Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanostructured single-atom catalysts derived from natural building blocks
Yajing
Zhang
 ab,
Guobin
Yang
ac,
Jin
Wang
ad,
Bin
Zhao
ab,
Guobin
Yang
ac,
Jin
Wang
ad,
Bin
Zhao
 e,
Yunxiang
He
e,
Yunxiang
He
 *ab and
Junling
Guo
*ab and
Junling
Guo
 *abf
*abf
aBMI Center for Biomass Materials and Nanointerfaces, College of Biomass Science and Engineering, Sichuan University, Chengdu, Sichuan 610065, China. E-mail: yxhe@scu.edu.cn; junling.guo@scu.edu.cn
bNational Engineering Laboratory for Clean Technology of Leather Manufacture, Sichuan University, Chengdu, Sichuan 610065, China
cWeihai Marine Organism & Medical Technology Research Institute, College of Marine Science and Technology, Harbin Institute of Technology, Weihai, Shandong 264209, China
dPritzker School of Molecular Engineering, The University of Chicago, Chicago, IL 60637, USA
eDepartment of Bioproducts and Biosystems, School of Chemical Engineering, Aalto University, Espoo, FI-02150, Finland
fBioproducts Institute, Department of Chemical and Biological Engineering, The University of British Columbia, Vancouver, BC V6T 1Z4, Canada. E-mail: junling.guo@ubc.ca
First published on 3rd January 2024
Abstract
Single-atom catalysts (SACs) exhibit maximized atomic utilization with individual metal atoms anchored on supporting materials, where the pursuit of high performance and low cost presents challenges. In this case, carbon provides structural versatility and customizable properties as a supporting material, which has been extensively studied. Biomass materials have emerged as promising precursors for the preparation of carbon-based SACs due to their renewable nature for sustainability, abundance for low cost, and high carbon content for advanced performance. In this review, representative synthesis strategies and advanced characterization techniques for biomass-derived CS-SACs are summarized, which facilitate the establishment of guidelines for the rational design and fabrication of biomass-derived SACs. In addition, we provide a timely and comprehensive discussion on the use of a broad range of natural biomass for SACs, with insights into the specific carbon nature of biomass resources, including their carbon structures, metal-carbon coordination environment, and center metal species. Furthermore, the application areas of biomass-derived CS-SACs in various catalytic processes are reviewed. Overall, the challenges and future perspectives of using biomass as precursors for SACs are outlined. We hope that this review can offer a valuable overview of the current knowledge, recent progress, and directions of biomass-derived SACs.
Broader contextSingle-atom catalysts (SACs), particularly carbon-supported single-atom catalysts (CS-SACs), have arguably emerged as the most active new frontier in catalysis. Carbon support materials play pivotal roles in the development of CS-SACs. However, most of the precursors for these support materials are predominantly derived from non-renewable sources, and their synthesis usually requires harsh and energy-intensive conditions. Predictably, the utilization of synthetic precursors can also exacerbate serious social issues, including energy shortages, climate warming, and environmental pollution. In nature, carbon is coupled with other elements to give rise to a wide array of life forms, including plants, animals, and microbes. Thus, the use of carbon-based biomass sources as renewable precursors for the controlled synthesis of CS-SACs represents a sustainable alternative that has the potential to substantially reduce our reliance on fossil reserves and accelerate the sustainable advancement of human society. To accelerate the development of biomass-derived CS-SACs, it is necessary to summarize the recent progress on their design principles and biomass sources. This review aims to provide a comprehensive summary of recent advances related to the synthesis of biomass-derived CS-SACs, focusing on their synthetic strategies and many types of biomass precursors. Additionally, the remaining challenges and perspectives in the future of these emerging catalysts are further discussed. |
1. Introduction
Single-atom catalysts (SACs) have emerged as a novel class of supported catalysts, which are characterized by catalytically active individual and isolated metal atoms anchored to suitable supports, enabling optimal metal utilization through the exposure of all metal atoms to the reactants and availability for catalytic reactions.1–3 SACs have attracted substantial attention in recent years due to their unique properties, including exceptional catalytic activity, selectivity, and stability in reactions such as ammonia synthesis, oxidation, and hydrogenation. These distinctive properties arise from the low-coordination environment for metal atoms, quantum size effect, and strong metal–support interaction, distinguishing them from traditional metal nanoparticles and mononuclear metal compounds.4,5The support materials largely determine the catalytic performance of SACs. Extensive research has demonstrated that the support materials not only provide strong stabilization for individual metal atoms but also play a crucial role in governing the local geometric and electronic structures of catalytic centers, resulting in exceptional retention of the isolated state and superior catalytic activity.6–8 To date, single atoms have been successfully dispersed on a variety of support materials including metals,9 metal oxides,10 metal hydroxides,11 sulfides,12 phosphides,13 zeolites,14 and carbon-based materials,15 enabling their widespread utilization as catalysts for thermochemical, electrochemical, and photochemical reactions.16,17 Among the reported support materials, carbon is prevalent due to the advantages of high conductivity, high specific area, and abundant carbon reserves.18–20 Furthermore, the structural diversity and designability of carbon-based materials (e.g., amorphous carbon, graphite, and diamond) allow a regulatory structure–performance correlation for the understanding of the catalytic mechanism and the resulting SACs with desired performance.21 In recent years, the precursors utilized for the synthesis of carbon-supported SACs (CS-SACs), comprising carbon and metal sources, have been elaborately designed but are limited by their complex structures and high production costs, including materials such as metal–organic frameworks (MOFs), covalent-organic frameworks (COFs), organometallic compounds, small molecular precursors, and functional polymers enriched with diverse heteroatoms.22–30 Moreover, the preparation of synthetic precursors requires organic solvents and multiple chemical components, which not only hinder the further development of CS-SACs, but also aggravate serious social problems, such as the energy crisis, global warming, and environmental pollution.31
Although numerous synthetic CS-SACs have been proposed and explored, the research focus has shifted from synthetic materials to biomass from renewable natural resources as precursors for the preparation of functional CS-SACs. Compared to artificially synthesized organics, biomass in nature offers inherent merits, including a wide distribution, fine structures, diverse compositions, abundance, low cost, non-toxicity, ease of modification, and biodegradability, endowing it with great potential to be adopted as a sustainable source for carbon-based materials.32,33 Owing to this fact, CS-SACs prepared from biomass are considered sustainable and environmentally friendly catalysts. To date, according to the different biomass sources, CS-SACs can be divided into raw biomass, waste biomass, and naturally biomass-derived chemicals. Firstly, the natural morphology and specific elemental compositions of raw or waste biomass precursors endow the derived carbon materials with very large surface areas, distinct porous structures, favorable electronic conductivities, and intrinsic receptiveness toward heteroatom doping.34,35 Secondly, notably, natural biomass-derived chemicals (e.g., macromolecules and small-molecule catecholamines) have increasingly been used as fundamental precursors for CS-SACs due to their diverse chemical structures and ease of modification. For instance, carbon-rich tannic acid (TA) and small molecules of dopamine have been employed for the preparation of precursors for CS-SACs based on the metal coordination properties of natural polyphenols.36–38 Alternatively, cheap functional polysaccharides, such as chitosan and starch, as carbon sources, enable the gram-scale, pervasive synthesis of multifunctional CS-SACs.39,40 The pursuit of utilizing naturally occurring materials, which are frequently regarded as “green” or “sustainable”, presents a promising opportunity to broaden the range of carbon carriers for the fabrication of CS-SACs.
Given the fast development and increasing research interest in biomass-derived CS-SACs, a timely and comprehensive review is essential. In this review, we focus on the utilization of natural building blocks from a broad range of bio-resources to construct CS-SACs, emphasizing the significance of CS-SACs in addressing critical hurdles encountered in diverse catalytic applications (Fig. 1). Firstly, general synthetic strategies and representative advanced characterization techniques for biomass-derived CS-SACs are briefly introduced, followed by the detailed examination of the advantages and disadvantages of each synthetic strategy.
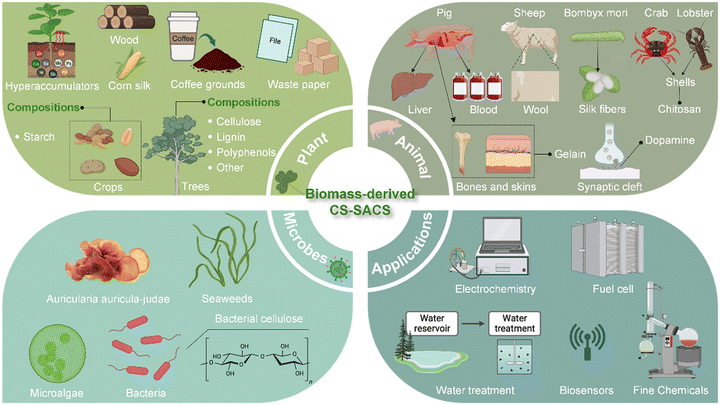 | ||
| Fig. 1 Construction and versatile applications of CS-SACs using natural building blocks from natural biomass resources. Created with BioRender.com. | ||
Subsequently, the natural building blocks from plants, animals, and microbes are introduced as precursors for the preparation of biomass-derived CS-SACs, including a discussion on their chemical composition, structural traits, catalytic performance, and inclusion in SAC design principles. In addition, by comparing the structure and function of natural building blocks and their potential for fabricating SACs, we provide some insights into the construction of biomass-derived CS-SACs and simply summarize their diverse applications at the present stage. Finally, we propose a short perspective on the current challenges and trends in the conversion of natural building blocks into SACs, which can further improve their utility in catalytic applications.
2. Synthetic strategies for biomass-derived CS-SACs
Challenges in the preparation of high-quality SACs are derived from the tendency of metal atoms to aggregate, which originates from the high surface energy of isolated single metal atoms on supports.41–43 The foundational principles and rules for the fabrication of CS-SACs lie in the construction of intense interactions between a single metal atom and the carbon support.44,45 Enormous efforts have been devoted to probing the various approaches for the fabrication of CS-SACs, where bottom-up and top-down methods are the two main categories with different integration modes of components.46 In the case of bottom-up methods such as coprecipitation, atomic layer deposition (ALD), and wet chemistry synthesis, metal-containing precursors are adsorbed/anchored, reduced, and confined by defects on oxides or carbon supports with plenty of N or O defects or vacancies.46,47 Alternatively, top-down methods (i.e., pyrolytic carbonization) have shown promising perspectives for practical production and large-scale preparation in industry, where well-organized nanostructures can be further tailored and engineered with desired properties.48–50As mentioned above, differing from synthetic carbon precursors, biomass materials have been increasingly acknowledged as promising precursors for CS-SACs due to their affordability, widespread accessibility, and eco-friendliness.33 In this section, we focus on the methods for preparing biomass-derived CS-SACs and their specific advantages/limitations (Table 1).
| Synthetic strategy | Category | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Carbonization strategy | Direct carbonization | Using inherent metal elements of biomass; sustainable and green | Low metal loading | 51–56 |
| Metal-salt-added carbonization | Tailoring different single metal centers; high metal loading | High cost | 57–59 | |
| Molten-salt-assisted carbonization | Much higher polarity; breaking chemical bonds; high yield | High cost; corrosive nature | 60–73 | |
| Other material-assisted carbonization | Adjustable microstructure; large-scale production | Dangerous etching reagent | 74–85 | |
| Ambient synthesis | — | Low energy consumption and cost | Depending on the functions of precursors | 86–88 |
2.1 Carbonization strategy
Carbonization is the most straightforward and extensively used method for synthesizing atomically dispersed materials, which is achieved through the high-temperature decomposition of various precursors under a gas atmosphere (such as N2, Ar, and NH3).89,90 This process can also be readily conducted in both laboratories and industrial factories. The biomass complexation strategy is a general approach for the preparation of catalyst precursors.39 It is known that carbon is insufficient to prevent the aggregation of metal atoms due to its weak coordination with metal, which raises concerns in the synthesis of biomass-derived CS-SACs.Heteroatoms are effective elements to stabilize isolated single atoms, typically, oxygen (O) and nitrogen (N) atoms. Accordingly, the oxygen-containing functional groups (e.g., –OH and –COOH) in biomass can effectively adsorb metal ions via electrostatic interactions to impede their aggregation and well-confine the metal atoms.91 N atoms have higher electronegativity and a similar atomic size as carbon (C), enabling their facile doping in N-poor biomass with internal or external N sources.16 For example, Spirulina contains significant amounts of inherent protein, which can be used as natural nitrogen precursors to in situ generate N-doped biochar.92 Also, the addition of external N sources such as urea, ammonium salts, nitric acid, melamine, and NH3 gas during the carbonization process is commonly employed to incorporate N elements.93 Moreover, besides O and N, phosphorus (P), sulfur (S), and chlorine (Cl) dopants have also been investigated to regulate the electronic structure of the active metal centers.94,95 It is worth mentioning that certain trace elements of P, S, and Cl present in biomass can effectively serve as coordinating or neighboring moieties for metal atoms.93,96 Du et al. employed reed bio-sorbent derivatives saturated with nitrate and sulfate to prepare Fenton-like catalysts for the decomposition of dinotefuran (DIN) by N and S co-doping, respectively, where S was doped in the crystalline Co3O4 phase due to the role of N as an anchor.97 Compared to the S-free sample, the S-doped samples exhibited 1.71-times greater activity for the decomposition of DIN. Due to the different origins of biomass materials and synthetic routes and approaches, diverse as-prepared single metal atoms with different types of carbon supports can be produced, where the metal atoms are presented with well-defined coordination environments. Given that they naturally contain rich N and O heteroatoms, biomass-derived SACs generally present the M–N4 and M–O3C1 configurations (Fig. 2). Different metal coordination configurations may lead to different performances in catalytic processes, which can assist in the selection of the carbon source for desired applications.
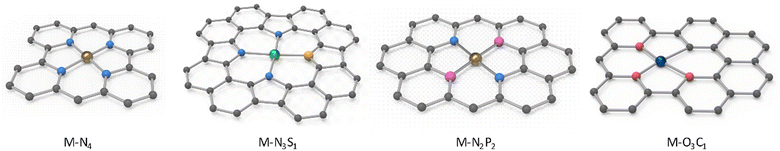 | ||
| Fig. 2 Representative coordination environments of as-prepared CS-SACs derived from biomass materials. | ||
The complex chemical composition of biomass makes the thermal transformation process intricate, but control of the procedure allows the engineering of the resulting biomass-derived CS-SACs with desirable qualities and properties.96 Based on the origin and carbonization environment of the biomass precursor in CS-SACs, this strategy can be classified into three categories, i.e., direct carbonization, metal-salt-added carbonization, molten-salt-assisted carbonization, and other material-assisted carbonization.
Besides the fundamental benefits outlined above, molten salt synthesis possesses other advantages compared to conventional solid-phase synthesis techniques, as follows: (1) the interaction between the molten salt and precursor enables better carbonization, leading to a significantly improved yield. (2) Molten salts possess a large heat capacity and thermal conductivity coefficient, promoting the controllable and fast formation of desired structures. (3) Molten salts are stable in a wide temperature range, which can be continuously adjusted from approximately 100 °C to more than 1000 °C.64,68,69 However, this strategy also presents certain challenges70 such as the spent salts are not always easily dissolved and removed by simple treatment, such as hot water. For example, carbonate salts tend to solidify to rock-like hardness after carbonization.71 Another challenge is cost. As is known, the common low-melting-point molten salts often contain expensive Li2CO3, but the regeneration of these salts is neither easy nor cheap.72 Moreover, the corrosive nature of many salts imposes specific demands on reaction equipment and leads to elevated expenses.73 Therefore, it becomes crucial to conform to the necessity before employing molten-salt-assisted carbonization for the synthesis of biomass-derived CS-SACs.
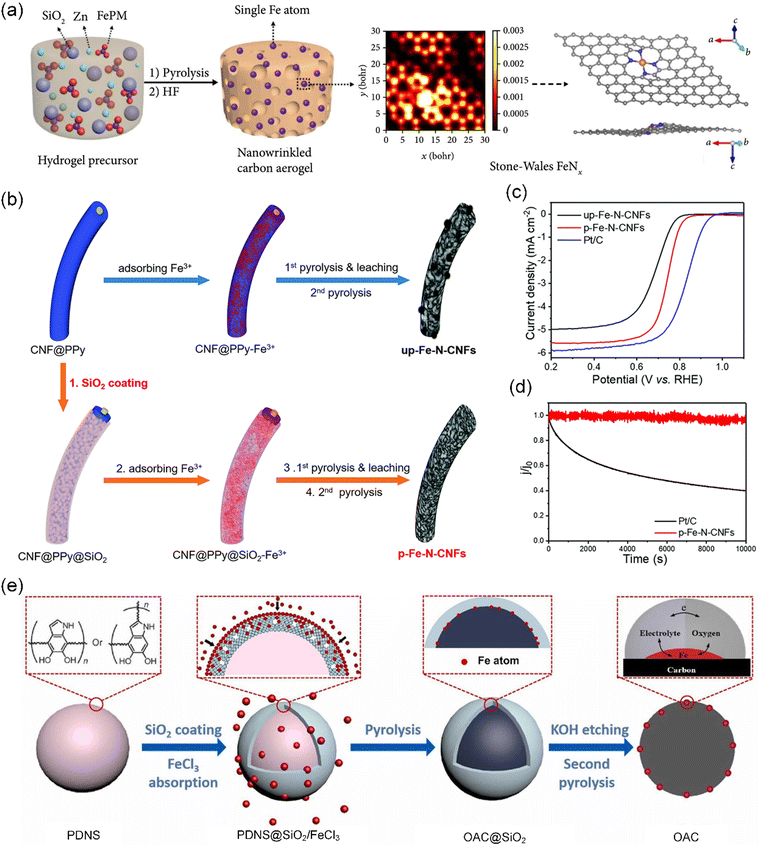 | ||
| Fig. 3 SiO2-assisted carbonization strategy. (a) Schematic representation of the synthesis of the NCAC–Zn/Fe carbon aerogel. Reproduced with permission from ref. 80 (Copyright 2019, AAAS). (b) Schematic illustration of the fabrication of up-Fe–N-CNF (top) and p-Fe–N-CNF (bottom) catalysts. (c) Linear sweep voltammetry (LSV) curve of up-Fe–N-CNFs, p-Fe–N-CNFs, and Pt/C. (d) Chronoamperometric test of p-Fe–N-CNFs and Pt/C at 0.45 V vs. RHE. (b)–(d) Reproduced with permission from ref. 81 (Copyright 2018, The Royal Society of Chemistry). (e) Schematic illustration of the preparation of CNS with single Fe atoms at the triple-phase boundary. Reproduced with permission from ref. 82 (Copyright 2022, ScienceDirect). | ||
Notably, the silica-protective-layer assisted strategy, using SiO2 as a coating material, can facilitate the reaction of N atoms with metal atoms during high-temperature carbonization to preferentially form catalytically active M–N4 sites.84,85 Based on this, Hu et al. successfully formed a uniform amorphous SiO2 shell around polypyrrole-coated carbonaceous nanofibers (CNF@PPy) via the base-catalyzed hydrolysis of tetraethyl orthosilicate (TEOS) (Fig. 3b).81 As designed, the SiO2 shell not only restricted the free migration of Fe species but also captured volatile gaseous compounds during carbonization, achieving the simultaneous optimization of both the surface functionalities and porous structures of CS-SACs. Meanwhile, the resultant catalysts exhibit a much-enhanced oxygen reduction reaction (ORR) activity in 0.1 M HClO4 with a half-wave potential of 0.74 V (vs. RHE), together with superior long-term stability (Fig. 3c and d). Nonetheless, not every single atomic site present in CS-SACs contributes to their ORR activity. Generally, the atomic M–Nx moieties located on the external surface of the catalyst exhibit a higher propensity to engage in the ORR. Subsequently, unlike the traditional silica-protective-layer assisted strategy, Deng et al. employed a silica-restricted diffusion strategy to first coat polydopamine nanospheres (PDAS) with a thin silica layer, followed by physically mixing with FeCl3, finally obtaining Fe atoms all anchored on the outermost surface of the carbon nanospheres (CNS) (Fig. 3e).82 In this strategy, Fe migrates across the silica shell by atomic exchange with silicon, which not only prevents the aggregation of Fe but also causes the Fe atoms to anchor on the surface. Obviously, these Fe-SACs showed excellent ORR activity with an onset potential of 0.98 V and an ideal 4e− pathway.
Although unique advantages of SiO2 have been explored to accelerate the fabrication of various biomass-derived CS-SACs, the process for the removal of SiO2 often requires a toxic solvent such as sodium hydroxide (NaOH) and hydrofluoric acid (HF), causing a variety of safety concerns. In addition, during the removal process, careful consideration must be given to its potential impact on the structural integrity and retention of the active metal sites in the final structure.
2.2 Ambient synthesis
The ambient synthetic strategy is a special preparation method for the synthesis of SACs under mild conditions, which is limited by the functions of the precursors. The development of ambient temperature synthesis techniques is primarily attributed to the excessive energy consumption and high production cost associated with pyrolytic synthesis methods, particularly when employing synthetic materials as precursors. Recently, Qu et al. elaborately designed an ambient synthetic strategy to directly transform easily accessible bulk metals such as metal foam (e.g., Fe, Co, Ni, and Cu) into SACs (M-SACs/GO, M = Fe, Co, Ni, and Cu) by the strong bonding from the surface dangling bonds of graphene oxide (GO) support (Fig. 4a).86 This synthesis at room temperature employing bulk metals was used to fabricate a series of M-SACs/GO, demonstrating its generality to access a variety of functional SACs. Subsequently, Ma et al. demonstrated a simple but robust carbonization-free route to prepare Co SACs by in situ wrapping an electrocatalytically active porphyrin-based thiophene-sulfur site-containing covalent organic polymer (PTS-COP) shell around a highly conductive multiwalled CNT (MWCNT) core, followed by accurately anchoring single-atom Co–N4 sites on the macrocyclic porphyrin structure (Fig. 4b).87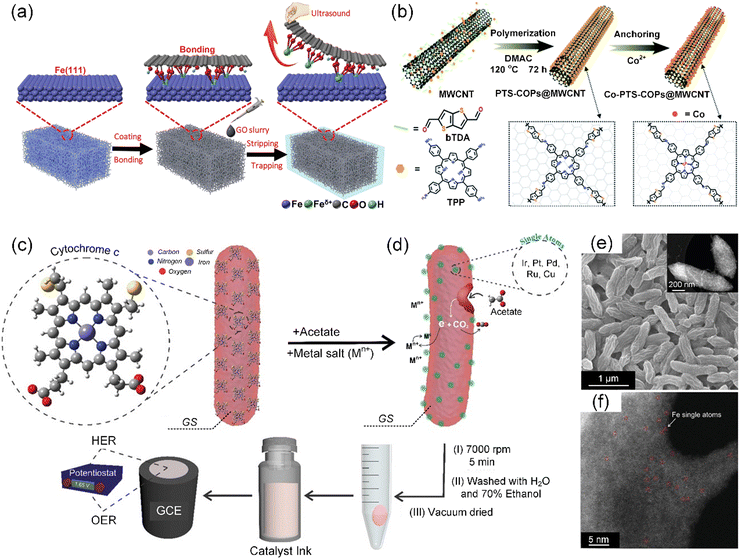 | ||
| Fig. 4 (a) Schematic illustration of the preparation of Fe-SACs/GO. Reproduced with permission from ref. 86 (Copyright 2019, Wiley). (b) Schematic illustration of the carbonization-free synthesis of Co-PTS-COP@MWCNT hybrid. Reproduced with permission from ref. 87 (Copyright 2022, The Royal Society of Chemistry). Schematic showing (c) GS cell with expressed heme-containing c-type cytochromes (c-Cyts) on the outer membrane of the cell, which are responsible for the extracellular electron transfer to the acceptor moieties. (d) Mechanism for the reduction of the metal ions (electron acceptors) on the surface of GS cell membrane. (e) SEM image, with the inset showing the TEM image of Fe-SACs/GS. (f) AC HAADF-STEM image of Fe-SACs/GS. Individual Fe atoms are highlighted by red circles. (c)–(f) Reproduced with permission from ref. 88 (Copyright 2021, Wiley). | ||
Considering that the aforementioned synthetic method cannot be applied to all transition metals including precious metals, Pedireddy et al. presented a nature-based facile method to prepare metal-based SACs (M-SACs, M = Fe, Ir, Pt, Ru, Cu, or Pd) in a single-step at ambient temperature, using the extracellular electron transfer capability of Geobacter sulfurreducens (GS) (Fig. 4c and d).88 Among them, the scanning electron microscopy (SEM) images of the representative Fe-SAC/GS displayed a crumpled morphology with many grooves on its surface, as shown in Fig. 4e, creating a high surface area for facilitating reactions. The transmission electron microscopy (TEM) image (Fig. 4e) and aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC HAADF-STEM) image (Fig. 4f) both indicated the absence of detectable Fe clusters/nanoparticles on the surface of GS. Notably, the AC HAADF-STEM images distinctly revealed the presence of individual Fe atomic sites, as highlighted by a red circle in Fig. 4f, on the surface of GS. Similarly, merely altering the metal precursors can enable the preparation of M-SACs for various applications. Alternatively, Pedireddy et al. incorporated the ambient synthetic strategy in a biomass system, thereby extending the technique for the in situ synthesis of SACs from biomass sources.
3. Advanced characterization techniques for biomass-derived CS-SACs
The advancement of CS-SACs is also highly reliant on the application of sophisticated characterization techniques to conduct comprehensive investigations. After the preparation of CS-SACs, it is essential to identify both their geometric structure and electronic structure to validate their successful synthesis. To date, representative advanced analytical methods have been applied for the characterization of CS-SACs, such as spherical aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC HAADF-STEM) and X-ray absorption fine spectroscopy (XAFS).98,993.1 AC HAADF-STEM
AC HAADF-STEM has a higher resolution compared to the conventional SEM or TEM techniques, enabling the clear characterization of sample microstructures with a resolution of 0.1 nm.100 Consequently, this technique allows for the direct imaging of individual metal atoms dispersed on a support, which facilitates the identification of its precise location in relation to the surface structures and the determination of the spatial distribution of single metal atoms.99,101 Currently, a variety of SACs (e.g., Fe, Co, Ni, Cu, Zn, Mn, Pb, and Ru) has been identified by AC HAADF-STEM. Although AC HAADF-STEM has become the most persuasive and intuitive approach to assess the presence of CS-SACs, there are some intrinsic limits to using AC HAADF-STEM, which can only image a tiny part of samples and cannot identify the target element.102 In particular, there are both single atoms and nanoparticles in as-prepared catalysts, while AC HAADF-STEM can miss key information due to the limited tested parts.3.2 XAFS
In contrast to AC HAADF-STEM, XAFS is a special adjunct method that has been widely used to determine the local chemical structures of CS-SACs. Generally, XAFS can be typically categorized into X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectroscopy.98,103 Specifically, XANES can reveal details about the electron and oxidation states, while EXAFS offers more information on the chemical bonding, interatomic distance, and coordination number of the target element by combining wavelet transform (WT) and Fourier transform (FT)-EXAFS analysis.89,104 Different XANES adsorption curves often show the central atoms with different coordination numbers or ligands, making it easy to identify SACs. Alternatively, XAFS can also be used in situ/operando to provide significant insights into the structural evolution of SACs under real catalytic conditions.102 This technique enables the monitoring of the dynamic electronic and local coordination structures of SACs during reactions. For example, in situ XAFS spectroscopy was performed during the ORR process to provide information regarding the successive structural transformations from Cu–N4 to Cu–N4/Cu-nanoclusters (NC), and then to Cu–N3/Cu–NC, which reflected the change in the structure of the Cu SAS in its true electrochemical environment.105 In summary, XAS and in situ XAS play a pivotal role in elucidating the atomic-level structural details of SACs, allowing for a deeper understanding of their reaction mechanisms.Besides the above-mentioned methods, some other characterization methods can also provide additional information for CS-SACs, such as scanning tunneling microscopy (STM), X-ray photoelectron spectroscopy (XPS), energy dispersive X-ray spectroscopy (EDX), electron energy loss spectroscopy (EELS), X-ray powder diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), and Raman spectroscopy. Moreover, density functional theory (DFT) calculations are often performed in conjunction with these characterization techniques for the structural detection of SACs. Considering the operational conditions of different instruments and the characteristics of SACs, it is essential to select appropriate characterization techniques.
4. CS-SACs from plant biomass
Plant biomass from trees, farm plants, and grasses is produced in huge quantities annually on a global scale and is an abundant renewable resource on Earth. Harnessing plant biomass, such as creating fires and building shields, is among the earliest wisdom of human civilization.106 To date, advances in plant utilization have been explored in the fabrication of CS-SACs. In this section, the typical plant-derived biomass precursors for CS-SACs are summarized, including structural materials [wood, hyperaccumulators, solid biowaste (corn silk, spent coffee grounds, and waste paper)], saccharide-containing functional molecules (starch and cellulose) and aromatic compounds (lignin and polyphenols).4.1 Structural materials
Wood-derived carbon materials can directly use the natural porosity of wood to create channels and fabricate “breathable” carbon materials with the preserved mechanical strength and inherent microchannels of wood,111 which inspires the preparation of SACs. Zhong et al. proposed a novel and effective strategy to form Fe-based SACs on plate wood-based porous carbon (Fe–N/WPC-SACs) in a two-in-one manner via a facile Lewis acid FeCl3 pretreatment and subsequent carbonization process (Fig. 5a–d).112 By using the Lewis acid FeCl3, the two-in-one approach created microchannels (Fig. 5e) and incorporated atomically dispersed Fe–N active species (Fig. 5f) in the hierarchical structure during cell wall pretreatment. Chang et al. obtained a self-supported single-atom electrocatalyst with a macroscopic structure by anchoring atomically dispersed Ni single atoms (Ni SAs) on N-doped carbonized wood (Ni–N/CW-SACs) via a two-step successive carbonization process (Fig. 5g).113 Benefitting from the NCW porous carrier (Fig. 5h), the Ni–N/WPC-SACs exhibited a high CO2-to-CO faradaic efficiency (FECO) of 92.1% for CO production with a current density of 11.4 mA cm−2 at −0.46 V vs. RHE (Fig. 5i).
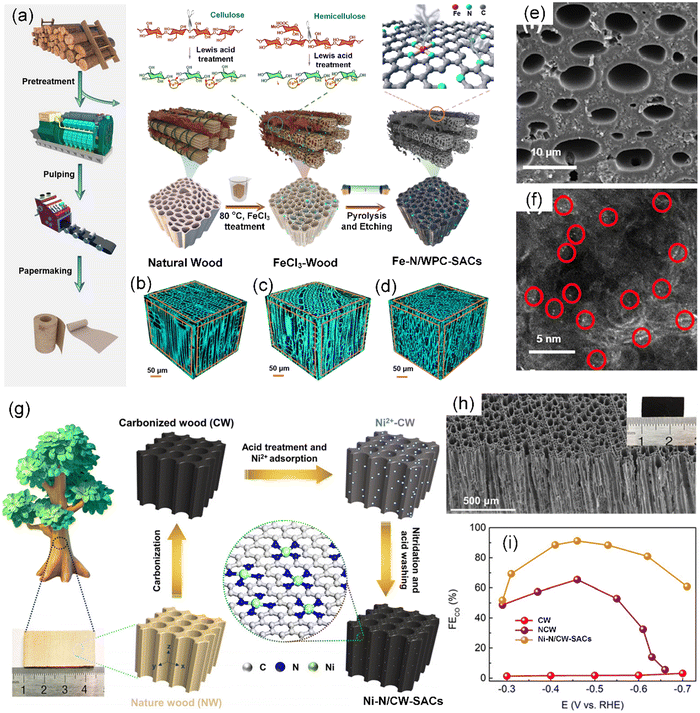 | ||
| Fig. 5 (a) Schematic illustration of the procedure for the fabrication of Fe–N/WPC-SACs. Microscopic three-dimensional (3D) structure of (b) natural wood, (c) FeCl3-wood, and (d) Fe–N/WPC-SACs. (e) SEM image of Fe–N/WPC-SACs. (f) AC HAADF-STEM image of Fe–N/WPC-SACs with the bright points representing Fe atoms. (a)–(f) Reproduced with permission from ref. 112 (Copyright 2021, American Chemical Society). (g) Schematic illustration of the preparation processes of Ni–N/CW-SACs. (h) SEM image of Ni–N/CW-SACs. Inset shows photograph of Ni–N/CW-SACs. (i) FE for CO production on CW, NCW, and Ni–N/CW-SACs electrodes at various applied potentials. (g)–(i) Reproduced with permission from ref. 113 (Copyright 2022, The Royal Society of Chemistry). | ||
P. americana, originating from North America, was introduced in China in 1935 due to its ornamental and medicinal applications.121 Unfortunately, it has become a present invader and has spread across China, especially in the central and southern regions.122 Nevertheless, the ability to accumulate high concentrations of Mn, enables P. americana to be employed as a natural precursor for developing Mn-based CS-SACs. Cui et al. and Yang et al. successfully synthesized novel Mn-SACs via the in situ carbonization of the root and stem of P. americana, respectively.56,123 The as-developed catalysts both formed an Mn–N4 structure, which could efficiently degrade and remove target organic pollutants, such as rhodamine B (RhB) and chloroquine phosphate.
M. aquaticum, a rooted emergent plant native to the Amazon River in South America, typically grows in freshwater streams, ponds, lakes, rivers, and canals.124 This species has been one of the main aquarium plant introduced and sold in China for the past 20 years. M. aquaticum as an ornamental plant offers the benefits of wide distribution, rapid growth, and effective purification ability, which has been successfully employed in constructed wetlands for water treatment.125 Recent studies demonstrated that M. aquaticum has potential to serve as a novel hyperaccumulator of Cd, demonstrating an enrichment capacity of 1.97 mg kg−1.126 Thus, considering its rich porous structure and abundant yield, Li et al. fabricated a novel M. aquaticum-derived Fe SAC via two-step carbonization, activation and graphitization, and chemical etching for peroxymonosulfate (PMS) activation to degrade organic pollutants.127
More strikingly, Fe-based SACs containing FeN4 active sites were also successfully fabricated by utilizing Fe-contaminated fern biomass harvested from the phytoremediation of iron mines, which achieved a high degradation of antibiotic pollutants under natural mild conditions.128
Corn silk, scientifically known as Maydis stigma, serves as a significant waste material from the production of maize crop and is usually collected and disposed as agricultural waste, manure, or animal feed.132 Alternatively, as a biomass raw material, corn silk is mainly composed of carbohydrates, proteins, and vitamins, and has been regarded as an effective precursor for carbon materials due to its natural hollow microtubule structure and abundant micro-channels.133,134 However, there are limited reports on the use of corn silk for the preparation of CS-SACs. It was shown that 3D corn silk-based porous carbon catalysts with dispersed Fe single atoms (Fe–N/CS-SACs) were developed for oxygen reduction/evolution reactions (ORR/OER) through the carbonization of corn silk with ZnCl2, Fe(NO3)3·9H2O, and melamine (Fig. 6a).135 In the simple strategy, ZnCl2 and melamine play two crucial roles in removing the lignin to create numerous micro-/meso-pores and incorporating extra N element with an ultrahigh N content (10 wt%), respectively. The as-prepared catalysts demonstrated a high peak power density of 101 mW cm−2 (Fig. 6b) and maintained a stable discharge–charge voltage gap of 0.73 V for over 44 h (Fig. 6c), showcasing their strong potential for application in zinc–air batteries. The unique hollow tubular structures of corn silk provide more accessible active sites to promote the mass transfer of reactants (Fig. 6d–f).
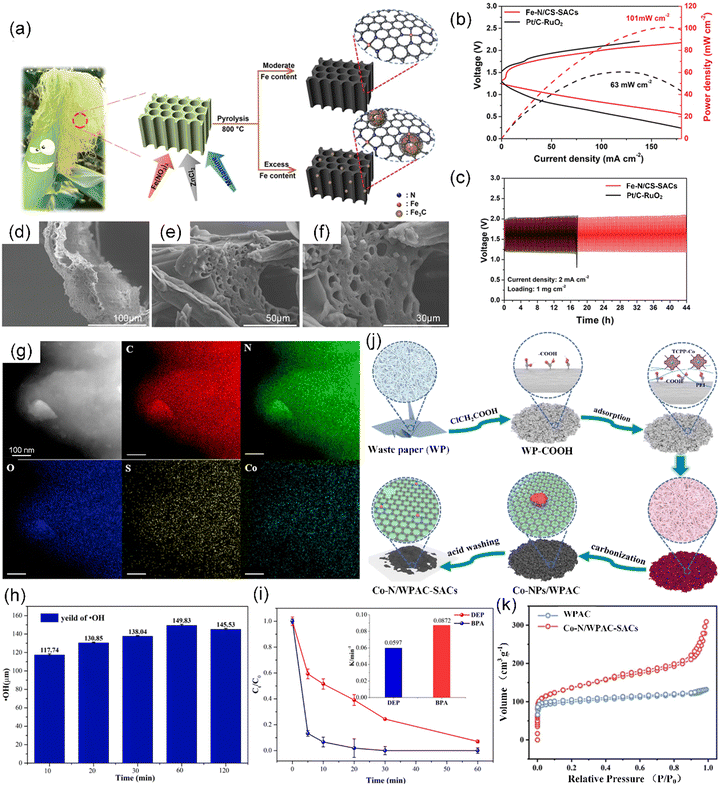 | ||
| Fig. 6 (a) Illustration of the synthesis of Fe–N/CS-SACs. (b) Charge and discharge polarization curves of Pt/C–RuO2 and Fe–N/CS-SACs air electrodes for a rechargeable Zn–air battery and the corresponding power density. (c) Cyclic stability at a current density of 2 mA cm−2. (d)–(f) SEM images of corn silk. (a)–(f) Reproduced with permission from ref. 135 (Copyright 2023, Wiley). (g) Elemental mapping of Co-NS/CS-SACs. (h) Yield of ·OH in the Co-NS/CS-SACs/PMS system, using BA as a probe to quantitatively calculate the yield of ·OH. (i) Diethyl phthalate (DEP) and bisphenol A (BPA) degradation by PMS activated with Co-NS/CS-SACs. (g)–(i) Reproduced with permission from ref. 136 (Copyright 2021, the American Chemical Society). (j) Schematic illustration of the fabrication of Co–N/WPAC-SACs. (k) N2 adsorption–desorption isotherms of Co–N/WPAC-SACs and WPAC. (j) and (k) Reproduced with permission from ref. 137 (Copyright 2023, ScienceDirect). | ||
Spent coffee grounds, the unutilized portion of the coffee beans left after the brewing process, tend to be discarded as solid waste without further utilization.138 The production of instant coffee and coffee brewing produce about 6 million tons of waste coffee grounds worldwide annually, which is estimated to represent 50% of the input mass of coffee feedstock.139 Even post-brewing, coffee grounds retain their inherent value as a valuable resource. Spent coffee grounds consist of significant quantities of organic compounds, including fatty acids, lignin, cellulose, hemicellulose, and various polysaccharides.140 These compounds present an opportunity for extracting and producing valuable products with added value, such as a source of sugar, a precursor for activated carbon, and a sorbent for metal removal.139 Spent coffee grounds have been reported to have a unique microporous structure with a high surface area of 300–1000 m2 g−1.141 In particular, besides micronutrients, used coffee grounds also contain a high content of macroelements, e.g., C, N, P, and K.142 For the first time, Cui et al. obtained novel cobalt–carbon-supported SACs (Co-NS/CS-SACs) via the one-step carbonization of Co-soaked spent coffee grounds, in which Co atoms were anchored on the developed N/S-doped biochar (Fig. 6g).136 The new type of SACs formed Co–N3S1 species via the coordination of Co atoms with N and S in the spent coffee grounds, exhibiting high efficiency in the activation of PMS to degrade various organic pollutants with a high efficiency of 90–100% (Fig. 6h and i).
Generally, waste paper refers to recovered paper, refused paper, scrap paper, secondary paper, discarded paper, used paper, and paper stock, which is a low-cost, renewable, and easily accessible material in daily life.143 Furthermore, the increase in paper consumption generates a large amount of paper waste. Globally, the use of paper and cardboard produces over 100 million tons of paper waste annually.144 Traditionally, waste paper, which typically contains 60–70% cellulose, plays an indispensable role in the circular economy as an essential alternative to wood and other plant-based fibers in the production of recycled paper. Currently, researchers have embarked on efforts aimed at developing multifunctional carbon-based materials from waste paper.145,146 In particular, waste paper-derived active carbon (WPAC) can also be used as a low-cost and robust support to anchor Co atoms for the preparation of Co–N/WPAC-SACs due to its large surface area (535.2 m2 g−1) and abundant pore structures (Fig. 6j and k).137
4.2 Saccharide-containing functional molecules
Polysaccharides, as the largest component of biomass, are an important type of polymeric carbohydrate molecules constructed by joining monosaccharides through glycosidic linkages.147,148 Estimably, more than 90% of carbohydrates in nature is found in the form of polysaccharides. As natural, non-toxic, and biodegradable polymers, polysaccharides are largely exploited from renewable biomass including terrestrial plants, animals, and microorganisms. Among the available biomass, plant polysaccharides are the predominant biomacromolecules in nature, with diverse roles including upholding the integrity of plant tissues as essential structural components (e.g., cellulose, hemicellulose, and pectin) and serving as repositories of energy in the form of starch and fructans.149 In the context of the circular economy, processing plant-derived polysaccharides has become a research opportunity. In this section, saccharide-containing functional molecules such as starch and cellulose in plant biomass will be introduced in the field of CS-SAC fabrication.Recently, due to its high carbon content, renewability, and low cost, starch has been widely developed and broadly used as a desirable carbon precursor in the preparation of various advanced carbon materials.154 For example, Cao et al. synthesized hierarchical porous carbon with a high specific surface area of approximately 2300 m2 g−1 and controlled porosity as an electrode material for supercapacitors by employing biomass starch as the carbon source.155 Hard carbon from starch was successfully prepared as an anode material for a sodium-based seawater battery, which exhibited an increased reversible capacity, current-rate capability, and cycling ability compared to commercial anodes.156 Moreover, Wu et al. further utilized low-cost starch as a carbon source to develop isolated single-atom site metal/N and B co-doped porous carbon (M–NB/PC-SACs, M = Co, Fe, or Ni) catalysts with multi-functional catalytic performance via biomass-assisted carbonization-etching-activation strategy (Fig. 7a).40 In the O-silylation reactions, the M–NB/PC-SACs (M = Co, Fe, or Ni) exhibited excellent catalytic activity of approximately 99% conversion and nearly 100% selectivity (Fig. 7b). Remarkably, starch, an affordable starting material, can be employed in the large-scale production of M–NB/PC-SACs, thereby establishing the foundation for crafting, designing, and synthesizing specific SACs for industrial applications.
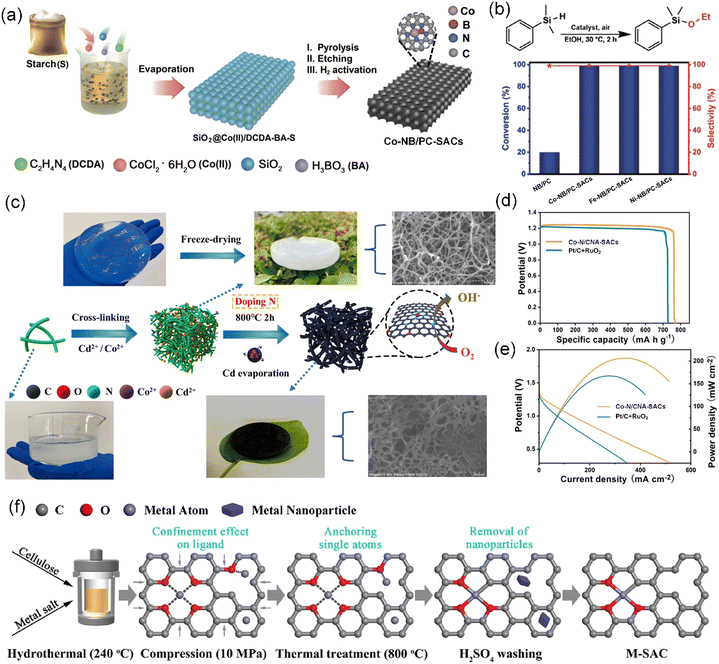 | ||
| Fig. 7 (a) Schematic illustration of the biomass-assisted PEA approach for the preparation of M–NB/PC-SACs (M = Co). (b) Catalysis of the O-silylation reactions of dimethylphenylmethane in the presence of ethanol by different catalysts. (a and b) Reproduced with permission from ref. 40 (Copyright 2022, Springer Nature). (c) Schematic diagram showing the preparation of Co–N/CNA-SACs. (d) Galvanostatic discharge plots and (e) polarization/power density plots of zinc–air batteries with Co–N/CNA-SACs and Pt/C + RuO2 air–cathode. (c)–(e) Reproduced with permission from ref. 157 (Copyright 2023, ScienceDirect). (f) Schematic diagram of the synthesis process of M-SAC. Reproduced with permission from ref. 158 (Copyright 2023, ScienceDirect). | ||
Carbonization is a common method to produce carbon materials from cellulose sources. Cellulose-derived Cu-SACs synthesized via simple one-step calcination displayed excellent catalytic activity and stability in the degradation of oxytetracycline.164 It was revealed that the Cu-SACs showed a high dispersion of Cu atoms. The employed α-cellulose possessed a size of ∼90 μm. Shen et al. effectively harnessed wood-based cellulose nanofibers (TOCNFs), which are rich in hydroxyl and carboxyl groups, to construct a TOCNF-Cd2+/Co2+ complexation aerogel precursor, enabling the successful atomic dispersion of cobalt on N-doped carbon nanofiber aerogel (Co–N/CNA-SACs) after direct carbonization (Fig. 7c).157 Cd salt facilitated the formation of a cross-linked aerogel, resulting in the generation of numerous internal micropores and defects, which in turn promoted the effective physical isolation of the Co atoms. Impressively, the as-designed Co–N/CNA-SACs not only showed superior ORR activity with the half-wave potential reaching 0.855 V but also a superior Zn–air battery performance with a high maximum power density (206 mW cm−2) and impressive specific capacity (769 mA h g−1) (Fig. 7d and e, respectively). In contrast to traditional carbonization methods, Li et al. introduced a novel approach that utilizes active O as anchoring sites and carbon from cellulose to obtain transition metal M-SACs (M = Fe, Co, Ni, Cu, and Zn) with a high metal content of 0.91–1.15 wt% through a combination of hydrothermal anchoring and compression confinement (Fig. 7f).158 This was the first study achieving the direct synthesis of transition metal SACs from raw biomass, capitalizing on the ample carbon and active oxygen species, without introducing other heteroatoms.
4.3 Aromatic compounds
Aromatic compounds are chemical compounds made up of conjugated planar rings with delocalized π-electron clouds instead of single alternating double and single bonds. In plants, a wide variety of aromatic compounds can be found, while the most prevalent are phenolic compounds, which are present in various forms, including flavonoids, phenolic acids, tannins, stilbenes, and lignans.165 Phenolic compounds, a class of chemicals with one or more hydroxyl groups attached to an aromatic ring, are ubiquitous secondary metabolites found in plant seeds, leaves, bark, and flowers, which have attracting increasing attention in recent years.166 In this section, as aromatic-containing functional compounds, lignin and polyphenols are highlighted in the preparation of biobased CS-SACs.Nevertheless, given its low price, high carbon content (>60%), and aromatic structure,171 lignin can be transformed into a variety of carbon materials via controllable carbonization and activation processes, thus achieving value-added applications. For instance, Zhou et al. reported a universal synthesis method to produce SACs on N-doped carbon (M–N/CS-SACs, where M = Fe, Co, Ni, Cu) by carbonizing the phenolic complex of lignin coordinated with transition metal ions (Fig. 8a and b).59 It was shown that lignin-based M–N/CS-SACs can effectively convert holocellulose-derived oxygenates into valuable building blocks and fragrance (Fig. 8c). The cleavage of C–N bonds is significant in organic and biochemical transformations, attracting considerable attention in recent years. Recently, a single Zn atom catalyst (ZnN4-SAC), which was formed by the carbonization of a lignin alkali-coordinated single Zn complex, was found to be a robust heterogeneous non-noble catalyst, enabling the oxidative cleavage of C–N bonds in N-alkylamines and N,N-dialkylamines with the presence of O2 (Fig. 8d and e).173 These technologies not only offer a facile, cost-effective, and scalable approach for producing lignin-based CS-SACs but also pave the way for advancing the utilization of lignin and promoting the growth of the biomass economy.
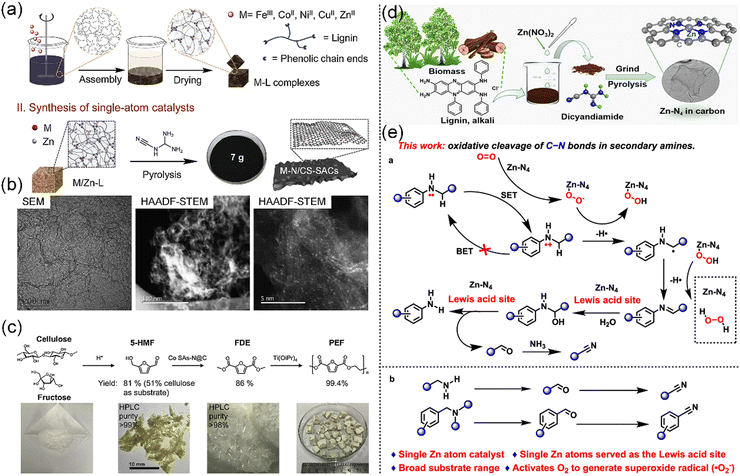 | ||
| Fig. 8 (a) Strategy for the synthesis of M–N/CS-SACs. (b) TEM and HAADF-STEM images of M–N/CS-SACs. (c) Synthesis of polyethylene furanoate (PEF) from C6 sugars. 5-HMF: 5-hydroxymethylfurfural and FDE: 2,5-furandicarboxylic acid dimethyl ester. (a)–(c) Reproduced with permission from ref. 59 (Copyright 2019, ScienceDirect). (d) Procedure for the preparation of the ZnN4-SAC catalyst. (e) Methods for the oxidative cleavage of C–N bonds in N-alkylamines in this work. (d) and (e) Reproduced with permission from ref. 173 (Copyright 2023, the American Chemical Society). | ||
Polyphenols consist of at least two hydroxyl groups and one or more aromatic rings. Common polyphenols usually contain dihydroxyphenyl (catechol) and/or trihydroxyphenyl (galloyl) groups. Particularly, catechol and/or galloyl groups can confer various covalent (e.g., metal ion coordination) and non-covalent interactions (e.g., hydrogen bonding, hydrophobic interactions, electrostatic interactions, π–π stacking and cation–π interactions) to drive the assembly of polyphenol-based materials (nanofilms, particles, and hydrogels) in multidisciplinary areas.174,178 Among them, the coordination between polyphenols and metal ions stands out as one of the extensively studied topics. Multivalent metal ions can trigger the crosslinking of polyphenols without any assistance from heat, electricity, and special solvents, ultimately resulting in the formation of a class of metal–organic materials, i.e., metal–phenolic networks, which are commonly referred to as MPNs.179,180 Due to the universal adherent properties of polyphenols, MPNs can be applied as coatings on diverse substrates or interfaces regardless of their structures and shapes (Fig. 9a and b).36,181,182 Of course, polyphenols and a variety of metal ions can also endow MPNs with different performance and functions (Fig. 9c).183 Furthermore, the metal-driven coordination of phenolic moieties also facilitates the interfacial assembly of colloidal building blocks on the secondary substrates. This polyphenol-based modular approach provides a versatile platform for the construction of complex functional supraparticles,184,185 therapeutically engineered cells,186,187 and photo-driven biohybrids for chemical production.188
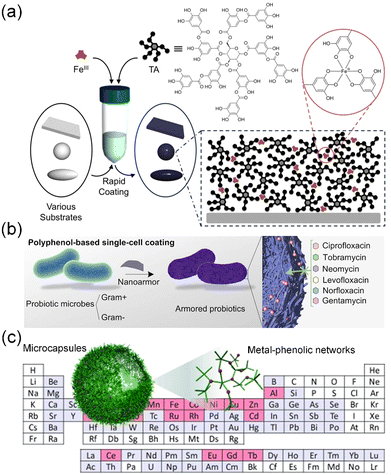 | ||
| Fig. 9 (a) Schematic illustration of MPN assembly by coating TA–Fe coordination complexes on different templates. Reproduced with permission from ref. 36 (Copyright 2013, American Association for the Advancement of Science). (b) Nanoarmor formed by MPN-based single-cell coating enables rapid and highly biocompatible single-cell encapsulation. Reproduced with permission from ref. 182 (Copyright 2022, Springer Nature). (c) One-step assembly process utilizing TA can effectively coordinate diverse metals, resulting in the creation of a broad library of MPN capsules. Reproduced with permission from ref. 183 (Copyright 2014, Wiley). | ||
Polyphenols are promising precursors for the preparation of CS-SACs due to their rich catechol and/or galloyl groups in structure and low price. As one of the most abundant plant polyphenols, TA possesses a highly branched structure resembling that of an octopus, where a central glucose molecule is linked to ten gallic acid units through five diacetyl ester groups. (Fig. 9a).37,181 It is the most widely used in CS-SACs because of the rich pyrogallol groups in its structure and low price. Owing to the absence of heteroatoms such as N element, metal–polyphenol complexes alone cannot be successfully converted into single atoms on polyphenol-derived carbon support.189 Consequently, the introduction of external heteroatoms can assist the fabrication of CS-SACs derived from polyphenols.
A universal synthesis technique of TA-based SACs was developed through one-step carbonization in an NH3 atmosphere, introducing external N heteroatoms, which is not limited to the substrate materials, dimensions, and sizes.37 In this strategy, TA is initially crosslinked with formaldehyde to form a uniform TA-formaldehyde (TAF) coating on various substrates, followed by chelation with metal ions, enabling the preparation of mono- and bi-metallic SACs (e.g., Co, Fe, Mn, Co–Mn, and Fe–Mn) on diverse substrates (e.g., carbon, SiO2, TiO2, and MoS2), dimensions (0D–3D), and sizes (50–5 cm) (Fig. 10a–e). In another example, novel covalently cross-linked poly(HCCP-TA-BPS) nanospheres (PSTA) with a tunable hollow structure were prepared by using hexachlorocyclotriphosphazene (HCCP), TA, and 4,4′-sulfonyldiphenol (BPS) as three comonomers, followed by further coordination of the unreacted catechol groups on TA with metal ions to obtain SACs with Co–N2P2 sites on N/P co-doped mesoporous carbon nanospheres with a small particle size (∼50 nm) and high specific surface area (411.60 m2 g−1) (Fig. 10f and g).189 As expected, the PAST-Co-1000 catalysts designed in this manner demonstrated remarkable electrocatalytic ORR activities in 0.1 M KOH, including high current density (6.4 mA cm−2), excellent durability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous cycles (slight shift in half-wave potential of ∼5 mV), and superior methanol tolerance (Fig. 10h and i).
000 continuous cycles (slight shift in half-wave potential of ∼5 mV), and superior methanol tolerance (Fig. 10h and i).
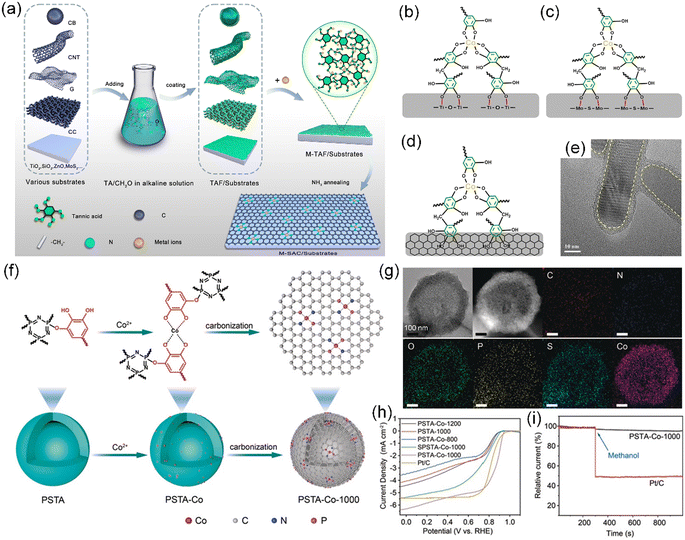 | ||
| Fig. 10 (a) Process for synthesizing SACs on various substrates based on TA chemistry. (b)–(d) Proposed chemical structures of Co-TAF/TiO2, Co-TAF/MoS2 and Co-TAF/carbon, respectively. (e) TEM image of Co-TAF/TiO2. (a)–(e) Reproduced with permission from ref. 37 (Copyright 2022, Wiley). (f) Preparation route to PSTA-Co-1000 hollow carbon nanospheres. (g) TEM image, HAADF-STEM image, and corresponding EDX element mapping of PSTA-Co-1000 nanospheres. (h) LSV curves of PSTA-Co-T (T = 800 °C, 1000 °C, and 1200 °C), PSTA-1000, SPSTA-Co-1000, and Pt/C at a rotating speed of 1600 rpm. (i) Tolerance to methanol by PSTA-Co-1000 compared with the Pt/C electrocatalyst, where the arrow indicates the injection time of methanol. (f)–(i) Reproduced with permission from ref. 189 (Copyright 2020, Wiley). | ||
Generally, plant-based precursors without doping mainly contain O as heteroatoms and can easily keep the original biomass structures in the final carbonized supports. Wood represents a hard biomass and contains a high content of crystalline cellulose, enabling the preservation of pristine structures and morphologies during carbonization. Sawdust and straws are the soft parts where the contained components are easy to hydrolyze into small pieces, further providing globular porous carbonaceous supports. High aromatic precursors such as lignin and polyphenols generally provide stable nonporous structures and a high degree of graphitization, while the cellulose and hemicellulose can undergo decomposition to afford abundant micropores due to the presence of abundant hydroxyl groups. Supports for CS-SACs can have tunable structures and designed properties by the selection of hard or soft plant sources with the corresponding components.
5. CS-SACs from animal biomass
There is an abundant variety of animals on Earth, which can provide biomass precursors for the synthesis of CS-SACs. Compared to plants, animal biomass as a raw material contains much more intricate compositions. In this section, several animal-derived biomass precursors used for the preparation of CS-SACs are summarized, including organ- or tissue-based materials (pig liver, pig blood, cocoon silk, and wool), macromolecular compounds (chitosan and gelatin) as well as small-molecule compounds (dopamine).5.1 Organ- or tissue-based materials
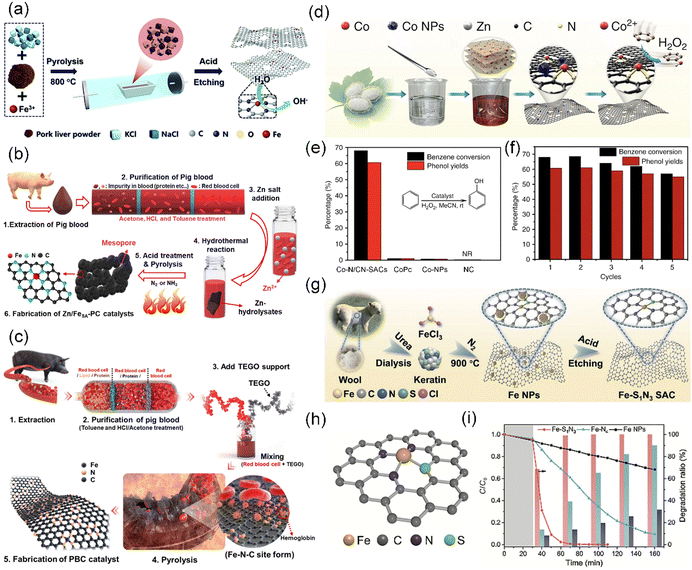 | ||
| Fig. 11 (a) Schematic showing the preparation of Fe–N/CS-SACs. Reproduced with permission from ref. 62 (Copyright 2021, The Royal Society of Chemistry). Schematic illustration of the preparation of (b) Zn-incorporated Fe single-atom porous carbon (designated Zn/FeSA-PC) catalyst and (c) pig blood-derived carbon (PBC) catalyst. (b) Reproduced with permission from ref. 190 (Copyright 2021, The Royal Society of Chemistry). (c) Reproduced with permission from ref. 191 (Copyright 2021, the American Chemical Society). (d) Synthetic process of Co–N/CN-SACs. (e) Comparison of benzene oxidation catalyzed by Co–N/CN-SACs, cobalt phthalocyanine (CoPc), Co nanoparticles/N-doped carbon (Co-NPs), and N-doped carbon (NC). (f) Recycling performance of Co–N/CN-SACs. (d)–(f) Reproduced with permission from ref. 192 (Copyright 2018, Springer Nature). (g) Schematic illustration of synthetic procedure for Fe–S1N3 SAC. (h) Atomic structure three-dimensional model of Fe–S1N3. (i) Removal efficiency of methylene blue (MB), RhB, and phenol using Fe–S1N3, Fe–N4 (single Fe atom supported on N-doped carbon prepared from natural silk fibroin carrier without S), and Fe NPs (Fe nanoparticles/N-doped carbon prepared from natural silk fibroin carrier). (g)–(i) Reproduced with permission from ref. 193 (Copyright 2023, Springer Nature). | ||
Animal blood, particularly pig blood, represents one of the most common byproducts in the meat industry in the world. To the best of our knowledge, this blood is predominantly used as fodder or and thrown away as waste in slaughterhouses, seriously debasing its value.194 It was noted that animal blood biomass contains a key component, namely, hemoglobin, which consists of four globular protein subunits joined by non-protein prosthetic heme groups.190,195 Specifically, the large amount of Fe-porphyrin contained in hemoglobin plays an important role in forming Fe–N–C active sites for the synthesis of SACs.191 Although Jiang et al. developed an efficient single atomic Fe–N–C electrocatalyst via the direct carbonization of pig blood inspired by the biological enzymes containing an Fe–N center,196 there are still difficulties in manufacturing SACs directly from animal blood. The phosphorus lipids and polypeptides in animal blood constituting the cell membrane in blood cells, pollute the catalytic active site during the carbonization process, leading to a reduced performance due to the sintering of the active components.191 Thus, it is important to remove the impurities from pig blood obtained in the initial slaughter before manufacturing CS-SACs through a direct carbonization process. Kim et al. prepared Fe-SACs dispersed on a hierarchically porous structure via a short heat treatment under NH3 conditions after purifying waste pig blood with an organic solvent (acetone, hydrochloric acid, and toluene) (Fig. 11b and c).190,191 Differently, in the carbonization process, Kim et al. added Zn salt as a sacrificial and activation template, while Lee et al. added additional thermal exfoliating graphene oxide with high porosity as a carbon support.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, and remarkable catalytic activity in OER.
000 cycles, and remarkable catalytic activity in OER.
Wool, a type of animal fiber, is mainly obtained by shearing fleece from living animals, such as goats. Wool fiber primarily consists of the animal protein keratin, which is a natural fiber rich in disulfide bonds.193,199 Wool keratin has become a hot topic in recent years considering the regulation of the local coordination environment of SACs on their performance. For instance, Fe–S1N3 SACs embedded in ultrathin 2D porous carbon nanosheets were prepared from natural keratin through precise S and N coordination of the active center via a three-step wool keratin chemical strategy. The Fe–S1N3 SACs could be used for the oxidative degradation of organic pollutant substrates (MB, RhB, and phenol) (Fig. 11g–i).193 The three steps in this wool keratin chemical strategy are as follows: (1) preparation of natural wool keratin, (2) addition of metal salts and carbonization and (3) acid etching.
5.2 Macromolecular compounds
It is imperative to explore innovative approaches to harness the chitosan resource for sustainable development due to the millions of tons of seafood waste disposed of annually. Zhu et al. designed a scalable approach to fabricate gram-scale Co-based SACs anchored on N-doped porous carbon nanobelt (Co–N/PCN-SACs) via the carbonization of chitosan for the selective oxidation of aromatic alkanes in water at room temperature (Fig. 12a).203 By using ZnCl2 and CoCl2 salts as effective activation–graphitization agents, a porous belt-like nanostructure (Fig. 12b) was achieved, as shown by the ultrahigh specific surface area (2513 m2 g−1), large pore volume (2.2 cm3 g−1), and high graphitization degree. In the oxidation of ethylbenzene, the Co–N/PCN-SACs showed a remarkable catalytic efficiency with 98% conversion and 99% acetophenone selectivity (Fig. 12c and d, respectively). By using NH4Cl salt as a foaming agent, Wang et al. controllably synthesized scalable Co-based SACs anchored on an N-doped 3D ultrathin porous carbon sheet derived from chitosan with a super-high specific surface area of 1977.9 m2 g−1.39 Liu et al. reported a highly dispersed Ru catalyst supported by N-doped carbon derived from chitosan (RuN/ZnO/C) with an extremely low loading, which showed an outstanding performance in the reductive catalytic fractionation of lignocellulose (Fig. 12e).204 This Ru catalyst afforded maximum yields of phenolic monomers (46.4 wt%) from lignin, while the cellulose and hemicellulose components were preserved. In this method, Zn species were introduced in the Ru catalyst as Lewis acid centers to promote the hydrogen hydrolysis of the β-O-4 unit.
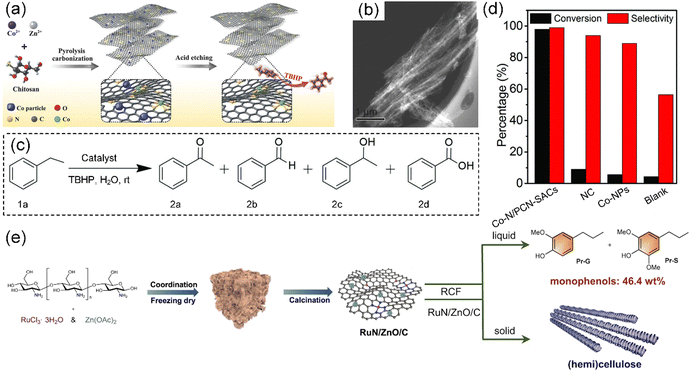 | ||
| Fig. 12 (a) Preparation of Co–N/PCN-SACs. (b) Typical STEM image of Co–N/PCN-SACs. (c) Reaction model of selective oxidation of ethylbenzene. (d) Catalytic activity for ethylbenzene oxidation by Co–N/PCN-SACs, NC (N-doped carbons), Co-NPs (Co nanoparticles), and blank condition. (a)–(d) Reproduced with permission from ref. 203 (Copyright 2018, Wiley). (e) Illustration of the fabrication process for RuN/ZnO/C catalyst and reductive catalytic fractionation of lignocellulose for RuN/ZnO/C catalyst. Reproduced with permission from ref. 204 (Copyright 2022, Springer Nature). | ||
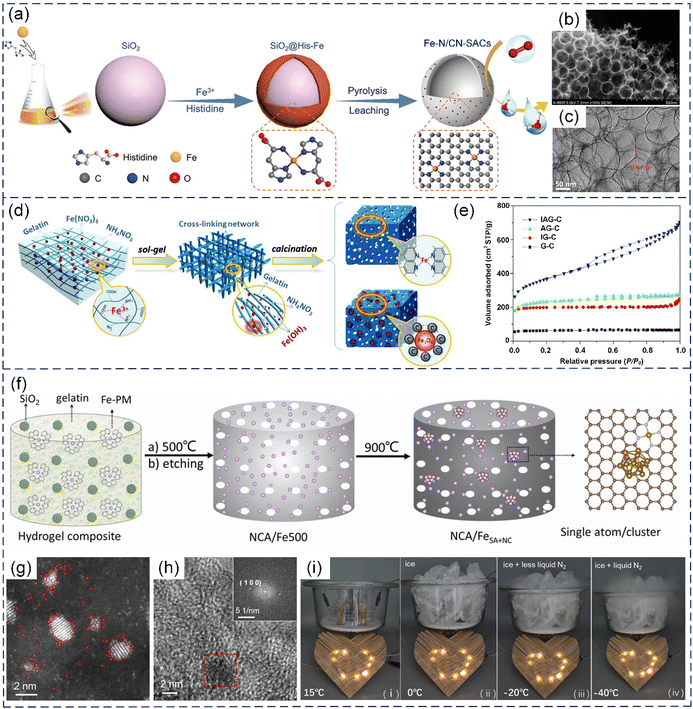 | ||
| Fig. 13 (a) Schematic illustration of the synthetic process, (b) SEM image, and (c) TEM image of Fe–N/CN-SACs. (a)–(c) Reproduced with permission from ref. 63 (Copyright 2018, Wiley). (d) Illustration of the procedure for the preparation of IAG-C catalysts (i.e., Fe–N–C catalyst: Fe(NO3)3, NH4NO3, and gelatin as precursor components) and Fe3O4@AGC (Fe3O4/N-doped carbon hybrid) electrode materials. (e) Nitrogen adsorption–desorption curves of four samples prepared with different precursors (AG-C: ammonium nitrate and gelatin; IG-C: iron nitrate and gelatin; and G-C: pure gelatin). (d) and (e) Reproduced with permission from ref. 207 (Copyright 2015, AAAS). (f) Schematic illustration of the preparation of NCA/FeSA+NC. (g) HAADF-STEM images of NCA/FeSA+NC. (h) High-resolution transmission electron microscopy (HRTEM) image of a typical metal cluster in NCA/FeSA+NC. The inset shows the Fourier transform of the red region. (i) Photographs of an LED pattern powered by two Zn//NCA/FeSA+NC batteries in series at different temperatures. (f)–(i) Reproduced with permission from ref. 210 (Copyright 2023, ScienceDirect). | ||
In addition, carbon aerogels derived from biobased hydrogels, which are 3D materials with rich porosity and nanowrinkles, have been extensively investigated for the preparation of SACs. The spatial confinement of the micropores and nanowrinkles in carbon aerogels not only facilitates the incorporation of isolated metal atoms but also boosts the intrinsic activity of the atomic sites.80,210,211 He et al. prepared RuNx-based SACs coupled with Ru nanoclusters that were embedded in carbon aerogels by using a gelatin-zinc hydrogel (GSi/Ru-PM) as the structural template and SiO2 nanoparticles as the porogen, while using a ruthenium-phenanthroline (Ru-PM) complex as the metal and N sources. The RuNx-based SACs showed a remarkable performance in the hydrogen evolution reaction (HER) in a broad range of solution pH.211 Subsequently, Fe-based SACs with adjacent Fe nanoclusters supported by N-doped carbon aerogels (NCA/FeSA+NC) were fabricated through the facile two-step carbonization of a gelatin hydrogel containing an Fe-phenanthroline (Fe-PM) complex, while nano-SiO2 was used as the template.210 The HAADF-STEM analysis showed that the NCA/FeSA+NC composites contained both metal nanoclusters (under 10 nm in diameter) and individual metal atoms (Fig. 13g and h). The Fe atomic centers displayed a high 3d electron density and reduced magnetic moment because of the adjacent metal nanoclusters, leading to favorable impacts on the electrocatalytic activity and oxidative stability in the ORR and OER. Remarkably, the Zn–air battery assembled using the NCA/FeSA+NC catalysts delivered a freeze-tolerant performance (Fig. 13i).
5.3 Small-molecule compounds
Dopamine (DA) is a type of catecholamine consisting of a 3,4-dihydroxy-substituted phenyl ring, which is widely found in animals, where it is known as a neurotransmitter.212 Marine mussels have a remarkable ability to adhere to nearly all inorganic and organic surfaces due to their adhesive proteins. DA, inspired by the catecholamines present in the adhesive proteins of marine mussels, was utilized for the synthesis of polydopamine (PDA) through a self-polymerization process under alkaline conditions, which could be coated on nearly all natural or synthetic substrates, e.g., metals, oxides, polymers, ceramics, bacteria, and cells (Fig. 14a and b).174,213 In addition, DA is also a type of polyphenol that consists of two hydroxyl groups, one aromatic ring and one ethylamine, possessing the ability to coordinate with metal ions, especially with transition metal ions (Fig. 14c).214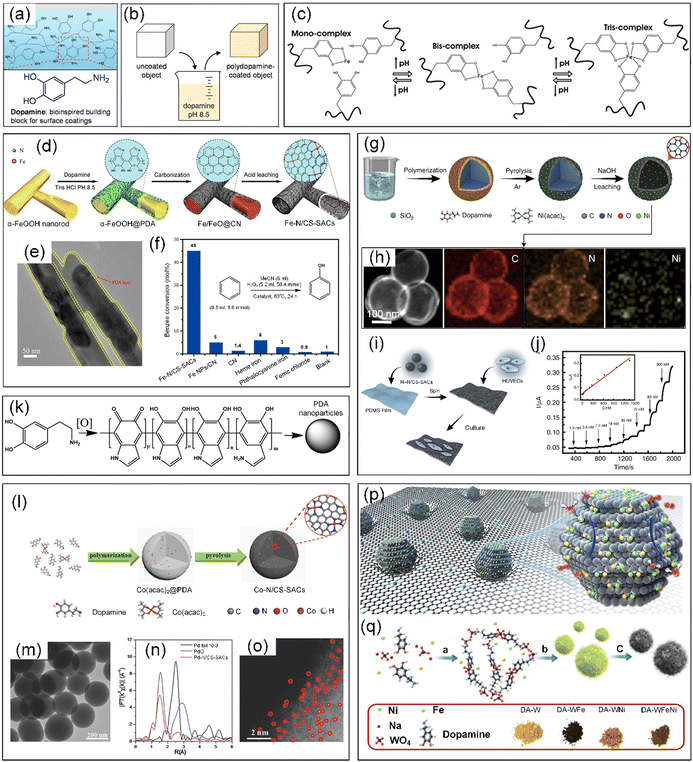 | ||
| Fig. 14 (a) Marine mussel-inspired DA building block for surface coating. (b) Schematic illustration of PDA thin film deposition by dip-coating an object in an alkaline DA solution. (a) and (b) Reproduced with permission from ref. 213 (Copyright 2007, AAAS). (c) Cross-linking reaction between catechol groups in DA and Fe3+ ions. Reproduced with permission from ref. 214 (Copyright 2014, the American Chemical Society). (d) Preparation of Fe–N/CS-SACs. (e) TEM image of α-FeOOH@PDA. (f) Benzene conversion was catalyzed by different materials. (d–f) Reproduced with permission from ref. 78 (Copyright 2017, the American Chemical Society). (g) Synthesis and (h) HAADF-STEM image and corresponding EDX element mapping of Ni–N/CS-SACs. (i) Schematic illustration of the fabrication of Ni–N/CS-SAC-based stretchable sensor for NO sensing and culturing HUVECs: PDMS film (polydimethylsiloxane) and HUVECs (human umbilical vein endothelial cells). (j) Amperometric response of the Ni–N/CS-SAC-based stretchable sensor (0.5 cm2) to the successive addition of NO at +0.80 V. Inset, calibration curve. (g)–(j) Reproduced with permission from ref. 215 (Copyright 2020, Springer Nature). (k) Scheme of the synthesis of PDA nanoparticles by oxidation of DA. Reproduced with permission from ref. 216 (Copyright 2018, the American Chemical Society). (l) Synthesis of Co–N/CS-SAC nanospheres. (m) TEM images of Co–N/CS-SAC nanospheres. (n) Pd K-edge of Pd–N/CS-SAC nanospheres, PdO, and Pd foil. (o) AC HAADF-STEM image of Pd–N/CS-SAC nanospheres. (l)–(o) Reproduced with permission from ref. 217 (Copyright 2018, Wiley). (p) Schematic illustration of WCx–FeNi catalyst. (q) Schematic illustration of the synthetic procedures. (p) and (q) Reproduced with permission from ref. 38 (Copyright 2021, Springer Nature). | ||
Inspired by the universal coating capability of PDA, Zhang et al. synthesized SACs dispersed on hollow N-doped carbon materials (Fe–N/CS-SACs) by carbonizing PDA-coated α-FeOOH nanorods (α-FeOOH@PDA) and subsequent acid leaching (Fig. 14d and e).78 By changing the metal precursors or polymers, N-doped carbon materials anchored by different metal single atoms (M–N/CS-SACs, M = Fe, Co, Ni, Mn, FeCo, FeNi, etc.) were successfully synthesized. Remarkably, the resultant Fe–N/CS-SACs exhibited a high conversion of 45% and high selectivity of 94% in the direct hydroxylation of benzene to produce phenol (Fig. 14f). SiO2 is also frequently used as a template for PDA coatings in the fabrication of CS-SACs.215,218 For instance, Zhang et al. obtained Ni-based SACs anchored on N-doped hollow carbon spheres (Ni–N/CS-SACs) through the carbonization of the formed core–shell SiO2@polydopamine/Ni(acac)2 structure by controlling the Ni loading and carbonization temperature, which were an excellent catalyst for electrochemical oxidation of NO (Fig. 14g).215 The EDX elemental analysis showed that the C, N, and Ni elements in the Ni–N/CS-SACs were homogeneously distributed over the entire architecture (Fig. 14h). Significantly, the electrocatalyst based on Ni–N/CS-SACs enabled the real-time detection of a trace amount of NO released from endothelial cells in response to drug and stretch stimulation (Fig. 14i and j).
In addition, DA can also self-assemble into PDA nanoparticles with a size ranging from several nanometres to hundreds of nanometres in aqueous alkaline solutions or after oxidation treatment, which was employed to manufacture CS-SACs (Fig. 14k).82,174,216,219 Han et al. prepared metal SACs supported by porous N-doped carbon nanospheres with a high surface area of ∼380 m2 g−1 through a facile polymer encapsulation strategy via simply mixing the corresponding metal acetylacetonate complexes with DA monomers (Fig. 14l and m).217 This approach can be used for the preparation of SACs employing both precious and non-precious metals (M-SACs, where M includes Co, Ni, Cu, Mn, and Pd) (Fig. 14n and o). Considering that the strong coordination between heteroatoms and catalytic metal sites affects the electronic environment (d-band center) of the metal atoms, and consequently the catalytic activity, Li et al. introduced a technique to construct atomic or bi-atomic metals (Fe, Ni, and FeNi) on transition metal carbides (TMCs) to yield highly efficient single-atom OER catalysts without heteroatom coordination (Fig. 14p).38 Specifically, WCx–Fe, WCx–Ni, and WCx–FeNi catalysts were obtained by the carbonization of a DA tungstate complex, which was formed by DA molecules with Fe, Ni, or FeNi ions and tungstate ions in a precipitation reaction because of the unique structure of tungsten carbides (Fig. 14q).
Animal-derived biomass possesses more complex compositions compared with plant-based precursors. N atoms are the most similar characteristics of the supports prepared from animal-based precursors, where chitin and proteins are the representative ones. The unpaired electrons in N atoms improve the thermal stability of CS-SACs and enhance the binding between the supports and metal atoms. The intrinsic presence of N avoids the utilization of an external N-source during carbonization, which is ideal at the industrial scale. Animal biomass derived from shells contains abundant N-acetyl groups, resulting in high thermal stability, but also requires deacetylation procedures during pre-carbonization to gain better control of the support structures for CS-SACs. Generally, hybridization is employed animal-based biomass to for structure inheritance after carbonization as precursors from plants. The hard inorganic templates (e.g., SiO2) guide the structure formation in the precursors, further leaving channels or patterns in the resulting supports after carbonization.
6. CS-SACs from microbial biomass
Microbial biomass, especially algae/seaweed-based species, is a promising precursor for the synthesis of SACs due to its high content of heteroatoms and metal ions.52–54,92 In this section, several microbial biomass precursors for the synthesis of CS-SACs are introduced, including eukaryotic organisms (macrofungi, macroalgae, and microalgae) as well as prokaryotic organisms (microalgae and bacteria).6.1 Eukaryotic organisms
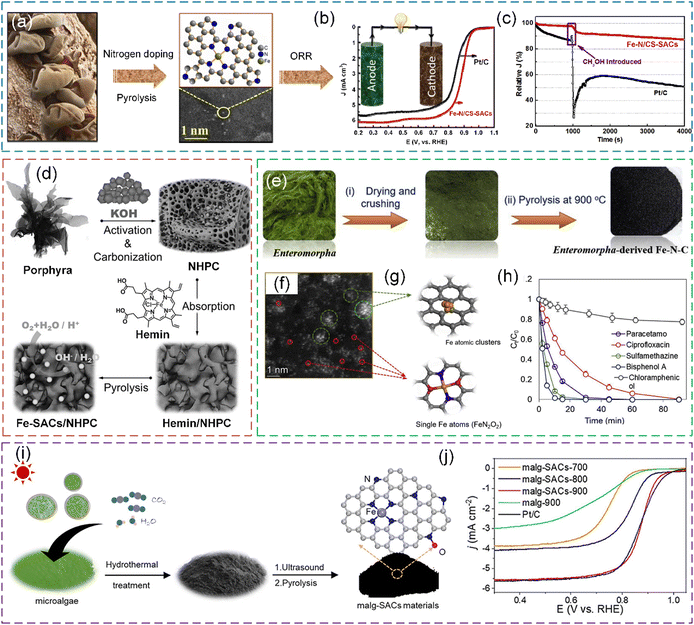 | ||
| Fig. 15 (a) Synthesis process of Fe–N/CS-SACs using AAJ as the precursor. (b) ORR polarization curves of Fe–N/CS-SACs and Pt/C. (c) Methanol resistance of Fe–N/CS-SACs and Pt/C in 0.1 M KOH saturated with O2 at 0.7 V. The purple rectangle shows the addition of 10% (v/v) methanol to the electrochemical cell. (a)–(c) Reproduced with permission from ref. 55 (Copyright 2023, the American Chemical Society). (d) Schematic illustration of the preparation process of Fe-SAC/NHPC electrocatalyst. Reproduced with permission from ref. 222 (Copyright 2017, Wiley). (e) Fabrication scheme and (f) HAADF-STEM image of Enteromorpha-derived Fe–N–C. (g) Simulated structures of Fe atomic clusters and single-atom Fe sites. (h) Degradation of different pollutants in Enteromorpha-derived Fe–N–C/PMS system. (e)–(h) Reproduced with permission from ref. 53 (Copyright 2021, ScienceDirect). (i) Schematic illustration of the procedure for the synthesis of malg-SACs-X from microalgae biomass. (j) Polarization curves for the malg-SACs products and Pt/C catalysts. (i) and (j) Reproduced with permission from ref. 223 (Copyright 2023, ScienceDirect). | ||
Porphyra has inherent heteroatom and abundant amino acids, which is a promising precursor for N-doped carbon227 and an outstanding precursor material for electrocatalyst preparation.228 Zhang et al. reported a strategy to prepare Fe–Nx moiety-modified N-doped hierarchically porous carbons derived from porphyra.227 The resultant electrocatalysts showed an excellent ORR performance. Zhang et al. also used Porphyra to synthesize Fe-based SACs supported by N-doped hierarchically porous carbon (Fe-SACs/NHPC) for the ORR, which exhibited outstanding catalytic activity and catalyst stability (Fig. 15d).222 Cao et al. designed a method to synthesize Fe/Pt single-atom electrocatalysts anchored on N-doped carbon derived from Porphyra biomass, which is potentially used for applications of hydrogen economy.229
Enteromorpha, a kind of green algae in the family Ulvaceae, has abundant nitrogenous compounds, e.g., polysaccharides, vitamins, proteins, and Fe elements.52,230 The high amounts of N and Fe in Enteromorpha can be incorporated in carbonaceous materials after in situ carbonization, converting Enteromorpha from a seawater pollutant into a carbon catalyst for the degradation of organics.231 Peng et al. reported that after the direct carbonization of Enteromorpha, Fe clusters and dispersed Fe–Nx sites derived from the intrinsic Fe in Enteromorpha were observed (Fig. 15e–g).53 Chen et al. designed a process to fabricate an N and Fe co-doped carbonaceous material (Fe–N@C) via the thermal treatment of Enteromorpha, and the as-prepared carbon-based Fe catalysts exhibited high stability, which allowed it to be used for environmental remediation.52 The PMS activation of Enteromorpha-derived carbocatalyst was investigated in the studies by Peng et al. and Chen et al. (Fig. 15h).
Scenedesmus obliquus NIES-2280, a typical freshwater chlorophyte with a rapid growth rate, possesses a robust uptake capacity of CO2 and nutrient elements (N and P).233 Particularly, the membrane-bound cells have abundant metal-containing biomolecules that could be used as metal sources.223,234 Ma et al. reported a method to prepare Fe-SACs for ORR through the direct conversion of Scenedesmus obliquus NIES-2280 using endogenous metals and introducing an exogenous N source from g-C3N4 (Fig. 15i and j).223 In the high-temperature carbonization, g-C3N4 plays a critical role in the formation of hierarchical porous structures and doping N heteroatom.
6.2 Prokaryotic organisms
Microbial biomass is an important non-negligible source for CS-SACs, typically algae, due to their rapid growth, good environmental tolerance, and solar energy storage. The rich nutrient contents enable the introduction of diverse heteroatoms and structures in the derived carbonaceous supports. These precursors are a promising sustainable energy feedstock and the alternatives for fossil fuels as the consumption of greenhouse gas during their growth. The main disadvantages also come from the high nutrients due the production of a wide product distribution. Extraction is preferred to remove the lipids from bio-oil products in the context of biorefinery. The disadvantages of microbes are the diverse and versatile characteristics and qualities of the generated CS-SACs from different species. Accordingly, pre-treatment such as extraction is recommended prior to carbonization.
7. Conclusions and perspective
Biomass found in nature offers not only captivating bio-inspired strategies for materials science but also a versatile toolbox of natural building blocks for the creation and development of innovative functional materials. The development of SACs using biomass sources as fundamental building blocks has accelerated the progress in the field of catalysis, opening up a new avenue for converting biomass into value-added products. Biomass-derived carbon materials provide an excellent support for SACs, enabling the in situ incorporation of metal atoms in the carbon matrix, while the inherent O, N, and S elements in many biomasses even waste biomass can serve as for anchoring the metal atoms. Moreover, naturally derived biomass, such as chitosan, starch, gelatin, cellulose, lignin, and polyphenol, can also be used as alternative ligands or an N-rich source for in situ doping and complexation during the synthesis of CS-SACs. This review highlighted the recent advancements related to the synthesis strategies, different types of biomass precursors, geometric and electronic properties and catalysis applications of various CS-SACs derived from biomass based on the considerable number of relevant published articles at the current stage. The various biomasses employed for the preparation of CS-SACs are summarized in Table 2. Particularly, these biomass-derived CS-SACs have wide potential in different application fields, including electrochemistry, fuel cells, water treatment, biosensors, and fine chemicals, which can realize the high-value utilization of biomass sources and are conducive to sustainable development.| Biomass | Metal source | Heteroatom source | SACs | Loading (wt%) | Configuration | Catalytic application | Ref. |
|---|---|---|---|---|---|---|---|
| Natural wood | FeCl3·6H2O | Ar-NH3 | SAC-FeN-WPC | 0.8 | Fe–N4 | ORR/OER/Zn–air batteries | 112 |
| Ni(NO3)2·6H2O | Urea | Ni SAs-NCW | 0.13 | Ni–N4 | CO2 reduction reaction | 113 | |
| P. americana | Accumulating Mn from MnCl2·4H2O | Urea | Mn SAC | 4.44, 1.13 | Mn–N4 | PMS-driven organics degradation | 56, 123 |
| M. aquaticum | K2FeO4 | — | ISA-Fe/MC | 2.4 | — | PMS-driven phenol degradation | 127 |
| Waste ferns | Fern contaminated by Fe mines | NH3·H2O | Fe SAC | 0.04 | Fe–N4 | PMS-driven quinolone antibiotic degradation | 128 |
| Corn silk | Fe(NO3)3·9H2O | Melamine | Fe SA/NCZ | 4.30 | Fe–N4 | ORR/OER/Zn–air batteries | 135 |
| Coffee grounds | CoCl2·6H2O | Intrinsic N, S | Co SAC | 1.13 | Co–N3S1 | PMS-driven organics degradation | 136 |
| Waste paper | TCPP-Co | Polyethyleneimine | Co–N3/WPAC | — | Co–N3 | Persulfate (PS)-driven sulfisoxazole degradation | 137 |
| Starch | Metal chlorides | Dicyandiamide, boric acid | ISAS M/NBPC (M = Co, Fe, or Ni) | 1.5–2.1 | M–N3B1 | Various catalytic reactions | 40 |
| Cellulose | Cu(NO3)2·3H2O | 1,10-Phenanthroline monohydrate (PM) | Cu-SACs | 4.08 | Cu–N4 | PMS-driven oxytetracycline degradation | 164 |
| Co(CH3COO)2·4H2O | NH4Cl | Co SAs/NCNA | 0.11 | Co–N4 | ORR/Zn–air batteries | 157 | |
| Metal nitrates | Inherent C and O | M-SACs (M = Fe, Co, Ni, Cu, and Zn) | 0.29–1.15 | M–O3C | Tetracycline photocatalytic removal | 158 | |
| Lignin | Metal nitrates | Dicyandiamide | M SAs-N@C (M = Fe, Co, Ni, Cu) | 1.47–5.44 | M–N3C | An integrated biorefinery | 59 |
| Zn(NO3)2 | Lignin alkali | ZnN4-SAC | 0.67 | Zn–N4 | Oxidative cleavage of C–N bonds | 173 | |
| Polyphenol | Metal nitrates | NH3 | M-SAC (M = Co, Fe, Mn, Co–Mn, Fe–Mn) | 2.09–5.50 | M–N4 | Oxygen reduction and photocatalytic activity | 37 |
| Co(OAc)2·4H2O | HCCP | PAST-Co-1000 | 10.12 | Co–N2P2 | ORR | 189 | |
| Pig liver | Fe(NO3)3 | N in protein | Fe–N/C-SAC | 2.60 | Fe–N4 | ORR | 62 |
| Pig blood | Fe-porphyrin | N in hemoglobin | Fe–N–C | — | Fe–Nx | ORR | 196 |
| — | — | Fe SACs | 0.5–1.3, 1.3–1.9 | Fe–N4 | Zn–air batteries/anion exchange membrane fuel cells | 190, 191 | |
| Silk fibers | Metal nitrates | N in silk fibroin | M-ISA/CNS (M = Fe, Co, Ni) | 0.6 | Co–N4 | Hydroxylation of benzene to phenol | 192 |
| FeCl3·6H2O | — | Fe–Nx–C | 0.12 | Fe–N4 | ORR | 198 | |
| Wool | FeCl3·6H2O | N and S in protein keratin | Fe–S1N3 SACs | 1.16 | Fe–S1N3 | Fenton-like catalysis | 193 |
| Chitosan | CoCl2·6H2O | Inherent N | Co-ISA/CNB | 0.1 | Co–N4 | Oxidation of ethylbenzene | 203 |
| Co(Ac)2·4H2O | — | Co SANC | 1.27 | Co–N4 | ORR/metal–air batteries | 39 | |
| RuCl3·3H2O | — | RuN/ZnO/C | 0.12 | Ru–N4 | Reductive catalytic fractionation of lignocellulose | 204 | |
| Na2MoO4·2H2O | — | Mo-SAs | 1.32 | Mo–N1C2 | HER | 206 | |
| Histidine | Fe(NO3)3·9H2O | Inherent N | Fe–N–C HNSs | 1.4 | Fe–N4 | ORR | 63 |
| Gelatin | Fe(NO3)3·9H2O | Inherent N | Fe–N–C | 0.14 | Fe–N4 | ORR | 207 |
| FeCl2·4H2O | Inherent N | NCAC–Zn/Fe | 0.72 | Fe–N4 | ORR/OER/Zn–air batteries | 80 | |
| RuCl3 | PM | NCAG/Ru | 0.47 | RuNxC4−–O | HER | 211 | |
| FeCl2·4H2O | PM | NCA/FeSA+NC | 1.73 | Fe–N4 | ORR/Zn–air batteries | 210 | |
| Dopamine | Metal(hydr)oxides | Inherent N | SA-M/CN (M = Fe, Co, Ni, Mn, FeCo, FeNi) | 0.9 | Fe–N4 | Hydroxylation of benzene to phenol | 78 |
| Metal phthalocyanine (MPc) | — | MPc@DNHCS-T (M = Fe, Co, Ni) | 0.63–0.77 | Co–N4 | CO2 electroreduction and Zn–CO2 batteries | 218 | |
| Ni(acac)2 | — | Ni SACs/N–C | 0.1 | Ni–N4 | Electrochemical oxidation of NO | 215 | |
| FeCl3 | — | Fe–N–C | 0.3 | Fe–N4 | ORR | 82 | |
| Metal acetylacetonates | — | M-ISAS/p-CN (M = Co, Ni, Cu, Mn, Pd, etc.) | 0.42 | M–N4 | ORR | 217 | |
| Metal nitrates | Na2WO4·2H2O | WCx–M (M = Fe, Ni, FeNi) | 0.62–1.64 | M–WCx | OER | 38 | |
| AAJ | Inherent Fe | Inherent N | Fe-ISA/NC | 0.10 | Fe–N4 | ORR | 55 |
| Sodium alginate | Metal nitrates | Melamine | A-M/r-GO (M = Co, Zn, Cu, Ni) | 3.6 | Co–N3C | ORR/Zn–air batteries | 226 |
| FeCl3 | Melamine | FeNC | 0.4 | Fe–N4 | ORR/Zn–air batteries | 225 | |
| Porphyra | Iron phthalocyanine (FePc) | Inherent amino acids | Fe–Nx/HPC | 0.35 | Fe–Nx | ORR | 227 |
| FeCl3·6H2O | — | Fe–NSDC | 0.32 | Fe–N3S | ORR/OER/Zn–air batteries | 228 | |
| Natural hemin | — | SA-Fe/NHPC | 0.48 | Cl–Fe–N4 | ORR | 222 | |
| PtCl4, inherent Fe | Urea | Fe1Pt1/NC | 0.166, 2.29 | Fe–N4, Pt–N4 | ORR/HER | 229 | |
| Enteromorpha | Inherent Fe | Inherent N | Fe–N–C | 0.84 | Fe–N2O2 | PMS-driven organics degradation | 53 |
| Inherent Fe | Inherent N | Fe–N@C | 0.60 | Fe–Nx | PMS-driven organics degradation | 52 | |
| Fe(NO3)3 | Melamine | FeSA-NEPBC | 1.3 | Fe–N4 | Peroxydisulfate (PDS)-driven organics degradation | 231 | |
| Microalgae | Co(NO3)2·6H2O | Inherent amino groups | Co–N/C-SAC | 3.1 | Co–N4 | ORR | 35 |
| Endogenous metals | g-C3N4 | malg-SACs | 1.98 | Fe–N4 | ORR/Zn–air batteries | 223 | |
| MnCl2·6H2O | Dicyandiamide | SA-Mn-NSC | 7.6 | Mn–N4 | PMS-driven organics degradation | 236 | |
| Inherent Fe | g-C3N4, inherent S | FeSA/FeONC/NSC | 0.25 | Fe–N4 | ORR/Zn–air batteries | 54 | |
| Bacteria | Inherent Fe, metal salts (Mn+ = Ir, Ru, Pt, Cu, or Pd) | — | SA-M (M = Fe, Ir, Pt, Ru, Cu, or Pd) | 1 | M–N3, Fe–N4, M–Fe | HER/OER | 88 |
| Co(SO4)3·7H2O, Fe2(SO4)3·10H2O | Oxygen groups | Fe–Co SAs | 0.10–0.22, 0.08–0.19 | Fe–O3, Co–O3, Fe–Co | Nitrogen reduction reaction (NRR) | 239 | |
The remarkable activity of SACs is predominantly attributed to the abundant presence of active sites. Despite the numerous natural biomasses that have recently been used to prepare CS-SACs, the synthesis of CS-SACs with a high metal loading (>10 wt%) remains an unsolved challenge. Generally, increasing the metal loading can further enhance the catalytic performance. Nevertheless, the high metal loading and stability of single atomic dispersions are usually mutually exclusive, which is an inherent dilemma in classical material design for SACs. The individual metal atoms easily undergo migration, agglomeration, and sintering during preparation and catalytic reactions due to their high surface energy, affecting the stability of SACs and reducing their overall catalytic activity. A high metal loading and long-term stability are the aim to realize ideal SACs. Natural biomass resources can open a new pathway for improving the stability of CS-SACs from their intrinsic structural and functional elements. Typically, the hard biomass can provide carbonaceous structures with abundant storage sites for metal species and fast transport paths for electrolyte ions via the regularly arranged fibers or stacked layers. The channels or porous structures increase the specific surface areas for active center exposure for both metal binding and mass transfer during catalysis. Purity is the first key factor to realize control of the structures and properties in prepared SACs. Biomass carbonaceous precursors contain versatile components, where activation by thermal treatment or chemical reagents enables the full exposure of the surface for active center attachments. However, their main drawback is the removal of the impurities generated during activation. Energy consumption is also a concern in green development.
Although biomass has been employed to fabricate abundant, low-cost, and highly porous CS-SACs to date, further research on the preparation of biomass-derived CS-SACs is still urgently needed to drive advancements across various applications.
(1) Biomass waste has been largely disposed of by landfills annually, where being a precursor for CS-SACs can facilitate the full advantages of its utilization. At present, a few types of biomass waste materials, such as corn silk, spent coffee grounds, waste paper, pig liver, and blood, have been reported to fabricate CS-SACs and can affect the ultimate structural morphology. Fibrous and lamellar structures are easily obtained from bamboo and wood after their simple pre-treatment with alkaline solutions. These structures provide improved specific surface area for single-atom attachment. Furthermore, plant-derived biomass possess advantages in constructing SACs with macroscopic shapes for large-scale catalytic applications. Protein-sourced biomass such as silks and hairs possess fibrous structures but are much shorter in length compared with cellulose-based biomass, which can undergo structure inheritance carbonization to preserve their high aspect ratio morphologies at the microscale. The restructuring strategy involves the decomposition of precursors into small parts or dissolution into molecular chains, providing chances to shape the precursors and ultimate carbonaceous structures into multiple morphologies for versatile applications such as microspheres, tubes, aerogels, and sponge-like networks.
(2) Despite the abundant biomass resources on Earth, most of the current research is still limited to the lab-scale. The high-temperature carbonization with high levels of energy consumption dominates the conventional preparation of biomass-derived CS-SACs, which is a major concern for future sustainability. Thus, to realize the industrialization of biomass-derived CS-SACs, less energy input is necessary and demanded. Deep exploration of the inherent capabilities of certain living biomass organisms for SAC preparation can potentially serve as alternatives to conventional carbonization methods. Using the extracellular electron transfer (EET) pathway of bacteria is a typical representation. The electroactive bacterium GS88 possesses a surface network of multiheme. Oxidation reactions occur in their cytoplasm and the electrons are transferred to the acceptors (e.g., metal ions) located outside their cells to afford reduced single atoms, which are further fixed and stabilized by the surface cytochromes. This highly efficient and green strategy has the potential to achieve industrialization in the future.
(3) Biomass-derived CS-SACs have attracted significant research attention in electrocatalysis, photocatalysis, and advanced oxidation processes. Thus, novel applications of these SACs are essential and promising to be explored. Enzymes are highly efficient and specific catalysts for bioreactions, where the enzyme-mimic characteristics of SACs show potential in biomedical applications, such as cancer therapy, antibacterial therapy, biosensing, oxidative-stress cytoprotection, and sepsis management. Biomass-derived precursors possess intrinsic biosafety, paving the way for their translation into medical devices or agents for bioreactions both in vitro and in vivo.
(4) Most CS-SACs derived from biomass primarily focus on the M–Nx–C sites, with limited research attention devoted to metal sites coordinated with other heteroatoms such as S, O, P, and B. Although the direct doping of heteroatoms in the biomass organism may appear uncomplicated, it often results in an unpredictable heteroatom doping content and an unclear doping mechanism. Some natural biomass-derived chemicals (e.g., polyphenol) possess well-defined chemical structures and abundant functional groups, making heteroatom groups easier to modify, and thus should be worthy of further development in the future.
(5) At present, there is still a lack of universal calculations to predict the composition, relative content of diverse biomass materials and components, synthesis conditions, and physicochemical properties of the resulting carbon materials (e.g., element type and functional groups). Consequently, obtaining ideal CS-SACs from biomass resources in a timely and precise manner remains a challenge. Incorporating machine learning techniques will further advance the systematic design and precise fabrication of biomass-derived CS-SACs. Although there have been some reports on the relationship between SAC structures and their corresponding applications, systematic information and analysis integrating the biomass types, carbon support structure, metal coordination configuration, carbonization conditions, and performance are still missing. We obtained qualitative evaluation on biomass and its derived SACs and collecting more detailed information will assist our understanding on the semi-quantitative and quantitative levels. Consequently, relevant information about biomass is effectively integrated, enabling the timely extraction of valuable insights to efficiently establish complex CS-SACs. It is essential to perform fundamental studies on the effects of the biomass chemical characteristics on the catalytic efficiency of CS-SACs. Finally, the employment of biomass waste may contribute to global waste minimization.
Conflicts of interest
The authors declare no competing interests.Acknowledgements
We acknowledge financial support from the National Key R&D Program of China (2022YFA0912800), National Excellent Young Scientists Fund (00308054A1045), National Natural Science Foundation of China (22178233, 22108181), Talents Program of Sichuan Province, Double First-Class University Plan of Sichuan University, State Key Laboratory of Polymer Materials Engineering (sklpme 2020-03-01), Tianfu Emei Program of Sichuan Province (2022-EC02-00073-CG), Postdoctoral special funding of Sichuan Province (TB2022063) and the Postdoctoral Funding of Sichuan University (2022SCU12099).Notes and references
- H. Jiang, W. Yang, M. Xu, E. Wang, Y. Wei, W. Liu, X. Gu, L. Liu, Q. Chen, P. Zhai, X. Zou, P. M. Ajayan, W. Zhou and Y. Gong, Nat. Commun., 2022, 13, 6863 CrossRef CAS PubMed.
- J. Y. Liu, ACS Catal., 2017, 7, 34–59 CrossRef CAS.
- L. Minmin, W. Linlin, Z. Kangning, S. Shanshan, S. Qinsi, Z. Lei, S. Xueliang, Z. Yufeng and Z. Jiujun, Energy Environ. Sci., 2019, 12, 2890–2923 RSC.
- G. Gao, Y. Jiao, E. R. Waclawik and A. Du, J. Am. Chem. Soc., 2016, 138, 6292–6297 CrossRef CAS PubMed.
- L. Lin, W. Zhou, R. Gao, S. Yao, X. Zhang, W. Xu, S. Zheng, Z. Jiang, Q. Yu, Y. W. Li, C. Shi, X. D. Wen and D. Ma, Nature, 2017, 544, 80–83 CrossRef CAS PubMed.
- X. F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Acc. Chem. Res., 2013, 46, 1740–1748 CrossRef CAS PubMed.
- J. Yang, B. Chen, X. Liu, W. Liu, Z. Li, J. Dong, W. Chen, W. Yan, T. Yao, X. Duan, Y. Wu and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 9495–9500 CrossRef CAS PubMed.
- C. Zhu, S. Fu, Q. Shi, D. Du and Y. Lin, Angew. Chem., Int. Ed., 2017, 56, 13944–13960 CrossRef CAS PubMed.
- Y. C. Yao, S. L. Hu, W. X. Chen, Z. Q. Huang, W. C. Wei, T. Yao, R. R. Liu, K. T. Zang, X. Q. Wang, G. Wu, W. J. Yuan, T. W. Yuan, B. Q. Zhu, W. Liu, Z. J. Li, D. S. He, Z. G. Xue, Y. Wang, X. S. Zheng, J. C. Dong, C. R. Chang, Y. X. Chen, X. Hong, J. Luo, S. Q. Wei, W. X. Li, P. Strasser, Y. E. Wu and Y. D. Li, Nat. Catal., 2019, 2, 304–313 CrossRef CAS.
- L. N. Cao and J. L. Lu, Catal. Sci. Technol., 2020, 10, 2695–2710 RSC.
- Y. F. Ma, X. H. Zhang, L. N. Cao and J. L. Lu, Catal. Sci. Technol., 2021, 11, 2844–2851 RSC.
- Y. Y. Feng, Y. X. Guan, H. J. Zhang, Z. Y. Huang, J. Li, Z. Q. Jiang, X. Gu and Y. Wang, J. Mater. Chem. A, 2018, 6, 11783–11789 RSC.
- K. Jiang, M. Luo, M. Peng, Y. Q. Yu, Y. R. Lu, T. S. Chan, P. Liu, F. M. F. de Groot and Y. W. Tan, Nat. Commun., 2020, 11, 2701 CrossRef CAS PubMed.
- Q. M. Sun, N. Wang, T. J. Zhang, R. Bai, A. Mayoral, P. Zhang, Q. H. Zhang, O. Terasaki and J. H. Yu, Angew. Chem., Int. Ed., 2019, 58, 18570–18576 CrossRef CAS PubMed.
- Y. Hou, M. Qiu, M. G. Kim, P. Liu, G. T. Nam, T. Zhang, X. D. Zhuang, B. Yang, J. Cho, M. W. Chen, C. Yuan, L. C. Lei and X. L. Feng, Nat. Commun., 2019, 10, 1392 CrossRef PubMed.
- M. M. Fan, J. W. Cui, J. J. Wu, R. Vajtai, D. P. Sun and P. M. Ajayan, Small, 2020, 16, 1906782 CrossRef CAS PubMed.
- H. M. Zhang, W. H. Liu, D. Cao and D. J. Cheng, iScience, 2022, 25, 104367 CrossRef CAS PubMed.
- Y. Chen, S. Ji, Y. Wang, J. Dong, W. Chen, Z. Li, R. Shen, L. Zheng, Z. Zhuang, D. Wang and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 6937–6941 CrossRef CAS PubMed.
- K. Gao, B. Wang, L. Tao, B. V. Cunning, Z. Zhang, S. Wang, R. S. Ruoff and L. Qu, Adv. Mater., 2019, 31, 1805121 CrossRef PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- Y. C. Yang, Y. W. Yang, Z. X. Pei, K. H. Wu, C. H. Tan, H. Z. Wang, L. Wei, A. Mahmood, C. Yan, J. C. Dong, S. L. Zhao and Y. Chen, Matter, 2020, 3, 1442–1476 CrossRef.
- D. N. Zhou, X. Y. Li, H. S. Shang, F. J. Qin and W. X. Chen, J. Mater. Chem. A, 2021, 9, 23382–23418 RSC.
- C. Liu, H. Li, F. Liu, J. S. Chen, Z. X. Yu, Z. W. Yuan, C. J. Wang, H. L. Zheng, G. Henkelman, L. Wei and Y. Chen, J. Am. Chem. Soc., 2020, 142, 21861–21871 CrossRef CAS PubMed.
- H. Fei, J. Dong, M. J. Arellano-Jiménez, G. Ye, N. Dong Kim, E. L. Samuel, Z. Peng, Z. Zhu, F. Qin, J. Bao, M. J. Yacaman, P. M. Ajayan, D. Chen and J. M. Tour, Nat. Commun., 2015, 6, 8668 CrossRef CAS PubMed.
- H. B. Yang, S. F. Hung, S. Liu, K. D. Yuan, S. Miao, L. P. Zhang, X. Huang, H. Y. Wang, W. Z. Cai, R. Chen, J. J. Gao, X. F. Yang, W. Chen, Y. Q. Huang, H. M. Chen, C. M. Li, T. Zhang and B. Liu, Nat. Energy, 2018, 3, 140–147 CrossRef CAS.
- X. Fu, P. Zamani, J. Y. Choi, F. M. Hassan, G. Jiang, D. C. Higgins, Y. Zhang, M. A. Hoque and Z. Chen, Adv. Mater., 2017, 29, 1604456 CrossRef PubMed.
- X. X. Wang, D. A. Cullen, Y. T. Pan, S. Hwang, M. Wang, Z. Feng, J. Wang, M. H. Engelhard, H. Zhang, Y. He, Y. Shao, D. Su, K. L. More, J. S. Spendelow and G. Wu, Adv. Mater., 2018, 30, 1706758 CrossRef PubMed.
- H. T. Chung, D. A. Cullen, D. Higgins, B. T. Sneed, E. F. Holby, K. L. More and P. Zelenay, Science, 2017, 357, 479–484 CrossRef CAS PubMed.
- W. Q. Zhang, X. H. Qin, T. R. Wei, Q. Liu, J. Luo and X. J. Liu, J. Colloid Interface Sci., 2023, 638, 650–657 CrossRef CAS PubMed.
- S. Liu, M. M. Jin, J. Q. Sun, Y. J. Qin, S. S. Gao, Y. Chen, S. S. Zhang, J. Luo and X. J. Liu, Chem. Eng. J., 2022, 437, 135294 CrossRef CAS.
- X. He, Q. He, Y. Deng, M. Peng, H. Chen, Y. Zhang, S. Yao, M. Zhang, D. Xiao, D. Ma, B. Ge and H. Ji, Nat. Commun., 2019, 10, 3663 CrossRef PubMed.
- Y. Wang, M. Zhang, X. Shen, H. Wang, H. Wang, K. Xia, Z. Yin and Y. Zhang, Small, 2021, 17, 2008079 CrossRef CAS PubMed.
- H. He, R. Zhang, P. Zhang, P. Wang, N. Chen, B. Qian, L. Zhang, J. Yu and B. Dai, Adv. Sci., 2023, 10, 2205557 CrossRef CAS PubMed.
- M. B. Gawande, P. Fornasiero and R. Zboril, ACS Catal., 2020, 10, 2231–2259 CrossRef CAS.
- D. Wu, J. Hu, C. Zhu, J. Zhang, H. Jing, C. Hao and Y. Shi, J. Colloid Interface Sci., 2021, 586, 498–504 CrossRef CAS PubMed.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. van Koeverden, G. K. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- H. F. Wang, X. P. Li, Y. Jiang, M. H. Li, Q. Xiao, T. Zhao, S. Yang, C. H. Qi, P. P. Qiu, J. P. Yang, Z. Jiang and W. Luo, Angew. Chem., Int. Ed., 2022, 61, e202200465 CrossRef CAS PubMed.
- S. Li, B. Chen, Y. Wang, M. Y. Ye, P. A. van Aken, C. Cheng and A. Thomas, Nat. Mater., 2021, 20, 1240–1247 CrossRef CAS PubMed.
- Y. Q. Wang, B. Y. Yu, K. Liu, X. T. Yang, M. Liu, T. S. Chan, X. Q. Qiu, J. Li and W. Z. Li, J. Mater. Chem. A, 2020, 8, 2131–2139 RSC.
- T. Wu, S. Li, S. Liu, W. C. Cheong, C. Peng, K. Yao, Y. Li, J. Wang, B. Jiang and Z. Chen, Nano Res., 2022, 15, 3980–3990 CrossRef CAS.
- Z. Q. Lin, M. Escudero-Escribano and J. Li, J. Mater. Chem. A, 2022, 10, 5670–5672 RSC.
- N. C. Cheng, L. Zhang, K. Doyle-Davis and X. L. Sun, Electrochem. Energy Rev., 2019, 2, 539–573 CrossRef.
- Y. J. Chen, S. F. Ji, C. Chen, Q. Peng, D. S. Wang and Y. D. Li, Joule, 2018, 2, 1242–1264 CrossRef CAS.
- Y. Chen, J. Lin, B. H. Jia, X. D. Wang, S. Y. Jiang and T. Y. Ma, Adv. Mater., 2022, 34, 2201796 CrossRef CAS PubMed.
- L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981–5079 CrossRef CAS PubMed.
- Q. Zhang and J. Guan, Adv. Funct. Mater., 2020, 30, 2000768 CrossRef CAS.
- Z. J. Li, D. H. Wang, Y. Wu and Y. D. Li, Natl. Sci. Rev., 2018, 5, 673–689 CrossRef CAS.
- L. W. Xing, Y. J. Jin, Y. X. Weng, R. Feng, Y. J. Ji, H. Y. Gao, X. Chen, X. W. Zhang, D. D. Jia and G. Wang, Matter, 2022, 5, 788–807 CrossRef CAS.
- G. F. Liao, L. Zhang, C. X. Li, S. Y. Liu, B. Z. Fang and H. M. Yang, Matter, 2022, 5, 3341–3374 CrossRef CAS.
- H. F. Xiong, A. K. Datye and Y. Wang, Adv. Mater., 2021, 33, 2004319 CrossRef CAS PubMed.
- W. J. Liu, H. Jiang and H. Q. Yu, Chem. Rev., 2015, 115, 12251–12285 CrossRef CAS PubMed.
- C. Chen, T. F. Ma, Y. N. Shang, B. Y. Gao, B. Jin, H. B. Dan, Q. Li, Q. Y. Yue, Y. W. Li, Y. Wang and X. Xu, Appl. Catal., B, 2019, 250, 382–395 CrossRef CAS.
- L. Peng, X. G. Duan, Y. N. Shang, B. Y. Gao and X. Xu, Appl. Catal., B, 2021, 287, 119963 CrossRef CAS.
- Y. Lei, F. W. Yang, H. M. Xie, Y. P. Lei, X. Y. Liu, Y. J. Si and H. H. Wang, J. Mater. Chem. A, 2020, 8, 20629–20636 RSC.
- X. L. Wang, J. Du, Q. H. Zhang, L. Gu, L. J. Cao and H. P. Liang, Carbon, 2020, 157, 614–621 CrossRef CAS.
- Q. Yang, W. X. Wang, Y. Y. Zhou, J. C. Hao, G. D. Fang, C. Liu, P. X. Cui and Y. J. Wang, ACS ES&T Engg, 2023, 3, 616–626 Search PubMed.
- X. Liang, N. H. Fu, S. C. Yao, Z. Li and Y. D. Li, J. Am. Chem. Soc., 2022, 144, 18155–18174 CrossRef CAS PubMed.
- Z. Pu, I. S. Amiinu, R. Cheng, P. Wang, C. Zhang, S. Mu, W. Zhao, F. Su, G. Zhang, S. Liao and S. Sun, Nano-Micro Lett., 2020, 12, 21 CrossRef CAS PubMed.
- H. Zhou, S. Hong, H. Zhang, Y. T. Chen, H. H. Xu, X. K. Wang, Z. Jiang, S. L. Chen and Y. Liu, Appl. Catal., B, 2019, 256, 117767 CrossRef.
- X. F. Liu, N. Fechler and M. Antonietti, Chem. Soc. Rev., 2013, 42, 8237–8265 RSC.
- M. M. Zhang, K. X. Huang, Y. Ding, X. Y. Wang, Y. L. Gao, P. F. Li, Y. Zhou, Z. Guo, Y. Zhang and D. P. Wu, Nanomaterials, 2022, 12, 4289 CrossRef CAS PubMed.
- D. Y. Wu, W. Liu, J. W. Hu, C. Zhu, H. Y. Jing, J. W. Zhang, C. Hao and Y. T. Shi, Mater. Chem. Front., 2021, 5, 3093–3098 RSC.
- Y. Chen, Z. Li, Y. Zhu, D. Sun, X. Liu, L. Xu and Y. Tang, Adv. Mater., 2019, 31, 1806312 CrossRef PubMed.
- N. N. Li, W. Liu, C. Zhu, C. Hao, J. Y. Guo, H. Y. Jing, J. W. Hu, C. C. Xin, D. Y. Wu and Y. T. Shi, J. Energy Chem., 2021, 54, 519–527 CrossRef CAS.
- M. Xiao, L. Zhang, B. Luo, M. Q. Lyu, Z. L. Wang, H. M. Huang, S. C. Wang, A. J. Du and L. Z. Wang, Angew. Chem., Int. Ed., 2020, 59, 7230–7234 CrossRef CAS PubMed.
- K. Wang, Z. J. Lu, J. Lei, Z. Y. Liu, Y. Z. Li and Y. L. Cao, ACS Nano, 2022, 16, 11944–11956 CrossRef CAS PubMed.
- J. W. Hu, D. Y. Wu, C. Zhu, C. Hao, C. C. Xin, J. W. Zhang, J. Y. Guo, N. N. Li, G. F. Zhang and Y. T. Shi, Nano Energy, 2020, 72, 104670 CrossRef CAS.
- W. Xia, J. Tang, J. Li, S. Zhang, K. C. Wu, J. He and Y. Yamauchi, Angew. Chem., Int. Ed., 2019, 58, 13354–13359 CrossRef CAS PubMed.
- J. Fu, Y. D. Hou, X. P. Liu, M. P. Zheng and M. K. Zhu, J. Mater. Chem. C, 2020, 8, 8704–8731 RSC.
- K. Zeng, J. Li, Y. P. Xie, H. P. Yang, X. Y. Yang, D. Zhong, W. X. Zhen, G. Flamant and H. P. Chen, Energy, 2020, 213, 118801 CrossRef CAS.
- S. Frangini and A. Masi, Int. J. Hydrogen Energy, 2016, 41, 18739–18746 CrossRef CAS.
- S. Raharjo, Y. Ueki, R. Yoshiie and I. Naruse, Energy Fuels, 2013, 27, 2762–2766 CrossRef CAS.
- S. Frangini, J. Power Sources, 2008, 182, 462–468 CrossRef CAS.
- Y. F. Chen, Z. J. Li, Y. B. Zhu, D. M. Sun, X. E. Liu, L. Xu and Y. W. Tang, Adv. Mater., 2019, 31, 1806312 CrossRef PubMed.
- H. Zhang, Y. Liu, T. Chen, J. Zhang, J. Zhang and X. W. D. Lou, Adv. Mater., 2019, 31, 1904548 CrossRef CAS PubMed.
- C. Zhu, S. Fu, J. Song, Q. Shi, D. Su, M. H. Engelhard, X. Li, D. Xiao, D. Li, L. Estevez, D. Du and Y. Lin, Small, 2017, 13, 1603407 CrossRef PubMed.
- J. C. Li, F. Xiao, H. Zhong, T. Li, M. J. Xu, L. Ma, M. Cheng, D. Liu, S. Feng, Q. R. Shi, H. M. Cheng, C. Liu, D. Du, S. P. Beckman, X. Q. Pan, Y. H. Lin and M. H. Shao, ACS Catal., 2019, 9, 5929–5934 CrossRef CAS.
- M. L. Zhang, Y. G. Wang, W. X. Chen, J. C. Dong, L. R. Zheng, J. Luo, J. W. Wan, S. B. Tian, W. C. Cheong, D. S. Wang and Y. D. Li, J. Am. Chem. Soc., 2017, 139, 10976–10979 CrossRef CAS PubMed.
- Y. Z. Li, R. Cao, L. B. Li, X. N. Tang, T. L. Chu, B. Y. Huang, K. Yuan and Y. W. Chen, Small, 2020, 16, 1906735 CrossRef CAS PubMed.
- T. He, B. Lu, Y. Chen, Y. Wang, Y. Zhang, J. L. Davenport, A. P. Chen, C. W. Pao, M. Liu, Z. Sun, A. Stram, A. Mordaunt, J. Velasco, Jr., Y. Ping, Y. Zhang and S. Chen, Research, 2019, 2019, 6813585 CAS.
- B. C. Hu, Z. Y. Wu, S. Q. Chu, H. W. Zhu, H. W. Liang, J. Zhang and S. H. Yu, Energy Environ. Sci., 2018, 11, 2208–2215 RSC.
- L. B. Deng, L. Qiu, R. Hu, L. Yao, Z. J. Zheng, X. Z. Ren, Y. L. Li and C. X. He, Appl. Catal., B, 2022, 305, 121058 CrossRef CAS.
- L. Jiao, R. Zhang, G. Wan, W. J. Yang, X. Wan, H. Zhou, J. L. Shui, S. H. Yu and H. L. Jiang, Nat. Commun., 2020, 11, 2831 CrossRef CAS PubMed.
- Y. J. Sa, D. J. Seo, J. Woo, J. T. Lim, J. Y. Cheon, S. Y. Yang, J. M. Lee, D. Kang, T. J. Shin, H. S. Shin, H. Y. Jeong, C. S. Kim, M. G. Kim, T. Y. Kim and S. H. Joo, J. Am. Chem. Soc., 2016, 138, 15046–15056 CrossRef CAS PubMed.
- M. Liu, J. J. Liu, Y. Song, Z. L. Li and F. Wang, Appl. Catal., A, 2019, 583, 117120 CrossRef CAS.
- Y. T. Qu, L. G. Wang, Z. J. Li, P. Li, Q. H. Zhang, Y. Lin, F. Y. Zhou, H. J. Wang, Z. K. Yang, Y. D. Hu, M. Z. Zhu, X. Y. Zhao, X. Han, C. M. Wang, Q. Xu, L. Gu, J. Luo, L. R. Zheng and Y. E. Wu, Adv. Mater., 2019, 31, 1904496 CrossRef CAS PubMed.
- R. Ma, X. Cui, Y. L. Wang, Z. Y. Xiao, R. Luo, L. K. Gao, Z. N. Wei and Y. K. Yang, J. Mater. Chem. A, 2022, 10, 5918–5924 RSC.
- S. Pedireddy, R. Jimenez-Sandoval, M. K. Ravva, C. Nayak, D. H. Anjum, S. N. Jha, K. P. Katuri and P. E. Saikaly, Adv. Funct. Mater., 2021, 31, 2010916 CrossRef CAS.
- Y. N. Shang, X. G. Duan, S. B. Wang, Q. Y. Yue, B. Y. Gao and X. Xu, Chin. Chem. Lett., 2022, 33, 663–673 CrossRef CAS.
- L. Y. Hu, W. R. Li, L. Wang and B. Wang, EnergyChem, 2021, 3, 100056 CrossRef CAS.
- Y. N. Shang, X. Xu, B. Y. Gao, S. B. Wang and X. G. Duan, Chem. Soc. Rev., 2021, 50, 5281–5322 RSC.
- S. H. Ho, Y. D. Chen, R. X. Li, C. F. Zhang, Y. M. Ge, G. L. Cao, M. Ma, X. G. Duan, S. B. Wang and N. Q. Ren, Water Res., 2019, 159, 77–86 CrossRef CAS PubMed.
- Z. H. Wan, Y. Q. Sun, D. C. W. Tsang, E. Khan, A. C. K. Yip, Y. H. Ng, J. Rinklebe and Y. S. Ok, Chem. Eng. J., 2020, 401, 126136 CrossRef CAS.
- J. Shan, C. Ye, Y. Jiang, M. Jaroniec, Y. Zheng and S. Z. Qiao, Sci. Adv., 2022, 8, eabo0762 CrossRef CAS PubMed.
- Q. H. Li, W. X. Chen, H. Xiao, Y. Gong, Z. Li, L. R. Zheng, X. S. Zheng, W. S. Yan, W. C. Cheong, R. A. Shen, N. H. Fu, L. Gu, Z. B. Zhuang, C. Chen, D. S. Wang, Q. Peng, J. Li and Y. D. Li, Adv. Mater., 2018, 30, 1800588 CrossRef PubMed.
- Y. D. Chen, Y. H. Yang, N. Q. Ren and X. G. Duan, Curr. Opin. Chem. Eng., 2023, 41, 100942 CrossRef.
- W. Y. Du, Q. Z. Zhang, Y. N. Shang, W. Wang, Q. Li, Q. Y. Yue, B. Y. Gao and X. Xu, Appl. Catal., B, 2020, 262, 118302 CrossRef CAS.
- Y. X. Wang, H. Y. Su, Y. H. He, L. G. Li, S. Q. Zhu, H. Shen, P. F. Xie, X. B. Fu, G. Y. Zhou, C. Feng, D. K. Zhao, F. Xiao, X. J. Zhu, Y. C. Zeng, M. H. Shao, S. W. Chen, G. Wu, J. Zeng and C. Wang, Chem. Rev., 2020, 120, 12217–12314 CrossRef CAS PubMed.
- W. X. Liu, J. X. Feng, T. R. Wei, Q. Liu, S. S. Zhang, Y. Luo, J. Luo and X. J. Liu, Nano Res., 2023, 16, 2325–2346 CrossRef CAS.
- C. Ye, N. Zhang, D. Wang and Y. Li, Commun. Chem., 2020, 56, 7687–7697 RSC.
- B. S. Chang, S. L. Wu, Y. Wang, T. L. Sun and Z. Cheng, Nanoscale Horiz., 2022, 7, 1340–1387 RSC.
- C. Gao, J. X. Low, R. Long, T. T. Kong, J. F. Zhu and Y. J. Xiong, Chem. Rev., 2020, 120, 12175–12216 CrossRef CAS PubMed.
- P. Qi, J. Wang, X. Djitcheu, D. H. He, H. M. Liu and Q. J. Zhang, RSC Adv., 2021, 12, 1216–1227 RSC.
- J. Z. Li, C. Chen, L. K. Xu, Y. Zhang, W. Wei, E. R. Zhao, Y. Wu and C. Chen, JACS Au, 2023, 3, 736–755 CrossRef CAS PubMed.
- G. Xing, M. Tong, P. Yu, L. Wang, G. Zhang, C. Tian and H. Fu, Angew. Chem., Int. Ed., 2022, 61, e202211098 CrossRef CAS PubMed.
- S. Zhou, K. Jin and M. J. Buehler, Adv. Mater., 2021, 33, 2003206 CrossRef CAS PubMed.
- W. Li, Z. Chen, H. Yu, J. Li and S. Liu, Adv. Mater., 2021, 33, 2000596 CrossRef CAS PubMed.
- H. Zhu, W. Luo, P. N. Ciesielski, Z. Fang, J. Y. Zhu, G. Henriksson, M. E. Himmel and L. Hu, Chem. Rev., 2016, 116, 9305–9374 CrossRef CAS PubMed.
- D. D. Li, Z. Han, K. Y. Leng, S. H. Ma, Y. Wang and J. B. Bai, J. Mater. Sci., 2021, 56, 12764–12774 CrossRef CAS.
- P. Wang, G. Zhang, X. Y. Wei, R. Liu, J. J. Gu and F. F. Cao, J. Am. Chem. Soc., 2021, 143, 3280–3283 CrossRef CAS PubMed.
- X. Peng, L. Zhang, Z. Chen, L. Zhong, D. Zhao, X. Chi, X. Zhao, L. Li, X. Lu, K. Leng, C. Liu, W. Liu, W. Tang and K. P. Loh, Adv. Mater., 2019, 31, 1900341 CrossRef PubMed.
- L. X. Zhong, C. Y. Jiang, M. T. Zheng, X. W. Peng, T. C. Liu, S. B. Xi, X. Chi, Q. H. Zhang, L. Gu, S. Q. Zhang, G. Shi, L. Zhang, K. Z. Wu, Z. H. Chen, T. Z. Li, M. Dahbi, J. Alami, K. Amine and J. Lu, ACS Energy Lett., 2021, 6, 3624–3633 CrossRef CAS.
- H. Chang, H. Pan, F. Wang, Z. Zhang, Y. Kang and S. Min, Nanoscale, 2022, 14, 10003–10008 RSC.
- R. D. Reeves, A. J. M. Baker, T. Jaffré, P. D. Erskine, G. Echevarria and A. van der Ent, New Phytol., 2018, 218, 407–411 CrossRef PubMed.
- N. Rascio and F. Navari-Izzo, Plant Sci., 2011, 180, 169–181 CrossRef CAS PubMed.
- J. F. Wang, Q. Y. Zhu, Y. G. Shan, Y. H. Wang, X. S. Song and X. H. Lei, Geoderma, 2018, 314, 1–7 CrossRef CAS.
- J. T. Li, H. K. Gurajala, L. H. Wu, A. van der Ent, R. L. Qiu, A. J. M. Baker, Y. T. Tang, X. E. Yang and W. S. Shu, Environ. Sci. Technol., 2018, 52, 119801 Search PubMed.
- C. Liu, W. S. Liu, A. van der Ent, J. L. Morel, H. X. Zheng, G. B. Wang, Y. T. Tang and R. L. Qiu, Chemosphere, 2021, 282, 131096 CrossRef CAS PubMed.
- Z. C. Liu, L. Z. Zhou, C. C. Gan, L. J. Hu, B. Pang, D. Zuo, G. Y. Wang, H. C. Wang and Y. L. Liu, Ecotoxicol. Environ. Saf., 2023, 254, 114757 CrossRef CAS PubMed.
- A. Sas-Nowosielska, R. Kucharski, E. Malkowski, M. Pogrzeba, J. M. Kuperberg and K. Krynski, Environ. Pollut., 2004, 128, 373–379 CrossRef CAS PubMed.
- D. F. Liu, Y. S. Liu, M. Y. Liu, Y. P. Geng, Y. J. Zhang, E. Siemann, B. Li and Y. Wang, J. Pest Sci., 2023, 183 DOI:10.1007/s10340-023-01654-0.
- D. F. Liu, L. Chen, C. Chen, X. K. An, Y. J. Zhang, Y. Wang and Q. J. Li, BMC Plant Biol., 2020, 20, 396 CrossRef CAS PubMed.
- P. Cui, C. Liu, X. Su, Q. Yang, L. Ge, M. Huang, F. Dang, T. Wu and Y. Wang, Environ. Sci. Technol., 2022, 56, 8034–8042 CrossRef CAS PubMed.
- X. Guo, Q. Z. Mu, H. Zhong, P. Li, C. J. Zhang, D. Wei and T. K. Zhao, Environ. Pollut., 2019, 254, 113101 CrossRef CAS PubMed.
- J. Cui, W. Wang, J. F. Li, J. M. Du, Y. J. Chang, X. J. Liu, C. Hu, J. W. Cui, C. Liu and D. R. Yao, Ecotoxicol. Environ. Saf., 2021, 213, 112032 CrossRef CAS PubMed.
- H. Guo, J. W. Jiang, J. Q. Gao, J. S. Zhang, L. Y. Zeng, M. Cai and J. L. Zhang, Ecotoxicol. Environ. Saf., 2020, 195, 110502 CrossRef CAS PubMed.
- Z. Li, K. Li, S. Ma, B. Dang, Y. Li, H. Fu, J. Du and Q. Meng, J. Colloid Interface Sci., 2021, 582, 598–609 CrossRef CAS PubMed.
- X. Li, K. Hu, Y. Z. Huang, Q. Q. Gu, Y. W. Chen, B. Yang, R. L. Qiu, W. H. Luo, B. M. Weckhuysen and K. Yan, J. Energy Chem., 2022, 69, 282–291 CrossRef CAS.
- A. Saravanan, S. Karishma, P. S. Kumar and G. Rangasamy, Fuel, 2023, 338, 127221 CrossRef CAS.
- D. M. C. Chen, B. L. Bodirsky, T. Krueger, A. Mishra and A. Popp, Environ. Res. Lett., 2020, 15, 074021 CrossRef CAS.
- S. Babu, S. S. Rathore, R. Singh, S. Kumar, V. K. Singh, S. K. Yadav, V. Yadav, R. Raj, D. Yadav, K. Shekhawat and O. A. Wani, Bioresour. Technol., 2022, 360, 127566 CrossRef CAS PubMed.
- P. Kaur, J. Singh, M. Kaur, P. Rasane, S. Kaur, J. Kaur, V. Nanda, C. M. Mehta and D. Sowdhanya, Waste Biomass Valorization, 2023, 14, 1413–1432 CrossRef CAS.
- Y. L. Zhang, R. Zhao, Y. Q. Li, X. X. Zhu, B. Zhang, X. Y. Lang, L. J. Zhao, B. Jin, Y. F. Zhu and Q. Jiang, J. Power Sources, 2021, 481, 228644 CrossRef CAS.
- Z. M. Zou, X. L. Luo, L. Wang, Y. Zhang, Z. J. Xu and C. H. Jiang, J. Energy Storage, 2021, 44, 103385 CrossRef.
- C. L. Jiao, Z. Xu, J. Z. Shao, Y. Xia, J. Tseng, G. Y. Ren, N. J. Zhang, P. F. Liu, C. X. Liu, G. S. Li, S. Chen, S. Q. Chen and H. L. Wang, Adv. Funct. Mater., 2023, 33, 2213897 CrossRef CAS.
- P. X. Cui, Q. Yang, C. Liu, Y. Wang, G. D. Fang, D. D. Dionysiou, T. L. Wu, Y. Y. Zhou, J. X. Ren, H. B. Hou and Y. J. Wang, ACS ES&T Engg, 2021, 1, 1460–1469 Search PubMed.
- M. Y. Qian, M. C. Lu, M. J. Yan, C. F. Chen, Y. W. Hu, Y. Li, J. R. Chen and X. L. Wu, J. Environ. Chem. Eng., 2023, 11, 109219 CrossRef CAS.
- J. McNutt and Q. He, J. Ind. Eng. Chem., 2019, 71, 78–88 CrossRef CAS.
- A. Colantoni, E. Paris, L. Bianchini, S. Ferri, V. Marcantonio, M. Carnevale, A. Palma, V. Civitarese and F. Gallucci, Sci. Rep., 2021, 11, 5119 CrossRef CAS PubMed.
- R. Campos-Vega, G. Loarca-Pina, H. A. Vergara-Castaneda and B. D. Oomah, Trends Food Sci. Technol., 2015, 45, 24–36 CrossRef CAS.
- A. Kovalcik, S. Obruca and I. Marova, Food Bioprod. Process., 2018, 110, 104–119 CrossRef CAS.
- A. Cervera-Mata, A. Fernandez-Arteaga, M. Navarro-Alarcon, D. Hinojosa, S. Pastoriza, G. Delgado and J. A. Rufian-Henares, J. Cleaner Prod., 2021, 328, 129548 CrossRef CAS.
- V. Kumar, P. Pathak and N. K. Bhardwaj, Waste Manage., 2020, 102, 281–303 CrossRef CAS PubMed.
- Z. J. Ma, Y. Yang, W. Q. Chen, P. Wang, C. Wang, C. Zhang and J. B. Gan, Environ. Sci. Technol., 2021, 55, 8492–8501 CrossRef CAS PubMed.
- X. D. Tang, G. Ran, J. J. Li, Z. Q. Zhang and C. X. Xiang, J. Hazard. Mater., 2021, 402, 123579 CrossRef CAS PubMed.
- J. F. Yang, Z. F. Ao, H. Wu, S. F. Zhang, C. C. Chi, C. Hou and L. W. Qian, Renewable Energy, 2020, 146, 477–483 CrossRef CAS.
- M. J. Amicucci, E. Nandita, A. G. Galermo, J. J. Castillo, S. Chen, D. Park, J. T. Smilowitz, J. B. German, D. A. Mills and C. B. Lebrilla, Nat. Commun., 2020, 11, 3963 CrossRef CAS PubMed.
- A. S. A. Mohammed, M. Naveed and N. Jost, J. Polym. Environ., 2021, 29, 2359–2371 CrossRef CAS PubMed.
- E. Nandita, N. P. Bacalzo, Jr., C. L. Ranque, M. J. Amicucci, A. Galermo and C. B. Lebrilla, Carbohydr. Polym., 2021, 257, 117570 CrossRef CAS PubMed.
- A. Apriyanto, J. Compart and J. Fettke, Plant Sci., 2022, 318, 111223 CrossRef CAS PubMed.
- T. Cai, H. B. Sun, J. Qiao, L. L. Zhu, F. Zhang, J. Zhang, Z. J. Tang, X. L. Wei, J. G. Yang, Q. Q. Yuan, W. Y. Wang, X. Yang, H. Y. Chu, Q. Wang, C. You, H. W. Ma, Y. X. Sun, Y. Li, C. Li, H. F. Jiang, Q. H. Wang and Y. H. Ma, Science, 2021, 373, 1523–1527 CrossRef CAS PubMed.
- F. G. Torres, O. P. Troncoso, A. Pisani, F. Gatto and G. Bardi, Int. J. Mol. Sci., 2019, 20, 5092 CrossRef CAS PubMed.
- L. C. Huang, H. Y. Tan, C. Q. Zhang, Q. F. Li and Q. Q. Liu, Plant Commun., 2021, 2, 100237 CrossRef CAS PubMed.
- M. Q. Li, Z. H. Bi, L. J. Xie, G. H. Sun, Z. Liu, Q. Q. Kong, X. X. We and C. M. Chen, ACS Sustainable Chem. Eng., 2019, 7, 14796–14804 CrossRef CAS.
- J. H. Cao, C. Y. Zhu, Y. Aoki and H. Habazaki, ACS Sustainable Chem. Eng., 2018, 6, 7292–7303 CrossRef CAS.
- Y. Kim, J. K. Kim, C. Vaalma, G. H. Bae, G. T. Kim, S. Passerini and Y. Kim, Carbon, 2018, 129, 564–571 CrossRef CAS.
- M. Shen, W. Hu, C. Duan, J. Li, S. Ding, L. Zhang, J. Zhu and Y. Ni, J. Colloid Interface Sci., 2023, 629, 778–785 CrossRef CAS PubMed.
- D. Li, F. Zhang, L. Luo, Y. W. Shang, S. S. Yang, J. X. Wang, W. X. Chen and Z. A. Liu, Chem. Eng. J., 2023, 461, 142104 CrossRef CAS.
- T. Li, C. J. Chen, A. H. Brozena, J. Y. Zhu, L. X. Xu, C. Driemeier, J. Q. Dai, O. J. Rojas, A. Isogai, L. Wagberg and L. B. Hu, Nature, 2021, 590, 47–56 CrossRef CAS PubMed.
- A. Plucinski, Z. Lyu and B. Schmidt, J. Mater. Chem. B, 2021, 9, 7030–7062 RSC.
- D. C. Wang, H. Y. Yu, D. M. Qi, Y. H. Wu, L. M. Chen and Z. H. Li, J. Am. Chem. Soc., 2021, 143, 11620–11630 CrossRef CAS PubMed.
- L. J. Xie, C. Tang, M. X. Song, X. Q. Guo, X. M. Li, J. X. Li, C. Yan, Q. Q. Kong, G. H. Sun, Q. Zhang, F. Y. Su and C. M. Chen, J. Energy Chem., 2022, 72, 554–569 CrossRef CAS.
- S. Zhang, S. F. Jiang, B. C. Huang, X. C. Shen, W. J. Chen, T. P. Zhou, H. Y. Cheng, B. H. Cheng, C. Z. Wu, W. W. Li, H. Jiang and H. Q. Yu, Nat. Sustainability, 2020, 3, 753–760 CrossRef.
- X. Liu, Y. Pei, M. B. Cao, H. B. Yang and Y. S. Li, Chem. Eng. J., 2022, 450, 138194 CrossRef CAS.
- A. M. Chiorcea-Paquim, T. A. Enache, E. D. Gil and A. M. Oliveira-Brett, Compr. Rev. Food Sci. Food Saf., 2020, 19, 1680–1726 CrossRef CAS PubMed.
- S. Khoshnoudi-Nia, N. Sharif and S. M. Jafari, Trends Food Sci. Technol., 2020, 95, 59–74 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 1246843 CrossRef PubMed.
- W. Zhang, J. Yin, C. Wang, L. Zhao, W. Jian, K. Lu, H. Lin, X. Qiu and H. N. Alshareef, Small Methods, 2021, 5, 2100896 CrossRef CAS PubMed.
- J. C. del Rio, J. Rencoret, A. Gutierrez, T. Elder, H. Kim and J. Ralph, ACS Sustainable Chem. Eng., 2020, 8, 4997–5012 CrossRef CAS.
- S. C. Wang, J. X. Bai, M. T. Innocent, Q. Q. Wang, H. X. Xiang, J. G. Tang and M. F. Zhu, Green Energy Environ., 2022, 7, 578–605 CrossRef CAS.
- W. Zhang, X. Qiu, C. Wang, L. Zhong, F. Fu, J. Zhu, Z. Zhang, Y. Qin, D. Yang and C. C. Xu, Carbon Res., 2022, 1, 14 CrossRef.
- H. Wang, P. Feng, F. Fu, X. Yu, D. Yang, W. Zhang, L. Niu and X. Qiu, Carbon Neutralization, 2022, 1, 277–297 CrossRef.
- J. Z. Qin, B. Han, X. M. Lu, J. B. Nie, C. S. Xian and Z. H. Zhang, JACS Au, 2023, 3, 801–812 CrossRef CAS PubMed.
- Y. Ju, H. Liao, J. J. Richardson, J. Guo and F. Caruso, Chem. Soc. Rev., 2022, 51, 4287–4336 RSC.
- X. Zhang, Z. Li, P. Yang, G. Duan, X. Liu, Z. Gu and Y. Li, Mater. Horiz., 2021, 8, 145–167 RSC.
- J. Guo, T. Suma, J. J. Richardson and H. Ejima, ACS Biomater. Sci. Eng., 2019, 5, 5578–5596 CrossRef CAS PubMed.
- J. J. Zhou, Z. X. Lin, Y. Ju, M. A. Rahim, J. J. Richardson and F. Caruso, Acc. Chem. Res., 2020, 53, 1269–1278 CrossRef CAS PubMed.
- Q. Dai, H. M. Geng, Q. Yu, J. C. Hao and J. W. Cui, Theranostics, 2019, 9, 3170–3190 CrossRef CAS PubMed.
- D. Wu, J. J. Zhou, M. N. Creyer, W. Yim, Z. Chen, P. B. Messersmith and J. V. Jokerst, Chem. Soc. Rev., 2021, 50, 4432–4483 RSC.
- Z. Zhang, L. S. Xie, Y. Ju and Y. L. Dai, Small, 2021, 17, 2100314 CrossRef CAS PubMed.
- H. Ejima, J. J. Richardson and F. Caruso, Nano Today, 2017, 12, 136–148 CrossRef CAS.
- J. Pan, G. Gong, Q. Wang, J. Shang, Y. He, C. Catania, D. Birnbaum, Y. Li, Z. Jia, Y. Zhang, N. S. Joshi and J. Guo, Nat. Commun., 2022, 13, 2117 CrossRef CAS PubMed.
- J. Guo, Y. Ping, H. Ejima, K. Alt, M. Meissner, J. J. Richardson, Y. Yan, K. Peter, D. von Elverfeldt, C. E. Hagemeyer and F. Caruso, Angew. Chem., Int. Ed., 2014, 53, 5546–5551 CrossRef CAS PubMed.
- J. Guo, B. L. Tardy, A. J. Christofferson, Y. Dai, J. J. Richardson, W. Zhu, M. Hu, Y. Ju, J. Cui, R. R. Dagastine, I. Yarovsky and F. Caruso, Nat. Nanotechnol., 2016, 11, 1105–1111 CrossRef CAS PubMed.
- Y. Zhang, J. Wang, Y. He, J. Pan, X. Jin, J. Shang, G. Gong, J. J. Richardson, I. Manners and J. Guo, Angew. Chem., Int. Ed., 2023, 62, e202303463 CrossRef CAS PubMed.
- Z. Zhao, D. C. Pan, Q. M. Qi, J. Kim, N. Kapate, T. Sun, C. W. Shields IV, L. L. Wang, D. Wu, C. J. Kwon, W. He, J. Guo and S. Mitragotri, Adv. Mater., 2020, 32, 2003492 CrossRef CAS PubMed.
- X. Liao, G. Gong, M. Dai, Z. Xiang, J. Pan, X. He, J. Shang, A. M. Blocki, Z. Zhao, C. W. T. Shields and J. Guo, Adv. Sci., 2023, 10, 2207488 CrossRef CAS PubMed.
- J. Guo, M. Suástegui, K. K. Sakimoto, V. M. Moody, G. Xiao, D. G. Nocera and N. S. Joshi, Science, 2018, 362, 813–816 CrossRef CAS PubMed.
- X. Wei, D. Zheng, M. Zhao, H. Z. Chen, X. Fan, B. Gao, L. Gu, Y. Guo, J. B. Qin, J. Wei, Y. L. Zhao and G. C. Zhang, Angew. Chem., Int. Ed., 2020, 59, 14639–14646 CrossRef CAS PubMed.
- H. S. Kim, J. Lee, J. H. Jang, H. Jin, V. K. Paidi, S. H. Lee, K. S. Lee, P. Kim and S. J. Yoo, Appl. Surf. Sci., 2021, 563, 150208 CrossRef CAS.
- J. Lee, H. S. Kim, J. H. Jang, E. H. Lee, H. W. Jeong, K. S. Lee, P. Kim and S. J. Yoo, ACS Sustainable Chem. Eng., 2021, 9, 7863–7872 CrossRef CAS.
- Y. Zhu, W. Sun, J. Luo, W. Chen, T. Cao, L. Zheng, J. Dong, J. Zhang, M. Zhang, Y. Han, C. Chen, Q. Peng, D. Wang and Y. Li, Nat. Commun., 2018, 9, 3861 CrossRef PubMed.
- Z. Y. Sun, Y. J. Wei, T. Cao, Z. Liu, R. Sui, X. Li, J. J. Pei, Z. Chen and S. Wang, Nano Res., 2023, 16, 9003–9011 CrossRef CAS.
- M. Borghei, J. Lehtonen, L. Liu and O. J. Rojas, Adv. Mater., 2018, 30, 1703691 CrossRef PubMed.
- J. Zheng, C. Z. Guo, C. Y. Chen, M. Z. Fan, J. P. Gong, Y. F. Zhang, T. X. Zhao, Y. L. Sun, X. F. Xu, M. M. Li, R. Wang, Z. L. Luo and C. G. Chen, Electrochim. Acta, 2015, 168, 386–393 CrossRef CAS.
- W. J. Jiang, W. L. Hu, Q. H. Zhang, T. T. Zhao, H. Luo, X. Zhang, L. Gu, J. S. Hu and L. J. Wan, Chem. Commun., 2018, 54, 1307–1310 RSC.
- C. Y. Wang, X. Li, E. L. Gao, M. Q. Jian, K. L. Xia, Q. Wang, Z. P. Xu, T. L. Ren and Y. Y. Zhang, Adv. Mater., 2016, 28, 6640–6648 CrossRef CAS PubMed.
- C. Wang, W. Chen, K. Xia, N. Xie, H. Wang and Y. Zhang, Small, 2019, 15, 1804966 CrossRef PubMed.
- X. Y. Xu, J. Y. Wu, Z. H. Meng, Y. R. Li, Q. L. Huang, Y. Qi, Y. F. Liu, D. Zhan and X. Y. Liu, ACS Appl. Nano Mater., 2018, 1, 5460v5469 Search PubMed.
- H. Amiri, M. Aghbashlo, M. Sharma, J. Gaffey, L. Manning, S. M. M. Basri, J. F. Kennedy, V. K. Gupta and M. Tabatabaei, Nat. Food, 2022, 3, 822–828 CrossRef PubMed.
- H. Ababneh and B. H. Hameed, Int. J. Biol. Macromol., 2021, 186, 314–327 CrossRef CAS PubMed.
- A. Khan, M. Goepel, J. C. Colmenares and R. Glaser, ACS Sustainable Chem. Eng., 2020, 8, 4708–4727 CrossRef CAS.
- Y. Q. Zhu, W. M. Sun, W. X. Chen, T. Cao, Y. Xiong, J. Luo, J. C. Dong, L. R. Zheng, J. Zhang, X. L. Wang, C. Chen, Q. Peng, D. S. Wang and Y. D. Li, Adv. Funct. Mater., 2018, 28, 1802167 CrossRef.
- Z. Liu, H. Li, X. Gao, X. Guo, S. Wang, Y. Fang and G. Song, Nat. Commun., 2022, 13, 4716 CrossRef CAS PubMed.
- G. Y. Zhang, X. Liu, L. Wang and H. G. Fu, J. Mater. Chem. A, 2022, 10, 9277–9307 RSC.
- W. X. Chen, J. J. Pei, C. T. He, J. W. Wan, H. L. Ren, Y. Q. Zhu, Y. Wang, J. C. Dong, S. B. Tian, W. C. Cheong, S. Q. Lu, L. R. Zheng, X. S. Zheng, W. S. Yan, Z. B. Zhuang, C. Chen, Q. Peng, D. S. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2017, 56, 16086–16090 CrossRef CAS PubMed.
- Z. L. Wang, D. Xu, H. X. Zhong, J. Wang, F. L. Meng and X. B. Zhang, Sci. Adv., 2015, 1, e1400035 CrossRef PubMed.
- N. Yang, R. Shao, Z. P. Zhang, M. L. Dou, J. Niu and F. Wang, Carbon, 2021, 178, 775–782 CrossRef CAS.
- J. Alipal, N. A. S. M. Pu'ad, T. C. Lee, N. H. M. Nayan, N. Sahari, H. Basri, M. I. Idris and H. Z. Abdullah, Mater. Today Proc., 2021, 42, 240–250 CrossRef CAS.
- Y. Chen, T. He, Q. M. Liu, Y. F. Hu, H. Gu, L. Deng, H. T. Liu, Y. C. Liu, Y. N. Liu, Y. Zhang, S. W. Chen and X. P. Ouyang, Appl. Catal., B, 2023, 323, 122163 CrossRef CAS.
- T. He, Y. Song, Y. Chen, X. Song, B. Lu, Q. Liu, H. Liu, Y. Zhang, X. Ouyang and S. Chen, Chem. Eng. J., 2022, 442, 136337 CrossRef CAS.
- J. A. Duce, S. Ayton, A. A. Miller, A. Tsatsanis, L. Q. Lam, L. Leone, J. E. Corbin, H. Butzkueven, T. J. Kilpatrick, J. T. Rogers, K. J. Barnham, D. I. Finkelstein and A. I. Bush, Mol. Psychiatry, 2013, 18, 245–254 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- M. Zhou, Y. Jiang, G. Wang, W. Wu, W. Chen, P. Yu, Y. Lin, J. Mao and L. Mao, Nat. Commun., 2020, 11, 3188 CrossRef CAS PubMed.
- Y. Meng, P. Liu, W. Zhou, J. Ding and J. Liu, ACS Nano, 2018, 12, 9070–9080 CrossRef CAS PubMed.
- A. J. Han, W. X. Chen, S. L. Zhang, M. L. Zhang, Y. H. Han, J. Zhang, S. F. Ji, L. R. Zheng, Y. Wang, L. Gu, C. Chen, Q. Peng, D. S. Wang and Y. D. Li, Adv. Mater., 2018, 30, 1706508 CrossRef PubMed.
- S. H. Gong, W. B. Wang, C. N. Zhang, M. H. Zhu, R. Q. Lu, J. J. Ye, H. Yang, C. D. Wu, J. Liu, D. W. Rao, S. Y. Shao and X. M. Lv, Adv. Funct. Mater., 2022, 32, 2110649 CrossRef CAS.
- X. L. Qi, Y. J. Huang, S. Y. You, Y. J. Xiang, E. Y. Cai, R. T. Mao, W. H. Pan, X. Q. Tong, W. Dong, F. F. Ye and J. L. Shen, Adv. Sci., 2022, 2106015 CrossRef CAS PubMed.
- E. C. Liu, Y. Ji, F. Zhang, B. J. Liu and X. H. Meng, J. Agric. Food Chem., 2021, 69, 1739–1750 CrossRef CAS PubMed.
- Y. H. Zhao, L. Wang, D. S. Zhang, R. Li, T. Y. Cheng, Y. B. Zhang, X. J. Liu, G. Wong, Y. G. Tang, H. Wang and S. Gao, Sci. Rep., 2019, 9, 78 CrossRef PubMed.
- Z. Zhang, X. Gao, M. Dou, J. Ji and F. Wang, Small, 2017, 13, 1604290 CrossRef PubMed.
- L. L. Ma, X. Hu, Y. Min, X. Y. Zhang, W. J. Liu, P. K. S. Lam, M. M. Jung, R. J. Zeng and R. Q. Ye, Carbon, 2023, 203, 827–834 CrossRef CAS.
- D. H. Li, D. J. Yang, X. F. Yang, Y. Wang, Z. Q. Guo, Y. Z. Xia, S. L. Sun and S. J. Guo, Angew. Chem., Int. Ed., 2016, 55, 15925–15928 CrossRef CAS PubMed.
- R. Hao, S. Gu, J. J. Chen, Z. Y. Wang, Q. M. Gan, Z. Q. Wang, Y. P. Huang, P. G. Liu, K. L. Zhang, K. Y. Liu, C. Liu and Z. G. Lu, Mater. Today Energy, 2021, 21, 100826 CrossRef CAS.
- L. J. Zhang, T. C. Liu, N. Chen, Y. Jia, R. S. Cai, W. Theis, X. F. Yang, Y. Z. Xia, D. J. Yang and X. D. Yao, J. Mater. Chem. A, 2018, 6, 18417–18425 RSC.
- Z. P. Zhang, X. J. Gao, M. L. Dou, J. Ji and F. Wang, J. Mater. Chem. A, 2017, 5, 1526–1532 RSC.
- J. Zhang, M. Zhang, Y. Zeng, J. Chen, L. Qiu, H. Zhou, C. Sun, Y. Yu, C. Zhu and Z. Zhu, Small, 2019, 15, 1900307 CrossRef PubMed.
- L. J. Cao, X. L. Wang, C. Yang, J. J. Lu, X. Y. Shi, H. W. Zhu and H. P. Liang, ACS Sustainable Chem. Eng., 2021, 9, 189–196 CrossRef CAS.
- B. Su, B. D. Ge, M. Z. Li, Y. Y. Chen, Y. M. Chen, J. H. Zhang, H. Chen and J. H. Li, Fuel, 2019, 237, 763–768 CrossRef CAS.
- Z. J. Wang, J. G. Bao, H. Z. He, S. Mukherji, L. T. Luo and J. K. Du, Chem. Eng. J., 2023, 458, 141513 CrossRef CAS.
- N. E. A. El-Naggar, M. H. Hussein, S. A. Shaaban-Dessuuki and S. R. Dalal, Sci. Rep., 2020, 10, 3011 CrossRef CAS PubMed.
- X. F. Shen, J. J. Liu, F. F. Chu, P. K. S. Lam and R. J. Zeng, Appl. Energy, 2015, 158, 348–354 CrossRef CAS.
- X. Hu, L. L. Ma, W. J. Liu, H. C. Li, M. M. Ma and H. Q. Yu, Sci. Total Environ., 2021, 782, 146844 CrossRef CAS.
- F. M. Morsy, Photochem. Photobiol., 2011, 87, 137–142 CrossRef PubMed.
- C. Zhou, Y. Liang, W. Xia, E. Almatrafi, B. Song, Z. Wang, Y. Zeng, Y. Yang, Y. Shang, C. Wang and G. Zeng, J. Hazard. Mater., 2023, 441, 129871 CrossRef CAS PubMed.
- K. Richter, M. Schicklberger and J. Gescher, Appl. Environ. Microbiol., 2012, 78, 913–921 CrossRef CAS PubMed.
- S. Kalathil, K. P. Katuri, A. S. Alazmi, S. Pedireddy, N. Kornienko, P. Costa and P. E. Saikaly, Chem. Mater., 2019, 31, 3686–3693 CrossRef CAS.
- S. B. Zhang, M. M. Han, T. F. Shi, H. M. Zhang, Y. Lin, X. S. Zheng, L. R. Zheng, H. J. Zhou, C. Chen, Y. X. Zhang, G. Z. Wang, H. J. Yin and H. J. Zhao, Nat. Sustainability, 2023, 6, 169–179 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |