Plasmon enabled Claisen rearrangement with sunlight†
Radha Krishna
Kashyap
 ,
Shreya
Tyagi
and
Pramod P.
Pillai
,
Shreya
Tyagi
and
Pramod P.
Pillai
 *
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Pune, Dr. Homi Bhabha Road, Pashan, Pune–411 008, India. E-mail: pramod.pillai@iiserpune.ac.in
First published on 9th October 2023
Abstract
Plasmonic-heat generated from the solar irradiation of gold nanoparticles is used as the thermal energy source for the Claisen rearrangement of allyl phenyl ether to 2-allylphenol, which is conventionally performed with electrical heating at 250 °C. The use of a closed reactor enables the physical separation of the reactants from the source of plasmonic-heat, thereby preventing the interference of the hot-charge carriers in the plasmon-driven Claisen rearrangement. In this way, the sole effect of plasmonic-heat in driving a high temperature organic transformation is demonstrated. Our study reveals the prospects of plasmonic nanostructures in conducting energy intensive chemical synthesis in a sustainable fashion.
The visible-light absorbing power of plasmonic nanoparticles (NPs) is exceptionally high (108–1010 M−1 cm−1) because of their unique excitation pathway based on surface plasmon resonance.1 A photoexcited NP can dissipate its excess energy through radiative and non-radiative pathways: ca. ∼5% and ∼95%, respectively, for NPs with size <25 nm.2 The main outcomes of the radiative relaxation pathway are scattering and emission; whereas, hot-charge carrier generation and heat-dissipation are the main outcomes of the non-radiative relaxation pathway.3 Both hot-charge carrier generation and heat-dissipation can bring out important chemical and physical transformations, which constitute the areas of plasmonic photocatalysis4 and thermoplasmonics,5 respectively. The majority of the photoexcitation energy in plasmonic NPs is dissipated in the form of heat because of the fast charge recombination dynamics associated with non-radiative relaxation processes (100 fs–10 ps; Landau damping, electron–electron, electron–phonon, and phonon–phonon scattering).3 In effect, the amount of heat dissipated by a photoexcited NP is huge because of the involvement of unique excitation and relaxation pathways (photothermal conversion efficiency is >90%).6 This heat dissipated from a photoexcited NP is termed as plasmonic-heat, which has found use in various photothermal applications including therapy,7 drug delivery,8 solar-vapor generation,9 azeotropic separation,10 and material synthesis.11
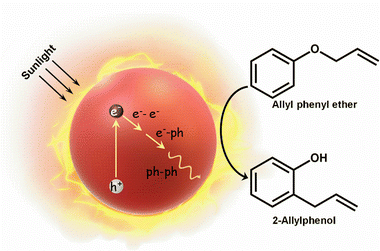
Recent studies have proved the suitability of plasmonic-heat in synthetic organic chemistry as well, with the aim of achieving sustainability in chemical synthesis.12 The idea here is to use the plasmonic-heat as the thermal energy source, instead of the conventional electrical heating, for performing high temperature organic reactions. However, it has been challenging to deconvolute the contribution of hot-charge carriers in a plasmonic-heat driven organic reaction, as both these outcomes are part of the same nonradiative decay process.13 In this work, the use of a proper reactor system enabled us to prevent the interference of the hot-charge carriers and study the sole effect of plasmonic-heat in high temperature organic reaction (Scheme 1). This was achieved by designing a thermodynamically closed reactor system, wherein the reaction mixture was physically separated from the plasmonic NPs (heating source). This will enable the exchange of energy (here, plasmonic-heat), whereas the exchange of matter (here, hot charge carriers) will be prevented. Our choice of the high temperature organic reaction was the thermally driven [3,3] sigmatropic Claisen rearrangement of allyl phenyl ether to 2-allylphenol, which is conventionally performed at 250 °C using electrical heating.14 Also, Claisen rearrangement is one of the commonly practiced reactions in synthetic organic chemistry to form carbon–carbon bonds, further justifying the importance and choice of the reaction in the present study.15 The plasmonic-heat dissipated from gold nanoparticles (AuNPs) was used as the thermal energy source for the Claisen rearrangement, which gave an excellent yield of ∼80% within 2 h of solar irradiation. Kinetic studies reveal that the rate of the reaction under plasmonic-heating was at least two times higher than the reaction performed with normal electrical heating, which can be attributed to the higher steady-state temperature achieved with plasmonic-heat.
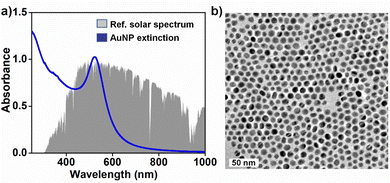
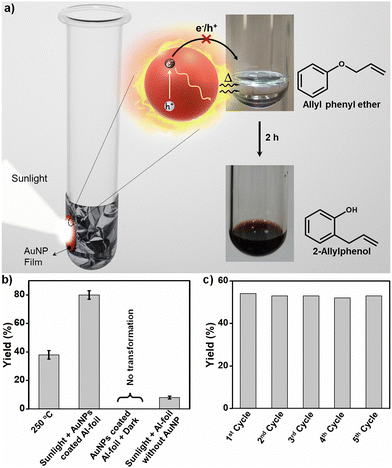
AuNPs were chosen as the plasmonic NP in the present study because of their strong absorption power in visible-light and high photostability under continuous solar irradiation.1 AuNPs of diameter 12.0 ± 0.4 nm were synthesized using a modified seed-mediated growth method16 (see Fig. 1 and Fig. S1, S2 in the ESI†). The surface of the AuNPs was functionalised with 11-mercaptoundecanoic acid (MUA) to enhance the colloidal stability of the NPs in the aqueous medium. This enabled the entire photothermal studies with AuNPs from the same batch. The rationale behind selecting ∼12 nm AuNPs was based on the knowledge that AuNPs in the size range of 10–24 nm show the highest photothermal conversion efficiency.17 The surface plasmon band of AuNPs (centred at ∼520 nm) has a strong overlap with the solar spectrum, enabling the use of sunlight as the irradiation source (Fig. 1a). Along with testing the suitability of plasmonic-heat in high temperature organic synthesis, our objective was to eliminate the contribution from hot-charge carriers in a plasmon-driven organic reaction. For this, a thin film of plasmonic AuNPs was coated on the surface of aluminium (Al) foil that was wrapped around the glass test tube (here, reactor) (see Fig. 2a and Fig. S3 in the ESI†). The Al-foil interface helped in achieving a stable coating of AuNPs on the test tube. Also, the high thermal conductivity of Al (205 W m−1 K−1) ensured the effective transfer of the plasmonic-heat to the reactants inside the glass test tube. The physical separation of the reactants from the plasmonic-heat source (here, AuNPs) prevented the transfer of hot-charge carriers to the reactant. Furthermore, the wrapping of the reactor with Al-foil will block the direct exposure of the reactants to sunlight, thereby overruling the interference of photochemical effects as well. Yet another advantage of the closed reactor is that the reaction mixture will not be contaminated with the plasmonic-heaters. In this way, a simple design of a closed reactor enabled us to study the sole effect of photothermal properties in a plasmon-driven organic reaction.
Claisen rearrangement was selected as the model reaction to study the potential of plasmonic-heat in performing high-temperature organic synthesis. The intramolecular ortho rearrangement of the allylic group in allyl phenyl ether to form 2-allylphenol via [3,3] sigmatropic rearrangement is an energy intensive thermal transformation, which is conventionally performed at 250 °C (Fig. 2a). Our idea was to replace the traditional electrical-heat with plasmonic-heat generated from the solar irradiation of AuNPs. In a typical photothermal experiment, the AuNP coated Al-foil wrapped test tube containing 1 mL of allyl phenyl ether was irradiated with focused sunlight (experimental details are provided in the ESI†). The colourless reactant turned to reddish yellow within 2 h of solar irradiation, indicating the thermal conversion of allyl phenyl ether to 2-allylphenol. The reaction mixture was purified, and the product was characterized using 1H-NMR and HRMS studies (Fig. S4 and S5 in the ESI†). All the yields were calculated from the 1H-NMR of the reaction mixture using the internal standard method (1,1,2,2-tetrachloroethane was used as the internal standard) (see Fig. S6 and S7 in the ESI† for details of yield calculation). The plasmonic-heat driven Claisen rearrangement resulted in a yield of ∼80% after 2 h of solar irradiation (Fig. 2b and Fig. S8 in the ESI†), which was at least double the yield obtained from normal thermal reaction performed at 250 °C under similar experimental conditions (Fig. 2b and Fig. S9 in the ESI†). The enhancement in the yield under solar irradiation can be explained as follows. The amount of energy absorbed by AuNPs upon solar irradiation is exceptionally high (∼108 M−1 cm−1), because of the phenomenon of surface plasmon resonance.1 The photoexcited AuNPs undergo a series of nonradiative relaxation processes (Landau damping, electron–electron, electron–phonon, and phonon–phonon interactions) to eventually dissipate the excess energy in the form of plasmonic-heat.3 It has been shown that the plasmonic-heat can raise the surface temperature of AuNPs to 600 °C,18 which will be finally dissipated to the surrounding medium. As a result, the temperature experienced by the surrounding medium close to the plasmonic AuNPs can be as high as ∼500 °C.12 In the present study too, a higher steady-state temperature (>250 °C)9b could have been experienced by the reactants because of the plasmonic-heat dissipated from the photoexcited AuNPs, leading to an improvement in the product yield under solar irradiation. Control experiments (i) in the dark at room temperature under similar experimental conditions with an AuNP film coated Al-foil wrapped test tube (Fig. 2b and Fig. S10 in the ESI†) and (ii) under sunlight illumination of allyl phenyl ether reactant in an Al-foil wrapped test tube without the AuNP film produced negligible yields (Fig. 2b and Fig. S11 in the ESI†), confirming the necessity of plasmonic-heat in performing the reaction. It is worth mentioning that the Al-foil completely blocks the focused sunlight from entering the test tube (Fig. S12, ESI†), which overrules the possibility of the thermal shielding effect arising from the confinement of light by Al-foil. Movie S1 (ESI†) clearly shows the vigorous boiling, along with a distinct color change to reddish yellow, when the AuNP coated test tube containing allyl phenyl ether reactant (b.p. = 191.7 °C) was irradiated with sunlight. In this particular experiment, the AuNP film was coated inside the glass test tube for the in-situ visualisation of the progress of the reaction (control experiments in the absence of AuNPs fail to show any boiling as well as conversion of the reactant. Details are given in the ESI†). All these control experiments confirm the sole-involvement of plasmonic-heat as the thermal energy source in the Claisen rearrangement of allyl phenyl ether to 2-allylphenol. The plasmonic-heaters based on AuNPs also showed excellent reusability with negligible loss in the photothermal reaction yield for at least five cycles (Fig. 2c and Fig. S13 in the ESI†), which can be attributed to their high photostability under continuous solar irradiation (the time of the reaction was reduced to 90 min to complete multiple cycles under similar solar irradiation power).
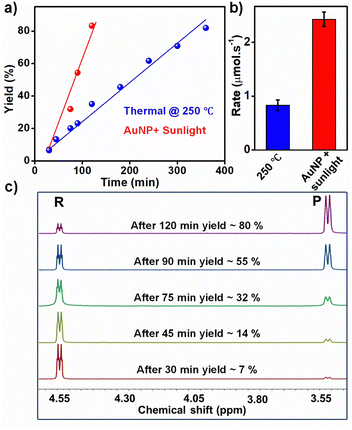
Finally, the rate of the Claisen rearrangement under plasmonic- and electrical-heating was compared. The progress of the reaction was monitored by analysing the product yield in aliquots collected at different time intervals (see ESI† for the experimental details). NMR studies revealed a gradual increase in the product yield with time (Fig. 3a and b), for both plasmonic and electrical-heating driven Claisen rearrangement. The NMR signal at 3.5 ppm corresponding to the product increased with time of heating, accompanied by a decrease in the reactant signal at 4.55 ppm (Fig. 3c and Fig. S14, S15 in the ESI†). Approximately 80% product was formed under the plasmonic-heating condition after 2 h of solar irradiation. In contrast, the normal electrical-heating at 250 °C required ∼6 h to yield ∼80% product formation (Fig. S15 in the ESI†). The rate of the reaction under plasmonic-heating conditions was at least two times higher than the one under electrical-heating, which confirms that the steady-state temperature achieved with plasmonic-heat is higher than 250 °C.
In conclusion, an energy intensive high temperature Claisen rearrangement was performed with an excellent yield of ∼80% using the plasmonic-heat generated from the solar irradiation of AuNPs. Control experiments conclusively prove the sole role of plasmonic-heat as the thermal energy source for driving the Claisen rearrangement. The high photostability of AuNPs ensured the constant supply of heat energy for performing the Claisen rearrangement, under continuous and repeated solar irradiations. More importantly, an appropriate design of a closed reactor enabled the physical separation of reactants from the source of the plasmonic-heat generator, which prevented the interference from the hot-charge carriers. In short, our study reveals the potential of plasmonic-heating as an alternative to electrical-heating for high-temperature organic reactions, thereby showcasing a way to achieve sustainability in high-temperature chemical synthesis.
R. K. K. prepared the AuNPs, and conducted and analysed the photothermal organic reactions. S. T. performed the characterization studies and analysed the data. P. P. P. conceived the project, wrote the manuscript with the assistance of all co-authors, and coordinated the research.
The authors acknowledge the financial support from MoE-STARS Grant No. MoE-STARS/STARS2/2023-0195. The authors thank U.S. Department of Energy (DOE)/NREL/ALLIANCE for the ASTM G173-03 AM1.5G reference spectrum. The authors thank Namitha Deepak and Mridul K. Vinod for helping with TOC, scheme preparation and sunlight experiments. R. K. K. thanks CSIR and S. T. thanks UGC for the PhD fellowships.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410–8426 CrossRef CAS
; (b) J. H. Hodak, I. Martini and G. V. Hartland, J. Phys. Chem. B, 1998, 102, 6958–6967 CrossRef CAS
; (c) C. F. Bohren, Am. J. Phys., 1983, 51, 323–327 CrossRef CAS
.
-
(a) C. Sönnichsen, T. Franzl, T. Wilk, G. von Plessen, J. Feldmann, O. Wilson and P. Mulvaney, Phys. Rev. Lett., 2002, 88, 077402 CrossRef PubMed
; (b) A. M. Brown, R. Sundararaman, P. Narang, W. A. I. Goddard and H. A. Atwater, ACS Nano, 2016, 10, 957–966 CrossRef CAS PubMed
.
-
(a) H. Inouye, K. Tanaka, I. Tanahashi and K. Hirao, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 11334–11340 CrossRef CAS
; (b) S. Link and M. A. El-Sayed, Annu. Rev. Phys. Chem., 2003, 54, 331–366 CrossRef CAS PubMed
; (c) M. L. Brongersma, N. J. Halas and P. Nordlander, Nat. Nanotechnol., 2015, 10, 25–34 CrossRef CAS PubMed
; (d) C. Boerigter, U. Aslam and S. Linic, ACS Nano, 2016, 10, 6108–6115 CrossRef CAS PubMed
; (e) V. Jain, R. K. Kashyap and P. P. Pillai, Adv. Opt. Mater., 2022, 10, 2200463 CrossRef CAS
.
-
(a) S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed
; (b) S. Mukherjee, F. Libisch, N. Large, O. Neumann, L. V. Brown, J. Cheng, J. B. Lassiter, E. A. Carter, P. Nordlander and N. J. Halas, Nano Lett., 2013, 13, 240–247 CrossRef CAS PubMed
; (c) S. Roy, S. Roy, A. Rao, G. Devatha and P. P. Pillai, Chem. Mater., 2018, 30, 8415–8419 CrossRef CAS
; (d) S. Roy, V. Jain, R. K. Kashyap, A. Rao and P. P. Pillai, ACS Catal., 2020, 10, 5522–5528 CrossRef CAS
; (e) V. Jain, I. N. Chakraborty, R. B. Raj and P. P. Pillai, J. Phys. Chem. C, 2023, 127, 5153–5161 CrossRef CAS
; (f) A. Dhankhar, V. Jain, I. N. Chakraborty and P. P. Pillai, J. Photochem. Photobiol. A, 2023, 437, 114472 CrossRef CAS
; (g) J. Kuno, N. Ledos, P.-A. Bouit, T. Kawai, M. Hissler and T. Nakashima, Chem. Mater., 2022, 34, 9111–9118 CrossRef CAS
; (h) R. Verma, R. Tyagi, V. K. Voora and V. Polshettiwar, ACS Catal., 2023, 13, 7395–7406 CrossRef CAS
.
-
(a) A. O. Govorov and H. H. Richardson, Nano Today, 2007, 2, 30–38 CrossRef
; (b) G. Baffou and R. Quidant, Laser Photonics Rev., 2013, 7, 171–187 CrossRef CAS
; (c) L. Jauffred, A. Samadi, H. Klingberg, P. M. Bendix and L. B. Oddershede, Chem. Rev., 2019, 119, 8087–8130 CrossRef CAS PubMed
; (d) M. J. Margeson, Y. E. Monfared and M. Dasog, ACS Appl. Opt. Mater., 2023, 1, 1004–1011 CrossRef CAS
; (e) B. Klemmed, L. V. Besteiro, A. Benad, M. Georgi, Z. Wang, A. Govorov and A. Eychmüller, Angew. Chem., Int. Ed., 2020, 59, 1696–1702 CrossRef CAS PubMed
; (f) M. Dhiman, A. Maity, A. Das, R. Belgamwar, B. Chalke, Y. Lee, K. Sim, J.-M. Nam and V. Polshettiwar, Chem. Sci., 2019, 10, 6594–6603 RSC
; (g) S. A. Mezzasalma, J. Kruse, S. Merkens, E. Lopez, A. Seifert, R. Morandotti and M. Grzelczak, Adv. Mater., 2023, 2302987 CrossRef CAS PubMed
; (h) S. Roy, R. K. Kashyap and P. P. Pillai, J. Phys. Chem. C, 2023, 127, 10355–10365 CrossRef CAS
.
-
(a) H. H. Richardson, M. T. Carlson, P. J. Tandler, P. Hernandez and A. O. Govorov, Nano Lett., 2009, 9, 1139–1146 CrossRef CAS PubMed
; (b) H. Breitenborn, J. Dong, R. Piccoli, A. Bruhacs, L. V. Besteiro, A. Skripka, Z. M. Wang, A. O. Govorov, L. Razzari, F. Vetrone, R. Naccache and R. Morandotti, APL Photonics, 2019, 4, 126106 CrossRef
.
-
(a) L. R. Hirsch, R. J. Stafford, J. A. Bankson, S. R. Sershen, B. Rivera, R. E. Price, J. D. Hazle, N. J. Halas and J. L. West, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 13549–13554 CrossRef CAS PubMed
; (b) X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed
.
-
(a) T. Sun, Y. S. Zhang, B. Pang, D. C. Hyun, M. Yang and Y. Xia, Angew. Chem., Int. Ed., 2014, 53, 12320–12364 CrossRef CAS PubMed
; (b) M. S. Strozyk, S. Carregal-Romero, M. Henriksen-Lacey, M. Brust and L. M. Liz-Marzán, Chem. Mater., 2017, 29, 2303–2313 CrossRef CAS
.
-
(a) O. Neumann, A. S. Urban, J. Day, S. Lal, P. Nordlander and N. J. Halas, ACS Nano, 2013, 7, 42–49 CrossRef CAS PubMed
; (b) R. K. Kashyap, I. Dwivedi, S. Roy, S. Roy, A. Rao, C. Subramaniam and P. P. Pillai, Chem. Mater., 2022, 34, 7369–7378 CrossRef CAS
.
- O. Neumann, A. D. Neumann, E. Silva, C. Ayala-Orozco, S. Tian, P. Nordlander and N. J. Halas, Nano Lett., 2015, 15, 7880–7885 CrossRef CAS PubMed
.
- R. Kamarudheen, G. Kumari and A. Baldi, Nat. Commun., 2020, 11, 3957 CrossRef CAS PubMed
.
-
(a) C. Fasciani, C. J. B. Alejo, M. Grenier, J. C. Netto-Ferreira and J. C. Scaiano, Org. Lett., 2011, 13, 204–207 CrossRef CAS PubMed
; (b) E. N. Van Burns and B. J. Lear, J. Phys. Chem. C, 2019, 123, 14774–14780 CrossRef CAS
; (c) J. Qiu and W. D. Wei, J. Phys. Chem. C, 2014, 118, 20735–20749 CrossRef CAS
; (d) C. Vázquez-Vázquez, B. Vaz, V. Giannini, M. Pérez-Lorenzo, R. A. Alvarez-Puebla and M. A. Correa-Duarte, J. Am. Chem. Soc., 2013, 135, 13616–13619 CrossRef PubMed
.
- L. Zhou, D. F. Swearer, C. Zhang, H. Robatjazi, H. Zhao, L. Henderson, L. Dong, P. Christopher, E. A. Carter, P. Nordlander and N. J. Halas, Science, 2018, 362, 69–72 CrossRef CAS PubMed
.
-
(a) L. Claisen, Ber. Dtsch. Chem. Ges., 1912, 45, 3157–3166 CrossRef CAS
; (b) A. M. Martín Castro, Chem. Rev., 2004, 104, 2939–3002 CrossRef PubMed
; (c) X. Han and D. W. Armstrong, Org. Lett., 2005, 7, 4205–4208 CrossRef CAS PubMed
.
-
M. Hiersemann and U. Nubbemeyer, The Claisen Rearrangement, Wiley-VCH, Weinheim, 2007 Search PubMed
.
-
(a) N. R. Jana and X. Peng, J. Am. Chem. Soc., 2003, 125, 14280–14281 CrossRef CAS PubMed
; (b) A. Rao, S. Roy, M. Unnikrishnan, S. S. Bhosale, G. Devatha and P. P. Pillai, Chem. Mater., 2016, 28, 2348–2355 CrossRef CAS
.
- R. K. Kashyap, M. J. Parammal and P. P. Pillai, ChemNanoMat, 2022, 8, e202200252 CrossRef CAS
.
- C. Wang, O. Ranasingha, S. Natesakhawat, P. R. Ohodnicki, M. Andio, J. P. Lewis and C. Matranga, Nanoscale, 2013, 5, 6968–6974 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental information, synthesis, additional characterization, and Movie S1. See DOI: https://doi.org/10.1039/d3cc04278b |
| This journal is © The Royal Society of Chemistry 2023 |