Open Access Article
Open Access ArticleUVC-based advanced oxidation processes for simultaneous removal of microcontaminants and pathogens from simulated municipal wastewater at pilot plant scale†
Isaac
Sánchez-Montes
 a,
Irene
Salmerón García
b,
Gracia
Rivas Ibañez
b,
José Mario
Aquino
a,
Irene
Salmerón García
b,
Gracia
Rivas Ibañez
b,
José Mario
Aquino
 a,
María Inmaculada
Polo-López
b,
Sixto
Malato
a,
María Inmaculada
Polo-López
b,
Sixto
Malato
 *b and
Isabel
Oller
*b
*b and
Isabel
Oller
*b
aDepartamento de Química, Universidade Federal de São Carlos, 13565-905, São Carlos (São Paulo), Brazil
bPlataforma Solar de Almería-CIEMAT, Ctra Senés km 4.5, 04200 Tabernas, Almería, Spain. E-mail: sixto.malato@psa.es; isabel.oller@psa.es
First published on 19th June 2020
Abstract
The challenge of providing good-quality reclaimed water free from contaminants of emerging concern, even at small concentrations, i.e., microcontaminants (MCs), and pathogens is one of the main hot topics worldwide. UVC-based advanced oxidation processes, using in situ production of strong oxidizing radicals, such as HO˙ and SO4˙−, have shown high oxidation rates for MCs; however, few studies have focused on the simultaneous removal of MCs and pathogens, like bacteria. Thus, the aim of this work was to assess the oxidation of six MCs, acetaminophen (ACT), caffeine, (CAF), carbamazepine (CBZ), trimethoprim (TMP), sulfamethoxazole (SMX), and diclofenac (DCF), in the presence of Escherichia coli, Enterococcus faecalis, and Salmonella enteritidis in a simulated effluent from a municipal wastewater treatment plant by the application of UVC/H2O2 and UVC/S2O82− processes at pilot plant scale. The concentration of MCs and bacteria was monitored along the oxidation processes as well as their regrowth after 24, 48, and 144 h. UVC-based processes were compared in terms of the required treatment time to remove at least 80% of the sum of MCs, regrowth assessment, and energy consumption. Despite the UVC/H2O2 and UVC/S2O82− processes showing similar results, even after using distinct molar concentrations, the UVC/H2O2 process did not exhibit bacterial regrowth under dark conditions. A simple model has also been proposed in this work with the main objective of calculating the minimum concentration of oxidants as a function of the radiation absorption at 254 nm in a given photo-reactor setup.
Water impactFresh water free from microcontaminants (MCs) and pathogens is one of the main hot topics worldwide. UVC advanced oxidation processes producing strong oxidizing radicals have shown high oxidation rates; however, few studies focused on the simultaneous removal of pathogens at pilot scale. These processes were compared in terms of the required treatment time to remove at least 80% of the sum of microcontaminants, regrowth assessment, and energy consumption. |
1. Introduction
Assurance of safe reclaimed water free of chemical and microbiological contaminants is a serious global concern that is increasing with population growth and uncertain climate changes. Wastewater effluents treated by conventional methods can contain a huge amount of microcontaminants (MCs) (pesticides, pharmaceuticals, personal care products, etc.) and pathogens (bacteria, viruses, protozoa, and parasites) that may lead to toxic effects in humans when reaching fresh water sources.1–4 In addition, water supplies from nontraditional sources, including treated municipal wastewater, have been proposed as feasible options in recent years.5,6 Unfortunately, consolidated tertiary treatments such as UVC radiation, ozonation and chlorination are not effective enough or present serious drawbacks in their application to remove MCs. UVC irradiation (200–280 nm) has been extensively used for water disinfection; however, serious limitations such as microbial regrowth (due mainly to the lack of residual effect) and mechanisms of self-repair of microorganisms' DNA were observed.7,8 In addition, depending on the chemical structure of the target molecule, UVC irradiation is not appropriate for MC elimination.9 Chlorination and ozonation can inactivate pathogens and remove MCs, though generation of toxic disinfection by-products, e.g., organochlorine, nitrosodimethylamine, and bromate, has been their main drawback.7,10–12 Other potential disinfection methods such as ultrasonication, hydrodynamic cavitation, membrane filtration, and electrochemical oxidation have been effectively used to inactivate or remove microorganisms from water as a low use of chemicals is needed;13–16 however, both physical and electrochemical technologies have common shortcomings including high energy consumption and operating costs as well as the requirement of designing sophisticated reactors.To overcome these problems, UVC-based advanced oxidation processes (UVC AOPs) represent a feasible alternative to conventional tertiary treatments.17,18 UVC AOPs have been investigated for the production of a huge variety of highly reactive free radicals, e.g., HO˙, Cl˙, SO4˙−, CO3˙−, that lead to high elimination rates of microorganisms and contaminants.19–22 In addition, commercial UVC reactors have already been used in water and wastewater treatment plants so they could be easily adapted for these processes.23 In comparison with other AOPs such as Fenton and photo-Fenton, these processes do not require pH adjustments and addition of Fe ions, which simplifies operational requirements.
The UVC/hydrogen peroxide (UVC/H2O2) process is one of the most disseminated AOPs used for organic compound degradation and disinfection.24,25 In this process, the non-selective HO˙ species can be produced from the homolytic cleavage of H2O2 by absorption of UVC radiation (mainly at 254 nm), with a quantum efficiency of 0.5 mol Es−1 (eqn (1)).26,27 Due to its high oxidizing power (E°(HO˙/H2O) = 1.8–2.7 V), HO˙ can cause irreversible damage in microorganisms having the advantage of reacting non-selectively with organic compounds through electron transfer, hydrogen atom abstraction or electrophilic addition.28
| H2O2 + hv → 2HO˙ (ϕ = 0.5 mol Es−1) | (1) |
| S2O82− + hv → 2SO4˙− (ϕ = 1.4 mol Es−1) | (2) |
| SO4˙− + H2O → HO˙ + SO42− + H+ | (3) |
In this context, this work aimed to investigate and compare the use of the UVC/H2O2 and UVC/S2O82− processes for the simultaneous removal of MCs and pathogens from a simulated municipal wastewater secondary effluent at pilot plant scale. Escherichia coli, Enterococcus faecalis, and Salmonella enteritidis were selected as target microorganisms because they are used as pathogen indicators in regulations and guidelines for wastewater disposal and reuse.38,39 Six MCs, acetaminophen (ACT), caffeine, (CAF), carbamazepine (CBZ), trimethoprim (TMP), sulfamethoxazole (SMX) and diclofenac (DCF), were chosen as target molecules since they are usually detected in municipal wastewater.40 In addition, this work intends to compare UVC/H2O2 and UVC/S2O82− processes in terms of treatment time, consumption of chemicals, and the influence of the oxidant residual concentration on the bacterial regrowth. Finally, a simple model based on the optical path length of the UVC radiation is proposed to determine the most suitable oxidant concentration to be used in these systems to simultaneously remove MCs and pathogens.
2. Experimental section
2.1 Chemicals
ACT, CBZ, TMP, SMX, and DCF were purchased from Sigma-Aldrich (>99%). Caffeine (CAF) was provided by Fluka (>99%). H2O2 (35%), Na2S2O8 (>98%), KI (>99.5%), Na2S2O3 (>99%), bovine liver catalase, phosphate-buffered saline, acetonitrile (UHPLC-grade), and formic acid (UHPLC-grade) were purchased from Sigma-Aldrich. All chemicals were used as received. The MC stock solution for experiments was prepared in methanol at 2.5 g L−1 each to avoid hydrolysis of MCs and allow spiking low volumes of water with very low quantities of methanol in the pilot plant. Simulated municipal wastewater (SMWW) secondary effluent was used as the wastewater model. The resulting physicochemical properties of the prepared SMWW effluent are shown in Table 1.| Parameters | |
|---|---|
| pH | 7.6 ± 0.3 |
| Conductivity (mS cm−1) | 1.4 ± 0.1 |
| Turbidity (NTU) | 3.4 ± 0.2 |
| DOC (mg L−1) | 15.5 ± 0.6 |
| DIC (mg L−1) | 13.5 ± 1.2 |
This matrix was prepared after adaption of the procedure described in Zhang et al.41 and in the APHA Standard Methods,42 using the following chemicals:
(i) Inorganics salts: NaHCO3 (96 mg L−1), MgSO4 (60 mg L−1), NaCl (580 mg L−1), and K2HPO4 (7.0 mg L−1) (Sigma-Aldrich); CaSO4·2H2O (60 mg L−1) and (NH4)2SO4 (23.6 mg L−1) (Panreac); KCl (4 mg L−1) (J.T. Baker).
(ii) Organic matter: beef extract (1.8 mg L−1) and peptone (2.7 mg L−1) (Biolife); humic acid (4.2 mg L−1), sodium lignin sulfonate (2.4 mg L−1) and sodium lauryl sulphate (0.9 mg L−1) (Sigma-Aldrich); tannic acid (4.2 mg L−1) and acacia gum powder (4.7 mg L−1) (Panreac).
It is important to remark that SMWW characteristics are highly similar to those of the actual MWWTP effluent and selected MCs and pathogens to be monitored were spiked at concentrations in the range of those actually found in such effluents.
2.2 Analyses
Other parameters were also monitored, such as pH (GLP 22 pH meter, CRISON), conductivity (GLP 31 conductometer, CRISON), and turbidity (2100 N turbidimeter, HACH). Dissolved organic carbon (DOC) and dissolved inorganic carbon (DIC) were measured using a TOC-VCSN analyzer (Shimadzu) in filtered samples through 0.45 μm nylon filter (AISIMO).
Ion chromatography was used to measure the concentration of various ions in samples previously filtered (0.45 μm nylon filter), using a Metrohm 850 Professional analyzer. For anion determination, a Metrosep A Supp 7150/4.0 column at 45 °C and 3.6 mmol L−1 sodium carbonate at 0.7 mL min−1 were used as the stationary and mobile phase, respectively. A Metrosep C6 150/4.0 column and 1.7 mmol L−1 solutions of nitric acid and dipicolinic acid at 1.2 mL min−1 were used for cation quantification.
The concentration of oxidants was determined by two different methods using a UV-vis Evolution 220 spectrophotometer (Thermo Scientific). H2O2 concentration was analyzed spectrophotometrically at 410 nm after adding 0.5 mL of titanium(IV) oxysulfate to 5 mL of a filtered sample (DIN 38402H15). S2O82− concentration was monitored by using an iodometric method adapted from Liang et al.43 Briefly, 3.5 mL of 50 g L−1 KI solution and 0.5 mL of 5 g L−1 NaHCO3 solution were added to 1 mL of previously filtered sample, allowed to react for 15 min, and then the absorbance at 352 nm was measured.
Bacterial quantification was performed by the standard plate counting method using specific culture media: Chromocult® (Merck), Slanetz–Bartley agar (1% TTC) (Scharlau), and Salmonella Shigella agar (Scharlau) for E. coli, E. faecalis, and S. enteritidis, respectively. When the bacterial concentration expected was lower than 2 × 102 CFU per 100 mL, samples were processed by the membrane filtration method. For each bacterium, 100 mL of sample were filtered using a 0.45 μm-pore-size cellulose nitrate membrane (Sartorius) and a Microfil filtration system (Millipore). Then, the obtained membranes were plated in the corresponding medium and incubated at 37 °C. E. coli colonies were counted after 24 h; E. faecalis and S. enteritidis samples were counted after 48 h. The detection limit (DL) of this technique is 1 CFU per 100 mL, taking into account the minimum disinfection level required by the Spanish legislation for reusing reclaimed wastewater (RD 1620/2007).44 A control sample (without treatment) for each bacterium was plated before and after the experiment to guarantee the strain's good quality.
When oxidant reagents were used, a proportional volume of bovine liver catalase solution (0.1 g L−1) or sodium thiosulfate (10 mmol L−1) was added to the samples in order to quench residual H2O2 and S2O82−, respectively. Regrowth of bacteria was quantified in predetermined samples stored at room temperature for 24, 48, and 144 h (6 days) (quencher was not added for this analysis). Disinfection experiments were carried out in duplicate and average values were plotted. The inactivation kinetics of each bacterium observed during the UVC-based treatments were calculated by using Chick–Watson's equation.45 The results were reproducible and the standard deviation of the replicates is shown in the graphs as error bars.
2.3 UVC pilot plant description and experimental procedure
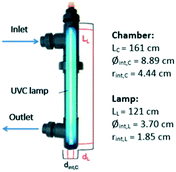
UVC, UVC/H2O2, and UVC/S2O82− experiments were carried out by using a UVC pilot plant previously described by Cerreta et al.46Fig. 1 shows a schematic configuration of the reactor containing the UVC lamp. The pilot plant consists of three medium pressure UVC lamps (230 W with radiation emission at 254 nm) protected by quartz tubes (Øint = 3.7 cm) and axially located in a stainless steel cylindrical photoreactor (Øint = 8.9 cm). The flexible design of the system allows the use of one, two or three lamps in batch or continuous flow mode. In this study, a single lamp was used in batch mode (recirculation flow rate 36 L min−1) with a total volume of 80 L.The total irradiated surface of the photoreactor (one lamp; Sp = 0.34 m2) and the illuminated volume (one lamp; Villu = 6.21 L) were calculated according to eqn (4) and (5) taking into account the specific characteristics of the lamp and its frame.
| Villu(L) = LLπ(rint,C2 − rint,L2) | (4) |
| Sp(m2) = 2πRint,CLL | (5) |
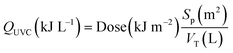 | (6) |
In UVC AOP experiments, the system's reservoir was filled with 80 L of SMWW secondary effluent and the required quantity of a stock solution of MCs was added to obtain an initial concentration of 100 μg L−1 of each compound. The sum of these concentrations for the six selected MCs is considered higher but very close to the range normally found in effluents of municipal wastewater treatment plants.47–50 Then, each bacterial stock was added to obtain 105 CFU per 100 mL per bacterium. After 15 min of homogenization (UVC lamp switched off), an initial sample was taken to check the initial concentration of both MCs and bacteria. Then, H2O2 (5, 15, 25, 35, and 50 mg L−1) or S2O82− (20, 40, and 100 mg L−1) was added to the reservoir tank. After homogenization (10 min), another sample was collected to verify the effect of the oxidants on the concentration of MCs and bacteria in the dark (any significant variation in initial concentrations was observed in either of the experiments performed, data not shown). Then the UVC lamp was switched on and the experiment started. Samples were collected at predetermined and regular time intervals to analyze simultaneously the degradation of MCs, inactivation of bacteria and reagent evolution along all the UVC-based experiments performed in this study.
3. Results and discussion
3.1 UVC treatment
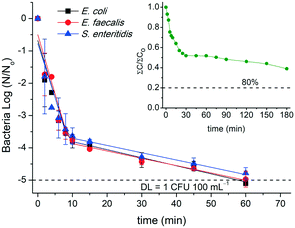
Results of simultaneous inactivation of bacteria and degradation of MCs using only UVC light in the SMWW secondary effluent are depicted in Fig. 2. A high inactivation rate of E. coli, E. faecalis, and S. enteritidis was obtained under UVC irradiation. The strong effect of the UVC light (mainly at 254 nm) observed on bacterial inactivation is based on the occurrence of very specific damage on DNA and other essential components such as proteins, lipids, membrane, etc., that inhibits its duplication and consequently bacterial reproduction. The typical UVC damage induces the formation of thymine–thymine cyclobutane cys–syn thymine–thymine photodimers and pyrimidine (6–4) pyrimidine photoproducts (TT (6–4) photoproducts).51 No significant differences in the irradiation time required (60 min) to reach the detection limit (DL) and in the calculated pseudo-first order kinetic constants (k) of bacterial inactivation were observed. A double log-linear kinetic characterized by a fast inactivation in the first stage (k1) followed by a slow second inactivation stage (k2) was observed for all bacteria (see Fig. 2). The k1 for E. coli, E. faecalis, and S. enteritidis were 3.5 ± 0.6 × 10−1, 3.6 ± 0.5 × 10−1, and 3.3 ± 0.7 × 10−1 min−1, respectively, while the second stage, k2, was around 0.23 ± 0.02 × 10−1 min−1 for all pathogens. Clearly, the inactivation process is governed by the first stage. In addition, for all bacteria, the accumulative UVC energy needed to reduce 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log the initial concentration (105 CFU 100 mL−1) was around 1.2 kJ L−1 and 0.09 kJ L−1 for a 3.5
log the initial concentration (105 CFU 100 mL−1) was around 1.2 kJ L−1 and 0.09 kJ L−1 for a 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (around 8 min of illumination). Similarly, Rodríguez-Chueca et al.17 did not observe differences in the energy consumption for E. coli and E. faecalis inactivation under UVC irradiation using real and simulated wastewater. In that paper, a 3.5
log reduction (around 8 min of illumination). Similarly, Rodríguez-Chueca et al.17 did not observe differences in the energy consumption for E. coli and E. faecalis inactivation under UVC irradiation using real and simulated wastewater. In that paper, a 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in bacteria concentration demanded 0.057 kJ L−1 of UVC energy, working in continuous flow mode but at laboratory scale. The upper energy consumption reported in the present work might come from the scaling-up to pilot plant scale as well as differences in the reactor design setup.
log reduction in bacteria concentration demanded 0.057 kJ L−1 of UVC energy, working in continuous flow mode but at laboratory scale. The upper energy consumption reported in the present work might come from the scaling-up to pilot plant scale as well as differences in the reactor design setup.
Increase of temperature resulting from the operation of the UVC lamp was also monitored during all experiments and the maximum-recorded T remained around 30 °C. This temperature range did not affect the bacterial viability since temperatures higher than 45 °C are necessary to generate thermal damage on the investigated bacteria.52,53 In addition, this temperature interval should not increase the efficiency of UVC/H2O2 or UVC/S2O82− processes, as temperatures higher than 40 °C and 50 °C, respectively, are normally required.54,55
Under certain conditions, UVC light has no effect on the cell wall as the mechanisms of self-repair of microorganisms reverse the DNA damage produced by light absorption.8 As the UVC process does not generate residual oxidants, reactivation of injured microorganisms is expected if favorable conditions are presented; such as the presence of nutrients related to wastewater (e.g., organic matter and inorganic salts) that provide a food source for bacteria, allowing them to metabolize and reproduce.56 Therefore, bacterial regrowth was analyzed in selected samples of experiments (shown in Fig. 2) stored in the dark after 24, 48, and 144 h (6 days) at room temperature. Although an apparent complete inactivation of E. coli, E. faecalis, and S. enteritidis was attained within 60 min under UVC irradiation, regrowth assessment in samples collected after 75 min of UVC treatment was carried out for all bacterial strains. E. coli had an exponential increase in the concentration of viable bacteria after 48 and 144 h in the dark, with values of 2.3 and 3.5 log, respectively. In contrast, the regrowth assessment for S. enteritidis decreased from 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log in 48 h to 0.2
log in 48 h to 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log after 144 h, probably due to the lack of essential nutrients for its viability. Regrowth assessment for E. faecalis was not done in the stored samples after 24 and 48 h, but it was observed (1
log after 144 h, probably due to the lack of essential nutrients for its viability. Regrowth assessment for E. faecalis was not done in the stored samples after 24 and 48 h, but it was observed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log) after 144 h. These regrowth tests offer a good evaluation of the effectiveness of a process and the ability to handle post-treated effluents, which could remain stored in the dark several days before its further reuse. In this sense, UVC technology is not fully recommended for municipal wastewater secondary effluent disinfection, even less for reclaimed final purposes.
log) after 144 h. These regrowth tests offer a good evaluation of the effectiveness of a process and the ability to handle post-treated effluents, which could remain stored in the dark several days before its further reuse. In this sense, UVC technology is not fully recommended for municipal wastewater secondary effluent disinfection, even less for reclaimed final purposes.
On the other hand, the inset in Fig. 2 shows the degradation profile for the sum of MC concentrations (∑Ct/∑C0) in the SMWW secondary effluent during the UVC process. For analysis purposes and considering, as an example, environmental regulations already established in Switzerland for MC elimination from MWWTPs, experiments were performed with the aim of removing 80% of total MCs.57,58 UVC radiation significantly decreased the total amount of MCs (60%) in the effluent after 180 min (3.8 kJ L−1 accumulated UVC radiation required); however, it was not enough to attain the degradation target of 80%. Clearly, some MCs demanded a longer irradiation time (and so higher accumulative UVC energy) to be oxidized than that required for reaching complete bacterial inactivation, but others were slightly affected by UVC irradiation. However, it is important to highlight that though complete elimination of the sum of MCs was not achieved, some of them attained degradation percentages higher than 75%. This behavior is in agreement with the different absorption capacities of UVC light by the organic compounds, measured by the quantum yield and the molar absorption coefficient at 254 nm. These two fundamental parameters govern the direct photolysis rate; thus, molecules with moderate values of these parameters will be more sensitive to degradation under UVC irradiation. The chemical structures, absorbance (at 254 nm), quantum yields, molar absorption coefficients, and UV absorption spectrum of these compounds can be seen in Table S2 and Fig. S2.† In this sense, DCF and SMX, with high values of these parameters at 254 nm, were substantially removed (<DL) in the beginning of the experiment, i.e., in 20 min (0.3 kJ L−1 accumulative UVC energy) and 30 min (0.6 kJ L−1 accumulative UVC energy), respectively. On the other hand, 75% of ACT was removed only after 180 min (3.8 kJ L−1 of accumulative UVC energy), while CBZ, CAF, and TMP showed the lowest degradation percentages (20%, 30%, and 40%, respectively) since they have low molar absorption and quantum yield. These results are in accordance with the findings of Yu et al.,9 which classified several pollutants based on their relative reactivity towards UVC direct photolysis. DCF and SMX were considered easily photodegraded by UVC light with no additional oxidants, whereas CAF, CBZ, and TMP were classified as photo-resistant but highly reactive with HO˙ radicals. Cerreta et al.18 also reported that SMX was almost completely removed in natural and distilled water after 30 min of treatment (90%; 0.7 kJ L−1 accumulative UVC energy) using only UVC, while only 18% of CBZ degradation was observed after 120 min (2.7 kJ L−1 accumulative UVC energy). Other parameters such as pH, conductivity, turbidity, DOC, DIC, and ion concentration were also monitored but had an insignificant variation throughout the experiments, as expected (data not shown). It is important to mention that specifically, a decrease in the organic load (measured by COD) is expected to provoke an increase of light penetration in the system. Consequently, this might result in an improvement of UVC-based AOP efficiency considering MC removal and bacterial inactivation.
3.2 UVC/H2O2 treatment for simultaneous bacterial inactivation and MC elimination
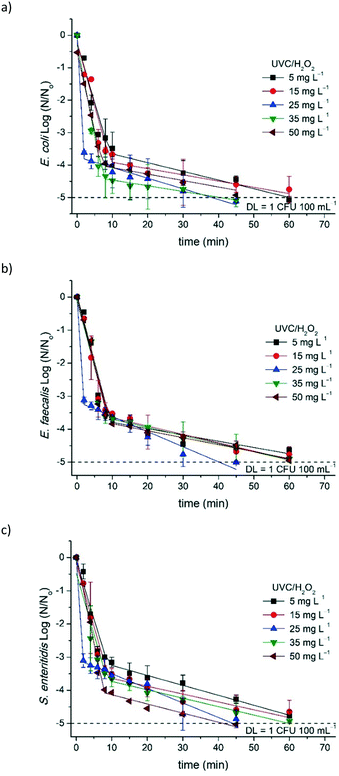
Fast inactivation rates of E. coli, E. faecalis, and S. enteritidis were observed for UVC/H2O2 experiments in all concentrations investigated (5–50 mg L−1), as it can be observed in Fig. 3. Similar to the UVC results, double log-linear kinetics was observed for all bacterial inactivation. However, in comparison with these results, the addition of H2O2 did not entail an improvement in the disinfection process since the pseudo-first-order inactivation kinetic constants (k1 and k2) did not show a significant increase (see Table 2). Only at higher H2O2 concentrations (25, 35, and 50 mg L−1), it was observed a slight increase in the inactivation kinetic constants and a reduction in the irradiation time to attain the DL. In particular, for 25 mg L−1 H2O2, bacterial inactivation was very fast in the first stage, so it was not possible to fit the curve to calculate k1. According to these results, the main inactivation mechanism came from the effect of UVC radiation rather than from damage produced by HO˙ generated through H2O2 photolysis.| Process | Bacteria – k1/k2 (10−1 min−1) | Total MCs – k (10−2 min−1) | ||||
|---|---|---|---|---|---|---|
| UVC/H2O2 (mg L−1) | E. coli | E. faecalis | S. enteritidis | (∑Ct/∑C0) | Timea (min) | Q UVC (kJ L−1) |
| a Values refer to the attainment of 80% removal of total MCs except for the UVC alone experiment, in which only 60% of total MC removal was attained. b Accumulative UVC energy required to attain 80% removal of total MCs. Values in parentheses refer to coefficient of determination (R2).ND = not determined. | ||||||
| 0 | 3.5 ± 0.6 (0.86)/0.24 ± 0.02 (0.97) | 3.6 ± 0.5 (0.90)/0.23 ± 0.02 (0.96) | 3.3 ± 0.7 (0.87)/0.22 ± 0.02 (0.97) | 0.5 (0.82) only 60% | 180 | 3.8 |
| 5 | 3.7 ± 0.5 (0.90)/0.27 ± 0.04 (0.90) | 4.0 ± 0.5 (0.93)/0.19 ± 0.04 (0.81) | 3.5 ± 0.5 (0.90)/0.30 ± 0.02 (0.98) | 1.2 (0.94) | 120 | 2.5 |
| 15 | 3.9 ± 0.6 (0.88)/0.19 ± 0.04 (0.77) | 3.9 ± 0.5 (0.91)/0.25 ± 0.04 (0.89) | 3.8 ± 0.4 (0.94)/0.24 ± 0.04 (0.86) | 3.0 (0.97) | 52 | 1.0 |
| 25 | ND/0.33 ± 0.03 (0.93) | ND/0.45 ± 0.4 (0.95) | ND/0.44 ± 0.04 (0.94) | 5.7 (0.99) | 24 | 0.4 |
| 35 | 5.6 ± 0.9 (0.93)/0.18 ± 0.02 (0.91) | 4.1 ± 0.7 (0.89)/0.25 ± 0.01 (0.98) | 3.8 ± 0.7 (0.87)/0.25 ± 0.02 (0.97) | 7.0 (0.99) | 21 | 0.4 |
| 50 | 4.8 ± 0.9 (0.88)/0.26 ± 0.03 (0.96) | 4.2 ± 0.5 (0.92)/0.21 ± 0.01 (0.97) | 5.3 ± 0.4 (0.97)/0.27 ± 0.03 (0.93) | 10.0 (0.99) | 17 | 0.3 |
Similarly, Pablos et al.19 and Yoon et al.59 suggested that the germicidal effect of UVC absorption by bacterial DNA in E. coli K12 and DH5α strains, respectively, is the main inactivation mechanism and no significant differences were observed in the values of k for UVC and UVC/H2O2. Moussavi et al.60 also reported that the enhancement on inactivation of E. coli by adding H2O2 was hardly noticeable compared to UVC in the treatment of hospital wastewater. In contrast, Rubio et al.61 reported a significant enhancement in E. coli K12 inactivation in natural water after addition of H2O2 compared to the use of only UVC light. The k increased 150% for the combined process. Moreover, Moreno-Andrés et al.62 indicated that the addition of H2O2 (10 mg L−1) to the UVC system improved the disinfection efficiency of E. faecalis in salty water. Probably, the sum of the effects of UVC irradiation and HO˙ species was the major route for bacterial inactivation in those studies, and in other cases HO˙ species compensated for the absorption of photons at 254 nm by H2O2.
The inactivation efficiency of microorganisms using this process may also depend on many factors, such as the hydrodynamic parameters of the photoreactor, power of the UVC lamp (or UVC energy dose), light path length of the photoreactor, water matrix, oxidant concentration, type of bacterial strains, etc. Moreno-Andrés62 also reported a detrimental effect on bacterial inactivation due to the addition of a high concentration of H2O2 (100 mg L−1) based on the competition for the absorption of photons at 254 nm between bacteria and the oxidant. This detrimental effect was not observed under the operating conditions used in this work, which would probably appear at higher concentrations of H2O2 or longer light path length in which the competition for photon absorption would be significantly higher (see section 3.4).
It is important to note that the photolysis percentage of H2O2 was only 10% after 60 min independently of the concentration used. This means a consumption rate of 0.008, 0.045, and 0.076 mg H2O2 L−1 min−1 for 5, 25 and 50 mg L−1, respectively, which explain the poor contribution of low H2O2 concentrations on bacterial inactivation in comparison with the UVC effect.
In this case, for concentrations between 25 and 50 mg L−1, high residual concentrations of this oxidant were present in the treated wastewater. This suggests that the slight enhancement on bacterial inactivation observed at high concentrations might be due to a small fraction of HO˙ generated or to the direct disinfectant effect of H2O2, since it is well known that at high concentrations this oxidant has a toxic effect on bacterial viability. Rodríguez-Chueca et al.52 reported 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log and 1.5
log and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction of E. coli and E. faecalis, respectively, after 180 min in the dark using 50 mg L−1 of H2O2. At lower concentrations (20 mg L−1) this effect is not significant on inactivation of E. coli O157:H7 and S. enteritidis, as reported by Nahim-Granados et al.53
log reduction of E. coli and E. faecalis, respectively, after 180 min in the dark using 50 mg L−1 of H2O2. At lower concentrations (20 mg L−1) this effect is not significant on inactivation of E. coli O157:H7 and S. enteritidis, as reported by Nahim-Granados et al.53
As discussed in the UVC reference experiment, bacterial regrowth was monitored in the treated samples due to their self-repair capacity in the dark. In the presence of H2O2, regrowth was not observed after the treatment (sample withdrawn after 75 min) under any of the conditions tested (5–50 mg L−1) and for all times analyzed (24, 48 and 144 h). H2O2 concentration in all experiments remained constant in the dark until 144 h of storage. Therefore, the remaining H2O2 has a possible further bacteriostatic effect, preventing bacterial repair/reproduction during the storage or through the distribution system. Other studies report assessment of bacterial regrowth but only after 24 and 48 h.17,63,64
The effect of H2O2 concentration on MC degradation was also evaluated (Fig. 4). The use of H2O2 in combination with UVC irradiation increased the removal of all target compounds compared with UVC alone, especially those that are photo-stable, CAF, CBZ, and TMP, resulting in a degradation rate of over 80% for the sum of MCs under all investigated conditions. This effect was mainly caused by the effect of HO˙ generated in H2O2 photolysis at 254 nm (see eqn (1)). Illumination time (and accumulative UVC energy) required to achieve 80% MC degradation decreased with the increase in H2O2 concentration, e.g., from 120 min (2.5 kJ L−1 accumulative UVC energy required) with 5 mg L−1 to 17 min (0.3 kJ L−1 accumulated UVC energy) with 50 mg L−1 H2O2 (Table 2).
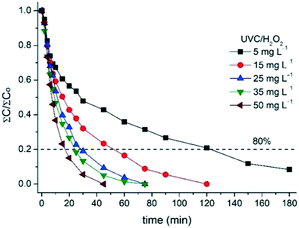 | ||
| Fig. 4 Effect of H2O2 concentration on the total MC degradation by UVC/H2O2as a function of treatment time in SMWW secondary effluent. Dashed line refers to 80% removal of total MCs (∑Ct/∑C0). | ||
Clearly, UVC/H2O2 at 5 mg L−1 required a higher UVC energy dose to degrade MCs than that required for reaching complete bacterial inactivation. Pseudo-first-order kinetic constants (k) corresponding to these experiments are also shown in Table 2. It was noticed that the degradation kinetic constants of this process were strongly dependent on the initial concentration of the oxidant. That behavior can be observed in Fig. S3,† which shows a linear relationship between kinetic constants and initial concentrations of H2O2. Despite this, it is important also to highlight that while kinetic constants increased proportionally with H2O2 till 0.1 min−1 for 50 mg L−1 H2O2, the illumination time required to reach 80% of degradation varied slightly when using 35 and 25 mg L−1 H2O2 and ranged from 21 to 24 min, respectively. In addition, oxidant residual concentrations after treatment were 49 and 23 mg L−1 for 50 and 25 mg L−1 H2O2, respectively, showing a limitation caused by the light path length and therefore by the photoreactor configuration, which will be addressed in section 3.4.
For a better understanding of the effect of the UVC/H2O2 process on MC removal, the degradation profile of each compound and the oxidant consumption are detailed in Fig. S4.† Moreover, Table S3† shows the calculated k for each contaminant as a function of H2O2 concentration used. CAF, TMP, and CBZ, which did not exhibit a high photodegradation percentage (20–40%), were significantly removed using 5 mg L−1 H2O2 under UVC irradiation, attaining removal rates of over 80% after 180 min and with a H2O2 consumption close to 1.0 mg L−1. For this condition, only 0.8 mg L−1 H2O2 and 2.5 kJ L−1 were required to eliminate 80% of the total MCs. A higher increase in the degradation rate was observed for UVC/H2O2 with 25 mg L−1, reaching 80% of removal in 24 min and consuming 2.3 mg L−1 of the oxidant. As expected, 80% of total MCs was quickly achieved using 50 mg L−1 (17 min; 0.3 kJ L−1 accumulated UVC energy) with a H2O2 consumption of 1.3 mg L−1. Similar values were also found by Miralles-Cuevas et al.65 for 90% removal of several MCs. The complete elimination (<DL) of all MCs was attained in 60 min (1.2 kJ L−1 accumulated UVC energy) and using 4.6 mg L−1 H2O2. It is important to note that for the same process, the degradation kinetic constant for the photo-stable compounds did not show significant differences, which confirm the non-selectivity of the generated HO˙ species by the H2O2 homolysis. On the other hand, DOC decreased around 10% only for high concentrations of H2O2 (25–50 mg L−1; data not shown).
3.3 UVC/S2O82− treatment for simultaneous bacterial inactivation and MC degradation
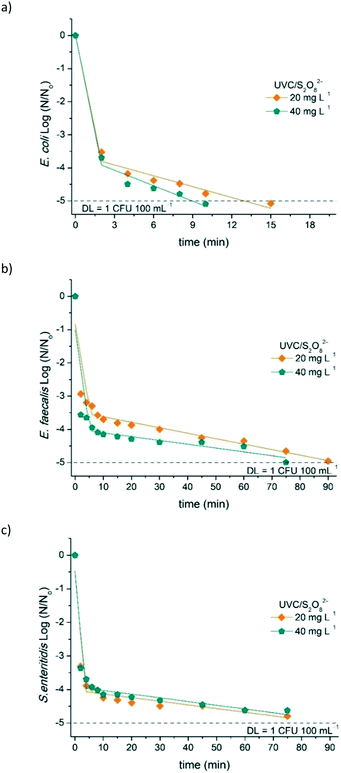
E. coli, E. faecalis, and S. enteritidis inactivation by the UVC/S2O82− process is shown in Fig. 5. As it can be observed, E. coli was quickly inactivated under the two investigated conditions, improving significantly the pseudo-first-order kinetic constants obtained with the UVC process (see Table 3). Due to the high rate of E. coli inactivation under these conditions, it was not possible to calculate the kinetic constants k1 (first stage). DL was achieved only after 10 min (0.1 kJ L−1 accumulated UVC energy) and 15 min (0.2 kJ L−1 accumulated UVC energy) for 40 and 20 mg L−1 S2O82−, respectively. Several studies attributed the bacterial inactivation in the UVC/S2O82− system to the selectivity and reactivity of generated SO4˙− species (see eqn (2)), which reacts with macromolecules that are present in the cell wall. Michael-Kordatou et al.66 reported a significant enhancement (around 200%) to reduced 5-log of E. coli in urban wastewater after the addition of S2O82−. Popova et al.67 also reported an increase (>130%) in the rate constant to eliminate E. coli when using the UVC/S2O82− process.| Process | Bacteria – k1/k2 (10−1 min−1) | Total MCs – k (10−2 min−1) | ||||
|---|---|---|---|---|---|---|
| UVC/S2O82− (mg L−1) | E. coli | E. faecalis | S. enteritidis | (∑Ct/∑C0) | Timea (min) | Q UVC (kJ L−1) |
| a Values refer to the attainment of 80% removal of total MCs except for the UVC alone experiment, in which only 60% of total MC removal was attained. b Accumulative UVC energy required to attain 80% removal of total MCs. Values in parentheses refer to coefficient of determination (R2).ND = not determined. NM = not measured. | ||||||
| 0 | 3.5 ± 0.6 (0.86)/0.24 ± 0.02 (0.97) | 3.6 ± 0.5 (0.90)/0.23 ± 0.02 (0.96) | 3.3 ± 0.7 (0.87)/0.22 ± 0.02 (0.97) | 0.5 (0.82) only 60% | 180 | 3.8 |
| 20 | ND/1.1 ± 0.1 (0.87) | 5.1 ± 2.1 (0.53)/0.2 ± 0.01 (0.95) | 6.2 ± 2.7 (0.59)/0.1 ± 0.02 (0.80) | 1.6 (0.99) | 90 | 1.8 |
| 40 | ND/1.5 ± 0.3 (0.84) | 6.0 ± 2.8 (0.52)/0.1 ± 0.01 (0.87) | 6.0 ± 2.7 (0.57)/0.1 ± 0.01 (0.92) | 4.2 (0.98) | 45 | 0.9 |
| 100 | NM | NM | NM | 11.6 (0.98) | 24 | 0.4 |
On the other hand, comparison with systems based on the generation of HO˙, such as UVC/H2O2, is difficult to address. From a purely chemical approach, the best option is to compare the efficiency of these systems using an equivalent molar ratio of both oxidants, but the high S2O82− molar mass implies very high concentrations in terms of mass per unit of volume.64 This would lead to a drastic increase in the operating costs of this process. In this sense, the focus of this study was to assess the behavior of this system under realistic conditions, rather than strictly compare kinetics with UVC/H2O2. It must be highlighted that H2O2 and S2O82- selected concentrations were in the range of those successfully studied in previous research works.17,30,64 In addition, other factors such as the stronger selective oxidation capability towards macromolecules/biomolecules of the cell membrane and the half-life of the produced radical can favor a stronger action of SO4˙−.
Wordofa et al.68 showed that exposure to SO4˙− promoted the loss of cell viability of E. coli O157:H7 5 times faster than when HO˙ was used. This unique feature of SO4˙− is possibly associated with its highly selective reactivity towards electron-rich moieties on the surface of E. coli O157:H7 cell membranes, such as flagella, proteins, and extracellular polymeric substances. Moreover, Serna-Galvis et al.69 also attributed the microorganism inactivation by UVC/S2O82− to the already commented high interaction of SO4˙− with organic macromolecules of the cell wall.
As can be seen in Fig. 5b and c, the UVC/S2O82− system was less effective on E. faecalis and S. enteritidis inactivation. Both bacteria were inactivated within 75–90 min (1.2–1.5 kJ L−1 accumulated UVC energy, respectively) for the two tested concentrations. Clearly, this result indicates a different inactivation mechanism, probably related to structural differences and cellular composition between these bacteria. In particular, E. faecalis, which required 90 and 75 min to attain the DL using 20 and 40 mg L–1 of S2O82−, respectively, has a structural difference with E. coli regarding the cell wall components. E. faecalis has a thicker cell wall in which the major component is the peptidoglycan layer. In contrast, E. coli has a thin layer of peptidoglycan together with an outer membrane that results in a more complex structure. These differences make Gram-negative bacteria (e.g., E. coli) more sensitive than Gram-positive (e.g., E. faecalis) with respect to UV-based treatments.63,70 Although both E. coli and S. enteritidis are Gram-negative bacteria, the latter one showed higher resistance to inactivation, which could be due to the presence of different sugars and sugar linkages that form the lipopolysaccharide,71 the major component of the Gram-negative bacterial outer membrane. Wordofa et al.68 also reported that the efficiency of these processes are dependent on the specific composition of macromolecules for each bacterial group. Another possible mechanism is related to the detrimental effect on bacteria inactivation based on the competition for the absorption of photons at 254 nm between bacteria and oxidant, since S2O82− has a high capacity of light absorption at this wavelength.26 While some fundamental investigations on the inactivation of different microorganisms by SO4˙− have been established, the molecular mechanisms of inactivation, particularly the interaction of SO4˙− with biomolecules, are far from complete comprehension.
Bacterial regrowth assessment was carried out after 24, 48, and 144 h after the treatment was finished. Regrowth for all bacteria was detected for 20 and 40 mg L−1 S2O82−, but much lower compared with that using only the UVC treatment. In particular, E. faecalis and S. enteritidis had a maximum regrowth of around 0.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log after 48 h under both conditions, but it was not detected (<DL) after 144 h of storage. In contrast, E. coli regrowth was significant, increasing the concentration of viable bacteria after 24 and 48 h in the dark, until 1.3 and 1.9
log after 48 h under both conditions, but it was not detected (<DL) after 144 h of storage. In contrast, E. coli regrowth was significant, increasing the concentration of viable bacteria after 24 and 48 h in the dark, until 1.3 and 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log, respectively, remaining almost constant after 144 h. This means that the residual concentration of S2O82−, 18 mg L−1 (for the initial concentration of 20 mg L−1) and 28 mg L−1 (for the initial concentration of 40 mg L−1) did not prevent bacterial regrowth since S2O82− has no bactericidal effect by itself. To check this, several experiments (data not shown) were carried out putting in contact bacteria with S2O82− in different concentrations (up to 50 mg L−1) and in the dark. No significant effect was observed on the viability of bacteria. This fact is explained due to the size and charge of S2O82−, which can limit the diffusion through the cell membrane, avoiding the inactivation via a Fenton-like reaction, as the case of H2O2.72 Moreno-Andrés et al.63 observed regrowth (after 48) for E. coli and E. faecalis bacteria in distilled water after UVC/S2O82− treatment, even when using a higher oxidant concentration (200 mg L−1) than the used in the present study.
log, respectively, remaining almost constant after 144 h. This means that the residual concentration of S2O82−, 18 mg L−1 (for the initial concentration of 20 mg L−1) and 28 mg L−1 (for the initial concentration of 40 mg L−1) did not prevent bacterial regrowth since S2O82− has no bactericidal effect by itself. To check this, several experiments (data not shown) were carried out putting in contact bacteria with S2O82− in different concentrations (up to 50 mg L−1) and in the dark. No significant effect was observed on the viability of bacteria. This fact is explained due to the size and charge of S2O82−, which can limit the diffusion through the cell membrane, avoiding the inactivation via a Fenton-like reaction, as the case of H2O2.72 Moreno-Andrés et al.63 observed regrowth (after 48) for E. coli and E. faecalis bacteria in distilled water after UVC/S2O82− treatment, even when using a higher oxidant concentration (200 mg L−1) than the used in the present study.
The UVC/S2O82− system was also effective in removing 80% of the sum of MCs (Fig. 6), but in slightly longer treatment times than those obtained with UVC/H2O2. This result could be justified by the use of S2O82− in a molar concentration lower than that of H2O2. Therefore, it is important to stress that similar treatment time (24 min; 0.4 kJ L−1 accumulated UVC energy) was required to eliminate 80% of the sum of MCs with 25 mg L−1 UVC/H2O2 and 100 mg L−1 UVC/S2O82−, that means 0.73 mmol L−1 and 0.52 mmol L−1, respectively. Starling et al.73 reported an increase in the removal rates for photo-stable compounds CAF and CBZ in 2 L of a real surface water by using UVC/S2O82− (1 mmol L−1), resulting in more than 90% degradation with a UVC energy of 5.9 and 11.8 J L−1, respectively. In contrast, DOC concentration had a very slight variation throughout the experiments (data not shown), even when using the higher S2O82− concentration (100 mg L−1).
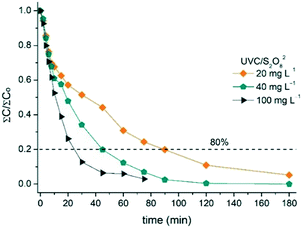 | ||
| Fig. 6 Effect of S2O82− concentration in the total MC degradation by UVC/S2O82− as a function of treatment time in SMWW secondary effluent. Dashed line refers to 80% removal of total MCs (∑Ct/∑C0). | ||
Similar to the UVC/H2O2 process, increase in the oxidant concentration also increased MC removal rates (k), (Table 3), achieving 80% of total degradation after 90 min (1.8 kJ L−1 accumulated UVC energy) when using 20 mg L−1 S2O82− and only 24 min (0.4 kJ L−1 accumulated UVC energy) for 100 mg L−1. This confirms that the generation of SO4˙− plays a major role in the degradation of the six MCs; Fig. S5† shows a linear relationship between the degradation kinetic constants with the initial concentration of S2O82−. In addition, Fig. S6† shows the degradation profile of each contaminant and the S2O82− consumption for all conditions studied.
Similar evolution curves for pseudo-first-order kinetic constants for MC removal were obtained compared to H2O2 tests (see Fig. S8, ESI†), confirming that it was not necessary to check more S2O82− concentrations.
As expected, the degradation curves had a similar profile as UVC/H2O2. No significant increase in the degradation rates of DCF and SFX were observed; however, TMP was slowly oxidized with regard to the rest of the MCs, contrary to what occurred when UVC/H2O2 was applied (see degradation kinetic constants in Table S4†). This behavior is explained by the different reaction rates of SO4˙− with specific functional groups of organic molecules. Wojnárovits et al.74 reported that electron-donating substituents increase the rate constants and electron-withdrawing substituents decrease it. In this sense, –OR and –NH2 (electron-withdrawing substituents) present in the molecular structure of TMP affect the efficiency of SO4˙− to degrade this compound. ACT also showed lower kinetic constants due to the effect of these substituents in its structure (–OH and –NHCOR). Once again, this confirms the selective character of generated SO4˙− species against the non-selective character of HO˙ generated in UVC/H2O2. As expected, consumption rates increased with S2O82− initial concentration, attaining 0.04, 0.15, and 0.20 mg S2O82− L−1 min−1 for 20, 40 and 100 mg L−1, respectively.
3.4 Preliminary model to determine the maximum yield of oxidant for UVC-based systems
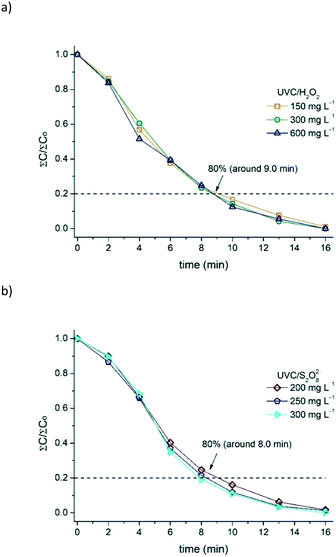
As reported in this study and in others from the literature, the UVC/H2O2 and UVC/S2O82− processes are very efficient for removing contaminants in aqueous medium. Nevertheless, critical oxidant concentrations seem to be attained and experiments carried out beyond these values are not effective in oxidizing organic compounds. Distinct critical concentrations of H2O2 have been reported as the most suitable since the optimum oxidant concentration is highly dependent on the nature of the target contaminant, water matrix, hydrodynamic parameters of the photoreactor, and power of the UVC lamp.In Fig. 7a and b, the illumination time required for the removal of 80% of the sum of MCs is shown to be close to 9 min, remaining constant with the increase in the concentration of the oxidant used (H2O2 and S2O82−).
Moreover, the first-order kinetic constant (k) showed that adding H2O2 and S2O82− above 150 and 200 mg L−1, respectively, did not produce any enhancement in the efficiency of the treatment (see Fig. S7 and S8†). This means that increasing the concentration of the oxidant is not always linked to a treatment improvement due to the self-scavenging reactions (see Table S5†).
On the other hand, the optical path length of the photo-reactor plays an important role in the efficiency of these processes, determining the amount of generated radicals. In this sense, the Beer–Lambert law that relates the absorbance with the optical path length and the oxidant concentration can be used to determine the most suitable oxidant quantity for a given photo-reactor setup, as shown in eqn (7):
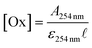 | (7) |
![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) is the optical path length of the photo-reactor (cm), and A is the absorbance of the solution (including the matrix effect). Here, A (0.186 measured at 254 nm) and
is the optical path length of the photo-reactor (cm), and A is the absorbance of the solution (including the matrix effect). Here, A (0.186 measured at 254 nm) and ![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) (2.595 cm, see Fig. 1 for further details, as
(2.595 cm, see Fig. 1 for further details, as ![[small script l]](https://www.rsc.org/images/entities/char_e146.gif) = rint,C − rint,L) are fixed parameters and depend on the nature of the oxidant.
= rint,C − rint,L) are fixed parameters and depend on the nature of the oxidant.
Taking these parameters into account, the H2O2 concentration calculated with eqn (7) is 126 mg L−1 (3.70 mmol L−1) using the molar absorptivity coefficient of H2O2 at 254 nm calculated for this system (5.7 × 10−4 mg−1 L cm−1). This represents a good approximation considering that the experimental concentration for the UVC/H2O2 process to attain saturation was 150 mg L−1.
As discussed above, the quantum yield of S2O82− is 2.8 times higher than that of H2O2, so the molar concentration to reach saturation using this oxidant is probably proportional to this factor or lower, i.e., 1.32 mmol L−1 (or 253 mg of S2O82− L−1). The obtained experimental concentration to attain saturation was 200 mg L−1 (or 1.04 mmol L−1). Additionally, there are many factors to be considered for modelling these systems, such as secondary reactions (including self-scavenging reactions), scavengers present in the matrix, nature of the target contaminants, etc. Therefore, the objective of this rough study was only to check the feasibility of a simple equation allowing us to predict the saturation concentration of the oxidants under the specific experimental conditions used in this investigation.
Conclusions
The simultaneous elimination of MCs and E. coli, E. faecalis, and S. enteritidis bacteria were attained at pilot plant scale by UVC/H2O2 and UVC/S2O82− processes under operation conditions quite close to actuality. UVC alone was not suitable due to subsequent bacteria regrowth accompanied by a very slow and incomplete removal of MCs.UVC/H2O2 led to a successful bacterial inactivation (without subsequent regrowth) and a simultaneous degradation of MCs up to 99%. By adding 25–50 mg L−1 H2O2, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log of E. coli, E. faecalis, and S. enteritidis bacterial inactivation and MC degradation rate higher than 80% were attained in less than 30 min. In the case of the UVC/S2O82− process, quicker E. coli inactivation was attained due to the possible reaction of generated SO4˙− with macromolecules in the cell wall. However, regrowth of bacteria was not prevented even when using 50 mg L−1 in the dark, possibly due to the limitation of S2O82− diffusion through the cell membrane. When using 20–40 mg L−1 S2O82−, 4
log of E. coli, E. faecalis, and S. enteritidis bacterial inactivation and MC degradation rate higher than 80% were attained in less than 30 min. In the case of the UVC/S2O82− process, quicker E. coli inactivation was attained due to the possible reaction of generated SO4˙− with macromolecules in the cell wall. However, regrowth of bacteria was not prevented even when using 50 mg L−1 in the dark, possibly due to the limitation of S2O82− diffusion through the cell membrane. When using 20–40 mg L−1 S2O82−, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log of E. coli, E. faecalis, and S. enteritidis bacterial inactivation was attained in less than 10 min, but achieving more than 80% MC degradation took a longer treatment time than in the UVC/H2O2 process. MCs exhibited removal rates proportional to the oxidant concentration used both for UVC/H2O2 and for UVC/S2O82−.
log of E. coli, E. faecalis, and S. enteritidis bacterial inactivation was attained in less than 10 min, but achieving more than 80% MC degradation took a longer treatment time than in the UVC/H2O2 process. MCs exhibited removal rates proportional to the oxidant concentration used both for UVC/H2O2 and for UVC/S2O82−.
The use of a simple model based on the Beer–Lambert law and taking into account the molar absorptivity of oxidants, as well as water absorbance (matrix effect) and optical length of the photo-reactor, enabled estimation of the maximum concentration of oxidants required to attain maximum oxidation rates in the specific UVC pilot plant used in this study. Further improvements, such as scavenging reactions and water matrix effects, must be considered for a better understanding of the UVC-based processes. Nevertheless, it has been demonstrated that UVC/H2O2 and UVC/S2O82− are able to produce an effluent with enough quality to be reused for several purposes, with agriculture as one of the most suitable end-uses as it is the highest consumer of freshwater worldwide. Nevertheless, it is important to highlight that UVC/S2O82− process would need a slight addition of bactericidal species to avoid bacterial regrowth along water storage or reclaimed water distribution systems.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 820718, and is jointly funded by the European Commission and the Department of Science and Technology of India (DST). Brazilian funding agencies (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq – grants #142350/2016-8 and #205818/2018-8, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES – Finance Code 001, and São Paulo Research Foundation – Fapesp) are also gratefully acknowledged for financial support and scholarships. The authors also wish to thank the Spanish Ministry of Science, Innovation and Universities (MCIU), AEI and FEDER for funding under the CalypSol Project (Reference: RTI2018-097997-B-C32).References
- J. M. Galindo-Miranda, C. Guízar-González, E. J. Becerril-Bravo, G. Moeller-Chávez, E. León-Becerril and R. Vallejo-Rodríguez, Occurrence of emerging contaminants in environmental surface waters and their analytical methodology – a review, Water Supply, 2019, 19, 1871–1884 CrossRef.
- M. Biel-Maeso, R. M. Baena-Nogueras, C. Corada-Fernández and P. A. Lara-Martín, Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain), Sci. Total Environ., 2018, 612, 649–659 CrossRef CAS PubMed.
- Q. Zhang, S. Yang, B. Xie, Z. Jian, C. Deng and R. Hu, Environmental and human health risks assessment of antibiotics residue in drinking water sources: case study of a fast-developing megacity-shenzhen, China, Water Supply, 2020, 20, 499–507 CrossRef.
- C. Caicedo, K. H. Rosenwinkel, M. Exner, W. Verstraete, R. Suchenwirth, P. Hartemann and R. Nogueira, Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review, Water Res., 2019, 149, 21–34 CrossRef CAS PubMed.
- P. Roccaro, Treatment processes for municipal wastewater reclamation: The challenges of emerging contaminants and direct potable reuse, Curr. Opin. Environ. Sci. Health, 2018, 2, 46–54 CrossRef.
- M. Salgot and M. Folch, Wastewater treatment and water reuse, Curr. Opin. Environ. Sci. Health, 2018, 2, 64–74 CrossRef.
- O. M. Lee, H. Y. Kim, W. Park, T.-H. Kim and S. Yu, A comparative study of disinfection efficiency and regrowth control of microorganism in secondary wastewater effluent using UV, ozone, and ionizing irradiation process, J. Hazard. Mater., 2015, 295, 201–208 CrossRef CAS PubMed.
- M.-T. Guo and C. Kong, Antibiotic resistant bacteria survived from UV disinfection: Safety concerns on genes dissemination, Chemosphere, 2019, 224, 827–832 CrossRef CAS PubMed.
- H.-W. Yu, M. Park, S. Wu, I. J. Lopez, W. Ji, J. Scheideler and S. A. Snyder, Strategies for selecting indicator compounds to assess attenuation of emerging contaminants during UV advanced oxidation processes, Water Res., 2019, 166, 115030 CrossRef CAS PubMed.
- K.-Y. Park, S.-Y. Choi, S.-H. Lee, J.-H. Kweon and J.-H. Song, Comparison of formation of disinfection by-products by chlorination and ozonation of wastewater effluents and their toxicity to Daphnia magna, Environ. Pollut., 2016, 215, 314–321 CrossRef CAS PubMed.
- C. Li, D. Wang, X. Xu and Z. Wang, Formation of known and unknown disinfection by-products from natural organic matter fractions during chlorination, chloramination, and ozonation, Sci. Total Environ., 2017, 587–588, 177–184 CrossRef CAS PubMed.
- X.-l. Zhang, H.-w. Yang, X.-m. Wang, J. Fu and Y. F. Xie, Formation of disinfection by-products: Effect of temperature and kinetic modeling, Chemosphere, 2013, 90, 634–639 CrossRef CAS PubMed.
- L. Jatzwauk, H. Schöne and H. Pietsch, How to improve instrument disinfection by ultrasound, J. Hosp. Infect., 2001, 48, S80–S83 CrossRef PubMed.
- P. R. Gogate, P. D. Thanekar and A. P. Oke, Strategies to improve biological oxidation of real wastewater using cavitation based pre-treatment approaches, Ultrason. Sonochem., 2020, 64, 105016 CrossRef CAS PubMed.
- T. Leiknes, The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review, J. Environ. Sci., 2009, 21, 8–12 CrossRef CAS.
- J. Radjenovic and D. L. Sedlak, Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water, Environ. Sci. Technol., 2015, 49, 11292–11302 CrossRef CAS PubMed.
- J. Rodríguez-Chueca, C. García-Cañibano, R. J. Lepistö, Á. Encinas, J. Pellinen and J. Marugán, Intensification of UV-C tertiary treatment: Disinfection and removal of micropollutants by sulfate radical based Advanced Oxidation Processes, J. Hazard. Mater., 2019, 372, 94–102 CrossRef PubMed.
- G. Cerreta, M. A. Roccamante, I. Oller, S. Malato and L. Rizzo, Contaminants of emerging concern removal from real wastewater by UV/free chlorine process: A comparison with solar/free chlorine and UV/H2O2 at pilot scale, Chemosphere, 2019, 236, 124354 CrossRef CAS PubMed.
- C. Pablos, J. Marugán, R. van Grieken and E. Serrano, Emerging micropollutant oxidation during disinfection processes using UV-C, UV-C/H2O2, UV-A/TiO2 and UV-A/TiO2/H2O2, Water Res., 2013, 47, 1237–1245 CrossRef CAS PubMed.
- I. J. S. Montes, B. F. Silva and J. M. Aquino, On the performance of a hybrid process to mineralize the herbicide tebuthiuron using a DSA® anode and UVC light: A mechanistic study, Appl. Catal., B, 2017, 200, 237–245 CrossRef CAS.
- R. Xiao, K. Liu, L. Bai, D. Minakata, Y. Seo, R. Kaya Göktaş, D. D. Dionysiou, C.-J. Tang, Z. Wei and R. Spinney, Inactivation of pathogenic microorganisms by sulfate radical: Present and future, Chem. Eng. J., 2019, 371, 222–232 CrossRef CAS.
- M. Sánchez-Polo, M. M. Abdeldaiem, R. Ocampo-Pérez, J. Rivera-Utrilla and A. J. Mota, Comparative study of the photodegradation of bisphenol A by HO, SO4− and CO3−/HCO3 radicals in aqueous phase, Sci. Total Environ., 2013, 463–464, 423–431 CrossRef PubMed.
- D. R. Hokanson, K. Li and R. R. Trussell, A photolysis coefficient for characterizing the response of aqueous constituents to photolysis, Front. Environ. Sci. Eng., 2016, 10, 428–437 CrossRef CAS.
- R. Guan, X. Yuan, Z. Wu, L. Jiang, Y. Li and G. Zeng, Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: A review, Chem. Eng. J., 2018, 339, 519–530 CrossRef CAS.
- D. B. Miklos, R. Hartl, P. Michel, K. G. Linden, J. E. Drewes and U. Hübner, UV/H2O2 process stability and pilot-scale validation for trace organic chemical removal from wastewater treatment plant effluents, Water Res., 2018, 136, 169–179 CrossRef CAS PubMed.
- W. Li, T. Jain, K. Ishida and H. Liu, A mechanistic understanding of the degradation of trace organic contaminants by UV/hydrogen peroxide, UV/persulfate and UV/free chlorine for water reuse, Environ. Sci.: Water Res. Technol., 2017, 3, 128–138 RSC.
- J. L. Weeks and M. S. Matheson, The Primary Quantum Yield of Hydrogen Peroxide Decomposition1, J. Am. Chem. Soc., 1956, 78, 1273–1278 CrossRef CAS.
- F. P. Tully, A. R. Ravishankara, R. L. Thompson, J. M. Nicovich, R. C. Shah, N. M. Kreutter and P. H. Wine, Kinetics of the reactions of hydroxyl radical with benzene and toluene, J. Phys. Chem., 1981, 85, 2262–2269 CrossRef CAS.
- S. Wacławek, H. V. Lutze, K. Grübel, V. V. T. Padil, M. Černík and D. D. Dionysiou, Chemistry of persulfates in water and wastewater treatment: A review, Chem. Eng. J., 2017, 330, 44–62 CrossRef.
- S. Guerra-Rodríguez, E. Rodríguez, N. D. Singh and J. Rodríguez-Chueca, Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review, Water, 2018, 10, 1828 CrossRef.
- E. Pelizzetti, V. Carlin, C. Minero and M. Graetzel, Enhancement of the Rate of Photocatalytic Degradation on TiO2 of 2- Chlorophenol, 2,7-Dichlorodibenzodioxin, and Atrazine by Inorganic Oxidizing Species, New J. Chem., 1991, 15, 351–359 CAS.
- Q. Yang, H. Choi, Y. Chen and D. D. Dionysiou, Heterogeneous activation of peroxymonosulfate by supported cobalt catalysts for the degradation of 2,4-dichlorophenol in water: The effect of support, cobalt precursor, and UV radiation, Appl. Catal., B, 2008, 77, 300–307 CrossRef CAS.
- S. Luo, Z. Wei, D. D. Dionysiou, R. Spinney, W.-P. Hu, L. Chai, Z. Yang, T. Ye and R. Xiao, Mechanistic insight into reactivity of sulfate radical with aromatic contaminants through single-electron transfer pathway, Chem. Eng. J., 2017, 327, 1056–1065 CrossRef CAS.
- R. Xiao, Z. Luo, Z. Wei, S. Luo, R. Spinney, W. Yang and D. D. Dionysiou, Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies, Curr. Opin. Chem. Eng., 2018, 19, 51–58 CrossRef.
- J. Sharma, I. M. Mishra and V. Kumar, Degradation and mineralization of Bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O82− oxidation systems, J. Environ. Manage., 2015, 156, 266–275 CrossRef CAS PubMed.
- E. Kudlek, M. Dudziak and J. Bohdziewicz, Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix, Water, 2016, 8, 532 CrossRef.
- J. A. Malvestiti and R. F. Dantas, Influence of industrial contamination in municipal secondary effluent disinfection by UV/H2O2, Environ. Sci. Pollut. Res., 2019, 26, 13286–13298 CrossRef PubMed.
- USEPA, Guidelines for Water Reuse, United States Environmental Protection Agency, Washington, DC, USA, 2012, EPA/600/R-12/618 Search PubMed.
- ISO, Guidelines for Treated Wastewater Use for Irrigation Projects, International Organization for Standardization, Geneva, Switzerland, 2015, vol. 16075 Search PubMed.
- B. Petrie, R. Barden and B. Kasprzyk-Hordern, A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring, Water Res., 2015, 72, 3–27 CrossRef CAS PubMed.
- R. Zhang, S. Vigneswaran, H. Ngo and H. Nguyen, A submerged membrane hybrid system coupled with magnetic ion exchange (MIEX®) and flocculation in wastewater treatment, Desalination, 2007, 216, 325–333 CrossRef CAS.
- Standard Methods for the Examination of Water and Wastewater, ed. A. D. Eaton and A. E. Greenberg, United Book Press Inc., American Public Health Association; American Waterworks Association, Water Environment Federation, Maryland, USA, 20th edn, 1998 Search PubMed.
- C. Liang, C.-F. Huang, N. Mohanty and R. M. Kurakalva, A rapid spectrophotometric determination of persulfate anion in ISCO, Chemosphere, 2008, 73, 1540–1543 CrossRef CAS PubMed.
- Royal Decree 1620/2007 (BOE No. 294, 2007), Concerns of the Legal Regime for the Reuse of Treated Water, 2007, Available at: http://www.boe.es/boe/dias/2007/12/08/pdfs/A50639-50661.pdf Search PubMed.
- J. Marugán, R. van Grieken, C. Sordo and C. Cruz, Kinetics of the photocatalytic disinfection of Escherichia coli suspensions, Appl. Catal., B, 2008, 82, 27–36 CrossRef.
- G. Cerreta, M. A. Roccamante, P. Plaza-Bolaños, I. Oller, A. Aguera, S. Malato and L. Rizzo, Advanced treatment of urban wastewater by UV-C/free chlorine process: Micro-pollutants removal and effect of UV-C radiation on trihalomethanes formation, Water Res., 2020, 169, 115220 CrossRef CAS.
- S. Nahim-Granados, G. Rivas-Ibáñez, J. A. Sánchez Pérez, I. Oller, S. Malato and M. I. Polo-López, Synthetic fresh-cut wastewater disinfection and decontamination by ozonation at pilot scale, Water Res., 2020, 170, 115304 CrossRef CAS PubMed.
- E. P. Costa, M. Roccamante, C. C. Amorim, I. Oller, J. A. Sánchez Pérez and S. Malato, New trend on open solar photoreactors to treat micropollutants by photo-Fenton at circumneutral pH: Increasing optical pathway, Chem. Eng. J., 2020, 385, 123982 CrossRef.
- A. B. Martínez-Piernas, M. I. Polo-López, P. Fernández-Ibáñez and A. Agüera, Validation and application of a multiresidue method based on liquid chromatography-tandem mass spectrometry for evaluating the plant uptake of 74 microcontaminants in crops irrigated with treated municipal wastewater, J. Chromatogr. A, 2018, 1534, 10–21 CrossRef PubMed.
- N. H. Tran, M. Reinhard and K. Y.-H. Gin, Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review, Water Res., 2018, 133, 182–207 CrossRef CAS PubMed.
- G. D. Harris, V. D. Adams, D. L. Sorensen and M. S. Curtis, Ultraviolet inactivation of selected bacteria and viruses with photoreactivation of the bacteria, Water Res., 1987, 21, 687–692 CrossRef CAS.
- J. Rodríguez-Chueca, M. I. Polo-López, R. Mosteo, M. P. Ormad and P. Fernández-Ibáñez, Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton, Appl. Catal., B, 2014, 150–151, 619–629 CrossRef.
- S. Nahim-Granados, J. A. Sánchez Pérez and M. I. Polo-Lopez, Effective solar processes in fresh-cut wastewater disinfection: Inactivation of pathogenic E. coli O157:H7 and Salmonella enteritidis, Catal. Today, 2018, 313, 79–85 CrossRef CAS.
- S. Yang, P. Wang, X. Yang, L. Shan, W. Zhang, X. Shao and R. Niu, Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide, J. Hazard. Mater., 2010, 179, 552–558 CrossRef CAS PubMed.
- R. L. Johnson, P. G. Tratnyek and R. O. B. Johnson, Persulfate Persistence under Thermal Activation Conditions, Environ. Sci. Technol., 2008, 42, 9350–9356 CrossRef CAS PubMed.
- S. Giannakis, E. Darakas, A. Escalas-Cañellas and C. Pulgarin, Elucidating bacterial regrowth: Effect of disinfection conditions in dark storage of solar treated secondary effluent, J. Photochem. Photobiol., A, 2014, 290, 43–53 CrossRef CAS.
- Federal Office of Environment, Gewässerqualität: Revision der Gewässerschutzverordnung, Switzerland, 2017, Available at: https://www.bafu.admin.ch/bafu/fr/home/themes/formation/communiques.msg-id-59323.html Search PubMed.
- M. Bourgin, B. Beck, M. Boehler, E. Borowska, J. Fleiner, E. Salhi, R. Teichler, U. von Gunten, H. Siegrist and C. S. McArdell, Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products, Water Res., 2018, 129, 486–498 CrossRef CAS PubMed.
- Y. Yoon, H. J. Chung, D. Y. Wen Di, M. C. Dodd, H.-G. Hur and Y. Lee, Inactivation efficiency of plasmid-encoded antibiotic resistance genes during water treatment with chlorine, UV, and UV/H2O2, Water Res., 2017, 123, 783–793 CrossRef CAS PubMed.
- G. Moussavi, E. Fathi and M. Moradi, Advanced disinfecting and post-treating the biologically treated hospital wastewater in the UVC/H2O2 and VUV/H2O2 processes: Performance comparison and detoxification efficiency, Process Saf. Environ. Prot., 2019, 126, 259–268 CrossRef CAS.
- D. Rubio, E. Nebot, J. F. Casanueva and C. Pulgarin, Comparative effect of simulated solar light, UV, UV/H2O2 and photo-Fenton treatment (UV–Vis/H2O2/Fe2+,3+) in the Escherichia coli inactivation in artificial seawater, Water Res., 2013, 47, 6367–6379 CrossRef CAS PubMed.
- J. Moreno-Andrés, L. Romero-Martínez, A. Acevedo-Merino and E. Nebot, Determining disinfection efficiency on E. faecalis in saltwater by photolysis of H2O2: Implications for ballast water treatment, Chem. Eng. J., 2016, 283, 1339–1348 CrossRef.
- J. Moreno-Andrés, R. Rios Quintero, A. Acevedo-Merino and E. Nebot, Disinfection performance using a UV/persulfate system: effects derived from different aqueous matrices, Photochem. Photobiol. Sci., 2019, 18, 878–883 RSC.
- F. Zeng, S. Cao, W. Jin, X. Zhou, W. Ding, R. Tu, S.-F. Han, C. Wang, Q. Jiang, H. Huang and F. Ding, Inactivation of chlorine-resistant bacterial spores in drinking water using UV irradiation, UV/Hydrogen peroxide and UV/Peroxymonosulfate: Efficiency and mechanism, J. Cleaner Prod., 2020, 243, 118666 CrossRef CAS.
- S. Miralles-Cuevas, D. Darowna, A. Wanag, S. Mozia, S. Malato and I. Oller, Comparison of UV/H2O2, UV/S2O82−, solar/Fe(II)/H2O2 and solar/Fe(II)/S2O82− at pilot plant scale for the elimination of micro-contaminants in natural water: An economic assessment, Chem. Eng. J., 2017, 310, 514–524 CrossRef CAS.
- I. Michael-Kordatou, M. Iacovou, Z. Frontistis, E. Hapeshi, D. D. Dionysiou and D. Fatta-Kassinos, Erythromycin oxidation and ERY-resistant Escherichia coli inactivation in urban wastewater by sulfate radical-based oxidation process under UV-C irradiation, Water Res., 2015, 85, 346–358 CrossRef CAS PubMed.
- S. Popova, G. Matafonova and V. Batoev, Simultaneous atrazine degradation and E. coli inactivation by UV/S2O82−/Fe2+ process under KrCl excilamp (222 nm) irradiation, Ecotoxicol. Environ. Saf., 2019, 169, 169–177 CrossRef CAS PubMed.
- D. Wordofa, S. Walker and H. Liu, Sulfate Radical-Induced Disinfection of Pathogenic Escherichia coli O157:H7 via Iron-Activated Persulfate, Environ. Sci. Technol. Lett., 2017, 4, 154–160 CrossRef CAS.
- E. A. Serna-Galvis, L. Salazar-Ospina, J. N. Jiménez, N. J. Pino and R. A. Torres-Palma, Elimination of carbapenem resistant Klebsiella pneumoniae in water by UV-C, UV-C/persulfate and UV-C/H2O2. Evaluation of response to antibiotic, residual effect of the processes and removal of resistance gene, J. Environ. Chem. Eng., 2020, 8, 102196 CrossRef CAS.
- R. van Grieken, J. Marugán, C. Pablos, L. Furones and A. López, Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganisms, Appl. Catal., B, 2010, 100, 212–220 CrossRef CAS.
- W. Wang, A. V. Perepelov, L. Feng, S. D. Shevelev, Q. Wang, S. Senchenkova, W. Han, Y. Li, A. S. Shashkov, Y. A. Knirel, P. R. Reeves and L. Wang, A group of Escherichia coli and Salmonella enterica O antigens sharing a common backbone structure, Microbiology, 2007, 153, 2159–2167 CrossRef CAS.
- S. Giannakis, M. I. Polo López, D. Spuhler, J. A. Sánchez Pérez, P. Fernández Ibáñez and C. Pulgarin, Solar disinfection is an augmentable, in situ-generated photo-Fenton reaction—Part 1: A review of the mechanisms and the fundamental aspects of the process, Appl. Catal., B, 2016, 199, 199–223 CrossRef CAS.
- M. C. V. M. Starling, P. P. Souza, A. Le Person, C. C. Amorim and J. Criquet, Intensification of UV-C treatment to remove emerging contaminants by UV-C/H2O2 and UV-C/S2O82−: Susceptibility to photolysis and investigation of acute toxicity, Chem. Eng. J., 2019, 376, 120856 CrossRef.
- L. Wojnárovits and E. Takács, Rate constants of sulfate radical anion reactions with organic molecules: A review, Chemosphere, 2019, 220, 1014–1032 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew00279h |
| This journal is © The Royal Society of Chemistry 2020 |