Polybutylene succinate, a potential bio-degradable polymer: synthesis, copolymerization and bio-degradation
K. S.
Savitha
b,
Bharatkumar
Ravji Paghadar
c,
M.
Senthil Kumar
 a and
R. L.
Jagadish
*b
a and
R. L.
Jagadish
*b
aAlumnus, Department of Chemistry, Indian Institute of Technology Madras, Chennai, India
bDepartment of Polymer Science, Sir M. V. P. G. Centre, Tubinakere, Mandya, India. E-mail: rljagadish@yahoo.com
cDepartment of Chemistry, MAHE, Manipal University, Manipal, India
First published on 10th May 2022
Abstract
Poly(butylene succinate) is one of the emerging bio-degradable polymers, which has huge potential to be employed in a wide range of applications. Furthermore, it is also recognized as one of the biopolymers of choice due to its environmentally benign nature and biodegradability. Even though PBS has various advantages, brittleness, thermal stability and a lack of the high molecular weight required for industrial applications restricts its commercial implementation. Various catalyst systems have been employed to produce high molecular weight PBS. In addition, various strategies like copolymerization and the preparation of composites and blends have been attempted to improve the physical and mechanical properties of PBS. However, the synthesis of high molecular weight PBS with enhanced properties still remains a challenging task. Herein, catalyst systems that have been employed towards the synthesis of PBS and its copolymers as well as its property enhancements (including bio-degradation) through copolymerization are covered in detail. This will provide readers with a detailed and systematic understanding of the catalysis, copolymerization and biodegradation of PBS.
Introduction
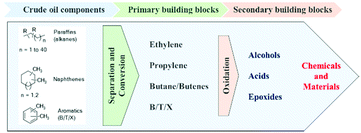
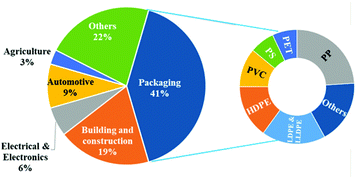
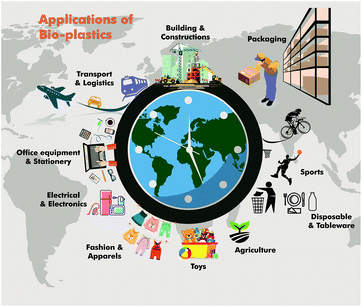
Hermann Staudinger (father of polymer science) was honored with the Nobel Prize for Chemistry for his pioneering research on macromolecules. His extensive research led to a basic understanding as well as designing the applications for polymers in every day life as commodities and specialty (special functional) polymers.1 In addition, the petrochemical revolution of the last century led to the production of polymers such as polyethylene (HDPE, LDPE, LLDPE), polypropylene (PP), poly(vinyl chloride) (PVC), poly(ethylene terephthalate) (PET), and polystyrene (PS) in large quantities from fossil resources (Fig. 1).2 Furthermore, they have gained significant importance as the material of choice for various applications such as food packaging, cosmetics, personal hygiene, building and construction, pharmaceuticals, automobiles, electrical devices and electronics, agriculture, etc., due to their low cost combined with excellent mechanical, thermodynamic and insulation properties (Fig. 2).3However, because of their durability and non-biodegradability, many synthetic petrochemical based polymers tend to accumulate in the environment rather than degrading, which has led to severe environmental plastic pollution. The presence of a considerable amount of aromatic moieties in polymers increases the durability and hence they are not biodegradable. At the beginning of the twenty first century, the accumulation of plastic waste and its negative environmental impact coupled with an increase in oil prices have led to the development of biodegradable polymers, which are generally referred to as synthetic or processed organic macromolecules derived from biological resources (Fig. 3).4
In recent years, the design and use of environmentally benign polymers from renewable resources are of significant interest among scientists in both academia and industry. Two different strategies can be employed for improving the ecological and sustainable aspects of polymeric materials. The first strategy mainly emphasizes the need for new bio-alternatives to petrochemical based polymers and the second one focuses on the end-of-life situation, which includes either bio-degradation, recycling, incineration, landfill or composting. Bio-based alternatives are of significant importance as they can save up to 315 million tons of CO2 equivalents annually.2,5
Furthermore, bioplastic alternatives have been developed for almost every conventional petroleum based polymer as well as for their corresponding applications (Fig. 4). In this regard, biodegradable polyesters, such as poly(lactic acid) (PLA), poly(butylene succinate) (PBS), polyhydroxyalkanoates (PHAs) and aliphatic–aromatic copolyesters, have gained increasing interest as bio-alternatives to traditional oil-based commodity polymers, such as polyolefins and poly(ethylene terephthalate) (PET).6 In addition, various renewable polymers, including thermoplastics,7 thermosets,8 and vitrimers,9 with good performances have also been developed to mimic traditional petroleum-based polymers.
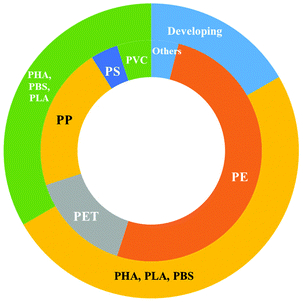
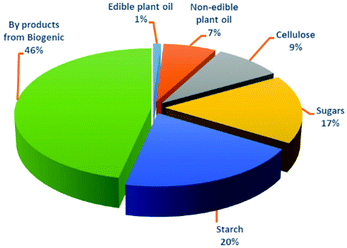
Moreover, the global production of bio-plastics (bio-based/non-biodegradable and biodegradable (Fig. 5) is expected to increase from 2.4 million tonnes in 2021 (which is <1% of the total volume of petroleum based polymers) to 7.59 million tonnes in 2026 with an annual growth rate (CAGR) of about 21% (Fig. 6a). In particular, the global production of PBS is expected to increase from 4% in 2021 to 16% in 2026 (Fig. 6b). Moreover, the segment wise global production of biodegradable polymers in 2021 is explained in Fig. 6b.2h
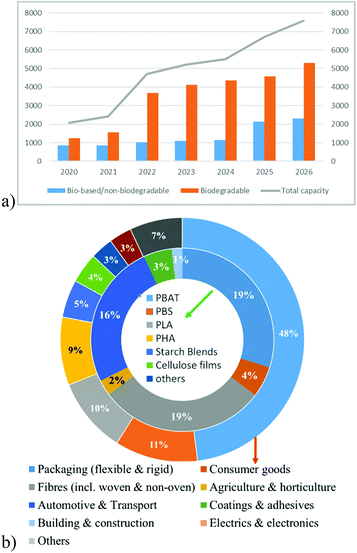 | ||
| Fig. 6 Global production of bioplastics in 2021 by (a) volume (in kilo tonnes) (b) polymer type (bio-degradable) and segment (non-biodegradable and biodegradable). | ||
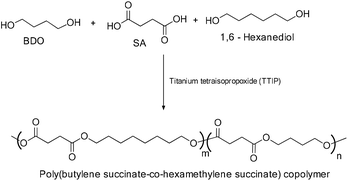
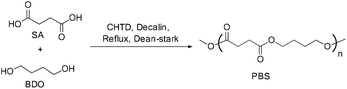
These bio-based polymers are capable of providing new macromolecular frameworks with superior properties.10 Among the bio-based polymers, poly(hydroxyalkanoate)s, poly(lactic acid), and poly(butylene succinate) (PBS) are of considerable interest and have already led to large scale production to cater to various applications such as packaging, agriculture, biomedical, automotive, electrical, pharmaceutical, etc. (Fig. 7). Poly(butylene succinate) belongs to the poly(alkylene dicarboxylate) family, which can be synthesized by the reaction of 1,4-butanediol with succinic acid.11
Bio-based synthesis of monomers (SA and BDO) for PBS
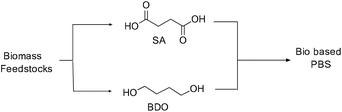
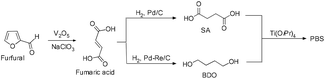
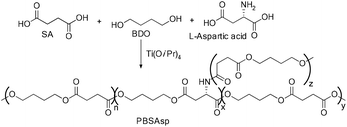
Recent developments in fermentation technology led to the production of bio-based monomers on an industrial scale. In the fermentation process, specific bacterial strains such as Actinobacillus succinogenes, Mannheimia succiniciproducens, Anaerobiospirillum succiniciproducens and recombinant Escherichia coli converts substrates such as corn starch, whey, cane molasses, glycerol, and wood hydrolysates into succinic acid in high yields, with lower amounts of by-products.12 This production of SA via a fermentation process is mainly an environmentally benign method which utilizes CO2 to convert the substrates into the products.13Moreover, succinic acid (SA) is one of the top twelve bio-based building blocks that are produced from carbohydrates, and thus has a potential market in the chemical industry.14 A bio-synthetic method for the production of 1,4-butanediol (BDO) also involves the fermentation of sugars, glucose, starch, xylose, etc. (Fig. 8) to yield SA and subsequent reduction of the SA leads to BDO. Conversely, the direct synthesis of BDO through the fermentation of sugars, by using engineered E. coli, has also been reported in the literature.15 The synthesis of both SA and BDO from biomass feedstocks appears to be particularly interesting, as it gives access to the production of fully bio-based PBS (Scheme 1).16
Synthesis of polybutylene succinate
Aliphatic polyesters are an important class of sustainable polymers under investigation due to the degradation of ester bonds under enzymatic conditions. Extensive research work has been carried out in developing biodegradable polymers, such as poly(caprolactone) (PCL), poly(butylene succinate) (PBS), poly(lactic acid) (PLA), and poly(butylene adipate-co-butylene terephthalate) (PBAT).2,13,17 In particular, PBS exhibits several interesting properties, such as barrier and thermal properties, melt processability, chemical resistance and most importantly biodegradability. Thus, it has gained considerable attention as a potential biodegradable polymer to mimic petroleum based polymers in various applications.2–6,15bIn the early 1930s, Carothers first initiated the preparation of industrially important polymers via the condensation reaction, which included polyamides and other aliphatic polyesters (Scheme 2).18 Nylon 66, of the polyamide family, was quickly identified during middle of the last century as one of the potential fiber-forming polymers. In copolyesters, the molecular weight of the obtained PBS polymer was very low and resulted in a weak and brittle polymer.19 Extensive research work is being carried out in developing efficient synthetic methods for the production of high molecular weight PBS. However, the preparation of high molecular weight aliphatic polyesters from dicarboxylic acids and diols still remains a challenging task due to their low thermal stability.20
Conventional method for the synthesis of PBS
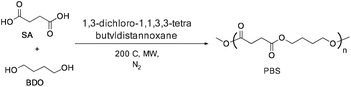
In general, the synthesis of PBS involves two steps, in which the first step is the esterification or trans-esterification (for production of oligomers) reaction at 160–180 °C followed by polycondensation (or) trans-esterification (for extension of the chain length) of the resulting oligomers at 230–250 °C under vacuum (Scheme 3).21 Various organo-metals, metal oxides, and metal halides have been used as catalysts to improve the efficacy of the polymerization reaction.22Titanium alkoxide based catalysts
Titanium tetrabutoxide (TBT) is the most widely used catalyst for the synthesis of PBS and it copolymers due to its catalytic efficiency.23Branched polybutylene succinates were prepared by a two-stage esterification and condensation polymerization strategy using 1,4-butanediol, 1,2-decanediol, and succinic acid in the presence of TBT as a catalyst (Scheme 4).24
PBS copolymers with a rigid C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C structure were also prepared by the copolymerization of BDO, SA and acetylene dicarboxylic acid using TBT catalyst. PBS and poly(butylene succinate-co-butylene acetylenedicarboxylate) (PBSAD) copolymers with Mn = 4.45 and 2.48 × 104 g mol−1 respectively were synthesized under the same reaction conditions (Scheme 5).25
C structure were also prepared by the copolymerization of BDO, SA and acetylene dicarboxylic acid using TBT catalyst. PBS and poly(butylene succinate-co-butylene acetylenedicarboxylate) (PBSAD) copolymers with Mn = 4.45 and 2.48 × 104 g mol−1 respectively were synthesized under the same reaction conditions (Scheme 5).25
A new copolymer, poly(butylene succinate/dithiodipropionate), P(BSBDTDP), was successfully synthesized using 3,3-dithiodiproprionic acid as a comonomeric unit in the presence of the TBT catalyst (Scheme 6).26
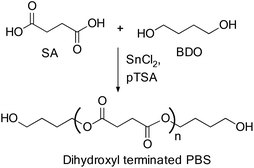
Also, dihydroxyl terminated PBS prepolymers, which are mainly used for chain extension and the copolymerization reaction, were also prepared by the reaction of BD and SA in the presence of the TBT catalyst (Scheme 7).27
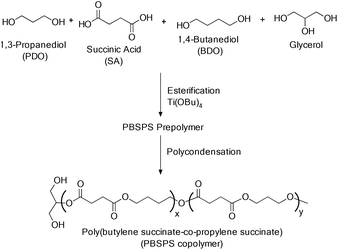
Poly(butylene succinate-co-propylene succinate) (PBSPS) copolymer was prepared by a bulk polymerization reaction in two steps using di-acid and diol in the presence of TBT as the catalyst (Scheme 8).28a Glycerol is used as a partial cross-linking agent to improve the thermal and mechanical properties of the copolymer. A polymer with Mn = 1.85 × 104 g mol−1, Mw = 9.84 × 104 g mol−1 and PDI = 5.32 was synthesized.28b
Aliphatic–aromatic bio-based poly(butylene succinate-co-furanoate) (PBSFc) copolymer was prepared by a melt polycondensation reaction of the corresponding esters in the presence of TBT as a catalyst. The number average molecular weights for PBS and PBSFc were 1.52 and 1.27 × 104 g mol−1 and the corresponding PDI values were 2.03 and 2.12, respectively (Scheme 9).29
Titanium tetrabutoxide as a modifier and compatibilizer
Interestingly, TBT was also employed as a modifier and compatibilizer in preparing polyester blends. Polymer blends prepared using poly(butylene succinate-co-adipate) and poly(ε-caprolactone) in the presence of the TBT catalyst demonstrated good compatibility and this might be due to the transesterification reaction between the polymers and, in addition, the viscosity of the blends tended to increase with an increase in catalyst concentration.30Titanium tetraisopropoxide as a catalyst
Titanium tetraisopropoxide is the next preferred catalyst for the synthesis of PBS and its copolymers. Numerous synthetic methods reported in the literature used titanium tetraisopropoxide as a catalyst for both esterification and polycondensation reactions.Poly(butylene succinate-ran-butylene adipate) random copolymers (PBSA)31 (Scheme 10) and high molecular weight poly[(butylene succinate)-ran-(ε-caprolactone)] copolyesters (PBS-ran-PCL)32 were prepared by melt polycondensation over a wide composition range using TTIP as a polycondensation catalyst. PBS with Mn = 7500, Mw = 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 and PDI = 2.9 was synthesized using this protocol (Scheme 11).32
470 and PDI = 2.9 was synthesized using this protocol (Scheme 11).32
Furfural is one of the most important bio-based compounds, which in turn can be readily produced from cellulose. Furthermore, furfural, on oxidation and subsequent reduction of the resulting fumaric acid, led to the formation of bio-based succinic acid and 1.4-butanediol. Tachibana and co-workers reported the titanium tetraisopropoxide mediated synthesis of PBS with Mn = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and Mw/Mn = 2.6. Furthermore, it was found that, both bio-based and petroleum based PBS synthesized using TTIP as a catalyst, were found to have similar properties (Scheme 12).33
000 and Mw/Mn = 2.6. Furthermore, it was found that, both bio-based and petroleum based PBS synthesized using TTIP as a catalyst, were found to have similar properties (Scheme 12).33
The synthesis of PBS (Mn = 6.49 × 104 g mol−1, Mw = 12.47 × 104 g mol−1 and PDI = 1.87) and its copolymers containing 1,4-cyclohexane dimethylene succinate (CHDMS) or butylene 1,4-cyclohexanedicarboxylate (BCHDA) units was further exploited via melt polycondensation using TTIP as a catalyst (Scheme 13).34
TTIP was further explored towards the synthesis of poly(butylene succinate-co-hexamethylene succinate) (P(BS-co-HS)) copolyesters. The intrinsic viscosity of the PBS homopolymer obtained was found to be 0.67 dl g−1 (Scheme 14).35
Efficiency of titanium based catalysts
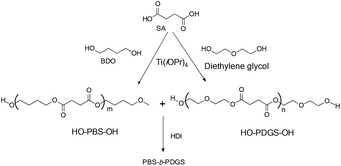
However, titanium based catalysts are sensitive to moisture and water is the by-product formed during the esterification reaction. Thus, it is observed that the addition of titanium based catalysts during the esterification reaction reduces the activity of the catalyst, when compared to the addition of the catalyst during the polycondensation step. This is directly related to the stability of the catalyst in the presence of the by-product (water) formed during the esterification step.36 Thus, to improve the catalytic efficiency, the catalyst was added during the polycondensation step (Scheme 15).2c,31Poly(butylene succinate-b-poly(diethylene glycol succinate) (PBS-b-PDGS) multiblock copolymers were synthesized using chain extenders via HO-PBS-OH and HO-PDGS-OH precursors, which in turn were prepared by using TTIP as a catalyst. During polymer precursor synthesis, the catalyst was added during the polycondensation step. PBS homopolymer with Mn = 3.5 × 10–4 g mol−1, Mw = 15.1 × 10–4 g mol−1 and PDI = 4.3 was synthesized under the same reaction conditions using chain extenders (Scheme 16).37
Poly[(butylene succinate)-co-poly(tetramethylene glycol)] (PBSTMG) copolymers were synthesized by a two step esterification and polycondensation reaction using SA, BD and poly(tetramethylene glycol) (PTMG) in the presence of the TBT catalyst (Scheme 17), in which the catalyst was added during the polycondensation step.38
Even in the few methods reported in the literature, the catalyst was added in parts in both the esterification and polycondensation steps to improve the conversion and achieve a high molecular weight of the polymer (Scheme 18).39
Titanium alkoxides in the presence of stabilizers
It is very well known that the synthesis of high molecular weight PBS still remains a challenging task due to its low thermal stability. Thus, the use of thermal stabilizers, in combination with a Lewis acid catalyst, has also been explored in producing high molecular weight copolyesters. As a result, titanium(IV) butoxide as a polycondensation catalyst, in combination with polyphosphoric acid as a thermal stabilizer, was utilized for the synthesis of poly(butylene succinate)/nano-boehmite composites40 (Scheme 19).The same catalyst combination was used to promote the polycondensation reaction towards the synthesis of poly(butylenesuccinate-co-fumarate) copolymer and poly-alkylene succinates with an odd and even number of methylene units.41 The addition of polyphosphoric acid (PPA) during the polycondensation step was also reported for the synthesis of poly(butylene-co-propylene succinate) copolymer. It is believed that the addition of PPA will reduce unwanted side reactions like etherification and polymer degradation.42
Qiu et al. demonstrated the use of a combination of titanium(IV) butoxide and diphenylphosphinic acid as a catalyst and co-catalyst system for the synthesis of poly(butylene succinate-co-decamethylene succinate) copolyesters (Scheme 20).43
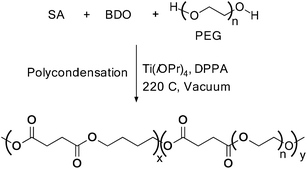
Similarly, Zhou et al. used titanium tetraisopropoxide, in combination with diphenylphosphinic acid (DPPA), as a catalyst for the polycondensation reaction to synthesize poly(butylene succinate) and poly(ethylene glycol) copolymers (Scheme 21). The formation of a high molecular weight copolymer, with Mw = 9.32 × 104 was observed when the ratio of BD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG2000 was around 95
PEG2000 was around 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.44
5.44
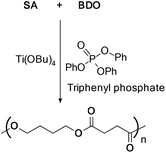
The combination of TBT as a catalyst and triphenyl phosphate as a stabilizer was also utilized for the synthesis of high molecular weight PBS with Mn = 6.3 × 104 g mol−1 and an intrinsic viscosity of 1.53 dL g−1 (Scheme 22).45
Tin based catalysts
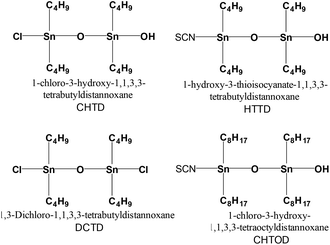
To find an efficient catalyst system, Ishii and co-workers screened various metal catalysts, like 1-chloro-3-hydroxy-1,1,3,3-tetrabutyldistannoxane, Ti(i-OPr)4, Al(i-OPr)3, Sn, and SnCl2, for PBS synthesis (Table 1). Moreover, they explored different distannoxane catalysts, such as CHTD, HTTD, DCTD and CHTOD, for the synthesis of PBS (Fig. 9) (Table 2).| Catalyst | □inh (dL g−1) |
|---|---|
| None | 0.49 |
| Ti(i-OPr)4 | 0.51 |
| Al(i-OPr)3 | 0.69 |
| Sn | 0.59 |
| SnCl2 | 0.69 |
| CHTD | 0.99 |
| Catalyst | □inh (dL g−1) |
|---|---|
| DCTD | 0.83 |
| HTTD | 0.76 |
| CHTD | 0.99 |
| CHTOD | 0.90 |
From the experimental screening, 1-chloro3-hydroxy-1,1,3,3-tetrabutyl-distannoxane (CHTD) exhibited better catalytic activity than the other catalysts and PBS thus obtained has an inherent viscosity of 0.99 dL g−1 and the number-average molecular weight (Mn) and weight-average molecular weight (Mw) values were 9.25 × 104 g mol−1 and 1.76 × 104 g mol−1 respectively (Scheme 23).46
The use of 1,3-dichloro-1,1,3,3-tetrabutyldistannoxane (DCTD) was explored as a catalyst for the synthesis of PBS with Mw of 1.02 × 104 g mol−1.47 The application of dibutyl tin oxide (DBTO) as a catalyst for both esterification and polycondensation steps was realized during the synthesis of poly(butylene succinate) (Mw = 11.2 × 104 g mol−1 and PDI = 2.2) and its ionomers (Scheme 24).48
DBTO was further used for the synthesis of PBS copolymers using the carbohydrate-based diol 2,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,5-di-O-methylene-D-mannitol (Manx). The DBTO catalyst is found to be mild and demonstrates a higher catalytic activity compared to TBT, which aids the polymerization of thermally sensitive sugar monomers at lower temperatures and also with reduced reaction times. The Mw of the resulting PManxS homopolyester was higher than 30
3,5-di-O-methylene-D-mannitol (Manx). The DBTO catalyst is found to be mild and demonstrates a higher catalytic activity compared to TBT, which aids the polymerization of thermally sensitive sugar monomers at lower temperatures and also with reduced reaction times. The Mw of the resulting PManxS homopolyester was higher than 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 with a PDI of 2.3.49
000 g mol−1 with a PDI of 2.3.49
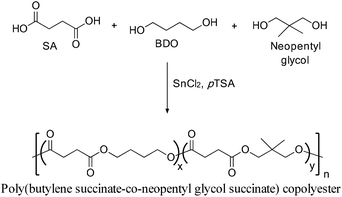
Stannous chloride and 4-methylbenzenesulfonic acid were used as a catalyst combination for the esterification reaction towards the synthesis of poly(butylene succinate-co-neopentyl glycol succinate) co-polyesters (Scheme 25).
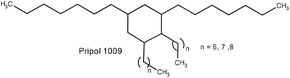
Furthermore, stannous chloride has also been used as a catalyst to promote the polycondensation reaction.50 Moreover, tin octoate was also explored as a catalyst for synthesis of dihydroxyl terminated PBS.51 High molecular weight PBS with Mn = 9.5 × 104 Da and Mw = 16.5 × 104 Da was prepared using Sn(Oct)2 catalyst via the ring opening polymerization of butyl succinate lactone, which in turn was prepared from PBS oligomers using zinc oxide as a catalyst (Scheme 26).52
Lee and co-workers employed tin octate as a catalyst in the presence of macro-initiators, such as polyethylene glycol (PEG), during the synthesis of PBS as well as its diblock (PEGPBS) and triblock (PBS-PEG-PBS) copolymers (Scheme 27).53
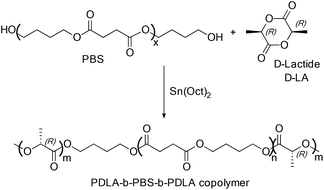
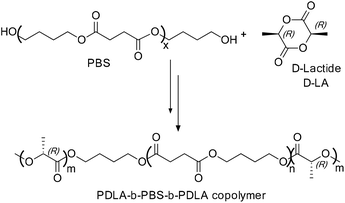
The PDLA-b-PBS-b-PDLA tri-block copolymer was synthesized by the ring opening polymerization of D-LA in the presence of hydroxyl capped PBS and tin(II) 2-ethylhexanoate (Sn(Oct)2) respectively as the macro-initiator and catalyst (Scheme 28).54
Furthermore, the efficiency of a metal catalyst, such as antimony(III) oxide (Sb2O3), tin(II) 2-ethylhexanoate (SnOct2) and titanium(IV) butoxide (Ti(OBu)4), for the polycondensation reaction was evaluated by Ferreira et al. (Table 3), and from their experimental results, it was realized that titanium(IV) butoxide (Ti(OBu)4) exhibited better catalytic activity than the tin and antimony based catalysts. Additional studies regarding the concentration of the catalyst towards the polycondensation reaction indicated that an increase in catalyst concentration and temperature would lead to unwanted side reactions via polymer degradation, which in turn resulted in the isolation of a polymer with a broad molecular weight distribution.55
| Catalyst | M w (KDa) | PDI |
|---|---|---|
| Sn(Oct)2 | 1.35 | 9.6 |
| Sb2O3 | 1.70 | 6.2 |
| Ti(OBu)4 | 1.80 | 7.1 |
Other (Zr, Sn, Hf, Sb, Bi and La) Lewis acid catalysts
Jacquel et al. reported the use of zirconium(IV) n-butoxide as a catalyst for the preparation of aromatic–aliphatic co-polyesters (poly(butylene succinate) copolymers) containing rigid bio-based comonomers like isosorbide (Fig. 10). During the synthesis, zirconium(IV) n-butoxide was added during the polycondensation step.56 PBS thus synthesized possessed Mn = 5.9 × 104 g mol−1 and the reduced viscosity was found to be 2.01 dL g−1.Rare earth metal triflates like Sc(OTf)3, Y(OTf)3, Sm(OTf)3, Yb(OTf)3, La(OTf)3 and scandium(III) trifluoromethanesulfonimide (Sc(NTf2)3) were also explored as catalysts for poly(butylene succinate) ionomer synthesis.57
Poly(butylene succinate-co-butylene terephthalate) was prepared by the reaction of BDO, SA and PTA using a rare earth metal as a catalyst under melt polycondensation reaction conditions. The weight average molecular weight and polydispersity index of the isolated polymer were found to be 9.4 × 104 Da (Mw) and 1.64 (PDI). The corresponding homopolymers, PBS (with Mw = 12.0 × 104 Da; PDI = 1.70) and PBT (with Mw = 11.5 × 104 Da; PDI = 1.66) were also synthesized using the same method.58
The efficiency of various organo metals like titanium(IV) n-butoxide, hafnium(IV) n-butoxide, tin 2-ethylhexanoate, zirconium(IV) n-butoxide, antinomy(III) n-butoxide, and bismuth neodecanoate, and metal oxides like germanium(IV) oxide and antimony(III) oxide were analysed as catalysts for PBS synthesis (Fig. 11). It was evident from their experimental results that the efficiency of the catalyst was found to be Ti ≫ Zr ∼ Sn > Hf > Sb > Bi. Conversely, metal oxides of germanium and antimony exhibited desirable catalytic activity in combination with hydroxy acids (lactic acid or glycolic acid), which could act as chelating agents.59
However, the overall transesterification rate decreased with respect to high concentrations of hydroxy acids. This is due to the substitution reaction of hydroxy acids with PBS during the polymerization reaction. Moreover, it was noticed that catalytic residues, especially titanium based catalysts, present in the polymer were found to promote the hydrolytic degradation of the polymer.59 Additional catalysts, such as triazabicyclodecene and tosic acid, were found to be less effective at producing high molecular weight polyesters.60
The efficiency of TBT and zinc acetate at synthesizing the aromatic/aliphatic copolyester (HBA/PBS) was further systematically investigated. From the results, TBT was found to be a better catalyst than zinc acetate and hydroxyl benzoic acid would undergo the transesterification reaction with PBS to yield PBS rich segment and HBA (p-hydroxybenzoic acid) rich segment polyesters. Furthermore, the reaction time tended to have some influence on polymerization but not on the crystal structure.61
Metal (Bi, Ca, Mg and Zn) chlorides and triflates
The importance of various metal salts (bismuth, calcium, magnesium and zinc) towards the synthesis of PBS was explored in the literature. The intrinsic viscosities of the synthesized polymers were found to be 0.36 to 0.37 dL g−1 using zinc chloride and 0.24 to 0.25 dL g−1 and 0.33 to 0.61 dL g−1 respectively using BiCl3 and MgCl2 as catalysts. Furthermore, it was realized that BiCl3 exhibited enhanced catalytic activity compared to other metal chlorides and triflates, such as ZnCl2, Zn triflate, MgCl2, Mg triflate and CaCl2.62Moreover, para-toluenesulfonic acid has also been explored as a polycondensation catalyst along with an inhibitor (hydroquinone) and stabilizer (phosphorous acid) for the synthesis of biodegradable unsaturated polyester (Scheme 29).63
Dual metal catalyst system
To improve the efficiency of the synthesis and also to achieve high molecular weight PBS, various Lewis acid combinations as a catalyst system were explored in the literature. Zinc acetate, in combination with titanium(IV) butoxide, was utilized as a catalyst system for the polycondensation reaction to synthesize high molecular weight poly(butylene succinate) containing rigid imide units (Scheme 30).64Furthermore, the use of the zinc acetate–titanium butoxide catalyst combination was exploited towards the esterification reaction to synthesize aliphatic–aromatic biodegradable copolyesters.65 In the zinc acetate–titanium butoxide catalyst combination, the use of zinc acetate as a trans-esterification catalyst and titanium butoxide as a catalyst for the polycondensation reaction has also been investigated towards the synthesis of PBS (Mw = 7.4 × 104 g mol−1, Mn = 1.6 × 104 g mol−1 and PDI = 2.2).66
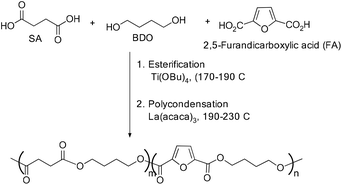
The use of titanium(IV) butoxide and lanthanum(III) acetylacetonate hydrate, respectively, as catalysts for esterification and polycondensation reactions have also been exploited to synthesize poly(butylene succinate-co-butylene furandicarboxylate) copolyesters (Scheme 31).67
Zhang and co-workers synthesized functional biodegradable poly(butylene succinate) with UV stabilizers covalently linked to the polymer backbone using conventional synthetic methods. However, they used titanium tetrabutoxide as an esterification catalyst and La(acac)3 as a catalyst for the polycondensation reaction towards the synthesis of functionalized PBS. The molecular weight of the PBS polymer thus obtained using La(acac)3 was found to be 10.4 × 104 g mol−1 with Mn of about 6.4 × 104 g mol−1. Furthermore, they varied the UV stabilizer ratio and found that a maximum Mw of 14.4 × 104 g mol−1 for the functional PBS was obtained when the molar ratio of HEPBP was about 0.5% (Scheme 32).68
A mixture of stannous octoate and stannous chloride as catalysts, in combination with 4-methylbenzenesulfonic acid as a co-catalyst, for the esterification reaction was reported for the synthesis of poly(butylene succinate-co-ε-caprolactone) copolymer.69
Dual catalyst system for PBS copolymer synthesis
In order to improve the efficacy of the PBS synthesis, different catalysts for the esterification and polycondensation reactions have been explored. Qu et al. reported the synthesis of poly(butylene-co-isosorbide succinate) using p-TSA as an esterification catalyst and titanium(IV) butoxide as a polycondensation catalyst (Scheme 33). However, TBT is found to be less effective with an increase in isosorbide content. Furthermore, they observed that steric hinderance was found to be an critical factor in deciding the efficiency of the catalyst. They explored Sb2O3 (antimony(III) oxide) and Sb2(OCH2CH2O)3 as catalysts for the synthesis of the PBIS copolymer. Due to lower steric hinderance, Sb2O3 is found to be more efficient than sterically crowded Sb2(OCH2CH2O)3.70Titanium tetraisopropoxide, in combination with magnesium hydrogen phosphate trihydrate as a catalyst component, has also been explored for the synthesis of PBS copolymers.71 It was observed that the co-catalyst magnesium hydrogen phosphate trihydrate accelerated the polycondensation reaction during PBS synthesis (Scheme 34).71,72
Titanium(IV) butoxide and dibutyl tin oxide respectively, in combination with the stabilizer Irganox 1010, were utilized as a catalyst system for producing PBS copolyesters using isosorbide and 2,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,5-di-O-methylene-D-glucitol (Glux-diol) co-monomers.73
3,5-di-O-methylene-D-glucitol (Glux-diol) co-monomers.73
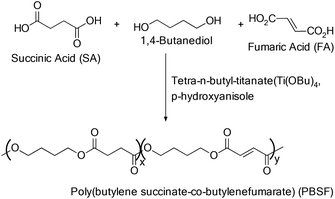
A combination of TBT and p-hydroxyanisole was employed as the catalyst and radical inhibitor respectively for the synthesis of poly(butylene succinate-co-butylene fumarate) copolyester (Scheme 35).74
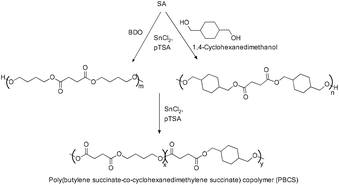
Poly(butylene succinate-co-cyclohexanedimethylene succinate)s (PBCSs) with varied compositions were prepared by the polycondensation reaction between cyclohexanedimethylene succinate (CS) and butylene succinate (BS) using anhydrous tin(II) chloride in combination with p-toluenesulfonic acid as a catalyst (Scheme 36).
Under the same reaction conditions, the corresponding homopolymers, PBS (with Mn = 4.1 × 104 g mol−1 and PDI 2.17) and PCS (with Mn = 2.8 × 104 g mol−1 with PDI 2.08) were prepared.75 The stannous chloride and 4-methylbenzenesulfonic acid catalyst combination was also used for the synthesis of dihydroxyl terminated PBS via a two step esterification and polycondensation reaction using SA and BDO (Scheme 37). The Mn and PDI values of the synthesized PBS under the reaction conditions are found to be 4.1 × 104 g mol−1 and 1.43 respectively.76
Heterogeneous catalysts
Most of the catalysts used for PBS synthesis are homogeneous in nature. The recovery and recycling of homogeneous catalysts is yet another challenging task and may lead to additional environmental pollution. Furthermore, the presence of the catalyst at ppm levels leads to degradation of the polymer during processing at high temperatures. Hence, removal of the catalyst from the final polymer will provide additional thermal stability to the polymer during processing.59 In general, heterogeneous catalysts are preferred over homogeneous catalysts due to the ease of recovery and recycling of the active catalyst.Recently, Sn-attapulgite was used as a heterogeneous catalyst for the synthesis of PBS with high intrinsic viscosity, high molecular weight and enhanced thermal properties. In addition, the Sn-attapulgite catalyst was found to exhibit better catalytic activity than that of SnCl2.77 Furthermore, Stȩpien et al. used a hydrolytically stable titanium dioxide/silicon dioxide co-precipitated C-94 catalyst for the synthesis of poly(butylene succinate–dilinoleic succinate) (PBS-DLS) copolymer under heterogeneous conditions. In addition, the use of thermal stabilizers was avoided during copolymer synthesis even at high temperatures (240 °C) (Scheme 38).78
Using the same C-94 catalyst system, new poly(butylene succinate–dilinoleic succinate–ethylene glycol succinate) (PBS-DLS-PEG) copolymers containing PBS as the hard segment and DLS and PEG as the soft segments were successfully synthesized through a two-step polycondensation reaction (Scheme 39).79
Suspension polymerization
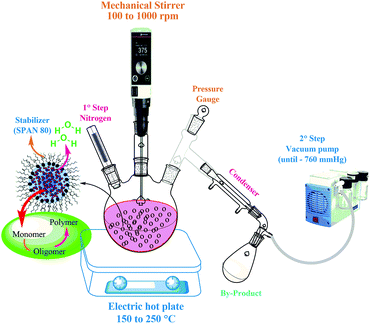
Polymerization in the bulk or solution phase has some inherent disadvantages, which include a long reaction time, high temperature and high viscosity of the reaction media. In general, due to low thermal stability, the polymers resulting from most of the melt-state polycondensations will have low molecular weights. Solution polymerization, in certain cases, can be considered as a potential alternative to overcome the disadvantages associated with bulk or solution phase polymerizations.In 1961, Lesek and Khromeček reported the first suspension polycondensation reactions for the preparation of ion exchange resins.80 Later, this methodology was extended to the synthesis of condensation polymers like poly(phenylene terephthalamide),81 polybenzimidazoles,82 polyimides,83etc. Recently, PBS microparticles, with Mw ranging from 0.32 to 8 × 104 g mol−1 and Mn = 0.12−7.2 × 104 g mol−1, were produced via suspension polycondensation, and the properties of the resulting polymer were suitable for personal care and cosmetics applications (Fig. 12).84
Solid state polymerization (SSP)
It is believed that limitations associated with melt polymerization, such as high reaction temperatures, color (from titanium based catalysts), low reactivity and possible side reactions can be overcome by the application of solid state polymerization. PBS with Mw = 7.2 × 104 g mol−1 and Mn = 4.4 × 104 g mol−1 was produced via the solid state polymerization technique. Moreover, it was found that there was an effective increase in Mw and thermal properties of PBS produced via SSP.85Microwave assisted synthesis
Velmathi and co-workers demonstrated the rapid synthesis of poly(butylene succinate) (PBS) with a weight-average molecular weight (Mw) of 2.35 × 104 g mol−1 using microwaves in the presence of 1,3-dichloro-l,1,3,3-tetrabutyldistannoxane as a catalyst (Scheme 40).47 Furthermore, they screened various catalysts such as Ti(OiPr)4, ZrCl4, SnCl2, p-TSA.H2O, HfCl4·2THF, Sc(OTf)3, and dibutyldichlorotin(IV) for PBS synthesis. From the experimental results, it was evident that SnCl2 was an efficient catalyst for the synthesis of PBS under microwave conditions. The generality of the methodology was further extended to synthesize other polyesters derived from various diols and diacids.86Similarly, Nagahata et al. also explored the synthetic utility of the microwave assisted synthesis of PBS and proposed that the increased reaction rate under microwave conditions was due to the efficient removal of by-products formed during the reaction.87 Furthermore, the synthesis of PBS using microwave irradiation was found to be more efficient than the corresponding conventional heating procedures.47,87
Enzyme mediated synthesis of PBS
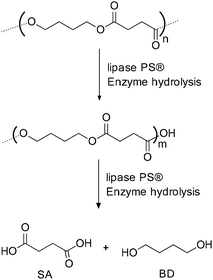
In addition to chemical synthesis, enzymatic methods for the production of PBS have also been explored. Lipase Novozym 435 (Candida antarctica) is used for the synthesis of polyesters under mild reaction conditions (Scheme 41).88The enzymatic method was further extended to the synthesis of poly(glycerol succinate) (Scheme 42). The molecular weight (Mw) and PDI of poly(butylene succinate) synthesized under enzymatic conditions were found to be 5.90 × 104 g mol−1 and 2.23 respectively.88
High molecular weight copolyesters, poly(butylene succinate-co-dilinoleic succinate), with both hard and soft segments were also prepared by the reaction of diethyl succinate, 1,4-butanediol, and dimer linoleic diol in the presence of Candida Antarctica lipase B (CAL-B) as a bio-catalyst in diphenyl ether as a solvent (Scheme 43). Copolymers with Mn ranging from 1.14 × 104 g mol−1 to 3.64 × 104 g mol−1 and Mw ranging from 2.85 × 104 g mol−1 to 7.33 × 104 g mol−1 were synthesized using enzyme mediated copolymerization.89,90
Enzyme mediated polymerization of divinyl adipate with 1,4-butanediol resulted in isolation of the corresponding polymer with a weight average molecular weight (Mw) of about 2.32 × 104 g mol−1 (Scheme 44).91 Interestingly, the use of divinyl adipate shifts the equilibrium of the polycondensation reaction towards the forward direction and this is due to the rapid tautomerization of the by-product vinyl alcohol into acetaldehyde.
Sugihara et al. recently developed a lipase Novozym 435 mediated green approach for the synthesis of high molecular weight PBS (Mw of 13 × 104 g mol−1) through ring opening polymerization of the cyclic oligomers (Scheme 45).92 Ren et al. introduced a two step protocol for polymerizing 1,4-butanediol and dimethyl succinate to give the corresponding PBS with molecular weights as high as Mw = 7.3 × 104 gmol−1, for which the catalyst Novozym 435 was added during the second polycondensation step.93
The generality of this method was further explored in synthesizing various PBS copolymers, such as poly(butylene succinate-co-butylene malate) (PBSM), poly(butylene succinate-co-butylene fumarate) (PBSFu), and poly(butylene succinate-co-butylene terephthalate) (PBST). The differences in the activities of the enzymes towards different di-acid monomers led to the formation of PBS copolymers with different molecular weights. Furthermore, from the literature it is evident that significant progress has been made in lipase-catalysed polymerization at temperatures below 90 °C.94
Poly(butylene succinate-ran-caprolactone) copolyesters (PBS-ran-PCL) with different compositions were synthesized by the ring opening polymerization of the corresponding cyclic oligomers of PBS and caprolactone using lipase CALB as a catalyst (Scheme 46). The Mn, Mw and PDI values of the PBS homopolymer produced under these enzymatic conditions were 4.7 × 104 g mol−1, 6.1 × 104 g mol−1 and 1.3 respectively.
Furthermore, the influence of the ring size on copolymerization was systemically investigated and, from the results, it is evident that, irrespective of the size of the oligomers, the rate of enzyme mediated ROP is almost the same and results in polymers without significant changes in their molecular weights. However, the molecular weights of polyesters generated by enzymatic ROP are greatly influenced by the polymerization conditions, concentration of the catalyst, monomer and reaction temperature.95
Copolymerization of 1,4-butanediol (1,4-BDO)/2,3-butanediol (2,3-BDO) and succinic acid in the presence of an enzyme, Novozym®435 (N435) (about 10 wt% of immobilized Candida antarctica lipase B resulted in the formation of the corresponding poly(1,4-butylene succinate-ran-2,3-butylene succinate) (PBBS) copolymer (Scheme 47).
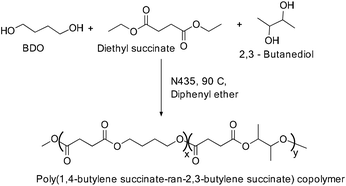 | ||
| Scheme 47 Enzyme mediated polymerization of 1,4-butanediol (1,4-BDO)/2,3-butanediol (2,3-BDO) and succinic acid. | ||
Solvent, temperature and concentration of the enzyme play a significant role in deciding the molecular weight of the polymer. Copolymerization using diphenyl ether as the solvent at 90 °C with the enzyme (10 wt%) afforded the corresponding co-polymer with high molecular weight (Mn = 4.3 g mol−1) and high 2,3-BDO contents. A decrease in Mw with an increase in 2,3-BDO content indicates the differences in enzymatic reactivity towards primary (1,4-DBO) and secondary (2,3-BDO) butane diols.96
Poly(p-dioxanone-co-butylene-co-succinate) (PPBS) copolyester was synthesized by the reaction of DES, BD and PDO under enzymatic conditions using 5 wt% of Novozym-435 as a catalyst in diphenyl ether as the solvent. Mn values of the synthesized homopolymer (PBS) and copolymer (PPBS) under the same reaction conditions were 1.31 × 104 g mol−1 and 1.9 × 104 g mol−1 respectively (Scheme 48).97
A truly green synthesis approach was employed for the synthesis of poly(butylene succinate-co-itaconate) (PBSI) copolymers using bio-based commercially available succinate, itaconate and 1,4-butanediol under enzymatic copolymerization conditions in the presence of Candida antarctica lipase B (CALB, in immobilized form as Novozym® 435) catalyst (Scheme 49). Moreover, diphenyl ether proved to be an efficient solvent system for this enzyme mediated copolymerization.98
 | ||
| Scheme 49 Green method for the synthesis of poly(butylene succinate-co-itaconate) (PBSI) copolymers. | ||
Enzymatic vs. Lewis acid mediated synthesis of PBS
Aromatic–aliphatic poly(butylene furandicarboxylate-co-succinate) copolyester was prepared by a ring opening polymerization reaction under both enzymatic and Lewis acid catalyzed conditions using CALB or Sn(Oct)2 respectively as the catalyst (Scheme 50). Copolyesters with Mw in the range of ∼5–6.5 × 104 g mol−1 and 1.5 × 104 and 4.5 × 104 g mol−1 were produced respectively under chemically and enzymatically catalyzed ROP.The ROP method is found to be a milder and more efficient method for producing poly(butylene 2,5-furandicarboxylate-co-succinate) copolyesters with high molecular weights when compared to the corresponding melt polycondensation method. The rate of enzyme mediated ROP is found to be high but produces copolyesters with low molecular weights. The copolymer composition can be easily controlled through ROP by controlling the feed ratio.99
PBS and its copolymers
In recent years, bio-based plastics and biopolymers have gained considerable interest and account for approximately 1% of global polymer production. Similarities in the physical and mechanical properties of bioplastics with respect to polyolefins, combined with the ease of processability using existing conventional equipment for polyolefins, has broadened their scope as potential alternatives to petroleum based non-biodegradable polymers.2a PBS is considered to be a potential alternative to fossil based polymers especially in packaging (films and boxes) and agriculture (mulch films) applications, where biodegradability is an added advantage. In addition, PBS has found increasing interest in biomedical applications. However, the relatively high cost and relatively lower physical and mechanical properties, with respect to conventional fossil fuel based polymers, largely restrict their applications in thermoplastic processing, foaming, filming, rigid packaging, and cosmetic and beverage bottles, where stiffness and thermal stability are critical requirements.15b,100To widen the application window of PBS towards performance materials, extensive research is being carried out to improve the thermal, mechanical and biodegradability properties of aliphatic polyesters. As a result, various chemical and physical methodologies, such as chain extension, copolymerization, forming cross-linking networks, and the use of polymer blends and composites, have been reported in the literature to improve the physical and chemical properties of PBS.6,75–81,101
Homo-polymers
Polyalkylene succinates
The crystallization and melting behaviour of poly(ethylene succinate) (PESu), poly(propylene succinate) (PPSu) and poly(butylene succinate) (PBS) were examined (Scheme 51). The formation of large spherulites and a linear increase in the spherulite radius with time was observed for PESu and PBS copolyesters. Conversely, small spherulites were observed for PPSu copolyesters; moreover, enzymatic hydrolysis is faster in the amorphous phase, especially between spherulites, than inside them. The crystallization half-times for PPSu are much longer than those of corresponding PBS. However, lower crystallinity, melting temperature and heat of fusion were observed for PPSu compared to those of PBS, which possesses a high crystallinity and melting temperature.102PBS is a semi-crystalline polymer, which exhibits a melting point around 115 °C and a heat distortion temperature (HDT) of about 97 °C. PBS exhibits two crystal forms, α- and β-forms, of which the α-form is more common, while the β-form exists only under strain, which, in turn, converts into the α-form after the removal of strain. Moreover, the physical properties of PBS can be varied by the incorporation of comonomers.15b
Co-polymers
In general, copolymerization is a widely known chemical method for tuning the physical, mechanical and thermal properties of the desired homopolymers. In recent years, extensive research was carried out to develop PBS based copolymers for various applications. As a result, numerous PBS based copolyesters with enhanced physical and mechanical properties have been reported in the literature.103 In this review, we tried our best to capture recent advancements in PBS copolymers.Copolymers via the chain extension reaction
Chain extension is a constructive approach to improve/increase the molecular weights of the polymer, which, in turn, will improve the mechanical and physical properties of the corresponding polymer.Multiblock poly(ester urethane) (PBESTU)
A novel PBESTU was synthesized via the chain extension reaction of hydroxyl terminated poly(butylene succinate) (PBS-OH) and poly(ethylene succinate-co-ethylene terephthalate) (PETS-OH) using toluene-2,4-diisocyanate (TDI) as the chain extender (Scheme 52).Interestingly, the two polymer segments in the copolymers are found to be compatible and exhibit a single glass transition temperature. Furthermore, it was observed that the PBS segment could enhance the crystallization capacity and thereby increase the mechanical properties of the polymers. Moreover, it is evident that the composition of the samples has no influence on the tensile strength and elongation at the break. However, an increase of the number of PBS segments tends to increase the Young's modulus and this is due to the better crystallization of PBESTUs.104
The PBESTU20 (PBS (20):PETS (80)) copolymer is found to be nearly amorphous, and its Tg is below its testing temperature (16.6 °C). Furthermore, its stress–strain curve is in agreement with that of a typical elastomer. In contrast, the stress–strain curves of PBEST40 (PBS (40):PETS (60)) and PBEST100 (PBS (100):PETS (0)) are found to be similar and in accordance with those of crystalline polymers. The thermal stability of the polymer slightly decreases with an increase in PBS content.
PBT-PBS copolymers
The chain extension reaction methodology under melt conditions was employed to synthesize a series of PBT-PBS copolyesters using dihydroxyl terminated PBT (HO-PBT-OH) and PBS (HO-PBS-OH) prepolymers in the presence of a chain extender (toluene-2,4-diisoyanate (TDI))(Scheme 53).A clear single glass transition was observed for all the PBT-PBS copolyesters between the Tg values of PBT (44.0 °C) and PBS (−28.7 °C), which clearly indicated the miscibility of non-crystallized phases of PBT and PBS. Furthermore, a single crystallization peak was observed for all the copolyesters. Moreover, an increase in PBS content in the copolymer resulted in a lowering of the crystallization temperature and induced broadness in the crystallization peak. This clearly indicates that an increase in the PBS content of the copolymer decreases the crystallization rate, which is believed to be due to increased flexibility of the molecular chains and restricted crystal formation of the PBS component. Additionally, an increase in the degree of randomness decreases the melting and crystallization temperatures and increases the Tg of the copolymer.
The molecular weights of HO-PBT-OH or HO-PBS-OH play a significant role in deciding the randomness of the synthesized copolyester. An increase in the molecular weight of either HO-PBT-OH or HO-PBS-OH increases the thermal stability and prevents the trans-esterification reaction.105
PBS-PCL copolymers
Copolyesters using PBS and PCL diols were synthesized via a chain extension reaction (Scheme 54). The resulting copolyesters exhibited a two-stage degradation behaviour (PCL followed by PBS segments), and copolymerization improved the thermal stability of PCL. Incompatibility between the PBS and PCL segments in the amorphous region resulted in separate melt and crystallizing properties. Thus, the PBS segment tended to crystallize first followed by the PCL segment. The incorporation of the PCL segment significantly enhanced the impact strength of the multiblock copolymers, and the copolymers became unbreakable with PCL contents higher than 10 wt%. Tensile strength, flexural strength, and the flexural modulus decrease with an increase in the PCL content and copolymers with PCL contents of 10–30 wt% display desirable tensile strength and excellent impact resistance properties.106PBS-b-PDGS copolymer
To enhance the properties of poly(butylene succinate), poly(diethylene glycol succinate) was introduced into the PBS backbone via the chain extension reaction of dihydroxyl-terminated PBS and PDGS precursors in the presence of a chain extender (hexamethylene diisocyanate (HDI)) (Scheme 55). PBS and PDGS units are found to be miscible in the copolymers. The incorporation of PDGS segments increases elongation at the break of PBS and a slight decrease in melting and crystallization temperatures is observed. Furthermore, the crystallization rate and spherulitic growth rate tend to decrease with an increase in PDGS content.In addition, the glass transition temperature and cold crystallization temperature were found to increase with an increase in PDGS content. However, the melt and melting crystallization temperatures as well as the crystallinity decrease with an increase in PDGS content. Finally, when compared to neat PBS, the PBS-b-PDGS copolyester displayed interesting mechanical properties with higher strength and toughness.37
PBS-PBF copolymers
Copolymers synthesized by the chain extension reaction of polybutylene fumarate diol and polybutylene succinate diol exhibited good mechanical properties similar to those of the corresponding homo-polymers, PBS and PBFu (Scheme 56). Tensile strength increases with an increase in PBS content, whereas elongation at the break ranges from 120% to 156%. Flexural strength, flexural modulus and elongation at the break remain almost unaltered with respect to composition.107PBS-b-PBSe copolymer
Novel PBS based, biodegradable and double crystalline poly(butylene succinate)-b-poly(butylene sebacate) multiblock copolymers were synthesized from PBS-diol and dihydroxyl terminated PBSe (PBSe-diol) using HDI as a chain extender (Scheme 57). The synthesized multiblock copolymers were found to be thermally stable and the thermal decomposition temperature was found to be above 340 °C. Both PBS and PBSe blocks crystallized separately during the cooling process. There was a gradual decease in the melt crystallization temperature and melt crystallization enthalpy of one block with an increase in the content of the other block. Furthermore, there was a slight and significant decrease in the crystallinity value for the PBS block and the PBSe block when compared to the corresponding homopolymers.Furthermore, the effect of PBS block crystals towards the crystallization of the PBSe block was found to be significant. The melting point temperatures of the two blocks were slightly lower than those of the corresponding homopolymers and these two blocks were found to be miscible in the amorphous phase. In addition, an enhancement in the mechanical properties was observed for the copolymers with respect to the PBS homopolymer, which was evident from the significant increase in the elongation at the break values (51.7 ± 0.8% for the PBS homopolymer to 299.2 ± 16.7% for PBS-b-PBSe2 (fPBS 77.4% and fPBSe 19.3%) and 673.6 ± 22.2% for PBS-b-PBSe4 (fPBS 56.5% and fPBSe 40%)). However, there is a slight decrease in the tensile strengths and Young's modulus values.27
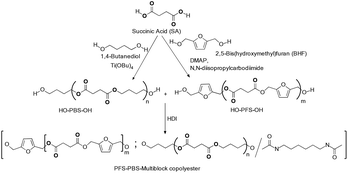
PFS-PBS multiblock copolymer
HO-PBS-OH (PBS diol) prepolymer was synthesized by the reaction of BDA and SA using TBT as a catalyst, which, in turn, was converted into the PFS-PBS multiblock copolymer by treatment with HO-PFS-OH (PFS diol) using HDI as a chain extender (Scheme 58). The PBS block exhibited better thermal stability than that of the PFS block. Two stages of thermal degradation (PFS followed by PBS) were observed for PFS-PBS copolyesters. The incorporation of the PFS blocks into PBS displayed a single Tg, which increased with an increase in the content of the PFS block. In addition, copolyesters showed a single crystallization peak and the crystallization of the PFS-PBS multiblock copolyesters was due to the formation of crystals from the PBS block.An increase in the PFS content decreases the crystallization rates and degree of crystallinity without affecting the crystallization mechanism. A gradual decrease in Tc was observed with an increase in the PFS content of the copolyesters. The exclusion of amorphous PFS blocks from crystalline PBS blocks during crystallization led to a decrease in the crystallinity of copolyesters.
In addition, the mechanical properties of the PFS-PBS copolyesters were found to be composition dependent and an increase in the PFS content resulted in a decrease of the modulus, tensile strength, Tc and crystallinity of the copolymer. However, Tg and elongation at the break tend to increase with an increase in PFS content. A single Tg was observed for all the synthesized PFS-PBS copolymers. Thus, PFS-PBS copolyesters could be tuned from crystalline thermoplastics to elastomer-like polyesters by changing the amorphous PFS content from low to high respectively in the copolyester.108
Copolymers with both hydrophilic and hydrophobic parts
A series of poly(ester ether urethane) (PEEU) block copolymers consisting of both a hydrophobic part (poly(butylene succinate) and a hydrophilic part (poly(ethylene glycol)) were prepared via the chain extension reaction using dihydroxyl terminated PBS and PEG in the presence of isophorone diisocyanate (IPDI) as a chain extender (Scheme 59). | ||
| Scheme 59 Synthesis of poly(ester ether urethane) (PEEU) block copolymers with both hydrophilic and hydrophobic parts. | ||
At a high concentration of PEG units in the copolymer, both the PBS segment and the PEG segment tended to form a single crystalline region and this behavior had a small impact on each segment within the copolymer. All the PEEU copolymers with different PEG contents exhibited ring-spherulite morphology, and then the size and distance between the spherulites increased with an increase in PEG content. The presence of PEG enhanced the crystallization ability of the PEEU copolymer without affecting the crystallization morphology. PEG and PBS segments were found to be miscible in the copolymer. In terms of the mechanical properties, an increase in the PEG content in the copolymer led to an increase in the tensile strength at first followed by a slight decrease. Whereas, the elongation at the break was found to increase gradually with an increase in PEG content. Flexibility of the PEG segment was greater than that of the PBS segment and therefore an increase in the PEG soft segment led to a gradual decrease in the tensile strength. On the other hand, a higher content of the PEG soft segment in the copolymer led to enhanced phase separation, which in turn increased the elongation at the break.76
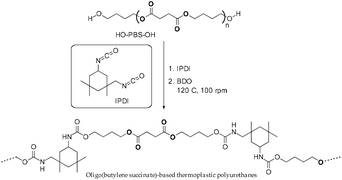
Oligo(butylene succinate)-based TPU
Higher strain at the break was observed for oligo(butylene succinate)-based thermoplastic polyurethanes (TPUs) (Scheme 60) when compared to that of the corresponding homopolymer (PBS). Hydrogen bonging increased with an increase in isocyanate content, which in turn increases the Young's modulus of the corresponding polymer without limiting the strain at the break. However, the introduction of chain extenders resulted in a reduction of the degree of crystallization and subsequent decrease in the storage modulus when compared to that of PBS. The presence of hard segments within TPUs acted as defects and reduced the crystallization ability of the PBS-based TPUs. A reduction in Tc and Tf for TPUs of about 10 °C was observed due to the irregularities induced by the hard segments.Thermal stability studies indicated that, due to a broader range of thermal degradation, PBS-based TPUs had a lower thermal stability compared to that of homo-PBS. However, the maximum rate of decomposition (Td max) was lower for TPUs than that of the corresponding homopolymer. Furthermore, TPUs with more urethane linkages (isocyanate index) displayed lower thermal stability.51
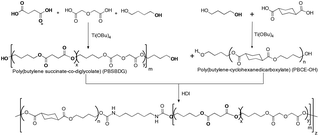
High molecular weight poly(ester urethane)s
High molecular weight poly(ester urethane)s were prepared by the chain extension reaction of the corresponding hydroxyl terminated poly(butylene cyclohexanedicarboxylate) (PBCE-OH) and poly(butylene succinate-co-diglycolate) random copolymers with two different compositions using hexamethylene diisocyanate (HDI) as a chain extender (Scheme 61).A higher amount of BDG content in the PBS-based blocks resulted in a lowering of the crystallizability of the PBS phase. Copolymers with equimolar concentrations (P(BS50BDG50) exhibited higher elongation at the break and a lower elastic modulus when compared to that of copolymers with a high PBS content P(BS70BDG30). Copolymers with equimolar concentrations exhibited lower crystallinity than that of the P(BS70BDG30) copolymer.109
PES-b-PBS multiblock copolymers
PES-b-PBS multiblock copolymers were prepared by the chain extension reaction of the corresponding dihydroxyl terminated prepolymers using HDI as a chain extending agent (Scheme 62).The miscibility of the copolymers was greatly enhanced by the block lengths of the corresponding copolymers. The miscibility of the PBS and PES blocks in the amorphous phase increased when the block lengths were smaller and the miscibility decreased with an increase in block length. Crystallization behaviors (crystal structure and morphologies) were not affected by copolymerization, but the crystallizability of the copolymer increased with an increase in block length. Thus, PES and PBS blocks in the copolymers were able to crystallize and the PES block crystallized within the interlamellar regions of PBS spherulites.110
Aliphatic copolymers
P(BS-co-HS) copolymers
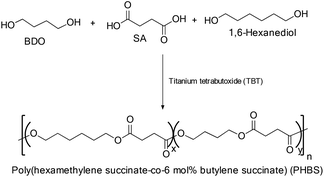
The synthesis of poly(butylene succinate-co-hexamethylene succinate) (P(BS-co-HS)) with various molar ratios of butylene succinate (BS)/hexamethylene succinate (HS) via direct melt poly-condensation was also reported in the literature (Scheme 63). The possibility of tuning the physical properties of the polymer by changing the monomer composition was explored in detail. All the synthesized polyesters exhibited good thermal stability and the degradation temperature ranged form 300 °C to 450 °C. Monomer composition played a significant role in enhancing the degradation temperature at 10% weight loss (Td – 10%) of the polyesters, which increased from 336 °C for neat PBS to 376 °C for PHS. In comparison with pure PBS, Td – 10% was enhanced by around 10 °C for BS-enriched copolyesters, and by around 30 °C for HS-enriched co-polyesters.Moreover, all the P(BS-co-HS) copolyesters exhibited only one Tg between those of neat PBS and PHS, which decreased with an increase of HS content in the copolyesters. However, the melting point temperature and equilibrium melting point temperature of P(BS-co-HS) were reduced significantly. Additional HS content initially decreased the Young's modulus and yield stress followed by an increase upon further addition of HS. Conversely, the elongation at the break tended to increase with an increase in comonomer content. Furthermore, P(BS-co-HS) copolyesters exhibited elastomeric behavior when compared to the brittle nature of pure PBS or ductile plastic nature of pure PHS.35
The crystal structures of P(BS-co-HS) copolyesters were found to be same with respect to that of neat PBS, which clearly indicated that the HS unit remained in the amorphous region, furthermore, incorporation of the HS comonomer reduced the crystallinity values compared to those in neat PBS. In addition, at the same crystallization temperature, the spherulitic growth rates of P(BS-co-HS) were smaller than that of neat PBS. It is also observed that the spherulitic growth rates of P(BS-co-HS) copolyester decreased with an increase in HS composition.111
Incorporation of the HS unit into neat PBS not only modified the crystal structures of PBS, but also slightly changed the crystal size and the degree of crystallinity (Scheme 63). Thus, copolyesters had bigger crystal sizes and lower degrees of crystallinity compared with those of neat PBS. Furthermore, the structure of the copolymer chains was found to be disordered, which resulted in the suppression of Tc, Tm and ΔHm. Furthermore, the elongation at the break for copolyesters increased with an increase in HS content compared to that of the corresponding homo-polymers. However, the Young's modulus of the copolyesters decreased with the introduction of HS units.112
PHBS copolymers
The thermal properties and crystallization behavior of novel poly(hexamethylene succinate-co-6 mol% butylene succinate) (PHBS) (Scheme 64) have been extensively studied and compared with those of the corresponding homopolymer PHS. The introduction of a small amount of PBS into PHS resulted in a reduction of the melting point and the equilibrium melting point of the copolymer but there was no significant change in the glass transition temperature.Both the homopolymer and the copolymer crystallized through the same crystallization mechanism and the crystallization rate of the copolymer was lower than that of the corresponding PHS homopolymer. It was also observed that a reduction in crystallization temperature increased the crystallization rate for both the homo- and copolymer. Spherulite morphologies were observed in both the homo- and copolymer, but the nucleation density was found to be smaller in the copolymer than that in the corresponding homopolymer.113
PP-r-BS and PPBA copolymers
Aliphatic copolyesters [poly(1,3-propylene succinate-ran-1,4-butylene succinate) (PP-r-BS) and poly(1,3-propylene adipate-ran-1,4-butylene adipate) (PPBA)] were synthesized using 1,3-propanediol (1,3-PDO) and 1,4-butanediol with different molar ratios and examined for their mechanical and thermal performances (Scheme 65).All the synthesized copolyesters exhibited good thermal stability below 260 °C and maximum degradation occurred above 315 °C and 325 °C respectively for PP-r-BS and PPBA copolyesters. The thermal stability of all adipate based copolyesters was found to be high and the thermal stability depended on the diacid monomer. Furthermore, the structural framework and length of the diol had no effect on the thermal stability. An isodimorphic co-crystallization behavior of both copolymers was very clear from the pseudo-eutectic melting behavior and the presence of only one crystalline phase, except for PPBA copolyesters with high 1,3-PDO contents. The succinic acid and 1,3-PDO contents in the copolyesters decreased the chain mobility and thereby increased the Tg of the corresponding copolyesters. The decrease in chain mobility might be due to an increase in the density of ester functional groups in the copolymer.114
PBSPS copolymer
The effect of glycerol on the crystallization growth rates in poly(butylene succinate-co-propylene succinate) (PBSPS) copolymers was systematically examined. Isothermal crystallization kinetic studies indicated that PBSPS copolymers grew three dimensionally; this was initiated by heterogeneous nucleation. Furthermore, the crystallization growth rates were faster in the presence of glycerol than in the absence of glycerol. The presence of glycerol in lower amounts (0.01%) in PBSPS formed a nucleation site and thereby increased the packing ability of the molecular chains. However, an increase in the glycerol content decreased the relative crystallinity due to restricted movement of the molecular chain. Thus, a small amount of glycerol could improve the relative crystallinity and crystallization rate of the PBSPS copolymer.115P(BS-BMS) copolymer
Copolymers of poly(butylene succinate-co-butylene 2-methylsuccinate) (P(BS-BMS)) were synthesized using succinic acid (SA), 2-methylsuccinic acid (MSA) and 1,4-butanediol (BDO) via a two step esterification and poly-condensation process (Scheme 66). | ||
| Scheme 66 Synthesis of poly(butylene succinate-co-butylene 2-methylsuccinate) (P(BS-BMS)) copolymers. | ||
The thermal stability of PBS and P(BS-BMS) was barely affected by the introduction of MSA and it was found that Td,5 (decomposition temperature at 5% weight loss) and Td,max (maximum rate) of pure PBS and P(BS-BMS) copolymers ranged from 337.1 to 340.0 °C and 397.6 to 399.4 °C, respectively. Furthermore, these copolymers possessed tunable melting temperatures and degrees of crystallinity. The crystallinity ranged from highly crystalline to amorphous in nature. An increase in the MSA content increased the amorphous region in the copolyester, due to destruction of the chain regularity and thereby this reduced the crystallinity.116
PBBS copolymer
The influence of regio isomers on the copolymers was studied using poly(1,4-butylene succinate-ran-2,3-butylene succinate) (PBBS) copolymer (Scheme 67). An increase in the 2,3-BDO content of the copolymers increased the Tg but decreased the chain mobility and degree of crystallinity of the corresponding copolyesters. Polymers with an amorphous nature were obtained when the 2,3-BDO content was higher than 47%. Interestingly, all copolyesters displayed excellent thermal stability.96PBSA copolymer
DSC and WAXS analysis of poly(butylene succinate-ran-butylene adipate) random copolymers (PBSA) (Scheme 68) indicated that PBSA random copolymers were isodimorphic in nature and each phase permitted the inclusion of the minority comonomer. In all copolymer compositions, the PBA-rich phase tended to crystallize in the β-PBA phase (kinetically favored) due to the inclusion of PBS repeating units in the cocrystals.21Additional investigations into the crystallization conditions for comonomer exclusion/inclusion balance in PBSA random copolymers indicated that fast crystallization (i.e., non-isothermal tests) favored BA inclusion inside the PBS phase crystals, whereas slow crystallization (i.e., isothermal test) strongly limited it. Interestingly, a combination of fast and slow crystallization conditions demonstrated intermediate behavior due to the inclusion of BA comonomers during the conditioning step, whereas the annealing steps permitted less incorporation. However, both fast and slow crystallization of PBA resulted in the formation of the β-PBA phase with BS unit inclusion irrespective of the cooling conditions. It was also observed that expansion of the PBS crystal unit cell permitted accommodation of the BA comonomeric unit. Consequently, BS inclusion expanded the β-PBA unit cell along both the a- and b-axes whereas the combination of both expansion and shrinkage along the a- and b-axes occurred in the α-PBA unit cells (Fig. 13).117
PBDS copolymer
An investigation of the thermal and mechanical properties as well as crystal structures of poly(butylene succinate-co-decamethylene succinate) (PBDS) copolyesters (Scheme 69) clearly indicated that the glass transition temperature, melting point temperature, nonisothermal melt crystallization peak temperature and equilibrium melting point temperature were lower than those of the corresponding PBS and tended to decrease further with an increase of the DS composition. Furthermore, an increase in DS composition significantly increased the elongation rate at the break of PBDS co-polyesters; however, there was a gradual decrease in yield stress, Young's modulus, and tensile strength.43P(BS-co-BAz) copolymers
Poly(butylenesuccinate-co-butyleneazelate) (P(BS-co-BAz)) copolyesters (Scheme 70) displayed good thermal stability up to 230 °C. All P(BS-co-BAz)s started to decompose in the range 230–260 °C and their thermal degradation followed a two-stage decomposition and ended at ∼490 °C. An increase in the number of BAz units decreased the weight loss in the first stage of decomposition and increased during the second stage of degradation. Furthermore, it was realized that increasing the BAz unit content in the copolymers decreased the elastic response of P(BS-co-BAz)s and increased the flexibility with a maximum close to 50 mol% BAz. This might be due to the formation of random copolymers with uniform composition along the chain.118(PBS-ran-PBAz) copolymers
The crystallization behavior of poly(butylene succinate-ran-butylene azelate) copolyesters (PBS-ran-PBAz) depended on the copolymer composition and it was found to be isodimorphic in nature, i.e., there was a small degree of comonomer inclusion in the crystalline phases that are formed in the copolymers. Thus, in PBS-ran-PBAz copolymers, where, PBS rich units are observed, a small amount of BAz units is present in PBS like crystalline units and vice versa. However, in a random copolymer that had a composition near the pseudo-eutectic point, the formation of two crystalline phases with the inclusion of a small amount of the second co-unit was observed. Furthermore, during crystallization, the amount of comonomer inclusion was much lower than the comonomer exclusion; this drives the fractionation ability of the copolymer.119An investigation of nucleation kinetics, spherulitic growth rates and overall crystallization kinetics of poly(butylene succinate-ran-butylene azelate) random copolyesters (PBS-ran-PBAz) indicated that the introduction of BAz-units into the PBS polymer chain significantly affected the crystallization of PBS-rich compositions. Furthermore, increasing difficulty in incorporating BAz units into the PBS crystals resulted in an increased density of nuclei and thereby decreased the spherulitic growth rate. However, in PBAz-rich compositions, the introduction of BS units did not affect crystallization and instead an anti-nucleating effect was observed. Moreover, it was concluded that the incorporation of comonomer within PBS crystals in the PBS rich composition was much lower when compared to PBAz-rich compositions.120
PBSC copolyesters
An array of 1,3/1,4-cyclohexanedimethanol based alicyclic/aliphatic copolyesters (PBSCs) at various molar ratios of monomers were also synthesized using SA, 1,4-BD and 1,3/1,4-CHDM (Scheme 71), to study the influence of these compositions on crystallinity, biodegradability and the mechanical properties of the copolyester. The glass transition temperature (Tg) of PBSCs was found to increase with respect to an increase in 1,3/1,4-CHDM content, due to restriction of the polymer chain movement by the bulky and cyclical moiety of 1,3/1,4-CHDM. Conversely, the melting temperature, degree of crystallinity, ultimate stress and initial modulus decreased significantly with an increase of the 1,3/1,4-CHDM content. Moreover, the strain at the break increased as the 1,3/1,4-CHDM content increased, indicating the influence of the alicyclic moiety in improving the ductility of the copolyester by reducing the degree of crystallinity.121The crystalline structure and morphology analysis revealed that the copolymer changed its morphology from ring-banded spherulites at a low cyclohexanedimethylene succinate (CS) content to a regular spherulite morphology at a high cyclohexanedimethylene succinate (CS) content. It was also evident that the cyclohexyl group of CHDM not only influenced crystal formation, but also altered the morphology of the copolymers during crystallization. The hot crystallization temperature (Tc) of PBCS copolymers decreased with an increase of CS content and no significant changes were observed with a further increase in CS content and this phenomenon could be due to the low crystallization rate. Furthermore, Tg of the copolymer increases with an increase in CS content and only one Tg was observed for all the copolymer compositions indicating that the copolymers were completely miscible at all compositions.121
Furthermore, an increase in CS up to 50% resulted in a decrease of the melting point, melting enthalpy and size of the spherulites for the PBCS copolymers and the opposite trend was observed for a further increase in the CS content above 50%. A comparison of temperature and shear rate on the viscosities of the copolymers indicated that the copolymers were more sensitive to temperature than the shear rate in the molten state.75
PBS-ran-PCL polymer
Poly[(butylene succinate)-ran-(ε-caprolactone)] copolyesters (PBS-ran-PCL) (Scheme 72) were found to be isodimorphic in nature and exhibited spherulitic superstructures, in which its nucleation depended on the copolymer composition. Properties such as Tm, Tc and lamellar thickness were insensitive to large molecular weight variations. Nevertheless, the degree of crystallinity and Tg varied significantly with respect to an increase in molecular weight. Moreover, the average length of the crystallizable sequences was controlled by comonomer exclusion and thus, a decrease in the average lamellar thickness (lc) at each side of the pseudoeutectic region was observed with an increase in comonomer addition.Furthermore, the amount of crystals that could be formed was also dictated by comonomer exclusion and hence, the degree of crystallinity decreased with an increase in comonomer content, while the amorphous layer thickness (la) increased on both sides of the pseudoeutectic region. The copolyester with BS45CL55 composition exhibited double crystalline properties, in which space filling spherulites were formed by the PBS rich phase at higher temperature and at lower temperatures, the PCL rich phase crystallized in the interlamellar amorphous regions.122
The composition of the copolymers strongly influenced the spherulitic texture and nucleation density. Thus, an increase in the PCL content in BS-rich copolymers resulted in an increased nucleation density and decreased spherulitic size. Conversely, an increase in PBS content in the copolymer resulted in larger sized spherulites with decreased nucleation density. From WAXS analysis at −60 °C, it was realized that copolymers with BS contents greater than 60% displayed unique PBS reflections and, on the other hand, copolymers with CL contents greater than 60% showed PCL reflections. However, copolymers in the pseudo-eutectic region with BS content (54%) and CL content (46%) displayed reflections corresponding to combined crystal types.95
PBSI copolymer
TGA and DSC analysis of the poly(butylene succinate-co-itaconate) (PBSI) copolymers indicated that the concentration of itaconate in the PBSI copolymer (Scheme 73) did not have any influence on Tg and the thermal stability of the copolymers. The glass transition temperatures of the homopolymer (PBS) and copolymer (PBSI) were found to be −35 and −38 °C respectively. Both the homopolymer and the copolymer degraded with a maximum at around 400 °C. Tm of the co-polyesters was significantly influenced by the itaconate content in PBSI and decreased almost linearly with respect to the mole percentage of itaconate.98PBSF copolymer
The introduction of trans-unsaturated comonomer (fumaric acid) units into saturated PBS polyester resulted in the isolation of poly(butylene succinate-co-butylene fumarate) (PBSFu) copolymer, which followed strict isomorphism by adopting the same crystal modification with slight differences in the crystal lattice parameters. A linear change in the melting point of the copolymers was observed with respect to copolymer composition and there was no significant change in the melting enthalpy. The introduction of trans-unsaturated comonomer units into PBS enhanced the total crystallization rate as well as the radial growth rate of the spherulites. In addition, PBF and PBSF tended to exhibit nucleating effects for PBS and its copolymers.74Poly(butylene succinate-co-butylene fumarate) (PBSFu) copolymers with various molar ratios of butylene fumarate (BFu) from 1% to 40% were synthesized (Scheme 74) and their corresponding single crystals were prepared using a self-seeding method. Single crystals of copolymers changed from a regular hexagonal shape to round with respect to an increase in BF content. However, the degree of supercooling of the PBSF single crystals with different BF contents increased with an increase of the BF content, which resulted in an increased crystalline density and a decrease in tee size of the single crystals.
Selected area electron diffraction (SAED) analysis of PBSFu single crystals indicated an isomorphism between butylene succinate and butylene fumarate in the PBSFu copolymer. Furthermore, a close similarity in crystalline structures with slight changes to the unit cell parameters was observed for PBS and the PBSFu copolymer. Ring banded spherulites were observed in PBSFu copolymers at suitable crystallization temperatures. The band spacing of PBSFu increased with the isothermal crystallization temperature and subsequently decreased with an increase in BF content at the same crystallization temperature. The crystallization temperature range for forming ring-banded spherulites broadened with an increase in the BFu molar ratios.123
P(BSBDTDP) copolymer
The hydrophobicity and thermal stability of poly(butylene succinate/dithiodipropionate) (P(BSBDTDP)) copolymer (Scheme 75) seemed to be unaltered, but the crystallinity of the copolymer was found to be lower when compared with the corresponding homopolymer. This reduced crystallinity resulted in faster degradation of the copolymer under physiological conditions.26PPBS copolymer
Good compatibility was realized between PBS and the PDO chains in the PPBS copolymer (Scheme 76), which was evident by the single Tg. Furthermore, PDO chains in the PPBS copolyester restricted the movement and packing of PBS units, which resulted in inhibited crystallization and decreased melting points (Tm) of the copolyester. A change/increase in the crystallization temperature for the PPBS copolyester resulted in the corresponding changes to the spherulite structures from a well-rounded shape to straight-stalk dendrites. The distance between the interlamellar region of the PPBS copolyester was highly affected by the crystallization temperature, which led to the segregation of the PBS chain and subsequent breakdown of faceted crystals into dendritic morphologies. All the copolyesters with different compositions exhibited dendritic morphologies irrespective of the thickness of the films produced from the respective copolyester.97PBS copolymers with hydroxy acid units
An investigation into the isothermal crystallization and subsequent melting behaviors of PDLA and PBS blocks clearly indicated that at higher crystallization temperatures, the incorporation of longer PDLA blocks into the copolymer increased the crystallization rate at first and then it decreased. Furthermore, longer PDLA blocks covalently bonded to PBS resulted in an increased crystallization enthalpy before levelling off accompanied by a gradual increase in melting temperature. In general, PDLA crystallized slower in the tri-block copolymer than in the corresponding PLA homopolymer. On the other hand, there was a gradual decrease in crystallization and melting enthalpies for the PBS block when a considerable amount of PDLA was incorporated into the copolymer (Scheme 77).54PBS copolymers with rigid monomeric units
PBIS copolymer
A series of PBS copolyesters (PBIS) were synthesized through two-step melt polymerization by using isosorbide (IS), one of the most significant carbohydrate-derived rigid and hydrophilic comonomers (Scheme 78). Because of its rigidity and chirality, incorporation of the IS unit into the PBS backbone linearly enhanced the Tg values of the copolyesters (PBIS). However, crystallinity, melting temperature and crystallization/melting enthalpies tended to decrease with an increase in IS content.73aThe use of rigid bio-based comonomers, namely, isosorbide and 2,5-furandicarboxylic acid, in PBS was also examined. Interestingly, all the copolymers exhibited an increased glass transition temperature. The largest increase was observed for 20 mol% of isosorbide in which the glass transition increased from −30 °C for the PBS homopolymer to −11 °C.
In general, a decrease in the melting temperature and crystallization temperature was observed with an increase in the comonomer content. This decrease is due to irregularities incorporated into the polymer chain. Furthermore, the crystalline content decreased with an increase in comonomer content, which resulted in reduced stickiness of the film. Although the incorporation of rigid units increased the melt strength of the polymer, its low crystallization rate restricted its processing application, especially in film extrusion blowing. However, a large increase in the elongation at the break of the polymer was observed, when considering the final film properties.56
PBISx and PBThSx copolymers
To improve the mechanical and barrier properties as well as biodegradability of PBS, two series of copolyesters with different rigid sugar units, isosorbide (IS) (Scheme 78) or 2,3-O-isopropylidene-L-threitol (ITh) (Scheme 79), as co-monomers were synthesized. The Mn values of PBISx and PBThSx were found to be 1.6–8.3 × 104 g mol−1 and 1.6–3.7 × 104 g mol−1 respectively and Mn values decreased with an increase in Is or ITh content owing to their low reactivity. It was also found that the thermal stability of the copolyesters was higher than that of PBS especially when the Is unit content was 5 or 11 mol%. However, a decrease in Tmax was observed, which could be due to low molecular weight. With respect to PBThSx copolyesters, Tmax was found to be lower than that of PBS and decreased with an increase in ITh content.Moreover, it is observed that copolyesters with bicyclic IS units exhibited similar thermal stability to that of PBS. Furthermore, the substitution of flexible butylene units with rigid bicyclic sugar units increased Tg of the copolyester, due to the fact that the bicyclic structure of IS conferred greater stiffness to the polymer chain and restricted its mobility. However, the influence of mono-cyclic ITh was slightly lower than that of bi-cyclic IS.
The incorporation of bicyclic sugar units increased the hydrophilicity of the copolyesters compared to the corresponding monocyclic sugar units. Incorporation of the IS comonomer unit increased the tensile strength and elongation at the break initially and a further increase in IS units led these to decrease. For a copolyester with ITh sugar units, the tensile strength decreased with an increase in ITh content; however, elongation at the break seems to be comparable to that of PBS.
It is evident that the introduction of the rigid bicyclic Is sugar unit into aliphatic polyesters led to better mechanical properties and decreased melting temperature, crystallinity, and crystallization rate compared to the corresponding monocyclic ITh sugar units. Furthermore, the introduction of the rigid IS comonomer, initially decreased and then increased the barrier properties of the copolyester.124
PBxManxyS and PBxGluxyS copolymers
Substitution of the butanediol units with 2,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,5-di-O-methylene-D-mannitol (Manx) in PBS (Scheme 80) increased Tg from −29 °C to 51 °C respectively for PB95Manx5S and PB30Manx70S copolymers. This increase in Tg of these copolymers would find wider application in the packaging industry. Insertion of Manx units into the PBS backbone significantly reduced Tm and ΔHm and dropped to a minimum for the copolymer with 30% Manx content. The crystallinity was reduced with an increase in Manx content, which resulted in the formation of copolymers with a narrower crystallization window (Tm − Tg). An increase in Manx content in the copolymer was associated with the steady increase in both the elastic modulus and tensile strength and a decrease in ductility values.49
3,5-di-O-methylene-D-mannitol (Manx) in PBS (Scheme 80) increased Tg from −29 °C to 51 °C respectively for PB95Manx5S and PB30Manx70S copolymers. This increase in Tg of these copolymers would find wider application in the packaging industry. Insertion of Manx units into the PBS backbone significantly reduced Tm and ΔHm and dropped to a minimum for the copolymer with 30% Manx content. The crystallinity was reduced with an increase in Manx content, which resulted in the formation of copolymers with a narrower crystallization window (Tm − Tg). An increase in Manx content in the copolymer was associated with the steady increase in both the elastic modulus and tensile strength and a decrease in ductility values.49
On the other hand, the introduction of 2,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,5-di-O-methylene-D-glucitol (Glux-Diol) resulted in a gradual decrease in Tm until the PBxGluxyS copolyesters became amorphous and this might be due to the asymmetric structure of Glux-diol. However, the PBxGluxyS copolymer exhibited a higher Tg due to its corrugated structure, which strongly hindered chain mobility of the polymers.73b
3,5-di-O-methylene-D-glucitol (Glux-Diol) resulted in a gradual decrease in Tm until the PBxGluxyS copolyesters became amorphous and this might be due to the asymmetric structure of Glux-diol. However, the PBxGluxyS copolymer exhibited a higher Tg due to its corrugated structure, which strongly hindered chain mobility of the polymers.73b
It is believed that the difference in Tm between PBxManxyS and PBxGluxyS copolymers might be due to the symmetric and asymmetric nature of the corresponding comonomers.
PBS copolymer with rigid imide units (PBSIr)
The introduction of rigid imide units into the PBS polymer chain (Scheme 81) decreased the melting temperature (Tm) of the copolyesters from 117.5 °C (PBS) to 90.6 °C. Furthermore, there was a small change in the decomposition temperature of PBSIr when compared to that of PBS (345.5 °C). The tensile strength of PBSIr decreased while the elongation at the break increased. Destruction of the chain regularity of the copolymers by the rigid imide units also reduced the crystallinity.64PBS copolymer with rigid acetylenedicarboxylic acid units
Furthermore, the introduction of a rigid acetylene dicarboxylic acid (AD) unit into the PBS polymer (Scheme 82) decreased the crystallization temperature and crystallinity without affecting the crystallization kinetics.Even at a low concentration, the presence of AD units significantly enhanced the primary nucleation and this nucleation capability constantly improved with respect to AD content, which in turn supported the recovery of the total crystallization rate of PBSAD copolymers under the same supercooling conditions. Furthermore, the existence of weak interactions between the AD units supported the melt memory effect at elevated temperatures. As the temperature decreased, this weak interaction between C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C units continuously strengthened, which resulted in the growth of a heterogeneous aggregation of AD units, and after certain point it induced the formation of a considerable amount of crystal nuclei. It was observed that the introduction of AD units enhanced nucleation and at the same time reduced the crystallinity.25
C units continuously strengthened, which resulted in the growth of a heterogeneous aggregation of AD units, and after certain point it induced the formation of a considerable amount of crystal nuclei. It was observed that the introduction of AD units enhanced nucleation and at the same time reduced the crystallinity.25
Aliphatic–aromatic PBS copolyesters
PBSFr copolymer
The introduction of aromatic rigid monomers into polyesters enhanced the mechanical and thermal properties. However, the biodegradability of the copolyester decreased significantly. The properties of aliphatic–aromatic random poly(butylene succinate-co-butylene furandicarboxylate) (PBSFr) copolyesters synthesized using 2,5-furandicarboxylic acid, succinic acid and 1,4-butane diol (Scheme 83) were investigated.Interestingly, copolyesters exhibited excellent thermal stability and the glass transition temperature, crystallizability and Tm of the copolyesters tended to decrease initially and then increase with an increase in butylene furandicarboxylate (BF) content (ϕBF). In general, both BS-rich and BF-rich copolyesters were crystalline in nature. Conversely, copolyesters with intermediate composition (ϕBF 40–60%) were close to being amorphous polymers. Furthermore, the tensile modulus and strength decreased with ϕBF in the 0–40% range, but increased again in the 50–100% range. However, the elongation at the break tended to follow the opposite trend with respect to the tensile modulus and strength.67
PBSFc copolymer
The presence of furanoate units in poly(butylene succinate-co-furanoate) (PBSFc) copolymer reduced the size of the spherulites and, in addition, retarded crystallization when compared to the corresponding homopolymers. A reduction in the size of the spherulites resulted in an increased nucleation density for the copolymer at all crystallization temperatures. Additionally, Tg (−29 °C) of the copolymer was found to be between the Tg of the corresponding homopolymers (Tg (PBS) = −36 °C and Tg (PBF) = 34 °C). A higher t1/2 for the PBSFc copolymer indicated that furanoate units in the copolymer reduced the crystallizability and influenced the anti-nucleation or retardation effect during the crystallization process.29PBST copolymer
Analysis of the structure–property relationship of poly(butylene succinate-co-butylene terephthalate) random copolymer (PBST) (Scheme 84) with a symmetrical composition clearly demonstrated a good balance between biodegradability and performance. Furthermore, the crystallizability of both components in the PBST copolymer was highly restricted. PBST copolymers with a symmetrical composition of PBT tended to possess a significantly higher Young's modulus (59 MPa), yield strength (8 MPa) and elongation to break ratio (1300%) than the corresponding widely used commercial PBAT with 44 mol% of PBT. Moreover, DSC and FTIR studies revealed that the crystals of PBS blocks formed by both quick stretching and slowly at room temperature for a non-deformed sample were immature in nature.58Aliphatic–aromatic PBS copolyesters via melt blending
A novel multi-block poly(butylene succinate)-multi-poly(butylene terephthalate) copolyester (m-PBST) was prepared by melt-blending of preformed PBS and PBT using tin octoate as a catalyst (Scheme 85). During melt-blend polymerization, a small amount of diisopropyl succinate was added to promote the ester-interchange reaction between the homopolymers to afford the corresponding required copolymer m-PBST.It was evident that films produced using r-PBST had a lower modulus and strength than those of the corresponding m-PBST, while the elongation at the break was found to be higher in r-PBST. Furthermore, m-PBST tended to mimic PBS and PBT in terms of strength and elongation and possessed average of these two homopolymers. Crystallization of the PBS segments in the m-PBST copolymer resulted in comparable elongation at the break with respect to the corresponding PBT homopolymer.125
Blended vs. synthesized copolymers
The difference in the mechanical properties of synthesized PBSAT15 (Scheme 86) and blended PBS/PBAT was also investigated in detail. Blended resin (PBS/PBAT) revealed a higher Young's modulus than that of the corresponding synthesized copolyester (PBSAT15) although the crystallinity of the blended resin was found to be higher than that of the synthesized copolyester. Furthermore, the increased elongation at the break value for the synthesized copolyester clearly indicated that the synthesized copolyester was more flexible than the blended resin. Specifically, the compatibility between resins influenced the elongation at the break for both the synthesized copolyester and blended resin. Compatibility between PBS and PBAT in the blended resin was found to be lower and the phases tended to separate when compared to that of synthesized PBSAT15, which had a smooth surface.126PBS copolyesters with cross-linking aids
Enhancement in the melting (Tm) and crystalline (Tc) temperatures was observed when pre-homopolymers of poly(butylene succinate) (PBS) and poly(butylene fumarate) (PBFu) were copolymerized (Scheme 87). The increase in the crystalline and melting temperatures was directly proportional to the copolymer fumarate content. Furthermore, the elastic modulus of the copolymers also increased with an increase in fumarate content; however, the tensile strength and elongation at the break tended to decrease with an increase in fumarate content.In addition, the properties of cross-linked and uncross-linked copolyesters were also investigated and it was found that rapidly cooled and cross-linked copolymer with 30% fumarate fraction exhibited the highest modulus of 393 MPa and lowest elongation at the break of 1.3% among all copolymers. In contrast, the rapidly cooled and uncross-linked copolymer with 10% fumarate fraction had the lowest modulus of 303 MPa and highest elongation at the break of 160%.41
PBS-PEG multiblock copolymers
The properties of poly(butylene succinate)–poly(ethylene glycol) multiblock copolymers, which were synthesized by a two step transesterification reaction, were investigated in detail (Scheme 88).An increase in the PEG segment chain length tended to increase the chain lengths of both PBS and PEG segments. A slight increase in tensile strength was observed with an increase in PEG segment content. The crystallinity of the PEG segment was significantly enhanced when the PEG segment content was more than 36 wt%. Conversely, there was a gradual increase in the elongation at the break with increasing FPEG segment content, which was due to greater phase separation with higher FPEG. The hydrophilicity of the copolymers increased with the incorporation of PEG segments. Furthermore, the chain length of the PEG segment also played a significant role in altering the water contact angle, and lower water contact angles were observed for PBSEGs possessing PEG segments with shorter chain length.127
PBSTMG copolymers
To enhance the properties of poly(butylene succinate) (PBS), an array of poly[(butylene succinate)-co-poly(tetramethylene glycol)]s (PBSTMGs) with different compositions of poly(tetramethylene glycol) (PTMG) were synthesized (Scheme 89). An increase in the comonomer content resulted in a gradual decrease in melting temperature, crystallization temperature, and crystallinity of the PBSTMG copolymers.Furthermore, the introduction of a third monomer into the PBS backbone resulted in a reduction of the sequence length of the crystallizable segments and comonomer units were excluded from the crystal lattice. However, increased incorporation of the comonomer reduced the thickness of the PBS lamella and hence the melting point and crystallinity of the copolymer decreased. Thermal degradation and the crystal lattice were not affected by copolymerization; however, an increase in comonomer content gradually decreased the crystallinity of the copolymer. A change in the copolymer composition had a significant influence on the storage modulus and Tg. An increase in the comonomer content decreased Tg and the presence of phase separation in the copolymers was also observed. The tensile strength of the copolymer was reduced but the impact strength and elongation at the break were improved, especially when the comonomer content was less than 10%.
Furthermore, the copolymer composition played a key role in reducing the size of the spherulites and crystallinity of the copolymer, which, in turn, promoted phase separation and thereby enhanced the toughness, ductility and impact strength of the copolymer. In addition to copolymerization, blends of PBS and PBSTMG copolymers also exhibited improved elongation at the break, toughness and impact strength when compared to that of the corresponding PBS homopolymer.38
PBS copolyesters using amino acid monomers
PBS copolymers of different amino acids were synthesized (Scheme 90) and the properties of the corresponding copolymers were investigated in detail. L-Aspartic acid displayed high reactivity when compared to other amino acids (glycine, L-alanine, L-aspartic acid, L-glutamic acid and L-phenylalanine). Furthermore, the time for the polymerization reaction increased with an increase in amino acid concentration, except for L-aspartic acid. There was no significant change in the thermal properties, such as the glass transition temperature (Tg), melting temperature (Tm), heat of fusion (ΔHm) and 2% thermal decomposition temperature (Td2). However, the mechanical properties of the copolymers, especially the break strain, tended to vary depending on the type of amino acid used. In particular, copolymers with alanine exhibited a slightly higher break strain than the corresponding PBS homopolymer.128In addition, PBS copolymers with various amount of L-aspartic acid were synthesized and investigated for their thermal and mechanical properties. The concentration of L-aspartic acid shortened the reaction time to produce copolymers with Mw of 24.7 × 104 g mol−1. Moreover, there was no remarkable change to the molecular weights and thermal properties when compared to that of PBS. However, the break strain increased from 289 to 548%, when the L-aspartic acid content was 0.3 mol% and a further increase in L-aspartic content led to a dramatic decrease in break strain.128
The effect of the incorporation of L-aspartic acid into PBS copolymers containing caprolactone co-monomer units was also investigated (Scheme 91). It was found that the molecular weight distribution, Mw/Mn, increased with an increase in L-aspartic acid content. No significant changes to the thermal properties of the copolymers were observed with and without L-aspartic acid. However, the break strain changed with respect to changes in the L-aspartic acid content. Furthermore, PBS copolymers with 3-octadecyloxy-1,2-propanediol and L-aspartic acid were synthesized. The reaction time for polymerization decreased with an increase in amino acid content, whereas the PDI (Mw/Mn value) tended to increase with an increase in amino acid concentration.128
PBS copolymers with long chain branching
Using 1,2-decane diol
Branched poly(butylene succinate) copolymers with 1,2-decane diol as a comonomer (Scheme 92) were synthesized and investigated for their thermal, mechanical and crystallization properties. The thermal stability of PBDS copolymers was similar to that of PBS. An increase in decane diol decreased the crystallizability, Tg, Tm, and of PBDS copolymers with respect to those of PBS.
of PBDS copolymers with respect to those of PBS.
However, regardless of the 1,2-decylene succinate (1,2-DS) content, the crystallization mechanism and crystal structure of the PBDS copolymers remained the same when compared to PBS. An increase in the 1,2-DS content increased branching in the polymer chain, which in turn increased the mobility of the polymer chain. Furthermore, an increase in the elongation at the break and a decrease in the Young's modulus were observed for PBDS copolymers with increased 1,2-DS content.24
Using 1,2,4-butanetriol
The influence of long-chain branching in PBS was also investigated using 1,2,4-butanetriol (1,2,4-BT) as a long chain branching agent (Scheme 93). From a systematic investigation of the physical and rheological properties, it was evident that an increase in the 1,2,4-BT segment resulted in a slight increase in crystallization and glass transition temperatures. A significant enhancement was observed with an increase in 1,2,4-BT segment content, which clearly indicated that long-chain branches would be advantageous towards enhancing the crystallization temperature and accelerating the crystallizability.However, a gradual decrease in the relative degree of crystallinity was observed. Furthermore, long-chain branched PBS exhibited a higher tensile strength without a remarkable decrease in the elongation at the break when compared with that of linear PBS. Furthermore, the addition of 1 mol% 1,2,4-BT segments improved the tensile strength of long-chain branched PBS by 31% when compared with that of the linear PBS, which clearly indicated that long-chain branches could enhance the stiffness of linear PBS. Moreover, it is evident that the increase in 1,2,4-BT segments resulted in the formation of a low branching reaction. Triol monomer could also promote possible cross-linking reactions.129
PBS-DLS-PEG copolymer
In addition, the introduction of the hydrophilic poly(ethylene glycol) unit with different molecular weights and amounts into poly(butylene succinate–dilinoleic succinate–ethylene glycol succinate) (PBS-DLS-PEG) copolymers (Scheme 94) resulted in different wettability and thus changed the hydrophobic copolymer to hydrophilic. Moreover, the presence of hard and soft segments resulted in low Tg and high Tm of the copolyesters. The presence of PEG units (up to 10%) in the copolymer improved the hydrophilicity without altering the thermal properties.79The incorporation of dimeric linoleic diol (DLAOH) into the PBS backbone resulted in the formation of copolyesters with hard and soft segments. The synthesized copolymers were processed into electrospun mats without defects. During copolymer processing, it was observed that solvent and concentration played crucial roles in obtaining electrospun fibres without any defects. Furthermore, the thermal and mechanical properties were found to be comparable to those of the bulk copolymer synthesized via organometallic catalysis. The presence of a higher amount of hard segments in PBS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DLS 70
DLS 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 copolymer electrospun fibre mats led to a high Young's modulus (24.2 MPa) and tensile strength (4.0 MPa). However, there was a lower elongation at the break (63%) when compared to the copolymer synthesized via organometallic catalysis.130
30 copolymer electrospun fibre mats led to a high Young's modulus (24.2 MPa) and tensile strength (4.0 MPa). However, there was a lower elongation at the break (63%) when compared to the copolymer synthesized via organometallic catalysis.130
Ternary random PBS copolyesters
Novel ternary or multiple random copolyesters containing various structural units were synthesized (Scheme 95), in order to understand the influence of chain length, aromaticity, symmetry, rigidity and short-chain branching of the structural units on crystallization, glass transition temperature and the tensile properties.All the synthesized copolyesters tended to exhibit one Tg, indicating that they were all random copolymers in nature. Furthermore, it was evident that the incorporation of shorter chain aliphatic diacids, aromatic diacids (asymmetric ones, in particular) or short-chain branched diol suppressed copolyester crystallization and increased the glass transition temperature. With respect to aromatic diacids, asymmetry and/or rigidity of the aromatic ring played a significant role in influencing the Tg. However, the incorporation of aliphatic diacids with longer alkylene chains reduced the glass transition temperature. Furthermore, it was clear that rigidity also contributed to higher Tg. The introduction of a terephthalate moiety into copolyesters improved the tensile properties; this was believed to be due to the presence of more intermolecular interactions between aromatic units. Furthermore, the introduction of isophthalate or furandicarboxylate tended to improve the elongation at the break because of lower crystallizability.131
Nucleating agents as crystallization aids
Major challenges associated with poly(butylene succinate) (PBS) are reduced mechanical properties, large spherulite size and relatively low crystallization rate. The use of nucleating agents to enhance the crystallization of poly(butylene succinate) (PBS), shorten the molding cycle as well as improve the mechanical properties has gained considerable interest for various applications. Extensive research has been carried out to develop suitable nucleating agents for PBS. As a result, various nucleating agents, such as organoclay, inorganic salts and oxides, carbon nanotubes, small organic molecules (cyclic aromatic or aliphatic acids), organo-phosphates, low-molecular-weight polyesters and α- or β-cyclodextrin, have been reported in the literature. However, the compatibility of all these nucleating agents with PBS was found lower and most of the nucleating agents were found to be larger in size (e.g., 10 μm), which resulted in the nonuniform dispersion of the nucleating agents in the polymer matrix.132Nevertheless, these nucleating agents increased the crystallization properties of PBS to some extent, but affected the most important mechanical properties, such as tensile strength and elongation at the break of the polymer. Recently, poly(butylene fumarate) and poly(butylene succinate-co-butylene fumarate) were also explored as polymeric nucleating agents to improve the crystallization of PBS with the aid of isomorphism.
Interestingly, the addition of nucleating agents like poly(vinyl butyral) (PVB) to the monomers prior to polymerization of poly(butylene succinate) (PBS), in order to accelerate crystallization and improve the mechanical properties, was also studied in detail. The addition of PVB to PBS significantly reduced the spherulitic sizes and increased the peak temperature of crystallization. An initial decrease in spherulitic size with an increase in PVB content was observed, followed by a minimum and then it increased finally. PVB with a low molecular weight resulted in smaller spherulitic sizes than the corresponding high molecular weight PVBs. Moreover, the nucleated PBS exhibited a significant improvement in tensile modulus (26%), tensile strength (30%), and elongation at the break (82%). It was observed that the size of the spherulites played a major role in deciding the tensile modulus, strength and elongation at the break. Enhanced mechanical properties were also observed when the spherulitic size was small.
Pre-dispersion of nucleating agents into the monomers before polymer synthesis is believed to be an effective and consistent approach to improve the crystallization properties when compared to that of corresponding post-polymerization melt blending of nucleating agents.132
Role of catalysts in copolymer synthesis
The influence of the catalyst on the crystal structure of poly[(butylene succinate)-co-(dilinoleic succinate)] was investigated in detail (Scheme 96). The catalyst played a significant role in determining the polymer's intrinsic properties. From GPC analysis, the number average molecular weights were found to be 5.1 × 104 g mol–1 and 2.5 × 104 g mol−1 respectively for C-94-catalyzed and CALB-catalyzed copolyesters.Thus, it ass clear that copolymerization under solvent free conditions resulted in the formation of high molecular weight polymers. In addition, a higher polydispersity index was observed for the CAL-B-catalyzed reaction (8.2) when compared to that of the C-94-catalyzed reaction (3.3). This difference in dispersity index values was due to the formation of more segmented block structures under enzyme mediated conditions, which led to the formation of the corresponding macromolecules.
The Tm, ΔHm and Tg values were comparable for both PBS-DLS 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30-E and PBS-DLS 70
30-E and PBS-DLS 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30_M catalyzed copolyesters. However, the Tc value for the C-94-catalyzed copolymer (33 °C) was found to be lower than that of the corresponding CAL-B-catalyzed copolymer (53 °C). This could be attributed to the higher Xc,h (crystalline phase content in the hard segments) and Xc,tot (total crystalline phase content in the polymer) for the enzyme catalyzed copolymer when compared to that of the metal catalyzed polymer (66.0 versus 62.7% and 44.9 versus 40.2%, respectively). Moreover, the average sequence length of the hard segments in the enzyme catalyzed reaction was found to be twice that of the metal catalyzed reaction. Furthermore, crystalline forms of the metal catalyzed copolymers were found to be denser and the phase change was 20% greater than that of the corresponding enzyme catalyzed copolymers. However, a homogeneous density distribution was observed in the enzyme mediated copolymers.39
30_M catalyzed copolyesters. However, the Tc value for the C-94-catalyzed copolymer (33 °C) was found to be lower than that of the corresponding CAL-B-catalyzed copolymer (53 °C). This could be attributed to the higher Xc,h (crystalline phase content in the hard segments) and Xc,tot (total crystalline phase content in the polymer) for the enzyme catalyzed copolymer when compared to that of the metal catalyzed polymer (66.0 versus 62.7% and 44.9 versus 40.2%, respectively). Moreover, the average sequence length of the hard segments in the enzyme catalyzed reaction was found to be twice that of the metal catalyzed reaction. Furthermore, crystalline forms of the metal catalyzed copolymers were found to be denser and the phase change was 20% greater than that of the corresponding enzyme catalyzed copolymers. However, a homogeneous density distribution was observed in the enzyme mediated copolymers.39
Differences in the thermal properties of the copolyesters produced with both an enzyme and a Lewis acid were negligible and could be correlated to the differences in the corresponding molecular weights.98
Thermoplastic elastomers with good processability were obtained by the incorporation of dimeric fatty acid soft segments into PBS (Scheme 97). Furthermore, an increase in the fatty acid soft segment resulted in lower water take up and mass loss, indicating slower degradation. Moreover, the effective surface area played a significant role in biodegradation.133
Thermal degradation of PBS copolymers
The thermal degradation mechanisms for poly(butylene succinate) (PBS) and PBS-rich poly(butylene succinate-co-propylene succinate) copolymers were investigated through FTIR analysis of the gases evolved from TGA (Scheme 98). The formation of olefin, ester, CO2 and anhydride was observed from the analysis. Based on this observation, a plausible mechanism was proposed, which involved the β-hydrogen bond cleavage of the ester groups followed by dehydration to form the corresponding anhydrides, olefins and CO2. Furthermore, the comonomer distribution was found to be random in the copolymer and this randomness did not alter the degradation mechanism.134Bio-degradation
The large dependence on polymer production from nonrenewable resources adds additional challenges to sustainable development. Conversely, chemical recycling of polymeric materials involves high energy consumption and longer downstream processing. Thus, the development of an efficient and ecofriendly method for recycling polymeric materials is much sought-after. As a result, research directed towards the synthesis, processing and degradability of biobased and/or biodegradable polymers has attracted increasing interest.Aliphatic polyesters exhibit remarkable physicochemical, mechanical and processability properties, which are comparable with those of conventional polyolefins. Polyesters such as polycaprolactone (PCL), poly(3-hydroxybutyrate) (PHB), poly(ethylene succinate) (PES), poly(butylene succinate) (PBS), poly(butylene adipate), poly(hexylene succinate) (PHS), and poly(hexylene adipate) are some of the bio-alternatives to conventional polyolefins with the greatest potential. Among them, PES, PBS, and PHS have favorable biodegradability and biocompatibility, and hence they have found widespread applications in biomaterials, packaging materials, agriculture, gene delivery, drug delivery, tissue engineering, and the pharmaceutical, medical and biomedical industries. Particularly, poly(butylene succinate) (PBS) has found widespread applications and achieved rapid growth due to its balanced thermal and mechanical properties.
However, the use of PBS in the packaging and agriculture industries, especially for short lived products, is limited due to its relatively low degradability rate as well as the low ratio of PBS degrading microorganisms in the environment.135
Firstly, it is necessary to understand the biodegradation mechanism in order to improve the biodegradability of PBS under different degradation environments. It is well documented in the literature that various factors, such as the chemical structure, repeating units, hydrophilic/hydrophobic balance, molecular weight, solid-state morphology (i.e. crystallinity, crystal size and distribution), molecular mobility, thermal characteristics and other physical factors, greatly influence the biodegradation rate of a polymer. The biodegradation rate of the amorphous region is much faster than that of the crystalline zone. Absolute biodegradability also directly related to the nature, relative quantity, and distribution of the co-monomeric units along the polymer chain.
Polyester degradation can be categorized into two processes: endo-type scission (ester cleavage occurs at random points along the polymer chain) and exo-type scission (ester cleavage occurs at ends of the polymer chains). In this review, we tried our best to cover the extensive research that has been carried out to improve the biodegradability of bio-based polymers.
Aliphatic PBS copolyesters
PBS, PHS and P(BS-co-HS) copolymers
From the bio-degradability studies of various aliphatic copolyesters, such as poly(butylene succinate) (PBS), poly(hexamethylene succinate) (PHS), and poly(butylene succinate-co-hexamethylene succinate)s (P(BS-co-HS)s) with various hexamethylene succinate (HS) contents, it was realized that the hexamethylene content had a strong influence on biodegradability. The copolyester with increased HS content degraded faster than the corresponding copolyester with a low HS content.112Co-polyesters tend to degrade faster than neat PHS and PBS. In particular, copolyesters with more of the HS component are more susceptible to lipase attack (Candida rugose) and degrade faster than copolyesters with more of the BS component. Furthermore, the mechanism of enzymatic degradation was found to be of the endo-type, which resulted in a steady decrease in molecular weight and also it was believed that the melting point and crystallinity were the main factors that controlled the degradation rate. However, since more equimolar P(BS-co-HS) (50/50) and (36/64) had Tm below the incubation temperature, there was likely to be a significant influence from chain motion and reorganization of the surface morphology.136
P(BS-co-CHDMS) and P(BS-co-BCHDA)
Enzymatic degradation of the homopolymer poly(butylene succinate) (PBS) and its copolymers containing 1,4-cyclohexane dimethylene succinate (CHDMS) or butylene 1,4-cyclohexanedicarboxylate (BCHDA) sequences with different molecular architectures (P(BS-co-CHDMS) and P(BS-co-BCHDA)) was investigated using Pseudomonas cepacia lipase (PCL) in chloroform. All the copolyesters degraded faster than pure PBS. However, the degradation rates were found to be higher in the presence of PCL in CHCl3.The introduction of monomers with a six-membered alicyclic group resulted in the destruction of the rigid spiral structure of PBS and thus improved the accessibility and stability of the copolyesters and enzyme (PCL) as well as enhancing the recognition of ester bonds by PCL, which in turn, accelerated enzymatic degradation. PBS modified by CHDM exhibited better enzymatic degradation than the corresponding PBS copolymer modified with CHDA. Molecular modeling studies have also shed some light on the interactions between residues at the active site of PCL and the polyester. It was very clear from the modeling studies that steric hindrance created by CHDA in the P(BS-co-BCHDA) copolymer reduced the accessibility of ester groups to the enzyme and thus inhibited the rate of enzymatic degradation.34
PBS, PBS-co-CL, PBS-co-CHDM and PBS-co-CL-co-CHDM copolymers
Investigation on catalytic degradation of poly(butylene succinate) (PBS) and its binary (PBS-co-CL), (PBS-co-CHDM) and ternary (PBS-co-CL-co-CHDM) and ternary copolymers by Immobilized Candida lipase (CA Novozym435), provided some mechanistic insights towards the formation of cyclic oligomers during the degradation (Scheme 99).Copolyesters with Mw greater than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 degraded into lower molecular weight copolymers. Enhanced degradation is observed in copolymers than the corresponding homopolymers. Copolymers with CHDM unit exhibits enhanced degradation due to increased gap between the polymer chains, which in turn provide easy access for enzymes to the corresponding ester bonds. Moreover, PBS modified with caprolactone also degrades faster than the corresponding PBS and this is due to the enhanced plasticity between the polymer chains. Degradation of CHDM-modified PBS copolymers and terpolymers of P(BS-co-CL-co-CHDM) exhibits small difference, which is due to the added advantage of both CHDM and CL units in the polymer backbone, which results in the formation of the cyclic oligomers.137
000 degraded into lower molecular weight copolymers. Enhanced degradation is observed in copolymers than the corresponding homopolymers. Copolymers with CHDM unit exhibits enhanced degradation due to increased gap between the polymer chains, which in turn provide easy access for enzymes to the corresponding ester bonds. Moreover, PBS modified with caprolactone also degrades faster than the corresponding PBS and this is due to the enhanced plasticity between the polymer chains. Degradation of CHDM-modified PBS copolymers and terpolymers of P(BS-co-CL-co-CHDM) exhibits small difference, which is due to the added advantage of both CHDM and CL units in the polymer backbone, which results in the formation of the cyclic oligomers.137
PBS-co-DEGS and PBS-co-BDGA copolymers
The importance of ether linkages at various positions in PBS towards biodegradability and the degradation mechanism was explored via enzymatic degradation of poly(butylene succinate-co-diethylene glycol succinate) (PBS-co-DEGS) and poly(butylene succinate-co-butylene diglycolic acid) (PBSco-BDGA) using lipase N435 in the THF/toluene mixed system. The rates of hydrolysis for all PBS-co-BDGAs were higher than that of PBS-co-DEGS, which clearly indicated that PBS-co-BDGA copolymers were more susceptible to hydrolysis. Except for PBS-co-BDGA15, the degradation rate increased with an increase in monomer content and the degradation rate of the PBS-co-BDGA copolymer was higher than that of PBS-co-DEGS. This was believed to be due to the presence of the ether linkage in the copolymer backbone. In PBS-co-BDGA, the ether bond was adjacent to the ester carbonyl bond of the diacids. Thus, it was more susceptible to hydrolysis than the corresponding PBS-co-DEGS, in which the ether bond was between the carbonyl groups of the diol part.138aMoreover, an increase in the DEGS content in the poly(butylene succinate-co-diethylene glycol succinate) (P(BS-co-DEGS)) copolymer resulted in an increase in the glass transition temperature, hydrophilicity, ductility and degradation rate of the polymer under hydrolytic conditions. However, the tensile strength and modulus, melting temperature, crystallizability and degree of crystallinity tended to decrease with an increase in the DEGS content of the copolymer. Interestingly, the thermal stability of the copolymer was found to be unaffected by the composition.138b
PEEU copolymers
Enzymatic hydrolysis of the PEEU copolymers was studied using Pseudomonas cepacia lipase at 40 °C. The rate of enzymatic degradation was found to increase with an increase in the hydrophilic PEG segment in the PEEU copolymer. Furthermore, the copolymer with better hydrophilic properties underwent faster degradation.76PES, PHS and PBS copolymers
The influence of hydroxy monomers on enzymatic degradation was examined using three different polymers, which were prepared using succinic acid and dihydric alcohols such as ethylene glycol (PES), 1,4-butanediol (PBS) and 1,6-hexanediol (PHS). From the experiment results, a very good correlation between the biodegradation behavior and the distance between ester groups (high methylene content), low crystallinity, low melt temperature and high hydrophilicity was realized. Thus, the biodegradability of the polymer PHS with six carbon atoms between the ester group exhibited maximum degradation and thus the order of enzymatic degradation was found to be PHS > PBS > PES.139PBS, PBS-co-HS and PBS-co-BA copolymers
The enzymatic degradation of poly(butylene succinate-co-hexamethylene succinate) (PBS-co-HS), poly(butylenesuccinate-co-butylene adipate) (PBS-co-BA), and poly(butylene succinate) (PBS) was investigated using Pseudomonas cepacia (PC) to understand the influence of butylene adipate (BA) and hexamethylene succinate (HS) units in the PBS backbone (Scheme 100).The PBS-co-HS copolymer degraded much faster and resulted in the formation of more oligomers than the corresponding PBS-co-BA copolymer. The formation of acyclic and cyclic oligomers was observed during the enzymatic degradation. Molecular modeling studies of enzymatic degradation indicated that the free energy of binding between the enzyme and the substrate in chloroform was higher for PBS-co-HS copolymers than those of the corresponding PBS-co-BA copolymer and PBS homopolymer. Moreover, the stability of the active sites of the enzyme containing HS units was greater than the other corresponding active sites.140
PBSCL copolymer
Lee et al. investigated the mechanism for the enzymatic degradation of PBS and poly(butylene succinate-co-6-hydroxycaproate) (PBSCL) using lipase PS® originating from Pseudomonas cepacia. The formation of 4-hydroxybutyl succinate (4HBS) during the enzymatic degradation of PBS indicated that bio-degradation was believed to be an exo-type hydrolysis mechanism (Scheme 101).135aAliphatic PBS copolyesters with unsaturated comonomers
PBSI copolymer
Biodegradability studies of poly(butylene succinate-co-butylene itaconate) copolymers indicated that copolymers with lower average molecular weight and lower degree of crystallinity degraded much faster than the other corresponding copolymers. Furthermore, it was observed that the introduction of an increased amount of unsaturated branched chain structure of the ITA monomer resulted in the reduced rate of biodegradation.141PBS and PBFu copolymer
The biodegradability of a new class of multiblock copolymers composed of poly(butylene fumarate) (PBFu) and poly(butylene succinate) was compared with those of the corresponding homo-polymers PBF and PBS. From the experimental investigation, it was found that PBS degraded much faster than PBF and the rate of degradation of the multiblock copolymer was significantly affected by the composition. An increase in the PBS content in the copolymer increased the biodegradability, especially when it was around or higher than 50 wt%. Molecular mobility, which determined the rigidity of the polymer chain, also played a significant role in biodegradation. Incorporation of the PBS segment into multiblock copolymers reduced the content of rigid carbon–carbon double bonds, and thus decreased the rigidity of the polymer. Thus, in the copolymer, the PBS segment was preferentially degraded and the PBS content decreased with the progress of degradation. Generally, the amorphous region degraded faster than the crystalline regions and thus, the PBS segment degraded preferentially compared to the PBF segment. Hence, the incorporation of PBS enhanced the degradation rate of the copolymers.142Aliphatic–aromatic copolyesters
In general, due to resistance to fungi or bacteria, aromatic polyesters with rigid benzene rings, such as poly(ethylene terephthalate) and poly(butylene terephthalate), are very difficult to degrade in the natural environment. However, the degradability of aromatic polyesters could be enhanced by introducing aliphatic polyester units into the polymer chain. Biodegradability studies of various aliphatic–aromatic copolyesters resulting from different ratios of diols (ethylene glycol, 1,4-butanediol and 1,6-hexanediol) and diacids (succinic acid and dimethyl terephthalate) indicated that all the synthesized aliphatic–aromatic copolyesters were degradable and the copolyester with the highest content of aliphatic units showed the best degradability.Moreover the biodegradability of copolyesters was also investigated through weight loss ratios and polymer morphology. Copolyesters with fewer aromatic units led to increased weight loss and thus, the degradation ability of copolyesters increased with an increase in the number of aliphatic units in the copolyesters. The importance of the aliphatic content to bio-degradation was realized during biodegradability studies on aliphatic–aromatic copolyesters. Copolymers with increased aliphatic content exhibited a higher biodegradation rate. Furthermore, the chemical structure of the copolyester played a more significant role in deciding the biodegradability than the degree of crystallinity of the copolyester.65
PBSFc copolymer
The bio-degradability of the aliphatic–aromatic copolyester poly(butylene succinate-co-furanoate) (PBSFc) clearly indicated that the amount of aromatic units influenced the degradation rate by enzymes. Thus, PBSFc copolyesters with a low furan dicarboxylic acid (FDCA) content of up to 15 mol% degraded as much as 90% in 180 days.56coPBFS copolymer
The degradation of poly(butylene 2,5-furandicarboxylate-co-succinate) (coPBFS) copolyesters in the presence and absence of enzymes, clearly indicated that biodegradation depended on the composition of the copolymer and the degradation rate increased with an increase in butylene succinate units in the copolymer. Furthermore, enhanced degradation was observed for the copolyesters under enzymatic conditions.99r- and m-PBST copolymers
Enzymatic hydrolysis of both r- and m-PBST (poly(butylene succinate)-multi-poly(butylene terephthalate)) increased with an increase in the number of butylene succinate units. Even at higher PBT concentrations, m-PBST exhibited a higher degradability than the corresponding r-PBST. This difference in degradation was due to the formation of a larger sized PBS domain in m-PBST than that in the corresponding r-PBST. Furthermore, the extent of degradation under enzymatic conditions was found to be greater than that in the absence of an enzyme.125PBS and PBSA copolymers
Furthermore, a yeast strain, Cryptococcus sp. MTCC 5455, was utilized for the hydrolysis of poly(butylene succinate) (PBS) and poly(butylene succinate-co-butylene adipate) (PBSA) copolymers. Complete degradation of the polymeric films was observed after 72 h and 16 h respectively.143 In addition, various microorganisms were examined for their activity towards the degradation of poly(butylene succinate-co-adipate) (PBSA). Among them, a newly isolated strain, S-32, was found to be more effective at degrading both solid and emulsified PBSA.143P(BS-co-PeD), P(BS-co-20%HDO) (75%) and P(BS-co-PDO)
The enzymatic hydrolysis of poly(butylene succinate) (PBS) based random copolyesters consisting of a third monomer, 1,3-propanediol (PDO), 1,5-pentanediol (PeD) and 1,6-hexanediol (HDO), was studied using Candida antarctica lipase B (CALB) enzyme. The degradation of P(BS-co-20%PeD) (85%) polyester was found to be highest followed by P(BS-co-20%HDO) (75%) and P(BS-co- 20%PDO) (23%) polyesters. It was assumed that the presence of a third monomer resulted in the destruction of the spiral chain structure of PBS and thereby decreased the degree of crystallinity. This offered easy access for enzymes to attack the free hydrolysable ester bond segments in the copolymers. The order of degradation rates was found to be P(BS-co-PeD) > P(BS-co-HDO) > P(BS-co-PDO) > PBS. Furthermore, increased ester bond density in the P(BS-co-PeD) copolyester compared to other copolyesters could offer more ester bonds to CALB and thus result in enhanced degradation. This was further supported by molecular modeling studies, in which PeDSPeD-CALB had the lowest free energy of binding, and thus enhanced the degradability of the copolyester P(BS-co-PeD).144PBS, PBA and PBAS copolymers
Enzyme mediated hydrolysis of the poly(butylene succinate-co-butylene adipate) copolyester was investigated by Nikolic et al., using Candida cylindracea lipase. It was observed that enzymes produced by microorganisms were endo-type enzymes and hence the bio-degradability was not dependent on the molecular weight of the polymers. It was very clear from the degradability studies that homopolymers of poly(butylene adipate) PBA and poly(butylene succinate) PBS, with a high degree of crystallinity, exhibited lower biodegradability than the corresponding copolyesters. The order of biodegradability was found to be PBAS-50 > PBAS-25 > PBAS-75. The copolyester with 50 mol% of butylene succinate units resulted in maximum biodegradability and this was believed to be due to a large decrease in the crystallinity of the corresponding copolyester.Furthermore, the melting temperatures and entropy of fusion played a crucial in deciding the biodegradability of copolyesters. Thus, PBAS-25 and PBAS-75 with similar degrees of crystallinity exhibited different rates of biodegradability. This could be attributed to the difference in the melting temperatures of the copolyesters. The copolyester PBAS-25 had a low melting temperature and high entropy of fusion, which greatly affected the rigidity of the chain backbone and thereby increased the flexibility of the polymer chain. Polymers with increased chain flexibility could easily fit into the enzyme's active sites and hence they were more susceptible to biodegradation.145
Copolymers with different diols and diacids
The bio-degradability of co-polyesters synthesized from 1,4-butanediol and diacids with different chain lengths (succinic acid, azelaic acid, and sebacic acid) was also examined. Satisfactory results for enzymatic hydrolysis at temperatures 10–20 °C lower than the polymer's melting point were observed. Furthermore, PBAz and PBSe degraded faster that the corresponding PBS and this was due to differences in the crystallinity of the co-polyesters. The copolyester with high crystallinity degraded at a lower rate.22aPBSTMG copolymer
Enzymatic and hydrolytic degradation of PBSTMG copolymers were found to be almost the same and the presence of the enzyme slightly enhanced the degradation of the copolymer. The introduction of the PTMG comonomer resulted in enhanced hydrolysis and degradation of the copolymer increased with an increase in PTMG content. This was due to increased hydrophilicity, chain flexibility and reduced crystallinity of the copolymer by the introduction of the soft PTMG segment.38Aerobic bio-degradation
Aerobic biodegradation of PPS, PBS, PPAd and PBA using cellulose material as a reference in soil revealed that PBA and PBS showed slightly higher degrees of degradation (44% and 40%, respectively) when compared to that of PPAd and PPS (35% and 40% respectively). Moreover, no correlation between biodegradability and degree of crystallinity or melting temperature was observed. Furthermore, an increase in the number of methylene units between the diols and diacids played a crucial role in determining the biodegradability. Thus, diols with an increase in chain length from 3 to 4 carbons exhibited an increased degradation rate. However, the increase in chain length of the diacids had little effect on the biodegradation rate. It was believed that the increase in the number of methylene units in the diols increased the chain flexibility and thereby reduced the degree of crystallinity, which in turn influenced the biodegradability rate.114Rigid hydrophilic units in bio-degradation
Both hydrolytic and enzymatic degradation of PBS copolyesters derived from isosorbide as a co-monomer (PBIS) were explored. PBIS degraded faster than the corresponding homopolymer under both hydrolytic and enzymatic conditions. The presence of the isosorbide unit in the PBIS copolymer increased the hydrophilicity and also reduced the crystallinity of the polymer. This facilitated the faster degradation of the PBIS copolyester than the corresponding homopolymer under enzymatic conditions. Furthermore, the degradation of polyesters derived from isosorbide units degraded substantially faster than other ester moieties derived from butane diol.73aBio-degradation by composting
Compost degradation experiments on poly(butylene succinate) based copolyesters with different ratios of Pripol 1009 moieties (Fig. 14) revealed that the biodegradability of copolymers was directly related to the incubation time and to the percentage of Pripol moieties in the copolymer. Interestingly, bacteria present in the compost attacked the butylene succinate units in the copolymer in a highly selective manner and thus resulted in an increased concentration of Pripol units in the decomposed polymer. The lower density of carboxylate groups in the copolymers with respect to PBS homopolymers might also be one of the reasons for the slower degradation of the Pripol segments.146Blends derived from soy protein and chemically modified poly(butylene succinate) were also investigated for their biodegradability in compost. Blends with an increased PBS content and increased molecular weight of PBS exhibited slow biodegradation rates. This could be correlated with the relatively low biodegradability of PBS. Furthermore, it was realized the biodegradation rate could be tuned by changing the structure and content of the PBS component.147
Biodegradation of the synthesized poly(ester urethane) copolymers under compost conditions indicated that crystallinity and surface hydrophilicity played key roles in determining the biodegradation of the co-polymer. Copolymers with high hydrophilicity and lower crystallinity (P(BS30BDG70)) underwent faster degradation than the other copolymer compositions. This further clearly indicated that polymers with more amorphous and labile functional groups that were easily accessible to the enzymes had increased biodegradability.109
Biodegradation of synthesized (PBSAT) and blended (PBS/PBT) aliphatic aromatic copolyester under compost conditions using microorganisms demonstrated that the monomer type and composition of the resins greatly influenced the biodegradability. Furthermore, PBS exhibited higher biodegradability due to its aliphatic segments, which were easily accessible to the microorganisms. The degrees of biodegradation of synthesized PBSAT10 and blended PBS/PBT were 19.9% and 28% respectively.126
Conclusion
In recent years, the availability of advanced technologies combined with increased interest in developing bio-alternatives to conventional petroleum based polymers have led to significant progress in the applications, development and industrialization of bio-based polyesters. Even though bio-degradable polymers are capable of mimicking synthetic polymers in various applications, only a few of them have found commercial importance. Among them, polybutylene succinate has gained significant importance owing to its improved mechanical and physical properties. However, cost and a lack of efficient methods for the synthesis of polybutylene succinate are the main challenges that restrict its application on a commercial scale. The development of cost effective methods for bio-based monomer synthesis as well as efficient and environmentally benign catalysts for polymerization are considered to be ideal solutions for commercializing polybutylene succinate.Various catalyst systems, such as Lewis acids, organic acids, organo- and organo-metallic catalysts, as well as the combination of catalysts with thermal stabilizers, have also been explored for the synthesis of polybutylene succinate. The lack of hydrolytic stability of the catalyst and high temperature reaction conditions restrict the synthesis of high molecular weight PBS.
Among various catalyst systems that have been explored towards PBS synthesis, titanium alkoxides are found to be more efficient in synthesizing PBS. Furthermore, it is observed that the addition of a catalyst during the polycondensation reaction is more effective than catalyst addition during an esterification reaction (Table 4). This is believed to be due to the hydrolytic instability of the titanium based catalysts during the esterification reaction, in which water is found to be one of the by-products formed. Conversely, antimony(III) oxide (Sb2O3) is found to be more efficient than titanium alkoxides towards the synthesis of copolymers using less reactive and sterically crowded comonomers such as isosorbide.70 Furthermore, under microwave conditions stannous chloride is found to be more efficient than titanium alkoxides.86
| Catalyst | M n × 104 g mol−1 | M w × 104 g mol−1 | PDI (Đ) | Ref. |
|---|---|---|---|---|
| a Catalyst added during the polycondensation step. b Dual catalyst system, in which one of the catalyst is added during the polycondensation step. | ||||
| TBT | 11.6 | 19.9 | 1.71 | 22a |
| TBTa | 17.64 | 36.27 | 2.06 | 38 |
| TBTa | 8.62 | 18.33 | 2.12 | 85 |
| TBTa | 4.8 | 14.2 | 2.95 | 129 |
| TBT/DPPA | — | 11.6 | — | 43 |
| CHTD | 9.2 | 17.6 | — | 46 |
| DBTO | — | 11.2 | 2.2 | 48 |
| Tin octoate | 9.64 | 16.4 | 1.82 | 53 |
| Zinc acetate/TBTb | 7 | 16 | 2.2 | 66 |
TBT/La(acac)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
6.4 | 10.48 | 1.62 | 68 |
| pTSA/TBTb | 8.8 | 21.3 | 2.41 | 70 |
In the literature, various other techniques like copolymerization and the preparation of blends and composites have also been explored to improve the physical, mechanical and bio-degradable properties of polybutylene succinate. In this regard, comonomers with aliphatic, aromatic, cyclic, acyclic and rigid backbones have been explored towards enhancing the properties of polybutylene succinate.
Although the polymerization of PBS with other comonomers led to an enhancement in either the physical or mechanical properties of the copolymer, there were compromises in its biodegradability (Table 5). Thus, the synthesis of high molecular weight PBS with balanced mechanical, physical and biodegradable properties still remains a challenging task. Thus, there is a lot of scope to develop mild and efficient polymerization catalysts as well as suitable comonomers to produce PBS with enhanced properties towards the commercialization of potential bio-degradable polymers, especially, polybutylene succinate.
| Copolymer | T g | T m | T c | Tensile strength | Elongation at break | Youngs modulus | Thermal stability | Bio-degradability | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| − Slight decrease; ++ increases; − − decreases; + − increases initially and then decreases; − + decreases initially and then increases; ++r increases with an increase in randomness; ** no significant change; − −** decreases and then no significant change; −** slight decrease and then no significant change; ** − − no significant change and decreases; ++b degradation in the amorphous region is much faster than that in the crystalline region; ## composition dependent. | |||||||||
| PBESTU | ++ | − − | ++ | − − | ** − − | − − | ** ++ | 104 | |
| PBT – PBS | ++r | − − | − − | 105 | |||||
| PBS-PCL | − − | − − | ** | − − | + + | — | − − | 106 | |
| PDGS | ** | − − | − − | + + | ++ | − − | 37 | ||
| PBSe | − − | − | − − | − | ++ | − | − | 27 | |
| PFS | ++ | − −** | − − | − − | ++ | − − | − − | 108 | |
| PEG | ** | − − | + − | ++ | − − | ++ | 76 | ||
| P(BSBDG)(Diglycolic acid) | ++ | − − | − − | − − | ++ | − − | − − | ++ | 109 |
| P(BS-co-HS) | − − | − − | − − | − −** | + − | −+ | − − | ++ | 111 and 112 |
| P(BS-BMS) | − − | − − | ** | ++b | 116 | ||||
| 2,3-BDO | ++ | − − | − − | − − | 96 | ||||
| (PBDS) | − − | − − | − − | − − | −+ | − − | ** | 43 | |
| P(BS-co-BAz) | − − | − − | − − | ++ | 118 | ||||
| PBS-ran-PBAz | − − | − − | − − | 119 | |||||
| PBSCs | ++ | − − | − − | ++ | − − | − − | ++ | 121 | |
| PBS-ran-PCL | − − | − − | − − | −** | 95 and 122 | ||||
| PBSI | ** | − − | − − | ** | − − | 98 and 141 | |||
| PBSFu | ++ | ++ | ++ | 74 and 123 | |||||
| P(BSBDTDP) | − − | − − | − − | − − | ++ | 26 | |||
| PBIS | ++ | − − | − − | ++ | − − | −+ | ++ | 73a | |
| PBST | ++ | − − | − − | −− | −− | 56 | |||
| PBSF | ++ | − − | − − | ++ | −** | 56 | |||
| PBISx & PBThSx | ++ | − − | − − | − − | ++ | − − | ** | ++ | 124 |
| PBSGlux | ++ | − − | − − | − + | − + | ++ | ++ | 73b | |
| PBSManx | ++ | − − | − − | ++ | − − | ++ | ++ | ++ | 49 |
| FCDA | ++ | − − | − | 29 | |||||
| PBSFr | ++ | − + | − + | − − | ++ | − − | −** | ## | 67 |
| PFS-PBS | ++ | − ** | − − | − − | ++ | − − | − − | 108 | |
| PBS-BFu(Cross linked) | ++ | ++ | ++ | − − | − − | − − | − − | − − | 41 |
| PBS-BFu(Uncross linked) | ++ | ++ | ++ | − − | ++ | ++ | − − | − − | 41 |
| PBSTMGs | − − | − − | − − | − − | ++ | − − | − − | ++ | 38 |
| PBSTMGs (Blends) | − − | − − | − − | +− | + − | 38 | |||
| 1,2-DD | − − | − − | − − | − − | ++ | − − | − − | ++ | 24 |
| 1,2,4-BT | ++ | ** | ++ | ++ | − − | 129 | |||
| Glycerol | − − | − − | − − | − − | − − | − − | 24 | ||
| 1,2-BDO | − − | − − | − − | ++ | 24 | ||||
The biodegradation of polymers depends on various parameters like chemical structure, repeating unit, hydrophilic/hydrophobic balance, molecular weight, solid-state morphology (i.e. crystallinity, crystal size and distribution), molecular mobility, and thermal characteristics. Moreover, amorphous and hydrophilic polymers degrade much faster than the corresponding crystalline and hydrophobic polymers. In addition, the nature, relative quantity, and distribution of the co-monomeric units along the polymer chain also have a significant influence on biodegradation.
Abbreviation
| PBS | Poly(butylene succinate) |
| CAGR | Compound annual growth rate |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| LLDPE | Linear low-density polyethylene |
| MMT | Million metric tonnes |
| SA | Succinic acid |
| BDO | 1,4-Butane diol |
| PBSAD | Poly(butylene succinate-co-butylene acetylenedicarboxylate) |
| p(BSBDTDP) | Poly(butylene succinate/dithiodipropionate) |
| TBT | Titanium tetrabutoxide |
| TTIP | Titanium tetraisopropoxide |
| PBSFu | Poly(butylene succinate-co-butylene fumarate) |
| PBFu | Poly(butylene fumarate) |
| PBSPS | Poly(butylene succinate-co-propylene succinate) |
| rPBSA | Poly(butylene succinate-ran-butylene adipate) random copolymers |
| PBS-ran-PCL | Poly[(butylene succinate)-ran-(ε-caprolactone)] copolyesters |
| BCHDA | Butylene 1,4-cyclohexanedicarboxylate |
| CHDMS | 1,4-Cyclohexane dimethylene succinate |
| P(BS-co-HS) | Poly(butylene succinate-co-hexamethylene succinate) |
| P(BS-co-BAz) | Poly(butylene succinate-co-butylene azelate) |
| PBS-b-PDGS | Poly(butylene succinate-b-poly(diethylene glycol succinate) |
| PBSTMGs | Poly[(butylene succinate)-co-poly(tetramethylene glycol)] |
| PTMG | Poly(tetramethylene glycol) |
| PESu | Poly(ethylene succinate) |
| PPSu | Poly(propylene succinate) |
| PPA | Polyphosphoric acid |
| DPPA | Diphenylphosphinic |
| HTTD | 1-Hydroxy-3-thioisocyanate-1,1,3,3-tetrabutyldistannoxane |
| DCTD | 1,3-Dichloro-1,1,3,3-tetrabutyldistannoxane |
| CHTD | 1-Chloro-3-hydroxy-1,1,3,3-tetrabutyldistannoxane |
| CHTOD | 1-Chloro-3-hydroxy1,1,3,3-tetraoctyldistannoxane |
| DBTO | Dibutyl tin oxide |
| PManxS | Bio-based PBS copolyesters obtained by partially replacing 1,4-butanediol in PBS with 2,4:3,5-di-O-methylene-D-mannitol |
| PEG | Polyethylene glycol |
| PDLA-b-PBS-b-PDLA | Poly(D-lactide)-block-poly(butylene succinate)-block-poly(D-lactide) |
| La(acac)3 | Lanthanum(III) acetylacetonate |
| HEPBP | 2-Hydro-4-(2,3-epoxypropoxy) benzophenone |
| p-TSA | p-Toluenesulfonic acid |
| CS | Cyclohexanedimethylene succinate |
| BS | Butylene succinate |
| PBS-DLS | Poly(butylene succinate–dilinoleic succinate) |
| PBS-DLS-PEG | Poly(butylene succinate–dilinoleic succinate–ethylene glycol succinate) |
| SSP | Solid state polymerization |
| PBSM | Poly(butylene succinate-co-butylene malate) |
| PBST | Poly(butylene succinate-co-butylene terephthalate) |
| PBBS | Poly(1,4-butylene succinate-ran-2,3-butylene succinate) |
| PPBS | Poly(p-dioxanone-co-butylene-co-succinate) |
| ROP | Ring opening polymerization |
| PBSI | Poly(butylene succinate-co-itaconate) |
| PBSIr | PBS copolymer with rigid imide units |
| PETS-OH | Hydroxyl terminated poly(ethylene succinate-co-ethylene terephthalate) |
| PBS-OH | Hydroxyl terminated poly(butylene succinate) |
| TDI | Toluene-2,4-diisocyanate |
| PBT-PBS | Poly(butylene terephthalate)–poly(butylene succinate) |
| HO-PBT-OH | Dihydroxyl terminated PBT |
| PDGS | Poly(diethylene glycol succinate) |
| PBF | Polybutylene fumarate |
| PBSe-diol | Dihydroxyl terminated poly(butylene sebacate) |
| PFS diol | Poly(2,5-furandimethylene succinate) |
| IPDI | Isophorone diisocyanate |
| PEEUs | Poly(ester ether urethane)s |
| TPU | Thermoplastic polyurethanes |
| PBCE-OH | Hydroxyl terminated poly(butylene cyclohexanedicarboxylate) |
| HDI | Hexamethylene diisocyanate |
| PES-b-PBS | Poly(ethylene succinate)-b-poly(butylene succinate) |
| PHBS | Poly(hexamethylene succinate-co-6 mol% butylene succinate) |
| PP-r-BS | Poly(1,3-propylene succinate-ran-1,4-butylene succinate) |
| PPBA | Poly(1,3-propylene adipate-ran-1,4-butylene adipate) |
| 1,3-PDO | 1,3-Propanediol |
| IDA | Imide dihydric alcohol |
| P(BS-BMS) | Poly(butylene succinate-co-butylene 2-methylsuccinate) |
| MSA | 2-Methylsuccinic acid |
| PBA | Poly(butylene adipate) |
| DS | Decamethylene succinate |
| BUP | Biodegradable unsaturated polyester |
| PBDS | Poly(butylene succinate-co-decamethylene succinate) |
| PBS-ran-PBAz | Poly(butylene succinate-ran-butylene azelate) copolyester |
| PBSCs | 1,3/1,4-Cyclohexanedimethanol based alicyclic/aliphatic copolyesters |
| PBCS | Poly(butylene succinate-co-cyclohexanedimethylene succinate) |
| PCS | Poly(cyclohexanedimethylene succinate) |
| WAXS | Wide-angle X-ray scattering |
| CL | Caprolactone |
| PCL | Poly(caprolactone) |
| SAED | Selected area electron diffraction |
| PDLA | Poly(D-lactic acid) |
| PBIS | PBS copolyester derived from isosorbide as a co-monomer |
| IS | Isosorbide |
| ITh | 2,3-O-Isopropylidene-lthreitol |
| Manx | 2,4:3,5-Di-O-methylene-D-mannitol |
| Glux-Diol | 2,4:3,5-Di-O-methylene-D-glucitol |
| AD | Acetylene dicarboxylic acid |
| BF | Butylene furandicarboxylate |
| PFS-PBS | Poly(2,5-furandimethylene succinate)-b-poly(butylene succinate) |
| PFS | Poly(2,5-furandimethylene succinate) |
| PBAT | Random copolymer of PBA and PBT |
| m-PBST | Multi-block poly(butylene succinate)–multi-poly(butylene terephthalate) copolyester |
| r-PBST | Random poly(butylene succinate)–multi-poly(butylene terephthalate) copolyester |
| PBSAT | Poly(butylene succinate-co-adipate-co-terephthalate) |
| PBSEG | Poly(butylene succinate)–poly(ethylene glycol) |
| PBSLAsp | PBS copolymer including e-caprolactone and aspartic acid |
| 1,2-DS | 1,2-Decylene succinate |
| 1,2,4-BT | 1,2,4-Butanetriol |
| DLAOH | Dimer linoleic diol |
| PVB | Poly(vinyl butyral) |
| FTIR | Fourier transform infrared spectroscopy |
| PHB | Poly(3-hydroxybutyrate) |
| PES | Poly(ethylene succinate) |
| PHS | Poly(hexylene succinate) |
| P(BS-co-CHDMS) | Poly(butylene succinate) (PBS) and its copolymers containing 1,4-cyclohexane dimethylene succinate |
| P(BS-co-BCHDA) | Poly(butylene succinate) (PBS) and its copolymers containing butylene 1,4-cyclohexanedicarboxylate (BCHDA) |
| CHCl3 | Chloroform |
| SDMS | 1,2-(Dimethoxycarbonyl)ethanesulfonate (sulfonated dimethyl succinate) |
| CHDM | 1,4-Cyclohexanedimethanol |
| CHDA | 1,4-Cyclohexanedicarboxylate |
| PBS-co-CL | Poly(butylene succinate-co-ε-caprolactone) |
| PBS-co-CHDM | Copolymer of PBS and 1,4-cyclohexane dimethanol |
| PBS-co-CL-co-CHDM | Ternary copolymer of PBS, PCL, and 1,4-cyclohexane dimethanol |
| PBS-co-DEGS | Poly(butylene succinate-co-diethylene glycol succinate) |
| PBS-co-BDGA | Poly(butylene succinate-co-butylene diglycolic acid) |
| HS | Hexamethylene succinate |
| BA | Butylene adipate |
| PBS-co-BA | Poly(butylenesuccinate-co-butylene adipate) |
| PBSCL | Poly(butylene succinate-co-6-hydroxycaproate) |
| FDCA | Furan dicarboxylic acid |
| PBT | Poly(butylene terephthalate) |
| PBSA | Poly(butylene succinate-co-butylene adipate) |
| PPAd | Poly(1,3-propylene adipate) |
| PBAS | Poly(butylene succinate-co-butylene adipate) |
| PBSFr | Poly(butylene succinate-co-butylene furandicarboxylate) |
| PBSFc | Poly(butylene succinate-co-furanoate) copolymer |
| PPS | Poly(1,3-propylene succinate) |
| PeD | 1,5-Pentanediol |
| HDO | 1,6-Hexanediol |
| CALB | Candida antarctica lipase B |
| P(BS-co-PeD) | PBS based random copolyester of a third monomer, 1,5-pentanediol (PeD) |
| P(BS-co-HDO) | PBS based random copolyester of a third monomer, 1,6-hexanediol (HDO) |
| P(BS-co-PDO) | PBS based random copolyester of a third monomer, 1,3-propanediol (PDO) |
| PBAz | Poly(butylene azelate) |
| PBSe | Poly(butylene sebacate) |
| (coPBFS) | Poly(butylene 2,5-furandicarboxylate-co-succinate) |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. S. Savitha Gwoda thanks the Sir M. Visvesvaraya Postgraduate Centre, Mandya, Karnataka, India, for providing research facilities.References
- S. Agarwal, Biodegradable Polymers: Present Opportunities and Challenges in Providing a Microplastic-Free Environment Macromol, Chem. Phys., 2020, 221, 2000017, DOI:10.1002/macp.v221.610.1002/macp.202000017.
- (a) A. Gandini, in Biocatalysis in Polymer Chemistry, ed. K. Loos, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011, pp. 1–33 Search PubMed; (b) O. Platnieks, S. Gaidukovs, V. K. Thakur, A. Barkane and S. Beluns, Bio-based poly (butylene succinate): Recent progress, challenges and future opportunities, Eur. Polym. J., 2021, 161, 110855, DOI:10.1016/j.eurpolymj.2021.110855; (c) S. A. Rafiqah, A. Khalina, A. S. Harmaen, I. A. Tawakkal, K. Zaman, M. Asim, M. N. Nurrazi and C. H. Lee, A Review on Properties and Application of Bio-Based Poly(Butylene Succinate), Polymers, 2021, 13, 1436, DOI:10.3390/polym13091436; (d) M. J. Mochane, S. L. Magagula, J. S. Sefadi and T. C. Mokhena, A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS), Polymers, 2021, 13, 1200, DOI:10.3390/polym13081200; (e) N. Chanthaset and H. Ajiro, Synthetic Biodegradable Polymers with Chain End Modification: Polylactide, Poly(butylene succinate), and Poly(hydroxyalkanoate), Chem. Lett., 2021, 50, 767–777, DOI:10.1246/cl.200859; (f) F. Xiao, G. Fontaine and S. Bourbigot, Recent developments in fire retardancy of polybutylene succinate, Polym. Degrad. Stab., 2021, 183, 109466, DOI:10.1016/j.polymdegradstab.2020.109466; (g) G.-Z. Yin and X.-M. Yang, Biodegradable polymers: a cure for the planet, but a long way to go, J. Polym. Res., 2020, 27, 38, DOI:10.1007/s10965-020-2004-1; (h) Bioplastics market development update 2021, https://www.european-bioplastics.org/news/publications/.
- (a) M. K. Huda and I. Widiastuti, Natural Fiber Reinforced Polymer in Automotive Application: A Systematic Literature Review, J. Phys.: Conf. Ser., 2021, 1808, 012015, DOI:10.1088/1742-6596/1808/1/012015; (b) A. L. Andrady and M. A. Neal, Applications and societal benefits of plastics, Philos. Trans. R. Soc., B, 2009, 364, 1977–1984, DOI:10.1098/rstb.2008.0304.
- I. Vroman and L. Tighzert, Biodegradable Polymers, Materials, 2009, 2, 307–344, DOI:10.3390/ma2020307.
- (a) S. Spierling, E. Knupffer, H. Behnsen, M. Mudersbach, H. Krieg, S. Springer, S. Albrecht, C. Herrmann and H. J. Endres, Bio-based plastics - a review of environmental, social and economic impact assessments, J. Cleaner Prod., 2018, 185, 476–491, DOI:10.1016/j.jclepro.2018.03.014; (b) Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang and L. Zhang, Bio-based polyesters: Recent progress and future prospects, Prog. Polym. Sci., 2021, 120, 101430, DOI:10.1016/j.progpolymsci.2021.101430; (c) S. Rameshkumar, P. Shaiju, K. E. O'Connor and R. B. Padamati, Bio-based and biodegradable polymers—State-of-the-art, challenges, and emerging trends, Curr. Opin. Green Sustainable Chem., 2020, 21, 75–81 CrossRef; (d) W. Sikorska, M. Musioł, B. Zawidlak-Węgrzyńska and J. Rydz, End-of-Life Options for (Bio)degradable Polymers in the Circular Economy, Adv. Polym. Technol., 2021, 6695140, DOI:10.1155/2021/6695140.
- (a) J. Xu and B. H. Guo, Poly(butylene succinate) and its copolymers: Research, development and industrialization, Biotechnol. J., 2010, 5, 1149–1163, DOI:10.1002/biot.201000136; (b) G. Guidotti, M. Soccio, V. Siracusa, M. Gazzano, E. Salatelli, A. Munari and N. Lotti, Novel Random PBS-Based Copolymers Containing Aliphatic Side Chains for Sustainable Flexible Food Packaging, Polymers, 2017, 9, 724, DOI:10.3390/polym9120724; (c) F. De Santis, V. Volpe and R. Pantani, Polym. Eng. Sci., 2016, 57, 306–311 CrossRef; (d) P. Scarfato, L. D. Maio, M. R. Milana, S. Giamberardini, M. Denaro and L. Incarnato, Performance properties, lactic acid specific migration and swelling by simulant of biodegradable poly(lactic acid)/nanoclay multilayer films for food packaging, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2017, 34, 1730–1742, DOI:10.1080/19440049.2017.1321786; (e) V. V. Jost, Packaging related properties of commercially available biopolymers—An overview of the status quo, eXPRESS Polym. Lett., 2018, 12, 429–435, DOI:10.3144/expresspolymlett.2018.36; (f) A. Z. Naser, I. Deiab and B. M. Darras, Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review, RSC Adv., 2021, 11, 17151 RSC; (g) European Bioplastics conference, bioplastics market development update 2021, https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf.
- Y. Tachibana, M. Yamahata, S. Kimura and K.-I. Kasuya, Synthesis, Physical Properties, and Biodegradability of Biobased Poly(butylene succinate-co-butylene oxabicyclate), ACS Sustainable Chem. Eng., 2018, 6, 10806–10814, DOI:10.1021/acssuschemeng.8b02112.
- (a) T. Liu, C. Hao, L. Wang, Y. Li, W. Liu, J. Xin and J. Zhang, Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability, Macromolecules, 2017, 50, 8588–8597, DOI:10.1021/acs.macromol.7b01889; (b) T. Liu, X. Guo, W. Liu, C. Hao, L. Wang, W. C. Hiscox, C. Liu, C. Jin, J. Xin and J. Zhang, Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation, Green Chem., 2017, 19, 4364–4372, 10.1039/C7GC01737E.
- (a) S. Zhang, T. Liu, C. Hao, L. Wang, J. Han, H. Liu and J. Zhang, Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives, Green Chem., 2018, 20, 2995–3000, 10.1039/C8GC01299G; (b) F. I. Altuna, V. Pettarin and R. J. J. Williams, Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution, Green Chem., 2013, 15, 3360–3366, 10.1039/C3GC41384E.
- C. Vilela, A. F. Sousa, A. Fonseca, A. Serra, J. Cohello, C. Freire and A. Silvestre, The quest for sustainable polyesters – insights into the future, Polym. Chem., 2014, 5, 3119–3141, 10.1039/C3PY01213A.
- (a) E. Pollet and L. Averous, Production, chemistry and properties of polyhydroxyalkanoates, in Biopolym. e New Mater. Sustain. Films Coat, ed. D. Plackett, John Wiley & Sons, Ltd, Chichester, 2011, pp. 65–86. (accessed February 16, 2016) Search PubMed; (b) L. Av_erous, Biodegradable Multiphase Systems Based on Plasticized Starch: A Review, J. Macromol. Sci., Part C, 2004, 44, 231–274, DOI:10.1081/MC-200029326; (c) M. Gigli, M. Fabbri, N. Lotti, R. Gamberini, B. Rimini and A. Munari, Poly(butylene succinate)-based polyesters for biomedical applications: A review, Eur. Polym. J., 2016, 75, 431–460, DOI:10.1016/j.eurpolymj.2016.01.016.
- (a) H. Song and S. Y. Lee, Production of succinic acid by bacterial fermentation, Enzyme Microb. Technol., 2006, 39, 352–361, DOI:10.1016/j.enzmictec.2005.11.043; (b) K.-K. Cheng, X.-B. Zhao, J. Zeng and J.-A. Zhang, Biotechnological production of succinic acid: current state and perspectives, Biofuels, Bioprod. Biorefin., 2012, 6, 302–318, DOI:10.1002/bbb.1327.
- G.-Q. Chen and M. K. Patel, Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review, Chem. Rev., 2012, 112, 2082–2099, DOI:10.1021/cr200162d.
- (a) V. Menon and M. Rao, Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept, Prog. Energy Combust. Sci., 2012, 38, 522–550, DOI:10.1016/j.pecs.2012.02.002; (b) B. Caulier and S. (Roquette Fre'res) Laurent, WO Patent2,010,092304 (A2), 2010 Search PubMed; (c) Top Value Added Chemicals from Biomass. Volume I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas, ed. T. Werpy and G. Petersen, US Department of Energy, Offfiice of Scientific and Technical Information, 2004, OSTI Id: 15008859 Search PubMed.
- (a) H. Yim, R. Haselbeck, W. Niu, C. Pujol-Baxley, A. Burgard, J. Boldt, J. Khandurina, J. D. Trawick, R. E. Osterhout, R. Stephen, J. Estadilla, S. Teisan, H. B. Schreyer, S. Andrae, T. H. Yang, S. Y. Lee, M. J. Burk and S. V. Dien, Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol, Nat. Chem. Biol., 2011, 7, 445–452, DOI:10.1038/nchembio.580; (b) L. Aliotta, M. Seggiani, A. Lazzer, V. Gigante and P. Cinelli, A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications, Polymers, 2022, 14, 844, DOI:10.3390/polym14040844.
- (a) B. Caulier and S. (Roquette Fre'res) Laurent, WO Patent2,010,092,304 (A2), August 19, 2010 Search PubMed; (b) T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass: Volume I – Results of Screening for Potential Candidates from Sugars and Synthesis Gas, 2004, OSTI Id: 15008859 Search PubMed; (c) D. P. Minh, M. Besson, C. Pinel, P. Fuertes and C. Petitjean, Aqueous-Phase Hydrogenation of Biomass-Based Succinic Acid to 1,4-Butanediol Over Supported Bimetallic Catalysts, Top. Catal., 2010, 53, 1270–1273, DOI:10.1007/s11244-010-9580-y; (d) R. M. Deshpande, V. V. Buwa, C. V. Rode, R. V. Chaudhari and P. L. Mills, Tailoring of activity and selectivity using bimetallic catalyst in hydrogenation of succinic acid, Catal. Commun., 2002, 3, 269–274, DOI:10.1016/S1566-7367(02)00119-X; (e) S. A. Rafiqah, A. Khalina, A. S. Harmaen, I. A. Tawakkal, K. Zaman, M. Asim, M. N. Nurrazi and C. H. Lee, A Review on Properties and Application of Bio-Based Poly(Butylene Succinate), Polymers, 2021, 13, 1436, DOI:10.3390/polym13091436; (f) R. Landucci, B. J. Goodman and C. E. Wyman, Methodology for evaluating the economics of biologically producing chemicals and materials from alternative feedstocks, Appl. Biochem. Biotechnol., 1994, 45–46, 677–696, DOI:10.1007/BF02941840; (g) J. G. Zeikus, M. K. Jain and P. Elankovan, Biotechnology of succinic acid production and markets for derived industrial products, Appl. Microbiol. Biotechnol., 1999, 51, 545–552, DOI:10.1007/s002530051431; (h) M. Shibata, Y. Inoue and M. Miyoshi, Mechanical properties, morphology, and crystallization behavior of blends of poly(l-lactide) with poly(butylene succinate-co-l-lactate) and poly(butylene succinate), Polymer, 2006, 47, 3557–3564, DOI:10.1016/j.polymer.2006.03.065; (i) T. E. Furia, Handbook of food additives, CRC-Press, Boca Raton, Florida, 1973, vol. 1 Search PubMed; (j) M. Kunioka, Possible incorporation of petroleum-based carbons in biochemicals produced by bioprocess, Appl. Microbiol. Biotechnol., 2010, 87, 491–497, DOI:10.1007/s00253-010-2630-3; (k) J. B. McKinlay, C. Vielle and J. G. Zeikus, Prospects for a bio-based succinate industry, Appl. Microbibol. Biotechnol., 2007, 76, 727–740, DOI:10.1007/s00253-007-1057-y; (l) CRC Handbook of Food Additives, ed. T. E. Furia, vol. I, ISBN: 0878195424; (m) J. E. Carnahan, T. A. Ford, W. F. Gresham, W. E. Grigsby and G. F. Hager, Ruthenium-catalyzed Hydrogenation of Acids to Alcohols, J. Am. Chem. Soc., 1955, 77, 3766–3768, DOI:10.1021/ja01619a025; (n) J. Xu and B.-H. Guo, in In Plastics from Bacteria: Natural Functions and Applications, ed. G. G. Q. Chen, Springer, Heiderberg, 2010, pp. 347–388 Search PubMed; (o) T. Mizukoshi, T. In, ICTABP2, Hangzhou, China, 2006, p. 123 Search PubMed; (p) S. Kato, T. Tsukahara, M. Kishimoto and I. Nagaya, In ICTABP2, Hangzhou, China, 2006, p. 96 Search PubMed; (q) N. Shitani and S. Kato, In GSC-AON, Tokyo, Japan, 2007, p. 235 Search PubMed.
- (a) E. Chiellini and R. Solaro, Biodegradable Polymeric Materials, Adv. Mater., 1996, 8, 305–313, DOI:10.1002/adma.19960080406; (b) Z. Gan, H. Abe, H. Kurokawa and Y. Doi, Solid-State Microstructures, Thermal Properties, and, Crystallization of Biodegradable Poly(butylene succinate) (PBS) and Its Copolyesters, Biomacromolecules, 2001, 2, 605–613, DOI:10.1021/bm015535e; (c) K. Fukushima and Y. Kimura, Stereocomplexed polylactides (Neo-PLA) as high-performance bio-based polymers: their formation, properties, and application, Polym. Int., 2006, 55, 626–642, DOI:10.1002/pi.2010; (d) A. A. Shah, F. Hasan, A. Hameed and S. Ahmed, Biological degradation of plastics: A comprehensive review, Biotechnol. Adv., 2008, 26, 246–265, DOI:10.1016/j.biotechadv.2007.12.005.
- (a) W. H. Carothers, Chem. Rev., 1931, 8, 353–426 CrossRef CAS . reprinted in Collected Papers of Wallace Hume Carothers on High Polymeric Substances, Mark, H., Whitby, G. S., Ed., Tnterscience: New York, 1940, pp 81–140; (b) Patents: Carothers, W. H. U.S. Pat. 2,071,250 (Feb. 16, 1937), U.S. Pat. 2,071,251 (Feb. 16, 1937), U.S. Pat. 2,130,523 (Sept. 30, 1938), U.S. Pat. 2,130,947 (Sept. 20, 1938), U.S. Pat. 2,130,948 (Sept. 20, 1938).
- (a) W. H. Carothers and J. A. Ariv, studies on polymerization and ring formation. Ii. Poly-esters, J. Am. Chem. Soc., 1929, 51, 2560–2570, DOI:10.1021/ja01383a042; (b) W. H. Carothers and J. W. Hill, studies of polymerization and ring formation. Xii. Linear superpolyesters, J. Am. Chem. Soc., 1932, 54, 1559–1566, DOI:10.1021/ja01343a048; (c) K. Chrissafis, K. M. Paraskevopoulos and D. N. Bikiaris, Effect of molecular weight on thermal degradation mechanism of the biodegradable polyester poly(ethylene succinate), Thermochim. Acta, 2006, 440, 166–175, DOI:10.1016/j.tca.2005.11.002; (d) J. Xu and B. H. Guo, Microbial Succinic Acid, Its Polymer Poly(butylene succinate), and Applications, Springer press, Heidelberg, 2010, p. 347 Search PubMed.
- (a) M. Mochizuki, K. Mukai, K. Yamada, N. Ichise, S. Murase and Y. Iwaya, Structural Effects upon Enzymatic Hydrolysis of Poly(butylene succinate-co-ethylene succinate)s, Macromolecules, 1997, 30, 7403–7407, DOI:10.1021/ma970036k; (b) H. R. Kricheldorf, D. Langanke, J. Spickermann and M. Schmidt, Macrocycles. 10. Macrocyclic Poly(1,4-butanediol−ester)s by Polycondensation of 2-Stanna-1,3-dioxepane with Dicarboxylic Acid Chlorides, Macromolecules, 1999, 32, 3559–3564, DOI:10.1021/ma981134o; (c) K. Ishihara, S. Ohara and H. Yamamoto, Direct Condensation of Carboxylic Acids with Alcohols Catalyzed by Hafnium(IV) Salts, Science, 2000, 290, 1140–1142, DOI:10.1126/science.290.5494.1140; (d) A. Takasu, Y. Ito, Y. Oishi, Y. Narukawa and T. Hirabayashi, Environmentally Benign Polyester Synthesis by Room Temperature Direct Polycondensation of Dicarboxylic Acid and Diol, Macromolecules, 2005, 38, 1048–1050, DOI:10.1021/ma0475378; (e) H. R. Kricheldorf, M. Rabenstein, M. Maskos and M. Schmidt, Macrocycles. 15. The role of cyclization in kinetically controlled polycondensations, Macromolecules, 2001, 34, 713–722, DOI:10.1021/ma001099x; (f) H. R. Kricheldorf, Q. Gao, P. Buzin and G. Schwarz, Aliphatic Polyesters of Butyne-1,4-Diol, Macromol. Chem. Phys., 2008, 209, 385–392, DOI:10.1002/macp.200700429; (g) S. Chatti, G. Behnken, D. Langanke and H. R. Kricheldorf, Biodegradable Polyesters Based on cis-1,4-Butenediol, Macromol. Chem. Phys., 2006, 207, 1474–1484, DOI:10.1002/macp.200600105; (h) P. Buzin, L. Mohammed, G. Schwarz and H. R. Kricheldorf, Aliphatic Polyesters by Bismuth Triflate-Catalyzed Polycondensations of Dicarboxylic Acids and Aliphatic Diols, Macromolecules, 2008, 41, 8491–8495, DOI:10.1021/ma8017662; (i) M. Lahcini, G. Schwarz and H. R. Kricheldorf, Bismuth halide-catalyzed polymerizations of ε-caprolactone, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7483–7490, DOI:10.1002/pola.23054; (j) H. Ito, N. Yamamoto, F. Hiroji and M. Jojima, Jap. Pat, 7 16-41, 1987 Search PubMed; (k) E. Takayama, I. Mikura and Y. Hatano, Jap. Pat, 1 89823, 1992 Search PubMed; (l) M. Miura, H. Watanabe and M. Fujiwara, Jap. Pat, 53 695, 1995 Search PubMed.
- K. Y. Choi and K. B. McAuley, Step-growth polymerization in Polymer Reaction Engineering, ed. J. M. Asua, Blackwell Publishing, Oxford, UK, 2007, ch. 7, pp. 273–314 Search PubMed.
- (a) X. Kong, H. Qi and J. M. Curtis, Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources, J. Appl. Polym. Sci., 2014, 131, 40579, DOI:10.1002/app.40579; (b) D. N. Bikiaris and D. Achilias, Synthesis of poly(alkylene succinate) biodegradable polyesters I. Mathematical modelling of the esterification reaction, Polymer, 2006, 47, 4851–4860, DOI:10.1016/j.polymer.2006.04.044; (c) C. Zhu, Z. Zhang, Q. Liu, Z. Wang and J. Jin, Synthesis and biodegradation of aliphatic polyesters from dicarboxylic acids and diols, J. Appl. Polym. Sci., 2003, 90, 982–990, DOI:10.1002/app.12722.
- (a) M. Garin, L. Tighzert, I. Vroman, S. Marinkovic and B. Estrine, The influence of molar mass on rheological and dilute solution properties of poly(butylene succinate), J. Appl. Polym. Sci., 2014, 131, 40887, DOI:10.1002/app.40887; (b) D. N. Bikiaris, G. Z. Papageorgiou and D. S. Achilias, Synthesis and Comparative Biodegradability Studies of Three Poly(Alkylene Succinates), Polym. Degrad. Stab., 2006, 91, 31–43, DOI:10.1016/j.polymdegradstab.2005.04.030; (c) D. N. Bikiaris and D. S. Achilias, Synthesis of poly(alkylene succinate) biodegradable polyesters, Part II: Mathematical modelling of the polycondensation reaction, Polymer, 2008, 49, 3677–3685, DOI:10.1016/j.polymer.2008.06.026; (d) M. S. Nikolic, D. Poleti and J. Djonlagic, Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene fumarate)s, Eur. Polym. J., 2003, 39, 2183–2192, DOI:10.1016/S0014-3057(03)00139-3.
- (a) Z. Sun, Z. Jiang and Z. Qiu, Thermal, crystallization and mechanical properties of branched Poly(butylene succinate) copolymers with 1,2-decanediol being the comonomer, Polymer, 2021, 213, 123197, DOI:10.1016/j.polymer.2020.123197; (b) H.-J. Jin, J.-K. Park, K.-H. Park, M.-N. Kim and J.-S. Yoon, Properties of Aliphatic Polyesters with n-Paraffinic Side Branches, J. Appl. Polym. Sci., 2000, 77, 547–555, DOI:10.1002/(SICI)1097-4628(20000718)77:3<547::AID-APP10>3.0.CO;2-E; (c) M. N. Kim, K. H. Kim, H.-J. Jin, J. K. Park and J. S. Yoon, Biodegradability of ethyl and n-octyl branched poly (ethylene adipate) and poly (butylene succinate), Eur. Polym. J., 2001, 37, 1843–1847, DOI:10.1016/S0014-3057(01)00003-9.
- Y. Li, G. Huang, C. Chen, X.-W. Wei, X. Dong, W. Zhao and H.-M. Ye, Poly(butylene succinate-co-butylene acetylenedicarboxylate): Copolyester with Novel Nucleation Behavior, Polymers, 2021, 13, 365, DOI:10.3390/polym13030365.
- G. Guidotti, M. Soccio, M. Gazzano, N. Bloise, G. Bruni, A. Aluigi, L. Visai, A. Munari and N. Lotti, Biocompatible PBS-based copolymer for soft tissue engineering: Introduction of disulfide bonds as winning tool to tune the final properties, Polym. Degrad. Stab., 2020, 182, 109403, DOI:10.1016/j.polymdegradstab.2020.109403.
- Y. Shang, Z. Jiang and Z. Qiu, Synthesis, thermal and mechanical properties of novel biobased, biodegradable and double crystalline poly(butylene succinate)-b-poly(butylene sebacate) multiblock copolymers, Polymer, 2021, 214, 123248, DOI:10.1016/j.polymer.2020.123248.
- (a) H.-I. Mao, L.-Y. Wang, C.-W. Chen, K.-H. Hsu, C.-H. Tsai, C.-J. Cho, Y.-Y. Yu, S.-P. Rwei and C.-C. Kuo, J. Polym. Res., 2021, 28, 1–14 CrossRef; (b) K.-H. Hsu, C.-W. Chen, L.-Y. Wang, H.-W. Chan, C.-L. He, C.-J. Cho and S.-P. Rwei, C-C Kuo;Bio-based thermoplastic poly(butylene succinate-co-propylene succinate) copolyesters: effect of glycerol on thermal and mechanical properties, Soft Matter, 2019, 9710–9720, 10.1039/C9SM01958H.
- G. Z. Papageorgiou and D. G. Papageorgiou, Solid-state structure and thermal characteristics of a sustainable biobased copolymer: poly(butylene succinate-co-furanoate), Thermochim. Acta, 2017, 656, 112–122, DOI:10.1016/j.tca.2017.09.004.
- M. V. B. Nicolino, A. D. A. Lucas and M. C. Branciforti, Reactive extrusion of poly (butylene succinate-co-adipate) and poly (ε-caprolactone) biodegradable blends through titanium-based transesterification catalyst, Polym. Degrad. Stab., 2020, 181, 109320, DOI:10.1016/j.polymdegradstab.2020.109320.
- (a) R. A. Pérez-Camargo, B. Fernández-d'Arlas, D. Cavallo, T. Debuissy, E. Pollet, L. Avérous and A. J. Müller, Tailoring the StructureMorphology, and Crystallization of Isodimorphic Poly(butylene succinate-ran-butylene adipate) Random Copolymers by Changing Composition and Thermal History, Macromolecules, 2017, 50, 597–608 CrossRef; (b) S. Wu, Y. Zhang, J. Han, Z. Xie, J. Xu and B. Guo, Copolymerization with Polyether Segments Improves the Mechanical Properties of Biodegradable Polyesters, ACS Omega, 2017, 2, 2639–2648, DOI:10.1021/acsomega.7b00517.
- M. Safari, L. L. Boigues, G. Shi, J. Maiz, G. Liu, D. Wang, C. Mijangos and A. J. Müller, Effect of Nanoconfinement on the Isodimorphic Crystallization of Poly(butylene succinate-ran-caprolactone) Random Copolymers, Macromolecules, 2020, 53, 6486–6497, DOI:10.1021/acs.macromol.0c01081.
- (a) Y. Tachibana, T. Masuda, M. Funabashi, K. Kasuya and M. Kunioka, Synthesis of biomass-based monomers from biomass-based furfural for polyesters and evaluation of their biomass carbon ratios, in Biobased Monomers, Polymers and Materials, ed. P. Smith and R. Gross, ACS Symp. Ser., Press 1105, American Chemical Society, 2012, pp. 91–110 Search PubMed; (b) Y. Tachibana, T. Masuda, M. Funabashi and M. Kunioka, Chemical Synthesis of Fully Biomass-Based Poly(butylene succinate) from Inedible-Biomass-Based Furfural and Evaluation of Its Biomass Carbon Ratio, Biomacromolecules, 2010, 11, 2760–2765, DOI:10.1021/bm100820y.
- J.-X. Qin, M. Zhang, C. Zhang, C.-T. Li, Y. Zhang, J. Song, H. M. Asif Javed and J. Qiu, New insight into the difference of PC lipase-catalyzed degradation on poly(butylenes succinate)-based copolymers from molecular levels, RSC Adv., 2016, 6, 17896–17905, 10.1039/C5RA13738A.
- B. Tan, S. Bi, K. Emery and M. J. Sobkowicz, Bio-based poly(butylene succinate-co-hexamethylene succinate) copolyesters with tunable thermal and mechanical properties, Eur. Polym. J., 2017, 86, 162–172, DOI:10.1016/j.eurpolymj.2016.11.017.
- A. Fradet and E. Marechal, Models for Polyesterification Kinetics. III. Preliminary Studies on the Catalysis by Titanium(IV) Tetraalkoxides, J. Macromol. Sci., Part A: Pure Appl.Chem., 1982, 17, 881–891, DOI:10.1080/0022233820806327.
- S.-L. Li, F. Wu, Y. Yang, Y.-Z. Wang and J.-B. Zeng, Synthesis, characterization and isothermal crystallization behavior of poly(butylene succinate)-b-poly(diethylene glycol succinate) multiblock copolymers, Polym. Adv. Technol., 2015, 26, 1003–1013, DOI:10.1002/pat.3519.
- S. Wu, Y. Zhang, J. Han, Z. Xie, J. Xu and B. Guo, Copolymerization with Polyether Segments Improves the Mechanical Properties of Biodegradable Polyesters, ACS Omega, 2017, 2, 2639–2648, DOI:10.1021/acsomega.7b00517.
- (a) M. Sokołowska, E. Stachowska, M. Czaplicka and M. El Fray, Effect of enzymatic versus titanium dioxide/silicon dioxide catalyst on crystal structure of ‘green’ poly[(butylene succinate)-co-(dilinoleic succinate)] copolymers, Polym. Int., 2021, 70, 514–526, DOI:10.1002/pi.6104; (b) M. Sokołowska, J. Nowak-Grzebyta, E. Stachowska and M. El Fray, Enzymatic Catalysis in Favor of Blocky Structure and Higher Crystallinity of Poly(Butylene Succinate)-co-(Dilinoleic Succinate) (PBS-DLS) Copolymers of Variable Segmental Composition, Materials, 2022, 15, 1132 CrossRef PubMed.
- S. Kahkesh and M. Rafizadeh, Flame retardancy and thermal properties of poly(butylene succinate)/nano–boehmite composites prepared via in situ polymerization, Polym. Eng. Sci., 2020, 60, 2262–2271, DOI:10.1002/pen.25468.
- (a) S. N. Sheikholeslami, M. Rafizadeh, F. A. Taromi, H. Shirali and E. Jabbari, Material properties of degradable Poly(butylene succinate-co-fumarate) copolymer networks synthesized by polycondensation of pre-homopolyesters, Polymer, 2016, 98, 70–79, DOI:10.1016/j.polymer.2016.06.012; (b) G. Z. Papageorgiou and D. N. Bikiaris, Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study, Polymer, 2005, 46, 12081–12092, DOI:10.1016/j.polymer.2005.10.073.
- G. Z. Papageorgiou and D. N. Bikiaris, Synthesis, Cocrystallization, and Enzymatic Degradation of Novel Poly(butylene-co-propylene succinate) Copolymers, Biomacromolecules, 2007, 8, 2437–2449, DOI:10.1021/bm0703113.
- X. Dai and Z. Qiu, Synthesis and properties of novel biodegradable poly(butylene succinate-co-decamethylene succinate) copolyesters from renewable resources, Polym. Degrad. Stab., 2016, 134, 305–310, DOI:10.1016/j.polymdegradstab.2016.11.004.
- X.-M. Zhou, Synthesis and characterization of polyester copolymers based on poly (butylene succinate) and poly(ethylene glycol), Mater. Sci. Eng., C, 2021, 32, 2459–2463, DOI:10.1016/j.msec.2012.07.025.
- T.-X. Jin, M. Zhou, S.-D. Hu, F. Chen, Q. Fu and Y. Fu, Effect of molecular weight on the properties of poly(butylene succinate), Chin. J. Polym. Sci., 2014, 32, 953–960, DOI:10.1007/s10118-014-1463-4.
- (a) M. Ishii, M. Okazaki, Y. Shibasaki, M. Ueda and T. Teranishi, Convenient Synthesis of Aliphatic Polyesters by Distannoxane-Catalyzed Polycondensation, Biomacromolecules, 2001, 2, 1267–1270, DOI:10.1021/bm015576a; (b) H. Takahashi, T. Hayakawa and M. Ueda, Convenient Synthesis of Poly(butylene succinate) Catalyzed by Distannoxane, Chem. Lett., 2000, 29, 684–685, DOI:10.1246/cl.2000.684.
- S. Velmathi, R. Nagahata, J.-I. Sugiyama and K. Takeuchi, A Rapid Eco-Friendly Synthesis of Poly(butylene succinate) by a Direct Polyesterification under Microwave Irradiation, Macromol. Rapid Commun., 2005, 26, 1163–1167, DOI:10.1002/marc.200500176.
- M. Bautista, A. Martínez de Ilarduya, A. Alla and S. Muñoz-Guerra, Poly(butylene succinate) Ionomers with Enhanced Hydrodegradability, Polymers, 2015, 7, 1232–1247, DOI:10.3390/polym7071232.
- (a) C. Lavilla, A. Alla, A. Martínez de Ilarduya and S. Muñoz-Guerra, High Tg Bio-Based Aliphatic Polyesters from Bicyclic d-Mannitol, Biomacromolecules, 2013, 14, 781–793, DOI:10.1021/bm301854c.
- W.-J. Xie and X.-M. Zhou, Non-isothermal crystallization kinetics and characterization of biodegradable poly(butylene succinate-co-neopentyl glycol succinate) copolyesters, Mater. Sci. Eng., C, 2014, 46, 366–373, DOI:10.1016/j.msec.2014.10.063.
- L. Poussard, A. Mecheri, J. Mariage, I. Barakat, L. Bonnaud, J.-M. Raquez and P. Dubois, Synthesis of Oligo(butylene succinate)-based Polyurethanes, J. Renewable Mater., 2014, 10, 13–22, DOI:10.7569/JRM.2013.634132.
- C. Labruyère, O. Talon, N. Berezina, E. Khousakounb and C. Jérôme, Synthesis of poly(butylene succinate) through oligomerization–cyclization–ROP route, RSC Adv., 2014, 4, 38643–38648, 10.1039/C4RA03373F.
- C. W. Lee, K. Masutani and Y. Kimura, Ring-opening polymerization of a macrocyclic lactone monomer isolated from oligomeric byproducts of poly(butylene succinate) (PBS): An efficient route to high-molecular-weight PBS and block copolymers of PBS, Polymer, 2014, 55, 5673–5679, DOI:10.1016/j.polymer.2014.08.028.
- C.-S. Feng, Y. Chen, J. Shao, G. Li and H.-Q. Hou, The Crystallization and Melting Behaviors of PDLA-b-PBS-b-PDLA Tri-block Copolymers, Chin. J. Polym. Sci., 2020, 38, 298–310, DOI:10.1007/s10118-020-2361-6.
- L. P. Ferreira, A. N. Moreira, J. C. Pinto and F. G. Souza Jr., Polym. Eng. Sci., 2015, 55, 1889–1896, DOI:10.1002/pen.24029.
- N. Jacquel, R. Saint-Loup, J. P. Pascault, A. Rousseau and F. Fenouillot, Bio-based alternatives in the synthesis of aliphatic–aromatic polyesters dedicated to biodegradable film applications, Polymer, 2015, 59, 234–242, DOI:10.1016/j.polymer.2014.12.021.
- (a) A. Takasu, Y. Oishi, Y. Iio, Y. Inai and T. Hirabayashi, Synthesis of Aliphatic Polyesters by Direct Polyesterification of Dicarboxylic Acids with Diols under Mild Conditions Catalyzed by Reusable Rare-Earth Triflate, Macromolecules, 2003, 36, 1772–1774, DOI:10.1021/ma021462v; (b) M. Bautista, A. M. Ilarduya, A. Alla and S. Munoz-Guerra, Poly(butylene succinate) ionomers and their use as compatibilizers in nanocomposites, Polym. Compos., 2016, 37, 2603–2610, DOI:10.1002/pc.23454; (c) A. Takasu, Y. Iio, T. Mimura and T. Hirabayashi, Room-Temperature Polycondensation of Dicarboxylic Acids and Diols Catalyzed by Water-Stable Lewis Acids, Polym. J., 2005, 37, 946–953, DOI:10.1295/polymj.37.946.
- Z. Cui, Z. Guixiang, S. Ying, L. Li-Zhi, R. Minqiao, Z. Wei and H. Ling, Crystallization, structures and properties of biodegradable poly (butylene succinate-co-butylene terephthalate) with a symmetric composition, Mater. Chem. Phys., 2021, 260, 124183, DOI:10.1016/j.matchemphys.2020.124183.
- N. Jacquel, F. Freyermouth, F. Fenouillot, A. Rousseau, J. P. Pascault, P. Fuertes and R. Saint-Loup, Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5301–5312, DOI:10.1002/pola.25009.
- C. A. DeRosa, X. Q. Kua, F. S. Bates and M. A. Hillmyer, Step-Growth Polyesters with Biobased (R)-1,3-Butanediol, Ind. Eng. Chem. Res., 2020, 59, 15598–15613, DOI:10.1021/acs.iecr.0c03009.
- A. Bian, F. Yang, J. Liu and Y. Wang, Copolymerization of P-Hydroxybenzoic Acid and Poly (Butylene Succinate): Effect of Catalysts, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 612(2), 022022, DOI:10.1088/1757-899X/612/2/022022.
- M. Lahcini, H. Qayouh, T. Yashiro, P. Simon and H. R. Kricheldorf, Syntheses of Poly(butylene succinate) by Means of Non-Toxic Catalysts, J. Macromol. Sci., Part A: Pure Appl.Chem., 2010, 47, 503–509, DOI:10.1080/10601321003741875.
- (a) Q. Wang, Y. Zai, D. Yang, L. Qiu and C. Niu, Bio-based elastomer nanoparticles with controllable biodegradability, RSC Adv., 2016, 6, 102142–102148, 10.1039/C6RA24336C; (b) D. K. Song and Y. K. Sung, Synthesis and characterization of biodegradable poly(1,4-butanediol succinate), J. Appl. Polym. Sci., 1995, 56, 1381–1395, DOI:10.1002/app.1995.070561102.
- W. He, J. Lan, X. Huang and J. Shang, Synthesis, characterization, and properties of biodegradable poly(butylene succinate) modified with imide dihydric alcohol, J. Appl. Polym. Sci., 2014, 131, 40807, DOI:10.1002/app.40807.
- X. Huang, R. Guo and J. Lan, Synthesis and characterization of biodegradable hexabasic aliphatic-aromatic copolyester, Polym. Sci., Ser. B, 2014, 56, 744–752, DOI:10.1134/S1560090414060098.
- G.-C. Liu, W.-Q. Zhang, X.-L. Wang and Y.-Z. Wang, Synthesis and performances of poly(butylene-succinate) with enhanced viscosity and crystallization rate via introducing a small amount of diacetylene groups, Chin. Chem. Lett., 2017, 28, 354–357, DOI:10.1016/j.cclet.2016.10.014.
- L. Wu, R. Mincheva, Y. Xu, J.-M. Raquez and P. Dubois, High Molecular Weight Poly(butylene succinate-co-butylene furandicarboxylate) Copolyesters: From Catalyzed Polycondensation Reaction to Thermomechanical Properties, Biomacromolecules, 2012, 13, 2973–2981, DOI:10.1021/bm301044f.
- Y. Zhang, J. Han, S. Wu, Z. Qi, J. Xu and B. Guo, Synthesis, physical properties and photodegradation of functional poly(butylene succinate) covalently linking UV stabilizing moieties in molecular chains, Colloids Surf., A, 2017, 524, 160–168 CrossRef CAS.
- Q. Liu and X.-M. Zhou, Preparation of Poly(butylene succinate)/poly(ε-caprolactone) Blends Compatibilized With Poly(butylene succinate-co-ε-caprolactone) Copolymer, J. Macromol. Sci., Part A: Pure Appl.Chem., 2015, 52, 625–629, DOI:10.1080/10601325.2015.1050634.
- D. Qu, S. Sun, H. Gao, Y. Bai and Y. Tang, Biodegradable copolyester poly(butylene-co-isosorbide succinate) as hot-melt adhesives, RSC Adv., 2019, 9, 11476–11483, 10.1039/C9RA01780A.
- A. Oishi, M. Zhang, K. Nakayama, T. Masuda and Y. Taguchi, Synthesis of Poly(butylene succinate) and Poly(ethylene succinate) Including Diglycollate Moiety, Polym. J., 2006, 38, 710–715, DOI:10.1295/polymj.PJ2005206.
- T. Masuda and A. Cao, Jpn. Kokai Tokkyo Koho, 2002, 356548 Search PubMed.
- (a) J. Qi, J. Wu, J. Chen and H. Wang, An investigation of the thermal and (bio)degradability of PBS copolyesters based on isosorbide, Polym. Degrad. Stab., 2019, 160, 229–241, DOI:10.1016/j.polymdegradstab.2018.12.031; (b) E. Zakharova, A. Alla, A. Martínez De Ilarduya and S. Muñoz-Guerra, Bio-based PBS copolyesters derived from a bicyclic d-glucitol, RSC Adv., 2015, 5, 46395–46404, 10.1039/C5RA03844H.
- H.-M. Ye, R.-D. Wang, J. Liu, J. Xu and B.-H. Guo, Isomorphism in poly(butylene succinate-co-butylene fumarate) and its application as polymeric nucleating agent for poly(butylene succinate), Macromolecules, 2012, 45, 5667–5675, DOI:10.1021/ma300685f.
- T. Wan, T. Du and S. Liao, Biodegradable poly (butylene succinate-co-cyclohexanedimethylene succinate): Synthesis, crystallization, morphology, and rheology, J. Appl. Polym. Sci., 2014, 131, 40103, DOI:10.1002/app.40103.
- X.-M. Zhou and W.-J. Xie, Synthesis and characterization of poly(ester ether urethane)s block copolymers based on biodegradable poly(butylene succinate) and Poly(ethylene glycol), Polym. Degrad. Stab., 2017, 140, 147–155, DOI:10.1016/j.polymdegradstab.2017.04.023.
- S. Lai, Y. Gao and L. Yue, Heterogeneous catalytic synthesis of poly(butylene succinate) by attapulgite-supported Sn catalyst, J. Appl. Polym. Sci., 2015, 132, 41729, DOI:10.1002/app.41729.
- K. Stȩpień, C. Miles, A. McClain, E. Wiśniewska, P. Sobolewski, J. Kohn, J. Puskas, H. D. Wagner and M. El Fray, Biocopolyesters of poly(butylene succinate) containing long-chain biobased glycol synthesized with heterogeneous titanium dioxide catalyst, ACS Sustainable Chem. Eng., 2019, 7, 10623–10632, DOI:10.1021/acssuschemeng.9b01191.
- M. Zarei and M. El Fray, Synthesis of Hydrophilic Poly(butylene succinate-butylene dilinoleate) (PBS-DLS) Copolymers Containing Poly(Ethylene Glycol) (PEG) of Variable Molecular Weights, Polymers, 2021, 13, 3177, DOI:10.3390/polym13183177.
- F. Lesek and R. Khromeček, Some problems of polycondensation in suspensions, J. Polym. Sci., 1961, 52, 251–256, DOI:10.1002/pol.1961.1205215733.
- P.-j. Wang, K. Wang, J.-s. Zhang and G.-s. Luo, Non-aqueous suspension polycondensation in NMP-CaCl2/paraffin system—A new approach for the preparation of poly(p-phenylene terephthalamide), Chin. J. Polym. Sci., 2015, 33, 564–575, DOI:10.1007/s10118-015-1607-1.
- T. Brock and D. C. Sherrington, Preparation of spherical polybenzimidazole particulates using a non-aqueous suspension methodology, Polymer, 1992, 33, 1773–1777, DOI:10.1016/0032-3861(92)91082-D.
- (a) J.-H. Ahn, J.-C. Kim, S.-K. Ihm, C.-G. Oh and D. C. Sherrington, Epoxidation of Olefins by Molybdenum(VI) Catalysts Supported on Functional Polyimide Particulates, Ind. Eng. Chem. Res., 2005, 44, 8560–8564, DOI:10.1021/ie040287z; (b) J. H. Ahn and D. C. Sherrington, Synthesis of functional polyimide beads and use as MoVI epoxidation catalyst supports, Chem. Commun., 1996, 643–644, 10.1039/CC9960000643; (c) T. Brock, D. C. Sherrington and J. Swindell, Synthesis and characterisation of porous particulate polyimides, J. Mater. Chem., 1994, 4, 229–236, 10.1039/JM9940400229.
- (a) L. Dutra, M. Nele de Souza and J. C. Pinto, Preparation of Polymer Microparticles through Nonaqueous Suspension Polycondensations. Part II—Effects of Operating Variables on Properties of Poly(butylene succinate), Macromol. React. Eng., 2018, 12, 1800039, DOI:10.1002/mren.201800039; (b) L. da Silva Dutra, M. N. de Souza and J. C. Pinto, Preparation of Polymer Microparticles Through Non-aqueous Suspension Polycondensations: Part IV—Effect of the Continuous Phase on the Characteristics of Final Poly(Butylene Succinate) Particles, J. Polym. Environ., 2021, 29, 219–229, DOI:10.1007/s10924-020-01869-7; (c) L. Dutra, B. F. Oechsler, A. L. T. Brandão, R. C. Lima, M. Nele and J. C. Pinto, Preparation of Polymer Microparticles through Nonaqueous Suspension Polycondensations: Part V—Modeling and Parameter Estimation for Poly(butylene succinate) Polycondensations, Macromol. React. Eng., 2020, 14, 2000007, DOI:10.1002/mren.202000007.
- (a) F. Jbilou, I.-N. Georgousopoulou, S. Marinkovic, S. Vouyiouka, C. D. Papaspyrides, B. Estrine, P. Dole, A. Cottaz and C. Joly, Intelligent monitoring of solid state polymerization via molecular rotors: The case of poly(butylene succinate), Eur. Polym. J., 2016, 78, 61–71, DOI:10.1016/j.eurpolymj.2016.03.005; (b) S. N. Vouyiouka, E. K. Karakatsani and C. D. Papaspyrides, Solid state polymerization, Prog. Polym. Sci., 2005, 30, 10–37, DOI:10.1016/j.progpolymsci.2004.11.001; (c) C. D. Papaspyrides, S. Vouyiouka, I.-N. Georgousopoulou, S. Marinkovic, B. Estrine, C. Joly and P. Dole, Feasibility of Solid-State Postpolymerization on Fossil- and Bio-Based Poly(butylene succinate) Including Polymer Upcycling Routes, Ind. Eng. Chem. Res., 2016, 55, 5832–5842, DOI:10.1021/acs.iecr.6b00588.
- (a) S. Velmathi, R. Nagahata and K. Takeuchi, Extremely Rapid Synthesis of Aliphatic Polyesters by Direct Polycondensation of 1:1 Mixtures of Dicarboxylic Acids and Diols Using Microwaves, Polym. J., 2007, 39, 841–844, DOI:10.1295/polymj.PJ2006248; (b) Z. Zeng, S. Wang and S. Yang, Synthesis and Characterization of PbS Nanocrystallites in Random Copolymer Ionomers, Chem. Mater., 1999, 11, 3365–3369, DOI:10.1021/cm990425o.
- R. Nagahata, T. Nakamura and K. Takeuchi, Microwave-assisted rapid synthesis of poly(butylene succinate): principal effect of microwave irradiation of accelerating the polycondensation reaction, Polym. J., 2018, 50, 347–354, DOI:10.1038/s41428-018-0024-z.
- S. A. Pasma, R. Daik and M. Y. Maskat, Enzymatic Synthesis of Biodegradable Polyesters using Succinic Acid Monomer Derived from Cellulose of Oil Palm Empty Fruit Bunch, J. Wood Chem. Technol., 2018, 38, 445–459, DOI:10.1080/02773813.2018.1533976.
- M. Sokołowska and M. E. Fray, Green Poly(Butylene Succinate-co-Dilinoleic Succinate) Copolymers Synthesized Using Candida antarctica Lipase B (CAL-B) as Biocatalyst, Proceedings, 2021, 69, 33, DOI:10.3390/CGPM2020-07221.
- A. Sonseca, A. McClain, J. E. Puskas and M. El Fray, Kinetic studies of biocatalyzed copolyesters of poly(butylene succinate)(PBS)containing fully bio-based dilinoleic diol, Eur. Polym. J., 2019, 116, 515–525, DOI:10.1016/j.eurpolymj.2019.04.038.
- A. K. Chaudhary, J. Lopez, E. J. Beckman and A. J. Russell, Biocatalytic Solvent-Free Polymerization To Produce High Molecular Weight Polyesters, Biotechnol. Prog., 1997, 13, 318–325, DOI:10.1021/bp970024i.
- S. Sugihara, K. Toshima and S. Matsumura, New Strategy for Enzymatic Synthesis of High-Molecular-Weight Poly(butylene succinate) via Cyclic Oligomers, Macromol. Rapid Commun., 2006, 27, 203–207, DOI:10.1002/marc.200500723.
- L. Ren, Y. Wang, J. Ge, D. Lu and Z. Liu, Enzymatic Synthesis of High-Molecular-Weight Poly(butylene succinate) and its Copolymers, Macromol. Chem. Phys., 2015, 216, 636–640, DOI:10.1002/macp.201400550.
- (a) A. Mahapatro and B. Kalra, B, A. Kumar, R. A. Gross, Lipase-Catalyzed Polycondensations: Effect of Substrates and Solvent on Chain Formation, Dispersity, and End-Group Structure, Biomacromolecules, 2003, 4, 544–551, DOI:10.1021/bm0257208; (b) A. Mahapatro, A. Kumar, B. Kalra and R. A. Gross, Solvent-Free Adipic Acid/1,8-Octanediol Condensation Polymerizations Catalyzed by Candida antartica Lipase B, Macromolecules, 2004, 37, 35–40, DOI:10.1021/ma025796w; (c) Y. Mei, L. Miller, W. Gao and R. A. Gross, Imaging the Distribution and Secondary Structure of Immobilized Enzymes Using Infrared Microspectroscopy, Biomacromolecules, 2003, 4, 70–74, DOI:10.1021/bm025611t; (d) H. Azim, A. Dekhterman, Z. Jiang and R. A. Gross, Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): shorter chain building blocks also work, Biomacromolecules, 2006, 7, 3093–3097, DOI:10.1021/bm060574h.
- C. Ciulik, M. Safari, A. Martínez de Ilarduya, J. C. Morales-Huerta, A. Iturrospe, A. Arbe, A. J. Müller and S. Muñoz-Guerra, Poly(butylene succinate-ran-ε-caprolactone) copolyesters: Enzymatic synthesis and crystalline isodimorphic character, Eur. Polym. J., 2017, 95, 795–808, DOI:10.1016/j.eurpolymj.2017.05.002.
- T. Debuissy, E. Pollet and L. Averous, Enzymatic synthesis of biobased poly(1,4-butylene succinate-ran-2,3-butylene succinate) copolyesters and characterization. Influence of 1,4- and 2,3-butanediol contents, Eur. Polym. J., 2017, 93, 103–115, DOI:10.1016/j.eurpolymj.2017.04.045.
- W.-C. Nie, Q. Xiao, J.-M. Wu, F. Song, X.-L. Wang and Y.-Z. Wang, Dendritic crystallization and morphology control of random poly(p-dioxanone-co-butylene-co-succinate) copolyesters, Eur. Polym. J., 2018, 108, 76–84, DOI:10.1016/j.eurpolymj.2018.08.027.
- Y. Jiang, A. J. J. Woortman, G. O. R. Van Ekenstein and K. Loos, Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources, Biomolecules, 2013, 3, 461–480, DOI:10.3390/biom3030461.
- J. C. Morales-Huerta, C. B. Ciulik, A. Martínez de Ilarduya and S. Muñoz-Guerra, Fully bio-based aromatic–aliphatic copolyesters: poly(butylene furandicarboxylate-co-succinate)s obtained by ring opening polymerization, Polym. Chem., 2017, 8, 748–760, 10.1039/C6PY01879C.
- (a) F. Luzi, L. Torre, J. M. Kenny and D. Puglia, Bio- and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure–Property Relationship, Materials, 2019, 12(3), 471, DOI:10.3390/ma12030471; (b) S. Nanda, B. R. Patra, R. Patel, J. Bakos and A. K. Dalai, Innovations in applications and prospects of bioplastics and biopolymers: a review, Environ. Chem. Lett., 2022, 20, 379–395, DOI:10.1007/s10311-021-0133.
- S. Y. Hwang, E. S. Yoo and S. S. Im, The synthesis of copolymers, blends and composites based on poly(butylene succinate, Polym. J., 2021, 44, 1179–1190, DOI:10.1038/pj.2012.157.
- G. Z. Papageorgiou and D. N. Bikiaris, Crystallization and melting behavior of three biodegradable poly(alkylene succinates). A comparative study, Polymer, 2005, 46, 12081–12092, DOI:10.1016/j.polymer.2005.10.073.
- Y.-R. Tang, Y. Zhang, Y. Liu, B.-H. Guo and J. Xu, Tuning the Properties of Biodegradable Poly(Butylene Succinate) Via Random and Block Copolymerization, J. Compos. Biodegrad. Polym., 2020, 8, 45–60, DOI:10.12974/2311-8717.2020.08.7.
- H.-B. Chen, X.-L. Wang, J.-B. Zeng, L.-L. Li, F.-X. Dong and Y.-Z. Wang, A Novel Multiblock Poly(ester urethane) Based on Poly(butylenesuccinate) and Poly(ethylene succinate-co-ethylene terephthalate), Ind. Eng. Chem. Res., 2011, 50, 2065–2072, DOI:10.1021/ie101798n.
- W.-D. Li, J.-B. Zeng, X.-J. Lou, J.-J. Zhang and Y.-Z. Wang, Aromatic-aliphatic random and block copolyesters: synthesis, sequence distribution and thermal properties, Polym. Chem., 2012, 3, 1344–1353, 10.1039/C2PY20068F.
- L. Zheng, C. Li, Z. Wang, J. Wang, Y. Xiao, D. Zhang and G. Guan, Novel Biodegradable and Double Crystalline Multiblock Copolymers Comprising of Poly(butylene succinate) and Poly(ε-caprolactone): Synthesis, Characterization, and Properties, Ind. Eng. Chem. Res., 2012, 51, 7264–7272, DOI:10.1021/ie300576z.
- S. Wu, L. Zheng, C. Li, S. Huo, Y. Xiao, G. Guan and W. Zhu, A facile and versatile strategy to efficiently synthesize sulfonated poly(butylene succinate), self-assembly behavior and biocompatibility, Polym. Chem., 2015, 6, 1495–1501, 10.1039/C4PY01305K.
- Y. Zhang, T. Li, Z. Xie, J. Han, J. Xu and B. Guo, Synthesis and Properties of Biobased Multiblock Polyesters Containing Poly(2,5-furandimethylene succinate) and Poly(butylene succinate) Blocks, Ind. Eng. Chem. Res., 2017, 56, 3937–3946, DOI:10.1021/acs.iecr.7b00516.
- L. Genovese, M. Soccio, M. Gigli, N. Lotti, M. Gazzano, V. Siracusa and A. Munari, Gas permeability, mechanical behaviour and compostability of fully-aliphatic bio-based multiblock poly(ester urethane)s, RSC Adv., 2016, 6, 55331–55342, 10.1039/C6RA08882A.
- J.-B. Zeng, Q.-Y. Zhu, X. Lu, Y.-S. He and Y.-Z. Wang, From miscible to partially miscible biodegradable double crystalline poly(ethylene succinate)-b-poly(butylene succinate) multiblock copolymers, Polym. Chem., 2012, 3, 399–408, 10.1039/C1PY00456E.
- G. Wang and Z. Qiu, Synthesis, Crystallization Kinetics, and Morphology of Novel Biodegradable Poly(butylene succinate-co-hexamethylene succinate) Copolyesters, Ind. Eng. Chem. Res., 2012, 51, 16369–16376, DOI:10.1021/ie302817k.
- J. Jing, L. Song, T. Su and Z. Wang, Effects of monomer composition on physical properties and enzymatic hydrolyzability of poly(butylene succinate-co-hexamethylene succinate)s, Polym. Eng. Sci., 2021, 61, 379–387, DOI:10.1002/pen.25581.
- K. Zhang, H. Yang and Z. Qiu, Thermal Properties and Crystallization Behavior of Novel Biodegradable Poly(hexamethylene succinate-co-6 mol% butylene succinate) and Poly(hexamethylene succinate), J. Polym. Environ., 2018, 26, 1320–1327, DOI:10.1007/s10924-017-1045-y.
- T. Debuissy, P. Sangwan, E. Pollet and L. Avérous, Study on the structure-properties relationship of biodegradable and biobased aliphatic copolyesters based on 1,3-propanediol, 1,4-butanediol, succinic and adipic acids’, Polymer, 2017, 122, 105–116, DOI:10.1016/j.polymer.2017.06.045.
- H.-I. Mao, L.-Y. Wang, C.-W. Chen, K.-H. Hsu, C.-H. Tsai, C.-J. Cho, Y.-Y. Yu, S.-P. Rwei and C.-C. Kuo, Enhanced crystallization rate of bio-based poly(butylene succinate-co-propylene succinate) copolymers motivated by glycerol, J. Polym. Res., 2021, 28, 92, DOI:10.1007/s10965-021-02460-x.
- J. Han, J. Shi, Z. Xie, J. Xu and B. Guo, Synthesis, Properties of Biodegradable Poly(Butylene Succinate-co-Butylene 2-Methylsuccinate) and Application for Sustainable Release, Materials, 2019, 12, 1507, DOI:10.3390/ma12091507.
- R. A. Perez-Camargo, G. Liu, D. Cavallo, D. Wang and A. J. Muller, Effect of the Crystallization Conditions on the Exclusion/Inclusion Balance in Biodegradable Poly(butylene succinate-ran-butylene adipate) Copolymers, Biomacromolecules, 2020, 21, 3420–3435, DOI:10.1021/acs.biomac.0c00847.
- R. Mincheva, A. Delangre, J.-M. Raquez, R. Narayan and P. Dubois, Biobased Polyesters with Composition-Dependent Thermomechanical Properties: Synthesis and Characterization of Poly(butylene succinate-co-butylene azelate), Biomacromolecules, 2013, 14, 890–899, DOI:10.1021/bm301965h.
- I. Arandia, A. Mugica, M. Zubitur, A. Iturrospe, A. Arbe, G. Liu, D. Wang, R. Mincheva, P. Dubois and A. J. Müller, Application of SSA thermal fractionation and X-ray diffraction to elucidate comonomer inclusion or exclusion from the crystalline phases in poly(butylene succinate-ran-butylene azelate) random copolymers, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 2346–2358, DOI:10.1002/polb.24146.
- I. Arandia, N. Zaldua, J. Maiz, R. A. Pérez-Camargo, A. Mugica, M. Zubitur, R. Mincheva, P. Dubois and A. J. Müller, Tailoring the isothermal crystallization kinetics of isodimorphic poly (butylene succinate-ran-butylene azelate) random copolymers by changing composition, Polymer, 2019, 183, 121863, DOI:10.1016/j.polymer.2019.121863.
- Y. Tsai, L.-C. Jheng and C.-Y. Hung, Synthesis, properties and enzymatic hydrolysis of biodegradable alicyclic/aliphatic copolyesters based on 1,3/1,4-cyclohexanedimethanol, Polym. Degrad. Stab., 2010, 95, 72–78, DOI:10.1016/j.polymdegradstab.2009.10.006.
- M. Safari, A. Martínez de Ilarduya, A. Mugica, M. Zubitur, S. Muñoz-Guerra and A. J. Müller, Tuning the Thermal Properties and Morphology of Isodimorphic Poly[(butylene succinate)-ran-(ε-caprolactone)] Copolyesters by Changing Composition, Molecular Weight, and Thermal History, Macromolecules, 2018, 51, 9589–9601, DOI:10.1021/acs.macromol.8b01742.
- Y. Zheng, X. Liu, F. Liu, J. Xue, J. Zhou, H. Huo and L. Li, Crystallization of isomorphic poly(butylene succinate-co butylene fumarate) biopolyester: Single crystals and ring-banded spherulites, Polym. Test., 2018, 68, 379–387, DOI:10.1016/j.polymertesting.2018.04.035.
- (a) R. T. Duan, Q. X. He, X. Dong, D. F. Li, X. L. Wang and Y. Z. Wang, Renewable Sugar-Based Diols with Different Rigid Structure: Comparable Investigation on Improving Poly(butylene succinate) Performance, ACS Sustainable Chem. Eng., 2016, 4, 350–362, DOI:10.1021/acssuschemeng.5b01335.
- C. W. Lee, M. Akashi, Y. Kimura and M. Kazunari, Synthesis and enzymatic degradability of an aliphatic/aromatic block copolyester: poly(butylene succinate)-multi-poly(butylene terephthalate), Macromol. Res., 2017, 25, 54–62, DOI:10.1007/s13233-017-5011-2.
- Y. J. Kim, G. D. Kang, K. C. Yoon and O. O. Park, Comparison of Mechanical Properties of Blended and Synthesized Biodegradable Polyesters, Macromol. Res., 2014, 22, 382–387, DOI:10.1007/s13233-014-2059-0.
- C.-L. Huang, L. Jiao, J.-J. Zhang, J.-B. Zeng, K.-K. Yang and Y.-Z. Wang, Poly(butylene succinate)-poly(ethylene glycol) multiblock copolymer: Synthesis, structure, properties and shape memory performance, Polym. Chem., 2012, 3, 800–808, 10.1039/C2PY00603K.
- A. Oishi, H. Iida and Y. Taguchi, Preparation of poly(butylene succinate) copolymers including L-aspartic acid, Polym. J., 2010, 42, 411–415, DOI:10.1038/pj.2010.16.
- G. Wang, B. Guo and R. Li, Synthesis, characterization, and properties of long-chain branched poly(butylene succinate), J. Appl. Polym. Sci., 2012, 124, 1271–1280, DOI:10.1002/app.34034.
- A. Sonseca and M. E. Fray, Enzymatic synthesis of an electrospinnable poly(butylene succinate-co-dilinoleic succinate) thermoplastic elastomer, RSC Adv., 2107, 7, 21258–21267, 10.1039/C7RA02509B.
- C. Pan, J. Lu, B. Wu, L. Wu and B.-G. Li, Effect of Monomer Structure on Crystallization and Glass Transition of Flexible Copolyesters, J. Polym. Environ., 2017, 25, 1051–1061, DOI:10.1007/s10924-016-0881-5.
- H. Sun, Y. Luo, B. Yang, H. Zhang and J. Huang, Non-isothermal crystallization of biopolyesters of poly(butylene succinate) formed via in situ polymerization in presence of poly(vinyl butyral), Polymer, 2018, 153, 378–390, DOI:10.1016/j.polymer.2018.08.035.
- A. Wcisłek, A. Sonseca Olalla, A. McClain, A. Piegat, P. Sobolewski, J. Puskas and M. El Fray, Enzymatic Degradation of Poly(butylene succinate) Copolyesters Synthesized with the Use of Candida antarctica Lipase B, Polymers, 2018, 10, 688, DOI:10.3390/polym10060688.
- S.-F. Lu, M. Chen and C. H. Chen, J. Appl. Polym. Sci., 2012, 123, 3610–3619, DOI:10.1002/app.34999.
- (a) C. W. Lee, Y. Kimura and J. D. Chung, Mechanism of Enzymatic Degradation of Poly(butylene succinate), Macromol. Res., 2008, 16, 651–658, DOI:10.1007/BF03218575; (b) Y. Tokiwa, B. P. Calabia, C. U. Ugwu and S. Aiba, Biodegradability of Plastics, Int. J. Mol. Sci., 2009, 10, 3722–3742, DOI:10.3390/ijms10093722; (c) B. Laycock, M. Nikolić, J. M. Colwell, E. Gauthier, P. Halley, S. Bottle and G. George, Lifetime prediction of biodegradable polymers, Prog. Polym. Sci., 2017, 71, 144–189, DOI:10.1016/j.progpolymsci.2017.02.004; (d) K. Shi, T. Su and Z. Wang, Comparison of poly(butylene succinate) biodegradation by Fusarium solani cutinase and Candida Antarctica lipase, Polym. Degrad. Stab., 2019, 164, 55–60, DOI:10.1016/j.polymdegradstab.2019.04.005.
- S. Bi, B. Tan, J. L. Soule and M. J. Sobkowicz, Enzymatic degradation of poly (butylene succinate-co-hexamethylene succinate), Polym. Degrad. Stab., 2018, 155, 9–14, DOI:10.1016/j.polymdegradstab.2018.06.017.
- M. Ding, M. Zhang, J. Yang and J-H. Qiu, Study on the enzymatic degradation of aliphatic polyester–PBS and its copolymers, J. Appl. Polym. Sci., 2012, 124, 2902–2907, DOI:10.1002/app.35347.
- (a) C. T. Li, M. Zhang, Y. X. Weng and J. X. Qin, Influence of ether linkage on the enzymatic degradation of PBS copolymers: Comparative study on poly (butylene succinate-co-diethylene glycol succinate) and poly (butylene succinate-co-butylene diglycolic acid), Int. J. Biol. Macromol., 2018, 118, 347–356, DOI:10.1016/j.ijbiomac.2018.06.062; (b) J.-B. Zeng, C.-L. Huang, L. Jiao, X. Lu, Y.-Z. Wang and X.-L. Wang, Synthesis and Properties of Biodegradable Poly(butylene succinateco-diethylene glycol succinate) Copolymers, Ind. Eng. Chem. Res., 2012, 51, 12258–12265, DOI:10.1021/ie300133a.
- Z. Bai, Y. Liu, T. Su and Z. Wang, Effect of Hydroxyl Monomers on the Enzymatic Degradation of Poly(ethylene succinate), Poly(butylene succinate), and Poly(hexylene succinate), Polymers, 2018, 10, 90, DOI:10.3390/polym10010090.
- C.-T. Li, M. Zhang, J.-X. Qin, Y. Zhang and J.-H. Qiu, Study on molecular modeling and the difference of PC lipase-catalyzed degradation of poly (butylene succinate) copolymers modified by linear monomers, Polym. Degrad. Stab., 2015, 116, 75–80, DOI:10.1016/j.polymdegradstab.2015.03.017.
- Q. Liu and X.-M. Zhou, Syntheses and Physical Characterization of Biodegradable Poly(butylene succinate-co-butylene itaconate) Copolymers, J. Macromol. Sci., Part A: Pure Appl.Chem., 2015, 52, 745–751, DOI:10.1080/10601325.2015.1063875.
- L. Zheng, Z. Wang, S. Wu, C. Li, D. Zhang and Y. Xiao, Novel Poly(butylene fumarate) and Poly(butylene succinate) Multiblock Copolymers Bearing Reactive Carbon–Carbon Double Bonds: Synthesis, Characterization, Cocrystallization, and Properties, Ind. Eng. Chem. Res., 2013, 52, 6147–6155, DOI:10.1021/ie303573d.
- (a) K. Thirunavukarasu, S. Purushothaman, J. Sridevi, M. Aarthy, M. K. Gowthaman, T. Nakajima-Kambe and N. R. Kamini, Degradation of poly(butylene succinate) and poly(butylene succinate-co-butylene adipate) by a lipase from yeast Cryptococcus sp. grown on agro-industrial residues, Int. Biodeterior. Biodegrad., 2016, 110, 99–107, DOI:10.1016/j.ibiod.2016.03.005; (b) N. Hemashenpagam, L. Growther, N. Murgalatha, S. V. Raj and S. S. Vimal, Effect of Lipase and esterase in Biodegrading Poly(butylene succinate co adipate) (PBSA), Indian J. Appl. Res., 2011, 3, 551–554, DOI:10.15373/2249555X/JULY2013/172.
- M. Zhang, X.-N. Ma, C.-T. Li, D. Zhao, Y.-I. Xing and J.-H. Qiu, A correlation between the degradability of poly(butylene succinate)-based copolyesters and catalytic behavior with Candida antarctica lipase B, RSC Adv., 2017, 7, 43052–43063, 10.1039/C7RA05553F.
- M. S. Nikolic and J. Djonlagic, Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene adipate)s, Polym. Degrad. Stab., 2001, 74, 263–270, DOI:10.1016/S0141-3910(01)00156-2.
- S. Quattrosoldi, M. Soccio, M. Gazzano, N. Lotti and A. Munari, Fully biobased, elastomeric and compostable random copolyesters of poly(butylene succinate) containing Pripol 1009 moieties: Structure-property relationship, Polym. Degrad. Stab., 2020, 178, 109189 CrossRef CAS.
- Y.-D. Li, J.-B. Zeng, W.-D. Li, K. K. Yang, X.-L. Wang and Y.-Z. Wang, Rheology, Crystallization, and Biodegradability of Blends Based on Soy Protein and Chemically Modified Poly(butylene succinate), Ind. Eng. Chem. Res., 2009, 48, 4817–4825, DOI:10.1021/ie801718f.
| This journal is © The Royal Society of Chemistry 2022 |