Effect of molecular weight and polymer composition on gallol-functionalized underwater adhesive†
Jinhong
Yu
,
Bohan
Cheng
 and
Hirotaka
Ejima
and
Hirotaka
Ejima
 *
*
Department of Materials Engineering, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku 113-8656, Japan. E-mail: ejima@material.t.u-tokyo.ac.jp
First published on 8th April 2020
Abstract
Despite high demands from various industries, strong adhesion in a wet environment remains challenging. We investigated the underwater adhesion of gallol-functionalized polymers as a function of molecular weight and gallol content. By optimizing these parameters, the underwater adhesion strength of aluminium substrates exceeded 4 MPa. Therefore, the biomimetic molecular design of phenolic polymers is effective for the development of strong underwater adhesives.
Adhesives play an essential role in our daily life; however, commercial glues are usually designed only for use under dry conditions.1 Under wet conditions, water forms an aqueous boundary layer between adhesives and adherents. This layer often makes the work of adhesion negative, which means underwater bonding is unfavourable.2 In contrast, many marine creatures can stick to wet surfaces even under turbulent environments. A marine mussel is a well-studied animal owing to its wet adhesion ability. The key is the use of peculiar proteins with high 3,4-dihydroxyphenylalanine (DOPA) contents. The catechol group in DOPA plays a crucial role in adhesion,3 which mediates various interactions, including hydrogen bondings,4 metal coordinations,5,6 cation–π interactions,7 π–π interactions,8 and covalent cross-linkings.9 Among mussel-inspired adhesives,1,10–16 poly(3,4-dihydroxystyrene-co-styrene) (P(VCat-co-St)) has shown the strongest adhesion strengths of 3.0 and 2.2 MPa in lap-shear and tensile tests, respectively, on aluminium substrates.16
In addition to the catechol-based adhesives whose high underwater adhesion capabilities have been established, another phenolic compound, viz. gallol group, is recently gaining increasing attention.17,18 As a member of the phenol family, gallol has three adjacent hydroxy groups attached to a benzene ring, and is often found in plant-derived polyphenols, including epigallocatechin19 and tannic acid.20 The gallol-functionalized copolymer, poly(3,4,5-trihydroxystyrene-co-n-butyl acrylate) (P(VGal-co-BA)) showed 7 times higher adhesion performance than its catechol-functionalized counterpart (P(VCat-co-BA)) under the same condition.21 However, the adhesion strength of P(VGal-co-BA) was 1.0 MPa,21 still lower than that of P(VCat-co-St).16 This can be attributed to the different choice of comonomer (i.e., BA and St).
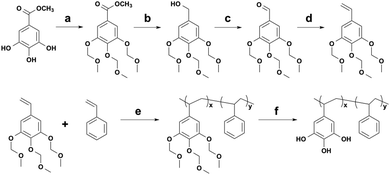
Here, we first synthesized P(VGal-co-St) and investigated the effect of molecular weight and composition on the wet adhesion strength. Both tensile and lap-shear tests were carried out and the adhesion strength exceeded 4 MPa under the optimized conditions.
The synthesis of PVGal via the methoxymethyl (MOM)-protection route was reported in our previous paper.21 We slightly modified this protocol to start from the inexpensive compound, methyl gallate (Scheme 1 and Fig. S1, S2 in ESI†). We chose St as a comonomer because it may increase cohesive interactions via π–π interactions. By changing the feed ratio of the initiator and monomer, the number-average molecular weight (Mn) of the obtained polymer was controlled between 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 111
000 and 111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (Table S1, ESI†).
000 g mol−1 (Table S1, ESI†).
The underwater adhesion strength was measured using tensile and lap-shear geometries. In the tensile tests, a polished aluminium rod (1.27 cm in diameter and 38 cm in height) was completely submerged underwater (Scheme 2). The polymer solution (20 μL, 0.3 mg mL−1) in a mixed solvent (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 9
methanol = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was deposited on the rod by using a pipette. A second aluminium rod was placed atop the first rod. A weight (25 g) was placed on the second rod and incubated for a certain period for setting. Samples were then removed from water bath and tested on SHIMAZU AGS-X 10 kN load cell with a crosshead speed of 10 mm min−1. The typical force–displacement curve is shown in Fig. S3 (ESI†). In the lap-shear test, aluminium plates (5 cm long × 1 cm wide) were used instead of the rods. The overlapped area of two plates was 1 cm2.
1, v/v) was deposited on the rod by using a pipette. A second aluminium rod was placed atop the first rod. A weight (25 g) was placed on the second rod and incubated for a certain period for setting. Samples were then removed from water bath and tested on SHIMAZU AGS-X 10 kN load cell with a crosshead speed of 10 mm min−1. The typical force–displacement curve is shown in Fig. S3 (ESI†). In the lap-shear test, aluminium plates (5 cm long × 1 cm wide) were used instead of the rods. The overlapped area of two plates was 1 cm2.
The effect of setting time for underwater adhesion is shown in Fig. 1. For these experiments, we used P(VGal-co-St) with a fixed gallol content of ∼10% and varied Mn from 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 103
000 to 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. As the solvent diffused out in a water bath, the chain mobility of the polymers decreased concomitantly. The polymer is finally glassified and set to bond the aluminium rods together. It took 72 h to reach its maximum adhesion strength, which is similar to the case of P(VCat-co-St).16
000 g mol−1. As the solvent diffused out in a water bath, the chain mobility of the polymers decreased concomitantly. The polymer is finally glassified and set to bond the aluminium rods together. It took 72 h to reach its maximum adhesion strength, which is similar to the case of P(VCat-co-St).16
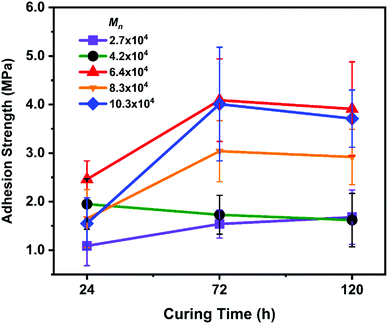 | ||
| Fig. 1 Adhesion strength with different curing times. Error bars indicate standard deviation, n ≥ 5. | ||
We then compared the adhesion strengths of polymers with different molecular weights (Fig. 2). In these tests, all the failure types were cohesive failures. This means that the adhesion between polymers and substrates is strong enough such that the adhesion strength is mainly determined by cohesive interactions inside a polymer layer. In general, the properties of adhesives are highly dependent on the molecular weight and chemical composition.22,23 In the regime of Mn < 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, adhesion strength increased with increasing Mn. This is due to the increase in entanglement of polymer chains, considering that the entanglement molecular weight (Me) of PSt is ∼17
000 g mol−1, adhesion strength increased with increasing Mn. This is due to the increase in entanglement of polymer chains, considering that the entanglement molecular weight (Me) of PSt is ∼17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1.24 Beyond 64
500 g mol−1.24 Beyond 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, the bonding strength of this underwater adhesive reached its plateau. The highest adhesion strength was achieved at Mn = 64
000 g mol−1, the bonding strength of this underwater adhesive reached its plateau. The highest adhesion strength was achieved at Mn = 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and the bonding strength was 4.09 ± 0.85 MPa in tensile tests. Further increase in Mn did not lead to any significant increase in adhesion strength. The adhesion strength is affected by the complicated balance of polymer–surface (adhesive) and polymer–polymer (cohesive) interactions. Polymer with low Mn has high chain mobility, such that it can easily establish polymer–surface interactions; however, polymer–polymer interactions are weaker because the few entanglements and other interactions, including π–π interactions and hydrogen bonding, exist between polymer chains. The low-Mn polymer was quicker to set within 24 h (Fig. S4, ESI†), and the adhesion strength at the plateau region was weaker (∼1.2 MPa) than that for polymers with higher Mn (∼3–4 MPa).
000, and the bonding strength was 4.09 ± 0.85 MPa in tensile tests. Further increase in Mn did not lead to any significant increase in adhesion strength. The adhesion strength is affected by the complicated balance of polymer–surface (adhesive) and polymer–polymer (cohesive) interactions. Polymer with low Mn has high chain mobility, such that it can easily establish polymer–surface interactions; however, polymer–polymer interactions are weaker because the few entanglements and other interactions, including π–π interactions and hydrogen bonding, exist between polymer chains. The low-Mn polymer was quicker to set within 24 h (Fig. S4, ESI†), and the adhesion strength at the plateau region was weaker (∼1.2 MPa) than that for polymers with higher Mn (∼3–4 MPa).
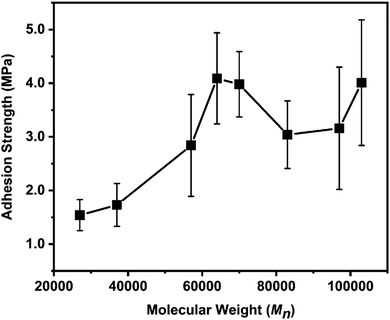 | ||
| Fig. 2 Adhesion strength as a function of number average molecular weight (Mn). Error bars indicate standard deviation, n ≥ 5. | ||
The adhesion strength of 4.09 ± 0.85 MPa (Mn = 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) obtained in this study is nearly two times higher than that of catechol-based underwater adhesives (2.2 MPa).16 The only difference is the number of phenolic hydroxy groups (i.e., three for gallol and two for catechol). The underwater adhesion strength of P(VGal-co-St) with optimized Mn was also measured to be 4.17 ± 0.47 MPa by using the lap-shear tests. This value is higher than that of P(VCat-co-St) (3.0 MPa),16 suggesting that the gallol group is more effective than catechol for developing strong underwater adhesives.
000) obtained in this study is nearly two times higher than that of catechol-based underwater adhesives (2.2 MPa).16 The only difference is the number of phenolic hydroxy groups (i.e., three for gallol and two for catechol). The underwater adhesion strength of P(VGal-co-St) with optimized Mn was also measured to be 4.17 ± 0.47 MPa by using the lap-shear tests. This value is higher than that of P(VCat-co-St) (3.0 MPa),16 suggesting that the gallol group is more effective than catechol for developing strong underwater adhesives.
Next, the effect of gallol content on adhesion strength was examined (Fig. 3). The polymer with 0% gallol content (PSt) has quite weak underwater adhesion, and usually fails during the tests. Interestingly, introducing a small amount of gallol groups (∼5%) dramatically enhanced the adhesion strength, which exceeded 4 MPa at 10% gallol content. In contrast, increasing the gallol content beyond 10% decreased the adhesion strength. This might be partly because the gallol groups interacted with each other via hydrogen bonding and the number of free gallol groups, which can participate in the gallol–surface interactions, decreased.
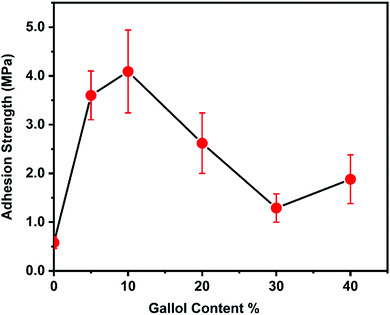 | ||
| Fig. 3 Effect of gallol content on underwater adhesion strength. The error bars indicate standard deviation, n ≥ 5. | ||
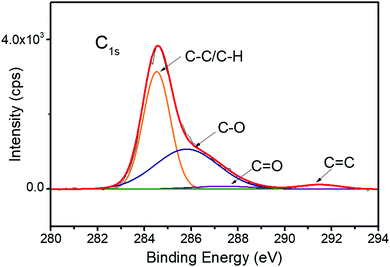
Similar to catechol groups, gallol groups are susceptible to oxidation.17,19 After the adhesion tests, the fractured samples were subjected to X-ray photoelectron spectroscopy (XPS) analysis. The C1s spectrum of the fractured surface (10% gallol content, Mn = 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) is shown in Fig. 4. The oxidation of gallol group forms quinone species, which increases the number of C
000 g mol−1) is shown in Fig. 4. The oxidation of gallol group forms quinone species, which increases the number of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. By comparing C–O and C
O bonds. By comparing C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak intensities, we estimated the oxidation degree of P(VGal10%-co-St90%). The peak areas of C–O and C
O peak intensities, we estimated the oxidation degree of P(VGal10%-co-St90%). The peak areas of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O accounted for 41.4% and 2.9%, respectively. Thus, ∼7% of hydroxy groups were estimated to be oxidized after 72 h curing in water. It is noted that the oxidation degree of fresh polymer before curing underwater was 0.2% (Fig. S5, ESI†). This low degree of oxidation ensured that the remaining adhesives were still soluble in organic solvents like acetone. The low gallol content (∼10%), low oxidation degree (∼7%), and high Mn (∼64
O accounted for 41.4% and 2.9%, respectively. Thus, ∼7% of hydroxy groups were estimated to be oxidized after 72 h curing in water. It is noted that the oxidation degree of fresh polymer before curing underwater was 0.2% (Fig. S5, ESI†). This low degree of oxidation ensured that the remaining adhesives were still soluble in organic solvents like acetone. The low gallol content (∼10%), low oxidation degree (∼7%), and high Mn (∼64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) ensured good balance between adhesive and cohesive interactions in the polymer.
000 g mol−1) ensured good balance between adhesive and cohesive interactions in the polymer.
In summary, we synthesized the gallol-functionalized copolymers, P(VGal-co-St), with different Mn and compositions. The effects of curing time, Mn, and gallol content on the underwater adhesion strength were investigated in detail by using tensile and lap shear tests. With increasing molecular weight, the adhesion strength increased when Mn was less than 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The introduction of ∼10% gallol units is sufficient to achieve high adhesion strength. Under the optimized conditions, the underwater adhesion strength reached 4.09 ± 0.85 MPa. This study demonstrates that the biomimetic molecular design of phenolic polymers focusing on the number of hydroxy groups is effective for developing strong underwater adhesive systems.
000. The introduction of ∼10% gallol units is sufficient to achieve high adhesion strength. Under the optimized conditions, the underwater adhesion strength reached 4.09 ± 0.85 MPa. This study demonstrates that the biomimetic molecular design of phenolic polymers focusing on the number of hydroxy groups is effective for developing strong underwater adhesive systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially supported by the Japan Society for the Promotion of Science (JSPS) through KAKENHI Grant Numbers 18K14000, Adaptable and Seamless Technology transfer Program through Target-driven R&D (A-STEP) from Japan Science and Technology Agency (JST), and the Ogasawara Foundation for the Promotion of Science & Engineering. H. E. acknowledges JSPS for its Leading Initiative for Excellent Young Researchers.Notes and references
- Y. Zhao, Y. Wu, L. Wang, M. Zhang, X. Chen, M. Liu, J. Fan, J. Liu, F. Zhou and Z. Wang, Nat. Commun., 2017, 8, 2218 CrossRef PubMed.
- B. P. Lee, P. B. Messersmith, J. N. Israelachvili and J. H. Waite, Annu. Rev. Mater. Res., 2011, 41, 99–132 CrossRef CAS PubMed.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed.
- S.-C. Li, J. Wang, P. Jacobson, X.-Q. Gong, A. Selloni and U. Diebold, J. Am. Chem. Soc., 2009, 131, 980–984 CrossRef CAS PubMed.
- T. Zhang, P. Wojtal, O. Rubel and I. Zhitomirsky, RSC Adv., 2015, 5, 106877 RSC.
- M. Krogsgaard, M. A. Behrens, J. S. Pedersen and H. Birkedal, Biomacromolecules, 2013, 14, 297–301 CrossRef CAS PubMed.
- D. S. Hwang, H. Zeng, Q. Lu, J. Israelachvili and J. H. Waite, Soft Matter, 2012, 8, 5640–5648 RSC.
- J. Saiz-Poseu, J. Mancebo-Aracil, F. Nador, F. Busqué and D. Ruiz-Molina, Angew. Chem., Int. Ed., 2019, 58, 696–714 CrossRef CAS PubMed.
- J. Yang, M. A. Cohen Stuart and M. Kamperman, Chem. Soc. Rev., 2014, 43, 8271–8298 RSC.
- M. Yu and T. J. Deming, Macromolecules, 1998, 31, 4739–4745 CrossRef CAS PubMed.
- T. H. Anderson, J. Yu, A. Estrada, M. U. Hammer, J. H. Waite and J. N. Israelachvili, Adv. Funct. Mater., 2010, 20, 4196–4205 CrossRef CAS PubMed.
- C. R. Matos-Pérez, J. D. White and J. J. Wilker, J. Am. Chem. Soc., 2012, 134, 9498–9505 CrossRef PubMed.
- L. Han, X. Lu, K. Liu, K. Wang, L. Fang, L.-T. Weng, H. Zhang, Y. Tang, F. Ren, C. Zhao, G. Sun, R. Liang and Z. Li, ACS Nano, 2017, 11, 2561–2574 CrossRef CAS PubMed.
- Z. Guo, S. Mi and W. Sun, J. Mater. Chem. B, 2018, 6, 6234–6244 RSC.
- S. Yan, W. Wang, X. Li, J. Ren, W. Yun, K. Zhang, G. Li and J. Yin, J. Mater. Chem. B, 2018, 6, 6377–6390 RSC.
- M. A. North, C. A. Del Grosso and J. J. Wilker, ACS Appl. Mater. Interfaces, 2017, 9, 7866–7872 CrossRef CAS PubMed.
- M. Shin, E. Park and H. Lee, Adv. Funct. Mater., 2019, 29, 1903022 CrossRef CAS.
- B. Cheng, K. Ishihara and H. Ejima, Polym. Chem., 2020, 11, 249–253 RSC.
- K. Zhan, H. Ejima and N. Yoshie, ACS Sustainable Chem. Eng., 2016, 4, 3857–3863 CrossRef CAS.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. A. Lau and P. B. Messersmith, Angew. Chem., Int. Ed., 2013, 52, 10766–10770 CrossRef CAS PubMed.
- K. Zhan, C. Kim, K. Sung, H. Ejima and N. Yoshie, Biomacromolecules, 2017, 18, 2959–2966 CrossRef CAS PubMed.
- C. L. Jenkins, H. J. Meredith and J. J. Wilker, ACS Appl. Mater. Interfaces, 2013, 5, 5091–5096 CrossRef CAS PubMed.
- G. Y. Choi, W. Zurawsky and A. Ulman, Langmuir, 1999, 15, 8447–8450 CrossRef CAS.
- M. Kapnistos, M. Lang, D. Vlassopoulos, W. Pyckhout-Hintzen, D. Richter, D. Cho, T. Chang and M. Rubinstein, Nat. Mater., 2008, 7, 997–1002 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tb00706d |
| This journal is © The Royal Society of Chemistry 2020 |