New strategies to prepare crystalline chalcogenides
Wei-Wei
Xiong
,
Guodong
Zhang
and
Qichun
Zhang
*
School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore. E-mail: qczhang@ntu.edu.sg
First published on 20th March 2014
Abstract
Because the structures and properties of crystalline metal chalcogenides are strongly dependent on their synthetic conditions, developing new methods to approach novel chalcogenides is highly desirable. This review will summarize the recent progress in growing crystalline chalcogenides through four new strategies: ionothermal, surfactant-thermal, hydrazine-thermal, and metal Zintl phase usage.
Introduction
In the past decades, extensive research interest has been raised in the investigation of crystalline chalcogenides due to their rich structure chemistry and unique properties ranging from classical photoelectric effect, thermoelectric behaviours, and photoluminescence to modern fast ion conductivity, selective ion exchange, photocatalysis, and gas adsorption.1–9 Obviously, novel chalcogenides with interesting structures and exciting properties strongly rely on the innovations in synthetic methodology. Traditionally, there are four methods to prepare chalcogenides: (1) high temperature solid-state processing, (2) room or lower temperature solution synthesis (e.g. diffusion, evaporation or recrystallization), (3) molten flux synthesis, and (4) hydro(solvo)thermal preparation. Generally, high temperature synthesis is used in preparing chalcogenides with high density10 and this type of reaction requires high thermal activation (T > 600 °C) to satisfy the solid–solid diffusion requirement. Normally, the products obtained under such synthetic conditions are usually the thermodynamically stable phases, and rational design of the desired products is very difficult owing to the limitation in kinetic control of the reactions. Lower temperature solution synthesis can solve the problems arising in solid-state reactions through using solvents as reaction media. Krebs reviewed the synthesis of a series of thio- and selenidometalates based on group 13–14 metals by reacting alkali metal chalcogenides with binary chalcogenides in water at ambient temperature.11 The crystalline structures of ternary M/14/16 chalcogenides that were isolated in water or methanol at room temperature have been summarized by Dehnen.12 Most of these compounds feature discrete anionic clusters with alkali metal cations, and the room temperature conditions allow the anionic clusters to remain intact in alkaline solution.13–16 As a consequence, self-assembly of extended chalcogenide networks from soluble precursors and transition metal ions can be performed in solution at room temperature.17–21 However, solution synthesis is heavily dependent on the usage of soluble molecular precursors, and the static room temperature prevents the thermodynamic control from the reactions.In order to involve both kinetic and thermodynamic control in the reaction process, two synthetic strategies (molten flux and hydro(solvo)thermal techniques), which involve molten salt or molecular solvents as strongly polarizing media at relatively low temperatures (T = 100–600 °C), have been introduced in the synthesis of chalcogenides.22,23 Compared with high temperature synthesis, the milder thermal regimes applied in these two synthetic methods allow the crystallization of metastable phases, which may not be possible at high temperature. In fact, molten flux has been proven to be a powerful synthetic technique that may be used to prepare new chalcogenides.24–29 Three types of molten salts have been employed as regents and solvents in the reaction system to lower the melting point: metal halides, metal chalcogenides, and alkali-metal polychalcogenides. Usually, low dimensional metal polychalcogenides are observed under molten flux conditions at intermediate temperatures, which are not accessible at high temperature due to the poor stability of polychalcogen ligands in those conditions. Several comprehensive reviews concerning the molten flux synthesis of polychalcogenides with unprecedented structures have been given by Kanatzidis and Kolis.30–32 Hydro(solvo)thermal synthesis represents a much milder and softer synthetic techniques by employing water, organic solvents, and organic amines as reaction media at relatively low temperature.33,34 The hydro(solvo)thermal synthesis of ternary and quaternary chalcogenides with alkali, alkaline earths, or organic cations has been discussed in several classical reviews.35,36 Since Bedard et al. used organic amines as structure-directing agents to prepare microporous chalcogenides under hydrothermal conditions,37 numerous research efforts have been directed towards employing organic amines as templates to direct the construction of open-framework chalcogenides.38–43 Such open-framework chalcogenides built up by tetrahedral clusters44–50 have been summarized by Feng et al.51 They also hydrothermally synthesized a series of microporous chalcogenides by using alkali and alkaline earth metal cations as templates.5 In addition, solvothermal synthesis can also be employed to construct extended inorganic–organic hybrid chalcogenides through covalently linking tetrahedral chalcogenide clusters with organic ligands.52–54 Besides these, the roles of chelating organic amines in the preparation of chalcogenides based on group 13–15 metals have been discussed in detail by Li and Dai in two review articles.55,56
It is clear that the exploration of new synthetic strategies always speeds up the advancement of crystalline chalcogenides. Besides popular approaches such as employing different reaction media in the synthesis to increase the solubility of the starting materials, lowering the reaction temperature to obtain metastable phases, optimally selecting soluble precursors for further assembling of extended networks, and using different organic or inorganic cations as templates to direct the formation of chalcogenides, recent research on ionothermal, surfactant-thermal, hydrazine-thermal, and metal Zintl phase usage to prepare novel chalcogenides also yielded very impressive results. In recent years, ionic liquids have been demonstrated as a new type of reaction medium (ionothermal) for synthesizing inorganic materials, such as zeolites, metal–organic frameworks, and chalcogenides.57–60 The specific ionic environment of ionic liquids not only increased the solubility of inorganic species, but also aided the formation of novel frameworks that are structurally directed by organic cations of ionic liquids. Therefore, ionothermal synthesis has been introduced as a new synthetic method for preparing crystalline chalcogenides and achieved considerable progress in chalcogenide chemistry.
Since surfactants have been extensively used as templates to control the size and shape of nanocrystals and the pore sizes and phases of porous materials,61–63 logically, surfactants could also act as reaction media to control the growth of bulk crystals. In fact, compared to ionic liquids, surfactants have several distinguishing features such as the Janus characteristics of surfactants containing both hydrophilic groups and hydrophobic groups which make it possible to increase the solubility of metal ions and organic ligands, negligible vapour pressure, and multifunctional properties (such as cationic, anionic, neutral, basic, acidic). For the first time, our group successfully prepared a variety of crystalline chalcogenides with novel structures and interesting properties through using various surfactants as reaction media.64–66 Furthermore, we extended this synthetic approach in the preparation of new metal–organic frameworks.67,68
In addition, this review will also cover the application of hydrazine and metal Zintl phases in preparing crystalline chalcogenides. It is well known that hydrazine is a basic solvent, has strong coordination with metal ions and displays high solubility to metal chalcogenides. Thus, hydrazine might be an excellent solvent to prepare diverse crystalline chalcogenides under solvothermal conditions. Beside the innovation of solvents in the reaction systems, the utilization of suitable precursors would also accelerate the reaction efficiently. Thus, we believe that metal Zintl phases could be employed as strong reducing agents to activate the unreactive element tellurium, leading to the generation of novel tellurides.
This article will cover the recent progress in the application of new synthetic strategies including ionothermal, surfactant-thermal, hydrazine-thermal, and metal Zintl phase usage for preparing crystalline chalcogenides. Their roles in the reaction process will also be discussed. Some results are summarized in Table 1.
| Compounds | Synthesis | Reactants | Solvent | d | Ref. |
|---|---|---|---|---|---|
| Abbreviations: hdz = hydrazine; en = ethylenediamine; ma = methylamine; taa = thioacetamide; py = pyridine; dma = dimethylamine; dpp = 1,3-di(4-pyridyl)propane; 4,4′-bpy = 4,4′-bipyridyl; dmmp = 2,6-dimethylmorpholine. | |||||
| [Sb7S8Br2][AlCl4]3 | Ionothermal | Sb + S | [EMIM]Br + AlCl3 | 0 | 59 |
| [M2Te2Br][AlCl4] (M = Sb, Bi) | Ionothermal | M + Te | [EMIM]Br + AlCl3 | 2 | 78 |
| [Bi2Se2Br][AlCl4] | Ionothermal | Bi + Se | [EMIM]Br + AlCl3 | 2 | 82 |
| [(Bi4Te4Br2)(Al2Cl5.46Br0.54)]Cl2 | Ionothermal | Bi + Te | [EMIM]Br + AlCl3 | 3 | 82 |
| [Sb10Se10][AlCl4]2 | RT ionic liquids | Sb + Se + SeCl4 | [BMIM]Cl + AlCl3 | 0 | 79 |
| Te4[Bi0.74Cl4] | RT ionic liquids | Te + TeCl4 + BiCl3 | [BMIM]Cl + AlCl3 | 0 | 81 |
| [Bi3GaS5]2[Ga3Cl10]2[GaCl4]2·S8 | Ionothermal | Bi + BiCl3 + GaCl3 + S | [BMIM]Cl | 0 | 80 |
| [BMIM]4[Sn9Se20] | Ionothermal | Sn + Se | [BMIM]Cl + hdz | 3 | 83 |
| [BMIM]4[Sn9Se20] | Ionothermal | [K4(H2O)4][SnSe4] | [BMIM][BF4] | 3 | 84 |
| [BMIM]4[Sn9Se20] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl4·5H2O | [BMMIM][BF4] + en | 3 | 86 |
| [BMMIM]4[Sn9Se19(Se2)0.9Se0.1] | Ionothermal | Sn + Se | [BMMIM]Cl + hdz | 3 | 83 |
| [PMMIM]4 [Sn9Se19(Se2)0.93Se0.07] | Ionothermal | Sn + Se | [PMMIM]Cl + hdz | 3 | 83 |
| [PMMIM]8[Sn17Se38] | Ionothermal | Sn + Se | [PMMIM]Cl + hdz | 2 | 83 |
| [PrMMIM]4[Sn9Se20] | Ionothermal | Sn + Se | [PrMMIM]Cl + ma | 2 | 89 |
| [PrMMIM]2[Sn3Se7] | Ionothermal | Sn + Se | [PrMMIM]Cl + en | 1 | 89 |
| [PrMMIM]2[Sn3Se7] | Ionothermal | Sn + Se | [PrMMIM]Cl + en | 2 | 89 |
| [BMMIM]2[Sn3Se7] | Ionothermal | Sn + Se | [BMMIM]Cl + en | 1 | 89 |
| [BMMIM]2[Sn3Se7] | Ionothermal | Sn + Se | [BMMIM]Cl + en | 2 | 89 |
| [BMMIM]7[AgSn12Se28] | Ionothermal | AgCl + Sn + Se | [BMMIM]Cl + hdz | 2 | 89 |
| [BMMIM]12[NH4][Cu5In30S52(SH)2Cl2] | Ionothermal | In2S3 + CuI + taa | [BMMIM]Cl + py + dma | 0 | 87 |
| [BMMIM]10[NH4]3[Cu5Ga30S52(SH)4] | Ionothermal | [enH]2[Ga4S7(en)2] + Cu(NO3)2·3H2O + taa + dpp | [BMMIM]Cl + dma | 0 | 87 |
| [BMMIM]8[NH4]3[Cu5Ga30S52(SH)2(Bim)2] | Ionothermal | [enH]2[Ga4S7(en)2] + Cu(NO3)2·3H2O + taa + 4,4′-bpy | [BMMIM]Cl + dma | 0 | 87 |
| [BMMIM]9.5[NH4]2[Cu5Ga30S52(SH)1.5Cl(Bim)1.5] | Ionothermal | [enH]2[Ga4S7(en)2] + Cu(NO3)2·3H2O + S + Na2CO3 | [BMMIM]Cl | 0 | 87 |
| [Cr7S8Cl2(NH3)14.5(H2O)1.5]Cl3·H2O | Ionothermal | CrCl3·6H2O + S + urea | [BMMIM]Cl + hdz | 0 | 88 |
| [EMIM]2[Sn2As2S4(S2)2Br2.43Cl1.56] | Ionothermal | Sn + S + As2S2 + EuCl3·6H2O + thiourea | [EMIM]Br + AlCl3 | 0 | 88 |
| [BMMIM]24[Sn36Ge24Se132] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl4·5H2O | [BMMIM][BF4] + dmmp | 0 | 85 |
| [BMIM]24[Sn32.5Ge27.5Se132] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl4·5H2O | [BMIM][BF4] + dmmp | 0 | 85 |
| [BMIM]2[Ge4Se9] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl4·5H2O | [BMIM][BF4] + dmmp | 1 | 86 |
| [BMMIM]2[Ge4SnSe10] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl2 | [BMMIM][BF4] + dmmp | 1 | 86 |
| [BMMIM]2[Ge0.83Sn3.17Se9.06] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl4·5H2O | [BMMIM][BF4] + dmmp | 2 | 86 |
| [BMMIM]8[Sn18Se40] | Ionothermal | [K4(H2O)3][Ge4Se10] + SnCl4·5H2O | [BMMIM][BF4] + en | 3 | 86 |
| [BMMIM]16[Sn24Se56] | Ionothermal | K2[Sn2Se5] | [BMMIM][BF4] + dmmp/en | 2 | 90 |
| [BMMIM]4[Sn6Se14] | Ionothermal | K2[Sn2Se5] | [BMMIM][BF4] | 1 | 90 |
| [BMMIM]3[dmmpH][Sn6Se14] | Ionothermal | K2[Sn2Se5] | [BMMIM][BF4] + dmmp | 1 | 90 |
| [EMIM]2[Ni(P2S8)2] | Ionothermal | Ni + P + S | [EMIM][BF4]/ [EMIM][CF3SO3] | 0 | 93 |
| [EMIM]3[Ni(P3S9)(P2S8)] | Ionothermal | Ni + P + S | [EMIM][BF4] | 0 | 93 |
| [EMIM]4[Ni(P3S9)2] | Ionothermal | Ni + P + S | [EMIM][BF4] | 0 | 93 |
| [EMIM]7[(NiP3S8)4(PS4)] | Ionothermal | Ni + P + S | [EMIM][BF4] | 0 | 93 |
| [NH4]8[Mn2As4S16] | Surfactant-thermal | Mn + As2S3 + S | PVP + hdz | 0 | 64 |
| [Mn(NH3)6][Mn2As2S8(N2H4)2] | Surfactant-thermal | Mn + As2S3 + S | PEG-400 + hdz | 1 | 64 |
| [enH][Cu3As2S5] | Surfactant-thermal | CuI + As2S3 + S | PEG-400 + en | 2 | 64 |
| [NH4][MnAs3S6] | Surfactant-thermal | Mn + As2S3 + S | [HMIM]Cl + hdz | 3 | 64 |
| [DBUH]2[Hg2Sn2Se6(Se2)] | Surfactant-thermal | HgCl2 + Sn + Se | PEG-400 + H2O + DBU | 1 | 65 |
| [DBUH]2[Hg2Sn2Se7] | Surfactant-thermal | HgCl2 + Sn + Se | PEG-400 + H2O + DBU | 1 | 65 |
| (N2H4)2Mn3Sb4S8(μ3-OH)2 | Surfactant-thermal | Mn + Sb2S5 + S | [HTBP]Br + hdz | 2 | 66 |
| (N2H4)ZnTe | Hydrazine-thermal | Zn(NO3)2·6H2O + Te | Hydrazine | 2 | 106 |
| Mn2SnS4(N2H4)2 | Hydrazine-thermal | Mn + Sn + S | Hydrazine + water | 3 | 112 |
| Mn2Sb2S5(N2H4)3 | Hydrazine-thermal | Mn + Sb2S3 + S | Hydrazine | 3 | 113 |
| Mn2Sb4S9(N2H4)2 | Hydrazine-thermal | Mn + Sb2S3 + S | Hydrazine + water | 3 | 114 |
| [In(en)3][In5Te8(en)2]·0.5en | Solvothermal | KIn2 + Te | en | 2 | 120 |
| [Mn(en)3]2[Ge5Te10] | Solvothermal | K4Ge9 + Te | en | 1 | 119 |
| [Ga(en)3]2(Ge2Te15) | Solvothermal | K4Ge9 + Ga2Te3 + Te | en | 1 | 121 |
Ionothermal method
Ionic liquids are a kind of molten salts with melting points below 100 °C. Their unique properties (such as low vapour pressure, high thermal stability, wide liquid temperature range, high ionic conductivity, and ability to dissolve organic or inorganic compounds) make them good alternatives to traditional molecular solvents in soft chemical material synthesis. Actually, ionic liquids have been extensively applied in solvent extraction, catalysis, and polymer science.69,70 Recently, ionothermal methods that employ ionic liquids as reaction media have become a new promising synthetic approach for the preparation of organic compounds, zeolites, metal–organic frameworks, clathrates, and nanomaterials.71–73 Although some known binary chalcogenide nanoparticles and nanorods have been prepared in ionic liquids,74–77 growing crystalline chalcogenides under ionothermal conditions remained elusive until Kanatzidis, Ruck, and other groups made a breakthrough in this field and synthesized a series of polycationic chalcogenides ranging from discrete clusters to three-dimensional frameworks in Lewis acidic ionic liquid media.59,78–82 Later on, Huang et al. and Dehnen et al. reported a variety of anionic chalcogenides through ionothermal synthesis, where various imidazolium cations acted as structure directing agents or charge balance agents in these structures.83–90Lewis acidic ionic liquids are prepared by mixing ionic liquids with Lewis acids or strong acceptors. Ionic liquids containing imidazolium cations are frequently used in this aprotic solvent system. Kanatzidis et al. first synthesized a chalcogenide, [Sb7S8Br2][AlCl4]3, by reacting Sb and S in a Lewis acidic ionic liquid, [EMIM]Br/AlCl3 (EMIM = 1-ethyl-3-methylimidazolium), with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 at 165 °C for 10 days.59 The crystal structure features a cationic [Sb7S8Br2]3+ cluster built up by linking two distorted cubic clusters [Sb4S4Br] on one corner (Sb atom), and the cationic cluster is surrounded by slightly distorted tetrahedral [AlCl4]− anions (Fig. 1a). The noncentrosymmetric space group P212121 of [Sb7S8Br2][AlCl4]3 offered opportunities in showing tunable SHG and DFG responses in the spectral range from 550 to 1000 nm. Thereafter, using the same Lewis acidic ionic liquid [EMIM]Br/AlCl3 with a molar ratio of 1
11 at 165 °C for 10 days.59 The crystal structure features a cationic [Sb7S8Br2]3+ cluster built up by linking two distorted cubic clusters [Sb4S4Br] on one corner (Sb atom), and the cationic cluster is surrounded by slightly distorted tetrahedral [AlCl4]− anions (Fig. 1a). The noncentrosymmetric space group P212121 of [Sb7S8Br2][AlCl4]3 offered opportunities in showing tunable SHG and DFG responses in the spectral range from 550 to 1000 nm. Thereafter, using the same Lewis acidic ionic liquid [EMIM]Br/AlCl3 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.8 as solvent or reaction medium, Kanatzidis et al. successfully synthesized a series of isostructural layered chalcogenide–halide compounds [M2Q2Br][AlCl4] (M = Bi, Sb; Q = Se, Te).78,82 The cationic infinite [M2Q2Br]+ layers were made up by connecting [M2Q2]2+ chains to Br− ions (Fig. 1b). The slightly distorted tetrahedral [AlCl4]− anions were located between the layers. [Bi2Te2Br][AlCl4] is a direct band gap, strongly anisotropic semiconductor. Recently, the same group reported a new chalcogenide, [(Bi4Te4Br2)(Al2Cl5.46Br0.54)]Cl2,82 prepared in the same Lewis acidic ionic liquid [EMIM]Br/AlCl3. The structure features a cationic three dimensional framework, [(Bi4Te4Br2)(Al2Cl5.46Br0.54)]2+ (Fig. 1c), which is set up by linking infinite layers of [Bi4Te4Br2]2+ with the neutral dimer Al2Cl5.46Br0.54. Seebeck coefficient measurements of single crystals of this chalcogenide confirm an n-type semiconductor.
4.8 as solvent or reaction medium, Kanatzidis et al. successfully synthesized a series of isostructural layered chalcogenide–halide compounds [M2Q2Br][AlCl4] (M = Bi, Sb; Q = Se, Te).78,82 The cationic infinite [M2Q2Br]+ layers were made up by connecting [M2Q2]2+ chains to Br− ions (Fig. 1b). The slightly distorted tetrahedral [AlCl4]− anions were located between the layers. [Bi2Te2Br][AlCl4] is a direct band gap, strongly anisotropic semiconductor. Recently, the same group reported a new chalcogenide, [(Bi4Te4Br2)(Al2Cl5.46Br0.54)]Cl2,82 prepared in the same Lewis acidic ionic liquid [EMIM]Br/AlCl3. The structure features a cationic three dimensional framework, [(Bi4Te4Br2)(Al2Cl5.46Br0.54)]2+ (Fig. 1c), which is set up by linking infinite layers of [Bi4Te4Br2]2+ with the neutral dimer Al2Cl5.46Br0.54. Seebeck coefficient measurements of single crystals of this chalcogenide confirm an n-type semiconductor.
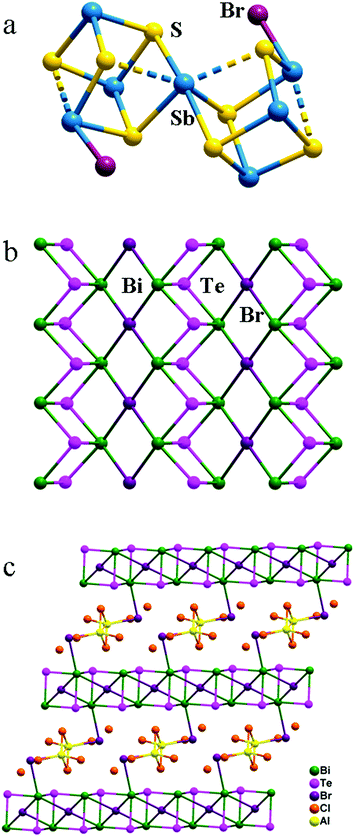 | ||
| Fig. 1 Crystal structures of cationic [Sb7S8Br2]3+ cluster (a), [Bi2Te2Br]+ layers (b), and [(Bi4Te4Br2)(Al2Cl5.46Br0.54)]2+ framework (c). | ||
Ruck et al. focused on the exploration of the synthesis of chalcogenides in ionic liquids at room temperature, and reported a heteronuclear polycyclic polycation [Sb10Se10]2+, which was synthesized by reacting Sb, Se, and SeCl2 in the Lewis acidic ionic liquid [BMIM]Cl/AlCl3 (BMIM = 1-n-butyl-3-methylimidazolium) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at room temperature.79 The polycation [Sb10Se10]2+ contains two realgar-like [Sb4Se4] cages linked by two Se atoms from a central [Sb2Se2] ring. When reacting BiCl3 with Te and TeCl4 in the Lewis acidic ionic liquid [BMIM]Cl/AlCl3 with a molar ratio of 1
2 at room temperature.79 The polycation [Sb10Se10]2+ contains two realgar-like [Sb4Se4] cages linked by two Se atoms from a central [Sb2Se2] ring. When reacting BiCl3 with Te and TeCl4 in the Lewis acidic ionic liquid [BMIM]Cl/AlCl3 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 at room temperature, the same group obtained a main-group salt, Te4[Bi0.74Cl4], featuring columnar structures of square tellurium polycations Te40.78+ with chloridobismuthate polyhedra.81 Interestingly, this compound exhibits superconductivity below 7.15 K. Feldmann et al. isolated a new compound, [Bi3GaS5]2[Ga3Cl10]2[GaCl4]2·S8, by reacting Bi, BiCl3, GaCl3, and S in the ionic liquid [BMIM]Cl at 150 °C for 10 days. The crystal structure consists of heterocubane-type [Bi3GaS5]2+ cations, trimeric star-shaped [Ga3Cl10]− anions, tetrahedral [GaCl4] anions, and neutral S8 rings.80
1.5 at room temperature, the same group obtained a main-group salt, Te4[Bi0.74Cl4], featuring columnar structures of square tellurium polycations Te40.78+ with chloridobismuthate polyhedra.81 Interestingly, this compound exhibits superconductivity below 7.15 K. Feldmann et al. isolated a new compound, [Bi3GaS5]2[Ga3Cl10]2[GaCl4]2·S8, by reacting Bi, BiCl3, GaCl3, and S in the ionic liquid [BMIM]Cl at 150 °C for 10 days. The crystal structure consists of heterocubane-type [Bi3GaS5]2+ cations, trimeric star-shaped [Ga3Cl10]− anions, tetrahedral [GaCl4] anions, and neutral S8 rings.80
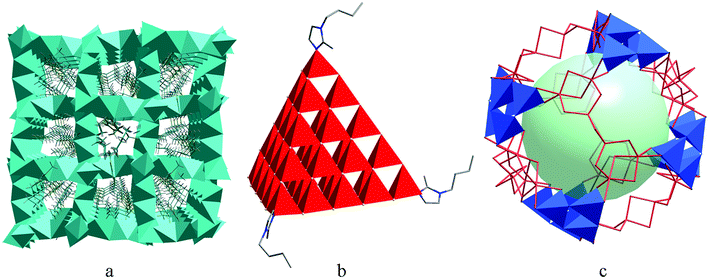
It is worth noting that the chalcogenide clusters and frameworks prepared in Lewis acidic ionic liquids are polycations, which may be related to the acidic reaction conditions. The ratio of ionic liquids to Lewis acids would significantly affect the formation of the final products. However, as we know, most chalcogenides with typical anionic nature were produced under basic reaction conditions. By using basic hydrazine monohydrate as auxiliary solvent in different ionic liquids, Huang et al. synthesized a series of selenidostannates featuring anionic open frameworks, whose channels were filled by various imidazolium cations from ionic liquids (Fig. 2a).83 Clearly, different imidazolium cations played a key role in directing the formation of the corresponding structures. In addition, hydrazine monohydrate also played an important role in the crystallization of these selenidostannates. When hydrazine monohydrate was removed from the reaction system, only SnSe2 powder or nanoparticles were obtained. Interestingly, varying the combination of ionic liquids and auxiliary amines (methylamine, ethylenediamine) as well as the reaction temperature and time, a series of selenidostannates with different one dimensional chains and two dimensional layers were observed.89 Remarkably, a heterometallic chalcogenide featuring anionic layers, [AgSn12Se28]7−, was isolated in ILs by linking the [Sn3Se7]n2n− double chains with linear two coordinate Ag+ ions. Furthermore, if more basic conditions were employed in the ionothermal synthesis, a series of supertetrahedral clusters or discrete T5 clusters with Cu–M–S constituents (M = Ga, In), which represent the largest molecular Tn clusters, were obtained.87 The anion–π interactions between S atoms of T5 clusters and imidazolium rings of [BMMIM]+ cations contribute greatly to the stabilization of discrete T5 clusters.91,92 It is also found that in situ decomposition of [BMMIM]Cl (BMMIM = 1-butyl-2,3-dimethyl-imidazolium) can generate BIM ligand (BIM = 1-butyl-2-methyl-imidazole) under ionothermal conditions, which further coordinates to some corner Ga3+ ions of T5 clusters to form inorganic–organic hybrid T5 clusters (Fig. 2b). The discrete Cu–Ga–S clusters exhibit broad emission bands from 500 to 800 nm. Recently, two chalcohalide clusters, [Cr7S8Cl2(NH3)14.5(H2O)1.5]Cl3·H2O and [EMIM]2[Sn2As2S4(S2)2Br2.43Cl1.56], have been synthesized under different ionothermal conditions by using hydrazine monohydrate and AlCl3 as additives, respectively.88
Beside the above mentioned factors (such as the size of ionic liquids, auxiliary amines, reaction temperature and time) that affect the composition and structure of final products obtained in ionothermal syntheses, optimal utilizations of various chalcogenide precursors also have significant influence on final materials. This was shown by Dehnen et al. in their successful ionothermal syntheses of a series of group 14 chalcogenides involving the utilization of different kinds of binary precursors. Directly reacting the precursor [K4(H2O)4][SnSe4] with ionic liquid [BMIM][BF4] at 130 °C for 7 days, they isolated red plate-like crystals of [BMIM]4[Sn9Se20] with three dimensional open-framework selenidostannates.84 If a larger precursor, [K4(H2O)3][Ge4Se10], was employed to react with SnCl4·5H2O in ionic liquids ([BMMIM][BF4], [BMIM][BF4]) containing a small amount of auxiliary amine (2,6-dimethylmorpholine), two ternary chalcogenides with the largest known discrete anionic cluster [Sn36−xGe24+xSe132]24− (x = 0, 3.5) that consists only of main-group elements (Fig. 2c) were obtained.85 Furthermore, the compound [BMMIM]24[Sn36Ge24Se132] can trap I2 molecules and induce heterolytic I–I bond cleavage. Under similar reaction conditions, a series of binary or ternary Ge/Sn/Se selenidostannates with anionic substructures ranging from one dimensional chains to three dimensional frameworks were synthesized by varying the ratios and the nature of the reactants.86 Dehnen et al. systematically investigated the crucial role of the amount and nature (basicity) of auxiliary amines on the generation of crystal structures. In addition to the binary precursors with discrete molecular [SnSe4]4− and [Ge4Se10]4− anions, the three dimensional anionic framework of K2[Sn2Se5] was also employed as binary precursor in ionothermal reactions. In particular, temperature-induced multistep phase transformations involving the dimensionalities of as-prepared frameworks from 3D to 1D and back to 3D were observed in the presence or absence of different amines.90 The obtained selenidostannates exhibit Schottky contact behaviour with absolute thresholds below 10 V in photoconductivity studies. Recently, Cody et al. introduced ionothermal methods in the synthesis of thiophosphates, and isolated four new nickel thiophosphate anions by reacting elements of Ni, P, S in [EMIM][BF4] or [EMIM][CF3SO3].93 Instead of using ionic liquids as solvents or reaction media in ionothermal synthesis, Dai et al. added ionic liquids as auxiliary agents ([BMIM]Br) into solvothermal reaction systems for synthesizing indium chalcogenides,94 and the auxiliary ionic liquids play an important role in the structural assembly of polymeric supertetrahedral InS clusters.
Surfactant-thermal method
Surfactants are usually organic compounds that consist of both hydrophilic groups and hydrophobic groups. They have been widely used in the synthesis of nanocrystals or mesoporous materials, and are recognized as efficient templates for tailoring the size, shape, and surface properties of nanoparticles, as well as controlling the pore sizes and phases of mesoporous frameworks.95–97 However, research involving the utilization of surfactants as solvents to direct the growth of crystalline chalcogenides remains unexplored. Actually, surfactants not only have the same characteristics as ionic liquids such as high thermal stability and negligible vapor pressure, but also possess some advantages (e.g. cheap price and multifunctional properties such as cationic, anionic, neutral, basic and acidic properties) which ionic liquids do not have. Therefore, surfactants can be used as promising reaction media for growing new crystalline materials with various structures and interesting properties. Kanatzidis and other groups reported room temperature solution processes of several crystalline binary chalcogenides containing discrete anionic clusters and charge-balancing surfactant cations.98–102 In these studies, the obtained inorganic phases were all discrete clusters of small T2 clusters [Ge4Q10]4− (Q = S, Se), P1 cluster [Cd8Se(SePh)12Cl4]2−, and [Sn2S6]4− dimer, while cationic or neutral surfactants (such as alkyl tri and dimethylammonium halides, alkyl quaternary ammonium chlorides, and aliphatic monoamines with different carbon chain lengths) functioned as reactants instead of reaction media in solution processes. Recently, our group first reported the application of surfactants as reaction media in the synthesis of crystalline metal chalcogenides with novel structures ranging from zero-dimensional cluster to three-dimensional framework.64–66 Furthermore, in continuation of our research work, we found that this surfactant-thermal method was also efficient in producing new metal–organic frameworks.67,68 Recently, Wang et al. also proved that surfactants could act as structure-directing agents or templates in controlling the pore size and shapes of crystalline zeolite frameworks.103 Thus, this recently-emerged surfactant-thermal method would offer more chances for synthesizing unprecedented crystalline materials (Fig. 3).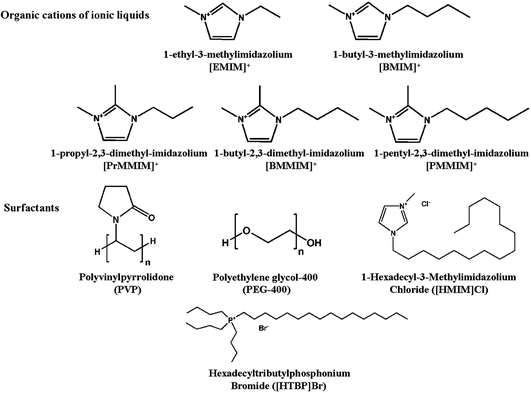 | ||
| Fig. 3 The organic cations of ionic liquids and surfactants used for the ionothermal and surfactant-thermal syntheses of chalcogenides, respectively. | ||
Clearly, using the surfactant-thermal method to prepare chalcogenides is still in its early stages. In our first investigation, three different surfactants (PVP = poly(vinylpyrrolidone), PEG-400 = poly(ethylene glycol)-400, and [Hmim]Cl = 1-hexadecyl-3-methylimidazolium chloride) were used as reaction media in the preparation of a series of thioarsenates ranging from zero-dimensional (0D) cluster to three-dimensional (3D) framework (Fig. 4).64 Either no crystals (for 0D, 1D, and 3D compounds) or a small amount of (less than 3% yield) crystals (for 2D compound) were obtained in the parallel experiments without surfactants. The results indicated that surfactants play an important role in controlling the crystal growth. Following this strategy, we synthesized two novel one-dimensional mercury selenidostannates, [DBUH]2[Hg2Sn2Se6(Se2)] (1) and [DBUH]2[Hg2Sn2Se7] (2) under surfactant-thermal conditions by using PEG-400 as reaction medium.65 It is interesting to point out that phase 1 is kinetically stable and can be transformed into thermodynamically stable phase 2 under the same reaction conditions with a long treatment time. However, when surfactant PEG-400 was removed or replaced by conventional organic solvents such as DMF, methanol and acetonitrile, only phase 2 was obtained at various reaction times, suggesting that PEG-400 can kinetically direct the formation of 1 in a relatively short time. Continual efforts were devoted to the exploration of surfactant-thermal synthesis of crystalline chalcogenides, and a new oxosulfide was isolated by reacting Mn, Sb2S5, S, and N2H4·H2O in a cationic surfactant (hexadecyltributylphosphonium bromide) medium at 175 °C for 7 days.66 The oxosulfide features a two-dimensional neutral layer, (N2H4)2Mn3Sb4S8(μ3-OH)2, which contains novel [Mn3(μ3-OH)2]n chains along the b-axis. This result clearly suggests that the cationic surfactant is the key for the formation of oxosulfide because no crystal can be produced if this cationic surfactant is removed or replaced by neutral surfactants (e.g. PEG). In addition, this compound could be used as a photocatalyst for H2 generation under visible-light irradiation without any co-catalyst.
Hydrazine-thermal method
Hydrazine is a basic solvent with strong coordination and reduction ability. Many chalcogenides can form good crystals in the presence of hydrazine.104,105 Thus, hydrazine might be a promising solvent to synthesize many novel chalcogenides with new architectures (from 1D chain to 3D framework) as well as interesting physical properties (photocatalytic, magnetic and photoelectric).The first chalcogenide compound prepared through hydrazine-thermal condition was two-dimensional layered telluride ZnTe(N2H4) in 2003.106 Later on, Mitzi et al. demonstrated that chalcogenides could also be prepared at ambient temperature and ambient pressure. Some of these chalcogenides are (N2H4)2ZnTe, (N2H5)4Sn2S6, (N2H4)3(N2H5)4Sn2Se6, (N2H5)4Ge2Se6, and N4H9Cu7S4.107–110 Both (N2H4)3(N2H5)4Sn2Se6 and (N2H5)4Ge2Se6 consist of M2Se64− (M = Sn, Ge) anions with edge-sharing MSe4 tetrahedra. In compound N2H5Ge2Se6, the charge of the Ge2Se64− anions was balanced by hydrazinium cations, while in compound (N2H4)3(N2H5)4Sn2Se6, both monoprotonated hydrazinium cations and neutral hydrazine molecules are found in the structure. As for the structure of compound N4H9Cu7S4, the Cu7S4− sheets are separated by hydrazinium cations and neutral hydrazine molecules. Interestingly, all these precursors may be fabricated to form films through spin coating technology in air at room temperature, which is an easier and cheaper way for large area and low-cost deposition.110,111 However, all of the compounds prepared in hydrazine at room temperature are simple binary chalcogenides with known structure, and these reactions cannot be thermodynamically controlled.
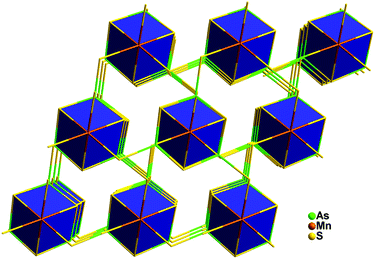
In 2009, Kanatzidis’ group firstly reported the synthesis of new chalcogenide materials Mn2SnS4(N2H4)2 under hydrazine-thermal conditions.112 The as-prepared crystal features a three-dimensional framework (Fig. 5), and hydrazine molecules act as linker to connect octahedral MnL6 (L = S, N) units to form a 3D structure. Also, a strong antiferromagnetic interaction was observed in this compound.
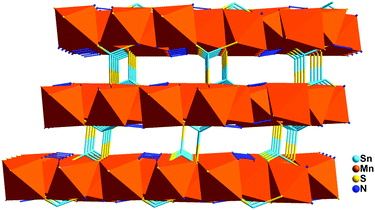
[Mn2Sb2S5(N2H4)3] and Mn2Sb4S8(N2H4)2, the first two examples in the Mn/pnictide–hydrazine chalcogenide family, were synthesized under hydrazine-thermal conditions in our group.113,114 Both compounds feature a three-dimensional framework with neutral H2N–H2N molecules as linkers. The oxidation states of Mn/Sb/S could be assigned as 2+/3+/2−. Here, neutral hydrazine molecules perform three different roles: (1) intra-layer bridged ligands, (2) inter-layer linkers, and (3) templates for the formation of 3D frameworks. The compound [Mn2Sb2S5(N2H4)3] has a similar Mn coordination geometry MnS4N2 to compounds [Mn2SnS4(N2H4)2] and Mn2Sb4S8(N2H4)2, while there are two Mn coordination geometries: MnN4S2 octahedron and MnS4 tetrahedron. Additionally, in compound [Mn2Sb2S5(N2H4)3], the Sb center adopts two configurations: SbS3 trigonal-pyramidal geometry and SbS4 four-fold coordination geometry. However, in compound Mn2Sb4S8(N2H4)2, the Sb center adopted SbS4 four-fold coordination and SbS5 square pyramidal geometry. Both compounds show strong antiferromagnetic interaction among Mn ions. The compound [Mn2Sb2S5(N2H4)3] also shows photocatalytic behavior with the generation of H2 at 4.64 mol h−1 g−1 under visible light irradiation.
Solvothermal synthesis based on Zintl anions/phases
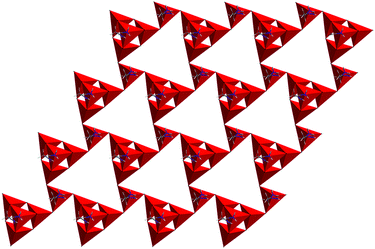
Metal Zintl phases usually exist as polyatomic clusters or network anions, which were produced by the reduction of p-element (post-transition) metals with alkali metals (or alkaline-earth metals).115–117 They are known to be strong reducing agents, and can be used to activate tellurium (Te) at room temperature.118 Kanatzidis et al. first performed this activation of tellurium with Zintl ions in solvothermal conditions, and three new tellurides, [In(en)3][In5Te9(en)2]·0.5en, {[Ga(en)3]2(Ge2Te15)}n, and [Mn(en)3]2(Ge5Te10) (en = ethylenediamine), were obtained by the reaction of Zintl anions with Te.119–121Compound {[In(en)3][In5Te9(en)2]·0.5en}n crystallizes in the hexagonal space group P61.120 It features layers of [In5Te9(en)2]3− in a new structural motif, which is formed by linking adamantine shaped [[In4Te9(en)2]6−] clusters (T2) with [InTe3(en)]3− tetrahedron (T1) by sharing the terminal Te atoms (Fig. 6). Three [In5Te9(en)2]3− anions create an In–Te window, and octahedral ΔMδλλ-[In(en)3]3+ complexes occupy the middle of the widows to balance the charge. In addition, this polar compound has good second harmonic generation response (approximately half that of AgGaS2) with type I phase matchability.
To prepare {[Mn(en)3]2(Ge5Te10)}n, K4Ge9 was used to activate the tellurium in solvothermal conditions. In this compound, [Ge5Te10]4− repeated to form 1/{[Ge5Te10]4−} infinite chains. Ge–Ge bonds formed in the compound. More interestingly, three different oxidation states of germanium centres containing Ge2+, Ge3+, and Ge4+ exist in the chains. As for compound {[Ga(en)3]2(Ge2Te15)}n, it has two unconventional polytelluride fragments: cross-shaped 36e− TeTe46− anions and boat-shaped 52e− Te84− rings. Interestingly, the new material is a p-type semiconductor.
Summary and outlook
In this article, four recently emerged new synthetic strategies (ionothermal, surfactant-thermal, hydrazine-thermal, and metal Zintl phase usage) to prepare crystalline metal chalcogenides have been reviewed. From the fast development of synthetic methods and as-prepared compounds with diverse structures and unique properties, we believe that more significant results will be discovered in this exciting field of research. It is noticeable that the innovations of solvents and precursors are important in the synthesis of new crystalline chalcogenides. Other factors such as counterion size and shape, reaction temperature and time can also influence the structural formation of the final products. In the ionothermal synthesis, the acidic or basic environment provided by auxiliaries (Lewis acid AlCl3 or amines) results in distinct cationic or anionic chalcogenides, respectively. Moreover, the current experiments in ionothermal synthesis of chalcogenides indicate that auxiliary solvents play a key role in the crystallization of chalcogenide frameworks. In the surfactant-thermal synthesis, the choice of different types of surfactants would heavily alter the solvent systems and kinetically control the crystal growth with varied structures. The strong reducing effects of hydrazine and Zintl phase would activate the reaction process. Although these new synthetic methods have been successfully used in the synthesis of crystalline chalcogenides, the research in this area is still at an early stage, and there are still questions to be answered, for example, (1) how ionic liquids or surfactants interact with metal and chalcogen ions, (2) how ionic liquids or surfactants control the nucleation and growth of crystalline chalcogenides, and (3) whether surfactants can be trapped in the structures of crystalline chalcogenides. Thus, the potential application of these new synthetic strategies deserves further exploration.Acknowledgements
QZ acknowledges financial support from AcRF Tier 1 (RG 16/12) and Tier 2 (ARC 20/12 and ARC 2/13) from MOE, and the CREATE program (Nanomaterials for Energy and Water Management) from NRF, Singapore.Notes and references
- A. K. Cheetham, G. Ferey and T. Loiseau, Angew. Chem., Int. Ed., 1999, 38, 3268–3292 CrossRef CAS.
- H. L. Li, A. Laine, M. O'Keeffe and O. M. Yaghi, Science, 1999, 283, 1145–1147 CrossRef CAS.
- K. Biswas, J. He, Q. Zhang, V. P. Dravid, G. Wang, C. Uher and M. Kanatzidis, Nat. Chem., 2011, 3, 160–166 CrossRef CAS PubMed.
- N. F. Zheng, X. G. Bu, B. Wang and P. Y. Feng, Science, 2002, 298, 2366–2369 CrossRef CAS PubMed.
- N. F. Zheng, X. H. Bu and P. Y. Feng, Nature, 2003, 426, 428–432 CAS.
- Q. Zhang, Y. Liu, X. Bu, T. Wu and P. Feng, Angew. Chem., Int. Ed., 2008, 47, 113–116 CrossRef CAS PubMed.
- M. L. Feng, D. N. Kong, Z. L. Xie and X. Y. Huang, Angew. Chem., Int. Ed., 2008, 47, 8623–8626 CrossRef CAS PubMed.
- M. G. Kanatzidis and N. Ding, Nat. Chem., 2010, 2, 187–191 CrossRef PubMed.
- T. Wu, R. Khazhakyan, L. Wang, X. H. Bu, S. T. Zheng, V. Chau and P. Y. Feng, Angew. Chem., Int. Ed., 2011, 50, 2536–2539 CAS.
- M. G. Kanatzidis and K. R. Poeppelmeier, Prog. Solid State Chem., 2008, 36, 1–133 CrossRef CAS PubMed.
- B. Krebs, Angew. Chem., Int. Ed., 1983, 22, 113–134 CrossRef PubMed.
- S. Dehnen and M. Melullis, Coord. Chem. Rev., 2007, 251, 1259–1280 CrossRef CAS PubMed.
- S. Dehnen and M. K. Brandmayer, J. Am. Chem. Soc., 2003, 125, 6618–6619 CrossRef CAS PubMed.
- M. K. Brandmayer, R. Clerac, F. Weigend and S. Dehnen, Chem. – Eur. J., 2004, 10, 5147–5157 CrossRef CAS PubMed.
- C. Zimmermann, M. Melullis and S. Dehnen, Angew. Chem., Int. Ed., 2002, 41, 4269–4272 CrossRef CAS.
- J. L. Mertz, N. Ding and M. G. Kanatzidis, Inorg. Chem., 2009, 48, 10898–10900 CAS.
- O. M. Yaghi, Z. Sun, D. A. Richardson and T. L. Groy, J. Am. Chem. Soc., 1994, 116, 807–808 CrossRef CAS.
- Z. P. Zheng, J. R. Long and R. H. Holm, J. Am. Chem. Soc., 1997, 119, 2163–2171 CrossRef CAS.
- H. Ahari, A. Garcia, S. Kirkby, G. A. Ozin, D. Young and A. J. Lough, J. Chem. Soc., Dalton Trans., 1998, 2023–2027 RSC.
- J. P. Lang, Q. T. Xu, R. X. Yuan and B. F. Abrahams, Angew. Chem., Int. Ed., 2004, 43, 4741–4745 CAS.
- Q. F. Xu, J. X. Chen, H. Zhang, Z. G. Ren, H. X. Li, Y. Zhang and J. P. Lang, Inorg. Chem., 2006, 45, 4055–4064 CrossRef CAS PubMed.
- A. Stein, S. W. Keller and T. E. Mallouk, Science, 1993, 259, 1558–1564 CAS.
- J. Gopalakrishnan, Chem. Mater., 1995, 7, 1265–1275 CAS.
- S. A. Sunshine, D. Kang and J. A. Ibers, J. Am. Chem. Soc., 1987, 109, 6202–6204 CAS.
- M. G. Kanatzidis and A. C. Sutorik, Prog. Inorg. Chem., 1995, 43, 151–265 CrossRef CAS PubMed.
- D. Kang and J. A. Ibers, Inorg. Chem., 1988, 27, 549–551 CrossRef CAS.
- T. J. Mccarthy and M. G. Kanatzidis, Chem. Mater., 1993, 5, 1061–1063 CrossRef CAS.
- M. G. Kanatzidis and Y. Park, Chem. Mater., 1990, 2, 99–101 CrossRef CAS.
- J. H. Liao, C. Varotsis and M. G. Kanatzidis, Inorg. Chem., 1993, 32, 2453–2462 CrossRef CAS.
- M. G. Kanatzidis, Chem. Mater., 1990, 2, 353–363 CAS.
- J. W. Kolis, Coord. Chem. Rev., 1990, 105, 195–219 CrossRef CAS.
- M. G. Kanatzidis and S. P. Huang, Coord. Chem. Rev., 1994, 130, 509–621 CrossRef CAS.
- A. Rabenau, Angew. Chem., Int. Ed., 1985, 24, 1026–1040 CrossRef PubMed.
- H. Schafer, Annu. Rev. Mater. Sci., 1985, 15, 1–41 CrossRef.
- W. S. Sheldrick and M. Wachhold, Angew. Chem., Int. Ed., 1997, 36, 207–224 Search PubMed.
- W. S. Sheldrick and M. Wachhold, Coord. Chem. Rev., 1998, 176, 211–322 CAS.
- R. L. Bedard, S. T. Wilson, L. D. Vail, J. M. Bennett, E. M. Flanigen, P. A. Jacobs and R. A. E. van Santen, Zeolites: Fact, Figure, Future, Proceedings of the 8th International Zeolite Conference, Elsevier, Amsterdam, 1989, p. 375 Search PubMed.
- J. B. Parise and Y. Ko, Chem. Mater., 1994, 6, 718–720 CrossRef CAS.
- C. L. Cahill and J. B. Parise, Chem. Mater., 1997, 9, 807–811 CrossRef CAS.
- C. L. Cahill, B. Gugliotta and J. B. Parise, Chem. Commun., 1998, 1715–1716 RSC.
- P. Vaqueiro, Inorg. Chem., 2006, 45, 4150–4156 CrossRef CAS PubMed.
- N. Pienack, A. Puls, C. Nather and W. Bensch, Inorg. Chem., 2008, 47, 9606–9611 CrossRef CAS PubMed.
- R. C. Zhang, H. G. Yao, S. H. Ji, M. C. Liu, M. Ji and Y. L. An, Chem. Commun., 2010, 46, 4550–4552 RSC.
- H. L. Li, M. Eddaoudi, A. Laine, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 1999, 121, 6096–6097 CrossRef CAS.
- H. L. Li, J. Kim, T. L. Groy, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2001, 123, 4867–4868 CrossRef CAS.
- Q. C. Zhang, X. H. Bu, J. Zhang, T. Wu and P. Y. Feng, J. Am. Chem. Soc., 2007, 129, 8412–8413 CrossRef CAS PubMed.
- P. Vaqueiro and M. L. Romero, J. Am. Chem. Soc., 2008, 130, 9630–9631 CrossRef CAS PubMed.
- Q. Zhang, X. Bu, L. Han and P. Feng, Inorg. Chem., 2006, 45, 6684–6687 CrossRef CAS PubMed.
- T. Wu, X. Q. Wang, X. H. Bu, X. Zhao, L. Wang and P. Y. Feng, Angew. Chem., Int. Ed., 2009, 48, 7204–7207 CrossRef CAS PubMed.
- X. Zhang, W. Luo, Y. P. Zhang, J. B. Jiang, Q. Y. Zhu and J. Dai, Inorg. Chem., 2011, 50, 6972–6978 CrossRef CAS PubMed.
- P. Y. Feng, X. H. Bu and N. F. Zheng, Acc. Chem. Res., 2005, 38, 293–303 CAS.
- (a) J. L. Xie, X. H. Bu, N. F. Zheng and P. Y. Feng, Chem. Commun., 2005, 4916–4918 RSC; (b) Q. Zhang, X. Bu, Z. Lin, T. Wu and P. Feng, Inorg. Chem., 2008, 47, 9724–9726 CrossRef CAS PubMed; (c) Y. Liu, M. J. W. Tan, F. Wei, Y. Tian, T. Wu, C. Kloc, F. Huo, Q. Yan, H. H. Hng, J. Ma and Q. Zhang, CrystEngComm, 2012, 14, 75–78 RSC.
- (a) N. F. Zheng, X. H. Bu, H. W. Lu, L. Chen and P. Y. Feng, J. Am. Chem. Soc., 2005, 127, 14990–14991 CrossRef CAS PubMed; (b) Y. Liu, L.-M. Yu, S. C. J. Loo, R. G. Blair and Q. Zhang, J. Solid State Chem., 2012, 191, 283–286 CrossRef CAS PubMed; (c) Y. Liu, F. Boey, L. L. Lao, H. Zhang, X. Liu and Q. Zhang, Chem. – Asian J., 2011, 6, 1004–1006 CrossRef CAS PubMed.
- P. Vaqueiro, Dalton Trans., 2010, 39, 5965–5972 RSC.
- J. Li, Z. Chen, R. J. Wang and D. M. Proserpio, Coord. Chem. Rev., 1999, 192, 707–735 Search PubMed.
- J. Zhou, J. Dai, G. Q. Bian and C. Y. Li, Coord. Chem. Rev., 2009, 253, 1221–1247 CrossRef CAS PubMed.
- Z. J. Lin, A. M. Z. Slawin and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 4880–4881 CrossRef CAS PubMed.
- E. R. Parnham and R. E. Morris, Acc. Chem. Res., 2007, 40, 1005–1013 CrossRef CAS PubMed.
- Q. Zhang, I. Chung, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, J. Am. Chem. Soc., 2009, 131, 9896–9897 CrossRef CAS PubMed.
- E. Ahmed, J. Breternitz, M. F. Groh and M. Ruck, CrystEngComm, 2012, 14, 4874–4885 RSC.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706–8715 CrossRef CAS.
- H. Yang, N. Coombs, I. Sokolov and G. A. Ozin, Nature, 1996, 381, 589–592 CAS.
- M. P. Pileni, Nat. Mater., 2003, 2, 145–150 CrossRef CAS PubMed.
- W. W. Xiong, E. U. Athresh, Y. T. Ng, J. F. Ding, T. Wu and Q. C. Zhang, J. Am. Chem. Soc., 2013, 135, 1256–1259 CrossRef CAS PubMed.
- W. W. Xiong, P. Z. Li, T. H. Zhou, A. L. Y. Tok, R. Xu, Y. L. Zhao and Q. C. Zhang, Inorg. Chem., 2013, 52, 4148–4150 CrossRef CAS PubMed.
- J. K. Gao, Q. L. Tay, P. Z. Li, W. W. Xiong, Y. L. Zhao, Z. Chen and Q. C. Zhang, Chem. – Asian J., 2014, 9, 131–134 CrossRef CAS PubMed.
- J. K. Gao, M. He, Z. Y. Lee, W. F. Cao, W. W. Xiong, Y. X. Li, R. Ganguly, T. Wu and Q. C. Zhang, Dalton Trans., 2013, 42, 11367–11370 RSC.
- J. K. Gao, K. Q. Ye, M. He, W. W. Xiong, W. F. Cao, Z. Y. Lee, Y. Wang, T. Wu, F. W. Huo, X. G. Liu and Q. C. Zhang, J. Solid State Chem., 2013, 206, 27–31 CAS.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- K. Biswas and C. N. R. Rao, Chem. – Eur. J., 2007, 13, 6123–6129 CrossRef CAS PubMed.
- Z. Ma, J. H. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS PubMed.
- D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050–11060 CAS.
- J. Jiang, S. H. Yu, W. T. Yao, H. Ge and G. Z. Zhang, Chem. Mater., 2005, 17, 6094–6100 CrossRef CAS.
- Y. Jiang and Y. J. Zhu, J. Phys. Chem. B, 2005, 109, 4361–4364 CrossRef CAS PubMed.
- H. Sakamoto, Y. Watanabe and T. Saito, Inorg. Chem., 2006, 45, 7028–7028 CrossRef CAS.
- X. D. Liu, J. M. Ma, P. Peng and W. J. Meng, Langmuir, 2010, 26, 9968–9973 CrossRef CAS PubMed.
- K. Biswas, Q. C. Zhang, I. Chung, J. H. Song, J. Androulakis, A. J. Freeman and M. G. Kanatzidis, J. Am. Chem. Soc., 2010, 132, 14760–14762 CAS.
- E. Ahmed, A. Isaeva, A. Fiedler, M. Haft and M. Ruck, Chem. – Eur. J., 2011, 17, 6847–6852 CAS.
- D. Freudenmann and C. Feldmann, Dalton Trans., 2011, 40, 452–456 RSC.
- E. Ahmed, J. Beck, J. Daniels, T. Doert, S. J. Eck, A. Heerwig, A. Isaeva, S. Lidin, M. Ruck, W. Schnelle and A. Stankowski, Angew. Chem., Int. Ed., 2012, 51, 8106–8109 CrossRef CAS PubMed.
- K. Biswas, I. Chung, J. H. Song, C. D. Malliakas, A. J. Freeman and M. G. Kanatzidis, Inorg. Chem., 2013, 52, 5657–5659 CrossRef CAS PubMed.
- J. R. Li, Z. L. Xie, X. W. He, L. H. Li and X. Y. Huang, Angew. Chem., Int. Ed., 2011, 50, 11395–11399 CrossRef CAS PubMed.
- Y. M. Lin and S. Dehnen, Inorg. Chem., 2011, 50, 7913–7915 CAS.
- Y. M. Lin, W. Massa and S. Dehnen, J. Am. Chem. Soc., 2012, 134, 4497–4500 CrossRef CAS PubMed.
- Y. M. Lin, W. Massa and S. Dehnen, Chem. – Eur. J., 2012, 18, 13427–13434 CrossRef CAS PubMed.
- W. W. Xiong, J. R. Li, B. Hu, B. Tan, R. F. Li and X. Y. Huang, Chem. Sci., 2012, 3, 1200–1204 RSC.
- K. Z. Du, M. L. Feng, J. R. Li and X. Y. Huang, CrystEngComm, 2013, 15, 5594–5597 RSC.
- J. R. Li, W. W. Xiong, Z. L. Xie, C. F. Du, G. D. Zou and X. Y. Huang, Chem. Commun., 2013, 49, 181–183 CAS.
- Y. M. Lin, D. W. Xie, W. Massa, L. Mayrhofer, S. Lippert, B. Ewers, A. Chernikov, M. Koch and S. Dehnen, Chem. – Eur. J., 2013, 19, 8806–8813 CrossRef CAS PubMed.
- P. Gamez, A. Robertazzi, F. Krull and E. W. Knapp, CrystEngComm, 2011, 13, 3293–3300 RSC.
- Z. Xu, N. J. Singh, S. K. Kim, D. R. Spring, K. S. Kim and J. Yoon, Chem. – Eur. J., 2011, 17, 1163–1170 CrossRef CAS PubMed.
- J. A. Cody, K. B. Finch, G. J. Reynders, G. C. B. Alexander, H. G. Lim, C. Nather and W. Bensch, Inorg. Chem., 2012, 51, 13357–13362 CAS.
- Y. H. Wang, J. B. Jiang, P. Wang, X. L. Sun, Q. Y. Zhu and J. Dai, CrystEngComm, 2013, 15, 6040–6045 RSC.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and Y. Q. Yan, Adv. Mater., 2003, 15, 353–389 CrossRef CAS PubMed.
- B. L. Cushing, V. L. Kolesnichenko and C. J. O'Connor, Chem. Rev., 2004, 104, 3893–3946 CrossRef CAS PubMed.
- Y. Yin and A. P. Alivisatos, Nature, 2005, 437, 664–670 CrossRef CAS PubMed.
- J. Q. Li, B. Marler, H. Kessler, M. Soulard and S. Kallus, Inorg. Chem., 1997, 36, 4697–4701 CrossRef CAS PubMed.
- F. Bonhomme and M. G. Kanatzidis, Chem. Mater., 1998, 10, 1153–1159 CAS.
- M. Wachhold and M. G. Kanatzidis, Chem. Mater., 2000, 12, 2914–2923 CrossRef CAS.
- A. Eichhofer, O. Hampe and M. Blom, Eur. J. Inorg. Chem., 2003, 1307–1314 CrossRef CAS PubMed.
- K. K. Rangan and M. G. Kanatzidis, Inorg. Chim. Acta, 2004, 357, 4036–4044 CrossRef CAS PubMed.
- H. Y. Lin, C. Y. Chin, H. L. Huang, W. Y. Huang, M. J. Sie, L. H. Huang, Y. H. Lee, C. H. Lin, K. H. Lii, X. H. Bu and S. L. Wang, Science, 2013, 339, 811–813 CrossRef CAS PubMed.
- J. R. Li and X. Y. Huang, Dalton Trans., 2011, 40, 4387–4390 RSC.
- H. Wiogo, M. Lim, P. Munroe and R. Amal, Cryst. Growth Des., 2011, 11, 1689–1696 CAS.
- X. Y. Huang, J. Li, Y. Zhang and A. Mascarenhas, J. Am. Chem. Soc., 2003, 125, 7049–7055 CrossRef CAS PubMed.
- D. B. Mitzi, L. L. Kosbar, C. E. Murray, M. Copel and A. Afzali, Nature, 2004, 428, 299–303 CrossRef CAS PubMed.
- D. B. Mitzi, Inorg. Chem., 2005, 44, 7078–7086 CrossRef CAS PubMed.
- D. B. Mitzi, Inorg. Chem., 2005, 44, 3755–3761 CrossRef CAS PubMed.
- D. B. Mitzi, Inorg. Chem., 2007, 46, 926–931 CrossRef CAS PubMed.
- D. J. Milliron, D. B. Mitzi, M. Cope and C. E. Murray, Chem. Mater., 2006, 18, 587–590 CrossRef CAS.
- M. J. Manos and M. G. Kanatzidis, Inorg. Chem., 2009, 48, 4658–4660 CrossRef CAS PubMed.
- Y. Liu, P. D. Kanhere, C. L. Wong, Y. F. Tian, Y. H. Feng, F. Boey, T. Wu, H. Y. Chen, T. J. White, Z. Chen and Q. C. Zhang, J. Solid State Chem., 2010, 183, 2644–2649 CAS.
- Y. Liu, Y. F. Tian, F. X. Wei, M. S. C. Ping, C. W. Huang, F. Boey, C. Kloc, L. Chen, T. Wu and Q. C. Zhang, Inorg. Chem. Commun., 2011, 14, 884–888 CrossRef CAS PubMed.
- H. Schafer, B. Eisenman and W. Muller, Angew. Chem., Int. Ed., 1973, 12, 694–712 CrossRef PubMed.
- J. D. Corbett, Chem. Rev., 1985, 85, 383–397 CrossRef CAS.
- J. D. Corbett, Angew. Chem., Int. Ed., 2000, 39, 670–690 CrossRef CAS.
- T. F. Fassler and U. Schutz, J. Organomet. Chem., 1997, 541, 269–276 CrossRef CAS.
- Q. C. Zhang, G. Armatas and M. G. Kanatzidis, Inorg. Chem., 2009, 48, 8665–8667 CrossRef CAS PubMed.
- Q. C. Zhang, I. Chung, J. I. Jang, J. B. Ketterson and M. G. Kanatzidis, Chem. Mater., 2009, 21, 12–14 CrossRef CAS.
- Q. C. Zhang, C. D. Malliakas and M. G. Kanatzidis, Inorg. Chem., 2009, 48, 10910–10912 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2014 |