Forming a composite electron blocking layer to enhance the performance of carbon-based CsPbI3 perovskite solar cells†
Yongfa
Song
a,
Weiping
Li
 *a,
Hailiang
Wang
a,
Huicong
Liu
*a,
Hailiang
Wang
a,
Huicong
Liu
 a,
Yue
Deng
a,
Qixian
Zhang
a,
Han
Rao
a,
Xiaoyu
Jiang
*b and
Haining
Chen
a,
Yue
Deng
a,
Qixian
Zhang
a,
Han
Rao
a,
Xiaoyu
Jiang
*b and
Haining
Chen
 *a
*a
aNo. 37 Xueyuan Road, Haidian District, Beijing 100191, People's Republic of China. E-mail: liweiping@buaa.edu.cn; chenhaining@buaa.edu.cn
bBeijing 100072, People's Republic of China. E-mail: jiangxiaoyu2007@gmail.com
First published on 3rd February 2023
Abstract
Carbon-based CsPbI3 perovskite solar cells (C-PSCs) have attracted much interest due to their high chemical stability. However, their efficiency still largely lags behind those achieved by conventional CsPbI3 PSCs because of the electron back transfer from the perovskite to the carbon electrode. Herein, we address the above issue by forming a composite electron blocking layer at the CsPbI3/carbon interface. The CsPbCl3 quantum dots (QDs) layer is first deposited on the CsPbI3 film by spin coating a QDs solution, while the Cs2PbI2Cl2 nanosheets are then generated on the surface by the post treatment of a CsCl solution. The composite blocking layer not only well suppresses electron back transfer but also passivates crystal defects. As a result, the efficiency of CsPbI3 C-PSCs is boosted from 12.51% to 16.10%.
Introduction
Perovskite solar cells (PSCs) have attracted intense attention in the past few years due to their high power conversion efficiency (PCE) based on solution-based processes.1–6 However, their low stability has greatly inhibited the commercial application of PSCs.7–10 Generally, their low stability mainly originates from the use of organic-inorganic hybrid perovskites, made from organic hole transport materials (HTMs) and metal electrodes.11–13 After replacing the HTM and metal electrode with a carbon electrode, carbon-based PSCs (C-PSCs) are made, and the device stability could be well improved because carbon materials are stable, inert to ion migration and inherently H2O-resistant.14–19Although C-PSCs have shown obvious enhancement in device stability, the organic components in organic-inorganic hybrid perovskites can be easily removed from the crystal structure, which still limits the device stability. In order to address the issue, researchers have introduced inorganic perovskites to replace the organic-inorganic hybrid perovskites as light absorbers in C-PSCs.20–25 Among various inorganic perovskites, CsPbI3 has shown great prospects duo to the suitable bandgap of 1.73 eV.26–32 So far, great progress has been made on CsPbI3 C-PSCs and over 16% PCE has been achieved.33
However, the above PCE still largely lags behind those achieved by the conventional CsPbI3 PSCs (about 21%).34 The low hole selectivity of the carbon electrode is the main cause because the photogenerated electrons tend to transfer to the carbon electrode for inducing serious recombination loss.11,35 To suppress the electron back transport, forming an electron blocking layer at the CsPbI3/carbon interface has been employed.36–38 For example, phenylethylamine iodide (PEAI)39 or CsCl40 have been used to treat the CsPbI3 layer to grow a 2D perovskite layer in situ acting as an electron blocking layer. However, the performance improvement is still limited for a single electron blocking layer and a new strategy is needed.
Herein, both CsPbCl3 QDs and 2D Cs2PbI2Cl2 were deposited on the CsPbI3 perovskite layer to form a composite electron blocking layer. Firstly, CsPbCl3 QDs were spin coated on the CsPbI3 perovskite layer by using a CsPbCl3 QDs cyclohexane solution. Then, a CsCl ethanol solution was also spin coated on the QDs-coated CsPbI3 perovskite layer to form 2D Cs2PbI2Cl2. Such architecture forms a favorable energy level alignment for electron blocking. In addition, crystal defects were also effectively passivated. As a result, the PCE of CsPbI3 C-PSCs was promoted from 12.51% to 16.10%.
Results and discussion
The CsPbCl3 QDs were synthesized using the thermal injection method described in the Experimental section.41,42 The TEM image in Fig. S1a (ESI†) shows that the CsPbCl3 QDs have a regular square structure with a size of about 12 nm. The X-ray diffraction (XRD) pattern in Fig. S1b (ESI†) indicates that all diffraction peaks match well with the tetragonal structure of the CsPbCl3 perovskite (JCPDS 18-0366).19 Therefore, CsPbCl3 QDs have been successfully prepared.For preparing the CsPbI3 perovskite films, a precursor solution containing CsI, PbI2 and DMAI was spin coated on the TiO2 substrate, followed by annealing at 220 °C for 5 min. After the deposition of the CsPbI3 perovskite films, three kinds of samples were obtained by the processes illustrated in Fig. 1. For the CsPbI3–CsCl film, the as-deposited CsPbI3 perovskite films were treated with a CsCl ethanol solution (1 mg ml−1) by spin coating. For the CsPbI3-QDs film, a CsPbCl3 QDs cyclohexane solution was spin-coated on the as-deposited CsPbI3 perovskite film. For the CsPbI3-QDs/CsCl film, the as-deposited CsPbI3 perovskite films were successively treated with a CsPbCl3 QDs solution and CsCl solution.
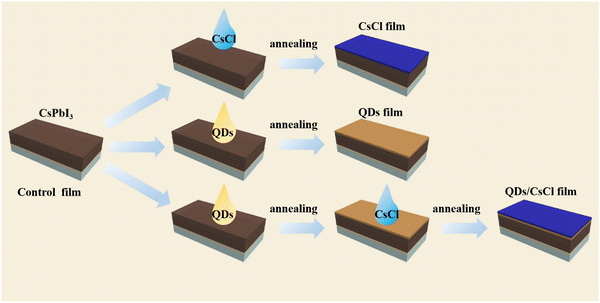 | ||
| Fig. 1 Schematic illustrating the deposition processes of the CsPbI3–CsCl, CsPbI3-QDs and CsPbI3-QDs/CsCl films. | ||
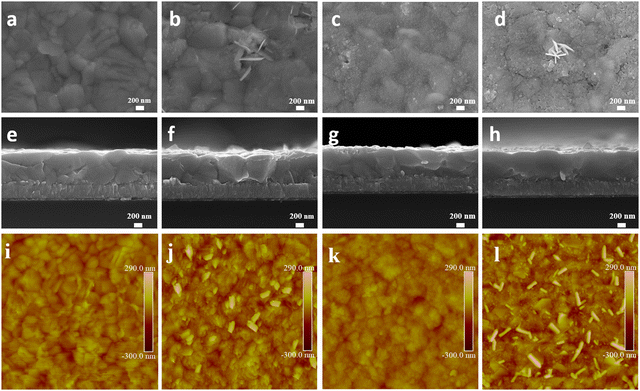
Scanning electron microscopy (SEM) was employed to evaluate the film morphology (Fig. 2 and Fig. S2, ESI†). The control CsPbI3 film exhibits uniform morphology (Fig. 2a). 2D nanosheets estimated to be around 200–350 nm in width appear on the surface of the CsPbI3–CsCl film (Fig. 2b and f). They are uniformly distributed on the surface and are perpendicular to the surface of the perovskite film. The CsPbCl3 QDs are uniformly distributed on the surface of the QDs film after spin-coating the CsPbCl3 QDs solution (Fig. 2c and g). After depositing the CsPbCl3 QDs, the CsPbI3-QDs film show a denser and more uniform surface morphology. For the CsPbI3-QDs/CsCl film, the CsPbCl3 QDs are uniformly dispersed and 2D nanosheets are also generated on the surface, forming a 2D/0D electron blocking layer. Atomic force microscopy (AFM) was further used to reveal the morphology change. The roughness of the QDs film is reduced from 41.8 to 28.6 nm compared with control CsPbI3 film, while the roughness of the CsPbI3-QDs/CsCl film is reduced from 72.5 to 48.1 nm compared with the control CsCl film, indicating that the CsPbCl3 QDs are more easily deposited at the grain boundary and reduce the surface roughness, which is conducive to the contact between CsPbI3 and the carbon electrode.
The X-ray diffraction (XRD) patterns in Fig. 3a reveal that the control CsPbI3 film exhibits two intense peaks at 14.3° and 28.8°, which are assignable to the (110) and (220) peaks of the CsPbI3 perovskite, respectively. After post treatment with CsCl solution, an additional peak is observed at 9.5°. As reported in our previous work, this peak corresponds to the (002) plane of 2D Cs2PbI2Cl2, resulting from the reaction between CsCl and CsPbI3.40,43 For the CsPbI3-QDs film, the peak corresponding to CsPbCl3 appear. As expected, both Cs2PbI2Cl2 and CsPbCl3 peaks are found for the CsPbI3-QDs/CsCl films. For more detailed observations in Fig. S3 (ESI†), the characteristic peaks of CsPbI3 perovskite shift to a larger angle after QDs or CsCl treatment, implying the partial replacement of I− ions with Cl− ions.44,45
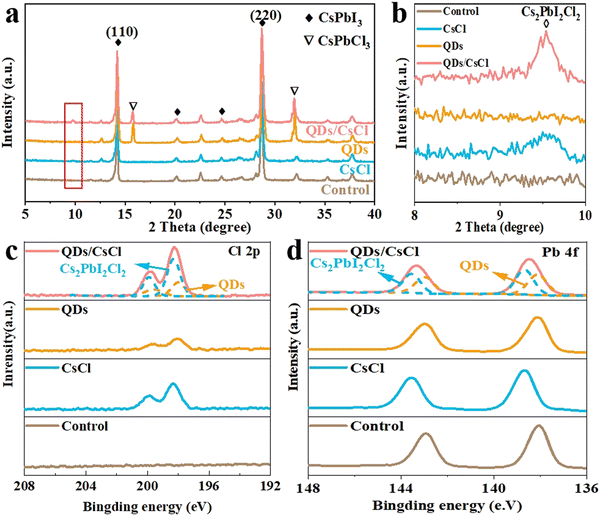 | ||
| Fig. 3 (a) XRD patterns and (b) magnified XRD patterns (in the 2θ ranges of 8–10°) of different CsPbI3 films. XPS spectra of (c) Cl 2p and (d) Pb 4f for different CsPbI3 films. | ||
X-ray photoelectron spectroscopy (XPS) measurements were further carried out to evaluate the composition of different CsPbI3 films. As shown in Fig. S4 (ESI†), compared with the control CsPbI3 film, the characteristic peaks of Cl 2p appeared in the CsPbI3–CsCl, -QDs and -QDs/CsCl films,45,46 indicating that Cl has been incorporated after post treatment. Due to the difference in the chemical environment of the Cl element, compared with the CsPbI3–CsCl film, the Cl 2p peak shifts to a lower binding energy for the CsPbI3-QDs film. As expected, the Cl 2p in the CsPbI3-QDs/CsCl films can be deconvolved into two sets of Cl 2p peaks, corresponding to the presence of CsPbCl3 QDs and Cs2PbI2Cl2 nanosheets. Similarly, the Pb 4f peaks can also be deconvolved into two sets of Pb 4f peaks. (Fig. 2d).
Ultraviolet-visible (UV-Vis) absorption spectra were subsequently measured for different films. As shown in Fig. 4a, the pristine CsPbI3 film shows the absorption onset at around 741 nm. Due to the partial replacement of I with Cl, the absorption onset of the CsPbI3–CsCl film blue shifts to about 737 nm. Similarly, a blue shift is also observed for the absorption onset of the CsPbI3-QDs film (about 735 nm). More obviously, the absorption onset of the CsPbI3-QDs/CsCl film blue shifts to about 734 nm. According to Tauc plots in Fig. S5 (ESI†), Eg for the pristine CsPbI3, CsPbI3–CsCl, CsPbI3-QDs and CsPbI3-QDs/CsCl films are calculated to be 1.671, 1.683, 1.686 and 1.688 eV, respectively. The confocal PL mappings in Fig. S6 (ESI†) further confirm the above phenomenon.
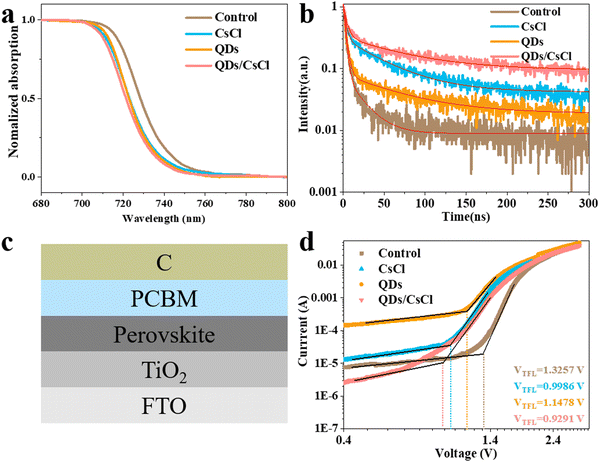 | ||
| Fig. 4 (a) UV-Vis, (b) TRPL spectra, (c) architecture of the electro-only device for SCLC, and (d) SCLC results for different CsPbI3 films. | ||
Time-resolved photoluminescence decay (TRPL) spectra were also taken for different films (Fig. 4b). By fitting the TRPL spectra with a double exponential decay model, the average lifetime (τave) of the different films could be calculated. As shown in Table S1 (ESI†), the τave of the control CsPbI3 film is 8.87 ns, while the τave of the CsPbI3–CsCl, -QDs and -QDs/CsCl films are obviously increased to 40.60 ns, 38.94 ns and 60.24 ns, respectively. Therefore, post treatment has well prolonged the carrier lifetime due to the reduced defect density, especially for the CsPbI3-QDs/CsCl film.47
Space charge limited current (SCLC) measurements were performed by constructing an electron-only device of FTO/TiO2/CsPbI3/PCBM/C (Fig. 4c). According to the dark J–V curve (Fig. 4d), the trap density (nt) can be calculated by the trap-filled limit voltage (VTFL).
| nt = 2ε0εVTFL/ed2 | (1) |
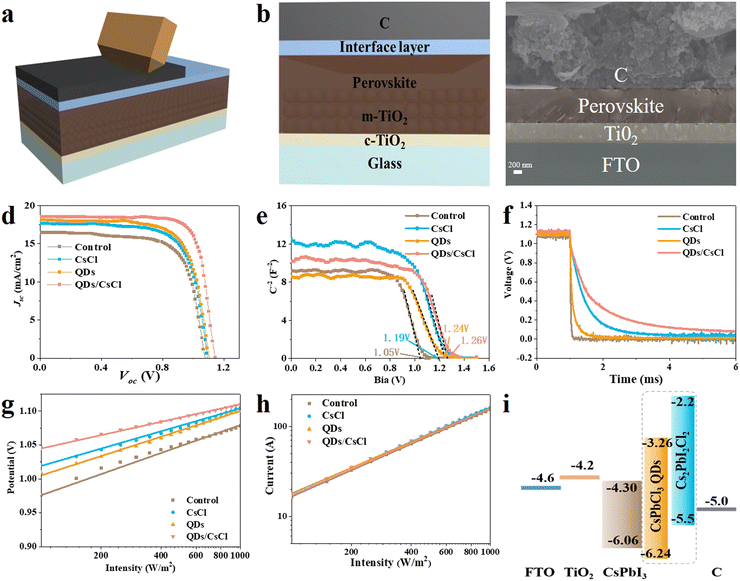
CsPbI3 C-PSCs were fabricated by directly painting commercial carbon paste on the perovskite films, followed by annealing 120 °C (Fig. 5a). All processes were carried out in dry air. Fig. 5b show the architecture of the CsPbI3 C-PSCs: FTO/c-TiO2/m-TiO2/CsPbI3/carbon and the cross-sectional SEM image in Fig. 5c indicates the close contact at the CsPbI3/carbon interface. The current density-voltage (J–V) curves in Fig. 5d and Table S2 (ESI†) indicate that the control device achieves a PCE of 12.51% with a Voc of 1.081 V, Jsc of 16.46 mA cm−2 and FF of 0.703. After treatment with CsCl, the CsPbI3–CsCl PSCs device displays a PCE of 13.65% with a Voc of 1.099, Jsc of 17.67 mA cm−2 and FF of 0.703. The CsPbI3-QDs device displays a PCE of 14.18% with a Voc of 1.105, Jsc of 18.14 mA cm−2 and FF of 0.708. Interestingly, the PCE of the CsPbI3-QDs/CsCl device is significantly improved to 16.10% with a Voc of 1.137 V, Jsc of 18.74 mA cm−2 and FF of 0.755, respectively. As noted, the large improvement in the PCE for the CsPbI3-QDs/CsCl device is mainly attributed to the increase in Voc and FF, which should originate from the improvement in energy level alignment and the reduction in the surface roughness. The J–V curves obtained from forward and reverse scans (Fig. S7, ESI†) and the steady-state power output for the CsPbI3-QDs/CsCl device demonstrate the small hysteresis and stable power output (Fig. S7, ESI†). The device stability was tested at 85 °C in N2 conditions (Fig. S8, ESI†). The QDs/CsCl device maintains over 95% of its initial PCE after 140 h, while the PCE of the pristine device declines to about 84%.
Mott–Schottky (M–S) plots (Fig. 5e) were taken for different devices to obtain the built-in potential (Vbi). The Vbi for the pristine CsPbI3 device is calculated to be about 1.06 V. After post treatment, all devices show increased Vbi and the highest Vbi of 1.26 V is achieved for the CsPbI3-QDs/CsCl device. The higher Vbi provides a larger driving force for carrier separation and collection, which is favorable for achieving higher Voc and FF. The transient photo-voltage decay (TPV) results in Fig. 5f indicate that the CsPbI3-QDs/CsCl device shows the slowest decay, further proving the most effective charge separation and the least charge recombination. The dark J–V curves of the pristine and QDs/CsCl devices are shown in Fig. S9 (ESI†). The QDs/CsCl device exhibits a much lower dark current density compared with the pristine device under a positive bias voltage, indicating that in the QDs/CsCl device the composite electron blocking layer well suppressed the back transfer of electrons from the perovskite to the carbon electrode.
The light intensity dependence of Jsc and Voc are shown in Fig. 5g and h, respectively. The recombination mechanism can be understood according to eqn (2) and (3).
| Jsc ∝ Iα (α ≤ 1) | (2) |
Voc = ε(KBT/e)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(I) + constant ln(I) + constant | (3) |
Conclusion
We have formed a composite electron blocking layer (CsPbCl3 QDs/Cs2PbI2Cl2) on the CsPbI3 film structure by successively treating the film with CsPbCl3 QDs and CsCl solutions. Such architecture forms a favorable energy level alignment for electron blocking. Furthermore, the crystal defects of the CsPbI3 film were also effectively passivated. As a result, the PCE of CsPbI3 C-PSCs was promoted from 12.51% to 16.10%.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51971012).References
- C. Liu, J. He, M. Wu, Y. Wu, P. Du, L. Fan, Q. Zhang, D. Wang and T. Zhang, All-Inorganic CsPbI2Br Perovskite Solar Cell with Open-Circuit Voltage over 1.3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V by Balancing Electron and Hole Transport, Sol. RRL, 2020, 4, 2000016 CrossRef CAS.
V by Balancing Electron and Hole Transport, Sol. RRL, 2020, 4, 2000016 CrossRef CAS. - Q. Tai, K.-C. Tang and F. Yan, Recent progress of inorganic perovskite solar cells, Energy Environ. Sci., 2019, 12, 2375–2405 RSC.
- M. Chen, M.-G. Ju, H. F. Garces, A. D. Carl, L. K. Ono, Z. Hawash, Y. Zhang, T. Shen, Y. Qi, R. L. Grimm, D. Pacifici, X. C. Zeng, Y. Zhou and N. P. Padture, Highly stable and efficient all-inorganic lead-free perovskite solar cells with native-oxide passivation, Nat. Commun., 2019, 10, 16 CrossRef CAS PubMed.
- A. Ho-Baillie, M. Zhang, C. F. J. Lau, F.-J. Ma and S. Huang, Untapped Potentials of Inorganic Metal Halide Perovskite Solar Cells, Joule, 2019, 3, 938–955 CrossRef CAS.
- Z. Chen, Q. Dong, Y. Liu, C. Bao, Y. Fang, Y. Lin, S. Tang, Q. Wang, X. Xiao, Y. Bai, Y. Deng and J. Huang, Thin single crystal perovskite solar cells to harvest below-bandgap light absorption, Nat. Commun., 2017, 8, 1890 CrossRef PubMed.
- L. Qiu, S. He, L. K. Ono, S. Liu and Y. Qi, Scalable Fabrication of Metal Halide Perovskite Solar Cells and Modules, ACS Energy Lett., 2019, 4, 2147–2167 CrossRef CAS.
- G. Tong, L. K. Ono and Y. Qi, Recent Progress of All-Bromide Inorganic Perovskite Solar Cells, Energy Technol., 2020, 8, 1900961 CrossRef CAS.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, High-Efficiency Perovskite Solar Cells, Chem. Rev., 2020, 120, 7867–7918 CrossRef CAS PubMed.
- H. Wang, Z. Dong, H. Liu, W. Li, L. Zhu and H. Chen, Roles of Organic Molecules in Inorganic CsPbX3 Perovskite Solar Cells, Adv. Energy Mater., 2021, 11, 2002940 CrossRef CAS.
- Y. Li, H. Xie, E. L. Lim, A. Hagfeldt and D. Bi, Recent Progress of Critical Interface Engineering for Highly Efficient and Stable Perovskite Solar Cells, Adv. Energy Mater., 2022, 12, 2102730 CrossRef CAS.
- H. Chen and S. Yang, Methods and strategies for achieving high-performance carbon-based perovskite solar cells without hole transport materials, J. Mater. Chem. A, 2019, 7, 15476–15490 RSC.
- D. Bogachuk, S. Zouhair, K. Wojciechowski, B. Yang, V. Babu, L. Wagner, B. Xu, J. Lim, S. Mastroianni, H. Pettersson, A. Hagfeldt and A. Hinsch, Low-temperature carbon-based electrodes in perovskite solar cells, Energy Environ. Sci., 2020, 13, 3880–3916 RSC.
- H. Chen and S. Yang, Carbon-Based Perovskite Solar Cells without Hole Transport Materials: The Front Runner to the Market?, Adv. Mater., 2017, 29, 1603994 CrossRef PubMed.
- X. Zhang, Z. Yu, D. Zhang, Q. Tai and X.-Z. Zhao, Recent Progress of Carbon-Based Inorganic Perovskite Solar Cells: From Efficiency to Stability, Adv. Energy Mater., 2022, 2201320 CrossRef.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D’Haen, L. D’Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca, E. Mosconi, F. D. Angelis and H.-G. Boyen, Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite, Adv. Energy Mater., 2015, 5, 1500477 CrossRef.
- J. K. Nam, S. U. Chai, W. Cha, Y. J. Choi, W. Kim, M. S. Jung, J. Kwon, D. Kim and J. H. Park, Potassium Incorporation for Enhanced Performance and Stability of Fully Inorganic Cesium Lead Halide Perovskite Solar Cells, Nano Lett., 2017, 17, 2028–2033 CrossRef CAS PubMed.
- P. Yu, W. Zhang, F. Ren, J. Wang, H. Wang, R. Chen, S. Zhang, Y. Zhang, Z. Liu and W. Chen, Strategies for highly efficient and stable cesium lead iodide perovskite photovoltaics: mechanisms and processes, J. Mater. Chem. C, 2022, 10, 4999–5023 RSC.
- Y. Zhao, J. Duan, Y. Wang, X. Yang and Q. Tang, Precise stress control of inorganic perovskite films for carbon-based solar cells with an ultrahigh voltage of 1.622 V, Nano Energy, 2020, 67, 104286 CrossRef CAS.
- X. Chang, W. Li, L. Zhu, H. Liu, H. Geng, S. Xiang, J. Liu and H. Chen, Carbon-Based CsPbBr3 Perovskite Solar Cells: All-Ambient Processes and High Thermal Stability, ACS Appl. Mater. Interfaces, 2016, 8, 33649–33655 CrossRef CAS PubMed.
- Z. Yao, W. Zhao and S. Liu, Stability of the CsPbI3 perovskite: from fundamentals to improvements, J. Mater. Chem. A, 2021, 9, 11124–11144 RSC.
- Z. Dong, W. Li, H. Wang, X. Jiang, H. Liu, L. Zhu and H. Chen, High-Temperature Perovskite Solar Cells, Sol. RRL, 2021, 5, 2100370 CrossRef CAS.
- W. Zhu, J. Ma, W. Chai, T. Han, D. Chen, X. Xie, G. Liu, P. Dong, H. Xi, D. Chen, J. Zhang, C. Zhang and Y. Hao, Intermediate Phase-Assisted Sequential Deposition Toward 15.24%-Efficiency Carbon-Electrode Cspbi2br Perovskite Solar Cells, Sol. RRL, 2022, 6, 2200020 CrossRef CAS.
- H. Wang, S. Xiang, W. Li, H. Liu, L. Zhu, S. Xiao, S. Yang and H. Chen, Skillfully deflecting the question: a small amount of piperazine-1,4-diium iodide radically enhances the thermal stability of CsPbI3 perovskite, J. Mater. Chem. C, 2019, 7, 11757–11763 RSC.
- C. Dong, B. Xu, D. Liu, E. G. Moloney, F. Tan, G. Yue, R. Liu, D. Zhang, W. Zhang and M. I. Saidaminov, Carbon-based all-inorganic perovskite solar cells: Progress, challenges and strategies toward 20% efficiency, Mater. Today, 2021, 50, 239–258 CrossRef CAS.
- S. Xiang, W. Li, Y. Wei, J. Liu, H. Liu, L. Zhu and H. Chen, The synergistic effect of non-stoichiometry and Sb-doping on air-stable α-CsPbI3 for efficient carbon-based perovskite solar cells, Nanoscale, 2018, 10, 9996–10004 RSC.
- R. J. Sutton, G. E. Eperon, L. Miranda, E. S. Parrott, B. A. Kamino, J. B. Patel, M. T. Hörantner, M. B. Johnston, A. A. Haghighirad, D. T. Moore and H. J. Snaith, Bandgap-Tunable Cesium Lead Halide Perovskites with High Thermal Stability for Efficient Solar Cells, Adv. Energy Mater., 2016, 6, 1502458 CrossRef.
- A. Miyata, A. Mitioglu, P. Plochocka, O. Portugall, J. T.-W. Wang, S. D. Stranks, H. J. Snaith and R. J. Nicholas, Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites, Nat. Phys., 2015, 11, 582–587 Search PubMed.
- Z. Shao, H. Meng, X. Du, X. Sun, P. Lv, C. Gao, Y. Rao, C. Chen, Z. Li, X. Wang, G. Cui and S. Pang, Cs4PbI6-Mediated Synthesis of Thermodynamically Stable FA0.15Cs0.85PbI3 Perovskite Solar Cells, Adv. Mater., 2020, 32, 2001054 CrossRef CAS PubMed.
- J. Liu, L. Zhu, S. Xiang, H. Wang, H. Liu, W. Li and H. Chen, Cs-Doped TiO2 Nanorod Array Enhances Electron Injection and Transport in Carbon-Based CsPbI3 Perovskite Solar Cells, ACS Sustainable Chem. Eng., 2019, 7, 16927–16932 CrossRef CAS.
- X. Fu, K. Zhou, X. zhou, H. Ji, Y. Min and Y. Qian, Surface passivation for enhancing photovoltaic performance of carbon-based CsPbI3 perovskite solar cells, J. Solid State Chem., 2022, 308, 122891 CrossRef CAS.
- R. Yin, K.-X. Wang, X.-N. Huo, Y.-S. Sun, W.-W. Sun, Y.-K. Gao, T.-T. You and P.-G. Yin, A One-Step Ionic Liquid Interface-to-Bulk Modification for Stable Carbon-Based CsPbI3 Perovskite Solar Cells with Efficiency Over 15%, Adv. Mater. Interfaces, 2022, 9, 2201488 CrossRef CAS.
- S. Xiang, W. Li, Y. Wei, J. Liu, H. Liu, L. Zhu, S. Yang and H. Chen, Natrium Doping Pushes the Efficiency of Carbon-Based CsPbI3 Perovskite Solar Cells to 10.7%, iScience, 2019, 15, 156–164 CrossRef CAS PubMed.
- H. Wang, H. Liu, Z. Dong, X. Wei, W. Li, L. Zhu, C. Zhu, Y. Bai and H. Chen, Moisture is not always bad: H2O accelerates the conversion of DMAPbI3 intermediate to CsPbI3 for boosting the efficiency of carbon-based perovskite solar cells to over 16%, Fundam. Res., 2022 DOI:10.1016/j.fmre.2022.07.005.
- Y. Cui, J. Shi, F. Meng, B. Yu, S. Tan, S. He, C. Tan, Y. Li, H. Wu, Y. Luo, D. Li and Q. Meng, A Versatile Molten-Salt Induction Strategy to Achieve Efficient CsPbI3 Perovskite Solar Cells with a High Open-Circuit Voltage >1.2 V, Adv. Mater., 2022, 2205028 CrossRef CAS PubMed.
- Z. Dong, W. Li, H. Wang, X. Jiang, H. Liu, L. Zhu and H. Chen, Carbon nanotubes in perovskite-based optoelectronic devices, Matter, 2022, 5, 448–481 CrossRef CAS.
- S. Zouhair, S.-M. Yoo, D. Bogachuk, J. P. Herterich, J. Lim, H. Kanda, B. Son, H. J. Yun, U. Würfel, A. Chahboun, M. K. Nazeeruddin, A. Hinsch, L. Wagner and H. Kim, Employing 2D-Perovskite as an Electron Blocking Layer in Highly Efficient (18.5%) Perovskite Solar Cells with Printable Low Temperature Carbon Electrode, Adv. Energy Mater., 2022, 12, 2200837 CrossRef CAS.
- X.-N. Huo, K.-X. Wang, R. Yin, W.-W. Sun, Y.-S. Sun, Y.-K. Gao, T.-T. You and P.-G. Yin, High-performance carbon-based all-inorganic CsPbI2Br perovskite solar cells via ethylammonium iodide and phenethylammonium iodide synergistic passivation, Sol. Energy Mater. Sol. Cells, 2022, 247, 111963 CrossRef CAS.
- J. Zhou, Z. Ye, J. Hou, J. Wu, Y.-Z. Zheng and X. Tao, Efficient ambient-air-stable HTM-free carbon-based perovskite solar cells with hybrid 2D–3D lead halide photoabsorbers, J. Mater. Chem. A, 2018, 6, 22626–22635 RSC.
- K. Wang, Z. Li, F. Zhou, H. Wang, H. Bian, H. Zhang, Q. Wang, Z. Jin, L. Ding and S. Liu, Ruddlesden–Popper 2D Component to Stabilize γ-CsPbI3 Perovskite Phase for Stable and Efficient Photovoltaics, Adv. Electron. Mater., 2019, 9, 1902529 CAS.
- H. Wang, H. Liu, Z. Dong, W. Li, L. Zhu and H. Chen, Composition manipulation boosts the efficiency of carbon-based CsPbI3 perovskite solar cells to beyond 14%, Nano Energy, 2021, 84, 105881 CrossRef CAS.
- A. De, N. Mondal and A. Samanta, Luminescence tuning and exciton dynamics of Mn-doped CsPbCl3 nanocrystals, Nanoscale, 2017, 9, 16722–16727 RSC.
- Y. Su, X. Chen, W. Ji, Q. Zeng, Z. Ren, Z. Su and L. Liu, Highly Controllable and Efficient Synthesis of Mixed-Halide CsPbX3 (X = Cl, Br, I) Perovskite QDs toward the Tunability of Entire Visible Light, ACS Appl. Mater. Interfaces, 2017, 9, 33020–33028 CrossRef CAS PubMed.
- X. Zhao, T. Liu, Q. C. Burlingame, T. Liu, R. Holley, G. Cheng, N. Yao, F. Gao and Y.-L. Loo, Accelerated aging of all-inorganic, interface-stabilized perovskite solar cells, Science, 2022, 377, 307–310 CrossRef CAS PubMed.
- S. Yang, W. Liu, Y. Han, Z. Liu, W. Zhao, C. Duan, Y. Che, H. Gu, Y. Li and S. Liu, 2D Cs2PbI2Cl2 Nanosheets for Holistic Passivation of Inorganic CsPbI2Br Perovskite Solar Cells for Improved Efficiency and Stability, Adv. Energy Mater., 2020, 10, 2002882 CrossRef CAS.
- T. V. Vu, A. A. Lavrentyev, B. V. Gabrelian, K. D. Pham, O. V. Parasyuk, N. M. Denysyuk and O. Y. Khyzhun, Electronic structure and optical constants of CsPbCl3: The effect of approaches within ab initio calculations in relation to X-ray spectroscopy experiments, Mater. Chem. Phys., 2021, 261, 124216 CrossRef CAS.
- Z. Zhao, W. Xu, G. Pan, Y. Liu, M. Yang, S. Hua, X. Chen, H. Peng and H. Song, Enhancing the exciton emission of CsPbCl3 perovskite quantum dots by incorporation of Rb+ ions, Mater. Res. Bull., 2019, 112, 142–146 CrossRef CAS.
- Y. Zhang, Z. Li, H. Chen, Y. Xu, Y. Lei, G. Peng, X. Zhou, Q. Wang and Z. Jin, Double-Layer Quantum Dots as Interfacial Layer to Enhance the Performance of CsPbI3 Solar Cells, Adv. Mater. Interfaces, 2022, 2200813 CrossRef CAS.
- V. M. Le Corre, E. A. Duijnstee, O. El Tambouli, J. M. Ball, H. J. Snaith, J. Lim and L. J. A. Koster, Revealing Charge Carrier Mobility and Defect Densities in Metal Halide Perovskites via Space-Charge-Limited Current Measurements, ACS Energy Lett., 2021, 6, 1087–1094 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qm01124g |
| This journal is © the Partner Organisations 2023 |