Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorisation of red beet waste: one-step extraction and separation of betalains and chlorophylls using thermoreversible aqueous biphasic systems†
Marguerita E.
Rosa
a,
Ana M.
Ferreira
 a,
Catarina M. S. S.
Neves
a,
Catarina M. S. S.
Neves
 a,
Mafalda R.
Almeida
a,
Rafael
Barros
bc,
Ana C.
Cristovão
c,
Ana C. A.
Sousa
bd,
Patrícia M.
Reis
a,
Mafalda R.
Almeida
a,
Rafael
Barros
bc,
Ana C.
Cristovão
c,
Ana C. A.
Sousa
bd,
Patrícia M.
Reis
 e,
Luís Paulo N.
Rebelo
e,
Luís Paulo N.
Rebelo
 e,
José M. S. S.
Esperança
e,
José M. S. S.
Esperança
 e,
João A. P.
Coutinho
e,
João A. P.
Coutinho
 a and
Mara G.
Freire
a and
Mara G.
Freire
 *a
*a
aCICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: maragfreire@ua.pt
bNuESA – Health and Environment Study Unit, Faculty of Health Sciences, University of Beira Interior, 6201-506 Covilhã, Portugal
cHealth Sciences Research Centre (CICS-UBI), University of Beira Interior, 6201-506 Covilhã, Portugal
dComprehensive Health Research Centre (CHRC) and Department of Biology, University of Évora, 7006-554 Évora, Portugal
eLAQV-REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa, 2829-516, Caparica, Portugal
First published on 14th February 2023
Abstract
Globally, up to 50% of root crops, fruits and vegetables produced is wasted. Beetroot stems and leaves fit into this scenario, with only a small fraction being used in cattle food. One way of approaching this problem is through their valorisation, by extracting and recovering valuable compounds present in this type of waste that could be used in other applications, while contributing towards a circular economy. In this work, a new integrated process using thermoreversible aqueous biphasic systems (ABS) composed of quaternary ammonium-based ionic liquids (ILs) and polypropyleneglycol 400 g mol−1 (PPG) is shown to allow the one-step extraction and separation of two pigment classes—betalains and chlorophylls—from red beet stems and leaves. The pigment extraction was carried out with a monophasic aqueous solution of the IL and PPG, whose phase separation was then achieved by a temperature switch, resulting in the simultaneous separation of chlorophylls and betalains into opposite phases. A central composite design was used to optimise the extraction parameters (time, temperature, and solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid (S/L) ratio) of both pigment extraction yields, reaching at 20 °C, 70 min and a S/L ratio of 0.12 a maximum extraction yield of 6.67 wt% for betalains and 1.82 wt% for chlorophylls (per weight of biomass). Moreover, it is shown that aqueous solutions of ILs better stabilise betalains than the gold standard solvent used for the extraction method. Among the studied systems, the ABS comprising the IL N-ethyl-N-methyl-N,N-bis(2-hydroxyethyl) bromide ([N21(2OH)(2OH)]Br) presented the best separation performance, with an extraction efficiency of 92% and 95% for chlorophylls and betalains, respectively, for opposite phases. The pigments were removed from the respective phases using affinity resins, with high recoveries: 96% for betalains and 98% for chlorophylls, further allowing the IL reuse. Finally, the cyto- and ecotoxicities of the quaternary ammonium-based ILs were determined. The obtained results disclosed low to negligible toxicity in the thousands of mg L−1 range, with [N21(2OH)(2OH)]Br being harmless from an ecotoxicological point of view. Overall, it is shown here that the developed process is an innovative approach for the one-step extraction and selective separation of pigments contributing to the valorisation of waste biomass.
liquid (S/L) ratio) of both pigment extraction yields, reaching at 20 °C, 70 min and a S/L ratio of 0.12 a maximum extraction yield of 6.67 wt% for betalains and 1.82 wt% for chlorophylls (per weight of biomass). Moreover, it is shown that aqueous solutions of ILs better stabilise betalains than the gold standard solvent used for the extraction method. Among the studied systems, the ABS comprising the IL N-ethyl-N-methyl-N,N-bis(2-hydroxyethyl) bromide ([N21(2OH)(2OH)]Br) presented the best separation performance, with an extraction efficiency of 92% and 95% for chlorophylls and betalains, respectively, for opposite phases. The pigments were removed from the respective phases using affinity resins, with high recoveries: 96% for betalains and 98% for chlorophylls, further allowing the IL reuse. Finally, the cyto- and ecotoxicities of the quaternary ammonium-based ILs were determined. The obtained results disclosed low to negligible toxicity in the thousands of mg L−1 range, with [N21(2OH)(2OH)]Br being harmless from an ecotoxicological point of view. Overall, it is shown here that the developed process is an innovative approach for the one-step extraction and selective separation of pigments contributing to the valorisation of waste biomass.
Introduction
Food waste has an enormous environmental and socioeconomic impact.1 Considering the food produced worldwide for human consumption, circa one-third is either lost or wasted.2 For root crops, fruits and vegetables, between 40 and 50% of the annual worldwide production is lost.3 These food products are amongst the most heavily wasted, including not only their content but also the tubers, peels and aerial parts, usually not used for human consumption.2 One way of approaching this problem is through the valorisation of food waste, by recovering valuable compounds that could be further used in different applications.Food waste contains several bioactive compounds, such as protein, dietary fibres, phenolics and pigments.4 Natural pigments can be used for both food and non-food applications. In food applications, they enhance food appearance through colour intensification, and provide nutritional benefits due to their anti-inflammatory, antiviral and antimicrobial effects.6 Regarding non-food applications, natural pigments can be used in the textile industry.6 Thus, there is high relevance in using natural pigments, especially when recovered from residues, such as food waste, to contribute towards a circular economy.
Conventional pigment extraction methods resort to organic solvents, such as petroleum ether and acetone, for hydrophobic pigments7 and water or aqueous solutions of ethanol, methanol or citric acid for the hydrophilic ones.8 However, the use of organic solvents, in spite of their easy accessibility and affordable price, may increase the environmental footprint of these processes, mainly due to their volatility and associated toxicity.
Ionic liquids (ILs) and their aqueous solutions have emerged as alternative solvents to overcome the drawbacks of conventional organic solvents. They are composed of a large organic cation and an inorganic/organic anion, with a unique set of properties: negligible vapour pressure and high thermal and chemical stability.9 Furthermore, ILs have several advantages over organic solvents; if properly designed, they may be non-toxic and biodegradable.10 They can be produced based on low cost and non-toxic cations, such as cholinium (or other quaternary ammonium-based ILs), thus opening doors for their application in biomolecules' extraction and further preservation.11 Moreover, aqueous solutions of ILs have shown a remarkable performance in the extraction of a plethora of biomolecules, such as alkaloids and flavonoids from complex sources like biomass.12
Biomass is a complex matrix, requiring several unit operations to extract and separate high-value compounds. However, there is a high demand to develop integrated extraction–separation processes, or stimuli-responsive ones, combining multiple unit operations that could make them more economical and sustainable. Temperature-responsive liquid–liquid systems that undergo reversible phase separation by temperature variation are one of the most exciting approaches for developing integrated platforms. Extraction or reaction can occur under homogeneous conditions, with further promotion of a biphasic regime by temperature changes to separate the extracted molecules or the reactants from the products. In this field, reversible aqueous biphasic systems (ABSs), if properly designed, are a sustainable platform for the extraction and separation of biomolecules. ABSs have a high water content and enable easy phase transition depending on the nature of the phase-forming components and their composition, temperature, pressure and pH.13 ABSs are obtained by mixing two different aqueous solutions comprising polymers, salts or ILs that become immiscible under certain conditions of temperature, pressure, pH, or concentration. In particular, huge interest has been devoted to IL-based ABSs due to their designer solvent ability, turning them more versatile for a variety of applications.13 So far, reversible IL-based ABSs composed of protic ILs have only been applied to extract model proteins, with no mixtures of proteins considered.14 Zwitterions have also been applied to develop thermoreversible ABS, yet applied in biocatalysis to separate the enzyme from the target product.15
Considering the need for cost-effective and more sustainable processes to extract and recover high-value bioactive compounds from food waste, we here investigated an integrated approach based on a series of thermoreversible IL-based ABSs to extract and separate betalains and chlorophylls from red beet stems and leaves. The annual worldwide production of beetroot (Beta vulgaris L.) exceeds 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons,16 generating a vast amount of stem and leaf residues. This waste is rich in betalains and chlorophylls. Betalains exhibit antioxidant, anti-lipidemic, antimicrobial, antitumoral, antiviral and anticancer activities.17 It is used as well as a natural colourant (E162) in food items (e.g. canned or bottled fruit, vegetables and breakfast cereals)18 being a relevant alternative to synthetic pigments.5 Chlorophylls display antioxidant properties and are used as a nutritional supplement for blood sugar reduction, detoxification and digestion.19 Due to its fixation properties, it is a promising compound to be used in the textile industry.20
000 tons,16 generating a vast amount of stem and leaf residues. This waste is rich in betalains and chlorophylls. Betalains exhibit antioxidant, anti-lipidemic, antimicrobial, antitumoral, antiviral and anticancer activities.17 It is used as well as a natural colourant (E162) in food items (e.g. canned or bottled fruit, vegetables and breakfast cereals)18 being a relevant alternative to synthetic pigments.5 Chlorophylls display antioxidant properties and are used as a nutritional supplement for blood sugar reduction, detoxification and digestion.19 Due to its fixation properties, it is a promising compound to be used in the textile industry.20
Recent studies have focused on the valorisation of red beet waste, namely leaves and stems,21 peel and pulp22 and roots,23 mainly addressing the recovery of phenolic compounds and betalains. To the best of our knowledge, there are no works reported on the simultaneous extraction of both pigments (betalains and chlorophylls) from red beet stems and leaves, neither their integrated separation, thus highlighting the novelty of this work.
We started by drawing the liquid–liquid ternary phase diagrams at different temperatures (25 °C, 35 °C and 45 °C) of the ABSs formed by quaternary ammonium-based ILs and polypropylene glycol 400 g mol−1 (PPG) to evaluate the possibility of these systems to change from monophasic to biphasic systems (and vice versa) by a proper choice of temperature. Afterwards, screening of these ABSs was carried out to extract the pigments in the monophasic region at 25 °C, with a subsequent increase of the temperature to 35 °C to induce phase separation, and thus selectively separate the pigments extracted. A response surface methodology (RSM) was used to optimise the pigments extraction yield (Y) by changing the operating conditions, namely the solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid (biomass
liquid (biomass![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent) ratio (S/L ratio), the extraction time (t) and the temperature (T). After separation by a temperature change, the pigments had their stability tested for 30 days. Furthermore, pigments recovery from the ABS phases was demonstrated using affinity resins, further allowing IL recovery and reuse. Foreseeing the potential application of the extracts, we further evaluated IL ecotoxicity and cytotoxicity towards human cell lines.
solvent) ratio (S/L ratio), the extraction time (t) and the temperature (T). After separation by a temperature change, the pigments had their stability tested for 30 days. Furthermore, pigments recovery from the ABS phases was demonstrated using affinity resins, further allowing IL recovery and reuse. Foreseeing the potential application of the extracts, we further evaluated IL ecotoxicity and cytotoxicity towards human cell lines.
Results and discussion
Phase diagrams
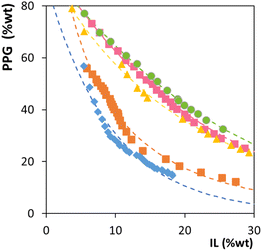
The ternary phase diagrams of water, ILs ([N1(2OH)(2OH)(2OH)]Cl, [N21(2OH)(2OH)]Br, [N1(2OH)(2OH)(2OH)]Br and [N2(2OH)(2OH)(2OH)]Br) and PPG were determined at 25 °C, 35 °C and 45 °C and atmospheric pressure. The definition of the acronyms of the studied ILs is provided as an endnote.‡ Choline chloride ([Ch]Cl or [N111(OH)]Cl) was also investigated as an ABS phase-forming component for comparison purposes. The liquid–liquid phase diagrams at 25 °C are shown in Fig. 1, in an orthogonal representation where the amount of water is not shown (being the amount required to reach 100 wt% in a given mixture point). Fig. 1 allows us to infer the IL's ability to create ABS with PPG. The detailed experimental weight fraction data and the representation of the phase diagrams for the remaining IL-based ABSs at different temperatures (25 °C, 35 °C and 45 °C) are presented in Tables S1–S3 and Fig. S1 and S2,† respectively, in the ESI.† For the studied systems, the experimental binodal data were further fitted, whose parameters are given in Tables S4–S6 in the ESI.† The phase diagrams were further characterised by the determination of several tie-lines (TLs), given in Table S7 in the ESI,† to infer the phase compositions for the given mixture compositions.For all phase diagrams, the compositions of IL and PPG above each binodal curve result in two-phase systems, whereas the mixture compositions below fall within the monophasic region. Phase diagrams with larger biphasic regions indicate that the corresponding IL and PPG combination has a higher ability to phase separate, requiring lower amounts of these phase-forming components to create an ABS. When analysing the binodal curves at a fixed temperature, as shown in Fig. 1, the IL ability to create ABS follows the order: [N111(OH)]Cl > [N1(2OH)(2OH)(2OH)]Cl > [N21(2OH)(2OH)]Br > [N1(2OH)(2OH)(2OH)]Br > [N2(2OH)(2OH)(2OH)]Br. Since PPG is considered a moderately hydrophobic polymer, more hydrophilic salts/ILs are required for easier ABS formation.24 In this sense, ILs composed of anions with lower hydrogen-bond basicity (Br−)—thus with a lower ability to accept protons and interact with water—are less capable of forming ABS, requiring higher amounts of phase-forming components for phase separation. An example of this effect is seen when comparing [N1(2OH)(2OH)(2OH)]Cl and [N1(2OH)(2OH)(2OH)]Br, in which the IL comprising the chloride anion is more able to induce the phase separation of PPG aqueous solutions. Furthermore, when comparing the effect of the cation alkyl side chain length, with the ILs [N1(2OH)(2OH)(2OH)]Br and [N2(2OH)(2OH)(2OH)]Br, an increase in the IL alkyl side chain length or hydrophobicity induces a decrease in the phase separation ability. However, the introduction of hydroxyl groups to the IL cation does not improve the phase separation ability, meaning that in addition to the IL ion hydration capacity, there are also some specific interactions occurring between the IL and PPG ruling the phase behaviour. This complexity of interactions has been previously shown in other polymer-IL-based ABS.24–27 In summary, the IL trend demonstrates that the anion hydrogen bond basicity strongly influences the ABS formation, followed by the influence of the cation aliphatic moieties.
After the previous assessment of the IL-PPG potential to form ABS at a common temperature (25 °C), these were appraised at 35 °C and 45 °C. At all analysed temperatures, the ABS formation trend is the same as that observed at 25 °C (Fig. S1 in the ESI†). These temperatures were chosen considering the final aim of extracting and separating pigments, namely betalains and chlorophylls. These temperatures are below their degradation temperatures. Nevertheless, it is important to mention that betalains are more thermosensitive than chlorophylls as they start to degrade at lower temperatures (>50 °C),28 while chlorophylls only degrade at temperatures >60 °C.29
Fig. 2 shows the ternary liquid–liquid phase diagrams at 25 °C, 35 °C and 45 °C for [N1(2OH)(2OH)(2OH)]Cl. Details of the experimental data and remaining ILs at different temperatures are provided in the ESI (Fig. S2†). IL-polymer ABS can present either upper critical solution temperature (UCST)-like or lower critical solution temperature (LCST)-like behaviours, depending on the IL-polymer pair.27 In this case, all phase diagrams for a given IL display an increase in the biphasic region with the increase in temperature. Therefore, the studied ABS follows an LCST-type behaviour, being a direct consequence of the PPG–water binary system LCST-type behaviour.30 Since PPG—a temperature-dependent polymer—increases its hydrophobic character with temperature, it is more readily displaced from the IL into a second, increasingly polymer-enriched phase.
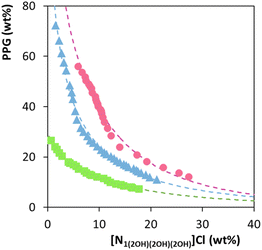 | ||
Fig. 2 Temperature effect on the phase diagrams of ternary systems composed of [N1(2OH)(2OH)(2OH)]Cl + PPG + H2O at 25 °C (pink ), 35 °C (blue ), 35 °C (blue ) and 45 °C (green ) and 45 °C (green  ). ). | ||
Pigment separation optimisation
The extraction of pigments from red beet stems and leaves was performed at 20 °C using a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in 2 h in the IL-based ABS monophasic regions. After the extraction, to move the system into the biphasic region, the temperature was increased to 35 °C. This allowed the two-phase system formation and the selective separation (partition for opposite phases) of chlorophylls and betalains. Moreover, since betalains are pH sensitive, the extraction of the pigments using IL-based ABS with pH adjustment was tested. According to the literature, betalains are stable between pH 3.5 and 7.0, with the optimum pH being around 6.0.31–34 Therefore, the pH of ABS was adjusted to this pH value. Fig. 3 and 4 depict the results regarding the selective separation of pigments from red beet stems and leaves using IL-based ABS with and without pH adjustment. In all the investigated systems, the chlorophylls partitioned predominantly to the PPG-rich phase (top phase), due to their high hydrophobicity; betalains, which are more hydrophilic, partitioned mainly to the IL-rich phase (bottom phase). This selective separation is explained by the log
10 in 2 h in the IL-based ABS monophasic regions. After the extraction, to move the system into the biphasic region, the temperature was increased to 35 °C. This allowed the two-phase system formation and the selective separation (partition for opposite phases) of chlorophylls and betalains. Moreover, since betalains are pH sensitive, the extraction of the pigments using IL-based ABS with pH adjustment was tested. According to the literature, betalains are stable between pH 3.5 and 7.0, with the optimum pH being around 6.0.31–34 Therefore, the pH of ABS was adjusted to this pH value. Fig. 3 and 4 depict the results regarding the selective separation of pigments from red beet stems and leaves using IL-based ABS with and without pH adjustment. In all the investigated systems, the chlorophylls partitioned predominantly to the PPG-rich phase (top phase), due to their high hydrophobicity; betalains, which are more hydrophilic, partitioned mainly to the IL-rich phase (bottom phase). This selective separation is explained by the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (logarithm of the octanol–water partition coefficient, a measure of hydrophobicity) values of the pigments, which provide indications on whether a substance has an affinity towards water (hydrophilic) or lipids (hydrophobic). Betanin (i.e. the type of betalain that is present in the highest amount in reed beets)28 has a log
Kow (logarithm of the octanol–water partition coefficient, a measure of hydrophobicity) values of the pigments, which provide indications on whether a substance has an affinity towards water (hydrophilic) or lipids (hydrophobic). Betanin (i.e. the type of betalain that is present in the highest amount in reed beets)28 has a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of −4.49, meaning a higher affinity for aqueous phases, and chlorophyll a and chlorophyll b have a log
Kow of −4.49, meaning a higher affinity for aqueous phases, and chlorophyll a and chlorophyll b have a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow of 14.73 and 13.93, respectively, indicating their higher hydrophobicity.35
Kow of 14.73 and 13.93, respectively, indicating their higher hydrophobicity.35
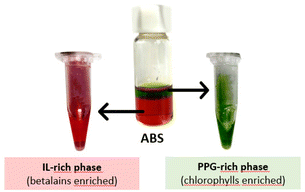 | ||
| Fig. 3 Representation of the phase separation after the extraction of the pigments from the beetroot biomass: the PPG-rich phase rich in chlorophylls and the IL-rich phase rich in betalains. | ||
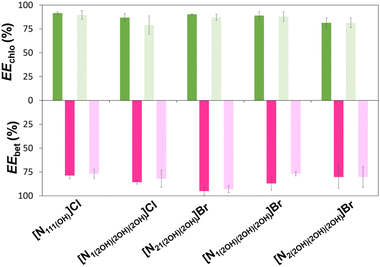
The extraction efficiencies of chlorophylls (EEchlo (%)) and betalains (EEbet (%)) to the PPG-rich and IL-rich phase, respectively, are provided in Fig. 4. The extraction efficiencies are defined as the percentage ratio of the total weight of pigment (betalains or chlorophylls) in one of the phases to that in the total mixture (detailed information is given in the Experimental section). The remarkable extraction efficiencies of chlorophylls to the PPG-rich phase, ranging between 79% and 93%, and of betalains to the IL-rich phase, ranging between 77% and 95%, were obtained in a single step (detailed data are provided in Table S8 in the ESI†). Despite the structural differences of the ILs used, and differences in their phase diagrams, no significant differences were observed for EEchlo (%) among all the studied ABS. Chlorophylls partition to the most hydrophobic phase (PPG-rich phase), where a reduced amount of ILs is present (between 0.82 wt% and 2.63 wt% according to the TLs—Table S7 in the ESI†). Therefore, no significant effect was observed on the chlorophyll separation when changing the IL. In addition, for EEbet (%), no significant differences were observed for the systems composed of [N1(2OH)(2OH)(2OH)]Cl, [N1(2OH)(2OH)(2OH)]Br, [N2(2OH)(2OH)(2OH)]Br and [N111(OH)]Cl. However, [N21(2OH)(2OH)]Br performed slightly better for betalains than the other systems tested.
Regarding the effect of ABS pH adjustment, the results obtained show that the pH adjustment is negligible over the EE (%) values. It should be noticed that the pH of the ABS without pH adjustment ranged between 3.5 (for the system composed of [N1(2OH)(2OH)(2OH)]Br) and 9.0 (for the system composed of [N21(2OH)(2OH)]Br). The absence of the pH effect on the extraction was also reported by other researchers,36 corroborating our results. However, if we consider the pigment stability with time, pH adjustment is important for betalains as, in the samples where the pH was not adjusted, the initial pink colour evolved to a yellow/orange colour (Fig. S3 in the ESI†) after 7 days, even when kept in the dark at 4 °C. The colour change was not observed in the samples where the pH was adjusted. This colour change is related to the degradation of betalains in the extract, likely due to the decarboxylation of betacyanin and the formation of degradation products.37 Hence, pH is a crucial factor for betalain stability as reported in the literature.32–34
Amongst the ABSs investigated, the one that allowed a higher selective separation of both pigments—higher EE (%) for opposite phases—was composed of [N21(2OH)(2OH)]Br. Therefore, further extractions were carried out using the ABS constituting [N21(2OH)(2OH)]Br with pH adjustment, in which the operating conditions for the extraction from biomass were optimised.
Extraction condition optimisation
A response surface methodology (RSM) was used to optimise the operating conditions to maximise the pigment extraction yields. RSM allowed the exploration of the relationship between the response (pigment extraction yield: Ybet (%) for the betalains and Ychlo (%) for the chlorophylls) and the independent variables that affect that value, in this case, the solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio (S/L ratio), temperature (T) and extraction time (t), using the ABS composed of [N21(2OH)(2OH)]Br with pH adjustment to 6. The extraction yield is defined as the percentage ratio of the weight of the pigment extracted (g) per biomass weight (g). The solid
liquid ratio (S/L ratio), temperature (T) and extraction time (t), using the ABS composed of [N21(2OH)(2OH)]Br with pH adjustment to 6. The extraction yield is defined as the percentage ratio of the weight of the pigment extracted (g) per biomass weight (g). The solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio was varied from 1
liquid ratio was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 to 1
25 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
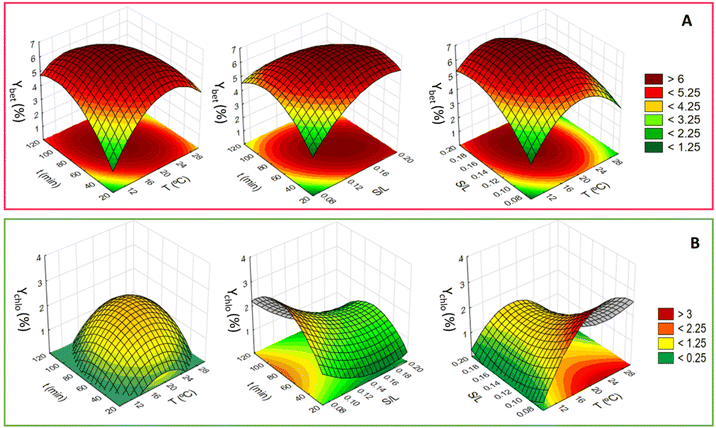
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the temperature from 10 to 30 °C, and the extraction time from 19 to 120 min (Tables S9 and S10 in the ESI†). The statistical analysis is shown in the ESI (Tables S11–S16†), and the respective data are depicted in Fig. 5. Variance analysis (ANOVA) was used to estimate the statistical significance of variables and the interactions between them. The obtained results were statistically analysed with a confidence level of 95%.
5, the temperature from 10 to 30 °C, and the extraction time from 19 to 120 min (Tables S9 and S10 in the ESI†). The statistical analysis is shown in the ESI (Tables S11–S16†), and the respective data are depicted in Fig. 5. Variance analysis (ANOVA) was used to estimate the statistical significance of variables and the interactions between them. The obtained results were statistically analysed with a confidence level of 95%.
When considering the extraction of betalains, the three variables studied are significant (solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio, extraction time and temperature), with the following variables being statistically significant: S/L, T2, S/L2 and t2, according to the Pareto chart provided in Fig. S4 in the ESI.† Nevertheless, the significant variables present a similar weight in the response, with all the quadratic ones presenting a negative effect, meaning that the maximum of betalain yield extraction is achieved at a moderate solid
liquid ratio, extraction time and temperature), with the following variables being statistically significant: S/L, T2, S/L2 and t2, according to the Pareto chart provided in Fig. S4 in the ESI.† Nevertheless, the significant variables present a similar weight in the response, with all the quadratic ones presenting a negative effect, meaning that the maximum of betalain yield extraction is achieved at a moderate solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio, extraction time and temperature.
liquid ratio, extraction time and temperature.
The second response that was evaluated was the yield of the extraction of chlorophylls. The statistically significant variables were S/L, T2, t2 and S/L2; here also the three variables in study were significant for the extraction. Moreover, from these parameters, S/L was the most significant parameter and had a negative impact (Fig. S5 in the ESI†). When analysing the surface plots obtained (Fig. 5), a shift in the optimum value is noticeable toward the lower values of solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio. Thus, higher values of solid
liquid ratio. Thus, higher values of solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio have a negative impact on chlorophyll extraction. On the other hand, the amount of extracted chlorophylls increases with time and temperature, reaching the maximum at 80 min. The solid
liquid ratio have a negative impact on chlorophyll extraction. On the other hand, the amount of extracted chlorophylls increases with time and temperature, reaching the maximum at 80 min. The solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio is also relevant, although with a behaviour that depends on other variables.
liquid ratio is also relevant, although with a behaviour that depends on other variables.
Forasmuch as the two pigment yields were under analysis, a compromise between the two values was achieved and the optimal conditions found were the following: an extraction temperature of 20 °C for 70 min using a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 0.12 (Fig. S6 in the ESI†). These extraction conditions gave an average value of (6.7 ± 0.5) wt% and (1.8 ± 0.5) wt% for Ybet (%) and Ychlo (%), respectively. Comparing the observed values with the predicted values according to multiple regression achieved with the RSM analysis, the values were 6.7 wt% and 1.8 wt%. Thus, the regression values obtained perfectly predict the experimental results obtained.
liquid ratio of 0.12 (Fig. S6 in the ESI†). These extraction conditions gave an average value of (6.7 ± 0.5) wt% and (1.8 ± 0.5) wt% for Ybet (%) and Ychlo (%), respectively. Comparing the observed values with the predicted values according to multiple regression achieved with the RSM analysis, the values were 6.7 wt% and 1.8 wt%. Thus, the regression values obtained perfectly predict the experimental results obtained.
To better understand the performance of the developed process, we compared our results with the available literature. Few studies used the same biomass source, and none was found with the goal of simultaneously extracting the two types of pigments. Koubaier et al.23 homogenised 50 g of red beet stems or roots with distilled water (250 mL) and macerated the materials for 3 days at room temperature. Extracts with a betanin concentration of (53 ± 4) mg g−1 and (11.0 ± 0.5) mg g−1 for roots and stems, respectively, were obtained. Moreover, Hernández-Aguirre et al.22 used different deep eutectic solvents at various pHs to extract betalains from red beet peels and pulp, obtaining a maximum yield of (4.0 ± 0.3) mg betalains per g of biomass (≈yield of 0.4%). Thus, from the gathered information, the betalains yield achieved in this work is higher than all the previously reported yields obtained using different approaches and, in some cases, from different biomass sources. Concerning the chlorophyll yields from red beet waste, to the best of our knowledge, no previous studies have been performed and hence, no comparison could be reported. Additionally, there is only one study that used the same biomass with pressurised liquid extraction to recover phenolic compounds.21
In summary, our work presents several novel aspects, not only due to the biomass source, which is one of the least studied (red beets' leaves and stems), but also due to the simultaneous extraction of both classes of pigments and their separation in one-step using thermoreversible ABS.
Pigment separation and recovery
Under the optimised conditions of extraction, the change in temperature from 20 °C to 35 °C allowed to simultaneously separate betalains and chlorophylls. The extraction efficiencies were 95% (betalains) and 92% (chlorophylls) for the IL-rich and PPG-rich phases, respectively. Fig. 3 shows the visual aspect of the integrated ABS after extraction and phase separation.Pigment recovery from the IL-rich phase and PPG-rich phase is a crucial task aimed at establishing the “real” utility of these systems as an integrated platform for extraction–separation. In this context, we further evaluated the possibility of separating the pigments (betalains and chlorophylls) from the IL- ([N21(2OH)(2OH)]Br) and PPG-rich phases. To this end, two affinity resins (solid-phase extraction approach) were used after the extraction and the selective separation of the pigments. The overall process is depicted in Fig. 6.
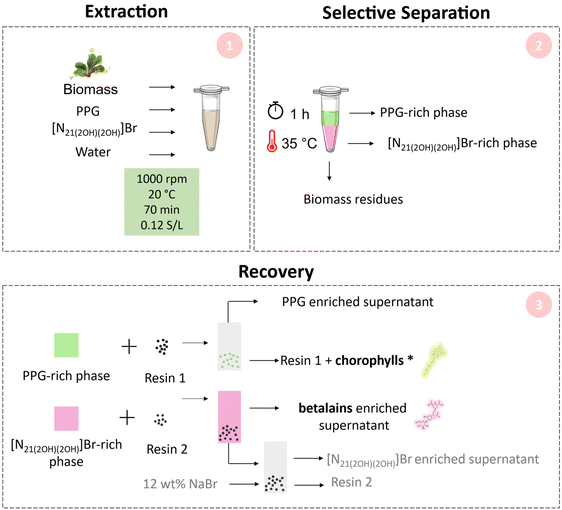 | ||
| Fig. 6 Schematic representation of the final process proposed in this work, which is composed of three main steps: Pigment extraction from the biomass (extraction) (1), pigment separation through ABS formation (selective separation) (2), and pigment recovery using resins (recovery) (3). * Recovery of the chlorophylls from resin 1 can be performed as described by Vaz et al.38 | ||
For betalains at the optimised conditions, we successfully removed 97% of the IL ([N21(2OH)(2OH)]Br) from the IL-rich phase of the ABS (betalains-rich). Most of the cation ([N21(2OH)(2OH)]+) was retained within the cation exchange resin, allowing a satisfactory cleaning-up step (Fig. S7 in the ESI†). Moreover, the IL cation previously retained was eluted using a 12 wt% NaBr aqueous solution, reaching a removal of 94%. In addition, after the recovery of the IL, we measured the concentration of betalains in the supernatant solution (removed after 30 min of contact), verifying that we have successfully recovered 96% of betalains. This technique is versatile as it allows a cleaning up of the IL from the betalain aqueous solution and its recovery, allowing it to be further used in new extractions and in the development of cost-effective and more sustainable technologies. Although the IL's reusability in a new ABS using fresh biomass was not carried out, different works by our group have already demonstrated this approach.39–41
The chlorophylls recovery was carried out by their adsorption within the resin Ambersep® 900 OH, with PPG being kept in the acetone solution. We successfully recovered 97% and 98% of chlorophylls a and b, respectively (Fig. S8 in the ESI†). Due to its low boiling temperature, acetone could be easily removed from PPG, which could be further used in new ABS-based extraction. In addition, it should be taken into account that in a previous work developed by Vaz et al.38 it was demonstrated that chlorophylls that remain trapped inside the Ambersep® 900 OH resin can be recovered through an approach based on the use of aqueous solutions of surface-active ILs, namely dodecyltrimethylammonium bromide ([N1,1,1,12]Br). Moreover, the authors showed that the resin could be regenerated with a solution of NaOH (4 wt%), demonstrating the possibility of having a pure ethanol solution of chlorophylls (without IL), while retaining the Ambersep® 900 OH resin integrity.
In summary, these results showed that both techniques applied for the two pigments allowed a satisfying polishing step, with 97% of IL removal from the betalains-rich phase and 94% of IL recovery from the resin. Despite the possible (negligible) contamination of the extracts with the IL, the IL toxicity is negligible as it will be shown below. As for chlorophylls, a 98% removal of these pigments from the PPG-rich phase was obtained. A specific resin was used to recover the chlorophylls, guaranteeing their high degree of purity, as confirmed by Vaz et al.38 Moreover, the biomass studied has a low protein content and is poor in lipids.42–44 Since aqueous solutions were used for the extraction, the probability of extracting lipids is indeed low.
Overall, it was shown that the extraction of chlorophylls and betalains can be carried out with an ABS formed by [N21(2OH)(2OH)]Br and PPG in the monophasic region, followed by phase separation by changing the temperature from 20 °C to 35 °C, thereby allowing the simultaneous separation of both pigments. These can be easily recovered using affinity resins.
Stability of the extracts
Pigments are sensitive to oxidation during storage, which affects their colour stability due to structural changes. Colour stability is a very important factor when using pigments as natural colourants. To better understand the advantage of the ABS used in the stability of pigments, a comparison was performed using two solvents: ethanol and water. These solvents were chosen because the literature suggested these as the gold standard solvents for extracting chlorophylls and betalains.38,45 They were applied to extract the pigments from red beet biomass under the same conditions as the ABS extraction (20 °C, for 70 min, using a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
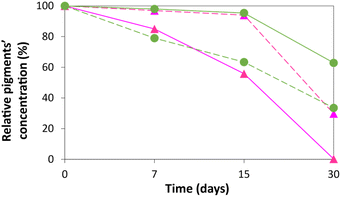
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 0.12). The calculated pigment relative concentrations (%) for each extractant during the 30 days of analysis are presented in Fig. 7 (detailed data are provided in Table S17 in the ESI†).
liquid ratio of 0.12). The calculated pigment relative concentrations (%) for each extractant during the 30 days of analysis are presented in Fig. 7 (detailed data are provided in Table S17 in the ESI†).
Our results show that the solvent that better preserved betalains is the IL-rich phase, keeping about 90% and 40% of the initial concentration after 15 and 30 days, respectively. In contrast, the water solvent led to a loss of concentration of 40% after 15 days and a total loss of betalains after 30 days. The IL-rich phase appears to retard the degradation of betalains in the extract, i.e. the change from red to yellow/orange colour of the extract. Thus, there is a positive impact of the IL on betalains stability. When looking at the chlorophylls, the impact is however different; the extraction using pure ethanol allowed to keep about 60% of the initial concentration after 30 days. However, the PPG-rich phase is able to retain the stability of chlorophylls for up to 15 days with negligible losses of stability, reinforcing their potential use in industry as well. Overall, these are good results as betalains are more prone to degradation than chlorophylls.31 This set of results opens the perspective of using the IL- or PPG-rich phases, after extraction and separation by a temperature change, as the preservation media of betalains and chlorophylls up to their recovery, further integrating one more step in the development of an integrated extraction–separation process.
IL toxicity
Considering the potential applications of the recovered pigments, it is crucial to evaluate the eco- and cytotoxicities of the used ILs. The ecotoxicity of the used ILs was investigated using Vibrio fischeri bioluminescent bacteria. Experimental EC50 values were determined using Microtox® bioassays after 5, 15 and 30 min exposure to IL. The respective results are provided in Fig. 8 (see Table S18 in the ESI† for detailed data). According to the Passino classification,46 the results show that all the studied quaternary ammonium-based ILs are harmless (EC50 > 1000 mg L−1, i.e., EC50 approximately higher than 0.4 mol L−1). Regarding [N21(2OH)(2OH)]Br, it is not toxic to the bacteria as it was not possible to calculate the EC50 value at a concentration of 60 mg L−1, thus highlighting the development of a greener process for the extraction and selective separation of pigments from food waste. Furthermore, these results also suggest that the cations studied play an important role in determining the toxicity of quaternary ammonium-based ILs. In addition, the quaternary ammonium-based ILs investigated are less toxic than [N111(OH)]Cl to the bacteria.47 Therefore, the investigated ILs have a lower environmental impact and potentially lower toxicity than the widely used and claimed non-toxic [N111(OH)]Cl.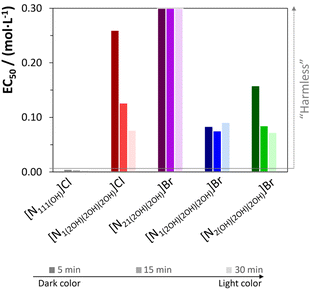 | ||
| Fig. 8 Average EC50 values obtained after 5, 15, and 30 min of exposure of the marine bacteria Vibrio fischeri to the different ILs. Values for [N111(OH)]Cl were obtained from Ventura et al.47 Line represents the upper limit for each IL, taking into account the Passino classification.46 | ||
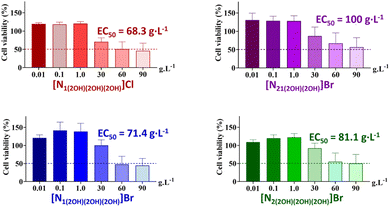
The cytotoxicity of the ILs was evaluated using the human colon carcinoma cell line (Caco-2). This cell line is widely used in the screening of the potential toxicity of new drugs, bioactive compounds, and formulations for human consumption. These cells are a good model of the human intestinal epithelial barrier by which nutrients and toxic compounds pass through.48,49Fig. 9 depicts the cytotoxic profiles of the synthesized ILs towards Caco-2 cells. The dose–response curves of each synthesised ILs are shown in Fig. S9 in the ESI.†
A reduction in cell viability was only observed when the cells were exposed to ILs at very high concentrations (from 30 g L−1 onwards). Such results clearly demonstrate the biocompatible nature of these ILs as the cells are unlikely to be exposed to concentrations higher than 1 g L−1. Overall, the cytotoxicity profile demonstrates that [N21(2OH)(2OH)]Br is the least toxic quaternary ammonium-based IL (EC50 > 100 g L−1) followed by [N2(2OH)(2OH)(2OH)]Br and [N1(2OH)(2OH)(2OH)]Br. The highest toxicity was observed for [N1(2OH)(2OH)(2OH)]Cl, which suggests that the IL anion plays an important role in the cytotoxicity of these ILs. Nevertheless, even for the most toxic IL, the EC50 values are extremely high, suggesting its low cytotoxic potential at concentrations in the thousands of mg L−1 order.
Experimental
Materials
Red beet stems and leaves(Beta vulgaris L.) samples were obtained from a local farmer (Aveiro, Portugal) and were stored at −20 °C. Before extraction, the red beet stems and leaves were frozen in liquid nitrogen, crushed with a mortar and pestle, and then ground using a coffee grinder (Qilive coffee grinder Q.5321) (Schematic representation of the biomass preparation process is shown in Fig. S10 in the ESI†). The PTFE 0.45 μm (13 mm) syringe filters used were acquired from ChromTech.The following reagents were used: polypropylene glycol with an average molecular weight of 400 g mol−1 (PPG), chlorophyll a (95% purity) and chlorophyll b (99% purity) standards, all acquired from Sigma Aldrich; acetonitrile and ethanol were purchased from Fisher Chemical (99.99% purity); ethyl acetate, methanol and acetone were acquired from Fisher Scientific (analytical grade); choline chloride ([N111(OH)]Cl) (98% purity) was from Acros Organics and betanin (red beet extract diluted with dextrin) standard was from TCI. Sodium bromide (NaBr) was acquired from BDH Chemicals (99.99% purity), hydrochloric acid (HCl) (37%) was from Honeywell and sodium hydroxide (NaOH) pellets (98.0% purity) were from Fisher. The resins AmberLite® HPR 1100 and Ambersep® 900 OH were both acquired from Sigma Aldrich. The water used in this work was ultrapure, distilled twice, passed through a reverse osmosis system and treated with a Milli-Q Integral 10 (Merck, Darmstadt, Germany) water purification device.
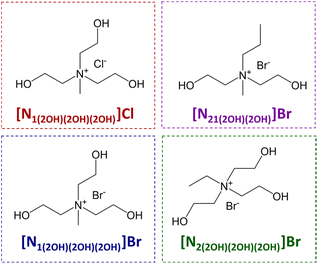
The ILs ([N1(2OH)(2OH)(2OH)]Cl, [N21(2OH)(2OH)]Br, [N1(2OH)(2OH)(2OH)]Br, and [N2(2OH)(2OH)(2OH)]Br) used in this work are shown in Fig. 10. They were synthesised according to the procedure described by de Ferro et al.50 Characterisation analysis by NMR and Elemental Analysis indicate an overall purity higher than 99 wt%. Each IL was dried under vacuum before any experiment.
The chemicals for ecotoxicity assays were acquired from Ambifirst. The human colon carcinoma cell line (Caco-2) used was obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). This commercial cell line is commonly used in toxicological studies and the cells were originally isolated from the colon tissue of a patient with colorectal adenocarcinoma.
Phase diagrams and tie-line determination
The phase diagrams for ternary mixtures composed of water, PPG and ILs were determined at 25 °C, 35 °C and 45 °C at atmospheric pressure. For this determination, the cloud point titration method51 was used, with a dropwise addition of the aqueous IL solution (50–80 wt%) to the aqueous solution of PPG (70–80 wt%). This addition was carried out under constant stirring until the detection of a cloudy solution (biphasic regime), followed by the dropwise addition of distilled water until reaching a clear solution (monophasic regime). Syringes were used to add the IL solutions and water to the PPG solution flask, which was kept at a fixed temperature in a water bath (Julabo ME-18V). The ternary system compositions were determined by weight quantification within ±10−3 g. Each binodal curve was then fit to the Merchuk52 equation. Tie-lines (TLs), which give the composition of each phase for a given mixture composition, were gravimetrically determined at 35 °C according to the method also reported by Merchuk et al.52 Further details regarding the phase diagrams and TL determination are given in the ESI (eqn (S1)–(S6)†).Pigment extraction
The ternary mixture compositions used in the extractions were chosen based on the phase diagrams previously determined: 10 wt% IL + 52 wt% PPG + 38 wt% water. Only for the system composed of [N1(2OH)(2OH)(2OH)]Cl a different mixture point was used: 5 wt% IL + 52 wt% PPG + 43 wt% water, due to the proximity between the mixture point and the binodal curve at 35 °C. The extraction was initially performed using a biomass solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 0.1 in each tube, containing the phase forming components in the previously defined wt%, using a water bath (Julabo ME-18V) at the desired temperature for a given time. During the extraction water-resistant stirrers (Thermo Scientific™ Cimarec™ i Micro Stirrers) at 1000 rpm were used for maximum contact between the biomass and the solution. The extraction tubes were covered in aluminium foil to avoid degradation of the pigments (Fig. S11 in the ESI†). Following the extraction, the tubes were centrifuged at 5000 rpm for 15 min (Thermo Fisher Scientific MEGAFUGE 16R) at 4 °C. Subsequently, the pellet (biomass) was discarded, and the supernatant was filtered using a PTFE syringe filter of 0.45 μm. The filtrate was collected in a new tube and then placed in a water bath at 35 °C to perform the desired phase separation. After 60 min, the phases were separated with a syringe and analysed by HPLC-DAD as described below. In the studied ABS, the top phase corresponds to PPG and water, while the bottom phase is mainly composed of the IL-rich aqueous phase.
liquid ratio of 0.1 in each tube, containing the phase forming components in the previously defined wt%, using a water bath (Julabo ME-18V) at the desired temperature for a given time. During the extraction water-resistant stirrers (Thermo Scientific™ Cimarec™ i Micro Stirrers) at 1000 rpm were used for maximum contact between the biomass and the solution. The extraction tubes were covered in aluminium foil to avoid degradation of the pigments (Fig. S11 in the ESI†). Following the extraction, the tubes were centrifuged at 5000 rpm for 15 min (Thermo Fisher Scientific MEGAFUGE 16R) at 4 °C. Subsequently, the pellet (biomass) was discarded, and the supernatant was filtered using a PTFE syringe filter of 0.45 μm. The filtrate was collected in a new tube and then placed in a water bath at 35 °C to perform the desired phase separation. After 60 min, the phases were separated with a syringe and analysed by HPLC-DAD as described below. In the studied ABS, the top phase corresponds to PPG and water, while the bottom phase is mainly composed of the IL-rich aqueous phase.
Pigment quantification
The quantification of the pigments, betalains and chlorophylls, was performed by HPLC-DAD (high performance liquid chromatography–diode array detector) (Shimadzu. model PROMINENCE), using an analytical C18 reversed-phase column (250 × 4.60 mm) Kinetex 5 μm C18 100 Å from Phenomenex. The mobile phase used was a gradient system of ultra-pure water (phase A), methanol (phase B) and ethyl acetate (phase C), previously degassed by ultrasonication. The separation was conducted in the following gradient mode: 0 min 10% (v/v) of A, 90% (v/v) of B, 0% (v/v) of C, 20 min 5% (v/v) of A, 45% (v/v) of B and 50% (v/v) of C and after 5 min returned to initial conditions for 20 min to ensure column stabilisation. The flow rate used was 1 mL min−1, with an injection volume of 20 μL. The DAD was set at 538 nm, 649 nm and 663 nm for betalains, chlorophyll a and chlorophyll b, respectively. The column oven was operated at a controlled temperature of 25 °C. The calibration curves were prepared using the commercial standards dissolved in organic solvents: ethanol for chlorophylls a and b and methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ultra-pure water 90
ultra-pure water 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) for betalains. Considering the retention time in the previous run conditions, betalains and chlorophylls a and b registered the following values: 2, 19 and 22 min. To better compare the results and evaluate the IL-based ABS efficiency, the following parameters were calculated: extraction efficiencies (EE)—(EEbet for betalains and EEchlo for chlorophylls)—and yields (Y)—Ybet for betalains and Ychlo for chlorophylls. The equations defining EE (%) and Y (%) are shown in the ESI (eqn (S7)–(S10)†).
10 (v/v) for betalains. Considering the retention time in the previous run conditions, betalains and chlorophylls a and b registered the following values: 2, 19 and 22 min. To better compare the results and evaluate the IL-based ABS efficiency, the following parameters were calculated: extraction efficiencies (EE)—(EEbet for betalains and EEchlo for chlorophylls)—and yields (Y)—Ybet for betalains and Ychlo for chlorophylls. The equations defining EE (%) and Y (%) are shown in the ESI (eqn (S7)–(S10)†).
Optimisation of the operational conditions by a response surface methodology (RSM)
A RSM with a central composite design (CCD) was used. A central point (zero level) was established together with the factorial points (1 and −1, level one) and the axial points defined by α (level α). To this end, α was calculated considering eqn (S11) in the ESI.† In a 2k RSM, there are k factors that contribute to a different response, and the data are treated according to a second-order polynomial equation in the ESI (eqn (S12)†), where k is the number of parameters being analysed. In this case, k = 3 since the analysed variables were the solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio (S/L), the temperature (T), and the time of extraction (t). RSM was applied to identify the most significant parameters that enhance the pigment extraction yields. The results were analysed with a confidence level of 95%, using Student's t-test to check the statistical significance of the adjusted data. The adequacy of the model was determined by evaluating the lack of fit, the regression coefficient (R), and the F-value obtained from the analysis of variance (ANOVA). The Statsoft Statistica 10.0© software was used for all statistical analyses and for representing the response surfaces and contour plots.
liquid ratio (S/L), the temperature (T), and the time of extraction (t). RSM was applied to identify the most significant parameters that enhance the pigment extraction yields. The results were analysed with a confidence level of 95%, using Student's t-test to check the statistical significance of the adjusted data. The adequacy of the model was determined by evaluating the lack of fit, the regression coefficient (R), and the F-value obtained from the analysis of variance (ANOVA). The Statsoft Statistica 10.0© software was used for all statistical analyses and for representing the response surfaces and contour plots.
Stability of the extracts
To determine the stability of the pigments in the ABS phases—betalains in the IL-rich phase and chlorophylls in the PPG-rich phase—both solutions were stored at 4 °C and protected from light for 30 days, and the concentration of the pigments was analysed through HPLC on days 0, 7, 15 and 30. This preservation temperature was chosen according to other studies that showed that temperatures below 10 °C were required to preserve betalains from degradation.45 Moreover, aiming to compare these results with the gold standard extraction methods for both pigments, the same extraction conditions optimised through CCD were applied to the biomass using water with the pH adjusted to 6 as a solvent for betalains and ethanol for chlorophylls, and the pigment stability was also followed for 30 days.Pigments and IL recovery
After the phase separation, the extracted pigments must be recovered from the ABS phases. For the betalains, since they partition to the IL-rich phase, AmberLite® HPR 1100 was used to retain the cation of the IL used in the extraction. For that purpose, 2.5 g of the resin was washed three times with 3 mL of distilled water, after which a diluted sample of the IL-rich phase of the ABS (3 mL) was added and left in contact with the resin for 30 min, under agitation. After that, the supernatant was collected and further analysed. The resin was then washed three times with 3 mL of distilled water to ensure no pigment was still within the resin, and eluted three times with 3 mL of a 12 wt% NaBr solution to remove the cations adsorbed in the resin. To understand the exact recovery percentage, all collected samples—elution and washing steps—were analysed through 1H NMR and the following equations were applied: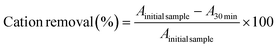 | (1) |
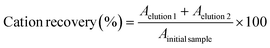 | (2) |
For chlorophylls recovery, Ambersep® 900 OH resin was used. A mass of 1.0 g of this resin was washed with distilled water, filtered, and dried in the oven (at 40 °C for circa 30 min). This step was followed by the addition of an extract of the top phase of the ABS: 0.5 mL of the real sample diluted with 9.5 mL of acetone. This extract was in contact with the resin for 60 min under agitation in the dark. The mass of chlorophylls of each sample was determined before and after the elution steps at 649 nm and 663 nm using the same HPLC procedure previously described. The chlorophyll recovery was calculated as:
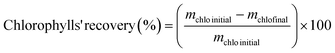 | (3) |
IL ecotoxicity
The ecotoxicity of the synthesised ILs was assessed by the standard microtox liquid phase assay. This test evaluates the inhibition of the luminescence of the marine bacteria Vibrio fischeri in the presence of IL aqueous solutions (from 0 to 82%), where 100% of the compound corresponds to a known concentration of a stock solution (approximately 10 g L−1). After 5-, 15-, and 30 min exposure to the IL, the luminous efficacy of the luminescent bacteria was determined and compared with the luminous efficacy of an empty control sample. Toxicity was assessed by the percent decrease in bacteria luminescence compared to that of the blank control sample. The result of this test is the EC50 parameter, which represents the effective concentration of a particular IL that causes a 50% inhibition of light emission. The analyses were performed using the MicrotoxOmniTM 211 software version 4.3.0.1.IL cytotoxicity
The cytotoxic potential of the synthesized ILs was evaluated using the human colon carcinoma cell line (Caco-2). The cells were maintained in high glucose Dulbecco's modified Eagle's medium (DMEM-HG) with 10% (v/v) fetal bovine serum (FBS), 100 units of penicillin, and 50 μg mL−1 streptomycin under a humidified atmosphere of 5% CO2 at 37 °C. When the cells reached a confluence of ca. 70–80%, they were trypsinized with a trypsin/EDTA solution and plated in 96 well culture plates (density of 1 × 104 cells per well). After 16 h, the cells were exposed for 24 h to different concentrations of the target ILs prepared by successive dilutions from a stock solution prepared in saline ([NaCl] = 0.9% (m/w)). Each concentration was tested in five replicate and three independent experiments were performed (n = 3). After 24 h of exposure, cell viability was evaluated using a Cell Counting Kit-8 (CCK-8). The CCK-8 kit allows the determination of the number of viable cells upon exposure to a given compound. This assay is based on the fact that when the cells are no longer viable, their dehydrogenase enzymes are unable to reduce the WST-8 (a water-soluble tetrazolium salt) into a water-soluble orange formazan dye. The changes in cell viability were evaluated by measuring the absorbance at 450 nm in a microplate spectrophotometer. Cell viability was calculated based on the relative absorbance compared with the control group (unexposed cells). The determination of the EC50, i.e., the concentration at which 50% of cells are viable, was performed with the GraphPad Prism V9 software using a non-linear model ([Inhibitor] vs. normalized response—variable slope equation using the least squares fitting method).Conclusions
This work successfully demonstrates the potential of thermoreversible ABS comprising ILs in integrated approaches for the simultaneous extraction and separation of two pigments – chlorophylls and betalains – from red beets waste. The system that allowed a higher extraction of both pigments is composed of 10 wt% [N21(2OH)(2OH)]Br and 52 wt% PPG, using as extraction conditions 20 °C, 70 min, and a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 0.12, optimised using a response surface methodology. After changing the temperature, this system allowed us to extract 95% of betalains to the IL-rich phase and 92% of chlorophylls to the PPG-rich phase. Pigments were finally recovered from the ABS phases using two affinity resins, allowing the recovery of 96% of betalains and 98% of chlorophylls. In addition to the integrated extraction–separation process developed, it was shown that the ABS phases can be used as preservation media for both pigments, at least up to 15 days, integrating one more step in the development of a process. Additionally, the studied quaternary ammonium-based ILs, particularly [N21(2OH)(2OH)]Br, exhibit low cytotoxic potential and are considered harmless from an ecotoxicological point of view.
liquid ratio of 0.12, optimised using a response surface methodology. After changing the temperature, this system allowed us to extract 95% of betalains to the IL-rich phase and 92% of chlorophylls to the PPG-rich phase. Pigments were finally recovered from the ABS phases using two affinity resins, allowing the recovery of 96% of betalains and 98% of chlorophylls. In addition to the integrated extraction–separation process developed, it was shown that the ABS phases can be used as preservation media for both pigments, at least up to 15 days, integrating one more step in the development of a process. Additionally, the studied quaternary ammonium-based ILs, particularly [N21(2OH)(2OH)]Br, exhibit low cytotoxic potential and are considered harmless from an ecotoxicological point of view.
Overall, this work brings new perspectives for the use of thermoreversible ABSs composed of polymers and quaternary ammonium-based ILs to extract, separate and stabilise chlorophylls and betalains from a complex food waste biomass source—stems and leaves of red beet—showing the versatility of the process and the possibility of its economic valorisation.
Author contributions
Marguerita E. Rosa: methodology, software, validation, writing – original draft, and writing – review & editing. Ana M. Ferreira: conceptualisation, methodology, software, writing – original draft, and writing – review & editing. Mafalda R. Almeida: methodology, software, and writing – review & editing. Rafael Barros: methodology and software. Ana C. Cristovão: methodology, supervision, resources, and writing – review & editing. Ana C. A. Sousa: methodology, software, supervision, and writing – review & editing. Catarina M. S. S. Neves: methodology and writing – review & editing. Patrícia M. Reis: methodology and writing – review & editing. Luís Paulo N. Rebelo: resources and writing – review & editing. José M. S. S. Esperança: resources and writing – review & editing. João A. P. Coutinho: conceptualisation, supervision, writing – review & editing, and funding acquisition. Mara G. Freire: conceptualisation, supervision, writing – review & editing, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Marguerita E. da Rosa acknowledges FCT—Fundação da Ciência e Tecnologia for the PhD grant FCT SFRH/BD/136995/2018. Catarina M. S. S. Neves also acknowledges FCT for the CEEC Individual contract (CEECIND/01975/2017). Patrícia M. Reis thanks FCT/MCTES for the financial support through an IF2014 researcher contract (IF/00621/2015). This work was partly developed within the scope of the projects CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020 and the Associate Laboratory for Green Chemistry-LAQV (UIDB/50006/2020, UIDP/50006/2020 and LA/P/0008/2020), financed by national funds through the FCT/MCTES (PIDDAC), the Health Sciences Research Center (CICS-UBI) (UIDB/00709/2020 and UIDP/00709/2020) and the Comprehensive Health Research Centre (CHRC) UIDP/04923/2020.References
- P. Sharma, V. K. Gaur, R. Sirohi, S. Varjani, S. Hyoun Kim and J. W. C. Wong, Bioresour. Technol., 2021, 325, 124684 CrossRef CAS PubMed.
- A. Székács, J. Agric. Environ. Ethics, 2017, 30, 153–170 CrossRef.
- Food and Agriculture Organization of the United Nations. https://www.fao.org/save-food/resources/keyfindings/en/. Accessed in April 2020.
- M. Sharma, Z. Usmani, V. K. Gupta and R. Bhat, Crit. Rev. Biotechnol., 2021, 41, 535–563 CrossRef CAS PubMed.
- F. A. Wani, R. Rashid, A. Jabeen, B. Brochier, S. Yadav, T. Aijaz, H. A. Makroo and B. N. Dar, Int. J. Food Sci. Technol., 2021, 56, 4823–4833 CrossRef CAS.
- K. Phan, K. Raes, V. Van Speybroeck, M. Roosen, K. De Clerck and S. De Meester, J. Cleaner Prod., 2021, 301, 126920 CrossRef CAS.
- B. H. Patel, in Handbook of Textile and Industrial Dyeing, Elsevier, 2011, vol. 1, pp. 395–424 Search PubMed.
- K. Ravichandran, N. M. M. T. Saw, A. A. A. Mohdaly, A. M. M. Gabr, A. Kastell, H. Riedel, Z. Cai, D. Knorr and I. Smetanska, Food Res. Int., 2013, 50, 670–675 CrossRef CAS.
- I. V. Pletnev, S. V. Smirnova, A. V. Sharov and Y. A. Zolotov, Russ. Chem. Rev., 2021, 90, 1109–1141 CrossRef.
- R. Md Moshikur, M. R. Chowdhury, M. Moniruzzaman and M. Goto, Green Chem., 2020, 22, 8116–8139 RSC.
- A. Le Donne and E. Bodo, Biophys. Rev., 2021, 13, 147–160 CrossRef CAS PubMed.
- A. I. Valente, A. M. Ferreira, M. R. Almeida, A. Mohamadou, M. G. Freire and A. P. M. Tavares, Sustainable Chem., 2021, 3, 1–18 CrossRef.
- M. Domínguez-Pérez, L. I. N. Tomé, M. G. Freire, I. M. Marrucho, O. Cabeza and J. A. P. Coutinho, Sep. Purif. Technol., 2010, 72, 85–91 CrossRef.
- H. Passos, A. Luís, J. A. P. Coutinho and M. G. Freire, Sci. Rep., 2016, 6, 20276 CrossRef CAS PubMed.
- A. M. Ferreira, H. Passos, A. Okafuji, A. P. M. Tavares, H. Ohno, M. G. Freire and J. A. P. Coutinho, Green Chem., 2018, 20, 1218–1223 RSC.
- R. Sharma, H. S. Oberoi and G. S. Dhillon, in Agro-Industrial Wastes as Feedstock for Enzyme Production, ed. G. S. Dhillon and S. Kaur, Elsevier, San Diego, 2016, pp. 23–59 Search PubMed.
- E. Madadi, S. Mazloum-Ravasan, J. S. Yu, J. W. Ha, H. Hamishehkar and K. H. Kim, Plants, 2020, 9, 1219 CrossRef CAS PubMed.
- S. Opinion, E. Panel, F. Additives and N. Sources, EFSA J., 2015, 13, 1–56 Search PubMed.
- Q. H. Tran, T. Q. Pham, H. T. Vu, D. X. Le, O. T. Tran, A. Q. Ngo, T. D. Nguyen, B. T. Hoang and S. T. Do, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 479, 012004 CrossRef CAS.
- A. Guesmi, N. Ladhari, N. Ben Hamadi, M. Msaddek and F. Sakli, J. Cleaner Prod., 2013, 39, 97–104 CrossRef CAS.
- H. F. Battistella Lasta, L. Lentz, L. G. Gonçalves Rodrigues, N. Mezzomo, L. Vitali and S. R. Salvador Ferreira, Biocatal. Agric. Biotechnol., 2019, 21, 101353 CrossRef.
- O. A. Hernández-Aguirre, C. Muro, E. Hernández-Acosta, Y. Alvarado and M. del C. Díaz-Nava, Molecules, 2021, 26, 6342 CrossRef PubMed.
- H. B. H. Koubaier, A. Snoussi, I. Essaidi, M. M. Chaabouni, P. Thonart and N. Bouzouita, Int. J. Food Prop., 2014, 17, 1934–1945 CrossRef.
- C. M. S. S. Neves, S. Shahriari, J. Lemus, J. F. B. Pereira, M. G. Freire and J. A. P. Coutinho, Phys. Chem. Chem. Phys., 2016, 18, 20571–20582 RSC.
- J. F. B. Pereira, L. P. N. Rebelo, R. D. Rogers, J. A. P. Coutinho and M. G. Freire, Phys. Chem. Chem. Phys., 2013, 15, 19580 RSC.
- J. F. B. Pereira, K. A. Kurnia, M. G. Freire, J. A. P. Coutinho and R. D. Rogers, ChemPhysChem, 2015, 16, 2219–2225 CrossRef CAS PubMed.
- F. A. e Silva, J. F. B. Pereira, K. A. Kurnia, S. P. M. Ventura, A. M. S. Silva, R. D. Rogers, J. A. P. Coutinho and M. G. Freire, Chem. Commun., 2017, 53, 7298–7301 RSC.
- I. Sadowska-Bartosz and G. Bartosz, Molecules, 2021, 26, 2520 CrossRef CAS PubMed.
- C. Sánchez, A. B. Baranda and I. M. De Marañón, Food Chem., 2014, 163, 37–45 CrossRef PubMed.
- M. Dilip, S. T. Griffin, S. K. Spear, H. Rodríguez, C. Rijksen and R. D. Rogers, Ind. Eng. Chem. Res., 2010, 49, 2371–2379 CrossRef CAS.
- A. F. Halwani, H. A. Sindi and H. A. Jambi, J. Biochem. Technol., 2018, 9, 10–14 CAS.
- L. Aztatzi-Rugerio, S. Y. Granados-Balbuena, Y. Zainos-Cuapio, E. Ocaranza-Sánchez and M. Rojas-López, J. Food Sci. Technol., 2019, 56, 3677–3686 CrossRef CAS PubMed.
- N. Kayın, D. Atalay, T. Türken Akçay and H. S. Erge, J. Food Sci. Technol., 2019, 56(11), 5097–5106 CrossRef PubMed.
- H. M. C. Azeredo, Int. J. Food Sci. Technol., 2009, 44, 2365–2376 CrossRef CAS.
- Chemaxon, https://chemaxon.com/chemicalize. Accessed in December 2022.
- R. Kushwaha, V. Kumar, G. Vyas and J. Kaur, Waste Biomass Valorization, 2018, 9, 1485–1494 CrossRef CAS.
- A. P. D. Costa, V. S. Hermes, A. O. Rios and S. H. Flôres, J. Food Sci. Technol., 2017, 54, 2050–2058 CrossRef PubMed.
- B. M. C. Vaz, M. Martins, L. M. de Souza Mesquita, M. C. Neves, A. P. M. Fernandes, D. C. G. A. Pinto, M. G. P. M. S. Neves, J. A. P. Coutinho and S. P. M. Ventura, Chem. Eng. J., 2022, 428, 131073 CrossRef CAS.
- E. V. Capela, M. V. Quental, P. Domingues, J. A. P. Coutinho and M. G. Freire, Green Chem., 2017, 19, 1850–1854 RSC.
- M. V. Quental, M. Caban, M. M. Pereira, P. Stepnowski, J. A. P. Coutinho and M. G. Freire, Biotechnol. J., 2015, 10, 1457–1466 CrossRef CAS PubMed.
- A. F. M. Cláudio, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, Green Chem., 2013, 15, 2002–2010 RSC.
- C. Liliana and N. Oana-Viorela, J. Nutr. Med. Diet Care, 2020, 6, 1–9 Search PubMed.
- P. B. F. Biondo, J. S. Boeing, É. O. Barizão, N. E. de Souza, M. Matsushita, C. C. de Oliveira, M. Boroski and J. V. Visentainer, Food Sci. Technol., 2014, 34, 94–101 CrossRef.
- P. Ebrahimi, D. Mihaylova, C. M. Marangon, L. Grigoletto and A. Lante, Molecules, 2022, 27, 8110 CrossRef CAS PubMed.
- G. S. N. Fernando, K. Wood, E. H. Papaioannou, L. J. Marshall, N. N. Sergeeva and C. Boesch, ACS Sustainable Chem. Eng., 2021, 9, 8736–8747 CrossRef CAS.
- D. R. M. Passino and S. B. Smith, Environ. Toxicol. Chem., 1987, 6, 901–907 CrossRef CAS.
- S. P. M. Ventura, F. A. e Silva, A. M. M. Gonçalves, J. L. Pereira, F. Gonçalves and J. A. P. Coutinho, Ecotoxicol. Environ. Saf., 2014, 102, 48–54 CrossRef CAS PubMed.
- V. Meunier, M. Bourrié, Y. Berger and G. Fabre, Cell Biol. Toxicol., 1995, 11, 187–194 CrossRef CAS PubMed.
- K. Verhoeckx, P. Cotter, I. López-Expósito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka and H. Wichers, The Impact of Food Bioactives on Health, Springer International Publishing, Cham, 2015 Search PubMed.
- A. M. de Ferro, P. M. Reis, M. R. C. Soromenho, C. E. S. Bernardes, K. Shimizu, A. A. Freitas, J. M. S. S. Esperança, J. N. Canongia Lopes and L. P. N. Rebelo, Phys. Chem. Chem. Phys., 2018, 20, 19307–19313 RSC.
- C. M. S. S. Neves, M. Figueiredo, P. M. Reis, A. C. A. Sousa, A. C. Cristóvão, M. B. Fiadeiro, L. P. N. Rebelo, J. A. P. Coutinho, J. M. S. S. Esperança and M. G. Freire, Front. Chem., 2019, 7, 1–12 CrossRef PubMed.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 711, 285–293 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Binodal weight fraction data, statistical analysis connected to the response surface methodology, and further experimental details. See DOI: https://doi.org/10.1039/d2gc04480c |
| ‡ Acronyms of ionic liquids: N-methyl-N,N,N-tris(2-hydroxyethyl)ammonium chloride, [N1(2OH)(2OH)(2OH)]Cl; N-ethyl-N-methyl-N,N-bis(2-hydroxyethyl)bromide [N21(2OH)(2OH)]Br; N-methyl-N,N,N-tris(2-hydroxyethyl)bromide [N1(2OH)(2OH)(2OH)]Br; and N-ethyl-N,N,N-tris(2-hydroxyethyl)bromide, [N2(2OH)(2OH)(2OH)]Br. |
| This journal is © The Royal Society of Chemistry 2023 |