Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development and application of a sensitive feeding assay for daphnids based on the ingestion of fluorescent microparticles
Maria
Giannouli
a,
Konstantinos
Panagiotidis
a,
Keith D.
Rochfort
b and
Konstantinos
Grintzalis
 *a
*a
aSchool of Biotechnology, Dublin City University, Republic of Ireland. E-mail: kgrintzalis@gmail.com; konstantinos.gkrintzalis@dcu.ie
bSchool of Nursing, Psychotherapy, and Community Health, Dublin City University, Republic of Ireland
First published on 29th August 2023
Abstract
The toxicological hazard and safety assessment of chemical substances relies on the outcome of animal testing. A combination of mortality, phenotypic and molecular endpoints are employed to assess this. However, animal welfare considerations, societal concerns, and regulatory action have initiated the need to use new approaches and methodologies in risk assessment. In this context, non-invasive tests and model species not categorized as “animals” can be used to reduce the use of higher animals according to the 3Rs principle. Additionally, such tests could provide comparative conclusions with faster and more economical approaches. Focusing on the freshwater ecosystem, daphnids have been extensively used for toxicological studies, and their feeding rate following exposure to pollutants is a common phenotypic endpoint in ecotoxicology assessment. Feeding impairment indicates early alterations in animal physiology, thus providing insight for further investigation. The feeding rate is usually assessed with extended incubation periods and large volumes of media, resulting in increased waste generation and use of animals, which highlights the need for improved methods. In this study, we developed a robust and sensitive approach based on tracking the ingestion of fluorescent microparticles that requires a low number of animals and incubation times. Parameters such as the total volume, the concentration of microparticles, and the number of daphnids were optimized to study the impact of a selection of pollutants. As indicated by the results, the animal number used per replicate had a significant impact (an increase in the animal number increased the ingestion) on the feeding rate rather than the assay volume and the concentration of microparticles. To assess the effect of exposure to chemicals on the feeding rate of daphnids, a range of metals (lithium chloride, zinc sulfate heptahydrate, zirconium chloride, aluminium sulfate hexadecahydrate, cobalt nitrate hexahydrate), pharmaceuticals (diltiazem hydrochloride, propranolol hydrochloride, diclofenac sodium, metformin), and one stimulant (nicotine) were selected. There was a concentration-dependent decrease in feeding rates for most of the pollutants used, particularly after exposure to metals, indicating their strong effect on the physiology of the animal. This method demonstrates an efficient means of assessing toxicology to guide future studies in working concentrations of chemicals and the assessment of water quality.
Environmental significanceWith the exacerbation of pollution in the environment, new approach methodologies hold great promise in providing useful information for chemical hazard and risk assessment. Although there are current tests available, new faster and more robust assays are required to generate meaningful conclusions. Focusing on the freshwater ecosystem, daphnids or commonly known as the water flea, have been highlighted among the eco-responsive model systems for pollution assessment. A key phenotypic endpoint is the filtering or feeding rate, which is usually monitored with methods relying on long duration tests and have flaws in their performance being not robust. Therefore, the need to develop new tools is eminent. Here we present a method to bridge this gap and assess feeding rate as a physiology endpoint. |
1 Introduction
Traditional approaches for water quality assessment are based on detecting chemicals and pollutants in the ecosystem. Such methods are limited by their respective spectrum of chemicals that they can detect and their sensitivity limits; thus, effect-based methods could provide an improved insight to support current methods aiming to assess the impact of pollutants in the aquatic environment. The latter provide mechanistic insight into the action of pollutants on organisms, aid more in developing coherent knowledge about contaminants, and are more sensitive tools for accurate predictions of pollution assessment.1 Focusing on the freshwater ecosystem, daphnids are key species used in freshwater ecology and ecotoxicology. Daphnia magna, similar to other sentinel species, is commonly used in aquatic toxicity testing due to the fact that it is sensitive to chemical and physical changes in the water environment; thus, it can reveal the sublethal effects of chemical pollution.2 Moreover, daphnids have a central role in food webs, and as filter feeders, they can be very responsive to pollutants.3 They also have many characteristics that make them easy to culture in the lab, such as their short life cycle and their parthenogenetic reproduction mode, allowing uniform responses among individuals in response to toxicants entering the aquatic environment.4Traditional approaches to water toxicity assessment often use lethality as an endpoint to evaluate the effects of a wide range of chemicals in the aquatic environment.5–11 Other studies focus on physiological changes in response to sublethal concentrations of pollutants such as growth, reproduction, respiration, swimming activity, and ingestion of food, which are more sensitive readouts than survival in freshwater organisms.12–17 Feeding activity, specifically, is impaired by various environmental changes, such as the availability of resources and the presence of pollutants. Changes in the feeding rate can also reveal population responses due to the fact that ingestion is connected with growth and reproduction.14,18 Several approaches are commonly employed to determine the feeding rate, such as algal cell counts,19 chlorophyll fluorescence, and radio-labelled algae, dyes, or beads.20 Each of the previously mentioned methods used presents its own limitations, such as using adult animals and high volumes, requiring continuous stirring to avoid algal sedimentation, being conducted under minimal light conditions to prevent algal growth, and being time-consuming.18,21,22 In an attempt to evaluate the impact of common toxicants on the feeding rate, it is essential to establish improved models in terms of convenience and efficiency that require low volumes and number of animals.
Daphnia magna can ingest particles with sizes between 1–50 μm due to its filter-feeding mode, and it has been shown that acute exposure to low concentrations of microparticles does not significantly affect the survival and growth of daphnids.23,24 Also, the method has high sensitivity due to the efficient tracking of fluorescent microparticles. With the focus on assessing the impact of toxicants on the feeding rate of daphnids, we used common pollutants such as pharmaceuticals, metals, and a stimulant (nicotine) that frequently occur in the aquatic environment and affect the physiology and several phenotypical endpoints of freshwater organisms.8,25–27 In this study, we developed a miniaturized feeding assay based on the ingestion of fluorescent microparticles. This approach aimed to improve on the previously stated limitations and provide an efficient method to assess the impact of pollutants on the feeding rate of Daphnia magna.
2 Materials and methods
2.1. Materials
All chemicals used in this study were of the highest analytical quality. Zinc sulfate heptahydrate (CAS: 7446-20-0), diclofenac sodium (CAS: 15307-79-6), cobalt(III) nitrate (CAS: 10026-22-9), 1,1-dimethylbiguanidine hydrochloride (metformin, CAS: 1115-70-4), DL-propranolol hydrochloride (CAS: 318-98-9), and L-nicotine (CAS: 54-11-5) were purchased from Acros Organics. Aluminium sulfate hexadecahydrate (CAS: 1628-11-8) and lithium chloride anhydrous (CAS: 7447-41-8) were purchased from ThermoFisher. Zirconium(IV) chloride (CAS: 10025-11-6) was purchased from Alfa Aesar. Diltiazem hydrochloride (CAS: 33286-22-5) and fluorescent microparticles (carboxylate-modified fluorescent latex beads, CAT number: L3030) were purchased from Sigma Aldrich.2.2. Culturing of daphnids for feeding assay development and toxicity exposures
Daphnids were maintained in glass beakers in aqueous media as described previously25 under a 16 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h of light
8 h of light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark photoperiod at 20 °C. For toxicity curves, neonates (<24 h) were collected from the third brood of their mothers and cultured until four days old with algal supplementation. Fifteen animals were used per replicate, with a minimum of four replicates, for each concentration and were exposed in 100 ml OECD media for 24 h in the absence of food. Toxicity curves were plotted using the four-parameter logistic model, and EC values were calculated. For the optimization of the feeding assay, four-day-old animals were incubated in media for 24 h to simulate the absence of food during the acute exposure to chemicals as described previously (Michalaki, 2022). For chemical exposures, sixty animals (four-day-old) were exposed to at 5, 10, and 20 mg l−1 of pollutants in density 1 animal/5 ml of media for 24 h in the absence of food. After 24 h, animals were divided in three replicates of fifteen animals each, and were incubated in fluorescent microparticles for 30 minutes, to assess the feeding rate.
dark photoperiod at 20 °C. For toxicity curves, neonates (<24 h) were collected from the third brood of their mothers and cultured until four days old with algal supplementation. Fifteen animals were used per replicate, with a minimum of four replicates, for each concentration and were exposed in 100 ml OECD media for 24 h in the absence of food. Toxicity curves were plotted using the four-parameter logistic model, and EC values were calculated. For the optimization of the feeding assay, four-day-old animals were incubated in media for 24 h to simulate the absence of food during the acute exposure to chemicals as described previously (Michalaki, 2022). For chemical exposures, sixty animals (four-day-old) were exposed to at 5, 10, and 20 mg l−1 of pollutants in density 1 animal/5 ml of media for 24 h in the absence of food. After 24 h, animals were divided in three replicates of fifteen animals each, and were incubated in fluorescent microparticles for 30 minutes, to assess the feeding rate.
Ethical review and approval were not required for this study, due to the fact that daphnids are regarded as “animals” in terms of being members of the kingdom Animalia, however, they are not “animals” as defined in regulation SI543 of 2012 on the protection of animals used for scientific purposes. Therefore, the study does not require authorization from the Health Products Regulatory Authority (HPRA), while is also in line with the aim of working under the 3Rs (reduce, refine, replacement) strategy, since daphnids are commonly used in ecology and ecotoxicology as replacements of more evolutionary advanced species (i.e., fishes), posing no ethical implications. However, the Biosafety Committee of the School of Biotechnology is aware and has authorized the procedures and experiments described.
2.3. Feeding quantification and visualization of microparticle ingestion by fluorescence
Fluorescent carboxy functionalized microparticles (Sigma L3030) were used in this study to assess the ingestion (feeding) rate of daphnids. These microparticles were initially tested to identify any toxic effects on daphnids. In the concentrations used and especially for the low exposure time to assess the feeding rate, were not toxic. Fluorescence was measured in the media at Ex/Em 560/590 nm using a TECAN plate reader, and the difference in fluorescence was converted to the amount of ingested microparticle using a standard curve and expressed as μg microparticle per animal and per minute. For the optimization of the feeding assay, the animals were incubated in a range of concentrations and volumes (from 2 to 10 ml in well plates) as described in the results section.To assess the impact of the selected chemicals on the feeding rate of daphnids, after the exposure period of 24 h, animals were transferred to clean media, and three replicates of 15 animals were incubated in falcon tubes using the optimized conditions for this feeding assay, of 18 ml total volume and 13 mg microparticles per l. After the incubation period of 30 minutes, animals were transferred to clean media in order to remove any microparticles sticking to their exoskeleton. The animals were also observed by fluorescence microscopy to confirm that only the ingested microparticle was measured. For the chemical exposures, the feeding rate of daphnids was measured after 30 minutes from the amount of ingested carboxy microparticles extracted from the animals following their homogenization. Specifically, fifteen animals (with a minimum of three replicates) were homogenized in 0.5 ml ddH2O with a microtube tissue grinder pestle, and the ingested microparticles were quantified by fluorescence. A separate pool of animals was incubated in the absence of microparticle as a negative control to account for the animal background fluorescence. The ingestion of microparticles was also confirmed with fluorescence microscopy using the TRITC filter.
2.4. Statistical analysis
Data were presented as mean ± standard deviation (SD) and were analyzed and plotted with the GraphPad Prism software. Statistically significant differences were identified with One-Way ANOVA corrected with post-hoc Dunnett's test.3 Results and discussion
3.1. Optimization of the feeding assay
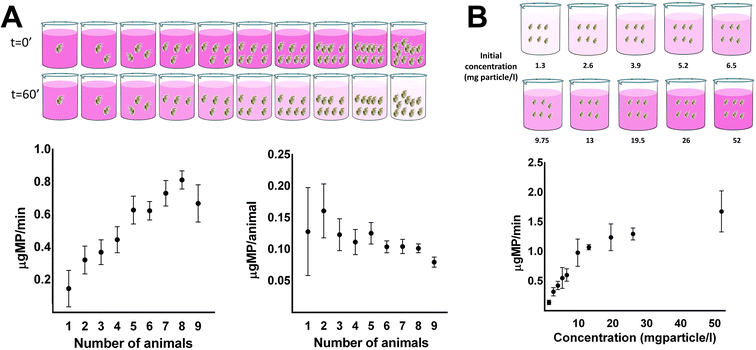
Acute toxicity experiments in daphnids are usually performed in neonates (<24 h); however, there have been many deviations for these guidelines, and many studies are performed in other ages. The general idea is that daphnids of different ages would exist in an actual population in the environment. In our study, we used four-day-old daphnids as they would have the chance to grow and be resistant to stress and they would provide a good pool of animals in relation to their feeding filtration performance to optimize the conditions of the feeding assay. Daphnids were incubated in media for 24 h prior to exposure to microparticle to simulate the absence of food during exposure to pollutants. For the development of the feeding assay, the impact of the media volume, the concentration of microparticle, and the number of animals were optimized.The first parameter assessed was the number of animals used in each replicate for the feeding assay (Fig. 1A). One to nine animals were incubated in a volume of 6 ml, and a concentration of microparticle at 13 mg l−1, and the linear range of ingestion of microparticle was measured at 10 minute intervals and followed over one hour. Keeping the volume and the concentration of the microparticle consistent per replicate, ingestion of microparticle and feeding rate per individual animal were determined in relation to the total animals used per well. Increasing the number of animals per replicate increased the consumption of microparticle when it was expressed per minute, as it was expected, while decreased the feeding rate expressed per individual animal (Fig. 1A). This indicates that a higher number of animals for the test would rapidly consume the microparticles in the media, decreasing the available microparticles, after monitoring their consumption for up to one hour. Consequently, this would result in the underestimation of the feeding rate as the microparticles would not be in excess during the assay. In addition, within the range of four to seven animals per replicate, the rate of ingestion of microparticles reached a plateau, meaning that ingestion was stable regardless of the animal number. In contrast, a low number of animals per replicate cannot consume a high amount of microparticles to observe significant differences. Furthermore, a low number of animals used per replicate increases the variance of consumption due to differences among individuals, which is essential to avoid during the optimization of the feeding assay. For the above reasons, the optimum number of daphnids per replicate to perform this test is between four to six animals. Taking also into account for example, that in some conditions of exposure to chemicals the animals may ingest slower than controls a number of six animals could be preferred to achieve a decrease at a shorter period similar to faster eating control unexposed animals.
The second parameter assessed was the impact of the concentration of microparticle. Keeping the volume of the media and the number of animals consistent, the effect of microparticle concentration on the feeding rate of daphnids was quantified. In a constant volume (6 ml) and number of animals (6 animals per replicate), the increase in the concentration of microparticle (from 1.3 to 52 mg microparticle per l) resulted in an increase in the feeding rate of daphnids (Fig. 1B). A maximum plateau was observed in the feeding rate for high concentrations of microparticles (>19.5 mg l−1). This can be explained because daphnids operate in a non-selective filtration manner for any particle in their media. Therefore, in low concentrations, all the amount of the microparticle was ingested fast, resulting in the absence of its excess in the media. In contrast, concentrations of microparticle above 19.5 mg l−1 had little to no effect on the feeding rate of the animals, considering that the food intake remained constant after a critical concentration. Subsequently, we chose 13 mg l−1 as the concentration to perform the following experiments for the optimization of the feeding assay, considering that a high feeding rate and minor variance are crucial.
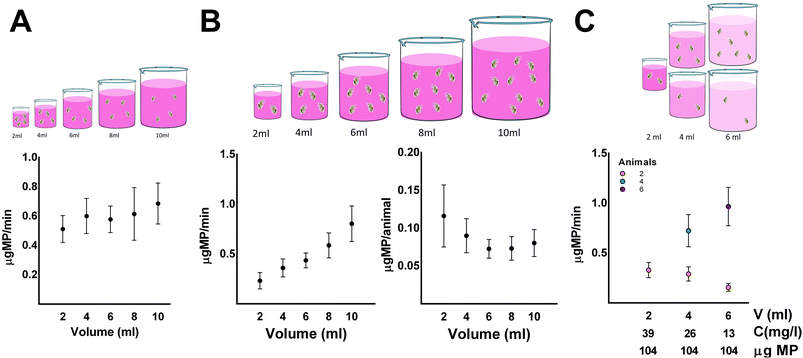
The impact of volume on the feeding rate of daphnids was also assessed. A constant number of animals and concentration of microparticles were used to determine the effect of the volume on the feeding rate of daphnids (Fig. 2A). Four animals per replicate were incubated for 30 minutes in different volumes (2 to 10 ml) at an initial concentration of microparticle of 13 mg l−1, and the concentration of microparticle in the supernatant was measured in 5 minute intervals. Increasing the total volume of the feeding assay results in a proportional increase of the absolute amount of microparticles as the concentration used was constant. The increase in volume had little to no effect on the feeding rate of daphnids. The impact of volume on the feeding rate was also assessed using a different number of animals with a volume-to-animal ratio equal to one. Two to ten animals were incubated in 2 to 10 ml of total volume, respectively, while the concentration of microparticle was constant (Fig. 2B). Increasing the volume at a constant concentration resulted in the absolute amount of microparticles being increased proportionally; hence there was a higher availability of microparticles in higher volumes. This increased the feeding rate by increasing the number of animals used per replicate, which appears to result from the animal number rather than the volume increase, as mentioned earlier. Nevertheless, in higher volumes, lower consumption per animal was observed. However, using lower volumes increases the variance, and thus volumes between four and six were selected to improve accuracy and minimize the variance of the feeding assay. Another approach to address the impact of the volume of microparticles in the feeding rate was to select varying total volumes and concentrations to achieve the same absolute amount of microparticles in every condition. First, two animals were incubated in 2, 4, and 6 ml of microparticle concentrations of 39, 26, and 13 mg microparticles per l, respectively. There was little to no effect on the feeding rate using the same number of animals and the same absolute amount of microparticles. When the volume-to-animal ratio was equal to one however, meaning that 2, 4, and 6 animals were incubated in 2, 4, and 6 ml of microparticle with concentrations of 39, 26, and 13 mg microparticles per l respectively, there was a significant difference in the feeding rate (Fig. 2C). As mentioned above, this result indicated that the number of animals is crucial in the feeding rate rather than the total volume of the assay and concentration of microparticle. Therefore, including the results from the previous experimental designs, it was preferred to use animals between four to six, with volumes between four to six, respectively. Finally, we chose a volume of 6 ml and five animals as the optimal conditions to assess the impact of the selected chemicals on the feeding rate of Daphnia magna.
3.2. The impact of pollutants on the feeding of daphnids
Four days old daphnids were exposed to a range of pollutants for 24 h, and EC values were calculated (Table 1). Their impact on the feeding rate was determined using sublethal concentrations (5, 10, 20 mg l−1) which were of similar comparable molecular concentrations, and prevented high mortality (Fig. 3).| Chemical | EC50 | (Min–max) | Hill slope | EC1 | EC5 | EC10 |
|---|---|---|---|---|---|---|
| Diltiazem hydrochloride | 80.82 | 78.99–82.70 | 16.37 | 61.04 | 67.52 | 70.67 |
| Propranolol hydrochloride | 83.62 | 78.04–89.61 | 3.864 | 25.46 | 39.03 | 47.35 |
| Diclofenac sodium | 84.86 | 81.01–88.89 | 5.292 | 35.61 | 48.65 | 56.03 |
| Metformin | 145 | 142.6–147.5 | 9.534 | 89.55 | 106.47 | 115.15 |
| L-Nicotine | 455 | 450.8–459.2 | 14.76 | 333.28 | 372.71 | 392.07 |
| Lithium chloride | 93.65 | 91.49–95.86 | 9.354 | 57.30 | 68.36 | 74.05 |
| Zinc sulfate heptahydrate | 29.75 | 27.26–32.47 | 3.858 | 9.04 | 13.87 | 16.83 |
| Zirconium chloride | 26.96 | 24.74–29.38 | 4.740 | 10.23 | 14.49 | 16.96 |
| Aluminium sulfate hexadecahydrate | 59.39 | 56.85–62.04 | 5.282 | 24.88 | 34.01 | 39.18 |
| Cobalt nitrate hexahydrate | 90.53 | 82.93–98.82 | 5.813 | 41.11 | 54.55 | 62.03 |
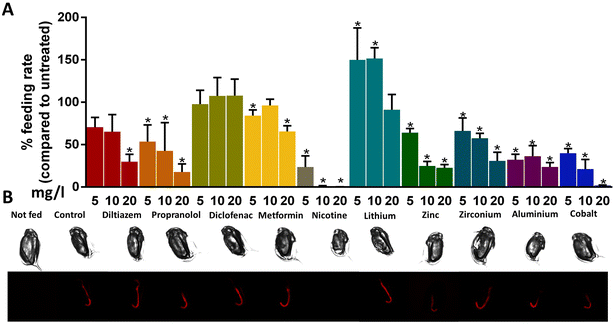
In this study, a range of commonly encountered pollutants was assessed for their impact on feeding in sublethal concentrations. In general, exposure to chemicals resulted in decreased feeding rates, except for diclofenac (Fig. 3A). Diclofenac is a non-steroidal anti-inflammatory drug (NSAID) that has been found to induce biochemical and behavioural changes in Daphnia magna; specifically, it reduced growth, reproduction, heart rate, and filtration and ingestion rate, revealing dose-dependent trends.28,29 Additionally, oxidative stress-related enzymes were also inhibited in response to exposure to diclofenac, which might be linked with decreased ingestion rates.30 Our results contradict previous studies, probably due to the different exposure periods and the age of individual animals as in this study, four days old animals were used.
Propranolol is a β-blocker often used to decrease the heart rate in patients with hypertension. It blocks the actions of the hormones adrenaline and noradrenaline and has been detected in the freshwater environment. Similar to its pharmaceutical action, propranolol reduced the heart rate of daphnids.31 Furthermore, several studies demonstrated that it impacts several biochemical markers in freshwater organisms32 and can affect physiological responses, such as swimming behavior and fertility of daphnids.26,33 In our study, exposure to 5, 10, and 20 mg l−1 of propranolol resulted in a concentration-dependent decrease of ingested microparticle per animal by 46%, 57%, and 82%, respectively (Fig. 3A).
On the other hand, exposure to diltiazem had a similar trend of decreasing feeding rate, which was significant only at the highest concentration (Fig. 3A). Diltiazem is a calcium channel blocker commonly used to treat patients with hypertension and rhythm disorders.34 The improper disposal of expired drugs results in their entering the freshwater environment through wastewater and can impact non-target aquatic organisms by inhibiting calcium channels, thus having a potential environmental risk.35 Especially in Daphnia magna, calcium plays a critical role in many physiological processes, such as the adjustment of the heart rate. Studies have shown that exposure to diltiazem impacts the physiology of daphnids by altering the levels of enzyme activities25 and increasing the heart rate and oxygen consumption.34
Acute exposure to 5 and 20 mg l−1 of metformin also had a significant impact on the feeding rate of daphnids. A 15% and 34% decrease were observed, respectively (Fig. 3A). Metformin is an antidiabetic drug used for patients with type 2 diabetes to lower blood glucose or with polycystic ovary syndrome and is widely prescribed worldwide.36 Previous studies determined that the concentrations of this chemical found in the aquatic environment are high compared to the rate of its removal through several processes. Due to that fact, the exposure concentrations are considerably high and can potentially impact the aquatic organisms.37,38 Specifically, acute and chronic exposure of Daphnia magna to metformin decreased the activity of various enzymes of the metabolism25 and increased the activity of several detoxification enzymes, respectively.39 The concentrations of pollutants tested in the developed feeding assay were notably lower compared to the effective concentrations reported in previous studies. This finding serves as compelling evidence for the high sensitivity of the method.40
Exposure to 5, 10, and 20 mg l−1 of nicotine resulted in a decrease in the feeding rate of daphnids by 76% (5 mg l−1) and 99% (10 and 20 mg l−1) (Fig. 3A). Nicotine is a drug and the main ingredient of tobacco commonly used as a lifestyle item of consumption; thus, it is widely found in wastewater and freshwater environments. It impacts the central nervous system and has several adverse effects on human health and aquatic organisms. Specifically, several studies suggested that it modifies the physiology of various marine species, even in sublethal concentrations. Specifically, acute exposure to nicotine affects the reproductive and nervous system of Daphnia magna by causing the production of male offspring and immobility.41,42 Other studies also demonstrated adverse effects on the heart rate and decreased activity of several enzymes.25,29 Additionally, neonicotinoids, insecticides that resemble nicotine, were found to reduce the swimming and thoracic limb activity of Daphnia magna.15 In our study, nicotine caused the most substantial reduction in the feeding rate out of the tested chemicals, probably due to its effect on swimming and thoracic limb activity, which contribute to ingestion.43
The impact on feeding rate was enhanced for daphnids exposed to metals compared to pharmaceuticals. The only exception was lithium chloride, where a significant increase in the ingestion of microparticles was observed. Specifically, exposure to 5 and 10 mg l−1 of lithium increased the feeding rate by 50% and 52%, respectively (Fig. 3A). Exposure to zinc sulfate decreased ingestion by 35%, 75%, and 77% for exposures 5, 10, and 20 mg l−1, and to zirconium chloride by 33%, 42%, and 69%, respectively, with a more gradual dose-dependent decrease in feeding rate (Fig. 3A). Finally, aluminium sulfate decreased the feeding rate by 67%, 63%, and 76%, and cobalt nitrate by 60%, 78%, and 98%, respectively, revealing a concentration-dependent reduction (Fig. 3A). These changes were also reflected in the fluorescence of animals visualized with microscopy localized in the intestine of the animals (Fig. 3B).
Metals can enter the aquatic environment from activities that are either natural (metal-containing rocks and volcanic eruptions), agricultural (fertilizers), or industrial effluents (mining or smelting). Essential heavy metals play an important role in the physiology of organisms at low concentrations, however, they can potentially be hazardous at high concentrations, while non-essential are toxic and can affect several mechanisms in humans and aquatic organisms. Both essential and non-essential metals can accumulate in aquatic organisms through transfer along the food chain, with adverse effects on human health and the aquatic environment. Especially for aquatic organisms, toxic heavy metals can affect their survival, growth, and populations.8,44,45
Even though the impact of metals has been previously examined, a wide range of studies focuses on survival as an endpoint to assess their toxicity on aquatic organisms. To our knowledge, limited studies have used other physiological endpoints, such as feeding behavior, to examine the impact of pollutants after exposure to sublethal concentrations. It has been shown that exposure to metals, even at lower concentrations, impacts phenotypical and physiological endpoints in Daphnia magna. Specifically, the activity of several enzymes was downregulated after exposure to aluminium and lithium,25 and the feeding rate of daphnids was decreased after exposure to low concentrations of zinc sulfate.12 Lithium also affected the growth, reproduction, and feeding rate of other freshwater organisms; nevertheless, there is no significant amount of published data about the effect of lithium on the feeding rate of Daphnia magna.46 Exposure to aluminium had an impact on the age of maturity and, when combined with another metal, had a strong interaction that also reduced growth, the production of neonates, and possibly the ingestion rate, while the highest concentrations of the metals were identified inside the gut of the animals.47 The results of our study corresponded with previous studies that examined the effect of metals on the feeding rate of Daphnia magna. In general, metals can potentially affect the ingestion of aquatic organisms, even at lower concentrations.
4 Conclusions
In summary, the results of this study provide a miniaturized and efficient assay to evaluate the feeding rate of Daphnia magna. This research aims to overcome the limitations of previous studies that examine the impact of pollutants on phenotypical endpoints, such as the feeding rate of aquatic organisms, taking into consideration the number of animals, the concentration of microparticle, and the total volume, to optimize the parameters of the feeding assay. This was based on the ability of daphnids to ingest any particle on their media, including fluorescent microparticles, which carries unique advantages that contribute to the efficacy and sensitivity of the developed assay. As indicated by the results, the number of animals used per replicate was crucial for the ingestion of microparticles in comparison to the volume of the media and the concentration of microparticle. Having defined the optimal parameters across the variables measured for this feeding assay, the effect of pollutants from two main categories (pharmaceuticals and metals) and one stimulant on the feeding rate of daphnids was examined. The concentrations of pollutants tested were mostly below the EC1 for the majority of the chemicals. Additionally, the concentrations of pollutants used in the developed feeding assay were lower (even up to 13 times), than the reported EC50 values in neonates.8,35,48 This emphasizes the high sensitivity of the feeding assay as a method for evaluating the impact on the physiology of freshwater organisms exposed to low concentrations of pollutants. Overall, metals had a more substantial decrease in the ingestion of microparticle than pharmaceuticals, and nicotine had the most notable decrease. Subsequently, the developed method is an efficient approach to assess the impact of pollutants in the ingestion of Daphnia magna and provides comparative results to traditional assays.Author contributions
MG and KP performed all experiments and processed the results. KG and KDR conceived the project and guided the study. All authors participated in the experimental design and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication has emanated from research conducted with the financial support of Science Foundation Ireland under grant number [18/SIRG/5563 Metabolomic approaches in mechanistic toxicology] and the Irish Research Council under grant number [GOIPD/2021/461 Nanoparticle metabolite coronas: a neglected feature with an essential contribution to toxicity]. The authors would like to acknowledge that the graphical abstract was created with https://www.biorender.com/.References
- W. Brack, S. A. Aissa, T. Backhaus, V. Dulio, B. I. Escher, M. Faust, K. Hilscherova, J. Hollender, H. Hollert, C. Müller, J. Munthe, L. Posthuma, T.-B. Seiler, J. Slobodnik, I. Teodorovic, A. J. Tindall, G. d. A. Umbuzeiro, X. Zhang and R. Altenburger, Effect-based methods are key. The European Collaborative Project SOLUTIONS recommends integrating effect-based methods for diagnosis and monitoring of water quality, Environ. Sci. Eur., 2019, 31, 10 CrossRef.
- M. Abdullahi, X. Li, M. A. Abdallah, W. Stubbings, N. Yan, M. Barnard, L. H. Guo, J. K. Colbourne and L. Orsini, Daphnia as a Sentinel Species for Environmental Health Protection: A Perspective on Biomonitoring and Bioremediation of Chemical Pollution, Environ. Sci. Technol., 2022, 56(20), 14237–14248 CrossRef CAS PubMed.
- S. I. Dodson and T. Hanazato, Commentary on effects of anthropogenic and natural organic chemicals on development, swimming behavior, and reproduction of Daphnia, a key member of aquatic ecosystems, Environ. Health Perspect., 1995, 103(Suppl 4), 7–11 CrossRef CAS PubMed.
- D. Ebert, Daphnia as a versatile model system in ecology and evolution, EvoDevo, 2022, 13, 16 CrossRef PubMed.
- V. Pinos-Vélez, G. S. Araujo, G. M. Moulatlet, A. Pérez-González, I. Cipriani-Ávila, P. Tripaldi and M. V. Capparelli, Acute Toxicity of Daphnia magna Neonates Exposed to Single and Composite Mixtures of Four Emerging Contaminants, Bull. Environ. Contam. Toxicol., 2022, 110, 14 CrossRef PubMed.
- A. A. Godoy, Á. C. d. Oliveira, J. G. M. Silva, C. C. d. J. Azevedo, I. Domingues, A. J. A. Nogueira and F. Kummrow, Single and mixture toxicity of four pharmaceuticals of environmental concern to aquatic organisms, including a behavioral assessment, Chemosphere, 2019, 235, 373–382 CrossRef CAS PubMed.
- OECD, Test No. 202: Daphnia sp., Acute Immobilisation Test, 2004 Search PubMed.
- A. Okamoto, M. Yamamuro and N. Tatarazako, Acute toxicity of 50 metals to Daphnia magna, J. Appl. Toxicol., 2015, 35, 824–830 CrossRef CAS PubMed.
- Z. Wang, K. W. Y. Yeung, G.-J. Zhou, M. M. N. Yung, C. E. Schlekat, E. R. Garman, F. Gissi, J. L. Stauber, E. T. Middleton, Y. Y. Lin Wang and K. M. Y. Leung, Acute and chronic toxicity of nickel on freshwater and marine tropical aquatic organisms, Ecotoxicol. Environ. Saf., 2020, 206, 111373 CrossRef CAS PubMed.
- J.-S. Bae and H. S. Freeman, Aquatic toxicity evaluation of new direct dyes to the Daphnia magna, Dyes Pigm., 2007, 73, 81–85 CrossRef CAS.
- Y. Verma, Acute toxicity assessment of textile dyes and textile and dye industrial effluents using Daphnia magna bioassay, Toxicol. Ind. Health, 2008, 24, 491–500 CrossRef CAS PubMed.
- E. Lari, P. Gauthier, E. Mohaddes and G. G. Pyle, Interactive toxicity of Ni, Zn, Cu, and Cd on Daphnia magna at lethal and sub-lethal concentrations, J. Hazard. Mater., 2017, 334, 21–28 CrossRef CAS PubMed.
- A. Agatz, T. A. Cole, T. G. Preuss, E. Zimmer and C. D. Brown, Feeding Inhibition Explains Effects of Imidacloprid on the Growth, Maturation, Reproduction, and Survival of Daphnia magna, Environ. Sci. Technol., 2013, 47, 2909–2917 CrossRef CAS PubMed.
- C. Barata, P. Alañon, S. Gutierrez-Alonso, M. C. Riva, C. Fernández and J. V. Tarazona, A Daphnia magna feeding bioassay as a cost effective and ecological relevant sublethal toxicity test for Environmental Risk Assessment of toxic effluents, Sci. Total Environ., 2008, 405, 78–86 CrossRef CAS PubMed.
- A. Bownik, Daphnia swimming behaviour as a biomarker in toxicity assessment: A review, Sci. Total Environ., 2017, 601–602, 194–205 CrossRef CAS PubMed.
- A. Bownik and B. Pawlik-Skowronska, Early indicators of behavioral and physiological disturbances in Daphnia magna (Cladocera) induced by cyanobacterial neurotoxin anatoxin-a, Sci. Total Environ., 2019, 695, 133913 CrossRef CAS PubMed.
- G. Bitton, K. Rhodes, B. Koopman and M. Cornejo, Short-term toxicity assay based on daphnid feeding behavior, Water Environ. Res., 1995, 67, 290–293 CrossRef CAS.
- J. L. Hite, A. C. Pfenning-Butterworth, R. E. Vetter and C. E. Cressler, A high-throughput method to quantify feeding rates in aquatic organisms: A case study with Daphnia, Ecol. Evol., 2020, 10, 6239–6245 CrossRef PubMed.
- K. Grintzalis, W. Dai, K. Panagiotidis, A. Belavgeni and M. R. Viant, Miniaturising acute toxicity and feeding rate measurements in Daphnia magna, Ecotoxicol. Environ. Saf., 2017, 139, 352–357 CrossRef CAS PubMed.
- Y.-H. Zhang, R.-Y. Gao, Z.-J. Wang, Q.-Q. Shao, Y.-W. Hu, H.-B. Jia, X.-J. Liu, F.-Q. Dong, L.-M. Fu and J.-P. Zhang, Daphnia magna uptake and excretion of luminescence-labelled polystyrene nanoparticle as visualized by high sensitivity real-time optical imaging, Chemosphere, 2023, 326, 138341 CrossRef CAS PubMed.
- P. A. Rublee and C. L. Gallegos, Use of fluorescently labelled algae (FLA) to estimate microzooplankton grazing, Mar. Ecol.: Prog. Ser., 1989, 51, 221–227 CrossRef.
- S. Reynaldi, S. Duquesne, K. Jung and M. Liess, Linking feeding activity and maturation of Daphnia magna following short-term exposure to fenvalerate, Environ. Toxicol. Chem., 2006, 25, 1826–1830 CrossRef CAS PubMed.
- P. M. Canniff and T. C. Hoang, Microplastic ingestion by Daphnia magna and its enhancement on algal growth, Sci. Total Environ., 2018, 633, 500–507 CrossRef CAS PubMed.
- Y. S. Eltemsah and T. Bohn, Acute and chronic effects of polystyrene microplastics on juvenile and adult Daphnia magna, Environ. Pollut., 2019, 254, 112919 CrossRef CAS PubMed.
- A. Michalaki, A. R. McGivern, G. Poschet, M. Buttner, R. Altenburger and K. Grintzalis, The Effects of Single and Combined Stressors on Daphnids-Enzyme Markers of Physiology and Metabolomics Validate the Impact of Pollution, Toxics, 2022, 10(10), 604 CrossRef CAS PubMed.
- L. L. de Oliveira, S. C. Antunes, F. Goncalves, O. Rocha and B. Nunes, Acute and chronic ecotoxicological effects of four pharmaceuticals drugs on cladoceran Daphnia magna, Drug Chem. Toxicol., 2016, 39, 13–21 CrossRef PubMed.
- K. A. De Schamphelaere, M. Canli, V. Van Lierde, I. Forrez, F. Vanhaecke and C. R. Janssen, Reproductive toxicity of dietary zinc to Daphnia magna, Aquat. Toxicol., 2004, 70, 233–244 CrossRef CAS PubMed.
- L. M. Gomez-Olivan, M. Galar-Martinez, S. Garcia-Medina, A. Valdes-Alanis, H. Islas-Flores and N. Neri-Cruz, Genotoxic response and oxidative stress induced by diclofenac, ibuprofen and naproxen in Daphnia magna, Drug Chem. Toxicol., 2014, 37, 391–399 CrossRef CAS PubMed.
- I. Fekete-Kertész, Z. Kunglne-Nagy and M. Molnár, Ecological impact of micropollutants on aquatic life determined by an innovative sublethal endpoint Daphnia Magna heartbeat rate, Carpathian J. Earth Environ. Sci., 2016, 11, 345–354 Search PubMed.
- M. Nkoom, G. Lu, J. Liu, H. Dong and H. Yang, Bioconcentration, behavioral, and biochemical effects of the non-steroidal anti-inflammatory drug diclofenac in Daphnia magna, Environ. Sci. Pollut. Res. Int., 2019, 26, 5704–5712 CrossRef CAS PubMed.
- T. Y. Jeong, D. Yoon, S. Kim, H. Y. Kim and S. D. Kim, Mode of action characterization for adverse effect of propranolol in Daphnia magna based on behavior and physiology monitoring and metabolite profiling, Environ. Pollut., 2018, 233, 99–108 CrossRef CAS PubMed.
- B. V. R. Pereira, G. N. Matus, M. J. Costa, A. Santos, E. C. M. Silva-Zacarin, J. B. do Carmo and B. Nunes, Assessment of biochemical alterations in the neotropical fish species Phalloceros harpagos after acute and chronic exposure to the drugs paracetamol and propranolol, Environ. Sci. Pollut. Res. Int., 2018, 25, 14899–14910 CrossRef CAS PubMed.
- M. E. Nielsen and P. Roslev, Behavioral responses and starvation survival of Daphnia magna exposed to fluoxetine and propranolol, Chemosphere, 2018, 211, 978–985 CrossRef CAS PubMed.
- D. Steinkey, E. Lari, S. G. Woodman, R. Steinkey, K. H. Luong, C. S. Wong and G. G. Pyle, The effects of diltiazem on growth, reproduction, energy reserves, and calcium-dependent physiology in Daphnia magna, Chemosphere, 2019, 232, 424–429 CrossRef CAS PubMed.
- Y. Kim, K. Choi, J. Jung, S. Park, P. G. Kim and J. Park, Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea, Environ. Int., 2007, 33, 370–375 CrossRef CAS PubMed.
- C. J. Bailey, Metformin: historical overview, Diabetologia, 2017, 60, 1566–1576 CrossRef CAS PubMed.
- G. A. Elizalde-Velazquez and L. M. Gomez-Olivan, Occurrence, toxic effects and removal of metformin in the aquatic environments in the world: Recent trends and perspectives, Sci. Total Environ., 2020, 702, 134924 CrossRef CAS PubMed.
- A. A. Godoy, I. Domingues, A. J. Arsenia Nogueira and F. Kummrow, Ecotoxicological effects, water quality standards and risk assessment for the anti-diabetic metformin, Environ. Pollut., 2018, 243, 534–542 CrossRef CAS PubMed.
- K. O’Rourke, B. Engelmann, R. Altenburger, U. Rolle-Kampczyk and K. Grintzalis, Molecular Responses of Daphnids to Chronic Exposures to Pharmaceuticals, Int. J. Mol. Sci., 2023, 24(4), 4100 CrossRef PubMed.
- M. Markiewicz, C. Jungnickel, S. Stolte, A. Białk-Bielińska, J. Kumirska and W. Mrozik, Ultimate biodegradability and ecotoxicity of orally administered antidiabetic drugs, J. Hazard. Mater., 2017, 333, 154–161 CrossRef CAS PubMed.
- K.-F. Chen, S.-Y. Huang, Y.-T. Chung, K.-S. Wang, C.-K. Wang and S.-H. Chang, Detoxification of nicotine solution using Fe0-based processes: toxicity evaluation by Daphnia magna neonate and embryo assays, Chem. Eng. J., 2018, 331, 636–643 CrossRef CAS.
- A. L. Oropesa, A. M. Floro and P. Palma, Toxic potential of the emerging contaminant nicotine to the aquatic ecosystem, Environ. Sci. Pollut. Res. Int., 2017, 24, 16605–16616 CrossRef PubMed.
- M. J. Beaton, D. Becker, J. K. Colbourne, M. Cordellier, E. Decaestecker, A. A. Kotov, C. M. Peters, M. E. Pfrender, J. R. Shaw, N. N. Smirnov, E. Turner, K. Van Damme and B. Zeis, in Physiology of the Cladocera, ed. N. N. Smirnov, Academic Press, 2nd edn, 2017, p. ix, DOI:10.1016/B978-0-12-805194-8.01002-3.
- H. Ali, E. Khan and I. Ilahi, Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation, J. Chem., 2019, 2019, 6730305 Search PubMed.
- X.-J. Liu, I. H. Ni and W.-X. Wang, Trophic transfer of heavy metals from freshwater zooplankton Daphnia magna to zebrafish Danio reiro, Water Res., 2002, 36, 4563–4569 CrossRef CAS PubMed.
- L. A. Kszos, J. J. Beauchamp and A. J. Stewart, Toxicity of Lithium to Three Freshwater Organisms and the Antagonistic Effect of Sodium, Ecotoxicology, 2003, 12, 427–437 CrossRef CAS PubMed.
- N. R. Brun, P. D. Fields, S. Horsfield, L. Mirbahai, D. Ebert, J. K. Colbourne and K. Fent, Mixtures of Aluminum and Indium Induce More than Additive Phenotypic and Toxicogenomic Responses in Daphnia magna, Environ. Sci. Technol., 2019, 53, 1639–1649 CrossRef CAS PubMed.
- M. Cleuvers, Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid, Ecotoxicol. Environ. Saf., 2004, 59, 309–315 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |