Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
ESIPT-based fluorescence probe for the ratiometric detection of superoxide†
Luling
Wu
 a,
Liyuan
Liu
a,
Liyuan
Liu
 a,
Hai-Hao
Han
a,
Hai-Hao
Han
 b,
Xue
Tian
b,
Xue
Tian
 a,
Maria L.
Odyniec
a,
Maria L.
Odyniec
 a,
Lei
Feng
a,
Lei
Feng
 ac,
Adam C.
Sedgwick
ac,
Adam C.
Sedgwick
 *d,
Xiao-Peng
He
*d,
Xiao-Peng
He
 b,
Steven D.
Bull
b,
Steven D.
Bull
 *a and
Tony D.
James
*a and
Tony D.
James
 *a
*a
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk; s.d.bull@bath.ac.uk
bKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Rd., Shanghai 200237, China
cCollege of Integrative Medicine, The National & Local Joint Engineering Research Center for Drug Development of Neurodegenerative Disease, College of Pharmacy, Dalian Medical University, Dalian 116044, China
dDepartment of Chemistry, University of Texas at Austin, 105 E 24th street A5300, Austin, TX 78712-1224, USA. E-mail: a.c.sedgwick@utexas.edu
First published on 23rd January 2019
Abstract
A simple ESIPT-based fluorescence probe (HMBT-LW) was developed for the detection of superoxide (O2˙−). HMBT-LW was synthesised over two steps and was shown to rapidly detect low concentrations of O2˙− (limit of detection = 7.4 μM), fully reacting within two minutes. Furthermore, HMBT-LW demonstrated excellent selectivity and sensitivity towards O2˙−.
Reactive oxygen species (ROS) are the transient by-products generated from the electron transport chain.1 More specifically, the ROS, superoxide (O2˙−) is a anion radical generated from the single electron reduction of molecular oxygen (O2), which means O2˙− is the precursor to most ROS.2 O2˙− is capable of reacting with nitric oxide (NO˙), which generates the highly reactive nitrogen species peroxynitrite (ONOO−), or with superoxide dismutase (SOD) to produce hydrogen peroxide (H2O2). Hydrogen peroxide can then be transformed into the highly reactive hydroxyl radical (˙OH) and hypochlorous acid (HOCl). These reactive oxygen species are associated with a number of pathological processes, including cardiomyopathy, autism, diabetes mellitus, cancer and neurodegenerative disorders (e.g., Alzheimer's disease and Parkinson's disease).3–6 Therefore, the development of a fluorescence probe for the real-time detection of O2˙− would further aid the understanding of O2˙− related diseases in living organisms.
Excited-state intramolecular proton transfer (ESIPT) is widely used in the design of fluorescent probes7 as ESIPT-based fluorescent probes display a number of favourable properties such as a large Stokes shift (∼200 nm) and the ability to undergo ratiometric sensing. The ratiometric detection of a target analyte is ideal as it enables the determination of the concentration of the target analyte directly without need of calibration.7–9
Within our research group, we have developed several ESIPT-based fluorescent probes for the detection of biological reactive oxygen species as well as biological thiols.10–13 Previously, we have developed a thiocarbamate functionalised methoxy-hydroxybenzothiazole (HMBT) fluorescent probe TCBT-OMe for the detection of HOCl/ClO− (Scheme 1). The addition of HOCl/ClO− to TCBT-OMe resulted in the rapid hydrolysis (<10 s) of the thiocarbamate linker, leading to a ratiometric change in fluorescence intensity.12
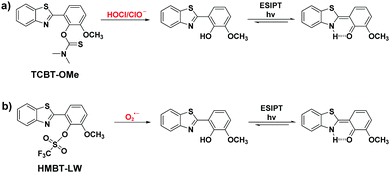 | ||
| Scheme 1 (a) Our previously reported ESIPT probe for the detection of HOCl/ClO−. (b) This work – a trifluoromethanesulfonate linker-based ESIPT HMBT-LW for the detection of O2˙−. | ||
Most fluorescent probes that are reported for the detection of O2˙− utilise its nucleophilicity to achieve excellent selectivity over other ROS.14–20 As a result of this, we believed the functionalisation of HMBT with the O2˙− reactive trifluoromethanesulfonate unit would result in a ratiometric fluorescent probe for the detection of O2˙− (Scheme 1).21
HMBT-LW was synthesized over two steps. The first step of the synthesis involved the addition of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aq H2O2/aq HCl solution to 2-aminothiophenol and o-vanilin in EtOH, which formed HMBT in good yield (68%).22–24 With HMBT in hand, trifluoromethanesulfonic anhydride was then added dropwise into a solution of HMBT in DCM at −78 °C under argon, NEt3 was subsequently added to the reaction. This reaction proceeded smoothly furnishing HMBT-LW in good yield (52%) (Scheme 2). The chemical structure of HMBT-LW was fully characterized by 1H NMR, 13C NMR and high-resolution mass spectrometry (HRMS).
1 aq H2O2/aq HCl solution to 2-aminothiophenol and o-vanilin in EtOH, which formed HMBT in good yield (68%).22–24 With HMBT in hand, trifluoromethanesulfonic anhydride was then added dropwise into a solution of HMBT in DCM at −78 °C under argon, NEt3 was subsequently added to the reaction. This reaction proceeded smoothly furnishing HMBT-LW in good yield (52%) (Scheme 2). The chemical structure of HMBT-LW was fully characterized by 1H NMR, 13C NMR and high-resolution mass spectrometry (HRMS).
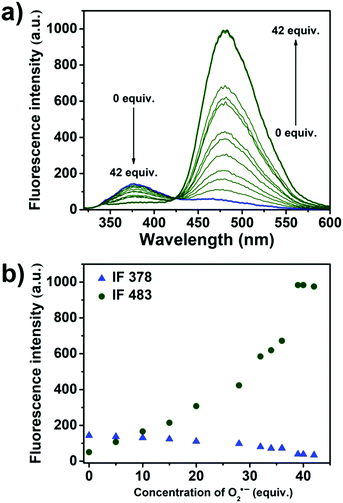
Next, we evaluated the UV-Vis properties of HMBT-LW (5 μM) with the addition of O2˙− (42 equivalents). This addition led to an increase in UV absorption between 200–400 nm indicating a change chemical structure (Fig. S1, ESI†). We then turned our attention towards the ability of HMBT-LW to detect O2˙− using fluorescence. Remarkably, HMBT-LW was shown to have a rapid response towards O2˙− with a significant increase in fluorescence intensity being observed within 2 minutes (Fig. S5, ESI†). Initially, a fluorescence emission intensity at 378 nm was only observed, since the ESIPT process is blocked by the trifluoromethanesulfonate group. However, in the presence of O2˙−, a notable increase in fluorescence emission intensity at 483 nm and a simultaneously decrease in fluorescence emission intensity at 378 nm was observed (Fig. 1) corresponding to the deprotection and release of the HMBT fluorophore enabling the ESIPT process to take place (Reaction mechanism confirmed by HRMS – see Fig. S6 and S7, ESI†). In addition a quantum yield of 0.508 was determined for HMBT under these measurement conditions.25
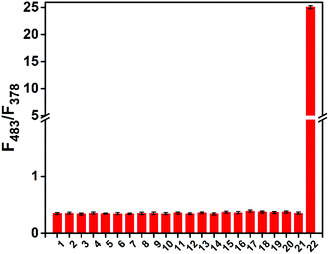
HMBT-LW was then shown to have good stability over a range of different pH 4–10, (Fig. S3, ESI†) and was capable of detecting low concentrations of O2˙− with a Limit of Detection (LoD) of 7.4 μM (Fig. S4, ESI†). Furthermore, HMBT-LW demonstrated excellent selectivity towards O2˙− over other ROS and biologically relevant analytes (Fig. 2).
With this research we have developed an ESIPT-based fluorescence probe (HMBT-LW) for the selective and sensitive detection of O2˙−. Sadly, the excitation wavelength for HMBT-LW is too short to enable its use in cellular imaging experiments. However, we are currently exploring related ESIPT based systems with longer excitation wavelengths that are more suitable for cellular imaging experiments. In summary HMBT-LW provides a platform on which it will be possible to develop long wavelength ESIPT-based fluorescent probes for the ratiometric selective and sensitive detection of O2˙−.
Conflicts of interest
No conflicts of interest.Acknowledgements
LW wishes to thank China Scholarship Council and the University of Bath for supporting his PhD work in the UK. We would like to thank the EPSRC and the University of Bath for funding. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award. NMR characterisation facilities were provided through the Material and Chemical Characterisation Facility (MC2) at the University of Bath (http://go.bath.ac.uk/mc2). The EPSRC UK National Mass Spectrometry Facility at Swansea University is thanked for analyses.Notes and references
- P. Held, An Introduction to Reactive Oxygen Species – Measurement of ROS in Cells, BioTek Instruments, 2010 Search PubMed.
- J. F. Turrens, J. Physiol., 2003, 552, 335–344 CrossRef CAS PubMed.
- S. Rose, S. Melnyk, O. Pavliv, S. Bai, T. G. Nick, R. E. Frye and S. J. James, Transl. Psychiatry, 2012, 2, e134 CrossRef CAS PubMed.
- R. A. Cairns, I. S. Harris and T. W. Mak, Nat. Rev. Cancer, 2011, 11, 85–95 CrossRef CAS PubMed.
- C. Gorrini, I. S. Harris and T. W. Mak, Nat. Rev. Drug Discovery, 2013, 12, 931–947 CrossRef CAS PubMed.
- M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185–4191 RSC.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- Y.-W. Wang, Y.-X. Hua, H.-H. Wu, X. Sun and Y. Peng, Chin. Chem. Lett., 2017, 28, 1994–1996 CrossRef.
- Z.-H. Fu, X. Han, Y. Shao, J. Fang, Z.-H. Zhang, Y.-W. Wang and Y. Peng, Anal. Chem., 2017, 89, 1937–1944 CrossRef CAS PubMed.
- L. Wu, H.-H. Han, L. Liu, J. E. Gardiner, A. C. Sedgwick, C. Huang, S. D. Bull, X.-P. He and T. D. James, Chem. Commun., 2018, 54, 11336–11339 RSC.
- L. Wu, Y. Wang, M. Weber, L. Liu, A. C. Sedgwick, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 9953–9956 RSC.
- L. Wu, Q. Yang, L. Liu, A. C. Sedgwick, A. J. Cresswell, S. D. Bull, C. Huang and T. D. James, Chem. Commun., 2018, 54, 8522–8525 RSC.
- A. C. Sedgwick, X. Sun, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2016, 52, 12350–12352 RSC.
- P. Li, W. Zhang, K. Li, X. Liu, H. Xiao, W. Zhang and B. Tang, Anal. Chem., 2013, 85, 9877–9881 CrossRef CAS PubMed.
- X. Han, R. Wang, X. Song, F. Yu, C. Lv and L. Chen, Biomaterials, 2018, 156, 134–146 CrossRef CAS PubMed.
- Y. Lv, D. Cheng, D. Su, M. Chen, B.-C. Yin, L. Yuan and X.-B. Zhang, Chem. Sci., 2018, 9, 7606–7613 RSC.
- J. J. Hu, N.-K. Wong, S. Ye, X. Chen, M.-Y. Lu, A. Q. Zhao, Y. Guo, A. C.-H. Ma, A. Y.-H. Leung, J. Shen and D. Yang, J. Am. Chem. Soc., 2015, 137, 6837–6843 CrossRef CAS PubMed.
- D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, Org. Lett., 2013, 15, 3946–3949 CrossRef CAS PubMed.
- D. Lu, L. Zhou, R. Wang, X.-B. Zhang, L. He, J. Zhang, X. Hu and W. Tan, Sens. Actuators, B, 2017, 250, 259–266 CrossRef CAS.
- Z. Zhang, J. Fan, Y. Zhao, Y. Kang, J. Du and X. Peng, ACS Sens., 2018, 3, 735–741 CrossRef CAS PubMed.
- A. Sytnik and M. Kasha, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 8627–8630 CrossRef CAS.
- X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690–10693 CrossRef CAS PubMed.
- J. Dey and S. K. Dogra, J. Photochem. Photobiol., A, 1992, 66, 15–31 CrossRef CAS.
- G. F. Kirkbright, D. E. M. Spillane, K. Anthony, R. G. Brown, J. D. Hepworth, K. W. Hodgson and M. A. West, Anal. Chem., 1984, 56, 1644–1647 CrossRef CAS.
- H. Li, Q. Yao, J. Fan, J. Du, J. Wang and X. Peng, Biosens. Bioelectron., 2017, 94, 536–543 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nj05656k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |