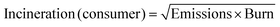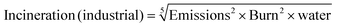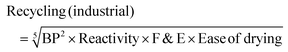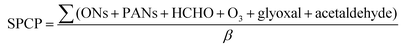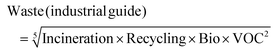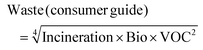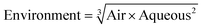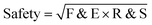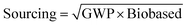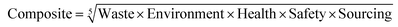Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a solvent sustainability guide for the paints and coatings industry†
Laura
Pilon
 a,
Daniel
Day
a,
Daniel
Day
 b,
Harry
Maslen
b,
Oliver P. J.
Stevens
b,
Harry
Maslen
b,
Oliver P. J.
Stevens
 b,
Nicola
Carslaw
b,
Nicola
Carslaw
 c,
David R.
Shaw
c,
David R.
Shaw
 c and
Helen F.
Sneddon
c and
Helen F.
Sneddon
 *b
*b
aPaint Research Association, Pera Business Park, Nottingham Road, Melton Mowbray, Leicestershire, LE13 0PB, UK. E-mail: l.pilon@pra-world.com
bGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: helen.sneddon@york.ac.uk
cDepartment of Environment and Geography, University of York, UK
First published on 27th June 2024
Abstract
A guide has been developed, highlighting various sustainability criteria of solvents used in the paints and coatings industry. The guide differs from previous, pharmaceutical-industry-focused guides both in the nature of the solvents included, and in the scoring of criteria, taking into account typical paints and coatings applications. The guide scores are, inevitably, subject to change, for example, if newer routes to biobased solvents become more widespread, or as further toxicology assessments are conducted, however it is intended as a tool to inform choices at R&D stages where full LCA may not be practical.
1. Introduction
In recent years solvent guides have been a key tool in influencing the overall nature of materials used and waste generated in the discovery, development and ultimately manufacture of active pharmaceutical ingredients (APIs) in the pharmaceutical industry.1However, such guides were designed with how solvents are typically used in pharmaceutical industry chemistry laboratories and manufacturing in mind. The relevance of the properties scored, and assumptions made about the conditions under which the solvents would be used, and how they might be disposed, do not necessarily translate to other use cases. For example, in pharmaceutical manufacturing, solvents are typically used in closed systems from which they can be recovered for reuse, recycling or incineration. In the case of consumer paints the solvent is part of the purchased product and its evaporation to the environment is integral to the successful use of the product. In addition, the nature of solvents used in other sectors is often significantly different from those solvents prevalent in the pharmaceutical industry.
Globally, use of solvents by the paints and coatings industry has long dwarfed solvent use by the pharmaceutical sector. The global solvents market has been estimated at 20 million tonnes per year, with 46 percent of all solvent being used in paints and coatings, compared to 9% in pharmaceuticals.2 Data suggests usage of around 9.2 million metric tonnes globally, or about 2.3 million metric tonnes in Europe, per year. The value of the global solvents market has been estimated at US$44 billion in 2020.3
The paints and coatings sector has been increasingly embracing waterborne and solventless compositions. The latter include radiation curing formulations and powder coating formulations, and reactive diluents – solvents that become chemically incorporated in the coating. Recent data from the British Coatings Federation and European Coatings respectively, revealed 84% of decorative paint sold in Britain is now water based,4 and the global water-based paints market size was approximately €62 billion in 2017.5 Water-based paints are expected to continue to grow globally owing to increasing awareness of air impacts of VOC emissions, and increasing regulation.
However, solvents remain essential for many paint and coating applications, particularly industrial ones where protection of the underlying substrate is of paramount importance. Frequently water can require more energy to remove in an industrial setting, and water-based industrial paint waste can be harder to dispose. Therefore solvents are still used both in the production and application of the paint or coating.
The role of solvents in the paints and coatings industry is mostly transient. They are intended to evaporate after application and until then they play an important role in binder and additive solubility/miscibility, dispersibility of pigments and fillers, and stability of all the components, throughout the preparation of the formulation, as well as during storage. Subsequently they are important for the coating application process via their influence on the paint rheology, and finally their influence on drying and film formation can impact the paint appearance and durability.
Whilst it could be argued that ideally, all coatings would be solvent-free,6 or water-based,7 and the paints industry is moving towards this goal, it is not always achievable with current technologies and it is expected that solvent use will continue for some time. Equally, small quantities of co-solvents are often included in water-based formulations for such purposes as improvement of ingredient compatibility, paint workability, and film formation. Therefore, it is important to ensure that continued solvent use has as little impact on our health and environment as practicable. A solvent guide, taking into account the needs of the paints and coatings industry, may represent one way to balance the sometimes-conflicting requirements of human health/environment/industry and communicate where and how potential substitutions can be made.
2. Solvents evaluated
In order to expedite the publication of a guide for solvent selection the number of solvents, and paint applications in which they are used was limited.77 solvents were assessed for this guide, 46 of which had previously been assessed by a related methodology in a pharmaceutical industry context,1b but which may also be of relevance in paints and coatings. 31 solvents were scored for the first time here (white spirit had previously been scored, but not as two separate grades, 1 and 2) (Fig. 1). These solvents were chosen as relevant based on their reporting in safety data sheets of industrial paints, or their use in guide formulations for industrial or decorative paints. An initial list was prepared and then cross-referenced against the solvents included in the CEPE (European Council of the Paint, Printing Ink and Artists’ Colours Industry) LCI project.8 Finally, the solvents were grouped into application groups, or “use cases”, where they are used and disposed of in similar ways to (i) allow customisation of the scoring methodology to suit the way they are used, and (ii) ensure fair comparisons across the group of solvents. For example, solvents used in high temperature applications such as coil coatings have higher boiling points and lower vapour pressures than solvents used in air-drying industrial coatings. If all were included in the same guide, the coil coating solvents might appear to have a safety advantage over the general industrial coating solvents, but would never be used in these applications due to their low evaporation rate at ambient application temperatures. The use cases presented in this article are:
1. Consumer decorative paints – paints designed to be applied by the general public in home environments. They are predominantly waterborne, but some trim paints and primers are still solvent borne. Drying takes place at ambient temperature.
2. Air-drying industrial paints – paints designed to be applied by professional applicators in factory environments with the protection of local exhaust ventilation and emissions capture. Drying takes place at ambient or slightly elevated temperature.
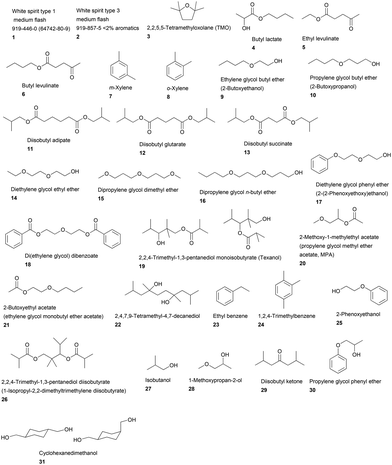 | ||
| Fig. 1 Names and structures of 31 paint and coating industry specific solvents assessed using the paints and coatings industry criteria. | ||
A direct comparison between selecting a solvent that would be recommended for a consumer product and one for an equivalent industrial use is not possible as there are too many different variables in both formulation and usage condition.
Additional use cases that could become the subject of future guides include:
• Oven-cured industrial paints
• Cleaning solvents (e.g. for manufacturing lines)
• Paint strippers (which represent one third of the total tonnage of solvents used by the paint industry)
3. Methodology
3.1 General methods for score calculation
An approach broadly comparable to that used in the GSK Solvent Sustainability Guide was applied.1b To summarise, each of the summary areas has multiple categories which are individually assessed and combined to give a summary score. Each category is itself dependent on multiple attributes. The data are assessed to provide individual scores ranging from 1 (least “green”) to 4 (most “green”); and the scores are then expanded to a 1–10 score from least to most “green” to give the category score. Differences in the scoring criteria for this guide are highlighted below, and in the ESI.†3.2 Assessment of waste score (comprising incineration, recycling (for industrial paints only), biotreatment and volatile organic compound emissions scores)
The ultimate fate of solvents used in the paints industry is complex and depends on the paint lifecycle stage and use case.• Paint manufacturing: VOC emissions from factories are tightly controlled and mitigated. In some cases solvents are burnt to fuel ovens for drying and curing activities (e.g. coil coatings). Wash solvents may be recycled, as might be solvents from surplus/out-of-specification products.
• Paint Application: For application in a factory environment, the mitigation above still applies. Wash solvents may be recycled. However, for consumer paints, the solvent vapours are simply released to the atmosphere (see section 3.3.2).
• Paint disposal: End-of-life paint is currently handled poorly in the UK. There are few collection points for surplus household paint, and where it is collected, the majority is landfilled or incinerated. Small schemes to re-use good quality paint do exist in some locations, such as Community RePaint,9 sponsored by Dulux®. Additionally, the British Coatings Federation “PaintCare” programme aims to increase the re-use or remanufacture rate of paints in the UK from 2% in 2022 to 75% by 2030.10 These schemes mainly focus on water-based paints however, so the ultimate fate of solvents from paints is likely to remain incineration or release to atmosphere for some time to come.
where BP = boiling point score, F&E = flammability and explosion potential score
The biotreatment score used was based on two areas:
• Treatability in aeration tanks which penalises those solvents which require greater oxidation as assessed by their theoretical oxygen demand (ThOD), the calculated amount of oxygen required to oxidize a compound to its final oxidation products, and additionally penalises nitrogen containing solvents because of the added demand that oxidation of nitrogenous solvents places on biotreatment and the risks (ecological stress, biodiversity loss) of affecting nitrogen content in the aqueous environment.
• Potential for a solvent to be present in an aqueous solution which penalises more miscible solvents.
Unlike the GSK Solvent Sustainability Guide, the paints and coatings biotreatment score did not include a component for release to air – in part because of the intended release to air for solvents in a paints and coatings application, and in part because this score previously rightly penalised halogenated solvents likely to give rise to problematic volatile organic compounds (VOCs), and efforts to remove halogenated solvents from paints and coatings11 have been successful to the extent that the 77 solvents assessed as part of this guide do not include halogenated compounds.
A comparable approach to that previously used was applied.1b VOC emissions may occur through intended use of the product, through spillages, or through the storage, transport, manufacture of the paint or coating itself. The score is primarily driven by vapour pressure, however solvents with particularly low boiling points are also penalised as this increases the likelihood of acute high emission rates.
3.3 Assessment of environmental impact score (comprising environmental impact – aqueous and environmental impact – air scores)
• Acute environmental toxicity, penalising known high toxicity against any aquatic species (fish, daphnia or algae);
• Chronic environmental toxicity, using partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW) as an indicator of chronic hazards, as well as relevant Globally Harmonized System (GHS) phrases;
KOW) as an indicator of chronic hazards, as well as relevant Globally Harmonized System (GHS) phrases;
• Biodegradation.
SPCP values were calculated using the INdoor CHEMical model in Python (INCHEM-Py v1.2), which uses the Master Chemical Mechanism (MCM v3.3.1) to provide predicted concentrations of indoor air pollutants over time.12 Model settings were used as published by Carslaw and Shaw, to investigate the development of indoor pollutants such as formaldehyde (and other aldehydes), nitrates and ozone in a residence in a polluted urban environment (Table 1).12,13 A baseline run was conducted to understand the evolution of the secondary products over time in the absence of the VOC, then a VOC concentration of 10 ppb was introduced to the expected baseline atmosphere and a 2-day period was simulated. The difference in concentration between the baseline run and the VOC run for each secondary product was calculated. Instantaneous SPCP at each data point was calculated using these difference values according to the below equation, and has units of ppb secondary products, per ppb of VOC added (β is the initially added VOC concentration, ONs are Organic Nitrates, PAN are peroxyacetylnitrates).
| Model parameter | Value |
|---|---|
| Temperature | 300 K |
| Relative humidity | 45% RH |
| Air change rate | 0.76 h−1 |
| Location (basis for outdoor concentrations of O3, NO, NO2 and PM2.5) | Urban Milan (August 2003 data) |
| Indoor light type | Incandescent |
| Light on times | Between 7 am and 7 pm each day |
| Window glass type | Glass C |
| Surface area to volume ratio | 0.02 cm−1 |
| Output species | Total organic nitrate (TOTORGNO3), total PAN (TOTPAN), ozone (O3), glyoxal (GLYOX), acetaldehyde (CH3CHO), formaldehyde (HCHO) |
The overall SPCP for each VOC was calculated as the average of the instantaneous SPCP between 8:00 and 18:00 on the second day.
To develop the scoring system used in our solvents guide, SPCP was directly calculated for as many of the solvents in our guide as possible. These results were then ranked, and the points at which the trend deviated from a linear relationship were identified. The highest values of SPCP were automatically scored 1, and the lowest values of SPCP were automatically scored 4. In-between, a linear regression was applied to the data to directly calculate the intermediate scores from the SPCP values. Where data were not available, near neighbour relationships were used to estimate the expected indoor air effect score (Table 2).
| Data range | Score |
|---|---|
| ≤0.005 | 4 |
| 0.006 to 0.079 | (−40 × SPCP) + 4.2 |
| ≥0.08 | 1 |
SPCP was used in calculation of the air score for consumer paints, due to their use in indoor environments with limited ventilation but was not used in the calculation of the air score for industrial paints due to higher ventilation requirements and standards in manufacturing environments. Interestingly a correlation could be seen between photochemical ozone creation potential (POCP) and SPCP values, with clear outliers observed for several alkyl substituted aromatics (see ESI†).
3.4 Assessment of health score (comprising health hazard and exposure potential scores)
3.5 Assessment of safety score (comprising flammability and explosion potential and reactivity and stability scores)
• Boiling point;
• Flash point;
• Auto ignition temperature;
• Electrical conductivity;
• Vapour pressure.
Explosion potential is assessed for the solvent itself, and not for potential byproducts such as peroxides, which are considered in the reactivity and stability score.
• Peroxide formation to penalise solvents which might form peroxides over time;
• Potential for self-reaction to penalise the potential for polymerisation or decomposition on heating;
• National Fire Protection Association (NFPA) Reactivity Rating to penalise solvents whose intrinsic reactivity increases the risk of fire;
• Acidity/Basicity to penalise solvents with the potential to react with acidic or basic components;
Unlike the earlier guide, the reactivity and stability score did not include a component for special hazards, to capture any special or unusual hazards such as pyrophoric or shock sensitive solvents, as unsurprisingly for solvents used in paints and coatings, none were found to meet this criterion.
3.6 Assessment of sourcing score
For the paints and coatings guide, Global Warming Potential (GWP), one aspect of LCA, was considered instead. Most solvents scored are considered to be “low GWP” with respect to key atmospheric pollutants such as refrigerants, however, it was still felt to be useful to be able to rank their impacts with the aim of generating improvements over time.
Cradle-to-gate global warming potential (GWP) values were obtained from CCalc 2 and other literature sources15–18 for a range of solvents relevant to the coatings industry. These were used to rank the solvents in order of increasing GWP. The data were split into quartiles and scores assigned as shown in Table 3.
| Data range | Score |
|---|---|
| Q1 (lowest GWP) | 4 |
| Q1 to median/Q2 | 3 |
| Median/Q2 to Q3 | 2 |
| Q4 (highest GWP) | 1 |
For solvents where GWP data were unavailable, a near neighbour approach was used to assign the score, taking into account factors that might affect the energy requirement during manufacture such as the effect of molar mass on ease of distillation and the complexity of the molecule (higher complexity/increased functionality tends to need a larger number of manufacturing steps).
| Assessment | Score |
|---|---|
| Current commercial whole structure biobased source | 4 |
| Potential commercial biobased source (unconfirmed) | 3 |
| Partially biobased (at least one component biobased) | 2.5 |
| Potential future commercial biobased source | 2 |
| No likely biobased source | 1 |
This is, inevitably, a potentially contentious assessment. It is inherently a crude assessment and one that may change over time. It cannot take into account whether or not a biobased source is sustainably sourced (e.g. from agricultural waste products), or whether it is actually lower in carbon footprint when bioderived than when petrochemically based. The “Biosourcing” tabs on the Excel files available in the ESI† contain comments as to the particular biobased route being considered at time of publication which should enable users to add additional context over time, for example if new routes become available.
3.7 Solvent colour coding and assessment of composite score
As with previous guides a major benefit is intended to lie in highlighting individual issues associated with certain solvents and in making detailed data available to enable informed choices to be made. However, it is acknowledged that there are also benefits to an at-a-glance green, amber, red traffic light inspired classification.For each general area of assessment (waste, environment, health, safety and sourcing), an overall summary score is defined through a combination of each of the relevant category scores. These calculations, in which it might be noted VOC emissions and Environmental impact – air are weighted more highly that in the GSK solvent sustainability guide, as a consequence of the differences in use-scenarios, are represented by the equations:
where Bio = biotreatment VOC = volatile organic compounds emissions score F&E = flammability and explosion potential score, R&S = reactivity and stability score and GWP = global warming potential score.
For each of these summary scores, as well as for the individual category scores used in the summary scores, the following colour designations of Green, Amber and Red were chosen (Table 5).
In addition, a composite score is defined as the geometric mean of the waste, environment, health, safety and sourcing scores, represented by the equation:
This is then used to rank order the solvents and assist in colour assignment.
3.8 Factors beyond the scope of this guide
There are many different factors which organisations and individuals may want to consider for their specific applications. Cost and availability are inevitably always of consideration but are variable over time and geography and so are excluded from this guide. Legislation and incentives might also change over time and may also differ between regions and countries. The intention is that new solvents can be readily added to this guide, and scoring can be modified in future as necessary.This guide does not cover the technology readiness levels (TRLs) of production of the biobased solvents, or scale of production, as such information is not always readily available, and is subject to rapid change. Whilst it is appreciated some users may wish to limit their solvent considerations to those solvents already available on scale, it is also to be hoped that consideration of scores on this guide may also be of value when considering new arrivals to the biobased solvent scene, and in positioning areas where these might yet be further explored.
This guide does not seek to address the wider sustainability impacts of paints and coatings – clearly protective coatings which reduce the need for equipment replacement or reflective coatings that reduce the need for air conditioning may have wider sustainability benefits beyond the scope of these considerations.
4. Discussion
4.1 Use of the guides
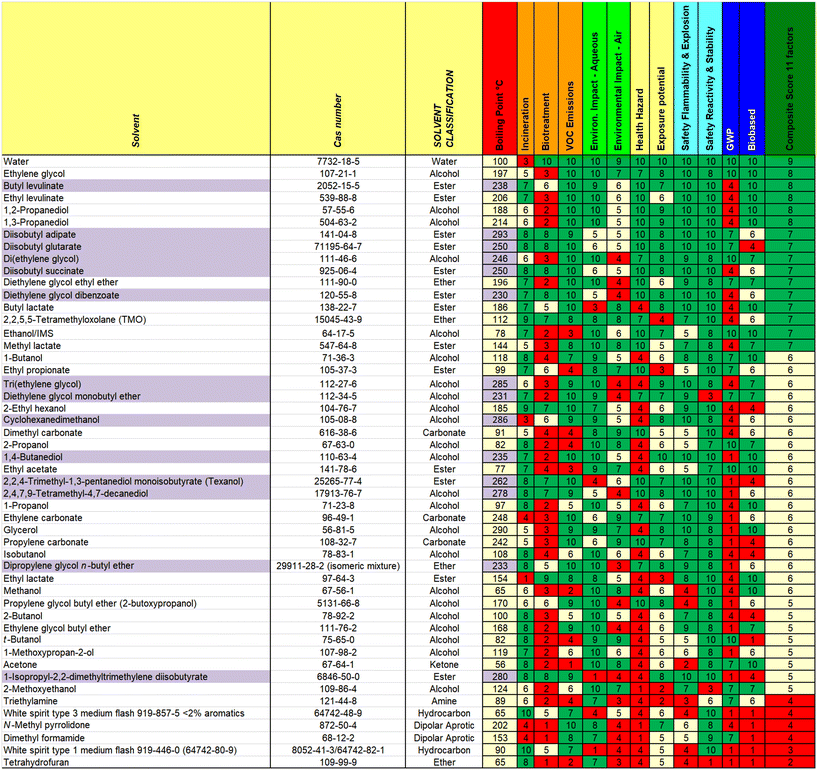
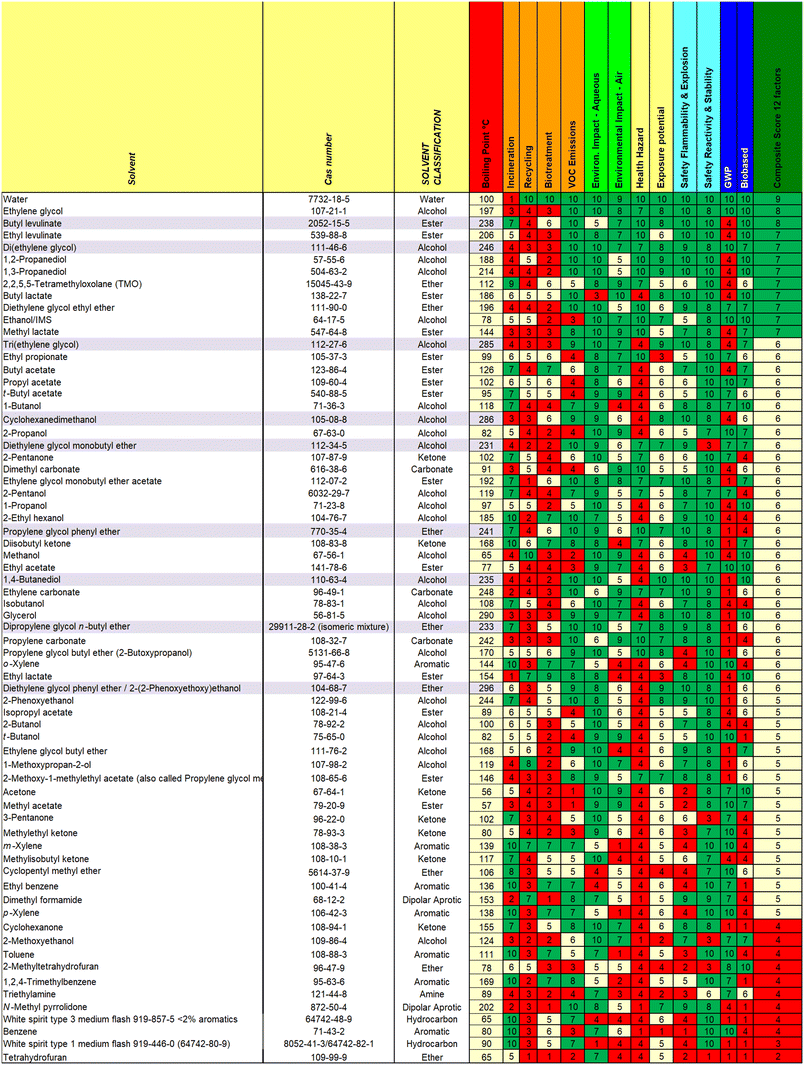
There are multiple ways in which the data can be displayed for the 77 solvents scored, or subsections of those solvents. We have prepared single page overviews of both the consumer relevant (Fig. 2) and industrially relevant (Fig. 3) solvents, as well as making the full tools available in Excel in the ESI.† The guides include a range of conventional solvents that are less widely used in paints and coatings, but are used in adjacent industries.NMP is included for comparison, but it should be noted as already having been phased out of use since its classification as reprotoxic.19 Another improvement already made in consumer paints is the replacement of type 3 white spirit (15–20% aromatics) (2) with type 1 white spirit (<2% aromatics) (known in the US as Stoddard solvent) (1).20
Guides can be used help to assess the solvents in use in a given facility against a single criterion, several criteria, or more holistically. This can be particularly instructive for solvent which may have acquired an “aura” of “greenness” or otherwise due to well-established drawbacks or advantages (e.g. ethylene glycol has well-documented mammalian toxicity21 and MeTHF is well-known as a biobased solvent).22 The holistic approach emphasises that the use of a single criterion does not form a good basis for selection of new solvents, and users of the guide are advised to consider which factors are most relevant for their applications.
The guide, and the data from the Excel Tables, can be used, at a glance, to compare solvents of similar boiling point, or in conjunction with other tools in assessing existing or new replacement solvents.
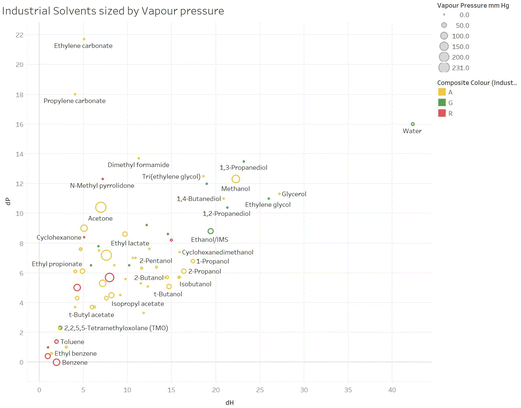
For example, we offer in Fig. 4 and 5, an example plot of δPvs. δH (relatively little variation in δD is seen for these solvents) sized by Vapour Pressure for respectively the Consumer and Industrial solvents.
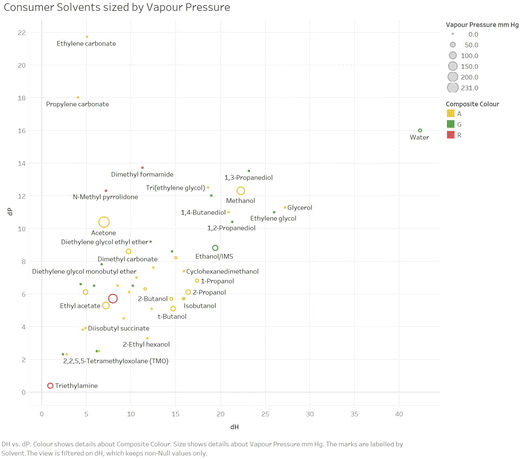 | ||
| Fig. 4 An example plot of δPvs. δH sized by vapour pressure for consumer solvents (white spirit omitted, as no one single δH or δP value). | ||
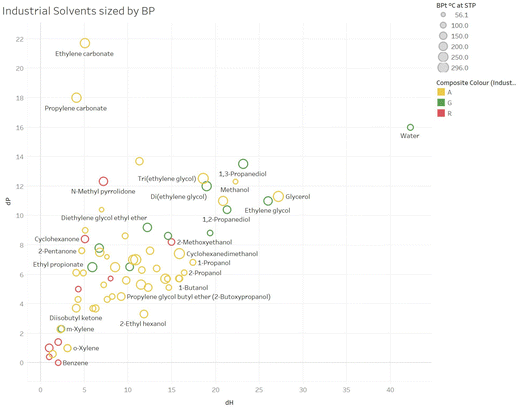
Fig. 6 and 7 show similar plots, sized by boiling point. These plots are colour coded by overall colour assignment for these solvents, but it is trivial to colour code by any particular sub-score of interest, and these plots represent a way in which these guides could be tailored to specific uses.
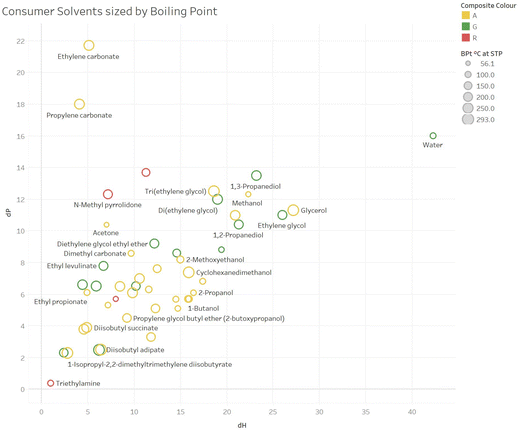 | ||
| Fig. 6 An example plot of δPvs. δH sized by boiling point for consumer solvents (white spirit omitted, as no one single δH or δP value). | ||
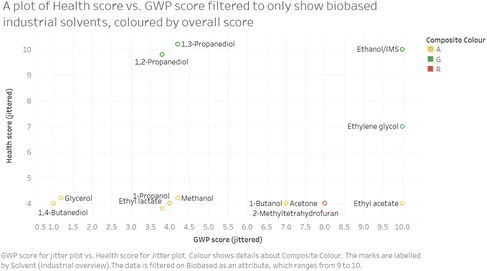
Fig. 8 shows how visualisations can be used to explore preconceptions. It shows a plot of health score vs. global warming potential for biobased industrial solvents, showing that not all biobased solvents perform well again these two criteria, or indeed, as seen by the overall colour coding, overall according to this scoring method.
Formulation for paints and coatings inevitably requires optimisation for specific properties, so like-for-like drop-in replacement recommendations are unlikely. However it is intended that these guides may provide useful information during research and development where full life cycle assessment of different options may not be practical. The guide can be used in combination with other tools to identify which solvents are poor performing across our range of criteria and highlight potential alternatives for further testing.
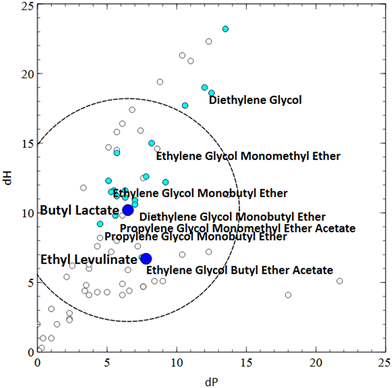
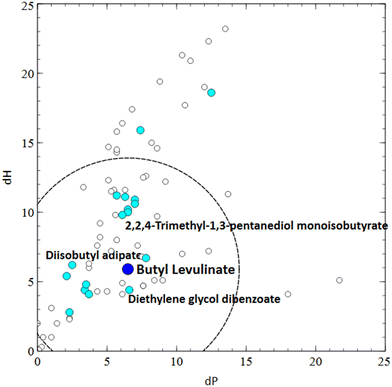
In order to explore a way in which this might be approached, 3 new solvents listed in the literature as potential greener alternatives for the paints and solvents industry were selected: tetramethyloxolane (TMO, 3) (Fig. 9),23 butyl lactate, 4, (Fig. 10)24 and alkyl levulinates 5 and 6 (Fig. 10 and 11).25 Hansen Solubility Parameters26 were compared for these solvents with the solvents from both the industrial and consumer paint solvents guides using the Hansen Solubility Parameters in Practice (HSPiP) software. To evaluate the relative energy difference (RED) of the new solvent from existing solvents, a radius of 8 was applied. This radius was used because it is typical of the distance of a marginal (θ) solvent from a polymer. Since solvent-based paints are at a basic level polymer solutions contained dispersed solids, this seemed to be an appropriate approximation. The smaller the resulting value of RED, the closer the 2 solvents are in their HSPs. The new solvents were then scored according to the guide and the criteria were then compared for the new and existing solvents, to highlight which hypothetical replacements might offer sustainability improvements based on these criteria. Selected physical properties differences (boiling, vapour pressure, relative evaporation rates) were also reviewed as such properties are critical when considering reformulation (Tables 6, 7 and 8). These new solvents are particularly interesting as they are proposed in literature as replacements for problematic solvents such as toluene/xylene and butyl glycol. These conventional solvents are high priorities for replacement due to their high hazards to health. It should be noted that health hazard classifications can change as improved toxicological information becomes available and this could affect future utilisation of the health hazard score within this guide. Whilst currently accurate, readers should make their own risk assessment on the accuracy of that data at the date of reading and consult suitable sources such as manufacturer's safety data sheets or ECHA for the most up to date classifications.
| Name | CAS/EC no. | bp (°C) | RER (nBuAc 1.0) | Density (g cm−3) | δ D | δ P | δ H | RED |
|---|---|---|---|---|---|---|---|---|
| TMO | 15045-43-9 | 112 | 0.4 | 0.81 | 15.6 | 2.3 | 2.4 | N/A |
| Toluene | 108-88-3 | 111 | 1.9 | 0.87 | 18 | 1.4 | 2 | 0.89 |
| p-Xylene | 106-42-3 | 138 | 0.6 | 0.86 | 17.8 | 1 | 3.1 | 0.84 |
| m-Xylene (7) | 108-38-3 | 139 | 0.6 | 0.86 | 18 | 2.3 | 2.3 | 0.87 |
| Low aromatic white spirit (LAWS) (2) | 919-446-0 | 151–200 | 0.15 | 0.78 | 16.1 | 0.1 | 0.1 | 0.61 |
| High aromatic white spirit (HAWS) (1) | 919-857-5 | 130–210 | 0.15 | 0.74–0.85 | 16.6 | 0.3 | 0.3 | 0.64 |
| Name | CAS no. | bp (°C) | RER (nBuAc 1.0) | Density (g cm−3) | δ D | δ P | δ H | RED |
|---|---|---|---|---|---|---|---|---|
| Butyl lactate (4) | 138-22-7 | 186 | 0.02 | 0.98 | 15.8 | 6.5 | 10.2 | N/A |
| Ethylene glycol butyl ether (9) | 111-76-2 | 171 | 0.06 | 0.9 | 16 | 5.1 | 12.3 | 0.64 |
| Propylene glycol butyl ether (10) | 5131-66-8 | 171 | 0.09 | 0.88 | 15.3 | 4.5 | 9.2 | 0.61 |
| Diethylene glycol butyl ether | 112-34-5 | 231 | 0.004 | 0.95 | 16 | 7 | 10.6 | 0.19 |
| Dipropylene glycol butyl ether (16) | 29911-28-2 | 230 | 0.006 | 0.91 | 15.7 | 6.5 | 10 | 0.07 |
| Propylene glycol methyl ether acetate (20) | 108-65-6 | 146 | 0.33 | 0.96 | 15.6 | 5.6 | 9.8 | 0.27 |
| Ethyl lactate | 97-64-3 | 154 | 0.09 | 1.1 | 16 | 7.6 | 12.5 | 0.65 |
| Name | CAS no. | bp (°C) | RER (nBuAc 1.0) | Density (g cm−3) | δ D | δ P | δ H | RED |
|---|---|---|---|---|---|---|---|---|
| Butyl levulinate (6) | 2052-15-5 | 238 | 0.003 | 0.97 | 15.7 | 9.7 | 5.8 | N/A |
| Ethyl levulinate (5) | 539-88-8 | 206 | 0.02 | 1.01 | 14.6 | 10.5 | 7 | 0.44 |
| Dipropylene glycol butyl ether (16) | 29911-28-2 | 230 | 0.001 | 0.95 | 15.7 | 6.5 | 10 | 0.88 |
| 2,2,4-Trimethyl-1,3-pentanediol monoisobutyrate (Texanol) (19) | 25265-77-4 | 244 | 0.002 | 0.95 | 15.1 | 6.1 | 9.8 | 0.92 |
| Diethylene glycol butyl ether | 112-34-5 | 230 | 0.004 | 0.95 | 16 | 7 | 10.6 | 0.92 |
| Propylene glycol methyl ether acetate (20) | 108-65-6 | 146 | 0.3 | 0.96 | 15.6 | 5.6 | 9.8 | 0.96 |
| Ethylene glycol methyl ether acetate | 110-49-6 | 145 | 0.4 | 1.01 | 15.9 | 5.5 | 11.6 | 1.2 |
| Ethylene glycol butyl ether acetate (21) | 112-07-2 | 192 | 0.04 | 0.94 | 15.3 | 7.5 | 6.8 | 0.28 |
| Diethylene glycol dibenzoate (18) | 120-55-8 | 236 | 0 | 1.18 | 18.3 | 6.6 | 4.4 | 1.04 |
| Diisobutyl adipate (11) | 141-04-8 | 293 | 0 | 0.96 | 16.7 | 2.5 | 6.2 | 1.25 |
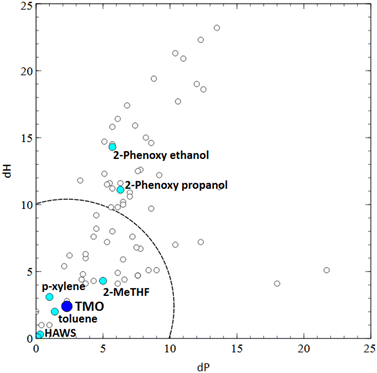
TMO (3) (b.p. 112 °C) has frequently been suggested as a potential replacement for toluene (b.p. 111 °C).27 It could also be seen to be close in Hansen space (low Relative Energy Difference – RED) to both para- and meta-xylene (7), and both high (1) aromatic and low (2) aromatic content white spirit. Aromatic solvents in particular are a key area for replacement in paints, due to their relatively high toxicity compared to other options. Progress has been made as already discussed, however, for low-polarity resins such as alkyds, it can be difficult to eliminate aromatics entirely. Aromatic solvents such as xylenes (e.g.7 and 8) are an integral part of the synthesis of alkyds through polycondensation, as they act as an azeotroping solvent to help remove water from the reaction, therefore the whole supply chain would need to transition to a new solvent for effective replacement of xylene. Link et al. recently reported the use of TMO (3) as an azeotroping solvent for the synthesis of polyester resins, which could allow the manufacture of truly xylene-free resins.28 Although TMO (3) has a lower boiling point (and therefore a higher evaporation rate) than xylenes and white spirit, it could then be used to formulate faster drying, aromatic solvent-free paints.
TMO (3) has an overall score of 7 in both the consumer and industrial guides in comparison to 3 (Type 1 (1)) or 4 (Type 3 (2)) for white spirit (consumer and industrial guides) and 5 (m- (7) or p-(8)) or 6 (o-) for xylene (industrial guide).
TMO can currently be produced on a commercial scale, but supplies to customers in the EU and UK are limited under REACH regulations as registration is not yet complete. Companies in these regions can receive evaluation quantities of up to 1 tonne per year, or larger quantities under Product and Process Oriented Research and Development (PPORD) exemptions.
Bio-based butyl lactate (4) has been proposed as a replacement for butyl glycol (ethylene glycol butyl ether) (9),24 which has undesirable toxicity (H331 – toxic if inhaled). In terms of RED, butyl lactate (4) is indeed close to butyl glycol (9) and propylene glycol butyl ether (10) in Hansen space, as well as ethylene glycol methyl ether acetate. Looking at boiling points and relative evaporation rates, butyl lactate (4) is slightly higher boiling so will be slower to evaporate. This could be mitigated by blending with lower boiling ethyl lactate, which would improve the RED match for butyl glycol (9) even further, however it should be noted that ethyl lactate has the H-statement “H318 – causes serious eye damage” therefore use of butyl lactate alone may still be preferred for some applications.
Looking at the overall guide rating for these two solvents, butyl lactate (4) scores 7, while butyl glycol (9) scores 5, in both the consumer and industrial guides. Butyl lactate (4) scores better in a number of areas, including biosourcing, GWP, reactivity, biotreatment and air impacts. It scores worse for incineration (due to its higher oxygen content) and impact on the aqueous environment. Due to its primary ester structure, its hydrolytic stability at the typical waterborne paint pH of 8–9 may need to be tested for use as a co-solvent in certain waterborne paints.
Bio-based butyl lactate is REACH registered and commercially available.
Ethyl (5) and butyl (6) levulinates have been proposed in the literature as potential replacements for D-limonene, dibasic esters (e.g.11–13) and glycol ethers (e.g.14–18).29D-Limonene has been excluded from examination in this guide due to its unfavourable effects on air quality (particularly indoors), while dibasic esters have boiling points higher than desirable for the air-drying industrial use case we have investigated. Looking at the Hansen parameters for levulinates in comparison to glycol ethers, their polarity is higher, but their hydrogen bonding is lower. Despite this, the RED of butyl (and ethyl) levulinate is sufficiently close to the glycol ethers and their acetates to be of interest as a potential replacement. In a paints and coatings context lower boiling ethyl levulinate might be expected to have washing/degreasing applications (cf. limonene, albeit with lower aldehyde formation potential) or substitute for the diglycol ethers in solvent borne coatings. The higher boiling point of butyl levulinate (6) (238 °C) is interesting, with its relative similarity to 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate (19) (244 °C) and other coalescents/high boiling glycol ethers suggesting it may be worthy of further investigation as a coalescent. Similarly to butyl lactate (4), due to its primary ester structure, its stability at pH of 8–9 may need examining. Use in higher temperature solvent borne paint applications such as coil coatings, while outside the scope of this paper, may also be relevant.
If we compare butyl levulinate (6) to Texanol (19) and dipropylene glycol butyl ether (16) (DPnB) in the consumer guide, we can see that butyl levulinate (6) scores the same or higher than both the conventional coalescents against most criteria. In particular, large improvements could be achieved in bio-content and GWP score by using butyl levulinate (6), with additionally improved biotreatment and reactivity scores over the glycol ether (16). As relatively new biobased products, further synthetic developments of the levulinate platform will be watched with interest for paint and coatings applications.
Both levulinate products are REACH registered and commercially available with production scales planned to be increased in the near future.
5. Conclusions
A solvent sustainability guide is offered for the paints and coatings industry. Whilst the methodology applied will not necessarily be appropriate for all solvent applications in this field, and we would encourage chemists to use their own judgement in applying the data provided by this tool, it is believed to be a better fit to most solvent use in paints and coatings than previous pharmaceutical industry-based guides. The ways in which solvents are used in the paint industry are varied, so we hope in future to expand the guide to other solvent use scenarios and evaluate a greater range of solvents, particularly in the up-and-coming bio-based sectors.The paints and coatings industry has increasingly been moving towards lower emissions (data from the U.S. Environmental Protection Agency (EPA) Toxic Release Inventory (TRI) in 2020 suggested a decrease in coatings manufacturing emissions since 1990,30) and as regulations on VOCs change the volatility of the solvents considered is anticipated to come under increasing scrutiny.
Particular customisations in the guide methodology for paints relative to pharmaceuticals have been inclusion of metrics to assess impact on indoor air quality during application of consumer paints, and addition of rankings for global warming potential and bio-sourcing. The latter measures are areas of increasing interest for paint manufacturers.
At this time, the sustainability focus from many paint manufacturers has understandably been on carbon footprint, with climate change and net zero targets being a pressing concern. However, the industry needs to be cautious of the risk of making regrettable substitutions if a single metric of sustainability is focussed on. It is encouraging therefore to see manufacturers including life cycle analysis in their sustainability plans and goals as this can help to highlight other risk areas. This solvent guide is proposed as another tool that can be used to mitigate this risk.
While the use of bio-sourced ingredients is only occasionally explicitly discussed in sustainability goals in the industry, interest in bio-based and recycled materials has been growing at paint industry conferences over the last few years. Several concerns relating to bio-based materials are acknowledged, such as their increased cost, lower availability, natural product variability and land/water use. However, the number of paint ingredients with bio-based content is increasing, and as demand increases, and scale up becomes possible, cost can be predicted to reduce. Use of waste products to produce these bio-based materials reduces the need to repurpose agricultural land from food production.
Whilst this guide has deliberately avoided considerations such as cost and availability, it is acknowledged that these are critical factors governing decision making. Cost and availability might be anticipated to potentially change depending on legislation, and scale of production, however at present biobased solvents are generally more expensive e.g. ethyl levulinate (5) retails at £371.00 per kg31 and diisobutyl adipate (11) retails at £17.80 per 250 mL.32 Whilst variation might be expected depending on purity, scale of purchase, geography, etc. for reference from the same supplier DCM currently retails at £128.00 per L33 and toluene at £81.90.34
While progress is slow due to the challenges of making such changes in a complex supply chain, we anticipate seeing more new “sustainable” solvents becoming available in the coming years and look forward to incorporating them into future iterations of this guide.
We have shown in this article how the assessment guide methodology can be used, in combination with simple solvent physical properties such as evaporation rates and Hansen Solubility Parameters, to find alternative solvents and compare their sustainability across a range of different metrics, or by focusing on specific goals. Practical examples have been given for specific problematic solvents currently used in the paints and coatings industry, such as the aromatic solvent xylene and the polar solvent butyl glycol. We hope to follow up on investigating the laboratory performance of such substitutions in the future.
Finally, it must be noted that new data continues to be discovered on solvents, and regulations continue to evolve. Therefore, it is essential to consult reliable sources such as the ECHA website35 to obtain the most current information, and only to use this guide in conjunction with such guidance.
Author contributions
Laura Pilon: conceptualization, data curation, funding acquisition, methodology, project administration, writing; Daniel Day: investigation; Harry Maslen: investigation; Oliver Stevens: investigation; Nicola Carslaw: investigation; David Shaw: investigation; Helen Sneddon: conceptualization, data curation, funding acquisition, methodology, project administration, supervision, writing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank PERA International and EPSRC Impact Acceleration Account – University of York 2022, Grant Ref: EP/X525856/1, for funding.The authors are indebted to the extensive work done over several iterations of the GSK solvent guide and wish to particularly acknowledge Alan Curzons, Rebecca De Leeuwe, John Hayler, Richard Henderson and Leanna Shuster. We are also appreciative of the assistance provided by Addible in facilitating the inclusion of additional data values for tetramethyloxolane.
References
- (a) D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC; D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2015, 17, 4848–4848 Search PubMed; (b) C. M. Alder, J. D. Hayler, R. K. Henderson, A. M. Redman, L. Shukla, L. E. Shuster and H. F. Sneddon, Green Chem., 2016, 18, 3879–3890 RSC; (c) K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC; (d) D. Prat, O. Pardigon, H. W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS; (e) L. J. Diorazio, D. R. J. Hose and N. K. Adlington, Org. Process Res. Dev., 2016, 20, 760–773 CrossRef CAS.
- British Coatings Federation data.
- Solvents Market Size, Growth & Trends|Report [2021–2028] (https://www.fortunebusinessinsights.com/industrial-solvents-market-102135, last accessed 29th June 2024).
- RSC Surface Coatings Conference, University of York, 10th Jan, 2023.
- Five facts on the water-borne coatings market – News and insights for the European coatings industry (https://www.european-coatings.com/news/markets-companies/five-facts-on-the-water_borne-coatings-market/, last accessed 29th June 2024), Waterborne coatings: A steady growing business – News and insights for the European coatings industry (https://www.european-coatings.com/news/raw-materials/waterborne-coatings-a-steady-growing-business/, last accessed 29th June 2024).
- M. Hilt and U. Christ, Eur. Coat. J., 2010, 12(82), 84–87 Search PubMed.
- A. Javadi, A. Cobaj and M. D. Soucek, Commercial waterborne coatings, in Handbook of Waterborne Coatings, ed. P. Zarras, M. D. Soucek and A. Tiwari, Elsevier, Amsterdam, 2020, ch. 12, pp. 303–344 Search PubMed.
- LCI Project – The European Council of the Paint, Printing Ink, and Artist's Colours Industry (CEPE) (https://www.cepe.org/sustainability/lci-project/, last accessed 29th June 2024).
- The Community RePaint network – Community RePaint (https://communityrepaint.org.uk/the-uks-paint-reuse-network/, last accessed 29th June 2024).
- Home – Paintcare (https://www.paintcare.org.uk, last accessed 29th June 2024).
- e.g. Source Reduction and Recycling of Halogenated Solvents in Paint Stripping (epa.gov).
- The chemical mechanistic information was taken from the Master Chemical Mechanism, MCM v3.3.1 ( M. E. Jenkin, S. M. Saunders and M. J. Pilling, Atmos. Environ., 1997, 31, 81 CrossRef CAS; S. M. Saunders, M. E. Jenkin, R. G. Derwent and M. J. Pilling, Atmos. Chem. Phys., 2003, 3, 161 CrossRef; M. E. Jenkin, S. M. Saunders, V. Wagner and M. J. Pilling, Atmos. Chem. Phys., 2003, 3, 181 CrossRef; C. Bloss, V. Wagner, M. E. Jenkin, R. Volkamer, W. J. Bloss, J. D. Lee, D. E. Heard, K. Wirtz, M. Martin-Reviejo, G. Rea, J. C. Wenger and M. J. Pilling, Atmos. Chem. Phys., 2005, 5, 641 CrossRef ), via website: https://www.mcm.york.ac.uk.
- N. Carslaw and D. Shaw, Environ. Sci.: Processes Impacts, 2019, 21, 1313–1322 RSC.
- D. Shaw and N. Carslaw, J. Open Source Softw., 2021, 6(63), 3224 CrossRef.
- CCaLC, Carbon Calculations over the Life Cycle of Industrial Activities accessed 12th March 2024.
- I. Ragazzi, M. Farley, K. Jeffery and I. Butnar, PLOS Sustain. Transform., 2023, 2(9), e0000080 CrossRef.
- I. Garcia-Herrero, R. M. Cuéllar-Franca, V. M. Enríquez-Gutiérrez, M. Alvarez-Guerra, A. Irabien and A. Azapagic, ACS Sustainable Chem. Eng., 2016, 4, 2088–2097 CrossRef CAS.
- E. Schols, M. North, W. Leitner, G. Mul, R. Heyn, D. Trambitas, O. Sigurbjornsson and S. Lucas, CyclicCO2R: Production of Cyclic Carbonates from CO2using renewable feedstocks. Project Final Report: Public Summary, 2016.
- European Chemicals Agency. Registry of Restriction Intentions. https://echa.europa.eu/registry-of-restriction-intentions/-/dislist/details/0b0236e1806abf64.
- Annex 1 – Background document to RAC opinion on white spirit. 21. Adopted_Annex 1_BD_for_white_spirit2.pdf (europa.eu) (accessed 20th April 2024).
- E. A. Friedman, J. B. Greenberg, J. P. Merrill and G. J. Dammin, Am. J. Med., 1962, 32(6), 891–902 CrossRef CAS PubMed.
- D. F. Aycock, Org. Process Res. Dev., 2007, 11(1), 156–159 CrossRef CAS; V. Pace, P. Hoyos, L. Castoldi, P. Dominguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369–1379 CrossRef PubMed.
- F. Byrne, B. Forier, G. Bossaert, C. Hoebers, T. J. Farmer, J. H. Clark and A. J. Hunt, Green Chem., 2017, 19, 3671–3678 RSC.
- PURASOLV® BL|Corbion accessed 18th April 2024, https://www.corbion.com/-/media/Corbion/Files/Biochemical-specialties/FCTS-AGRO-PURASOLV-BL-ENG-0923.pdf.
- The Use Of Levulinates As Coalescing Agents in Water-based Coatings (paint.org) accessed 18th April 2024, https://www.paint.org/coatingstech-magazine/articles/the-use-of-levulinates-as-coalescing-agents-in-water-based-coatings/.
- S. Abbott, C. M. Hansen and H. Yamamoto, Hansen Solubility Parameters in Practice – Complete with software, data and examples, 5th edn, 2015. ISBN: 9780955122026 Search PubMed.
- F. Byrne, W. Hodds, S. Shimizu, T. Farmer and A. Hunt, J. Cleaner Prod., 2019, 240, 118175 CrossRef CAS.
- T. Link, J. Sherwood, D. Day, M. Kluge, T. J. Farmer and T. Robert, Ind. Eng. Chem. Res., 2024, 63(15), 6609–6614 CrossRef CAS.
- NXT SOLV 200 – NXTLEVVEL (specialchem.com) accessed 18th April 2024.
- American Coatings Association, Sustainability Report 2022.
- Price as of Sigma-Aldrich catalogue, Ethyl levulinate natural, =98, FG 539-88-8 (sigmaaldrich.com) accessed 7th June 2024.
- Price as of Sigma-Aldrich catalogue Diisobutyl adipate 99 141-04-8 (sigmaaldrich.com) accessed 7th June 2024.
- Price as of Sigma-Aldrich catalogue Dichloromethane anhydrous, =99.8, amylene 40–150 ppm stabilizer 75-09-2 (sigmaaldrich.com) accessed 7th June 2024.
- Price as of Sigma-Aldrich catalogue Toluene|Sigma-Aldrich (sigmaaldrich.com) accessed 7th June 2024.
- Homepage – ECHA (europa.eu) (accessed 17th June 2024).
Footnote |
| † Electronic supplementary information (ESI) available: Excel files of both the consumer and industrial guides. See DOI: https://doi.org/10.1039/d4gc01962h |
| This journal is © The Royal Society of Chemistry 2024 |