Open Access Article
Open Access ArticleAn anthropocene-framed transdisciplinary dialog at the chemistry-energy nexus†
Mathieu S.
Prévot‡
 a,
Valeria
Finelli‡
a,
Valeria
Finelli‡
 bc,
Xavier
Carrier‡
bc,
Xavier
Carrier‡
 d,
Gabriele
Deplano‡
d,
Gabriele
Deplano‡
 b,
Margherita
Cavallo‡
b,
Margherita
Cavallo‡
 b,
Elsje Alessandra
Quadrelli‡
b,
Elsje Alessandra
Quadrelli‡
 ae,
Juliette
Michel‡
f,
Marie-Hélène
Pietraru‡
g,
Clément
Camp‡
ae,
Juliette
Michel‡
f,
Marie-Hélène
Pietraru‡
g,
Clément
Camp‡
 h,
Giulia
Forghieri‡
h,
Giulia
Forghieri‡
 i,
Anna
Gagliardi‡
jk,
Sebastian
Seidel‡
i,
Anna
Gagliardi‡
jk,
Sebastian
Seidel‡
 l,
Antoine
Missemer‡
l,
Antoine
Missemer‡
 m,
Bertrand
Reuillard‡
m,
Bertrand
Reuillard‡
 n,
Barbara
Centrella‡
b,
Silvia
Bordiga‡
n,
Barbara
Centrella‡
b,
Silvia
Bordiga‡
 b,
María Grace
Salamanca González‡
o,
Vincent
Artero‡
b,
María Grace
Salamanca González‡
o,
Vincent
Artero‡
 n,
Keanu V. A.
Birkelbach‡
l and
Niklas
von Wolff‡
n,
Keanu V. A.
Birkelbach‡
l and
Niklas
von Wolff‡
 p
p
aUniversité Claude Bernard Lyon 1, CNRS, Institut de Recherche sur la catalyse et l'environnement (IRCELYON, UMR 5256), 2 Av. A. Einstein, F-69626 Villeurbanne Cedex, France. E-mail: transdisciplinarychem@protonmail.com
bDepartment of Chemistry, NIS and INSTM Reference Centre, University of Turin, Via P. Giuria 7 I-10125 and Via G. Quarello 15/A, I-10135, Turin, Italy
cUniversity School for Advanced Studies IUSS Pavia, Palazzo del Broletto, Piazza della Vittoria 15, I-27000, Pavia, Italy
dSorbonne Université, CNRS, Laboratoire de Réactivité de Surface, LRS, F-75005 Paris, France
eCPELYON, 43 Boulevard du 11 Novembre 1918, F-69616, Villeurbanne, France
fUniversité de Lyon, Ecole Urbaine de Lyon, France
gUniversité Paris-Saclay, CEA, CNRS, NIMBE, F-91191, Gif-sur-Yvette, France
hUniversité de Lyon, Institut de Chimie de Lyon, Laboratory of Catalysis, Polymerization, Processes & Materials, CP2M UMR 5128 CNRS-UCB Lyon 1-CPE Lyon, 43 Bd du 11 Novembre 1918, F-69616, Villeurbanne, France
iCATMAT Lab, Department of Molecular Sciences and Nanosystems, Ca'Foscari University and INSTM-RU Venice, Via Torino 155, I-30172 Venice, Italy
jDipartimento di Chimica Industriale “Toso Montanari”, viale del Risorgimento 4, Bologna 40136, Italy
kCenter for Chemical Catalysis-C3, University of Bologna, viale del Risorgimento 4, Bologna 40136, Italy
lRWTH Aachen University, Institute of Technical and Macromolecular Chemistry ITMC, Worringerweg 2, D-52074 Aachen, Germany
mCNRS, CIRED - Centre International de Recherche sur l'Environnement et le Développement, 45bis Avenue de La Belle Gabrielle, F-94736 Nogent-sur-Marne, France
nUniv. Grenoble Alpes, CNRS, CEA, IRIG, Laboratoire de Chimie et Biologie des Métaux UMR-5250, 17 rue des Martyrs, F-38054 Grenoble, France
oConsejo Nacional de Humanidades, Ciencia y Tecnología, CONAHCYT, Mexico
pLaboratoire d'Électrochimie Moléculaire, LEM UMR 7591, Université Paris Cité, CNRS, F-75006 Paris, France
First published on 2nd May 2024
Abstract
At the energy-chemistry nexus, key molecules include carbon dioxide (CO2), hydrogen (H2), methane (CH4), and ammonia (NH3). The position of these four molecules and that of the more general family of synthetic macromolecular polymer blends (found in plastics) were cross-analyzed with the planetary boundary framework, and as part of five scientific policy roadmaps for the energy transition. According to the scenarios considered, the use of some of these molecular substances will be drastically modified in the coming years. Ammonia, which is currently almost exclusively synthesized as feedstock for the fertilizer industry, is envisioned as a future carbon-free energy vector. “Green hydrogen” is central to many projected decarbonized chemical processes. Carbon dioxide is forecast to shift from an unavoidable byproduct to a valuable feedstock for the production of carbon-based compounds. In this context, we believe that interdisciplinary elements from history, economics and anthropology are relevant to any attempted cross-analysis. Distinctive and crucial insights drawn from elements of humanities and social sciences have led us to formulate or re-raise open questions and possible blind-spots in main roadmaps, which were developed to guide, inter alia, chemical research toward the energy transition. We consider that these open questions are not sufficiently addressed in the academic arena around chemical research. Nevertheless, they are relevant to our understanding of the current planetary crisis, and to our capacity to properly assess the potential and limitations of chemical research addressing it. This academic perspective was written to share this understanding with the broader academic community. This work is intended not only as a call for a larger interdisciplinary method, to develop a sounder scientific approach to broader scenarios, but also – and perhaps mostly – as a call for the development of radically transdisciplinary routes of research. As scientists with different backgrounds, specialized in different disciplines and actively involved in contributing to shape solutions by means of our research, we bear ethical responsibility for the consequences of our acts, which often lead to consequences well beyond our discipline. Do our research and the knowledge it produces respond, perpetuate or even aggravate the problems encountered by society?
1. Introduction
1.1. Anthropocene: the overarching context
The term Anthropocene, popularized by Paul J. Crutzen (an atmospheric chemist) around 2000,1 is becoming established both among scientists and the wider public. It reflects how Earth's biochemical processes have been profoundly altered by humanity's way of inhabiting the environment (housing, agriculture, industry, transportation, etc.). The effects extend to the composition of the Earth's crust and its atmosphere. Consequently, our activities have become an Earth-altering force of geological magnitude. The term was devised to refer to our current age, considering that the Earth had moved beyond the stable periods of the Holocene (the temperate period of the last 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years, following the last Pleistocene glaciation) and the striving age of humanity (with the beginning of agriculture around 11
000 years, following the last Pleistocene glaciation) and the striving age of humanity (with the beginning of agriculture around 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years ago). The Anthropocene age is characterized by fluctuations and uncertainty, which are further exacerbated by the “great acceleration” suggested by the analysis of several socio-economic and Earth Systems trends performed by scientists from the Stockholm Resilience Center.2 With others, P. J. Crutzen and W. Steffen contributed to the definition of the “planetary boundaries”-framework aiming to assess the extent and consequences of our impact on nine Earth system processes.3–14
000 years ago). The Anthropocene age is characterized by fluctuations and uncertainty, which are further exacerbated by the “great acceleration” suggested by the analysis of several socio-economic and Earth Systems trends performed by scientists from the Stockholm Resilience Center.2 With others, P. J. Crutzen and W. Steffen contributed to the definition of the “planetary boundaries”-framework aiming to assess the extent and consequences of our impact on nine Earth system processes.3–14
The challenges posed by the Anthropocene will need to be addressed, and science, including chemistry, is expected to be part of this process. Indeed, options for change, and their implementations to help either avoid or mitigate the multidimensional ecological crisis humanity faces will need to be scientifically informed. Among the pressing open questions, scientists must provide new knowledge to help the deployment of solutions to achieve an equilibrium between our energy and resource consumption and the planet's human habitability. Several sustainability-driven systemic frameworks have been proposed for chemistry, e.g., the green and sustainable periodic table,15 one-world-chemistry,16 or “circular chemistry for circular economy”.17 Implementing sustainability has also been advocated for in chemistry education, through the teaching – among other principles – of systems thinking.18–20
In this paper, we particularly explore the importance of Anthropocene-spurred interdisciplinary connections between the social sciences, chemistry and the humanities as part of the search for the sustainability-driven balance at the chemistry-energy nexus.
1.2. About the authors
Chemistry's connections with ethical, social, economic, and political aspects have always been present, since chemistry both shapes and is being shaped by them. At the same time, these connections become more explicit in the Anthropocene, since the Anthropocene questions the roles of human societies in natural dynamics. The Anthropocene therefore explicitly calls for a dialog between, and even an interweaving of, the social and natural sciences in order to scientifically consider the state of the planet and its future course.Since interweaving social and natural sciences is a defining stance of this manuscript, it seemed necessary to adopt aspects of the social sciences in our writing, even though they are rarely used in the natural sciences. We will therefore start by stating “from where we write”. Indeed, social scientists have demonstrated that scientific discourse – including that in the natural science field – is rarely neutral. Despite all the methodological precautions, all scientific discourse is historically and culturally situated.21,22 This contrasts with the idea of science as neutral and objective, which is a recurrent trope in the natural and physical sciences. Therefore, as a prelude to any attempt at interdisciplinarity of chemistry-centered academic research, it seemed necessary to make this teaching our own, and to specify aspects of our own situations and backgrounds (details in ESI, Section SI-1.1†). Briefly, we are a group of scholars with different profiles (age, career trajectory, nationality) and disciplinary backgrounds, including chemistry, economy, history, and ethics. We share a common viewpoint, that a narrow disciplinary approach cannot successfully guide chemistry-centered research in the context of the Anthropocene due to the complexity of the associated interdependencies. This shared viewpoint led us to formulate two questions: do we consider that the way chemists are called upon to work on the energy transition by some, if not most, leading research-shaping authorities satisfactorily aligns with Anthropocene-related challenges? Can interdisciplinarity help us to shape tools to answer this question?
1.3. Outline and scope of the paper
After the introduction, the paper is developed in three sections. In Section 2, elements of the planetary framework are presented through five representative molecular substances: carbon dioxide (CO2), hydrogen (H2), ammonia (NH3), methane (CH4), and (bio)polymers. For each of these substances, Section 3 presents an analysis of a selection of recently published scenarios produced by leading organizations guiding policy makers in Europe, where most of the authors are based (more details in the ESI, Section SI-1†), and at the global level. These include scenarios by the IPCC (Intergovernmental Panel on Climate Change),25 the IEA (International Energy Agency),24 Dechema,25 Sunergy,26 and Shell.27 Section 4 builds upon a selection of insights provided by other disciplines, in particular drawing in the social sciences and humanities (history, economics, political science, ethics), to highlight some limitations that emerge when attempting to bring together the elements presented in the two previous sections. We then conclude and share our current perspectives on the topic.Our focus on the chemistry-energy nexus, which is our main center of academic expertise, necessarily obscures other aspects for which chemistry is central. Indeed, we make no claims to exhaustivity nor to have selected the most important possible subfields. At the same time, this approach allowed us to define a perimeter, the energy transition, which attracts or even monopolizes a large segment of public and scientific discourse, thus justifying the scope, relevance, and timeliness of this manuscript. Our paper is intended to all scientists not familiar with transdisciplinary research: PhD students, early career researchers and more senior scholars, in chemical sciences or trained in other fields and working on the energy transition, who do not regularly have opportunities to experience what substantial integration of other disciplines can bring to their own research. Our aim is to share with our peers some of our thoughts and concerns about the transdisciplinary challenges associated with our research.
2. Five sample molecular substances at the energy/chemistry/planetary boundaries nexus
2.1. Why these five molecular substances?
The nine Earth system processes and their Planetary Boundaries (PB) scientific framework was developed in tight association with the Anthropocene concept in 2009 by authors from the Stockholm Resilience Centre and others. These PB correspond to quantitative limits associated with essential Earth system processes.4 The framework attempts to define quantitative boundaries within which humanity can continue to evolve safely in the long-term with the stability that characterized the Holocene. PB have been quantified for many years for seven of these nine Earth system processes3–11 – freshwater use, land-system change, biosphere integrity, climate change, stratospheric ozone depletion, ocean acidification, and biogeochemical flows – and are updated regularly,12,13 whereas estimation of the remaining two – atmospheric aerosol loading and novel entities – has only started recently.12,14 All these Earth system processes have aspects which are at the heart of the chemical-energy nexus and are therefore closely related to the chemical sciences.Here, we have chosen four molecules to focus on: carbon dioxide (CO2), hydrogen (H2), methane (CH4), and ammonia (NH3). We selected these molecules not only because their consumption and production are central to the fabric of modern society, but also because they have been repeatedly identified as key pieces of the global transition needed to wane from excessive fossil resources consumption. They are projected to fill crucial niches in alternative carbon-free or carbon-neutral processes. As such, they appear central to most energy-driven transition scenarios proposed by governments, scientific consortia, and companies in their roadmaps for the coming three decades, all of which emphasize the objective of “Net-Zero-Emissions by 2050”, as called for by the COP2021 Paris agreement,28 and the United Nations.29 We have added to the four molecules listed above the broader family of synthetic macromolecules represented by polymer blends found in plastics. According to the IEA, “petrochemicals are rapidly becoming the largest driver of global oil demand”,30 and nowadays, 90% of these petrochemicals are used for the synthesis of polymers.31 Plastics are thus currently tightly dependent on fossil resources: their inclusion in our scope aimed at introducing an indicator of fossil-based chemical production not directly embedded in energy-driven scenarios (Fig. 1).
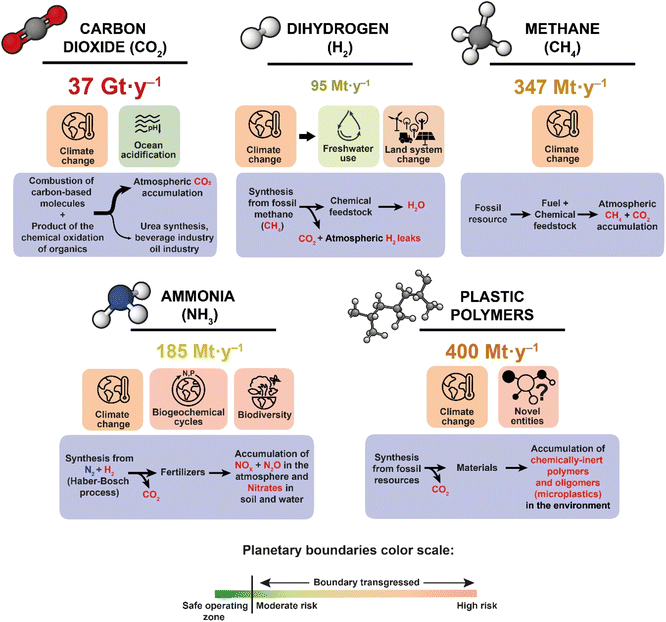 | ||
| Fig. 1 The four molecules (CO2, H2, CH4 and NH3) and plastic polymers, chosen here as illustrative molecular substances sitting at the crux of the chemistry-energy nexus. For each of these substances, are reported: their current annual global production (in 2022), and the main Earth system processes with which they interact. In the case of dihydrogen, the shift in affected Earth system processes entailed by a shift towards production of “green hydrogen” (see Scheme 1 for definition) is materialized by an arrow. The current level of transgression of each planetary boundary estimated in literature is represented by its color, according to the caption at the bottom of the figure. Finally, current mode of production, main usage, and issues associated with end-of-life considerations are described in the blue boxes. More details in text and in ESI, Table SI-3.† | ||
Below, we present an overview of the space occupied by these five molecular substances in the current global chemical landscape, in particular in relation to energy, and how they can be linked to the Planetary Boundaries framework.
2.2. Carbon dioxide
Anthropic CO2 overwhelmingly remains a waste product of oxidative fossil fuel use as a heat source, or for other energy generation. Four sectors were responsible for 94% of the 36.8 Gt of CO2 released worldwide in 2022: power generation (14.65 Gt), industry (9.15 Gt), transportation (7.98 Gt), and building (2.97 Gt).32 Among industrial processes, most emissions are linked to the production of cement, iron, and steel. In 2022, 4.16 Gt of cement were produced,33 releasing 2.4 Gt of CO2 into the atmosphere (0.58 t of CO2 produced per ton of cement).34 During the same year, 1.88 Gt of steel and iron were produced,35 associated with 2.6 Gt of CO2 (1.41 t of CO2 per ton of steel).36 Chemical and petrochemical production emitted 1.33 Gt of CO2 in 2022.37 This included the production of ammonia (419.80 Mt of CO2 emitted), methanol (261.25 Mt of CO2 emitted), and other high-value chemicals (i.e., ethylene, propylene, benzene, toluene, xylenes, 255.29 Mt of CO2 emitted in total).38 Although the chemical sector ranks third in terms of direct CO2 emissions (25% due to processes, 75% due to fuel combustion), it is the primary consumer of energy: 50% of primary petrochemicals are used as actual energy-source to fuel the processes, and 50% are used as feedstock – and are thus no longer available for energy generation.The current main uses of CO2 as a feedstock are for food and beverage purposes (230 Mt per year),39 enhanced oil recovery (80 Mt per year),39 and in the synthesis of key compounds: urea (130 Mt per year), methanol (2 Mt per year), salicylic acid (30 kt per year), and cyclic carbonate (40 kt per year).26 Overall, these processes consume up to 422 Mt per year, which is only slightly more than 1% of the annual anthropogenic CO2 emissions.
2.3. Hydrogen
Historically, large-scale hydrogen use was first developed in the 18th century with aerial transportation (Zeppelin airships). It expanded in the 20th century as part of massive industrial processes (mainly ammonia and methanol production and oil refining, Fig. 2, right), and also found niche applications in space propulsion or lubrication. Some consider the end of the 1990s as the period when the idea of a hydrogen-based society emerged, with applications expected in both transportation and energy.47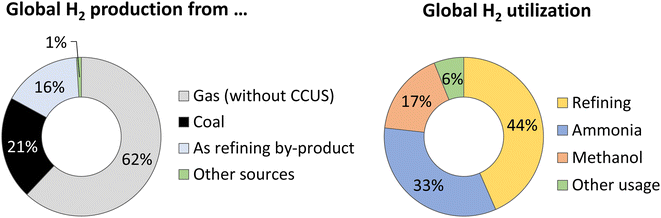 | ||
| Fig. 2 Production (left) and utilization (right) of H2 in 2022, according to IEA.48 “Other sources” in the H2 production panel include oil (0.5%), fossil fuels coupled to CCUS (0.6%), and water electrolysis (0.1%). “Other usage” in the H2 utilization panel is mostly linked to the iron and steel industry (DRI, Direct Reduced Iron). | ||
Global hydrogen production reached 95 Mt in 2022![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 of which less than 0.7% is low-emission hydrogen, i.e., mostly from fossil fuel coupled to carbon capture, utilization and storage (CCUS) technologies (0.6%), or through water electrolysis (0.1%): in 2022, only 100 kt of H2 was produced by electrolysis.48 The remaining >99% was produced through fossil fuel-based industrial processes, primarily from natural gas through steam reforming (Fig. 2, left). The demand for hydrogen is mainly related to refining processes (e.g., for the removal of sulfur from crude oil) and base chemical production, like key small molecules (e.g., ammonia), chemical intermediates (e.g., methanol), and fine chemicals (e.g., for the food and pharmaceutical industries). These numerous applications reveal how hydrogen connects fossil resources to several global production processes.
49 of which less than 0.7% is low-emission hydrogen, i.e., mostly from fossil fuel coupled to carbon capture, utilization and storage (CCUS) technologies (0.6%), or through water electrolysis (0.1%): in 2022, only 100 kt of H2 was produced by electrolysis.48 The remaining >99% was produced through fossil fuel-based industrial processes, primarily from natural gas through steam reforming (Fig. 2, left). The demand for hydrogen is mainly related to refining processes (e.g., for the removal of sulfur from crude oil) and base chemical production, like key small molecules (e.g., ammonia), chemical intermediates (e.g., methanol), and fine chemicals (e.g., for the food and pharmaceutical industries). These numerous applications reveal how hydrogen connects fossil resources to several global production processes.
2.4. Methane
Most of the methane currently used in the industry and energy sector is of fossil origin, as it is a major component of natural and shale gas. Between 2010 and 2021, worldwide methane consumption rose by 28% to 4038 billion cubic meters (bcm) (132 EJ, 4.3 × 1013 kW h).50 Methane is mainly used as an energy source through combustion. Compared to coal and oil, the combustion of methane is more carbon efficient: natural gas-powered plants emit about 50% less CO2 than coal-powered plants51 (see Scheme 1 for a relative comparison based on hydrogen production rather than fuel combustion).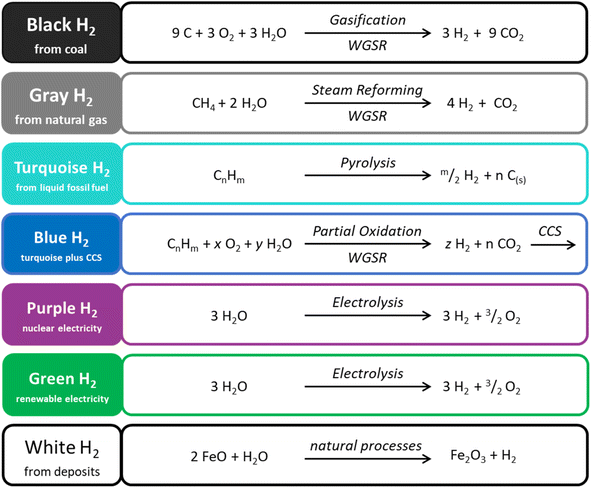 | ||
| Scheme 1 Simplified chemical reactions (only one possible representative reaction is shown) associated with current fossil-based and projected hydrogen syntheses, with CO2 emissions decreasing top to bottom and the corresponding “color” scheme used in literature. WGSR: water–gas shift reaction. CCS: carbon capture and storage.78–80 | ||
About 15% of methane is used as a chemical feedstock.52 Current upgrading routes of methane progress via steam methane reforming and the water-gas shift reaction (CO2/CO/H2 mixtures). Direct routes for methane upgrading (either under oxidative or non-oxidative conditions) have been proposed as alternatives, yet limited advances have been made in converting methane into chemicals other than syngas. Given methane's important role in the current energy infrastructure, biomass methanization processes are being developed, and other biological, photo-, electro- or photoelectro-chemical routes are explored (power-to-methane, either via direct electrolysis of captured CO2 or via the intermediate production of green H2, for example; see Scheme 1 for the definition of “green H2”).
In 2021, 144 bcm (4.7 EJ, 1.5 × 1012 kW h) of natural gas were flared: besides being a waste of fuel, flaring also releases CO2, soot, and other greenhouse gases in the atmosphere. In 2022, a 3% decrease in flaring was recorded.53 More ambitiously, the World Bank's Zero Routine Flaring by 2030 initiative,54 which is in line with the Net-Zero Emissions by 2050 scenario24 calls for a complete stop to this practice.
Large distances often separate up- and downstream processing sites of methane, necessitating extensive and vulnerable infrastructures, particularly pipelines and tankers to transport liquefied natural gas (LNG). For instance, methane emissions from local distribution in the United States have been estimated at 0.69 Mt per year.55 Methane leaks at various points in the natural gas supply chain can have detrimental environmental consequences.56
2.5. Ammonia
The Haber–Bosch process is at the root of industrial ammonia production. It converts atmospheric N2 to NH3, while consuming about 1.2% of the world's energy (about 39.3 GJ required per ton of ammonia produced). Current annual industrial ammonia production is 185 Mt.57 This represents the amount of anthropogenic elemental nitrogen that enters the reactive nitrogen (Nr) pool every year: 75–80% in the form of fertilizers and 10% as explosives. Fertilizers include urea (NH2)2CO, ammonium sulfate (AS), diammonium phosphate (DAP), monoammonium phosphate (MAP), ammonium nitrate (AN), and calcium ammonium nitrate (CAN). The nitrates among these can be further combined with phosphates to generate NPK (nitrogen, phosphorus, potassium) fertilizers. When used, urea (∼60% in N mass) will eventually release CO2 back into the atmosphere.15% in N mass of ammonia is converted to nitric acid (HNO3) through the Ostwald process, en route to nitrates.58,59 During the Ostwald process, 6–9 kg of N2O, a potent GHG (298 CO2eq.), are produced along each ton of nitric acid, although abatement measures may reduce this value down to 0.12 kg.60 This main route to nitrates which starts with dinitrogen reduction via Haber–Bosch to ammonia followed by oxidative Ostwald process is a chemical detour and thus energy intensive.
Further down the “value chain” of ammonia utilization as fertilizer, the ammonia-to-eaten food arc has a deplorable nitrogen use efficiency.61 Overall, between 86 and 96% of the nitrogen fixed as ammonia is not eaten, which represents a significant waste of energy and excess CO2 emissions during NH3 manufacturing, transformation, transport, and dispersal.62 A significant fraction of losses occurs through denitrification of NH3 to N2: the non-denitrified “run-off” nitrogen excess contributes substantially to major pollution events such as eutrophication, marine anoxia, and fine particle emissions (PM2.5, particulate matter <2.5 μm), leading to loss of biodiversity. Among these run-off nitrogen compounds, 3 to 5% of ammonia degraded through biological denitrification is released as the GHG N2O.63 In fact, if only 10% of annual ammonia production were biologically denitrified, the CO2-equivalent footprint of N2O byproducts would be comparable to that of the whole Haber–Bosch process, thus doubling the carbon footprint of ammonia synthesis.
In summary, ammonia is produced, dispersed, and chemically transformed in quantities and ways that are difficult for Earth Systems to “metabolize” without entailing severe effects on Earth system processes, causing most planetary boundaries to be transgressed.
2.6. Plastics
The definition of term “plastics” varies depending on the source.65 The term generally refers to materials which contain as an essential ingredient synthetic polymers of high molecular mass. Plastics include some of the most widespread synthetic carbon-based materials. The roughly 400 Mt of plastics produced in 2022 are mainly constituted of the following polymers: polypropylene (PP) counts for 18.9% of the production, followed by low density and linear low-density polyethylene (PE-LD, PE-LLD) with 14.1%, polyvinyl chloride (PVC) at 12.7% and high density and medium density polyethylene (PE-HD, PE-MD) at 12.2%.66 The remainder share of production is mainly constituted by polyethylene terephthalate (PET), polyurethanes (PUR), and polystyrene (PS), together with the contribution of other less common polymers. More than 90% of the total amount of polymers is obtained from the polymerization of petrochemicals and are therefore fossil-based. “Circular” plastic (i.e. recycled or bio-based) is estimated to account for the remaining 10%.66 The vast majority of plastics produced are used in the packaging industry (44%) followed by the building and construction industry (18%).67Global plastics cumulative production has risen exponentially from 1950 to 2019 (Fig. 3, left), to the point where the total amount of plastics now accounts for twice as much as the total mass of animals on the planet,68 contributing to 2020 being a landmark year: it is the date when the combined weight of man-made elements started exceeding that of all living biomass (Fig. 3, right).
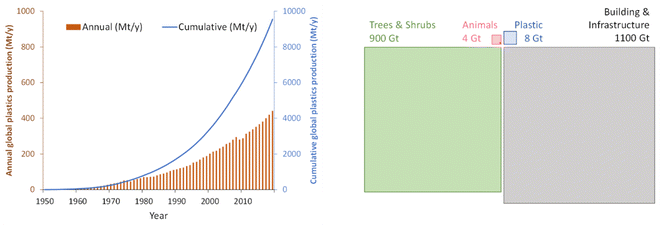 | ||
| Fig. 3 Left: annual (brown bars) and cumulative (blue line) global plastic production between 1950 and 2019 (data by OurWorldinData, Licence CC-BY).69 Right: comparison between the mass of animals (pink), trees & shrubs (green), plastic (blue), and building & infrastructure (gray). The areas of the squares are proportional to the total mass estimated for each group.68 The red dot in the “Animals” square represents at scale the mass of all human beings (ca. 0.06 Gt of carbon).70 Assumptions in mass accounting made in different studies explain the differences in absolute numbers between the left and right parts of the figure; the overall message remains the same in the two parts of the figure, with respect to the general trend discussed here. | ||
Furthermore, synthetic polymers traditionally combine high chemical stability with the absence of any natural biogeological cycle, leading to long residence times in most environments. As such, their massive introduction at accelerating rates has recently been presented as the main evidence of transgression of the “Novel Entity” Earth system process.14 Despite difficulties in accurately quantifying the PB related to this process, the claim that the boundary has been transgressed is linked to the fact that the rate of production and release of plastics is so rapid that it has outstripped our ability to assess safety and monitor adverse environmental effects. To support this, there is increasing evidence that plastic and microplastic ingestion and accumulation can lead to environmental and health damage,72 affecting the biosphere integrity and the freshwater use Earth system processes.
3. Selected scenarios and projections through the prism of these molecular substances
3.1. Selected scenarios
The five molecular substances under discussion in this article are among key levers of the energy transition. Strategies for their production or management have been the subject of modelling efforts by a wide range of institutions ranging from intergovernmental, international, national, and private bodies to industry leaders.These institutional and private stakeholders provide data and advice to policy makers, so that they can implement actions to meet the objectives and define regulations surrounding the production and use of chemicals on their territory and, by extension, globally. It is clear that many factors can influence the vision presented in these roadmaps, starting with the identity of the authors. Given our Europe-centered composition, using representative European examples, we will explore the visions most often presented to political leaders and policy makers, resulting in policy programs shaping the research objectives chemists are expected to meet.
Among the available scenarios, we selected five bodies that cover a part of this institutional diversity while largely maintaining a focus on United Nations-level, trans-Atlantic and Europe-centered institutions, organizations, and companies.
- The Intergovernmental Panel on Climate Change (IPCC):23 the United Nations body assessing the science of climate change from a global perspective. IPCC reports are based on a diverse array of data and research from member countries and aim to provide policy makers with scientific assessments of climate change and to propose appropriate mitigation and adaptation approaches.
- The International Energy Agency (IEA):24 this intergovernmental organization provides reports, data, and analyses of the global energy sector. In particular, it publishes policy recommendations to reach targets set by the political leadership of its members. It has 31 member-countries and 13 association countries, which overall represent 75% of the global energy demand. As an autonomous body under the umbrella of the Office of Community Economic Development (OECD), spurred from post-world war II Marshall plan, its membership reflects this trans-Atlantic barycenter. The current members, for example, do not include Africa, Russia, most of Asia and Middle East (Japan, South Korea, Turkey, and Israel are members).
- Dechema, the German Society for Chemical Engineering and Biotechnology:25 a German non-profit organization representing over 5000 chemists, biotechnologists, and engineers, working at the interface between academic science, industry, economics, and the general public. Dechema has notably published several roadmaps for the use of chemicals in the European Union in the coming years.
- The Sunergy Initiative26 powered by the EU-funded SUNER-C coordination and support action: this community groups together more than 300 academic and industrial stakeholders, with the aim of studying and promoting the development of breakthrough technologies to allow circular energy models at the European level.
- Private stakeholders, whose businesses involve the use or production of the substances of interest here, have regularly published data and perspectives on their activities. As a representative example, we selected the British–Dutch oil company Shell,27 which is one of the largest multinationals. Shell regularly publishes reports on the future of fossil fuel and frequently lobbies policy makers regarding regulations on fossil fuel resource extraction, management, and use.
As summarized in Table 1, these scenarios are not synoptic. Their authors have vastly different roles in society, which results in a multitude of approaches and ultimately different individual goals. At the same time, we posit that a dominant picture for each of the five molecular substances described above can be proposed from a comparative analysis of these scenarios. While nuances or even differences exist between these scenarios, our goal is precisely to explore the possibility that a shared picture can be extracted, which surpasses the fragmented vision offered by focusing on the specificities of each scenario.
| IPCC | Dechema | Shell | IEA | Sunergy | |
|---|---|---|---|---|---|
| a NPK: nitrogen, phosphorus, potassium fertilizer; Nr: reactive nitrogen; TRL: technology readiness level; CCUS: carbon capture, utilization, and storage; STEPS: stated policies scenario; APS: announced pledges scenario; NZE: net-zero-emissions; DAC: direct air capture; SNG: synthetic natural gas. See Scheme 1 and Fig. 4 for definitions of “green H2” and “green NH3”. | |||||
| CO2 | - 1.5–2 °C warming is inevitable, measures should aim to manage this new reality | - Zero emissions 2050 | - Net-zero 2050, 50% reduction by 2030 | -3 scenarios: (1) STEPS: 32 GtCO2 per year in 2050, +2.5 °C. (2) APS: 12 GtCO2 per year in 2050, +1.7 °C. (3) NZE: 0 Gt per year in 2050, +1.4 °C | - CO2 as a feedstock for localized on-demand production of chemicals |
| - CCUS is a potential solution but with its own negative environmental impact | - Point sources (via CCUS) and biomass as dual feedstocks for industry | - Switch to renewables, geological storage and reforestation | - Need to decouple CO2 emissions from chemical value chains, requires massive, immediate investments | - Sourced from DAC | |
| -4 scenarios described | -0.4 Mt CO2 in CCS in 2021, up to 25 Mt in 2035 | - Notes huge discrepancy between required and realized CCS capacity | - DAC currently not at CCUS level, low TRL | ||
| H2 | - Low-carbon hydrogen required (e.g., electrolysis of water) | - Hydrogen from different types of electrolyzers | - Investment in increased H2 production | - H2 mostly produced by electrolysis of water, but also from fossil resources, coupled with CCUS | - Solar to hydrogen technologies, photo(electro)chemical technologies |
| - Low-carbon hydrogen as an energy carrier when electrification not possible | - Poor electrolyzer scaling makes direct large-scale industrial use difficult due to land occupancy | - Maritime and terrestrial transportation | - Low carbon H2: 90 Mt in 2030, and 450 Mt in 2050 | - Water electrolysis | |
| - Development of refueling stations | |||||
| CH4 | - CH4 fugitive emissions from transportation of fossil fuels could be avoided to reduce the GHG emissions linked to CH4 | - Potential use as H2 vector if methane is green, but CH4 pyrolysis is low TRL | - Reduction of methane emissions and methane flaring | - All non-emergency flaring must cease by 2030 | - CH4 as a solar fuel |
| - SNG as potential energy storage for renewables (CO2 hydrogenation) | - Biomethane production to double by 2025, either by biogas upgrading or gasification of biomass | - DAC and subsequent upgrading of CO2 to CH4 using solar energy | |||
| - SNG synthesis has to deal with H2O byproduct and energy costs | - Beneficial to US and Europe with well-established gas grids | ||||
| NH3 | - Reduction of fertilizer consumption through innovation and best practice | - Production in current infrastructure but with green H2 | - Use as maritime fuel | - Emissions from NH3 synthesis need to drop despite growth in demand | - Precision farming to reduce Nr pollution |
| - NPK recovery from wastewater | - Most important lever is cost of “green” (as in CO2-neutral) energy | - Envisions NH3 as low-emission H2 fuel | - Multiple “green” pathways toward NH3, but all low TRL | ||
| - Will consume 25% of “greener” H2 in 2050 | - Requires significant improvements to Haber–Bosch to become viable | ||||
| - NH3 and H2 make up 45% of energy use in shipping 2050 | - Emphasis on decentralized NH3 | ||||
| - H2/NH3 for seasonal storage of renewables, NH3 to transport H2 by sea | |||||
| Plastics | - Switch from fossil to biomass as carbon source | -4 scenarios for more sustainability: 1) CO2 to MeOH. 2) from biomass. 3) circular economy. 4) CO2 copolymers | — | - Switch toward biopolymers to improve footprint | - Incorporation of bio-based feedstocks into chemical industry |
| - In addition: Improvements in recycling | - Main emphasis on recycling of existing polymer | - CO2 as building block for polymers | |||
| - Highly durable biopolymers act as a carbon-negative technology | |||||
3.2. Dominant projected future for carbon dioxide
All scenarios consider anthropogenic CO2 emissions to be the main cause of climate change, and share common views of future CO2-related technologies. To reduce the concentration of CO2 in the atmosphere it should be captured either from air (Direct Air Capture, DAC) or from point sources, where CO2 is very concentrated (e.g., industrial exhaust fumes). The captured CO2 should then either be used as a starting material to produce fuels and primary chemicals (CCU, Carbon Capture and Utilization), or stored through geological storage or mineralization (CCS, Carbon Capture and Storage). All published scenarios share a demand for large-scale and low-cost “green energy” to power these processes, “green energy” ranging from energy from renewable sources (solar, wind, …) to energy production routes with lower carbon impact with respect to current dominant ones.Potential solutions (carbon storage vs. carbon utilization) vary between the different authors involved – academia, policy makers or industry. As CO2 is the key driver of climate change, the IPCC proposes a 2030 goal of a 45% reduction in CO2 emissions. Shell sees CO2 as an unavoidable byproduct of energy generation: to maintain the level of production of (petro-sourced) energy, Shell suggests that the emitted CO2 should therefore be captured and kept in geological storage by the (petro)chemical industry itself. The required CCS (Carbon Capture and Storage) technologies should be powered by wind and solar energy, to decrease total CO2 emissions by 50% in 2030.27 The other scenarios analyzed here – Dechema and Sunergy – focus on either CO2 captured from air or sequestered from point sources and its use as a key feedstock in the chemical value chain. To allow the maturation of the novel required processes, the significant emission-reduction goals in their scenarios are set only after 2030.25,26
Below we will discuss technologies directly related to CO2, although some of them rely on other processes such as the production of “green” hydrogen (see Scheme 1) or the generation of decarbonized electricity. The level of detail available on the technologies to be promoted differs depending on the scenario.
Sunergy has reviewed various solutions for carbon capture. The most mature technology is amine-based CO2 absorption: large plants are expected to capture from a point source up to 0.4 MtCO2 per year at an energy cost of about 3.5–3.8 GJ/tCO2, and an economic cost of less than 50 € per tCO2. Higher modularity could be achieved by implementing membrane adsorption.26 Direct air capture (DAC) is also possible, but poses the additional problem of separation and purification of the low-abundant CO2 in air, if it is to be subsequently exploited. Today, each ton of CO2 treated by DAC costs €300–600 and consumes 5–9 GJ of energy. Nevertheless, the IEA estimates that following scale-up and optimization, DAC costs could fall to less than 100 € per tCO2.24 Sunergy also proposes a long-term solution based on the direct capture of CO2 from the air in small-scale, decentralized plants.26 Dechema, in contrast, considers harnessing point sources to be the most appealing strategy as it would allow easier capture of highly concentrated CO2 streams.25
The scenario proposed by the IEA involves large-scale facilities capable of capturing about 1200 MtCO2 per year in 2050. In 2022, about 45 Mt of CO2 were captured, and based on the TRL of future planned plants, the IEA estimates that about 390 Mt year could be captured in 2030, i.e., less than a third of the Net Zero Emissions, NZE, goal.74 Carbon dioxide removal technologies would only have a significant impact if emissions could be first reduced to ∼10–20% of their current levels.75
While Dechema sees CCU as a technology to be integrated into existing large-scale industrial infrastructure, Sunergy aims to decentralize the entire value chain, thanks to small-scale DAC setups with subsequent valorization via microplants producing chemicals for local needs. Sunergy also presents various strategies to exploit CO2, starting with biohybrid systems, involving biocatalytic conversion (e.g., by bacteria or enzymes), and extending to electrochemical CO2 reduction, powered by green electrons from solar energy, or, ideally, artificial photosynthesis and photoelectrochemical conversion of CO2. Once again, the TRLs of the solutions proposed by Sunergy range from low to medium, which means that they may not be ready to implement in time to address the most urgent challenges.26 This concern is shared by the IEA scenario,24 for which more than 60 of the required technologies are at the prototype or demonstration stage. In the long-term, Sunergy aims to harness processes encompassing both CO2 capture and its conversion. Direct air capture and conversion (DACC) would selectively yield valuable chemicals – such as syngas, hydrocarbons, or methanol – through the formation of carbonate or carbamate intermediates. Thermally-powered DACC to syngas is envisioned by 2030, with a photoelectrochemical process projected by 2040. The ideal processes would be insensitive to oxygen and nitrogen present in captured air, to allow immediate conversion of CO2 without the need for its purification from the air stream – which would have advantages in energy terms.26
Notwithstanding its possible limitations,76 the concept of the carbon footprint – the analysis of the positive or negative emissions caused by chemical processes – has become one of the major criteria by which the sustainability of chemical processes is assessed. This choice affects the four other molecular substances considered here (H2, CH4, NH3, plastics), and drives research in academia and industry into modifying processes accordingly.
3.3. Dominant projected future for hydrogen
Hydrogen is presented as one of the most promising short-term fossil-free and carbon-free energy carriers in all the scenarios studied. With a stored energy per weight of 120 MJ kg−1, it surpasses all other chemicals (e.g., 50 MJ kg−1 for methane). This has led to massive policy incentives for production of so-called green H2 at individual country and international levels.The next section will help clarify the chemical definition of the color associated with hydrogen. More broadly, the term “green” will be used hereafter with the same ambiguity found in common literature: while it can, in some context, be associated with the idea of sustainability and absence of detrimental environmental impact, the term green can also more prosaically mean less impactful than current routes (mostly in terms of overall CO2 emissions, ideally tending toward CO2-neutrality). Water electrolysis powered by renewable electricity is the current technology of choice for green H2 production. Thus, the European Strategic Roadmap envisions a rapid increase in green H2 production, from 6 GW to 40 GW of available electrolyzer power in the 2024–2030 period.77 Some further details on green H2 production infrastructure technologies are reported in ESI, Section SI-4.† Importantly, beyond its potential role as an energy vector, H2 remains a central reducing chemical involved in a multitude of large-scale chemical processes (see Fig. 2), for which a switch to green H2 could directly reduce CO2 emissions.
As of yet, production of green hydrogen (median cost 3.64 $ per kg) is more expensive than production of gray hydrogen (median cost 1.66 $ per kg for steam reforming of methane without CCUS).81 Several projections phase out gray hydrogen by 2050,82 but with non-fossil H2 production remaining below 50% of total hydrogen production. The scientific literature does not unanimously agree on the benefits of transitioning through blue H2 due to limitations in CCS technologies (see Section 3.2), and arguments have been advanced for greater investment in renewables (i.e., green hydrogen) in the midterm.81
The IEA roadmap24 anticipates an explosive rise in blue and green H2 demand from about 0.3 Mt in 2021 to about 420 Mt in 2050, with 13% of this demand coming from the energy sector, 30% from industry, 26% from transportation, and 26% for use as feedstock in the production of other low-carbon fuels. In this scenario, hydrogen would represent a 20% share of the yearly global energy supply. This scenario contrasts with another projection set out in the same IEA report, the Stated Policies Scenario (STEPS), which is based on a trajectory modeled on today's global policy settings. The latter predicts a lower demand for blue and green H2 of only 24 Mt by 2050. This illustrates the gap to be bridged if we are to transition to a green hydrogen economy capable of net-zero CO2 emissions. For comparison, current demand for H2, which is met by gray H2, is about 95 Mt.
As the electrification of the chemical industry progresses, the IPCC scenarios envisage hydrogen primarily as an energy carrier. The limited development of a hydrogen infrastructure, compared to the electricity grid, supports this trend. Nevertheless, green hydrogen may be adopted by some large industrial sectors, for example as fuel for furnaces in the glass industry, which need very high temperatures and stable operation conditions.86
Overall, the role of hydrogen as a fuel in the IEA global energy picture is projected to have a relatively low impact. Thus, in the NZE 2050 scenario, that is “Net Zero Emissions by 2050” scenario (one of the more optimistic scenarios when it comes to fossil resource and H2 use), H2 will account for a total share of roughly 6% by 2050.87
3.4. Dominant projected future for methane
Methane is attributed a particular role in energy transition in cases where existing infrastructure can be easily switched from coal to gas.90 In the Net-Zero-Emission scenario by 2050, a 75% reduction of energy-related emissions is projected by 2030, down to 30 Mt, and reaching 10 Mt in 2050. Indeed, due to the increased global warming potential of methane (80 times that of CO2 in a 20 years timeframe), its emissions must be substantially reduced in the near future by diminishing fossil fuel consumption, applying new emission-reduction measures throughout the fossil technology supply chain,91 and implementing leak-detection programs impacting on fossil methane's climate footprint.90 Measures to reduce these leaks and rapidly phase out all non-emergency flaring are included in all the scenarios, as they address both the strained gas markets (prone to geopolitical tension) and the need to reduce GHG emissions. According to Shell Sustainability Report 2021,92 routine flaring contributed to 7% of Shell's global GHG emissions in 2021 (4.5 MtCO2eq.); and it was reduced by 33% from 2020 to 2021 (0.2 Mt).Methane could be used as a low carbon-footprint feedstock for hydrogen production during its controlled thermal decomposition.25 Synthetic natural gas (SNG) production from CO2 could be considered as a means to store energy, if green H2 is employed to reduce CO2. Using CO2 in this way could help with the transition from fossil fuels to sustainable and renewable fuels, in accordance with the Paris Agreement and the SUNRISE Technological Roadmap for the development of negative emissions technologies (NETs), which are supposed to be optimized by the 2030s.26
Methane and fuels in general could also be produced by reducing CO2 in photoelectrochemical devices, which should allow a more versatile, local, and thoughtful on-demand production. Working with autonomous systems that only depend on sunlight would obviously represent a great advantage.26
Even though in 2020 biomethane only accounted for 0.1% of methane demand, sustainable biomethane production could be a turning point in the context of circular use of energy resources. Since it is chemically equivalent to fossil-derived methane, biomethane can be used directly to produce electricity and heat, and is fully compatible with natural gas infrastructures and gas-powered vehicles.94 Around 60% of plants are currently operational or under conversion to include biomethane in the gas distribution network.94 Biomethane combines the advantages of natural gas with a lower carbon footprint.90 It is thus a valuable tool to harness agricultural/municipal waste, since it can generally be produced in small-scale plants located near the end-use site. Adoption of biomethane could require a drastic modification of the distribution network to allow for the injection of excess biomethane produced in summer into the underground reserves as well as a change in production scale, from large to smaller, in particular for rural and farming communities. Such a change could limit transportation issues and geopolitical tensions. Hence, the benefits of biomethane might extend even beyond the defossilization issue.
Current worldwide bio-LNG and synthetic LNG production is 2.2 bcm per year (0.07 EJ per year, 2.3 × 1010 kW h per year). Biomethane potentials are in fact much higher and, in principle, could provide nearly 60 bcm per year (2 EJ per year, 6.3 × 1011 kW h per year) in the EU-27 for production only through thermal gasification.95 This value has almost doubled compared to the data provided for 2019, in line with the REPowerEU 2030 target plans.96 Biomethane potentials in EU-27 by 2030 are estimated at 41 bcm per year (1.3 EJ year, 4.3 × 1011 kW h per year), considering both biomethane produced from anaerobic digestion and from thermal gasification.96 Even if biomethane is more expensive than natural gas, its cost-effectiveness and competitiveness could increase over time. Nevertheless, it is quite difficult to evaluate the CO2 footprint of biomethane because it depends on the region, type of biomass and technology used. The IEA Sustainable Development Scenario underlines that consuming biomethane will avoid the emission of around 1000 Mt of GHG in 2040, including CO2 emissions related to natural gas and CH4 emissions from biomass decomposition.90
3.5. Dominant projected future for ammonia
The amount of anthropogenic reactive nitrogen species (Nr) has increased constantly since the Haber–Bosch process was initially developed in the early 20th century as a result of its use for agriculture (see Section 2.5). Production will accelerate in the near future if NH3 use is extended to energy applications.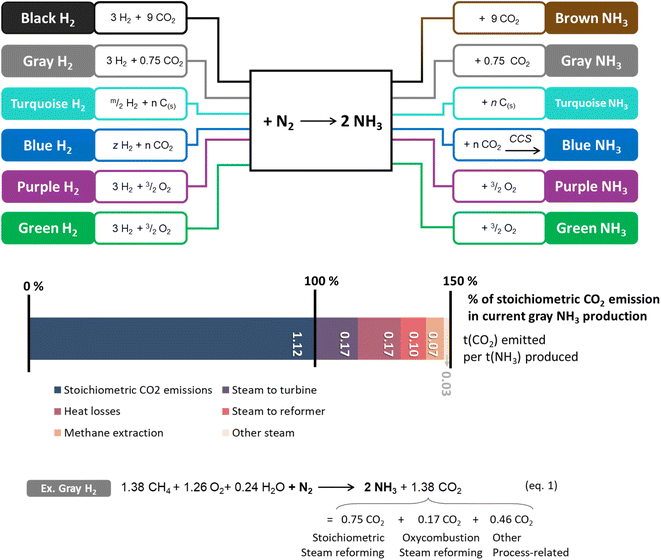 | ||
| Fig. 4 Upper panel: schematic representation of current fossil-based and projected ammonia synthesis processes with decreasing CO2 emissions (top to bottom) and respective ammonia “color”. The CO2 reported is only the stoichiometric quantity. See Scheme 1 for hydrogen syntheses. Since in ammonia plants, on-site hydrogen production is integrated with ammonia synthesis, the two hydrogen routes in Scheme 1 and here differ. Lower panel: differences between stoichiometric quantity (blue) and additional process-related CO2 emissions (other colors) expressed in tCO2/tNH3 in modern optimized methane-powered Haber–Bosch processes for gray ammonia, leading to overall eqn (1) (see more on eqn (1) in Scheme SI-1†).98 | ||
Therefore, overall efficiencies do not leave much room for improvement on that front, and indeed they have remained largely constant over the last two decades. In fact, most of the carbon footprint of the Haber–Bosch process is linked to H2 production via natural gas reforming (Fig. 4).97 A modern methane-powered Haber–Bosch process generates at best around 0.6 tNH3/tCO2. It is less in practice, resulting in effective global emissions of 450 Mt per year of CO2. This makes current industrial ammonia a fossil chemical in all but name.
Current decarbonation approaches are based on the deployment of carbon dioxide capture and storage technologies at ammonia plants (blue ammonia) or use of decarbonized H2 from water electrolysis powered by renewable electricity (green ammonia) (Fig. 4).
3.6. Dominant projected future for plastics
In the roadmaps studied here, the impact of plastic production and use in relation to Earth system processes is almost exclusively tied to the high CO2 emissions generated by the production of monomers by oil cracking in refineries. To mitigate this issue, three main low-carbon-emission circular alternatives are advanced. (i) Producing bioplastics from biomass, the largest reservoir of contemporary carbon, by breaking down and refining biopolymers into biosourced monomers. Dechema suggests that this path should be preferentially followed to obtain monomers that maintain the functional units of the feedstock molecules, (e.g., oxygen-rich and carbonated molecules), rather than hydrocarbons such as ethylene or propylene, due to the low efficiency of biomass use for the production of the latter. Biomass is also recommended for the production of methanol and ethanol for further exploitation. (ii) Using CO2 and green hydrogen as feedstock to produce syngas, followed by Fischer–Tropsch inspired production of olefins. This route should reduce CO2 emissions, but can be energy intensive. (iii) Alternatively, CO2 can be hydrogenated to produce methanol, which can then be converted into light olefins or aromatic compounds through established catalytic “methanol-to-olefins” and “methanol-to-aromatics” processes.The European Green Deal includes a plan on increased regulations regarding the use and production of microplastics, and highlights the need for risk assessments to include the entire life cycle of materials, which is relevant to remaining within the planetary boundary related to Novel Entities. Importantly, the EU also voiced concerns with the global trade of plastics, and in particular the export of plastic waste. This strategy has led to plastic waste accumulation in landfills in South-East Asia, progressively contaminating freshwater sources, and ultimately large ocean areas in the form of a loosely agglomerated floating mass three times the size of France. This mass was infamously named the “seventh continent”.100 A recent European Green Deal101 act implements stricter measures on plastic consumption, adopting a reduce-reuse-recycle strategy. Attention is drawn to a more transparent labeling system, especially with regard to the use of “bioplastics” terminology, making consumers active players in the energy transition.
Among the scenarios analyzed, the main solutions proposed for the manufacture of sustainable and defossilized plastics include the macro-topic of bioplastics to replace conventional plastics and the use of CO2 to produce building blocks as part of a sustainable polymer industry. Less emphasis is placed on end-of-life of synthetic polymers, which is linked to the issue of environmental accumulation. When tackling this aspect, scenarios often encourage investment in the recycling chain, and only touch on biodegradability, even though this is a hot topic in polymer research. Furthermore, replacement of conventional plastics with biodegradable plastics has been proposed or is under implementation in several countries.102
3.7. Conclusion on the scenarios section
In summary, the five European/UN/OECD scenarios reviewed share some viewpoints. The existence of climate change and its consequences are central: the aim is to develop energy systems that are more carbon-sober. This system should be scaled to respond to projected increases in demand in most economic and industrial sectors. While all the scenarios generally agree that the energy transition envisioned should be powered by low-carbon electricity, different technical solutions, or distributions over various solutions are proposed. The general consensus is that some new or currently low-maturity technologies will be needed, and must be deployed at scale to reach some of the goals. This vision shapes most research-orienting frameworks and research-funding opportunities at the chemistry/energy nexus.Open questions remain, as pointed out in the IPCC report, for example: “most models and studies fail to address the systemic impacts of the widespread development of new technology” including “material and resources”. “Systemic solutions are also not being sufficiently discussed, such as low-carbon materials; making buildings, transport, and industrial equipment lighter-weight; promoting a circular economy, recyclability and reusability, and addressing the food-energy-water nexus”.23
4. Interdisciplinary considerations
The scenarios presented above set out scientific and technological orientations mostly informed by engineering and data processing tools (such as techno-economic analysis, life cycle analysis, etc.). In addition, the scenarios have been almost exclusively elaborated to address the transgression of the planetary boundary causing climate change, with little to no mention of possible ripple effects impacting other Earth system processes. In this section, through selected examples, we will attempt to show that extension toward other fields of knowledge (game-theory, history, ethics, ecology, earth systems, etc.) can help bring to focus aspects that the scenarios might appear to overlook but that remain relevant when seeking to assess the sustainability of the proposed research directions.We will showcase six examples related to the substances discussed above and some other underlying concepts mobilized by the energy transition/chemistry/Earth system processes nexus addressed in this manuscript:
- CO2: how (economics-derived) game-theory considerations moderate the hopes that research into the use of CO2 with a view to industrial transfer will substantially contribute to mitigating climate change.
- CH4 and H2: how political science can bring forward the tensions and potential conflicts surrounding the use of land and water resources for the deployment of methane- or hydrogen-based “energy transition solutions”.
- NH3: how Earth system analysis, based on the planetary boundaries framework, raises questions as to the suitability of ammonia as a major contributor to the energy transition.
- Plastics: how decolonial studies can bring into focus the dynamics of waste recycling at a global scale.
- Related issue of the requirement for materials: how cultural studies of science can help deconstruct part of the implicit model in the scenarios proposed, whereby access to natural resources is taken for granted.
- Within the overarching concept of the energy transition: how history raises questions on the use of the term “transition” subsuming the scenarios proposed.
4.1. CO2 as a raw material: can we really scale up?
The direct catalytic conversion of CO2 to valuable chemicals has attracted considerable research interest over the past few years.103 As detailed above, from a fundamental research perspective, like for many other challenging reactions, there is a curiosity-driven interest in understanding how such a stable and relatively inert molecule can be activated in an energy-efficient way to selectively produce carbon-based molecules.104 In a more climate-related context, any such process could also theoretically contribute to reducing atmospheric CO2 concentrations – either by replacing fossil fuels with CO2-derived “solar” and “e-fuels,” or by producing useful fossil-free carbon-based chemical – thus helping to “close the carbon cycle”.105 These approaches are often referred to by acronyms such as “CCU”, “CCUS” and “CDU” (for, respectively, “carbon capture and utilization”, “carbon capture, utilization and storage” and “carbon dioxide utilization”). Furthermore, these “emerging” technologies/processes can be viewed as possible contributors to the value chain in the chemical industry.75,106 Carbon capture technologies are thus represented both as an economic opportunity and as an environmental solution, with verification of total global warming impact provided by life cycle analysis (LCA).107Even if the current technological hurdles could be overcome, substantial economic and social obstacles might arise.108 Game-theory can be an interesting tool to help analyze and understand how decision-making is influenced on a global scale. In public economics, two criteria are generally used to characterize goods: rivalry and exclusion. Rivalry refers to the fact that a product can only be consumed once. Exclusion refers to the fact that some individuals may not have access to the market. Based on these criteria, four categories of goods are defined: private goods, club goods, common goods, public goods (Table 2).
| Rivalry | Non-rivalry | |
|---|---|---|
| Exclusion | Private goods e.g., open-market products | Club goods e.g., products accessible only to a restricted group of individuals |
| Non-exclusion | Common goods e.g., fish or game stocks in freely-accessible fishing/hunting grounds | Public goods e.g., public lighting, broadcasted radio programs, open-access scientific literature |
In this economic framework, the reduction of CO2 emissions can be considered a public good, as it counteracts a public menace – CO2 emissions – that has a negative impact on climate stability. The reduction of CO2 emissions is subject to what economists call free riding: the tendency of individuals to benefit from collective efforts without having to contribute to them. Within this analysis, to mitigate climate change, every country tends to make the minimum effort. In game-theory, economists also refer to a sub-optimal equilibrium, or Nash equilibrium: the resulting strategy is that each country keeps polluting, whereas, the optimal strategy would be to reduce emissions (Table 3).
| Relative gains | Country B reduces | Country B pollutes |
|---|---|---|
| Country A reduces | 5, 5 | 2, 6 |
| Country A pollutes | 6, 2 | 3, 3 |
Research aiming to reduce CO2 through chemical transformation could be included in these public good-oriented activities. In our current situation, it is difficult to imagine the potential scale-up of CO2-based industry. Indeed, in the case of free riding, unless the technological solutions found are price-competitive in the current (possibly subsidized, more below) market, they will not be adopted.
Even assuming an absence of free riding (if, for example, a binding international climate agreement were adopted), the success of CO2-based chemicals would not be guaranteed if fossil-based materials and fuels remained cheaper and widely available. The argument that CO2-based chemicals have a double advantage – of creating new value and reducing the amount of CO2 in the atmosphere – could be strengthened by quantifying what economists call the co-benefits of a policy. Co-benefits refer to subsidiary gains from a policy initially targeting a specific objective. In environmental policy, co-benefits are commonplace: e.g., the promotion of active forms of transport (walking, cycling) not only reduces polluting motorized traffic but also has long-term health benefits.109 In the case of CO2-emission reduction, the co-benefits of CO2-based chemicals could be measured by estimating the values of carbon emissions – there are many estimates of the social value of carbon that reflect the economic and social gains associated with reducing emissions.110 This social value could then be added to the private value of the chemicals, and this might actually reverse the co-benefit comparison relative to fossil-derived chemicals. To achieve this reversal, the co-benefits must not only be theoretically quantified, but strong political measures are needed to support them, e.g., forcing fossil-based products to bear an additional cost through a carbon tax. However, the economics literature shows that the capacity of industrial and political trade-offs to take into account the co-benefits associated with environmental measures remains weak for the moment.111 Hindrances and obstacles, including lobbying, are important, and difficult to quantify. It is therefore far from certain that the environmental co-benefits associated with the development of CO2-based chemicals would tip the balance in favor of these products, especially as a full assessment would also include potential drawbacks or hidden costs of these products, such as the risks of rent capture by a few private actors, like industrial companies capable of deploying the technology at the targeted scale, to the detriment of social welfare.
At the same time, the co-development of waste-handling strategies (e.g., geological storage, CO2-based carbon cap-and-trade programs, synthesis of stable CO2-based molecules as a form of long-term chemical storage, etc.) are already underway. The emerging players for the most part respond to the dynamics of market economies (large established industrial groups, start-up companies whose business model revolves around CCUS-related services, etc.). These players have therefore found solvent markets for their activity. Without going into the open question raised by the possible rebound effect, also known as Jevons effect, of a downstream “solution” to emissions, it can be relevant to connect that these solvent payers are mostly public bodies. The cost of such remediation actions is therefore to be borne by public assets. Noteworthy, the private profits made by the oil sector over the last 50 years is estimated at 3 billion dollars a day over 50 years,112 with no foreseeable substantial and specific redistribution of such private wealth. It is also in this context, that the interest in carbon capture and re-use policies to safeguard fossil production and trading-related profits can be analyzed, especially in the case that fossil assets were to become stranded assets due to fuel extraction bans.113
In summary, some elements of economics (like the sub-Nash equilibrium linked to the risk of cooperation failure and the fragility of schemes requiring environmental co-benefit profits), suggest that chemical research aimed at industrialization of CO2-mitigating solutions as a means to tackle climate change might face the same market-driven economic pressures as research that has no such CO2-mitigating ambitions, such as the requirement that they be cheaper, possibly after public subsidy, than fossil-based solutions in order to displace them.
4.2. Methane and hydrogen: are conflicts over control and usage taken into consideration?
The technical, social and political issues around hydrogen were already questioned by R. S. Cherry in 2004 in a paper entitled “A hydrogen utopia?”.116 Cherry stressed that most of the issues related to the development of hydrogen as an energy carrier were centered on three main topics, i.e., safety, storage and cost, while externalities (side-effects) were very rarely considered. For example, he mentioned energy equity, where remote and less populated areas may encounter difficulties in accessing a competitive hydrogen infrastructure. Indeed, most projects involving a hydrogen society focus on two major bottlenecks: economy (price of hydrogen) and technology (production efficiency). This approach masks other aspects and sources of potential conflicts that nevertheless need to be addressed. Here, we will take two examples: land use and water impact.
If 70 Mt of hydrogen were produced by PV in Europe alone, the land area required would increase to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 km2 (i.e., about 0.5% of the total land covered by EU28, or about 4% of mainland France). Despite the apparent modesty of these figures, it should be kept in mind that, in densely populated areas, or in places where agriculture and forestry are already vying for space, adding large PV harvesting stations might be problematic, and the difficulty should not be underestimated. For this reason, the Sunergy technological roadmap targets higher solar-to-hydrogen conversion efficiencies of 30%. Recent social and political setbacks in the Netherlands related to the objectives for renewable installation at the local level119 illustrate the potential conflicting situations arising from high hydrogen-production goals. Occupation of land may also lead to poor social acceptance, due to inequalities and non-inclusive development processes for renewable energies, leading to unwanted land uses, unfair compensation for expropriation or forced relocations. History shows that these would particularly affect vulnerable and marginalized communities (such as indigenous communities), and examples abound in Morocco, Mexico, and South Africa, where local communities were exposed to a power imbalance with energy developers.120 Land use is definitely a place where technology development must interact with the social sciences to ensure independent local assessment of the impact of massive renewable energy projects.
500 km2 (i.e., about 0.5% of the total land covered by EU28, or about 4% of mainland France). Despite the apparent modesty of these figures, it should be kept in mind that, in densely populated areas, or in places where agriculture and forestry are already vying for space, adding large PV harvesting stations might be problematic, and the difficulty should not be underestimated. For this reason, the Sunergy technological roadmap targets higher solar-to-hydrogen conversion efficiencies of 30%. Recent social and political setbacks in the Netherlands related to the objectives for renewable installation at the local level119 illustrate the potential conflicting situations arising from high hydrogen-production goals. Occupation of land may also lead to poor social acceptance, due to inequalities and non-inclusive development processes for renewable energies, leading to unwanted land uses, unfair compensation for expropriation or forced relocations. History shows that these would particularly affect vulnerable and marginalized communities (such as indigenous communities), and examples abound in Morocco, Mexico, and South Africa, where local communities were exposed to a power imbalance with energy developers.120 Land use is definitely a place where technology development must interact with the social sciences to ensure independent local assessment of the impact of massive renewable energy projects.
The Sunergy analysis26 – focused on Europe – calculated that if the current hydrogen production was to rely entirely on water electrolysis, this would demand 3700 TWh of electricity annually, and the freshwater requirement would amount to 630 Mt per year. This is 0.7% of the annual EU28 freshwater consumption, and remains lower (one third) than the volume of freshwater used by the service industry (2400 Mt per year). Considering that the demand for hydrogen in Europe is about 15% of the global demand, the Sunergy analysis appears to confirm that the use of freshwater is not a major barrier to the development of hydrogen through electrolysis. This figure may be underestimated: additional water may be needed due to the other processes involved in water electrolysis, such as cooling of the electrolyzers, or treatment of the input water to meet the purity standards for electrolyzers, furthermore, this is a global scale analysis, and care must be taken in areas where freshwater is not easily accessible. Desalination could be an option, and in this case Beswick et al.122 calculated that the energy and cost additions would be minimal. However, desalination adds penalties in terms of (i) CO2 emissions when the required energy is produced from fossil fuels, and (ii) brines (salt-rich mixtures potentially contaminated with chemicals), which must be disposed of in the oceans, and that may impact the marine ecosystem. Other options, still at the research level, would be to use ambient humidity for hydrogen production.123 In addition, water stress (the ratio between the fresh water withdrawals and the total renewable freshwater resources) will need to be assessed locally as it varies considerably from one country to another (Fig. 6). The IRENA (International RENewable Agency) foresees that more than 70% of planned electrolyzer projects will be in water-stressed regions, with most water provided through desalination.124 Again, conflicts of usage over water and other resources will not be easy to resolve if they arise at the local level. In 2022, social struggles over water reservoirs for agriculture125 and the cooling of nuclear plants in Southern France126 show that local obstacles due to limited water supply that the generalization of large-scale hydrogen production could induce locally should not be underestimated. The NOOR I solar thermal power plant in Ouarzazate (Morocco) is another example where the constraints on water resources were experienced as a negative impact for the local communities.127 Attempt to scale H2 production must therefore be analyzed also in the context of practices' fairness.
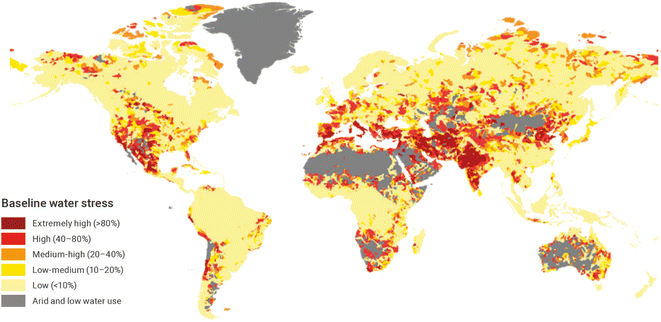 | ||
| Fig. 6 Annual baseline water stress. Source: WRI (2019). Licence Creative Commons Attribution 4.0 International (CC by 4.0).128 | ||
In summary, this quick overview of possible increasing conflicts linked to land and water usage upon deployment of scenarios relying heavily on hydrogen or methane-based solutions reveals non-zero risks of creating or reinforcing existing geopolitical tensions, as well as triggering local social unrest.
4.3. Ammonia: should a molecule which is today a major environmental burden be scaled up and become an important energy vector in the future?
Ammonia is advanced in the scenarios analyzed as a green hydrogen carrier, or even a fuel in itself, mostly for the maritime sector (see Section 3.5). Replacing the current use of diesel for shipping by ammonia would consume approximately an additional 586 million tons of ammonia per year, i.e., four times the current annual production.99 For comparison, the planetary boundary for reactive nitrogen species is estimated at around 62 million tons per year at most.12 Wolfram et al. recently pointed out that overstepping this boundary to power maritime shipping will necessarily cause major disruption in the global nitrogen cycle.99 Moreover, they estimated that if just 0.4% of ammonia fuel were converted and released into the atmosphere as N2O, it would completely offset the GHG emission benefits resulting from switching from diesel fuel. They point out that it is likely that leakage in extended NH3 supply lines will result in additional N2O emissions through natural denitrification, which will likely prove difficult to monitor and mitigate.Beyond the projected usage, currently ammonia is mainly converted to urea and nitrates, for use as fertilizers. The scenarios reviewed identify two main approaches: blue and green ammonia (see Fig. 4). These approaches consider ammonia production through the Haber–Bosch process as an item to be improved, mostly at the level of upstream CO2 emissions (see eqn (1) in Fig. 4), rather than as a major disruptor in ecological cycles. As a result, these approaches fail to address the severe ecological issues linked to disproportionate ammonia dispersion in the environment.12,129
Because of the N2O emissions linked to poor Nitrogen Use Efficiency (NUE), even just from a climate change mitigation perspective, “green and blue” approaches are not a suitable answer when the full impact is considered. While the IPCC report points toward ways of improving NUE through nutrient recycling, or changes in agricultural practices (agroforestry, cover crops, hydroponics), it also points out that climate change is having a negative effect on the protein content of crops, leading to a compensatory increase in fertilizer use.
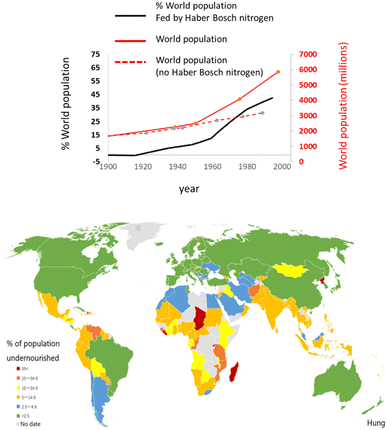
The argument that “ammonia feeds half of the world”62 is regularly used to help justify the magnitude of our current reliance on it, but the argument loses some of its power and becomes tainted with condescendence in the context of global North-South food inequality (Fig. 7). The next question could be phrased: if ammonia really does feed “half of the world”, which half would that be? This serves to exemplify the recurring deception that global world-average statistics can induce with respect to disparities, geographical or other. Meaningful disparities can become invisible under such global averages.
 | ||
| Fig. 7 Top: Evolution of world population over time (historical data) and projected world population without the development of the Haber–Bosch process, adapted from J. W. Erisman, et al.62 Right: the Food Hunger Map provided by the Food and Agricultural Organization of the United Nations.130 Global data make a clear causality link between the use of fertilizers and a growing population, but inequalities in access to food remain nonetheless. If ammonia really does feed “half of the world”, which half would that be? | ||
By questioning the chemical strategy linking ammonia to fertilizers, it is interesting to note that the reduction of elemental nitrogen (N0 in N2) to NH3 (NIII−) consumes costly dihydrogen to ultimately oxidize it to NV+ in nitrates through the NOx-emissive Ostwald process. It was the most efficient way to secure reactive nitrogen in Europe when the continent could no longer easily access South-American guano at the dawn of the 20th century.131 At the time, CO2 emissions and the downstream environmental impact of nitrogen use were almost completely ignored. But in the current framework of Anthropocene leading to the crossing of planetary boundaries related to most of the Earth system processes, this choice calls for a wider multidisciplinary assessment.
In summary, some elements taken from the scenarios for the possible role for ammonia in the energy transition combined with the current transgression in terms of reactive nitrogen release raise serious questions: Do these projections account for perturbation of the nitrogen cycle at a global scale? How could a ten-fold increase in ammonia production not have major environmental consequences, when even today – before any such increase – the environment appears severely compromised by the current production and usage?
4.4. Plastics: are we really prepared to take the consequences in our own back yards?
By contrast to CO2, which is diluted and invisible, plastic waste is more visible and easily identifiable. It has thus elicited waste-handling strategies. Even though plastic waste trade involves a complicated network, the general flow can be identified. Worldwide waste has been transferred from Europe and North America toward Asia since the late 1980s, and China's ban in 2017 shifted the destination toward Southeast Asian countries.132 This practice was considered as a win–win policy where the exporter gets rid of their waste, while the importer gains access to valuable material to enrich their economy,133 resulting in an overall saving of energy and resources.134 This type of commercial exchange, where one group's waste is sent across the world can be considered a NIMBY (“not in my backyard”) attitude according to social science. One could say that, besides the plastic waste itself, the object of the commercial transaction is to shift formal responsibility for plastic pollution. The plastic waste trade, among other things, moves the burden of responsibility from the actual users and producers of waste (exporters) to the final collector.In 2019, The European Green Deal acknowledged the issue by suggesting “that the EU should stop exporting its waste outside of the EU”. The report stated that this would be tackled by revisiting “the rules on waste shipments and illegal exports”, which by 2020 still accounted for more than 10% of all plastic waste produced (3.789 Mt out of 37.068 Mt).135 Since 2021, the shipment of unsorted and hazardous waste from the EU to non-OECD countries has been banned, and only clean, non-hazardous waste can be exported for the specific purpose of recycling.136 However, is it really sustainable, and fair, to export (even part of) our plastic waste to other parts of the planet? Regarding sustainability, the consequences of the Anthropocene will ultimately affect the global North, notwithstanding the original destination of the waste. Moreover, regarding fairness, from the importers' point of view, plastic waste trade can be considered an unwanted remnant of colonial ideology, contributing to the exploitation of populations in the global South.137
A partial solution to this situation, the third R of the “Reduce, Reuse and Recycle” strategy is to create new waste treatment facilities within our borders, on a scale compatible with the size of the problem to be treated. In other words, one more technology-intensive industrial infrastructure must be added to European material needs (steel, cement, metals, etc.) to handle its transition to better waste-handling.
The production of bio-sourced conventional bioplastics will not solve the recycling question, as these plastics are still in large part non-biodegradable and not always easy to recycle. On the other hand, a fraction of bioplastics are also biodegradable. This difference must be highlighted to avoid confusion caused by the prefix “bio”. However, biodegradability remains a general concept. Biodegradable materials are defined as those that break down into natural elements or small molecules under the influence of microbial activity in a relatively short timeframe and in naturally occurring conditions.138 Although it is mostly accepted that the ultimate products of such degradation processes are water, CO2, methane and biomass,139,140 biodegradable biopolymers are quite often only broken down into oligomers, called metabolites, which then enter the catabolism of nature.141 Assuming that the eventual biodegradation of plastics in the amounts currently produced would not cause imbalance in the ecosystem is a risky bet. The open questions on the end-of-life effects of bio-sourced plastics on the environment remain.
4.5. What does the scalability requirement say about cultural representations?
The need to scale-up emerging technologies is central to the vast majority of “Net-Zero-Emissions” roadmaps. The benefits of this intensification are clearly presented in terms of reduced GHG emissions and through the traditional levers of the economy of scale to power the energy transition and the electrification of the chemical industry, with the related costs and hurdles generally being also partly discussed.Solar and wind energy, while ubiquitous, are naturally intermittent, low-density sources of energy. Consequently, collecting and storing them will take up much more space and require far more units than current power plants.142,143 Moreover, direct electrification has been prioritized due to the excellent energy yield it offers (especially for transportation with electric vehicles). At the same time, transport and storage of electricity will require considerable construction of novel infrastructure, which explains in part the projected increases in mineral resource consumption.144 Even replacing fossil fuels with “green fuels”, such as hydrogen, relies on significant critical metal availability (see ESI, Section SI-5†). This rampant increase in resource consumption is not new. In fact, it has been a defining aspect of the Anthropocene, and it remains a dynamic on which the energy transition is being constructed. Indeed, the IEA forecasts a six-fold increase in mineral demand by 2040 to meet the target of NZE by 2050, mostly driven by steel, rare earths, graphite, copper, aluminum, zinc, cobalt, nickel, silicon, and lithium.144 The extraction of these resources is mainly performed in open-pit mines with a high land footprint and causing extensive ecosystem disruption.145–147 Moreover, extracting these non-renewable resources will itself consume significant energy, and generate substantial pollution at the site of extraction. Is it reasonable to keep labeling some scenarios as “sustainable solutions”, notwithstanding these (generally less addressed) aspects (with respect to carbon footprint, for example)?
Associated with this increased demand for mineral resources is the fact that in our current globalized world, the targeted elements from the mineral resources are often not used where they are produced. North America and Europe exhibit by far the highest domestic material consumption per capita, but Europe in particular generates the lowest amount of ore per capita, with the exception of West Asia.148 For instance, the vast majority of the world's cobalt comes from the Democratic Republic of Congo, while nickel is mainly produced in Indonesia, Iran, and the Philippines, and the biggest producer of copper is Chile.144,149 Much like with plastic waste management, the outsourcing of metal supply directly transfers pollution to places where environmental and health fallouts are less controlled and regulated, particularly in the context of the increasing development of artisanal mining operations.150–152 In addition, the scale of disruption expected for the energy transition is enormous, as fossil resource mining operations are expected to shut down, while exploitation of other resources is expected to surge rapidly.153,154 Due to the urgency imposed by the accelerating climate crisis, such a drastic and fast-paced shift is bound to lead to numerous “casualties” on the social and human front.155,156 How are the interdependencies between countries and their dramatically different circumstances taken into account? Is it a mistake to continue to adopt a neutral and universal viewpoint when describing the problems and their possible solutions? Is considering the Earth in terms of “resources” part of the problem?
Overall, it appears to us that the proposed pathways toward a low-carbon economy are essentially built on most of the same mechanisms and dominant beliefs that led to the very advent of the Anthropocene: consuming ever-increasing amounts of energy, ultimately requiring most likely increasing natural resources that are incompatible with Earth system processes, while at the same time failing to set the social implications of the desired transition as a guiding principle. It is notable that, historically, the interests of indigenous peoples have suffered as a result of large-scale resource extraction or energy production projects. There seems to be high probability of conflicts that will emerge from the intensification of resource extraction and the building of large-scale installations. Some of the places concerned are already the most threatened by the ongoing climate crisis, and socio-political problems have already started, such as forced migrations or unconsidered health problems, for example caused by mining. Is the projected energy transition really going to be “clean”? Does this transition deal with different cultural representations?
4.6. Does the notion of energy transition really make sense? And if so, how?
The common narrative associated with the history of energy transitions is a succession of dominant energy sources and regimes, particularly since the advent of the Industrial Revolution at the end of the 18th century. Western society moved from an organic energy regime, based on photosynthesis and brute (animal) force, to a fossil energy regime, the consolidation of which occurred in the 19th century. Starting with coal, then petroleum and natural gas, societies underwent successive energy changes, especially following the development of electricity in the 20th century. According to this narrative, the energy transition in the 21st century would constitute a new, albeit ambitious, phase in history, with the replacement of dominant CO2-emissive energy sources by renewables and other non-emissive sources.However, this narrative is hardly correct: our modern energy scenario has not so much evolved through transitions and shifts, as through addition and accumulation.157 The massive development of coal extraction in the 19th century did not lead to the disappearance of so-called organic sources of energy, especially animal strength. During the 19th century, the number of horses in France increased by 50% and water and wind mills continued to meet the need of a large part of the European population.158,159 In the mid-20th century, the rapid increase of oil supply did not lead to an equivalent decline in coal extraction; nor did the later development of nuclear power. Today, according to the IEA, the world's coal, oil, and natural gas supply is at more than 490![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TJ, more than ever before in modern history.160
000 TJ, more than ever before in modern history.160
Furthermore, these energy layers have not been added as mere juxtapositions. Energy sources have, throughout history, been combined with each other, and with material resources. An example is provided by the massive use of wood in the coal age, to consolidate mine shafts, build railway tracks, as cable supports for the emerging telegraph, etc. Thus, coal only developed thanks to the parallel use of wood and steel. This is what has been called energy and material “symbioses”.161
Through this historical lens, the challenges for the 21st-century energy transition increase immeasurably: the complete replacement of CO2-emissive energies by non-CO2-emissive energies would be unprecedented. It will not be simple to achieve as it will require significant reconfiguration of current energy and material symbioses – see the need for rare earths or lithium for batteries intended for electric, low-carbon mobility.144
In chemical terms, it is therefore hardly possible to tackle today's challenges by reasoning in silos, molecule by molecule, without considering from the outset that molecules combine, not only in the literal, microscopic sense – through catalysis, for example – but also in the figurative, macroscopic sense, in material and socio-technical configurations that must be kept in mind. To produce NH3 with low GHG emissions, we need to think about green hydrogen and about all the materials needed to store and transport it. We also need to think about all the energy and material combinations on which this NH3 and hydrogen could be grafted, to be sure that they will not support other GHG-emissive technologies or symbioses – a major issue that is not really addressed in the scenarios mentioned above. This will require chemists to constantly broaden how they view their own field of specialization.
History also teaches us that energy is never only a matter of source or technology. Indeed, energy systems have always required infrastructure (e.g., distribution networks such as pipelines),162 business models (e.g., large vs. small companies, rent distribution, marginal pricing strategies for electricity),163,164 institutional rules (e.g., anti-trust laws, energy policies),165 and even cultural representations and practices (e.g., pressing a button to get light in no way resembles switching on a paraffin lamp and having to ensure the availability of fuel).166 If the notion of transition might be meaningful from a historical perspective, it is not just in relation to energy sources or technologies per se, but when energy systems as a whole are considered.
For researchers in chemistry, this has two further implications. First, it means that when working on a molecule or a specific reaction, chemists do not work only on specific materials or related technologies; they also indirectly tap into broader components of the energy or material system, including infrastructure, business models, and policies, but also cultural representations, and habits.167 For instance, turning CO2 into a raw material would certainly change what is now a waste product into a valuable material, but it would also mean changing the representations associated with CO2. Why should we strive to reduce emissions if CO2 is now a “good” thing? This might have detrimental effects if as a result emissions remain too high – no longer being avoided, with the public thinking that a high atmospheric CO2 concentration is no longer an issue – compared to the absorption and transformation capacities of new technologies.75 This threat can be exemplified by the recent German request to postpone the termination of commercialization of internal combustion engine vehicles, set by the EU for 2035, on the basis, among other considerations, that by that time we will be able to produce CO2-neutral e-fuels.168 It is therefore legitimate for chemists to wonder about the systemic effects of the chemical processes they work out and to act so that no misleading or detrimental cultural representations emerge or is strengthened thanks to their discoveries.
The second implication is that in energy systems the difficulties with changing long-lasting technological trajectories and habits – or path dependencies – have not only material (or chemical) and economic (related to profitability) explanations, but also institutional and cultural ones. This might seem obvious, but it may not be so obvious when faced with social demand for (technical and profitable) solutions to the ecological crisis from fundamental and applied sciences, as if the technological lever was the cornerstone of future changes. It is worth remembering that levers other than technology exist (e.g., energy demand modulation, new spatial planning, reduced packaging, better sharing of existing resources) to ensure the transition to a non–CO2–emissive energy system.
4.7. Discussion
As a first example of the just mentioned risks, focused on potential environmental impacts, several Earth system processes, which are connected to the interdependencies outlined (e.g. freshwater Earth system process for hydrogen and water conflicts, and Land Use Earth system process for hydrogen and land use conflicts, Novel Entities Earth for the section on plastics) are generally either ignored or overlooked by the scenarios analyzed. Returning within some of the identified planetary boundaries is not explicitly stated as a goal of the scenarios, and little evidence is provided to project that the transgression of these boundaries could eventually be reversed. While the main focus of the scenarios we have chosen is, by construction, one particular Earth system process (i.e., dominance of climate change-tailored actions through the recurrent notion of the carbon footprint), other interconnected consequences of a move away from fossil fuels are seldom adequately treated (e.g., land, water and resource management connected to the energy transition; effect on biodiversity; consequences of the introduction of novel entities). On the contrary, in some cases, the energy transition scenarios analyzed carry a serious risk of further exacerbating the overstepping of a planetary boundary, even when the situation is already dire (e.g., ammonia).
As a second example of the possible risks, focused on the potential geopolitical and social impacts of the proposed scenarios, (some) people are overlooked by scenarios. Many of the proposed strategies are blind to North-South inequalities, and the scenarios may contribute to maintaining or even exacerbating conflicts linked to extractivism, productivism, commodification of resources, and waste management (e.g., through extra-European mining activities connected with metal resources required to establish fossil fuel-decoupled European mobility).
Concurrently, this interdisciplinary analysis of scenarios, examined through the prism of five molecular substances at the energy-chemistry nexus, also highlights one invariant between the present time and the projected transition(s): the hierarchy of values.
Even though the triple-bottom line of sustainability – the “three Ps”, People, Planet, and Profit, i.e., economically viable, socially just, and ecologically sound – is mentioned throughout some scenarios and prospective sustainability exercises, these are generally established and projected in a hierarchical order. Profitability appears as a necessary condition, with scalability and commodification being the main – if not the only– reasonable strategy, while at the same time de facto omitting important aspects of the Planet and People bottom lines.155,169 This will potentially have problematic consequences in terms of environmental and social justice.
Notwithstanding possible intrinsic limits to the efficiency of some industry-targeted research, as discussed in the context of proposed energy-transition-related solutions (e.g., sub-optimal Nash equilibria), the strategies analyzed overwhelmingly retain private profit as a necessary principal driver. This driver has a structural tendency to increase production and/or exclude less profitable beneficiaries, thus echoing and substantiating the two aspects of concern highlighted above: supply the world with commodities beyond its planetary limits and/or prosper by excluding or exploiting some groups of people or resources. Consequently, the trajectory associated with the development and scaling-up of several novel technologies could potentially mimic some aspects of the dynamics that led to the Anthropocene in the first place. For this review, we considered scenarios requiring the creation of and need for new entities, with an expectation of a dramatically-expanding market: non-fossil fuels and technologies, and commodities, infrastructure, etc., associated with their production. We are poised to see a considerable acceleration of new variables, potentially representing additional energy sources rather than complete substitutions for current fuels. The question relates less to the common objective (to decrease fossil fuel consumption) and the possible means to achieve it (alternative energy sources, increased efficiency, etc.) than to the overall arc traced by these scenarios, and the method(s) that will reveal it. This question is further complexified by the fact that through interdisciplinary dialog, we are able to catch a glimpse of the non-scientific – some philosophers have used the term “imaginary”170 in contexts akin to the one under study here – dimensions underlaying our scientific developments. This interdisciplinary dialog has helped show that, in order to build up and justify our scientific choices, we rely on “cultural axioms”, that we mostly do not question nor explicitly mention.
We propose that including inter/transdisciplinary thinking as part of a broader and more systemic vision of the energy transition, relying not only on productivity and CO2 emission numbers, could produce more balanced and equitable roadmaps (see ESI, Section SI-1.3† for our distinction between interdisciplinarity and transdisciplinarity). For instance, established and measurable global descriptors, such as planetary boundaries, could be used to assess and teach171,172 the impact of any proposed approach, along with systems thinking. In addition, notions of social and environmental justice, that go beyond the use of measurable descriptors, could be included when considering societal organization, resource use, and waste disposal. In ecological economics for instance, i.e. a research arena gathering both economists and environmental scientists, some initiatives do exist to cross indicators and recommendations for a more sustainable future, with varying degree of heuristics power. Kate Raworth's doughnut economics, combining social thresholds and ecological ceilings, is one example.173 Richard Norgaard's coevolutionary economics, examining the interrelation between socio- and eco-systems, is another one.174
Developing transdisciplinary tools to explore and analyze the complex ramifications of the global shift in economic, social, and technological models represented by the energy transition appears to us not only beneficial, but necessary, to break the hegemonic view currently put forward and to generate novel paradigms for the chemistry-energy nexus. We note that alternatives have been and continue to be proposed.175 The relevance of non-hegemonic frameworks may need to be assessed to widen the narrow and potentially dangerous path currently set out by the dominant scenarios analyzed. We mentioned Profit, Planet and People: examples of existing scientific and minoritarian frameworks in non-mainstream economics (ecological economics, degrowth/post-growth, political ecology) and in critiques of dominant power structures, including economic ones, are all relevant in this quest to deconstruct the current implicit hierarchy of values that could very problematically guide the future of research at the chemistry-energy nexus.
In summary, the take-home message is two-fold: the path offered by the scenarios is narrow and is associated with numerous pitfalls. This suggests that alternatives based on a broader, transdisciplinary method of analysis with closer attention to historically overshadowed – or politically suppressed – theories can be revisited by chemists as a source of inspiration.
In 1972, twelve scenarios were presented by Meadows' team, in the “limit to growth” report,176 regarding the trends for five key interconnected indicators (resources, population, food per capita, pollution, industrial output per capita) between 1900 and 2100. Modeling their interdependencies and carve system-based scenarios were termed “wicked problems” by the Club of Rome. In 2021, almost 50 years later, current analyses156 after a 30 years update178 suggest that among all the scenarios considered, the one that is statistically the furthest from empirical data is the “Stabilize the World” scenario (see Fig. 8). While this scenario contributed to the UN “sustainable development” definition that still permeates some current discourse, other scenarios like “Business As Usual” and “Comprehensive Technology” are on the contrary the most compatible with current data (see ESI, Fig. SI-4†). Pointedly, the “comprehensive technology” scenario assumes “exceptionally high technological development and adoption rates”. This, compounded with the feasibility doubts mentioned by technology-driven proponents mentioned above, show that there is an active possibility for a path to the most unstable future which entails brutal collapse of population and resources (see “business as usual” models, Fig. SI-4†).
 | ||
| Fig. 8 SW (stabilize the world) scenario for the projected evolution of population (blue line), pollution (orange line), food per capita (red line), non-renewable resources (green line), ecological footprint (brown line), human welfare (pink line), industrial output (gray line), and death rate (black line), and comparison with corresponding empirical data (dots), adapted from Herrington177 and Meadows.178 SW is referred as the sustainable model: indicators are stabilized on the long-term thanks to meaningful policy changes. Data for three other scenarios (Business As Usual and Comprehensive Technology) are available in ESI, Section SI-6 and Fig. SI-4.† | ||
To return to the topic of this paper, there is still a chance that the common elements of the five “energy transition” scenarios discussed above (Section 3.7) could be enacted, if the incessant, increasing, and here-to-fore only very partially addressed calls for substantial changes were finally met. However, even if these calls were met, some of the flaws consubstantial with the scenarios mentioned above would become a reality (e.g., recurrent disregard of the global South and disregard of Earth system processes (other than CO2)-related problems).
Therefore, the urgency is evident: we must make sure that we have the appropriate tools to avoid the aforementioned failure to change, but we must also make sure that we have the appropriate tools to avoid the risks associated with implementation of some aspects of the dominant scenarios discussed above.
5. General conclusion and perspectives
5.1. Broader scenarios needed
We are living in the Anthropocene, and as such the quality of human life on Earth is jeopardized by certain choices, and modeled and institutionalized under some ideas of how we should inhabit Earth. These choices and ideas permeate the research horizons and the specific research subjects we as scientists must treat.One of the pervasive messages surrounding the energy transition, included in the chemistry-oriented responses analyzed through the pivotal molecules dealt with in this paper, is that the energy transition is designed to avoid future crises that remain to be faced. However, this minimizes the fact that the planet is already in crisis. We further showed that this crisis was potentially caused by some aspects that are still present in some of the responses that are currently proposed as solutions to implement the energy transition. We also showed that this crisis presents multiple aspects that are not addressed by the response specifically tailored to the energy transition challenge. We therefore propose as first conclusion to this paper that there is a potential incongruity between the problem stated and several of the scientific research avenues proposed in the scenarios analyzed here.
The second conclusion is based on the disciplinary fragmentation we face in our academic practice. This is a complicating factor when trying to address such types of incongruity. In contrast, transdisciplinarity appears to help circumvent deadlocks emerging from proposing disciplinary approaches to systemic problems. Indeed, one lead from this paper is that a radically interdisciplinary dialog – between the natural and social sciences – must be explored. Progress in natural science has the power to facilitate a change in society, just as much as the pressures exerted by a changing society or politics can lead to intensified efforts in some specific areas of research in natural science. Echoing a classification proposed by German philosopher Dilthey,179 chemistry is a science that focuses on mastering how transformations occur, while social sciences and humanities describe and interpret why transformations occur. This is not to say that social sciences can explain why the transformation of matter and energy occurs in physical or chemical terms, but that, since the transformation of technologies and production processes also has social motivations and implications, social scientists provide critical information about the connection between, on one side, these motivations and implications and, on the other side, the technological and material change itself: What are the social motivations and implication that contribute to the chemists being asked to search for CO2-based materials? What sort of (social and economic) hypotheses are implicitly embarked in their research? etc. As a consequence, social scientists should be involved in chemistry projects, not as side contributors but more granularly throughout the projects. In a sense, the project that led to this paper explored precisely this suggestion. Intertwining aspects of the chemical sciences, relevant to the transformation of matter at the energy-chemistry nexus, through the prism of five molecular substances, with aspects of social sciences and philosophy, connected to transformations of societies at the same nexus, seems to offer a relevant perspective on the transition scenarios during the Anthropocene crisis.
The third conclusion to this work relates to the utility of turning to wider scientific approaches that have been historically/politically suppressed (non-orthodox economics, decolonial and post-colonial studies, gender studies, see Section 4) and of investigating their capacity to inspire chemistries beyond the currently hegemonic one which is, at least partly, problematic. For example, academic chemists' agenda is for now mostly framed, often implicitly, by economic and political ideas such as, for instance, priority given to research likely to lead to economic growth and profitability and toward the quest for groundbreaking technological progress. By looking at other economic, social, and political framings, inspired for instance by ecological economics173 and post-growth theories,180,181 or decolonial studies,182,183 chemists could become inspired in investigating other, less conventional research avenues. For example, community energy projects that could exemplarily contribute to mitigation strategies while inspiring the rise of other energy communities elsewhere,184 as well as low-tech and nature-inspired approach developed in the global South using a non-hegemonic framing,185 could inspire global North initiatives to develop low-tech as a way to rethink technology for sobriety, conviviality, and sustainability, options in academia,186 there included in chemistry research.
5.2. How do research topics align with researchers' values?
Beyond interdisciplinarity and transdisciplinarity interactions with other disciplines, as well as devoting attention to suppressed portions of disciplines, the questioning can be further extended to the forms of knowledge themselves. The production of knowledge that by academic construction rewards and sustains a narrow path (with problematic invariants) is also part of the equation. What values collectively lead us to define research excellence, or, on the contrary, irrelevance? What values are encouraged, allowed or suppressed by the scenarios shaping our research projects? Do these reflect our personal values, and ensure Earth will remain habitable while emphasizing the need for social justice?In the context of the Anthropocene, this leads to an immediate overarching question: What is our responsibility with respect to the research horizons and the specific subjects we treat as scientists, when we suspect such incongruity? Some may think this has nothing to do with our job as scientists and that we should leave the problem to international organizations, or governments – that we can act only as individuals with individual responsibilities. On the contrary, following in the footsteps of eminent thinkers (Box 1), we believe that there is a social dimension of responsibility. Quoting the ethical group advising the CNRS, the largest French research body, “the specific purpose [of research], which is to produce knowledge in the service of society, confers on research[ers] the particular responsibility of also questioning the use that can be made of their contributions […] and the way that this use can help respond positively to the problems encountered by society or, on the contrary, perpetuate or even aggravate them.” We also believe, although we each have our own specialization, that we bear an ethical responsibility for the consequences of our acts, which often lead to consequences well beyond our disciplines. Do our research and the knowledge it produces respond to, perpetuate, or aggravate the problems raised?
To explore our own invitation to mobilize concepts produced in the global South as a source of inspiration, the problem might be less related to what we know and what we can do, but more to what we do not know and what we are willingly ignoring.187–189 We suggest that overcoming disciplinary isolation, paying attention to critical scientific theories and reaching out to knowledge-producing activities beyond academic circles should be explored to help deflect the ominous course of the Anthropocene through our research activities. Even if we are scientists, the Anthropocene is neither just a matter of academic discussion nor a question of agreement or disagreement. Our posture cannot be that of academic practitioners undertaking compartmentalized disciplinary research based on scenarios that only partially reflect our values.
Author contributions
More information on the authors' contributions can be found in section “SI-1.2 Our Authorship policy” of the ESI.†Conflicts of interest
We have no conflict of interests to declare. In spirit of transparency, we wish to mention here that VA contributed to the Sunergy technological roadmap discussed in Section 3.Acknowledgements
The content above is under the sole responsibility of us, the authors, and does not imply endorsement by the authors' affiliations' institutions nor the people or bodies mentioned here below. However, since we all met during the CatEnerChem Winter School which sparked this collective perspective work, we wish to acknowledge the institutions and structures that sponsored that event (see ESI, Fig. SI-2†). All the organizers, speakers and participants at that event are also gratefully acknowledged for nourishing the early stages of this work. The list of can be found at http://catenerchem.cpe.fr/. We also wish to thank all the people that read intermediate versions and gave us constructive feedback. The reasons why we all wish to be all considered as first co-authors and that a collective email is used as corresponding address, are given in ESI, Section SI-1.2.†References
- P. J. Crutzen, Geology of Mankind, Nature, 2002, 415(6867), 23, DOI:10.1038/415023a.
- W. Steffen, W. Broadgate, L. Deutsch, O. Gaffney and C. Ludwig, The Trajectory of the Anthropocene: The Great Acceleration, Anthropocene Rev., 2015, 2(1), 81–98, DOI:10.1177/2053019614564785.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. De Vries, C. A. De Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sörlin, Planetary Boundaries: Guiding Human Development on a Changing Planet, Science, 2015, 347(6223), 1259855, DOI:10.1126/science.1259855.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. I. Chapin, E. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. De Wit, T. Hughes, S. Van Der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. Foley, Planetary Boundaries: Exploring the Safe Operating Space for Humanity, Ecol. Soc., 2009, 14(2), art32, DOI:10.5751/ES-03180-140232.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin, E. F. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. A. Foley, A Safe Operating Space for Humanity, Nature, 2009, 461(7263), 472–475, DOI:10.1038/461472a.
- S. R. Carpenter and E. M. Bennett, Reconsideration of the Planetary Boundary for Phosphorus, Environ. Res. Lett., 2011, 6(1), 014009, DOI:10.1088/1748-9326/6/1/014009.
- S. W. Running, A Measurable Planetary Boundary for the Biosphere, Science, 2012, 337(6101), 1458–1459, DOI:10.1126/science.1227620.
- W. de Vries, J. Kros, C. Kroeze and S. P. Seitzinger, Assessing Planetary and Regional Nitrogen Boundaries Related to Food Security and Adverse Environmental Impacts, Curr. Opin. Environ. Sustain., 2013, 5(3), 392–402, DOI:10.1016/j.cosust.2013.07.004.
- D. Gerten, H. Hoff, J. Rockström, J. Jägermeyr, M. Kummu and A. V. Pastor, Towards a Revised Planetary Boundary for Consumptive Freshwater Use: Role of Environmental Flow Requirements, Curr. Opin. Environ. Sustain., 2013, 5(6), 551–558, DOI:10.1016/j.cosust.2013.11.001.
- G. M. Mace, B. Reyers, R. Alkemade, R. Biggs, F. S. Chapin, S. E. Cornell, S. Díaz, S. Jennings, P. Leadley, P. J. Mumby, A. Purvis, R. J. Scholes, A. W. R. Seddon, M. Solan, W. Steffen and G. Woodward, Approaches to Defining a Planetary Boundary for Biodiversity, Global Environ. Change, 2014, 28, 289–297, DOI:10.1016/j.gloenvcha.2014.07.009.
- W. L. Steffen, J. Rockström and R. Costanza, How Defining Planetary Boundaries Can Transform Our Approach to Growth, Solutions: For A Sustainable & Desirable Future, 2011, 2(3), 59–65 Search PubMed.
- K. Richardson, W. Steffen, W. Lucht, J. Bendtsen, S. E. Cornell, J. F. Donges, M. Drüke, I. Fetzer, G. Bala, W. von Bloh, G. Feulner, S. Fiedler, D. Gerten, T. Gleeson, M. Hofmann, W. Huiskamp, M. Kummu, C. Mohan, D. Nogués-Bravo, S. Petri, M. Porkka, S. Rahmstorf, S. Schaphoff, K. Thonicke, A. Tobian, V. Virkki, L. Wang-Erlandsson, L. Weber and J. Rockström, Earth beyond Six of Nine Planetary Boundaries, Sci. Adv., 2023, 9(37), eadh2458, DOI:10.1126/sciadv.adh2458.
- L. Wang-Erlandsson, A. Tobian, R. J. van der Ent, I. Fetzer, S. te Wierik, M. Porkka, A. Staal, F. Jaramillo, H. Dahlmann, C. Singh, P. Greve, D. Gerten, P. W. Keys, T. Gleeson, S. E. Cornell, W. Steffen, X. Bai and J. A. Rockström, Planetary Boundary for Green Water, Nat. Rev. Earth Environ., 2022, 3(6), 380–392, DOI:10.1038/s43017-022-00287-8.
- L. Persson, B. M. Carney Almroth, C. D. Collins, S. Cornell, C. A. De Wit, M. L. Diamond, P. Fantke, M. Hassellöv, M. MacLeod, M. W. Ryberg, P. Søgaard Jørgensen, P. Villarrubia-Gómez, Z. Wang and M. Z. Hauschild, Outside the Safe Operating Space of the Planetary Boundary for Novel Entities, Environ. Sci. Technol., 2022, 56(3), 1510–1521, DOI:10.1021/acs.est.1c04158.
- P. T. Anastas and J. B. Zimmerman, The Periodic Table of the Elements of Green and Sustainable Chemistry, Green Chem., 2019, 21(24), 6545–6566, 10.1039/C9GC01293A.
- S. A. Matlin, G. Mehta, H. Hopf and A. Krief, One-World Chemistry and Systems Thinking, Nat. Chem., 2016, 8(5), 393–398, DOI:10.1038/nchem.2498.
- T. Keijer, V. Bakker and J. C. Slootweg, Circular Chemistry to Enable a Circular Economy, Nat. Chem., 2019, 11(3), 190–195, DOI:10.1038/s41557-019-0226-9.
- P. G. Mahaffy, S. A. Matlin, T. A. Holme and J. MacKellar, Systems Thinking for Education about the Molecular Basis of Sustainability, Nat. Sustain., 2019, 2(5), 362–370, DOI:10.1038/s41893-019-0285-3.
- A. Redman and A. Wiek, Competencies for Advancing Transformations Towards Sustainability, Front. Educ., 2021, 6, 785163, DOI:10.3389/feduc.2021.785163.
- Systems Thinking in Chemistry for Sustainability: Toward 2030 and Beyond (STCS 2030+), IUPAC | International Union of Pure and Applied Chemistry. https://iupac.org/project/, accessed 2024-03-23 Search PubMed.
- D. Haraway, Situated Knowledges: The Science Question in Feminism and the Privilege of Partial Perspective, Fem. Stud., 1988, 14(3), 575–599, DOI:10.2307/3178066.
- D. M. Kahan, Climate-Science Communication and the Measurement Problem, Polit. Psychol., 2015, 36(S1), 1–43, DOI:10.1111/pops.12244.
- Climate Change 2022 – Mitigation of Climate Change: Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. Cambridge University Press, Intergovernmental Panel on Climate Change (IPCC), Cambridge, 2023, DOI:10.1017/9781009157926.
- International Energy Agency, Net Zero by 2050 - A Roadmap for the Global Energy Sector, International Energy Agency, Paris, 2021 Search PubMed.
- A. M. Bazzanella, F. Ausfelder and DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V, Low Carbon Energy and Feedstock for the European Chemical Industry, DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V., 2017 Search PubMed.
- C. Faber, Y. Allahverdiyeva-Rinne, Vi. Artero, L. Baraton, A. Barbieri, H. Bercegol, M. Fleischer, H. Huynhthi, J. Kargul, H. Lepaumier, L. Lopez, A. Magnuson and A. Roth, SUNRISE - Solar Energy for a Circular Economy - Technological Roadmap, 2019. https://sunergy-initiative.eu/project/sunrise-technological-roadmap/.
- Shell, The Energy Transformation Scenarios, 2021 Search PubMed.
- United Nations, The Paris Agreement, United Nations. https://www.un.org/en/climatechange/paris-agreement, accessed 2023-11-18 Search PubMed.
- United Nations, Net Zero Coalition, United Nations, https://www.un.org/en/climatechange/net-zero-coalition, accessed 2023-11-18 Search PubMed.
- The Future of Petrochemicals – Analysis, IEA, https://www.iea.org/reports/the-future-of-petrochemicals, accessed 2024-03-23 Search PubMed.
- J.-P. Lange, Towards Circular Carbo-Chemicals – the Metamorphosis of Petrochemicals, Energy Environ. Sci., 2021, 14(8), 4358–4376, 10.1039/D1EE00532D.
- International Energy Agency, CO2 Emissions in 2022, 2023 Search PubMed.
- International Energy Agency, Global cement production in the Net Zero Scenario, 2010-2030 – Charts – Data & Statistics, IEA, https://www.iea.org/data-and-statistics/charts/global-cement-production-in-the-net-zero-scenario-2010-2030-5260, accessed 2023-11-14 Search PubMed.
- International Energy Agency, Direct emissions intensity of cement production in the Net Zero Scenario, 2015-2030 – Charts – Data & Statistics, IEA, https://www.iea.org/data-and-statistics/charts/direct-emissions-intensity-of-cement-production-in-the-net-zero-scenario-2015-2030-2, accessed 2023-11-14 Search PubMed.
- December 2022 crude steel production and 2022 global crude steel production totals, https://worldsteel.org/, https://worldsteel.org/media-centre/press-releases/2023/december-2022-crude-steel-production-and-2022-global-totals/, accessed 2023-11-14.
- International Energy Agency, Direct CO2 intensity of the iron and steel sector in the Net Zero Scenario, 2010-2030 – Charts – Data & Statistics, IEA, https://www.iea.org/data-and-statistics/charts/direct-co2-intensity-of-the-iron-and-steel-sector-in-the-net-zero-scenario-2010-2030, accessed 2023-11-14 Search PubMed.
- International Energy Agency, Direct CO2 emissions from industry in the Net Zero Scenario, 2000-2030 – Charts – Data & Statistics, IEA. https://www.iea.org/data-and-statistics/charts/direct-co2-emissions-from-industry-in-the-net-zero-scenario-2000-2030-2, accessed 2023-11-14 Search PubMed.
- International Energy Agency, Direct CO2 emissions from primary chemical production in the Net Zero Scenario, 2010-2030 – Charts – Data & Statistics, IEA, https://www.iea.org/data-and-statistics/charts/direct-co2-emissions-from-primary-chemical-production-in-the-net-zero-scenario-2010-2030-2, accessed 2023-11-14 Search PubMed.
- International Energy Agency, CO2 Capture and Utilisation - Energy System, IEA, https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage/co2-capture-and-utilisation, accessed 2023-11-14 Search PubMed.
- P. M. Forster, C. J. Smith, T. Walsh, W. F. Lamb, R. Lamboll, M. Hauser, A. Ribes, D. Rosen, N. Gillett, M. D. Palmer, J. Rogelj, K. Von Schuckmann, S. I. Seneviratne, B. Trewin, X. Zhang, M. Allen, R. Andrew, A. Birt, A. Borger, T. Boyer, J. A. Broersma, L. Cheng, F. Dentener, P. Friedlingstein, J. M. Gutiérrez, J. Gütschow, B. Hall, M. Ishii, S. Jenkins, X. Lan, J.-Y. Lee, C. Morice, C. Kadow, J. Kennedy, R. Killick, J. C. Minx, V. Naik, G. P. Peters, A. Pirani, J. Pongratz, C.-F. Schleussner, S. Szopa, P. Thorne, R. Rohde, M. Rojas Corradi, D. Schumacher, R. Vose, K. Zickfeld, V. Masson-Delmotte and P. Zhai, Indicators of Global Climate Change 2022: Annual Update of Large-Scale Indicators of the State of the Climate System and Human Influence, Earth Syst. Sci. Data, 2023, 15(6), 2295–2327, DOI:10.5194/essd-15-2295-2023.
- P. Friedlingstein, M. O'Sullivan, M. W. Jones, R. M. Andrew, L. Gregor, J. Hauck, C. Le Quéré, I. T. Luijkx, A. Olsen, G. P. Peters, W. Peters, J. Pongratz, C. Schwingshackl, S. Sitch, J. G. Canadell, P. Ciais, R. B. Jackson, S. R. Alin, R. Alkama, A. Arneth, V. K. Arora, N. R. Bates, M. Becker, N. Bellouin, H. C. Bittig, L. Bopp, F. Chevallier, L. P. Chini, M. Cronin, W. Evans, S. Falk, R. A. Feely, T. Gasser, M. Gehlen, T. Gkritzalis, L. Gloege, G. Grassi, N. Gruber, Ö. Gürses, I. Harris, M. Hefner, R. A. Houghton, G. C. Hurtt, Y. Iida, T. Ilyina, A. K. Jain, A. Jersild, K. Kadono, E. Kato, D. Kennedy, K. Klein Goldewijk, J. Knauer, J. I. Korsbakken, P. Landschützer, N. Lefèvre, K. Lindsay, J. Liu, Z. Liu, G. Marland, N. Mayot, M. J. McGrath, N. Metzl, N. M. Monacci, D. R. Munro, S.-I. Nakaoka, Y. Niwa, K. O'Brien, T. Ono, P. I. Palmer, N. Pan, D. Pierrot, K. Pocock, B. Poulter, L. Resplandy, E. Robertson, C. Rödenbeck, C. Rodriguez, T. M. Rosan, J. Schwinger, R. Séférian, J. D. Shutler, I. Skjelvan, T. Steinhoff, Q. Sun, A. J. Sutton, C. Sweeney, S. Takao, T. Tanhua, P. P. Tans, X. Tian, H. Tian, B. Tilbrook, H. Tsujino, F. Tubiello, G. R. van der Werf, A. P. Walker, R. Wanninkhof, C. Whitehead, A. Willstrand Wranne, R. Wright, W. Yuan, C. Yue, X. Yue, S. Zaehle, J. Zeng and B. Zheng, Global Carbon Budget 2022, Earth Syst. Sci. Data, 2022, 14(11), 4811–4900, DOI:10.5194/essd-14-4811-2022.
- S. A. Matlin, S. E. Cornell, A. Krief, H. Hopf and G. Mehta, Chemistry Must Respond to the Crisis of Transgression of Planetary Boundaries, Chem. Sci., 2022, 13(40), 11710–11720, 10.1039/D2SC03603G.
- L. Jiang, R. A. Feely, B. R. Carter, D. J. Greeley, D. K. Gledhill and K. M. Arzayus, Climatological Distribution of Aragonite Saturation State in the Global Oceans, Global Biogeochem. Cycles, 2015, 29(10), 1656–1673, DOI:10.1002/2015GB005198.
- European Environment Agency, Ocean acidification, https://www.eea.europa.eu/ims/ocean-acidification, accessed 2023-09-13.
- K. Calvin, D. Dasgupta, G. Krinner, A. Mukherji, P. W. Thorne, C. Trisos, J. Romero, P. Aldunce, K. Barrett, G. Blanco, W. W. L. Cheung, S. Connors, F. Denton, A. Diongue-Niang, D. Dodman, M. Garschagen, O. Geden, B. Hayward, C. Jones, F. Jotzo, T. Krug, R. Lasco, Y.-Y. Lee, V. Masson-Delmotte, M. Meinshausen, K. Mintenbeck, A. Mokssit, F. E. L. Otto, M. Pathak, A. Pirani, E. Poloczanska, H.-O. Pörtner, A. Revi, D. C. Roberts, J. Roy, A. C. Ruane, J. Skea, P. R. Shukla, R. Slade, A. Slangen, Y. Sokona, A. A. Sörensson, M. Tignor, D. Van Vuuren, Y.-M. Wei, H. Winkler, P. Zhai, Z. Zommers, J.-C. Hourcade, F. X. Johnson, S. Pachauri, N. P. Simpson, C. Singh, A. Thomas, E. Totin, P. Arias, M. Bustamante, I. Elgizouli, G. Flato, M. Howden, C. Méndez-Vallejo, J. J. Pereira, R. Pichs-Madruga, S. K. Rose, Y. Saheb, R. Sánchez Rodríguez, D. Ürge-Vorsatz, C. Xiao, N. Yassaa, A. Alegría, K. Armour, B. Bednar-Friedl, K. Blok, G. Cissé, F. Dentener, S. Eriksen, E. Fischer, G. Garner, C. Guivarch, M. Haasnoot, G. Hansen, M. Hauser, E. Hawkins, T. Hermans, R. Kopp, N. Leprince-Ringuet, J. Lewis, D. Ley, C. Ludden, L. Niamir, Z. Nicholls, S. Some, S. Szopa, B. Trewin, K.-I. Van Der Wijst, G. Winter, M. Witting, A. Birt, M. Ha, J. Romero, J. Kim, E. F. Haites, Y. Jung, R. Stavins, A. Birt, M. Ha, D. J. A. Orendain, L. Ignon, S. Park, Y. Park, A. Reisinger, D. Cammaramo, A. Fischlin, J. S. Fuglestvedt, G. Hansen, C. Ludden, V. Masson-Delmotte, J. B. R. Matthews; K. Mintenbeck, A. Pirani, E. Poloczanska, N. Leprince-Ringuet and C. Péan, IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, ed. H. Lee and J. Romero, IPCC; Intergovernmental Panel on Climate Change (IPCC), Geneva, Switzerland, First, 2023, DOI:10.59327/IPCC/AR6-9789291691647.
- International Energy Agency, CO2 Emissions in 2023, 2024 Search PubMed.
- Hydrogen Production and Energy Transition, ed. M. H. van de Voorde, Energy, environment and new materials, De Gruyter, Berlin, Boston, 2021 Search PubMed.
- International Energy Agency, Global Hydrogen Review 2023, 2023 Search PubMed.
- IEA – International Energy Agency, Global hydrogen production by technology in the Net Zero Scenario, 2019-2030 – Charts – Data & Statistics, International Energy Agency, https://www.iea.org/data-and-statistics/charts/global-hydrogen-production-by-technology-in-the-net-zero-scenario-2019-2030-3, accessed 2023-11-14 Search PubMed.
- Bp Statistical Review of World Energy 2022, 2022 Search PubMed.
- United Nations Economic Commission for Europe, Carbon Neutrality in the UNECE Region: Integrated Life-Cycle Assessment of Electricity Sources, ECE Energy Series; United Nations, 2022, DOI:10.18356/9789210014854.
- J. S. Valente, R. Quintana-Solórzano, H. Armendáriz-Herrera and J.-M. M. Millet, Decarbonizing Petrochemical Processes: Contribution and Perspectives of the Selective Oxidation of C 1 –C 3 Paraffins, ACS Catal., 2023, 13(3), 1693–1716, DOI:10.1021/acscatal.2c05161.
- World Bank Publications, Global Gas Flaring Tracker Reports, 2023 Search PubMed.
- Zero Routine Flaring by 2030 (ZRF), https://www.worldbank.org/en/programs/zero-routine-flaring-by-2030, accessed 2023-09-17.
- Z. D. Weller, S. P. Hamburg and J. C. Von Fischer, A National Estimate of Methane Leakage from Pipeline Mains in Natural Gas Local Distribution Systems, Environ. Sci. Technol., 2020, 54(14), 8958–8967, DOI:10.1021/acs.est.0c00437.
- T. Lauvaux, C. Giron, M. Mazzolini, D. Shindell and P. Ciais, Global Assessment of Oil and Gas Methane Ultra-Emitters, Science, 2022, 375, 557–561 CrossRef CAS PubMed.
- K. Rouwenhorst and G. Castellanos, Innovation Outlook: Renewable Ammonia, IRENA and AEA - Innovation Outlook: Renewable Ammonia, 2022 Search PubMed.
- M. Thiemann, E. Scheibler and K. W. Wiegand, Nitric Acid, Nitrous Acid, and Nitrogen Oxides, In Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, Ltd, 2000, DOI:10.1002/14356007.a17_293.
- M. C. E. Groves, Nitric Acid, In Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Ltd, 2020, pp. 1–37, DOI:10.1002/0471238961.1409201803120118.a01.pub3.
- Ammonia Technology Roadmap – Analysis, IEA. https://www.iea.org/reports/ammonia-technology-roadmap, accessed 2024-04-02 Search PubMed.
- K. A. Congreves, O. Otchere, D. Ferland, S. Farzadfar, S. Williams and M. M. Arcand, Nitrogen Use Efficiency Definitions of Today and Tomorrow, Front. Plant Sci., 2021, 12, 637108, DOI:10.3389/fpls.2021.637108.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, How a Century of Ammonia Synthesis Changed the World, Nat. Geosci., 2008, 1(10), 636–639, DOI:10.1038/ngeo325.
- C. Scheer, K. Fuchs, D. E. Pelster and K. Butterbach-Bahl, Estimating Global Terrestrial Denitrification from Measured N2O:(N2O + N2) Product Ratios, Curr. Opin. Environ. Sustain., 2020, 47, 72–80, DOI:10.1016/j.cosust.2020.07.005.
- S. Rigal, V. Dakos, H. Alonso, A. Auniņš, Z. Benkő, L. Brotons, T. Chodkiewicz, P. Chylarecki, E. De Carli, J. C. Del Moral, C. Domşa, V. Escandell, B. Fontaine, R. Foppen, R. Gregory, S. Harris, S. Herrando, M. Husby, C. Ieronymidou, F. Jiguet, J. Kennedy, A. Klvaňová, P. Kmecl, L. Kuczyński, P. Kurlavičius, J. A. Kålås, A. Lehikoinen, Å. Lindström, R. Lorrillière, C. Moshøj, R. Nellis, D. Noble, D. P. Eskildsen, J.-Y. Paquet, M. Pélissié, C. Pladevall, D. Portolou, J. Reif, H. Schmid, B. Seaman, Z. D. Szabo, T. Szép, G. T. Florenzano, N. Teufelbauer, S. Trautmann, C. Van Turnhout, Z. Vermouzek, T. Vikstrøm, P. Voříšek, A. Weiserbs and V. Devictor, Farmland Practices Are Driving Bird Population Decline across Europe, Proc. Natl. Acad. Sci. U.S.A., 2023, 120(21), e2216573120, DOI:10.1073/pnas.2216573120.
- Preparatory Materials for the Plastics Treaty INC-2 (May 2023), Center for International Environmental Law, https://www.ciel.org/wp-content/uploads/2023/05/Compilation-of-Key-Terms-Relevant-for-the-Negotiation-of-a-Treaty-to-End-Plastic-Pollution_FINAL.pdf, accessed 2024-03-23 Search PubMed.
- Plastics – the fast Facts 2023 Plastics Europe, Plastics Europe. https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/, accessed 2024-03-23 Search PubMed.
- Plastics - the Facts 2022 Plastics Europe, Plastics Europe, https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/, accessed 2023-09-13 Search PubMed.
- E. Elhacham, L. Ben-Uri, J. Grozovski, Y. M. Bar-On and R. Milo, Global Human-Made Mass Exceeds All Living Biomass, Nature, 2020, 588(7838), 442–444, DOI:10.1038/s41586-020-3010-5.
- FAQs on Plastics, Our World in Data, https://ourworldindata.org/faq-on-plastics, accessed 2023-12-10.
- Y. M. Bar-On, R. Phillips and R. Milo, The Biomass Distribution on Earth, Proc. Natl. Acad. Sci. U. S. A., 2018, 115(25), 6506–6511, DOI:10.1073/pnas.1711842115.
- OCDE, Plastics Flows and Their Impacts on the Environment, OCDE, Paris, 2022, DOI:10.1787/71a51317-en.
- K. Bucci, M. Tulio and C. M. Rochman, What Is Known and Unknown about the Effects of Plastic Pollution: A Meta-Analysis and Systematic Review, Ecol. Appl., 2020, 30(2), e02044, DOI:10.1002/eap.2044.
- International Energy Agency, IEA CCUS Projects Explorer, https://www.iea.org/data-and-statistics/data-tools/ccus-projects-explorer, accessed 2023-09-03.
- Capacity of current and planned large-scale CO2 capture projects vs. the Net Zero Scenario, 2020-2030 – Charts – Data & Statistics – IEA, https://www.iea.org/data-and-statistics/charts/capacity-of-current-and-planned-large-scale-co2-capture-projects-vs-the-net-zero-scenario-2020-2030, accessed 2024-04-02.
- D. T. Ho, Carbon Dioxide Removal Is Not a Current Climate Solution — We Need to Change the Narrative, Nature, 2023, 616(7955), 9, DOI:10.1038/d41586-023-00953-x.
- J. M. Turner, Counting Carbon: The Politics of Carbon Footprints and Climate Governance from the Individual to the Global, Global Environ. Polit., 2014, 14(1), 59–78, DOI:10.1162/GLEP_a_00214.
- European Union, European Strategic Roadmap, 2023, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0301.
- Casale HQ Switzerland, Green and Blue Technologies, https://www.casale.ch/green-and-blue-solutions/green-and-blue-technologies, accessed 2023-09-03 Search PubMed.
- J. Diab, L. Fulcheri, V. Hessel, V. Rohani and M. Frenklach, Why Turquoise Hydrogen Will Be a Game Changer for the Energy Transition, Int. J. Hydrogen Energy, 2022, 47(61), 25831–25848, DOI:10.1016/j.ijhydene.2022.05.299.
- J. Incer-Valverde, A. Korayem, G. Tsatsaronis and T. Morosuk, “Colors” of Hydrogen: Definitions and Carbon Intensity, Energy Convers. Manage., 2023, 291, 117294, DOI:10.1016/j.enconman.2023.117294.
- T. Longden, F. J. Beck, F. Jotzo, R. Andrews and M. Prasad, Clean’ Hydrogen? – Comparing the Emissions and Costs of Fossil Fuel versus Renewable Electricity Based Hydrogen, Appl. Energy, 2022, 306, 118145, DOI:10.1016/j.apenergy.2021.118145.
- P. Schembri, Transition Énergétique et Défi Climatique : Quelle Place Pour l’hydrogène Vert ?, In Énergies « nouvelles » et société. La transition énergétique actuelle à la croisée des chemins et des savoirs, MSH Paris-Saclay Éditions, 2021, pp 85–113, DOI:10.52983/EWUS7854.
- International Energy Agency, Global EV Outlook 2022, International Energy Agency, Paris, 2022, https://www.iea.org/reports/global-ev-outlook-2022, accessed 2023-09-03 Search PubMed.
- M. D. L. N. Camacho, D. Jurburg and M. Tanco, Hydrogen Fuel Cell Heavy-Duty Trucks: Review of Main Research Topics, Int. J. Hydrogen Energy, 2022, 47(68), 29505–29525, DOI:10.1016/j.ijhydene.2022.06.271.
- N. Gray, S. McDonagh, R. O'Shea, B. Smyth and J. D. Murphy, Decarbonising Ships, Planes and Trucks: An Analysis of Suitable Low-Carbon Fuels for the Maritime, Aviation and Haulage Sectors, Adv. Appl. Energy, 2021, 1, 100008, DOI:10.1016/j.adapen.2021.100008.
- A. Keeley and M. Haden, Energy: Using Hydrogen for Glass, The Chemical Engineer, https://www.thechemicalengineer.com/features/energy-using-hydrogen-for-glass/, accessed 2023-09-03 Search PubMed.
- International Energy Agency, World Energy Outlook 2022, International Energy Agency, Paris, 2022, https://www.iea.org/reports/world-energy-outlook-2022 Search PubMed.
- International Energy Agency, Electrolysers - Energy System, https://www.iea.org/energy-system/low-emission-fuels/electrolysers, accessed 2023-09-03.
- M. Sand, R. B. Skeie, M. Sandstad, S. Krishnan, G. Myhre, H. Bryant, R. Derwent, D. Hauglustaine, F. Paulot, M. Prather and D. Stevenson, A Multi-Model Assessment of the Global Warming Potential of Hydrogen, Commun. Earth Environ., 2023, 4(1), 1–12, DOI:10.1038/s43247-023-00857-8.
- International Energy Agency, Clean Energy Innovation; Energy Technology Perspectives, International Energy Agency, Paris, 2020 Search PubMed.
- Intergovernmental Panel On Climate Change, Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, 1st edn, 2023, DOI:10.1017/9781009157896.
- Shell, Shell Sustainability Report 2021, Shell plc, 2021, https://reports.shell.com/sustainability-report/2021/services/downloads.html, accessed 2023-09-03 Search PubMed.
- International Energy Agency, Gas 2020, International Energy Agency, Paris, 2020, https://www.iea.org/reports/gas-2020 Search PubMed.
- International Energy Agency, Outlook for Biogas and Biomethane: Prospects for Organic Growth, International Energy Agency, Paris, 2020, https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth, accessed 2023-09-03 Search PubMed.
- Shell, Shell LNG Outlook 2022, 2022, https://www.shell.com/energy-and-innovation/natural-gas/liquefied-natural-gas-lng/lng-outlook-2022.html#iframe=L3dlYmFwcHMvTE5HX291dGxvb2tfMjAyMi8 Search PubMed.
- M. Decorte, A. Sainz and V. Perot, EBA - Statistical Report 2022 Tracking Biogas and Biomethane Deployment across Europe, European Biogas Association, Brussels, Belgium, Series edn., 2022 Search PubMed.
- P. Sun, B. Young, A. Elgowainy, Z. Lu, M. Wang, B. Morelli and T. Hawkins, Criteria Air Pollutants and Greenhouse Gas Emissions from Hydrogen Production in U.S. Steam Methane Reforming Facilities, Environ. Sci. Technol., 2019, 53(12), 7103–7113, DOI:10.1021/acs.est.8b06197.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape, Energy Environ. Sci., 2020, 13(2), 331–344, 10.1039/C9EE02873K.
- P. Wolfram, P. Kyle, X. Zhang, S. Gkantonas and S. Smith, Using Ammonia as a Shipping Fuel Could Disturb the Nitrogen Cycle, Nat. Energy, 2022, 7(12), 1112–1114, DOI:10.1038/s41560-022-01124-4.
- L. Lebreton and F. Ferrari, Evidence That the Great Pacific Garbage Patch Is Rapidly Accumulating Plastic, Sci. Rep., 2018, 8, 4666, DOI:10.1038/s41598-018-22939-w.
- European Green Deal: Putting an end to wasteful packaging, European Commission - European Commission, https://ec.europa.eu/commission/presscorner/detail/en/ip_22_7155, accessed 2023-09-17 Search PubMed.
- L. Godfrey, Waste Plastic, the Challenge Facing Developing Countries—Ban It, Change It, Collect It?, Recycling, 2019, 4(1), 3, DOI:10.3390/recycling4010003.
- O. S. Bushuyev, P. D. Luna, C. T. Dinh, L. Tao, G. Saur, J. Lagemaat, S. O. Kelley and E. H. Sargent, What Should We Make with CO2 and How Can We Make It?, Joule, 2018, 2(5), 825–832, DOI:10.1016/j.joule.2017.09.003.
- R. Francke, B. Schille and M. Roemelt, Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts, Chem. Rev., 2018, 118(9), 4631–4701, DOI:10.1021/acs.chemrev.7b00459.
- P. De Luna, C. Hahn, D. Higgins, S. A. Jaffer, T. F. Jaramillo and E. H. Sargent, What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes?, Science, 2019, 364(6438), eaav3506, DOI:10.1126/science.aav3506.
- M. Peplow, The Race to Upcycle CO2 into Fuels, Concrete and More, Nature, 2022, 603(7903), 780–783, DOI:10.1038/d41586-022-00807-y.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment, Chem. Rev., 2018, 118(2), 434–504, DOI:10.1021/acs.chemrev.7b00435.
- A. Caparrós and J.-C. Péreau, Multilateral versus Sequential Negotiations over Climate Change, Oxf. Econ. Pap., 2017, 69(2), 365–387 CrossRef.
- P. Barban, A. De Nazelle, S. Chatelin, P. Quirion and K. Jean, Assessing the Health Benefits of Physical Activity Due to Active Commuting in a French Energy Transition Scenario, Int. J. Public Health, 2022, 67, 1605012, DOI:10.3389/ijph.2022.1605012.
- C. Guivarch, A. Méjean, A. Pottier and M. Fleurbaey, Social Cost of Carbon: Global Duty, Science, 2016, 351(6278), 1160–1161, DOI:10.1126/science.351.6278.1160-b.
- M. Karlsson, E. Alfredsson and N. Westling, Climate Policy Co-Benefits: A Review, Clim. Pol., 2020, 20(3), 292–316, DOI:10.1080/14693062.2020.1724070.
- D. Carrington, Oil Sector's ‘Staggering’ $3bn-a-Day Profits for Last 50 Years, The Guardian, ed. D. C. E. Revealed, 2022 Search PubMed.
- L. Daumas, Financial Stability, Stranded Assets and the Low-Carbon Transition – A Critical Review of the Theoretical and Applied Literature, J. Econ. Surv., 2023, 1–116, DOI:10.1111/joes.12551.
- EuChemS, The Carbon Element – the Good, the Bad and the Ugly, https://www.euchems.eu/wp-content/uploads/2021/11/211103Press-Release_Periodic-Table.pdf.
- Element Scarcity - EuChemS Periodic Table, EuChemS, https://www.euchems.eu/euchems-periodic-table/, accessed 2024-03-26 Search PubMed.
- R. S. Cherry, A Hydrogen Utopia?, Int. J. Hydrogen Energy, 2004, 29(2), 125–129, DOI:10.1016/S0360-3199(03)00121-6.
- B. C. R. Ewan and R. W. K. Allen, A Figure of Merit Assessment of the Routes to Hydrogen, Int. J. Hydrogen Energy, 2005, 30(8), 809–819, DOI:10.1016/j.ijhydene.2005.02.003.
- A. Wijk, Hydrogen Key to a Carbon-Free Energy System, In Hydrogen key to a carbon-free energy system, De Gruyter, 2021, pp. 43–104, DOI:10.1515/9783110596250-005.
- C. W. Klok, A. F. Kirkels and F. Alkemade, Impacts, Procedural Processes, and Local Context: Rethinking the Social Acceptance of Wind Energy Projects in the Netherlands, Energy Res. Social Sci., 2023, 99, 103044, DOI:10.1016/j.erss.2023.103044.
- L. Cremonese, G. K. Mbungu and R. Quitzow, The Sustainability of Green Hydrogen: An Uncertain Proposition, Int. J. Hydrogen Energy, 2023, 48(51), 19422–19436, DOI:10.1016/j.ijhydene.2023.01.350.
- A. M. Oliveira, R. R. Beswick and Y. Yan, A Green Hydrogen Economy for a Renewable Energy Society, Curr. Opin. Chem. Eng., 2021, 33, 100701, DOI:10.1016/j.coche.2021.100701.
- R. R. Beswick, A. M. Oliveira and Y. Yan, Does the Green Hydrogen Economy Have a Water Problem?, ACS Energy Lett., 2021, 6(9), 3167–3169, DOI:10.1021/acsenergylett.1c01375.
- Solar Energy for Carbon-Free Liquid Fuel | Sun-To-X Project | Fact Sheet | H2020, CORDIS | European Commission, https://cordis.europa.eu/project/id/883264, accessed 2023-09-06 Search PubMed.
- International Energy Agency, Geopolitics of the Energy Transformation: The Hydrogen Factor, 2022 Search PubMed.
- Water “mega-basins” stir up turmoil in western France, Le Monde.fr, https://www.lemonde.fr/en/france/article/2022/11/28/water-mega-basins-stir-up-turmoil-in-western-france_6005917_7.html, accessed 2023-11-18 Search PubMed.
- F. Crellin, Warming Rivers Threaten France's Already Tight Power Supply, Warming rivers threaten France’s already tight power supply, Reuters. July 15, 2022, https://www.reuters.com/business/energy/warming-rivers-threaten-frances-already-tight-power-supply-2022-07-15/, accessed 2023-11-18.
- J. Terrapon-Pfaff, T. Fink, P. Viebahn and E. M. Jamea, Social Impacts of Large-Scale Solar Thermal Power Plants: Assessment Results for the NOORO I Power Plant in Morocco, Renew. Sustain. Energy Rev., 2019, 113, 109259, DOI:10.1016/j.rser.2019.109259.
- UNESCO, UN World Water Development Report 2023 : Partnerships and Cooperation for Water, 2023 Search PubMed.
- C. J. Stevens, Nitrogen in the Environment, Science, 2019, 363(6427), 578–580, DOI:10.1126/science.aav8215.
- FAO Hunger Map, https://www.fao.org/fileadmin/templates/SOFI/2022/docs/map-pou-print.pdf, accessed 2023-11-18.
- L. Kinsley, The Significance of Peruvian Guano in British Fertilizer History (c.1840–1880), Agric. Hist. Rev., 2022, 70(2), 219–240 Search PubMed.
- C. Wang, L. Zhao, M. K. Lim, W.-Q. Chen and J. W. Sutherland, Structure of the Global Plastic Waste Trade Network and the Impact of China's Import Ban, Resour. Conserv. Recycl., 2020, 153, 104591, DOI:10.1016/j.resconrec.2019.104591.
- Y. Bai and J. Givens, Ecologically Unequal Exchange of Plastic Waste? A Longitudinal Analysis of International Trade in Plastic Waste, J. World Syst. Res., 2021, 27(1), 265–287, DOI:10.5195/jwsr.2021.1026.
- A. Yoshida, China's Ban of Imported Recyclable Waste and Its Impact on the Waste Plastic Recycling Industry in China and Taiwan, J. Mater. Cycles Waste Manag., 2022, 24(1), 73–82, DOI:10.1007/s10163-021-01297-2.
- W.-T. Hsu, T. Domenech and W. McDowall, How Circular Are Plastics in the EU?: MFA of Plastics in the EU and Pathways to Circularity, Clean. Environ. Syst., 2021, 2, 100004, DOI:10.1016/j.cesys.2020.100004.
- Plastic waste shipments: new EU rules on importing and exporting plastic waste, https://environment.ec.europa.eu/news/plastic-waste-shipments-new-eu-rules-importing-and-exporting-plastic-waste-2020-12-22_en, accessed 2023-08-29.
- S. Fuller, T. Ngata, S. B. Borrelle and T. Farrelly, Plastics Pollution as Waste Colonialism in Te Moananui, J. Polit. Ecol., 2022, 29(1), 534–560, DOI:10.2458/jpe.2401.
- K. Tian and M. Bilal, Chapter 15 - Research Progress of Biodegradable Materials in Reducing Environmental Pollution, In Abatement of Environmental Pollutants, ed. P., Singh, A., Kumar and A., Borthakur, Elsevier, 2020, pp 313–330, DOI:10.1016/B978-0-12-818095-2.00015-1.
- E. Balestri, V. Menicagli, F. Vallerini and C. Lardicci, Biodegradable Plastic Bags on the Seafloor: A Future Threat for Seagrass Meadows?, Sci. Total Environ., 2017, 605–606, 755–763, DOI:10.1016/j.scitotenv.2017.06.249.
- T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society, Angew. Chem., Int. Ed., 2019, 58(1), 50–62, DOI:10.1002/anie.201805766.
- S. Karlsson and A. Albertsson, Biodegradable Polymers and Environmental Interaction, Polym. Eng. Sci., 1998, 38(8), 1251–1253, DOI:10.1002/pen.10294.
- K. Bucci, M. Tulio and C. M. Rochman, What Is Known and Unknown about the Effects of Plastic Pollution: A Meta-analysis and Systematic Review, Ecol. Appl., 2020, 30(2), e02044, DOI:10.1002/eap.2044.
- D.-J. van de Ven, I. Capellan-Peréz, I. Arto, I. Cazcarro, C. de Castro, P. Patel and M. Gonzalez-Eguino, The Potential Land Requirements and Related Land Use Change Emissions of Solar Energy, Sci. Rep., 2021, 11(1), 2907, DOI:10.1038/s41598-021-82042-5.
- International Energy Agency, The Role of Critical Minerals in Clean Energy Transitions - World Energy Outlook Special Report, 2021 Search PubMed.
- B. Lottermoser, Mine Wastes: Characterization, Treatment and Environmental Impacts, 2010, p 400 Search PubMed.
- J. Phillips, Climate Change and Surface Mining: A Review of Environment-Human Interactions & Their Spatial Dynamics, Appl. Geogr., 2016, 74, 95–108, DOI:10.1016/j.apgeog.2016.07.001.
- J. J. Leppänen, J. Weckström and A. Korhola, Multiple Mining Impacts Induce Widespread Changes in Ecosystem Dynamics in a Boreal Lake, Sci. Rep., 2017, 7(1), 10581, DOI:10.1038/s41598-017-11421-8.
- H. Schandl, M. Fischer-Kowalski, J. West, S. Giljum, M. Dittrich, N. Eisenmenger, A. Geschke, M. Lieber, H. Wieland, A. Schaffartzik, F. Krausmann, S. Gierlinger, K. Hosking, M. Lenzen, H. Tanikawa, A. Miatto and T. Fishman, Global Material Flows and Resource Productivity - Assessment Report for the UNEP International Resource Panel, 2016 Search PubMed.
- Statista - The Statistics Portal: Mining, Metals & Minerals, Statista, https://www.statista.com/markets/410/topic/954/mining-metals-minerals/, accessed 2023-08-31 Search PubMed.
- C. N. Boocock, Environmental Impacts of Foreign Direct Investment in the Mining Sector in Sub-Saharan Africa - OECD Global Forum on International Investment, 2022 Search PubMed.
- A. L. Gulley, China, the Democratic Republic of the Congo, and Artisanal Cobalt Mining from 2000 through 2020, Proc. Natl. Acad. Sci. U. S. A., 2023, 120(26), e2212037120, DOI:10.1073/pnas.2212037120.
- C. Banza Lubaba Nkulu, L. Casas, V. Haufroid, T. De Putter, N. D. Saenen, T. Kayembe-Kitenge, P. Musa Obadia, D. Kyanika Wa Mukoma, J.-M. Lunda Ilunga, T. S. Nawrot, O. Luboya Numbi, E. Smolders and B. Nemery, Sustainability of Artisanal Mining of Cobalt in DR Congo, Nat. Sustain., 2018, 1(9), 495–504, DOI:10.1038/s41893-018-0139-4.
- K. Svobodova, J. R. Owen, D. Kemp, V. Moudrý, É. Lèbre, M. Stringer and B. K. Sovacool, Decarbonization, Population Disruption and Resource Inventories in the Global Energy Transition, Nat. Commun., 2022, 13(1), 7674, DOI:10.1038/s41467-022-35391-2.
- K. Svobodova, J. R. Owen and J. Harris, The Global Energy Transition and Place Attachment in Coal Mining Communities: Implications for Heavily Industrialized Landscapes, Energy Res. Social Sci., 2021, 71, 101831, DOI:10.1016/j.erss.2020.101831.
- S. Carley and D. M. Konisky, The Justice and Equity Implications of the Clean Energy Transition, Nat. Energy, 2020, 5(8), 569–577, DOI:10.1038/s41560-020-0641-6.
- J. R. Owen, D. Kemp, A. M. Lechner, J. Harris, R. Zhang and É. Lèbre, Energy Transition Minerals and Their Intersection with Land-Connected Peoples, Nat. Sustain., 2023, 6(2), 203–211, DOI:10.1038/s41893-022-00994-6.
- J.-B. Fressoz, Sans Transition: Une Nouvelle Histoire de l’énergie, Seuil, Paris, 2024 Search PubMed.
- J.-P. Digard, Éric Baratay, La société des animaux. De la Révolution à la Libération, ed. de La Martinière, Etudes rurales, EHESS, 2009 Search PubMed.
- F. Jarrige and A. Vrignon, Face à la puissance : une histoire des énergies alternatives à l’âge industriel, La Découverte, 2020 Search PubMed.
- H. Ritchie, P. Rosado and M. Roser, Fossil Fuels. Our World in Data. https://ourworldindata.org/fossil-fuels, accessed 2023-11-18.
- J.-B. Fressoz, Pour une histoire des symbioses énergétiques et matérielles, Annales des Mines - Responsabilité et environnement, 2021, 101(1), 7–11, DOI:10.3917/re1.101.0007.
- C. F. Jones, Routes of Power: Energy and Modern America, Harvard University Press, Cambridge, MA, 2016 Search PubMed.
- A. Missemer, Les Économistes et La Fin Des Énergies Fossiles (1865-1931), Classiques Garnier, 2017, DOI:10.15122/isbn.978-2-406-06254-7.
- G. Yon, Building a National Machine: The Pricing of Electricity in Postwar France, Hist. Polit. Econ., 2020, 52(S1), 245–269, DOI:10.1215/00182702-8718035.
- C. D. Goodwin, Energy Policy in Perspective: Today's Problems, Yesterday’ Solution, Brookings Institution, Washington, 1981 Search PubMed.
- A. Beltran and P. Carré, La Vie Électrique. Histoire et Imaginaire (XVIIIe-XXIe Siècle), Paris: Belin, 2016 Search PubMed.
- M. Schmelzer and M. Büttner, Fossil Mentalities: How Fossil Fuels Have Shaped Social Imaginaries, Geoforum, 2024, 150, 103981, DOI:10.1016/j.geoforum.2024.103981.
- D. Keating, Did Germany just kill the electric car?https://www.energymonitor.ai/sectors/transport/did-germany-kill-the-electric-car/, accessed 2023-11-18.
- J. Rockström, J. Gupta, D. Qin, S. J. Lade, J. F. Abrams, L. S. Andersen, D. I. Armstrong McKay, X. Bai, G. Bala, S. E. Bunn, D. Ciobanu, F. DeClerck, K. Ebi, L. Gifford, C. Gordon, S. Hasan, N. Kanie, T. M. Lenton, S. Loriani, D. M. Liverman, A. Mohamed, N. Nakicenovic, D. Obura, D. Ospina, K. Prodani, C. Rammelt, B. Sakschewski, J. Scholtens, B. Stewart-Koster, T. Tharammal, D. van Vuuren, P. H. Verburg, R. Winkelmann, C. Zimm, E. M. Bennett, S. Bringezu, W. Broadgate, P. A. Green, L. Huang, L. Jacobson, C. Ndehedehe, S. Pedde, J. Rocha, M. Scheffer, L. Schulte-Uebbing, W. de Vries, C. Xiao, C. Xu, X. Xu, N. Zafra-Calvo and X. Zhang, Safe and Just Earth System Boundaries, Nature, 2023, 619(7968), 102–111, DOI:10.1038/s41586-023-06083-8.
- The Imaginary Institution of Society, MIT Press, https://mitpress.mit.edu/9780262031349/the-imaginary-institution-of-society/, accessed 2023-12-14 Search PubMed.
- R. P. MacDonald, A. N. Pattison, S. E. Cornell, A. K. Elgersma, S. N. Greidanus, S. N. Visser, M. Hoffman and P. G. Mahaffy, An Interactive Planetary Boundaries Systems Thinking Learning Tool to Integrate Sustainability into the Chemistry Curriculum, J. Chem. Educ., 2022, 99(10), 3530–3539, DOI:10.1021/acs.jchemed.2c00659.
- P. G. Mahaffy, A. Krief, H. Hopf, G. Mehta and S. A. Matlin, Reorienting Chemistry Education through Systems Thinking, Nat. Rev. Chem., 2018, 2, 0126, DOI:10.1038/s41570-018-0126.
- K. Raworth, Doughnut Economics: Seven Ways to Think Like a 21st-Century Economist, Chelsea Green Publishing, 2017 Search PubMed.
- R. B. Norgaard, Development Betrayed: The End of Progress and a Coevolutionary Revisioning of the Future, Routledge, 1994 Search PubMed.
- M. P. Vianna Franco and A. Missemer, A History of Ecological Economic Thought, Routledge: London, 2023, DOI:10.4324/9780429345623.
- D. H. Meadows, D. L. Meadows, J. Randers and W. W. III, The Limits to Growth, Club of Rome, 1972 Search PubMed.
- G. Herrington, Update to Limits to Growth: Comparing the World3 Model with Empirical Data, J. Ind. Ecol., 2021, 25(3), 614–626, DOI:10.1111/jiec.13084.
- D. H. Meadows, J. Randers and D. L. Meadows, The Limits to Growth: The 30-Year Update, Revised edition, Earthscan, London, 2004 Search PubMed.
- W. Dilthey, Gesammelte Schriften. 1, Einleitung in die Geisteswissenschaften : Versuch einer Grundlegung für das Studium der Gesellschaft und der Geschichte…[Wilhelm Dilthey], 1922 Search PubMed.
- T. Jackson, Prosperity without Growth: Economics for a Finite Planet, Earthscan, London ; Sterling, VA, 2009 Search PubMed.
- G. Kallis, C. Kerschner and J. Martinez-Alier, The Economics of Degrowth, Ecol. Econ., 2012, 84, 172–180, DOI:10.1016/j.ecolecon.2012.08.017.
- A. Escobar, J. Aste Daffós, M. Tabra, C. J. Echave, M. Giraud, S. G. Bunker, L. S. Wagner, M. V. Secreto, H. Alimonda, H. Machado Aráoz, M. Pablo-Romero, P. del, M. Pérez Negrete, M. Palacín Quispe, G. A. Palacio Castañeda, M. Svampa, M. J. Lamberti and H. A. Alimonda, La naturaleza colonizada: ecología política y minería en América Latina, Latin American Social Sciences Council, Colección Grupos de Trabajo, CLACSO: Buenos Aires, Argentina, 2011 Search PubMed.
- B. Escobar Andrae and N. Arellano Escudero, Green Innovation from the Global South: Renewable Energy Patents in Chile, 1877–1910, Bus. Hist. Rev., 2019, 93(02), 379–395, DOI:10.1017/S000768051900062X.
- M. Rezaei and H. Dowlatabadi, Off-Grid: Community Energy and the Pursuit of Self-Sufficiency in British Columbia's Remote and First Nations Communities, Local Environ., 2016, 21(7), 789–807, DOI:10.1080/13549839.2015.1031730.
- S. Baruah, J. Dutta and G. Hornyak, Poor Man's Nanotechnology—From the Bottom Up (Thailand), In Nanotechnology and Global Sustainability; Perspectives in Nanotechnology, CRC Press, 2011, vol. 20113230, pp 141–154, DOI:10.1201/b11299-9.
- P. Bihouix, L’Âge des low tech. Vers une civilisation techniquement soutenable, Seuil, Paris, 2014 Search PubMed.
- H. Alimonda, C. T. Pérez and F. Martín, Ecología Política Latinoamericana, CLACSO: Universidad Autónoma Metropolitana, Ciudad Autónoma de Buenos Aires, 2017 Search PubMed.
- S. Castro-Gómez, Ciencias sociales, violencia epistémica y el problema de la “invención del otro”, CLACSO, Buenos Aires, Argentina, 2000 Search PubMed.
- E. M. Conway and N. Oreskes, Merchants of Doubt, Bloomsbury Press, 2010 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc00099d |
| ‡ All authors are to be considered as co-first authors. See Section SI-1.2‡ for more details. |
| This journal is © The Royal Society of Chemistry 2024 |