Biological applications of lipoic acid-based polymers: an old material with new promise
Qing
Yu
ab,
Zhiyue
Fang
ab,
Shifang
Luan
 ab,
Lei
Wang
ab,
Lei
Wang
 *a and
Hengchong
Shi
*a and
Hengchong
Shi
 *ab
*ab
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: leiwang@ciac.ac.cn; shihc@ciac.ac.cn
bSchool of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China
First published on 16th April 2024
Abstract
Lipoic acid (LA) is a versatile antioxidant that has been used in the treatment of various oxidation–reduction diseases over the past 70 years. Owing to its large five-membered ring tension, the dynamic disulfide bond of LA is highly active, enabling the formation of poly(lipoic acid) (PLA) via ring-opening polymerization (ROP). Herein, we first summarize disulfide-mediated ROP polymerization strategies, providing basic routes for designing and preparing PLA-based materials. PLA, as a biologically derived, low toxic, and easily modified material, possesses dynamic disulfide bonds and universal non-covalent carboxyl groups. We also shed light on the biomedical applications of PLA-based materials based on their biological and structural features and further divide recent works into six categories: antibacterial, anti-inflammation, anticancer, adhesive, flexible electronics, and 3D-printed tissue scaffolds. Finally, the challenges and future prospects associated with the biomedical applications of PLA are discussed.
Introduction
Lipoic acid (LA), a naturally occurring fatty acid with a five-membered disulfide ring, exhibits both amphiphilicity and antioxidant properties. As a crucial enzyme cofactor in the human body, it is involved in the coordination of mitochondrial energy metabolism, body detoxification, resistance to skin inflammation, and maintaining blood sugar levels.1,2 Ever since it was first isolated from hepatocytes in 1951 by Reed et al.,3 LA with various biological activities has been widely used in the treatment of many oxidation–reduction (REDOX) diseases, such as diabetes,4,5 atherosclerosis,6 and cancer therapy.7–9 Owing to its unique pharmacological characteristics and biomass sources, the clinical applications and disease treatment mechanisms of LA have been extensively studied for the effective treatment of diabetic peripheral neuropathy (DPN),10 and its global market demand exceeds 3000 tons annually.LA is easily activated owing to its high five-membered ring tension and low dynamic disulfide bond energy. A high concentration of LA molecules can undergo dynamic covalent ring-opening polymerization (ROP) triggered by photo/thermal effects. Free radical polymerization initiated by heat is one of the conventional methods to obtain PLA. In the solvent-free molten state, LA can yield cyclic polymers.11 In recent years, there have been significant research studies on achieving the controlled polymerization of LA and its derivatives for the precise structural control of PLA. Among these, the most popular method is to add inert organic bases and mercaptan. It is possible to achieve topological control of PLA through the use of protonic solvents such as thiols, which depend on the nucleophilicity of thiols.12 PLA can also introduce non-covalent interactions, such as hydrogen bonds, metal-carboxy coordination, and van der Waals forces, to construct highly ordered self-assembled supramolecular network structures with side chains of carboxyl groups.13–15 Additionally, the hydrophilicity and hydrophobicity of PLA can be regulated by modification of the side-chain carboxyl group with chemical reactions such as esterification, amidation, and deprotonation. Dynamic supramolecular polymers mediated by disulfide bonds and side-chain engineering via non-covalent crosslinking offer broad range of application prospects for PLA, including sustainable plastics,16 self-healing hydrogels,17–19 cell-penetrating peptides,20 and intelligent biomaterials.21,22
Currently, there have been some works23,24 aiming to introduce PLA-based dynamic covalent materials combining self-assembly and reversible crosslinking to provide advanced biocompatible templates. Consequently, these have been extensively studied in biomedical applications, including protein modification,25,26 tumor treatment,27,28 biosensing,29–31 and tissue scaffold 3D-printing.32 Most reviews33,34 have primarily focused on the properties and clinical applications of small-molecule LA, while limited attention is given to the synthesis strategies and biological applications of PLA-based systems. In this work, after fully considering the impact of specific structures on biological performances, we highlight the latest advancements of PLA in polymerization methods and biomedical applications, and the prospects for further development in the future (Scheme 1).
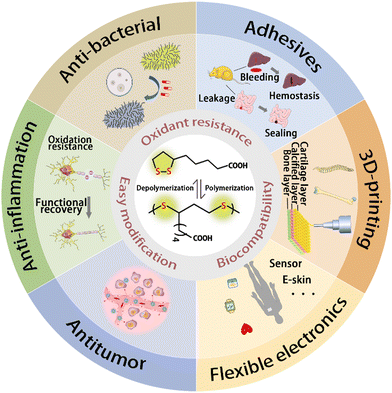 | ||
| Scheme 1 Polymerization and depolymerization of LA molecules, and features and biomedical applications of PLAs. | ||
Biological features of free LA molecules
LA contains three structural units: a lipophilic five-membered disulfide ring, a hydrophilic/acidic functionalized carboxyl group, and a hydrophobic alkyl chain. There are two enantiomers of free LA molecules for the chiral carbon atom C6, where the bioactive one is (R)-LA.35LA is described as a “universal antioxidant”, which can reduce lipid peroxidation damage induced by H2O2 or Fe2+ and Cu2+ through direct the scavenging of free radicals6 or metal chelation (Fig. 1B).36 LA can be readily reduced into dihydrolipoic acid (DHLA) under oxidizing conditions (Fig. 1A). Both LA and DHLA possess potent antioxidant properties and play a synergistic effect in vivo, similar to the relationship between glutathione (GSH) and the oxidized form of glutathione (GSSG). Attributed to its biological origin, good biocompatibility, easy modification, it is widely used in the treatment or prevention of various chronic diseases related to oxidative stress, including anti-aging nutritional supplements,37 and even to suppress the appetite.38
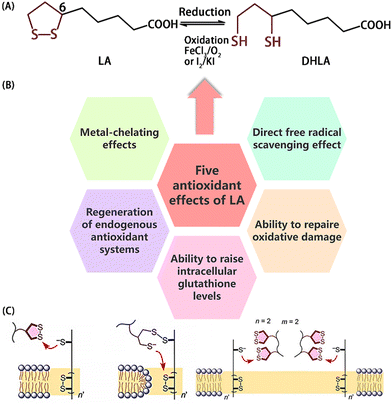 | ||
| Fig. 1 (A) Mutual transformation of LA and DHLA. (B) Antioxidant mechanism of LA. (C) Scheme of LA promoting cell endocytosis. Reprinted from ref. 39 with permission. Copyright 2020, Wiley. | ||
Simultaneously, because of its amphipathic nature, it can be easily absorbed and transported through cell membranes to penetrate tissues, such as the nervous system and the heart, that are mainly composed of fat and water. Disulfide-mediated uptake can be achieved through dynamic covalent exchange of disulfide and mercaptan on the cell surface. Covalent binding to the cell surface may occur via several uptake mechanisms, including fusion, endocytosis, or direct translocation into the cytoplasm along the disulfide orbits and micellar pores (Fig. 1C).39 However, the biological applications of free LA molecules are limited due to its rapid metabolism, poor bioavailability, and short biological half-life. Carbone et al.40 nano-encapsulated LA derivatives and prepared hydrophilic nanocapsules as well as lipophilic-nanostructured lipid carriers to address these issues. However, there are still problems that need to be solved.
ROP polymerization of LA molecules
In 1956, Thomas and Reed observed a “viscous liquid” during the oxidation of LA, which was later reported to be PLA.41 LA exhibits instability under photoconditions with oligomer formation in dilute solutions exposed to long wavelength ultraviolet light. Besides, upon reaching the melting point (59–62 °C) of LA, it rapidly polymerizes to form higher molecular weight polymers. Presently, the most common method of LA polymerization is still free radical polymerization, initiated by ultraviolet light (Fig. 2A) or heat (Fig. 2B). The resulting PLA has a ring interlocking structure.42 Interestingly, the total activation energy for this process is very low and is concentration- and temperature-dependent.11 It is challenging to obtain the polymer at below the melting point or under certain concentrations. Conversely, once the temperature exceeds the melting point of LA, polymerization occurs more readily. However, the bulk thermal polymerization process for obtaining PLA without any initiator is reversible, yielding amorphous polymers. When LA molecules are sufficiently close in ethanol at a high concentration, intermolecular disulfide exchange occurs, leading to PLA with alternating disulfide bonds in the main chain with a lower polymerization temperature (Fig. 2C). Nevertheless, the resulting PLA is metastable at room temperature, and after ethanol evaporation, it quickly reverts back to semicrystalline oligomers due to retro-cyclic depolymerization. To address these issues, the controlled thermal polymerization of PLA can be enhanced by leveraging the synergistic effects of both covalent and non-covalent bonds. On the one hand, chemical reactions of the carboxyl group via esterification, guanidinylation, amidation, etc. can introduce different functional groups and facilitate hydrogen bonds. Zhang et al.43 reported a water-soluble ABA triblock copolymer; whereby a hydrophilic poly(ethylene oxide) B block and hydrophobic A block, derived from a carbonate with LA, self-assembled to form a flower-like microgel in water medium. Concentration induction led to physical crosslinking, while the addition of a telechelic dithiol triggered chemical crosslinking. Lu et al.25 utilized sulfhydryl protein residues to initiate methoxy oligoethylene glycol-modified LA polymerization, achieving protein-PDS graft synthesis at physiological temperature and reducing the polymerization concentration of LA. Zhang et al.13 constructed a supramolecular polymer network using three different types of dynamic chemical bonds (chemical crosslinking, hydrogen bonding, and complexation) in a single polymer system. On the other hand, ionic liquid could be added to prevent the depolymerization of PLA. Wang et al.44 achieved a concentration-induced ring-opening polymerization of LA at room temperature and obtained stable PLA ion sols in air by adding an ionic solution of 1-ethyl-3-methylimidazolium ethyl sulfate. Additionally, the carboxyl group can be complexed with various metal ions (Fe3+, Cu2+, Zn2+, Mn2+, etc.). These complexations often offer an opportunity to modulate the optical and electrical properties of inorganic surfaces, including quantum dots (QDs), plasma materials, and perovskites.45,46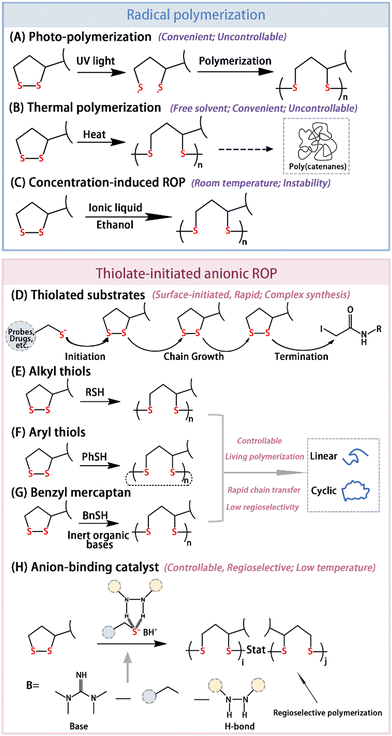 | ||
| Fig. 2 Two mechanisms for the ring-opening polymerization for PLA: radical polymerization and thiolate-initiated anionic ROP. (A) Photoinduced ring-opening polymerization for PLA. (B) Thermal polymerization for PLA. (C) Ring-opening polymerization induced by the concentration in ethanol solution. (D) Process of substrate-initiated polymerization: chain initiation, chain growth, and chain termination. Reprinted from ref. 20 with permission. Copyright 2013 Elsevier. (E)–(G) Effect of mercaptan nucleophilicity on the topological structures of PLA. (H) Regioselective ring-opening polymerization for PLA by anion-binding catalysis. Reprinted from ref. 49 with permission. Copyright 2023, Elsevier. | ||
Although free radical polymerization is convenient, the polymerization of LA often results in a mixture of PLA and a small number of cyclic monomers. To achieve precise structural control and a low dispersion, researchers have explored living anionic polymerization triggered by mercaptan initiators. Bang et al.20 utilized thiolated probes or drugs as initiators for the anion polymerization of guanidinylated LA derivatives at room temperature, with iodoacetamides acting as terminators (Fig. 2D). Notably, PLA is susceptible to reduction and depolymerization,47 making it a promising candidate for non-invasive drug delivery.48 Some studies have shown that the structures of PLA depend on the nucleophilicity of the initiators.12 Alkyl mercaptans tend to yield linear polymers (Fig. 2E), while aryl mercaptans favor ring polymers (Fig. 2F). Linear PLA was successfully obtained using benzyl mercaptan with inert bases (Fig. 2G). What's more, achieving regionally selective PLA remains challenging due to the highly nucleophilic chain ends of PLA, which may react with S–S bond monomers or polymers to induce chain transfer and chemical irregularities. Du et al.49 addressed this issue using an anion-binding catalyst (Fig. 2H). By regulating the nucleophilicity of mercaptan through hydrogen bond interactions, they reduced the negative charge at the chain ends and improved the regional selectivity of the ROP. However, further investigations are needed to enhance the regional selectivity of PLA synthesis to align with diverse application requirements. As the most controllable way, thiolate-initiated anionic ROP remains problematic in certain applications, such as in inert conditions and non-initiating monomers with active protons. Qu et al.50 reported an acid-catalyzed cationic ROP, which resulted in high-molecular-weight (over 1000 kDa) poly(disulfide)s under open air without inert protection.
This material exhibits significant potential in the biomedical field due to its interaction between dynamic covalent bonds and dynamic non-covalent sites, as well as its non-toxicity, biological origin, closed-loop recyclability, and other notable characteristics.
Biomedical applications
Antibacterial
So far, most antibacterial polymers lack the capability for reactive degradation. These chemically stable polymers can accumulate in the human body or the environment, potentially leading to significant cytotoxicity and inducing bacterial resistance.51 The biological origin of PLA enables its biodegradability and biocompatibility in vivo, allowing for its rapid degradation by in vivo reducing agents. What's more, because of the synergistic effect of dynamic disulfide bonds and dynamic non-covalent sites, as well as the presence of modifiable carboxyl groups, this material holds promise as a responsive degradable antibacterial agent.Zhao et al.52 coated poly(TA-Zn) on an alkali heat-treated ZE21B alloy scaffold for enhancing the corrosion resistance and blood compatibility of the scaffold (Fig. 3A). Poly(TA-Zn) scavenged free radicals because the released TA molecules could lower 2,2-diphenyl-1-picrylhydrazyl (DPPH) content by electron transfer or hydrogen. The complex interactions between Zn2+ and carboxyl groups replace a part of the weak hydrogen bond interactions, thereby enhancing the network stability. Furthermore, REDV peptide was coupled to the scaffold through amidation reactions to promote the re-endothelialization. PLA rich in guanidine groups can be utilized for efficient drug delivery. The cationic groups facilitate the initial binding of the polymer to the negatively charged bacterial cell membrane, followed by the entry of lipophilic groups into the membrane to exert their function. Poly(TA-Zn) samples could significantly inhibit the growth of Staphylococcus aureus, where the average size of the inhibition ring was 1.56 ± 0.27 mm, and the hemolysis rate of blood cells was less than 5%. Mu et al.53 functionalized PLA with guanidine groups and grafted it with dextran to serve as an antibiotic adjuvant (Fig. 3B). The PLAs could be depolymerized via GSH, preventing the accumulation of non-degradable polycations in vivo and effectively reducing cellular toxicity. This approach reduces bacterial resistance to antibiotics by decreasing the bacterial cell membrane permeability or disrupting the efflux pump functions. In the presence of Dex-g-PSS30, neither MDR-AB nor MRSA cells cultured for more than 30 generations developed resistance to various antibiotics, including levofloxacin and tobramycin.
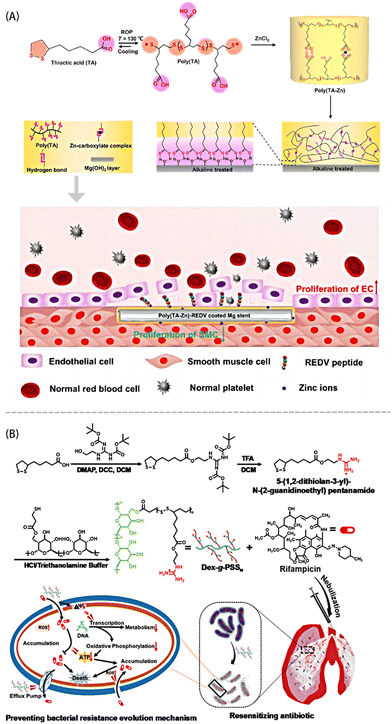 | ||
| Fig. 3 (A) Schematic diagram of the material design of poly(TA-Zn)-REDV and the function of its enhanced re-endothelialization and suppressed SMC overgrowth. Reprinted from ref. 52 with permission. Copyright 2023 Elsevier. (B) Synthesis route of Dex-g-PSSn and preventative mechanisms toward bacterial resistance. Reprinted from ref. 53 with permission. Copyright 2023, Wiley. | ||
Anti-inflammation
ROS can oxidize low-density lipoprotein (LDL) to form oxidized LDL, which in turn transforms macrophages into foam cells and induces an inflammatory response.54,55 PLA has disulfide bonds, which enable its remarkable electrophilicity and free radical reaction ability, such as the removal of ˙OH, ˙NO, ˙ONOO in vivo, or H2O2 and HClO. The tightly crosslinked disulfide bonds and negative carboxylate-rich surface of PLA can effectively prevent blood dilution and serum protein adhesion.6 However, the stability and hydrophobicity of PLA limit the release of bioactive LA monomers in water, compromising its biological functions. It has been discovered that LA-Na not only retains the polymerization properties of LA but also rapidly decomposes into small LA sodium salts when exposed to water.56Cui et al.57 prepared a binary synergistic elastomer adhesive patch made from PLA-Na/PLA by mixing an ethanol solution of PLA with an aqueous solution of PLA-Na, followed by evaporation at 37 °C (Fig. 4). This patch exhibited long-term wet-adhesion and anti-inflammatory properties for treating oral ulcers. The carboxyl groups of PLA could achieve firm tissue adhesion through multiple hydrogen bonds and electrostatic interactions with the amino groups on the tissue surface. Additionally, the hydrophobicity of PLA limited water absorption by the patch, and disrupted the orderly arrangement of PLA-Na. The introduction of PLA via multi-component –COOH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– hydrogen bonds reduce the dissociation rate of PLA-Na, enabling a slow and sustained release of LA-Na. Immunohistochemical staining further demonstrated that the patch significantly downregulated the expressions of pro-inflammatory cytokines IL-6 and TNF-α in mini-pig oral mucosa tissue.
C– hydrogen bonds reduce the dissociation rate of PLA-Na, enabling a slow and sustained release of LA-Na. Immunohistochemical staining further demonstrated that the patch significantly downregulated the expressions of pro-inflammatory cytokines IL-6 and TNF-α in mini-pig oral mucosa tissue.
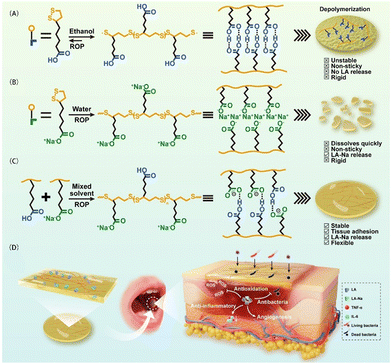 | ||
| Fig. 4 Schematic diagram of the preparation and application of a PLA-based binary synergistic elastomer adhesive patch. Reprinted from ref. 57 with permission. Copyright 2023, Springer. | ||
Antitumor
The tumor microenvironment plays a crucial role in the initiation, proliferation, invasion, and metastasis of tumor cells. Numerous studies have been conducted to develop therapeutic strategies targeting the acidic environment (with a higher GSH concentration than normal cells), H2O2 overexpression, hypoxia, and severe inflammation.27 Disulfide bonds exhibit minimal cytotoxicity toward tumor cells. PLA can undergo rapid in vivo biodegradation by reducing agents such as GSH,20,58 resulting in the formation of small molecules possessing antioxidant properties, and it exhibits non-conventional thiol-mediated covalent translocation with the cell membrane.39 Therefore, incorporating disulfide bonds into prodrugs allows for their reduction to sulfhydryl groups within the tumor cytoplasm at high GSH concentrations.28,59 This enhances the hydrophilicity of the drug system, promotes adjacent chemical bond hydrolysis (such as ester bonds), and facilitates drug release.Zhu et al.60 utilized amidation to attach guanidylated lysine derivatives to LA and then polymerized them with methoxypolyethylene glycol mercaptan to obtain bioreducible cell-penetrating polyguanidine (mPEG225-b-PSSn) for delivering plasmids KillerRed-p53 (PSR-p53) in antitumor therapy (Fig. 5). Among them, the agarose gel electrophoresis results showed that disulfide bonds within mPEG225-b-PSS26 framework were decomposed in the presence of 8 mmol L−1 GSH and greatly facilitated DNA release. The immunohistochemical staining results indicated that the complex formed with pKR-p53 could enter tumor cells through mercaptan mediation and successfully express KillerRed and p53 proteins upon GSH-mediated plasmid release.
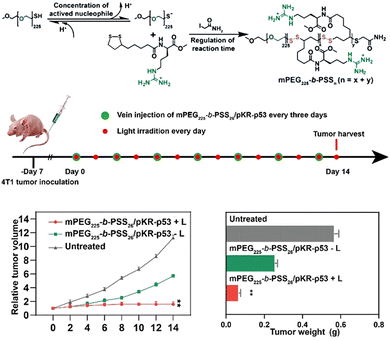 | ||
| Fig. 5 Schematic diagram for the preparation and antitumor therapy application of mPEG225-b-PSSn. Reprinted from ref. 60 with permission. Copyright 2022, Wiley. | ||
Adhesion
Polymer-based biomedical tissue adhesives have attracted widespread attention in the medical field due to their excellent performances. They are suitable for closing and regenerating various tissue injuries. However, their applications are somewhat restricted due to their weak wet adhesive and lack of biological functionality. Adhesive hydrogels have been extensively utilized in biomedical applications,61–63 such as wound dressings, sutures, and strain sensors, owing to their high-water content and resemblance to biological soft tissues. Self-healing hydrogels are typically engineered by incorporating dynamic covalent or reversible non-covalent bonds, including disulfide bonds, acylhydrazones, hydrogen bonds, coordination bonds, guest–host interactions, and hydrophobic interactions. PLA possesses disulfide bonds that can strongly bind to tissues, and can introduce a variety of dynamic bonds to fulfill the requirements of adhesive hydrogels.64 The natural biocompatible polymer skeleton also facilitates its biological applications.Chai et al.61 achieved covalent bonding between polydopamine (PDA) and PLA through a Michael addition (reaction between the catechol groups of PDA and the thiols of PLA), while also establishing non-covalent bonding through hydrogen bonds. By introducing PLA as a natural biocompatible skeleton and utilizing PDA as a crosslinking agent, they demonstrated an enhanced adhesion capability and antiswelling ability. The synergistic effects resulting from the combination of covalent bonds and non-covalent interactions between PLA and PDA imparted a high stretchability, self-healing ability, and toughness to the hydrogels. These hydrogels could be employed as wound dressings for skin defects. Additionally, the presence of PLA in the hydrogel provides antioxidant properties that help eliminate free radicals and inhibit inflammation. Furthermore, PDA's catechol groups can establish strong contact with proteins and cells, thereby promoting cell attachment and proliferation. Cui et al.65 created a self-stabilized deep eutectic supramolecular polymer adhesive by heating a mixture of LA and LA-Na (S-, R-isomer, racemate) with LA in one step. When the LA/LA-Na ratio was 2, the adhesion strength of the adhesive was increased significantly in water, from 137 kPa to 1.07 Mpa after soaking in water for 1 day. As a tissue sealant, the adhesive could replace surgical sutures and enhanced wound healing of a rat skin incision.
Flexible electronics
Flexible electronic materials possess both high electrical conductivity and material flexibility, making them highly promising for applications in wearable devices and flexible energy-storage systems. Additionally, to meet the diverse requirements of biomedicine, flexible electronic devices need to exhibit specific properties, such as surface adhesion, permeability, biocompatibility, and long-term stability. Various ionic conductive gel materials, including ionic gels and hydrogels, have been employed for the development of flexible sensors.66 Hydrogels offer adjustable mechanical properties and extensibility, but their practical application is often limited due to their poor environmental stability. Ionic gels demonstrate a non-combustible property with low volatility along with excellent chemical, thermal, and electrochemical stability, which makes them highly suitable for sensing, energy conversion, and separation.67–69 Nevertheless, most ionic gels suffer from inadequate mechanical properties. By strategically combining soft segments with hard segments possessing different chemical structures, as well as arranging specific covalent bonds or intermolecular forces to form various concentrated state structures, the overall material properties can effectively be regulated.In addition to permanent covalent crosslinkers, PLA polymer networks can also introduce disulfide bonds, ionic crosslinks, electrostatic interactions, and hydrogen bonds to provide the desired flexibility, adhesion, and self-healing characteristics required for ideal wearable electronic devices. Moreover, the presence of disulfide bonds within the main chain imparts additional thermal/photosensitivity while promoting remoldability and recyclability. Wang et al.70 incorporated an ionic liquid into ethanol-induced polymerized PLA to fabricate an ionic gel, which could then serve as a flexible sensor for monitoring the physiological temperature signals of firefighters. The weak hydrogen bond interactions between the ions and the carboxyl groups on the molecular chain promoted molecular chain deformation, facilitating the movement of positive/negative ions within the polymer composite under strain. Consequently, this led to obvious changes in resistance within the composite material. Pei et al.71 synthesized a zwitterionic dynamic elastomer from the copolymerization of LA, 1-ethyl-3-methylimidazo-lipoic acid ([Emim][LA]), and 1-ethyl-3-methylimidazole acetate ([Evim][Ac]) to detect weak physiological activity in the wrist. Dang et al.72 added acrylic acid (AA), choline chloride (CCl), and Fe3+ during the heating polymerization of PLA to obtain a novel LA-based ionic conductor for monitoring human joint activity. In addition to adding iron ions to PLA to introduce coordination bonds to provide good stretchability, Zhang et al.31 also introduced a mussel-like activity to ensure that the material had high intrinsic adhesion on both wet and dry skin surfaces. The material could be used to prepare breathable skin electrode arrays for stable 12-lead clinical eletrocardiography (ECG) monitoring. Khan et al.73 prepared an entirely gel-based triboelectric nanogenerator (TENG) with high stretchability, self-healing, and anti-freezing capabilities to harvest energy by sandwiching a conductive self-healable organohydrogel (CSO) as an electrode between two symmetric non-conductive self-healable organogel (NSO)-based piezoelectric layers (Fig. 6). Besides adding an ionic liquid into the matrix for electrical conductivity, Qu et al.74 reported an intrinsically conductive assembled network combining reversible disulfide with β-sheet-inspired H-bonding. The amphipathic LA-derived small molecular system could assemble into an ordered network via an evaporation-induced polymerization and exhibited high efficiency in ion transport (3.56 × 10−3 S cm−1).
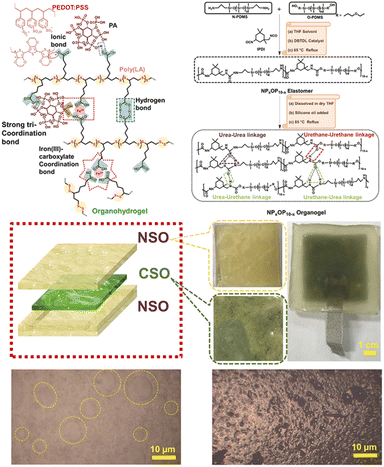 | ||
| Fig. 6 Synthetic structures of organohydrogels and organogels and diagram of a TENG device. Reprinted from ref. 73 with permission. Copyright 2023, Elsevier. | ||
Tissue scaffold 3D-printing
3D printing technology holds great potential in tissue engineering and regenerative medicine. It allows for the precise fabrication of personalized scaffold structures, tailored to match specific shapes, sizes, pore structures, and porosities.75 In tissue engineering, 3D printing scaffolds must provide structural support and create a suitable microenvironment to support cellular behavior. In recent years, the 3D printing of supramolecular materials with hydrogels as the main object has been realized. Cai et al.76 found that PLA obtained by LA thermally activated dynamic polymerization exhibited a unique time-dependent self-reinforcing pattern (after 3D printing, the mechanical capabilities and stiffness of PLA and LA continued to increase over time). Therefore, LA can be used as an initial material for 3D-fused deposition modeling (3D-FDM).To achieve biomedical and tissue engineering applications, 3D printing materials need to be biodegradable, biocompatible, and self-repairable, but also need to have sufficient printing capacity, stent reliability, and mechanical properties. Tran et al.77 found that a poly(malate-co-propylene oxide) copolymer capped with LA and grafted by UV irradiation of the LA-capped backbone polymer could control the printable, mechanical, and cell adhesion of the gel, as well as the reliability of the printed scaffold during 3D printing to obtain a printing scaffold with high resolution and mechanical properties. After that, they then found that adding cell adhesion proteins, such as gelatin and albumin, to the gel made it possible to print biologically functional 3D scaffolds (Fig. 7).32 In in vivo studies, four weeks after implantation in mice, protein-loaded scaffolds showed great biocompatibility and increased angiogenesis without an inflammatory response, demonstrating the promise as a printable tough hydrogel ink for tissue engineering.
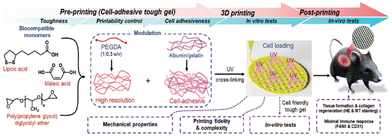 | ||
| Fig. 7 Process and application scenario of a 3D printing biocompatible protein support scaffold. Reprinted from ref. 32 with permission. Copyright 2023, Wiley. | ||
Conclusions and perspective
In summary, PLA polymerization possesses several advantages, including a simple preparation process, mild conditions, low cost, and easy modification. Polymer materials with dynamic disulfide bonds have exhibited good compatibility, degradation, flexibility, rapid self-healing, and reversible adhesion. Additionally, PLA has demonstrated an antioxidant capacity and can be applied in REDOX diseases, such as for its antitumor effects, antibacterial properties, and anti-inflammatory effects.This review summarized the polymerization strategies of LA, particularly focusing on two polymerization methods: free radical polymerization and thiol-initiated anionic polymerization. Additionally, this work summarized the use of modifiable carboxyl groups to introduce functional moieties or supramolecular interactions for achieving the ordered self-assembly of polymers. Furthermore, leveraging the dynamic disulfide bonds and non-covalent binding sites in PLA cancan promote enhanced performance and broaden its applications. We also highlight recent advancements in biomedical applications (Table 1), especially in emerging fields, such as hydrogels, biosensors, and 3D-printed tissue scaffolds. Moreover, due to the low toxicity and ease of depolymerization, PLA can serve as a suitable polymer substrate for constructing functional microenvironments. For instance, PLA-based nanoparticles can facilitate the transfer of active molecules, such as mRNA, or construct vesicles for drug-delivery purposes.6,25,78 It also offers a viable approach for molecular engineering hybrid materials by regulating optical/electrical properties on inorganic surfaces, like QDs, plasma materials, and perovskites.79
| Bio-applications | Components | Polymeric mechanism | Material properties | Ref. |
|---|---|---|---|---|
| PXBP: Dex-g-PSSn: dextran-graft-poly(5-(1,2-dithiolan-3-yl)-N-(2-guanidinoethyl)pentanamide). PLL: poly(lipoic acid-co-sodium lipoate). SEBS: styrene ethylene butylene styrene. Emim: 1-ethyl-3-methylimidazole. Evim: 1-ethyl-3-vinylimidazole. PEDOT:PSS: poly(3,4-ethylenedioxythiophene) polystyrene sulfonate. MPLE: poly(maleate-propylene oxide)-lipoatepoly(ethylene oxide). PEGDA: poly(ethylene glycol) diacrylate. | ||||
| Antibacterial | Poly(LA-Zn); ZE21B | Thermal ROP | Self-healing; anti-coagulant; hemocompatibility; promoting re-endothelialization | 52 |
| Dex-g-PSSn; rifampicin | Thiolate-mediated ROP | Preventing bacterial resistance; biological absorption; degradation | 53 | |
| Anti-inflammation | PLL hydrogels; NaHCO3; LiCl | Concentration-induced ROP | Facile injectability; adequate adhesiveness; self-healing; conductivity | 56 |
| Poly LA-Na/poly LA | Concentration-induced ROP | Flexibility; adhesion; wet-resistance | 57 | |
| Antitumor | mPEG225-b-PSS26; pKR-p53 or pEGF | Thiolate-mediated ROP | Biocompatibility; degradability; transfection | 60 |
| Adhesives | PPA-PLA hydrogel | Thermal ROP | Stretchability; resilience; antiswelling; self-healing; repeatable adhesion; capacity; cell affinity; biodegradation | 61 |
| PLA/PLA-Na | Thermal ROP | Wet-adhesion; biocompatibility; oxidation resistance; antibacterial | 65 | |
| Flexible electronics | P(LA-co-AM)/[EE] ionogel | Thiolate-mediated ROP | Tensile strength; toughness; stretchability; transparency; shape-memory designability | 70 |
| PLTA-Fe3+; SEBS/Au | Thermal ROP | Stretchability; adhesion; water and air permeability | 31 | |
| P(LA-co-[Emim][LA]-co-[Evim][Ac]; AlCl3) | Thermal ROP | Transparency; adhesion; self-healing; remoldability; recyclability; stretchability; sensing durability; conductivity | 71 | |
| Electrode(PLA; PA; FeCl3·H2O; PEDOT:PSS); tribolayers(NPxOP10−x organogel) | Thiolate-mediated ROP | Self-healability; stretchability; anti-freezing; energy-harvesting | 73 | |
| PLA; AA; CCl; Fe3+ | Thermal ROP | Transparency; stretchability; sensing; recyclability | 72 | |
| Poly(STGly-n) | Thermal ROP | Efficient cation conductivity; chemical recyclability | 74 | |
| 3D printing | PLA/LA | Thermal ROP | Self-reinforcing; time-dependent assembly | 76 |
| MPLE gel; PEDGA; gelatin; albumin | Condensation polymerization; radical graft polymerization | Bioactivity; biofunctionality; cytocompatibility; bioadhesion; cell spreading; printability; long-term stability | 32 | |
| PEDGA(LA-capped); MPLE gel | Condensation polymerization; radical graft polymerization | 3D printability; biocompatibility; adhesiveness; self-healing; tunable swell-ability; degradation | 77 | |
However, there are still many challenges in the design and synthesis of PLA-based biomaterials, including around the following themes:
(1) Polymerization methods and mechanisms: currently, the main methods for PLA are still free radical polymerization and thiol-initiated anionic polymerization. However, more diverse polymerization approaches are needed to achieve controlled PLA polymerization in both equilibrium-controlled and non-equilibrium systems. The dynamic properties of this material, such as self-healing ability, stimulus responsiveness, and recyclability, are mostly governed by thermodynamic equilibrium control.
(2) Precise molecular control: achieving structurally controlled PLA relies on a thiol-initiated anionic ROP. However, most initiators, such as bases and thiols, need to remain inert. Achieving structural control, such as controlling the relative molecular weight and distribution, is crucial for enhancing the material performance. For example, in tissue engineering, 3D-printing tissue scaffolds require materials with excellent mechanical properties and specific biological functionality. Additionally, synthesizing stereoselective polymers remains challenging.
(3) Clinical translation and industrial-scale synthesis: current research on PLA-based materials is primarily at the laboratory stage. Before clinical adoption, a series of biocompatibility assessments and in vitro/vivo tests are necessary. The large-scale production of PLA still faces challenges, especially considering its depolymerization reversibility, which complicates storage.
(4) New applications: with technological advancements, the synergistic use of various tools and materials can broaden the material scales to match different application needs. For instance, simulating and screening synthetic pathways and mechanisms using big data tools, like artificial intelligence, can accelerate material development rates and reduce costs.
LA, an endogenous small molecule, has been widely used in clinical treatments for conditions such as diabetes, Alzheimer's disease, and cancer over the past seventy years.33 In the latest 10 years, because of its strong antioxidant and rich biological sources, it has attracted widespread attention and experienced rapid development. Despite the remaining unresolved problems and the fact there is a long way to go in terms of clinical applications, further optimization through various of scientific tools is expected to stimulate its application in material design and other cutting-edge fields.
Abbreviations
| DHLA | Dihydrolipoic acid |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| REDOX | Oxidation–reduction |
| DPN | Diabetic peripheral neuropathy |
| QDs | Quantum dots |
| PDS | Polydimethylsiloxane |
| LDL | Low-density lipoprotein |
| Emim | 1-Ethyl3-methylimidazo |
| Evim | 1-Ethyl-3-methylimidazole |
| ECG | Eletrocardiography |
| TENG | Triboelectric nanogenerator |
| CSO | Conductive self-healable organohydrogel |
| NSO | Non-conductive self-healable organogels |
| 3D-FDM | 3D-fused deposition modeling |
Author contributions
Qing Yu: investigation, methodology, formal analysis, writing – original draft. Zhiyue Fang: writing – review & editing, formal analysis. Shifang Luan: writing – review & editing, conceptualization. Lei Wang: investigation, methodology, project administration, supervision, funding acquisition, writing – review & editing. Hengchong Shi: supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (Grant No. 2021YFC2101700), the Jilin Provincial Key Research and Development Plan Project (Project No. 20230204105YY), the National Natural Science Foundation of China (Project No. 52373049, 52293384, and 52293380), Youth Innovation Promotion Association of CAS (Grant No. Y2021066), and Chinese Academy of Sciences-Wego Group Hightech Research & Development Program.Notes and references
- M. D. Spalding and S. T. Prigge, Microbiol. Mol. Biol. Rev., 2010, 74, 200–228 CrossRef CAS PubMed.
- K. Kofuji, T. Isobe and Y. Murata, Food Chem., 2008, 115, 483–487 CrossRef.
- J. L. Reed, G. B. DeBusk, C. I. Gunsalus and S. C. Hornberger, Science, 1951, 114, 93–94 CrossRef PubMed.
- J. M. May, Z.-c Qu and D. J. Nelson, Clin. Biochem., 2007, 40, 1135–1142 CrossRef CAS PubMed.
- S. K. Azzam, H. Jang, M. C. Choi, H. Alsafar, S. Lukman and S. Lee, Mol. Pharmaceutics, 2018, 15, 2098–2106 CrossRef CAS PubMed.
- X. Lu, Z. He, X. Xiao, X. Wei, X. Song and S. Zhang, Small, 2023, 19, 2303459 CrossRef CAS PubMed.
- M. J. Jeon, W. G. Kim, S. Lim, H.-J. Choi, S. Sim, T. Y. Kim, Y. K. Shong and W. B. Kim, Mol. Cell. Endocrinol., 2015, 419, 113–123 CrossRef PubMed.
- P. P. Phiboonchaiyanan and P. Chanvorachote, Cell. Oncol., 2017, 40, 497–510 CrossRef CAS PubMed.
- J. Tripathy, A. Tripathy, M. Thangaraju, M. Suar and S. Elangovan, Life Sci., 2018, 207, 15–22 CrossRef CAS PubMed.
- D. Ziegler, M. Reljanovic, H. Mehnert and F. A. Gries, Exp. Clin. Endocrinol. Diabetes, 1999, 107, 421–430 CrossRef CAS PubMed.
- A. Kisanuki, Y. Kimpara, Y. Oikado, N. Kado, M. Matsumoto and K. Endo, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5247–5253 CrossRef CAS.
- Y. Liu, Y. Jia, Q. Wu and J. S. Moore, J. Am. Chem. Soc., 2019, 141, 17075–17080 CrossRef CAS PubMed.
- Q. Zhang, C.-Y. Shi, D.-H. Qu, Y.-T. Long, B. L. Feringa and H. Tian, Sci. Adv., 2018, 4, eaat8192 CrossRef CAS PubMed.
- Y. Deng, Q. Zhang, C. Shi, R. Toyoda, D.-H. Qu, H. Tian and B. L. Feringa, Sci. Adv., 2022, 8, eabk3286 CrossRef CAS PubMed.
- C.-Y. Shi, D.-D. He, B.-S. Wang, Q. Zhang, H. Tian and D.-H. Qu, Angew. Chem., Int. Ed., 2022, 62, e202214422 CrossRef PubMed.
- K. R. Albanese, P. T. Morris, J. Read de Alaniz, C. M. Bates and C. J. Hawker, J. Am. Chem. Soc., 2023, 145, 22728–22734 CrossRef CAS PubMed.
- J. Gao, Q. Zhang, B. Wu, X. Gao, Z. Liu, H. Yang, J. Yuan and J. Huang, Small, 2023, 19, 2207334 CrossRef CAS PubMed.
- K. X. Hou, S. P. Zhao, D. P. Wang, P. C. Zhao, C. H. Li and J. L. Zuo, Adv. Funct. Mater., 2021, 31, 2107006 CrossRef CAS.
- D. Chao, S. Changyou, L. Hongchen, C. Yian and Q. Haisong, Nano Energy, 2021, 90, 106619 CrossRef.
- E.-K. Bang, G. Gasparini, G. Molinard, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2013, 135, 2088–2091 CrossRef CAS PubMed.
- S. Huang, Y. Shen, H. K. Bisoyi, Y. Tao, Z. Liu, M. Wang, H. Yang and Q. Li, J. Am. Chem. Soc., 2021, 143, 12543–12551 CrossRef CAS PubMed.
- C. Dang, F. Zhang, Y. Li, Z. Jin, Y. Cheng, Y. Feng, X. Wang, C. Zhang, Y. Chen, C. Shao, Q. Zheng and H. Qi, Small, 2022, 18, 2200421 CrossRef CAS PubMed.
- Q. Zhang, D.-H. Qu, B. L. Feringa and H. Tian, J. Am. Chem. Soc., 2022, 144, 2022–2033 CrossRef CAS PubMed.
- C. D. Vo, G. Kilcher and N. Tirelli, Macromol. Rapid Commun., 2009, 30, 299–315 CrossRef CAS PubMed.
- J. Lu, Z. Xu, H. Fu, Y. Lin, H. Wang and H. Lu, J. Am. Chem. Soc., 2022, 144, 15709–15717 CrossRef CAS PubMed.
- M. C. Montasell, P. Monge, S. Carmali, L. M. Dias Loiola, D. G. Andersen, K. B. Løvschall, A. B. Søgaard, M. M. Kristensen, J. M. Pütz and A. N. Zelikin, Nat. Commun., 2022, 13, 4861 CrossRef CAS PubMed.
- Z. Zhang, Q. Cao, Y. Xia, C. Cui, Y. Qi, Q. Zhang, Y. Wu, J. Liu and W. Liu, Bioact. Mater., 2023, 31, 408–421 Search PubMed.
- D. Zhong, H. Wu, Y. Wu, Y. Li, X. Xu, J. Yang and Z. Gu, Nanoscale, 2019, 11, 15091–15103 RSC.
- D. Pei, S. Yu, P. Liu, Y. Wu, X. Zhang, Y. Chen, M. Li and C. Li, Adv. Funct. Mater., 2022, 35, 2204257 CrossRef.
- Y. Wang, Y. Zhang, Z. Zhang, T. Li, J. Jiang, X. Zhang, T. Liu, J. Qiao, J. Huang and W. Dong, ACS Nano, 2022, 16, 3394–3403 CrossRef CAS PubMed.
- Z. Jun, H. Mingshuang, Z. Xinran, W. Huiquan, L. Xiangxiang, Z. Xiaoying and Y. Hui, Adv. Mater. Technol., 2023, 8, 2300243 CrossRef.
- H. N. Tran, I. G. Kim, J. H. Kim, A. Bhattacharyya, E.-J. Chung and I. Noh, Macromol. Biosci., 2023, 23, 2300316 CrossRef CAS PubMed.
- S.-Y. Lv, S. He, X.-L. Ling, Y.-Q. Wang, C. Huang, J.-R. Long, J.-Q. Wang, Y. Qin, H. Wei and C.-Y. Yu, Int. J. Pharm., 2022, 627, 122201 CrossRef CAS PubMed.
- M. D. M. Viana, P. S. S. Lauria, A. A. D. Lima, L. C. F. Opretzka, H. R. Marcelino and C. F. Villarreal, Antioxidants, 2022, 11, 2420 CrossRef CAS PubMed.
- M. H. Brookes, B. T. Golding, D. A. Howes and A. T. Hudson, J. Chem. Soc., Chem. Commun., 1983, 1051–1053 RSC.
- L. M. Gaetke and C. K. Chow, Toxicology, 2003, 189, 147–163 CrossRef CAS PubMed.
- T. M. Hagen, R. T. Ingersoll, J. Lykkesfeldt, J. Liu, C. M. Wehr, V. Vinarsky, J. C. Bartholomew and A. B. Ames, FASEB J., 1999, 13, 411–418 CrossRef CAS PubMed.
- C. H. Park, K.-U. Lee, J.-Y. Park, E.-H. Koh, H.-S. Kim and J. Lee, Pharmazie, 2010, 65, 580–584 CAS.
- R. Martinent, D. Du, J. López-Andarias, N. Sakai and S. Matile, ChemBioChem, 2020, 22, 253–259 CrossRef PubMed.
- C. Carbone, E. Arena, V. Pepe, O. Prezzavento, I. Cacciatore, H. Turkez, A. Marrazzo, A. Di Stefano and G. Puglisi, Colloids Surf., B, 2017, 155, 238–247 CrossRef CAS PubMed.
- R. C. Thomas and L. J. Reed, J. Am. Chem. Soc., 1956, 78, 6148–6149 CrossRef CAS.
- K. Endo, T. Shiroi and N. Murata, Polym. J., 2005, 37, 512–516 CrossRef CAS.
- X. Zhang and R. M. Waymouth, J. Am. Chem. Soc., 2017, 139, 3822–3833 CrossRef CAS PubMed.
- Y. Wang, S. Sun and P. Wu, Adv. Funct. Mater., 2021, 31, 2101494 CrossRef CAS.
- H. Chen, T. Liu, P. Zhou, S. Li, J. Ren, H. He, J. Wang, N. Wang and S. Guo, Adv. Mater., 2020, 32, 1905661 CrossRef CAS PubMed.
- Z. Chen, Q. Cheng, H. Chen, Y. Wu, J. Ding, X. Wu, H. Yang, H. Liu, W. Chen, X. Tang, X. Lu, Y. Li and Y. Li, Adv. Mater., 2023, 35, 2300513 CrossRef CAS PubMed.
- M. M. Kristensen, K. B. Løvschall and A. N. Zelikin, ACS Macro Lett., 2023, 12, 955–960 CrossRef CAS PubMed.
- H. Yang, W. Shen, W. Liu, L. Chen, P. Zhang, C. Xiao and X. Chen, Biomacromolecules, 2018, 19, 4492–4503 CrossRef CAS PubMed.
- T. Du, B. Shen, J. Dai, M. Zhang, X. Chen, P. Yu and Y. Liu, J. Am. Chem. Soc., 2023, 145, 27788–27799 CrossRef CAS PubMed.
- B.-S. Wang, Q. Zhang, Z.-Q. Wang, C.-Y. Shi, X.-Q. Gong, H. Tian and D.-H. Qu, Angew. Chem., Int. Ed., 2023, 62, e202215329 CrossRef CAS PubMed.
- M. Haktaniyan and M. Bradley, Chem. Soc. Rev., 2022, 51, 8584–8611 RSC.
- Z. Zhao-Qi, T. Pei-Duo, W. Li, Q. Zheng-Hui, L. Jing-An, L. Hang, G. Shao-Kang, L. Cun-Guo and W. Hong-Yan, Chem. Eng. J., 2022, 451, 139096 Search PubMed.
- S. Mu, Y. Zhu, Y. Wang, S. Qu, Y. Huang, L. Zheng, S. Duan, B. Yu, M. Qin and F.-J. Xu, Adv. Mater., 2022, 34, 2204065 CrossRef CAS PubMed.
- R. Salvayre, A. Negre-Salvayre and C. Camaré, Biochimie, 2016, 125, 281–296 CrossRef CAS PubMed.
- H. Xu, P. She, B. Ma, Z. Zhao, G. Li and Y. Wang, Biomaterials, 2022, 288, 121734 CrossRef CAS PubMed.
- W. Fang, D. Jiaqiang, Q. Hao, L. Dongfan, G. Dong, C. Jinjin, Z. Yanfeng, C. Yilong and H. Xijing, Chem. Eng. J., 2023, 466, 143071 CrossRef.
- C. Cui, L. Mei, D. Wang, P. Jia, Q. Zhou and W. Liu, Nat. Commun., 2023, 14, 7707 CrossRef CAS PubMed.
- G. Gasparini, E. K. Bang, G. Molinard, D. V. Tulumello, S. Ward, S. O. Kelley, A. Roux, N. Sakai and S. Matile, J. Am. Chem. Soc., 2014, 136, 6069–6074 CrossRef CAS PubMed.
- C. Wang, B. Wang, S. Zou, B. Wang, G. Liu, F. Zhang, Q. Wang, Q. He and L. Zhang, Biomater. Sci., 2021, 5977–5987 RSC.
- Y. Zhu, M. Lin, W. Hu, J. Wang, Z.-G. Zhang, K. Zhang, B. Yu and F.-J. Xu, Angew. Chem., Int. Ed., 2022, 61, e202200535 CrossRef CAS PubMed.
- C. Chai, Y. Guo, Z. Huang, Z. Zhang, S. Yang, W. Li, Y. Zhao and J. Hao, Langmuir, 2020, 36, 10448–10459 CrossRef CAS PubMed.
- L. Caikun, L. Ruilin, J. Mengqi, X. Xiao, C. Yun, L. Pengfei and Z. Shiyong, Chem. Mater., 2023, 35, 2588–2599 CrossRef.
- X. Ke, S. Tang, Z. Dong, K. Ren, P. Yu, X. Xu, J. Yang, J. Luo and J. Li, Chem. Eng. J., 2022, 443, 136206 CrossRef.
- C.-Y. Shi, D.-D. He, Q. Zhang, F. Tong, Z.-T. Shi, H. Tian and D.-H. Qu, Natl. Sci. Rev., 2023, 10, nwac139 CrossRef CAS PubMed.
- C. Cui, Y. Sun, X. Nie, X. Yang, F. Wang and W. Liu, Adv. Funct. Mater., 2023, 33, 2307543 CrossRef CAS.
- L. Hu, P. L. Chee, S. Sugiarto, Y. Yu, C. Shi, R. Yan, Z. Yao, X. Shi, J. Zhi, D. Kai, H.-D. Yu and W. Huang, Adv. Mater., 2023, 35, 2205326 CrossRef CAS PubMed.
- Y. Ren, Z. Liu, G. Jin, M. Yang, Y. Shao, W. Li, Y. Wu, L. Liu and F. Yan, Adv. Mater., 2021, 33, 2008486 CrossRef CAS PubMed.
- G. Sun, P. Wang, Y. Jiang, H. Sun, T. Liu, G. Li, W. Yu, C. Meng and S. Guo, Nano Energy, 2023, 110, 108367 CrossRef CAS.
- D. Gao, G. Thangavel, J. Lee, J. Lv, Y. Li, J.-H. Ciou, J. Xiong, T. Park and P. S. Lee, Nat. Commun., 2023, 14, 1990 CrossRef CAS PubMed.
- J. Wang, Y. Zheng, T. Cui, T. Huang, H. Liu, J. Zhu, L. Song and Y. Hu, Adv. Funct. Mater., 2024, 34, 2312383 CrossRef CAS.
- D. Pei, S. Yu, X. Zhang, Y. Chen, M. Li and C. Li, Chem. Eng. J., 2022, 445, 136741 CrossRef CAS.
- C. Dang, M. Wang, J. Yu, Y. Chen, S. Zhou, X. Feng, D. Liu and H. Qi, Adv. Funct. Mater., 2019, 29, 1902467 CrossRef.
- K. Amir, G. Sreekanth, W. Chia-Hua, L. Hong-Wei, P. Yi-Fang, I. W. Judy, G. Dipti, L. Ying-Chih and L. Hong-Cheu, Nano Energy, 2021, 90, 106525 CrossRef.
- C.-Y. Shi, Q. Zhang, B.-S. Wang, D.-D. He, H. Tian and D.-H. Qu, CCS Chem., 2022, 5, 1422–1432 CrossRef.
- C. Wang, W. Huang, Y. Zhou, L. He, Z. He, Z. Chen, X. He, S. Tian, J. Liao, B. Lu, Y. Wei and M. Wang, Bioact. Mater., 2020, 5, 82–91 Search PubMed.
- C. Cai, S. Wu, Y. Zhang, F. Li, Z. Tan and S. Dong, Adv. Sci., 2022, 9, 2203630 CrossRef CAS PubMed.
- H. N. Tran, I. G. Kim, J. H. Kim, E.-J. Chung and I. Noh, Biomater. Res., 2022, 26, 75 CrossRef CAS PubMed.
- Q. Zhang, Y.-X. Deng, H.-X. Luo, C.-Y. Shi, G. M. Geise, B. L. Feringa, H. Tian and D.-H. Qu, J. Am. Chem. Soc., 2019, 141, 12804–12814 CrossRef CAS PubMed.
- W. Fang, Z. Mu, Y. He, K. Kong, K. Jiang, R. Tang and Z. Liu, Nature, 2023, 619, 293–299 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |

