole-based host material for solution processable green phosphorescent organic light emitting diodes, RSC Adv., 2021, 11, 29115–29123 RSC.
- V. V. Patil, J. Lim and J. Y. Lee, C2-, C3- spirobifluorene fused carbazole modified triazine as an electron transport type host of exciplex, Dyes Pigm., 2021, 189, 109247 CrossRef CAS.
- X.-H. Zheng, J.-W. Zhao, T.-T. Huang, X. Chen, C. Cao, G.-X. Yang, Z.-H. Lin, Q.-X. Tong, S.-L. Tao and D. Liu, Versatile Host Materials for Highly-Efficient Green, Red Phosphorescent and White Organic Light-Emitting Diodes, ChemElectroChem, 2019, 6, 5810–5818 CrossRef CAS.
- Y. Hiraga, R. Kuwahara and T. Hatta, Novel indolo[3,2,1-jk]carbazole-based bipolar host material for highly efficient thermally activated delayed-fluorescence organic light-emitting diodes, Tetrahedron, 2021, 94, 132317 CrossRef CAS.
- A. Arai, H. Sasabe, K. Nakao, Y. Masuda and J. Kido, π-Extended Carbazole Derivatives as Host Materials for Highly Efficient and Long-Life Green Phosphorescent Organic Light-Emitting Diodes, Chem. – Eur. J., 2021, 27, 4971–4976 CrossRef CAS.
- J. M. Choi, J. H. Kim, Y. J. Kang and J. Y. Lee, Development of an exciplex type mixed host using a pyrrolocarbazole type material for extended lifetime in green phosphorescent organic light-emitting diodes, Org. Electron., 2017, 49, 393–399 CrossRef CAS.
- P. L. dos Santos, J. S. Ward, A. S. Batsanov, M. R. Bryce and A. P. Monkman, Optical and Polarity Control of Donor–Acceptor Conformation and Their Charge-Transfer States in Thermally Activated Delayed-Fluorescence Molecules, J. Phys. Chem. C, 2017, 121, 16462–16469 CrossRef CAS.
- K. Wang, C.-J. Zheng, W. Liu, K. Liang, Y.-Z. Shi, S.-L. Tao, C.-S. Lee, X.-M. Ou and X.-H. Zhang, Avoiding Energy Loss on TADF Emitters: Controlling the Dual Conformations of D–A Structure Molecules Based on the Pseudoplanar Segments, Adv. Mater., 2017, 29, 1701476 CrossRef PubMed.
- D.-G. Chen, T.-C. Lin, C.-L. Chen, Y.-T. Chen, Y.-A. Chen, G.-H. Lee, P.-T. Chou, C.-W. Liao, P.-C. Chiu, C.-H. Chang, Y.-J. Lien and Y. Chi, Optically Triggered Planarization of Boryl-Substituted Phenoxazine: Another Horizon of TADF Molecules and High-Performance OLEDs, ACS Appl. Mater. Interfaces, 2018, 10, 12886–12896 CrossRef CAS PubMed.
- K. Masui, H. Nakanotani and C. Adachi, Analysis of exciton annihilation in high-efficiency sky-blue organic light-emitting diodes with thermally activated delayed fluorescence, Org. Electron., 2013, 14, 2721–2726 CrossRef CAS.
- C. S. Oh, H. L. Lee, S. H. Han and J. Y. Lee, Rational Molecular Design Overcoming the Long Delayed Fluorescence Lifetime and Serious Efficiency Roll-Off in Blue Thermally Activated Delayed Fluorescent Devices, Chem. – Eur. J., 2019, 25, 642–648 CrossRef CAS PubMed.
- R. K. Konidena, K. R. J. Thomas and J. W. Park, Recent Advances in the Design of Multi-Substituted Carbazoles for Optoelectronics: Synthesis and Structure–Property Outlook, ChemPhotoChem, 2022, 6, e202200059 CrossRef CAS.
- D. Zhang, X. Song, M. Cai, H. Kaji and L. Duan, Versatile Indolocarbazole-Isomer Derivatives as Highly Emissive Emitters and Ideal Hosts for Thermally Activated Delayed Fluorescent OLEDs with Alleviated Efficiency Roll-Off, Adv. Mater., 2018, 30, 1705406 CrossRef PubMed.
- K. Sato, K. Shizu, K. Yoshimura, A. Kawada, H. Miyazaki and C. Adachi, Organic Luminescent Molecule with Energetically Equivalent Singlet and Triplet Excited States for Organic Light-Emitting Diodes, Phys. Rev. Lett., 2013, 110, 247401 CrossRef PubMed.
- Q. Zhang, H. Kuwabara, W. J. Potscavage, S. Huang, Y. Hatae, T. Shibata and C. Adachi, Anthraquinone-Based Intramolecular Charge-Transfer Compounds: Computational Molecular Design, Thermally Activated Delayed Fluorescence, and Highly Efficient Red Electroluminescence, J. Am. Chem. Soc., 2014, 136, 18070–18081 CrossRef CAS PubMed.
- D. Karthik, S. Y. Lee, D. H. Ahn, H. Lee, J. Y. Lee, J. H. Kwon and J. H. Park, Comparative analysis of various indolocarbazole-based emitters on thermally activated delayed fluorescence performances, Org. Electron., 2019, 74, 282–289 CrossRef CAS.
- M. Kim, S. K. Jeon, S.-H. Hwang, S.-S. Lee, E. Yu and J. Y. Lee, Correlation of Molecular Structure with Photophysical Properties and Device Performances of Thermally Activated Delayed Fluorescent Emitters, J. Phys. Chem. C, 2016, 120, 2485–2493 CrossRef CAS.
- H. J. Lee, H. L. Lee, S. H. Han and J. Y. Lee, Novel secondary acceptor based molecular design for superb lifetime in thermally activated delayed fluorescent organic light-emitting diodes through high bond energy and fast up-conversion, Chem. Eng. J., 2022, 427, 130988 CrossRef CAS.
- J. Wang, J. Zhang, C. Jiang, C. Yao and X. Xi, Effective Design Strategy for Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence Emitters Achieving 18% External Quantum Efficiency Pure-Blue OLEDs with Extremely Low Roll-Off, ACS Appl. Mater. Interfaces, 2021, 13, 57713–57724 CrossRef CAS PubMed.
- M. R. Bryce, Aggregation-induced delayed fluorescence (AIDF) materials: a new break-through for nondoped OLEDs, Sci. China: Chem., 2017, 60, 1561–1562 CrossRef CAS.
- J. Guo, X.-L. Li, H. Nie, W. Luo, R. Hu, A. Qin, Z. Zhao, S.-J. Su and B. Z. Tang, Robust Luminescent Materials with Prominent Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence for High-Performance Organic Light-Emitting Diodes, Chem. Mater., 2017, 29, 3623–3631 CrossRef CAS.
- D. Liu, J. Y. Wei, W. W. Tian, W. Jiang, Y. M. Sun, Z. Zhao and B. Z. Tang, Endowing TADF luminophors with AIE properties through adjusting flexible dendrons for highly efficient solution-processed nondoped OLEDs, Chem. Sci., 2020, 11, 7194–7203 RSC.
- J. H. Kim, M. Eum, T. H. Kim and J. Y. Lee, A novel pyrrolocarbazole donor for stable and highly efficient thermally activated delayed fluorescent emitters, Dyes Pigm., 2017, 136, 529–534 CrossRef CAS.
- W.-L. Tsai, M.-H. Huang, W.-K. Lee, Y.-J. Hsu, K.-C. Pan, Y.-H. Huang, H.-C. Ting, M. Sarma, Y.-Y. Ho, H.-C. Hu, C.-C. Chen, M.-T. Lee, K.-T. Wong and C.-C. Wu, A versatile thermally activated delayed fluorescence emitter for both highly efficient doped and non-doped organic light emitting devices, Chem. Commun., 2015, 51, 13662–13665 RSC.
- J. G. Yu, S. H. Han, H. L. Lee, W. P. Hong and J. Y. Lee, A novel molecular design employing a backbone freezing linker for improved efficiency, sharpened emission and long lifetime in thermally activated delayed fluorescence emitters, J. Mater. Chem. C, 2019, 7, 2919–2926 RSC.
- H. Kang, S. O. Jeon, Y. S. Chung, M. Sim, J. S. Kim, J. Kim, H. Lee, S.-G. Ihn, S. Kim and J. Hong, High-efficiency blue organic light-emitting Diodes using emissive carbazole-triazine-based donor-acceptor molecules with high reverse intersystem crossing rates, Org. Electron., 2019, 75, 105399 CrossRef CAS.
- X. Zheng, F. Cao, C. Wang, T. Tsuboi, Y. Zhu, Q. Ai, C. Deng, D. Wang, L. Su, Z. Liu and Q. Zhang, Expanding the hole delocalization range in excited molecules for stable organic light-emitting diodes employing thermally activated delayed fluorescence, J. Mater. Chem. C, 2020, 8, 10021–10030 RSC.
- Z. Zhang, E. Crovini, P. L. dos Santos, B. A. Naqvi, D. B. Cordes, A. M. Z. Slawin, P. Sahay, W. Brütting, I. D. W. Samuel, S. Bräse and E. Zysman-Colman, Efficient Sky-Blue Organic Light-Emitting Diodes Using a Highly Horizontally Oriented Thermally Activated Delayed Fluorescence Emitter, Adv. Opt. Mater., 2020, 8, 2001354 CrossRef CAS.
- E. Crovini, Z. Zhang, Y. Kusakabe, Y. Ren, Y. Wada, B. A. Naqvi, P. Sahay, T. Matulaitis, S. Diesing, I. D. W. Samuel, W. Brütting, K. Suzuki, H. Kaji, S. Bräse and E. Zysman-Colman, Effect of a twin-emitter design strategy on a previously reported thermally activated delayed fluorescence organic light-emitting diode, Beilstein J. Org. Chem., 2021, 17, 2894–2905 CrossRef CAS PubMed.
- D. H. Ahn, S. W. Kim, H. Lee, I. J. Ko, D. Karthik, J. Y. Lee and J. H. Kwon, Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors, Nat. Photonics, 2019, 13, 540–546 CrossRef CAS.
- Y. H. Lee, S. Park, J. Oh, S.-J. Woo, A. Kumar, J.-J. Kim, J. Jung, S. Yoo and M. H. Lee, High-Efficiency Sky Blue to Ultradeep Blue Thermally Activated Delayed Fluorescent Diodes Based on Ortho-Carbazole-Appended Triarylboron Emitters: Above 32% External Quantum Efficiency in Blue Devices, Adv. Opt. Mater., 2018, 6, 1800385 CrossRef.
- A. Shuto, T. Kushida, T. Fukushima, H. Kaji and S. Yamaguchi, π-Extended Planarized Triphenylboranes with Thiophene Spacers, Org. Lett., 2013, 15, 6234–6237 CrossRef CAS PubMed.
- Y. Kitamoto, T. Namikawa, D. Ikemizu, Y. Miyata, T. Suzuki, H. Kita, T. Sato and S. Oi, Light blue and green thermally activated delayed fluorescence from 10H-phenoxaborin-derivatives and their application to organic light-emitting diodes, J. Mater. Chem. C, 2015, 3, 9122–9130 RSC.
- Y. Kitamoto, T. Namikawa, T. Suzuki, Y. Miyata, H. Kita, T. Sato and S. Oi, Dimesitylarylborane-based luminescent emitters exhibiting highly-efficient thermally activated delayed fluorescence for organic light-emitting diodes, Org. Electron., 2016, 34, 208–217 CrossRef CAS.
- K. Suzuki, S. Kubo, K. Shizu, T. Fukushima, A. Wakamiya, Y. Murata, C. Adachi and H. Kaji, Triarylboron-Based Fluorescent Organic Light-Emitting Diodes with External Quantum Efficiencies Exceeding 20%, Angew. Chem., Int. Ed., 2015, 54, 15231–15235 CrossRef CAS PubMed.
- M. Numata, T. Yasuda and C. Adachi, High efficiency pure blue thermally activated delayed fluorescence molecules having 10H-phenoxaborin and acridan units, Chem. Commun., 2015, 51, 9443–9446 RSC.
- J. Hwang, C. W. Koh, J. M. Ha, H. Y. Woo, S. Park, M. J. Cho and D. H. Choi, Aryl-Annulated[3,2-a]Carbazole-Based Deep-Blue Soluble Emitters for High-Efficiency Solution-Processed Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes with CIEy < 0.1, ACS Appl. Mater. Interfaces, 2021, 13, 61454–61462 CrossRef CAS PubMed.
- J. Hwang, H. Kang, J.-E. Jeong, H. Y. Woo, M. J. Cho, S. Park and D. H. Choi, Donor engineered Deep-Blue emitters for tuning luminescence mechanism in Solution-Processed OLEDs, Chem. Eng. J., 2021, 416, 129185 CrossRef CAS.
- G. Meng, H. Dai, Q. Wang, J. Zhou, T. Fan, X. Zeng, X. Wang, Y. Zhang, D. Yang, D. Ma, D. Zhang and L. Duan, High-efficiency and stable short-delayed fluorescence emitters with hybrid long- and short-range charge-transfer excitations, Nat. Commun., 2023, 14, 2394 CrossRef CAS PubMed.
- D. Zhang, C. Zhao, Y. Zhang, X. Song, P. Wei, M. Cai and L. Duan, Highly Efficient Full-Color Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes: Extremely Low Efficiency Roll-Off Utilizing a Host with Small Singlet–Triplet Splitting, ACS Appl. Mater. Interfaces, 2017, 9, 4769–4777 CrossRef CAS PubMed.
- X. Lv, R. Huang, S. Sun, Q. Zhang, S. Xiang, S. Ye, P. Leng, F. B. Dias and L. Wang, Blue TADF Emitters Based on Indenocarbazole Derivatives with High Photoluminescence and Electroluminescence Efficiencies, ACS Appl. Mater. Interfaces, 2019, 11, 10758–10767 CrossRef CAS PubMed.
- M. Inoue, T. SereviĿius, H. Nakanotani, K. Yoshida, T. Matsushima, S. JuršĿnas and C. Adachi, Effect of reverse intersystem crossing rate to suppress efficiency roll-off in organic light-emitting diodes with thermally activated delayed fluorescence emitters, Chem. Phys. Lett., 2016, 644, 62–67 CrossRef CAS.
- Y. Im, M. Kim, Y. J. Cho, J.-A. Seo, K. S. Yook and J. Y. Lee, Molecular Design Strategy of Organic Thermally Activated Delayed Fluorescence Emitters, Chem. Mater., 2017, 29, 1946–1963 CrossRef CAS.
- G. H. Kim, R. Lampande, J. B. Im, J. M. Lee, J. Y. Lee and J. H. Kwon, Controlling the exciton lifetime of blue thermally activated delayed fluorescence emitters using a heteroatom-containing pyridoindole donor moiety, Mater. Horiz., 2017, 4, 619–624 RSC.
- J. S. Ward, R. S. Nobuyasu, M. A. Fox, A. S. Batsanov, J. Santos, F. B. Dias and M. R. Bryce, Bond Rotations and Heteroatom Effects in Donor–Acceptor–Donor Molecules: Implications for Thermally Activated Delayed Fluorescence and Room Temperature Phosphorescence, J. Org. Chem., 2018, 83, 14431–14442 CrossRef CAS PubMed.
- Z. Chen, F. Ni, Z. Wu, Y. Hou, C. Zhong, M. Huang, G. Xie, D. Ma and C. Yang, Enhancing Spin–Orbit Coupling by Introducing a Lone Pair Electron with p Orbital Character in a Thermally Activated Delayed Fluorescence Emitter: Photophysics and Devices, J. Phys. Chem. Lett., 2019, 10, 2669–2675 CrossRef CAS PubMed.
- K. Matsuo and T. Yasuda, Blue thermally activated delayed fluorescence emitters incorporating acridan analogues with heavy group 14 elements for high-efficiency doped and non-doped OLEDs, Chem. Sci., 2019, 10, 10687–10697 RSC.
- X.-K. Chen, B. W. Bakr, M. Auffray, Y. Tsuchiya, C. D. Sherrill, C. Adachi and J.-L. Bredas, Intramolecular Noncovalent Interactions Facilitate Thermally Activated Delayed Fluorescence (TADF), J. Phys. Chem. Lett., 2019, 10, 3260–3268 CrossRef CAS PubMed.
- T. Agou, K. Matsuo, R. Kawano, I. S. Park, T. Hosoya, H. Fukumoto, T. Kubota, Y. Mizuhata, N. Tokitoh and T. Yasuda, Pentacyclic Ladder-Heteraborin Emitters Exhibiting High-Efficiency Blue Thermally Activated Delayed Fluorescence with an Ultrashort Emission Lifetime, ACS Mater. Lett., 2020, 2, 28–34 CrossRef CAS.
- D. R. Lee, S.-H. Hwang, S. K. Jeon, C. W. Lee and J. Y. Lee, Benzofurocarbazole and benzothienocarbazole as donors for improved quantum efficiency in blue thermally activated delayed fluorescent devices, Chem. Commun., 2015, 51, 8105–8107 RSC.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- D. R. Lee, J. M. Choi, C. W. Lee and J. Y. Lee, Ideal Molecular Design of Blue Thermally Activated Delayed Fluorescent Emitter for High Efficiency, Small Singlet–Triplet Energy Splitting, Low Efficiency Roll-Off, and Long Lifetime, ACS Appl. Mater. Interfaces, 2016, 8, 23190–23196 CrossRef CAS PubMed.
- X. Zhang, C. Fuentes-Hernandez, Y. Zhang, M. W. Cooper, S. Barlow, S. R. Marder and B. Kippelen, High performance blue-emitting organic light-emitting diodes from thermally activated delayed fluorescence: A guest/host ratio study, J. Appl. Phys., 2018, 124, 55501 CrossRef.
- H. Abroshan, E. Cho, V. Coropceanu and J.-L. Brédas, Suppression of Concentration Quenching in Ortho-Substituted Thermally Activated Delayed Fluorescence Emitters, Adv. Theory Simul., 2020, 3, 1900185 CrossRef CAS.
- D. J. Shin, J. Lim and J. Y. Lee, Combinatorial donor engineering for highly efficient blue thermally activated delayed fluorescence emitters with low efficiency roll-off, J. Mater. Chem. C, 2021, 9, 15276–15283 RSC.
- D. S. Kim, S. J. Yoon, K. H. Lee, J. Y. Lee and W. P. Hong, Starburst Type Benzofuroindolocarbazole Donor for High Efficiency and Long Lifetime in Thermally Activated Delayed Fluorescence Emitters, Adv. Opt. Mater., 2021, 9, 2001432 CrossRef CAS.
- Q. Zhang, S. Xiang, Z. Huang, S. Sun, S. Ye, X. Lv, W. Liu, R. Guo and L. Wang, Molecular engineering of pyrimidine-containing thermally activated delayed fluorescence emitters for highly efficient deep-blue (CIEy < 0.06) organic light-emitting diodes, Dyes Pigm., 2018, 155, 51–58 CrossRef CAS.
- M. Cai, M. Auffray, D. Zhang, Y. Zhang, R. Nagata, Z. Lin, X. Tang, C.-Y. Chan, Y.-T. Lee, T. Huang, X. Song, Y. Tsuchiya, C. Adachi and L. Duan, Enhancing spin–orbital coupling in deep-blue/blue TADF emitters by minimizing the distance from the heteroatoms in donors to acceptors, Chem. Eng. J., 2021, 420, 127591 CrossRef CAS.
- D. J. Shin, S. J. Hwang, J. Lim, C. Y. Jeon, J. Y. Lee and J. H. Kwon, Reverse intersystem crossing accelerating assistant dopant for high efficiency and long lifetime in red hyperfluorescence organic light-emitting diodes, Chem. Eng. J., 2022, 446, 137181 CrossRef CAS.
- J. Lee, U. Jo and J. Y. Lee, Suppression of Dexter Energy Transfer through Modulating Donor Segments of Thermally Activated Delayed Fluorescence Assistant Dopants, ACS Appl. Mater. Interfaces, 2023, 15, 21261–21269 CrossRef CAS PubMed.
- J.-A. Seo, Y. Im, S. H. Han, C. W. Lee and J. Y. Lee, Unconventional Molecular Design Approach of High-Efficiency Deep Blue Thermally Activated Delayed Fluorescent Emitters Using Indolocarbazole as an Acceptor, ACS Appl. Mater. Interfaces, 2017, 9, 37864–37872 CrossRef CAS PubMed.
- Y. Im, S. H. Han and J. Y. Lee, Deep blue thermally activated delayed fluorescent emitters using CN-modified indolocarbazole as an acceptor and carbazole-derived donors, J. Mater. Chem. C, 2018, 6, 5012–5017 RSC.
- P. L. dos Santos, J. S. Ward, D. G. Congrave, A. S. Batsanov, J. Eng, J. E. Stacey, T. J. Penfold, A. P. Monkman and M. R. Bryce, Triazatruxene: A Rigid Central Donor Unit for a D–A3 Thermally Activated Delayed Fluorescence Material Exhibiting Sub-Microsecond Reverse Intersystem Crossing and Unity Quantum Yield via Multiple Singlet–Triplet State Pairs, Adv. Sci., 2018, 5, 1700989 CrossRef PubMed.
- T. J. Penfold and J. Eng, Mind the GAP: quantifying the breakdown of the linear vibronic coupling Hamiltonian, Phys. Chem. Chem. Phys., 2023, 25, 7195–7204 RSC.
- P. L. dos Santos, D. de Sa Pereira, J. Eng, J. S. Ward, M. R. Bryce, T. J. Penfold and A. P. Monkman, Fine-Tuning the Photophysics of Donor–Acceptor (D–A3) Thermally Activated Delayed Fluorescence Emitters Using Isomerisation, ChemPhotoChem, 2023, 7, e202200248 CrossRef.
- K. J. Kim, G. H. Kim, R. Lampande, D. H. Ahn, J. B. Im, J. S. Moon, J. K. Lee, J. Y. Lee, J. Y. Lee and J. H. Kwon, A new rigid diindolocarbazole donor moiety for high quantum efficiency thermally activated delayed fluorescence emitter, J. Mater. Chem. C, 2018, 6, 1343–1348 RSC.
- L. Chen, C. Li, E. Fu, M. Li, Y. Kuboi, Z.-Y. Li, Z. Chen, J. Chen, X. Liu, X. Tang, L. Frédéric, F. Maurel, C. Adachi, F. Mathevet and S. Zhang, A Donor–Acceptor Cage for Thermally Activated Delayed Fluorescence: toward a New Kind of TADF Exciplex Emitters, ACS Mater. Lett., 2023, 5, 1450–1455 CrossRef CAS.
- Y. Chen, S. Wang, X. Wu, Y. Xu, H. Li, Y. Liu, H. Tong and L. Wang, Triazatruxene-based small molecules with thermally activated delayed fluorescence, aggregation-induced emission and mechanochromic luminescence properties for solution-processable nondoped OLEDs, J. Mater. Chem. C, 2018, 6, 12503–12508 RSC.
- Y. Liu, X. Wu, Y. Chen, L. Chen, H. Li, W. Wang, S. Wang, H. Tian, H. Tong and L. Wang, Triazatruxene-based thermally activated delayed fluorescence small molecules with aggregation-induced emission properties for solution-processable nondoped OLEDs with low efficiency roll-off, J. Mater. Chem. C, 2019, 7, 9719–9725 RSC.
- Y. Liu, Y. Chen, H. Li, S. Wang, X. Wu, H. Tong and L. Wang, High-Performance Solution-Processed Red Thermally Activated Delayed Fluorescence OLEDs Employing Aggregation-Induced Emission-Active Triazatruxene-Based Emitters, ACS Appl. Mater. Interfaces, 2020, 12, 30652–30658 CrossRef CAS PubMed.
- S. K. Pathak, H. Liu, C. Zhou, G. Xie and C. Yang, Triazatruxene based star-shaped thermally activated delayed fluorescence emitters: modulating the performance of solution-processed non-doped OLEDs via side-group engineering, J. Mater. Chem. C, 2021, 9, 7363–7373 RSC.
- N. Aizawa, Y.-J. Pu, Y. Harabuchi, A. Nihonyanagi, R. Ibuka, H. Inuzuka, B. Dhara, Y. Koyama, K. Nakayama, S. Maeda, F. Araoka and D. Miyajima, Delayed fluorescence from inverted singlet and triplet excited states, Nature, 2022, 609, 502–506 CrossRef CAS PubMed.
- H. L. Lee, C. S. Oh, K. H. Lee, J. Y. Lee and W. P. Hong, Lifetime-Extending 3-(4-Phenylbenzo[4,5]thieno[3,2-d]pyrimidin-2-yl)benzonitrile Acceptor for Thermally Activated Delayed Fluorescence Emitters, ACS Appl. Mater. Interfaces, 2021, 13, 2908–2918 CrossRef CAS PubMed.
- J. H. Maeng, R. Braveenth, Y. H. Jung, S. J. Hwang, H. Lee, H. L. Min, J. Y. Kim, C. W. Han and J. H. Kwon, Efficiency enhancement in orange red thermally activated delayed fluorescence OLEDs by using a rigid di-indolocarbazole donor moiety, Dyes Pigm., 2021, 194, 109580 CrossRef CAS.
- Q. Zhang, S. Sun, W. J. Chung, S. J. Yoon, Y. Wang, R. Guo, S. Ye, J. Y. Lee and L. Wang, Highly efficient TADF OLEDs with low efficiency roll-off based on novel acridine–carbazole hybrid donor-substituted pyrimidine derivatives, J. Mater. Chem. C, 2019, 7, 12248–12255 RSC.
- Q. Zhang, S. Sun, W. Liu, P. Leng, X. Lv, Y. Wang, H. Chen, S. Ye, S. Zhuang and L. Wang, Integrating TADF luminogens with AIE characteristics using a novel acridine–carbazole hybrid as donor for high-performance and low efficiency roll-off OLEDs, J. Mater. Chem. C, 2019, 7, 9487–9495 RSC.
- D. Yang, J.-S. Huh and J.-I. Hong, Spiro−type TADF emitters based on acridine donors and anthracenone acceptor, Dyes Pigm., 2022, 197, 109873 CrossRef CAS.
- Y. Fu, H. Liu, B. Z. Tang and Z. Zhao, Realizing efficient blue and deep-blue delayed fluorescence materials with record-beating electroluminescence efficiencies of 43.4%, Nat. Commun., 2023, 14, 2019 CrossRef CAS PubMed.
- H. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta and T. Hatakeyama, One-Step Borylation of 1,3-Diaryloxybenzenes Towards Efficient Materials for Organic Light-Emitting Diodes, Angew. Chem., Int. Ed., 2015, 54, 13581–13585 CrossRef CAS PubMed.
- K. Stavrou, A. Danos, T. Hama, T. Hatakeyama and A. Monkman, Hot Vibrational States in a High-Performance Multiple Resonance Emitter and the Effect of Excimer Quenching on Organic Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2021, 13, 8643–8655 CrossRef CAS PubMed.
- S. Oda, W. Kumano, T. Hama, R. Kawasumi, K. Yoshiura and T. Hatakeyama, Carbazole-Based DABNA Analogues as Highly Efficient Thermally Activated Delayed Fluorescence Materials for Narrowband Organic Light-Emitting Diodes, Angew. Chem., Int. Ed., 2021, 60, 2882–2886 CrossRef CAS PubMed.
- Y. Zhang, D. Zhang, J. Wei, Z. Liu, Y. Lu and L. Duan, Multi-Resonance Induced Thermally Activated Delayed Fluorophores for Narrowband Green OLEDs, Angew. Chem., Int. Ed., 2019, 58, 16912–16917 CrossRef CAS PubMed.
- X.-F. Luo, H.-X. Ni, H.-L. Ma, Z.-Z. Qu, J. Wang, Y.-X. Zheng and J.-L. Zuo, Fused π-Extended Multiple-Resonance Induced Thermally Activated Delayed Fluorescence Materials for High-Efficiency and Narrowband OLEDs with Low Efficiency Roll-Off, Adv. Opt. Mater., 2022, 10, 2102513 CrossRef CAS.
- Y. Zhang, D. Zhang, T. Huang, A. J. Gillett, Y. Liu, D. Hu, L. Cui, Z. Bin, G. Li, J. Wei and L. Duan, Multi-Resonance Deep-Red Emitters with Shallow Potential-Energy Surfaces to Surpass Energy-Gap Law, Angew. Chem., Int. Ed., 2021, 60, 20498–20503 CrossRef CAS PubMed.
- X.-F. Luo, S.-Q. Song, H.-X. Ni, H. Ma, D. Yang, D. Ma, Y.-X. Zheng and J.-L. Zuo, Multiple-Resonance-Induced Thermally Activated Delayed Fluorescence Materials Based on Indolo[3,2,1-jk]carbazole with an Efficient Narrowband Pure-Green Electroluminescence, Angew. Chem., Int. Ed., 2022, 61, e202209984 CAS.
- M. Yang, I. S. Park and T. Yasuda, Full-Color, Narrowband, and High-Efficiency Electroluminescence from Boron and Carbazole Embedded Polycyclic Heteroaromatics, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed.
- Y. Zhang, G. Li, L. Wang, T. Huang, J. Wei, G. Meng, X. Wang, X. Zeng, D. Zhang and L. Duan, Fusion of Multi-Resonance Fragment with Conventional Polycyclic Aromatic Hydrocarbon for Nearly BT.2020 Green Emission, Angew. Chem., Int. Ed., 2022, 61, e202202380 CAS.
- Y.-C. Cheng, X.-C. Fan, F. Huang, X. Xiong, J. Yu, K. Wang, C.-S. Lee and X.-H. Zhang, A Highly Twisted Carbazole-Fused DABNA Derivative as an Orange-Red TADF Emitter for OLEDs with Nearly 40% EQE, Angew. Chem., Int. Ed., 2022, 61, e202212575 CAS.
- Y. Sano, T. Shintani, M. Hayakawa, S. Oda, M. Kondo, T. Matsushita and T. Hatakeyama, One-Shot Construction of BN-Embedded Heptadecacene Framework Exhibiting Ultra-narrowband Green Thermally Activated Delayed Fluorescence, J. Am. Chem. Soc., 2023, 145, 11504–11511 CrossRef CAS PubMed.
- H. Lee, R. Braveenth, J. Do Park, C. Y. Jeon, H. S. Lee and J. H. Kwon, Manipulating Spectral Width and Emission Wavelength towards Highly Efficient Blue Asymmetric Carbazole Fused Multi-Resonance Emitters, ACS Appl. Mater. Interfaces, 2022, 14, 36927–36935 CrossRef CAS PubMed.
- E. Ravindran, H. E. Baek, H. W. Son, J. H. Park, Y.-H. Kim and M. C. Suh, Steric Rooted Multi-Resonant Thermally Activated Delayed Fluorescent Emitters for Pure Blue Organic Light Emitting Diodes with Ultralow Efficiency Roll-Off, Adv. Funct. Mater., 2023, 2213461 CrossRef.
- Y.-T. Lee, C.-Y. Chan, M. Tanaka, M. Mamada, K. Goushi, X. Tang, Y. Tsuchiya, H. Nakanotani and C. Adachi, Tailor-Made Multi-Resonance Terminal Emitters toward Narrowband, High-Efficiency, and Stable Hyperfluorescence Organic Light-Emitting Diodes, Adv. Opt. Mater., 2022, 10, 2200682 CrossRef CAS.
- I. S. Park, H. Min and T. Yasuda, Ultrafast Triplet–Singlet Exciton Interconversion in Narrowband Blue Organoboron Emitters Doped with Heavy Chalcogens, Angew. Chem., Int. Ed., 2022, 61, e202205684 CAS.
- X. Cao, K. Pan, J. Miao, X. Lv, Z. Huang, F. Ni, X. Yin, Y. Wei and C. Yang, Manipulating Exciton Dynamics toward Simultaneous High-Efficiency Narrowband Electroluminescence and Photon Upconversion by a Selenium-Incorporated Multiresonance Delayed Fluorescence Emitter, J. Am. Chem. Soc., 2022, 144, 22976–22984 CrossRef CAS PubMed.
- F. Liu, Z. Cheng, Y. Jiang, L. Gao, H. Liu, H. Liu, Z. Feng, P. Lu and W. Yang, Highly Efficient Asymmetric Multiple Resonance Thermally Activated Delayed Fluorescence Emitter with EQE of 32.8% and Extremely Low Efficiency Roll-Off, Angew. Chem., Int. Ed., 2022, 61, e202116927 CAS.
- X. Xiong, Y.-C. Cheng, K. Wang, J. Yu and X.-H. Zhang, A comparative study of two multi-resonance TADF analogous materials integrating chalcogen atoms of different periods, Mater. Chem. Front., 2023, 7, 929–936 RSC.
- Y. X. Hu, J. Miao, T. Hua, Z. Huang, Y. Qi, Y. Zou, Y. Qiu, H. Xia, H. Liu, X. Cao and C. Yang, Efficient selenium-integrated TADF OLEDs with reduced roll-off, Nat. Photonics, 2022, 16, 803–810 CrossRef CAS.
- D. Hall, K. Stavrou, E. Duda, A. Danos, S. Bagnich, S. Warriner, A. M. Z. Slawin, D. Beljonne, A. Köhler, A. Monkman, Y. Olivier and E. Zysman-Colman, Diindolocarbazole – achieving multiresonant thermally activated delayed fluorescence without the need for acceptor units, Mater. Horiz., 2022, 9, 1068–1080 RSC.
- H. L. Lee, S. O. Jeon, I. Kim, S. C. Kim, J. Lim, J. Kim, S. Park, J. Chwae, W.-J. Son, H. Choi and J. Y. Lee, Multiple-Resonance Extension and Spin-Vibronic-Coupling-Based Narrowband Blue Organic Fluorescence Emitters with Over 30% Quantum Efficiency, Adv. Mater., 2022, 34, 2202464 CrossRef CAS PubMed.
- Q. Peng, W. Yang, N. Li, S. Gong, X. Gao, C. Ye, Y. Zou and C. Yang, Indolocarbazole-based deep-blue multiple-resonance narrowband emitters and efficient organic light-emitting diodes, Chem. Eng. J., 2023, 466, 143423 CrossRef CAS.
- G. Meng, D. Zhang, J. Wei, Y. Zhang, T. Huang, Z. Liu, C. Yin, X. Hong, X. Wang, X. Zeng, D. Yang, D. Ma, G. Li and L. Duan, Highly efficient and stable deep-blue OLEDs based on narrowband emitters featuring an orthogonal spiro-configured indolo[3,2,1-de]acridine structure, Chem. Sci., 2022, 13, 5622–5630 RSC.
- A. Pershin, D. Hall, V. Lemaur, J.-C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Highly emissive excitons with reduced exchange energy in thermally activated delayed fluorescent molecules, Nat. Commun., 2019, 10, 597 CrossRef CAS.
- S. K. Jeon, H. L. Lee, K. S. Yook and J. Y. Lee, Recent Progress of the Lifetime of Organic Light-Emitting Diodes Based on Thermally Activated Delayed Fluorescent Material, Adv. Mater., 2019, 31, 1803524 CrossRef PubMed.
- S. Sudheendran Swayamprabha, D. K. Dubey, Shahnawaz, R. A. K. Yadav, M. R. Nagar, A. Sharma, F.-C. Tung and J.-H. Jou, Approaches for Long Lifetime Organic Light Emitting Diodes, Adv. Sci., 2021, 8, 2002254 CrossRef CAS PubMed.
- C. Li, A. K. Harrison, Y. Liu, Z. Zhao, F. B. Dias, C. Zeng, S. Yan, M. R. Bryce and Z. Ren, TADF dendronized polymer with vibrationally enhanced direct spin-flip between charge-transfer states for efficient non-doped solution-processed OLEDs, Chem. Eng. J., 2022, 435, 134924 CrossRef CAS.
- S. Liu, Y. Tian, L. Yan, S. Wang, L. Zhao, H. Tian, J. Ding and L. Wang, Color Tuning in Thermally Activated Delayed Fluorescence Polymers with Carbazole and Tetramethylphenylene Backbone, Macromolecules, 2023, 56, 876–882 CrossRef CAS.
- Y. Liu, Y. Xie, L. Hua, X. Tong, S. Ying, Z. Ren and S. Yan, Realizing External Quantum Efficiency over 25% with Low Efficiency Roll-Off in Polymer-Based Light-Emitting Diodes Synergistically Utilizing Intramolecular Sensitization and Bipolar Thermally Activated Delayed Fluorescence Monomer, CCS Chem., 2022, 5, 1005–1017 CrossRef.
- P. Khammultri, P. Chasing, C. Chitpakdee, S. Namuangruk, T. Sudyoadsuk and V. Promarak, Red to orange thermally activated delayed fluorescence polymers based on 2-(4-(diphenylamino)-phenyl)-9H-thioxanthen-9-one-10,10-dioxide for efficient solution-processed OLEDs, RSC Adv., 2021, 11, 24794–24806 RSC.
- X.-Y. Zeng, Y.-Q. Tang, X.-Y. Cai, J.-X. Tang and Y.-Q. Li, Solution-processed OLEDs for printing displays, Mater. Chem. Front., 2023, 7, 1166–1196 RSC.
- N. R. Paisley, C. M. Tonge and Z. M. Hudson, Stimuli-Responsive Thermally Activated Delayed Fluorescence in Polymer Nanoparticles and Thin Films: Applications in Chemical Sensing and Imaging, Front. Chem., 2020, 8, 229 CrossRef CAS PubMed.
- 7th International TADF Workshop, 2022, https://www.tadf.jp.
- 13th International Conference on Electroluminescence, 2022, https://icel2022.org.
- O. Graydon, OLEDs are more than just displays, Nat. Photonics, 2023, 17, 216–217 CrossRef CAS.
- W. Ma, Y. Su, Q. Zhang, C. Deng, L. Pasquali, W. Zhu, Y. Tian, P. Ran, Z. Chen, G. Yang, G. Liang, T. Liu, H. Zhu, P. Huang, H. Zhong, K. Wang, S. Peng, J. Xia, H. Liu, X. Liu and Y. M. Yang, Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging, Nat. Mater., 2022, 21, 210–216 CrossRef CAS.
- M. Jian, Z. Song, X. Chen, J. Zhao, B. Xu and Z. Chi, Afterglows from the indolocarbazole families, Chem. Eng. J., 2022, 429, 132346 CrossRef CAS.
| This journal is © the Partner Organisations 2023 |