Micro/nanomotors for neuromodulation
Yulin
Huang
and
Fei
Peng
 *
*
School of Materials Science and Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: pengf26@mail.sysu.edu.cn
First published on 31st March 2024
Abstract
Micro–nanomotors (MNMs) are micro/nanoscale intelligent devices with vast potential in the fields of drug delivery, precision medicine, biosensing, and environmental remediation. Their primary advantage lies in their ability to convert various forms of external energy (such as magnetic, ultrasonic, and light energy) into their own propulsive force. Additionally, MNMs offer high controllability and modifiability, enabling them to navigate in the microscopic world. Importantly, recent research has harnessed the unique advantages of MNMs to synergize their capabilities in neuromodulation. This mini-review presents the significant progress and pioneering achievements in the use of MNMs for neuromodulation, with the aim of inspiring readers to explore the broader biomedical applications of these MNMs. Through continuous innovation and diligent exploration, MNMs show promise to have a profound impact on the field of biomedicine.
1 Introduction
The film Fantastic Voyage released in 1966 vividly shows a microsubmarine sailing through blood and treating diseases, attracting worldwide researchers to focus on the research of micro- and nanoscale machineries. Half a century later, the Nobel Prize in Chemistry 2016 was jointly awarded to Jean-Pierre Sauvage, Fraser Stoddart and Ben Feringa for their pioneering contributions in the field of molecular machines.1 Inspired by molecular machines and benefitting from the rapid advancement of nanotechnology,2,3 various micro/nanomotors have been developed, which can convert chemical energy and other energy into mechanical energy at the micro/nanoscale, enabling them to move in a directional manner through their asymmetric structure, thereby performing more complex tasks.4Micro/nanomotors, also known as micro/nanomachines, micro/nanorobots and micro/nanoswimmers, are generally tens of nanometers to tens of micrometers in size, and many researchers have worked on using various ways to make them move. Because the Reynolds number of MNMs in water is extremely low, driving such a tiny device is a laborious task. In this case, the motion of motors is completely dominated by viscous force, which is similar to one swimming in a pool full of viscous syrup.5 Due to the time reversal symmetry of a low Reynolds number fluid, many macro-feasible propulsion methods are not applicable,6,7 such as breaststroke. Therefore, scientists have developed some novel propulsion mechanisms to drive MNMs. These mechanisms can be divided into two categories: (1) self-propulsion: achieved by creating an asymmetric field around the motor using chemical or physical reactions to induce fluid flow or by generating bubbles and direct mechanical forces to propel the motor. Self-propulsion can be subdivided into bubble propulsion,8–12 self-electrophoresis,13–15 self-diffusiophoresis,16–19 self-thermophoresis20–23 and biological agent propulsion (directly utilizing natural motile microorganisms to drive motors, such as bacteria or spermatozoa).24 (2) External propulsion: utilizing external energy for propulsion, including light, electricity, ultrasound, and particularly magnetic field,25,26 which is regarded as a facile method for clinical settings. Among these driving mechanisms, a common idea is to make physical or chemical reactions occur asymmetrically on the motor to generate uneven forces to propel itself.
Thanks to their ultra-small size, micro/nanomotors can perform operations in the microscale that cannot be reached by traditional devices, so they have a particularly profound impact on biomedicine.27,28 For instance, MNMs can be employed in microfluidic chips for micromanipulation,29,30 they can navigate in the natural lumen of an organism for medical imaging,31–33 and they can even have potential as label-free biomechanical probes.34,35 MNMs can also work together, and one can control the MNM groups and reach the target to release drugs.36,37
In the past few years, with the vigorous development of micro/nanomotors, reviews on different applications of motors have sprung up, which reflects researchers’ enthusiasm for more extensive application of MNMs. In recent years, our research group has revealed the immense potential of MNMs in neuromodulation, but this function has not attracted enough attention in industry. It is time to summarize the current research progress of MNMs in neuromodulation, which have not been reviewed yet.
2 Micro/nanomotors for neuromodulation
Neuromodulation refers to biomedical engineering technologies that utilize invasive or non-invasive techniques to modulate the activity of the nervous system through physical or chemical stimulus, resulting in specific functions.38 The development of neuromodulation techniques is not only an important pathway for basic neuroscience research, but also an effective option for treating neurological disorders, such as Alzheimer's disease, gastrointestinal diseases, Parkinson's disease, muscle tension disorders, obsessive-compulsive disorder, etc.Micro/nanomotors as emerging tools for non-invasive neuromodulation, have begun to receive attention from researchers due to their advantages of limited trauma and low cost.39 According to different mechanisms, MNMs for neuromodulation are divided into two categories in this article: chemical and physical pathways. The two have a common characteristic that they actually have the ability of nerve stimulation through direct electrical activation of the corresponding ion channels (such as thermosensitive ion channels, mechanosensitive ion channels, and specific ion channels activated by reactive oxygen species), making them particularly suitable for the treatment of neurological related diseases, which will be elaborated in the following sections.
2.1 Physical pathways
Physical stimulation (mechanical, thermal, acoustic, optical and electrical stimulus) is the most traditional neuromodulation option.40 In this section, we summarized the applications of cross-modal neuromodulation (such as magnetism-electric and ultrasonic-electric synergistic stimulation). It is worth noting that considering the long history and wide application of electrical stimulation, we present detailed introductions to self-established electricity and piezoelectric effects here. However, besides utilizing electricity, mechanical stimulation, thermal stimulation, and non-thermal stimulation using mid-infrared light have also been emerging neuromodulation methods in recent years.One approach to addressing the reliance on wired power supply is by utilizing the self-generated electric field produced by micro/nanomotors (MNMs). To this end, our research group has developed a water-driven Ni–Zn tubular MNM capable of magnetically controlling its direction for the purpose of directional differentiation of neural stem cells.46 The aim of this study is to propose new treatment strategies for neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease. The motor is fabricated using the electrochemical deposition method and serves as a typical representative of metal-based micro/nanomotors.47 The motor's motion mechanism primarily relies on the reaction between the Zn segment and water: Zn + 2H+ → Zn2+ + H2, which increases the concentration of Zn2+ ions in the zinc segment, consequently creating a self-generated electric field towards the Ni segment and inducing the motor's movement towards the Zn segment. It is noteworthy that the motion of the motor does not generate any bubbles. Consequently, when compared to previous chemically driven micromotors, the likelihood of gas blockage is eliminated. Under magnetic field control, the motor can achieve an average speed of 27.7 ± 1.5 μm s−1 in deionized water. Leveraging its self-propulsion and directional capability, motors can reach the region where endogenous neural stem cells reside, facilitating the exchange of bioelectric signals and intracellular communication with neural stem cells through the self-generated electrochemical fields. As a result, the neural stem cells can undergo differentiation into neurons. In vitro experiments involving the cocultivation of the motor and neural stem cells have shown significant differentiation of neural stem cells into neurons. Furthermore, in vivo experiments have provided evidence of the ability of the motor to guide neuronal regeneration. This study offers compelling evidence of the neuronal regeneration capabilities exhibited by micro/nanomotors, unveiling new possibilities for exploration in the field of minimally invasive precision medicine (Fig. 1).
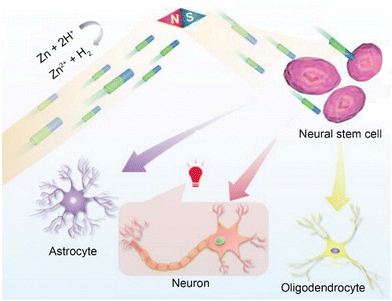 | ||
| Fig. 1 The schematic diagram illustrating the motion behavior of Ni–Zn micromotors, targeted activation and directed cell differentiation of NSCs. This figure has been reproduced from ref. 46 with permission from John Wiley and Sons, copyright 2023. The Ni–Zn micromotor approach targets NSCs under magnetic guidance. With a self-generated chemical electric field, the micromotors further activate voltage-gated ion channels in the cells, thus guiding the differentiation fate of the target NSCs. | ||
Additionally, our research group has also conducted other in-depth studies on the use of self-generated electricity for neural stimulation. For example, we have successfully developed motors based on Au–TiO2 and explored their application in modulating neuronal activity. Firstly, we synthesized the TiO2–Au-nanowire-based motor,48 which converts ultraviolet light energy into mechanical motion through photoelectric conversion, achieving highly controllable movement in a biological environment. By locally generating electric fields, motors were able to activate calcium ion channels and achieve precise manipulation and regulation of individual neurons. In addition to using MNMs for neural stimulation of individual neurons, the collective behavior of nanomotors has also intrigued us in the field of neuromodulation. Therefore, we further described the black (B)-TiO2@N/Au nanorobots.49 The main purpose of studying this motor is to explore collective intelligent systems that effectively interact with biological entities. When these nanomotors were exposed to program controlled near-infrared (NIR) light, they showcased periodic swarming oscillation behavior due to electrophoresis induced by photolytic water decomposition. It has been discovered that this oscillating electrochemical field leads to the activation of nearby neurons in vitro and triggers resonant oscillation of neural populations without any synaptic contact. This research explores the connection between oscillating nanomotor populations and biological rhythms, particularly neuronal activity, while effectively demonstrating the stability and biocompatibility of the nanomotor populations in various cellular environments. Ultimately, the significance of this research lies in the innovative collective resonant nanomotors which facilitate the transmission of neural signals beyond the millimeter scale, thus offering fresh and practical avenues for regulating neural activity and information transmission. Moreover, these findings have potential to contribute to the advancement of bioelectronics by providing valuable insights and potential applications (Fig. 2).
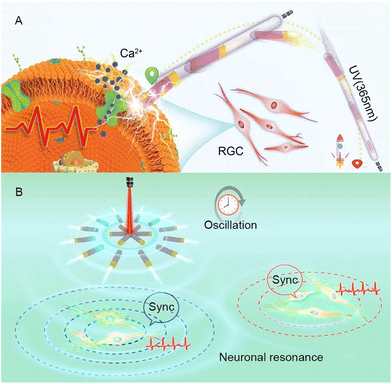 | ||
| Fig. 2 (A) TiO2–Au nanowire-based motors are capable of targeting neuronal retinal ganglion cells under ultraviolet irradiation. They generate electricity transmitted as a signal to the target cells and trigger cell activation. This figure has been reprinted from ref. 48 with permission from John Wiley and Sons, copyright 2020. (B) Under the programmed near-infrared light, the B-TiO2@N/Au nanorobot swarm demonstrates periodic swarm oscillations and triggers resonant oscillations of neuron populations without synaptic contacts (approximately 2 mm apart). This figure has been reproduced from ref. 49 with permission from the American Chemical Society, copyright 2023. | ||
It is essential to acknowledge that, despite significant progress being made in in vitro and animal experiments, there are still many obstacles to overcome on the path to clinical conversion. One such challenge involves the programmability of electric fields, which is critical for effective implementation.50,51
BaTiO3 is a piezoelectric material with a long history. As early as the 1950s, it was regarded as a promising candidate for applications in piezoelectric transducers.55 In recent years, it has emerged as a viable alternative to lead zirconate titanate [Pb(Zr,Ti)O3(PZT)], which is the most widely used piezoelectric ceramic material in the fields of neural modulation and neural tissue engineering. This substitution aims to mitigate the severe toxicity associated with lead.56 Our research group proposed a strategy that combines the piezoelectric effect of BaTiO3 with micro/nanomotors for neural stem-like cell stimulation. To this end, we have designed and fabricated a magnetic and piezoelectric nanomotor, denoted as S. platensis@Fe3O4@tBaTiO3, which serves as a highly controllable platform for neural stem cell stimulation.57 The preparation of this nanomotor involved the coating of Fe3O4 and BaTiO3 nanoparticles onto S. platensis. The Fe3O4 component endows the nanomotor with the capability to autonomously migrate towards the target neural stem cell position under the influence of a magnetic field. And BaTiO3 exhibits the property of converting ultrasonic energy into electrical signals, thereby inducing the differentiation of the desired target cells. The research team has further investigated the functionalities of the nanomotor. By varying the intensity of ultrasonic stimulation, neural stem cells can be directed by the nanomotor to differentiate into astrocytes, functional neurons, and oligodendrocytes.58 Notably, our study also demonstrates the differentiation of neural stem cells into dopamine neurons and cholinergic neurons, offering potential remedies for disorders associated with the degeneration of these specific neuron types (Fig. 3).
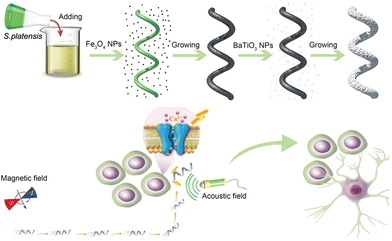 | ||
| Fig. 3 The schematic diagram depicting the assembly of S. platensis@Fe3O4@tBaTiO3 micromotors, and the motors precisely stimulate the differentiation of neural stem cell-like cells under magnetic driving. This figure has been adapted from ref. 57 with permission from John Wiley and Sons, copyright 2020. | ||
In general, piezoelectric micro/nanomotors have broad prospects for applications in the field of neuroscience. Other piezoelectric materials, such as molybdenum disulfide (MoS2), have recently been reported as adjustable artificial synapses, providing new inspiration for the development of more piezoelectric micro–nanomotors.59 However, there are still some issues in research that cannot be ignored, such as the biocompatibility of piezoelectric materials in synergy with MNMs. Taking the classic piezoelectric material zinc oxide nanoparticles (ZnO NPs) as an example, although ZnO NPs were previously considered to have low toxicity, recent research has reported various toxicities and mechanisms of ZnO NPs.60 In addition, ultrasound, as the signal to trigger the piezoelectric effect in MNMs, may face attenuation issues inside the human body. Nevertheless, excessive increase in ultrasound intensity may lead to side effects such as excessive thermal and ultrasonic cavitation effects.
In addition to nerve regulation through electricity, other physical stimulation has expanded the toolkit and provided valuable inspiration for subsequent research. Regarding thermal stimulation, recent research by Wu et al. utilized infrared light within the 400–1800 nm range to implement deep brain stimulation in mice via the photothermal conversion of nanoparticles.61 However, given the limitations associated with using light-thermal stimulation, a growing number of researchers are turning their focus towards non-thermal methods of nerve stimulation. For instance, Wu et al.34 developed nanoparticles that directly employ blue light excited by near-infrared light for neuronal stimulation, taking an important step towards non-invasive deep brain stimulation.62 Furthermore, mechanically sensitive ion channels have received much interest. Collier et al. designed a magnetically-driven magnetic microdisk (MMD) with a diameter of 4 μm and thickness of 100 nm for activating hippocampal neurons and cortical neurons, showcasing high efficiency and prolonged stimulation characteristics.63
2.2 Chemical pathways
Not only in the field of micro/nanomotors, previous research has devoted an excessive amount of attention to the application of electrical stimulation in the field of neuromodulation. The majority of commercially available neuromodulation methods rely on electrical stimulation, while current studies on neuromodulation using chemical approaches primarily concentrate on drug design or passive targeting strategies utilizing nanoparticles.54 Therefore, there is a need for increased focus on actively targeted neuromodulation methods that involve principles of chemistry.Reactive oxygen species (ROS) have a significant impact on the nervous system. ROS is a natural byproduct of oxygen metabolism and plays crucial roles in cell signaling and homeostasis. ROS have been demonstrated to activate particular ion channels, including the mammalian transient receptor potential (TRP) channels.64 Significantly, the ROS and calcium pathways have the ability to interact,65 rendering them suitable for neural regulation. However, exposure to environmental pressures such as ultraviolet radiation, heat, and trauma can cause a sharp increase in ROS levels, leading to severe damage to cell structures, which is known as oxidative stress.66 Numerous researchers in the field of micro/nanomotors are dedicated to counteracting ROS via various strategies to minimize their harmful effects on the nervous system.67–69 Interestingly, ROS generation can also be harnessed to eliminate proteins responsible for neurodegenerative diseases in the nervous system.70–72 Due to the effects of ROS on normal cells including oxidative stress and carcinogenic risk, many ROS probes have been developed to prevent damage to normal tissues. In addition, methods for regulating ROS concentration have also emerged. For example, Sun's team developed a titanium oxide electrocatalyst,73 which promotes ROS generation at low ROS concentrations but exhibits inhibitory effects at high concentrations. It can be expected that this technology can be applied in the field of micro/nanomotors, such as for photodynamic therapy and neural modulation. However, due to the complexity of ROS composition, it remains a challenge to be addressed for biomedical applications of ROS. This section aims to present recent research trends over the past three years and elucidate the extensive development possibilities of ROS as a typical chemical neuromodulation approach, in combination with MNMs.
To address this, Xue's team proposed Janus catalysis-driven nanomotors (JCNs) using a plasma-induced alloying technique and sputtering-caused half-coating strategy, successfully solving the challenge of rapid and deep penetration into brain tissue.68 Theoretical calculations and experimental results demonstrate that heteroatom-doped alloy engines endow JCNs with higher catalytic activity in the removal of reactive oxygen species (ROS) and reactive nitrogen species (RNS) compared to common Pt-based motors. When JCNs are dropped onto the surface of a fractured skull, they effectively catalyze endogenous hydrogen peroxide, as fuel to promote JCNs to infiltrate deep brain injuries. This allows for further nanocatalyst-mediated cascade-blocking therapy to clear ROS and RNS, such as hydroxyl radicals, superoxide radicals, and peroxynitrite ions. Ultimately, JCNs reversed various behavioral impairments and significantly reduced the mortality rate of mice with sTBI. In summary, this work provides a revolutionary nanomotor-based strategy for sensing brain injuries and clearing oxidative stress, demonstrating significant therapeutic potential in assisting emergency TBI.
In addition, spinal cord injury (SCI) is an external trauma that brings heavy physical and economic burden to patients. The pathological process of SCI is complex, and once it occurs, it leads to the loss or severe impairment of motor, sensory, and autonomic functions.76,77 The existence of the blood–spinal cord barrier (BSCB) creates challenges in facilitating the access of numerous drugs employed for SCI treatment to the specific site of spinal cord injury. Consequently, the selection of an effective drug delivery system to improve treatment outcomes has become a hot topic in current research.
Recently, Mao and Wan's research group have provided a new strategy for treating SCI through the design of a poly(2-methacryloyloxyethyl phosphorylgallylcholine) (PMPC)/L-arginine (PMPC/A)-based nanomotor.67 This nanomotor can release nitric oxide (NO), load the nerve growth factor (NGF), and inducible nitric oxide synthase inhibitor 1400W. With its high drug loading capacity and strong ability to penetrate pathological tissues, it shows great potential in SCI treatment. Researchers have also discovered that this nanomotor can lower oxidative damage and inhibit the expression of nitric oxide synthase by regulating the levels of ROS, leading to the suppression of inflammatory reactions. Additionally, the nanomotor can effectively penetrate the BSCB, then by regulating the internal environment and releasing therapeutic drugs, restore the motor function in a rat SCI model (Fig. 4).
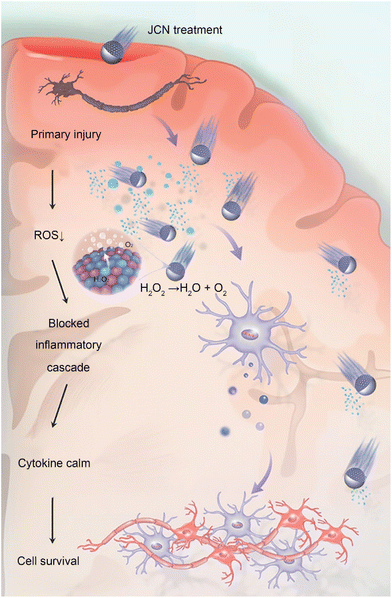 | ||
| Fig. 4 JCNs decompose excessive ROS to generate an asymmetric pressure wave to propel themselves forward, and the decomposition of ROS can also block the inflammatory cascade reaction induced by oxidative stress, thus preventing secondary damage to brain tissues. This figure has been reproduced from ref. 68 with permission from John Wiley and Sons, copyright 2022. | ||
These studies have demonstrated the potential of MNMs in the treatment of acute neurological disorders. They provide new ideas for developing novel therapeutic strategies, improving treatment efficiency, and shortening treatment duration. However, these studies are still in the laboratory stage and require further clinical research and validation to ensure safety and efficacy.
To enhance the dissociation effect, the Mayorga-Burrezo team has designed a light-driven micromotor based on concave BiVO4 microspheres.70 Experimental results demonstrate that this micromotor can generate reactive ROS, such as superoxide radicals and hydroxyl radicals, to induce protein fiber disaggregation. The study also reveals that the helical trajectory of the motor enhances this effect through the uniform distribution of ROS. This motor not only exhibits great potential in dismantling protein fibers, but also has the potential to be extended to photodynamic therapies, such as lung cancer or skin cancer, which can utilize ROS for treatment. This work provides crucial insights into the application of novel strategies in the treatment of neurodegenerative disorders, filling a gap in the application of MNMs for protein fibril disaggregation.
Although this is an exciting new discovery, the detrimental impact of ROS on normal tissues cannot be disregarded. Elevated ROS levels can lead to damage in proteins, lipids, and DNA, resulting in oxidative stress and ultimately contributing to the development of neurodegenerative diseases.79 In addition, the potential adverse effects of ROS produced by MNMs on cells or animals have not been definitively established, creating a controversial issue. Therefore, it is imperative to precisely regulate the concentration range of ROS generation to ensure that it does not induce severe consequences while effectively eliminating pathological proteins. Furthermore, Hajipour et al. propose that shorter fibrils generated after nanoparticle disaggregation may exhibit more potent pathogenic effects compared to longer fibrils. This observation could be attributed to their smaller size and increased mobility, enabling them to insert and disrupt membrane environments.80 As a result, the traditional assumption that protein fibril dissolution will lead to laxation in Alzheimer's disease must be reevaluated. It is important to acknowledge that while this research presents a groundbreaking application, there is still a long way to go before clinical implementation and it requires further efforts (Fig. 5).
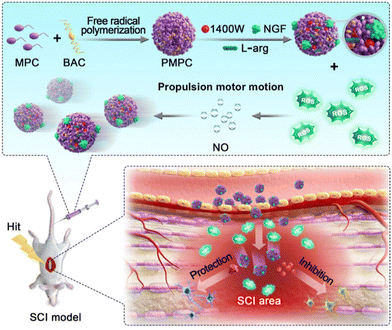 | ||
| Fig. 5 L-Arginine loaded on PMPC/A/1400W/NflF nanomotors can react with reactive oxygen species in the injured neural microenvironment to produce NO, thereby propelling the motors. The motors can effectively penetrate the BSCB and restore motion function by regulating the internal environment of the rat SCI model and the release of therapeutic drugs. This figure has been adapted from ref. 67 with permission from the American Chemical Society, copyright 2023. | ||
3 Conclusions and outlook
The field of micro/nanomotors has witnessed significant advancements, leading to the development and application of motors with diverse mechanisms and structures in various biomedical scenarios. Despite these advancements, there remain untapped potential areas that are suitable for exploration. Thus, this article aims to review representative emerging examples of MNMs in the field of neuromodulation within the last three years, shedding light on their applications in this field. As a newly emerging application scenario in recent years, neuromodulation demonstrates the revolutionary potential of MNMs in the field of nanoscience for practical applications. Science is limitless, and we believe that in addition to the several mechanisms mentioned in this minireview, more physical or chemical properties proposed in fundamental research on MNMs will be applied to neuromodulation. We discuss the challenges faced by this field and its future development direction in this section in order to provide new inspiration for relevant researchers and urging attention to conflicts in upstream theories in future research. This is the purpose of this minireview.(1) The advantages revealed by MNMs serve as a source of inspiration for this field. MNMs possess a high spatial resolution due to their minuscule size, enabling precise stimulation of neurons through accurate positioning. This characteristic is particularly beneficial for achieving high spatial resolution in neuroregulation with the use of piezoelectric nanomotors. MNMs share the strengths of non-invasiveness, controllability, and multifunctionality. Compared with traditional methods of electrical or chemical stimulation, the use of MNMs is less invasive, minimizing damage to tissues and neurons. By modifying the shape, size, and composition of motors, the properties and intensity of piezoelectric effects can be controlled, facilitating various modes of neuromodulation to meet the diverse requirements and provide customized treatment approaches. Furthermore, the mechanisms of MNMs are often not singular, which is known as cross-modal neuromodulation, further augmenting their multifunctionality.54 This attribute positions MNMs as a highly promising tool for neuromodulation, capable of selectively exerting their effects in different application scenarios.
(2) Focus is required on the challenges currently faced in the field of micro/nanomotors and further promoting their practical biomedical applications. Biocompatibility is a crucial consideration, as studies have shown that nanomaterials can induce adverse effects such as immune reactions, cell toxicity, and inflammatory responses. Safety is paramount when utilizing MNMs for neuromodulation due to the sensitivity and significance of the nervous system. For the biocompatibility of neurobiological materials, it is required that the material has no adverse effects on neurons during degradation. In addition to the toxicity during the degradation process, in terms of physical compatibility, materials in contact with nerves need to be compatible biomechanically, otherwise inflammatory reactions can be induced.81 However, several factors persist regarding the potential long-term risks of MNM utilization in the nervous system, including the stability, distribution, and metabolic pathways of nanoparticles. Additionally, the lack of standardization and regulation hampers the progress of research and applications in the field of neural modulation using MNMs. Conducting more comprehensive investigations to determine the optimal dosage, actuation duration, frequency, and other parameters of nanomotors is essential to ensure the feasibility of practical implementations. Subsequent research should give priority to addressing these challenges to promote the further development and application of MNMs in neural modulation.
(3) Attention to current upstream research enables the discovery of new possibilities for nanomotors. For instance, significant advancements have been achieved in optogenetics involving model organisms like Caenorhabditis elegans, which are relevant to neuromodulation.82,83 In addition, there is a long research history on related nanoparticles, such as upconversion nanoparticles (UCNPs).84,85 However, the spatiotemporal accuracy of these nanoparticles is hindered by the scattering of light in biological tissues and their inadequate targeting efficiency. MNMs offer potential solutions to address this issue. It is crucial to acknowledge that genetic modifications necessary for this technology continue to be constrained within human and non-human primate species, resulting in a challenging path towards clinical trials.
In the past two decades, we have witnessed the emergence of the first micro/nanomotor and its rapid proliferation, showcasing its value in addressing various complex problems. In the future, with the close integration of medicine, materials, chemistry, biology, and nanotechnology, micro/nanomotors are destined to evolve into powerful tools for fundamental neuroscience research and clinical neuromodulation.
Author contributions
Yulin Huang: investigation and writing – original draft. Fei Peng: conceptualization, funding acquisition, supervision, and writing – review and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22375224, 51973241, and 22175083), Guangdong Provincial Science Foundation for Distinguished Young Scholars (Grant No. 2018B030306007) and National Key Research and Development Program of China (2022YFA1206900).Notes and references
- V. Richards, Nat. Chem., 2016, 8, 1090–1090 CrossRef CAS PubMed.
- T. J. Huang and B. K. Juluri, Nanomedicine, 2008, 3, 107–124 CrossRef CAS PubMed.
- W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2006, 1, 25–35 CrossRef CAS PubMed.
- S. Sánchez, L. Soler and J. Katuri, Angew. Chem., Int. Ed., 2015, 54, 1414–1444 CrossRef PubMed.
- T. Liu, L. Xie, C.-A. H. Price, J. Liu, Q. He and B. Kong, Chem. Soc. Rev., 2022, 51, 10083–10119 RSC.
- A. Najafi and R. Golestanian, J. Phys.: Condens. Matter, 2005, 17, S1203 CrossRef CAS.
- E. M. Henley, Int. J. Mod. Phys. E, 2013, 22, 1330010 CrossRef.
- R. F. Ismagilov, A. Schwartz, N. Bowden and G. M. Whitesides, Angew. Chem., Int. Ed., 2002, 41, 652–654 CrossRef CAS.
- A. A. Solovev, Y. Mei, E. Bermúdez Ureña, G. Huang and O. G. Schmidt, Small, 2009, 5, 1688–1692 CrossRef CAS PubMed.
- Y. Mei, A. A. Solovev, S. Sanchez and O. G. Schmidt, Chem. Soc. Rev., 2011, 40, 2109–2119 RSC.
- A. A. Solovev, S. Sanchez, M. Pumera, Y. F. Mei and O. G. Schmidt, Adv. Funct. Mater., 2010, 20, 2430–2435 CrossRef CAS.
- K. M. Manesh, M. Cardona, R. Yuan, M. Clark, D. Kagan, S. Balasubramanian and J. Wang, ACS Nano, 2010, 4, 1799–1804 CrossRef CAS PubMed.
- R. Liu and A. Sen, J. Am. Chem. Soc., 2011, 133, 20064–20067 CrossRef CAS PubMed.
- W. F. Paxton, A. Sen and T. E. Mallouk, Chem. – Eur. J., 2005, 11, 6462–6470 CrossRef CAS PubMed.
- W. Wang, W. Duan, S. Ahmed, T. E. Mallouk and A. Sen, Nano Today, 2013, 8, 531–554 CrossRef CAS.
- S. Ahmed, W. Wang, L. Bai, D. T. Gentekos, M. Hoyos and T. E. Mallouk, ACS Nano, 2016, 10, 4763–4769 CrossRef CAS PubMed.
- Y. Hong, M. Diaz, U. M. Córdova-Figueroa and A. Sen, Adv. Funct. Mater., 2010, 20, 1568–1576 CrossRef CAS.
- J. Palacci, S. Sacanna, A. P. Steinberg, D. J. Pine and P. M. Chaikin, Science, 2013, 339, 936–940 CrossRef CAS PubMed.
- T. Liu, L. Xie, J. Zeng, M. Yan, B. Qiu, X. Wang, S. Zhou, X. Zhang, H. Zeng, Q. Liang, Y. He, K. Liang, J. Liu, E. Velliou, L. Jiang and B. Kong, ACS Appl. Mater. Interfaces, 2022, 14, 15517–15528 CrossRef CAS PubMed.
- Z. Li, S. Fu, H. Li, B. Chen, D. Xie, D. Fu, Y. Feng, C. Gao, S. Liu, D. A. Wilson, Y. Tu and F. Peng, Chem. Eng. J., 2023, 468, 143393 CrossRef CAS.
- X. Han, Z. Chen, Y. Liu, B. Song, H. Zhang and B. Dong, ChemistrySelect, 2023, 8, e202203888 CrossRef CAS.
- W. Liu, W. Wang, X. Dong and Y. Sun, ACS Appl. Mater. Interfaces, 2020, 12, 12618–12628 CrossRef CAS PubMed.
- Y. Xing, M. Zhou, X. Liu, M. Qiao, L. Zhou, T. Xu, X. Zhang and X. Du, Chem. Eng. J., 2023, 461, 142142 CrossRef CAS.
- Y. Alapan, O. Yasa, B. Yigit, I. C. Yasa, P. Erkoc and M. Sitti, Annu. Rev. Control Robot. Auton. Syst., 2019, 2, 205–230 CrossRef.
- M. Yang, Y. Zhang, F. Mou, C. Cao, L. Yu, Z. Li and J. Guan, Sci. Adv., 2023, 9, eadk7251 CrossRef CAS PubMed.
- Z. Yu, L. Li, F. Mou, S. Yu, D. Zhang, M. Yang, Q. Zhao, H. Ma, W. Luo, T. Li and J. Guan, InfoMat, 2023, 5, e12464 CrossRef CAS.
- W. Wang, J. Pang, J. Su, F. Li, Q. Li, X. Wang, J. Wang, B. Ibarlucea, X. Liu, Y. Li, W. Zhou, K. Wang, Q. Han, L. Liu, R. Zang, M. H. Rümmeli, Y. Li, H. Liu, H. Hu and G. Cuniberti, InfoMat, 2022, 4, e12262 CrossRef.
- J. Wang and W. Gao, ACS Nano, 2012, 6, 5745–5751 CrossRef CAS PubMed.
- L. Baraban, D. Makarov, R. Streubel, I. Mönch, D. Grimm, S. Sanchez and O. G. Schmidt, ACS Nano, 2012, 6, 3383–3389 CrossRef CAS PubMed.
- J. Burdick, R. Laocharoensuk, P. M. Wheat, J. D. Posner and J. Wang, J. Am. Chem. Soc., 2008, 130, 8164–8165 CrossRef CAS PubMed.
- S. Zheng, Y. Wang, S. Pan, E. Ma, S. Jin, M. Jiao, W. Wang, J. Li, K. Xu and H. Wang, Adv. Funct. Mater., 2021, 31, 2100936 CrossRef CAS.
- G. T. van Moolenbroek, T. Patiño, J. Llop and S. Sánchez, Adv. Intell. Syst., 2020, 2, 2000087 CrossRef.
- C. Gao, Y. Wang, Z. Ye, Z. Lin, X. Ma and Q. He, Adv. Mater., 2021, 33, 2000512 CrossRef CAS PubMed.
- M. Pal, N. Somalwar, A. Singh, R. Bhat, S. M. Eswarappa, D. K. Saini and A. Ghosh, Adv. Mater., 2018, 30, 1800429 CrossRef PubMed.
- S. Ahmed, W. Wang, L. Bai, D. T. Gentekos, M. Hoyos and T. E. Mallouk, ACS Nano, 2016, 10, 4763–4769 CrossRef CAS PubMed.
- D. Xu, Y. Wang, C. Liang, Y. You, S. Sanchez and X. Ma, Small, 2020, 16, 1902464 CrossRef CAS PubMed.
- H. Choi, B. W. Hwang, K. M. Park, K. S. Kim and S. K. Hahn, Part. Part. Syst. Charact., 2020, 37, 1900418 CrossRef CAS.
- S. Luan, I. Williams, K. Nikolic and T. G. Constandinou, Front. Neuroeng., 2014, 7, 1662–6443 Search PubMed.
- S. Chen, Y. Chen, M. Fu, Q. Cao, B. Wang, W. Chen and X. Ma, J. Mater. Chem. B, 2022, 10, 7099–7107 RSC.
- P. M. Lewis, R. H. Thomson, J. V. Rosenfeld and P. B. Fitzgerald, Neuroscientist, 2016, 22, 406–421 CrossRef CAS PubMed.
- Y. Temel and A. Jahanshahi, Science, 2015, 347, 1418–1419 CrossRef PubMed.
- X. Yang, T. Zhou, T. J. Zwang, G. Hong, Y. Zhao, R. D. Viveros, T.-M. Fu, T. Gao and C. M. Lieber, Nat. Mater., 2019, 18, 510–517 CrossRef CAS PubMed.
- R. Feiner and T. Dvir, Nat. Rev. Mater., 2017, 3, 17076 CrossRef.
- J. Deng, H. Yuk, J. Wu, C. E. Varela, X. Chen, E. T. Roche, C. F. Guo and X. Zhao, Nat. Mater., 2021, 20, 229–236 CrossRef CAS PubMed.
- Y. Zhang, L. Zhou, X. Gao, C. Liu, H. Chen, H. Zheng, J. Gui, C. Sun, L. Yu and S. Guo, Nano Energy, 2021, 89, 106319 CrossRef CAS.
- Y. Feng, C. Gao, D. Xie, L. Liu, B. Chen, S. Liu, H. Yang, Z. Gao, D. A. Wilson, Y. Tu and F. Peng, Adv. Mater., 2023, 35, 2301736 CrossRef CAS PubMed.
- J. Zhang, Z. Chen, R. K. Kankala, S.-B. Wang and A.-Z. Chen, Int. J. Pharm., 2021, 596, 120275 CrossRef CAS PubMed.
- B. Chen, L. Liu, K. Liu, F. Tong, S. Wang, D. Fu, J. Gao, J. Jiang, J. Ou, Y. Ye, D. A. Wilson, Y. Tu and F. Peng, Adv. Funct. Mater., 2021, 31, 2008667 CrossRef CAS.
- B. Chen, H. Tan, M. Ding, L. Liu, S. Wang, X. Peng, H. Tian, J. Jiang, J. Gao, W. Huang, H. Li, Y. Ye, F. Wang, D. A. Wilson, Y. Tu and F. Peng, ACS Nano, 2023, 17, 13826–13839 CrossRef CAS PubMed.
- C. M. Lam, U. Latif, A. Sack, S. Govindan, M. Sanderson, D. T. Vu, G. Smith, D. Sayed and T. Khan, Bioengineering, 2023, 10, 185 CrossRef PubMed.
- C. H. Knowles, S. de Wachter, S. Engelberg, P. Lehur, K. E. Matzel, L. Zirpel and The European SNM Expert Group, Colorectal Dis., 2021, 23, 592–602 CrossRef PubMed.
- T. Sun, J. Wright and T. Datta-Chaudhuri, Bioelectron. Med., 2020, 6, 16 CrossRef PubMed.
- T. Zhang, H. Liang, Z. Wang, C. Qiu, Y. B. Peng, X. Zhu, J. Li, X. Ge, J. Xu, X. Huang, J. Tong, J. Ou-Yang, X. Yang, F. Li and B. Zhu, Sci. Adv., 2022, 8, eabk0159 CrossRef CAS PubMed.
- X. Yang, E. McGlynn, R. Das, S. P. Paşca, B. Cui and H. Heidari, Adv. Mater., 2021, 33, 2103208 CrossRef CAS PubMed.
- H. Jaffe, Ind. Eng. Chem., 1950, 42, 264–268 CrossRef CAS.
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti, Jr. and J. Rödel, Appl. Phys. Rev., 2017, 4, 041305 Search PubMed.
- L. Liu, B. Chen, K. Liu, J. Gao, Y. Ye, Z. Wang, N. Qin, D. A. Wilson, Y. Tu and F. Peng, Adv. Funct. Mater., 2020, 30, 1910108 CrossRef CAS.
- L. Liu, J. Wu, S. Wang, L. Kun, J. Gao, B. Chen, Y. Ye, F. Wang, F. Tong, J. Jiang, J. Ou, D. A. Wilson, Y. Tu and F. Peng, Nano Lett., 2021, 21, 3518–3526 CrossRef CAS PubMed.
- W. Huh, D. Lee, S. Jang, J. H. Kang, T. H. Yoon, J.-P. So, Y. H. Kim, J. C. Kim, H.-G. Park, H. Y. Jeong, G. Wang and C.-H. Lee, Adv. Mater., 2023, 35, 2211525 CrossRef CAS PubMed.
- C. T. Ng, L. Q. Yong, M. P. Hande, C. N. Ong, L. E. Yu, B. H. Bay and G. H. Baeg, Int. J. Nanomed., 2017, 12, 1621–1637 CrossRef CAS PubMed.
- X. Wu, Y. Jiang, N. J. Rommelfanger, F. Yang, Q. Zhou, R. Yin, J. Liu, S. Cai, W. Ren, A. Shin, K. S. Ong, K. Pu and G. Hong, Nat. Biomed. Eng., 2022, 6, 754–770 CrossRef CAS PubMed.
- S. Chen, A. Z. Weitemier, X. Zeng, L. He, X. Wang, Y. Tao, A. J. Y. Huang, Y. Hashimotodani, M. Kano, H. Iwasaki, L. K. Parajuli, S. Okabe, D. B. L. Teh, A. H. All, I. Tsutsui-Kimura, K. F. Tanaka, X. Liu and T. J. McHugh, Science, 2018, 359, 679–684 CrossRef CAS PubMed.
- C. Collier, N. Muzzio, R. Thevi Guntnur, A. Gomez, C. Redondo, R. Zurbano, I. K. Schuller, C. Monton, R. Morales and G. Romero, Adv. Healthcare Mater., 2022, 11, 2101826 CrossRef CAS PubMed.
- A. V. Zholos, Curr. Neuropharmacol., 2015, 13, 279–291 CrossRef CAS PubMed.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- J.-C. Preiser, J. Parenter. Enteral Nutr., 2012, 36, 147–154 CrossRef CAS PubMed.
- T. Bao, N. Li, H. Chen, Z. Zhao, J. Fan, Y. Tao, C. Chen, M. Wan, G. Yin and C. Mao, ACS Appl. Mater. Interfaces, 2023, 15, 32762–32771 CrossRef CAS PubMed.
- H. Wang, X. Chen, Y. Qi, C. Wang, L. Huang, R. Wang, J. Li, X. Xu, Y. Zhou, Y. Liu and X. Xue, Adv. Mater., 2022, 34, 2206779 CrossRef CAS PubMed.
- W. Venegoni, Q. Shen, A. R. Thimmesch, M. Bell, J. B. Hiebert and J. D. Pierce, J. Adv. Nurs., 2017, 73, 1331–1338 CrossRef PubMed.
- P. Mayorga-Burrezo, C. C. Mayorga-Martinez and M. Pumera, Adv. Funct. Mater., 2022, 32, 2106699 CrossRef CAS.
- F. Zeng, K. Peng, L. Han and J. Yang, ACS Biomater. Sci. Eng., 2021, 7, 3573–3585 CrossRef CAS PubMed.
- C. L. Bigarella, R. Liang and S. Ghaffari, Development, 2014, 141, 4206–4218 CrossRef CAS PubMed.
- Y. Sun, D. Lu, H. Zhang, G. Liu, Y. Hu, H. Xie and J. Ma, Environ. Sci. Technol., 2023, 57, 13226–13235 CrossRef CAS PubMed.
- S. Kalra, R. Malik, G. Singh, S. Bhatia, A. Al-Harrasi, S. Mohan, M. Albratty, A. Albarrati and M. M. Tambuwala, Inflammopharmacology, 2022, 30, 1153–1166 CrossRef CAS PubMed.
- E. Prasetyo, Crit. Care Shock, 2020, 23, 4–13 Search PubMed.
- M. Karsy and G. Hawryluk, Curr. Neurol. Neurosci. Rep., 2019, 19, 65 CrossRef PubMed.
- T. N. Bryce, V. Huang and M. X. Escalon, Braddom's Phys. Med. Rehabil, Elsevier, 2021, pp. 1049–1100 Search PubMed.
- D. M. Wilson 3rd, M. R. Cookson, L. Van Den Bosch, H. Zetterberg, D. M. Holtzman and I. Dewachter, Cell, 2023, 186, 693–714 CrossRef PubMed.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS PubMed.
- M. J. Hajipour, H. Mohammad-Beigi, I. Nabipour, N. Mahmoudi, M. Azhdarzadeh, H. Derakhshankhah, D. E. Dawud, R. Mohammadinejad and D. E. Otzen, Nano Today, 2020, 35, 100983 CrossRef CAS.
- S. Bao, Y. Lu, J. Zhang, L. Xue, Y. Zhang, P. Wang, F. Zhang, N. Gu and J. Sun, Nanoscale, 2023, 15, 3532–3541 RSC.
- Y. Tsukada and I. Mori, in Optogenetics in Caenorhabditis elegans, ed. H. Yawo, H. Kandori, A. Koizumi and R. Kageyama, Springer Singapore, Singapore, 2021, pp. 321–334 Search PubMed.
- X. Dong, S. Kheiri, Y. Lu, Z. Xu, M. Zhen and X. Liu, Sci. Robot., 2021, 6, eabe3950 CrossRef PubMed.
- K. Huang, Q. Dou and X. J. Loh, RSC Adv., 2016, 6, 60896–60906 RSC.
- J. Guo, L. Chen, F. Xiong, Y. Zhang, R. Wang, X. Zhang, Q. Wen, S. Gao and Y. Zhang, Nanoscale, 2023, 15, 7845–7853 RSC.
| This journal is © The Royal Society of Chemistry 2024 |


