Caroline T. Saouma, Chih-Chin Tsou, Sarah Richard, Rob Ameloot, Frederik Vermoortele, Simon Smolders, Bart Bueken, Antonio G. DiPasquale, Werner Kaminsky, Carolyn N. Valdez, Dirk E. De Vos and James M. Mayer
Chem. Sci., 2019,10, 1322-1331
DOI:
10.1039/C8SC04138E,
Edge Article


 Open Access
Open Access 
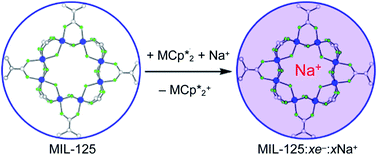
 (M = Cr, Co) are described. The two MOFs contain clusters with Ti8O8 rings: Ti8O8(OH)4(bdc)6; bdc = terephthalate (MIL-125) and Ti8O8(OH)4(bdc-NH2)6; bdc-NH2 = 2-aminoterephthalate (NH2-MIL-125). The stoichiometry of the redox reactions was probed using solution NMR methods. The extent of reduction is greatly enhanced by the presence of Na+, which is incorporated into the bulk of the material. The roughly 1 : 1 stoichiometry of electrons and cations indicates that the storage of e− in the MOF is tightly coupled to a cation within the architecture, for charge balance.
(M = Cr, Co) are described. The two MOFs contain clusters with Ti8O8 rings: Ti8O8(OH)4(bdc)6; bdc = terephthalate (MIL-125) and Ti8O8(OH)4(bdc-NH2)6; bdc-NH2 = 2-aminoterephthalate (NH2-MIL-125). The stoichiometry of the redox reactions was probed using solution NMR methods. The extent of reduction is greatly enhanced by the presence of Na+, which is incorporated into the bulk of the material. The roughly 1 : 1 stoichiometry of electrons and cations indicates that the storage of e− in the MOF is tightly coupled to a cation within the architecture, for charge balance.