Open Access Article
Open Access ArticleHigh performance, but low cost and environmental impact? Integrated techno-economic and life cycle assessment of polyoxazolidinone as a novel high-performance polymer†
Marvin
Bachmann‡
 a,
Annika
Marxen‡
b,
Reinhard
Schomäcker
b and
André
Bardow
a,
Annika
Marxen‡
b,
Reinhard
Schomäcker
b and
André
Bardow
 *c
*c
aInstitute of Technical Thermodynamics, RWTH Aachen University, Schinkelstr. 8, 52062 Aachen, Germany
bDepartment of Reaction Engineering, Technische Universität Berlin, TC 8, TU-Berlin, Str. des 17. Juni 124, 10623 Berlin, Germany
cEnergy and Process Systems Engineering, Department of Mechanical and Process Engineering, ETH Zurich, Tannenstrasse 3, Zurich 8092, Switzerland. E-mail: abardow@ethz.ch
First published on 8th November 2022
Abstract
High-performance thermoplastic polymers (HPTs) are increasingly used in advanced applications such as aviation or electronics due to their superior chemical and mechanical properties at elevated temperatures. However, producing HPTs is resource- and energy-intensive, resulting in high environmental impacts and production costs. Polyoxazolidinone (POX) has been proposed as a novel HPT with potential environmental and economic benefits compared to reference HPTs by increased process efficiency and readily available inputs. By a combined techno-economic and life-cycle assessment, we show that POX reduces environmental impacts while being cost-competitive compared to reference HPTs polyetherimide, polyethersulfone, and polysulfone. For fossil-based production, POX reduces GHG emissions by 34–45%. Bio-based production combined with renewable energy further reduces GHG emissions of HPTs by 55–78% but leads to environmental trade-offs. The economic evaluation of POX suggests a 26–35% price reduction compared to reference HPTs, and potential markups over 100% in the HPT market. Our results demonstrate how enhanced process efficiency of novel products such as POX can contribute to the decarbonizing polymer industry.
Introduction
Thanks to their versatile properties, plastics have become an indispensable part of our everyday lives. However, plastic production is expected to be the largest driver of oil consumption and a major source of greenhouse gas (GHG) emissions in the future.1 To reduce GHG emissions of commodity plastics such as polyethylene or polypropylene, multiple cost-efficient options are available, e.g., increasing process efficiency, recycling, or switching to an alternative resource basis such as biomass or CO2.1–4However, the properties of commodity plastics are not sufficient for advanced applications, e.g., in the aviation or electronics industry.5 Such advanced applications require a more specialized property profile, combining low density and high thermal stability with high elasticity modulus and chemical resistance.6 In contrast to commodity plastics, these characteristics are provided by high-performance thermoplastic polymers (HPTs).6,7 HPTs can be classified into semi-crystalline polymers, such as polyphenylene sulfide and polyether ether ketone, and amorphous polymers, such as polyetherimide (PEI), polyethersulfone (PES), and polysulfone (PSU) (see Fig. 1).5 HPTs have superior chemical and mechanical properties at temperatures higher than 150 °C resulting from the high aromatic content in the polymer backbone.5,7–10
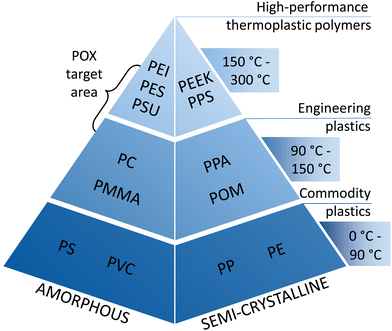 | ||
| Fig. 1 Polymer pyramid adapted from de Leon et al., 2021.9 Temperature ranges given correspond to typical values of heat distortion temperatures and continuous use temperatures of the polymers.10 For simplicity, only a few polymers are shown by their common abbreviation. | ||
Commonly used HPTs are often produced from complex monomers and via multi-step synthesis. The more complex production of HPTs increases production costs compared to commodity plastics.5 For example, the costs of PEI are about five to ten times, and the sales revenues are even up to twenty times higher than those for polyethylene (1–2 € per kg).11
The multi-step production also leads to high environmental impacts.12 HPTs have a significantly higher carbon footprint than commodity plastics.12 Accordingly, HPTs offer great leverage for reducing GHG emissions. Still, the environmental impacts of HPTs has been neglected in recent studies of global polymer production due to their relatively small production volumes compared to commodity plastics.1–4 However, HPT production volumes have risen sharply and will continue to rise due to increasing demands, e.g., in the electronics industry. For instance, the production volume of PEI is expected to increase by 4.5% and that of PES by 5.9% in the coming years.13 In comparison, the global plastic market is expected to grow at an annual growth rate of 3.4%.14 Besides improving current HPTs, the development of novel HPTs with low costs and environmental impacts seems promising.
Recent advancements in catalyst technology and process engineering have enabled the production of polyoxazolidinone (POX) as a new HPT.15–18 POX has a similar chemical structure and mechanical and chemical properties within the same range as commercial amorphous HPTs PEI, PES, and PSU (see the ESI for details†). Thus, we define PEI, PES, and PSU as reference HPTs for POX in this study.
Compared to the reference HPTs, POX has key advantages during production of increased process efficiency due to a 1-step polyaddition without by-products and highly available inputs.15–18 Thus, POX provides opportunities to reduce environmental impacts and costs compared to reference HPTs. Furthermore, in contrast to the reference HPTs, POX production is suitable for extrusion-based and solvent-free downstream processing (downstreaming).19
In addition, POX can be produced to a large extent from bio-based feedstocks. Bio-based production offers additional climate benefits, as already shown for commodity plastics.20 In addition, Das has shown that bio-based production also reduces GHG emissions of other advanced materials, such as carbon-fiber-reinforced plastics, by about 20% compared to the fossil reference.21 Thus, bio-based production of HPTs may offer additional savings in GHG emissions. However, bio-based production may result in environmental trade-offs, i.e., reducing GHG emissions but increasing other environmental impacts due to agriculture.22 These potential trade-offs have to be analyzed by a sound environmental assessment.
This study investigates the potential environmental impacts and production costs for a recently developed industrial-scale production process of POX.23 We apply a comparative Life Cycle Assessment (LCA) based on ISO 14040/14044 and a Techno-economic Assessment (TEA).24,25 Thus, we assess whether POX reduces environmental impacts compared to reference HPTs while being cost-competitive. Furthermore, we evaluate the environmental impacts of integrating bio-based feedstocks in the supply chain of POX and its reference products.
Goal and scope definition
The presented study aims to compare the potential environmental impacts of fossil-based POX production to reference HPTs. Furthermore, the study assesses potential future environmental impacts based on renewable energy and biomass as feedstock. For this purpose, we conduct a comparative Life Cycle Assessment of POX and its reference HPTs PEI, PES, and PSU. We follow the recommended procedure of Walker et al. and include all mandatory steps of the Product Environmental Footprint guidance in our assessment.26Furthermore, POX can only achieve the potential environmental benefits if it is cost-competitive compared to the reference HPTs, as commercialization largely depends on economic performance. Therefore, the TEA analyzes the cost of POX compared to the production costs of reference products by conducting a factorial-based cost estimation.
The following section first defines the functional unit of the LCA and TEA. Afterward, the scope of the assessments is described.
Functional unit
We choose PEI, PES, and PSU as reference products for POX due to their similar properties (see the ESI†). However, HPTS can be varied in essentially infinite chemical ways due to their flexibility in monomer and catalyst selection.13 Thus, the considered polymers should rather be regarded as families of materials with a few common chemical characteristics than single products.As the functional unit for comparison, we choose 1 kg of HPT. We model the production of the base resin without any additives since compounding depends on the application. We choose a mass-based functional unit since in TEA materials are usually compared per unit of mass. However, HPTs are also frequently replaced on a molded part-specific basis so that volume rather than mass can be the decisive unit. The density of POX (1.2 g cm−3)19 is lower than its reference products (1.24–1.37 g cm−3),10 while the mechanical and chemical stability is on par (details in the ESI†). Thus, less material could be required for the same molded part when substituting the reference products with POX. Therefore, a mass-based functional unit allows for a conservative assessment of the reduction potential of POX.
Scope of life cycle assessment
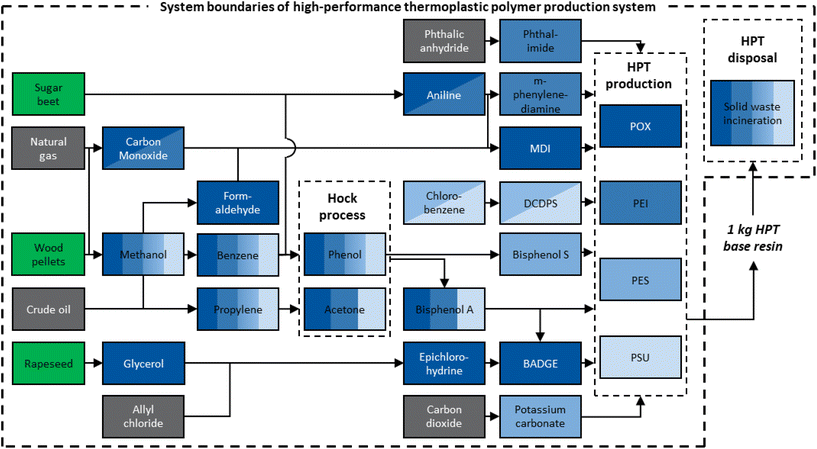
For comparative LCA, we apply cradle-to-grave system boundaries, including the supply chain, production, and final disposal (see Fig. 2). The use phase is assumed to be similar and is thus neglected from the assessment. However, depending on the HPT application, the use phase may have a significant influence on the life-cycle environmental impacts. Therefore, please note that the absolute environmental impacts of HPTs are higher when considering the entire life cycle. At the same time, HPTs might replace other materials that are environmentally more harmful, leading to environmental benefits from the use phase. This analysis needs to be carried out for each application. Accordingly, in this study, relative savings refer only to the system boundaries of HPT production and disposal.As the so-called foreground system, we modeled the fossil-based supply chain and included options to integrate bio-based chemicals: methanol, carbon monoxide, aniline, ethanol, and glycerol.
For the background system, we used aggregated GaBi data sets. If available, we used data sets for the region of Germany. Otherwise, we used European data. Furthermore, we neglect plant construction in the LCA's foreground system because the environmental impacts of plant construction are typically small for chemical products and probably similar for all HPTs.27 However, we consider the capital investment of plant construction in the TEA.
For bio-based chemicals, we account for the CO2 absorbed from the atmosphere during the biomass growth phase. The amount of CO2 absorbed depends on the carbon content of the biomass. For absorbing 1 kg of CO2, we give a credit of 1 kg CO2-equivalent emissions (CO2-eq.) as negative GHG emissions. Furthermore, we consider land-use change emission using aggregated datasets from the LCA database GaBi.12 In addition, we conduct a sensitivity analysis on land-use change emissions in the ESI.†
For the environmental impacts of PEI, an aggregated dataset is available in GaBi.12 However, aggregated datasets do not provide insights into a product's production and supply chain. Thus, the aggregated dataset cannot be applied to fulfill the goal of this study and assess the bio-based production of PEI. Furthermore, for PES, no data sets are available in commercial databases. A dataset for PSU is available in Ecoinvent.28 However, the dataset is modeled based on stoichiometry and, therefore, only represents a rough estimate of the environmental impacts of PSU. Thus, the PSU dataset does not meet the required technical appropriateness for a consistent assessment of all HPTs. Accordingly, we modeled the production of PEI, PES, and PSU to identify environmental hotspots, enable insights into their supply chains and ensure a high and consistent data quality.
The life cycle inventories of HPT production are based on patents and experimentally validated process simulations conducted by Covestro Deutschland AG and NexantECA.13,19 According to Parvatker et al., process simulations are the most accurate methods to generate life cycle inventories if actual plant data are missing.29 Therefore, the data quality is regarded as sufficient to assess the environmental impacts of the considered HPTs.
The supply of process steam and electricity in HPT production and supply chains assumes a natural gas boiler with an efficiency of 95% and the 2019 electricity grid mix from GaBi.12 Additionally, we assess the environmental impacts of future HPT production by assuming low-carbon power for electricity supply represented by the current wind power and process steam production via electric boiler with 95% efficiency as a best-case assumption for GHG emissions. Furthermore, we use biogas as a renewable alternative to natural gas if high-temperature heat is required. Accordingly, this study assesses four scenarios of HPT production, summarized in Table 1.
| Scenario | Feedstock type | Electricity source | High-temperature heat source |
|---|---|---|---|
| Conventional | Fossil | Grid mix | Natural gas |
| Biomass | Biomass | ||
| Renewable energy | Fossil | Low-carbon (represented by wind power) | Biogas |
| Renewable carbon & energy | Biomass |
The production of POX does not result in any by-products. However, both the reference HPT production and the supply chains yield by-products, thus making these processes multifunctional. We solve the multifunctionality problem by giving credit for the avoided conventional production whenever possible. If no conventional process exists or sufficient data on the conventional process are not available, we apply mass allocation. For the given product system, the impact of this allocation is expected to be small.
POX has a lower melting temperature (∼170 °C) compared to the reference products (∼180–220 °C), which promises lower environmental impacts in further processing steps, e.g., by injection molding. However, detailed modeling of further processing requires defining the application, as it influences crucial process parameters such as the number of pieces and storage conditions. Due to the variety of HPT applications, this study avoids determining a single application and focuses on the materials. Therefore, we do not consider further processing in this study.
Depending on the application, the use phase may determine the life cycle emissions of HPTs, e.g., if used in lightweight construction. Here, HPTs compete with other advanced materials that may emit more GHGs during production but further reduce the weight of the final product compared to HPTs. Thus, use phase emissions of HPTs might be higher compared to other materials, resulting in trade-offs between life cycle phases.30 These trade-offs strongly depend on the application and other parameters like the lifetime of the materials. However, to the best of the authors’ knowledge, no study exists that quantifies the life cycle emissions of HPTs compared to other advanced materials. We assume that the environmental impacts of the use phases of POX and reference HPTs are similar and can thus be neglected in a comparative LCA. The similar density and mechanical and chemical properties of HPTs support this assumption.
We do not consider recycling in our assessment due to the poor data availability on the recyclability of HPTs. However, by applying simplified assumptions for the recycling efficiency of PSU, Schwarz et al. found that HPTs have the lowest environmental impacts if primary recycling (dissolution or closed-loop mechanical recycling) is used due to the high environmental impacts of the PSU production phase.31 Primary recycling requires either pure or well-sorted PSU mono streams. Decreasing sorting efficiency negatively affects the environmental impacts of PSU recycling to the extent that primary recycling may perform worse than other recycling technologies.31 However, the collection and sorting efficiencies of HPTs are limited, as HPTs are primarily applied in smaller quantities compared to commodity plastics. Thus, depending on the application, the environmental impacts from the collection and separation of HPTs may outweigh the environmental benefits of HPT recycling.
As end-of-life treatment, we consider incineration. We adopted the incineration model from Meys et al.,32 which was modeled according to Doka.33,34 The model accounts for all environmental impacts of flue gas emissions, flue gas cleaning, and disposal of residuals. Potential energy production from incineration is not considered, and all emissions are allocated to waste treatment representing a worst-case assumption.33,34 Furthermore, we assume that non-usable by-products and wastes from HPT production and their supply chains are treated by incineration. Accordingly, we apply the incineration model to close the mass balances for all unit processes.
As impact assessment methods, we use all methods recommended by the Joint Research Center in the framework of the Environmental Footprint 3.0.35 In the main article, we show the impact category of climate change as a primary driver for the development of novel HPT. Furthermore, bio-based processes, in particular, tend to shift environmental burdens from climate change to other impact categories, e.g., acidification and eutrophication.22 Consequentially, we also assess acidification and eutrophication in the main article. All other environmental impacts can be found in the ESI.†
Scope of techno-economic assessment
POX must be economically viable to compete in the world market.36 Accordingly, we conduct a TEA to quantify POX economic performance and compare it with the reference products. The scope of TEA assumes a base case scenario that encompasses an 8 kt stand-alone plant producing 8000 h per year in Germany in 2021 with a lifetime of ten years.For the TEA, the subject of analysis is HPT production. Therefore, the system boundaries are the gates of the manufacturing company. However, the TEA considers input market prices and, thus, all upstream costs. Accordingly, the scope of the TEA is equivalent to cradle-to-gate system boundaries in LCA. Downstream costs for disposal and recycling are expected to be similar for all HPTs and usually not attributed to the manufacturer. Thus, downstream costs are neglected in this analysis.
The TEA considers capital and production costs to obtain the total cost of production per kilogram of polymer, i.e., no compounding, packing, or shipping is considered. For this analysis, the methodology and terminology of Sinnott and Towler are used unless otherwise specified.37
Capital costs include fixed capital investment and working capital. The fixed capital investment comprises the costs of building the actual plant, called inside battery limit (ISBL) costs, all additional offsite costs for site infrastructure modifications called outside battery limit (OSBL) costs, design and engineering costs, and contingency charges. ISBL costs are derived from major equipment costs and respective installation factors. OSBL costs depend on existing site infrastructure and can vary from 10% to 100% of ISBL. We used 40% of ISBL as a common practice.37 Design and engineering costs range between 30% of ISBL and OSBL costs for small and 10% for larger projects. We assumed 25% of ISBL and OSBL costs due to a relatively small production capacity of 8 kt/year for all HPTs. Contingency charges account for unforeseen events and are typically 10% of ISBL and OSBL costs. Working capital is the capital needed for plant start-up and operation until it generates income and comprises seven weeks of cost of production, two weeks of feedstock costs, and 1% of fixed capital investment.37
Several static and dynamic methods exist to calculate the annual capital charge from capital costs. Following the analysis of NexantECA, static costs are calculated, i.e., no periodic cash flows are considered. Thus, capital costs are divided by the plant lifetime of ten years, assuming erection and start-up to be in period zero, i.e., a linear depreciation over ten years is applied as a common value in the petrochemical industry. Working capital is not included in the static profit calculation since it is recovered at the end of the plant lifetime. Furthermore, financing and taxes are not considered. An overview of fixed capital investment and annual capital charge calculations can be found in the ESI.†
The production costs consist of variable and fixed costs of production. Variable costs of production comprise materials, utilities, effluent disposal, packaging, and shipping. Materials and utilities required for the synthesis are valued according to their industry bulk purchasing prices (details in the ESI†). Materials include reactants and consumables. Effluent disposal is not considered due to a lack of data. Additionally, packaging and shipping are out of the scope of this analysis.
Fixed costs of production (COPs) do not vary with the plant output rate, e.g., personnel, maintenance, or general overhead charges. Fixed COPs are derived from factors on estimated labor and capital costs (see the ESI for details†).
No ISO Norm exists for conducting a cost estimation in the chemical industry. However, there are many established methods for allocating the aforementioned cost positions.37–44 To ensure a consistent comparison with the reference polymers, the analysis is aligned with those of PEI, PES, and PSU by NexantECA, i.e., assumptions and methodology were harmonized where reasonable, and adjusted to the approach of Sinnott and Towler.37
For the reference HPTs, the ISBL costs are adopted from NexantECA. NexantECA does not provide detailed information about the ISBL estimation. However, the description of the ISBL cost items in the NexantECA report is similar to the ISBL calculation proposed by Sinnott and Towler.37 Thus, the ISBL costs of POX and reference HPT are expected to be comparable. Given the ISBL costs, the OSBL costs are derived as stated above. Design and engineering costs and contingency charges are included in the ISBL and OSBL costs for the reference HPTs.
Calculating the fixed capital investment of POX requires an estimation of the major equipment costs of the preliminary process design. In the early stages of process design, data availability and uncertainty limit the assessment methodology pool. For POX, a detailed factorial American Association of Cost Engineers class 4, which corresponds to a technology readiness level (TRL) 5 to 6 method, is applied.45 Typical uncertainty ranges of fixed capital investment at these project levels are −30% and +50%, depending on technology type and complexity, data availability, and contingency considerations.46 Based on a preliminary process design and equipment list,19 equipment costs for the POX process are calculated using the process cost correlations from Sinnott and Towler and further assumptions (details in the ESI†).37 The equipment is assumed to be in stainless steel 304. ISBL costs are derived by applying installation factors from reference37 to the equipment cost (details in the ESI†). OSBL costs, design and engineering costs, and contingency charges are calculated using the abovementioned factors.
The ISBL plant cost by NexantECA is based on a 10 kt capacity and converted to an 8 kt by the “six-tenths rule” to facilitate comparison.37 Using the six-tenths rule, the ISBL costs of the smaller capacity are approximated by multiplying the costs of the higher capacity with the capacity ratio to the power of an exponent n. The exponent n varies from 0.4 to 0.9 depending on the scale and instrumentation degree of the process. We use a factor of 0.6 as the average within the chemical industry.37
NexantECA cost data for the benchmark polymers is provided in U.S. Dollars in the first quarter of 2021 and based on facilities, materials, utilities, and labor in Western Europe. ISBL costs are converted to Euro using the exchange rate of U.S. Dollars to Euro in Q1 2021. A list of all material and utility prices is available in the ESI.†
Production of high-performance thermoplastics
To assess POX's environmental and economic performance compared to the reference HPTs, Covestro Deutschland AG provided us with energy and material requirements for an industrial-scale POX process.19 Each step of the POX process has already been proven on a lab or pilot scale. Thus, the present inventory data is regarded suitable for evaluating the potential impacts of an up-scaled industrial process.The production of the reference products PEI, PES, and PSU is modeled using process data from NexantECA.13 The NexantECA data contains detailed information about reactants and utilities such as electricity, heat, and cooling demands. However, the data do not always include information about auxiliary materials such as chain stoppers. Therefore, the data were checked for consistency and adjusted or extended if necessary. In addition, the reference products are standard commercially available HPTs without additives, thus ensuring a consistent comparison with POX. The life cycle inventories of POX and reference HPTs are listed in Table 2.
| Function | Input | Value | Unit | Modeled as | |||
|---|---|---|---|---|---|---|---|
| POX | PEI | PES | PSU | ||||
| Product | Polyoxazolidinone | 1000 | — | — | — | kg | Modeled using industry data |
| Polyetherimide | — | 1000 | — | — | kg | Modeled using data from NexantECA, details in ESI† | |
| Polyethersulfone | — | — | 1000 | — | kg | Modeled using data from NexantECA, details in ESI† | |
| Polysulfone | — | — | — | 1000 | kg | Modeled using data from NexantECA, details in ESI† | |
| By-products | Dilute nitric acid (60%) | — | 2200 | — | — | kg | GaBi – DE: nitric acid (60%) |
| Reactants | Bisphenol A diglycidyl ether | −549 | — | — | kg | Modeled using data from NexantECA, details in ESI† | |
| Methylene diphenyl diisocyanate | −420 | — | — | kg | Modeled using data from NexantECA, details in ESI† | ||
| Bisphenol A | — | −412 | — | −526 | kg | Modeled using data from NexantECA, details in ESI† | |
| Phthalic anhydride | — | −550 | — | — | kg | GaBi – DE: Phthalic anhydride | |
| n-Methyl phthalimide | — | −70 | — | — | kg | Modeled based on stoichiometry, details in ESI† | |
| m-Phenylenediamine | — | −192 | — | — | kg | p-Phenylenediamine used as a proxy, details in ESI† | |
| Nitric acid | — | −2500 | — | — | kg | GaBi – DE: nitric acid (98%) | |
| Caustic soda | — | −300 | — | −105 | kg | GaBi – DE: sodium hydroxide (caustic soda) mix (100%) | |
| 4,4′-Dichlorodiphenyl sulfone | — | — | −631 | −662 | kg | Modeled using data from NexantECA, details in ESI† | |
| 4,4′-Dihydroxydiphenyl sulfone | — | — | −550 | — | kg | Modeled using data from NexantECA, details in ESI† | |
| Potassium carbonate | — | — | −286 | — | kg | Modeled using data from ecoinvent | |
| Chain stopper | p-tert-Butylphenyl glycidyl ether | −28 | — | — | — | kg | BADGE used as a proxy |
| Triethylamine | — | −11 | — | — | kg | Modeled using data from ecoinvent | |
| Methyl chloride | — | — | −6 | −7 | kg | Modeled based on stoichiometry | |
| Catalyst | Others | −3 | — | — | — | kg | POX catalyst is confidential, other catalysts are not considered |
| Solvents | Benzonitrile | −10 | — | — | — | kg | Modeled based on stoichiometry, details in ESI† |
| Utilities | Electricity | −2036 | −6545 | −6160 | −6160 | MJ | GaBi – DE: electricity grid mix (2019)/DE: electricity from wind power |
| Steam, medium pressure | −6648 | −22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
−20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
kg | GaBi – DE: process steam from natural gas 95% or modeled separately, details in ESI† | |
| Cooling water | −116 | −732 | −628 | −610 | m3 | GaBi – DE: Tap water from surface water | |
| Fuel gas | 0 | −651 | −56 | −51 | kg | GaBi – DE: natural gas mix | |
| Inert gas | 0 | −0.1 | −0.1 | −0.1 | kg | GaBi – DE: nitrogen (gaseous) | |
The modeling of POX is described in detail below. For the reference products see the ESI.† POX can be produced via many routes, the most promising being the 1-step polyaddition of diisocyanates and di-epoxides.15,47 The main limitation of this route is the required chemoselectivity since trimerization of isocyanates in POX polymerization leads to the formation of insoluble products. Recent developments have identified a catalyst system and reaction conditions for a highly selective formation of the oxazolidinone group via polyaddition.15,47 This development enables the production of linear POX with a high molecular weight, which can be thermally processed in subsequent steps.
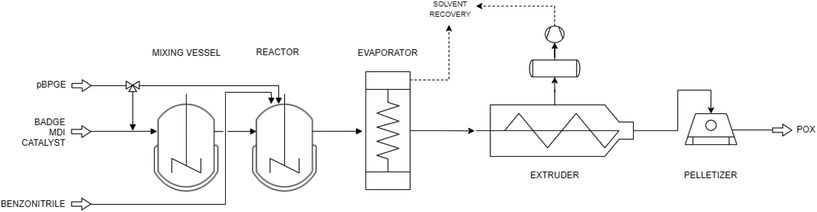
We modeled POX production as a 1-step polyaddition of bisphenol A diglycidyl ether (BADGE) and methylene diphenyl diisocyanate (MDI) with p-tert-butyl phenyl glycidyl ether (pBPGE) as the chain terminator (see Fig. 3 and 4). The mass balance is derived from the reaction stoichiometry for a POX composition with a molecular weight of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. Deriving the mass balance from reaction stoichiometry and assuming complete conversion is reasonable since no by-product and other residues are expected in industrial practice. Benzonitrile is used as the reaction solvent. After heated premixing of BADGE the catalyst, benzonitrile, and the first charge of the chain terminator, the mixture is passed to a reactor where MDI is added, and polymerization is initiated. Polymerization is ended by adding a second charge of the chain terminator.
000 g mol−1. Deriving the mass balance from reaction stoichiometry and assuming complete conversion is reasonable since no by-product and other residues are expected in industrial practice. Benzonitrile is used as the reaction solvent. After heated premixing of BADGE the catalyst, benzonitrile, and the first charge of the chain terminator, the mixture is passed to a reactor where MDI is added, and polymerization is initiated. Polymerization is ended by adding a second charge of the chain terminator.
 | ||
| Fig. 3 Simplified process flowsheet of the POX synthesis and the extrusion-based downstream processing. | ||
POX is purified by extrusion-based downstreaming, which enables a high processing temperature and, thus, a low viscosity of the reactor slurry. Therefore, extrusion-based downstreaming allows for a high solid reaction content of 50 wt% between reactants and benzonitrile. For benzonitrile recovery, we assumed a solvent recovery rate of 99% as a standard value in industrial practice.19 The provided energy requirements for the extrusion-based process are based on process simulations conducted using the commercial flowsheeting software Aspen Plus®.19
The reactants of POX production result from a complex supply chain that causes a high share of POX's overall environmental impact (see Fig. 2). Accordingly, changes in the supply chain from fossil-based to renewable feedstock may reduce the overall impact. Thus, we modeled the supply chain of POX and assessed the environmental impact of their bio-based production. We considered the following bio-based chemicals since they are applicable in the HPT supply chain, and sufficient data for modeling were available: aniline, methanol, carbon monoxide, glycerol, and ethanol.
Aniline, methanol, and carbon monoxide are used to produce MDI.48 For aniline, a bio-based process was recently developed.49 Bio-based methanol is produced via the gasification of wood chips and the subsequent conversion to methanol. Methanol can be integrated into the POX supply chain by the methanol-to-olefins and methanol-to-aromatics processes to produce propylene and benzene, respectively. Propylene and benzene are used as feedstocks in the Hock process to produce phenol and acetone, the feedstocks for bisphenol A. Bisphenol A, in turn, is the primary feedstock for BADGE. Furthermore, the gas produced from the gasification process can be separated into carbon monoxide and hydrogen.
The second feedstock necessary for producing BADGE is epichlorohydrin.50,51 For bio-based production, we considered epichlorohydrin from glycerol. Bio-based production of epichlorohydrin has increased since glycerol became a cheap feedstock alternative as a by-product of biodiesel production.50,52 Furthermore, bio-based ethanol is used in the PEI supply chain. Since ethanol is nowadays mainly produced in a bio-based manner, we did not assess fossil-based ethanol.
More information on the reference products, the supply chain modeling, and a list of all LCA datasets can be found in the ESI.† Furthermore, we added a list of process yields for the most important chemical intermediates to the ESI.†
Environmental impacts of high-performance thermoplastics
In the following section, we first quantify the climate change impacts of POX in comparison to the reference HPTs. Here, we assess four scenarios differing in feedstock type and the supply of electricity, process steam, and fuel gas. Furthermore, we show acidification and eutrophication in the main article to analyze potential burden-shifting, i.e., shifting environmental impacts from climate change to other impact categories. All other environmental impacts are shown in the ESI.†Climate change
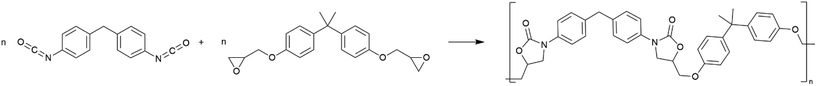
In the conventional scenario, fossil-based production leads to 9.3 kgCO2-eq. per kg POX and 14.4–16.8 kgCO2-eq. per kg reference HPTs (Fig. 5). For POX production, 51% of GHG emissions result from feedstock supply, 17% from the energy supply, and 3% from the chain terminator and solvent supply and disposal. The end-of-life (EoL) treatment emits the remaining 30%.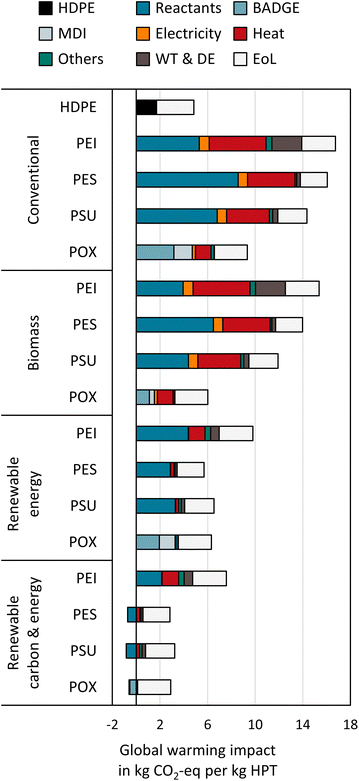 | ||
| Fig. 5 Global warming impact of 1 kg of high-performance thermoplastic polymers (HPT) under four scenarios: (1) fossil-based feedstock with fossil energy (conventional), (2) bio-based feedstock with fossil energy (biomass), (3) fossil-based feedstock with renewable energy using wind power and biogas (renewable energy), and (4) bio-based feedstock with renewable energy using wind power and biogas (renewable carbon & energy). We additionally show high-density polyethylene (HDPE) as a reference.53 Further abbreviations: BADGE = bisphenol A diglycidyl ether, MDI = methylene diphenyl diisocyanate, WT & DE = waste treatment and direct emissions, EoL = end-of-life treatment. | ||
Compared to the reference HPTs, POX production achieves an 11–45% reduction in GHG emissions from feedstock supply and 64–72% savings in energy supply. Savings in energy supply mainly result from POX's lower process steam demand compared to the reference HPTs. For PEI, the higher process steam demand results from the more complex production requiring four process steps compared to the 1-step polyaddition in POX production. For PES and PSU, the production complexity is lower than that for PEI, but the supply of the reactants already emits 31–45% more GHGs than the supply of MDI and BADGE. The high GHG emissions from the PES and PSU supply chain arise from the production of organosulfur compounds bisphenol S and DCDPS.
To investigate the impact of process yields, we added a sensitivity analysis to the ESI,† elaborating on the influence of key chemical intermediates and utilities on the climate change impacts of HPT. The sensitivity analysis shows that especially increased process steam demands and decreasing process yields can have a significant influence of up to 23% on the GHG emissions of HPT. Still, POX remains climate beneficial compared to its reference HPT, even under unfavorable process conditions.
Due to the solvent-free downstream processing via extrusion, the direct emissions from waste treatment are 90–99% lower for POX than for the reference HPTs. Direct emissions are particularly high for PEI due to the high amount of fuel gas burned in PEI production. The EoL emissions of the reference HPTs are 12–18% lower for PES and PSU than for POX due to their lower carbon contents. EoL treatment of PEI emits equal amounts of GHGs as POX. The EoL emissions of HPTs are quantified assuming complete combustion. Recycling HPTs could potentially reduce EoL emissions and substitute virgin production. However, as mentioned above, HPTs are applied in lower quantities compared to commodity plastics. Thus, HPT recycling would either require separate reverse logistics concepts or lead to high sorting efforts. Both approaches may outweigh the environmental benefits of HPT recycling compared to incineration.
Compared to commodity plastics like high-density polyethylene, GHG emissions of HPTs are about 2–3 times higher from cradle-to-grave and even 4–8 times higher from cradle-to-gate.53 These substantially higher GHG emissions stress the need for GHG mitigation options discussed below.
In the biomass scenario, POX production leads to GHG emissions of 6.0 kgCO2-eq. per kg, thus 35% less compared to fossil-based production. These reductions are due to the reduced impact of bio-based BADGE and MDI. Bio-based BADGE reduces 65% and MDI 73% of their GHG emissions compared to their fossil counterparts.
The bio-based production of reference HPTs reduces GHG emissions by 8–17%. Savings result mainly from bio-based bisphenol A for PEI and PSU and bio-based phenol in bisphenol S production for PES. For PEI, in particular, GHG emissions could be reduced further by using bio-based xylene to produce phthalic anhydride in the supply chain. However, no data of sufficient quality were available for modeling bio-based xylene. In addition, LUC emissions only increase GHG emissions from bio-based production to a minor extent of 1–8% (see the ESI for details†).
The utilization of wind power and biogas in the renewable energy scenario saves about 96% of GHG emissions from the energy supply in POX production. Additionally, 31% savings from feedstock supply can be achieved due to reduced emissions in the supply chain. For the reference HPTs, using renewable energy in HPT production reduces 75–93% of GHG emissions from energy supply. An additional 17–67% of GHG emissions from feedstock supply can be reduced by using renewable energy in the HPT supply chain. The higher savings from feedstock supply for PES and PSU results from the energy-intensive production of DCDPS and bisphenol S. For DCDPS and bisphenol S, GHG emissions are reduced by 53% and 85%, respectively. Overall, using renewable energy results in PES exhibiting the lowest GHG emissions of all HPTs, followed by POX and PSU.
In the renewable carbon and energy scenario, POX production emits 2.3 kgCO2-eq. per kg GHGs, corresponding to a saving of 75% compared to fossil-based production. POX's cradle-to-gate impact is even negative, meaning that more bio-based carbon stored in POX than fossil-based carbon is emitted in production.
Bio-based production with renewable energy reduces GHG emissions of reference HPTs to 2.1–7.6 kgCO2-eq. per kg. The remaining GHG emissions of PEI are mainly related to the supply of m-phenylenediamine and phthalic anhydride. For m-phenylenediamine, low-carbon ammonia could further reduce GHG emissions.
In general, producing HPTs based on bio-based feedstocks and renewable energies reduces 55–87% GHG emissions compared to fossil-based production. In the renewable carbon and energy scenario, POX emits only slightly more (<10%) GHGs than PES and PSU, even though best-case assumptions were made for the reference HPTs without considering solvent and catalyst consumption. Thus, POX is expected to substantially reduce GHG emissions compared to the benchmark HPTs for fossil- and bio-based production, while leading to similar climate impacts as the best benchmark HPTs in low-carbon energy scenarios. However, especially bio-based feedstocks bear the risk of burden-shifting from GHG emissions to other environmental impacts. Therefore, we assess these other environmental impacts in the following section.
Acidification
PEI has the highest impact on acidification in all scenarios, followed by PES, PSU, and POX (see Fig. 6). The high impact of fossil-based PEI results from m-phenylenediamine production (25%) and direct emissions of nitrogen-containing compounds from waste treatment (35%). In the renewable energy scenarios, the incineration of biogas as a fuel gas substitute for natural gas further increases PEI's acidification potential due to higher nitrogen oxides and sulfur dioxide emissions.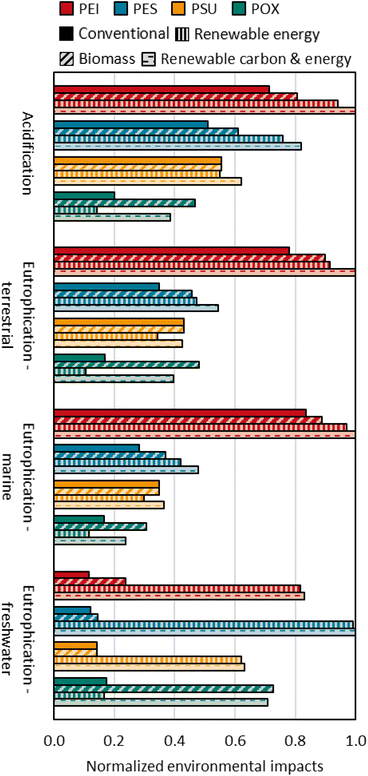 | ||
| Fig. 6 Environmental impacts of high-performance thermoplastic polymers normalized to the maximum environmental impact in each category. The scenarios are shown as patterns. | ||
For fossil-based PES and PSU, the highest impact on acidification results from the supply of organosulfur compounds (63–75%). POX has the lowest impact on acidification in all scenarios, reducing impacts by 15–74% compared to the best HPT. However, the bio-based production of POX increases acidification due to bio-based glycerol in epichlorohydrin production. The acidification from the aggregated glycerol process mainly results from direct ammonia emission to the air.12
Eutrophication
Eutrophication is classified into terrestrial, marine, and freshwater eutrophication (Fig. 6). For HPT production, terrestrial eutrophication follows the same trend as acidification since both impacts result mainly from inorganic, nitrogen-containing emissions to the air. Marine eutrophication also shows similar results to acidification and terrestrial eutrophication. Thus, POX has the lowest impact on marine eutrophication in all scenarios and reduces impacts by 12–61% compared to the best HPT. However, only 64–76% of PEI's marine eutrophication is related to nitrogen-containing emissions to air, and the other 30% is caused by nitrate and ammonium emissions to freshwater. For the other HPTs, the share of marine eutrophication from freshwater emissions ranges between 10 and 17% for POX and 6 and 31% for PES and PSU.For freshwater eutrophication, fossil-based POX shows the highest impact compared to the fossil-based production of the reference HPTs. The higher impact results mainly from catalyst production. However, please note that catalyst production is not considered for the reference HPTs leading to a worst-case benchmarking study for POX (details in the ESI†). Thus, considering catalysts for reference HPTs might also increase freshwater eutrophication. Furthermore, switching to bio-based feedstocks and biogas increases freshwater eutrophication by up to 4 times for POX and up to 8 times for the reference HPTs.
Other environmental impacts
Considering the other environmental impact categories in the conventional scenario, POX reduces environmental impacts in 23 out of 25 categories compared to the reference HPTs (details in the ESI†). However, the catalyst in POX production increases ozone depletion. In the renewable energy scenarios, the supply of bio-based glycerol for POX production emits lead and mercury, which increase human toxicity. However, due to the high uncertainties, the Joint Research Center assigned the human toxicity categories with a recommendation level III (“recommended, but to be applied with caution”), and should therefore be interpreted with caution.Overall, compared to the reference products, POX shows only a minor shifting of environmental impacts from GHG emissions to other environmental impacts. Nevertheless, large-scale production of POX requires a detailed regional assessment of all environmental impacts.
Economic evaluation of high-performance thermoplastics
This section elaborates on the capital costs, material and utility costs, and total COP of POX and the reference HPTs PEI, PES, and PSU.Capital costs
The comparison of capital costs between HPTs is discussed via ISBL costs only since OSBL costs, design and engineering costs, and contingency charges are calculated based on ISBL costs. POX ISBL costs derive from the equipment costs of major process steps, i.e., mixing and reaction, evaporation, extrusion, and pelletizing, and amount to 26 Mio € (see Fig. 7). Accordingly, ISBL costs are 74% lower for POX than for PEI and 60% lower than for PES and PSU. ISBL costs of PES and PSU are the same according to Nexant13 and account for 64 Mio €, while ISBL costs of PEI are the highest at 99 Mio €. High PEI ISBL costs result from a multi-step process with integrated monomer production and several loop cycles for recovery and recycling cycles of solvents and reactants. | ||
| Fig. 7 Estimated capital costs of POX, PEI, PES, and PSU. Exact numbers of capital cost estimation for all HPTs can be found in the ESI.† Further abbreviations: D&E = design and engineering costs, WC = working capital, FCI = fixed capital investment. | ||
POX has a capital cost advantage of 91 Mio € compared to PEI and 44 Mio € and 42 Mio € compared to PES and PSU, respectively. The cost advantage results from the extrusion-based process design that avoids large recycling steps from precipitation or washing. However, equipment costs for the POX process are not derived from price quotations or detailed purchased equipment lists. Instead, POX equipment costs are estimated by applying cost correlations and therefore must be interpreted carefully.
Working capital as a function of feedstock costs, variable production costs, and fixed capital investment is the highest for PES due to cost-intensive monomers.
Material costs
Material costs for all HPTs are shown in Fig. 8 (data in the ESI†). Monomer costs account for the largest share of material costs for all HPTs. The epoxide monomer BADGE and the diisocyanate monomer MDI represent 93% of POX material costs which sum up to 3.25 € per kg. The highest monomer and material costs of 4.45 € per kg include that of PES due to the high-priced organosulfur compounds bisphenol S and DCDPS. Together with bisphenol A, high-priced DCDPS is also used as a monomer in PSU synthesis resulting in overall material costs of 3.05 € per kg. PEI has the lowest material costs of 1.95 € per kg since the monomer synthesis is integrated into PEI production, and no high-priced monomers must be purchased.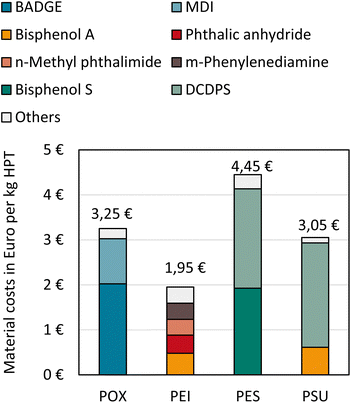 | ||
| Fig. 8 Material costs for POX, PEI, PES, and PSU per kg product. Exact numbers of capital cost estimation for all HPTs can be found in the ESI.† | ||
Monomer and other chemical costs are subject to fluctuations. However, major increases or decreases in relevant cost items are only observed in times when oil prices also show larger fluctuations. Thus, the material costs of all HPTs are likely to fluctuate in constant proportions to the oil price.54 Furthermore, substantial increases in feedstock prices are passed on to consumers resulting in approximately constant margins so that only short-term margin losses are expected when feedstock prices rise rapidly.37
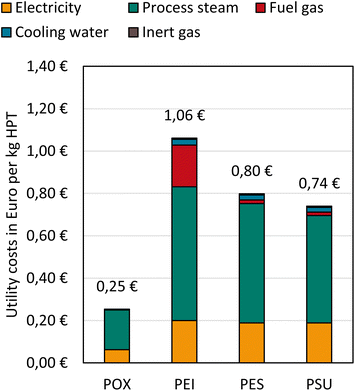
Utility costs
For HPT production, the main utility costs include energy, process steam, cooling water, fuel, and inert gas (see Fig. 9). Assumed industry prices and reference data are shown in the ESI.† There is no data available for POX inert gas consumption, but we expect only a minor impact on the overall utility costs. Furthermore, we assume that no fuel gas is consumed in POX production.Process steam costs comprise the highest utility share for all HPTs followed by electricity costs. For POX, 74% of utility costs are attributed to process steam and 24% to electricity.
PEI has the highest utility costs of 1.06 € per kg due to an elaborated and energy-intensive process with integrated monomer production. PEI has the highest fuel gas consumption, constituting 18% of total utility costs. PES and PSU have similar overall utility costs of 0.80 € per kg and 0.74 € per kg, respectively.
The overall utility costs of POX are the lowest due to the less resource and energy-intensive production of extrusion-based, solvent-free processing.
Total cost of production
The total COP comprises material and utility costs, fixed COP, and the annual capital charge (see Fig. 10). The fixed COP, comprising mainly of labor, overhead, and maintenance, are derived via factors of labor or capital cost items (see details in the ESI†). Since the applied factors are the same for all HPTs and depend linearly on labor and capital costs, the results of the fixed COP are not discussed in detail. Total fixed COP amounts to 0.98 € per kg for POX, 1.46 € per kg for PSU and PES, and 2.11 € per kg for PEI. Since the largest share of the fixed COP derives from factors applied to capital costs, it is apparent that PEI, having the highest ISBL costs, also has the highest fixed COP compared to the other HPTs.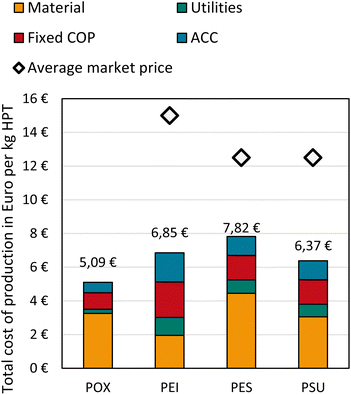 | ||
| Fig. 10 Total cost of production of POX, PEI, PES, and PSU. Market prices are taken from Biron 2018.55 Further abbreviations: FCOP = fixed cost of production, ACC = annual capital charge. | ||
The total COP of POX is 5.09 € per kg resulting in a cost advantage of 26% over PEI, 35% over PES, and 31% over PSU. POX's material costs account for 64% of the total COP, followed by 19% fixed COP and 12% annual capital charge.
PEI costs amount to 6.85 € per kg, and the largest shares are the fixed COP with 31% of the total COP, followed by 28% material costs. Due to monomer process integration and low-priced reactants, a relatively large amount (25%) of PEI costs is capital charges. Among all HPTs, the energy costs of PEI are the highest and makeup 15% of the total COP. The market price of PEI is about two times the total COP, indicating a possible markup of more than 100%.55 The disproportionally high markup may result from the fact that SABIC, the dominating PEI manufacturer, is likely to determine PEI's market price. Furthermore, PEI is used in special applications such as aviation, where its superior performance leads to a dependency on PEI.
PES total COP is the highest amongst the HPTs at 7.82 € per kg. Material costs make up 57%, followed by 19% fixed COP, 14% annual capital charge, and 10% utility costs. PSU's total COP amounts to 6.37 € per kg, comprising 48% material costs, 23% fixed COP, 18% annual capital charge, and 12% utility costs. The markups of PES and PSU range between 60 and 96%. The analysis shows that POX production via extrusion is highly cost-competitive against reference HTPs with 26–35% cost advantages. Accordingly, our analysis indicates possible markups for POX between 145 and 195% if similar market prices can be achieved.
POX's net present value (NPV) is 277 Mio € for a 10-year plant lifetime, a 1.35% interest rate, and a market price of about 10 € per kg corresponding to a markup of 97% (see the ESI for details†). To account for uncertainty in economic viability, we vary the interest rates and market prices of POX and reference HPTs in a sensitivity analysis (see the ESI†). The sensitivity analysis shows that POX has the highest NPV among all HPTs in every scenario. The NPV of reference HPTs ranges between -214 Mio € for PES in the least favorable scenario and 599 Mio € for PSU in the most favorable scenario. In comparison, POX has an NPV range from 29 to 694 Mio €. The high NPV of POX is a solid positive indication of an economically viable project.
Conclusion
High-performance thermoplastic polymers have become an essential building block for the industry due to their specialized property profile and high mechanical and thermal stability. However, the production of HPTs results in high environmental impacts and costs, which were, therefore, holistically investigated in this study. For this purpose, we conducted an LCA and TEA on the recently developed, amorphous HPT POX and its reference products PEI, PES, and PSU.For fossil-based production, POX reduces 35–45% of GHG emissions compared to the reference HPTs. Please note that relative savings refer to HPT production and the end-of-life treatment by incineration, whereas the use phase is not considered. POX reduces GHG emissions in the feedstock supply and by saving process energy. Savings in process energy result mainly from POX's simplified, extrusion-based downstreaming.
By switching to bio-based production with renewable energy, GHG emissions of POX decrease by 75%, and those of reference HPTs by 55–87%. GHG emissions may decrease further by implementing circular production and disposal systems based on recycling. In particular, these systems are promising for larger volume applications such as battery cases for electric vehicles. In these applications, HPTs are easily accessible for reverse logistics, leading to only low environmental impacts from collection and sorting, provided they are not mixed or combined with other materials.
Other environmental impacts such as freshwater eutrophication increase by 4–8 times when bio-based products are used. Therefore, environmental trade-offs must be considered in detail before large-scale implementation.
We used conservative assumptions to evaluate POX environmental impacts compared to the reference HPTs, i.e., a mass-based functional unit, assessing catalyst consumption for POX and neglecting it for the reference HPTs, and an ideal solvent recovery rate of 100% for the reference products. Furthermore, the sensitivity analyses of key process parameters for POX and reference HPT production show that POX is climate beneficial, even under unfavorable process conditions (see the ESI for details†). Accordingly, we are confident that the environmental benefits of POX compared to those of reference HPTs can be achieved when POX is produced on an industrial scale.
From the economic perspective, POX is highly cost-competitive compared to reference HPTs. The estimation suggests a 26–35% price reduction per kg compared to those of PEI, PES, and PSU. Furthermore, high markups of more than 100% could be achieved on the market if POX is sold at similar prices to the reference products. However, the economic performance of POX is highly dependent on the fluctuations of the materials costs and the revenue that can be achieved on the market via sales volume and price. Still, a sensitivity analysis of the net present value has proven the economic viability under uncertainty. Increasing TRL can reduce this uncertainty, leading to more accurate cost estimates. The presented study provides a green light to advance TRL for POX. Overall, POX is a promising new HPT with environmental and economic potential and thus provides the next step towards a decarbonized polymer industry.
Author contributions
M. B. and A. M. worked on conceptualization, methodology, and data curation and wrote the original draft and the manuscript. R. S. and A. B. supervised the project, worked on conceptualization and methodology, and assisted in writing the manuscript. All authors contributed to discussions and to finalizing the manuscript.Abbreviations
| ACC | Annual capital charge |
| BADGE | Bisphenol A diglycidyl ether |
| D&E | Design and engineering costs |
| COP | Cost of production |
| FCOP | Fixed cost of production |
| EoL | End-of-Life |
| FCI | Fixed capital investment |
| LCA | Life cycle assessment |
| HPTs | High-performance thermoplastic polymers |
| ISBL | Inside battery limits |
| NPV | Net present value |
| MDI | Methylene diphenyl diisocyanate |
| OSBL | Outside battery limits |
| pBPGE | p-tert-Butylphenyl glycidyl ether |
| PEI | Polyetherimide |
| PES | Polyethersulfone |
| POX | Polyoxazolidinone |
| PSU | Polysulfone |
| TEA | Techno-economic assessment |
| TRL | Technology readiness level |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge funding from the German Federal Ministry of Education and Research (BMBF project ID: 033R199B). Furthermore, the authors thank Covestro Deutschland AG for providing the process data and Sohajl Movahhed, Stefan Westhues, and Benedikt Winter for their valuable comments on the manuscript.References
- J. Zheng and S. Suh, Nat. Clim. Change, 2019, 9, 374–378 CrossRef.
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef PubMed.
- I. Posen, P. Jaramillo, A. E. Landis and W. M. Griffin, Environ. Res. Lett., 2017, 12, 34024 CrossRef.
- R. Meys, A. Kätelhön, M. Bachmann, B. Winter, C. Zibunas, S. Suh and A. Bardow, Science, 2021, 71–76 CrossRef CAS.
- D. Parker, J. Bussink, H. T. van de Grampel, G. W. Wheatley, E.-U. Dorf, E. Ostlinning, K. Reinking, F. Schubert, O. Jünger and R. Wagener, Ullmann's Encyclopedia of Industrial Chemistry, 2012 Search PubMed.
- S. M. Rangappa, J. Parameswaranpillai, S. Siengchin and L. Kroll, Lightweight Polymer Composite Structures. Design and Manufacturing Techniques, CRC Press, 2020 Search PubMed.
- H.-G. Elias and R. Mülhaupt, Ullmann's encyclopedia of industrial chemistry, Wiley, Chichester, 2010, pp. 1–70 Search PubMed.
- P. M. Hergenrother, High Performance Polymers, 2003 Search PubMed.
- A. C. C. de Leon, I. G. M. da Silva, K. D. Pangilinan, Q. Chen, E. B. Caldona and R. C. Advincula, React. Funct. Polym., 2021, 162, 104878 CrossRef CAS.
- D. Kyriacos, Brydson's plastics materials, ed. M. Gilbert, Butterworth-Heinemann is an imprint of Elsevier, Amsterdam, Boston, Heidelberg und 9 weitere, 2017, pp. 545–615 Search PubMed.
- M. Biron, Thermoplastics and Thermoplastic Composites, Elsevier/William Andrew, Amsterdam, 2nd edn, 2013 Search PubMed.
- GaBi 10.6.0.110: Sphera Solutions GmbH, Software-System and Database for Life Cycle Engineering, Leinfelden-Echterdingen, Germany, 2022 Search PubMed.
- Nexant Inc., Amorphous High Temperature Engineering Thermoplastics. TECH Report, 2021 Search PubMed.
- Grand View Research, GVR Report cover Plastic Market Size, Share & Trends Report Plastic Market Size, Share & Trends Analysis Report By Product (PE, PP, PU, PVC, PET, Polystyrene, ABS, PBT, PPO, Epoxy Polymers, LCP, PC, Polyamide), By Application, By End-use, By Region, And Segment Forecasts, 2021–2028, available at: https://www.grandviewresearch.com/industry-analysis/global-plastics-market.
- A. Prokofyeva, H. Laurenzen, D. J. Dijkstra, E. Frick, A. M. Schmidt, C. Guertler, C. Koopmans and A. Wolf, Polym. Int., 2017, 66, 399–404 CrossRef CAS.
- T. E. Müller, C. Gürtler, S. Basu, I. Latorre, C. Rangheard and W. Leitner, WO/2015/173111, 2015.
- T. E. Müller, C. Gürtler, S. Basu and W. Leitner, WO/2014/076024, 2015.
- T. E. Müller, C. Gürtler, S. Basu, C. Rangheard, D. Rivillo, W. Leitner and B. Köhler, WO/2016/128380, 2016.
- Covestro Deutschland AG, personal communication.
- M. Weiss, J. Haufe, M. Carus, M. Brandão, S. Bringezu, B. Hermann and M. K. Patel, J. Ind. Ecol., 2012, 16, S169–S181 CrossRef CAS.
- S. Das, Int. J. Life Cycle Assess., 2011, 16, 268–282 CrossRef CAS.
- F. Brentrup, J. Küsters, H. Kuhlmann and J. Lammel, Eur. J. Agron., 2004, 20, 247–264 CrossRef.
- Fraunhofer Institute for Systems and Innovation Research ISI, r+Impuls - Innovative technologies for resource efficiency – impulses for industrial resource efficiency. DreamCompoundConti – Resource-efficient process to produce a new high-performance thermoplastic, available at: https://www.r-plus-impuls.de/rplus-en/joint-projects/projects/ongoing/Dream-compound-conti.php Search PubMed.
- Deutsches Institut für Normung, Environmental Management – Life Cycle Assessment –Principles and Framework, Beuth Verlag, 2006 Search PubMed.
- Deutsches Institut für Normung, Environmental Management – Life Cycle Assessment – Requirements and Guidelines, Beuth Verlag, 2006 Search PubMed.
- S. Walker and R. Rothman, J. Cleaner Prod., 2020, 261, 121158 CrossRef CAS.
- C. Bauer, N. Heck, N. Jungbluth and T. Nemecek, The environmental relevance of capital goods in life cycle assessments of products and services, 2007 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, The ecoinvent database version 3 (part I): overview and methodology, available at: https://link.springer.com/10.1007/s11367-016-1087-8, accessed 06/2021.
- A. G. Parvatker and M. J. Eckelman, ACS Sustainable Chem. Eng., 2019, 7, 350–367 CrossRef CAS.
- M. Delogu, L. Zanchi, C. A. Dattilo, S. Maltese, R. Riccomagno and M. Pierini, Take-Home Messages from the Applications of Life Cycle Assessment on Lightweight Automotive Components, SAE Technical Paper 2018-37-0029, 2018 Search PubMed.
- A. E. Schwarz, T. N. Ligthart, D. Godoi Bizarro, P. de Wild, B. Vreugdenhil and T. van Harmelen, Waste Manage., 2021, 121, 331–342 CrossRef CAS PubMed.
- R. Meys, A. Kätelhön and A. Bardow, Green Chem., 2019, 21, 3334–3342 RSC.
- G. Doka, Life cycle inventories of waste treatment services, 2003 Search PubMed.
- G. Doka, Updates to Life Cycle Inventories of Waste Treatment Services-part II. Waste incineration, Doka Life Cycle Assessments, Zurich, 2013 Search PubMed.
- European Commission, Joint Research Centre, S. Sala, V. de Laurentiis, L. Zampori, E. Diaconu and S. Fazio, Supporting information to the characterisation factors of recommended EF Life Cycle Impact Assessment methods version 2. from ILCD to EF 3.0, Publication Office, 2019.
- J. Wunderlich, K. Armstrong, G. A. Buchner, P. Styring and R. Schomäcker, J. Cleaner Prod., 2021, 287, 125021 CrossRef CAS.
- R. Sinnott and G. Towler, Chemical Engineering Design, Elsevier, 2020 Search PubMed.
- I. V. Klumpar, R. F. Brown and J. W. Fromme, Rapid capital estimation based on process modules, 1983 Search PubMed.
- M. S. Peters, K. D. Timmerhaus and R. E. West, Plant design and economics for chemical engineers, 2003 Search PubMed.
- E. A. Stallworthy, Engineering and Process Economics, 1977, vol. 2, pp. 78–79 Search PubMed.
- J. K. Maund, Engineering and Process Economics, 1976, vol. 1, pp. 241–243 Search PubMed.
- J. S. Page, Conceptual Cost Estimating Manual, Gulf Professional Publishing, 1996 Search PubMed.
- D. E. Garrett, Chemical Engineering Economics, Springer Netherlands, Dordrecht, 2012 Search PubMed.
- K. K. Humphreys, Project and Cost Engineers’ Handbook, CRC Press, 2004 Search PubMed.
- G. A. Buchner, K. J. Stepputat, A. W. Zimmermann and R. Schomäcker, Industrial & Engineering Chemistry Research, 2019 Search PubMed.
- J. Bates, C. D. J. Burton, R. C. Creese, J. K. Hollmann, K. K. Humphreys, D. F. McDonald, C. A. Miller, B. A. Pietlock, W. R. Querns and D. L. Short, Cost estimate classification system, 2013 Search PubMed.
- D. Braun and J. Weinert, Angew. Makromol. Chem., 1979, 78, 1–19 CrossRef CAS.
- Nexant Inc., Nitrobenzene/Aniline/MDI. PERP Report, 2011 Search PubMed.
- B. Winter, R. Meys and A. Bardow, J. Cleaner Prod., 2021, 290, 125818 CrossRef CAS.
- Nexant Inc., Epichlorohydrin. PERP Report, 2011 Search PubMed.
- Nexant Inc., Epoxy resins. PERP Report, 2015 Search PubMed.
- F. H. Isikgor and C. Remzi Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- Plastics Europe, Eco-profiles for determining environmental impacts of plastics, 2016 Search PubMed.
- UN Comtrade Database, available at: https://comtrade.un.org/, accessed 05/22.
- Thermoplastics and thermoplastic composites, ed M. Biron, William Andrew Applied Science Publishers, Oxford, United Kingdom, Cambridge, MA, United States, 2018 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc02400d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |