Tuning the optical properties in CsPbBr3 quantum dot-doped glass by modulation of its network topology
Zhousu
Xu†
 a,
Tao
Chen†
a,
Duoduo
Zhang
b,
Guojun
Zheng
d,
Zhuo
Wang
d,
Zhijun
Ma
a,
Tao
Chen†
a,
Duoduo
Zhang
b,
Guojun
Zheng
d,
Zhuo
Wang
d,
Zhijun
Ma
 f,
Jinhua
Yan
a,
Xianwei
Wang
f,
Jinhua
Yan
a,
Xianwei
Wang
 a,
Xiaofeng
Liu
a,
Xiaofeng
Liu
 *bc and
Jianrong
Qiu
*de
*bc and
Jianrong
Qiu
*de
aInstitute of Intelligent Optoelectronic Technology, Zhejiang University of Technology, Hangzhou, 310014, China
bSchool of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027, China. E-mail: xfliu@zju.edu.cn
cWuhan National Laboratory for Optoelectronics, Wuhan, 430074, China
dState Key Laboratory of Modern Optical Instrumentation, College of Optical Science and Engineering, Zhejiang University, Hangzhou, 310027, China. E-mail: qjr@zju.edu.cn
eCAS Center for Excellence in Ultra-intense Laser Science, Jiading, Shanghai 201800, China
fState Key Laboratory of Luminescent Materials and Devices, and Institute of Optical Communication Materials, South China University of Technology, Guangzhou 510641, China
First published on 28th April 2021
Abstract
All inorganic metal halide perovskite quantum dots (QDs) exhibit excellent optical properties promising for diverse applications, while their inherent frangibility remains an enormous challenge for both scientific research and practical applications. Recently emerged oxide glasses doped with such QDs exhibit high stability against degradation and are thus highly promising for photonic applications. Here, we demonstrate the tuning of the linear and nonlinear optical properties of perovskite QD-doped glass through modulation of its network topology. By introducing a metal fluoride network modifier, we show that the precipitation of the QDs can be promoted as a result of the enhanced ion mobility during heat treatment. The QD-doped glasses show strong one-photon and two-photon upconversion photoluminescence (PL). Also, the PL intensity can be enhanced by up to 800% for the sample with an optimal local structure. In addition, the control of QD precipitation by modulating the glass network topology could allow for the observation of a cross-over from saturable absorption to reverse saturable absorption. The methodology for tuning the optical properties of QD-in-glass composites studied here might be applied for the design of photonic glasses for relevant applications.
1. Introduction
Owing to the excellent tunable photoluminescence (PL) emission covering the entire visible spectral region (400–700 nm) with high photoluminescence quantum yield (PLQY), all-inorganic cesium lead halide (CsPbX3, X = Cl, Br, and I) perovskite quantum dots (QDs) have attracted tremendous attention,1–3 as these unique features could endow CsPbX3 QDs with great potential for applications in various fields, such as solar cells, displays, lasers, and light-emitting diodes (LEDs).4–8 The synthesis of these QDs has been realized by both chemical and physical processes, such as the spin-coating method,9–11 sol–gel method,12–14 co-deposition in solution method,15,16 and molecular beam epitaxy method.17–19 Recently, all-inorganic perovskite QDs have also been precipitated in different oxide glasses by a conventional melt–quenching–annealing method.20–34 The oxide glass matrix offers strong protection needed by the incorporated perovskite QDs against thermal, chemical, and optical degradation. In addition, this method provides a convenient way for precipitating all-inorganic perovskite QDs in glasses with a controllable concentration and size, which allows for tuning their optical performances.The use of a glass matrix for QDs offers additional benefits as the composition and properties of the glass can be carefully tuned to control the formation and the optical properties of the precipitated QDs. Similar to the wet-chemistry route, the control of the growth conditions such as the temperature and duration could allow for the modulation of the QD size and concentration, which strongly affects the optical properties of QDs. On the other hand, the glass matrix itself could be designed to control the growth of QDs by modulating its network topology. For instance, the introduction of alkali earth metal fluoride could result in the modification of the network structure as F− ions can replace the bridge oxygen position, making the bridge oxygen into non-bridge oxygen and breaking the tight glass network, which is beneficial for the movement of the ions and the nucleation and growth of crystals inside the glass.35–38 Moreover, the low vibration energy of the metal fluorine bond compared to that of the metal–oxygen bond could result in the reduction of phonon energy of the glass host, so that the non-radiative transition probability is reduced, and the PL intensity and PLQY are increased.39–41
In this investigation, we study the tuning of the optical performance of CsPbBr3 perovskite QDs precipitated in a borosilicate glass by modulating the network topology based on the conventional melt–quenching–annealing technique. CaF2 is employed as the network modifier which strongly modifies the connectivity of oxygen atoms and the local structure of the examined glass. The results indicate that the formation of QDs and their linear and nonlinear optical properties are strongly dependent on the glass structure. The methodology demonstrated in the present work could be further exploited for the development of glass-based photonic materials.
2. Experiment
2.1. Synthesis of CsPbBr3 QD-doped glass
CsPbBr3 QD-doped glasses were prepared using the conventional melting–quenching method. The molar composition of CsPbBr3 QD-doped glass is 38SiO2–34B2O3–11ZnO–6Cs2CO3–2PbO–9NaBr–xCaF2 (x = 0, 0.5, 1, 2, 3, 4, and 5), and raw powder materials were mixed thoroughly and melted at 1100 °C for 30 min to form precursor glass samples. Then, the precursor glasses were heat-treated at 440–480 °C for 10 h to precipitate CsPbBr3 perovskite QDs. Eventually, all the glass samples were cut and polished for optical measurements. The glass samples were denoted as CPBx–y–z, where x is the molar fraction of the added CaF2, and y and z are the heat treatment temperature and duration, respectively.2.2. Characterization
X-ray diffraction (XRD) patterns were recorded using an X’Pert PRO powder diffractometer with Cu Kα radiation (PANalytical, Holland). Microstructure characterization was performed with a Tecnai G2 F30 S-Twin high-resolution transmission electron microscope (HRTEM) (Philips-FEI Instruments, Holland). Fourier transform infrared (FTIR) spectra were recorded by using a Nicolet 6700 spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Raman spectra were recorded for the glass samples by using an InVia Reflex Raman spectrometer (Renishaw, UK) using a 532 nm diode laser as the excitation source. Differential scanning calorimetry (DSC) curves were recorded by using SDT Q600 simultaneous thermal analyzers (TA Instruments, USA). Absorption spectra were recorded with a UV-3600 UV-VIS-NIR spectrophotometer (Shimadzu Instruments, Japan). PL spectra were recorded by using a FLS980 fluorescence spectrometer (Edinburgh Instruments, UK). The PL quantum yield (QY) was measured by using an absolute photoluminescence quantum yield measurement system (Quantaurus-QY Plus C13534-35, Japan). The refractive index of the sample was measured by using a Model 2010/M prism coupler (Metricon Instruments).2.3. Z-scan measurement
An open aperture Z-scan system was employed to investigate the NLO properties of the CsPbBr3 QD-doped glass, using a femtosecond laser (central wavelength: 1030 nm, pulse duration: 226 fs, and repetition rate: 10 kHz) as the excitation source.3. Results and discussion
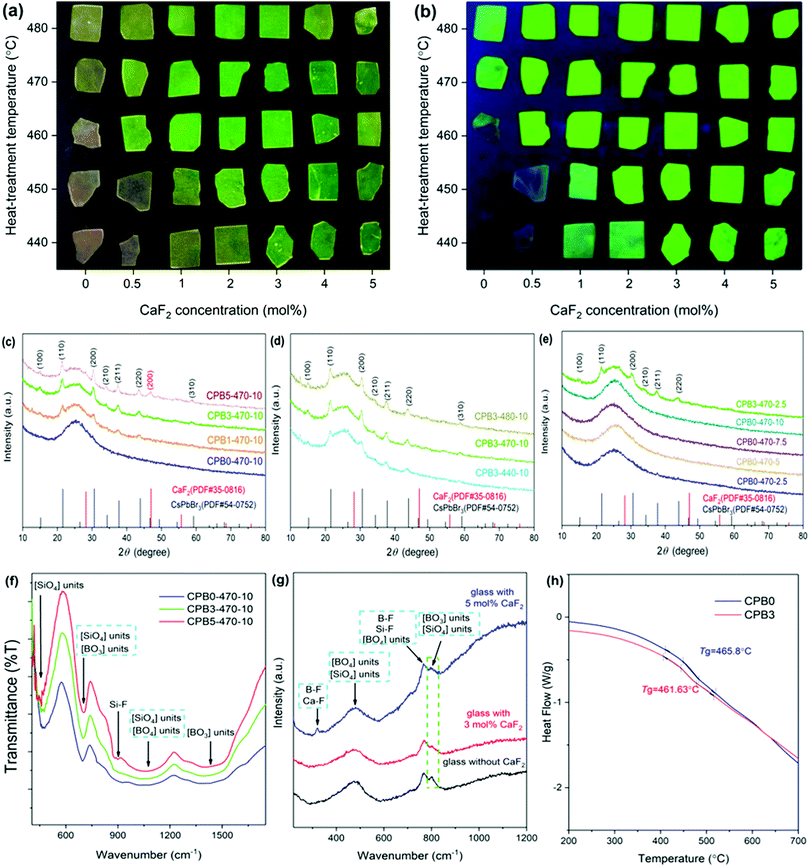
We use a borosilicate glass as the matrix as it can be melted at a temperature of 1100 °C, at which the metal halide reactive species suffer moderate evaporation loss. CaF2, a stable fluoride with a melting temperature (1423 °C) close to the glass melt, was introduced to this glass as an additional network modifier that breaks the oxygen bridges and facilitates the mobility of ions needed for the formation of the QDs.As can be seen from Fig. 1(a), the color of the samples changes from light green to yellow, depending on the CaF2 concentration and heat-treatment temperature. Under irradiation from a 365 nm ultraviolet (UV) lamp, strong PL is observed and the PL intensity is high for samples with a green color (Fig. 1(b)). Fig. 1(c) shows the X-ray diffraction (XRD) patterns of the glass samples CPBx-470-10 (x = 0, 1, 3, and 5). Several diffraction peaks are observed at angles (2θ) of approximately 21.4°, 30.4°, 37.5°, 43.5°, and 58.9° for the samples CPB1-470-10, CPB3-470-10, and CPB5-470-10, corresponding to the (110), (200), (211), (220) and (310) planes of CsPbBr3 QDs (PDF#54-0752), respectively, which indicates the successful precipitation of CsPbBr3 perovskite QDs in the glasses. Compared with the sample CPB0-470-10, the samples CPB1-470-10 and CPB3-10-2.5 show strong diffraction peaks, as shown in Fig. 1(c) and (e). The XRD patterns show that the addition of CaF2 is favourable for the crystallization of CsPbBr3 QDs in the glass. Sample CPB5-470-10 has an additional diffraction peak at 46.9°, corresponding to the (200) plane of the CaF2 crystal (PDF#35-0816), which indicates that the CaF2 crystal was also precipitated in the glass matrix. The XRD patterns of the glass samples obtained at different heat-treatment temperatures were also recorded, as shown in Fig. 1(d). The result shows that the intensity of the diffraction peak of glass samples CPB3-y-10 increases with the increase of heat-treatment temperature from 440 °C to 480 °C, which implies the enhancement of crystallinity and concentration of CsPbBr3 QDs in the glasses. To understand the network structure of the glass samples, Fourier transform infrared (FTIR) spectra and Raman spectra were recorded. To avoid self-fluorescence of the CsPbBr3 QDs in the Raman spectrum measurement, the glass samples without CsPbBr3 QDs were prepared by removal of PbO from the glass compositions. Four main absorption bands can be observed in the FTIR spectra, as shown in Fig. 1(f). The absorption bands of the [BO3] triangle (located at 695–705 cm−1 and 1380–1400 cm−1), [BO4] tetrahedron (1020–1060 cm−1), and [SiO4] tetrahedron (695–705 cm−1 and 1020–1060 cm−1) can be clearly observed.42 These groups are also captured in the Raman spectra, while the intensities are different,43,44 as shown in Fig. 1(g). The FTIR and Raman spectra results indicate that the borosilicate glass network mainly consists of a [BO3] triangle, [BO4] tetrahedron and [SiO4] tetrahedron through the interconnection of apical oxygen atoms. With the addition of CaF2 into the glass, an extra absorption band appears at approximately 900 cm−1 in the FT-IR spectra, which can be ascribed to the Si–F bond.45,46 Moreover, the formation of B–F and Ca–F bonds is also evidenced from the Raman spectra.47,48 With the increase in the CaF2 concentration, the Raman peak intensity at around 800 cm−1 due to bridged oxygen decreases, while the peak at 768 cm−1 contributed from both bridge oxygen (B–O) and non-bridge oxygen bonds (B–F, Si–F) remains almost unchanged.43,44,49–51 This observation implies that the added F− ions break the strong oxygen bridge (i.e., O–Si–O and O–B–O) to produce non-bridge oxygen bonds (Si–F, B–F) and thus change the network topology structure, which is beneficial for the movement of the ions and the nucleation and growth of the QDs inside the glass during heat treatment. This result is consistent with the change in the thermal stability of the samples. Fig. 1(h) shows the differential scanning calorimetry (DSC) curves of the glass samples with different CaF2 concentrations before heat-treatment. The glass transition temperature (Tg) of 461.63 °C with the addition of 3 mol% CaF2 is lower than that of the sample without CaF2 (465.8 °C), indicating that the addition of CaF2 can effectively reduce the Tg as a result of the change in the glass topology structure.
The precipitation of CsPbBr3 QDs in the glass was further confirmed by transmission electron microscopy (TEM) observation, as shown in Fig. 2. Fig. 2(a) and (b) clearly show that a large number of nanoparticles (NPs) are dispersed in the glass. As shown in Fig. 2(c) and (d), the plane spacing values are approximately 0.261 nm and 0.291 nm, which correspond to the (210) and (200) crystal planes of CsPbBr3 NCs (PDF#54-0752), respectively. Fig. 2(e) and (f) show the selected area electron diffraction (SAED) patterns of CPB0-470-10 and CPB3-470-10, respectively. The diffraction rings of CPB0-470-10 and CPB3-470-10 correspond to the (211) plane and the (200) plane of CsPbBr3 nanocrystals (NCs), respectively. These results confirm that CsPbBr3 QDs are precipitated in the glass matrix.
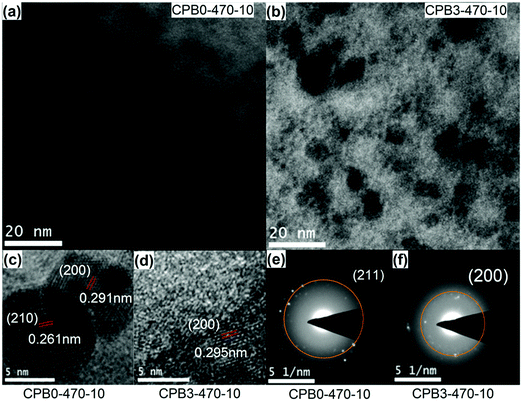 | ||
| Fig. 2 (a and b) TEM images of glass samples CPB0-470-10 and CPB3-470-10. (c and d) HRTEM images of glass samples CPB0-470-10 and CPB3-470-10. (e and f) SAED images of CPB0-470-10 and CPB3-470-10. | ||
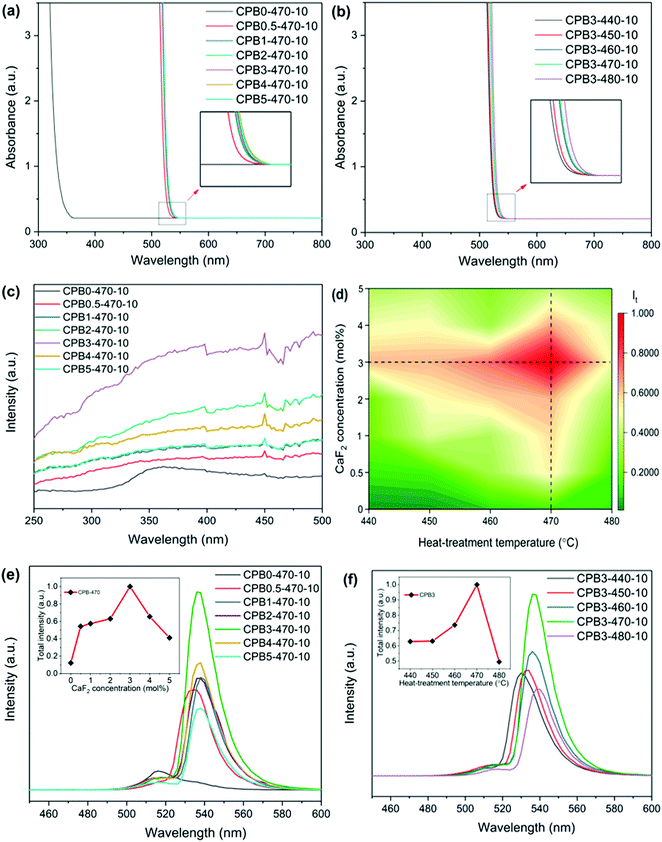
Fig. 3(a) and (b) show the absorption spectra of glass samples CPBx-470-10 and CPB3-y-10. After doping with CaF2, the absorption edge shifts from 340 nm to 530 nm. In addition, the absorption edge shows a small red-shift with the increase in the heat-treatment temperature. According to the XRD results (Fig. 1(c) and (d)), we know that the addition of the appropriate amount of CaF2 and the enhancement of heat-treatment temperature could promote the crystallization of CsPbBr3 QDs, which leads to the red-shift of the absorption edge. The excitation spectra of the glass samples with different CaF2 concentrations are shown in Fig. 3(c). Broad excitation bands are observed in the visible wavelength region. With the increase of the CaF2 concentration, the intensity of the excitation spectra first increases then decreases, implying a change in the QD size and concentration.
The emission spectra under excitation at 400 nm are shown in Fig. 3 (d)–(f), and the characteristics of the emission spectra are given in Tables 1 and 2. As the concentration of CaF2 increases, we observe a clear red-shift in the PL peak from 516 nm to 538 nm, which is mainly attributed to the increase of the QD size due to a high growth rate in samples with higher CaF2 content. The growth process of the CsPbBr3 QDs is mainly attributed to Oswald ripening, in which the small QDs gradually attach to the large QDs, resulting in the increase of the QDs’ average size.52–54 The accelerated growth of the QDs in glass with CaF2 can be attributed to the enhanced ion mobility due to the decreased connectivity in the glass network topology. As the heat treatment temperature increases, the PL peak wavelength red-shifts from 530 nm to 538 nm, which is also attributed to the increase of the QD size at high heat treatment temperatures. The PL intensity reaches the maximum value for the sample CPB3-470-10, which is 8 times greater than that of the glass sample without CaF2, as shown in Table 1. Fig. 3(e) shows that the PL intensity first increases and then decreases with the increase of the CaF2 concentration. There are two main factors for the enhancement of PL intensity after the addition of CaF2. First, an appropriate amount of fluoride additive is favorable for the crystallization of CsPbBr3 perovskite QDs in the oxide glass as the Tg is reduced due to the decrease in the network connectivity. Second, the addition of metal fluoride can effectively reduce the phonon energy of the glass and decrease the rate of nonradiative transition. Fig. 3(f) shows that the PL intensity first increases and then decreases with the increase of heat-treatment temperature. As a higher heat-treatment temperature could lead to an increase of precipitated QD concentration (also observed by absorption), the self-absorption and the coupling among QDs could prohibit the further increase of PL intensity.
| Sample | λ p (nm) | I t (a.u.) | QY (%) |
|---|---|---|---|
| Note: λp denotes the PL peak wavelength. It denotes the PL total intensity, which is obtained by calculating the spectral integral area. | |||
| CPB0-470-10 | 516 | 0.12 | 15.4 |
| CPB0.5-470-10 | 534 | 0.54 | 20.1 |
| CPB1-470-10 | 538 | 0.57 | 27.4 |
| CPB2-470-10 | 536 | 0.63 | 36.7 |
| CPB3-470-10 | 536 | 1 | 40.4 |
| CPB4-470-10 | 538 | 0.65 | 30.5 |
| CPB5-470-10 | 538 | 0.41 | 22.5 |
| Sample | λ p (nm) | I t (a.u.) | QY (%) |
|---|---|---|---|
| Note: λp denotes the PL peak wavelength. It denotes the PL total intensity, which is obtained by calculating the spectral integral area. | |||
| CPB3-440-10 | 530 | 0.62 | 28.0 |
| CPB3-450-10 | 534 | 0.63 | 31.4 |
| CPB3-460-10 | 536 | 0.73 | 37.6 |
| CPB3-470-10 | 536 | 1 | 40.4 |
| CPB3-480-10 | 538 | 0.46 | 27.2 |
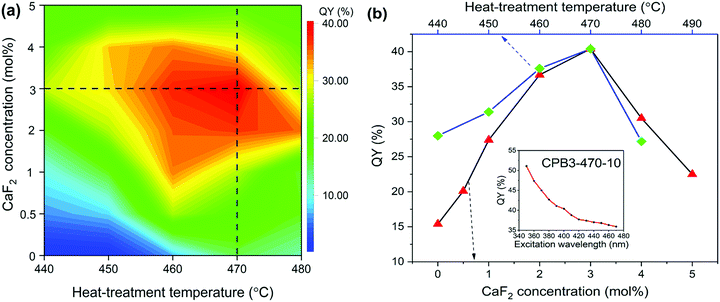
In order to further investigate the PL properties, we measured the QY of glass samples, as given in Fig. 4(a) and (b), Tables 1 and 2. It is found that the QY first increases and then decreases with the increase of heat-treatment temperature or CaF2 concentration, as shown in Fig. 4(a) and (b), which is similar to the change of PL intensity. Compared with the QY of the glass sample CPB0-470-10 without CaF2, the QY of CBP3-470-10 increases by around 300% under optimized fabrication conditions. In addition, the QY values decrease with the red-shift of the excitation wavelength due to reduced absorption.
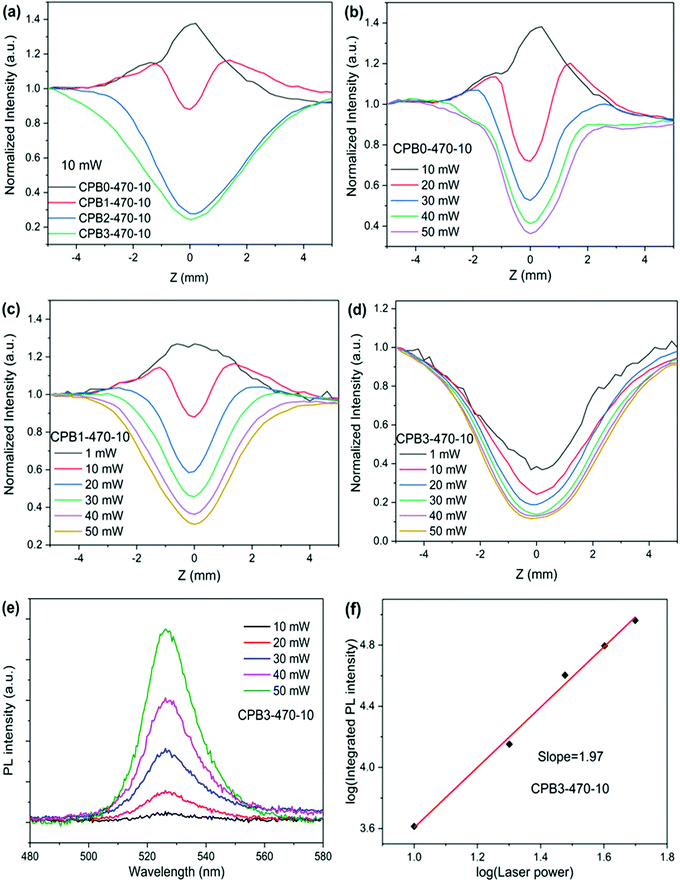
The manipulation of the network topology by introducing CaF2 into the glass also enables the control of the nonlinear optical (NLO) absorption and nonlinear PL of the precipitated QDs. To examine the NLO response of the CsPbBr3 perovskite QD-doped glasses, we first measured the NLO absorption using the Z-scan technique under femtosecond pulsed laser excitation at 1030 nm. The open-aperture (OA) Z-scan transmittance curves are shown in Fig. 5. The experimental results show that the CsPbBr3 QD-doped borosilicate glass exhibits a clear nonlinear absorption (NLA) response. With the increase of the CaF2 concentration, the NLA response of the glass samples CPBx-470-10 changes from the saturable absorption (SA) process to the reverse saturable absorption (RSA) process, as shown in Fig. 5 (a). With the laser power increasing from 10 mW to 50 mW, the NLA response of the glass sample CPB0-470-10 also changes from the SA process to the RSA process, as shown in Fig. 5 (b). Similar laser intensity-dependent transformation from the SA to the RSA has been previously reported in other semiconductor nanomaterials or nanomaterials, such as GaAs semiconductors, WS2 two-dimensional (2D) semiconductors, Cu2–xSe NPs, Pd NPs, black phosphorus nanoplatelets and SnSe Nanosheets.55–60 Generally, the change from the SA process to the RSA process indicates a transition from single-photon absorption (SPA) to two-photon absorption (TPA).
According to the NLO theory,61–64 we can calculate the parameters related to NLA of glass sample CPB3-470-10 by fitting the OA Z-scan transmittance curves, including nonlinear absorption coefficient (β) and the imaginary part of the third-order NLO susceptibility (Im![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) χ(3)). The refractive index of the glass sample CPB3-470-10 is 1.52. The calculated results are given in Table 3. The β and Imχ(3) values of sample CBP3-470-10 decrease with the increase of laser power, as shown in Table 3, which indicates that the RSA response becomes saturated. The highest value of Imχ(3) is about 8.29 × 10−13 esu, which is close to the previously reported results for colloidal CsPbBr3 QDs in ref. 65 and 66.
χ(3)). The refractive index of the glass sample CPB3-470-10 is 1.52. The calculated results are given in Table 3. The β and Imχ(3) values of sample CBP3-470-10 decrease with the increase of laser power, as shown in Table 3, which indicates that the RSA response becomes saturated. The highest value of Imχ(3) is about 8.29 × 10−13 esu, which is close to the previously reported results for colloidal CsPbBr3 QDs in ref. 65 and 66.
| P (mW) | 1 | 10 | 20 | 30 | 40 | 50 |
| β (10−12 m W−1) | 10.99 | 1.32 | 0.71 | 0.50 | 0.38 | 0.31 |
| Imχ(3) (10−13 esu) | 8.29 | 0.99 | 0.53 | 0.37 | 0.29 | 0.23 |
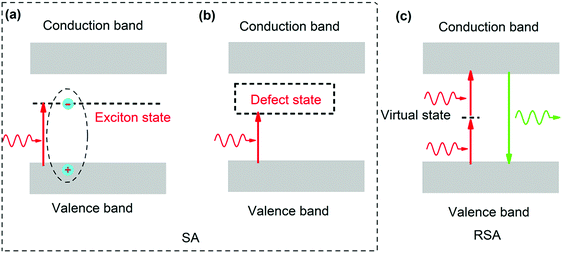
According to the linear absorption spectra given in Fig. 3(a) and (b), the absorption edges are located at approximately 530 nm and the calculated energy of the direct bandgap is about 2.33 eV, which is much larger than the energy of the fs laser photon (1.20 eV) in our measurement. For semiconductor QD materials, when the energy of laser photons is lower than the energy of the direct bandgap, the SA nonlinear process is mainly attributed to the absorption by transitions involving excitons and the defect state located within the bandgap.60,67–71 Due to the effect of quantum confinement, there is an exciton state in the bandgap and its energy is slightly lower than the energy of the direct bandgap,72–74 as shown in Fig. 6(a). In addition, the presence of defects, e.g., dangling bonds, on the QD surfaces may introduce a defect state within the bandgap,75,76 as shown in Fig. 6(b). Generally, two-photon absorption (TPA) mediated by a virtual state dominates the RSA process for the perovskite QDs,64,77–79 as shown in Fig. 6(c). Because the energy of the fs laser photon is much lower than the energy of the direct bandgap in our measurement, the defect state absorption plays a dominant role in the SA nonlinear process. According to the XRD results in Fig. 1(c), with the increase of the CaF2 concentration, the quality of the QDs increases, and the defects on the QD surface are passivated, thereby reducing the defect state absorption, which leads to weakening of the SA process and eventually transitions to the RSA process, as shown in Fig. 5(a). Under the fs laser excitation with low pulse energy, the observed SA process dominates the NLA process for the samples CPB0-470-10 and CPB1-470-10, as shown in Fig. 5(b) and (c). With the increase of laser power, the SA process becomes saturated and the TPA process mediated by a virtual state begins to dominate the NLA process for the samples CPB0-470-10 and CPB1-470-10, resulting in the change from the SA process to the RSA process, as shown in Fig. 5(b) and (c).
In order to explore the RSA mechanism of CsPbBr3 QD-doped glass, the excitation energy-dependent PL spectrum was recorded under excitation by the fs laser (∼1030 nm), as shown in Fig. 5(e) and (f). The PL intensity increases with the increase in the laser pulse energy. A nearly linear relationship between the logarithm of excitation energy and the logarithm of integrated PL intensity is obtained, giving a slope of 1.97 for the sample CPB3-470-10, which indicates that TPA dominates the RSA process in the CsPbBr3 perovskite QD-doped glass.
4. Conclusion
We have demonstrated the tuning of linear and nonlinear optical properties of CsPbBr3 perovskite QD-doped glass by modulating the network topology through the introduction of a network modifier, CaF2. The precipitation of the QDs and the optical properties of the samples are strongly affected by the introduced metal fluoride network modifier and the PLQY values could be increased by over 300% under optimal conditions. In addition, the modification of the glass network structure also allows for the control of absorptive optical nonlinearity between saturable absorption and reverse saturable absorption. The strategy demonstrated in the present work can be further exploited for tailoring the optical properties of photonic glasses.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work is financially supported by the Natural Science Foundation of Zhejiang Province (grant no. LY21F050005, LR21E020005), the National Natural Science Foundation of China (Grant No. U20A20211, 51772270, 61775192), Open funds of the State Key Laboratory of High Field Laser Physics, Shanghai Institute of Optics and Fine Mechanics, Chinese Academy of Sciences and the Fundamental Research Funds for the Central Universities.References
- Y. T. Dong, Y. K. Wang, F. L. Yuan, A. Johnston, Y. Liu, D. X. Ma, M. J. Choi, B. Chen, M. Chekini, S. W. Baek, L. K. Sagar, J. Fan, Y. Hou, M. J. Wu, S. Lee, B. Sun, S. Hoogland, R. Quintero-Bermudez, H. Ebe, P. Todorovic, F. Dinic, P. C. Li, H. T. Kung, M. I. Saidaminov, E. Kumacheva, E. Spiecker, L. S. Liao, O. Voznyy, Z. H. Lu and E. H. Sargent, Nat. Nanotechnol., 2020, 15, 668–674 CrossRef CAS PubMed
.
- X. Luo, R. Lai, Y. Li, Y. Han, G. Liang, X. Liu, T. Ding, J. Wang and K. Wu, J. Am. Chem. Soc., 2019, 141, 4186–4190 CrossRef CAS PubMed
.
- X. Huang, Q. Guo, D. Yang, X. Xiao, X. Liu, Z. Xia, F. Fan, J. Qiu and G. Dong, Nat. Photonics, 2020, 14, 82–88 CrossRef CAS
.
- K. B. Lin, J. Xing, L. N. Quan, F. P. G. De Arquer, X. W. Gong, J. X. Lu, L. Q. Xie, W. J. Zhao, D. Zhang, C. Z. Yan, W. Q. Li, X. Y. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. H. Xiong and Z. H. Wei, Nature, 2018, 562, 245–248 CrossRef CAS PubMed
.
- Y. Wang, M. I. Dar, L. K. Ono, T. Y. Zhang, M. Kan, Y. W. Li, L. J. Zhang, X. T. Wang, Y. G. Yang, X. Y. Gao, Y. B. Qi, M. Gratzel and Y. X. Zhao, Science, 2019, 365, 591–595 CrossRef CAS PubMed
.
- J. S. Yao, J. Ge, B. N. Han, K. H. Wang, H. B. Yao, H. L. Yu, J. H. Li, B. S. Zhu, J. Z. Song, C. Chen, Q. Zhang, H. B. Zeng, Y. Luo and S. H. Yu, J. Am. Chem. Soc., 2018, 140, 3626–3634 CrossRef CAS PubMed
.
- T. Chiba, S. Ishikawa, J. Sato, Y. Takahashi, H. Ebe, S. Ohisa and J. Kido, Adv. Opt. Mater., 2020, 8, 2000289 CrossRef CAS
.
- R. H. Liu, J. Q. Zhang, H. Zhou, Z. H. Song, Z. N. Song, C. R. Grice, D. J. Wu, L. P. Shen and H. Wang, Adv. Opt. Mater., 2020, 8, 1901735 CrossRef CAS
.
- Y. H. Lin, N. Sakai, P. Da, J. Y. Wu, H. C. Sansom, A. J. Ramadan, S. Mahesh, J. L. Liu, R. D. J. Oliver, J. Lim, L. Aspitarte, K. Sharma, P. K. Madhu, A. B. Morales-Vilches, P. K. Nayak, S. Bai, F. Gao, C. R. M. Grovenor, M. B. Johnston, J. G. Labram, J. R. Durrant, J. M. Ball, B. Wenger, B. Stannowski and H. J. Snaith, Science, 2020, 369, 96–102 CrossRef CAS PubMed
.
- I. Poli, U. Hintermair, M. Regue, S. Kumar, E. V. Sackville, J. Baker, T. M. Watson, S. Eslava and P. J. Cameron, Nat. Commun., 2019, 10, 2097 CrossRef PubMed
.
- C. J. Qin, A. S. D. Sandanayaka, C. Y. Zhao, T. Matsushima, D. Z. Zhang, T. Fujihara and C. Adachi, Nature, 2020, 585, 53–57 CrossRef CAS PubMed
.
- P. Y. Cao, B. B. Yang, F. Zheng, L. Wang and J. Zou, Ceram. Int., 2020, 46, 3882–3888 CrossRef CAS
.
- J. M. Hoffman, J. Strzalka, N. C. Flanders, I. Hadar, S. A. Cuthriell, Q. Zhang, R. D. Schaller, W. R. Dichtel, L. X. Chen and M. G. Kanatzidis, Adv. Mater., 2020, 32, 2002812 CrossRef CAS PubMed
.
- X. Zhang, X. Y. Gao and X. Meng, J. Alloys Compd., 2019, 810, 151943 CrossRef CAS
.
- Z. Y. Ni, C. X. Bao, Y. Liu, Q. Jiang, W. Q. Wu, S. S. Chen, X. Z. Dai, B. Chen, B. Hartweg, Z. S. Yu, Z. Holman and J. S. Huang, Science, 2020, 367, 1352–1358 CrossRef CAS PubMed
.
- V. V. Belykh, D. R. Yakovlev, M. M. Glazov, P. S. Grigoryev, M. Hussain, J. Rautert, D. N. Dirin, M. V. Kovalenko and M. Bayer, Nat. Commun., 2019, 10, 1746 CrossRef PubMed
.
- J. Lapano, M. Brahlek, L. Zhang, J. Roth and R. Engel-Herbert, Nat. Commun., 2019, 10, 2464 CrossRef PubMed
.
- F. M. Li, B. A. Davidson, R. Sutarto, H. Shin, C. Liu, I. Elfimov, K. Foyevtsova, F. Z. He, G. A. Sawatzky and K. Zou, Phys. Rev. Mater., 2019, 3, 100802 CrossRef CAS
.
- H. T. Zhang, L. R. Dedon, L. W. Martin and R. Engel-Herbert, Appl. Phys. Lett., 2015, 106, 233102 CrossRef
.
- C. Y. Wang, H. Lin, X. Q. Xiang, Y. Cheng, Q. M. Huang, Y. Gao, X. S. Cui and Y. S. Wang, J. Mater. Chem. C, 2018, 6, 9964–9971 RSC
.
- S. N. Chen, J. Mater. Sci.: Mater. Electron., 2019, 30, 19536–19540 CrossRef CAS
.
- Y. Ye, W. C. Zhang, Z. Y. Zhao, J. Wang, C. Liu, Z. Deng, X. J. Zhao and J. J. Han, Adv. Opt. Mater., 2019, 7, 1801663 CrossRef
.
- Y. Zhou, C. Liu, Z. Y. Zhao, W. C. Zhang, K. Li, Y. Ye, C. F. Zhu and X. G. Meng, J. Alloys Compd., 2020, 827, 154349 CrossRef CAS
.
- Z. S. Xu, X. F. Liu, J. R. Qiu and C. Cheng, Opt. Lett., 2019, 44, 5626–5629 CrossRef CAS PubMed
.
- K. Zhang, D. C. Zhou, J. B. Qiu, Z. W. Long, R. Zhu, Q. Wang, J. N. Lai, H. Wu and C. C. Zhu, J. Am. Ceram. Soc., 2020, 103, 2463–2470 CrossRef CAS
.
- S. S. Li, L. J. Nie, S. M. Ma, G. P. Yao, F. M. Zeng, X. Y. Wang, C. Y. Sun, G. X. Hu and Z. M. Su, J. Eur. Ceram. Soc., 2020, 40, 3270–3278 CrossRef CAS
.
- Y. Q. Zhang, J. M. Liu, H. L. Zhang, Q. Y. He, X. J. Liang and W. D. Xiang, J. Eur. Ceram. Soc., 2020, 40, 6023–6030 CrossRef CAS
.
- P. P. Li, Y. M. Duan, Y. Lu, A. Xiao, Z. Y. Zeng, S. Q. Xu and J. J. Zhang, Nanoscale, 2020, 12, 6630–6636 RSC
.
- E. Erol, O. Kibrisli, M. C. Ersundu and A. E. Ersundu, Chem. Eng. J., 2020, 401, 126053 CrossRef CAS
.
- Y. Liu, W. Chen, J. Zhong and D. Chen, J. Eur. Ceram. Soc., 2019, 39, 4275–4282 CrossRef CAS
.
- D. Chen, S. Yuan, J. Chen, J. Zhong and X. Xu, J. Mater. Chem. C, 2018, 6, 12864–12870 RSC
.
- Y. Wang, R. Zhang, Y. Yue, S. Yan, L. Zhang and D. Chen, J. Alloys Compd., 2020, 818, 152872 CrossRef CAS
.
- P. P. Li, W. Q. Xie, W. Mao, Y. Tian and J. J. Zhang, J. Alloys Compd., 2019, 817, 153338 CrossRef
.
- X. Z. Zhang, L. Z. Guo, Y. H. Zhang, C. H. Cheng, Y. Cheng, X. P. Li, J. S. Zhang, S. Xu, Y. Z. Cao, J. S. Sun, L. H. Cheng and B. J. Chen, J. Am. Ceram. Soc., 2020, 103, 5028–5035 CrossRef CAS
.
- D. Chen, Y. Liu, C. Yang, J. Zhong, S. Zhou, J. Chen and H. Huang, Nanoscale, 2019, 11, 17216–17221 RSC
.
- G. Lakshminarayana, E. M. Weis, B. L. Bennett, A. Labouriau, D. J. Williams, J. G. Duque, M. Sheik-Bahae and M. P. Hehlen, Opt. Mater., 2012, 35, 117–125 CrossRef CAS
.
- X. Tian, S. Lian, C. Ji, Z. Huang, J. Wen, Z. Chen, H. Peng, S. Wang, J. Li, J. Hu and Y. Peng, J. Alloys Compd., 2019, 784, 628–640 CrossRef CAS
.
- R. E. Youngman and M. J. Dejneka, J. Am. Ceram. Soc., 2002, 85, 1077–1082 CrossRef CAS
.
- K. X. Han, P. Zhang, S. B. Wang, Y. Y. Guo, D. C. Zhou and F. X. Yu, Sci. Rep., 2016, 6, 31207 CrossRef CAS PubMed
.
- A. Maaoui, M. Haouari, A. Bulou, B. Boulard and H. Ben Ouada, J. Lumin., 2018, 196, 1–10 CrossRef CAS
.
- Y. P. Peng, X. Q. Yuan, L. Zhang, P. G. Yan, W. F. Zhang and S. C. Ruan, J. Rare Earths, 2019, 37, 487–491 CrossRef CAS
.
- Z. S. Xu, X. F. Liu, C. Jiang, D. W. Ma, J. J. Ren, C. Cheng and J. R. Qiu, J. Am. Ceram. Soc., 2018, 101, 1508–1515 CrossRef CAS
.
- S. L. Kang, X. Q. Song, X. J. Huang, J. R. Qiu and G. P. Dong, Opt. Mater. Express, 2016, 6, 2351–2359 CrossRef CAS
.
- A. H. Mir, I. Monnet, B. Boizot, C. Jégou and S. Peuget, J. Nucl. Mater., 2017, 489, 91–98 CrossRef CAS
.
- L. G. Jacobsohn, D. F. Franceschini, I. V. Afanasyev-Charkin, D. W. Cooke, L. L. Daemen, R. D. Averitt and M. Nastasi, Surf. Coat. Technol., 2006, 200, 6079–6082 CrossRef CAS
.
- J. Wu, Y. L. Wang and C. T. Kuo, Surf. Coat. Technol., 2006, 200, 3303–3308 CrossRef CAS
.
- J. OJensen, J. Mol. Struct.: THEOCHEM, 2004, 676, 193–202 CrossRef
.
- X. W. Miao, Z. T. Bai, X. T. Huo, M. Guo, F. Q. Cheng and M. Zhang, Ceram. Int., 2019, 45, 8510–8517 CrossRef CAS
.
- Y. Suzuki, T. Park, K. Hachiya and T. Goto, J. Fluorine Chem., 2020, 238, 109616 CrossRef CAS
.
- Z. G. Zhang, H. Wang and M. Zhu, Int. J. Hydrogen Energy, 2011, 36, 8203–8208 CrossRef CAS
.
- I. S. Peretyazhko, S. Z. Smirnov, A. R. Kotelnikov and Z. A. Kotelnikova, Russ. Geol. Geophys., 2010, 51, 349–368 CrossRef
.
- T. Wang, Z. Yang, L. L. Yang, X. Yu, L. T. Sun, J. B. Qiu, D. C. Zhou, W. Lu, S. F. Yu, Y. Lin and X. H. Xu, J. Phys. Chem. Lett., 2020, 11, 4618–4624 CrossRef CAS PubMed
.
- K. S. Hun, K. D. Park and S. L. Hong, Energies, 2021, 14, 275 CrossRef
.
- J. B. Zhang, L. W. Fan, J. L. Li, X. F. Liu, R. W. Wang, L. Wang and G. L. Tu, Nano Res., 2019, 12, 121–127 CrossRef CAS
.
- R. H. Xu, S. Z. Zhao, K. J. Yang, G. Q. Li, T. Li and D. C. Li, Opt. Express, 2018, 26, 8542–8549 CrossRef CAS PubMed
.
- X. Zheng, Y. W. Zhang, R. Z. Chen, X. A. Cheng, Z. J. Xu and T. Jiang, Opt. Express, 2015, 23, 15616–15623 CrossRef CAS PubMed
.
- M. A. Ali, Y. H. Xian, X. F. Liu and J. R. Qiu, J. Phys. Chem. C, 2019, 123, 9394–9399 CrossRef CAS
.
- G. H. Fan, S. L. Qu, Q. A. Wang, C. J. Zhao, L. Zhang and Z. G. Li, J. Appl. Phys., 2011, 109, 023102 CrossRef
.
- X. Zheng, R. Z. Chen, G. Shi, J. W. Zhang, Z. J. Xu, X. A. Cheng and T. Jiang, Opt. Lett., 2015, 40, 3480–3483 CrossRef CAS PubMed
.
- Y. T. Ye, Y. H. Xian, J. W. Cai, K. C. Lu, Z. Z. Liu, T. C. Shi, J. Du, Y. X. Leng, R. F. Wei, W. Q. Wang, X. F. Liu, G. Bi and J. R. Qiu, Adv. Opt. Mater., 2019, 7, 1800579 CrossRef
.
- J. Z. Li, H. X. Dong, B. Xu, S. F. Zhang, Z. P. Cai, J. Wang and L. Zhang, Photonics Res., 2017, 5, 457–460 CrossRef CAS
.
- Z. S. Xu, Q. B. Guo, C. Liu, Z. J. Ma, X. F. Liu and J. R. Qiu, Appl. Phys. B: Lasers Opt., 2016, 122, 259 CrossRef
.
- Q. B. Guo, Y. D. Cui, Y. H. Yao, Y. T. Ye, Y. Yang, X. M. Liu, S. Zhang, X. F. Liu, J. R. Qiu and H. Hosono, Adv. Mater., 2017, 29, 1700754 CrossRef PubMed
.
- Z. S. Xu, T. Chen, D. D. Zhang, G. J. Zheng, J. H. Wu, J. H. Yan, X. F. Liu and J. R. Qiu, J. Eur. Ceram. Soc., 2021, 41, 729–734 CrossRef CAS
.
- W. G. Lu, C. Chen, D. Han, L. Yao, J. Han, H. Zhong and Y. Wang, Adv. Opt. Mater., 2016, 4, 1732–1739 CrossRef CAS
.
- M. Jin, X. Liang, H. Zhang, J. Liu and Y. Song, J. Eur. Ceram. Soc., 2020, 40, 4140–4147 CrossRef CAS
.
- D. Mao, S. Zhang, Y. Wang, X. Gan, W. Zhang, T. Mei, Y. Wang, Y. Wang, H. Zeng and J. Zhao, Opt. Express, 2015, 23, 27509–27519 CrossRef CAS PubMed
.
- S. Wang, H. Yu, H. Zhang, A. Wang, M. Zhao, Y. Chen, L. Mei and J. Wang, Adv. Mater., 2014, 26, 3538–3544 CrossRef CAS PubMed
.
- G. W. Liang, L. H. Zeng, Y. H. Tsang, L. L. Tao, C. Y. Tang, P. K. Cheng, H. Long, X. Liu, J. Li, J. L. Qu and Q. Wen, J. Mater. Chem. C, 2018, 6, 7501–7511 RSC
.
- L. Zhang, S. Fahad, H. R. Wu, T. T. Dong, Z. Z. Chen, Z. Q. Zhang, R. T. Liu, X. P. Zhai, X. Y. Li, X. Fei, Q. W. Song, Z. J. Wang, L. C. Chen, C. L. Sun, Y. Peng, Q. Wang and H. L. Zhang, Nanoscale Horiz., 2020, 5, 1420–1429 RSC
.
- S. L. Jiang, J. Yang, Y. P. Shi, J. Zhao, C. Y. Xie, L. Y. Zhao, J. T. Fu, P. F. Yang, Y. H. Huan, Q. Xie, H. C. Jiang, Q. Zhang, X. L. Wang, F. H. Su and Y. F. Zhang, Nano Res., 2020, 13, 667–675 CrossRef CAS
.
- Q. J. Han, W. Z. Wu, W. L. Liu, Q. X. Yang and Y. Q. Yang, Opt. Mater., 2018, 75, 880–886 CrossRef CAS
.
- M. L. De Giorgi, A. Perulli, N. Yantara, P. P. Boix and M. Anni, J. Phys. Chem. C, 2017, 121, 14772–14778 CrossRef CAS
.
- Y. H. Qiu, F. Nan, Q. Wang, X. D. Liu, S. J. Ding, Z. H. Hao, L. Zhou and Q. Q. Wang, J. Phys. Chem. C, 2017, 121, 6916–6923 CrossRef CAS
.
- J. Kang and L. W. Wang, J. Phys. Chem. Lett., 2017, 8, 489–493 CrossRef CAS PubMed
.
- R. Brakkee and R. M. Williams, Appl. Sci., 2020, 10, 3061 CrossRef CAS
.
- R. Ketavath, N. K. Kattur, S. G. Ghugal, H. K. Kolli, T. Swetha, V. R. Soma and B. Murali, J. Phys. Chem. Lett., 2019, 10, 5577–5584 CrossRef CAS PubMed
.
- K. Wei, Z. J. Xu, R. Z. Chen, X. Zheng, X. G. Cheng and T. Jiang, Opt. Lett., 2016, 41, 3821–3824 CrossRef CAS PubMed
.
- J. Zhang, T. Jiang, X. Zheng, C. Shen and X. A. Cheng, Opt. Lett., 2017, 42, 3371–3374 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |