Open Access Article
Open Access ArticleRecommendations for replacing PET on packaging, fiber, and film materials with biobased counterparts
Andreia F.
Sousa
 *ab,
Rafael
Patrício
a,
Zoi
Terzopoulou
*ab,
Rafael
Patrício
a,
Zoi
Terzopoulou
 cd,
Dimitrios N.
Bikiaris
cd,
Dimitrios N.
Bikiaris
 c,
Tobias
Stern
e,
Julia
Wenger
c,
Tobias
Stern
e,
Julia
Wenger
 e,
Katja
Loos
e,
Katja
Loos
 f,
Nadia
Lotti
f,
Nadia
Lotti
 g,
Valentina
Siracusa
h,
Anna
Szymczyk
g,
Valentina
Siracusa
h,
Anna
Szymczyk
 i,
Sandra
Paszkiewicz
i,
Sandra
Paszkiewicz
 i,
Konstantinos S.
Triantafyllidis
i,
Konstantinos S.
Triantafyllidis
 c,
Alexandra
Zamboulis
c,
Marija S.
Nikolic
c,
Alexandra
Zamboulis
c,
Marija S.
Nikolic
 j,
Pavle
Spasojevic
k,
Shanmugam
Thiyagarajan
j,
Pavle
Spasojevic
k,
Shanmugam
Thiyagarajan
 l,
Daan S.
van Es
l,
Daan S.
van Es
 l and
Nathanael
Guigo
m
l and
Nathanael
Guigo
m
aCICECO—Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: andreiafs@ua.pt
bCentre for Mechanical Engineering, Materials and Processes, Department of Chemical Engineering, University of Coimbra Rua Sílvio Lima − Polo II, 3030-790 Coimbra, Portugal
cDepartment of Chemistry, Aristotle University of Thessaloniki, GR5424 Thessaloniki, Greece
dDepartment of Chemistry, University of Ioannina, GR45110 Ioannina, Greece
eInstitute of Systems Sciences, Innovation and Sustainability Research, University of Graz, Austria
fMacromolecular Chemistry & New Polymeric Materials, Zernike Institute for Advanced Materials, University of Groningen, Groningen, The Netherlands
gUniversity of Bologna, Department of Civil, Chemical, Environmental, and Materials Engineering, Via Terracini 28, 40131 Bologna, Italy
hUniversity of Catania, Chemical Science Department, Viale A. Doria 6, 95125 Catania, Italy
iFaculty of Mechanical Engineering and Mechatronics, West Pomeranian University of Technology, Al. Piastów 19, Szczecin, PL 70-310, Poland
jFaculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, Belgrade, Serbia
kFaculty of Technical Sciences, University of Kragujevac, Svetog Save 65, Cacak, Serbia
lWageningen Food & Biobased Research, P.O. Box 17, 6700 AA Wageningen, The Netherlands
mInstitut de Chimie de Nice, Université Côte d'Azur, CNRS, UMR 7272, 06108 Nice, France
First published on 10th November 2021
Abstract
This review sheds light on urgent questions that arise from the need to replace a polymer resin,–poly(ethylene terephthalate), which represents 7.7% market-share in the global plastic demand (Plastics–the Facts 2019), by renewable alternatives. The main question that this review will address is: what are the most promising PET replacements made from biomass? Currently, under debate is naturally its biobased counterpart bio-PET (or even recycle rPET), as well as other aromatic key-players with comparable thermo-mechanical performance and enhanced barrier properties, such as poly(ethylene 2,5-furandicarboxylate) (PEF) and poly(trimethylene 2,5-furandicarboxylate) (PTF). They are most adequate for packaging, but not restricted to. Additional alternatives are the miscellaneous of lignin-based thermoplastic polymers, although the technology involved in this latter case is still premature. (Bio)degradable aliphatic polyesters, despite their typical inferior thermo-mechanical properties, can also play a role e.g., among PET fiber industry applications. Poly(lactic acid) (PLA) is the most developed renewable polyester, already a commercial reality. All biobased polymers reviewed face a major hindrance for their wider deployment their cost-competitiveness. A pertinent question arises then: Are these alternatives, or will they be, economically feasible? Social, political and legal frameworks together with supportive financial schemes are boosting rapid changes. In the future, most probably more than one polymer will come to the market and will be used in some of the panoply of PET applications. This evaluation overviews sustainability issues, including perspectives on their green synthesis. Moreover, this review does also not neglect the accumulation of plastics waste in the environment and the inherent challenges of polymers’ end-of-life. Approximately 8 M tons of polymers waste leaks into the environment each year, a fact not disconnected to PET's non-biodegradability and still insufficient collection and recycling rates.
 Andreia F. Sousa | Andreia F. Sousa's (PhD) research work is mainly focused on the design of sustainable polymers (renewable, biodegradable, and recyclable) at CICECO of the University of Aveiro. |
 Rafael Patrício | Rafael Patrício (MSc) investigates the development of sustainable polymers based on 2,5-furandicarboxylic acid and their biodegradation at CICECO of the University of Aveiro. |
 Zoi Terzopoulou | Zoi Terzopoulou (PhD) works on biomaterials for tissue engineering, biobased polymers, and nanocomposites. |
 Dimitrios N. Bikiaris | Dimitrios N. Bikiaris’ (Dr) research interests include the synthesis and characterization of polyesters, their molecular weight increase, recyclability, and their biodegradation. |
 Tobias Stern | Tobias Stern (Prof.) investigates bioeconomy-related innovations and transitions at the Institute of Systems Science, Innovation and Sustainability Research at the University of Graz. |
 Julia Wenger | Julia Wenger (M.Sc.) investigates environmental and economic aspects of forest-based biorefinery innovations at the University of Graz. |
 Katja Loos | Katja Loos investigates the synthesis and properties of biobased monomers and polymers at The University of Groningen, The Netherlands. |
 Nadia Lotti | Nadia Lotti's research activity regards the synthesis and characterization of polyesters at the University of Bologna. |
 Valentina Siracusa | Valentina Siracusa is professor of Chemistry for Engineering, working on gas barrier and environmental behavior of biobased and biodegradable polymers. |
 Anna Szymczyk | Anna Szymczyk investigates polymer systems based on renewable resources at the FMEaM of West Pomeranin Univeristy of Technology in Szczecin. |
 Alexandra Zamboulis | Alexandra Zamboulis’ (PhD) research interests revolve around the design and synthesis of novel bio-based polymeric materials at the Aristotle University of Thessaloniki. |
 Pavle Spasojevic | Dr Pavle Spasojevic investigates the feasibility of fully bio-based polyester production at the University of Belgrade. |
 Shanmugam Thiyagarajan | Shanmugam Thiyagarajan is a Senior Scientist working on the Sustainable and Circular Chemistry at Wageningen Food & Biobased Research. |
 Nathanael Guigo | Nathanaël Guigo is associate professor working on physical-chemistry of polymers (especially furanics) at University Côte d'Azur. |
1. Introduction
Poly(ethylene terephthalate), widely known as PET, is a popular thermoplastic polyester, produced from the fossil-based terephthalic acid and ethylene glycol. PET is globally consumed for countless applications, especially among packaging, mostly in plastic bottles, thermoformed trays, and, to a lesser extent, in flexible packaging.1 PET is also widely applied to textile fibers, historically commercialized under the tradename Dacron (or Terylene, its British equivalent) for the demand of an ever-growing fashion industry.2 The other main application field of PET is for films. For example, biaxially-oriented PET films (Melinex tradename) are used in flexible packaging and electronics, among others. Therefore, it is not surprising that the global trade market (primary form) of PET has reached over 10 million tons per annum since 2018 and has been growing steadily in the last decades.3Nevertheless, in the last few years, the growth in the PET market decelerated, both for the traded amount and in terms of the production capacity and consumption, thus denoting some saturation.3 This is due to an interplay of factors to which the regulation scenario and the social pressure related to the global awareness regarding the finite nature of fossil resources, as well as fluctuations in oil prices or the geopolitical instability of regions where these resources are typically located. This has fostered the renascence of renewable polymers.4,5 In particular, a quest within the academia and the industry for biobased renewable alternatives to replace PET has started.
Several potential PET alternatives, derived from renewable biomass sources, has emerged in the polymer scene as the most promising. A few even reached the commercial stage, including poly(lactic acid) (PLA), poly(butylene succinate) (PBS), and partially renewable PET. Another one, i.e., poly(ethylene-2,5-furandicarboxylate) (PEF), is expected to be available at a commercial scale by 2023.6 But the brisk activity in the field has given a rise to countless biobased polymers, for example, poly(trimethylene-2,5-furandicarboxylate) (PTF) (and many other furan-based polyesters),4 100% biobased PET (bio-PET), and lignin-based polymers. So far, there has not been a clear winner in the challenge to (partially) replace PET in a variety of applications.
To tackle this challenging task, the renewable origin of a potential PET replacement has to be assessed in conjunction with several other performance (properties) and sustainability criteria to identify the most suitable candidate (or candidates) that could phase out PET in packaging, fibers, and film materials.
The adequate candidate should suppress or at least match the irreprehensible PET thermal and mechanical properties, namely, a glass transition temperature (Tg) of about 80 °C (reaching 125 °C upon stretching), a melting temperature (Tm) of about 245 °C, a yield stress of ca. 55 MPa, and an elongation at break that reaches 200%. Besides, PET films also have low haze and are optically transparent, together with being inert to many chemical and biological environments (Table 1). These intrinsic properties of PET have permitted the development of suitable and optimal conditions for material formation such as blowing, injection moulding, thermoforming, and fiber spinning. The adaptability of PET to different forming processes has thus allowed its large industrial implementation. Another tremendous advantage of PET is its relatively low permeability to gas (water, CO2, and O2), beside the fact that it allows the fabrication of lightweight PET containers with enough barrier properties, avoiding the use of glass or aluminium and therewith significantly light-weighting the packaging solution. Should an ideal renewable replacement candidate for PET have all these properties? Or should specific properties be envisioned for specific applications?
| Property | Value/unit | Ref. |
|---|---|---|
| Density | 1.333–1.455 g cm−3 | 25 |
| Melting temperature | 245–280 °C | 26–31 |
| Glass transition temperature | 69–125 °C | 26–31 |
| Heat of fusion ΔH, DSC | 24.1 kJ mol−1 | 25, 26, 28 and 31 |
| Thermal expansion coefficient | 0.00016 K−1 (30–60 °C), 0.00037 K−1 (90–190 °C) | 25 and 26 |
| Thermal conductivity | 0.147 W m−1 K−1 | 25 |
| Deflection temperature | 63 °C at 264 psi, 71 °C at 66 psi | 26, 30 and 31 |
| Flammability, UL94 | HB-V-0 | 32 |
| Surface tension, 25 °C, solid/liq. | 39.5 mN m−1 | 25 |
| Intrinsic viscosity | 0.72–0.85 dl g−1 | 25 |
| Yield stress | 55 MPa | 33 |
| Young's modulus | 1700 MPa | 26, 30 and 31 |
| Elongation at break | 40–200% | 26, 30 and 31 |
| Elongation at yield | 4–6% | 26, 30 and 31 |
| Hardness, (Rockwell) | R105 | 26, 30 and 31 |
| Impact strength, Notched Izold | 90 J m−1 | 26, 30 and 31 |
| Flexural yield strength | 60–121 MPa | 26, 30 and 31 |
| Flexural modulus | 1.90–3.31 GPa | 26, 30 and 31 |
| Water absorption (after 1 week) | 0.8% | 25 |
| Dielectric constant, | 3.3 (60 Hz), 3.25 (1 kHz), 3 (1 MHz) | 25 |
| Dielectric strength | 295 kV mm−1 (60 Hz) | 25 |
| Dissipation factor, 106 Hz | 0.0025 (60 Hz), 0.005 (1 kHz); 0.0016 (1 MHz) | 25 |
| Refractive index | 1.57–1.64 | 25 |
| Haze | 0.300–40.0% | 34 |
| Gloss | 108–166% | 34 |
| Transmission, visible | 67.0–99.0% | 34 |
| O2 permeability coefficient | 0.0257–0.044 × 10–13 cm3 cm cm−2 s−1 Pa−1 | 25 |
| CO2 permeability coefficient | 0.118–0.227 × 10–13 cm3 cm cm−2 s−1 Pa−1 | 25 |
| Water vapor permeability coefficient | 13 × 10−13 cm3 cm cm−2 s−1 Pa−1 | 25 |
Certainly, the previous high-performance properties (or some of them) must go hand-in-hand with a set of sustainability requirements and not exclusively the renewability of the resources used to prepare the building-block monomers but also the catalysts and solvents. Furthermore, the sustainability of a biobased polymer depends on the biomass feedstock used, as shown by the food-vs.-fuel debate (‘1st generation’ food crops vs. ‘2nd and 3rd generation’ non-edible crops, by-products, residues, and wastes), as well as crop cultivation conditions (required arable land, pesticides, fertilizers).7,8
On the production level, the application of green chemistry and material-development principles should be followed in terms of, for example:9–11
• Usage of green synthesis routes that are clean and prevent waste or hazardous chemicals exposure,
• Production of useful by-products,
• And the necessity of high energy efficiency of the manufacturing processes and applications.
This is further described elsewhere.7,12
The rigorous recommendation for a biobased PET replacement should also consider its potential to reuse, recycle, and biodegrade. End-of-Life (EoL) solutions should be foreseen to ensure the environmentally sound management of wastes.13 The added value and benefits for all the actors in the supply chain as well as the absence of negative social and/or environmental impacts are other criteria that should be balanced.8
Due to the countless number of renewable PET counterparts developed so far (and future ones to come) and the economic value involved, as well as the implications on the sustainability of the adoption of one of these polymers (or several), there is an urgent need to define a strategic vision for PET replacements envisaging the global packaging, textile fibers, and films sectors. Despite some existing previous evaluations, summarizing biobased polymers’ synthesis and properties, several on biomass to platform chemicals,14 and even one specifically on the role of polyesters for packaging,4,5,15,16 to the best of our knowledge, this is the first evaluation to address this pertinent question from the perspective of these three specific applications and with such a holistic approach—which sustainable polymer(s) should be recommended to replace PET on packaging, fiber, and film materials?
2. Short overview on PET
PET arrived at the polymer science and technology scene in 1941, when Dickson and Whinfield, from the Calico Printers Association (United Kingdom), patented their work on the synthesis of a new polymer from terephthalic acid (TPA) and ethylene glycol (EG).17 The patent rights were granted afterward to ICI that introduced, in 1947, the Terylene fibers and later, the ‘Melinex’ film. Patent rights were also granted to DuPont that branded, in 1951, the PET fibers ‘Dacron’. A great boost to the PET high volume applications came later, in 1977, when oriented controlled structure-blown moulded bottles were introduced in the USA. Currently, the global production capacity was estimated at 30 million tons per annum (2017)18 and the global trade market over 10 million tons per annum (2018).3Today, PET is industrially produced from high-purity fossil-based TPA (or, to a less extent, based on its dimethyl ester), obtained through the oxidation of p-xylene through the Amoco process and EG. These building-block monomers react via bis(hydroxyethyl) terephthalate (BHET) intermediate synthesis, mainly (still) using the carcinogenic antimony(III) oxide (Sb2O3) as the catalyst. In this regard, however, several works have focused on Sb-free catalysts using non-toxic metals (e.g., titanium, aluminium, magnesium, germanium, and phosphorus).19–23 After the polycondensation stage, PET with a degree of polymerization (DP) of 100, suitable for textile applications, is produced. When high molecular weights (MW) are desired, as in the case for bottle grade PET resins, solid-state polymerization (SSP) has also to be carried out to increase the DP up to 150. Typically, PET resin manufacturing uses the same reaction path depicted schematically in Scheme 1.
PET popularity and wide use is not dependent of a variety of ways in which it can be processed, including injection moulding, extrusion, blow moulding, thermoforming and, more recently, additive manufacturing.24 But of course, the wide acceptance of PET is mostly related with a unique set of properties suitable for packaging and textiles, as well as for automotive, electronics, and medical industries. PET offers resistance to alcohols, oils, greases, solvents, and diluted acids; has low gas permeability; excellent gas (oxygen, carbon dioxide) and moisture barrier properties; and high strength (Table 1).
In addition, PET is (mechanically) recyclable and indeed, it is the most recycled plastic worldwide.35
2.1 The downside of PET
PET has several unsolved problems, the most relevant of which is being based on fossil resources. This fact has been demonstrated to be environmentally disadvantageous, both regarding greenhouse gas (GHG) emissions and energy inputs (fossil fuel consumption).36As already mentioned previously, PET production is typically based on the use of Sb2O3, which is classified as a carcinogen and some forms are even potentially endocrine disrupting.37 It has been found that a certain amount of antimony compounds may leach into the food from PET packaging.38
Also, PET EoL remains quite challenging (despite its recyclability), with incineration ranking first, besides landfill accumulation and the uncontrolled waste that leaks into the environment causing pollution debris.1 On another perspective, PET does not organically biodegrade under relevant environmental conditions, being instead inert. However, when exposed to the environment, the PET waste can cause micro(nano)plastics debris with an impact on the health and the environment.39,40 Interesting, the PET waste can be itself considered as a PET feedstock but this is still under-valorized and underexploited. In this regard, mechanical recycling technologies (with a higher level of maturity) as well as chemical recycling (less evolved) could prompt this waste valorization and contribute to the global demand of PET.
These issues remain unsolved at the local or global scale for PET and other polymers, in general. Therefore, the introduction of renewable alternatives has emerged driven by the aim of improving the environmental sustainability41 but still conserving the high-performance properties at competitive costs.
Some of the most important PET alternatives are disclosed below, namely, biosourced PEF, PPF, PBF, and lignin-derived polymers, as well as the role claimed by some aliphatic polyesters, namely, PLA and PBS. The unique role of 100% biobased PET (bio-PET) is also discussed subsequently.
3. Bio-PET
100% renewable PET, the so-called bio-PET, is perhaps the most obvious candidate to replace fossil PET itself since both the properties and the technology to produce, process, and manufacture bio-PET into packages, fibers, or films are already well-known and established. An additional argument in favor of bio-PET is regarding recycling and the inherent mono-waste stream and no need for adaptations or modification of processes and technologies.However, bio-PET development is far from being a viable alternative to fossil-based PET yet. Although the PET monomer, ethylene glycol, can already be industrially produced from, e.g., biobased ethylene or from the hydrogenolysis of platform molecules, such as sorbitol or glucose, accounting for 30 wt% of current biobased PET (i.e., the biobased content is currently limited to 30 wt%); there are still substantial challenges in the efficient synthesis of biobased terephthalic acid. No large-scale commercial process for renewable TPA has been launched to date, although several routes are being investigated and developed. Subsequently, on this evaluation, we will provide an overview on the TPA technologies.
TPA technologies can be summarized into two main categories, those based on either the use of p-xylene (pX) as the intermediate or via a non-pX route. Although different levels of technological maturity have been reached for those routes, bio-TPA (or bio-PET in this regard) remains challenging. Technology Readiness Levels (TRL) [based on European Union definitions]28 are proposed in Table 2 based on criteria such as feedstock availability, number of steps (including operating conditions and costs involved), and atom and mass economy (yield and selectivity).
| Technology developer | Biomass feedstock | Technology | Intermediate precursor | Number of steps to TPA | Yield (%) | TRL | Criteriaa | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From biomass feedstock | From intermediate compound | Intermediate precursor compound | TPA | TRL | FA | S | A&M E | |||||
| TRL = Technology readiness levels according to EU definitions,99 estimated by the authors based on open-source available information, FA = feedstock availability, S = number of steps and possible scale-up options, A&M E = atom and mass economy defines yield and selectivity of the products obtained in individual and/or overall yield of the entire process, pX = p-xylene, NR = not reported, p-TALD = p-tolualdehyde, Ref = reference.a The symbols used in the criteria section (estimated by the authors based on open-source available information) stand for: + = high (or) attractive, − = low (or) less attractive, ± = average (or) not convincing. | ||||||||||||
| p-Xylene route | ||||||||||||
| Virent Inc. | C5 and C6 carbohydrates | Catalytic reforming | pX | 4 | 1 | 23 (pX) | 9 | + | + | — | 42 and 43 | |
| Anellotech Inc | Lignocellulosic biomass (sugarcane, wood and corn stover) | Pyrolysis | pX5 | 4 | 1 | NR6 | 9 | + | + | — | 44–47 | |
| Ensyn | Lignocellulosic biomass (wood) | Pyrolysis | pX | 4 | 1 | <20 (pX) | 8–9 | + | + | — | 98 | |
| Gevo Inc. | C5 and C6 sugars (maize) | Fermentation | Isobutanol | 5 | 4 | 18.7 (pX) | 9 | + | ± | — | 48 | |
| Univ North Carolina/BASF | Biomass | Diels–Alder | Ethanol | 6 | 4–5 | 56 (pX) | 4–5 | + | ± | ± | 49 and 50 | |
| Univ Delaware | C6 sugars | Diels–Alder | 5-HMF | 7–8 | 4–5 | NR6 | 3–4 | + | — | ± | 51–53 | |
| Micromidas | C6 sugars | Diels–Alder | CMF | 7–8 | 4–5 | NR6 | 8–9 | ± | ± | — | 54 and 55 | |
| Micromidas | C6 sugars | Diels–Alder | pX | 7–8 | 1 | 99 pX | 8–9 | + | ± | ± | 55 | |
| Univ California Toste | C6 sugars | Diels–Alder | DMF, Acrolein | 6–8 | 4 | 34 pX | 3–4 | + | − | − | 56 | |
| Non-p-xylene route | ||||||||||||
| BP Corporation | C6 sugars | Diels–Alder | 5-HMF | 3–4 | 2–3 | — | 0.14 | 3–4 | + | + | — | 57 |
| Furanix | C6 sugars | Diels–Alder | 5-HMF | 3–4 | 2–3 | — | 11.6–16.5 | 3–5 | + | + | — | 58 |
| Univ York | C6 sugars | Diels–Alder | FDCA (ethyl ester) | 3–4 | 1 | — | 59 (PTA-Et ester) | 3–4 | + | + | ± | 61 |
| Michigan State Univ and Draths Corp (now Amyris) | C6 sugars | Fermentation/Diels–Alder | Muconic acid (cis/trans isomers) | 4 | 2–3 | — | 81 (PTA-Et ester) | 8–9 | + | + | ± | 59, 60 and 62 |
| Univ Bologna/IFP Energies Nouvelles | Non-edible berries, glucose | Diels–Alder | Sorbic acid/acrylic acid | 4 | 3 | — | 86 | 3–4 | ± | ± | + | 63 |
| Iowa State University | Sugars | Fermentation/Diels–Alder | Methyl coumalate/2-methoxyacrylate | 4+ | 1 | — | 95 (PTA-Me ester) | 3–4 | ± | + | + | 64 and 65 |
| Univ Florida | Biomass | Diels–Alder | Acrolein/Isoprene | 5+/6+ | 3 | 33 (p-TALD)7 | 3–4 | ± | ± | — | 66 | |
| Michigan State Univ | Biomass | Diels–Alder | Acrolein/Propiolic acid | 5+/ | 3 | 61 | 3–4 | + | ± | ± | 67 | |
| Michigan State Univ | Biomass/glucose | Diels–Alder | Acrylic acid/Isoprene | 5/6+ | 3 | 68 | 3–4 | + | ± | ± | 68 | |
| Gunma Univ | Biomass/C5 sugars | Diels–Alder/isomerization | Furfural/furan | 9–10 | 7–8 | 20 | 3–4 | + | — | — | 69 | |
| Dow Global Technologies | C6 sugars | Claisen condensation/Dieckmann cyclization | Succinic acid | 4–5 | 3 | 15 (pTA-Et ester) | 3–4 | + | ± | — | 72 | |
| Institute of Chemical and Engineering Sciences | Lignin | Multiple | Vanillic acid/syringic acid | 4+ | 2 | NR | 3–4 | + | ± | — | 73 | |
| SABIC Innovative Plastics | Limonene | Dehydrogenation/oxidation | p-Cymene | 3–4 | 2 | 80 | 3–5 | — | ± | — | 74, 75 and 77 | |
pX route to bio-TPA. In this category, pX is first obtained through (i) direct or (ii) indirect approaches. Once renewable pX is obtained, it can be fed directly into the existing processes for TPA production based on Amoco oxidation.
In the case of the direct synthesis of pX (i), several companies (Virent (USA), Anellotech (USA), and Ensyn (Canada)) developed catalytic pyrolysis technology for converting renewable feedstocks (e.g., cellulose, hemicellulose, sugarcane, wood, and corn stover) into hydrocarbons/bio-oil and subsequently into bio-BTX, similar to the refining process currently used in petroleum industries.42–47 From the industrial practice point of view, the use of various biomass feedstocks for the production of bio-BTX, from either catalytic reforming or pyrolysis technologies, is interesting. However, from a commercial point of view, none of these technologies appear to have appreciable selectivities toward pX product (limited to approximately 20%), thereby requiring full valorization of the many co-products.
The indirect synthesis of pX (ii) has been addressed by several companies making use of different biobased starting materials. For example, the USA company Gevo Inc. has developed a four-step process (fermentation, dehydration, dimerization, and dehydrocyclization) to convert carbohydrate-derived isobutanol into pX.48 Alternatively, a process developed by BASF is based on (bio)ethylene trimerization, disproportionation, and Diels–Alder (DA) cycloaddition reactions.49–53 Micromidas, another USA company, established a process to produce pX via 2,5-dimethylfuran (2,5-DMF) from 5-chloromethylfurfural (5-CMF).54,55,55 The process of converting 2,5-DMF to pX via DA cycloaddition with either ethylene or acrolein as the dienophiles, respectively, using a range of acid/hetero-homogeneous catalysts, were investigated by several academic and industrial researchers.56 Unlike direct synthesis, the indirect method to obtain pX results in comparatively higher yields. In particular, the DA reaction of 2,5-DMF with ethylene, in the presence of various catalyst systems, has been extensively investigated, claiming selectivity values up to >90% pX. In these reactions, high ethylene pressures are often required to achieve maximum substrate conversion. For this specific approach to become industrially viable, a significant increase in the efficient and cost-effective conversion of carbohydrates to 2,5-DMF is required.
Non-pX based routes to bio-TPA. Besides the drop-in strategies described above, several authors reported other approaches that do not involve pX as an intermediate. These include (iii) Diels–Alder cycloaddition reaction of biomass-derived substrates to directly yield TPA (or its esters);57–68 (iv) isomerization of phthalic acid;69–71 (v) cyclization of dimethyl succinate,72 and using (vi) lignin,73 or (vii) terpenes.74–78 A detailed overview of the feedstock, technologies used, and number of steps to either the starting material or TPA is given in Table 2. It should be noted that the technologies mentioned in the table are predominantly based on abundant carbohydrate feedstocks (such as glucose or fructose). Still, this implies that for the production of 60–80 million tons of bio-TPA, at least a two to three-fold (150–250 million tons) of carbohydrates is required, underlining the necessity for efficient PET recycling in order to reduce the demand for carbohydrate feedstock. This is a strategic vision, which may bring advantages compared to bio-PET (please refer to EoL strategies toward TPA section above).
The use of DA reaction (iii) to synthesize TPA is an interesting strategy, especially the technology developed by Draths (now Amyris) and the Michigan State University using muconic acid and ethylene, and acrylic acid and isoprene.59,60,62 These starting materials can be (efficiently) produced from carbohydrates, and the reported overall yields range between 68–81%, which is significantly higher and appealing compared to other reported approaches.
The other approaches (iv–vii) are more challenging. For example, Tachibana et al. reported a 5-step methodology to convert furfural into phthalic acid, and subsequently employed a Henkel-type isomerization reaction to obtain TPA.69 However, one of the reasons for the phase out of the Henkel-type technology was based on the use of the toxic cadmium catalyst typically used to achieve high conversions. Therefore, for this technology to be revived, significant innovations with respect to effective and benign catalyst systems would be required.
EoL strategies toward TPA. An interesting opportunity to obtain TPA (or its derivatives) is by means of chemical recycling via PET depolymerization approaches (e.g., hydrolysis, glycolysis, and aminolysis). In fact, instead of incinerating or simply landfill accumulation of the Mtons of PET waste produced each year,79 it could be used as a feedstock to obtain virgin quality PET.80 Although this is not a biobased source of TPA (and consequently also of PET), its implementation would contribute to part of the need of virgin-quality PET. Nevertheless, the depolymerization reactions are classically a high energy-demanding process, carried out at high temperature and/or pressure, and in the presence of strong acids or bases. In this regard, several efforts have been reported for rendering the process less-energy demanding by employing organocatalysis, enzymes, and ‘green’ solvents such as Ionic Liquids (ILs) and Deep Eutectic Solvents (DES) using milder conditions, and in some cases, short reaction times.81–86 Despite the promising results, most of the technologies developed so far, especially compared to mechanical recycling, which is well-established industrially, chemical depolymerization industrialization is still at the lab scale, or, at most, at the pilot stage. Ioniqa Technologies, a spin-off from the Technical University Eindhoven (The Nederland),87 and PerPETual Global Technologies88 based in the UK, are some of the industrial players.
3.1 Environmental sustainability
From an environmental sustainability perspective, studies making use of life-cycle assessments (LCA), focused on the production of bio-PET for bottles or its TPA precursor,36,89–91 support replacing fossil-based PET by bio-PET, despite several issues that still remain.For example, Benavides et al.36 found that both corn-stover-based PET and recycled PET (rPET) bottles can be environmentally advantageous compared to commercial fossil PET bottles, both regarding fossil fuel consumption and GHG emissions. Indeed, in terms of GHG emissions, bio-PET (specially the rPET) generally performed better than the standard fossil-based counterpart,92,93 although emissions were strongly affected by transportation and factory location (Semba et al.,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 2018). Benavides et al.36 recommended optimized processes due to the high-water amount requirements and energy inputs of these routes.36
92 2018). Benavides et al.36 recommended optimized processes due to the high-water amount requirements and energy inputs of these routes.36
Other studies90 evidenced mixed and assumption-sensitive results; for instance, lignocellulosic biomass might be preferable in terms of global warming potential and fossil-fuel use but performs worse in other categories such as ecotoxicity and ozone depletion.90 Investigating different routes for TPA production, Volanti et al.91 found that biobased routes derived from agro-waste residues (e.g., orange peel) could be environmentally competitive with the fossil-based alternative; however, this could not be guaranteed for the use of dedicated crops.91 Some authors, in LCA analyses, when comparing the cultivation of dedicated crops, such as corn, to other feedstocks showed adverse sustainability impacts in some of the investigated categories (e.g., on human health and ecosystem quality due to the use of pesticides, consumption of land, and consumption of water for production).94–96 These impacts could, in principle, be reduced using other feedstocks such as residues.97 Simply using renewable plant-based feedstock (especially, when dedicated crops are used) does not necessarily mean that products are more sustainable on the whole.
4. FDCA-based polyesters
4.1 FDCA production
Several routes for the production of FDCA from biomass have been reported but the catalytic oxidation of 5-hydroxymethyl furfural (5-HMF) prevails.100 5-HMF is in turn produced from simple C6 sugars such as fructose.101 Avantium has already demonstrated their FDCA production technology called YXY Technology® by a pilot plant with a capacity of 40 tonnes per year, and is in the process of constructing a reference plant (TRL8) with a capacity of 5000 tonnes per year.102 DuPont and ADM are the key producers of the dimethyl ester of FDCA (DMFDC), with their 60 tonnes per year pilot plant, starting from corn fructose.101 AVA Biochem produces 5-HMF using hydrothermal processing technology and is also offering high purity DMFDC.101Economic perspective. After life cycle assessment studies and techno-economic analyses on FDCA and HMF production, Davidson et al. concluded that the production of polymers from either one of these chemicals could lead to a reduction of GHG emissions ranging from 24 to 79%.100 In order to make HMF and FDCA production more favorable than conventional fossil-based chemicals, it is suggested that the yield and process efficiency should be improved, organic solvents should be avoided, and the sites, as well as the equipment for large scale production must be chosen carefully to minimize energy consumption.100 One great challenge still prevails in these technologies related to the use of edible sources of starting sugars. The use of alternative sources and the use of wastes are highly recommended.
4.2 PEF
Poly(ethylene-2,5-furandicarboxylate) is currently considered by several among industrial and academic communities the best available renewable candidate to replace PET,106,107 especially for films and bottles. The main arguments in favor of replacement relies on PEF's unique set of properties, besides its renewability and impeccable sustainable performance.Even if the potential of renewable furan derivatives as monomers for the production of polymers was mentioned many decades ago,108 the importance of 2,5-furandicarboxylic acid (PEF precursor) was widely recognized only after the US Department of Energy included it in the top 10 of value added chemicals list, in 2004, due to its potential in replacing TPA.109 Since then, research on PEF polyesters bloomed,15,110–113 starting with the pioneering work of A. Gandini who synthesized PEF from different starting monomers106,114,115 and the publication of the related patents on PEF, almost 70 years after it was first known.116–120 At the same time, Avantium B.V., a chemical technology company based in The Netherlands recognized the huge potential of PEF and started producing it in on a pilot plant scale in 2011,121 with plans to commercialize it by 2023, which was followed by several other players such as the Swedish Stora Enso.
In the case of FDCA, several routes have been reported for the production of FDCA from biomass, as reviewed previously, but the catalytic oxidation of 5-hydroxymethyl furfural (5-HMF) is the most exploited.100
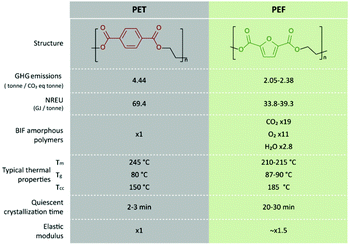
Because of the rigidity and polarity of the furan ring, PEF has reduced O2, CO2, and H2O permeability,122–124 which is low enough such that PEF bottles do not require an additional gas barrier layer as PET ones do and can even act as a barrier layer in PET. Amorphous PEF is ca. 11 times less permeable to O2,123 19 times to CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125 and up to 2.8 times to H2O126 than PET. Moreover, crystallization enhances its barrier properties even more.127 Indicatively, the barrier improvement factor (BIF) of oxygen permeability of amorphous PEF vs. semi-crystalline PET is 2–6 but that of semi-crystalline PEF is 8–10.127 The furan ring is rigid because, in contrast with terephthalate moieties, carbonyl motions and ring flipping are strongly hindered because of the non-linear axis of furan's rotation and its polarity.122,128 This suppressed chain mobility makes the diffusion of gases through PEF films more difficult.128 The polarity of furan also contributes to the permeability of PEF as the polar ring has increased affinity toward, for e.g., CO2.124
125 and up to 2.8 times to H2O126 than PET. Moreover, crystallization enhances its barrier properties even more.127 Indicatively, the barrier improvement factor (BIF) of oxygen permeability of amorphous PEF vs. semi-crystalline PET is 2–6 but that of semi-crystalline PEF is 8–10.127 The furan ring is rigid because, in contrast with terephthalate moieties, carbonyl motions and ring flipping are strongly hindered because of the non-linear axis of furan's rotation and its polarity.122,128 This suppressed chain mobility makes the diffusion of gases through PEF films more difficult.128 The polarity of furan also contributes to the permeability of PEF as the polar ring has increased affinity toward, for e.g., CO2.124
PEF exhibits similar mechanical properties to PET, with slightly higher elastic modulus (2 450 ± 220 MPa) and yield stress (98.2 ± 1.2 MPa) because of the rigidity of its structure, and smaller elongation at break with brittle fracture behavior.33,129–131
While the melting temperature of PEF is 210–215 °C, significantly lower than that of PET's, which could potentially result in energy saving during its melt processing, its glass transition temperature is higher (∼87 °C instead of 80 °C for PET).104 The rigidity of the furan ring and the reduced mobility of its chains is the underlying reason for the higher glass transition temperature. Its thermal stability is slightly lower but it is still stable up to 350 °C.104,132 The quiescent crystallization rate of PEF is slower than that of PET both from the glass and from the melt.103,104,133,134 Both these differences are important to take into account the industrial processes where these types of quiescent crystallization are applied; crystallization from the glass is performed on pellets and SSP, where fast rates are required, and crystallization from the melt needs to be carefully controlled during injection stretch blow moulding for the manufacturing of films and bottles.135 In fact, the hazing of PET preforms is a common problem that arises from its melt crystallization and/or during the heating of the preform prior to blowing into a bottle. However, this issue can be circumvented since, as reported by Sbirrazzuoli et al.,133 the rate of quiescent melt crystallization of PEF can be improved by the nucleating effect of, for e.g., calcium, which can be subjected to stretching, both uniaxial136–141 and biaxial,131 a process necessary for the production of mechanical robust films, fibers, and bottles, as explained below.
Regarding containers, it has the ability to replace the usual additional barrier layers used in polymers such as PET,146 as described in a patent currently assigned to Coca Cola Co., therefore enabling their recycling and reducing the costs of production. In addition, PEF bottles can be manufactured with improved geometrical structure and reduced weight of the preforms in comparison with PET,147 allowing the manufacturing of smaller bottles (<300 mL). They have excellent shrinkage behavior.148
PEF can be used to produce air-tight, heat-sealable laminate films.149 Films are being used in different packaging applications, including food, pharmaceuticals, and electronics. Because of their improved barrier properties, PEF packaging containers extend the shelf life of the products they contain.
PEF/PET blend bottles are also possible by injection moulding to manufacture the preform, followed by stretch blow moulding to make the bottle itself, in conditions similar to those of PET.150
Transparent layered films containing a layer of biaxially-oriented PEF and a thin layer (10–200 nm) of inorganic compounds (aluminium and silicon oxide) with excellent mechanical and gas barrier properties have been fabricated. The inorganic layer is commonly used to reduce the permeability of the polymer films.151 Multilayer recyclable PET container with semicrystalline PEF as an inner barrier layer127 has been prepared by injection moulding, either in one step or with over moulding. Using semicrystalline PEF allows the reduction of the barrier layer's thickness of thermoforming containers and, therefore, the total weight of an all-PET bottle was reduced up to 5% for every weight percent of PEF added, together with the improvement of the shelf-life of the container.127
With respect to the processing of PEF into final articles, there are some technical challenges despite the same processing technologies and equipment applied as that for PET. The most notable change in the processing conditions is the difference in the processing temperature, arising from PEF's lower Tm and higher Tg compared to that of PET. Another issue is caused by its higher natural draw ratio, caused by its lower entanglement density. Very recently, the time/temperature superposition principle has been demonstrated for PEF for different set of stretching conditions, which allows for transposition to industrial processes.141 In the same way as that for PET, lower stretch ratio can be obtained for PEF by increasing the equivalent strain rate.141 For the injection stretch blow moulding (ISBM) in bottle application, this would imply an adaptation of the processing conditions but it remains realistic in the current PET assets. The successful implementation of PEF in different applications will, therefore, require development and training efforts, which does not apply to the drop-in bio-PET solution.
The discoloration of PEF is likely the most significant practical issue that needs to be overcome to extend these polymer applications, especially for packaging applications. This market needs transparent, haze-free products to satisfy consumer needs. From early on, it was recognized that the typical polymerization of FDCA (based on a two-step esterification (or transesterification), followed by the polytransesterification reaction (at high temperature and applying vacuum), yielded a dark brown polymer from a yellow one.130,132,153,155,156 The factors that affect the final color of PEF were early identified in an in-depth study by Gruter et al.157 and further studied by Gubbels et al.,158viz., monomer purity and thermal stability, type of catalyst, and reaction conditions.
Since FDCA is sourced from biomass, even the smallest amounts of impurities can affect PEF color. Even when FDCA with 99.5% purity was used, the final product was still discolored.156 Thus, much of the future success of PEF (or not!) may rely on the FDCA production technology.
The relatively low thermal stability of FDCA that can be subjected to decarboxylation reactions during PEF synthesis, thus affecting its color, has been circumvented using FDCA dimethyl ester157 as it has improved thermal stability and can be purified more easily than FDCA. However, the dimethyl ester route does have its challenges in scaling up also because most of the current polymersation assets in place for PET use the diacid route.
In addition, to avoid extensive discoloration, polycondensation temperatures can be adjusted below 240 °C and the reaction times can be limited.153,157 Regarding catalysts, Ti-based ones commonly yield colored products, usually more colored than Sb derivatives (Fig. 1(a)).152,153 Zn and Al catalysts also yielded less discolored PEF films than Ti-based ones Fig. 1(b).153
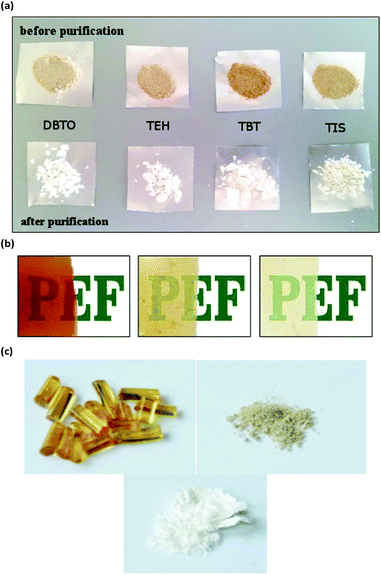 | ||
| Fig. 1 The appearance of PEF (a) prepared with different catalysts after 2 h melt polycondensation at 230 °C,152 reproduced by permission of The Royal Society of Chemistry, (b) prepared with different catalysts TBT, ZnAcO, and Al(acac)3, reprinted (adapted) with permission from ref. 153 (Copyright 2019 American Chemical Society), (c) derived from polycondensation (left) and ROP (middle and right) (CC BY 4.0) (adapted with permission from ref. 154). | ||
Overall, PEF with high molecular weight and no discoloration can be obtained with (i) very high purity DMFDC as the starting monomer; (ii) maximum polycondensation temperature of Tm + 30 °C; and (iii) preferred catalysts organotin(IV) salts for the transesterification stage and tin(II) salts for the polycondensation stage. The tin(IV) salt is reduced to a tin(II) salt with the use of a reducing compound in the melt of the prepolymer, which can successfully prevent discoloration.116
In this context, rapid ring-opening polymerization (ROP) technique (Scheme 2) was used to prepare bottle-grade PEF (>95% conversion,  , uncolored), avoiding the usual degradation and discoloration (Fig. 1(c)).154,159 As for PET, the ROP-derived process applied to PEF showed similar superiority to polycondensation-derived PEF: a higher glass transition temperature; a lower melting point, a higher yield stress, and a higher Young's modulus. Compared to conventional polycondensation, in which vast energy is required due to the high vacuum power over the long reaction times, the authors established a greener and economically competitive ROP route. In addition, the successful synthesis of the corresponding cyclic oligomers as the PEF precursor, using a non-metal catalyst, was reported.160,161
, uncolored), avoiding the usual degradation and discoloration (Fig. 1(c)).154,159 As for PET, the ROP-derived process applied to PEF showed similar superiority to polycondensation-derived PEF: a higher glass transition temperature; a lower melting point, a higher yield stress, and a higher Young's modulus. Compared to conventional polycondensation, in which vast energy is required due to the high vacuum power over the long reaction times, the authors established a greener and economically competitive ROP route. In addition, the successful synthesis of the corresponding cyclic oligomers as the PEF precursor, using a non-metal catalyst, was reported.160,161
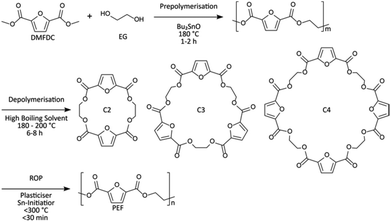 | ||
| Scheme 2 Polyethylene furanoate (PEF) synthesis via rapid ring-opening polymerization (ROP).154 | ||
Qu and coworkers recently undertook different approaches based on the use of imidazolium ionic Liquids (ILs).162 They found that the solubility between the ILs and monomers was the main factor that affects the catalytic activity. PEF, with the highest molecular weight, was obtained using [C2MIM]BF4. In addition, Wu et al. investigated PEF synthesis using an organic non-metal catalyst (1,8-diazabicyclo[5.4.0]undec-7-ene, DBU) via polycondensation and achieved a moderate molecular weight (intrinsic viscosity 0.54 dL g−1).163 It has to be noted that ILs bear potential risks to the environment164 (and in particular imidazolium), which renders this reaction potentially non-green. This process should be re-visited once new non-ecotoxic green solvents are reported.
The processes described before show a fundamental background to design better pathways for sustainable biobased PEF production in the future. However, these processes are still far from being implemented in the industry.
Concerning the sorting process, PEF can be separated from PET by its different infrared (IR) absorption profile, which will allow the mechanical recycling of PEF on its own. Replacing PET with PEF will also help to partially overcome the most serious problem in current PET recycling (rPET), i.e., contaminations originating, among others, from additional barrier layers, usually polyamide. On another perspective, if PET is not entirely replaced by PEF but is used instead of polyamide or EVOH as a barrier material for PET, the deterioration of rPET stream quality will be circumvented as PEF has a much better compatibility with PET and can actually be transesterified with it,167 resulting in haze-free high performance rPET products.
When PEF was subjected to 3 consecutive remelting cycles, its properties deteriorated as it decomposed, including the reduction of its intrinsic viscosity and thermal stability, and discoloration. This deterioration depended on the catalyst used for its synthesis, with titanium catalysts decomposing PEF faster than tin catalysts.168 However, its molecular weight can be increased efficiently with the use of commercially available chain extenders.169
PEF can undergo methanolysis to recover back its monomers at a much faster rate than PET.170 The chemical glycolysis of PEF was examined as an EoL option with the use of an organic catalyst.171 The depolymerization gave bis(hydroxyethyl)-2,5-furandicarboxylate monomer and its dimer as the products, which were successfully re-polymerized to PEF but needed extensive SSP times to reach their initial molecular weight and thermal properties. PEF can also be hydrolyzed by Cutinase 1 from Thermobifida cellulosilytica and Humicola insolens cutinase and yields the monomer FDCA, suggesting that it can be recycled enzymatically.172,173 In fact, PEF was hydrolyzed 1.7 faster than PET.25
Avantium and Organic Waste Systems recently reported that non-weathered and weathered PEF biodegrade 90% after 385 and 240 days, respectively, under industrial composting conditions. In comparison, PET degraded only up to 10%. In addition, they are performing field degradation experiments on PEF with the University of Amsterdam, and preliminary results showed the attachment of various microorganisms onto the films.174 However, it was argued that the slow biodegradation of PEF cannot be considered as an EoL option but is a valuable property that might minimize the accumulation of PEF microplastics in the environment.174 Finally, PEF can be 3D-printed with fused deposition modelling in successive cycles, making reuse a viable alternative option for the reduction of PEF waste.155
This potential environmental impact of even partially replacing PET by PEF is one of the critical factors that brought PEF to the forefront of the search for better sustainability performance polymers.
Nevertheless, for replacing PET by PEF, at a large scale, cost competitiveness is an issue. A techno-economic study carried out by Eerhart et al.177 shed some light on the way forward to PEF becoming a real bio-alternative. This study points out that the production of PEF is only feasible at a minimum production scale of 80 kt per year, with a wheat straw cost maximum of 150 USD per tonne, and at certain by-products’ prices. Capital costs and discount rate are further sensitive factors identified. To increase the competitiveness, the way forward was identified to focus on higher-value uses for the lignin by-product, further process optimization, and technological learning over time.177
4.3 PTF and other FDCA-based polyesters
PET replacement by furan counterparts is not limited to PEF, despite its prominent position. Indeed, the ‘whole family’ of furan-based polyesters (hereinafter, simply, referred to as FBP), for example poly(trimethylene-2,5-furandicarbocylate) (PTF), poly(butylene-2,5-furandicarbocylate) (PBF), poly(pentamethylene-2,5-furandicarboxylate) (PPeF), or poly(hexamethylene-2,5-furandicarboxylate) (PHF), have a wide range of tuneable thermal and mechanical properties (dependent on the number of methylene groups of the glycol moiety)178 (Fig. 2), which could enable their use in typical PET applications, such as in rigid or flexible packaging or in fibers.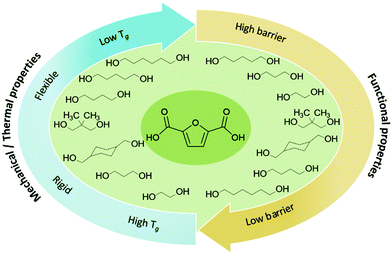 | ||
| Fig. 2 Overview on polyesters from FDCA and different glycol moieties, and their potential to tune the functional properties of the resulting materials. | ||
In particular, PTF has become an object of industrial interest by the hand of the giant DuPont and ADM. In this regard, they will use DuPont's proprietary bio-PDO (biobased 1,3-propanediol) and exploit the production of DMFDC from fructose, as developed by both companies. The first plant to produce DMFDC monomer in the USA, with a capacity of 60 tons per year, has already been installed.179
PTF exhibits enhanced mechanical properties compared to PET and PTT, with an elastic modulus of 1.6–2.7 GPa, tensile stress at break of 79 MPa, and 3% elongation at break with brittle fracture behavior.105,181
The melting temperature of PTF is at about 172–176 °C, which is lower than that of PET or even PEF, which could result in energy saving during its melt processing.105 Its Tg is ca. 20 °C higher than that of PET and 31 °C lower than that of PEF.105 PTF is able to crystallize but the rate is significantly lower than that for PTT and PET, as already observed for PEF. The DSC trace of PTF displays a nearly imperceptible melting event when the sample is heated at 10 °C min−1, indicating reduced ability to crystallize at this heating rate. Also, during cooling from the molten state, at the rate of 10 °C min−1, no crystallization was detected. Therefore, unlike PET, the addition of a comonomer to reduce the heat-induced crystallization rate is not needed.
PTF is characterized by polymorphism when crystallized from the bulk and from the melt, exhibiting three different crystalline forms (α′, α, and β).182
PTF is thermally stable in air and inert atmosphere up to 350 °C.178 Hence, it is expected that it will not be needed to introduce antioxidants into PTF to stabilize it against thermal oxidation during processing.
Most of the studies on PTF have almost exclusively focused on the preparation of the PTF films, while its spinnability and fibrous properties have been largely neglected, with a noticeable exception of the recent work by Chen et al.178 This group found that the PTF fibers exhibited lower modulus, lower tensile strength, and lower crystallinity but high elongation at break compared to that of the PTT fibers. It was also established that the PTF fibers exhibited better hydrolytic degradation behavior under various conditions compared to that of the PTT fibers due to the furan ring and the lower crystallinity of the PTF fibers.178 This study confirmed that PTF fibers possess great potential to be widely used in eco-friendly textile industries and can replace PET or PTT fibers in some applications.
PBF is a semicrystalline polymer with a Tg of 31–40 °C and a Tm of about 170 °C,4 which can crystallize during non-isothermal cooling from the melt in the wide temperature window from 150 to 60 °C.183 The mechanical and thermal properties and crystal structures of PBF are similar to its direct fossil homologue PBT.184 PBF films are 7 and 4 times less permeable to CO2 and O2 with respect to PET,178 respectively. Its gas barrier performances are similar to those of PEF, which endow it with a huge potential for food packaging applications, with the advantage over PEF of being less brittle.
PPeF is also quite a promising FBP despite being an amorphous polymer; in the rubbery state, it can be easily processed as a freestanding flexible film, characterized by exceptional thermal stability (maximum degradation temperature at 414 °C, while for PET, it is about 440 °C), mechanical response to tensile test typical of elastomeric materials with an instant recovery of the initial shape after breaking,185 and unexpected exceptional barrier properties to both O2 and CO2, comparable to those of the ethylene-vinyl alcohol copolymer with 32% ethylene.186
Poly(hexamethylene-2,5-furandicarboxylate) (PHF) could be employed for rigid packaging to retain an atmosphere rich in O2 and poor in CO2, which is ideal for decreasing the metabolism of packed products or spoilage activity, thus maintaining or prolonging the desired shelf life.178 Despite the potential of these two polymers among many other FBPs (the interested reader can refer to specialized reviews on the topic4,15), they are, to the best of our knowledge, still a long way off form reaching the market and replacing polymers such as PET. PTF is an exception, as mentioned above, having attracted the attention of two giants, namely, Dupont and ADM.
One typical limitation of PTF polyester is its relatively long crystallization time in compression-moulded samples. Nevertheless, this can be overcome using the industrial techniques for manufacturing mono- or bi-oriented films that can induce much faster crystallization under appropriate conditions. Such a drawback can also be solved by introducing one or more plasticizers, nucleating agents, and copolymerizing PTF with more flexible co-monomers.187
Another issue that needs to be overcome for PTF is its synthesis optimization in order to control the MW and its distribution. Hence, to obtain consistency in its properties, the cyclic dimer and end-group type should be identified and quantified. However, low level of cyclic diesters and the high barrier performances of PTF can reduce the problems encountered by polyesters such as sublimation, deposits, and migration during polymerization and post processing.
5. Lignin-based thermoplastic polymers
The interest in (hetero-)aromatic polyesters relies on their typical enhanced mechanical and thermal properties, which are hardly achieved through aliphatic counterparts. Therefore, it is not surprising that PEF or PPF are in the spotlight today; despite this, as reviewed before, several technical challenges still remain, as well as the dependence on primary feedstocks costs and land. In this regard, ubiquitous lignin (highly abundant as, for example, a by-product of the pulp and paper industry) can also play a role as the source of different aromatic building-block monomers,219,220 especially the commercially produced vanillin.221 In this context, both vanillin and vanillic acid are the two most used compounds on thermoplastics and are the focus of the present evaluation.222,223 Ferulic acid derivatives have only been mentioned briefly. Scheme 4 illustrates the multitude of structurally different thermoplastic polymers that have been prepared starting from vanillin (1–8), vanillic acid (9–18), or ferulic acid (19–23).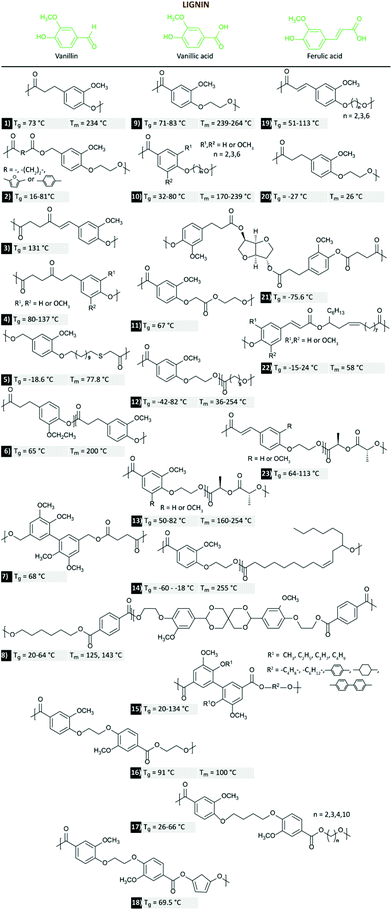 | ||
| Scheme 4 Representative multitude of vanillin-(1,197 2,198 3 & 4,199 5,200 6,201 7,202 8203), vanillic acid-(9,204–206 10,204,207 11,208 12,205,209 13,209 14,210 15,211,212 16,213 17,208 18214), and ferulic acid-(19,215 20,216 21,217 22,218 23209) based thermoplastic polyesters. | ||
Despite early initial reports, ‘vanillics’ were only thrown in the limelight in 2010 by Miller's group, who elegantly circumvented the low reactivity of the phenolic hydroxyl group by ‘activation’ through primary hydroxyl group addition by derivatization.197 Starting from vanillin, poly(dihydroferulic acid) (PDHF) was synthesized through a polycondensation reaction in the bulk ((Fig. 3) and Scheme 5 (1)), exhibiting excellent thermal characteristics such as a slightly lower melting temperature than PET (234 °C) but a comparable Tg (73 °C).197 The molecular weight attained was, however, relatively modest (<15.5 kDa) and no mechanical properties were reported. Since this pioneer study, ‘vannillics’ have attracted an ever-growing interest.197
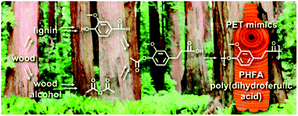 | ||
| Fig. 3 Synthetic root of poly(dihydroferulic acid) (PDHF) from biorenewable feedstocks vanillin and acetic anhydride (reproduced from ref. 197 with permission from the Royal Society of Chemistry). | ||
 | ||
| Scheme 5 Synthesis of poly(ethylene vanillate).204,206 | ||
Other lignin-polyesters comprise fully renewable poly(ester ketone)s (Scheme 4 (e.g., 3–4)), with satisfactory molecular weights and Tgs (80–137 °C);199 polymers with medium/long aliphatic segments (Scheme 4 (e.g., 5, 8, 10, 12–15, 17, and 19, 22–23) with Tg, Tm (for semi-crystalline polymers), and thermal stability generally decreasing with the relative amount of aliphatic moieties;204,208,209,212,224 or poly(ethylene vanillate) (Scheme 4 (9) and Scheme 5), structurally similar to both PDHF and PET and with comparable Tg (71–83 °C) and Tm (239–264 °C).204–206 This latter polyester was thermally stable up to 327 °C, while nanoindentation studies evidenced a hardness close to that of PET and an elastic modulus comparable to that of PP.206
Vanillin has also been dimerized (enzymatically) or bridged through ether or ester linkages to afford divanillic monomers and polymers thereof (Scheme 4 (e.g., 7 and 15–18)).202,203,211–213 For example, the polymer obtained with dimethyl succinate (Scheme 4 (7)) exhibited a Tg similar to PET (68 °C) and a storage modulus of 5.1 GPa.202
Mankar et al. designed a spiro-diol vanillin-based monomer, whose synthesis remarkably produced lower GHG emissions than biobased 1,3-propanediol and afforded a transparent material after polymerization (Scheme 4 (8)).203 Finally, it is noteworthy that polymer 16 from Scheme 4 exhibited better mechanical properties than PET.213 Apart from a few exceptions, all the aforementioned polymers are stable up to at least 300 °C.
Besides vanillin, other lignin-derived monomers have been studied for the preparation of thermoplastics, though to a lesser extent.215,216,218,220,225–230
5.1 Scientific and technical drawbacks
To conclude this section, although lignin-derived monomers afforded interesting polymers with a wide range of Tgs, comparable to that of PET or even higher, the mechanical properties are often neglected or too poor to be reported.In addition, for the time being, to the best of our knowledge, EoL solutions have been barely addressed. There are only a few studies dealing with the degradability/recyclability of these novel materials,203,213,225,230 and it is definitely an aspect that needs to be looked into for further deployment.
Furthermore, the low reactivity of the OH-phenolic groups still places lignin-based polyesters as challenging polymers, which are in some cases under-exploited. Thus, emphasis must be given on simple, straightforward synthetic methods and the promotion of green polymerization procedures (favoring bulk polymerization instead of solution polymerization, for example).
Economic perspective. From this perspective, lignin has also several constraints, especially in terms of vision and market exploitation. A recent review, from some of us, points out precisely that lignin literature repeatedly refers to a rather small set of general statements on lignin applications and market aspects, namely, ‘lignin is abundant’, ‘potentially cheap’, ‘sustainable’, and ‘underutilized’.231 Moreover, a detailed analysis of the related techno-economic and socio-economic studies indicated that lignin research is mainly focused on internal (direct) factors that influence the success of commercialization (e.g., improving the technology, performance, reducing costs, and optimization processes), while paying little attention to external (indirect) factors, where substitution markets, demands, and regulatory issues are important to address.
6. Aliphatic polyesters
One of the most important argument in favor of PET replacement by aliphatic polyesters are their inherent (bio)degradability. Generally speaking, aliphatic polyesters, due to their labile ester bonds, are prone to chain scission (either through simple hydrolysis or by the catalytic action of enzymes) and can (bio)degrade. However, only a few examples of renewable aliphatic polyesters meet satisfactory thermo-mechanical properties for applications among packaging, films, or as fibers.232 The most investigated and commercialized ones are poly(lactic acid) and poly(butylene succinate) (PBS) (Fig. 4). Poly(glycolic acid) (PGA), despite its interesting properties and commercial availability, is not considered in the present evaluation due to its fossil origin. Also, poly(hydroxyalkanoate)s (PHAs) due to being synthesized by microorganisms, were also not considered. The interested reader should instead refer elsewhere for in-depth reviews on the subject.233,234Biodegradable polyesters (or polymers in general) need to be carefully evaluated regarding the risks and benefits because biodegradability can also bring specific challenges, such as ecotoxicity of the degradation products and influence on the surrounding biota, GHG emissions, energy lost, and the perfect match between the biodegradation timescale and the shelf life, besides the fact that biodegradation under relevant natural scenarios does not always occur,235,236 even when it does in (industrially) composting conditions. Nevertheless, biodegradability is highly recommended in some particular applications among disposable plastics as an EoL solution, or in tissue-engineering and in biomedicine in general, whereas controlled degradation and/or reabsorption is needed (out of scope of the current evaluation).
6.1 PLA
PLA is undoubtedly the most important biobased aliphatic polyester, which is industrially produced by the ring-opening (ROP) polymerization of the lactide (cyclic dimer of lactic acid) at an estimated capacity of 400 kton per year (2020). The ROP reaction is typically carried out under a solvent-free process, which contributes to the green perspective of PLA.However, hazardous tin(II)-2-ethylhexanoate catalyst is usually used, which cause severe concerns, especially among the biomedical field. In this regard, there are several research efforts toward the development of greener alternatives, such as those based on organocatalysis or using other metal-based catalysts.237
The building-block monomer of PLA, lactic acid, can be advantageously prepared from bio-sources (such as wheat, corn, whey, starch, or their residues) by fermentation process, or instead by chemical processes using petro-based chemicals (acetaldehyde).238,239
Lactic acid contains chiral carbon and can exist as the D- or L-isomer; the latter is naturally occurring. Commercially available PLA is mainly based on the L-enantiomer as the dominant isomer. The overall properties of PLA depend strongly on its optical purity, i.e., on the content of L- and D-enantiomers in the polyester chain. For example, optically-pure PLLA (composed of L-enantiomer) has a Tm of about 175–180 °C, whereas it shows a fall of 5 °C for the incorporation of 1% D-enantiomeric units,240 and a decrease in the crystallinity was reported. In the same context, the mechanical and barrier properties of PLA depend on the degree of crystallinity as well as the thermal history.241
The Tg for PLA with different L- and D-contents ranges between 55–65 °C. These properties are still far from those of PET (Table 4), though they are appropriate for many applications, namely, in medical parts or textile fibers.
Despite the abovementioned limitations, PLA is seen as a polymer that can be broadly used for general packaging (compostable tableware and plastics bags) and agriculture (mulch films). Also, PET applications in the fiber industry can be advantageously replaced by PLA, namely, in disposable garments and home textiles, since it has acceptable properties and can be easily processed using conventional equipment.249–251 Indeed, PLA fibers are already a commercial reality.252,253 For example, NatureWorks commercializes PLA under the tradename of Ingeo™ specially designed as fibers.252
However, the cultivation of dedicated crops such as corn is still an issue since they show adverse sustainability impacts in terms of human health and ecosystem quality (due to the use of pesticides), as well as on land use and water consumption for production.94–96 This could be mitigated using other feedstocks, such as residues.97 Regarding the production processes, further optimization has been recommended (e.g., regarding different energy issues, such as using renewables);97,254 transportation can also lead to significant impacts.95,255
The EoL options also play major roles for the sustainability performance of the PLA products,94,259 whereby in most studies, recycling scenarios were found to be the most beneficial option, followed by composting (in particular, when compared to landfilling and/or incineration scenarios).257,260–262 Nevertheless, general statements about the overall sustainability of PLA products are difficult to make essentially due to the uncertain EoL scenarios and respective conditions.259 Developing a circular economy for plastics, EoL management strategies as well as more research on these are very important issues.8,259
6.2 PBS
PBS can be 100% biobased (as well as only partially), and readily synthesized through the classical two-step approach using succinic acid and 1,4-butanediol.263 Chain extension through coupling agents, e.g., using hazard diisocyanates, is also applied in order to further increase the MW and to obtain a material suitable for processing by, for example, film blowing.264,265 Also, there are studies on the greener synthesis of PBS (and its copolyesters) by enzyme catalyzed reactions. Issues to be overcome, mainly related to the need to improve MWs and the use of solvents, have been put forward for the possible industrialization of such processes.266,267In terms of properties, PBS is a semi-crystalline polyester with a Tm of ca. 114 °C and a Tg of approximately −32 °C. Regarding the mechanical properties, PBS resembles polyolefins (but drifting away from PET) with the elongation at break reaching up to 560%, elastic modulus of 0.3–0.5 GPa, and the tensile strength equal to ca. 30–35 MPa.265
To modulate the biodegradability as well as the crystallization rate, copolymerization and blending are frequently employed.266,268 In 1994, PBS and its copolyesters with (fossil-based) adipic acid (PBSA) were commercialized by Showa Denko (Japan) under the trade name of Bionolle.269
6.3 Economic perspective
A techno-economic (cost) perspective on the eco-efficiency of PLA and PBS (as well as on PHAs and PP) in Thailand by Changwichan et al.257 concluded that PLA was cheaper than PBS but the cheapest was PP.257The economic performance of a PLA biorefinery situated in Poland per kg of product and per ha of land showed the occurrence of the net costs. However, a system with PLA from short rotation wood and with the production of a high-value fiber product (or high quantity of recycled PLA) could lead to net benefits. The market price of PLA products played an important role in the profitability, while the land costs did not play such a major role. However, if biorefineries are implemented on a larger scale, the own-price elasticity of the demand for land could be of significant importance.273
In addition, to replace currently used commodity PET, in some packaging applications of PLs (and in particular of PBSs), some of their shortcomings, such as poor barrier and thermo-mechanical properties, brittleness, and/or cost-effective production, have to be overcome. However, PLA is already a commercial reality252,253 and is used in traditional PET applications, such as in fibers but not limited to them. PBS is currently being developed for flexible packaging. We do foresee that PLA, specially PBS, will not straightforwardly replace PET in all broad range of applications that PET has but in some, they have already been implemented, as discussed previously.
7. General considerations on biobased PET alternatives
The biobased polymers considered so far can be understood as substitutes274 for PET when they have comparable performance characteristics, aim at the same applications, and are aimed to be sold in global markets (Table 5).274 Therefore, on the one hand, we have performance and applications, and, on the other hand, we have market/bioeconomic aspects.| Biobased polymer | Main application(s) | Challenges | Main EoL options | Maturity stage | Main industrial player |
|---|---|---|---|---|---|
| Bio-PET | As PET | Increase in the efficiency of TPA root | As PET | TPA synthetic roots can reach demonstration scale | BASF |
| MICROMIDAS | |||||
| DOW | |||||
| SABIC | |||||
| PEF | Thermoformed packaging containers, bottles, films or fibers | - Discoloration issues | Recycling | Pilot-scale achieved | Avantium B.V. |
| - Long crystallization rates | Stora Enso | ||||
| - Elongation can be only 4% | |||||
| Circumvented by adequate control of polymerization conditions or processing | |||||
| - PEF production must be 80 kt/year, wheat straw cost maximum of 150 $/tonne, certain by-products’ prices to be feasable | |||||
| PTF | Similar to PEF | - Discoloration issues | - | DMFDC precursor Pilot-scale achieved | DuPont & ADM |
| - Long crystallization rates | |||||
| Miscellaneous lignin-based thermoplastics | high Tg polymers | - Low reactivity of the OH-phenolic groups | Mostly unknown | - Polymers at bench or Lab Scale | — |
| - Commercial vanillin and vanillic acid monomers | |||||
| PLA | - Fibers | Poor barrier properties | - Recycling | Commercial scale | NatureWorks |
| - General packaging (compostable tableware and plastics bags), agriculture (mulch films) | - Composting | Toray | |||
| PBS | - Flexible packaging | - Inferior tensile strength and elastic modulus than PET | Commercial scale | Mitsubishi Chemical Corporation | |
| - Poor barrier properties | |||||
| Common adverse impacts | |||||
| Sustainability impacts | |||||
| - Cultivation of dedicated crops such as corn | |||||
| - Non-renewable energy issues | |||||
| - Transportation | |||||
| - EoL options | |||||
| Bioeconomic obstacles | |||||
| - Highly-standardized PET | |||||
| - Cheap PET | |||||
| - Bio-based processes are not cost competitive | |||||
Performance. Firstly, let us take a look at PET alternatives from the properties and applications perspectives. The current PET applications have many requirements in terms of mechanical performances, shelf-life, barrier properties, thermal stability, end-of-life, price, annual volume, and sorting, which shows that one global solution meeting all these requirements is not realistic. Therefore, each PET application might find advantageous biobased replacers that have specific value propositions for that particular application against the incumbent PET solution, possibly along with reducing the environmental impact (renewability, EoL options). Taking the example of the bottle application, relatively long shelf life (>six months), good barrier properties, and enhanced mechanical performance along with recyclability are mandatory. Therefore, choosing composting polyesters bearing relatively poor barrier properties, such as PLA, maybe not the right solution. On the other hand, a biobased polyester such as PEF (or PTF), presenting higher barrier properties than PET, comparable mechanical properties, and recyclability in the PET stream, is undoubtedly a better solution, giving some new advantages (e.g., avoid the use of multilayers) even when compared to bio-PET. However, when long shelf-life or high barrier properties are not strictly necessary (disposable garments, home textiles, etc.), and rather biodegradability/compostability would be a plus, then, PLA is in the spotlight. PBS is more appropriate for flexible packaging.
To conclude, (new) sustainable polyesters do not have to sensu stricto mimic PET (in terms of the structure, properties, processability, impact, etc.) but it is rather the (new) applications that need to be adapted to the existing (and future) large portfolio of biobased polyesters. Finally, proposing binary polymer blends, taking the advantages of each resin that would be mechanically recyclable in the same stream, could also be a win–win strategy. Polymer blends, for instance bio-PET/PEF, rPET/PEF, and PLA/PBS, are also interesting solutions (at least to some proportion where neat polymers properties are not substantially affected and if the proportions can be controlled) to move forward higher sustainability without compromising the constant quality requirements demanded by end-users and consumers.
Bioeconomic aspects. Concomitant to the properties and applications, the PET substitution process also needs to consider techno-economic assessments. To understand the substitution processes, economists usually investigate the interaction of supply and demand between the substitutes by calculating the cross-price elasticities of demand.275,276 A cross-price elasticity of the demand captures the responsiveness of the quantity demanded of one good to a change in the price of another good. However, such neo-classical economic approaches are not (yet) common in the case of biobased polymers and PET, with only a few economic studies existing so far.100,231
A huge obstacle in introducing new biobased polymers is PET itself because it is a highly standardized intermediate product and relatively cheap. In contrast, biobased polymers of competitive PET-properties are currently more expensive to produce than PET.277–279 The question posed earlier in this review ‘Are PET alternatives, or will they be, economically feasible?’ will be answered in two parts.
Part 1: Currently the bio-based processes are not cost competitive if one does not consider the sustainability assets (costs of pollution and climate change impacts). Indeed, it is more than questionable if competing with PET in terms of current production costs will ever lead to a solution, without bring into the equation the negative environmental externalities of crude oil.231 In this context, environmental economics intend to consider according to policy interventions (e.g., carbon taxing). Following this approach, it is not sufficient to look at the biobased PET alternatives alone but at the environmental problem to solve and all corresponding routes and options (e.g., 9R framework).
As a mitigation to this challenge to overcome the large price differences during the gradual scale up of production volumes, the biobased alternatives will need to target the applications where they excel in their properties. In some cases, these technical and/or societal value propositions can lead to a cost benefit on a product level even at (much) higher resin price levels, creating a willingness to change.
Part 2: In the future, a different perspective may be reached that is quite dependent on the used feedstock/upscaling parameters/price developments, as well as on the future market developments, future policies and regulations, among other socio-economic factors that are relevant for potential market uptake.
The biobased PET alternatives discussed in this evaluation may contribute to a PET solution but avoiding and reducing packaging or textiles, or refund systems are also needed to be addressed by all (including consumers).280 Hence, we must assume that biobased solutions may get a chance in the future where other solutions cannot be implemented. This support (e.g., European Green Deal)281 will, however, not favor the one material over the other (the so-called technology neutral policy).282 Therefore, in the future, PET will certainly be replaced by more than one option, as the analysis of the properties and applications has already pointed out. Moreover, in our opinion, future research and related public and private funds should:
• investigate the future demand for non-standardized polymers packaging, fibers, and films;
• assess the competitiveness of biobased polymers compared to other reusable, recyclable, or compostable materials in terms of technical and economic properties;
• assess the environmental impacts of biobased polymers compared to these alternative solutions.
8. Conclusions
To emphasize, replacing–an exceptional polyester such as PET, partly or totally, is not a simple issue, particularly because PET is well implemented in the market for almost forty years. If one ideal solution would have existed, this review article would not have been necessary. The approach that consists of simply replacing/substituting PET by another more sustainable polyester may not be the most adaptable to the situation. Indeed, the angle of attack should be rather focused on the applications instead of the materials while considering the environmental, sustainability, and economic aspects. In this regard, PEF and PTF will have a major role to play in packaging, avoiding the additional gas barrier layer of PET bottles or even acting as a barrier layer in PET. In addition, PLA can be an important fiber for disposable textiles, although PEF can also be used in fibers. PBS is most suitable for flexible packaging.PET replacement by alternatives such as PEF, PTF, PLA, and PBS will stimulate sustainable growth. Despite this, a global solution to the negative impact of polymers on the environment cannot be detached from our social behavior, where reducing consumption habits should be at the core of our daily lifestyles. Rephrasing this idea, what future do we aim at achieving?
Author contributions
AFS – conceptualization, supervision and writing – original draft and – review & editing. RP – data curation, writing – review & editing.DNB, TS, NL and NG – conceptualization, writing – original draft and – review & editing. ZT, JW, VS, SP, AZ and DSvE – writing – original draft and – review & editing. KL, AS, KST, MSN, PS and ST– validation, writing – original draft and – review & editing.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Roy Visser for fruitful reading of this review. This publication is based upon work from COST Action FUR4Sustain- European network of FURan based chemicals and materials FOR a Sustainable development, CA18220, supported by COST (European Cooperation in Science and Technology). This work was supported within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 & UIDP/50011/2020, financed by national funds through the FCT– Fundação para a Ciência e a Tecnologia/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. This research is also sponsored by FEDER funds through the program COMPETE—Programa Operacional Factores de Competitividade—and by national funds through FCT under the project UID/EMS/00285/2020. FCT is also acknowledged for the research contract under Scientific Employment Stimulus to AFS (CEECIND/02322/2020). SP is acknowledged for funding this research by the National Science Centre within project SONATA no 2018/31/D/ST8/00792. MSN acknowledge support from the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract No. 451-03-9/2021-14/200135). ST and D.S.v.Es. acknowledge for funding by the Knowledge Base programme “Towards a circular and climate positive society” of Wageningen University & Research (WUR), in the project “Biobased materials and chemicals for relieving and replacing the fossil feedstock system” (KB-34-010-001). The WUR Knowledge Base programme is financed by the Dutch Ministry of Agriculture, Nature and Food Quality.Notes and references
- Plastics–the Facts 2020: An analysis of European plastics production, demand and waste data, 2020.
- Z. Ma, M. W. Ryberg, P. Wang, L. Tang and W. Q. Chen, ACS Sustainable Chem. Eng., 2020, 8, 16861–16868 CrossRef CAS.
- UN Comtrade Database, https://comtrade.un.org, (accessed 30 November 2020).
- A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G.-J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961–5983 RSC.
- C. Vilela, A. F. Sousa, A. C. Fonseca, A. C. Serra, J. F. J. Coelho, C. S. R. Freire and A. J. D. Silvestre, Polym. Chem., 2014, 5, 3119–3141 RSC.
- E. Bioplastics and nova-Institute , Bioplastics facts and figures.
- C. R. Álvarez-Chávez, S. Edwards, R. Moure-Eraso and K. Geiser, J. Cleaner Prod., 2012, 23, 47–56 CrossRef.
- C. L. Reichert, E. Bugnicourt, M.-B. Coltelli, P. Cinelli, A. Lazzeri, I. Canesi, F. Braca, B. M. Martínez, R. Alonso, L. Agostinis, S. Verstichel, L. Six, S. De Mets, E. C. Gómez, C. Ißbrücker, R. Geerinck, D. F. Nettleton, I. Campos, E. Sauter, P. Pieczyk and M. Schmid, Polymers, 2020, 12, 1558 CrossRef CAS PubMed.
- G. Papageorgiou, Polymers, 2018, 10, 952 CrossRef PubMed.
- S. RameshKumar, P. Shaiju, K. E. O'Connor and R. B. P, Curr. Opin. Green Sustainable Chem., 2020, 21, 75–81 CrossRef.
- R. Mülhaupt, Macromol. Chem. Phys., 2013, 214, 159–174 CrossRef.
- K. Kümmerer, J. H. Clark and V. G. Zuin, Science, 2020, 367, 369–370 CrossRef PubMed.
- UN, Sustainable Development Goals, https://www.un.org/sustainabledevelopment/sustainable-development-goals/, (accessed 8 February 2020).
- R. A. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- G. Z. Papageorgiou, D. G. Papageorgiou, Z. Terzopoulou and D. N. Bikiaris, Eur. Polym. J., 2016, 83, 202–229 CrossRef CAS.
- M. Rabnawaz, I. Wyman, R. Auras and S. Cheng, Green Chem., 2017, 19, 4737–4753 RSC.
- W. J. Rex and D. James Tennant, US2465319A, 1945.
- Polyethylene Terephthalate (PET): Production, Price, Market and its Properties, https://www.plasticsinsight.com/resin-intelligence/resin-prices/polyethylene-terephthalate/, (accessed 30 November 2020).
- K. C. Cannon, R. R. Dirkx and S. R. Seshadri, EPA, EP0970983A2, 2000.
- D. A. Schiraldi, U S Pat Tradem Off, US5922828, 1999.
- D. E. Putzig, U S Pat Tradem Off, 6166170, 2000.
- D. E. Putzig, U S Pat Tradem Off, US6080834.
- W. MacDonald, Polym. Int., 2002, 51, 923–930 CrossRef CAS.
- H. Gu, F. AlFayez, T. Ahmed and Z. Bashir, Polymers, 2019, 11, 2041 CrossRef CAS PubMed.
- Polymer Handbook, ed. E. A. G. J. Brandrup and E. H. Immergut, Wiley, New York, 1999 Search PubMed.
- Encyclopedia of Polymer Science and Engineering, ed. H. F. Mark, N. M. Bikales, C. G. Overberger and G. Menges, Wiley, 1985, vol. 12 Search PubMed.
- W. L. Hergenrother, J. Polym. Sci., Part A: Polym. Chem., 1974, 12, 875–883 CAS.
- L. H. Sperling, Introduction to Physical Polymer Science, Wiley, 2005 Search PubMed.
- F. Rodriguez, C. Cohen, C. K. Ober and L. Archer, Principles of Polymer Systems, CRC Press, 2014 Search PubMed.
- L. H. Palys and P. J. Phillips, J. Polym. Sci., Polym. Phys. Ed., 1980, 18, 829–852 CrossRef CAS.
- I. I. Rubin, Handbook of plastic materials and technology, 1990 Search PubMed.
- M. Hough and R. Dolbe, The Plastics Compendium: Key properties and sources, Rapra Technology, 1995 Search PubMed.
- J. G. Van Berkel, N. Guigo, H. A. Visser and N. Sbirrazzuoli, Macromolecules, 2018, 51, 8539–8549 CrossRef CAS.
- International Plastics Selector, Inc., Films, sheets, and laminates, International Plastics Selector, San Diego, 1979 Search PubMed.
- M. J. Forrest, Recycling of polyethylene terephthalate, De Gruyter, Berlin, 2nd edn., 2019 Search PubMed.
- P. T. Benavides, J. B. Dunn, J. Han, M. Biddy and J. Markham, ACS Sustainable Chem. Eng., 2018, 6, 9725–9733 CrossRef CAS.
- A. Saerens, M. Ghosh, J. Verdonck and L. Godderis, Int. J. Environ. Res. Public Health, 2019, 16, 1–24 Search PubMed.
- F. Welle and R. Franz, Food Addit. Contam., Part A, 2011, 28, 115–126 CrossRef CAS PubMed.
- U. Pirc, M. Vidmar, A. Mozer and A. Kržan, Environ. Sci. Pollut. Res., 2016, 23, 22206–22211 CrossRef CAS PubMed.
- A. L. Andrady, Mar. Pollut. Bull., 2017, 119, 12–22 CrossRef CAS PubMed.
- G. Bhagwat, K. Gray, S. P. Wilson, S. Muniyasamy, S. G. T. Vincent, R. Bush and T. Palanisami, J. Polym. Environ., 2020, 28, 3055–3075 CrossRef CAS.
- R. D. Cortright and P. G. Blommel, U S Pat Tradem Off, US7977517B, 2011.
- R. D. Cortright and J. A. Dumesic, U S Pat Tradem Off, US5736478A, 1998.
- G. W. Huber, Y.-T. Cheng, T. Carlson, T. Vispute, J. Jae and G. Tompsett, U S Pat Tradem Off, US8277643B, 2012.
- Bio-TCat™ for Renewable Chemicals & Fuels | Anellotech, Inc | Cost Competitive Bio-Sourced Chemicals and Fuels.
- G. W. Huber, Y.-T. Cheng, Z. Wang and W. Fan, U S Pat Tradem Off, US20130324772A1, 2013.
- G. W. Huber, H. Zhang and T. Carlson, U S Pat Tradem Off, US20130060070A1, 2013.
- M. W. Peters, J. D. Taylor, M. Jenni, L. Manzer and D. Henton, U S Pat Tradem Off, US20110087000A1, 2011.
- T. W. Lyons, D. Guironnet, M. Findlater and M. Brookhart, J. Am. Chem. Soc., 2012, 134, 15708–15711 CrossRef CAS PubMed.
- M. Brookhart, M. Findlater, D. Guironnet and T. Warren Lyons, U S Pat Tradem Off, 20130237732A1, 2013.
- Z. Lin, V. Nikolakis and M. Ierapetritou, Ind. Eng. Chem. Res., 2014, 53, 10688–10699 CrossRef CAS.
- Y. Román-Leshkov, C. J. Barrett, Z. Y. Liu and J. a. Dumesic, Nature, 2007, 447, 982–985 CrossRef PubMed.
- Z. Lin, V. Nikolakis and M. Ierapetritou, Ind. Eng. Chem. Res., 2015, 54, 2366–2378 CrossRef CAS.
- M. N. Masuno, D. Cannon, J. Bissell, R. L. Smith, M. Foster, A. B. Wood, P. B. Smith and D. A. Hucul, WIPO, WO2013040514A1, 2013.
- M. N. Masuno, P. B. Smith, D. A. Hucul, A. Dumitrascu, K. Brune, R. L. Smith, J. Bissell and M. Foster, U S Pat Tradem Off, US 2013/0245316A1, 2013 Search PubMed.
- M. Shiramizu and F. D. Toste, Chem. – Eur. J., 2011, 17, 12452–12457 CrossRef CAS PubMed.
- W. H. Gong, U S Pat Tradem Off, US8299278B2, 2012.
- B. Wang, G. J. M. Gruter, M. A. Dam and R. M. Kriegel, U S Pat Tradem Off, 20160137579A1, 2016.
- United States Patent and Trademark Office, US8367858B2, 2013.
- J. W. Frost, A. Miermont, D. Schweitzer, V. Bui, E. Paschke and D. A. Wicks, US Pat Tradem Off, 20110288263A1, 2011.
- J. K. Ogunjobi, T. J. Farmer, C. R. McElroy, S. W. Breeden, D. J. Macquarrie, D. Thornthwaite and J. H. Clark, ACS Sustainable Chem. Eng., 2019, 7, 8183–8194 CrossRef CAS.
- J. W. Frost, A. Miermont, D. Schweitzer, V. Bui and D. A. Wicks, U S Pat Tradem Off, US20110288311A1, 2011.
- M. B. Banella, C. Gioia, M. Vannini, M. Colonna, A. Celli and A. Gandini, ChemSusChem, 2016, 9, 942–945 CrossRef CAS PubMed.
- J. J. Lee and G. A. Kraus, Green Chem., 2014, 16, 2111–2116 RSC.
- J. J. Lee and G. A. Kraus, Tetrahedron Lett., 2013, 54, 2366–2368 CrossRef CAS.
- F. Wang and Z. Tong, ChemistrySelect, 2016, 1, 5538–5541 CrossRef CAS.
- P. Zhang, V. Nguyen and J. W. Frost, ACS Sustainable Chem. Eng., 2016, 4, 5998–6001 CrossRef CAS.
- K. K. Miller, P. Zhang, Y. Nishizawa-Brennen and J. W. Frost, ACS SustainableChem. Eng., 2014, 2, 2053–2056 CrossRef CAS.
- Y. Tachibana, S. Kimura and K. Kasuya, Sci. Rep., 2015, 5, 8249 CrossRef PubMed.
- B. Raecke, Angew. Chemie, 1958, 70, 1–5 CrossRef CAS.
- Y. Dôzen, J. Synth. Org. Chem., Jpn., 1961, 19, 570–581 CrossRef.
- J. Kruper, C. L. Rand and D. C. Molzahn, WIPO,WO2012125218A1, 2012.
- Z. Bai, W. C. Phuan, J. Ding, T. H. Heng, J. Luo and Y. Zhu, ACS Catal., 2016, 6, 6141–6145 CrossRef CAS.
- D. Buhl, D. M. Roberge and W. F. Hölderich, Appl. Catal., A, 1999, 188, 287–299 CrossRef CAS.
- Hull Washington, U S Pat Tradem Off, US2272711A, 1942.
- M. Colonna, C. Berti, M. Fiorini, E. Binassi, M. Mazzacurati, M. Vannini and S. Karanam, Green Chem., 2011, 13, 2543 RSC.
- S. Lundmark, M. Kangas and B. Häggman, WIPO, WO2014133433A1, 2014.
- E. Jongedijk, F. van der Klis, R. de Zwart, D. S. van Es and J. Beekwilder, ChemistryOpen, 2018, 7, 201–203 CrossRef CAS PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, 1–5 Search PubMed.
- E. Barnard, J. J. Rubio Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- D. Stanica-Ezeanu and D. Matei, Sci. Rep., 2021, 11, 4431 CrossRef CAS PubMed.
- Y. Wang, Y. Zhang, H. Song, Y. Wang, T. Deng and X. Hou, J. Cleaner Prod., 2019, 208, 1469–1475 CrossRef CAS.
- E. Sert, E. Yılmaz and F. S. Atalay, J. Polym. Environ., 2019, 27, 2956–2962 CrossRef CAS.
- Q. Wang, X. Yao, Y. Geng, Q. Zhou, X. Lu and S. Zhang, Green Chem., 2015, 17, 2473–2479 RSC.
- S. Choi and H. M. Choi, Fibers Polym., 2019, 20, 752–759 CrossRef CAS.
- R. Koshti, L. Mehta and N. Samarth, J. Polym. Environ., 2018, 26, 3520–3529 CrossRef CAS.
- ioniqa, A clean-tech spinoff from the Eindhoven University of Technology (NL), specialized in creating value out of PET waste by using its proprietary circular technology, https://ioniqa.com, (accessed 5 August 2021).
- S. Devraj, United States Patent Office, 9234074B2, 2016.
- Y. Akanuma, S. E. M. Selke and R. Auras, Int. J. Life Cycle Assess., 2014, 19, 1238–1246 CrossRef CAS.
- L. Chen, R. E. O. Pelton and T. M. Smith, J. Cleaner Prod., 2016, 137, 667–676 CrossRef CAS.
- M. Volanti, D. Cespi, F. Passarini, E. Neri, F. Cavani, P. Mizsey and D. Fozer, Green Chem., 2019, 21, 885–896 RSC.
- T. Semba, Y. Sakai, T. Sakanishi and A. Inaba, J. Cleaner Prod., 2018, 195, 932–938 CrossRef CAS.
- L. Shen, E. Worrell and M. K. Patel, Biofuels, Bioprod. Biorefin., 2012, 6, 625–639 CrossRef CAS.
- F. Gironi and V. Piemonte, Energy Sources, Part A, 2011, 33, 1949–1959 CrossRef CAS.
- C. Ingrao, M. Gigli and V. Siracusa, J. Cleaner Prod., 2017, 150, 93–103 CrossRef CAS.
- U. Suwanmanee, V. Varabuntoonvit, P. Chaiwutthinan, M. Tajan, T. Mungcharoen and T. Leejarkpai, Int. J. Life Cycle Assess., 2013, 18, 401–417 CrossRef CAS.
- E. T. H. Vink, K. R. Rábago, D. A. Glassner and P. R. Gruber, Polym. Degrad. Stab., 2003, 80, 403–419 CrossRef CAS.
- ENSYN, http://www.ensyn.com/technology/overview/, (accessed 17 February 2015).
- M. Héder, From NASA to EU: the evolution of the TRL scale in Public Sector Innovation, 2017, vol. 22 Search PubMed.
- M. G. Davidson, S. Elgie, S. Parsons and T. J. Young, Green Chem., 2021, 23, 3154–3171 RSC.
- A. D. K. Deshan, L. Atanda, L. Moghaddam, D. W. Rackemann, J. Beltramini and W. O. S. Doherty, Front. Chem., 2020, 8, 659 CrossRef CAS PubMed.
- Avantium N.V, Avantium reaches key commercial milestone for its FDCA flagship plant with commitments for >50% of its output.
- A. Codou, N. Guigo, J. van Berkel, E. de Jong and N. Sbirrazzuoli, Macromol. Chem. Phys., 2014, 215, 2065–2074 CrossRef CAS.
- G. Z. Papageorgiou, V. Tsanaktsis and D. N. Bikiaris, Phys. Chem. Chem. Phys., 2014, 16, 7946–7958 RSC.
- X. Fei, X. Fei, X. Fei, J. Wang, J. Wang, J. Zhu, J. Zhu, X. Wang, X. Liu and X. Liu, ACS Sustainable Chem. Eng., 2020, 8, 8471–8485 CrossRef CAS.
- A. Gandini, A. J. D. Silvestre, C. P. Neto, A. F. Sousa and M. Gomes, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 295–298 CrossRef CAS.
- Avantium, YXY Technology, https://www.avantium.com/technologies/yxy, (accessed 7 February 2020).
- W. J. McKillip, in Adhesives from Renewable Resources, ACS Publications, 1989, pp. 408–423 Search PubMed.
- J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- C. J. Zhang, M. Hu, Q. F. Ke, C. X. Guo, Y. J. Guo and Y. P. Guo, J. Hazard. Mater., 2020, 386, 121999 CrossRef CAS PubMed.
- Z. Terzopoulou, L. Papadopoulos, A. Zamboulis, D. G. Papageorgiou, G. Z. Papageorgiou and D. N. Bikiaris, Polymers, 2020, 12, 1209–1259 CrossRef CAS PubMed.
- J. Zhang, Y. Liu, Z. Qi, L. He and L. Peng, BioResources, 2020, 15(2), 4502–4527 Search PubMed.
- K. Loos, R. Zhang, I. Pereira, B. Agostinho, H. Hu, D. Maniar, N. Sbirrazzuoli, A. J. D. Silvestre, N. Guigo and A. F. Sousa, Front. Chem., 2020, 8, 1–18 CrossRef PubMed.
- A. Gandini, D. Coelho, M. Gomes, B. Reis and A. Silvestre, J. Mater. Chem., 2009, 19, 8656 RSC.
- M. Gomes, A. Gandini, A. J. D. Silvestre, B. Reis, N. Gomes, A. Gandini, A. J. D. Silvestre and B. Reis, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3759–3768 CrossRef CAS.
- L. Sipos, European Patent Office, 2370496A1, 2017.
- K. Matsuda, Japan Patent Office, 2009215467A, 2008.
- E. C. Boswell, D. I. Collias, R. E. Magness, D. A. Zimmerman, J. M. Layman, J. A. McDaniel, H. B. Rauckhorst, A. B. Watson, A. J. Burns, B. M. Dunphy and A. E. Neltner, European Patent Office, 2668105A1, 2011.
- C. Saywell, S. Mai and C. Capelas Romeu, WO, 2013097013A1, 2013 Search PubMed.
- T. Ghosh, K. Mahajan, S. Narayan-Sarathy, M. Naceur Balgacem and P. Goparakrishnan, US Pat Trad Off, 20130171397A1, 2013.
- E. De Jong, M. A. Dam, L. Sipos and G. J. M. Gruter, ACS Symp. Ser., 2012, 1105, 1–13 CrossRef CAS.
- S. K. Burgess, J. E. Leisen, B. E. Kraftschik, C. R. Mubarak, R. M. Kriegel and W. J. Koros, Macromolecules, 2014, 47, 1383–1391 CrossRef CAS.
- S. K. Burgess, O. Karvan, J. R. Johnson, R. M. Kriegel and W. J. Koros, Polymers, 2014, 55, 4748–4756 CrossRef CAS.
- L. Sun, J. Wang, S. Mahmud, Y. Jiang, J. Zhu and X. Liu, Eur. Polym. J., 2019, 118, 642–650 CrossRef CAS.
- S. K. Burgess, R. M. Kriegel and W. J. Koros, Macromolecules, 2015, 48, 2184–2193 CrossRef CAS.
- S. K. Burgess, D. S. Mikkilineni, B. Y. Daniel, D. J. Kim, C. R. Mubarak, R. M. Kriegel and W. J. Koros, Polymers, 2014, 55, 6870–6882 CrossRef CAS.
- J. G. Van Berkel and J. J. Kolstad, U S Pat Tradem Off, US20200283587A1, 2020.
- C. F. Araujo, M. M. Nolasco, P. J. Ribeiro-Claro, S. Rudić, A. J. Silvestre, P. D. Vaz and A. F. Sousa, Macromolecules, 2018, 51, 3515–3526 CrossRef CAS.
- X. Fei, J. Wang, J. Zhu, X. Wang and X. Liu, ACS Sustainable Chem. Eng., 2020, 8(23), 8471–8485 CrossRef CAS.
- R. J. I. Knoop, W. Vogelzang, J. van Haveren and D. S. van Es, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4191–4199 CrossRef CAS.
- J. G. van Berkel, N. Guigo, J. J. Kolstad and N. Sbirrazzuoli, Macromol. Mater. Eng., 2018, 303, 1700507 CrossRef.
- M. Jiang, Q. Liu, Q. Zhang, C. Ye and G. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1026–1036 CrossRef CAS.
- J. G. Van Berkel, N. Guigo, J. J. Kolstad, L. Sipos, B. Wang, M. A. Dam and N. Sbirrazzuoli, Macromol. Mater. Eng., 2015, 300, 466–474 CrossRef CAS.
- G. Stoclet, G. G. Du Sart, B. Yeniad, S. De Vos and J. M. Lefebvre, Polymers, 2015, 72, 165–176 CrossRef CAS.
- N. Guigo, E. Forestier and N. Sbirrazzuoli, in Advances in Polymer Science, Springer, Berlin, Heidelberg, 2019, pp. 19–51 Search PubMed.
- G. Stoclet, J. M. Lefebvre, B. Yeniad, G. G. du Sart and S. De Vos, Polymers, 2018, 134, 227–241 CrossRef CAS.
- Y. Mao, D. G. Bucknall and R. M. Kriegel, Polymers, 2018, 139, 60–67 CrossRef CAS.
- Y. Mao, D. G. Bucknall and R. M. Kriegel, Polymers, 2018, 143, 228–236 CrossRef CAS.
- E. Forestier, N. Guigo, C. Combeaud, N. Billon and N. Sbirrazzuoli, Macromolecules, 2020, 53, 8693–8703 CrossRef CAS.
- E. Forestier, C. Combeaud, N. Guigo, N. Sbirrazzuoli and N. Billon, Polymers, 2020, 203, 122755 CrossRef CAS.
- E. Forestier, C. Combeaud, N. Guigo, G. Monge, J.-M. Haudin, N. Sbirrazzuoli and N. Billon, Polymers, 2020, 187, 122126 CrossRef CAS.
- J.-P. Besson, M.-B. Bouffand and P. Reutenauer, U S Pat Tradem Off, US9713897B2, 2017.
- R. M. Kriegel, R. D. Moffitt, M. W. Schultheis, Y. Shi and X. You, WIPO, WO 2015/031907Al, 2015.
- L. Sipos, G. J. M. Gruter, J. J. Kolstad and M. A. Dam, U S Pat Tradem Off, US9527954, 2016.
- L. Sipos, G. J. M. Gruter, J. J. Kolstad and M. A. Dam, U S Pat Tradem Off, US10030097B2, 2018.
- R. D. Moffitt, J. Kaur, T. E. Freeman, R. Kriegel, Y. Shi, M. S. Morales and V. Nagpal, U S Pat Tradem Off, US20180022866A1, 2018.
- M.-B. Bouffand, A. Colloud and P. Reutenauer, World Intellectual Property Organization, 2014032730A1, 2014 Search PubMed.
- H. A. Visser and J. G. Van Berkel, World Intellectual Property Organization, 2020/013694, 2020.
- S. Hayakawa, Y. Numata, K. Ito, J. Inagaki and J. G. Van Berkel, U S Pat Tradem Off, US 2019/0389189A1, 2019.
- A. J. Duncan and P. Fagan, US Pat Trad Off, 2016200653A1, 2018.
- J. Inagaki, Y. Numata and J. G. Van Berkel, U S Pat Tradem Off, US 2020/0269559Al, 2020.
- Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, V. Tsanaktsis, N. Nikolaidis, M. Kostoglou, G. Z. Papageorgiou, D. A. Lambropoulou and D. N. Bikiaris, Polym. Chem., 2017, 8, 6895–6908 RSC.
- M. B. Banella, J. Bonucci, M. Vannini, P. Marchese, C. Lorenzetti and A. Celli, Ind. Eng. Chem. Res., 2019, 58, 8955–8962 CrossRef CAS.
- J. G. Rosenboom, D. K. Hohl, P. Fleckenstein, G. Storti and M. Morbidelli, Nat. Commun., 2018, 9, 1–7 CrossRef CAS PubMed.
- F. A. Kucherov, E. G. Gordeev, A. S. Kashin and V. P. Ananikov, Angew. Chemie, 2017, 129, 16147–16151 CrossRef.
- J.-G. Wang, X.-Q. Liu and J. Zhu, Chin. J. Polym. Sci., 2018, 36, 720–727 CrossRef CAS.
- G.-J. M. Gruter, L. Sipos and M. Adrianus Dam, Comb. Chem. High Throughput Screening, 2012, 15, 180–188 CrossRef CAS PubMed.
- E. Gubbels, L. Jasinska-Walc, B. A. J. Noordover and C. E. Koning, Eur. Polym. J., 2013, 49, 3188–3198 CrossRef CAS.
- J. C. Morales-Huerta, A. M. de Ilarduya and S. Muñoz-Guerra, Polymers, 2016, 87, 148–158 CrossRef.
- J. Carlos Morales-Huerta, A. Martínez De Ilarduya and S. Muñoz-Guerra, Polymers, 2016, 87, 148–158 CrossRef CAS.
- A. Martínez De Ilarduya and S. Muñoz Guerra, Polym. Chem., 2020, 11, 4850–4860 RSC.
- X. ling Qu, M. Jiang, B. Wang, J. Deng, R. Wang, Q. Zhang, G. yuan Zhou and J. Tang, ChemSusChem, 2019, 1–10 Search PubMed.
- J. Wu, H. Xie, L. Wu, B.-G. Li and P. Dubois, RSC Adv., 2016, 6, 101578–101586 RSC.
- T. P. Thuy Pham, C. W. Cho and Y. S. Yun, Water Res., 2010, 44, 352–372 CrossRef PubMed.
- M. Niaounakis, Eur. Polym. J., 2019, 114, 464–475 CrossRef CAS.
- European Pet Bottle Platform and EBPB, European PET bottle platform technical opinion: Synvina–poly(ethylene 2,5-furandicarboxylate) resin, 2017 Search PubMed.
- D. G. Papageorgiou, I. Tsetsou, R. O. Ioannidis, G. N. Nikolaidis, S. Exarhopoulos, N. Kasmi, D. N. Bikiaris, D. S. Achilias and G. Z. Papageorgiou, Polymers, 2021, 13(7), 1070 CrossRef CAS PubMed.
- Z. Terzopoulou, E. Karakatsianopoulou, N. Kasmi, M. Majdoub, G. Z. Papageorgiou and D. N. Bikiaris, J. Anal. Appl. Pyrolysis, 2017, 126, 357–370 CrossRef CAS.
- H. Nakajima, J. Gabriël van Berkel and J. J. Kolstad, WIPO, WO2017023175A1, 2017.
- L. Sipos and M. L. Olson, U S Pat Tradem Off, US9073886, 2015.
- E. Gabirondo, B. Melendez-Rodriguez, C. Arnal, J. M. Lagaron, A. Martínez de Ilarduya, H. Sardon, S. Torres Giner and S. Torres-Giner, J. Biotechnol., 2016, 235, 47–53 CrossRef PubMed.
- A. Pellis, K. Haernvall, C. M. Pichler, G. Ghazaryan, R. Breinbauer and G. M. Guebitz, J. Biotechnol., 2016, 235, 47–53 CrossRef CAS PubMed.
- S. Weinberger, K. Haernvall, D. Scaini, G. Ghazaryan, M. T. Zumstein, M. Sander, A. Pellis and G. M. Guebitz, Green Chem., 2017, 19, 5381–5384 RSC.
- CORBION, The future of plastics is biobased, https://www.corbion.com/bioplastics/fdca, (accessed 10 May 2019).
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407–6422 RSC.
- L. Jiang, A. Gonzalez-Diaz, J. Ling-Chin, A. Malik, A. P. Roskilly and A. J. Smallbone, Nat. Sustainability, 2020, 3, 761–767 CrossRef.
- A. J. J. E. Eerhart, M. K. Patel and A. P. C. Faaij, Biofuels, Bioprod. Biorefin., 2015, 9, 307–325 CrossRef CAS.
- S. Chen, R. Zou, L. Li, J. Shang, S. Lin and J. Lan, J. Appl. Polym. Sci., 2021, 138, e50345 Search PubMed.
- A. Tullo, C&EN, 2016, 94, 6–6 Search PubMed.
- M. Vannini, P. Marchese, A. Celli and C. Lorenzetti, Green Chem., 2015, 17, 4162–4166 RSC.
- A. Zubkiewicz, S. Paszkiewicz and A. Szymczyk, Polym. Eng. Sci., 2021, 61, 1536–1545 CrossRef CAS.
- M. C. Righetti, P. Marchese, M. Vannini, A. Celli, C. Lorenzetti, D. Cavallo, C. Ocando, A. J. Müller and R. Androsch, Biomacromolecules, 2020, 21, 2622–2634 CrossRef CAS PubMed.
- G. Z. Papageorgiou, V. Tsanaktsis, D. G. Papageorgiou, S. Exarhopoulos, M. Papageorgiou and D. N. Bikiaris, Polymers, 2014, 55, 3846–3858 CrossRef CAS.
- J. Zhu, J. Cai, W. Xie, P.-H. Chen, M. Gazzano, M. Scandola and R. A. Gross, Macromolecules, 2013, 46, 796–804 CrossRef CAS.
- G. Guidotti, M. Soccio, M. C. García-Gutiérrez, E. Gutiérrez-Fernández, T. A. Ezquerra, V. Siracusa, A. Munari and N. Lotti, ACS Sustainable Chem. Eng., 2019, 7, 17863–17871 CrossRef CAS.
- L. W. McKeen, in Permeability Properties of Plastics and Elastomers, Elsevier, 2012, pp. 145–193 Search PubMed.
- G. M. Cohen, F. Nederberg and B. Rajagopalan, EP3083800B1, 2020.
- H. Hu, R. Zhang, J. Wang, W. Bin Ying and J. Zhu, Eur. Polym. J., 2018, 102, 101–110 CrossRef CAS.
- M. J. Soares, P.-K. K. Dannecker, C. Vilela, J. Bastos, M. A. R. Meier and A. F. Sousa, Eur. Polym. J., 2017, 90, 301–311 CrossRef CAS.
- K. Haernvall, S. Zitzenbacher, H. Amer, M. T. Zumstein, M. Sander, K. McNeill, M. Yamamoto, M. B. Schick, D. Ribitsch and G. M. Guebitz, Biotechnol. J., 2017, 12, 1600741 CrossRef PubMed.
- Y. Jiang and K. Loos, Polymers, 2016, 8, 243 CrossRef PubMed.
- Y. Jiang, G. O. R. A. van Ekenstein, A. J. J. Woortman and K. Loos, Macromol. Chem. Phys., 2014, 215, 2185–2197 CrossRef CAS.
- D. Maniar, Y. Jiang, A. J. J. Woortman, J. van Dijken and K. Loos, ChemSusChem, 2019, 12, 990–999 CrossRef CAS PubMed.
- Y. Jiang, A. J. J. Woortman, G. O. R. Alberda van Ekenstein, D. M. Petrović and K. Loos, Biomacromolecules, 2014, 15, 2482–2493 CrossRef CAS PubMed.
- A. Pellis, S. Weinberger, M. Gigli, G. M. Guebitz and T. J. Farmer, Eur. Polym. J., 2020, 130, 109680 CrossRef CAS.
- Y. Jiang, A. J. J. Woortman, G. O. R. Alberda van Ekenstein and K. Loos, Polym. Chem., 2015, 6, 5198–5211 RSC.
- L. Mialon, A. G. Pemba and S. A. Miller, Green Chem., 2010, 12, 1704 RSC.
- C. Zhao, C. Huang, Q. Chen, I. D. V. Ingram, X. Zeng, T. Ren and H. Xie, Polymers, 2020, 12, 1–15 Search PubMed.
- Q. Chen, T. Ren, Y. Chai, Y. Guo, I. D. V. Ingram, M. North, H. Xie and Z. Kent Zhao, ChemCatChem, 2018, 10, 5377–5381 CrossRef CAS.
- M. Firdaus and M. a. R. Meier, Eur. Polym. J., 2013, 49, 156–166 CrossRef CAS.
- G. Kurt and E. Gokturk, J. Polym. Res., 2020, 27, 1–6 CrossRef.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Polym. Chem., 2015, 6, 6058–6066 RSC.
- S. V. Mankar, M. N. Garcia Gonzalez, N. Warlin, N. G. Valsange, N. Rehnberg, S. Lundmark, P. Jannasch and B. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 19090–19103 CrossRef CAS.
- L. Mialon, R. Vanderhenst, A. G. Pemba and S. A. Miller, Macromol. Rapid Commun., 2011, 32, 1386–1392 CrossRef CAS PubMed.
- C. Gioia, M. B. Banella, P. Marchese, M. Vannini, M. Colonna and A. Celli, Polym. Chem., 2016, 7, 5396–5406 RSC.
- A. Zamboulis, L. Papadopoulos, Z. Terzopoulou, D. N. Bikiaris, D. Patsiaoura, K. Chrissafis, M. Gazzano, N. Lotti and G. Z. Papageorgiou, Polymers, 2019, 11, 1672 CrossRef CAS PubMed.
- E. Xanthopoulou, Z. Terzopoulou, A. Zamboulis, L. Papadopoulos, K. Tsongas, D. Tzetzis, G. Z. Papageorgiou and D. N. Bikiaris, ACS Sustainable Chem. Eng., 2021, 9, 1383–1397 CrossRef CAS.
- C. Pang, J. Zhang, Q. Zhang, G. Wu, Y. Wang and J. Ma, Polym. Chem., 2015, 6, 797–804 RSC.
- H. T. H. Nguyen, G. N. Short, P. Qi and S. A. Miller, Green Chem., 2017, 19, 1877–1888 RSC.
- C. Gioia, M. B. Banella, G. Totaro, M. Vannini, P. Marchese, M. Colonna, L. Sisti and A. Celli, J. Renewable Mater., 2018, 6, 126–135 CrossRef CAS.
- Y. Zhang, Y. Enomoto and T. Iwata, Polymers, 2020, 203, 122751 CrossRef CAS.
- Y. Enomoto and T. Iwata, Polymers, 2020, 193, 122330 CrossRef CAS.
- S. Zhang, Z. Cheng, S. Zeng, G. Li, J. Xiong, L. Ding and M. Gauthier, J. Appl. Polym. Sci., 2020, 137, 49189 CrossRef CAS.
- N. Kasmi, L. Papadopoulos, Y. Chebbi, G. Z. Papageorgiou and D. N. Bikiaris, Polym. Degrad. Stab., 2020, 181, 109315 CrossRef CAS.
- H. T. H. Nguyen, M. H. Reis, P. Qi and S. A. Miller, Green Chem., 2015, 17, 4512–4517 RSC.
- O. Kreye, S. Oelmann and M. A. R. Meier, Macromol. Chem. Phys., 2013, 214, 1452–1464 CrossRef CAS.
- F. Pion, P.-H. Ducrot and F. Allais, Macromol. Chem. Phys., 2014, 215, 431–439 CrossRef CAS.
- A. Yamamoto, K. Nemoto, M. Yoshida, Y. Tominaga, Y. Imai, S. Ata, Y. Takenaka, H. Abe and K. Sato, RSC Adv., 2020, 10, 36562–36570 RSC.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Macromol. Rapid Commun., 2016, 37, 9–28 CrossRef CAS PubMed.
- A. C. Fonseca, M. S. Lima, A. F. Sousa, A. J. Silvestre, J. F. J. Coelho and A. C. Serra, Polym. Chem., 2019, 10, 1696–1723 RSC.
- EuroVanillin. The home of sustainable vanillin, https://vanillin.com, (accessed 6 April 2021).
- M. Fache, E. Darroman, V. Besse, R. Auvergne, S. Caillol and B. Boutevin, Green Chem., 2014, 16, 1987–1998 RSC.
- M. Fache, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 68, 488–502 CrossRef CAS.
- C. Gioia, M. B. Banella, P. Marchese, M. Vannini, M. Colonna and A. Celli, Polym. Chem., 2016, 7, 5396–5406 RSC.
- T. Kaneko, T. H. Thi, D. J. Shi and M. Akashi, Nat. Mater., 2006, 5, 966–970 CrossRef CAS PubMed.
- W. Dong, H. Li, M. Chen, Z. Ni, J. Zhao, H. Yang and P. Gijsman, J. Polym. Res., 2011, 18, 1239–1247 CrossRef CAS.
- H. Sun, S. Kanehashi, K. Tsuchiya and K. Ogino, Chem. Lett., 2016, 45, 439–441 CrossRef CAS.
- A. F. Reano, F. Pion, S. Domenek, P. H. Ducrot and F. Allais, Green Chem., 2016, 18, 3334–3345 RSC.
- L. Longe, G. Garnier and K. Saito, J. Polym. Sci., 2020, 58, 540–547 CrossRef CAS.
- S. Curia, A. Biundo, I. Fischer, V. Braunschmid, G. M. Gübitz and J. F. Stanzione, ChemSusChem, 2018, 11, 2529–2539 CrossRef CAS PubMed.
- J. Wenger, V. Haas and T. Stern, Curr. Foresty Rep., 2020, 6, 294–308 CrossRef.
- A. Larrañaga and E. Lizundia, Eur. Polym. J., 2019, 121 Search PubMed.
- P. K. Samantaray, A. Little, D. M. Haddleton, T. McNally, B. Tan, Z. Sun, W. Huang, Y. Ji and C. Wan, Green Chem., 2020, 22, 4055–4081 RSC.
- K. W. Meereboer, M. Misra and A. K. Mohanty, Green Chem., 2020, 22, 5519–5558 RSC.
- M. Nazareth, M. R. C. Marques, M. C. A. Leite and Í. B. Castro, J. Hazard. Mater., 2019, 366, 714–722 CrossRef CAS PubMed.
- R. T. Martin, L. P. Camargo and S. A. Miller, Green Chem., 2014, 16, 1768–1773 RSC.
- L. Mezzasalma, A. P. Dove and O. Coulembier, Eur. Polym. J., 2017, 95, 628–634 CrossRef CAS.
- A. Kumar, A. Thakur and P. S. Panesar, Rev. Environ. Sci. Biotechnol., 2019, 18, 823–853 CrossRef.
- R. Datta and M. Henry, J. Chem. Technol. Biotechnol., 2006, 81, 1119–1129 CrossRef CAS.
- S. Saeidlou, M. A. Huneault, H. Li and C. B. Park, Prog. Polym. Sci., 2012, 37, 1657–1677 CrossRef CAS.
- S. Farah, D. G. Anderson and R. Langer, Adv. Drug Delivery Rev., 2016, 107, 367–392 CrossRef CAS PubMed.
- U. Sonchaeng, F. Iñiguez-Franco, R. Auras, S. Selke, M. Rubino and L. T. Lim, Prog. Polym. Sci., 2018, 86, 85–121 CrossRef CAS.
- M. Matos, A. F. Sousa, A. C. Fonseca, C. S. R. Freire, J. F. J. Coelho and A. J. D. Silvestre, Macromol. Chem. Phys., 2014, 215, 2175–2184 CrossRef CAS.
- S. Domenek, A. Louaifi, A. Guinault and S. Baumberger, J. Polym. Environ., 2013, 21, 692–701 CrossRef CAS.
- Y. L. Chung, J. V. Olsson, R. J. Li, C. W. Frank, R. M. Waymouth, S. L. Billington and E. S. Sattely, ACS Sustainable Chem. Eng., 2013, 1, 1231–1238 CrossRef CAS.
- A. Nagardeolekar, M. Ovadias, K. T. Wang and B. Bujanovic, ChemSusChem, 2020, 13, 4702–4721 CrossRef CAS PubMed.
- N. Wang, C. Zhang and Y. Weng, J. Appl. Polym. Sci., 2021, 138, 50199 CrossRef CAS.
- W. Yang, E. Fortunati, F. Dominici, G. Giovanale, A. Mazzaglia, G. M. Balestra, J. M. Kenny and D. Puglia, Int. J. Biol. Macromol., 2016, 89, 360–368 CrossRef CAS PubMed.
- L.-T. Lim, R. Auras and M. Rubino, Prog. Polym. Sci., 2008, 33, 820–852 CrossRef CAS.
- R. Auras, B. Harte and S. Selke, Macromol. Biosci., 2004, 4, 835–864 CrossRef CAS PubMed.
- H. Nakajima, P. Dijkstra and K. Loos, Polymers, 2017, 9(10), 523 CrossRef PubMed.
- What is Ingeo, https://www.natureworksllc.com/What-is-Ingeo, (accessed 3 June 2021).
- I. MINIFIBERS, MINIFIBERS, INC., https://www.minifibers.com/our-company/about-fibers/hts-code-numbers/, (accessed 4 August 2021).
- P. Bałdowska-Witos, W. Kruszelnicka, R. Kasner, A. Tomporowski, J. Flizikowski, Z. Kłos, K. Piotrowska and K. Markowska, Polymers, 2020, 12, 388 CrossRef PubMed.
- S. Madival, R. Auras, S. P. Singh and R. Narayan, J. Cleaner Prod., 2009, 17, 1183–1194 CrossRef CAS.
- S. Papong, P. Malakul, R. Trungkavashirakun, P. Wenunun, T. Chom-in, M. Nithitanakul and E. Sarobol, J. Cleaner Prod., 2014, 65, 539–550 CrossRef CAS.
- K. Changwichan, T. Silalertruksa and S. H. Gheewala, Sustainability, 2018, 10, 1–15 CrossRef.
- I. D'Adamo, P. M. Falcone, E. Imbert and P. Morone, Ecol. Econ., 2020, 178, 106794 CrossRef.
- P. T. Benavides, U. Lee and O. Zarè-Mehrjerdi, J. Cleaner Prod., 2020, 277, 124010 CrossRef CAS.
- J. Beigbeder, L. Soccalingame, D. Perrin, J.-C. Bénézet and A. Bergeret, Waste Manage., 2019, 83, 184–193 CrossRef CAS PubMed.
- M. F. Cosate de Andrade, P. M. S. Souza, O. Cavalett and A. R. Morales, J. Polym. Environ., 2016, 24, 372–384 CrossRef CAS.
- D. Maga, M. Hiebel and N. Thonemann, Resour., Conserv. Recycl., 2019, 149, 86–96 CrossRef.
- I. Bechthold, K. Bretz, S. Kabasci, R. Kopitzky and A. Springer, Chem. Eng. Technol., 2008, 31, 647–654 CrossRef CAS.
- J. Xu and B.-H. Guo, Biotechnol. J., 2010, 5, 1149–1163 CrossRef CAS PubMed.
- M. Gigli, M. Fabbri, N. Lotti, R. Gamberini, B. Rimini and A. Munari, Eur. Polym. J., 2016, 75, 431–460 CrossRef CAS.
- T. Debuissy, E. Pollet and L. Avérous, Eur. Polym. J., 2017, 87, 84–98 CrossRef CAS.
- T. Yagihara and S. Matsumura, Polymers, 2012, 4, 1259–1277 CrossRef.
- Y. Ichikawa and T. Mizukoshi, in Synthetic Biodegradable Polymers, ed. B. Rieger, A. Künkel, G. W. Coates, R. Reichardt, E. Dinjus and T. A. Zevaco, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011, vol. 245, pp. 285–313 Search PubMed.
- T. Fujimaki, Polym. Degrad. Stab., 1998, 59, 209–214 CrossRef CAS.
- J. Nilsen-Nygaard, E. N. Fernández, T. Radusin, B. T. Rotabakk, J. Sarfraz, N. Sharmin, M. Sivertsvik, I. Sone and M. K. Pettersen, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1333–1380 CrossRef PubMed.
- MITSUBISHI CHEMICAL, BioPBS™, https://www.m-chemical.co.jp/en/products/departments/mcc/sustainable/product/1201025_7964.html.
- P. Tecchio, P. Freni, B. De Benedetti and F. Fenouillot, J. Cleaner Prod., 2016, 112, 316–325 CrossRef CAS.
- V. Dornburg, A. Faaij, M. Patel and W. C. Turkenburg, Resour., Conserv. Recycl., 2006, 46, 377–409 CrossRef.
- S. O. Hansson, L. Molander and C. Rudén, Regul. Toxicol. Pharmacol., 2011, 59, 454–460 CrossRef PubMed.
- L. Sun and K. Niquidet, For. Policy Econ., 2017, 81, 83–87 CrossRef.
- D. Jochem, N. Janzen and H. Weimar, J. Cleaner Prod., 2016, 137, 1216–1227 CrossRef.
- M. Brodin, M. Vallejos, M. T. Opedal, M. C. Area and G. Chinga-Carrasco, J. Cleaner Prod., 2017, 162, 646–664 CrossRef CAS.
- N.-S. Lau, D. H.-E. Ch'ng, K.-H. Chia, Y.-M. Wong and K. Sudesh, J. Biobased Mater. Bioenergy, 2014, 8, 118–129 CrossRef CAS.
- H. Xu and Y. Yang, in ACS Symposium Series, American Chemical Society, 2012, vol. 1114, pp. 113–140 Search PubMed.
- N. Escobar, S. Haddad, J. Börner and W. Britz, Environ. Res. Lett., 2018, 13, 125005 CrossRef CAS.
- The European Green Deal, https://ec.europa.eu/info/sites/default/files/european-green-deal-communication_en.pdf, (accessed 9 June 2021).
- H. Hellsmark and P. Söderholm, Biofuels, Bioprod. Biorefin., 2016, 11, 28–40 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |