Inorganic engineered nanoparticles in drinking water treatment: a critical review
Konstantinos
Simeonidis
*a,
Stefanos
Mourdikoudis
*bc,
Efthimia
Kaprara
d,
Manassis
Mitrakas
d and
Lakshminarayana
Polavarapu
*ef
aDepartment of Mechanical Engineering, University of Thessaly, 38334, Volos, Greece. E-mail: ksime@physics.auth.gr
bSorbonne Universités, UPMC Univ Paris 06, UMR 8233, MONARIS, F-75005, Paris, France. E-mail: stefanos.mourdikoudis@upmc.fr
cCNRS, UMR 8233, MONARIS, F-75005, Paris, France
dDepartment of Chemical Engineering, Aristotle University of Thessaloniki, 54124, Thessaloniki, Greece
ePhotonics and Optoelectronics Group, Department of Physics and CeNS, Ludwig-Maximilians-Universität München, D-80799, Munich, Germany. E-mail: l.polavarapu@physik.uni-muenchen.de
fNanosystems Initiative Munich (NIM), D-80799, Munich, Germany
First published on 14th August 2015
Abstract
This review summarizes the recent research in the field of inorganic engineered nanoparticle development with direct or potential interest for drinking water treatment. The incorporation of engineered nanoparticles into drinking water treatment technologies against the removal of heavy metals, microorganisms and organic pollutants appears as a very dynamic branch of nanotechnology. Nanoparticles owe their potential to the high specific surface area and surface reactivity compared to conventional bulk materials. Depending on the mechanism of uptake, nanoparticles can be designed to establish high selectivity against specific pollutants and provide the required efficiency for application. However, despite early encouraging results, nanoparticles meet a number of limitations to get promoted and become part of large-scale water treatment plants. The most important is their availability in the required large quantities and their efficiency to fulfil the strict regulations for drinking water consumption and environmental safety. Both deal with the particle preparation cost and the cost of treatment operation with respect to the increase in supplied water price for the consumers. Under this view, this work attempts to evaluate reported studies according to their possibility to meet the reliable requirements of water technology and also suggests an experimental approach to allow validation of tested nanoparticles.
Water impactThe interest of the scientific community in the potential applications of inorganic engineered nanoparticles for drinking water treatment is continuously increasing. This review paper is an up-to-date summary of the recent research progress in this field with respect to the removal of heavy metals, microorganisms and organic pollutants. In parallel, a critical investigation on the applicability of developed nanomaterials is attempted, considering the economic viability, environmental safety and sustainability of the proposed processes. On this, the manuscript discusses issues such as the stability and fate of engineered nanoparticles during and after use, whereas it suggests a generalized approach for excluding reliable and comparable results by laboratory experiments. |
1. Introduction
Following the principles and the discoveries related to the evolution of nanosciences during the past two decades, a wide variety of technological fields have been promoted.1 The impact was immediate and more obvious to the so-called high-technology applications where the demand for a dimension decrease combined with the novel electronic, optical, magnetic and mechanical properties of nanomaterials resulted in the development of new devices and methods.2,3 The expansion of nanotechnology, in these first stages, is mainly referred to the field of electronics and health sciences,4–8 whereas its incorporation into more traditional fields of technology was limited. For instance, the adoption of nanomaterials in conventional everyday products (clothes, shoes, cosmetics, and dyes) and in industrial, agricultural and environmental protection processes9–11 encounters more skepticism based on the large quantity demands combined with their relatively high cost, the need for redesign and reconstruction of process lines and the uncertainty arising from the fate and the effect of nanomaterials on the direct or indirect environmental receptors.12–16Nevertheless, numerous products nowadays claim their innovation on the addition of nanomaterials which improve their physical properties. In addition, there is a significant and intense research effort on the development and optimization of nanomaterials aiming for antimicrobial or catalytic activities with high potential for environmental applications.17,18 The decontamination of flue gases from heavy metals and aromatics,19,20 the treatment of municipal and industrial wastewater for the removal of various pollutants21,22 and the purification of drinking water23,24 or recirculating blood25,26 are the major sections of investigation. Among them, the treatment of natural water for drinking purposes appears as the most challenging field directly dealing with human nutrition and health.27 In this field, any applied method should also comply with the extremely low pollutant concentrations met in natural water and the strict international legislation for human health and environmental safety. In particular, nanomaterials have been tested as media for purification, disinfection, removal of heavy metals, degradation of organic compounds and pharmaceuticals.28–31
Since the beneficiary use of nanomaterials in drinking water treatment is considered as an achievement of high importance, they should be designed to maintain the highest possible specific surface area in order to maximize surface reactivity, effective contact and uptake capacity. For this reason, the class of engineered nanoparticles, and more specifically, inorganic nanoparticles which combine relatively enhanced purification properties and high stability in water, should be preferred for water treatment. Inorganic engineered nanoparticles optimized for water treatment usually act as reaction catalysts causing the degradation, oxidation and reduction or as adsorbents which form strong bonds with specific compounds in a non-reversible way. Therefore, apart from the requirement of a high surface area, the selectivity of nanoparticles for specific water purification processes should stand on the chemical affinity, the surface charge density and the electron transfer ability. However, the main drawbacks for nanoparticle use in water treatment are not directly related to their efficiency but to technical, economical and safety limitations which complicate the replacement of conventional methods.
This review presents the recent laboratory research related to the application of engineered nanoparticles consisting of inorganic phases in water treatment and their classification in the fields of heavy metal removal, antimicrobial activity and organic compound degradation. The reported results are discussed not only according to their potential for application in different drinking water treatment processes but also from a critical consideration of the possibility to scale-up in technologically viable methods and become competitive with existing techniques and conventional materials. As one of the main limitations in the effort to evaluate the efficiency of nanomaterials from different authors is the absence of a unified procedure that enables direct comparison of results, this work suggests an experimental methodology working with reliable conditions and parameter ranges of drinking water treatment and generating proper indices for the validation of performance.
2. Synthesis methods of nanoparticles
A wide variety of methods have been used to produce nanoparticles based on traditional and modern chemical or mechanical procedures. Chemical methods usually generate nanoparticles in dispersions following the gradual size increment of small nuclei after the deposition of atoms or ions released by a chemical reaction (bottom-up).32 Nanoparticles are formed as a result of oversaturation of soluble phases when a change in their solubility occurs. Depending on the source of solubility modification, chemical methods are classified as precipitation (acidity variations),33 thermal decomposition (high temperatures),34 solvothermal (high pressure),35 sonication (supersonics)36 and electrodeposition (redox potential).37 On the opposite, in mechanical preparation routes, nanoparticles are obtained after splitting large-dimension materials in smaller units (top-down). High-energy ball-milling is the main mechanical preparation method for size reduction and preparation of single- or multiple-phase nanoparticle systems.38 Finally, spray techniques may produce nanoparticles in the vapor phase by thermal or laser assisted chemical reactions.39In general, structural and chemical stabilization of nanoparticles are the most important requirements for a successful synthetic approach. The existence of such features ensures high surface-to-volume ratio, sufficient resistance against phase changes (e.g. oxidation) and appearance of nanoscale effects. For this reason, high-quality nanoparticle preparation methods are usually based on the use of surfactants or inorganic coatings to ensure good isolation and a series of size separation and classification procedures to minimize polydispersity. However, most of these processes are not compatible with environmental applications, being even less compatible with water purification for drinking purposes.
As already mentioned, due to the high volumes of water to be treated, water purification demands proportionally high availability in nanoparticle quantities when those are qualified for the application. Therefore, the preparation cost for nanoparticles may dominate the overall cost of the treatment process. This implies that synthesis methods based on expensive reactants or working at high temperatures are not so favorable. In addition, the high toxicity and the incompatibility with aqueous processes are serious drawbacks for the adoption of methods using organic metal precursors, reactants or solvents. It should be mentioned that in terms of industrial production, the accomplishment of strict environmental conditions does not only concern the obtained nanoparticles and their application in water treatment but their large-scale production line as well.
Since water treatment reactions usually take place in active sites on the surface of the solid, another limitation in the preparation approach is related to the need to keep the surface free of surfactants or coating layers which are usually employed to isolate or protect nanoparticles. On the contrary, the formation of nanoparticles without surfactants facilitates agglomeration and a consequent loss in effective specific surface area. Such restrictions indicate that in principle only low-cost, easily scalable, aqueous compatible and potentially environmentally friendly methods may provide nanoparticles suitable for water technology. According to previous analysis, it is concluded that chemical precipitation and mechanical size reduction should be initially considered and developed to obtain nanoparticles for water treatment. More expensive methods should be followed for secondary and selective stages when small quantities of nanoparticles are required.
3. Applications in water treatment
3.1. Removal of heavy metals
The presence of heavy metals in aqueous systems is considered a major worldwide problem related to many harmful effects on the health of humans and other life forms.40–42 The main threats are associated with the consumption of elements such as arsenic, lead, chromium, mercury, antimony, cadmium and nickel, some of which appear in the form of soluble oxy-ions in natural water. Traditional removal methods for heavy metals are classified as relatively selective (chemical coagulation/filtration, adsorption) and non-selective (nanofiltration, reverse osmosis). Among these methods, adsorption is considered as one of the most promising methods because metal-loaded adsorbents are more compact and generally form stronger bonds. For this reason, the use of consumable adsorbents is nowadays the dominant trend, since it is the simplest removal method. The qualification of the proper adsorbent for an individual heavy metal is based on a number of conditions defined by the uptake mechanism of its species. High chemical affinity, stabilization of positive or negative surface charge and incorporation of ion or electron exchange potential are described as possible directions of optimization. A large variety of nanostructured materials, usually in the form of inorganic engineered nanoparticles, have been studied as adsorbents for the removal of heavy metals. The main research efforts concern the use of inorganic nanoparticles such as zero-valent iron (ZVI), iron oxides (Fe3O4, γ-Fe2O3) and oxy-hydroxides (FeOOH), some other metal oxides (Al2O3, TiO2, MnO2, ZrO2, ZnO, MgO, CeO2) and metals or alloys (Au, Ag, Pd). Few of them were already promoted as commercial products in water treatment technology. The goal of this process is the reduction of residual concentration below the regulation limit set by international organizations. Depending on the established risk of each heavy metal, its concentration must comply with a different tolerance limit. For instance, the regulation limit for arsenic in E.U. countries is 10 μg L−1, while the corresponding one for mercury is 1 μg L−1.43Iron-based nanoparticles are the most widely applied systems for the uptake of heavy metals in water.44 The combination of properties like chemical affinity to targeted oxy-ions, surface charge and redox potential together with their stability and low-cost enable their use for various cases. In addition, the magnetic behavior of phases such as Fe3O4, γ-Fe2O3 and ZVI facilitates their recovery after application. In particular, ZVI nanoparticles are studied for their potential to work as an agent which catalyzes the reduction of some heavy metal forms to a lower oxidation state. For instance, this approach is important in the case of hexavalent chromium where its reduction to the trivalent form results in the separation of Cr as insoluble hydroxides.45 However, the main drawbacks of nanoscale ZVI are the release of soluble iron ions and its susceptibility in surface oxidation. To overcome the latter issue, a protective layer for the iron nanoparticle surface was employed. Chitosan-coated ZVI nanoparticles were reported for their potential to remove Cr(VI) from water by its reduction to Cr(III) and the simultaneous formation of a precipitate with Fe(III).46,47 Carboxymethyl cellulose, polyphenols and starch were also mentioned as stabilizers for ZVI nanoparticles oriented to Cr(VI) uptake,48–51 whereas particles supported on carbon nanotubes or graphene were also reported in an effort to avoid their aggregation.52,53 Another way to protect nanoscale ZVI and preserve its reducing ability for Cr(VI) was by their preparation into orange peel pith in sizes 20–80 nm (ref. 54) or shear-thinning gels of xanthan gum.55 The coating of ZVI nanoparticles by a thin layer of another metal or the formation of bimetallic systems was also tested. ZVI nanoparticles coated by Ag or Cu were found to enhance their stability against corrosion,56,57 whereas the galvanic coupling of Fe with Ag, Pd, Ni, Al, Cu, Co or Zn is able to increase the reduction rate of Cr(VI).58 Magnetite-stabilized ZVI nanoparticles also facilitate Cr(VI) reduction reaction.59 Nevertheless, very stable uncoated Fe nanoparticles at the size range of 45–80 nm with significant Cr(VI) removal capacity at low concentrations were prepared by physical vapor deposition using solar beam.60 Low-cost ZVI nanoparticles prepared by scrap iron and steel pickling waste liquor were also suggested as an alternative for the complete removal of Cr(VI) from water.61,62 In general, the high reactivity of ZVI nanoparticles explains the improvement of efficiency and kinetics in Cr(VI) removal compared to granular ZVI63,64 although specific interferences may play an important role.65 For this reason, ZVI nanoparticles are promoted for the remediation of polluted groundwater.66–69 Integrated systems for large-scale environmental remediation based on nanoscale ZVI have already become commercially available. Nanofer25 produced by NanoIron has been tested for the removal of Cr(VI), U(VI) and chlorinated hydrocarbons from contaminated soil and groundwater.70 The reduction potential of ZVI nanoparticles has also been reported for the uptake of U(VI) and Se(VI) from water.71–73
Zero-valent iron in the form of nanoparticles is examined for the treatment of a variety of other heavy metals. Arsenic removal is favored after taking its surface oxidation and corrosion as an advantage for the adsorption of As(III) and As(V). During water treatment, ZVI gets oxidized forming in situ oxy-hydroxides and oxides with enhanced surface area, charge density and reactivity (Fig. 1). Nanoscale ZVI was used as synthesized,74 embedded on activated carbon, bentonite or chitosan nanospheres75–77 or surface modified by sodium dodecyl sulfate78 or humic acid.79 The mechanisms of adsorption under various pH conditions, the gradual modification of ZVI during the process and the possible interferences have been reported separately for As(III) and As(V).80–83 The treatment of As-polluted groundwater under aerobic and anaerobic conditions was also investigated.84,85 A significant number of studies suggest the use of nanoscale ZVI for the adsorption of Pb2+.86–89 Kaolin-supported ZVI nanoparticles were found similarly efficient to remove Ni2+ and Cd2+ together with Pd2+.90 Treatment of water with high concentrations in Cu2+,91,92 Hg2+,93 Co2+,94 Ba2+ (ref. 95) and Zn2+ (ref. 96) is also considered. Their good performance for multiple heavy metals was also demonstrated for water systems polluted by Cr(VI), Mo(VI), Cu2+, Cd2+, and U(VI).97–100
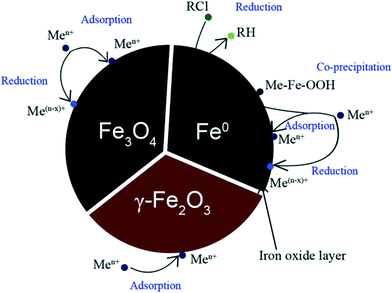 | ||
| Fig. 1 Possible mechanisms of pollutant removal during water treatment by magnetic iron-based nanoparticles.101 | ||
Iron oxide nanoparticles may also provide reducing properties like those of ZVI but also surpass the drawback of soluble iron release. At the same time, they present higher stability against structural and chemical transformation, while their chemical affinity to many heavy metal oxy-ions is significant (Fig. 1). The last characteristic is important for adsorption processes where pollutants may form covalent bonds through oxygen bridges and be captured in a non-reversible way. In addition, magnetite (Fe3O4) and maghemite (γ-Fe2O3) nanoparticles provide the opportunity for their recovery and handling in water systems due to their magnetic response. Arsenic removal is the most studied case of magnetic iron oxide application in water treatment. The efficiency of oleic acid-coated Fe3O4 nanoparticles for the uptake of As(III) and As(V) was examined for various sizes in the range of 12–300 nm.102 In these studies, the adsorption capacity was getting higher following the decrease in the dimensions of nanoparticles. Such observation should be related not only to the increase in specific surface area but also to the easier oxidation of the nanoparticle surface to γ-Fe2O3 which is more efficient to arsenic adsorption than Fe3O4. The same group suggested the recovery of used nanoparticles using a column high-gradient magnetic separator.103 In an effort to overcome the high preparation cost of nanoparticles in these studies, an alternative method for the synthesis of low-cost and environmentally friendly magnetite nanoparticles based on everyday ingredients was examined.104 However, the coating of Fe3O4 by organic surfactants was found to inhibit the adsorption process. In particular, 30 nm magnetite nanoparticles prepared by surfactant-assisted ball-milling were inefficient to reduce As(III) and As(V) concentrations below 200 μg L−1.105 The same work indicated that hematite-coated Fe3O4 may combine improved arsenic removal capacity and the required magnetic properties for their easy recovery. Similar conclusions were obtained for nanocrystalline magnetite produced by a mechanical process and tested as an arsenic adsorbent.106 A series of publications deals with the kinetics of arsenic adsorption on magnetite or mixed magnetite/maghemite nanoparticles and the role of particle concentration, pH and interfering ions in the process. The results suggest that As(III) and As(V) adsorption follows a first-order rate equation slightly affected by the ionic strength and temperature, while efficiency is presented to be almost constant in the pH range 6–8 of typical natural water sources, reaching its maximum values below pH 4.107–109 As observed for magnetite/maghemite mixed nanoparticles, contact time and initial arsenic concentration determine the spontaneous adsorption process which requires at least 3 h to reach equilibrium.110 Among the various interferences tested, only phosphate and nitrate ions at relatively high concentrations may significantly inhibit the adsorption of arsenic on magnetite nanoparticles.111,112
Several efforts were focused on the preparation of functionalized or composite magnetite nanoparticles. Graphene oxide modified with magnetite and MnO2 nanoparticles was used as an equally efficient adsorbent for both As(III) and As(V) providing high surface area and magnetic properties.113 Magnetite nanoparticles modified by cetyltrimethylammonium bromide (CTAB),114 starch-bridged,115 ascorbic acid-coated116 or supported on boron nitride nanotubes,117 hydrotalcite,118 multiwalled carbon nanotubes119 and activated microfibrillated cellulose120 were also evaluated for arsenic removal. The introduction of Fe3O4 nanoparticles obtained by wastes was another field of investigation to capture As(III) and As(V) aqueous species.121,122
Maghemite has a similar crystal structure to magnetite, but the fact that iron appears exclusively as Fe3+ and the frequent stabilization of its nanoparticles at smaller dimensions explain its higher affinity to As(V).123 Electrochemically synthesized γ-Fe2O3 nanoparticles with sizes 11–23 nm indicated an endothermic As(V) adsorption limited by the mass transfer of As(V) and described by a pseudo first-order model.124 An enhancement in the removal efficiency was observed at lower particle dimensions (6 nm) where the oxidation of As(III) was favorable.125 Furthermore, the adsorption stability of arsenic on maghemite nanoparticles was demonstrated.126
Nanoparticles consisting of magnetic iron oxides have been widely tested for Cr(VI) removal. An overall study on the potential of Fe3O4 nanoparticles to be incorporated in drinking water technology was recently reported.127 The results indicate that magnetite nanoparticles may combine sufficient cost, chemical stability, improved Cr(VI) reduction ability and environmental safety after use. An integrated system for the treatment and magnetic recovery of the nanoparticles was also suggested. Preliminary adsorption kinetics of the process at various temperatures were presented elsewhere.128 Removal of Cr(VI) by mixed magnetite–maghemite nanoparticles is an endothermic process which follows an oxidation–reduction mechanism as revealed by Raman and X-ray photoelectron spectroscopy measurements.129 Such systems have also been prepared on-site by an electrocoagulation process using iron electrodes into the polluted water.130 Adsorption of Cr(VI) on maghemite nanoparticles occurs in two steps: external surface diffusion followed by intra-particle diffusion.131 The effects of pH, temperature, initial concentration and the presence of coexisting ions were examined in ref. 132. Equilibrium could be reached even after 15 min of contact. A number of publications describe modified magnetite nanoparticle systems applied for Cr(VI) removal. Engineered biogenic magnetite nanoparticles proposed for a remediation process133 showed a high ability to reduce Cr(VI) in its trivalent nontoxic form which is stabilized in a spinel layer of the iron oxide.134 Additional studies discussing the efficiency of chitosan-coated135,136 or humic acid-coated137 magnetite nanoparticles, polydopamine138 or δ-FeOOH coated maghemite nanoparticles,139 montmorillonite,140 diatomite141 or carbon nanotube142 supported iron oxide nanoparticles were also reported. In another case, Fe3O4 nanoparticles produced by a hydrothermal approach were successfully tested for Cr(VI) and Pb2+ removal from wastewater.143
Furthermore, iron oxide magnetic nanoparticles were employed for the uptake of different heavy metals. The reduction mechanisms of Hg2+ by Fe3O4 nanoparticles were examined in ref. 144 and 145. Magnetite nanoparticles were also tested for the uptake of U(VI) (ref. 146) and Se(IV).147 Functionalized Fe3O4 nanoparticles by amines,148–150 cyclodextrin,151 polymers152 and polyacrylic acid153 were used for the adsorption of Cu2+, Cd2+, Pb2+, Ni2+, and Cr(VI), whereas the combination with polypyrrole,154 silica155,156 or humic acid157 was investigated for the uptake of Cr(VI), U(VI), Hg2+, Pb2+, Cd2+, and Cu2+. On the other hand, Mo(VI), Cu2+, Zn2+, Pb2+, Cd2+, Ni2+, Se(IV) and Hg2+ were successfully adsorbed from water using maghemite-based nanoparticles.158–166
A number of publications for the removal of As, Cr(VI), Cu2+, Se and Co2+ suggest ferrite nanoparticles as a magnetically activated system for water treatment. MnFe2O4,167–170 MgFe2O4,171,172 ZnFe2O4,173 CoFe2O4,174 NiFe2O4 (ref. 175) and CuFe2O4 (ref. 176) have been studied in these works although the easy leaching of their components is an important drawback for application.
Non-magnetic iron oxides and hydroxides are another interesting class of nanoscale materials for the removal of heavy metals from water. Hematite (α-Fe2O3) nanoparticles were reported for their adsorption ability against bivalent ionic forms of heavy metals including Zn2+, Cd2+, Cu2+, and Pb2+.177 The mechanism of Zn2+ adsorption was further investigated by EXAFS spectroscopy revealing the existence of adsorption complexes.178 Their efficiency was also examined for the removal of U(VI) (ref. 179) and As(V).180 Combinations of hematite nanoparticles with magnetite or scoria were also presented as adsorbents for bivalent heavy metals as well as As(III) and Sb(III).181–183 Iron oxyhydroxides and hydrated oxides in the form of granules are the most important category of heavy metal adsorbents in the market. More specifically, the high adsorption efficiency of arsenates (H2AsO42−) by iron oxy-hydroxides is explained by the affinity of arsenic to iron combined with the positive surface charging obtained after the preparation method.184 However, only few studies describe the use of these phases as nanoparticles. In some of them, goethite nanoparticles were applied as arsenic adsorbents,185–187 whereas ferrihydrite, akaganeite, lepidocrocite and hydrated iron oxides were evaluated for similar applications in water purification.188,189 Graphene oxide supported schwertmannite nanocomposites were used for the synergistic Sb(V) removal in another case.190
The preparation of iron oxyhydroxides under controllable slightly acidic conditions and high redox potential in a continuous flow reactor allowed the formation of hollow nanostructures consisting of schwertmannite with enhanced adsorption capacity for As(V).191 Some of the authors of this review discovered that the incorporation of Mn4+ into the oxyhydroxide structure provides an oxidizing mediation mechanism which facilitates an equally high removal for As(III) using this Mn-feroxyhyte nanoadsorbent (Fig. 2).192,193 Recently, the corresponding commercial product optimized for arsenic treatment (AquAsZero) has become available in the market.194 The performance of these nanoadsorbents was attributed to the positive surface charge and the ion exchange possibility of arsenic species with adsorbed sulfate ions.195 The successful removal of Hg2+ and U(VI) by single iron and binary iron/manganese oxyhydroxide nanostructures was also reported.196,197 In this case, the uptake of mercury species is favored by negatively charged adsorbents. Iron sulfide nanoparticles also showed a high potential for the adsorption of mercury as well as Mn2+.198–201
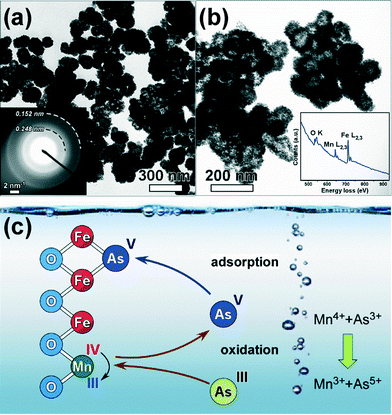 | ||
| Fig. 2 Transmission electron microscopy images of hollow Fe oxyhydroxide (a) and Mn feroxyhyte nanostructures (b). Mechanism of Mn4+ mediated adsorption of As(III) by Fe/Mn binary oxyhydroxides (c).191,192 | ||
The ability of manganese oxide (MnO2) to favor intermediate oxidation reactions is an important property especially for the removal of As(III) species. Nanoscale MnO2 has been tested as a coating agent for modified polyether ether ketone (PEEK-WC) nanostructured capsules202 and magnetite nanoparticles203 introducing a high removal capacity for both As(III) and As(V). MnO2 nanorods combined with maghemite nanoparticles and embedded on a perlite carrier were found efficient for As(V) adsorption.204
In the same direction, the photocatalytic activity of nanoscale titanium dioxide when exposed to UV radiation was exploited for the removal of various heavy metals (Fig. 3).205–207 Regarding arsenic, nanocrystalline TiO2 was reported to be an efficient adsorbent for As(V) and a very good photocatalyst for the oxidation of As(III) to As(V) in the presence of oxygen in sunlight.208 The mechanisms of arsenic species adsorption and the oxidation extent of As(III) have been studied at various pH values.209 Anatase nanoparticles (20–60 nm) were prepared by the sol–gel method and effectively used for the removal of heavy metals, namely Pb2+, Cu2+ and As(III), from water.210 By a similar preparation route, pure and iron-doped TiO2 nanoparticles were optimized for arsenic removal under various conditions of air and light.211 The effect of particle size in the range of 6.6–30.1 nm has been reported elsewhere indicating a decrease in efficiency above 15 nm.212 A partial decrease in photooxidation rate in As(III) was observed as the size increases. Arsenite removal through a simultaneous photooxidation–adsorption process was reported for γ-Fe2O3/TiO2 nanoparticles.213 Remediation of groundwater from organic and inorganic arsenic was also performed by nanocrystalline TiO2.214 The adsorption affinity of TiO2 nanoparticles with arsenic oxy-ions was studied through the increased accumulation of As(V) in carp fish.215,216 In addition, Alizarin red S(ARS)-sensitized colloidal TiO2 nanoparticles were employed in surface enhanced Raman scattering (SERS) technology to determine Cr(VI) in water. It was found that the strong coupling interaction between the dye molecules and TiO2 leads to the formation of charge-transfer complexes, therefore yielding a new electronic transition pathway for the charge-transfer process.217 Zhang et al. reported that Fe3O4@SiO2@TiO2 magnetic nanoparticles could be applied for the solid phase extraction of trace amounts of Cd2+, Cr(III), Mn2+ and Cu2+ from environmental water samples. In their approach, a light-induced hydroxide ion emitter, malachite green carbinol base, was employed to adjust the pH of the sample solution for quantitative adsorption.218 Importantly, aggregated TiO2 nanoparticles were commercially promoted (MetSorb,219 ADSORBSIA220) for the treatment of water from As, Cr and Se.221,222 Zinc and tin oxide nanoparticles were also mentioned to act as photocatalysts for the removal of Cr(VI).223
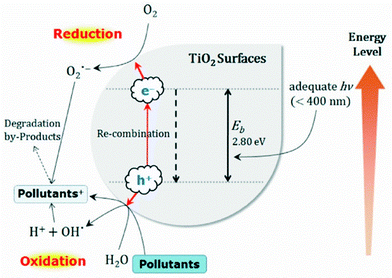 | ||
| Fig. 3 Scheme depicting the pollutant removal through the formation of photoinduced charge carriers (e−/h+) in TiO2 nanoparticle surfaces.205 | ||
Many other metal oxide nanoparticles have been suggested for heavy metal removal from water at lower occurrences. Nanostructured MgO is an excellent low-cost adsorbent for As(III), As(V) and Cr(VI).224–226 Its activity is mostly based on the hydration of the external surface to Mg(OH)2. However, MgO/Mg(OH)2 nanoadsorbents have the disadvantage of dissolution when working at pH values below 9. Cerium oxide (CeO2) has the potential to be used for the removal of Cd2+, Pb2+, Cu2+, As(III), As(V) and Cr(VI) either in the form of individual nanoparticles,227–230 associated with MnO2,231 nanowires232 or in porous nanospheres with ZrO2 (ref. 233). Zirconium oxide nanoparticles (also introduced as a commercial product234) with sizes of 6–10 nm were able to successfully capture As(III) and As(V) in a strong inner-sphere surface complex.235–237 The well-known efficiency of granulated alumina in As(III) and As(V) uptake was attempted to be transferred in nanostructured materials. In particular, γ-Al2O3 nanocomposites238 and alumina-supported ZVI239 were used for As(V) removal, whereas γ-Al2O3 catalyzed by H2O2 showed good performance in the oxidation/removal of As(III).240 Batch and column tests were performed to validate the potential applicability of CuO nanoparticles in arsenic removal under realistic conditions of groundwater treatment in a flow-through reactor.241 The kinetic, thermodynamic and adsorption mechanisms in this process were also analyzed.242,243
Besides, noble metal nanoparticles have also been widely tested in sensing and task-specific applications for heavy metal capture from water despite the high cost. One of the most important applications of Au nanocrystals in water treatment is the removal of mercury. Pradeep and co-workers reported the use of alumina-supported gold nanoparticles for the removal of Hg through the amalgamation between both metals.244 An approach for the ultrasensitive selective detection of Hg2+ and CH3Hg+ based on fluorescence quenching of gold nanoclusters is reported in ref. 245. Similarly, the speciation between mercury and methylmercury ions was achieved by employing a SERS-sensing platform consisting of a monolayer of mercaptopyridine able to bind with gold nanoparticles anchored onto polystyrene microbeads. The coordination with mercury species took place via the nitrogen of the pyridine moiety in water.246 Another process based on amalgam formation has been used to eliminate Hg2+ from both deionized water and natural-like water with citrate-coated Au nanoparticles (Fig. 4). Although their approach was mainly effective for relatively low Hg concentrations, in certain cases they were able to reduce further the mercury concentration down to the levels specified for drinking water.247 More recently, a simple, fast and cost-effective route to capture Hg has been demonstrated in tap water through its amalgamation with Au. Their method was eco-friendly due to the use of non-toxic ascorbic acid as a reducing agent for CTAB-coated Au nanostructures.248 Decontamination of mercury by carbon-coated magnetic Co nanoparticles was also reported.249 The selective determination of Cr(III) and Cr(VI) in tap water and wastewater was developed by another simple, economical and relatively ‘green’ method, based on the fluorescence quenching of glutathione-stabilized gold nanoclusters.250 Palladium-modified titanium oxide nanoparticles have also been described as excellent materials for the removal of most kinds of pollutants from potable water. TiON/PdO nanoparticles displayed a significant photocatalytic activity for the removal of As(III) from water due to the strong optoelectronic coupling between PdO and TiON, under visible light conditions.251 Pd nanoparticles were also used as catalytic centers for the removal of heavy metals through reduction reactions like in the case of Cr(VI) using formic acid.252
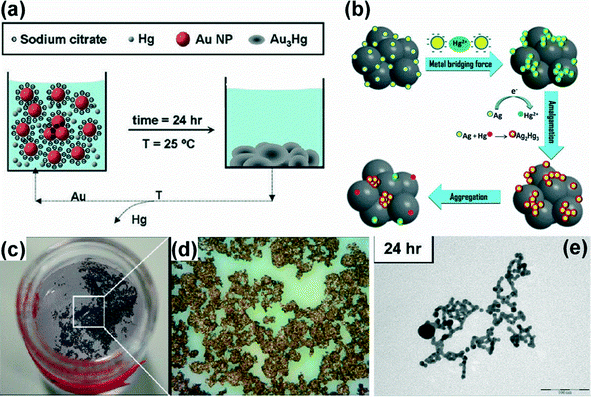 | ||
| Fig. 4 Mercury elimination process through amalgamation using Au nanoparticles (a, c, d, e) and SiO2/Ag nanoparticles (b).247,262 | ||
The remarkable optical properties and high extinction cross-section of Ag nanoparticles allowed them to be used as colorimetric sensors as well as for the removal of toxic ions such as Hg and As species from water.253–260 Detection of toxic ions using Ag nanoparticles is based on the functionalization of nanoparticles with specific molecules that can bind to target ions.254–259 For example, mercaptosuccinic acid (MSA) capped Ag nanoparticles supported on activated alumina have been used as an adsorbent for the removal of Hg2+ ions present in contaminated waters. Hg2+ ions could be removed from water by amalgamation with metals (as mentioned above) and by complexation with head groups of the monolayer surface present on the nanoparticles; therefore, the detection sensitivity depends on the density of functional groups on the surface. In another study, p-phenylenediamine (p-PDA) functionalized Ag nanoparticles were used for the detection of Hg2+and Fe3+ ions in aqueous medium through the aggregation of nanoparticles upon their addition.261 Furthermore, biomolecules such as DNA (having thymine nucleotides), glutathione and cysteine have also been used as capping molecules to selectively bind with specific ions.254 For example, Wu et al. reported the preparation of oligonucleotide-functionalized silver nanoparticles for a sensitive and selective detection system for Hg2+ ions via thymine (T) and Hg2+ interactions.260 Thymine molecules have a strong tendency to interact with Hg2+via T–Hg2+–T formation and such interaction has been widely investigated in various systems.257 Similarly, Li et al. reported SERS-based selective detection of As(III) ions in aqueous media using glutathione functionalized Ag nanoparticles.255 Upon the addition of As(III), Ag nanoparticles tend to aggregate and the extent of aggregation depends on the concentration of As(III), which can be easily monitored by SERS using a Raman tag 4-mercaptopyridine (4-MPY). Besides, unfunctionalized Ag nanoparticles have also been used to remove Hg2+ ions through the formation of amalgam. For example, silica spheres decorated with Ag nanoparticles have been used as an effective sorbent for the removal of mercury from water via an amalgamation process (Ag2Hg3) (Fig. 4b).262
3.2. Antimicrobial activity—disinfection
A variety of inorganic nanoparticles were proved to be toxic against a number of microorganisms.263–266 Such biocidal activity could turn into an advantage for antimicrobial processes and disinfection of drinking water. Among all inorganic engineered nanostructures, Ag nanoparticles have drawn a special attention due to their excellent antibacterial and antifungal activity.267–278 The main advantage of silver nanoparticles is the combination of high selectivity for specific microorganisms and easy penetration in biological entities due to their small size. Therefore, the relatively high cost of Ag nanoparticles can be counterbalanced by the provided accuracy, efficiency and limitation in by-products compared to traditional water disinfectants. More particularly, their antimicrobial activity could be applied for the preventive disinfection of drinking water treatment infrastructures (e.g. filters, column beds) or sites with limited sanitation care and high risk of pathogen development (third world countries).Since ancient times, silver has been widely used for the fabrication of utensils, which allows the preservation of food products and disinfection of water.279–281 The high specific surface area provided by nanoparticles and the fact they can penetrate deeper and thus can purify water from pathogens such as bacteria, fungi and viruses282 triggered the development of nanoscale research in this direction.283 Over the years, a great amount of research has been carried out by researchers around the globe to understand the antibacterial activity of zero-valent Ag nanoparticles and Ag+ ions and to implement them in a broad range of products, including medical devices, food products, clothing, cosmetics, sunscreens, paints and in wastewater treatment plants. For example, Ag nanoparticles have been extensively used in biomedical products for wound dressing, treatment of burns and bacterial infections, diagnostics and surgery. The majority of the Ag nanoparticle-based products that we use in our daily lives are based on their excellent antimicrobial activity. During the past decade, significant research efforts have been devoted toward the development of Ag nanoparticle-based products for the purification of drinking water.207,279,284–296 In this section, we review the proposed mechanisms for the excellent antimicrobial activity of Ag nanoparticles and recent advances made toward the development of various Ag nanoparticle-based products for drinking water purification.
The mechanism behind the antibacterial activity has been well investigated, and various mechanistic paths have been proposed.267,272,276 Compared to metal ions, metal nanoparticles have a strong tendency to interact with the cell surface and pass through the cell membrane. In addition, the high surface area of nanoparticles allows to functionalize them with antibodies and target specific cell types in order to inhibit the growth of infected cells without affecting the normal cells. Several studies have shown that the generation of reactive oxygen species (ROS) and oxidative stress cells were the two major mechanisms responsible for the toxicity or antibacterial activity of Ag nanoparticles (Fig. 5).272 The presence of Ag nanoparticles leads to the breakage of the cell membrane at endocytosis and other parts, through which nanoparticles or ions penetrate into the cells. Subsequently, Ag nanoparticles react with other species in the cell matrix, such as proteins or glutathione to generate ROS or hydroxyl radicals via Fenton reactions. The excess of generated ROS eventually leads to cell death through the destruction of several species inside the cell matrix, as shown in Fig. 5.272 For instance, the generated ROS could damage the DNA by the inhibition of cell growth, by the activation of signaling cascades in mitochondrial pathway or by lipid peroxidation.272 The mechanism of antibacterial activity at the molecular level has been further investigated by the identification of biomolecules in comparison with model compounds through SERS. The SERS results suggest that there is a significant interaction of Ag nanoparticles with proteins through metabolic processes of purine.276 Besides, Li et al. investigated the mechanism for the antibacterial activity of Ag nanoparticles on Escherichia coli using transmission electron microscopy.267 They have found that the presence of Ag nanoparticles leads to the destruction of the cell membrane and hinders the activity of some enzymes, which cause the bacteria to eventually die.
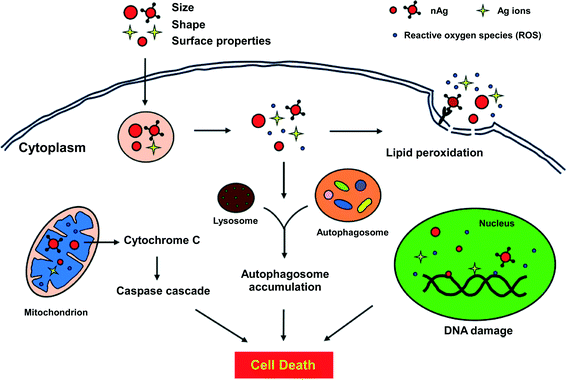 | ||
| Fig. 5 Possible antimicrobial interactions of Ag nanoparticles in cells.272 | ||
In general, antibacterial activity of Ag nanoparticles depends on their size, morphology and surface chemistry.271,273,275,276,297–299 Agnihotri et al. have studied the size dependent antibacterial efficiency of Ag nanoparticles of size ranging from 5 to 100 nm and they found that particles smaller than 10 nm showed significant performance, with the 5 nm particles showing the highest efficiency.271 They have also found that Escherichia coli MTCC 443 and Staphylococcus aureus NCIM 5201 were the most and least sensitive strains to Ag nanoparticles, respectively, regardless of their size. In addition, the surface chemistry of nanoparticles plays an important role in their antibacterial activity as well as toxicity. For instance, Kora et al. studied the antibacterial efficiency of Ag nanoparticles prepared with two natural plant gums (gum ghatti and gum olibanum) against Gram-negative and Gram-positive bacteria and they found that antibacterial efficiency and cytotoxicity of the Ag nanoparticles prepared with gum ghatti was higher than the particles prepared with gum olibanum.274 In another study, the role of surface chemistry on cytotoxicity of Ag nanoparticles was investigated revealing that the capping molecules, the type of surface facets and sample aging could influence the toxicity of Ag nanoparticles.297 In addition, the incorporation of Ag nanoparticles into other materials such as graphene oxide, carbon and polymers induced a higher antibacterial efficiency.270,300 On this, Bao et al. reported the antibacterial properties of Ag nanoparticle–graphene oxide nanosheet composites against Escherichia coli and Staphylococcus aureus bacterial strains270 concluding that the composites exhibit higher activity than pure graphene oxide. Similarly, the synthesis of polyvinyl alcohol/aminopropyltriethoxysilane hybrid materials with embedded silver nanoparticles by the sol–gel method for antimicrobial applications was reported.301 The advantage of such composite materials is that they can be easily deposited on a ceramic membrane to be used for water disinfection. In addition, an optimum amount of Ag could be embedded into the hybrid materials in order to reduce the cytotoxicity while preserving the antimicrobial efficiency. For instance, Ag nanoparticles decorated on carbon nanotubes showed effective antibacterial activity to prevent the bacterial growth while they maintained minimum cytotoxicity.302 As a result of significant efforts from the researchers worldwide, it is very clear that Ag nanoparticles are promising candidates for antibacterial applications. However, one should also take into account that the increase in the use of Ag nanoparticle-based products can affect human health due to high cytotoxicity. A recent review by Liu and co-workers has explained the mechanism of dose- (low and high) dependent toxic effects of Ag nanoparticles with more emphasis on sublethal effects (low doses).272 So, it is very important to consider this issue and try to reduce the side effects of Ag nanoparticle-based antibacterial agents.
Over the past decade, significant progress has been devoted to the development of various kinds of Ag nanoparticle-based water purifiers and some of the related products have been commercialized. In order to develop such products, Ag nanoparticles have been incorporated into robust and porous supports, including paper, ceramics, biopolymers, calcium carbonate and carbon.207,279,280,284–294,296,297,303 Among all of them, ceramic materials have been widely used as a support material for low-cost point-of-use water purification as they are relatively cheap and easy to prepare.207,288,291,293,304 A review by Kim and Van der Bruggen discussed the fabrication and use of nanoparticles in polymeric and ceramic membrane structures for water purification.207 For example, cylindrical colloidal-silver-impregnated ceramic filters for household (point-of-use) water treatment have been manufactured.291 The filters were porous (diameters ranging from 0.02 to 15 μm) and they were able to remove nearly 100% bacteria (E. coli) upon water filtration. Very recently, a porous ceramic tablet impregnated with Ag nanopatches for house-hold water purification has been reported. Such ceramic tablets could be dropped into a water storage container to release Ag ions that kills bacteria.293 It was found that the amount of Ag released was repeatable for 10 L of water daily for 154 days and the amount was well below the World Health Organization drinking water standard for silver (0.1 mg L−1). Elsewhere, biopolymers have been used as support materials to reduce cytotoxicity effects. A water filter based on biopolymer-reinforced synthetic granular nanocomposites for point-of-use water purification was developed (Fig. 6).279 The prepared nanocomposites exhibiting river sand-like properties were easy to prepare in water itself and constantly release Ag ions. Such composites can effectively remove E. coli, Fe2+, Pb2+, and As5+ from water, and they have been used to manufacture an affordable water purifier providing clean drinking water at US $2.5 year per family. Besides, cellulose materials have been extensively used as low cost support materials to Ag nanoparticles for water purification. For instance, Dankovich et al. designed low-cost bactericidal paper embedded with Ag nanoparticles for point-of-use water purification (Fig. 6c).294 Such paper was used as a water filter and it was able to remove bacteria completely, while the silver release from the Ag nanoparticles was below 0.1 ppm. Similarly, woven fabric microfiltration membranes embedded with Ag nanoparticles have also been used as water filters.287 As shown in Fig. 6d, the membranes turn into brown-yellow after the incorporation of Ag nanoparticles. The coated membranes were more hydrophilic showing higher water permeability and 100% removal of bacterial load from drinking water. Besides, Ag nanoparticles could be incorporated into cellulose paper by microwave irradiation (Fig. 6e).290 Such paper sheets could be fixed to containers of different models to filter the contaminated water providing excellent antibacterial activity against Escherichia coli and Enterococcus faecalis bacteria in deionized water or suspensions (Fig. 6e). Overall, the reported studies indicate that the supported-Ag nanoparticles can be easily incorporated into drinking water purifiers so as to deliver safe and clean water at low cost. Nevertheless, the large demands in nanoparticle availability and the high microbial loading during disinfection of drinking water still limits Ag nanoparticles either for household (point-of-use) water treatment or when specialized treatment is required.
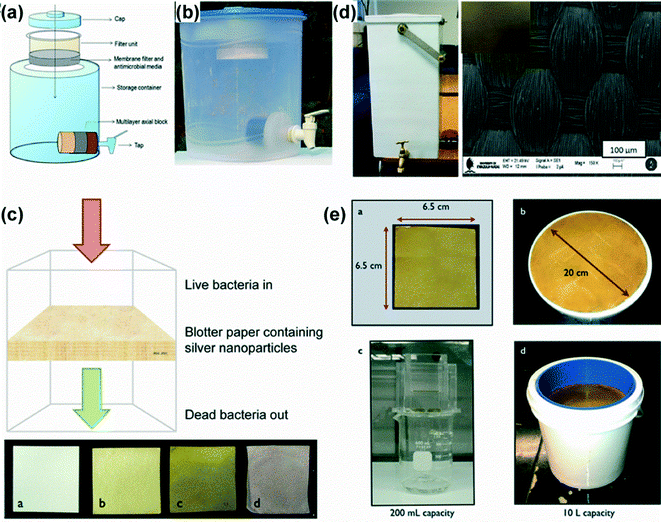 | ||
| Fig. 6 Water purification systems using supported Ag nanoparticles into biopolymer-reinforced synthetic granular nanocomposites (a, b),279 bactericidal paper impregnated with Ag nanoparticles (c),294 a woven fabric microfiltration gravity filter (d),287 and Ag nanoparticle-doped paper (e).290 | ||
The antimicrobial activity of several other types of nanostructures has also been highlighted.305 He et al. reported the combination of Au nanoparticles with ZnO ones to form hybrid nanostructures with enhanced photocatalytic and antimicrobial activity. The enhancement effect might be assigned to a higher efficiency of electron transport and charge carrier separation induced by Au nanoparticles.306 Additionally, the synthesis of Au nanoparticles on the surface of R. oryzae fungus provided active nanoparticles against different bacteria and yeasts being able to absorb organophosphorous pesticides.307 On the other hand, a colorimetric assay using gold nanoparticles for the fast detection of Salmonella was developed.308 In another case, Au nanoparticles were decorated on the surface of Fe3O4@TiO2 microspheres, enhancing their photocatalytic activity, thus yielding a better ability for organic molecule degradation and antibacterial yield in water.309
The antimicrobial activity of TiO2 nanostructures is also widely demonstrated in the literature. Alrousan et al. have compared the photocatalytic inactivation rate of Escherichia coli between samples of surface and distilled water using immobilized titania nanoparticle films. The presence of organic and inorganic species at surface water was responsible for a decreased disinfection rate in that case.310E. coli was used as a model organism in a systematic study on the toxicity of titania nanoparticles, studying the influence of a range of sizes, crystal structures and water chemistry conditions.311 In addition to bacteria, virus inactivation in drinking water has also been achieved using TiO2-based nanoparticles. More specifically, silica-decorated TiO2 nanocomposites inactivated the MS2 bacteriophage virus via a simple, low-cost and green photocatalytic process.312 Actually, a promising antibacterial activity against E. coli was also demonstrated using TiO2 nanoparticle-decorated cellulose fibers. These paper matrices displayed in addition an excellent photocatalytic performance on the degradation of methyl orange dye.313 Elsewhere, cellulose acetate membranes were coated with TiO2 nanoparticles, thus forming composite materials able to remove turbidity and color in drinking water in a satisfactory extent.314 The role of TiO2, ZnO and other nanostructure systems as antimicrobial agents in drinking water treatment together with a discussion on the combination of conventional approaches with nanotechnology has been described by Alvarez and colleagues.264
The combination of Ag with TiO2 has been reported to often provide excellent nanomaterials with enhanced photocatalytic properties due to arising synergistic effects from both components. The use of Ag-doped TiO2 nanoparticles for the inactivation of MS2 and the increased photocatalytic activity were attributed to a possible synergetic effect between silver and titania.315 The visible-light-assisted antimicrobial activity of Ag nanoparticle–chitosan–TiO2 composites was deeply studied, suggesting also some insights into the antimicrobial mechanism taking place.316 In agreement with the existence of such synergistic effects, Ag-TiO2 nanoparticles were reported to perform better than their single counterparts in the inactivation of putida and subtilis bacteria, either in the absence or in the presence of light.317 In fact, the modification of titania nanoparticles with silver as a dopant enables the use of visible light, due to the fact that individual TiO2 can be photo-activated only in wavelength values below 387 nm. This was also exploited by Younas and colleagues who found that nanoscale 1% Ag-TiO2 was very effective in the photocatalysis of E. coli by simply applying visible light.318 Similar conclusions were deduced by investigating the bactericidal ability of TiO2 and Ag-TiO2 prepared by the co-precipitation method.319 In addition, immobilized Ag-TiO2 nanoparticles onto polystyrene waste were used as cheap and environmentally benign agents for the removal of E. coli and Aspergillus niger together with Cr(VI) and methylene blue from water.320 Actually, mesoporous anatase TiO2 modified with Ag nanoparticles was also reported to possess a great and recyclable capacity to degrade Rhodamine B dye and E. coli under UV light irradiation, using relatively low silver concentrations.304
Several iron-based nanoparticles have been studied for their influence in microbe inactivation. Zero-valent iron nanoparticles appeared to be toxic against a number of bacterial cells including Gram-negative Escherichia coli,321 Gram-positive Bacillus subtilis,322 Gram-negative Pseudomonas fluorescens, and the fungus Aspergillus versicolor.323 Their antimicrobial activity lies on the corrosion of particle surface and the release of Fe2+ ions which react with intracellular oxygen and hydrogen peroxide following a Fenton reaction.324,325 In some cases, a direct interaction between microorganisms and the ZVI particle surface was also observed.326 The bactericidal effect of ZVI nanoparticles was investigated under reliable natural conditions to evaluate the influence of particle aging, adsorption of polyelectrolytes or natural organic matter and pH variations.322,327,328 Iron oxide nanoparticles were also applied for the inactivation of Staphylococcus aureus329 and Escherichia coli.330 In the last case, the adsorption rates for various particle sizes were examined revealing faster kinetics for larger nanoparticles. Magnetite nanoparticles functionalized by carboxyl, amine and thiol groups were able to capture and remove bacteria from water.331
Another important kind of nanomaterials known for their antimicrobial properties is some other metal oxide nanoparticles namely CuO, MgO and ZnO. Copper oxide nanoparticles showed significant antibacterial activity against Escherichia coli and Pseudomonas aeruginosa.332 The size dependence of their efficiency was investigated for both Gram-positive and negative bacterial strains333,334 with better results observed for nanoparticles smaller than 5 nm. Similarly, MgO nanoparticles presented enhanced bactericidal behavior against pathogens such as Escherichia coli, Salmonella stanley,335Bacillus subtilis and Staphylococcus aureus.336 Their efficiency was also evaluated in comparison to other metal oxides (Al2O3, Fe2O3, CeO2, ZrO2),337 supported on Al2O3 (ref. 338) or combined with halogen adducts.339
Many publications deal with the antimicrobial and antifungal ability of ZnO nanoparticles.340 Among other metal oxides, ZnO nanoparticles were found advantageous for the treatment of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis.341 Various synthetic conditions and particles sizes were examined for their role in the bactericidal properties.342–347 The selective antibacterial effect against Campylobacter jejuni was also proved,348 while ZnO nanoparticles present antifungal activity for Botrytis cinerea, Penicillium expansum,349Candida albicans350 and Aspergillus brasiliensi when applied on polyurethane membranes.351 Photoactivated ZnO nanoparticles suggest a higher antimicrobial potential when exposed to light.352 Nanocomposites with cellulose nanocrystals were also tested.353 Finally, commercially available ZnO nanoparticles were evaluated in comparison to Ag and CuO ones for their antibacterial efficiency and found toxic even for the beneficial environmental microbe Pseudomonas putida KT2440.354
Copper nanoparticles are another class of nanomaterials studied for their antimicrobial and antifungal properties. A variety of representative microorganisms were examined to test the efficiency of Cu nanoparticles whether coated by starch,355 CTAB,356 alkylamines357 or combined with soda-lime glass358 and hydrosol.359 Other examples of reported antimicrobial nanoparticles are hybrid CdS/Pt–TiO2 nanotubes,360 nitrogen-doped TiO2 and ZrO2 nanoparticles,361 bimetallic Fe–Ag nanoparticles362 and Pd–cellulose nanohybrids.363
3.3. Organic pollutants
Common organic pollutants found in natural water are usually of anthropogenic source and include toxic organic compounds as chlorinated and non-chlorinated aliphatic and aromatic molecules, dyes, detergents and surfactants, pesticides, pharmaceuticals, volatile organic compounds (VOCs) and natural organic matter (NOM). Organohalogen pesticides, the most common class of organic contaminants in water, are implicated with several diseases, chronic damages and carcinogenicity.280 In order to remove pesticides from water, conventional methods like chemical precipitation, chemical oxidation, adsorption on powdered activated carbon and reverse osmosis are widely used. The mechanisms of removal lie on the degradation of pesticide molecules or their separation by adsorption. However, the revealing of the health risks related to organic compounds and the continuous trend for minimizing maximum contaminant levels in the sub-ppb range raise the need for the development of removal methods with high selectivity and efficiency. The size, the high reactivity and the novel mechanisms introduced by nanoparticles enable their use as a promising technology for future application. The research is focused directly on the successful treatment of organic pollutants by nanoparticles (photocatalysis, degradation, and adsorption) as well as on their detection by means of selective nanoparticle forms. An important task that should be always addressed during the treatment of organic compounds is the chemical analysis to detect secondary by-products that could appear even more toxic. In this case, nanoparticles able to completely degrade initial pollutants or provide adsorption sites for the secondary formed molecules should be preferred. Nanoparticles with photocatalytic properties are first discussed. Monometallic Au nanoparticles were applied in a SERS-based strategy for a fast and selective detection of bisphenol A in river water and Gatorade drink.364 Besides, pesticides can be also detected and in some cases removed by the use of Au nanoparticles. For instance, a process for the detection of endosulfan, chlorpyrifos and malathion via spectrophotometry using either gold or silver nanoparticles was published. The extraction of pesticides from water was also feasible through their interaction with the nanoparticle surfaces.365,366 In fact, some by-products during the decomposition of pesticides might be more toxic than the initial compound. For this reason, Bootharaju and Pradeep tried to elucidate the degradation mechanism of the pesticide chlorpyrifos by using Au and Ag nanoparticles in either supported or unsupported forms.367 Au–ZnO nanocomposites prepared by a one-pot protocol exhibited an increased photocatalytic activity for the degradation of cationic and anionic dyes under sunlight irradiation.368 Special attention concerns antibiotics, as their incomplete removal from wastewaters could affect the microbial communities that occur in water ecosystems. The elimination of the antibiotic chloramphenicol (CAP) in water was achieved by using resin-supported Au–Pd nanoparticles which cleaved the carbon–halogen bond of CAP while keeping the nitro-group unaffected and this resulted in less toxic degradation products.369 Furthermore, Wong et al. illustrated the ability of bimetallic Au–Pd nanoparticles to act as superior catalysts for the hydrodechlorination of trichloroethene in groundwater.370 Gold nanoparticles were also combined with magnetic materials aiming to form another class of hybrid materials with improved features for water treatment. For instance, the role of Au shell in Fe3O4@Au nanoparticles used for magnetic solid phase extraction was to provide a universal intermediate platform for thiol ligands with various head groups.371The Au-doping was effective also in the case of the simultaneous removal of nitrate ions and cadmium, by using zero-valent Fe nanoparticles. In particular, 1% doping with gold significantly reduced the nitrite yield ratio, while maintaining an equally high Cd2+ removal capacity.372 In another approach, Au nanoparticles were conjugated with yttrium hydroxide fluoride nanotubes to produce nanocomposites with good SERS properties, capable of quantitatively detecting and removing the Congo red dye in wastewater.373 Moreover, a porous foam of poly(dimethylsiloxane) incorporated with Au nanoparticles was developed. The resulting nanocomposite combined the properties of each component showing a high efficiency at the absorbance/removal of organic solvents, oil spills and thioanisole from water in a recyclable manner.374 More details on the role of gold nanoparticles to clean water from various pollutants were reviewed by Qian et al.375 and Wang and Yu376 while Pradeep and Anshup wrote a comprehensive article to describe the utility of all types of suitable noble metal nanoparticles for water purification.280
Titanium dioxide nanoparticles have been also proven efficient for the treatment of organic pollutants in water. In many instances, the enhancement of photocatalytic properties in the visible region has been achieved by metal implantation (Au), non-metal doping (N, C) and surface organic modification.377 The combination of adsorption and TiO2 nanoparticle-based heterogeneous photocatalysis has been employed for the degradation of 2-chlorophenol. The adsorbent used was hexadecylpyridinium chloride-treated montmorillonite.378 The simultaneous use of montmorillonite and TiO2 nanocomposite films also facilitated the removal of methylene blue.379 Moreover, nanostructured glass-supported N-doped TiO2 prepared by the sol–gel synthesis was applied for the photocatalytic decolorization of methylene blue and the removal of eriochrome black-T dyes in aqueous solution.380 In fact, azo-dyes constitute around half the amount of dyes used in the textile industry, so the effluent streams generated from textile factories should be treated for the removal of such toxic residues which would otherwise be released in the environment. In this context, Filice and colleagues published the photodegradation of the azo-dye methyl orange using graphene oxide and titania nanoparticle hybrid Nafion membranes.381 The synergistic effects of adsorption, filtration and photocatalytic degradation were also illustrated for the decoloration of methylene blue in a prototype membrane reactor with TiO2 nanobelts.382 In addition, it was shown that TiO2-modified poly(vinyl alcohol)/poly(vinylidene fluoride) hollow fiber composite membranes exhibited higher dye separation efficiency and better thermal stability in comparison to their ‘undoped’ counterparts.383 Another study was focused on the activity of sulfur- and nitrogen-doped and undoped titania nanoparticles in the presence of inorganic anions on the degradation of dyes such as Rhodamine B.384 On the other hand, TiO2 anatase-phase nanobelts synthesized with a hydrothermal method were found not only active against the decomposition of the dye malachite green but also at the degradation of pharmaceutical and personal care products.385
A mixture of 22 organic pollutants (iopamidol, iopromide, diatrizoic acid, diclofenac, triclosan, etc.) was successfully treated in both ultrapure water and wastewater by using nano-sized TiO2 supported on single-wall carbon nanotubes.386 Furthermore, volatile organic compounds (VOCs) such as methanol, acetone and benzene were photocatalytically decomposed using N-doped TiO2 under visible and sunlight irradiation.387 The same group compared the photocatalytic performance of nanoscale titania synthesized by several sol–gel pathways (acid route, alcohol route, surfactant route) on the decomposition of mixed pesticides in drinking water concluding that the surfactant-route prepared anatase TiO2 was more suitable for this application. Their studies involved glass-supported titanium dioxide for the pesticides lindane, dichlorvos and methyl parathion.388 Doping of TiO2 nanoparticles with Zn was used to enhance the efficiency for azo dye degradation.389 Natural organic matter consists of the decomposition products of plant and animal residues. An example of NOM treatment is the degradation of fulvic acid by employing nano- and micro-scale TiO2 in a submerged membrane photocatalytic reactor. In this work, acidic pH conditions favored the degradation of fulvic acid.390 Another acid compound, formic acid, was effectively decomposed using titanium dioxide nanoparticles, as described by Abd Elrady et al.391 Their particles were also efficient for the inactivation of coliform bacteria in water.
Pharmaceuticals and personal care products (PPCPs) are regarded as a harmful sub-category of water contaminants as their incomplete treatment during wastewater remediation has resulted in their occurrence in surface and groundwater. This may comprise a danger for potable water as many drinking water treatment plants use source water impacted by wastewater.392 Hu et al. synthesized highly entangled titania nanowires by the hydrothermal method on Ti substrates and such porous materials were effective in the degradation of pharmaceuticals such as trimethoprim and other PPCP pollutants.393 A more recent study has reported a better behavior of TiO2 nanowires in the degradation of several pharmaceuticals compared to commercial TiO2 nanoparticles. Besides, they also confirmed that anatase-phase TiO2 nanowires were more effective for the majority of the pharmaceutical compounds tested; however, for a couple of them, the rutile-phase TiO2 showed a better performance. Therefore, it was concluded that the photocatalytic degradation yield varies on a PPCP and nanomaterial-specific basis.394 In fact, the presence of pharmaceutical compounds such as antibiotics in surface water could disturb the natural elementary cycles and present a potential danger when surface waters are destined for use as sources of drinking water.395 Another study presenting the efficient treatment of such compounds has to do with the photocatalytic decomposition of the antibiotic oxolinic acid in water in a range of catalyst concentrations and pH values. Such treatment was found fast and efficient while the residual antimicrobial activity of the by-products that remain after the photocatalytic process was limited.396
Methylene blue was also successfully decomposed using hybrid Pd–F-doped TiO2 nanoparticles under LED visible light. The lowering of the band gap in the hybrid material was attributed to the insertion of palladium nanoparticles, while the variation in the quantity of fluorine did not cause any important changes in the band gap values.397 The synergistic effects of all components were also considered as responsible for the high photocatalytic performance of Pd-modified N-doped nanoscale TiO2 for the degradation of NOM.398
It should be mentioned that the promotion of TiO2 nanoparticles in water treatment meets technological barriers related to their efficiency and application process design. For instance, as mentioned above, the activation of their photocatalytic potential requires an external source of radiation or sunlight. Therefore, such processes should not be preferred in countries with low sunshine and close-packed configurations which reduce active illumination.399 Another serious issue deals with the post-recovery of nanoparticles at the end of the treatment. In order to avoid the introduction of an additional separation unit, TiO2 and other kinds of nanoparticles are immobilized on glass, polymeric, ceramic and metallic substrates or supports with high specific surface areas such as activated carbon, graphene oxide, silica, alumina, fibers, clays and zeolites.27,310,377,400–412 For example, glass substrates are supposed to provide transparency and, as a result, enhance the photo-oxidative efficiency of nanoparticles.413,414 Another advantage of immobilized TiO2 nanoparticles is the avoidance of aggregation as well as the possibility to increase the contact with hydrophobic contaminants. Nevertheless, immobilized TiO2 nanoparticles are usually less efficient compared to slurry reactors due to the reduction in specific surface and mass transfer rate.415–417
Iron nanoparticles are another important category studied for their efficiency in the degradation of specific organic pollutants.418 Nanoscale ZVI has been widely tested for the dechlorination of trichloroethylene in water or the remediation of contaminated sites.419–421 This process was reported to leave no chlorinated intermediates.422 The rate, mechanism and efficiency of dechlorination were further investigated in ref. 423 and 424, whereas the effect of ZVI nanoparticle presence in biological degradation of trichloroethylene was also evaluated.425 ZVI nanoparticles, pure or doped by Pd, were also tested after their immobilization on alginate beads.426 In addition, bimetallic Fe–Pd nanoparticles were examined under batch laboratory experiments targeting to clarify the effect of sorption, surfactants and dissolved organic matter in the degradation of soil-sorbed trichloroethylene427 or in the field assessment for chlorinated aliphatic hydrocarbons.428 The ability of bimetallic Ni/Fe nanoparticles to degrade DDT and the effect of pH in the observed efficiency was the subject of another study.429 Similarly, other chlorinated compounds such as lindane, atrazine, alachlor, atrazine, perchlorate or chlorophenols were successfully treated by ZVI-based nanoparticles.430–434 The remediation of ibuprofen,435 the removal of AB24 dye436 and the oxidative degradation of herbicides437 were other reported cases of ZVI nanoparticle application. Importantly, the field performance of emulsified ZVI nanoparticles for the treatment of chlorinated volatile organic compounds and solvents was monitored, providing encouraging results.438,439
Pesticides can be also removed by means of iron oxide nanoparticles. However, these nanomaterials were mostly tested for their ability to adsorb organic dyes and selectively detect other organic pollutants for analysis purposes. The affinity of magnetic nanoparticles consisting of iron oxides was validated in a number of cases for organochlorine or organophosphorus pesticides,440–442 triazine herbicide443 and bromelain.444 Humic acid coated Fe3O4 nanoparticles were found efficient for the removal of sulfathiazole445 from water, whereas Fe3O4@TiO2 nanoparticles may function as a sensing agent for pesticide biomarkers.446 Several research studies examined the adsorption of cationic, anionic and azo-dyes from aqueous solutions. In brief, Fe3O4 nanoparticles conjugated with carboxymethyl-β-cyclodextrin,447 coated by CTAB,448 humic acid,449 polymers450 or chitosan451 and combined with carbon452,453 were able to adsorb a variety of representative dyes. The adsorption ability for dyes was also described for maghemite nanoparticle-based systems as well.454,455
Finally, a number of individual cases with various inorganic nanoparticles have been reported for the potential uptake or adsorption of organic pollutants from water. In particular, iron sulfide and ZrO2 nanoparticles were tested for the removal of organochlorine and organophosphate pesticides, respectively.456–458 The suitability of manganese oxide hollow nanostructures and MgO nanoparticles to remove dyes was reported in ref. 459 and 460, while the photocatalytic performance of Mn-doped ZnO and CdS nanoparticles against organic dyes was described elsewhere.461,462
4. Fate of used nanoparticles
Engineered nanoparticles used in water technology are usually considered as consumables even if large quantities are required. Their productive life cycle should be determined by the working period during which an affordable efficiency in terms of pollutant removal and process cost is guaranteed. Since the small size, the high reactivity, the mobility and the relative stability of inorganic nanoparticles in aqueous conditions imply the possibility of toxic effects to the environment and the living organisms, consumed nanoparticles should be properly handled while the possible effects of their leakage should be well known. For this reason, it is important in each application to study whether regeneration, recovery and safe disposal can be achieved. In addition, regarding their loading with toxic pollutants, any leaching or degradation behavior and chemical transformation of nanoparticles in the environment should also be assessed when designing a water treatment process.The application of adsorbents with particle units lying in the nanoscale may introduce unknown health effects to humans and other living organisms in the accidental case of release or during their disposal. During the past years, many theoretical and experimental research efforts have focused on the behavior of inorganic engineered nanoparticles in aqueous or soil environment and their interaction with living organisms as a source of ecotoxicity.463–467 In parallel, a continuous effort to develop reproducible and standardized hazard testing methods and evaluate the human and environmental exposure to manufactured nanoparticles is still under progress.468 The role of particle size, shape, degree of aggregation and dissolution has been examined by introducing a combination of analytical techniques.469,470 The effect of environmental factors including the pH, the salinity and the presence of organic matter was also pronounced.471 An even larger number of works discuss the cytotoxicity of nanoparticles corresponding to human exposure enhancing the knowledge for their behavior in biological entities.472,473 Nevertheless, the conclusions are controversial, and in most cases, it is unclear which characteristics are responsible for cell uptake and toxicity effects.474 In general, gold nanoparticles appear as the more stable but less toxic system when tested with algal and freshwater fish cells.475 Depending on their coating, Au nanoparticles could be adsorbed easier in fish cells. It has been observed that TiO2 nanoparticles provoke clastogenicity, genotoxicity, oxidative DNA damage and inflammation in vivo in mice.476 In another study, it was demonstrated that the presence of titania nanoparticles causes an increased metabolism of pentachlorophenol in zebrafish larvae, thus inducing enhanced oxidative damage in early developing fish.477 In this context, some recent research works have focused on the identification of the fate of nanoparticles employed for water purification. Schwab and colleagues have spiked TiO2, Ag and ZnO nanoparticles in five types of water (groundwater, freshwater and so on) and they noticed that although techniques such as membrane filtration resulted in high removals of nanoparticles, finished waters still contained detectable metal concentrations that may pose hazards to human health.478 Engineered ZnO nanoparticles may act toxic after the dissolution of Zn ions and the generation of active oxygen, whereas photo-induced reactions may also contribute in this process.479 Clustering of CeO2 around algae cells has been mentioned as another mechanism of toxicity.480 The phytotoxicity and interactions of nanoparticles with plants during seedling is another important issue for nanoparticles side effects.481–484 The risk assessment after the use of zero-valent iron and iron oxide nanoparticles has been also reported in many publications concerning their phytotoxicity and seed germination.485–487 Surface oxidation of ZVI nanoparticles is a critical parameter for both aggregation tendency and toxicity.488
The fate of nanoparticles when released to the environment is another field of research related to their effect in several living species (Fig. 7). Most of the approaches deal with theoretical models for the transport and the transformations of nanoparticles.489,490 Gold nanoparticles were found to strongly interact with natural organic matter which causes their rapid aggregation especially under high ionic strengths.491 However, the coating of nanoparticles is responsible for the stability and removal from aquatic systems as concluded for nanoscale Ag.492 Loading of ZVI nanoparticles with As or Cr after water treatment was found to enhance aggregation tendency.493 Modelling of the environmental fate of TiO2 nanoparticles in Rhine River suggests that a significant downstream transport of nanoparticles is possible with the role of suspended particulate matter being the major one in their heteroaggregation procedure.494 Importantly, the release of engineered nanoparticles in wastewaters could provide the proper substrate for their transformation to less toxic products.495 For instance, the sulfidation of Ag nanoparticles which is a common process in wastewater provides an insoluble and non-toxic form of silver.496,497
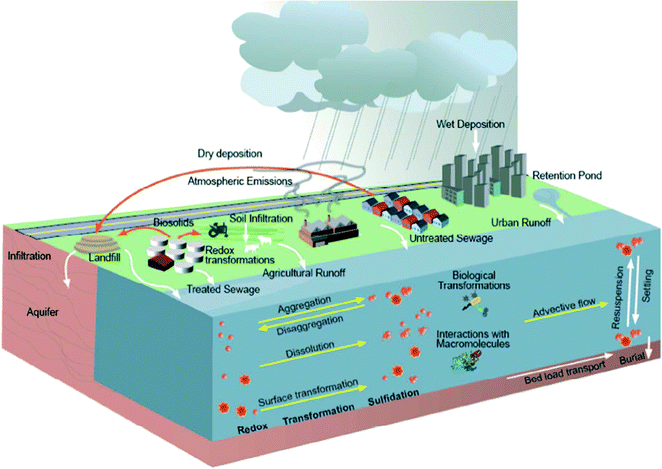 | ||
| Fig. 7 Schematic representation of sources and flow of nanomaterials in the environment and the key processes determining the fate and behavior of nanomaterials in the aquatic environments.489 | ||
The non-predictable behavior of nanoparticles in the environment initiates the demand for an integrated strategy for the potential risk management.498 A limited number of conventional, membrane and sorption technologies have been reported for the removal of engineering nanomaterials during water treatment.27 However, the best way to reduce potential environmental effects of nanoparticle disposal is to minimize the quantities of solids used in the water treatment. It is self-evident that obtaining nanoparticles optimized and specialized in the targeted process should be the primary approach to fulfill this requirement. However, the complete or even partial regeneration of nanoparticles back to their initial state enabling their multiple reuse is a critical matter able to define the validity of the whole process. The possibility for nanoparticle recycling depends on many parameters. Among them, the mechanism of pollutant's removal (adsorption, precipitation, degradation, photocatalysis) is the most important. For instance, when the nanoparticle surface is gradually covered by adsorbed molecules and ions or precipitates, a regeneration process using proper chemical reagents (e.g. NaOH) should be carried out. In this case, a secondary problem which has to be addressed is the treatment of the regeneration reagent which contains a concentrated quantity of the pollutant.499 When nanoparticles act as reaction catalysts, the periodical mild refreshment of surface properties is sufficient to maintain a high process yield. There are also processes where nanoparticles do not reach saturation in pollutant's loading after their usage cycle. Such systems should be designed to allow their recovery and continuous recirculation in the treatment site until saturation occurs.
Recovery of spent nanoparticles is another essential step when studying nanoparticle application in water technology. The motivation for this procedure is to ensure the absence of nanoscale solids not only in the purified water supplied to the consumers but also to the waste streams of the treatment process. Many times, the collection of dispersed nanoparticles is a very difficult task as a consequence of their small dimensions. In addition, in order to ensure their complete separation from treated water, an expensive filtration step of nanofiltration should be included.500 A rather more ‘conventional’ approach for the removal of nanoparticles from water is the coagulation process: nanoparticles can be enmeshed by the coagulate floc as it sediments out of the water. Coagulating agents can influence the stability of nanoparticles through the production of charged hydrolytic species that neutralize surface charges on nanoparticles. This procedure generates large nanoparticle aggregates due to the mitigation of electrostatic repulsion.478 The role of coagulant type, dose and TiO2 nanoparticle concentration was investigated upon the removal of such nanoparticles during primary water treatment.501 On the other hand, the aggregation behavior of ZnO and TiO2 nanoparticles in wastewater and their adverse impact on the oxygen uptake of activated sludge after an exposure time of a few hours was studied.502 A more sophisticated approach to overcome this issue is the incorporation of nanoparticles with a magnetic response to external fields.127 For this reason, ZVI and iron oxide nanoparticles are preferred to be used individually or as substrates for other active phases. Magnetic separation of nanoparticles from aqueous dispersions using high-gradient fields is a widely examined subject providing many possibilities of process design.102,503–505
However, recovery is not the last concern of nanoparticle application. Saturated nanoparticles from water treatment are a highly toxic solid waste that needs to be safely disposed against future leakage of pollutants.493 Failure to cover this part implies a dramatic increase in the whole cost of the procedure since a specialized treatment of the spent nanoparticles will have to be followed. For this reason, it is critical to investigate the leaching behavior of captured pollutants following the experimental protocols that characterize solid wastes as inert, non-hazardous or toxic according to the standard test EN 12457 (ref. 506) or the U.S. Toxicity Characteristic Leaching Procedure (TCLP).507 The research on the behavior of ZnO, TiO2 and Ag nanoparticles indicated that their effect in the biological landfill processes is insignificant, while the leachate of metal ions is primary associated with other components.508 When the strength of pollutant adsorption is enhanced, nanoparticles appear very stable against leaching even under intense conditions similar to that of a landfill. After that, saturated nanoparticles may be considered and handled as an inert waste. In the opposite case, other solutions for the inertization should be developed. An example is their application as additives in building materials, ceramics or their vitrification.
5. Technological evaluation
An overview of the reported research efforts dealing with the design and development of engineered nanoparticles for water treatment indicates the absence of a generalized approach for the evaluation of their efficiency. Furthermore, there are important issues related to the inappropriate methodology followed to validate their potential for drinking water purification. In general, a number of important testing conditions and quality indices should be established to allow the classification of nanoparticle properties among competitive treatment methods.As already mentioned, drinking water purification faces much lower initial pollutant concentrations compared to wastewater treatment but also demands proportionally lower or even zero residual concentrations. For this reason, the examination of nanoparticle efficiency should be carried out in the concentration range defined by the common natural water levels of the pollutant and the regulation limits for drinking water. For instance, studies for arsenic removal from drinking water should be focused on concentrations not higher than around 100 μg L−1, whereas residual concentrations below the maximum contaminant level of 10 μg L−1 should be considered after treatment. In addition, proper investigation of the corresponding kinetic rates and the presence of residual solid or toxic by-products determine the dimensions of a treatment unit and the demand for post-treatment stages.
Another important condition to obtain reliable results during laboratory experiments is the adjustment of treatment conditions very close to those met in natural water sources. The pH value of water during the treatment by nanoparticles appears as the most critical parameter which significantly determines the efficiency of the process. It is very common in laboratory research to perform experiments for pollutant removal at relatively acidic pH values (below 5) where observed efficiencies are maximized. However, working at pH values out of the range of 6–8 is not a compatible approach for drinking water treatment technology. In particular, significant variations of pH from its initial values may affect the physicochemical characteristics of natural water and introduce the need for an extra treatment step with chemical additives to recover drinkability for humans. On the same aspect, the coexistence of common constituents of natural water may interfere with the nanoparticle performance. Depending on the form of pollutants and the mechanism of their removal by nanoparticles, some of these anions (HCO3−, Cl−, SO42−, SiO2−, PO43−) and cations (Ca2+, Mg2+, Na+) act as competitors or inhibitors of the process. In some reports, the interfering of common ions is discussed individually for each of them. However, their collective influence specifically at the concentrations usually found in natural water is rarely reported. Using natural-like water instead of distilled water has been found to decrease the pollutant removal efficiency by more than 50%.127 Therefore, a study on nanoparticle performance should also include this information in order to provide an integrated view of their potential qualification for technological applications.
Apart from the described inconsistencies among research studies of nanoparticle consideration in water treatment, an obvious objection in the development of reported results is the absence of universal and absolute indices for the evaluation and comparison of treatment efficiency. The usual procedure, especially in heavy metal treatment, is to provide adsorption or removal isotherms after batch tests as a proof for the ability of nanoparticles to decrease pollutant concentration. Such diagrams indicate the removal capacity (Q) of the nanomaterial in terms of the quantity of pollutant removed per quantity of nanoparticles added. However, authors usually point on the maximum Q value, which refers to extremely high residual concentrations, as a criterion of sufficient treatment. In some other cases, the percentage of removal (residual-to-initial concentration ratio) is used to support high efficiency potential. Again, this kind of evaluation often refers to high residual concentrations from the point of drinking water demands, but the quantity of added nanoparticles is not considered at all. For instance, decreasing an initial pollutant concentration from 10 mg L−1 to 0.5 mg L−1 corresponds to a 95% removal, but still the residual concentration is many times above the common contaminant levels for drinking water (Fig. 8, curve C). On the contrary, a reduction of an initial pollutant concentration of 40 μg L−1 by 70% could be acceptable. A better way to monitor nanoparticle efficiency is by the removal capacity which corresponds to the residual concentration equal to the regulation limit of each pollutant, which is actually the target value in water treatment. This index (QRL) is derived after the projection of the regulation level to the adsorption or removal isotherm. Under common experimental conditions, the QRL index may directly provide an estimation of the expected efficiency of nanoparticles according to the demands of the designed process (Fig. 8, curves A and B).
However, other experimental approaches can predict nanoparticle performance more accurately than batch removal tests. Depending on the application type, the design and study of a continuous flow system for pollutant removal by dispersing nanoparticles or rapid-scale column tests when using granulated nanomaterials have been reported in this direction.127,193 This aspect is a challenge for future studies since there is not enough ‘know-how’ available on the design of water purification units based on nanoparticles. Considering that the main forms in which nanoparticles are employed in such processes are (i) water dispersed (slurry), (ii) immobilized on supports and (iii) granulated aggregates, a general scheme of the treatment process should be the following: In large water treatment facilities, the nanoparticle unit should be located after the primary treatment processes and just before the disinfection stage. This ensures a relatively good quality of water in the inflow of the pollutant-specific treatment by nanoparticles. A stage for the recovery or the separation of escaped nanoparticles should be also included (nanofilter, magnetic separator). In point-of-use and point-of-entry home solutions, nanoparticles should be preferably used in compact forms (grains, immobilized) as a part of filtration properly sequenced by other treatment stages so as to minimize possible interferences.
At the end, the evaluation of nanoparticles under realistic conditions is not the ultimate criterion for nanoparticle incorporation into the drinking water technology. As explained, the ratio of efficiency per nanoparticle cost determines the competitiveness with other existing technologies and the possibility for commercial promotion. But still there are secondary constraints able to reject nanoparticle use in water treatment. The most important is their compliance to environmental limitations of disposal after effective life cycle. According to these, the saturated nanoparticles should overcome successive leaching tests for solid wastes. Taking also in account their small dimensions, their safe recovery and handling should be essential information given during their study.
6. Summary
The summary of reported studies related with the application of inorganic engineered nanoparticles in water treatment indicates the increasing interest for their development as novel products with improved performance in drinking water purification processes. Overall most of them report fundamental approaches without getting deeper in potential practical applications while some other report practical aspects without focusing on process development and only few study both sides. Depending on the properties provided by each class of nanoscale materials, the research is mainly focused on their optimization as adsorbents for heavy metals, disinfectants against microorganisms or catalysts for the degradation of organic pollutants. As widely stated, the efficiency of nanoparticles in water technology is proportional to their specific area and the reactivity of their surface, i.e. the characteristics that differentiate them from materials with conventional dimensions. Therefore, one of the critical tasks in the design of engineered nanoparticles and incorporation into the technological field is to ensure stability against aggregation and chemical transformations during storage, handling and use. However, high efficiency is only one of the requirements being able to validate the competitiveness of nanoparticles in the market. Considering the strict legislation which regulates drinking water processes for the protection of human health and environment, a parallel examination of the side effects of nanoparticle application should be successfully carried out. This corresponds to the accomplishment of a risk assessment with respect to consumer's use manner, safe disposal and potential effects in their release to the environment towards the benefits of using qualified nanoparticles in water treatment. Following the first encouraging results of nanoparticle use in a variety of water treatment applications, future research efforts should be better oriented in the evaluation of engineered nanoparticles under more reliable conditions of field application to assist their potential commercialization in large scale.Acknowledgements
L. Polavarapu greatly acknowledges the financial support from Alexander von Humboldt Foundation. The study was also implemented within the framework of the Action “Supporting Postdoctoral Researchers” of the Operational Program “Education and Lifelong Learning” (Action's Beneficiary: General Secretariat for Research and Technology) and is co-financed by the European Social Fund and the Greek State PE8 (135).References
- J.-L. Pautrat, C. R. Phys., 2011, 12, 605–613 CrossRef CAS.
- X. Huang and M. A. El-Sayed, J. Adv. Res., 2010, 1, 13 CrossRef.
- M. Hasan, M. F. Huq and Z. H. Mahmood, SpringerPlus, 2013, 2, 151 CrossRef PubMed.
- B. Yu and M. Meyyappan, Solid-State Electron., 2006, 50, 536–544 CrossRef CAS.
- N. Collaert, A. Alian, H. Arimura, G. Boccardi, G. Eneman, J. Franco, T. Ivanov, D. Lin, R. Loo, C. Merckling, J. Mitard, M. A. Pourghaderi, R. Rooyackers, S. Sioncke, J. W. Sun, A. Vandooren, A. Veloso, A. Verhulst, N. Waldron, L. Witters, D. Zhou, K. Barla and A. V.-Y. Thean, Microelectron. Eng., 2015, 132, 218–225 CrossRef CAS.
- C. E. Handford, M. Dean, M. Spence, M. Henchion, C. T. Elliott and K. Campbell, Food Control, 2015, 57, 24–34 CrossRef.
- P. Boisseau and B. Loubaton, C. R. Phys., 2011, 12, 620–636 CrossRef CAS.
- A. Wicki, D. Witzigmann, V. Balasubramanian and J. Huwyler, J. Controlled Release, 2015, 200, 138–157 CrossRef CAS PubMed.
- F. Sanchez and K. Sobolev, Constr. Build. Mater., 2010, 24, 2060–2071 CrossRef.
- A. K. Hussein, Renewable Sustainable Energy Rev., 2015, 42, 460–476 CrossRef CAS.
- A. Mihranyan, N. Ferraz and M. Strømme, Prog. Mater. Sci., 2012, 57, 875–910 CrossRef CAS.
- C.-F. Chau, S.-H. Wu and G.-C. Yen, Trends Food Sci. Technol., 2007, 18, 269–280 CrossRef CAS.
- B. Kim, C.-S. Park, M. Murayama and M. F. Hochella, Environ. Sci. Technol., 2010, 44, 7509 CrossRef CAS PubMed.
- S. Seal and B. Karn, Saf. Sci., 2014, 63, 217–225 CrossRef.
- R. Owen and M. Depledge, Mar. Pollut. Bull., 2005, 50, 609–612 CrossRef CAS PubMed.
- T. Sheetz, J. Vidal, T. D. Pearson and K. Lozano, Technol. Soc., 2005, 27, 329–345 CrossRef.
- M. L. W. Knetsch and L. H. Koole, Polymers, 2011, 3, 340–366 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- B.-A. Dranga, L. Lazar and H. Koeser, Catalysts, 2012, 2, 139–170 CrossRef CAS.
- Y. Liu, D. J. A. Kelly, H. Yang, C. C. H. Lin, S. M. Kuznicki and Z. Xu, Environ. Sci. Technol., 2008, 42, 6205–6210 CrossRef CAS PubMed.
- D. K. Tiwari, J. Behari and P. Sen, Carbon Nanotube, 2008, 3, 417–433 Search PubMed.
- M. Hua, S. Zhang, B. Pan, W. Zhang, L. Lv and Q. Zhang, J. Hazard. Mater., 2012, 211–212, 317–331 CrossRef CAS PubMed.
- R. Narayan, Mater. Today, 2010, 13, 44–46 CrossRef CAS.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed.
- I. K. Herrmann, R. E. Bernabei, M. Urner, R. N. Grass, B. Beck-Schimmer and W. J. Stark, Nephrol., Dial., Transplant., 2011, 26, 2948–2954 CrossRef CAS PubMed.
- I. K. Herrmann, A. Schlegel, R. Graf, C. M. Schumacher, N. Senn, M. Hasler, S. Gschwind, A.-M. Hirt, D. Gunther, P.-A. Clavien, W. J. Stark and B. Beck-Schimmer, Nanoscale, 2013, 5, 8718–8723 RSC.
- G. R. Boyd, M. E. Tuccillo, A. Sandvig, M. Pelaez, C. Han and D. D. Dionysiou, J. - Am. Water Works Assoc., 2013, 105, E699–708 CrossRef.
- I. Ali, Chem. Rev., 2012, 112, 5073–5091 CrossRef CAS PubMed.
- P. Xu, G. M. Zeng, D. L. Huang, C. L. Feng, S. Hu, M. H. Zhao, C. Lai, Z. Wei, C. Huang, G. X. Xie and Z. F. Liu, Sci. Total Environ., 2012, 424, 1–10 CrossRef CAS PubMed.
- A. Ayati, A. Ahmadpour, F. F. Bamoharram, B. Tanhaei, M. Manttari and M. Sillanpaa, Chemosphere, 2014, 107, 163 CrossRef CAS PubMed.
- J. Gómez-Pastora, E. Bringas and I. Ortiz, Chem. Eng. J., 2014, 256, 187–204 CrossRef.
- C. N. R. Rao, S. R. C. Vivekchand, K. Biswas and A. Govindaraj, Dalton Trans., 2007, 3728 RSC.
- K. Petcharoen and A. Siriva, Mater. Sci. Eng., B, 2012, 177, 421 CrossRef CAS.
- M. E. F. Brollo, R. Lopez-Ruiz, D. Muraca, S. J. A. Figueroa, K. R. Pirota and M. Knobel, Sci. Rep., 2014, 4, 6839 CrossRef CAS PubMed.
- J. Lai, W. Niu, R. Luque and G. Xu, Nano Today, 2015, 10, 240–267 CrossRef CAS.
- A. Esmaielzadeh Kandjani, M. Farzalipour Tabriz and B. Pourabbas, Mater. Res. Bull., 2008, 43, 645 CrossRef.
- U. S. Mohanty, J. Appl. Electrochem., 2011, 41, 257 CrossRef CAS.
- J. E. Munoz, J. Cervantes, R. Esparza and G. Rosas, J. Nanopart. Res., 2007, 9, 945 CrossRef CAS.
- F. Iskandar, Adv. Powder Technol., 2009, 20, 283 CrossRef CAS.
- International Agency for Research on Cancer, IARC Monographs on the evaluation of carcinogenic risks to humans: Some drinking-water disinfectants and contaminants, Including Arsenic, Lyon, France, 2004, vol. 84 Search PubMed.
- F. Fernández-Luqueño, F. López-Valdez, P. Gamero-Melo, S. Luna-Suárez, E. N. Aguilera-González, A. I. Martínez, M. D. S. García-Guillermo, G. Hernández-Martínez, R. Herrera-Mendoza, M. A. Álvarez-Garza and I. R. Pérez-Velázquez, Afr. J. Environ. Sci. Technol., 2013, 7, 567–584 Search PubMed.
- C. H. Wang, C. K. Hsiao, C. L. Chen, L. I. Hsu, H. Y. Chiou, S. Y. Chen, Y. M. Hsueh, M. M. Wu and C. J. Chen, Toxicol. Appl. Pharmacol., 2011, 222, 315–326 CrossRef PubMed.
- European Commission, Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption.
- L. Li, M. Fan, R. C. Brown, J. H. Van Leeuwen, J. Wang, W. Wang, Y. Song and P. Zhang, Crit. Rev. Environ. Sci. Technol., 2006, 36, 405–431 CrossRef CAS.
- Y. T. He and S. J. Traina, Environ. Sci. Technol., 2005, 39, 4499–4504 CrossRef CAS PubMed.
- B. Geng, Z. Jin, T. Li and X. Qi, Sci. Total Environ., 2009, 407, 4994–5000 CrossRef CAS PubMed.
- B. Geng, Z. Jin, T. Li and X. Qi, Chemosphere, 2009, 75, 825–830 CrossRef CAS PubMed.
- Q. Wang, H. Qian, Y. Yang, Z. Zhang, C. Naman and X. Xu, J. Contam. Hydrol., 2010, 114, 35–42 CrossRef CAS PubMed.
- D. V. Franco, L. M. Da Silva and W. F. Jardim, Water, Air, Soil Pollut., 2009, 197, 49–60 CrossRef CAS.
- C. Mystrioti, N. Papassiopi, A. Xenidis, D. Dermatas and M. Chrysochoou, J. Hazard. Mater., 2015, 281, 64–69 CrossRef CAS PubMed.
- L. Alidokht, A. R. Khataee, A. Reyhanitabar and S. Oustan, Desalination, 2011, 270, 105–110 CrossRef CAS.
- X. Lv, J. Xu, G. Jiang and X. Xu, Chemosphere, 2011, 85, 1204–1209 CrossRef CAS PubMed.
- H. Jabeen, V. Chandra, S. Jung, J. W. Lee, K. S. Kim and S. Bin Kim, Nanoscale, 2011, 3, 3583–3585 RSC.
- G. López-Téllez, C. E. Barrera-Díaz, P. Balderas-Hernández, G. Roa-Morales and B. Bilyeu, Chem. Eng. J., 2011, 173, 480–485 CrossRef.
- S. Comba and R. Sethi, Water Res., 2009, 43, 3717–3726 CrossRef CAS PubMed.
- K. P. Singh, A. K. Singh, S. Gupta and S. Sinha, Desalination, 2011, 270, 275–284 CrossRef CAS.
- C. Y. Hu, S. L. Lo, Y. H. Liou, Y. W. Hsu, K. Shih and C. J. Lin, Water Res., 2010, 44, 3101–3108 CrossRef CAS PubMed.
- M. Rivero-Huguet and W. D. Marshall, J. Hazard. Mater., 2009, 169, 1081–1087 CrossRef CAS PubMed.
- Y. Wu, J. Zhang, Y. Tong and X. Xu, J. Hazard. Mater., 2009, 172, 1640–1645 CrossRef CAS PubMed.
- K. Simeonidis, M. Tziomaki, M. Angelakeris, C. Martinez-Boubeta, L. Balcells, C. Monty, M. Mitrakas, G. Vourlias and N. Andritsos, EPJ Web Conf., 2013, 40, 08007 CrossRef CAS.
- P. V. V. V. Prasad, C. Das and A. K. Golder, Can. J. Chem. Eng., 2011, 89, 1575–1582 CrossRef CAS.
- Z. Fang, X. Qiu, R. Huang, X. Qiu and M. Li, Desalination, 2011, 280, 224–231 CrossRef CAS.
- B. Karn, T. Kuiken and M. Otto, Environ. Health Perspect., 2009, 117, 1823–1831 Search PubMed.
- M. Gheju, Water, Air, Soil Pollut., 2011, 222, 103–148 CrossRef CAS.
- Q. Wang, N. Cissoko, M. Zhou and X. Xu, Phys. Chem. Earth, 2011, 36, 442–446 CrossRef.
- X. Li, D. W. Elliott and W. Zhang, Crit. Rev. Solid State Mater. Sci., 2006, 31, 111–122 CrossRef CAS.
- Y. Xu and D. Zhao, Water Res., 2007, 41, 2101–2108 CrossRef CAS PubMed.
- W. X. Zhang, J. Nanopart. Res., 2003, 5, 323–332 CrossRef CAS.
- S.-F. Niu, Y. Liu, X.-H. Xu and Z.-H. Lou, J. Zhejiang Univ., Sci., B, 2005, 6, 1022–1027 CrossRef PubMed.
- http://www.nanoiron.cz .
- R. A. Crane, M. Dickinson, I. C. Popescu and T. B. Scott, Water Res., 2011, 45, 2931–2942 CrossRef CAS PubMed.
- Z.-J. Li, L. Wang, L.-Y. Yuan, C.-L. Xiao, L. Mei, L.-R. Zheng, J. Zhang, J.-H. Yang, Y.-L. Zhao, Z.-T. Zhu, Z.-F. Chai and W.-Q. Shi, J. Hazard. Mater., 2015, 290, 26–33 CrossRef CAS PubMed.
- L. Ling, B. Pan and W. Zhang, Water Res., 2015, 71, 274–281 CrossRef CAS PubMed.
- S. R. Kanel, J. M. Greneche and H. Choi, Environ. Sci. Technol., 2006, 40, 2045–2050 CrossRef CAS PubMed.
- H. Zhu, Y. Jia, X. Wu and H. Wang, J. Hazard. Mater., 2009, 172, 1591–1596 CrossRef CAS PubMed.
- L. N. Shi, X. Zhang and Z. L. Chen, Water Res., 2011, 45, 886–892 CrossRef CAS PubMed.
- A. Gupta, M. Yunus and N. Sankararamakrishnan, Chemosphere, 2012, 86, 150–155 CrossRef CAS PubMed.
- K. R. Kim, B. T. Lee and K. W. Kim, J. Geochem. Explor., 2012, 113, 124–129 CrossRef CAS.
- A. B. M. Giasuddin, S. R. Kanel and H. Choi, Environ. Sci. Technol., 2007, 41, 2022–2027 CrossRef CAS PubMed.
- S. R. Kanel, B. Manning, L. Charlet and H. Choi, Environ. Sci. Technol., 2005, 39, 1291–1298 CrossRef CAS PubMed.
- H. Dong, X. Guan and I. M. C. Lo, Water Res., 2012, 46, 4071–4080 CrossRef CAS PubMed.
- V. Tanboonchuy, N. Grisdanurak and C. H. Liao, J. Hazard. Mater., 2012, 205–206, 40–46 CrossRef CAS PubMed.
- V. Tanboonchuy, J. C. Hsu, N. Grisdanurak and C. H. Liao, J. Hazard. Mater., 2011, 186, 2123–2128 CrossRef CAS PubMed.
- H. Sun, L. Wang, R. Zhang, J. Sui and G. Xu, J. Hazard. Mater., 2006, 129, 297–303 CrossRef CAS PubMed.
- S. Comba, A. Di Molfetta and R. Sethi, Water, Air, Soil Pollut., 2011, 215, 595–607 CrossRef CAS.
- Y. Xi, M. Mallavarapu and R. Naidu, Mater. Res. Bull., 2010, 45, 1361–1367 CrossRef CAS.
- S. A. Kim, S. Kamala-Kannan, K. J. Lee, Y. J. Park, P. J. Shea, W. H. Lee, H. M. Kim and B. T. Oh, Chem. Eng. J., 2013, 217, 54–60 CrossRef CAS.
- S. M. Ponder, J. G. Darab and T. E. Mallouk, Environ. Sci. Technol., 2000, 34, 2564–2569 CrossRef CAS.
- X. Zhang, S. Lin, Z. Chen, M. Megharaj and R. Naidu, Water Res., 2011, 45, 3481–3488 CrossRef CAS PubMed.
- X. Zhang, S. Lin, X. Q. Lu and Z. L. Chen, Chem. Eng. J., 2010, 163, 243–248 CrossRef CAS.
- S. Xiao, H. Ma, M. Shen, S. Wang, Q. Huang and X. Shi, Colloids Surf., A, 2011, 381, 48–54 CrossRef CAS.
- A. Ayob, N. Ismail, T. T. Teng and A. Z. Abdullah, Environ. Prot. Eng., 2012, 38, 119–131 CAS.
- T. Liu, Z.-L. Wang, X. Yan and B. Zhang, Chem. Eng. J., 2014, 245, 34–40 CrossRef CAS.
- Ç. Üzüm, T. Shahwan, A. E. Eroǧlu, I. Lieberwirth, T. B. Scott and K. R. Hallam, Chem. Eng. J., 2008, 144, 213–220 CrossRef.
- O. Çelebi, Ç. Üzüm, T. Shahwan and H. N. Erten, J. Hazard. Mater., 2007, 148, 761–767 CrossRef PubMed.
- N. Kržišnik, A. Mladenovič, A. S. Škapin, L. Škrlep, J. Ščančar and R. Milačič, Sci. Total Environ., 2014, 476–477, 20–28 CrossRef PubMed.
- T. B. Scott, I. C. Popescu, R. A. Crane and C. Noubactep, J. Hazard. Mater., 2011, 186, 280–287 CrossRef CAS PubMed.
- T. Liu, X. Yang, Z. L. Wang and X. Yan, Water Res., 2013, 47, 6691–6700 CrossRef CAS PubMed.
- P. Huang, Z. Ye, W. Xie, Q. Chen, J. Li, Z. Xu and M. Yao, Water Res., 2013, 47, 4050–4058 CrossRef CAS PubMed.
- M. Dickinson and T. B. Scott, J. Hazard. Mater., 2010, 178, 171–179 CrossRef CAS PubMed.
- S. C. N. Tang and I. M. C. Lo, Water Res., 2013, 47, 2613–2632 CrossRef CAS PubMed.
- C. T. Yavuz, J. T. Mayo, W. W. Yu, A. Prakash, J. C. Falkner, S. Yean, L. Cong, H. J. Shipley, A. Kan, M. Tomson, D. Natelson and V. L. Colvin, Science, 2006, 314, 964–967 CrossRef PubMed.
- J. T. Mayo, C. Yavuz, S. Yean, L. Cong, H. Shipley, W. Yu, J. Falkner, A. Kan, M. Tomson and V. L. Colvin, Sci. Technol. Adv. Mater., 2007, 8, 71–75 CrossRef CAS.
- C. T. Yavuz, J. T. Mayo, C. Suchecki, J. Wang, A. Z. Ellsworth, H. D'Couto, E. Quevedo, A. Prakash, L. Gonzalez, C. Nguyen, C. Kelty and V. L. Colvin, Environ. Geochem. Health, 2010, 32, 327–334 CrossRef CAS PubMed.
- K. Simeonidis, T. Gkinis, S. Tresintsi, C. Martinez-Boubeta, G. Vourlias, I. Tsiaoussis, G. Stavropoulos, M. Mitrakas and M. Angelakeris, Chem. Eng. J., 2011, 168, 1008–1015 CrossRef CAS.
- Z. Bujňáková, P. Baláž, A. Zorkovská, M. J. Sayagués, J. Kováč and M. Timko, J. Hazard. Mater., 2013, 262, 1204–1212 CrossRef PubMed.
- H. J. Shipley, S. Yean, A. T. Kan and M. B. Tomson, Environ. Toxicol. Chem., 2009, 28, 509–515 CrossRef CAS PubMed.
- H. J. Shipley, S. Yean, A. T. Kan and M. B. Tomson, Environ. Sci. Pollut. Res., 2010, 17, 1053–1062 CrossRef CAS PubMed.
- S. Luther, N. Borgfeld, J. Kim and J. G. Parsons, Microchem. J., 2012, 101, 30–36 CrossRef CAS.
- S. R. Chowdhury, E. K. Yanful and A. R. Pratt, Environ. Earth Sci., 2011, 64, 411–423 CrossRef CAS.
- A. Khodabakhshi, M. M. Amin and M. Mozaffari, J. Environ. Health Sci. Eng., 2011, 8, 189–200 CAS.
- S. R. Chowdhury and E. K. Yanful, J. Environ. Manage., 2010, 91, 2238–2247 CrossRef CAS PubMed.
- X. Luo, C. Wang, S. Luo, R. Dong, X. Tu and G. Zeng, Chem. Eng. J., 2012, 187, 45–52 CrossRef CAS.
- Y. Jin, F. Liu, M. Tong and Y. Hou, J. Hazard. Mater., 2012, 227–228, 461–468 CrossRef CAS PubMed.
- B. An, Q. Liang and D. Zhao, Water Res., 2011, 45, 1961–1972 CrossRef CAS PubMed.
- L. Feng, M. Cao, X. Ma, Y. Zhu and C. Hu, J. Hazard. Mater., 2012, 217–218, 439–446 CrossRef CAS PubMed.
- R. Chen, C. Zhi, H. Yang, Y. Bando, Z. Zhang, N. Sugiur and D. Golberg, J. Colloid Interface Sci., 2011, 359, 261–268 CrossRef CAS PubMed.
- T. Türk and I. Alp, J. Ind. Eng. Chem., 2014, 20, 732–738 CrossRef.
- A. K. Mishra and S. Ramaprabhu, J. Phys. Chem. C, 2010, 114, 2583–2590 CAS.
- S. Hokkanen, E. Repo, S. Lou and M. Sillanpää, Chem. Eng. J., 2015, 260, 886–894 CrossRef CAS.
- S. Lunge, S. Singh and A. Sinha, J. Magn. Magn. Mater., 2014, 356, 21–31 CrossRef CAS.
- I. Akin, G. Arslan, A. Tor, M. Ersoz and Y. Cengeloglu, J. Hazard. Mater., 2012, 235–236, 62–68 CrossRef CAS PubMed.
- T. Tuutijärvi, J. Lu, M. Sillanpää and G. Chen, J. Hazard. Mater., 2009, 166, 1415–1420 CrossRef PubMed.
- H. Park, N. V. Myung, H. Jung and H. Choi, J. Nanopart. Res., 2009, 11, 1981–1989 CrossRef CAS.
- M. Auffan, J. Rose, O. Proux, D. Borschneck, A. Masion, P. Chaurand, J. L. Hazemann, C. Chaneac, J. P. Jolivet, M. R. Wiesner, A. Van Geen and J. Y. Bottero, Langmuir, 2008, 24, 3215–3222 CrossRef CAS PubMed.
- T. Tuutijärvi, R. Vahala, M. Sillanpää and G. Chen, Environ. Technol., 2012, 33, 1927–1936 CrossRef PubMed.
- K. Simeonidis, E. Kaprara, T. Samaras, M. Angelakeris, N. Pliatsikas, G. Vourlias, M. Mitrakas and N. Andritsos, Sci. Total Environ., 2015, 535, 61–68 CrossRef CAS PubMed.
- J. Hu, I. M. C. Lo and G. Chen, Water Sci. Technol., 2004, 50, 139–146 CAS.
- S. R. Chowdhury, E. K. Yanful and A. R. Pratt, J. Hazard. Mater., 2012, 235–236, 246–256 CrossRef CAS PubMed.
- K. L. Dubrawski, C. M. van Genuchten, C. Delaire, S. E. Amrose, A. J. Gadgil and M. Mohseni, Environ. Sci. Technol., 2015, 49, 2171–2179 CrossRef CAS PubMed.
- W. Jiang, M. Pelaez, D. D. Dionysiou, M. H. Entezari, D. Tsoutsou and K. O'Shea, Chem. Eng. J., 2013, 222, 527–533 CrossRef CAS.
- J. Hu, G. Chen and I. M. C. Lo, Water Res., 2005, 39, 4528–4536 CrossRef CAS PubMed.
- D. E. Crean, V. S. Coker, G. Van Der Laan and J. R. Lloyd, Environ. Sci. Technol., 2012, 46, 3352–3359 CrossRef CAS PubMed.
- N. D. Telling, V. S. Coker, R. S. Cutting, G. Van Der Laan, C. I. Pearce, R. A. D. Pattrick, E. Arenholz and J. R. Lloyd, Appl. Phys. Lett., 2009, 95, 163701 CrossRef.
- N. N. Thinh, P. T. B. Hanh, L. T. T. Ha, L. N. Anh, T. V. Hoang, V. D. Hoang, L. H. Dang, N. Van Khoi and T. D. Lam, Mater. Sci. Eng., C, 2013, 33, 1214–1218 CrossRef CAS PubMed.
- X. Liu, Q. Hu, Z. Fang, X. Zhang and B. Zhang, Langmuir, 2009, 25, 3–8 CrossRef CAS PubMed.
- W. Jiang, Q. Cai, W. Xu, M. Yang, Y. Cai, D. D. Dionysiou and K. E. O'Shea, Environ. Sci. Technol., 2014, 48, 8078–8085 CrossRef CAS PubMed.
- A. Nematollahzadeh, S. Seraj and B. Mirzayi, Chem. Eng. J., 2015, 277, 21–29 CrossRef CAS.
- J. Hu, I. M. C. Lo and G. Chen, Sep. Purif. Technol., 2007, 58, 76–82 CrossRef CAS.
- P. Yuan, M. Fan, D. Yang, H. He, D. Liu, A. Yuan, J. Zhu and T. Chen, J. Hazard. Mater., 2009, 166, 821–829 CrossRef CAS PubMed.
- P. Yuan, D. Liu, M. Fan, D. Yang, R. Zhu, F. Ge, J. Zhu and H. He, J. Hazard. Mater., 2010, 173, 614–621 CrossRef CAS PubMed.
- C. Wu, J. Fan, J. Jiang and J. Wang, RSC Adv., 2015, 5, 47165–47173 RSC.
- L. Wang, J. Li, Q. Jiang and L. Zhao, Dalton Trans., 2012, 41, 4544 RSC.
- H. A. Wiatrowski, S. Das, R. Kukkadapu, E. S. Ilton, T. Barkay and N. Yee, Environ. Sci. Technol., 2009, 43, 5307–5313 CrossRef CAS PubMed.
- T. S. Pasakarnis, M. I. Boyanov, K. M. Kemner, B. Mishra, E. J. O'Loughlin, G. Parkin and M. M. Scherer, Environ. Sci. Technol., 2013, 47, 6987–6994 CAS.
- D. M. Singer, S. M. Chatman, E. S. Ilton, K. M. Rosso, J. F. Banfield and G. A. Waychunas, Environ. Sci. Technol., 2012, 46, 3821–3830 CrossRef CAS PubMed.
- R. L. D. A. Loyo, S. I. Nikitenko, A. C. Scheinost and M. Simonoff, Environ. Sci. Technol., 2008, 42, 2451–2456 CrossRef PubMed.
- S. H. Huang and D. H. Chen, J. Hazard. Mater., 2009, 163, 174–179 CrossRef CAS PubMed.
- J. Wang, S. Zheng, Y. Shao, J. Liu, Z. Xu and D. Zhu, J. Colloid Interface Sci., 2010, 349, 293–299 CrossRef CAS PubMed.
- Y.-M. Hao, C. Man and Z.-B. Hu, J. Hazard. Mater., 2010, 184, 392–399 CrossRef CAS PubMed.
- A. Z. M. Badruddoza, A. S. H. Tay, P. Y. Tan, K. Hidajat and M. S. Uddin, J. Hazard. Mater., 2011, 185, 1177–1186 CrossRef CAS PubMed.
- F. Ge, M.-M. Li, H. Ye and B.-X. Zhao, J. Hazard. Mater., 2012, 211–212, 366–372 CrossRef CAS PubMed.
- A. R. Mahdavian and M. A. S. Mirrahimi, Chem. Eng. J., 2010, 159, 264–271 CrossRef CAS.
- M. Bhaumik, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 190, 381–390 CrossRef CAS PubMed.
- F.-L. Fan, Z. Qin, J. Bai, W.-D. Rong, F.-Y. Fan, W. Tian, X.-L. Wu, Y. Wang and L. Zhao, J. Environ. Radioact., 2012, 106, 40–46 CrossRef CAS PubMed.
- O. Hakami, Y. Zhang and C. J. Banks, Water Res., 2012, 46, 3913–3922 CrossRef CAS PubMed.
- J. Liu, Z. Zhao and G. Jiang, Environ. Sci. Technol., 2008, 42, 6949–6954 CrossRef CAS PubMed.
- A. Afkhami and R. Norooz-Asl, Colloids Surf., A, 2009, 346, 52–57 CrossRef CAS.
- A. Roy and J. Bhattacharya, Chem. Eng. J., 2012, 211–212, 493–500 CrossRef CAS.
- N. C. Feitoza, T. D. Gonçalves, J. J. Mesquita, J. S. Menegucci, M. K. M. S. Santos, J. A. Chaker, R. B. Cunha, A. M. M. Medeiros, J. C. Rubim and M. H. Sousa, J. Hazard. Mater., 2014, 264, 153–160 CrossRef CAS PubMed.
- J. Hu, G. Chen and I. M. C. Lo, J. Environ. Eng., 2006, 132, 709–715 CrossRef CAS.
- J. Song, H. Kong and J. Jang, J. Colloid Interface Sci., 2011, 359, 505–511 CrossRef CAS PubMed.
- S. R. Chowdhury and E. K. Yanful, J. Environ. Manage., 2013, 129, 642–651 CrossRef CAS PubMed.
- N. Jordan, A. Ritter, A. C. Scheinost, S. Weiss, D. Schild and R. Hübner, Environ. Sci. Technol., 2014, 48, 1665–1674 CrossRef CAS PubMed.
- N. Jordan, A. Ritter, H. Foerstendorf, A. C. Scheinost, S. Weiß, K. Heim, J. Grenzer, A. Mücklich and H. Reuther, Geochim. Cosmochim. Acta, 2013, 103, 63–75 CrossRef CAS.
- Y. Feng, J.-L. Gong, G.-M. Zeng, Q.-Y. Niu, H.-Y. Zhang, C.-G. Niu, J.-H. Deng and M. Yan, Chem. Eng. J., 2010, 162, 487–494 CrossRef CAS.
- S. Garcia, S. Sardar, S. Maldonado, V. Garcia, C. Tamez and J. G. Parsons, Microchem. J., 2014, 117, 52–60 CrossRef CAS PubMed.
- Y. Meng, D. Chen, Y. Sun, D. Jiao, D. Zeng and Z. Liu, Appl. Surf. Sci., 2015, 324, 745–750 CrossRef CAS.
- J. Hu, I. M. C. Lo and G. Chen, Langmuir, 2005, 21, 11173–11179 CrossRef CAS PubMed.
- J. Hu, I. M. C. Lo and G. Chen, Sep. Purif. Technol., 2007, 56, 249–256 CrossRef CAS.
- V. Srivastava, Y. C. Sharma and M. Sillanpää, Appl. Surf. Sci., 2015, 338, 42–54 CrossRef CAS.
- W. Tang, Y. Su, Q. Li, S. Gao and J. K. Shang, Water Res., 2013, 47, 3624–3634 CrossRef CAS PubMed.
- A. Meidanchi and O. Akhavan, Carbon, 2014, 69, 230–238 CrossRef CAS.
- A. Dey, R. Singh and M. K. Purkait, Journal of Water Process Engineering, 2014, 3, 1–9 CrossRef.
- N. D. Phu, P. C. Phong, N. Chau, N. H. Luong, L. H. Hoang and N. H. Hai, J. Exp. Nanosci., 2009, 4, 253–258 CrossRef CAS.
- W. Sun, W. Pan, F. Wang and N. Xu, Chem. Eng. J., 2015, 273, 353–362 CrossRef CAS.
- V. A. Grover, J. Hu, K. E. Engates and H. J. Shipley, Environ. Toxicol. Chem., 2012, 31, 86–92 CrossRef CAS PubMed.
- J. Ha, T. P. Trainor, F. Farges and G. E. Brown, Langmuir, 2009, 25, 5574–5585 CrossRef CAS PubMed.
- H. Zeng, A. Singh, S. Basak, K. U. Ulrich, M. Sahu, P. Biswas, J. G. Catalano and D. E. Giammar, Environ. Sci. Technol., 2009, 43, 1373–1378 CrossRef CAS PubMed.
- H. J. Shipley, K. E. Engates and A. M. Guettner, J. Nanopart. Res., 2010, 13, 2387–2397 CrossRef.
- J. S. Kwon, S. T. Yun, J. H. Lee, S. O. Kim and H. Y. Jo, J. Hazard. Mater., 2010, 174, 307–313 CrossRef CAS PubMed.
- M. A. Ahmed, S. M. Ali, S. I. El-Dek and A. Galal, Mater. Sci. Eng. B Solid State Mater. Adv. Technol., 2013, 178, 744–751 CrossRef CAS.
- C. Shan, Z. Ma and M. Tong, J. Hazard. Mater., 2014, 268, 229–236 CrossRef CAS PubMed.
- K. Henke, Arsenic: Environmental Chemistry, Health Threats and Waste Treatment, John Wiley & Sons, West Sussex, United Kingdom, 2009 Search PubMed.
- M. K. Ghosh, G. E. J. Poinern, T. B. Issa and P. Singh, Korean J. Chem. Eng., 2012, 29, 95–102 CrossRef CAS.
- A. Manasse and C. Viti, Environ. Geol., 2007, 52, 1365–1374 CrossRef CAS.
- K. Matis and A. Zouboulis, Water, Air, Soil Pollut., 1999, 297–316 CrossRef CAS.
- M. Mohapatra and S. Anand, Int. J. Eng. Sci. Res. Technol., 2010, 2, 127–146 Search PubMed.
- B. Pan, H. Qiu, B. Pan, G. Nie, L. Xiao, L. Lv, W. Zhang, Q. Zhang and S. Zheng, Water Res., 2010, 44, 815–824 CrossRef CAS PubMed.
- S. Dong, X. Dou, D. Mohan, C. U. Pittman Jr. and J. Luo, Chem. Eng. J., 2015, 270, 205–214 CrossRef CAS.
- S. Tresintsi, K. Simeonidis, G. Vourlias, G. Stavropoulos and M. Mitrakas, Water Res., 2012, 46, 5255–5267 CrossRef CAS PubMed.
- S. Tresintsi, K. Simeonidis, S. Estradé, C. Martinez-Boubeta, G. Vourlias, F. Pinakidou, M. Katsikini, E. C. Paloura, G. Stavropoulos and M. Mitrakas, Environ. Sci. Technol., 2013, 47, 9699–9705 CrossRef CAS PubMed.
- S. Tresintsi, K. Simeonidis and M. Mitrakas, Chem. Eng. J., 2014, 251, 192–198 CrossRef CAS.
- http://www.infimet.com/ .
- S. Tresintsi, K. Simeonidis, N. Pliatsikas, G. Vourlias, P. Patsalas and M. Mitrakas, J. Solid State Chem., 2014, 213, 145–151 CrossRef CAS.
- E. Kokkinos, K. Simeonidis, A. Zouboulis and M. Mitrakas, Desalin. Water Treat., 2014, 54, 2082–2090 Search PubMed.
- V. Dimiropoulos, I. A. Katsoyiannis, A. I. Zouboulis, F. Noli, K. Simeonidis and M. Mitrakas, Journal of Water Process Engineering, 2015, 7, 227–236 CrossRef.
- H. Y. Jeong, B. Klaue, J. D. Blum and K. F. Hayes, Environ. Sci. Technol., 2007, 41, 7699–7705 CrossRef CAS PubMed.
- J. Liu, K. T. Valsaraj, I. Devai and R. D. DeLaune, J. Hazard. Mater., 2008, 157, 432–440 CrossRef CAS PubMed.
- Z. Xiong, F. He, D. Zhao and M. O. Barnett, Water Res., 2009, 43, 5171–5179 CrossRef CAS PubMed.
- T. Arakaki and J. W. Morse, Geochim. Cosmochim. Acta, 1993, 57, 9–14 CrossRef CAS.
- A. Criscuoli, S. Majumdar, A. Figoli, G. C. Sahoo, P. Bafaro, S. Bandyopadhyay and E. Drioli, J. Hazard. Mater., 2012, 211–212, 281–287 CrossRef CAS PubMed.
- Z. Zhao, J. Liu, F. Cui, H. Feng and L. Zhang, J. Mater. Chem., 2012, 22, 9052–9057 RSC.
- D. Nguyen Thanh, M. Singh, P. Ulbrich, N. Strnadova and F. Štěpánek, Sep. Purif. Technol., 2011, 82, 93–101 CrossRef.
- S. Y. Lee and S.-J. Park, J. Ind. Eng. Chem., 2013, 19, 1761 CrossRef CAS.
- R. K. Upadhyay, N. Soin and S. S. Roy, RSC Adv., 2014, 4, 3823 RSC.
- J. Kim and B. Van der Bruggen, Environ. Pollut., 2010, 158, 2335 CrossRef CAS PubMed.
- M. E. Pena, G. P. Korfiatis, M. Patel, L. Lippincott and X. Meng, Water Res., 2005, 39, 2327 CrossRef CAS PubMed.
- G. Jegadeesan, S. R. Al-Abed, V. Sundaram, H. Choi, K. G. Scheckel and D. D. Dionysiou, Water Res., 2010, 44, 965–973 CrossRef CAS PubMed.
- Z. O. Kocabas-Atakli and Y. Yurum, Chem. Eng. J., 2013, 225, 625 CrossRef CAS.
- D. Nabi, I. Aslam and I. A. Qazi, J. Environ. Sci., 2009, 21, 402–408 CrossRef CAS.
- Z. Xu and X. Meng, J. Hazard. Mater., 2009, 168, 747–752 CrossRef CAS PubMed.
- L. Yu, X. Peng, F. Ni, J. Li, D. Wang and Z. Luan, J. Hazard. Mater., 2013, 246–247, 10–17 CrossRef CAS PubMed.
- C. Y. Jing, X. G. Meng, E. Calvache and G. B. Jiang, Environ. Pollut., 2009, 157, 2514–2519 CrossRef CAS PubMed.
- H. Sun, X. Zhang, Q. Niu, Y. Chen and J. C. Crittenden, Water, Air, Soil Pollut., 2007, 178, 245–254 CrossRef CAS.
- H. Sun, X. Zhang, Z. Zhang, Y. Chen and J. C. Crittenden, Environ. Pollut., 2009, 157, 1165–1170 CrossRef CAS PubMed.
- W. Ji, Y. Wang, I. Tanabe, X. Han, B. Zhao and Y. Ozaki, Chem. Sci., 2015, 6, 342 RSC.
- N. Zhang, H. Peng and B. Hu, Talanta, 2012, 94, 278 CrossRef CAS PubMed.
- http://gravertech.com/pr_overview_metsorb.html .
- http://www.dowwaterandprocess.com/en/Products/A/ADSORBSIA_AS600 .
- K. Hristovski, A. Baumgardner and P. Westerhoff, J. Hazard. Mater., 2007, 147, 265–274 CrossRef CAS PubMed.
- K. Hristovski, P. Westerhoff and J. Crittenden, J. Hazard. Mater., 2008, 156, 604–611 CrossRef CAS PubMed.
- K. Y. Kumar, H. B. Muralidhara, Y. A. Nayaka, J. Balasubramanyam and H. Hanumanthappa, Powder Technol., 2013, 246, 125–136 CrossRef CAS.
- Y. Liu, Q. Li, S. Gao and J. K. Shang, J. Am. Ceram. Soc., 2011, 94, 217–223 CrossRef CAS.
- X. Y. Yu, T. Luo, Y. Jia, Y. X. Zhang, J. H. Liu and X. J. Huang, J. Phys. Chem. C, 2011, 115, 22242–22250 CAS.
- W. Liu, F. Huang, Y. Wang, T. Zou, J. Zheng and Z. Lin, Environ. Sci. Technol., 2011, 45, 1955–1961 CrossRef CAS PubMed.
- A. R. Contreras, A. García, E. González, E. Casals, V. Puntes, A. Sánchez, X. Font and S. Recillas, Desalin. Water Treat., 2012, 41, 296–300 CrossRef CAS.
- S. Recillas, A. García, E. González, E. Casals, V. Puntes, A. Sánchez and X. Font, Desalination, 2011, 277, 213–220 CrossRef CAS.
- R. Li, Q. Li, S. Gao and J. K. Shang, Chem. Eng. J., 2012, 185–186, 127–135 CrossRef CAS.
- W. Sun, Q. Li, S. Gao and J. K. Shang, Chem. Eng. J., 2012, 185–186, 136–143 CrossRef CAS.
- K. Gupta, S. Bhattacharya, D. Chattopadhyay, A. Mukhopadhyay, H. Biswas, J. Dutta, N. R. Ray and U. C. Ghosh, Chem. Eng. J., 2011, 172, 219–229 CrossRef CAS.
- X.-F. Yu, J.-W. Liu, H.-P. Cong, L. Xue, M. N. Arshad, H. A. Albar, T. R. Sobahi, Q. Gao and S.-H. Yu, Chem. Sci., 2015, 6, 2511–2515 RSC.
- W. Xu, J. Wang, L. Wang, G. Sheng, J. Liu, H. Yu and X. J. Huang, J. Hazard. Mater., 2013, 260, 498–507 CrossRef CAS PubMed.
- http://www.zrpure.com/drinking-water/isolux_media.asp .
- C. Hang, Q. Li, S. Gao and J. K. Shang, Ind. Eng. Chem. Res., 2012, 51, 353–361 CrossRef CAS.
- H. Cui, Y. Su, Q. Li, S. Gao and J. K. Shang, Water Res., 2013, 47, 6258–6268 CrossRef CAS PubMed.
- K. D. Hristovski, P. K. Westerhoff, J. C. Crittenden and L. W. Olson, Environ. Sci. Technol., 2008, 42, 3786–3790 CrossRef CAS PubMed.
- L. Önnby, C. Svensson, L. Mbundi, R. Busquets, A. Cundy and H. Kirsebom, Sci. Total Environ., 2014, 473–474, 207–214 CrossRef PubMed.
- H. Ahmad, Y. Abbas, M. Hussain, N. Akhtar, T. Ansari, M. Zuber, K. Zia and S. Arain, Korean J. Chem. Eng., 2014, 31, 284–288 CrossRef CAS.
- L. Önnby, P. S. Kumar, K. G. V. Sigfridsson, O. F. Wendt, S. Carlson and H. Kirsebom, Chemosphere, 2014, 113, 151–157 CrossRef PubMed.
- K. J. Reddy, K. J. McDonald and H. King, J. Colloid Interface Sci., 2013, 397, 96–102 CrossRef CAS PubMed.
- A. Goswami, P. K. Raul and M. K. Purkait, Chem. Eng. Res. Des., 2012, 90, 1387–1396 CrossRef CAS.
- C. A. Martinson and K. J. Reddy, J. Colloid Interface Sci., 2009, 336, 406–411 CrossRef CAS PubMed.
- K. P. Lisha, Anshup and T. Pradeep, Gold Bull., 2009, 42, 144 CrossRef CAS.
- Y.-H. Lin and W.-L. Tseng, Anal. Chem., 2013, 82, 9194 CrossRef PubMed.
- L. Guerrini, I. Rodriguez-Loureiro, M. A. Correa-Duarte, Y. H. Lee, X. Y. Ling, F. J. Garcia de Abajo and R. A. Alvarez-Puebla, Nanoscale, 2014, 6, 8368 RSC.
- I. Ojea-Jimenez, X. Lopez, J. Arbiol and V. Puntes, ACS Nano, 2012, 6, 2253 CrossRef CAS PubMed.
- L.-H. Jin and C.-S. Han, Sens. Actuators, B, 2014, 195, 239 CrossRef CAS.
- S. Fernandes, C. M. Eichenseer, P. Kreitmeier, J. Rewitzer, V. Zlateski, R. N. Grass, W. J. Stark and O. Reiser, RSC Adv., 2015, 5, 46430–46436 RSC.
- H. Zhang, Q. Liu, T. Wang, Z. Yun, G. Li, J. Liu and G. Jiang, Anal. Chim. Acta, 2013, 770, 140 CrossRef CAS PubMed.
- Q. Li, N. J. Easter and J. K. Shang, Environ. Sci. Technol., 2009, 43, 1534 CrossRef CAS PubMed.
- M. A. Omole, I. O. K'Owino and O. A. Sadik, Appl. Catal., B, 2007, 76, 158–167 CrossRef CAS.
- Y. Chen, L. Wu, Y. Chen, N. Bi, X. Zheng, H. Qi, M. Qin, X. Liao, H. Zhang and Y. Tian, Microchim. Acta, 2012, 177, 341–348 CrossRef CAS.
- C. Jiang, Z. Guan, S. Y. Rachel Lim, L. Polavarapu and Q.-H. Xu, Nanoscale, 2011, 3, 3316–3320 RSC.
- J. Li, L. Chen, T. Lou and Y. Wang, ACS Appl. Mater. Interfaces, 2011, 3, 3936–3941 CAS.
- M. Liu, Z. Wang, S. Zong, H. Chen, D. Zhu, L. Wu, G. Hu and Y. Cui, ACS Appl. Mater. Interfaces, 2014, 6, 7371–7379 CAS.
- L. Polavarapu, J. Perez-Juste, Q.-H. Xu and L. M. Liz-Marzan, J. Mater. Chem. C, 2014, 2, 7460–7476 RSC.
- E. Sumesh, M. S. Bootharaju, Anshup and T. Pradeep, J. Hazard. Mater., 2011, 189, 450–457 CrossRef CAS PubMed.
- Y. Wang, F. Yang and X. Yang, ACS Appl. Mater. Interfaces, 2010, 2, 339–342 CAS.
- Z.-H. Wu, J.-H. Lin and W.-L. Tseng, Biosens. Bioelectron., 2012, 34, 185–190 CrossRef PubMed.
- S. Bothra, J. N. Solanki and S. K. Sahoo, Sens. Actuators, B, 2013, 188, 937–943 CrossRef CAS.
- T. Yordanova, P. Vasileva, I. Karadjova and D. Nihtianova, Analyst, 2014, 139, 1532–1540 RSC.
- A. Herman and A. P. Herman, J. Nanosci. Nanotechnol., 2014, 14, 946–957 CrossRef CAS PubMed.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Water Res., 2008, 42, 4591–4602 CrossRef CAS PubMed.
- H. Schwegmann and F. H. Frimmel, in Nanoparticles in the Water Cycle: Properties, Analysis and Environmental Relevance, 2010, pp. 165–182 Search PubMed.
- N. von Moos and V. I. Slaveykova, Nanotoxicology, 2014, 8, 605–630 CrossRef CAS PubMed.
- W.-R. Li, X.-B. Xie, Q.-S. Shi, H.-Y. Zeng, Y.-S. Ou-Yang and Y.-B. Chen, Appl. Microbiol. Biotechnol., 2010, 85, 1115–1122 CrossRef CAS PubMed.
- R. Kaegi, A. Voegelin, B. Sinnet, S. Zuleeg, H. Hagendorfer, M. Burkhardt and H. Siegrist, Environ. Sci. Technol., 2011, 45, 3902–3908 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- Q. Bao, D. Zhang and P. Qi, J. Colloid Interface Sci., 2011, 360, 463–470 CrossRef CAS PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, RSC Adv., 2014, 4, 3974–3983 RSC.
- Z. Wang, T. Xia and S. Liu, Nanoscale, 2015, 7, 7470–7481 RSC.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS PubMed.
- A. J. Kora and R. B. Sashidhar, J. Antibiot., 2015, 68, 88–97 CrossRef CAS PubMed.
- M. Ahamed, M. Karns, M. Goodson, J. Rowe, S. M. Hussain, J. J. Schlager and Y. L. Hong, Toxicol. Appl. Pharmacol., 2008, 233, 404–410 CrossRef CAS PubMed.
- L. Cui, P. Y. Chen, S. D. Chen, Z. H. Yuan, C. P. Yu, B. Ren and K. S. Zhang, Anal. Chem., 2013, 85, 5436–5443 CrossRef CAS PubMed.
- T. Hennebel, B. De Gusseme, N. Boon and W. Verstraete, Trends Biotechnol., 2009, 27, 90–98 CrossRef CAS PubMed.
- J. S. Kim, E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D. H. Jeong and M.-H. Cho, Nanomedicine, 2007, 3, 95–101 CrossRef CAS PubMed.
- M. U. Sankar, S. Aigal, S. M. Maliyekkal, A. Chaudhary, Anshup, A. A. Kumar, K. Chaudhari and T. Pradeep, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 8459–8464 CrossRef CAS PubMed.
- T. Pradeep and Anshup, Thin Solid Films, 2009, 517, 6441–6478 CrossRef CAS.
- M. Ahamed, M. S. AlSalhi and M. K. J. Siddiqui, Clin. Chim. Acta, 2010, 411, 1841–1848 CrossRef CAS PubMed.
- V. S. Srivastava, Arch. Appl. Sci. Res., 2010, 2, 82 Search PubMed.
- M. T. Amin, A. A. Alazba and U. Manzoor, Adv. Mater. Sci. Eng., 2014, 2014, 825910 Search PubMed.
- T. A. Dankovich and J. A. Smith, Water Res., 2014, 63, 245–251 CrossRef CAS PubMed.
- M. Ben-Sasson, X. Lu, E. Bar-Zeev, K. R. Zodrow, S. Nejati, G. Qi, E. P. Giannelis and M. Elimelech, Water Res., 2014, 62, 260–270 CrossRef CAS PubMed.
- W.-L. Chou, D.-G. Yu and M.-C. Yang, Polym. Adv. Technol., 2005, 16, 600–607 CrossRef CAS.
- C. A. Mecha and V. L. Pillay, J. Membr. Sci., 2014, 458, 149–156 CrossRef CAS.
- Y. Lv, H. Liu, Z. Wang, S. Liu, L. Hao, Y. Sang, D. Liu, J. Wang and R. I. Boughton, J. Membr. Sci., 2009, 331, 50–56 CrossRef CAS.
- R. Bandyopadhyaya, M. V. Sivaiah and P. A. Shankar, J. Chem. Technol. Biotechnol., 2008, 83, 1177–1180 CrossRef CAS.
- T. A. Dankovich, Environ. Sci.: Nano, 2014, 1, 367–378 RSC.
- V. A. Oyanedel-Craver and J. A. Smith, Environ. Sci. Technol., 2008, 42, 927–933 CrossRef CAS PubMed.
- D. He, M. Kacopieros, A. Ikeda-Ohno and T. D. Waite, Environ. Sci. Technol., 2014, 48, 12320–12326 CrossRef CAS PubMed.
- B. Ehdaie, C. Krause and J. A. Smith, Environ. Sci. Technol., 2014, 48, 13901–13908 CrossRef CAS PubMed.
- T. A. Dankovich and D. G. Gray, Environ. Sci. Technol., 2011, 45, 1992–1998 CrossRef CAS PubMed.
- P. Jain and T. Pradeep, Biotechnol. Bioeng., 2005, 90, 59–63 CrossRef CAS PubMed.
- V. Apalangya, V. Rangari, B. Tiimob, S. Jeelani and T. Samuel, Appl. Surf. Sci., 2014, 295, 108–114 CrossRef CAS.
- Y. J. Xiong, M. Brunson, J. Huh, A. R. Huang, A. Coster, K. Wendt, J. Fay and D. Qin, Small, 2013, 9, 2628–2638 CrossRef CAS PubMed.
- H. Zhang, J. A. Smith and V. Oyanedel-Craver, Water Res., 2012, 46, 691–699 CrossRef CAS PubMed.
- M. V. D. Z. Park, A. M. Neigh, J. P. Vermeulen, L. J. J. de la Fonteyne, H. W. Verharen, J. J. Briedé, H. van Loveren and W. H. de Jong, Biomaterials, 2011, 32, 9810–9817 CrossRef CAS PubMed.
- D. Gangadharan, K. Harshvardan, G. Gnanasekar, D. Dixit, K. M. Popat and P. S. Anand, Water Res., 2010, 44, 5481–5487 CrossRef CAS PubMed.
- R. Bryaskova, N. Georgieva, D. Pencheva, Z. Todorova, N. Lazarova and T. Kantardjiev, Colloids Surf., A, 2014, 444, 114–119 CrossRef CAS.
- Y. Seo, J. Hwang, J. Kim, Y. Jeong, M. P. Hwang and J. Choi, Int. J. Nanomed., 2014, 9, 4621–4629 Search PubMed.
- N. Savage and M. Diallo, J. Nanopart. Res., 2005, 7, 331–342 CrossRef CAS.
- Z. Xiong, J. Ma, W. J. Ng, T. D. Waite and X. S. Zhao, Water Res., 2011, 45, 2095–2103 CrossRef CAS PubMed.
- V. R. Ravisankar and A. B. Januna, in Science against microbial pathogens: communicating current research ad technological advances, ed. A. Mendez-Vilas, Formatex Research Center, Badajoz, Spain, 2011 Search PubMed.
- W. He, H.-K. Kim, W. G. Wamer, D. Melka, J. H. Callahan and J.-J. Yin, J. Am. Chem. Soc., 2014, 136, 750 CrossRef CAS PubMed.
- S. K. Das, A. R. Das and A. K. Guha, Langmuir, 2009, 25, 8192 CrossRef CAS PubMed.
- D. Prasad, Shankaracharya and A. S. Vidyarthi, World J. Microbiol. Biotechnol., 2011, 27, 2227 CrossRef CAS.
- C. Li, R. Younesi, Y. Cai, Y. Zhu, M. Ma and J. Zhu, Appl. Catal., B, 2014, 156–157, 314 CrossRef CAS.
- D. M. A. Alrousan, P. S. M. Dunlop, T. A. McMurray and J. A. Byrne, Water Res., 2009, 43, 47 CrossRef CAS PubMed.
- X. Lin, J. Li, S. Ma, G. Liu, K. Yang, M. Tong and D. Lin, PLoS One, 2014, 9, e110247 Search PubMed.
- M. V. Liga, S. J. Maguire-Boyle, H. R. Jafry, A. R. Barron and Q. Li, Environ. Sci. Technol., 2013, 47, 6463 CAS.
- I. Chauhan and P. Mohanty, Cellulose, 2015, 22, 507 CrossRef CAS.
- R. Bergamasco, F. V. da Silva, F. S. Arakawa, N. U. Yamaguchi, M. H. M. Reis, C. J. Tavares, M. T. P. S. de Amorim and C. R. G. Tavares, Chem. Eng. J., 2011, 174, 102–109 CrossRef CAS.
- M. V. Liga, E. L. Bryant, V. L. Colvin and Q. Li, Water Res., 2011, 45, 535 CrossRef CAS PubMed.
- G. Xiao, X. Zhang, W. Zhang, S. Zhang, H. Su and T. Tan, Appl. Catal., B, 2015, 170–171, 255 CrossRef CAS.
- M. Li, M. E. Noriega-Trevino, N. Nino-Martinez, C. Marambio-Jones, J. Wang, R. Damoiseaux, F. Ruiz and E. M. V. Hoek, Environ. Sci. Technol., 2011, 45, 8989 CrossRef CAS PubMed.
- H. Younas, I. A. Qazi, I. Hashmi, M. A. Awan, A. Mahmood and H. A. Qayyum, Environ. Sci. Pollut. Res., 2014, 21, 740 CrossRef CAS PubMed.
- R. Liu, H. S. Wu, R. Yeh, C. Y. Lee and Y. Hung, Int. J. Photoenergy, 2012, 2012, 640487 Search PubMed.
- I. Altin and M. Sokmen, Water, Air, Soil Pollut., 2014, 225, 1786 CrossRef.
- M. Auffan, W. Achouak, J. Rose, M. A. Roncato, C. Chanéac, D. T. Waite, A. Masion, J. C. Woicik, M. R. Wiesner and J. Y. Bottero, Environ. Sci. Technol., 2008, 42, 6730–6735 CrossRef CAS PubMed.
- J. Chen, Z. Xiu, G. V. Lowry and P. J. J. Alvarez, Water Res., 2011, 45, 1995–2001 CrossRef CAS PubMed.
- M. Diao and M. Yao, Water Res., 2009, 43, 5243–5251 CrossRef CAS PubMed.
- A. Sevcu, Y. S. El-Temsah, E. J. Joner and M. Cernik, Microbes Environ., 2011, 26, 271–281 CrossRef PubMed.
- C. Lee, J. Y. Kim, W. Il Lee, K. L. Nelson, J. Yoon and D. L. Sedlak, Environ. Sci. Technol., 2008, 42, 4927–4933 CrossRef CAS PubMed.
- J. Y. Kim, C. Lee, D. C. Love, D. L. Sedlak, J. Yoon and K. L. Nelson, Environ. Sci. Technol., 2011, 45, 6978–6984 CrossRef CAS PubMed.
- R. J. Barnes, C. J. van der Gast, O. Riba, L. E. Lehtovirta, J. I. Prosser, P. J. Dobson and I. P. Thompson, J. Hazard. Mater., 2010, 184, 73–80 CrossRef CAS PubMed.
- Z. Li, K. Greden, P. J. J. Alvarez, K. B. Gregory and G. V. Lowry, Environ. Sci. Technol., 2010, 44, 3462–3467 CrossRef CAS PubMed.
- N. Tran, A. Mir, D. Mallik, A. Sinha, S. Nayar and T. J. Webster, Int. J. Nanomed., 2010, 5, 277–283 CAS.
- W. Zhang, B. Rittmann and Y. Chen, Environ. Sci. Technol., 2011, 45, 2172–2178 CrossRef CAS PubMed.
- S. Singh, K. C. Barick and D. Bahadur, J. Hazard. Mater., 2011, 192, 1539–1547 CrossRef CAS PubMed.
- D. Das, B. C. Nath, P. Phukon and S. K. Dolui, Colloids Surf., B, 2013, 101, 430–433 CrossRef CAS PubMed.
- A. Azam, A. S. Ahmed, M. Oves, M. S. Khan and A. Memic, Int. J. Nanomed., 2012, 7, 3527–3535 CrossRef CAS PubMed.
- V. V. T. Padil and M. Černík, Int. J. Nanomed., 2013, 8, 889–898 Search PubMed.
- T. Jin and Y. He, J. Nanopart. Res., 2011, 13, 6877–6885 CrossRef CAS.
- L. Huang, D. Q. Li, Y. J. Lin, M. Wei, D. G. Evans and X. Duan, J. Inorg. Biochem., 2005, 99, 986–993 CrossRef CAS PubMed.
- S. Ravikumar, R. Gokulakrishnan and P. Boomi, Asian Pac. J. Trop. Dis., 2012, 2, 85–89 CrossRef CAS.
- Y. J. Lin, D. Q. Li, G. Wang, L. Huang and X. Duan, J. Mater. Sci.: Mater. Med., 2005, 16, 53–56 CrossRef CAS PubMed.
- P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, Langmuir, 2002, 18, 6679–6686 CrossRef CAS.
- P. J. P. Espitia, N. F. F. Soares, J. S. R. Coimbra, N. J. de Andrade, R. S. Cruz and E. A. A. Medeiros, Food Bioprocess Technol., 2012, 5, 1447–1464 CrossRef CAS.
- A. Azam, A. S. Ahmed, M. Oves, M. S. Khan, S. S. Habib and A. Memic, Int. J. Nanomed., 2012, 7, 6003–6009 CrossRef CAS PubMed.
- R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M. F. Benedetti and F. Fiévet, Nano Lett., 2006, 6, 866–870 CrossRef CAS PubMed.
- R. Colonia, J. L. Solís and M. Gómez, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2013, 5, 015008 CrossRef.
- C. O. Dimkpa, J. E. McLean, D. W. Britt and A. J. Anderson, BioMetals, 2013, 26, 913–924 CrossRef CAS PubMed.
- S. Gunalan, R. Sivaraj and V. Rajendran, Prog. Nat. Sci.: Mater. Int., 2012, 22, 693–700 CrossRef.
- N. Padmavathy and R. Vijayaraghavan, Sci. Technol. Adv. Mater., 2008, 9, 035004 CrossRef.
- D. Sharma, J. Rajput, B. S. Kaith, M. Kaur and S. Sharma, Thin Solid Films, 2010, 519, 1224–1229 CrossRef CAS.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. Shi, Appl. Environ. Microbiol., 2011, 77, 2325–2331 CrossRef CAS PubMed.
- L. He, Y. Liu, A. Mustapha and M. Lin, Microbiol. Res., 2010, 166, 207–215 CrossRef PubMed.
- A. Lipovsky, Y. Nitzan, A. Gedanken and R. Lubart, Nanotechnology, 2011, 22, 105101 CrossRef PubMed.
- S. Vlad, C. Tanase, D. Macocinschi, C. Ciobanu, T. Balaes, D. Filip, I. N. Gostin and L. M. Gradinaru, Dig. J. Nanomater. Bios., 2012, 7, 51–58 Search PubMed.
- K. Kairyte, A. Kadys and Z. Luksiene, J. Photochem. Photobiol., B, 2013, 128, 78–84 CrossRef CAS PubMed.
- S. Azizi, M. Ahmad, M. Mahdavi and S. Abdolmohammadi, BioResources, 2013, 8, 1841–1851 CrossRef.
- P. Gajjar, B. Pettee, D. W. Britt, W. Huang, W. P. Johnson and A. J. Anderson, J. Biol. Eng., 2009, 3, 9 CrossRef PubMed.
- M. Valodkar, P. S. Rathore, R. N. Jadeja, M. Thounaojam, R. V. Devkar and S. Thakore, J. Hazard. Mater., 2012, 201–202, 244–249 CrossRef CAS PubMed.
- P. Kanhed, S. Birla, S. Gaikwad, A. Gade, A. B. Seabra, O. Rubilar, N. Duran and M. Rai, Mater. Lett., 2014, 115, 13–17 CrossRef CAS.
- Y. Wei, S. Chen, B. Kowalczyk, S. Huda, T. P. Gray and B. A. Grzybowski, J. Phys. Chem. C, 2010, 114, 15612–15616 CAS.
- L. Esteban-Tejeda, F. Malpartida, A. Esteban-Cubillo, C. Pecharromán and J. S. Moya, Nanotechnology, 2009, 20, 505701 CrossRef CAS PubMed.
- U. Bogdanović, V. Lazić, V. Vodnik, M. Budimir, Z. Marković and S. Dimitrijević, Mater. Lett., 2014, 128, 75–78 CrossRef.
- Q. Kang, Q. Z. Lu, S. H. Liu, L. X. Yang, L. F. Wen, S. L. Luo and Q. Y. Cai, Biomaterials, 2010, 31, 3317–3326 CrossRef CAS PubMed.
- Y. Liu, J. Li, X. Qiu and C. Burda, J. Photochem. Photobiol., A, 2007, 190, 94–100 CrossRef CAS.
- Z. Marková, K. M. Šišková, J. Filip, J. Čuda, M. Kolář, K. Šafářová, I. Medřík and R. Zbořil, Environ. Sci. Technol., 2013, 47, 5285–5293 CrossRef PubMed.
- S. Y. Park, J. W. Chung, R. D. Priestley and S. Y. Kwak, Cellulose, 2012, 19, 2141–2151 CrossRef CAS.
- J.-Q. Xue, D.-W. Li, L.-L. Qu and Y.-T. Long, Anal. Chim. Acta, 2013, 777, 57 CrossRef CAS PubMed.
- A. S. Nair, R. T. Tom and T. Pradeep, J. Environ. Monit., 2003, 5, 363 RSC.
- A. S. Nair and T. Pradeep, J. Nanosci. Nanotechnol., 2007, 7, 1871–1877 CrossRef CAS PubMed.
- M. S. Bootharaju and T. Pradeep, Langmuir, 2012, 28, 2671 CrossRef CAS PubMed.
- C. Mondal, J. Pal, M. Ganguly, A. K. Sinha, J. Jana and T. Pal, New J. Chem., 2014, 38, 2999 RSC.
- X. Wang, Y.-X. Wang, B. Yuan, H.-J. Cui and M.-L. Fu, RSC Adv., 2015, 5, 18806 RSC.
- M. S. Wong, P. J. J. Alvarez, Y.-I. Fang, N. Akcin, M. O. Nutt, J. T. Miller and K. N. Heck, J. Chem. Technol. Biotechnol., 2009, 84, 158 CrossRef CAS.
- Y. Li, L. Qi, Y. Shen and H. Ma, Anal. Chim. Acta, 2014, 811, 36 CrossRef CAS PubMed.
- Y. Su, A. S. Adeleye, Y. Huang, X. Sun, C. Dai, X. Zhou, Y. Zhang and A. A. Keller, Water Res., 2014, 63, 102 CrossRef CAS PubMed.
- D.-Q. Zhang, T.-Y. Sun, X.-F. Yu, Y. Jia, M. Chen, J.-H. Wang, H. Huang and P. K. Chu, Mater. Res. Bull., 2014, 52, 122 CrossRef CAS.
- R. Gupta and G. U. Kulkarni, ChemSusChem, 2011, 4, 737 CrossRef CAS PubMed.
- H. Qian, L. A. Pretzer, J. C. Velazquez, Z. Zhao and M. S. Wong, J. Chem. Technol. Biotechnol., 2013, 88, 735 CrossRef CAS.
- C. Wang and C. Yu, Rev. Anal. Chem., 2013, 32, 1 CrossRef.
- H. Dong, G. Zeng, L. Tang, C. Fan, C. Zhang, X. He and Y. He, Water Res., 2015, 79, 128–146 CrossRef CAS PubMed.
- I. Ilisz, A. Dombi, K. Mogyorosi and I. Dekany, Colloids Surf., A, 2004, 230, 89 CrossRef.
- M. Rastgar, A. R. Zolfaghari, H. R. Mortaheb, H. Sayahi and H. R. Naderi, J. Adv. Oxid. Technol., 2013, 16, 292 CAS.
- V. Vaiano, O. Sacco, D. Sannino and P. Ciambelli, Appl. Catal., B, 2015, 170–171, 153 CrossRef CAS.
- S. Filice, D. D'Angelo, S. Libertino, I. Nicotera, V. Kosma, V. Privitera and S. Scalese, Carbon, 2015, 82, 489 CrossRef CAS.
- A. Hu, R. Liang, X. Zhang, S. Kurdi, D. Luong, H. Huang, P. Peng, E. Marzbanrad, K. D. Oakes, Y. Zhou and M. R. Servos, J. Photochem. Photobiol., A, 2013, 256, 7 CrossRef CAS.
- X. Li, Y. Chen, X. Hu, Y. Zhang and L. Hu, J. Membr. Sci., 2014, 471, 118 CrossRef CAS.
- A. M. Asiri, M. S. Al-Amoudi, S. A. Bazaid, A. A. Adam, K. A. Alamry and S. Anandan, J. Saudi Chem. Soc., 2014, 18, 155 CrossRef.
- R. Liang, A. Hu, W. Li and Y. N. Zhou, J. Nanopart. Res., 2013, 15, 1990 CrossRef.
- S. Murgolo, F. Petronella, R. Ciannarella, R. Comparelli, A. Agostiano, M. L. Curri and G. Mascolo, Catal. Today, 2015, 240, 114 CrossRef CAS.
- V. S. Priya and L. Philip, Int. J. Environ. Sci. Dev., 2015, 6, 286 CrossRef.
- J. Senthilnathan and L. Philip, Water, Air, Soil Pollut., 2010, 210, 143 CrossRef CAS.
- P. Singla, M. Sharma, O. P. Pandey and K. Singh, Appl. Phys. A: Mater. Sci. Process., 2014, 116, 371–378 CrossRef CAS.
- F. U. Jian-Feng, J. I. Min and A. N. Ding-Nian, J. Environ. Sci., 2005, 17, 942 Search PubMed.
- A. A. Abd Elrady, M. A. Salman Hassan and A. M. Kamal, Nanosci. Nanotechnol., 2013, 3, 90 Search PubMed.
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Environ. Sci. Technol., 2009, 43, 597 CrossRef CAS PubMed.
- A. Hu, X. Zhang, K. D. Oakes, P. Peng, Y. N. Zhou and M. R. Servos, J. Hazard. Mater., 2011, 189, 278 CrossRef CAS PubMed.
- A. Hu, X. Zhang, D. Luong, K. D. Oakes, M. R. Servos, R. Liang, S. Kurdi, P. Peng and Y. Zhou, Waste Biomass Valorization, 2012, 3, 443 CrossRef CAS.
- Water Treatment, in Waste Water Treatment Methods, ed. W. Elshorbagy and R. K. Chowdhury, InTech, 2013, ch. 4 ISBN: 978-953-51-0928-0 Search PubMed.
- A. L. Giraldo, G. A. Penuela, R. A. Torres-Palma, N. J. Pino, R. A. Palominos and H. D. Mansilla, Water Res., 2010, 44, 5158 CrossRef CAS PubMed.
- S. Lazaro-Navas, S. Prashar, M. Fajardo and S. Gomez-Ruiz, J. Nanopart. Res., 2015, 17, 94 CrossRef.
- T. I. Nkambule, A. T. Kuvarega, R. W. M. Krause, J. Haarhoff and B. B. Mamba, Environ. Sci. Pollut. Res., 2012, 19, 4120 CrossRef CAS PubMed.
- J. Grzechulska and A. W. Morawski, Appl. Catal., B, 2003, 46, 415–419 CrossRef CAS.
- M. Lazar, S. Varghese and S. Nair, Catalysts, 2012, 2, 572–601 CrossRef CAS.
- M. S. Vohra and K. Tanaka, Water Res., 2003, 37, 3992–3996 CrossRef CAS PubMed.
- T. Tanaka, K. Teramura, T. Yamamoto, S. Takenaka, S. Yoshida and T. Funabiki, J. Photochem. Photobiol., A, 2002, 148, 277–281 CrossRef CAS.
- E. P. Reddy, L. Davydov and P. Smirniotis, Appl. Catal., B, 2003, 42, 1–11 CrossRef CAS.
- C. Ooka, H. Yoshida, M. Horio, K. Suzuki and T. Hattori, Appl. Catal., B, 2003, 41, 313–321 CrossRef CAS.
- J. Araña, J. M. Doña-Rodríguez, E. Tello Rendón, C. Garriga, I. Cabo, O. González-Díaz, J. A. Herrera-Melián, J. Pérez-Peña, G. Colón and J. A. Navío, Appl. Catal., B, 2003, 44, 161–172 CrossRef.
- C. H. Ao and S. C. Lee, Appl. Catal., B, 2003, 44, 191–205 CrossRef CAS.
- G. Balasubramanian, D. D. Dionysiou, M. T. Suidan, I. Baudin and J. M. Laîné, Appl. Catal., B, 2004, 47, 73–84 CrossRef CAS.
- N. Baram, D. Starosvetsky, J. Starosvetsky, M. Epshtein, R. Armon and Y. Ein-Eli, Electrochim. Acta, 2009, 54, 3381–3386 CrossRef CAS.
- G. R. M. Echavia, F. Matzusawa and N. Negishi, Chemosphere, 2009, 76, 595–600 CrossRef CAS PubMed.
- N. Miranda-García, S. Suárez, B. Sánchez, J. M. Coronado, S. Malato and M. I. Maldonado, Appl. Catal., B, 2011, 103, 294–301 CrossRef.
- B. Tryba, J. Hazard. Mater., 2008, 151, 623–627 CrossRef CAS PubMed.
- A. H. Fostier, M. D. S. S. Pereira, S. Rath and J. R. Guimarães, Chemosphere, 2008, 72, 319–324 CrossRef CAS PubMed.
- G. R. R. A. Kumara, F. M. Sultanbawa, V. P. S. Perera, I. R. M. Kottegoda and K. Tennakone, Sol. Energy Mater. Sol. Cells, 1999, 58, 167–171 CrossRef CAS.
- J. C. Lee, M. S. Kim and B. W. Kim, Water Res., 2002, 36, 1776–1782 CrossRef CAS PubMed.
- I.-H. Cho, J.-H. Park and Y.-G. Kim, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2005, 40, 1033–1044 CrossRef CAS.
- J. Senthilnathan and L. Philip, J. Environ. Sci. Health, Part B, 2009, 44, 262–270 CrossRef CAS PubMed.
- R. van Grieken, J. Marugán, C. Sordo, P. Martínez and C. Pablos, Appl. Catal., B, 2009, 93, 112–118 CrossRef CAS.
- W. Zhang and D. W. Elliott, Biorem. J., 2006, 16, 7–21 CrossRef.
- Z. M. Xiu, Z. H. Jin, T. L. Li, S. Mahendra, G. V. Lowry and P. J. J. Alvarez, Bioresour. Technol., 2010, 101, 1141–1146 CrossRef CAS PubMed.
- L. Wu, M. Shamsuzzoha and S. M. C. Ritchie, J. Nanopart. Res., 2005, 7, 469–476 CrossRef CAS.
- C. B. Wang and W. X. Zhang, Environ. Sci. Technol., 1997, 31, 2154–2156 CrossRef CAS.
- H. Song and E. R. Carraway, Appl. Catal., B, 2008, 78, 53–60 CrossRef CAS.
- Y. Liu, T. Phenrat and G. V. Lowry, Environ. Sci. Technol., 2007, 41, 7881–7887 CrossRef CAS PubMed.
- Y. Liu, S. A. Majetich, R. D. Tilton, D. S. Sholl and G. V. Lowry, Environ. Sci. Technol., 2005, 39, 1338–1345 CrossRef CAS PubMed.
- R. J. Barnes, O. Riba, M. N. Gardner, A. C. Singer, S. A. Jackman and I. P. Thompson, Chemosphere, 2010, 80, 554–562 CrossRef CAS PubMed.
- H. Kim, H. J. Hong, J. Jung, S. H. Kim and J. W. Yang, J. Hazard. Mater., 2010, 176, 1038–1043 CrossRef CAS PubMed.
- M. Zhang, F. He, D. Zhao and X. Hao, Water Res., 2011, 45, 2401–2414 CrossRef CAS PubMed.
- D. W. Elliott and W. X. Zhang, Environ. Sci. Technol., 2001, 35, 4922–4926 CrossRef CAS PubMed.
- H. Tian, J. Li, Z. Mu, L. Li and Z. Hao, Sep. Purif. Technol., 2009, 66, 84–89 CrossRef CAS.
- S. H. Joo and D. Zhao, Chemosphere, 2008, 70, 418–425 CrossRef CAS PubMed.
- D. W. Elliott, H.-L. Lien and W.-X. Zhang, J. Environ. Eng., 2009, 135, 317–324 CrossRef CAS.
- A. N. Bezbaruah, J. M. Thompson and B. J. Chisholm, J. Environ. Sci. Health, Part B, 2009, 44, 518–524 CrossRef CAS PubMed.
- Z. Xiong, D. Zhao and G. Pan, Water Res., 2007, 41, 3497–3505 CrossRef CAS PubMed.
- R. Cheng, J. Wang and W. Zhang, Front. Environ. Sci. Eng. China, 2008, 2, 103–108 CrossRef.
- S. Machado, W. Stawiński, P. Slonina, A. R. Pinto, J. P. Grosso, H. P. A. Nouws, J. T. Albergaria and C. Delerue-Matos, Sci. Total Environ., 2013, 461–462, 323–329 CrossRef CAS PubMed.
- Y. T. Lin, C. H. Weng and F. Y. Chen, Sep. Purif. Technol., 2008, 64, 26–30 CrossRef CAS.
- S. H. Joo, A. J. Feitz and T. D. Waite, Environ. Sci. Technol., 2004, 38, 2242–2247 CrossRef CAS PubMed.
- C. Su, R. W. Puls, T. A. Krug, M. T. Watling, S. K. O'Hara, J. W. Quinn and N. E. Ruiz, Water Res., 2012, 46, 5071–5084 CrossRef CAS PubMed.
- F. He, D. Zhao and C. Paul, Water Res., 2010, 44, 2360–2370 CrossRef CAS PubMed.
- S. Ozcan, A. Tor and M. E. Aydin, J. AOAC Int., 2012, 95, 1343–1349 CrossRef CAS PubMed.
- G. Guan, L. Yang, Q. Mei, K. Zhang, Z. Zhang and M. Y. Han, Anal. Chem., 2012, 84, 9492–9497 CrossRef CAS PubMed.
- X. Zheng, L. He, Y. Duan, X. Jiang, G. Xiang, W. Zhao and S. Zhang, J. Chromatogr. A, 2014, 1358, 39–45 CrossRef CAS PubMed.
- Z. He, P. Wang, D. Liu and Z. Zhou, Talanta, 2014, 127, 1–8 CrossRef CAS PubMed.
- D. H. Chen and S. H. Huang, Process Biochem., 2004, 39, 2207–2211 CrossRef CAS.
- H. Niu, D. Zhang, S. Zhang, X. Zhang, Z. Meng and Y. Cai, J. Hazard. Mater., 2011, 190, 559–565 CrossRef CAS PubMed.
- X. Ge, W. Zhang, Y. Lin and D. Du, Biosens. Bioelectron., 2013, 50, 486–491 CrossRef CAS PubMed.
- A. Z. M. Badruddoza, G. S. S. Hazel, K. Hidajat and M. S. Uddin, Colloids Surf., A, 2010, 367, 85–95 CrossRef CAS.
- G. R. Chaudhary, P. Saharan, A. Kumar, S. K. Mehta, S. Mor and A. Umar, J. Nanosci. Nanotechnol., 2013, 13, 3240–3246 CrossRef CAS PubMed.
- X. Zhang, P. Zhang, Z. Wu, L. Zhang, G. Zeng and C. Zhou, Colloids Surf., A, 2013, 435, 85–90 CrossRef CAS.
- F. Ge, H. Ye, M.-M. Li and B.-X. Zhao, Chem. Eng. J., 2012, 198–199, 11–17 CrossRef CAS.
- Z. Zhou, S. Lin, T. Yue and T. C. Lee, J. Food Eng., 2014, 126, 133–141 CrossRef CAS.
- M. H. Do, N. H. Phan, T. D. Nguyen, T. T. S. Pham, V. K. Nguyen, T. T. T. Vu and T. K. P. Nguyen, Chemosphere, 2011, 85, 1269–1276 CrossRef CAS PubMed.
- Z. Zhang and J. Kong, J. Hazard. Mater., 2011, 193, 325–329 CrossRef CAS PubMed.
- S. Qu, F. Huang, S. Yu, G. Chen and J. Kong, J. Hazard. Mater., 2008, 160, 643–647 CrossRef CAS PubMed.
- A. Afkhami, M. Saber-Tehrani and H. Bagheri, Desalination, 2010, 263, 240–248 CrossRef CAS.
- K. M. Paknikar, V. Nagpal, A. V. Pethkar and J. M. Rajwade, Sci. Technol. Adv. Mater., 2005, 6, 370–374 CrossRef CAS.
- D. Du, X. Ye, J. Zhang, Y. Zeng, H. Tu, A. Zhang and D. Liu, Electrochem. Commun., 2008, 10, 686–690 CrossRef CAS.
- G. Liu and Y. Lin, Anal. Chem., 2005, 77, 5894–5901 CrossRef CAS PubMed.
- J. Fei, Y. Cui, X. Yan, W. Qi, Y. Yang, K. Wang, Q. He and J. Li, Adv. Mater., 2008, 20, 452–456 CrossRef.
- G. Moussavi and M. Mahmoudi, J. Hazard. Mater., 2009, 168, 806–812 CrossRef CAS PubMed.
- R. Ullah and J. Dutta, J. Hazard. Mater., 2008, 156, 194–200 CrossRef CAS PubMed.
- R. K. Upadhyay, M. Sharma, D. K. Singh, S. S. Amritphale and N. Chandra, Sep. Purif. Technol., 2012, 88, 39–45 CrossRef CAS.
- T. M. Scown, R. van Aerle and C. R. Tyler, Crit. Rev. Toxicol., 2010, 40, 653–670 CrossRef CAS PubMed.
- H. Weinberg, A. Galyean and M. Leopold, TrAC, Trends Anal. Chem., 2011, 30, 72–83 CrossRef CAS.
- M. N. Moore, Environ. Int., 2006, 32, 967–976 CrossRef CAS PubMed.
- R. Barrena, E. Casals, J. Colón, X. Font, A. Sánchez and V. Puntes, Chemosphere, 2009, 75, 850–857 CrossRef CAS PubMed.
- E. Navarro, A. Baun, R. Behra, N. B. Hartmann, J. Filser, A. J. Miao, A. Quigg, P. H. Santschi and L. Sigg, Ecotoxicology, 2008, 17, 372–386 CrossRef CAS PubMed.
- E. J. Petersen, S. A. Diamond, A. J. Kennedy, G. G. Goss, K. Ho, J. Lead, S. K. Hanna, N. B. Hartmann, K. Hund-Rinke, B. Mader, N. Manier, P. Pandard, E. R. Salinas and P. Sayre, Environ. Sci. Technol., 2015, 49, 9532–9547 CrossRef CAS PubMed.
- K. Tiede, M. Hassellöv, E. Breitbarth, Q. Chaudhry and A. B. A. Boxall, J. Chromatogr. A, 2009, 1216, 503–509 CrossRef CAS PubMed.
- A. Simon-Deckers, S. Loo, M. Mayne-L'Hermite, N. Herlin-Boime, N. Menguy, C. Reynaud, B. Gouget and M. Carriere, Environ. Sci. Technol., 2009, 43, 8423–8429 CrossRef CAS PubMed.
- R. D. Handy, F. Von Der Kammer, J. R. Lead, M. Hassellöv, R. Owen and M. Crane, Ecotoxicology, 2008, 17, 287–314 CrossRef CAS PubMed.
- N. Lewinski, V. Colvin and R. Drezek, Small, 2008, 4, 26–49 CrossRef CAS PubMed.
- A. Albanese and W. C. W. Chan, ACS Nano, 2011, 5, 5478–5489 CrossRef CAS PubMed.
- A. Menard, D. Drobne and A. Jemec, Environ. Pollut., 2011, 159, 677–684 CrossRef CAS PubMed.
- K. Van Hoecke, K. A. C. De Schamphelaere, Z. Ali, F. Zhang, A. Elsaesser, P. Rivera-Gil, W. J. Parak, G. Smagghe, C. V. Howard and C. R. Janssen, Nanotoxicology, 2011, 1–11 Search PubMed.
- B. Trouiller, R. Reliene, A. Westbrook, P. Solaimani and R. H. Schiestl, Cancer Res., 2009, 69, 8784–8789 CrossRef CAS PubMed.
- Q. Fang, X. Shi, L. Zhang, Q. Wang, X. Wang, Y. Guo and B. Zhou, J. Hazard. Mater., 2015, 283, 897 CrossRef CAS PubMed.
- T. E. A. Chalew, G. S. Ajmani, H. Huang and K. J. Schwab, Environ. Health Perspect., 2013, 121, 1161 Search PubMed.
- H. Ma, P. L. Williams and S. A. Diamond, Environ. Pollut., 2013, 172, 76–85 CrossRef CAS PubMed.
- K. Van Hoecke, J. T. K. Quik, J. Mankiewicz-Boczek, K. A. C. De Schamphelaere, A. Elsaesser, P. Van Der Meeren, C. Barnes, G. Mckerr, C. V. Howard, D. Van De Meent, K. Rydzyński, K. A. Dawson, A. Salvati, A. Lesniak, I. Lynch, G. Silversmit, B. De Samber, L. Vincze and C. R. Janssen, Environ. Sci. Technol., 2009, 43, 4537–4546 CrossRef CAS PubMed.
- D. Lin and B. Xing, Environ. Sci. Technol., 2008, 42, 5580–5585 CrossRef CAS PubMed.
- X. Ma, J. Geiser-Lee, Y. Deng and A. Kolmakov, Sci. Total Environ., 2010, 408, 3053–3061 CrossRef CAS PubMed.
- D. Lin and B. Xing, Environ. Pollut., 2007, 150, 243–250 CrossRef CAS PubMed.
- P. Miralles, T. L. Church and A. T. Harris, Environ. Sci. Technol., 2012, 46, 9224–9239 CrossRef CAS PubMed.
- K. D. Grieger, A. Fjordbøge, N. B. Hartmann, E. Eriksson, P. L. Bjerg and A. Baun, J. Contam. Hydrol., 2010, 118, 165–183 CrossRef CAS PubMed.
- X. Ma, A. Gurung and Y. Deng, Sci. Total Environ., 2013, 443, 844–849 CrossRef CAS PubMed.
- Y. S. El-Temsah and E. J. Joner, Environ. Toxicol., 2012, 27, 42–49 CrossRef CAS PubMed.
- T. Phenrat, T. C. Long, G. V. Lowry and B. Veronesi, Environ. Sci. Technol., 2009, 43, 195–200 CrossRef CAS PubMed.
- A. L. Dale, E. A. Casman, G. V. Lowry, J. R. Lead, E. Viparelli and M. Baalousha, Environ. Sci. Technol., 2015, 49, 2587–2593 CrossRef CAS PubMed.
- G. Lowry and E. Casman, Nanomaterials, 2009, 125–137 Search PubMed.
- S. Diegoli, A. L. Manciulea, S. Begum, I. P. Jones, J. R. Lead and J. A. Preece, Sci. Total Environ., 2008, 402, 51–61 CrossRef CAS PubMed.
- C. O. Hendren, A. R. Badireddy, E. Casman and M. R. Wiesner, Sci. Total Environ., 2013, 449, 418–425 CrossRef CAS PubMed.
- K. Yin, I. M. C. Lo, H. Dong, P. Rao and M. S. H. Mak, J. Hazard. Mater., 2012, 227–228, 118–125 CrossRef CAS PubMed.
- A. Praetorius, M. Scheringer and K. Hungerbühler, Environ. Sci. Technol., 2012, 46, 6705–6713 CrossRef CAS PubMed.
- S. K. Brar, M. Verma, R. D. Tyagi and R. Y. Surampalli, Waste Manage., 2010, 30, 504–520 CrossRef CAS PubMed.
- C. Levard, E. M. Hotze, B. P. Colman, A. L. Dale, L. Truong, X. Y. Yang, A. J. Bone, G. E. Brown, R. L. Tanguay, R. T. Di Giulio, E. S. Bernhardt, J. N. Meyer, M. R. Wiesner and G. V. Lowry, Environ. Sci. Technol., 2013, 47, 13440–13448 CrossRef CAS PubMed.
- C. Levard, E. M. Hotze, G. V. Lowry and G. E. Brown, Environ. Sci. Technol., 2012, 46, 6900–6914 CrossRef CAS PubMed.
- I. Bhatt and B. N. Tripathi, Chemosphere, 2011, 82, 308–317 CrossRef CAS PubMed.
- S. Tresintsi, K. Simeonidis, M. Katsikini, E. C. Paloura, G. Bantsis and M. Mitrakas, J. Hazard. Mater., 2014, 265, 217–225 CrossRef CAS PubMed.
- S.-A. Lee, K.-H. Choo, C.-H. Lee, H.-I. Lee, T. Hyeon, W. Choi and H.-H. Kwon, Ind. Eng. Chem. Res., 2001, 40, 1712 CrossRef CAS.
- R. J. Honda, V. Keene, L. Daniels and S. L. Walker, Environ. Eng. Sci., 2014, 31, 127 CrossRef CAS PubMed.
- Z. Xiao-hong, H. Bao-cheng, Z. Tao, L. Yan-chen and S. Han-chang, Chemosphere, 2015, 119, 568 CrossRef PubMed.
- G. D. Moeser, K. A. Roach, W. H. Green, T. A. Hatton and P. E. Laibinis, AIChE J., 2004, 50, 2835–2848 CrossRef CAS.
- A. Ditsch, S. Lindenmann, P. E. Laibinis, D. I. C. Wang and T. A. Hatton, Ind. Eng. Chem. Res., 2005, 44, 6824–6836 CrossRef CAS.
- J. Sun, R. Xu, Y. Zhang, M. Ma and N. Gu, J. Magn. Magn. Mater., 2007, 312, 354–358 CrossRef CAS.
- European Standard EN 12457-4, 2002. Characterization of waste – leaching – compliance test for leaching of granular waste materials and sludge – Part 1: One stage batch test at a liquid to solid ratio of 10 L/kg for materials with high solid content and with particle size below 10 mm (without or with size reduction).
- U.S. EPA, Test methods for evaluating solid wastes, Toxicity Characteristic Leaching Procedure (TCLP), Method 1311 SW-846, 3rd. edn., Washington, DC, USA, 1986 Search PubMed.
- S. C. Bolyard, D. R. Reinhart and S. Santra, Environ. Sci. Technol., 2013, 47, 8114–8122 CAS.
| This journal is © The Royal Society of Chemistry 2016 |