 Open Access Article
Open Access ArticleHuman skin oil: a major ozone reactant indoors†
Charles J.
Weschler
 ab and
William W
Nazaroff
ab and
William W
Nazaroff
 c
c
aEnvironmental and Occupational Health Sciences Institute, Rutgers University, Piscataway, NJ, USA. E-mail: weschlch@rwmjs.rutgers.edu
bInternational Centre for Indoor Environment and Energy, Technical University of Denmark, Lyngby, Denmark
cDepartment of Civil and Environmental Engineering, University of California, Berkeley, CA, USA
First published on 22nd February 2023
Abstract
Human skin oil is rich in ozone-reactive compounds, including squalene and unsaturated acyl groups of free fatty acids, glycerols, and wax esters. Squalene and unsaturated acyl groups are each responsible for about half of the double bonds in skin oil. When there are no indoor sources, ozone concentrations are smaller indoors than outdoors, chiefly because ozone reacts with indoor surfaces. Ozone reacts rapidly with skin oils on occupants' exposed skin, hair, and clothing. Also, skin oil and its unsaturated oxidation products are transferred to indoor surfaces. A recent study of an occupied residence inferred that the average surface density of skin oil double bonds on inanimate indoor surfaces was approximately 5 μmol m−2. Estimates suggest that about 15% of outdoor ozone transported into residences is removed by skin oil or its byproducts. This percentage increases with occupant density indoors. In classrooms, the proportion of ozone removal attributable to skin oil may be in the range 35–55%. Further measurements of skin oil on off-body surfaces in a variety of indoor environments are needed to improve such estimates. In occupied indoor environments, the amount of ozone and ozone-derived products that occupants inhale is materially affected by the extent to which ozone reacts with skin-oil constituents. Toxicities of the products of ozone reactions with skin oil warrant further attention. The relative contribution of other fugitive sources (e.g., cooking oils, paints, and pesticides), as well as constituents inherent to building materials and furnishings, to ozone-reactive compounds on indoor surfaces remains unknown and also merits attention.
Environmental significanceOzone-reactive compounds reduce indoor ozone concentrations while generating both transient and stable oxidized species. Human skin oil is a source of ozone-reactive chemicals. Skin oil is present on occupant surfaces, including skin, hair, and clothing. Skin oil constituents also redistribute to nonoccupant surfaces via desquamation, partitioning, and contact transfer. Available evidence indicates that skin oil is a major ozone reactant in occupied environments. Humans inhale, ingest, and dermally absorb products resulting from ozone reactions with skin oil. Better defining the occurrence of ozone chemistry with skin oil on occupant and nonoccupant surfaces furthers our understanding of the ways in which occupant emissions influence human chemical exposures indoors. |
1 Introduction
Ozone is commonly present at lower concentrations indoors than outdoors, largely because of ozone-reactive compounds found indoors. Unsaturated organic compounds tend to dominate these ozone sinks. Their indoor sources include building materials, furnishings, cleaning products, personal care products, cooking, and the occupants themselves. Ozone-reactive compounds and their abundances vary among indoor environments and may also change with time as new sources are introduced, old sources dissipate, and activities wax and wane. Typically, substantially more ozone is removed from indoor air by reactions on surfaces than by reactions in air, a finding supported by both experiments and modelling.1–4In addition to reacting with unsaturated organic compounds, ozone can react rapidly with nitric oxide. Unvented combustion appliances, such as gas cooktops, are common. Nitric oxide can be a meaningful ozone sink whenever unvented combustion occurs. However, nitric oxide reactions generally comprise a small part of the time-averaged loss rate of indoor ozone.4 Ozone can also be deliberately removed from indoor air through use of air-cleaning technologies, such as activated carbon filters.5 However, the use of such control measures is relatively uncommon. In the interest of simplicity, in this paper, we focus on the most prominent aspect of ozone loss indoors: removal via reactions with unsaturated organic molecules on indoor surfaces.
Although not widely recognized, a potentially important source of ozone-reactive compounds in regularly occupied spaces is human skin oil, both on occupants and on nonoccupant surfaces.6–8 A prior review examined the many ways in which human occupants influence indoor chemistry.9 The present paper probes deeply into one important aspect of this influence. We compile and analyse evidence from the literature, much of it published since the earlier review, to investigate the extent to which human skin oil constituents contribute to ozone removal in regularly occupied indoor environments.
2 Background
2.1 Skin surface lipids
Skin oils consist of a mix of compounds from sebum and from the epidermis, with sebum making the larger contribution. Sebum comprises squalene, triglycerides, free fatty acids, wax and sterol esters, and free sterols. Cholesterol is the major skin oil constituent contributed by the epidermis. Bacteria on the skin promote the hydrolysis of tri-, di- and monoglycerides to free fatty acids. As reported by Nicolaides,10 human skin oil consists of squalene (10% by mass), free fatty acids (25%), triacyl glycerols (25%), di- and monoacyl glycerols (10%), wax esters (22%), sterols/sterol esters (4%), and assorted other species (4%). Downing and Strauss,11 summarizing surface lipid constituents from five different studies, report similar percentages. The most abundant unsaturated fatty acids are cis-hexadec-6-enoic acid (sapienic acid; 5–6% of skin oil) and cis-octadec-8-enoic acid (2%). Oleic acid, which has many other indoor sources including cooking, is present at about 0.5% in human skin oil.Squalene (Fig. 1) is responsible for about half of the double bonds in skin oil. Unsaturated bonds in the acyl groups of free fatty acids, glycerols, and wax esters comprise the remainder. Pandrangi and Morrison12 estimated that skin oil contains an average of 0.92 unsaturated –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds per molecule.
C– bonds per molecule.
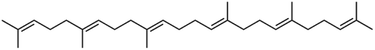 | ||
Fig. 1 Squalene (C30H50) is a triterpene with six –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds. Ozone can react at any of the double bond sites. C– bonds. Ozone can react at any of the double bond sites. | ||
Adults typically excrete sebum at a rate of ∼33 μg cm−2 h−1.13–15 This mass flux corresponds to ∼1000 μmol of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds per m2 per h, assuming an average molecular weight of 280 g mol−1 and an average of 0.9 double bonds per molecule for sebum. Sebum mass excretion rates from dry skin are about half the baseline value and are as much as four times larger than baseline in people with seborrhoea.15
C– bonds per m2 per h, assuming an average molecular weight of 280 g mol−1 and an average of 0.9 double bonds per molecule for sebum. Sebum mass excretion rates from dry skin are about half the baseline value and are as much as four times larger than baseline in people with seborrhoea.15
2.2 Desquamation
The stratum corneum is the outer layer of the epidermis and is “sloughed off continually as new cells take its place, but this shedding process slows down with age. Complete cell turnover occurs every 28 to 30 days in young adults, while the same process takes 45 to 50 days in elderly adults.” (https://training.seer.cancer.gov/melanoma/anatomy/layers.html). The skin cells that are shed are called squames and the shedding of skin cells is referred to as desquamation. The desquamation rate for adult humans is roughly 1000 cells cm−2 h−1 or 10 to 30 mg h−1.16–19 When first shed, squames contain about 1% squalene by weight.20 Squalene's molecular weight is 411 g mol−1, so humans shed roughly 0.2 to 0.7 μmol of squalene per hour through desquamation. Given that squalene contains six double bonds and that, on a molar basis, total double bonds in skin oil are about twice those in squalene, humans shed double bonds at a rate of 3 to 9 μmol of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds per hour. In a single-occupancy room with 10 m2 of upward-facing horizontal surface, the expected upper bound at which desquamation from a single occupant contributes –C
C– bonds per hour. In a single-occupancy room with 10 m2 of upward-facing horizontal surface, the expected upper bound at which desquamation from a single occupant contributes –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds to these surfaces would be 0.3 to 0.9 μmol m−2 h−1. Although clothing is expected to capture a fraction of the squames that a human sheds, the friction between clothing and skin also promotes desquamation.21,22 The net balance between these competing processes remains to be determined.
C– bonds to these surfaces would be 0.3 to 0.9 μmol m−2 h−1. Although clothing is expected to capture a fraction of the squames that a human sheds, the friction between clothing and skin also promotes desquamation.21,22 The net balance between these competing processes remains to be determined.
It is instructive to compare the above estimate for the flux of occupant-derived double bonds (0.3 to 0.9 μmol m−2 h−1) to the flux of ozone to surfaces. Assuming a typical indoor ozone concentration of 5 ppb and a deposition velocity of ozone to upward facing horizontal surfaces of 1.4 m h−1, then the ozone flux would be 0.3 μmol m−2 h−1. Hence, the estimate for the flux of human-derived –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds to upward-facing horizontal surfaces is comparable to the flux of ozone to those surfaces at an ozone level of 5 ppb. These estimates illustrate the potential of human-derived double bonds to contribute to off-body ozone loss in indoor environments.
C– bonds to upward-facing horizontal surfaces is comparable to the flux of ozone to those surfaces at an ozone level of 5 ppb. These estimates illustrate the potential of human-derived double bonds to contribute to off-body ozone loss in indoor environments.
2.3 Reactions of ozone with double bonds on indoor surfaces
The reaction of ozone on an indoor surface can be viewed as occurring in two steps: transport to the surface and chemical interaction at the surface. The flux of ozone to a surface is influenced by airflow adjacent to the surface and by the concentrations of ozone-reactive compounds on the surface. Transport rates vary with indoor air movement and with surface orientation, surface topography, and location of the surface in the indoor environment.23–26 Depending on circumstances, either transport or kinetic reaction steps may be the slower process and therefore rate-determining. When ozone arrives at an indoor surface, it reacts with chemicals that are intrinsically part of the surface or with chemicals that have accumulated on (soiled) the surface.The abundance of double bonds in organic molecules on a surface (quantified in units of μmol m−2) can be used to characterize the surface's ozone reactivity. This abundance is dynamically influenced by the balance between the rate at which double bonds arrive at the surface and the rate at which they are lost, e.g., by reacting with ozone. The balance is influenced by both the net flux of double bonds to the surface from indoor sources and the indoor concentration of ozone. When evaluating the contribution of skin oil on off-body surfaces to net ozone removal indoors, one must consider the occupant-driven flux of double bonds to indoor surfaces compared to the co-occurring flux of double bonds from non-occupant sources. An upper bound estimate can be made for the former (see Section 2.2); however, we currently have little information regarding the magnitude of the flux of double bonds from nonoccupant sources. Migration of double bonds to surfaces from within certain materials appears to contribute to the abundance of double bonds on surfaces. Evidence is seen in the regeneration of the ozone-scavenging potential of common indoor surfaces that are first exposed and then isolated from ozone exposure.27–31 With regard to reactivity attenuation, the residual level of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds on a surface should depend inversely on the rate of ozone reaction on a surface, which would scale with indoor ozone concentration.
C– bonds on a surface should depend inversely on the rate of ozone reaction on a surface, which would scale with indoor ozone concentration.
Deming and Ziemann32 measured extractable double-bond density using surface wipes of vertical glass and painted surfaces sampled in offices, classrooms, and other buildings on a university campus. On vertically oriented glass and painted surfaces, the respective mean values were 1.4 ± 1.0 and 0.9 ± 0.7 μmol m−2. Furthermore, the researchers found that the lifetime of double bonds owing to their reaction with ozone was on the order of an hour “indicating the highly dynamic” nature of ozone reactivity on these surfaces.
The average concentration of double bonds on upward-facing horizontal surfaces is expected to be larger than those on vertical surfaces, influenced by the preferential deposition via settling of coarse particles (including squames) onto upward-facing surfaces.33 At present, there are no published measurements of double bond density for upward-facing indoor surfaces sampled using surface wipes. Gall and Rim34 measured the ozone reactivity of initially clean glass plates that were horizontally exposed for varying time intervals in a mechanically ventilated office and in a naturally ventilated residence in Singapore. After 56 days, the net accumulated mass density on the glass plates was 0.52 g m−2 in the office and 0.78 g m−2 in the residence. Using ozone reactivities for multiple substrates exposed over different time intervals, a linear correlation was found between the moles of ozone removed by a glass plate and the mass of material accumulated on that plate (office: 2.4 μmol g−1; residence: 4.3 μmol g−1). The product of these terms — accreted mass density (g m−2) times the ozone removal intensity (μmol g−1) indicates the double bond surface density (μmol m−2) as determined at the end of the sampling period, assuming moles of O3 consumed during the subsequent exposures corresponds to moles of double bonds present. Hence, after 56 days, the soiled substrates from the office and the residence had double bond densities of 1.2 and 3.4 μmol m−2, respectively. As was the case for the double bonds on vertical surfaces in university buildings, the lifetime of the double bonds in the material accumulated on the Gall and Rim substrates would be relatively short – on the order of hours at typical indoor ozone concentrations. Consequently, these results should be indicative of the final net abundance of reactive bonds on the collection surfaces, rather than the rate at which such bonds are deposited.
2.4 Products of ozone reacting with unsaturated skin surface lipids
When ozone reacts with skin oil, prominent products include acetone, 6-methyl-5-hepten-2-one (6-MHO), decanal, geranyl acetone (GA), 4-oxopentanal (4-OPA), and 1,4-butanedial.6,12,28,35–39 The stable volatile and condensed-phase products that result from ozone reacting specifically with the skin oil constituent squalene are summarized in Coffaro and Weisel.40Recent studies have contributed valuable new knowledge about the products of ozone reactions with human surfaces, emphasizing the role of skin oils. The gas-phase products of ozone reacting with skin oil constituents from twenty volunteers were measured using a PTR-ToF-MS and a flow reactor affixed to the volunteers' skin.41 The average yields of the various products, relative to ozone loss, were reported. Major primary products of ozone reacting with skin oil included 6-MHO, geranyl acetone, and decanal; the average yield of 6-MHO (0.22 moles of product per mole of ozone consumed) was somewhat larger than that of geranyl acetone (0.16). The total net yield of gas-phase products ranged from 0.33 to 0.93, with substantial differences among volunteers. Using a similar instrument, emissions of volatile organic compounds (VOCs) from four volunteers were measured in a chamber during exposures to air without and with ozone.42 Experiments were conducted at different temperatures and relative humidity levels and with the volunteers wearing either long or short clothes. Altogether, five different groups of volunteers took part in these chamber experiments. The whole-body emission rate of VOCs more than doubled in the presence of ozone – from 2180 ± 620 μg h−1 per person (no O3) to 4600 ± 500 μg h−1 per person (with 37 ppb O3). When more skin was covered by clothing, the emission rate of ozone/skin-oil-derived products was somewhat lower. The emission rate increased with relative humidity. Lakey et al.43 applied a kinetic model that incorporates a new mechanism for O3 reactions with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds to further explore the influence of relative humidity on gas-phase squalene ozonolysis products. Other measurements of whole-body VOC emission rates are discussed in S1 of the ESI.†
C– bonds to further explore the influence of relative humidity on gas-phase squalene ozonolysis products. Other measurements of whole-body VOC emission rates are discussed in S1 of the ESI.†
A novel analytical approach, secondary electrospray ionization high-resolution mass spectrometry, was used to identify products formed when ozone reacts with skin oil on the hands of three volunteers.44 The ions that were detected suggest reaction pathways leading to various primary and secondary ozonides that in turn may react with ammonia emitted from human skin. As summarized in Coffaro and Weisel,40 considerable progress has been made recently in identifying the condensed phase products of the chemical interactions of ozone with skin oil.45–52
2.5 Surface chemistry vs. gas-phase chemistry
Substantially more ozone is removed by reactions on indoor surfaces than by reactions in air.1–4 Gas-phase reactions with organic compounds typically contribute less than 10% to ozone loss. For example, Price et al.53 estimated a total ozone gas-phase reactivity of 3 × 10−5 s−1 (0.1 h−1) in an average residence, whereas the total ozone reactivity on indoor surfaces (excluding occupants) is much higher, about 6 × 10−4 s−1 (2 h−1).4 This feature also applies for ozone loss to skin oil and its unsaturated derivatives. The initial reactions of ozone with squalene and the unsaturated acyl groups in skin oil occur almost exclusively on surfaces. Only three of the more abundant unsaturated acyl groups in skin oil contain two double bonds (C18:2delta5,8, C18:2delta9,12, and C20:2delta7,10), and the secondary reactions of ozone with their products also occur chiefly on surfaces. Hence, the primary and secondary reactions of ozone with unsaturated acyl groups, which constitute about half of the double bonds in skin oil on a molar basis, are almost all surface reactions.In the case of squalene, six primary ozonides are formed via surface reactions with ozone. These primary ozonides decay to twelve carbonyls and their respective Criegee intermediates. Ten of these carbonyls contain double bonds and participate in secondary reactions with ozone. Noteworthy among these are 4,8,13,17,21-tetramethyl-octadeca-4,8,12,16,20-pentaenal (TOP; five double bonds), 4,9,13,17-tetramethyl-octadeca-4,8,12,16-tetraenal (TOT; four double bonds), and 5,9,13-trimethyl-tetradeca-4,8,12-trienal (TTT; three double bonds). Of the initial products of primary chemistry, only 6-MHO reacts chiefly in the gas phase. The other nine carbonyls react with ozone chiefly on surfaces. In summary, six primary reactions of ozone with squalene occur on surfaces and 28 out of 30 secondary reactions of ozone with carbonyl products occur on surfaces. (This analysis ignores both the products formed by decay of Criegee intermediates and possible differences in the rates at which the various primary and secondary reactions occur.)
A recent study by Zhang et al.54 supports the inference that surface reactions dominate over gas-phase reactions when considering the interaction of ozone with human skin oils. The researchers simulated off-body reactions of ozone with squalene and its initial products in an indoor environment. They estimated that the first-order rate constant for the net loss of ozone via primary and secondary reactions with squalene and its derivatives on off-body surfaces was 0.18 h−1, whereas the first-order rate constant for the net loss of ozone via gas-phase reaction with 6-MHO and GA was an order of magnitude smaller, 0.017 h−1.
3 Contribution of skin oil on occupant surfaces to ozone removal indoors
3.1 Ozone removal by the human envelope
Concern about ozone exposures indoors dates back a half-century, as represented by the influential work of Shair and Heitner.1 At that time, ozone loss indoors was thought to occur primarily by reaction on inanimate surfaces.55 Two decades later, Liu et al.56 were the first to identify the existence of an ozone concentration gradient in the vicinity of human occupants indoors. However, that work did not make clear the specific connection between skin oil and ozone depletion. We now know that ozone reacts rapidly on the hair, skin, and clothing of building occupants, and, to a lesser extent, with unsaturated gaseous products of skin oil ozonolysis.Starting about two decades ago, the role of humans as ozone sinks and skin oil as the specific source of important ozone-reactive chemicals in occupied indoor environments was documented in several chamber and laboratory studies.6,12,28,36,37,57,58 These investigations began with an interest in the fate of ozone in the passenger cabins of aircraft, motivated by a concern about ozone being naturally present along the flight path and introduced into the cabin with ventilation air.59 The first reports of skin oil ozonolysis products in indoor environments came from experiments in which previously worn t-shirts were exposed to ozone in a simulated aircraft cabin.36,58 Subsequently, human volunteers were exposed to ozone in the same simulated aircraft. In a typical experiment, when 16 passengers entered the cabin (volume = 28.5 m3), the ozone concentration decreased from 130 ppb to 80 ppb.37 In this densely occupied chamber, measurements indicated that about 55% of ozone loss was directly attributable to reactions on the human occupants. Coleman et al.28 identified skin oil oxidation products emitted by soiled fabrics exposed to ozone. Pandrangi and Morrison12 quantified the products emitted by unwashed and washed hair exposed to ozone. Using sorbent sampling and GC-MS analysis, Weisel et al.60 measured selected products of ozone/skin oil chemistry in actual aircraft cabins during fifty-two flights. Decanal and 6-MHO were detected at sub- to low-ppb levels, and the 6-MHO concentration roughly varied with the percentage of seats occupied.
Even in much less densely occupied office environments, loss to human surfaces can substantially affect indoor ozone levels. Wisthaler and Weschler6 reported that when two people entered a 30 m3 simulated office ventilated at an air-change rate of 1 h−1, the ozone concentration decreased from 33 ppb to 17 ppb. The occupants removed ozone with an effective volumetric air cleaning rate of 25–30 m3 h−1 per person. The authors estimated that a single occupant, in a 30 m3 room would “… contribute between 10 and 25% to the overall ozone removal (i.e., the sum of the first-order rate constants for removal by air exchange, room surfaces, gas phase chemistry, and a single occupant …).” Considering only the chemical sinks for ozone in such a room, a single occupant would remove about a third of the ozone while the inanimate indoor surfaces would account for the other two-thirds.
Destaillats et al.61 passed ozone through ventilation-system filters collected from buildings at two San Francisco locations and monitored the downstream air using a PTR-MS. They identified oxidation products that appeared to come from occupants' skin flakes and/or skin oil present in the filter cakes. In experiments in a school, Fischer et al.62 examined ozone removal by a teacher and 24 pupils (age 11 years) in a classroom (volume = 182 m3). They found the classroom concentration of ozone to be anticorrelated with carbon dioxide, as would be expected with occupants being a source of CO2 and a sink for ozone. After occupants entered at the start of a class period, the ozone concentration quickly decreased while the CO2 concentration increased. The opposite occurred when the occupants left at the end of a teaching period. In that study, the mainly juvenile occupants removed ozone with an effective volumetric rate of 16 ± 4 m3 h−1 per person.
Additional evidence has emerged during the past decade, showing clearly that ozone reactions with occupants affect the composition of nearby air. Veres et al.63 used a PTR-MS to measure human emissions before, during, and after a soccer game in an open-air arena attended by ∼31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fans. When fans began to enter the stadium, the level of ozone decreased while that of CO2, 6-MHO, and decanal increased. When fans exited at the end of the game, ozone levels increased and the levels of CO2, 6-MHO, and decanal decreased. In separate studies at two universities, Liu et al.64 and Tang et al.65 measured a decrease in ozone concentration and an increase in products derived from ozone reactions with skin oil when students entered a classroom. Identified ozone/skin oil products included 6-MHO and 4-OPA, with lesser amounts of geranyl acetone, hydroxy acetone, and 1,4-butanedial. Avery et al.66 found a linear correlation between the ozone removal rate constant and the change in carbon dioxide concentration in a classroom. They also measured higher aerosol mass with occupancy and, using mass spectral analysis, identified fragment ions consistent with products of ozone/squalene chemistry “indicative of the secondary nature of the aerosol mass.” Finewax et al.67 made real-time measurements of airborne organic compounds at a university athletic centre and observed a correlation between the per-person 6-MHO emission factor (μg h−1) and the per-person CO2 emission factor (g h−1). They also observed a correlation between the per-person 6-MHO emission factor (μg h−1) and the per-person ozone consumption rate (μg h−1). Using space- and time-resolved measurements of volatile organic compounds in an Oakland, CA, home, Liu et al.8 examined ozone chemistry that occurred in that home during eight weeks of sampling. Also interpreting sampling data from that study, Zhang et al.68 estimated that, when occupied, the occupants themselves removed ozone with a first-order rate constant of 0.09 h−1. Given that one or two occupants were commonly present in the 350 m3 home, this loss rate is equivalent to an effective volumetric removal rate for ozone of 16–32 m3 h−1 per person, roughly consistent with prior studies in other types of indoor settings. Additional results and implications from this study are presented in Section 4.2. Deng et al.69 made real-time measurements of naturally occurring nitrous acid, nitrogen oxides, ozone, and organics in an apartment room in Guangzhou, China. In this study, researchers used scripted activities (e.g., opening/closing windows, and mopping) and deliberately varied the number of occupants in the room. Ozone levels were affected by occupancy. Numerous ions with mass-to-charge ratios indicative of compounds derived from ozone reactions with skin oils (e.g., 6-MHO, and geranyl acetone) were higher during periods of occupancy.
000 fans. When fans began to enter the stadium, the level of ozone decreased while that of CO2, 6-MHO, and decanal increased. When fans exited at the end of the game, ozone levels increased and the levels of CO2, 6-MHO, and decanal decreased. In separate studies at two universities, Liu et al.64 and Tang et al.65 measured a decrease in ozone concentration and an increase in products derived from ozone reactions with skin oil when students entered a classroom. Identified ozone/skin oil products included 6-MHO and 4-OPA, with lesser amounts of geranyl acetone, hydroxy acetone, and 1,4-butanedial. Avery et al.66 found a linear correlation between the ozone removal rate constant and the change in carbon dioxide concentration in a classroom. They also measured higher aerosol mass with occupancy and, using mass spectral analysis, identified fragment ions consistent with products of ozone/squalene chemistry “indicative of the secondary nature of the aerosol mass.” Finewax et al.67 made real-time measurements of airborne organic compounds at a university athletic centre and observed a correlation between the per-person 6-MHO emission factor (μg h−1) and the per-person CO2 emission factor (g h−1). They also observed a correlation between the per-person 6-MHO emission factor (μg h−1) and the per-person ozone consumption rate (μg h−1). Using space- and time-resolved measurements of volatile organic compounds in an Oakland, CA, home, Liu et al.8 examined ozone chemistry that occurred in that home during eight weeks of sampling. Also interpreting sampling data from that study, Zhang et al.68 estimated that, when occupied, the occupants themselves removed ozone with a first-order rate constant of 0.09 h−1. Given that one or two occupants were commonly present in the 350 m3 home, this loss rate is equivalent to an effective volumetric removal rate for ozone of 16–32 m3 h−1 per person, roughly consistent with prior studies in other types of indoor settings. Additional results and implications from this study are presented in Section 4.2. Deng et al.69 made real-time measurements of naturally occurring nitrous acid, nitrogen oxides, ozone, and organics in an apartment room in Guangzhou, China. In this study, researchers used scripted activities (e.g., opening/closing windows, and mopping) and deliberately varied the number of occupants in the room. Ozone levels were affected by occupancy. Numerous ions with mass-to-charge ratios indicative of compounds derived from ozone reactions with skin oils (e.g., 6-MHO, and geranyl acetone) were higher during periods of occupancy.
The flux of ozone to a surface normalized by the freestream ozone concentration is referred to as ozone's deposition velocity, vh, with units of m h−1.70 In the present paper, in addition to considering ozone's deposition velocity to the human body, we will use “volumetric ozone removal rate per person,” Qh, which is analogous to the “clean air delivery rate” (m3 h−1) used to evaluate the efficacy of stand-alone air cleaners. These two parameters are linked by the apparent surface area of the human envelope, Ah, such that Qh = Ah × vh. A typical value for an adult is Ah = 1.8 m2.71Table 1 summarizes reported values for the “effective” deposition velocity (vh) and volumetric ozone removal rate per person (Qh), as measured in different indoor environments. Reported deposition velocities range from 7.2 to 8.3 m h−1 per person in a simulated aircraft cabin58 to 18.5 ± 0.5 m h−1 per person in a chamber.72 The corresponding values of Qh are 13–15 m3 h−1 in the aircraft cabin study and 33.3 ± 0.9 m3 h−1 in the chamber study. In the aircraft cabin study, the occupant density was high, the occupants were in aircraft seats the entire time, and air flow around the body differed from the other studies summarized in Table 1. Such features may influence the net reaction rate of ozone on occupants.
| Location | Conditions | v d (m h−1) | Q h (m3 h−1) | Reference |
|---|---|---|---|---|
| a Surface area of 1 m2 per pupil used to calculate deposition velocity to children. b Nine-hour average. c T = 27–28 °C. | ||||
| Simulated aircraft cabin | Four experiments, each with 16 occupants | 7.2–8.3 | 13–15 | 58 |
| Simulated office | Two experiments, each with two occupants | 14, 18 | 25, 32 | 6 |
| Simulated office | Six experiments, each with 18–20 occupants | 18 ± 4 | 32 ± 7 | 73 |
| Classroom | Teacher and 24 pupilsa | 16 ± 4 | 16 ± 4 | 62 |
| Classroom | 15–64 occupants on the day of measurement | 9b | 16b | 74 |
| Bedrooms | 52 measurements in 5 bedrooms with 1–3 occupants | 15 ± 10 | 27 ± 18 | 75 |
| Chamber | 11 experiments, 4 occupants each — long clothesc | 16.8 ± 1.7 | 30 ± 3 | 72 |
| Chamber | 5 experiments, 4 occupants each — short clothesc | 18.5 ± 0.5 | 33 ± 0.9 | 72 |
| Occupied home | Two occupants, eight weeks of sampling | 9–18 | 16–32 | 68 |
The chamber study by Bekö et al.72 included 11 experiments in which the 4 occupants wore long shirts, pants, and socks (vh = 16.8 ± 1.7 m h−1) and 5 experiments in which they wore t-shirts, shorts, and short socks (vh = 18.5 ± 0.5 m h−1). The difference in deposition velocities between these conditions was small but statistically significant (p = 0.0003). The statistical significance disappears (p = 0.3) if the comparison is restricted to studies that used the same volunteers (4 experiments with long clothing (vh = 18.0 ± 0.8 m h−1) and 4 experiments with short clothing (vh = 18.5 ± 0.5 m h−1)). This evidence suggests that the amount of exposed skin has little influence on the ozone deposition velocity averaged over the human envelope. Temperature and relative humidity were also varied during these chamber studies. The largest deposition velocity (vh = 23.3 m h−1) was measured at 32.5 °C and 62% RH as compared to 17.3 m h−1 at 28 °C and ∼25% RH. Taken together, the studies listed in Table 1 suggest ozone deposition velocities to the human envelope indoors are in the range vh = 10–20 m h−1, corresponding to volumetric ozone removal rates per person of Qh = 18–36 m3 h−1. Approximate central tendency values are vh = 15 m h−1 per person or Qh = 25 m3 h−1 per person, numbers that we'll utilize in illustrative calculations later in this paper.
The deposition velocities of ozone to human surfaces listed in Table 1 are remarkably large when compared to the deposition velocity of ozone to inanimate indoor surfaces (∼1 m h−1).4 A key reason for this difference is the abundance of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds in skin oils on the human body envelope, including on hair and clothing.
C– bonds in skin oils on the human body envelope, including on hair and clothing.
The measured ozone deposition velocities to a human surface are larger than those calculated by some (∼8–10 m h−1),76–78 but not all (∼22 m h−1)79 computational fluid dynamic simulations. Airflow conditions would influence the effective deposition velocity of ozone to humans. Seemingly large values may reflect the generally higher airflows associated with the personal convective boundary layer.80 The buoyancy-induced volumetric air flow above a standing person may be as high as 60 L s−1.81 Furthermore, the airflow around a human body in carefully controlled chamber experiments and in numerical simulations may differ from that in actual indoor environments. Contributions to the large deposition velocity values to humans may also derive from ozone reacting with skin flakes shed from human surfaces,82 either on the inanimate surfaces adjacent to an occupant or in the envelope of air that surrounds an occupant. Although representing relatively small sinks based on measured emission rates,42 ozone would also react with gaseous 6-MHO and other unsaturated products emitted by ozone/skin surface chemistry on humans. Perhaps it is a combination of such factors, in addition to the direct reaction of ozone with skin and clothing surfaces, that contribute to the large “effective” deposition velocities (and correspondingly large volumetric removal rates) associated with indoor occupants.
A first-order loss-rate coefficient for ozone because of its reaction with the human envelope (kh) in an indoor environment can be estimated as the quotient of ozone's volumetric removal rate per person (Qh) and the volume of the occupied space (V):
| kh = Qh/V | (1) |
For example, if the volumetric removal rate due to a single occupant (Qh) is 25 m3 h−1 and the volume of the occupied space (V) is 30 m3, then the occupant removes ozone with a rate constant of 0.85 h−1, a value similar to that measured by Wisthaler and Weschler.6 One should expect the removal of ozone by human occupants to vary with occupant density. For the conditions just outlined, two adults in a 30 m3 room would remove ozone with a rate constant of 1.7 h−1 while one adult in a 60 m3 room would remove ozone with a rate constant of 0.4 h−1.
Further relevant evidence is available from studies of the influence of ozone exposure on skin-oil composition. Using glass capillaries touched by human fingers, Zhou et al.51 have demonstrated a significant chemical change in the composition of skin oil over timescales of hours at typical indoor and outdoor ozone concentrations. In vivo, ozone exposure is known to alter the relative level of skin-oil constituents. For example, in the elevated ozone environment of Mexico City, the average squalene/cholesterol ratio in skin wipes of 96 volunteers was smaller (7.6 μg μg−1) than in skin wipes from 93 volunteers in the less polluted environment of Cuernavaca (11.7 μg μg−1).83 In a related study, squalene averaged 12% of total lipids for skin wipes from 79 volunteers in the more polluted air of the Xu Jia Hui district of Shanghai as compared to 16% of lipids in wipes from 80 volunteers in the cleaner air of Chong Ming.84 In recent chamber experiments in which four volunteers were exposed to 35 ppb ozone for approximately three hours, the ratio of squalene to cholesterol in skin wipes taken after the exposure decreased by about 35% relative to the pre-exposure ratio.85
At some point, do the ozone-reactive compounds present on an occupant's skin, hair, and clothing become depleted? When considering this question, it is informative to compare the secretion rate of double bonds found in sebum with ozone flux to the human envelope.12 As estimated in Section 2.1, adults excrete double bonds in sebum at a rate of approximately 1000 μmol of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds per m2 per h. At a typical indoor ozone concentration of 5 ppb and a deposition velocity of 15 m h−1 to the human envelope, the flux of ozone to an occupant's surface would be 3 μmol m−2 h−1. In chamber experiments,85 the ozone flux to humans was 20 μmol m−2 h−1, while outdoors in Mexico City and Shanghai, ozone flux likely was somewhat larger than 60 μmol m−2 h−1. Hence, whereas squalene depletion has been observed in skin wipes of individuals exposed to elevated ozone levels, the rate at which the sebaceous glands secrete double bonds is much larger than the rate at which ozone is anticipated to consume double bonds in skin surface lipids. One may infer from this evidence that occupants would continue to be important ozone sinks throughout extended periods of exposure.
C– bonds per m2 per h. At a typical indoor ozone concentration of 5 ppb and a deposition velocity of 15 m h−1 to the human envelope, the flux of ozone to an occupant's surface would be 3 μmol m−2 h−1. In chamber experiments,85 the ozone flux to humans was 20 μmol m−2 h−1, while outdoors in Mexico City and Shanghai, ozone flux likely was somewhat larger than 60 μmol m−2 h−1. Hence, whereas squalene depletion has been observed in skin wipes of individuals exposed to elevated ozone levels, the rate at which the sebaceous glands secrete double bonds is much larger than the rate at which ozone is anticipated to consume double bonds in skin surface lipids. One may infer from this evidence that occupants would continue to be important ozone sinks throughout extended periods of exposure.
3.2 Influence of clothing
The human body is commonly clothed. Ozone reacts irreversibly on clothing. Some portion of the reactivity may be attributable to the clothing fabrics themselves.28,86 However, ozone also reacts with skin oils that accumulate on clothing during wear. This feature was first revealed when previously worn t-shirts were placed over seat backs in a simulated aircraft cabin and organic emissions were measured with and without ozone added to ventilation air.36 Products derived from the oxidation of human skin lipids were observed when ozone was present. Ozone reactivity is much higher on previously worn clothing than on freshly laundered fabrics. Through contact transfer, clothing acquires skin oil relatively quickly. Cotton t-shirts36 as well as cotton, wool, and polyester fabrics28 that had been in contact with skin overnight were found to be strong emitters of skin-oil oxidation products when exposed to ozone. In chamber experiments, Rai et al.86 observed that a t-shirt worn for a few hours consumed more ozone and generated more VOCs than did a freshly laundered t-shirt. The first-order rate constant for ozone removal in a chamber increased from 0.4 to 1.2 h−1 when a freshly laundered t-shirt was introduced to a previously empty chamber. The rate increased to 1.3 h−1 when a t-shirt worn for 2 hours was introduced and to 1.9 h−1 when a t-shirt worn for 6 hours was introduced. There was no further increase in the ozone removal rate constant for a shirt that had been worn for 12 h.Laundered clothing impedes ozone/skin oil chemistry. In experiments in which four volunteers entered a chamber with freshly laundered long-sleeve shirts, long pants, and calf socks and were exposed to ozone 3.5 hours later, the emission rate of products characteristic of skin oil was ∼2400 μg h−1 per person. In an analogous experiment, when the volunteers wore shorts, t-shirts, and ankle socks the emission rate was ∼3500 μg h−1 per person.42 Notably, overall ozone consumption by the four volunteers was similar whether wearing short clothing (with more exposed skin) or long clothing. Although the emission rates of skin oil-derived products decreased with long clothing, those of certain products were higher, suggesting a contribution from reactions between ozone and clothing-associated chemicals that did not originate from skin oil. When two volunteers, who had sat in the chamber for 1.5 h without a shirt, put on freshly laundered t-shirts, the concentrations of 6-MHO and 4-oxopentanal (4-OPA) immediately dropped, indicating that ozone/skin oil chemistry had been suppressed. Concomitantly, the concentrations of nonanal and nonenal increased, presumably because precursors of these compounds were present in the clothing fabrics. These experiments demonstrate that recently laundered clothing decreases the emission of products generated by ozone/skin oil chemistry. The extent to which clothing reduces the loss of ozone to skin oil is expected to depend on the fraction of skin covered by clothing, how long the clothing has been worn, and the thickness of the fabric. In calculations that follow we assume that between half and all (50–100%) of ozone loss on occupant surfaces is due to skin oil, recognizing that this is a poorly known parameter.
4 Contribution of skin oil on nonoccupant surfaces to ozone removal indoors
4.1 Preamble
Skin oil on nonoccupant surfaces can contribute to ozone removal in indoor environments. Knowledge regarding ozone loss to skin oil on off-body surfaces is scant compared to what has been learned about ozone loss to skin oil on people. To some extent, knowledge of surface soiling can be applied to bound the extent to which skin oil contributes to ozone loss on off-body surfaces. Indoor surfaces are soiled, in part, by airborne particles and dust. This section begins with a summary of squalene, indicative of skin oil, measured in airborne particles and settled dust. We then examine mechanisms by which skin oil might be transported to off-body surfaces and discuss differences anticipated for the accumulation of skin oil on horizontal versus vertical surfaces. We next summarize direct evidence for ozone loss to skin oil on inanimate surfaces that was obtained during field studies in an Oakland home8 and an Indiana office.87 We conclude this section with a summary of important indirect evidence: decanal generated by ozone-surface chemistry as an indicator of skin oil on indoor surfaces.4.2 Squalene in airborne particles and settled dust
Squalene, and, by inference, skin oil, has been identified and quantified in airborne particles and settled dust, but only in a few studies. Clark and Shirley20 measured the mass fraction of squalene in size-fractionated airborne particles collected from indoor locations in greater London and used these measurements to estimate the percentage of skin in the particles. Table 2 summarizes their results for squalene. For airborne particles with diameters between 0.4 and 6 μm, collected in either a home or in a laboratory corridor, the measured squalene mass fraction ranged between 40 and 100 μg g−1. For particles between 0.3 and 5.5 μm diameter, collected in the London Underground, the measured squalene mass fraction was an order of magnitude higher, ∼1000 μg g−1 (i.e., 0.1%). Coupled with an expected squalene abundance of 1% in squames, these measurements suggest a remarkable finding, that 10% of the airborne particles collected in the London Underground originated as skin from transit riders.| Mass fraction in airborne particles, in relation to particle diameter, dp | |||||
|---|---|---|---|---|---|
| Indoor location | Sample | d p = 0.4–0.7 μm | d p = 0.7–2 μm | d p = 2–6 μm | Average |
| House | Air | 80 | 70 | 40 | 63 |
| Laboratory corridor | Air | 50 | 50 | 100 | 67 |
| d p = 0.3–2 μm | d p = 2–3.5 μm | d p = 3.5–5.5 μm | Average | ||
| London underground | Air | 1000 | 1000 | 1000 | 1000 |
| Mass fraction in dust | |||||
|---|---|---|---|---|---|
| Indoor location | Sample | GM (GSD) | 25th percentile | 75th percentile | AM |
| Children's bedrooms | Dust (N = 495) | 32 (4.3) | 19 | 73 | 56 |
| 0.015–0.045 occupants per m3 | Dust (N = 165) | 29 | |||
| 0.045–0.067 occupants per m3 | Dust (N = 165) | 29 | |||
| 0.067–0.247 occupants per m3 | Dust (N = 165) | 41 | |||
| Daycare facilities | Dust (N = 151) | 11.5 (4.3) | 6 | 26 | 26 |
Weschler et al.82 measured the mass fraction of squalene in settled dust collected from 495 children's bedrooms and 151 day-care facilities in Denmark. The findings are summarized in Table 2. The arithmetic means were 56 μg g−1 for dust from bedrooms and 26 μg g−1 for dust from day-care facilities. The distributions could be described reasonably well by lognormal fits with large variances. Homes had a geometric mean (GM) of 32 μg g−1 with a geometric standard deviation (GSD) of 4.3; day-care facilities had GM = 11.5 μg g−1 and GSD = 4.3. Homes with larger occupant densities tended to have a larger mass fraction of squalene in dust. Because squalene comprises only 10% of skin oil, the mass fractions of skin oil in airborne particles and settled dust are anticipated to be an order of magnitude larger than those of squalene.
From the time that squames were initially shed from human skin until the time that airborne particles or dust samples were collected and analysed, double bonds from squalene and other unsaturated skin oil constituents could be consumed by reactions with ozone. Consequently, the mass fractions of skin oil in fresh airborne particles or settled dust are expected to be higher than those listed in Table 2.
4.3 Transport of unsaturated skin oil constituents to surfaces
Nonoccupant surfaces may accumulate skin oil via direct deposition of squames and airborne particles containing skin oil. Contributions may also arise via resuspension of dust-containing skin oil followed by surface deposition. Additionally, skin oil constituents or products of ozone/skin oil chemistry (e.g., geranyl acetone) can partition from the gas phase to indoor surfaces. By such processes, double bonds in skin oil can become redistributed throughout indoor environments. Humans also transfer skin oils directly to the objects they touch, including clothes, bedding, tables, chairs, and countertops. Cohen Hubal et al.88,89 studied the contact transfer of chemicals from surfaces to hands, but this process is certainly a two-way exchange. Fingerprints demonstrate contact transfer of skin oils to indoor surfaces.90 In summary, ozone-reactive compounds in skin oil, including its unsaturated reaction products, are anticipated to be commonly present on indoor surfaces.To what extent do ozone-reactive compounds in skin oil partition to indoor surfaces? Based on its vapor pressure, (3.7 ± 1.3) × 10−7 Pa at 25 °C,91 squalene's redistribution from a surface at room temperature to other indoor surfaces via the vapor phase is expected to be small (see S2 in the ESI†). However, some indoor surfaces are occasionally warmed, e.g., during cooking, heating, bathing, or via sunshine. The resulting increases in surface temperature and in the vapor pressure of surface-accumulated skin oil constituents could promote the redistribution of squalene to other indoor surfaces. This transport mechanism may explain the detection by Lim and Abbatt92 of what appears to be a set of squalene-derived oxidation products in surface films that had accumulated on vertically oriented glass tubes exposed indoors for about two weeks. Signals obtained with their DART-MS in negative mode are consistent with levulinic acid (m/z = 115), succinic acid (117), a C-14 aldehydic acid (237), a C-14 diacid (253), and a C-17 acid (263). (See Table S7† of Lim and Abbatt.92). While some of these mass-to-charge ratios are not unique markers of squalene oxidation, taken together they represent reasonable evidence for the presence of squalene oxidation products on exposed indoor surfaces. The question remains whether these squalene oxidation products derived from the in situ oxidation of squalene, which was not itself detected on the glass tubes, or were transported to the glass tubes following their generation on indoor surfaces elsewhere. Other skin oil constituents were detected on the glass tubes. These include pyroglutamic acid, palmitic acid, and stearic acid, but some of these have indoor sources in addition to skin oil. In summary, based on theoretical considerations, coupled with the measurements of surface film constituents by Lim and Abbatt, it appears that squalene may migrate to indoor vertical surfaces via the gas-phase at a slow rate. Rapid oxidation by ozone would preclude its substantial accumulation.
At 25 °C, unsaturated fatty acids in skin oil have vapor pressures in the range 5–100 × 10−6 Pa and are 10–250 times more volatile than squalene.93 Nevertheless, when these fatty acids redistribute from skin or inanimate room temperature surfaces, their fluxes to other surfaces are expected to be smaller than ozone's. When redistributing from heated surfaces, such unsaturated fatty acids are more likely than squalene to have fluxes to other surfaces that approach those of ozone.
Unsaturated skin oil oxidation products (e.g., 6-MHO and geranyl acetone) occur with much larger average concentrations in indoor air than squalene or unsaturated fatty acids. In the Oakland home monitored for an extended summer period, the average indoor 6-MHO concentration was 0.35 ppb during occupancy.8 In an Indiana office, also monitored for an extended period, it was often in the range of 0.2 to 0.3 ppb during occupancy.87 Geranyl acetone (GA) concentrations tend to be smaller than 6-MHO, but GA also has a substantially larger octanol/air partition coefficient (Koa) and a correspondingly greater tendency to sorb on indoor nonpolar surfaces. The low water–air (Kwa) partition coefficients would limit the partitioning of 6-MHO and GA to polar sorptive reservoirs.94 (For 6-MHO, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koa = 5.0, log
Koa = 5.0, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kwa = 2.5; for GA, log
Kwa = 2.5; for GA, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koa = 7.1, log
Koa = 7.1, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kwa = 2.3 as calculated for 25 °C using SPARC.) This evidence hints that transport from the vapor phase of unsaturated skin oil oxidation products may contribute to skin oil double bonds on indoor surfaces – not just in the instance of geranyl acetone but also for other higher molecular weight unsaturated ozonolysis products such as 5,9,13-trimethyl-tetradeca-4,8,12-trienal (TTT).
Kwa = 2.3 as calculated for 25 °C using SPARC.) This evidence hints that transport from the vapor phase of unsaturated skin oil oxidation products may contribute to skin oil double bonds on indoor surfaces – not just in the instance of geranyl acetone but also for other higher molecular weight unsaturated ozonolysis products such as 5,9,13-trimethyl-tetradeca-4,8,12-trienal (TTT).
Ultrafine particles (UFP) rich in unsaturated skin oil oxidation products22,95 can also soil indoor surfaces. However, calculations using what we know about such processes suggest that the flux of UFP rich in skin oils to indoor surfaces would be too small for this transport mechanism to constitute a major surface-reaction sink for indoor ozone (see S3 in the ESI†).
4.4 Influence of surface orientation
Coarse particles derived from skin flakes predominantly deposit by means of gravitational settling, which means onto upward oriented surfaces. In general, coarse particle deposition rates to upward-facing surfaces are much faster than to vertical surfaces.96,97 We have previously estimated33 that indoor particle deposition and dust fall contribute organic matter to upward surfaces at a rate of 600–9000 μg m−2 day−1 and to vertical surfaces at a rate of 0.3–4 μg m−2 day−1. The large difference in these ranges suggests that double bonds from skin oil associated with particles and dust accumulate at relatively slow rates on vertical surfaces. Limited countervailing experimental results98 and recent modelling assessments99 suggest higher deposition rates of coarse particles to vertical surfaces than predicted by simple models. In small to medium rooms (30–50 m3), upward-facing surfaces nominally comprise only about 20% of the total indoor surface area. Deposition to vertical surfaces might be large enough to matter, especially when one considers that settled dust on floors can be resuspended via disturbances such as footfalls multiple times, thereby presenting repeated opportunities for deposition to other surfaces. In addition, the vapor-phase transport of skin oil constituents and their unsaturated ozonolysis products may contribute to double bonds on vertical and downward-facing surfaces to a degree that would be comparable to upward surface deposition from the vapor phase. Also, skin oil can be transferred directly to vertical surfaces by physical contact. However, if transfer from human contact is a major pathway by which skin oil accumulates on vertical surfaces, then the distribution of skin oil on vertical surfaces should be more broadly heterogeneous than would be the case if airborne transport dominates.Wang and Morrison100 sampled both horizontal and vertical surfaces in four occupied homes. They measured a range of aldehydes emitted because of ozone reacting with these surfaces. Decanal was among the identified products, which is noteworthy since decanal resulting from ozone-initiated surface chemistry is due almost exclusively to reactions with skin oil constituents (see Section 4.6). The mean decanal yield from upward-facing horizontal surfaces (living room carpets, kitchen floors and rugs, and kitchen countertops) was 0.013 ± 0.013. Measurements on vertical surfaces (living room walls) show low decanal yields (0.00 and 0.01 ± 0.0027) in two homes but high and highly variable yields in a third (0.12 ± 0.43). No measurement is reported for the fourth home. The elevated and variable yields on the wall of the third home are suggestive of direct touch as a skin-oil transfer mechanism. These few measurements hint that the decanal yields on vertical surfaces may be only fractionally smaller than those on horizontal surfaces. If this were to be confirmed in a larger sampling of homes, that would suggest a greater degree of influence of skin-oil-associated ozone reactivity on vertical surfaces than can be presently accounted for by partitioning of unsaturated skin oil constituents and their unsaturated products or by deposition of particles containing such skin-oil derived species. With respect to ozone removal by skin oil, even if upward surfaces had an area-normalized rate that was as much as five times higher than that on vertical surfaces, aggregate scavenging by the larger net area of vertical surfaces could still be a substantial fraction of the total, given the relative proportion of surface orientations.
4.5 Field evidence
Early evidence that skin oils influence ozone reactivity of off-body surfaces emerged from the study by Fischer et al.62 of ozone chemistry in a Swedish classroom. The investigators measured elevated concentrations of 4-OPA when the classroom had been vacant for at least an hour and speculated that ozone might be reacting with “soiled furniture, books and other objects that were handled by the pupils and teacher during the workday ….” More definitive evidence is found in Liu et al.,8 who analysed continuous measurements of ozone and ozonolysis products over an eight-week summer period in an Oakland, CA home with two occupants. Among the ozone/skin oil products monitored were 6-MHO, 4-OPA, and decanal. During normal occupancy, the measured net yield (moles of product per mole of O3 consumed) for these three species was bounded to be >6.6% (see Table 1 of Liu et al.). On average, 6-MHO, 4-OPA, and decanal have been found to constitute 35–45% of the gas-phase products resulting from ozone/skin oil chemistry.41,42 Assuming these percentages apply at the Oakland study site, we estimate that 15–19% of ozone loss by chemical reaction in the occupied home was attributable to reactions with skin oil on occupant and nonoccupant surfaces. Liu et al.8 estimated that during the initial hours of vacancy, off-body skin oil was responsible for a 6-MHO production rate that was 80% of its production rate during occupancy (see S4 in the ESI†). Assuming that off-body skin oil was responsible for 80% of the total production of gaseous products derived from ozone/skin oil chemistry, we estimate that 12–15% of reactive ozone loss during occupancy of this Oakland home was due to reactions with skin oil on nonoccupant surfaces.Analysing the same primary dataset, Zhang et al.54 characterized “off-body squalene ozonolysis on indoor surfaces” in the Oakland home using a previously developed model.68 Zhang et al.54 estimated a nonoccupant surface density of 2.7 μmol m−2 for double bonds contributed by squalene plus three polyunsaturated aldehydes produced by squalene ozonolysis (TOP, TOT, and TTT). The density of double bonds from other skin oil constituents is expected to be similar to those of squalene and unsaturated products derived from squalene. Hence, the level of double bonds from skin oil in this house would have been approximately 5.4 μmol m−2 during normal occupancy. This value is approximately five times the mean value for double bonds from all sources on vertical surfaces sampled in university buildings32 and somewhat larger than the residual level of double bonds from all sources on horizontally oriented substrates sampled in an office and a residence (see Section 2.4).34 Even recognizing likely differences in indoor ozone levels and known differences in surface orientation, each of which affects the abundance of double bonds on a surface, the comparison suggests that skin oil contributed substantially to the net level of double bonds on nonoccupant surfaces in the Oakland home.
Using continuous, real-time measurements in an occupied office, indoor ozone was associated with an average net yield for 6-MHO, 4-OPA, and decanal of 9%.87 Assuming 6-MHO, 4-OPA, and decanal constitute 35–45% of the gas-phase products resulting from ozone/skin oil chemistry, we estimate that 20–25% of ozone loss by chemical reaction in this occupied office was attributable to reactions with skin oil on occupant and nonoccupant surfaces. Wu et al.87 erroneously reported that ozonolysis of off-body skin lipids was not an important source of skin oil ozonolysis products in their study, a consequence of miscalculating the ozone loss rate to indoor surfaces, as acknowledged in personal correspondence. Our analysis of data from this study suggests that a large fraction of what Wu et al. ascribed to desorption of 6-MHO, 4-OPA, and decanal from surfaces is instead attributable to ozone reactions with skin oil on off-body surfaces (see S5 in the ESI†).
These field studies are the only direct published evidence that skin oil on nonoccupant surfaces consumes indoor ozone.
4.6 Estimates based on decanal yields
Nominally, an acyl group is an entity that results when an “OH” is removed from an oxoacid. An acyl group that contains a double bond located ten carbon atoms from the terminal methyl group can be described as an ω-10 acyl group (i.e., CH3(CH2)8CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–). Decanal is a major product when O3 reacts with the double bond in an ω-10 acyl group. The three most abundant unsaturated fatty acids in human skin surface lipids contain ω-10 acyl groups. These are cis-hexadec-6-enoic acid (sapienic acid, Fig. 2), 22% of the total fatty acids by weight; cis-octadec-8-enoic acid, 9%; and cis-15-methylpentadec-6-enoic acid, 4%.10 Together these three decanal precursors constitute 35% of the fatty acids in skin-surface lipids.
CH–). Decanal is a major product when O3 reacts with the double bond in an ω-10 acyl group. The three most abundant unsaturated fatty acids in human skin surface lipids contain ω-10 acyl groups. These are cis-hexadec-6-enoic acid (sapienic acid, Fig. 2), 22% of the total fatty acids by weight; cis-octadec-8-enoic acid, 9%; and cis-15-methylpentadec-6-enoic acid, 4%.10 Together these three decanal precursors constitute 35% of the fatty acids in skin-surface lipids.
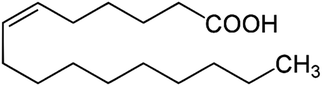 | ||
| Fig. 2 Sapienic acid (cis-hexadec-6-enoic acid, C16H30O2) is the most abundant unsaturated fatty acid in human skin oil (20–25% of fatty acids and 5–6% of skin oil).10 | ||
Other decanal precursors in skin oil include octadeca-5,8-dienoic acid (sebaleic acid), 1% of the total fatty acids, and the most common polyunsaturated lipid in skin oil; cis-eicos-10-enoic acid, 0.5%; and cis-eicos-7,10-dienoic acid, 0.5%. The acyl groups present in the fatty acids that are decanal precursors are also present in the triglycerides, diglycerides, monoglycerides, and wax esters that occur in skin oil. Table 3 summarizes the decanal precursors in skin oil and lists their molar fraction of total unsaturations, assuming that the distribution of acyl groups in wax esters and tri-, di-, and monoglycerides is like that in free fatty acids.12 Altogether, on a molar basis, the decanal precursors constitute about a third of the total –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds in skin-surface lipids. Squalene, in comparison, constitutes close to half (45%) of the total –C
C– bonds in skin-surface lipids. Squalene, in comparison, constitutes close to half (45%) of the total –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bonds.
C– bonds.
| Fatty acid, wax esters, and tri-, di-, and monoglycerides containing the following acyl groupsa | Molecular weight of acyl group (g mol−1) | Molar fraction of unsaturationsb |
|---|---|---|
| a An acyl group is formed when an “OH” is removed from an oxoacid. b See Tables 1 and 4 of Nicolaides10 and Table S1 in Pandrangi and Morrison.12 | ||
| Decanal precursors | ||
cis-Hexadec-6-enoyl: CH3(CH2)8C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)4C(O)– C(CH2)4C(O)– |
237.4 | 0.20 |
cis-Octadec-8-enoyl: CH3(CH2)8C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)6C(O)– C(CH2)6C(O)– |
265.5 | 0.074 |
cis-Methylpentadec-6-enoyl: CH3(CH2)8C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)C C(CH2)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)3C(O)– C(CH2)3C(O)– |
237.4 | 0.038 |
Octadeca-5,8-dienoyl: CH3(CH2)8C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)C C(CH2)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)3C(O)– C(CH2)3C(O)– |
263.4 | 0.019 |
cis-Eicos-10-enoyl: CH3(CH2)8C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)8C(O)– C(CH2)8C(O)– |
293.5 | 0.004 |
cis-Eicos-7,10-dienoyl: CH3(CH2)8C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)C C(CH2)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)5C(O)– C(CH2)5C(O)– |
291.5 | 0.008 |
| Total | 0.34 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Nonanal precursors | ||
cis-Octadec-9-enoyl: CH3(CH2)7C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)7C(O)– C(CH2)7C(O)– |
265.5 | 0.016 |
cis-Heptadec-8-enoyl: CH3(CH2)7C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2)6C(O)– C(CH2)6C(O)– |
251.4 | 0.008 |
| Total | 0.024 | |
Remarkably, the occurrence of sapienic acid, which by itself is responsible for 20% of the double bonds in skin oil, is otherwise relatively rare in nature. This feature was called out a half-century ago by Nicolaides.10 More recently, Prouty and Pappas101 have reiterated this point: “Unique among the complexity of sebaceous lipids is sapienic acid, a 16-carbon monounsaturated fatty acid with an extremely rare position of the double bond, located between carbons 6 and 7 from the carboxyl terminal. Human sebum is the only documented location in the animal kingdom where sapienic acid is abundant and naturally occurring.”
Available evidence from the literature suggests that skin oil is the chief substance commonly found indoors whose ozonolysis generates decanal. Sapienic acid and other decanal precursors have a negligible presence in cooking oils.102,103 In contrast, oleic acid (octadec-9-enoic acid), linoleic acid (octadeca-9,12-dienoic acid), and palmitoleic acid (exadic-9-enoic acid), which react with ozone to produce nonanal, hexanal, and heptanal, respectively, are present in many cooking oils. Decanal precursors are produced in insufficient amounts to be included in various chemical production inventories, whereas oleic acid had an aggregate production volume of 100–200 million pounds in 2016.104 There are reports in the literature of decanal resulting from the ozonolysis of carpets,105,106 but such findings could conceivably reflect inadvertent contributions from skin oil ozonolysis. Fruekilde et al.35 have shown that the “accidental touching of material which later comes into contact with ozone can lead to strong artifact formation” including decanal. In what follows, the emission of decanal when ozone reacts with indoor surfaces has been used as a quantitative indicator of the abundance of skin oils on these surfaces. The reader is cautioned that these findings depend on the assumption that skin oil constituents are the dominant source of decanal when ozone reacts with an indoor surface.
Decanal from the ozonolysis of human skin oil was first reported by Fruekilde et al.35 In that study, the investigators rubbed glass wool between fingers and then exposed it to 100 ppb ozone in a flow reactor. After 10 minutes, the accumulated products, based on Tenax sampling, included 500 ng of decanal, 900 ng of 6-MHO, and 1000 ng of geranyl acetone. Decanal has subsequently been identified as a skin oil ozonation product in numerous studies (see Section 2.4). It is noteworthy that decanal has been reported as a product when samples of indoor floor dust from office buildings, schools, and homes were exposed to ozone.107
The recent study of ozone chemistry in an occupied home8 included plots of the source strength of an ion (C8H15O+) characteristic of 6-MHO as a function of indoor ozone concentration and occupancy. Those images are reproduced as Fig. 3A and B in the present paper. Also displayed (Fig. 3C) is a plot of this 6-MHO ion as a function of time during an unoccupied period when the indoor ozone concentration was between 2 and 4 ppb.
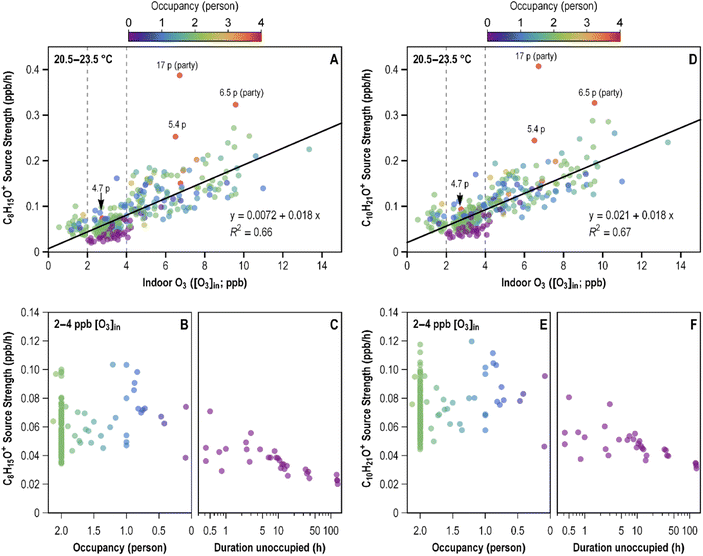 | ||
| Fig. 3 Source strengths of 6-MHO and decanal in an occupied home. Source strength of (A) 6-MHO ion and (D) decanal ion as a function of indoor ozone concentration. Source strength of (B) 6-MHO ion and (E) decanal ion as a function of occupancy when indoor ozone was in the range 2–4 ppb. Source strength of (C) 6-MHO ion and (F) decanal ion as a function of duration that the home was unoccupied. The 6-MHO plots are reproduced from Liu et al.8 The decanal plots, not previously published, were prepared from the same dataset by Y Liu (personal communication). | ||
Analogous plots are newly presented here for an ion characteristic of decanal (C10H21O+) (Fig. 3D–F). The plots for 6-MHO and decanal are clearly similar. As shown in Fig. 3A and D, the source strength of both the 6-MHO ion and the decanal ion is linearly correlated with the indoor ozone concentration, and the intercepts for both the 6-MHO ion and the decanal ion are close to zero, consistent with ozone chemistry being the dominant source for both species. Furthermore, for both 6-MHO and decanal, the source strength increases when the number of occupants increases (orange markers), reinforcing the inference that skin oil ozonolysis is a source of both 6-MHO and decanal.
Fig. 3C and F reveal that, during a five-day unoccupied period, the source strength of both 6-MHO and decanal decay by roughly half; the source strength of 6-MHO decreased from 0.045 to 0.023 ppb h−1 and that of decanal decreased from 0.088 to 0.043 ppb h−1. Taken together, the data displayed in these figures indicate that ozonolysis of skin oil constituents, with negligible contributions from other species, is the major pathway producing decanal (and 6-MHO) in the Oakland home.
For what follows, we define the decanal yield when ozone reacts with an indoor surface as moles of volatilized decanal produced per mole of ozone consumed. The yield of decanal depends on the amounts of other ozone-reactive compounds on a surface. If all else is equal, the yield of decanal will decrease as the fraction of ozone-reactive compounds on a surface from sources other than skin oil increases. Hence, when ozone reacts with room surfaces, the decanal yield can be used to estimate the fraction of ozone removed by skin oil on that surface. The fraction of O3 removed by skin oil on an inanimate surface (O3-Lossskin_oil_off-body) is approximately the ratio of the decanal yield when ozone reacts with that surface (DecYieldoff-body) to the decanal yield when ozone reacts with the surface of human skin (DecYieldon-body).
| O3-lossskin_oil_off-body = DecYieldoff-body/DecYieldon-body | (2) |
Decanal is especially well suited for estimating the fraction of ozone removed by skin oil on off-body surfaces. Its yields are less sensitive to environmental conditions than is the case for secondary reaction products such as 6-MHO or 4-OPA. Decanal's “effective yield” and “surface yield” are almost identical. Unlike 6-MHO, decanal is a terminal product; it does not react with ozone. Table 4 presents estimates of decanal yields resulting from ozone reactions with on-body and off-body surfaces as reported in various studies. Further discussion of the underlying studies is presented in S6 in the ESI.† These yields have been calculated disregarding sorptive uptake of decanal to surfaces. That is a reasonable approximation given that log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koa = 5.5 and log
Koa = 5.5 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kwa = 1.5 for decanal at 25 °C (as calculated using SPARC). The moderate octanol-air (Koa) and low water–air (Kwa) partition coefficients would respectively limit the partitioning of decanal to nonpolar and polar sorptive reservoirs.94
Kwa = 1.5 for decanal at 25 °C (as calculated using SPARC). The moderate octanol-air (Koa) and low water–air (Kwa) partition coefficients would respectively limit the partitioning of decanal to nonpolar and polar sorptive reservoirs.94
| Decanal yield | Measurement location and method | Reference |
|---|---|---|
| a Initial yield. b Aggregate mean and median across all surfaces reported for four houses (N = 13). c Mean yields measured over three seasons in five homes. See Fig. 3 in cited publication. | ||
| Hair, skin surfaces, skin oil on glass wool | ||
| 0.072 ± 0.029 | Unwashed human hair in a flow reactor (N = 6); Tenax-TA sample tubes analysed using a thermal desorber followed by GC-MS | 12 |
| Forearm: 0.051a | (i) Ozone passing through a flow reactor placed on the forearm or forehead; (ii) ozone passing through a tube containing glass wool soiled with skin oil; PTR-MS detection | 6 |
| Forehead: 0.051a | ||
| Skin oil on glass wool: 0.060a | ||
| Mean: 0.016; interquartile range: 0.012–0.020 | Ozone passing through a flow reactor placed on the forearm; Vocus PTR-ToF-MS detection | 41 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Occupied chambers | ||
| Mean: 0.025; range: 0.016–0.036 | Simulated aircraft cabin with 16 occupants | 37 |
| 0.063 | Chamber (simulated office) with two occupants | 6 |
| 30% RH: 0.012; 70% RH: 0.029 | Chamber experiments with four occupants; PTR-ToF-MS | 42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Isolated room surfaces | ||
| Living room carpet, kitchen floor, kitchen counter, kitchen rug: mean ± standard deviation = 0.013 ± 0.013; median and IQR = 0.01 and 0.00–0.01b | Horizontal off-body surfaces in the living room and kitchen in three houses and one apartment; measured during summer; Tenax sorption and GC-FID detection | 100 |
| Bedroom carpet: 0.015c; living room carpet: 0.016c; kitchen floor: 0.022c; kitchen counter: 0.018c | Horizontal off-body surfaces in five homes over two summers and one winter; Tenax sorbent sampling with GC-MS analysis | 108 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| All room surfaces | ||
| ≥0.018 | Off-body surfaces in a normally occupied home at the beginning of an unoccupied period; PTR-ToF-MS | 8 |
| 0.0071 ± 0.0023 | Eleven days monitored with different fractions of recirculated air; off-body surfaces in occupied office at the beginning of the unoccupied period; PTR-MS | 87 |
Table 5 lists estimates for the fraction of ozone removed by reactions with skin oil on off-body surfaces. These estimates have been calculated using eqn (2), yields for ozone reacting with various off-body surfaces (DecYieldoff-body) from the lower section of Table 4, and assuming the decanal yield when ozone reacts with human surfaces (DecYieldon-body) is 0.06. We have chosen the value 0.06 since it is close to the yields from the two studies in Table 4 that used authentic decanal standards for calibration,6,12 coupled with the fact that potential fragmentation of decanal during PTR-MS analysis appears to be less likely in the Wisthaler and Weschler study. With a smaller value for DecYieldon-body, as reported in Morrison et al.41 or Wang et al.,42 then the calculated ozone loss due to skin oil on off-body surfaces is correspondingly larger. Note that it is reasonable to expect that off-body ozone consumption by skin oils would vary among indoor environments with ozone levels (more ozone would correspond to less skin-oil-induced consumption) and with the history of occupancy (higher occupant density would be associated with more skin-oil-induced consumption).
| Fraction removed by skin oil | Measurement location | Reference |
|---|---|---|
| a Aggregate mean ± standard deviation across all surfaces reported for four houses (N = 13). b Based on mean yields measured over three seasons in five homes. See Fig. 3 in cited publication. | ||
| Isolated room surfaces | ||
| Living room carpet, kitchen floor, kitchen counter, kitchen rug: 0.22 ± 0.22a | Horizontal off-body surfaces in the living room and kitchen in three houses and one apartment; vertical surface (wall) in one house; measured during summer | 100 |
| Bedroom carpet: 0.25b; living room carpet: 0.27b; kitchen floor: 0.37b; kitchen counter: 0.30b | Horizontal off-body surfaces in five homes over two summers and one winter | 108 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| All room surfaces | ||
| ≥ 0.30 | Off-body surfaces in a normally occupied home at the beginning of an unoccupied period | 8 |
| 0.12 ± 0.04 | Ten days with different fractions of recirculated air; off-body surfaces in an occupied office at the beginning of the unoccupied period | 87 |
The estimates in Table 5, coupled with the field studies discussed in Section 4.5, suggest that skin oil constituents and their unsaturated products are important sinks for ozone on off-body surfaces in occupied environments. However, we caution that the scope of these investigations is modest. Specifically, measurements on isolated room surfaces have been conducted in just five homes, and the field measurements that pertain to all surfaces have been made in one occupied residence and one occupied office.
5 Synthesizing the evidence
5.1 Method
We define kh-all as the first-order loss-rate coefficient attributable to people, obtained by multiplying the number of occupants per volume of the indoor space (N/V) by the effective volumetric removal rate for ozone per person:| kh-all = (N/V) × Qh | (3) |
First-order loss rate coefficients for total ozone removal by reactions on inanimate upward-facing indoor surfaces (kd-up) are obtained as the product of a deposition velocity (vd-up) and the surface-to-volume ratio of the surfaces in the indoor environment (Aup/V):
| kd-up = vd-up × Aup/V | (4) |
Similarly, the first-order loss rate coefficient for ozone removal by reactions on other nonoccupant surfaces (kd-other) is given by:
| kd-other = vd-other × Aother/V | (5) |
First-order loss rate coefficients for total ozone removal by reactions on all indoor surfaces (ksum) can then be apportioned as reactions on occupant surfaces (kh-all) and off-body surfaces (kd-up + kd-other):
| ksum = kh-all + kd-up + kd-other | (6) |
The fraction of ozone removed by reactions on these different surfaces is evaluated with the following equations:
| fh = kh-all/ksum | (7) |
| fup = (vd-up × Aup/V)/ksum | (8) |
| fother = (vd-other × Aother/V)/ksum | (9) |
For each of these surface categories, a fraction of the ozone loss is attributable to reactions with skin oils. Those fractions are denoted as SOh, SOup, and SOother for human surfaces, upward-facing inanimate surfaces, and other indoor surfaces, respectively. Given estimates for these skin-oil reaction fractions, combined with the loss apportionments defined in eqn (7)–(9), one can estimate the aggregate proportion of ozone loss on indoor surfaces that is attributable to reactions with skin oils:
| SOsum = SOh × fh + SOup × fup + SOother × fother | (10) |
5.2 Illustrative calculations of indoor ozone loss attributable to skin oil
In the numerical estimates that follow, we assume that ozone's deposition velocity to upward horizontal surfaces (vd-up) is 1.4 m h−1,24 and to all other nonoccupant surfaces (vd-other) it is 0.7 m h−1.4 We assume that upward horizontal surface area is 20% of total nonoccupant surface area. We assume that the volumetric removal rate of ozone on occupant surfaces is Qh = 27 m3 h−1 (which corresponds to a deposition velocity of 15 m h−1 and an occupant surface area of 1.8 m2; see Table 1 and Nazaroff and Weschler).4 For occupants, we assume that SOh is in the range 0.5–1, allowing that clothing fibres and other chemicals sorbed to clothing contribute some of the ozone reactivity on occupant surfaces (see Section 3.2). Based on evidence summarized in Section 4.6, we estimate that SOup is in the range 0.1–0.35, whereas SOother probably lies within 0.02–0.1. In the calculations that follow, we will use values of SOh = 0.75, SOup = 0.25, and SOother = 0.05. We emphasize that these are not suggested as “best estimates” but rather as reasonable values for illustrative calculations.Table 6 reports the proportion of indoor ozone lost to skin oils on occupant and nonoccupant surfaces for various indoor environments. It also lists representative air-change rates (λ) for the various indoor environments, based on measurements for residences110 or derived from minimum ventilation rates for offices and classrooms as listed in ANSI/ASHRAE Standard 62.1.111 Given the air-change rate and the rate constant for net O3 removal on indoor surfaces (ksum), the ratio of the concentrations of indoor and outdoor ozone can be estimated; the time-averaged I/O ratio for ozone is well approximated as λ × (λ + ksum)−1. The results presented in Table 6 support the discussion that follows.
| Indoor environment | Number of occupants (volume) | Percent O3 loss to skin oil on occupantsb | Percent O3 loss to skin oil on inanimate surfacesc | Percent O3 loss to skin oil on allindoor surfaces | Total rate constant for O3 removal on surfaces (ksum) | Air-change rate (λ)d | Ozone I/O ratio |
|---|---|---|---|---|---|---|---|
| a Relative to total ozone removed by indoor surfaces, percent lost to skin oil on occupant surfaces, to skin oil on nonoccupant surfaces, and the sum (i.e., to skin oil on all indoor surfaces). Results are for occupied conditions. b Calculated assuming 75% of ozone loss on occupant surface is due to skin oil and Qh = 27 m3 h−1 (adults) or 15 m3 h−1 (children aged 5–8 years). c Estimated assuming (Aup + Aother)/V = 3.5 m2 m−3,109vd-up = 1.4 m h−1, vd-other = 0.7 m h−1, SOup = 25%, SOother = 5%. d Air-change rate for the residence is the geometric mean from Nazaroff;110 the air-change rates for the office and classrooms are derived from the default minimum ventilation rates listed in Table 6.1 of ANSI/ASHRAE Standard 62.1.111 e Occupancy and volumes are means for US houses and apartments.112 f Representative occupant densities taken from Table 6.1 of ANSI/ASHRAE Standard 62.1 (ref. 111) for offices and various classrooms with 100 m2 floor area; 2.7 m ceiling height assumed. | |||||||
| US residencee | 2.6 (485 m3 ) | 4% | 11% | 15% | 3.1 h−1 | 0.5 h−1 | 0.14 |
| Officef | 5 (270 m3) | 11% | 10% | 21% | 3.4 h−1 | 0.6 h−1 | 0.15 |
| Classroom, 5–8 yearsf | 25 (270 m3) | 25% | 8% | 32% | 4.4 h−1 | 2.5 h−1 | 0.36 |
| Classroom, high schoolf | 35 (270 m3) | 41% | 5% | 46% | 6.4 h−1 | 3.1 h−1 | 0.32 |
| Lecture roomf | 65 (270 m3) | 52% | 4% | 55% | 9.4 h−1 | 3.7 h−1 | 0.28 |
In Section 3.1, we suggested that the surface of a single occupant in a 30 m3 room could be responsible for approximately 1/3 of ozone's loss indoors via chemical reactions. We also noted that this result depended on occupant density. Table 6 reports estimates for the percentage of ozone surface removal resulting from reactions with skin oil constituents on occupants' surfaces in common indoor environments with varying occupant densities. In the US, residences tend to have low occupant densities. In the illustrative calculations for an average residence, 11% of the reactive ozone loss occurs on skin oil on inanimate surfaces, with an additional 4% of the overall ozone loss attributable to skin oil on occupants. In a 270 m3-office with five workers, the relative amount of ozone loss to skin oil on inanimate surfaces (10%) is like the result for residences, whereas the estimated loss on occupants (11%) is three times larger. In 270 m3 classrooms, with much larger occupant densities, ozone loss attributable to skin oil occurs primarily on occupants, with proportions ranging from 25% of total reactive loss for a classroom with 25 children to 52% for a lecture room with 65 young adults. For these classrooms, ozone loss to skin oil on inanimate surfaces contributes an additional 4–8%.
In the Oakland home discussed previously, ozone removal by skin oil on non-occupant surfaces was about four times larger than removal by skin oil on occupant surfaces.8 In the U.S. residence entry in Table 6, the ratio of skin oil removal on off-body to on-body surfaces is similar – about a factor of three. That comparison is not circular. The percent ozone lost to skin oil on occupant surfaces depends primarily on the deposition velocity to the human envelope and occupant density in the residence, while the percent ozone lost on nonoccupant surfaces depends primarily on parameter values derived from Wang and Morrison100,108 coupled with the ratio of “net body surface area to total inanimate surface area”.
We have used the yield of decanal associated with ozone-driven surface chemistry to estimate the fraction of ozone loss to skin oil on indoor surfaces. While there are indoor sources of decanal other than ozone/skin oil chemistry (e.g., certain essential oils, perfumes, and flavorings), ozonolysis of skin oil may be the major source of decanal in indoor residences. A simple exercise tests the likelihood of this conjecture. Consider a residence in Toronto with an average indoor ozone concentration of 4 ppb.113 Assuming an average air-change rate110 of 0.5 h−1 and an indoor/outdoor ozone concentration ratio of 0.14 (Table 6), then the outdoor ozone concentration would be 29 ppb, and the difference between outdoor and indoor ozone concentration (ozone loss) would be 25 ppb. The fractional ozone loss to skin oil on all indoor surfaces is estimated to be 15% (Table 6), and the decanal yield for ozone reacting with skin oil is approximately 6%.6 Consequently, the indoor concentration of decanal attributable to ozone/skin oil chemistry would be 0.23 ppb (25 ppb × 15% × 6%). At 293 K, 1 ppb equals 6.5 μg m−3 of decanal. Hence, in this hypothetical Toronto residence, the concentration of decanal resulting from ozone/skin oil chemistry is estimated to be 1.5 μg m−3. This value is remarkably close to the measured arithmetic mean concentration of decanal reported for over 3000 Canadian homes by Zhu et al.114 of 1.45 μg m−3, suggesting that ozonolysis is, indeed, a major source of decanal in homes. That inference is reinforced by decanal concentration data from other large-scale surveys of volatile organic compounds in residences conducted in Canada, Japan, and Germany during the years 2012–2017. (See Table S1† in the ESI.†).
The illustrative calculations summarized in Table 6 suggest that, in indoor settings with low occupant densities, skin oil on occupants is responsible for perhaps 15% of total ozone loss via chemical reactions on indoor surfaces. Conversely, in densely occupied settings, skin oil on occupants is responsible for a much larger fraction of ozone loss. Skin oil on inanimate surfaces influences ozone loss in residences and offices more than in densely occupied settings, such as classrooms. These estimates have been calculated using the same values for the fraction of ozone removed by skin oil on off-body surfaces in residences, offices, and classrooms. However, it is reasonable to expect that skin oil soiling on classroom surfaces might be greater than in residences because of the higher occupant densities in classrooms. If true, then ozone loss to skin oil on off-body surfaces in classrooms would be larger than the estimates shown.
Ten parameters were used for the estimates presented in Table 6. The values for seven of these are known well or moderately well: N, N/V, vh, Ah, vd-up, vd-other, and Aup/Aother. The values for the three terms representing the fractional loss on different surfaces attributable to skin oil have large associated uncertainties: SOh, SOup, and SOother. This comparison illustrates a broader point. Experimental evidence from a small number of homes has been used to inform skin-oil-associated ozone loss on inanimate surfaces in residences as well as non-residences. Likewise, although evidence supports the inference that most ozone loss on occupants is attributable to skin oils, the actual proportion isn't well established. Consequently, the values in Table 6 should be applied with caution.
The rate constant for net ozone removal on indoor surfaces, ksum, increases with increasing occupant density. Table 6 provides a sense of this parameter's influence. If the air-change rate were the same in each of the hypothetical settings, the I/O ratio for ozone would vary inversely with occupant density. However, the minimum ventilation rate recommended by various standards and guidelines also increases with occupant density. If these guidelines are followed, then the increase in ventilation rate can be large enough to offset the increase in the net rate of ozone removal on surfaces. For conditions in Table 6, the predicted I/O ratios for ozone in residences and in offices are in the vicinity of 0.15, whereas for more densely occupied classrooms, the estimated ratio is 0.3–0.4. These I/O ratios are generally consistent with empirical evidence for these types of indoor environments.4
The calculated values in Table 6 are for periods when the respective indoor environment is occupied. When occupants leave, in the initial period of vacancy, ozone removal by skin oil would persist on inanimate indoor surfaces. As the period of vacancy increases, ozone removal by skin oil on surfaces would decline. In a matter of days to weeks, depending on the indoor ozone concentration and the amount of initially unreacted skin oil on surfaces, skin oil would cease to make a meaningful contribution to ozone removal indoors.
The ozone loss values in Table 6 may seem surprisingly large, but they reflect the fact that skin oil is particularly effective at reacting with ozone. The number of double bonds per mass of skin oil tends to be larger than the number of double bonds per mass of cooking oil or other non-human derived organic matter that soils indoor surfaces. Skin oils are also relatively “fresh” compared to gaseous and particle-phase organics that originated outdoors and have partially reacted with ozone prior to entering and depositing on an indoor surface. Considering common indoor sources of unsaturated organics, substantially more cooking oil or cleaning product residue would need to accumulate on an indoor surface to remove the same quantity of ozone as is removed by a specific amount of fresh skin oil.
6 Other considerations
Ozone removal through reaction with human skin oil has two contrasting outcomes. On the one hand, it reduces indoor ozone concentrations and, hence, exposure to ozone. On the other hand, it increases exposure to products derived from ozone-initiated indoor chemistry. There are reasons to believe that inhalation of both ozone and the by-products of ozone-initiated chemistry have adverse health consequences.115 The health endpoints affected by ozone exposure may be different from those affected by exposure to products of ozone chemistry. Ozone is less likely to penetrate deep into the lung116 than are many of the products of ozone/skin oil chemistry (e.g., SOA, 4-OPA, decanal, and organic peroxides), suggesting that ozone adversely affects the upper airways while certain oxidation products are a greater threat to the deep lung. A recent epidemiological study found that biomarkers indicative of oxidative stress in the nasal cavity were associated with ozone exposure while biomarkers indicative of pulmonary and systemic oxidative stress were associated with exposure to ozone oxidation products.117When ozone-initiated reactions occur on body surfaces, the products are generated within a rising thermal plume that can bring reaction products close to the breathing zone.76,118 The combined effects of ozone/skin oil chemistry and near-occupant airflow conditions can lead to the chemical composition of inhaled air being somewhat different from the room average condition, with diminished ozone concentrations and correspondingly increased concentrations of reaction by-products in the breathing zone.
Information about the identity and emission rates for stable products that result from ozone/skin oil chemistry has increased substantially in the past decade.40,42,119 Much less is known about the various short-lived products derived from such chemistry, including stabilized Criegee intermediates, hydro- and methyl peroxy radicals, other organic peroxy radicals, and secondary ozonides.48,49,120–123 Whether considering stable products or short-lived products, the toxicity of most of these compounds is poorly known.119 Given the routine inhalation of such products in all occupied environments, they should be targeted for more thorough toxicity evaluations.
In a manner not widely recognized, exposures to ozone and to the by-products of ozone reactions with skin oils can be materially influenced by the occupancy level and ventilation rates in buildings, two parameters that may themselves be coupled. Increasing occupant density while holding the air-change rate constant would reduce ozone exposure and increase by-product exposures. Increasing the air-change rate while holding occupancy constant would increase indoor ozone exposure and reduce exposure to ozone/skin oil by-products. Increasing air-change rates while holding occupancy constant and removing ozone from the ventilation air with activated carbon filters124–126 would decrease exposure to both indoor ozone and to the by-products of ozone-initiated chemistry.
Recent research reveals that ozone reactions with skin oils can influence indoor air chemistry more broadly than detailed in this review. For example, the reaction of ozone with skin oil influences the total hydroxy radical (OH) reactivity of human emissions.127,128 In experiments with ozone absent from chamber air, isoprene from the breath of four occupants was the major OH-consuming chemical. In contrast, with ozone present (35 ppb), but otherwise identical chamber conditions, the total OH reactivity was twice as large. Carbonyl products derived from ozone/skin oil chemistry contributed roughly 60% to total OH consumption.128
Although the rate of OH removal increases when ozone is introduced to an occupied indoor environment, ozone/skin oil chemistry increases the rate of OH production even more. In some of the same chamber studies used to measure total OH reactivity, Zannoni et al.118 reported increased OH concentrations when people were exposed to ozone versus when ozone was absent. Key to the elevated levels of hydroxy radicals in these experiments was 6-MHO, which is both a sink for OH by reacting directly with it and a source of OH via its reactions with ozone. The net increase in OH concentration, initiated by ozone reactions on human surfaces, is also expected to occur when ozone reacts with skin oil on off-body surfaces. This inference is consequential since ozone principally oxidizes organic compounds that have unsaturated carbon–carbon bonds, whereas the hydroxyl radical oxidizes almost all types of organic compounds. In other words, the net production of OH radicals via ozone reactions with skin oil results in the indoor oxidation of a much broader array of organic compounds than are oxidized by ozone alone. In a similar fashion, Criegee intermediates generated by ozone/skin oil chemistry may serve as less discriminating indoor oxidants than ozone itself. Palmitic acid, the most common saturated acid in skin oil,10 contains no double bonds and does not react directly with ozone. Nonetheless, Zhou et al.51,52 have reported that its concentration decreased by more than 60% when skin oil was exposed to gaseous ozone. They speculated that the consumption of palmitic acid may be due to its reaction with Criegee intermediates to form secondary ozonides,129 consistent with the known reaction between Criegee intermediates and carboxylic acids.
The focus of this review has been ozone removal through reaction with human skin oil. The extent to which other indoor and outdoor sources are responsible for ozone-reactive chemicals on indoor surfaces remains to be examined in a quantitative fashion. Double bonds may be inherent to wood used in flooring, panelling, and furnishings, as appears to be the case for certain floor and wallcoverings (e.g., carpets, floor waxes, and vinyl wallpaper). Double bonds also occur in specific SVOCs and particles that soil indoor surfaces. SVOCs with double bonds originate in cleaning products and cooking oils, while particles whose constituents contain double bonds result from cooking and smoking, among other sources. The relative contribution of these other sources to ozone-reactive compounds on indoor surfaces is poorly characterized and warrants further study.
7 Conclusions
A better understanding of the reactive chemicals that attenuate indoor ozone concentrations can improve strategies designed to protect the public from air pollution today and in a warmer future that may have even higher outdoor ozone concentrations. Indoor ozone levels are substantially lower than outdoor levels primarily because of chemical reactions on indoor surfaces. In central tendency, indoor ozone concentrations for residences are about a quarter of outdoor concentrations.4 The larger the difference between indoor and outdoor ozone concentrations, the larger the indoor concentration of the resulting gas-phase products. During occupied periods, evidence suggests that reactions with skin oil are responsible for a large proportion of total indoor ozone loss. Such reactions occur not only on the body envelopes of occupants, but also, because of skin-oil transfer mechanisms, on inanimate indoor surfaces.The proportion of indoor ozone that is removed by reactions with skin oils varies with occupant density. Even in residences, with relatively low occupant densities, the fraction of ozone removed by reactions with skin oil appears to be greater than 10%. In densely occupied indoor environments (e.g., classrooms, aircraft cabins, and call centres), 30–55% of ozone loss may be due to human skin oil. The evidence supporting the extent of on-body ozone loss is stronger than that for off-body ozone loss. It appears that skin oil on vertical surfaces makes a somewhat smaller contribution to ozone removal indoors than does skin oil on upward-facing horizontal surfaces. However, this inference is based on only a few measurements in a small number of homes. When occupants leave an indoor environment, skin oil constituents on off-body surfaces are gradually consumed, with lifetimes varying between hours and weeks depending on the relative scale of indoor ozone concentration in relation to the degree of surface soiling by skin oils and their reactive products. Knowledge of the proportion of ozone removed by skin oil on indoor surfaces can aid us in understanding the potential consequences of ozone reactive loss following its transport indoors.
Future research investigating human skin oil as an indoor ozone sink might address several areas with large uncertainties. To better refine estimates of ozone consumed by skin oil on inanimate indoor surfaces, more sampling is needed of skin oil constituents and their oxidation products on surfaces in a variety of indoor environments with different occupant densities. Wipes could be taken from surfaces of all orientations and analysed for both skin oil constituents and ozone-reactive products (e.g., squalene, sapienic acid, pyroglutamic acid, TOP, TOT, and TTT). Different skin oil constituents react with ozone at different rates or not at all. Rate constants for ozone reactions with the more abundant unsaturated skin oil constituents or products, including unsaturated triglycerides and wax esters, are needed. It would be valuable to know how the distribution of skin oil constituents on off-body surfaces changes from that on a human body. Multiple wipes from a vertical surface would provide a picture of the heterogeneity of skin oil on that surface and the relative importance of contact transfer versus airborne transport of skin oil constituents. It would be informative to conduct studies analogous to those executed by Gall and Rim,34 but with vertical as well as horizontally oriented substrates and targeted measurements of skin oil constituents and their oxidation products on the substrates at regular time intervals over a period spanning months. Such studies might be complemented by experiments like those of Wang and Morrison,100,108 in which air containing ozone would be passed over different vertical and horizontal room surfaces and into a highly sensitive, time-resolved monitoring instrument. Use of authentic standards for decanal, 6-MHO, 4-OPA, 1,4-butanedial, and other key oxidation products would increase the reliability of such measurements.
There are numerous additional questions to address in future research: What is the fractional loss of ozone to skin oil (SOh) on typically clothed residents, office workers, and students? How do room temperature, surface temperature, ventilation rate, and indoor ozone concentration influence the accumulation of skin oil on off-body surfaces? How do levels of skin oil on off-body surfaces vary with occupant density (e.g., a residence compared to a classroom)? How does the frequency of cleaning affect these levels? What are the gas-phase concentrations of sapienic acid and other unsaturated fatty acids in densely occupied environments (e.g., classrooms)? During unoccupied conditions, what is the lifetime of squalene, sapienic acid, and other skin oil constituents on off-body surfaces? During occupied periods, what is the rate at which off-body surfaces are recharged with skin oil? To what extent do reservoirs of skin oil accumulate on indoor surfaces? Widespread utilization of sensitive, time-resolved monitoring equipment in actual indoor environments during occupied and unoccupied conditions promises to reduce uncertainty regarding the occurrence, nature, and significance of ozone/skin oil chemistry on occupant and non-occupant surfaces. In effect, the future research possibilities enumerated above illustrate the limitations of the present review and highlight areas where additional information is needed to firm up conclusions suggested by the results reported in Table 6. Such research is warranted. If there is ozone present in an occupied indoor environment, inhalation of the products of ozone/skin oil chemistry is inescapable.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Glenn Morrison for valuable discussions regarding several items in this study. We are grateful to Yingjun Liu for preparing Fig. 3D–F, including the relevant data analysis. CJW acknowledges the support of NIH-NIEHS P30 ES005022 during the preparation of this manuscript.References
- F. H. Shair and K. L. Heitner, Theoretical Model for Relating Indoor Pollutant Concentrations to Those Outside, Environ. Sci. Technol., 1974, 8, 444–451 CrossRef CAS.
- C. J. Weschler, Ozone in Indoor Environments: Concentration and Chemistry, Indoor Air, 2000, 10, 269–288 CrossRef CAS PubMed.
- M. Kruza, A. C. Lewis, G. C. Morrison and N. Carslaw, Impact of surface ozone interactions on indoor air chemistry: a modeling study, Indoor Air, 2017, 27, 1001–1011 CrossRef CAS PubMed.
- W. W. Nazaroff and C. J. Weschler, Indoor ozone: concentrations and influencing factors, Indoor Air, 2022, 32, e12942 CAS.
- M. Tang, J. A. Siegel, R. L. Corsi and A. Novoselac, Evaluation of ozone removal devices applied in ventilation systems, Build. Environ., 2022, 225, 109582 CrossRef.
- A. Wisthaler and C. J. Weschler, Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 6568–6575 CrossRef CAS PubMed.
- M. von Domaros, P. S. J. Lakey, M. Shiraiwa and D. J. Tobias, Multiscale Modeling of Human Skin Oil-Induced Indoor Air Chemistry: Combining Kinetic Models and Molecular Dynamics, J. Phys. Chem. B, 2020, 124, 3836–3843 CrossRef CAS PubMed.
- Y. Liu, P. K. Misztal, C. Arata, C. J. Weschler, W. W. Nazaroff and A. H. Goldstein, Observing ozone chemistry in an occupied residence, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2018140118 CrossRef CAS PubMed.
- C. J. Weschler, Roles of the human occupant in indoor chemistry, Indoor Air, 2016, 26, 6–24 CrossRef CAS PubMed.
- N. Nicolaides, Skin Lipids: Their Biochemical Uniqueness, Science, 1974, 186, 19–26 CrossRef CAS PubMed.
- D. T. Downing and J. S. Strauss, Synthesis and Composition of Surface Lipids of Human Skin, J. Invest. Dermatol., 1974, 62, 228–244 CrossRef CAS.
- L. S. Pandrangi and G. C. Morrison, Ozone interactions with human hair: ozone uptake rates and product formation, Atmos. Environ., 2008, 42, 5079–5089 CrossRef CAS.
- D. T. Downing, M. E. Stewart and J. S. Strauss, Estimation of Sebum Production Rates in Man by Measurement of the Squalene Content of Skin Biopsies, J. Invest. Dermatol., 1981, 77, 358–360 CrossRef CAS PubMed.
- D. T. Downing, A. M. Stranieri and J. S. Strauss, The Effect of Accumulated Lipids on Measurements of Sebum Secretion in Human Skin, J. Invest. Dermatol., 1982, 79, 226–228 CrossRef CAS PubMed.
- G. Honari and H. Maibach, Skin Structure and Function, in Applied Dermatotoxicology: Clinical Aspects, ed. H. Maibach and G. Honari, Academic Press, Boston, 2014, pp. 1–10 Search PubMed.
- H. Baker and A. M. Kligman, Technique for Estimating Turnover Time of Human Stratum Corneum, Arch. Dermatol., 1967, 95, 408–411 CrossRef CAS.
- H. A. Gowadia and G. S. Settles, The Natural Sampling of Airborne Trace Signals from Explosives Concealed upon the Human Body, J. Forensic Sci., 2001, 46, 1324–1331 CAS.
- L. M. Milstone, Epidermal desquamation, J. Dermatol. Sci., 2004, 36, 131–140 CrossRef PubMed.
- D. Roberts and R. Marks, The Determination of Regional and Age Variations in the Rate of Desquamation: A Comparison of Four Techniques, J. Invest. Dermatol., 1980, 74, 13–16 CrossRef CAS PubMed.
- R. P. Clark and S. G. Shirley, Identification of Skin in Airborne Particulate Matter, Nature, 1973, 246, 39–40 CrossRef CAS PubMed.
- G. S. Hall, C. A. Mackintosh and P. N. Hoffman, The dispersal of bacteria and skin scales from the body after showering and after application of a skin lotion, J. Hyg., 1986, 97, 289–298 CrossRef CAS PubMed.
- S. Yang, G. Bekö, P. Wargocki, J. Williams and D. Licina, Human Emissions of Size-Resolved Fluorescent Aerosol Particles: Influence of Personal and Environmental Factors, Environ. Sci. Technol., 2021, 55, 509–518 CrossRef CAS PubMed.
- J. A. Cano-Ruiz, D. Kong, R. B. Balas and W. W. Nazaroff, Removal of reactive gases at indoor surfaces: combining mass transport and surface kinetics, Atmos. Environ., 1993, 27A, 2039–2050 CrossRef CAS.
- G. C. Morrison and W. W. Nazaroff, The rate of ozone uptake on carpet: mathematical modeling, Atmos. Environ., 2002, 36, 1749–1756 CrossRef CAS.
- G. C. Morrison, P. Zhao and L. Kasthuri, The spatial distribution of pollutant transport to and from indoor surfaces, Atmos. Environ., 2006, 40, 3677–3685 CrossRef CAS.
- G. Pei, Y. Xuan, G. Morrison and D. Rim, Understanding Ozone Transport and Deposition within Indoor Surface Boundary Layers, Environ. Sci. Technol., 2022, 56, 7820–7829 CrossRef CAS PubMed.
- G. C. Morrison and W. W. Nazaroff, The Rate of Ozone Uptake on Carpets: Experimental Studies, Environ. Sci. Technol., 2000, 34, 4963–4968 CrossRef CAS.
- B. K. Coleman, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics, Atmos. Environ., 2008, 42, 642–654 CrossRef CAS.
- C. P. Hoang, K. A. Kinney and R. L. Corsi, Ozone removal by green building materials, Build. Environ., 2009, 44, 1627–1633 CrossRef.
- D. Rim, E. T. Gall, R. L. Maddalena and W. W. Nazaroff, Ozone reaction with interior building materials: influence of diurnal ozone variation, temperature and humidity, Atmos. Environ., 2016, 125, 15–23 CrossRef CAS.
- J. Shen and Z. Gao, Ozone removal on building material surface: a literature review, Build. Environ., 2018, 134, 205–217 CrossRef.
- B. L. Deming and P. J. Ziemann, Quantification of alkenes on indoor surfaces and implications for chemical sources and sinks, Indoor Air, 2020, 30, 914–924 CrossRef CAS PubMed.
- C. J. Weschler and W. W. Nazaroff, Growth of organic films on indoor surfaces, Indoor Air, 2017, 27, 1101–1112 CrossRef CAS PubMed.
- E. T. Gall and D. Rim, Mass accretion and ozone reactivity of idealized indoor surfaces in mechanically or naturally ventilated indoor environments, Build. Environ., 2018, 138, 89–97 CrossRef.
- P. Fruekilde, J. Hjorth, N. R. Jensen, D. Kotzias and B. Larsen, Ozonolysis at vegetation surfaces: a source of acetone, 4-oxopentanal, 6-methyl-5-hepten-2-one, and geranyl acetone in the troposphere, Atmos. Environ., 1998, 32, 1893–1902 CrossRef CAS.
- A. Wisthaler, G. Tamás, D. P. Wyon, P. Strøm-Tejsen, D. Space, J. Beauchamp, A. Hansel, T. D. Märk and C. J. Weschler, Products of Ozone-Initiated Chemistry in a Simulated Aircraft Environment, Environ. Sci. Technol., 2005, 39, 4823–4832 CrossRef CAS PubMed.
- C. J. Weschler, A. Wisthaler, S. Cowlin, G. Tamás, P. Strøm-Tejsen, A. T. Hodgson, H. Destaillats, J. Herrington, J. Zhang and W. W. Nazaroff, Ozone-Initiated Chemistry in an Occupied Simulated Aircraft Cabin, Environ. Sci. Technol., 2007, 41, 6177–6184 CrossRef CAS PubMed.
- J. R. Wells, G. C. Morrison and B. K. Coleman, Kinetics and Reaction Products of Ozone and Surface-Bound Squalene, J. ASTM Int., 2008, 5, JAI101629 CrossRef.
- L. Petrick and Y. Dubowski, Heterogeneous oxidation of squalene film by ozone under various indoor conditions, Indoor Air, 2009, 19, 381–391 CrossRef CAS PubMed.
- B. Coffaro and C. P. Weisel, Reactions and Products of Squalene and Ozone: A Review, Environ. Sci. Technol., 2022, 56, 7396–7411 CrossRef CAS PubMed.
- G. C. Morrison, A. Eftekhari, F. Majluf and J. E. Krechmer, Yields and Variability of Ozone Reaction Products from Human Skin, Environ. Sci. Technol., 2021, 55, 179–187 CrossRef CAS PubMed.
- N. Wang, L. Ernle, G. Bekö, P. Wargocki and J. Williams, Emission Rates of Volatile Organic Compounds from Humans, Environ. Sci. Technol., 2022, 56, 4838–4848 CrossRef CAS PubMed.
- P. S. J. Lakey, A. Zuend, G. C. Morrison, T. Berkemeier, J. Wilson, C. Arata, A. H. Goldstein, K. R. Wilson, N. Wang, J. Williams, J. P. D. Abbatt and M. Shiraiwa, Quantifying the impact of relative humidity on human exposure to gas phase squalene ozonolysis products, Environ. Sci.: Atmos., 2023, 3, 49 CAS.
- J. Zeng, M. Mekic, X. Xu, G. Loisel, Z. Zhou, S. Gligorovski and X. Li, A Novel Insight into the Ozone–Skin Lipid Oxidation Products Observed by Secondary Electrospray Ionization High-Resolution Mass Spectrometry, Environ. Sci. Technol., 2020, 54, 13478–13487 CrossRef CAS PubMed.
- S. Enami, Fates of Organic Hydroperoxides in Atmospheric Condensed Phases, J. Phys. Chem. A, 2021, 125, 4513–4523 CrossRef CAS PubMed.
- D. Fu, C. Leng, J. Kelley, G. Zeng, Y. Zhang and Y. Liu, ATR-IR Study of Ozone Initiated Heterogeneous Oxidation of Squalene in an Indoor Environment, Environ. Sci. Technol., 2013, 47, 10611–10618 CrossRef CAS PubMed.
- J. A. Garrido, S. Parthasarathy, C. Moschet, T. M. Young, T. E. McKone and D. H. Bennett, Exposure Assessment for Air-To-Skin Uptake of Semivolatile Organic Compounds (SVOCs) Indoors, Environ. Sci. Technol., 2019, 53, 1608–1616 CrossRef CAS PubMed.
- N. Heine, F. A. Houle and K. R. Wilson, Connecting the Elementary Reaction Pathways of Criegee Intermediates to the Chemical Erosion of Squalene Interfaces during Ozonolysis, Environ. Sci. Technol., 2017, 51, 13740–13748 CrossRef CAS PubMed.
- N. Heine, C. Arata, A. H. Goldstein, F. A. Houle and K. R. Wilson, Multiphase Mechanism for the Production of Sulfuric Acid from SO2 by Criegee Intermediates Formed During the Heterogeneous Reaction of Ozone with Squalene, J. Phys. Chem. Lett., 2018, 9, 3504–3510 CrossRef CAS PubMed.
- M. I. Jacobs, B. Xu, O. Kostko, N. Heine, M. Ahmed and K. R. Wilson, Probing the Heterogeneous Ozonolysis of Squalene Nanoparticles by Photoemission, J. Phys. Chem. A, 2016, 120, 8645–8656 CrossRef CAS PubMed.
- S. Zhou, M. W. Forbes, Y. Katrib and J. P. D. Abbatt, Rapid Oxidation of Skin Oil by Ozone, Environ. Sci. Technol. Lett., 2016, 3, 170–174 CrossRef CAS.
- S. Zhou, M. W. Forbes and J. P. D. Abbatt, Kinetics and Products from Heterogeneous Oxidation of Squalene with Ozone, Environ. Sci. Technol., 2016, 50, 11688–11697 CrossRef CAS PubMed.
- D. J. Price, D. A. Day, D. Pagonis, H. Stark, L. B. Algrim, A. V. Handschy, S. Liu, J. E. Krechmer, S. L. Miller, J. F. Hunter, J. A. de Gouw, P. J. Ziemann and J. L. Jimenez, Budgets of Organic Carbon Composition and Oxidation in Indoor Air, Environ. Sci. Technol., 2019, 53, 13053–13063 CrossRef CAS PubMed.
- M. Zhang, Y. Gao and J. Xiong, Characterization of the off-body squalene ozonolysis on indoor surfaces, Chemosphere, 2022, 291, 132772 CrossRef CAS PubMed.
- R. H. Sabersky, D. A. Sinema and F. H. Shair, Concentrations, Decay Rates, and Removal of Ozone and Their Relation to Establishing Clean Indoor Air, Environ. Sci. Technol., 1973, 7, 347–353 CrossRef CAS.
- L. J. S. Liu, M. P. Olson III, G. A. Allen, P. Koutrakis, W. F. McDonnell and T. R. Gerrity, Evaluation of the Harvard Ozone Passive Sampler on Human Subjects Indoors, Environ. Sci. Technol., 1994, 28, 915–923 CrossRef CAS PubMed.
- Z. Bakó-Biró, C. J. Weschler, P. Wargocki and P. O. Fanger, Effects of indoor pollution sources and ventilation rate on ozone's surface removal rate and the occurrence of oxygenated VOCs in an office space, in, Indoor Air 2005: Proceedings of the 10th International Conference on Indoor Air Quality and Climate, eds. X. Yang, B. Zhao and R. Zhao, Tsinghua University Press, Beijing, 2005, vol. II, pp. 2320–2324 Search PubMed.
- G. Tamás, C. J. Weschler, Z. Bakó-Biró, D. P. Wyon and P. Strøm-Tejsen, Factors affecting ozone removal rates in a simulated aircraft cabin environment, Atmos. Environ., 2006, 40, 6122–6133 CrossRef.
- S. Bhangar and W. W. Nazaroff, Atmospheric ozone levels encountered by commercial aircraft on transatlantic routes, Environ. Res. Lett., 2013, 8, 014006 CrossRef CAS.
- C. Weisel, C. J. Weschler, K. Mohan, J. Vallarino and J. D. Spengler, Ozone and Ozone Byproducts in the Cabins of Commercial Aircraft, Environ. Sci. Technol., 2013, 47, 4711–4717 CrossRef CAS PubMed.
- H. Destaillats, W. Chen, M. G. Apte, N. Li, M. Spears, J. Almosni, G. Brunner, J. Zhang and W. J. Fisk, Secondary pollutants from ozone reactions with ventilation filters and degradation of filter media additives, Atmos. Environ., 2011, 45, 3561–3568 CrossRef CAS.
- A. Fischer, E. Ljungström and S. Langer, Ozone removal by occupants in a classroom, Atmos. Environ., 2013, 81, 11–17 CrossRef CAS.
- P. R. Veres, P. Faber, F. Drewnick, J. Lelieveld and J. Williams, Anthropogenic sources of VOC in a football stadium: assessing human emissions in the atmosphere, Atmos. Environ., 2013, 77, 1052–1059 CrossRef CAS.
- S. Liu, R. Li, R. J. Wild, C. Warneke, J. A. de Gouw, S. S. Brown, S. L. Miller, J. C. Luongo, J. L. Jimenez and P. J. Zieman, Contribution of human-related sources to indoor volatile organic compounds in a university classroom, Indoor Air, 2016, 26, 925–938 CrossRef CAS PubMed.
- X. Tang, P. K. Misztal, W. W. Nazaroff and A. H. Goldstein, Volatile Organic Compound Emissions from Humans Indoors, Environ. Sci. Technol., 2016, 50, 12686–12694 CrossRef CAS PubMed.
- A. M. Avery, M. S. Waring and P. F. DeCarlo, Human occupant contribution to secondary aerosol mass in the indoor environment, Environ. Sci.: Processes Impacts, 2019, 21, 1301–1312 RSC.
- Z. Finewax, D. Pagonis, M. S. Claflin, A. V. Handschy, W. L. Brown, O. Jenks, B. A. Nault, D. A. Day, B. M. Lerner, J. L. Jimenez, P. J. Ziemann and J. A. de Gouw, Quantification and source characterization of volatile organic compounds from exercising and application of chlorine-based cleaning products in a university athletic center, Indoor Air, 2021, 31, 1323–1339 CrossRef CAS PubMed.
- M. Zhang, J. Xiong, Y. Liu, P. K. Misztal and A. H. Goldstein, Physical-Chemical Coupling Model for Characterizing the Reaction of Ozone with Squalene in Realistic Indoor Environments, Environ. Sci. Technol., 2021, 55, 1690–1698 CrossRef CAS PubMed.
- H. Deng, X. Xu, K. Wang, J. Xu, G. Loisel, Y. Wang, H. Pang, P. Li, Z. Mai, S. Yan, X. Li and S. Gligorovski, The Effect of Human Occupancy on Indoor Air Quality through Real-Time Measurements of Key Pollutants, Environ. Sci. Technol., 2022, 56, 15377–15388 CrossRef CAS PubMed.
- W. W. Nazaroff, A. J. Gadgil and C. J. Weschler, Critique of the Use of Deposition Velocity in Modeling Indoor Air Quality, in Modeling of Indoor Air Quality and Exposure, ASTM STP 1205, ed. N. L. Nagda, American Society for Testing and Materials, Philadelphia, 1993, pp. 81–104 Search PubMed.
- J. Verbraecken, P. Van de Heyning, W. De Backer and L. Van Gaal, Body surface area in normal-weight, overweight, and obese adults. A comparison study, Metab., Clin. Exp., 2006, 55, 515–524 CrossRef CAS PubMed.
- G. Bekö, P. Wargocki, N. Wang, M. Li, C. J. Weschler, G. Morrison, S. Langer, L. Ernle, D. Licina, S. Yang, N. Zannoni and J. Williams, The Indoor Chemical Human Emissions and Reactivity (ICHEAR) project: Overview of experimental methodology and preliminary results, Indoor Air, 2020, 30, 1213–1228 CrossRef PubMed.
- M. O. Fadeyi, C. J. Weschler, K. W. Tham, W. Y. Wu and Z. M. Sultan, Impact of Human Presence on Secondary Organic Aerosols Derived from Ozone-Initiated Chemistry in a Simulated Office Environment, Environ. Sci. Technol., 2013, 47, 3933–3941 CrossRef CAS PubMed.
- J. Xiong, Z. He, X. Tang, P. K. Misztal and A. H. Goldstein, Modeling the Time-Dependent Concentrations of Primary and Secondary Reaction Products of Ozone with Squalene in a University Classroom, Environ. Sci. Technol., 2019, 53, 8262–8270 CrossRef CAS PubMed.
- M. Yao, L. Ke, Y. Liu, Z. Luo and B. Zhao, Measurement of ozone deposition velocity onto human surfaces of Chinese residents and estimation of corresponding production of oxidation products, Environ. Pollut., 2020, 266, 115215 CrossRef CAS PubMed.
- D. Rim, A. Novoselec and G. Morrison, The influence of chemical interactions at the human surface on breathing zone levels of reactants and products, Indoor Air, 2009, 19, 324–334 CrossRef CAS PubMed.
- D. Rim, E. T. Gall, S. Ananth and Y. Won, Ozone reaction with human surfaces: influences of surface reaction probability and indoor air flow condition, Build. Environ., 2018, 130, 40–48 CrossRef.
- G. Pei and D. Rim, Quality control of computational fluid dynamics (CFD) model of ozone reaction with human surface: effects of mesh size and turbulence model, Build. Environ., 2021, 189, 107513 CrossRef.
- J. He, Y. Lin, J. Pei, Y. Sun, Z. Liu, Q. Chen and X. Yang, A model to evaluate ozone distribution and reaction byproducts in aircraft cabin environments, Indoor Air, 2022, 32, e13178 CAS.
- W. W. Nazaroff, Indoor aerosol science aspects of SARS-CoV-2 transmission, Indoor Air, 2022, 32, e12970 CAS.
- D. Licina, J. Pantelic, A. Melikov, C. Sekhar and K. W. Tham, Experimental investigation of the human convective boundary layer in a quiescent indoor environment, Build. Environ., 2014, 75, 79–91 CrossRef.
- C. J. Weschler, S. Langer, A. Fischer, G. Bekö, J. Toftum and G. Clausen, Squalene and Cholesterol in Dust from Danish Homes and Daycare Centers, Environ. Sci. Technol., 2011, 45, 3872–3879 CrossRef CAS PubMed.
- M.-A. Lefebvre, D.-M. Pham, B. Boussouira, D. Bernard, C. Camus and Q.-L. Nguyen, Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico, Int. J. Cosmet. Sci., 2015, 37, 329–338 CrossRef CAS PubMed.
- M.-A. Lefebvre, D.-M. Pham, B. Boussouira, H. Qiu, C. Ye, X. Long, R. Chen, W. Gu, A. Laurent and Q.-L. Nguyen, Consequences of urban pollution upon skin status. A controlled study in Shanghai area, Int. J. Cosmet. Sci., 2016, 38, 217–223 CrossRef PubMed.
- S. Langer, A. Sjöblom, G. Giovanoulis, G. Bekö, P. Wargocki, G. Morrison, C. J. Weschler and J. Williams, Squalene in skin wipes: dependence on ozone, indoor climate and skin coverage, Indoor Air 2022, Kuopio, Finland, 2022, Presentation 1162 Search PubMed.
- A. C. Rai, B. Guo, C.-H. Lin, J. Zhang, J. Pei and Q. Chen, Ozone reaction with clothing and its initiated VOC emissions in an environmental chamber, Indoor Air, 2014, 24, 49–58 CrossRef CAS PubMed.
- T. Wu, A. Tasoglou, H. Huber, P. S. Stevens and B. E. Boor, Influence of Mechanical Ventilation Systems and Human Occupancy on Time-Resolved Source Rates of Volatile Skin Oil Ozonolysis Products in a LEED-Certified Office Building, Environ. Sci. Technol., 2021, 55, 16477–16488 CrossRef CAS PubMed.
- E. A. Cohen Hubal, J. C. Suggs, M. G. Nishioka and W. A. Ivancic, Characterizing residue transfer efficiencies using a fluorescent imaging technique, J. Exposure Anal. Environ. Epidemiol., 2005, 15, 261–270 CrossRef CAS PubMed.
- E. A. Cohen Hubal, M. G. Nishioka, W. A. Ivancic, M. Morara and P. P. Egeghy, Comparing Surface Residue Transfer Efficiencies to Hands using Polar and Nonpolar Fluorescent Tracers, Environ. Sci. Technol., 2008, 42, 934–939 CrossRef CAS PubMed.
- S. Cadd, M. Islam, P. Manson and S. Bleay, Fingerprint composition and aging: a literature review, Sci. Justice, 2015, 55, 219–238 CrossRef PubMed.
- A. Zafar and J. Chickos, The vapor pressure and vaporization enthalpy of squalene and squalane by correlation gas chromatography, J. Chem. Thermodyn., 2019, 135, 192–197 CrossRef CAS.
- C. Y. Lim and J. P. Abbatt, Chemical Composition, Spatial Homogeneity, and Growth of Indoor Surface Films, Environ. Sci. Technol., 2020, 54, 14372–14379 CrossRef CAS PubMed.
- J. A. Wilson and J. S. Chickos, Vapor Pressures and Vaporization, Sublimation, and Fusion Enthalpies of Some Fatty Acids, J. Chem. Eng. Data, 2013, 58, 322–333 CrossRef CAS.
- C. Wang, D. B. Collins, C. Arata, A. H. Goldstein, J. M. Mattila, D. K. Farmer, L. Ampollini, P. F. DeCarlo, A. Novoselac, M. E. Vance, W. W. Nazaroff and J. P. D. Abbatt, Surface reservoirs dominate dynamic gas-surface partitioning of many indoor air constituents, Sci. Adv., 2020, 6, eaay8973 CrossRef CAS PubMed.
- S. Yang, D. Licina, C. J. Weschler, N. Wang, N. Zannoni, M. Li, J. Vanhanen, S. Langer, P. Wargocki, J. Williams and G. Bekö, Ozone Initiates Human-Derived Emission of Nanocluster Aerosols, Environ. Sci. Technol., 2021, 55, 14536–14545 CrossRef CAS PubMed.
- A. C. K. Lai and W. W. Nazaroff, Modeling indoor particle deposition from turbulent flow onto smooth surfaces, J. Aerosol Sci., 2000, 31, 463–476 CrossRef CAS.
- J. D. Sinclair, L. A. Psota-Kelty, C. J. Weschler and H. C. Shields, Measurement and modeling of airborne concentrations and indoor surface accumulation rates of ionic substances at Neenah, Wisconsin, Atmos. Environ., 1990, 24A, 627–638 CrossRef CAS.
- A. C. K. Lai and W. W. Nazaroff, Supermicron particle deposition from turbulent chamber flow onto smooth and rough vertical surfaces, Atmos. Environ., 2005, 39, 4893–4900 CrossRef CAS.
- L. Lv and B. Zhao, Deposition of non-spherical particles on indoor surfaces: modification of diffusion coefficient, Aerosol Sci. Technol., 2022, 56, 1190–1200 CrossRef CAS.
- H. Wang and G. C. Morrison, Ozone-Initiated Secondary Emission Rates of Aldehydes from Indoor Surfaces in Four Homes, Environ. Sci. Technol., 2006, 40, 5263–5268 CrossRef CAS PubMed.
- S. M. Prouty and A. Pappas, Sapienic Acid: Species-Specific Fatty Acid Metabolism of the Human Sebaceous Gland, in Lipids and Skin Health, ed. A. Pappas, Springer, New York, 2015, pp. 139–157 Search PubMed.
- R. C. Zambiazi, R. Przybylski, M. W. Zambiazi and C. B. Mendonça, Fatty acid composition of vegetable oils and fats, Bol. Cent. Pesqui. Process. Aliment., 2007, 25, 111–120 CAS.
- V. Kostik, S. Memeti and B. Bauer, Fatty acid composition of edible oils and fats, J. Hyg. Eng. Des., 2013, 4, 112–116 Search PubMed.
- U.S. Environmental Protection Agency, Chemical Data Reporting under the Toxic Substances Control Act, https://www.epa.gov/chemical-data-reporting, last accessed on Jan. 8, 2023 Search PubMed.
- G. C. Morrison and W. W. Nazaroff, Ozone Interactions with Carpet: Secondary Emissions of Aldehydes, Environ. Sci. Technol., 2002, 36, 2185–2192 CrossRef CAS PubMed.
- M. Nicolas, O. Ramalho and F. Maupetit, Reactions between ozone and building products: Impact on primary and secondary emissions, Atmos. Environ., 2007, 41, 3129–3138 CrossRef CAS.
- A. Vibenholt, P. A. Clausen and P. Wolkoff, Ozone reaction characteristics of indoor floor dust examined in the emission cell “FLEC”, Chemosphere, 2014, 107, 230–239 CrossRef CAS PubMed.
- H. Wang and G. Morrison, Ozone-surface reactions in five homes: surface reaction probabilities, aldehyde yields, and trends, Indoor Air, 2010, 20, 224–234 CrossRef CAS PubMed.
- W. W. Nazaroff and C. J. Weschler, Indoor acids and bases, Indoor Air, 2020, 30, 559–644 CrossRef CAS PubMed.
- W. W. Nazaroff, Residential air-change rates: a critical review, Indoor Air, 2021, 31, 282–313 CrossRef PubMed.
- ANSI/ASHRAE Standard 62.1, Ventilation for Acceptable Indoor Air Quality, ASHRAE, Atlanta, GA, 2022 Search PubMed.
- U.S. Census Bureau, American Housing Survey, 2017, https://www.census.gov/programs-surveys/ahs.html, last accessed Aug 12, 2022 Search PubMed.
- L.-J. S. Liu, P. Koutrakis, J. Leech and I. Broder, Assessment of Ozone Exposures in the Greater Metropolitan Toronto Area, J. Air Waste Manage. Assoc., 1995, 45, 223–234 CrossRef CAS PubMed.
- J. Zhu, S. L. Wong and S. Cakmak, Nationally Representative Levels of Selected Volatile Organic Compounds in Canadian Residential Indoor Air: Population-Based Survey, Environ. Sci. Technol., 2013, 47, 13276–13283 CrossRef CAS PubMed.
- C. J. Weschler, Ozone's Impact on Public Health: Contributions from Indoor Exposures to Ozone and Products of Ozone-Initiated Chemistry, Environ. Health Perspect., 2006, 114, 1489–1496 CrossRef CAS PubMed.
- W. A. Pryor, How far does ozone penetrate into the pulmonary air/tissue boundary before it reacts?, Free Radical Biol. Med., 1992, 12, 83–88 CrossRef CAS PubMed.
- L. He, C. J. Weschler, Y. Zhang, F. Li, M. H. Bergin, M. Black and J. Zhang, Ozone Reaction Products Associated with Biomarkers of Cardiorespiratory Pathophysiology, Am. J. Respir. Crit. Care Med., 2023 DOI:10.1164/rccm.202212-2203LE.
- N. Zannoni, P. S. J. Lakey, Y. Won, M. Shiraiwa, D. Rim, C. J. Weschler, N. Wang, L. Ernle, M. Li, G. Bekö, P. Wargocki and J. Williams, The human oxidation field, Science, 2022, 377, 1071–1077 CrossRef CAS PubMed.
- National Academies of Sciences, Engineering, and Medicine, Why Indoor Chemistry Matters, The National Academies Press, Washington, DC, 2022. DOI:10.17226/26228.
- M. Ge, S. Tong, W. Wang, W. Zhang, M. Chen, C. Peng, J. Li, L. Zhou, Y. Chen and M. Liu, Important Oxidants and Their Impact on the Environmental Effects of Aerosols, J. Phys. Chem. A, 2021, 125, 3813–3825 CrossRef CAS PubMed.
- Z. Hassan, M. Stahlberger, N. Rosenbaum and S. Bräse, Criegee Intermediates Beyond Ozonolysis: Synthetic and Mechanistic Insights, Angew. Chem., Int. Ed., 2021, 60, 15138–15152 CrossRef CAS PubMed.
- B. Long, J. L. Bao and D. G. Truhlar, Rapid unimolecular reaction of stabilized Criegee intermediates and implications for atmospheric chemistry, Nat. Commun., 2019, 10, 2003 CrossRef PubMed.
- M. Zeng, N. Heine and K. R. Wilson, Evidence that Criegee intermediates drive autoxidation in unsaturated lipids, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 4486–4490 CrossRef CAS PubMed.
- J. R. Aldred, E. Darling, G. Morrison, J. Siegel and R. L. Corsi, Benefit-cost analysis of commercially available activated carbon filters for indoor ozone removal in single-family homes, Indoor Air, 2016, 26, 501–512 CrossRef CAS PubMed.
- G. Bekö, M. O. Fadeyi, G. Clausen and C. J. Weschler, Sensory pollution from bag-type fiberglass ventilation filters: conventional filter compared with filters containing various amounts of activated carbon, Build. Environ., 2009, 44, 2114–2120 CrossRef.
- H. C. Shields, C. J. Weschler and D. Naik, Ozone removal by charcoal filters after continuous extensive use (5 to 8 years), Proceedings of the 8th International Conference on Indoor Air Quality and Climate, 1999, vol. 4, pp. 49–54 Search PubMed.
- N. Wang, N. Zannoni, L. Ernle, G. Bekö, P. Wargocki, M. Li, C. J. Weschler and J. Williams, Total OH Reactivity of Emissions from Humans: In Situ Measurement and Budget Analysis, Environ. Sci. Technol., 2021, 55, 149–159 CrossRef CAS PubMed.
- N. Zannoni, M. Li, N. Wang, L. Ernle, G. Bekö, P. Wargocki, S. Langer, C. J. Weschler, G. Morrison and J. Williams, Effect of Ozone, Clothing, Temperature, and Humidity on the Total OH Reactivity Emitted from Humans, Environ. Sci. Technol., 2021, 55, 13614–13624 CrossRef CAS PubMed.
- S. Zhou, S. Joudan, M. W. Forbes, Z. Zhou and J. P. D. Abbatt, Reaction of Condensed-Phase Criegee Intermediates with Carboxylic Acids and Perfluoroalkyl Carboxylic Acids, Environ. Sci. Technol. Lett., 2019, 6, 243–250 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ea00008g |
| This journal is © The Royal Society of Chemistry 2023 |


