Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stabilization of phenanthrene anions in helium nanodroplets
Siegfried
Kollotzek
 a,
Farhad
Izadi
a,
Miriam
Meyer
a,
Farhad
Izadi
a,
Miriam
Meyer
 a,
Stefan
Bergmeister
a,
Stefan
Bergmeister
 a,
Fabio
Zappa
a,
Fabio
Zappa
 a,
Stephan
Denifl
a,
Stephan
Denifl
 a,
Olof
Echt
a,
Olof
Echt
 b,
Paul
Scheier
b,
Paul
Scheier
 a and
Elisabeth
Gruber
a and
Elisabeth
Gruber
 *a
*a
aInstitut für Ionenphysik und Angewandte Physik, Universität Innsbruck, A-6020 Innsbruck, Austria. E-mail: E.Gruber@uibk.ac.at
bDepartment of Physics, University of New Hampshire, Durham, NH 03824, USA
First published on 4th May 2022
Abstract
It has been debated for years if the polycyclic aromatic hydrocarbon phenanthrene exists in its anionic form, or, in other words, if its electron affinity (EA) is positive or negative. In this contribution we confirm that the bare phenanthrene anion Ph− created in a binary collision with an electron at room temperature has a lifetime shorter than microseconds. However, the embedding of neutral phenanthrene molecules in negatively charged helium nanodroplets enables the formation of phenanthrene anions by charge transfer processes and the stabilization of the latter in the ultracold environment. Gentle shrinking of the helium matrix of phenanthrene-doped HNDs by collisions with helium gas makes the bare Ph− visible by high-resolution mass spectrometry. From these and previous measurements we conclude, that the EA of phenanthrene is positive and smaller than 24.55 meV.
Introduction
Polycyclic aromatic hydrocarbon (PAH) molecules are hydrocarbon compounds with multiple six-membered aromatic rings which are abundantly present in the interstellar medium in the form of neutral closed shell-molecules, molecular radicals, and ionized species. In the past few decades, anions of PAHs have received considerable attention as in regions with high electron densities and low UV photon flux, PAHs are considered to exist predominantly as negatively charged ions and influence substantially the chemical reactions in dense interstellar clouds.1 The stability of the anionic form relative to its neutral counterpart is governed by the electron affinity (EA). As a rule of thumb, an EA above 0.5 eV is needed for the formation of long-lived PAH anions, i.e. anions with lifetimes above 1 μs upon attachment of thermal electrons to PAHs.2–5 A negative EA means that the anionic form is not stable and isolated species of negative charge do not exist.The EA of PAHs generally increases with increasing molecular size. Thus, benzene (C6H6) and naphthalene (C10H8) have a negative EA, while anthracene (C14H10, An) is the smallest unsubstituted PAH with a positive EA. A recent experimental value deduced from photoelectron spectroscopy (PES) of An− showed an EA of 0.532(3) eV,6 in good agreement with earlier PES results7–9 and values obtained with other experimental approaches10–12 as well as theoretical calculations.13–20 A benchmark theoretical study accounting for zero-point vibrational energies and geometry relaxation effects results in an EA of 0.526(6) eV,21 matching the experimental value perfectly. An− readily forms upon attachment of low-energy electrons to An molecules in the gas phase.2,6–9,22,23
The situation for phenanthrene (Ph), a bent isomer of An, is less satisfying. Several attempts to form the parent anion Ph− by attaching low-energy electrons to Ph in the gas phase have failed so far,2,22,24 indicating that its EA is less than that of An. Several experimental studies using the electron-capture detector technique which is based on the temperature dependent kinetics of electron attachment have reported adiabatic electron affinities (AEAs) of Ph close to 0.3 eV.10–12,25–27 However, the validity of these data has been questioned,5,14,15,28 partly because the mass of the formed anions has not been measured, and partly because theoretical values for the AEA of Ph are well below 0.3 eV, or even negative.13,17,29,30 A benchmark study that includes structural relaxation and zero-point vibrational energy corrections places the AEA of Ph at −0.08 eV.29 To our knowledge, so far, only Lee et al. reported the observation of a small signal due to the parent anion, Ph−, by performing photoelectron spectroscopy.30,31 A potential complication in the detection of Ph− in their setup with a mass resolution of only 1/200 is the fact that copious amounts of dehydrogenated Ph, (Ph-H)−, are formed by dissociative electron attachment at a resonance between 7 and 8 eV,2 contributing to the mass spectrum, as well as (Ph-H)− ions that contain one 13C (its ion yield is 15% of the main isotopologue).
A promising approach to study the properties of a molecule such as Ph with a small or even negative EA is to study complexes of Ph with an adduct M with a vanishingly small EA. The ion-induced dipole interaction between the excess electron and M may increase the EA of MPh− or MnPh− clusters, and stabilize the corresponding anion. This approach has been applied by Tschurl et al. who reported photoelectron spectra of (H2O)nPh− with 1 < n < 3.24 The authors did not observe the bare Ph−, but extrapolation to n = 0, with correction for the anticipated increase in the solvation shift upon addition of the first solvent water molecule, resulted in an EA of −0.01(4) eV.
Recently, our group studied complexes of Ph with various ligands M with negative (He, H2, H2O) or extremely small EA (Ca, EA = 24.55 meV) embedded in helium nanodroplets (HNDs).32 We showed that long-lived HenPh− are formed in HNDs and fragment by low-energy collisions with Ar atoms into HenPh− with n > 0. Bare Ph− was not observed. Through competition between helium evaporation and electron detachment of HenPh− clusters, a lower limit of the vertical detachment energy (VDE) of Ph− of about −3 meV was determined. In case of CaPh− complexes, collision with Ar atoms produces Ca− but no Ph−, indicating that the EA of Ph− is below that of Ca, i.e. below 24.55 meV.32
In this contribution, first, we confirm by performing electron attachment measurements that Ph− is not observed isolated in the gas phase, and secondly, we show that bare Ph− can be stabilized in the HND environment by a suitable choice of parameters, albeit in very small amounts. Nonetheless, from these results we conclude, that Ph has a small, but positive EA.
Experimental part
Three different experimental setups were used to study the formation of Ph (purchased from Sigma Aldrich, sublimed grade, ≥99.5%) anions. To test the experimental setups, the measurements were repeated with An (purchased from Sigma Aldrich, ReagentPlus, 99%). For all measurements, the sample was vaporized in ultrahigh vacuum at temperatures in the range of 60–70 °C. The recorded mass spectra of anions were analyzed with the software package IsotopeFit, which accounts for isotopic patterns, isobaric ions and the background.37Resonance electron mass spectrometry
The crossed electron-molecular beam setup Wippi combined with a quadrupole mass spectrometer (QMS), described in detail elsewhere,33,34 was used in the present study of electron attachment in the gas phase (see Fig. 1a). An and Ph were vaporized in an oven at 71 and 65 °C, respectively. The vapor entered the interaction chamber of a hemispherical electron monochromator (HEM) through a 1 mm-diameter, stainless-steel and copper capillary, where it crossed an electron beam. The HEM is composed of three parts: a hairpin tungsten filament (heated by applying a current of 2.35 A) used as an electron source, two concentric hemispheres at different electric potentials that function as an energy filter, and two columns forming a series of electrostatic lenses. The latter is used to direct the electron beam from the source to the hemisphere and from the hemisphere to the interaction region. The HEM was tuned to generate the electron beam with an energy resolution of 100 meV (full-width-at-half-maximum, FWHM). The anions were extracted into the quadrupole mass analyzer (QMS) by a weak electrostatic field and detected by a channeltron operated in single ion counting mode. The ion yield of a given mass-selected anion was measured as a function of the incident electron energy. The electron energy scale and energy resolution were determined by measuring the well-known resonances for the formation of SF6− from SF6 and Cl− from CCl4 at 0 eV. The electron current after the interaction region was monitored by using a picoamperemeter.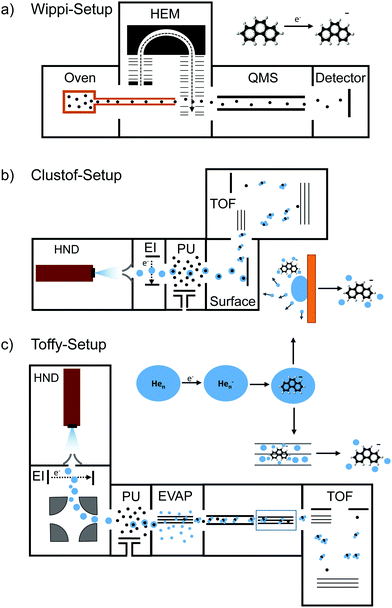 | ||
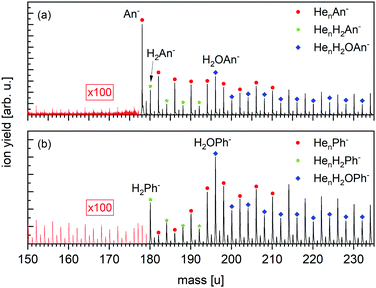
| Fig. 1 Schematics of the three used experimental setups: (a) Wippi-setup33,34 for performing resonance electron mass spectrometry, (b) Clustof-setup35 and (c) Toffy-setup36 for performing mass spectrometry of ions embedded in HNDs. Details about the setups are described in the main text. | ||
Mass spectrometry of PAH ions in anionic HNDs
The mass spectrometric measurements of An and Ph anions embedded in HNDs were performed at two different setups, Clustof35 and Toffy.36 In both setups, HNDs are produced via supersonic expansion by expanding ultrapure helium (Messer, purity 99.9999%) with a stagnation pressure of 28 bar through a 5 μm pinhole nozzle into ultrahigh vacuum. The nozzle is cooled with a closed-cycle cryocooler (Sumitomo Heavy Industries) to 8.8 K. According to Gomez et al.38 the resulting average droplet size is between 2.5 × 105 and 106 He atoms for the present conditions. To prevent destruction of HNDs by collisions with shock fronts, the resulting jet of He is then passed through a 0.5 mm skimmer (Beam Dynamics, Inc) located about 10 mm after the nozzle, producing a supersonic molecular beam. Afterwards, the HNDs are ionized by passing through a Nier-type electron impact ionization unit, operating at an electron energy of about 22 eV and an electron current of about 200 μA.In the Clustof setup, the beam of ionized HNDs propagates into the pick-up chamber, where evaporated An or Ph molecules are captured. The doped HNDs collide with an orthogonal metal surface, where the cold droplet “splashes” and evaporates away.35 The bare ions with some tens of He atoms attached are extracted and guided towards the time-of-flight (TOF) detector system.
In the Toffy setup, the beam of ionized HNDs is first mass-to-charge selected in a quadrupole bender and then guided into the pickup chamber filled with Ph vapor. In contrast to the Clustof setup, a gentle shrinking of the surrounding He matrix is here enabled by collisions of doped HNDs with He atoms in a subsequent evaporation cell at tunable He pressure. The resulting ionic clusters, optionally decorated with some He atoms, are analyzed in a TOF mass spectrometer (Q-TOF Ultima Waters/Micromass).
Results
Electron attachment to bare anthracene or phenanthrene
Attachment of low-energy electrons to An produces the parent anion An− as shown in Fig. 2a. The very weak resonance which appears in Fig. 2a near 8 eV is not due to An− but to (An-H)− ions that contain one 13C atom. The FWHM of the 0 eV resonance is 105 meV, limited by the energy resolution of the primary electron beam. Between 6 and 10 eV dehydrogenated An anions, (An-H)−, appear (see Fig. 2b). The resonance was previously observed by Tobita et al. who attributed it to a two-particle-one-hole resonance in which capture of the incident electron is accompanied by simultaneous electronic excitation, leading to two electrons in normally unoccupied molecular orbitals.2 The onset and the maximum of the resonance reported in their work is indicated by dashed lines; the agreement with our data is excellent.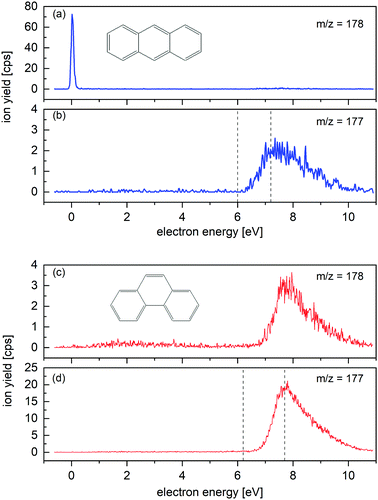 | ||
| Fig. 2 Energy dependence of the ion yield measured for m/z = 178 and m/z = 177 upon electron attachment to An (a) and (b) and Ph (c) and (d) in the gas phase. An− parent ions are clearly formed (a), whereas no Ph− parent ions are observed (c). (An-H)− and (Ph-H)− are resonantly formed around 8 eV. Dashed lines in (b) and (d) indicate the onset and the maximum of the yield of dehydrogenated anions reported by Tobita et al.2 The resonances near 8 eV in (a) and (c) are due to the dehydrogenated anions which contain one 13C atom. | ||
Attachment of low-energy electrons to Ph does not produce the parent ion, in agreement with the report by Tobita et al.2 (Ph-H)− is formed resonantly around 8 eV, see Fig. 2d. The onset and maximum of the observed resonance are in good agreement with the values reported by Tobita et al. which are indicated in Fig. 2d by dashed lines.2 The 8 eV resonance appearing in Fig. 2c is due to (Ph-H)− anions containing one 13C isotope, rather than to isotopically pure Ph−.
Close inspection of the data in Fig. 2c reveals another broad resonance in the Ph− signal around 3 eV. It is weaker than the 8 eV resonance by a factor of 20. Electron transmission measurements through An reveal two-particle-one-hole resonances in this energy range,39 but we are not aware of similar experiments involving Ph. Note that the energy threshold for formation of (Ph-H)− + H is below 3 eV.2 The detected signal around 3 eV may correspond to a metastable Ph− parent anion, or to the dissociative electron attachment ion yield from an heavier impurity of the sample. A detectable contamination of the Ph sample with the isomer An is excluded, as no signal is registered at the 0 eV resonance in Fig. 2c.
Anions extracted from doped HNDs
Fig. 3 displays a negative-ion mass spectrum of HNDs doped with An and Ph, respectively, using the Clustof setup. The strongest mass peak in Fig. 3a is due to An− at mass 178 u, which is to be expected as it is known that An forms long-lived anions upon the capture of low-energy electrons2,7,40 and has a positive EA of 0.53 eV. Moreover, three homologous ion series, namely HenAn− (n ≥ 0), HenH2An− (n ≥ 0) and HenH2OAn− (n ≥ 0) are clearly visible in the spectrum. Each of these mass peaks is followed by a satellite peak due to ions that contain one 13C (natural abundance 1.07%; their yield is 15% of the main isotopologues). Contributions from ions that contain two 13C atoms are negligible (1%). The mass peaks, which are isotopically pure (containing only 1H, 4He, and 12C) are marked. Beginning at mass m = 196 u, the HenH2An− (n ≥ 4) series cannot be distinguished anymore from the HenH2OAn− (n ≥ 0) series. The presence of anions that contain one or more helium atoms attests to their very low vibrational temperature. Water is a contaminant whose appearance cannot be avoided when growing very large HNDs that contain 106 atoms. Moreover, these HNDs have very large collision cross sections and will readily pickup residual gas atoms or molecules in the vacuum system. Likewise, the H2 impurities are probably the product of collisions between the HND and residual hydrogen. Turbomolecular pumps that are used in our system have a particularly low compression ratio for hydrogen gas.The negative ion mass spectrum of Ph in Fig. 3b covers the same mass range as in Fig. 3a. All analogues of ions identified in Fig. 3a are observed, namely HenPh− (n ≥ 1), HenH2Ph− (n ≥ 0) and HenH2OPh− (n ≥ 0), except for the absence of the bare parent ion Ph− which would appear at 178 u.
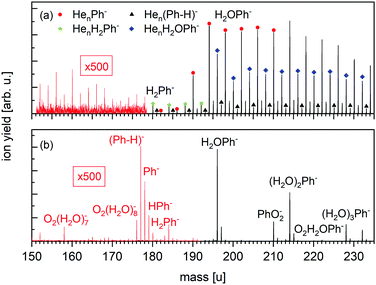
The negative ion mass spectrum of Ph in Fig. 4 was obtained by using the Toffy setup. While the ionization and doping of the HNDs proceed in the same way as in Clustof, the dopant ions are made accessible for mass spectrometry in a different way. Instead of being collided with a surface, the He matrix is softly removed by collisions with He atoms. The tuning of the He pressure in the evaporation chamber enables to control the size of the He matrix, in which the dopant ions are embedded. Fig. 4a shows the mass spectrum for an evaporation pressure of 0.1 Pa and Fig. 4b for an evaporation pressure of 0.2 Pa (the measured pressure was corrected by taking the gas correction factor of He into account). Fig. 4a looks similar to the mass spectrum of Fig. 3a obtained with the Clustof setup. The dominant peaks can be linked to HenPh− (n ≥ 1), Hen(Ph-H)− (n ≥ 1), HenH2Ph− (n ≥ 0), and HenH2OPh− (n ≥ 0). A zoom-in of the lower mass range <180 u (ion yield multiplied by a factor of 500 to increase the visibility of the mass peaks), does not show a clear signal of the bare parent ion Ph− here either. The observed peaks can be linked to He series attached to impurities. The situation changes when the He matrix is further reduced (Fig. 4b). Again, the most prominent peaks are due to clustering of Ph with impurities (H2O, O2, H2) followed by a satellite peak due to isotopologues that contain one 13C. Nevertheless, a closer look at masses <180 u (the ion yield is multiplied again by a factor of 500 to increase the visibility of the mass peaks), shows not only a weak mass peak at 177 u due to dehydrogenated Ph anions, (Ph-H)−, but also another peak at 178 u which is more intense than the expected contribution from the 13C-containing isotopologue of (Ph-H)−. For further inspection, Fig. 5 shows a zoom-in of this mass section with the expected isotopic patterns. 15% of the measured ion yield at mass 178 u (the mass of the parent Ph anion) arises from the dehydrogenated anion (Ph-H)− that contains one 13C and 15% of the measured ion yield at mass 179 u arises from the bare Ph anion, contributing to the protonated Ph peak. In contrast to the stabilization of the transient molecule SF6+ in HNDs by the formation of SF5+F clusters,41,42 in the present case, the ultracold HND environment enables the stabilization of bare as well as dehydrogenated Ph anions.
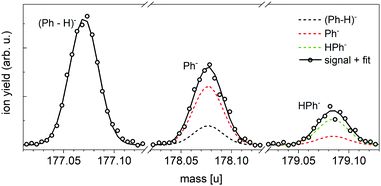 | ||
| Fig. 5 Expanded view of the anion mass spectrum of Fig. 4b recorded at PEvap = 0.2 Pa. Dehydrogenated Ph anions (Ph-H)− as well as bare Ph− and HPh− are visible. The dashed lines show the contribution of these ions under consideration of the minor isotopes with one 13C. 15% of the measured ion yield at mass 178 u arises from the dehydrogenated anion (Ph-H)− that contains one 13C, and 15% of the measured ion yield at mass 179 u arises from the bare Ph anion that contains one 13C. The sum of all isotopic contributions results into the solid line, which fits the measured ion yield. | ||
Discussion
While for the resonant electron-attachment measurements in the gas phase at room temperature no Ph− was detected, a small amount of the latter became visible in the HND environment, after gently removing the surrounding He matrix by collisions with He atoms. Each collision will transfer, on average, 0.05 eV to the doped HND, ∼80 times the evaporation energy of bulk helium, leading to partial or complete evaporation of the He matrix. By selecting suitable parameters for the evaporation pressure, Ph− becomes detectable. Under the assumption that the bare Ph anion is born in the evaporation chamber, a minimum lifetime of several ms is necessary to detect the anion in the TOF. In previously performed collision induced dissociation (CID) measurements we collided Ar atoms with HenPh− (8 ≥ n > 0) complexes. Only HenPh− (7 ≥ n > 0) fragments, but no bare Ph− were detected.32 The “non-detection” of the bare anion might be explainable by the higher transferred collision energy of Ar atoms in comparison to He atoms. Moreover, the measurements were aggravated by larger noise/poorer counting statistics.An even more unexpected observation of the measurements presented in Fig. 4 is the formation of Hen(Ph-H)− in the HND environment. We know from gas-phase electron-attachment measurements, that an electron energy of 7–8 eV is necessary to cause dehydrogenation. To retrace the underlying process inside the HND, we have to revisit the formation of HND anions and charge transfer to the embedded dopants.43
The formation of negatively charged HNDs can proceed via two mechanisms. In one case, low energy electrons of about 2 eV overcome the HND surface barrier (between 0.6 and 1.1 eV), will thermalize and be trapped in the HND forming a so-called electron bubble, a void with a radius of 1.7 nm. In the second case, penetrating electrons at around 22 eV may lead to the excitation of a helium atom (the first three excited states are at 19.8 eV, 20.9 eV and at 22.7 eV) and subsequent capture of the scattered incident electron. The process terminates in the formation of He*−, which is solvated in the droplet and may interact with another helium atom to form He2*−.44 In the following, He*− (or He2*−) may de-excite into the ground state, transferring the released energy to the ‘attached’ electron. Another possible, but less probable de-excitation path is the interaction of He*− or He2*− with a dopant M or another excited He atom, leading to Penning ionization He*− + M → He + M+ + 2e− or He*− + He* → He + He+ + 2e−, delivering two free electrons. The electrons from these de-excitation channels may eventually interact with the dopants while moving through the HND. Investigations on Penning ionization of acene molecules in EUV-excited HNDs have shown a broad spectrum of kinetic energies of the Penning electron, indicating that the emitted electrons are severely affected by collective electron helium interactions.45 Thus, electrons of a broad range of kinetic energies are formed and may interact with the dopants. The parent anion Ph− as well as the dehydrogenated anion (Ph-H)− may be formed by this sequence of events.
As long as the dopant anions are embedded in a He matrix, the formation of HenPh− dominates over the formation of the Hen(Ph-H)− channel. This is nicely observed in Fig. 4a and also in Fig. 3, even though the water and hydrogen impurities are the more dominating contributions. The He matrix seems to efficiently quench the hydrogen-loss channel, the only channel observed for electron attachment to bare Ph in the gas phase (see Fig. 2c and d). A complete evaporation of the helium matrix means the removal of the fragmentation quencher, and the formation of dehydrogenated anions (Ph-H)− dominates over the stabilization of the bare parent anion Ph−, as seen in Fig. 4b and 5.
Conclusion
In this contribution, we have confirmed the non-detection of Ph− in the gas-phase after binary collision with an electron, and the stabilization of the latter in the HND environment, even though the measured ion yield was minor in comparison to Ph anions complexed with other atoms or molecules. From these and previous observations we conclude, that Ph has a small (<24.55 meV), but positive EA. Due to the small electron affinity, it can be expected that bare Ph anions are hardly found in interstellar clouds. However, complexation of Ph with other molecules like H2 and H2O as well as the clustering to larger Ph complexes (dimers, trimers, etc.) leads to a considerable increase of the electron affinity. These complexes might be abundantly present in the interstellar medium and play an important role for the interstellar chemistry.Besides bare Ph anions, we also detected dehydrogenated Ph anions (Ph-H)− in the HND environment. The formation of the latter was rationalized by retracing the formation of HND anions, the de-excitation paths of excited He*− and He2*− as well as associated charge transfer processes to the embedded dopants. Once more, it has been shown that helium nanodroplets generate a versatile and suitable environment to stabilize and study metastable molecular ions.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The work by S. K., S. B., O. E., M. M. and E. G. was supported by the FWF Projects I4130, W1259, P31149 and T1181.Notes and references
- V. Wakelam and E. Herbst, Astrophys. J., 2008, 680, 371 CrossRef CAS.
- S. Tobita, M. Meinke, E. Illenberger, L. G. Christophorou, H. Baumgärtel and S. Leach, Chem. Phys., 1992, 161, 501–508 CrossRef CAS.
- L. D. Betowski, M. Enlow and D. H. Aue, Int. J. Mass Spectrom., 2006, 255–256, 123–129 CrossRef CAS.
- R. V. Khatymov, R. F. Tuktarov and M. V. Muftakhov, JETP Lett., 2011, 93, 437–441 CrossRef CAS.
- R. V. Khatymov, M. V. Muftakhov and P. V. Shchukin, Rapid Commun. Mass Spectrom., 2017, 31, 1729–1741 CrossRef CAS PubMed.
- S. J. Kregel, G. K. Thurston and E. Garand, J. Chem. Phys., 2018, 148, 234306 CrossRef PubMed.
- J. Schiedt and R. Weinkauf, Chem. Phys. Lett., 1997, 266, 201–205 CrossRef CAS.
- J. K. Song, N. K. Lee, J. H. Kim, S. Y. Han and S. K. Kim, J. Chem. Phys., 2003, 119, 3071–3077 CrossRef CAS.
- N. Ando, M. Mitsui and A. Nakajima, J. Chem. Phys., 2007, 127, 234305 CrossRef PubMed.
- R. S. Becker and E. Chen, J. Chem. Phys., 1966, 45, 2403–2410 CrossRef CAS PubMed.
- L. Wojnárovits and G. Földiák, J. Chromatogr. A, 1981, 206, 511–519 CrossRef.
- G. Chen and R. G. Cooks, J. Mass Spectrom., 1995, 30, 1167–1173 CrossRef CAS.
- G. Malloci, G. Mulas, G. Cappellini, V. Fiorentini and I. Porceddu, Astron. Astrophys., 2005, 432, 585–594 CrossRef CAS.
- L. Betowski, M. Enlow and D. H. Aue, Int. J. Mass Spectrom., 2006, 255, 123–129 CrossRef.
- L. D. Betowski, M. Enlow, L. Riddick and D. H. Aue, J. Phys. Chem. A, 2006, 110, 12927–12946 CrossRef CAS PubMed.
- E. S. Kadantsev, M. Stott and A. Rubio, J. Chem. Phys., 2006, 124, 134901 CrossRef PubMed.
- A. Modelli and L. Mussoni, Chem. Phys., 2007, 332, 367–374 CrossRef CAS.
- A. Kukhta, I. Kukhta, N. Kukhta, O. Neyra and E. Meza, J. Phys. B, 2008, 41, 205701 CrossRef.
- F. Carelli, F. A. Gianturco, M. Satta and F. Sebastianelli, Int. J. Mass Spectrom., 2014, 365, 377–383 CrossRef.
- R. M. Richard, M. S. Marshall, O. Dolgounitcheva, J. V. Ortiz, J.-L. Bredas, N. Marom and C. D. Sherrill, J. Chem. Theory Comput., 2016, 12, 595–604 CrossRef CAS PubMed.
- B. Hajgató, M. Deleuze, D. Tozer and F. De Proft, J. Chem. Phys., 2008, 129, 084308 CrossRef PubMed.
- K. Steinfelder and R. Tümmler, Angew. Chem., 1961, 73, 136–142 CrossRef.
- A. Lietard, G. Mensa-Bonsu and J. R. R. Verlet, Nat. Chem., 2021, 13, 737–742 CrossRef CAS PubMed.
- M. Tschurl, U. Boesl and S. Gilb, J. Chem. Phys., 2006, 125, 194310 CrossRef PubMed.
- E. Chen and W. Wentworth, Mol. Cryst. Liq. Cryst., 1989, 171, 271–285 CrossRef.
- R. S. Ruoff, K. M. Kadish, P. Boulas and E. Chen, J. Phys. Chem., 1995, 99, 8843–8850 CrossRef CAS.
- E. C. Chen and E. S. Chen, J. Chromatogr. A, 2018, 1573, 1–17 CrossRef CAS PubMed.
- K. M. Ervin, I. Anusiewicz, P. Skurski, J. Simons and W. C. Lineberger, J. Phys. Chem. A, 2003, 107, 8521–8529 CrossRef CAS.
- M. Huzak, B. Hajgató and M. Deleuze, Chem. Phys., 2012, 406, 55–64 CrossRef CAS.
- S. H. Lee, N. Kim, D. G. Ha and J. K. Song, RSC Adv., 2013, 3, 17143 RSC.
- S. H. Lee, J. K. Song and S. K. Kim, Chem. Phys. Lett., 2015, 626, 63–68 CrossRef CAS.
- E. Gruber, S. Kollotzek, S. Bergmeister, F. Zappa, M. Ončák, P. Scheier and O. Echt, Phys. Chem. Chem. Phys., 2022, 24, 5138–5143 RSC.
- S. Denifl, S. Ptasinska, B. Sonnweber, P. Scheier, D. Liu, F. Hagelberg, J. Mack, L. T. Scott and T. D. Mark, J. Chem. Phys., 2005, 123, 104308 CrossRef CAS PubMed.
- M. Mahmoodi-Darian, A. Mauracher, A. Aleem, S. Denifl, B. Rittenschober, A. Bacher, M. Probst, T. Mark and P. Scheier, J. Phys. Chem. A, 2009, 113, 14923–14929 CrossRef CAS PubMed.
- P. Martini, S. Albertini, F. Laimer, M. Meyer, M. Gatchell, O. Echt, F. Zappa and P. Scheier, Phys. Rev. Lett., 2021, 127, 263401 CrossRef CAS PubMed.
- L. Tiefenthaler, J. Ameixa, P. Martini, S. Albertini, L. Ballauf, M. Zankl, M. Goulart, F. Laimer, K. von Haeften, F. Zappa and P. Scheier, Rev. Sci. Instrum., 2020, 91, 033315 CrossRef CAS PubMed.
- S. Ralser, J. Postler, M. Harnisch, A. M. Ellis and P. Scheier, Int. J. Mass Spectrom., 2015, 379, 194–199 CrossRef CAS PubMed.
- L. F. Gomez, E. Loginov, R. Sliter and A. F. Vilesov, J. Chem. Phys., 2011, 135, 154201 CrossRef PubMed.
- P. Burrow, J. Michejda and K. Jordan, J. Chem. Phys., 1987, 86, 9–24 CrossRef CAS.
- M. V. Ardenne, K. Steinfelder and R. Tummler, Angew. Chem., 1961, 73, 136–142 CrossRef CAS.
- S. Albertini, S. Bergmeister, F. Laimer, P. Martini, E. Gruber, F. Zappa, M. OncÌŒák, P. Scheier and O. Echt, J. Phys. Chem. Lett., 2021, 12, 4112–4117 CrossRef CAS PubMed.
- E. Zunzunegui-Bru, E. Gruber, S. Bergmeister, M. Meyer, F. Zappa, M. Bartolomei, F. Pirani, P. Villarreal, T. González-Lezana and P. Scheier, Phys. Chem. Chem. Phys., 2022, 24, 2004–2014 RSC.
- A. Mauracher, O. Echt, A. Ellis, S. Yang, D. Bohme, J. Postler, A. Kaiser, S. Denifl and P. Scheier, Phys. Rep., 2018, 751, 1–90 CrossRef CAS.
- S. Albertini, E. Gruber, F. Zappa, S. Krasnokutski, F. Laimer and P. Scheier, Mass Spectrom. Rev., 2021, 1–39 Search PubMed.
- M. Shcherbinin, A. LaForge, M. Hanif, R. Richter and M. Mudrich, J. Phys. Chem. A, 2018, 122, 1855–1860 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2022 |