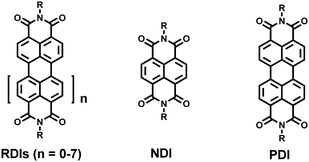
Naphthalene diimide- and perylene diimide-based supramolecular cages
Qing-Hui
Ling
a,
Jun-Long
Zhu
a,
Yi
Qin
*a and
Lin
Xu
 *ab
*ab
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200241, China. E-mail: qinyihuaxue@126.com; lxu@chem.ecnu.edu.cn
bState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, P. R. China
First published on 9th September 2020
Abstract
During the past few years, the construction of naphthalene diimide (NDI)- and perylene diimide (PDI)-based cages has attracted increasing attention due to the unique photo- and electrochemical properties and widespread applications of these materials in catalysis, chemical separation, sensing, etc. Thus, it is the right time to summarize the recent development of NDI-/PDI-based cages. In this review, the preparations, properties, and applications of NDI-/PDI-based cages in catalysis, chemical separation, and sensing will be discussed.
1. Introduction
Since the discovery of crown ethers and cryptands by Lehn et al., the construction of new supramolecular hosts, including macrocycles and cages, has attracted increasing interest.1 Compared to two-dimensional macrocycles such as pillar[n]arene2 and metal-coordinated macrocycles,3 which possess an open cavity and allow guests to fill a hole or bind on the surface of the macrocycle, three-dimensional cages possess enclosed cavities in which guests are entirely encapsulated in the confined space in general. Due to the efficient encapsulation of various guests, cages could thus be applied in many fields, such as molecular recognition, catalysis, chemical transfer and separation, and stabilization of reactive intermediates.4 During the past few decades, a variety of cages with structural complexity, including Platonic and Archimedes polyhedrons, were reported and exhibited unique properties and functions,5 which promoted the development of modern supramolecular chemistry.Rylene diimides (RDIs, n = 0–7) are a series of aromatic diimides; for example, n = 0 or 1, corresponded to naphthalene diimide (NDI) and perylene diimide (PDI), respectively (Scheme 1). NDI and PDI have attracted extensive attention due to their large planar structure, electron-deficient nature, photochemical and electrochemical properties (strong absorption in the visible region, high quantum yield and redox activity), and easy functionalization.6 Therefore, in the past few decades, a large number of NDI and PDI derivatives have been prepared with applications in sensing, light harvesting, photocatalysis and supramolecular self-assembly.7 For example, some NDI- and PDI-based macrocycles were successfully constructed through covalent synthesis or non-covalent self-assembly.8 Since NDI and PDI derivatives exhibit excellent photochemical and electrochemical properties, integrating NDI or PDI subunits into a cage may result in distinctive properties and functions: (1) the electron-deficient nature of NDI/PDI could endow the cage with a strong affinity for electron-rich guests such as C60; (2) the switchable redox activity of NDI/PDI endows the cage with catalytic performance; and (3) a cage with strong absorption in the visible region may serve as a visible-light photocatalyst. In recent decades, a series of NDI-/PDI-based cages were constructed, and their properties and applications were investigated in detail. It should be noted that among the NDI/PDI cages, metal-coordinated cages are in the majority since coordination-driven self-assembly has proven to be a powerful tool to construct various supramolecular architectures, including three-dimensional metal-coordinated cages.9 In addition, due to the linear structure of NDI/PDI units, tetrahedral cages were readily prepared through coordination-driven self-assembly.10
This review will focus on the preparation strategies, photochemical and electrochemical properties of NDI/PDI-based cages as well as their applications. For clarity, this review will be classified into NDI-based cages and PDI-based cages.
2. Naphthalene diimide-based cages
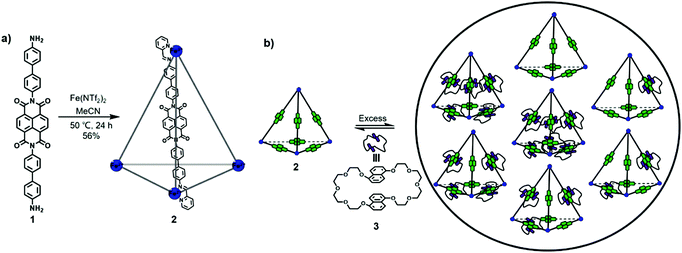
Naphthalene diimide (NDI) derivatives are planar and electron-deficient as well as π-acidic and can be used in anion–π catalysis.11 Typically, NDI derivatives could undergo two reversible one-electron reduction under chemical or electrochemical conditions with half-wave potentials of −1.10 V and −1.51 V vs. Fc/Fc+ in CH2Cl2.6a Based on the outstanding features of NDI units and the confined space of cages, NDI-based cages show unique physical and chemical properties and can be applied in various fields, such as catalysis, sensing, and separation of anions.Nitschke and co-workers have made significant contributions in the field of NDI-based cages. In 2013, Nitschke, Schalley and Sanders et al. reported the synthesis of a tetrahedral NDI cage and investigated its mechanically interlocked behaviour with bis-1,5-(dinaphtho)-[38]crown-10.12 As shown in Fig. 1, by treating NDI-containing diamine 1, 2-formylpyridine and Fe(NTf2)2 in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in acetonitrile at 50 °C for 24 h, an M4L6-type NDI cage 2 was obtained through subcomponent self-assembly. The NDI cage 2 was well characterized by 1H NMR, ESI-MS, COSY, and NOESY NMR spectroscopy, and the broadening and overlap of the signals in the 1H NMR spectra indicated the existence of various diastereomers with T, S4, and C3 symmetry of the cage in solution. Due to their planar structure and electron-deficient properties, NDI derivatives readily form donor–acceptor complexes with aromatic species. Thus, NDI cage 2 was further treated with bis-1,5-(dinaphtho)-[38]crown-10 (10 equiv.) 3, and three-dimensional polycatenanes were formed. The 1H NMR spectrum of the polycatenanes showed two sets of signals, in which one signal corresponded to the crown ether-bound NDI cage (an upfield shift in 1H NMR relative to the initial NDI cage 2) and the other signal corresponded to the free NDI cage. The above result also suggested the slow exchange process between the bound and free crown ether on the NMR timescale. In addition, the mixtures of polycatenanes cannot be distinguished in NMR spectra due to the overlapping signals. Furthermore, treatment of NDI cage 2 with a large excess of 3 (38 equiv.) provided saturated [7]catenane. DOSY spectra of the polycatenanes also showed two groups of resonances corresponding to the crown ether-bound NDI cages and the free crown ether, consistent with the 1H NMR spectra. An infrared multiphoton dissociation (IRMPD) tandem MS experiment was further performed to confirm the specific binding between the NDI cages and crown ethers, and the results indicated that [M4L6C7]8+ (C = catenated crown ether 3) showed non-specific binding between NDI cage 2 and crown ether 3, while [M4L6C6]8+ ions maintained their tetrahedral structure with six catenated crown ethers. Furthermore, the binding isotherm revealed that the binding of the six crown ethers to the NDI cage was not cooperative due to the relatively large space for threading, and the binding constant was determined to be 794 ± 34 L mol−1. In conclusion, this work successfully constructed three-dimensional polycatenanes through three reversible interactions (metal–ligand coordination, imine bond, and donor–acceptor interactions) and studied the dynamic systems in detail, laying the foundation for the construction of new molecular machines with various applications.
4 in acetonitrile at 50 °C for 24 h, an M4L6-type NDI cage 2 was obtained through subcomponent self-assembly. The NDI cage 2 was well characterized by 1H NMR, ESI-MS, COSY, and NOESY NMR spectroscopy, and the broadening and overlap of the signals in the 1H NMR spectra indicated the existence of various diastereomers with T, S4, and C3 symmetry of the cage in solution. Due to their planar structure and electron-deficient properties, NDI derivatives readily form donor–acceptor complexes with aromatic species. Thus, NDI cage 2 was further treated with bis-1,5-(dinaphtho)-[38]crown-10 (10 equiv.) 3, and three-dimensional polycatenanes were formed. The 1H NMR spectrum of the polycatenanes showed two sets of signals, in which one signal corresponded to the crown ether-bound NDI cage (an upfield shift in 1H NMR relative to the initial NDI cage 2) and the other signal corresponded to the free NDI cage. The above result also suggested the slow exchange process between the bound and free crown ether on the NMR timescale. In addition, the mixtures of polycatenanes cannot be distinguished in NMR spectra due to the overlapping signals. Furthermore, treatment of NDI cage 2 with a large excess of 3 (38 equiv.) provided saturated [7]catenane. DOSY spectra of the polycatenanes also showed two groups of resonances corresponding to the crown ether-bound NDI cages and the free crown ether, consistent with the 1H NMR spectra. An infrared multiphoton dissociation (IRMPD) tandem MS experiment was further performed to confirm the specific binding between the NDI cages and crown ethers, and the results indicated that [M4L6C7]8+ (C = catenated crown ether 3) showed non-specific binding between NDI cage 2 and crown ether 3, while [M4L6C6]8+ ions maintained their tetrahedral structure with six catenated crown ethers. Furthermore, the binding isotherm revealed that the binding of the six crown ethers to the NDI cage was not cooperative due to the relatively large space for threading, and the binding constant was determined to be 794 ± 34 L mol−1. In conclusion, this work successfully constructed three-dimensional polycatenanes through three reversible interactions (metal–ligand coordination, imine bond, and donor–acceptor interactions) and studied the dynamic systems in detail, laying the foundation for the construction of new molecular machines with various applications.
 | ||
| Fig. 1 (a) Synthesis of tetrahedral cage 2. (b) Graphical representation of the polycatenanes formed upon the addition of excess crown ether 3 into cage 2. | ||
The exploration of synthetic chemical systems that respond to different stimuli can offer insight into the signalling events and new means to synthesize useful stimuli-responsive systems and materials. Nitschke, Schalley and Sanders et al. reported NDI- and porphyrin-based mixed-ligand tetrahedral cages and studied their stimulus-responsive behaviour upon catenation and guest encapsulation.13 As shown in Fig. 2a, two different cages, 5 and 7, based on NDI and porphyrin, respectively, were readily obtained through subcomponent self-assembly. The crystal structures of 5 and 7 confirmed their tetrahedral structure, and the NDI ligand and the porphyrin ligand showed almost the same length (18.4 Å), suggesting the possibility of ligand exchange upon mixing of the two cages. In addition, interactions between the NDI and porphyrin cages with crown ether 3 and C70 were investigated (Fig. 2b). The results indicated that at most two equivalents of crown ethers 3 could thread onto the NDI moiety in cage 5, which was less than its analogue 2 (at most 6 crown ethers), probably due to the steric strain between the adjacent NDI moieties resulting from the smaller space in cage 5. In addition, 5 showed no host–guest interaction with C70 due to the existence of large pores in its external surface, whereas porphyrin cage 7 could form a host–guest complex with C70. Furthermore, in order to investigate the structural reconstitutions through catenation and guest encapsulation, NDI-based cage 5 and porphyrin-containing cage 7 were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 25 °C for 24 h and a dynamic combinatorial library (DCL) of mixed-ligand tetrahedral cages was obtained (Fig. 2c). Because the species in the DCL had similar ESI-response factors, ESI-MS could be used to evaluate the content of each species. The results indicated that upon simple mixing, various species were observed, and species 4462 and 4165 (the subscripts indicate the number of ligands in one cage) were in the majority, which deviated from the principle of Pascal's triangle, revealing that minor differences in the ligand will largely affect the final results of self-assembly and that the ligands tend to form homoleptic assemblies through self-sorting. In addition, upon addition of C70 into the DCL, the final composition was almost complete for 5 and C70⊂7 species because the host–guest adduct C70⊂7 was thermodynamically stable. Upon the addition of crown ether 3 into the DCL, 4462 and 4165 appeared with the highest abundance to meet the requirement of the maximum degree of catenation, thus maximizing the number of π-interactions between NDI and naphthalene. This work constructed an elaborate dynamic system that showed a response to catenation and host–guest encapsulation, which was helpful for understanding the stimulus-responsive behaviours of biological systems.
1 ratio at 25 °C for 24 h and a dynamic combinatorial library (DCL) of mixed-ligand tetrahedral cages was obtained (Fig. 2c). Because the species in the DCL had similar ESI-response factors, ESI-MS could be used to evaluate the content of each species. The results indicated that upon simple mixing, various species were observed, and species 4462 and 4165 (the subscripts indicate the number of ligands in one cage) were in the majority, which deviated from the principle of Pascal's triangle, revealing that minor differences in the ligand will largely affect the final results of self-assembly and that the ligands tend to form homoleptic assemblies through self-sorting. In addition, upon addition of C70 into the DCL, the final composition was almost complete for 5 and C70⊂7 species because the host–guest adduct C70⊂7 was thermodynamically stable. Upon the addition of crown ether 3 into the DCL, 4462 and 4165 appeared with the highest abundance to meet the requirement of the maximum degree of catenation, thus maximizing the number of π-interactions between NDI and naphthalene. This work constructed an elaborate dynamic system that showed a response to catenation and host–guest encapsulation, which was helpful for understanding the stimulus-responsive behaviours of biological systems.
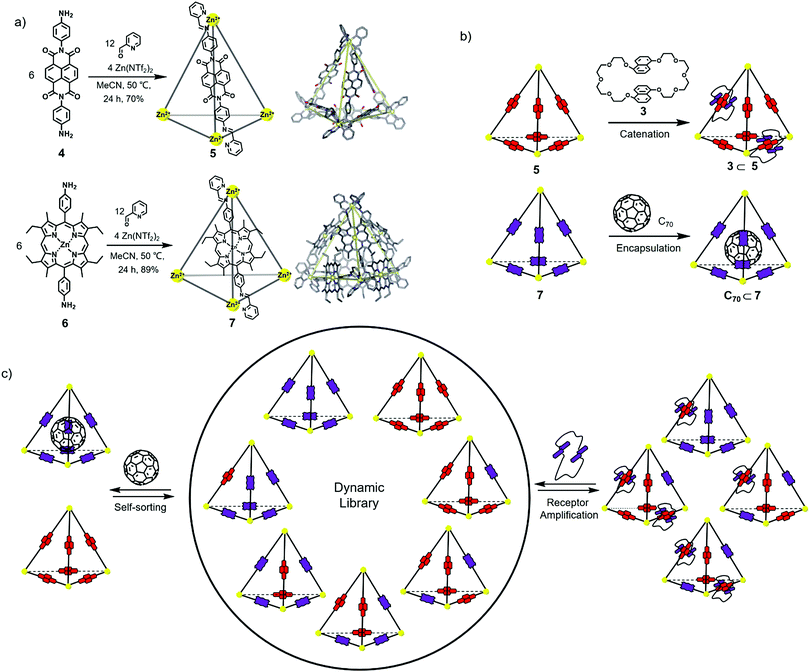 | ||
Fig. 2 (a) Synthesis of tetrahedral cages 5 and 7 and their crystal structures. (b) Host–guest behaviour of cages 5 and 7 upon the addition of 3 and C70, respectively. (c) Schematic representation of the different templation effects observed in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DCL formed upon mixing of 5 and 7. Reproduced from ref. 13 with permission from Royal Society of Chemistry, copyright 2016. 1 DCL formed upon mixing of 5 and 7. Reproduced from ref. 13 with permission from Royal Society of Chemistry, copyright 2016. | ||
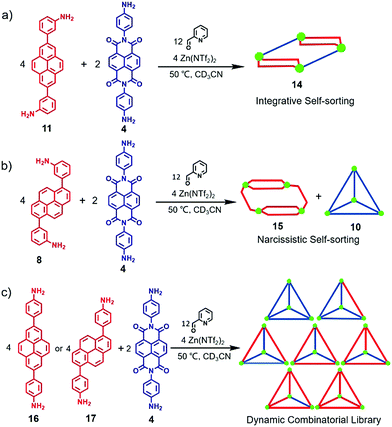
In 2015, Nitschke and co-workers reported the construction of an NDI-based heteroleptic metal–organic cage through the donor–acceptor interaction of pyrene and NDI.14 As shown in Fig. 3, reaction of the pyrene-containing diamine 8 or 11, 2-formylpyridine, and metal ions (Fe2+, Zn2+ or Co2+) in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 afforded two MII4L6 type cages, 9 and 12, respectively. X-ray diffraction analysis indicated that cage 9 showed a C2-symmetric rhomboidal structure with mer metal coordination and that cage 12 showed a C3-symmetric pseudotetrahedron with fac metal coordination. These structures were obviously different from the common tetrahedral MII4L6 cages, which was ascribed to the π-stacking of pyrene subunits. In addition, the reaction of pyrene-containing diamine 11, 2-formylpyridine and metal ions (Zn2+ or Co2+) in a ratio of 6
4 afforded two MII4L6 type cages, 9 and 12, respectively. X-ray diffraction analysis indicated that cage 9 showed a C2-symmetric rhomboidal structure with mer metal coordination and that cage 12 showed a C3-symmetric pseudotetrahedron with fac metal coordination. These structures were obviously different from the common tetrahedral MII4L6 cages, which was ascribed to the π-stacking of pyrene subunits. In addition, the reaction of pyrene-containing diamine 11, 2-formylpyridine and metal ions (Zn2+ or Co2+) in a ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 provided a box-like structure 13 that could encapsulate electron-deficient guests such as NDI, which inspired the authors to construct heteroleptic cages through donor–acceptor interactions. By mixing box 13 with additional NDI-based diamine 4 or the reaction of pyrene-containing diamine 11, NDI-based diamine 4, 2-formylpyridine and metal ions (Fe2+, Zn2+ or Co2+) in a ratio of 4
6 provided a box-like structure 13 that could encapsulate electron-deficient guests such as NDI, which inspired the authors to construct heteroleptic cages through donor–acceptor interactions. By mixing box 13 with additional NDI-based diamine 4 or the reaction of pyrene-containing diamine 11, NDI-based diamine 4, 2-formylpyridine and metal ions (Fe2+, Zn2+ or Co2+) in a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 afforded heteroleptic cage 14. X-ray diffraction analysis indicated that cage 14 adopted an elongated sandwich-like structure due to the internal pyrene–pyrene–NDI stacks. This donor–donor–acceptor arrangement was complementary to the common donor–acceptor interactions. Further self-sorting experiments were performed to investigate the universality of the construction of heteroleptic cages. As shown in Fig. 4, due to the thermodynamic stability of 14, it was readily obtained via integrative self-sorting. However, a mixture of 8 and 4, 2-formylpyridine and Zn(NTf2)2 in CD3CN resulted in homoleptic cages Zn-15 and Zn-10, which revealed that the process was narcissistic self-sorting. In addition, by treatment of 16 (or 17) and 4 under the same conditions, a mixture of seven assemblies was observed, and a dynamic combinatorial library (DCL) was formed (similar to the results by mixing cages 5 and 7 without templation effects), which was ascribed to the absence of π-stacking between pyrene and NDI units. In conclusion, an NDI-based heteroleptic cage was successfully constructed through donor–acceptor interactions, which provided a new method to construct metal–organic cages with more complexity.
4 afforded heteroleptic cage 14. X-ray diffraction analysis indicated that cage 14 adopted an elongated sandwich-like structure due to the internal pyrene–pyrene–NDI stacks. This donor–donor–acceptor arrangement was complementary to the common donor–acceptor interactions. Further self-sorting experiments were performed to investigate the universality of the construction of heteroleptic cages. As shown in Fig. 4, due to the thermodynamic stability of 14, it was readily obtained via integrative self-sorting. However, a mixture of 8 and 4, 2-formylpyridine and Zn(NTf2)2 in CD3CN resulted in homoleptic cages Zn-15 and Zn-10, which revealed that the process was narcissistic self-sorting. In addition, by treatment of 16 (or 17) and 4 under the same conditions, a mixture of seven assemblies was observed, and a dynamic combinatorial library (DCL) was formed (similar to the results by mixing cages 5 and 7 without templation effects), which was ascribed to the absence of π-stacking between pyrene and NDI units. In conclusion, an NDI-based heteroleptic cage was successfully constructed through donor–acceptor interactions, which provided a new method to construct metal–organic cages with more complexity.
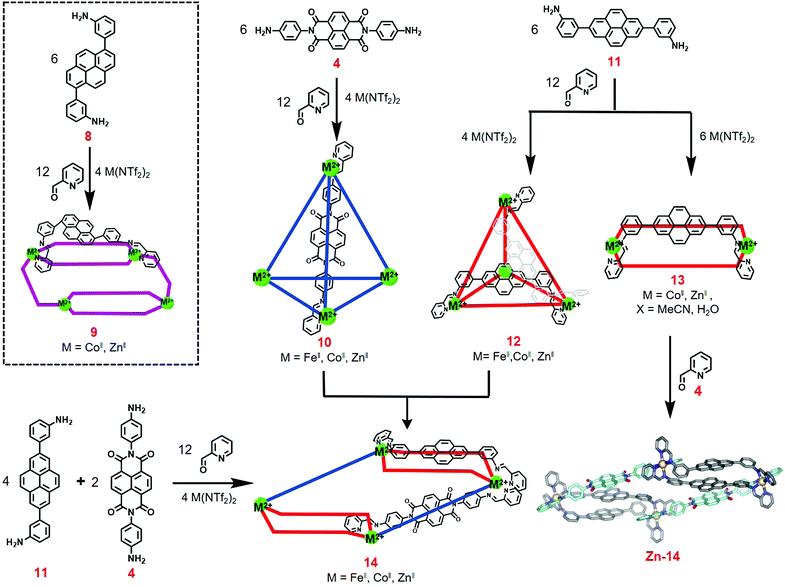 | ||
| Fig. 3 Synthesis of cages 9, 10, 12, 13, and 14 and crystal structure of cage Zn-14. Reproduced from ref. 14 with permission from American Chemical Society, copyright 2015. | ||
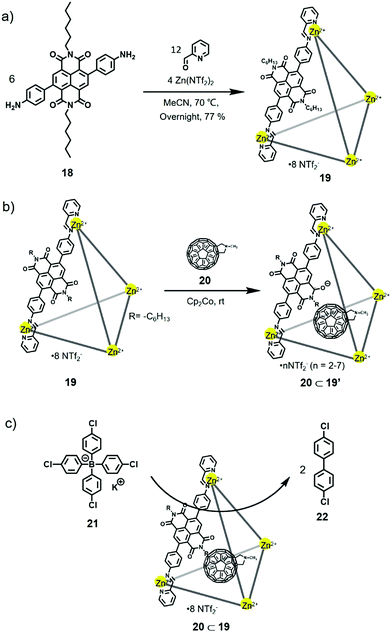
Supramolecular catalysts, such as metal coordinated cages, have been widely applied in molecular synthesis.15 In 2019, Nitschke et al. reported the synthesis of an NDI-based tetrahedral cage with redox-switchable behaviour and could serve as a catalyst for C–C bond coupling.16 As shown in Fig. 5, treating NDI-containing diamine 18 and 2-formylpyridine with Zn(NTf2)2 in acetonitrile afforded NDI cage 19 with a yield of 77%. NDI cage 19 was well characterized by NMR and ESI-MS spectra. Furthermore, cyclic voltammetry (CV) experiments were conducted to investigate the redox properties of 19, and the results indicated that 19 showed two reduction peaks at −1.1 V and −1.52 V vs. Fc/Fc+, corresponding to the NDI moieties. In addition, upon the addition of cobaltocene (Cp2Co) and tetrachloro-1,4-benzoquinone, the cage could be reversibly reduced and re-oxidized without disturbing the cage's framework. 1H NMR spectra indicated that the cage could form a host–guest complex with C60 derivative 20. Moreover, tetrakis(4-chlorophenyl)borate 21 was used in place of Cp2Co as the reductant, and the 1H NMR and EPR spectra revealed the generation of radical species. Interestingly, the formation of 4,4′-dichlorobiphenyl was also observed during the reduction, which inspired the authors to investigate the cage as a catalyst for the C–C bond coupling of organoborates. Further experiments indicated that the host–guest complex 20⊂19 exhibited a two-fold increase in yield during the catalytic process relative to cage 19 alone, and the control compound, such as the NDI ligand, showed no catalysis. In addition, complex 20⊂19 could catalyse the coupling of organoborates with both electron-withdrawing and electron-donating groups, which was complementary to common coupling catalysis. EPR experiments were performed to investigate the catalytic mechanism, and the results indicated that C60 derivative 20 could serve as a radical-stabilizing agent to stabilize the radical species of the cage. Thus, the catalytic performance occurred on the surface of the cage, and C60 acted as a co-catalyst to enhance the catalytic activity for the oxidative coupling of organoborates, which afforded a new view into supramolecular catalysis.
Molecular sensing plays an important role in supramolecular chemistry. In 2020, Valiyaveettil et al. reported the construction of a NDI-based water-soluble macrocycle which contains quaternary ammonium groups.8i The macrocycle exhibited high affinity for aromatic carboxylate anions such as perylene tetracarboxylate tetrapotassium salt (Ksv = 1.6 × 105 M−1) and biphenyl-4,4′-dicarboxylic acid dipotassium salt (Ksv = 4.3 × 104 M−1), due to the electrostatic interactions between cationic macrocycle and anionic guests, size matching effect and the interaction between electron-deficient NDI units and aromatic perylene. Considering the redox-active properties of NDI, at the same year, Nitschke and co-workers reported the synthesis of a NDI-based cage with redox-responsive guest uptake and release behaviour.17 Notably, the addition of excess reductant Cp2Co into cage 19 led to the formation of precipitation resulting from charge neutralization (the negatively charged NDI panels were compensable to the cationic charges of Zn2+ vertices), which hampered the further study in solution. Thus, quaternary ammonium-functionalized NDI ligand 23 in place of ligand 18 was prepared to construct the new NDI cage 24 (Fig. 6). X-ray crystallographic analysis indicated that the cage adopted the typical tetrahedral structure, and three carborate anions were encapsulated in the cage due to the multiple positive charges of 24. CV experiments indicated that cage 24 showed two reduction peaks at −0.81 V and −1.19 V vs. Fc/Fc+ and two oxidation peaks at −1.29 V and −0.78 V. It should be noted that the reduction peaks were reversible, while they were irreversible for the NDI ligand 23, indicating the self-assembled cages enhanced the robustness of NDI units under redox process. Thus, the cage could be reversibly reduced and re-oxidized upon the addition of Cp2Co and AgNTf2. In addition, 1H NMR experiments demonstrated that cage 24 could bind only anion species such as BF4−, PhBF3− and CB11H12− instead of neutral species due to its multiple positive charges. Furthermore, the redox-responsive guest uptake and release behaviour of the cage was investigated, and the results indicated that the guest PhBF3− was ejected from the cage upon reduction with Cp2Co, while with the subsequent addition of AgNTf2, the guest could be re-taken up (Fig. 6b). It should be noted that cage 24 exhibited a suitable volume for binding with fullerenes, which was similar to the analogous polyaromatic cages.1n Thus, interestingly, the reduced form of cage 24 showed obvious affinity for C60, probably due to the partially neutralized charges upon reduction (Fig. 6c). Further addition of AgNTf2 into the complex led to the release of C60, which required more than 30 days due to the kinetically trapped nature of the complex. Moreover, when the C60⊂24 complex was treated in MeCN/EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with aqueous Na2SO4, cage 24 was transferred into the water phase, and C60 was released, which could be applied in chemical purification due to its simplicity and recyclability. In conclusion, this research realized redox-switchable guest uptake and release behaviour within an NDI cage, which could be helpful for designing supramolecular systems for chemical separations and transport.
1) with aqueous Na2SO4, cage 24 was transferred into the water phase, and C60 was released, which could be applied in chemical purification due to its simplicity and recyclability. In conclusion, this research realized redox-switchable guest uptake and release behaviour within an NDI cage, which could be helpful for designing supramolecular systems for chemical separations and transport.
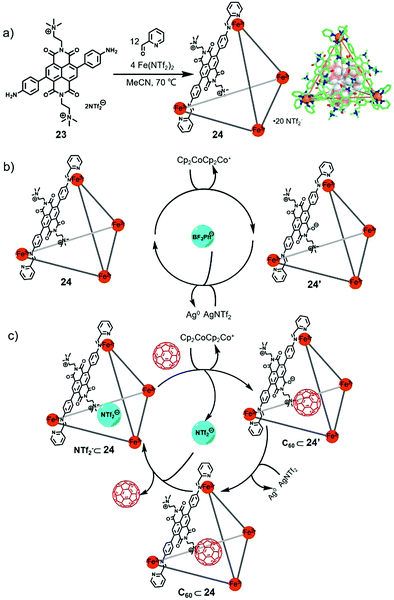 | ||
| Fig. 6 (a) Synthesis of NDI-based tetrahedral cage 24 and its crystal structure. (b) Redox control of KBF3Ph binding within 24. (c) The encapsulation and release of C60 within cage 24 upon reduction with Cp2Co and subsequent re-oxidation with AgNTf2. Reproduced from ref. 17 with permission from Royal Society of Chemistry, copyright 2020. | ||
In 2018, Chand and co-workers reported the preparation of an NDI-based Pd2L4-type metal–organic cage, which can form metallogels with multi-stimulus-responsive properties and anionic dye removal ability.18 As shown in Fig. 7, NDI-containing ligand 25 reacted with Pd(NO3)2 in DMSO or MeCN/H2O to afford cylindrical-drum-shaped NDI cage 26. Interestingly, adding cage 26 in DMSO or MeCN/H2O led to the formation of gels, and the concentration, solvent, counteranion and metal ions had a considerable impact on the formation of gels. UV-Vis spectral studies showed a hyperchromic shift with increasing concentrations, probably due to the aggregation of cage 26 in solution. Rheology studies confirmed the formation of gels and revealed that the strength of the gel increased with increasing temperature or concentration. In addition, the gel also exhibited multi-stimulus-responsive properties including a thixotropic nature and chemical-stimulus-responsive behaviour. For example, upon vigorous shaking or addition of TBABr or DMAP, the metallogel changed into a solution state and could be recovered upon subsequent standing or addition of AgNO3 or TsOH, indicating that the stimulus-responsive property was reversible (Fig. 7b). Moreover, UV-Vis spectral experiments indicated that the gel could uptake pyrene due to the electron-deficient nature of the NDI ligand and the suitable cavity of the cage. Furthermore, as shown in Fig. 7c, the gel could be used for dye removal, and the results showed that the gel could efficiently remove anionic dyes instead of cationic dyes due to the positive charge character of the NDI cage.
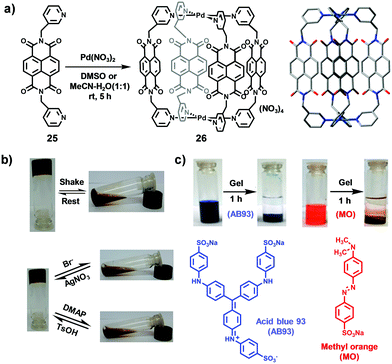 | ||
| Fig. 7 (a) Synthesis of NDI-based cage 26 and its optimized structure. (b) Stimulus-responsive behaviour of the metallogel upon shaking/resting and the addition of TBABr/AgNO3 or DMAP/TsOH pairs. (c) Removal of anion dyes AB93 and MO with the metallogel. Reproduced from ref. 18 with permission from American Chemical Society, copyright 2018. | ||
3. Perylene diimide-based cages
Relative to the NDI derivatives, perylene diimide (PDI) derivatives have a larger planar structure and π-conjugation and intense absorption in the visible region. In general, PDI derivatives exhibit two reversible one-electron reduction with half-wave potentials of −0.96 V and −1.22 V vs. Fc/Fc+ in CH2Cl2.6d Compared with a large number of reported PDI-based macrocycles,8 PDI-based cages have rarely been investigated. Herein, we introduce some PDI-based cages, including metal-coordinated and covalent cages, and the photochemical and electrochemical properties of the cages as well as their applications in molecular sensing and photocatalysis.Würthner and co-workers reported several PDI-based metal–organic cages. In 2013, they reported the preparation of giant tetrahedral PDI cages and investigated their photo- and electrochemical properties and host–guest behaviour.19 As shown in Fig. 8, treatment of the bipyridine-functionalized PDI ligand 27 with FeX2 (X = BF4−, OTf−) in a solution of CH3CN/CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) provided tetrahedral cages 28–29. The cages were well characterized by 1H NMR, 2D DOSY and ESI-MS spectra. Furthermore, UV-vis spectroscopy and cyclic voltammetry (CV) were performed to investigate the photo- and electrochemical properties of the cages. UV-Vis spectral studies indicated that cages 28–29 exhibited two main absorption bands at 542 and 580 nm in the visible region (corresponding to the PDI subunits) with molar absorption coefficients of 212
1) provided tetrahedral cages 28–29. The cages were well characterized by 1H NMR, 2D DOSY and ESI-MS spectra. Furthermore, UV-vis spectroscopy and cyclic voltammetry (CV) were performed to investigate the photo- and electrochemical properties of the cages. UV-Vis spectral studies indicated that cages 28–29 exhibited two main absorption bands at 542 and 580 nm in the visible region (corresponding to the PDI subunits) with molar absorption coefficients of 212![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and 268
700 and 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1, respectively, almost six times as high as the PDI ligand 27. In addition, the peak of the bipyridine units at 297 nm showed a redshift of approximately 20 nm, and the relative intensities of the peaks at 542 and 580 nm also varied due to the metal-to-ligand charge-transfer of [Fe(bipyridine)3]2+ complexes (Fig. 8b). CV experiments indicated that the cages showed two reversible oxidation waves at 0.95 (PBI/PBI+) and 0.73 V (Fe2+/Fe3+) vs. Fc/Fc+ and five reversible reduction waves at −0.94 (PBI/PBI−), −1.11 (PBI−/PBI2−), −1.65, −1.84 and −2.08 V (bpy/bpy−) vs. Fc/Fc+. The higher oxidation potential of Fe2+/Fe3+ was probably due to the Fe2+ metal centre being close to the electron-deficient PDI moieties, and the easier reduction of cage 28 could be ascribed to the preorganized framework and highly porous nature of the PDI cage. In addition, the counteranions (BF4− or OTf−) had a negligible influence on the photo- and electrochemical properties of the cages. Moreover, the results of 13C NMR and ESI-TOF-MS spectra indicated that the cage could efficiently encapsulate C60, and the UV-Vis absorption spectra suggested that each cage bound 1.9 C60 on average. Considering the volume of C60 (597 Å3) and the PDI cage (950–2150 Å3 due to the rotatable OPhtBu substituents), the host–guest behaviour of the cage is consistent with the principle of the empirical 55% rule established by Mecozzi and Rebek.20 Additionally, the results of molecular stimulations indicated that when the cage bound one C60, C60 was located in almost the centre of the cavity, and there was no interaction between the cage and C60 (Fig. 8c). However, when the cage bound two C60, the guests were located in the corner of the cage to afford enough space for binding both guests. Notably, 28 or 29 displayed the ability of binding two C60 in one cage, which was different from the common polyaromatic cages1n that could only bind one C60, probably due to its larger internal cavity. This research successfully constructed PDI cages with affinity for C60, which afforded a guideline for designing functional cages for guest encapsulation and reaction within the cavity.
500 M−1 cm−1, respectively, almost six times as high as the PDI ligand 27. In addition, the peak of the bipyridine units at 297 nm showed a redshift of approximately 20 nm, and the relative intensities of the peaks at 542 and 580 nm also varied due to the metal-to-ligand charge-transfer of [Fe(bipyridine)3]2+ complexes (Fig. 8b). CV experiments indicated that the cages showed two reversible oxidation waves at 0.95 (PBI/PBI+) and 0.73 V (Fe2+/Fe3+) vs. Fc/Fc+ and five reversible reduction waves at −0.94 (PBI/PBI−), −1.11 (PBI−/PBI2−), −1.65, −1.84 and −2.08 V (bpy/bpy−) vs. Fc/Fc+. The higher oxidation potential of Fe2+/Fe3+ was probably due to the Fe2+ metal centre being close to the electron-deficient PDI moieties, and the easier reduction of cage 28 could be ascribed to the preorganized framework and highly porous nature of the PDI cage. In addition, the counteranions (BF4− or OTf−) had a negligible influence on the photo- and electrochemical properties of the cages. Moreover, the results of 13C NMR and ESI-TOF-MS spectra indicated that the cage could efficiently encapsulate C60, and the UV-Vis absorption spectra suggested that each cage bound 1.9 C60 on average. Considering the volume of C60 (597 Å3) and the PDI cage (950–2150 Å3 due to the rotatable OPhtBu substituents), the host–guest behaviour of the cage is consistent with the principle of the empirical 55% rule established by Mecozzi and Rebek.20 Additionally, the results of molecular stimulations indicated that when the cage bound one C60, C60 was located in almost the centre of the cavity, and there was no interaction between the cage and C60 (Fig. 8c). However, when the cage bound two C60, the guests were located in the corner of the cage to afford enough space for binding both guests. Notably, 28 or 29 displayed the ability of binding two C60 in one cage, which was different from the common polyaromatic cages1n that could only bind one C60, probably due to its larger internal cavity. This research successfully constructed PDI cages with affinity for C60, which afforded a guideline for designing functional cages for guest encapsulation and reaction within the cavity.
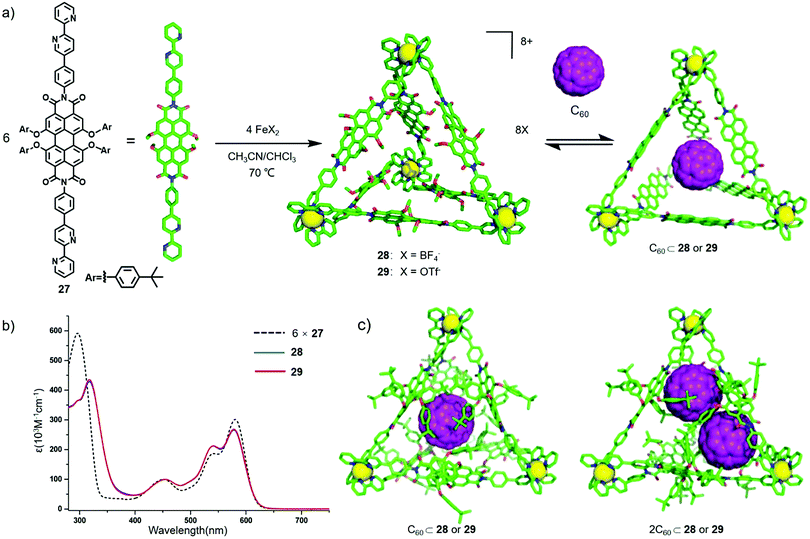 | ||
| Fig. 8 (a) Synthesis of PDI-based tetrahedral cages 28–29 and graphical representation of the complexation of 28–29 with C60. (b) UV-Vis absorption spectrum of 27–29. (c) Optimized structure of C60⊂28 or 29 and 2C60⊂28 or 29. Reproduced from ref. 19 with permission from American Chemical Society, copyright 2013. | ||
Even though PDI cages 28–29 showed efficient bonding with C60, the cages were non-emissive because the coordinated Fe metal atom facilitated intersystem crossing (ISC) and subsequent non-radiative decay, which hampered their fluorescence sensing.21 Hence, Würthner and co-workers reported the synthesis of a highly fluorescent tetrahedral PDI cage and studied its host–guest behaviour in 2015.22 As shown in Fig. 9, the reaction of PDI-containing ligand 30, Zn(NTf2)2 and tris(2-aminoethyl)amine (TREN) under microwave heating at 100 °C provided tetrahedral PDI cage 31 in 92% yield. It should be noted that Zn was vital for the formation of the fluorescent cage. Cage 31 was well characterized by 1H NMR, 2D NMR and ESI-MS spectra. Variable-temperature 1H NMR experiments showed a diastereomeric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 for the T, S4, and C3 diastereomers of the cage. 2D DOSY NMR indicated that the hydrodynamic radius of cage 31 was 2.2 nm, in good agreement with the result from theoretical simulations (1.9 nm). In addition, the results of the MMFF geometry optimization model indicated that the length of the edge of cage 31 was 3.8 nm and the internal volume was approximately 1300 Å3 (Fig. 9b). Further investigations on the photophysical properties revealed that 31 showed two main absorption bands at 488 nm and 523 nm corresponding to the absorption of PDI units and two emission peaks at 538 and 578 nm with a quantum yield of 0.67 ± 0.02 (Fig. 9c). The decrease in the quantum yield relative to that of the PDI ligand (almost unity) was probably due to excitonic coupling between the adjacent PDI chromophore. The fluorescence lifetime of cage 31 was also calculated and determined to be 4.8 ns. Furthermore, the host–guest behaviour of the cage was studied, and 1H NMR titrations indicated that cage 31 could form a 1
0.7 for the T, S4, and C3 diastereomers of the cage. 2D DOSY NMR indicated that the hydrodynamic radius of cage 31 was 2.2 nm, in good agreement with the result from theoretical simulations (1.9 nm). In addition, the results of the MMFF geometry optimization model indicated that the length of the edge of cage 31 was 3.8 nm and the internal volume was approximately 1300 Å3 (Fig. 9b). Further investigations on the photophysical properties revealed that 31 showed two main absorption bands at 488 nm and 523 nm corresponding to the absorption of PDI units and two emission peaks at 538 and 578 nm with a quantum yield of 0.67 ± 0.02 (Fig. 9c). The decrease in the quantum yield relative to that of the PDI ligand (almost unity) was probably due to excitonic coupling between the adjacent PDI chromophore. The fluorescence lifetime of cage 31 was also calculated and determined to be 4.8 ns. Furthermore, the host–guest behaviour of the cage was studied, and 1H NMR titrations indicated that cage 31 could form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 complex with perylene or a 1
3 complex with perylene or a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with coronene (Fig. 9d and e). Furthermore, the host–guest complexation induced a decrease in the absorption and emission intensity of 31, probably due to the photoinduced charge transfer from the PDI units to the guests (Fig. 9f and g). In addition, the phenomenon of amplified emission quenching (one guest caused the quenching of 2.1 PBI units) could be ascribed to the close contact of the cage and guests. In conclusion, this work realized fluorescence sensing based on a highly emissive PDI cage, which laid the foundation for constructing fluorescent self-assembly hosts for molecular recognition.
2 complex with coronene (Fig. 9d and e). Furthermore, the host–guest complexation induced a decrease in the absorption and emission intensity of 31, probably due to the photoinduced charge transfer from the PDI units to the guests (Fig. 9f and g). In addition, the phenomenon of amplified emission quenching (one guest caused the quenching of 2.1 PBI units) could be ascribed to the close contact of the cage and guests. In conclusion, this work realized fluorescence sensing based on a highly emissive PDI cage, which laid the foundation for constructing fluorescent self-assembly hosts for molecular recognition.
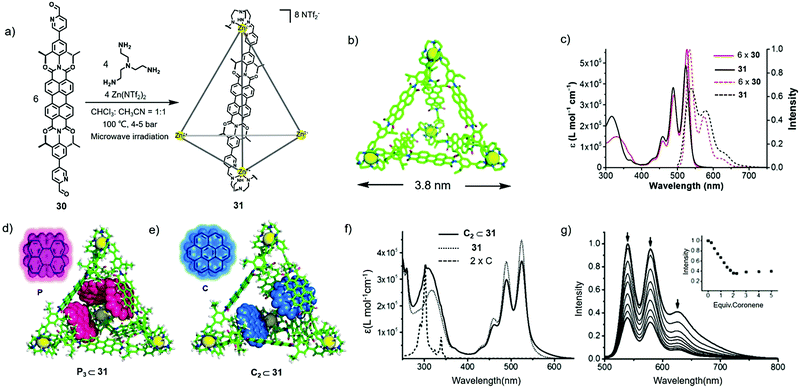 | ||
| Fig. 9 (a) Synthesis of PDI-based tetrahedral cage 31. (b) Optimized structure of cage 31. (c) Absorption and emission spectra of 30 and 31 in CH3CN (λex = 490 nm). (d) Optimized structure of the host–guest complex P3⊂31 (P = perylene). (e) Optimized structure of the host–guest complex C2⊂31 (C = coronene). (f) Absorption spectrum of 31, C and C2⊂31. (g) Emission spectra upon the addition of C into 31. Reproduced from ref. 22 with permission from Wiley-VCH, copyright 2015. | ||
Similarly, in 2015, Würthner and co-workers reported the synthesis of a large PDI-based tetrahedron and investigated its supramolecular transformation at various concentrations.23 As shown in Fig. 10, PDI cage 32 was obtained via subcomponent self-assembly of PDI-containing ligand 30, p-anisidine with Zn(NTf2)2 under microwave conditions at 100 °C. The NMR, ESI-MS and atomic force microscopy (AFM) results confirmed the formation of the M4L6-type tetrahedral cage. However, the concentration had a great influence on the structure of the self-assembly product. 1H NMR and UV/Vis spectral studies indicated that the initial M4L6 tetrahedron disassembles into an M2L3-type helicate with some fragments when the concentration was below 8.7 × 10−6 mol L−1. In addition, similar to 31, the cage also showed fluorescence upon Zn coordination.
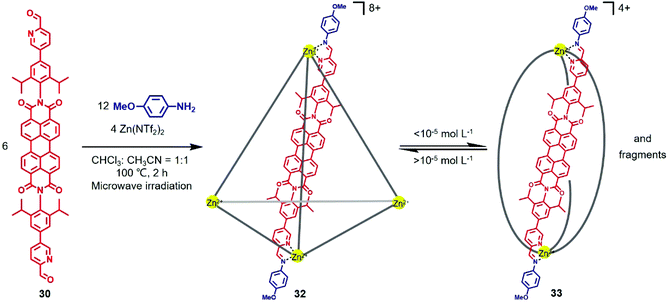 | ||
| Fig. 10 Synthesis of PDI-based tetrahedral cage 32 and its concentration-dependent supramolecular transformation. | ||
In 2019, Zhang et al. reported the preparation of chiral imine cages based on PDI and investigated their host–guest and photocatalytic behaviour.24 As shown in Fig. 11, treatment of PDI-containing diamine 34 (R or S) and tetraphenylethene (TPE)-containing tetraaldehyde 35 provided PDI-TPE cage 36 (R or S). The cages were well characterized by 1H NMR, 2D NMR and ESI-MS spectra. Circular dichroism (CD) spectra of the two cages displayed signals with complete mirror symmetry, demonstrating that the cages were enantiomeric. UV-Vis absorption of cage R-36 exhibited a maximum absorption peak at 526 nm, consistent with the PDI ligand, revealing no interaction between the PDI units in the cage. The fluorescence spectra of cage R-36 indicated that J-type aggregation occurred at higher concentrations resulting from charge transfer between TPE and PDI units. In addition, CV experiments of the cage showed two reduction peaks at −0.84 V and −1.05 V (vs. SCE), which were assigned to the formation of PDI*− and PDI2−, respectively. Furthermore, the host–guest behaviour of cages with polycyclic aromatic hydrocarbons (PAHs), such as perylene and naphthalene, was investigated. The fluorescence of the guests declined dramatically upon the addition of cage R-36, probably due to the aromatic π–π stacking interaction between the planar PAHs and PDI subunits (Fig. 11c). Fluorescence titration experiments indicated that the cage could bind 18–22 small PAH molecules, such as naphthalene and pyrene, or 8–10 larger PAH molecules, such as perylene and 9,10-diphenylanthracene. In addition, the fluorescence of the cage itself was enhanced upon guest binding because a large number of guests occupied the space of the cage and decreased the structural relaxation of the PDI units. Since cage R-36 had strong absorption in the visible range and could efficiently encapsulate a series of guest molecules, it could further serve as a visible-light photocatalyst. The results indicated that cage R-36 could efficiently transform 2-aryloxybenzoic acids 37 into aryl salicylates 38 (Smiles rearrangement) under a blue LED (Fig. 11d). The higher catalytic efficiency of the cage relative to the PDI ligand was ascribed to intermolecular photoinduced electron transfer from TPE to PDI units, indicating the importance of the existence of the imine cage. Moreover, different substituents on the substrate had an important effect on the catalytic activity, and an electron-donating group in the ortho position in 37 could improve the yield. In conclusion, this research constructed a PDI-based imine cage for visible-light photocatalysis, affording a guideline for designing photoactive supramolecular hosts for photochemical reactions.
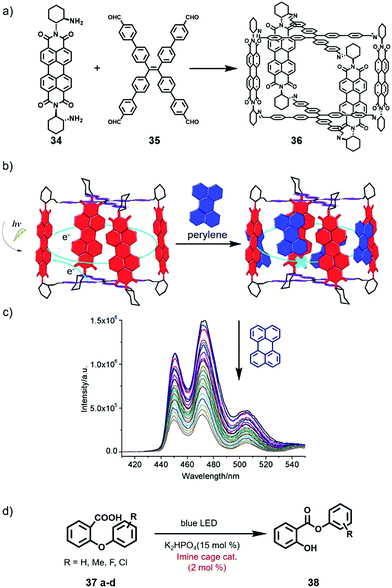 | ||
| Fig. 11 (a) Synthesis of PDI-based imine cage 36 from PDI-containing diamine 34 and TPE-containing tetraaldehyde 35. (b) The host–guest behaviour of cage R-36 with perylene. (c) Emission spectrum of perylene following the titration of R-36. (d) Smiles rearrangement under the photolysis of cage R-36. Reproduced from ref. 24 with permission from Wiley-VCH, copyright 2019. | ||
4. Conclusions
NDI-/PDI-based cages, including metal coordination and dynamic covalent cages, which possess different properties and functions, have been investigated during the past few years. First, due to the planar structure and electron-deficient nature of NDI/PDI, the cages could bind various electron-rich guests, which were further applied in molecular sensing and constructing a dynamic combinatorial library (DCL). Second, the cages with redox-switchable properties resulting from NDI/PDI units showed unique functions, including controllable uptake and release and catalysis for C–C bond coupling. In addition, the NDI/PDI cages could also be applied in dye removal or photocatalysis, indicating their large potential. Even so, there are still some issues that need to be addressed in this field. For example, the types of catalytic reaction of the NDI/PDI cages are very limited. On the other hand, due to the utilization of Fe2+ as vertices for constructing metal coordination cages, most of the NDI/PDI cages were non-fluorescent even though NDI/PDI ligands were highly emissive, which hampered their development of fluorescent materials.Thus, in our view, some improvement should be taken into consideration in further developing NDI/PDI cages. On one hand, NDI/PDI ligands should be designed reasonably. According to some unpublished results in our group, we could make use of NDI/PDI ligands with larger π-conjugation to construct cages that bind more sophisticated guests, thus further extending their catalytic reactions. On the other hand, more kinds of metals should be utilized to construct cages with various shapes and topological architectures. This may not only prevent the fluorescence quenching of NDI/PDI ligands to some extent but also extend their applications, such as photocatalysis. In conclusion, NDI-/PDI-based cages will be considered to play more important roles in a wide range of fields, including supramolecular chemistry and materials science, in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 21922506, 21871092, and 21672070), the Fundamental Research Funds for the Central Universities, the Opening Projects of Shanghai Key Laboratory of Green Chemistry and Chemical Processes, and State Key Laboratory of Fine Chemicals (KF 1801).Notes and references
- (a) J.-M. Lehn, Cryptates: inclusion complexes of macropolycyclic receptor molecules, Pure Appl. Chem., 1978, 50, 871–892 CAS; (b) S. Tashiro and M. Shionoya, Novel Porous Crystals with Macrocycle-Based Well-Defined Molecular Recognition Sites, Acc. Chem. Res., 2020, 53, 632–643 CrossRef CAS; (c) X. Ji, M. Ahmed, L. Long, N. M. Khashab, F. Huang and J. L. Sessler, Adhesive supramolecular polymeric materials constructed from macrocycle-based host–guest interactions, Chem. Soc. Rev., 2019, 48, 2682–2697 RSC; (d) P. S. Bols and H. L. Anderson, Template-Directed Synthesis of Molecular Nanorings and Cages, Acc. Chem. Res., 2018, 51, 2083–2092 CrossRef CAS; (e) Y. Jin, Q. Wang, P. Taynton and W. Zhang, Dynamic Covalent Chemistry Approaches Toward Macrocycles, Molecular Cages, and Polymers, Acc. Chem. Res., 2014, 47, 1575–1586 CrossRef CAS; (f) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Stimuli-Responsive Metal–Ligand Assemblies, Chem. Rev., 2015, 115, 7729–7793 CrossRef CAS; (g) M.-X. Wang, Nitrogen and Oxygen Bridged Calixaromatics: Synthesis, Structure, Functionalization, and Molecular Recognition, Acc. Chem. Res., 2012, 45, 182–195 CrossRef CAS; (h) M. Yoshizawa and L. Catti, Bent Anthracene Dimers as Versatile Building Blocks for Supramolecular Capsules, Acc. Chem. Res., 2019, 52, 2392–2404 CrossRef CAS; (i) K. Acharyya and P. S. Mukherjee, Organic Imine Cages: Molecular Marriage and Applications, Angew. Chem., Int. Ed., 2019, 58, 8640–8653 CrossRef CAS; (j) F. Würthner, C.-C. You and C. R. Saha-Möller, Metallosupramolecular squares: from structure to function, Chem. Soc. Rev., 2004, 33, 133–146 RSC; (k) W.-X. Gao, H.-N. Zhang and G.-X. Jin, Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages, Coord. Chem. Rev., 2019, 386, 69–84 CrossRef CAS; (l) H. Zhu, L. Shangguan, B. Shi, G. Yu and F. Huang, Recent progress in macrocyclic amphiphiles and macrocyclic host-based supra-amphiphiles, Mater. Chem. Front., 2018, 2, 2152–2174 RSC; (m) Y. Qin, X. Liu, P.-P. Jia, L. Xu and H.-B. Yang, BODIPY-based macrocycles, Chem. Soc. Rev., 2020, 49, 5678–5703 RSC; (n) M. Yoshizawa and M. Yamashina, Coordination-driven Nanostructures with Polyaromatic Shells, Chem. Lett., 2017, 46, 163–171 CrossRef CAS; (o) K. Yazaki, L. Catti and M. Yoshizawa, Polyaromatic molecular tubes: from strategic synthesis to host functions, Chem. Commun., 2018, 54, 3195–3206 RSC.
- (a) T. Kakuta, T.-A. Yamagishi and T. Ogoshi, Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes, Acc. Chem. Res., 2018, 51, 1656–1666 CrossRef CAS; (b) H. Zhu, J. Liu, B. Shi, H. Wang, Z. Mao, T. Shan and F. Huang, Pillararene-based host–guest recognition facilitated magnetic separation and enrichment of cell membrane proteins, Mater. Chem. Front., 2018, 2, 1475–1480 RSC; (c) Z. Zhang, K. Sun, S. Li and G. Yu, A pillar[5]arene-based molecular grapple of hexafluorophosphate, Chin. Chem. Lett., 2019, 30, 957–960 CrossRef CAS.
- (a) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Coordination assemblies from a Pd(II)-cornered square complex, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS; (b) L.-J. Chen, H.-B. Yang and M. Shionoya, Chiral metallosupramolecular architectures, Chem. Soc. Rev., 2017, 46, 2555–2576 RSC; (c) Z. Zhang, Y. Li, B. Song, Y. Zhang, X. Jiang, M. Wang, R. Tumbleson, C. Liu, P. Wang, X.-Q. Hao, T. Rojas, A. T. Ngo, J. L. Sessler, G. R. Newkome, S. W. Hla and X. Li, Intra- and intermolecular self-assembly of a 20 nm-wide supramolecular hexagonal grid, Nat. Chem., 2020, 12, 468–474 CrossRef CAS; (d) J. Zhu, X. Liu, J. Huang and L. Xu, Our expedition in the construction of fluorescent supramolecular metallacycles, Chin. Chem. Lett., 2019, 30, 1767–1774 CrossRef CAS; (e) Y.-X. Hu, X. Hao, L. Xu, X. Xie, B. Xiong, Z. Hu, H. Sun, G.-Q. Yin, X. Li, H. Peng and H.-B. Yang, Construction of Supramolecular Liquid-Crystalline Metallacycles for Holographic Storage of Colored Images, J. Am. Chem. Soc., 2020, 142, 6285–6294 CrossRef CAS; (f) J.-L. Zhu, L. Xu, Y.-Y. Ren, Y. Zhang, X. Liu, G.-Q. Yin, B. Sun, X. Cao, Z. Chen, X.-L. Zhao, H. Tan, J. Chen, X. Li and H.-B. Yang, Switchable Organoplatinum Metallacycles with High Quantum Yields and Tunable Fluorescence Wavelengths, Nat. Commun., 2019, 10, 4285 CrossRef; (g) M. L. Saha, X. Yan and P. J. Stang, Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and its Applications, Acc. Chem. Res., 2016, 49, 2527–2539 CrossRef CAS; (h) C.-B. Huang, L. Xu, J.-L. Zhu, Y.-X. Wang, B. Sun, X. Li and H.-B. Yang, Real-Time Monitoring the Dynamics of Coordination-Driven Self-Assembly by Fluorescence-Resonance Energy Transfer, J. Am. Chem. Soc., 2017, 139, 9459–9462 CrossRef CAS; (i) L. Ma, T. Yang, Z. Zhang, S. Yin, Z. Song, W. Shi, D. Chu, Y. Zhang and M. Zhang, Cyanostilbene-based near-infrared emissive platinum(II) metallacycles for cancer theranostics, Chin. Chem. Lett., 2019, 30, 1942–1946 CrossRef CAS.
- (a) S. Pullen and G. H. Clever, Mixed-ligand metal–organic frameworks and heteroleptic coordination cages as multifunctional scaffolds—a comparison, Acc. Chem. Res., 2018, 51, 3052–3064 CrossRef CAS; (b) A. M. Castilla, W. J. Ramsay and J. R. Nitschke, Stereochemistry in subcomponent self-assembly, Acc. Chem. Res., 2014, 47, 2063–2073 CrossRef CAS; (c) L.-X. Cai, S.-C. Li, D.-N. Yan, L.-P. Zhou, F. Guo and Q.-F. Sun, Water-Soluble Redox-Active Cage Hosting Polyoxometalates for Selective Desulfurization Catalysis, J. Am. Chem. Soc., 2018, 140, 4869–4876 CrossRef CAS; (d) X. Zhang, X. Dong, W. Lu, D. Luo, X.-W. Zhu, X. Li, X.-P. Zhou and D. Li, Fine-Tuning Apertures of Metal–Organic Cages: Encapsulation of Carbon Dioxide in Solution and Solid State, J. Am. Chem. Soc., 2019, 141, 11621–11627 CrossRef CAS; (e) J. Guo, Y.-Z. Fan, Y.-L. Lu, S.-P. Zheng and C.-Y. Su, Visible-Light Photocatalysis of Asymmetric [2+2] Cycloaddition in Cage-Confined Nanospace Merging Chirality with Triplet-State Photosensitization, Angew. Chem., Int. Ed., 2020, 59, 8661–8669 CrossRef CAS; (f) J. Wei, L. Zhao, C. He, S. Zheng, J. N. H. Reek and C. Duan, Metal–Organic Capsules with NADH Mimics as Switchable Selectivity Regulators for Photocatalytic Transfer Hydrogenation, J. Am. Chem. Soc., 2019, 141, 12707–12716 CrossRef CAS; (g) L. Xu, D. Zhang, T. K. Ronson and J. R. Nitschke, Improved Acid Resistance of a Metal–Organic Cage Enables Cargo Release and Exchange between Hosts, Angew. Chem., Int. Ed., 2020, 59, 7435–7438 CrossRef CAS; (h) L. Zhang, R. Das, C.-T. Li, Y.-Y. Wang, F. E. Hahn, K. Hua, L.-Y. Sun and Y.-F. Han, C 3-Symmetric Assemblies from Trigonal Polycarbene Ligands and MI Ions for the Synthesis of Three-Dimensional Polyimidazolium Cations, Angew. Chem., Int. Ed., 2019, 58, 13360–13364 CrossRef CAS.
- (a) W. Cullen, M. C. Misuraca, C. A. Hunter, N. H. Williams and M. D. Ward, Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage, Nat. Chem., 2016, 8, 231–236 CrossRef CAS; (b) P. Howlader, B. Mondal, P. C. Purba, E. Zangrando and P. S. Mukherjee, Self-assembled Pd(II) barrels as containers for transient merocyanine form and reverse thermochromism of spiropyran, J. Am. Chem. Soc., 2018, 140, 7952–7960 CrossRef CAS; (c) D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Self-assembly of tetravalent Goldberg polyhedra from 144 small components, Nature, 2016, 540, 563–566 CrossRef CAS; (d) Q.-Q. Wang, S. Gonell, S. H. A. M. Leenders, M. Dürr, I. Ivanović-Burmazović and J. N. H. Reek, Self-assembled nanospheres with multiple endohedral binding sites pre-organize catalysts and substrates for highly efficient reactions, Nat. Chem., 2016, 8, 225–230 CrossRef CAS; (e) M. Yamashina, Y. Tanaka, R. Lavendomme, T. K. Ronson, M. Pittelkow and J. R. Nitschke, An antiaromatic-walled nanospace, Nature, 2019, 574, 511–515 CrossRef CAS; (f) K. Li, L.-Y. Zhang, C. Yan, S.-C. Wei, M. Pan, L. Zhang and C.-Y. Su, Stepwise assembly of Pd6(RuL3)8 nanoscale rhombododecahedral metal–organic cages via metalloligand strategy for guest trapping and protection, J. Am. Chem. Soc., 2014, 136, 4456–4459 CrossRef CAS; (g) L. Catti, N. Kishida, T. Kai, M. Akita and M. Yoshizawa, Polyaromatic nanocapsules as photoresponsive hosts in water, Nat. Commun., 2019, 10, 1948 CrossRef; (h) H. Duan, Y. Li, Q. Li, P. Wang, X. Liu, L. Cheng, Y. Yu and L. Cao, Host-Guest Recognition and Fluorescence of a Tetraphenylethene-Based Octacationic Cage, Angew. Chem., Int. Ed., 2020, 59, 10101–10110 CrossRef CAS; (i) Z. Sun, P. Li, S. Xu, Z.-Y. Li, Y. Nomura, Z. Li, X. Liu and S. Zhang, Controlled Hierarchical Self-Assembly of Catenated Cages, J. Am. Chem. Soc., 2020, 142, 10833–10840 CrossRef CAS; (j) Z. Zhang, Z. Zhao, L. Wu, S. Lu, S. Ling, G. Li, L. Xu, L. Ma, Y. Hou, X. Wang, X. Li, G. He, K. Wang, B. Zou and M. Zhang, Emissive Platinum(II) Cages with Reverse Fluorescence Resonance Energy Transfer for Multiple Sensing, J. Am. Chem. Soc., 2020, 142, 2592–2600 CrossRef CAS; (k) D. Zhang, T. K. Ronson, L. Xu and J. R. Nitschke, Transformation Network Culminating in a Heteroleptic Cd6L6L2′ Twisted Trigonal Prism, J. Am. Chem. Soc., 2020, 142, 9152–9157 CrossRef CAS; (l) P. Li, S. Xu, C. Yu, Z.-Y. Li, J. Xu, Z.-M. Li, L. Zou, X. Leng, S. Gao, Z. Liu, X. Liu and S. Zhang, De Novo Construction of Catenanes with Dissymmetric Cages by Space-Discriminative Post-Assembly Modification, Angew. Chem., Int. Ed., 2020, 59, 7113–7121 CrossRef CAS.
- (a) M. A. Kobaisi, S. V. Bhosale, K. Latham, A. M. Raynor and S. V. Bhosale, Functional naphthalene diimides: synthesis, properties, and applications, Chem. Rev., 2016, 116, 11685–11796 CrossRef CAS; (b) S. V. Bhosale, C. H. Janiab and S. J. Langford, Chemistry of naphthalene diimides, Chem. Soc. Rev., 2008, 37, 331–342 RSC; (c) Z. Yang and X. Chen, Semiconducting perylene diimide nanostructure: multifunctional phototheranostic nanoplatform, Acc. Chem. Res., 2019, 52, 1245–1254 CAS; (d) W. Jiang, Y. Li and Z. Wang, Tailor-made rylene arrays for high performance n-channel semiconductors, Acc. Chem. Res., 2014, 47, 3135–3147 CrossRef CAS; (e) J. Li, Y.-H. Hu, C.-W. Ge, H.-G. Gong and X.-K. Gao, The role of halogen bonding in improving OFET performance of a naphthalenediimide derivative, Chin. Chem. Lett., 2018, 29, 423–428 CrossRef CAS; (f) L. Li, J. Wang, M. Chen, Y. Chen, W. Xiao, D. Chen and M. Lin, The impact of vertical π-extension on redox mechanisms of aromatic diimide dyes, Chin. Chem. Lett., 2019, 30, 2254–2258 CrossRef CAS; (g) S.-L. Suraru and F. Würthner, Strategies for the synthesis of functional naphthalene diimides, Angew. Chem., Int. Ed., 2014, 53, 7428–7448 CrossRef CAS.
- (a) Y. Wu, S. Schneider, C. Walter, A. H. Chowdhury, B. Bahrami, H.-C. Wu, Q. Qiao, M. F. Toney and Z. Bao, Fine-tuning semiconducting polymer self-aggregation and crystallinity enables optimal morphology and high-performance printed all-polymer solar cells, J. Am. Chem. Soc., 2020, 142, 392–406 CrossRef CAS; (b) A. Sarkar, T. Behera, R. Sasmal, R. Capelli, C. Empereur-mot, J. Mahato, S. S. Agasti, G. M. Pavan, A. Chowdhury and S. J. George, Cooperative supramolecular block copolymerization for the synthesis of functional axial organic heterostructures, J. Am. Chem. Soc., 2020, 142, 11528–11539 CrossRef CAS; (c) A. L. Jones, J. Jiang and K. S. Schanze, Excitation-wavelength-dependent photoinduced electron transfer in a π-conjugated diblock oligomer, J. Am. Chem. Soc., 2020, 142, 12658–12668 CrossRef CAS; (d) M. Mahl, K. Shoyama, A.-M. Krause, D. Schmid and F. Würthner, Base-assisted imidization: a synthetic method for the introduction of bulky imide substituents to control packing and optical properties of naphthalene and perylene imides, Angew. Chem., Int. Ed., 2020, 59, 13401–13405 CrossRef CAS; (e) A. Zhang, W. Jiang and Z. Wang, Fulvalene-embedded perylene diimide and its stable radical anion, Angew. Chem., Int. Ed., 2020, 59, 752–757 CrossRef CAS; (f) T. A. Barendt, L. Ferreira, I. Marques, V. Félix and P. D. Beer, Anion- and solvent-induced rotary dynamics and sensing in a perylene diimide [3]catenane, J. Am. Chem. Soc., 2017, 139, 9026–9037 CrossRef CAS; (g) L. Guo, Z. Zhang and Y. Tang, Cationic conjugated polymers as signal reporter for label-free assay based on targets-mediated aggregation of perylene diimide quencher, Chin. Chem. Lett., 2018, 29, 305–308 CrossRef CAS; (h) A. I. Oliva, B. Ventura, F. Würthner, A. Camara-Campos, C. A. Hunter, P. Ballester and L. Flamigni, Self-assembly of double-decker cages induced by coordination of perylene bisimide with a trimeric Zn porphyrin: study of the electron transfer dynamics between the two photoactive components, Dalton Trans., 2009, 4023–4037 RSC.
- (a) A. Mizuno, Y. Shuku, R. Suizu, M. M. Matsushita, M. Tsuchiizu, D. R. Mañeru, F. Illas, V. Robert and K. Awaga, Discovery of the K4 structure formed by a triangular π radical anion, J. Am. Chem. Soc., 2015, 137, 7612–7615 CrossRef CAS; (b) L. He, L.-X. Cai, M.-H. Li, G. Zhang, L. Zhou, T. Chen, M.-J. Lin and Q.-F. Sun, Designing highly stable coordination-driven metallacycle for imaging-guided photodynamic cancer theranostics, Chem. Sci., 2020, 11, 7940–7949 RSC; (c) S. K. M. Nalluri, J. Zhou, T. Cheng, Z. Liu, M. T. Nguyen, T. Chen, H. A. Patel, M. D. Krzyaniak, W. A. Goddard III, M. R. Wasielewski and J. F. Stoddart, Discrete dimers of redox-active and fluorescent perylene diimide-based rigid isosceles triangles in the solid state, J. Am. Chem. Soc., 2019, 141, 1290–1303 CrossRef; (d) S. K. M. Nalluri, Z. Liu, Y. Wu, K. R. Hermann, A. Samanta, D. J. Kim, M. D. Krzyaniak, M. R. Wasielewski and J. F. Stoddart, Chiral redox-active Isosceles triangles, J. Am. Chem. Soc., 2016, 138, 5968–5977 CrossRef CAS; (e) T. A. Barendt, W. K. Myers, S. P. Cornes, M. A. Lebedeva, K. Porfyrakis, I. Marques, V. Félix and P. D. Beer, The green box: an electronically versatile perylene diimide macrocyclic host for fullerenes, J. Am. Chem. Soc., 2020, 142, 349–364 CrossRef CAS; (f) M. L. Ball, B. Zhang, Q. Xu, D. W. Paley, V. C. Ritter, F. Ng, M. L. Steigerwald and C. Nuckolls, Influence of molecular conformation on electron transport in giant, conjugated macrocycles, J. Am. Chem. Soc., 2018, 140, 10135–10139 CrossRef CAS; (g) W. Wang, L. Wang, B. J. Palmer, G. J. Exarhos and A. D. Q. Li, Cyclization and catenation directed by molecular self-assembly, J. Am. Chem. Soc., 2006, 128, 11150–11159 CrossRef CAS; (h) P. Spenst and F. Würthner, A perylene bisimide cyclophane as a “turn-on” and “turn-off” fluorescence probe, Angew. Chem., Int. Ed., 2015, 54, 10165–10168 CrossRef CAS; (i) T. Türel and S. Valiyaveettil, A Naphthalene Diimide Based Macrocycle Containing Quaternary Ammonium Groups: An Electron-Deficient Host for Aromatic Carboxylate Derivatives, ChemPlusChem, 2020, 85, 1430–1437 CrossRef.
- T. R. Cook and P. J. Stang, Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS.
- D. Zhang, T. K. Ronson and J. R. Nitschke, Functional capsules via subcomponent self-assembly, Acc. Chem. Res., 2018, 51, 2423–2436 CrossRef CAS.
- M. Paraja and S. Matile, Primary anion-π catalysis of epoxide-opening ether cyclization into rings of different sizes: access to new reactivity, Angew. Chem., Int. Ed., 2020, 59, 6273–6277 CrossRef CAS.
- S. P. Black, A. R. Stefankiewicz, M. M. J. Smulders, D. Sattler, C. A. Schalley, J. R. Nitschke and J. K. M. Sanders, Generation of a dynamic system of three-dimensional tetrahedral polycatenanes, Angew. Chem., Int. Ed., 2013, 52, 5749–5752 CrossRef CAS.
- S. P. Black, D. M. Wood, F. B. Schwarz, T. K. Ronson, J. J. Holstein, A. R. Stefankiewicz, C. A. Schalley, J. K. M. Sanders and J. R. Nitschke, Catenation and encapsulation induce distinct reconstitutions within a dynamic library of mixed-ligand Zn4L6 cages, Chem. Sci., 2016, 7, 2614–2620 RSC.
- T. K. Ronson, D. A. Roberts, S. P. Black and J. R. Nitschke, Stacking interactions drive selective self-assembly and self-sorting of pyrene-based MII4L6 architectures, J. Am. Chem. Soc., 2015, 137, 14502–14512 CrossRef CAS.
- C. M. Hong, R. G. Bergman, K. N. Raymond and F. D. Toste, Self-assembled tetrahedral hosts as supramolecular catalysts, Acc. Chem. Res., 2018, 51, 2447–2455 CrossRef CAS.
- Z. Lu, R. Lavendomme, O. Burghaus and J. R. Nitschke, A Zn4L6 capsule with enhanced catalytic C–C bond formation activity upon C60 binding, Angew. Chem., Int. Ed., 2019, 58, 9073–9077 CrossRef CAS.
- Z. Lu, T. K. Ronson and J. R. Nitschke, Reversible reduction drives anion ejection and C60 binding within an FeII4L6 cage, Chem. Sci., 2020, 11, 1097–1101 RSC.
- S. Ganta and D. K. Chand, Multi-stimuli-responsive metallogel molded from a Pd2L4-type coordination cage: selective removal of anionic dyes, Inorg. Chem., 2018, 57, 3634–3645 CrossRef CAS.
- K. Mahata, P. D. Frischmann and F. Würthner, Giant electroactive M4L6 tetrahedral host self-assembled with Fe(II) vertices and perylene bisimide dye edges, J. Am. Chem. Soc., 2013, 135, 15656–15661 CrossRef CAS.
- S. Mecozzi and J. Rebek, Jr., The 55% solution: a formula for molecular recognition in the liquid state, Chem. – Eur. J., 1998, 4, 1016–1022 CrossRef CAS.
- A. Kaloudi-Chantzea, N. Karakostas, C. P. Raptopoulou, V. Psycharis, E. Saridakis, J. Griebel, R. Hermann and G. Pistolis, Coordination-driven self-assembly of a brilliantly fluorescent rhomboid cavitand composed of bodipy-dye subunits, J. Am. Chem. Soc., 2010, 132, 16327–16329 CrossRef CAS.
- P. D. Frischmann, V. Kunz and F. Würthner, Bright fluorescence and host–guest sensing with a nanoscale M4L6 tetrahedron accessed by self-assembly of zinc–imine chelate vertices and perylene bisimide edges, Angew. Chem., Int. Ed., 2015, 54, 7285–7289 CrossRef CAS.
- P. D. Frischmann, V. Kunz, V. Stepanenko and F. Würthner, Subcomponent self-assembly of a 4 nm M4L6 tetrahedron with ZnII vertices and perylene bisimide dye edges, Chem. – Eur. J., 2015, 21, 2766–2769 CrossRef CAS.
- X. Feng, P. Liao, J. Jiang, J. Shi, Z. Ke and J. Zhang, Perylene diimide based imine cages for inclusion of aromatic guest molecules and visible-light photocatalysis, ChemPhotoChem, 2019, 3, 1014–1019 CrossRef CAS.
| This journal is © the Partner Organisations 2020 |