Förster resonance energy transfer (FRET) paired carbon dot-based complex nanoprobes: versatile platforms for sensing and imaging applications
Shihai
Miao
a,
Kang
Liang
 bc and
Biao
Kong
bc and
Biao
Kong
 *a
*a
aDepartment of Chemistry, Collaborative Innovation Center of Chemistry for Energy Materials (iChEM), Laboratory of Advanced Materials, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Shanghai 200433, P. R. China. E-mail: bkong@fudan.edu.cn; Web: https://softnanolab.fudan.edu.cn Fax: +86-21-31249176; Tel: +86-21-31249176
bSchool of Chemical Engineering, Graduate School of Biomedical Engineering, The University of New South Wales, Sydney NSW 2052, Australia
cAustralian Centre for NanoMedicine, The University of New South Wales, Sydney NSW 2052, Australia
First published on 21st October 2019
Abstract
As one of the most promising carbon-based photoluminescent materials, carbon dots (CDots) have recently attracted great attention for many potential applications owing to their excellent optical, electrical, and chemical properties. Förster Resonance Energy Transfer (FRET) is a highly sensitive spectroscopic technique that has been widely utilized for all applications of fluorescence. In this review, we provide an updated roadmap of CDot-based FRET systems for nanoprobes. Several CDot-based FRET systems obtained by co-assembling different fluorescent molecules are surveyed. Furthermore, the synthesis strategies for CDot-based FRET systems and their applications in nanoprobes, bioimaging, and biomedicine are reviewed. We further provide an outlook toward the future development of CDot-based FRET systems. This article summarizes the latest progress in CDot-based FRET systems and looks forward to further developments in these exciting nanocomposites.
1. Introduction
Förster resonance energy transfer (FRET) is the mechanism by which non-radiative energy is transferred between a luminescent donor and an energy acceptor within a close range of approximately 10 to 100 Å.1–3 FRET can be easily measured in standard bulk fluorescence experiments. FRET can also be monitored using fluorescence microscopy techniques, including single-molecule fluorescence spectroscopy. FRET serves as a highly sensitive spectroscopic technique that has been widely utilized in all applications of fluorescence, including medical diagnosis, biological analysis, and optical imaging.4–7 FRET-based dual-emission nanoprobes for ratiometric fluorescence sensing are achieved by synthesizing nanoparticles from two or more fluorescent molecules with different emission bands. One fluorophore serves as the reference, while the other acts as a response molecule, allowing the FRET system to be used as a ratiometric fluorescence nanoprobe. To date, many excellent studies and reviews of FRET have been conducted. In 2013, Chen et al. reviewed fluorescent nanosensors based on FRET. This review introduced the design and applications of sensitive and selective FRET-based ratiometric nanoprobes in detail. This review also classified nanosensors based on the type of nanoparticle and demonstrated the design and application of FRET-based fluorescent nanosensors for the detection of metal ions, small molecules, DNA, and other analytes.17 In 2014, Prevo et al. reviewed FRET and kinesin motor proteins. The physical basis of FRET and how to apply FRET to biological molecules was systematically introduced. Prevo et al. focused on the application of different FRET methods in motor protein drivers that had undergone several conformational changes under the action of enzymes. This FRET system moves unidirectionally along the microtubule filament, driving active intracellular transport.18 In 2014, Algar et al. examined the applications of FRET systems based on semi-conductor quantum dots. They reviewed the characterization of non-traditional structures of quantum dot-based FRET systems and their applications in biosensors, energy conversion, and optoelectronic device manufacturing in recent years.19 Although many applications of FRET have been reported, it is still an analytical tool that must continue to be developed. For example, the study of new energy-transfer donor and receptor pairs in the FRET system is important to improve FRET efficiency and analytical performance.Carbon dots (CDots) are newly emerging carbon-based fluorescent nanomaterials that have received considerable attention in recent years. Owing to their high optical stability, ease of synthesis and surface functionalization, good biocompatibility, and adjustable composition, CDots exhibit great potential in various applications such as catalysis, nanoprobes, optoelectronic devices, bioimaging, and biomedicine.20–25 The first discovery of carbon-based fluorescent nanomaterials was in 2004, when Xu et al. synthesized single-walled carbon nanotubes via an electrophoretic and purification process using arc soot as a raw material. Surprisingly, fluorescent carbon was found separate from the mixture of reactants.26 The first report of CDots as a carbon-based fluorescent nanomaterial was in 2006, when Sun et al. synthesized quantum-sized CDots through the laser ablation of a carbon target in the presence of water vapor using argon as a carrier gas. As the excitation wavelength increased, the emission wavelength of the CDots showed a continuous red shift.27 Since then, CDot research and reports have expanded rapidly. In 2012, Wei et al. showed the possibility and importance of CDots as energy donors to build FRET systems for the first time. The fluorescence of CDots can be quenched by graphene via the FRET process and then restored upon the introduction of K+.28 In 2013, Tang et al. reported an efficient CDot-based FRET system for the real-time monitoring of drug delivery and bioimaging. In this system, CDots served as both a FRET donor and a drug carrier, and doxorubicin served as an acceptor.9 In 2016, Yu et al. reported FRET between CDots and rhodamine B based on an up-conversion process spanning three components, resulting in the efficient utilization of the apparently wasted portion of sunlight (200–800 nm).29 In 2019, Su et al. described a straightforward strategy for preparing nanocomposites via the co-assembly of CDots and boron dipyrromethene based on FRET. The nanocomposite exhibited high water solubility, good biocompatibility, and photodynamic therapy efficiency.30
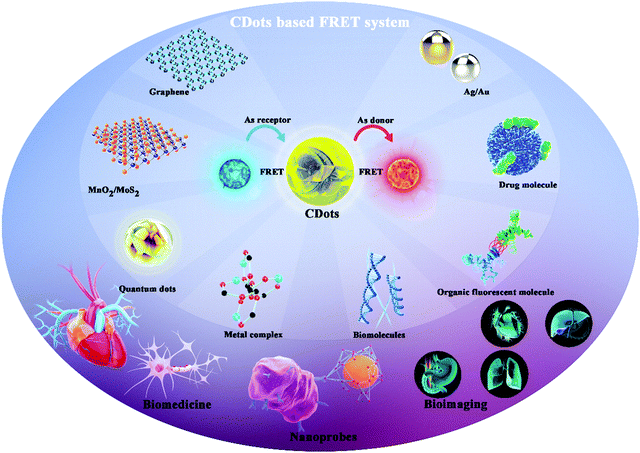
Many research efforts have attempted to build Cdot-based FRET systems as nanoprobes. Fig. 1 shows a timeline highlighting recent research efforts on Cdot-based FRET systems.8–16 Many fluorescent molecules can be employed to construct FRET systems with CDots. Generally, a FRET system contains two kinds of fluorescent molecules with different emission wavelengths. When CDots act as donors, they transfer energy to another molecule to construct the FRET system. Conversely, when the CDots act as receptors, they accept energy from other molecules, achieving ratiometric luminescence via FRET. Previous reports demonstrate that paired CDot-based FRET systems exhibit high sensitivity and have been widely utilized in all fluorescence-based applications, including medical diagnosis, biological analysis, and optical imaging. As CDots and FRET have been detailed in many excellent reviews, this review focuses on Cdot-based FRET systems with respect to their construction strategies, beneficial properties, and potential in a variety of applications.
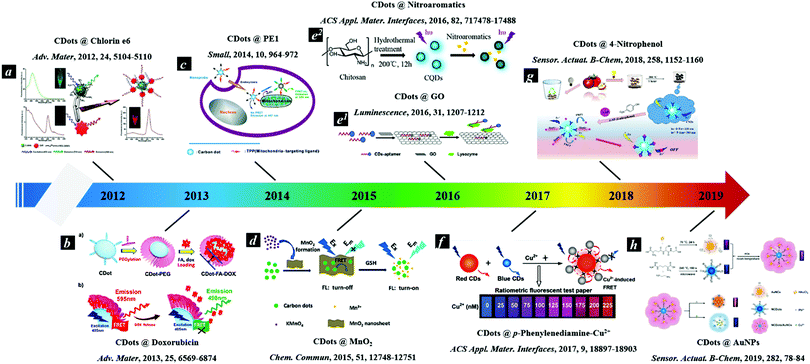 | ||
| Fig. 1 Timeline showing recent studies on CDot-based FRET systems.8–16 Reprinted with permission ref. 9–17. Copyright 2012 Wiley, Copyright 2013 Wiley, Copyright 2014 Wiley, Copyright 2015 The Royal Society of Chemistry, Copyright 2016 John Wiley and Sons Ltd, Copyright 2016 American Chemical Society, Copyright 2017 American Chemical Society, Copyright 2018 Elsevier, Copyright 2019 Elsevier. | ||
In this review, we present details on several Cdot-based FRET systems co-assembled from different classes of fluorescent materials, including organic fluorescent molecules, drug molecules, metal complexes, and nanomaterials. Based on the different fluorescent molecules, different combinations can be used to construct hetero-interfaces with CDots. These hetero-interfaces are mainly based on covalent binding and surface functionalization/coupling, thus resulting in different FRET effects. We also review the synthetic strategies for Cdot-based FRET systems and the applications of these systems in nanoprobes, bioimaging, and biomedicine.
2. Nanoprobes based on CDots and fluorescent molecules
2.1 CDots & fluorescent molecules
Fluorescent molecules exhibit characteristic fluorescence in the ultraviolet–visible–near-infrared region, and their fluorescence properties (excitation and emission wavelengths, intensity, lifetime, polarization, etc.) can be sensitively changed based on the properties of the environment (e.g., polarity, refractive index, and viscosity). Based on the excellent and diverse properties of fluorescent molecules, many promising applications have been demonstrated. Unsurprisingly, many studies have focused on FRET between CDots and fluorescent molecules. Table 1 presents a survey of recent works on FRET systems based on fluorescent molecules and CDots along with their applications.| Coupling molecule | Application | Ref. | Coupling molecule | Application | Ref. |
|---|---|---|---|---|---|
| Chlorin e6 | Bioimaging, biomedicine | 8 | Naphthalimide | H2S nanoprobe | 31 |
| Naphthalimide | NO nanoprobe | 32 | Isothiocyanate | PH nanoprobe | 33 |
| NBD-PE; BODIPY-PH | Bioimaging | 34 | Rhodamine B | Fe3+ nanoprobe | 35 |
| Boronate protected fluorescein (PFl) | H2O2 nanoprobe, bioimaging | 10 | Trinitrophenol | Bioimaging | 36 |
| Nitroaromatics | Nanosensor | 13 | NBD-PE | Nanoprobe, bioimaging | 37 |
| Rhodamine B | Up-conversion properties | 29 | Chlorin e6 | Bioimaging | 38 |
| Rhodamine 6G | Fe3+ nanoprobe | 39 | TNB− | DDVP nanoprobe | 40 |
| Naphthalimide | Nanoprobe, bioimaging | 41 | Ethidium bromide | DNA nanosensor | 42 |
| Glyphosate | Gly nanoprobe | 43 | 2,3-Diaminophenazine | I− nanoprobe | 44 |
| Fluorescein | NH3 nanoprobe | 45 | Isothiocyanate | PH nanoprobe | 46 |
| Acridone derivate | RNA nanoprobe | 47 | Naphthalimide | Tyrosinase nanoprobe | 48 |
| Rhodamine123 | Bioimaging | 49 | Nitrophenol | Nanoprobe, bioimaging | 15 |
| Acriflavine | SEB nanoprobe | 50 | Fluorescein (FAM) | Zn+ nanoprobe, bioimaging | 51 |
| Boron dipyrromethene | Bioimaging | 30 | Naphthalimide | Cys nanoprobe, bioimaging | 52 |
In 2012, Huang et al. reported a CDot–chlorin e6 FRET system based on a theranostic platform. Fig. 2a shows the FRET process between the CDots and chlorin Ce6, which combined through covalent binding. FRET occurs from the CDots to chlorin e6, and the CDots and CDots–chlorin e6 displayed green and red fluorescence, respectively. The CDots–Ce6 FRET system shows promise for applications in near-infrared fluorescence imaging-guided photodynamic therapy due to its excellent imaging and tumor-homing properties.8 In 2013, Du et al. synthesized a ratiometric fluorescent nanoprobe with low cytotoxicity and high reversibility based on FRET for pH sensing (Fig. 2b). In this nanoprobe, fluorescein isothiocyanate was assembled onto the CDots, which acted as the energy-transfer donor and carrier. The nanoprobe was successfully used to image live cells and reveal intracellular pH gradients.33 In 2014, Du et al. reported a FRET-based ratiometric nanoprobe for mitochondrial H2O2 in living cells (Fig. 2c). The FRET from the CDots (donor) to boronate-protected fluorescein (PF1; acceptor), which is covalently linked to the CDots, results the blue emission of the CDots. As H2O2 is added, FRET occurs between the CDots and PF1, resulting in green fluorescence. The, the ratiometric detection of H2O2 is realized.10 In 2018, Chatzimarkou et al. synthesized a sensitive and selective FRET-based nanoprobe for 4-nitrophenol (Fig. 2d). The synthesized N-CDots exhibited blue fluorescence, which can effectively be quenched by 4-nitrophenol through the FRET process from CDots to 4-nitrophenol. The CDots/4-nitrophenol showed bright luminescence and low toxicity, demonstrating promise for cell imaging and other biosensing applications.15
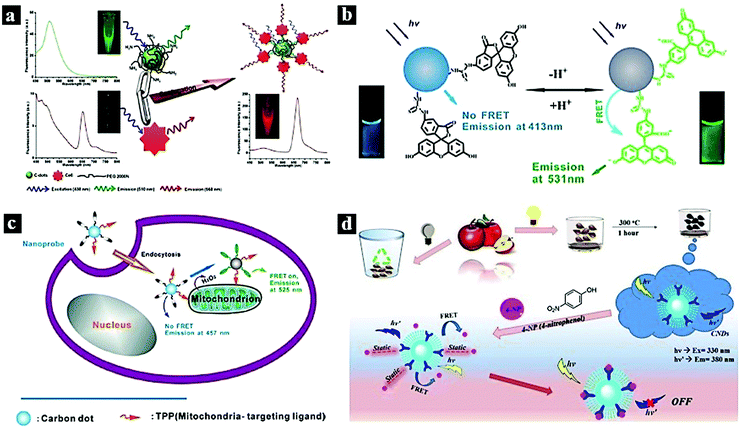 | ||
| Fig. 2 (a) FRET process between CDots and chlorin e6.8 Reprinted with permission ref. 8. Copyright 2012 Wiley. (b) Schematic illustration of a Cdot-based FRET nanoprobe for pH sensing.33 Reprinted with permission ref. 33. Copyright 2013 IOP Publishing Ltd. (c) Nanoprobe using FRET-based H2O2 ratiometric sensing in a living cell.10 Reprinted with permission ref. 10. Copyright 2014 Wiley-VCH Verlag. (d) Schematic illustration of the synthetic process of CDots and the FRET process between CDots and 4-nitrophenol.15 Reprinted with permission ref. 15. Copyright 2018 Elsevier. | ||
2.2 CDots & drug molecules
Drug molecules play a vital role in processes related to human health, such as nucleic acid repair, cell apoptosis, and tumor suppression. When coupled with CDots, certain drugs can be utilized to construct CDot–drug FRET systems, which show promising applications in nanoprobes, bioimaging, and biomedicine. Drug molecules commonly used in FRET include doxorubicin,9,56,57 porphyrins,58,59 vitamins,53,55,60 curcumin,61 rhodizonate,62 and cisplatin(IV) prodrug.54In 2013, Tang et al. reported an efficient CDot-based FRET system for the real-time monitoring of drug delivery and bioimaging. Fig. 3a shows the assembly process of a FRET–CDots drug delivery system (DDS) via direct surface coupling and the proposed mechanism of the FRET-CDots DDS for drug delivery. In this system, the CDots serve as both a donor and a drug carrier, and doxorubicin serves as an acceptor. As doxorubicin is released from the surfaces of the CDots, the FRET between doxorubicin and the CDots is terminated, restoring the fluorescence of the CDots.9 In 2015, Wang et al. reported a Cdot-based FRET system with high quantum yield (QY) for the detection of biologically significant vitamin B12. Fig. 3b shows the thermal reduction process of the high-QY CDots and the FRET process between the CDots and vitamin B12. The low-QY CDots exhibited emission wavelengths at 445 nm; after thermal treatment, the CDots showed high QY with an emission wavelength of 431 nm. The CDots were used to detect vitamin B12 based on the FRET between the CDots and vitamin B12.53 In 2017, Feng et al. reported a CDot-based system for the real-time ratiometric monitoring of anticancer prodrug activation in living cells. Fig. 3c shows the FRET process between the CDots and cisplatin(IV) prodrug. The quencher unit attached to the CDot surfaces through amide condensation. The CDots showed blue, green, and red emission under different excitation conditions as a drug nanocarrier. Based on the FRET between the CDots and cisplatin(IV) prodrug, the blue emission of the CDots was quenched. Under reductive conditions, the nanocarrier released the dabsyl unit and active Pt(II), causing the blue emission of the CDots to recover with time; meanwhile, the intensity of green and red fluorescence remained almost unchanged.54 In 2015, Wang et al. reported a ratiometric fluorescent nanoprobe based on a N,S-CDots FRET system that was used for the determination of eriboflavin in aqueous solutions (Fig. 3d). The N,S-CDots exhibited blue emission at 465 nm; through the FRET process from N,S-CDots to riboflavin, the blue emission intensity decreased monotonically, while the green emission intensity increased.55
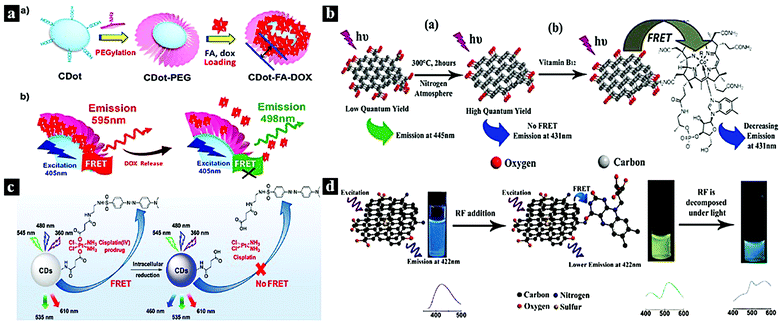 | ||
| Fig. 3 (a) Assembly process of FRET–CDots–DDS and the proposed mechanism of FRET–CDots–DDS for drug delivery.9 Reprinted with permission ref. 9. Copyright 2013 Wiley. (b) Thermal reduction of high-QY CDots and the FRET process between CDots and vitamin B12.53 Reprinted with permission ref. 53. Copyright 2015 The Royal Society of Chemistry. (c) FRET process between CDots and cisplatin(IV) prodrug.54 Reprinted with permission ref. 54. Copyright 2017 American Chemical Society. (d) Schematic diagram of a riboflavin probe based on N,S-CDots and the FRET process.55 Reprinted with permission ref. 55. Copyright 2015 Elsevier. | ||
2.3 CDots & metal complexes
The monitoring of metal ions is meaningful in the fields of human health and environmental protection. FRET systems based on CDot–metal complexes can act as efficient and sensitive nanoprobes for the detection of certain metal ions. The most investigated metal complexes include rhodamine–Al3+,63 cobalt oxyhydroxide,64,65 quercetin–Zn2+,66 cyclam–Cu2+,67p-phenylenediamine–Cu2+,14 ruthenium complex,68 and HCM–Cu2+.69In 2015, Kim et al. designed a CDot–rhodamine–Al3+ FRET-based nanoprobe for Al3+ detection in aqueous solution. Fig. 4a schematically illustrates the FRET process between the CDots and rhodamine–Al3+, which are covalently combined. The CDots act as an energy donor, while rhodamine–Al3+ serves as an energy acceptor in the presence of Al3+ ions. The emission of the CDots can be tuned from blue to green based on the FRET process from the CDots to rhodamine–Al3+.63 In 2017, Liu et al. synthesized dual optical CDots-based ratiometric fluorescent nanoprobe for Cu2+. Fig. 4b schematically illustrates the FRET process from the blue CDots to the p-phenylenediamine–Cu2+ complex. As Cu2+ can coordinate with –COOH and p-phenylenediamine, this led to blue CDots joining to the surface of red CDots. FRET occurred from the blue CDots to p-phenylenediamine–Cu2+ on the surfaces of the red CDots, leading to the fluorescence quenching of the blue CDots. The emission color could be tuned from blue to red based on the FRET process by adjusting the Cu2+ doping content.14 In 2015, Yang et al. reported fluorescent CDots as a sensitive nanoprobe for Zn2+. Fig. 4c schematically illustrates the detection of Zn2+ based on a FRET system comprising CDots and pentahydroxyflavone–Zn2+ complex. Upon excitation at 360 nm, FRET occurred from the CDots to pentahydroxyflavone–Zn2+, while pentahydroxyflavone–Zn2+ accepted the energy and exhibited fluorescence emission at 480 nm.66 In 2018, Yan et al. reported a FRET-based ratiometric fluorescent nanoprobe for Cu2+ using 7-diethylaminocoumarin-3-carbohydrazide (CMH)-functionalized CDots (Fig. 4d). In this system, CMH is covalently bound to the surfaces of the CDots, and FRET occurs from the CDots to CMH. However, the FRET process was inhibited by adding Cu2+; meanwhile, the fluorescence intensity increased at 400 nm and decreased at 458 nm. The emission can be tuned from blue to blue-green in the presence and absence of Cu2+ under UV light.69
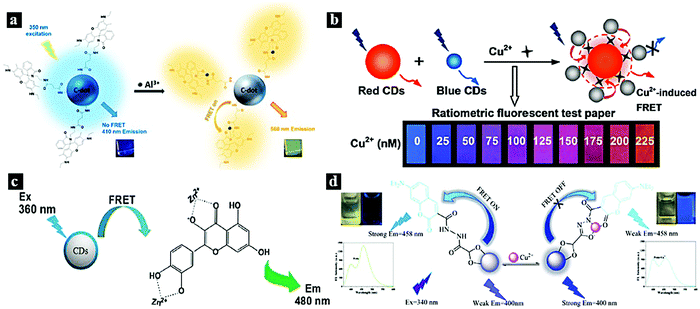 | ||
| Fig. 4 (a) Schematic illustration of the FRET process between CDots and rhodamine–Al3+.63 Reprinted with permission ref. 63. Copyright 2015 American Chemical Society. (b) Schematic illustration of the FRET process from blue CDots to p-phenylenediamine–Cu2+.14 Reprinted with permission ref. 14. Copyright 2017 American Chemical Society. (c) Schematic illustration of the detection of Zn2+ based on a CDots/pentahydroxyflavone–Zn2+ FRET system.66 Reprinted with permission ref. 64. Copyright 2015 Springer-Verlag Wien. (d) Schematic illustration of a FRET system based on CDots and CMH–Cu2+.69 Reprinted with permission ref. 65. Copyright 2018 Elsevier. | ||
2.4 CDots & biomolecules
Biomolecules are an important part of higher flora and fauna due to their indispensable functions, such as helping cells transmit signals and controlling physiological and pathological processes. The biomolecules commonly applied in FRET include nucleic acids,70 tryptophan,71 and dopamine.72In 2017, Khakbaz et al. reported a nanoprobe for micro-RNA sensing based on a FRET system. The FRET occurs between CDots and carboxyfluorescein-labeled DNA, and the CDot fluorescence is quenched. The nanoprobe can be used for micro-RNA detection and the early detection of breast cancer.70 In 2017, Dang et al. synthesized a Cdot-based FRET system via a one-step method using tryptophan and β-cyclodextrin as precursors. FRET was found to occur between tryptophan and the CDots, and this system could be used as a sensitive probe for Fe3+ detection.71 In 2017, Zhang et al. reported a FRET system based on polymerized dopamine (PDA) and CDots for bioimaging (Fig. 5). The FRET effect occurs between the CDots and PDA, resulting in highly efficient and tunable emission. The cell imaging ability and cytotoxicity of the CDot–PDA system were also explored, indicating good biocompatibility and strong prospects for biotechnological applications.72
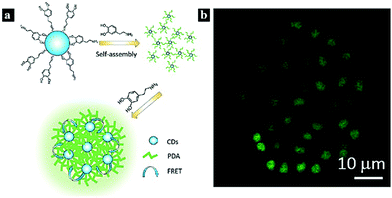 | ||
| Fig. 5 (a) Schematic illustration of a nanoprobe based on FRET between CDots and PDA. (b) Confocal fluorescence images of MCF-7 cells under 405 nm laser excitation. Reprinted with permission ref. 76. Copyright 2017 The Royal Society of Chemistry. | ||
3. Nanoprobes based on CDots and nanomaterials
3.1 CDots & MnO2/MoS2
Layered transition-metal disulphides or dioxides (e.g., MnO2, WS2, and MoS2) are a class of two-dimensional nanomaterials that show excellent properties, including large surface areas along with semiconducting and energy-harvesting characteristics, giving them widespread applications. Because of their excellent optical absorption capability and fast electron transfer rate, these materials are promising fluorescence quenchers and have attracted considerable attention in the construction of FRET-based sensing platforms.MnO2 is one of the most stable manganese oxides and possesses excellent chemical and physical properties. As a transition metal oxide, it attracts considerable attention in batteries, supercapacitors, and even catalysis driven by visible light. MnO2 shows broad absorption in the range of 250–550 nm, which overlaps with the fluorescence excitation of CDots. This phenomenon allows the occurrence of FRET between CDots and MnO2.11,73–79 The transition metal oxide MoS2 has a sandwich structure that is bonded by weak van der Waals forces and can be regarded as S–Mo–S. MoS2 possesses a large intrinsic band gap that depends largely on the number of layers. In addition, most transition metal ions have inherent fluorescence quenching characteristics. Therefore, many FRET systems based on CDots and MoS2 have been reported.80–83
In 2015, Wang et al. reported a FRET system between CDots and MnO2 for glutathione (GSH) sensing in human whole blood samples (Fig. 6a). Due to the FRET from the CDots to MnO2 through MnO2-surface functionalization with CDots, the fluorescence emission of the CDots was quenched. After the introduction of GSH, the fluorescence of the CDots was restored.11 In 2018, Yan et al. synthesized a CDots–MnO2 FRET platform and applied it in the sensitive detection of organophosphorus pesticides (Fig. 6b). The fluorescence intensity of the CDots can be tuned off via FRET from CDots to the energy acceptor MnO2. Moreover, the quenching effect induced by MnO2 nanosheets can be restored by adding acetylthiocholine and butyrylcholinesterase to the FRET system.74 In 2015, Yang et al. reported a turn-on fluorescence nanoprobe for GSH in aqueous solutions based on a CDots–MnO2 FRET system (Fig. 6c). The CDots–MnO2 complex is easily synthesized via surface functionalization and exhibits a stable FRET process. The MnO can be reduced in the presence of GSH introduced into the system, thereby inhibiting the FRET process between the CDots and MnO2 and restoring the fluorescence intensity.75 In 2017, Jana et al. reported a CDots–MnO2 FRET system for molecular logic operations using N-acetyl-L-cysteine (NAC) and H+ as inputs (Fig. 6d). The FRET process occurs from the CDots to MnO2, resulting in fluorescence quenching; the fluorescence can be restored by adding NAC.77 In 2017, Wang et al. synthesized a CDots–MoS2 FRET platform and used it for the sensitive detection of the antibiotic kanamycin (Fig. 6e). Through van der Waals forces, the CDots can be assembled onto the MoS2 surface, thus quenching the fluorescence due to the FRET from the CDots to MoS2.81
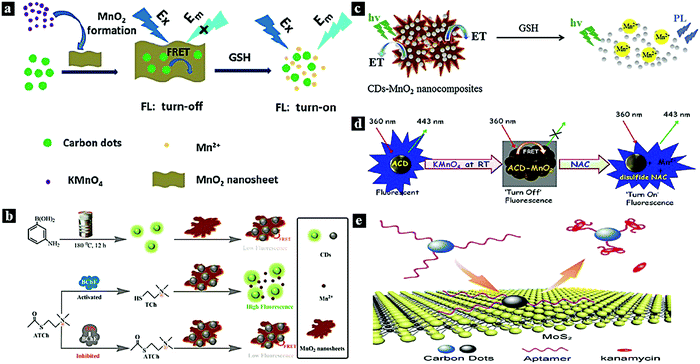 | ||
| Fig. 6 (a) Schematic illustration of the preparation of a nanoprobe for GSH based on a CDots–MnO2 FRET system.11 Reprinted with permission ref. 11. Copyright 2015 The Royal Society of Chemistry. (b) Schematic illustration of a nanoprobe for organophosphorus pesticides based on a CDots–MnO2 FRET system.74 Reprinted with permission ref. 70. Copyright 2018 American Chemical Society. (c) Schematic illustration of a nanoprobe for GSH based on a CDots–MnO2 FRET system.75 Reprinted with permission ref. 71. Copyright 2015 Elsevier. (d) Schematic illustration of an NAC nanoprobe based on a FRET system.77 (e) Schematic illustration of a kanamycin sensing strategy based on a CDots–MoS2 FRET system.81 Reprinted with permission ref. 73. Copyright 2017 Springer-Verlag Wien. | ||
3.2 CDots & Au/Ag
Metal nanoparticles and particularly Ag and Au have attracted great research interest due to their physicochemical and photoelectric properties. Therefore, they are widely used in catalytic, photovoltaic, and biological applications. Au and Ag nanoparticles (AuNPs and AgNPs) can also be used as efficient acceptors for most fluorescent groups due to their high extinction coefficients and absorption bandwidths. Therefore, AuNPs89–95 and AgNPs96–100 are ideal nano-quenching agents to establish FRET-based nanoprobes due to their large extinction coefficients and wide absorption spectra, which overlap with the fluorescent emission of CDots.In 2017, Shen et al. synthesized a fluorescent nanoprobe for the sensitive detection of adenosine based on FRET. Fig. 7a schematically illustrates the nanoprobe for adenosine based on FRET between CDots and AuNPs. Through the hybridization between single-strand DNA (ssDNA)-functionalized CDots and aptamer-functionalized AuNPs, fluorescence quenching was observed due to FRET from the CDots to the AuNPs. When adenosine was present, the binding between adenosine and aptamer released the ssDNA-functionalized CDots from the AuNP surfaces, resulting in fluorescence recovery.84 In 2017, Li et al. reported a CDots–Au nanocluster FRET system combined via a carbodiimide-activated coupling reaction as a ratiometric fluorescence nanoprobe for the bioimaging of H2O2 (Fig. 7b). The synthesized CDots–Au system showed a dual-emission fluorescent property with 40% FRET efficiency from the CDots to Au. Furthermore, the red emission of the CDots–Au system could be quenched by H2O2, while the blue emission was used as a reference signal to provide built-in correction.85 In 2018, Ge et al. synthesized a selective and sensitive nanoprobe for the antibiotic D-penicillamine (D-PA) based on a FRET system between CDots and AuNPs combined via electrostatic interaction (Fig. 7c). In this system, AuNPs act as a colorimetric indicator and a fluorescence quencher. The CDot fluorescence can be quenched through the FRET process from the CDots to the AuNPs. Moreover, the quenching effect induced by the AuNPs can be restored by adding acetylthiocholine and D-PA to the FRET system.86 In 2019, Wang et al. synthesized a CDots–AuNCs FRET-based ratiometric fluorescent nanoprobe for Pb2+/Cu2+ (Fig. 7d). The probe was simply prepared by mixing AuNCs and N-CDots in aqueous solution. The probe can interact selectively with Pb2+ and Cu2+, leading to a shift in the fluorescent signal. The system showed good detection sensitivity at 0.5 and 0.15 μM for Pb2+ and Cu2+, respectively.16 In 2018, He et al. reported a CDots–AuNCs FRET-based dual-emission ratiometric fluorescence nanoprobe for dopamine sensing (Fig. 7e). The FRET-based nanoprobe consists of two fluorophores (AuNCs as the acceptor and CDots as the energy donor) and exhibits dual emission wavelengths of 610 and 420 nm under excitation at 380 nm. The AuNCs were subsequently conjugated to the CDots via simple surface functionalization. The fluorescence of the AuNCs can be quenched by dopamine, inhibiting the FRET process from the CDots to Au.87 In 2019, Dong et al. reported a nanoprobe for the ratiometric detection of L-cysteine based on FRET between CDots and AuNPs (Fig. 7f). FRET was established from the energy donor (CDots) to the acceptor (AuNPs), which were combined via electrostatic interaction, effectively inhibiting the fluorescence emission of the CDots. After the addition of L-cysteine to the system, the fluorescence of the NAC-AuNPs was quenched, while the emission intensity of the CDots remained almost constant.88
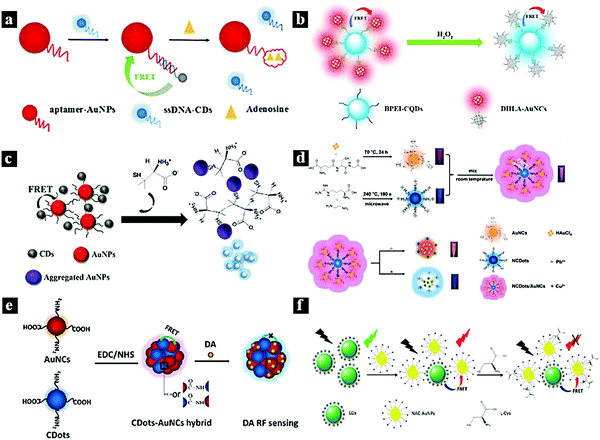 | ||
| Fig. 7 (a) Schematic illustration of a nanoprobe for adenosine based on FRET between CDots and AuNPs.84 Reprinted with permission ref. 84. Copyright 2017 The Royal Society of Chemistry. (b) Schematic illustration of a FRET-based nanoprobe for H2O2.85 Reprinted with permission ref. 85. Copyright 2017 Elsevier. (c) Schematic illustration of a FRET-based nanoprobe for D-PA.86 Reprinted with permission ref. 86. Copyright 2018 Springer New York. (d) Schematic illustration of a ratiometric nanoprobe for Pb2+/Cu2+ based on a CDots–Au FRET system.16 Reprinted with permission ref. 16. Copyright 2019 Elsevier. (e) Schematic illustration of a dopamine nanoprobe based on a CDots–Au FRET system.87 Reprinted with permission ref. 87. Copyright 2018 Elsevier. (f) Schematic illustration of a nanoprobe for L-cysteine based on a FRET system between CDots and AuNPs.88 Reprinted with permission ref. 88. Copyright 2019 Elsevier. | ||
3.3 CDots & graphene oxide
Graphene oxide (GO) is a two-dimensional graphitic carbon system with monoatomic thickness and oxygen-containing functional groups. Due to its good water solubility, large specific surface area, high electronic conductivity, and long-range nanoscale energy transfer characteristics, GO has attracted significant research attention. Recently, it has been used as a good energy acceptor to quench the fluorescence of CDots based on the FRET process.12,28,101–104In 2012, Wei et al. reported a metal-ion nanoprobe with high selectivity and tunable dynamic range based on FRET from CDots to GO. Fig. 8a shows a schematic illustration of the nanoprobe for K+ sensing based on the CDots–GO FRET system, in which the CDots and GO are combined via a specific cation–ligand complexation reaction. The fluorescence of the CDots can be quenched by GO based on the FRET process; however, after the introduction of K+, which competes with ammonium functionalized on GO, the fluorescence of the CDots was restored.28 In 2016, Wu et al. synthesized a photoluminescent nanoprobe for the detection of lysozyme based on FRET from CDots to GO (Fig. 8b). Upon the addition of GO, the photoluminescence of the aptamer-conjugated CDots was effectively quenched because of the FRET from CDots to GO. However, in the presence of the target lysozyme, the CDots bound strongly to lysozyme via the aptamer–lysozyme interaction and desorbed from the GO surface, causing the photoluminescence of the CDots to recover.12 In 2015, Cui et al. synthesized a fluorescent nanoprobe for Hg2+ detection based on FRET between CDots and graphene oxide (Fig. 8c). The fluorescent signal of the CDots was quenched upon binding to GO based on the FERT process. However, when Hg2+ was present, oligodeoxyribonucleotide-functionalized CDots selectively bound to Hg2+ ions in solution and initiated the adsorption of CDots from GO, causing the CDot fluorescence to recover.101 In 2018, Cheng et al. synthesized a fluorometric nanoprobe for adenosine triphosphate (ATP) sensing based on FRET between CDots and GO (Fig. 8d). The CDots and GO were combined through π stacking and hydrophobic interactions. CDots and GO acted as the energy donor and acceptor, respectively, resulting the quenching of the CDot fluorescence. When ATP was present, the aptamer bound strongly to ATP, causing the CDots to unbind from GO and the CDot fluorescence to recover.102
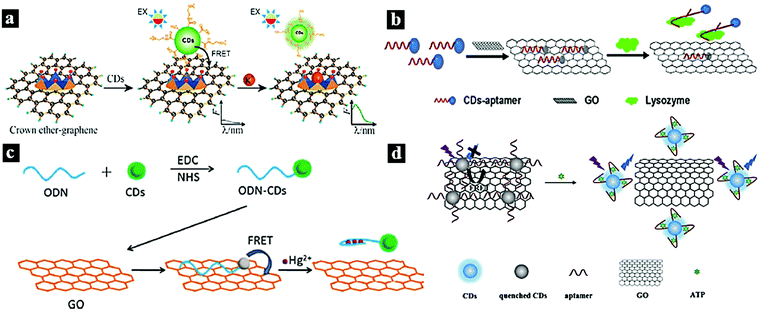 | ||
| Fig. 8 (a) Schematic illustration of a nanoprobe for K+ based on a CDots–GO FRET system.28 Reprinted with permission ref. 28. Copyright 2012 The Royal Society of Chemistry. (b) Schematic illustration of a nanoprobe for lysozyme based on a CDots–GO FRET system.12 Reprinted with permission ref. 12. Copyright 2016 John Wiley and Sons Ltd. (c) Schematic illustration of a FRET system for Hg2+ detection.101 Reprinted with permission ref. 101. Copyright 2015 Elsevier Ltd. (d) Schematic illustration of a nanoprobe for ATP based on FRET between CDots and GO.102 Reprinted with permission ref. 102. Copyright 2018 Springer-Verlag Wien. | ||
3.4 CDots & quantum dots
Fluorescence resonance energy transfer between CDots and quantum dots has also been widely studied. The main studied quantum dots include CdTe,105 CDots,106 graphene quantum dots.107 In 2014, Tao et al. reported a nanoprobe for chlortoluron detection in water based on a FRET system between CDots and CdTe quantum dots. The FRET occurs from the CDots (donor) to CdTe (acceptor), greatly enhancing the fluorescence intensity of CdTe. After the addition of chlortoluron, the strong interaction between CdTe and chlortoluron leads to the quenching of CdTe fluorescence by the formation of the chlortoluron–CdTe ground state complex.105 In 2016, Wang et al. reported a nanoprobe for volatile organic compounds based on intra-particle FRET in Mn–CDots. The FRET occurs from the surface-related energy donor to the metal-related energy acceptor in the Mn–CDots. The FRET is not sensitive to proximity; instead, it is sensitive to polarity resulting from the inherent fixed position of the two fluorescent groups on the CDot backbone. Therefore, based on the sensitivity of the FRET, the Mn–CDots have extraordinary sensing ability for some small molecules.106 In 2019, Chini et al. reported a nanoprobe for sensing heavy metal ions based on a FRET system between CDots and graphene quantum dots. In this FRET system, the graphene quantum dots serve as the energy donor, while the CDots are the acceptor. After the addition of heavy metals such as mercury (Hg2+) and arsenic (As5+) into this nanoprobe system, the FRET signal was significantly reduced.1074. Conclusions & perspectives
CDots have recently emerged as a new class of fluorescent carbon nanomaterials and gained tremendous attention due to their excellent physicochemical properties, which distinguish them from traditional fluorescent materials. The major advantages of Cdot-based FRET systems include high sensitivity, adjustable composition, and good biocompatibility, which make them promising materials for nanoprobes, bioimaging, and biomedicine. In comparison with conventional FRET systems, CDot-based FRET systems overcome drawbacks such as spectral crosstalk, photobleaching, and direct acceptor excitation. CDot-based FRET systems also provide increased sensitivity, for single function and multifunction use. As a new type of nanomaterial with excellent optical properties, CDots can serve as an energy donor and receptor, greatly improving the application range and efficacy of CDot-based FRET systems. Perspectives for potential future applications of CDot-based FRET systems are summarized in Fig. 9.Research on the design and fabrication of CDot-based FRET systems has become of great interest in chemical and biological sciences along with engineering fields. With the development of FRET-based nanoprobes, the functional units of FRET have been expanded from simple molecules to macromolecule and nanoparticles, and the research focus has shifted from single measurements to multifunctional and interdisciplinary applications. CDot-based FRET nanoprobes show promise for achieving high sensitivity, high selectivity, portability, fast response, and biocompatibility, which are important in applications in biotechnology, the environment, and medicine. For example, all-in-one diagnostic platforms for recognition, imaging, and therapy have received increasing attention. However, a great number of challenges and opportunities remain.108 For example, while numerous optical light-emitting diode devices have been reported, further research in the field of biological devices is needed. This review summarized recent progress in the research and development of CDot-based FRET systems with a focus on the different classifications of fluorescent molecules and potential applications in nanoprobes. We reviewed several fluorescent molecules in detail, including organic fluorescent molecules, drug molecules, metal complexes, biomolecules, MnO2/MoS2, Au/Ag, graphene/GO, and quantum dots. We also presented extensive applications of CDot-based FRET systems as highly sensitive nanoprobes for diverse analytes.
Although many applications of CDot-based FRET systems have been reported, challenges remain. First, for CDots acting as the energy donor, new energy receptors are needed to facilitate efficient energy transfer and increase detection sensitivity. Second, for CDots acting as the energy receptor, new energy donors should be explored. Thus, CDots can be enabled to exhibit superior optical performance for nanoprobe and biological applications. Third, novel CDots based on heteroatom doping and surface functionalization should be explored to allow the FRET process to be modulated by preparing CDots with different properties. In summary, exploring new energy transfer donor and receptor pairs for FRET systems is important to improve FRET efficiency and analytical performance. CDot-based FRET systems will undoubtedly overcome the limitations of traditional luminescent materials and have a bright future in nanoprobes, bioimaging, biomedicine and other applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFA0206901, 2017YFA0206900), the NSF of China (21705027), the Major Scientific and Technological Innovation Project of Shandong (2017CXGC0501), the Natural Science Foundation of Shandong Province (ZR2017PEM004, and ZR2017BEM020), the Recruitment Program of Global Experts of China and the Recruitment Program of Global Experts of Shanghai, and the Australia National Health and Medical Research Council (NHMRC APP1163786).References
- A. T. Aron, M. O. Loehr, J. Bogena and C. J. Chang, J. Am. Chem. Soc., 2016, 138, 14338–14346 CrossRef CAS.
- R. Vafabakhsh, J. Levitz and E. Y. Isacoff, Nature, 2015, 524, 497 CrossRef CAS.
- J. Guo, X. Qiu, C. Mingoes, J. R. Deschamps, K. Susumu, I. L. Medintz and N. Hildebrandt, ACS Nano, 2019, 13, 505–514 CrossRef CAS.
- J. Liu and Y. Lu, J. Am. Chem. Soc., 2002, 124, 15208–15216 CrossRef CAS.
- E. Galperin, V. V. Verkhusha and A. Sorkin, Nat. Methods, 2004, 1, 209–217 CrossRef CAS.
- Y. Yang, H. Liu, M. Han, B. Sun and J. Li, Angew. Chem., Int. Ed., 2016, 55, 13538–13543 CrossRef CAS.
- S. Basu, L.-M. Needham, D. Lando, E. J. R. Taylor, K. J. Wohlfahrt, D. Shah, W. Boucher, Y. L. Tan, L. E. Bates, O. Tkachenko, J. Cramard, B. C. Lagerholm, C. Eggeling, B. Hendrich, D. Klenerman, S. F. Lee and E. D. Laue, Nat. Commun., 2018, 9, 2520 CrossRef PubMed.
- H. Peng, L. Jing, W. Xiansong, W. Zhe, Z. Chunlei, H. Meng, W. Kan, C. Feng, L. Zhiming and S. Guangxia, Adv. Mater., 2012, 24, 5104–5110 CrossRef.
- T. Jing, K. Biao, W. Hao, X. Ming, W. Yongcheng, W. Yanli, Z. Dongyuan and Z. Gengfeng, Adv. Mater., 2013, 25, 6569–6574 CrossRef.
- F. Du, Y. Min, F. Zeng, C. Yu and S. Wu, Small, 2014, 10, 964–972 CrossRef CAS PubMed.
- W. Yuhui, J. Kai, Z. Jiali, Z. Ling and L. Hengwei, Chem. Commun., 2015, 51, 12748–12751 RSC.
- J. Wu, Y. Hou, P. Wang, Z. Wang, Y. Li, S. Wang and M. Yang, Luminescence, 2016, 31, 1207–1212 CrossRef CAS.
- Z. Liang, M. Kang, G. F. Payne, X. Wang and R. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 17478–17488 CrossRef CAS.
- C. Liu, D. Ning, C. Zhang, Z. Liu, R. Zhang, J. Zhao, T. Zhao, B. Liu and Z. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 18897–18903 CrossRef CAS PubMed.
- A. Chatzimarkou, T. G. Chatzimitakos, A. Kasouni, L. Sygellou, A. Avgeropoulos and C. D. Stalikas, Sens. Actuators, B, 2018, 258, 1152–1160 CrossRef CAS.
- L. Wang, H.-X. Cao, Y.-S. He, C.-G. Pan, T.-K. Sun, X.-Y. Zhang, C.-Y. Wang and G.-X. Liang, Sens. Actuators, B, 2019, 282, 78–84 CrossRef CAS.
- G. Chen, F. Song, X. Xiong and X. Peng, Ind. Eng. Chem. Res., 2013, 52, 11228–11245 CrossRef CAS.
- B. Prevo and E. J. G. Peterman, Chem. Soc. Rev., 2014, 43, 1144–1155 RSC.
- W. R. Algar, H. Kim, I. L. Medintz and N. Hildebrandt, Coord. Chem. Rev., 2014, 263-264, 65–85 CrossRef CAS.
- J. Tang, Y. Zhang, B. Kong, Y. Wang, P. Da, J. Li, A. A. Elzatahry, D. Zhao, X. Gong and G. Zheng, Nano Lett., 2014, 14, 2702–2708 CrossRef CAS.
- B. Kong, J. Tang, Y. Zhang, T. Jiang, X. Gong, C. Peng, J. Wei, J. Yang, Y. Wang, X. Wang, G. Zheng, C. Selomulya and D. Zhao, Nat. Chem., 2015, 8, 171 CrossRef PubMed.
- S. Tao, S. Lu, Y. Geng, S. Zhu, S. A. T. Redfern, Y. Song, T. Feng, W. Xu and B. Yang, Angew. Chem., Int. Ed., 2018, 57, 2393–2398 CrossRef CAS PubMed.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- L. Wang, X. Wu, S. Guo, M. Han, Y. Zhou, Y. Sun, H. Huang, Y. Liu and Z. Kang, J. Mater. Chem. A, 2017, 5, 2717–2723 RSC.
- B. Kong, A. Zhu, C. Ding, X. Zhao, B. Li and Y. Tian, Adv. Mater., 2012, 24, 5844–5848 CrossRef CAS.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS.
- Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS.
- W. Wei, C. Xu, J. Ren, B. Xu and X. Qu, Chem. Commun., 2012, 48, 1284–1286 RSC.
- S. Yu, Y. L. Su, H. N. Umh and J. Yi, Nano Energy, 2016, 26, 479–487 CrossRef CAS.
- Y. Su, S. Lu, P. Gao, M. Zheng and Z. Xie, Mater. Chem. Front, 2019, 3, 1747–1753 RSC.
- Y. Changmin, L. Xizhen, Z. Fang, Z. Fangyuan and W. Shuizhu, Chem. Commun., 2013, 49, 403–405 RSC.
- C. Yu, Y. Wu, Z. Fang and S. Wu, J. Mater. Chem. B, 2013, 1, 4152 RSC.
- F. Du, Y. Ming, F. Zeng, C. Yu and S. Wu, Nanotechnology, 2013, 24, 365101 CrossRef.
- N. Sukhendu, M. Ravit, P. K. Kaviya, M. Yelena, K. Sofiya and J. Raz, Chem. Commun., 2014, 50, 10299–10302 RSC.
- S. Hu, Q. Zhao, Q. Chang, J. Yang and J. Liu, RSC Adv., 2014, 4, 41069–41075 RSC.
- D. Shi, F. Yan, T. Zheng, Y. Wang, X. Zhou and C. Li, RSC Adv, 2015, 5, 98492–98499 RSC.
- S. Nandi, R. Malishev, S. K. Bhunia, S. Kolusheva, J. Jopp and R. Jelinek, Biophys. J., 2016, 110, 2016–2025 CrossRef CAS.
- K. Yang, L. Fan, W. Y. Che, X. Hu and T. Feng, RSC Adv., 2016, 6, 101447–101451 RSC.
- M. Deng, W. Sha, C. Liang, H. Shang and S. Jiang, RSC Adv., 2016, 6, 26936–26940 RSC.
- J. Hou, Z. Tian, H. Xie, Q. Tian and S. Ai, Sens. Actuators, B, 2016, 232, 477–483 CrossRef CAS.
- J. S. Sidhu, A. Singh, N. Garg and N. Singh, ACS Appl. Mater. Interfaces, 2017, 9, 25847–25856 CrossRef CAS.
- J. Kudr, L. Richtera, K. Xhaxhiu, D. Hynek, Z. Heger, O. Zitka and V. Adam, Biosens. Bioelectron., 2017, 92, 133–139 CrossRef CAS.
- Y. Yuan, J. Jiang, S. Liu, J. Yang, Z. Hui, J. Yan and X. Hu, Sens. Actuators, B, 2017, 242, 545–553 CrossRef CAS.
- H. Wang, Q. Lu, Y. Liu, H. Li, Y. Zhang and S. Yao, Sens. Actuators, B, 2017, 250, 429–435 CrossRef CAS.
- H. Chao-Peng, H. Zahralsaadat, A. Efe, Z. Shanyu, S. Michel, Z. Haijiang, G. Huizhang, W. Lukas, R. M. Rossi and M. M. Koebel, Sens. Actuators, B, 2017, 253, 714–722 CrossRef.
- H. Liu, C. Xu, Y. Bai, L. Liu, D. Liao, J. Liang, L. Liu and H. Han, Spectrochim. Acta, Part A, 2017, 171, 311–316 CrossRef CAS.
- Y. Xia, L. Wang, J. Li, X. Chen and J. H. Chen, Anal. Chem., 2018, 90, 8969–8976 CrossRef CAS.
- J. S. Sidhu and N. Singh, J. Mater. Chem. B, 2018, 6, 4139–4145 RSC.
- A. Das, D. Roy, M. Mandal, C. Jaiswal, M. Ta and P. K. Mandal, J. Phys. Chem. Lett., 2018, 9, 5092–5099 CrossRef CAS.
- S. Kumari, M. Tiwari and P. Das, Sens. Actuators, B, 2019, 279, 393–399 CrossRef CAS.
- Y. Shu, N. Zheng, A.-Q. Zheng, T.-T. Guo, Y.-L. Yu and J.-H. Wang, Anal. Chem., 2019, 91, 4157–4163 CrossRef CAS.
- J. S. Sidhu, A. Singh, N. Garg, N. Kaur and N. Singh, Sens. Actuators, B, 2019, 282, 515–522 CrossRef CAS.
- J. Wang, J. Wei, S. Su and J. Qiu, New J. Chem., 2015, 39, 501–507 RSC.
- T. Feng, H. J. Chua and Y. Zhao, ACS Biomater. Sci. Eng., 2017, 3, 1535–1541 CrossRef CAS.
- J. Wang, S. Su, J. Wei, R. Bahgi, L. Hope-Weeks, J. Qiu and S. Wang, Physica E, 2015, 72, 17–24 CrossRef CAS.
- H. Chen, Z. Wang, S. Zong, P. Chen, D. Zhu, L. Wu and Y. Cui, Nanoscale, 2015, 7, 15477–15486 RSC.
- S. Liu, IOP Conf. Ser., 2018, 382, 022055 Search PubMed.
- F. Colin, N. Nikolitsa, A. P. Mchale, M. C. Bridgeen and J. F. Callan, Chem. Commun., 2013, 49, 8934–8936 RSC.
- J. Wang, Z. Zhang, S. Zha, Y. Zhu, P. Wu, B. Ehrenberg and J. Y. Chen, Biomaterials, 2014, 35, 9372–9381 CrossRef CAS.
- L. Lin, Y. Wang, Y. Xiao and X. Chen, Anal. Bioanal. Chem., 2019, 411, 2803–2808 CrossRef CAS.
- Y. Shi, C. Li, S. Liu, Z. Liu, J. Zhu, J. Yang and X. Hu, RSC Adv, 2015, 5, 64790–64796 RSC.
- M. Ganiga and J. Cyriac, Sens. Actuators, B, 2016, 225, 522–528 CrossRef CAS.
- Y. Kim, G. Jang and T. S. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 15649–15657 CrossRef CAS.
- L. Linbo, W. Chao, L. Kangyu, W. Yuhan, L. Kun and L. Yuqing, Anal. Chem., 2015, 87, 3404–3411 CrossRef.
- G. Li, W. Kong, M. Zhao, S. Lu, P. Gong, G. Chen, L. Xia, H. Wang, J. You and Y. Wu, Biosens. Bioelectron., 2016, 79, 728–735 CrossRef CAS.
- M. Yang, W. Kong, L. Hao, J. Liu, H. Hui, L. Yang and Z. Kang, Micro. Acta, 2015, 182, 2443–2450 CrossRef CAS.
- J. Chen, Y. Li, K. Lv, W. Zhong, H. Wang, Z. Wu, P. Yi and J. Jiang, Sens. Actuators, B, 2016, 224, 298–306 CrossRef CAS.
- D. Yang, S. Guan, Y. Niu, Z. Xie, S. Zhou and X. Qu, J. Mater. Chem. B, 2018, 6, 2315–2322 RSC.
- F. Yan, Z. Bai, Y. Chen, F. Zu, X. Li, J. Xu and L. Chen, Sens. Actuators, B, 2018, 275, 86–94 CrossRef CAS.
- F. Khakbaz and M. Mahani, Anal. Biochem., 2017, 523, 32–38 CrossRef CAS.
- Y. Q. Dang, Y. J. Zhou, J. T. Cai, G. Y. Liu, Y. T. Zhang and J. S. Qiu, J. Nano Res., 2017, 45, 134–141 CAS.
- T. Zhang, H. Xu, W. He, J. Zhu, Z. Yue, B. Xue, B. Dong and H. Song, RSC Adv, 2017, 7, 28987–28993 RSC.
- Q. Y. Cai, J. Li, J. Ge, L. Zhang, Y. L. Hu, Z. H. Li and L. B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef CAS.
- X. Yan, Y. Song, C. Zhu, H. Li, D. Du, X. Su and Y. Lin, Anal. Chem., 2018, 90, 2618–2624 CrossRef CAS.
- C. Yang, W. Deng, H. Liu, S. Ge and Y. Mei, Sens. Actuators, B, 2015, 216, 286–292 CrossRef CAS.
- Y. Hu, L. Zhang, X. Geng, J. Ge, H. Liu and Z. Li, Anal. Methods, 2017, 9, 5653–5658 RSC.
- J. Jana, T. Aditya, M. Ganguly and T. Pal, Sens. Actuators, B, 2017, 246, 716–725 CrossRef CAS.
- J. Yang, Z. Huang, Y. Hu, J. Ge, J. Li and Z. Li, New J. Chem., 2018, 42, 15121–15126 RSC.
- D. Garg, A. Mehta, A. Mishra and S. Basu, Spectrochim. Acta, Part A, 2018, 192, 411–419 CrossRef CAS.
- K. Srinivasan, K. Subramanian, K. Murugan and K. Dinakaran, Analyst, 2016, 141, 6344–6352 RSC.
- Y. Wang, T. Ma, S. Ma, Y. Liu, Y. Tian, R. Wang, Y. Jiang, D. Hou and J. Wang, Micro. Acta, 2017, 184, 203–210 CrossRef CAS.
- S. Hamd-Ghadareh, A. Salimi, S. Parsa and F. Fathi, Int. J. Biol. Macromol., 2018, 118, 617–628 CrossRef CAS.
- S. Gogoi and R. Khan, Phys. Chem. Chem. Phys., 2018, 20, 16501–16509 RSC.
- X. Shen, L. Xu, W. Zhu, B. Li, J. Hong and X. Zhou, New J. Chem., 2017, 41, 9230–9235 RSC.
- Z. Li, G. Song, Z. Yuan and L. Chao, Sens. Actuators, B, 2017, 241, 821–827 CrossRef CAS.
- H. Ge, K. Zhang, H. Yu, J. Yue, L. Yu, X. Chen, T. Hou, K. A. Alamry, H. M. Marwani and S. Wang, J. Fluoresc., 2018, 28, 1405–1412 CrossRef CAS.
- Y.-S. He, C.-G. Pan, H.-X. Cao, M.-Z. Yue, L. Wang and G.-X. Liang, Sens. Actuators, B, 2018, 265, 371–377 CrossRef CAS.
- W. Dong, R. Wang, X. Gong, W. Liang and C. Dong, Spectrochim. Acta, Part A, 2019, 213, 90–96 CrossRef CAS.
- Y. Shi, Y. Pan, H. Zhang, Z. Zhang, M.-J. Li, C. Yi and M. Yang, Biosens. Bioelectron., 2014, 56, 39–45 CrossRef CAS.
- H. Dai, S. Yan, Y. Wang, Y. Sun, J. Hu, P. Ni and L. Zhuang, Sens. Actuators, B, 2014, 202, 201–208 CrossRef CAS.
- M. Xu, Z. Gao, Z. Qian, Y. Lin, M. Lu and D. Tang, Biosens. Bioelectron., 2016, 86, 978–984 CrossRef CAS.
- X. Wu, Y. Song, X. Yan, C. Zhu, Y. Ma, D. Du and Y. Lin, Biosens. Bioelectron., 2017, 94, 292 CrossRef CAS.
- J. Korram, L. Dewangan, R. Nagwanshi, I. Karbhal, K. K. Ghosh and M. L. Satnami, New J. Chem., 2019, 43, 6874–6882 RSC.
- S. Xu, F. Zhang, L. Xu, X. Liu, P. Ma, Y. Sun, X. Wang and D. Song, Sens. Actuators, B, 2018, 273, 1015–1021 CrossRef CAS.
- Y. Yang, D. Huo, H. Wu, X. Wang, J. Yang, M. Bian, Y. Ma and C. Hou, Sens. Actuators, B, 2018, 274, 296–303 CrossRef CAS.
- M. Amjadi, Z. Abolghasemi-Fakhri and T. Hallaj, J. Photochem. Photobiol., A, 2015, 309, 8–14 CrossRef CAS.
- P. A. Sajid, S. S. Chetty, S. Praneetha, A. V. Murugan, Y. Kumar and L. Periyasamy, RSC Adv., 2016, 6, 103482–103490 Search PubMed.
- S. Li, J. Wang, W. Sheng, W. Wen, Y. Gu and S. Wang, Micro. Acta, 2018, 185, 388 CrossRef.
- C. Wang, R. Tan and D. Chen, Talanta, 2018, 182, 363–370 CrossRef CAS.
- J.-T. Cao, W.-S. Zhang, H. Wang, S.-H. Ma and Y.-M. Liu, New J. Chem., 2019, 43, 1424–1430 RSC.
- X. Cui, L. Zhu, J. Wu, Y. Hou, P. Wang, Z. Wang and M. Yang, Biosens. Bioelectron., 2015, 63, 506–512 CrossRef CAS.
- X. Cheng, Y. Cen, G. Xu, F. Wei, M. Shi, X. Xu, M. Sohail and Q. Hu, Micro. Acta, 2018, 185, 144 CrossRef.
- Y. Ding, J. Ling, H. Wang, J. Zou, K. Wang, X. Xiao and M. Yang, Anal. Methods, 2015, 7, 7792–7798 RSC.
- X. Fu, L. Sheng, Y. Yu, M. Ma, Z. Cai and X. Huang, Sens. Actuators, B, 2018, 269, 278–287 CrossRef CAS.
- H. Tao, X. Liao, C. Sun, X. Xie, F. Zhong, Z. Yi and Y. Huang, Spectrochim. Acta, Part A, 2015, 136, 1328–1334 CrossRef CAS.
- Y. Wang, H. Meng, M. Jia, Y. Zhang, H. Li and L. Feng, Nanoscale, 2016, 8, 17190–17195 RSC.
- M. K. Chini, V. Kumar, A. Javed and S. Satapathi, Nano-Struct. Nano-Objects, 2019, 19, 100347 CrossRef CAS.
- J. Wang, F. Zhang, Y. Wang, Y. Yang and X. Liu, Carbon, 2018, 126, 426–436 CrossRef CAS.
| This journal is © the Partner Organisations 2020 |