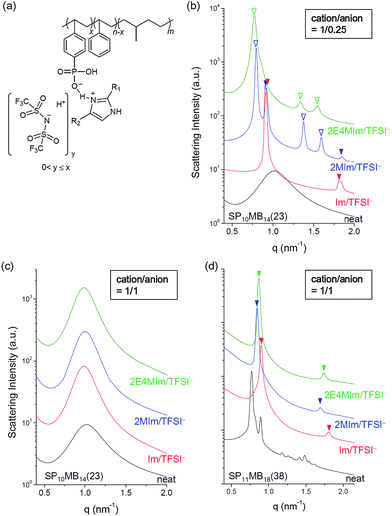
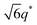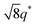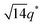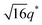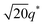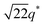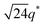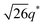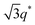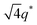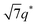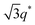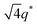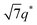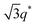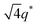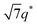Thermodynamics and phase behavior of acid-tethered block copolymers with ionic liquids
Ha Young
Jung
a and
Moon Jeong
Park
*ab
aDepartment of Chemistry, Pohang University of Science and Technology (POSTECH), Pohang, 790-784 Korea. E-mail: moonpark@postech.edu
bDivision of Advanced Materials Science, Pohang University of Science and Technology (POSTECH), Pohang, 790-784 Korea
First published on 7th June 2016
Abstract
We investigate the phase behavior of acid-tethered block copolymers with and without ionic liquids. Two phosphonated block copolymers and their sulfonated analogs were synthesized by fine-tuning the degree of polymerization and the acid content. The block copolymers carrying acid groups with ionic liquids exhibited rich phase sequences, i.e., disorder–lamellae (LAM), gyroid–LAM, gyroid–hexagonal cylinder (HEX), and gyroid–A15 lattice, and the cation/anion ratio in the ionic liquid exerted profound effects on the segregation strength and topology of the self-assembled structures. Additionally, using ionic liquids with excessive cation content was found to enhance the effective Flory–Huggins interaction parameter, χeff, of the samples. However, as the anion content of the ionic liquids increased the segregation strength decreased. This is attributed to the packing frustration accompanied by the prevailing repulsive electrostatic interactions of the anions in the ionic liquid and the polymer matrix. As the hydrophobicity of the ionic liquids increased, well-defined ordered phases emerged in the phosphonated block copolymers with increased anion content, contrary to the disordered phases of the sulfonated samples. Thus, the balance between solvation energy of the anions and the electrostatic interactions is a key determinant of the thermodynamics of acid-tethered block copolymers containing ionic liquids.
Introduction
Recently, ion-containing polymers have attracted substantial attention for applications in energy-storage and conversion systems.1–3 Ionic block copolymers composed of ion-dissolving blocks and mechanically robust ionophobic blocks have emerged as particularly promising materials for the synergistic achievement of high ionic conductivity and mechanical integrity with salt-doping.4,5 These positive prospects stem from the unique self-assembly behavior of block copolymers,6 which enables ions to be confined into nanoscale domains without affecting the mechanical strength of the ionophobic blocks.7–10The most extensively studied ionic block copolymers are based on poly(ethylene oxide) (PEO), which is capable of dissolving metal salts.11–14 Decades of studies on PEO-containing block copolymers, i.e., poly(styrene-b-ethylene oxide) (PS-b-PEO)4,15,16 and poly(methyl methacrylate-b-oligooxyethylene methacrylate) (PMMA-b-POEM),17,18 have revealed that the addition of metal salts significantly affects these polymers' self-assembly.19–21 For example, lithium salt-doping altered the morphologies of PEO-containing block copolymers because of favorable molecular interactions between lithium salts and ether oxygens, thereby changing the interfaces between the PEO and ionophobic phases.15,19 Furthermore, the ordered phases of block copolymers were found to be stabilized by lithium salts, as shown by the disorder-to-order phase transitions19,22,23 and substantially increased order-to-disorder transition (ODT) temperatures.18,19,24,25 This effect was attributed to increases in the effective Flory–Huggins interaction parameter (χeff) resulting from the addition of lithium salts.16,24–26
Amphiphilic block copolymers containing ionic liquids27,28 have also been the subject of considerable interest in recent years because of ionic liquids' unique features such as negligible vapor pressure, good electrochemical stability, and high ionic conductivity.29 Several studies have described the lyotropic phase transitions of block copolymers with ionic liquids30–32 and have shown that their physics is very similar to that of lithium salt-doped block copolymers. Indeed, the addition of ionic liquids increases the χeff value and alters the microphase-separated morphology.28,31 Examples of the ionic liquid-dissolving amphiphilic block copolymers investigated include PS-b-PEO,33 poly(butadiene-b-ethylene oxide) (PB-b-PEO),34 poly(styrene-b-methyl methacrylate) (PS-b-PMMA),31,32,35,36 and poly(styrene-b-2-vinylpyridine) (PS-b-P2VP).8,37,38
In addition to the amphiphilic block copolymers mentioned above, acid-tethered block copolymers are worth investigating to gain valuable insights into the thermodynamics and phase behaviors of ionic liquid-containing polymers.7,39–41 In such polymers, the specific molecular interactions of the ionic phases can be precisely controlled by independently varying the cations and anions of the ionic liquids and the tethered acid groups of the polymers. Various parameters such as the degree of ionic liquid dissociation in the ionic phases42–44 and the binding affinity between ionic liquids and polymer matrices45,46 should exert decisive influences on the phase behaviors of ionic liquid-containing block copolymers. Nevertheless, the current thermodynamic understanding of the phase behaviors of acid-tethered block copolymers with ionic liquids remains lacking, partly because synthesizing well-defined acid-tethered block copolymers is difficult.
Herein, we investigate the thermodynamics and phase behavior of acid-tethered block copolymers with ionic liquids. A set of phosphonated block copolymers and sulfonated block copolymers were synthesized with fine-tuned molecular weights and acid contents. The neat block copolymers were found to exhibit various morphologies of lamellae (LAM), gyroid, and disorder. Rich phase sequences were obtained with the addition of ionic liquids with a range of cation/anion compositions, allowing us to draw full phase diagrams for ionic liquid-containing acid-tethered block copolymers. Specifically, we found that the cation/anion ratio of the ionic liquid profoundly affects the segregation strength and topology of self-assembled structures. This effect was ascribed to the balance between the ionic liquid's solvation energy and the repulsive electrostatic interactions of the anions in the ionic liquid with the acid groups of the polymer. The χeff parameter of each sample was quantitatively determined by fitting the small-angle X-ray scattering (SAXS) profiles of disordered phases using random phase approximation (RPA) theory based on Leibler's mean-field theory.47
Results and discussion
Phase transitions of phosphonated block copolymers with imidazoles
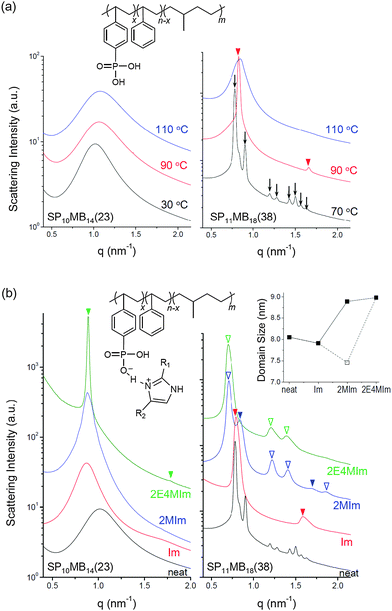
Two PSP-b-PMB block copolymers composed of randomly phosphonated PS (PSP) and poly(methylbutylene) (PMB) were synthesized by varying the molecular weight and the phosphonation level (PL). The molecular structure of PSP-b-PMB is shown in Fig. 1a, where the PL is defined as x/n. Hereafter, the PSP-b-PMB samples are labeled according to the degree of polymerization (N) of each block (subscript) and the PL value (number in parenthesis), i.e., SP10MB14(23) and SP11MB18(38).The phase behavior of neat SP10MB14(23) and SP11MB18(38) was first investigated by SAXS experiments in the temperature range of 25–150 °C, as presented in Fig. 1a. The neat SP10MB14(23) showed disordered phases throughout the temperature window, attributed to the low N and low PL. Heating SP10MB14(23) gradually decreased its scattering intensities, which is indicative of the upper critical solution temperature (UCST) behavior. Increasing the N and PL values of the PSP-b-PMB block copolymer, i.e., SP11MB18(38), produced a gyroid structure with the Ia3d space group, which was identified by a series of Bragg peaks indexed to the (211), (220), (321), (400), (420), (332), (422), and (431) planes. The domain size (d211) was 8.0 nm at 70 °C. The scattering data for the neat SP11MB18(38) further indicated a gyroid-to-LAM transition at 90 ± 10 °C followed by an ODT at 110 ± 10 °C. All of the phase transitions were thermally reversible.
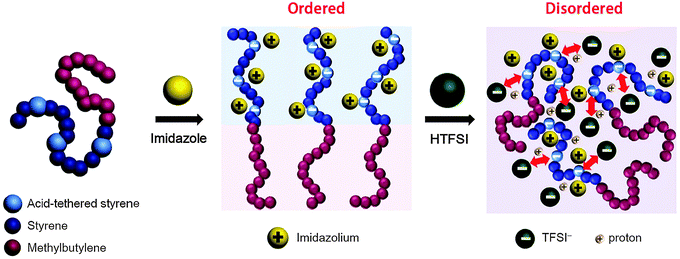
SP10MB14(23) and SP11MB18(38) were doped with various imidazoles, i.e., imidazole (Im), 2-methylimidazole (2MIm), and 2-ethyl-4-methylimidazole (2E4MIm). The molar ratio of imidazole and phosphonic acid in the polymer was fixed at 1/1. A representative molecular structure of the samples doped with imidazole is illustrated in Fig. 1b (R1, R2 = H for Im; R1 = CH3, R2 = H for 2MIm; and R1 = CH2CH3, R2 = CH3 for 2E4MIm). The SAXS profiles in Fig. 1b, which were measured at 70 °C, indicated that imidazole-doping enhanced the segregation strength of SP10MB14(23) in the following order: 2E4MIm > 2MIm > Im. The appearance of Bragg peaks (▼) at 1q* and 2q* with q* = d100 and the narrow full-width at half maximum (FWHM) of the first Bragg peak of the 2E4MIm-doped sample suggest that the alkyl-substituents in imidazole contribute to increasing χeff because of the favorable hydrophobic interactions of the PSP phases. Because the embedded amounts of Im, 2MIm, and 2E4MIm in SP10MB14(23) were small (7 ± 1 wt%), the morphological changes shown in Fig. 1b are notable.
For SP11MB18(38) doped with imidazoles (1/1 molar ratio of imidazole and –PO3H2), various phase sequences were observed, including a gyroid-to-LAM transition with Im and a gyroid-to-hexagonal cylinder (HEX) transition with 2E4MIm. All of the observed morphologies were independent of the temperature. Thus, the addition of 2E4MIm caused more effective swelling of the PSP chains than Im. The LAM and HEX phases coexist in the 2MIm-containing sample, as indicated by Bragg peaks at 1q*, 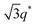 ,
, 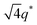 , and
, and 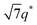 (▽) and at 1q* and 2q* (▼), respectively. The domain spacing of the HEX phase is approximately 18% larger than that of the LAM phase. This coexistence likely arises from the different partitioning of 2MIm between the LAM and HEX phases, although its origin remains unclear. It should be noted that because of the high PL value of SP11MB18(38), the amount of imidazoles required to achieve a 1/1 molar ratio with the phosphonic acid groups is increased by 13 ± 3 wt%.
(▽) and at 1q* and 2q* (▼), respectively. The domain spacing of the HEX phase is approximately 18% larger than that of the LAM phase. This coexistence likely arises from the different partitioning of 2MIm between the LAM and HEX phases, although its origin remains unclear. It should be noted that because of the high PL value of SP11MB18(38), the amount of imidazoles required to achieve a 1/1 molar ratio with the phosphonic acid groups is increased by 13 ± 3 wt%.
Phase transitions of phosphonated block copolymers with ionic liquids
We now elucidate the phase transitions of PSP-b-PMB block copolymers with ionic liquids. By combining imidazoles and bis(trifluoromethanesulfonyl)imide (HTFSI), Im/TFSI−, 2MIm/TFSI−, and 2E4MIm/TFSI− were synthesized with various compositions. The chemical structure given in Fig. 2a describes the SP10MB14(23) block copolymers containing non-stoichiometric ionic liquids.Introducing ionic liquids into SP10MB14(23) with a cation/anion/–PO3H2 molar ratio of 1/0.25/1, as shown in Fig. 2b, resulted in the development of well-defined ordered morphologies of LAM, coexisting LAM + HEX, and HEX for Im/TFSI−, 2MIm/TFSI−, and 2E4MIm/TFSI−, respectively. The Bragg peaks of the HEX structure at 1q*, 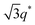 ,
, 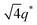 , and
, and 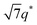 (▽) and those of LAM at 1q* and 2q* (▼) are indicated in the figure. These results suggest that the segregation strength between the PSP and PMB phases can be further enhanced by the presence of TFSI− anions (the morphologies were ill-defined when only imidazoles were added, Fig. 1b) upon mediating the long-ranged intermolecular interactions in the PSP phases. Another probable reason is the free protons (donated from super-acidic HTFSI) in PSP containing non-stoichiometric ionic liquids, which can strengthen the ionic characteristics of PSP phases. The smallest change in the domain size was found for Im/TFSI−, whereas the 2E4MIm/TFSI− sample exhibited a substantially increased domain size.
(▽) and those of LAM at 1q* and 2q* (▼) are indicated in the figure. These results suggest that the segregation strength between the PSP and PMB phases can be further enhanced by the presence of TFSI− anions (the morphologies were ill-defined when only imidazoles were added, Fig. 1b) upon mediating the long-ranged intermolecular interactions in the PSP phases. Another probable reason is the free protons (donated from super-acidic HTFSI) in PSP containing non-stoichiometric ionic liquids, which can strengthen the ionic characteristics of PSP phases. The smallest change in the domain size was found for Im/TFSI−, whereas the 2E4MIm/TFSI− sample exhibited a substantially increased domain size.
The most unexpected results were found for the incorporation of stoichiometric ionic liquids into SP10MB14(23) with a cation/anion/–PO3H2 molar ratio of 1/1/1. As shown in Fig. 2c, the re-emergence of disordered phases with large reductions in the domain size was observed in all samples as a result of ionic liquid embedding. This is intriguing because the loading amounts of stoichiometric ionic liquids were as high as 26 ± 1 wt%. We suppose that the existence of TFSI− anions in the samples leads to the competition between attractive (imidazolium cation/polymer) and repulsive (TFSI− anion/polymer) electrostatic interactions, and increasing the TFSI− content in ionic liquids eventually causes the dominant electrostatic repulsion with the polymer matrix, thereby decreasing the segregation of the PSP and PMB blocks.
Analogous thermodynamic behavior was identified for SP11MB18(38) containing stoichiometric ionic liquids. As shown in Fig. 2d, all three samples exhibited LAM phases at 70 °C, which underwent ODTs at 140 ± 10 °C, whereas in the presence of the cation only, temperature-insensitive LAM, LAM + HEX coexistence, and HEX structures were found (Fig. 1b). The substantially reduced domain sizes (7.0–7.4 nm) despite the high ionic liquid loading (39 ± 1 wt%) are particularly noteworthy.
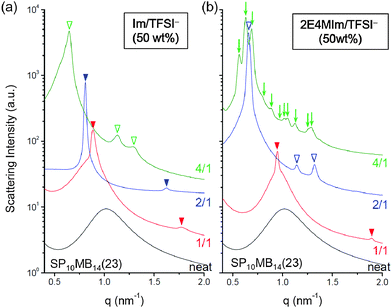
This result prompted us to further investigate the phase behaviors of ionic liquid-containing PSP-b-PMB block copolymers over a range of cation/anion compositions. To rule out the effects of the amount of the ionic liquid on the lyotropic phase transitions, the ionic liquid loading was fixed at 50 wt%. The morphologies of SP10MB14(23) samples containing Im/TFSI− and 2E4MIm/TFSI− are compared in Fig. 3a and b, respectively. The cation/anion molar ratios employed are noted in the figure. Clearly, although the amount of ionic liquid remained constant, the resulting self-assembled morphologies and domain sizes varied greatly depending on the cation/anion composition. Increasing the anion content in the ionic liquids decreased the segregation strength and reduced the swelling, in good agreement with the conclusions drawn in Fig. 2.
After Im/TFSI− addition, as shown in Fig. 3a, HEX and LAM phases were observed for SP10MB14(23) at the cation/anion composition of 4/1 and 2/1, respectively, and markedly different domain sizes were observed. Intriguingly, Fig. 3b shows that the SP10MB14(23) containing 4/1 2E4MIm/TFSI− displayed a series of Bragg peaks (↓), which were indexed to the (200), (210), (211), (220), (310), (222), (320), (321), (400), (420), and (421) planes of the A15 lattice with space group Pm3n, whereas the use of 2/1 2E4MIm/TFSI− yielded a HEX structure. The observed A15 lattice is noteworthy because it has been shown to be an unstable phase for linear diblock copolymer melts and solutions. It should be noted that the formation of a thermally reversible, equilibrium A15 phase bordered by HEX and disordered phases was previously demonstrated in our very recent study on a different set of PSP-b-PMB block copolymers containing 2/1 2E4MIm/TFSI− and 2/1 2MIm/TFSI−.41
When 50 wt% stoichiometric ionic liquids (corresponding to a cation/anion/–PO3H2 molar ratio of 3/3/1) were used, SP10MB14(23) showed an analogous ordered LAM structure coexisting with disordered phases, regardless of the cation in the ionic liquid (Fig. 3a and b), unlike the disordered morphology obtained with stoichiometric ionic liquids (cation/anion/–PO3H2 molar ratio of 1/1/1) (Fig. 2c). Thus, for stoichiometric ionic liquids, the microphase separation of SP10MB14(23) eventually occurs at high ionic liquid loadings. The microphase separation of acid-tethered polymers as a result of ionic liquid addition is consistent with the literature.44,45,48–50
Based on the results obtained thus far, we drew the following conclusions: (1) ionic liquids containing excessive cations enhance the segregation strength in PSP-b-PMB block copolymers containing ionic liquids. (2) Alkyl-substituted imidazoles increase the selectivity for PSP chains because of their favorable hydrophobic interactions. (3) The range of accessible self-assembled PSP-b-PMB block copolymer morphologies can be greatly expanded by adding ionic liquids with various compositions.
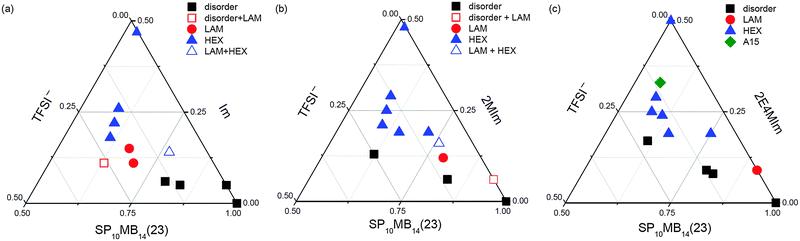
Phase diagrams of the ionic liquid-containing PSP-b-PMB block copolymers are shown in Fig. 4. As representative examples, we only present the phase diagrams of SP10MB14(23) samples. The volume fraction (ϕ) of each component was calculated using the pure component densities (ρPS = 1.05 g cm−3, ρPSP = 1.44 g cm−3, ρPMB = 0.86 g cm−3, ρIm = 1.12 g cm−3, ρ2MIm = 1.06 g cm−3, ρ2E4MIm = 1.00 g cm−3, and ρTFSI− = 1.94 g cm−3), ignoring the volume changes in mixing.41 Most observed morphologies were found to be temperature insensitive, except for a few samples that showed thermally reversible ODTs at high temperatures.
For SP10MB14(23)/Im/TFSI−, as shown in Fig. 4a, the disordered phases were stabilized when 0.80 < ϕSP10MB14(23) < 1.00, whereas when 0.65 < ϕSP10MB14(23) < 0.80 and 0.10 < ϕIm < 0.15, the samples adopted a LAM structure. The LAM and disorder were found to coexist in the phase boundaries. As the Im/TFSI− content was further increased, a large portion of the phase diagram became occupied by HEX phases. It should be noted that because of the coarse ionic liquid composition employed to study the phase behavior, other morphologies such as gyroid and perforated LAM may exist at the phase boundaries between the LAM and HEX phases.
Using 2MIm/TFSI− and 2E4MIm/TFSI− produced analogous phase diagrams, Fig. 4b and c, although the HEX phases occupied relatively large areas of the phase diagrams. Additionally, the LAM window became increasingly narrow for imidazoles with long alkyl substituents. Notably, SP10MB14(23)/2E4MIm/TFSI− can self-assemble into A15 lattices at a high ionic liquid loading, as shown in Fig. 4c. Because A15 lattices are stabilized in the high χN windows of phase diagrams compared to HEX phases,48 SP10MB14(23)/2MIm/TFSI− and SP10MB14(23)/Im/TFSI− samples may also adopt A15 lattices at high ionic liquid loadings with excessive cation compositions.
Thermodynamic underpinnings of the phase behavior of acid-tethered block copolymers with ionic liquids
To achieve an in-depth understanding of the phase behaviors of PSP-b-PMB block copolymers containing ionic liquids, we employed a theoretical approach—Leibler's theory based on the RPA given in eqn (1)47—to estimate the χeff values of the samples with various cation/anion compositions.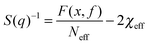 | (1) |
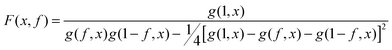 | (2) |
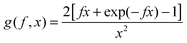 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 by assuming ideal mixing, as in eqn (4).31
41 by assuming ideal mixing, as in eqn (4).31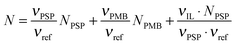 | (4) |
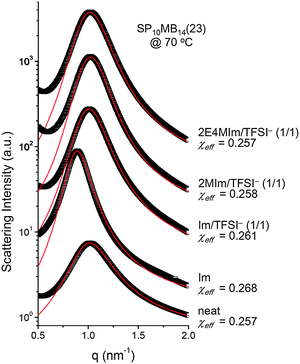
Fig. 5 shows the SAXS profiles of SP10MB14(23) measured at 70 °C in the absence and presence of various ionic liquids. The curves represent the best least-squares RPA fits (eqn (1)) with Rg and χ as the adjustable parameters. The neat SP10MB14(23) had a χeff of 0.257, which increased to 0.268 upon doping with Im. However, the χeff value decreased to 0.261 with 1/1 Im/TFSI−. Among the samples comprising Im/TFSI−, 2MIm/TFSI−, and 2E4MIm/TFSI−, the lowest χeff value was determined with 2E4MIm/TFSI−. This is noteworthy because the 2E4MIm-doped sample had the strongest segregation, exceeding those of the Im- and 2MIm-doped samples (Fig. 1b). We suppose that the relatively weak binding energy between 2E4MIm and the PSP chains (419 kJ mol−1, cf. 459 kJ mol−1 for Im)41 may enhance the repulsive interactions between TFSI− and the phosphonic acid groups. Qualitative agreement was found between the χeff value of 0.257 for the neat SP10MB14(23) determined by the RPA analysis and those obtained using Leibler theory (χeff,ODT = 0.276)47 and Fredrickson–Helfand theory (χeff,ODT = 0.597).51
Furthermore, we noted that the temperature dependence of χ was affected by the addition of ionic liquids. Analyzing the SAXS data collected at different temperatures (data and RPA fits not shown here) gave χeff = 0.1974 + 20.076/T for the neat SP10MB14(23), whereas the addition of 1/1 Im/TFSI− to SP10MB14(23) yielded a weak temperature dependence of χeff = 0.2252 + 12.174/T. Consequently, the differences in χeff values between the neat SP10MB14(23) and Im/TFSI−-doped SP10MB14(23) become great as the temperature is increased, i.e., 0.244 and 0.254 at 150 °C.
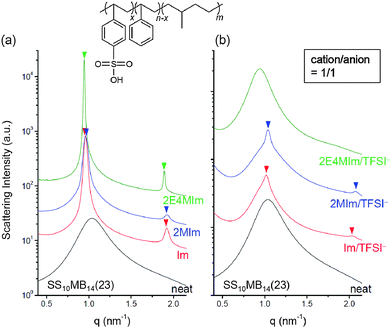
Finally, we investigated the phase behaviors of ionic liquid-containing block copolymers carrying different acid groups. Sulfonated block copolymers with molecular weights and acid content equal to those of SP10MB14(23), i.e., SS10MB14(23), were synthesized for this purpose. As shown in Fig. 6a, the neat SS10MB14(23) also exhibited disordered morphology, which transformed into ordered LAM phases with the addition of Im, 2MIm, and 2E4MIm in a 1/1 molar ratio to the –SO3H group. Overall, stronger phase separation was found in SS10MB14(23) doped with imidazoles than in the phosphonated analogs.
Adding 1/1 Im/TFSI−, 1/1 2MIm/TFSI−, and 1/1 2E4MIm/TFSI− to SS10MB14(23) (molar ratios relative to the number of moles of –SO3H groups) disrupted the microphase separation (Fig. 6b), in line with the results obtained for the SP10MB14(23) samples (Fig. 2c). However, remnant traces of the ordered LAM phases were observed for SS10MB14(23) doped with Im/TFSI− and 2MIm/TFSI− whereas the 2E4MIm/TFSI−-containing SS10MB14(23) retained no long-range order. This finding is in good agreement with the results of the RPA analysis provided in Fig. 5, where the greatest reduction in the χeff value caused by increasing the anion loading was found for 2E4MIm/TFSI−.
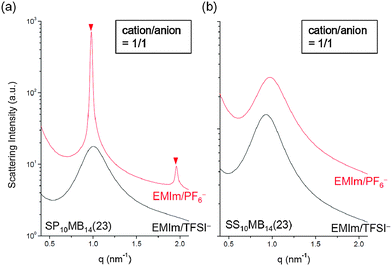
The major discrepancy between SP10MB14(23) and SS10MB14(23) resulted from varying the anions in the ionic liquids. As shown in Fig. 7a and b, the addition of a hydrophobic ionic liquid, i.e., 1/1 EMIm/PF6−, to SP10MB14(23) yielded sharp Bragg peaks (▼) at 1q* and 2q* with q* = 2π/d100 and d100 = 6.4 nm, indicating well-defined LAM morphology instead of the fully disordered phases exhibited by SS10MB14(23) containing 1/1 EMIm/PF6−. Without a doubt, this difference arises because PSP chains are fundamentally more hydrophobic than PSS, therefore, the PSP blocks have relatively high selectivity for EMIm/PF6−. This finding leads us to conclude that the balance between the solvation energy of ionic liquids in acid-tethered polymers and the repulsive electrostatic interactions between the anions and the polymer matrix is the key determinant of the phase behaviors of acid-tethered block copolymers containing ionic liquids.
The thermodynamics of acid-tethered block copolymers with ionic liquids are schematically depicted in Fig. 8. The imidazoles were incorporated into the acid-tethered polymers to enhance the thermodynamic incompatibility with the ionophobic polymer chains and thereby achieve microphase-separated morphologies. Adding ionic liquids composed of imidazoles and counter-anions further enhanced the segregation strength by creating long-ranged intermolecular interactions in the ionic phases. However, as the anion content increased, the prevailing repulsive interactions between the anions and the polymer matrix disrupted the ordered microdomains. The complete ionic liquid-solvation mechanisms in polymers, which involve hydrogen bonding, electrostatic interactions, and hydrophobic interactions, remain unknown and will be the subject of future studies.
Conclusions
Full phase diagrams of block copolymers carrying acid groups were constructed after doping with imidazolium ionic liquids. Using a range of cation/anion compositions, various equilibrium morphologies, including disorder, LAM, HEX, gyroids, and A15 lattices, were observed. RPA analysis based on Leibler theory allowed us to quantitatively estimate the samples' χeff values. These results indicated that imidazole-doping decreases the miscibility of acid-tethered polymers and ionophobic polymers and that the χeff value is sensitive to the ring structure of imidazoles. In contrast, increasing the anion content of the added ionic liquids likely caused the disruption of the ordered microdomains of the block copolymers. This effect is attributable to the dominant repulsive electrostatic interactions of the anions in the ionic liquids with the acid groups of the polymers. Intriguingly, the thermodynamics was found to be substantially influenced by the solvation energies of anions. Therefore, the use of hydrophobic ionic liquids results in opposing trends in the phase behaviors of phosphonated block copolymers and their sulfonated analogs.Experimental
Synthesis of PSP-b-PMB block copolymers
Poly(styrene phosphonate-b-methylbutylene) (PSP-b-PMB) block copolymers were synthesized by bromination and subsequent palladium-catalyzed phosphonation of PS-b-PMB block copolymers using procedures described in ref. 41. The precursor PS-b-PMB block copolymers were prepared by sequencial anionic polymerization of PS and polyisoprene (PI), followed by selective hydrogenation of PI.7 The absolute molecular weights of precursor PS-b-PMB block copolymers were determined by gel permeation chromatography (GPC, Waters Breeze 2 HPLC) using a light scattering detector, where the dn/dc value of PS-b-PMB was calculated based on the block composition. The PLs of the PSP-b-PMB block copolymers were determined by 1H nuclear magnetic resonance spectroscopy (1H-NMR, Bruker AVB-300, in CDCl3) based on the signals of the phosphonic ethyl ester groups (in the ester form), which were confirmed after hydrolysis by titration with 0.02 M NaOH aqueous solution. The polydispersity indices of PSP-b-PMB block copolymers in the ester form, as measured by GPC with polystyrene standards in THF, were lower than 1.09.Synthesis of PSS-b-PMB block copolymers
Using the same PS-b-PMB precursor block copolymers employed for the preparation of PSP-b-PMB, poly(styrene sulfonate-b-methylbutylene) (PSS-b-PMB) block copolymers with controlled SL values were synthesized by using acetic sulfate in 1,2-dichloroethane. Details regarding the synthetic procedures are given elsewhere.10 The SLs were determined by 1H NMR (in d6-acetone) based on aromatic protons of PS.Imidazoles and ionic liquids
Im (≥99.5%), 2MIm (≥99.0%), 2E4MIm (≥95.0%), and HTFSI (≥95%) were purchased from Sigma-Aldrich and used as received. Ionic liquids in a range of cation and anion compositions were prepared by blending predetermined amounts of imidazoles and HTFSI, followed by heating above the melting temperature of ionic liquids for 10 h. The final compositions of the prepared ionic liquids were determined by 1H NMR.Preparation of ionic liquid-doped polymers
Inhibitor-free anhydrous THF (≥99.9%) was used without further purification and methanol was degassed two times prior to use. Predetermined quantities of ionic liquids were added to the PSP-b-PMB or PSS-b-PMB block copolymers using 50/50 vol% THF and methanol mixtures, and stirred overnight at room temperature. Membranes were prepared by solvent casting under an argon atmosphere for 2 days, followed by vacuum drying at 70 °C for 7 days. To avoid water contamination of hygroscopic samples, sample preparation was performed in an argon-filled glove box with oxygen and moisture concentration below 0.1 ppm.Small angle X-ray scattering (SAXS)
Synchrotron SAXS measurements on PSP-b-PMB and PSS-b-PMB block copolymers with and without ionic liquids were performed using the 9A SAXS beamline at the Pohang Light Source (PLS-II). The samples were packed into an air-tight sample cell consisting of an aluminum spacer, two Kapton windows, O-rings, and aluminum covers. The sample temperature was controlled within ±0.2 °C using a sample stage provided by the PLS-II. The wavelength (λ) of the incident X-ray beam was 0.073 nm (Δλ/λ = 10−4), and a sample-to-detector distance of 2.0 m was used yielding a scattering wave vector q (q = 4πsin(θ/2)/λ, where θ is the scattering angle) in the range 0.1–2.5 nm−1. The scattering data were corrected for the CCD dark current and the scattering from air and Kapton windows. The resulting two-dimensional scattering data were averaged azimuthally to obtain intensity versus q.Acknowledgements
This work was financially supported by the National Nuclear R&D Program (2011-0031931), the Global Frontier R&D program on Center for Multiscale Energy System funded by the National Research Foundation under the Ministry of Science, ICT & Future, Korea.References
- X. Wang, M. Goswami, R. Kumar, B. S. Sumpter and J. Mays, Soft Matter, 2012, 8, 3036–3052 RSC.
- N. Li and M. D. Guiver, Macromolecules, 2014, 47, 2175–2198 CrossRef CAS.
- M. Tanaka, K. Fukasawa, E. Nishion, S. Yamaguchi, K. Yamada, H. Tanaka, B. Bae, K. Miyatake and M. Watanabe, J. Am. Chem. Soc., 2011, 133, 10646–10654 CrossRef CAS PubMed.
- M. Singh, O. Odusanya, G. M. Wilmes, H. B. Eitouni, E. D. Gomez, A. J. Patel, V. L. Chen, M. J. Park, P. Fragouli, H. Iatrou, N. Hadjichristidis, D. Cookson and N. P. Balsara, Macromolecules, 2007, 40, 4578–4585 CrossRef CAS.
- J. D. McIntosh, T. Kubo and T. P. Lodge, Macromolecules, 2014, 47, 1090–1098 CrossRef.
- F. S. Bates and G. H. Fredrickson, Phys. Today, 1999, 52, 32–38 CrossRef CAS.
- S. Y. Kim, S. Kim and M. J. Park, Nat. Commun., 2010, 1, 88 Search PubMed.
- M. L. Hoarfrost, M. S. Tyagi, R. A. Segalman and J. A. Reimer, Macromolecules, 2015, 45, 3112–3120 CrossRef.
- E. D. Gomez, A. Panday, E. H. Feng, V. Chen, G. M. Stone, A. M. Minor, C. Kisielowski, K. H. Downing, O. Borodin, G. D. Smith and N. P. Balsara, Nano Lett., 2009, 9, 1212–1216 CrossRef CAS PubMed.
- J. B. Gilbert, M. Luo, C. K. Shelton, M. F. Rubner, R. E. Cohen and T. H. Epps, ACS Nano, 2015, 9, 512–520 CrossRef CAS PubMed.
- T. H. Epps, T. S. Bailey, R. Waletzko and F. S. Bates, Macromolecules, 2003, 36, 2873–2881 CrossRef CAS.
- G. Jo, H. Jeon and M. J. Park, ACS Macro Lett., 2015, 4, 225–230 CrossRef CAS.
- A. Ghosh, C. Wang and P. Kofinas, J. Electrochem. Soc., 2010, 157, A846–A849 CrossRef CAS.
- J. Ji, J. Keen and W. H. Zhong, J. Power Sources, 2011, 196, 10163–10168 CrossRef CAS.
- G. Jo, H. Ahn and M. J. Park, ACS Macro Lett., 2013, 2, 990–995 CrossRef CAS.
- W. S. Young, J. N. L. Albert, A. B. Schantz and T. H. Epps, Macromolecules, 2011, 44, 8116–8123 CrossRef CAS.
- B. Huang, C. C. Cook, S. Mui, P. P. Soo, D. H. Staelin, A. M. Mayes and D. R. Sadoway, J. Power Sources, 2001, 97, 674–676 CrossRef.
- A. V. G. Ruzette, P. P. Soo, D. R. Sadoway and A. M. Mayes, J. Electrochem. Soc., 2001, 148, A537–A543 CrossRef CAS.
- N. S. Wanakule, J. M. Virgili, A. A. Teran, Z. G. Wang and N. P. Balsara, Macromolecules, 2010, 43, 8282–8289 CrossRef CAS.
- W. S. Young, P. J. Brigandi and T. H. Epps, Macromolecules, 2008, 41, 6276–6279 CrossRef CAS.
- E. F. Ioannou, G. Mountrichas, S. Pispas and E. I. Kamitsos, Macromolecules, 2008, 41, 6183–6190 CrossRef CAS.
- N. S. Wanakule, A. Panday, S. A. Mullin, E. Gann, A. Hexemer and N. P. Balsara, Macromolecules, 2009, 42, 5642–5651 CrossRef CAS.
- I. Nakamura, N. P. Balsara and Z. G. Wang, ACS Macro Lett., 2013, 2, 478–481 CrossRef CAS.
- A. A. Teran and N. P. Balsara, J. Phys. Chem. B, 2014, 118, 4–17 CrossRef CAS PubMed.
- I. Nakamuara, N. P. Balsara and Z. G. Wang, Phys. Rev. Lett., 2001, 107, 198301(5) Search PubMed.
- I. Nakamura and Z. G. Wang, Soft Matter, 2012, 8, 9356–9367 RSC.
- E. P. Grishina, L. M. Ramenskaya and A. N. Mudrov, Eur. Polym. J., 2014, 59, 247–253 CrossRef CAS.
- G. Zardalidis, E. F. Ionaaou, K. D. Gatsouli, S. Pispas, E. I. Kamitsos and G. Floudas, Macromolecules, 2015, 48, 1473–1482 CrossRef CAS.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621–629 CrossRef CAS PubMed.
- P. Madhavan, R. Sougrat, A. R. Behzad, K. V. Peinemann and S. P. Nunes, J. Membr. Sci., 2015, 492, 568–577 CrossRef CAS.
- T. M. Bennett, K. S. Jack, K. J. Thurecht and I. Blakey, Macromolecules, 2016, 49, 205–214 CrossRef CAS.
- L. Gwee, J. H. Choi, K. I. Winey and Y. A. Elabd, Polymer, 2010, 51, 5516–5524 CrossRef CAS.
- P. M. Simone and T. P. Lodge, ACS Appl. Mater. Interfaces, 2009, 1, 2812–2820 Search PubMed.
- P. M. Simone and T. P. Lodge, Macromolecules, 2008, 41, 1753–1759 CrossRef CAS.
- M. L. Hoarfrost and R. A. Segalman, ACS Macro Lett., 2012, 1, 937–943 CrossRef CAS.
- S. Sharick, J. Koski, R. A. Riggleman and K. I. Winey, Macromolecules, 2016, 49, 2245–2256 CrossRef CAS.
- J. M. Virgili, A. Hexemer, J. A. Pople, N. P. Balsara and R. A. Segalman, Macromolecules, 2009, 42, 4604–4613 CrossRef CAS.
- J. M. Virgili, M. L. Hoarfrost and R. A. Segalman, Macromolecules, 2010, 43, 5417–5423 CrossRef CAS.
- S. S. Sekhon, J. S. Park and Y. W. Choi, Phys. Chem. Chem. Phys., 2010, 12, 13763–13769 RSC.
- S. Y. Kim, E. Yoon, T. Joo and M. J. Park, Macromolecules, 2011, 44, 5289–5298 CrossRef CAS.
- H. Y. Jung, S. Y. Kim, O. Kim and M. J. Park, Macromolecules, 2015, 48, 6142–6152 CrossRef CAS.
- J. Hou, Z. Zhang and L. A. Madson, J. Phys. Chem. B, 2011, 115, 4576–4582 CrossRef CAS PubMed.
- I. Nakamura, Soft Matter, 2014, 10, 9596–9600 RSC.
- S. Y. Kim, J. Lee and M. J. Park, Macromolecules, 2014, 47, 1099–1108 CrossRef CAS.
- O. Kim, S. Y. Kim, H. Ahn, C. W. Kim, Y. M. Rhee and M. J. Park, Macromolecules, 2012, 45, 8702–8713 CrossRef CAS.
- C. Jangu, J. H. H. Wang, D. Wang, G. Fahs, J. R. Heflin, R. B. Moore, R. H. Colby and T. E. Long, J. Mater. Chem. C, 2015, 3, 3891–3901 RSC.
- L. Leibler, Macromolecules, 1980, 13, 1603–1617 CrossRef.
- H. Y. Jung, O. Kim and M. J. Park, Macromol. Rapid Commun., 2016 DOI:10.1002/marc.201600036.
- S. Yi, F. Zhang, W. Li, C. Huang, H. Zhang and M. Pan, J. Membr. Sci., 2011, 366, 349–355 CrossRef CAS.
- S. S. Sekhon, J.-S. Park, J.-S. Baek, S.-D. Yim, T.-H. Yang and C.-S. Kim, Chem. Mater., 2010, 22, 803–812 CrossRef CAS.
- G. H. Fredrickson and E. Helfand, J. Chem. Phys., 1987, 87, 697–705 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |