Synthesis of 3-aryl-1H-indazoles via iridium-catalysed C–H borylation and Suzuki–Miyaura coupling†
Ben A. Egan and
Paul M. Burton*
Syngenta Ltd., Jealott's Hill International Research Centre, Bracknell, Berkshire, RG42 6EY, UK. E-mail: Paul.Burton@syngenta.com
First published on 11th June 2014
Abstract
The regioselective iridium-catalysed C3-borylation of 1H-indazoles has been achieved. Subsequent Suzuki–Miyaura coupling of the boronate esters with aryl chlorides, bromides and iodides affords 3-aryl-1H-indazoles in good yields.
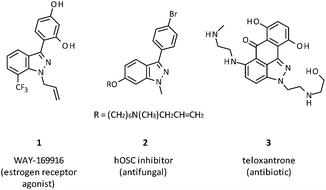
Indazoles are rare in nature but are an important class of heterocycles in medicinal and agrochemical discovery programs due to their isosterism with indoles.1 In particular, 3-aryl-1H-indazoles feature in the estrogen receptor agonist 1,2 the antifungal agent 2 (ref. 3) and the antibiotic teloxantrone (3) (Fig. 1).
Previously reported approaches to 3-aryl-1H-indazoles include the condensation of hydrazines with fluorobenzophenones,2–4 diazomethane cycloaddition with benzynes,5 C–H amidation of aryl hydrazones6 or catalytic N–N bond formation.7 However, these methods all suffer from requiring the use of either explosive or toxic reagents. In addition, the diversity of substitution on both the indazole and the aryl group must be introduced relatively early on in the synthetic sequence when employing these conditions.
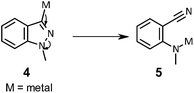
The synthesis of 3-aryl-1H-indazoles via the cross coupling of 1H-indazoles metalated in the 3-position to aryl halides is particularly attractive from a discovery-chemistry viewpoint because it enables the late-stage attachment of an indazole moiety to an aryl halide intermediate, thus allowing the rapid access to a variety of substitution patterns. However, this reaction is hampered by the propensity of a 3-metallated, 1H-indazole 4 to undergo ring-opening to the corresponding 2-aminophenylnitrile 5 (Scheme 1).
Knochel and co-workers reported an elegant solution to this problem in 2012 by zincation at the 3-position of 1-alkyl-1H-indazoles employing TMPZnCl, followed by subsequent Negishi cross-coupling with aryl iodides.8 Subsequent reports from the Itami, Yu and Guillaumet groups have demonstrated the direct C–H arylation of 1H-indazoles with aryl iodides.9–11 However, the conditions reported require high temperatures (up to 150 °C) and they fail when the cheaper and more readily available aryl chlorides are used as the coupling partner. Furthermore, the examples of aryl halides reported are limited to those unsubstituted at the ortho positions.
In an attempt to broaden the substrate-scope of 1H-indazole C3-arylation to include both aryl chlorides and bromides and also aryl halides that possess one or two ortho substituents, we turned to the Ir-catalysed C–H borylation reaction,12 followed by Suzuki–Miyaura cross coupling. The results of this investigation are reported herein.
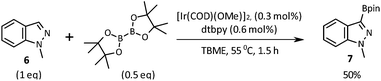
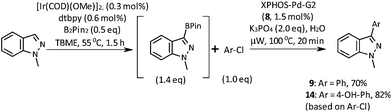
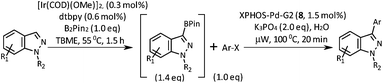
Our initial investigations were focussed on the borylation of 1-methyl-1H-indazole 6 employing the [Ir(OMe)(COD)]2/di-tert-butyl bipyridine (dtbpy) catalyst system pioneered by Ishiyama, Takagi, Hartwig and Miyaura and subsequently applied by other researchers to the C–H borylation of a wide variety of aromatics and heteroaromatics over the last decade.12–15 We were pleased to observe that when employing 0.5 equivalents of bis(pinacolato)diboron (B2Pin2) in tert-butyl methyl ether (TBME) at 55 °C for 1.5 h, selective borylation at the 3-position of 6 gave 7 in 50% yield after chromatographic purification (Scheme 2). Boronate 7 was stored in a freezer without evidence of degradation for 6 months. The high C3-regioselectivity can be attributed to a combination of steric factors and the C–H acidity at this position.16 To the best of our knowledge, 3-boronic esters of 1-alkyl-1H-indazoles have not previously been reported in the literature.17
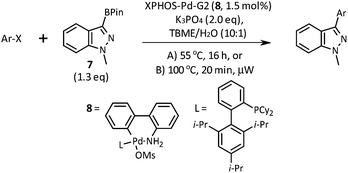
A short screen of conditions for the Suzuki–Miyaura arylation of boronic ester 7 was concluded with the discovery that the Buchwald second-generation XPhos precatalyst 8 in TBME and aqueous potassium phosphate effects the cross coupling with most of the aryl halides we tried (Table 1).18 This cross coupling could be performed thermally (55 °C for 16 h, conditions A) or under microwave irradiation (100 °C for 20 min, conditions B).
| Entry | Ar–X | Product | Yielda (%) |
|---|---|---|---|
a Isolated yields after chromatography.b Reaction conditions: a solution of the aryl halide (1.0 eq., typically 0.50 mmol), 7 (1.3 eq.), XPhos-Pd-G2 (0.015 eq.) and K3PO4 (2.0 eq.) in TBME–H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, typically 0.1 M) was heated in a RBF at 55 °C for 16 h (conditions A) or in a microwave reactor at 100 °C for 20 min (conditions B). 1, typically 0.1 M) was heated in a RBF at 55 °C for 16 h (conditions A) or in a microwave reactor at 100 °C for 20 min (conditions B). |
|||
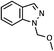
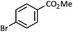
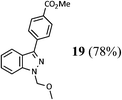
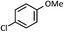
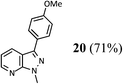
| 1 |  |
 |
72 (A), 74 (B) |
| 2 | 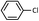 |
9 | 71 (A), 66 (B) |
| 3 | 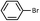 |
9 | 86 (A), 68 (B) |
| 4 | 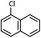 |
 |
63 (A), 97 (B) |
| 5 | 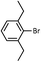 |
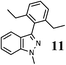 |
90 (B) |
| 6 | 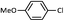 |
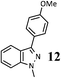 |
76 (B) |
| 7 | 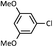 |
 |
91 (B) |
| 8 | 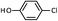 |
 |
84 (B) |
| 9 | 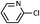 |
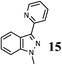 |
87 (B) |
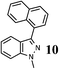
| 10 | 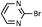 |
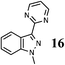 |
97 (B) |
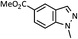
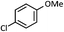
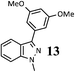
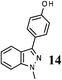
As shown in Table 1, aryl iodides, chlorides and bromides all react in good yields (entries 1–3). The coupling is tolerant of electron donating substituents (entries 6 and 7). Highly hindered electrophiles such as 2,6-diethylphenyl bromide also perform well (entry 5). The reaction is also tolerant of free alcohols (entry 8) and will work on 2-pyridinyl and 2-pyrimidyl halides (entries 9 and 10).
We next turned our attention to combining the indazole borylation and Suzuki–Miyaura coupling into a one-pot procedure.19 We were pleased to find that this was possible without a work-up or solvent swap.20 It was possible to simply monitor the borylation by GCMS and, once this indicated the consumption of starting material, the palladium precatalyst 8, the aryl halide, potassium phosphate and water could be added to the reaction mixture (Scheme 3).
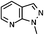
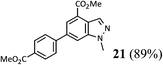
We next investigated varying the substitution on the indazole employing this one-pot procedure (Table 2). The commercially available indazole bearing a methyl ester at the 5-position (entry 1) was smoothly borylated using the conditions described in Scheme 3. When using more highly functionalised indoles, we found it preferential to use 1 equivalent of B2Pin2 for the borylation, as doing so increased the reaction rate (entries 1, 2, 3 and 5). When a methyl ester is in the 4-position, borylation is instead directed to the 6-position (entry 5), presumably due to the steric bulk of the 4-substituent. 1N-methoxymethyl protected indazole also performs well in the 3-borylation-Suzuki sequence (entries 2 and 3). Furthermore, the azaindazole in entry 4 is well tolerated.21
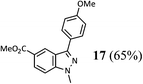
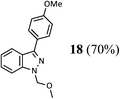
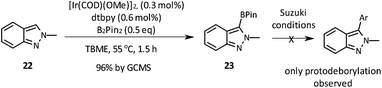
Finally, the borylation of the isomeric 2-methyl-2H-indazole 22 was attempted (Scheme 4). Despite smooth borylation at the 3-position, the resulting pinacol boronate 23 proved to be unstable in our hands. All attempts to employ it in a Suzuki–Miyaura reaction employing either the Pd–XPhos precatalyst or other standard catalysts for this reaction (e.g. PdCl2(dppf)/CsF or Pd(OAc)2/SPhos/K3PO4) resulted in proto-deborylation and no desired product formation.
In conclusion, we have demonstrated a novel route to 3-aryl-1H-indazoles employing Ir-catalysed C–H borylation at the 3-position, followed by Suzuki–Miyaura coupling with aryl halides. In particular, the wide substrate scope of the aryl halide coupling partner in combination with the high yielding and operationally simple procedure allows a diverse selection of 3-aryl-1H-indazoles to be rapidly furnished as part of a discovery chemistry program.
Notes and references
- N. A. Ali, B. A. Dar, V. Pradhan and M. Farooqui, Mini-Rev. Med. Chem., 2013, 1792 Search PubMed.
- J. B. Bruning, A. A. Parent, G. Gil, M. Zhao, J. Nowak, M. C. Pace, C. L. Smith, P. V. Afonine, P. D. Adams, J. A. Katzenellenbogen and K. W. Nettles, Nat. Chem. Biol., 2010, 837 CrossRef CAS PubMed.
- H. Dehmlow, J. D. Aebi, S. Jolidon, Y.-H. Ji, E. M. von der Mark, J. Himber and O. H. Morand, J. Med. Chem., 2003, 3354 CrossRef CAS PubMed.
- (a) R. J. Steffan, E. Matelan, M. A. Ashwell, W. J. Moore, W. R. Solvibile, E. Trybulski, C. C. Chadwick, S. Chippari, T. Kenney, A. Eckert, L. Borges-Marcucci, J. C. Keith, Z. Xu, L. Mosyak and D. C. Harnish, J. Med. Chem., 2004, 6435 CrossRef CAS PubMed; (b) I. A. Murray, G. Krishnegowda, B. C. DiNatale, C. Flaveny, C. Chiaro, J.-M. Lin, A.-K. Sharma, S. Amin and G. H. Perdew, Chem. Res. Toxicol., 2010, 955 CrossRef CAS PubMed.
- (a) T. Jin and Y. Yamamoto, Angew. Chem., Int. Ed., 2007, 3323 CrossRef CAS PubMed; (b) C. Spiteri, S. Keeling and J. E. Moses, Org. Lett., 2010, 3368 CrossRef CAS PubMed; (c) P. Li, J. Zhao, C. Wu, R. C. Larock and F. Shi, Org. Lett., 2011, 3340 CrossRef CAS PubMed.
- (a) K. Inamoto, T. Saito, M. Katsuno, T. Sakamoto and K. Hiroya, Org. Lett., 2007, 2931 CrossRef CAS PubMed; (b) T. Zhang and W. Bao, J. Org. Chem., 2013, 1317 CrossRef CAS PubMed; (c) X. Li, L. He, H. Chen, W. Wu and H. Jiang, J. Org. Chem., 2013, 3636 CrossRef CAS PubMed; (d) I. Thome, C. Besson, T. Kleine and C. Bolm, Angew. Chem., Int. Ed., 2013, 7509 CrossRef CAS PubMed.
- (a) D.-G. Yu, M. Suri and F. Glorius, J. Am. Chem. Soc., 2013, 8802 CrossRef CAS PubMed.
- A. Unsinn and P. Knochel, Chem. Commun., 2012, 2680 RSC.
- K. Hattori, K. Yamaguchi, J. Yamaguchi and K. Itami, Tetrahedron, 2012, 7605 CrossRef CAS PubMed.
- M. Ye, A. J. F. Edmunds, J. A. Morris, D. Sale, Y. Zhang and J.-Q. Yu, Chem. Sci., 2013, 2374 RSC.
- A. Ben-Yahia, M. Naas, S. E. Kazzouli, E. M. Essassi and G. Guillaumet, Eur. J. Org. Chem., 2012, 7075 CrossRef CAS.
- For a review on C–H borylation, see: I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy and J. F. Hartwig, Chem. Rev., 2010, 890 CrossRef CAS PubMed.
- For the C–H borylation of arenes, see: (a) T. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi and J. F. Hartwig, J. Am. Chem. Soc., 2002, 390 CrossRef CAS PubMed; (b) T. Ishiyama, J. Takagi, J. F. Hartwig and N. Miyaura, Angew. Chem., Int. Ed., 2002, 3056 CrossRef CAS; (c) T. M. Boller, J. M. Murphy, M. Hapke, T. Ishiyama, N. Miyaura and J. F. Hartwig, J. Am. Chem. Soc., 2005, 14263 CrossRef CAS PubMed; (d) D. N. Coventry, A. S. Batsanov, A. E. Goeta, J. A. K. Howard, T. B. Marder and R. N. Perutz, Chem. Commun., 2005, 2172 RSC; (e) T. A. Boebel and J. F. Hartwig, J. Am. Chem. Soc., 2008, 7534 CrossRef CAS PubMed; (f) A. Joliton and E. M. Carreira, Org. Lett., 2013, 5147 CrossRef CAS PubMed.
- For the borylation of heteroarenes, see: (a) J. Takagi, K. Sato, J. F. Hartwig, T. Ishiyama and N. Miyaura, Tetrahedron Lett., 2002, 5649 CrossRef CAS; (b) T. Ishiyama, J. Takagi, Y. Yonekawa, J. F. Hartwig and N. Miyaura, Adv. Synth. Catal., 2003, 1103 CrossRef CAS; (c) I. A. I. Mkhalid, D. N. Coventry, D. Albesa-Jove, A. S. Batsanov, J. A. K. Howard, R. N. Perutz and T. B. Marder, Angew. Chem., Int. Ed., 2006, 489 CrossRef CAS PubMed; (d) S. Paul, G. A. Chotana, D. Holmes, R. C. Reichle, R. E. Maleczka Jr and M. R. Smith III, J. Am. Chem. Soc., 2006, 15552 CrossRef CAS PubMed; (e) W. F. Lo, H. M. Kaiser, A. Spannenberg, M. Beller and M. K. Tse, Tetrahedron Lett., 2007, 371 CrossRef CAS PubMed; (f) G. A. Chotana, V. A. Kallepalli, R. E. Maleczka Jr and M. R. Smith III, Tetrahedron, 2008, 6103 CrossRef CAS PubMed; (g) M. Klecka, R. Pohl, B. Klepetarova and M. Hocek, Org. Biomol. Chem., 2009, 866 RSC; (h) V. A. Kallepalli, F. Shi, S. Paul, E. N. Onyeozili, R. E. Maleczka Jr and M. R. Smith III, J. Org. Chem., 2009, 9199 CrossRef CAS PubMed; (i) S. Kawamorita, H. Ohmiya and M. Sawamura, J. Org. Chem., 2010, 3855 CrossRef CAS PubMed; (j) D. W. Robbins and J. F. Hartwig, Org. Lett., 2012, 4266 CrossRef CAS PubMed; (k) S. M. Preshlock, D. L. Plattner, P. E. Maligres, S. W. Krska, R. E. Maleczka Jr and M. R. Smith III, Angew. Chem., Int. Ed., 2013, 12915 CrossRef CAS PubMed; (l) M. A. Larsen and J. F. Hartwig, J. Am. Chem. Soc., 2014, 4287 CrossRef CAS PubMed.
- More recently, the electrophilic borylation of indoles has been reported: (a) A. Prokofjevs, J. W. Kampf and E. Vedejs, Angew. Chem., Int. Ed., 2011, 2098 CrossRef CAS PubMed; (b) T. Stahl, K. Müther, Y. Ohki, K. Tatsumi and M. Oestreich, J. Am. Chem. Soc., 2013, 10978 CrossRef CAS PubMed.
- H. Tajuddin, P. Harrisson, B. Bitterlich, J. C. Collings, N. Sim, A. S. Batsanov, M. S. Cheung, S. Kawamorita, A. C. Maxwell, L. Shukla, J. Morris, Z. Lin, T. B. Marder and P. G. Steel, Chem. Sci., 2012, 3505 RSC.
- There is one example of 1H-indazole, 3-pinacolboronate being used in a cross coupling reaction, though the reference does not explain how it was prepared: S. Huang, R. Li, P. J. Connolly, S. Emanuel, A. Fuentes-Pesquara, M. Adams, R. H. Gruninger, J. Seraj, S. A. Middleton, J. M. Davis and D. F. C. Moffat, Bioorg. Med. Chem. Lett., 2007, 2179 CrossRef CAS PubMed.
- T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2010, 14073 CrossRef CAS PubMed.
- For examples of one-pot borylation/Suzuki–Miyaura sequences, see: (a) J.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka Jr and M. 1R. Smith III, Science, 2002, 305 CrossRef CAS PubMed; (b) T. Ishiyama, Y. Nobuta, J. F. Hartwig and N. Miyaura, Chem. Commun., 2003, 2924 RSC; (c) T. Kikuchi, Y. Nobuta, J. Umeda, Y. Yamamoto, T. Ishiyama and N. Miyaura, Tetrahedron, 2008, 4967 CrossRef CAS PubMed; (d) P. Harrisson, J. Morris, P. G. Steel and T. B. Marder, Synlett, 2009, 147 CAS.
- See ref. 19d for the use of TBME in borylation/Suzuki–Miyaura sequences.
- 1H-indazole itself, lacking N-substitution, does not react under these conditions.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and all spectra of compounds synthesised. See DOI: 10.1039/c4ra04235b |
| This journal is © The Royal Society of Chemistry 2014 |