Open Access Article
Open Access ArticleMetallic nanostructure-based aptasensors for robust detection of proteins
Navid
Rabiee
 ab,
Sepideh
Ahmadi
c,
Kamal
Rahimizadeh
ab,
Suxiang
Chen
ab and
Rakesh N.
Veedu
ab,
Sepideh
Ahmadi
c,
Kamal
Rahimizadeh
ab,
Suxiang
Chen
ab and
Rakesh N.
Veedu
 *ab
*ab
aCentre for Molecular Medicine and Innovative Therapeutics, Health Futures Institute, Murdoch University, Perth, WA 6150, Australia. E-mail: r.veedu@murdoch.edu.au
bPrecision Nucleic Acid Therapeutics, Perron Institute for Neurological and Translational Science, Perth, WA 6009, Australia
cStudent Research Committee, Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
First published on 13th December 2023
Abstract
There is a significant need for fast, cost-effective, and highly sensitive protein target detection, particularly in the fields of food, environmental monitoring, and healthcare. The integration of high-affinity aptamers with metal-based nanomaterials has played a crucial role in advancing the development of innovative aptasensors tailored for the precise detection of specific proteins. Aptamers offer several advantages over commonly used molecular recognition methods, such as antibodies. Recently, a variety of metal-based aptasensors have been established. These metallic nanomaterials encompass noble metal nanoparticles, metal oxides, metal-carbon nanotubes, carbon quantum dots, graphene-conjugated metallic nanostructures, as well as their nanocomposites, metal–organic frameworks (MOFs), and MXenes. In general, these materials provide enhanced sensitivity through signal amplification and transduction mechanisms. This review primarily focuses on the advancement of aptasensors based on metallic materials for the highly sensitive detection of protein targets, including enzymes and growth factors. Additionally, it sheds light on the challenges encountered in this field and outlines future prospects. We firmly believe that this review will offer a comprehensive overview and fresh insights into metallic nanomaterials-based aptasensors and their capabilities, paving the way for the development of innovative point-of-care (POC) diagnostic devices.
Introduction
The development of appropriate methods to detect specific proteins in biological samples with great sensitivity and precision is very important since their quantification can be complex, and time-consuming, and the information is crucial for biosensing applications. Biosensors are greatly sensitive and accurate diagnostic tools and they are relatively cheap to produce, highly portable, and can be mass-produced.1 For many years, biosensors have been developed and commercialized for various applications including measuring blood glucose, thrombin, lactate, etc. However, the sensitivity, accuracy, stability, and design of biosensors need to be improved considering the broad commercial potential. Towards this, metallic nanoparticle-based aptamer sensors (metallic aptasensors) could offer great potential.Aptamers are short single-strand DNA or RNA molecules with a great affinity to their targets because of their capability to form three-dimensional structures in solution.2 Aptamers, often termed chemical antibodies, can be used as an alternative to antibodies, and possess some obvious advantages over antibodies including high stability, freedom to incorporate multiple chemical modifications without changing their binding properties, long shelf life, and easy laboratory-based development and production in large amounts.2 In addition, aptamers can discriminate between chiral molecules and can identify a certain epitope of a target molecule. Aptamers are formed by a reiterative selection process in vitro mentioned as the Systematic Evolution of Ligands by EXponential enrichment (SELEX), and aptamers can be selected for almost any desired target ranging from small molecules to complex proteins (Fig. 1).3 Aptamers are generally used for several applications, such as diagnostics development, drug development, and drug delivery.4–12 The biosensing potential of aptamers (aptasensors) can be strengthened by incorporating them with various metal-based nanomaterials.13,14 The use of metallic nanomaterials can increase the optical, thermal, and electrochemical properties of the transducers, resulting in enhanced aptamer–ligand interactions. In fact, the coupling of nanomaterials with aptamers is a quickly emerging field where various aptamer–material conjugates via outstanding sensing efficacies have been developed.15 Metal-based nanomaterials, including carbon-based nanomaterials, metal nanoparticles (NPs), metal–organic frameworks (MOFs), and MXenes, have been developed to fabricate biosensing systems according to their exclusive properties.16,17 Besides the above-mentioned properties, metallic nanomaterials might be used as aptamer carriers in biosensing systems.
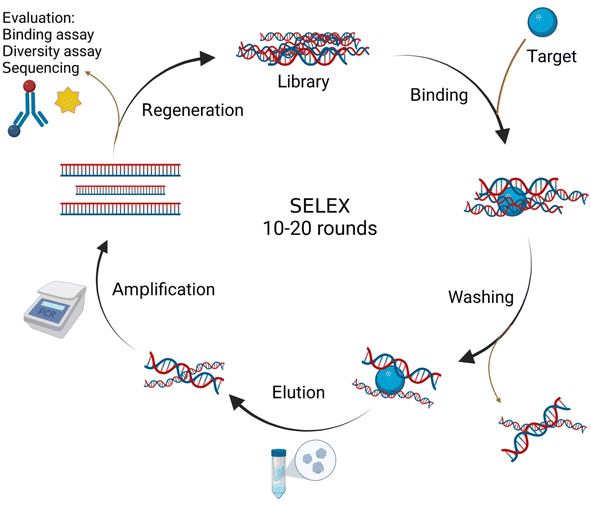 | ||
| Fig. 1 Schematic illustration of the SELEX process. This figure was drawn by using https://BioRender.com. | ||
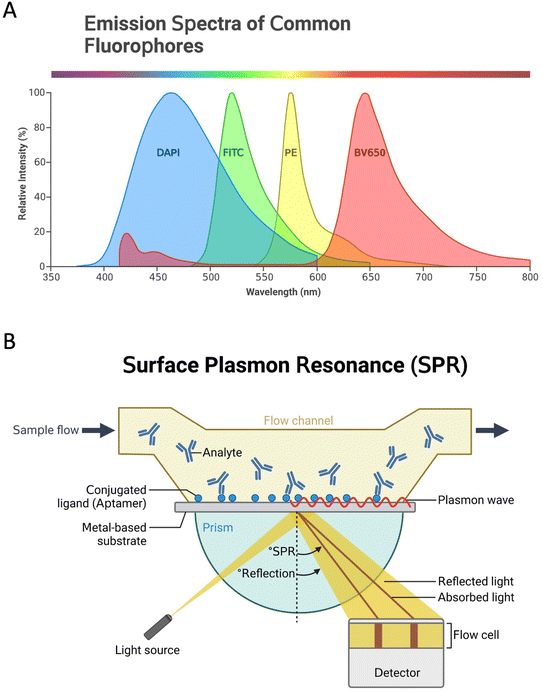
Since the aptamer is immobilized onto the transducer surface, using an effective transducer is paramount for the performance of aptasensors. Therefore, transducers have a crucial role in the aptasensors development to record the signal and exchange it for a detectable output in different plans.18 In fact, based on the type of transducer, sensors can be classified into electrochemical, optical, and thermal biosensors.19 Optical aptasensors comprise aptamers labeled with NPs, luminophores, and fluorophores (Fig. 2A), or label-free aptamers as observed in surface plasmon resonance (SPR) (Fig. 2B) and surface-enhanced Raman spectroscopy (SERS) sensors. Most targets can be optically identified by using fluorescence, chemiluminescence, or SPR.20 In electrochemical aptasensors (ECAs), the surface of the electrode is used as a substrate to immobilize the aptamer, and changes in the electrochemical current are investigated. High sensitivity and low synthesis cost are the advantages of the electrochemical signaling.21 ECAs include labeled and label-free systems.22,23 Electrochemical signal changes can be analyzed qualitatively and quantitatively to investigate target molecules. Labels can be electroactive materials, enzymes, and some NPs such as quantum dots (QDs).23 In label-free systems, the detection of analytes is performed by the recognition of the analytes by the specific aptamers via electrostatic interaction.22 Detection with the high specificity of proteins, such as enzymes, growth factors, etc., can be done by this type of aptasensors.
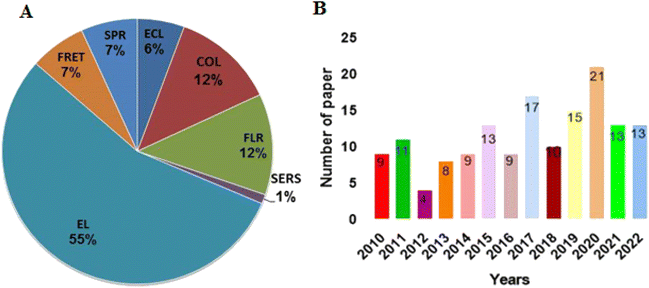
Several studies related to the role of metallic nanomaterials-based aptasensors in high-sensitivity detection of protein targets have been published in recent years, which reflects a higher importance of metal NPs than other nanomaterials in this field. Fig. 3 displays the status of research articles published in the area of metal nanomaterials-based aptasensors for protein detection over the last dozen years. According to the figure, there is a gradual increase in the number of studies during this period, indicating an increasing awareness of the importance of metallic nanomaterials for the design of aptasensors. Although different latest review articles have been published on the role of metal-based nanomaterials in aptasensors, none has provided a thorough investigation of these aptasensors in detecting proteins.24,25 In our estimation, there is a certain need for a comprehensive review of this topic. Thus, we herein review the relevant studies and finally present the challenges and prospects for the future development of metallic nanomaterials-based aptasensors.
The commercialization of aptamer-based biosensors (i.e., aptasensors) has the potential to generate significant economic benefits for various industries. The global biosensors market was valued at US$21.2 billion in 2020 and is anticipated to develop at a compound annual growth rate (CAGR) of 8.8% from 2021 to 2028, driven by the demand for accurate and reliable diagnostic tools. The healthcare sector is the largest consumer of biosensors, with a market share of over 40%. The market for aptasensors is expected to grow rapidly, mainly owing to their great specificity and sensitivity, and low production cost. According to a study conducted by the National Institutes of Health (NIH), the cost of developing a biosensor can vary between US$100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and US$1 million, depending on the level of innovation and the required regulatory approvals. Although costly, once commercialized, the potential revenue generated by a biosensor on the market can far exceed the initial investment. In fact, several government agencies and private organizations have been providing funding to maintain the development and commercialization of sensors. For instance, the National Science Foundation (NSF) offers grants of up to US$1.5 million for biosensor research and development (R&D) projects. The NIH also offers funding opportunities for biosensor research, including Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants. In addition, private investors and venture capitalists are interested in funding biosensor startups as well, with over US$2 billion in venture capital investments in the biosensor industry in 2020 alone. In summary, the cost of developing and commercializing aptamer-based biosensors varies depending upon the complexity of the biosensor design and their target application; and it is expected that the commercialization of aptasensors will generate significant economic return, as the biosensors market is projected to grow rapidly in the coming years. In consistence with this, government agencies and private investors are financially supporting biosensor R&D. Simply put, the potential economic benefits of biosensors make them a hopeful tool for revolutionizing healthcare, food safety, and environmental monitoring.26–28
000 and US$1 million, depending on the level of innovation and the required regulatory approvals. Although costly, once commercialized, the potential revenue generated by a biosensor on the market can far exceed the initial investment. In fact, several government agencies and private organizations have been providing funding to maintain the development and commercialization of sensors. For instance, the National Science Foundation (NSF) offers grants of up to US$1.5 million for biosensor research and development (R&D) projects. The NIH also offers funding opportunities for biosensor research, including Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants. In addition, private investors and venture capitalists are interested in funding biosensor startups as well, with over US$2 billion in venture capital investments in the biosensor industry in 2020 alone. In summary, the cost of developing and commercializing aptamer-based biosensors varies depending upon the complexity of the biosensor design and their target application; and it is expected that the commercialization of aptasensors will generate significant economic return, as the biosensors market is projected to grow rapidly in the coming years. In consistence with this, government agencies and private investors are financially supporting biosensor R&D. Simply put, the potential economic benefits of biosensors make them a hopeful tool for revolutionizing healthcare, food safety, and environmental monitoring.26–28
The advancement of compact, chip-based point-of-care (POC) diagnostic devices represents a crucial aspect of global healthcare progress. These devices are designed to swiftly and accurately detect various target analytes responsible for serious diseases.29 They play a pivotal role in advancing treatment, prevention, and optimal pharmaceutical dosing. POC testing systems are characterized by their simplicity and user-friendliness, providing diagnostics directly to patients without the required for specialized preparation. Consequently, these devices can reduce analysis costs, save time, and empower underserved regions with restricted resources to conduct diagnostics.30 Precise and accurate diagnosis is pivotal in ensuring the most effective treatments and improving recovery chances, even for life-threatening diseases with high mortality rates. Hence, in addition to miniaturization and portability, considerable emphasis must be placed on biosensor research to enhance the speed, accuracy, precision, and reliability of diagnostic tools.31 These devices face significant challenges on their path to commercialization. The factors behind the commercialization of ECAs are market size, instrument ease, and cost considerations, such as conservation expenses. Despite the rapid growth of aptamer-related markets, the aptasensors market in diagnostics has to experience significant expansion, despite its considerable potential in the analytical sector. In the past few decades, nanomaterial-based ECAs had a profound effect on bioanalysis according to their high sensitivity.32 Nevertheless, a comprehensive discussion is required to address the barriers to aptasensor commercialization. Hence, a comprehensive discussion has centered on aptamer recognition layer-based electrochemical sensors. This discussion initially identifies existing gaps and challenges, followed by a brief exploration of the necessary efforts to translate laboratory findings into practical uses for ECA commercialization. The barriers to ECA commercialization can be classified as technical (e.g., limits of detection, dynamic range, sensitivity, stability, accuracy) and commercial (e.g., workable manufacturing of POC devices, automation, and digitalization). Thus, optimization of these parameters is essential before commercialization. While the primary focus of ECA devices is to create robust, accurate, and cost-effective tools, additional crucial factors must be considered. These include analytical work, analyte type, concentration, sample requirements, sample preparation, sample matrix composition, pH, temperature, and humidity.33,34 Specimen type, especially for solid samples like fish, chicken, pork, eggs, vegetables, and infant formula, requires particular attention due to additional pre-treatment steps compared to liquid specimens (e.g., clipping, mixing, blending, centrifuging, and distillation). In contrast, liquid specimens (e.g., milk, fruit juice, serum, and water) necessitate simple sample preparation steps. Aptasensors have made remarkable strides in the past decade, demonstrating their potential in bioanalysis. However, successful aptasensor market penetration requires efficient resolution of both technical and commercial issues. These challenges include the high costs of research and development, access to low cost, the lack of a high-quality aptamer generation methodology, multiplexed detection in samples without interfering, and the replacement of common methods, such as addressing legal aspects. These obstacles significantly hinder the promotion of ECAs in the market. With quick progress in nanotechnology, it is expected that the progress of analytical tools combining aptamers and nanomaterials will continue to evolve, addressing the limitations in POC diagnostics. There is a crucial requirement for a discovery procedure and aptamer generation methodology to overcome these issues.35–38 Microfluidic paper-based analytical devices (μPADs) and electrochemical sensors offer multiplexed testing capabilities, and show potential as diagnostic tools. Progress in materials, microfabrication techniques, and electronics is facilitating the rapid commercialization of biosensor technologies, mitigating material-related cost and quality barriers. Despite aptamers' appeal as recognition elements and graphene's potential as a signal transduction component, integrating them effectively into complex matrices remains a significant challenge for POC systems. Emphasis should be placed on interdisciplinary research to develop more efficient, and high-throughput systems applicable in real-world scenarios. In the context of emerging medical Internet of Things (IoT) applications, hopeful ECAs must be integrated into POC biosensors, the internet, and healthcare providers to offer comprehensive healthcare monitoring. The increasing number of proof-of-concept systems holds promise for market availability.
Aptamer-decorated metal nanomaterials: conjugation strategies, synthesis, and properties
Conjugation of aptamers with metal nanomaterials to generate aptamer-decorated metallic nanomaterials can considerably increase biosensor sensitivity, selectivity, and stability. The large surface area of metallic nanomaterials can provide more active sites to anchor aptamers. Different covalent and non-covalent functionalization methods are established to cross-link aptamers with the metal nanomaterials with various structures and functions.Non-covalent functionalization
Conjugation of aptamer with metal nanomaterials can be performed by non-covalent functionalization strategies, which depend on adsorption by non-covalent interactions, such as halogen/hydrogen bonding, electrostatic and van der Waals interactions.39 DNA interacts with graphene oxide (GO) by hydrogen bonds and π–π stacking. Moreover, the phosphate backbone of nucleic acid can also anchor metal oxides-based nanomaterials.40 Researchers developed an assay to detect DNA via a non-covalent method based on hydrogen bonds to connect DNA and MOFs. They used amine-functionalized MOFs, UiO-66-NH2, which had a conjugated π-electron system and formed hydrogen bonds from cross-linking between MOFs and single-stranded DNA (ssDNA). This biosensor can detect the fluorescence of the ssDNA.41Covalent functionalization
Covalent methods are also applied in functionalizing nanomaterials, which form more stable linkages compared to the non-covalent functionalization.42 Covalent thiol linkages are among the most important connections between aptamers and nanomaterials. In a study, researchers developed thiol-containing aptamers (–SH) immobilized on the gold (Au) surface of a nanocages-modified carbon electrode via an Au–S chemical reaction. In addition to –SH–terminated aptamers, disulfide-terminated or other mercaptoethanol-linked sulfur groups are also effectively used for functionalizing gold nanomaterials. Amine, hydroxyl, and carboxylic acid are traditional groups on the surface of most materials. Carboxyl and hydroxyl groups exist on the surface of noble metal NPs and GO.43 Covalent immobilization often relies on amide bonds formed between aptamers and the organic ligand on MOFs. This binding is very stable and necessary for sensitive biosensing. For example, aptamer-MOFs were developed based on UiO-66-NH2 for the recognition of let-7a microRNA. This system was based on the formation of amide bonds among the amino groups on UiO-66 organic ligands and carboxyl groups on the ends of aptamers. In addition, aptamers can be encapsulated in MOFs as well; however, this process is different from the adsorption or covalent binding in that interaction between aptamer and nanomaterials does not occur.44Synthesis strategies
Different synthesis methods can characterize metal-based nanomaterials by offering useful information about their morphology and shapes, size, roughness, and physiochemical and electric properties, which are critical to their in vivo activity. Synthesis of noble metal materials follows a top-down or bottom-up approach. The top-down process is based on thermolysis, lithography, and pyrolysis of material, breaking it into small pieces. In contrast, in the bottom-up method, NPs are synthesized from atoms and related biomolecules using biological and chemical methods. This approach has more advantages because it offers more control over the formation of the product with a more homogeneous size distribution, morphology, and chemical composition. The bottom-up method is usually a wet chemical synthesis method, including sonochemical, and green assays.45,46Different methods, such as solvothermal, electrochemical, microwave, and sonochemical methods, have been developed for the synthesis of MOFs. Usually, MOFs are crystallized from a solution. Water and organic solvents fill the pores with unreacted residues of the initial compounds. Thus, the encapsulation of external molecules into the porous structure and the production of inorganic units have a significant role in forming MOFs.47 Various methods, including exfoliation, ultrasonication, and liquid exfoliation, can be used to synthesize two-dimensional (2D) MOF nanosheets. Researchers showed that 2D MOFs could be applied as a new class of materials for the chemoresistive sensing of volatile organic compounds.48
With the synthesis of the first Ti3C2Tx MXene in 2011, the group of 2D metal carbides and carbonitrides has expanded considerably to comprise single- and multi-element MXenes. The properties of MXene and the improvement of their characteristics require a deeper mechanistic understanding of their synthesis procedures.49 MXenes are fabricated by etching the “A” group metal layer from the Mn+1AXn (MAX) phase structure, wherever M refers to transition metals. Although bottom-up approaches have been applied to synthesize MXenes, they are often formed by top-down approaches, with their structure and composition originating from their nitride precursors.50 These top-down approaches are based on hydrofluoric (HF) etching of the MAX phase.49 In general, the properties of MXenes can be engineered during the synthesis process. They are predisposed by various parameters, such as the precursor MAX phase, temperature, and the etchant. However, regulating these parameters is challenging to acquire suitably designed MXenes with wanted properties.51Table 1 shows different metal-based nanomaterials used in aptasensors, their synthesis, and their physical properties.
| Metal-based materials | Synthesis methods | Desired physical properties |
|---|---|---|
| Noble metal/metal oxide | • Hydrothermal | • High optical and electrical conductivity |
| • Electrochemical | • Easy synthesis method | |
| • In situ reduction | • Non-toxicity | |
| Carbon materials | • Chemical vapor deposition (CVD) | • Large surface area |
| • Laser ablation | • Excellent electrical and mechanical properties | |
| • High stretchability | ||
| Metal organic framework (MOFs) | • Surfactant-assisted synthesis | • Porosity |
| • Exfoliation | • Stability | |
| • Ultrasonication | • Synthetic tunability | |
| • Liquid exfoliation | ||
| MXenes | • Hydrofluoric acid etching | • High electrical conductivity |
| • Fluoride-free etching | • High biocompatibility | |
| • Molten salt etching | • Excellent electrical properties |
Metal-based aptasensors for protein detection
Noble metal/metal oxide-based aptasensors
Metal-based nanomaterials with different sizes have interesting physicochemical and optoelectronic properties.52,53 They are being progressively used as transducing materials in biosensors for the analysis of biomolecules. Noble metal NPs are beneficial over other nanomaterials due to the simplicity of chemical modifications, great surface area, exclusive optical, redox, and luminescence properties, and their capability to respond optically and electrochemically to external stimuli.54 With the enhancement of the surface area in metal nanomaterials, the optical, electronic, chemical, and thermal properties of the materials are altered. The choice of synthesis approach and compounds and conditions used for the synthesis are key to the formation of structures with the appropriate sizes and shapes.Noble metal nanomaterials with exclusive physiochemical properties can increase the sensitivity and selectivity of biosensors. Utilizing aptamers as the recognition elements, these sensing platforms hold great promise in achieving economic, quick, and highly sensitive detection of proteins.13 The key roles of metal nanomaterials in biosensors are immobilizing receptors, catalyzing bioreactions, electron transfer, and amplifying mass change.55
Au nanostructures have been extensively used in studies including the construction of biosensors, which can be attributed to their great compatibility, simplicity in their synthesis, and stability in the synthesis procedures.56 To greatly increase the sensitivity of aptamer-based biosensors in protein detection, an efficient signal amplification method is needed. Oligonucleotide-modified AuNPs have been broadly used as signal-amplifying tags in new sensing systems since they can connect biological molecules and have excellent electrochemical performance. Each AuNP can be loaded within DNA strands.57 In contrast, aptamers can provide a high level of control over the interaction between the aptamer and protein in AuNPs-based aptasensors. One of the important questions is, what effect will aptamer density have on AuNPs. In a study, the kinetics of interaction among the IFN-γ and AuNPs-aptamer is investigated through fluorescence spectroscopy. The connection of IFN-γ with AuNPs-aptamer alters the construction of aptamer and enhances the fluorescence signal. Furthermore, the number of aptamers on the surface of AuNPs affects the binding reaction between aptamer and protein.58 The AuNPs-aptamer system with the greatest surface coverage is advantageous in biosensing systems due to its high sensitivity. These outcomes expand our knowledge of the aptamer–protein interaction on the AuNPs surface, which is critical to enhancing the application of AuNPs-aptamer-based biosensors.
Thrombin is one of the important proteins in blood coagulation, as well as tissue repair. Thrombin can control platelet aggregation and another crucial responses in the vascular system.59 A photoelectrochemical (PEC) aptasensor developed to detect different concentrations of thrombin. The transducer is a glassy carbon electrode (GCE), and a layer of AuNPs was deposited on its surface (Fig. 4A and B). This electrode was functionalized with perylene tetracarboxylic acid (PTCA), and a new layer of AuNPs was deposited on the surface of the transducer. With the immobilization of amine-modified thrombin-specific aptamer on the surface of AuNPs, a structure comprising GCE/AuNPs/PTCA/AuNPs/aptamer was formed. Subsequently, a sandwich structure was formed based on the stabilization of the analyte from both sides through the aptamer on the transducer surface. Glutaraldehyde was added as a cross-linking agent, while C3N4@C60 core–shell was added as a quencher, and the PEC signal yielded by the aptasensor was measured under different analyte concentrations. Here, a competition between C3N4 and PTCA for light absorption in the existence of the electron-donating ascorbic acid delivered various photocurrents when the sandwich conformation existed or the thrombin was absent. This quenched signal of the PEC aptasensor could sensitively detect the mentioned biomarker with an LOD of 1.5 fM.60 Therefore, the important point is to choose a signal amplification strategy to obtain a lower LOD in electrochemical systems. Another study showed an electrochemical sandwich aptasensor containing two thrombin aptamers, AuNPs, and [Ru(NH3)6]3+ to signal alteration. In the presence of thrombin, aptamers connect to the thrombin, and their complexes lead to the fabrication of a sandwich structure, causing more [Ru(NH3)6]3+ adsorbed on the phosphate backbone of the aptamers, and enhancing the sensitivity for pulse voltammetric measurements. The LOD of this detection was 0.14 fM. This aptasensor showed good selectivity and was used to detect thrombin.57
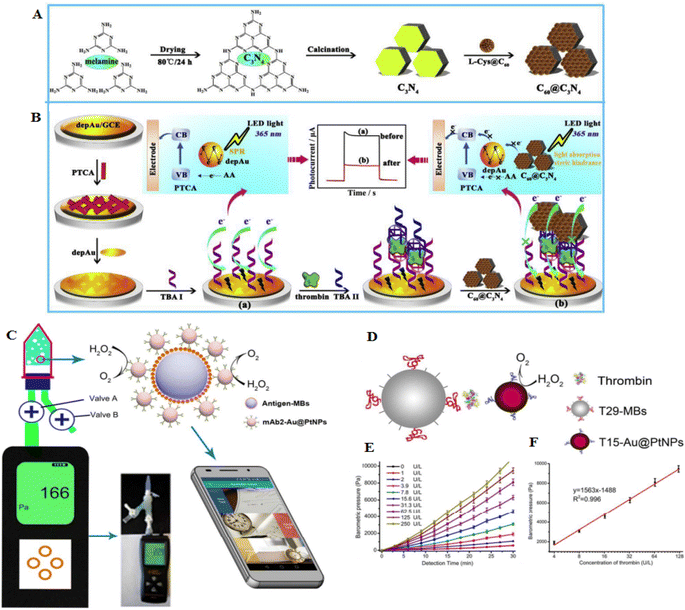 | ||
| Fig. 4 An electrochemical sensor for recognition of thrombin by using C60@C3N4 nanocomposites-based electrodes. (A) The formation of C60@C3N4 composites. (B) C60@C3N4 nanocomposites stimulate signal-off PEC aptasensor. Reproduced from ref. 60 with permission from Elsevier, Copyright 2019. (C) The barometer-based sensor is based on the measurement of oxygen production using a precise barometer. (D) Diagnosis of thrombin using the barometer-based aptasensor. (E) Concentration-dependent barometric pressures to thrombin detection. (F) The calibration curve of the thrombin, reproduced from ref. 61 with permission from American Chemical Society, Copyright 2017. | ||
Au@PtNPs were developed as probes since they showed catalytic stability for the decomposition of H2O2 and were highly sensitive in the diagnosis of carcinoembryonic antigen (CEA) and ractopamine (RAC) in urine samples (Fig. 4C). To detect thrombin, aptamer 1 (T29) was conjugated with molecular beacons (MBs), and aptamer 2 (T15) was labeled with Au@PtNPs. With the binding of T15-Au@PtNPs to the T29-MBs, a high concentration of thrombin was detected (LOD of 0.025 ng mL−1) (Fig. 4D–F). A smartphone was applied to detect and transmit the results. Moreover, the aptasensor showed great specificity to CEA, RAC, and thrombin in serum, urine, and serum samples, respectively.61
Tumor necrosis factor α (TNFα) is one of the most important proteins involved in the signaling pathways, which have an important role in inflammation as well as inhibition of tumor progression. An electrochemical aptasensor was established for the recognition of TNF-α. At first, the electrode modification print with graphite plate by cobalt hexacyanoferrate (CoHCF) was done. Its modification was also done with colloidal gold NPs. Then, a thiolated aptamer was immobilized on the electrode surface modified with CoHCF/AuNPs, and the aptamer was connected to the AuNPs surface by Au–S bonds. TNF-α was captured through aptamer on one side, and an antibody absorbed TNF-α on the other side. This platform is capable of quantifying TNF-α. Also, the LOD of the said aptasensor was found to be about 0.52 pg mL−1.62
Silver nanomaterials have high electrical conductivity, which can immobilize molecules through an Ag–S bond. Therefore, silver nanowires (AgNWs) and AgNPs can be recognized as good substrate materials for biosensor fabrication.63 Using AgNPs increases the film's conductivity and raises the surface area of the GCE. Instead, AgNPs were fixed in a polymer matrix and showed significant antimicrobial activity.64 AgNWs can provide high diffusion ways to electrons, which can intensely increase the electrochemical activity of the biosensors. Fig. 5A shows a sensitive electrochemical aptasensor based on AgNWs electrode and signal amplification of ZnFe2O4 for thrombin detection. Here, AgNWs@PCs are applied as the electrode and substrate to bind aptamer. Deposited AgPCs can effectively increase the soldering of AgNWs, which improves the conductivity of the electrode. This type of sensor has a LOD of 0.01 pM, which shows suitable performance in selectivity and reproducibility and is effective in identifying proteins.63
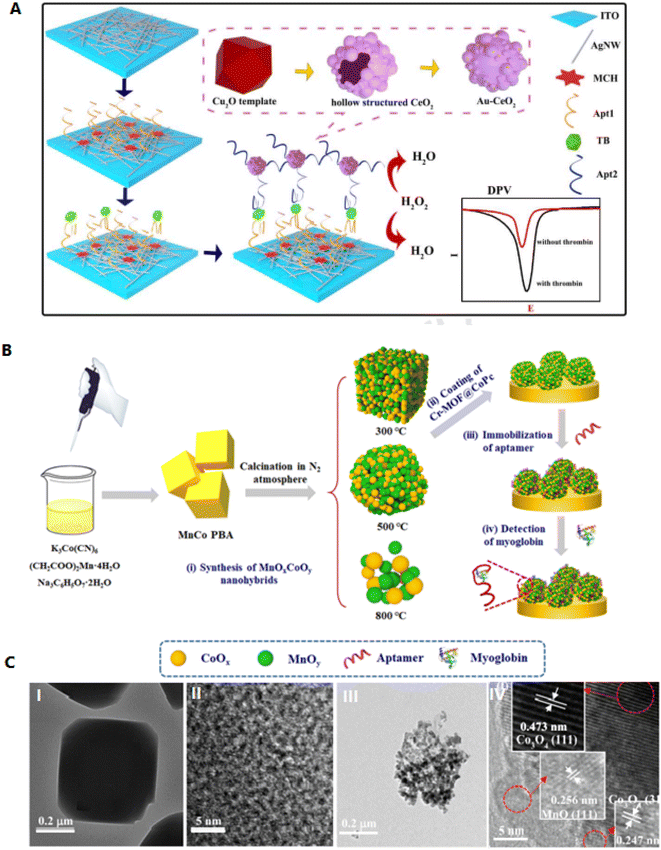 | ||
| Fig. 5 Designing a new type of aptasensor for highly efficient detection of myoglobin. (A) Schematic illustration of decorated AgNWs on the cleaned indium tin oxide (ITO) surface. These hierarchical networks cause electron transfer and act as an excellent conducting substrate and a free electrochemical sensor. Reproduced from ref. 63 with permission from Elsevier, Copyright 2021. (B) Fabrication of MnOxCoOy-based aptasensor. (C) Transmission electron microscopy (TEM) image of the (I, II) MnCo PBA as well as (III, IV) MnOxCoOy. Reproduced from ref. 67 with permission from Elsevier, Copyright 2020. | ||
A study showed that polydopamine (PDA) films act as an intermediate to inhibit the interaction of sulfhydryl-polyethylene glycol (SH-PEG) to AgNPs, as it results in the delamination of the silver layer.64 PDA-AgNPs can be applied as perfect electrode substrates to electrochemical biosensors. Here, deposition of PDA has occurred on the GCE through self-polymerization, and the thrombin-specific aptamer was grafted onto the PDA-modified GCE. In the presence of the thrombin, the electrode's electrochemical impedance is progressively enhanced. The results showed a LOD of 36 fM in human samples. The selective method detects thrombin in diluted human serum.65 Despite the signal from AgNPs, the AgNPs aggregates offer a highly amplified electrochemical signal three times and display high merits in the electrochemical biosensing.66 An aptasensor based on amperometric assay using AgNPs on a gold electrode is designed for glycoprotein detection. Aggregation is performed by adding 4-mercaptophenylboronic acid (MPBA) according to the production of Ag–S bonds. A thiolated aptamer was connected to the electrode to absorb the glycoprotein. As a result, after absorption, the prespore-specific antigen (PSA) glycoprotein reacts with MPBA by creating boronated esters. Subsequently, the electrochemical signal is amplified through the synthesis of an AgNPs network that acts as a redox reporter. The LOD of PSA is 0.2 pg mL−1, which is used as a strong aptamer to detect all types of glycoproteins.66
One of the metal oxides is manganese oxides (MnOx), including MnO2, Mn3O4, Mn2O3,etc. MnOx can be used as active electrodes according to their great precise capacitances via the structures controlled in growth procedures. The electron transfer of MnOx can be appropriated for different applications, such as biosensing systems.67 MnOxCoOy were formed from MnCo-based Prussian blue analog through calcination at various temperatures, and this impedimetric aptasensor showed precise detection of myoglobin (Myo) (Fig. 5B). Under a high temperature of 500 °C, the homogeneously dispersed MnOx and CoOy NPs were attained, increasing the electrochemical conductivity. MnOxCoOy, showed high sensitivity than the MnCo-phenylboronic acid (PBA)-based aptasensor in the detection of Myo, with a LOD of 0.56 fg mL−1. This approach creates a critical system for building an aptasensor that is possible for Myo recognition in a real human serum sample.67 Co3O4 nanomaterial, as a semiconductor, was applied to detect the cardiac troponin. The diagnosis of cardiac troponin T is performed via immobilizing its DNA aptamer. The LOD of the biosensor was 0.1 μg mL−1, which was enhanced by about 250% compared with pure Co3O4 nanorods (NRs). However, the limitation of this aptasensor is its LOD ability, which can be improved by changing the fabrication procedure. This DNA aptasensor is new and can be established in a combined and high-throughput system.68Table 2 shows the role of noble metal/metal oxide nanomaterials applied as transducers to detect proteins.
| Nanomaterials | Aptamer | Biosensing principle | Target protein | Properties | LOD | Ref. |
|---|---|---|---|---|---|---|
| a PL: photoluminescence. EL: electrochemical. FL: fluorescence. LSPR: localized surface plasmon resonance. | ||||||
| AuHCF/AuNPs | TGGTGGATGGCGCAGTCGGCGACAA | EL | Tumor necrosis factor alpha (TNF-α) | High sensitivity detection even at very low concentrations | 5.5 pg mL−1 | 69 |
| Fern leaves-like gold nanostructure (FLGN) | TTTTTTTTTTGGGAAUUCGAGCUCGGUACCUUUACCGUAAGGCCUGUCUUCGUUUGACAGCGGCUUGUUGACCCUCACACUUUGUACCUGCUGCCAACUGCAGGCAUGCAAGCUUGG | EL | Amyloid beta (Aβ) | Administration of Alzheimer's disease at early stages | 0.4 pg mL−1 | 70 |
| AuNPs | ATACCAGCTTATTCAATTACAGTAGTGAGG GGTCCGTCGTGGGGTAGTTGGGTCGTGGAGATAGTAAGTGCAATCT | LSPR | Retinol-binding protein 4 (RBP4) | Rapid, high sensitivity | 90.7 nM | |
| AgNCs | CAGGCTACGGCACGTAGAGCATCACCATGATCC TGATTAGTTCCTCAGCATCAGG(P1) | FL | Platelet-derived growth factor B-chain homodimer (PDGF-BB) | High selectivity toward specific protein | 0.37 nM | 71 |
| CCCACCCTCCCACCTGATGCTGAGGGT(P2) | ||||||
| ZrCo-MOF | CCACGATACCAGCTTATTCAATTCGTGG | EL | CEA | High selectivity, good reproducibility, high stability | 0.35 fg mL−1 | 72 |
| QDs | N/A | EL | p24-HIV protein | Early detection of HIV infection with a wide linear range, high selectivity | 51.7 pg mL−1 | 73 |
| CdSe/CdS/ZnS QDs | GCCTGTGTTGGGGCGGGTGCG (AβO) | PL | AβO and tau protein | High flexibility, high sensitivity | AβO: 50 pM, Tau: 20 pM | 74 |
| GCGGAGCGTGGCAGG (Tau) | ||||||
An invention presents a portable system for detecting cardiac biomarkers in a patient's bodily fluids using impedimetric techniques. The system is composed of a conductive material interface that includes an epoxy substrate and a vertically aligned platinum wires. A sensor agent is used to the surface of the conductive material interface, which consists of an immobilization agent and one aptamer that binds to the cardiac biomarkers of interest. The aptamer generates an electrochemical impedance signal when it binds to the biomarkers, which is indicative of their presence or absence in the bodily fluid sample. The platinum wires works as the point of contact in order to transducing the electrochemical impedance signals into an interpretable output. The system can detect multiple cardiac biomarkers simultaneously, including C-reactive protein, troponin T, myoglobin, brain natriuretic peptide, and D-dimer. To detect cardiac biomarkers using the system, a fluid sample is obtained from the patient and is then brought into contact with the detection device. The detection device is formed by casting the platinum wires in the epoxy substrate and polishing the surface of the conductive material interface. Then, the electrochemical impedance signal generated by the aptamer binding to the cardiac biomarkers is transduced and displayed using a hand-held device. Alternatively, the detection system can take the form of a test strip, and the method involves correlating visual changes in the test strip with a chart or key to determine the presence or absence of cardiac biomarkers. The surface of the conductive material interface is polished to provide a surface roughness inside the range of about 320 to 2400 grit.75
Magnetic-based aptasensors
Magnetic NPs show paramagnetic properties, which are highly capable of separating target molecules from other mixtures through a magnetic field, causing them gorgeous materials to manufacture sensors.76,77 One of the applications of this type of material is as an electrode modifier to increase electron conductivity (an inherent feature of metal oxide NPs). Also, their combination with aptamers can separate biomolecules and increase the signal.78 Binary transition metal oxides (BTMOs) provide different benefits than single metal oxides. They can offer a high potential, greater conductivity, extra active sites, and good stability, which are critical to increasing biosensor activity. MFe2O4 (M = Ni, Co, Zn, or Cd) are a group of BTMOs. In addition to the magnetic characteristics, spinel ferrites such as ZnFe2O4 are critical in proposing high redox reaction electrochemistry. An ZnFe2O4 NPs-based aptasensor using laser-scribed graphene (LSG) electrodes as a polyimide substrate was developed. LSG-ZnFe2O4 electrodes showed an increased sensitivity and electrocatalytic activity of about 30% higher than the unmodified electrode. ZnFe2O4 is conjugated with aptamer by Zn–S and Fe–S bonds, which can produce a greatly stabilized electron transfer bonding way. This aptasensor can detect the cardiac troponin I (cTn-I) with a LOD of 0.001 ng mL−1. The fabricated aptasensor has greater to detecting cTn-I and slight cross-reactivity with other biomolecules. Fig. 6A shows the mechanism of the LSG-ZnFe2O4 electrochemical aptasensor to detect cTn-I.78 Another magnetic composite consists of Fe3O4@Au nanomaterials, magnetic field-focused self-assembly, and selected aptamer with great specificity to thrombin. Here, in the existence of protein, aptamer connected with thrombin and showed complementary strands; thus the electrical signal was enhanced (Fig. 6B). Consequently, the electrochemical signal increased by three times through the catalyzed cleavage of surface modification by Pb2+-related DNAzyme.79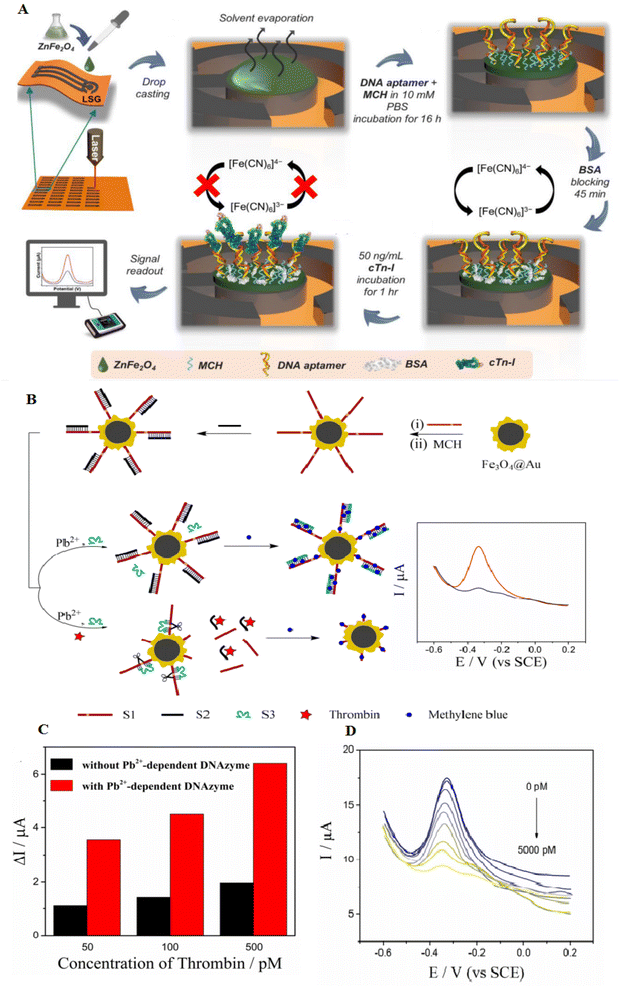 | ||
| Fig. 6 Designing novel laser-scribed graphene (LSG) electrodes using ZnFe2O4 surface modification for the detection of thrombin. (A) The ZnFe2O4 modified LSG-based cTn-I aptasensor and fabrication assay, reproduced from ref. 78 with permission from Elsevier, Copyright 2021. (B) The synthesis of Fe3O4@Au nanocomposites to easily separate magnetic thrombin. (C) The reduced electrochemical signal of Fe3O4@Au-S1/S2 under various concentrations of thrombin. (D) Differential Pulse Voltammetry (DPV) of the aptasensor after adding various concentrations of thrombin. The LOD was 0–5000 pmol L−1, and the weakening of DPV response was observed with increased thrombin, reproduced from ref. 79 with permission from Elsevier, Copyright 2019. | ||
Developing a label-free electrochemical aptasensor based on a modified glassy carbon electrode (MGCE) modified with Fe3O4/Fe2O3@AuNPs was used to detect VEGF165 highly. Fe3O4/Fe2O3@AuNPs act as the signal amplifier, which is critical to detecting biological analytes. The aptamers' binding to the nanomaterial was performed by Au–S bonds and is precisely known as VEGF165. Under optimal detection conditions, a virtuous linear association was attained with the aptasensor. The LOD was 0.01 pg mL−1, and this aptasensor showed high stability. The outcomes exhibited that the aptasensor was hopeful to offer an effective, precise, and cost-effective detection assay to the early investigation of VEGF165-related diseases, which showed significant biomedical applications for the study of medicine accuracy.80
Due to the establishment of magnetic Fe3O4 NPs to reduce the inherent peroxidase-like activity, colorimetric detection has appealed to enhance the consideration of recycling and clearance from the system with progress in the magnetic separation.81 Au@Fe3O4 NPs were synthesized by the solvothermal method. This platform showed great peroxidase-like activity according to the synergistic effect. Besides, the hydroxyl groups (–OH) on the NPs enable their binding with aptamer.81 A new colorimetric sensor was synthesized through the connection of peroxidase-mimicking DNAzyme with magnetic NPs for high-sensitivity detection of target proteins. This complex was used as the peroxidase-mimicking DNAzyme, wherein hemin was conjugated to G-quadruplex (G4)–DNA to double into a catalytic structure and perform as an enzyme. These aptasensors are formed from magnetic NPs, cDNA modified with biotin, as well as ssDNA comprising the aptamer and DNAzyme. The trivalent DNAzyme has high catalytic activity, which catalyzed the H2O2-related oxidation of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS). Besides, this aptasensor was used to detect mucin 1 (MUC1), which showed LOD of 5.08 and 5.60 nM. This aptasensor with high selectivity and good anti-interference capability has high potential in disease diagnoses.82Table 3 shows the role of noble magnetic nanomaterials applied as transducers to detect proteins.
| Nanomaterials | Aptamer | Biosensing principle | Target protein | Properties | LOD | Ref. |
|---|---|---|---|---|---|---|
| a SERS: surface-enhanced Raman spectroscopy. ECL: electrochemiluminescence. FL: fluorescence. EL: electrochemical. | ||||||
| Fe3O4@SiO2@Ag | N/A | SERS | cTnI | High specificity and sensitivity | 10 ng mL−1 | 83 |
| Magnetic NPs | GGGATGGGTTTAGTCCGTGGTAGGGCAGGTTGGGGTGACTTTACTGCCTGACTTTCTTTTTTGGGTTGGG | Optical | Thrombin | Sensitive detection of thrombin in complex samples | 0.5 nM | 84 |
| Fe3O4 | N/A | ECL | HBsAg | Rapid magnetic separation, highly sensitive detection | 0.05 ng mL−1 | 85 |
| Magnetic NPs | N/A | FL | CD63 exosome | High specificity | 100 particles per mL | 86 |
| AuMNPs | GGTTGGTGTGGTTGG | EL | Thrombin | High sensitivity detection | 5.5 fM | 87 |
Carbon-based aptasensors
Carbon nanomaterials appeal to attention according to their exclusive chemical, and electrical properties. They are broadly applied in photovoltaic, electronic, optical, and sensing systems. These exceptional properties considerably rely on the functional structures of carbon nanomaterials but how they interact with another materials, including AuNPs, SiO2, etc.88 Besides, virtuous electrical conductivity, thermal properties, and great electrocatalytic activity permit carbon nanomaterials to be promising carriers for aptamers.89 Carbon nanomaterials for fabricating ECAs include carbon nanotubes (CNTs), graphene oxide (GO), carbon nanocomposites, etc.90CNTs are one-dimensional nanomaterials made of hybridized carbon atoms connecting via C–C interaction. Commonly, CNTs include SWCNTs and MWCNTs. CNTs have exclusive thermal, mechanical and electrochemical properties, which have an important role in the electron transfer of various electroactive materials.91,92 CNTs have good adjustable properties that can be functionalized with various chemical groups according to specific requirements. Thus excellent characteristics of CNTs would be turned up, such as compatibility and solubility.89 SWCNTs can selectively adsorb aptamers. ssDNA can be adsorbed, although dsDNA cannot. A study showed that SWCNTs-based electrochemical sensors are designed based on the use of ssDNA.93 Progress of an electrochemical biosensing system by SWCNTs-screen-printed electrodes (SWCNT-SPEs) modified with a redox-tagged DNA aptamer can be connected to the SARS-CoV-2 spike protein S1. It controls a folding-based apparatus that outcomes in major changes in the amperometric under the connecting of the S1 protein to the aptamer (Fig. 7). The aptasensor can detect S1 protein with a LOD of 7 nM. This protein is specifically detectable in a buffer solution, and a synthetic viral transport medium is broadly applied for collecting nasopharyngeal swabs. This electrochemical aptasensor based on SWCNT-SPE can detect other proteins.94
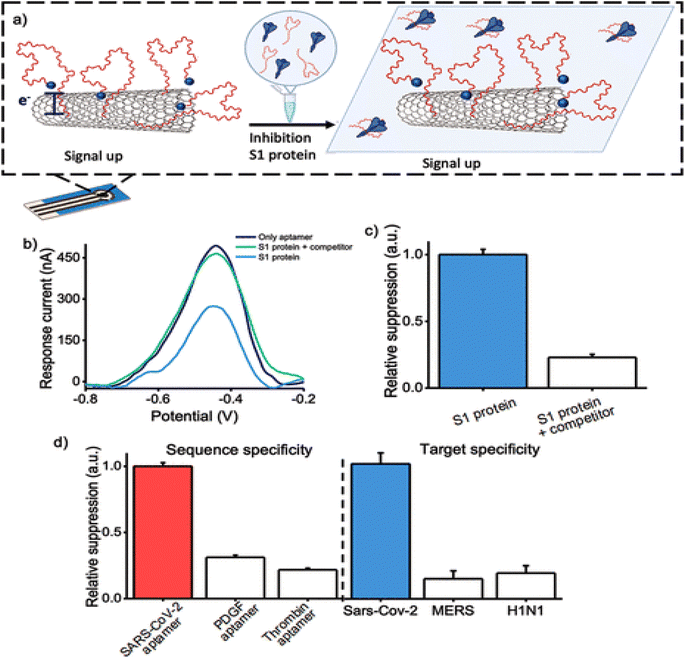 | ||
| Fig. 7 Inhibition of S1 protein with its mechanism on the surface of the MWCNTs, as a hopeful approach for the recognition of coronavirus via a novel aptasensor. (a) The aptasensor activity when treating the S1 protein with the COVID-19 aptamer. (b) DPV voltammograms without any target protein, in the existence of S1 protein, threatened with increased participant aptamer. (c) Signal suppression was gained when measuring the amperometric in the existence of the S1 protein. (d) The specificity of the biosensor was assessed by using various aptamer sequences immobilized onto the electrode surface in the existence of the S1 protein, reproduced from ref. 94 with permission from American Chemical Society, Copyright 2022. | ||
The development of metal oxide/carbon-based aptasensors based on CNTs showed more benefits than other CNTs-based biosensors. For example, a biosensor based on integrating a composite of titanium dioxide (TiO2), chitosan, MWCNT, and a synthetic Schiff base is suggested by a hydrothermal assay and applied for thrombin detection. This aptasensor has important advantages to biomolecule immobilization, according to the great surface area, π-conjugation structure, high conductivity produced by MWCNT, a porous structure of TiO2 nanocrystals, as well as great amounts of amino/hydroxyl groups, and positively charged chitosan. If thrombin is present, the aptamer is placed on the absorbent layer, preventing electrons from flowing. This leads to the inhibition of electron transfer and increases the impedance signals of TiO2-MWCNT/chitosan-Schiff base (CHIT-SB). This aptasensor works exclusively and does not include other proteins. It also has a LOD of 1.0 fmol L−1 for thrombin. Also, the clinical application was carried out by examining thrombin levels in the blood using an aptasensor and commercial enzyme-linked immunosorbent assay (ELISA) kit, and the outcomes show a promising system for the clinical analysis of the thrombin.95
In addition to CNTs, carbon quantum dots (CQDs) can also enhance the immobilization of aptamer on the surfaces of the materials due to their good stability, hydrophilicity, biodegradability, non-toxicity, and high conductivity.96 An MWCNT/poly(diallyldimethylammonium chloride) (PDDA)/CQD nanocomposite is an efficient electrochemical biosensing used to detect lysozyme. The lysozyme-aptamers immobilized on the composite through covalent binding among the amino groups of the aptamer and the carboxy groups of the composite. This electrode was considered through electrochemical impedance spectroscopy and cyclic voltammetry. Increased sensitivity and enhancement of the electrochemical signal were obtained by this aptasensor (LOD of 12.9 fmol L−1). This aptasenor is different from other ECAs for aptamers immobilization, such as: (1) the presence of high amounts of amino and carboxy groups in CQD; (2) high surface area in the existence of CQDs and MWCNTs; and (3) quick electron-transfer potential in the presence of MWCNTs.97 The limitations of this study are the difficulties and expensive synthesis of NPs and CQDs; however, its high sensitivity and chemical stability make it useful for developing and improving ECAs.
Graphene, as a new carbon-based material, has a significant role in biosensors with outstanding optical, electrochemical as well as magnetic properties.98 Graphene oxide (GO) has exclusive properties such as virtuous aqueous solubility, great surface area, and good fluorescence quenching capability. GO is considered a good energy receptor in fluorescence resonance energy transfer (FRET), which causes GO to have an extensive use in fluorescence-based aptasensor. Furthermore, GO can connect to aptamers through π–π stacking interactions.99,100 A non-label fluorescence aptasensor based on GO showed highly sensitive thrombin detection. The aptamer can be bound to its target and be separated from GO, and robust fluorescence was observed (LOD of 0.37 nM).101 A fluorescent aptasensor based on a GO can be used to detect extremely sensitive troponin I (cTnI). Thus, GO connects to the anti-cTnI aptamer and reduces its fluorescence. In fact, in the existence of cTnI, the anti-cTnI aptamer greeneries the GO combines with cTnI and then renovates the fluorescence of the aptamer. This aptasensor showed highly sensitive analytical measurement with a LOD of 0.07 ng mL−1. The formed diagnosis platform suggested a high-potential instrument for cTnI detection in serum samples.102
A novel hydrothermal assay was applied for the production of electrochemiluminescence (ECL) nanographene oxide covering TiO2. The nGO@TiO2 NLPs showed a core shell shape. This platform was applied to manufacture the ECL to target recognition, ECL signal generation, and amplification. If cTnI is present, the aptamer remains from the electrode surface due to its severity, which leads to a decrease in the charge transfer resistance of the electrode and an increase in the ratio of the two ECL signals of the NGO@TiO2 nanoluminophore (NLP) (Fig. 8A–C). As a result, according to the increase in the ECL ratio, cTnI can be detected by the aptasensor with a detection limit of 4 × 10−14 mol L−1. This method has more advantages than most of the reported electrochemical methods. This ECL approach displayed high potential in clinical diagnosis with its self-regulation ability and quick analytical performance.103 The synthesis of noble NPs, such as AgNPs decorated GO, can provide novel electrocatalytic properties according to their inherent catalytic activities. This type of composite can increase the catalytic activities of metal NPs and attain various applications. For example, AgNP-GO was formed through a chemical reduction assay, and one anti-thrombin aptamer (TBA1), was immobilized onto AgNP-GO. Besides, the second anti-thrombin aptamer (TBA2), keeping a thiol group at the 3′ ends, was self-assembled onto the electrode surface. The formed sandwich conjugate runs to a robust oxidation signal of Ag. Then, this voltammetry signal of AgNPs was documented to display the thrombin (LOD of 0.03 nmol L−1). From the characteristics of this aptasensor, the use of catalytic signal amplification caused by AgNP-GO, high-efficiency electrode, and also high sensitivity in detection by the aptamer-thrombin sandwich binding model, a sensitive electrochemical technique to the recognition of thrombin in samples was established104 (Fig. 8D).
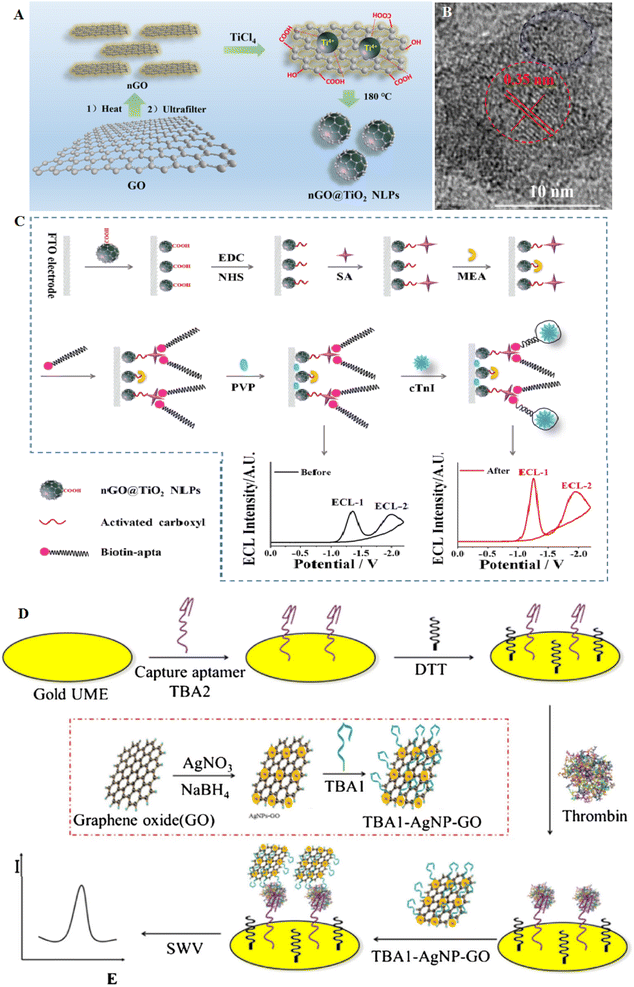 | ||
| Fig. 8 A carbon-based aptasensor for highly efficient recognition of thrombin. (A) The formation of nGO@TiO2 NLPs via hydrothermal assay. (B) TEM image of the nGO@TiO2 NLPs with the size of 6 nm. (C) Schematic of the ratiometric ECL aptasensor to cTnI detection based on nGO@TiO2 NLPs, reproduced from ref. 103 with permission from American Chemical Society, Copyright 2019. (D) Scheme of the electrochemical aptasensor for thrombin at gold microelectrode using TBA1-AgNP-GO as signal probe, reproduced from ref. 104 with permission from Elsevier, Copyright 2018. | ||
Conductive polymers, such as poly(3,4-ethylenedioxythiophene) (PEDOT), have great electrical conductivity and high electrochemical stability and are deliberated as gorgeous sensing materials according to their biocompatibility.105 Graphene-based ternary nanocomposite comprises conductive polymers and displays greater activity over binary composites. The presence of conductive polymers, according to their high electrical conductivity and stability, can make them important in developing great-performance biosensors.106 AuNPs/GO-doped PEDOT was deposited onto the surface of fluorine tin oxide glass at a potential of 1.0 V. MUC1 is a transmembrane glycoprotein, which is known as a biomarker that has a prognostic value in breast cancer. MUC1 aptamer was immobilized onto the composite film via a biotin–avidin interaction approach. This biosensor displayed a LOD of 1 fg mL−1. This aptasensor also recovered about 85–93% during the detection of MUC1 (ref. 106) (Fig. 9A).
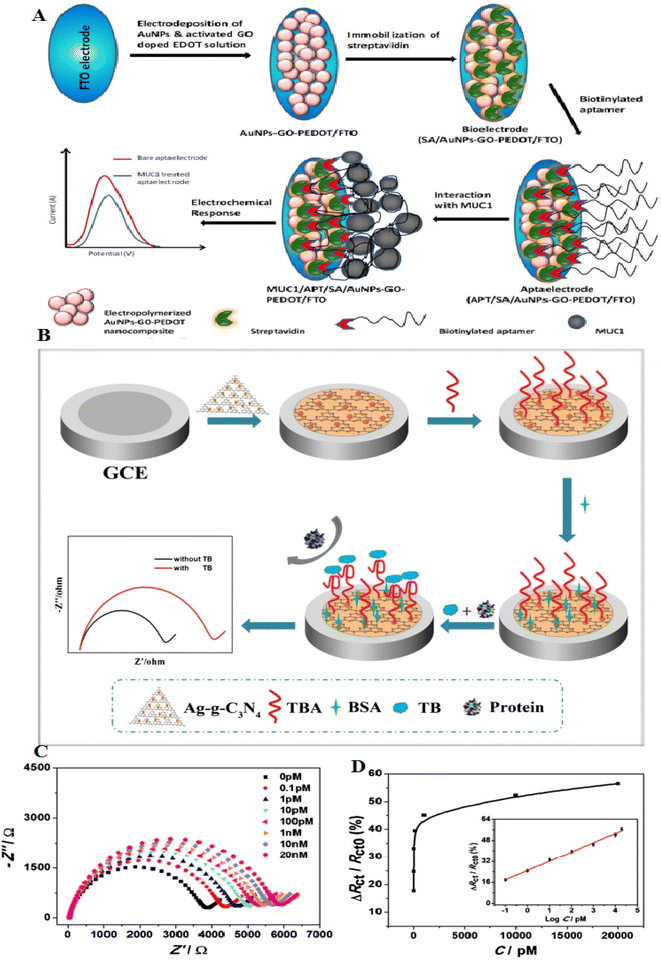 | ||
| Fig. 9 A novel electrochemical aptasensor platform for detection of a wide range of analytes, including thrombin, with a new mechanism and recoverable electrodes. (A) Schematic illustration of the formation of APT/SA/AuNPs-GO-PEDOT coated FTO electrode in order to breast cancer diagnosis, reproduced from ref. 106 with permission from Elsevier, Copyright 2018. (B) Schematic of the aptasensor construction process. (C) EIS signal of the aptasensor incubated with variable thrombin concentrations, from 0.1 pM to 20 nM. (D) Charge-transfer resistance changes of various concentrations of thrombin, reproduced from ref. 110 with permission from Elsevier, Copyright 2020. | ||
Graphite-like carbon nitride (g-C3N4) is beneficial in photocatalysts and biosensors according to the large surface and cost-effectiveness.107,108 g-C3N4, a 2D semiconductor, holds an exclusive electronic band structure, high compatibility, and great thermal stability.109 They have uniform nitrogen, making them easily decorated with metal NPs. However, one of their challenges is the poor conductivity of g-C3N4, which restricts them from electrochemical biosensing. It remains a limitation to increase the use of the g-C3N4 in electrochemical biosensors. Many strategies can overcome these limitations, including modification with metal NPs and polymers. g-C3N4 can be modified with AgNPs, which can be considered a photocatalyst. An electrochemical aptasensor, Ag-g-C3N4, showed a great surface and high compatibility. A glass carbon electrode (GCE) modified with Ag-g-C3N4 can immobilize many thrombin-binding aptamers. This aptasensor displays an LOD of 38 fM. The assay was used to detect thrombin, and the recoveries changed from 97–103% (ref. 110) (Fig. 9B–D). Table 4 shows the role of carbon-based nanomaterials-based aptasensors in protein detection.
| Nanomaterials | Aptamer | Biosensing principle | Target protein | Properties | LOD | Ref. |
|---|---|---|---|---|---|---|
| a EL: electrochemical. | ||||||
| PEI-rGO | ATCCGTCACACCTGCTCTTAATTACAGGCAGTTCCACTTAGACAGACACACGAATGGTGTTGGCTCCCGTAT | EL | Cardiac myoglobin (cMb) | High specificity, high reproducibility | 0.97 pg mL−1 | 22 |
| g-C3N4 | GGT TGG TGT GGT TGG | EL | Thrombin | Wide linear range, high selectivity, reproducibility | 38 fM | 110 |
| rGO/GCE | N/A | EL | Carcinoembryonic antigen (CEA) | Without immobilization of bioprobe, high sensitivity | 80 pg mL−1 | 111 |
| MWCNTs | CCCTCCTTTCCTTCGACGTAGATCTGCTGCGTTGTTCCGA | EL | Myoglobin | Greatly specific recognition capability of the aptamer | 2.2 pM | 112 |
Metal–organic framework (MOF)-based aptasensors
Metal–organic frameworks (MOFs) were fabricated from metal ions and organic linkers, have established high consideration, and have been extensively applied in catalysis and separation according to their flexible structures, and exclusive porosity.113 MOFs-based materials can be applied to design different electrochemical sensing systems, such as electrochemical and photoelectrochemical biosensors.114 MOFs-MIL-53(Al) showed high thermal and chemical stability.115 Researchers have described that Pd NPs coated MIL-53 were used to create an electrochemiluminescence immunosensor for the diagnosis of amyloid-β proteins.116 The combination of aptamers with MOFs causes the formation of nanostructure with properties of both aptamer and MOFs.117,118 MOFs with large surface areas permit an extensive range of ssDNA or RNA to adhere to the surface. Additionally, MOFs with adjustable pore diameters permit various nucleic acids to be immobilized on their surface. According to these properties, different types of MOFs-based aptasensors have been established, each with advantages such as great sensitivity and visual detection activity.119 Currently, there is no clear roadmap to select desirable MOFs for different biosensor applications; however, with the current trends in the literature, it is vividly predictable that in the near future, a pattern to choose the desired MOF for specific biosensor applications is possible.The fabrication of an electrochemical aptasensor based on the MIL-53(Al) decorated with Au@Pt NPs can be used to identify SARS-CoV-2 nucleocapsid protein through the catalysis of the nanomaterials. First, the immobilization of two thiol-modified aptamers on the surface of the gold electrode occurred; at that time, the Au@Pt/MIL-53 (Al) was decorated through horseradish peroxidase (HRP) and hemin/G-quadruplex DNAzyme as signal nanoprobe. This aptasensor has a high potential for detecting nCoV-NP, demonstrating the results with an extensive linear range from 0.02–50 ng mL−1 and the LOD of 8.3 pg mL−1 for SARS-CoV-2. The MOFs-based aptasensor showed high potential in the early detection of selectivity markers associated to the virus (Fig. 8).114
Magnetic MOFs are a class of MOFs with magnetic ability, which can be refined and separated throughout the synthesis by presenting an external magnetic field for protein separation.120 Sensitive detection of cTnI was performed by a voltammetric aptasensor based on dual-aptamer (dAPT) attached to nanotetrahedron (NTH) DNA.121 A meaningful increase in cTnI detection was observed according to the great binding affinity among the aptamer and cTnI. Owing to the advantage of the great surface area of MOFs, the nanoprobes can immobilize DNAzyme, HRP enzyme, and Au@PtNPs, intensifying the electrochemical signal. This aptasensor creates an excellent activity in the detection of cTnI with great specificity, high stability, and LOD of about 5 pg mL−1. Since this aptasensor usage still has several limitations, including the time-consuming fabrication procedure, new technologies such as microfluidics is needed to improve its performance in clinical diagnosis.
Developing a dual-signal sensing system is critical in bioanalysis according to the likelihood of using both simultaneously. Each method has benefits and limitations that can complement each other's abilities in two-manner detection. As a result, being stronger and more resistant is observed in the case of sensors with dual-mode detection features. For example, a biosensor was formed to detect C-reactive protein (CRP) using the two modes of the optical transducer, fluorometry and colorimetry. In this aptasensor, the combination of RNA on Cu-MOF was done. Cu-MOF can exhibit fluorescence and peroxidase enzymatic activity, which can be applied as signal transduction. CRP binding RNA as a greatly sensitive recognition element immobilized on the Cu-MOF (Fig. 10). Besides, with the addition of CRP to the RNA/Cu-MOF, the deliver of RNA from the Cu-MOF surface has occurred. Here, the LOD of this aptasensor was 40 pg mL−1 in fluorescence sensing and LOD of 240 pg mL−1 in the colorimetry approach. This LOD is lesser than the LOD of nephelometric methods applied in clinical applications and CRP assays with 1 μg mL−1. The MOF-aptasensor was effectively used to diagnose CRP in COVID-19 patients.122
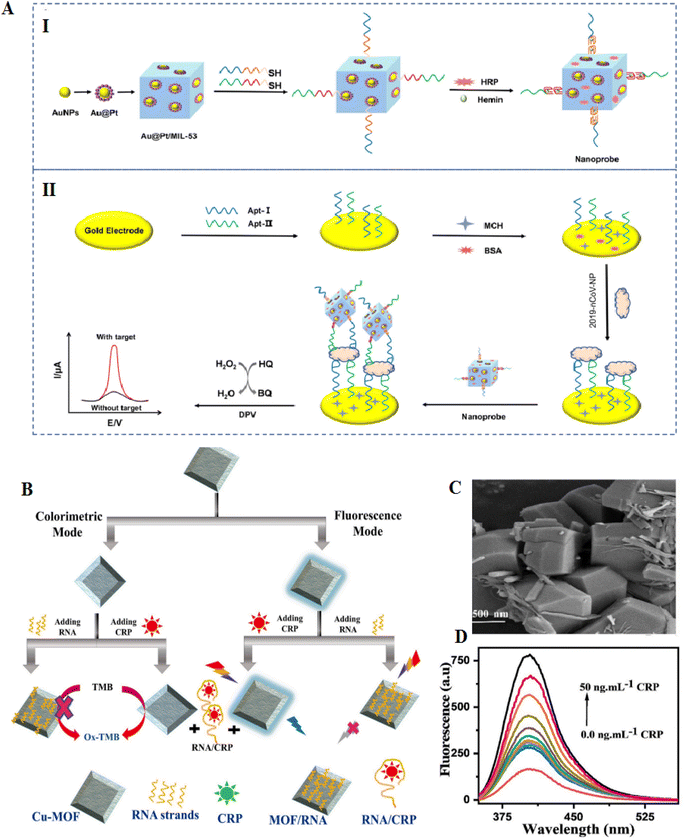 | ||
| Fig. 10 MOF-based electrodes for the detection of coronavirus via aptamer-surface decoration. (A) (I) manufacture procedure of the nanoprobes (II) and the aptasensor to recognition of COVID-19. The Au@Pt NPs were connected to the MOFs NH2-MIL-53(Al) NPs via amino groups. The catalysis of HRP and GQH DNAzyme nanoprobe after the decorated HRP onto the NPs, reproduced from ref. 114 with permission from Elsevier, Copyright 2021. (B) The procedures for CRP aptasensor based on Cu-MOF. (C) FESEM of Cu-MOF, the materials have polydisperse decahedral crystal forms. (D) Fluorescence spectra of MOF-RNA probe treated with various concentrations of CRP, reproduced from ref. 122 with permission from Elsevier, Copyright 2022. | ||
The objective of the disclosed method (US Patent) was to provide a low-cost, and greatly sensitive way of preparing a ratiometric electrochemical aptasensor for vanillin. The method involved several steps, including dissolving 2-methylimidazole (Hmim) in ethanol, adding ferrocene and Ketjenblack, stirring well, adding zinc nitrate, and then transferring to a high-pressure reactor to form a ferrocene-Ketjenblack-ZIF-8 composite. The nanocomposite was applied to modify a glassy carbon electrode, which is immersed in a buffer electrolyte solution and chloroauric acid to generate gold NPs in situ by one-step electrodeposition, thereby obtaining a gold NP-deposited ZIF-8 nanocomposite. Then, the aptamer DNA of vanillin was connected to the surface of the gold NP-deposited ZIF-8 nanocomposite to obtain an aptamer-modified Au NP-ZIF-8 composite-modified electrode. The modified electrode was then applied as a working electrode, and the electrochemical square-wave voltammetry curves are measured in the existence of various vanillin concentrations. The ratios of the peak intensities of ferrocene and vanillin were applied as a reference signal and a response signal, respectively, to construct the ratiometric electrochemical aptasensor for vanillin. The method had several parameters that can be adjusted for optimal results for commercializations. In step (1), the reaction temperature, reaction time, and mass concentration ratio of Hmim, ferrocene, Ketjenblack to zinc nitrate can be varied. In step (2), the dosage of Nafion, mass concentration of the ZIF-8 nanocomposite, the concentration of chloroauric acid, potential range, scanning rate, and scanning number can be adjusted. In step (3), the concentration of the aptamer of vanillin and the incubation time can be varied. In step (4), the linear detection range of vanillin concentration and the LOD can be adjusted. Compared to the prior art, the disclosed method was advantageous because it is simple, low-cost, and uses easily obtainable raw materials. The ratiometric signal had a strong anti-interference ability, making it highly sensitive and accurate. The method can be developed into a new ratiometric electrochemical aptasensor in order to the efficient recognition of vanillin.123
The objective of another invention was to provide a low-cost, sensitive, and selective assay for making an electrochemical miR3123 aptasensor based on a Cu-MOF nanocomposite codoped with black phosphorus nanosheets (BPNSs) and thionine (TH). To achieve this objective, a method was developed, which included the following steps: formation of a TH/Cu-MOF composite, preparation of a BPNSs/TH/Cu-MOF composite, preparation of an aptamer-BPNSs/TH/Cu-MOF composite, and using the nanocomposite modified glassy carbon electrode. The resulting sensor had a linear detection concentration of 1 nM to 10 μM and a LOD of 1–5 nM. The method involved reacting TH and Cu-MOF precursor to prepare a TH/Cu-MOF composite, which was then coated with BPNSs dispersion to prepare a BPNSs/TH/Cu-MOF composite. The composite was coated on the surface of a glassy carbon electrode to construct a composite-modified glassy carbon electrode. A ferrocene (Fc)-labeled ssDNA aptamer was adsorbed on the BPNSs to form an aptamer-BPNSs/TH/Cu-MOF nanocomposite. Target molecule miR3123 was specifically bound to the aptamer, causing Fc-DNA to escape from BPNSs, resulting in weakened electrochemical signals of Fc, while the TH signals remained unaffected. By using the TH as a reference and Fc as a signal response unit, a ratio electrochemical miR3123 aptamer sensor was constructed.124
MXene-based aptasensors
Compared to 2D materials, MXenes show great surface area and good electrical conductivity.125 MXene has a general formula of Mn+1XnTx (M: transition metal, X: carbon, and Tx: functional groups on the surface of MXenes). They are formed through liquid stripping methods and etching of layer A atoms from the MAX phase. They have several properties, such as unique surface chemistry, electrochemical behavior, conductivity, and high biocompatibility, making them suitable for both treatment and diagnostic systems.126–129The mixture of 2D MXenes with great affinity aptamers showed new aptasensing assays for protein diagnosis. Despite many studies on the role of metal nanomaterials-aptasensors in protein detection, these studies on the role of MXene are much less due to their novelty. The development of an MXene aptasensor needs the immobilization of aptamer on the surface of MXene. Therefore, various covalent and non-covalent procedures have been investigated for aptamer immobilization on MXene. MXene has many hydroxyl groups on its surface, which facilitates their activation. Also, another method involves coupling with zwitterions, which makes it possible to modify the MXene surface.130 Wang and coworkers applied MXene to form an aptasensor detecting MUC1. MXene was primarily modified with a ferrocene-labeled cDNA biosensor to enhance the detection signals against MUC1 detection. The probe was immobilized with aptamer over the electrode to construct an MXene-based aptasensor. The recognition assay to bind MUC1 aptamer with a probe or MUC1 increased the sensitivity, as presented in Fig. 11A. The detection of MUC1 in blood serum showed that the biosensor keeps a high activity for recognizing the MUC1 biomarker in real samples.126 The formed electrochemical aptasensor offers a LOD of 0.3 pM, which is hopeful for clinical diagnosis.
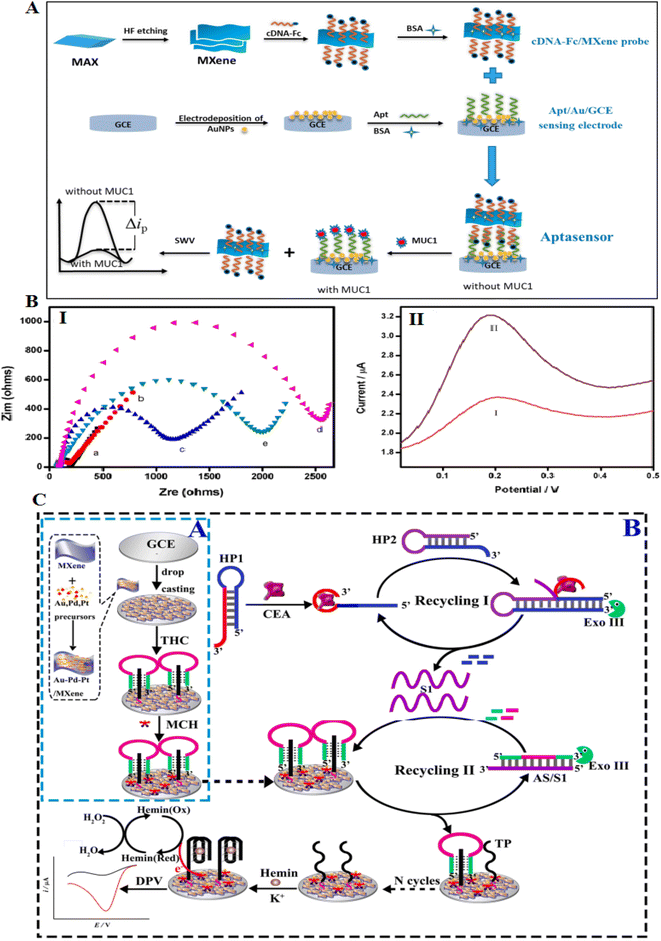 | ||
| Fig. 11 MXene-based aptasensors for recognition of low concentrations of CEA. (A) Schematic of the formation process of the competitive electrochemical aptasensor. (B) (I) EIS of various modified electrodes in 5 mM K3[Fe(CN)6]/K4[Fe(CN)6] solution. (II) SWV of various electrodes in PBS buffer (0.1 M): ((I) cDNA-Fc/Apt/Au/GCE), ((II) cDNA-Fc/MXene/Apt/Au/GCE), reproduced from ref. 126 with permission from Elsevier, Copyright 2020. (C) (A) The synthesis assay for formed nanocomposite. (C) The Exo III-related dual recycling amplifications to catalytic CEA detection, reproduced from ref. 132 with permission from Elsevier, Copyright 2022. | ||
Carcinoembryonic antigen (CEA) is a protein generally present in the blood. However, the CEA blood level can be enhanced in colon, breast, and lung cancers.131 Researchers developed a label-free sensor to detect CEA that combines an Au–Pd–Pt/Ti3C2Tx nanocomposite with a dual amplification system to fabricate a modified electrode. The CEA molecules can be conjugated with the aptamer comprising hairpin probes to stimulate cleavage of the hairpins via exonuclease III to release the ssDNAs (Fig. 11B and C). Exo III causes the cleaving of the duplexes to release several sequences that can restrict hemin on the electrode surface. This aptasensor showed two advantages for the detection of CEA: first, the Exo III could help the signal amplification in the combination of catalytic activity of the Au–Pd–Pt/Ti3C2Tx nanomaterial to attain signal enhancement to increase the sensitivity; then, this aptasensor is label-free to evade the contribution of labeling the DNA probes by electroactive types.132
Incorporating an electrochemical biosensor with a microfluidic system can increase the sensitivity and specificity of diagnostic systems.133 Microfluidic technology is a powerful technique to control fluids in a micrometer-sized channel. It can complete the sample separation, which acts as a rapid technique.134 Zhao and coworkers133 developed a biosensor using a carbon electrode modified with a hemin-coated carboxylic CNT decorated Ti3C2 nanosheets (NSs) [He@carboxylated carbon nanotube (CCNT)/Ti3C2]. Then, the vertical fluid flows through a microfluidic chip prepared with a fishbone structure to the biosensor interface, which can increase the affinity between the aptamer and the CEA ligand. When CEA is captured, developing an insulating protein layer decreases the efficacy of electron transport (Fig. 12). This sensing technology showed different advantages than common detection models, such as: (1) the combination of electrochemical sensing with a microfluidic device critically increases the movability of the sensing platform, and (2) the whole procedure of activity performed on one integrated chip. The LOD of CEA detection was 2.8 pg mL−1.
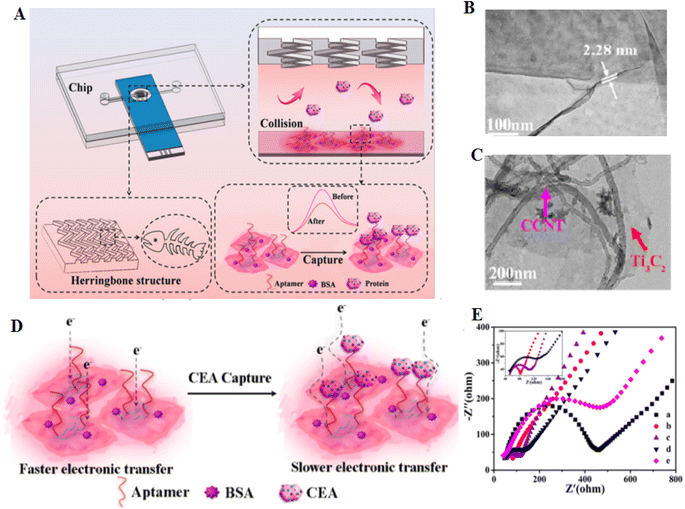 | ||
| Fig. 12 This is a novel system for capturing and detection of CEA, based on microfluidics and aptamer. (A) Microfluidic electrochemical system for detection of CEA. (B) FESEM image of Ti3C2 nanosheets and (C) He@CCNT/Ti3C2. (D) Diagram of electron transfer before and after CEA capture. (E) The decreases of the Ret value of He@CCNT/Ti3C2/SPCE (curve b) compared with that of bare SPCE (curve a), endorsing the good conductivity of He@CCNT/Ti3C2, reproduced from ref. 133 with permission from American Chemical Society, Copyright 2021. | ||
As a result, the electrochemical/microfluidic platform displays excellent application in the diagnosis.133 MXene-based aptasensors seem very useful according to their sensitivity ranging from picomolar to femtomolar. However, despite their benefits, they have several challenges, including: (1) differences in signal transduction according to the quality and conditions of MXene synthesis; (2) their intrinsic instability and expensive manufacture; (3) scalability of fabrication procedures. Table 5 shows the role of MXenes applied as transducers for detecting proteins.
| Nanomaterials | Aptamer | Biosensing principle | Target protein | Properties | LOD | Ref. |
|---|---|---|---|---|---|---|
| a ECL: electrochemiluminescence. EC: electrochemical. FRET: fluorescence resonance energy transfer. | ||||||
| Ti3C2Tx/ZIF-8/GCE/Apt | AGTCAGTGTGGAAAATCTCTAGC | ECL | HIV protein | Good ECL response, improved electrical conductivity | 0.3 fM | 135 |
| AuNPs/Ti3C2Tx/Apt | N/A | ECL | CD63 protein | High sensitivity and selectivity to exosome and their proteins, accurate detection | 30 particle per μL | 136 |
| Ti3C2/PAMAM-Au NPs/CD63-Apt/GCE | TTTTTTCACCCCACCTCGCTCCCGTGACACTAATGCTA | EC | CD63 protein | High specificity even in serum samples, excellent conductivity | 229 particle per μL | 137 |
| Cy3-CD63-Apt/Ti3C2 | CACCCCACCTCGCTCCCGTGACACTAATGCTA | FRET | CD63 protein | Detection of multiple biomarkers on the exosome surface | 1.4 × 103 particles per mL | 138 |
| Apt/He@CCNT/Ti3C2/SPCE | N/A | EC | CEA | Increases the movability of the sensing platform | 2.88 pg mL−1 | 133 |
Metallic nanostructures-based aptasensors: the road of commercialization
Aptasensors have revealed immense potential as a diagnostic tool for a wide range of diseases and disorders, such as cancer, infectious diseases, and autoimmune disorders. One of the most promising types of aptasensors is the metallic nanostructures-based aptasensor. These sensors are built using metallic NPs such as gold, silver, and platinum, and can detect a variety of target analytes with high sensitivity and specificity. Despite their potential, there are several challenges that must be addressed before metallic nanostructures-based aptasensors can be successfully commercialized.(1) Stability and reproducibility: one of the biggest challenges facing metallic nanostructures-based aptasensors is achieving stability and reproducibility. Metallic NPs are known to be sensitive to changes in their environment, such as pH, temperature, and ionic strength. These changes can cause the NPs to aggregate or change shape, which can affect their optical and electrical properties, and thus, their ability to detect target analytes. In addition, the synthesis of metallic NPs can be variable, leading to differences in their size, shape, and surface chemistry, which can also affect their performance as aptasensors. To address these challenges, researchers have developed methods to stabilize metallic NPs and improve their reproducibility. For example, the use of biocompatible coatings such as polyethylene glycol (PEG) or silica can prevent aggregation and stabilize the NPs in solution. In addition, the use of template-assisted synthesis or microfluidic techniques can control the size and shape of the NPs, leading to more reproducible synthesis.
(2) Sensitivity and selectivity: another challenge facing metallic nanostructures-based aptasensors is achieving high sensitivity and selectivity. Metallic NPs can enhance the detection of target analytes through localized surface plasmon resonance (LSPR) and surface-enhanced Raman scattering (SERS). However, the performance of these sensors can be affected by factors such as the size and shape of the NPs, the distance between the NPs, and the type and density of functional groups on the surface of the NPs. To address these challenges, researchers have developed strategies to optimize the performance of metallic nanostructures-based aptasensors. For example, the use of different shapes of NPs such as nanospheres, nanorods, and nanostars can enhance the LSPR effect and improve the sensitivity of the sensor. In addition, the use of functionalized ligands such as thiolated DNA can improve the selectivity of the sensor by specifically binding to the target analyte.
(3) Manufacturing and scalability: another challenge facing metallic nanostructures-based aptasensors is manufacturing and scalability. The synthesis of metallic NPs is often carried out in small batches using specialized equipment, which can be time-consuming and expensive. In addition, the functionalization of the NPs with aptamers requires careful optimization to ensure their specificity and stability. To address these challenges, researchers have developed methods for large-scale synthesis and functionalization of metallic NPs. For example, the use of microwave-assisted synthesis or continuous flow reactors can increase the yield and reduce the time required for synthesis. In addition, the use of automated functionalization platforms can improve the consistency and reproducibility of aptamer immobilization on the surface of the NPs.
(4) Regulatory approval: finally, one of the most significant challenges facing metallic nanostructures-based aptasensors is obtaining regulatory approval for commercialization. In many countries, medical devices such as aptasensors must undergo rigorous testing and regulatory approval before they can be marketed to the public. This process can be time-consuming and expensive, requiring extensive clinical trials and documentation of safety and efficacy. To address these challenges, researchers and manufacturers must work closely with regulatory agencies to ensure that their aptasensors meet the required standards for safety and effectiveness. This may involve consulting with regulatory experts, conducting pre-clinical and clinical trials, and providing detailed documentation of the device's performance and safety. In addition to regulatory approval, the marketing and distribution of aptasensors also face challenges related to intellectual property and market competition. Companies must protect their intellectual property and secure patents for their technology to prevent infringement by competitors. They must also develop effective marketing strategies to promote their products and differentiate them from other similar devices in the market.
Apart from the challenges mentioned above, there are several other obstacles that need to be overcome for the commercialization of metallic nanostructures-based aptasensors. One such issue is the reproducibility of the sensor response. The variability in the size, shape, and composition of metallic NPs used in the synthesis of the sensor can lead to variations in the signal response. Therefore, to achieve reproducible results, standardized synthesis and fabrication protocols need to be established. Another challenge is the stability of the sensor over time. The metallic NPs used in the sensor fabrication can undergo aggregation or oxidation, leading to a decrease in the sensor's sensitivity and stability. Moreover, the aptamers used in the sensor can undergo denaturation or degradation, leading to a reduction in their affinity and specificity towards the target analyte. Therefore, the development of strategies to increase the stability of both the NPs and aptamers is crucial for the successful commercialization of the sensor. Another critical issue is the cost-effectiveness of the sensor. The synthesis and fabrication of metallic NPs are relatively expensive compared to other materials used in sensor fabrication. Moreover, the use of sophisticated instrumentation for the detection of the signal response can also add to the overall cost of the sensor. Therefore, efforts should be made to reduce the cost of the sensor without compromising its performance. Despite these challenges, metallic nanostructures-based aptasensors keep high potential for the commercialization of novel sensing technologies. The ability of these sensors to detect analytes with high sensitivity, selectivity, and accuracy makes them an attractive option for a wide range of applications, such as clinical diagnostics, and environmental monitoring. Moreover, the versatility of the aptamer sequences allows the development of sensors for a wide range of analytes, including small biomolecules, proteins, and cells. In conclusion, the commercialization of aptasensors presents several challenges that require to be overcome for their effective integration into the market. The development of reliable, stable, and cost-effective sensors with high selectivity and sensitivity towards the target analyte is crucial for their acceptance by end-users. The advancement of technologies for the synthesis and fabrication of metallic nanostructures, as well as the optimization of signal detection methods, can pave the way for the successful commercialization of aptasensors. With the current pace of research and development in this field, it is expected that aptasensors will become an integral part of the diagnostic and monitoring tools used in healthcare and other industries in the near future.
Conclusion and future perspective
This review discussed recent progress in developing aptasensors based on metal nanomaterials as a transducer for the specific detection of proteins. The integration of metal nanomaterials indicates a remarkable improvement in analytical activity in aptasensors, in line with progress in biosensors based on metal nanomaterials. Nanomaterials add to the sensitivity of the biosensors by providing a great surface area for aptamer immobilization and by deliberating their physical and electrochemical properties to the biosensor. Metal nanomaterials-based aptasensors seem highly beneficial due to their detection sensitivity ranging from picomolar to femtomolar, which is greater than common methods, such as chromatography. Noble metal/metal oxide nanomaterials, such as AgNPs, AuNPs, QDs, etc., have unique properties for accurate and sensitive biosensors. The use of composite nanomaterials and the mixture use of two or more nanomaterials shows the current tendency toward synergetic effects on sensitivity and stability enhancement in aptasensors. MXene, with its exclusive properties, functionalization, and conductivity, shows greater electrochemical properties than other materials; therefore, ample functional groups on their surface are vital for developing increased sensors.Despite these benefits, aptasensors are still in their beginning stages compared to other biosensing techniques. However, aptasensors based on metal nanomaterials could be suitable when critical challenges are addressed:
(1) Aptamers have a short half-life, which is a serious problem for the aptasensor industry. As a result, there is a need for modification and changes in aptamers to increase their half-life, such as the modification of the aptamer with 2′-O-methylation, which may considerably enhance the aptamer's half-life, without compromising their affinity.139
(2) Mass production and additional costs, including the scalability of manufacturing processes, can limit aptasensors based on metal NPs, including MOF and MXene. This type of aptasensors was made for laboratory-scale production and cannot be used for mass construction. Thus, aptasensors must be marketed on a large scale, and manufacturing techniques must be developed to build a large supply of aptasensors that are produced using inexpensive cost-effective precursors.
(3) One of the important challenges is the oxidation of metal nanomaterials, such as noble metal nanomaterials and MXenes in aptasensors, which affects the stability of sensors in hot environments; thus, the investigation at the molecular level, as well as the interactions among different metal NPs and aptamer is essential. One suggestion would be the use of natural products and leaf extracts as capping agents and surface decoration agents.
(4) It is vital to investigate the toxicity and compatibility of metal nanomaterials-based aptasensor systems, especially in cells or in vivo. However, selecting flexible metal NPs composites allows researchers to build customizable aptasensors with the desired functionality. For instance, since fluorine is toxic, fluorine-free MXene Ti3C2Tx is better than HF-etched MXene when used in in vivo detection.140 Since these theories need to be tested widely, we encourage researchers to focus on these topics to address the current challenges.
(5) A more comprehensive study of aptamer immobilization approaches on different metal NP-based aptasensors is needed for cost-effective sensing of multiple molecules, especially for POC systems.
Commercialization requires inventions to confirm that these techniques can be applied regularly. Continuous benefits in all these metal nanomaterials-autosensing are possible by joining multiple methods. Thus, future hard work should be focused on the building of less erudite technologies that can be employed via non-professional people. The assessment of metal nanomaterials-based aptasensors and future propensity are predicted to further discover prospects for biomedical sensing developments, especially for protein detection.
Author contributions
N. R., S. A., K. R., S. C., and R. N. V. wrote the main manuscript, conceptualized and finalized the manuscript. All authors reviewed and approved the manuscript.Conflicts of interest
The authors declare they have no conflict of interest.References
- A. Ghasemi, N. Rabiee, S. Ahmadi, S. Hashemzadeh, F. Lolasi, M. Bozorgomid, A. Kalbasi, B. Nasseri, A. Shiralizadeh Dezfuli and A. R. Aref, et al., Optical assays based on colloidal inorganic nanoparticles, Analyst, 2018, 143(14), 3249–3283, 10.1039/C8AN00731D.
- J. Zhou and J. Rossi, Aptamers as targeted therapeutics: current potential and challenges, Nat. Rev. Drug Discovery, 2017, 16(3), 181–202, DOI:10.1038/nrd.2016.199.
- H. Y. Kong and J. Byun, Nucleic Acid aptamers: new methods for selection, stabilization, and application in biomedical science, Biomol. Ther., 2013, 21(6), 423–434, DOI:10.4062/biomolther.2013.085.
- M. Chakravarthy, H. AlShamaileh, H. Huang, R. K. Tannenberg, S. Chen, S. Worrall, P. R. Dodd and R. N. Veedu, Development of DNA aptamers targeting low-molecular-weight amyloid-β peptide aggregates in vitro, Chem. Commun., 2018, 54(36), 4593–4596, 10.1039/c8cc02256a.
- M. Chakravarthy, S. Chen, P. R. Dodd and R. N. Veedu, Nucleic Acid-Based Theranostics for Tackling Alzheimer’s Disease, Theranostics, 2017, 7(16), 3933–3947, DOI:10.7150/thno.21529.
- S. L. Edwards, V. Poongavanam, J. R. Kanwar, K. Roy, K. M. Hillman, N. Prasad, R. Leth-Larsen, M. Petersen, M. Marušič, J. Plavec, J. Wengel and R. N. Veedu, Targeting VEGF with LNA-stabilized G-rich oligonucleotide for efficient breast cancer inhibition, Chem. Commun., 2015, 51(46), 9499–9502, 10.1039/c5cc02756j.
- Q. W. Hughes, B. T. Le, G. Gilmore, R. I. Baker and R. N. Veedu, Construction of a Bivalent Thrombin Binding Aptamer and Its Antidote with Improved Properties, Molecules, 2017, 22(10), 1770, DOI:10.3390/molecules22101770.
- J. R. Kanwar, K. Roy, N. G. Maremanda, K. Subramanian, R. N. Veedu, R. Bawa and R. K. Kanwar, Nucleic acid-based aptamers: applications, development and clinical trials, Curr. Med. Chem., 2015, 22(21), 2539–2557, DOI:10.2174/0929867322666150227144909.
- K. Rahimizadeh, H. AlShamaileh, M. Fratini, M. Chakravarthy, M. Stephen, S. Shigdar and R. N. Veedu, Development of Cell-Specific Aptamers: Recent Advances and Insight into the Selection Procedures, Molecules, 2017, 22(12), 2070, DOI:10.3390/molecules22122070.
- B. Sriramoju, R. Kanwar, R. N. Veedu and J. R. Kanwar, Aptamer-targeted oligonucleotide theranostics: a smarter approach for brain delivery and the treatment of neurological diseases, Curr. Top. Med. Chem., 2015, 15(12), 1115–1124, DOI:10.2174/1568026615666150413153928.
- T. Wang, C. Chen, L. M. Larcher, R. A. Barrero and R. N. Veedu, Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development, Biotechnol. Adv., 2019, 37(1), 28–50, DOI:10.1016/j.biotechadv.2018.11.001.
- T. Wang, L. Chen, A. Chikkanna, S. Chen, I. Brusius, N. Sbuh and R. N. Veedu, Development of nucleic acid aptamer-based lateral flow assays: A robust platform for cost-effective point-of-care diagnosis, Theranostics, 2021, 11(11), 5174–5196, DOI:10.7150/thno.56471.
- Y. He, C. Y. Wen, Z. J. Guo and Y. F. Huang, Noble metal nanomaterial-based aptasensors for microbial toxin detection, J. Food Drug Anal., 2020, 28(4), 508–520, DOI:10.38212/2224-6614.1155.
- R. Yazdian-Robati, N. Hedayati, M. Ramezani, K. Abnous and S. M. Taghdisi, Colorimetric gold nanoparticles-based aptasensors, Nanomedicine, 2018, 5(1), 1–5, DOI:10.22038/nmj.2018.05.001.
- G. Wang, Y. Wang, L. Chen and J. Choo, Nanomaterial-assisted aptamers for optical sensing, Biosens. Bioelectron., 2010, 25, 1859–1868, DOI:10.1016/j.bios.2009.11.012.
- W. Cheng, X. Tang, Y. Zhang, D. Wu and W. Yang, Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances, Trends Food Sci. Technol., 2021, 112, 268–282, DOI:10.1016/j.tifs.2021.04.004.
- R. Eivazzadeh-Keihan, E. Bahojb Noruzi, E. Chidar, M. Jafari, F. Davoodi, A. Kashtiaray, M. Ghafori Gorab, S. Masoud Hashemi, S. Javanshir and R. Ahangari Cohan, et al., Applications of carbon-based conductive nanomaterials in biosensors, Chem. Eng. J., 2022, 442, 136183, DOI:10.1016/j.cej.2022.136183.
- E. O. Polat, M. M. Cetin, A. F. Tabak, E. Bilget Güven, B. Ö. Uysal, T. Arsan, A. Kabbani, H. Hamed and S. B. Gül, Transducer Technologies for Biosensors and Their Wearable Applications, Biosensors, 2022, 12(6), 385 CrossRef CAS PubMed.
- T. Phumlani, S. Poslet Morgan and N.-T. Zikhona, Biosensors: Design, Development and Applications, in Nanopores, ed. Sadia, A., Akhtar, M. S. and Hyung-Shik, S., IntechOpen, 2021, ch. 3 Search PubMed.
- A. Sassolas, L. J. Blum and B. D. Leca-Bouvier, Optical detection systems using immobilized aptamers, Biosens. Bioelectron., 2011, 26(9), 3725–3736 CrossRef CAS PubMed.
- R. Abd-Ellatief and M. R. Abd-Ellatief, Electrochemical Aptasensors: Current Status and Future Perspectives, Diagnostics, 2021, 11(1), 104, DOI:10.3390/diagnostics11010104.
- A. Sharma, J. Bhardwaj and J. Jang, Label-Free, Highly Sensitive Electrochemical Aptasensors Using Polymer-Modified Reduced Graphene Oxide for Cardiac Biomarker Detection, ACS Omega, 2020, 5(8), 3924–3931, DOI:10.1021/acsomega.9b03368.
- J. Zhang, X. Zhang and S. Bi, Two-Dimensional Quantum Dot-Based Electrochemical Biosensors, Biosensors, 2022, 12(4), 254, DOI:10.3390/bios12040254.
- H. Sun, N. Wang, L. Zhang, H. Meng and Z. Li, Aptamer-Based Sensors for Thrombin Detection Application, Chemosensors, 2022, 10(7), 255 CrossRef CAS.
- H. Kaur and M. Shorie, Nanomaterial based aptasensors for clinical and environmental diagnostic applications, Nanoscale Adv., 2019, 1(6), 2123–2138, 10.1039/C9NA00153K.
- L. Huang, S. Tian, W. Zhao, K. Liu, X. Ma and J. Guo, Aptamer-based lateral flow assay on-site biosensors, Biosens. Bioelectron., 2021, 186, 113279 CrossRef CAS PubMed.
- J. H. Luong, K. B. Male and J. D. Glennon, Biosensor technology: technology push versus market pull, Biotechnol. Adv., 2008, 26(5), 492–500 CrossRef CAS PubMed.
- P. B. Deroco, D. Wachholz Junior and L. T. Kubota, Recent Advances and Future Trends in Bioanalytical Chemistry, Tools and Trends in Bioanalytical Chemistry, 2022, pp. 543–558 Search PubMed.
- B. Nasseri, N. Soleimani, N. Rabiee, A. Kalbasi, M. Karimi and M. R. Hamblin, Point-of-care microfluidic devices for pathogen detection, Biosens. Bioelectron., 2018, 117, 112–128 CrossRef CAS PubMed.
- W. Li, W. Luo, M. Li, L. Chen, L. Chen, H. Guan and M. Yu, The impact of recent developments in electrochemical POC sensor for blood sugar care, Front. Chem., 2021, 9, 723186 CrossRef CAS PubMed.
- R. Zeng, H. Gong, Y. Li, Y. Li, W. Lin, D. Tang and D. Knopp, CRISPR-Cas12a-derived photoelectrochemical biosensor for point-of-care diagnosis of nucleic acid, Anal. Chem., 2022, 94(20), 7442–7448 CrossRef CAS PubMed.
- Y. Aslan, M. Atabay, H. K. Chowdhury, I. Göktürk, Y. Saylan and F. Inci, Aptamer-based point-of-care devices: Emerging technologies and integration of computational methods, Biosensors, 2023, 13(5), 569 CrossRef CAS PubMed.
- Y. Zhao, S. Bu, C. Wang, C. Ma, Z. Li, W. Zhang and J. Wan, Dual aptamer-copper (II) phosphate nanocomposite-based point-of-care biosensor for the determination of Escherichia coli O157: H7 through pressure monitoring with a hand-held barometer, Anal. Lett., 2020, 54(10), 1603–1615 CrossRef.
- Q. Li, B. Liang, W. Li, W. Li, J. Sun, S. Jiao, S. Wang, Y. Jin, T. Zheng and J. Li, A capillary device made by aptamer-functionalized silica photonic crystal microspheres for the point-of-care detection of Ochratoxin A, Sens. Actuators, B, 2021, 330, 129367 CrossRef CAS.
- J. Wang, Q. Wang, Y. Zhong, D. Wu and N. Gan, A sandwich-type aptasensor for point-of-care measurements of low-density lipoprotein in plasma based on aptamer-modified MOF and magnetic silica composite probes, Microchem. J., 2020, 158, 105288 CrossRef CAS.
- A. Jaisankar, S. Krishnan and L. Rangasamy, Recent developments of aptamer-based lateral flow assays for point-of-care (POC) diagnostics, Anal. Biochem., 2022, 114874 CrossRef CAS PubMed.
- M. Prante, E. Segal, T. Scheper, J. Bahnemann and J. Walter, Aptasensors for point-of-care detection of small molecules, Biosensors, 2020, 10(9), 108 CrossRef PubMed.
- N. Rabiee, S. Chen, S. Ahmadi and R. N. Veedu, Aptamer-engineered (nano) materials for theranostic applications, Theranostics, 2023, 13(15), 5183 CrossRef CAS PubMed.
- V. Georgakilas, J. N. Tiwari, K. C. Kemp, J. A. Perman, A. B. Bourlinos, K. S. Kim and R. Zboril, Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications, Chem. Rev., 2016, 116(9), 5464–5519, DOI:10.1021/acs.chemrev.5b00620.
- B. Liu, S. Salgado, V. Maheshwari and J. Liu, DNA adsorbed on graphene and graphene oxide: Fundamental interactions, desorption and applications, Curr. Opin. Colloid Interface Sci., 2016, 26, 41–49, DOI:10.1016/j.cocis.2016.09.001.
- H.-T. Zhang, J.-W. Zhang, G. Huang, Z.-Y. Du and H.-L. Jiang, An amine-functionalized metal–organic framework as a sensing platform for DNA detection, Chem. Commun., 2014, 50(81), 12069–12072, 10.1039/C4CC05571C.
- S. Zhang, S. Malik, N. Ali, A. Khan, M. Bilal and K. Rasool, Covalent and Non-covalent Functionalized Nanomaterials for Environmental Restoration, Top. Curr. Chem., 2022, 380(5), 44, DOI:10.1007/s41061-022-00397-3.
- A. Y. S. Eng, C. K. Chua and M. Pumera, Refinements to the structure of graphite oxide: absolute quantification of functional groups via selective labelling, Nanoscale, 2015, 7(47), 20256–20266, 10.1039/C5NR05891K.
- W. Xu, L. Jiao, H. Yan, Y. Wu, L. Chen, W. Gu, D. Du, Y. Lin and C. Zhu, Glucose Oxidase-Integrated Metal-Organic Framework Hybrids as Biomimetic Cascade Nanozymes for Ultrasensitive Glucose Biosensing, ACS Appl. Mater. Interfaces, 2019, 11(25), 22096–22101, DOI:10.1021/acsami.9b03004.
- G. Habibullah, J. Viktorova and T. Ruml, Current Strategies for Noble Metal Nanoparticle Synthesis, Nanoscale Res. Lett., 2021, 16(1), 47, DOI:10.1186/s11671-021-03480-8.
- E. Celikbas, S. Balaban, S. Evran, H. Coskunol and S. Timur, A Bottom-Up Approach for Developing Aptasensors for Abused Drugs: Biosensors in Forensics, Biosensors, 2019, 9(4), 118, DOI:10.3390/bios9040118.
- V. Butova, M. Soldatov, A. Guda, K. Lomachenko and C. Lamberti, Metal-organic frameworks: structure, properties, methods of synthesis, and characterization, Russ. Chem. Rev., 2016, 85, 280–307, DOI:10.1070/RCR4554.
- F. Cao, M. Zhao, Y. Yu, B. Chen, Y. Huang, J. Yang, X. Cao, Q. Lu, X. Zhang and Z. Zhang, et al., Synthesis of Two-Dimensional CoS1.097/Nitrogen-Doped Carbon Nanocomposites Using Metal–Organic Framework Nanosheets as Precursors for Supercapacitor Application, J. Am. Chem. Soc., 2016, 138(22), 6924–6927, DOI:10.1021/jacs.6b02540.
- K. R. G. Lim, M. Shekhirev, B. C. Wyatt, B. Anasori, Y. Gogotsi and Z. W. Seh, Fundamentals of MXene synthesis, Nat. Synth., 2022, 1(8), 601–614, DOI:10.1038/s44160-022-00104-6.
- A. VahidMohammadi, J. Rosen and Y. Gogotsi, The world of two-dimensional carbides and nitrides (MXenes), Science, 2021, 372(6547), eabf1581, DOI:10.1126/science.abf1581.
- A. Parihar, A. Singhal, N. Kumar, R. Khan, M. A. Khan and A. K. Srivastava, Next-Generation Intelligent MXene-Based Electrochemical Aptasensors for Point-of-Care Cancer Diagnostics, Nano-Micro Lett., 2022, 14(1), 100, DOI:10.1007/s40820-022-00845-1.
- A. A. Yaqoob, H. Ahmad, T. Parveen, A. Ahmad, M. Oves, I. M. I. Ismail, H. A. Qari, K. Umar and M. N. Mohamad Ibrahim, Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications, A Review, Front. Chem., 2020, 8, 341, DOI:10.3389/fchem.2020.00341.
- S. Ahmadi, N. Rabiee, M. Bagherzadeh, F. Elmi, Y. Fatahi, F. Farjadian, N. Baheiraei, B. Nasseri, M. Rabiee and N. T. Dastjerd, et al., Stimulus-responsive sequential release systems for drug and gene delivery, Nano Today, 2020, 34, 100914, DOI:10.1016/j.nantod.2020.100914.
- M. Pan, J. Yang, K. Liu, Z. Yin, T. Ma, S. Liu, L. Xu and S. Wang, Noble Metal Nanostructured Materials for Chemical and Biosensing Systems, Nanomaterials, 2020, 10, 209, DOI:10.3390/nano10020209.
- H. Malekzad, P. S. Zangabad, H. Mirshekari, M. Karimi and M. R. Hamblin, Noble metal nanoparticles in biosensors: recent studies and applications, Nanotechnol. Rev., 2017, 6(3), 301–329, DOI:10.1515/ntrev-2016-0014.
- J. Khan, Y. Rasmi, K. K. Kırboğa, A. Ali, M. Rudrapal and R. R. Patekar, Development of gold nanoparticle-based biosensors for COVID-19 diagnosis, Beni-Suef Univ. J. Basic Appl. Sci., 2022, 11(1), 111, DOI:10.1186/s43088-022-00293-1.
- Y. Chen, J. Xiang, B. Liu, Z. Chen and X. Zuo, Gold nanoparticle-engineered electrochemical aptamer biosensor for ultrasensitive detection of thrombin, Anal. Methods, 2020, 12(29), 3729–3733, 10.1039/D0AY01163K.
- X. Chen, F. Lisi, P. Bakthavathsalam, G. Longatte, S. Hoque, R. D. Tilley and J. J. Gooding, Impact of the coverage of aptamers on a nanoparticle on the binding equilibrium and kinetics between aptamer and protein, ACS Sens., 2021, 6(2), 538–545, DOI:10.1021/acssensors.0c02212.
- Y. Liu, X. Jiang, W. Cao, J. Sun and F. Gao, Detection of thrombin based on fluorescence energy transfer between semiconducting polymer dots and BHQ-labelled aptamers, Sensors, 2018, 18(2), 589 CrossRef PubMed.
- L. Yang, X. Zhong, L. Huang, H. Deng, R. Yuan and Y. Yuan, C60@C3N4 nanocomposites as quencher for signal-off photoelectrochemical aptasensor with Au nanoparticle decorated perylene tetracarboxylic acid as platform, Anal. Chim. Acta, 2019, 1077, 281–287, DOI:10.1016/j.aca.2019.05.058.
- Q. Fu, Z. Wu, D. Du, C. Zhu, Y. Lin and Y. Tang, Versatile Barometer Biosensor Based on Au@Pt Core/Shell Nanoparticle Probe, ACS Sens., 2017, 2(6), 789–795, DOI:10.1021/acssensors.7b00156.
- M. Hosseini Ghalehno, M. Mirzaei and M. Torkzadeh-Mahani, Electrochemical aptasensor for tumor necrosis factor α using aptamer–antibody sandwich structure and cobalt hexacyanoferrate for signal amplification, J. Iran. Chem. Soc., 2019, 16(8), 1783–1791, DOI:10.1007/s13738-019-01650-1.
- Q. Zhang, W. Li, F. Zhao, C. Xu, G. Fan, Q. Liu, X. Zhang and X. Zhang, Electrochemical sandwich-type thrombin aptasensor based on silver nanowires& particles decorated electrode and the signal amplifier of Pt loaded hollow zinc ferrite, Colloids Surf., A, 2021, 611, 125804, DOI:10.1016/j.colsurfa.2020.125804.
- T. S. Sileika, H.-D. Kim, P. Maniak and P. B. Messersmith, Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components, ACS Appl. Mater. Interfaces, 2011, 3(12), 4602–4610, DOI:10.1021/am200978h.
- Q. Xu, G. Wang, M. Zhang, G. Xu, J. Lin and X. Luo, Aptamer based label free thrombin assay based on the use of silver nanoparticles incorporated into self-polymerized dopamine, Mikrochim. Acta, 2018, 185(5), 253, DOI:10.1007/s00604-018-2787-5.
- N. Xia, C. Cheng, L. Liu, P. Peng, C. Liu and J. Chen, Electrochemical glycoprotein aptasensors based on the in situ aggregation of silver nanoparticles induced by 4-mercaptophenylboronic acid, Microchim. Acta, 2017, 184(11), 4393–4400, DOI:10.1007/s00604-017-2488-5.
- M. Kang, Z. Li, M. Hu, O. Oderinde, B. Hu, L. He, M. Wang, G. Fu, Z. Zhang and M. Du, Bimetallic MnCo oxide nanohybrids prepared from Prussian blue analogue for application as impedimetric aptasensor carrier to detect myoglobin, Chem. Eng. J., 2020, 395, 125117, DOI:10.1016/j.cej.2020.125117.
- S. G. Surya, S. M. Majhi, D. K. Agarwal, A. A. Lahcen, S. Yuvaraja, K. N. Chappanda and K. N. Salama, A label-free aptasensor FET based on Au nanoparticle decorated Co3O4 nanorods and a SWCNT layer for detection of cardiac troponin T protein, J. Mater. Chem. B, 2020, 8(1), 18–26, 10.1039/C9TB01989H.
- M. H. Ghalehno, M. Mirzaei and M. Torkzadeh-Mahani, Aptamer-based determination of tumor necrosis factor α using a screen-printed graphite electrode modified with gold hexacyanoferrate, Microchim. Acta, 2018, 185(3), 165, DOI:10.1007/s00604-018-2704-y.
- M. Negahdary and H. Heli, An ultrasensitive electrochemical aptasensor for early diagnosis of Alzheimer's disease, using a fern leaves-like gold nanostructure, Talanta, 2019, 198, 510–517, DOI:10.1016/j.talanta.2019.01.109.
- J. J. Liu, X. R. Song, Y. W. Wang, A. X. Zheng, G. N. Chen and H. H. Yang, Label-free and fluorescence turn-on aptasensor for protein detection via target-induced silver nanoclusters formation, Anal. Chim. Acta, 2012, 749, 70–74, DOI:10.1016/j.aca.2012.09.002.
- Y. Song, K. Chen, S. Li, L. He, M. Wang, N. Zhou and M. Du, Impedimetric aptasensor based on zirconium–cobalt metal–organic framework for detection of carcinoembryonic antigen, Microchim. Acta, 2022, 189, DOI:10.1007/s00604-022-05427-x.
- J. L. Gogola, G. Martins, A. Gevaerd, L. Blanes, J. Cardoso, F. K. Marchini, C. E. Banks, M. F. Bergamini and L. H. Marcolino-Junior, Label-free aptasensor for p24-HIV protein detection based on graphene quantum dots as an electrochemical signal amplifier, Anal. Chim. Acta, 2021, 1166, 338548, DOI:10.1016/j.aca.2021.338548.
- X. Lu, X. Hou, H. Tang, X. Yi and J. Wang, A High-Quality CdSe/CdS/ZnS Quantum-Dot-Based FRET Aptasensor for the Simultaneous Detection of Two Different Alzheimer’s Disease Core Biomarkers, Nanomaterials, 2022, 12(22), 4031 CrossRef CAS PubMed.
- P. N. Kumta and M. S. Patil, Development and parameter assessment for vertically aligned platinum wire aptasensor arrays for impedimetric detection of cardiac biomarkers, US Pat. 11156609, 2021 Search PubMed; European Pat., 3359968, 2018 Search PubMed.
- A. Mittal, I. Roy and S. Gandhi, Magnetic Nanoparticles: An Overview for Biomedical Applications, Magnetochemistry, 2022, 8(9), 107 CrossRef CAS.
- S. M. Dadfar, D. Camozzi, M. Darguzyte, K. Roemhild, P. Varvarà, J. Metselaar, S. Banala, M. Straub, N. Güvener and U. Engelmann, et al., Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance, J. Nanobiotechnol., 2020, 18(1), 22, DOI:10.1186/s12951-020-0580-1.
- S. Rauf, V. Mani, A. A. Lahcen, S. Yuvaraja, T. Beduk and K. N. Salama, Binary transition metal oxide modified laser-scribed graphene electrochemical aptasensor for the accurate and sensitive screening of acute myocardial infarction, Electrochim. Acta, 2021, 386, 138489, DOI:10.1016/j.electacta.2021.138489.
- C. Zhu, W. Zhu, L. Xu and X. Zhou, A label-free electrochemical aptasensor based on magnetic biocomposites with Pb2+-dependent DNAzyme for the detection of thrombin, Anal. Chim. Acta, 2019, 1047, 21–27, DOI:10.1016/j.aca.2018.09.040.
- Y. Zhang, M. Liu, S. Pan, L. Yu, S. Zhang and R. Liu, A magnetically induced self-assembled and label-free electrochemical aptasensor based on magnetic Fe3O4/Fe2O3@Au nanoparticles for VEGF165 protein detection, Appl. Surf. Sci., 2022, 580, 152362, DOI:10.1016/j.apsusc.2021.152362.
- C. Wang, J. Qian, K. Wang, X. Yang, Q. Liu, N. Hao, C. Wang, X. Dong and X. Huang, Colorimetric aptasensing of ochratoxin A using Au@Fe3O4 nanoparticles as signal indicator and magnetic separator, Biosens. Bioelectron., 2016, 77, 1183–1191, DOI:10.1016/j.bios.2015.11.004.
- S. Liu, N. Xu, C. Tan, W. Fang, Y. Tan and Y. Jiang, A sensitive colorimetric aptasensor based on trivalent peroxidase-mimic DNAzyme and magnetic nanoparticles, Anal. Chim. Acta, 2018, 1018, 86–93, DOI:10.1016/j.aca.2018.01.040.
- R. S. Alves, F. A. Sigoli and I. O. Mazali, Aptasensor based on a flower-shaped silver magnetic nanocomposite enables the sensitive and label-free detection of troponin I (cTnI) by SERS, Nanotechnology, 2020, 31(50), 505505, DOI:10.1088/1361-6528/abb84f.
- D. Zhu, J. Luo, X. Rao, J. Zhang, G. Cheng, P. He and Y. Fang, A novel optical thrombin aptasensor based on magnetic nanoparticles and split DNAzyme, Anal. Chim. Acta, 2012, 711, 91–96, DOI:10.1016/j.aca.2011.10.053.
- Z. Xi, Q. Gong, C. Wang and B. Zheng, Highly sensitive chemiluminescent aptasensor for detecting HBV infection based on rapid magnetic separation and double-functionalized gold nanoparticles, Sci. Rep., 2018, 8(1), 9444, DOI:10.1038/s41598-018-27792-5.
- H. Zhang, Y. Zhou, D. Luo, J. Liu, E. Yang, G. Yang, G. Feng, Q. Chen and L. Wu, Immunoassay-aptasensor for the determination of tumor-derived exosomes based on the combination of magnetic nanoparticles and hybridization chain reaction, RSC Adv., 2021, 11, 4983–4990, 10.1039/D0RA10159A.
- J. Zhao, F. Lin, Y. Yi, Y. Huang, H. Li, Y. Zhang and S. Yao, Dual amplification strategy of highly sensitive thrombin amperometric aptasensor based on chitosan–Au nanocomposites, Analyst, 2012, 137(15), 3488–3495, 10.1039/C2AN35340G.
- T. Laurila, S. Sainio and M. A. Caro, Hybrid carbon based nanomaterials for electrochemical detection of biomolecules, Prog. Mater. Sci., 2017, 88, 499–594, DOI:10.1016/j.pmatsci.2017.04.012.
- Z. Wang, J. Yu, R. Gui, H. Jin and Y. Xia, Carbon nanomaterials-based electrochemical aptasensors, Biosens. Bioelectron., 2016, 79, 136–149, DOI:10.1016/j.bios.2015.11.093.
- N. K. Dandu, C. G. Chandaluri, K. Ramesh, D. Saritha, N. Mahender Reddy and G. V. Ramesh, Chapter 11 - Carbon nanomaterials: Application as sensors for diagnostics, in Advanced Nanomaterials for Point of Care Diagnosis and Therapy, ed. Dave S., Das J. and Ghosh S., Elsevier, 2022, pp. 211–248 Search PubMed.
- M. Musameh, J. Wang, A. Merkoçi and Y. Lin, Low-Potential Stable NADH Detection at Carbon-Nanotube-Modified Glassy Carbon Electrodes, Electrochem. Commun., 2002, 4, 743–746, DOI:10.1016/S1388-2481(02)00451-4.
- H. Zare, S. Ahmadi, A. Ghasemi, M. Ghanbari, N. Rabiee, M. Bagherzadeh, M. Karimi, T. J. Webster, M. R. Hamblin and E. Mostafavi, Carbon Nanotubes: Smart Drug/Gene Delivery Carriers, Int. J. Nanomed., 2021, 16, 1681–1706, DOI:10.2147/ijn.S299448.
- X. Zhao and J. K. Johnson, Simulation of Adsorption of DNA on Carbon Nanotubes, J. Am. Chem. Soc., 2007, 129(34), 10438–10445, DOI:10.1021/ja071844m.
- F. Curti, S. Fortunati, W. Knoll, M. Giannetto, R. Corradini, A. Bertucci and M. Careri, A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein, ACS Appl. Mater. Interfaces, 2022, 14(17), 19204–19211, DOI:10.1021/acsami.2c02405.
- E. Heydari-Bafrooei, M. Amini and M. H. Ardakani, An electrochemical aptasensor based on TiO2/MWCNT and a novel synthesized Schiff base nanocomposite for the ultrasensitive detection of thrombin, Biosens. Bioelectron., 2016, 85, 828–836, DOI:10.1016/j.bios.2016.06.012.
- S. Y. Lim, W. Shen and Z. Gao, Carbon quantum dots and their applications, Chem. Soc. Rev., 2015, 44(1), 362–381 RSC.
- B. Rezaei, H. R. Jamei and A. A. Ensafi, Lysozyme aptasensor based on a glassy carbon electrode modified with a nanocomposite consisting of multi-walled carbon nanotubes, poly(diallyl dimethyl ammonium chloride) and carbon quantum dots, Microchim. Acta, 2018, 185(3), 180, DOI:10.1007/s00604-017-2656-7.
- L. Xu, Y. Wen, S. Pandit, V. R. S. S. Mokkapati, I. Mijakovic, Y. Li, M. Ding, S. Ren, W. Li and G. Liu, Graphene-based biosensors for the detection of prostate cancer protein biomarkers: a review, BMC Chem., 2019, 13(1), 112, DOI:10.1186/s13065-019-0611-x.
- L. Gao, Q. Li, R. Li, L. Yan, Y. Zhou, K. Chen and H. Shi, Highly sensitive detection for proteins using graphene oxide-aptamer based sensors, Nanoscale, 2015, 7(25), 10903–10907 RSC.
- T.-T. Chen, X. Tian, C.-L. Liu, J. Ge, X. Chu and Y. Li, Fluorescence activation imaging of cytochrome c released from mitochondria using aptameric nanosensor, J. Am. Chem. Soc., 2015, 137(2), 982–989 CrossRef CAS PubMed.
- Y. Wei, L. Wang, Y. Zhang and Y. Dong, An Enzyme- and Label-Free Fluorescence Aptasensor for Detection of Thrombin Based on Graphene Oxide and G-Quadruplex, Sensors, 2019, 19(20), 4424 CrossRef CAS PubMed.
- D. Liu, X. Lu, Y. Yang, Y. Zhai, J. Zhang and L. Li, A novel fluorescent aptasensor for the highly sensitive and selective detection of cardiac troponin I based on a graphene oxide platform, Anal. Bioanal. Chem., 2018, 410(18), 4285–4291, DOI:10.1007/s00216-018-1076-9.
- Z. Han, J. Shu, X. Liang and H. Cui, Label-Free Ratiometric Electrochemiluminescence Aptasensor Based on Nanographene Oxide Wrapped Titanium Dioxide Nanoparticles with Potential-Resolved Electrochemiluminescence, Anal. Chem., 2019, 91(19), 12260–12267, DOI:10.1021/acs.analchem.9b02318.
- B. Qin and K. Yang, Voltammetric aptasensor for thrombin by using a gold microelectrode modified with graphene oxide decorated with silver nanoparticles, Mikrochim. Acta, 2018, 185(9), 407, DOI:10.1007/s00604-018-2924-1.
- Y. Zhang, S. Liu, L. Wang, Y. Luo, J. Tian, A. M. Asiri, A. O. Al-Youbi and X. Sun, Novel Use of Poly(3,4-ethylenedioxythiophene) Nanoparticles for Fluorescent Nucleic Acid Detection, ACS Comb. Sci., 2012, 14(3), 191–196, DOI:10.1021/co2001394.
- P. Gupta, A. Bharti, N. Kaur, S. Singh and N. Prabhakar, An electrochemical aptasensor based on gold nanoparticles and graphene oxide doped poly(3,4-ethylenedioxythiophene) nanocomposite for detection of MUC1, J. Electroanal. Chem., 2018, 813, 102–108, DOI:10.1016/j.jelechem.2018.02.014.
- X. Liu, J. Zhang, J. Di, Y. Long, W. Li and Y. Tu, Graphene-like carbon nitride nanosheet as a novel sensing platform for electrochemical determination of tryptophan, J. Colloid Interface Sci., 2017, 505, 964–972 CrossRef CAS PubMed.
- D. Fan, C. Guo, H. Ma, D. Zhao, Y. Li, D. Wu and Q. Wei, Facile fabrication of an aptasensor for thrombin based on graphitic carbon nitride/TiO2 with high visible-light photoelectrochemical activity, Biosens. Bioelectron., 2016, 75, 116–122 CrossRef CAS PubMed.
- S. Ansari, M. S. Ansari, H. Devnani, S. Satsangee and R. Jain, CeO2/g-C3N4 nanocomposite: a perspective for electrochemical sensing of anti-depressant drug, Sens. Actuators, B, 2018, 273, 1226–1236 CrossRef CAS.
- H. Xu, T. Zhang, Y. Gu, X. Yan, N. Lu, H. Liu, Z. Xu, Y. Xing, Y. Song and Z. Zhang, et al., An electrochemical thrombin aptasensor based on the use of graphite-like C3N4 modified with silver nanoparticles, Microchim. Acta, 2020, 187(3), 163, DOI:10.1007/s00604-020-4111-4.
- L. Ge, W. Wang, X. Sun, T. Hou and F. Li, Affinity-Mediated Homogeneous Electrochemical Aptasensor on a Graphene Platform for Ultrasensitive Biomolecule Detection via Exonuclease-Assisted Target-Analog Recycling Amplification, Anal. Chem., 2016, 88(4), 2212–2219, DOI:10.1021/acs.analchem.5b03844.
- N. G. Nia and A. Azadbakht, Nanostructured aptamer-based sensing platform for highly sensitive recognition of myoglobin, Microchim. Acta, 2018, 185, 333 CrossRef PubMed.
- H. Wang, Y. Jian, Q. Kong, H. Liu, F. Lan, L. Liang, S. Ge and J. Yu, Ultrasensitive electrochemical paper-based biosensor for microRNA via strand displacement reaction and metal-organic frameworks, Sens. Actuators, B, 2018, 257, 561–569 CrossRef CAS.
- J. Tian, Z. Liang, O. Hu, Q. He, D. Sun and Z. Chen, An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein, Electrochim. Acta, 2021, 387, 138553, DOI:10.1016/j.electacta.2021.138553.
- T. Ahnfeldt, D. Gunzelmann, T. Loiseau, D. Hirsemann, J. Senker, G. Férey and N. Stock, Synthesis and modification of a functionalized 3D open-framework structure with MIL-53 topology, Inorg. Chem., 2009, 48(7), 3057–3064, DOI:10.1021/ic8023265.
- J. Fang, G. Zhao, X. Dong, X. Li, J. Miao, Q. Wei and W. Cao, Ultrasensitive electrochemiluminescence immunosensor for the detection of amyloid-β proteins based on resonance energy transfer between g-C(3)N(4) and Pd NPs coated NH(2)-MIL-53, Biosens. Bioelectron., 2019, 142, 111517, DOI:10.1016/j.bios.2019.111517.
- O. B. A. Shatery and K. M. Omer, Selectivity Enhancement for Uric Acid Detection via In Situ Preparation of Blue Emissive Carbon Dots Entrapped in Chromium Metal–Organic Frameworks, ACS Omega, 2022, 7(19), 16576–16583, DOI:10.1021/acsomega.2c00790.
- X.-J. Xing, X.-G. Liu, Y. He, Y. Lin, C.-L. Zhang, H.-W. Tang and D.-W. Pang, Amplified Fluorescent Sensing of DNA Using Graphene Oxide and a Conjugated Cationic Polymer, Biomacromolecules, 2013, 14(1), 117–123, DOI:10.1021/bm301469q.
- G. K. Mishra, A. Barfidokht, F. Tehrani and R. K. Mishra, Food Safety Analysis Using Electrochemical Biosensors, Foods, 2018, 7(9), 141, DOI:10.3390/foods7090141.
- M. Kurmoo, Magnetic metal–organic frameworks, Chem. Soc. Rev., 2009, 38(5), 1353–1379 RSC.
- Z. Luo, D. Sun, Y. Tong, Y. Zhong and Z. Chen, DNA nanotetrahedron linked dual-aptamer based voltammetric aptasensor for cardiac troponin I using a magnetic metal-organic framework as a label, Microchim. Acta, 2019, 186(6), 374, DOI:10.1007/s00604-019-3470-1.
- G. K. Ali and K. M. Omer, Ultrasensitive aptamer-functionalized Cu-MOF fluorescent nanozyme as an optical biosensor for detection of C-reactive protein, Anal. Biochem., 2022, 658, 114928, DOI:10.1016/j.ab.2022.114928.
- G. U. I. Rijun, Y. Sun, H. Jin and X. Jiang, Method for preparing ratiometric electrochemical aptasensor for Vaniline based on nanocomposite modified electrode, US Pat., 10895552, 2021 Search PubMed; China Pat., 109187693B, 2019 Search PubMed; WO, 2020093638A1WIPO (PCT), 2020 Search PubMed.
- G. U. I. Rijun, Y. Sun, H. Jin and X. Jiang, Method for preparing ratiometric electrochemical miR3123 aptasensor based on metal-organic framework composite. US Pat., 11099150, 2021 Search PubMed; WO, 2021035653A1WIPO (PCT) 2021 Search PubMed; China Pat., 110455896, 2020 Search PubMed.
- M. R. Farani, B. N. Khiarak, R. Tao, Z. Wang, S. Ahmadi, M. Hassanpour, M. Rabiee, M. R. Saeb, E. C. Lima and N. Rabiee, 2D MXene nanocomposites: electrochemical and biomedical applications, Environ. Sci.: Nano, 2022, 9(11), 4038–4068, 10.1039/D2EN00527A.
- H. Wang, J. Sun, L. Lu, X. Yang, J. Xia, F. Zhang and Z. Wang, Competitive electrochemical aptasensor based on a cDNA-ferrocene/MXene probe for detection of breast cancer marker Mucin1, Anal. Chim. Acta, 2020, 1094, 18–25, DOI:10.1016/j.aca.2019.10.003.
- M. R. Farani, B. N. Khiarak, R. Tao, Z. Wang, S. Ahmadi, M. Hassanpour, M. Rabiee, M. R. Saeb, E. C. Lima and N. Rabiee, 2D MXene nanocomposites: electrochemical and biomedical applications, Environ. Sci.: Nano, 2022, 9, 4038–4068 RSC.
- N. Rabiee, M. Bagherzadeh, M. Jouyandeh, P. Zarrintaj, M. R. Saeb, M. Mozafari, M. Shokouhimehr and R. S. Varma, Natural polymers decorated MOF-MXene nanocarriers for co-delivery of doxorubicin/pCRISPR, ACS Appl. Bio Mater., 2021, 4(6), 5106–5121 CrossRef CAS PubMed.
- M. Tavakolizadeh, M. Atarod, S. J. S. Tabaei, S. Sojdeh, E. N. Zare, M. Rabiee and N. Rabiee, Green modified-UiO-66/MXene sandwich composites for gene-chemotherapy synergistic cancer suppression: Co-delivery of doxorubicin and pCRISPR, Alexandria Eng. J., 2023, 80, 144–154 CrossRef.
- J. Ji, L. Zhao, Y. Shen, S. Liu and Y. Zhang, Covalent stabilization and functionalization of MXene via silylation reactions with improved surface properties, FlatChem, 2019, 17, 100128, DOI:10.1016/j.flatc.2019.100128.
- Y.-J. Fang, L. J.-H. Lee, K.-H. Luo, P.-S. Fang, C.-C. Yang and H.-Y. Chuang, The Association of Carcinoembryonic Antigen (CEA) and Air Pollutants—A Population-Based Study, Atmosphere, 2022, 13(3), 466 CrossRef CAS.
- X. Song, H. Gao, R. Yuan and Y. Xiang, Trimetallic nanoparticle-decorated MXene nanosheets for catalytic electrochemical detection of carcinoembryonic antigen via Exo III-aided dual recycling amplifications, Sens. Actuators, B, 2022, 359, 131617, DOI:10.1016/j.snb.2022.131617.
- P. Zhao, J. Zheng, Y. Liang, F. Tian, L. Peng, D. Huo and C. Hou, Functionalized Carbon Nanotube-Decorated MXene Nanosheet-Enabled Microfluidic Electrochemical Aptasensor for Carcinoembryonic Antigen Determination, ACS Sustain. Chem. Eng., 2021, 9(46), 15386–15393, DOI:10.1021/acssuschemeng.1c03597.
- S. Ahmadi, N. Rabiee, M. Bagherzadeh and M. Karimi, Chapter 6 - Microfluidic devices for pathogen detection, in Biomedical Applications of Microfluidic Devices, ed. Hamblin, M. R. and Karimi, M., Academic Press, 2021, pp. 117–151 Search PubMed.
- Y. Wang, W. Sun, Y. Li, X. Zhuang, C. Tian, F. Luan and X. Fu, Imidazole metal-organic frameworks embedded in layered Ti3C2Tx Mxene as a high-performance electrochemiluminescence biosensor for sensitive detection of HIV-1 protein, Microchem. J., 2021, 167, 106332, DOI:10.1016/j.microc.2021.106332.
- H. Zhang, Z. Wang, F. Wang, Y. Zhang, H. Wang and Y. Liu, In Situ Formation of Gold Nanoparticles Decorated Ti3C2 MXenes Nanoprobe for Highly Sensitive Electrogenerated Chemiluminescence Detection of Exosomes and Their Surface Proteins, Anal. Chem., 2020, 92(7), 5546–5553, DOI:10.1021/acs.analchem.0c00469.
- H. Zhang, Z. Wang, F. Wang, Y. Zhang, H. Wang and Y. Liu, Ti3C2 MXene mediated Prussian blue in situ hybridization and electrochemical signal amplification for the detection of exosomes, Talanta, 2021, 224, 121879, DOI:10.1016/j.talanta.2020.121879.
- Q. Zhang, F. Wang, H. Zhang, Y. Zhang, M. Liu and Y. Liu, Universal Ti(3)C(2) MXenes Based Self-Standard Ratiometric Fluorescence Resonance Energy Transfer Platform for Highly Sensitive Detection of Exosomes, Anal. Chem., 2018, 90(21), 12737–12744, DOI:10.1021/acs.analchem.8b03083.
- L. J. Talbot, Z. Mi, S. D. Bhattacharya, V. Kim, H. Guo and P. C. Kuo, Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy in reversing growth of human breast cancer cells, Surgery, 2011, 150(2), 224–230, DOI:10.1016/j.surg.2011.05.015.
- T. Li, L. Yao, Q. Liu, J. Gu, R. Luo, J. Li, X. Yan, W. Wang, P. Liu and B. Chen, et al., Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment, Angew. Chem., Int. Ed., 2018, 57(21), 6115–6119, DOI:10.1002/anie.201800887.
| This journal is © The Royal Society of Chemistry 2024 |